Abstract
BACKGROUND
Perinatal HIV transmission has dramatically decreased with combination antiretroviral (ARV) regimens, but complications among children who are HIV-exposed but uninfected (HEU), such as microcephaly, warrant ongoing surveillance. The goal of our study was to evaluate whether individual in utero ARV exposures were associated with increased risk of microcephaly based on long-term follow up of HEU infants and children.
METHODS
We evaluated HEU children enrolled in the Surveillance Monitoring for ART Toxicities (SMARTT) study, a prospective cohort study conducted by the PHACS network. Microcephaly was defined using 2000 CDC Growth z-scores for head circumference (HC) measurements obtained at 6–36 months of age (z-score<−2) and using Nellhaus standards (< 2nd percentile) after age 3 (“SMARTT” criteria), or using Nellhaus standards across all ages. Modified Poisson regression models were fit to obtain relative risks (RRs) for associations between in utero ARV exposure and microcephaly status, adjusted for potential confounders. Neurodevelopmental functioning was compared between HEU children with versus without microcephaly.
FINDINGS
Among 3055 SMARTT participants enrolled as of April 2017 with a HC measurement, the cumulative incidence of microcephaly over a median of 5·1 years of follow-up (IQR=3·0, 7·2 years) was 159 (5·2%, 95%CI: 4·4–6·1%) by Nellhaus criteria and 70 (2·3%, 95%CI: 1·8–2·9%) by SMARTT criteria. In adjusted models, in utero exposure to efavirenz (4·7% exposed) was associated with increased risk of microcephaly by both Nellhaus standards (adjusted RR (aRR)=2·02, 95%CI: 1·16, 3·51) and SMARTT criteria (aRR=2·56, 95%CI: 1·22, 5·37). These associations were more pronounced among children exposed to combination regimens of efavirenz which included zidovudine+lamivudine than those including tenofovir+emtricitabine. Protective associations were observed for darunavir exposure (aRR=0·50; 95%CI: 0·24, 1·00). HEU children with microcephaly had lower mean scores on neurodevelopmental assessments at age 1 and 5 years and higher prevalence of impairment than those without microcephaly.
INTERPRETATION
These findings support consideration of alternatives to efavirenz as part of first line ARV therapy for pregnant women.
Keywords: Antiretroviral, pregnancy, HIV-exposed, safety, infants, head circumference, neurodevelopment
INTRODUCTION
Despite the success of combination antiretroviral (ARV) regimens used during pregnancy in reducing HIV transmission, concerns remain regarding adverse consequences of in utero exposure to ARVs.1–3 Neurological conditions are of particular concern, given the potential for effects of ARV exposures on the developing brain and central nervous system. One specific neurologic condition warranting attention is microcephaly, which generally reflects poor brain growth and common among infants with HIV encephalopathy.4,5 Microcephaly is multifactorial in origin, and can either present congenitally, with or without other defects, or be acquired postnatally.6,7 Although multiple possible etiologies may cause microcephaly with different long-term prognoses, microcephaly has generally been linked to poorer neurodevelopmental outcomes, including among infants infected with HIV.7–9 However, microcephaly has received less attention among HIV-exposed but uninfected (HEU) infants and children who were exposed to ARVs in utero, and its relationship to neurodevelopment among HEU children has not been investigated.
Head circumference (HC) has been noted to be significantly lower among HEU infants than among children born to women without HIV infection, particularly in low and middle income countries.10,11 The pre-ZIKA rate of microcephaly among HEU infants in Latin America was 7·5%, exceeding that expected based on WHO standards for the general infant population.12 Studies in the U.S. have less often identified differences in HC between HEU children and those in the general population, although comparisons of microcephaly rates across studies have been hindered by differences in standards used to define microcephaly.13 Despite their high observed high rate of microcephaly, the Latin American study observed no association with ARV drugs taken during pregnancy. However, in utero exposure to the specific ARV drug tenofovir disoproxil fumarate (TDF) was linked to lower HC in 1-year olds in the U.S.14 A comprehensive assessment of associations of maternal ARV use with microcephaly has not been conducted in higher resource settings.
We report on the evaluation of microcephaly with maternal ARV drugs and regimens based on a prospective cohort study conducted by the Pediatric HIV/AIDS Cohort Study (PHACS) network. We designed the PHACS Surveillance Monitoring for ART Toxicities (SMARTT) Study to identify potential adverse effects of ARV exposures in infants born to HIV-infected women, and to evaluate associations with ARV combinations and specific ARV drugs in order to help inform treatment guidelines for HIV-infected pregnant women.15 In this paper, we evaluate the association of ARV exposures with microcephaly based on long-term follow up of HEU infants and children enrolled in the SMARTT study.
METHODS
Study Design and Study Population
The study population for this analysis includes children in the SMARTT Static and Dynamic cohorts who enrolled prior to April 1, 2017 and had HC measured at least once by August 1, 2017. The Static Cohort children and their mothers (or caregivers) enrolled at age 1–12 years and had maternal ARV use information from prior studies. The Dynamic Cohort enrolled HIV-infected women and their infants during gestation or <1 week after delivery. Both cohorts opened to participating sites in the United States including Puerto Rico in March 2007; details have been previously described.15 The SMARTT protocol [NCT01310023] was approved by Human Subject research review boards at each participating site and the Harvard T.H. Chan School of Public Health. We obtained written informed consent from the parent or legal guardian at each research site.
Procedures
At study entry, we obtained clinical diagnoses and dates of prenatal ARV use from medical charts and participant interview. Birth characteristics were abstracted and maternal HIV disease characteristics during pregnancy were collected including plasma HIV RNA concentration (viral load, VL), absolute CD4+ lymphocyte (CD4) cell counts, and CD4%. Trimester-specific information on substance use during pregnancy was obtained by self-reported questionnaire, including alcohol, tobacco, marijuana, opiates, and other substances, which has been validated using meconium samples.16 Caregivers of participating children completed questionnaires on household composition, education and income levels, and other information related to family environment. After enrollment, we followed children and their mothers or caregivers at annual study visits, in which we conducted a complete physical examination including anthropometric assessments, and abstracted diagnoses and clinical information from the medical chart or by interview.
Head (occipitofrontal) circumference measurements were obtained annually in triplicate by clinical staff using a non-stretchable tape measure according to a standardized protocol. Microcephaly was defined using HC measurements obtained at least 6 months after birth to avoid measurement error due to molding that may occur during delivery. Microcephaly was evaluated using two definitions: (1) HC Z-score <−2 based on U.S. Centers for Disease Control and Prevention (CDC) 2000 growth standards through age 3 and below the annual thresholds published by Nellhaus (1968) for children over age 3 (designated the “SMARTT” criteria),17,18 or (2) HC <2nd percentile based on the Nellhaus standards using monthly cutoff values across all ages (designated the Nellhaus criteria). For both definitions, we required at least one HC measure (defined as the average of the three measurements obtained at each visit) meeting criteria. We considered microcephaly defined by Nellhaus criteria as the primary outcome and included results for microcephaly by SMARTT criteria in the Appendix. For both definitions, we adjusted for prematurity when applicable by subtracting the number of weeks born preterm from the age at assessment through age 18 months. Children with Down syndrome were compared to specific HC standards for that syndrome.19 For comparison with other studies, we also calculated the rates of microcephaly using WHO standards.20 Quality control measures were implemented to confirm or exclude extreme Z-score values based on concurrent anthropometric measures and within-subject longitudinal trends.
The primary exposure of interest was in utero ARV exposure. We classified children according to exposure to ARV agents by drug class, to individual ARV agents, and by trimester of initiation. The reference group for each exposure consisted of children unexposed to the specific ARV drug class or drug being considered either overall or within the trimester of interest. We evaluated each individual ARV drug among a targeted subset of participants who could have been exposed, based on calendar years in which the drug had been approved and was used. More specifically, we restricted models to children with known exposure status based on chart review and/or interview, and born after 2007 for darunavir, raltegravir, fosamprenavir, and integrase inhibitors (IIs, as drug class), after 2011 for rilpivirine, and after 2013 for dolutegravir and elvitegravir; all other ARV drugs were evaluated in the full SMARTT cohort. ARV drugs with fewer than 50 exposed infants were not considered.
We identified potential confounders based on past literature and descriptive statistics from our cohort, under the guidance of a directed acyclic graph. We considered the following maternal covariates to be potential confounders: age at delivery, race, pre-pregnancy BMI, chronic health conditions such as pre-gestational diabetes, HIV VL and CD4 counts early in pregnancy, and first trimester substance use (including alcohol, tobacco, marijuana, cocaine, and opiates). In addition, socioeconomic measures were evaluated including household income and caregiver education levels. We descriptively summarized pregnancy outcome [low birth weight (LBW, <2500g), preterm birth (<37 weeks gestation), small for gestational age (SGA, <10th percentile), and mode of birth (vaginal or Cesarean delivery)] by microcephaly status, but excluded these characteristics from adjusted models since they may be on the causal pathway. Among all covariates identified as likely confounders, those with p<0·20 in unadjusted models for case status were included in initial multivariable models, and those with p<0·10 in multivariable models (without any ARV exposures) were retained in final models for all ARVs.
Statistical analysis
We estimated the rates of microcephaly by each of the two definitions and their corresponding exact 95% confidence intervals (CIs) under the binomial distribution. Due to the low event rates, we fit modified Poisson regression models to obtain relative risks (RRs) for associations between ARV exposures by drug class and to specific ARV agents, overall and for first trimester exposure.21 We present both unadjusted and adjusted RRs for each ARV drug. Because the effects of individual ARV drugs may depend on interactions with other ARV drugs, we also examined associations with microcephaly accounting for other drugs in the same regimen. First, we evaluated relative associations when ARV drugs were used together with different NRTI backbones. Secondly, to account for all ARV drugs used in the same regimen, we fit a hierarchical log-binomial regression model following the approach of Correia and Williams22, in which individual drugs are modeled as random effects within drug classes; this approach has been shown to result in fewer false positives and reduced bias in estimating associations as compared to evaluating each ARV drug in separate models.
We conducted several sensitivity analyses to assess the robustness of findings and reduce possible sources of bias, including: 1) restricting analysis to the Dynamic cohort (children followed from birth); 2) restricting follow-up to the first two years of life; 3) excluding children with major congenital anomalies, and 4) restricting to mothers who received combination ART (cART) during pregnancy (defined as ≥3 drugs from ≥2 drug classes, or ≥3 NRTIs). We conducted stratified analyses by preterm birth, LBW, SGA, maternal VL and CD4, substance use, and birth cohort to evaluate whether associations for specific ARV drugs were driven by vulnerable subgroups. We evaluated the impact of timing of exposure by comparing pre-conception versus post-conception risks. We also conducted analyses of the incidence of microcephaly using Poisson models to estimate the incidence rate ratio (IRR) accounting for person-time of follow up. In addition, we conducted analyses accounting for the correlation among children at the same clinical research site and among children from the same family. Multiple imputation with 5 imputed datasets was used to confirm select findings.
Finally, to better understand clinical implications of microcephaly, we compared neurodevelopmental functioning between children with and without microcephaly, within the separate subset with assessments at age 1 with the Bayley Scales of Infant and Toddler Development, Version III (Bayley-III) and at age 5 with the Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III).23,24 These assessments are scheduled to be conducted among all children at 1 and 5 year-old visits, respectively, although not all children attended these study visits depending on age at study entry and duration of follow up. These descriptive comparisons were based on differences by microcephaly status in mean subscale scores, as well as percent with neurodevelopmental impairment as defined previously.15 All statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
Role of the funding source
The funders of the study were involved in the study design of the SMARTT study, and as coauthors were involved in the data interpretation and writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Among 3759 SMARTT participants enrolled as of April 2017 and with a study visit by August 2017, 3557 (95%) had at least one HC measurement obtained, and 3055 (81%) had one 6 months or more after birth (Appendix, p.2). Over a median follow-up of 5·1 years (IQR=3·0, 7·2) for these 3055 children, 159 (5·20%, 95% CI: 4·44–6·05%) had microcephaly identified by Nellhaus criteria and 70 (2·29%, 95% CI: 1·79–2·89%) by SMARTT criteria. Using WHO standards, 2·15% had microcephaly (95% CI: 1·61, 2·81). The median number of annual HC measures per child was 3 (range: 1–8); the distribution of HC measures by age is provided in the Appendix (p.3).
The characteristics of the 3055 children are shown in Table 1, overall and by microcephaly status according to Nellhaus criteria. Just over half were male (52%), 67% Black, and 32% Hispanic. Most mothers (93%) received cART during pregnancy, and all but 2% (mostly zidovudine monotherapy prior to 2002) received at least 2 ARV drugs. The percent on ART at conception increased from 18% for infants born <2002 to 53% for those born 2015–2017 (Appendix, p.4–5). Microcephaly cases were more often identified in households with lower annual income and caregiver education than non-cases, and mothers of cases more often reported substance use during pregnancy, particularly alcohol (Table 1). Maternal HIV disease measures were similar for children with and without microcephaly. In multivariable models, low education of the caregiver (aRR=1·94), low household income level (aRR=1·96), maternal alcohol use during pregnancy (aRR=1·75), and birth cohort were all confirmed as independent predictors of microcephaly (see Appendix, p.6).
Table 1.
Demographic and Maternal Characteristics of SMARTT Study Participants by Microcephaly Status
| Microcephaly by Nellhaus Criteria |
|||
|---|---|---|---|
| Characteristic | No (N=2896) | Yes (N=159) | Total (N=3055) |
| Cohort | |||
| Dynamic | 1,888 (65%) | 135 (85%) | 2,023 (66%) |
| Static | 1,008 (35%) | 24 (15%) | 1,032 (34%) |
| Birth Cohort | |||
| < 2002 | 311 (11%) | 3 (2%) | 314 (10%) |
| 2002–2006 | 533 (18%) | 11 (7%) | 544 (18%) |
| 2007–2010 | 937 (32%) | 69 (43%) | 1,006 (33%) |
| 2011–2014 | 824 (28%) | 62 (39%) | 886 (29%) |
| 2015–2017 | 291 (10%) | 14 (9%) | 305 (10%) |
| Female sex of infant | 1,408 (49%) | 72 (45%) | 1,480 (48%) |
| Race/origin | |||
| White | 775 (27%) | 42 (26%) | 817 (27%) |
| Black/African American | 1,940 (67%) | 105 (66%) | 2,045 (67%) |
| Other | 24 (1%) | 3 (2%) | 27 (1%) |
| Not known/not reported | 157 (5%) | 9 (6%) | 166 (5%) |
| Latino/Hispanic | 934 (32%) | 46 (29%) | 980 (32%) |
| Birth characteristics | |||
| Low birth weight (<2500g) | 496 (17%) | 46 (29%) | 542 (18%) |
| Preterm birth (<37 weeks gestation) | 542 (19%) | 32 (20%) | 574 (19%) |
| Small for gestational age (<10th percentile) | 509 (18%) | 57 (36%) | 566 (19%) |
| C-section at delivery | 1,570 (55%) | 88 (56%) | 1,658 (55%) |
| Caregiver/Household Characteristics | |||
| Household income<$20,000 | 1,930 (71%) | 127 (85%) | 2,057 (72%) |
| Caregiver not high school graduate | 946 (33%) | 77 (49%) | 1,023 (34%) |
| Maternal Characteristics | |||
| ARV Regimen during Pregnancy | |||
| No ARVs | 57 (2%) | 3 (2%) | 60 (2%) |
| Monotherapy | 62 (2%) | 1 (1%) | 63 (2%) |
| Dual therapy | 70 (2%) | 0 (0%) | 70 (2%) |
| cART | 2,615 (93%) | 151 (97%) | 2,766 (93%) |
| On ARV regimen at conception | 1,009 (36%) | 59 (38%) | 1,068 (36%) |
| PI-containing regimen | 2,026 (72%) | 114 (73%) | 2,140 (72%) |
| NNRTI-containing regimen | 506 (18%) | 34 (22%) | 540 (18%) |
| II-containing regimen | 250 (9%) | 14 (9%) | 264 (9%) |
| Age and Marital Status | |||
| >35 years at birth of child | 525 (18%) | 27 (17%) | 552 (18%) |
| Single, never married | 1,865 (65%) | 112 (72%) | 1,977 (65%) |
| Maternal Immunologic and Virologic Health During Pregnancy | |||
| VL > 1000 copies/mL at delivery | 362 (13%) | 20 (13%) | 382 (13%) |
| VL > 1000 copies/mL early in pregnancy | 1,399 (50%) | 84 (54%) | 1,483 (51%) |
| CD4<250 cells/μL at delivery | 387 (14%) | 29 (19%) | 416 (14%) |
| CD4<250 cells/μL early in pregnancy | 497 (18%) | 35 (23%) | 532 (18%) |
| Maternal Substance Use During Pregnancy | |||
| Marijuana | 203 (7%) | 16 (10%) | 219 (8%) |
| Hard drug use (cocaine/opiate) | 64 (2%) | 9 (6%) | 73 (3%) |
| Alcohol use | 224 (8%) | 22 (14%) | 246 (9%) |
| Tobacco use | 500 (18%) | 36 (23%) | 536 (19%) |
cART= combination antiretroviral treatment, defined as ≥3 drugs from ≥2 classes or ≥3 NRTIs, NRTI=nucleoside reverse transcriptase inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor, II=integrase inhibitor, PI=protease inhibitor, VL=HIV viral load.
Data on certain characteristics were not available for some participants, including ethnicity (n=3), birth characteristics (n=14 for birth weight, 24 for gestational age, 32 for delivery by Cesarean section), maternal age at delivery (n=22), marital status (n=24), household income (n=191), caregiver education (n=24), cART regimen (n=96), drug class exposures (n=72), maternal CD4 measures (n=130) and HIV RNA viral load (n=124), and maternal substance use (n=161); percentages are calculated based on those with available data.
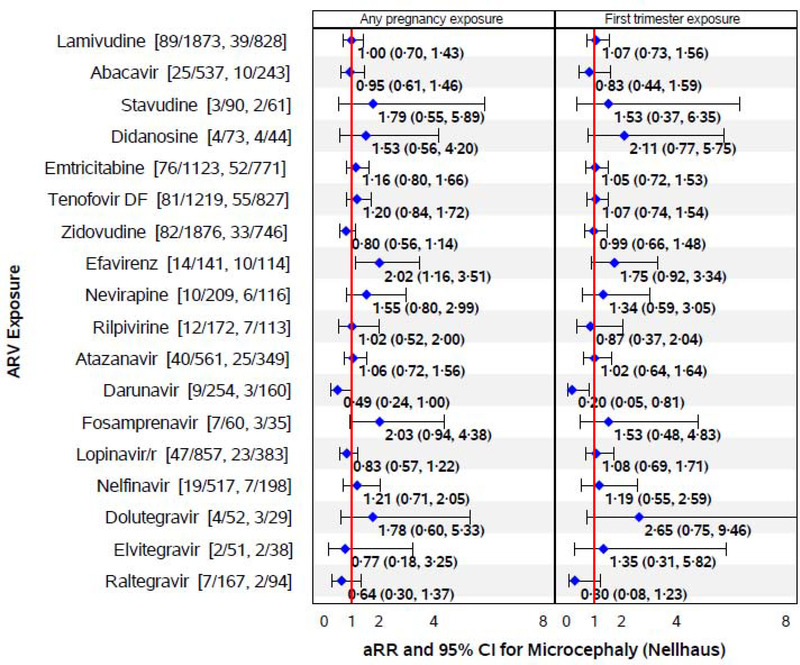
The associations of in utero ARV exposures with microcephaly based on unadjusted and adjusted Poisson regression models are summarized in Table 2 for microcephaly defined by Nellhaus criteria (Appendix, p.7–8 for microcephaly by SMARTT criteria). In adjusted models, efavirenz exposure was associated with two-fold higher risk of microcephaly by Nellhaus standards (Table 2, Figure 1). Among the 2983 with ARV exposure information available, 9·9% of EFV-exposed had microcephaly as compared to 5·0% of EFV-unexposed. Demographic and maternal characteristics by efavirenz exposure status are shown in the Appendix (p.9). While microcephaly by SMARTT criteria was less common, efavirenz exposure was again associated with significantly higher risk (aRR=2·56, 95% CI: 1·22, 5·37, Appendix, p.7–8). Use of WHO standards for defining HC Z-scores yielded a similarly elevated risk of microcephaly for efavirenz-exposed children (6·5%) compared to efavirenz-unexposed children (2·0%, aRR=3·69, 95% CI: 1·77, 7·.70). The associations were less pronounced for 1st trimester efavirenz exposure (Figure 1, Appendix, p.8). Fosamprenavir also showed higher risk of microcephaly by Nellhaus but not SMARTT standards. Darunavir showed a protective association with microcephaly based on Nellhaus criteria, both overall and for 1st trimester exposure (Figure 1), but not by SMARTT standards (Appendix, p.8).
Table 2.
Association of in utero ARV Exposure with Microcephaly by Nellhaus Criteria
| Percent of Cases |
Unadjusted Model |
Adjusted Model* |
||||||
|---|---|---|---|---|---|---|---|---|
| # Evaluated | Percent Exposed | Exposed | Unexposed | RR (95% CI) | P-value | RR (95% CI) | P-value | |
| By ARV Drug Class or Regimen | ||||||||
| NRTIs | 2983 | 97·6 | 153/2912 (5·3%) | 3/71 (4·2%) | 1·24 (0·40, 3·90) | 0·71 | 1·41 (0·35, 5·72) | 0·63 |
| NNRTIs | 2983 | 18·1 | 34/540 (6·3%) | 122/2443 (5·0%) | 1·26 (0·86, 1·84) | 0·23 | 1·45 (0·97, 2·15) | 0·068 |
| PIs | 2983 | 71·7 | 114/2140 (5·3%) | 42/843 (5·0%) | 1·07 (0·75, 1·52) | 0·71 | 0·84 (0·57, 1·22) | 0·35 |
| IIs | 1976 | 13·4 | 14/264 (5·3%) | 118/1712 (6·9%) | 0·77 (0·44, 1·34) | 0·35 | 0·89 (0·50, 1·57) | 0·68 |
| Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | ||||||||
| Lamivudine | 2983 | 62·8 | 89/1873 (4·8%) | 67/1110 (6·0%) | 0·79 (0·57, 1·08) | 0·14 | 1·00 (0·70, 1·43) | 1·00 |
| Abacavir | 2983 | 18·0 | 25/537 (4·7%) | 131/2446 (5·4%) | 0·87 (0·57, 1·33) | 0·52 | 0·95 (0·61, 1·46) | 0·81 |
| Stavudine | 2983 | 3·0 | 3/90 (3·3%) | 153/2893 (5·3%) | 0·63 (0·20, 1·98) | 0·43 | 1·79 (0·55, 5·89) | 0·34 |
| Didanosine | 2983 | 2·4 | 4/73 (5·5%) | 152/2910 (5·2%) | 1·05 (0·39, 2·83) | 0·92 | 1·53 (0·56, 4·20) | 0·41 |
| Emtricitabine | 2983 | 37·6 | 76/1123 (6·8%) | 80/1860 (4·3%) | 1·57 (1·15, 2·15) | 0·005 | 1·16 (0·80, 1·66) | 0·43 |
| Tenofovir | 2983 | 40·9 | 81/1219 (6·6%) | 75/1764 (4·3%) | 1·56 (1·14, 2·14) | 0·005 | 1·20 (0·84, 1·72) | 0·32 |
| Zidovudine | 2983 | 62·9 | 82/1876 (4·4%) | 74/1107 (6·7%) | 0·65 (0·48, 0·90) | 0·008 | 0·80 (0·56, 1·14) | 0·21 |
| Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs) | ||||||||
| Efavirenz | 2983 | 4·7 | 14/141 (9·9%) | 142/2842 (5·0%) | 1·99 (1·15, 3·44) | 0·014 | 2·02 (1·16, 3·51) | 0·013 |
| Nevirapine | 2983 | 7·0 | 10/209 (4·8%) | 146/2774 (5·3%) | 0·91 (0·48, 1·73) | 0·77 | 1·55 (0·80, 2·99) | 0·19 |
| Rilpivirine | 927 | 18·6 | 12/172 (7·0%) | 52/755 (6·9%) | 1·01 (0·54, 1·90) | 0·97 | 1·02 (0·52, 2·00) | 0·95 |
| Protease Inhibitors (PIs) | ||||||||
| Atazanavir | 2983 | 18·8 | 40/561 (7·1%) | 116/2422 (4·8%) | 1·49 (1·04, 2·13) | 0·030 | 1·06 (0·72, 1·56) | 0·75 |
| Darunavir | 1976 | 12·9 | 9/254 (3·5%) | 123/1722 (7·1%) | 0·50 (0·25, 0·98) | 0·042 | 0·49 (0·24, 1·00) | 0·050 |
| Fosamprenavir | 2983 | 3·0 | 7/60 (11·7%) | 125/1916 (6·5%) | 1·79 (0·84, 3·83) | 0·13 | 2·03 (0·94, 4·38) | 0·071 |
| Lopinavir | 2983 | 28·7 | 47/857 (5·5%) | 109/2126 (5·1%) | 1·07 (0·76, 1·51) | 0·70 | 0·83 (0·57, 1·22) | 0·35 |
| Nelfinavir | 2983 | 17·3 | 19/517 (3·7%) | 137/2466 (5·6%) | 0·66 (0·41, 1·07) | 0·091 | 1·21 (0·71, 2·05) | 0·49 |
| Saquinavir | 2983 | 2·3 | 1/70 (1·4%) | 155/2913 (5·3%) | 0·27 (0·04, 1·92) | 0·19 | 0·35 (0·05, 2·55) | 0·30 |
| Integrase Inhibitors (IIs) | ||||||||
| Dolutegravir | 506 | 10·3 | 4/52 (7·7%) | 28/454 (6·2%) | 1·25 (0·44, 3·56) | 0·68 | 1·78 (0·60, 5·33) | 0·30 |
| Elvitegravir | 506 | 10·1 | 2/51 (3·9%) | 30/455 (6·6%) | 0·59 (0·14, 2·49) | 0·48 | 0·77 (0·18, 3·25) | 0·72 |
| Raltegravir | 1976 | 8·5 | 7/167 (4·2%) | 125/1809 (6·9%) | 0·61 (0·28, 1·30) | 0·20 | 0·64 (0·30, 1·37) | 0·25 |
Adjusted model includes low education, low household income, alcohol use during pregnancy, and birth cohort (2007–2010, 2011–2014 and 2015–2017 vs <2007). Models restricted to children born after 2007 (N=1976) for darunavir, raltegravir, fosamprenavir, and IIs (as drug class), after 2011 (N=927) for rilpivirine, and after 2013 (N=506) for dolutegravir and elvitegravir; all other ARV drugs are evaluated in the full SMARTT cohort (N=2983). Associations with p<0·05 are shown in bold-face.
Figure 1. Associations of in utero ARV Exposures with Microcephaly by Nellhaus criteria.
Numbers in brackets after each drug name indicate number of microcephaly cases among those exposed to the specific ARV drug during pregnancy, and number of cases among those exposed to that ARV drug during the first trimester. Adjusted relative risks (aRRs) are based on modified Poisson regression models adjusting for low education, low household income, alcohol use during pregnancy, and birth cohort (2007–2010, 2011–2014 and 2015–2017 vs <2007). Models based on 1976 children born after 2007 for darunavir, raltegravir, fosamprenavir, and IIs (as drug class), 927 children born after 2011 for rilpivirine, and 506 children born after 2013 for dolutegravir and elvitegravir; all other ARV drugs are evaluated in the full SMARTT cohort (N=2983).
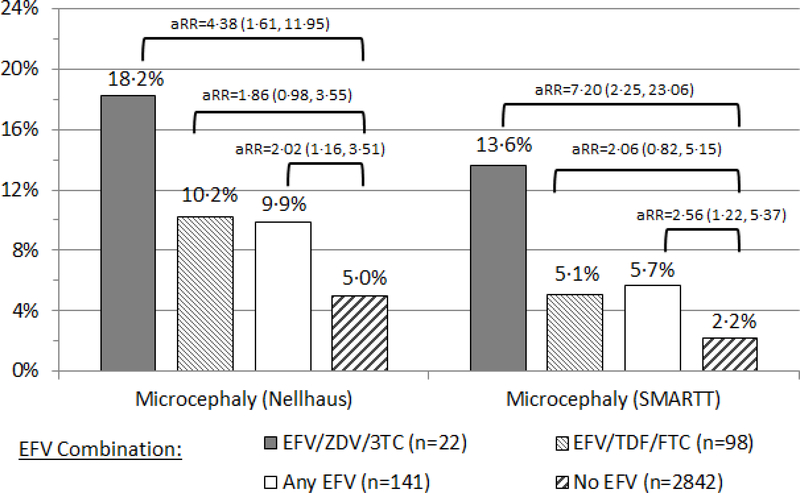
Additional evaluations were conducted to examine typical nucleoside reverse transcriptase inhibitor (NRTI) backbones used with efavirenz. The association was more pronounced when efavirenz was used in combination with zidovudine+lamivudine than with TDF+emtricitabine, with corresponding adjusted RRs of 4·38 and 1·86 for microcephaly by Nellhaus criteria and 7·20 and 2·06 by SMARTT criteria (Figure 2). Adjusted models including all ARV exposures simultaneously, either as individual fixed effects or using a hierarchical approach which includes ARV drugs as random effects within drug classes, also provided consistent findings. In these models, efavirenz was the only ARV drug associated with an increased risk of microcephaly by SMARTT criteria (aRR=3·17; 95% CI: 1·41, 7·12 for the fixed effect model, aRR=2·31; 95% CI: 1·15, 4·63 for the hierarchical model) even after accounting for all other ARV drugs used in the same regimen (Appendix, p.11).
Figure 2.
Percent of HEU Infants with Microcephaly by efavirenz (EFV)-containing Maternal ARV Regimen, with Adjusted Risk Ratios (aRRs) and 95% Confidence Intervals
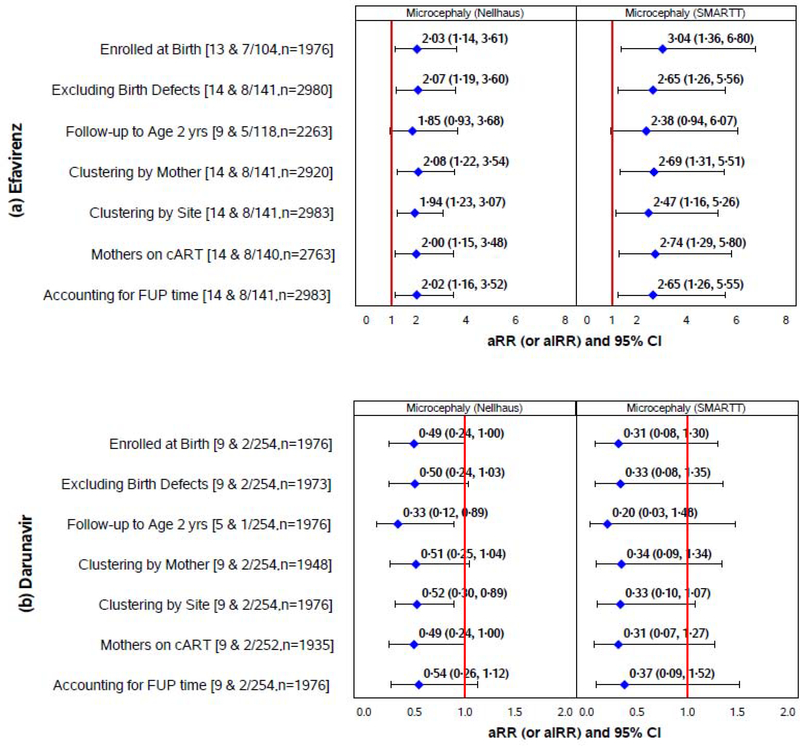
Sensitivity analyses were conducted to confirm the robustness of findings, and generally confirmed the association of efavirenz with increased risk of microcephaly by both SMARTT criteria and Nellhaus thresholds, with adjusted RRs ranging from 2–3. In particular, associations with efavirenz persisted when restricted to children followed from birth, including only the first two years of life, excluding cases of microcephaly occurring among infants with other congenital defects, when restricted to children whose mothers received cART during pregnancy, and accounting for follow-up time (Figure 3(a)). Associations also persisted in models stratifying by birth characteristics (LBW, preterm, SGA), maternal VL and CD4, substance use, and birth cohort (Appendix, p.12), and when considering pre-conception versus post-conception timing of efavirenz exposure (Appendix, p.13). Within the Dynamic cohort, exposure to nevirapine was also associated with higher risk of microcephaly (aRR=2·20; 95% CI: 1·11, 4·36 by Nellhaus criteria, aRR=4·34; 95% CI: 1·82, 10·39 by SMARTT criteria). The protective association of darunavir was also observed in many of the sensitivity analyses (Figure 3(b)).
Figure 3. Sensitivity Analyses for Associations of efavirenz and darunavir with Microcephaly in HEU Infants and Children.
Numbers in brackets indicate the number of microcephaly cases among those exposed to efavirenz (panel (a)) or darunavir (panel (b)), by the Nellhaus and SMARTT criteria, respectively; the total sample size for each sensitivity analysis is also provided.
Adjusted relative risks (aRRs) and adjusted incidence rate ratios (aIRRs) are based on modified Poisson regression models adjusting for low education, low household income, alcohol use during pregnancy, and birth cohort (2007–2010, 2011–2014 and 2015–2017 vs <2007) for microcephaly by Nellhaus criteria, and adjusting for low education, 1st trimester tobacco, and birth cohorts (2007–2010, 2011–2014 and 2015–2017 vs <2007) for microcephaly by SMARTT criteria. Models accounting for clustering within site or within the same mother/family are fit using generalized estimating equation models with an assumed exchangeable correlation structure. Incidence rates are estimated based on person-time from birth or study entry to the first date of documented microcephaly or latest study visit without microcephaly. FUP=follow-up, cART= combination ARV regimen.
Among the 3055 children included in our analysis, 1555 had Bayley-III assessments at age 1 and 1218 had WPPSI-III assessments at age 5. Bayley_III scores for cognitive, motor, and language domains were significantly lower among those with than without microcephaly, with mean differences of 5 to 12 points (Table 3). Similarly, mean WPPSI-III scores were significantly lower for children with than without microcephaly for most subscales. Among the 2089 with any neurodevelopmental assessment, the percent impaired was 14·8% vs 4·6% for those with versus without microcephaly by Nellhaus criteria, and 25·0% vs 4·7% for those with versus without microcephaly by SMARTT criteria.
Table 3.
Neurodevelopmental Functioning Measures by Microcephaly Status, among those with Both Head Circumference Measures and Neurodevelopmental Assessments
| Microcephaly (Nellhaus) |
Microcephaly (SMARTT) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Neurodevelopmental Score | No |
Yes |
Difference |
No |
Yes |
Difference |
||
| Mean (SD) | Mean (SD) | Mean (SE) | P-Value | Mean (SD) | Mean (SD) | Mean (SE) | P-Value | |
| Bayley-III at age 1 year | ||||||||
| N=1405–1449 | N=97–99 | N=1462–1506 | N=40–41 | |||||
| Cognitive score | 103·1 (14·1) | 97·5 (17·4) | 5·58 (1·50) | 0·011 | 103·1 (14·1) | 90·9 (20·5) | 12·2 (2·26) | <·001 |
| Motor score | 97·5 (13·1) | 92·3 (16·3) | 5·17 (1·40) | 0·002 | 97·5 (13·0) | 84·6 (20·0) | 12·8 (2·11) | <·001 |
| Language score | 94·4 (14·1) | 90·8 (14·9) | 3·57 (1·50) | 0·043 | 94·4 (14·1) | 85·7 (16·9) | 8·67 (2·27) | 0·003 |
| General adaptive score | 94·6 (14·9) | 91·0 (17·4) | 3·59 (1·58) | 0·075 | 94·6 (14·8) | 85·8 (20·6) | 8·84 (2·40) | 0·002 |
| Social-emotional score | 101·5 (17·9) | 98·0 (16·7) | 3·47 (1·87) | 0·064 | 101·5 (17·9) | 94·0 (16·5) | 7·49 (2·86) | 0·029 |
| WPPSI-III at age 5 years | ||||||||
| N=1124–1141 | N=70–72 | N=1148–1188 | N=23–25 | |||||
| Full Scale IQ | 94·8 (14·9) | 89·8 (16·5) | 5·06 (1·84) | 0·006 | 94·7 (14·9) | 85·4 (15·7) | 9·36 (3·02) | 0·002 |
| Verbal IQ score | 92·5 (13·8) | 89·4 (15·0) | 3·05 (1·69) | 0·072 | 92·4 (13·8) | 87·3 (15·3) | 5·08 (2·80) | 0·070 |
| Performance IQ Score | 97·2 (15·4) | 92·3 (16·1) | 4·92 (1·88) | 0·009 | 97·1 (15·5) | 87·8 (15·7) | 9·31 (3·13) | 0·003 |
| Processing Speed Score | 95·8 (15·6) | 91·3 (16·1) | 4·53 (1·94) | 0·020 | 95·7 (15·6) | 86·5 (14·6) | 9·22 (3·29) | 0·005 |
P-value by Wilcoxon Test
SD=standard deviation, SE=standard error, WPPSI=Wechsler Preschool and Primary Scales of Intelligence
DISCUSSION
Our results were generally reassuring in not identifying an increased risk of microcephaly for most individual ARVs or drug classes. The key exception to these overall findings was the robust association of in utero efavirenz exposure with two to three-fold higher risk of microcephaly. The magnitude of this effect was similar to that for factors reflecting low socioeconomic status, and slightly stronger than that for fetal alcohol exposure. We also noted a protective effect of darunavir and increased risk with fosamprenavir for microcephaly based on Nellhaus criteria, but these associations were less robust. While microcephaly defined by CDC or WHO criteria was less common, the association with efavirenz appeared stronger (aRRs of 2·4–3·7) than when using Nellhaus thresholds (aRRs of 1·8–2·1). These associations persisted across a broad range of sensitivity analyses conducted to evaluate the potential for bias; stratified analyses by birth characteristics and other maternal risk factors suggested that associations were not primarily attributable to vulnerable subgroups of the population. Although the estimated RR for 1st trimester efavirenz was elevated, consistent with other estimated RRs for pre- and post-conception efavirenz exposure, it was not statistically significant due to the relatively infrequent first trimester use. In addition, we did not find that pre-conception initiation of efavirenz exposure had more pronounced effects on microcephaly than post-conception initiation. We observed a more pronounced association of efavirenz with microcephaly when used in combination with zidovudine+lamivudine than with TDF+emtricitabine, although these findings were based on small numbers and warrant confirmation. We employed novel approaches to account for other ARV drugs in the same regimen, and obtained consistent findings of elevated risk for efavirenz. Efavirenz passes through the placenta, and animal studies have demonstrated reduced body weight and changes in the brain motor cortex in offspring after perinatal efavirenz exposure; these changes have been attributed to the targeted effects of efavirenz and its metabolites on the central nervous system, particularly the serotonergic system.25 Preliminary findings from our SMARTT study also suggest increased risk of neurologic conditions in general with efavirenz exposure.26
We observed a low rate of 2·3% with microcephaly (by SMARTT criteria) in our population of HEU children born to mothers with HIV infection, consistent with CDC standards for U.S. children. We used CDC standards for HC to define microcephaly since our study was conducted solely within the U.S., but WHO standards yielded similar overall rates and findings of elevated risk for efavirenz-exposed children. We also considered the criteria for microcephaly proposed by Nellhaus,18 often used in clinical practice since CDC growth standards are not available for children over 3 years. Using the Nellhaus criteria, we observed a slightly higher rate of microcephaly of 5·2%, but still lower than the 7·5% rate reported for HEU infants followed through 6 months of age in Latin America.12 Differences in these estimates may be related to the background population; for example, substance use is less common among women enrolled in SMARTT than reported in Latin America. The higher rate observed in their study may also be due to their two evaluations in the first 6 months of life, when microcephaly is most often identified. Other surveillance programs have reported much lower rates for microcephaly, of 4–15 cases per 10,000 births, but have typically focused on microcephaly in the context of birth defects surveillance using more extreme criteria.27,28 Identifying a background prevalence of microcephaly among HEU children may be important in recognizing potential increases with co-infection by Zika virus, cytomegalovirus, or other congenital infections.28
The implications of microcephaly on longer term outcomes have been evaluated in the general population but have been understudied among HEU children. We demonstrated significant neurodevelopmental deficits at 1 and 5 years of age among children with microcephaly. Mean differences in Bayley-III and WPPSI-III outcomes were of clinical significance, translating to a three- to five-fold higher proportion of children with neurodevelopmental impairment among those with than without microcephaly.
Our study has limitations inherent with those of an observational cohort study; while we attempted to control for confounding by indication in our analyses, residual confounding may still remain. This is particularly important for a drug like efavirenz, which was subject to intensive scrutiny as a potential teratogen several decades ago and may have preferentially been avoided by some clinicians for mothers with shared risk factors for microcephaly in their infants.29 Recent studies and systematic reviews have not identified increased risk of adverse birth outcomes for women receiving efavirenz during pregnancy, and these studies have considered populations where a higher percentage of women received efavirenz as a first-line treatment.29–32 Another limitation of our analysis is that we were unable to account for other important congenital infections, such as cytomegalovirus. The annual visits in our study design meant that early post-natal measurements of HC were not collected, which prevented distinction of congenital from acquired microcephaly.
However, our analysis had several key strengths, including its well-characterized cohort with collection of many potential confounding factors, and the long-term follow up of HEU children which is a recognized advantage of the SMARTT study but may be challenging in lower-resource settings. The median follow-up of over 5 years from birth in this study allowed the identification of microcephaly cases which may not have been captured in infancy, and others have noted that HC is a strong predictor of brain volume even for pre-school and older children.4 Other strengths of our study included an approach which tailored the examination of each individual ARV drug to the time period appropriate for that medication, given its approval date and use in our cohort. Finally, while many safety studies have examined ARV drugs individually, we accounted for other ARV medications taken concurrently in the same regimen, and also employed a comparative safety approach to examine relative risks when efavirenz was used with specific NRTI backbones.
In conclusion, our findings are generally reassuring in supporting the use of combination ART in pregnant women for their own health and to reduce risk of perinatal HIV transmission. However, the increased risk of microcephaly observed with efavirenz use during pregnancy warrants closer examination in other settings. Efavirenz-based ART is currently recommended by the WHO as the preferred first-line regimen for adults and adolescents3,32, and is consequently taken by thousands of women during pregnancy each year. Recent reports of potential adverse birth outcomes (neural tube defects) with use of dolutegravir may result in increased usage of efavirenz.32 The implications of our findings thus have broad global implications in lower resource settings where efavirenz is used more widely, and underscore the need for continued monitoring of long-term outcomes of new and existing ARVs.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Elevated rates of mitochondrial dysfunction have been reported in children born to mothers with HIV who took antiretroviral (ARV) medications during pregnancy compared to ARV-unexposed children, and neurological conditions comprised a substantial proportion of these cases. One particular neurologic condition of concern is microcephaly, which generally reflects poor brain growth and has been linked to poorer neurodevelopmental outcomes. To identify studies which evaluated microcephaly among children born to mothers with HIV, we searched PubMed on 11/20/2018, using the terms “microcephaly”, “HIV”, and “pregnancy”. The search was unrestricted by language or publication date and resulted in 20 papers. We eliminated those specifically related to microcephaly as a result of ZIKA infection, which led to 12 papers. After full-text screening of these papers and the references therein, we identified three relevant studies. One observational study conducted in Latin American reported higher rates of microcephaly among HIV-exposed uninfected (HEU) children than in the general population, but observed no association with maternal ARVs used during pregnancy. Another study evaluated children born in Zimbabwe to HIV-infected mothers in the era prior to ARV use, and observed microcephaly more often in infected than uninfected children, but also lower head circumferences in HEU children than HIV-unexposed children. The third study compared growth measures including head circumference of HEU children under 2 years old in the U.S. to HIV-unexposed children, and observed no difference.
Added value of this study
We present the first comprehensive assessment of maternal ARV use during pregnancy for women with HIV infection and association of specific ARVs with microcephaly in a higher resource setting, based on a longitudinal cohort study which provided longer term follow-up than previous studies. We also evaluated the implications of microcephaly on neurodevelopmental functioning, which has not been addressed in HEU children.
Implications of all available evidence
Our findings suggest that maternal use of efavirenz during pregnancy may increase the risk of microcephaly in their children. Careful consideration should be given to whether efavirenz should be included as part of first-line treatment.
ACKNOWLEDGEMENTS
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Program Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
FUNDING: Eunice Kennedy Shriver National Institute of Child Health and Human Development
Footnotes
Declaration of interests: We declare no competing interests.
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2017, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Patricia A. Garvie, James Blood; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Marsha Vasserman; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Nicolas Rosario, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Stephan Kohlhoff, Ava Dennie, Ady Ben-Israel, Jean Kaye; Tulane University School of Medicine: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Paige Hickman, Dan Marullo; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado, Denver: Elizabeth McFarland, Emily Barr, Christine Kwon, Carrie Chambers; University of Florida, Center for HIV/AIDS Research, Education and Service: Mobeen Rathore, Kristi Stowers, Saniyyah Mahmoudi, Nizar Maraqa, Laurie Kirkland; University of Illinois, Chicago: Karen Hayani, Lourdes Richardson, Renee Smith, Alina Miller; University of Miami: Gwendolyn Scott, Sady Dominguez, Jenniffer Jimenez, Anai Cuadra; Keck Medicine of the University of Southern California: Toni Frederick, Mariam Davtyan, Guadalupe Morales-Avendano, Janielle Jackson-Alvarez; University of Puerto Rico School of Medicine, Medical Science Campus: Zoe M. Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Paige L. Williams, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, MA
Cenk Yildirim, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, MA.
Ellen G. Chadwick, Northwestern University Feinberg School of Medicine, Chicago IL
Russell B. Van Dyke, Tulane University School of Medicine, New Orleans, LA
Renee Smith, University of Illinois at Chicago, IL.
Katharine F. Correia, Amherst College, Amherst, MA
Alexandria DiPerna, Frontier Science Technology and Research Foundation (FSTRF), Amherst, NY.
George R. Seage, III, Center for Biostatistics in AIDS Research, Harvard T. H. Chan School of Public Health, Boston, MA.
Rohan Hazra, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD.
Claudia S. Crowell, Seattle Children’s Hospital and University of Washington, Seattle, WA
References
- 1.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz D, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1 infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2005; 29(5):484–494. [DOI] [PubMed] [Google Scholar]
- 2.Zash RM, Williams PL, Sibiude J, Lyall H, Kakkar F. Surveillance monitoring for safety of in utero antiretroviral therapy exposures: current strategies and challenges. Expert Opin Drug Saf. 2016. (11):1501–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV. Geneva, Switzerland: WHO; 2018. https://www.who.int/hiv/pub/guidelines/ARV2018update/en/, Accessed 5/2/2019. [Google Scholar]
- 4.Rollins JD, Collins JS, Holden KR. United States head circumference growth reference charts: birth to 21 years. J Pediatr 2010; 156: 907–913. [DOI] [PubMed] [Google Scholar]
- 5.Epstein LG, Gelbard HA. HIV-induced neuronal injury in the developing brain. J Leukoc Biol. 1999; 65:453–457. [DOI] [PubMed] [Google Scholar]
- 6.Rosman NP, Tarquinio DC, Datseris M, Hou W, Mannheim GB, Emigh CE et al. Postnatal onset microcephaly: pathogenesis, patterns of growth, and prediction of outcome. Pediatrics 2011; 127:665–671. [DOI] [PubMed] [Google Scholar]
- 7.Baxter PS, Rigby AS, Rotsaert MH, Wright I. Acquired microcephaly: causes, patterns, motor and IQ effects, and associated growth changes. Pediatrics. 2009; 124(2): 590–595. [DOI] [PubMed] [Google Scholar]
- 8.Cheong JL, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, et al. Head growth in preterm infants: correlation with magnetic resonance imagining and neurodevelopmental outcome. Pediatrics. 2008; 121:e1534–e1540. [DOI] [PubMed] [Google Scholar]
- 9.Macmillan C, Magder LS, Brouwers P, Chase C, Hittelman J, Lasky T, et al J. Head growth and neurodevelopment of infants born to HIV-1-infected drug-using women. Neurology. 2001; 57(8):1402–11. [DOI] [PubMed] [Google Scholar]
- 10.Evans C, Chasekwa B, Ntozini R, Humphrey JH, Prendergast AJ. Head circumference of children born to HIV-infected and HIV-uninfected mothers in Zimbabwe during the preantiretroviral therapy area. AIDS. 2016; 30: 2323–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez C, Archila ME, Rugeles C, Carrizosa J, Rugeles MT, Cornejo JW. A prospective study of neurodevelopment of uninfected children born to human immunodeficiency virus type 1 positive mothers. Rev Neurol. 2009; 48: 287–291. [PubMed] [Google Scholar]
- 12.Spaulding AB, Yu Q, Civitello L, Mussi-Pinhata, Pinto J, Gomes IM,, et al. ; NISDI LILAC Study Team. Neurologic outcomes in HIV-exposed/uninfected infants exposed to antiretroviral drugs during pregnancy in Latin America and the Caribbean. AIDS Res Human Retrov. 2016; 32(4): 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neri D, Somarriba A, Schaefer NN, Chaparro AI, Scott GW, Lopez Mitnik G et al. Growth and body composition of uninfected children exposed to human immunodeficiency virus: comparison with a contemporary cohort and the United States national standards. J Pediatr. 2013; 163: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siberry GK, Williams PL, Mendez H, Seage GR III, Jacobson D, et al. for the Pediatric HIV/AIDS Cohort Study. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012; 26:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PL, Seage GR, Van Dyke RB, Siberry GK, Griner R, et al. for the Pediatric HIV/AIDS Cohort Study. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol. 2012; 175(9):950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tassiopoulos K, Read JS, Brogly S, Rich K, Lester B, Spector SA Yogev R, Seage GR. Substance use in HIV-infected women during pregnancy: self-report versus meconium analysis. AIDS Behav. 2010; 14(6):1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Clinical growth charts. Available at: https://www.cdc.gov/growthcharts/clinical_charts.htm. Accessed on February 10, 2019.
- 18.Nellhaus G Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968; 41(1):106–14. [PubMed] [Google Scholar]
- 19.Zemel BS, Pipan M, Stallings VA Growth charts for children with Down syndrome in the United States. Pediatrics. 2015; 136:e1204–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization: WHO child growth standards. Available at https://www.who.int/childgrowth/standards/en/. Accessed June 18, 2019.
- 21.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 22.Correia K, Williams PL. A hierarchical modeling approach for assessing the safety of exposure to complex antiretroviral drug regimens during pregnancy. Stat Methods Med Res. 2017; doi: 10.1177/0962280217732597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bayley N Bayley Scales of Infant and Toddler Development. Third Edition. San Antonio: Harcourt Assessment Inc.; 2006. [Google Scholar]
- 24.Wechsler Preschool and Primary Scales of Intelligence — Third Edition. San Antonio: The Psychological Corporation; 2002. [Google Scholar]
- 25.Van der Wijer, Garcia LP, Hanswijk SL, Rando J, Middelman A et al. Neurodevelopmental and behavioral consequences of perinatal exposure to the HIV drug efavirenz in a rodent study. Translational Psych 2019; 9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowell C, Williams P, Yildirim C, Van Dyke R, Smith R, Chadwick E, Seage G III, Hazra R for the Pediatric HIV/AIDS Cohort Study. Safety of In Utero Antiretroviral (ARV) Exposure: Neurologic Outcomes in HIV-Exposed, Uninfected Children. ID Week Conference, October 3 – 7, 2018, San Francisco, California. [Google Scholar]
- 27.Cragan JD, Isenburg JL, Parker SE, Alverson CJ, Meyer RE, Stallings EB, et al. ; National Birth Defects Prevention Network. Population-based microcephaly surveillance in the United States, 2009 to 2013: An analysis of potential sources of variation. Birth Defects Res A Clin Mol Teratol. 2016; 106(11):972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orioli IM, Dolk H, Lopez-Camelo JS, Mattos D, Poletta FA, et al. Prevalence and clinical profile of microcephaly in South America pre-Zika, 2005–14: prevalence and case-control study. BMJ. 2017; 359:j5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford N, Mofenson L, Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, Shaffer N, Renaud F. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2014; 28 Suppl 2:S123–31. [DOI] [PubMed] [Google Scholar]
- 30.Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health. 2018; 6(7):e804–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanters S, Vitoria M, Doherty M, Socias ME, Ford N, Forrest JI, et al. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV. 2016; 3(11):e510–e520. [DOI] [PubMed] [Google Scholar]
- 32.Dugdale CM, Ciaranello AL, Bekker L-G, Stern ME, Myer L, et al. Risks and benefits of dolutegravir- and efavirenz-based strategies for South African women of child bearing potential. Ann Int Med 2019; doi: 10.7326/M18-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.