Abstract
Environmental stimuli are perceived and transduced inside the cell through the activation of signaling pathways. One common type of cell signaling transduction network is initiated by G-proteins. G-proteins are activated by G-protein-coupled receptors (GPCRs) and transmit signals from hormones, neurotransmitters, and other signaling factors, thus controlling a number of biological processes that include synaptic transmission, visual photoreception, hormone and growth factors release, regulation of cell contraction and migration, as well as cell growth and differentiation. G-proteins mainly act as heterotrimeric complexes, composed of alpha, beta, and gamma subunits. In the last few years, whole exome sequencing and biochemical studies have shown causality of disease-causing variants in genes encoding G-proteins and human genetic diseases. This review focuses on the G-protein β subunits and their emerging role in the etiology of genetically inherited rare diseases in humans.
Keywords: heterotrimeric G-proteins, β subunits, neurodevelopmental disorders, human genetic diseases
1. G-Protein-Coupled Receptors (GPCRs) and Heterotrimeric G-Proteins
The G-Protein-Coupled Receptor (GPCR) superfamily includes over 800 members in humans [1] and is the largest group of cell-surface seven-transmembrane receptors [2]. They translate the signal from extracellular ligands into intracellular responses [3]. The GPCRs have a ligand-binding pocket, with seven motif α-helices, in the extracellular region, and a cytoplasmic domain engaged in G-proteins binding, guanosine triphosphate (GTP)-binding heterotrimers, consisting of α, β, and γ subunits [4,5].
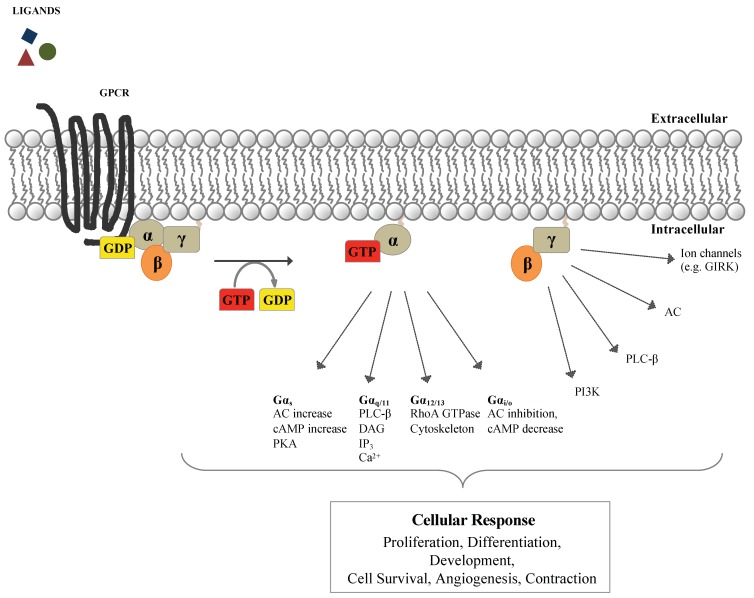
When inactive, the G-protein α subunit is linked to guanosine diphosphate (GDP). Ligand-activated GPCRs catalyze the exchange of GDP with GTP on Gα, promoting its dissociation from Gβγ (Figure 1). The Gαβγ dissociation, in turn, promotes the activation of the Gα and Gβγ units that activate downstream factors, thus regulating an array of cellular functions such as cell contraction, excitability, migration, cell growth, and differentiation [6,7]. Notably, the combinatorial association of the distinct G-protein subunit subtypes, comprising at least 20 Gα, 5 Gβ, and 13 Gγ subunits [8,9], provides the level of selectivity that is needed to generate the wide range of signals governed by G-proteins and their cognate GPCRs (Figure 1) [10,11,12].
Figure 1.
G-protein-coupled receptors signalosome. In the resting state, G-proteins are heterotrimers of alpha bound to guanosine diphosphate (GDP, yellow), beta, and gamma subunits. When activated by an extracellular ligand through G-protein coupled receptors (GPCRs, black), they undergo a conformational change that permits the GDP exchange with GTP (red) on Gα, which then dissociates from Gβγ. In the active state, Gα-GTP and Gβγ regulate various effectors. According to functional and structural homologies of their α subunit, heterotrimeric G-proteins are divided into four types (Gαs, Gαi/o, Gαq/11, and Gα12/13). Each Gα defines the unique Gαβγ mediated cellular responses [1,13,14,15,16]. Gαs and Gαi subfamily members are involved in the modulation of the intracellular second-messenger cAMP levels, either stimulating (Gs) or inhibiting (Gi) the production of cAMP by AC activity. Gαq/11 induces the activation of PLC-β, promoting the production of the intracellular messenger DAG and IP3 which activate the PKC and calcium signaling. Gα12/13 plays a role in the activation of the RhoA GTPase and of phospholipase D in regulating cell shape and motility [14,17,18]. Adenylyl cyclase (AC); cyclic adenosine monophosphate (cAMP); protein kinase A (PKA); phospholipase Cβ (PLC-β); diacylglycerol (DAG); inositol (1,4,5) trisphosphate (IP3); protein kinase C (PKC); intracellular concentration of free Ca2+ (Ca2+); Ras homolog family member A GTPase (RhoA GTPase); phosphatidylinositol-3-kinase (PI3K); G-protein–gated inwardly rectifying potassium channels (GIRK).
The propagation of the GPCR signaling cascade is restricted by the Regulators of G-protein Signaling (RGS) proteins, which limit the active Gα subunit lifetime and accelerate its GTP hydrolysis with a consequent re-association with the Gβγ dimer [19,20,21,22,23,24].
Here, we review the Gβ subunits and their contribution to the etiology of rare human genetic conditions. In the last six years, the outbreak of Next Generation Sequencing (NGS) technologies has assisted us to reach the description of a tapestry of human genetic conditions caused by the pathogenic variants in Gβ subunits, and disease manifestations mainly involving neuronal and cardiac systems associated with ophthalmic pathology.
2. Gβ Subunits: Genes and Proteins Structure
The human genome contains five genes (GNB1 to GNB5) encoding the different Gβ subunits [25]. Chromosomal locations, genes structure and exons content of each of the five subunits are summarized in Table 1. The Gβ1-4 subunits share between 80 and 90% sequence identity and are widely expressed throughout the tissues [26,27]; the Gβ5 exhibits much less homology (~50%) and is preferentially expressed in the brain and nervous system [28], while the Gβ5 longer isoform, Gβ5L, has restricted expression in retinal photoreceptor outer segments [9,29].
Table 1.
Gene content and major features of the five genes encoding the Gβ subunits. Gene names are reported according to the Hugo Gene Nomenclature Committee (HGNC, [33]); Ensembl gene and transcript IDs, information on transcript/protein length as well as number of exons were retrieved to the Ensembl 97 and Ensembl Genomes 44 release, and, finally, genomic coordinates are specified on the GRCh38.p13 genome assembly. Uniprot identifiers rely on the UniProt release 2019_06 (published July 3, 2019) [34]. MIM IDs and phenotype MIM numbers are as in OMIM (Online Mendelian Inheritance in Men) database.
| Gene Name (HGNC) | Description | Ensembl ID | RefSeq ID | Ensembl Transcript ID | Transcritp Length (bp) | Protein length (aa) | Uniprot | Cytogenetic Location | Genomic Coordinates (GRCh38, from Ensembl) | Strand | Nr. of Exons | Nr. of Coding Exons | MIM ID | Phenotype MIM Number(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GNB1 | G protein subunit beta 1 | ENSG00000078369.18 | NM_002074 | ENST00000378609.9 | 3163 | 340 | P62873 | 1p36.33 | 1:1,785,285-1,891,117 | reverse strand | 12 | 9 | *139380 | #616973 |
| GNB2 | G protein subunit beta 2 | ENSG00000172354.10 | NM_005273 | ENST00000303210.9 | 1664 | 340 | Q6FHM2 | 7q22.1 | 7:100,673,567-100,679,174 | forward strand | 10 | 9 | *139390 | - |
| GNB3 | G protein subunit beta 3 | ENSG00000111664.10 | NM_002075 | ENST00000229264.7 | 1923 | 340 | P16520 | 12p13.31 | 12:6,840,211-6,847,393 | forward strand | 11 | 9 | *139130 | #617024 |
| GNB4 | G protein subunit beta 4 | ENSG00000114450.10 | NM_021629 | ENST00000232564.8 | 6315 | 340 | Q9HAV0 | 3q26.33 | 3:179,396,088-179,451,476 | reverse strand | 10 | 9 | *610863 | #615185 |
| GNB5 | G protein subunit beta 5 | ENSG00000069966.18 | NM_006578 | ENST00000358784.11 | 1735 | 353 | O14775 | 15q21.2 | 15:52,122,206-52,180,001 | reverse strand | 11 | 11 | *604447 | #617173, #617182 |
At the protein level, iconic is the beta-propeller structure of the Gβ subunits, characterized by seven regular WD40-repeats [30] and a coiled coil domain at the N-terminus end. The WD40 domain is one of the most abundant and interacting domain in the eukaryotic proteome; each domain is approximately 40 amino acids long and is characterized by a conserved tryptophan (W)-aspartic (D) acid pair, hence the name WD40 [29,31]. With its β-propeller architecture, the WD40 domain provides extensive surface exposure for protein-protein or protein-DNA interaction, that coordinate downstream cellular events including signal transduction, autophagy, and apoptosis [32].
3. Gβ proteins and Human Diseases
3.1. G Protein Subunit Beta 1 (GNB1, Gβ1)
In humans, heterozygous GNB1 (MIM 139380) missense, splice-site and frameshift pathogenic variants cause an autosomal dominant neurodevelopmental disorder, named MRD42 (Mental Retardation, Autosomal Dominant 42; MIM#616973). The phenotype observed across individuals with MRD42 include global developmental delay (GDD)/intellectual disability (ID), hypotonia often associated with limb hypertonia, various types of seizures, and poor overall growth [35,36,37]. Strabismus, nystagmus, cortical visual impairment, attention deficit hyperactivity disorder, and autistic features may also be present [38]. Less frequent and variable symptoms are ataxia, dystonia, hydronephrosis, acute lymphoblastic leukemia [35,37,38,39,40], and cutaneous mastocytosis [41,42].
GNB1 was found as one of the five genes deleted in five patients with 200 to 823-kb overlapping interstitial deletions of chromosome 1p36.33 (MIM#607872) affected by ID, developmental delay, seizures and muscular hypotonia together with characteristic dysmorphic features, and behavior abnormalities [43,44]. Functional evidence of GNB1 involvement in neurodevelopmental delay is also corroborated by the study of homozygous Gnb1 mutant mice, demonstrating that Gnb1 is essential for normal embryonic neurogenesis. Forty percent of Gnb1 knock-out embryos were neonatal lethal and showed defects in neural tube closure and neural progenitor cell proliferation associated to exencephaly (Table 2); embryos without neural tube defects presented microencephaly and died after birth [45]. Moreover, Gnb1 heterozygous mice exhibited abnormal retina morphology with progressive degeneration (http://www.informatics.jax.org/marker/MGI:95781), thus supporting the ophthalmic manifestations reported in MRD42 affected individuals.
Table 2.
GNB genes have been studied in different model organisms. The table lists phenotypic manifestations resulting from complete (knock-out, KO) or partial (knock-down, KD) lack of each of the five GNB genes. “HET” refers to mouse models carrying only one functional copy of the gene, and “Dup” concerns the presence of three copies. Of note, in Zebrafish each of the genes has two paralogs, as a result of an ancient genome duplication event. In Drosophila melanogaster and C. elegans only two definite homologues have been identified, one corresponding to human GNB1-4 and one corresponding to human GNB5, in each species. NA indicates “Not Available” model.
| Gene Name (HGNC) | Annotated Terms in Animal Models | |||
|---|---|---|---|---|
| M. Musculus | Zebrafish | D. Melanogaster | C. elegans | |
| GNB1 | (Gnb1): abnormal brain morphology and size (KO) [52] | (gnb1a/gnb1b): altered regulation of neutrophil migrations and posterior lateral line neuromast primordium migration (KD) [54] | (CG10545): abnormal spindle size (KD and overexpression) [55] | (gpb1): essential for embryo development (50–80% embryonic lethality); uncoordinated phenotype in surviving adult worms; functions in establishment of mitotic spindle orientation; expressed in alimentary system, body wall musculature, epithelial system, nervous system and reproductive organs (KD) [56,57] |
| GNB2 | (Gnb2): abnormal behavioral response to light, increased heart rate, shortened PQ interval, shortened RR interval, shortened ST segment (KO) [52] | NA | ||
| GNB3 | (Gnb3): abnormal eye electrophysiology, mild bradycardia (KO); weight gain (Dup) [58,59,60] | (gnb3a): expressed throughout development; (gnb3b) expressed in the cones of the dorsal and medial retina (KD) [61] | ||
| GNB4 | (Gnb4): enlarged heart and spleen (KO) [52] | NA | ||
| GNB5 | (Gnb5): pre-weaning lethality with incomplete penetrance, decreased body size, slow post-natal weigh gain and abnormal vision (KO); increased body weight, adiposity, insulin resistance and liver steatosis (HET) [62,63,64,65] | (gnb5a/gnb5b): abnormal heart contraction, optokinetic behavior and swimming behavior (KO) [66] | (CG10763): pain responsive defective (KD) [67] | (gpb-2): behavioral defects, e.g., delayed egg laying, locomotion, and pharingeal pumping (KO and overexpression) [68] |
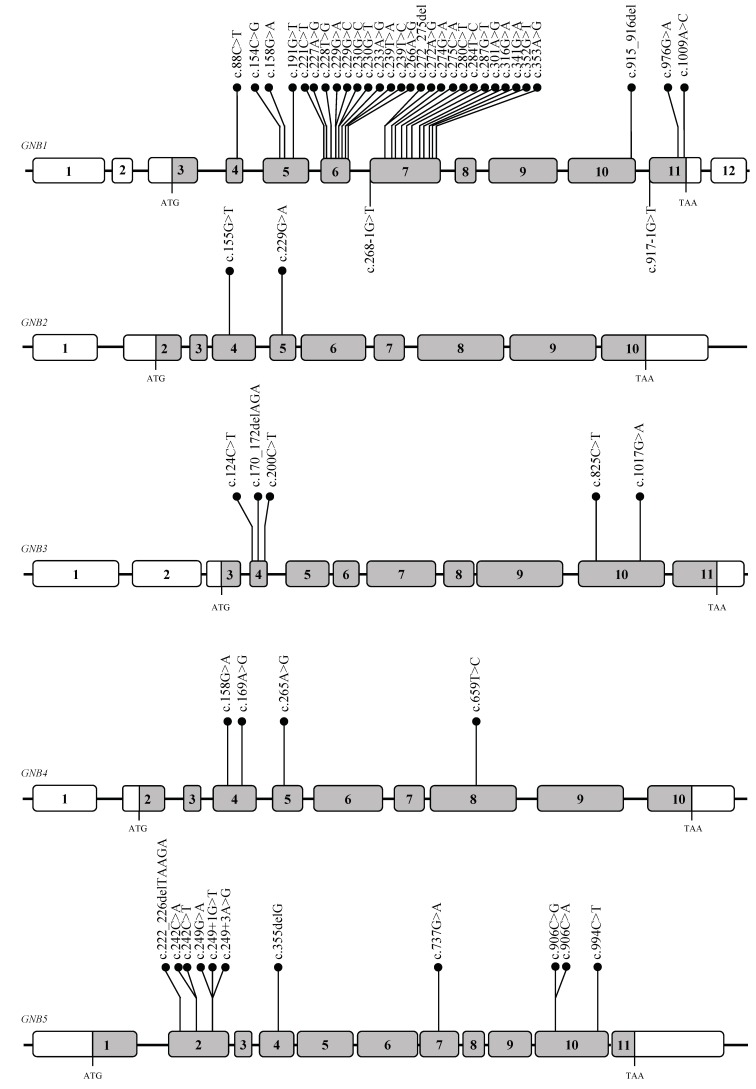
To date, twenty eight de novo and four with undefined inheritance GNB1 variants have been reported in 53 affected individuals; of these 28 are missense, 2 frameshift, and 2 splice-site variants (Figure 2) [35,36,37,38,39,40,41,42].
Figure 2.
Variants distribution across the entire GNB1-GNB5 genes. Genomic coordinates are specified on the GRCh37.p13 genome assembly. Coding exons are indicated by grey boxes, while untranslated regions are displayed in white. Variants annotations refer to NM_002074 for GNB1, NM_005273 for GNB2, NM_002075 for GNB3, NM_021629 for GNB4, NM_006578 for GNB5.
Overall, 24/32 (~75%) GNB1 variants affect residues coded by exon 6 (11/24) or exon 7 (13/24) (Figure 2). This small part of the gene encodes for a protein region forming the Gα and Gβγ interaction surface [46]. Accordingly, three GNB1 likely benign missense variants (c.88C>T, p.(Leu30Phe); c.272A>G, p.(His91Arg); c.1009A>C, p.(Lys337Gln) are located distantly from the interaction site and no impaired Gβ1 functionality has been shown [40].
3.2. G Protein Subunit Beta 2 (GNB2, Gβ2)
Heterozygous GNB2 (MIM 139390) c.155G>T, p.(Arg52Leu) (Figure 2) missense variant has been identified in 11 individuals of a family composed of 25 members. Carriers were affected by an autosomal dominant form of Sinus Node Dysfunction (SND) occurring in combination with atrioventricular conduction dysfunction and atrial fibrillation, in the absence of heart structural problems [47]. Crystal structure model of the mammalian G-protein–coupled inwardly rectifying potassium channel 2 (GIRK2) with β1γ2 G-protein complex, showed that Arg52 lays at the binding interface with GIRK [48], a data confirmed also for GIRK1/4 and Gβ2 [47]. Therefore, the presence of the mutant residue is predicted to decrease the steric interaction at the GIRK-Gβ2 surface. Functional studies revealed that the heterozygous variant has an impact on the rectification of the GIRK channel with a consequent increase of ACh-activated K+ current (IK,ACh) [47], thus displaying a gain-of-function effect. Of note, the cardiac GIRK channels are directly switched on by the Gβγ units and are involved in the negative chronotropic effect of the parasympathetic nervous system, thus controlling heart rate and cellular electrical excitability [49,50]. A recent study of 52 unrelated patients with idiopathic SND uncovered a nonsynonymous substitution (c.303G>C, p.(Trp101Cys)) in the KCNJ5 gene, encoding the Kir3.4 subunit of the GIRK channel. The mutation leads to a sustained activation of the cardiac GIRK channel [51]. Finally, further examples of the connection between GNB2 and heart disease are provided by the Gnb2 knock-out mice, generated by the International Mouse Phenotyping Consortium (IMPC; https://www.mousephenotype.org/data/genes/MGI:95784) [52]. Null Gnb2 mice showed an increased heart rate, and abnormal electrocardiogram line, revealing shortened RR interval, PQ interval, and ST segment (Table 2).
Additionally, an individual with global developmental delay, intellectual disability, muscle hypotonia and dysmorphisms carrying a de novo GNB2 missense variant (c.229G>A, p.(Gly77Arg)) (Figure 2), predicted to impair protein function, was recently described in [53]. This study highlights that GNB2 variants not only associate to cardiac manifestations, but cause developmental delay too [53].
3.3. G Protein Subunit Beta 3 (GNB3, Gβ3)
Homozygous and compound heterozygous GNB3 (MIM 139130) disease-causing variants were described in three individuals of a large Lebanese–Armenian family affected by Congenital Stationary Night Blindness type 1H (CSNB1H, MIM#617024) [8] and in a fourth sporadic case [69]. CSNB refers to a group of clinically heterogeneous retinal disorders caused by genetic defects of the retinoid metabolism in the retinal pigment epithelium (RPE), phototransduction, or signal transmission through the bipolar cells (BCs) [70,71]. Based on BCs ability to either initiate or terminate light stimuli, BCs can be either ON- or OFF-type. Specifically, while cone photoreceptors can connect both ON- and OFF-BCs, the rods are served largely by the ON-BCs [70].
The three first identified GNB3 variants lie in the first (c.170_172delAGA, p.(Lys57del); c.200C>T, p.(Ser67Phe)) and seventh (c.1017G>A, p.(Trp339*)) WD40 repeat of the encoded Gβ3 protein, respectively (Figure 2). Homology model studies of Gβ3 protein structure, pointed out that each variant would impact interactions abilities as well as the formation of effective G-protein complexes [8]. A fourth GNB3 variant (c.124C>T, p.(Arg42Ter)) was found in a patient with distinct early-onset inherited retinal disease, characterized by nystagmus, normal funduscopic exam, full-field electroretinography abnormalities, and mild disturbance of the central macula [69]. The Arg42 variant, located in exon 4 of the gene, gives rise to a premature stop codon, which is expected to be a substrate of the nonsense–mediate decay pathway [69].
Gβ3 is expressed at higher levels in the eyes, in particular in the cone photoreceptors and ON-BCs of the retina in mammals and additional species [72,73,74]. In the eye, Gβ3 modulates cone transducing function and cone and rod ON-bipolar signaling [8].
Similar to humans [8], abnormal light ON bipolar response and reduced cone sensitivity was also found in a Gnb3 knock-out mouse model [75,76], while retinopathy globe enlarged (rge) phenotype was reported in the chicken carrying a 3-bp homozygous deletion of the GNB3 homolog [77]. Interestingly, ablation of the Gnb3 gene in mice causes mild bradycardia [58], thus suggesting a possible additional role of GNB3 in heart rate regulation.
Beyond the role of GNB3 pathogenic variants in the etiology of CSNB1H, [8] Siffert and colleagues [78], described the c.825C>T (rs5443) polymorphism in exon 10 of the gene as linked to the expression of a shortened splice variant, Gβ3s, whose translated protein is characterized by the deletion of 41 amino acids, responsible of enhanced G-proteins signal transduction [78]. The c.825C>T polymorphism is associated with an increased risk of hypertension [78,79,80,81], obesity [59,82], diabetes [83], metabolic syndrome component [84,85], depression [86,87], seasonal variations in mood and behaviors [88], functional dyspepsia [89,90], stroke [91,92], arrhythmia [93], coronary artery disease [94,95], and other cardiovascular phenotypes [96,97,98,99]. In addition, duplication and overexpression of GNB3 gene is responsible for a syndromic form of childhood obesity [59,100].
3.4. G Protein Subunit Beta (GNB4, Gβ4)
Heterozygous pathogenic variants in GNB4 gene (MIM 610863) (Figure 2) have been reported as causative of intermediate Charcot–Marie–Tooth disease F (CMTDIF, MIM#615185), an autosomal dominant form of CMT. CMT is a neurologic disorder characterized by progressive distal muscle atrophy and weakness and variable nerve conduction velocities ranging from the demyelinating to the axonal range [101]. Heterozygous c.158G>A, p.(Gly53Asp) GNB4 missense variant was reported in six affected family members. An unrelated case carried the heterozygous c.265A>G; p.(Lys89Glu) de novo missense variant [101]. The pathogenicity of the variants and the importance of GPCR signaling in peripheral-nerve function in humans were supported by the reduced Gβ4 immunostaining in the axon and Schwann cells of peripheral nerves of affected individuals. Moreover, in vitro studies demonstrated that both variants altered the bradykinin induced GPCR signaling [101].
More recently, the description of one Czech patient presenting the c.169A>G, p.(Lys57Glu) variant [102], and one Japanese family, for which axonal neuropathy has been reported, and segregating with c.659T>C, p.(Gln220Arg) [103], confirmed the pathogenic role of GNB4 as causal gene of CMTIDF.
All the GNB4 pathogenic variants described so far are located in the first (p.(Gly53Asp) and p.(Lys57Glu)) [101,102], in the second (p.(Lys89Glu)) [101], and in the fifth WD40 domain (p.(Gln220Arg)) [103], respectively (Figure 2). The Gly53 and Lys89 are important residues for the architecture of the WD40 β-propeller structure [104]. Functional characterization of p.(Gly53Asp) and p.(Lys89Glu) showed an impaired GPCR signaling via a dominant-negative effect, and resulting in reduced PLCβ2 activity [104,105] followed by inhibition of IP3 production and moderate increase in cytosolic calcium (Ca2+) level [101,106], a universal second messenger that regulates the transmission of the depolarizing signal and neuronal synaptic activity.
Similar to Gβ2, Gβ4 is known to influence the activity of the cardiac GIRK channel, which regulates the heart rhythm through the acetylcholine-dependent activation of the muscarinic M2-receptor present in the sinoatrial node [107,108,109,110]. Although this gene has been reported in human hereditary neuropathy, genome-wide association studies have revealed association of the GNB4 locus with variation in heart rate [47,111,112]. This suggests that GNB4 variation may also impact heart rate.
3.5. G Protein Subunit Beta (GNB5, Gβ5)
The GNB5 gene (MIM 604447), encoding the subunit β5 of the heterotrimeric G-proteins, is a divergent member of the Gβ family with distinct biochemical properties. Differently from Gβ1–4, Gβ5 forms irreversible dimer with the G-protein γ-like (GGL) domain [113] present in the R7 regulator group of G-protein signaling proteins (R7 RGS) [64,114,115,116,117,118,119]. Interaction of the GGL domain and the atypical Gβ5 is a general requirement for stabilization of the whole R7 protein subfamily.
Homozygous or compound heterozygous variants in the GNB5 gene have been associated with either IDDCA (Intellectual Developmental Delay with Cardiac Arrhythmia, MIM#617173) or LADCI (Language delay and ADHD/Cognitive Impairment with or without cardiac arrhythmia, MIM#617182) human syndromes [66,120,121,122,123,124,125]. Homozygous carriers of the recurrent missense variant c.242C>T, p.(Ser81Leu), present with LADCI syndrome, characterized by mild intellectual disability in combination with language delay, attention-deficit/hyperactivity disorder, with or without cardiac arrhythmia [66,125]. The substitution of the evolutionary conserved Serine 81 with the hydrophobic Leucine was predicted to compromise protein folding and/or stability as well as impair the binding kinetics of RGS proteins [66] and their capacity to deactivate G-protein signaling initiated by dopamine receptors [125]. By contrast, homozygous or compound heterozygous carriers of GNB5 Loss of Function alleles presented IDDCA, whose phenotypic spectrum includes epileptic seizures, severe intellectual disability, drastic impairment in speech and language skills, vision problems (which mainly include nystagmus and retinal abnormalities), hypotonia, and sick sinus syndrome [66,120,121,122,123,124]. Among the GNB5 pathogenic variants described so far [66,120,121,122,123,124,125], a mutational hot spot in exon 2, encoding the first WD40 domain and containing 58% of described variants, has been identified (Figure 2). The evidence of the GNB5 involvement in neuronal and cardiac signaling was confirmed in Gnb5-null zebrafish and mouse models that resulted in neuronal and cardiac phenotypes reminiscent of those of IDDCA patients [63,66,126,127].
Gnb5-null mouse models displayed marked neurobehavioral abnormalities, impaired gait and motor learning, hyperactivity [62,63,64,65], defective visual adaptation with perturbed development and functioning of retinal bipolar cells [127,128,129]. Moreover, targeted deletion of one or two copies of the Gnb5 gene had distinct effects on body weight and behavior in mice [62]. Although the cardiac phenotype of Gnb5-null mouse has never been studied, it is interesting to observe that bradycardia and heart rate responses to the cholinergic stimulation were exhibited by mice lacking Rgs6, the Gnb5-dependent RGS protein in the heart [130,131,132]. The gnb5 knock-out zebrafish model also recapitulated the phenotypic spectrum of affected individuals, highlighting the involvement of GNB5 in the control of motor capacity, vision and heart rate [66]. Several model organisms have been characterized regarding GNB5; information of additional animal models is included in Table 2.
4. Concluding Remarks
Heterotrimeric G-protein signaling is one of the most important mechanisms of cellular communication. They are involved in a vast array of cellular processes required for the normal growth and development of cells. The Gβ proteins, representing one of the components of the heterotrimeric G-proteins, are specifically expressed in different tissues and elicit a wide range of specialized cellular responses. It is not surprising that mutations altering the G-proteins function, compromise cellular responses and associate with aberrant physiological functions, resulting in disease.
We anticipate that unravelling the role of Gβ proteins in neurodevelopmental and cardiac conditions may help to provide targeted strategies to effectively modulate their pathogenesis and to shed light on possible future therapeutic approach.
Acknowledgments
We thank Utsa Bhaduri for editing the whole text for English grammar, word choice and sentence construction.
Author Contributions
N.M. and G.M. conceived this review. N.M. and P.D.N. reviewed the literature and wrote the manuscript along with G.M. All authors contributed to the final version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health (Ricerca Corrente), the Daunia Plast and Stuppiello’s Family (Private Donors) to GM.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fredriksson R., Lagerstrom M.C., Lundin L.G., Schioth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharm. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 2.Kamato D., Burch M.L., Osman N., Zheng W., Little P.J. Therapeutic implications of endothelin and thrombin G-protein-coupled receptor transactivation of tyrosine and serine/threonine kinase cell surface receptors. J. Pharm. 2013;65:465–473. doi: 10.1111/j.2042-7158.2012.01577.x. [DOI] [PubMed] [Google Scholar]
- 3.de Oliveira P.G., Ramos M.L.S., Amaro A.J., Dias R.A., Vieira S.I. Gi/o-Protein coupled receptors in the aging brain. Front. Aging Neurosci. 2019;11:89. doi: 10.3389/fnagi.2019.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lagerstrom M.C., Schioth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 5.Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 6.Gilman A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 7.Smrcka A.V. G protein βγ subunits: Central mediators of G protein-coupled receptor signaling. Cell Mol. Life Sci. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent A., Audo I., Tavares E., Maynes J.T., Tumber A., Wright T., Li S., Michiels C., Consortium G.N.B., Condroyer C., et al. Biallelic mutations in GNB3 cause a unique form of autosomal-recessive congenital stationary night blindness. Am. J. Hum. Genet. 2016;98:1011–1019. doi: 10.1016/j.ajhg.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson A.J., Aragay A.M., Slepak V.Z., Simon M.I. A novel form of the G protein β subunit Gβ5 is specifically expressed in the vertebrate retina. J. Biol. Chem. 1996;271:28154–28160. doi: 10.1074/jbc.271.45.28154. [DOI] [PubMed] [Google Scholar]
- 10.Khan S.M., Sleno R., Gora S., Zylbergold P., Laverdure J.P., Labbe J.C., Miller G.J., Hebert T.E. The expanding roles of Gβγ subunits in G protein-coupled receptor signaling and drug action. Pharm. Rev. 2013;65:545–577. doi: 10.1124/pr.111.005603. [DOI] [PubMed] [Google Scholar]
- 11.Dupre D.J., Robitaille M., Rebois R.V., Hebert T.E. The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharm. Toxicol. 2009;49:31–56. doi: 10.1146/annurev-pharmtox-061008-103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robishaw J.D., Berlot C.H. Translating G protein subunit diversity into functional specificity. Curr. Opin. Cell Biol. 2004;16:206–209. doi: 10.1016/j.ceb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Azzi M., Charest P.G., Angers S., Rousseau G., Kohout T., Bouvier M., Pineyro G. Β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yudin Y., Rohacs T. Inhibitory Gi/O-coupled receptors in somatosensory neurons: Potential therapeutic targets for novel analgesics. Mol. Pain. 2018;14 doi: 10.1177/1744806918763646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajagopal S., Kim J., Ahn S., Craig S., Lam C.M., Gerard N.P., Gerard C., Lefkowitz R.J. Β-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. PNAS Proc. Natl. Acad. Sci. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nobles M., Benians A., Tinker A. Heterotrimeric G proteins precouple with G protein-coupled receptors in living cells. PNAS Proc. Natl Acad Sci. 2005;102:18706–18711. doi: 10.1073/pnas.0504778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milligan G., Kostenis E. Heterotrimeric G-proteins: A short history. Br. J. Pharm. 2006;147:S46–S55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hepler J.R., Berman D.M., Gilman A.G., Kozasa T. RGS4 and GAIP are GTPase-activating proteins for Gqα and block activation of phospholipase Cβ by γ-thio-GTP-Gqα. PNAS Proc. Natl. Acad. Sci. 1997;94:428–432. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozasa T., Jiang X., Hart M.J., Sternweis P.M., Singer W.D., Gilman A.G., Bollag G., Sternweis P.C. P115 RhoGEF, a GTPase activating protein for Gα12 and Gα13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 21.Hunt T.W., Fields T.A., Casey P.J., Peralta E.G. RGS10 is a selective activator of Gαi GTPase activity. Nature. 1996;383:175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 22.Berman D.M., Wilkie T.M., Gilman A.G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996;86:445–452. doi: 10.1016/S0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- 23.De Vries L., Zheng B., Fischer T., Elenko E., Farquhar M.G. The regulator of G protein signaling family. Annu. Rev. Pharm. Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 24.Neer E.J. Heterotrimeric G proteins: Organizers of transmembrane signals. Cell. 1995;80:249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- 25.Downes G.B., Gautam N. The G protein subunit gene families. Genomics. 1999;62:544–552. doi: 10.1006/geno.1999.5992. [DOI] [PubMed] [Google Scholar]
- 26.Lindorfer M.A., Myung C.S., Savino Y., Yasuda H., Khazan R., Garrison J.C. Differential activity of the G protein β5γ2 subunit at receptors and effectors. J. Biol. Chem. 1998;273:34429–34436. doi: 10.1074/jbc.273.51.34429. [DOI] [PubMed] [Google Scholar]
- 27.Gautam N., Downes G.B., Yan K., Kisselev O. The G-protein βγ complex. Cell Signal. 1998;10:447–455. doi: 10.1016/S0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- 28.Howlett A.C., Gray A.J., Hunter J.M., Willardson B.M. Role of molecular chaperones in G protein β5/regulator of G protein signaling dimer assembly and G protein βγ dimer specificity. J. Biol. Chem. 2009;284:16386–16399. doi: 10.1074/jbc.M900800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson A.J., Katz A., Simon M.I. A fifth member of the mammalian G-protein β-subunit family. Expression in brain and activation of the β2 isotype of phospholipase C. J. Biol. Chem. 1994;269:22150–22156. [PubMed] [Google Scholar]
- 30.Li D., Roberts R. WD-repeat proteins: Structure characteristics, biological function, and their involvement in human diseases. Cell Mol. Life Sci. 2001;58:2085–2097. doi: 10.1007/PL00000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sondek J., Bohm A., Lambright D.G., Hamm H.E., Sigler P.B. Crystal structure of a G-protein βγ dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 32.Jain B.P., Pandey S. WD40 repeat proteins: Signalling scaffold with diverse functions. Protein J. 2018;37:391–406. doi: 10.1007/s10930-018-9785-7. [DOI] [PubMed] [Google Scholar]
- 33.Yates B., Braschi B., Gray K.A., Seal R.L., Tweedie S., Bruford E.A. Genenames.org: the HGNC and VGNC resources in 2017. Nucleic Acids Res. 2017;45:D619–D625. doi: 10.1093/nar/gkw1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinrucke S., Lohmann K., Domingo A., Rolfs A., Baumer T., Spiegler J., Hartmann C., Munchau A. Novel GNB1 missense mutation in a patient with generalized dystonia, hypotonia, and intellectual disability. Neurol. Genet. 2016;2:e106. doi: 10.1212/NXG.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo W., Ikemoto S., Togashi N., Miyabayashi T., Nakajima E., Hamano S.I., Shibuya M., Sato R., Takezawa Y., Okubo Y., et al. Phenotype-genotype correlations in patients with GNB1 gene variants, including the first three reported Japanese patients to exhibit spastic diplegia, dyskinetic quadriplegia, and infantile spasms. Brain Dev. 2019 doi: 10.1016/j.braindev.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Brett M., Lai A.H., Ting T.W., Tan A.M., Foo R., Jamuar S., Tan E.C. Acute lymphoblastic leukemia in a child with a de novo germline gnb1 mutation. Am. J. Med. Genet. A. 2017;173:550–552. doi: 10.1002/ajmg.a.38026. [DOI] [PubMed] [Google Scholar]
- 38.Petrovski S., Kury S., Myers C.T., Anyane-Yeboa K., Cogne B., Bialer M., Xia F., Hemati P., Riviello J., Mehaffey M., et al. Germline de novo mutations in GNB1 cause severe neurodevelopmental disability, hypotonia, and seizures. Am. J. Hum. Genet. 2016;98:1001–1010. doi: 10.1016/j.ajhg.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones H.F., Morales-Briceno H., Barwick K., Lewis J., Sanchis-Juan A., Raymond F.L., Stewart K., Waugh M.C., Mahant N., Kurian M.A., et al. Myoclonus-dystonia caused by GNB1 mutation responsive to deep brain stimulation. Mov. Disord. 2019;34:1079–1080. doi: 10.1002/mds.27708. [DOI] [PubMed] [Google Scholar]
- 40.Lohmann K., Masuho I., Patil D.N., Baumann H., Hebert E., Steinrucke S., Trujillano D., Skamangas N.K., Dobricic V., Huning I., et al. Novel GNB1 mutations disrupt assembly and function of G protein heterotrimers and cause global developmental delay in humans. Hum. Mol. Genet. 2017;26:1078–1086. doi: 10.1093/hmg/ddx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemati P., Revah-Politi A., Bassan H., Petrovski S., Bilancia C.G., Ramsey K., Griffin N.G., Bier L., Cho M.T., Rosello M., et al. Refining the phenotype associated with GNB1 mutations: Clinical data on 18 newly identified patients and review of the literature. Am. J. Med. Genet. A. 2018;176:2259–2275. doi: 10.1002/ajmg.a.40472. [DOI] [PubMed] [Google Scholar]
- 42.Szczaluba K., Biernacka A., Szymanska K., Gasperowicz P., Kosinska J., Rydzanicz M., Ploski R. Novel GNB1 de novo mutation in a patient with neurodevelopmental disorder and cutaneous mastocytosis: Clinical report and literature review. Eur. J. Med. Genet. 2018;61:157–160. doi: 10.1016/j.ejmg.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Rosenfeld J.A., Crolla J.A., Tomkins S., Bader P., Morrow B., Gorski J., Troxell R., Forster-Gibson C., Cilliers D., Hislop R.G., et al. Refinement of causative genes in monosomy 1p36 through clinical and molecular cytogenetic characterization of small interstitial deletions. Am. J. Med. Genet. Part A. 2010;152A:1951–1959. doi: 10.1002/ajmg.a.33516. [DOI] [PubMed] [Google Scholar]
- 44.Shaffer L.G., Lupski J.R. Molecular mechanisms for constitutional chromosomal rearrangements in humans. Annu. Rev. Genet. 2000;34:297–329. doi: 10.1146/annurev.genet.34.1.297. [DOI] [PubMed] [Google Scholar]
- 45.Okae H., Iwakura Y. Neural tube defects and impaired neural progenitor cell proliferation in Gβ1-deficient mice. Dev. Dyn. 2010;239:1089–1101. doi: 10.1002/dvdy.22256. [DOI] [PubMed] [Google Scholar]
- 46.Ford C.E., Skiba N.P., Bae H., Daaka Y., Reuveny E., Shekter L.R., Rosal R., Weng G., Yang C.S., Iyengar R., et al. Molecular basis for interactions of G protein βγ subunits with effectors. Science. 1998;280:1271–1274. doi: 10.1126/science.280.5367.1271. [DOI] [PubMed] [Google Scholar]
- 47.Stallmeyer B., Kuss J., Kotthoff S., Zumhagen S., Vowinkel K., Rinne S., Matschke L.A., Friedrich C., Schulze-Bahr E., Rust S., et al. A mutation in the G-protein gene GNB2 causes familial sinus node and atrioventricular conduction dysfunction. Circ. Res. 2017;120:e33–e44. doi: 10.1161/CIRCRESAHA.116.310112. [DOI] [PubMed] [Google Scholar]
- 48.Whorton M.R., MacKinnon R. X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature. 2013;498:190–197. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gehrmann J., Meister M., Maguire C.T., Martins D.C., Hammer P.E., Neer E.J., Berul C.I., Mende U. Impaired parasympathetic heart rate control in mice with a reduction of functional G protein βγ-subunits. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H445–H456. doi: 10.1152/ajpheart.00565.2001. [DOI] [PubMed] [Google Scholar]
- 50.Logothetis D.E., Kurachi Y., Galper J., Neer E.J., Clapham D.E. The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 51.Kuss J., Stallmeyer B., Goldstein M., Rinne S., Pees C., Zumhagen S., Seebohm G., Decher N., Pott L., Kienitz M.C., et al. Familial sinus node disease caused by a gain of GIRK (G-protein activated inwardly rectifying K+ channel) channel function. Circ. Genom. Precis. Med. 2019;12:e002238. doi: 10.1161/CIRCGEN.118.002238. [DOI] [PubMed] [Google Scholar]
- 52.Dickinson M.E., Flenniken A.M., Ji X., Teboul L., Wong M.D., White J.K., Meehan T.F., Weninger W.J., Westerberg H., Adissu H., et al. High-throughput discovery of novel developmental phenotypes. Nature. 2016;537:508–514. doi: 10.1038/nature19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fukuda T., Hiraide T., Yamoto K., Nakashima M., Kawai T., Yanagi K., Ogata T., Saitsu H. Exome reports A de novo GNB2 variant associated with global developmental delay, intellectual disability, and dysmorphic features. Eur. J. Med. Genet. 2019:103804. doi: 10.1016/j.ejmg.2019.103804. [DOI] [PubMed] [Google Scholar]
- 54.Ke W., Ye D., Mersch K., Xu H., Chen S., Lin F. Gβ1 is required for neutrophil migration in zebrafish. Dev. Biol. 2017;428:135–147. doi: 10.1016/j.ydbio.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuse N., Hisata K., Katzen A.L., Matsuzaki F. Heterotrimeric G proteins regulate daughter cell size asymmetry in Drosophila neuroblast divisions. Curr. Biol. 2003;13:947–954. doi: 10.1016/S0960-9822(03)00334-8. [DOI] [PubMed] [Google Scholar]
- 56.Simmer F., Moorman C., van der Linden A.M., Kuijk E., van den Berghe P.V., Kamath R.S., Fraser A.G., Ahringer J., Plasterk R.H. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1:E12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dubaj Price M., Hurd D.D. WormBase: A model organism database. Med. Ref. Serv. Q. 2019;38:70–80. doi: 10.1080/02763869.2019.1548896. [DOI] [PubMed] [Google Scholar]
- 58.Ye Y., Sun Z., Guo A., Song L.S., Grobe J.L., Chen S. Ablation of the GNB3 gene in mice does not affect body weight, metabolism or blood pressure, but causes bradycardia. Cell Signal. 2014;26:2514–2520. doi: 10.1016/j.cellsig.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozdemir A.C., Wynn G.M., Vester A., Weitzmann M.N., Neigh G.N., Srinivasan S., Rudd M.K. GNB3 overexpression causes obesity and metabolic syndrome. PLoS ONE. 2017;12:e0188763. doi: 10.1371/journal.pone.0188763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldlust I.S., Hermetz K.E., Catalano L.M., Barfield R.T., Cozad R., Wynn G., Ozdemir A.C., Conneely K.N., Mulle J.G., Dharamrup S., et al. Mouse model implicates GNB3 duplication in a childhood obesity syndrome. Proc. Natl. Acad. Sci. 2013;110:14990–14994. doi: 10.1073/pnas.1305999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lagman D., Callado-Perez A., Franzen I.E., Larhammar D., Abalo X.M. Transducin duplicates in the zebrafish retina and pineal complex: Differential specialisation after the teleost tetraploidisation. PLoS ONE. 2015;10:e0121330. doi: 10.1371/journal.pone.0121330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q., Levay K., Chanturiya T., Dvoriantchikova G., Anderson K.L., Bianco S.D., Ueta C.B., Molano R.D., Pileggi A., Gurevich E.V., et al. Targeted deletion of one or two copies of the G protein β subunit Gβ5 gene has distinct effects on body weight and behavior in mice. FASEB J. 2011;25:3949–3957. doi: 10.1096/fj.11-190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J.H., Pandey M., Seigneur E.M., Panicker L.M., Koo L., Schwartz O.M., Chen W., Chen C.K., Simonds W.F. Knockout of G protein β5 impairs brain development and causes multiple neurologic abnormalities in mice. J. Neurochem. 2011;119:544–554. doi: 10.1111/j.1471-4159.2011.07457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie K., Ge S., Collins V.E., Haynes C.L., Renner K.J., Meisel R.L., Lujan R., Martemyanov K.A. Gβ5-RGS complexes are gatekeepers of hyperactivity involved in control of multiple neurotransmitter systems. Psychopharmacology (Berl) 2012;219:823–834. doi: 10.1007/s00213-011-2409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C.K., Eversole-Cire P., Zhang H., Mancino V., Chen Y.J., He W., Wensel T.G., Simon M.I. Instability of GGL domain-containing RGS proteins in mice lacking the G protein β-subunit Gβ5. PNAS Proc. Natl. Acad. Sci. 2003;100:6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lodder E.M., De Nittis P., Koopman C.D., Wiszniewski W., Moura de Souza C.F., Lahrouchi N., Guex N., Napolioni V., Tessadori F., Beekman L., et al. GNB5 mutations cause an autosomal-recessive multisystem syndrome with sinus bradycardia and cognitive disability. Am. J. Hum. Genet. 2016;99:704–710. doi: 10.1016/j.ajhg.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thurmond J., Goodman J.L., Strelets V.B., Attrill H., Gramates L.S., Marygold S.J., Matthews B.B., Millburn G., Antonazzo G., Trovisco V., et al. FlyBase 2.0: The next generation. Nucleic Acids Res. 2019;47:D759–D765. doi: 10.1093/nar/gky1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robatzek M., Niacaris T., Steger K., Avery L., Thomas J.H. Eat-11 encodes GPB-2, a Gβ5 ortholog that interacts with Goα and Gqα to regulate C. elegans behavior. Curr. Biol. 2001;11:288–293. doi: 10.1016/S0960-9822(01)00074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arno G., Holder G.E., Chakarova C., Kohl S., Pontikos N., Fiorentino A., Plagnol V., Cheetham M.E., Hardcastle A.J., Webster A.R., et al. Recessive retinopathy consequent on mutant G-protein β subunit 3 (GNB3) JAMA Ophthalmol. 2016;134:924–927. doi: 10.1001/jamaophthalmol.2016.1543. [DOI] [PubMed] [Google Scholar]
- 70.Das R.G., Becker D., Jagannathan V., Goldstein O., Santana E., Carlin K., Sudharsan R., Leeb T., Nishizawa Y., Kondo M., et al. Genome-wide association study and whole-genome sequencing identify a deletion in LRIT3 associated with canine congenital stationary night blindness. Sci. Rep. 2019;9:14166. doi: 10.1038/s41598-019-50573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeitz C., Robson A.G., Audo I. Congenital stationary night blindness: An analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog. Retin. Eye Res. 2015;45:58–110. doi: 10.1016/j.preteyeres.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Ritchey E.R., Bongini R.E., Code K.A., Zelinka C., Petersen-Jones S., Fischer A.J. The pattern of expression of guanine nucleotide-binding protein β3 in the retina is conserved across vertebrate species. Neuroscience. 2010;169:1376–1391. doi: 10.1016/j.neuroscience.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee R.H., Lieberman B.S., Yamane H.K., Bok D., Fung B.K. A third form of the G protein β subunit. 1. Immunochemical identification and localization to cone photoreceptors. J. Biol. Chem. 1992;267:24776–24781. [PubMed] [Google Scholar]
- 74.Peng Y.W., Robishaw J.D., Levine M.A., Yau K.W. Retinal rods and cones have distinct G protein β and γ subunits. Proc. Natl. Acad. Sci. 1992;89:10882–10886. doi: 10.1073/pnas.89.22.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nikonov S.S., Lyubarsky A., Fina M.E., Nikonova E.S., Sengupta A., Chinniah C., Ding X.Q., Smith R.G., Pugh E.N., Jr., Vardi N., et al. Cones respond to light in the absence of transducin β subunit. J. Neurosci. 2013;33:5182–5194. doi: 10.1523/JNEUROSCI.5204-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dhingra A., Ramakrishnan H., Neinstein A., Fina M.E., Xu Y., Li J., Chung D.C., Lyubarsky A., Vardi N. Gβ3 is required for normal light ON responses and synaptic maintenance. J. Neurosci. 2012;32:11343–11355. doi: 10.1523/JNEUROSCI.1436-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tummala H., Ali M., Getty P., Hocking P.M., Burt D.W., Inglehearn C.F., Lester D.H. Mutation in the guanine nucleotide-binding protein β-3 causes retinal degeneration and embryonic mortality in chickens. Invest. Ophthalmol. Vis. Sci. 2006;47:4714–4718. doi: 10.1167/iovs.06-0292. [DOI] [PubMed] [Google Scholar]
- 78.Siffert W., Rosskopf D., Siffert G., Busch S., Moritz A., Erbel R., Sharma A.M., Ritz E., Wichmann H.E., Jakobs K.H., et al. Association of a human G-protein β3 subunit variant with hypertension. Nat. Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 79.Sousa A.C., Reis R.P.D., Pereira A., Borges S., Gouveia S., Spinola A., Freitas A.I., Guerra G., Gois T., Rodrigues M., et al. The genetic variant C825T of the β3 subunit of G protein is associated with hypertension in a Portuguese population. Rev. Port. Cardiol. 2018;37:499–507. doi: 10.1016/j.repc.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 80.Rong S.L., Zheng J.Z., Wang X.L., Zhang C.Y., Su J., Li B. Association of G-protein β3 subunit C825T polymorphism with essential hypertension: Evidence from 63,729 subjects. J. Hum. Hypertens. 2017;31:511–514. doi: 10.1038/jhh.2017.31. [DOI] [PubMed] [Google Scholar]
- 81.Hengstenberg C., Schunkert H., Mayer B., Doring A., Lowel H., Hense H.W., Fischer M., Riegger G.A., Holmer S.R. Association between a polymorphism in the G protein β3 subunit gene (GNB3) with arterial hypertension but not with myocardial infarction. Cardiovasc. Res. 2001;49:820–827. doi: 10.1016/S0008-6363(00)00292-3. [DOI] [PubMed] [Google Scholar]
- 82.Moselhy S.S., Alhetari Y.A., Iyer A., Huwait E.A., Al-Ghamdi M.A., Al-Ghamdi S., Balamash K.S., Basuni A.A., Alama M.N., Kumosani T.A., et al. Analysis of SNPs of MC4R, GNB3 and FTO gene polymorphism in obese Saudi subjects. Afr. Health Sci. 2017;17:1059–1069. doi: 10.4314/ahs.v17i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rizvi S., Raza S.T., Rahman Q., Mahdi F. Role of GNB3, NET, KCNJ11, TCF7L2 and GRL genes single nucleotide polymorphism in the risk prediction of type 2 diabetes mellitus. 3 Biotech. 2016;6:255. doi: 10.1007/s13205-016-0572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen P.S., Chang H.H., Huang C.C., Lee C.C., Lee S.Y., Chen S.L., Huang S.Y., Yang Y.K., Lu R.B. A longitudinal study of the association between the GNB3 C825T polymorphism and metabolic disturbance in bipolar II patients treated with valproate. Pharm. J. 2017;17:155–161. doi: 10.1038/tpj.2015.96. [DOI] [PubMed] [Google Scholar]
- 85.Prystupa L.N., Moiseyenko I.O., Garbuzova V.Y., Kmyta V.V., Dudchenko I.A. Association of metabolic syndrome components with the genotypes of the C825T polymorphism in the G protein β3-subunit gene (GNB3) Wiadomosci Lekarskie. 2018;71:1242–1249. [PubMed] [Google Scholar]
- 86.Ma J., Wang L., Yang Y., Qiao Z., Fang D., Qiu X., Yang X., Zhu X., He J., Pan H., et al. GNB3 and CREB1 gene polymorphisms combined with negative life events increase susceptibility to major depression in a Chinese Han population. PLoS ONE. 2017;12:e0170994. doi: 10.1371/journal.pone.0170994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zill P., Baghai T.C., Zwanzger P., Schule C., Minov C., Riedel M., Neumeier K., Rupprecht R., Bondy B. Evidence for an association between a G-protein β3-gene variant with depression and response to antidepressant treatment. Neuroreport. 2000;11:1893–1897. doi: 10.1097/00001756-200006260-00018. [DOI] [PubMed] [Google Scholar]
- 88.Nam Y.J., Cho C.H., Kim L., Lee H.J. Association of G-protein β3 subunit C825T polymorphism with seasonal variations in mood and behavior. Psychiatry Investig. 2018;15:200–204. doi: 10.30773/pi.2017.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song Y.Z., You H.Y., Zhu Z.H., Wen Z.D., Xu H.Y., Chen B.C., Chen Z.J., Huang Q.K. The C825T polymorphism of the G-protein β3 gene as a risk factor for functional dyspepsia: A meta-analysis. Gastroenterol. Res. Pr. 2016;2016:5037254. doi: 10.1155/2016/5037254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Triantafyllou K., Kourikou A., Gazouli M., Karamanolis G.P., Dimitriadis G.D. Functional dyspepsia susceptibility is related to CD14, GNB3, MIF, and TRPV1 gene polymorphisms in the Greek population. Neurogastroenterol. Motil. 2017;29 doi: 10.1111/nmo.12913. [DOI] [PubMed] [Google Scholar]
- 91.Morrison A.C., Doris P.A., Folsom A.R., Nieto F.J., Boerwinkle E. G-protein β3 subunit and α-adducin polymorphisms and risk of subclinical and clinical stroke. Stroke. 2001;32:822–829. doi: 10.1161/01.STR.32.4.822. [DOI] [PubMed] [Google Scholar]
- 92.Zhang L., Zhang H., Sun K., Song Y., Hui R., Huang X. The 825C/T polymorphism of G-protein β3 subunit gene and risk of ischaemic stroke. J. Hum. Hypertens. 2005;19:709–714. doi: 10.1038/sj.jhh.1001883. [DOI] [PubMed] [Google Scholar]
- 93.Schreieck J., Dostal S., von Beckerath N., Wacker A., Flory M., Weyerbrock S., Koch W., Schomig A., Schmitt C. C825T polymorphism of the G-protein β3 subunit gene and atrial fibrillation: Association of the TT genotype with a reduced risk for atrial fibrillation. Am. Heart J. 2004;148:545–550. doi: 10.1016/j.ahj.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 94.Zhu W., Li J., Sun X., Hua Q. Association of G-protein β3 subunit gene C825T polymorphism with cardiac and cerebrovascular events in Chinese hypertensive patients. Clin. Exp. Hypertens. 2017;39:80–84. doi: 10.1080/10641963.2016.1210621. [DOI] [PubMed] [Google Scholar]
- 95.Eba A., Raza S.T., Abbas M., Rizvi S., Rajput M., Mahdi F. Association of SDF1β (G801A) and GNB3 (C825T) polymorphisms with the incidence and severity of coronary artery disease. Br. J. Biomed. Sci. 2019;76:49–51. doi: 10.1080/09674845.2018.1527802. [DOI] [PubMed] [Google Scholar]
- 96.Nakao R., Tanaka H., Takitani K., Kajiura M., Okamoto N., Kanbara Y., Tamai H. GNB3 C825T polymorphism is associated with postural tachycardia syndrome in children. Pediatr. Int. 2012;54:829–837. doi: 10.1111/j.1442-200X.2012.03707.x. [DOI] [PubMed] [Google Scholar]
- 97.Frey U.H., Moebus S., Mohlenkamp S., Kalsch H., Bauer M., Lehmann N., Nothen M., Muhleisen T.W., Stang A., Erbel R., et al. GNB3 gene 825 TT variant predicts hard coronary events in the population-based Heinz Nixdorf Recall study. Atherosclerosis. 2014;237:437–442. doi: 10.1016/j.atherosclerosis.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 98.Wascher T.C., Paulweber B., Malaimare L., Stadlmayr A., Iglseder B., Schmoelzer I., Renner W. Associations of a human G protein β3 subunit dimorphism with insulin resistance and carotid atherosclerosis. Stroke. 2003;34:605–609. doi: 10.1161/01.STR.0000058159.63950.EA. [DOI] [PubMed] [Google Scholar]
- 99.Casiglia E., Tikhonoff V., Boschetti G., Bascelli A., Saugo M., Guglielmi G., Caffi S., Rigoni G., Giordano N., Grasselli C., et al. The C825T GNB3 polymorphism, independent of blood pressure, predicts cerebrovascular risk at a population level. Am. J. Hypertens. 2012;25:451–457. doi: 10.1038/ajh.2011.257. [DOI] [PubMed] [Google Scholar]
- 100.D’Angelo C.S., Varela M.C., de Castro C.I.E., Otto P.A., Perez A.B.A., Lourenco C.M., Kim C.A., Bertola D.R., Kok F., Garcia-Alonso L., et al. Chromosomal microarray analysis in the genetic evaluation of 279 patients with syndromic obesity. Mol. Cytogenet. 2018;11:14. doi: 10.1186/s13039-018-0363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Soong B.W., Huang Y.H., Tsai P.C., Huang C.C., Pan H.C., Lu Y.C., Chien H.J., Liu T.T., Chang M.H., Lin K.P., et al. Exome sequencing identifies GNB4 mutations as a cause of dominant intermediate Charcot-Marie-Tooth disease. Am. J. Hum. Genet. 2013;92:422–430. doi: 10.1016/j.ajhg.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lassuthova P., Safka Brozkova D., Neupauerova J., Krutova M., Mazanec R., Seeman P. Confirmation of the GNB4 gene as causal for Charcot-Marie-Tooth disease by a novel de novo mutation in a Czech patient. Neuromuscul. Disord. 2017;27:57–60. doi: 10.1016/j.nmd.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 103.Miura S., Morikawa T., Fujioka R., Noda K., Kosaka K., Taniwaki T., Shibata H. A novel missense variant (Gln220Arg) of GNB4 encoding guanine nucleotide-binding protein, subunit β-4 in a Japanese family with autosomal dominant motor and sensory neuropathy. Eur. J. Med. Genet. 2017;60:474–478. doi: 10.1016/j.ejmg.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 104.Wall M.A., Posner B.A., Sprang S.R. Structural basis of activity and subunit recognition in G protein heterotrimers. Structure. 1998;6:1169–1183. doi: 10.1016/S0969-2126(98)00117-8. [DOI] [PubMed] [Google Scholar]
- 105.Gaudet R., Bohm A., Sigler P.B. Crystal structure at 2.4 angstroms resolution of the complex of transducin βγ and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/S0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- 106.Khan S.M., Min A., Gora S., Houranieh G.M., Campden R., Robitaille M., Trieu P., Petrin D., Jacobi A.M., Behlke M.A., et al. Gβ4γ1 as a modulator of M3 muscarinic receptor signalling and novel roles of Gβ1 subunits in the modulation of cellular signalling. Cell Signal. 2015;27:1597–1608. doi: 10.1016/j.cellsig.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Chandler N.J., Greener I.D., Tellez J.O., Inada S., Musa H., Molenaar P., Difrancesco D., Baruscotti M., Longhi R., Anderson R.H., et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation. 2009;119:1562–1575. doi: 10.1161/CIRCULATIONAHA.108.804369. [DOI] [PubMed] [Google Scholar]
- 108.Ruiz-Velasco V., Ikeda S.R., Puhl H.L. Cloning, tissue distribution, and functional expression of the human G protein β4-subunit. Physiol. Genom. 2002;8:41–50. doi: 10.1152/physiolgenomics.00085.2001. [DOI] [PubMed] [Google Scholar]
- 109.Fleischmann B.K., Duan Y., Fan Y., Schoneberg T., Ehlich A., Lenka N., Viatchenko-Karpinski S., Pott L., Hescheler J., Fakler B. Differential subunit composition of the G protein-activated inward-rectifier potassium channel during cardiac development. J. Clin. Invest. 2004;114:994–1001. doi: 10.1172/JCI200415925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rosskopf D., Nikula C., Manthey I., Joisten M., Frey U., Kohnen S., Siffert W. The human G protein β4 subunit: Gene structure, expression, Gγ and effector interaction. FEBS Lett. 2003;544:27–32. doi: 10.1016/S0014-5793(03)00441-1. [DOI] [PubMed] [Google Scholar]
- 111.den Hoed M., Eijgelsheim M., Esko T., Brundel B.J., Peal D.S., Evans D.M., Nolte I.M., Segre A.V., Holm H., Handsaker R.E., et al. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat. Genet. 2013;45:621–631. doi: 10.1038/ng.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smolock E.M., Ilyushkina I.A., Ghazalpour A., Gerloff J., Murashev A.N., Lusis A.J., Korshunov V.A. Genetic locus on mouse chromosome 7 controls elevated heart rate. Physiol. Genom. 2012;44:689–698. doi: 10.1152/physiolgenomics.00041.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patil D.N., Rangarajan E.S., Novick S.J., Pascal B.D., Kojetin D.J., Griffin P.R., Izard T., Martemyanov K.A. Structural organization of a major neuronal G protein regulator, the RGS7-Gβ5-R7BP complex. eLIFE. 2018;7:e42150. doi: 10.7554/eLife.42150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie K., Masuho I., Brand C., Dessauer C.W., Martemyanov K.A. The complex of G protein regulator RGS9-2 and Gβ5 controls sensitization and signaling kinetics of type 5 adenylyl cyclase in the striatum. Sci. Signal. 2012;5:ra63. doi: 10.1126/scisignal.2002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Witherow D.S., Slepak V.Z. A novel kind of G protein heterodimer: The G β5-RGS complex. Recept. Channels. 2003;9:205–212. [PubMed] [Google Scholar]
- 116.Sanchez-Blazquez P., Rodriguez-Diaz M., Lopez-Fando A., Rodriguez-Munoz M., Garzon J. The Gβ5 subunit that associates with the R7 subfamily of RGS proteins regulates mu-opioid effects. Neuropharmacology. 2003;45:82–95. doi: 10.1016/S0028-3908(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 117.Nini L., Zhang J.H., Pandey M., Panicker L.M., Simonds W.F. Expression of the Gβ5/R7-RGS protein complex in pituitary and pancreatic islet cells. Endocrine. 2012;42:214–217. doi: 10.1007/s12020-012-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sondek J., Siderovski D.P. Gγ-like (GGL) domains: New frontiers in G-protein signaling and β-propeller scaffolding. Biochem. Pharm. 2001;61:1329–1337. doi: 10.1016/S0006-2952(01)00633-5. [DOI] [PubMed] [Google Scholar]
- 119.Xie K., Allen K.L., Kourrich S., Colon-Saez J., Thomas M.J., Wickman K., Martemyanov K.A. Gβ5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat. Neurosci. 2010;13:661–663. doi: 10.1038/nn.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao Z., Tumber A., Maynes J., Tavares E., Kannu P., Heon E., Vincent A. Unique retinal signaling defect in GNB5-related disease. Doc. Ophthalmol. 2019 doi: 10.1007/s10633-019-09735-1. [DOI] [PubMed] [Google Scholar]
- 121.Poke G., King C., Muir A., de Valles-Ibanez G., Germano M., Moura de Souza C.F., Fung J., Chung B., Fung C.W., Mignot C., et al. The epileptology of GNB5 encephalopathy. Epilepsia. 2019;60 doi: 10.1111/epi.16372. [DOI] [PubMed] [Google Scholar]
- 122.Malerba N., Towner S., Keating K., Squeo G.M., Wilson W., Merla G. A NGS-targeted autism/ID Panel reveals compound heterozygous GNB5 variants in a novel patient. Front. Genet. 2018;9:626. doi: 10.3389/fgene.2018.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vernon H., Cohen J., De Nittis P., Fatemi A., McClellan R., Goldstein A., Malerba N., Guex N., Reymond A., Merla G. Intellectual developmental disorder with cardiac arrhythmia syndrome in a child with compound heterozygous GNB5 variants. Clin. Genet. 2018;93:1254–1256. doi: 10.1111/cge.13194. [DOI] [PubMed] [Google Scholar]
- 124.Turkdogan D., Usluer S., Akalin F., Agyuz U., Aslan E.S. Familial early infantile epileptic encephalopathy and cardiac conduction disorder: A rare cause of SUDEP in infancy. Seizure. 2017;50:171–172. doi: 10.1016/j.seizure.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 125.Shamseldin H.E., Masuho I., Alenizi A., Alyamani S., Patil D.N., Ibrahim N., Martemyanov K.A., Alkuraya F.S. GNB5 mutation causes a novel neuropsychiatric disorder featuring attention deficit hyperactivity disorder, severely impaired language development and normal cognition. Genome Biol. 2016;17:195. doi: 10.1186/s13059-016-1061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Veerman C.C., Mengarelli I., Koopman C.D., Wilders R., van Amersfoorth S.C., Bakker D., Wolswinkel R., Hababa M., de Boer T.P., Guan K., et al. Genetic variation in GNB5 causes bradycardia by augmenting the cholinergic response via increased acetylcholine-activated potassium current (IK,ACh) Dis. Model. Mech. 2019;12 doi: 10.1242/dmm.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krispel C.M., Chen C.K., Simon M.I., Burns M.E. Novel form of adaptation in mouse retinal rods speeds recovery of phototransduction. J. Gen. Physiol. 2003;122:703–712. doi: 10.1085/jgp.200308938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tian M., Zallocchi M., Wang W., Chen C.K., Palczewski K., Delimont D., Cosgrove D., Peng Y.W. Light-induced translocation of RGS9-1 and Gβ5L in mouse rod photoreceptors. PLoS ONE. 2013;8:e58832. doi: 10.1371/journal.pone.0058832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rao A., Dallman R., Henderson S., Chen C.K. Gβ5 is required for normal light responses and morphology of retinal ON-bipolar cells. J. Neurosci. 2007;27:14199–14204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kulkarni K., Xie X., Marron Fernandez de Velasco E., Anderson A., Martemyanov K.A., Wickman K., Tolkacheva E.G. Correction: The influences of the M2R-GIRK4-RGS6 dependent parasympathetic pathway on electrophysiological properties of the mouse heart. PLoS ONE. 2018;13:e0200553. doi: 10.1371/journal.pone.0200553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Posokhova E., Wydeven N., Allen K.L., Wickman K., Martemyanov K.A. RGS6/Gβ5 complex accelerates IKACh gating kinetics in atrial myocytes and modulates parasympathetic regulation of heart rate. Circ. Res. 2010;107:1350–1354. doi: 10.1161/CIRCRESAHA.110.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maity B., Stewart A., Yang J., Loo L., Sheff D., Shepherd A.J., Mohapatra D.P., Fisher R.A. Regulator of G protein signaling 6 (RGS6) protein ensures coordination of motor movement by modulating GABAB receptor signaling. J. Biol. Chem. 2012;287:4972–4981. doi: 10.1074/jbc.M111.297218. [DOI] [PMC free article] [PubMed] [Google Scholar]