Abstract
The hippocampus is one of the most important brain areas of cognition. This region is particularly sensitive to hypoxia and ischemia. Neuregulin-1 (NRG1) has been shown to be able to protect against focal cerebral ischemia. The aim of the present study was to investigate the neuroprotective effect of NRG1 in primary hippocampal neurons and its underlying mechanism. Our data showed oxygen-glucose deprivation (OGD)-induced cytotoxicity and overexpression of ErbB4 in primary hippocampal neurons. Moreover, pretreatment with NRG1 could inhibit OGD-induced overexpression of ErbB4. In addition, NRG1 significantly attenuated neuronal death induced by OGD. The neuroprotective effect of NRG1 was blocked in ischemic neurons after pretreatment with AG1478, an inhibitor of ErbB4, but not after pretreatment with AG879, an inhibitor of ErbB2. These results indicate an important role of ErbB4 in NRG1-mediated neuroprotection, suggesting that endogenous ErbB4 might serve as a valuable therapeutic target for treating global cerebral ischemia.
Keywords: NRG1, ErbB4, Global cerebral ischemia, Oxygen-glucose deprivation, Cell death
Introduction
Global cerebral ischemia is a multifaceted disorder in which brain tissue is subjected to reduced levels of oxygen and glucose due to impairment in blood supply to the entire brain. However, numerous studies have focused on the hippocampus as this important region is one of areas injured [1,2]. One of the main features of transient global cerebral ischemia is delayed death of pyramidal neurons of the hippocampal CA1 region that occurs hours to days after the insult [2]. After this time-window, neurons can undergo apoptosis/necrosis or survive by maintaining cellular homeostasis [3,4].
Neuregulin-1 (NRG1) is a family of proteins containing an epidermal growth factor (EGF)-like motif that mediates important functions in the nervous system [5,6,7]. NRG1 signaling regulates nerve cell differentiation, neuron migration, neuronal survival, neurite outgrowth, and synaptic activity [6,8]. NRG1 and its receptor ErbB tyrosine kinases are expressed not only in the developing nervous system, but also in adult brains [9,10]. Its receptors are tyrosine kinases ErbB2, ErbB3, and ErbB4. ErbB2 and ErbB4, but not ErbB3, can be activated by NRG1. ErbB4 is expressed at synapses [11,12,13]. It has been implicated in synaptic plasticity [10,12,14]. Previous studies have demonstrated that NRG1 can prevent apoptosis of PC12 and SH-SY5Y cells induced by serum deprivation and H2O2 [15,16,17]. Treatment with NRG1 immediately after oxygen-glucose deprivation (OGD) can significantly increase neuronal survival in rat B-35 neuroblastoma cells and cortical neurons [18,19,20]. A number of recent reports have shown that pre-administration of NRG1 could reduce delayed ischemic cortical damage following transient middle cerebral artery occlusion and permanent focal cerebral ischemia in rats [21,22,23,24]. However, the involvement of NRG1 pathway for the selective vulnerability of hippocampal neurons to global ischemia has not been fully understood yet. To identify NRG1 effect elicited by ischemic insults, primary hippocampal cultures were subjected to OGD as an in vitro model for global ischemia. Our study demonstrates an important role of ErbB4 in NRG1-mediated neuroprotection, suggesting that endogenous ErbB4 can serve as a novel target for global ischemic therapy.
Materials and Methods
Reagents and antibodies
The NRG1 used in this study was a recombinant polypeptide containing the entire EGF domain of the β-type NRG1 from PROSPEC (East Brunswick, NJ, USA). Antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA; ErbB4, sc-283, sc-8050; β-actin, sc-47778; HRP-conjugated anti-rabbit IgG, sc-2004; and HRP-conjugated anti-mouse IgG, sc-2005). AG1478 (Calbiochem, Darmstadt, Germany) and AG879 (Calbiochem) as inhibitors of ErbB4 and ErbB2, respectively, were dissolved in dimethylsulfoxide (DMSO) (Sigma, Louis, MO, USA). The final concentration of DMSO was 0.001% or less when applied to cells.
Primary hippocampal neuronal culture
Primary hippocampal neurons were cultured as described previously [25] . Briefly, the hippocampus was removed from Sprague-Dawley rat embryos (E18) and dissociated by gentle trituration in PBS (Gibco, Carlsbad, CA, USA). Cells were seeded onto poly-L-lysine-coated 12-well plates and cultured in Neurobasal media (Gibco). Experiments were performed at 14 days after seeding (DIV14).
Lactate dehydrogenase release assay
The extent of cell death was assessed by determining the activity level of lactate dehydrogenase (LDH) released into the culture medium. LDH activity was determined using a Cytotox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Results are expressed as a percentage of the maximum LDH release obtained upon complete cell lysis.
Terminal deoxynucleotidyl transferase dUTP nick end labeling staining
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was performed using an in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's protocol. Apoptotic primary hippocampal neurons were labeled with TMR red and nuclei were labeled with 10 µM Hoechst dye. The number of apoptotic cells was counted in five random fields using an LSM 510 Meta system (Zeiss LSM 510 laser scanning microscope, Carl Zeiss, Oberkochen, Germany).
Immunofluorescence
Immunostaining of rat hippocampal neurons (DIV14) was performed as described previously [13]. Briefly, neurons were fixed with 4% paraformaldehyde and 4% sucrose in PBS for 20 min. These cells were permeabilized by incubation in PBS containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100 for 30 minutes at room temperature. After washing, neurons were incubated in buffer containing antibodies against mouse ErbB4 (1:100) overnight at 4℃. These neurons were washed and incubated with an appropriate fluorescein isothiocyanate-conjugated secondary antibody for anti-ErbB4. Nuclei were labeled with 10 µM Hoechst dye. Images were visualized using a LSM 510 Meta system (Zeiss LSM 510 laser scanning microscope, Carl Zeiss).
Western blotting
Western blotting was performed as previously described [25]. Samples were resolved using sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were then transferred to nitrocellulose membranes followed by blocking with TBS that contained 5% BSA and 0.05% Tween 20 at room temperature for 1 hour. Membranes were then incubated with anti-ErbB4 (1:1,000, mouse, Santa Cruz Biotechnology) and anti-β-actin (rabbit, 1:5,000, Santa Cruz Biotechnology) antibodies at 4℃ overnight. After washing, blots were developed with horseradish peroxidase-conjugated secondary antibodies and enhanced using a chemiluminescence system (Amersham Pharmacia, California, CA, USA).
Statistical analysis
Data are presented as mean±SEM of three or more independent experiments. For multiple group comparisons, statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Bonferroni's post-hoc test. Student's paired t test was used for comparisons of means between two groups of cells in a single experiment. Values of P<0.05 were considered statistically significant.
Results
OGD induces neurotoxicity and overexpression of ErbB4 receptor in primary hippocampal neurons
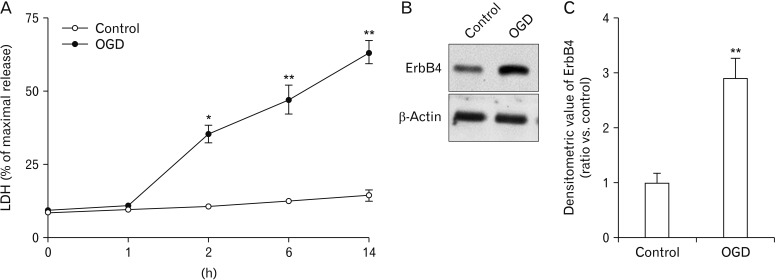
OGD is widely used to study mechanisms associated with global ischemic condition in vitro. First, effects of OGD-induced cytotoxicity in primary hippocampal neurons (DIV 14) were examined. Hippocampal neurons will die when oxygen and glucose are deprived of the medium [26]. This can be monitored by LDH that is produced and released into the medium. LDH assays showed that OGD resulted in a significant increase in hippocampal neuronal death. In particular, compared to the control group, OGD significantly increased cell death following exposure for more than 2 hours, although LDH release continued to increase in each subsequent time interval (0, 1, 2, 6, 14 hours) (Fig. 1A). Therefore, in all forthcoming experiments, cells were subjected to OGD for 2 hours. As shown in Fig. 1B and C, OGD for 2 hours induced ErbB4 receptor overexpression in primary hippocampal neurons.
Fig. 1. Effect of oxygen-glucose deprivation (OGD) on neurotoxicity and protein expression of ErbB4. (A) Neuron cell death was detected based on the activity of lactate dehydrogenase (LDH) following OGD or serum free medium for indicated time periods. Effects of OGD for 0, 1, 2, 6, and 14 hours on LDH release in cultured neuronal cells were determined (n=5, *P<0.05, **P<0.01). (B) Western blotting demonstrating that OGD affected ErbB4 protein expression levels. ErbB4 protein expression was significantly increased by OGD in primary hippocampal neurons. (C) Quantification of data in B. Densitometry values are shown as ratios relative to values of the control group (n=4, **P<0.01).
NRG1 attenuates apoptosis induced by OGD
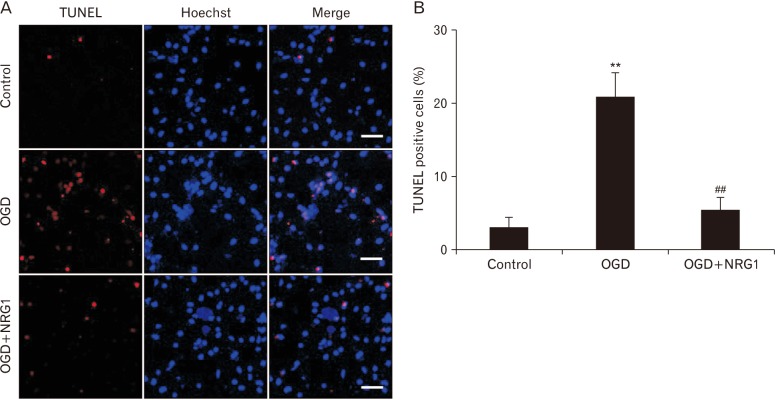
Next, whether NRG1 affected OGD-induced apoptosis in primary hippocampal neurons was examined using TUNEL staining. NRG1 reduced the proportion of apoptotic nuclei in primary hippocampal neurons induced by OGD (Fig. 2A, B). In control and OGD, percentages of apoptotic cells were 3.00%±1.53% and 21.03%±3.22% of total cells, respectively (n=6, P<0.01) (Fig. 2B). Treatment with 5 nM NRG1 for 2 hours significantly reduced the proportion of TUNEL-positive cells (OGD, 21.03%±3.22% vs. OGD+NRG1, 5.33%±1.86%, n=6; P<0.01) (Fig. 2B). The effect of NRG1 on the number of TUNEL-positive cells provided support that NRG1 could attenuate OGD-induced apoptosis in primary hippocampal neurons.
Fig. 2. Neuregulin-1 (NRG1) attenuates apoptosis induced by oxygen-glucose deprivation (OGD) in primary hippocampal neurons. (A) After 2 hours, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (red) was performed to determine the amount of apoptosis that occurred after OGD in primary hippocampal neurons treated with either vehicle or 5 nM NRG1. Hoechst staining (blue) was used as a counterstain. Scale bars=50 µm. (B) Numbers of TUNEL-positive cells (red) were quantified in 16 photographs from three independent experiments. The apoptotic neuronal cell ratio was expressed as a percent of TUNEL-positive cells to the total number of DAPI-positive cells (n=6, **P<0.01, ##P<0.01).
NRG1 pretreatment reduces OGD-induced overexpression of ErbB4 receptor in primary hippocampal neurons
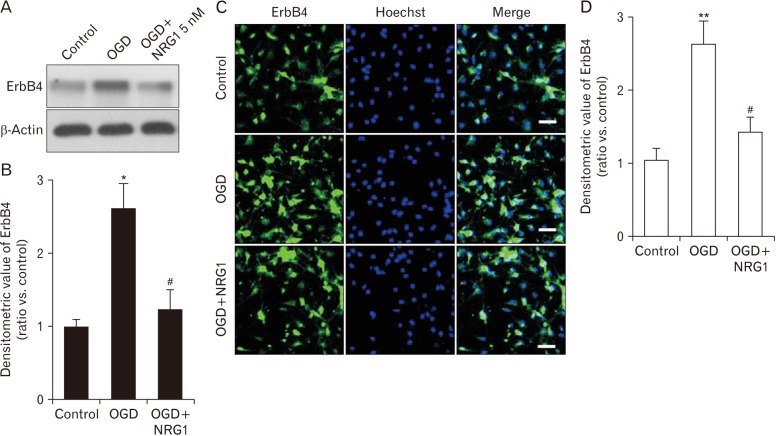
To investigate whether NRG1 could affect OGD-induced increase of ErbB4 receptor expression, cells were pretreated with 5 nM NRG1 followed by 2 hours of OGD. Western blotting was then performed to examine levels of ErbB4 in primary hippocampal neurons. Results confirm that pretreatment with 5 nM NRG1 for 2 hours attenuated the increase of ErbB4 expression induced by OGD (OGD, 2.61±0.34; OGD+NRG1, 1.24±0.26, n=5; P<0.05, P<0.05) (Fig. 3A, B). The immunoreactivity of ErbB4 was then measured via immunofluorescence in primary hippocampal neurons. To examine effects of NRG1 on neurons, cells were pretreated with 5 nM NRG1 and then subjected to OGD 15 minutes later. OGD for 2 hours significantly upregulated ErbB4 expression in comparison with the control (CON, 1.04±0.17; OGD, 2.63±0.31, n=6; P<0.01). Treatment with 5 nM NRG1 for 2 hours attenuated the increase in ErbB4 immunoreactivity induced by OGD (OGD, 3.63±0.31; OGD+NRG1, 1.43±0.21, n=6; P<0.05) (Fig. 3C, D). These results of immunoreactivity were consistent with those of Western blot assay.
Fig. 3. Neuregulin-1 (NRG1) inhibits oxygen-glucose deprivation (OGD)-induced overexpression of ErbB4 in primary hippocampal neurons. (A) NRG1 prevents the elevation of ErbB4 receptor in OGD-induced cultured hippocampal neurons. Neurons were incubated with or without OGD for 2 hours in the presence or absence of pretreatment with NRG1 (5 nM) at 15 minutes prior to OGD. (B) Quantification of data in A. Densitometry values are shown as ratios relative to values of the control group (n=5, *P<0.05, #P<0.05). (C) Expression levels of ErbB4 after 2 hours of incubation under control, OGD alone, or OGD with pretreatment with 5 nM NRG1 in primary hippocampal neurons by immunofluorescence. Cells were double-labeled with DAPI (blue) and anti-ErbB4 (green) and then analyzed by fluorescence microscopy. Scale bars=50 µm. (D) The fluorescence density of ErbB4 was measured in each group (n=6, **P<0.01, #P<0.05).
ErbB4 is involved in the effect of NRG1 on OGD-induced cell death
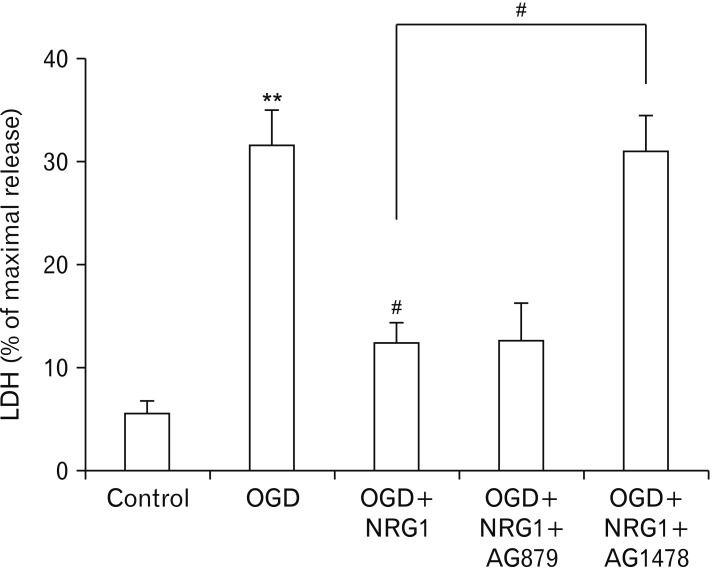
NRG1 can activate three ErbB kinases. However, only ErbB2 and ErbB4, but not ErbB3, are catalytically active [27]. To determine which ErbB was involved in the protective effect of NRG1, hippocampal neurons were treated with AG879 (5 µM) and AG1478 (5 µM), known inhibitors of ErbB2 and ErbB4, respectively [24].
However, the protective effect of NRG1was inhibited in neurons after pretreatment with AG1478, suggesting that NRG1 might protect hippocampal neurons by activating ErbB4 kinase. This effect was specific because NRG1 was able to protect neurons after pretreatment with AG879 (Fig. 4). Taken together, these results indicate a role for ErbB4, not ErbB2, in NRG1 neuroprotection.
Fig. 4. Neuregulin-1 (NRG1) attenuates primary hippocampal cell death induced by oxygen-glucose deprivation (OGD). NRG1 (5 nM) attenuates cell death induced by OGD in primary hippocampal neurons. After 2 hours, degrees of cell death were assessed by lactate dehydrogenase activity in the medium (n=6, **P<0.01, #P<0.05). LDH, lactate dehydrogenase.
Discussion
Results of this study demonstrate that ErbB4 is necessary for NRG1-mediated protection of primary hippocampal neurons in in vitro models. When ErbB4 was inhibited, the protective effect of NRG1 on cultured hippocampal neurons after OGD was attenuated. Moreover, expression levels of ErbB4 receptor following OGD were examined. Our results showed a dramatic increase in ErbB4 expression after OGD in cultured neurons. However, pretreatment with NRG1 significantly inhibited OGD-induced abnormal overexpression of ErbB4 receptor in primary hippocampal neurons.
NRG1 and their receptors, ErbB kinases, are continually expressed in mature brain. They are mostly likely to function in neurotransmission and neuroplasticity. NRG1 and ErbB4 are widely expressed in adult brains, including the hippocampus, the cerebellum, and the prefrontal cortex [9,13,28].
Previous studies have indicated that ErbB4 can activate synaptic transmission in the central nervous system. Overexpression of erbB4 enhances AMPA receptor-mediated excitatory postsynaptic currents in rodent CA1 hippocampal pyramidal cells evoked by stimulation of Schaffer collaterals from CA3 [10]. NRG1 enhances γ-aminobutyric acid release from interneurons in the prefrontal cortex [13]. Thus, the neural protective effect of NRG1 might be due to its regulation of synaptic plasticity. In addition, phosphoinositide 3-kinase/Akt signaling pathways might mediate the survival effect of NRG, a critical survival pathway in neurons and most cell types [29,30]. Moreover, global cerebral ischemia risk factors promote the production of reactive oxygen species. Oxidative stress can promote inflammation, activate apoptosis signaling pathway and disrupt synaptic activity. NRG1 could attenuate the release of free radicals [17] and protected hippocampal neuronal cells from OGD-induced stress.
On the other hand, high levels of ErbB4 might be pathogenic. Both reduced and increased ErbB4 levels have been associated with schizophrenia [31,32,33]. ErbB4 expression after closed head injury is elevated in neurons. It occurs before its elevation in activated microglia/macrophage [34]. Consistent with this notion is the observation that ErbB4 is upregulated in injured neurons and macrophages/microglia in the penumbra following middle cerebral artery occlusion [35]. Recently, it has been reported that protein levels of ErbB4 are drastically increased in human symptomatic epileptogenic tissues [36].
Several lines of evidence collectively suggest that NRG1 exerts a protective role in neurons against neurotoxic stimuli including ischemic insult and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine [21,37]. Previously, our reports have shown that NRG1 signaling plays a neuroprotective role against Swedish mutant and C-terminal fragments of amyloid precursor protein and amyloid beta-peptide Aβ1-42 [38,39,40]. We have also demonstrated that NRG1/ErbB4 can prevent Aβ1-42-induced impairment of long-term potentiation through the phosphoinositide 3-kinase pathway [41]. Taken together, our results suggest that NRG1/EbB4 signaling can protect from oxygen-glucose deprivation insult. Further study is needed to clarify whether pharmacologically targeting ErbB4 in vivo can reduce histological and behavioral abnormalities associated with cerebral ischemia.
Acknowledgements
This work was partly supported by grants (NRF-2019R1H1A2079060 awarded to R.-S. Woo and NRF-2016R1A2B4010574 and 2017R1D1A1B03028729 awarded to J.-H. Lee) of the National Research Foundation funded by the Ministry of Education, Science, and Technology, Republic of Korea.
Footnotes
- Conceptualization: RSW.
- Data acquisition: JYY, HBK, SYY.
- Data analysis or interpretation: HIY, TKB, DYS.
- Drafting of the manuscript: JYY, RSW.
- Critical revision of the manuscript: JHL, RSW.
- Approval of the final version of the manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Calle PA, Bogaert MG, Van Reempts JL, Buylaert WA. Neurological damage in a cardiopulmonary arrest model in the rat. J Pharmacol Methods. 1989;22:185–195. doi: 10.1016/0160-5402(89)90013-2. [DOI] [PubMed] [Google Scholar]
- 2.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 3.Kass IS, Lipton P. Protection of hippocampal slices from young rats against anoxic transmission damage is due to better maintenance of ATP. J Physiol. 1989;413:1–11. doi: 10.1113/jphysiol.1989.sp017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi T, Kuroda S, Tada M, Houkin K, Iwasaki Y, Abe H. Calcium-induced mitochondrial swelling and cytochrome c release in the brain: its biochemical characteristics and implication in ischemic neuronal injury. Brain Res. 2003;960:62–70. doi: 10.1016/s0006-8993(02)03767-8. [DOI] [PubMed] [Google Scholar]
- 5.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 6.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 7.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014;83:27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 9.Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 13.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, Lai C, Xiong WC, Gao TM, Mei L. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Akasofu S, Kosasa T, Kimura M, Kubota A. Protective effect of donepezil in a primary culture of rat cortical neurons exposed to oxygen-glucose deprivation. Eur J Pharmacol. 2003;472:57–63. doi: 10.1016/s0014-2999(03)01865-x. [DOI] [PubMed] [Google Scholar]
- 15.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2. Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 16.Erlich S, Goldshmit Y, Lupowitz Z, Pinkas-Kramarski R. ErbB-4 activation inhibits apoptosis in PC12 cells. Neuroscience. 2001;107:353–362. doi: 10.1016/s0306-4522(01)00350-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Yoo JY, Kim HB, Yoo HI, Song DY, Min SS, Baik TK, Woo RS. Neuregulin1 attenuates H2O2-induced reductions in EAAC1 protein levels and reduces H2O2-induced oxidative stress. Neurotox Res. 2019;35:401–409. doi: 10.1007/s12640-018-9965-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croslan DR, Schoell MC, Ford GD, Pulliam JV, Gates A, Clement CM, Harris AE, Ford BD. Neuroprotective effects of neuregulin-1 on B35 neuronal cells following ischemia. Brain Res. 2008;1210:39–47. doi: 10.1016/j.brainres.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan YF, Wu CY, Fang YY, Zeng YN, Luo ZY, Li SJ, Li XW, Zhu XH, Mei L, Gao TM. Neuregulin 1 protects against ischemic brain injury via ErbB4 receptors by increasing GABAergic transmission. Neuroscience. 2015;307:151–159. doi: 10.1016/j.neuroscience.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 20.Lu YM, Gao YP, Tao RR, Liao MH, Huang JY, Wu G, Han F, Li XM. Calpain-Dependent ErbB4 Cleavage Is Involved in Brain Ischemia-Induced Neuronal Death. Mol Neurobiol. 2016;53:2600–2609. doi: 10.1007/s12035-015-9275-2. [DOI] [PubMed] [Google Scholar]
- 21.Guo WP, Wang J, Li RX, Peng YW. Neuroprotective effects of neuregulin-1 in rat models of focal cerebral ischemia. Brain Res. 2006;1087:180–185. doi: 10.1016/j.brainres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Xu Z, Ford GD, Croslan DR, Cairobe T, Li Z, Ford BD. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Res. 2007;1184:277–283. doi: 10.1016/j.brainres.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Jiang J, Ford G, Ford BD. Neuregulin-1 is neuroprotective and attenuates inflammatory responses induced by ischemic stroke. Biochem Biophys Res Commun. 2004;322:440–446. doi: 10.1016/j.bbrc.2004.07.149. [DOI] [PubMed] [Google Scholar]
- 24.Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004;25:935–944. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Yu HN, Park WK, Nam KH, Song DY, Kim HS, Baik TK, Woo RS. Neuregulin 1 controls glutamate uptake by up-regulating excitatory amino acid carrier 1 (EAAC1) J Biol Chem. 2015;290:20233–20244. doi: 10.1074/jbc.M114.591867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes J, Vieira M, Carreto L, Santos MA, Duarte CB, Carvalho AL, Santos AE. In vitro ischemia triggers a transcriptional response to down-regulate synaptic proteins in hippocampal neurons. PLoS One. 2014;9:e99958. doi: 10.1371/journal.pone.0099958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 28.Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 30.Baik TK, Kim YJ, Kang SM, Song DY, Min SS, Woo RS. Blocking the phosphatidylinositol 3-kinase pathway inhibits neuregulin-1-mediated rescue of neurotoxicity induced by Aβ1-42. J Pharm Pharmacol. 2016;68:1021–1029. doi: 10.1111/jphp.12563. [DOI] [PubMed] [Google Scholar]
- 31.Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu RC, Gingrich JA, Small S, Moore H, Dwork AJ, Talmage DA, Role LW. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 33.Joshi D, Fullerton JM, Weickert CS. Elevated ErbB4 mRNA is related to interneuron deficit in prefrontal cortex in schizophrenia. J Psychiatr Res. 2014;53:125–132. doi: 10.1016/j.jpsychires.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Erlich S, Shohami E, Pinkas-Kramarski R. Closed head injury induces up-regulation of ErbB-4 receptor at the site of injury. Mol Cell Neurosci. 2000;16:597–608. doi: 10.1006/mcne.2000.0894. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Ford BD. Upregulation of erbB receptors in rat brain after middle cerebral arterial occlusion. Neurosci Lett. 2005;375:181–186. doi: 10.1016/j.neulet.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 36.Zhu JM, Li KX, Cao SX, Chen XJ, Shen CJ, Zhang Y, Geng HY, Chen BQ, Lian H, Zhang JM, Li XM. Increased NRG1-ErbB4 signaling in human symptomatic epilepsy. Sci Rep. 2017;7:141. doi: 10.1038/s41598-017-00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depboylu C, Rösler TW, de Andrade A, Oertel WH, Höglinger GU. Systemically administered neuregulin-1β1 rescues nigral dopaminergic neurons via the ErbB4 receptor tyrosine kinase in MPTP mouse models of Parkinson's disease. J Neurochem. 2015;133:590–597. doi: 10.1111/jnc.13026. [DOI] [PubMed] [Google Scholar]
- 38.Ryu J, Yu HN, Cho H, Kim HS, Baik TK, Lee SJ, Woo RS. Neuregulin-1 exerts protective effects against neurotoxicities induced by C-terminal fragments of APP via ErbB4 receptor. J Pharmacol Sci. 2012;119:73–81. doi: 10.1254/jphs.12057fp. [DOI] [PubMed] [Google Scholar]
- 39.Woo RS, Lee JH, Kim HS, Baek CH, Song DY, Suh YH, Baik TK. Neuregulin-1 protects against neurotoxicities induced by Swedish amyloid precursor protein via the ErbB4 receptor. Neuroscience. 2012;202:413–423. doi: 10.1016/j.neuroscience.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Ryu J, Hong BH, Kim YJ, Yang EJ, Choi M, Kim H, Ahn S, Baik TK, Woo RS, Kim HS. Neuregulin-1 attenuates cognitive function impairments in a transgenic mouse model of Alzheimer's disease. Cell Death Dis. 2016;7:e2117. doi: 10.1038/cddis.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Min SS, An J, Lee JH, Seol GH, Im JH, Kim HS, Baik TK, Woo RS. Neuregulin-1 prevents amyloid β-induced impairment of long-term potentiation in hippocampal slices via ErbB4. Neurosci Lett. 2011;505:6–9. doi: 10.1016/j.neulet.2011.05.246. [DOI] [PubMed] [Google Scholar]