Key Points
Sirolimus provides similar day 28 complete/partial response rates as prednisone in initial therapy of standard-risk acute GVHD.
Sirolimus therapy was associated with reduced steroid exposure, greater immune suppression discontinuation, and improved quality of life.
Abstract
Clinical- and biomarker-based tools may identify a lower-risk acute graft-versus-host disease (GVHD) population amenable to novel, reduced-intensity treatments. Previous data suggest sirolimus may rival standard of care prednisone. We conducted a National Heart, Lung, and Blood Institute/National Cancer Institute-funded Blood and Marrow Transplant Clinical Trials Network multicenter, open-label, randomized phase 2 trial to estimate the difference in day 28 complete response (CR)/partial response (PR) rates for sirolimus vs prednisone as initial treatment of patients with standard risk (SR) acute GVHD as defined by the Minnesota (MN) GVHD Risk Score and Ann Arbor (AA1/2) biomarker status. A total of 127 MN-SR patients were randomized (1:1), and 122 were AA1/2 (sirolimus, n = 58; prednisone, n = 64). Others were AA3 (n = 4), or AA status missing (n = 1). The day 28 CR/PR rates were similar for sirolimus 64.8% (90% confidence interval [CI], 54.1%-75.5%) vs 73% (90% CI, 63.8%-82.2%) for prednisone. The day 28 rate of CR/PR with prednisone ≤0.25 mg/kg/day was significantly higher for sirolimus than prednisone (66.7% vs 31.7%; P < .001). No differences were detected in steroid-refractory acute GVHD, disease-free survival, relapse, nonrelapse mortality, or overall survival. Sirolimus was associated with reduced steroid exposure and hyperglycemia, reduced grade 2 to 3 infections, improvement in immune suppression discontinuation and patient-reported quality of life, and increased risk for thrombotic microangiopathy. For patients with clinical- and biomarker-based SR acute GVHD, sirolimus demonstrates similar overall initial treatment efficacy as prednisone. In addition, sirolimus therapy spares steroid exposure and allied toxicity, does not compromise long-term survival outcomes, and is associated with improved patient-reported quality of life. This trial was registered at www.clinicaltrials.gov as #NCT02806947.
Visual Abstract
Introduction
Acute graft-versus-host disease (GVHD) frequently occurs after allogeneic hematopoietic cell transplantation (HCT) despite commonly used immune suppression prophylaxis.1,2 As an important source of early post-HCT mortality,3,4 this complication threatens the otherwise curative potential of HCT. The mainstay of treatment of acute GVHD is high-dose corticosteroids, which are effective in about half of patients, but have significant toxicities.5-7
Recently, clinical and blood biomarker-based tools have been developed to estimate the likelihood of initial response to steroid treatment and subsequent risk for mortality in acute GVHD. The Minnesota (MN) GVHD Risk Score uses acute GVHD organ involvement and severity to identify standard-risk (SR) vs high-risk (HR) status at onset of GVHD.8-10 The Ann Arbor (AA) biomarker risk uses 2 serum biomarkers (REG3: regenerating islet-derived 3-α; ST2: suppression of tumorigenicity 2) at acute GVHD onset to generate a score from 1 to 3, with higher scores indicating greater mortality risk. The algorithm, derived and validated in multicenter studies,11,12 is now also used in clinical practice: In 2019, more than 40 HCT centers sent hundreds of samples for analysis (VIRACOR, personal communication). Independent groups have demonstrated association of ST2 with GVHD and nonrelapse mortality as well.13-15 Although published clinical trials have examined varied initial steroid doses according to acute GVHD severity,16,17 prospective multicenter trials employing novel clinical and biomarker tools for risk-adapted GVHD therapy have not been performed.
The National Heart, Lung, and Blood Institute and National Cancer Institute (NHLBI and NCI) funded the Blood and Marrow Transplant Clinical Trials Network (BMT CTN), which aimed to develop a portfolio of risk-adapted acute GVHD therapy trials using these clinical and biomarker tools.18 Prior single-center data suggested sirolimus had activity in initial GVHD therapy.19 Although intriguing, these initial data required prospective, randomized confirmation.
The BMT CTN 1501 trial examined the activity of sirolimus vs standard prednisone (2 mg/kg/day) therapy in the initial treatment of patients with a combined SR (MN-SR; AA1/2 biomarker risk) acute GVHD profile. The primary objective of the trial was to estimate the difference in day 28 treatment response rates between the study groups. Secondary objectives encompassed the full extent of treatment efficacy measures, toxicity, potential steroid-sparing effects of sirolimus, and long-term outcomes.
Methods
Study design
The trial (NCT02806947) was designed as a multicenter, randomized, phase 2 trial comparing sirolimus vs standard prednisone therapy among patients with newly diagnosed MN-SR, AA biomarker 1/2 acute GVHD. Randomization was performed in a 1:1 ratio, using permuted blocks and stratified by HCT center. Patients meeting initial eligibility by MN-SR criteria had a 5-mL blood sample collected before randomization to centrally assess biomarker status, with expected results within 48 to 72 hours after randomization. Randomized therapy began within 24 hours of randomization, based on MN-SR eligibility (randomized therapy did not wait until biomarker results were available). A total of 120 MN-SR AA1/2 subjects were required for assessment of the primary end point. Once biomarkers resulted, those with AA1/2 status continued their randomized therapy and informed all primary and secondary outcome analyses. Those with AA3 or missing status were excluded from the primary analysis, and further therapy was at the discretion of the treating physician. The National Marrow Donor Program Institutional Review Board was offered as a central institutional review board, but centers could use their local boards if preferred. An NHLBI Data and Safety Monitoring Board monitored the trial.
Objectives and end points
The primary objective was to estimate the difference in day 28 complete response (CR)/partial response (PR) rates. A predefined key secondary end point was the rate of day 28 CR/PR with prednisone dose 0.25 mg/kg/day or less. Prednisone use after randomization to sirolimus was considered a failure for the primary outcome, but not for the key secondary outcome, provided it remained below the threshold. Response definitions were as follows: CR, score of 0 in all GVHD organs (skin, gastrointestinal [GI], liver); PR, improvement in target organ or organs without progression in others; mixed response (MR), improvement in organ or organs with worsening in another involved organ or new organ involvement; and progressive disease (PD), worsening in organ or organs without improvement in others. An Endpoint Review Committee adjudicated day 28 and day 56 response, as well as success for the key day 28 secondary outcome (supplemental Methods, available on the Blood Web site).
Secondary objectives were to assess serial acute GVHD treatment responses; treatment failure (inclusive of no response [NR], PD, use of an additional line of systemic therapy for GVHD beyond randomized therapy, or death); National Institutes of Health chronic GVHD incidence20; systemic infections; event-free survival, defined as freedom from acute GVHD progression; chronic GVHD, malignancy relapse, and death; disease-free and overall survival; and nonrelapse mortality.
Exploratory objectives evaluated prednisone exposure, use of topical acute GVHD agents, discontinuation of immunosuppressive therapy, Epstein-Barr virus requiring therapy, hyperglycemia (random glucose >200 mg/dL or fasting level >126 mg/dL, or use of diabetes medications), myopathy assessments (hip flexor and quadriceps strength, 2-minute walk test, 5 time sit-to-stand, and adult myopathy assessment tool), hyperlipidemia (values elevated above normal or use of lipid-lowering medications), thrombotic microangiopathy (TMA),21 cytomegalovirus (CMV) reactivation requiring therapy, and patient-reported outcomes (PRO) (MD Anderson Symptom Inventory,22 FACT-BMT,23 SF-36,24 PedsQL25 for pediatric subjects).
Eligibility
Patients had to have MN-SR acute GVHD not previously treated with systemic acute GVHD therapy. No restriction was placed on age or prior HCT characteristics. Eligible subjects needed to be able to tolerate oral or enterically delivered medications and have an absolute neutrophil count higher than 500/μL. Excluded were prior therapy with sirolimus within 14 days of enrollment, relapsed malignancy post-HCT, prior donor lymphocyte infusion, TMA within 7 days of enrollment, uncontrolled infections, chronic GVHD, pregnancy or breast feeding, men or women of child-bearing potential unwilling to use effective birth control for the duration of the study, dialysis, mechanical ventilation, severe hepatic sinusoidal obstruction syndrome, and history of hypersensitivity to sirolimus.
Treatment description
Sirolimus (as tablet or oral solution) was given as a loading dose (6 mg orally once for those aged >12 years, or 5 mg/m2 orally once for those aged ≤12 years), followed by maintenance to continue therapeutic levels through at least 56 days (10-14 ng/mL until acute GVHD resolution, then 5-10 ng/mL after resolution until at least day 56). A recommended taper schedule was provided that would discontinue sirolimus by 3 months from the day 56 point. Recommended targets for concurrent calcineurin inhibitors were provided (tacrolimus, 3-7 ng/mL; cyclosporine, 120-200 ng/mL). Prednisone (or prednisone dose-equivalent) was initiated at 2 mg/kg/day and required to remain at this dose for 3 days. This starting prednisone dose was chosen on the basis of precedent in prior BMT CTN acute GVHD therapy trials5,26 to address the potential effect of heterogeneity in starting prednisone dose if not standardized, based on consensus among BMT CTN Steering Committee members in protocol development. A suggested taper was provided that decreased to 1 mg/kg/day after 7 days, 0.5 mg/kg/day at week 2, 0.25 mg/kg/day at week 3, 0.2 mg/kg/day at week 4, 0.1 mg/kg/day at week 5, 0.1 mg/kg/day every other day at week 6, and off at week 7. This suggested taper would allow patients with treatment-responsive GVHD in the prednisone group of the trial to reach less than 0.25 mg/kg by the day 28 assessment. Safety monitoring was performed (supplemental Methods).
Statistical methods
The targeted sample size (n = 120 MN-SR; AA1/2 patients) was selected to achieve a 90% confidence interval (CI) half width of 15% for the difference in day 28 CR/PR rates between groups. The sample size was chosen in this manner to attain a desirable level of precision for the treatment effect from this phase 2 study, rather than targeting a specific effect size for a hypothesis test of superiority or noninferiority. Baseline characteristics and study outcomes are summarized by treatment group, with patients classified according to the intention-to-treat principle. For day 28 CR/PR (primary end point), rates and 90% Wald CIs were estimated. For other end points, categorical outcomes are described using proportions and 95% Wald or exact CIs and compared between groups using Z tests of binomial proportions, Barnard’s exact unconditional tests, Fisher’s exact tests, and logistic regression models. Continuous outcomes are described using means, standard errors of means, medians, ranges, and interquartile ranges (IQRs) and compared within groups using 1-sample t-tests and Wilcoxon signed rank tests, and between groups using independent sample t-tests and Wilcoxon rank sum tests. Time to event outcomes used Kaplan-Meier and Aalen-Johansen estimators and their variance estimators and were compared by log-rank and Gray’s tests. Missing clinical and patient-reported outcome assessments were considered missing completely at random. All statistical tests are 2-sided at a .05 significance level. Analyses were completed using SAS version 9.4 and R version 3.4.0.
Results
Enrolled and randomized subjects
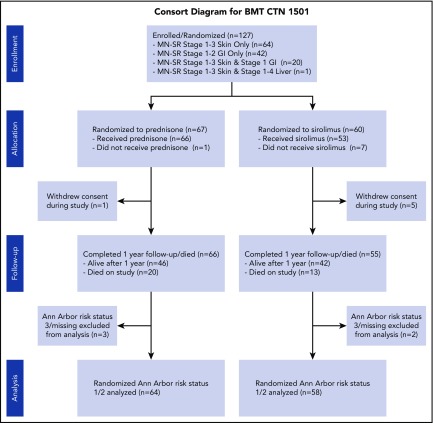
A total of n = 127 subjects were enrolled from 21 BMT CTN centers, with n = 122 identified as AA1/2 risk status (prednisone, n = 64; sirolimus, n = 58; Figure 1). Trial accrual opened 31 October 2016, with first enrollment on 15 November 2016 and last enrollment occurring on 14 February 2018. The number of patients screened for the trial at enrolling centers was not tracked. Four patients were AA3, and 1 had missing AA biomarker status. Baseline characteristics of the primary analysis population are presented in Table 1. Skin and upper GI were common in both study groups. Lower GI involvement was less common, and was greater in the prednisone group. Comprehensive data on biopsy of acute GVHD target organs is presented in supplemental data (supplemental Table 7). Among 31 subjects with isolated upper GI acute GVHD, 12 had biopsies performed, and all of these had pathologic evidence of acute GVHD. A total of 14 subjects randomized and treated with sirolimus did not complete 56 days of therapy as planned, for reasons including toxicity (n = 1), acute GVHD progression or flare (n = 6), insufficient response (n = 1), malignancy relapse/progression (n = 2), physician decision (n = 1), mixed chimerism (n = 1), anemia and thrombocytopenia (n = 1), and elevated sirolimus level (n = 1). Blood sirolimus levels achieved at serial points on trial were similar for those with or without GI involvement (supplemental Table 8).
Figure 1.
Consort diagram.
Table 1.
Baseline characteristics
| Prednisone (n = 64), N (%) | Sirolimus (n = 58), N (%) | Total (n = 122), N (%) | |
|---|---|---|---|
| AA score | |||
| AA1 | 45 (70.3%) | 38 (65.5%) | 83 (68.0%) |
| AA2 | 19 (29.7%) | 20 (34.5%) | 39 (32.0%) |
| Male sex | 33 (51.6%) | 37 (63.8%) | 70 (57.4%) |
| Age, y | |||
| Median (range) | 52.4 (0.9-72.7) | 58.0 (7.8-74.7) | 54.6 (0.9-74.7) |
| Pediatric (<18 y) | 3 (4.7%) | 1 (1.7%) | 4 (3.3%) |
| Median days of follow-up since enrollment (range) | 365.0 (13.0-365.0) | 365.0 (1.0-365.0) | 365.0 (1.0-365.0) |
| Skin GVHD stage at enrollment* | |||
| 0 | 19 (29.7%) | 20 (34.5%) | 39 (32.0%) |
| 1 | 11 (17.2%) | 8 (13.8%) | 19 (15.6%) |
| 2 | 13 (20.3%) | 14 (24.1%) | 27 (22.1%) |
| 3 | 21 (32.8%) | 16 (27.6%) | 37 (30.3%) |
| Upper GI GVHD stage at enrollment† | |||
| 0 | 36 (56.3%) | 32 (55.2%) | 68 (55.7%) |
| 1 | 28 (43.8%) | 26 (44.8%) | 54 (44.3%) |
| Lower GI GVHD stage at enrollment‡ | |||
| 0 | 56 (87.5%) | 56 (96.6%) | 112 (91.8%) |
| 1 | 7 (10.9%) | 2 (3.4%) | 9 (7.4%) |
| 2 | 1 (1.6%) | 0 (0.0%) | 1 (0.8%) |
| Liver GVHD stage at enrollment¶ | |||
| 0 | 63 (98.4%) | 58 (100.0%) | 121 (99.2%) |
| 1 | 1 (1.6%) | 0 (0.0%) | 1 (0.8%) |
| MN risk category | |||
| Stage 1-3 Skin | 32 (50.0%) | 31 (53.4%) | 63 (51.6%) |
| Stage 1-2 GI | 19 (29.7%) | 20 (34.5%) | 39 (32.0%) |
| Stage 1-3 Skin and stage 1 GI | 12 (18.8%) | 7 (12.1%) | 19 (15.6%) |
| Stage 1-3 Skin and stage 1-4 Liver | 1 (1.6%) | 0 (0.0%) | 1 (0.8%) |
| Acute GVHD grade at enrollment (consensus conference) | |||
| I | 16 (25.0%) | 16 (27.6%) | 32 (26.2%) |
| II | 47 (73.4%) | 42 (72.4%) | 89 (73.0%) |
| III | 1 (1.6%) | 0 (0.0%) | 1 (0.8%) |
| Organ involvement at enrollment | |||
| Skin stage 1 only | 6 (9.4%) | 5 (8.6%) | 11 (9.0%) |
| Skin stage 2 only | 8 (12.5%) | 11 (19.0%) | 19 (15.6%) |
| Skin stage 3 only | 18 (28.1%) | 15 (25.9%) | 33 (27.0%) |
| Upper GI only | 13 (20.3%) | 18 (31.0%) | 31 (25.4%) |
| Lower GI only | 1 (1.6%) | 1 (1.7%) | 2 (1.6%) |
| Multiple organs | 18 (28.1%) | 8 (13.8%) | 26 (21.3%) |
| Patients using topical steroids at enrollment | 36 (56.3%) | 32 (55.2%) | 68 (55.7%) |
| Donor type | |||
| Related BM or PB | 31 (48.4%) | 23 (39.7%) | 54 (44.3%) |
| Unrelated BM or PB | 29 (45.3%) | 32 (55.2%) | 61 (50.0%) |
| Unrelated Cord Blood | 4 (6.3%) | 3 (5.2%) | 7 (5.7%) |
| Karnofsky Performance Score | |||
| 90-100 | 42 (65.6%) | 26 (44.8%) | 68 (55.7%) |
| <90 | 21 (32.8%) | 32 (55.2%) | 53 (43.4%) |
| Missing | 1 (1.6%) | 0 (0.0%) | 1 (0.8%) |
| Primary disease | |||
| Acute leukemias | 34 (53.1%) | 28 (48.3%) | 62 (50.8%) |
| Hodgkin and non-Hodgkin lymphomas | 9 (14.1%) | 3 (5.2%) | 12 (9.8%) |
| Plasma cell disorder/multiple myeloma | 2 (3.1%) | 2 (3.4%) | 4 (3.3%) |
| Other leukemias | 7 (10.9%) | 6 (10.3%) | 13 (10.7%) |
| Nonmalignant disorders | 2 (3.1%) | 0 (0.0%) | 2 (1.6%) |
| Myelodysplastic/myeloproliferative disorders | 10 (15.6%) | 19 (32.8%) | 29 (23.8%) |
| Conditioning regimen intensity | |||
| Myeloablative | 35 (54.7%) | 32 (55.2%) | 67 (54.9%) |
| NMA/RIC | 29 (45.3%) | 26 (44.8%) | 55 (45.1%) |
| HLA match score | |||
| 8/8 BM or PB | 46 (71.9%) | 46 (79.3%) | 92 (75.4%) |
| 7/8 BM or PB | 2 (3.1%) | 3 (5.2%) | 5 (4.1%) |
| ≤6/8 BM or PB | 12 (18.8%) | 6 (10.3%) | 18 (14.8%) |
| Cord blood | 4 (6.3%) | 3 (5.2%) | 7 (5.7%) |
| Graft source | |||
| Bone marrow | 14 (21.9%) | 8 (13.8%) | 22 (18.0%) |
| Peripheral blood | 46 (71.9%) | 47 (81.0%) | 93 (76.2%) |
| Cord blood | 4 (6.3%) | 3 (5.2%) | 7 (5.7%) |
| GVHD prophylaxis | |||
| CNI-based | 49 (76.6%) | 44 (75.9%) | 93 (76.2%) |
| Post-CY + other(s) | 15 (23.4%) | 14 (24.1%) | 29 (23.8%) |
| Donor-recipient CMV status | |||
| ± | 18 (28.1%) | 25 (43.1%) | 43 (35.2%) |
| Others | 42 (65.6%) | 30 (51.7%) | 72 (59.0%) |
| Cord blood | 4 (6.3%) | 3 (5.2%) | 7 (5.7%) |
| Donor-recipient sex matching | |||
| Female donor, male recipient | 12 (18.8%) | 9 (15.5%) | 21 (17.2%) |
| Others | 52 (81.3%) | 49 (84.5%) | 101 (82.8%) |
BM, bone marrow; BSA, body surface area; CB, cord blood; CNI, calcineurin inhibitor; CMV, cytomegalovirus; CY, cyclophosphamide; GI, gastrointestinal; GVHD, graft-versus-host disease; NMA/RIC, nonmyeloablative/reduced-intensity conditioning; PB, peripheral blood.
Stage 0 = no active (erythematous) GVHD rash, stage 1, maculopapular rash <25% BSA; stage 2, maculopapular rash 25% to 50% BSA; stage 3, maculopapular rash >50% BSA.
Stage 0, no or intermittent nausea, vomiting, or anorexia; stage 1, persistent nausea, vomiting, or anorexia.
Stage 0, diarrhea, adult: 0 to 499 mL/day, 0 to 2 episodes/day; child: 0 to 9 mL/kg/day, 0 to 3 episodes/day; stage 1, diarrhea, adult: 500 to 999 mL/day, 3 to 4 episodes/day; child: 10 to 19.9 mL/kg/day, 4 to 6 episodes/day; stage 2, diarrhea, adult: 1000 to 1500 mL/day, 5 to 7 episodes/day; child: 20 to 30 mL/kg/day, 7 to 10 episodes/day
Stage 0, bilirubin <2.0 mg/dL; stage 1, bilirubin 2.0-3.0 mg/dL.
Acute GVHD therapy response
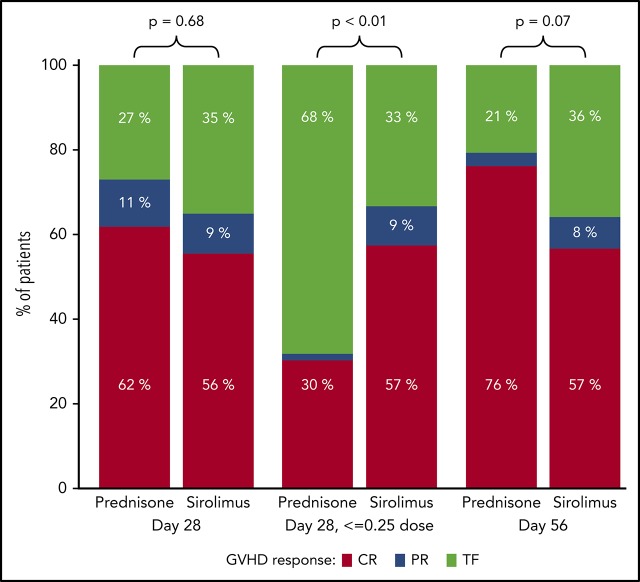
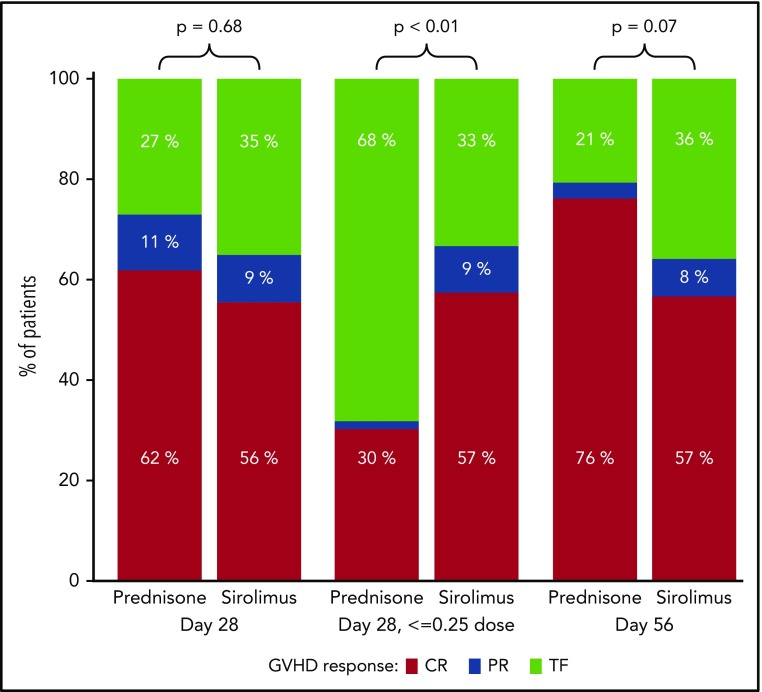
A total of n = 117 (from the n = 122 AA1/2 subjects) were evaluable for the primary end point analysis; 5 subjects were unevaluable because of withdrawal from study before day 28. Of the n = 117 subjects, 64.8% in the sirolimus group and 73.0% in the prednisone group had a CR/PR at day 28 (Figure 2), an estimated difference of −8.2% (90% CI, −22.3%-5.9%). Subgroup analyses according to GVHD and HCT features demonstrated similar findings (supplemental Figure 1), but suggested greater response for prednisone for acute GVHD after HLA-mismatched HCT. In a post hoc analysis, we excluded those with skin stage 1 to 2 or isolated upper GI disease to evaluate a potentially higher-risk subgroup within the study population. We found that CR/PR rates were comparable for sirolimus (n = 24) and prednisone (n = 37) at day 28 (73.9% [95% CI, 56.0%-91.9%] vs 70.3% [95% CI, 55.6%-85.0%]) and at day 56 (73.9% [95% CI, 56.0%-91.9%] vs 75.7% [95% CI, 61.9%-89.5%]). By day 56, n = 116 subjects were evaluable; nonevaluable subjects included withdrawal from study (n = 5) and missing GVHD staging information at day 56 (n = 1). Among evaluable subjects, CR/PR was achieved by 64.2% in the sirolimus group and 79.4% in the prednisone group, with an observed difference of −15.2% (95% CI, −31.5%-1.1%). Detailed response categories (CR, PR, MR, NR, progression) at days 28 and 56 are presented in supplemental Table 1. Nonresponse was significantly greater at day 56 in the sirolimus group. A total of 23 patients in the sirolimus group received systemic steroids after sirolimus during the first 56 days of follow-up on trial, with a median starting dose of 1 mg/kg/day (interquartile range, 0.96-1.80 mg/kg/day). Among these 23 patients, the GVHD response before steroid start varied (n = 7 with steroid initiated within week 1 on trial [hence no prior response data available]; n = 4 with CR; n = 3 with PR; n = 2 with MR; n = 5 with NR; and n = 2 with PD). Reasons for initiating steroids among those with preceding CR included GVHD persistence/flare (n = 2), pure red cell aplasia resulting from ABO incompatibility (n = 1), and anemia and thrombocytopenia (n = 1). For those with preceding PR, steroids were started for GHVD persistence/flare (n = 2) or unknown cause (n = 1). Among these 23 patients, the subsequent day 56 acute GVHD response was n = 13 with CR, n = 4 with PR, n = 2 with MR, and n = 4 with NR. There was no evidence of excess steroid-refractory acute GVHD among sirolimus-treated subjects: A total of n = 23 (39.7%) in the sirolimus group received any systemic steroid therapy, of whom n = 2 (3.4% of total, 8.7% of steroid recipients) received subsequent immune suppressive therapy. In contrast, n = 63 (98.4%) in the prednisone group were treated with prednisone per intended randomized therapy, and 14 (21.9% of total, 22.2% of steroid recipients) received additional immune suppressive therapy.
Figure 2.
Comparison of treatment responses at day 28 and 56 for prednisone vs sirolimus. TF (treatment failure) includes MR, NR, and progression. Prednisone use in the sirolimus group was considered TF for day 28 response and day 56 response.
Steroid-sparing effect of sirolimus therapy
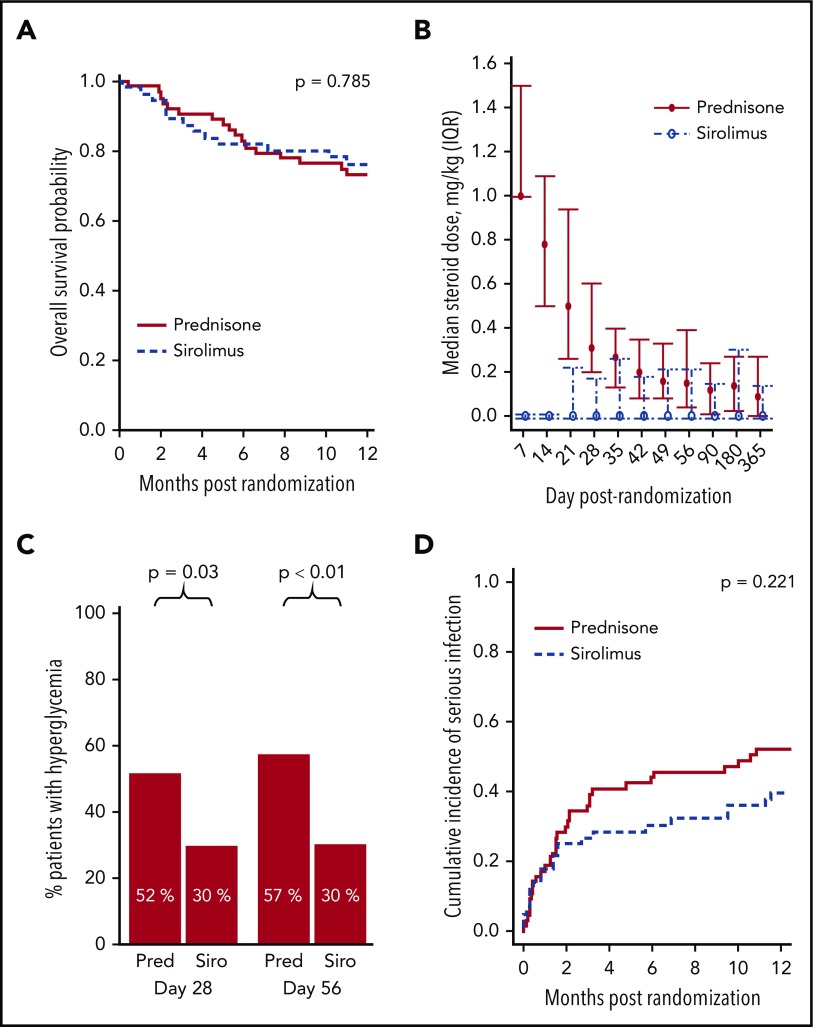
The key secondary end point (day 28 CR/PR with ≤0.25 mg/kg/day prednisone) was significantly improved in the sirolimus group: 66.7% for sirolimus vs 31.7% for prednisone (P < .001). Steroid exposure was lower in the sirolimus group (Figure 3B). The average daily dose of steroid through 56 days was lower for sirolimus (median, 0.00 mg/kg; IQR, 0.00-0.28 mg/kg) vs prednisone (median, 0.46 mg/kg; IQR, 0.00-2.50 mg/kg; P < .001). At randomization, 55.2% of sirolimus patients and 56.3% of prednisone patients were receiving topical steroids. Among those who were not, 50% of the sirolimus patients and 75% of the prednisone patients initiated topical therapy after randomization (P = .056).
Figure 3.
Sirolimus therapy was associated with similar overall survival, yet improved steroid exposure, hyperglycemia, and serious infections. (A) Overall survival. (B) Steroid dose from baseline through day 365 (median, IQR). (C) Hyperglycemia status (random glucose >200 mg/dL or fasting level >126 mg/dL, or use of diabetes medications) at day 28 and 56. (D) Cumulative incidence of serious (grade II-III) infections.
Toxicity and infectious complications
Toxicities and causes of death are presented in supplemental Table 2. Hyperglycemia was lower in the sirolimus group (Figure 3C): Adjusting for baseline hyperglycemia, sirolimus was associated with lower odds at day 28 (0.40; 95% CI, 0.18-0.91; P = .029) and day 56 (0.33; 95% CI, 0.15-0.74; P = .007). No significant difference was observed at day 180 (odds ratio, 0.54; 95% CI, 0.23-1.3; P = .16). Adjusting for baseline hyperlipidemia status, there was no evidence in logistic regression models of a significant effect of treatment group on day 28, 56, or 180 for any of the lipid profile measures or medication use (P > .05 for all). The rate of TMA within 6 months of randomization was higher in the sirolimus group vs the prednisone group (10.3% vs 1.6%; P = .041). Maximum TMA grade for sirolimus was I (n = 3), III (n = 2), or IV (n = 1), and for prednisone it was I (n = 1).
Frequency, time of onset, and severity of infections are presented in supplemental Table 3. Infection grading followed the BMT CTN technical manual of procedures. The cumulative incidence of serious (grade 2-3) infections did not differ significantly between groups (Figure 3D; P = .221). For those at risk (prior donor and/or recipient CMV seropositivity), the rate of CMV reactivation requiring therapy by 6 months was 9.1% for sirolimus vs 2.2% for prednisone (P = .172). Epstein-Barr virus requiring therapy or development of posttransplant lymphoproliferative disease was uncommon: By 6 months, the cumulative incidence was 3.6% (95% CI, 0.7%-11%) for sirolimus vs 1.6% (95% CI, 0.1%-7.4%) for prednisone (P = .126).
Long-term HCT outcomes
No differences were detected in 12-month outcomes between the sirolimus and prednisone groups, respectively (overall survival, 76.3% [95% CI, 62.7%-85.5%] vs 73.2% [95% CI, 60.4%-82.4%; Figure 3A); disease-free survival, 61.6% [95% CI, 47.4%-73.0%] vs 70.2% [95% CI, 57.3%-79.8%]; event-free survival, 35.9% [95% CI, 23.4%-48.5%] vs 31.2% [95% CI, 20.4%-42.7%]; nonrelapse mortality, 16.5% [95% CI, 8.1%-27.6%] vs 14.2% [95% CI, 6.9%-24.0%]; relapse, 21.9% [95% CI, 12.0%-33.7%] vs 15.7% [95% CI, 8.0%-25.7%]; chronic GVHD, 31.4% [95% CI, 19.5%-44.0%] vs 40.6% [95% CI, 28.4%-52.4%]; GVHD-free survival, 50.9% [95% CI, 37.5%-64.4%] vs 46.0% [95% CI, 33.7%-58.3%]). Complete discontinuation of immune suppressive therapy was significantly improved in the sirolimus group (Figure 4A; P = .049).
Figure 4.
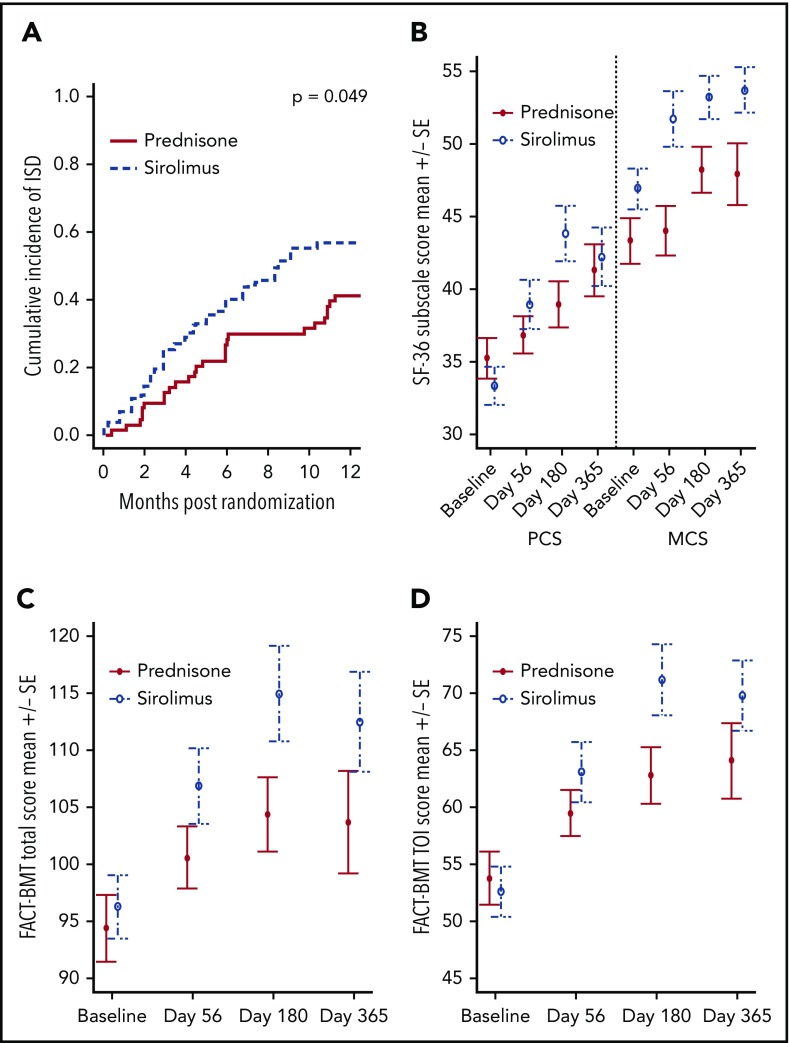
Sirolimus treatment resulted in improved immune suppression discontinuation and improved patient-reported quality of life. (A) Cumulative incidence of complete discontinuation of all systemic immune suppressive medications, with death as competing risk event. (B) SF-36 physical component score (PCS) and mental component score (MCS) changes over time. (C) FACT-BMT total score over time. (D) FACT-BMT trial outcome index (TOI) score over time. Change from baseline to day 180 was significantly greater for sirolimus vs prednisone for SF-36 PCS (P = .012) and for FACT-BMT TOI (P = .034).
Functional measures and patient-reported outcomes
Change in myopathy assessments from baseline to days 56 and 180 were evaluated and compared across study groups (supplemental Tables 4 and 5). For the 2-minute walk test, the walking distance increased from baseline to day 56 for both the prednisone and sirolimus groups (P = .006 and .001, respectively). Other within- and between-group comparisons were not statistically significant (P > .05 for all).
Changes in patient-reported outcome measures from baseline to days 56, 180, and 365 were examined within treatment groups and compared across groups (Figure 4B-D; supplemental Table 6). Improvement in SF-36 PCS, FACT-BMT total, and FACT-BMT TOI scores from baseline to day 180 were statistically and clinically significantly greater for the sirolimus vs prednisone group.
AA3/missing subjects
A total of n = 4 subjects (n = 3 for prednisone, n = 1 for sirolimus) with AA3 status were enrolled and their outcomes followed in a descriptive manner. Outcome was poor, with all receiving additional immune suppressive therapy beyond randomized therapy and death occurring in all cases, ranging from 18 to 122 days postenrollment. The 1 case with missing biomarker status (sirolimus group) survived the 1-year study follow-up period.
Discussion
This randomized multicenter phase 2 trial is the first to prospectively demonstrate comparable efficacy of sirolimus vs 2 mg/kg/day prednisone for initial therapy of SR acute GVHD. As well, it for the first time demonstrates the feasibility of clinically and centrally assessed biomarker-based risk-stratified acute GVHD therapy. The data support that approximately two-thirds of patients with this SR phenotype (MN-SR, AA1/2 biomarker acute GVHD) can achieve durable CR/PR with sirolimus alone, and that the remainder can be salvaged with prednisone with no excess risk of developing steroid-refractory disease. Sirolimus as a first-line therapy is associated with reduction in steroid exposure and allied steroid-related complications, including hyperglycemia, which has been previously associated with adverse outcome in this population.27,28 Importantly, use of prednisone after sirolimus was at the discretion of the treating clinician, and not controlled by the protocol. These data support that sirolimus primary therapy does not adversely affect post-HCT survival, nonrelapse mortality, or risk for malignancy relapse and, except the known risk for TMA, is not associated with increased risk for toxicity. Impressively, sirolimus therapy was associated with significant improvements in complete discontinuation of immune suppressive therapy and patient-reported quality of life, which are critical long-term measures of successful HCT outcome.29-31
These data are largely generalizable to patients with SR GVHD as defined by the MN GVHD Risk Score. We note that the trial eligibility was open to the full extent of MN-SR disease, and that all enrolled subjects were deemed appropriate by their treating physician for systemic immune suppressive therapy. However, screening of patients for the trial at individual centers was not tracked, and thus we cannot describe the total population of patients considered (vs enrolled) for the trial. As well, in prior analyses that developed9 and validated10 the MN acute GVHD risk score, 80% (grade I, 25%; grade II, 55%) to 64% (grade I, 11%; grade II, 53%) of subjects studied respectively received high-dose systemic steroid therapy for overall initial grade I to II acute GVHD. These data speak to common use of systemic steroid therapy in this context. Comparing the enrolled MN-SR population with that previously reported,8,9 these are comparable except that stage 1 to 2 GI involvement was overrepresented with a greater proportion of isolated upper GI disease in this trial. We note that isolated upper GI disease in the prior analysis had greater likelihood of day 28 CR/PR and decreased nonrelapse mortality. In the previously published MN-SR population,9 approximately 20% of subjects had any lower GI acute GVHD (alone or in combination with skin or upper GI GVHD), whereas among the population enrolled on this trial, 8% had lower GI involvement with an overrepresentation in the prednisone group vs the sirolimus group. Importantly, randomization on this trial was stratified for center, but not for GVHD, organ involvement. On the basis of the limited number of sirolimus-treated subjects with lower GI GVHD, additional study is needed to permit more definitive conclusions in this subgroup. Finally, liver involvement was low, in keeping with the expected 4% involvement in the prior data.8-10
The quality-of-life differences observed are important and deserve further detailed study, including attention to specific patient- and treatment-level effects. As well, detailed long-term follow-up through the Center for International Blood and Marrow Transplant Research will facilitate study of long-term immune suppression discontinuation beyond the 1-year trial follow-up period. In prior work,32,33 later post-HCT follow-up and detailed attention to immune suppression discontinuation failure was needed to model this outcome. Next, our steroid myopathy measures were not sensitive to the reduction in steroid exposure across the study groups, suggesting further study is needed. We note that the trial had limited representation of pediatric subjects, acute GVHD occurring after mismatched unrelated or umbilical cord blood HCT, or acute GVHD occurring after diverse initial GVHD prevention practices (although nearly 25% had previously received post-HCT cyclophosphamide-based prophylaxis).
In conclusion, these data support that among MN-SR, AA1/2 acute GVHD, sirolimus achieves comparable responses as 2 mg/kg/day prednisone, spares steroid exposure, and results in similar long-term survival outcomes. A definitive randomized phase 3 noninferiority trial is needed, however, both to confirm these findings and to further explore treatment efficacy in SR acute GVHD subgroups.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Adam Mendizabal, Amelia Langston, Amy Foley, Anne Renteria, Betty Hamilton, Eric Leifer, George Morales, Greg Yanik, Hisham Abdel-Azim, Iris Gersten, John McCarty, John Wingard, Kate Bickett, Lauren Kunz, Mitchel Horwitz, Monzr Al Malki, Muna Qayed, Scott Solomon, Sunil Abhyankar, Vincent Ho, and William Hogan. Centers participating in this trial: Blood and Marrow Transplant program at Northside Hospital, Children's Healthcare of Atlanta, Children’s Hospital Los Angeles, City of Hope National Medical Center, Cleveland Clinic Foundation, Duke University, Emory University, Fred Hutchinson Cancer Research Center, H. Lee Moffitt Cancer Center, Mayo Clinic-Rochester, Medical College of Wisconsin, The Icahn School of Medicine at Mount Sinai, The Ohio State University, University of Florida College of Medicine, University of Kansas Hospital, University of Michigan, University of Minnesota, University of Texas/MD Anderson Cancer Center, Vanderbilt University Medical Center, Virginia Commonwealth University, and Washington University. Samples were tested for the Ann Arbor acute GVHD risk score at the Biomarker Laboratory of the Icahn School of Medicine at Mount Sinai.
The Blood and Marrow Transplant Clinical Trials Network is supported in part by grants #U10HL069294 and #U24HL138660 from the National Heart, Lung, and Blood Institute and the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also acknowledge Center for International Blood and Marrow Transplantation Research support from Amgen, Magenta, Gamida Cell, and Medac.
Footnotes
For original data, please contact the corresponding author.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.P. and M.L.M. designed the research, performed the research, analyzed data, and wrote the paper; M.H., P.D., M.M., A.M.A., M.J., Y.A.E., S.C., I.P., S.G.H., J.L.M.F., J.E.L., M.M., C.A., J.H.A., J.B.-M., A.H., B.R.L., E.S.L., T.S.P., and M.M.H. contributed to the design and conduct of the research and contributed to analysis of data and manuscript writing.
Conflict-of-interest disclosure: M.H. received research funding from Takeda, Otsuka, Spectrum, Astellas, and Incyte, and served on the advisory board of Pharmacyclics. M.J. served on the advisory board for and had a consultancy with Incyte; received research funding from Mallinckrodt and Janssen; had a consultancy with Kadmon: and served on the advisory board for Incyte. Y.A.E. received research funding from Celgene; served on the speaker’s bureau for Janssen and Akcea, and served on the independent review committee and speaker’s bureau for Takeda. S.G.H. served on the advisory board for Incyte and BMS. J.L.M.F. had a consultancy and intellectual property rights with Viracor and a consultancy with Xenikos. J.E.L. has royalties and intellectual property rights with Viracor and a consultancy with Therakos, Novartis, Ironwood, Incyte, and Bluebird. M.M.H. receives research funding from Celgene and Novartis. The remaining authors declare no competing financial interests.
Correspondence: Joseph Pidala, Blood and Marrow Transplantation and Cellular Immunotherapy, H Lee Moffitt Cancer Center and Research Institute, 12902 Magnolia Dr, FOB 3308, Tampa, FL 33612; e-mail: joseph.pidala@moffitt.org.
REFERENCES
- 1.Anasetti C, Logan BR, Lee SJ, et al. ; Blood and Marrow Transplant Clinical Trials Network . Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cutler C, Logan B, Nakamura R, et al. . Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratwohl A, Brand R, Apperley J, et al. ; Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (CLWP-EBMT) . Graft-versus-host disease and outcome in HLA-identical sibling transplantations for chronic myeloid leukemia. Blood. 2002;100(12):3877-3886. [DOI] [PubMed] [Google Scholar]
- 4.Levine JE, Logan B, Wu J, et al. ; Blood and Marrow Transplant Clinical Trials Network . Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolaños-Meade J, Logan BR, Alousi AM, et al. . Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124(22):3221-3227, quiz 3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alousi AM, Weisdorf DJ, Logan BR, et al. . Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacMillan ML, Weisdorf DJ, Wagner JE, et al. . Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387-394. [DOI] [PubMed] [Google Scholar]
- 8.MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157(6):732-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacMillan ML, Robin M, Harris AC, et al. . A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacMillan ML, DeFor TE, Holtan SG, Rashidi A, Blazar BR, Weisdorf DJ. Validation of Minnesota acute graft-versus-host disease Risk Score [published online ahead of print 18 July 2019]. Haematologica. doi:10.3324/haematol.2019.220970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine JE, Braun TM, Harris AC, et al. ; Blood and Marrow Transplant Clinical Trials Network . A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21-e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartwell MJ, Özbek U, Holler E, et al. . An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2(3):e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu Zaid M, Wu J, Wu C, et al. . Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood. 2017;129(2):162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive value of measurements before GVHD onset and treatment. Blood. 2015;126(1):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponce DM, Hilden P, Mumaw C, et al. . High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125(1):199-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mielcarek M, Storer BE, Boeckh M, et al. . Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113(13):2888-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mielcarek M, Furlong T, Storer BE, et al. . Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100(6):842-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appelbaum FR, Anasetti C, Antin JH, et al. . Blood and marrow transplant clinical trials network state of the Science Symposium 2014. Biol Blood Marrow Transplant. 2015;21(2):202-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pidala J, Tomblyn M, Nishihori T, et al. . Sirolimus demonstrates activity in the primary therapy of acute graft-versus-host disease without systemic glucocorticoids. Haematologica. 2011;96(9):1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. . National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945-956. [DOI] [PubMed] [Google Scholar]
- 21.Ho VT, Cutler C, Carter S, et al. . Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571-575. [DOI] [PubMed] [Google Scholar]
- 22.Cleeland CS, Mendoza TR, Wang XS, et al. . Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634-1646. [DOI] [PubMed] [Google Scholar]
- 23.McQuellon RP, Russell GB, Cella DF, et al. . Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19(4):357-368. [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Ware JE Jr., Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247-263. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. [DOI] [PubMed] [Google Scholar]
- 26.Alousi AM, Weisdorf DJ, Logan BR, et al. ; Blood and Marrow Transplant Clinical Trials Network . Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pidala J, Kim J, Kharfan-Dabaja MA, et al. . Dysglycemia following glucocorticoid therapy for acute graft-versus-host disease adversely affects transplantation outcomes. Biol Blood Marrow Transplant. 2011;17(2):239-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammer MJ, Casper C, Gooley TA, O’Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2009;15(3):344-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pidala J, Lee SJ, Quinn G, Jim H, Kim J, Anasetti C. Variation in management of immune suppression after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(10):1528-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jim HS, Quinn GP, Gwede CK, et al. . Patient education in allogeneic hematopoietic cell transplant: what patients wish they had known about quality of life. Bone Marrow Transplant. 2014;49(2):299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pidala J, Martens M, Carreras J, et al. . Multi-state modeling identifies determinants of successful immune suppression discontinuation: Secondary analysis of BMT CTN 0201 and 0402 trials. Biol Blood Marrow Transplantation. 2017;23(3):S88-S90. [Google Scholar]
- 33.Pidala J, Martens M, Anasetti C, et al. . Factors associated with successful discontinuation of immune suppression after allogeneic hematopoietic cell transplantation [published online ahead of print 26 September 2019]. JAMA Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.