Abstract
Background
In PARADIGMS, a double-blind phase III trial in 215 paediatric patients with multiple sclerosis (MS) (10 to <18 years), fingolimod administered for up to 2 years significantly reduced the annualised relapse rate (ARR) and rate of new/newly enlarged T2 (n/neT2) lesions compared with interferon (IFN) β-1a.
Objectives
To investigate (1) differences between treatment groups across subpopulations (treatment-naïve, younger/prepubertal patients); (2) disability progression.
Methods
ARRs at 10, 11 and 12 years were estimated based on predefined modelling extrapolations. Changes in Expanded Disability Status Scale (EDSS), and in 3 month (3M) and 6 month (6M) confirmed disability progression (CDP) were evaluated post hoc.
Results
In the treatment-naïve subpopulation, fingolimod reduced ARR and n/neT2 lesions by 85.8% and 53.4%, respectively versus INF β-1a (both p<0.001), compared with 81.9% and 52.6% in the overall population. Model-based ARR reductions in younger patients (≤12 years) were 91.9%–94.6%. Twice as many IFN β-1a-treated than fingolimod-treated patients had worse EDSS scores at study end (20.6% vs 10.5%, p=0.043). Risk reductions in 3M-CDP and 6M-CDP were 77.2% (p=0.007) and 80.2% (p=0.040), respectively.
Conclusions
Fingolimod in paediatric MS was associated with consistent control of disease activity versus IFN β-1a (including treatment-naïve and younger patients) and resulted in less disability progression for up to 2 years.
Trial registration number
Keywords: fingolimod, paediatric multiple sclerosis, disability progression, PARADIGMS, gilenya
Introduction
Paediatric-onset multiple sclerosis (MS) compared with adult-onset MS is characterised by increased clinical and radiological disease activity suggestive of a more inflammatory initial course.1 2 This observation supports the rationale for prompt initiation of effective immunomodulatory therapies in children and adolescents with MS. However, efficacy, safety and tolerability data of available therapies in the paediatric MS population so far have been derived from retrospective and open-label studies rather than randomised controlled trials. Until recently, treatment options were limited to injectable agents such as β-interferons and glatiramer acetate, which are approved by the European Medicines Agency for use in children over 12 years of age,3 4 but in the absence of randomised controlled trial data. These agents are not approved for paediatric relapsing MS in the USA. Therapeutic choices in paediatric MS should be based on controlled trials and be expanded to include newer and more effective agents.5 6
PARADIGMS, the first randomised controlled trial completed in paediatric patients with MS, assessed the efficacy and safety of the oral sphingosine 1-phosphate receptor modulator fingolimod compared with intramuscular interferon beta-1a (IFN β-1a) administered for up to 2 years in 215 children and adolescents from 10 to <18 years of age.7 The primary efficacy end point (annualised relapse rate (ARR)) and key secondary end point (annualised rate of new/newly enlarged T2 (n/neT2) lesions) were met, demonstrating superior efficacy of fingolimod versus IFN β-1a (81.9% reduction in ARR and 52.6% reduction in n/neT2 lesions with fingolimod vs IFN β-1a). As a result, fingolimod became the first Food and Drug Administration-approved drug for the treatment of relapsing MS in paediatric patients in the USA; fingolimod has also been approved for paediatric use in other regions, including the European Union.8 9
Here, we present analyses that assess the robustness of treatment effects, and provide further insights on the efficacy of fingolimod in paediatric subpopulations. These include predefined supportive and sensitivity analyses of the primary and key secondary outcomes: (1) excluding patients with major protocol deviations (ie, predefined deviations that could confound the interpretation of the analyses), (2) excluding patients in the INF treatment group that are positive for IFN antibodies, (3) evaluating treatment-naïve patients. Treatment effects on confirmed relapses in patients ≤12 years of age, in whom data are limited, are also explored. A similar focus is given to prepubertal versus pubertal patients, as puberty and associated hormonal changes are suspected to influence the course of the disease.10–12 Finally, we conducted post hoc analyses to explore the effect of fingolimod on the Expanded Disability Status Scale (EDSS) and disability progression up to 2 years.
Methods
PARADIGMS study design and participants
Study design
PARADIGMS was a double-blind, double-dummy, active-controlled, parallel-group, multicentre study involving 87 centres across 26 countries from July 2013 to August 2017. The trial was conducted in accordance with the provisions of the International Conference on Harmonization Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki.13 Full review details are available from Novartis on request. The legal guardian or legally acceptable representative of each patient gave written informed consent before any study-related procedures were performed. In addition, the patient provided his/her written consent (in accordance with local ethical/regulatory requirements).
Participants
PARADIGMS design and inclusion/exclusion criteria have been published previously7 in accordance with the Consolidated Standards of Reporting Trials guidelines. To summarise, the study included paediatric patients (aged 10 to <18 years) with a diagnosis of MS as defined by the revised consensus definition for paediatric MS,14 and who had experienced at least one MS relapse in the year preceding screening, or more than one relapse in the 2 years preceding screening, or had evidence of at least one gadolinium-enhancing lesion on T1-weighted MRI in the 6 months before randomisation (including screening MRI). Participants had an EDSS score of 0.0–5.5, inclusive.
Baseline demographic and disease characteristics of the 215 randomised patients were generally comparable between treatment groups and as expected in relatively newly diagnosed young patients (see Chitnis et al for full baseline characteristics).7 Mean baseline EDSS score for the combined groups was low (1.54) and over 63% of patients (n=136) were treatment naïve. Twenty-two (10.2%) patients were aged ≤12 years and 10 (4.7%) were prepubertal (Tanner stage 1).
Supportive and sensitivity analyses of key end points
All analyses—including supportive, sensitivity and subgroup analyses—tested the difference between treatments on the primary efficacy outcome (annualised rate of confirmed relapses) and/or on the key secondary end point (annualised rate of n/neT2 lesions) using negative binomial models. The difference in the annualised rate of confirmed and unconfirmed relapses was also assessed. Region and pubertal status, as randomisation stratification factors, were included as covariates along with treatment and number of relapses within the previous 2 years. The primary analysis was performed on the intent-to-treat population (full analysis set (FAS)) including 214 (99.5%) patients. All the analyses presented in this section were predefined.
Primary end point (ARR)
These predefined analyses were conducted to assess the primary outcome: (1) in the FAS population after the exclusion of data following drug discontinuation; (2) in the per-protocol set (PPS), which excludes patients with major protocol deviations (ie, deviations that could confound the interpretation of analyses conducted on the FAS); (3) in the FAS population excluding patients in the IFN β-1a group who were positive for IFN β-1a neutralising antibodies (NAbs; as per single sample collected at end-of-study visit). Supportive analyses were also done to evaluate the annualised rate of all relapses (confirmed and unconfirmed) in both the FAS and the PPS populations.
In order to assess the impact of prior treatment status, we analysed the subpopulation of paediatric patients with MS who did not have previous disease-modifying therapy (DMT) (DMT-naïve patients).
A negative binomial regression modelling approach was used to extrapolate fingolimod-treatment effect on ARR (confirmed relapse) in the small subgroup of younger patients as a function of age at randomisation/first dose, including 10, 11 and 12 years of age. Similarly, a Bayesian-negative binomial model was fit to extrapolate efficacy from the pubertal patients to the small subgroup of prepubertal patients.
Key secondary end point (n/neT2 lesions)
The key secondary efficacy variable, analysed on the FAS population from month 0 to end of study,7 was further tested (1) on the FAS from months 0–6, 0–12, 0–18 and 0–24; (2) on the PPS; (3) on the FAS after excluding patients in the IFN β-1a arm who were IFN NAbs-positive. The impact of prior treatment status on the key secondary end point was also assessed as a predefined supportive analysis focusing on the DMT-naïve subgroup.
Analyses of disability
Expanded Disability Status Scale
The EDSS score and its change from baseline were assessed every 3 months until month 24 as a predefined analysis. EDSS ratings were performed as far as possible by the same certified EDSS raters to minimise inter-rater variability, and only EDSS changes not related to acute relapse events were considered. Categorical change in EDSS from baseline was assessed at each visit, with patients assigned to one of three groups: improving, stable and worsening (also predefined). For patients with baseline EDSS 5.0 or less, change of ≤−1 point was defined as improvement, change from −0.5 to 0.5 as stable, change of ≥1 point as deterioration. For patients with baseline EDSS >5.0, change of ≤−0.5 point was defined as improvement, zero change as stable, change of ≥0.5 point as deterioration. Inferential testing based on χ2 test was performed as a post hoc analysis.
Confirmed disability progression (post hoc)
Kaplan-Meier (KM) survival analyses were used to study the times to 3 month and 6 month confirmed disability progression (3M-CDP and 6M-CDP, defined as 3 month and 6 month sustained increase in EDSS score by ≥1.5 if null at baseline, by ≥1.0 if 1–5 at baseline and by ≥0.5 if ≥5.5 at baseline, respectively). It was required that 3M-CDP and 6M-CDP be confirmed by EDSS at a scheduled visit 3 months and 6 months, respectively, after the onset of disability progression and in the absence of a relapse.
KM estimates at 12 and 24 months, together with 95% CIs, were calculated and a log-rank test of the treatment difference in KM estimates was performed. A Cox proportional hazards regression model adjusted for treatment, region, pubertal status and baseline EDSS was fit as a supportive analysis. To assess the robustness of the results, we analysed time to 3M-CDP sustained until the last observation using the same method.
Results
Supportive and sensitivity analyses of key end points
Primary end point
Findings from all predefined supportive and sensitivity analyses were consistent with the primary efficacy results (ie, 81.9% reduction in ARR in the fingolimod group compared with the IFN β-1a group7 and demonstrated consistent reductions at study end (up to Month 24) in the annualised rate of confirmed relapses (all p<0.001, unless otherwise specified; table 1).
Table 1.
Supportive and sensitivity analyses of primary end points at end of study (up to Month 24)*
| Fingolimod (n=107) |
Interferon β-1a (n=107) |
Rate ratio or between-group difference (95% CI) | Rate reduction | P value | |
| Estimated annualised relapse rate (95% CI)† | |||||
| FAS | 0.12 (0.08 to 0.19) | 0.67 (0.52 to 0.89) | 0.18 (0.11 to 0.30) | 81.9% | <0.001 |
| Excluding data after SDD | 0.12 (0.08 to 0.20) | 0.73 (0.55 to 0.96) | 0.17 (0.10 to 0.29) | 83.1% | <0.001 |
| PPS | N’=96 0.12 (0.07 to 0.20) |
N’=101 0.72 (0.53 to 0.97) |
0.17 (0.10 to 0.30) | 83.2% | <0.001 |
| Excluding IFN NAbs-positive patients | N’=107 0.12 (0.08 to 0.20) |
N’=98 0.67 (0.50 to 0.89) |
0.19 (0.11 to 0.31) | 81.5% | <0.001 |
| Treatment-naïve patients | N’=69 0.07 (0.04 to 0.15) |
N’=67 0.50 (0.34 to 0.74) |
0.14 (0.06 to 0.32) | 85.8% | <0.001 |
| Observed annualised relapse rate | |||||
| Patients ≤12 years | N’=13 0.10 |
N’=9 0.72 |
See model | ||
| Prepubertal patients | N’=7 0.20 |
N’=3 1.49 |
See model | ||
| Pubertal patients | N’=98 0.13 |
N’=104 0.73 |
See model | ||
| Model-based annualised relapse rate based on extrapolation from all paediatric patients (95% CI) | |||||
| Younger patients‡ | |||||
| Overall FAS | 0.12 (0.07 to 0.19) | 0.73 (0.56 to 0.97) | 0.16 (0.1 to 0.28) | 83.8% | <0.001 |
| 10 years | 0.04 (0.01 to 0.21) | 0.76 (0.29 to 2.03) | 0.05 (0.01 to 0.36) | 94.6% | 0.003 |
| 11 years | 0.05 (0.01 to 0.20) | 0.76 (0.33 to 1.71) | 0.07 (0.01 to 0.33) | 93.4% | <0.001 |
| 12 years | 0.06 (0.02 to 0.19) | 0.75 (0.39 to 1.44) | 0.08 (0.02 to 0.30) | 91.9% | <0.001 |
| Prepubertal patients§ | N’=7 | N’=3 | Pos-Prob¶: | ||
| 0.16 (0.00 to 0.89) | 1.97 (0.20 to 10.53) | 0.26 (0.00 to 1.82) | 73.9% | 93.8% | |
| Pubertal patients | N’=98 | N’=104 | |||
| 0.08 (0.04 to 0.15) | 0.72 (0.54 to 0.96) | 0.12 (0.05 to 0.22) | 88.2% | 100.0% | |
| All relapses (confirmed and unconfirmed) | |||||
| FAS | FAS 0.18 (0.13 to 0.26) | 0.80 (0.64 to 1.01) | 0.23 (0.15 to 0.35) | 77.4% | <0.001 |
| PPS | N’=96 0.17 (0.11 to 0.26) |
N’=101 0.81 (0.63 to 1.04) |
0.21 (0.13 to 0.34) | 78.6% | <0.001 |
End of study defined as the last assessment taken on or before the final study phase visit date.
*Unless otherwise stated.
†Between-treatment comparison obtained from fitting a negative binomial regression model adjusted as described in methodology.
‡Estimated at a given age at baseline by fitting a negative binomial regression model adjusted for treatment, age at baseline, region, pubertal status (stratified variable), number of relapses within the previous 2 years before randomisation as well as age and treatment interaction.
§Obtained from fitting a Bayesian-negative binomial regression model with mixture priors adjusted for treatment, age, number of relapses within the previous 2 years before randomisation as well as age and treatment interaction.
¶Pos-Prob indicates the posterior probability of the rate ratio is <1, ie, the probability of the ARR with fingolimod being lower than the ARR with IFN β-1a.
ARR, annualised relapse rate; FAS, full analysis set; IFN, interferon; N’, total number of subjects with available result and included in the analysis; NAbs, neutralising antibodies; PPS, per-protocol set; SDD, study drug discontinuation.
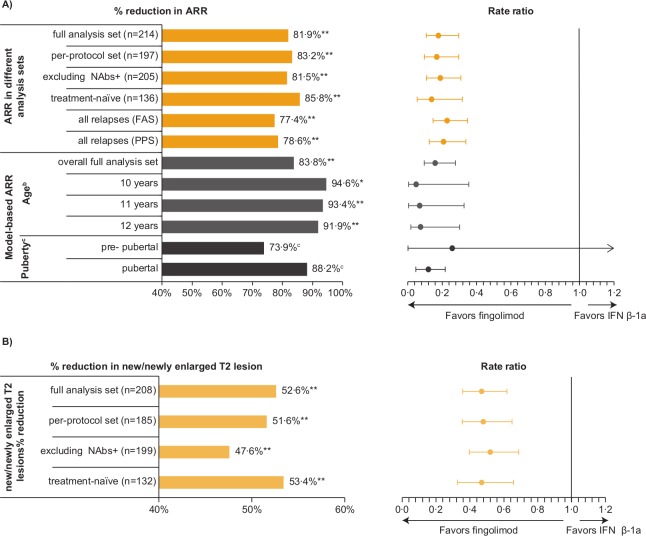
Excluding data after study drug discontinuation (8 patients on fingolimod and 26 on IFN β-1a prematurely discontinued study drug), patients with major protocol deviations (n=17) and IFN NAbs-positive patients (n=9) yielded similar reductions as observed for the overall population. Of the nine NAbs-positive IFN patients, eight completed the study. The ARR reduction in treatment-naïve patients was 85.8% vs 81.9% in the overall population. ARR reductions in all relapses (confirmed and unconfirmed) were also observed in the fingolimod versus the IFN β-1a group (see figure 1A and table 1 for an overview of all results).
Figure 1.
Supportive and sensitivity analyses of primary and key secondary end points: percentage reductions in ARR (A) and in new or newly enlarged T2 lesions (B) with fingolimod vs INF β-1a at study end (up to month 24). Fingolimod vs IFN β-1a comparison: *p=0.03, **p<0.001. aThe relapses considered for the analyses are confirmed relapses unless stated ‘all relapses’. bEstimated at a given age at baseline by fitting a negative binomial regression model adjusted for treatment, age at baseline, region, pubertal status (stratified variable), number of relapses within the previous 2 years before randomisation as well as age and treatment interaction. cObtained from fitting a Bayesian-negative binomial regression model with mixture priors adjusted for treatment, age, number of relapses within the previous 2 years before randomisation as well as age and treatment interaction; post-prob=100% (pubertal patients) and 93.8% (prepubertal patients). ARR, annualised relapse rate; FAS, full analysis set; IFN, interferon; NAbs+, neutralising antibodies-positive; post-prob, posterior probability of rate ratio <1; PPS, per-protocol set.
Younger and prepubertal patients
As shown in table 1, ARR reductions in younger patients estimated from the negative binomial regression modelling extrapolation were 94.6% (p=0.003), 93.4% and 91.9% for 10, 11 and 12 years of age, respectively vs 83.8% for the overall FAS population (also estimated by the model). Observed ARR in the subpopulation of patients ≤12 years are displayed in table 1 along with model-based ARR.
Based on the Bayesian extrapolation model, the ARR reduction in prepubertal patients was estimated at 73.9% vs 88.2% in the pubertal patients (table 1). The posterior probabilities (ie, the probabilities of ARR being lower with fingolimod than with IFN β-1a) were 100% in pubertal patients and 93.8% in prepubertal patients.
Key secondary endpoint
Predefined analyses (ie, in the PPS population, excluding IFN NAbs-positive patients, and in treatment-naïve patients) were all consistent with the key secondary efficacy results, which demonstrated a 52.6% reduction in the annualised rate of n/neT2 lesions with fingolimod versus IFN β-1a (table 2).7 We observed consistent reductions in n/neT2 lesions with fingolimod compared with IFN β-1a. All annualised rates for n/neT2 lesions were also significantly lower from months 0–6, 0–12, 0–18 and 0–24 in the fingolimod group than in the IFN β-1a group, noting that due to the study design fewer patients were included on the month 0–24 analysis (all p<0.001, unless otherwise specified; figure 1B and table 2).
Table 2.
Supportive and sensitivity analyses of the key secondary end point at end of study (up to month 24)
| Estimated annualised rate of n/ne T2 lesions (95% CI)* | |||||
| Fingolimod (n=107) |
IFN β-1a (n=107) |
Rate ratio or between-group difference (95% CI) | Rate reduction (%) | P value | |
| FAS | |||||
| Month 0–end of study | N’=106 4.39 (3.62 to 5.34) |
N’=102 9.27 (7.66 to 11.21) |
0.47 (0.36 to 0.62) | 52.6 | <0.001 |
| Month 0–6 | N’=104 9.29 (7.43 to 11.62) |
N’=100 17.80 (14.32 to 22.12) |
0.52 (0.38 to 0.71) | 47.8 | <0.001 |
| Month 0–12 | N’=98 5.18 (4.24 to 6.33) |
N’=90 10.44 (8.57 to 12.72) |
0.50 (0.37 to 0.66) | 50.4 | <0.001 |
| Month 0–18 | N’=71 5.14 (4.10 to 6.44) |
N’=53 8.48 (6.57 to 10.94) |
0.61 (0.43 to 0.85) | 39.4 | 0.004 |
| Month 0–24 | N’=35 3.48 (2.49 to 4.85) |
N’=24 10.97 (7.46 to 16.13) |
0.32 (0.19 to 0.54) | 68.3 | <0.001 |
| PPS | N’=92 4.75 (3.86 to 5.85) |
N’=93 9.83 (8.04 to 12.01) |
0.48 (0.36 to 0.65) | 51.6 | <0.001 |
| Excluding IFN NAbs-positive patients | N’=106 4.34 (3.58 to 5.26) |
N’=93 8.29 (6.80 to 10.10) |
0.52 (0.40 to 0.69) | 47.6 | <0.001 |
| Treatment-naïve patients | N’=68 3.90 (3.06 to 4.97) |
N’=64 8.38 (6.59 to 10.65) |
0.47 (0.33 to 0.66) | 53.4 | <0.001 |
End of study defined as the last assessment taken on or before the final study phase visit date.
*Between-treatment comparison obtained from fitting a negative binomial regression model adjusted as described in methodology.
FAS, full analysis set; IFN, interferon; N’, total number of subjects with available result and included in the analysis; NAbs, neutralising antibodies; PPS, per-protocol set.
Analyses of disability
Expanded Disability Status Scale
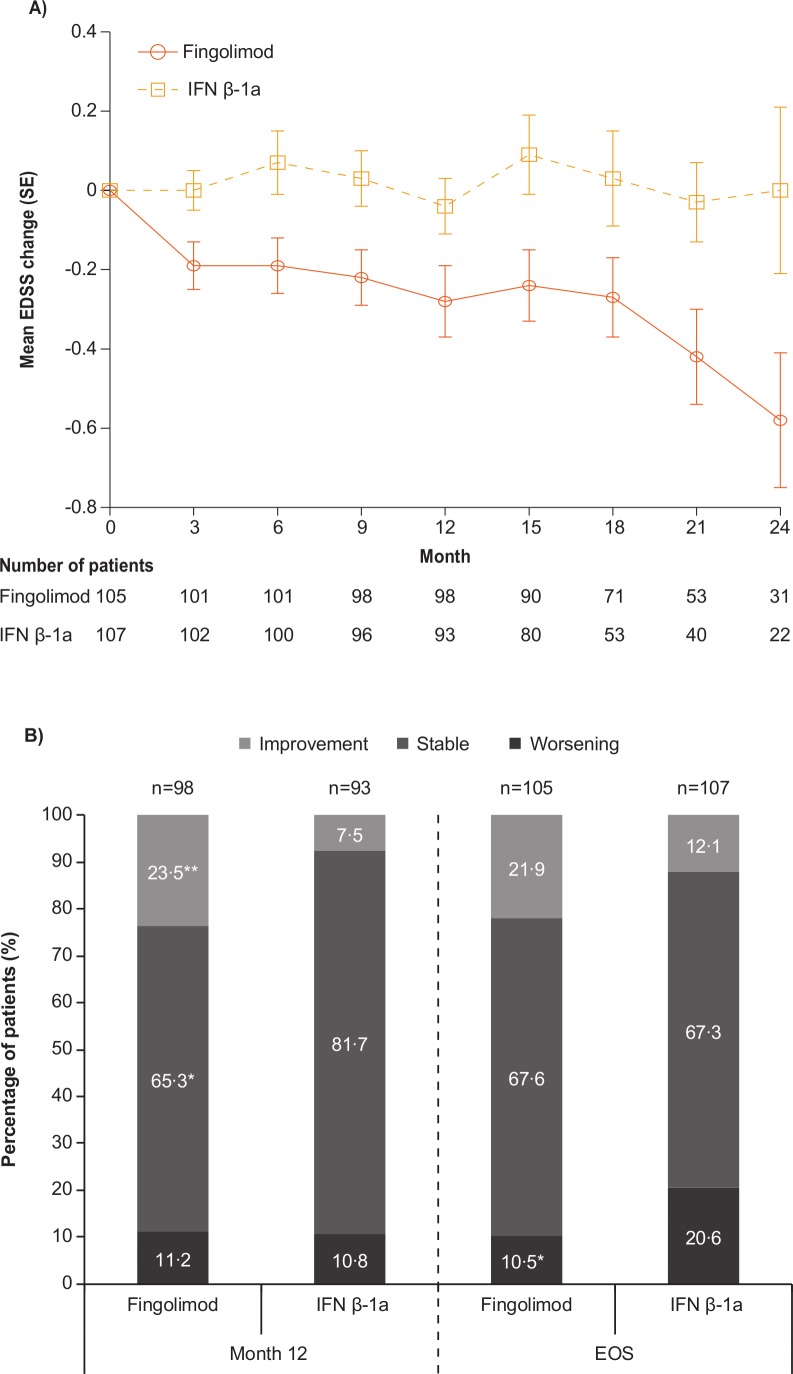
The mean EDSS score (SD) at baseline was 1.5 (1.14) in the fingolimod group and 1.6 (0.89) in the IFN β-1a group (figure 2A). In the predefined analysis, the mean change from baseline in EDSS score at the end of study was numerically negative (−0.23) (improvement) in the fingolimod group and positive (0.22) (worsening) in the IFN β-1a group. This improvement in the fingolimod group was apparent from month 3 onwards and consistently observed every 3 months up to month 24, whereas numeric increases in EDSS score or stable values were observed at each corresponding time-point in the IFN β-1a group (figure 2A).
Figure 2.
Changes in EDSS from baseline (A) mean EDSS changes; (B) categorical EDSS changes†. *P<0.05; **p<0.01. †Patients with baseline ≤EDSS 5.0: change of ≤−1 point defined as improvement, change from −0.5 to 0.5 as stable, change of ≥1 point as deterioration; patients with baseline. EDSS >5.0: change of ≤−0.5 point defined as improvement, zero change as stable, change of ≥0.5 point as deterioration. EDSS, Expanded Disability Status Scale; EOS, end of study, defined as the last assessment taken on or before the final study phase visit date; IFN, interferon; n, number of patients.
At all time-points, more fingolimod-treated patients had categorically improved EDSS scores (ie, lower EDSS scores) and fewer patients had categorical worsening (ie, higher EDSS scores; figure 2B) compared with INF β-1a-treated patients. At the study end, nearly twice as many fingolimod-treated compared with IFN β-1a-treated patients demonstrated a trend towards categorical improvement from baseline in EDSS (21.9% vs 12.1%, respectively, p=0.059; p values calculated post hoc) while only half as many had categorical worsening (10.5% vs 20.6%, respectively, p=0.043). A similar proportion of patients in both groups showed stable EDSS (67.6% vs 67.3%; p=0.959).
Disability progression (post hoc)
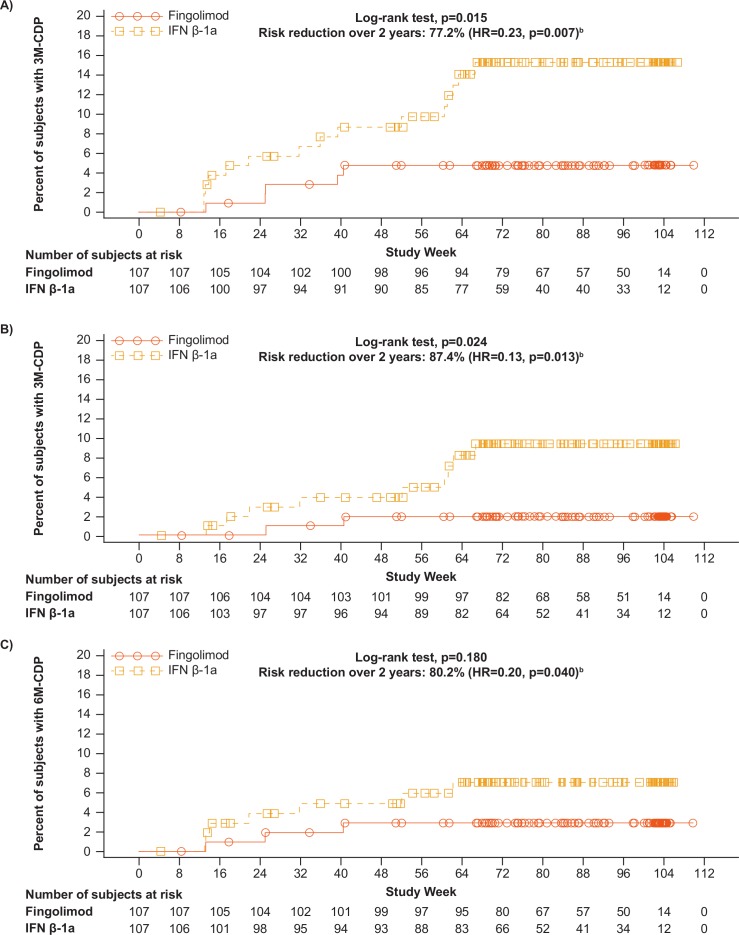
In a post hoc analysis, fingolimod significantly delayed the time to 3M-CDP compared with IFN β-1a (log-rank test, p=0.015) (figure 3A), with KM estimates shown in table 3. A supportive analysis indicated a risk reduction of 77.2% in 3M-CDP up to 2 years for fingolimod compared with IFN β-1a (p=0.007) (table 3).
Figure 3.
Kaplan-Meier plot for (A) time to 3M-CDP; (B) time to 3M-CDP sustained until last observationa; (C) time to 6M-CDP. aPost hoc analysis of full analysis set. bEstimated using a Cox regression model adjusted for treatment, pubertal status and baseline EDSS. 3M-CDP, 3 month confirmed disability progression; 6M-CDP, 6 month confirmed disability progression; EDSS, Expanded Disability Status Scale; IFN, interferon.
Table 3.
Risk of confirmed disability progression (FAS)
| Outcome | Treatment | Patients without progression (95% CI)† |
Between-treatment comparison* | ||
| Risk reduction (%) | HR (95% CI) | P value | |||
| 3M-CDP | Fingolimod n/N=5/107 | 95.2% (91.1 to 99.3) | 77.2 | 0.23 (0.08 to 0.66) | 0.007 |
| INF β-1a n/N=15/107 | 84.7% (77.5 to 91.9) | ||||
| Sustained 3M-CDP‡ | Fingolimod n/N=2/107 | 98.1% (95.5 to 100.0) | 87.4 | 0.13 (0.02 to 0.64) | 0.013 |
| INF β-1a n/N=9/107 | 90.6% (84.7 to 96.5) | ||||
| 6M-CDP | Fingolimod n/N=3/107 | 97.1% (93.9 to 100.0) | 80.2% | 0.20 (0.04 to 0.93) | 0.040 |
| INF β-1a n/N=7/107 | 93.0% (88.0 to 98.0) | ||||
*Performed on time to event using a Cox regression model adjusted as described in methodology.
†Estimated at month 24 from Kaplan-Meier analysis.
‡3M-CDP sustained until last observation.
FAS, full analysis set; IFN, interferon; 3M-CDP, 3 month confirmed disability progression; 6M-CDP, 6 month confirmed disability progression; N, total number of patients included in the analysis; n, total number of events included in the analysis.
A similar outcome was observed when the analysis was repeated for time to 3M-CDP sustained until the last observation. Fingolimod significantly delayed such progression, compared with IFN β-1a (log-rank test, p=0.024) (figure 3B). The KM estimate of the percentage of patients free of 3M-CDP sustained until the last observation was higher for fingolimod (98.1%) compared with IFN β-1a (90.6%), with a risk reduction of 87.4% (p=0.013 for this supportive analysis) (table 3).
The results of the post hoc analysis of time to the onset of 6M-CDP show very few events, as expected with a flexible duration study design with mean duration <2 years: 3/107 (2.8%) patients in the fingolimod group and 7/107 (6.5%) patients in IFN β-1a group had 6M-CDP during the study. The KM estimate of the percentage of patients free of 6M-CDP up to month 24 was 97.1% in the fingolimod group compared with 93.0% in the IFN β-1a group (figure 3C, table 3). The log-rank test (p=0.180) indicates no significant difference in delaying time to onset of 6M-CDP for fingolimod compared with IFN β-1a due to small number of events in both arms and thus low power for the comparison. The Cox proportional hazard model nevertheless shows that fingolimod reduced the risk of 6M-CDP over up to 24 months by 80.2% compared with IFN β-1a (HR=0.20, p=0.040).
Discussion
The supportive and sensitivity analyses of this first phase III trial in paediatric MS are all consistent with the primary and key secondary efficacy results. Fingolimod consistently reduced ARRs and the formation of n/neT2 lesions across all analysis sets compared with IFN β-1a in paediatric patients treated for up to 2 years. Depending on the subpopulation considered, the reductions ranged from 81.5% to 85.8% for confirmed relapses and 47.6% to 53.4% for n/neT2 lesions.
Excluding patients in the IFN β-1a arm who were positive for IFN antibody at the end of the study had no impact on the results. In addition, strong reductions both in ARR and n/neT2 lesions (85.8% and 53.4%, respectively) were observed in treatment-naïve patients, which represented the majority (63%) of patients.
Excluding patients with major protocol deviations as well as patients who discontinued the study drug had no impact on the primary and key secondary results. This last observation is notable because more patients discontinued treatment on IFN β-1a than on fingolimod, and half of IFN β-1a-treated patients who discontinued treatment did so due to lack of efficacy.7
Efficacy in younger and prepubertal patients were comparable to the overall population. Due to limited sample size, statistical models were applied to quantify the relationship between ARR and age or pubertal status; this allowed us to extrapolate relapse rates for children ≤12 years and for prepubertal children. The negative binomial extrapolation supported the high efficacy of fingolimod compared with IFN β-1a in patients ≤12 years of age. The magnitude of the estimated effect of fingolimod on ARR reduction was inversely proportional with age, with higher reduction observed in the younger paediatric population. Results from a post hoc analysis of fingolimod phase III trials in adults, using a similar modelling extrapolation, had already suggested a stronger effect of fingolimod on ARR in younger adult patients (20 and 30 years of age), with the youngest adults showing the highest reduction.15 These findings in adults are consistent with what we observed in the paediatric population of PARADIGMS, supporting the trajectory of greater benefits of fingolimod on ARR in younger patients. The higher clinical and radiological activity in paediatric MS, suggesting a more inflammatory course of the disease,1 2 could explain the higher efficacy of fingolimod, which is a stronger immunomodulatory treatment than IFN.
Results from the extrapolation based on the Bayesian-negative binomial model suggest that both prepubertal and pubertal patients are benefiting from the effect of fingolimod on relapses. The posterior probabilities of observing a high reduction in ARR with fingolimod compared with IFN β-1a treatment remained high, 100% in pubertal patients and 93.8% in prepubertal patients. While paediatric MS is already infrequent, MS in prepubertal patients is rare and onset before 10–12 years of age is estimated to account for <1% of all MS cases.16 17 In PARADIGMS, however, due to specific enrolment efforts, close to 5% of enrolled patients were prepubertal. Although the use of the applied model mitigates the uncertainty associated with low numbers, caution is still required when interpreting this finding.
Post hoc analyses of disability progression suggest that the benefits observed on disease activity in paediatric patients may translate into benefits on disability progression over the treatment period of up to 2 years. It is important to note that while the EDSS score changes were small (mean changes of <0.5 in any direction and may not be clinically meaningful), findings were statistically significant. The overall EDSS scores remained very low (median EDSS scores remained <2), and therefore, the changes in EDSS scores should be interpreted with caution. EDSS scores of 1 or 2 often reflect minor findings on examination, and rarely indicate actual disability. Although the direction of EDSS changes in favour of fingolimod are supported by the magnitude of the primary results, and are also consistent with the findings from the adult fingolimod trials,18–20 the long-term implications are yet to be determined. Note also that the categorical change in EDSS from baseline at each visit may reflect relapse-induced transient change in EDSS rather than sustained disability progression, which is to be confirmed by subsequent EDSS assessments such as 3M-CDP and 6M-CDP.
The positive effect on 3M-CDP is consistent across supportive and sensitivity analyses, with various tests and outcome definitions, increasing the confidence in this retrospective analysis. This applies to 3M-CDP sustained until the last observation, which does not include patients who may partly recover before study end. There was no significant difference in delaying the time to onset of 6M-CDP for fingolimod compared with IFN β-1a based on the log-rank test; the difference, however, reached significance in a supportive Cox regression analysis adjusting for covariates. The lack of significance based on the log-rank test is likely due to the small number of 6M-CDP events, resulting in a low statistical power for the comparison. A number of factors have probably contributed to the low number of 6M-CDP events. First, paediatric patients with patients rarely accrue disability in the first 10 years of disease; and by definition, 6M-CDP has a lower incidence than does 3M-CDP because of the stricter confirmation procedure: 6M-CDP requires that disability progression is confirmed 6 months after onset and that all interim EDSS assessments must meet the definition of disability progression. This is also true for studies in the pivotal phase III clinical trials of fingolimod in adult MS.18–20 Second, because of the flexible follow-up design, not all patients with a 3-month follow-up EDSS assessment to confirm disability progression also had the 6-month EDSS assessment, resulting in fewer 6M-CDP events than 3M-CDP events. As mentioned, given that paediatric patients with MS rarely accrue physical disability early in their disease course, a study duration of 2 years is underpowered to detect disability progression. In an analysis of 394 patients with disease onset before the age of 16, time to reach an EDSS score of 4 was 20 years as compared with 8 years for adult-onset patients.21
The longer time to disability progression in paediatric patients occurs despite more frequent and severe relapses compared with adult patients, and it has been suggested to be due to the greater potential paediatric patients have for complete recovery from relapses, particularly early during the disease course.22–25 This greater potential for complete recovery has been proposed to be due to a greater potential for remyelination and repair in younger patients, consistent with studies in animal demyelination models, which show a decreasing capacity for remyelination with increasing age.26 27 Paediatric patients with MS have usually not yet acquired significant spinal cord lesional damage, which contributes to motor impairment in adult-onset MS. Finally, important comorbidities such as cerebral vascular disease, severe obesity and lung disease, all of which reduce physical functioning, are much less prevalent in paediatric MS.
A recent meta-analysis of 38 clinical MS trials supports the hypothesis that a greater effect of immunomodulatory DMTs on disease activity may translate into greater benefits on disability progression in younger patients. The results show that the efficacy of immunomodulatory DMTs on disability progression strongly decreases with advancing age.28 The meta-analysis also found that the difference in the effect on disability between higher and lower efficacy therapies is greater in younger patients. The stronger effect on disability progression in younger patients is likely due to the stronger effect on relapse, resulting in less residual postrelapse symptoms. With fewer relapses, reorganising and repairing damage processes can proceed uninterrupted. In addition, inflammation interferes with plasticity, hence targeting the high level of inflammation present in the young brain may help maintain reorganising capabilities and support potential recovery from damage29; in line with this observation, it has been shown that more pronounced acute axonal damage is observed in inflammatory demyelinating lesions in children compared with adults.30
PARADIGMS 5-year open-label extension will assess how the robust effects of fingolimod in paediatric MS demonstrated in the core study are sustained over time. We hope that these findings will help initiate a paradigm shift towards the tailored use of more efficacious DMT options earlier on in children and adolescents with MS, thereby preventing a detrimental disease course and addressing the serious unmet needs of this challenging population.
Acknowledgments
The authors would like to thank all patients for their participation and commitment. The authors would like to thank all of the principal investigators as well as the Novartis clinical trial team of the PARADIGMS study and the trial team partners in all the participating countries. The authors would also like to thank Marie-Catherine Mousseau (Novartis Ireland Limited, Dublin, Ireland) for developing the first draft, referencing, preparing tables and figures, coordinating authors’ reviews and editorial assistance under the guidance of all authors.
Footnotes
Contributors: KD, PH, BB, TC, JG, LK and EW were principal investigators, involved in the design and/or ongoing conduct of the study, responsible for data collection and involved in the data analyses. TS, GLP and MM are Novartis associates and were involved in study design, study conduct and analyses. All authors contributed to writing and critical review at all stages of manuscript preparation and approved final content for publication.
Funding: This work was supported by Novartis Pharma AG, Basel, Switzerland.
Competing interests: KD received personal compensation for speaker activities from Novartis. PH received compensation for serving on a scientific advisory board from Novartis, and for speaking from Bayer Health care. BB has served as a remunerated central MRI reviewer for the present trial (Novartis). EW volunteers on an advisory board for a Novartis trial. She is a site PI for clinical trials with Roche and Novartis. JG in the last 3 years has received honoraria for lectures and consultancy fees from Bayer, Teva and Novartis. LK has received personal compensation for activities as a speaker, consultant and/or participant on an advisory board from Biogen Idec, Novartis, Teva Neurosciences and Multicell. In addition, LK has received royalty or licence fees from ER Squibb & Sons, Avenir, Johnson & Johnson and Osmotica, and has received research support from Novartis, Biogen Idec, Celgene Corporation and Genentech. TC has received personal compensation for advisory boards/consulting for F. Hoffman-La Roche, Biogen and Novartis; TC has also received financial support for research activities from Biogen, Merck Serono, Verily and Novartis. TS, GLP and MM are employees of Novartis.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by an institutional review board or ethics committee at each study site; the central institutional review board was Quorum Review IRB, Seattle, Washington, file number 28233/1.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Once the 5-year open-label extension has been completed, the reader will be able to request the raw data (anonymized) and related documents (e.g., protocol, reporting and analysis plan, clinical study report) that underlie the results reported in this article by connecting to https://www.clinicalstudydatarequest.com and signing a Data Sharing Agreement with Novartis. These will be made available to qualified external researchers, with requests reviewed and approved by an independent review panel on the basis of scientific merit.
References
- 1. Gorman MP, Healy BC, Polgar-Turcsanyi M, et al. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 2009;66:54–9. 10.1001/archneurol.2008.505 [DOI] [PubMed] [Google Scholar]
- 2. Scalfari A, Lederer C, Daumer M, et al. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler 2016;22:1750–8. 10.1177/1352458516630396 [DOI] [PubMed] [Google Scholar]
- 3. Jancic J, Nikolic B, Ivancevic N, et al. Multiple sclerosis in pediatrics: current concepts and treatment options. Neurol Ther 2016;5:131–43. 10.1007/s40120-016-0052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ghezzi A, Amato MP, Makhani N, et al. Pediatric multiple sclerosis: conventional first-line treatment and general management. Neurology 2016;87(9 Suppl 2):S97–102. 10.1212/WNL.0000000000002823 [DOI] [PubMed] [Google Scholar]
- 5. Brenton JN, Banwell BL. Therapeutic approach to the management of pediatric demyelinating disease: multiple sclerosis and acute disseminated encephalomyelitis. Neurotherapeutics 2016;13:84–95. 10.1007/s13311-015-0396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chitnis T, Ghezzi A, Bajer-Kornek B, et al. Pediatric multiple sclerosis: escalation and emerging treatments. Neurology 2016;87(9 Suppl 2):S103–9. 10.1212/WNL.0000000000002884 [DOI] [PubMed] [Google Scholar]
- 7. Chitnis T, Arnold DL, Banwell B, et al. Trial of fingolimod versus interferon beta-1a in pediatric multiple sclerosis. N Engl J Med 2018;379:1017–27. 10.1056/NEJMoa1800149 [DOI] [PubMed] [Google Scholar]
- 8. Novartis Pharmaceuticals Corporation Gilenya® (fingolimod) full prescribing information. East Hanover, New Jersey, USA: Novartis Pharmaceuticals Corporation, 2018. [Google Scholar]
- 9. European Commission Community register of medicinal products for human use , 2018. Available: http://ec.europa.eu/health/documents/community-register/html/h677.htm [Accessed 14 Dec 2018].
- 10. Chitnis T. Role of puberty in multiple sclerosis risk and course. Clin Immunol 2013;149:192–200. 10.1016/j.clim.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 11. Huppke B, Ellenberger D, Rosewich H, et al. Clinical presentation of pediatric multiple sclerosis before puberty. Eur J Neurol 2014;21:441–6. 10.1111/ene.12327 [DOI] [PubMed] [Google Scholar]
- 12. Lulu S, Graves J, Waubant E. Menarche increases relapse risk in pediatric multiple sclerosis. Mult Scler 2016;22:193–200. 10.1177/1352458515581873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Declaration of Helsinki: ethical principles for medical research involving human subjects. Ferney-Voltaire FWMA, 2018. Available: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [Accessed 30 Jan 2018].
- 14. Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 2013;19:1261–7. 10.1177/1352458513484547 [DOI] [PubMed] [Google Scholar]
- 15. Gärtner J, Chitnis T, Ghezzi A, et al. Relapse rate and MRI activity in young adult patients with multiple sclerosis: a post hoc analysis of phase 3 fingolimod trials. Mult Scler J Exp Transl Clin 2018;4 10.1177/2055217318778610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldman A, Ness J, Pohl D, et al. Pediatric multiple sclerosis: clinical features and outcome. Neurology 2016;87(9 Suppl 2):S74–81. 10.1212/WNL.0000000000003028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langer-Gould A, Brara SM, Beaber BE, et al. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology 2013;80:548–52. 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kappos L, Radue E-W, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 19. Calabresi PA, Radue E-W, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (freedoms II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol 2014;13:545–56. 10.1016/S1474-4422(14)70049-3 [DOI] [PubMed] [Google Scholar]
- 20. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–15. 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- 21. Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med 2007;356:2603–13. 10.1056/NEJMoa067597 [DOI] [PubMed] [Google Scholar]
- 22. O'Mahony J, Marrie RA, Laporte A, et al. Recovery from central nervous system acute demyelination in children. Pediatrics 2015;136:e115–23. 10.1542/peds.2015-0028 [DOI] [PubMed] [Google Scholar]
- 23. Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord 2014;3:186–93. 10.1016/j.msard.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 24. Fay AJ, Mowry EM, Strober J, et al. Relapse severity and recovery in early pediatric multiple sclerosis. Mult Scler 2012;18:1008–12. 10.1177/1352458511431725 [DOI] [PubMed] [Google Scholar]
- 25. Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol 2009;66:967–71. 10.1001/archneurol.2009.135 [DOI] [PubMed] [Google Scholar]
- 26. Zhao C, Li W-W, Franklin RJM. Differences in the early inflammatory responses to toxin-induced demyelination are associated with the age-related decline in CNS remyelination. Neurobiol Aging 2006;27:1298–307. 10.1016/j.neurobiolaging.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 27. Franklin RJM, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol 2008;255(Suppl. 1):19–25. 10.1007/s00415-008-1004-6 [DOI] [PubMed] [Google Scholar]
- 28. Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol 2017;8:577 10.3389/fneur.2017.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tomassini V, d'Ambrosio A, Petsas N, et al. The effect of inflammation and its reduction on brain plasticity in multiple sclerosis: MRI evidence. Hum Brain Mapp 2016;37:2431–45. 10.1002/hbm.23184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfeifenbring S, Bunyan RF, Metz I, et al. Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann Neurol 2015;77:655–67. 10.1002/ana.24364 [DOI] [PMC free article] [PubMed] [Google Scholar]