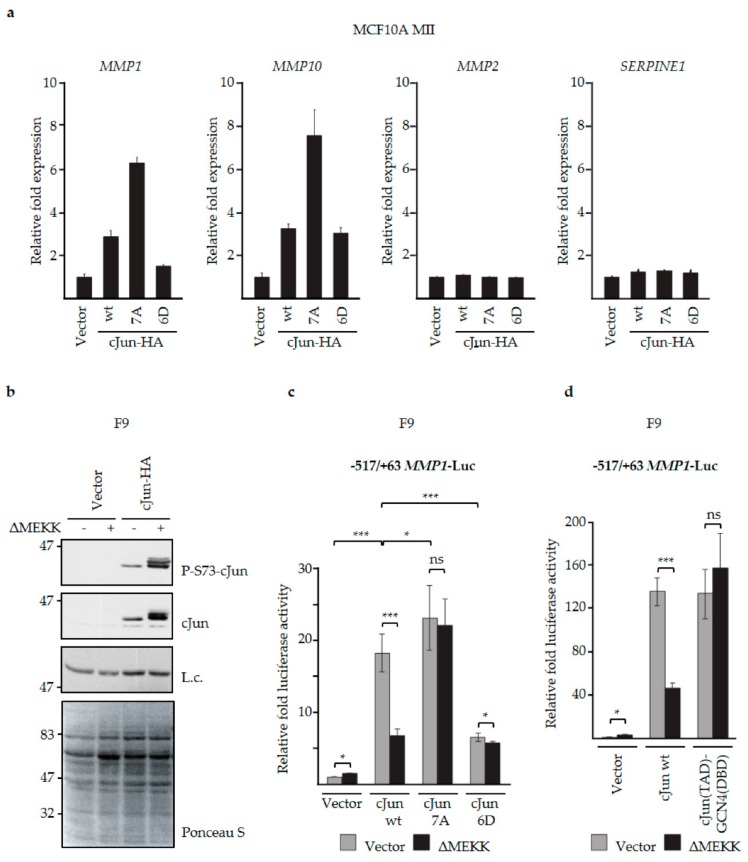
Figure 4.
N-terminal cJun phosphorylation negatively affected the activation of MMP1 and MMP10. (a) MCF10A MII cells were transfected with expression vectors for wt cJun as well as cJun-6D and -7A mutants, and MMP1, MMP10, MMP2, and SERPINE1 mRNA levels were analyzed by qPCR and normalized to GAPDH, which was not significantly affected by the treatments; (b) F9 cells were transfected with expression vectors for constitutively active ΔMEKK and cJun-HA as indicated. After 6 h, cell extracts were prepared for immunoblotting analysis with antibodies specific for phosphorylated cJun-Ser73 or total cJun. A background band of the cJun blot (L.c.) and Ponceau S staining were included as loading control; (c) F9 cells were transfected with the -517/+63 pGL3 MMP1 luciferase reporter and expression vectors for constitutively active ΔMEKK and wt cJun, and cJun-6D or -7A mutants, as indicated. After 6 h, cell extracts were prepared and luciferase activity was analyzed; *p < 0.05, ***p < 0.001. (d) F9 cells were transfected with the -517/+63 pGL3 MMP1 luciferase reporter and expression vectors for constitutively active ΔMEKK and cJun wt or cJun-GCN4—a chimeric protein in which the DNA-binding domain (DBD) of cJun is replaced by the corresponding DNA-binding domain of GCN4, as indicated (TAD: transactivation domain). After 16 h, cell extracts were prepared and luciferase activity was analyzed.