Abstract
Chlamydomonas reinhardtii is a well-established model system for basic research questions ranging from photosynthesis and organelle biogenesis, to the biology of cilia and basal bodies, to channelrhodopsins and photoreceptors. More recently, Chlamydomonas has also been recognized as a suitable host for the production of high-value chemicals and high-value recombinant proteins. However, basic and applied research have suffered from the inefficient expression of nuclear transgenes. The combined efforts of the Chlamydomonas community over the past decades have provided insights into the mechanisms underlying this phenomenon and have resulted in mutant strains defective in some silencing mechanisms. Moreover, many insights have been gained into the parameters that affect nuclear transgene expression, like promoters, introns, codon usage, or terminators. Here I critically review these insights and try to integrate them into design suggestions for the construction of nuclear transgenes that are to be expressed at high levels.
Keywords: algal synthetic biology, Golden Gate cloning, modular cloning, algal biotechnology, transcriptional gene silencing, histone modifications
1. Chlamydomonas reinhardtii—A Versatile Model System
Chlamydomonas reinhardtii is a unicellular green microalga living in the soil and in the pelagic zone of lakes [1]. Chlamydomonas has emerged as a valuable model organism for basic research e.g., on photosynthesis and chloroplast biogenesis [2], the biology of cilia and basal bodies [3], the cell cycle [4], the plant heat stress response [5], or the circadian clock [6]. Moreover, Chlamydomonas has received much attention because of its ability to produce molecular hydrogen [7] and lipids [8], both with promise as biofuels. Important developments for major research fields in life sciences have their origin in basic Chlamydomonas research. Prominent examples are the insights into the structure and function of cilia for the understanding of human diseases [9], the recently made connection between photoreceptors and the quenching of excess excitation energy in photosynthesis [10], or the discovery of channelrhodopsins, which have founded the field of optogenetics [11]. Many molecular tools have been developed for Chlamydomonas and these have been comprehensively reviewed previously [8,12,13].
More recently, Chlamydomonas has been recognized as a host for the production of high-value chemicals and high-value recombinant proteins [14,15]. For the latter, the Chlamydomonas chloroplast is a suitable expression platform with yields reported to range between 0.5% and 5% of total soluble protein [16]. The disadvantage of the chloroplast as expression platform is that recombinant proteins need to be purified from whole-cell extracts and will not be glycosylated. Transgenes expressed in the nucleus, however, can be targeted for secretion, allowing recombinant proteins to be glycosylated and secreted into the medium, from where purification can be achieved more easily [17,18]. First reports comparing the efficiency of secretion signals and introducing tags with serine-proline repeats to improve secretion by enhanced glycosylation have been published [19,20]. However, the major limitation of nuclear transgene expression in Chlamydomonas has been the low level of expression achieved. Here, I provide an overview to the history of nuclear transformation and the pitfalls encountered regarding the expression of nuclear transgenes. I review approaches taken by the Chlamydomonas community to understand the mechanisms underlying inefficient nuclear transgene expression and to overcome this problem. Finally, I try to integrate the insights gained into design suggestions for the construction of nuclear transgenes that are to be expressed at high levels.
2. Nuclear Transformation of Chlamydomonas Is Robust and Easy
Nuclear transformation of Chlamydomonas was established in the late 1980s. This was demonstrated by the successful complementation of mutant lines defective in the genes encoding argininosuccinate lyase and nitrate reductase with the respective wild-type genes [21,22]. In both reports, DNA was delivered with a particle gun, leading to the stable integration of multiple transgene copies into the genome with rather low transformation efficiency (max. 25 transformants per µg DNA) and low transformation frequency (1 × 10−8 to 2 × 10−6). The particle gun was soon replaced by a protocol based on the agitation with glass beads, which results in the stable integration of fewer transgene copies with higher transformation efficiency (500 transformants per µg DNA) and frequency (~2 × 10−5) [23]. Eventually, electroporation was robustly developed to deliver transgene DNA into Chlamydomonas nuclei, with transformation efficiencies of up to ~1.9 × 105 transformants per µg DNA and a transformation frequency of 2.6 × 10−3 [24]. Protocols for both agitation with glass beads and electroporation require that cells lack cell walls, which is accomplished either by treating them with autolysin before transformation, or by using cell wall deficient mutants. The introduction of a square electric pulse-generating electroporator enabled electroporation also of walled Chlamydomonas cells with a transformation efficiency of up to ~4000 transformants per µg DNA and a transformation frequency of ~10−3 [25].
The first dominant selectable marker allowing direct transformation of any Chlamydomonas strain was the Chlamydomonas CRY1 gene, which encodes a mutated version of the ribosomal S14 protein and confers resistance to the translation inhibitors emetine and cryptopleurine [26]. Soon, other dominant selectable markers followed, including the ble gene from Streptoalloteichus hindustanus, conferring resistance to phleomycin [27,28]; the eubacterial aadA gene, conferring resistance to spectinomycin and streptomycin [29,30]; the aphVIII gene from Streptomyces rimosus, conferring resistance to paromomycin [31]; or the aph7″ gene from Streptomyces hygroscopicus, conferring resistance to hygromycin B [32]. Currently, the dominant selectable markers based on enzymatic activities (aph7″, aphVIII, and aadA) are the most widely used ones because they robustly allow for high transformation rates and do not have side effects like double strand breakages potentially induced by phleomycin.
With the exception of few hot and cold spots, the integration of transforming DNA was found to occur randomly [33]. Transforming DNA, like a resistance cassette, can be subject of endonucleolytic cleavage during transformation [30,33,34,35]. Moreover, its uptake by a cell may be accompanied by the uptake of DNA from lysed cells, which also can be cleaved by the endonuclease. The resulting mix of DNA fragments either is concatenated and inserted into one site, or the fragments are inserted at independent sites into the genome [29,33,35]. Frequently, deletions and inversions of genomic DNA flanking the insertion site of the transforming DNA occur [34,35,36,37]. It was proposed that large deletions occur more frequently when the transforming DNA is large, plasmid-derived, and delivered by agitation with glass beads as compared to when it is small, PCR-derived, and delivered by electroporation [36].
3. Mechanisms of Transcriptional Gene Silencing in Chlamydomonas
If a nuclear transgene is constantly under selection pressure, it will be expressed in Chlamydomonas. However, if the selection pressure is relieved, Chlamydomonas readily silences transgenes [29,38]. Moreover, transgenes may never be expressed, even if they are located next to the selection marker [39,40]. Unfortunately, in Chlamydomonas this is the rule rather than the exception and it is not known why transgene silencing is so efficient in this organism.
Transgene silencing in Chlamydomonas is caused by transcriptional or post-transcriptional mechanisms [29]. Post-transcriptional gene silencing usually is the result of inadequate transgene design, i.e., when no or too few introns have been inserted [41], the Chlamydomonas codon usage has not been respected [42], or when transgenes contain inverted repeats that give rise to double-stranded RNA [43,44,45,46]. Guidelines for proper transgene design are provided in Section 12 of this review. Transcriptional gene silencing is the main cause for transgene silencing in Chlamydomonas [38] and is largely mediated by protein factors that place specific histone modifications onto nucleosomes at the transgene loci to trigger the formation of a repressive chromatin structure—a mechanism that may have evolved to protect the genome from invading DNA [47,48,49,50].
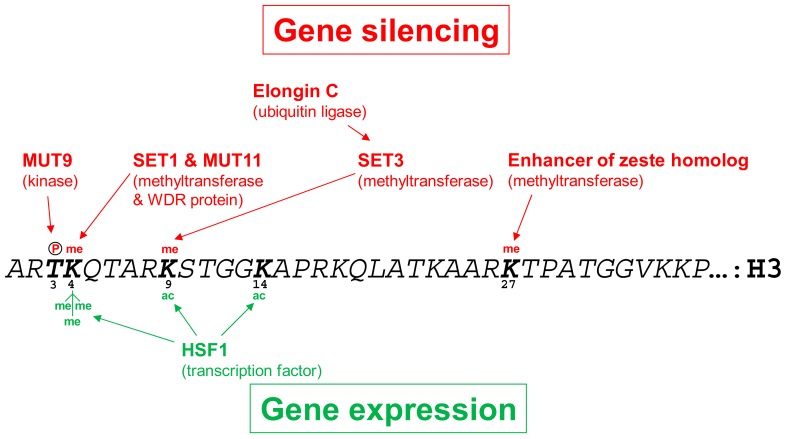
Nucleosomes consist of four different types of histones, H2A, H2B, H3, and H4, with each of them contributing two copies to form a histone octamer [51]. Especially histones H3 and H4 are subject of extensive post-translational modifications, largely occurring at their unstructured N-termini. These modifications constitute the histone code, which is read out by specific factors that recruit machineries for the remodeling of chromatin structure and thereby promote gene silencing or gene expression [52,53] (Figure 1). Histone marks that are known to occur on nucleosomes in promoter regions of silent genes in Chlamydomonas are histone H3 lysine 4 (H3K4) monomethylation [43,47,54], H3K9 monomethylation [42,43,45,49,55], H3K27 mono- and dimethylation [48], and H3T3 phosphorylation [47]. Histone marks generally found on nucleosomes at promoter regions of active genes are H3K4 trimethylation [48,49] and the acetylation of multiple lysine residues on histones H3 and H4 [42,43,45,48,49,56].
Figure 1.
Effect of modifications at the N-terminus of histone H3 on gene expression in Chlamydomonas. Shown in black is the amino acid sequence of the unstructured N-terminus of histone H3 from Chlamydomonas reinhardtii. Letters in bold designate residues known to be modified with consequences on gene expression, with those shown in red on top of the sequence promoting gene silencing and those in green below the sequence promoting gene expression. me—methylation; ac—acetylation; P—phosphorylation. The protein factors involved in setting the modifications are indicated. See main text for details.
A likely scenario is that histones in nucleosomes forming de novo on foreign DNA upon its integration into the genome are specifically modified to tag and control this locus [49]. H3K9me1 has been found to be strongly enriched in nucleosomes on transgenic Chlamydomonas promoters, while this modification was virtually absent in nucleosomes on the respective native promoters. Hence, H3K9me1 is a good candidate for a modification set when new nucleosome arrays form on foreign DNA. Since the source of DNA (plasmid- or PCR-derived) had no influence on transgene silencing, it appears likely that the chromatin modifiers tagging foreign DNA are directly associated with the machinery involved in their integration into the genome and/or with the repair of double strand breaks [49]. The maintenance of the initial repressive marks during the cell cycle could then also be achieved by other chromatin modifiers. A lack of the initial modifiers is expected to have no consequences for cells kept in the laboratory but would render them more susceptible to invading DNA in the wild. The lack of modifiers that maintain histone modifications is expected to have more severe consequences, as the expression of regular genes would get deregulated.
Several factors involved in the maintenance and perhaps also the initial setting of repressive histone modifications have been identified in Chlamydomonas (Figure 1). One of them is MUT11, a homolog of the human WDR5 protein, which presents H3K4 for methylation [57]. In the Chlamydomonas mut11 knock-out mutant, silenced single-copy transgenes and dispersed transposons get activated and the mutant is more sensitive to DNA damaging agents [58,59]. MUT11 was shown to interact with SET domain histone methyltransferases and RNAi-mediated suppression of SET1, a trithorax-like H3K4 histone methyltransferase, resulted in reduced levels of H3K4 monomethylation and the relief of silencing of a single-copy transgene and of TOC1 retrotransposons [54].
Another silencing factor is the SU(VAR)3-9-related protein SET3. Suppression of SET3 by RNAi released the transcriptional silencing of tandemly repeated transgenes and correlated with a partial reduction of levels of monomethylated lysine 9 at histone H3 (H3K9), while repressed, single-copy euchromatic transgenes and dispersed transposable elements were not reactivated [55].
The MUT9 kinase phosphorylates threonine 3 at histone H3 and residues at histone H2A and is required for long-term, heritable gene silencing. In the Chlamydomonas mut9 knock-out mutant, silenced single-copy transgenes and several transposons are derepressed and the mutant is more sensitive to DNA damaging agents. Reduced levels of H3T3 phosphorylation in mut9 correlate with reduced levels of H3K4 monomethylation and suggest crosstalk between these histone modifications [47,59].
The Chlamydomonas enhancer of zeste homolog (EZH) catalyzes the methylation of lysine 27 at histone H3. RNAi-mediated suppression of EZH in Chlamydomonas resulted in a global increase in levels of histone H3K4 trimethylation and H4 acetylation, both characteristic for active chromatin, thus leading to the release of retrotransposons and of silenced, tandemly repeated transgenes [48].
Finally, the silencing of the transgenic Rubisco small subunit 2 (RBCS2) promoter, driving the expression of an inverted repeat construct, was found to be associated with low levels of histone H3 acetylation and high levels of H3K9 monomethylation at the transgenic promoter [43]. Deletion of the Elongin C gene, which is a component of E3 ubiquitin ligase complexes, relieved silencing of the transgenic RBCS2 promoter. The activated promoter was characterized by high levels of H3 acetylation and low levels of H3K9 monomethylation [45].
4. Expression Strains Allow Efficient Nuclear Transgene Expression in Chlamydomonas
All the above-mentioned factors involved in the maintenance/setting of repressive chromatin marks have been identified in an endeavor to understand the mechanisms underlying transcriptional gene silencing in Chlamydomonas. Mutants lacking these factors have not yet been exploited for the generation of expression strains. Exactly for this purpose, Neupert et al. performed a UV mutagenesis screen, using as starting point a transformant with a weakly expressed transgenic copy of the Chlamydomonas CRY1 gene, conferring resistance only to low concentrations of emetine [50]. This strain (Elow47) was subjected to UV mutagenesis. The two resulting mutants, UVM4 and UVM11, were not only resistant to high concentrations of emetine, but also expressed another, newly transformed, heterologous transgene at high frequency to high levels [50]. Although it is not yet known which gene(s) is affected in the UVM4/11 strains, these data suggest that the affected factor(s) plays a role in the setting of negative chromatin marks at transgenic promoters. Importantly, the UVM4/11 expression strains have successfully been used for the high-level expression of various nuclear transgenes [20,41,42,60,61,62,63,64]. Therefore, they can be considered as an important breakthrough for basic research with Chlamydomonas, e.g., for the generation of overexpressing lines, and for promoting this organism as a workhorse for algal biotechnology.
Based on studies with Volvox carteri [65,66], Kong et al. suspected that a maintenance-type DNA methyltranferase (MET1) might be an additional silencing factor and indeed reported an increased frequency of high-level transgene expression in a Chlamydomonas met1 insertion mutant [67]. With the goal to generate a super-expression strain, they started out from the met1 mutant and subjected it to a similar UV mutagenesis screen as was used for generating the UVM4/11 strains [50]. Indeed, they obtained UV mutants that expressed transgenes with higher frequency and to higher levels than the UVM4 strain [68]. It will be interesting to know whether the same gene(s) is affected in UVM4/11 and the new expression strains.
5. Transcriptional Transgene Silencing Can Be Relieved to Some Extent by Specific Transcription Factors
All the factors described above mediate transcriptional transgene silencing in Chlamydomonas and, consequently, their inactivation resulted in the reactivation of transgenes (Figure 1). Interestingly, some factors can also actively counteract transgene silencing, at least to some extent. This is the case for the Chlamydomonas HSP70A promoter (abbreviated as A promoter). When transgene expression is driven directly by the A promoter, or when the A promoter is fused upstream of other Chlamydomonas promoters, like those from genes RBCS2 (abbreviated as R promoter), β2TUB, or HSP70B, transgene expressing transformants were found at high frequency [39,69]. While transformation of the ble gene driven by the AR fusion promoter (AR-ble) construct resulted in about two-fold higher numbers of zeocin-resistant transformants than transformation with R-ble, ble mRNA levels in pools of zeocin-resistant transformants were about the same. This apparent contradiction was resolved in experiments where AR-ble and R-ble constructs were co-transformed with the ARG7 gene and selection was on arginine prototrophy. Here, the fraction of co-transformants expressing AR-ble was more than three-fold higher than that expressing R-ble, indicating that the A promoter increased the fraction of expressing transgenes by counteracting transcriptional gene silencing [70].
In line with this conclusion was the observation that histones H3 and H4 in nucleosomes on transgenic R promoters contained higher acetylation levels, if the R promoter was preceded by an A promoter. Also, H3K4 trimethylation levels were higher, while H3K9 monomethylation was reduced [49]. While levels of H3K4 trimethylation at transgenic R promoters preceded by an A promoter were comparable to those at the native R promoter, levels of H3/4 acetylation were still below and levels of H3K9 monomethylation still far above those detected at the native R promoter. Hence, the A promoter alleviated transgene silencing, but did not fully overcome it [49].
Two regions within the A promoter were mapped that independently counteract R-ble transgene silencing, and the responsible cis-acting motifs were identified as heat shock element 1, TATA-box, and heat shock element 4 [49,70,71]. DNase I hypersensitive sites were detected on both motifs under ambient conditions, indicating the constitutive binding of a trans-acting factor [72]. This trans-acting factor turned out to be heat shock factor 1 (HSF1), as the inducible depletion of HSF1 relieved the activating effect of the A promoter on the R promoter in an AR-ble transgene [49] (Figure 1). HSF1 is the only canonical HSF of the two HSFs encoded by the Chlamydomonas genome and, since it forms trimers constitutively, HSF1 can potentially bind heat shock elements also under non-stress conditions [73]. Indeed, HSF1 was found to constitutively occupy the A promoter and was proposed to organize a scaffold, presumably containing mediator, TFIID, A, H, and E, that serve in recruiting RNA polymerase II to transcriptional start sites at the downstream promoter [49,56]. This scenario would explain why the spatial setting between A and R promoter is crucial for the activating effect of A [49,70,71]. Interestingly, upon the inducible depletion of HSF1, histone H4 acetylation at the R promoter in an AR-ble transgene declined with much slower kinetics than HSF1 occupancy and ble transcript abundance [49]. Hence, active chromatin marks like H4 acetylation per se are insufficient to promote promoter activity, it is the active recruitment of RNA polymerase that makes the difference.
6. Nucleosome Positioning and the Strength of Transcriptional Activators Might Affect Promoter Activity in Different Transgene Contexts
High-level transgene expression requires promoters that work robustly with all kinds of transgenes. In many organisms this is achieved with viral promoters that have evolved to overcome cellular constraints on the expression of foreign genes. Unfortunately, there are no reports on viruses infecting Chlamydomonas, perhaps because of the very efficient mechanisms for the silencing of foreign DNA sequences described above. Viral promoters commonly used in other organisms were at most only weakly active in Chlamydomonas [27,40,74,75,76,77].
Therefore, researchers went for native Chlamydomonas promoters driving genes whose gene products are known to accumulate at high levels. The outcome of this approach was rather heterogenous and an ill-understood dependence on the respective transgene construct was often observed. For example, a genomic copy of the α-tubulin gene was barely expressed in transgenic Chlamydomonas when it was driven by its own promoter, but strongly expressed when it was driven by the R promoter [78]. The R promoter was also functional in the context of the ble gene, even in the absence of selective pressure [27,28,70]. However, the R promoter was ineffective when it was supposed to drive expression of a genomic copy of the HSP70B gene, as was the native HSP70B promoter or the β2TUB promoter, while the A promoter worked well [39]. But the β2TUB promoter efficiently drove expression of the native arylsulfatase gene [79].
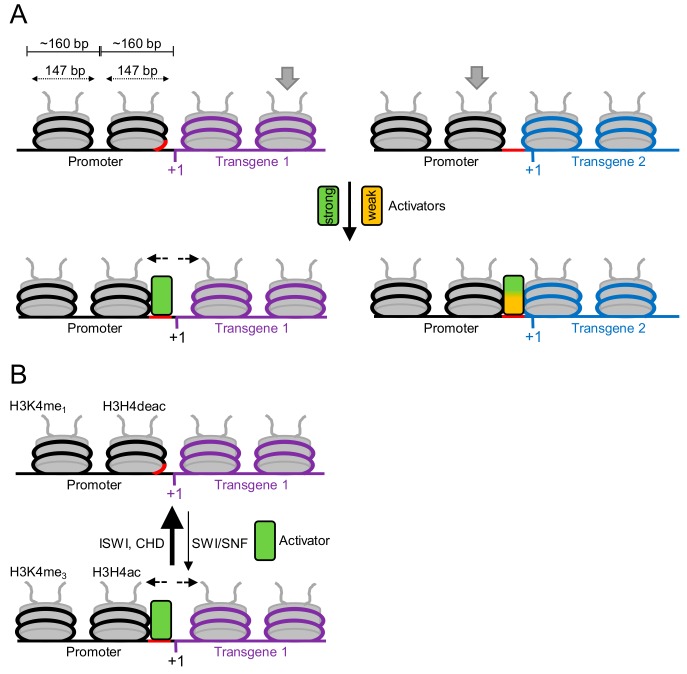
We learn that in Chlamydomonas the native promoter activity is not necessarily maintained in a transgene setting. This can be explained by the accessibility of cis-regulatory promoter sequences within the nucleosome array that has formed on the transgenic DNA (Figure 2). The covering of cis-regulatory sequences by nucleosomes may render them inaccessible to trans-activators and therefore results in an inactive promoter [80].
Figure 2.
Hypothetical model explaining the different performances of transgenic promoters in Chlamydomonas. Shown is a nucleosome array formed on the promoter–transgene junction in the context of two different transgenes (purple and blue). (A) The positions of the nucleosomes in the array are influenced by transgene sequences such that cis-regulatory sequences (red) are inaccessible because covered by a nucleosome (transgene 1, purple), or accessible because located in the linking DNA (transgene 2, blue). Occluded cis-regulatory sequences can still be recognized by strong activators (green) that can alter nucleosome positions by themselves or by recruiting chromatin remodelers of the SWI/SNF-type. Weak activators (orange) can only bind to accessible cis-regulatory sequences in linker DNA. (B) Chromatin at transgene loci in Chlamydomonas is characterized by low levels of histone H3/4 acetylation and high levels of histone H3 monomethylation at lysine 4 (H3K4me1). Deacetylated nucleosomes are recognized by ISWI- and CHD-type of chromatin remodelers that consume ATP to evenly distribute nucleosomes and thereby occlude cis-regulatory sequences. In contrast, acetylated histones recruit SWI/SNF-type chromatin remodelers that consume ATP to slide away nucleosomes and expose cis-regulatory sequences. This allows access for activators (green). Modified from [80] and [81].
In the chromatin regions containing the Chlamydomonas A and R promoters, the nucleosome repeat length is ~160 bp [72]. With 147 bp of DNA wrapped around each nucleosome, only ~13 bp of linker DNA remain, i.e., the likelihood that cis-regulatory sequences at Chlamydomonas promoters are occluded by nucleosomes is high (Figure 2A). Different DNA sequences have different affinities for the histone octamer because the free energy required to bend the DNA sharply around the histone octamer depends on the sequence [82]. Moreover, N6-methyldeoxyadenosine (6mA) modifications were found to be enriched in linker DNA at transcriptionally active genes in Chlamydomonas and have been proposed to contribute to the precise positioning of nucleosomes [83]. Hence, if sequences and/or 6mA sites in the transgene determine nucleosome positioning, this could lead to unfavorable nucleosome positions on the transgene-driving promoter that render cis-regulatory sequences inaccessible (Figure 2A, top left). If sequences and/or 6mA sites in the promoter determine nucleosome positioning, cis-regulatory sequences are accessible and the promoter is active, independent of the transgene context (Figure 2A, top right).
A promoter may become independent of unfavorable nucleosome positions (and therefore of the transgene context) if it is served by a ‘strong’ trans-activator that can get access to cis-regulatory sequences even if they are covered by nucleosomes (Figure 2A bottom and Figure 2B). This is most likely achieved by the recruitment of chromatin remodeling factors of the SWI/SNF type that consume ATP to slide away or eject nucleosomes [52,80,84]. This activity is enhanced by acetylated histone H3. SWI/SNF remodelers have the ISWI/CHD-type of remodelers as counterplayers, which consume ATP to re-establish a dense nucleosome array, thereby promoting the occlusion of cis-regulatory sequences and thus gene silencing. The activity of these remodelers is enhanced by unmodified histone tails. Therefore, the low levels of histone acetylation frequently observed at transgenic promoters in Chlamydomonas [43,49] points to the occlusion of cis-regulatory promoter sequences by ISWI/CHD-like activities as one mechanism underlying transgene silencing in this organism.
Even for transgenes equipped with an efficient promoter, high variation in transgene expression levels is observed between different transformants generated with the same transgene [28,39]. This phenomenon is also referred to as position effect, as transgene expression is considered to be affected by the chromosomal integration site [85]. Position effects often make it necessary to screen dozens of transformants for a few with high expression levels. Moreover, they make it necessary to pool hundreds of transformants to make comparisons of promoter strengths meaningful [49,71]. Position effects were claimed to be overcome in the UVM4/11 strains [50], but this view was challenged previously [63]. A position effect was proposed to be due to the distance in 3D nuclear space that a transgenic promoter has to a nuclear region (‘factory’) containing the appropriate factors necessary for the transcription of that particular promoter [86]. This idea is attractive, but it does not explain why two transgenes harboring the same promoter and placed next to each other on the same piece of transgenic DNA are expressed at very different levels (our unpublished observation)—these promoters would have about the same distance to the next ‘factory’. Hence, more mechanisms must be at work that cause position effects. For example, nucleosomes formed de novo on the transgenic DNA might affect the accessibility of cis-regulatory sequences more at one transgene than at the other. Furthermore, if the initial setting of repressive chromatin marks on nucleosomes formed on the transgenic DNA upon its integration into the genome is a stochastic process, it might affect one transgene promoter more than another.
7. Tricks to Surmount Poor Nuclear Transgene Expression
As mentioned above, transgenes do get expressed in Chlamydomonas if selection pressure is maintained. This circumstance was exploited by using the foot and mouth disease virus (FMDV) 2A peptide [87,88,89]. The 2A peptide consists of 19 to 39 amino acids that mediate ribosome skipping during translation to give rise to two proteins originating from a single ORF. Most of the 2A peptide sequence remains fused to the C-terminus of the first protein product, whereas the following protein contains only one amino acid of the 2A peptide at its N-terminus (this is always a proline). Rasala et al. fused the ORF coding for the Bleomycin protein (Ble) in frame with the 2A peptide and the protein of interest. Since resistance to phleomcyin is based on the stoichiometric binding of Ble to the drug, high level Ble expression is required. This in turn also leads to high-level expression of the protein encoded by the second ORF. The first protein product on the common ORF must not necessarily encode a protein conferring resistance, it only needs to be well expressed. This, for example, is the case for the IFT25 protein when its gene contains all three native introns [76]. The 2A peptide-based system was used to co-express several proteins at high levels, including fluorescent proteins targeted to various cellular subcompartments, a secreted fungal xylanase, mCerulean-tagged α-tubulin, the Cpf1 endonuclease, squalene synthase, RBCS2, and FKB12 [67,76,87,88,89,90,91,92,93].
A small disadvantage of this system is that the efficiency of ribosome skipping varies for unknown reasons between transformants expressing different fusion proteins and even between different transformants expressing the same fusion protein. Therefore, unpredictable amounts of non-processed fusion protein accumulate in addition to the processed one [67,76,87,88,91,92]. Although the efficiency of ribosome skipping increased when the extended 2A peptide with 39 amino acids was used instead of the minimal 19-amino acids version, residual fusion protein was always detected [76,89]. The latter might give misleading results in localization studies, e.g., when the first ORF encodes Ble, which is targeted to the nucleus [94].
The use of bicistronic transcripts is an approach resembling the ribosome skipping approach but avoiding the accumulation of fusion proteins. Here, the ORF of the gene of interest (GOI) is placed on the same transcript upstream of the ORF encoding the APHVIII selection marker [95]. The stop codon of the former is separated by four nucleotides from the start codon of the latter (GOI-TAGccatATG-APHVIII). This setup leads to regular translation termination of the first ORF, followed by translation reinitiation at the close-by start codon of the second ORF [95]. The efficiency of this post-termination reinitiation is low, which is why it only works with the APHVIII marker that obviously confers resistance to paromomycin at low expression levels. With this system, paromomycin-resistant transformants accumulate the product of the GOI at high frequency to high levels, therefore reducing screening efforts.
8. Determinants of Nuclear Transgene Expression—Promoters
As outlined above, the activity of a Chlamydomonas promoter in a transgenic context may vary strongly with the transgene to be expressed. Chlamydomonas promoters that have robustly enabled constitutive high-level expression of several different transgenes and therefore must be served by strong trans-activators are the AR fusion promoter and the PSAD promoter [39,96]. While HSF1 is the strong trans-activator of the AR promoter [49], the identity of the trans-activator serving the PSAD promoter is not known. Although both promoters routinely showed strong activity, the PSAD promoter sometimes performed better than the AR promoter [62,97], sometimes no difference was observed between the two promoters [41,95], and sometimes the AR promoter outperformed the PSAD promoter [91,95,98]. These ambivalent results are likely due to ill-defined versions of the AR promoter used, since the stimulating effect by the A promoter depends on its length (it should comprise at least 467 bp upstream of the start codon) and its spatial setting versus the R promoter [49,70,71]. Moreover, some of these comparisons were done with an AR promoter harboring the first RBCS2 intron close to the translational start site, which contains an enhancer sequence that would have stimulated also the PSAD promoter [41] (see Section 10). Finally, it is likely that the performance also of these promoters depends on the transgene context.
Several studies have compared the activities of other constitutive Chlamydomonas promoters with those of the AR and PSAD promoters as benchmarks. Again, these comparisons often used setups that introduced additional parameters, like different numbers/types of introns, different terminators, or ill-defined versions of the AR promoter, and often were limited to one or few reporters. For example, the AR promoter fusion employed by Lauersen et al. contains 20 additional nucleotides between both promoters when compared with the optimal fusion [70,71,99], and the A promoter employed lacks heat shock element 4, which increased expression of the ble transgene three-fold [49]. It is likely that both differences impair the fusion promoter’s performance. Hence, the outcomes of studies comparing promoter activities should be taken with caution. Nevertheless, the constitutive promoters analyzed displayed strong activities in the context of certain transgenes and are promising, especially to avoid repetitions of the AR and PSAD promoters in constructs harboring multiple transgenes. These promoters were the actin promoter [97], promoters RPL23, RPL35a, and FDX1 [91], the ARG7 promoter [100], and the IFT25 promoter [76]. The latter could not drive expression of a GFP reporter gene to detectable levels but drove expression of its own gene (including all native introns) to very high levels and did so, too, when various ORFs were translationally fused downstream.
Several Chlamydomonas promoters have been reported to effectively drive the conditional expression of transgenes. The conditional expression of transgenes is desirable when the gene product creates a burden for cellular physiology. Conditional promoters are those from genes CAH1 and CAH4, which are activated under low CO2 concentrations (air) in the light [101,102]; the NIT1 promoter, which is inactive in the presence of ammonium in the medium and gets activated upon the exchange of ammonium by nitrate [103,104]; heat shock promoters like that of the HSP70A gene, which gets strongly induced upon a shift from 23 °C to 40 °C [39,89]; the FEA1 promoter, which is induced by the depletion of iron from the medium by medium exchange, growth, or the addition of the iron chelator deferroxamine [92,93]; the CYC6 promoter, which is induced by the addition of nickel or cobalt to the medium, or by the depletion of copper from the medium by medium exchange, growth, or the addition of the copper chelator TETA [105,106,107]; the METE promoter, which is repressed in the presence of vitamin B12 in the medium and activated in its absence [108]. Inducible expression of transgenes also can be achieved by including the riboswitch-containing THI4 5′ UTR between a constitutive promoter and the ORF of the transgene. Through riboswitch-mediated alternative splicing, the transgene’s ORF is translated only when thiamine (vitamin B1) is absent from the growth medium [109]. Note that the inducing cues are often associated with changes in cellular physiology that need to be accounted for. Moreover, since most Chlamydomonas ‘wild type’ strains cannot use nitrate as nitrogen source, the NIT1 promoter cannot be used in these strains.
Like constitutive promoters, conditional promoters suffer from position effects. In our hands, position effects not only affect the maximum expression levels achieved for a transgene after induction, but also affect promoter tightness. For example, transgenes driven by the NIT1 promoter tend to be expressed to some level even in the presence of ammonium [110]. Hence, extensive screening is necessary to identify transformants that do not express the transgene under repressive conditions but do so at high levels after induction. What might be causing the position-dependent leaky expression of conditional promoters? One possibility is that promoter activity is regulated at the level of chromatin structure, such that chromatin structure is remodeled by factors activated by the inducing signal which render cis-regulatory sequences accessible for general transcription factors. Such regulation might be impaired if the nucleosome array formed on the conditional promoters is dictated by DNA sequences surrounding the transgene integration site. Another possibility is that the transgenic conditional promoter gets located so close in the 3D nuclear space to a proper ‘factory‘ [86] that regulatory constraints are overridden.
9. Determinants of Nuclear Transgene Expression—Codon Usage
The first synthetic gene adopted to the codon usage of Chlamydomonas nuclear genes encoded GFP and was synthesized already in 1999. The codon-optimized GFP gene allowed for the first localization study employing a fluorescent protein in Chlamydomonas [94]. Codon optimization was stimulated by the finding that the Streptoalloteichus hindustanus ble gene with GC-content and codon usage similar to that of Chlamydomonas was stably expressed in Chlamydomonas [27,28], while the aadA gene with unbiased codon usage was poorly expressed and expression was unstable [29,38]. While the mechanisms behind this finding remained unresolved, it promoted the synthesis of more reporter genes with Chlamydomonas codon usage, including Renilla luciferase [111], Gaussia luciferase [74,112], NanoLuc [62], and several other fluorescent proteins [20,42,64,88,95]. Since gene synthesis has become cheap, the synthesis of all kinds of foreign genes with optimal Chlamydomonas codon usage has become routine [30,41,60,67,76,87,113,114,115].
In a systematic study to elucidate the mechanisms responsible for the improved expression of codon-optimized transgenes in Chlamydomonas, Barahimipour et al. employed four YFP-encoding sequences with different codon usage and GC-content, but driven by the same promoter and terminator [42]. It turned out that high-level YFP expression depended on an optimal codon usage, while a high GC-content itself was not enough. YFP protein levels correlated with transcript levels and, interestingly, also with active chromatin marks on nucleosomes at the promoter (higher levels of H4 acetylation and lower levels of H3K9 monomethylation). These findings indicate that transcripts with improper codon usage are less translated and therefore more prone to degradation. Degraded transcripts might then give rise to small RNAs that affect the chromatin state at the transgene locus via post-transcriptional gene silencing [116]. Definitely, codon optimization is crucial for high-level expression of nuclear transgenes in Chlamydomonas [117].
10. Determinants of Nuclear Transgene Expression—Introns
Introns can strongly increase gene expression in eukaryotes. This is achieved by two mechanisms. First, introns may contain enhancer sequences that increase rates of transcription initiation. Second, the mere presence of introns can increase transcript levels by a process termed intron mediated enhancement (IME). The mechanism proposed for IME is that efficient transcript elongation depends on the interaction of RNA polymerase II with the spliceosome. If this interaction is not provided, RNA polymerase aborts transcription and the immature transcript is degraded. By this mechanism, transcripts arising from real genes can be distinguished from those originating from the intergenic space or from invading DNA, both lacking introns [118]. With 7.3 introns per gene, Chlamydomonas genes are intron-rich relative to other unicellular eukaryotes and land plants; 92% of the genes contain introns with an average size of 373 bp, while the average size of exons is 190 bp [119]. Hence, primary transcripts in Chlamydomonas undergo intensive processing and there is evidence that enhancers and IME both contribute to gene expression in Chlamydomonas.
Clear evidence for the presence of an enhancer sequence in the first intron of the RBCS2 gene was provided by the demonstration that this intron leads to higher transgene expression when placed upstream of the transgene driving R promoter in any orientation [28]. This enhancer appears not to be specific for the R promoter, because it enhanced expression of the Pogostemon cablin patchoulol synthase gene to a similar extent when it was driven by the AR promoter or the PSAD promoter [41]. Moreover, the first RBCS2 intron also enhanced expression of the Renilla luciferase gene driven by the CYC6 promoter when it was placed upstream of the promoter in 3′ to 5′ orientation [106]. Evidence for IME in Chlamydomonas comes from the finding that the second RBCS2 intron also increased transgene expression although it lacks enhancer activity—it did not increase transgene expression when placed upstream of the R promoter [18,28,41,120].
Whether mediated by enhancers or IME, there is no doubt that introns dramatically increase transgene expression in Chlamydomonas, as the presence of introns has increased the expression of various transgenes when compared with transgene variants lacking these introns [28,32,41,62,70,76,95,106,120]. This was true also for transgenes driven by the PSAD promoter [41], although this promoter was proposed not to depend on introns [96]. In systematic studies, Baier et al. and Eichler-Stahlberg et al. inserted RBCS2 introns into various codon-optimized transgenes and could show that transgene expression is enhanced by the regular insertion of introns (leaving exon sequences of <500 bp). Highest expression was achieved when two, or better, all three RBCS2 introns were inserted in their native order [18,41]. These findings clearly demonstrate that intron engineering into transgenes is indispensable for high-level transgene expression in Chlamydomonas.
11. Determinants of Nuclear Transgene Expression—Terminators
Only few studies have investigated the role of gene terminator sequences for transgene expression in Chlamydomonas. However, these provide evidence that terminators have a strong impact: Kumar et al. detected up to ~40-fold differences in LUC reporter activity when the LUC gene was driven by the same promoter but different terminators. Here, the terminator of the PSAD gene performed best when compared to those of the β2TUB and CCP1 genes [97]. Similarly, Lopez-Pas et al. found strong variation in LUC reporter gene expression depending on the terminator used [91]. Among the four terminators tested, those from genes FDX1 and RPL23 performed best. Given that the RPL23 terminator contains an intron while the FDX1 terminator does not, the strong performance of the FDX1 terminator is remarkable. The lowest reporter activity was observed with the frequently used RBCS2 terminator. This might be due to a promoter activity within the RBCS2 3′ UTR transcribing in antisense orientation and potentially giving rise to dsRNA that might initiate post-transcriptional gene silencing [31].
12. Design Suggestions for Nuclear Transgenes
For the construction of nuclear transgenes, I strongly recommend using the Chlamydomonas Modular Cloning (MoClo) kit [62]. This kit is available at the Chlamydomonas resource center and comprises 119 standardized genetic parts, i.e., promoters, coding sequences, tags, untranslated regions, etc. (https://www.chlamycollection.org/). These parts adhere to the syntax for Golden Gate-based modular cloning established for plant synthetic biology [121]. Using the Type IIS restriction enzyme BsaI and ligase, multiple genetic parts (level 0) can directionally be assembled into transcription units (level 1) in a single reaction. Using the Type IIS restriction enzyme BpiI and DNA ligase, several transcription units can then simultaneously and directionally be assembled into multigene constructs encoding entire metabolic pathways (level 2) [122].
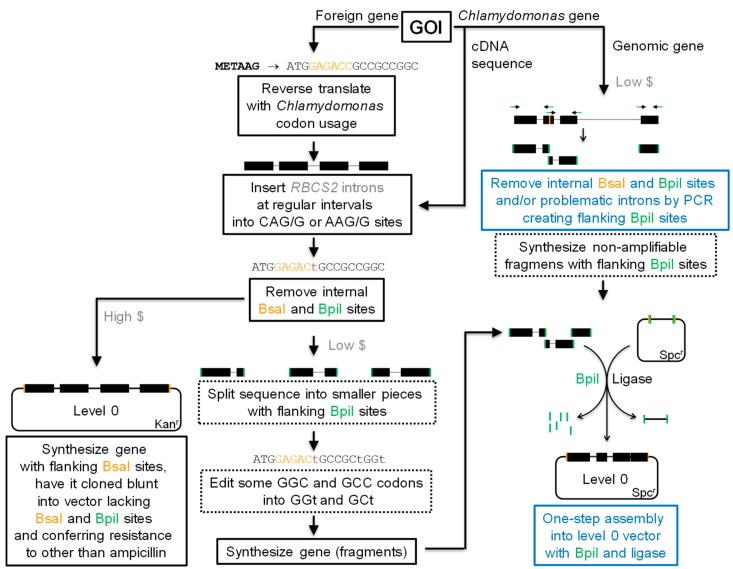
Hence, for the construction of nuclear transgenes via the MoClo strategy, only a level 0 genetic part needs to be generated that contains the ORF of the GOI (Figure 3). If the GOI is a foreign gene, it needs to be synthesized de novo adopting the Chlamydomonas codon usage. For this, the amino acid sequence is reverse-translated using, e.g., the tool offered at http://www.bioinformatics.org/sms2/rev_trans.html [123] based on the Chlamydomonas codon usage provided by the Kazusa DNA Research Institute at https://www.kazusa.or.jp/codon/cgi-bin/showcodon.cgi?species=3055. Next, introns need to be inserted and internal BsaI and BpI recognition sites removed from the resulting sequence. We routinely insert the three RBCS2 introns with regular spacing at CAG/G or AAG/G sequences (the slash is where the intron is). Although these flanking sequences differ from those in the RBCS2 gene, they do correspond to the canonical sequences around Chlamydomonas introns [124] and match those determined experimentally to support efficient intron splicing [41]. Finally, it appears advisable to eliminate cryptic intron splice sites and to increase the folding energy of mRNA secondary structures around the start codon by introducing synonymous mutations into the designed sequence [117]. Commercial companies offer de novo synthesis and cloning of the designed sequence. In this case, the sequence should be flanked with BsaI recognition sites generating the four nucleotide overhangs assigned to that particular part, which depends on where in the final transcriptional unit it is positioned [121]. Moreover, it is required that the destination vector lacks internal BsaI and BpiI sites and confers resistance to an antibiotic other than ampicillin. These design steps can be performed conveniently with the recently released Intronserter software [120].
Figure 3.
Workflow for the construction of nuclear transgenes as level 0 parts for Modular Cloning. Black text boxes indicate in silico steps or synthesis steps that are carried out by commercial companies. Blue text boxes indicate experimental steps that need to be conducted in the own laboratory and dotted text boxes indicate optional steps. Solid black boxes represent coding sequences of the gene of interest (GOI) and thin grey lines represent introns. Green and orange lines indicate recognition sites for Type IIS restriction enzymes BpiI and BsaI, respectively. Kanr—kanamycin resistance; Spcr—spectinomycin resistance. See main text for details.
Companies offering gene synthesis at low cost (i.e., as linear, double-stranded, and non-clonal gene blocks) will complain about regions with very high GC content and repeats, which typically occur when using the optimal Chlamydomonas codon usage. This problem is somewhat alleviated with the three inserted RBCS2 introns, which have a lower than average GC content (58% GC for the first and second intron, 61% GC for the third intron). The problem with repeats, e.g., resulting from the insertion of several copies of the same RBCS2 intron, can be solved by splitting the GOI into several shorter pieces. In this case, each piece is synthesized independently with flanking BpiI recognition sites that generate unique four-nucleotide overhangs. The problem with very GC-rich regions can only be solved by editing several preferred GC-rich codons into less preferred ones containing A and T. In our hands, the editing of the third nucleotide of codons for Ala (GCC) and Gly (GGC) from C to T was tolerated, presumably because these codons are more common in Chlamydomonas genes than codons for other amino acids with T at the third position. Nevertheless, if maximum gene expression is the goal, this editing is not recommendable [42] and more expensive synthesis platforms should be consulted. The synthesized pieces of the GOI can be assembled into a level 0 vector in a single reaction step with BpiI and DNA ligase.
If the GOI is a Chlamydomonas gene with too many, too large, or too complex introns, it can be synthesized de novo based on the cDNA sequence with RBCS2 introns inserted at regular intervals [41] (Figure 3). Internal BsaI and BpiI recognition sites again need to be removed. If the GOI is a Chlamydomonas gene with no or only few problematic introns (very long or with highly repetitive sequences), the gene can be amplified by PCR. If the sequence contains internal BsaI and BpiI recognition sites, these need to be removed for domestication [121]. This is achieved by introducing silent point mutations via primers covering the respective site that are flanked by BpiI recognition sites. This strategy can also be employed to remove problematic introns. Non-amplifiable pieces can be synthesized de novo as gene blocks, also with flanking BpiI sites (Figure 3). Using a MoClo destination vector for the particular part, several gene fragments can then be assembled directionally in a single reaction to yield the desired level 0 module [122].
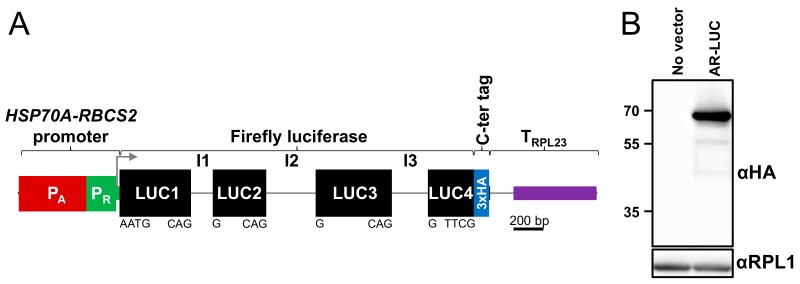
In our hands, nuclear transgenes designed with this workflow routinely give rise to high-level expression in Chlamydomonas. An example is shown in Figure 4. Here we have followed the left side of the workflow given in Figure 3 to generate a level 0 module encoding firefly luciferase harboring all three RBCS2 introns. The level 1 construct consists of the optimal AR promoter, the luciferase ORF, a 3xHA tag, and the intron-containing RPL23 terminator; the latter two are part of the current MoClo kit. This construct expressed the ~68 kDa foreign protein to the highest levels we have obtained until now.
Figure 4.
Example for a foreign gene encoding firefly luciferase that has been brought into the MoClo context for nuclear expression. (A) Level 1 construct. The red box depicts the HSP70A promoter harboring 467 nt upstream of the start codon in optimal spacing toward the RBCS2 promoter (green box) harboring 217 nt upstream of its start codon. Black boxes represent the codon-optimized coding sequences of firefly luciferase (LUC) interrupted by RBCS2 introns 1 to 3 (I1-I3, grey lines). Letters below are the respective flanking sequences. The blue box stands for a 3x hemagglutinin (HA) tag. The purple box indicates the 3′ UTR of the RPL23 gene, which contains an intron (grey line). The elements are drawn to scale. (B) Immunoblot analysis of the untransformed UVM4 recipient strain (no vector) and a UVM4 transformant harboring the AR-LUC level 1 construct. The expected mass of LUC-3xHA is 67.8 kDa. Figure courtesy of Miriam Schulz-Raffelt.
13. Outlook
The combined efforts of the Chlamydomonas community have dramatically improved the capacity of Chlamydomonas to express nuclear transgenes at high levels. This has paved the path for this organism as a chassis for synthetic biology and biotechnology [14]. With the tools available, a properly designed transgene in our hands can achieve expression levels reaching those of highly expressed native Chlamydomonas genes. However, expression levels achieved from nuclear transgenes are still below those achieved by chloroplast expression or by other eukaryotic expression platforms like Pichia pastoris or insect cells using the baculovirus system. Hence, further improvements must be achieved by identifying and eliminating more factors mediating transgene silencing at the transcriptional and post-transcriptional levels to generate expression strains even better than UVM4/11. For example, we need to identify the methyltransferase(s) responsible for setting the H3K9me1 mark on nucleosomes forming on foreign DNA [49]. To this end, CRISPR/Cas mediated genome editing will be of great value [125,126].
Moreover, we need to further investigate the roles of regulatory sequences like promoters, enhancers, introns, and terminators and systematically analyze their performance in the context of various transgenes. To this end, the MoClo system is ideally suited, not only because it allows rapid cycles of construction and testing, but also because it promotes the exchange of defined, standardized genetic parts across the community [62].
Finally, a yet insufficiently investigated problem is the endonucleolytic activity on transforming DNA [33,35], which is likely to cause problems when constructs containing several genes are delivered. Such constructs can now easily be generated with the MoClo strategy [62]. Here, the events occurring upon the integration of such large constructs into the genome need to be investigated in detail.
Acknowledgments
I would like to thank Miriam Schulz-Raffelt for the immunoblot shown in Figure 4B.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB/TRR175, project C02).
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Sasso S., Stibor H., Mittag M., Grossman A.R. From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. eLife. 2018;7 doi: 10.7554/eLife.39233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eberhard S., Finazzi G., Wollman F.A. The dynamics of photosynthesis. Annu. Rev. Genet. 2008;42:463–515. doi: 10.1146/annurev.genet.42.110807.091452. [DOI] [PubMed] [Google Scholar]
- 3.Wingfield J.L., Lechtreck K.F. Chlamydomonas Basal Bodies as Flagella Organizing Centers. Cells. 2018;7 doi: 10.3390/cells7070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross F.R., Umen J.G. The Chlamydomonas cell cycle. Plant J. 2015;82:370–392. doi: 10.1111/tpj.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroda M., Hemme D., Mühlhaus T. The Chlamydomonas heat stress response. Plant J. 2015;82:466–480. doi: 10.1111/tpj.12816. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo T., Ishiura M. Chlamydomonas reinhardtii as a new model system for studying the molecular basis of the circadian clock. FEBS Lett. 2011;585:1495–1502. doi: 10.1016/j.febslet.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Melis A., Zhang L.P., Forestier M., Ghirardi M.L., Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122:127–135. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong F., Yamaoka Y., Ohama T., Lee Y., Li-Beisson Y. Molecular Genetic Tools and Emerging Synthetic Biology Strategies to Increase Cellular Oil Content in Chlamydomonas reinhardtii. Plant Cell Physiol. 2019 doi: 10.1093/pcp/pcz022. [DOI] [PubMed] [Google Scholar]
- 9.Ostrowski L.E., Dutcher S.K., Lo C.W. Cilia and models for studying structure and function. Proc. Am. Thorac. Soc. 2011;8:423–429. doi: 10.1513/pats.201103-027SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petroutsos D., Tokutsu R., Maruyama S., Flori S., Greiner A., Magneschi L., Cusant L., Kottke T., Mittag M., Hegemann P., et al. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature. 2016;537:563–566. doi: 10.1038/nature19358. [DOI] [PubMed] [Google Scholar]
- 11.Rost B.R., Schneider-Warme F., Schmitz D., Hegemann P. Optogenetic Tools for Subcellular Applications in Neuroscience. Neuron. 2017;96:572–603. doi: 10.1016/j.neuron.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Jinkerson R.E., Jonikas M.C. Molecular techniques to interrogate and edit the Chlamydomonas nuclear genome. Plant J. 2015;82:393–412. doi: 10.1111/tpj.12801. [DOI] [PubMed] [Google Scholar]
- 13.Mussgnug J.H. Genetic tools and techniques for Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2015;99:5407–5418. doi: 10.1007/s00253-015-6698-7. [DOI] [PubMed] [Google Scholar]
- 14.Scaife M.A., Nguyen G.T., Rico J., Lambert D., Helliwell K.E., Smith A.G. Establishing Chlamydomonas reinhardtii as an industrial biotechnology host. Plant J. 2015;82:532–546. doi: 10.1111/tpj.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scranton M.A., Ostrand J.T., Fields F.J., Mayfield S.P. Chlamydomonas as a model for biofuels and bio-products production. Plant J. 2015;82:523–531. doi: 10.1111/tpj.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taunt H.N., Stoffels L., Purton S. Green biologics: The algal chloroplast as a platform for making biopharmaceuticals. Bioengineered. 2018;9:48–54. doi: 10.1080/21655979.2017.1377867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauersen K.J., Huber I., Wichmann J., Baier T., Leiter A., Gaukel V., Kartushin V., Rattenholl A., Steinweg C., von Riesen L., et al. Investigating the dynamics of recombinant protein secretion from a microalgal host. J. Biotechnol. 2015;215:62–71. doi: 10.1016/j.jbiotec.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Eichler-Stahlberg A., Weisheit W., Ruecker O., Heitzer M. Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta. 2009;229:873–883. doi: 10.1007/s00425-008-0879-x. [DOI] [PubMed] [Google Scholar]
- 19.Molino J.V.D., de Carvalho J.C.M., Mayfield S.P. Comparison of secretory signal peptides for heterologous protein expression in microalgae: Expanding the secretion portfolio for Chlamydomonas reinhardtii. PLoS ONE. 2018;13:e0192433. doi: 10.1371/journal.pone.0192433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos-Martinez E.M., Fimognari L., Sakuragi Y. High-yield secretion of recombinant proteins from the microalga Chlamydomonas reinhardtii. Plant Biotechnol. J. 2017;15:1214–1224. doi: 10.1111/pbi.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debuchy R., Purton S., Rochaix J.D. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: An important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 1989;8:2803–2809. doi: 10.1002/j.1460-2075.1989.tb08426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindle K.L., Schnell R.A., Fernandez E., Lefebvre P.A. Stable nuclear transformation of Chlamydomonas using the Chlamydomonas gene for nitrate reductase. J. Cell Biol. 1989;109:2589–2601. doi: 10.1083/jcb.109.6.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kindle K.L. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 1990;87:1228–1232. doi: 10.1073/pnas.87.3.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimogawara K., Fujiwara S., Grossman A., Usuda H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics. 1998;148:1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamano T., Iguchi H., Fukuzawa H. Rapid transformation of Chlamydomonas reinhardtii without cell-wall removal. J. Biosci. Bioeng. 2013;115:691–694. doi: 10.1016/j.jbiosc.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Nelson J.A., Savereide P.B., Lefebvre P.A. The CRY1 gene in Chlamydomonas reinhardtii: Structure and use as a dominant selectable marker for nuclear transformation. Mol. Cell. Biol. 1994;14:4011–4019. doi: 10.1128/MCB.14.6.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens D.R., Rochaix J.D., Purton S. The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol. Gen. Genet. 1996;251:23–30. doi: 10.1007/BF02174340. [DOI] [PubMed] [Google Scholar]
- 28.Lumbreras V., Stevens D.R., Purton S. Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J. 1998;14:441–447. doi: 10.1046/j.1365-313X.1998.00145.x. [DOI] [Google Scholar]
- 29.Cerutti H., Johnson A.M., Gillham N.W., Boynton J.E. A eubacterial gene conferring spectinomycin resistance on Chlamydomonas reinhardtii: Integration into the nuclear genome and gene expression. Genetics. 1997;145:97–110. doi: 10.1093/genetics/145.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meslet-Cladiere L., Vallon O. Novel shuttle markers for nuclear transformation of the green alga Chlamydomonas reinhardtii. Eukaryot. cell. 2011;10:1670–1678. doi: 10.1128/EC.05043-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sizova I., Fuhrmann M., Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/S0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 32.Berthold P., Schmitt R., Mages W. An engineered Streptomyces hygroscopicus aph 7” gene mediates dominant resistance against hygromycin B in Chlamydomonas reinhardtii. Protist. 2002;153:401–412. doi: 10.1078/14344610260450136. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R., Patena W., Armbruster U., Gang S.S., Blum S.R., Jonikas M.C. High-Throughput Genotyping of Green Algal Mutants Reveals Random Distribution of Mutagenic Insertion Sites and Endonucleolytic Cleavage of Transforming DNA. Plant Cell. 2014;26:1398–1409. doi: 10.1105/tpc.114.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dent R.M., Haglund C.M., Chin B.L., Kobayashi M.C., Niyogi K.K. Functional genomics of eukaryotic photosynthesis using insertional mutagenesis of Chlamydomonas reinhardtii. Plant Physiol. 2005;137:545–556. doi: 10.1104/pp.104.055244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Zhang R., Patena W., Gang S.S., Blum S.R., Ivanova N., Yue R., Robertson J.M., Lefebvre P.A., Fitz-Gibbon S.T., et al. An Indexed, Mapped Mutant Library Enables Reverse Genetics Studies of Biological Processes in Chlamydomonas reinhardtii. Plant Cell. 2016;28:367–387. doi: 10.1105/tpc.15.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Ballester D., Pootakham W., Mus F., Yang W., Catalanotti C., Magneschi L., de Montaigu A., Higuera J.J., Prior M., Galvan A., et al. Reverse genetics in Chlamydomonas: A platform for isolating insertional mutants. Plant Methods. 2011;7:24. doi: 10.1186/1746-4811-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tam L.W., Lefebvre P.A. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993;135:375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerutti H., Johnson A.M., Gillham N.W., Boynton J.E. Epigenetic silencing of a foreign gene in nuclear transformants of Chlamydomonas. Plant Cell. 1997;9:925–945. doi: 10.1105/tpc.9.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroda M., Blocker D., Beck C.F. The HSP70A promoter as a tool for the improved expression of transgenes in Chlamydomonas. Plant J. 2000;21:121–131. doi: 10.1046/j.1365-313x.2000.00652.x. [DOI] [PubMed] [Google Scholar]
- 40.Blankenship J.E., Kindle K.L. Expression of chimeric genes by the light-regulated cabII-1 promoter in Chlamydomonas reinhardtii: A cabII-1/nit1 gene functions as a dominant selectable marker in a nit1- nit2- strain. Mol. Cell. Biol. 1992;12:5268–5279. doi: 10.1128/MCB.12.11.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baier T., Wichmann J., Kruse O., Lauersen K.J. Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res. 2018;46:6909–6919. doi: 10.1093/nar/gky532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barahimipour R., Strenkert D., Neupert J., Schroda M., Merchant S.S., Bock R. Dissecting the contributions of GC content and codon usage to gene expression in the model alga Chlamydomonas reinhardtii. Plant J. 2015;84:704–717. doi: 10.1111/tpj.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamasaki T., Miyasaka H., Ohama T. Unstable RNAi effects through epigenetic silencing of an inverted repeat transgene in Chlamydomonas reinhardtii. Genetics. 2008;180:1927–1944. doi: 10.1534/genetics.108.092395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koblenz B., Lechtreck K.F. The NIT1 promoter allows inducible and reversible silencing of centrin in Chlamydomonas reinhardtii. Eukaryot. cell. 2005;4:1959–1962. doi: 10.1128/EC.4.11.1959-1962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamasaki T., Ohama T. Involvement of Elongin C in the spread of repressive histone modifications. Plant J. 2011;65:51–61. doi: 10.1111/j.1365-313X.2010.04400.x. [DOI] [PubMed] [Google Scholar]
- 46.Fuhrmann M., Stahlberg A., Govorunova E., Rank S., Hegemann P. The abundant retinal protein of the Chlamydomonas eye is not the photoreceptor for phototaxis and photophobic responses. J. Cell Sci. 2001;114:3857–3863. doi: 10.1242/jcs.114.21.3857. [DOI] [PubMed] [Google Scholar]
- 47.Casas-Mollano J.A., Jeong B.R., Xu J., Moriyama H., Cerutti H. The MUT9p kinase phosphorylates histone H3 threonine 3 and is necessary for heritable epigenetic silencing in Chlamydomonas. Proc. Natl. Acad. Sci. USA. 2008;105:6486–6491. doi: 10.1073/pnas.0711310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaver S., Casas-Mollano J.A., Cerny R.L., Cerutti H. Origin of the polycomb repressive complex 2 and gene silencing by an E(z) homolog in the unicellular alga Chlamydomonas. Epigenetics. 2010;5:301–312. doi: 10.4161/epi.5.4.11608. [DOI] [PubMed] [Google Scholar]
- 49.Strenkert D., Schmollinger S., Schroda M. Heat shock factor 1 counteracts epigenetic silencing of nuclear transgenes in Chlamydomonas reinhardtii. Nucleic Acids Res. 2013;41:5273–5289. doi: 10.1093/nar/gkt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neupert J., Karcher D., Bock R. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 2009;57:1140–1150. doi: 10.1111/j.1365-313X.2008.03746.x. [DOI] [PubMed] [Google Scholar]
- 51.Luger K. Structure and dynamic behavior of nucleosomes. Curr. Opin. Genet. Dev. 2003;13:127–135. doi: 10.1016/S0959-437X(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 52.Clapier C.R., Iwasa J., Cairns B.R., Peterson C.L. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol. 2017;18:407–422. doi: 10.1038/nrm.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nature reviews. Genetics. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 54.Van Dijk K., Marley K.E., Jeong B.R., Xu J., Hesson J., Cerny R.L., Waterborg J.H., Cerutti H. Monomethyl histone H3 lysine 4 as an epigenetic mark for silenced euchromatin in Chlamydomonas. Plant Cell. 2005;17:2439–2453. doi: 10.1105/tpc.105.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casas-Mollano J.A., van Dijk K., Eisenhart J., Cerutti H. SET3p monomethylates histone H3 on lysine 9 and is required for the silencing of tandemly repeated transgenes in Chlamydomonas. Nucleic. Acids Res. 2007;35:939–950. doi: 10.1093/nar/gkl1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strenkert D., Schmollinger S., Sommer F., Schulz-Raffelt M., Schroda M. Transcription factor dependent chromatin remodeling at heat shock and copper responsive promoters in Chlamydomonas reinhardtii. Plant Cell. 2011;23:2285–2301. doi: 10.1105/tpc.111.085266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruthenburg A.J., Wang W., Graybosch D.M., Li H., Allis C.D., Patel D.J., Verdine G.L. Histone H3 recognition and presentation by the WDR5 module of the MLL1 complex. Nat. Struct. Mol. Biol. 2006;13:704–712. doi: 10.1038/nsmb1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang C., Wu-Scharf D., Jeong B.R., Cerutti H. A WD40-repeat containing protein, similar to a fungal co-repressor, is required for transcriptional gene silencing in Chlamydomonas. Plant J. 2002;31:25–36. doi: 10.1046/j.1365-313X.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 59.Jeong B.R., Wu-Scharf D., Zhang C., Cerutti H. Suppressors of transcriptional transgenic silencing in Chlamydomonas are sensitive to DNA-damaging agents and reactivate transposable elements. Proc. Natl. Acad. Sci. USA. 2002;99:1076–1081. doi: 10.1073/pnas.022392999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barahimipour R., Neupert J., Bock R. Efficient expression of nuclear transgenes in the green alga Chlamydomonas: Synthesis of an HIV antigen and development of a new selectable marker. Plant Mol. Biol. 2016;90:403–418. doi: 10.1007/s11103-015-0425-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauersen K.J., Berger H., Mussgnug J.H., Kruse O. Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii. J. Biotechnol. 2013;167:101–110. doi: 10.1016/j.jbiotec.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Crozet P., Navarro F.J., Willmund F., Mehrshahi P., Bakowski K., Lauersen K.J., Perez-Perez M.E., Auroy P., Gorchs Rovira A., Sauret-Gueto S., et al. Birth of a photosynthetic chassis: A MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas reinhardtii. Acs Synth. Biol. 2018;7:2074–2086. doi: 10.1021/acssynbio.8b00251. [DOI] [PubMed] [Google Scholar]
- 63.Kong F., Yamasaki T., Ohama T. Expression levels of domestic cDNA cassettes integrated in the nuclear genomes of various Chlamydomonas reinhardtii strains. J. Biosci. Bioeng. 2014;117:613–616. doi: 10.1016/j.jbiosc.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 64.Lauersen K.J., Kruse O., Mussgnug J.H. Targeted expression of nuclear transgenes in Chlamydomonas reinhardtii with a versatile, modular vector toolkit. Appl. Microbiol. Biotechnol. 2015;99:3491–3503. doi: 10.1007/s00253-014-6354-7. [DOI] [PubMed] [Google Scholar]
- 65.Babinger P., Kobl I., Mages W., Schmitt R. A link between DNA methylation and epigenetic silencing in transgenic Volvox carteri. Nucleic Acids Res. 2001;29:1261–1271. doi: 10.1093/nar/29.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babinger P., Volkl R., Cakstina I., Maftei A., Schmitt R. Maintenance DNA methyltransferase (Met1) and silencing of CpG-methylated foreign DNA in Volvox carteri. Plant Mol. Biol. 2007;63:325–336. doi: 10.1007/s11103-006-9091-1. [DOI] [PubMed] [Google Scholar]
- 67.Kong F., Yamasaki T., Kurniasih S.D., Hou L., Li X., Ivanova N., Okada S., Ohama T. Robust expression of heterologous genes by selection marker fusion system in improved Chlamydomonas strains. J. Biosci. Bioeng. 2015;120:239–245. doi: 10.1016/j.jbiosc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Kurniasih S.D., Yamasaki T., Kong F., Okada S., Widyaningrum D., Ohama T. UV-mediated Chlamydomonas mutants with enhanced nuclear transgene expression by disruption of DNA methylation-dependent and independent silencing systems. Plant Mol. Biol. 2016;92:629–641. doi: 10.1007/s11103-016-0529-9. [DOI] [PubMed] [Google Scholar]
- 69.Schroda M., Vallon O., Wollman F.A., Beck C.F. A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell. 1999;11:1165–1178. doi: 10.1105/tpc.11.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schroda M., Beck C.F., Vallon O. Sequence elements within an HSP70 promoter counteract transcriptional transgene silencing in Chlamydomonas. Plant J. 2002;31:445–455. doi: 10.1046/j.1365-313X.2002.01371.x. [DOI] [PubMed] [Google Scholar]
- 71.Lodha M., Schulz-Raffelt M., Schroda M. A new assay for promoter analysis in Chlamydomonas reveals roles for heat shock elements and the TATA box in HSP70A promoter-mediated activation of transgene expression. Eukaryot. cell. 2008;7:172–176. doi: 10.1128/EC.00055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lodha M., Schroda M. Analysis of chromatin structure in the control regions of the Chlamydomonas HSP70A and RBCS2 genes. Plant Mol. Biol. 2005;59:501–513. doi: 10.1007/s11103-005-0450-0. [DOI] [PubMed] [Google Scholar]
- 73.Schulz-Raffelt M., Lodha M., Schroda M. Heat shock factor 1 is a key regulator of the stress response in Chlamydomonas. Plant J. 2007;52:286–295. doi: 10.1111/j.1365-313X.2007.03228.x. [DOI] [PubMed] [Google Scholar]
- 74.Ruecker O., Zillner K., Groebner-Ferreira R., Heitzer M. Gaussia-luciferase as a sensitive reporter gene for monitoring promoter activity in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genom.: Mgg. 2008;280:153–162. doi: 10.1007/s00438-008-0352-3. [DOI] [PubMed] [Google Scholar]
- 75.Day A., Debuchy R., Vandillewijn J., Purton S., Rochaix J.D. Studies on the Maintenance and Expression of Cloned DNA Fragments in the Nuclear Genome of the Green-Alga Chlamydomonas-Reinhardtii. Physiol. Plant. 1990;78:254–260. doi: 10.1111/j.1399-3054.1990.tb02089.x. [DOI] [Google Scholar]
- 76.Dong B., Hu H.H., Li Z.F., Cheng R.Q., Meng D.M., Wang J., Fan Z.C. A novel bicistronic expression system composed of the intraflagellar transport protein gene ift25 and FMDV 2A sequence directs robust nuclear gene expression in Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2017;101:4227–4245. doi: 10.1007/s00253-017-8177-9. [DOI] [PubMed] [Google Scholar]
- 77.Díaz-Santos E., de la Vega M., Vila M., Vigara J., León R. Efficiency of different heterologous promoters in the unicellular microalga Chlamydomonas reinhardtii. Biotechnol. Prog. 2013;29:319–328. doi: 10.1002/btpr.1690. [DOI] [PubMed] [Google Scholar]
- 78.Kozminski K.G., Diener D.R., Rosenbaum J.L. High level expression of nonacetylatable alpha-tubulin in Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton. 1993;25:158–170. doi: 10.1002/cm.970250205. [DOI] [PubMed] [Google Scholar]
- 79.Davies J.P., Weeks D.P., Grossman A.R. Expression of the arylsulfatase gene from the beta 2-tubulin promoter in Chlamydomonas reinhardtii. Nucleic. Acids Res. 1992;20:2959–2965. doi: 10.1093/nar/20.12.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cairns B.R. The logic of chromatin architecture and remodelling at promoters. Nature. 2009;461:193–198. doi: 10.1038/nature08450. [DOI] [PubMed] [Google Scholar]
- 81.Voss T.C., Hager G.L. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nature reviews. Genetics. 2014;15:69–81. doi: 10.1038/nrg3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chereji R.V., Clark D.J. Major Determinants of Nucleosome Positioning. Biophys. J. 2018;114:2279–2289. doi: 10.1016/j.bpj.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fu Y., Luo G.Z., Chen K., Deng X., Yu M., Han D., Hao Z., Liu J., Lu X., Dore L.C., et al. N(6)-methyldeoxyadenosine marks active transcription start sites in chlamydomonas. Cell. 2015;161:879–892. doi: 10.1016/j.cell.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swinstead E.E., Paakinaho V., Presman D.M., Hager G.L. Pioneer factors and ATP-dependent chromatin remodeling factors interact dynamically: A new perspective: Multiple transcription factors can effect chromatin pioneer functions through dynamic interactions with ATP-dependent chromatin remodeling factors. Bioessays. 2016;38:1150–1157. doi: 10.1002/bies.201600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis E.B. The phenomenon of position effect. Adv. Genet. 1950;3:73–115. doi: 10.1016/s0065-2660(08)60083-8. [DOI] [PubMed] [Google Scholar]
- 86.Feuerborn A., Cook P.R. Why the activity of a gene depends on its neighbors. Trends Genet. 2015;31:483–490. doi: 10.1016/j.tig.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Rasala B.A., Lee P.A., Shen Z., Briggs S.P., Mendez M., Mayfield S.P. Robust expression and secretion of Xylanase1 in Chlamydomonas reinhardtii by fusion to a selection gene and processing with the FMDV 2A peptide. PLoS ONE. 2012;7:e43349. doi: 10.1371/journal.pone.0043349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rasala B.A., Barrera D.J., Ng J., Plucinak T.M., Rosenberg J.N., Weeks D.P., Oyler G.A., Peterson T.C., Haerizadeh F., Mayfield S.P. Expanding the spectral palette of fluorescent proteins for the green microalga Chlamydomonas reinhardtii. Plant J. 2013;74:545–556. doi: 10.1111/tpj.12165. [DOI] [PubMed] [Google Scholar]
- 89.Plucinak T.M., Horken K.M., Jiang W., Fostvedt J., Nguyen S.T., Weeks D.P. Improved and versatile viral 2A platforms for dependable and inducible high-level expression of dicistronic nuclear genes in Chlamydomonas reinhardtii. Plant J. 2015;82:717–729. doi: 10.1111/tpj.12844. [DOI] [PubMed] [Google Scholar]
- 90.Rasala B.A., Chao S.S., Pier M., Barrera D.J., Mayfield S.P. Enhanced genetic tools for engineering multigene traits into green algae. PLoS ONE. 2014;9:e94028. doi: 10.1371/journal.pone.0094028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-Paz C., Liu D., Geng S., Umen J.G. Identification of Chlamydomonas reinhardtii endogenous genic flanking sequences for improved transgene expression. Plant J. 2017;92:1232–1244. doi: 10.1111/tpj.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barjona do Nascimento Coutinho P., Friedl C., Heilmann M., Buchholz R., Stute S.C. Validated Nuclear-Based Transgene Expression Regulated by the Fea1 Iron-Responsive Promoter in the Green Alga Chlamydomonas reinhardtii. Mol. Biotechnol. 2019;61:305–316. doi: 10.1007/s12033-018-00148-0. [DOI] [PubMed] [Google Scholar]
- 93.Barjona do Nascimento Coutinho P., Friedl C., Buchholz R., Stute S.C. Chemical regulation of Fea1 driven transgene expression in Chlamydomonas reinhardtii. Algal Research. 2017;26:323–329. doi: 10.1016/j.algal.2017.08.006. [DOI] [Google Scholar]
- 94.Fuhrmann M., Oertel W., Hegemann P. A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J. 1999;19:353–361. doi: 10.1046/j.1365-313X.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 95.Onishi M., Pringle J.R. Robust Transgene Expression from Bicistronic mRNA in the Green Alga Chlamydomonas reinhardtii. G3 (Bethesda) 2016;6:4115–4125. doi: 10.1534/g3.116.033035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischer N., Rochaix J.D. The flanking regions of PsaD drive efficient gene expression in the nucleus of the green alga Chlamydomonas reinhardtii. Mol. Genet. Genom. 2001;265:888–894. doi: 10.1007/s004380100485. [DOI] [PubMed] [Google Scholar]
- 97.Kumar A., Falcao V.R., Sayre R.T. Evaluating nuclear transgene expression systems in Chlamydomonas reinhardtii. Algal Res. 2013;2:321–332. doi: 10.1016/j.algal.2013.09.002. [DOI] [Google Scholar]
- 98.Heitzer M., Zschoernig B. Construction of modular tandem expression vectors for the green alga Chlamydomonas reinhardtii using the Cre/lox-system. Biotechniques. 2007;43:324, 326, 328 passim. doi: 10.2144/000112556. [DOI] [PubMed] [Google Scholar]
- 99.Schmollinger S., Schulz-Raffelt M., Strenkert D., Veyel D., Vallon O., Schroda M. Dissecting the heat stress response in Chlamydomonas by pharmaceutical and RNAi approaches reveals conserved and novel aspects. Mol. Plant. 2013;6:1795–1813. doi: 10.1093/mp/sst086. [DOI] [PubMed] [Google Scholar]
- 100.Specht E.A., Nour-Eldin H.H., Hoang K.T., Mayfield S.P. An improved ARS2-derived nuclear reporter enhances the efficiency and ease of genetic engineering in Chlamydomonas. Biotechnol. J. 2015;10:473–479. doi: 10.1002/biot.201400172. [DOI] [PubMed] [Google Scholar]
- 101.Kucho K., Ohyama K., Fukuzawa H. CO(2)-responsive transcriptional regulation of CAH1 encoding carbonic anhydrase is mediated by enhancer and silencer regions in Chlamydomonas reinhardtii. Plant Physiol. 1999;121:1329–1338. doi: 10.1104/pp.121.4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Villand P., Eriksson M., Samuelsson G. Carbon dioxide and light regulation of promoters controlling the expression of mitochondrial carbonic anhydrase in Chlamydomonas reinhardtii. Biochem. J. 1997;327:51–57. doi: 10.1042/bj3270051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohresser M., Matagne R.F., Loppes R. Expression of the arylsulphatase reporter gene under the control of the nit1 promoter in Chlamydomonas reinhardtii. Curr. Genet. 1997;31:264–271. doi: 10.1007/s002940050204. [DOI] [PubMed] [Google Scholar]
- 104.Schmollinger S., Strenkert D., Schroda M. An inducible artificial microRNA system for Chlamydomonas reinhardtii confirms a key role for heat shock factor 1 in regulating thermotolerance. Curr. Genet. 2010;56:383–389. doi: 10.1007/s00294-010-0304-4. [DOI] [PubMed] [Google Scholar]
- 105.Quinn J.M., Merchant S. Two copper-responsive elements associated with the Chlamydomonas Cyc6 gene function as targets for transcriptional activators. Plant Cell. 1995;7:623–628. doi: 10.1105/tpc.7.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrante P., Catalanotti C., Bonente G., Giuliano G. An optimized, chemically regulated gene expression system for Chlamydomonas. PLoS ONE. 2008;3:e3200. doi: 10.1371/journal.pone.0003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Quinn J.M., Kropat J., Merchant S. Copper response element and Crr1-dependent Ni(2+)-responsive promoter for induced, reversible gene expression in Chlamydomonas reinhardtii. Eukaryot. cell. 2003;2:995–1002. doi: 10.1128/EC.2.5.995-1002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Helliwell K.E., Scaife M.A., Sasso S., Araujo A.P., Purton S., Smith A.G. Unraveling vitamin B12-responsive gene regulation in algae. Plant Physiol. 2014;165:388–397. doi: 10.1104/pp.113.234369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Croft M.T., Moulin M., Webb M.E., Smith A.G. Thiamine biosynthesis in algae is regulated by riboswitches. Proc. Natl. Acad. Sci. USA. 2007;104:20770–20775. doi: 10.1073/pnas.0705786105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Theis J., Lang J., Spaniol B., Ferte S., Niemeyer J., Sommer F.K., Zimmer D., Venn B., Mehr S.F., Muhlhaus T., et al. The Chlamydomonas deg1c mutant accumulates proteins involved in high light acclimation. Plant Physiol. 2019 doi: 10.1104/pp.19.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuhrmann M., Hausherr A., Ferbitz L., Schodl T., Heitzer M., Hegemann P. Monitoring dynamic expression of nuclear genes in Chlamydomonas reinhardtii by using a synthetic luciferase reporter gene. Plant Mol. Biol. 2004;55:869–881. doi: 10.1007/s11103-005-2150-1. [DOI] [PubMed] [Google Scholar]
- 112.Shao N., Bock R. A codon-optimized luciferase from Gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga Chlamydomonas reinhardtii. Curr. Genet. 2008;53:381–388. doi: 10.1007/s00294-008-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baier T., Kros D., Feiner R.C., Lauersen K.J., Muller K.M., Kruse O. Engineered Fusion Proteins for Efficient Protein Secretion and Purification of a Human Growth Factor from the Green Microalga Chlamydomonas reinhardtii. ACS Synth. Biol. 2018;7:2547–2557. doi: 10.1021/acssynbio.8b00226. [DOI] [PubMed] [Google Scholar]
- 114.Lauersen K.J., Vanderveer T.L., Berger H., Kaluza I., Mussgnug J.H., Walker V.K., Kruse O. Ice recrystallization inhibition mediated by a nuclear-expressed and -secreted recombinant ice-binding protein in the microalga Chlamydomonas reinhardtii. Appl. Microbiol. Biotechnol. 2013;97:9763–9772. doi: 10.1007/s00253-013-5226-x. [DOI] [PubMed] [Google Scholar]
- 115.Lauersen K.J., Baier T., Wichmann J., Wordenweber R., Mussgnug J.H., Hubner W., Huser T., Kruse O. Efficient phototrophic production of a high-value sesquiterpenoid from the eukaryotic microalga Chlamydomonas reinhardtii. Metab. Eng. 2016;38:331–343. doi: 10.1016/j.ymben.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 116.Schroda M. RNA silencing in Chlamydomonas: Mechanisms and tools. Curr. Genet. 2006;49:69–84. doi: 10.1007/s00294-005-0042-1. [DOI] [PubMed] [Google Scholar]
- 117.Weiner I., Atar S., Schweitzer S., Eilenberg H., Feldman Y., Avitan M., Blau M., Danon A., Tuller T., Yacoby I. Enhancing heterologous expression in Chlamydomonas reinhardtii by transcript sequence optimization. Plant J. 2018;94:22–31. doi: 10.1111/tpj.13836. [DOI] [PubMed] [Google Scholar]
- 118.Rose A.B. Intron-mediated regulation of gene expression. Curr. Top. Microbiol. Immunol. 2008;326:277–290. doi: 10.1007/978-3-540-76776-3_15. [DOI] [PubMed] [Google Scholar]
- 119.Merchant S.S., Prochnik S.E., Vallon O., Harris E.H., Karpowicz S.J., Witman G.B., Terry A., Salamov A., Fritz-Laylin L.K., Marechal-Drouard L., et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jaeger D., Baier T., Lauersen K.J. Intronserter, an advanced online tool for design of intron containing transgenes. Algal Res. 2019;42:101588. doi: 10.1016/j.algal.2019.101588. [DOI] [Google Scholar]
- 121.Patron N.J., Orzaez D., Marillonnet S., Warzecha H., Matthewman C., Youles M., Raitskin O., Leveau A., Farre G., Rogers C., et al. Standards for plant synthetic biology: A common syntax for exchange of DNA parts. New phytol. 2015;208:13–19. doi: 10.1111/nph.13532. [DOI] [PubMed] [Google Scholar]
- 122.Weber E., Engler C., Gruetzner R., Werner S., Marillonnet S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE. 2011;6:e16765. doi: 10.1371/journal.pone.0016765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques. 2000;28:1102–1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- 124.Silflow C.D. The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas. Springer; Dordrecht, The Netherlands: 1998. [Google Scholar]
- 125.Greiner A., Kelterborn S., Evers H., Kreimer G., Sizova I., Hegemann P. Targeting of photoreceptor genes in Chlamydomonas reinhardtii via zinc-finger nucleases and CRISPR/Cas9. Plant Cell. 2017;29:2498–2518. doi: 10.1105/tpc.17.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ferenczi A., Pyott D.E., Xipnitou A., Molnar A. Efficient targeted DNA editing and replacement in Chlamydomonas reinhardtii using Cpf1 ribonucleoproteins and single-stranded DNA. Proc. Natl. Acad. Sci. USA. 2017;114:13567–13572. doi: 10.1073/pnas.1710597114. [DOI] [PMC free article] [PubMed] [Google Scholar]