Abstract
Impact of Plerixafor (P) mobilized stem cells on immune reconstitution, such as absolute lymphocyte count at day 30 (ALC30), and on long-term outcomes of Multiple Myeloma (MM) patients undergoing autologous stem cell transplant (ASCT) has not been well established. We evaluated total of 469 patients mobilized with G-CSF (G) alone and 141 patients mobilized with G-CSF plus plerixafor (G+ P). Patients only received plerixafor if they had peripheral blood CD34+ve blood count < 20/uL on first planned day of collection. Primary endpoint, ALC30, was 1.3 K/uL (range, 0.1–4.5) and 1.2 K/uL (range, 0.1–5.1) for G and G+P, respectively (p=0. 61). The median PFS was 2.5 years (95% CI, 2.1–3.2) and 2.8 years (95% CI, 2.0–3.3) for G and G + P, respectively (HR: 1.13; 95% CI, 0.84–1.50; p=0. 42). The median OS was 6.1 years (95% CI, 4.6-NR) for G group compared to 3.7 years (95% CI, 3.2-NR) for the G+P group (HR: 1.64; 95% CI, 1.12–2.40; p=0. 01). The median follow-up time for OS was 2.53 years (95% CI, 2.13–2.99) and 1.59 years (95% CI, 1.17–2.02) for G and G+ P group, respectively. In this large retrospective analysis of MM patients mobilized with G-CSF vs G-CSF + P, there was no significant difference in lymphocyte recovery or PFS. There was an overall survival difference in patients who were poor mobilizers and could not be mobilized with G-CSF alone.
Keywords: G-CSF, Plerixafor, Multiple Myeloma, Autologous stem cell transplant
Introduction
High-dose therapy followed by autologous stem cell transplantation (ASCT) remains a standard of care in eligible patients undergoing treatment for multiple myeloma (MM). Approximately 20% of the MM patients undergoing mobilization of peripheral blood stem cells (PBSC) with granulocyte colony stimulating factor (G-CSF) alone or G-CSF plus chemotherapy are not able to mobilize the minimum number of CD-34+ stem cells (2 X 106 CD34 cells/kg) recommended for transplant [1, 2]. Plerixafor (P) is a reversible direct antagonist of CXCR4/SDF-1, which prevents binding of its ligand CXCL12 and induces mobilization of cells expressing this receptor, including hematopoietic stem and progenitor cells (HSPCs) [3]. Based on a pivotal randomized phase-3 study comparing G-CSF vs G-CSF plus plerixafor, plerixafor has been approved for mobilization of PBSCs in MM by the Food and Drug Administration [4]. However, because the true benefit of plerixafor use is mainly seen in patients who are poor mobilizers and increased cost associated with its use, a risk-adapted approach for plerixafor use (using plerixafor in only poor mobilizers such as patients who after 5 days of G-CSF have a peripheral CD34+ve cells count of < 20 cells/μL) is in practice at many centers, including ours [5, 6].
Peripheral blood stem cell grafts collected with plerixafor contain more natural killer (NK) cells, T cells (CD3, CD4), primitive CD-34 cells, mature lymphocytes, dendritic cells and repopulating cells compared to grafts collected with G-CSF alone [7, 8]. Higher amounts of CD-34+ cells infused at transplant have been associated with improved survival in multiple studies [9, 10]. It is also hypothesized that the use of plerixafor can improve lymphocyte recovery post-transplant through mobilization of more mature lymphocytes [11, 12]. Early lymphocyte recovery (absolute lymphocyte count, ALC-30, of > 1,000/μL at day 30 after transplant) has been shown to predict improved PFS in MM patients [12–14]. No major difference has been observed in terms of engraftment of neutrophils and platelets in MM patients undergoing mobilization with G-CSF plus plerixafor compared to G-CSF alone in randomized studies [4, 15, 16]; however, ALC-30 has not been well studied in the context of plerixafor use for MM.
Long term follow-up of the patients from the pivotal phase-3 trial comparing the G and G+P groups showed similar 5-year OS and PFS in MM [17]. However, it is unclear if the long-term outcomes (PFS and OS) would be different if the mobilization agent is determined based on a risk-adapted approach. With this study, we retrospectively evaluated the short-term outcomes such as engraftment including lymphocyte recovery (ALC-30) and long-term outcomes of PFS and OS in a large group of MM patients undergoing ASCT with PBSCs mobilized with G-CSF (G) and G-CSF plus Plerixafor (G+P) where plerixafor was administered when the peripheral CD-34+ cell-count was < 20/uL on day 5 of G-CSF use.
Methods
Patients
We conducted a retrospective study of patients who underwent their first ASCT for diagnosis of MM with either G-CSF or G-CSF plus plerixafor mobilized stem cells at Karmanos Cancer Institute. Patients in whom GM-CSF was used for mobilization were excluded from this study. This study was approved by Wayne State University Intuitional Review Board.
Karmanos Cancer Center Blood and Marrow Stem Cell Transplant Program prospectively collected relevant data in all patients who received stem cell transplantation for reporting to the Center for International Blood and Marrow Transplant Research (CIBMTR). This study was the outcome analysis of retrospective retrieval of collected details regarding patient demographics, disease status and transplant outcomes. International Staging System (ISS) was used for initial staging and International Myeloma Working Group (IMWG) Uniform Response criteria was used to group patients in CR, VGPR, PR, SD or PD at the time of transplant [18]. Patients who had CR, VGPR or PR were considered to have “response” and patients with SD and PD were considered to have “no response”. Information regarding significant co-existing disease (defined as more than one or multiple chronic or long-term diseases/conditions), total lines of treatment before transplant (one or more), utilization of a doublet vs triplet therapy approach and post-transplant maintenance was also collected. No patients in this study received a tandem transplant.
Mobilization and Preparative Regimen
Per institutional guidelines, the goal for mobilization was to collect PBSCs for two autologous transplants with the minimum collection of 4 X 106 CD34 cells/kg. All patients received G-CSF 10mcg/kg/day for five days subcutaneously until the first planned day of apheresis. Plerixafor 240 mcg/kg was administered when the peripheral CD-34+ cell-count was < 20/ uL on day 5 of G-CSF use. All patients received Melphalan conditioning either at 140 or 200 mg/m2 at treating physician’s discretion based on co-morbidities and functional status. Per institutional policy, Melphalan140 mg/m2 was used for pts > 65 years, those with significant comorbidities and imapired renal function.
Engraftment
Neutrophil engraftment was defined as the first of the 3 consecutive days with absolute neutrophil count greater than 0.5 X109/L (ANC500) without growth factor support and platelet engraftment was defined as platelet value of greater than 20 X 109/L (PLT20) without transfusion support for 7 consecutive days per Center for International Blood and Marrow transplant Research (CIBMTR) definitions. ALC-30 was defined as absolute lymphocyte count at day 30 (+/−7days).
Outcome Measures
The primary end point was to compare the absolute lymphocyte count at day 30 post-transplant (ALC-30) between the G and G+P groups. Secondary end points were to compare the PFS and OS between the two groups and to evaluate the associations between predefined factors (age, race, stage at diagnosis, melphalan dose, disease status at transplant, ALC30, post-transplant maintenance, pre-transplant induction with doublet or triplet therapy, lines of treatment prior to transplant) and outcomes (PFS and OS). The distribution for the cause of death was compared between the two groups.
Statistical Analysis
Baseline patient characteristics were summarized using count and percentage for categorical variables and median and range for continuous variables. Patient baseline characteristics were further compared between two groups of patients with G and G+P. Kruskal-Wallis tests were used to compare two groups for continuous variables and Chi-squared or Fisher’s exact tests for categorical variables. OS was calculated as the time from the date of transplantation to death from any cause. Patients who were alive were considered censored at the date of last observation. PFS was calculated as the time from the date of transplantation to the date of progression or death from any cause. Patients who were alive without progression were considered censored at the date of last observation. Univariable and multivariable Cox proportional hazards regression models were fit to assess associations between nine pre-defined predictors (age, race, stage at diagnosis, melphalan dose, disease status at transplant, ALC30, post-transplant maintenance, doublet/triplet therapy, lines of treatment, G vs G+P group) and outcomes (PFS and OS). The proportional hazards assumption was assessed, and no violation was found.
Results
Baseline Characteristics
Six-hundred and ten patients underwent ASCT between January 2008 and December 2016. Mobilization agents used were G-CSF alone (n= 469) or G-CSF plus plerixafor (n= 141) (Table 1). Median age of patients who were mobilized with G+P was older than those who were mobilized with G only (62 vs. 60 years, p = 0.006). Distribution of patients with respect to sex, race (Caucasian vs non-Caucasians), co-morbidities, ISS Staging (I, II, III) and response to induction therapy before transplant was similar between the two groups (Table 1). Triplet induction therapy (82 vs. 72%, p = 0.015) and more lines of treatment (33 vs. 20%, p = 0.003) were more common in the G+P group (Table 1). Use of 200mg/ m2 dose of Melphalan (69 vs. 58%, p = 0.020) and a higher dose of CD-34 cells infused (3.19 X 106 cells/kg vs. 2.88 X 106 cells/kg, p = 0.001) were more common in the G group (Table 1). Post-transplant maintenance treatment use was similar between the two groups. However, use of post-transplant lenalidomide use was less (46 vs 57%, p = 0.034) in the G+P group.
Table 1.
Baseline characteristics of G-CSF vs G-CSF + Plerixafor patients
| G-CSF (N = 469) |
G-CSF + Plerixafor (N = 141) |
All (N = 610) |
Signif. | |
|---|---|---|---|---|
| Age at transplant, year - median (range) | 60 (30–76) | 62 (41–76) | 61 (30–76) | 0.006 |
| Sex – no. (%) | 0.770 | |||
| Male | 274 (58) | 85 (60) | 359 (59) | |
| Female | 195 (42) | 56 (40) | 251 (41) | |
| Race - no. (%) | 0.445 | |||
| Caucasian | 330 (70) | 107 (76) | 437 (72) | |
| African-American | 129 (28) | 32 (23) | 161 (26) | |
| Others | 10 (2) | 2 (1) | 12 (2) | |
| Stage at Diagnosis – no. (%) | >0.99 | |||
| 1 | 70 (15) | 20 (14) | 90 (15) | |
| 2 | 110 (23) | 32 (23) | 142 (23) | |
| 3 | 269 (57) | 77 (55) | 346 (57) | |
| Unknown | 20 (4) | 12 (9) | 32 (5) | |
| Disease status at transplant – no. (%) | 0.575 | |||
| CR | 77 (16) | 21 (15) | 98 (16) | |
| VGPR | 153 (33) | 47 (33) | 200 (33) | |
| PR | 163 (35) | 44 (31) | 207 (34) | |
| SD | 47 (10) | 21 (15) | 68 (11) | |
| PD | 28 (6) | 8 (6) | 36 (6) | |
| Sig CoExist Disease - no. (%) | 0.147 | |||
| Yes | 413 (88) | 117 (83) | 530 (87) | |
| No | 54 (12) | 23 (16) | 77 (13) | |
| Doublet vs Triplet Therapy – no. (%) | 0.015 | |||
| Doublet | 132 (28) | 25 (18) | 157 (26) | |
| Triplet | 337 (72) | 116 (82) | 453 (74) | |
| Lines of Tx - median (range) | 1 (1–6) | 1 (1–5) | 1 (1–6) | 0.002 |
| Lines of Tx - no. (%) | 0.003 | |||
| ≤1 | 374 (80) | 95 (67) | 469 (77) | |
| >1 | 95 (20) | 46 (33) | 141 (23) | |
| Melphalan (mg/ m2) - median (range) | 0.020 | |||
| 140 | 146 (31) | 59 (42) | 205 (34) | |
| 200 | 323 (69) | 82 (58) | 405 (66) | |
| Infused CD34 - median X 106(range) | 3.19 (1.77–16.9) | 2.88 (1.71–7.2) | 3.125 (1.71–16.9) | 0.001 |
| ANC500 (day) - median (range) | 12 (10–27) | 12 (11–18) | 12 (10–27) | 0.810 |
| ALC30 (K/uL) - median (range) | 1.3 (0.1–4.5) | 1.2 (0.1–5.1) | 1.3 (0.1–5.1) | 0.608 |
| ALC30 (K/uL) - no. (%) | 0.369 | |||
| ≤1 | 173 (37) | 46 (33) | 219 (36) | |
| >1 | 296 (63) | 95 (67) | 391 (64) | |
| Post-transplant maintenance - no. (%) | 0.892 | |||
| Yes | 323 (69) | 83 (59) | 406 (67) | |
| No | 81 (17) | 22 (16) | 103 (17) | |
| Unknown | 65 (14) | 36 (26) | 101 (17) | |
| Post-transplant Lenalidomide - no. (%) | 0.034 | |||
| Yes | 265 (57) | 65 (46) | 330 (54) | |
| No | 204 (43) | 76 (54) | 280 (46) | |
Engraftment Outcomes
The median ALC30 was 1.3 K/uL (range, 0.1–4.5) and 1.2 K/uL(range, 0.1–5.1) for G and G+P groups, respectively (p = 0.608) (Table 1). In addition, absolute monocyte count was similar between the two groups with AMC30 of 0.7 K/uL in both group (p = 0.237). Median day to neutrophil recovery was similar in both groups (ANC 500 at Day 12, p = 0.810) (Table 1). Median day to platelet recovery (PLT20) was 21 days for the G group and 20 days for G+P group (p=.238).
Survival Outcomes
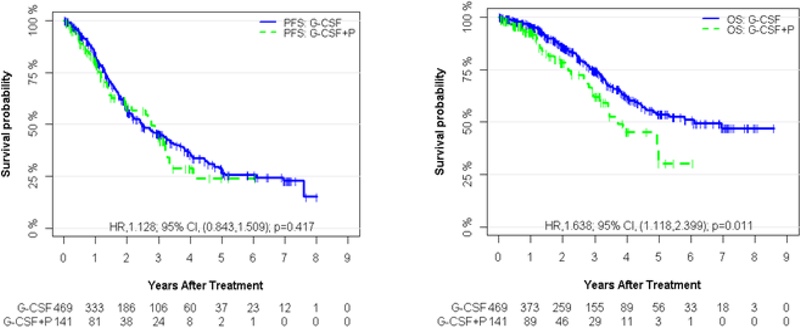
The median PFS was 2.46 years (95% CI, 2.14– 3.15) and 2.77 years (95% CI, 1.99–3.27) for G and G +P, respectively (HR: 1.13; 95% CI, 0.84–1.50; p=0. 417) (Figure 1a). The median follow-up time for PFS was 2.99 years (95% CI, 2.48–3.13) in the G and 1.92 years (95% CI, 1.20–2.53) in the G + P group, respectively. The median OS was 6.09 years (95% CI, 4.55-NR) for G group compared to 3.73 years (95% CI, 3.20-NR) for the G+P group (HR: 1.64; 95% CI, 1.12–2.40; p=0. 011) (Figure 1b). The median follow-up time for OS was 2.53 years (95% CI, 2.13–2.99) and 1.59 years (95% CI, 1.17–2.02) for G and G+ P group, respectively. 115 of 469 patients (25%) in the G group and 35 of 141 patients (25%) in the G+P group had died at the time of the data analysis and the causes of death were similar between the two groups, with disease-related cause being the most common (77% vs 66%), followed by infections (5% vs 1%) and secondary malignancies (4% vs 0%) (Table 2) with a p value of 0.116.
Figure 1.
PFS and OS of G-CSF vs G-CSF + Plerixafor groups. a) Kaplan-Meier curves of progression-free survival (PFS) according to G-CSF and GCSF + Plerixafor (G-CSF+P). The median PFS is 2.46 years (95% CI, 2.14 to 3.15) and 2.77 years (95% CI, 1.99 to3.27) for G-CSF and G-CSF+P, respectively. b) Kaplan-Meier curves of overall survival (OS) according to G-CSF and G-CSF + Plerixafor (G-CSF+P). The median OS is 6.09 years (95% CI, 4.55 to NR) and 3.73 years (95% CI, 3.20 to NR)for G-CSF and G-CSF+P, respectively.
Table 2.
Univariable and multivariable Cox regression analyses of factors associated with PFS and OS
| PFS | OS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted* | Adjusted$ | Unadjusted* | Adjusted$ | ||||||
| HR (95% CI) | Signif. | HR (95% CI) | Signif. | HR (95% CI) | Signif. | HR (95% CI) | Signif. | ||
| Age | |||||||||
| <=60 >60 |
Reference 0.988 (0.785,1.244) |
0.919 | Reference 0.897 (0.691,1.165) |
0.416 | Reference 1.519 (1.095,2.107) |
0.012 | Reference 1.293 (0.891,1.877) |
0.176 | |
| Race | |||||||||
| Caucasian Others |
Reference 1.251 (0.974,1.608) |
0.080 | Reference 1.125 (0.851,1.487) |
0.410 | Reference 1.077 (0.757,1.53) |
0.681 | Reference 1.016 (0.684,1.508) |
0.939 | |
| Stage at diagnosis | |||||||||
| 1 2 3 |
Reference 1.209 (0.781,1.87) 1.826 (1.256,2.655) |
0.394 0.002 |
Reference 1.435 (0.903,2.28) 1.896 (1.27,2.83) |
0.126 0.002 |
Reference 2.1 (1.02,4.326) 3.25 (1.701,6.209) |
0.044 <0.001 |
Reference 2.475 (1.153,5.314) 3.355 (1.679,6.704) |
0.020 0.001 |
|
| Melphalan dose | |||||||||
| 200 <200 |
Reference 1.370 (1.077,1.743) |
0.010 | Reference 1.238 (0.939,1.631) |
0.130 | Reference 2.025 (1.465,2.798) | <0.001 | Reference 1.527 (1.049,2.223) |
0.027 | |
| Disease status at transplant | |||||||||
| Response Non-Response |
Reference 1.346 (1.067,1.699) |
0.012 | Reference 1.387 (1.07,1.799) |
0.014 | Reference 1.39 (1.002,1.93) |
0.049 | Reference 1.454 (1.006,2.099) |
0.046 | |
| ALC30 | |||||||||
| >1 ≤1 |
Reference 1.023 (0.806,1.298) |
0.854 | Reference 0.983 (0.759,1.273) |
0.896 | Reference 1.114 (0.8,1.549) |
0.523 | Reference 0.991 (0.694,1.414) |
0.959 | |
| Post-transplant Maintenance | |||||||||
| Yes No |
Reference 1.378 (1.005,1.888) |
0.046 | Reference 1.467 (1.051,2.049) | 0.024 | Reference 1.775 (1.151,2.737) |
0.009 | Reference 2.075 (1.303,3.304) |
0.002 | |
| Doublet vs Triplet Therapy | |||||||||
| Triplet Doublet |
Reference 1.115 (0.872,1.427) |
0.385 | Reference 1.126 (0.864,1.469) |
0.380 | Reference 1.159 (0.831,1.617) |
0.385 | Reference 1.224 (0.853,1.755) |
0.273 | |
| Lines of treatment | |||||||||
| ≤1 >1 |
Reference 1.659 (1.288,2.137) |
<0.001 | Reference 1.413 (1.065,1.874) |
0.017 | Reference 1.695 (1.204,2.386) | 0.002 | Reference 1.292 (0.884,1.888) |
0.186 | |
| Group | |||||||||
| G-CSF G-CSF+P |
Reference 1.128 (0.843,1.509) |
0.417 | Reference 1.084 (0.785,1.498) |
0.623 | Reference 1.638 (1.118,2.399) |
0.011 | Reference 1.651 (1.089,2.503) |
0.018 | |
Abbreviations: PFS, progression-free survival; OS, overall survival; HR, Hazard ratio; CI, Confidence interval.
, Univariable Cox regression analysis;
, Multivariable Cox regression.
Multivariable Analysis
In multivariable analysis (MVA) with predermined variables as defined in the statistics section, higher stage at diagnosis, less than PR (SD or PD) before ASCT, and no post-transplant maintenance therapy were associated with worse PFS and OS (Table 3). In addition, more than one line of treatment prior to transplant was associated with worse PFS. Use of G+P for mobilization and Melphalan dose of 140 mg/ m2 were associated with worse OS. ALC-30 >1,000/μL was not associated with difference in PFS or OS in our analysis (Table 3).
Table 3.
PFS and OS in G-CSF vs G-CSF + P groups in other studies
| Study | (G-CSF) PFS | (G-CSF) OS | (G-CSF + P) PFS | (G-CSF + P) OS |
|---|---|---|---|---|
| Moreb et al Ref [23] |
- | - | 22 months (n=8) | 40 months (n=8) |
| Moreb et al Ref [24] |
44 months (n=232) | 93 months (n=232) | 21 months (n=7) | 53 months (n=7) |
| Valtola et al Ref [8] |
13 months (n=20) | - | 19 months (n=7) | - |
| Our Study | 30 months (n=469) | 73 months (n=469) | 33.6 months (n=161) | 44 months (n=161) |
Discussion
This study represents a large retrospective study looking at engraftment and survival differences between patients undergoing ASCT for MM with stem cell grafts mobilized with G-CSF vs Poor mobilizers who required use of plerixafor in addition to G-CSF. ALC30, the primary end point of this study, was similar between the two groups. We conclude that the use of plerixafor for augmentation of stem cell mobilization did not alter the ALC30, contrary to the hypothesis that plerixafor can mobilize more lymphocytes [11, 12]. The patient population that received plerixafor in our study was composed of poor mobilizers which could have negated the effect of plerixafor on the mobilization of the lymphocytes as observed in a previous study [19]. An alternative explanation could be that the kinetics of lymphocyte mobilization may be altered due to the use of G-CSF in the previous days. It would be interesting to further analyze the subset of lymphocytes such as natural killer (NK) cells to see whether the combination of G+P can mobilize more NK cells than G alone, as more NK cells have been associated with better survival after transplant [19, 20].
Overall, 141 of 610 (23%) patients in this study were poor mobilizers who required plerixafor as a secondary mobilization agent. This rate of failure of mobilization with G-CSF is similar to previous studies [1, 2]. The extent of prior chemotherapy (time of exposure and lines of treatment) and radiation have been associated with poor stem cell mobilization [21]. In our study, patients who received plerixafor were more likely to have received more than one line of treatment as well as triplet therapy compared to those who received G alone. In addition the median time from diagnosis to transplant was longer in the group that required use of plerixafor by 44 days (274 days vs. 230 days). Older age is a well-recognized risk factor known to cause low yield of PBSCs [22]. Patients who were in G+P group were on average two years older (62 vs 60); however, the difference of 2 years might not be clinically significant.
PFS was similar between the G and G+P groups (30 vs 33.6 months) in this study, despite the fact that patients who required plerixafor had a lower median dose of Melphalan and more previous chemotherapy. Compared to previous small retrospective studies that looked at use of plerixafor in poor-mobilizers (PFS of 22, 21, and 19 months in other studies), the PFS of 33.6 months in our patients who used G+P is superior [8, 23, 24] (Table 4). This observation could be related to rigorous use of maintenance therapy in our study (at least 67% of the patients).
Table 4.
PFS and OS in G-CSF vs G-CSF + P groups in other studies
| Study | (G-CSF) PFS | (G-CSF) OS | (G-CSF + P) PFS | (G-CSF + P) OS |
|---|---|---|---|---|
| Moreb et al Ref [23] |
- | - | 22 months (n=8) | 40 months (n=8) |
| Moreb et al Ref [24] |
44 months (n=232) | 93 months (n=232) | 21 months (n=7) | 53 months (n=7) |
| Valtola et al Ref [8] |
13 months (n=20) | - | 19 months (n=7) | - |
| Our Study | 30 months (n=469) | 73 months (n=469) | 33.6 months (n=161) | 44 months (n=161) |
Infused-CD34 cells were lower by only 0.3 X 106 (3.19 X 106 vs 2.88 X 106) cells/kg after the use of plerixafor in the G+P group which is not be clinically meaningful. Our data supports the use of plerixafor for augmentation in poor-mobilizers, allowing for sufficient CD-34 cells to be collected for the transplant, and these patients can expect similar PFS.
Overall survival of 44 months in this study in the G+P group is comparable to 40 and 53 months seen in previous small studies [23, 24](Table 4). However, OS in our study was worse for group of patients who were poor mobilizers compared to G group (44 months vs 73 months). Melphalan dosing correlated with OS in our multivariable analysis, with improved OS seen in patients receiving 200 mg/ m2 rather than 140 mg/ m2 of melphalan. Although use of plerixafor was associated with worse OS in our patient population; this has not been observed in previous studies and could be related to risk-based selection of poor mobilizers for the plerixafor group [8, 23, 24]. Cause of death did not differ between those receiving plerixafor and those who did not. The number of patients in the G+P group who received melphalan does of 200 mg/m2 and lenalidomide maintenance were lower which have been associated with worse OS. In addition, information regarding treatment at the time of post-transplant progression, which could influence OS, was not available. No patient in the G+P group received a second transplant while 11 patients in the G group received a second transplant
Limitations of this study include its retrospective nature and variation in baseline characteristics of the two groups of patients. Also lack of data on post-transplant progression treatment should be noted, and the association of plerixafor use with OS should be interpreted with caution. Follow-up periods were different for the two groups due to study’s retrospective nature and patients could have been exposed to different post-transplant treatments based on drug approvals and practice.
In conclusion, our experience highlights the feasibility of using the day 5 peripheral blood CD-34 count to determine the need for plerixafor in a risk adapted strategy. This strategy is cost effective and provides acceptable PFS in patient who otherwise may not have been able to undergo ASCT due to poor mobilization.
Supplementary Material
Acknowledgments
AD designed the research; HS and PS collected the data, SK analyzed the data, HS and AD wrote the manuscript. All authors reviewed and approved the final version of the manuscript.
Footnotes
Declarations of interest: None
References
- 1.Gertz MA, et al. , Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant, 2010. 45(9): p. 1396–403. [DOI] [PubMed] [Google Scholar]
- 2.Pusic I, et al. , Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant, 2008. 14(9): p. 1045–1056. [DOI] [PubMed] [Google Scholar]
- 3.Domingues MJ, Nilsson SK, and Cao B, New agents in HSC mobilization. Int J Hematol, 2017. 105(2): p. 141–152. [DOI] [PubMed] [Google Scholar]
- 4.DiPersio JF, et al. , Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood, 2009. 113(23): p. 5720–6. [DOI] [PubMed] [Google Scholar]
- 5.Nademanee AP, et al. , Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial. Biol Blood Marrow Transplant, 2012. 18(10): p. 1564–72. [DOI] [PubMed] [Google Scholar]
- 6.Micallef IN, et al. , Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biol Blood Marrow Transplant, 2013. 19(1): p. 87–93. [DOI] [PubMed] [Google Scholar]
- 7.Devine SM, et al. , Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood, 2008. 112(4): p. 990–8. [DOI] [PubMed] [Google Scholar]
- 8.Valtola J, et al. , Blood graft composition and post-transplant recovery in myeloma patients mobilized with plerixafor: a prospective multicenter study. Leuk Lymphoma, 2019. 60(2): p. 453–461. [DOI] [PubMed] [Google Scholar]
- 9.Toor AA, et al. , Favourable results with a single autologous stem cell transplant following conditioning with busulphan and cyclophosphamide in patients with multiple myeloma. British Journal of Haematology, 2004. 124(6): p. 769–776. [DOI] [PubMed] [Google Scholar]
- 10.Bolwell BJ, et al. , Patients mobilizing large numbers of CD34+ cells (‘super mobilizers’) have improved survival in autologous stem cell transplantation for lymphoid malignancies. Bone Marrow Transplant, 2007. 40(5): p. 437–41. [DOI] [PubMed] [Google Scholar]
- 11.Tanhehco YC, et al. , The evolving role of plerixafor in hematopoietic progenitor cell mobilization. Transfusion, 2013. 53(10): p. 2314–26. [DOI] [PubMed] [Google Scholar]
- 12.Porrata LF et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood.98, 579–586 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Rueff J, et al. , Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant, 2014. 20(6): p. 896–9. [DOI] [PubMed] [Google Scholar]
- 14.Hiwase DK, et al. , Higher infused lymphocyte dose predicts higher lymphocyte recovery, which in turn, predicts superior overall survival following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant, 2008. 14(1): p. 116–24. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann T, et al. , Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev, 2015(10): p. CD010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spoerl S, et al. , Patients’ outcome after rescue plerixafor administration for autologous stem cell mobilization: a single-center retrospective analysis. Transfusion, 2017. 57(1): p. 115–121. [DOI] [PubMed] [Google Scholar]
- 17.Micallef IN, et al. , Plerixafor Plus Granulocyte Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma and Multiple Myeloma: Long-Term Follow-Up Report. Biol Blood Marrow Transplant, 2018. 24(6): p. 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S, et al. , International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. The Lancet Oncology, 2016. 17(8): p. e328–e346. [DOI] [PubMed] [Google Scholar]
- 19.Varmavuo V, et al. , Blood graft lymphocyte subsets after plerixafor injection in non-Hodgkin’s lymphoma patients mobilizing poorly with chemotherapy plus granulocyte-colony-stimulating factor. Transfusion, 2012. 52(8): p. 1785–91. [DOI] [PubMed] [Google Scholar]
- 20.Porrata LF, et al. , Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant, 2008. 14(7): p. 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shpall EJ, Champlin R. & Glaspy JA Effect of CD34 peripheral blood progenitor cell dose on hematopoietic recovery. 92, 84–92 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Bailen R, et al. , Factors predicting peripheral blood progenitor cell mobilization in healthy donors in the era of related alternative donors: Experience from a single center. J Clin Apher, 2019. [DOI] [PubMed] [Google Scholar]
- 23.Moreb JS, et al. , Long-Term Outcome after Autologous Stem Cell Transplantation with Adequate Peripheral Blood Stem Cell Mobilization Using Plerixafor and G-CSF in Poor Mobilizer Lymphoma and Myeloma Patients. Adv Hematol, 2011. 2011: p. 517561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreb JS, et al. , Poor peripheral blood stem cell mobilization affects long-term outcomes in multiple myeloma patients undergoing autologous stem cell transplantation. J Clin Apher, 2018. 33(1): p. 29–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.