Abstract
High salinity is a challenging environmental stress for organisms to overcome. Unicellular photosynthetic microalgae are especially vulnerable as they have to grapple not only with ionic imbalance and osmotic stress but also with the generated reactive oxygen species (ROS) interfering with photosynthesis. This review attempts to compare and contrast mechanisms that algae, particularly the eukaryotic Chlamydomonas microalgae, exhibit in order to immediately respond to harsh conditions caused by high salinity. The review also collates adaptation mechanisms of freshwater algae strains under persistent high salt conditions. Understanding both short-term and long-term algal responses to high salinity is integral to further fundamental research in algal biology and biotechnology.
Keywords: high salt stress, green algae, adaptation, transcriptome, salinity, Chlamydomonas
1. Introduction
Algae refer to a broad group of micro- and macroorganisms capable of oxygenic photosynthesis, yet show striking differences compared to land plants. The traditional and common definition of algae includes prokaryotic cyanobacteria and eukaryotic algae belonging to highly different phylogenetic clades. Eukaryotic microalgae are polyphyletic in origin and have an extensive ecological niche and evolutionary history. They are ubiquitous across most environments as primary producers, including extreme environments like soda pans and salt lakes. Soda pans are highly saline, shallow, and intermittent aquatic systems that are highly alkaline due to the high concentration of sodium and carbonate ions. Salt lakes represent another great example of hypersaline environments where green algae grow, and in some cases, thrive [1]. These environments have greater and often changing salt concentrations than that of seawater. Despite these extreme conditions, eukaryotic green algae remain primary producers in these ecosystems alongside cyanobacteria and euglenophytes [2]. Eukaryotic algae possess great plasticity and adaptability to most abiotic stresses. Some species belonging to the same genus are capable of growing in both fresh and saline water.
However, most freshwater strains show reduced growth and survival in high salinity environments and are generally unable to survive beyond a low threshold of salinity. In this review we will use the terminology of salt sensitive algae to refer to freshwater algae strains that have limited viability under high salt stress and salt tolerant algae to refer to marine or brackish algae strains that can easily cope with high salinity. Later on in the review, we will also be discussing the adaptation of salt sensitive strains to high salinity conditions and we will refer to these as salt adapted strains. We propose to use this unified terminology since multiple studies using different terminologies are being discussed and compared in this review.
Studying properties of salt tolerant algae species is a good first step in understanding how photosynthetic organisms cope with high salt. This is because unlike their freshwater counterparts, the machinery to tackle with salinity is engraved in the genome of marine algae as a result of centuries of evolutionary selection. In contrast, freshwater species have to devise mechanisms to cope with high salinity stress they are not accustomed to. This requires drastic changes in morphology and osmolyte concentrations in the short-term and accumulation of advantageous mutations in the long run. More than four decades of research has shaped our understanding of how microalgae respond, acclimatize, and grow under such challenging stress. However, there are few reviews tabulating all the multitude of changes that occur. Further, some of these changes are strongly species-specific. Thus, a comparison across different species can provide a more complete map of the different mechanisms used. Finally, a lot of commercially viable products are produced by microalgae to improve their survival under highly saline conditions. Exploitation of these mechanisms is particularly sought after in multiple fields. However, successful exploitation can only occur with a thorough understanding of these mechanisms and the propensity of the mechanisms to change under long-term adaptation.
Unfortunately, only a few species of salt tolerant Chlamydomonas are described. Chlamydomonas pulsatilla is a marine species isolated in Canada. Growth studies for this species have shown salinities of 10% artificial seawater (ASW) to be optimal for high growth rate, while reduced growth rate was observed at higher salinities. The growth rate at 200% ASW was 69% of the growth rate at 10% ASW [3]. This strain can utilize acetate as a carbon source, but it is incapable of utilizing nitrate or urea as nitrogen source. However, it can utilize several different amino acids for nitrogen [4]. Salt tolerant marine strains are valuable models to identify and characterize genes associated with salinity resistance. Chlamydomonas W80 is a marine Chlamydomonas species, isolated from the Japanese coast [5]. A number of studies using cDNA libraries built from C. W80 have successfully characterized a small group of genes that provided increased salinity tolerance in transformed E. coli cells. Genes from eukaryotic microalgae that are experimentally characterized as salt tolerance-related genes are shown in Table 1.
Table 1.
Genes with experimentally observed functional response to salt stress.
| Gene Name | Organism From | Organism Transformed | Effect Observed | Reference |
|---|---|---|---|---|
| Cyclophilins (CYP) PsCYP1 | Pyropia seriata (marine red algae) | Chlamydomonas sp. | Heat and salt tolerance | [6] |
| Photosynthetic ferredoxin (PETF) and ferredoxin-5 gene (FDX5) | Chlamydomonas sp. | Chlamydomonas sp. | Salt tolerance | [7] |
| Gene with unknown function | Chlamydomonas W80 | E. coli | Salt and cadmium tolerance | [8] |
| Anti-stress genes | Chlamydomonas W80 | E. coli | Salt stress protection | [9] |
| Breast basic conserved gene (bbc1) | Chlamydomonas W80 | E. coli | Salt stress protection | [10] |
| Glutathione peroxidase | Chlamydomonas W80 | Tobacco plant | Salt stress protection | [11] |
| Group-3 late embryogenesis abundant protein gene (cw80lea3) | Chlamydomonas W80 | Synechococcus PCC7942 | Salt and cold stress protection | [12] |
2. Effect of High Salt Concentration on Green Algae
There are multiple morphological and molecular changes that occur simultaneously that improve survival of salt sensitive algae under high salt stress. These are described in greater detail below.
2.1. Basic Morphological Changes
Stress caused by high salt concentration slows down cell division, reduces size, ceases motility, and triggers palmelloid formation in Chlamydomonas species [13,14,15,16]. Growth rate is immediately and directly impacted and can be easily detected. Chlamydomonas reinhardtii cells under high salt stress have lower growth rate compared to untreated cells. Untreated (control) salt sensitive algal cells reached an optical density (OD) of 1 at 750 nm in four days while it took the salt-treated cells six days to achieve the same OD. The cells were smaller in the dividing stage across all the salt concentrations [16]. Increasing salt concentrations negatively affected the growth of other freshwater algae species such as Chlorella vulgaris, Chlorella salina, Chlorella emersonii [17], and Scenedesmus opoliensis [18]. Figure 1 provides an overview of all the short term acclimatization responses that occur when Chlamydomonas is exposed to hypersaline conditions.
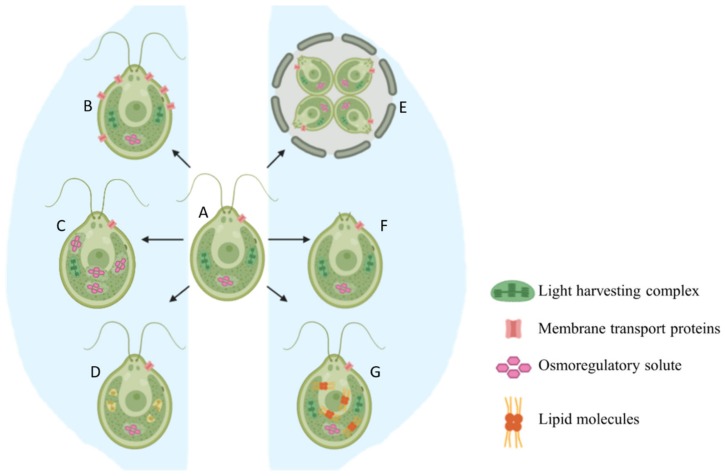
Figure 1.
Conceptual image detailing the morphological changes that occur when normal cell (A) is exposed to saline conditions (B–G). (A) A C. reinhardtii cell under no stress, (B) Upregulation of membrane transport proteins, (C) Accumulation of osmoregulatory solutes, (D) Degradation of light harvesting complexes, (E) Palmelloid formation, (F) Flagellar loss and reduction of motility, (G) Accumulation of lipids.
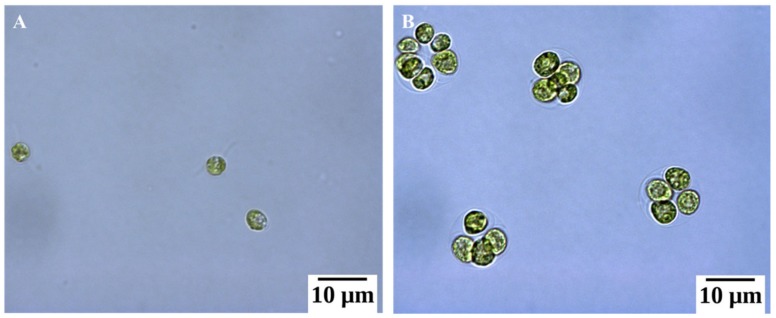
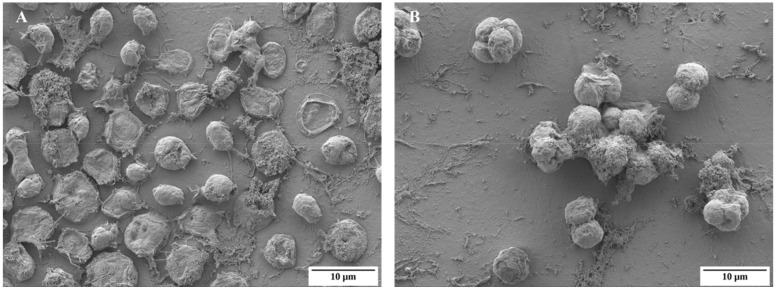
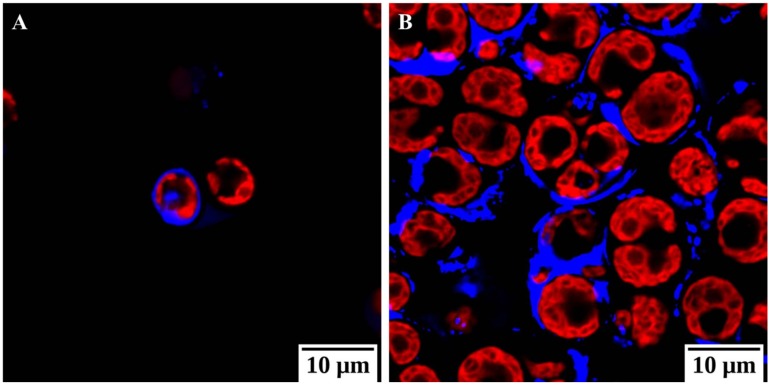
Chlamydomonas cells exposed to unfavorable conditions enter a temporary stage termed as “palmelloid”. Multiple structural changes occur in the palmelloid form including (i) loss of flagella, (ii) clustering of cells with a minimum of two cells per cluster, (iii) increased secretion of exopolysaccharide (EPS), (iv) cells surrounded by an EPS matrix sharing a common membrane, and (v) thickening of individual cell walls (Figure 1). The palmelloids increase steadily in number until 24 h after exposure to high salinity. A proteomic analysis identified expansin, Wall Stress-responsive Component (WSC) domain protein, pheophorin-C5, Vegetative Storage Protein rich protein (VSP4), and Cathepsin-Z-like proteins to be putatively linked to palmelloid formation [14]. The palmelloid structure of C. reinhardtii is highly interesting, the multicellular structures can be clearly visualized under microscope (Figure 2, Figure 3 and Figure 4, unpublished in-house data).
Figure 2.
Optical microscopy images of Chlamydomonas reinhardtii cc124 under normal condition (A) and under salt stressed (150 mM NaCl) condition (B) (unpublished in-house data).
Figure 3.
Electron microscopy images of Chlamydomonas reinhardtii cc124 under normal condition (A) and under salt stressed (150 mM NaCl) condition (B) (unpublished in-house data).
Figure 4.
Confocal microscopy images of Chlamydomonas reinhardtii cc124 under normal condition (A) and under salt stressed (150 mM NaCl) condition (B). Calcofluor white stains cellulose and chitin and is blue in color while photosystem II was excited and visualized in red. These images show an increase in polysaccharides as an integral event of the palmelloid formation (unpublished in-house data).
Under favorable conditions, the EPS matrix is degraded and the cells dissociate from their palmelloid form to enter the medium. Accumulation of a protein with a PAN/APPLE like domain in the post-stress spent media [14] backs up this phenomenon because such domains facilitate protein–carbohydrate interactions [19]. The same trend was observed for metalloproteinases Matrix Metallopeptidase 13 (MMP13) and Matrix Metallopeptidase 3 (MMP3), which are calcium-dependent endopeptidases involved in the degradation of EPS matrix such as mucilage [20].
Although the EPS matrix consists of different polysaccharides, the exact composition of EPS varies from species to species and environmental conditions. The production of EPS is energy intensive. However, the protection offered by the EPS matrix allows stressed cells to survive under adverse conditions by sequestering stressed cells away from the environment. Salt sensitive Chlamydomonas cells placed in a medium with 100–150 mM NaCl produced 3–6-fold more EPS compared to that in a medium without increased salt [14]. This implies that the increase in salinity modulated the increase of EPS production.
Algal genera such as Dunaliella and Chlorella do not form palmelloids and show different mechanisms to tackle with salt stress. Chlorella cells have a rigid cell wall, thus limiting its ability to change cell volume. Hence, osmoregulation through production of organic solutes and accumulation of inorganic ions are used to maintain osmotic homeostasis. Furthermore, cations in the media are bound within intracellular spaces reducing the osmotic activity [21]. In contrast, Dunaliella lacks a rigid cell wall which allows the cells to rapidly change the volume during high salinity stress by adjusting intracellular ion and glycerol concentration eventually restoring the cells turgor pressure [22]. A study on cell size changes in D. salina caused by high salt stress showed cell volumes continuously fluctuate for ten days, finally stabilizing at a cell size that was slightly larger compared to unstressed conditions [23].
2.2. Production of Osmoregulatory Solutes
Algal cells with rigid cell walls have limited ability to change cell volume and thus depend heavily on organic solutes for osmoregulation. These solutes, also termed as compatible solutes, are typically small organic molecules with neutral charge and low toxicity at high concentration [24]. Furthermore, compatible solutes at high concentrations allow for efficient functioning of different enzymes. Compatible solutes accumulate in the cytosol and balance the osmotic stress between the outside medium and the cytosol.
Glycerol is a good example of a common but effective compatible solute that is produced by most salt sensitive algal species under high saline stress. Glycerol is highly soluble and chemically inert, therefore it is non-toxic. It is an end-product metabolite and thus production and accumulation does not interfere with other metabolic pathways. Finally, glycerol production from starch is not expensive energetically and is not nitrogen-dependent [25].
In Chlamydomonas HS-5, glycerol accumulation corresponded to salt concentration with higher salt concentration causing higher glycerol content [26]. A similar effect was recorded for C. reinhardtii [27], C. mexicana [28], Chlamydomonas sp. JSC4 [29], and C. pulsatilla [4]. A study in euryhaline Chlorella autotrophica showed similar increase in glycerol content in response to increasing salinity [21]. Glycerol plays a similar role in various Dunaliella, Scenedesmus, and Micrasterias species among other eukaryotic microalgae. Salt stressed Dunaliella cells accumulate massive amounts of glycerol and the level of intracellular glycerol was found to be proportional and osmotically equivalent to the external salt concentration [30]. The high concentration of glycerol also allows salt stressed Dunaliella cells to resume original cell volume even under extreme salt stress. Finally, starch degradation corresponded closely with glycerol accumulation in C. pulsatilla indicating that glycerol was synthesized through degradation of starch [31].
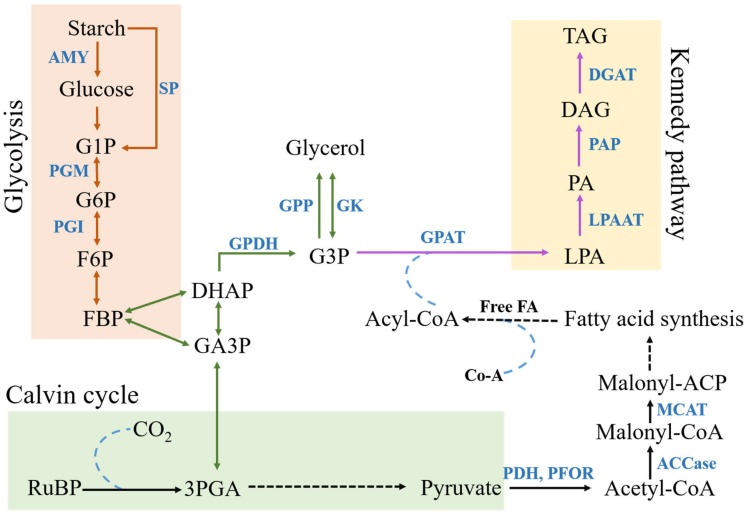
Glycerol biosynthesis (Figure 5) can occur through starch degradation or by using photosynthetic products [32]. Glucose produced through photosynthesis or starch hydrolysis is converted to fructose 1,6-bisphosphate and next to dihydroxyacetone-phosphate (DHAP), which is converted to glycerol-3-phosphate (G3P) by glycerol-3-phosphate dehydrogenase (G3PDH). G3PDH is the best studied enzyme in this biosynthetic pathway. G3PDH reduces DHAP to yield G3P, which is converted to glycerol by the action of a G3P phosphatase (GPP) or potentially through the reversible reaction of a glycerol kinase [33]. There are five isoforms of G3PDH enzyme in C. reinhardtii. Of these, GPDH2 and GPDH3 play integral roles in glycerol production and lipid synthesis [34].
Figure 5.
Schematic diagram of Glycerol and TAG synthesis. Metabolites: G1P- glucose 1-phosphate, G6P- glucose 6-phosphate, F6P- fructose 6-phosphate, FBP-, DHAP- dihydroxyacetone phosphate, GA3P- glyceraldehyde 3- phosphate, G3P- glycerol 3-phosphate, RuBP- ribulose 1,5-bisphosphate carboxylase/oxygenase, 3PGA- 3-phosphoglycerate, LPA-, PA-, DAG-, TAG-. Enzymes: AMY- α-amylase, SP- starch phosphorylase, PGM- phosphoglucomutase, PGI- phosphoglucoisomerase, GPDH- glycerol 3-phosphate dehydrogenase, GPAT- glycerol-3-phosphate acyltransferase, LPAAT- lysophosphatidic acid acyltransferase, PAP- phosphatidate phosphatase, DGAT- Diacylglycerol acyltransferase, MCAT- Malonyl CoA-acyl carrier protein transacylase, ACCase- AcCoA carboxylase, PDH- pyruvate dehydrogenase, PFOR- pyruvate-ferredoxin oxidoreductase, GK- glycerol kinase, GPP- glycerol-3-phosphate phosphatase. Others: Free FA- free fatty acids, Co-A- Coenzyme A.
While most strains produce glycerol and maintain it within the cell, some have the capacity to continuously leak significant quantities of glycerol into the surrounding environment. Releasing large quantities of photosynthetic products leads to an increase in photosynthetic rate in higher plants. Arabidopsis with extremely large sugar exporting veins can triple its photosynthetic capacity. Thus, it seems like there is an interplay between photosynthetic rate and the limited ability of the organism to use, export, or store the photosynthetically fixed carbon [35,36]. NADPH is continuously consumed to make up for released glycerol, thus, any inhibition in photosynthetic rate due to accumulation of NADPH and glycerol is prevented. Further, production and release of glycerol is linearly proportional to light intensity and exudation of glycerol led to increased growth rates in C. reinhardtii [37]. This is yet another pathway that algae can use to counter high salt stress and is observed especially in freshwater and marine Chlamydomonas cells [5,37] and in a single Dunaliella species [38].
Proline is another osmoregulatory solute that linearly increases in concentration with increase in salinity across higher plants and algae [39,40]. Like glycerol, it is low in molecular weight, neutrally charged and highly soluble. Exogenous application of proline reduces detrimental effects of high salinity by reducing accumulation of Na+ and Cl− in C. reinhardtii [41] and plants [42]. Apart from proline, other amino acids such as lysine and leucine have been implicated in promoting Chlamydomonas growth under highly saline conditions [4]. However, not many studies are carried out on the specific roles of these amino acids in osmoregulation. Up-regulation of genes involved in proline synthesis has been recorded for Picochlorum oklahomensis [43] as well as Picochlorum SE3 [44] during salt stress. Unlike in Chlamydomonas sp. and D. salina, where the major osmolyte is glycerol and starch degradation increases, proline is the principle osmolyte in Picochlorum species and starch synthesis is upregulated while starch degradation is limited [44,45,46].
Besides proline and glycerol, trehalose also has an established role in stabilization of proteins by increasing the transition temperature of proteins and as an osmoregulatory molecule [47]. High salt stress in Chlamydomonas [48], Chlorella, and Scytonema was shown to lead to an increased production of trehalose [49]. In maize, trehalose was observed to reduce the negative effects of high saline stress as an osmoprotectant [50]. Other polyols such as sorbitol and mannitol are also important in osmoregulation [44]. Platymonas suecica is a salt tolerant algae and shows a linear accumulates mannitol with increasing levels of salinity [51]. Stichococcus chloranthus and Stichococcus bacillaris both show an accumulation of sorbitol with increasing salinities [40,52]. Sorbitol and mannitol have not been detected in appreciable quantities in other algal species indicating that this might be a mechanism specific to these species.
2.3. Reorganization of Membrane Transport Proteins
Efficient uptake and export of ions through the cell membrane is another important strategy to cope with high salt stress by maintaining the intracellular ion balance. According to certain studies in plants, high concentration of Na+ can interfere with uptake of other cations, especially K+ [53,54]. Since K+ participates in a plethora of physiological functions in plants, it is integral to maintain the cytosolic K+/Na+ ratio under highly saline conditions.
Upregulation of membrane transport proteins can confer tolerance to high salinity in halotolerant algae species by actively transporting K+ ions through membrane transport proteins. Salt adapted mutants of C. reinhardtii showed enhanced expression of multiple membrane transport proteins [55]. Genes for K+ ion transport were significantly upregulated when salt sensitive C. reinhardtii cells were under salt stress [48], possibly compensating the disruption in K+ uptake caused due to high concentration of Na+ ions.
Intracellular ion content data revealed that C. pulsatilla had a remarkable capacity to increase sodium and chloride, and, to a lesser extent, potassium and magnesium in response to increased salinity, although accumulation of glycerol remained the principal approach to cope with salinity stress [3]. Further, salt tolerant strains of Dunaliella maintain intracellular Na+ concentrations below that of the outside medium [56]. The Na+/H+ antiporter catalyzes influx of Na+, followed by an export of Na+ through the Na+-ATPase and plasma membrane electron transport system. Thus, this Na+ extrusion in Dunaliella is an evolved, adaptive mechanism in hypersaline environments to maintain Na+ concentrations within cells. Thus, it seems that unlike marine or hypersaline algae, freshwater or salt sensitive strains are unable to maintain the intracellular ion concentration by preventing uptake of Na+, by accumulating K+ or pumping out Na+ ions to restore the cells to their normal or nearly normal homeostatic state.
Apart from membrane transport proteins, the amount of two plasma membrane proteins P150 and P60 were greatly elevated with increasing salt concentration in D. salina [57,58]. Moreover, immediately after a drastic hyperosmotic shock, the induction of these proteins coincided with increased growth suggesting their role in conferring salt tolerance. P150 is a 150 kD transferrin-like plasma membrane protein involved in iron uptake, thus it helps the cells overcome any possible limitation of iron availability under high salinity [58]. However, this protein was not detected in other algae and the most similar protein is found only in animals. We also carried out a basic blast analysis using the cDNA sequence of P150 (accession number: AAF72064.1, data not shown) and found no matches in any other algal species, indicating that this protein and mechanism is fully specific to D. salina. However, proteins related to iron uptake were upregulated in salt adapted C. reinhardtii CC-503 indicating that iron uptake under high salt stress might be limiting to C. reinhardtii as well [55]. P60 is a novel type of carbonic anhydrase and is potentially involved in CO2 fixation within cells growing under hypersaline conditions [57]. The upregulation of three types of carbonic anhydrases was observed in salt tolerant Chlorella pyrenoidosa 820 [59]. However, no such relationships have been reported in any type of Chlamydomonas species, despite the presence of 12 different carbonic anhydrases in Chlamydomonas reinhardtii [60].
2.4. Lipid Accumulation
Most green algae under salt stress show characteristic accumulation of lipids. In fact, this response to high salinity has been studied for the production of biofuel [28]. Lipids are produced as high energy storage compounds, synthesized when algal cells are under unfavorable environmental conditions. Once the algal cells are moved to optimal conditions, these lipid molecules are utilized. Lipids are synthesized by assimilating carbon from three different, independently regulated metabolic pathways. The first is direct incorporation of photosynthetically fixed CO2 into fatty acids within the chloroplast. The second involves degradation of starch. The third is through degradation of polar lipids [61]. However, no study has quantified the contribution of these pathways under normal conditions.
Chlamydomonas sp. JSC4 is a salt tolerant strain isolated from a marine environment and shows high lipid accumulation under high salt stress. This strain was utilized to identify the mechanism of lipid biosynthesis under high salt stress [29,62]. Chlamydomonas sp. JSC4 shows a highly specific switch from starch synthesis to lipid synthesis under salt stress [29]. Presumably, the brackish water heritage of Chlamydomonas sp. JSC4 selected for and adapted this algal strain to cope with higher salinity as the strain moved from brackish water to marine conditions. The switch from starch to lipid synthesis ensures the maintenance of energy reserves, thereby ensuring long-term survival. Degradation of accumulated starch leads to the production of an extremely important precursor of lipid production; G3P [62]. G3P is also an extremely important precursor for glycerol synthesis in eukaryotic green algae, as discussed earlier. Finally, all the glycerol accumulated can also be converted back to G3P through glycerol kinase [63]. However, no study has investigated this possibility in green algae so far.
However, it is important to note that lipid accumulation is not a strategy that is identified in all algae. This is especially true for salt adapted algae. A few studies have explored the evolution of salt resistance in green algae under progressively increasing saline conditions. This selection strategy was used to generate salt adapted mutants from salt sensitive C. reinhardtii [64], salt sensitive Chlorella sp. AE10 [65], and a salt tolerant marine Chlamydomonas JSC4 [66]. Transcriptome analyses of all the adapted mutants showed decreased lipid accumulation. Furthermore, the salt adapted (originally salt tolerant) Chlamydomonas JSC4 also lost its switching capability from starch synthesis to lipid synthesis. There was only one adaptation experiment where adaptation to high salinity also led to increased lipid accumulation. A salt tolerant Schizochytrium sp. was adapted to increased salt stress, and the adapted mutant displayed increased lipid accumulation [67]. These studies clearly show that salt adaptation can occur in multiple different, species specific ways.
2.5. Impact of High Salt Stress on Photosynthesis
Most photosynthetic organisms show a significant decrease in photosynthetic activity under high salt stress. This reduction in photosynthetic activity can be attributed to deficiency in different cations, production of reactive oxygen species (ROS) and osmotic stress, which interfere with various biochemical and physiological processes [68]. Pigment analysis in C. reinhardtii has demonstrated that the photosystem I (PSI) light harvesting complexes (LHCs) are damaged by reactive oxygen species (ROS) at high salt conditions, and photosystem II (PSII) proteins involved in oxygen evolution are also impaired [16,69]. Salinity stress seems to divert the resources from PSII’s D1 protein turnover to the intensive process of maintaining cell homeostasis [16]. Transcriptome studies in C. reinhardtii have shown impairment of photosynthesis, with several PSI LHC genes being significantly down-regulated, e.g., PSI light harvesting complex genes LHCA2, LHCA3, and LHCA5. Furthermore, the levels of most of the chloroplast encoded transcripts (e.g., psaA, B, C, J, M) in PSI were relatively unchanged while the nuclear genes (e.g., psaD, E, G, F, H) were down-regulated under highly saline conditions [48].
Several transcriptomic studies have led to the implication that salt stressed cells upregulate different genes to resist harsh conditions and loss in photosynthetic activity. Transcriptome study of salt stressed C. reinhardtii cells showed significant upregulation of genes involved in eliminating ROS including plastid Fe superoxide dismutase 1 (SOD), thioredoxins, glutathione transferase, and heat shock factor binding proteins [64]. Pigments such as carotenoids play important functional roles as antioxidants. Carotenoids are lipid soluble antioxidants and are mainly located inside the chloroplast envelope and protects the LHC against ROS induced damage. Dunaliella sp. are good examples of algal strains that can produce high quantities of carotenoids in response to salt stress and have been industrially exploited for carotenoid production [70]. Another study on salt sensitive C. reinhardtii and C. vulgaris showed increased carotenoid production at moderate levels of salt stress (0.05 M–0.15 M).
Other species cope with salt stress in a different fashion. The cells of salt tolerant D. salina increase photosynthetic activity by significantly increasing Chlorophyll-a (Chl a) content in response to salt stress [17]. This is consistent with another study in salt tolerant D. salina that showed Chl a/b ratio slightly increased with increased salt concentration (3 M NaCl) [71]. In salt sensitive C. vulgaris, chlorophyll content was increased at lower salt concentrations (0.2 M) but reduced at higher salt concentrations (0.3 M–0.4 M) which slowed growth [72]. A slight increase in photosynthetic activity is only seen under moderate levels of salt stress and is presumably done in order to enhance energy generation to produce energetically expensive molecules that can protect salt stressed cells (e.g., EPS and pigments), or to drive energetically expensive Na+ exclusion or to increase amount of storage molecules like lipids. Thus, salt sensitive strains and salt tolerant strains seem to have different mechanisms of tackling with salt stress. The increase in photosynthetic activity for salt tolerant strains at moderately increased salinities is likely a regulatory response since these strains often need to deal with variable levels of salinity.
2.6. Glycolysis
Glycolysis is considered to play an important role in plant development and adaptation to multiple abiotic stresses, such as cold, salt, and drought [73,74]. In a transcriptome study of salt stressed C. reinhardtii, a significantly increased expression of genes participating in the metabolism of carbohydrates, such as starch, sucrose, soluble sugar, and glucose was observed. Furthermore, salt stressed cells showed an upregulation of 31 genes involved in glycolytic processes, including plastidic pyruvate kinase PKP-ALPHA and PKP-BETA1 [48]. This is consistent with the results observed in salt adapted Chlorella sp. S30 [66] and in plant studies [75] where high salt stress significantly increased the main carbohydrate contents. Carbohydrates are involved not only in osmotic adjustment, but also can be used as protective agents for homeostasis as osmoprotective solutes as previously described.
High saline stress negatively impacts photosynthetic efficiency in sensitive cells, which often inhibits transport of carbohydrates leading to an accumulation of excess starch or sucrose. C. reinhardtii enhanced glycolysis to decrease carbohydrate accumulation in cells, which would promote the respiratory metabolism and mitochondrial electron transport. Thus reducing the effects of ionic toxicity and osmotic stress caused by excess salt [48]. Instead the carbon from glycolysis is used in lipid production and ATP generated could be directed into energy intensive mechanisms described above to improve survival under salt stress. Further, as mentioned in Section 2.2, glycolysis is the major pathway that produces osmoregulatory solutes like glycerol. Glycolysis products can also be utilized in lipid production. Thus, regulation of this pathway is highly important under high salt stress (Figure 5).
2.7. Role of Acetate
Studies have demonstrated the use of acetate in the medium as an alternative source of energy to compensate for the lowered efficiency in photosynthesis [76]. Transcriptome studies of high salt stressed C. reinhardtii cells show an upregulation of acetyl-CoA synthetase encoding gene, which combines acetate and CoA to form acetyl-CoA [64]. Upregulation of acetate metabolism related genes like acetyl-CoA carboxylase and dehydrogenase a were also observed in transcriptomic studies of the halophyte Prymnesium parvum, along with homologs to Type I and Type III polyketide synthases [77]. Salt adapted mutants of C. reinhardtii showed an improved growth, when salt stressed cells were growing with acetate. More importantly, a greater reduction in growth was observed when the mutants were grown without acetate in salt free media. Further there was significant upregulation of TCA related genes [64]. These results suggest that acetate is introduced into energy generation pathways such as TCA cycle in salt stressed algal cells to compensate for reduction in photosynthesis.
3. Development of Salt Tolerant Freshwater Algae
Directed experimental evolution by selecting for survivors under a progressively increasing stress has been successfully applied to generate salt adapted algae from salt sensitive progenitor cells. Salt adapted algae strains were selected through multiple generations of cultivation under saline stress. Salt tolerance was developed by the 1255th cycle and by the 46th cycle in C. reinhardtii [64] and Chlorella sp. AE10 [65], respectively. Transcriptomic data revealed that responses to salt stress were different in salt sensitive progenitor and salt adapted evolved mutants. The progenitor salt sensitive C. reinhardtii cells displayed a reduction in photosynthesis, upregulation of glycerophospholipid signaling, and upregulation of transcription and translation machinery. In contrast, salt adapted C. reinhardtii cells showed downregulation of genes responsible for lipid accumulation and the transcription/translation mechanisms. Similar results were observed in salt adapted Chlorella sp. AE10 strains [65].
Directed experimental evolution coupled with random mutagenesis through irradiation has been applied in Chlamydomonas sp. JSC4 to improve biomass production under saline conditions [66]. Irradiation using heavy ion beam was performed to induce mutations before long-term and continuous cultivation. Heavy ion beams have been observed to be effective mutagens for application in mutational breeding because they induce several types of mutations such as single and short nucleotide alterations as well as large insertions/deletions in the genomic DNA [78]. Nevertheless, spontaneous mutations resulting from long-term cultivation also proved to be efficient, however, not to the same extent as the irradiation-induced mutagenesis. No increase in biomass or lipid accumulation was identified in these salt adapted mutants [66]. Taken together, the lack of lipid accumulation in salt adapted algae is a highly interesting and important result, especially for those studies looking to utilize salt stress induced algal lipid accumulation for biofuel purposes.
Transformation of salt tolerance conferring genes is a feasible way of enhancing freshwater strains ability to cope with high salt stress. While it has been successfully applied for cyanobacteria where the stress tolerance genes were simply overexpressed [79], limited studies have carried out transformation using specific genes to confer salt tolerance in eukaryotic algae (Table 1).
Other methods such as genome shuffling through selective sexual breeding have also been successfully used to develop salt adapted algae strains. This is a promising alternative to quickly generate salt adapted algae. Unfortunately, so far only a single study has utilized this approach to generate a salt adapted C. reinhardtii strain [80]. By comparing the genomes of the newly evolved salt adapted strain to the genome of its parental salt sensitive strain, genes that might confer protection under salt stress could be identified. Sexual breeding pathways were identified in various Scenedesmus [81], Chlamydomonas [82], and Dunaliella species [83]. Coupling random mutagenesis approaches along with sexual breeding of selected mutants is a promising combination method to develop salt adapted strains in multiple, biotechnologically important algal strains. It is mainly the result of selective breeding strategies that we currently have a diverse pool of agricultural crops with tolerance to different abiotic stresses. These techniques could be applied to algal species as well in order to generate economically viable algal variants.
4. Perspectives of Salt Stress Utilization in Biotechnological Applications
Salt stress can be utilized to generate a plethora of valuable products. Multiple studies have been carried out on the exploitation of high salinity condition as a methodology to improve algal lipid production. Overall, salt stress promotes lipid and β-carotene production which is a phenomenon that can be exploited and commercialized. Such phenomenon was observed for β-carotene production in D. salina [70,84]. It was observed that pairing nitrogen deficiency and high salt stress could be a feasible way of enhancing glycerol production using microalgae [85].
However, it is important to differentiate between processes that occur in salt sensitive algae, salt adapted mutants and salt tolerant strains. Studies have shown that gene expression patterns change as algae adapt to high salt stress. Furthermore, gene expression patterns under high salt stress are strikingly different in salt tolerant algae strains and salt adapted mutants. As previously mentioned, salt adapted mutants of C. reinhardtii do not show the characteristic lipid accumulation response to high salt stress as observed in salt sensitive and in salt tolerant strains. This observation indicates that there are various adaptation pathways to high salt stress. Further, adaptation to salt stress is species specific and depends greatly on the life history of the strain. In many cases salt adapted algae might not be suitable candidates for biotechnological applications such as biodiesel production, because adaptation of a salt sensitive, lipid producing strain might just produce a mutant that is salt tolerant but may lose its lipid accumulation capacity. Thus, we propose to utilize salt tolerant strains coupled with sexual breeding approaches to improve lipid production rather than to harness adaptation of salt sensitive strains. Sexual breeding approaches might be better suited to select and conserve pathways of algal lipid accumulation compared to non-specific mutational approaches. A lot of commercial algae cultivations prefer indoor and closed cultivation techniques to avoid fatal contaminations, thereby strongly increasing the cost of cultivation and technology required. The use of salinity as a crop protection mechanism is also feasible. It has been applied as an effective protection method for reducing the microbial contaminants in Picochlorum (Nannochloris) atomus freshwater green algae cultures [86]. Reliance on salinity as a control measure allows the harvesting of high quality biomass, which reduces economic losses pertaining to culture re-establishment and end-product loss. Picochlorum SE3, isolated from a shallow mesophilic brackish-water lagoon, can tolerate a hypervariable environment making it a suitable candidate for large-scale open-pond cultivation [44]. Similarly, various mutant eukaryotic green algae with evolved salt tolerance features [65,66] can be grown in saline media with no reduction in biomass, thus reducing the dependency on freshwater sources. Hence, high quality algae biomass with limited contamination can be cultivated using either salt tolerant and salt adapted algal strains.
5. Conclusions
More than four decades of research has drastically enriched our understanding of the various mechanisms used by microalgae to cope with salt stress. Initial studies exploring accumulation of osmolyte concentration provided us with an understanding of what kinds of osmolytes are accumulated (such as glycerol) and why these osmolytes are important. Physiological changes such as C. reinhardtii cells entering the palmelloid stage or increased production of EPS and cell size changes are all integral to further improve osmoregulation. Contemporary differential expression studies have begun to probe the pathways that are differentially regulated under optimal and high salt stressed conditions. Most algal cells show a characteristic accumulation of lipids under salt stress. Lipids act as storage reserves for salt stressed cells and can be immediately degraded when stressed cells are introduced to optimal conditions. These studies have also improved our understanding of how products from pathways such as glycolysis, Kennedy pathway, and Calvin cycle are all used to improve both osmolyte production and reserves of storage molecules, mostly lipids (Figure 5). Studying diverse algal species such as Prymnesium parvum [77] and salt adapted mutants of C. reinhardtii [64] showed that algae can adapt to loss in photosynthetic efficiency by taking up acetate to improve energy generation and carbon assimilation. Finally, differential expression analysis studies have also enhanced our understanding of how transport proteins are involved in osmoregulation.
However, there is much uncovered ground in understanding how algae signal each other to begin the cascade of pathways that will lead to improved survival. One such example is the study of volatile organic compounds (VOCs) that are released when algal cells are under stress. A preliminary study has shown that VOCs like hexanal and longifolene are released from salt stressed C. reinhardtii cells. When these compounds are collected and exposed to algal cells growing under optimal conditions, a reduction in cell density, a slight increase in chlorophyll and an increase in antioxidant enzyme activity was observed [87]. Presumably, the VOCs signal unstressed cells to prepare for an oncoming stressful condition. Thus, future studies need to concentrate on signaling pathways that begin the cascade of physiological and metabolic changes that are described in this review. Further, while there have been a handful of studies on directed experimental evolution to improve salt tolerance, none of these have characterized the changes that are occurring in the genomes of these adapted organisms. Such studies coupled with next generation sequencing strategies and directed experimental evolution approaches will continue to further improve and deepen our understanding of how algae respond and adapt to stressful conditions.
Acknowledgments
We thank Attila Farkas for the microscopic analyses.
Author Contributions
P.S., M.M.G. and G.M. collected and thoroughly discussed the relevant literature and all participated in composing the manuscript.
Funding
This work was supported by the following international and domestic funds: NKFI-FK-123899 (GM), GINOP-2.3.2-15-2016-00011, János Bolyai Research Scholarship of the Hungarian Academy of Sciences (GM), and by a Bolyai+ grant UNKP-19-4-SZTE-70 (GM).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Brock T.D. Salinity and the ecology of Dunaliella from Great Salt Lake. Microbiology. 1975;89:285–292. doi: 10.1099/00221287-89-2-285. [DOI] [Google Scholar]
- 2.Costelloe J.F., Powling J., Reid J.R.W., Shiel R.J., Hudson P. Algal diversity and assemblages in arid zone rivers of the Lake Eyre Basin, Australia. River Res. Appl. 2005;21:337–349. doi: 10.1002/rra.851. [DOI] [Google Scholar]
- 3.Ahmad I., Hellebust J.A. The role of glycerol and inorganic ions in osmoregulatory responses of the euryhaline flagellate Chlamydomonas pulsatilla Wollenweber. Plant Physiol. 1986;82:406–410. doi: 10.1104/pp.82.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellebust J.A., Le Gresley S.M. Growth characteristics of the marine rock pool flagellate Chlamydomonas pulsatilla Wollenweber (Chlorophyta) Phycologia. 1985;24:225–229. doi: 10.2216/i0031-8884-24-2-225.1. [DOI] [Google Scholar]
- 5.Miyasaka H., Ohnishi Y., Akano T., Fukatsu K., Mizoguchi T., Yagi K., Isamu M., Yoshiaki I., Hiroyo M., Norio S., et al. Excretion of glycerol by the marine Chlamydomonas sp. strain W-80 in high CO2 cultures. J. Ferment. Bioeng. 1998;85:122–124. doi: 10.1016/S0922-338X(97)80367-4. [DOI] [Google Scholar]
- 6.Lee H.N., Kim S.H., Han Y.J., Im S., Jeong W.J., Park E.J., Mi S.H., Choi D.W. PsCYP1 of marine red alga Pyropia seriata (Bangiales, Rhodophyta) confers salt and heat tolerance in Chlamydomonas. J. Appl. Phycol. 2017;29:617–625. doi: 10.1007/s10811-016-0934-0. [DOI] [Google Scholar]
- 7.Huang L.F., Lin J.Y., Pan K.Y., Huang C.K., Chu Y.K. Overexpressing ferredoxins in Chlamydomonas reinhardtii increase starch and oil yields and enhance electric power production in a photo microbial fuel cell. Int. J. Mol. Sci. 2015;16:19308–19325. doi: 10.3390/ijms160819308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka S., Suda Y., Ikeda K., Ono M., Miyasaka H., Watanabe M., Ken S., Hirata K. A novel gene with antisalt and anticadmium stress activities from a halotolerant marine green alga Chlamydomonas sp. W80. FEMS Microbiol. Lett. 2007;271:48–52. doi: 10.1111/j.1574-6968.2007.00696.x. [DOI] [PubMed] [Google Scholar]
- 9.Miyasaka H., Kanaboshi H., Ikeda K. Isolation of several anti-stress genes from the halotolerant green alga Chlamydomonas by simple functional expression screening with Escherichia coli. World J. Microbiol. Biotechnol. 2000;16:23–29. doi: 10.1023/A:1008982332139. [DOI] [Google Scholar]
- 10.Tanaka S., Ikeda K., Miyasaka H. Enhanced tolerance against salt-stress and freezing-stress of Escherichia coli cells expressing algal bbc1 gene. Curr. Microbiol. 2001;42:173–177. doi: 10.1007/s002840010199. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura K., Miyao K., Gaber A., Takeda T., Kanaboshi H., Miyasaka H., Shigeoka S. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. Plant J. 2004;37:21–33. doi: 10.1046/j.1365-313X.2003.01930.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka S., Ikeda K., Miyasaka H. Isolation of a new member of group 3 late embryogenesis abundant protein gene from a halotorelant green alga by a functional expression screening with cyanobacterial cells. FEMS Microbiol. Lett. 2004;236:41–45. doi: 10.1111/j.1574-6968.2004.tb09624.x. [DOI] [PubMed] [Google Scholar]
- 13.Hema R., Senthil-Kumar M., Shivakumar S., Reddy P.C., Udayakumar M. Chlamydomonas reinhardtii, a model system for functional validation of abiotic stress responsive genes. Planta. 2007;226:655–670. doi: 10.1007/s00425-007-0514-2. [DOI] [PubMed] [Google Scholar]
- 14.Khona D.K., Shirolikar S.M., Gawde K.K., Hom E., Deodhar M.A., D’Souza J.S. Characterization of salt stress-induced palmelloids in the green alga, Chlamydomonas reinhardtii. Algal Res. 2016;16:434–448. doi: 10.1016/j.algal.2016.03.035. [DOI] [Google Scholar]
- 15.Nakamura K.A.Z.U.O., Bray D.F., Wagenaar E.B. Ultrastructure of Chlamydomonas eugametos palmelloids induced by chloroplatinic acid treatment. J. Bacteriol. 1975;121:338–343. doi: 10.1128/jb.121.1.338-343.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neelam S., Subramanyam R. Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J. Photochem. Photobiol. B Biol. 2013;124:63–70. doi: 10.1016/j.jphotobiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Talebi A.F., Tabatabaei M., Mohtashami S.K., Tohidfar M., Moradi F. Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not. Sci. Biol. 2013;5:309–315. doi: 10.15835/nsb539114. [DOI] [Google Scholar]
- 18.Demetriou G., Neonaki C., Navakoudis E., Kotzabasis K. Salt stress impact on the molecular structure and function of the photosynthetic apparatus—The protective role of polyamines. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007;1767:272–280. doi: 10.1016/j.bbabio.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Tordai H., Bányai L., Patthy L. The PAN module: The N-terminal domains of plasminogen and hepatocyte growth factor are homologous with the apple domains of the prekallikrein family and with a novel domain found in numerous nematode proteins. FEBS Lett. 1999;461:63–67. doi: 10.1016/S0014-5793(99)01416-7. [DOI] [PubMed] [Google Scholar]
- 20.Verma R.P., Hansch C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q) SARs. Bioorg. Med. Chem. 2007;15:2223–2268. doi: 10.1016/j.bmc.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad I., Hellebust J.A. Osmoregulation in the extremely euryhaline marine micro-alga Chlorella autotrophica. Plant Physiol. 1984;74:1010–1015. doi: 10.1104/pp.74.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaçka A., Dönmez G. Isolation of Dunaliella spp. from a hypersaline lake and their ability to accumulate glycerol. Bioresour. Technol. 2008;99:8348–8352. doi: 10.1016/j.biortech.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Fu W., Paglia G., Magnúsdóttir M., Steinarsdóttir E.A., Gudmundsson S., Palsson B.Ø., Brynjólfsson S. Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microb. Cell Factories. 2014;13:3. doi: 10.1186/1475-2859-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown A.D., Simpson J.R. Water relations of sugar-tolerant yeasts: The role of intracellular polyols. Microbiology. 1972;72:589–591. doi: 10.1099/00221287-72-3-589. [DOI] [PubMed] [Google Scholar]
- 25.Läuchli A., Lüttge U. Salinity: Environment-Plants-Molecules. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. [Google Scholar]
- 26.Miyasaka H., Ikeda K. Osmoregulating mechanism of the halotolerant green alga Chlamydomonas, strain HS-5. Plant Sci. 1997;127:91–96. [Google Scholar]
- 27.León R., Galván F. Halotolerance studies on Chlamydomonas reinhardtii: Glycerol excretion by free and immobilized cells. J. Appl. Phycol. 1994;6:13–20. doi: 10.1007/BF02185898. [DOI] [Google Scholar]
- 28.Salama E.S., Kim H.C., Abou-Shanab R.A., Ji M.K., Oh Y.K., Kim S.H., Jeon B.H. Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst. Eng. 2013;36:827–833. doi: 10.1007/s00449-013-0919-1. [DOI] [PubMed] [Google Scholar]
- 29.Ho S.H., Nakanishi A., Kato Y., Yamasaki H., Chang J.S., Misawa N., Yuu H., Jun M., Tomohisda H., Kondo A. Dynamic metabolic profiling together with transcription analysis reveals salinity-induced starch-to-lipid biosynthesis in alga Chlamydomonas sp. JSC4. Sci. Rep. 2017;7:45471. doi: 10.1038/srep45471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Amotz A., Avron M. The role of glycerol in the osmotic regulation of the halophilic alga Dunaliella parva. Plant Physiol. 1973;51:875–878. doi: 10.1104/pp.51.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellebust J.A., Lin Y.-H. Regulation of glycerol and starch metabolism in Chlamydomonas pulsatilla in response to changes in salinity. Plant Cell Environ. 1989;12:621–627. doi: 10.1111/j.1365-3040.1989.tb01230.x. [DOI] [Google Scholar]
- 32.Chen H., Lu Y., Jiang J.G. Comparative analysis on the key enzymes of the glycerol cycle metabolic pathway in Dunaliella salina under osmotic stresses. PLoS ONE. 2012;7:e37578. doi: 10.1371/journal.pone.0037578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driver T., Trivedi D.K., McIntosh O.A., Dean A.P., Goodacre R., Pittman J.K. Two glycerol-3-phosphate dehydrogenases from Chlamydomonas have distinct roles in lipid metabolism. Plant Physiol. 2017;174:2083–2097. doi: 10.1104/pp.17.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casais-Molina M.L., Peraza-Echeverria S., Echevarría-Machado I., Herrera-Valencia V.A. Expression of Chlamydomonas reinhardtii CrGPDH2 and CrGPDH3 cDNAs in yeast reveals that they encode functional glycerol-3-phosphate dehydrogenases involved in glycerol production and osmotic stress tolerance. J. Appl. Phycol. 2016;28:219–226. doi: 10.1007/s10811-015-0588-3. [DOI] [Google Scholar]
- 35.Cohu C.M., Muller O., Stewart J.J., Demmig-Adams B., Adams III W.W. Association between minor loading vein architecture and light-and CO2-saturated rates of photosynthetic oxygen evolution among Arabidopsis thaliana ecotypes from different latitudes. Front. Plant Sci. 2013;4:264. doi: 10.3389/fpls.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams W.W., III, Cohu C.M., Muller O., Demmig-Adams B. Foliar phloem infrastructure in support of photosynthesis. Front. Plant Sci. 2013;4:194. doi: 10.3389/fpls.2013.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burch T.A., Adams W.W., Degrenne B.L., Englert C.H., Mines B.R., Nash P.C., Emma C.B., Demmig-Adams B. Environmental manipulation of growth and energy carrier release from freshwater and marine Chlamydomonas species. J. Appl. Phycol. 2015;27:1127–1136. doi: 10.1007/s10811-014-0433-0. [DOI] [Google Scholar]
- 38.Grizeau D., Navarro J.M. Glycerol production by Dunaliella tertiolecta immobilized within Ca-alginate beads. Biotechnol. Lett. 1986;8:261–264. doi: 10.1007/BF01030509. [DOI] [Google Scholar]
- 39.Liu J., Zhu J.K. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1997;114:591–596. doi: 10.1104/pp.114.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown L.M., Hellebust J.A. Sorbitol and proline as intracellular osmotic solutes in the green alga Stichococcus bacillaris. Can. J. Bot. 1978;56:676–679. doi: 10.1139/b78-074. [DOI] [Google Scholar]
- 41.Reynoso G.T., De Gamboa B.A. Salt tolerance in the freshwater algae Chlamydomonas reinhardii: Effect of proline and taurine. Comp. Biochem. Physiol. Part A Physiol. 1982;73:95–99. doi: 10.1016/0300-9629(82)90098-6. [DOI] [Google Scholar]
- 42.Aggarwal M., Sharma S., Kaur N., Pathania D., Bhandhari K., Kaushal N., Ramanpreet K., Kamaljit S., Alok S., Nayyar H. Exogenous proline application reduces phytotoxic effects of selenium by minimising oxidative stress and improves growth in bean (Phaseolus vulgaris L.) seedlings. Biol. Trace Elem. Res. 2011;140:354–367. doi: 10.1007/s12011-010-8699-9. [DOI] [PubMed] [Google Scholar]
- 43.Henley W.J., Hironaka J.L., Guillou L., Buchheim M.A., Buchheim J.A., Fawley M.W., Fawley K.P. Phylogenetic analysis of the ‘Nannochloris-like’algae and diagnoses of Picochlorum oklahomensis gen. et sp. nov. (Trebouxiophyceae, Chlorophyta) Phycologia. 2004;43:641–652. doi: 10.2216/i0031-8884-43-6-641.1. [DOI] [Google Scholar]
- 44.Foflonker F., Ananyev G., Qiu H., Morrison A., Palenik B., Dismukes G.C., Bhattacharya D. The unexpected extremophile: Tolerance to fluctuating salinity in the green alga Picochlorum. Algal Res. 2016;16:465–472. doi: 10.1016/j.algal.2016.04.003. [DOI] [Google Scholar]
- 45.Goyal A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol. Biochem. 2007;45:705–710. doi: 10.1016/j.plaphy.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Xia B.B., Wang S.H., Duan J.B., Bai L.H. The relationship of glycerol and glycolysis metabolism pathway under hyperosmotic stress in Dunaliella salina. Cent. Eur. J. Biol. 2014;9:901–908. [Google Scholar]
- 47.Kaushik J.K., Bhat R. Why is trehalose an exceptional protein stabilizer? An analysis of the thermal stability of proteins in the presence of the compatible osmolyte trehalose. J. Biol. Chem. 2003;278:26458–26465. doi: 10.1074/jbc.M300815200. [DOI] [PubMed] [Google Scholar]
- 48.Wang N., Qian Z., Luo M., Fan S., Zhang X., Zhang L. Identification of salt stress responding genes using transcriptome analysis in green alga Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2018;19:3359. doi: 10.3390/ijms19113359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.del Pilar Bremauntz M., Torres-Bustillos L.G., Cañizares-Villanueva R.O., Duran-Paramo E., Fernández-Linares L. Trehalose and sucrose osmolytes accumulated by algae as potential raw material for bioethanol. Nat. Resour. 2011;2:173. [Google Scholar]
- 50.Zeid I.M. Trehalose as osmoprotectant for maize under salinity-induced stress. Res. J. Agric. Biol. Sci. 2009;5:613–622. [Google Scholar]
- 51.Hellebust J.A. Effect of salinity on photosynthesis and mannitol synthesis in the green flagellate Platymonas suecica. Can. J. Bot. 1976;54:1735–1741. doi: 10.1139/b76-187. [DOI] [Google Scholar]
- 52.Brown L.M., Hellebust J.A. Some new taxonomic characteristics applied to Stichococcus bacillaris (Chlorophyceae) Can. J. Bot. 1980;58:1405–1411. doi: 10.1139/b80-171. [DOI] [Google Scholar]
- 53.Chakraborty K., Bose J., Shabala L., Shabala S. Difference in root K+ retention ability and reduced sensitivity of K+-permeable channels to reactive oxygen species confer differential salt tolerance in three Brassica species. J. Exp. Bot. 2016;67:4611–4625. doi: 10.1093/jxb/erw236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shabala S. Regulation of potassium transport in leaves: From molecular to tissue level. Ann. Bot. 2003;92:627–634. doi: 10.1093/aob/mcg191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sithtisarn S., Yokthongwattana K., Mahong B., Roytrakul S., Paemanee A., Phaonakrop N., Yokthongwattana C. Comparative proteomic analysis of Chlamydomonas reinhardtii control and a salinity-tolerant strain revealed a differential protein expression pattern. Planta. 2017;246:843–856. doi: 10.1007/s00425-017-2734-4. [DOI] [PubMed] [Google Scholar]
- 56.Pick U., Karni L., Avron M. Determination of ion content and ion fluxes in the halotolerant alga Dunaliella salina. Plant Physiol. 1986;81:92–96. doi: 10.1104/pp.81.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher M., Gokhman I., Pick U., Zamir A. A salt-resistant plasma membrane carbonic anhydrase is induced by salt in Dunaliella salina. J. Biol. Chem. 1996;271:17718–17723. doi: 10.1074/jbc.271.30.17718. [DOI] [PubMed] [Google Scholar]
- 58.Fisher M., Gokhman I., Pick U., Zamir A. A structurally novel transferrin-like protein accumulates in the plasma membrane of the unicellular green alga Dunaliella salina grown in high salinities. J. Biol. Chem. 1997;272:1565–1570. doi: 10.1074/jbc.272.3.1565. [DOI] [PubMed] [Google Scholar]
- 59.Sun X., Wang W., Shen J., Xu N. Transcriptome sequencing of the marine microalga, Chlorella pyrenoidosa (Chlorophyta), and analysis of carbonic anhydrase expression under salt stress. Bot. Mar. 2014;57:403–412. doi: 10.1515/bot-2014-0013. [DOI] [Google Scholar]
- 60.Moroney J.V., Ma Y., Frey W.D., Fusilier K.A., Pham T.T., Simms T.A., Robert J.D., Jing Y., Bratati M. The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: Intracellular location, expression, and physiological roles. Photosynth. Res. 2011;109:133–149. doi: 10.1007/s11120-011-9635-3. [DOI] [PubMed] [Google Scholar]
- 61.Goncalves E.C., Wilkie A.C., Kirst M., Rathinasabapathi B. Metabolic regulation of triacylglycerol accumulation in the green algae: Identification of potential targets for engineering to improve oil yield. Plant Biotechnol. J. 2016;14:1649–1660. doi: 10.1111/pbi.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho S.H., Nakanishi A., Ye X., Chang J.S., Hara K., Hasunuma T., Kondo A. Optimizing biodiesel production in marine Chlamydomonas sp. JSC4 through metabolic profiling and an innovative salinity-gradient strategy. Biotechnol. Biofuels. 2014;7:97. doi: 10.1186/1754-6834-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Jaeger L., Carreres B.M., Springer J., Schaap P.J., Eggink G., Dos Santos V.A.M., Rene H.W., Dirk E.M. Neochloris oleoabundans is worth its salt: Transcriptomic analysis under salt and nitrogen stress. PLoS ONE. 2018;13:e0194834. doi: 10.1371/journal.pone.0194834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perrineau M.M., Zelzion E., Gross J., Price D.C., Boyd J., Bhattacharya D. Evolution of salt tolerance in a laboratory reared population of Chlamydomonas reinhardtii. Environ. Microbiol. 2014;16:1755–1766. doi: 10.1111/1462-2920.12372. [DOI] [PubMed] [Google Scholar]
- 65.Li X., Yuan Y., Cheng D., Gao J., Kong L., Zhao Q., Wei W., Sun Y. Exploring stress tolerance mechanism of evolved freshwater strain Chlorella sp. S30 under 30 g/L salt. Bioresour. Technol. 2018;250:495–504. doi: 10.1016/j.biortech.2017.11.072. [DOI] [PubMed] [Google Scholar]
- 66.Kato Y., Ho S.H., Vavricka C.J., Chang J.S., Hasunuma T., Kondo A. Evolutionary engineering of salt-resistant Chlamydomonas sp. strains reveals salinity stress-activated starch-to-lipid biosynthesis switching. Bioresour. Technol. 2017;245:1484–1490. doi: 10.1016/j.biortech.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 67.Sun X.M., Ren L.J., Bi Z.Q., Ji X.J., Zhao Q.Y., Huang H. Adaptive evolution of microalgae Schizochytrium sp. under high salinity stress to alleviate oxidative damage and improve lipid biosynthesis. Bioresour. Technol. 2018;267:438–444. doi: 10.1016/j.biortech.2018.07.079. [DOI] [PubMed] [Google Scholar]
- 68.Sudhir P., Murthy S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica. 2004;42:481–486. doi: 10.1007/S11099-005-0001-6. [DOI] [Google Scholar]
- 69.Subramanyam R., Jolley C., Thangaraj B., Nellaepalli S., Webber A.N., Fromme P. Structural and functional changes of PSI-LHCI supercomplexes of Chlamydomonas reinhardtii cells grown under high salt conditions. Planta. 2010;231:913–922. doi: 10.1007/s00425-009-1097-x. [DOI] [PubMed] [Google Scholar]
- 70.Ye Z.-W., Jiang J.G., Wu G.H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008;26:352–360. doi: 10.1016/j.biotechadv.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Mishra A., Mandoli A., Jha B. Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J. Ind. Microbiol. Biotechnol. 2008;35:1093. doi: 10.1007/s10295-008-0387-9. [DOI] [PubMed] [Google Scholar]
- 72.Hiremath S., Mathad P. Impact of salinity on the physiological and biochemical traits of Chlorella vulgaris Beijerinck. J. Algal Biomass Utln. 2010;1:51–59. [Google Scholar]
- 73.Plaxton W.C. The organization and regulation of plant glycolysis. Annu. Rev. Plant Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- 74.Capriotti A.L., Borrelli G.M., Colapicchioni V., Papa R., Piovesana S., Samperi R., Serena S., Aldo L. Proteomic study of a tolerant genotype of durum wheat under salt-stress conditions. Anal. Bioanal. Chem. 2014;406:1423–1435. doi: 10.1007/s00216-013-7549-y. [DOI] [PubMed] [Google Scholar]
- 75.Espartero J., Sánchez-Aguayo I., Pardo J.M. Molecular characterization of glyoxalase-I from a higher plant; upregulation by stress. Plant Mol. Biol. 1995;29:1223–1233. doi: 10.1007/BF00020464. [DOI] [PubMed] [Google Scholar]
- 76.Heifetz P.B., Förster B., Osmond C.B., Giles L.J., Boynton J.E. Effects of Acetate on Facultative Autotrophy in Chlamydomonas reinhardtii Assessed by Photosynthetic Measurements and Stable Isotope Analyses. Plant Physiol. 2000;122:1439–1446. doi: 10.1104/pp.122.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Talarski A., Manning S.R., La Claire J.W. Transcriptome analysis of the euryhaline alga, Prymnesium parvum (Prymnesiophyceae): Effects of salinity on differential gene expression. Phycologia. 2016;55:33–44. doi: 10.2216/15-74.1. [DOI] [Google Scholar]
- 78.Tanaka A., Shikazono N., Hase Y. Studies on biological effects of ion beams on lethality, molecular nature of mutation, mutation rate, and spectrum of mutation phenotype for mutation breeding in higher plants. J. Radiat. Res. 2010;51:223–233. doi: 10.1269/jrr.09143. [DOI] [PubMed] [Google Scholar]
- 79.Su H.Y., Chou H.H., Chow T.J., Lee T.M., Chang J.S., Huang W.L., Chen H.J. Improvement of outdoor culture efficiency of cyanobacteria by over-expression of stress tolerance genes and its implication as bio-refinery feedstock. Bioresour. Technol. 2017;244:1294–1303. doi: 10.1016/j.biortech.2017.04.074. [DOI] [PubMed] [Google Scholar]
- 80.Takouridis S.J., Tribe D.E., Gras S.L., Martin G.J. The selective breeding of the freshwater microalga Chlamydomonas reinhardtii for growth in salinity. Bioresour. Technol. 2015;184:18–22. doi: 10.1016/j.biortech.2014.10.120. [DOI] [PubMed] [Google Scholar]
- 81.Trainor F.R. Reproduction in Scenedesmus. Algae Korean J. Phycol. 1996;11:183–201. [Google Scholar]
- 82.Adair W.S., Hwang C., Goodenough U.W. Identification and visualization of the sexual agglutinin from the mating-type plus flagellar membrane of Chlamydomonas. Cell. 1983;33:183–193. doi: 10.1016/0092-8674(83)90347-1. [DOI] [PubMed] [Google Scholar]
- 83.Oren A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005;1:2. doi: 10.1186/1746-1448-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pisal D.S., Lele S.S. Carotenoid production from microalga, Dunaliella salina. Indian J. Biotechnol. 2005;4:476–483. [Google Scholar]
- 85.Afify A.E.M.M., Shalaby E.A., Shanab S.M. Enhancement of biodiesel production from different species of algae. Grasas Y Aceites. 2010;61:416–422. [Google Scholar]
- 86.von Alvensleben N., Stookey K., Magnusson M., Heimann K. Salinity tolerance of Picochlorum atomus and the use of salinity for contamination control by the freshwater cyanobacterium Pseudanabaena limnetica. PLoS ONE. 2013;8:e63569. doi: 10.1371/journal.pone.0063569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zuo Z.J., Zhu Y.R., Bai Y.L., Wang Y. Volatile communication between Chlamydomonas reinhardtii cells under salt stress. Biochem. Syst. Ecol. 2012;40:19–24. doi: 10.1016/j.bse.2011.09.007. [DOI] [Google Scholar]