Abstract
Introduction
In contemporary oncology drug development, implementation of novel early-phase designs with the ability to address multiple research objectives is needed to better refine regimens. This paper describes an adaptive design strategy for identifying a range of optimal regimens based on two endpoints within multiple cohorts. The proposed design was developed to address objectives in an early-phase trial of cancer vaccines in combination with agonistic antibodies to CD40 and CD27.
Materials and methods
We describe a model-based design strategy that was developed for a trial evaluating the safety and immunogenicity of vaccination with (1) peptides plus CD40 antibody and TLR3 ligand, (2) systemic administration of an agonistic CD27 antibody, and (3) to assess immune response from (1) and (2) compared to optimal controls in participants with stage IIB-IV melanoma.
Results and conclusions
The proposed design is a practical adaptive method for use with combined immunotherapy regimens with multiple objectives within multiple cohorts of interest. Further advances in the effectiveness of cancer immunotherapies will require new approaches that include redefining optimal strategies to take multiple regimens forward into later phases, incorporating additional endpoints in the dose selection process and testing drug combination therapies to improve efficacy and reduce toxicity. Our goal is to facilitate the acceptance and application of more novel designs in contemporary early development trials.
Electronic supplementary material
The online version of this article (10.1007/s00262-019-02442-5) contains supplementary material, which is available to authorized users.
Keywords: Early-phase, Adaptive design, Cancer vaccines, Combination
Introduction
In recent years, the paradigm of early-phase clinical trial design has shifted in an attempt to address the numerous challenges offered by the current landscape of oncology drug development [1]. Further advances in assessing the safety and clinical efficacy of novel agents and combination of agents require different design assumptions to appropriately determine the maximum tolerated dose (MTD) or optimal biologic dose (OBD) in Phase I trials, compared to the historical assumptions made to assess general cytotoxic agents (e.g., chemotherapy). The traditional approach to Phase I clinical trial design is ill-suited for generalization to more contemporary studies [2, 3]. There is an expanding need to conduct novel study designs that focus on the clinical issues and statistical considerations emerging from new treatment paradigms [4]. Modern early-phase studies are asking multiple research questions that potentially involve simultaneously accounting for numerous factors. For instance, it may of interest to carry forward into middle development a variety of combination regimens in various participant cohorts based on multiple endpoints (i.e., toxicity and activity). This is a substantial departure from the problem of identifying the MTD of a single, cytotoxic agent. In the presence of such complexities, it is unlikely that there will exist a dose-finding method that is directly applicable to the mixture of research questions being studied. Rather than attempting to reduce the problem to a setting in which one could straightforwardly apply an “off-the-shelf” method, potentially missing the opportunity to answer promising and relevant research questions, there is an increasing demand to tailor early-phase trial designs to the research questions being posed to treat study participants as efficiently and pragmatically as current information dictates.
This article describes the design of an early-phase, prospective trial evaluating the safety and biologic activity of combination regimens in concurrent studies of two cohorts, with the goal of recommending a range of regimens for further testing in each cohort. The design was motivated by a proposed study at the University of Virginia (UVA) Cancer Center investigating vaccination with (a) peptides plus CD40 antibody and TLR3 ligand, (b) systemic administration of an agonistic CD27 antibody, and (c) to assess immune response from (a) and (b) compared to controls in participants with stage IIB-IV melanoma. The clinical success of checkpoint blockade agents in multiple cancers has established a clear platform on which to build the next generation of more potent cancer immunotherapies. Cancer vaccines inducing antigen-specific T cell responses are emerging as a component of combination immunotherapy. After several decades of development and optimization [5–9], there is now evidence that some cancer vaccines may improve clinical outcomes, in particular in combination with other active therapy [10–13]. The frequency and severity of adverse events observed in cancer vaccine approaches necessitate a change in the way early-phase clinical trials of these treatment strategies are designed and conducted [14–17]. There are no existing dose-finding methods that could directly address the multitude of challenges presented by the wide range of study research objectives, so our team needed to adapt relevant components of existing methods in developing a flexible design strategy. Funding of the clinical trial was secured but changes in access to one of the study agents ultimately lead to further design modifications with a more limited scope. The tailoring of the original design, described herein, to the complex research objectives being posed in the study provides the framework via example to demonstrate how adaptive designs can be modified within a single trial to address multiple study specific objectives to advance early development of novel treatment regimens. Thus, the aim of this article is to provide a blueprint on how to approach complex early-phase trials with multiple objectives in various cohorts.
Methods
Design considerations
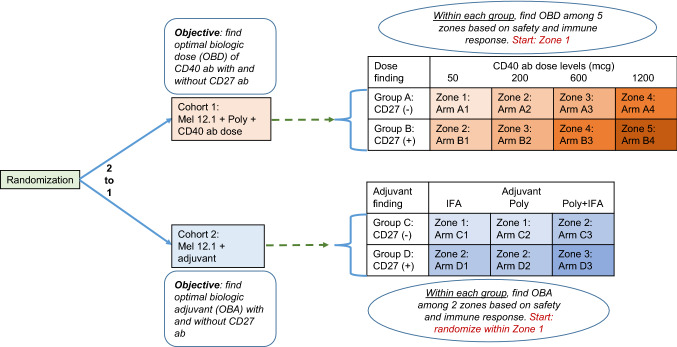
This paper describes the proposed design of an open-label, randomized, phase I-II study to test the safety and immunogenicity of vaccination with 12 class I major histocompatibility complex–restricted melanoma peptides plus tetanus helper peptide (Mel12.1) [18–20] with specified combinations of the TLR3 agonist polyICLC [21], incomplete Freund’s adjuvant (IFA) [22], agonistic CD27 antibody, and agonistic CD40 antibody. The complex design structure is provided in Fig. 1. The term ‘cohort’ represents the inclusion or exclusion of CD40 antibody, the term ‘group’ represents the inclusion or exclusion of CD27 antibody within a cohort, the term ‘dose level’ represents the various doses of CD40 antibody (in mcg), the term ‘arm’ represents each specified treatment combination, the term ‘adjuvant’ represents control adjuvants including IFA alone, polyICLC alone, and IFA + polyICLC, and the term ‘zone’ represents sets of arms in which the safety profile ordering is assumed to be unknown. Participants in Groups A and B would have received the various doses of CD40 antibody (+ Mel12.1 + polyICLC), with and without CD27 antibody, respectively, and they would have been tested in cohort 1 (Fig. 1). Participants in Groups C and D would have received Mel12.1 with adjuvants (IFA, polyICLC, or IFA + polyICLC), with and without CD27 antibody, respectively, and they would have been tested in cohort 2 (Fig. 1). The arms in Fig. 1 are grouped into “zones” based on the dose levels (cohort 1) and the number of adjuvants (cohort 2). The trial was designed to find the optimal biologic doses (OBD) of CD40 antibody among arms within each set of predefined Groups A and B, and the optimal biologic adjuvant (OBA) among arms within each of the control Groups C and D. The primary outcomes for determination of the OBDs and OBAs included the frequency of treatment-related dose-limiting toxicities (DLTs) and frequency of immunologic response. Although few DLTs are expected with these vaccines, we still must monitor for safety and protect against the unexpected. Within each group, the first goal was to determine the set of possible optimal arms, where an optimal arm is one that was estimated to have an acceptable toxicity profile as measured by DLTs, and a high rate of immunologic response as defined below. A DLT is defined as any unexpected adverse event that is possibly, probably or definitely related and (1) ≥ Grade 3, (2) ≥ Grade 1 ocular adverse events, (3) ≥ Grade 2 allergic/autoimmune reactions. The primary immunological response of interest was the CD8 + T cell response to the defined minimal peptides epitopes represented by Mel12.1, assessed primarily by ELIspot assay. Assessment of immunologic response was based upon a fold-increase over the maximum of the two negative controls. Evaluation of T cell responses was based on the following definitions: Nvax = number CD8 + T cells responding to vaccine peptide; Nneg = number CD8 + T cells responding to maximum negative control; Rvax = Nvax/Nneg. A participant was considered to have a positive CD8 + T cells response to vaccination (binary yes/no), only if all of the following criteria were met at any post-vaccination time point: (1) Nvax exceeded Nneg by at least 20 cells/100,000 CD8 + PBMC, (2) (Nvax − 1 SD) ≥ (Nneg + 1 SD), and (3) Rvax after vaccination ≥ 2 × Rvax pre-vaccine. Pre-vaccine Rvax values less than one (e.g., control counts exceed number of responding CD8 + T cells) were set equal to one to indicate no response and to prevent overinflating adjusted fold-increases due to pre-vaccine ratios less than one, or division by zero. A secondary objective was, if more than one arm was contained within the range of optimal arms, to estimate the difference in immunologic response rates among the range of optimal arms.
Fig. 1.
Schema of illustrating the proposed trial design. Cohort, group, arm, and zone designation for the proposed study to test safety and immunogenicity of vaccination with the 12 class I major histocompatibility complex-restricted melanoma peptides (Mel12.1) with specified combinations of polyICLC (poly), incomplete Freund’s adjuvant (IFA), CD40 antibody, and with/without CD27 antibody (±)
Estimation
Within cohort, it assumed that, as the dose level of CD40 antibody increases (cohort 1) or the number of adjuvants increases (cohort 2), the probability of DLT does not increase. Thus, arms in higher zones are assumed to have probabilities of DLT that are higher than or equal to probabilities of arms in lower zones. Whether arms within the same zone have higher or lower DLT probabilities than one another is assumed to be unknown. For instance, in cohort 1, it may be that arm A2 < arm B1 or that arm B1 < arm A2 with respect to their true probability of DLT. The relationship between arm A2 and arm B1 corresponds to a decrease in the dose of CD40 antibody but increase of CD27 antibody. Similar statements could be made about the relationship between other arms, such as arms C3 and D2, for example. Multiple one-parameter models that convey various possible relationships among the probabilities of DLT were used to account for the toxicity uncertainty among arms. A model selection procedure was employed to sequentially choose the model that is most consistent with the accumulating data [23]. One of the models recommended by the continual reassessment method (CRM; [24]) is expressed through a set of pre-specified constants, termed the ‘skeleton’ of the model, raised to an exponent , where is a parameter to be adaptively estimated by the accumulating data [25]. The method described in Lee and Cheung [26] was used to produce the skeleton values for each model, which were chosen to generate robust operating characteristics in a wide spectrum of scenarios. Estimation of DLT probabilities relied upon a selected set of working models corresponding to possible “shifts” between the arms that define the acceptable set in each group within each cohort [27, 28]. Based on assumed DLT probability relationships between arms, the set of arms that is considered “acceptable” in one group within a cohort may be shifted zero (Model 1), one (Model 2), or two (Model 3) dose levels or adjuvants away from those considered acceptable in the other group within that cohort. Table 1 illustrates shifts models for cohort 1, and Table 2 illustrates the shift models for cohort 2. The shift models for cohort 2 are constructed under two possible adjuvant-toxicity relationships; (1) IFA is more toxic than polyICLC, or (2) polyICLC is more toxic than IFA. Based on data from previous studies, it is assumed that the combination of polyICLC and IFA does not have a lower DLT probability than each adjuvant alone [29]. Therefore, we set up models that represented the three possible shifts under each of these possible relationships. Upon accrual of each participant into the trial, the model with the largest likelihood, indicating that it best fits the data, within each cohort, is selected and DLT probability estimates are estimated for each arm using this model. A set of “acceptable” arms, defined as any arm with estimated DLT probability less than or equal to 33%, is specified based on these estimates.
Table 1.
Shift models for the DLT probabilities in Cohort 1
| Mel12.1 and poly + | Model 1 (shift = 0) | Model 2 (shift = 1) | Model 3 (shift = 2) | ||||
|---|---|---|---|---|---|---|---|
| CD27 (−) | CD27 (+) | CD27 (−) | CD27 (+) | CD27 (−) | CD27 (+) | ||
| CD40 dose | 50 | ||||||
| 200 | |||||||
| 600 | |||||||
| 1200 | |||||||
|
↓ Group A |
↓ Group B |
↓ Group A |
↓ Group B |
↓ Group A |
↓ Group B |
||
For each model, the highest acceptable arm in each group is denoted in bold
Table 2.
Shift models for the DLT probabilities in cohort 2
| Mel12.1 + | Model 1 (shift = 0) IFA < PolyICLC | Model 2 (shift = 1) IFA < PolyICLC | Model 3 (shift = 2) IFA < PolyICLC | |||
|---|---|---|---|---|---|---|
| CD27 (−) | CD27 (+) | CD27 (−) | CD27 (+) | CD27 (−) | CD27 (+) | |
| IFA | ||||||
| Poly | ||||||
| Poly + IFA | ||||||
|
↓ Group C |
↓ Group D |
↓ Group C |
↓ Group D |
↓ Group C |
↓ Group D |
|
| Mel12.1 + | Model 4 (shift = 0) IFA > PolyICLC | Model 5 (shift = 1) IFA > PolyICLC | Model 6 (shift = 2) IFA > PolyICLC | |||
|---|---|---|---|---|---|---|
| CD27 (−) | CD27 (+) | CD27 (−) | CD27 (+) | CD27 (−) | CD27 (+) | |
| IFA | ||||||
| Poly | ||||||
| Poly + IFA | ||||||
|
↓ Group C |
↓ Group D |
↓ Group C |
↓ Group D |
↓ Group C |
↓ Group D |
|
For each model, the highest acceptable arm in each group is denoted in bold
Randomization and arm allocation
There were two planned levels of randomization, among cohorts and within cohorts. The first level of randomization (among cohorts) allows for a more efficient study of the research objectives within the two cohorts, concurrently in a single trial, rather than sequentially in two separate studies. The second level of randomization (to zones within cohorts) allows for adequate exploration of the regimens to obtain information more broadly in determining the optimal regimens. In general, eligible participants were to be randomized 2:1 among cohorts 1 and 2, with the initial allocation constraint that the first three study participants were randomized with equal allocation to regimens in zone 1. Randomization within cohort was planned based upon the following allocation rules. Arm allocation was to occur in two stages.
Within group allocation The initial stage was planned to accrue eligible participants in sets of one in each escalating zone until a participant experiences a DLT. The escalation plan for the first stage was based on the grouping of arms into “zones” as previously described. With this dose-escalation design participants could have been accrued and assigned to other open arms within a zone but escalation would not have occurred outside the zone until the minimum follow-up period was observed for the first participant accrued to an arm and all arms within a zone were tried or a DLT was observed. The overall strategy was to monitor safety data continuously to adaptively update a set of acceptable (safe) arms in each group, with which to make allocation decisions based on immune response. The second stage was designed to accrue eligible participants in sets of one and to use the estimation procedure described above to estimate DLT probabilities in each arm. Randomization was based on equal allocation among allowable arms unless a weighted allocation scheme was triggered. A weighted allocation scheme was to be triggered if late responses were observed or to balance arms by participants with tumor accessible for biopsy at study entry. Participants were required to be observed for a minimum of 3 weeks after the initial vaccine for initial escalation between zones, and between the 1st and 2nd participants within an arm. In the absence of DLTs, participants could continue to be entered onto acceptable arms until a DLT occurs (upon which the modeling stage begins), or until at least three participants were treated at every arm in the cohort, at which point allocation was to be based on which arm had the highest immune response.
Arm recommendation Based on the set of acceptable arms, the number of participants accrued to the study at the time a decision is to be made determines the recommended arm for the next participant. The recommended arm for the next participant accrued to the study was chosen at random from the acceptable arms if less than three participants had been treated on any acceptable combination. The recommended arm for the next accrued participant was the acceptable combination with the highest observed immune response rate if at least three participants had been treated on every acceptable arm. The procedure for recommending and allocating an arm for a participant was repeated for the accrual of each new participant and the study was designed to terminate once the stopping rules described below were triggered and enough data about the optimal range of regimens was acquired.
Sample size and accrual
Maximum sample size was driven by the goal of obtaining an adequate amount of data to evaluate the objective of selecting the arm with the highest immune response rate within a group for comparisons, assuming at least one optimal arm had been found. Maximum sample size was set at 105 participants, but the study would have been stopped and the OBD and OBA chosen in a group when a 13th participant was assigned to an arm that has already accrued 12 participants. This decision rule allowed for 12 participants to be accrued to the OBD and OBA within each group. For comparisons between groups, the minimum of 12 was based upon being able to detect at least a fivefold improvement in peak magnitude of immune response on the log scale assuming a coefficient of variation of 1.8 from our historical data [18] (peak magnitude of response 8.4, 15.2) with at least 80% power and a 2-sided 2.5% level test. Alpha was set at 2.5% to adjust for the main paired comparisons of CD40 versus CD27, and each versus control.
Results
We illustrate the behavior of the design described in this article under a set of hypothesized DLT and immune response probabilities, which serve as Scenario 1 in our simulation studies (Supplementary Table S1). They indicate arms A3 and B3 to be the OBDs in Groups A and B, respectively, and they indicate arms C3 and D3 to be the OBAs in Groups C and D, respectively. These arms all have true DLT probabilities under the 33% safety threshold and maximize the immune response rate. The true underlying DLT probabilities are consistent with a shift of 0 between the groups in each cohort. For the sake of brevity, only the data from the first 13 participants in the simulated trial are provided in Table 3. The first eligible participant is randomized to arm C1 (i.e., Mel12.1 + IFA without CD27 antibody) in Group C of cohort 2, and he/she does not experience a DLT. The second eligible participant is randomized to arm A1 in Group A of cohort 1, and he/she does not experience a DLT. Within each cohort and group, escalation proceeds without DLT until participant 6 in cohort 2 (overall participant 10) experiences a DLT on arm C3 in Group C. At this point in the study, the observed data in cohort 2 consist of at least one DLT and one non-DLT, so the modeling stage begins in cohort 2. In Group C, the observed DLT data at arms C1–C3, respectively, are (0/1, 0/1, 1/1). In Group D, the observed DLT data at arms D1–D3, respectively, are (0/1, 0/1, 0/1). Based on these data, the shift models in Table 2 are fit, and Model 4 (shift = 0; polyICLC < IFA) is indicated to be most consistent with the data. Based on this model, the estimated DLT probabilities for both Group C and Group D are = (0.14, 0.08, 0.22), indicating that all arms have estimated DLT probabilities under the 33% threshold and are acceptable in terms of safety. The minimum number of participants (i.e., n = 3) has not yet been accrued to each available arm; so, the next participant accrued to cohort 2 will be randomized with equal probability among the acceptable arms. In cohort 1, escalation proceeds without DLT until participant seven (overall participant 13) experiences a DLT on arm A4 in Group A. At this point in the study, the observed data in cohort 1 consist of at least one DLT and one non-DLT, so the modeling stage begins in cohort 1. In Group A, the observed DLT data at arms A1–A4, respectively, are (0/1, 0/1, 0/1, 1/1). In Group B, the observed DLT data at arms B1–B4, respectively, are (0/1, 0/1, 0/1, 0/0). Based on these data, the shift models in Table 1 are fit, and Model 1 (shift = 0) is indicated to be most consistent with the data. Based on this model, the estimated DLT probabilities in Groups A and B are = (0.04, 0.09, 0.16, 0.24), indicating that all arms have estimated DLT probabilities under the 33% threshold and are acceptable in terms of safety. The minimum number of participants has not yet been accrued to each available arm, so the next participant accrued to cohort 1 will be randomized with equal probability among the acceptable arms. Once at least three participants have been treated at every acceptable arm, the recommended combination for the next participant entered is defined as the “acceptable” combination with the highest observed immune response rate. This process of estimation and allocation continues until the stopping rules described above are triggered. Simulation results demonstrating the operating characteristics of this design based on 1000 simulated trials are provided in Supplementary Tables S1–S4.
Table 3.
Simulated data for the first 13 participants illustrating the described design
| Participant | Cohort | Group | Arm | DLT | Rsp |
|---|---|---|---|---|---|
| 1 | 2 | C | C1 | No | No |
| 2 | 1 | A | A1 | No | No |
| 3 | 2 | D | D1 | No | Yes |
| 4 | 2 | C | C2 | No | No |
| 5 | 1 | B | B1 | No | No |
| 6 | 1 | A | A2 | No | No |
| 7 | 2 | D | D2 | No | No |
| 8 | 2 | D | D3 | No | Yes |
| 9 | 1 | B | B2 | No | Yes |
| 10 | 2 | C | C3 | Yes | Yes |
| 11 | 1 | A | A3 | No | Yes |
| 12 | 1 | B | B3 | No | Yes |
| 13 | 1 | A | A4 | Yes | No |
Discussion
More complex research questions are being posed in early-phase oncology clinical trials, which necessitate design strategies that are tailored to the multiple objectives of the study. Rule-based methods intended for MTD-based dose-finding are inflexible and lack the ability to account for additional complexity presented by contemporary early development trials [30, 31]. We have presented an adaptive design strategy for a proposed early-phase trial of novel vaccination strategies for participants with melanoma, with the aim of identifying a range of combination regimens to carry forward in multiple cohorts based on two binary endpoints. Although the design is presented using cancer vaccines as an illustrative example, the approach described is not specific to early-phase vaccination trials and could be applied to other multiple cohort combination studies with well-defined binary toxicity and activity endpoints. The use of more innovative approaches is being encouraged by the FDA and by others [2, 3, 32, 33]. The description of the general design strategy and thought-process for implementation is the type of information that improves understanding, acceptance, and approval of novel designs [34, 35]. Details of study designs often are not found on sites such as clinicaltrials.gov, therefore, current clinical trials lack the transparency needed to support the timely implementation of novel designs. The aim of this article is to bring to light published examples of novel design strategies that address current study objectives as a means of augmenting the implementation of innovative designs in the future and to demonstrate the flexibility of adaptive designs in satisfying changing design conditions. Display and publication of current or proposed trials that use novel designs are needed to overcome barriers of infrequent implementation of innovative design strategies in early phase trials, so we believe that the current work can aid in the uptake of novel design use. In addition, given the often lengthy timeline between study concepts to protocol completion it is valuable to present design considerations that have a broad application. It is worth noting that even after study completion, journals do not require complete protocols as supplemental material for dose-finding trials, and final clinical trial publications do not have sufficient room to describe the details of novel designs. The proposed study adds to a growing number of trials that have recommended adaptive design strategies to address specific research objectives presented by contemporary early-phase cancer trials [36–38]. This support for adaptive designs will augment efficient early-phase trial design in drug combination studies [30]. Well-performing dose-finding designs can have a tremendous impact on the drug development process [39].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Editor and two reviewers for their comments that lead to an improved paper.
Abbreviations
- DLT
Dose-limiting toxicity
- FDA
Food and drug administration
- IFA
Incomplete Freund’s adjuvant
- Mel12.1
12 Class I MHC-restricted melanoma peptides plus tetanus helper peptide
- MTD
Maximum tolerated dose
- OBA
Optimal biologic adjuvant
- OBD
Optimal biologic dose
- UVA
University of Virginia
Author contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Nolan A. Wages and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Dr. Wages is supported by Grant Number K25CA181638 from the National Cancer Institute. Dr. Petroni is partially supported by Grant Number R01CA142859 from the National Cancer Institute. Drs. Slingluff and Petroni are supported by a Team Science Award from the Melanoma Research Alliance. Supported in part by the Biostatistics Shared Resource, University of Virginia Cancer Center, University of Virginia (P30CA044579).
Compliance with ethical standards
Conflict of interest
Dr. Slingluff is an inventor on patents of peptides for use in clinical trials of cancer vaccines; these patents are held by the University of Virginia Licensing and Ventures Group. He also is on and the University receives funding for his roles as external advisory board member for Immatics, CureVac., and as PI for a clinical trial of a cell-based vaccine sponsored by Polynoma. He also receives support for investigator-sponsored cancer immunotherapy clinical trials from GlaxoSmithKline, Celldex, 3 M, and Merck. All remaining authors have declared no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hobbs B, Barata P, Kanjanapan Y, Paller C, Perlmutter J, Pond GR, Prowell T, Rubin E, Seymour L, Wages NA, Yap T, Feltquate D, Garrett-Mayer E, Grossman W, Hong D, Ivy P, Siu L, Reeves S, Rosner G. Seamless designs: current practice and considerations for early phase drug development in oncology. J Natl Cancer Inst. 2019;111:118–128. doi: 10.1093/jnci/djy196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paoletti X, Ezzalfani M, Le Tourneau C. Statistical controversies in clinical research: requiem for the 3 + 3 design for phase I trials. Ann Oncol. 2015;26:1808–1812. doi: 10.1093/annonc/mdv266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nie L, Rubin EH, Mehrotra N, et al. Rendering the 3 + 3 design to rest: more efficient approaches to oncology dose-finding trials in the era of targeted therapy. Clin Cancer Res. 2016;22:2623–2629. doi: 10.1158/1078-0432.CCR-15-2644. [DOI] [PubMed] [Google Scholar]
- 4.Wages NA, Chiuzan C, Panageas KS. Design considerations for early-phase clinical trials of immune-oncology agents. J Immunother Cancer. 2018;6:81. doi: 10.1186/s40425-018-0389-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Bernatchez C, Sharma P, Radvanyi LG, Hwu P. Advances in the development of cancer immunotherapies. Trends Immunol. 2012;34:90–98. doi: 10.1016/j.it.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikora AG, Jaffarzad N, Hailemichael Y, Gelbard A, Stonier SW, Schluns KS, et al. IFN-alpha enhances peptide vaccine-induced CD8 + T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 10.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hailemichael Y, Woods A, Fu T, et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J Clin Invest. 2018;128:1338–1354. doi: 10.1172/JCI93303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel PM, Ottensmeier CH, Mulatero C, et al. Targeting gp100 and TRP-2 with a DNA vaccine: Incorporating T cell epitopes with a human IgG1 antibody induces potent T cell responses that are associated with favourable clinical outcome in a phase I/II trial. Oncoimmunology. 2018;7:e1433516. doi: 10.1080/2162402X.2018.1433516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillman RO, Cornforth AN, Nistor GI, et al. Randomized phase II trial of autologous dendritic cell vaccines versus autologous tumor cell vaccines in metastatic melanoma: 5-year follow up and additional analyses. J Immunother Cancer. 2018;6:19. doi: 10.1186/s40425-018-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petroni GR. Design issues for early-stage clinical trials. In: Disis M, editor. Immunotherapy of cancer. Totowa: Human Press; 2006. pp. 479–485. [Google Scholar]
- 15.Zohar S, Baldi I, Forni G, Merletti F, Masucci G, Gregori D. Planning a Bayesian early-phase phase I/II study for human vaccines in HER2 carcinomas. Pharm Stat. 2011;10:218–226. doi: 10.1002/pst.450. [DOI] [PubMed] [Google Scholar]
- 16.Cunanan KM, Koopmeiners JS. A Bayesian adaptive phase I-II trial design for optimizing the schedule of therapeutic cancer vaccines. Stat Med. 2017;36:43–53. doi: 10.1002/sim.7087. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Rosner GL, Roden RBS. A Bayesian design for phase I cancer therapeutic vaccine trials. Stat Med. 2018;38:1170–1189. doi: 10.1002/sim.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29:2924–2932. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slingluff CL, Jr, Lee S, Zhao F, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602) Clin Cancer Res. 2013;19:4228–4238. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Nishimura F, Sasaki K, et al. Toll like receptor-3 ligand poly-ICLC promotes the efficacy of peripheral vaccinations with tumor antigen-derived peptide epitopes in murine CNS tumor models. J Transl Med. 2007;5:10. doi: 10.1186/1479-5876-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonhoure F, Gaucheron J. Montanide ISA 51 VG as adjuvant for human vaccines. J Immunother. 2006;29:647–648. [Google Scholar]
- 23.Wages NA, Conaway MR, O’Quigley J. Continual reassessment method for partial ordering. Biometrics. 2011;67:1555–1563. doi: 10.1111/j.1541-0420.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase I clinical trials in cancer. Biometrics. 1990;46:33–48. doi: 10.2307/2531628. [DOI] [PubMed] [Google Scholar]
- 25.Paoletti X, Kramar A. A comparison of model choices for the continual reassessment method in phase I clinical trials. Stat Med. 2009;28:3012–3028. doi: 10.1002/sim.3682. [DOI] [PubMed] [Google Scholar]
- 26.Lee SM, Cheung YK. Model calibration in the continual reassessment method. Clin Trials. 2009;6:227–238. doi: 10.1177/1740774509105076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Quigley J. Theoretical study of the continual reassessment method. J Stat Plan Infer. 2006;136:1765–1780. doi: 10.1016/j.jspi.2005.08.003. [DOI] [Google Scholar]
- 28.Horton BJ, Wages NA, Conaway MR. Shift models for dose-finding in partially ordered groups. Clin Trials. 2019;16:32–40. doi: 10.1177/1740774518801599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbatini P, Tsuji T, Ferran L, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res. 2012;18:6497–6508. doi: 10.1158/1078-0432.CCR-12-2189. [DOI] [PubMed] [Google Scholar]
- 30.Riviere MK, Le Tourneau C, Paoletti X, et al. Designs of drug-combination phase I trials in oncology: a systematic review of the literature. Ann Oncol. 2015;26:1036–1037. doi: 10.1093/annonc/mdu516. [DOI] [PubMed] [Google Scholar]
- 31.Iasonos A, O’Quigley J. Adaptive dose-finding studies: a review of model-guided phase I clinical trials. J Clin Onc. 2014;32:2505–2511. doi: 10.1200/JCO.2013.54.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FDA USDoHaHS, Administration FaD, Research CfBEa. Guidance for Industry Clinical Considerations for Therapeutic Cancer Vaccines (2011). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-considerations-therapeutic-cancer-vaccines
- 33.Rahma OE, Gammoh E, Simon RM, Khleif SM. Is the “3 + 3” dose escalation phase I clinical trial design suitable for therapeutic cancer vaccine development? a recommendation for alternative design. Clin Cancer Res. 2014;20:4758–4767. doi: 10.1158/1078-0432.CCR-13-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iasonos A, Gönen M, Bosl GJ. Scientific review of phase I protocols with novel dose-escalation designs: how much information is needed? J Clin Onc. 2015;33:2221–2225. doi: 10.1200/JCO.2014.59.8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petroni GR, Wages NA, Paux G, Dubois F. Implementation of adaptive methods in early-phase clinical trials. Stat Med. 2017;36:215–224. doi: 10.1002/sim.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wages NA, Slingluff CL, Jr, Petroni GR. Statistical controversies in clinical research: early-phase adaptive design for combination immunotherapies. Ann Oncol. 2017;35:696–701. doi: 10.1093/annonc/mdw681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wages NA, Slingluff CL, Petroni GR. A phase I/II adaptive design to determine the optimal treatment regimen from a set of combination immunotherapies. Contemp Clin Trials. 2015;41:172–179. doi: 10.1016/j.cct.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melssen MM, Petroni GR, Chianese-Bullock KA, et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. J Immunother Cancer. 2019;7:163. doi: 10.1186/s40425-019-0625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conaway MR, Petroni GR. The impact of early phase trial design in the drug development process. Clin Cancer Res. 2019;19:819–827. doi: 10.1158/1078-0432.CCR-18-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.