Abstract
Background
Competitions might encourage people to undertake and/or reinforce behaviour change, including smoking cessation. Competitions involve individuals or groups having the opportunity to win a prize following successful cessation, either through direct competition or by entry into a lottery or raffle.
Objectives
To determine whether competitions lead to higher long‐term smoking quit rates. We also aimed to examine the impact on the population, the costs, and the unintended consequences of smoking cessation competitions.
Search methods
This review has merged two previous Cochrane reviews. Here we include studies testing competitions from the reviews ‘Competitions and incentives for smoking cessation' and ‘Quit & Win interventions for smoking cessation'. We updated the evidence by searching the Cochrane Tobacco Addiction Group Specialized Register in June 2018.
Selection criteria
We considered randomized controlled trials (RCTs), allocating individuals, workplaces, groups within workplaces, or communities to experimental or control conditions. We also considered controlled studies with baseline and post‐intervention measures in which participants were assigned to interventions by the investigators. Participants were smokers, of any age and gender, in any setting. Eligible interventions were contests, competitions, lotteries, and raffles, to reward cessation and continuous abstinence in smoking cessation programmes.
Data collection and analysis
For this update, data from new studies were extracted independently by two review authors. The primary outcome measure was abstinence from smoking at least six months from the start of the intervention. We performed meta‐analyses to pool study effects where suitable data were available and where the effect of the competition component could be separated from that of other intervention components, and report other findings narratively.
Main results
Twenty studies met our inclusion criteria. Five investigated performance‐based reward, where groups of smokers competed against each other to win a prize (N = 915). The remaining 15 used performance‐based eligibility, where cessation resulted in entry into a prize draw (N = 10,580). Five of these used Quit & Win contests (N = 4282), of which three were population‐level interventions. Fourteen studies were RCTs, and the remainder quasi‐randomized or controlled trials. Six had suitable abstinence data for a meta‐analysis, which did not show evidence of effectiveness of performance‐based eligibility interventions (risk ratio (RR) 1.16, 95% confidence interval (CI) 0.77 to 1.74, N = 3201, I2 = 57%). No trials that used performance‐based rewards found a beneficial effect of the intervention on long‐term quit rates.
The three population‐level Quit & Win studies found higher smoking cessation rates in the intervention group (4% to 16.9%) than the control group at long‐term follow‐up, but none were RCTs and all had important between‐group differences in baseline characteristics. These studies suggested that fewer than one in 500 smokers would quit because of the contest.
Reported unintended consequences in all sets of studies generally related to discrepancies between self‐reported smoking status and biochemically‐verified smoking status. More serious adverse events were not attributed to the competition intervention.
Using the GRADE system we rated the overall quality of the evidence for smoking cessation as ‘very low', because of the high and unclear risk of bias associated with the included studies, substantial clinical and methodological heterogeneity, and the limited population investigated.
Authors' conclusions
At present, it is impossible to draw any firm conclusions about the effectiveness, or a lack of it, of smoking cessation competitions. This is due to a lack of well‐designed comparative studies. Smoking cessation competitions have not been shown to enhance long‐term cessation rates. The limited evidence suggesting that population‐based Quit & Win contests at local and regional level might deliver quit rates above baseline community rates has not been tested adequately using rigorous study designs. It is also unclear whether the value or frequency of possible cash reward schedules influence the success of competitions. Future studies should be designed to compensate for the substantial biases in the current evidence base.
Plain language summary
Do competitions help smokers to quit in the medium to long term?
Background
In competitions designed to help people to quit smoking, participants are encouraged to quit while also having the chance to win a reward if they are successful in doing so. Some contests allow groups of smokers to compete against each other directly, with the group in which the most smokers quit winning a prize. Alternatively, a person who quits smoking might be entered into a lottery to win a prize, such as cash payments, vouchers, salary bonuses, promotional items (t‐shirts, pens or bags), holidays, or luxury goods (cars or boats). A particular type of stop smoking competition called the ‘Quit & Win' contest took place internationally until 2006.
Study characteristics
This review has merged studies from two previous reviews. One of the reviews was of competitions and incentives for quitting smoking. The studies that investigated incentives are now in a separate review. Here we include the studies which investigated competitions, alongside the studies originally included in our review of Quit & Win contests. We also searched for more recent relevant studies that were published up to June 2018. We include 20 studies of more than 11,000 participants that investigated competitions to encourage people to quit smoking. In five of these studies, groups of smokers recruited from workplaces competed directly against each other. In the other 15 studies, successful quitters were entered into prize draws.
Key results and the quality of the evidence
None of the studies in which groups of smokers competed against each other directly found that more people quit than in similar groups of smokers who were not entered into a competition. Combining the results of randomized controlled trials of lottery‐type competitions, which provide the best evidence, did not show evidence that competitions increase rates of quitting smoking. Three Quit & Win contests did find that people who were in the contest had higher quit rates than people in a comparison community, who did not take part. However, these studies were of low quality and appeared to have very little effect on the overall smoking rates in the community, as fewer than one in 500 smokers appeared to quit because of the Quit & Win contest.
Fourteen of the 20 studies included were randomized controlled trials, but many of these did not describe their methods well enough for us to decide whether they were of high quality. Overall, we judged the quality of the evidence included in this review to be very low, so we can draw no strong conclusions from the findings. It is important that any future research in this area is designed to be of high quality and is reported in detail, so that we can increase the confidence we have in our findings.
Summary of findings
Summary of findings for the main comparison. Effects of smoking cessation competitions on smoking abstinence.
| Effects of smoking cessation competitions on smoking abstinence | |||||
|
Patient or population: tobacco smokers Settings: any Intervention: smoking cessation competitions Comparison: no intervention or non‐competition based smoking cessation intervention Outcome: long‐term smoking abstinence (six month+ follow‐up) | |||||
| Outcomes | Illustrative comparative cessation rates* (95% CI) | Risk Ratio (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed cessation rate | Corresponding cessation rate | ||||
| Control | Intervention | ||||
| Smoking cessation: performance‐based eligibility competitions versus alternative cessation intervention | 9.3% | 10.8% (7.2%, 16.2%) | 1.16 (0.77, 1.74) | 3201 (6 RCTs)** | ⊕⊝⊝⊝ VERY LOW1,2,3 |
| Smoking cessation: performance‐based reward competitions versus alternative cessation intervention | Unable to estimate effectiveness of intervention. None of the included studies reported meaningful differences in the quit rates reported in the intervention and control groups. | 915 (2 RCTs; 3 CTs) | ⊕⊝⊝⊝ VERY LOW1,2,4 |
||
| *The assumed risk is based on the overall control cessation rate in the six included studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the control group and the relative effect of the intervention (and its 95% CI). ** Additionally, the 3 CTs (2000 participants) of population Quit & Win competitions found differences in the one‐year quit rates of the experimental and control participants. RCT: randomized controlled trial; CT: controlled trial, CI: confidence interval | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1 Downgraded one level due to high risks of bias identified across studies: the majority of studies had high or unclear risk of bias in at least one of the domains assessed.
2 Downgraded one level for inconsistency: substantial methodological and clinical heterogeneity that could not be accounted for in analyses.
3 Downgraded one level for imprecision: the number of studies is limited and quit rates vary across these. The reason for this is not obvious, which limits our confidence in the existence or strength of an effect.
4 Downgraded one level due to indirectness: competitions were only assessed in participants recruited from their workplace, so can only be applied to this limited population.
Background
Description of the condition
Tobacco use kills more than seven million people a year worldwide (WHO 2018). As a result, many smokers would like to quit. It is important that services are provided to help them to do so and to remove some of the burden on health services (WHO 2017).
Description of the intervention
Competitions and incentives routinely feature in many smoking cessation programmes, as a way to support the quitting process. Although they are similar interventions, incentives work differently from competitions by providing participants with guaranteed prespecified rewards for participating and/or successfully quitting, and are covered by a companion review (Cahill 2015). This review focuses on competitions or contests (used interchangeably), defined as interventions that offer individual participants or groups a chance, but not a guarantee, of winning a particular reward if they successfully quit. A variety of rewards has been used for these purposes, including cash payments, vouchers, salary bonuses, promotional items such as T‐shirts, pens and bags, holidays, and luxury goods such as cars or boats.
This review is a modified version of our previous review Competitions and incentives for smoking cessation (Cahill 2011). As the emphasis in public health has shifted in recent years away from cessation competitions, and towards contingent and non‐contingent incentive programmes, we have now split the review into two. In doing so we have also merged this review with another previous review,Quit and win contests for smoking cessation (Cahill 2008b).
Studies of competition interventions for smoking cessation fall into two broad categories.
1) Studies in which groups of participants competing directly against each other, with the team or teams achieving the best outcome (such as the highest proportion of participants stopping smoking) winning a reward, either given to their workplace or shared among the participating individuals. These studies, which typically pitch different workplaces in competition with each other, we term ‘performance‐based reward'.
2) Studies in which participants who quit smoking are entered into chance‐based competition such as a prize draw, lottery or raffle. Usually participants compete on an individual basis, and so prizes are not guaranteed for successful quitters. We term this category ‘performance‐based eligibility'.
Although many variations of the performance‐based eligibility design have been adopted, the one that has received the most widespread and prolonged attention internationally is the Quit & Win contest. This smoking cessation competition was first developed by the Minnesota Heart Health Program in the early 1980s, using mass media and posters and brochures distributed to schools, workplaces and medical facilities (Lando 1994; Pechacek 1994). Participants competed individually, and those who stopped smoking were eligible to be entered into a prize draw. For example, in the first iteration of the contest, participants who achieved biochemically‐validated cessation at one month post‐programme were entered into a raffle to win a holiday to Disneyworld. A validated quit rate of 32% was achieved at one month, although 16% of those originally claiming to have quit did not turn out to be smoke‐free, and relapse rates at 12 months were high. The programme became the model for many recruitment and cessation campaigns, particularly in the USA (O'Connor 2006).
Quit & Win competitions have since been extended worldwide. In 1994, the first international Quit & Win contest was conducted under the auspices of the World Health Organization, with 13 countries participating. The contest ran every two years, growing rapidly so that 84 countries and 700,000 smokers participated in the final contest in 2006 (WHO 2007). Estimated quit rates at one‐year follow‐up showed great variation across countries, with year 2000 continuous abstinence rates reported to range from 5% in Argentina to 44% in regions of China (Sandström 2002). The 2006 contest awarded a grand prize of US$10,000 and regional prizes of US$2,500 each, drawn from among the national winners (Malta Independent 2006). More recently, a series of annual ‘Quit to Win' contests began in Hong Kong in 2009. Although the success of these contests has not been evaluated in controlled trials, they have acted as recruitment tools for add‐on studies of other smoking cessation interventions (Chan 2012; Cheung 2013; Wang 2014; Wang 2015; Wang 2016; Wang 2017; Wang 2018).
How the intervention might work
The mechanisms by which competitions might influence behaviour change are likely to be complex and multifaceted. Incentivization, in a broad sense, could contribute to cessation efforts by (1) increasing or improving motivations to quit; (2) increasing or improving action to quit; and/or (3) increasing or improving maintenance of an effort to quit (Leeks 2010). The available evidence about incentives for smoking cessation suggests that they may be effective, although the extent of their success is likely to depend on the amount and nature of the incentive offered (Cahill 2015; Halpern 2015; Volpp 2009). The primary mechanism may be via the instigation of a quit attempt, which does not necessarily lead to long‐term behaviour change (Aveyard 2011).
A key component of the rationale behind the Quit & Win model was that the possibility of winning a large prize could offset the discomforts of quitting, and could attract large numbers of smokers to make the attempt. This must be interpreted in the context of ‘temporal discounting' ‐ that most individuals value immediate rewards more highly than the prospect of future rewards ‐ which has been observed widely in relation to both financial gain and substance addiction (Bickel 2007). In the case of competition interventions, an additional consideration is that the prize or its value is not guaranteed, which might plausibly serve to either reduce motivation to quit via the uncertain nature of the reward, or conversely to increase motivation provided the prize offered is sufficiently large. There is some evidence that individuals may prefer the chance of winning a larger, non‐guaranteed reward to a smaller, guaranteed reward when the required behaviour change is difficult or time‐consuming, so as to have a chance of receiving a prize commensurate with the effort expended (Haisley 2008; Kivetz 2003). They may also place disproportionate utility on high rewards, such as might be offered in a lottery, when the chance of winning is low (Stuart 2016).
Research in this field has increasingly turned from a single prize at the end of a programme towards multiple incentives over time being available as part of contingency management for smoking cessation. This approach aligns with longstanding research on operant conditioning for behaviour change, as reviewed by Donatelle 2004 and Higgins 2012a. Again, whether this approach is beneficial when the rewards offered are not guaranteed even for participants who achieve ongoing cessation is not clear.
Any enhanced participation rate that competitions may deliver should be weighed against the stability of the long‐term quit rates that are achieved. This review uses longest follow‐up as the primary outcome, with an inclusion criterion of a minimum of six months of follow‐up. This is important for competition interventions to ensure that cessation is maintained beyond the period of the competition, a concern which has been raised in studies of incentive interventions (Gneezy 2011). The possibility that incentivization might reduce intrinsic motivation to achieve behaviour change has also been considered, although little evidence has been put forward either to support or to refute this definitively for smoking cessation (Promberger 2013).
A possible negative consequence of competition interventions is that they may lead to increased rates of deception, either by participants falsely claiming to be abstinent, or by non‐smokers taking part and then claiming to have quit. This was a major concern in earlier population‐level contests in relation to the accuracy of the biochemical validation used (Chapman 1994). In addition, individuals who elect to take part in a cessation programme that offers material rewards may be differently motivated from those who sign up to more conventional cessation methods, and this may be reflected in differential relapse rates. The Minnesota Heart Health Program, for example, has demonstrated that achieved quit rates cannot be assessed in isolation from community participation rates (Lando 1990). For population‐level competition programmes, there is likely to be an interdependence between participation and cessation rates in any assessment of the success of the programme, for example, in populations where the participation rate is low, those who do choose to participate may be those who are more motivated to quit. For this reason we propose to include an assessment of population impact to evaluate these studies, where the data will support it.
Why it is important to do this review
The use of competition prizes may increase the costs of running a smoking cessation programme, although this may be offset by savings in the delivery of the intervention itself compared to programmes that use counselling, for example. Therefore, it is important that the outlay is justified by the benefits that the intervention delivers. Conversely, competitions may target large numbers of smokers as part of a single programme, and so the potential population benefit is large. It is necessary to quantify how much, if at all, competitions enhance long‐term quit rates.
Objectives
To assess the effects of competitions as aids to smoking cessation. We aimed to address the following questions.
Do competitions, contests and prizes affect smoking cessation rates?
Does the amount and type of prize affect cessation?
What is the population impact of population‐based smoking cessation competitions?
Does the amount and type of prize affect the population impact of population‐based competitions?
What are the cost implications, to employers and to the community, of competitions?
Are there unintended consequences arising from the use of competitions, such as false claims or ineligible applicants?
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) allocating individuals, communities, workplaces or groups within workplaces to intervention or to control conditions.
We also included non‐randomized controlled trials that assessed post‐intervention outcomes, provided allocation to at least one of the study groups was assigned by the investigators. This is consistent with previous versions of the review and acknowledges the difficulty in conducting formally randomized trials for certain types of competition intervention. Purely observational studies are excluded.
Types of participants
Smokers, of either gender, in any setting. In this review update, trials conducted in adolescent smokers and those conducted in pregnant smokers, as described in other Cochrane Reviews (Fanshawe 2017 and Chamberlain 2013, respectively) are eligible for inclusion.
Types of interventions
Contests, competitions, lotteries, and raffles, including population‐based Quit & Win contests at local, national and international levels, to reward cessation or continuous abstinence in smoking cessation programmes. Studies that offered prizes or rewards for participation only, and not for cessation, are excluded. Interventions are required to include a chance element, rather than a guarantee of a specific reward for achieving cessation. Both studies in which the competition comprises the entire intervention and those in which the competition is offered alongside other intervention components, such as counselling, are included. We have not included reports of the effectiveness of rewards to healthcare workers for the delivery of smoking cessation interventions. We have also excluded reimbursement to patients for smoking cessation treatment costs, as these are covered in another Cochrane Review (van den Brand 2017).
Types of outcome measures
Primary outcomes
Our primary outcome is smoking cessation rate at longest follow‐up, including point prevalence, prolonged and/or continuous abstinence, for a minimum of six months from the start of the intervention, whether or not biochemically validated (Hughes 2003). The gold standard is biochemically‐verified continuous abstinence for at least six months. Trials which did not report cessation rates and those with shorter follow‐up are excluded.
Secondary outcomes
Population impact, calculated as participation rate × cessation rate due to intervention, where data were available (for trials of population‐based interventions only)
Costs of the intervention
Any unintended consequences of the intervention
Search methods for identification of studies
We searched the Cochrane Tobacco Addiction Group Specialized Register, which includes studies identified by systematic electronic searches of multiple databases, handsearching of specialist journals, and ‘grey' literature (conference proceedings and unpublished reports not normally covered by most electronic indexing systems). In addition, we used specifically developed strategies to search two clinical trials registries, ClinicalTrials.gov, and the ICTRP. Search terms included incentive*, competition*, contest*, lotter*, reward*, prize*, contingent payment*, deposit contract*, quit and win, quit to win. The most recent searches were performed on 19 June 2018. Studies performed in adolescents and pregnant women had been excluded from previous versions of this review. We therefore also searched the reference lists of the two relevant Cochrane Reviews (Fanshawe 2017 and Chamberlain 2013) that cover interventions for these specific groups for earlier studies that may now be suitable for inclusion. Likewise, we checked the reference list of Cahill 2015 for interventions containing competitions with non‐guaranteed prizes that may previously have been classified as incentives.
Data collection and analysis
Selection of studies
In this update, two review authors (TRF & JHB) screened all search results (abstracts) for possible inclusion. The same review authors independently assessed relevant studies for inclusion and resolved discrepancies by consensus. The other review authors were available to resolve any persistent disagreements. We noted reasons for the non‐inclusion of studies assessed at the full‐text stage.
Data extraction and management
For this update, all data extraction was conducted in duplicate. For previous versions of this review, one review author extracted data, and the second review author checked them. The other review authors were available to resolve any persistent disagreements. The following information was extracted for each eligible study:
Report citations
Setting and location
Details of randomization (whether randomized, unit of randomization, allocation concealment method)
Details of any blinding
Number of participants and allocations
Participant characteristics and baseline equivalence across study groups
Intervention details, including method of potential reward
Outcomes
Attrition and loss to follow‐up
Assessment of risk of bias in included studies
Two review authors (TRF & JHB) assessed each study according to the presence and quality of the randomization process including concealment of allocation (selection bias), whether or not outcome assessors were ‘blinded' (detection bias), the description and level of withdrawals and dropouts (attrition bias), and other potential sources of bias, such as whether the analysis was appropriate to the study design and whether group‐level outcome data were reported in sufficient detail. We did not assess performance bias, as the behavioural nature of the interventions assessed meant that blinding of participants and trialists was impossible. We assigned a grade (low, high, or unclear) for risk of bias for each of the domains. We resolved any disagreements through discussion with another review author (NL).
Measures of treatment effect
We analyzed dichotomous data by calculating the risk ratio (RR), using the longest follow‐up data reported. For cessation, we calculated the RR as (number of events in intervention condition/intervention denominator)/(number of events in control condition/control denominator) with a 95% confidence interval (CI). For studies with more than two groups, we used the group comparison that best estimated the effect of the competition intervention component, for example competition plus counselling versus counselling alone in the case of a study that provided additional behavioural support for some groups.
The population impact of a given intervention was measured for each study that used a population‐based intervention, where possible. This is calculated by multiplying the achieved quit rate by the percentage of smokers who participated in the contest, and allows a comparison between different events in different communities.
Costs and unintended consequences of the interventions were extracted and summarized narratively.
Unit of analysis issues
Where studies were cluster‐randomized, we assessed the appropriateness of analyses in the associated reports. If clustering had not been accounted for and no intraclass correlation coefficient (ICC) was reported, we applied the estimated ICC reported by Martinson 1999 (ICC for percentage quit smoking, 0.01049) to obtain an adjusted estimate of the effect size.
Dealing with missing data
Wherever possible, and whether or not the trialists themselves used this approach, we have used an intention‐to‐treat (ITT) analysis, using the number of participants originally randomized to intervention or control as the denominator. For smoking cessation, we treated participants with missing data as still smoking, as is standard in the field. For some studies this approach may have led to the quit rates reported in this review differing from those presented in the study reports.
Assessment of heterogeneity
We assessed the clinical and methodological diversity between studies to guide our decision as to whether data should be pooled in the first instance. We also assessed statistical heterogeneity using the Chi2 test and the I2 statistic. An I2 > 50% was deemed to indicate substantial heterogeneity.
Assessment of reporting biases
Where possible we planned to assess reporting bias using funnel plots, where 10 or more RCTs contributed to an outcome. There are currently insufficient studies in this review to support this approach. We searched trial registries to identify unpublished studies.
Data synthesis
The method of synthesizing the studies depended on the type, quality, design and heterogeneity of studies identified. We planned to perform meta‐analyses of RCTs within each of the two main categories of studies that competition interventions fall into (performance‐based reward and performance‐based eligibility, as described in Description of the intervention). Where possible, we combined eligible RCTs of competitions for smoking cessation with RR as the effect size measure. A random‐effects model was used because of variation in the nature of the interventions and the value of prizes available. We discuss narratively the results of non‐randomized studies and studies that did not present sufficient data to enter in meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Within performance‐based eligibility studies, we aimed to perform subgroup analyses of studies conducted in pregnant women, studies conducted in adolescents, and studies that used Quit & Win competitions, although we decided the latter was unfeasible because the Quit & Win studies were highly clinically heterogeneous and in most cases not RCTs. We also considered grouping studies dependent on other common features of the competitions tested, such as the amount and type of prize offered; however we decided that the studies in this review were insufficient to support this approach.
Sensitivity analysis
We planned to carry out a sensitivity analysis based on the assessed risk of bias of studies included in meta‐analyses. However there are currently insufficient high‐quality studies in the review to support this approach.
'Summary of findings' table
Following standard Cochrane methodology, we created a ‘Summary of findings' table for the primary smoking cessation outcome. We did this separately for the studies of performance‐based reward and performance‐based eligibility. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome, and to draw conclusions about the quality of evidence.
Results
Description of studies
Results of the search
The most recent updated search returned 185 records. After screening the titles and abstracts for eligibility, we obtained the full text of 16 records, reporting 12 studies. We judged two of these studies as eligible for inclusion and identified one ongoing study that may be eligible but is not yet completed. As a result of checking the list of studies in other reviews in relation to the modified inclusion criteria of this review (to include studies in pregnant and young people), we identified four additional eligible studies and one ongoing study from Chamberlain 2013 and no studies from Fanshawe 2017. Hawk 2006 and Lando 1991a were included in previous versions of this review but are now excluded (see Differences between protocol and review). The search of trial registries returned 76 records, all of which were excluded at title and abstract screening except for two that were duplicates of existing publications and seven that were excluded at full‐text review. See Figure 1 for more information.
1.
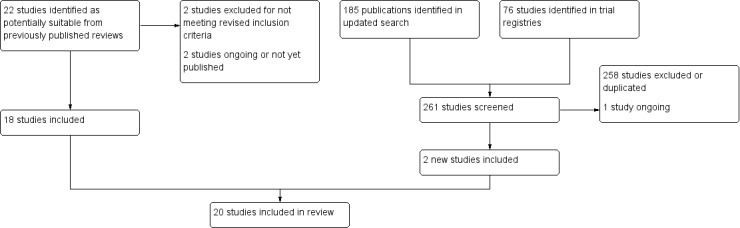
Flow diagram.
Included studies
This update includes 20 studies that met our inclusion criteria (18 from previous reviews, and two new studies). Five were studies of performance‐based reward, in which groups of participants, usually from different workplaces, competed directly against each other (Gomel 1993; Klesges 1986; Klesges 1987; Koffman 1998; Maheu 1989; N = 915). Fifteen studies used performance‐based eligibility, such that cessation resulted in entry into a lottery, raffle or prize draw (N = 10,580). Five of these studies were of Quit & Win contests (Bains 2000; Hahn 2005; McAlister 2000; Parker 2007; Thomas 2016; N = 4282), and the remaining 10 used other prize draw interventions or intervention components (Alessi 2014; Alessi 2017; Crowley 1995; Glasgow 1993; Hennrikus 2002; Ledgerwood 2014; Lillington 1995; Sexton 1984; Walsh 1997; Winhusen 2014; N = 6298). All but six studies (Bains 2000; Hahn 2005; Klesges 1986; Koffman 1998; Maheu 1989; McAlister 2000) were RCTs. These six were all ‘quasi‐randomized' or controlled trials without random allocation.
Worksite competition settings included ambulance stations; banks/saving and loan companies; manufacturing; the aerospace industry; and, in one study, a variety of work settings (Klesges 1987). The Quit & Win contests of Bains 2000, Hahn 2005 and McAlister 2000 recruited from the community. The remaining studies recruited from clinics or healthcare settings, including those for prenatal care (Alessi 2014; Crowley 1995; Ledgerwood 2014; Lillington 1995; Parker 2007; Sexton 1984; Walsh 1997) and substance abuse (Winhusen 2014); other worksites (Glasgow 1993; Hennrikus 2002), universities (Thomas 2016) or from the community (Alessi 2017). Fifteen studies were based in the USA (Alessi 2014; Alessi 2017; Crowley 1995; Glasgow 1993; Hahn 2005; Hennrikus 2002; Klesges 1986; Klesges 1987; Ledgerwood 2014; Lillington 1995; Maheu 1989; Parker 2007; Sexton 1984; Thomas 2016; Winhusen 2014), two in Australia (Gomel 1993; Walsh 1997), one in Russia (McAlister 2000), one in Canada (Bains 2000) and one in the USA and Canada (Koffman 1998).
Participants
All included studies recruited current adult smokers, either self‐reported or biochemically verified, with no other age restriction. Four studies were targeted at pregnant women (Lillington 1995; Parker 2007; Sexton 1984; Walsh 1997). Alessi 2014 was aimed at men only and the remaining studies recruited both sexes. In some studies, individuals with certain serious health conditions, substance use disorders and/or a history of pathological gambling were specifically excluded (Alessi 2014; Alessi 2017; Crowley 1995; Ledgerwood 2014; Thomas 2016), while one recruited only individuals with substance use disorder (Winhusen 2014).
Prizes
Twelve studies used lotteries or raffles with cash rewards (Bains 2000; Crowley 1995; Glasgow 1993; Gomel 1993; Hahn 2005; Hennrikus 2002; Lillington 1995; Maheu 1989; Parker 2007; Sexton 1984; Thomas 2016; Walsh 1997), with the monetary value of prizes ranging from US$30 (Sexton 1984) to US$5000 (Thomas 2016). Many of these studies offered a small guaranteed cash sum as an incentive for quitting, in addition to entry into the prize draw. Lillington 1995 and Sexton 1984 additionally offered prize draws for inexpensive non‐cash items. Three studies offered prizes with a range of cash values (Alessi 2014; Alessi 2017; Ledgerwood 2014), typically, a large number of small prizes such as toiletries and a small number of large prizes such as televisions. One study involved quitters being entered into a monthly lottery to win a holiday (McAlister 2000), and four studies split participants into teams and the team with the most quitters won a prize (Klesges 1986; Klesges 1987; Koffman 1998; Maheu 1989). In three cases these prizes were solely monetary (Klesges 1987; Koffman 1998; Maheu 1989), but in one case the monetary prize was in addition to a catered meal for the winning team, served by executives of the losing institutions (Klesges 1986). Maheu 1989 and McAlister 2000 also included lottery draws for smoking ‘buddies' or ‘sponsors', who supported smokers trying to quit.
Although all studies rewarded smoking cessation as the primary outcome, some added incentives for other performance indicators. Participation and/or compliance, irrespective of smoking status, were rewarded by Alessi 2014, Alessi 2017, Klesges 1986, Klesges 1987, Koffman 1998, Ledgerwood 2014, Maheu 1989, Sexton 1984 and Thomas 2016. Koffman 1998 also paid those smokers who reduced their cigarettes to no more than 80 in the first month of the programme, as a preparation for stopping completely.
Cessation interventions and comparators
The included studies varied widely in the extent and form of additional smoking cessation support offered alongside the competition component. Six studies (Bains 2000; Hahn 2005; Lillington 1995; Sexton 1984; Walsh 1997; Winhusen 2014) offered a substantial amount of additional support only to the intervention group, and not to the control group, making isolating the effect of the competition component difficult. Bains 2000 offered a ‘Quit Kit' of support materials, including cessation advice, maintenance tips, a list of local cessation programmes, and a fridge magnet with the number of a health information unit. Hahn 2005 supplied weekly mailed postcards giving gender‐specific advice throughout the contest period, access to a cessation web site and a toll‐free quit line and workplace support. Lillington 1995 provided bilingual health educators for individual counselling, and a self‐help guide. Sexton 1984 also offered self‐help information and counselling, both individually and in group sessions, as well as the chance to undergo hypnosis, although this was discontinued because of low uptake. Walsh 1997 provided various options for cessation support, including midwife and doctor counselling, social support and a self‐help manual. The intervention package used by Winhusen 2014 included bupropion, a nicotine inhaler and cessation counselling as well as a prize draw based on contingency management. In each of these studies the comparison group received either usual care or a much reduced form of this cessation support.
The remaining studies offered similar additional support to both intervention and control groups, as follows. Alessi 2014 and Alessi 2017 offered brief counselling and/or self‐help. Gomel 1993, Hennrikus 2002, Klesges 1986 and Klesges 1987 offered individual or group counselling. Bains 2000, Hahn 2005, Koffman 1998 and Parker 2007 offered a self‐help programme, with or without additional counselling. Maheu 1989 used aversive smoking alongside nicotine replacement therapy as part of a multi‐component programme, and McAlister 2000 used what they described as ‘behavioural journalism', which consisted of role model stories in the local newspaper and on promotional leaflets. Crowley 1995 supplied nicotine gum to all three study groups. Ledgerwood 2014 offered four weeks of daily carbon monoxide (CO) and cotinine monitoring in addition to twice‐daily brief counselling. Glasgow 1993 provided little or no additional quitting support.
The three population‐based Quit & Win studies (Bains 2000; Hahn 2005; McAlister 2000) all compared entrants with non‐participant controls. In Bains 2000 the controls were smokers selected by random digit dialling who had not entered the contest but who lived in the same area or in two adjacent counties, while both Hahn 2005 and McAlister 2000 compared contest participants to smokers outside the contest areas.
Outcomes
Primary outcome
Nine trials followed up participants for a maximum of six months (Alessi 2014; Alessi 2017; Crowley 1995; Hennrikus 2002; Klesges 1986; Klesges 1987; Ledgerwood 2014; Thomas 2016; Winhusen 2014), six for 12 months (Bains 2000; Gomel 1993; Hahn 2005; Koffman 1998; Maheu 1989; McAlister 2000), and one for two years (Glasgow 1993). Klesges 1986 and Klesges 1987 delivered their final cessation rewards six months into the programme, which was also the end of the designated follow‐up period; thereby confounding the intervention rewards with testing at the longest follow‐up.
Among the studies of pregnant women, Lillington 1995 and Parker 2007 reported at six weeks postpartum, corresponding approximately to six‐month follow‐up for participants recruited at the start of the second trimester of pregnancy, and Walsh 1997 at six to 12 weeks postpartum. Sexton 1984 reported at eight months gestation among participants who remained pregnant, corresponding approximately to six‐month follow‐up for participants recruited early in pregnancy.
All studies except for Bains 2000 attempted to use some form of validation procedure to confirm smoking cessation. Twelve tested levels of cotinine in blood, saliva or urine (Alessi 2014; Alessi 2017; Crowley 1995; Glasgow 1993; Gomel 1993; Hahn 2005; Hennrikus 2002; Ledgerwood 2014; Lillington 1995; Maheu 1989; Parker 2007; Walsh 1997). Eleven studies tested CO levels (Alessi 2014; Alessi 2017; Crowley 1995; Glasgow 1993; Klesges 1986; Klesges 1987; Koffman 1998; Ledgerwood 2014; Maheu 1989; McAlister 2000; Winhusen 2014). Crowley 1995 measured blood oxygen saturation, and Klesges 1986, Klesges 1987, Maheu 1989 and Sexton 1984 saliva thiocyanate. Thomas 2016 used a combination of the NicCheck and NicAlert tests, and anatabine/anabasine. Three studies assessed smoke‐free status via the testimony of a nominated ‘buddy', friend or family member (Bains 2000; Hahn 2005; Hennrikus 2002).
Secondary outcomes
We only aimed to assess population impact for trials of population‐based interventions. This applied to only three Quit & Win studies (Bains 2000; Hahn 2005; McAlister 2000).
Information about costs was provided by three studies (Parker 2007; Thomas 2016; Walsh 1997).
Alessi 2014, Alessi 2017, Crowley 1995, Glasgow 1993, Hennrikus 2002, Lillington 1995, Sexton 1984 and Walsh 1997 all provided some information on misreporting of smoking cessation status and/or adverse events, although not specifically in relation to the competition component of the intervention.
Ongoing studies
Two potentially relevant studies were identified that were not completed at the time of the search, one of a contingency management intervention in socially‐disadvantaged minority pregnant women (Accornero 2014), and the other of a contingency management intervention in people living with HIV/AIDS (Ledgerwood 2015). One further study, although completed, is also listed in Ongoing studies as it has not yet been published and from the description of the intervention provided it is unclear whether a competition intervention was used (Horgan 2016).
Excluded studies
Our list of excluded studies includes 70 studies. These studies were excluded for one or more of the following reasons: they studied the use of incentives rather than being a competition, and therefore are included in Cahill 2015; there was no comparison group; the follow‐up was shorter than six months; the study was a population‐based survey rather than a trial; the competition in the study was an extra component of the intervention, which did not differ between groups, and therefore was not being tested.
Risk of bias in included studies
Assessments of the risk of bias for the following domains in each study are given in the Characteristics of included studies tables and summarized in Figure 2.
2.
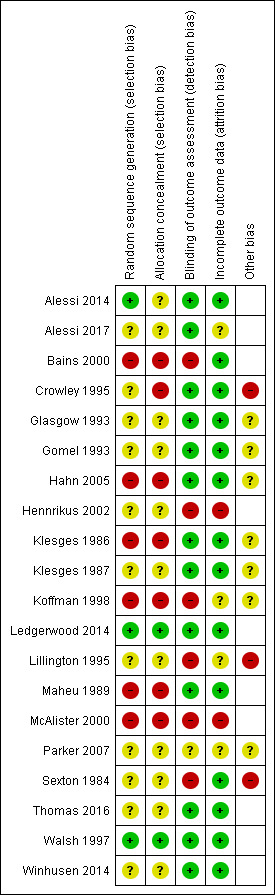
Risk of bias summary.
Allocation
Six of the included studies were not randomized and so were deemed to be at high risk of bias for this domain (Bains 2000; Hahn 2005; Klesges 1986; Koffman 1998; Maheu 1989; McAlister 2000). Of the randomized studies, only three gave sufficient detail of random sequence generation and/or allocation concealment for the integrity of the randomization to be assessed and classified as low risk (Alessi 2014; Ledgerwood 2014; Walsh 1997).
Blinding (detection bias)
Detection bias was deemed to be low if the study used an adequate form of biochemical verification to confirm smoking cessation. Six studies were deemed at a high risk of detection bias on this basis: Bains 2000 used only ‘buddy' verification, four studies (Hennrikus 2002; Koffman 1998; Lillington 1995; McAlister 2000) did not use verification consistently in all study groups, or verified only a subset of participants, and Sexton 1984 used salivary thiocyanate for biochemical verification, which is no longer recommended as it is not specific to tobacco use (Benowitz 2002). Parker 2007 reported conflicting information about the number of participants who underwent biochemical verification and so received a rating of ‘unclear' for this domain.
Incomplete outcome data
Two studies were rated as having high risk of bias in this domain: in McAlister 2000 attrition rates were high (187/378, 49.5%), and substantially higher in the control region than in the Quit & Win region, and in Hennrikus 2002 there was also a differential level of follow‐up depending on group allocation. Four studies were rated as having ‘unclear' risk of bias in this domain because of inadequate or ambiguous reporting of the rate of attrition (Alessi 2017; Koffman 1998; Lillington 1995; Parker 2007).
Other potential sources of bias
Additional potential for bias was identified in three of the included studies: Crowley 1995 failed to report outcome data by study group; Lillington 1995 reported baseline inequality in current smoking rates and did not adjust for clustering effects; in Sexton 1984, the effect size may have been biased by participants who quit between initial recruitment and randomization. Several other studies were rated as having ‘unclear' risk of bias in this domain for differing reasons described in Characteristics of included studies (Glasgow 1993; Gomel 1993; Hahn 2005; Klesges 1986; Klesges 1987; Koffman 1998; Parker 2007).
Effects of interventions
See: Table 1
First, we present the results of studies with performance‐based reward and studies of performance‐based eligibility in relation to the primary smoking cessation outcome, and then we present the results for secondary outcomes. To aid readability, we do not present numeric results for individual studies in the text; these can be found in Analysis 3.1 and Analysis 3.2.
3.1. Analysis.
Comparison 3 Tables of included studies, Outcome 1 Competitions using performance‐based rewards.
| Competitions using performance‐based rewards | ||||||
|---|---|---|---|---|---|---|
| Study | Abstinence definition | Time point | Validation | N randomized | Quit rate (N quit / N followed up) | Comments |
| Gomel 1993 | Continuous | 12 months | Cotinine < 100 ng/mL | BCI 95 BC 124 |
BCI 1/30 (3%) BC 3/30 (10%) |
Other two groups had zero quit rates. |
| Klesges 1986 | PPA | 6 months | CO < 8ppm SCN | I 91 C 16 |
I 18% C 14% | Numbers not reported. |
| Klesges 1987 | PPA | 6 months | CO ≤ 10ppm | 136 | I 8/66 (12%) C 7/61 (11%) | |
| Koffman 1998 | 7 days abstinence | 12 months | CO (level not stated) | 185 | MI 37% M 30% C 11% | N = 185 followed up at 6 m. Numbers per group not reported. Statistically significant difference reported for Control versus each of the two Intervention groups. |
| Maheu 1989 | 7 days abstinence | 12 months | CO ≤ 10ppm | I 32 C 24 |
I 50% (16/32) C 25% (6/24) | |
3.2. Analysis.
Comparison 3 Tables of included studies, Outcome 2 Competitions using performance‐based eligibility.
| Competitions using performance‐based eligibility | ||||||
|---|---|---|---|---|---|---|
| Study | Abstinence definition | Time point | Validation | N randomized | Quit rate (N quit / N followed up) | Comments |
| Population Quit & Win studies | ||||||
| Bains 2000 | 6‐month continuous abstinence | 12 months | 'Buddy' confirmation | I 231 C 385 |
I 39/200 (19.5%) C 4/325 (1.2%) |
|
| Hahn 2005 | 7‐day PPA | 12 months | Urinary cotinine + buddy confirmation | I 494 C 512 |
I 36/494 (7.3%) C 3/512 (0.6%) | Confirmed % quit declined from 14.0% (3m) to 7.3% (12m) in I, roughly stable over time in C. Statistically significant difference between groups reported. |
| McAlister 2000 | PPA | 12 months | Self‐report with only potential winners tested (expired CO) | I 176 C 202 |
I 26/102 (26%) C 2/85 (2%) |
Statistically significant difference between groups reported. |
| Other | ||||||
| Alessi 2014 | 7‐day PPA | 24 weeks | CO < 6 ppm, urinary cotinine < 30 ng/mL | I 24 C 21 |
I 3/24 (12%) C 5/21 (24%) |
|
| Alessi 2017 | Continuous abstinence | 24 weeks | CO ≤ 6 ppm, urinary cotinine ≤ 30 mg/mL | I 45 C 45 |
I 7/38 (18%) C 7/43 (14%) |
|
| Crowley 1995 | PPA | 6 months | Expired CO, urinary cotinine, finger pulse oximetry | E 18 CSR 16 C 15 |
5/36 (14%) across 3 groups combined | Quit rate not reported separately by group. |
| Glasgow 1993 | PPA | 24 months | CO < 9 ppm, salivary cotinine < 25 mg/mL | I 474 C 623 |
I 49/344 (14%) C 49/426 (12%) |
Confirmed % quit declined from 14.0% (3m) to 7.3% (12m) in I, roughly stable over time in C. |
| Hennrikus 2002 | 7‐day PPA | 24 months | Salivary cotinine < 10 ng/mL | 2402 | 19.4% | Quit rate not reported separately by group. |
| Ledgerwood 2014 | PPA | 6 months | Cotinine ≤ 100 ng/mL and CO ≤ 6 ppm | ECM 36 TCM 28 SC 17 |
ECM 1/36 (2.8%) TCM 3/28 (10.7%) SC 1/17 (5.9%) |
TCM and SC groups included in meta‐analysis. |
| Lillington 1995 | PPA | 6 weeks postpartum | Saliva cotinine < 20 ng/mL (minority) | 768 | I 20/79 (25%) C 17/146 (12%) |
Authors state significant difference between groups. Most samples not biochemically verified. |
| Parker 2007 | 30‐day abstinence | 32 weeks gestation, 6 weeks postpartum |
Urinary cotinine < 80 ng/mL (minority) | I1 329 I2 358 C 378 |
I1 51/329 (16%) I2 76/358 (21%) C 72/378 (19%) |
Time point at which outcome reported is unclear. Most samples not biochemically verified. |
| Sexton 1984 | Unclear | 8 months gestation | Salivary thiocyanate | I 463 C 472 |
I 167/388 (43%) C 79/395 (20%) |
I 393 and C 397 of randomized sample were still pregnant at 8 months |
| Thomas 2016 | Continuous abstinence | 6 months | Urine NicCheck/NicAlert, anatabine/anabasine | MC 615 SC 602 |
MC 48/615 (7.8%) SC 23/602 (3.8%) |
2x2 factorial trial; results presented as pairs of groups combined. |
| Walsh 1997 | PPA and "consecutive cessation" | 6‐12 weeks postpartum | Urinary cotinine < 500 nmol/L | I 148 C 145 |
I 8/127 (6%) C 0/125 (0%) |
|
| Winhusen 2014 | 7‐day PPA | 6 months | CO < 8 ppm | I 267 C 271 |
I 35/267 (13.1%) C 10/271 (3.7%) |
Statistically significant difference between groups reported. |
Smoking cessation
Studies with performance‐based reward
Two of the five studies in this subgroup were randomized controlled trials (RCTs) (Gomel 1993; Klesges 1987). The risk ratios (RRs) for each study, calculated from their long‐term abstinence data, are shown in Analysis 1.1, adjusted for clustering, as both were cluster‐randomized. We judged that these studies were too heterogeneous to pool in a meta‐analysis. Neither study showed a benefit of competitions as smoking cessation interventions on quit rates, and the quit rates in both studies were very low (absolute numbers are reported in Analysis 3.1). The 12‐month quit rates for Gomel 1993 were very low (1% to 2%) when expressed in relation to the number of participants randomized, while six‐month point prevalence abstinence (PPA) rates for Klesges 1987 were higher (around 11%), but similar in the two study arms.
1.1. Analysis.
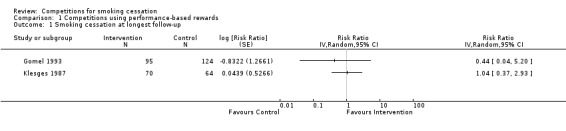
Comparison 1 Competitions using performance‐based rewards, Outcome 1 Smoking cessation at longest follow‐up.
The other three studies were ‘quasi‐randomized' (Klesges 1986; Koffman 1998; Maheu 1989). None of these studies indicated a difference between the relevant comparator groups (Analysis 3.1). Koffman 1998 used two intervention groups (consisting of a multi‐component intervention, with or without a reward element) and a control group of participants who received only a self‐help manual and limited group support. In this study, a similar elevation in quit rate was observed in both intervention groups compared to control, and so this effect could not be attributed to the competition component of the intervention.
Studies with performance‐based eligibility
Five studies carried out in groups other than pregnant women were suitable for inclusion in a meta‐analysis (Analysis 2.1; Analysis 3.2; Alessi 2014; Alessi 2017; Glasgow 1993; Ledgerwood 2014; Thomas 2016). The pooled RR estimate from these studies was 1.40 (95% CI 0.97 to 2.03, n = 2494, I2 = 21%). The largest study, Thomas 2016, reported a substantially higher intervention effect than most of the other studies (RR 2.04, 95% CI 1.26 to 3.31). In Thomas 2016, the control group consisted of a single prize draw, as opposed to the opportunity to enter multiple prize draws (three in total) in the intervention group, rather than usual care or no intervention without prize draws, as was the case in Alessi 2014, Alessi 2017, Glasgow 1993 and Ledgerwood 2014. Thomas 2016 was not unique in offering the opportunity to enter multiple prize draws. Alessi 2014, Alessi 2017 and Ledgerwood 2014 offered participants in the intervention group the opportunity to enter multiple prize draws, as many as 195 possible draws in the case of the contingency management approach adopted by Ledgerwood 2014, with both a greater chance for participants who had stopped smoking to win prizes and greater expected winnings over the course of the study. Additionally, although Thomas 2016 found an effect of multiple contests for the more stringent six‐month continuous abstinence outcome (shown in Analysis 2.1), this study did not find an intervention effect for its primary outcome, 30‐day PPA (quit rate 13.5% (83/615) in multiple contests group, 11.7% (reported as 70/602) in single contest group), even though this was also measured at six months.
2.1. Analysis.
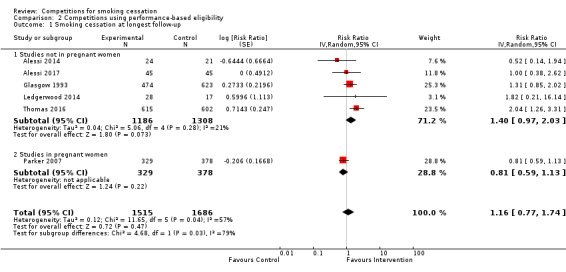
Comparison 2 Competitions using performance‐based eligibility, Outcome 1 Smoking cessation at longest follow‐up.
Only one study in pregnant women was suitable for inclusion in this meta‐analysis (Analysis 2.1; Parker 2007). This study, whose results are only available via a conference abstract, had a RR estimate of 0.81 (95% CI 0.59 to 1.13), with methodological weaknesses (inadequate biochemical verification and unclear reporting of outcomes). When combined with the five studies in non‐pregnant participants, the pooled RR (reported above) was attenuated to 1.16 (95% CI 0.77 to 1.74, n = 3201, I2 = 57%), and statistical heterogeneity increased.
Three further studies in pregnant women could not be included in the meta‐analysis as the effect of the competition could not be separated from other intervention components. Sexton 1984 provided telephone self‐help to intervention group participants, although it was not clear whether control group participants received similar support, and both Lillington 1995 and Walsh 1997 offered various extra cessation support measures to participants in the intervention group, including self‐help materials and counselling. Similarly, Winhusen 2014, which recruited participants from substance use clinics, was excluded from the meta‐analysis as the intervention included multiple components, and although a beneficial effect of the intervention was found, this could not be attributed to the competition element.
We did not attempt meta‐analysis of the three included population‐based Quit & Win studies (Analysis 3.2; Bains 2000; Hahn 2005; McAlister 2000) because of methodological heterogeneity. All three found differences in the one‐year quit rates between intervention and control group participants, but these results need to be considered alongside issues of potential bias associated with these non‐randomized studies. Each is described in further detail below.
Bains 2000 reported substantial differences in quit rates between the experimental group (39/231, 16.9%) and the random survey control group (4/385, 1%). The authors urge caution in interpreting these results, since the experimental group, comprising contestants in a local Quit & Win contest, were on average younger, heavier smokers, better educated, and more likely to be employed than control group participants. In the intervention group, 86.8% of participants were already in the process of quitting smoking at baseline, compared with 2.3% of participants in the survey group, most of whom were at an earlier stage of considering quitting. The use of random telephoning to assemble a control group therefore induced “systematic differences” between smokers who chose to enter the Quit & Win contests and those who did not. Finally, this study relied entirely on self‐report and buddy testimony to assess smoking status, with no biochemical verification. Hahn 2005 reported a 7.3% (36/494) biochemically‐validated quit rate at 12 months for the Quit & Win group, and a similarly low quit rate for the controls (3/512, 0.6%). In this study, in which the control group was selected by random digit telephone dialling, there were also substantial differences between the groups in baseline characteristics, including gender, education and income. Again, competition participants were much more likely to be intending to quit imminently or already quitting. The final eligible Quit & Win study (McAlister 2000) reported a 15% (26/176) cessation rate in the Quit & Win community compared with 1% (2/202) in comparison community participants, who did not receive any smoking cessation intervention. This study was borderline for inclusion in this review as intervention and control groups did not originate from the same base population, but from adjacent districts that the authors regarded as “very similar”. The result should be viewed with some caution as follow‐up rates were low and disparate, biochemical verification was not used in the control region, and insufficient information about baseline characteristics was reported in the study paper to make a detailed assessment of the comparability of the individuals in the two study regions.
Two further studies with performance‐based eligibility could not be included in meta‐analysis because they did not report outcome data by study group. Crowley 1995 was a small study of 49 participants randomized to three groups, two of which allowed for lottery ticket rewards for cessation. Only five participants achieved cessation at six months, and the authors concluded that the cessation rates “did not differ” between groups. Hennrikus 2002 was a factorial, cluster‐randomized workplace‐based study (24 workplaces, 2402 participants) that provided both a guaranteed cash reward and entry into a grand prize lottery for intervention group participants who stopped smoking. Counselling was also available to some participating workplaces, depending on group allocation. The authors did not report quit rates per group in a form that allowed data extraction, but concluded that “incentives did not have an effect on quit rates”. It is therefore likely that had it been possible to include these two studies in the meta‐analysis in Analysis 3.1, the effect size estimate would have moved further towards the null.
Secondary outcomes
Population impact
Bains 2000 reported a population impact of 0.17%, based on a participation rate of 0.83% of adult smokers. In practical terms, and assuming there is a causal link between entering the contest and quitting, this would mean that 1 in 588 smokers within this Canadian community might be expected to achieve long‐term abstinence because of the Quit & Win contest. A similar calculation for the McAlister 2000 study gave a population impact of 0.21%, based on the reported participation rate of about 3% of smokers in the Quit & Win contest; in other words, about 1 in every 500 smokers in the community might be expected to achieve abstinence through taking part in the contest. For Hahn 2005, the authors estimated the contest reached 1% of the target adult smokers, implying a population impact of around 0.07%, or 1 in 1370.
Costs
Few studies reported information relating to cost beyond the costs of providing the competition prizes themselves. Parker 2007 provided an assessment of the telephone counselling component of their intervention, but not the Quit & Win component. Walsh 1997 estimated costs per abstainer to be around US$121 in the intervention group and US$38 in the control group, but it was not possible to attribute this difference to the competition component of the intervention.
The one study to provide a detailed cost‐effectiveness analysis was Thomas 2016, in a follow‐up paper published in 2018. They estimated the cost per additional quit (using a six‐month continuous abstinence outcome) of multiple Quit & Win contests compared to a single contest to be US$1275, which they regarded as cost‐saving, but acknowledged that this has not been compared to a strategy without Quit & Win contests.
Unintended consequences
Some of the included studies reported on inconsistencies between self‐reported and biochemically‐verified smoking status. Crowley 1995 calculated a ‘Corrected CO' index to compare CO levels with number of cigarettes reported, and suggest greater dissimulation in the group of participants who were awarded the lottery ticket reward based on self‐reported cessation than in the group that received the reward based on biochemically‐verified cessation. There was also an increased tendency for participants to misreport after the first baseline measurements. Glasgow 1993 stated that they could not confirm abstinence in the self‐reports of 27% of participants, either through the participant admitting relapse when asked to make an appointment for biochemical validation, failure to attend the appointment, or (for one participant only) failing verification. Similarly, in Hennrikus 2002, only 3% of 128 analyzed samples failed verification, but 21 individuals admitted relapse when asked to provide a sample. Sexton 1984 stated that only 5% to 10% of participants would be classified differently by self‐report and thiocyanate verification.
By contrast, in Lillington 1995, 23% of 111 saliva cotinine samples among self‐reported quitters exceeded the threshold for smoking cessation, and 143 other self‐reported quitters failed to provide samples. Walsh 1997 stated that 52% of cotinine tests in the control group and 12% in the experimental group were inconsistent with self‐report. In Hahn 2005, 12‐month self‐reported abstinence rates (24.6% and 8.1% in the two study groups) were much higher than the biochemically‐validated quit rates (7.3% and 0.6%, respectively). As almost all studies used some form of biochemical verification, the opportunity to win competition prizes as a result of incorrect self‐report was limited in most included studies.
Few studies reported the occurrence of adverse events. Alessi 2014 reported one participant being hospitalized for alcohol‐related heart, liver and lung problems, considered by the trialists not to be associated with the intervention programme. Alessi 2017 reported one overnight hospitalization for food poisoning, 34 occurrences of minor physical complaints related to transdermal nicotine, 25 occurrences of sleep disturbance possibly or probably related to transdermal nicotine, and 27 emergency room visits and physical complaints “unrelated to study participation”. Two of the studies carried out in pregnant women reported adverse events: 35 miscarriages, 20 stillbirths, seven neonatal deaths in hospital and one automobile fatality in Sexton 1984, and 20 abortions or miscarriages and seven preterm deliveries in Walsh 1997. Winhusen 2014 reported a total of 23 serious adverse events and 329 other adverse events, with the majority attributed either to medications delivered to intervention group participants or reasons unrelated to study participation. In no studies was there any indication that adverse events were related to the competition component of the intervention.
Discussion
Summary of main results
Heterogeneity between interventions classified as contests or competitions led us to consider these interventions within subgroups in this review.
Performance‐based reward
Of the five studies using interventions classified as performance‐based reward, in which groups of smokers from different workplaces competed directly against each other, none provided direct evidence of the beneficial effect of the competition intervention component on quit rates. A meta‐analysis was not performed due to methodological heterogeneity. These studies tended to be small in both the number of individual participants and the number of participating workplaces, which makes their findings difficult to generalize. We have found no eligible studies of performance‐based rewards since the non‐randomized study of Koffman 1998, and so this no longer appears to be an active research area. The effect of the ‘grand prize' lottery that formed part of the incentivization used in one study arm in Koffman 1998 could not be separated from that of guaranteed incentive payments for continuous cessation, and follow‐up studies have not been performed to explore this further.
Performance‐based eligibility
The remaining 15 studies used performance‐based eligibility, such that quitters entered a prize draw or lottery. In a meta‐analysis of six RCTs in this group that provided analyzable data and for which for the effect of the competition could be estimated, overall cessation rates in participants allocated to competitions were not clearly higher than those in non‐competition participants. Among these studies, of particular note is Thomas 2016, which was the only one that appeared to demonstrate a clear benefit of the competition intervention. This study appeared to show an increase in cessation rates for the group eligible for two additional competition draws during follow‐up, compared to a group eligible for a single draw. Two further studies that did not provide sufficient data to be included in a meta‐analysis did not demonstrate a benefit of competitions (Crowley 1995; Hennrikus 2002).
Quit & Win
We looked at the Quit & Win contest in isolation as this particular form of smoking cessation competition gained widespread adoption in the 1990s and early 2000s, although interest has waned in recent years and the international competition has not run since 2006. Despite its previous popularity, we identified only three eligible studies of the Quit & Win contest as applied at the population level (Bains 2000; Hahn 2005; McAlister 2000), with two further studies using Quit & Win‐type contests in specific recruited groups of participants (Parker 2007; Thomas 2016). Although Bains 2000, Hahn 2005 and McAlister 2000 appeared to indicate that Quit & Win was associated with substantially increased cessation rates, none of these studies was an RCT and all suffered from major baseline differences in important confounders between intervention and comparator groups. At the effect size estimated in these studies, the population impact of the Quit & Win contest would require at least 500 smokers in the population in each case to achieve one smoker quitting.
It is important to consider, when taking these findings into account, that our certainty in the above findings is low due to substantial limitations in the evidence base, which are discussed further below.
Population subgroups
There was very limited evidence investigating the effectiveness of competition interventions in adolescents and pregnant women. In adolescents, there were no eligible studies, while of the four studies conducted in pregnant women, only one allowed the effectiveness of the competition component to be estimated (Parker 2007). The results of this study have only been published via a conference abstract and did not show the competition to be beneficial, although the available prize of US$100 via a lottery draw was low in value compared with those in other studies in this review.
Overall completeness and applicability of evidence
There was insufficient evidence to assess whether the amount and type of prize affected cessation or population impact. There was also insufficient evidence to say anything definitive on cost implications. There was little evidence that levels of deception varied between experimental and control participants or that competition participants were at increased risk of other unintended consequences.
The considerations below relate to cessation and population impact outcomes.
Study design
The evidence assessing smoking cessation competitions is limited. In the case of Quit & Win competitions, this is at least partly due to the fact that, population‐based interventions are less amenable to the RCT design than those aimed at specific participant groups, since comparison communities free from contamination are difficult to find (Bains 1998; Chapman 1993). This has led some authors to consider alternative designs such as modified time‐series (Tillgren 1995), and discuss the prohibitive logistics of setting up a community‐based RCT to avoid selection bias. However, this does not account for the lack of high‐quality studies assessing performance‐based reward in the workplace setting, or those using performance‐based eligibility that are not population‐level interventions.
In contrast with the earlier studies in this field, more recent studies such as Winhusen 2014 have increasingly used multicomponent or complex intervention designs in which the competition was just one component of a larger intervention. Another example is Walsh 1997, where participants rated the lottery component as the least helpful of the seven components that made up the experimental protocol. Designs that use an approach based on contingency management, in which participants may be entered into multiple prize draws to reward continuous cessation over repeated follow‐ups (Alessi 2014; Thomas 2016), also appear to be becoming more popular. In this approach, designs that use guaranteed rewards (reviewed by Cahill 2015) have received greater prominence than those based around lotteries or prize draws. Overviews of this area have also tended to focus on guaranteed payouts as opposed to lotteries or prize draws (Marteau 2009).
Prizes
The use of tangible rewards is a trade‐off between maximizing participation and attracting smokers who may be motivated more by the rewards than by the wish to stop smoking, and therefore may be less likely to achieve long‐term cessation. The form and magnitude of the prize have been considered critical elements in the design of a cessation programme offering prizes or incentives, although these are just two of nine domains used to classify incentive schemes in the framework developed by Adams 2014. Given that non‐guarantee of a prize is a defining characteristic of the studies in this review, the level of certainty (or probability) with which participants receive a prize is another key consideration. The perceived value of the reward therefore balances the chance of winning it with its intrinsic value, and may also vary according to the socioeconomic circumstances of the participants (Lynagh 2013).
These features varied widely among the included studies, for example a small chance of winning a relatively large prize of $US2500 in the Quit & Win study of Hahn 2005, as opposed to multiple chances of winning generally more modest prizes as part of contingency management in Alessi 2017. There were insufficient data to explore the effect of these factors on intervention success as part of the analysis of the review, although the trial that showed the largest intervention effect offered a large grand prize of US$5000 (Thomas 2016). Studies varied in the nature of rewards offered: many used cash prizes, but gift vouchers, gift items of a specified cash value, holidays and, in one study (Klesges 1986), a catered meal, also featured. The available evidence is not sufficient to distinguish between these prize types.
Participants and non‐participants
In non‐randomized studies of competition interventions, smokers who choose to participate may have different characteristics from those who do not. From the included and excluded Quit & Win studies in this review, people who register for a contest are more likely to be female (Altman 1987; Bains 2000; Cummings 1990; Hahn 2005; HEA 1991; Korhonen 1992; O'Connor 2006; Roberts 1993; Tillgren 1992), younger (Altman 1987; Bains 2000; Cummings 1990; Hahn 2005; Hawk 2006; HEA 1991; Korhonen 1999; O'Connor 2006), better educated Bains 2000; Cummings 1990; Hahn 2005; Hawk 2006; Korhonen 1999; Lando 1995a), smoking more cigarettes per day (Bains 2000; Cummings 1990; Hawk 2006; Korhonen 1999; O'Connor 2006), more likely to be intending to stop smoking in the near or very near future (Bains 2000; Hahn 2005; Lando 1991b; Resnicow 1997; Roberts 1993), and having made more previous quit attempts than those smokers who do not enter the contest (Korhonen 1999). This confounding clearly limits the conclusions that can be drawn about population‐level competitions.
Quality of the evidence
Fourteen of the 20 included studies were RCTs. Only one included study was judged to be at low risk of bias (see Risk of bias in included studies). Overall, we rated the certainty of evidence for smoking cessation in this review as ‘very low' for both performance‐based reward and performance‐based eligibility competitions (Table 1). Particular concerns included risk of bias (as previously mentioned), imprecision, and heterogeneity in participants and interventions. For performance‐based reward competitions, there was also concern about lack of generalizability across populations, as studies were conducted only in participants recruited from particular workplaces.
Most studies did however use some form of verification to confirm the smoking status of those claiming abstinence. This is important in studies of competitions to ensure that those eligible to win prizes are truly smokers at entry, and truly abstinent at the evaluation points (Chapman 1994). In practice, this is difficult to achieve and may be prohibitively expensive in population‐based contests, while for RCTs, it would now be expected as part of good trial design (Benowitz 2002). Although several studies reported discrepancies between self‐reported and biochemically‐verified quit rates (Crowley 1995; Glasgow 1993; Hahn 2005; Lillington 1995; Walsh 1997), which were in some cases larger than typical rates of misreport found in a systematic review of biochemical verification (Patrick 1994), the fact that verification is now routinely performed in such studies suggests this is unlikely to have a major influence on which participants received prizes.
Potential biases in the review process
We have followed standard Cochrane methods to identify and evaluate the studies contributing to this review. We have sought missing or incomplete data, and have contacted authors where possible to clarify our interpretation of their work.
In this review we have included only controlled studies that specified smoking cessation as a primary outcome, and which restricted eligibility of rewards to participants who achieved abstinence. We have not considered observational studies, nor those that may have encouraged smokers to reduce consumption without quitting entirely. Studies that looked at increasing participation rates in cessation programmes were also excluded. It is plausible therefore that potential rewards may have value as a mechanism for recruiting participants into the cessation process, as distinct from their role in aiding or enhancing cessation.
We hoped to assess the population impact of population‐based competitions, the cost of smoking cessation competitions and any adverse consequences. Unfortunately, very few studies eligible for inclusion reported clearly on these, which limits the conclusions of this review, although the frequency of adverse events directly caused by competitions appears low. Population impact may be better estimated using data from observational studies, which were excluded from this review. Most studies did not report detailed information on costs of delivering the intervention or cost‐effectiveness of competitions interventions. This information would be useful in future trials in this area.
Agreements and disagreements with other studies or reviews
This review has updated and combined information about studies testing smoking cessation competitions that were originally included in a review which jointly addressed incentives and competitions for smoking cessation (Cahill 2011) and one that focused solely on Quit & Win contests (Cahill 2008b). As a result of clarifying certain inclusion criteria (see Differences between protocol and review), the set of included studies does not exactly match those used in the previous reviews, but the conclusions are broadly similar in that there remains little high‐quality evidence for the success of smoking cessation competition.
International Quit & Win
In the case of Quit & Win, our conclusions match those of Cahill 2008b. We were not able to identify any RCTs of international Quit & Win; these contests no longer regularly occur, and it seems unlikely in practice that any such studies could be implemented. As no new eligible studies of this intervention have emerged in the last decade, we refer to Cahill 2008b for a more detailed discussion of its history, including a summary of reports of related international contests such as those run in Finland (Korhonen 2000; Sandström 2001; Sandström 2002), China (Sun 2000) and Iran (Pourshams 2000; Sarraf Zadegan 2006), some of which demonstrated high quit rates in uncontrolled assessments. Although the social marketing aspect of the Quit & Win contest has been reviewed in glowing terms, with a call to enhance participation rates (Lavack 2007), it appears that public health resources for smoking cessation have been channeled away from competitions in favour of other population‐level interventions. The only remaining comparable annual contest appears to be the Hong Kong ‘Quit to Win' competition. The effect of this competition on quit rates has not been widely reported and the competition has been used as a means of recruiting smokers to other interventions such as telephone counselling (Wang 2015).
Incentives for smoking cessation
Studies investigating guaranteed incentives to promote smoking cessation are now included in a separate review (Cahill 2015). The incentives review contains 21 studies in mixed populations and nine in pregnant smokers, and a meta‐analysis containing 17 studies and 7715 participants found a positive effect of incentives for smoking cessation at long‐term follow‐up in comparison to control (odds ratio (OR) = 1.42; 95% confidence interval (CI) 1.19 to 1.69). This result was heavily influenced by the results of two studies (Volpp 2009; White 2013) conducted in very different settings (the workplace of an American multinational company, and rural communities in Thailand, respectively) that both showed large positive effects. The results in Cahill 2015 also suggest the positive effect was more pronounced in pregnant smokers (also discussed in the earlier review Donatelle 2004), with an analysis including eight studies giving an OR at longest follow‐up of 3.60 (95% CI 2.39 to 5.43). The authors conclude that incentives do appear to boost cessation rates whilst they are in place. On this basis, the benefits of guaranteed incentives appear more promising than those of competitions that do not guarantee a reward even if participants successfully comply with a programme or achieve cessation.
A review by Leeks 2010 suggested a positive effect on smoking cessation, based on a mixed sample of worksite‐based incentives and competitions, but were unable to isolate the effect of the incentive or competition from other intervention components, so this finding is difficult to interpret. Other reviews or overviews of incentives that discuss smoking cessation do not focus specifically on the delivery of non‐guaranteed incentives (Giles 2014; Higgins 2012b; Higgins 2016; Sigmon 2012).
It is important to note concerns raised by the quality of the evidence in Cahill 2015. The evidence contributing to the analysis of the mixed population was judged ‘Low' quality according to the GRADE standard, and that contributing to the analysis of pregnant smokers was judged to be of ‘Moderate' quality. In addition, due to the low quality of the evidence contained in our review, it is impossible to be sure whether competitions for smoking cessation truly have limited efficacy, or whether the evidence is not of a high enough quality to detect beneficial effects.
Authors' conclusions
Implications for practice.
Overall, there is not clear evidence for the success of competition interventions for smoking cessation, where smokers are incentivized by the prospect of a reward for quitting that is not guaranteed.
Interventions in which participants from different workplaces compete directly against each other do not generally appear to enhance long‐term cessation rates, but this finding is limited by the scarcity and low quality of the contributing evidence.
Controlled studies of population‐based Quit & Win competitions specifically suggest that there is an increase in quit rates among participants compared to control populations, but this finding is also limited by the scarcity and low quality of the contributing evidence.
Calculations of the population impact of Quit & Win studies suggest that fewer than one in 500 smokers in targeted communities quit as a result of the contest.
There was little evidence that levels of deception varied between experimental and control participants or that competition participants were at increased risk of other unintended consequences.
There are insufficient data to draw conclusions about the cost‐effectiveness of smoking cessation competitions.
Implications for research.
Further research investigating the efficacy of smoking cessation competitions needs to be designed with the weaknesses of the existing studies in mind. Conducting studies of a higher quality will improve the confidence we can have in our conclusions.
The relative lack of success of workplace contests and international prize draw competitions, and the lack of new publications in these areas, suggest that these are no longer active research areas.
Future studies of competition interventions, if conducted, should instead seek to evaluate the impact of the form, value and frequency of competition prizes on long‐term smoking cessation outcomes, preferably alongside a cost‐effectiveness assessment. Final follow‐up should preferably be longer than the period of the competition.
Studies might also compare competition interventions against those with guaranteed incentives, for which the evidence is currently stronger.
The stability of successful payment schedules needs to be tested in varying populations of smokers, from different socio‐economic, regional and ethnic backgrounds.
What's new
| Date | Event | Description |
|---|---|---|
| 12 September 2018 | New citation required but conclusions have not changed | Searches updated June 2018, four new studies added. Conclusions not changed. |
| 12 September 2018 | New search has been performed | Merged two reviews: now contains Quit & Win contests and other competitions (which were previously included in the review 'Competitions and incentives for smoking cessation'). |
History
Review first published: Issue 2, 2019
| Date | Event | Description |
|---|---|---|
| 14 April 2011 | Amended | Minor typographical errors corrected |
| 24 November 2010 | New search has been performed | 15 new trials added: 2 included, 13 excluded. |
| 24 November 2010 | New citation required and conclusions have changed | New included study (Volpp 2009) found long‐term positive effects of their incentive‐based trial. Risk of bias tables added for all studies. |
| 6 August 2008 | Amended | Source of support added |
| 29 April 2008 | New citation required but conclusions have not changed | Name change for first author |
| 2 April 2008 | Amended | Converted to new review format. |
| 2 April 2008 | New search has been performed | Two new included studies, nine new excluded studies, conclusions unchanged. |
Acknowledgements
We would like to thank Suzanne Colby, Sandra Gallagher, Leonard Jason, Susan McMahon, Erin Rotherham‐Fuller, Damaris Rohsenow, Steven Shoptaw, Tracy Tevyaw and Kevin Volpp for supplying additional data or clarification, and Harry Lando and Esteve Salto for commenting on an earlier version of the original ‘Competitions and Incentives' review. We would like to thank Per Tillgren for supplementary details and documents supplied for the 2008 update of the ‘Quit & Win' review. We also thank Namrata Bains, Chris Roberts and Patrick Sandström (International Quit & Win Finland) for additional information on an earlier version of the ‘Quit & Win' review, and Anne Lavack and Corinne Husten for comments.
For the present version of this review we gratefully acknowledge the advice of Professor Pekka Puska on the time of the last Quit & Win contest carried out for the ‘Background' of the review. We also thank Professor Payam Sheikattari for information relating to Sheikhattari 2015, Dr Catherine Chamberlain for helping us locate one of the studies included in Chamberlain 2013, Professor Paul Aveyard for background information on the theoretical basis of the intervention, and Clare Miles for assistance with data extraction.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. Outside of this work, JHB also receives funding from the NIHR Oxford Biomedical Research Centre (BRC). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care.
Data and analyses
Comparison 1. Competitions using performance‐based rewards.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 2 | Risk Ratio (Random, 95% CI) | Totals not selected |
Comparison 2. Competitions using performance‐based eligibility.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 6 | 3201 | Risk Ratio (Random, 95% CI) | 1.16 [0.77, 1.74] |
| 1.1 Studies not in pregnant women | 5 | 2494 | Risk Ratio (Random, 95% CI) | 1.40 [0.97, 2.03] |
| 1.2 Studies in pregnant women | 1 | 707 | Risk Ratio (Random, 95% CI) | 0.81 [0.59, 1.13] |
Comparison 3. Tables of included studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Competitions using performance‐based rewards | Other data | No numeric data | ||
| 2 Competitions using performance‐based eligibility | Other data | No numeric data | ||
| 2.1 Population Quit & Win studies | Other data | No numeric data | ||
| 2.2 Other | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alessi 2014.
| Methods | Country: USA Setting: residential substance use disorder clinics, southern Connecticut Design: randomized controlled trial |
|
| Participants | 45 participants (24 Intervention, 21 Control). All were men aged 18+, smoking 10+ cpd with interest in quitting smoking, who were entering long‐term (> 6 months) residential substance use disorder treatment. Average age 37; 84% “non‐Hispanic”; average 11.4 years education; average 18.6 cpd at baseline with groups similar at baseline. |
|
| Interventions | All participants received 2 quit‐smoking preparation sessions, the first consisting of CO measurement, 30‐minutes counselling and a self‐help quit guide, with a review of progress and a quit date set in the second session 4 days later. All participants received US$15 at intake, US$25 per follow‐up and a US$1 gift certificate/snacks/gum for each CO and cotinine sample, irrespective of smoking status. 1. Control: additionally received a brief monitoring intervention consisting of 5‐minute individualized support/feedback Monday‐Friday for 4 weeks. CO measured and cpd tracked at each session. Cotinine measured weekly. 2. Intervention: received the control intervention plus entry into multiple prize draws, contingent on abstinence, as follows: Week 1: a “guaranteed prize” bowl with 70 cards, of which 64 had a US$1 prize (e.g. toiletries, sports drink, gum), 5 for a US$20 prize (e.g. exercise weights, portable games, Barnes and Noble gift cards), and 1 for a US$100 prize (linens, TV, and DVD player), with 1 to 5 draws available depending on number of consecutive CO tests abstinent. Weeks 2 to 4: a “standard prize” bowl with 500 cards, 50% of which had a prize (219 US$1 prizes, 30 US$20 prizes and 1 US$100 prize). A cotinine‐negative test gave 5 bonus draws. Overall, 190 draws were available for negative CO tests (mean earnings US$426.56) and 15 for negative cotinine tests (mean earnings US$46.43). |
|
| Outcomes | 7‐day PPA at 4, 8, 12, 24 weeks, biochemically verified twice‐daily by CO (< 6 ppm) and weekly by urinary cotinine (< 30 ng/mL) % reduction in cpd, self‐efficacy, non‐nicotine substance use |
|
| Notes | Also in ISC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization to one of two conditions occurred using an urn procedure.” |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few losses; 2 participants left the treatment centre before completion. |
Alessi 2017.
| Methods | Country: USA Setting: community Design: randomized controlled trial |
|
| Participants | 90 (45 Intervention, 45 Control) regular smokers (10+ cigarettes daily, verified by CO ≥ 8 ppm). No past‐year abstinence exceeding 3 months. Intent to quit within 3 weeks. Age 18+. Mailing address and photo ID. Exclusions: past month behavioural or pharmacotherapy for smoking, serious and unstable psychiatric illness or disease, contraindication for transdermal nicotine, pregnant, breastfeeding, nursing a child, not using effective contraception if female, ongoing use of monoamine oxidase inhibitors, antipsychotics, mood stabilizers, bupropion or naltrexone, not English‐speaking. 52 (58%) female, 67 (74%) European American, 12 (13%) African American, 11 (12%) Asian/Multiple ethnicity, average 18.8 cpd within last 30 days. Groups balanced in baseline characteristics |
|
| Interventions | Both groups: 2 in‐person behavioural counselling sessions to set a target quit date. Brief telephone counselling scheduled twice weekly for 4 weeks. 8‐week supply of transdermal nicotine (typically patches) with use encouraged but not required. Incentives were US$25 for in‐person intake, US$35 for each in‐person follow‐up at 4, 12 and 24 weeks, US$10 per cotinine sample, US$50 for returning study equipment. Participants in both arms received US$1 for each CO sample submitted and a US$10 bonus for submitting all CO samples in a week. Control (Usual Care with Abstinence Monitoring ‐ mHealth Monitoring): participants prompted by cell phone to complete video‐recorded CO self‐test up to 3 times daily for 4 weeks at irregular intervals. Staff compared test results against reports to confirm accuracy. Results were discussed with participants during counselling sessions in weeks 1 to 4. Intervention (mHealth Monitoring Plus Reinforcement): as control, with prize draws for negative CO tests. In weeks 1 to 2, a prize was guaranteed contingent on negative test with amount randomly selected with chance 65/70 of winning a “small” US$1 prize (e.g. gum, mints), chance 4/70 to win a “large” US$20 prize (e.g. gift card) and chance 1/70 to win a “jumbo” prize (e.g. bluetooth headset). In weeks 3 to 4, 50% of draws won prizes: 130/280 “small”, 9/280 “large”, 1/280 “jumbo”. Number of prize draws awarded were escalated for consecutive negative tests and reset for missed or positive tests, with a maximum of 190 draws available. Expected prize value for perfect record of abstinence was approx. US$502. |
|
| Outcomes | Continuous abstinence to 24 weeks and 7‐day PPA at 24 weeks, verified by CO ≤ 6 ppm and urinary cotinine (Accutest NicAlert) ≤ 30 mg/mL. In 4‐week intervention period, thrice‐daily CO test allows estimation of % negative CO tests and longest duration of prolonged abstinence. Patient satisfaction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Using an urn procedure” but exact method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number with biochemically‐verified follow‐up at 24 weeks may be 33/45 (73%, Intervention) versus 43/45 (96%, Control), allowing possibility of large differential between study groups, although numbers are not clearly reported. |
Bains 2000.
| Methods | Country: Canada Setting: four counties in Eastern Ontario; entrants to a 1995 Q&W contest in 2 counties (Frontenac, Lennox and Addington), and a random sample of non‐entrant smokers from all 4 counties (Frontenac, Lennox and Addington, Hastings, Prince Edward). Design: Quasi‐randomized; Intervention group were all entrants to the Q&W contest; controls were selected by random telephone dialling (full details obtained from authors). | |
| Participants | 616 participants (231 Intervention, 385 Control). All had to be 18+, daily smokers of at least 10 cpd. Baseline differences: Intervention group higher % female (59.4% vs 54%), younger, more highly educated, more likely to be employed, more likely to be in a professional or semi‐professional job. Significant differences also in average cpd, average years smoking, quit attempts in past year, number of smoking friends, working in a smoke‐free workplace, number of smoking co‐workers, and stage of change. | |
| Interventions | 1. Intervention: entry into a locally publicized Q&W contest. ‘Quit Kit' supplied to each entrant (letter of encouragement, cessation info, list of local cessation programmes, tips on maintenance, fridge magnet with health unit info phone number). Winners were entered into a lottery draw with a grand prize of C$1000 and secondary prizes of lesser values. 2. Control: no cessation support, only baseline and 1‐year telephone interview | |
| Outcomes | Contest winners (smoke‐free for month prior to the draw) not biochemically validated; verification was from ‘buddy' testimony. Unvalidated self‐report of 6 months continuous abstinence at 1‐year follow‐up. Stage of change. |
|
| Notes | Quit rates were not reported as ITT, but it is possible to discern these from the paper. Originally in Q&W. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention group were all entrants to the Q&W contest; controls were selected by random telephone dialling. Therefore, a sequence was not generated and the intervention group were self‐selected. Members of the intervention group, compared with the random survey group, were younger, more highly educated, more likely to be employed, more likely to be working as a semi‐professional or professional, had fewer friends or co‐workers who smoked and more often worked in a smoke‐free environment. In order to be eligible to win the Q&W contest, respondents had to be smoke‐free in the month before its conclusion. As a result, a very high proportion of the intervention group (87%) were actively trying to quit at the time of the baseline interview. Thus the intervention group were more likely to be in the action or preparation stages of change. |
| Allocation concealment (selection bias) | High risk | Intervention group were all entrants to the Q&W contest; controls were selected by random telephone dialling. Therefore, the intervention group was self‐selected. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation (a ‘buddy' was used for verification). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Of those recruited at baseline, 86.5% (n = 200) of the intervention group were re‐contacted successfully after one year using a follow‐up telephone call, and 84.4% (n = 325) of the random survey group were re‐contacted.” Therefore, follow‐up rates were high and comparable between trial conditions. |
Crowley 1995.
| Methods | Country: USA Setting: outpatient smokers at a Denver COPD clinic Design: 3‐arm randomized controlled trial |
|
| Participants | 49 smokers (18 experimental group, 16 cigarette self‐report group, 15 control group), age at least 35 with COPD, breath CO > 14 ppm and FEV < 70% of FVC. Exclusion criteria: absence from study region, job exposure to high CO, pregnancy, other serious health or dental conditions, elevated bilirubin or blood urea nitrogen. 12 (24%) female, average age 61.4, average FEV 49.5% of normal value. No baseline imbalances. |
|
| Interventions | All participants had daily CO monitoring performed by a study technician over 85 days, + brochure + nicotine gum. At start of study participants were given 3 Colorado Lottery tickets to quote: “throw cigarettes down the toilet”. All were given 1 lottery ticket per day for quote: “time and effort”. Experimental group: received lottery ticket reward for every CO test < 10 ppm. Cigarette self‐report group: received lottery ticket reward for each self‐reported abstinence since previous visit. Control group: each pt received (irrespective of their own abstinence) the same reward as an experimental participant with whom they had been paired. Measurement intervals and payment schedules were changed frequently. |
|
| Outcomes | Verified 24‐hour PPA, measured at 6 months (CO corrected for air pollution < 10 ppm, also verified using urinary cotinine and blood oxygen saturation). Corrected CO relative to number of cigarettes reported. |
|
| Notes | Also in ISC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | High risk | Participants stratified by sex and FEV and groups allocated randomly, such that within each stratum, a participant was always assigned to the experimental group before a participant could be assigned to the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used in addition to self‐report. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout: experimental group 4/18; cigarette self‐report group 4/16; control group 1/15. 4/40 moved away or died. An ITT analysis was conducted. |
| Other bias | High risk | Paper does not report 6‐month cessation outcome per group, but only figures for all groups combined after noting non‐significant differences. |
Glasgow 1993.
| Methods | Country: USA Setting: 18 worksites employing 100 to 1000 employees each, in Salem, Oregon and Portland, Oregon Design: cluster‐randomized controlled trial |
|
| Participants | 8 intervention (including two which were combined) and 10 control worksites, stratified by number of employees and estimated smoking prevalence, comprising 1097 individuals (474 intervention, 623 control). Participants needed to have smoked quote: “even a puff” in the last 7 days. 63% female, average age 40.5, average Duncan SES 4.4, average quote: “educational level” 4.0, average cpd 18.5. Significant but small differences in educational level at baseline (average 4.1 intervention, 3.9 control). |
|
| Interventions | Intervention: Health Incentives Program (HIP). Received US$10 for each monthly PPA over 1 year of programme + monthly worksite lottery (US$5 to US$20 in first 6 months, then minimum US$50 for second 6 months). 12 months sweepstake for US$200, US$100 and US$50 at each worksite. A “good buddy” nonsmokers' lottery prize was also available. No formal quitting support Control: No intervention, surveys at 1 year and 2 years. |
|
| Outcomes | PPA measured at 24 months, verified by CO < 9 ppm and salivary cotinine < 25 mg/mL | |
| Notes | 344 (73%) of the intervention group and 426 (68%) of the control group remained in the study at 24 months. Main reason for dropout appears to be the participant leaving the worksite. However, all participants are retained for ITT analysis in this review, consistent with other studies. The authors report the estimated ICC for this study to be quote: “less than 0.005” for biochemical abstinence, so a value of 0.005 is assumed in calculating the relative risk adjusted for clustering effects. Also in ISC. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar dropout by group (27% of intervention and 32% of control group left by 24 months). |
| Other bias | Unclear risk | Data extrapolated from percentages. |
Gomel 1993.
| Methods | Country: Australia
Setting: 28 ambulance stations, Sydney Design: 4‐arm cluster‐randomized controlled trial |
|
| Participants | 431 smokers; average age 32, 17% female, 59% married or cohabiting, average cpd 17.9. Significant baseline difference between groups on job description. | |
| Interventions | 1. Health risk assessment (HRA): risk factor profile feedback (10 stations, 130 participants) 2. Risk factor education (RFE): as 1 + advice, brochure, videos (8 stations, 82 participants) 3. Behavioural counselling (BC): as 2 + individual counselling (6 stations, 124 participants) 4. Behavioural counselling + incentives (BCI): as 3 + life‐style change manual + counselling + incentives, i.e. 2 lottery draws for A$40 over 10 weeks period, + 5 draw tickets for 1 week cessation; At 3 months A$40 voucher for achieved targets. Station achieving highest % of participants meeting 6 months goals won A$1000. (4 stations, 95 participants) | |
| Outcomes | Continuous cessation at 3, 6 and 12 months (cotinine validation). Cardiovascular risk factor modifications. |
|
| Notes | Only Groups 3 and 4 used in the comparison as this estimates effect of lottery component of intervention.
Study was funded by the Commonwealth Department of Health, the National Heart Foundation of Australia, and the New South Wales Government Employees Assistance to Medical Research Fund. Originally in C&I. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | quote: “Twenty‐eight stations... were randomly selected”. Method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence was biochemically validated so judged to be at low risk of differential misreport. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 94%, 86%, and 84% of baseline participants completed the 3, 6 and 12 month assessments, respectively. Therefore dropout rates were not high and did not differ significantly between groups. |
| Other bias | Unclear risk | Quote: “Movement and transfer of individuals between the different ambulance stations did occur”: possible contamination. ICC used in the analysis for other risk factors, but not for smoking, as numbers too small. |
Hahn 2005.
| Methods | Country: USA Setting: Bluegrass Kentucky 2001 Q&W contest, Lexington‐Fayette county Design: Two‐group quasi‐randomized study | |
| Participants | 1006 adults (age 18+) who had used tobacco within the last 30 days. 1. Intervention: 494 volunteer registrants in a Q&W contest (56% of all entrants); average age 38, 68% female, 47% married, 89% white, 65% college education, 48% earning > US$25,000, 98% smoked cigarettes. 2. Control: 512 current smokers selected by random digit dialling from outside the contest area (8.5% of contacted households). average age 42.8, years, 56% female, 54% married, 91% white. 37% college education, 39% earning > US$25,000, 92% smoked cigarettes. |
|
| Interventions | Intervention: community quit date; weekly gender‐specific cessation information by post throughout contest; online quit assistance; toll‐free phone quit assistance; media campaign; support through worksites, physicians, health professionals, community leaders. Registrants declared tobacco status, and nominated a tobacco‐free ‘buddy'. Lottery draw for cash prizes (grand prize US$2500 and 5 prizes of US$500) for all validated quitters. Control: baseline and follow‐up surveys only. | |
| Outcomes | 7‐day PP. Abstinence at contest end validated by ‘buddy' testimony. Follow‐up telephone interviews at 3 months, 6 months and 12 months, with urinary cotinine test for all quitters at all follow‐up points. Predictors of quitting. |
|
| Notes | In the 2005 version of our review, this trial reported on low‐income smokers only. The current version reports full trial data.
ITT analysis, with missing or non‐negative urines and dropouts counted as continuing smokers. Originally in Q&W. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intervention group were all entrants to the Q&W contest; controls were selected by random telephone dialling. Therefore, a sequence was not generated and the intervention group were self‐selected. Treatment group participants were significantly younger than those in the control group, the percentage of females was significantly greater. Nearly two‐thirds of the treatment group had at least some college education, while nearly the same percentage of the control group had at most a high school education. A significantly lower percentage of the control group had household incomes of US$25,000 or more and a higher percentage of treatment group participants smoked cigarettes at baseline than those in the control group. Compared to the control group, significantly fewer members of the treatment group used smokeless tobacco. 70% of the treatment group were at the preparation stage of change or higher, compared with only 16% of those in the control group. |
| Allocation concealment (selection bias) | High risk | Intervention group were all entrants to the Q&W contest; controls were selected by random telephone dialling. Therefore, the intervention group were self‐selected. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence was biochemically validated so judged to be at low risk of differential misreport. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the treatment group, the retention at 3, 6 and 12 months was 85%, 75% and 63%, respectively.The retention rate for the control group at 3, 6 and 12 months was 78%, 66% and 60%, respectively. The dropout rate was judged to not differ significantly between groups. |
| Other bias | Unclear risk | The following was noted and may have affected the results of the study: Quote: “The findings of this study show that smokers in the control group who were in preparation may have been prompted to quit merely because they were repeatedly interviewed about their tobacco use.” |
Hennrikus 2002.
| Methods | Country: USA Setting: 24 worksites in Minneapolis and St Paul, Minnesota, each employing 300‐1000 employees at a single site Design: 2 x 3 factorial cluster‐RCT |
|
| Participants | 2402 individuals in 24 worksites (4 for each of 6 arms) without a smoking cessation programme. 56% female, average age 39 years, 93% white, 20% had college degree. Groups differed at baseline in age, sex, education and occupational level, marital status, ethnicity, % whose first cigarette was within 30 minutes of waking, and confidence in ability to quit. |
|
| Interventions | 6 arms encompassed all combinations of programme (Group/Phone/Choice) and incentive (Yes/No). Group: participants received 13 group counselling sessions delivered over 2 months. Phone: participants received printed self‐help materials and 3 to 6 telephone counselling sessions. Choice: participants had a free choice between the Group and Phone programmes. Incentive: Quitters at 1 month received a guaranteed US$20 and entry into a grand prize lottery (1 prize of US$500 at 5 sites, 2 prizes of US$250 at 6 sites, and 4 prizes of US$125 at 1 site). Draws took place 3 times at each site in total (roughly every 6 months until 18 months after baseline). |
|
| Outcomes | 7‐day PPA at 12 months and 24 months, self‐report countersigned by friend or family member. Grand prize winners and a random sample of 188 participants at 24 months were contacted for salivary cotinine (< 10 ng/mL) verification. | |
| Notes | Also in ISC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only a sample of those reporting quitting were contacted for biochemical validation, and 60/188 of those contacted did not have samples analyzed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout 18.3% at 24 months but method of follow‐up differed by arm (Group participant dropouts not followed up, Phone participant dropouts followed by phone and letter). |
Klesges 1986.
| Methods | Country: USA Setting: 4 banks and a savings and loan company in Fargo, North Dakota Design: ‘quasi‐experimental' non‐randomized cluster design | |
| Participants | Baseline equivalence on age, sex, socio‐economic status and smoking prevalence, but demographics not reported. Intervention smokers had significantly higher levels of nicotine dependence than controls. 91 smokers in 4 intervention sites (banks), 16 in control site (savings and loan company). | |
| Interventions | Control: basic smoking programme (SP): 6 weeks CBT programme. Intervention: SP+competition: cash prizes for institutions with the best participation (US$100), greatest CO reductions at 6 weeks (US$150) and at 6 months (US$250). Individual awards of certificates, public recognition. Also catered meal for bank with highest cessation rate at 6 months follow‐up, served to winning bank by executives of the losing banks. Participant badges distributed throughout competition sites, and ‘smoking barometer' in each bank. | |
| Outcomes | PPA at 6 weeks and 6 months, verified by CO < 8 ppm and SCN samples. CO reduction in non‐quitters. |
|
| Notes | Smoking reduction was also a measured outcome.
Study was funded by National Institutes of Health. Originally in C&I. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assignment by worksite type, not randomization, described as “quasi‐experimental”. Quote: “A quasi‐experimental design was employed in which the savings and loan was assigned to a basic treatment program and the four banks were assigned to a competition plus basic treatment program.” |
| Allocation concealment (selection bias) | High risk | Participants were not randomized. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No difference in attrition rates between the two conditions through treatment. It was possible to contact 97% of participants who completed the program for follow‐up. |
| Other bias | Unclear risk | Allocation was by worksite, but analysis by individual participant. The risk of bias through ICC was not assessed. 6 months assessment was the main incentive point, so could more accurately be seen as end‐of‐program, rather than true follow‐up. |
Klesges 1987.
| Methods | Country: USA
Setting: 8 worksites (4 Fargo, North Dakota; 4 Eugene, Oregon) Design: cluster‐RCT with 2x2 factorial design |
|
| Participants | 136 smokers (127 completed the programme). average age 38 years, 47% female, average cpd 28, average years smoking, 19. | |
| Interventions | 2 x 2 design with factors Competition (Yes/No) and Relapse Prevention training (Yes/No). Basic Programme: 6‐week group CBT, aimed at brand‐switching and reduction, aiming for final quitting or reduced % of each cigarette smoked. Also information on maintenance and RP. Competition: as Basic Programme, + within‐site team competitions. Weekly feedback on team performance, ‘smoking barometer', prizes for team with the highest % completing treatment (˜ US$5 per team member), for team with highest % of quitters at the end of the programme (˜ US$10 per member), and for the team with the highest abstinence rate at 6 months follow‐up (˜ US$15 per member). Relapse Prevention: As Basic Programme, with 1‐ or 2‐monthly meetings to discuss, role‐play, encouragement to quit again, develop relapse prevention skills. |
|
| Outcomes | PPA at 6 months. Validation: CO < 10 ppm and SCN at baseline. CO preferred to SCN at 6‐month follow‐up. Relapse prevention training. Changes in smoking habits among non‐quitters. |
|
| Notes | Analysis found no interaction effects on cessation outcomes. Combined Competition versus No Competition is therefore the comparison used in this review. Rewards were paid for team performance, not individual success or failure. Study was funded by National Heart, Lung, and Blood Institute. Originally in C&I. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The four worksites in each community were randomly assigned.” No further information specified. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were not differential across conditions and 93% overall completed treatment (127/136). 96% of these participants (122/127) were available for the 6‐month follow‐up ‐ this is not split by condition; however numbers are so low it is unlikely that dropout was different across groups. |
| Other bias | Unclear risk | Allocation was by worksite, but analysis by individual. The risk of bias through ICC was not assessed. Quit rates were not reported as ITT (denominator was based on those who completed treatment); it is possible to discern total number randomized from the paper, but not number per group. 6‐month assessment was the main incentive point, so could more accurately be seen as end‐of‐program, rather than true follow‐up. |
Koffman 1998.
| Methods | Countries: USA and Canada
Setting: aerospace manufacturing worksites in Pomona, Rancho Cucamonga and Ontario Design: 3‐arm quasi‐experimental cluster design with no randomization |
|
| Participants | Participation rate not known (185 participants followed up at 6 months). Average age 41, average years smoking 22.7, 41% female. Significant baseline difference in age, years at the company, job description, working with chemicals, years smoking, addiction level. | |
| Interventions | The 3 interventions were assigned to one worksite each. 1. Multicomponent package (M): Self‐help ALA package + group cessation sessions in teams of 5 to 7 + monthly telephone counselling for 12 months and maintenance sessions for weight, fitness and stress management. 2. Incentives (MI): as 1, + incentives: US$15 for abstinence each month during the 5‐month programme; US$5 for ‘fading' (smoking no more than 80 cigarettes) in 1st month. Participants organized into teams, and any US$15 forfeited by an individual was added to US$2500 “super grand prize”. 3 top teams (posted on ‘smoking barometer') at end of programme shared super grand prize (by then US$3960), with the top team winning 50% and 2 teams tied for second each winning 25%. 3. Traditional: Self‐help ACS ‘Fresh Start' manual + 5 x 90‐minute group support sessions + videos. |
|
| Outcomes | PPA at 6 months, 12 months. Validation: CO (groups 1 and 2 only) | |
| Notes | Control participants paid out US$20 deposit, refundable on programme completion; incentive participants paid out US$50 non‐refundable initiation fee. Suggests that the multicomponent element may be the key factor for efficacy, though confounded by more thorough evaluation at baseline for multicomponent and incentive than for the traditional group. Relevant comparison for this review is MI versus M. Study was funded by General Dynamics, Air Defense Division (participant companies), and materials donated by ALA and ACS. Originally in C&I. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomized. Allocation was “quasi‐experimental”, as 1 worksite would not accept randomization. |
| Allocation concealment (selection bias) | High risk | See above. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Biochemical validation was not consistently used across groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Other bias | Unclear risk | Allocation was by worksite, but analysis by individual participant. Incentives participants paid a non‐refundable US$50 initiation fee; this might have skewed or deterred participation. 6‐month assessment took place around the final phase of the reward program, so could more accurately be seen as end‐of‐program, rather than true follow‐up. The traditional group received a different initial assessment from the other intervention groups; however further details of the nature of this difference were not given. |
Ledgerwood 2014.
| Methods | Country: USA Setting: University clinic, Michigan Design: 3‐arm randomized controlled trial |
|
| Participants | 81 participants (17 Standard Care, 28 Traditional CM, 36 Early‐Treatment Enhanced CM). Participants were adult nicotine‐dependent smokers with Fagerström score ≥ 4 and English literacy. Exclusions: uncontrolled psychiatric disorders, substance dependence (except for nicotine and caffeine), in recover for pathological gambling, already receiving smoking cessation treatment. 50 (62%) female, average age 45 years, 27 (33%) European American, 53 (65%) African American, 1 (1%) Other ethnicity, average education 14 years, average cpd 16.6. Around average 1 year more education in CM participants than Standard Care participants. |
|
| Interventions | Standard Care: in weeks 2 to 5, CO and cotinine monitoring + 5 minutes counselling twice daily, 5 days a week. Participants received US$1 per sample submitted + bonus of US$20 for submitting all 10 samples in a week. Traditional CM: as Standard Care, + entry into prize draws. Prize urn contained 250 slips of paper, 50% with a reward: 44.8% Small (worth around US$1, e.g. snacks, toiletries), 4.8% Large (worth around US$20, e.g. gift certificates, electronics), 0.4% Jumbo (worth US$100, e.g. DVD player, gift certificate). On Day 1, participant drew for a prize if CO down by at least 3 ppm, and on subsequent days if CO down by at least 6 ppm. Number of draws available increased by 1 per day for every day abstinent, to a maximum of 5 at the end of the week. 5 bonus draws were awarded for Monday cotinine level ≤ 100 ng/mL in weeks 3 to 5. In total 180 regular draws and 15 bonus draws were possible. Enhanced CM: As for Traditional CM, but in the first week of prize draws a prize was guaranteed if test was negative. Cotinine‐negative tests also received a guaranteed prize. The guaranteed prize urn contained 91.2% Small, 8% Large, 0.8% Jumbo prizes. Subsequent weeks used a regular prize urn with 30% Small, 4% Large, 0.2% Jumbo prizes. |
|
| Outcomes | PPA, cotinine (≤ 100 ng/mL) and CO (≤ 6 ppm) verified, at 2 months and 6 months. Prize money won, attendance at CM schedules. |
|
| Notes | Traditional CM versus Standard Care is the comparison used in this review. Also in ISC. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician‐prepared sequentially numbered randomization envelopes concealed group assignment until assigned. |
| Allocation concealment (selection bias) | Low risk | As above. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6m dropout by group: Standard Care 2/17, Traditional CM7/28, Enhanced CM 10/36. Differences not significant and ITT analyses performed. |
Lillington 1995.
| Methods | Country: USA Setting: 4 women, infant and children sites in south‐central Los Angeles, California Design: cluster‐RCT |
|
| Participants | 768 pregnant women, 18+, who had smoked in the previous year, attending any of 4 clinic sites (2 experimental, 2 control) from similar neighbourhoods that were paired on ethnic mix. Of 555 women followed up, average age 26.8 years, 291 (53.0%) African‐American, 234 (42.6%) Hispanic, 20 (3.6%) Caucasian, 4 (0.7) Other ethnicity, 225 (40.5%) current smokers and 330 (59.5%) ex‐smokers at baseline. Participants in experimental group were more likely to be current smokers (51.0% versus 36.5%) and less likely to be in 3rd trimester (22.1% versus 39.5%) than controls. |
|
| Interventions | Experimental group: assessment of smoking motivation and intention to quit. Bilingual (Spanish/English) health educators provided 15 minutes individual counselling including risk information and quit messages or reinforcement. Participants selected a quit date and nominated a “quit buddy”. Participants received a self‐help guide (“Time for a change”) with behavioural counselling. Weekly prize draws were available for completing activity sheets. Prizes were inexpensive baby items (e.g. baby toys, infant clothing) and a grand prize of US$100. Participants received a booster postcard after 1 month. Control group: “Usual care”, including printed information about the risks of smoking during pregnancy and a group quit‐smoking message. |
|
| Outcomes | PPA at 6 weeks postpartum (self‐report + salivary cotinine < 20 ng/mL, although majority of self‐reported quitters did not provide a saliva sample). | |
| Notes | Study sample contains both current smokers and ex‐smokers at baseline. Length of follow‐up differs between participants, depending on length of gestation at time of recruitment. Also in PIP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only 111/254 of those who self‐reported smoking provided a saliva sample, and quote: “the number of saliva samples was too small to permit analysis for the baseline smokers”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 28% attrition in intervention group and 25% in control group, combining both baseline smokers and ex‐smokers. Attrition not reported separately for baseline smokers. |
| Other bias | High risk | Analysis did not allow for clustering or matching of clusters. Baseline inequality in important characteristics such as % currently smoking. |
Maheu 1989.
| Methods | Country: USA Setting: 2 aerospace worksites (Competition: ˜4500 employees; No Competition: ˜12000 employees) in San Diego, California. Both sites had recent smoking bans in public areas. Design: non‐randomized comparative study, cluster design | |
| Participants | Total 56 (Competition: 32, No Competition: 24). Significant differences on age and years smoking. Av 29.9 cpd, 41% female, 12% blue‐collar workers. Recruitment at Competition site was 2% of eligible smokers, versus 0.6% at No Competition site (P < 0.01). | |
| Interventions | Both sites received 9 x 2‐hour class meetings (self‐monitoring, aversive ‘smoke‐holding', nicotine gum) and 9 x 1‐hour maintenance meetings over 14 weeks. Strategies included stress and weight management, relaxation and RP. Participants all paid a US$50 tuition fee, of which they could win US$35 back for attendance and abstinence. A buddy system was promoted where pairs or triads of participants could contact each other for 3 months after the quit day. 1. Competition participants were divided into 3 teams; team with most abstainers at 3 months won pooled prize of US$160. Also a site‐wide raffle, and a participants' raffle for attendance at meetings. Non‐participants could sponsor a smoker who they could contact as desired. Non‐participants whose sponsored smokers were confirmed abstinent at 3 months were given 5 tickets for a US$150 travel voucher raffle. 2. No Competition participants abstinent at 3 months received pooled prize (US$120) divided equally. | |
| Outcomes | Continuous abstinence CO < 10 ppm from weeks 5, and at 3 months and 1 year. SCN also collected at 3 months in ‘bogus pipeline' procedure. ‘Buddy' supportiveness. Number of sick days. |
|
| Notes | Allocation was by worksite, but analysis by individual participant. Intervention being tested and rewarded was group co‐operation and competition. All participants received partial refunds for programme attendance, and for attending 1‐year follow‐up. Study was funded by Merrell Dow Pharmaceuticals (Nicorette products). Originally in C&I. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized. |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were followed up. |
McAlister 2000.
| Methods | Country: Russia Setting: Pitkäranta and Suojärvi, a comparable neighbouring district, both in Russian Karelia Design: quasi‐experimental panel study, with baseline and 1‐year smoking surveys | |
| Participants | 176 daily smokers in Pitkäranta (experimental) and 202 in Suojärvi (control). Baseline comparisons not discussed, but communities “very similar”. Smoking prevalence estimated to be 47% for men and 6.3% for women in Pitkäranta, and 55% and 8.3%, respectively in Suojärvi. | |
| Interventions | 1. Intervention: 6 months rolling Q&W contest, monthly draws for holidays for quitters and their nominated supporters. Newspaper and leaflet support throughout the campaign. 2. Control: Surveys only, no cessation programme or contest. | |
| Outcomes | PP at 12 months on ITT basis, and on responders‐only basis. CO validation for potential winners. | |
| Notes | Baseline measure was taken from a large international study of adult populations in different countries in Eastern and Western Europe. 1‐year follow‐up was an ad hoc survey by the study team. Originally in Q&W. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study was described as quasi‐experimental. Participants were not randomly assigned to an intervention; however their selection from the population was reported to be “random”. |
| Allocation concealment (selection bias) | High risk | The study was not randomized. The Q&W competition was implemented in one geographic area and a comparison area where the competition was not being held was selected. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | CO validation was carried out in the Q&W group only. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The panel of smokers consisted of 176 persons in the Q&W area and 202 in the control area. Follow‐up surveys were conducted with 102 and 89 of the daily smokers in the Q&W and control areas, respectively. The higher follow‐up rate in Pitkäranta probably reflects the more intense activity and interest in smoking issues among health workers in that area. |
Parker 2007.
| Methods | Country: USA Setting: 22 urban prenatal care clinics in Connecticut, Rhode Island and Massachusetts Design: 3‐arm randomized controlled trial |
|
| Participants | 1065 pregnant women (Control 378, Intervention‐1 329, Intervention‐2 358). Participants were required to have smoked in the past 30 days, were no more than 26 weeks pregnant, had telephone access and could speak English or Spanish. In a subsample in which baseline characteristics were reported, average age 25, 62% White, 27% Black, 18% Hispanic, 3% Other ethnicity, 41% had ≤ 11 years education, mean cpd 8.1. Groups comparable at baseline. |
|
| Interventions | Control: Self‐help materials including a quit kit and video. Intervention‐1: Quit kit + enrolment in a Q&W lottery. US$100 prize was draw was available for smokers with 30‐day abstinence confirmed by urinary cotinine. Intervention‐2: Additionally received up to 3 motivational interviewing telephone calls. |
|
| Outcomes | Self‐reported abstinence within the past 30 days, verified by urinary cotinine < 80 ng/mL (verification only possible for 23 samples), measured at 32 weeks gestation and 6 months postpartum. Number of telephone calls, cost‐effectiveness. |
|
| Notes | Intervention‐1 versus Control is the comparison used in this review. Also in PIP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Two study reports give different information about the proportion of participants who had biochemical verification at follow‐up. Number without biochemical verification may be high and possibility of differential verification between groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear which time point conference abstract reports on and therefore unable to assess attrition. |
| Other bias | Unclear risk | Unclear which time point conference abstract reports on and therefore possibility of publication bias as no complete published report could be found. |
Sexton 1984.
| Methods | Country: USA Setting: Large university hospital obstetric clinic in Baltimore, Maryland Design: Randomized controlled trial |
|
| Participants | 935 pregnant women (Control 472, Intervention 463) who were smoking ≥ 10 cpd immediately prior to pregnancy, enrolled at < 18 weeks gestation. Average age 24.9 years, 40.8% Black, 59.2% White/Other, average education 12.3 years, average cpd 11.2. |
|
| Interventions | Control: not clearly specified. Intervention: at least 1 in‐person visit and additional telephone contacts from a health educator. Self‐help information was mailed every 2 weeks, and a monthly newsletter was mailed in the last year of the study. Group sessions were available. Hypnosis was offered but discontinued because of low uptake. Intervention continued until end of pregnancy. A monthly lottery ran from the first newsletter until the end of the intervention period. Participants not smoking in the previous 2 weeks entered a prize draw for a prize (e.g. perfume, makeup) worth around US$30. Participants in both groups received a US$20 gift certificate at the end of the study for participation. A bonus of a US$10 gift certificate was introduced in the last year of the study to participants 2 weeks after they stopped smoking. |
|
| Outcomes | Cessation measure not clearly stated, but recorded at 8 months gestation among women who were still pregnant. Verification used SCN. | |
| Notes | Participants not still pregnant at 8 months were included in the study but not included in the results. Also in PIP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Inadequate biochemical verification, outcome mainly based on self‐report with possible differential misreport given different levels of support in different study arms. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout before 8 months was primarily due to early delivery or miscarriage and little difference in rate between groups. |
| Other bias | High risk | 16% of Intervention and 17% of Control quit between recruitment and randomization which might bias effect size estimate among baseline smokers. |
Thomas 2016.
| Methods | Country: USA Setting: Universities in Minnesota, Ohio, Texas and Wisconsin Design: 2 x 2 factorial randomized controlled trial |
|
| Participants | 1217 adult undergraduates enrolled in participating universities who smoked at least one cigarette on 10 or more days in the last 30 days. Participants needed to be willing to set a quit date within 1 month of eligibility assessment, had telephone and internet access and were willing to receive telephone coaching. Pregnancy, pathological gambling and use of current smoking cessation medication/counselling were exclusion criteria. 668 (55%) female, average age 26.3 years, 85% white, average 11.5 cpd, no clear imbalance between groups at baseline. |
|
| Interventions | Conditions in this factorial trial were ‘Standard contest/Multiple contest' and ‘Counselling protocol' (yes/no). Standard contest: single Q&W contest lasting 30 days. Participants asked to abstain from tobacco, received free 2‐week supply of NRT patches with dose determined by cpd and days smoked on baseline survey. Those completing survey in final week of contest received a US$25 gift card. Those with confirmed abstinence were entered into a “lottery‐based contest prize” (a trip to the Caribbean worth US$3000 or the equivalent value in gift cards) (602 participants). Multiple contests: same as Standard contest + entry into two additional subsequent 30‐day contests. Prize available was contingent upon the number of contests in which the winning participant had confirmed abstinence (US$3000 for 1 month abstinence, US$4000 for 2 months, US$5000 for 3 months). Those completing surveys after contests 2 and 3 received gift cards for US$25 and US$35, respectively. Counselling protocol: up to 6 telephone‐administered MAPS (Motivation and Problem Solving) counselling sessions over 12 weeks (615 participants). Counsellors contacted participants to schedule first call approx. 10 days prior to quit date, remaining five sessions scheduled at end of each session at discretion of participants. Encouraged to spend at least 20 minutes per call. Counsellors received 40 hours of training. Biochemical verification used a urine NicCheck and/or NicAlert test, with those reporting NRT use analyzed for anatabine/anabasine. |
|
| Outcomes | Continuous abstinence at 6 months, 30‐day PPA at 4 months, 6 months, cost‐effectiveness | |
| Notes | Paper reports results pooled over each design factor but found no interaction effects on cessation outcomes. Combined Standard versus Multiple contest is therefore the comparison used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Used biochemical validation so differential misreport judged unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Around 19% of participants did not complete or were lost to follow‐up with similar proportions in each arm. Authors tested impact in sensitivity analyses and state it did not affect conclusions. |
Walsh 1997.
| Methods | Country: Australia Setting: public hospital antenatal clinic in Newcastle, New South Wales Design: randomized controlled trial |
|
| Participants | 239 pregnant women (Control 145, Intervention 148) attending first prenatal clinic appointment and self‐reporting current smoking, ≤ 26 weeks gestation. Quote: “Too ill or psychologically disturbed” excluded. No demographic characteristics reported. | |
| Interventions | Control: doctor and midwife advice to stop smoking. Participants received a sticker, pamphlet and 2‐page cessation guide. Intervention: risk information from doctor. 14‐minute video. 10‐minute standardized information from midwife with negotiation of a quit date where possible. Self‐help manual and 4 packets of confectionery gum. Chance to enter a lottery draw (4 prizes of approximately US$75 each) for biochemically‐validated abstainers at a second visit. Social support from an accompanying adult + a manual. Chart reminder (sticker in medical record). A letter was sent within 10 days. Brief midwife and doctor counselling was available at the second visit and at the 34 to 36 weeks visit, where continued smokers were advised to attend an external cessation course. |
|
| Outcomes | PPA and consecutive cessation (defined as abstinence at 2 or 3 consecutive visits) measured at 6 to 12 weeks postpartum, verified by urinary cotinine < 500 nmol/L. | |
| Notes | Quote: “Only 42% of the women strongly agreed/agreed that the lottery encouraged them to quit” (the lowest of all the intervention components). Also in PIP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Quote: “Precoded questionnaires contained in manila envelopes.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical verification used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Around 25% dropout, similar between groups. |
Winhusen 2014.
| Methods | Country: USA Setting: 12 Community Treatment Programs aimed at treating individuals with stimulant dependence Design: randomized controlled trial |
|
| Participants | 538 adults (Control 271, Intervention 267) undergoing treatment for substance use disorder (cocaine 56%, methamphetamine 39%, both 5%). 258 (48%) female, average age 36.4 years, 32% African‐American and 60% Caucasian, average education 11.9 years, average 16.3 cpd, pregnant women excluded, 241 (45%) also had diagnosis of alcohol/other non‐stimulant disorder. |
|
| Interventions | Treatment as usual: participants received substance use disorder treatment as typically provided by the study site (consisting of at least 1 treatment session per week for 10 weeks) Treatment as usual + Smoking Cessation Treatment intervention: extended‐release bupropion (150 mg/day in days 1 to 3, 300 mg/day in days 4 to 10), nicotine inhaler, smoking cessation counselling (10‐minute sessions weekly for 10 weeks using the ‘Smoke Free and Living It’ manual), prize‐based contingency management using “a fishbowl from which chips were drawn” to reinforce negative CO (< 4 ppm). Number of draws escalated with each consecutive week of abstinence and reset if evidence of smoking observed. Maximum draws 110, corresponding to approx. $380 of prizes (nature of prizes not specified). |
|
| Outcomes | 7‐day PPA measured at 10 weeks (end of treatment phase), 3 months and 6 months (self‐report and CO < 8 ppm). Continuous smoking abstinence in post‐quit days 15 to 42 stated in protocol as a secondary outcome but not reported. Primary outcome of study was being stimulant‐free (stimulant‐negative drug screens + self‐report in weeks 1 to 10). |
|
| Notes | Previously listed in ISC. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reports quote: “randomized 1:1 at a centralized site” but method of generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Used CO verification. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80.4% (control) and 78.7% iIntervention) completed 6‐month follow‐up and participants who did not were assumed to be smokers. |
ACS: American Cancer Society
ALA: American Lung Association
CBT: cognitive behavioural therapy
C&I: competitions and incentives
CM: contingency management
CO: carbon monoxide
COPD: chronic obstructive pulmonary disease
CPD: cigarettes per day
ICC: intra‐class correlation
ISC: Incentives for Smoking Cessation
ITT: intention‐to‐treat
FEV: forced expiratory volume
FVC: forced vital capacity
NRT: Nicotine Replacement Therapy
PIP: Psychosocial Interventions in Pregnancy
PPA: point prevalence abstinence
ppm: parts per million
Q&W: Quit and Win
RP: relapse prevention
SCN: saliva thiocyanate
SES: socioeconomic status
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashbury 2006 | Random sample (347 completed surveys) follow up of participants 12 months post‐contest. No comparison group. |
| Chan 2012 | Follow‐on study recruiting from the Hong Kong Quit to Win series. |
| Chapman 1993 | Four‐month follow‐up of a contest, no control group |
| Cheung 2013 | Follow‐on study recruiting from the Hong Kong Quit to Win series. |
| Croghan 2001 | Before‐and‐after population‐based survey, without a control group |
| Cummings 1990 | Population‐based survey, without a control group |
| Donatelle 2000a | Target is pregnant smokers (covered by reviews in Childhood and Pregnancy Group) |
| Donatelle 2000b | Studies the use of incentives; included study in Cahill 2015 |
| Donatelle 2000c | Studies the use of incentives; included study in Cahill 2015 |
| Donatelle 2002 | Studies the use of incentives; included study in Cahill 2015 |
| Drummond 2014 | Studies the use of incentives; included study in Cahill 2015 |
| Elder 1991 | No comparison group, followed up for only 2 months. |
| Gallagher 2007 | Studies the use of incentives; included study in Cahill 2015 |
| Giné 2010 | Studies the use of incentives; included study in Cahill 2015 |
| Glasgow 1985 | Cross‐sectional survey of participants one week post‐contest; no control group |
| Gomez‐Zamudio 2004 | Interventions being tested were pharmacological aids, social support and cessation materials. No comparison group |
| Halpern 2015 | Studies the use of incentives; included study in Cahill 2015 |
| Hawk 2006 | Observational study |
| HEA 1991 | Population‐based survey, no control group |
| Heil 2008a | Studies the use of incentives; included study in Cahill 2015 |
| Higgins 2004 | Trial of contingent versus non‐contingent rewards for abstinence on pregnancy and postpartum. |
| Higgins 2004a | Studies the use of incentives; included study in Cahill 2015 |
| Higgins 2014 | Studies the use of incentives; included study in Cahill 2015 |
| Jason 1995 | Studies the use of incentives; included study in Cahill 2015 |
| King 1987 | No details of comparison community |
| Kinoshita 2004 | Overview of 3 Osaka Quit & Win contests 1998‐2000, no control groups. |
| Kira 2016 | Not a competition |
| Kollins 2010 | Not a randomized trial, and participants were followed for 24 days. |
| Korhonen 1992 | Inter‐contest comparison of TV groups; no non‐intervention control group |
| Korhonen 1993 | No non‐intervention comparison group |
| Korhonen 1998 | Evaluation of 1994 contests in Finland, Russia, Catalonia; no comparison groups |
| Korhonen 1999 | No comparison group |
| Lai 2000 | Before‐and‐after population‐based survey, without a control group |
| Lando 1990 | Comparison of 2 Minnesota contests, but only 4 to 5 months follow‐up |
| Lando 1991a | Intervention did not contain a competition. Although some participants received the Quit & Win self‐help materials, no prize was available. |
| Lando 1991b | Survey data, followed up at 3 to 4 months, without a control group |
| Lando 1995a | No comparison group |
| Lefebvre 1990 | No non‐intervention comparison group |
| Leinweber 1994 | 6‐week follow‐up, no comparison group |
| NCT01983150 | Not a competition |
| O'Connor 2006 | 11 contests in New York 2001‐2004, 4‐ to 6‐month follow‐up. No control groups |
| Ondersma 2012 | Studies the use of incentives; included study in Cahill 2015 |
| Paxton 1980 | Studies the use of incentives; included study in Cahill 2015 |
| Paxton 1981 | Studies the use of incentives; included study in Cahill 2015 |
| Paxton 1983 | Studies the use of incentives; included study in Cahill 2015 |
| Pirie 1997 | Intervention being tested was social support, not the contest itself |
| Quintiliani 2015 | Not a competition |
| Rand 1989 | Studies the use of incentives; included study in Cahill 2015 |
| Resnicow 1997 | Quit & Win surveyed as part of a multicomponent intervention, no non‐intervention control group reported |
| Roberts 1993 | Follow‐up of a pilot contest, surveyed at 4 months |
| Rooney 2005 | No non‐intervention comparison group |
| Sarraf Zadegan 2006 | International Quit & Win contests in Iran; participation and self‐reported quit rates |
| Secades‐Villa 2014 | Studies the use of incentives; included study in Cahill 2015 |
| Sheikhattari 2015 | Not a competition |
| Shoptaw 2002 | Studies the use of incentives; included study in Cahill 2015 |
| Stotts 2013 | Four‐month follow‐up |
| Tappin 2015 | Studies the use of incentives; included study in Cahill 2015 |
| Tevyaw 2009 | Studies the use of incentives; included study in Cahill 2015 |
| Tillgren 1992 | No non‐intervention control group |
| Tillgren 2000 | Contest for smoking mothers, 12‐month follow‐up, no control group |
| Tuten 2012 | Studies the use of incentives; included study in Cahill 2015 |
| Volpp 2006 | Studies the use of incentives; included study in Cahill 2015 |
| Volpp 2009 | Studies the use of incentives; included study in Cahill 2015 |
| Wang 2014 | Follow‐on study recruiting from the Hong Kong Quit to Win series |
| Wang 2015 | Follow‐on study recruiting from the Hong Kong Quit to Win series |
| Wang 2016 | Follow‐on study recruiting from the Hong Kong Quit to Win series |
| Wang 2017 | Follow‐on study recruiting from the Hong Kong Quit to Win series |
| Wang 2018 | Follow‐on study recruiting from the Hong Kong Quit to Win series |
| White 2013 | Studies the use of incentives; included study in Cahill 2015 |
| Windsor 1988 | Studies the use of incentives; included study in Cahill 2015 |
CO: carbon monoxide
Characteristics of ongoing studies [ordered by study ID]
Accornero 2014.
| Trial name or title | Quit Smoking Now |
| Methods | Randomized controlled trial (two arms) |
| Participants | Highly disadvantaged minority pregnant women (current daily smokers) recruited from outpatient obstetric clinics at a large teaching hospital, Miami‐Dade County, Florida, USA |
| Interventions | Participants received either a standard pyschoeducational intervention (Quit Smoking Now) only, or the same intervention plus contingency management based on biochemically‐verified abstinence (prize draws for prizes ranging from US$1 to US$100) |
| Outcomes | Primary outcomes include smoking abstinence from the quit date until 6 months postpartum, measured by self‐report, carbon monoxide, salivary and urine cotinine |
| Starting date | October 2015 |
| Contact information | Veronica Accornero, University of Miami (vaccornero@med.miami.edu) |
| Notes |
Horgan 2016.
| Trial name or title | Pro‐Change Smoking Cessation Intervention |
| Methods | Randomized controlled trial (two arms) |
| Participants | Current adult smokers |
| Interventions | Usual care with behavioural economics incentives (nature of incentives not specified), or usual care with minimal incentives |
| Outcomes | Smoking cessation at six months, engagement in treatment |
| Starting date | January 2016 |
| Contact information | Karen Horgan, VAL Health |
| Notes | Clinicaltrials.gov record suggests trial is complete but not yet unpublished, and insufficient information is available to classify as a competition intervention |
Ledgerwood 2015.
| Trial name or title | Sequential Multiple Assignment Randomized Trial (SMART) |
| Methods | Two‐phase randomized controlled trial |
| Participants | Current adult smokers enrolled in an HIV clinic with a diagnosis of HIV/AIDS |
| Interventions | Phase 1: participants are randomized to receive either standard care (brief counselling and bupropion) or standard care and entry into a prize draw (contingency management) Phase 2a: non‐responders from Phase 1 are randomized to receive continued counselling and monitoring support, with or without a prize draw Phase 2b: responders from Phase 1 are randomized to receive either no additional treatment, or continued monitoring + a prize draw |
| Outcomes | Primary outcomes: urinary cotinine, longest duration of continuous abstinence, 7‐day self‐reported point prevalence, carbon monoxide at follow‐up times up to 12 months |
| Starting date | August 2013 |
| Contact information | Lisa Sulkowski, Wayne State University (lsulkows@med.wayne.edu) David Ledgerwood, Wayne State University |
| Notes |
Differences between protocol and review
For this 2018 update, we have separated out the competitions trials from the incentives trials, and now present the findings as two separate updates. We have also added the studies from the ‘Quit and Win contests for smoking cessation' review (Cahill 2008b) to the competitions trials, and withdrawn that review. Therefore, this review incorporates competition interventions, including ‘Quit & Win' contests, only. We took this decision because competition‐based programmes are now a rarely‐used intervention, while incentives and contingency management programmes are increasingly being developed and deployed, so that the research agenda continues to grow and change in this area. In making this review distinct from the separate review of incentives, we have clarified the inclusion criteria, which has resulted in two previously‐included studies (Hawk 2006 and Lando 1991a) now being excluded. In Hawk 2006, participants decided which of the intervention conditions they wanted to join, and so is not a controlled trial with group allocation determined by the investigators. Lando 1991a was included in Cahill 2008b on the basis that it used self‐help materials taken from the Quit & Win programme, but did not offer a prize or include a competition element, and is therefore ineligible for this review. In common with other reviews in this review group, we have presented effect sizes in the current update as risk ratios where possible, and so some results differ from previous review versions in which results were presented as odds ratios.
Contributions of authors
For previous versions of the ‘Competitions and Incentives' and ‘Quit & Win' reviews, data extraction was carried out by Kate Cahill and RP. Kate Cahill wrote the original review, with comments from RP, who also advised on and conducted statistical analyses. NL combined the ‘Competitions' elements of Cahill 2011 and Cahill 2008b into one review for the current version. TRF and JHB carried out study screening and data extraction of new studies for the current version, and TRF updated the text and the analyses, with the other authors reviewing drafts. All review authors approved the final version of this review.
Sources of support
Internal sources
Department of Primary Health Care, University of Oxford, UK.
National School for Health Research School for Primary Care Research, UK.
External sources
National Institute for Health Research, UK.
Declarations of interest
TRF: none known
JHB: none known
RP: none known
NL is employed by the University of Oxford to work as a Managing Editor for the Cochrane Tobacco Addiction Review Group. Core infrastructure funding for the Cochrane Tobacco Addiction Group is provided by the NIHR to the University of Oxford. She was a co‐applicant and collaborator on a research grant awarded by the NIHR HTA programme (09/110/01), investigating the use of pre‐quit nicotine patches for smoking cessation. The excess treatment provided for this research were nicotine patches, supplied free of charge by GlaxoSmithKline (GSK). However, GSK had no further involvement in the research, and this had no impact on the reported work. This trial was completed in 2016.
New
References
References to studies included in this review
Alessi 2014 {published data only}
- Alessi SM, Petry NM. Smoking reductions and increased self‐efficacy in a randomized controlled trial of smoking abstinence ‐ contingent incentives in residential substance abuse treatment patients. Nicotine & Tobacco Research 2014;16(11):1436‐45. [DOI: 10.1093/ntr/ntu095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alessi 2017 {published data only}
- Alessi SM, Rash CJ. Treatment satisfaction in a randomized clinical trial of mHealth smoking abstinence reinforcement. Journal of Substance Abuse Treatment 2017;72:103‐10. [DOI: 10.1016/j.jsat.2016.06.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Rash CJ, Petry NM. A randomized trial of adjunct mHealth abstinence reinforcement With transdermal nicotine and counseling for smoking cessation. Nicotine & Tobacco Research 2017;19(3):290‐8. [DOI: 10.1093/ntr/ntw155] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bains 2000 {published data only}
- Bains N, Pickett W, Laundry B, Mecredy D. Predictors of smoking cessation in an incentive‐based community intervention. Chronic Diseases in Canada 2000;21(2):54‐61. [PubMed] [Google Scholar]
- Pickett W, Bains N. Staging of adult smokers according to the transtheoretical model of behavioural change: analysis of an Eastern Ontario cohort. Canadian Journal of Public Health 1998;89(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowley 1995 {published data only}
- Crowley TJ, Macdonald MJ, Walter MI. Behavioral anti‐smoking trial in chronic obstructive pulmonary disease patients. Psychopharmacology 1995;119(2):193‐204. [DOI] [PubMed] [Google Scholar]
Glasgow 1993 {published data only}
- Glasgow RE, Hollis JF, Ary DV, Boles SM. Results of a year‐long incentives‐based worksite smoking‐cessation program. Addictive Behaviors 1993;18:455‐64. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Hollis JF, Ary DV, Lando HA. Employee and organizational factors associated with participation in an incentive‐based worksite smoking cessation program. Journal of Behavioral Medicine 1990;13(4):403‐18. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Hollis JF, Pettigrew L, Foster L, Givi MJ, Morrisette G. Implementing a year‐long worksite‐based incentive program for smoking cessation. American Journal of Health Promotion 1991;5(3):192‐9. [DOI] [PubMed] [Google Scholar]
Gomel 1993 {published data only}
- Gomel M, Oldenburg B, Simpson JM, Owen N. Work‐site cardiovascular risk reduction: a randomized trial of health risk assessment, education, counseling, and incentives. American Journal of Public Health 1993;83(9):1232‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 2005 {published data only}
- Hahn EJ, Rayens MK, Chirila C, Riker CA, Paul TP, Warnick TA. Effectiveness of a quit and win contest with a low‐income population. Preventive Medicine 2004;39:543‐50. [DOI] [PubMed] [Google Scholar]
- Hahn EJ, Rayens MK, Kirsch KL, Passik SD. Brief report: pain and readiness to quit smoking cigarettes. Nicotine & Tobacco Research 2006;8(3):473‐80. [DOI] [PubMed] [Google Scholar]
- Hahn EJ, Rayens MK, Warnick TA, Chirila C, Rasnake RT, Paul TP, et al. A controlled trial of a Quit and Win contest. American Journal of Health Promotion 2005;20(2):117‐26. [DOI] [PubMed] [Google Scholar]
Hennrikus 2002 {published data only}
- Hennrikus DJ, Jeffery RW, Lando HA, Murray DM, Brelje K, Davidann B, et al. The SUCCESS project: the effect of program format and incentives on participation and cessation in worksite smoking cessation programs. American Journal of Public Health 2002;92(2):274‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando HA, Thai DT, Murray DM, Robinson LA, Jeffery RW, Sherwood NE, et al. Age of initiation, smoking patterns, and risk in a population of working adults. Preventive Medicine 1999;29(6 Pt 1):590‐8. [DOI] [PubMed] [Google Scholar]
- Martinson BC, Murray DM, Jeffery RW, Hennrikus DJ. Intraclass correlation for measures from a worksite health promotion study: estimates, correlates and applications. American Journal of Health Promotion 1999;13(6):347‐57. [DOI] [PubMed] [Google Scholar]
- Sherwood NE, Hennrikus DJ, Jeffery RW, Lando HA, Murray DM. Smokers with multiple behavioral risk factors: how are they different?. Preventive Medicine 2000;31(4):299‐307. [DOI] [PubMed] [Google Scholar]
Klesges 1986 {published data only}
- Hessol NA. Worksite smoking modification competitions: long‐term vs short‐term success. American Journal of Public Health 1986;76(7):819‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges RC, Vasey MM, Glasgow RE. A worksite smoking modification competition: potential for public health impact. American Jounral of Public Health 1986;76(2):198‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klesges 1987 {published data only}
- Klesges RC, Glasgow RE, Klesges LM, Morray K, Quayle R. Competition and relapse prevention training in worksite smoking modification. Health Education Research 1987;2(1):5‐14. [Google Scholar]
Koffman 1998 {published data only}
- Koffman DM, Lee JW, Hopp JW, Emont SL. The impact of including incentives and competition in a workplace smoking cessation program on quit rates. American Journal of Health Promotion 1998;13(2):105‐11. [DOI] [PubMed] [Google Scholar]
- Matson D M. The impact of an incentive/competition program on participation and smoking cessation among high‐risk smokers at the worksite. Proquest Digital Dissertations AAT 9300218, DAI‐B 53/12 1992.
Ledgerwood 2014 {published data only}
- Ledgerwood DM, Arfken CL, Petry NM, Alessi SM. Prize contingency management for smoking cessation: a randomized trial. Drug and Alcohol Dependence 2014;140:208‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lillington 1995 {published data only}
- Lillington L, Royce J, Novak D, Ruvalcaba M, Chlebowski R. Evaluation of a smoking cessation program for pregnant minority women. Cancer Practice 1995;3(3):157‐63. [PubMed] [Google Scholar]
Maheu 1989 {published data only}
- Maheu MM, Gervitz RN, Sallis JF, Schneider NG. Competition/cooperation in worksite smoking cessation using nicotine gum. Preventive Medicine 1989;18(6):867‐76. [DOI] [PubMed] [Google Scholar]
McAlister 2000 {published data only}
- McAlister AL, Gumina T, Urjanheimo E‐L, Laatikainen T, Uhanov M, Oganov R, et al. Promoting smoking cessation in Russian Karelia: a 1‐year community‐based program with quasi‐experimental evaluation. Health Promotion International 2000;15(2):109‐12. [Google Scholar]
Parker 2007 {published data only}
- Parker DR, Roberts MB, Windsor RA, Lasater TM. Telephone‐based smoking cessation interventions effective in high‐risk, underserved pregnant women. Joint Conference of SRNT and SRNT‐Europe; 2009 April 27‐30; Dublin, Ireland 2009.
- Parker DR, Windsor RA, Roberts MB, Hecht J, Hardy NV, Strolla LO, et al. Feasibility, cost, and cost‐effectiveness of a telephone‐based motivational intervention for underserved pregnant smokers. Nicotine & Tobacco Research 2007;9(10):1043‐51. [DOI] [PubMed] [Google Scholar]
Sexton 1984 {published data only}
- Fox NL, Sexton M, Hebel JR, Thompson B. The reliability of self‐reports of smoking and alcohol consumption by pregnant women. Addictive Behaviors 1989;14(2):187‐95. [DOI] [PubMed] [Google Scholar]
- Fox NL, Sexton MJ, Hebel JR. Alcohol consumption among pregnant smokers: effects of a smoking cessation intervention program. American Journal of Public Health 1987;77(2):211‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BH. Estimating treatment effects in randomized clinical trials with non‐compliance: the impact of maternal smoking on birthweight. Health Economics 2001;10(5):399‐410. [DOI] [PubMed] [Google Scholar]
- Hebel JR, Nowicki P, Sexton M. The effect of antismoking intervention during pregnancy: an assessment of interactions with maternal characteristics. American Journal of Epidemiology 1985;122(1):135‐48. [DOI] [PubMed] [Google Scholar]
- Nowicki P, Gintzig L, Hebel JR, Latham R, Miller V, Sexton M. Effective smoking intervention during pregnancy. Birth 1984;11(4):217‐24. [DOI] [PubMed] [Google Scholar]
- Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA 1984;251(7):911‐5. [PubMed] [Google Scholar]
- Sexton M, Nowicki P, Hebel JR. Verification of smoking status by thiocyanate in unrefrigerated, mailed saliva samples. Preventative Medicine 1986;15(1):28‐34. [DOI] [PubMed] [Google Scholar]
Thomas 2016 {published data only}
- Popp J, Nyman JA, Luo X, Bengtson J, Lust K, An L, et al. Cost‐effectiveness of enhancing a Quit‐and‐Win smoking cessation program for college students. European Journal of Health Economics 2018;19(9):1319‐33. [DOI: 10.1007/s10198-018-0977-z] [DOI] [PubMed] [Google Scholar]
- Thomas JL, Bengtson JE, Wang Q, Luo X, Marigi E, Ghidei W, et al. Abstinence rates among college cigarette smokers enrolled in a randomized clinical trial evaluating Quit and Win contests: The impact of concurrent hookah use. Preventive Medicine 2015;76:20‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Luo X, Bengtson J, Wang Q, Ghidei W, Nyman J, et al. Enhancing Quit & Win contests to improve cessation among college smokers: a randomized clinical trial. Addiction 2016;111:331‐9. [DOI: 10.1111/add.13144] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Reitzel LR, Figueroa PY, Velasquez MM, Mazas CA, Ninciripini PM, et al. Motivation and Problem Solving (MAPS): motivationally based skills training for treating substance use. Cognitive and Behavioral Practice 2013;20(4):501‐16. [DOI: 10.1016/j.cbpra.2011.11.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walsh 1997 {published data only}
- Walsh RA, Redman S, Brinsmead MW, Byrne JM, Melmeth A. A smoking cessation program at a public antenatal clinic. American Journal of Public Health 1997;87(7):1201‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RA, Redman S, Byrne JM, Melmeth A, Brinsmead MW. Process measures in an antenatal smoking cessation trial: another part of the picture. Health Education Research 2000;15(4):469‐83. [DOI] [PubMed] [Google Scholar]
Winhusen 2014 {published data only}
- Winhusen T, Stitzer M, Woody G, Brigham G, Kropp F, Ghitza U, et al. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant‐dependent individuals. Contemporary Clinical Trials 2012;33:197‐205. [DOI: 10.1016/j.cct.2011.09.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Adihoff B, Lewis DF, Brigham GS, Gardin JG 2nd, Sonne SC, et al. A tale of two stimulants: mentholated cigarettes may play a role in cocaine, but not methamphetamine, dependence. Drug and Alcohol Dependence 2013;133(3):845‐51. [DOI: 10.1016/j.drugalcdep.2013.09.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG 2nd, Penn P, et al. A randomized trial of concurrent smoking‐cessation and substance use disorder treatment in stimulant‐dependent smokers. Journal of Clinical Psychiatry 2014;75(4):336‐43. [DOI: 10.4088/JCP.13m08449] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Kropp F, Theobald J, Lewis DF. Achieving smoking abstinence is associated with decreased cocaine use in cocaine‐dependent patients receiving smoking cessation treatment. Drug and Alcohol Dependence 2014;134:391‐5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ashbury 2006 {published data only}
- Ashbury FD, Cameron C, Finlan C, Holmes R, Villareal E, Decoste Y, et al. The impact of a quit smoking contest on smoking behaviour in Ontario. Chronic Diseases in Canada 2006;27(2):77‐84. [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan SS‐c. Promoting smoking cessation in the community via Quit to Win contest 2012. https://clinicaltrials.gov/ct2/show/NCT01670864.
Chapman 1993 {published data only}
- Chapman S, Smith W, Mowbray G, Hugo C, Egger G. Quit and win smoking cessation contests: how should effectiveness be evaluated?. Preventive Medicine 1993;22:423‐32. [DOI] [PubMed] [Google Scholar]
Cheung 2013 {published data only}
- Cheung LWH. Promoting smoking cessation in the community via Quit to Win contest 2013. https://clinicaltrials.gov/ct2/show/NCT01928251.
Croghan 2001 {published data only}
- Croghan IT, O'Hara MR, Schroeder DR, Patten CA, Croghan GA, Hays JT, et al. A community‐wide smoking cessation program: Quit and Win 1998 in Olmsted County. Preventive Medicine 2001;33:229‐38. [DOI] [PubMed] [Google Scholar]
- Spencer L. A community‐wide smoking cessation program: Quit and Win 1998 in Olmsted County. American Journal of Health Promotion 2005;19(3):205‐6. [Google Scholar]
Cummings 1990 {published data only}
- Cummings KM, Kelly J, Sciandra R, DeLoughry T, Francois F. Impact of a community‐wide stop smoking contest. American Journal of Health Promotion 1990;4(6):429‐34. [DOI] [PubMed] [Google Scholar]
Donatelle 2000a {published data only}
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Support (SOS) program. Tobacco Control 2000;9(Supplement 3):iii67‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donatelle 2000b {published data only}
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Suppl 2):S163‐79. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Support (SOS) program. Tobacco Control 2000;9(Supplement III):iii67‐iii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donatelle 2000c {published data only}
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Suppl 2):S163‐79. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Using social support, biochemical feedback, and incentives to motivate smoking cessation during pregnancy: comparison of three intervention trials. Poster at American Public Health Association meeting, Boston MA 2000.
Donatelle 2002 {published data only}
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Suppl 2):S163‐79. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Hudson D, Lichtenstein E. Using 5 A's and incentives to promote prenatal smoking cessation [presentation to National Conference of Tobacco or Health, 2002]. ncth.confex.com/ncth/responses/2002/257.ppt retrieved August 10 2004. 2002.
Drummond 2014 {published data only}
- Drummond MB, Astemborski J, Lambert AA, Goldberg S, Stitzer ML, Merlo CA, et al. A randomized study of contingency management and spirometric lung age for motivating smoking cessation among injection drug users. BMC Public Health 2014;14:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elder 1991 {published data only}
- Elder JP, Campbell NR, Mielchen SD, Hovell MF, Litrownik AJ. Implementation and evaluation of a community‐sponsored smoking cessation contest. American Journal of Health Promotion 1991;5(3):200‐7. [DOI] [PubMed] [Google Scholar]
Gallagher 2007 {published data only}
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. Journal of Psychoactive Drugs 2007;39(4):487‐97. [DOI] [PubMed] [Google Scholar]
Giné 2010 {published and unpublished data}
- Giné X, Karlan D, Zinman J. Put your money where your butt is: a commitment contract for smoking cessation. American Economic Journal: Applied Economics 2010;2:213‐35. [Google Scholar]
Glasgow 1985 {published data only}
- Glasgow RE, Klesges RC, Mizes JS, Pechacek TF. Quitting smoking: strategies used and variables associated with success in a stop‐smoking contest. Journal of Consulting and Clinical Psychology 1985;53(6):905‐12. [DOI] [PubMed] [Google Scholar]
Gomez‐Zamudio 2004 {published data only}
- Gomez‐Zamudio M, Renaud L, Labrie L, Massé R, Pineau G, Gagnon L. Role of pharmacologic aids and social supports in smoking cessation associated with Quebec's 2000 Quit and Win campaign. Preventive Medicine 2004;38:662‐7. [DOI] [PubMed] [Google Scholar]
Halpern 2015 {published data only}
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain‐McGovern J, et al. A randomized trial of four financial‐incentive programs for smoking cessation. New England Journal of Medicine 2015;372(22):2108‐117. [DOI: 10.1056/NEJMoa1414293] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain‐McGovern J, et al. Randomized trial of four financial‐incentive programs for smoking cessation. Journal of Vascular Surgery 2016;63(2 Supplement):553‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawk 2006 {published data only}
- Hawk LW, Higbee C, Hyland A, Alford T, O'Connor R, Cummings KM. Concurrent Quit & Win and nicotine replacement therapy voucher giveaway programs: participant characteristics and predictors of smoking abstinence. Journal of Public Health Management and Practice 2006;12(1):52‐9. [DOI] [PubMed] [Google Scholar]
- Hyland A, Higbee C, Bauer J, Brown A, Giovino G, Cummings KM. Erie‐Niagara Adult Tobacco Use Survey‐2. Buffalo, New York: Roswell Park Cancer Institute, 2004. [Google Scholar]
HEA 1991 {published data only}
- Health Education Authority. Quit and Win UK 1990: Evaluation summary. HEA Research Report Series 1994.
Heil 2008a {published data only}
- Heil SH, Higgins ST. Characterizing nicotine withdrawal and craving in pregnant cigarette smokers. 66th Annual Meeting of the Collegeon Problems of Drug Dependence; June 12‐17; San Juan Puerto Rico. 2004.
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher‐based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 2008;103(6):1009‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Solomon LJ, Lynch ME, McHale L, Dumeer A, et al. Voucher‐based incentives for abstinence from cigarette smoking in pregnant and postpartum women. Society for Research on Nicotine and Tobacco 13th Annual Meeting; Feb 21 ‐ 24; Austin, TX, USA. 2007.
- Heil SH, Tidey JW, Holmes HW, Badger GJ, Higgins ST. A contingent payment model of smoking cessation: effects on abstinence and withdrawal. Nicotine & Tobacco Research 2003;5(2):205‐13. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher‐based contingency management on birth outcomes. Addiction 2010;105(11):2023‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Mongeon JA, Solomon LJ, McHale L, et al. Biochemical verification of smoking status in pregnant and recently postpartum women. Experimental and Clinical Psychopharmacology 2007;15(1):58‐66. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking‐cessation outcomes in pregnant women. Drug and Alcohol Dependence 2006;85(2):138‐41. [DOI] [PubMed] [Google Scholar]
- Jones C, Gaalema D, Shephard DS, Erten M, Stoeckel M, Day S, et al. Incentive‐based treatments to promote smoking abstinence during pregnancy: findings from the Vermont Center on Behavior and Health. Value in Health 2014;17(3):A217. [Google Scholar]
- Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviors 2009;34(8):705‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon LJ, Higgins ST, Heil SH, Badger GJ, Thomas CS, Bernstein IM. Predictors of postpartum relapse to smoking. Drug and Alcohol Dependence 2007;90(2‐3):224‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology 2007;15(2):176‐86. [DOI] [PubMed] [Google Scholar]
Higgins 2004 {published data only}
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug & Alcohol Dependence 2009;104:s100‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking‐cessation outcomes in pregnant women. Drug and Alcohol Dependence 2006;85(2):138‐41. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher‐based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research 2004;6(6):1015‐20. [DOI] [PubMed] [Google Scholar]
- Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine & Tobacco Research 2010;12(5):483‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology 2007;15(2):176‐86. [DOI] [PubMed] [Google Scholar]
Higgins 2004a {published data only}
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher‐based contingency management on birth outcomes. Addiction 2010;105(11):2023‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug & Alcohol Dependence 2009;104 Suppl 1:s100‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking‐cessation outcomes in pregnant women. Drug and Alcohol Dependence 2006;85(2):138‐41. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher‐based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research 2004;6(6):1015‐20. [DOI] [PubMed] [Google Scholar]
- Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine & Tobacco Research 2010;12(5):483‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology 2007;15(2):176‐86. [DOI] [PubMed] [Google Scholar]
Higgins 2014 {published data only}
- Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon LJ, Lynch ME, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Preventive Medicine 2014;68:51‐7. [DOI: 10.1016/j.ypmed.2014.03.024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jason 1995 {published data only}
- Jason LA, McMahon SD, Salina D, Hedeker D, Stockton M, Dunson K, et al. Assessing a smoking cessation intervention involving groups, incentives, and self‐help manuals. Behavior Therapy 1995;26:393‐408. [Google Scholar]
- Jason LA, Salina D, McMahon SD, Hedeker D, Stockton M. A worksite smoking intervention: a 2 year assessment of groups, incentives, and self‐help. Health Education Research 1997;12(1):129‐38. [DOI] [PubMed] [Google Scholar]
- McMahon SD, Jason LA. Social support in a worksite smoking intervention. Behavior Modification 2000;24(2):184‐201. [DOI] [PubMed] [Google Scholar]
- McMahon SD, Jason LA. Stress and coping in smoking cessation: a longitudinal examination. Anxiety, Stress and Coping 1998;11:327‐43. [Google Scholar]
- McMahon SD, Jason LA, Salina D. Stress, coping, and appraisal in a smoking cessation intervention. Anxiety, Stress and Coping 1994;7(2):161‐71. [Google Scholar]
King 1987 {published data only}
- King AC, Flora JA, Fortmann SP, Taylor CB. Smokers' challenge: immediate and long‐term findings of a community smoking cessation contest. American Journal of Public Health 1987;77(10):1340‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinoshita 2004 {published data only (unpublished sought but not used)}
- Kinoshita T, Nakamura M, Mizuta I, Oshima A. Evaluation of a “stop smoking” contest in Japan [Japanese]. Nippon Koshu Eisei Zasshi 2004;51(5):357‐70. [PubMed] [Google Scholar]
Kira 2016 {published data only}
- Kira A, Glover M, Walker N, Bauld L. Recruiting pregnant indigenous women who smoke into a high contact incentivized cessation trial: a feasibility study. Nicotine & Tobacco Research 2016;18(10):2036‐40. [DOI] [PubMed] [Google Scholar]
Kollins 2010 {published data only}
- Kollins SH, McClernon FJ, Voorhees EE. Monetary incentives promote smoking abstinence in adults with attention deficit hyperactivity disorder (ADHD). Experimental and Clinical Psychopharmacology 2010;18(3):221‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korhonen 1992 {published data only}
- Korhonen HJ, Niemensivu H, Piha T, Koskela K, Wiio J, Johnson CA, et al. National TV smoking cessation program and contest in Finland. Preventive Medicine 1992;21:74‐87. [DOI] [PubMed] [Google Scholar]
Korhonen 1993 {published data only}
- Korhonen HJ, Puska P, Lipand A, Kasmel A. Combining mass media and contest in smoking cessation: an experience from a series of national activities in Finland. Hygie 1993;XII(1):15‐7. [PubMed] [Google Scholar]
Korhonen 1998 {published data only}
- Korhonen T, Kamardina T, Salto E, Korhonen HJ, Puska P. Quit and Win contest 1994. European Journal of Public Health 1998;8(2):150‐3. [Google Scholar]
Korhonen 1999 {published data only}
- Korhonen T, Urjanheimo E‐L, Mannonen P, Korhonen HK, Utela A, Puska P. Quit and Win campaigns as a long‐term anti‐smoking intervention in North Karelia and other parts of Finland. Tobacco Control 1999;8:175‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2000 {published data only}
- Lai KQ, McPhee SJ, Jenkins CN, Wong C. Applying the Quit and Win contest model in the Vietnamese community in Santa Clara county. Tobacco Control 2000;9, Supp II:ii56‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lando 1990 {published data only}
- Lando HA, Loken B, Howard‐Pitney B, Pechacek T. Community impact of a localized smoking cessation contest. American Journal of Public Health 1990;80(5):601‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lando 1991a {published data only}
- Lando HA, Pirie PL, McGovern PG, Pechacek TF, Swim J, Loken B. A comparison of self‐help approaches to smoking cessation. Addictive Behaviors 1991;16:183‐93. [DOI] [PubMed] [Google Scholar]
Lando 1991b {published data only}
- Lando HA, Hellerstedt WL, Pirie PL, Fruetel J, Huttner P. Results of a long‐term community smoking cessation contest. American Journal of Health Promotion 1991;5(6):420‐5. [DOI] [PubMed] [Google Scholar]
Lando 1995a {published data only}
- Lando HA, Pirie PL, Dusich KH, Elsen C, Bernards J. Community incorporation of quit and win contests in Bloomington, Minnesota. American Journal of Health Promotion 1995;85(2):263‐5. [PMC free article] [PubMed] [Google Scholar]
Lefebvre 1990 {published data only}
- Elder JP, McGraw SA, Rodrigues A, Lasater TM, Ferreira A, Kendall L, et al. Evaluation of two community‐wide smoking cessation contests. Preventive Medicine 1987;16:221‐34. [DOI] [PubMed] [Google Scholar]
- Lefebvre RC, Cobb GD, Goreczny AJ, Carleton RA. Efficacy of an incentive‐based community smoking cessation program. Addictive Behaviors 1990;15:403‐11. [DOI] [PubMed] [Google Scholar]
Leinweber 1994 {published data only}
- Leinweber CE, Macdonald JM, Campbell HS. Community smoking cessation contests: an effective public health strategy. Canadian Journal of Public Health 1994;85(2):95‐8. [PubMed] [Google Scholar]
NCT01983150 {published data only}
- A randomized controlled trial to test the effect of a smartphone quit smoking intervention on young adult smokers. https://clinicaltrials.gov/ct2/show/nct01983150.
O'Connor 2006 {published data only}
- O'Connor R, Fix B, Celestino P, Carlin‐Menter S, Hyland A, Cummings KM. Financial incentives to promote smoking cessation: evidence from 11 Quit and Win contests. Journal of Public Health Management and Practice 2006;12(1):44‐51. [DOI] [PubMed] [Google Scholar]
Ondersma 2012 {published data only}
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer‐based motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment 2005;28(4):305‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Lam PK, Connors‐Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer‐delivered brief intervention and low‐intensity contingency management for smoking during pregnancy. Nicotine & Tobacco Research 2012;14(3):351‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paxton 1980 {published data only}
- Paxton R. The effects of a deposit contract as a component in a behavioural programme for stopping smoking. Behaviour Research and Therapy 1980;18(1):45‐50. [DOI] [PubMed] [Google Scholar]
Paxton 1981 {published data only}
- Paxton R. Deposit contracts with smokers: varying frequency and amount of repayments. Behaviour Research and Therapy 1981;19(2):117‐23. [DOI] [PubMed] [Google Scholar]
Paxton 1983 {published data only}
- Paxton R. Prolonging the effects of deposit contracts with smokers. Behaviour Research and Therapy 1983;21(4):425‐33. [DOI] [PubMed] [Google Scholar]
Pirie 1997 {published data only}
- Pirie PL, Rooney BL, Pechacek TF, Lando HA, Scmid LA. Incorporating social support into a community‐wide smoking‐cessation contest. Addictive Behaviors 1997;22(1):131‐7. [DOI] [PubMed] [Google Scholar]
Quintiliani 2015 {published data only}
- Quintiliani LM, Russinova ZL, Bloch PP, Truong V, Xuan Z, Pbert L, et al. Patient navigation and financial incentives to promote smoking cessation in an underserved primary care population: a randomized controlled trial protocol. Contemporary Clinical Trials 2015;45:449‐57. [DOI: 10.1016/j.cct.2015.09.005] [DOI] [PubMed] [Google Scholar]
Rand 1989 {published data only}
- Rand CS, Stitzer ML, Bigelow GE, Mead AM. The effects of contingent payment and frequent workplace monitoring on smoking abstinence. Addictive Behaviors 1989;14(2):121‐8. [DOI] [PubMed] [Google Scholar]
Resnicow 1997 {published data only}
- Resnicow K, Royce J, Vaughan R, Orlandi MA, Smith M. Analysis of a multicomponent smoking cessation project: what worked and why. Preventive Medicine 1997;26:373‐81. [DOI] [PubMed] [Google Scholar]
- Royce JM, Ashford A, Resnicow K, Freeman HP, Caesar AA, Orlandi MA. Physician‐ and nurse‐assisted smoking cessation in Harlem. Journal of the National Medical Association 1995;87(4):291‐300. [PMC free article] [PubMed] [Google Scholar]
Roberts 1993 {published data only}
- Roberts C, Smith C, Catford J. Quit and Win Wales: an evaluation of the 1990 pilot contest. Tobacco Control 1993;2:114‐9. [Google Scholar]
Rooney 2005 {published data only}
- Rooney BL, Silha P, Gloyd J, Kreutz R. Quit and Win smoking cesssation contest for Wisconsin college students. Wisconsin Medical Journal 2005;104(4):45‐9. [PubMed] [Google Scholar]
Sarraf Zadegan 2006 {published data only}
- Sarraf Zadegan N, Shahrokhi S, Kelishadi R, Rooh Afza HR. Trends in one month and one year abstinence rates among smokers in four anti‐smoking campaigns (1998‐2004) [POS 102‐80]. 13th World Conference on Tobacco OR Health, July 2006, Washington DC. 2006.
Secades‐Villa 2014 {published data only}
- Secades‐Villa R, García‐Rodríguez O, López‐Núñez C, Alonso‐Pérez F, Fernández‐Hermida JR. Contingency management for smoking cessation among treatment‐seeking patients in a community setting. Drug and Alcohol Dependence 2014;140:63‐8. [DOI] [PubMed] [Google Scholar]
Sheikhattari 2015 {published data only}
- Sheikhattari P, Wagner FA. Cease quit smoking; a successful CBPR trial. Drug and Alcohol Dependence 2015;146:e97. [DOI: 10.1016/j.drugalcdep.2014.09.631] [DOI] [Google Scholar]
Shoptaw 2002 {published data only}
- Shoptaw S, Rotheram‐Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, et al. Smoking cessation in methadone maintenance. Addiction 2002;97(10):1317‐28. [DOI] [PubMed] [Google Scholar]
Stotts 2013 {published data only}
- Stotts AL, Northrup TF, Schmitz JM, Green C, Tyson J, Velasquez MM, et al. Baby's Breath II protocol development and design: a secondhand smoke exposure prevention program targeting infants discharged from a neonatal intensive care unit. Contemporary Clinical Trials 2013;35:97‐105. [DOI: 10.1016/j.cct.2013.02.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tappin 2015 {published data only}
- Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskill S, et al. Financial incentives for smoking cessation during pregnancy: randomised controlled trial. Lancet 2014;350:134. [DOI] [PubMed] [Google Scholar]
- Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskill S, et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ 2015;350:h134. [DOI] [PubMed] [Google Scholar]
- Tappin DM, Bauld L, Tannahill C, Caestecker L, Radley A, McConnachie A, et al. The Cessation in Pregnancy Incentives Trial (CPIT): study protocol for a randomized controlled trial. Trials 2012;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tevyaw 2009 {published data only}
- Tevyaw TO, Colby SM, Tidey JW, Kahler CW, Rohsenow DJ, Barnett NP, et al. Contingency management and motivational enhancement: a randomized clinical trial for college students. Nicotine & Tobacco Research 2009;11(6):739‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tillgren 1992 {published data only}
- Tillgren P, Hagkund BJA, Gilljam H, Holm L‐E. A tobacco quit and win model in the Stockholm cancer prevention programme. European Journal of Cancer Prevention 1992;1:361‐6. [DOI] [PubMed] [Google Scholar]
- Tillgren P, Haglund BJA, Ainetdin T, Holm L‐E. Who is a successful quitter? One‐year follow‐up of a national tobacco Quit and Win contest in Sweden. Scandinavian Journal of Social Medicine 1995;23(3):193‐201. [DOI] [PubMed] [Google Scholar]
- Tillgren P, Rosen M, Haglund BJ, Ainetdin T, Lindholm L, Holm L‐E. Cost‐effectiveness of a tobacco ‘Quit and Win' contest in Sweden. Health Policy 1993;26(1):43‐53. [DOI] [PubMed] [Google Scholar]
Tillgren 2000 {published data only}
- Eriksson L, Guldbrandsson K, Reimers A, Spiik A, Tillgren P, Stjerna ML, et al. A report on “Women Quitting Smoking” ‐ a smoking cessation contest for mothers with children 0‐6 years within the South‐West Health‐Care region, 1995‐1996 (Swedish) [En rapport om “Fimpa tjejer” ‐ en rökslutstävling för småbarnsmammor i Sydvästra sjukvårdsområdet 1995‐1996]. Sydvästra Sjukvårdsområdet 1997.
- Johansson PM, Tillgren PE, Guldbrandsson KA, Lindholm LA. A model for cost‐effectiveness analyses of smoking cessation interventions applied to a Quit‐and‐Win contest for mothers of small children. Scandinavian Journal of Public Health 2005;33:343‐52. [DOI] [PubMed] [Google Scholar]
- Tillgren P, Eriksson L, Guldbrandsson K, Spiik M. Impact of direct mail as a method to recruit smoking mothers into a “Quit and Win” contest. Journal of Health Communication 2000;5(4):293‐303. [DOI] [PubMed] [Google Scholar]
Tuten 2012 {published data only}
- Tuten M, Fitzsimons H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone‐maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction 2012;107(10):1868‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpp 2006 {published data only}
- Volpp KG, Levy AG, Asch DA, Berline JA, Murphy JJ, Gomez A, et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiology, Biomarkers and Prevention 2006;15(1):12‐8. [DOI] [PubMed] [Google Scholar]
Volpp 2009 {published data only}
- Kim A, Kamyab K, Zhu J, Volpp K. Why are financial incentives not effective at influencing some smokers to quit? Results of a process evaluation of a worksite trial assessing the efficacy of financial incentives for smoking cessation. Journal of Occupational and Environmental Medicine 2011;53(1):62‐7. [DOI] [PubMed] [Google Scholar]
- Kim AE, Towers A, Renaud J, Zhu J, Shea JA, Galvin R, et al. Application of the RE‐AIM framework to evaluate the impact of a worksite‐based financial incentive intervention for smoking cessation. Journal of Occupational and Environmental Medicine 2012;54(5):610‐4. [DOI] [PubMed] [Google Scholar]
- Troxel AB, Volpp KG. Effectiveness of financial incentives for longer‐term smoking cessation: evidence of absence or absence of evidence?. American Journal of Health Promotion 2012;26(4):204‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Das A. Comparative effectiveness ‐ thinking beyond medication A versus medication B. New England Journal of Medicine 2009;361(4):331‐3. [DOI] [PubMed] [Google Scholar]
- Volpp KG, Galvin R. Reward‐based incentives for smoking cessation: how a carrot became a stick. JAMA 2014;311(9):909‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine 2009;360(7):699‐709. [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang MPK. Promoting smoking cessation in the community via QTW 2014. https://clinicaltrials.gov/ct2/show/NCT02188433.
Wang 2015 {published data only}
- Wang MP. Promoting smoking cessation in the community via QTW 2015. https://clinicaltrials.gov/ct2/show/NCT02539875.
- Wang MP, Suen YN, Li WH, Lam CO, Wu SY, Kwong AC, et al. Intervention with brief cessation advice plus active referral for proactively recruited community smokers: a pragmatic cluster randomized clinical trial. JAMA Internal Medicine 2017;177(12):1790‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang MP. Building capacity and promoting smoking cessation in the community via “Quit to Win” contest 2016. https://clinicaltrials.gov/ct2/show/NCT02804880.
Wang 2017 {published data only}
- Wang MP. Promoting smoking cessation in the community via “Quit to Win” contest 2017. https://clinicaltrials.gov/ct2/show/NCT03182790.
Wang 2018 {published data only}
- Wang MP. Building capacity and promoting smoking cessation in the community via “Quit to Win” contest 2018. https://clinicaltrials.gov/ct2/show/NCT03565796.
White 2013 {published data only}
- White JS, Dow WH, Rungruanghiranya S. Commitment contracts and team incentives: a randomized controlled trial for smoking cessation in Thailand. American Journal of Preventive Medicine 2013;45(5):533‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Windsor 1988 {published data only}
- Windsor RA, Lowe JB. Behavioral impact and cost analysis of a worksite self‐help smoking cessation program. Progress in Clinical and Biological Research 1989;392:231‐42. [PubMed] [Google Scholar]
- Windsor RA, Lowe JB, Bartlett EE. The effectiveness of a worksite self‐help smoking cessation program: a randomized trial. Journal of Behavioral Medicine 1988;11(4):407‐21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Accornero 2014 {published data only}
- Accornero V. Contingency management for smoking cessation in pregnant minority women. https://clinicaltrials.gov/ct2/show/NCT02195570.
Horgan 2016 {published data only}
- Horgan K, Volpp K, Asch D. Pro‐change smoking cessation intervention. https://clinicaltrials.gov/ct2/show/NCT02299076.
Ledgerwood 2015 {published data only}
- Ledgerwood D. Smoking cessation for people living with HIV/AIDS. https://clinicaltrials.gov/ct2/show/NCT01965405. [DOI] [PMC free article] [PubMed]
- Ledgerwood DM, Lundahl LH, Greenwald M, Arfken CL, Tancer ME, Cohn JA. Prize contingency management for smoking cessation among people living with HIV/AIDS: preliminary analysis. Drug and Alcohol Dependence 2015;156:e124‐5. [Google Scholar]
Additional references
Adams 2014
- Adams J, Giles EL, McColl E, Sniehotta FF. Carrots, sticks and health behaviours: a framework for documenting the complexity of financial incentive interventions to change health behaviours. Health Psychology Review 2014;8(3):286‐95. [DOI: 10.1080/17437199.2013.848410] [DOI] [PubMed] [Google Scholar]
Altman 1987
- Altman DG, Flora JA, Fortmann SP, Farquhar JW. The cost‐effectiveness of three smoking cessation programs. American Journal of Public Health 1987;77(2):162‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aveyard 2011
- Aveyard P, Bauld L. Incentives for promoting smoking cessation: what we still do not know. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.ED000027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bains 1998
- Bains N, Pickett W, Hoey J. The use and impact of incentives in population‐based smoking cessation programs: a review. American Journal of Health Promotion 1998;12(5):397‐20. [DOI] [PubMed] [Google Scholar]
Benowitz 2002
- SRNT Subcommittee on Biochemical Verification [Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, et al]. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research 2002;4(2):149‐59. [DOI] [PubMed] [Google Scholar]
Bickel 2007
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug and Alcohol Dependence 2007;90S:S85‐S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2015
- Cahill K, Hartmann‐Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD004307.pub5] [DOI] [PubMed] [Google Scholar]
Chamberlain 2013
- Chamberlain C, O'Mara‐Eves A, Oliver S, Caird JR, Perlen SM, Eades SJ, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001055.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1994
- Chapman S, Smith W. Deception among quit smoking lottery Entrants. American Journal of Health Promotion 1994;8(5):328‐30. [DOI] [PubMed] [Google Scholar]
Donatelle 2004
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: Status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Supp. 2):S163‐S179. [DOI] [PubMed] [Google Scholar]
Fanshawe 2017
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone‐Banks J, Hartmann‐Boyce J. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD003289.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giles 2014
- Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta‐analysis. PLOS One 2014;9(3):e90347. [DOI: 10.1371/journal.pone.0090347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gneezy 2011
- Gneezy U, Meier S, Rey‐Biel P. When and why incentives (don't) work to modify behavior. Journal of Economic Perspectives 2011;25(4):191‐210. [Google Scholar]
Haisley 2008
- Haisley E. The appeal of lotteries and their use in incentive design. The appeal of lotteries and their use in incentive design [doctoral thesis]. Tepper School of Business, Carnegie Mellon University, 2008. [Google Scholar]
Higgins 2012a
- Higgins ST, Silverman K, Sigmon SC, Naito NA. Incentives and health: an introduction. Preventive Medicine 2012;55:S2‐S6. [DOI: 10.1016/j.ypmed.2012.04.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2012b
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Preventive Medicine 2012;55:S33‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2016
- Higgins ST, Solomon LJ. Some recent developments on financial incentives for smoking cessation among pregnant and newly postpartum women. Current Addiction Reports 2016;3(1):9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2003
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine and Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
Kivetz 2003
- Kinetz R. The effects of effort and motivation on intrinsic choice. Marketing Science 2003;22(4):477‐502. [Google Scholar]
Korhonen 2000
- Korhonen T, McAlister A, Laaksonen M, Laaktikainen T, Puska P. International Quit and Win 1996: standardized evaluation in selected campaign countries. Preventive Medicine 2000;31(6):742‐51. [DOI] [PubMed] [Google Scholar]
Lando 1994
- Lando HA, Pechacek TF, Fruetel J. The Minnesota Health Heart Program community Quit and Win contests. American Journal of Health Promotion 1994;9(2):85‐7. [DOI: 10.4278/0890-1171-9.2.85] [DOI] [PubMed] [Google Scholar]
Lavack 2007
- Lavack AM, Watson L, Markwart J. Quit and Win contests: a social marketing success story. Social Marketing Quarterly 2007;13(1):31‐52. [Google Scholar]
Leeks 2010
- Leeks KD, Hopkins DP, Soler RE, Aten A, Chattopadhyay SK, the Task Force on Community Preventive Services. Worksite‐based incentives and competitions to reduce tobacco use: a systematic review. American Journal of Preventative Medicine 2010;38(2S):S263‐S274. [DOI] [PubMed] [Google Scholar]
Lynagh 2013
- Lynagh MC, Sanson‐Fisher RW, Bonevski B. What’s good for the goose is good for the gander. Guiding principles for the use of financial incentives in health behaviour change. International Journal of Behavioral Medicine 2013;20:114‐20. [DOI: 10.1007/s12529-011-9202-5] [DOI] [PubMed] [Google Scholar]
Malta Independent 2006
- Malta Independent. International Quit And Win 2006 competition. http://www.independent.com.mt/articles/2006‐04‐18/news/international‐quit‐and‐win‐2006‐competition‐90090/ (Accessed 06/09/2018) 2006.
Marteau 2009
- Marteau TM, Ashcroft RE, Oliver A. Using financial incentives to achieve healthy behaviour. BMJ 2009;338:b1415. [DOI: ] [DOI] [PubMed] [Google Scholar]
Martinson 1999
- Martinson BC, Murray DM, Jeffery RW, Hennrikus DJ. Intraclass correlation for measures from a worksite health promotion study: estimates, correlates, and applications. American Journal of Health Promotion 1999;13(6):347‐57. [DOI] [PubMed] [Google Scholar]
Patrick 1994
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self‐reported smoking: a review and meta‐analysis. American Journal of Public Health 1994;84(7):1086‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pechacek 1994
- Pechacek TF, Lando HA, Northweyr F, Lichtenstein E. Quit and Win: a community‐wide approach to smoking cessation. Tobacco Control 1994;3(3):236‐41. [Google Scholar]
Pourshams 2000
- Pourshams A, Mohammadifard N, Asgary S, Golshadi I, Sarraf‐zadegan N. Evaluation of the international “Quit and Win” contest 1998 in Isfahan, Iran. Annals of Iranian Medicine 2000;3(2). [Google Scholar]
Promberger 2013
- Promberger M, Marteau TM. When do financial incentives reduce intrinsic motivation? comparing behaviors studied in psychological and economic literatures. Health Psychology 2013;32(9):950‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandström 2001
- Sandström P, Korhonen T, Mannonen P, Vartiainen ER, Pyykonen M, Puska P. International Quit and Win 1998. Helsinki, Finland: KTL ‐ National Public Health Institute 2001.
Sandström 2002
- Sandström P, Vartiainen ER, Pyykonen M, Nissinen A, Puska P. International Quit and Win 2000. Helsinki: KTL National Public Health Institute 2002.
Sigmon 2012
- Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Preventive Medicine 2012;55(Suppl):S24‐S32. [DOI: 10.1016/j.ypmed.2012.04.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stuart 2016
- Stuart, LC. Behavioral economics: lessons for lotteries. Public Gaming International November/December 2016:62‐4.
Sun 2000
- Sun S, Korhonen T, Uutela A, Korhonen HJ, Puska P, Jun Y, et al. International Quit and Win 1996: comparative evaluation study in China and Finland. Tobacco Control 2000;9(3):303‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tillgren 1995
- Tillgren P, Haglund B, Ainetdin T, Thornqvist E, Uhrbom E, Holm LE. Effects of different intervention strategies in the implementation of a nationwide tobacco Quit and Win contest in Sweden. Tobacco Control 1995;4(4):344‐50. [Google Scholar]
van den Brand 2017
- Brand FA, Nagelhout GE, Reda AA, Winkens B, Evers SM, Kotz D, et al. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD004305.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- World Health Organisation. The European Tobacco Control Report: 2007. http://www.euro.who.int/__data/assets/pdf_file/0005/68117/E89842.pdf (Accessed 07/09/2018) 2007.
WHO 2017
- WHO report on the global tobacco epidemic, 2017. World Health Organization 2017.
WHO 2018
- World Health Organisation. Tobacco: Fact sheet No. 339. World Health Organisation Media centre: http://www.who.int/mediacentre/factsheets/fs339/en/ (Accessed 07/09/2018) Updated March 2018.
References to other published versions of this review
Cahill 2005
- Cahill K, Perera R. Quit and Win contests for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004986.pub2] [DOI] [Google Scholar]
Cahill 2008a
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004307.pub3] [DOI] [PubMed] [Google Scholar]
Cahill 2008b
- Cahill K, Perera R. Quit and Win contests for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004986.pub3] [DOI] [PubMed] [Google Scholar]
Cahill 2011
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD004307.pub4] [DOI] [PubMed] [Google Scholar]
Hey 2003
- Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004307] [DOI] [PubMed] [Google Scholar]
Hey 2004
- Hey K, Perera R. Quit and Win contests for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004986] [DOI] [PubMed] [Google Scholar]
Hey 2005
- Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004307.pub2] [DOI] [PubMed] [Google Scholar]


