Abstract
The late (L) domain sequence used by mouse mammary tumor virus (MMTV) remains undefined. Similar to other L domain-containing proteins, MMTV p8 and p14NC proteins are monoubiquitinated, suggesting L domain function. Site-directed mutagenesis of p8, PLPPV, and p14NC, PLPPL, sequences in MMTV Gag revealed a requirement only for the PLPPV sequence in virion release in a position-dependent manner. Electron microscopy of a defective Gag mutant confirmed an L domain budding defect morphology. The equine infectious anemia virus (EIAV) YPDL core L domain sequence and PLPPV provided L domain function in reciprocal MMTV and EIAV Gag exchange mutants, respectively. Alanine scanning of the PLPPV sequence revealed a strict requirement for the valine residue but only minor requirements for any one of the other residues. Thus, PLPPV provides MMTV L domain function, representing a fourth type of retroviral L domain that enables MMTV Gag proteins to co-opt cellular budding pathways for release.
Introduction
Retroviruses assemble into virions that are released from the cell by budding through the cellular membrane, the last step in particle formation. The critical step in this process is the pinching off of the neck of the virus bud to release the virion from the cell. This process requires the use of cellular proteins and pathways to resolve this structure (Bieniasz, 2006; Hurley and Cada, 2018; Johnson, Bleck, and Simon, 2018; Martin-Serrano and Neil, 2011; Pincetic and Leis, 2009; Sette et al., 2013; Votteler and Sundquist, 2013).
In the absence of the cellular assistance, the retroviruses remain attached to either their budding membrane, forming lollipop-like structures (Gottlinger et al., 1991; Huang et al., 1995), or to one another in long strings or arrays of virions released from the cell (Demirov, Orenstein, and Freed, 2002). The Gag polyprotein is the only viral protein strictly required to produce particles from the cell and is the major structural protein of the virion. Gag contains one or more late (L) domain sequences that commandeer cellular budding pathways by binding cellular proteins which ultimately sequester components of the endosomal sorting complex required for transport III (ESCRT III) to the site of viral budding. Here they act with vacuolar protein sorting-associated complex protein 4 (VPS4) to complete the budding process by resolving the neck structure and releasing the virion (Baumgartel et al., 2011; Hurley and Cada, 2018; Johnson, Bleck, and Simon, 2018; Jouvenet et al., 2011; Morita et al., 2011; Muziol et al., 2006; Stuchell-Brereton et al., 2007; Votteler and Sundquist, 2013).
There are currently three recognized retroviral L domain motifs by which retroviruses gain access to the release machinery: PPXY recognized by HECT domain containing E3 ubiquitin ligases, including Nedd4 and Nedd4-like ligases (Heidecker et al., 2007; Jadwin et al.,; Medina et al., 2005), PTAP/PSAP bound by Tsg101 (Garrus et al., 2001; Martin-Serrano, Zang, and Bieniasz, 2001; VerPlank et al., 2001), and YPXnL, recognized by Alix (Strack et al., 2003; von Schwedler et al., 2003). In many cases L domains can act in a positionally independent manner (Li et al., 2002; Parent et al., 1995; Xaing et al., 1996) and can also be exchanged between viruses (Li et al., 2002; Medina et al., 2005; Parent et al., 1995; Shehu-Xhilaga et al., 2004). However, heterologous L domain sequences do not always function in HIV-1 Gag (Martin-Serrano, Perez-Caballero, and Bieniasz, 2004; Ott et al., 2005; Shehu-Xhilaga et al., 2004; Xaing et al., 1996).
The study of L domains and their cognate protein partners that allow them to access the cellular budding and release machinery has increased our understanding of the production of both retroviruses (Bieniasz, 2009; Morita and Sundquist, 2004; Weiss and Gottlinger, 2010) as well as of other enveloped RNA viruses (Craven et al., 1999; Martin-Serrano, Zang, and Bieniasz, 2001; Schmitt et al., 2005). Despite the large body of work in this area, the L domain sequence for mouse mammary tumor virus (MMTV) has not been identified. Here, we identify and characterize an L domain-like sequence used by MMTV Gag to produce virus-like particles and show it is able to provide L domain function to equine infectious anemia virus Gag (EIAV).
Materials and methods
Gag expression constructs and mutant construction
All mutations were introduced into construct using either direct PCR or overlap extension (Horton et al., 1990). MMTV Gag mutants were constructed using the MMTV expression construct pSMt-HYBΔPro (Zabransky et al., 2009), a kind gift of Iva Pichova (Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Prague, Czech Republic) which contains a large internal deletion that eliminates Pol expression from the modified proviral construct. We modified this construct by adding an OLLAS epitope tag (SGFANELGPRLMGK) to the C-terminus of Gag (Park et al., 2008). Addition of this tag had no discernable effect on virus-like particle (VLP) production (data not shown). Mutants constructed in pSMtΔproOLLAS are: p8, the PLPPV at amino acid (aa) positions 246–250 changed to ASVA; p14, the PLPPL at aa positions 577–581 changed to ASVA; p8–p14, the p8 and p14 mutations combined; p8PLPPL, valine at aa 250 changed to L; p8PLPPL–p14, the p8PLPPL and p14 mutation combined, p14PLPPV, the leucine at position 581 changed to valine in the p8 mutant; p8YPDL, a replacement of PLPPV (aa 246–250) with a variant EIAV sequence containing the EIAV L domain core sequence (underlined): QNLYPDLSKIK; p8YPDL–p14, the p8YPDL mutant combined with the p14 mutant; p14YPDL, a replacement of PLPPL (aa 577–581) with the EIAV QNLYPDLSKIK sequence; p8P1–A, proline aa 246 replaced with alanine, p8L2–A; p8P3–A, proline aa 248 replaced with alanine; p8P4–A, proline aa 249 replaced with alanine, p8V–A, valine at aa 250 replaced with alanine; and p8P1–A/P3–A, p8P1–A and P3–A combined. EIAV Gag expression constructs were engineered using pGag.PRE (Patnaik et al., 2002) that contained a 7-amino acid deletion (p9Gag aa 450–457) and an Sbf1 restriction site in the DNA just 3′ of the region encoding the YPDL that inserts 4 residues between aa 461 and aa 464. The release properties of this modified EIAV Gag were indistinguishable from the parent pGag.PRE (data not shown), consistent with the prior finding, of the p9Gag sequence, that YPDL is a main contributor to L domain function (Li et al., 2002; Puffer, Watkins, and Montelaro, 1998). The EIAV plasmid was then used to produce the following constructs: E-PLPPV, the YPDL (aa 457–461, according to the original EIAV Gag sequence) replaced with PLPPV; E-PLxtnd, the YPDL replaced with aa 243–254 of MMTV, RRKPLPPVGFAG. The ΔYPDL mutant, pGag.PRE with replacement of YPDL with SRSA was a kind gift of Eric Freed (Drug Resistance Program, NCI-Frederick, Frederick, MD) and placed in our EIAV Gag background.
Cell culture and lysate preparation
HEK293T cells were cultured in Dulbecco’s modified Eagles medium supplemented with 10% fetal calf serum, 2 mM glutamine, and 100 U per ml penicillin and streptomycin. Transfections were carried out using the TransIT-293® transfection reagent (Mirus Bio, Madison, WI) according to the manufacturer’s recommendations and supernatant and cells were harvested 48 h post-transfection. VLP from cell supernatants were isolated by density centrifugation and prepared for immunoblot analysis as previously described (Ott et al., 1999). Cells were washed once with Dulbecco’s phosphate-buffered saline solution without calcium or magnesium and with 1 mM EDTA, then lysed with 10 mM Tris HCl pH 7.5, 0.1 % SDS, and 40 U/ml of Omnicleave® nuclease (Epicentre Biotechnologies, Madison, WI), and incubated for 2 h at 4°C; then treated with an equal volume of SDS-PAGE gel loading buffer (250 mM Tris-Cl pH 6.8, 8% sodium dodecyl sulfate, 20% vol/vol β-mercaptoethanol, 40% vol/vol glycerol, and 0.02% bromophenol brilliant blue).
Near infrared (nIR) immunoblots
Samples were separated by SDS-PAGE electrophoresis and blotted onto PDVF-FL membranes (Millipore, Billerica, MA) using a semi-dry apparatus as previously described (Ott et al., 1999). Blots were blocked for at least 1 h in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE), then incubated in blocking buffer with one or two primary antiserum(a)/antibody(ies) for at least 2 h. Blots were washed twice with blocking buffer for 10 min then incubated with the appropriate Donkey IRDye 800CW and/or IR Dye680 LT fluorescently labeled secondary antibodies (LI-COR) at a 1:10,000 ratio vol/vol in blocking buffer for at least 2 h. Blots were washed five times for 10 m in blocking buffer, and then the image was captured with an Odyssey infrared imaging system using a laser intensity of between 1 and 5 and analyzed by Odyssey software. All blotting steps were carried out at room temperature. Goat antiserum against EIAV p15MA was produced internally within our program and goat antiserum against MMTV p27CA was obtained from (ViroMed Biosafety Products, Camden, NJ). The actin monoclonal used was obtained from the former Amersham Life Science company. Release factors for the VLPs were determined by dividing the measured MMTV Gag VLP fluorescence values (arbitrary units) by the total Gag signal (VLP + cellular Gag). The cellular Gag values were corrected for loading and cell extract processing differences by normalizing signals from actin staining with a second color. Immunoblots for figures 2B, 4, 5, 6 and 7, are presented in Fig. S1.
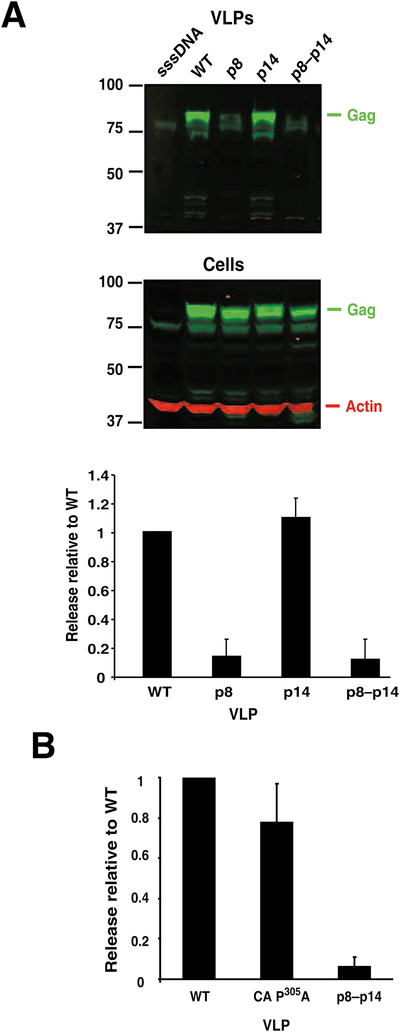
Fig. 2.
Relative release factors of motif mutants. A. An image of an nIR-immunoblot analysis of VLP and cellular lysates from 293T cells transfected with sheared salmon sperm (sssDNA), WT, p8, p14, or p8–p14 constructs is presented with Gag staining pseudocolored in green and actin staining in red. Molecular mass markers are indicated at left and reacting bands identified at right. A graphical summary of our experiments (at least three independent experiments) is presented below the blot. B. A graphical summary of average data from 3 independent experiments with the CA P305A is presented. Standard deviations are indicated by error bars.
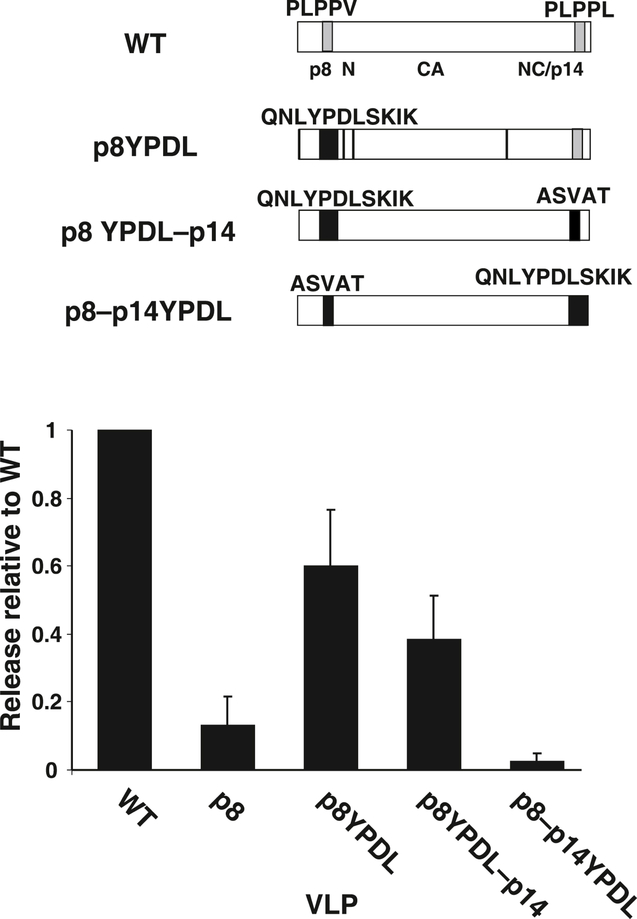
Fig. 4.
Release analysis of YPDL transfer into MMTV Gag. A diagram of mutants tested is presented above a graph of the relative release factors of mutant VLPs from at least 3 independent experiments. Standard deviations are indicated by error bars.
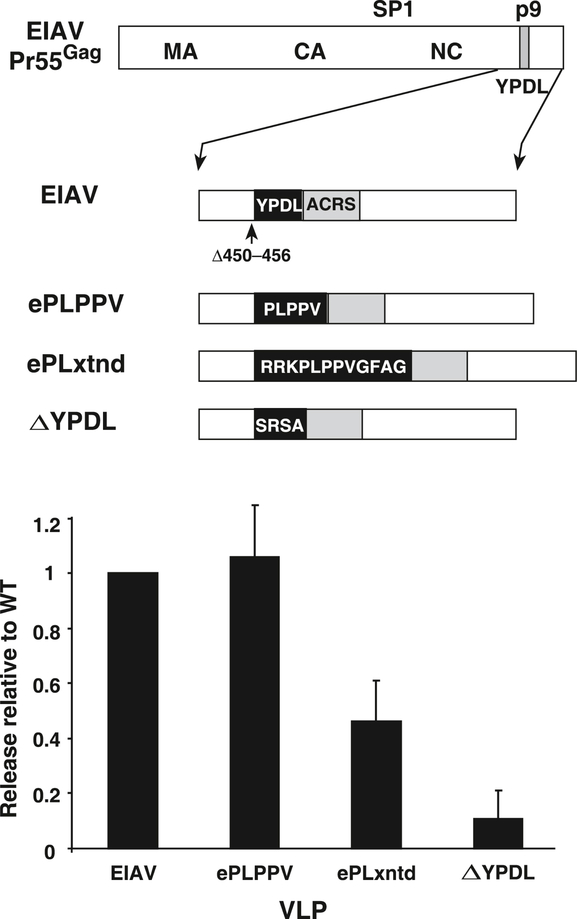
Fig. 5.
Release analysis of PLPPV transfer into EIAV Gag. A diagram of mutants tested is presented above a graph of the relative release factors of mutant VLPs from at least 3 independent experiments. Standard deviations are indicated by error bars.
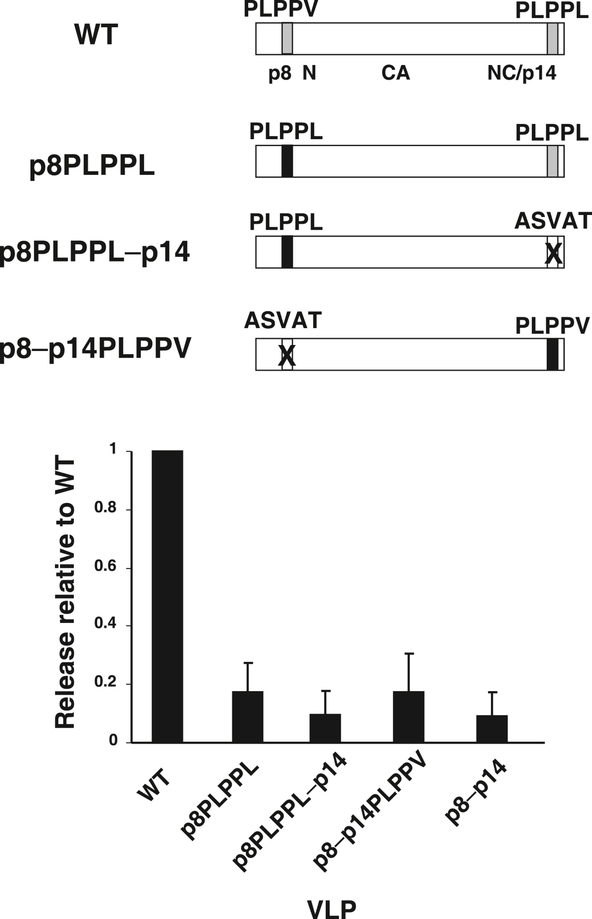
Fig. 6.
Release analysis of position-dependence mutants. A diagram of mutants tested is presented above a graph of the relative release factors of mutant VLPs from at least 3 independent experiments. Standard deviations are indicated by error bars.
Fig. 7.
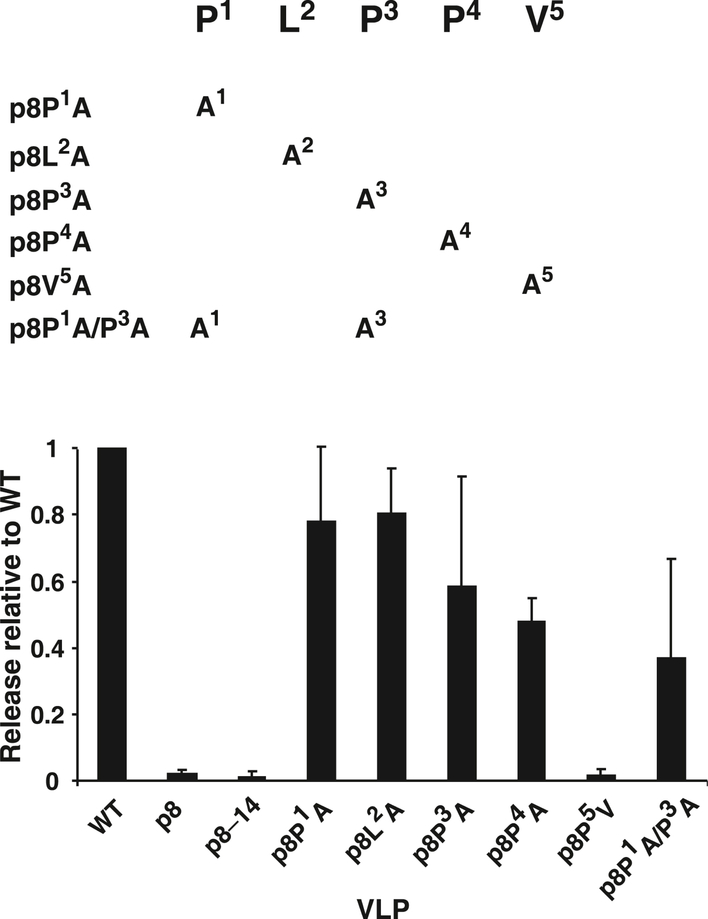
Release analysis of PLPPV fine-mapping mutants. A diagram of mutants tested is presented above a graph of the relative release factors of mutant VLPs from at least 3 independent experiments. Standard deviations are indicated by error bars.
Transmission electron microscopy
Transmission electron micrographs of thin section VLP samples were obtained as previously described (Dussupt et al., 2011).
Results
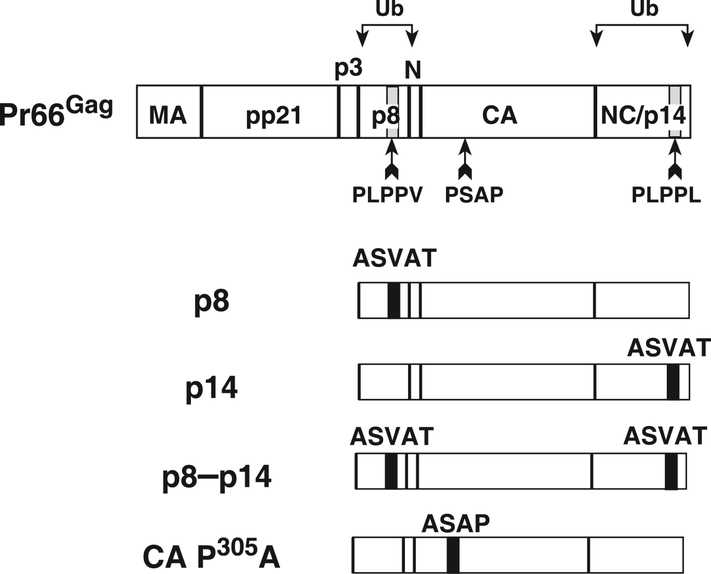
Our prior studies and those of others have shown that the virions of most retroviruses contain a small amount of a mature Gag protein that is monoubiquitinated near its L domain (Heidecker et al., 2004; Ott et al., 2000; Ott et al., 1998; Ott et al., 2003; Ott et al., 2002). We had previously found that MMTV virions contain small amounts of monoubiquitinated mature p8 and p14NC proteins (Ott et al., 2003), Fig. 1), making these regions in Gag prime candidates to harbor MMTV L domain sequences. Furthermore, since L domain core sequences (and other low affinity protein-binding motifs, e.g., WW and SH3 domain-binding sites) typically rely on the unique structure of proline for specificity (Zarrinpar, Bhattacharyya, and Lim, 2003), we examined the MMTV Gag sequences near p8 and p14NC for sequences rich in proline and identified two PLPPΦ motifs (Φ, any hydrophobic amino acid (Aasland et al., 2002)) in p8 and p14NC (Fig. 1). Additionally, another potential L domain sequence in MMTV Gag is a PSAP sequence in CA, a variant of the PTAP motif that can bind Tsg101 (Pornillos et al., 2002).
Fig. 1.
A diagram of mutants. MMTV Gag is shown at top with the various PLPPΦ mutations made in the p8-to-NC (p14) to the MMTV expression construct indicated below.
To examine the potential L domain function of these sequences, we produced a series of constructs in the MMTV Gag-expressing construct pSMt-HYBΔPro (Zabransky et al.), a Pol-deleted version of the MMTV provirus that contains a 5′ Mason-Pfizer monkey virus LTR in lieu of its hormone-dependent LTR. Earlier attempts to carry out these studies in the MMTV-HYB proviral clone (Shackleford and Varmus, 1988) were abandoned, due to the clone’s poor expression in both human and mouse cell line transfections even in the presence of dexamethasone. To test the importance of the PLPPΦ motifs this motif was changed to ASVAT in either p8 (PLPPV, Gag amino acids 246–250) to produce the p8 construct or p14NC (PLPPL, Gag amino acids 577–581) to produce the p14 construct (Fig. 1). These two mutations were also combined to produce a double mutant, p8–p14, that removes both PLPPΦ motifs. To determine the effect of these mutations on VLP production, these mutant constructs were transfected into HEK293T cells, and purified VLP and cell lysates were produced and examined by nIR immunoblotting using the LI-COR Odyssey imaging system. The results showed that the signal intensity of the Gag bands in the p8 and p8–p14 mutant VLP samples were markedly reduced from those in the wild-type sample (Fig. 2A). In contrast, the Gag band in the VLP sample from the p14NC single mutant, p14, had a similar intensity as the wild-type band (Fig. 2A). The release factor, the amount of Gag signal detected in the VLP sample divided by the sum of the signal in the VLP and actin normalized cell samples, was calculated from the signal density of the nIR immunoblot scans for each sample. Release factors from at least three independent experiments revealed that mutating the PLPPV site in p8, either alone or in combination with p14 in the p8–14 construct, significantly reduced VLP production (Fig. 2A), greater than 5- to 10-fold in this assay system. In contrast, the loss of the PLPPL site in p14NC had no discernable effect on release.
The PSAP motif is dispensable for MMTV VLP production
To determine the importance of the PSAP motif, an MMTV Gag mutant, CA P305A, was constructed with a proline-to-alanine substitution at position 305 of Gag (Fig. 1), effectively interrupting the potential of this motif to bind Tsg101 and function as an L domain (Garrus et al., 2001; Huang et al., 1995; Pornillos et al., 2002). Our nIR immunoblot analysis of CA P305A transfections found only a slight decrease in the relative release factor, compared to wild-type (Fig. 2B). Similar to the results above, the p8-p14 double mutant had a marked reduction in virion release. Thus, this putative Tsg101 binding site is not required for MMTV Gag VLP release.
Defective mutant VLPs exhibit an L domain morphology
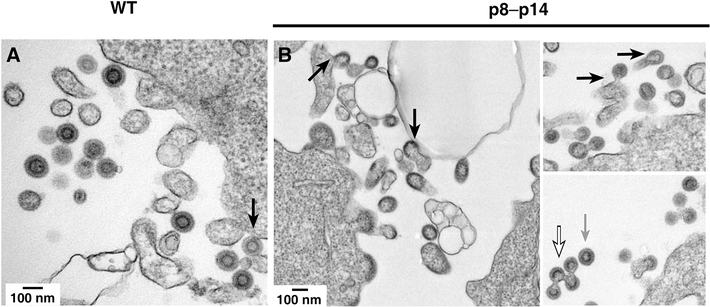
A hallmark phenotype of L domain mutations is a marked arrest in the release of budding forms from the surface of the virus-producing cells (Gottlinger et al., 1991; Gottwein et al., 2003; Huang et al., 1995) and, in some systems, accumulation of cell-free structures that contain multiple immature Gag particles held together by plasma membrane (Demirov, Orenstein, and Freed, 2002). To visualize the budding phenotype of our L domain mutant, we analyzed transfected HEK293T cells expressing either wild-type or mutant p8-p14 Gag by thin-section electron transmission microscopy (Fig. 3). Micrographs of cells expressing wild-type MMTV Gag contained mostly free VLPs with a complete concentric electron dense ring (Fig. 3A), typical of immature MMTV virions (Bernhard, 1958; de Harven, 1974; Tanaka and Moore, 1967), with some plasma membrane budding forms with electron dense partial rings (arrow in Fig. 3A). In contrast, micrographs of p8-p14 transfected cells contained only a few observable free VLPs with the vast majority of virus-like structures present as either arrested budding forms attached to the membrane or complexes of cell-free VLPs that appear linked together by membrane (black-bordered white arrow, Fig. 3B). In many cases the arrested budding forms appeared to be associated with ruffles or cellular filopodia adjacent to the main cell body in the thin sections (Fig. 3B, black arrows). Those few free single VLP forms observed did have a complete well-resolved morphology identical to the VLP in the wild-type samples (grey arrow, Fig. 3B), inconsistent with an assembly defect. Taken together, these results show that the PLPPV mutant exhibits a clear L domain morphology, being arrested late in the assembly process, during budding.
Fig. 3.
Thin section transmission electron microscopy. Micrographs of (A) MMTV Gag and (B) p8–p14 mutant VLPs are presented.
Replacement of PLPPV with EIAV YPDL restores MMTV Gag release
To confirm our electron microscopy results indicating that the mutation of PLPPV results in an L domain effect, we exploited one of the key properties of L domains, the ability to be functionally exchanged for other L domains (Li et al., 2002; Parent et al., 1995; Puffer et al., 1997). We constructed a mutant, p8YPDL (Fig. 4), that contained a replacement of the PLPPV sequence in p8 with an EIAV sequence that contains the YPDL L domain which has been used successfully in L domain exchange experiments (Li et al., 2002; Puffer et al., 1997). The nIR immunoblot analysis of these mutants expressed by transfection of HEK293T cells found that the YPDL exchange mutant dramatically released VLPs better than the p8 mutant (Fig. 4), showing that the EIAV sequences could functionally replace the PLPPV sequences in MMTV Gag though not at wild-type levels. This result rules out the defect being a failure of the p8 mutant to form VLP due to inappropriate Gag-Gag interactions, since it is improbable that the exchanged EIAV sequence would be able to rescue a structural MMTV assembly defect in this context. Interestingly, a p8YPDL–p14 mutant that combined the p8YPDL mutant with the p14 mutation of PLPPL was consistently less efficient at release than just the YPDL exchange (Fig. 4). This was unexpected since mutating the p14 PLPPL sequence had no effect on VLP release in the presence of p8. Perhaps the p14 PLPPL sequence can mildly assist the YPDL-using Gag in a way that is not apparent in the p8 mutation context. To determine if the EIAV sequence could function in the p14NC position, a p8–p14YPDL mutant that combined the p8 mutation and the replacement of PLPPL with the EIAV sequence (Fig. 4) was constructed. The release of p8–p14YPDL from transfected cells was quite low, demonstrating that YPDL was unable to rescue MMTV budding when placed in NC. The ability of a heterologous L domain to functionally replace the p8 PLPPV sequence suggests that the p8 sequence supplies an L domain function rather than a required structural role in particle formation.
PLPPV can replace the EIAV L domain
To determine if PLPPV alone or with some of its flanking sequences could provide L domain function in a heterologous context, we replaced the YPDL sequence in an EIAV Gag expression construct with either PLPPV, ePLPPV, or an extended PLPPV sequence containing p8 flanking sequences (RRKPLPPVGFAG), ePLxtnd. We chose EIAV Gag for these experiments as prior studies have shown that more types of foreign L domains can function it the EAIV context, especially more so than HIV-1, which has limited tolerance in L domain exchange constructs (Martin-Serrano, Perez-Caballero, and Bieniasz, 2004; Ott et al., 2005; Shehu-Xhilaga et al., 2004). Also, EIAV appears to have only one known L domain, while other routinely studied retroviruses contain two or more functioning core L domain sequences that complicate exchange experiments, including HIV-1 (Gottlinger et al., 1991; Huang et al., 1995; Strack et al., 2003), human T-cell leukemia virus (Bouamr et al., 2003), Mason-Pfizer monkey virus (Gottwein et al., 2003), murine leukemia virus (Segura-Morales et al., 2005), and Rous sarcoma virus (Dilley et al., 2010; Wills et al., 1994). Testing these EIAV-MMTV chimeras in our release analysis system found that PLPPV could readily restore L domain function to EIAV Gag (Fig. 5). However, inclusion of some of the MMTV flanking sequences around the PLPPV sequence reduced the efficiency of particle release over the PLPPV alone, possibly due to an incompatibility of these extra MMTV sequences with EIAV Gag. Nevertheless, these results show that PLPPV can function as an L domain in EIAV.
The MMTV L domain is position-dependent
Even though they are quite similar, both having a PLPPΦ motif, the motif in the p8 sequence provides release function while the motif in p14NC is dispensable. This could be due to either the sequence difference between them– the p8 motif has a valine while the p14NC motif contains a leucine– or the difference in position, its presence in p8 or p14NC. To examine these possibilities, we altered the p8 PLPPV sequence to PLPPL, creating p8PLPPL that expresses Gag with two PLPPL motifs and also introducing the same change in the p14 mutant, forming p8PLPPL–p14 which contains PLPPL only in the p8 position (Fig. 6). Both constructs failed to release particles efficiently, a defect comparable to that of the p8-p14 mutant which lacks both motifs (Fig. 6). Therefore, the p14NC motif fails to function in the p8 position, indicating a requirement for valine in position 5.
To examine positional dependence, we converted the p14 PLPPL to PLPPV in the p8 mutant, forming p8–p14 PLPPV (Fig. 6). Testing of its release properties revealed the same profound defect in release as the other defective mutants (Fig. 6). Thus, the L domain of MMTV is both positionally and sequence-dependent: efficient release requires PLPPV in p8, similar to the p8YPDL chimera.
Fine mapping of the MMTV L domain
To better understand the importance of the individual residues in the PLPPV sequence, we produced a series of mutants that replaced amino acids in this region of MMTV Gag with alanine and examined the release properties of the mutants (Fig. 7). Similar to the results presented above, in which PLPPV and PLPPL were exchanged, a valine-to-alanine substitution eliminated the release function (p8P5V, Fig. 7). However, singularly altering the leucine or prolines in the motif resulted in a slight or modest reduction in release efficiency, revealing a flexibility in the motif. A p8 P1A /P3A double mutant also exhibited a greater reduction than either P1 or P3 alone. Thus, the only strictly required amino acid in the motif is valine.
Discussion
Here, we identify that a PLPPV sequence in the p8 Gag protein provides an L domain function for MMTV Gag, enabling efficient release of VLP. This sequence represents a fourth type of L domain motif used by retroviruses for release from the plasma membrane. PLPPV exhibited key properties of L domains. Mutation of the sequence produced an arrested budding phenotype where particles formed but failed to efficiently release from the cell and separate from one another. However, unlike the case with some retroviruses, the MMTV L domain sequence was positionally dependent in Gag, functioning only within its natural context in p8: ectopic placement of PLPPV at the PLPPL-containing site in p14NC failed to provide release function. The PLPPV sequence functionally substituted for the YPDL L domain sequence in EIAV Gag. Similarly, an EIAV sequence containing YPDL was only able to function in the p8 position but not in the p14NC position. Thus, the both PLPPV and YPDL sequences only provided release function in the p8 site, suggesting that MMTV Gag has a more stringent positional requirement than other retroviruses.
Other putative L domain sequences present in MMTV, PLPPL in p14NC and PSAP in CA, did not appear to provide any significant release function for MMTV. Mutating the p14NC PLPPL sequence readily produced particles and placing the PLPPL sequence in lieu of the PLPPV in p8 failed to efficiently release virus-like particles. Furthermore, mutating both p8 PLPPV and p14NC PLPPL in MMTV Gag produced only a marginally lower release defect over the p8 PLPPV mutation alone, suggesting, at best, a minor influence of PLPPL on budding. However, we did observe a minor contribution of PLPPL to the release of MMTV Gag with the YPDL L domain sequence exchange mutant. Perhaps PLPPL in its p14 position binds some complex that assists only YPDL-mediated release.
Since both a small amount of p8 and p14NC in MMTV virions is monoubiquitinated, it is likely that both sequences interact with complexes that possess ubiquitin ligase activity. However, since only PLPPV is sufficient for release, PLPPL might interact with a different complex or one that is inactive in release function.
While the p8 PLPPV sequence is critical for budding, another unidentified sequence could also be providing some additional L domain function for MMTV. Many retroviruses have two sequences which additively provide L domain (Dilley et al., 2010; Gottlinger et al., 1991; Gottwein et al., 2003; Heidecker et al., 2004; Segura-Morales et al., 2005; Strack et al., 2003), typically with one providing the majority of the release activity, and the other playing an auxiliary role. While the severity of the p8 PLPPV mutation on release is clear, we cannot exclude this possibility
Alanine scanning of the PLPPV found that the valine was the only clearly required sequence in the motif, consistent with our PLPPL motif exchange result. Mutation of any one of the other residues in the motif produced only a mild reduction in VLP release, however mutation of P1 and P3 did have some additive decrease on release. Other proline-rich sequences can have relatively flexible sequence requirements for protein binding, where any one proline in a motif seems able to compensate for the loss of any other (Li, 2005), which seems to be the case here.
One of the difficulties in examining Gag mutants by the presence of particles in the supernatant is that a mutation in Gag might result in virus-like particle production defects due to an interruption of Gag-Gag assembly interactions, rather than to a failure to release. It is especially important to differentiate between these two possibilities since the deletion of p8 interrupts particle production (Zabransky et al., 2010). Our data strongly support PLPPV acting purely as an L domain. First, our microscopy reveals an accumulation of a classic attached lollipop structure on the surface of cells and virions that fail to separate from one another, the latter presumably being released from the cell en masse rather than by individual budding. A purely Gag-Gag interaction mutant would fail to produce these types of arrested forms. Second, the ability of the EIAV L domain-containing sequences to replace the PLPPV sequence in MMTV Gag strongly suggests that the PLPPV motif mutation did not alter any critical Gag-Gag interactions, since it is highly unlikely that EIAV sequences would fortuitously provide an interaction that would complement the missing MMTV sequences to rescue a putative Gag-Gag assembly defect. Third, the ability of PLPPV to provide release function to EIAV clearly demonstrates that this sequence is an L domain.
Zabransky et al. recently reported that the deletion of the PLPPVGFAG sequence in p8 produces both a particle release and spherical particle assembly defect (Zabransky et al., 2010). Together with our results, it appears that this region has both an L domain and an undefined assembly domain. In their report, the authors noted that the deleted sequence contains a PPXXF EVH1 motif which is associated with actin dynamics (Zabransky et al., 2010). The loss of this site could be an important contributor to the assembly defect portion of this region. It is important to note that several of our mutants that interrupt this site had little or only very modest negative effects on release. In contrast, our valine mutants (p8P5V and p8PLPPL) have a severe release reduction, yet do not interrupt the core EVH1 motif. An MMTV Gag construct carrying a phenylalanine-to-threonine mutation, which removes a critical part of the EVH1 motif, appears to be unstable in cells, so we are unable to directly test the significance of this motif in MMTV assembly (data not shown).
The cellular protein that functionally interacts with PLPPV is not clear. This sequence could be a ligand for a SH3 or WW domain-containing protein, binding consensuses PXXP or PΦPΦ, respectively (Li, 2005; Mayer, 2001; Zarrinpar, Bhattacharyya, and Lim, 2003). While PLPPV resembles the PPLP ligand recognized by a specialized WW-domain present in formin-containing proteins (Chan, Bedford, and Leder, 1996; Ermekova et al., 1997), our data show that the required leucine in this motif is dispensable for efficient release in the MMTV sequence. Nevertheless, a WW domain protein might be recognizing this motif. NEDD4-like HECT ubiquitin ligases, which contain WW-domains, have been implicated in the release pathways of retroviruses and Ebola virus (Freed, 2002; Morita and Sundquist, 2004).
At its core, retroviral budding and release requires cellular proteins and systems. Studies with HIV-1 and EIAV have implicated ESCRT III, most notably members of the CHMP4 and CHMP 2 families and VPS4 as key proteins that pinch off the plasma membrane neck allowing for virus release (Baumgartel et al., 2011; Hurley and Cada, 2018; Johnson, Bleck, and Simon, 2018; Jouvenet et al., 2011; Morita et al., 2011; Muziol et al., 2006; Stuchell-Brereton et al., 2007; Votteler and Sundquist, 2013). It seems likely that retroviruses share this common mechanism yet use different cellular pathways to approach this shared point, dictated by their respective L domains (Votteler and Sundquist, 2013). Our identification of a fourth L domain opens up the possibility that MMTV connects to this shared point from a unique direction that does not depend on proteasome function (Ott et al., 2003). Thus, the identification of this pathway should greatly assist our understanding of retroviral budding.
Supplementary Material
Fig. S1. Supplemental immunoblot data for release graphs in Fig. 2B, 4, 5, 6 and 7 are presented with each blot identified at top left by its corresponding figure number. Sample-containing wells are labeled above their respective lanes and molecular weight markers and Gag/actin fluorescence are labeled at left and right, respectively.
Acknowledgements
We thank Iva Pichova for the kind gift of pSMt-HYBdelpro and pSMt-HYB and Teresa Shatzer for expert technical assistance. This project has been funded with federal funds from the National Cancer Institute, National Institutes of Health, under Contracts Nos. HHSN261200800001E and N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. government. Declarations of interest: none.
Footnotes
I wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
I confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. I further confirm that the order of authors listed in the manuscript has been approved by all.
I confirm that I have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, I confirm that we have followed the regulations of our institutions concerning intellectual property.
I understand that as the Corresponding Author I am the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). I am responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. I confirm that I have been provided current and correct email addresses from which I can accept email from all authors of this publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aasland R, Abrams C, Ampe C, Ball LJ, Bedford MT, Cesareni G, Gimona M, Hurley JH, Jarchau T, Lehto VP, Lemmon MA, Linding R, Mayer BJ, Nagai M, Sudol M, Walter U, and Winder SJ (2002). Normalization of nomenclature for peptide motifs as ligands of modular protein domains. FEBS Lett. 513(1), 141–144. [DOI] [PubMed] [Google Scholar]
- Baumgartel V, Ivanchenko S, Dupont A, Sergeev M, Wiseman PW, Krausslich HG, Brauchle C, Muller B, and Lamb DC (2011). Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat. Cell Biol 13(4), 469–474. [DOI] [PubMed] [Google Scholar]
- Bernhard W (1958). Electron microscopy of tumor cells and tumor viruses; a review. Cancer Res. 18(5), 491–509. [PubMed] [Google Scholar]
- Bieniasz PD (2006). Late budding domains and host proteins in enveloped virus release. Virology 344(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD (2009). The cell biology of HIV-1 virion genesis. Cell Host Microbe 5(6), 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouamr F, Melillo JA, Wang MQ, Nagashima K, de Los Santos M, Rein A, and Goff SP (2003). PPPYEPTAP motif Is the late domain of human T-cell leukemia virus type 1 Gag and mediates its functional interaction with cellular proteins Nedd4 and Tsg101. J. Virol 77(22), 11882–11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC, Bedford MT, and Leder P (1996). Formin binding proteins bear WWP/WW domains that bind proline-rich peptides and functionally resemble SH3 domains. Embo J. 15(5), 1045–1054. [PMC free article] [PubMed] [Google Scholar]
- Craven RC, Harty RN, Paragas J, Palese P, and Wills JW (1999). Late domain function identified in the vesicular stomatitis virus M protein by use of rhabdovirus-retrovirus chimeras. J. Virol 73(4), 3359–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Harven E (1974). Remarks on the ultrastructure of type A, B, and C virus particles. Adv. Virus. Res 19, 221–264. [DOI] [PubMed] [Google Scholar]
- Demirov DG, Orenstein JM, and Freed EO (2002). The late domain of human immunodeficiency virus type 1 p6 promotes virus release in a cell type-dependent manner. J. Virol 76(1), 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley KA, Gregory D, Johnson MC, and Vogt VM (2010). An LYPSL late domain in the gag protein contributes to the efficient release and replication of Rous sarcoma virus. J. Virol 84(13), 6276–6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussupt V, Sette P, Bello NF, Javid MP, Nagashima K, and Bouamr F (2011). Basic residues in the nucleocapsid domain of Gag are critical for late events of HIV-1 budding. J. Virol 85(5), 2304–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermekova KS, Zambrano N, Linn H, Minopoli G, Gertler F, Russo T, and Sudol M (1997). The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophila enabled. J. Biol. Chem 272(52), 32869–32877. [DOI] [PubMed] [Google Scholar]
- Freed EO (2002). Viral late domains. J. Virol 76(10), 4679–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, Myszka DG, and Sundquist WI (2001). Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107(1), 55–65. [DOI] [PubMed] [Google Scholar]
- Gottlinger HG, Dorfman T, Sodroski JG, and Haseltine WA (1991). Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. U. S. A 88(8), 3195–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Bodem J, Muller B, Schmechel A, Zentgraf H, and Krausslich HG (2003). The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release. J. Virol 77(17), 9474–9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G, Lloyd PA, Fox K, Nagashima K, and Derse D (2004). Late assembly motifs of human T-cell leukemia virus type 1 and their relative roles in particle release. J. Virol 78(12), 6636–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G, Lloyd PA, Soheilian F, Nagashima K, and Derse D (2007). The role of WWP1-Gag interaction and Gag ubiquitination in assembly and release of human T-cell leukemia virus type 1. J. Virol 81(18), 9769–9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Cai Z, Ho SN, and Pease LR (1990). Gene splicing by overlap extension: Tailor-made genes using polymerase chain reaction. BioTechniques 8, 528–535. [PubMed] [Google Scholar]
- Huang M, Orenstein JM, Martin MA, and Freed EO (1995). p6gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol 69(11), 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley JH, and Cada AK (2018). Inside job: how the ESCRTs release HIV-1 from infected cells. Biochem. Soc. Trans 46(5), 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadwin JA, Rudd V, Sette P, Challa S, and Bouamr F (2010). Late domainindependent rescue of a release-deficient Moloney murine leukemia virus by the ubiquitin ligase itch. J. Virol 84(2), 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DS, Bleck M, and Simon SM (2018). Timing of ESCRT-III protein recruitment and membrane scission during HIV-1 assembly. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N, Zhadina M, Bieniasz PD, and Simon SM (2011). Dynamics of ESCRT protein recruitment during retroviral assembly. Nat. Cell Biol 13(4), 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chen C, Puffer BA, and Montelaro RC (2002). Functional replacement and positional dependence of homologous and heterologous L domains in equine infectious anemia virus replication. J. Virol 76(4), 1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SS (2005). Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction. Biochem. J 390(Pt 3), 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J, and Neil JD (2011). Host factors invovled in retroviral budding and release. Nat. Rev,. Microbiol 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Perez-Caballero D, and Bieniasz PD (2004). Context-dependent effects of L domains and ubiquitination on viral budding. J. Virol 78(11), 5554–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Serrano J, Zang T, and Bieniasz PD (2001). HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med 7(12), 1313–1319. [DOI] [PubMed] [Google Scholar]
- Mayer BJ (2001). SH3 domains: complexity in moderation. J. Cell Sci 114(Pt 7), 1253–1263. [DOI] [PubMed] [Google Scholar]
- Medina G, Zhang Y, Tang Y, Gottwein E, Vana ML, Bouamr F, Leis J, and Carter CA (2005). The functionally exchangeable L domains in RSV and HIV-1 Gag direct particle release through pathways linked by Tsg101. Traffic 6(10), 880–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, Sandrin V, McCullough J, Katsuyama A, Baci Hamilton I, and Sundquist WI (2011). ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe 9(3), 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita E, and Sundquist WI (2004). Retrovirus budding. Annu. Rev. Cell Dev. Biol 20, 395–425. [DOI] [PubMed] [Google Scholar]
- Muziol T, Pineda-Molina E, Ravelli RB, Zamborlini A, Usami Y, Gottlinger H, and Weissenhorn W (2006). Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell 10(6), 821–830. [DOI] [PubMed] [Google Scholar]
- Ott DE, Chertova EN, Busch LK, Coren LV, Gagliardi TD, and Johnson DG (1999). Mutational analysis of the hydrophobic tail of the human immunodeficiency virus type 1 p6(Gag) protein produces a mutant that fails to package its envelope protein. J. Virol 73(1), 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott DE, Coren LV, Chertova EN, Gagliardi TD, and Schubert U (2000). Ubiquitination of HIV-1 and MuLV Gag. Virology 278(1), 111–121. [DOI] [PubMed] [Google Scholar]
- Ott DE, Coren LV, Copeland TD, Kane BP, Johnson DG, Sowder RC II, Yoshinaka Y, Oroszlan S, Arthur LO, and Henderson LE (1998). Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type-1 and simian immunodeficiency virus and the p12Gag protein of Moloney murine leukemia virus. J. Virol 72, 2962–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott DE, Coren LV, Gagliardi TD, and Nagashima K (2005). Heterologous late-domain sequences have various abilities to promote budding of human immunodeficiency virus type 1. J. Virol 79(14), 9038–9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott DE, Coren LV, Sowder RC II, Adams J, and Schubert U (2003). Retroviruses have differing requirements for proteasome function in the budding process. J. Virol 77(6), 3384–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott DE, Coren LV, Sowder RC II, Adams J, Nagashima K, and Schubert U (2002). Equine infectious anemia virus and the ubiquitin-proteasome system. J. Virol 76(6), 3038–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent LJ, Bennett RP, Craven RC, Nelle TD, Krishna NK, Bowzard JB, Wilson CB, Puffer BA, Montelaro RC, and Wills JW (1995). Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol 69(9), 5455–5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Cheong C, Idoyaga J, Kim JY, Choi JH, Do Y, Lee H, Jo JH, Oh YS, Im W, Steinman RM, and Park CG (2008). Generation and application of new rat monoclonal antibodies against synthetic FLAG and OLLAS tags for improved immunodetection. J. Immunol. Methods 331(1–2), 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik A, Chau V, Li F, Montelaro RC, and Wills JW (2002). Budding of equine infectious anemia virus is insensitive to proteasome inhibitors. J. Virol 76(6), 2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincetic A, and Leis J (2009). The mechanism of budding of retroviruses from cell membranes. In “Advances in Virology”, Vol. Article ID 623969, pp. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos O, Alam SL, Davis DR, and Sundquist WI (2002). Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nat. Struct. Biol 9(11), 812–817. [DOI] [PubMed] [Google Scholar]
- Puffer BA, Parent LJ, Wills JW, and Montelaro RC (1997). Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol 71, 6541–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puffer BA, Watkins SC, and Montelaro RC (1998). Equine infectious anemia virus gag polyprotein late domain specifically recruits cellular AP-2 adapter protein complexes during virion assembly. J. Virol 72(12), 10218–10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AP, Leser GP, Morita E, Sundquist WI, and Lamb RA (2005). Evidence for a new viral late-domain core sequence, FPIV, necessary for budding of a paramyxovirus. J. Virol 79(5), 2988–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Morales C, Pescia C, Chatellard-Causse C, Sadoul R, Bertrand E, and Basyuk E (2005). Tsg101 and Alix interact with murine leukemia virus Gag and cooperate with Nedd4 ubiquitin ligases during budding. J. Biol. Chem 280(29), 27004–27012. [DOI] [PubMed] [Google Scholar]
- Sette P, Nagashima K, Piper RC, and Bouamr F (2013). Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding. Retrovirology 10, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford GM, and Varmus HE (1988). Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc. Natl. Acad. Sci U S A 85(24), 9655–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehu-Xhilaga M, Ablan S, Demirov DG, Chen C, Montelaro RC, and Freed EO (2004). Late domain-dependent inhibition of equine infectious anemia virus budding. J. Virol 78(2), 724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack B, Calistri A, Craig S, Popova E, and Gottlinger HG (2003). AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114(6), 689–699. [DOI] [PubMed] [Google Scholar]
- Stuchell-Brereton MD, Skalicky JJ, Kieffer C, Karren MA, Ghaffarian S, and Sundquist WI (2007). ESCRT-III recognition by VPS4 ATPases. Nature 449(7163), 740–744. [DOI] [PubMed] [Google Scholar]
- Tanaka H, and Moore DH (1967). Electron microscopic localization of viral antigens in mouse mammary tumors by ferritin-labeled antibody. I. The homologous systems. Virology 33(2), 197–214. [DOI] [PubMed] [Google Scholar]
- VerPlank L, Bouamr F, LaGrassa TJ, Agresta B, Kikonyogo A, Leis J, and Carter CA (2001). Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci. U S A 98(14), 7724–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, Scott A, Krausslich HG, Kaplan J, Morham SG, and Sundquist WI (2003). The protein network of HIV budding. Cell 114(6), 701–713. [DOI] [PubMed] [Google Scholar]
- Votteler J, and Sundquist WI (2013). Virus budding and the ESCRT pathway. Cell Host Microbe. 14(3), 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss ER, and Gottlinger H (2010). The role of cellular factors in promoting HIV budding. J. Mol. Biol 410(4), 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills JW, Cameron CE, Wilson CB, Xaing Y, Bennett RP, and Leis J (1994). An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol 63, 4331–4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaing Y, Cameron CE, Wills JW, and Leis J (1996). Fine mapping and characterization of the Rous sarcoma virus Pr76gag late assembly domain. J. Virol 70, 5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabransky A, Hadravova R, Stokrova J, Sakalian M, and Pichova I (2009). Premature processing of mouse mammary tumor virus Gag polyprotein impairs intracellular capsid assembly. Virology 384(1), 33–37. [DOI] [PubMed] [Google Scholar]
- Zabransky A, Hoboth P, Hadravova R, Stokrova J, Sakalian M, and Pichova I (2010). The noncanonical Gag domains p8 and n are critical for assembly and release of mouse mammary tumor virus. J. Virol 84(21), 11555–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Bhattacharyya RP, and Lim WA (2003). The structure and function of proline recognition domains. Sci. STKE 2003(179), RE8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Supplemental immunoblot data for release graphs in Fig. 2B, 4, 5, 6 and 7 are presented with each blot identified at top left by its corresponding figure number. Sample-containing wells are labeled above their respective lanes and molecular weight markers and Gag/actin fluorescence are labeled at left and right, respectively.