Abstract
Lung Protective mechanical ventilation (MV) of critically ill adults and children is lifesaving but it may decrease diaphragm contraction and promote Ventilator Induced Diaphragm Dysfunction (VIDD). An ideal MV strategy would balance lung and diaphragm protection. Building off a Phase I pilot study, we are conducting a Phase II controlled clinical trial that seeks to understand the evolution of VIDD in critically ill children and test whether a novel computer-based approach (Real-time Effort Driven ventilator management (REDvent)) can balance lung and diaphragm protective ventilation to reduce time on MV. REDvent systematically adjusts PEEP, FiO2, inspiratory pressure, tidal volume and rate, and uses real-time measures from esophageal manometry to target normal levels of patient effort of breathing. This trial targets 300 children with pulmonary parenchymal disease. Patients are randomized to REDvent vs. usual care for the acute phase of MV (intubation to first Spontaneous Breathing Trial (SBT)). Patients in either group who fail their first SBT will be randomized to REDvent vs usual care for weaning phase management (interval from first SBT to passing SBT). The primary clinical outcome is length of weaning, with several mechanistic outcomes. Upon completion, this study will provide important information on the pathogenesis and timing of VIDD during MV in children and whether this computerized protocol targeting lung and diaphragm protection can lead to improvement in intermediate clinical outcomes. This will form the basis for a larger, Phase III multi-center study, powered for key clinical outcomes such as 28-day ventilator free days.
Keywords: MeSH Terms: Ventilator-Induced Lung Injury, ventilators, Mechanical, Ventilator Weaning, Work of Breathing, Pediatrics
Introduction
Mechanical Ventilation (MV) necessitates careful attention to prevent complications. Over the past 30 years, lung-protective ventilation has been a focus, particularly for adults and children with Acute Respiratory Distress Syndrome (ARDS). This includes: Positive End Expiratory Pressure (PEEP) to prevent atelectrauma, limiting tidal volume and driving pressure to prevent over-distension, and use of permissive hypercapnia. To achieve lung protection, patients are often sedated or undergo neuromuscular blockade. This results sub-physiologic levels of patient effort and diaphragm contraction, which contributes to Ventilator Induced Diaphragm Dysfunction (VIDD). VIDD is associated with longer length of ventilation, higher rates of weaning and extubation failure, and higher post-Intensive Care Unit (ICU) morbidity and mortality. (1–7)
Alternatively, when patients have very high degrees of effort and diaphragm contraction, lung injury can be exacerbated through a variety of mechanisms together termed patient self-inflicted lung injury. These mechanisms relate to large negative swings in pleural pressures resulting in atelectrauma, high total transpulmonary driving pressure, mechanical stretch of the alveoli, patient-ventilator asynchrony, and regional maldistribution of ventilation.(8) Furthermore, supra-physiologic patient effort also appears to have negative effects on diaphragm architecture and function.(3, 9) Ideally, MV strategies should provide lung protective levels of PEEP, driving pressure, and tidal volume while still promoting physiologic levels of patient effort of breathing.
However, implementing a MV strategy that is both lung and diaphragm protective is challenging. Computerized decision support (CDS) offers advantages in circumstances where complex decisions need to be made to weigh potentially competing risks, depending on the physiologic state of the patient. We employ such an approach using a CDS tool that promotes lung protective ventilation protocols for PEEP, tidal volume, and inspiratory pressure coupled with a target of maintaining patient effort of breathing in a physiologic range (as measured by esophageal manometry) whenever spontaneous breathing is permitted. This intervention is called Real-time Effort Driven ventilator management (REDvent), and in initial phases is targeting children with acute respiratory failure, although the principles and techniques will likely be applicable to adults.
Preliminary Studies
During usual care ventilation, there are lost opportunities to promote lung protective ventilation (10, 11) and reduce ventilator settings.(11),(12) Ventilator driving pressures are often higher than necessary and respiratory alkalosis is common, preventing meaningful patient effort. (13),(14–17) Furthermore, while practitioners frequently target spontaneous breathing during the weaning phase, the level of ventilator assistance is often high, and patient breathing effort is 2–4 times below the normal physiologic range of extubated patients. (3, 18) This sub-physiologic patient effort likely potentiates diaphragm weakness.
Accurate quantification of the severity of diaphragm dysfunction is understudied in pediatrics. While diaphragm ultrasound can provide corroborative data about architectural changes, ultrasound does not directly measure strength. (3, 19–22) Direct measures of respiratory muscle strength using maximal inspiratory pressure during airway occlusion (PiMax) either of the airway or esophagus are regarded as the most appropriate tests in adults.(23, 24) When patients are intubated, there is divergence in the literature as to whether maximal voluntary efforts can be guaranteed(2, 23, 25–28). Alternatively, some investigators advocate twitch stimulation of the phrenic nerve, measuring change in airway pressure in response to a standardized brief stimulation. This provides a measure of diaphragm strength, although the stimulation is not sufficient for maximal activation and therefore is not directly measuring PiMax (23, 29). Although twitch stimulation has been applied in a limited capacity in young children, it has high variability and limited reproducibility.(30–35) The measurement of PiMax can provide insight into respiratory muscle function. In an analysis of 409 patients at the time of extubation, we found that low PiMax (both airway (aPiMax) and esophageal (ePiMax)) measured during airway occlusion while the child is breathing spontaneously, was associated with re-intubation. (36) A trained provider performed airway occlusion maneuvers, ensuring that the child was at end-exhalation and that the airway remained occluded for 3–5 consecutive breaths.(28) There was a dose response relationship between lower aPiMax and re-intubation risk, and children with aPiMax < 30cm H20 (35% of the population) had a nearly 3 fold higher risk of intubation.(37) This suggests that respiratory muscle weakness is common in children and is associated with adverse outcome.
The CDS tool used by REDvent is an electronic protocol that makes recommendations at user-set time intervals, using a pediatric modification of the Acute Respiratory Distress Syndrome Network (ARDSNet) protocol for pressure control ventilation.(38) We have demonstrated that pediatric critical care practitioners generally agree on the recommendations generated by the modified protocol.(39, 40) If the patient has spontaneous efforts, then this protocol also implements a rule set using esophageal manometry that adjusts recommendations to promote physiologic levels of patient effort. The feasibility of this REDvent protocol was tested in 32 MV children with Pediatric ARDS (PARDS) as part of a Phase I intervention only pilot study. This study identified that protocol recommendations had high rates of adherence (> 75%). REDvent patients compared to historical controls had PEEP managed more in line with the ARDSNet low PEEP/FiO2 grid, had lower tidal volume, and lower delta pressure (peak inspiratory pressure-PEEP). Patients managed with the REDvent protocol had on average 3 fewer days on MV with no significant difference in survival or re-intubation compared to matched historical controls.(41)
Study Methods for the REDvent Phase II Randomized Controlled Trial (RCT)
Main Study Aims
This is a single-center Phase II RCT (150 children per arm) using REDvent (intervention) as compared with usual care (control) ventilator management including a standardized daily Spontaneous Breathing Trial (SBT) (Funding: NIH/NHLBI R01HL124666). The central hypothesis is that REDvent will reduce VIDD, leading to shorter time on MV. The primary question is to determine if REDvent acute and/or weaning phase protocols can shorten the duration of weaning from MV (primary outcome). We expect that patients randomized to receive REDvent will experience a shorter duration of weaning compared to usual care. Secondary outcomes include 28-day ventilator free days and extubation failure.
Additional research questions surround (1) how changes to direct measures of respiratory muscle strength, load, effort, and architecture throughout the duration of MV are related to weaning outcomes, and if these factors differ between control and intervention groups as well as (2) to determine if patient effort of breathing during both acute and weaning phases of MV is independently associated with the development of VIDD. Enrollment began in late 2017 and is anticipated to continue through 2022.
Inclusion Criteria
Children > 1 month (at least 44 weeks corrected gestational age) and ≤ 18 years of age AND
Supported on MV for pulmonary parenchymal disease (radiographic evidence of alveolar or interstitial opacifications with a clinical risk factor for lung disease e.g. pneumonia, acute respiratory distress syndrome, aspiration, etc.) with Oxygen Saturation Index (OSI= (FiO2 * Mean Airway Pressure*100)/SpO2) ≥ 5 or Oxygenation Index (OI= FiO2 * Mean Airway Pressure*100)/PaO2)) ≥4 (42) AND
Within 48 hours of initiation of invasive MV (72 hours if transferred from another institution)
Exclusion Criteria
Contraindications to an esophageal catheter (i.e. severe mucosal bleeding, nasal encephalocele, trans-sphenoidal surgery) OR
Contraindications to use of Respiratory Inductance Plethysmography (RIP) bands (i.e. omphalocele, chest immobilizer or cast) OR
Conditions precluding diaphragm ultrasound measurement (i.e. abdominal wall defects, pregnancy) OR
Conditions precluding conventional methods of weaning (i.e., status asthmaticus, severe lower airway obstruction, critical airway, intracranial hypertension, Extra Corporeal Life Support (ECLS), limitation of care, severe chronic respiratory failure, spinal cord injury above lumbar region, cyanotic heart disease (unrepaired or palliated) OR
Primary Attending physician refusal
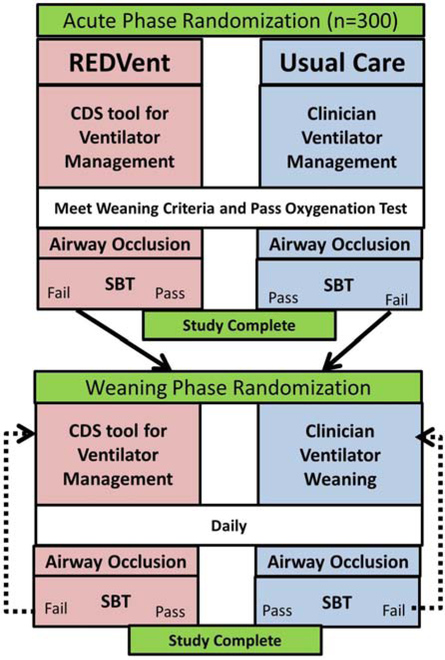
This study seeks to understand whether it is important to implement these lung and diaphragm protective protocols near initiation of MV (acute phase) or if there is also benefit during the weaning phase of ventilation. As such, acute phase randomization occurs upon study enrollment, and patients who fail the first SBT undergo a weaning phase randomization (Figure 1).
Figure 1:
High level overview of study protocol in both acute and weaning phases.
Acute Phase
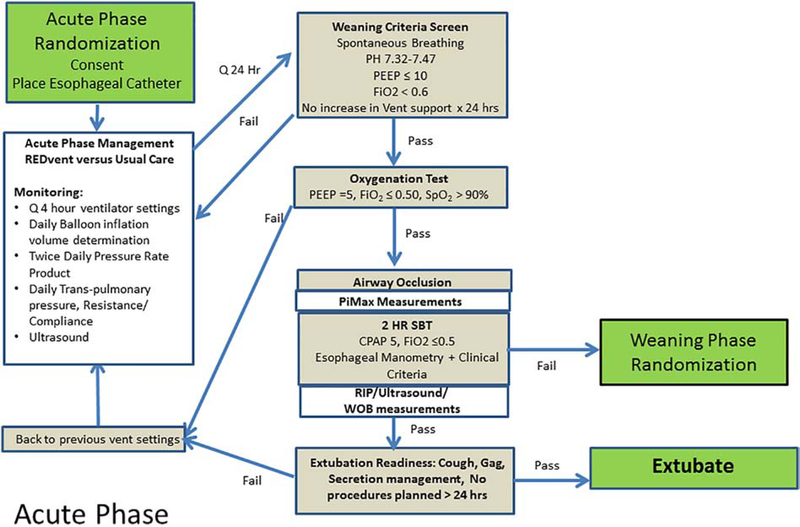
The acute phase is defined as the time from intubation until the patient meets weaning criteria,(43, 44) and passes the initial oxygenation test (decrease PEEP to 5 cmH2O and FiO2 to 0.5, Figure 2). All patients are screened daily to determine if they meet weaning criteria, and if they are eligible for the oxygenation test.
Figure 2:
Acute vs. weaning phase interventions and monitoring for both intervention and control patients.
Intervention Arm (REDvent-acute):
Patients will be managed in a synchronized intermittent mandatory ventilation mode with pressure control plus Pressure Support (PS) with a CDS tool recommending changes to ventilator settings every 4 hours or with a new blood gas. Details of the rules behind the actual protocols are in the appendix. PEEP/FiO2 management is based on the low PEEP/FiO2 table from the ARDSNet protocol.(38) Ventilation is changed based on pH range, peak inspiratory pressure, and ventilator rate. When the patient is breathing spontaneously during the acute phase, the Pressure Rate Product (PRP, peak to trough change in esophageal pressure*respiratory rate) from esophageal manometry is incorporated into the algorithm, targeting maintaining PRP in a physiologic range of 200–400.
This PRP range was established based on the typical range (25–75%) for patient effort of breathing 60 minutes after successful extubation, using data from approximately 400 mechanically ventilated critically ill children.(45, 46) This range of PRP corresponds to a pressure-time product range between 75 and 200. In addition, we have also found this range is significantly lower than the typical PRP range for infants with bronchiolitis who are maintained on high flow nasal cannula (> 75% have PRP over400), and slightly lower than the typical PRP range for infants on nasal CPAP (47, 48). Therefore, this range appears to result in effort of breathing even lower than what most critical care clinicians would deem acceptable for critically ill children who are not on mechanical ventilation. While it is possible that respiratory muscle oxygen consumption could be increased near the upper limit of this range, in our previous pilot study we found that lower targets or a more narrow range (i.e. keeping PRP close 200) resulted in very frequent titrations of ventilator support which was difficult to implement in clinical practice. Furthermore, normal variation in breathing pattern and in response to stimulation in infants and young children often results in PRP changes of 100–200, making it important to maintain a slightly larger range for clinical acceptability.
The esophageal balloon is inflated to a prescribed volume every 4 hours prior to assessment, based on an optimal filling volume algorithm. The median PRP over 10–20 breaths during calm periods of breathing (i.e. not agitated, not recently suctioned) is entered into the CDS tool. High Frequency Oscillatory (HFO) Ventilation can be used as a rescue therapy as per the decision of the bedside clinicians, but HFO management is protocolized using the HFOV CDS tool (expected use: 10–15% in this cohort). This HFO protocol has a MAP/FiO2 table, and also recommends alterations in Power (Amplitude) and Frequency (Hertz) based on pH.
Control Arm:
Ventilator management will be per usual care until the patient meets weaning criteria and passes the oxygenation test (Figure 2).
Regardless of treatment group, patients will have an esophageal manometry catheter placed. Daily measures of patient effort (PRP), transpulmonary pressures, and diaphragm thickness and contractile activity will occur. Once the patient passes the oxygenation test, an airway occlusion maneuver will be performed to measure neuromuscular strength (PiMax), using previously validated techniques which consist of continuous airway occlusion at end-exhalation for 3–5 breath attempts (37, 49, 50). Patients will subsequently undergo an SBT, during which RIP bands will be placed to monitor thoraco-abdominal asynchrony. The primary study outcome (length of the weaning phase) is defined as the time from initiation of the first SBT until successful passing of an SBT (or extubation, whichever comes first). Patients who fail the initial SBT will move on to the weaning phase, and will undergo the weaning phase randomization to allocate treatment or control arms for weaning management.
Spontaneous Breathing Trials (SBTs)
SBTs are performed on Continuous Positive Airway Pressure (CPAP) of 5 cm H20 (without PS), with FiO2 ≤ 0.5. If any failure criteria are met during the 2-hour SBT (Table 1), the patient will be labeled as failing the SBT for study purposes. At this point, the clinical team may choose to stop the SBT, and the patient is returned to the previous ventilator settings, and weaning phase randomization will occur. The clinical team may alternatively choose to continue the SBT or to extubate the patient. If the patient is not extubated within 6 hours of SBT failure, then weaning phase randomization will occur.
Table 1:
Spontaneous breathing trial passage criteria
| Variable | Failure within 2 hours |
|---|---|
| pH (arterial or capillary) | < 7.32 |
| End tidal CO2 | ↑10 mmHg from baseline |
| Oxygenation | FiO2 >0.5 and SpO2 <90% on PEEP = 5 cmH2O |
| Heart rate | ↑ > 40 BPM over baseline |
| Rapid Shallow Breathing Pattern (RSBI) (bpm/ml/kg) | ≥ 12 |
| Pressure Rate Product (PRP) | >500 |
| Retractions | Moderate or Severe Retractions |
If the patient successfully passes the SBT, the primary outcome is achieved. However, the decision to extubate is left to the clinical team. If the patient is not extubated within 6 hours of passing the SBT, acute phase management (to whichever group they were randomized) is resumed until extubation. An SBT is repeated daily until extubation. The reasons for not extubating after passage of an SBT are collected (i.e. inadequate cough, gag, handling secretions, feedings not interrupted, procedures planned).
Weaning Phase
The weaning phase is defined as the time from the first SBT until the patient successfully passes an SBT. SBTs are performed daily during the weaning phase, along with measurement of PiMax with airway occlusion maneuvers.
Intervention Arm (REDvent-weaning):
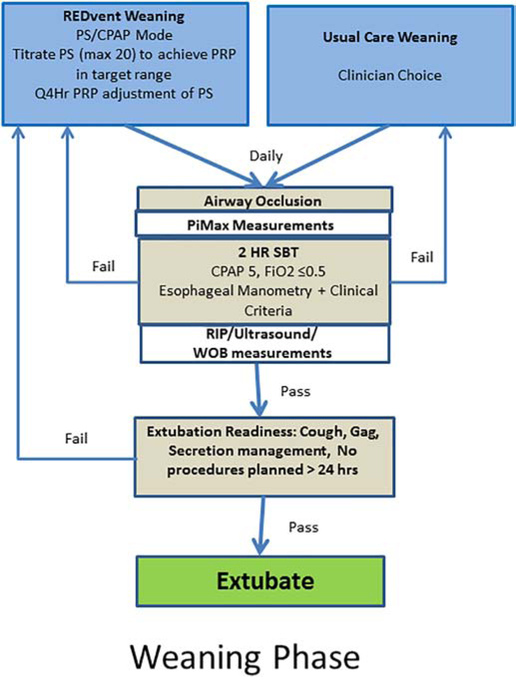
Patients will be managed in a PS/CPAP mode of ventilation and PRP will be monitored continuously, adjusting PS (to a max of 20 cmH20) every 4 hours to maintain PRP in the target range (Figure 3). PEEP may be adjusted between 5 and 10 cmH20. Weaning phase intervention continues until extubation.
Figure 3:
Weaning phase interventions
Control Arm:
Ventilator management will be per usual care as determined by the treating clinical team until the patient is extubated.
The decision to use non-invasive ventilation and other post-extubation management will not be protocolized, regardless of study arm. Suspension of the study intervention is permitted for up to 12 hours for invasive procedures, or rapid changes in patient status. If the intervention is suspended for > 12 hours, it is considered a protocol violation and continued participation in the study will be discussed with the primary attending physician. For patients in the weaning phase who have suspension of the protocol and no longer meet weaning criteria at the 12-hour mark (Figure 2), the acute phase management is resumed. The patient will be managed as per their pre-assigned acute phase group. Once weaning criteria are met, an SBT will again be performed. If the patient fails the SBT, then the weaning phase intervention to which they were previously randomized is resumed. If the patient has not passed the SBT by day 28 after enrollment, study interventions and daily measurements will be terminated. All ventilator management will be as per usual care. Clinical outcomes will continue to be followed. Patients who develop exclusion criteria after study enrollment that preclude continuation of the study interventions (i.e. use of ECLS, new condition requiring removal of esophageal catheter) will have the study protocol and measurements terminated, but clinical outcomes will continue to be followed and analysis will be as per Intention-To-Treat (ITT).
Data Collection
Demographics, clinical variables, and outcomes will be measured as detailed in Table 2. To ensure the adequacy of randomization and understand the risk factors that may contribute to neuromuscular weakness and weaning failure, we will gather detailed, serial data for variables that may be related.(1–7) Identical measurements of respiratory parameters will occur for all patients enrolled in the study (regardless of study arm) (1) serially during both acute and weaning phases, (2) during airway occlusion prior to SBTs, and (3) during SBTs.
Table 2:
Data collection timeline
| Enrollment | Acute (Daily) | Weaning (Daily) | SBT | Post-Extubation | |
|---|---|---|---|---|---|
| Demographics (age, race, gender, past medical history, primary diagnoses, comorbidities, PRISM-III-12 severity of illness scores, height, weight) | |||||
| Clinical Variables (total dose of sedatives, analgesics, highest/lowest/modal sedation scores, pain scores, corticosteroids, aminoglycosides, fluid balance, caloric and protein intake, hypoxemia markers, dead space markers, ventilator type and settings (q6h), blood gasses (all), inotropes/vasopressors, organ failure scores (PELOD), procedures) | |||||
| Effort of Breathing and Respiratory Load | |||||
| Esophageal Manometry: PRP, PTP, TTI, Pi | |||||
| Respiratory Inductance Plethysmography (51): Phase Angle (PA) | |||||
| Ultrasound: Diaphragm Contractile Activity (DCA) | |||||
| Spirometry: Resistance, Dynamic and Static Compliance | |||||
| Respiratory Muscle Strength (during airway occlusion) | |||||
| Esophageal Manometry: Esophageal PiMax (ePiMax) | |||||
| Airway Pressure: Airway PiMax (aPiMax) | |||||
| Ultrasound: DCA during airway occlusion | |||||
| Diaphragm Architecture (Thickness on Exhalation) | |||||
| Outcomes (Weaning Duration, VFDs and components, re-intubation, Non-Invasive Ventilation use and duration, ICU, Hospital, 90-day mortality) | |||||
Co-interventions
Sedation management is the same in both arms and includes targeted sedation guided by the State Behavioral Scale (SBS).(52) Inhaled nitric oxide is permitted as per the discretion of the clinical team. For patients in the intervention group, weaning of nitric oxide will be recommended once FiO2 is reduced below 0.6, which is in line with the standard clinical practice in the PICU.
Statistical Considerations
Randomization Strategy and Blinding
For the acute phase, subjects will be block randomized to either arm stratified by age group: infant (30 days −365 days), child (366 days to ≤8 years), and older child/adolescent (9–18 years); and immune suppression (congenital or acquired conditions that result in marked inability to respond to antigenic stimuli). Block randomization will be based on random block sizes of 4, 6 and 8 within the strata above, and are loaded into a central database for implementation. Weaning phase randomization is block randomized by acute phase group, and age, using the same methodology. Although blinding is not possible given the open label nature of the intervention, analysis will be blinded to treatment groups.
Analysis Plan
Baseline characteristics at the time of randomization will be computed for each treatment group (REDvent and usual care) and within each phase (Acute and Weaning). For all analyses, assumptions for data distribution will be assessed, and transformations of the data or non-parametric analysis will be performed as necessary. The mean and standard deviation will be reported for normally distributed continuous variables, the median and interquartile ranges will be reported for non-normally distributed continuous variables. Given this is an RCT, we anticipate balanced baseline characteristics. To evaluate this, we will calculate effect sizes for measures of central tendency (Cohen d, Cramer v) between and across groups. For non-parametric variables, effect sizes defined for the Mann-Whitney U will be calculated to produce r. For frequency counts and percentages the rate ratio will be evaluated to calculate the effect size. Variables that have more than a small effect size (d > 0.2, v > 0.01, or r>0.1, categorical effect size >1.2) indicate potential imbalances in baseline characteristics between groups. These variables will be included in sensitivity analyses after the primary ITT analyses are performed.
Retention, adherence, and missing data will be compared between and across groups. High levels of missing data are not anticipated given the nature of the study. If the missingness of data is determined to be related to the outcome (not missing at random) or related to group or a covariate (missing at random), we will explore the impact of these biases in sensitivity analyses after the primary ITT analyses using multiple imputation processes. Our primary approach to imputing missing data is the Markov Chain Monte Carlo (MCMC) simulation. Statistical Analysis Software (SAS) protocols will be used.
The primary analysis seeks to address if there is difference in length of weaning between: 1) REDvent-acute compared to usual care-acute, 2) REDvent-weaning compared to usual care-weaning, and 3) REDvent-acute and weaning (combined) compared to usual care acute and weaning (combined). Analyses of these aims will follow the ITT principle. The primary analyses will compare median weaning duration between groups using a Mann-Whitney U test, or a t-test with transformation as necessary. The effect size (r) will also be computed to assess the magnitude of treatment effect. If imbalances in baseline characteristics are found between or across randomized treatment groups, a Cox proportional hazard ratio will be performed to adjust for covariates. The estimates (mean, median, or hazard ratio) and the associated 95% confidence interval, as well as the p-values, will be presented for interpretation.
Power analysis:
For the primary outcome (weaning duration), a 1-day change in length of weaning is considered clinically significant. It is anticipated that up to 20% of patients may not achieve the primary outcome (successful passage of an SBT or extubation due to death or dropout), and these patients will not be included in the primary outcome analysis, but will be included in secondary outcomes. We are targeting an overall sample size of 300 patients, with a minimum of 240 patients (120 per arm) available for analysis of the primary outcome. Using the two planned statistical tests above, this sample size would be able to detect a ≥ 1-day change in weaning duration with a two-sided alpha of 0.05 and power of 0.9, or a relative hazard ratio of 1.5 (ratio between control/intervention group) with a power of 0.9 or a hazard ratio of 1.4 with a power of 0.8. Patients who fail the initial SBT will undergo the weaning phase randomization. From our pilot data, approximately 25% of patients exposed to the intervention passed the initial SBT, corroborating previous studies.(43) Anticipating 180 patients (90 per arm) will receive weaning phase interventions, there will be adequate power to detect a ≥ 1 day change in the length of the weaning phase, or a hazard ratio of 1.5 with an alpha of 0.05 and power of 0.8.
The analytic approach for secondary aims and outcomes such as mortality and ventilator free days (Table 2) will follow those described above. For categorical data, a χ2 or Fisher’s exact test will be used to compare difference between groups. Logistic regression will be used to assess dichotomous outcomes, and a multinomial/ordinal logistic regression will be used for categorical outcomes (more than 2 categories) while adjusting for covariates. To assess the association between 2 continuous variables, a Pearson or Spearman correlation will be used, and analysis of covariance (ANCOVA) will be used to adjust for covariates. Generalized Estimating Equation (GEE) will be used when necessary to control for repeated measures. Sensitivity, specificity, and overall discrimination of predictive models will also be reported. Analyses will be performed using the appropriate recent version of the SAS statistical software (SAS Institute Inc, Cary, NC). Power analysis and more detailed analytic plans are provided in the appendix.
Human Subjects and Data Monitoring
The subjects of this study are intubated infants and children who will not be able to consent for their own participation in this study. Parents will be informed about the study and given an opportunity to voluntarily give their permission for their child to participate. Assent of subjects of appropriate age and capacity will be obtained after sedation has cleared to ensure permission for continued data collection until hospital discharge. The protocol has been approved by the Children’s Hospital Los Angeles (CHLA) Institutional Review Board, as well as an independent Data Safety and Monitoring Board.
All outcomes are independently extracted and verified by at least two members of the research team. A vast majority of the data are collected through automated electronic feeds. A trained study data collector uses extracts from the electronic feeds in conjunction with data in the electronic health care record to populate study specific Case Report Forms (CRFs). Data collection occurs in real-time. Respiratory measurements are entered into web-based CRFs at the time of study measurements. In addition, raw data from esophageal manometry and RIP are recorded during each measurement, and the calculations are post-processed to verify the real-time data entry. Ultrasound images are interpreted in real-time and calculations entered into the web-based CRF. In addition, all ultrasound images are uploaded to a secure server, and undergo blinded interpretation by a single provider. Data validity and quality checks are performed weekly. All application software is hosted securely on the CHLA/University of Southern California network, which is protected by several firewalls and security is monitored and audited regularly.
Conclusions
Completion of this Phase II clinical trial will provide important information on whether this computerized protocol targeting lung and diaphragm protection can lead to improvement in physiologic outcomes and intermediate clinical outcomes. This will form the basis for a larger, Phase III multi-center study.
Supplementary Material
Funding Sources:
NIH/NHLBI R01HL124666
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration:
References
- 1.Daniel Martin A, Smith BK, Gabrielli A. Mechanical ventilation, diaphragm weakness and weaning: a rehabilitation perspective. Respir Physiolo Neurobiol 2013; 189: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doorduin J, van Hees HW, van der Hoeven JG, Heunks LM. Monitoring of the respiratory muscles in the critically ill. Am J Respir Crit Care Med 2013; 187: 20–27. [DOI] [PubMed] [Google Scholar]
- 3.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, Rittayamai N, Lanys A, Tomlinson G, Singh JM, Bolz SS, Rubenfeld GD, Kavanagh BP, Brochard LJ, Ferguson ND. Evolution of Diaphragm Thickness during Mechanical Ventilation. Impact of Inspiratory Effort. Am J Respir Crit Care Med 2015; 192: 1080–1088. [DOI] [PubMed] [Google Scholar]
- 4.Heunks LM, Doorduin J, van der Hoeven JG. Monitoring and preventing diaphragm injury. Curr Opin Crit Care 2015; 21: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooijman PE, Beishuizen A, Witt CC, de Waard MC, Girbes AR, Spoelstra-de Man AM, Niessen HW, Manders E, van Hees HW, van den Brom CE, Silderhuis V, Lawlor MW, Labeit S, Stienen GJ, Hartemink KJ, Paul MA, Heunks LM, Ottenheijm CA. Diaphragm muscle fiber weakness and ubiquitin-proteasome activation in critically ill patients. Am J Respir Crit Care Med 2015; 191: 1126–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sieck GC. Muscle weakness in critical illness. Am J Respir Crit Care Med 2015; 191: 1094–1096. [DOI] [PubMed] [Google Scholar]
- 7.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care 2013; 17: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017; 195: 438–442. [DOI] [PubMed] [Google Scholar]
- 9.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, Vorona S, Sklar MC, Rittayamai N, Lanys A, Murray A, Brace D, Urrea C, Reid WD, Tomlinson G, Slutsky AS, Kavanagh BP, Brochard LJ, Ferguson ND. Mechanical Ventilation-induced Diaphragm Atrophy Strongly Impacts Clinical Outcomes. Am J Respir Crit Care Med 2018; 197: 204–213. [DOI] [PubMed] [Google Scholar]
- 10.Newth CJ, S K, K RG, D JM. Variability In Usual Care Mechanical Ventilation For Pediatric Acute Lung Injury. American Journal of Respiratory and Critical Care Medicine 2014; 189: A2613. [Google Scholar]
- 11.Khemani RG, Sward K, Morris A, Dean JM, Newth CJL, (CPCCRN) NCPCCRN. Variability in usual care mechanical ventilation for pediatric acute lung injury: the potential benefit of a lung protective computer protocol. Intensive Care Med 2011; 37: 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll C, Flori H, Tasker RC, Rimensberger P, Randolph A, PALIVE Investigators, PALISI Network, ESPNIC. Acute lung injury in children:therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 2010; 11: 681–689. [DOI] [PubMed] [Google Scholar]
- 13.Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator-induced diaphragmatic dysfunction--human studies confirm animal model findings! Crit Care 2011; 15: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk DJ, Deruisseau KC, Van Gammeren DL, Deering MA, Kavazis AN, Powers SK. Mechanical ventilation promotes redox status alterations in the diaphragm. J Appl Physiol 2006; 101: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 15.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 2008; 358: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 16.Powers SK, DeCramer M, Gayan-Ramirez G, Levine S. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care 2008; 12: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futier E, Constantin JM, Combaret L, Mosoni L, Roszyk L, Sapin V, Attaix D, Jung B, Jaber S, Bazin JE. Pressure support ventilation attenuates ventilator-induced protein modifications in the diaphragm. Crit Care 2008; 12: R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emeriaud G, Larouche A, Ducharme-Crevier L, Massicotte E, Flechelles O, Pellerin-Leblanc AA, Morneau S, Beck J, Jouvet P. Evolution of inspiratory diaphragm activity in children over the course of the PICU stay. Intensive Care Med 2014; 40: 1718–1726. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin CE, Paratz JD, Bersten AD. Diaphragm and peripheral muscle thickness on ultrasound: intra-rater reliability and variability of a methodology using non-standard recumbent positions. Respirology 2011; 16: 1136–1143. [DOI] [PubMed] [Google Scholar]
- 20.DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014; 69: 423–427. [DOI] [PubMed] [Google Scholar]
- 21.Matamis D, Soilemezi E, Tsagourias M, Akoumianaki E, Dimassi S, Boroli F, Richard JC, Brochard L. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 2013; 39: 801–810. [DOI] [PubMed] [Google Scholar]
- 22.Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, Brochard L. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 2012; 38: 796–803. [DOI] [PubMed] [Google Scholar]
- 23.ATS ERS. ATS/ERS Statement on Respiratory Muscle Testing. American Journal of Respiratory and Critical Care Medicine 2002; 166: 518–624. [DOI] [PubMed] [Google Scholar]
- 24.Benditt JO. Esophageal and gastric pressure measurements. Respir Care 2005; 50: 68–75. [PubMed] [Google Scholar]
- 25.Gozal D, Shoseyov D, Keens TG. Inspiratory pressures with CO2 stimulation and weaning from mechanical ventilation in children. Am Rev Respir Dis 1993; 147: 256–261. [DOI] [PubMed] [Google Scholar]
- 26.Harikumar G, Egberongbe Y, Nadel S, Wheatley E, Moxham J, Greenough A, Rafferty GF. Tension-time index as a predictor of extubation outcome in ventilated children. Am J Respir Crit Care Med 2009; 180: 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen AS, Woo MS, Keens TG. How many maneuvers are required to measure maximal inspiratory pressure accurately. Chest 1997; 111: 802–807. [DOI] [PubMed] [Google Scholar]
- 28.Harikumar G, Moxham J, Greenough A, Rafferty GF. Measurement of maximal inspiratory pressure in ventilated children. Pediatr Pulmonol 2008; 43: 1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson AC, Hughes PD, Louise Harris M, Hart N, Ware RJ, Wendon J, Green M, Moxham J. Measurement of twitch transdiaphragmatic, esophageal, and endotracheal tube pressure with bilateral anterolateral magnetic phrenic nerve stimulation in patients in the intensive care unit. Crit Care Med 2001; 29: 1325–1331. [DOI] [PubMed] [Google Scholar]
- 30.Dimitriou G, Greenough A, Moxham J, Rafferty GF. Influence of maturation on infant diaphragm function assessed by magnetic stimulation of phrenic nerves. Pediatr Pulmonol 2003; 35: 17–22. [DOI] [PubMed] [Google Scholar]
- 31.Luo YM, Hart N, Mustfa N, Man WD, Rafferty GF, Polkey MI, Moxham J. Reproducibility of twitch and sniff transdiaphragmatic pressures. Respir Physiolo Neurobiol 2002; 132: 301–306. [DOI] [PubMed] [Google Scholar]
- 32.Man WD, Luo YM, Mustfa N, Rafferty GF, Glerant JC, Polkey MI, Moxham J. Postprandial effects on twitch transdiaphragmatic pressure. Eur Respir J 2002; 20: 577–580. [DOI] [PubMed] [Google Scholar]
- 33.Polkey MI, Kyroussis D, Hamnegard CH, Hughes PD, Rafferty GF, Moxham J, Green M. Paired phrenic nerve stimuli for the detection of diaphragm fatigue in humans. Eur Respir J 1997; 10: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 34.Rafferty GF, Greenough A, Dimitriou G, Kavadia V, Laubscher B, Polkey MI, Harris ML, Moxham J. Assessment of neonatal diaphragm function using magnetic stimulation of the phrenic nerves. Am J Respir Crit Care Med 2000; 162: 2337–2340. [DOI] [PubMed] [Google Scholar]
- 35.Rafferty GF, Mustfa N, Man WD, Sylvester K, Fisher A, Plaza M, Davenport M, Blaney S, Moxham J, Greenough A. Twitch airway pressure elicited by magnetic phrenic nerve stimulation in anesthetized healthy children. Pediatr Pulmonol 2005; 40: 141–147. [DOI] [PubMed] [Google Scholar]
- 36.Sekayan T, Hotz J, Flink R, Iyer NP, Newth CJ, Khemani R. Risk Factors for pediatric extubation failure: the importance of respiratory muscle strength. Crit Care Med 2017; Abstact 2017 Conference: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khemani RG, Sekayan T, Hotz J, Flink RC, Rafferty GF, Iyer N, Newth CJL. Risk Factors for Pediatric Extubation Failure: The Importance of Respiratory Muscle Strength. Critical care medicine 2017; 21: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ARDSnet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. New England Journal of Medicine 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 39.Sward K, N CJL, K RG, D JM. Data-driven knowledge base evaluation: Translating an adult CDS tool for use in pediatric care (Abstract). Journal of the American Medical Informatics Association (JAMIA) 2015; 2015 Annual conference [Google Scholar]
- 40.Sward K, Newth CJ, Page K, Dean JM. Pediatric Intensivist Attitudes About a Ventilator Management Computer Protocol. C57 NEWS FROM THE NICU AND PICU: American Thoracic Society; 2015. p. A4772–A4772. [Google Scholar]
- 41.Khemani R, Hotz J, Newth C. The feasibility and potential benefit of using a computerized real time effort driven ventilator management protocol for children with ARDS. American Journal of Respiratory and Critical Care Medicine 2017; 195: A2823. [Google Scholar]
- 42.Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Group ftPALICC. Pediatric Acute Respiratory Distress Syndrome: Definition, Incidence, and Epidemiology: Proceedings From the Pediatric Acute Lung Injury Consensus Conference. Pediatric Critical Care Medicine 2015; 16: S23–S40. [DOI] [PubMed] [Google Scholar]
- 43.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH, Pediatric Acute Lung I, Sepsis Investigators N. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. Jama 2002; 288: 2561–2568. [DOI] [PubMed] [Google Scholar]
- 44.Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, Pollack M, Zimmerman J, Anand KJ, Carcillo JA, Nicholson CE, Eunice Shriver Kennedy National Institute of Child H, Human Development Collaborative Pediatric Critical Care Research N. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med 2009; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khemani RG, Sekayan T, Hotz J, Flink RC, Rafferty GF, Iyer N, Newth CJL. Risk Factors for Pediatric Extubation Failure: The Importance of Respiratory Muscle Strength. Crit Care Med 2017; 45: e798–e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khemani RG, Hotz J, Morzov R, Flink RC, Kamerkar A, LaFortune M, Rafferty GF, Ross PA, Newth CJ. Pediatric extubation readiness tests should not use pressure support. Intensive Care Med 2016; 42: 1214–1222. [DOI] [PubMed] [Google Scholar]
- 47.Weiler T, Kamerkar A, Hotz J, Ross PA, Newth CJL, Khemani RG. The Relationship between High Flow Nasal Cannula Flow Rate and Effort of Breathing in Children. Journal of Pediatrics 2017; 29: 29. [DOI] [PubMed] [Google Scholar]
- 48.Kamerkar A, Hotz J, Morzov R, Newth CJ, Ross PA, Khemani RG. Comparison of Effort of Breathing for Infants on Nasal Modes of Respiratory Support. Journal of Pediatrics 2017; 30: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khemani RG, Hotz J, Morzov R, Flink R, Kamerkar A, Ross PA, Newth CJ. Evaluating Risk Factors for Pediatric Post-extubation Upper Airway Obstruction Using a Physiology-based Tool. Am J Respir Crit Care Med 2016; 193: 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khemani R, PA R, Newth CJ. Validation of Respiratory Inductance Plethysmography and Esophageal Manometry to Detect Upper Airway Obstruction in Children: An Animal Model. Am J Respir Crit Care Med 2012. [Google Scholar]
- 51.Pandharipande PP, Shintani AK, Hagerman HE, St Jacques PJ, Rice TW, Sanders NW, Ware LB, Bernard GR, Ely EW. Derivation and validation of Spo2/Fio2 ratio to impute for Pao2/Fio2 ratio in the respiratory component of the Sequential Organ Failure Assessment score. Crit Care Med 2009; 37: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curley MA, Wypij D, Watson RS, Grant MJ, Asaro LS, Cheifetz IM, Dodson BL, Franck LS, Gedeit RG, Angus DC, Matthay MA, RESTORE, Network P. Protocolized Sedation vs. Usual Care in Pediatric Patients Mechanically Ventialted for Acute Respiratory Failure. Jama 2015; 313: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.