Abstract
Objective
Common variants near TMEM106B associate with risk of developing frontotemporal dementia (FTD). Emerging evidence suggests a role for TMEM106B in neurodegenerative processes beyond FTD. The objective of this study is to evaluate the effect of TMEM106B genotype on cognitive decline across multiple neurogenerative diseases.
Methods
870 subjects with diagnoses of Parkinson’s disease (PD, N=179), FTD (N=179), Alzheimer’s disease (AD, N=300), or memory-predominant mild cognitive impairment (MCI, N=75), and neurologically-normal control subjects (NC, N=137) were followed longitudinally at the University of Pennsylvania (UPenn). All participants had annual MMSE (median follow-up duration 3.0 years), and were genotyped at TMEM106B index SNP rs1990622. Genotype effects on cognition were confirmed by extending analyses to additional cognitive instruments (Mattis Dementia Rating Scale-2 (DRS-2) and Montreal Cognitive Assessment (MoCA)) and to an international validation cohort (Parkinson’s Progression Markers Initiative (PPMI), N=371).
Results
The TMEM106B rs1990622T allele, linked to increased risk of FTD, associated with greater MMSE decline over time in PD subjects, but not in AD or MCI subjects. For FTD subjects, rs1990622T associated with more rapid decrease in MMSE only under the minor-allele, rs1990622C, dominant model. Among PD patients, rs1990622T carriers from the UPenn cohort demonstrated more rapid longitudinal decline in DRS-2 scores. Finally, in the PPMI cohort, TMEM106B risk allele carriers demonstrated more rapid longitudinal decline in MoCA scores.
Interpretation
Irrespective of cognitive instrument or cohort assessed, TMEM106B acts as a genetic modifier for cognitive trajectory in PD. Our results implicate lysosomal dysfunction in the pathogenesis of cognitive decline in two different proteinopathies.
INTRODUCTION
Neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and frontotemporal dementia (FTD), are a major cause of morbidity and mortality. Our understanding of these diseases as distinct clinical entities is evolving. Each neurodegenerative disease is characterized by the aggregation of abnormally folded protein inclusions, with a distinct distribution and morphology, defining the signature “proteinopathy” of the disease. Thus, AD is defined by the presence of tau-positive neurofibrillary tangles and amyloid-beta plaques, PD is defined by alpha-synuclein-containing Lewy bodies (LB), and amyotrophic lateral sclerosis (ALS) as well as ~50% of FTD cases are defined by hyperphosphorylated inclusions of TAR DNA protein of 43-kDa (TDP-43, encoded by TARDBP); this neuropathological subtype of FTD is termed FTLD-TDP.1 However, overlap exists among the neurodegenerative diseases, as exemplified by the co-existence of multiple proteinopathies in nearly half of all neurodegenerative disease subjects.2
Common genetic variants at the TMEM106B locus were first linked to risk for developing FTLD-TDP by genome-wide association studies.3 Noncoding genetic variation at this locus may alter expression of TMEM106B through differential recruitment of the chromatin organizing protein CTCF.4 Changes in TMEM106B expression, in turn, impact the function of lysosomes, to which the TMEM106B protein localizes in many cell types, including neurons.5,6 In cell culture and animal studies, TMEM106B has been linked mechanistically to both progranulin (the protein product of GRN, an autosomal dominant cause of FTD characterized by FTLD-TDP neuropathologically) and the protein product of C9orf72 (expansions of which are also an autosomal dominant cause of FTD characterized by FTLD-TDP neuropathologically).5,7
Echoing the mechanistic findings linking TMEM106B, GRN, and C9orf72 are human genetic studies demonstrating that TMEM106B genotype modifies disease severity in FTD patients with GRN mutations8 or C9orf72 expansions.9 Growing evidence from human genetics moreover suggests a role for TMEM106B in neurodegenerative processes beyond FTD. Specifically, TMEM106B genotypes influence risk for cognitive symptoms in the TDP-43 proteinopathy ALS.10 Furthermore, TMEM106B genotype affects the risk of developing TDP-43 pathology even in diseases that are not primarily characterized by TDP-43 inclusions: genetic variation in TMEM106B associates with the burden of TDP-43 pathology in cases of AD,11 hippocampal sclerosis (HpScl) with or without concomitant AD pathology,12 and LB disease with HpScl.13 Moreover, in two genome-wide analyses, TMEM106B emerged as a locus associated with general cognition among elderly individuals without a neurodegenerative disease,14 and with a pattern of frontal cortex gene expression enriched in aging.14
Despite suggestions that TMEM106B plays a broader role in neurodegenerative processes, the question of whether TMEM106B impacts the clinical manifestation of the major neurodegenerative diseases AD and PD remains unanswered. Demonstration of modifier effects on disease progression in AD or PD would widen the scope of patients in which therapeutic targeting of TMEM106B might be beneficial. To answer this question, we studied 870 subjects with PD, FTD, AD, and memory-predominant MCI, as well as neurologically normal controls (NC), followed in prospective cohort studies at UPenn. The effect of TMEM106B genotype on longitudinal rate of cognitive decline was assessed, with positive findings replicated using additional cognitive instruments and cohorts.
METHODS
Subjects
Subjects were enrolled at the University of Pennsylvania (UPenn) within specific research studies across multiple disease centers (Udall Center for Parkinson’s Disease Research, Parkinson’s Disease and Movement Disorders Clinic, Alzheimer’s Disease Research Center, and Frontotemporal Disease Dementia Center). Clinical disease categorization followed published clinical diagnostic criteria for AD, PD, memory-predominant MCI, and FTD, inclusive of behavioral and language variants of FTD, as previously reported.15
For verification of genetic influences on PD progression, we used the Parkinson’s Progression Markers Initiative (PPMI) cohort, an international, multi-site, prospective, longitudinal cohort study. Overall study aims, methods and details of assessments for PPMI have been previously described,16 and are available on the PPMI website (http://www.ppmi-info.org).
Subjects were included in the analysis if (1) they had at least two research visits assessing cognition a minimum of 0.8 years apart, (2) had genetic material available for analysis, and (3) had normal cognition, MCI, or mild dementia defined as a baseline Mini Mental State Exam (MMSE) score ≥18.17 In subsequent analyses, a total Mattis Dementia Rating Scale-2 (DRS-2) score of ≥130 or Montreal Cognitive Assessment (MoCA) score of ≥20 at baseline was an inclusion criterion to exclude any subject with PD dementia.18 A minimum of 0.8 years of follow up time was chosen to approximate 1 year of follow up time allowing for accommodations in scheduling research visits.
Standard Protocol and Informed Consent
This study was approved by the UPenn Institutional Review Board. For the PPMI cohort, IRB approval was also obtained at the University of Rochester, and independently at each participating site. Informed consent was obtained at study enrollment.
Clinical Assessments
Clinical assessments and neuropsychiatric testing were performed by trained research staff. At baseline and at each subsequent visit clinical information was obtained and MMSE,19 DRS-2,20 or MoCA21 scales were administered at least at yearly intervals. In the PD test cohort, the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III)22 was administered at yearly intervals.
Genotyping
At enrollment, blood was obtained from all participants, and genomic DNA was extracted. Genotype at rs1990622 was determined using real-time allelic discrimination with applied Biosystems TaqMan probes, as previously described.3 In the PPMI cohort, genotyping arrays were performed, as previously described.23 In the PPMI cohort, rs3173615 genotype was used as a proxy for rs1990622 (R2=1.0 in CEU population).
Statistical Analysis
All statistical analysis was performed in R (http://www.r-project.org); R-scripts are available upon request. Descriptive statistics were calculated for baseline demographics and cognitive testing utilizing available data. Cochran-Armitage trend tests were used to assess association of disease with TMEM106B genotype in codominant models.
Linear mixed-effects models24 were used to test for associations between TMEM106B genotype and cognitive score over time (in years), with covariates of age, sex, years of education, and baseline cognitive test score in allele-dominant and codominant genetic models. The mixed effects model controls for variable follow-up time and variable intervals between follow-up visits. A random intercept was included in each mixed-effects model to account for correlations among repeated measures. A comparison of Akaike Information Criterion (AIC) between models including a random intercept or combined random intercept and slope was performed to determine the best fit. Unless noted, models are presented with random intercepts. For initial investigations, statistical tests were two-sided, and alpha was set at 0.05. In confirmatory analyses with predicted directions of change, we report both two-sided and one-sided p-values, and alpha was set at 0.05.
Role of the funding source
The funding source did not have any role in the study design, collection, analysis, or interpretation of the data, writing the report, or decision to submit for publication.
RESULTS
Patient cohorts
Two-hundred seventeen PD, 195 FTD, 380 AD, 75 MCI and 137 NC research participants met inclusion criteria from August, 1990 to March, 2018. Thirteen percent of participants were excluded for low MMSE score. For those included in the analysis, the median age at disease diagnosis was 69 years (IQR 64–75) for PD, 64 years (IQR 58–69) for FTD, 73 years (IQR 67–78) for AD, 73 years (IQR 66–78) for MCI. At enrollment, NC had a median age of 68 years (IQR 63–72). Demographic summaries and baseline characteristics of each group are shown in Table 1. Among FTD patients, the clinical subtypes in our cohort were 53% (N=95) behavioral variant FTD (bvFTD), 15% (N= 26) nonfluent agrammatic primary progressive aphasia (naPPA), 15.5% (N=28) logopenic variant PPA (lvPPA), 15.5% (N=28) semantic variant PPA (svPPA), and 1% (N=2) nonspecific PPA (nPPA). The median age at enrollment was 59 (IQR 55–65), 65.5 (IQR 58–70), 63 (IQR 54.5–70), 61.5 (IQR 54.5–66), and 66.5 (IQR 65–68), and the baseline MMSE was 27 (IQR 24–28), 25 (IQR 22–27), 26 (IQR 23–27.5), 27 (IQR 25–28), and 28 (IQR 27–29) for bvFTD, naPPA, lvPPA, svPPA, and nPPA groups, respectively.
Table 1.
Descriptive statistics of neurodegenerative disease cohorts. All values are represented as median (IQR) unless otherwise specified. NC=Neurologically normal control, PD=Parkinson’s disease, FTD=Frontotemporal dementia, MCI=Mild cognitive impairment, AD=Alzheimer’s disease.
| NC | FTD | PD | MCI | AD | |
|---|---|---|---|---|---|
| N | 137 | 179 | 179 | 75 | 300 |
| Baseline Age, yr | 68 (63–72) | 64 (58–69) | 69 (64–75) | 73 (66–78) | 73 (67–78) |
| Sex (%) M | 40 | 43 | 67 | 49 | 41 |
| F | 59 | 57 | 33 | 50 | 59 |
| Race (%) White | 77.37 | 98.32 | 96.64 | 84 | 89 |
| Black | 19.71 | 1.12 | 1.12 | 13.33 | 10.65 |
| Asian | 0.73 | 0.56 | 1.68 | 0 | 0.35 |
| Multiracial | 2.19 | 0 | 0.56 | 2.67 | 0 |
| Baseline Disease Duration, yr | N/A | 2 (1–4) | 8 (5–12) | 1 (0–3) | 2 (1–3) |
| Education, yr | 16 (16–18) | 16 (12–18) | 16 (14–18) | 16 (14–18) | 16 (12–18) |
| Follow up duration, yr | 4.7 (2.1–8.0) |
2.2 (1.4–3.7) |
2.2 (1.2–4.1) |
3.5 (2.0–5.5) |
3.6 (2.1–5.3) |
| Baseline MMSE | 29 (29–30) | 26 (23–28) | 29 (27–29) | 28 (27–29) | 24 (21–26) |
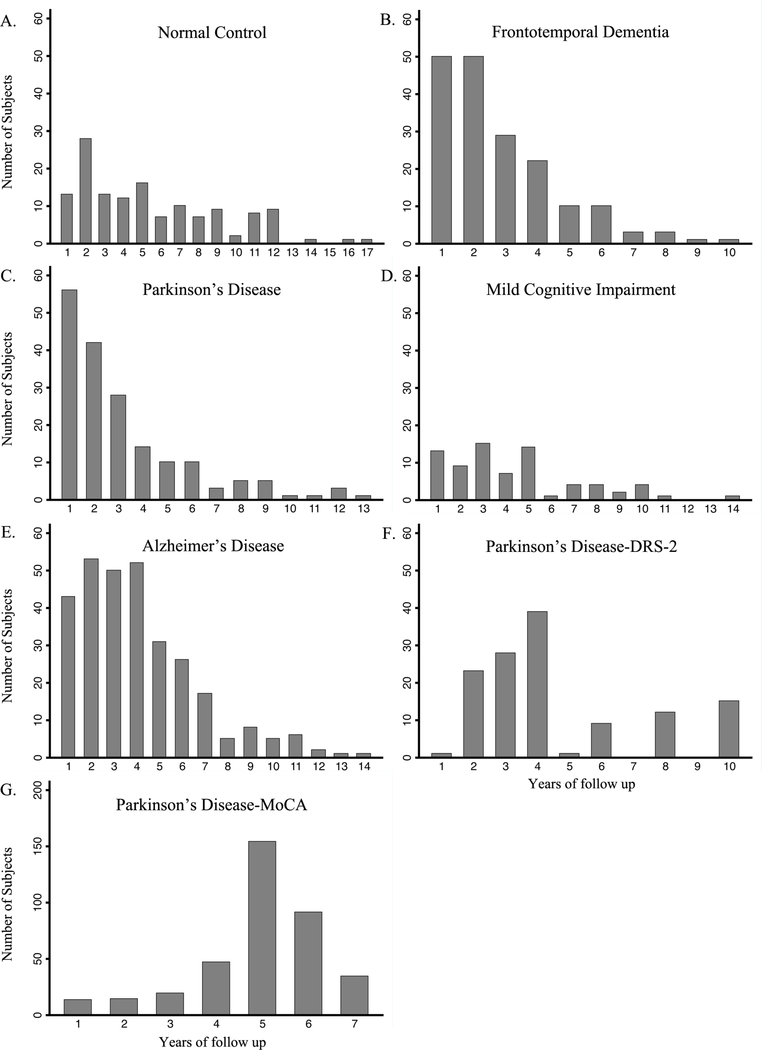
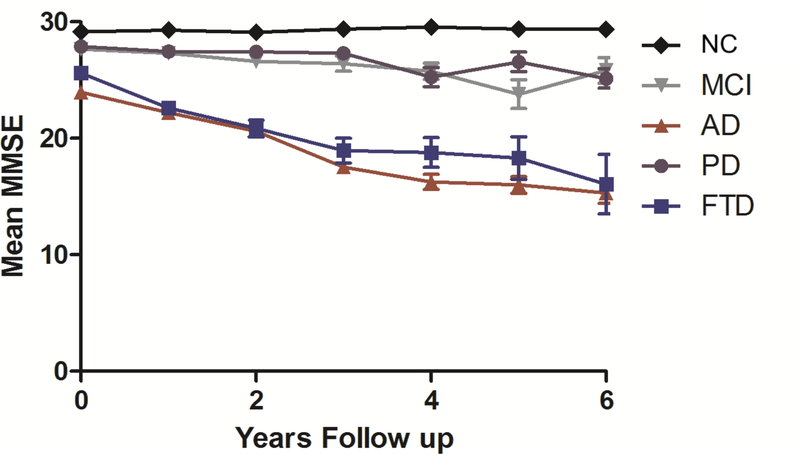
The subjects were followed for up to 17.3 years, with median follow-up of 3.0 (IQR 1.8–5.2) years overall. Within each disease, median follow up was 2.2 years (IQR 1.2–4.1) for PD, 2.2 years (IQR 1.4–3.7) for FTD, 3.6 years (IQR 2.1–5.3) for AD, 3.5 years (IQR 2.0–5.5) for MCI and 4.7 years (IQR 2.1–8.0) for NC. The number of subjects followed at each yearly interval for each disease group is shown in Figure 1A–E. The MMSE was administered to each subject at yearly intervals, and data for the first six years are displayed in Figure 2. As expected, AD and FTD patients showed greater cognitive decline than PD and MCI subjects.
Figure 1.
Number of subjects followed at each year of follow-up for each cohort.
Figure 2.
Mini Mental State Examination (MMSE) scores over time across neurologically normal and neurodegenerative disease states for the first 6 years of follow up. Data points reflect mean MMSE (± SEM) for all subjects evaluated at each time point. For sample size at each time point see Figure 1. NC=Neurologically normal control, PD=Parkinson’s disease, FTD=Frontotemporal dementia, MCI=Mild cognitive impairment, AD=Alzheimer’s disease.
TMEM106B genotype frequencies do not differ between disease cohorts and controls.
To investigate the association of TMEM106B genotype with disease status, Cochrane-Armitage tests of trend were performed between disease groups and NC. Genotype frequencies were in Hardy-Weinberg equilibrium and did not differ between NC and MCI, AD, PD, and FTD groups (Table 2).
Table 2.
TMEM106B rs1990622 genotype frequencies among neurologically normal controls (NC) and neurodegenerative disease groups. Reported as N (% of disease group). MAF = minor allele frequency. Results shown for association testing between TMEM106B rs1990622 genotype and disease state (relative to NC) by Cochran-Armitage trend tests. PD=Parkinson’s disease, FTD=Frontotemporal dementia, MCI=Mild cognitive impairment, AD=Alzheimer’s disease, ns=nonsignificant.
| NC | FTD | PD | MCI | AD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs1990622 | TT | 37(27) | 56(31) | ns | 54(30) | ns | 25(33) | ns | 87(29) | ns |
| TC | 65(47) | 89(50) | 92(51) | 35(47) | 147(49) | |||||
| CC | 35(26) | 34(19) | 33(19) | 15(20) | 66(22) | |||||
| MAF | 0.49 | 0.44 | 0.44 | 0.43 | 0.47 | |||||
TMEM106B genotype predicts rate of change in MMSE in PD and FTD
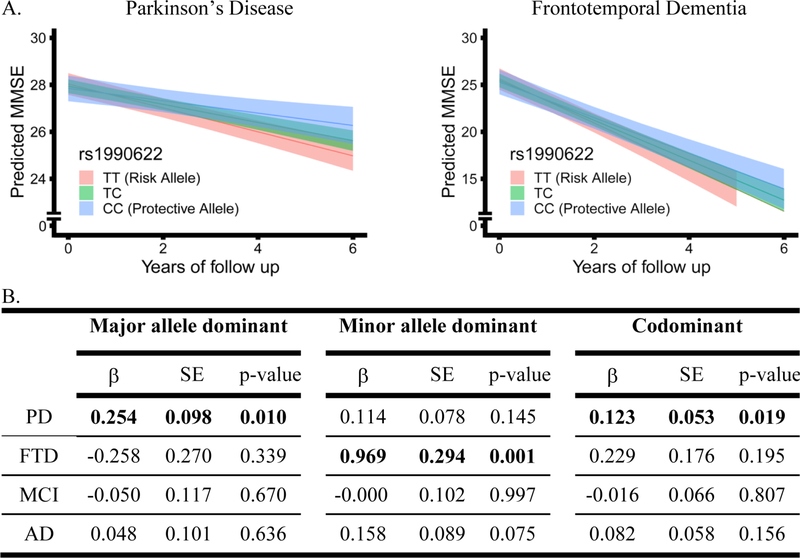
Linear mixed-effects models were used to assess the relationship between TMEM106B genotype and change in MMSE over time within each disease group, adjusting for age, sex, and baseline MMSE score (Figure 3). Education had no effect on MMSE over time in our cohort, and its inclusion as a covariate did not change results.
Figure 3.
Effects of TMEM106B genotype on longitudinal Mini Mental State Examination (MMSE) performance in each disease, assessed with linear mixed effects models adjusting for age, sex, and baseline MMSE. In panel A, genotypes are indicated by color, and bands represent 95% confidence intervals for PD and FTD in codominant models. In panel B, coefficients (β), standard error (SE), and p-values for TMEM106B genotype*time interaction shown, with time in years. PD=Parkinson’s disease, FTD=Frontotemporal dementia, MCI=Mild cognitive impairment, AD=Alzheimer’s disease.
The only clinical group in which the TMEM106B index SNP rs1990622 exerted a significant effect in codominant models was PD (β=0.123 p=0.019), with rs1990622T carriers declining more rapidly. In PD, rs1990622T also associated with more rapid cognitive decline under a major allele (rs1990622T)-dominant model (β=0.254 p= 0.010). This effect was specific to cognition in PD, as TMEM106B genotype did not associate with differences in rate of change in motor function measured by Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III, data not shown).
Among all clinical FTD patients, rs1990622 genotype associated with cognitive decline only under a minor allele (rs1990622C)-dominant model (β=0.969 p=0.001). However, in the bvFTD subgroup (n=95), rs1990622 genotype associated with cognitive decline under both minor-allele (β=1.322 p=0.001) and codominant (β=0.763 p=0.001) models. Since other clinical FTD subgroups had <30 cases each, we did not perform subgroup analyses for naPPA, lvPPA, svPPA, and nPPA. TMEM106B genotype did not associate with differences in rate of change in MMSE in AD or MCI subjects under any genetic model; NC subjects did not cognitively decline during the follow-up period (Figure 2). Covariates were assessed independently using linear mixed-effects models to examine their association with change in MMSE over time (Table 3). Across disease groups, 84% to 98% of patients self-reported their race as white. Repeating our analysis with just those individuals self-reporting as white did not change results (data not shown).
Table 3.
Effects of covariates on longitudinal cognitive testing performance in each disease, assessed with mixed linear effects models. Coefficients (β), standard error (SE), and p-values for covariate*time interaction shown, with time in years. Cognitive scale is Mini Mental State Examination (MMSE), except for PD-DRS-2 (Mattis Dementia Rating Scale −2) and PD-MoCA (Montreal Cognitive Assessment). Reported as beta coefficients with time in years.
| Age*time | Sex*time | Baseline cognitive test*time |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | |
| NC | −0.003 | 0.001 | 0.007 | −0.006 | 0.020 | 0.760 | −0.034 | 0.011 | 0.002 |
| FTD | −0.012 | 0.013 | 0.333 | 0.534 | 0.266 | 0.045 | −0.075 | 0.042 | 0.072 |
| PD | −0.029 | 0.005 | 0.000 | −0.057 | 0.098 | 0.558 | −0.023 | −0.192 | 0.223 |
| MCI | 0.012 | 0.005 | 0.027 | 0.063 | 0.096 | 0.514 | 0.097 | 0.024 | 0.000 |
| AD | 0.078 | 0.005 | 0.000 | 0.074 | 0.085 | 0.382 | 0.007 | 0.013 | 0.584 |
| PD-DRS-2 | −0.037 | 0.157 | 0.020 | −0.343 | 0.221 | 0.121 | 0.030 | 0.030 | 0.299 |
| PD-MoCA | −0.012 | 0.002 | 0.000 | −0.073 | 0.048 | 0.132 | −0.050 | 0.010 | 0.000 |
In PD, TMEM106B genotypes predict rates of cognitive decline across multiple cognitive tests
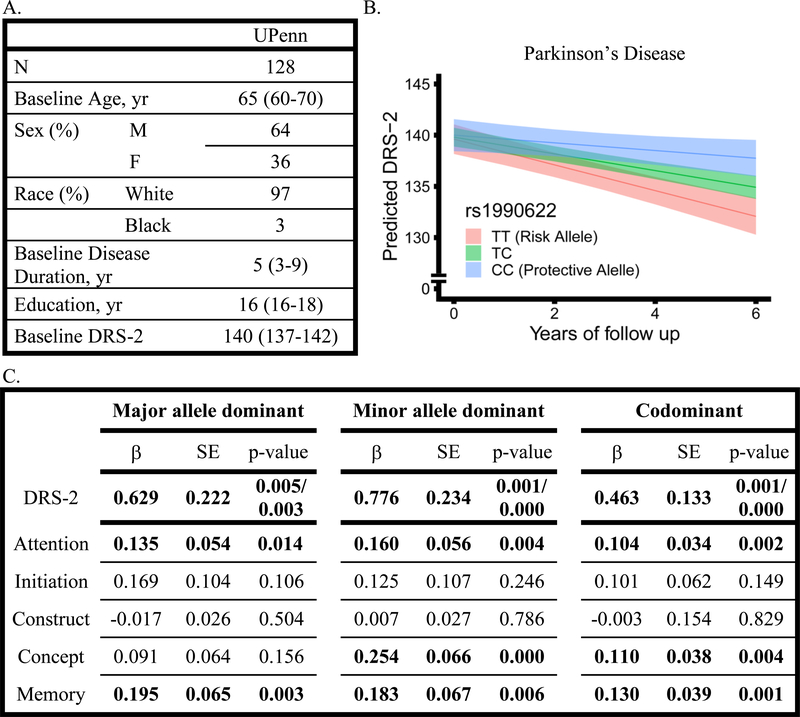
To confirm our findings of association between TMEM106B genotypes and cognitive decline in PD, we investigated 128 PD subjects followed at UPenn with the DRS-2, a detailed 144-point cognitive scale.20 This cohort was followed for up to 10.4 years with a median follow up time of 4.0 years (IQR 3.0–6.0). The number of subjects followed at each yearly interval for each disease group are shown in Figure 1F, and 36% (46 of 128) of this cohort overlaps with the individuals used in the MMSE analysis above. The median age at baseline was 65 years (IQR 60–70), baseline disease duration was 5 years (IQR 3–9), and individuals had a median of 16 years of education (IQR 16–18).
Regardless of genetic model used, rs1990622T was associated with faster rate of decline in DRS-2 (β=0.629 p=0.005, β=0.776 p=0.001, β=0.463 p=0.001 in major allele-dominant, minor allele-dominant, and codominant models, Figure 4). Best-fit models include random intercepts and slopes. Predicted values with 95% confidence interval in the codominant model are depicted in Figure 4B. Models were adjusted for age, sex, and baseline DRS score; inclusion of education as an additional covariate had no effect on our results. Covariates were assessed independently using linear mixed-effects models to examine their association with change in DRS-2 over time (Table 3).
Figure 4.
A. Demographics and baseline characteristics of the University of Pennsylvania cohort who were administered the Mattis Dementia Rating Scale-2 (DRS-2). All values represent median (IQR) unless otherwise specified. B and C. Effects of TMEM106B genotype on longitudinal DRS-2 total and cognitive domain performance in Parkinson’s disease patients, assessed with linear mixed effects models adjusting for age, sex, and baseline DRS-2 total or domain score. In panel B, predicted DRS-2 scores are shown by TMEM106B genotype, indicated by color, and bands represent 95% confidence intervals. In panel C, coefficients (β), standard error (SE), and p-values (two-sided/one-sided) for TMEM106B genotype*time interaction shown, with time in years.
We further examined the relationship between TMEM106B genotype and decline in DRS-2 scores in individual cognitive domains over time in the UPenn PD cohort. We used linear mixed-effects models adjusting for age, sex and baseline DRS-2 domain score. We found that rs1990622T was associated with a faster rate of domain-specific decline in attention (β=0.104 p=0.002), conceptualization (β=0.110 p=0.004), and memory (β=0.130 p=0.001) in codominant models (Figure 4C); results were minimally affected by changes in genetic model. We found no significant rs1990622T effects on initiation or construction domains. Thus, TMEM106B genotype effects on cognitive decline in PD extend across multiple cognitive domains.
TMEM106B genotype predicts rate of cognitive decline in an international PD cohort
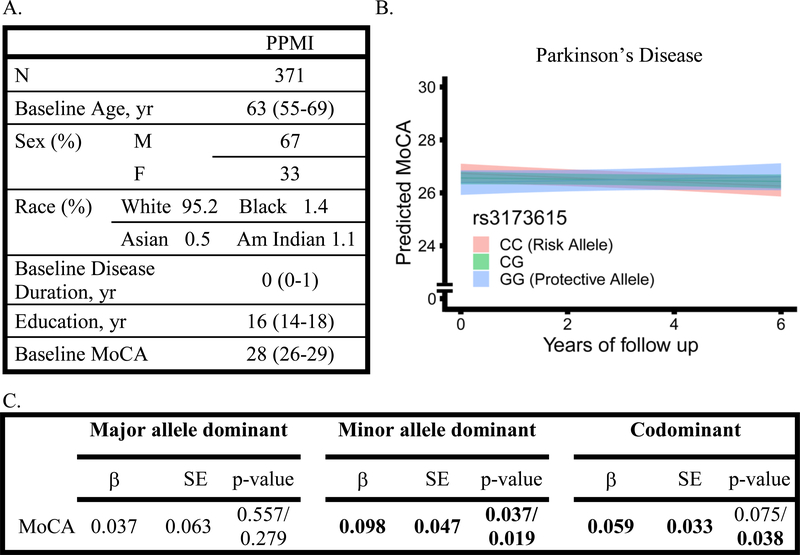
Having demonstrated that TMEM106B genotypes associate with rate of cognitive decline in PD irrespective of cognitive instrument used, we next tested our results for replication in an international PD cohort of early stage disease. The PPMI cohort enrolled newly-diagnosed PD patients from 33 clinical sites in 11 countries, with a median follow-up time of 5.1 years (IQR 4.4–6.1). Of 406 possible PD subjects from PPMI, one was omitted from the analysis for lack of follow-up data, three for baseline MoCA less than 20, and 31 for lack of genetic data, leaving 371 available for analysis. The number of subjects followed at each yearly interval for the PPMI cohort is shown in Figure 1G. The median age at baseline was 63 years (IQR 55–69), baseline disease duration was 0 years (IQR 0–1), with a median of 16 years of education (IQR 14–18).
In both codominant models and a minor-allele-dominant model the risk allele, rs3173615C, associated with faster rates of cognitive decline, (β=0.059, p=0.038 and β=0.098 p=0.019, Figure 5). Predicted values with 95% confidence interval in the codominant model are depicted in Figure 5B. Models were adjusted for age, sex, and baseline MoCA score, and include random intercepts and slopes; additionally, co-varying for education did not affect results. Covariates were assessed independently using linear mixed-effects models to examine their association with change in MoCA over time (Table 3).
Figure 5.
A. Demographics and baseline characteristics of the PPMI cohort who were administered the Montreal Cognitive Assessment (MoCA). All values are represented as median (IQR) unless otherwise specified. Race is not reported for N=7 subjects. B and C. Effects of TMEM106B genotype on longitudinal MoCA performance in Parkinson’s disease patients, assessed with linear mixed effects models adjusting for age, sex, and baseline MoCA. In panel B, predicted MoCA scores are shown by TMEM106B genotype, indicated by color, and bands represent 95% confidence intervals. In panel C, coefficients (β), standard error (SE), and p-values (two-sided/one-sided) for TMEM106B genotype*time interaction shown, with time in years.
DISCUSSION
We leveraged 870 longitudinally-followed AD, PD, FTD, MCI and NC subjects enrolled at a single center, as well as 371 longitudinally-followed PD subjects from a 33-site international cohort, in order to dissect the effect of common genetic variation in the lysosomal gene TMEM106B across the neurodegenerative disease spectrum. We found evidence for TMEM106B genetic modifier effects on longitudinal cognitive decline in FTD, the disease for which TMEM106B is known to confer susceptibility. Importantly, we also found a novel genetic modifier effect for TMEM106B in PD cognitive decline in both test and validation cohorts.
In 2010, common variants at the TMEM106B locus were linked by genome-wide association study (GWAS) with risk for frontotemporal lobar degeneration with TDP-43 proteinopathy (FTLD-TDP), the most common neuropathological substrate of the clinical disease FTD, an umbrella term encompassing both multiple neuropathological subtypes, and multiple clinical presentations.3 The rs1990622T allele demonstrated an odds ratio of ~1.6 for development of FTLD-TDP,3 and subsequent cross-sectional studies have investigated associations between the rs1990622T allele (or its proxies) and development of various diseases, with mixed results. Notably, while TMEM106B variants do not associate with risk for developing the TDP-43 proteinopathy ALS, they do associate with cognitive impairment among individuals with manifest ALS.10 Neuropathological studies have also implicated TMEM106B in the development of TDP-43 proteinopathy both for the neuropathological entities defined by TDP-43 proteinopathy and for those in which TDP-43 proteinopathy may be a secondary feature.3,25
We find here that TMEM106B variants that increase risk for FTLD-TDP may also associate with a faster rate of cognitive decline in clinically-diagnosed FTD patients, with positive findings in the bvFTD subgroup as well. While this result may seem intuitive, our study is the first to formally assess effects on cognitive change in a longitudinally-followed FTD cohort. In this context, we point out that other genetic variants associated with increased risk for developing a disease have been associated with both faster progression of that disease (e.g. APOE4 alleles in AD)26 and slower progression (e.g. LRRK2 G2019S in PD),27 underlining the importance of formal evaluation in longitudinal studies.
More surprising is our demonstration that TMEM106B variants that increase risk for FTLD-TDP also associate with faster rates of cognitive decline in PD. Our results are unlikely to be spurious, as they are found in both our UPenn cohort of established PD patients and the international PPMI cohort of newly-diagnosed PD patients. Moreover, we observe the same association between TMEM106B risk alleles and faster longitudinal cognitive decline whether we ascertain cognition by MMSE, DRS-2, or MoCA. While all three tests assess global cognition, domains of emphasis as well as psychometric properties for these scales do differ. Thus, the robustness of this association to differences in clinical site, stage of PD, cognitive instrument, and genetic model increases confidence in our finding. Indeed, the association of TMEM106B genotypes with longitudinal rates of cognitive decline is arguably more robust in PD than FTD in our study, since in the latter case, we only see significant associations under a minor allele-dominant genetic model.
Cortical TDP-43 proteinopathy is not a common neuropathological feature of PD, with <10% of Lewy body disease cases demonstrating neocortical TDP-43 proteinopathy in one neuropathological series.2 Thus, our finding raises the question of whether TMEM106B might exert effects on neurodegenerative disease processes through TDP-43-independent mechanisms. While few studies from the human genetics literature address this question, our biological understanding of TMEM106B as a protein localized to lysosomes,7 whose dysregulation can result in lysosomal and autolysosomal dysfunction,5 could certainly encompass both TDP-43-dependent and TDP-43-independent pathways. When viewed from the lens of those neurodegenerative diseases in which lysosomal dysfunction is most likely to play an important role, the fact that we observe TMEM106B genetic modifier effects in FTD and PD is less surprising. Indeed, FTD and PD share the fact that Mendelian genetic subgroups of patients are heterozygous carriers of mutations in genes (GBA in PD, GRN in FTD) whose homozygous loss results in a lysosomal storage disorder (Gaucher’s disease for GBA,28 neuronal ceroid lipofuscinosis for GRN).29
The ability to study multiple neurodegenerative diseases longitudinally, using shared clinical metrics and molecular characterization, positions our UPenn cohort well for analyses across the neurodegenerative spectrum, as evidenced by prior neuropathological studies.2 Despite this considerable strength, limitations of our work should be acknowledged. First, the fact that we did not find significant differences in TMEM106B allele frequencies for any of our disease groups, including FTD, compared to NC, is likely due to Type II error stemming from inadequate sample sizes for a disease association analysis. Our aim in this study was not to associate TMEM106B variants with disease class, which has been done previously, but rather to investigate effects on the longitudinal progression of disease, which has not been done. This goal guided our choice of a longitudinally-followed cohort of hundreds vs. a cross-sectional sample of thousands. Second, we chose to use one cognitive instrument – the MMSE – across multiple longitudinally-followed disease groups in the first part of this study in order to compare results across neurodegenerative diseases. However, we recognize that the use of a relatively simple cognitive instrument for this purpose may limit interpretation with respect to more domain-specific aspects of cognition.
Third, the lack of association between TMEM106B variants and longitudinal rates of cognitive decline in AD or MCI, as well as our failure to detect a significant association between TMEM106B genotype and cognitive decline in FTD under some genetic models, could be related to sample sizes. That is, a true effect may exist, which our study is underpowered to detect. This may be particularly salient for our MCI group, which only numbered 75 individuals. With respect to AD, however, we can state with some confidence that TMEM106B genetic modifier effects on cognition, if they exist, have smaller effect sizes than in PD and FTD, since the sample size of our AD group (N=300) was larger than either comparator group, with the extent of cognitive decline equivalent to that in FTD and greater than in PD. Fourth, follow-up times in our study were variable across individuals and across disease groups. However, the overall median follow-up time was 3.0 years, ranging from 2.2 years in PD and FTD to 4.7 years in NC, suggesting that follow-up periods are adequate for detecting cognitive decline. In addition, our use of linear mixed-effects models for analysis optimizes the use of data with variable follow-up times. Fifth, our patient cohorts ranged from 84% (in MCI) to 98% (in FTD) white, by self-report. Thus, we could not address the question of whether TMEM106B effects on cognition generalize across multiple racial groups. Finally, we chose throughout our analyses to present nominal p-values for associations, as many analyses (e.g., considerations of major allele-dominant vs. minor allele-dominant vs. codominant genetic model; investigations of different, but related, cognitive tests in PD) are not independent, making the problem of how many hypotheses for which to correct prone to arbitrary answers. We note, however, that after Bonferroni correction for four different disease groups (PD, FTD, MCI, and AD) in the MMSE analysis, significant associations between TMEM106B genotype and longitudinal rate of change persist in the PD and FTD groups. Moreover, the association between TMEM106B genotype and longitudinal cognitive decline in PD is corroborated by multiple additional analyses.
Limitations notwithstanding, our study newly identifies TMEM106B as a genetic modifier of cognitive decline in PD. More generally, it exemplifies a cross-disease approach that can delineate disease-specific vs. common features of AD, PD, FTD, and ALS, important for both diagnostic and therapeutic development purposes. Indeed, our current findings extend the potential scope of benefit for therapeutic strategies targeting the lysosomal protein TMEM106B beyond FTD to PD. Since long-term cognitive decline in PD is highly prevalent and disabling,30 and both symptomatic and disease-modifying therapies for this indication are largely lacking, finding new therapeutic targets for PD cognition is a top priority.
Acknowledgements:
The authors would like to acknowledge our patients for their generous participation in this study, Travis Unger for technical support, and the clinical research associates at the University of Pennsylvania. Data used in the preparation of this article were obtained from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. This research was supported by the NIH-NINDS (R01 NS082265, P50 NS06284, T32 NS091008), Institute for Translational Medicine and Therapeutics of the Perelman School of Medicine at the University of Pennsylvania, Benaroya Fund. ACP is additionally supported by the Parker Family Chair.
Footnotes
Conflicts of Interest: Authors do not report any known conflicts of interest related to this study.
References
- 1.Arnold SE, Toledo JB, Appleby DH, et al. Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. [Internet]. J. Comp. Neurol 2013;521(18):4339–55.[cited 2018 Sep 29] Available from: http://doi.wiley.com/10.1002/cne.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated [Internet]. Brain 2018;141(7):2181–2193.[cited 2018 Sep 29] Available from: https://academic.oup.com/brain/article/141/7/2181/5033683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Deerlin VM, Sleiman PMA, Martinez-Lage M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions [Internet]. Nat. Genet 2010;42(3):234–239.[cited 2018 Sep 28] Available from: http://www.nature.com/articles/ng.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher MD, Posavi M, Huang P, et al. A Dementia-Associated Risk Variant near TMEM106B Alters Chromatin Architecture and Gene Expression. [Internet]. Am. J. Hum. Genet 2017;101(5):643–663.[cited 2018 Oct 9] Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002929717303713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen-Plotkin AS, Unger TL, Gallagher MD, et al. TMEM106B, the Risk Gene for Frontotemporal Dementia, Is Regulated by the microRNA-132/212 Cluster and Affects Progranulin Pathways [Internet]. J. Neurosci 2012;32(33):11213–11227.Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0521-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady OA, Zheng Y, Murphy K, et al. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function [Internet]. Hum. Mol. Genet 2013;22(4):685–695.[cited 2018 Oct 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23136129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch JI, Unger TL, Jain N, et al. Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes. Hum. Mol. Genet 2016;25(13):2681–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch N, Carrasquillo MM, Baker M, et al. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers [Internet]. Neurology 2011;76(5):467–474.[cited 2018 Sep 30] Available from: http://www.neurology.org/cgi/doi/10.1212/WNL.0b013e31820a0e3b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher MD, Suh E, Grossman M, et al. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions [Internet]. Acta Neuropathol. 2014;127(3):407–418.[cited 2018 Sep 28] Available from: http://link.springer.com/10.1007/s00401-013-1239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vass R, Ashbridge E, Geser F, et al. Risk genotypes at TMEM106B are associated with cognitive impairment in amyotrophic lateral sclerosis. [Internet]. Acta Neuropathol. 2011;121(3):373–80.[cited 2018 Sep 29] Available from: http://link.springer.com/10.1007/s00401-010-0782-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutherford NJ, Carrasquillo MM, Li M, et al. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease [Internet]. Neurology 2012;79(7):717–718.[cited 2018 Sep 29] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22855871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias [Internet]. Acta Neuropathol. 2014;128(3):411–421.[cited 2018 Sep 29] Available from: http://www.ncbi.nlm.nih.gov/pubmed/24899141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki N, Murray ME, Ogaki K, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A [Internet]. Acta Neuropathol. 2015;129(1):53–64.[cited 2018 Sep 29] Available from: http://www.ncbi.nlm.nih.gov/pubmed/25367383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhinn H, Abeliovich A. Differential Aging Analysis in Human Cerebral Cortex Identifies Variants in TMEM106B and GRN that Regulate Aging Phenotypes. [Internet]. Cell Syst. 2017;4(4):404–415.e5.[cited 2018 Oct 9] Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405471217300534 [DOI] [PubMed] [Google Scholar]
- 15.Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank [Internet]. Alzheimer’s Dement. 2014;10(4):477–484.e1.[cited 2018 Sep 28] Available from: https://linkinghub.elsevier.com/retrieve/pii/S1552526013024679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marek K, Jennings D, Lasch S, et al. The Parkinson Progression Marker Initiative (PPMI) [Internet]. Prog. Neurobiol. 2011;95(4):629–635.[cited 2018 Sep 28] Available from: https://www.sciencedirect.com/science/article/pii/S0301008211001651?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tombaugh TN, Mclntyre NJ. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 18.Skorvanek M, Goldman JG, Jahanshahi M, et al. Global scales for cognitive screening in Parkinson’s disease: Critique and recommendations. Mov. Disord. 2018;33(2):208–218. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. [Internet]. J. Psychiatr. Res 1975;12(3):189–98.[cited 2018 Sep 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/1202204 [DOI] [PubMed] [Google Scholar]
- 20.Lucas JA, Smith GE, Bohac DL, et al. Normative Data for the Mattis Dementia Rating Scale [Internet]. J. Clin. Exp. Neuropsychol. (Neuropsychology, Dev. Cogn. Sect. A) 1998;20(4):536–547.[cited 2016 Dec 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/9892057 [DOI] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment [Internet]. J. Am. Geriatr. Soc 2005;53(4):695–699.[cited 2018 Sep 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15817019 [DOI] [PubMed] [Google Scholar]
- 22.Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale. In: Recent developments in Parkinson’s disease. 1987. p. 153–63. [Google Scholar]
- 23.Nalls MA, Keller MF, Hernandez DG, et al. Baseline genetic associations in the Parkinson’s Progression Markers Initiative (PPMI) [Internet]. Mov. Disord. 2016;31(1):79–85.[cited 2018 Sep 28] Available from: http://doi.wiley.com/10.1002/mds.26374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laird NM, Ware JH. Random-effects models for longitudinal data. [Internet]. Biometrics 1982;38(4):963–74.[cited 2016 Dec 28] Available from: http://www.ncbi.nlm.nih.gov/pubmed/7168798 [PubMed] [Google Scholar]
- 25.Yu L, De Jager PL, Yang J, et al. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 2015;84(9):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eldholm RS, Barca ML, Persson K, et al. Progression of Alzheimer’s Disease: A Longitudinal Study in Norwegian Memory Clinics [Internet]. J. Alzheimer’s Dis. 2018;61(3):1221–1232.[cited 2018 Oct 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/29254085 [DOI] [PubMed] [Google Scholar]
- 27.Saunders-Pullman R, Mirelman A, Alcalay RN, et al. Progression in the LRRK2 -Associated Parkinson Disease Population [Internet]. JAMA Neurol. 2018;75(3):312[cited 2018 Oct 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/29309488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jmoudiak M, Futerman AH. Gaucher disease: Pathological mechanisms and modern management. Br. J. Haematol 2005;129(2):178–188. [DOI] [PubMed] [Google Scholar]
- 29.Smith KR, Damiano J, Franceschetti S, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage [Internet]. Am. J. Hum. Genet 2012;90(6):1102–1107.Available from: 10.1016/j.ajhg.2012.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hely MA, Reid WGJ, Adena MA, et al. The Sydney Multicenter Study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord 2008;23(6):837–844. [DOI] [PubMed] [Google Scholar]