Description of the invention
The majority of allergic diseases, including asthma, atopic dermatitis and food allergy, are caused by allergen-specific IgE production and Th2-polarized immune responses. Allergic sensitization is caused by DC activation of naïve T cells to generate and expand Th2-biased cellular immune responses, resulting in the production of cytokines such as IL-4, IL-5 and IL-13. Th2 cytokines induce the generation and expansion of allergen-specific IgE by B cells as well as accumulation of mast cells in tissues (Figure 1). Because of the central role Th2 immune responses play in the genesis of allergic disease, it is important to focus on therapeutic approaches capable of suppressing or redirecting these Th2 immune responses.
Figure 1. Modulation of allergic immunity by NE adjuvant.
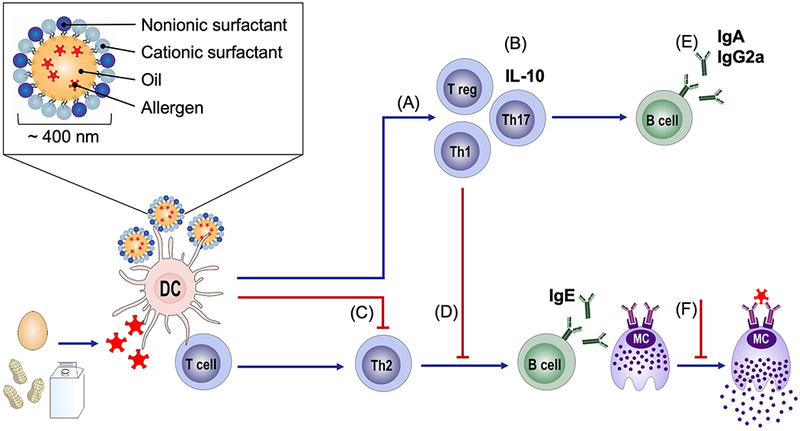
Allergic sensitization results in the differentiation of Th2 cells as well as IgE-producing B cells. Immunization with NE (A) induces Th1 and Th17 cells and (B) Tregs and IL-10-producing cells and (C) inhibits Th2 cells. NE immunization (D) reduces production of allergen-specific IgE while (E) increasing allergen-specific IgA and IgG2a antibodies. Modulation of the allergic immune responses by NE results in protection from reaction upon allergen exposure through (F) decreases in mast cell infiltration into tissues as well as prevention of mast cell degranulation upon allergen exposure. (Inset), Structure of oil-in-water nanoemulsion droplet.
The patent described herein (PCT/US2015/054943) covers the use of nanoemulsion compositions for the stimulation of immune responses capable of suppressing Th2-polarized immunity and reducing allergic disease.1 Nanoemulsions (NE) are nanoscale oil-in-water emulsions composed of a combination of surfactants, oil, ethanol and water. Our group has developed NE adjuvant formulations consisting of soybean oil and a combination of cationic and nonionic surfactants with an average droplet size of 400-500 nm (Figure 1A).2 These formulations provide adjuvant activity by enhancing the delivery of antigens as well as activating innate immune responses to boost immunity. We have previously reported that NE adjuvants can be formulated with a wide variety of antigen types and induce potent Th1 and Th17-polarized mucosal, humoral and cellular immune responses after intranasal administration in both animal models and humans.3,4
The NE-based vaccine adjuvants covered in this patent are capable of suppressing and redirecting established Th2-polarized immunity. Supporting evidence for this patent application was found in murine models of food allergy where we discovered that therapeutic immunization of peanut-sensitized mice with NE and peanut led to protection from allergic reactivity upon exposure to peanut. Based on these observations, we claimed the use of NE compositions for the suppression of allergic disease through the suppression of Th2-polarized immune responses.
Path leading to the invention and recent developments
Vaccines have made significant impacts in global health and although vaccines typically aim to generate or boost immune responses, recent work has demonstrated the ability of adjuvants to modulate pre-existing immunity or even suppress detrimental immune responses. The NE adjuvants described here were initially developed at the University of Michigan as broad-spectrum antimicrobial agents, but NEs proved to be effective mucosal adjuvants. A wide variety of antigen types (recombinant proteins, virus-like particles, whole virus or bacteria) have been formulated into NE droplets through simple mixing, resulting in association of antigen materials with the lipid phase of the droplets. This incorporation increases stability of the immunogen, as antigens are protected from denaturation and degradation.5,6
MF59 and AS03 are other emulsion adjuvants currently used in human vaccines. These adjuvant formulations are composed of squalene and are neutral or negatively charged, in contrast to NE adjuvant which contains soybean oil and is positively charged due to cationic surfactants. These physiochemical differences may drive immune activation via distinct mechanisms of action for these emulsion adjuvants.
While characterizing a NE-adjuvanted hepatitis B (HepB) vaccine we fortuitously discovered that in mice with pre-existing Th2 HepB immunity from an intramuscular alum-adjuvanted HepB vaccine, a single i.n. immunization with NE-adjuvanted HepB induced Th1-associated responses while reducing Th2 responses. The immunization with NE modulated the magnitude and kinetics of the antibody and cellular response indicating that NE was able to redirect established Th2 polarized immunity towards Th1. This led to the hypothesis that immunization with NE and allergens could also be used to suppress the aberrant Th2 immune responses associated with allergic hypersensitivity. This redirection of the allergen-specific immune response away from the harmful Th2-polarized response would be enhanced by induction of cellular responses associated with protection from allergic disease, including IFN-γ and IL-10. Our hypothesis is supported by studies from other investigators that have demonstrated that allergen immunotherapy in grass pollen allergic individuals increases allergen-specific IFN-γ producing cells, correlating with reduced symptoms.7 Additionally, proliferating allergen-specific T cells have been found in healthy non-allergic controls, suggesting that some non-allergic individuals may be protected from allergic manifestations due to the presence of Th1 producing allergen-specific cells.8 Together, this suggests that the induction of allergen-specific Th1-polarized immune responses along with the suppression of Th2 responses may induce long-term protective immunity against clinical allergies.
Next, we evaluated the ability of NE to modulate the Th2 and IgE immune responses responsible for allergic diseases in mouse models of food allergy. NE, when formulated with specific food allergens and administered intranasally three times at monthly intervals, was effective to suppress allergic responses on oral or systemic allergen challenge, providing protection from anaphylaxis in multiple murine models, as reflected by reduction in clinical symptoms, mast cell degranulation, and temperature change upon challenge (Figure 2). This is associated with reduction of Th2 and IgE allergen-specific responses and activation of Th1, Th17 and regulatory T cells as well as IL-10-producing cells (Figure 1 and 2).9 Of interest, the NE significantly enhances mucosal immunity, including IgA antibodies that may neutralize allergen. NE-mediated suppression of Th2 allergic responses and protection against anaphylaxis could be partially blocked by anti-IL10, suggesting a role for IL-10 associated regulatory cells.
Figure 2. Therapeutic immunization with NE modulates allergic immune responses and protects from allergen challenge.9.
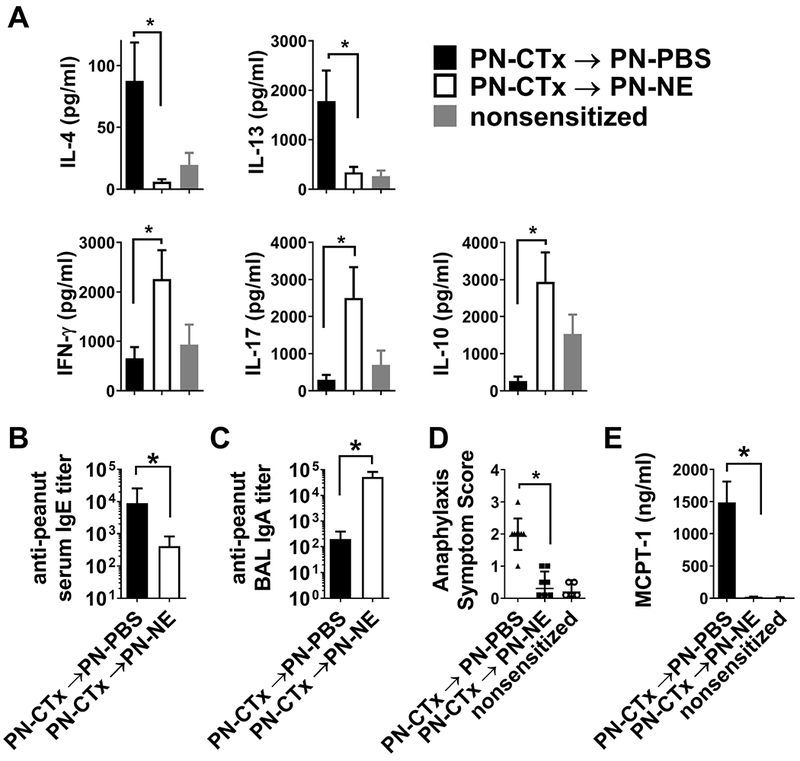
Mice were sensitized orally with peanut and cholera toxin (PN-CTx). (A) Cytokine secretion by mesenteric lymph node cells and (B peanut-specific IgE and IgA were determined after 3 immunizations with with peanut in NE (PN-NE) or peanut in PBS (PN-PBS). Reactivity to oral challenge was determined by (D) scoring of clinical symptoms and (E) quantification of mast cell degranulation as measured by release of MCPT-1 into serum.
Comprehensive evaluation of acute and chronic effects of NE formulations have been performed to assess the safety of the intranasal route of delivery of NE adjuvants. No inflammation has been observed in the nasal cavity in safety studies in mice, rats, guinea pigs and dogs.6 The association of antigen with the oil core of the NE droplets which are taken up by mucosal epithelial and dendritic cells should limit interaction between allergen and mast cells to improve the safety of the intranasal route of allergen immunotherapy.
Conclusion
Allergic disease has become a significant burden on global public health. Until recently, most drugs used to treat allergies simply managed symptoms. New advances in allergen immunotherapy and biological therapies have demonstrated the ability to suppress IgE and the allergic response. Unfortunately, to this point, only a few approaches have demonstrated the persistent modulation of the Th2-polarized immune responses that drive allergic disease, suggesting an absence of immunological memory for the tolerant state. This is especially true for food allergies where allergic reactivity typically returns just weeks after cessation of therapy. In contrast to these studies, we have demonstrated in multiple vaccine models that immunization with NE adjuvant induces long-term immunological memory responses, that could be able to more permanently redirect the immune system away from Th2 immunity, thereby preventing allergic hypersensitivity. Additionally, adjuvant-driven immune modulation could offer a less complex therapeutic regimen compared to current immunotherapies that require daily administration over extended periods of time. As the supporting data for this patent were generated in mouse models, future clinical studies will be necessary to determine if these same effects occur in humans. Because the NE vaccine platform can be combined with virtually any antigen, it provides a platform to investigate any allergic disease associated with aberrant Th2 immune responses. It also could provide an approach to clinical investigations of suppression of allergic disease as the NE adjuvant has successfully completed safety studies in humans.3 The technology described here will advance the understanding of mechanisms to modulate established Th2 immunity and may provide significant therapeutic benefit in alleviating food allergic patients and their families of the burdens of disease through long-lasting suppression of allergy.
Acknowledgments
Grant support: This project has been funded by the National Institute for Allergy and Infectious Disease, National Institutes of Health under Contract No. HHSN272200900031C United States; the Department of Defense Award W81XWH-14-PRMRP-DA, and a Food Allergy Research and Education New Investigator Award to JJO.
References
- 1.Baker JR Jr., Bielinska, Anna U, Smith, Douglas, Makidon, Paul E, O’Konek, Jessica J, Inventor. Nanoemulsion compositions for preventing, suppressing or eliminating allergic and inflammatory disease. Patent WO 2016/057921A1, EP 3204039A4, JP 2017/534608A, US 2017/0246294 A12. [Google Scholar]
- 2.Wong PT, Leroueil PR, Smith DM, et al. Formulation, high throughput in vitro screening and in vivo functional characterization of nanoemulsion-based intranasal vaccine adjuvants. PLoS One . 2015;10(5):e0126120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanberry LR, Simon JK, Johnson C, et al. Safety and immunogenicity of a novel nanoemulsion mucosal adjuvant W805EC combined with approved seasonal influenza antigens. Vaccine . 2012;30(2):307–316. [DOI] [PubMed] [Google Scholar]
- 4.Bielinska AU, Makidon PE, Janczak KW, et al. Distinct pathways of humoral and cellular immunity induced with the mucosal administration of a nanoemulsion adjuvant. J Immunol . 2014;192(6):2722–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamouda T, Sutcliffe JA, Ciotti S, Baker JR Jr. Intranasal immunization of ferrets with commercial trivalent influenza vaccines formulated in a nanoemulsion-based adjuvant. Clin Vaccine Immunol . 2011;18(7):1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makidon PE, Bielinska AU, Nigavekar SS, et al. Pre-clinical evaluation of a novel nanoemulsion-based hepatitis B mucosal vaccine. PLoS One . 2008;3(8):e2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulten V, Tripple V, Sidney J, et al. Association between specific timothy grass antigens and changes in TH1- and TH2-cell responses following specific immunotherapy. J Allergy Clin Immunol . 2014;134(5):1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinz D, Seumois G, Gholami AM, et al. Lack of allergy to timothy grass pollen is not a passive phenomenon but associated with the allergen-specific modulation of immune reactivity. Clin Exp Allergy . 2016;46(5):705–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Konek JJ, Landers JJ, Janczak KW, et al. Nanoemulsion adjuvant-driven redirection of TH2 immunity inhibits allergic reactions in murine models of peanut allergy. J Allergy Clin Immunol . 2018;141(6):2121–2131. [DOI] [PubMed] [Google Scholar]


