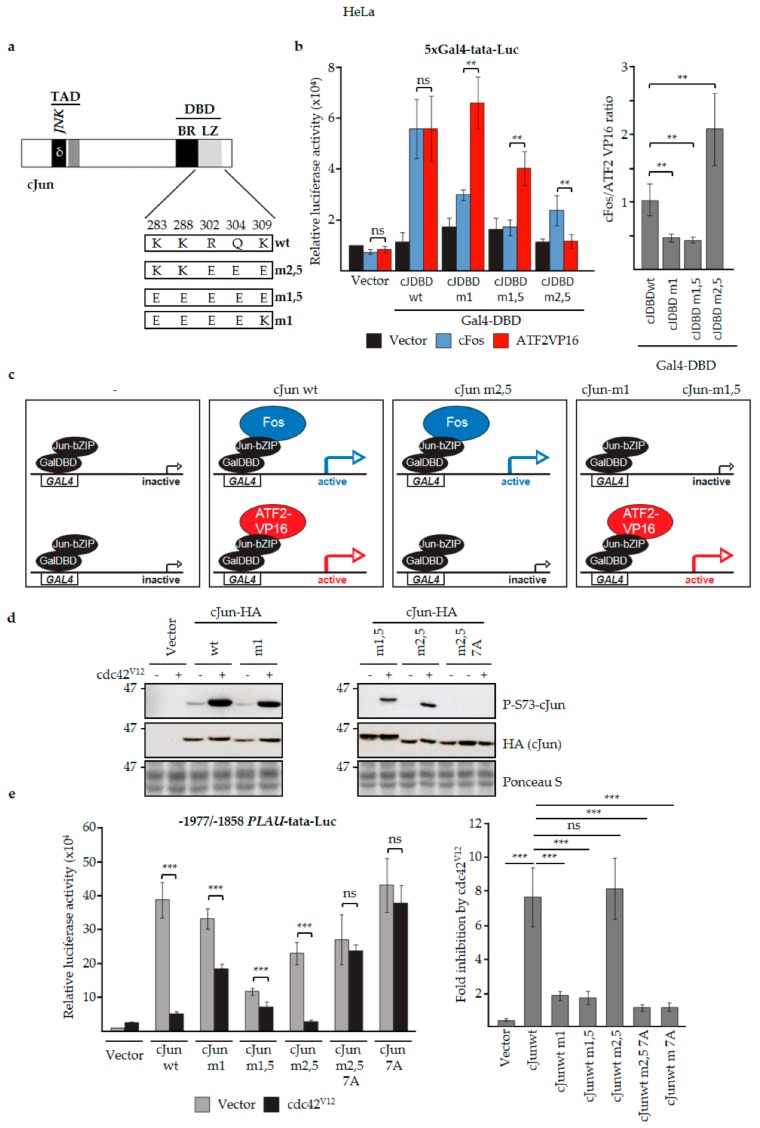
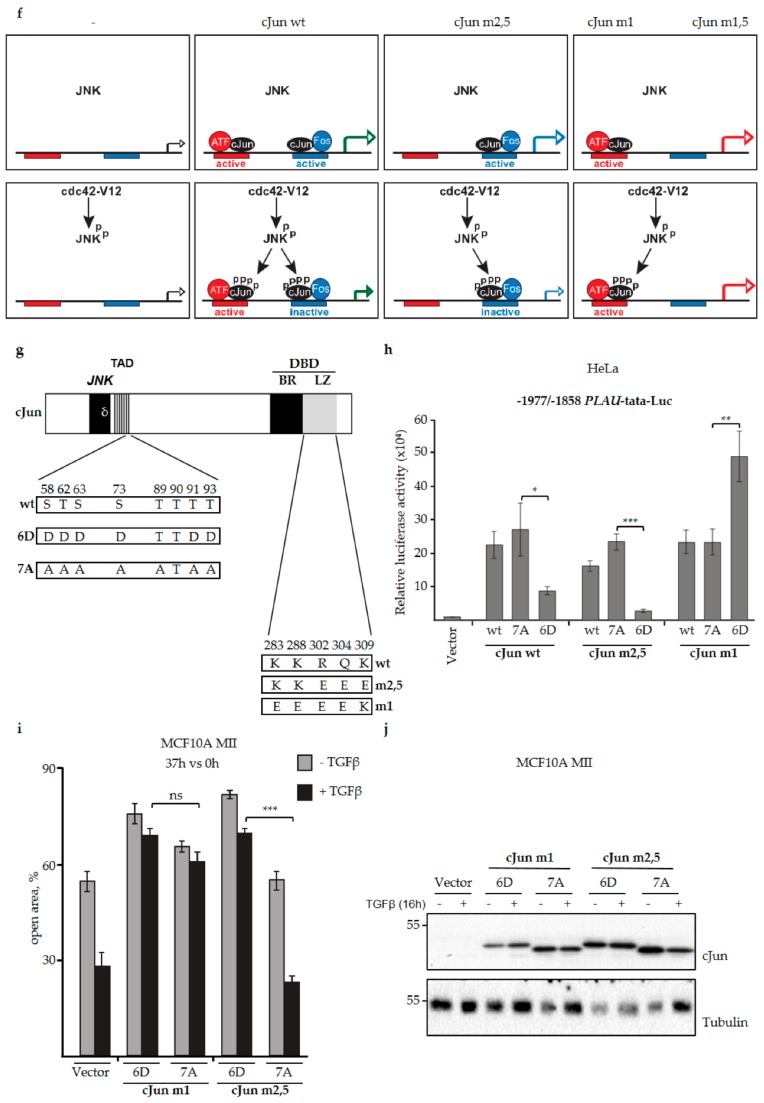
Figure 5.
JNK-dependent hyperphosphorylation of cJun specifically inhibits gene activation and migration by Jun/Fos dimers. (a) cJun mutants with altered Jun/Fos and Jun/ATF2 dimerization specificities. Schematic representation showing the changes in the mutants m2,5, m1,5, and m1 compared to wt cJun. Numbers correspond to the positively charged cJun amino acid residues at the e and g positions of the cJun leucine zipper (LZ) α-helix that stabilize cJun/Fos heterodimers [60,74], some of which were mutated to negatively charged amino acid residues. (b) Left: HeLa thymidine kinase (tk)− cells were cotransfected with the 5xGal4-tata luciferase reporter, the indicated wt and mutant GAL4DBD-cJunDBD (cJDBD) fusion constructs and/or ATF2-VP16 or cFos vectors, and luciferase activity was measured. Right: the luciferase activity ratio of the fold activation by Fos and ATF2VP16 on the indicated Jun constructs. cJDBD, the basic region-leucine zipper (bZIP) DNA-binding domains of the cJun mutants m2,5, m1,5, and m1 or wt cJun fused to the Gal4 DBD, as depicted schematically in panel (c); *p < 0.05, **p < 0.01, ***p < 0.001. (c) Schematic representation of the cJun DBD mammalian-one-hybrid analysis [60] described in panel (b). GAL4 represents one of the five GAL4 transcription factor binding sites of the 5xGal4-tata luciferase reporter; GalDBD-JunbZIP represents the transcriptionally inactive wt and mutant GAL4DBD-cJunDBD (cJDBD) fusion constructs used in (b) which bind to the GAL4 site but by themselves do not activate (left panels). The upper panels show the activation of the reporter by GalDBDJunbZIP-wt and -m2,5 when cFos is co-expressed; the lower panels show the activation by GalDBDJunbZIP-wt, -m1, and -m1,5 when ATF2VP16 is co-expressed. ATF2VP16 is used because ATF2 wt is not active in these assays. (d) HeLa tk- cells were transfected with expression vectors for cdc42V12 (a constitutively active upstream activator of the JNK pathway) and wt and mutant cJun-HA, as indicated. After 16 h, cell extracts were prepared for immunoblot analysis with antibodies specific for phosphorylated cJun-Ser73 or cJun-HA. (e) Left: HeLa tk- cells were transfected with the -1977/-1858 PLAU tata luciferase reporter, cdc42V12, and the (HA-tagged) cJun vectors, as indicated. After 16 h, cell lysates were prepared. Right: the fold inhibition by cdc42V12 on the different cJun constructs; average of at least six independent biological replicates. (f) Schematic representation of the regulation of the -1977/-1858-PLAU enhancer by cJun/Fos and cJun/ATF2 dimers in the absence and presence of cdc42V12, a constitutively active upstream activator of the JNK pathway. The upper panels depict the observed enhancer activation by cJun/Fos (formed by cJun wt and cJun m,2,5) and cJun/ATF (formed by cJun wt, cJun m1, and cJun m,1,5) in the absence of cdc42V12. The lower panels show the observed enhancer activation upon phosphorylation of JNK and cJun in response to cdc42V12.. Under these conditions, enhancer activation by cJun/Fos (formed by cJun wt and cJun m,2,5) was severely reduced. (g) Schematic representation of the combined phospho-site (Figure 1a) and dimerization (Figure 5a) mutants of cJun used. (h) HeLa tk- cells were transfected with the -1977/-1858 PLAU tata luciferase reporter and HA-tagged wt cJun, and the cJun-7A and -6D mutants, as indicated. After 16 h cell lysates were prepared. (i,j) Migration of MCF10A MII cells stably overexpressing the HA-tagged Fos- (m2,5) or ATF- (m1) preferring variants of cJun-6D and cJun-7A, as measured by wound-healing assays for 37 h (i) and immunoblot validation of (HA) expression levels (j).