Abstract
Background
Nipple-sparing mastectomy (NSM) is increasingly performed for invasive breast cancer. Growing evidence supporting NSM’s oncologic safety has led to its widespread use and broadened indications. Here we examine indications, complications, and long-term outcomes of therapeutic NSM.
Methods
From 2003–2016, women undergoing NSM for invasive cancer or ductal carcinoma in situ (DCIS) were identified from a prospectively maintained database. Patient and disease characteristics were compared by procedure year. Complications were compared by procedure year using generalized mixed effects models accounting for a random surgeon effect. Overall survival and time to recurrence were examined.
Results
Of the 467 therapeutic NSMs, 337 (72%) were invasive cancer, 126 (27%) were DCIS, and 4 (1%) were phyllodes tumors. Median age was 45 years (range 24–75); median follow-up among survivors was 39.4 months. 357 (76.4%) cases were performed in 2011 or after. When comparing NSMs performed before and after 2011, there was a significant increase in NSMs performed for invasive tumors (58% vs 77%, p<.001). There was no difference in family history, genetic mutations, smoking status, neoadjuvant chemotherapy, prior radiation, nodal involvement, or tumor subtype. 21 (4.5%) nipple excisions were performed; of which 14 were performed for cancer at the nipple margin. 44 breasts (9.4%) had complications that required re-operation. 15 patients had locoregional recurrence or distant metastasis.
Conclusions
NSM use for invasive carcinoma has doubled at our institution since 2011, while postoperative complications and recurrence rates remain low. Our experience supports the selective use of NSM in the malignant setting with careful patient selection.
Keywords: nipple-sparing mastectomy, breast cancer, ductal carcinoma in situ, invasive carcinoma
INTRODUCTION
Many patients who have total mastectomy for invasive cancer or ductal carcinoma in situ (DCIS) undergo immediate breast reconstruction. In this setting, skin-sparing mastectomy (SSM) or nipple-sparing mastectomy (NSM) are performed. SSM was first described by Toth and Lappert,1 and is performed by removing the entire breast parenchyma through a small ellipse of skin that encompasses the nipple-areola complex. NSM was first described by Freeman,2 and preserves the skin envelope and nipple-areolar complex while all glandular breast tissue is removed. Preserving the skin envelope in either SSM or NSM allows the breast to be reconstructed immediately, with implant-based or tissue-transfer techniques
The oncologic safety of SSM has been established, with equivalent recurrence rates compared to a standard total mastectomy,3–11 and it is accepted as the standard mastectomy procedure without increased risk of local recurrence. Unlike SSM, NSM leaves a small amount of ductal tissue behind the nipple, and this has raised oncologic concerns of potentially increasing the risk of local recurrence.12,13 Due to this oncologic concern, a section of the retroareolar tissue (also called “nipple-margin”) is usually examined routinely to exclude cancer involvement. Nipple-areolar complex involvement on women undergoing therapeutic NSM ranges from 8% to 33%, with the majority at 25% in mastectomy specimens,12,14,15 and multiple studies with follow-up ranging from 10–101 months have demonstrated low rates of locoregional recurrence.12,16,17
In 2009 we reported our initial NSM experience at Memorial Sloan Kettering Cancer Center (MSK); at the time, most cases were risk-reduction prophylactic mastectomies.18 As the use of NSM has become more widespread, and as evidence supporting the oncologic safety of NSM has increased, its indications have broadened over time to the point where more NSMs are being performed for cancer. This study reviews and examines the indications, complications, and long-term outcomes of therapeutic NSM performed at our institution.
METHODS
Following institutional review board approval, we queried MSK’s prospectively maintained breast cancer database to identify patients who underwent NSM for invasive cancer or DCIS with or without a contralateral prophylactic NSM between 2003–2016. We excluded all patients undergoing bilateral prophylactic or risk-reduction NSM with no cancer diagnosis.
All electronic medical records were reviewed to update the follow-up status for each patient. The following variables were collected: age at diagnosis; family history of breast/ovarian cancer (≥ 1 first- or second-degree family member with breast cancer); genetic testing, if performed, with mutations detected; smoking status at the time of diagnosis; pathologic T and N stage; histologic grade; estrogen receptor status; progesterone receptor status; HER2 status; use of neoadjuvant and adjuvant chemotherapy, radiation therapy, hormonal therapy, or trastuzumab.
Compared to our previous report, NSM selection criteria were expanded to only exclude those patients with locally advanced breast cancer, extensive disease in the periphery of the breast (where limited exposure can become an obstacle to appropriately remove the breast tissue), direct invasion of the nipple, and tumors ≤ 1 cm from nipple on imaging. Patients with increased risk factors for nipple necrosis, such as, prior radiation, smoking, cup size ≥ C were also not considered eligible. All procedures were performed by a surgeon from the MSK breast surgical service in coordination with a plastic surgeon. We also reviewed the type of reconstruction procedure (implant/expander or autologous) performed in each case. The following complication incidences were recorded: residual cancer in the nipple margin, whether on frozen section or final pathology, requiring removal of the nipple-areolar complex; nipple or areola loss; re-operation; infection; expander/implant removal; seroma; hematoma; skin desquamation (defined as partial-thickness skin loss not requiring surgical debridement); or mastectomy flap necrosis (defined as full-thickness skin loss requiring surgical debridement).
Each mastectomy was considered an individual event in patients with bilateral breast malignancies, and contralateral prophylactic mastectomies (CPMs) were recorded as such. Patient and disease characteristics were summarized using the median (minimum, maximum) when continuous, and the frequency (%) when categorical. Patients were classified as having surgery before 2011, or during 2011 and after. Patient and disease characteristics were compared according to year of surgical procedure using the Kruskal-Wallis test for continuous variables and Fisher’s exact test for categorical variables. Complications were compared according to year of surgical procedure using a generalized mixed effects model that incorporated a random intercept to account for correlation among procedures done by the same surgeon. Because of the small number of patients with bilateral therapeutic procedure (n = 18), statistical methods to account for correlation between these dual observations were not utilized.
Overall survival (OS) was estimated from date of surgery to date of death. Time to recurrence (TTR) was estimated from date of surgery to date of first local recurrence or metastasis. Patients without the event of interest were censored at their date of last follow-up. The Kaplan-Meier method estimated OS and TTR. The log-rank test was used for comparisons according to year of surgical procedure.
A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using R version 3.4.1 (R Core Development Team, Vienna, Austria).
RESULTS
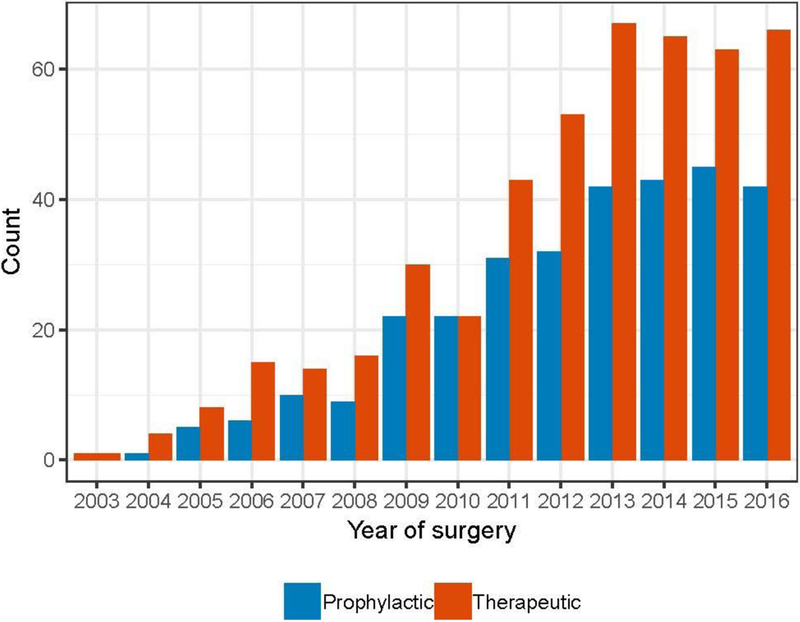
449 women underwent 777 NSMs between January 2003 and December 2016. Of these, 467 (60.1%) were performed for treatment of breast cancer and 310 (39.9%) were CPMs. Fig. 1 shows the institutional trends over time of therapeutic and contralateral prophylactic NSMs.
Fig. 1.
Institutional trends in nipple-sparing mastectomy from 2003–2016.
Patient characteristics at presentation and by surgery year are described in Table 1. The median age for the entire cohort was 45 years (range 24–75), and the median follow-up time among survivors was 39.4 months (range 0.4–150). Of the 449 patients, 118 (26.3) had ≥ 1 first-degree relative with breast cancer and 11 (2.4%) had ≥ 1 first-degree relative with ovarian cancer. Genetic testing was performed in 270 (60.1%) patients; 28 (10.4%) were positive for a deleterious BRCA1 mutation and 18 (6.7%) were positive for the BRCA2 mutation. 326 (72.6%) patients had never smoked, 98 (21.8%) were former smokers, and only 25 (5.6%) were current smokers. 32 (7.1%) had received prior radiation either for prior breast cancer or mantle radiation. Preoperative MRI was performed in 294 (65.5%) patients. 344 (76.6%) patients were operated on after 2011. There was no difference in patient characteristics before and after 2011 regarding family history, genetic mutations, smoking status, prior radiation, or rate of CPM. There was an increased use of preoperative MRI (p < .001) (Table 1).
TABLE 1.
Patient Characteristics by Surgery Year
| Overall (n = 449) | NSM before 2011 (n = 105) | NSM after 2011 (n = 344) | p-value | |
|---|---|---|---|---|
| Median Age, years (range) | 45 (24–75) | 44 (25–64) | 45 (24–75) | 0.51 |
| Family History | ||||
| Breast Cancer (≥ 1st Degree) | 118 (26.3%) | 27 (25.7%) | 90 (26.2%) | 0.90 |
| Ovarian Cancer (≥ 1st Degree) | 11 (2.4%) | 6 (5.7%) | 5 (1.5%) | 0.03a |
| Genetic Testing | 0.07 | |||
| No | 179 (39.9%) | 50 (47.6%) | 129 (37.5%) | |
| Yes | 270 (60.1%) | 55 (52.4%) | 215 (62.5%) | |
| Mutations Detected (n = 270) | ||||
| BRCA1 | 28 (10.4%) | 6 (10.9%) | 22 (10.2%) | 0.81 |
| BRCA2 | 18 (6.7%) | 5 (9.1%) | 13 (6.0%) | 0.38 |
| Smoking Status | 0.83 | |||
| Current Smoker | 25 (5.6%) | 6 (5.7%) | 19 (5.5%) | |
| Never Smoker | 326 (72.6%) | 74 (70.5%) | 252 (73.3%) | |
| Former Smoker | 98 (21.8%) | 25 (23.8%) | 73 (21.2%) | |
| Prior Radiation | 0.08 | |||
| Yes | 32 (7.1%) | 12 (11.4%) | 20 (5.8%) | |
| No | 417 (92.9%) | 93 (88.6%) | 324 (94.2%) | |
| Preoperative MRI | < .00 lb | |||
| Yes | 294 (65.5%) | 43 (41.0%) | 251 (73.0%) | |
| No | 155 (34.5%) | 62 (59.0%) | 93 (27.0%) | |
| Contralateral Prophylactic NSM | 0.63 | |||
| Yes | 310 (69.0%) | 75 (71.4%) | 235 (68.3%) | |
| No | 139 (31.0%) | 30 (28.6%) | 109 (31.7%) |
NSM nipple-sparing mastectomy
bold increased frequency of family history of ovarian cancer in cases before 2011
bold denotes increased use of preoperative MRI
Tumor histology and management by surgery year are described in Table 2. Of the 467 therapeutic NSMs, 337 (72.2%) were performed for invasive cancer, 126 (27.0%) were DCIS, and 4 (0.9%) were phyllodes tumors. Among all therapeutic cases, 123 (26.3%) were stage 0, 230 (49.3%) were stage I, 83 (17.8%) were stage II, 18 (3.9%) were stage III, and 13 (2.8%) were of unknown stage. The median tumor size was 1.2 cm (range 0–9) for all invasive tumors. Sentinel lymph node biopsy (SLNB) was the only axillary procedure performed in 388 (83.1%) of all therapeutic NSM breast cancer cases, and axillary lymph node dissection (ALND) was performed in 54 (11.6%). When comparing NSMs performed before and after 2011, there was an increased use of SLNB (p < .001) and a significant increase in the number of therapeutic NSMs performed for invasive tumor histology after 2011 (58% versus 77%, p < .001) (Table 2). Patients treated after 2011 also tended to have a higher T stage (p = 0.009) and higher overall TNM stage (p = 0.005) (Table 2).
TABLE 2.
Tumor Histology and Management of Therapeutic Procedures by Surgery Year
| Overall (n = 467) | NSM before 2011 (n = 110) | NSM after 2011 (n = 357) | p-value | |
|---|---|---|---|---|
| Tumor Histology | < .001a | |||
| Invasive Cancer | 337 (72.2%) | 64 (58.2%) | 273 (76.5%) | |
| DCIS | 126 (27.0%) | 44 (40.0%) | 82 (23.0%) | |
| Phyllodes | 4 (0.9%) | 2 (1.8%) | 2 (0.6%) | |
| Invasive Tumor Subtype (n = 337) | 0.08 | |||
| HR+/HER2− | 232 (68.8%) | 42 (65.6%) | 190 (69.6%) | |
| HR+/HER2+ | 38 (11.3%) | 4 (6.2%) | 34 (12.5%) | |
| HR−/HER2− | 35 (10.4%) | 12 (18.8%) | 23 (8.4%) | |
| HR−/HER2+ | 17 (5.0%) | 3 (4.7%) | 14 (5.1%) | |
| Unknown | 15 (4.5%) | 3 (4.7%) | 12 (4.4%) | |
| Median Tumor Size (cm, range) | 1.2 (0–9) | 1.2 (0.1–8.2) | 1.2 (0–9) | 0.65 |
| T Category | 0.009b | |||
| Tis | 126 (27.0%) | 44 (40.0%) | 82 (23.0%) | |
| T1 | 268 (57.4%) | 52 (47.3%) | 216 (60.5%) | |
| T2 | 57 (12.2%) | 10 (9.1%) | 47 (13.2%) | |
| T3 | 2 (0.4%) | - | 2 (0.6%) | |
| Unknown/Others | 14 (3.0%) | 4 (3.6%) | 10 (2.8%) | |
| Nodal Status | 1.0 | |||
| Node Negative | 390 (83.5%) | 92 (83.6%) | 298 (83.5%) | |
| Node Positive | 77 (16.5%) | 18 (16.4%) | 59 (16.5%) | |
| Breast Cancer Stage | 0.005c | |||
| 0 | 123 (26.3%) | 43 (39.1%) | 80 (22.4%) | |
| I | 230 (49.3%) | 42 (38.2%) | 188 (52.7%) | |
| II | 83 (17.8%) | 16 (14.5%) | 67 (18.8%) | |
| III | 18 (3.9%) | 4 (3.6%) | 14 (3.9%) | |
| Unknown | 13 (2.8%) | 5 (4.5%) | 8 (2.2%) | |
| Axillary Procedure | < .001d | |||
| SLNB | 388 (83.1%) | 75 (68.2%) | 313 (87.7%) | |
| ALND | 54 (11.6%) | 21 (19.1%) | 33 (9.2%) | |
| None | 25 (5.4%) | 14 (12.7%) | 11 (3.1%) | |
| Neoadjuvant Chemotherapy (n = 449) | 0.14 | |||
| Yes | 33 (7.3%) | 4 (3.8%) | 29 (8.4%) | |
| No | 416 (92.7%) | 101 (96.2%) | 315 (91.6%) | |
| Adjuvant Chemotherapy (n = 449) | 0.72 | |||
| Yes | 150 (33.4%) | 37 (35.2%) | 113 (32.8%) | |
| No | 295 (65.7%) | 68 (64.8%) | 227 (66%) | |
| Unknown | 4 (0.9%) | 0 (0%) | 4 (1.2%) | |
| PMRT (n = 467) | 0.55 | |||
| Yes | 37 (7.9%) | 7 (6.4%) | 30 (8.4%) | |
| No | 428 (91.6%) | 103 (93.6%) | 325 (91.0%) | |
| Unknown | 2 (0.4%) | - | 2 (0.6%) | |
| Adjuvant Endocrine Therapy (n=449) | 0.001e | |||
| Yes | 233 (51.9%) | 40 (38.1%) | 193 (56.1%) | |
| No | 210 (46.8%) | 65 (61.9%) | 145 (42.2%) | |
| Unknown | 6 (1.3%) | 0 (0%) | 6 (1.7%) |
NSM nipple-sparing mastectomy, DCIS ductal carcinoma in situ, SLNB sentinel lymph node biopsy, ALND axillary lymph node dissection, PMRT postmastectomy radiation therapy
bold denotes there was a significant increase in the number of therapeutic NSMs performed for invasive tumor histology after 2011
bold denotes patients after 2011 tended to have a higher T stage
bold denotes patients after 2011 tended to have a higher overall TNM stage
bold denotes patients after 2011more frequently received SLNB
bold denotes patients after 2011 more frequently received adjuvant endocrine therapy
Of the 449 patients undergoing therapeutic NSMs, 150 (33.4%) received adjuvant chemotherapy and 233 (51.9%) received adjuvant endocrine therapy; only 33 (7.3%) received neoadjuvant chemotherapy. Only 37 (7.9%) of all 467 therapeutic NSMs received postmastectomy radiation therapy (PMRT). Patients treated after 2011 more frequently received adjuvant endocrine therapy (p = 0.001) (Table 2).
Almost all 449 patients underwent breast reconstruction procedures, of which 391 (87.1%) were tissue expander/implant based. 45 (10.0%) went straight to implant, and 10 (2.2%) had autologous flaps. Only 3 (0.7%) had no reconstruction due to small breast size.
The nipple-areolar complex was entirely preserved in 446 therapeutic mastectomies (95.5%). There were a total of 21 (4.5%) nipple excisions, with 14 performed due to cancer at the nipple margin (Table 3). 186 (39.8%) breasts had some degree of skin desquamation at follow-up, but most of these were mild and resolved fully without intervention; 38 (8.1%) had skin necrosis requiring debridement. 44 (9.4%) had a complication that required a re-operation, including implant removal in 16 breasts (3.4%). 28 patients (6.0%) were treated for infection. There was no significant difference in the rate or type of complications between NSMs performed before or after 2011 (all p > 0.05) (Table 3).
TABLE 3.
Complications Among Therapeutic Procedures by Surgery Year. P-Values from Mixed Effects Model Accounting for Random Surgeon Effect
| Overall (n = 467) | NSM before 2011 (n = 110) | NSM after 2011 (n = 357) | p-value | |
|---|---|---|---|---|
| Any Complication | 221 (47.3%) | 52 (47.3%) | 169 (47.3%) | 0.91 |
| Nipple Excision | 21 (4.5%) | 7 (6.4%) | 14 (3.9%) | 0.37 |
| Re-operation for Complication | 44 (9.4%) | 12 (10.9%) | 32 (9.0%) | 0.54 |
| Skin Desquamation | 186 (39.8%) | 42 (38.2%) | 144 (40.3%) | 0.73 |
| Skin Necrosis | 38 (8.1%) | 7 (6.4%) | 31 (8.7%) | 0.45 |
| Hematoma | 13 (2.8%) | 3 (2.7%) | 10 (2.8%) | 0.97 |
| Implant/Expander Removal | 16 (3.4%) | 3 (2.7%) | 10 (2.8%) | 0.65 |
| Infection | 28 (6.0%) | 5 (4.5%) | 23 (6.4%) | 0.47 |
NSM nipple-sparing mastectomy
Of the 310 CPMs performed, 24 (7.7%) harbored occult malignancies; 6 (1.9%) were invasive cancers, and 18 (5.8%) were DCIS only.
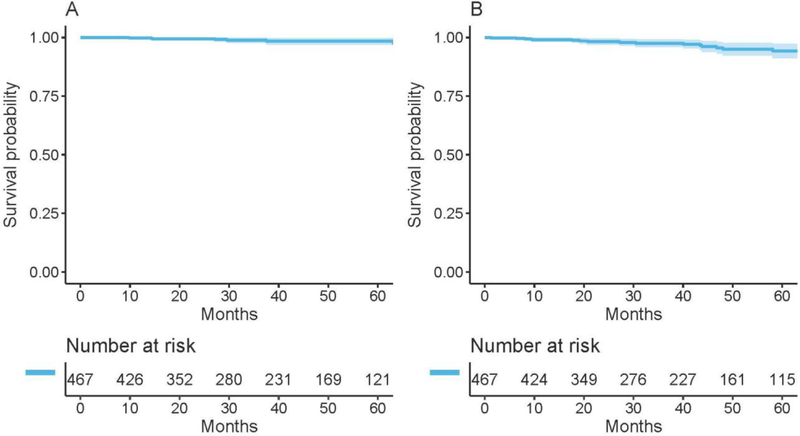
At a median follow-up of 39.4 months (range 0.4–150), 7 patients had died (Fig. 2A) and 15 patients had recurrence (Fig. 2B). Of these, 3 were locoregional recurrences (LRRs) only, 11 were distant metastasis only, and 1 was an LRR and distant metastasis. None of the LRRs involved the nipple-areolar complex.
Fig. 2.
Overall survival (A) and time to recurrence (B) among therapeutic procedures.
DISCUSSION
Preservation of the nipple-areolar complex enhances the cosmetic results of the mastectomy procedure and has been shown to positively contribute to postmastectomy body image.19–24 Patients with preserved nipple-areolar complexes reported higher levels of psychosocial well-being.25,26 Even in instances when patients reported nipple sensation and arousal as “fair” with NSM,27–29 studies have shown that women still reported better body image and improved self-esteem. In an earlier report from MSK, cosmetic results of NSM were reported as “excellent” in 30 of 42 (71.4%) patients, and “good” in 7 of 42 (16.7%) patients.18
Unilateral and contralateral prophylactic mastectomy rates have been increasing despite growing evidence that long-term survival is equivalent regardless of whether BCS or mastectomy is performed, even among women who are considered appropriate candidates for BCS at presentation.30,31 In our cohort, 69% of patients elected to have a CPM. BRCA1/BRCA2 testing, increased use of preoperative MRI, and increased anxiety have all been implicated in the increasing rates of mastectomy (in patients who are candidates for BCS) and CPM. 60.1% of women in our cohort had genetic testing, 17.1% tested positive for BRCA1/BRCA2, and 65.5% had a preoperative MRI. Paralleling the increase in bilateral mastectomies, there has been an increase in the rate of NSM for therapeutic purposes in the United States.32 In our study, 72% of the NSMs were performed for invasive cancer; the remainder was performed for in-situ disease. This correlates with national and international trends in which two-thirds of NSMs are performed for invasive cancer.32,33
The use of NSM has expanded from risk-reduction procedures to the treatment of breast cancer. In our early experience, NSM was selectively recommended for patients undergoing risk-reduction procedures and mastectomy for DCIS.18 National Comprehensive Cancer Network guidelines34 recommend selecting patients with early-stage and biologically favorable invasive cancers or in situ cancers for NSM. In our more recent cohort, more patients with invasive cancer underwent NSM; however, the majority were stage I-II. We have also selectively offered NSM to patients with more aggressive subtypes; 16.1% of invasive cancers were HER2 positive and 10.2% were triple negative (Table 2). This reflects the expanded indications of NSMs to cancer patients, as evidence supporting the oncologic safety has increased.
Single-institution studies have reported similarly on the expansion of the eligibility criteria for NSM over time to include women with larger breasts, more advanced disease, larger tumors or node-positive disease, and prior radiation therapy (breast or mantle), and even in cases that might require PMRT without compromising oncologic outcomes.35–38 In a single retrospective study examining outcomes of NSM after neoadjuvant chemotherapy, after 3 years of follow-up, the LRR was 1.6% and none of those recurrences involved the nipple.37 Despite concerns regarding NSM use in the setting of prior radiation, our study shows that it can be used selectively with good results. 32 (7.1%) patients in our cohort of 449 women proceeded with NSM after previous radiation without increased postoperative complications or nipple loss. These low complication rates associated with NSM after radiation have been similarly demonstrated in prior studies.39,40 PMRT was administered to 37 (7.9%) breasts in our cohort without increasing the complication rates or compromising their nipples; notably, 4 of those patients had nipple excision for invasive cancer at the nipple margin. Regardless of whether SSM or NSM is used, complication rates are 3 times what is reported when PMRT is not used.41 Overall, rates are comparable by stage, regardless of preservation of the nipple, and therefore PMRT could be considered for NSM.42,43
Our single-institution experience is important, as it reviews our experience with NSM over time while considering the broadening eligibility criteria for this procedure. At 39.4 months median follow-up, 4 of 449 patients in our series developed locoregional recurrence, and none of those recurrences involved the nipple-areolar complex. The data are encouraging, but longer follow-up is needed to support the expanding indications for NSM and to conclusively determine its oncologic safety. With appropriate follow-up, LRRs after SSM range from 5.5–6.2% over 25–78.1 months,11,44 while LRRs with NSM range from 0–4.6% over 10–60 months.16,33,35,36,45,46 Variation in the rate of the LRR can be explained by the different inclusion criteria in each of those studies.
Involvement of the nipple-areolar complex is associated with tumor size, nodal involvement, tumor distance to the nipple, tumor grade, lymphovascular invasion (LVI), and HER2 overexpression.47 Studies have also suggested that LRR is associated with tumor biology rather than preservation of the nipple-areolar complex during NSM.48 In our study of 467 NSMs, there were a total of 21 (4.5%) nipple excisions, of which 14 were performed due to cancer at the nipple margin (4 invasive cancers and 10 DCIS). The nipple-areolar complex was entirely preserved in 446 mastectomies (95.5%).
Strengths of our study include its evaluation of a relatively large cohort of NSM patients over an extended time period. Our findings suggest that evolving indications over time did not affect oncologic outcomes. Limitations of our study include its retrospective nature. Additionally, we recognize that selection bias may have resulted in NSM being offered disproportionately to patients with a good prognosis. However, the selection criteria for NSM have expanded compared with our previous report to include higher stage and more aggressive subtypes. The presence of a small number of cases with local or regional recurrence during our median follow-up period of 39 months suggests that NSM is oncologically safe in this patient group; however, longer follow-up is needed to establish the procedure’s long-term safety.
Conclusions
The use of NSM for invasive carcinoma has doubled at our institution since 2011, while postoperative complications and recurrence rates have remained low. Despite this encouraging experience, it is important to realize that long-term outcomes are needed to confirm the safety of the procedure in selected patients with breast cancer. Our experience supports the selective use of NSM in the malignant setting with careful patient selection.
Synopsis:
Here we examine indications, complications, and long-term outcomes of therapeutic nipple-sparing mastectomy. Our experience supports selective use of nipple-sparing mastectomy in the malignant setting with careful patient selection.
ACKNOWLEDGEMENTS
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the 71st Society of Surgical Oncology Annual Cancer Symposium, March 21-24, 2018, Chicago, IL Dr. Monica Morrow has received honoraria from Genomic Health and Roche. Dr. Andrea Pusic is a co-developer of the BREAST-Q and receives royalties when the questionnaire is used in for-profit industry-sponsored clinical trials.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Toth BA, Lappert P. Modified skin incisions for mastectomy: the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87(6):1048–53. [PubMed] [Google Scholar]
- 2.Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull. 1962;30:676–82. [DOI] [PubMed] [Google Scholar]
- 3.Eldor L, Spiegel A. Breast reconstruction after bilateral prophylactic mastectomy in women at high risk for breast cancer. Breast J. 2009;15 Suppl 1:S81–9. [DOI] [PubMed] [Google Scholar]
- 4.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev. 2010(11):CD002748. [DOI] [PubMed] [Google Scholar]
- 5.Morrow M, Mehrara B. Prophylactic mastectomy and the timing of breast reconstruction. Br J Surg. 2009;96(1):1–2. [DOI] [PubMed] [Google Scholar]
- 6.Peled AW, Irwin CS, Hwang ES, Ewing CA, Alvarado M, Esserman LJ. Total skin-sparing mastectomy in BRCA mutation carriers. Ann Surg Oncol. 2014;21(1):37–41. [DOI] [PubMed] [Google Scholar]
- 7.Warren Peled A, Foster RD, Stover AC, et al. Outcomes after total skin-sparing mastectomy and immediate reconstruction in 657 breasts. Ann Surg Oncol. 2012;19(11):3402–9. [DOI] [PubMed] [Google Scholar]
- 8.Lanitis S, Tekkis PP, Sgourakis G, Dimopoulos N, Al Mufti R, Hadjiminas DJ. Comparison of skin-sparing mastectomy versus non-skin-sparing mastectomy for breast cancer: a meta-analysis of observational studies. Ann Surg. 2010;251(4):632–9. [DOI] [PubMed] [Google Scholar]
- 9.Kroll SS, Schusterman MA, Tadjalli HE, Singletary SE, Ames FC. Risk of recurrence after treatment of early breast cancer with skin-sparing mastectomy. Ann Surg Oncol. 1997;4(3):193–7. [DOI] [PubMed] [Google Scholar]
- 10.Kroll SS, Khoo A, Singletary SE, et al. Local recurrence risk after skin-sparing and conventional mastectomy: a 6-year follow-up. Plast Reconstr Surg. 1999;104(2):421–5. [DOI] [PubMed] [Google Scholar]
- 11.Newman LA, Kuerer HM, Hunt KK, et al. Presentation, treatment, and outcome of local recurrence afterskin-sparing mastectomy and immediate breast reconstruction. Ann Surg Oncol. 1998;5(7):620–6. [DOI] [PubMed] [Google Scholar]
- 12.Gerber B, Krause A, Dieterich M, Kundt G, Reimer T. The oncological safety of skin sparing mastectomy with conservation of the nipple-areola complex and autologous reconstruction: an extended follow-up study. Ann Surg. 2009;249(3):461–8. [DOI] [PubMed] [Google Scholar]
- 13.De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall Survival, Disease-Free Survival, Local Recurrence, and Nipple-Areolar Recurrence in the Setting of Nipple-Sparing Mastectomy: A Meta-Analysis and Systematic Review. Ann Surg Oncol. 2015;22(10):3241–9. [DOI] [PubMed] [Google Scholar]
- 14.Crowe JP, Patrick RJ, Yetman RJ, Djohan R. Nipple-sparing mastectomy update: one hundred forty-nine procedures and clinical outcomes. Arch Surg. 2008;143(11):1106–10; discussion 10. [DOI] [PubMed] [Google Scholar]
- 15.Lagios MD, Gates EA, Westdahl PR, Richards V, Alpert BS. A guide to the frequency of nipple involvement in breast cancer. A study of 149 consecutive mastectomies using a serial subgross and correlated radiographic technique. Am J Surg. 1979;138(1):135–42. [DOI] [PubMed] [Google Scholar]
- 16.de Alcantara Filho P, Capko D, Barry JM, Morrow M, Pusic A, Sacchini VS. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol. 2011;18(11):3117–22. [DOI] [PubMed] [Google Scholar]
- 17.Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol. 2012;23(8):2053–8. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Etienne CA, Cody Iii HS 3rd, Disa JJ, Cordeiro P, Sacchini V. Nipple-sparing mastectomy: initial experience at the Memorial Sloan-Kettering Cancer Center and a comprehensive review of literature. Breast J. 2009;15(4):440–9. [DOI] [PubMed] [Google Scholar]
- 19.Morimoto T, Komaki K, Inui K, et al. Involvement of nipple and areola in early breast cancer. Cancer. 1985;55(10):2459–63. [DOI] [PubMed] [Google Scholar]
- 20.Luttges J, Kalbfleisch H, Prinz P. Nipple involvement and multicentricity in breast cancer. A study on whole organ sections. J Cancer Res Clin Oncol. 1987;113(5):481–7. [DOI] [PubMed] [Google Scholar]
- 21.Santini D, Taffurelli M, Gelli MC, et al. Neoplastic involvement of nipple-areolar complex in invasive breast cancer. Am J Surg. 1989;158(5):399–403. [DOI] [PubMed] [Google Scholar]
- 22.Vyas JJ, Chinoy RF, Vaidya JS. Prediction of nipple and areola involvement in breast cancer. Eur J Surg Oncol. 1998;24(1):15–6. [DOI] [PubMed] [Google Scholar]
- 23.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34(2):143–8. [DOI] [PubMed] [Google Scholar]
- 24.Petit JY, Veronesi U, Rey P, et al. Nipple-sparing mastectomy: risk of nipple-areolar recurrences in a series of 579 cases. Breast Cancer Res Treat. 2009;114(1):97–101. [DOI] [PubMed] [Google Scholar]
- 25.Nahabedian MY, Tsangaris TN. Breast reconstruction following subcutaneous mastectomy for cancer: a critical appraisal of the nipple-areola complex. Plast Reconstr Surg. 2006;117(4):1083–90. [DOI] [PubMed] [Google Scholar]
- 26.Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat. 2009;118(3):623–33. [DOI] [PubMed] [Google Scholar]
- 27.Djohan R, Gage E, Gatherwright J, et al. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: an 8-year outcome study. Plast Reconstr Surg. 2010;125(3):818–29. [DOI] [PubMed] [Google Scholar]
- 28.Yueh JH, Houlihan MJ, Slavin SA, Lee BT, Pories SE, Morris DJ. Nipple-sparing mastectomy: evaluation of patient satisfaction, aesthetic results, and sensation. Ann Plast Surg. 2009;62(5):586–90. [DOI] [PubMed] [Google Scholar]
- 29.Dossett LA, Lowe J, Sun W, et al. Prospective evaluation of skin and nipple-areola sensation and patient satisfaction after nipple-sparing mastectomy. J Surg Oncol. 2016;114(1):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA. 2014;312(9):902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–9. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal S, Agarwal S, Neumayer L, Agarwal JP. Therapeutic nipple-sparing mastectomy: trends based on a national cancer database. Am J Surg. 2014;208(1):93–8. [DOI] [PubMed] [Google Scholar]
- 33.Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: Surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016;25:75–81. [DOI] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network (NCCN). NCCN Guidelines Version 2.2016. Invasive breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed March 26, 2019).
- 35.Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol. 2013;20(10):3218–22. [DOI] [PubMed] [Google Scholar]
- 36.Krajewski AC, Boughey JC, Degnim AC, et al. Expanded Indications and Improved Outcomes for Nipple-Sparing Mastectomy Over Time. Ann Surg Oncol. 2015;22(10):3317–23. [DOI] [PubMed] [Google Scholar]
- 37.Santoro S, Loreti A, Cavaliere F, et al. Neoadjuvant chemotherapy is not a contraindication for nipple sparing mastectomy. Breast. 2015;24(5):661–6. [DOI] [PubMed] [Google Scholar]
- 38.Smith BL, Tang R, Rai U, et al. Oncologic Safety of Nipple-Sparing Mastectomy in Women with Breast Cancer. J Am Coll Surg. 2017;225(3):361–5. [DOI] [PubMed] [Google Scholar]
- 39.Alperovich M, Choi M, Frey JD, et al. Nipple-sparing mastectomy in patients with prior breast irradiation: are patients at higher risk for reconstructive complications? Plast Reconstr Surg. 2014;134(2):202e–6e. [DOI] [PubMed] [Google Scholar]
- 40.Tang R, Coopey SB, Colwell AS, et al. Nipple-Sparing Mastectomy in Irradiated Breasts: Selecting Patients to Minimize Complications. Ann Surg Oncol. 2015;22(10):3331–7. [DOI] [PubMed] [Google Scholar]
- 41.Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol. 2013;20(10):3294–302. [DOI] [PubMed] [Google Scholar]
- 42.Janssen S, Holz-Sapra E, Rades D, Moser A, Studer G. Nipple-sparing mastectomy in breast cancer patients: The role of adjuvant radiotherapy (Review). Oncol Lett. 2015;9(6):2435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reish RG, Lin A, Phillips NA, et al. Breast reconstruction outcomes after nipple-sparing mastectomy and radiation therapy. Plast Reconstr Surg. 2015;135(4):959–66. [DOI] [PubMed] [Google Scholar]
- 44.Carlson GW, Styblo TM, Lyles RH, et al. The use of skin sparing mastectomy in the treatment of breast cancer: The Emory experience. Surg Oncol. 2003;12(4):265–9. [DOI] [PubMed] [Google Scholar]
- 45.Boneti C, Yuen J, Santiago C, et al. Oncologic safety of nipple skin-sparing or total skin-sparing mastectomies with immediate reconstruction. J Am Coll Surg. 2011;212(4):686–93; discussion 93–5. [DOI] [PubMed] [Google Scholar]
- 46.Lohsiriwat V, Martella S, Rietjens M, et al. Paget’s disease as a local recurrence after nipple-sparing mastectomy: clinical presentation, treatment, outcome, and risk factor analysis. Ann Surg Oncol. 2012;19(6):1850–5. [DOI] [PubMed] [Google Scholar]
- 47.Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol. 2009;27(30):4948–54. [DOI] [PubMed] [Google Scholar]
- 48.Voltura AM, Tsangaris TN, Rosson GD, et al. Nipple-sparing mastectomy: critical assessment of 51 procedures and implications for selection criteria. Ann Surg Oncol. 2008;15(12):3396–401. [DOI] [PubMed] [Google Scholar]