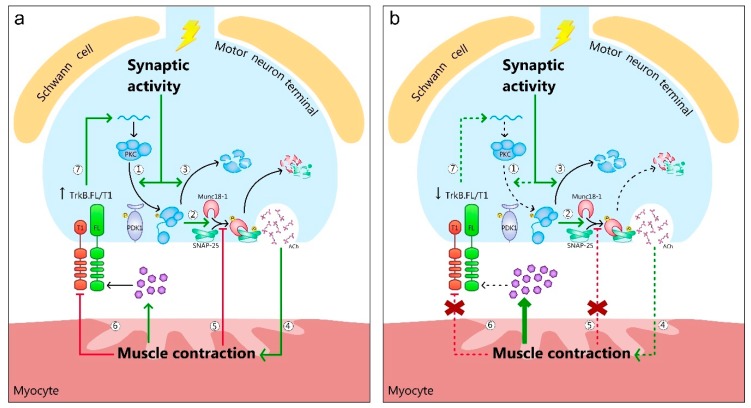
Figure 2.
The BDNF/TrkB feedback signaling at the NMJs of healthy and amyotrophic lateral sclerotic (ALS) animals. (a) Healthy animals. Synaptic activity promotes PKC phosphorylation in the membrane through PDK1 (1). This results in the phosphorylation of PKC targets implicated in exocytosis, such as Munc18-1 and SNAP-25 (2). Once PKC has executed its action, it is typically degraded, decreasing its protein level (3). Consequently, ACh is released to reach its receptors in the junctional folds and trigger muscle contraction (4). Muscle contraction functions as a feedback loop to inhibit PKC phosphorylating activity over Munc18-1 and SNAP-25 (5). Moreover, it increases TrkB.FL signaling by secreting BDNF and decreasing TrkB.T1 levels and its dominant negative way to decrease TrkB.FL signaling (6). Therefore, an increased ratio TrkB.FL/T1 stimulates PKC synthesis in the presynaptic terminal, restoring its levels after activity-induced consumption (7). (b) ALS animals. Synaptic activity-induced PKC phosphorylation in the membrane through PDK1 is decreased (1). However, the levels of phosphorylated PKC targets implicated in exocytosis, such as Munc18-1 and SNAP-25 are increased (2), indicating that low PKC activity is functional. Moreover, PKC total levels are decreased, suggesting a minor synthesis or an increased degradation activity (3). Despite of the enhanced levels of phosphorylated exocytotic molecules, ACh release, and consequently, muscle contraction are decreased (4), suggesting a non-functional accumulation of these molecules. Therefore, muscle contraction loses the feedback loop to inhibit PKC’s phosphorylating activity (5), further contributing to the increase of Munc18-1 and SNAP-25 phosphorylated levels. Finally, despite of increased BDNF levels, maybe as a compensatory mechanism for TrkB misbalance, muscle contraction decreases the TrkB.FL signaling because the inhibiting action over TrkB.T1 is lost (6). Therefore, TrkB.FL’s effect on PKC synthesis is decreased, (7) contributing to the decrease of PKC levels.