Abstract
Citrus species contain significant amounts of flavonoids that possess antioxidant activities; furthermore, dietary citrus is not associated with adverse effects or cytotoxicity in healthy individuals. Hesperidin, which is an abundant flavanone glycoside in the peel of citrus fruits, possesses a variety of biological capabilities that include antioxidant and anti-inflammatory actions. Over the last few decades, many studies have been investigated the biological actions of hesperidin and its aglycone, hesperetin, as well as their underlying mechanisms. Due to the antioxidant effects of hesperidin and its derivatives, the cardioprotective and anti-cancer effects of these compounds have been widely reviewed. Although the biological activities of hesperidin in neurodegenerative diseases have been evaluated, its potential involvement in a variety of central nervous system (CNS) disorders, including autoimmune demyelinating disease, requires further investigation in terms of the underlying mechanisms. Thus, the present review will focus on the potential role of hesperidin in diverse models of CNS neuroinflammation, including experimental autoimmune encephalomyelitis, with special consideration given to its antioxidant and anti-inflammatory effects in neurodegenerative disease models. Additionally, current evidence provides information regarding the nutraceutical use of hesperidin to prevent various CNS disorders.
Keywords: Hesperidin, Neuroinflammation, Antioxidant, Demyelination, Hippocampus
Introduction
Hesperidin (4′-methoxy-7-O-rutinosyl-3′,5-dihydroxyflavanone; hesperetin 7-O-rutinoside) is a naturally occurring flavanone glycoside found in citrus [1,2]. Hesperidin is hydrolyzed by the intestinal microflora when ingested orally and then absorbed in the large intestine, and hesperetin (4′-methoxy-5,7,3′-trihydroxyflavanone), aglycone form of hesperidin, is mainly absorbed in the small intestine [3]. Hesperidin exerts immunoregulatory properties via the modification of lymphocyte composition in the intestinal mucosa [4] and in gut-associated lymphoid tissues [5]. The chemical structures of both hesperidin and hesperetin (Fig. 1) have been well-described in previous studies [3,6].
Fig. 1. Chemical structures of hesperetin and hesperidin.
Due to its antioxidant and anti-inflammatory properties, hesperidin possesses a variety of biological effects in models of cardiovascular disease [7,8] and diabetes [2,9] as well for the prevention of cancer [8]. Additionally, the antioxidant and anti-inflammatory effects of hesperidin ameliorate symptoms in animal models of the neurodegenerative diseases of Alzheimer's disease [10], Parkinson's disease [11,12], Huntington's disease [13,14], depression [15,16], neuroimmunological multiple sclerosis (MS) [17,18], brain ischemia-reperfusion injury [19], and traumatic injury in central nervous system (CNS) tissues [20]. Although the etiologies of the abovementioned neuroinflammatory diseases are varied and distinct, oxidative stress and inflammatory responses are common and consistent findings in CNS tissues affected by these disorders. However, these changes are occasionally reversed by antioxidant flavonoids.
The beneficial effects of citrus flavonoids, including hesperidin, have been reviewed in the context of their neuropharmacological properties [3] and neuroprotective capabilities [21,22,23] with a special focus on anti-depressive actions and protection against learning and memory deficits. Moreover, updated data on the effects of hesperidin in animal models of neurological disorders remain to be further analyzed. The present review will provide updates on recent publications assessing the effects of hesperidin in models of oxidative stress-related neuroinflammation including lipopolysaccharide (LPS)-induced endotoxemia, brain ischemia-reperfusion injury, and chemical-induced hippocampal dysfunction. Because the etiologies of autoimmune CNS diseases are distinct from those of other neurodegenerative diseases, this review will discuss the potential role that hesperidin plays in autoimmune diseases.
For this review, the following terms were searched for in the PubMed database: “hesperidin and antioxidant and inflammation” (79 articles); “hesperidin and central nervous system” (66 articles); “hesperidin and autoimmune” (9 articles); and “hesperidin and demyelination” (5 articles). For the data extraction, all authors of the present review determined the eligibility of the articles.
Molecular Mechanisms of Hesperidin (Antioxidation and Anti-inflammation)
The antioxidant activities of hesperidin have been previously reported [1,7]. Briefly, the beneficial effects of hesperidin are largely dependent on its radical scavenging activities and its augmentation of cellular antioxidant mechanisms [7]. In terms of scavenging radicals, hesperidin restores deficits in the activity of antioxidant enzymes, including glutathione peroxidase, glutathione reductase, catalase, and superoxide dismutase, that are downregulated in the brain in experimental models of stroke [24], irradiation [25], and LPS-induced endotoxicity [26].
Similar to its antioxidant effects, hesperidin also activates cellular protection machinery, including nuclear factor-erythroid 2-related factor-2 and heme oxygenase-1, which are centrally involved in cell survival against oxidative stress [27,28]. Regarding the signal cascades associated with cell protection, hesperidin stimulates peroxisome proliferator-activated receptor-gamma, which is centrally involved in the mediation of anti-inflammatory and antioxidant effects in models of stress-induced gastric ulcers [29], ischemic heart disease [30], and cyclophosphamide-induced hepatotoxicity [31].
The anti-inflammatory activities of hesperidin are indispensable to its antioxidant activities [7]. For example, hesperidin reduces the production of pro-inflammatory mediators, such as tumor necrosis factor α (TNF-α), in a model of aluminum chloride-induced neuroinflammation in the hippocampus [32] and in N-methyl-D-aspartate-induced excitotoxicity in the retina [33]. Furthermore, intraperitoneal injections of hesperidin to normal animals decrease the level of phosphorylated extracellular signal-regulated kinase-1 and -2, which are important signals in cell activation in the cerebral cortex, cerebellum, and hippocampus [34]. Thus, it can be postulated that, similar to other antioxidant flavonoids, hesperidin activates radical scavenging activity and subsequently attenuates the inflammatory response to be ultimately protective against cell death.
Bioactivity of Hesperidin in the Nervous System
Effects of hesperidin on neuroinflammation
In animal models, LPS triggers neuroinflammation in conjunction with the development of cognitive deficits and septic shock [26]. Additionally, LPS-induced neuroinflammation in mice is associated with upregulated levels of the mediators of oxidative stress and inflammation, including TNF-α, whereas hippocampal acetylcholinergic activity decreases due to the activation of acetylcholinesterase [26]. Hesperidin reverses the phenomena induced by LPS treatment by increasing the generation of radical scavenging enzymes, including superoxide dismutase and catalase. In LPS-treated animals, hesperidin also reduces the level of pro-inflammatory cytokines, including interleukin (IL)-1β, IL-6, and TNF-α, in the brain by promoting activation of the miRNA-132 pathway [35]. Even though the cellular sources of pro-inflammatory cytokines in LPS-induced neuroinflammation models have yet to be validated, it has been postulated that neuroglial cells, including microglia and astrocytes, are potential sources of these mediators.
In terms of the effects of hesperidin on neuroglial cells, a conditioned medium from hesperidin-treated astrocytes increases neuronal progenitor cells [36], which suggests that hesperidin-exposed astrocytes indirectly influence neuronal cell differentiation. Regarding the permeability of hesperidin through the blood-brain barrier, hesperidin may be absorbed in the large intestine due to its turnover to hesperetin via microflora [3] and then enter the blood circulation to, ultimately, penetrate the brain through the blood-brain barrier [37]. The production of nitric oxide and pro-inflammatory mediators, including TNF-α and IL-1β, are significantly suppressed by hesperidin in the RAW246.7 macrophage cell line [38]. Thus, there is a consensus that hesperidin suppresses the biological activity of astrocytes, microglia, or both, in multiple ways.
Taken together, these data suggest that hesperidin interferes with the initiation of inflammation in non-neuronal systems as well as the brain, possibly, via reductions in proinflammatory cytokines, enhancing the activation of radical scavenging enzymes, and restoring neuronal activity.
Effects of hesperidin on demyelinating diseases in the CNS
Effects of hesperidin in autoimmune demyelinating diseases
The types of neuroinflammation associated with autoimmune demyelinating diseases, such as MS and its animal models, as well as experimental autoimmune encephalomyelitis (EAE) are distinct from that of neurodegenerative diseases, such as Alzheimer's disease [39]. EAE is characterized by the generation of autoimmune T cells in the peripheral lymphoid tissues and the infiltration of these autoimmune T cells into the CNS parenchyma, which result in demyelination and reactive gliosis [40]. Thus, the suppression of T-cell proliferation during the induction stage of EAE is a primary target for the prevention of this disease. Additionally, the interruption of inflammatory cell migration into CNS tissues is another key step in preventing damage to CNS cells, including glial cells and neurons. Finally, the suppression of oxidative stress and inflammatory mediators might be an alternative method that can inhibit EAE progression [41]. Blocking at least one of these three processes would be applicable for the amelioration of EAE. Previous studies have shown that the antioxidant apigenin, which is a natural flavonoid, inhibits the progression of EAE via one of the processes described above [42].
In models of autoimmune CNS diseases, hesperidin improves EAE-induced paralysis when administered orally (50, 100, and 200 mg/kg [18]) or via subcutaneous injection (50 mg/kg/day for 7 consecutive days [17]). The mechanisms underlying the effects of hesperidin on EAE are largely dependent on its strong antioxidant and anti-inflammatory properties [17,18]. Furthermore, hesperidin is involved in the suppression of autoimmune T cell proliferation, the activation of regulatory T cells, reductions in microglial cells in EAE lesions, and decreased demyelination [18], which could possibly lead to the M2 phenotype macrophage bias in EAE [41]. Thus, the beneficial effects of hesperidin on EAE appear to be largely dependent on suppressing the inflammatory response and oxidative stress in the peripheral immune system and EAE target tissues, which would lead to reductions in demyelination.
However, a study investigating the effects of hesperetin, which is a flavanone and an aglycone of hesperidin, reported that hesperetin treatment (approximately 10 mg/day/each in drinking water) delayed the recovery of paralysis in mice with proteolipid protein-induced EAE [43], which suggests that hesperetin has a detrimental effect in EAE. It is possible that the differential effects of hesperidin-related compounds on EAE depend on the treatment dose and different immunization antigens (myelin oligodendrocyte glycoprotein vs. proteolipid protein) because it is widely accepted that flavones, including luteolin and apigenin, inhibit antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells in vitro [51].
Regarding the role of radicals in models of autoimmune disease, nitric oxide occasionally suppresses the proliferation of T cells and also induces cell apoptosis [52]. Therefore, reductions in nitric oxide levels may facilitate the proliferation of autoimmune T cells and interfere with the apoptotic elimination of inflammatory cells in target organs. This finding is further supported by studies that assessed treatment of the nitric oxide carrier in EAE [53,54]. Thus, the hesperidin-induced reduction of radicals might be case-sensitive and/or inflammation stage–dependent in models of autoimmune CNS diseases.
Effects of hesperetin in a model of chemical-induced demyelination
Demyelination can be visualized via decreases in myelin, which can be caused by either the degradation of myelin-forming oligodendrocytes or direct myelin loss without the loss of oligodendrocytes [55]. A model of lysolecithin-induced focal demyelination [56] showed that this process is distinct from that of autoimmune demyelination in EAE, which is associated with the infiltration of inflammatory cells into the CNS parenchyma [41]. Lysolecithin-induced demyelination is characterized by myelin loss and glial activation without the infiltration of hematogenous cells and can be ameliorated by hesperetin treatment [57]. The beneficial effects of hesperetin (in the present review article, the term “hesperetin” will be used rather than “hersperitin” according to the generic name of the structural formula of the compound) have been attributed to its direct cytoprotective and antioxidant effects in CNS tissues, which is similar to the ability of the flavonoid quercetin regarding the repair of myelin [58]. This finding suggests that hesperetin can permeate through the bloodbrain barrier to influence neurons and neuroglial cells directly in damaged regions.
Effects of hesperidin in hippocampal dysfunction
The hippocampus is centrally involved in cognitive and memory functions as well as the progression of MS and the animal model of EAE. Currently, there is a consensus that MS patients suffer from cognitive dysfunction [59,60] and that EAE mice exhibit hippocampal atrophy in conjunction with increased radical activity and the activation of microglial cells [61,62]. In models of EAE, hesperidin exerts neuroprotective and other beneficial effects that, possibly, occur through the suppression of microglial cells. It is known that hesperidin protects neurons against death and promotes the survival of neuronal progenitor cells through activation of the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways [63]. Even though the biochemical activity of neurons in models of EAE has yet to be precisely evaluated following hesperidin treatment, it is possible that hesperidin may protect neurons against glutamate excitotoxicity in EAE-affected brains [64], as it does in models of neurodegeneration [3,22].
The neuroprotective effects of hesperidin against hippocampal excitotoxicity were assessed in a model of aluminum chloride-induced excitotoxicity that leads to cell death with the concurrent hyperactivation of microglial cells [65]. The cytoprotective effects of hesperidin are largely dependent on its antioxidant and anti-inflammatory activities [32,47], which indicates that cognitive impairments are ameliorated by hesperidin treatment. Hesperidin treatment is also associated with decreased levels of nitrate/nitrite and increased levels of brain-derived neurotrophic factor in the mouse hippocampus [45] as well as the inhibition of glutamate release following kainic acid-induced excitotoxicity in the rat hippocampus [49]. Furthermore, hesperidin improves memory consolidation in mice with streptozotocin-induced cognitive impairments by modulating acetylcholinesterase activity [48] and protects neurons against cell death [63]. Taken together, these findings indicate that hesperidin exerts protective effects in the hippocampus.
As a model of stroke, brain ischemia-reperfusion injury is characterized by cell loss and reactive gliosis in the hippocampus of adult [24] and neonatal [50] rats. However, hesperidin significantly reverses reductions in the activities of antioxidant enzymes, such as glutathione peroxidase, glutathione reductase, catalase, and superoxide dismutase, and ameliorates glutathione content following middle cerebral artery occlusion. Furthermore, hesperidin reduces the production of various inflammatory mediators, including TNF-α and IL-1β, and the expression of inducible nitric oxide synthase. Thus, it can be postulated that hesperidin-induced neuroprotection is partly dependent on the suppression of inflammatory responses in the hippocampus after middle cerebral artery occlusion [19,24] and that hesperidin contributes to neuronal survival [63].
Effects of hesperidin on depressive-like behaviors
Hesperidin may also have beneficial effects on depressivelike behaviors because a majority of studies have found that treatment with hesperidin ameliorates depression, irrespective of the causative agent [3]. For example, hesperidin reduces immobility time in the forced swimming test [44,45] and, possibly, promotes neuronal activity in the hippocampus. Additionally, a recent study reported that the mechanisms underlying the antidepressant-like effects of hesperidin include the modulation of serotonergic 5-HT1A receptors [46] and kappaopioid receptors in the hippocampus [44]. The neuroprotective activity and modulation of serotonergic neurons following hesperidin treatment also alter a variety of depressive-like behaviors in LPS-injected mice with endotoxemia [35], mice with traumatic brain injury [20], streptozotocin-induced diabetic rats [16], a 6-hydroxydopamine model of Parkinson's disease [12], and mice following an olfactory bulbectomy [15]. Thus, it can be postulated that the antidepressant-like effects of dietary citrus containing hesperidin are non-specifically obtained via a broad spectrum of neuroprotection, as described above [3,22,23].
Conclusion
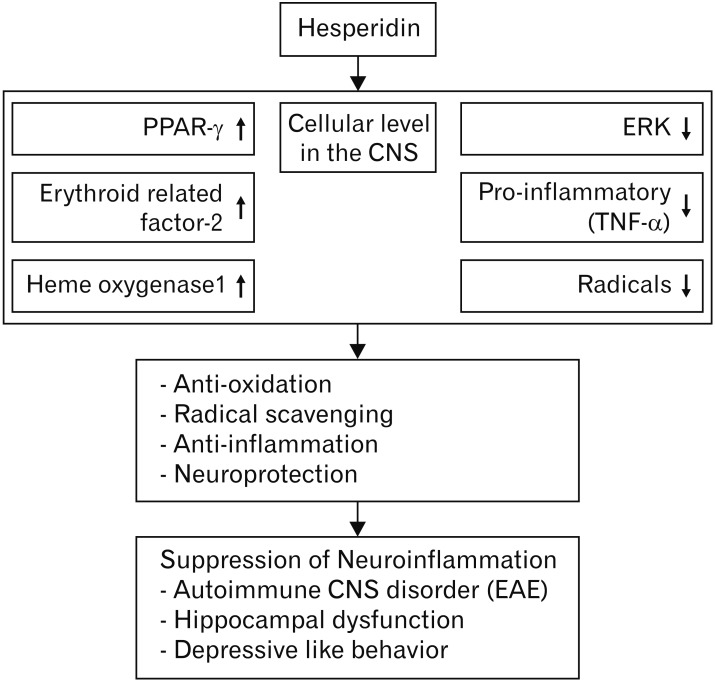
In general, the molecular mechanisms that underlie the beneficial effects of the flavone glycoside hesperidin on CNS disease remain unclear. However, citrus is known to exert positive effects because it contains a variety of non-toxic antioxidants. Decades of research on these antioxidant activities have provided experimental evidence showing that hesperidin originating from citrus plays an important neuroprotective role through its antioxidant and anti-inflammatory activities (Table 1). Moreover, the molecular mechanisms of hesperidin, underlying it effects in nervous tissues and immune cells, are largely dependent on the suppression of cell activation signals and radical scavenging effects (Fig. 2). In terms of nutraceutical effects, citrus without toxic effects can provide beneficial effects by preventing and curing CNS disorders, independently of inflammation type, in different lesions of CNS tissues. Therefore, the antioxidant and anti-inflammatory activities of hesperidin will be able to encourage the clinical trials for neurodegenerative diseases. Further studies are necessary to develop medicine and/or medical supply for effective absorption of hesperidin, which owns various functions.
Table 1. Bioactivity properties of hesperidin and their underlying mechanisms in animal models.
| Model | Compound and treatment protocol | Results and underlying mechanism | References |
|---|---|---|---|
| Normal status | |||
| Swiss albino mice | Hesperidin (10 mg/kg) per B.W., i.p. | Reduction in the phosphorylation state of ERK 1/2, in the cerebral cortex, cerebellum and hippocampus | [34] |
| Swiss albino mice | Hesperidin (0.1, 0.3 and 1 mg/kg, B.W.), i.p. | Decreasing the immobility time in the forced swimming test | [44] |
| Kappa-opioid, but not with the δ-opioid, μ-opioid or adenosinergic receptors mediate the antidepressant-like activity of hesperidin | |||
| Swiss albino mice | Hesperidin or hesperetin (0.1, 0.3 and 1 mg/kg, B. W.), i.p. | Significantly decreasing in nitrate/nitrite levels in the hippocampus | [45] |
| Increasing of BDNF level in the hippocampus | |||
| Inhibition of L-arginine-nitric oxide-cGMP pathway in hippocampus | |||
| Swiss albino mice | Hesperidin (0.3 mg/kg, B.W.), i.p. | Decreasing the immobility time in the tail suspension test and forced swimming time | [46] |
| Decreasing the antidepressant-like activity of hesperidin in mice with selective 5HT1A receptor antagonist | |||
| Learning and memory deficit model | |||
| Wistar rat, AlCl3-induced cognitive impairment | Hesperidin (100 mg/kg, B.W.), orally, 60 days | Prevention of cognitive deficits, biochemical anomaly and apoptosis induced by AlCl3 | [47] |
| Wistar rat, AlCl3-induced cognitive impairment | Hesperidin (100 mg/kg, B.W.), orally, 60 days | Reduction of inflammatory markers (GFAP, Iba-1, NF-κB, COX-2, IL-1β, TNF-α, iNOS) and apoptotic markers (cytosolic cytochrome c, caspase-3, -8, and -9) | [32] |
| Increasing of phospho-Akt and phospho-GSK3β | |||
| Swiss albino mice | Hesperidin (100 and 200 mg/kg, B.W.), orally, 15 days, pretreatment | Prevention of the cognitive impairment | [48] |
| STZ injection-induced cognitive impairment | Improvement of memory consolidation process as tested by Morris water maze possibly through modulation of AChE activity | ||
| Inhibition in the overexpression of inflammatory markers, including NF-κB, iNOS, COX-2, and GFAP-positive astrocytes | |||
| Sprague-Dawley rat | Hesperidin (10 or 50 mg/kg, i.p.), pretreatment | Inhibition of glutamate release | [49] |
| KA-induced neurotoxicity (Seizure model) | Protection of CA3 neurons against excitotoxicity induced by KA | ||
| Inhibition of pro-inflammatory molecules by microglia | |||
| PD model | |||
| C57BL/6 mice | Hesperidin (50 mg/kg), orally, 28 days | Improvement of memory impairment and depressive-like behavior | [12] |
| Intracerebroventricular injection of 6-OHDA–induced PD | Attenuation of reduction in glutathione peroxidase and catalase activity, total reactive antioxidant potential and the dopamine and its metabolite levels in the striatum of aged mice | ||
| Depression model | |||
| C57BL/6 mice | Hesperidin (50 mg/kg), orally, 14 days | Reverses cognitive and depressive disorder behaviorally | [15] |
| Depression induced by olfactory bulbectomy | Reduction of pro-inflammatory cytokines and AChE activity in hippocampus | ||
| Upregulation of BDNF and NGF in hippocampus | |||
| Wistar rat | Hesperidin (25, 50, and 100 mg/kg B.W.), orally, 21 days | Reversed the STZ-induced increase in immobility duration in the forced swimming test | [16] |
| Depressive-like behavior in STZ-induced diabetic rats | Attenuation of hyperglycaemia and malondialdehyde | ||
| Decreasing of IL-6 | |||
| Increasing of BDNF level | |||
| Amelioration of STZ-induced neurochemical alterations, as indicated by upregulation of monoamines (norepinephrine, dopamine, and serotonin) in the brain | |||
| NMRI mice | Ten days after mild TBIinduction, mice received oral hesperidin treatment (50 mg/kg/14 days) | Attenuation of depression-related symptoms behaviorally through sucrose preference test, forced swimming test, novelty-suppressed feeding test, and tail suspension test. | [20] |
| Depressive-like behaviors in mice with mild traumatic brain injury | Decreasing of neuro-inflammation and oxidative damage | ||
| Increasing of BDNF level in the hippocampus | |||
| Depressive-like behavior in LPS-injected mice | Hesperidin (25, 50, and 100 mg/kg) for 7 days, pretreatment | Downregulation of the serum corticosterone | [35] |
| Reduction of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) via the miRNA-132 pathway (upregulation) in the brain | |||
| Stroke model (brain ischemia-reperfusion injury) | |||
| Sprague-Dawley rat (neonatal) | Hesperidin (50 mg/kg/day), orally, 3 days after injury | Prevent an increase in intracellular reactive oxygen species and lipid peroxide levels | [50] |
| Hypoxia-ischemic brain jury | Activation of the key survival signaling kinase including Akt | ||
| Wistar rat | Hesperidin (50 mg/kg B.W.), orally, 15 days before MCAO | Reduced the neurological deficits behaviorally | [24] |
| MCAO-induced brain ischemia | Upregulation of the antioxidant enzymes | ||
| Reduced the induction of pro-inflammatory cytokines | |||
| Wistar rat | Hesperidin (50 and 100 mg/kg) orally, 7 days, pretreatment | Improved neurobehavioral alterations, oxidative defense and mitochondrial complex enzyme activities in hippocampus | [19] |
| Memory dysfunction in brain I/R injury | Inhibition of L-arginine-nitric oxide signaling pathway | ||
| Amelioration of memory dysfunction and biochemical alterations | |||
| Autoimmune CNS disease model | Hesperidin (50, 100, and 200 mg/kg), orally, daily for 25 days | Suppression of the incidence and severity of EAE. | [18] |
| C57BL/6 mice | Decrease IL-17 and IL-6 | ||
| MOG-induced EAE | Increase IL-1β and TGF-β | ||
| Increase Treg cells in spleen and lymph node | |||
| C57BL/6 mice | Hesperidin (50 mg/kg B.W.), subcutaneously for 7 days | Decrease in lipid peroxidation | [17] |
| MOG-induced EAE | Increase in elements of the antioxidant defense systems in brain tissue | ||
| Decrease in serum levels of TNF-α and IL-1β | |||
| SJL/J mice | Hesperetin (aglycone form of hesperidin, 10 mg/kg), orally, 14 or 25 days | Fail to beneficially influence the course of EAE | [43] |
| Proteolipid protein-induced | Suppress recovery from acute inflammatory damage | ||
| EAE |
B.W., body weight; i.p., intraperitoneally; ERK, extracellular signal-regulated kinases; BDNF, brain-derived neurotrophic factor; AlCl3, aluminum chloride; GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor molecule-1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; COX, cyclooxygenase; IL, interleukin; TNF, tumor necrosis factor; iNOS, inducible nitric oxide synthase; GSK, glycogen synthase kinase; STZ, streptozotocin; AChE, acetylcholine esterase; KA, kainic acid; PD, Parkinson's disease; 6-OHDA, 6-hydroxydopamine; NGF, nerve growth factor; TBI, traumatic brain injury; LPS, lipopolysaccharide; MCAO, middle cerebral artery occlusion; I/R, ischemic/reperfusion; CNS, central nervous system; MOG, myelin oligodendrocyte glycoprotein; EAE, experimental autoimmune encephalomyelitis; TGF, transforming growth factor; Treg, regulatory T cell.
Fig. 2. Illustration of hesperidin involvement in models of neuroinflammation. PPAR, peroxisome proliferator-activated receptor; CNS, central nervous system; TNF-α, tumor necrosis factor α; EAE, experimental autoimmune encephalomyelitis.
Acknowledgements
This work was supported by a research grant from Jeju National University in 2019.
Footnotes
- Conceptualization: JK, TS.
- Data acquisition: JK, MA.
- Data analysis or interpretation: JK, MBW, MA.
- Drafting of the manuscript: JK, MBW, TS.
- Critical revision of the manuscript: MBW, AT, HM, TS.
- Approval of the final version of the manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 2.Umeno A, Horie M, Murotomi K, Nakajima Y, Yoshida Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules. 2016;21:E708. doi: 10.3390/molecules21060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin: a mini-review. Life Sci. 2014;113:1–6. doi: 10.1016/j.lfs.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Camps-Bossacoma M, Franch À, Pérez-Cano FJ, Castell M. Influence of hesperidin on the systemic and intestinal rat immune response. Nutrients. 2017;9:E580. doi: 10.3390/nu9060580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estruel-Amades S, Massot-Cladera M, Pérez-Cano FJ, Franch À, Castell M, Camps-Bossacoma M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients. 2019;11:E324. doi: 10.3390/nu11020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testai L, Calderone V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients. 2017;9:E502. doi: 10.3390/nu9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 8.Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Homayouni F, Haidari F, Hedayati M, Zakerkish M, Ahmadi K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes: a randomized double-blind controlled clinical trial. Phytother Res. 2018;32:1073–1079. doi: 10.1002/ptr.6046. [DOI] [PubMed] [Google Scholar]
- 10.Sawikr Y, Yarla NS, Peluso I, Kamal MA, Aliev G, Bishayee A. Neuroinflammation in Alzheimer's disease: the preventive and therapeutic potential of polyphenolic nutraceuticals. Adv Protein Chem Struct Biol. 2017;108:33–57. doi: 10.1016/bs.apcsb.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Jung UJ, Kim SR. Beneficial effects of flavonoids against Parkinson's disease. J Med Food. 2018;21:421–432. doi: 10.1089/jmf.2017.4078. [DOI] [PubMed] [Google Scholar]
- 12.Antunes MS, Goes AT, Boeira SP, Prigol M, Jesse CR. Protective effect of hesperidin in a model of Parkinson's disease induced by 6-hydroxydopamine in aged mice. Nutrition. 2014;30:1415–1422. doi: 10.1016/j.nut.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Kumar P, Kumar A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide. Behav Brain Res. 2010;206:38–46. doi: 10.1016/j.bbr.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 14.Menze ET, Tadros MG, Abdel-Tawab AM, Khalifa AE. Potential neuroprotective effects of hesperidin on 3-nitropropionic acidinduced neurotoxicity in rats. Neurotoxicology. 2012;33:1265–1275. doi: 10.1016/j.neuro.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Antunes MS, Jesse CR, Ruff JR, de Oliveira Espinosa D, Gomes NS, Altvater EE, Donato F, Giacomeli R, Boeira SP. Hesperidin reverses cognitive and depressive disturbances induced by olfactory bulbectomy in mice by modulating hippocampal neurotrophins and cytokine levels and acetylcholinesterase activity. Eur J Pharmacol. 2016;789:411–420. doi: 10.1016/j.ejphar.2016.07.042. [DOI] [PubMed] [Google Scholar]
- 16.El-Marasy SA, Abdallah HM, El-Shenawy SM, El-Khatib AS, El-Shabrawy OA, Kenawy SA. Anti-depressant effect of hesperidin in diabetic rats. Can J Physiol Pharmacol. 2014;92:945–952. doi: 10.1139/cjpp-2014-0281. [DOI] [PubMed] [Google Scholar]
- 17.Ciftci O, Ozcan C, Kamisli O, Cetin A, Basak N, Aytac B. Hesperidin, a citrus flavonoid, has the ameliorative effects against Experimental Autoimmune Encephalomyelitis (EAE) in a C57BL/J6 Mouse Model. Neurochem Res. 2015;40:1111–1120. doi: 10.1007/s11064-015-1571-8. [DOI] [PubMed] [Google Scholar]
- 18.Haghmorad D, Mahmoudi MB, Salehipour Z, Jalayer Z, Momtazi Brojeni AA, Rastin M, Kokhaei P, Mahmoudi M. Hesperidin ameliorates immunological outcome and reduces neuroinflammation in the mouse model of multiple sclerosis. J Neuroimmunol. 2017;302:23–33. doi: 10.1016/j.jneuroim.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Gaur V, Kumar A. Hesperidin pre-treatment attenuates NOmediated cerebral ischemic reperfusion injury and memory dysfunction. Pharmacol Rep. 2010;62:635–648. doi: 10.1016/s1734-1140(10)70321-2. [DOI] [PubMed] [Google Scholar]
- 20.Kosari-Nasab M, Shokouhi G, Ghorbanihaghjo A, Abbasi MM, Salari AA. Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury. Life Sci. 2018;213:198–205. doi: 10.1016/j.lfs.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Hajialyani M, Hosein Farzaei M, Echeverría J, Nabavi SM, Uriarte E, Sobarzo-Sánchez E. Hesperidin as a neuroprotective agent: a review of animal and clinical evidence. Molecules. 2019;24:E648. doi: 10.3390/molecules24030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang SL, Shih PH, Yen GC. Neuroprotective effects of citrus flavonoids. J Agric Food Chem. 2012;60:877–885. doi: 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- 23.Cirmi S, Ferlazzo N, Lombardo GE, Ventura-Spagnolo E, Gangemi S, Calapai G, Navarra M. Neurodegenerative diseases: might citrus flavonoids play a protective role? Molecules. 2016;21:E1312. doi: 10.3390/molecules21101312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raza SS, Khan MM, Ahmad A, Ashafaq M, Khuwaja G, Tabassum R, Javed H, Siddiqui MS, Safhi MM, Islam F. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res. 2011;1420:93–105. doi: 10.1016/j.brainres.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 25.Said UZ, Saada HN, Abd-Alla MS, Elsayed ME, Amin AM. Hesperidin attenuates brain biochemical changes of irradiated rats. Int J Radiat Biol. 2012;88:613–618. doi: 10.3109/09553002.2012.694008. [DOI] [PubMed] [Google Scholar]
- 26.Rotimi SO, Bankole GE, Adelani IB, Rotimi OA. Hesperidin prevents lipopolysaccharide-induced endotoxicity in rats. Immunopharmacol Immunotoxicol. 2016;38:364–371. doi: 10.1080/08923973.2016.1214142. [DOI] [PubMed] [Google Scholar]
- 27.Chen B, Lu Y, Chen Y, Cheng J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol. 2015;225:R83–R99. doi: 10.1530/JOE-14-0662. [DOI] [PubMed] [Google Scholar]
- 28.Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H(2)O(2)-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother. 2017;88:124–133. doi: 10.1016/j.biopha.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 29.Elshazly SM, Abd El Motteleb DM, Ibrahim IA. Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats. Chem Biol Interact. 2018;291:153–161. doi: 10.1016/j.cbi.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Agrawal YO, Sharma PK, Shrivastava B, Ojha S, Upadhya HM, Arya DS, Goyal SN. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS One. 2014;9:e111212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoud AM. Hesperidin protects against cyclophosphamideinduced hepatotoxicity by upregulation of PPARγ and abrogation of oxidative stress and inflammation. Can J Physiol Pharmacol. 2014;92:717–724. doi: 10.1139/cjpp-2014-0204. [DOI] [PubMed] [Google Scholar]
- 32.Justin-Thenmozhi A, Dhivya Bharathi M, Kiruthika R, Manivasagam T, Borah A, Essa MM. Attenuation of aluminum chlorideinduced neuroinflammation and caspase activation through the AKT/GSK-3β pathway by hesperidin in Wistar rats. Neurotox Res. 2018;34:463–476. doi: 10.1007/s12640-018-9904-4. [DOI] [PubMed] [Google Scholar]
- 33.Maekawa S, Sato K, Fujita K, Daigaku R, Tawarayama H, Murayama N, Moritoh S, Yabana T, Shiga Y, Omodaka K, Maruyama K, Nishiguchi KM, Nakazawa T. The neuroprotective effect of hesperidin in NMDA-induced retinal injury acts by suppressing oxidative stress and excessive calpain activation. Sci Rep. 2017;7:6885. doi: 10.1038/s41598-017-06969-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez MC, Fernandez SP, Loscalzo LM, Wasowski C, Paladini AC, Marder M, Medina JH, Viola H. Hesperidin, a flavonoid glycoside with sedative effect, decreases brain pERK1/2 levels in mice. Pharmacol Biochem Behav. 2009;92:291–296. doi: 10.1016/j.pbb.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Shao H, Zhang X, Qin B. Hesperidin alleviates lipopolysaccharide-induced neuroinflammation in mice by promoting the miRNA-132 pathway. Inflammation. 2016;39:1681–1689. doi: 10.1007/s10753-016-0402-7. [DOI] [PubMed] [Google Scholar]
- 36.Nones J, Spohr TC, Gomes FC. Effects of the flavonoid hesperidin in cerebral cortical progenitors in vitro: indirect action through astrocytes. Int J Dev Neurosci. 2012;30:303–313. doi: 10.1016/j.ijdevneu.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem. 2003;85:180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Zug C, Qu H, Schluesener H, Zhang Z. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav Brain Res. 2015;281:32–42. doi: 10.1016/j.bbr.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz M, Deczkowska A. Neurological disease as a failure of brain-immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol. 2016;37:668–679. doi: 10.1016/j.it.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Shin T, Kojima T, Tanuma N, Ishihara Y, Matsumoto Y. The subarachnoid space as a site for precursor T cell proliferation and effector T cell selection in experimental autoimmune encephalomyelitis. J Neuroimmunol. 1995;56:171–178. doi: 10.1016/0165-5728(94)00144-d. [DOI] [PubMed] [Google Scholar]
- 41.Shin T, Ahn M, Matsumoto Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anat Cell Biol. 2012;45:141–148. doi: 10.5115/acb.2012.45.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginwala R, McTish E, Raman C, Singh N, Nagarkatti M, Nagarkatti P, Sagar D, Jain P, Khan ZK. Apigenin, a natural flavonoid, attenuates EAE severity through the modulation of dendritic cell and other immune cell functions. J Neuroimmune Pharmacol. 2016;11:36–47. doi: 10.1007/s11481-015-9617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbeek R, van Tol EA, van Noort JM. Oral flavonoids delay recovery from experimental autoimmune encephalomyelitis in SJL mice. Biochem Pharmacol. 2005;70:220–228. doi: 10.1016/j.bcp.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 44.Filho CB, Del Fabbro L, de Gomes MG, Goes AT, Souza LC, Boeira SP, Jesse CR. Kappa-opioid receptors mediate the antidepressant-like activity of hesperidin in the mouse forced swimming test. Eur J Pharmacol. 2013;698:286–291. doi: 10.1016/j.ejphar.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Donato F, de Gomes MG, Goes AT, Filho CB, Del Fabbro L, Antunes MS, Souza LC, Boeira SP, Jesse CR. Hesperidin exerts antidepressant-like effects in acute and chronic treatments in mice: possible role of l-arginine-NO-cGMP pathway and BDNF levels. Brain Res Bull. 2014;104:19–26. doi: 10.1016/j.brainresbull.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Souza LC, de Gomes MG, Goes AT, Del Fabbro L, Filho CB, Boeira SP, Jesse CR. Evidence for the involvement of the serotonergic 5-HT(1A) receptors in the antidepressant-like effect caused by hesperidin in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:103–109. doi: 10.1016/j.pnpbp.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Justin Thenmozhi A, William Raja TR, Manivasagam T, Janakiraman U, Essa MM. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer's disease. Nutr Neurosci. 2017;20:360–368. doi: 10.1080/1028415X.2016.1144846. [DOI] [PubMed] [Google Scholar]
- 48.Javed H, Vaibhav K, Ahmed ME, Khan A, Tabassum R, Islam F, Safhi MM, Islam F. Effect of hesperidin on neurobehavioral, neuroinflammation, oxidative stress and lipid alteration in intracerebroventricular streptozotocin induced cognitive impairment in mice. J Neurol Sci. 2015;348:51–59. doi: 10.1016/j.jns.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 49.Chang CY, Lin TY, Lu CW, Huang SK, Wang YC, Chou SS, Wang SJ. Hesperidin inhibits glutamate release and exerts neuroprotection against excitotoxicity induced by kainic acid in the hippocampus of rats. Neurotoxicology. 2015;50:157–169. doi: 10.1016/j.neuro.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Rong Z, Pan R, Xu Y, Zhang C, Cao Y, Liu D. Hesperidin pretreatment protects hypoxia-ischemic brain injury in neonatal rat. Neuroscience. 2013;255:292–299. doi: 10.1016/j.neuroscience.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 51.Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol. 2004;68:621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 52.van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;1:1491–1500. doi: 10.1016/s1567-5769(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 53.Nath N, Morinaga O, Singh I. S-nitrosoglutathione a physiologic nitric oxide carrier attenuates experimental autoimmune encephalomyelitis. J Neuroimmune Pharmacol. 2010;5:240–251. doi: 10.1007/s11481-009-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willenborg DO, Staykova M, Fordham S, O'Brien N, Linares D. The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:16–25. doi: 10.1016/j.jneuroim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Osorio-Querejeta I, Sáenz-Cuesta M, Muñoz-Culla M, Otaegui D. Models for studying myelination, demyelination and remyelination. Neuromolecular Med. 2017;19:181–192. doi: 10.1007/s12017-017-8442-1. [DOI] [PubMed] [Google Scholar]
- 56.Plemel JR, Michaels NJ, Weishaupt N, Caprariello AV, Keough MB, Rogers JA, Yukseloglu A, Lim J, Patel VV, Rawji KS, Jensen SK, Teo W, Heyne B, Whitehead SN, Stys PK, Yong VW. Mechanisms of lysophosphatidylcholine-induced demyelination: a primary lipid disrupting myelinopathy. Glia. 2018;66:327–347. doi: 10.1002/glia.23245. [DOI] [PubMed] [Google Scholar]
- 57.Baradaran S, Hajizadeh Moghaddam A, Ghasemi-Kasman M. Hesperetin reduces myelin damage and ameliorates glial activation in lysolecithin-induced focal demyelination model of rat optic chiasm. Life Sci. 2018;207:471–479. doi: 10.1016/j.lfs.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Naeimi R, Baradaran S, Ashrafpour M, Moghadamnia AA, Ghasemi-Kasman M. Querectin improves myelin repair of optic chiasm in lyolecithin-induced focal demyelination model. Biomed Pharmacother. 2018;101:485–493. doi: 10.1016/j.biopha.2018.02.125. [DOI] [PubMed] [Google Scholar]
- 59.Planche V, Panatier A, Hiba B, Ducourneau EG, Raffard G, Dubourdieu N, Maitre M, Lesté-Lasserre T, Brochet B, Dousset V, Desmedt A, Oliet SH, Tourdias T. Selective dentate gyrus disruption causes memory impairment at the early stage of experimental multiple sclerosis. Brain Behav Immun. 2017;60:240–254. doi: 10.1016/j.bbi.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 60.Mandolesi G, Grasselli G, Musumeci G, Centonze D. Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeneration. Neurol Sci. 2010;31(Suppl 2):S255–S259. doi: 10.1007/s10072-010-0369-3. [DOI] [PubMed] [Google Scholar]
- 61.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. 2010;90:774–786. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurkowska-Jastrzębska I, Swiątkiewicz M, Zaremba M, Cudna A, Piechal A, Pyrzanowska J, Widy-Tyszkiewicz E, Członkowska A. Neurodegeneration and inflammation in hippocampus in experimental autoimmune encephalomyelitis induced in rats by one: time administration of encephalitogenic T cells. Neuroscience. 2013;248:690–698. doi: 10.1016/j.neuroscience.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 63.Nones J, E Spohr TC, Gomes FC. Hesperidin, a flavone glycoside, as mediator of neuronal survival. Neurochem Res. 2011;36:1776–1784. doi: 10.1007/s11064-011-0493-3. [DOI] [PubMed] [Google Scholar]
- 64.Levite M. Glutamate, T cells and multiple sclerosis. J Neural Transm (Vienna) 2017;124:775–798. doi: 10.1007/s00702-016-1661-z. [DOI] [PubMed] [Google Scholar]
- 65.Jovanova-Nesic K, Shoenfeld Y, Spector NH. Aluminum excytotoxicity and neuroautotoimmunity: the role of the brain expression of CD32+ (FcγRIIa), ICAM-1+ and CD3ξ in aging. Curr Aging Sci. 2012;5:209–217. doi: 10.2174/1874609811205030007. [DOI] [PubMed] [Google Scholar]