Abstract
Mammalian target of rapamycin complex 1 (mTORC1) promotes cell growth and proliferation in response to nutrients and growth factors. Amino acids induce lysosomal translocation of mTORC1 via the Rag GTPases. Growth factors activate Ras homolog enriched in brain (Rheb), which in turn activates mTORC1 at the lysosome. Amino acids and growth factors also induce the phospholipase D (PLD)–phosphatidic acid (PA) pathway, required for mTORC1 signaling through mechanisms that are not fully understood. Here, using human and murine cell lines, along with immunofluorescence, confocal microscopy, endocytosis, PLD activity, and cell viability assays, we show that exogenously supplied PA vesicles deliver mTORC1 to the lysosome in the absence of amino acids, Rag GTPases, growth factors, and Rheb. Of note, pharmacological or genetic inhibition of endogenous PLD prevented mTORC1 lysosomal translocation. We observed that precancerous cells with constitutive Rheb activation through loss of tuberous sclerosis complex subunit 2 (TSC2) exploit the PLD–PA pathway and thereby sustain mTORC1 activation at the lysosome in the absence of amino acids. Our findings indicate that sequential inputs from amino acids and growth factors trigger PA production required for mTORC1 translocation and activation at the lysosome.
Keywords: mTOR complex (mTORC), phosphatidic acid, phospholipase D, phospholipid vesicle, lysosome, amino acid, cancer biology, cancer therapy, growth factor
Introduction
mTORC18 is a conserved (1) serine/threonine catalytic complex that integrates signals from nutrients and growth factors to regulate cell growth, proliferation, survival, and metabolism (2). Activation of mTORC1 is a two-step process whereby amino acids induce Rag-dependent translocation of mTORC1 from the cytoplasm to the lysosome, followed by mTOR kinase activation by the lysosomal small GTPase Rheb upon growth factor stimulation (3).
Phospholipase D (PLD) and its product, the signaling lipid phosphatidic acid (PA) play a role in mTORC1 activation in response to amino acids and growth factors. Amino acids induce lysosomal translocation of PLD1 (4). Once on the lysosome, PLD1 binds to Rheb, which activates PLD1 in response to growth factors (5). PLD1 is widely expressed in mammals and converts the most abundant membrane phospholipid phosphatidylcholine to choline and PA (6). Conserved basic amino acids in the FKBP12–rapamycin binding (FRB) domain of mTOR (7–9) lead to proton dissociation to generate PA with two negative charges. This locks mTOR onto deprotonated PA (10), promoting mTORC1 assembly and stability (11, 12).
We report here that PA with an unsaturated fatty acid stimulates lysosomal translocation and activation of mTORC1 in the absence of amino acids, Rag GTPases, growth factors, or Rheb. Our work provides a unifying model showing that PA is critical for translocation and full activation of mTORC1 at the lysosome in response to sequential signals provided by amino acids and growth factors.
Results
PA with a monounsaturated fatty acid drives mTOR to the lysosome in the absence of amino acids
We previously reported that the amino acid–induced activation of mTORC1 depends on PLD (13). Amino acids induce the translocation of mTOR from the cytoplasm to the lysosome. PLD and PA play critical roles in cellular vesicle trafficking (14). Thus, PLD and PA could play a role in the translocation of mTOR from the cytoplasm to the lysosome. To test this hypothesis, we performed amino acid depletion and restimulation experiments in the presence of PA, followed by confocal microscopy detection of LAMP2 (lysosomal marker) and mTOR (Fig. 1A). In DU145 prostate cancer cells we saw a pronounced amino acid–induced mTOR lysosomal translocation. We previously reported that activation of mTOR by PA generated by exogenously supplied fatty acids was achieved with oleic acid (15). We therefore used a PA species with the unsaturated fatty acid oleic acid, PA-18:1, and a PA species without unsaturated fatty acids, PA-16:0 (Fig. 1B). PA-18:1 is the most abundant species of PA in mouse brain, whereas PA-16:0 has low abundance (16). PA-18:1 induced mTOR lysosomal translocation similar to amino acids (Fig. 1A). If we added back both amino acids and PA-18:1, LAMP2/mTOR colocalization was even stronger, indicating a synergy between amino acids and PA. In contrast, PA-16:0 had no effect on mTOR translocation (Fig. 1A). Fig. 1C depicts the quantification of two parameters of Fig. 1A: the percentage of cells with LAMP2/mTOR colocalization and the Pearson's correlations of LAMP2/mTOR-labeled pixels. Next, we determined the levels of mTORC1 activity toward the targets ribosomal subunit S6 kinase (S6K), at Thr-389, and eukaryotic initiation factor 4E–binding protein 1 (4E-BP1), at Thr-37/46 (Fig. 1D). PA-18:1, but not PA-16:0, increased the phosphorylation of S6K (P-S6K) and 4E-BP1 (P-4E-BP1) to levels similar to amino acid stimulation. These results suggest that PA-18:1 drives mTOR to the lysosome in the absence of amino acids and promotes mTORC1 activity.
Figure 1.
PA-18:1 drives mTOR to the lysosome and activates mTORC1 in the absence of amino acids. A, IF detection of LAMP2/mTOR in DU145 cells treated as indicated (region of interest (ROI), amplification of 2 × 2-μm areas of relevance). B, structure, conventional nomenclature, and abbreviated nomenclature of the two PA species used in A. PA-18:1 is a high-abundance PA species, whereas PA-16:0 is a low-abundance PA species. C, LAMP2/mTOR percentage of colocalization and Pearson's correlation for images shown in A (mean of triplicate experiments ± S.E.; comparisons relative to −AA). D, Western blotting analysis of mTORC1 activity of cells shown in A. E, Western blotting analysis of the dose-curve of PA-18:1 activation of mTORC1 in DU145 cells. MM, molecular marker.
In our experiments with PA, we prepared small unilamellar vesicles (SUVs). To form SUVs, the concentration of PA has to be above the critical micelle concentration (CMC). Because different CMCs could account for the differences between PA species, we determined the CMC (17) of PA-18:1 (63.6 μm) and PA-16:0 (78.8 μm) (Fig. S1). These indicate that, at a final concentration of 300 μm, both PA species form vesicles. To show that PA-18:1 can only activate mTORC1 in a vesicle format, we performed a dose-curve experiment in the absence of amino acids (Fig. 1E). We observed mTORC1 activation if the concentration of PA-18:1 was above 50 μm. 50 μm is very close to the CMC determined here: 63.6 μm. The CMC was determined in PBS, so it is possible that PA will form vesicles at a slightly lower concentration in culture medium. Our findings suggest that PA-18:1 can only activate mTORC1 once it forms vesicles.
PA-18:1 vesicles enter the cell via macropinocytosis and rapidly reach the lysosome
To understand how exogenously supplied PA-18:1 vesicles enter the cell and promote mTORC1 translocation, we performed an endocytosis assay (18) of fluorescently labeled PA (Fig. 2A). DU145 cells were given PA-18:1–NBD or PA-16:0–NBD (50 μm because of high background levels) for 30 min at 10 °C, a temperature that inhibits endocytosis. The cells were then shifted to 37 °C for 3 min to allow endocytosis to proceed. Both PA-18:1–NBD and PA-16:0–NBD entered the cell through endocytosis (intracellular puncta at 37 °C). Phospholipid SUVs are typically 50 nm in diameter (19) and enter the cell through pinocytosis (macropinocytosis, considering the size of the SUVs). Therefore, we investigated whether PA-18:1 enters the cell through macropinocytosis. For that, we depleted DU145 cells of amino acid for 1 h, after which we added 300 μm of PA-18:1 in the absence or the presence of 75 μm 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), a macropinocytosis inhibitor (20). EIPA prevented mTOR translocation to the lysosome induced by PA-18:1 (Fig. 2B). These findings were confirmed by the quantification of LAMP2/mTOR percentage of colocalization and Pearson's correlation values (Fig. 2C). Considering that both PA-18:1 and PA-16:0 vesicles enter the cell but behave very differently regarding mTORC1 translocation and activation, we determined whether either of the PA species colocalized with mTOR at the lysosome (Fig. 2D). We observed a strong colocalization between PA-18:1–NBD and mTOR but not with PA-16:0–NBD. These findings were supported by colocalization percentages and by Pearson's correlation values (Fig. 2E). These results suggest that even though both types of vesicles can enter the cell, only vesicles made of PA-18:1 drive mTOR to the lysosome. In addition, we generated evidence that rules out involvement of the lysophosphatidic acid receptor during mTORC1 translocation and activation by PA-18:1. In the presence of a short species of PA that ends right before the kink of PA-18:1 and that is known to inhibit several lysophosphatidic acid receptors (21), we were still able to induce mTORC1 translocation and activation (Fig. S2).
Figure 2.
PA-18:1 vesicles enter the cell by macropinocytosis and rapidly reach the lysosome. A, endocytosis assay with fluorescently labeled PA species: PA-18:1–NBD and PA-16:0–NBD. DU145 cells were incubated at 10 °C for 30 min with 50 μm PA-18:1–NBD or PA-16:0–NBD and then transferred to 37 °C for 3 min. B, IF detection of LAMP2/mTOR in DU145 cells upon inhibition of macropinocytosis with 75 μm EIPA (ROI, amplification of 2 × 2-μm areas of relevance). C, LAMP2/mTOR percentage of colocalization and Pearson's correlation of cells shown in B (mean of triplicate experiments ± S.E., comparisons relative to untreated). D, confocal microscopy detection of PA-18:1–NBD, PA-16:0–NBD, and mTOR in DU145 cells (ROI, amplification of 2 × 2-μm areas of relevance). E, PA/mTOR percentage of colocalization and Pearson's correlations of cells shown in D (mean of triplicate experiments ± S.E., comparisons relative to PA-18:1–NBD).
Pharmacological and genetic inhibition of PLD prevents mTOR translocation to the lysosome
We propose that mTOR translocation and activation by PA-18:1 vesicles mimics an endogenous mechanism of translocation and activation of mTOR triggered by amino acids. To investigate this hypothesis, we used PLD inhibitors during amino acid stimulation. In Fig. 3A we show that PLD1/2 inhibitors (20 μm each) (22) or 1-butanol (BuOH) (0.8%) (11) abolished mTOR translocation to the lysosome in response to amino acids. The doses of the PLD inhibitors were chosen based on a dose curve (Fig. S3). These results were supported by colocalization percentages and Pearson's correlations (Fig. 3B). Furthermore, biochemical analysis of mTORC1 activity revealed that both PLD inhibitors and 1-butanol inhibit P-S6K (Fig. 3C) in response to amino acids. These data suggest that endogenous PLD-generated PA is required for amino acid–induced mTOR transport to the lysosome.
Figure 3.
PLD inhibition prevents amino acid–induced mTOR translocation to the lysosome. A, DU145 cells were grown as in Fig. 1A. Amino acid deprivation and restimulation was performed in the presence of PLD1/2 inhibitors (PLDi, 20 μm each) or 1-butanol (BuOH) (0.8%). Low-dose (200 nm) and high-dose (20 μm) rapamycin (RAPA) were used as controls (ROI, amplification of 2 × 2-μm areas of relevance). B, LAMP2/mTOR percentage of colocalization and Pearson's correlation of cells shown in A (mean of triplicate experiments ± S.E., comparisons relative to +AA). C, Western blotting analysis of mTORC1 activation for cells treated in A. t-buOH, tertiary BuOH, is a control for 1-BuOH; MM, molecular marker.
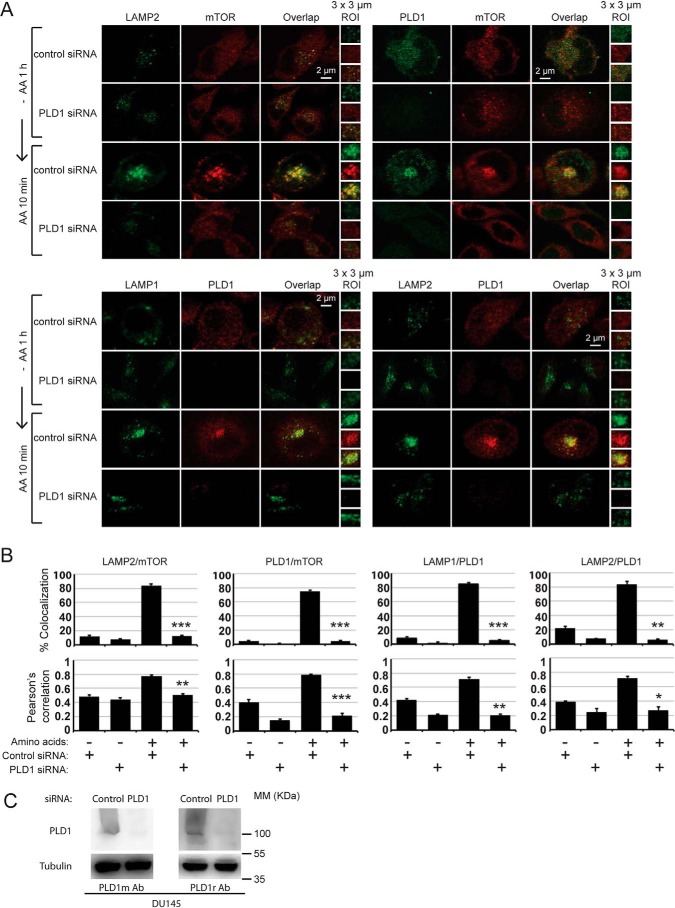
Of the several PLD genes in mammalian cells, PLD1 and PLD2 are the most widely expressed PLD proteins (6). We have found that double inhibition of PLD1/2 leads to the greatest suppression of mTORC1 (11, 13). In the context of amino acid induction of mTORC1, two other studies have shown a PLD1-specific requirement (4, 23). To assess a possible role of PLD1 in the amino acid–induced mTOR translocation to the lysosome, we performed PLD1 siRNA experiments in DU145 cells, after which we challenged the cells with amino acid deprivation/restimulation (Fig. 4A). PLD1 knockdown (96 h) prevented mTOR translocation to the lysosome upon amino acid stimulation, PLD1 and mTOR colocalization at the lysosome upon amino acid stimulation (4), and lysosomal aggregation into a large structure. We used two different antibodies specific for PLD1 and saw the same results with both (Fig. 4A). These findings were supported by percentage of colocalization and Pearson's correlation quantifications (Fig. 4B). We could barely detect PLD1 protein after 96 h of siRNA treatment (Fig. 4C). Our data demonstrate that PLD1 is required for amino acid–induced translocation of mTOR to the lysosome.
Figure 4.
Genetic ablation of PLD1 prevents amino acid–induced mTOR translocation to the lysosome. A, IF detection of LAMP2/mTOR, PLD1/mTOR, LAMP1/PLD1, and LAMP2/PLD1 in DU145 cells transfected with control or PLD1 siRNAs (ROI, amplification of 3 × 3-μm areas of relevance). B, percentage of colocalization and Pearson's correlations of cells and markers shown in A (mean of triplicate experiments ± S.E., comparisons relative to +AA + ctr siRNA). C, Western blotting analysis of PLD1 knockdown and detection used in A. rAb, rabbit antibody; MM, molecular marker.
Pharmacological and genetic inhibition of PLD prevents lysosomal retention of mTOR in cells with constitutive Rheb activation
It was previously shown that Rheb interacts with PLD1 in a GTP-dependent manner to promote PLD1 activity (5). Thus, we hypothesized that cancer cells with constitutive Rheb activation elevate PLD activity and increase production of PA, preventing mTOR from leaving the lysosome in the absence of amino acids. To test our hypothesis we used tuberous sclerosis complex 2 (TSC2)–null mouse embryonic fibroblasts (MEFs). TSC2-null MEFs have constitutive Rheb activation because they lack the TSC complex, which is a GTPase-activating protein for Rheb (24) (Rheb inhibitor). These cells represent benign human tumors (hamartomas) originated from loss of the TSC complex in the human syndrome TSC. However, to avoid senescence induced by the loss of TSC2 in culture conditions, p53 had to be deleted in these cells. In that sense, TSC2-null MEFs are closer to cancer cells than cells found in human hamartomas. We show that TSC2-null MEFs have residual retention of mTOR at the lysosome in the absence of amino acids (25) (Fig. 5A). We then tested whether this phenomenon depended on PLD and PA. We deprived cells of amino acids for 1 h and then added PLD inhibitors for 1 h. We used this time point because a 10-min amino acid treatment in TSC2-null MEFs does not elevate mTORC1 activity (Fig. 5B). PA-18:1 could also elevate P-S6K at 1 h (Fig. 5C). PLD inhibitors abolished the residual retention of mTOR at the lysosome (Fig. 5A). The addition of PA-18:1 simultaneously with PLD inhibitors completely rescued the lysosomal localization of mTOR (Fig. 5A). Unlike PLD inhibitors, high-dose rapamycin did not remove mTOR from the lysosome (Fig. 5A). These findings were supported by the quantification of percentages of colocalization and Pearson's correlations (Fig. 5D). They were also supported by Western blotting analysis of P-S6K (Fig. 5E). The data show that TSC2-null MEFs fail to remove mTOR from the lysosome in the absence of amino acids because of PLD and PA. To show that PLD activity is increased in these cells, we performed PLD activity assays (26) (Fig. 5F). TSC2-null MEFs have significantly higher PLD activity compared with WT MEFs in complete medium (CM). Upon amino acid stimulation, PLD activity in WT MEFs elevated and reached the same levels of PLD activity of TSC2-null MEFs. Importantly, PLD activity in TSC2-null MEFs did not increase with amino acids, suggesting that the main event induced by amino acids—PLD translocation to the lysosome leading to Rheb activation of PLD (4, 5)—is already in place in these cells. Next, we wanted to show that TSC2-null MEFs exploit this mechanism through elevated PLD production of PA, to survive and thrive in nutrient-poor conditions. For that, we treated WT and TSC2-null MEFs with PLD1/2 inhibitors (20 μm each) for 24 h (Fig. 5G). This treatment led to 60% of cell death in TSC2-null MEFs but only 20% in WT MEFs, confirming that TSC2-null MEFs require PLD and PA for survival.
Figure 5.
Cells with constitutive Rheb activation elevate PLD activity and fail to remove mTOR from the lysosome during amino acid starvation (residual retention). A, IF detection of LAMP1/mTOR in Tsc2−/− MEFs treated as indicated (ROI, amplification of 3 × 3-μm areas of relevance). B, Western blotting analysis of mTORC1 activation kinetics by amino acids in Tsc2−/− MEFs. MM, molecular marker. C, Western blotting analysis of mTORC1 activation by PA and amino acids in Tsc2−/− and WT MEFs. WT MEFs were used to show mTORC1 hyperactivity in Tsc2−/− MEFs. D, LAMP1/mTOR percentage of colocalization and Pearson's correlation of cells shown in A (mean of triplicate experiments ± S.E., comparisons: −AA versus −AA + PLDi; −AA + PLDi versus −AA + PLDi + PA-18:1). E, Western blotting analysis of mTORC1 activity in Tsc2−/− MEFs shown in A. F, TLC isolation followed by radioactive PA quantification (PLD activity assay) in Tsc2−/− and WT MEFs (mean of triplicate experiments ± S.E.; comparisons: WT CM versus Tsc2−/− CM; p > 0.05 for Tsc2−/− CM versus Tsc2−/− −AA + AA; WT CM versus WT −AA + AA). G, trypan blue exclusion cell viability assay of WT and Tsc2−/− MEFs grown in CM and treated with DMSO, PLD inhibitors (PLD1/2, 20 μm each), or rapamycin (RAPA, 20 μm) for 24 h (mean of triplicate experiments ± S.E.).
To assess a possible specific role for PLD1 in the retention of mTORC1 at the lysosome in TSC2-null MEFs, we performed PLD1 knockdown experiments, after which we subjected the cells to amino acid deprivation for 1 h or kept them in CM (control) (Fig. S4A). PLD1 knockdown abolished LAMP1/mTOR colocalization both in CM (large perinuclear rings) and in medium lacking amino acids (residual retention), lysosomal organization around the nucleus, and Rheb/mTOR colocalization both in CM and in medium without amino acids. We verified PLD1/mTOR colocalization in large perinuclear rings if cells were grown in CM or in smaller perinuclear globules if cells were deprived of amino acids (residual retention; Fig. S4A). We observed a similar PLD1/Rheb colocalization pattern (Fig. S4A). These results indicate that PLD1 fails to leave the lysosome upon amino acid withdrawal in cells with hyperactive Rheb, and this in turn is required for mTOR residual lysosomal retention. These results were supported by the quantification of percentages of colocalization and Pearson's correlations for each pair of markers (Fig. S4B).
Exogenously supplied PA-18:1 drives mTOR to the lysosome in the absence of Rheb or Rag GTPases
To fully validate the Rheb-PLD-PA-mTOR pathway, we investigated whether the residual retention of mTOR at the lysosome in TSC2-null MEFs depends on hyperactive Rheb. For, that we performed Rheb siRNA experiments, after which we deprived cells of amino acids for 1 h and then added PA-18:1 for 1 h (Fig. 6A). Rheb knockdown induced loss of residual retention of mTOR at the lysosome (25), which was rescued by exogenously supplied PA-18:1. Rheb knockdown, similar to PLD1 knockdown, induced lysosomal dispersal. Considering that the amino acid–induced Rag pathway of mTOR lysosomal translocation is very well-established (3), and residual lysosomal retention of mTOR happens in the absence of amino acids, we knocked down the Rag GTPases as a control. To prevent compensation between redundant Rag proteins (27), we knocked down both RagC and RagD simultaneously. We did not expect Rag knockdown to abolish mTOR residual lysosomal retention. However, that was not the case. In the absence of both RagC and D, mTOR lysosomal retention was lost, but PA-18:1 could rescue this effect and drive mTOR to the lysosome (Fig. 6A). This suggests that PA can bring mTOR to the lysosome regardless of the Rag GTPases. These results were supported by quantifications of marker colocalizations and Pearson's correlations (Fig. 6B). In addition, they were also supported by biochemical analysis of mTORC1 activity toward S6K (Fig. 6C). Next, we wanted to show that the residual retention of mTOR at the lysosome lost with Rheb knockdown was due to loss of PLD activity. In the absence of Rheb, PLD activity in TSC2-null cells deprived of amino acids suffered a 60% reduction (Fig. 6D), showing that Rheb activates PLD and production of PA. Upon Rag C + D knockdown, we observed a 20% reduction in PLD activity (Fig. 6D), suggesting that PLD and the Rags are not directly linked. To further dissect the role of PLD and PA relative to the Rags, we used RagAGTP/GTP MEFs, which have constitutive mTOR activity refractory to amino acid depletion. The levels of P-S6K, however, are lower than the ones seen in RagA+/+ MEFs upon amino acid induction (28). We confirmed these findings in our experiments, where we challenged RagA+/+ and RagAGTP/GTP MEFs to inhibition of PLD1, PLD2, or both (Fig. 6E). In RagA+/+ cells, we observed increased P-S6K levels upon amino acid stimulation. This was abrogated by PLD1 inhibition or inhibition of both PLD1/2, confirming that PLD1 does travel to the lysosome upon amino acid stimulation and promotes mTORC1 translocation to the lysosome in an amino acid–dependent pathway. RagAGTP/GTP behaved differently. PLD1 or PLD2 inhibition reduced the levels of P-S6K. However, only simultaneous PLD1/2 inhibition completely abrogated P-S6K, suggesting that mTORC1 is not accessible to Rheb activation in the complete absence of PLD activity, even if the Rags are constitutively activated.
Figure 6.
Exogenously supplied PA-18:1 drives mTOR to the lysosome in TSC2-null MEFs lacking Rheb or the Rag GTPases. A, IF detection of LAMP1/mTOR in Tsc2−/− MEFs transfected with control, Rheb, or RagC+D siRNAs (ROI, amplification of 4 × 4-μm areas of relevance). B, percentage of colocalization and Pearson's correlation of cells shown in A (mean of triplicate experiments ± S.E., comparisons relative to −AA ctr siRNA). C, Western blotting analysis of mTORC1 activity of cells shown in A. MM, molecular marker. D, PLD activity of cells shown in A (mean of triplicate experiments ± S.E.; comparison relative to ctr siRNA). E Western blotting analysis of mTORC1 activity in RagA+/+ and RagAGTP/GTP MEFs treated with PLD1 (20 μm), PLD2 (20 μm), or both inhibitors (20 μm each).
Discussion
Our data support a model (Fig. 7) where PLD1, similar to mTORC1, acts like a coincidence detector and effector of both amino acids and growth factors. Amino acids induce PLD activity and production of PA. Exogenously supplied PA vesicles enter the cell through endocytosis and drive mTOR to the lysosome, suggesting that amino acids induce production of PA-containing endosomes that carry mTOR to the lysosome. In agreement, inhibition of endogenous PLD prevented mTOR translocation to the lysosome in response to amino acids. Amino acid–induced RagA/B–GTP RagC/D–GDP heterodimers provide a parallel pathway that locks mTOR on the lysosome. Amino acids also induce the translocation of PLD1 from cytoplasmic puncta to the lysosome. Once on the lysosome, PLD1 binds to Rheb. Growth factors activate Rheb, which then activates PLD1. Lysosomal PA production promotes further binding of PA to mTOR to allow complex stability and activation.
Figure 7.

Model of amino acid and growth factor activation of mTORC1 mediated by PLD1-PA. Amino acids induce elevation of cytoplasmic PLD activity and PA production. PA-containing endosomes promote the transport of mTORC1 to the lysosome (Figs. 1 and 2). It is not clear how exogenously supplied PA recruits mTORC1 to the lysosome as indicated by the question marks, but the PLD requirement for amino acid-induced mTORC1 translocation (Fig. 3) suggests a role for PA in both the trafficking and activation of mTORC1. On the lysosome, mTORC1 binds to amino acid–activated Rags, which lock mTORC1 at the subcellular compartment. Amino acids also induce translocation of PLD 1 from the cytoplasm to the lysosome where it colocalizes with mTOR and Rheb (Fig. 4). Upon growth factor stimulation, Rheb is activated, binds to, and elevates PLD activity. Increased PA production at the lysosome prevents mTOR from leaving the compartment and allows complete activation of mTORC1 (Fig. 5 and Fig. 6). Precancerous cells caused by loss of the TSC complex exploit permanent activation of the pathway Rheb–PLD–PA–mTORC1 at the lysosome to thrive in nutrient-poor conditions (Fig. 5 and Fig. 6). Full activation of mTORC1 in response to amino acids and growth factors happens when both Rags and PA operate simultaneously (Fig. 6).
Previous studies showed that exogenously supplied PA induced mTORC1 activation in the presence but not in the absence of amino acids (4). Here, we were able to induce mTORC1 translocation to the lysosome and mTORC1 activity with exogenously supplied PA-18:1 vesicles in the absence of amino acids. The main difference between the two studies is that we performed amino acid and serum deprivation (to prevent contamination of amino acids present in serum) for 1 h, followed by amino acid stimulation for 10 min. In contrast, Yoon et al. (4) performed amino acid starvation for 2 h after overnight serum starvation, followed by amino acid stimulation for 30 min.
Previous findings suggest that mTORC1 assembles before reaching the lysosome because binding of mTOR to the Rag GTPases requires the mTORC1 component raptor (29). PA promotes mTORC1 assembly and stability (11). PA-containing endosomes carrying mTORC1 to the lysosome is therefore an attractive model in which PA would allow mTORC1 formation and stability. We show that exogenously supplied PA can drive mTOR to the lysosome and induce mTORC1 activity in TSC2-null MEFs where RagC and D were genetically ablated. Therefore, we propose that delivery of mTORC1 to the lysosome does not require the Rags. However, we did find that residual retention of mTOR at the lysosome in TSC2-null MEFs was lost upon RagC and D knockdown. This suggests that the Rags operate in parallel to PA to lock mTORC1 on the lysosome, after mTORC1 delivery by PA. We show that genetic ablation of PLD1 induced lysosomal scattering. This favors the idea that PA-containing endosomes carry mTORC1 to the lysosome, fuse with the lysosome, and increase its size.
Exogenously supplied PA vesicles induce mTORC1 translocation and activation in the absence of Rheb, indicating that the key step in Rheb activation of mTORC1 is increased PLD1 activity and PA production. Consistent with this observation, the Rheb association with mTOR is independent of GTP loading (30), whereas the Rheb association with PLD1 depends on GTP loading (5). Additionally, we found that PLD1/2 inhibitors, in combination, abolished mTORC1 activity in RagAGTP/GTP MEFs, indicating that PA production is required for complex assembly and stability. If mTORC1 is not intact, then constitutive Rag activation is lost. Thus, the effect of PA on mTORC1 is downstream of Rheb and parallel to Rag GTPases.
Genetic deletion of mTOR in mice is embryonic lethal (31). Unlike mTOR, mice with genetic deletion of PLD1 (32), PLD2 (33), or both (34) are viable, suggesting that PLD and PA may not be required for steady state but rather acute activation of mTOR. PLD inhibitors preferentially killed TSC-null MEFs, whereas rapamycin did not. This suggests an advantage in terms of cancer therapeutics because targeting PLD may selectively target cancer cells, with minimal side effects. PLD inhibitors were developed from halopemide (22), a psychotropic drug extensively used in humans without toxicities. Thus, PLD inhibition might be a viable alternative to current therapies in cancers with mTORC1 hyperactivation that requires PLD-generated PA.
Experimental procedures
Cell lines
We used the DU145 prostate cancer cell line (ATCC HTB-81), Tsc2−/− p53−/− and Tsc2+/+ p53−/− (control) mouse embryonic fibroblasts from David Kwiatkowski (35, 36) and RagAGTP/GTP and RagA+/+ (control) mouse embryonic fibroblasts provided by Alejo Efeyan and David Sabatini (28). All cells were grown at 37 °C with 5% CO2, in Dulbecco's modified Eagle's medium (DMEM): high glucose, with l-glutamine (Sigma D6429–1L) supplemented with 10% fetal bovine serum (FBS) (SAFC 12306C-500 ml), and 1× penicillin and streptomycin (pen/strep) (Gemini Bio-Products, 400-101).
Amino acid depletion and replenishment experiments
The cells were seeded 24 h prior to experiments in 6-cm dishes for cell lysis or 3-cm dishes containing fibronectin-coated microscope coverslips (BioCoat 354088) for immunofluorescence. We used 1 × 106 DU145 cells, 0.5 × 106 Tsc2−/− p53−/− cells, 0.7 × 106 Tsc2+/+ p53−/− cells, 1 × 106 RagAGTP/GTP cells, and 1 × 106 RagA+/+ cells, per 6-cm dish in 3 ml of medium. We seeded 0.5 × 106 DU145 cells and 0.25 × 106 Tsc2−/− p53−/− cells per 3-cm dish in 2 ml of medium. 24 h later we discarded the growth medium, washed the cells once in RPMI 1640 without amino acids (US Biologicals R8999-04A), and then added RPMI 1640 without amino acids for 1 h (3 ml per 6-cm dish or 2 ml per 3-cm dish). 1 h later, the cells were stimulated with amino acids for 10 min (DU145 cells) or for 1 h (Tsc2−/− p53−/− cells) by adding a liquid solution of RPMI 50× amino acids (Sigma, R7131) to a final concentration of 1× (20 μl of 50× amino acid solution per ml). RPMI 1640 without amino acids was bought as a powder and made fresh before experiments. After adjusting the pH to 7.4, the medium was filter-sterilized (0.22 μm). Serum was absent in all amino acid depletion and replenishment experiments to prevent serum-containing amino acid contamination. These experimental conditions are identical to the ones described by Sancak et al. (29), with the exception that we used a 50× RPMI amino acid solution instead of a 10× amino acid mixture.
Preparation of PA SUVs
We bought all our phospholipids from Avanti Polar Lipids: PA-18:1, 1-palmitoyl-2-oleoyl-sn-glycero-3P, catalog no. 840857, 10 mg/ml, chloroform, MW 696.910; PA-16:0, 1,2-dipalmitoyl-sn-glycero-3P, catalog no. 830855, 25 mg, powder, MW 670.873; diC8-PA, 1,2-dioctanoyl-sn-glycero-3P, catalog no. 830842, 10 mg/ml, chloroform, MW 446.448; PA-18:1–NBD, 1-oleoyl-2-{12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3P, catalog no. 810176, 1 mg/ml, chloroform, MW 813.465; and PA-16:0–NBD, 1-palmitoyl-2-{12-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]dodecanoyl}-sn-glycero-3P, catalog no. 810174, 1 mg/ml, chloroform, MW 787.921. Lipids were stored at −20 °C, unopened until use. After opening the vial, lipids in chloroform were transferred to a sterile glass vial with a lid (scintillation vial). Before closing the lid, vial atmosphere was replaced with N2 (g) to prevent lipid oxidation. We sealed vials with parafilm and immediately stored them at −20 °C. We prepared 6.9 mm PA small unilamellar vesicles in DPBS without Ca2+ and Mg2+ (Gibco 14190–144). For that, we placed 63 μl of PA-18:1, 0.6 mg of PA-16:0 and 40 μl of diC8-PA into Eppendorf tubes and dried PA-18:1 and diC8-PA under a N2 gas thin outlet to remove chloroform. Then we added 130 μl of DPBS and vortexed the tube until a cloudy suspension was formed. To make small unilamellar vesicles, we sonicated the lipid suspensions with an ultrasonicator (QSonica Q700) with the following settings: 3 × 30 s at amplitude 60, with 30-s intervals between pulses. After sonication, lipid suspensions were kept at room temperature and used immediately. We added 130 μl of SUVs per 3 ml of growth medium to reach a final concentration of 300 μm. For PA-18:1–NBD and PA-16:0—NBD, we used a final concentration of 50 μm in 100 μl of growth medium in a coverslip previously labeled with a hydrophobic pen (Dako, S2002). For that we dried 4 μl of each lipid, after which we added 25 μl of DPBS and sonicated the suspensions. We added 25 μl of 0.2 mm SUVs directly to a coverslip with 75 μl of medium.
Immunofluorescence
The cells were grown in 3-cm dishes containing fibronectin-coated glass microscope coverslips (BD Biocoat 354088) where we previously placed a hydrophobic barrier with a Dako pen (Dako S2002). For that, we made a circle around the coverslip and allowed it to dry for 5 min. After the experiments were complete, the cells were rinsed in PBS and fixed in 4% paraformaldehyde (Alfa Aesar, 43368) for 15 min. Immunofluorescence labeling took place at room temperature. After fixation, the cells were washed in PBS and permeabilized for 5 min in PBS with 0.05% Triton X-100, (Fisher Scientific, BP151). The cells were then washed three times in PBS or until reconstitution of the hydrophobic barrier. After that cells were incubated for 1 h in PBS with 5% BSA with primary antibodies. The cells were rinsed four times in PBS before the addition of secondary antibodies in a PBS with 5% BSA solution. Incubation with secondary antibodies took place in the dark for 30 min. Then the cells were washed four times in PBS, and coverslips were mounted onto microscope slides with a drop of Prolong gold antifade mounting medium with DAPI (Molecular Probes P36931).
Confocal microscopy
Image acquisition was done with a PerkinElmer Ultra View Spinning disk confocal microscope coupled to a Nikon camera and equipped with Volocity 3D image capture and analysis software. We used a 60× oil immersion objective to acquire 0.5-μm interval stacks (z-stacks). After manually defining the center of the stack (center of the cell), we set the range −5 to +5 μm for DU145 cells and −3 to +3 μm for Tsc2−/− p53−/− MEFs because these cells are very flat. For each stack, we acquired the green channel followed by simultaneous acquisition of the red and blue channels. We captured images with 1344 × 1024 pixels and 155.78 × 118.69 microns. Five random samples of each experimental condition were acquired. We used the same exposure time and magnification for all our samples. For each sample, a cell density of 35–65 cells per field was achieved, unless the cells were treated with drugs known to lift cells off the plates or kill cells. The images were analyzed with ImageJ. File format conversion was done with the LOCI bioformats importer plugin. Colocalization analysis was performed in two different ways. First, we visually scored the percentage of cells with marker colocalization relative to the total number of cells. In all our images, marker colocalization is shown in yellow and orange. Then we computed colocalization correlation coefficients (Pearson's correlation) in ImageJ with the plugin JACoP. Pearson's correlations were based on pixel intensity after applying Costes's thresholds. We used threshold intensities to exclude the background signal in siRNA-treated samples. In all our figures, we show representative cells and the respective cell population percentage of colocalization and Pearson's correlations. In Fig. 7, we show whole images with the purpose of allowing comparability with (25).
Endocytosis assay
To study endocytosis (18) of exogenously supplied PA vesicles, the cells were seeded in DMEM with 10% FBS 1× pen/strep onto a microscope coverslip in a 3-cm dish. 24 h later we changed the growth medium to 2 ml of DMEM and added SUVs of fluorescently labeled PA analogs (PA-18:1–NBD and PA-16:0–NBD) to a final concentration of 50 μm. The cells were then incubated at 10 °C for 30 min to label the plasma membrane, after which the medium was replaced with 2 ml DMEM. The cells were then shifted to 37 °C for 3 min to allow endocytosis to proceed. After that, we washed cells with PBS and then removed excess fluorescent PA remaining at the outer leaflet of the plasma membrane by BSA back exchange, in which cells were washed five times with 2 ml of a 5% fatty acid–free BSA (Sigma, A9205) solution made with PBS at 10 °C for 10 min.
PLD activity assay
Our PLD activity assays were slightly modified from (37) and (26). DU145 and Tsc2−/− p53−/− cells were seeded in 6-well plates as described above for 3-cm dishes. 24 h later cell growth medium was replaced with 2 ml of fresh medium per well, and cellular phosphatidylcholine was labeled at 37 °C for 4 h with 3.0 μCi (1.5 μl/ml) of [3H]myristic acid (PerkinElmer, NET830005MC). 1 h before labeling completion (3 h), we shifted the cells to RPMI without amino acids with 3.0 μCi (1.5 μl/ml) of [3H]myristic acid. 20 min later (3 h, 40 min labeling), we added 1-butanol (Sigma–Aldrich, B7906) to a final concentration of 0.8%. 20 min later (total 4 h labeling), we placed the plates on ice, discarded the radioactive media appropriately, and washed cells twice with 2 ml of ice-cold PBS. To collect cells we placed 1 ml of medium from plate (with cells) in an Eppendorf tube and spun 5 min at 3000 rpm at 4 °C. We discarded the supernatant, and then we added the remaining 1 ml left in the plate after scraping the plate. We spun the tube again for 5 min at 3000 rpm at 4 °C. After discarding the supernatant, we washed the pellet in ice-cold PBS twice and centrifuged for 5 min at 3000 rpm at 4 °C after each wash. Finally, we removed the supernatant and resuspended the pellet in 500 μl of acidified methanol (methanol with 6 n HCl, 50:2). We then transferred the cells into polypropylene tubes kept on ice with the first extraction buffer: 155 μl of 1 m NaCl and 500 μl of chloroform. We vortexed the tubes and then centrifuged them for 3 min at 13300 rpm at 4 °C, after which we discarded the top layer. We placed the bottom layer (avoiding middle layer) into polypropylene tubes kept on ice with the second extraction buffer: 350 μl of distilled H2O, 115 μl of methanol, and 115 μl of 1 m NaCl. The tubes were then centrifuged for 3 min at 13,300 rpm at 4 °C. These tubes were stored at −80 °C for no longer than 1 week. Upon thawing, the tubes were spun again to reseparate the layers. Equal amounts of volume from the bottom layer were added to a new tube along with 10 μl of phosphatidylbutanol standard solution (Avanti Polar Lipids, 860203C, 10 mg/ml). We dried the samples with nitrogen for 10 min. All of the dried samples were resuspended in 30 ml of spotting solution (9:1 chloroform:methanol) and applied to a TLC plate (Millipore Sigma, 1.11798.0001). The plates were run in a chamber containing 100 ml of the upper mobile-phase solution of ethyl acetate:isooctane:glacial acetic acid:water, 88:40:16:80, for 2 h 30 min. The plates were air-dried for 10 min and then placed in an iodine chamber for 5 min to detect the presence of lipids. We marked the spots with a pencil and let the plate sit for an hour to allow iodine to leave. At this stage, the plates can be left at room temperature overnight before scraping. Lipid bands corresponding to phosphatidylbutanol were scraped and placed in 3 ml of scintillation fluid (PerkinElmer, 6013681) and quantified (cpm) in a scintillation counter. All our experiments were performed in triplicate and normalized to protein levels determined with extra plates subjected to identical conditions until cell lysis.
Cell viability assay
We seeded three 6-cm dishes with 0.5 × 106 Tsc2−/− p53−/− cells, and three 6-cm dishes with 0.7 × 106 Tsc2+/+ p53−/− cells. 24 h later we started drug treatments and allowed the drugs to act for 24 h, after which we collected medium into a 15-ml Falcon tube (to include dead cells). We collected cells with 0.5 ml of 1× trypsin (Sigma T4174) into the respective 15 ml Falcon tube and added DMEM 10% FBS 1× pen/strep to each tube to a total of 14 ml. We centrifuged cells at 1500 rpm for 5 min at 4 °C. We discarded supernatants and resuspended cellular pellets in 2 ml of DMEM 10% FBS 1× pen/strep. We prepared Eppendorf tubes for each condition with 60 μl of DMEM 10% FBS 1× pen/strep, 20 μl of cellular suspension (1/4 dilution factor), and 8 μl of a 0.4% trypan blue solution (Sigma T8154). We allowed trypan blue incorporation for 5 min. Then we loaded 10 μl into a cell-counting hemocytometer chamber and counted nonviable cells (blue) and viable cells (not blue) per condition. We performed three cellular counts per condition.
Quantification and statistical analysis
Statistical comparisons of two different treatments in the same cell type were performed with Student's t test (nonpaired, two tails, equal variance) with R programming in R studio or Excel. All figures describe significant differences found in t test comparisons performed with means of triplicate experiments per condition: ***, p < 0.001; **, p < 0.01; and *, p < 0.05.
Supporting “Experimental procedures”
Reagents, antibodies, CMC determination, siRNAs, transient transfection of siRNAs, cell lysis, and Western blotting can be found in the supporting text.
Author contributions
M. A. F., S. M., D. M., and D. A. F. conceptualization; M. A. F. formal analysis; M. A. F. and D. A. F. supervision; M. A. F., S. M., A. W., and M. U. investigation; M. A. F. and D. A. F. visualization; M. A. F., S. M., E.L., and D. A. F. methodology; M. A. F. writing-original draft; D. A. F. resources; D. A. F. funding acquisition; D. A. F. writing-review and editing; M. A. F. co-corresponding author.
Supplementary Material
Acknowledgments
We thank the Foster Lab for insightful discussions and support for this work. The Tsc2−/− p53−/− and Tsc2+/+ p53−/− MEFs were from the Kwiatkowski laboratory. We thank Alejo Efeyan and David Sabatini for the RagAGTP/GTP and RagA+/+ MEFs.
This study was supported by National Institutes of Health Grants R01-CA046677 and R01-CA179542 (to D. A. F.), a pilot project award from the Research Centers in Minority Institutions, and Award RP-03037 from the National Center for Research Resources of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains text and Figs. S1–S4.
- mTORC1
- mTOR complex 1
- mTOR
- mammalian target of rapamycin
- AA
- amino acid
- BuOH
- 1-butanol
- CM
- complete medium
- CMC
- critical micelle concentration
- ctr
- control
- DMEM
- Dulbecco's modified Eagle's medium
- DPBS
- Dulbecco's phosphate buffered saline
- EIPA
- 5-(N-ethyl-N-isopropyl)-amiloride
- FBS
- fetal bovine serum
- IF
- immunofluorescence
- NBD
- nitro-benzoxadiazol
- S6K
- ribosomal subunit S6 kinase
- SUV
- small unilamellar vesicle
- PA
- phosphatidic acid
- PA-18:1
- 1-palmitoyl-2-oleoyl-PA
- PA-16:0
- dipalmitoyl-PA
- PLD
- phospholipase D
- PLDi
- PLD inhibitor
- ROI
- region of interest
- TSC
- tuberous sclerosis complex
- MEF
- mouse embryonic fibroblast
- pen/strep
- penicillin and streptomycin
- MW
- molecular weight.
References
- 1. González A., and Hall M. N. (2017) Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 36, 397–408 10.15252/embj.201696010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dibble C. C., and Manning B. D. (2013) Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat. Cell Biol. 15, 555–564 10.1038/ncb2763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Efeyan A., Zoncu R., and Sabatini D. M. (2012) Amino acids and mTORC1: from lysosomes to disease. Trends Mol. Med. 18, 524–533 10.1016/j.molmed.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoon M. S., Du G., Backer J. M., Frohman M. A., and Chen J. (2011) Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J. Cell Biol. 195, 435–447 10.1083/jcb.201107033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun Y., Fang Y., Yoon M. S., Zhang C., Roccio M., Zwartkruis F. J., Armstrong M., Brown H. A., and Chen J. (2008) Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc. Natl. Acad. Sci. U.S.A. 105, 8286–8291 10.1073/pnas.0712268105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frohman M. A. (2015) The phospholipase D superfamily as therapeutic targets. Trends. Pharmacol. Sci. 36, 137–144 10.1016/j.tips.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang Y., Vilella-Bach M., Bachmann R., Flanigan A., and Chen J. (2001) Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294, 1942–1945 10.1126/science.1066015 [DOI] [PubMed] [Google Scholar]
- 8. Veverka V., Crabbe T., Bird I., Lennie G., Muskett F. W., Taylor R. J., and Carr M. D. (2008) Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene 27, 585–595 10.1038/sj.onc.1210693 [DOI] [PubMed] [Google Scholar]
- 9. Foster D. A. (2013) Phosphatidic acid and lipid-sensing by mTOR. Trends Endocrinol. Metab. 24, 272–278 10.1016/j.tem.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shin J. J., and Loewen C. J. (2011) Putting the pH into phosphatidic acid signaling. BMC Biol. 9, 85 10.1186/1741-7007-9-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toschi A., Lee E., Xu L., Garcia A., Gadir N., and Foster D. A. (2009) Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol. Cell. Biol. 29, 1411–1420 10.1128/MCB.00782-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foster D. A., Salloum D., Menon D., and Frias M. A. (2014) Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J. Biol. Chem. 289, 22583–22588 10.1074/jbc.R114.566091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu L., Salloum D., Medlin P. S., Saqcena M., Yellen P., Perrella B., and Foster D. A. (2011) Phospholipase D mediates nutrient input to mammalian target of rapamycin complex 1 (mTORC1). J. Biol. Chem. 286, 25477–25486 10.1074/jbc.M111.249631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roth M. G. (2008) Molecular mechanisms of PLD function in membrane traffic. Traffic 9, 1233–1239 10.1111/j.1600-0854.2008.00742.x [DOI] [PubMed] [Google Scholar]
- 15. Menon D., Salloum D., Bernfeld E., Gorodetsky E., Akselrod A., Frias M. A., Sudderth J., Chen P. H., DeBerardinis R., and Foster D. A. (2017) Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J. Biol. Chem. 292, 6303–6311 10.1074/jbc.M116.772988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Triebl A., Trötzmüller M., Eberl A., Hanel P., Hartler J., and Köfeler H. C. (2014) Quantitation of phosphatidic acid and lysophosphatidic acid molecular species using hydrophilic interaction liquid chromatography coupled to electrospray ionization high resolution mass spectrometry. J. Chromatogr. A 1347, 104–110 10.1016/j.chroma.2014.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Vendittis E., Palumbo G., Parlato G., and Bocchini V. (1981) A fluorimetric method for the estimation of the critical micelle concentration of surfactants. Anal. Biochem. 115, 278–286 10.1016/0003-2697(81)90006-3 [DOI] [PubMed] [Google Scholar]
- 18. Lipids. (2008) Current Protocols in Cell Biology, Chapter 24, pp. 24.20.21–24.20.22, John Wiley and Sons, Inc., Wiley Interscience [Google Scholar]
- 19. Szoka F. Jr., and Papahadjopoulos D. (1980) Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu. Rev. Biophys. Bioeng. 9, 467–508 10.1146/annurev.bb.09.060180.002343 [DOI] [PubMed] [Google Scholar]
- 20. Commisso C., Davidson S. M., Soydaner-Azeloglu R. G., Parker S. J., Kamphorst J. J., Hackett S., Grabocka E., Nofal M., Drebin J. A., Thompson C. B., Rabinowitz J. D., Metallo C. M., Vander Heiden M. G., and Bar-Sagi D. (2013) Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 10.1038/nature12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischer D. J., Nusser N., Virag T., Yokoyama K., Wang D., Baker D. L., Bautista D., Parrill A. L., and Tigyi G. (2001) Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol. Pharmacol. 60, 776–784 [PubMed] [Google Scholar]
- 22. Scott S. A., Selvy P. E., Buck J. R., Cho H. P., Criswell T. L., Thomas A. L., Armstrong M. D., Arteaga C. L., Lindsley C. W., and Brown H. A. (2009) Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat. Chem. Biol. 5, 108–117 10.1038/nchembio.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoon M. S., Son K., Arauz E., Han J. M., Kim S., and Chen J. (2016) Leucyl-tRNA synthetase activates Vps34 in amino acid–sensing mTORC1 signaling. Cell Rep. 16, 1510–1517 10.1016/j.celrep.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Y., Gao X., Saucedo L. J., Ru B., Edgar B. A., and Pan D. (2003) Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat. Cell Biol. 5, 578–581 10.1038/ncb999 [DOI] [PubMed] [Google Scholar]
- 25. Demetriades C., Doumpas N., and Teleman A. A. (2014) Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 156, 786–799 10.1016/j.cell.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernfeld E., Menon D., Vaghela V., Zerin I., Faruque P., Frias M. A., and Foster D. A. (2018) Phospholipase D–dependent mTOR complex 1 (mTORC1) activation by glutamine. J. Biol. Chem. 293, 16390–16401 10.1074/jbc.RA118.004972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Efeyan A., Schweitzer L. D., Bilate A. M., Chang S., Kirak O., Lamming D. W., and Sabatini D. M. (2014) RagA, but not RagB, is essential for embryonic development and adult mice. Dev. Cell 29, 321–329 10.1016/j.devcel.2014.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Efeyan A., Zoncu R., Chang S., Gumper I., Snitkin H., Wolfson R. L., Kirak O., Sabatini D. D., and Sabatini D. M. (2013) Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493, 679–683 10.1038/nature11745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., and Sabatini D. M. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Long X., Lin Y., Ortiz-Vega S., Yonezawa K., and Avruch J. (2005) Rheb binds and regulates the mTOR kinase. Curr. Biol. 15, 702–713 10.1016/j.cub.2005.02.053 [DOI] [PubMed] [Google Scholar]
- 31. Murakami M., Ichisaka T., Maeda M., Oshiro N., Hara K., Edenhofer F., Kiyama H., Yonezawa K., and Yamanaka S. (2004) mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 24, 6710–6718 10.1128/MCB.24.15.6710-6718.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elvers M., Stegner D., Hagedorn I., Kleinschnitz C., Braun A., Kuijpers M. E., Boesl M., Chen Q., Heemskerk J. W., Stoll G., Frohman M. A., and Nieswandt B. (2010) Impaired αIIbβ3 integrin activation and shear-dependent thrombus formation in mice lacking phospholipase D1. Science signaling 3, ra1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Norton L. J., Zhang Q., Saqib K. M., Schrewe H., Macura K., Anderson K. E., Lindsley C. W., Brown H. A., Rudge S. A., and Wakelam M. J. (2011) PLD1 rather than PLD2 regulates phorbol-ester-, adhesion-dependent and Fcγ-receptor–stimulated ROS production in neutrophils. J. Cell Sci. 124, 1973–1983 10.1242/jcs.082008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thielmann I., Stegner D., Kraft P., Hagedorn I., Krohne G., Kleinschnitz C., Stoll G., and Nieswandt B. (2012) Redundant functions of phospholipases D1 and D2 in platelet α-granule release. J. Thromb. Haemost. 10, 2361–2372 10.1111/j.1538-7836.2012.04924.x [DOI] [PubMed] [Google Scholar]
- 35. Kwiatkowski D. J., Zhang H., Bandura J. L., Heiberger K. M., Glogauer M., el-Hashemite N., and Onda H. (2002) A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 11, 525–534 10.1093/hmg/11.5.525 [DOI] [PubMed] [Google Scholar]
- 36. Zhang H., Cicchetti G., Onda H., Koon H. B., Asrican K., Bajraszewski N., Vazquez F., Carpenter C. L., and Kwiatkowski D. J. (2003) Loss of Tsc1/Tsc2 activates mTOR and disrupts PI3K-Akt signaling through downregulation of PDGFR. J. Clin. Invest. 112, 1223–1233 10.1172/JCI200317222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Utter M., Chakraborty S., Goren L., Feuser L., Zhu Y. S., and Foster D. A. (2018) Elevated phospholipase D activity in androgen-insensitive prostate cancer cells promotes both survival and metastatic phenotypes. Cancer Lett. 423, 28–35 10.1016/j.canlet.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.