Abstract
Background
Mechanical methods were the first methods developed to ripen the cervix and induce labour. During recent decades they have been substituted by pharmacological methods. Potential advantages of mechanical methods, compared with pharmacological methods may include reduction in side effects that could improve neonatal outcomes. This is an update of a review first published in 2001, last updated in 2012.
Objectives
To determine the effectiveness and safety of mechanical methods for third trimester (> 24 weeks' gestation) induction of labour in comparison with prostaglandin E2 (PGE2) (vaginal and intracervical), low‐dose misoprostol (oral and vaginal), amniotomy or oxytocin.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP), and reference lists of retrieved studies (9 January 2018). We updated the search in March 2019 and added the search results to the awaiting classification section of the review.
Selection criteria
Clinical trials comparing mechanical methods used for third trimester cervical ripening or labour induction with pharmacological methods.
Mechanical methods include: (1) the introduction of a catheter through the cervix into the extra‐amniotic space with balloon insufflation; (2) introduction of laminaria tents, or their synthetic equivalent (Dilapan), into the cervical canal; (3) use of a catheter to inject fluid into the extra‐amniotic space (EASI).
This review includes the following comparisons: (1) specific mechanical methods (balloon catheter, laminaria tents or EASI) compared with prostaglandins (different types, different routes) or with oxytocin; (2) single balloon compared to a double balloon; (3) addition of prostaglandins or oxytocin to mechanical methods compared with prostaglandins or oxytocin alone.
Data collection and analysis
Two review authors independently assessed trials for inclusion and assessed risk of bias. Two review authors independently extracted data and assessed the quality of the evidence using the GRADE approach.
Main results
This review update includes a total of 113 trials (22,373 women) contributing data to 21 comparisons. Risk of bias of trials varied. Overall, the evidence was graded from very‐low to moderate quality. All evidence was downgraded for lack of blinding and, for many comparisons, the effect estimates were too imprecise to make a valid judgement.
Balloon versus vaginal PGE2: there may be little or no difference in vaginal deliveries not achieved within 24 hours (average risk ratio (RR) 1.01, 95% confidence interval (CI) 0.82 to 1.26; 7 studies; 1685 women; I² = 79%; low‐quality evidence) and there probably is little or no difference in caesarean sections (RR 1.00, 95% CI 0.92 to 1.09; 28 studies; 6619 women; moderate‐quality evidence) between induction of labour with a balloon catheter and vaginal PGE2. A balloon catheter probably reduces the risk of uterine hyperstimulation with fetal heart rate (FHR) changes (RR 0.35, 95% CI 0.18 to 0.67; 6 studies; 1966 women; moderate‐quality evidence), serious neonatal morbidity or perinatal death (RR 0.48, 95% CI 0.25 to 0.93; 8 studies; 2757 women; moderate‐quality evidence) and may slightly reduce the risk of aneonatal intensive care unit (NICU) admission (RR 0.82, 95% CI 0.65 to 1.04; 3647 women; 12 studies; low‐quality evidence). It is uncertain whether there is a difference in serious maternal morbidity or death (RR 0.20, 95% CI 0.01 to 4.12; 4 studies; 1481 women) or five‐minute Apgar score < 7 (RR 0.74, 95% CI 0.49 to 1.14; 4271 women; 14 studies) because the quality of the evidence was found to be very low and low, respectively.
Balloon versus low‐dose vaginal misoprostol: it is uncertain whether there is a difference in vaginal deliveries not achieved within 24 hours between induction of labour with a balloon catheter and vaginal misoprostol (RR 1.09, 95% CI 0.85 to 1.39; 340 women; 2 studies; low‐quality evidence). A balloon catheter probably reduces the risk of uterine hyperstimulation with FHR changes (RR 0.39, 95% CI 0.18 to 0.85; 1322 women; 8 studies; moderate‐quality evidence) but may increase the risk of a caesarean section (average RR 1.28, 95% CI 1.02 to 1.60; 1756 women; 12 studies; I² = 45%; low‐quality evidence). It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death (RR 0.58, 95% CI 0.12 to 2.66; 381 women; 3 studies), serious maternal morbidity or death (no events; 4 studies, 464 women), both very low‐quality evidence, and five‐minute Apgar score < 7 (RR 1.00, 95% CI 0.50 to 1.97; 941 women; 7 studies) and NICU admissions (RR 1.00, 95% CI 0.61 to 1.63; 1302 women; 9 studies) both low‐quality evidence.
Balloon versus low‐dose oral misoprostol: a balloon catheter probably increases the risk of a vaginal delivery not achieved within 24 hours (RR 1.28, 95% CI 1.13 to 1.46; 782 women, 2 studies, and probably slightly increases the risk of a caesarean section (RR 1.17, 95% CI 1.04 to 1.32; 3178 women; 7 studies; both moderate‐quality evidence) when compared to oral misoprostol. It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes (RR 0.81, 95% CI 0.48 to 1.38; 2033 women; 2 studies), serious neonatal morbidity or perinatal death (RR 1.11, 95% CI 0.60 to 2.06; 2627 women; 3 studies), both low‐quality evidence, serious maternal morbidity or death (RR 0.50, 95% CI 0.05 to 5.52; 2627 women; 3 studies), very low‐quality evidence, five‐minute Apgar scores < 7 (RR 0.71, 95% CI 0.38 to 1.32; 2693 women; 4 studies) and NICU admissions (RR 0.82, 95% CI 0.58 to 1.17; 2873 women; 5 studies) both low‐quality evidence.
Authors' conclusions
Low‐ to moderate‐quality evidence shows mechanical induction with a balloon is probably as effective as induction of labour with vaginal PGE2. However, a balloon seems to have a more favourable safety profile. More research on this comparison does not seem warranted.
Moderate‐quality evidence shows a balloon catheter may be slightly less effective as oral misoprostol, but it remains unclear if there is a difference in safety outcomes for the neonate. When compared to low‐dose vaginal misoprostol, low‐quality evidence shows a balloon may be less effective, but probably has a better safety profile.
Future research could be focused more on safety aspects for the neonate and maternal satisfaction.
Plain language summary
Mechanical methods for induction of labour
We set out to determine from randomised controlled trials the effectiveness and safety of mechanical methods to bring on labour in the third trimester of pregnancy (> 24 weeks' gestation). Use of a balloon to stretch the cervix (the lower end of the uterus) was compared with prostaglandin E2 (PGE2), low‐dose misoprostol or oxytocin.
What is the issue?
Induction is carried out generally when the risk of continuing pregnancy outweighs the benefits, or at the request of pregnant women.
Mechanical methods for induction promote cervical ripening and onset of labour by stretching the cervix. They are amongst the oldest methods used to initiate labour. During the last decades, medication such as PGE2, misoprostol and oxytocin have partly replaced mechanical methods.
Why is this important?
More and more women have labour induced and indications are often not urgent. This means that the safety aspects of induction methods become more important, although this could be at the expense of effectiveness. Mechanical methods could have advantages over pharmacological methods as they are widely available, low in cost and may have fewer side effects, such as excessive contractions of the uterus (uterine hyperstimulation). This could potentially be safer for the baby because if contractions are too long or very close together, the baby may not receive sufficient oxygen.
What evidence did we find?
For this review we included a total of 113 randomised controlled trials involving 22,373 women who were scheduled for induction of labour for different indications. The data contributed to 21 different comparisons and 20 subgroup comparisons. Overall, the evidence was graded from very low to moderate quality. For many comparisons there were too few women in the trials to determine any clear differences in serious illness for mothers and babies.
Twenty‐eight trials (6619 women) showed mechanical induction with a balloon is as effective as vaginal PGE2 as there may be little or no difference in vaginal deliveries within 24 hours and there probably is little or no difference in caesarean sections between groups. However, a balloon appears to be safer for the neonate as it probably reduces the risk of uterine hyperstimulation with an abnormal heart rate of the baby, serious illness or death of the baby and may slightly reduce the risk for a neonatal intensive care unit admission. It was unclear if there was a difference in serious illness or death of the mother or in the five‐minute Apgar score less than seven.
Thirteen trials (1818 women) compared induction of labour with a balloon with vaginal misoprostol and showed a balloon probably reduces the risk of uterine hyperstimulation with an abnormal heart rate of the baby, but may increase the risk of a caesarean section. It was unclear if there was a difference in vaginal deliveries within 24 hours, serious illness or death of the baby, serious illness or death of the mother, five‐minute Apgar score less than seven or neonatal intensive care unit admissions.
Seven trials (3178 women) showed a balloon may be less effective than oral misoprostol as a balloon probably increases the risk of a vaginal delivery not achieved within 24 hours and probably slightly increases the risk of a caesarean section. Data on safety are still unclear as it is uncertain whether there is a difference in uterine hyperstimulation with an abnormal heart rate of the baby, serious illness or death of the baby, serious illness or death of the mother, five‐minute Apgar score less than seven or neonatal intensive care unit admissions.
What does this mean?
Mechanical induction with a balloon is probably as effective as induction of labour with vaginal PGE2. However, a balloon seems to have a more favourable safety profile for the baby. More research on this comparison does not seem warranted.
A balloon catheter may be slightly less effective as oral misoprostol, but It remains unclear if there is a difference in safety outcomes for the baby. When compared to low‐dose vaginal misoprostol, a balloon catheter may be less effective, but probably has a better safety profile for the baby.
Future research could focus more on safety aspects for the baby and maternal satisfaction.
Summary of findings
Background
The previous version of this review formed one of a series of reviews of methods for induction of labour that followed a standardised published ’generic’ protocol (Hofmeyr 2009). These reviews were initially developed to help inform the recommendations of the National Institute for Health and Care Excellence (NICE) clinical practice guidelines on induction of labour (NICE 2008). This review no longer strictly follows the original protocol and has been updated with the intention of being a stand‐alone review. This is an update of a review first published in 2001 (Boulvain 2001), and last updated in 2012 (Jozwiak 2012).
Description of the condition
Labour induction is a common obstetric procedure, which is generally carried out when the risk of continuing pregnancy outweighs the benefits. Also, induction of labour is being used more and more at the request of pregnant women to shorten the duration of pregnancy or to time the birth of the baby according to the convenience of the mother and/or healthcare workers (WHO 2011). In the USA, approximately one in four women are induced and in the last decade, the induction rate in the UK has risen up to almost 30% (NICE 2008; NHS 2017). Although rates are generally lower in developing countries, in some settings they can be as high as those observed in developed countries (WHO 2011). To maximise the success of induction of labour in women with an unfavourable cervix, various ripening methods are available.
Description of the intervention
Mechanical methods were the first methods developed to ripen the cervix and induce labour (Thiery 1989). Devices that were used in this context include various type of catheters and laminaria tents, introduced into the cervical canal or through the cervix into the extra‐amniotic space. During recent decades they were partly substituted by pharmacological methods, including various prostaglandin E2 (PGE2) preparations (vaginal gel, tablets, inserts, intracervical gel), prostaglandin E1 (PGE1; misoprostol tablets, applied either orally or vaginally) and oxytocin. Pharmacological methods however, have a variety of effects at different sites and receptors in the body that can lead to unwanted side effects when used, such as uterine hyperstimulation (excessive contractions of the uterus) and as result, fetal distress. Therefore, mechanical induction methods are gaining in popularity as it has the potential to have a better safety profile compared to pharmacological methods, however possibly at the cost of a longer duration of labour. These factors need to be considered to determine the most appropriate methods depending on the clinical situation, with impact on labour duration possibly being of secondary importance as more women have labour induced for less urgent indications.
How the intervention might work
The goal of mechanical induction methods is to ripen the cervix, which can be achieved directly through dilatation of the canal, indirectly by increasing prostaglandin or oxytocin secretion, or both (Keirse 1983). In addition to the local effect, mechanisms which involve neuro‐endocrine reflexes (the Ferguson reflex) may promote the onset of contractions, leading to labour onset (Krammer 1995b).
The standard Foley urinary catheter can be used, as well as a specially developed 'Atad' double‐balloon catheter (Atad 1996) or Cook balloon. The catheter is introduced through the cervical canal to reach the extra‐amniotic space. The balloon is then inflated to keep the catheter in place. Traction is applied to the catheter in some cases. Another method involving catheters consists of infusing saline solution or prostaglandins through a catheter inserted, via the cervical canal, in the extra‐amniotic space (EASI).
Laminaria tents, made from sterile sea‐weed or synthetic hydrophilic materials (e.g. Lamicel), are introduced into the cervical canal. These devices increase in diameter because of their hydrophilic properties. This achieves a gradual stretching of the cervix.
Digital stripping or sweeping of the membranes is evaluated in a different review (Boulvain 2005).
Why it is important to do this review
Mechanical methods were never completely abandoned, but were substituted by pharmacological methods in recent decades. However, as induction rates rise and indications are often less urgent, the safety aspects of induction methods become more important, although this could be at the expense of effectiveness. Apart for being widely available and low in cost, potential advantages of mechanical methods over pharmacological ones may include a reduction in side effects, such as uterine hyperstimulation, thereby having the potential to improve neonatal outcomes.
Objectives
To determine the effectiveness and safety of mechanical methods for third trimester (> 24 weeks' gestation) induction of labour in comparison with prostaglandin E2 (PGE2) (vaginal and intracervical), low‐dose misoprostol (oral and vaginal), amniotomy or oxytocin.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials, comparing mechanical methods for cervical ripening or labour induction with other induction methods. Quasi‐randomised controlled trials and trials only reported as abstract were eligible for inclusion. Cluster‐randomised trials are unlikely to be conducted in this area, however, if identified by a future search, they will be handled with appropriate methods.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus.
Predefined subgroup comparisons were: previous caesarean section or not, nulliparity or multiparity. Only those outcomes with data appear in the analyses tables.
Types of interventions
Different types of intervention have been considered as mechanical methods: (1) the introduction of a catheter (Foley single balloon, Atad/Cook double balloon or other type), through the cervix into the extra‐amniotic space, either with or without traction; (2) introduction of laminaria tents, or their synthetic equivalent (Dilapan), into the cervical canal; (3) use of a catheter to inject fluids, usually saline water, in the extra‐amniotic space (EASI).
Mechanical methods were compared with other induction methods (i.e. vaginal PGE2, intracervical PGE2, intravenous oxytocin, amniotomy, vaginal and oral misoprostol). For this update, the comparison with placebo/no treatment was left out. When the protocol for reviews of induction methods was designed, it was relevant to know if cervical ripening before actual induction of labour (rupturing the membranes, and if needed, administer of oxytocin) was beneficial. Since we already know the advantages of cervical ripening in case of an unfavourable cervix, no future trials will be done to study the effect of cervical ripening with a mechanical method versus no ripening. Also, in the case of pharmacological methods, it is possible to perform a placebo‐controlled study, but with mechanical methods of labour, this is not possible. Studies which do make this comparison between mechanical induction and no treatment, explore other objectives rather than the ones relevant for his review (induction of labour versus expectant management to improve birth outcome). Therefore, the choice was made to depart from the original research protocol and leave out this pre‐specified comparison. For this update, we also chose only to include low‐dose misoprostol (defined as ≤ 50 mcg every ≥ 4 hours) as evidence suggests low‐dose misoprostol is superior to high‐dose misoprostol regarding safety outcomes and being equally effective (Alfirevic 2014; Hofmeyr 2010).
In addition, other comparisons were made: (1) a single balloon compared to a double balloon; (2) laminaria tent compared to other hygroscopic dilatators; (3) addition of prostaglandins or oxytocin to mechanical methods compared with prostaglandins or oxytocin alone. These comparisons were not pre‐specified in the generic protocol of induction of labour reviews (Hofmeyr 2009).
Types of outcome measures
We included all clinically relevant outcomes for trials of methods of cervical ripening/labour induction as had been pre‐specified by two authors of the generic protocol for labour induction reviews (Justus Hofmeyr and Zarko Alfirevic). We added six more outcomes to the list of the original protocol. Differences were settled by discussion.
Primary outcomes
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications. Subgroup comparisons were limited to the primary outcomes:
vaginal delivery not achieved within 24 hours (from start cervical ripening);
uterine hyperstimulation with fetal heart rate (FHR) changes;
caesarean section;
serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood);
serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction in mainly term pregnancies, this is unlikely. All these events are rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. The incidence of individual components were explored as secondary outcomes (see below).
Secondary outcomes relate to measures of effectiveness, complications and satisfaction.
Measures of effectiveness:
cervix unfavourable/unchanged after 12 to 24 hours;
oxytocin augmentation.
Complications:
uterine hyperstimulation without FHR changes;
uterine rupture;
epidural analgesia;
instrumental vaginal delivery;
meconium‐stained liquor;
Apgar score less than seven at five minutes;
neonatal intensive care unit (NICU) admission;
neonatal encephalopathy;
perinatal death;
disability in childhood;
maternal side effects (all);
maternal nausea;
maternal vomiting;
maternal diarrhoea;
other maternal side effects;
postpartum haemorrhage (as defined by the trial authors);
serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture);
maternal death.
Measures of satisfaction:
woman not satisfied;
caregiver not satisfied.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the review, we use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (9 January 2018). We updated this search on 19 March 2019 and added the results to Studies awaiting classification for consideration in the next update.
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics) and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (19 March 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeJozwiak 2012.
For this update, the following methods were used for assessing the 247 reports that were identified as a result of the updated search. The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (Marieke de Vaan and Mieke ten Eikelder) independently assessed all potential studies identified as a result of the search strategy for inclusion. Any disagreement was resolved through discussion, or if required, by involving a third review author (Marta Jozwiak).
Data extraction and management
We designed a form to extract data. For eligible studies, two groups of two review authors (Marieke de Vaan, Marta Jozwiak, Ben Willem Mol and Kirsten Palmer) extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. Data were entered into Review Manager software (RevMan 2014) and checked by a second review author for accuracy.
When information regarding any of the above was unclear, we contacted authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (Marieke de Vaan and Mieke ten Eikelder) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor (Marta Jozwiak).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
Assessment of the quality of the evidence using the GRADE approach
For this update, the quality of the evidence was assessed for the comparisons relating to the most frequently used methods of cervical ripening (i.e. vaginal prostaglandin E2 (PGE2), vaginal misoprostol, and oral misoprostol) using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes.
Vaginal delivery not achieved within 24 hours
Uterine hyperstimulation with FHR changes
Caesarean section
Serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood)
Serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia)
Neonatal intensive care unit admission
Apgar score less than seven at five minutes
For the main comparisons we used GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
No continuous data were analysed in this update. If outcomes using continuous data are included in future versions of this review, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome but use different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials are eligible for inclusion in the analyses along with individually‐randomised trials. None have currently been identified. If in the future such trials are identified, we will adjust their standard errors using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not eligible for inclusion.
Other unit of analysis issues
Trials in pregnancy and childbirth may include outcomes for multiple pregnancies, but the trials identified to date have included singleton pregnancies only. Trials with multiple pregnancy will be included, but the outcomes relating to the babies will have to take account of clustering of events, as outlined in the Pregnancy and Childbirth Group Methodological Guidelines and the Handbook (Higgins 2011).
Some trials are multi‐arm studies, where this occurs only the intervention arms relevant to this review were included and this is noted in the Characteristics of included studies table.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. In the case of substantial heterogeneity (above 30%), if possible, we explored it by subgroup analyses.
Assessment of reporting biases
When there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and the clinical implications of treatment effects differing between trials is discussed. If the average treatment effect was not clinically meaningful, we did not combine trials. When random‐effects analyses were used, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out formal subgroup analysis to investigate heterogeneity, but carried out additional analyses of subgroups of trials based on the following.
Previous caesarean section or not
Nulliparity or multiparity
The following outcomes were used in the subgroups.
Vaginal delivery not achieved within 24 hours
Uterine hyperstimulation with FHR changes
Caesarean section
Serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood)
Serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia)
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
See: Figure 1.
1.
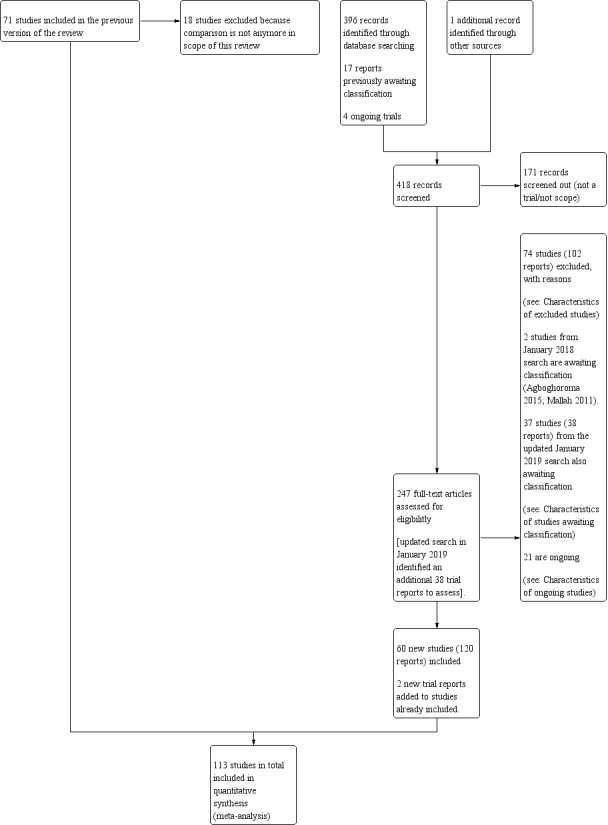
Study flow diagram.
For this update, we identified 418 trial reports to assess in the search of 9 January 2018. One study (Pineda Rivas 2016) was retrieved through other sources. When exploring the included trial registration of this study, we found out that an abstract of this study was published.
We also reassessed the 17 reports awaiting classification and the four ongoing studies in the previous version of the review (Jozwiak 2012). One hundred and seventy‐one reports were screened out because they did not meet the scope for this review or were not randomised controlled trials. We then assessed trial reports which related to 166 new trials (247 reports). We included 60 new trials (120 reports), added two trial reports to already included studies and excluded 74 trials (102 reports). Two trials from the January 2018 search are awaiting classification (Agboghoroma 2015; Mallah 2011), and 21 are ongoing (Argilagos 2016; Beckmann 2013; Bekele 2017; Berndl 2016; Bhide 2017; Eser 2016; Goli 2017; Goonewardene 2016; Gupta 2016; Hassanzadeh 2017; Igwe 2017; Lacarin 2017; Lauterbach 2017; Levy 2016; Osoti 2016; Park 2012; Perrotin 2016; Tagore 2015; Viteri 2015; Wise 2016; Yildirim 2017).
Of the 71 previous included studies, we excluded 18 trials because they were no longer within the scope of this review. Four studies were excluded because they compared a mechanical method with a placebo or no cervical ripening (De Oliveira 2003; Gilson 1996; Gower 1982; Lackritz 1979), 11 studies because of the use of high‐dose misoprostol (Adeniji 2005b; Barrilleaux 2002a; Buccellato 2000; Chung 2003; Greybush 2001; Hill 2009; Kashanian 2006; Owolabi 2005; Rust 2001; Sciscione 2001; Vengalil 1998), two studies compared extra‐amniotic space infusion (EASI) versus induction with a balloon or laminaria (El‐Torkey 1995; Lin 1995), and one study compared a balloon versus prostaglandin F2alpha (Mawire 1999).
In the updated search of 19 March 2019, we identified an additional 38 trial reports which were added to Studies awaiting classification for consideration in the next update. The references have been assessed but not incorporated into the review. Only seven of these trials are likely to contribute data for this review and are mainly small trials (Khatib 2019; Lim 2018; Osoti 2018; Souizi 2018; ten Eikelder 2017; Tulek 2018; Viteri 2019). We imputed the data for these trials and this resulted in no changes in terms of the direction or strength of the evidence. We will incorporate these studies fully at the next update.
Included studies
Altogether, this review now comprises 113 included studies, 105 of which contributed data. The studies that contributed data involved 22,373 women (see Characteristics of included studies). Trials with more than two arms may be included in more than one comparison. No cluster‐randomised trials were identified by the search.
Eight studies did not contribute any data to this review because the outcomes of interest were not reported, or reported in a format that could not be included in this review (Biron‐Shental 2004; Deo 2013; Hughes 2002; Jalilian 2011; Peedicayil 1998; Qamar 2012; Thiery 1981; Zahoor 2014). These studies are therefore not included in the descriptions of study details and 'Risk of bias' assessment below.
Design
All included studies were randomised controlled trials although the randomisation method was not always well described and in three studies the allocation process was not truly random (Jagani 1982; Kandil 2012; Roztocil 1998). All studies involved two trial arms except for Aduloju 2016, Allouche 1993, Atad 1996, Browne 2011, Cromi 2011, Deo 2012, Dionne 2011, El Khouly 2017, Guinn 2000, Matonhodze 2003, Lewis 1983, Orhue 1995, Pennell 2009, Prager 2008, Saleem 2006, Sheikher 2009 and Yuen 1996, which had three arms. Gelisen 2005, Lyndrup 1989 and Roberts 1986 had four arms, and Jagani 1982 had five arms. Not all comparisons in these studies were relevant for this review and therefore one or more arms in the studies of Gelisen 2005, Jagani 1982, Lewis 1983 and Roberts 1986 were excluded.
Setting
Nine studies were multicentre studies (Edwards 2014c; Guinn 2000; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Lokkegaard 2015; Mundle 2017; Sarreau 2016; ten Eikelder 2016), the remaining studies were single‐centre studies.
All studies took place in a hospital setting, except for Henry 2013, in which the period of cervical ripening took place in an outpatient setting.
The included studies were conducted in the following countries: Australia (Henry 2013; Pennell 2009), Brazil (Filho 2002; Oliveira 2010, Canada (Lemyre 2006; Pineda Rivas 2016; St Onge 1995), Czech Republic (Roztocil 1998), China (Wang 2012; Wang 2014; Wu 2017; Yuen 1996), Denmark (Lokkegaard 2015; Lyndrup 1989; Lyndrup 1994), Egypt (Ahmed 2016; El Khouly 2017; Kandil 2012), Finland (Kruit 2016), France (Allouche 1993; Sarreau 2016;), India (Chavakula 2015; Dalui 2005; Deo 2012; Deshmukh 2011; Goonewardene 2014; Gunawardena 2012; Joshi 2016; Kuppulakshmi 2016; Laddad 2013; Lanka 2014; Meetei 2015; Mundle 2017; Sheikher 2009), Iran (Moini 2003; Niromanesh 2003; Roudsari 2011; Sharami 2005) Italy (Cromi 2011; Cromi 2012), Israel (Atad 1996; Barda 2018; Ophir 1992; Shechter‐Maor 2015; Salim 2011; Solt 2009), Jordan (Al‐Taani 2004; Khamaiseh 2012), the Netherlands (Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016), Nigeria (Aduloju 2016; Garba 2016; Orhue 1995; Tabowei 2003), Norway (Haugland 2012), Pakistan (Husain 2017; Matonhodze 2003; Mazhar 2003; Saleem 2006), Russia (Glagoleva 1999), Rwanda (Gilson 2017), South Africa (Bagratee 1990; Jeeva 1982; Ntsaluba 1997), Singapore (Chua 1997), Sri Lanka (Rudra 2012; Somirathne 2017; Tan 2015), Sweden (Hemlin 1998; Prager 2008), Tunis (Benzineb 1996), Turkey (Gelisen 2005), the UK (Dionne 2011; Guinn 2000; Hay 1995; Johnson 1985; Lewis 1983), the USA (Al‐Ibraheemi 2018; Amorosa 2017; Blumenthal 1990; Browne 2011; Carbone 2013; Casey 1995; Culver 2004; Edwards 2014c; Hibbard 1998; Hoppe 2016; Hudon 1999; Jagani 1982; Krammer 1995a; Mackeen 2018; Mullin 2002; Perry 1998; Ridgway 1991; Roberts 1986; Rouben 1993; Sanchez‐Ramos 1992; Sciscione 1999; Suffecool 2014; Sullivan 1996; Tita 2006; Turnquest 1997).
Dates
The study of Blumenthal 1990 and Sanchez‐Ramos 1992 took place between 1980 and 1989; the studies of Allouche 1993, Guinn 2000, Hemlin 1998, Hibbard 1998, Khamaiseh 2012, Lyndrup 1994, Orhue 1995, Perry 1998, Roudsari 2011, Roztocil 1998, Sciscione 1999, St Onge 1995, Sullivan 1996 and Turnquest 1997 between 1990 and 1999; the studies of Tabowei 2003, Culver 2004 and Mullin 2002 between 1998 and 2001; the studies of Al‐Taani 2004, Cromi 2011, Deshmukh 2011, Dionne 2011, Filho 2002, Joshi 2016, Jozwiak 2012, Jozwiak 2013, Krammer 1995a, Lokkegaard 2015, Matonhodze 2003, Mazhar 2003, Moini 2003, Niromanesh 2003, Oliveira 2010, Pennell 2009, Prager 2008, Roudsari 2011, Rudra 2012, Saleem 2006, Sharami 2005 and Tita 2006 between 2000 and 2009; the studies of Jozwiak 2014 and Salim 2011 between 2008 and 2011; and the studies of Aduloju 2016, Ahmed 2016, Al‐Ibraheemi 2018, Amorosa 2017, Barda 2018, Browne 2011, Carbone 2013, Chavakula 2015, Cromi 2012, Edwards 2014c, El Khouly 2017, Garba 2016, Goonewardene 2014, Haugland 2012, Henry 2013, Hoppe 2016, Husain 2017, Kandil 2012, Kruit 2016, Kuppulakshmi 2016, Laddad 2013, Mundle 2017, Noor 2015, Sarreau 2016, Somirathne 2017, Suffecool 2014, ten Eikelder 2016, Wang 2014 and Wu 2017 between 2010 and the present day.
For the remaining studies, no study period was reported (Atad 1996; Bagratee 1990; Benzineb 1996; Casey 1995; Chua 1997; Dalui 2005; Deo 2012; Gelisen 2005; Gilson 2017; Glagoleva 1999; Gunawardena 2012; Hay 1995; Hudon 1999; Jagani 1982; Jeeva 1982; Johnson 1985; Lanka 2014; Lanka 2014; Lewis 1983; Lyndrup 1989; Ntsaluba 1997; Ophir 1992; Pineda Rivas 2016; Ridgway 1991; Roberts 1986; Rouben 1993; Solt 2009; Shechter‐Maor 2015; Sheikher 2009; Tan 2015; Wang 2012; Yuen 1996).
Participants
Most studies included both nulliparous and multiparous women. Nine studies included only nulliparous women (Culver 2004; Deshmukh 2011; Gunawardena 2012; Johnson 1985; Kandil 2012; Pennell 2009; Sharami 2005; Suffecool 2014; Wang 2012) and two studies included only multiparous women (Al‐Taani 2004; Garba 2016).
Thirteen studies included women with a specific indication for labour induction or specific patient groups, i.e. women with a hypertensive disease (Mundle 2017), women with a body mass index (BMI) greater than 30 (Pineda Rivas 2016), post‐date pregnancies (Gelisen 2005; Goonewardene 2014; Gunawardena 2012; Kandil 2012; Somirathne 2017), oligohydramnios (Shechter‐Maor 2015; Wang 2014) or pre labour rupture of membranes (PROM; Amorosa 2017; Kruit 2016; Mackeen 2018; Tita 2006). Most authors specified that only women with intact membranes were included, except for Prager 2008, in which this was not an exclusion criteria. Orhue 1995, Roudsari 2011 and Roztocil 1998 reported nothing on membrane status, so it was not clear if women with ruptured membranes could be included.
Most studies excluded women with a past history of caesarean section, although four studies only included women with a past history of caesarean section (Joshi 2016; Meetei 2015; Sarreau 2016; Tabowei 2003). Three studies did not exclude women with a past history of caesarean section, but did not specify the outcomes for this subgroup of women separately (Mackeen 2018; Tabowei 2003; Tita 2006). Benzineb 1996, Cromi 2011, Deo 2012, Guinn 2000, Haugland 2012, Lyndrup 1994, Pineda Rivas 2016, Rouben 1993, and Wu 2017 reported nothing on previous caesarean section in their inclusion and exclusion criteria.
The majority of studies included women with a gestational age beyond 37 weeks, except for Edwards 2014c and Hemlin 1998 who reported a minimal gestational age of 36 weeks, Amorosa 2017, Chavakula 2015, Cromi 2011, Cromi 2012, Mackeen 2018Matonhodze 2003, Pennell 2009; Roudsari 2011 and Sharami 2005 of 34 weeks, Dalui 2005 of 33 weeks, Lokkegaard 2015 of 32 weeks, Culver 2004, Lanka 2014 and El Khouly 2017 of 28 weeks, Browne 2011 of 26 weeks, Carbone 2013 of 24 weeks and Mundle 2017 of 20 weeks, although in this last study, no women with a gestational age below 28 weeks were included.
Twenty‐four studies were not clear on their inclusion and exclusion criteria: Gilson 2017, Jeeva 1982 and Kuppulakshmi 2016 reported no inclusion or exclusion criteria. Jagani 1982, Rudra 2012 and Turnquest 1997 only reported that women with intact membranes were included. Glagoleva 1999 only reported that women with a previous caesarean section were excluded. Bagratee 1990, Dionne 2011, Johnson 1985, Lyndrup 1989, Ridgway 1991, Solt 2009; Sullivan 1996 reported that only women with an indication for labour induction with an unfavourable cervix were included. Hemlin 1998 reported nothing on membrane status or previous caesarean section. Casey 1995, Garba 2016, Hudon 1999, Krammer 1995a, Lemyre 2006, Lewis 1983 and Saleem 2006 reported nothing on fetal presentation, membrane status or previous caesarean section. Chua 1997 and Ophir 1992 reported nothing on gestational age, fetal presentation, membrane status or previous caesarean section.
Interventions and comparisons
The protocol of administration in the intervention and in the control groups varied between studies. Different mechanical devices were evaluated (i.e. balloon catheter, laminaria tents, and extra‐amniotic infusion). Prostaglandins (intracervical or intravaginal PGE2, and oral or vaginal misoprostol) were used with different protocols of administration. We regrouped these protocols as follows: (1) balloon catheter versus other interventions; (2) laminaria tent versus other interventions: (3) extra‐amniotic infusion versus other interventions; (4) any mechanical method combined with other (non‐mechanical) intervention versus other interventions. For this last group of comparisons, we considered both PGE2 (intracervical or intravaginal PGE2) and misoprostol (oral or vaginal misoprostol) as a single intervention. The information on comparisons made in each trial, used device and balloon size is summarised below.
Studies evaluating laminaria or Dilapan were considered together, irrespective of the number of devices inserted. Similarly, evaluations of a Foley catheter (regardless of sizes and amount of liquid used to inflate the balloon and traction applied on the catheter) and a specially designed double‐balloon catheter (ATAD or Cook catheter), we considered as similar interventions. However, when a catheter was used to perform extra‐amniotic saline infusion (EASI), we considered these studies separately. Despite having regrouped similar interventions, this review still includes a large number of comparisons.
Most of the studies included in the review examined a balloon and compared it with either vaginal PGE2 or with vaginal or oral misoprostol. A smaller number of studies examined a balloon versus either intracervical PGE2 or oxytocin. Since the last update, no more studies have been published about induction of labour with a Laminaria tent or with EASI. None of the included studies examined the combination of a mechanical method with amniotomy.
The following comparisons were made in this review.
1. Balloon comparisons
Balloon (Foley or ATAD) versus vaginal prostaglandin E2
PGE2 tablets: Al‐Taani 2004 (50 cc); Atad 1996 (double balloon); Barda 2018 (80 cc); Khamaiseh 2012 (50 cc to 60 cc); Lokkegaard 2015 (double balloon); Niromanesh 2003 (30 cc); Ophir 1992 (40 cc); Pennell 2009 (30 cc and double balloon); Tan 2015 (double balloon).
PGE2 gel: Browne 2011 (40 cc); Deo 2012 (30 cc); Deshmukh 2011 (balloon size unknown); Henry 2013 (30 cc); Jozwiak 2012 (30 cc); Orhue 1995 (30 cc); Prager 2008 (30 cc); Rouben 1993 (30 cc); Rudra 2012 (40 cc).
PGE2 vaginal insert:Cromi 2011 (50 cc; for this comparison the two groups of Foley catheter (12 hours and 24 hours) were combined); Cromi 2012 (double balloon); Edwards 2014c (30 cc); Jozwiak 2013 (30 cc); Lewis 1983 (30 cc); Lyndrup 1994 (30 cc); Pineda Rivas 2016 (balloon size unknown); Saleem 2006 (40 cc to 50cc); Shechter‐Maor 2015 (double balloon); Suffecool 2014 (double balloon); Wang 2012 (80 cc); Wang 2014 (double balloon); Yuen 1996 (double balloon).
Balloon (Foley or ATAD) versus intracervical prostaglandin E2
PGE2 intracervical gel:Allouche 1993 (50 cc); gel: Benzineb 1996 (40 cc); Dalui 2005 (30 cc); Gunawardena 2012 (balloon size unknown); Hudon 1999 (40 cc); Kuppulakshmi 2016 (30 cc); Laddad 2013: (balloon size unknown); Moini 2003 (30 cc); Ntsaluba 1997 (30 cc); Sciscione 1999 (30 cc); St Onge 1995 (30 cc); Yuen 1996 (double balloon).
Balloon (Foley or ATAD) versus low‐dose vaginal misoprostol
Misoprostol tablets: Aduloju 2016 (30 cc); Chavakula 2015 (30 cc); Filho 2002 (30 cc); Jozwiak 2014 (30 cc); Kandil 2012 (30 cc); Lemyre 2006 (balloon size unknown); Noor 2015 (50 cc); Oliveira 2010 (30 cc); Prager 2008 (30 cc); Roudsari 2011 (50 cc); Sheikher 2009 (30 cc); Tabowei 2003 (50 cc).
Balloon (Foley or ATAD) versus low‐dose oral misoprostol
Misoprostol tablets:Goonewardene 2014 (balloon size unknown); Kruit 2016 (50 cc to 60 cc); Mundle 2017 (30 cc); Saleem 2006 (40 cc to 50 cc); Sheikher 2009 (30 cc); Somirathne 2017 (60 cc);ten Eikelder 2016 (30 cc). misoprostol solution:Matonhodze 2003 (50 cc).
Balloon (Foley or ATAD) versus oxytocin
Amorosa 2017 (60 cc); Atad 1996 (double balloon); El Khouly 2017 (30 cc); Gelisen 2005 (50 cc); Jagani 1982 (70 to 80 cc); Joshi 2016; (30 cc); Meetei 2015 (30 cc); Orhue 1995 (30 cc); Sarreau 2016 (50 cc).
Balloon (Foley or ATAD) versus amniotomy
Jagani 1982 (70 cc to 80 cc).
Single balloon (Foley versus double balloon (ATAD)
Ahmed 2016 (50 cc); Haugland 2012 (size unknown); Hoppe 2016 (30 cc); Pennell 2009 (30 cc); Salim 2011 (60 cc); Solt 2009 (balloon size unknown).
No studies were found for the comparison of a balloon versus oxytocin with amniotomy.
2. Laminaria comparisons
Laminaria tent versus vaginal prostaglandin E2
PGE2 tablets:Bagratee 1990 (Lamicel); Hay 1995 (Dilapan); Jeeva 1982; (laminaria).
PGE2 gel:Johnson 1985 (Lamicel); Roudsari 2011 (Dilapan); Sanchez‐Ramos 1992 (Dilapan).
Laminaria tent versus intracervical prostaglandin E2
PGE2 intracervical gel:Chua 1997 (Dilapan); Glagoleva 1999 (Dilapan); Krammer 1995a; (Dilapan); Roztocil 1998 (Dilapan).
Laminaria tent versus oxytocin
Jagani 1982 (70 to 80 cc); Roberts 1986 (Lamicel).
Laminaria tent versus amniotomy
Jagani 1982 (70 to 80 cc).
Laminaria tent versus other hygroscopic dilator
Blumenthal 1990 (Dilapan versus laminaria tent).
No studies were found for the comparison of laminaria tent versus oxytocin with amniotomy or laminaria tent versus vaginal or oral misoprostol.
3. EASI comparisons
The only studies which were found compared EASI with PGE2.
EASI versus vaginal prostaglandin E2
Vaginal insert:Mazhar 2003.
EASI versus intracervical prostaglandin E2
Intracervical gel:Hemlin 1998.
4. Any mechanical combined with prostaglandin E2 comparisons
Any mechanical method combined with prostaglandin E2 versus prostaglandin E2 alone
PGE2 intracervical gel:Allouche 1993 (50 cc); Casey 1995 (50 cc); Ridgway 1991 (Lamicel); Sullivan 1996 (50 cc).
PGE2 vaginal gel:Browne 2011 (40 cc); Hibbard 1998 (Dilapan); Lyndrup 1989; (Lamicel); Turnquest 1997 (Laminaria)
Any mechanical method combined with prostaglandin E2 versus low‐dose misoprostol alone
Vaginal misoprostol:Perry 1998.
Any mechanical method combined with prostaglandin E2 versus oxytocin alone
Lyndrup 1989 (Lamicel).
No studies were found which compared a mechanical method combined with PGE2 with amniotomy or oxytocin with amniotomy
5. Any mechanical combined with low‐dose misoprostol comparisons
Any mechanical method combined with low‐dose misoprostol versus prostaglandin E2 alone
Oral misoprostol:Matonhodze 2003.
Any mechanical method combined with low‐dose misoprostol versus low‐dose misoprostol alone
Vaginal misoprostol:Aduloju 2016 (30 cc); Al‐Ibraheemi 2018 (60 cc); Carbone 2013 (60 cc); Dionne 2011 (balloon size and dosage of misoprostol unknown); Lanka 2014 (30 cc).
Oral misoprostol:Husain 2017 (30 cc); Matonhodze 2003 (50 cc).
No studies were found which compared a mechanical method combined with low‐dose misoprostol with amniotomy, oxytocin or oxytocin with amniotomy.
6. Any mechanical method combined with oxytocin comparisons
Any mechanical method combined with oxytocin versus prostaglandin E2 alone
PGE2 intracervical gel:Guinn 2000 (laminaria + oxytocin and EASI + oxytocin); Lyndrup 1989 (Lamicel); Sharami 2005 (EASI).
Any mechanical method combined with oxytocin versus low‐dose misoprostol alone
Vaginal misoprostol:Culver 2004 (30 cc); Dionne 2011 (balloon size unknown); Gilson 2017 (30 cc); Garba 2016 (balloon size and dosage of misoprostol unknown); Mullin 2002.
Any mechanical method combined with oxytocin versus oxytocin alone
El Khouly 2017 (30 cc); Lyndrup 1989 (Lamicel); Mackeen 2018 (30 cc); Tita 2006 (balloon size unknown); Wu 2017 (double balloon).
No studies were found which compared a mechanical method combined with oxytocin to amniotomy or oxytocin with amniotomy.
Outcomes
The study authors frequently reported on continuous outcome measures such as change in the cervical status or time to onset of labour, but also mean Apgar score after five minutes and mean pH in the umbilical artery. As these were not pre‐specified in our protocol, we have not included these results in the review. In several studies, the only pre‐specified result available was the number of women delivered by caesarean section. Maternal or neonatal death were infrequently pre‐specified by the authors and therefore not specifically reported. Therefore, these outcomes could not be included in this review.
Maternal satisfaction was reported in seven studies (Ahmed 2016; Chavakula 2015; Gilson 2017; Henry 2013; Lyndrup 1994; Mundle 2017; Shechter‐Maor 2015). Of these seven studies, only three studies contributed data for the meta‐analysis (Gilson 2017; Lyndrup 1994; Mundle 2017). The other four studies reported on maternal satisfaction with continuous data. Because of the importance of this outcome, we decided to report these results in narrative form.
Source of trial funding
Only 14 trials provided details for their funding sources: Filho 2002 received financial support from CAPES. Guinn 2000 reported that UpJohn Pharmaceuticals provided funds to purchase study drugs. Kruit 2016 received a grand from the Finnish medical society Duodecim and Helsinky university central hospital. Lokkegaard 2015 reported the randomisation procedure was funded by Snedkermester Sophus Jacobsen & Astrid Jacobsens fond and the Danish Toyota Foundation. Mackeen 2018 received a small internal grant to assist with the conduct and statistical analyses for the entire study. Mundle 2017 received funding from the Department for International Development, Medical Research Council, and Wellcome Trust Joint Global Health Trials Scheme. The study of Pennell 2009 was supported by a grant from the Women and Infants Research Foundation and Adeza Biomedical Corporation contributed support for the fetal fibronectin test kits. Roberts 1986 and Sullivan 1996 stated they were supported by the Vicksburg hospital medical foundation. Salim 2011 received funding from the Emek medical centre. Tan 2015 reported that the double balloons were provided by Cook medical. ten Eikelder 2016 received funding from Fonds Nuts Ohra. Wang 2014 received financial support of The People’s Liberation Army. Wu 2017 received a grant from the Nature Science Foundation of China.
Thirteen studies reported they received no funding (Aduloju 2016; El Khouly 2017; Garba 2016; Hoppe 2016; Husain 2017; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Laddad 2013; Lanka 2014; Meetei 2015; Shechter‐Maor 2015; Somirathne 2017). All other studies did not provide information on received funding.
Declarations of interest
Thirty‐five studies declared no conflict of interest (Aduloju 2016; Ahmed 2016; Al‐Ibraheemi 2018; Amorosa 2017; Barda 2018; Chavakula 2015; Cromi 2012; Edwards 2014c; El Khouly 2017; Filho 2002; Garba 2016; Goonewardene 2014; Henry 2013; Hoppe 2016; Husain 2017; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Kandil 2012; Kruit 2016; Laddad 2013; Lanka 2014; Lewis 1983; Lokkegaard 2015; Mackeen 2018; Meetei 2015; Noor 2015; Pennell 2009; Salim 2011; Shechter‐Maor 2015; Somirathne 2017;Tan 2015; ten Eikelder 2016; Wang 2014; Wu 2017).
Two studies reported they had conflicts of interest. Atad 1996 stated that the first author has a patent licensing arrangement for Atad ripening device and thus has the potential gain from its sales. Mundle 2017 reported that one of the authors was a scientific adviser to Azanta, a Danish pharmaceutical company.
The remaining studies did not report whether any conflicts of interest were present.
Excluded studies
In total, 138 studies were excluded (see Characteristics of excluded studies), 74 studies (102 reports) in this update. In this update, most of the excluded trials (54 studies) made comparisons not within the scope of this review (Ahmad 2015; Arsenijevic 2012; Arshad 2016; Caughey 2007; Connolly 2016; Connolly 2017; Demirel 2015; Edwards 2017; El‐Khayat 2016; El Sharkwy 2017; Forgie 2016; Forooshani 2011; Fruhman 2017; Gadel 2015; Ghanaei 2009; Ghanaie 2013; Gibson 2013; Gu 2015; Haghighi 2015; Hallak 2008; He 2000; Hill 2013; Hussein 2012; Ifnan 2006; Jonsson 2011; Kehl 2012; Kehl 2015; Lam 2006; Leong 2017; Levine 2016; Lutgendorf 2012; Manish 2016; Mattingly 2015; McGee 2016; Mei‐Dan 2012a; Mei‐Dan 2014; Movahed 2016; Mullin 2014; Neethurani 2013; Rameez 2007; Rezk 2014; Saad 2016; Salmeen 2012; Sandberg 2017; Schoen 2017; Sharma 2015a; Sharma 2017; Siddiqui 2013; Torbenson 2015;Walfisch 2015; Wickramasinghe 2014; Wilkinson 2015; Yaddehige 2015; Zakaria 2017). Four studies were not randomised trials (Du 2015; Miller 2015; Naseem 2007; Nasir 2012) and one study did a cross‐over after 24 hours (Ugwu 2013). Thirteen trial registration were excluded because they exceeded the participated end date by more than two years and it was presumed the trial was terminated before enrolment (Anabosy 2014; Baacke 2006; Behrashi 2013; Cullimore 2009; Dias 2008EUCTR 2012; Kamilya 2011; Mei‐Dan 2012; Park 2011Pathiraja 2014; Reif 2012; Yazdani 2011; Zhang 2014). For more information, see Characteristics of excluded studies.
Risk of bias in included studies
The quality assessments are graphically summarised in Figure 2 and Figure 3.
2.
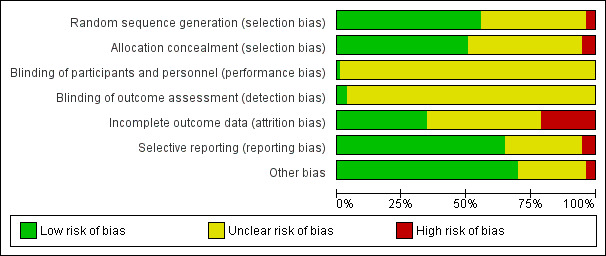
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
This review update includes nine comparisons with more than 10 studies, of which we constructed funnel plots (Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11; Figure 12). Visual inspection of one funnel plot (Figure 5) was somewhat asymmetrical suggesting some form of publication bias for this outcome (oxytocin augmentation) for the comparison of a balloon versus vaginal PGE2. Visual assessment of the other funnel plots did not show asymmetry, suggesting there is no publication bias for these comparisons.
4.
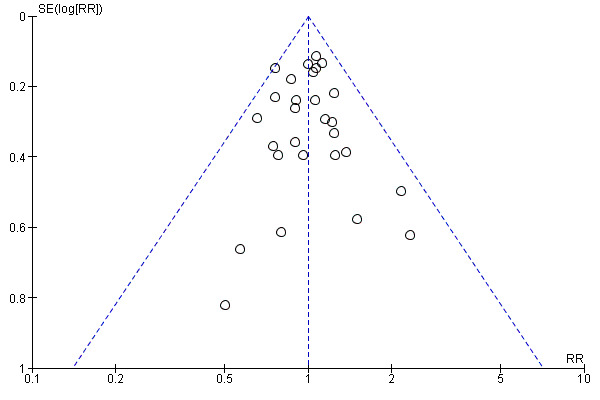
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.3 Caesarean section.
5.
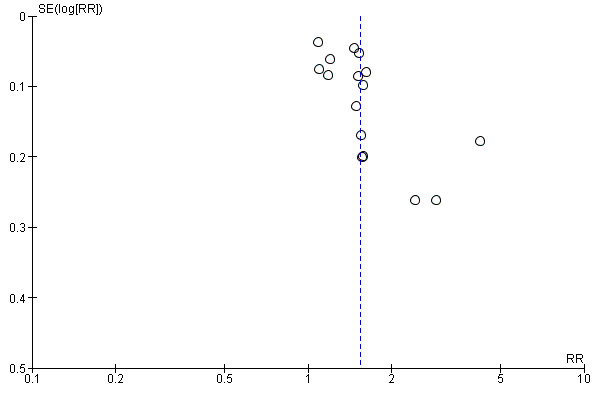
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.6 Oxytocin augmentation.
6.
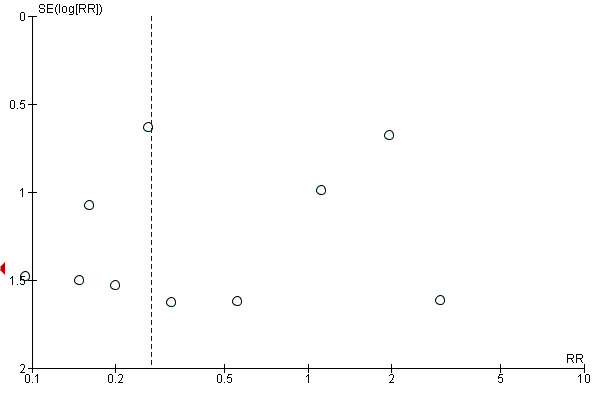
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.7 Uterine hyperstimulation without fetal heart rate changes.
7.
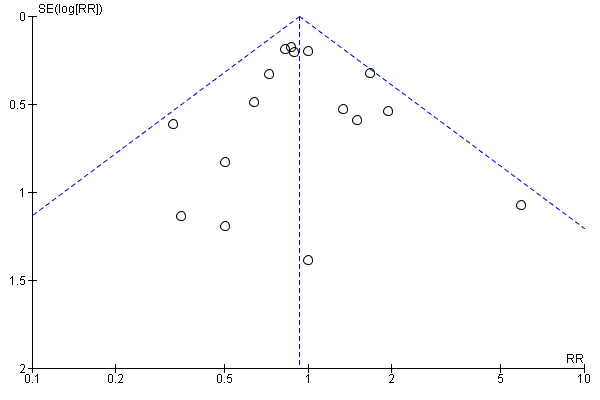
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.10 Instrumental vaginal delivery.
8.
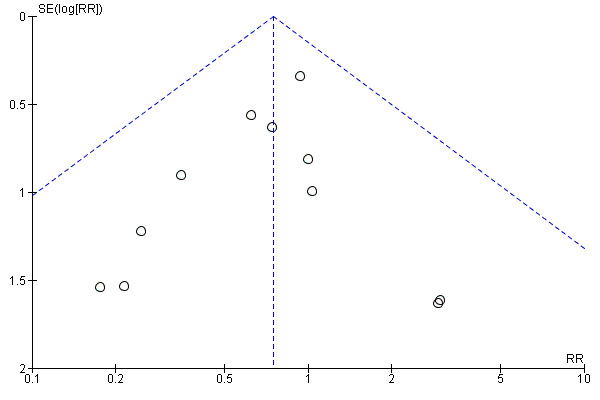
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.12 Apgar score < 7 at 5 minutes.
9.
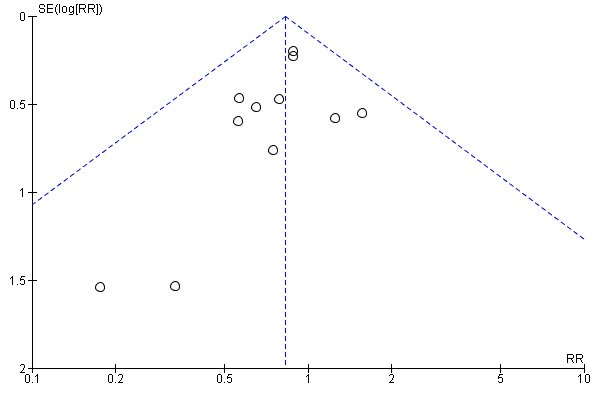
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.13 Neonatal intensive care unit admission.
10.
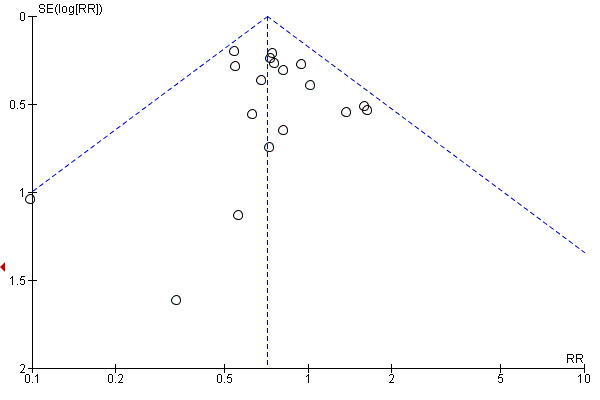
Funnel plot of comparison: 1 Balloon (Foley or ATAD) versus vaginal Prostaglandin E2: all women, outcome: 1.21 Fetal distress.
11.
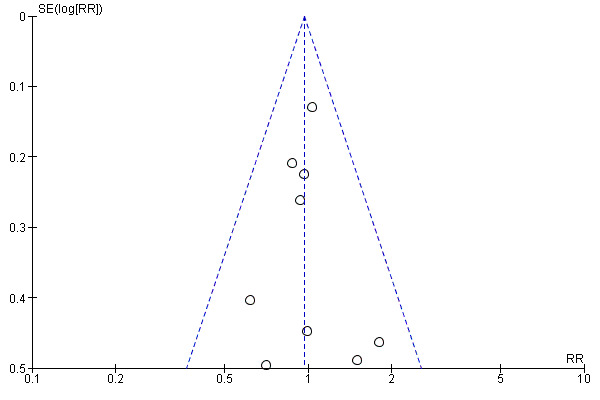
Funnel plot of comparison: 4 Balloon (Foley or ATAD) versus intracervical Prostaglandin E2: all women, outcome: 4.3 Caesarean section.
12.
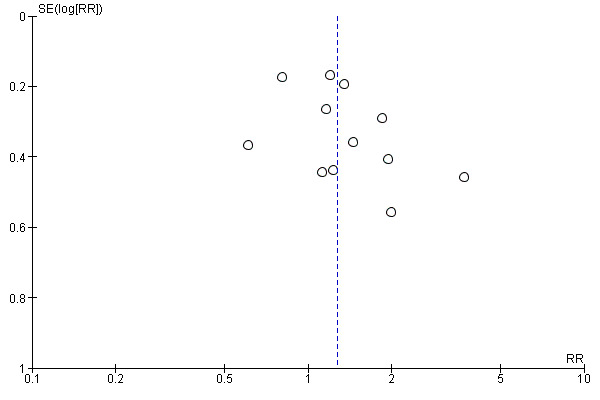
Funnel plot of comparison: 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, outcome: 7.3 Caesarean section.
Allocation
Sequence generation
We judged 62 trials to be at low risk of selection bias, reporting some form of adequate random sequencing such as a computer‐generated sequence or a list of random numbers (Aduloju 2016; Ahmed 2016; Al‐Ibraheemi 2018; Al‐Taani 2004; Amorosa 2017; Atad 1996; Bagratee 1990; Blumenthal 1990; Browne 2011; Carbone 2013; Chavakula 2015; Chua 1997; Cromi 2011; Cromi 2012; Culver 2004; Deo 2012; Edwards 2014c; El Khouly 2017; Filho 2002; Garba 2016; Gelisen 2005; Goonewardene 2014; Guinn 2000; Henry 2013; Hibbard 1998; Husain 2017; Johnson 1985; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Khamaiseh 2012; Krammer 1995a; Lanka 2014; Lokkegaard 2015; Mackeen 2018; Matonhodze 2003; Mazhar 2003; Meetei 2015; Mullin 2002; Mundle 2017; Niromanesh 2003; Oliveira 2010; Ophir 1992; Orhue 1995; Perry 1998; Prager 2008; Rouben 1993; Salim 2011; Sanchez‐Ramos 1992; Sciscione 1999; Sharami 2005; Shechter‐Maor 2015; Solt 2009; Somirathne 2017; St Onge 1995; Suffecool 2014; Tabowei 2003; Tan 2015; ten Eikelder 2016; Turnquest 1997; Wang 2012; Yuen 1996).
Three trials were classified as high risk because they were quasi‐randomised trials. Jagani 1982 randomised by last digit of the chart number, Kandil 2012 randomised by odd or even admission date and Roztocil 1998 randomised by week of admission.
We judged the remaining 40 trials to be at unclear risk of selection bias, as they did not report on how a random sequence was generated (Allouche 1993; Barda 2018; Benzineb 1996; Casey 1995; Dalui 2005; Deshmukh 2011; Dionne 2011; Gilson 2017; Glagoleva 1999; Gunawardena 2012; Haugland 2012; Hay 1995; Hemlin 1998; Hoppe 2016; Hudon 1999; Jeeva 1982; Joshi 2016; Kruit 2016; Kuppulakshmi 2016; Laddad 2013; Lemyre 2006; Lewis 1983; Lyndrup 1989; Lyndrup 1994; Moini 2003; Noor 2015; Ntsaluba 1997; Pennell 2009; Pineda Rivas 2016; Ridgway 1991; Roberts 1986; Roudsari 2011; Rudra 2012; Saleem 2006; Sarreau 2016; Sheikher 2009; Sullivan 1996;Tita 2006; Wang 2014; Wu 2017).
Allocation concealment
Fifty‐five studies reported a method of allocation concealment likely to have a low risk of bias, either by central randomisation or sequentially numbered, sealed, opaque envelopes (Aduloju 2016; Ahmed 2016; Al‐Ibraheemi 2018; Amorosa 2017; Blumenthal 1990; Browne 2011; Carbone 2013; Chavakula 2015; Cromi 2012; Culver 2004; Deo 2012; Edwards 2014c; El Khouly 2017; Filho 2002; Gelisen 2005; Goonewardene 2014; Guinn 2000; Hemlin 1998; Henry 2013; Hibbard 1998; Hoppe 2016; Husain 2017; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Kruit 2016; Lanka 2014; Lokkegaard 2015; Lyndrup 1989; Lyndrup 1994; Matonhodze 2003; Mullin 2002; Mundle 2017; Niromanesh 2003; Ntsaluba 1997; Oliveira 2010; Orhue 1995; Pennell 2009; Perry 1998; Prager 2008; Roberts 1986; Rouben 1993; Salim 2011; Sciscione 1999; Sharami 2005; Somirathne 2017; St Onge 1995; Suffecool 2014; Sullivan 1996; Tabowei 2003; Tan 2015; ten Eikelder 2016; Turnquest 1997; Wang 2014; Yuen 1996).
Five studies were judged to be high risk. In the quasi‐randomised trials of Jagani 1982, Kandil 2012 and Roztocil 1998 no measures were taken to conceal the allocation; Mackeen 2018 stated that the allocation was not concealed and Ophir 1992 allocated women by odd or even randomisation number.
The remaining 45 studies did not report a method for concealing allocation and were judged as being at unclear risk of bias (Allouche 1993; Al‐Taani 2004; Atad 1996; Bagratee 1990; Barda 2018; Benzineb 1996; Casey 1995; Chua 1997; Cromi 2011; Dalui 2005; Deshmukh 2011; Dionne 2011; Garba 2016; Gilson 2017; Glagoleva 1999; Gunawardena 2012; Haugland 2012; Hay 1995; Hudon 1999; Jeeva 1982; Johnson 1985; Joshi 2016; Khamaiseh 2012; Krammer 1995a; Kuppulakshmi 2016; Laddad 2013; Lemyre 2006; Lewis 1983; Mazhar 2003; Meetei 2015; Moini 2003; Noor 2015; Pineda Rivas 2016; Ridgway 1991; Roudsari 2011; Rudra 2012; Saleem 2006; Sanchez‐Ramos 1992; Sarreau 2016; Shechter‐Maor 2015; Sheikher 2009; Solt 2009; Tita 2006; Wang 2012; Wu 2017).
Blinding
Performance bias
Given the nature of the intervention (mechanical methods for induction of labour) and comparison (pharmacological methods for induction of labour), it was not possible for women or clinicians to be blinded to the treatment group in any of the trials. For the more objective outcomes such as perinatal death, the lack of blinding is unlikely to be a major source of bias. Therefore, risk of performance bias was judged as unclear in all studies, but was a reason to downgrade the quality of evidence from high to moderate.
Detection bias
It would have been possible for outcome assessment to have been undertaken by someone blinded to allocation groups. However, only four trials reported blinded outcome assessment (rated as low risk of bias). Gelisen 2005 blinded only for the outcome of hyperstimulation. In the studies of Pennell 2009 and Gelisen 2005, data were collected by research midwives who were blinded to the intervention. Rudra 2012 and Haugland 2012 both stated they performed a double blind‐trial but provided too little information to assess how this was done. The remaining 101 trials did not detail whether outcome assessment was blinded, and thus we judged risk of detection bias to be unclear. Measurement of outcomes such as perinatal death are unlikely to be biased by lack of blinding.
Incomplete outcome data
We considered 38 studies to be at low risk of attrition bias with data analyses according to intention‐ to‐treat and minimal/no loss to follow‐up or exclusion of women (Aduloju 2016; Al‐Ibraheemi 2018; Al‐Taani 2004; Amorosa 2017; Atad 1996; Carbone 2013; Chavakula 2015; Chua 1997; Cromi 2011; Culver 2004; Dalui 2005; Deshmukh 2011; Edwards 2014c; El Khouly 2017; Filho 2002; Guinn 2000; Henry 2013; Jeeva 1982; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Lanka 2014; Lokkegaard 2015; Mackeen 2018; Mullin 2002; Mundle 2017; Noor 2015; Ntsaluba 1997; Oliveira 2010; Pennell 2009; Perry 1998; Prager 2008; Roberts 1986; Roztocil 1998; Suffecool 2014; Sullivan 1996; ten Eikelder 2016; Wu 2017).
Forty‐three studies were judged to be at unclear risk of attrition bias, mainly because it was not clear if intention‐to‐treat analyses was used (Allouche 1993; Benzineb 1996; Garba 2016; Gelisen 2005; Hemlin 1998; Hibbard 1998; Hoppe 2016; Jagani 1982; Johnson 1985; Joshi 2016; Khamaiseh 2012; Laddad 2013; Lewis 1983; Matonhodze 2003; Meetei 2015; Niromanesh 2003; Roudsari 2011; Salim 2011; Sanchez‐Ramos 1992; Sharami 2005; Shechter‐Maor 2015; Somirathne 2017; St Onge 1995), or there was too little information to judge attrition bias (Barda 2018; Casey 1995; Dionne 2011; Gilson 2017; Glagoleva 1999; Gunawardena 2012; Haugland 2012; Hay 1995; Hudon 1999; Kuppulakshmi 2016; Lemyre 2006; Mazhar 2003; Moini 2003; Pineda Rivas 2016; Ridgway 1991; Rudra 2012; Saleem 2006; Sarreau 2016; Solt 2009; Tabowei 2003).
Twenty‐four studies were classified as high risk for attrition bias. In the studies of Ahmed 2016, Cromi 2012 and Wang 2014, women were excluded because of failed placement of the balloon. Kandil 2012 also excluded nine patients because of failed placement of the Foley catheter, but replaced them with women who did receive a Foley catheter. Deo 2012 analysed data as treated and also four cases went missing without a given explanation. Husain 2017, Kruit 2016, Lyndrup 1989, Sciscione 1999, Tan 2015, Turnquest 1997, Wang 2012 and Yuen 1996 excluded cases because of protocol violation and Krammer 1995a reported they analysed intention‐to‐treat, but eventually excluded women because of protocol violation or if they delivered within six hours after induction had started. Goonewardene 2014 also excluded women if they went into spontaneous labour after the intervention. Lyndrup 1994 excluded women if they delivered after 48 hours of induction had started. Orhue 1995 excluded women if they had an unfavourable cervix after 12 hours of induction. Rouben 1993 excluded women after failed induction. The studies of Bagratee 1990, Blumenthal 1990, Browne 2011, Ophir 1992, Sheikher 2009, Tita 2006 were judged to be of high risk for attrition bias because cases were missing without a given explanation.
Selective reporting
Seventy‐two studies were judged to be at low risk of reporting bias as all pre‐specified outcomes were reported (Aduloju 2016; Al‐Ibraheemi 2018; Al‐Taani 2004; Amorosa 2017; Atad 1996; Bagratee 1990; Barda 2018; Blumenthal 1990; Carbone 2013; Chavakula 2015; Chua 1997; Cromi 2011; Cromi 2012; Culver 2004; Dalui 2005; Deo 2012; Deshmukh 2011; Edwards 2014c; El Khouly 2017; Filho 2002; Garba 2016; Gelisen 2005; Goonewardene 2014; Guinn 2000; Hemlin 1998; Henry 2013; Hibbard 1998; Hoppe 2016; Husain 2017; Jagani 1982; Johnson 1985; Joshi 2016; Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; Kandil 2012; Khamaiseh 2012; Krammer 1995a; Kruit 2016; Kuppulakshmi 2016; Lanka 2014; Lokkegaard 2015; Lyndrup 1994; Mackeen 2018; Matonhodze 2003; Mazhar 2003; Meetei 2015; Mullin 2002; Mundle 2017; Noor 2015; Ntsaluba 1997;Oliveira 2010; Ophir 1992; Orhue 1995; Pennell 2009; Perry 1998; Prager 2008; Rouben 1993; Roztocil 1998; Salim 2011; Sciscione 1999; Sharami 2005; Solt 2009; Somirathne 2017; St Onge 1995; Suffecool 2014; Sullivan 1996; Tabowei 2003; ten Eikelder 2016; Turnquest 1997; Wang 2012;Wu 2017; Yuen 1996). It is important to note that not all studies had a trial protocol available and therefore it was not possible to check if there were other pre‐specified outcomes not reported in the method section of the article.
Twenty‐eight studies were judged to be of unclear risk of reporting bias. In 10 studies no outcomes were pre‐specified in the methods section (Allouche 1993; Benzineb 1996; Jeeva 1982; Laddad 2013; Lewis 1983; Lyndrup 1989; Roberts 1986; Sanchez‐Ramos 1992; Tan 2015; Wang 2014 and in 18 studies there was too little information to judge reporting bias (Casey 1995; Dionne 2011; Gilson 2017; Glagoleva 1999; Guinn 2000; Gunawardena 2012; Haugland 2012; Hay 1995; Hudon 1999; Lemyre 2006; Moini 2003; Niromanesh 2003; Pineda Rivas 2016; Ridgway 1991; Roudsari 2011; Rudra 2012; Saleem 2006; Sarreau 2016).
The studies of Ahmed 2016, Browne 2011, Shechter‐Maor 2015, Sheikher 2009 and Tita 2006 were judged as high risk as not all pre‐specified outcomes were reported in the results section.
Other potential sources of bias
For 24 studies it was not clear if there was another source of bias and these were therefore judged as unclear. For one study (Barda 2018), only a manuscript with no tables was available. Two trials (Browne 2011; Tita 2006) were not published, but the results of the primary outcome and adverse events were reported in the trial registration. Guinn 2000 stopped recruiting women for one arm of the study without an explanation. Mullin 2002 calculated a sample size of 140 women but included 200 women without explanation. Prager 2008 included patients who did not meet inclusion criteria. Eighteen studies were only published as abstracts, or there was too little information provided and so it was not possible to judge the risk of bias (Casey 1995; Dionne 2011; Garba 2016; Gilson 2017; Glagoleva 1999; Haugland 2012; Hay 1995; Hudon 1999; Lemyre 2006; Oliveira 2010; Pineda Rivas 2016; Ridgway 1991; Rudra 2012; Sarreau 2016; Shechter‐Maor 2015; Solt 2009; Tabowei 2003; Wang 2012).
The studies of Culver 2004, Hibbard 1998, and Kruit 2016 were judged as high risk for other potential sources of bias as they were terminated early before the required sample size was recruited.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Balloon (Foley or ATAD) compared to vaginal prostaglandin E2 for third trimester labour induction in women with a viable fetus.
| Balloon (Foley or ATAD) compared to vaginal prostaglandin E2 for third trimester labour induction in women with a viable fetus | ||||||
| Patient or population: third trimester labour induction in women with a viable fetus Setting: Australia, China, Denmark, Iran, Jordan, India, Italy, Israel, Nigeria, Pakistan, Singapore, Sweden, the Netherlands, USA, UK Intervention: balloon (Foley or ATAD) Comparison: vaginal prostaglandin E2 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with vaginal prostaglandin E2 | Risk with balloon (Foley or ATAD) | |||||
| Vaginal delivery not achieved in 24 hours | Study population | RR 1.01 (0.82 to 1.26) | 1685 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 528 per 1000 | 533 per 1000 (433 to 665) | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 0.35 (0.18 to 0.67) | 1966 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 31 per 1000 | 11 per 1000 (6 to 21) | |||||
| Caesarean section | Study population | RR 1.00 (0.92 to 1.09) | 6619 (28 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 238 per 1000 | 238 per 1000 (219 to 260) | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 0.48 (0.25 to 0.93) | 2757 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 20 per 1000 | 9 per 1000 (5 to 18) | |||||
| Serious maternal morbidity or death | Study population | RR 0.20 (0.01 to 4.12) | 1481 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 3 per 1000 | 1 per 1000 (0 to 11) | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 0.74 (0.49 to 1.14) | 4271 (14 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 22 per 1000 | 16 per 1000 (11 to 25) | |||||
| Neonatal intensive care unit admission | Study population | RR 0.82 (0.65 to 1.04) | 3647 (12 RCTs) | ⊕⊕⊝⊝ LOW 1 4 | ||
| 74 per 1000 | 60 per 1000 (48 to 77) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded (1) level for serious limitation in study design due to lack of blinding (although not feasible due to nature of event)
2We downgraded (1) level for serious inconsistency due to evidence of statistical heterogeneity (I2 = >30%)
3We downgraded (2) levels for very serious imprecision due to wide CI crossing the line of no effect and small number of events
4We downgraded (1) level for serious imprecision due to wide CI crossing the line of no effect
Summary of findings 2. Balloon (Foley or ATAD) compared to low‐dose vaginal misoprostol for third trimester induction of labour in women with a viable fetus.
| Balloon (Foley or ATAD) compared to low‐dose vaginal misoprostol for third trimester induction of labour in women with a viable fetus | ||||||
| Patient or population: third trimester induction of labour in women with a viable fetus Setting: Brazil, Egypt, India, Iran, Nigeria, the Netherlands, Sweden Intervention: balloon (Foley or ATAD) Comparison: low‐dose vaginal misoprostol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low‐dose vaginal misoprostol | Risk with balloon (Foley or ATAD) | |||||
| Vaginal delivery not achieved in 24 hours | Study population | RR 1.09 (0.85 to 1.39) | 340 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 412 per 1000 | 449 per 1000 (350 to 573) | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 0.39 (0.18 to 0.85) | 1322 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 33 per 1000 | 13 per 1000 (6 to 28) | |||||
| Caesarean section | Study population | RR 1.28 (1.02 to 1.60) | 1756 (12 RCTs) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 243 per 1000 | 311 per 1000 (247 to 388) | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 0.58 (0.12 to 2.66) | 381 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 | ||
| 21 per 1000 | 12 per 1000 (2 to 55) | |||||
| Serious maternal morbidity or death | Study population | not estimable | 464 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 5 | no events occurred in included studies | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.00 (0.50 to 1.97) | 941 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 30 per 1000 | 30 per 1000 (15 to 59) | |||||
| Neonatal intensive care unit admission | Study population | RR 1.00 (0.61 to 1.63) | 1302 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 2 6 | ||
| 47 per 1000 | 47 per 1000 (29 to 77) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded (1) level for serious limitation in study design due to lack of blinding (although not feasible due to nature of event)
2We downgraded (1) level for serious imprecision due to wide CI crossing the line of no effect
3We downgraded (1) level for serious inconsistency due to evidence of statistical heterogeneity (I2 = >30%)
4We downgraded (2) levels for very serious imprecision due to wide CI crossing the line of no effect and small number of events
5 We downgraded (2) levels for very serious imprecision due to wide CI crossing the line of no effect and no events reported in included studies
6 Although there was some evidence suggesting small‐study effect we did not downgrade for publication bias because individual studies did not reach statistical significance and there was low heterogeneity across all studies for this outcome. Also, no difference was found between fixed‐effect or random‐effect analyses
Summary of findings 3. Balloon (Foley or ATAD) compared to low‐dose oral misoprostol for third trimester induction of labour in women with a viable fetus.
| Balloon (Foley or ATAD) compared to low‐dose oral misoprostol for third trimester induction of labour in women with a viable fetus | ||||||
| Patient or population: third trimester induction of labour in women with a viable fetus Setting: Finland, India, Pakistan, Sri Lanka, the Netherlands Intervention: balloon (Foley or ATAD) Comparison: low‐dose oral misoprostol | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with low‐dose oral misoprostol | Risk with balloon (Foley or ATAD) | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.28 (1.13 to 1.46) | 782 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 476 per 1000 | 609 per 1000 (538 to 695) | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 0.81 (0.48 to 1.38) | 2033 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 29 per 1000 | 24 per 1000 (14 to 40) | |||||
| Caesarean section | Study population | RR 1.17 (1.04 to 1.32) | 3178 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 3 | ||
| 222 per 1000 | 259 per 1000 (230 to 293) | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 1.11 (0.60 to 2.06) | 2627 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 2 4 | ||
| 14 per 1000 | 16 per 1000 (9 to 30) | |||||
| Serious maternal morbidity or death | Study population | RR 0.50 (0.05 to 5.52) | 2627 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 5 | ||
| 2 per 1000 | 1 per 1000 (0 to 8) | |||||
| Apgar score < 7 after 5 minutes | Study population | RR 0.71 (0.38 to 1.32) | 2693 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 4 | ||
| 18 per 1000 | 13 per 1000 (6 to 28) | |||||
| Neonatal intensive care unit admission | Study population | RR 0.82 (0.58 to 1.17) | 2873 (5 RCTs) | ⊕⊕⊝⊝ LOW 1 2 4 | ||
| 46 per 1000 | 37 per 1000 (26 to 53) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded (1) level for serious limitation in study design due to lack of blinding (although not feasible due to nature of event)
2We downgraded (1) level for serious imprecision due to wide CI crossing the line of no effect
3 Trial of Mundle 2017 did not meet the pre‐specified population as pregnancies with a non viable fetus were included. Sensitivity analyses did not alter the estimated effect size. Therefore we did not downgrade
4 Trial of Mundle 2017 did not meet the pre‐specified population as pregnancies with a non viable fetus were included. Sensitivity analysis did not change the direction of the effect size and numbers of events were not higher compared to other trials. Therefore we did not downgrade.
5 We downgraded (2) levels for very serious imprecision due to wide CI crossing the line of no effect and small number of events
Balloon (single or double) versus vaginal prostaglandin E2 (28 trials involving 6619 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
There may be little or no difference in vaginal deliveries not achieved within 24 hours between induction of labour with a balloon catheter and vaginal PGE2 (average risk ratio (RR) 1.01, 95% confidence interval (CI) 0.82 to 1.26; 7 studies; 1685 women; low‐quality evidence; Analysis 1.1), although there was substantial heterogeneity for this outcome (Tau² = 0.06; Chi² = 29.06, df = 6 (P =< 0.0001); I² = 79%).
1.1. Analysis.
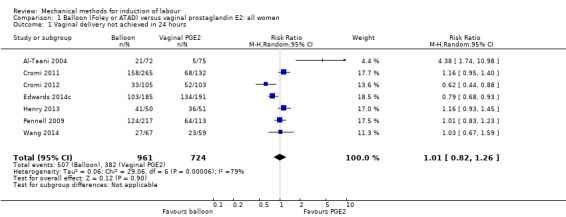
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
A sensitivity analysis, after eliminating the two trials assessed as having a potentially higher risk of concealment or attrition bias (Cromi 2012; Wang 2014), did not change the effect observed, despite the result becoming less precise (average RR 1.10, 95% CI 0.86 to 1.41; 1351 women; 5 studies; I² = 82%).
The same result was seen on a subgroup comparison for primiparous women (RR 1.01, 95% CI 0.83 to 1.23; 330 women; 1 study; Analysis 2.1). While for multiparous women, a balloon catheter may increase the risk of a vaginal delivery not being achieved within 24 hours (RR 4.38, 95% CI 1.74 to 10.98; 147 women; 1 study; Analysis 3.1).
2.1. Analysis.
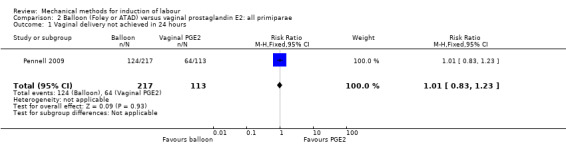
Comparison 2 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
3.1. Analysis.
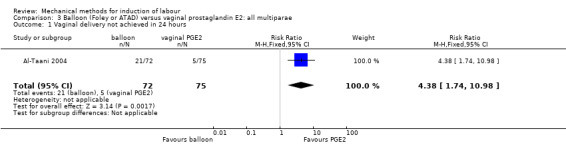
Comparison 3 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all multiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
Uterine hyperstimulation with fetal heart rate (FHR) changes
A balloon catheter probably reduces the risk of uterine hyperstimulation with FHR changes when compared to vaginal PGE2 (RR 0.35, 95% CI 0.18 to 0.67; 6 studies; 1966 women; moderate‐quality evidence; Analysis 1.2), the absolute effect being 21 less per 1000 deliveries.
1.2. Analysis.
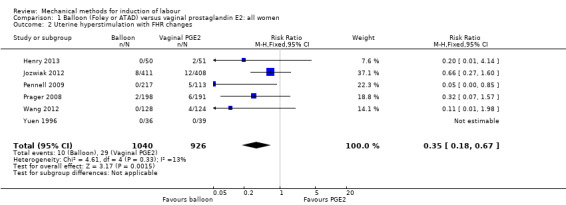
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
The same result was seen on a subgroup comparison for primiparous women (RR 0.05, 95% CI 0.00 to 0.85; 330 women; 1 study; Analysis 2.2). For multiparous women, no outcomes were reported.
2.2. Analysis.
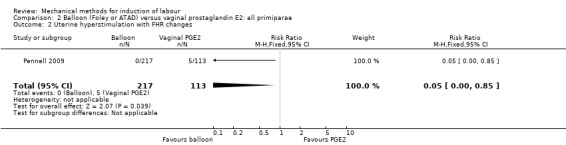
Comparison 2 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
Caesarean section
There probably is little or no difference in caesarean sections between both induction methods (RR 1.00, 95% CI 0.92 to 1.09; 28 studies; 6619 women; moderate‐quality evidence; Analysis 1.3). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 4).
1.3. Analysis.
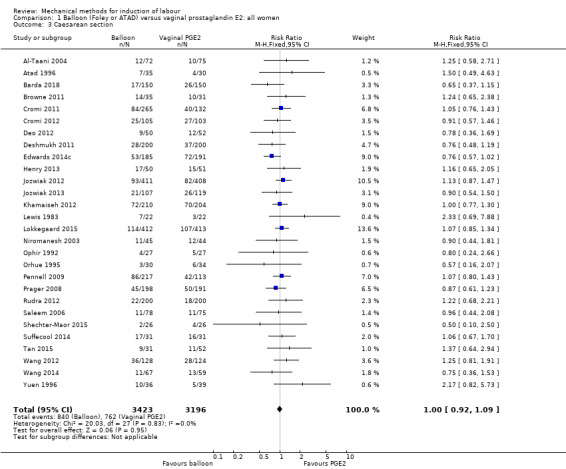
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 3 Caesarean section.
It is uncertain whether there is a difference in caesarean sections between both induction methods on subgroups for both primiparous women (average RR 0.89, 95% CI 0.59 to 1.33; 828 women; 5 studies; Analysis 2.3) and multiparous women (RR 1.31, 95% CI 0.65 to 2.63; 180 women; 2 studies; Analysis 3.2) as the results of these outcomes were imprecise. Furthermore, for the primiparous group, there was also substantial heterogeneity (Tau² = 0.11; Chi² = 10.01, df = 4 (P = 0.04); I² = 60%).
2.3. Analysis.
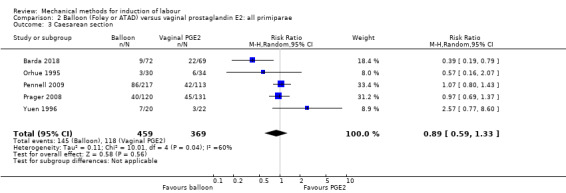
Comparison 2 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all primiparae, Outcome 3 Caesarean section.
3.2. Analysis.
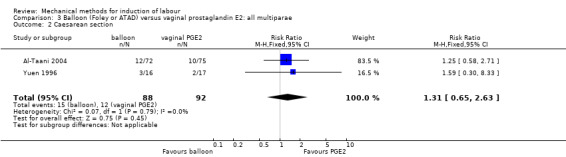
Comparison 3 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all multiparae, Outcome 2 Caesarean section.
Serious neonatal morbidity or perinatal death
A balloon catheter probably reduces the risk of serious neonatal morbidity or perinatal death when compared to vaginal PGE2 (RR 0.48, 95% CI 0.25 to 0.93; 8 studies; 2757 women; moderate‐quality evidence; Analysis 1.4). However, numbers are low (12/1483 versus 25/1274, respectively) and almost all of these numbers were cases of birth asphyxia. Only two perinatal deaths were reported, both in the PGE2 group (Edwards 2014c). No heterogeneity was seen for this outcome.
1.4. Analysis.
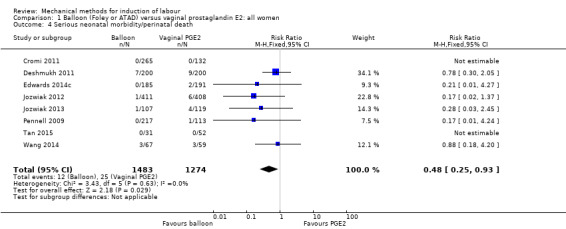
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 4 Serious neonatal morbidity/perinatal death.
For primiparous women, it is uncertain whether there is a difference in effect as the result for this outcome was imprecise (RR 0.17, 95% CI 0.01 to 4.24; 330 women; 1 study; Analysis 2.4). For multiparous women, no outcomes were reported.
2.4. Analysis.
Comparison 2 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all primiparae, Outcome 4 Serious neonatal morbidity/perinatal death.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (RR 0.20, 95% CI 0.01 to 4.12; 4 studies; 1481 women; very low‐quality evidence; Analysis 1.5). Of all the 28 studies included for this comparison, only four studies reported on this composite outcome. No events were reported in the balloon group. One author (Jozwiak 2012) reported two events in the PGE2 group, both events being uterine rupture.
1.5. Analysis.
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 5 Serious maternal morbidity or death.
Only one study (60 women) reported on this outcome in primiparous women, in which no events were seen (Analysis 2.5). For multiparous women, no outcomes were reported.
2.5. Analysis.
Comparison 2 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all primiparae, Outcome 5 Serious maternal morbidity or death.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
Induction of labour with a balloon catheter may increase the risk of oxytocin augmentation when compared to vaginal PGE2 (average RR 1.54, 95% CI 1.35 to 1.76; 4828 women; 16 studies; Analysis 1.6), although there was substantial heterogeneity for this outcome (Tau² = 0.05; Chi² = 141.47, df = 15 (P = < 0.0001); I² = 89%). Visual inspection of the funnel plot was somewhat asymmetrical, suggesting some form of publication bias (Figure 5).
1.6. Analysis.
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 6 Oxytocin augmentation.
A sensitivity analysis, after eliminating the five trials assessed as having a potentially higher risk of allocation or attrition bias (Cromi 2012; Deo 2012; Tan 2015; Wang 2012; Wang 2014), did not alter the result, nor did it lower heterogeneity (average RR 1.37, 95% CI 1.21 to 1.54; 4005 women; 11 studies; I² = 87%).
Uterine hyperstimulation without FHR changes
A balloon catheter may reduce the risk of uterine hyperstimulation without FHR changes when compared to vaginal PGE2 (average RR 0.27, 95% CI 0.11 to 0.66; 2444 women; 15 studies; Analysis 1.7), although there was moderate heterogeneity for this outcome (Tau² = 1.13; Chi² = 22.28, df = 12 (P = 0.03); I² = 46%). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 6).
1.7. Analysis.
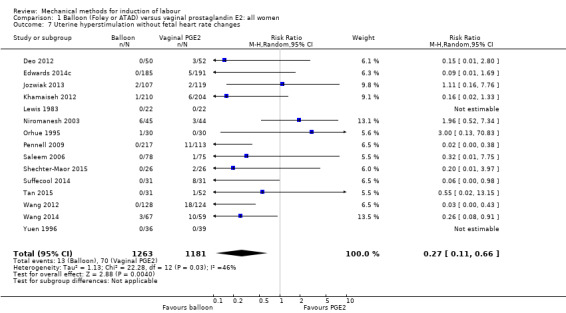
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 7 Uterine hyperstimulation without fetal heart rate changes.
A sensitivity analysis, after eliminating the seven trials assessed as having a potentially higher risk of allocation or attrition bias (Deo 2012; Orhue 1995; Shechter‐Maor 2015; Tan 2015; Wang 2012; Wang 2014; Zahoor 2014), made this result less precise and therefore raises uncertainty as to whether there is a difference in uterine hyperstimulation without FHR changes (average RR 0.26, 95% CI 0.06 to 1.05; 1694 women; 8 studies; I² = 62%).
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (RR 0.20, 95% CI 0.01 to 4.12; 1045 women; 2 studies; Analysis 1.8). Only two cases of uterine rupture were reported, both in the PGE2 group in the study of Jozwiak 2012. Uterine rupture was defined by the authors as a separation of the uterine wall, and in one case this was caused by inserting an intrauterine pressure catheter.
1.8. Analysis.
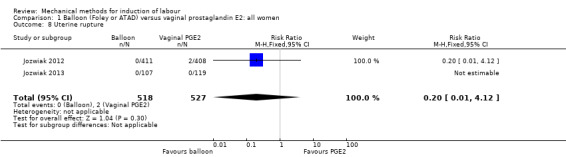
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 8 Uterine rupture.
Epidural analgesia
A balloon catheter may slightly increase the use of epidural analgesia during labour when compared to vaginal PGE2 (average RR 1.14, 95% CI 1.00 to 1.29; 2828 women; 8 studies; Analysis 1.9). However, there was substantial heterogeneity for this outcome (Tau² = 0.02; Chi² = 32.09, df = 7 (P = < 0.0001); I² = 78%).
1.9. Analysis.
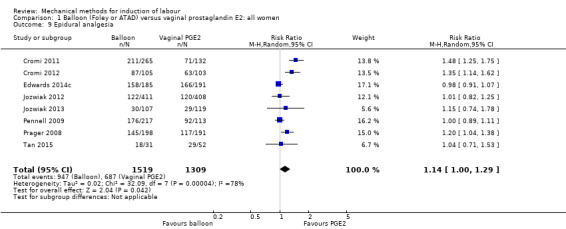
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 9 Epidural analgesia.
A sensitivity analysis, after eliminating the two trials assessed as having a potentially higher risk of allocation or attrition bias (Cromi 2012; Tan 2015), did not alter the result, nor did it lower heterogeneity (average RR 1.11, 95% CI 0.97 to 1.28; 2537 women; 6 studies; I² = 80%).
Instrumental vaginal delivery
There probably is little or no difference in instrumental vaginal deliveries between both induction methods (RR 0.93, 95% CI 0.79 to 1.09; 4514 women; 16 studies; Analysis 1.10). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 7).
1.10. Analysis.
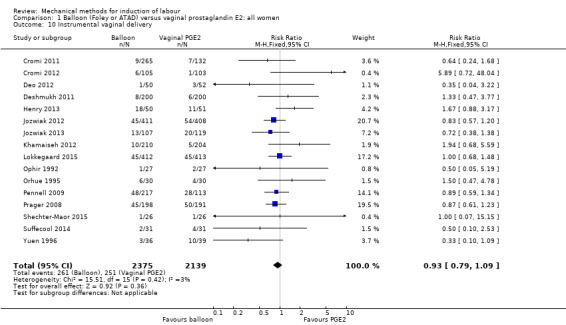
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 10 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.89, 95% CI 0.67 to 1.19; 964 women; 4 studies; Analysis 1.11).
1.11. Analysis.
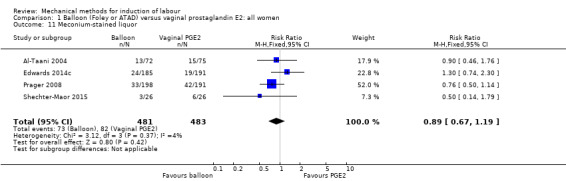
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 11 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar score less than seven at five minutes between both induction methods (RR 0.74, 95% CI 0.49 to 1.14; 4271 women; 14 studies; low‐quality evidence; Analysis 1.12). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 8).
1.12. Analysis.
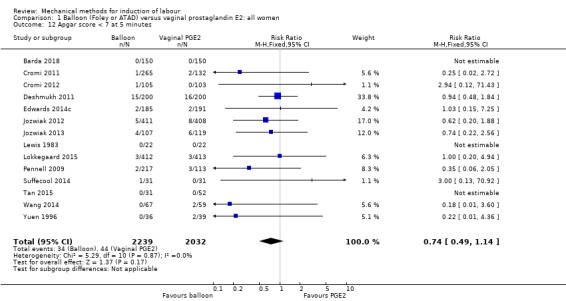
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 12 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit (NICU) admission
A balloon catheter may reduce the risk of a NICU admission when compared to vaginal PGE2 (RR 0.82, 95% CI 0.65 to 1.04; 3647 women; 12 studies; low‐quality evidence; Analysis 1.13), the absolute effect being 15 fewer NICU admission per 1000 deliveries. Although it should be noted that there is a wide range of treatment effects that are compatible with the data, from a very small increase in risk to very large decrease. Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 9).
1.13. Analysis.
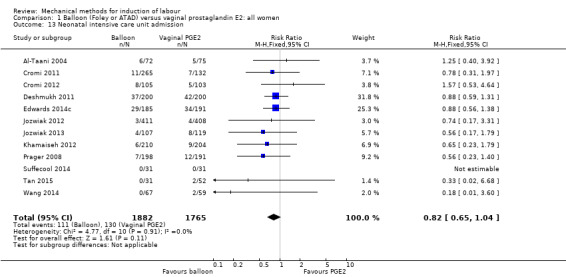
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 13 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both induction methods (RR 0.21, 95% CI 0.01 to 4.27; 1036 women; 5 studies; Analysis 1.14). Only two cases of perinatal death were reported by Edwards 2014c, both being cases of neonatal death and born to women randomised to vaginal PGE2. The authors describe that in both cases the neonates died as a result of complications related to a congenital diaphragmatic hernia and were unrelated to the induction method.
1.14. Analysis.
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 14 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 0.82, 95% CI 0.63 to 1.06; 2215 women; 8 studies; Analysis 1.15).
1.15. Analysis.
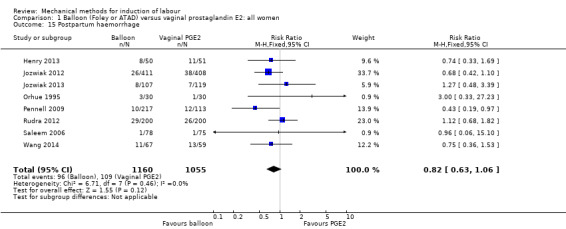
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 15 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
A balloon catheter may reduce the amount of women not being satisfied with the induction method when compared to prostaglandin E2 (RR 0.61, 95% CI 0.39 to 0.97; 93 women; 1 study; Analysis 1.16), the absolute effect being 224 fewer women not satisfied per 1000 deliveries. This outcome was reported by Henry 2013 by asking the women if they would choose the randomised induction method again. Patient satisfaction was also reported by Shechter‐Maor 2015, but could not be included in the meta‐analysis. In this study women were asked to score their satisfaction with the induction process on a five‐point Likert scale. No difference in satisfaction was seen between both induction methods (3.41 (± 1.3) versus 3.33 (± 1.2), respectively; P = 0.860).
1.16. Analysis.
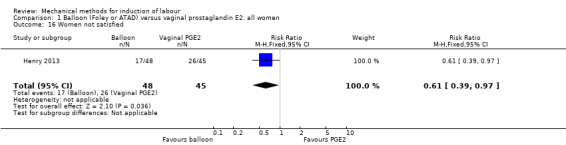
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 16 Women not satisfied.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
It is uncertain whether there is a difference in maternal fever during labour between both methods (RR 0.87, 95% CI 0.65 to 1.17; 2362 women; 7 studies; Analysis 1.17).
1.17. Analysis.
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 17 Maternal fever during labour.
Antibiotics during labour
It is uncertain whether there is a difference in antibiotics during labour between both methods (RR 1.43, 95% CI 0.89 to 2.29; 330 women; 1 study; Analysis 1.18).
1.18. Analysis.
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 18 Antibiotics during labour.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 0.69, 95% CI 0.32 to 1.49; 376 women; 1 study; Analysis 1.19).
1.19. Analysis.
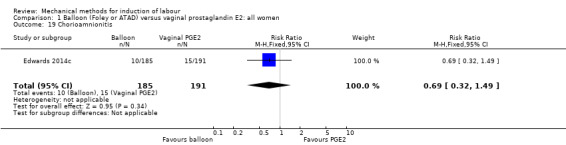
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 19 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 0.49, 95% CI 0.19 to 1.27; 706 women; 2 studies; Analysis 1.20).
1.20. Analysis.
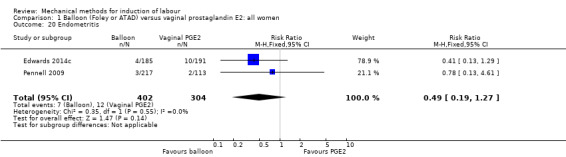
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 20 Endometritis.
Fetal distress
A balloon catheter probably reduces the risk of fetal distress for which a caesarean section is indicated when compared to vaginal PGE2 (RR 0.71, 95% CI 0.60 to 0.83; 4753 women; 20 studies; Analysis 1.21). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 10).
1.21. Analysis.
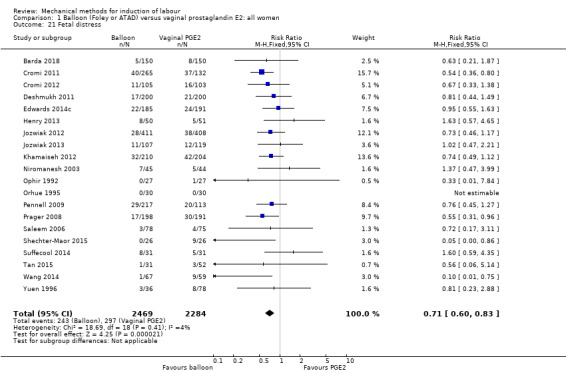
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 21 Fetal distress.
Umbilical artery pH < 7.10
A balloon catheter probably reduces the risk of an umbilical artery pH less than 7.10 directly postpartum when compared to vaginal PGE2 (RR 0.65, 95% CI 0.44 to 0.94; 2675 women, 8 studies; Analysis 1.22). However, numbers occurred infrequently in both groups (35 per 1000 versus 56 per 1000, respectively).
1.22. Analysis.
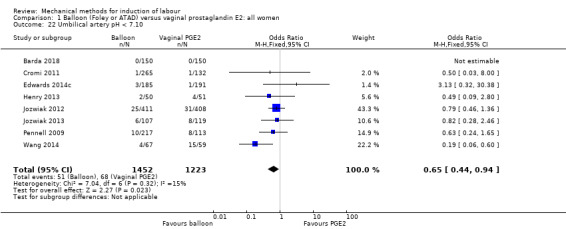
Comparison 1 Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women, Outcome 22 Umbilical artery pH < 7.10.
Balloon (single or double) versus cervical prostaglandin E2 (10 trials involving 1428 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
It is uncertain whether there is a difference in vaginal deliveries achieved within 24 hours between induction of labour with a balloon catheter and cervical PGE2 (average RR 1.01, 95% CI 0.35 to 2.91; 200 women; 2 studies; Analysis 4.1). There also was substantial heterogeneity for this outcome (Tau² = 0.53; Chi² = 10.35, df = 1 (P = 0.001); I² = 90%). Even though data were pooled, both studies may be incompatible as no overlap of CIs is present. No sensitivity analysis was conducted as no potential high‐risk studies were included for this outcome.
4.1. Analysis.
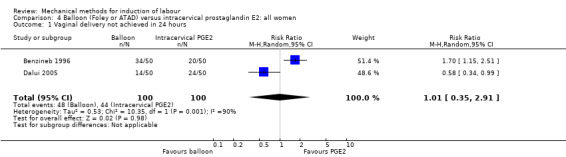
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (RR 0.37, 95% CI 0.02 to 8.90; 447 women; 4 studies; Analysis 4.2).
4.2. Analysis.
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
Only one small study (53 women) reported this outcome for the subgroups of primiparous and multiparous women. No events were reported in primiparous women (Analysis 5.1). For multiparous women, it is uncertain whether there is a difference for this outcome between both induction methods (RR 0.30, 95% CI 0.01 to 7.02; 53 women; 1 study; Analysis 6.1).
5.1. Analysis.
Comparison 5 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all primiparae, Outcome 1 Uterine hyperstimulation with FHR changes.
6.1. Analysis.
Comparison 6 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all multiparae, Outcome 1 Uterine hyperstimulation with FHR changes.
Caesarean section
There probably is little or no difference in caesarean sections between both induction methods (RR 0.97, 95% CI 0.81 to 1.15; 1309 women; 9 studies; Analysis 4.3). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 11).
4.3. Analysis.
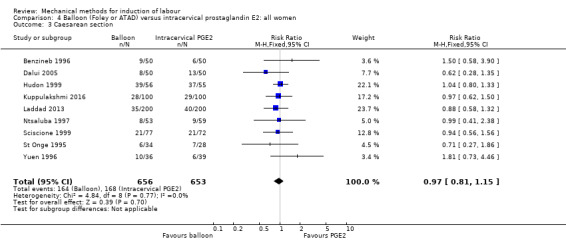
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 3 Caesarean section.
It is uncertain whether there is a difference in caesarean sections between both induction methods on subgroup comparisons for both primiparous women (RR 1.30, 95% CI 0.86 to 1.95; 245 women; 3 studies; Analysis 5.2) and multiparous women (average RR 0.66, 95% CI 0.16 to 2.78; 136 women; 3 studies; Analysis 6.2) as the results for both comparisons were imprecise. For the multiparous group, there also was substantial heterogeneity (Tau² = 0.90; Chi² = 4.78, df = 2 (P = 0.09); I² = 58%).
5.2. Analysis.
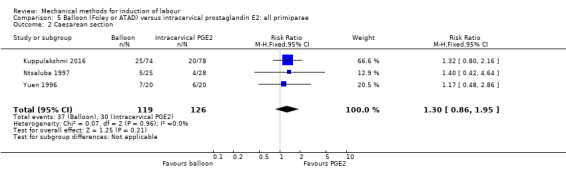
Comparison 5 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all primiparae, Outcome 2 Caesarean section.
6.2. Analysis.
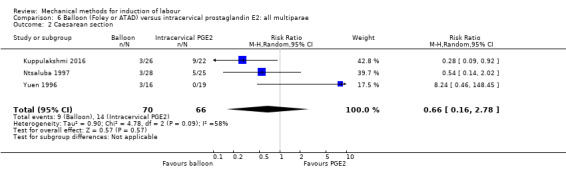
Comparison 6 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all multiparae, Outcome 2 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 0.78, 95% CI 0.29 to 2.05; 500 women; 2 studies; Analysis 4.4). Of the 10 studies included for this comparison, two studies (Benzineb 1996; Laddad 2013) reported on this composite outcome. All reported events in these studies were cases of perinatal death.
4.4. Analysis.
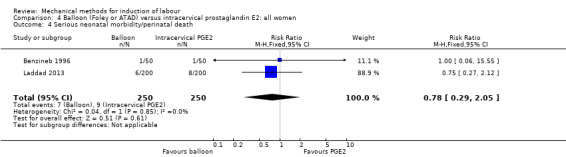
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 4 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
Not reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
It is uncertain whether there is a difference in an unfavourable cervix after 24 hours between both induction methods (RR 0.96, 95% CI 0.70 to 1.34; 219 women; 2 studies; Analysis 4.5).
4.5. Analysis.
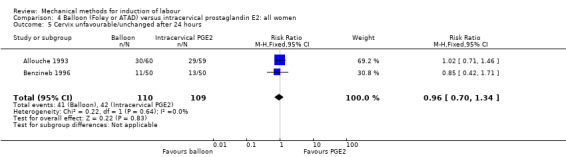
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 5 Cervix unfavourable/unchanged after 24 hours.
Oxytocin augmentation
There may be little or no difference in oxytocin augmentation between both induction methods (RR 1.08, 95% CI 0.93 to 1.26; 400 women; 1 study; Analysis 4.6).
4.6. Analysis.
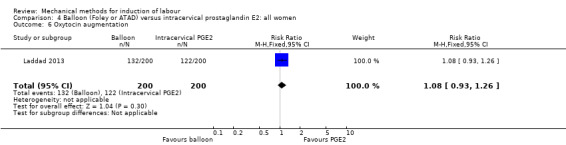
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 6 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (average RR 0.99, 95% CI 0.09 to 10.38; 654 women; 5 studies; Analysis 4.7). Also, there was substantial heterogeneity for this outcome (Tau² = 2.92; Chi² = 6.33, df = 2 (P = 0.04); I² = 68%).
4.7. Analysis.
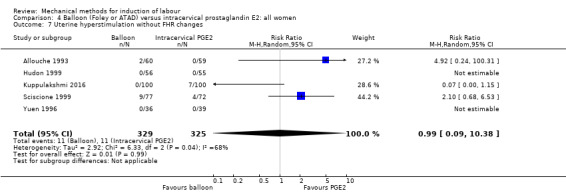
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 7 Uterine hyperstimulation without FHR changes.
A sensitivity analysis, after eliminating the two trials assessed as having a potentially higher risk of allocation or attrition bias (Sciscione 1999; Yuen 1996) did not alter the result, nor did it lower heterogeneity (average RR 0.56, 95% CI 0.01 to 39.31; 430 women; 3 studies; I² = 76%).
Uterine rupture
Not reported.
Epidural analgesia
There may be little or no difference in epidural analgesia during labour between both induction methods (RR 0.91, 95% CI 0.81 to 1.02; 149 women; 1 study; Analysis 4.8).
4.8. Analysis.
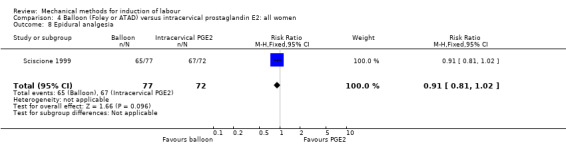
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 8 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 1.18, 95% CI 0.68 to 2.05; 337 women; 3 studies; Analysis 4.9).
4.9. Analysis.
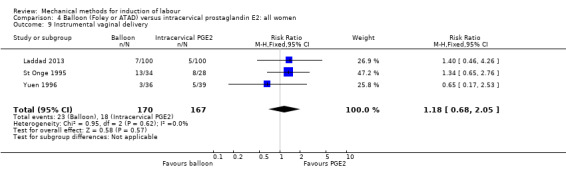
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 9 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 1.17, 95% CI 0.42 to 3.26; 118 women; 1 study; Analysis 4.10).
4.10. Analysis.
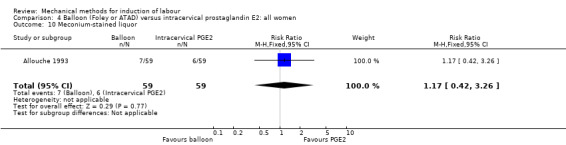
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 10 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes (RR 0.79, 95% CI 0.41 to 1.53; 475 women; 2 studies; Analysis 4.11).
4.11. Analysis.
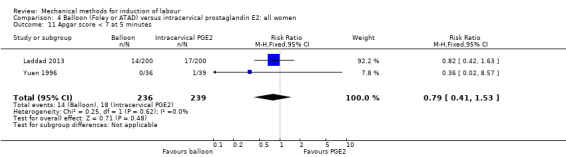
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 11 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both methods (RR 0.88, 95% CI 0.60 to 1.31; 400 women; 1 study; Analysis 4.12).
4.12. Analysis.
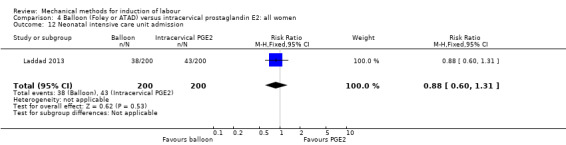
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 12 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is difference in perinatal death between both induction methods (RR 0.78, 95% CI 0.29 to 2.05; 500 women; 2 studies. Analysis 4.13). Noteworthy, there was a relatively high number of neonatal deaths reported in the study of Laddad 2013 for the balloon group (6/200), as well as in the cervical PGE2 group (8/200), for which no explanation was given by the authors.
4.13. Analysis.
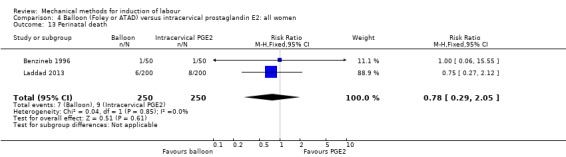
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 13 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects
It is uncertain whether there is a difference in maternal side effects (RR 0.15, 95% CI 0.02 to 1.24; 211 women; 2 studies; Analysis 4.14). The nature of the side effects was not specified in both included studies.
4.14. Analysis.
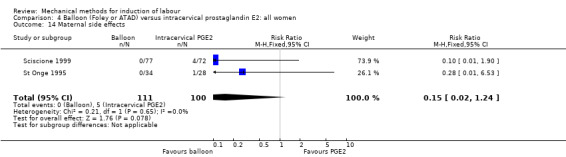
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 14 Maternal side effects.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 0.20, 95% CI 0.01 to 4.06; 100 women; 1 study; Analysis 4.15).
4.15. Analysis.
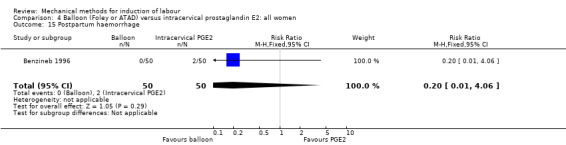
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 15 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 1.00, 95% CI 0.21 to 4.75; 118 women; 1 study; Analysis 4.16).
4.16. Analysis.
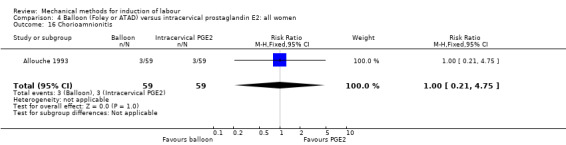
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 16 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 1.00, 95% CI 0.06 to 15.61; 118 women; 1 study; Analysis 4.17).
4.17. Analysis.
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 17 Endometritis.
Fetal distress
A balloon catheter probably reduces the risk of fetal distress for which a caesarean section is indicated when compared to cervical PGE2 (RR 0.61, 95% CI 0.42 to 0.89; 1023 women; 6 studies; Analysis 4.18).
4.18. Analysis.
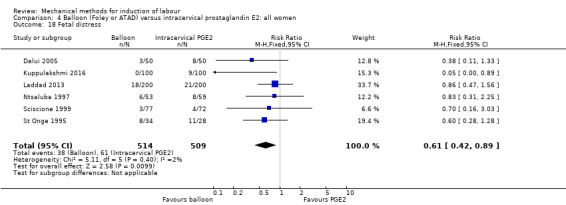
Comparison 4 Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women, Outcome 18 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Balloon (single or double) versus low‐dose vaginal misoprostol (13 trials involving 1818 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
It is uncertain whether there is a difference in vaginal deliveries not achieved within 24 hours between induction of labour with a balloon catheter and vaginal misoprostol (RR 1.09, 95% CI 0.85 to 1.39; 340 women; 2 studies; low‐quality evidence; Analysis 7.1).
7.1. Analysis.
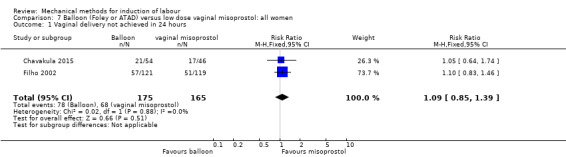
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
A balloon catheter probably reduces the risk of uterine hyperstimulation with FHR changes when compared to vaginal misoprostol (RR 0.39, 95% CI 0.18 to 0.85; 1322 women; moderate‐quality evidence; 8 studies; Analysis 7.2), the absolute effect being 22 fewer cases per 1000 deliveries.
7.2. Analysis.
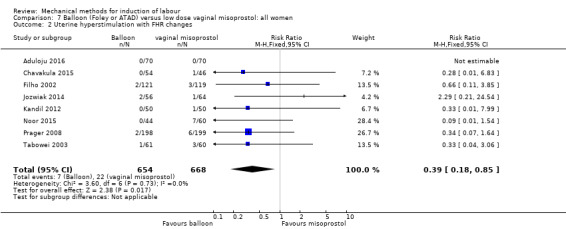
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
A balloon catheter may increase the risk of a caesarean section when compared to vaginal misoprostol (average RR 1.28, 95% CI 1.02 to 1.60; 1756 women; 12 studies; low‐quality evidence; Analysis 7.3), the absolute effect being 53 more caesarean sections per 1000 deliveries. However, there was moderate heterogeneity for this outcome (Tau² = 0.06; Chi² = 19.86, df = 11 (P = 0.05); I² = 45%).
7.3. Analysis.
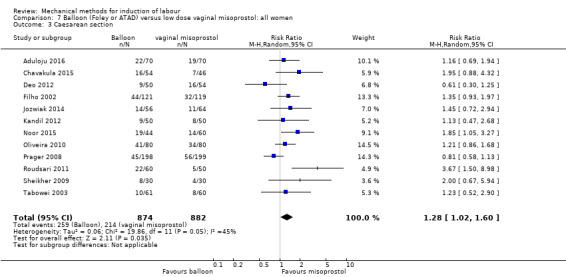
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 3 Caesarean section.
A sensitivity analysis, after eliminating the three trials assessed as having a potentially higher risk of allocation or attrition bias (Deo 2012; Kandil 2012; Sheikher 2009), did not alter the result, nor did it lower heterogeneity (average RR 1.34, 95% CI 1.05 to 1.71; 1492 women; 10 studies; I² = 48%). Visual inspection of the funnel plot associated with this outcome does not suggest any evidence of publication bias (Figure 12).
For the subgroup of primiparous women, it is uncertain whether there is a difference in caesarean sections between both induction methods (RR 0.82, 95% CI 0.59 to 1.13; 255 women; 1 study; Analysis 8.1). For multiparous women, no outcomes were reported.
8.1. Analysis.
Comparison 8 Balloon (Foley or ATAD versus low dose vaginal misoprostol: all primiparae, Outcome 1 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 0.58, 95% CI 0.12 to 2.66; 381 women; 3 studies; very low‐quality evidence; Analysis 7.4). All of the cases included for this composite outcome were cases of perinatal asphyxia (2/187 versus 4/194, respectively).
7.4. Analysis.
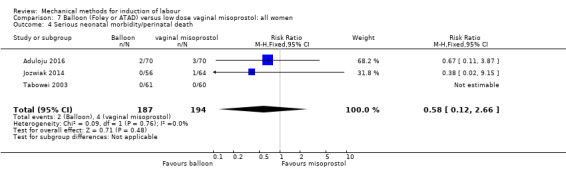
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 4 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (very low‐quality evidence; Analysis 7.5). Of the 13 studies included for this comparison, four studies (464 women) reported on this composite outcome. No events of maternal morbidity or death occurred in one of these studies.
7.5. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 5 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 12 hours
It is uncertain whether there is a difference in an unfavourable cervix after 12 hours between both induction methods (average RR 2.66, 95% CI 0.60 to 11.89; 200 women; 2 studies; Analysis 7.6). Also, there was moderate heterogeneity for this outcome (Tau² = 0.63; Chi² = 1.56, df = 1 (P = 0.21); I² = 36%). No studies reported on a time period of 24 hours.
7.6. Analysis.
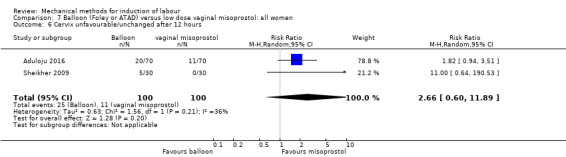
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 6 Cervix unfavourable/unchanged after 12 hours.
A sensitivity analysis, after eliminating one trial assessed as having a potentially higher risk of allocation or attrition bias (Sheikher 2009), did not change the result, but did narrow the CI (RR 1.82, 95% CI 0.94 to 3.51; 1 study).
Oxytocin augmentation
A balloon catheter probably increases the risk of oxytocin augmentation when compared to vaginal misoprostol (average RR 1.62, 95% CI 1.38 to 1.90; 911 women; 9 studies; Analysis 7.7), although there was substantial heterogeneity for this outcome (Tau² = 0.03; Chi² = 21.93, df = 8 (P = 0.005); I² = 64%).
7.7. Analysis.
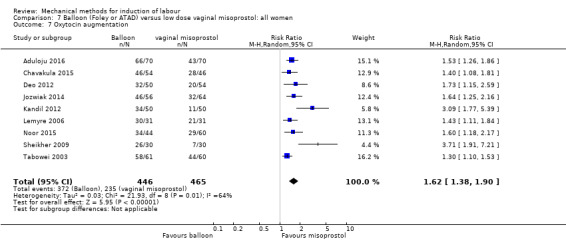
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 7 Oxytocin augmentation.
In the sensitivity analysis, after eliminating two trial assessed as having a potentially higher risk of allocation or attrition bias (Kandil 2012 and Sheikher 2009), heterogeneity was lost without altering the effect observed (average RR 1.50, 95% CI 1.36 to 1.64; 751 women, 7 studies; I² = 0%).
Uterine hyperstimulation without FHR changes
A balloon catheter probably reduces the risk of uterine hyperstimulation without FHR changes when compared to vaginal misoprostol (RR 0.25, 95% CI 0.14 to 0.44; 1139 women; 9 studies; Analysis 7.8).
7.8. Analysis.
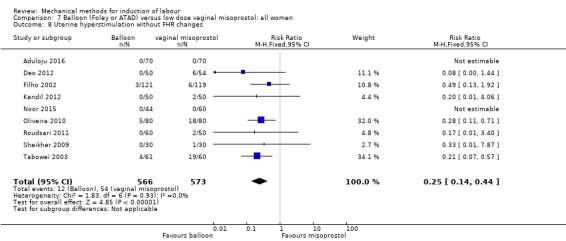
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 7.9). Of the 13 studies included for this comparison, only three studies (364 women) reported on this outcome. No events of uterine rupture occurred in one of these studies.
7.9. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 9 Uterine rupture.
Epidural analgesia
A balloon catheter probably slightly increases the use of epidural analgesia during labour when compared to vaginal misoprostol (RR 1.22, 95% CI 1.06 to 1.41; 517 women; 2 studies; Analysis 7.10).
7.10. Analysis.
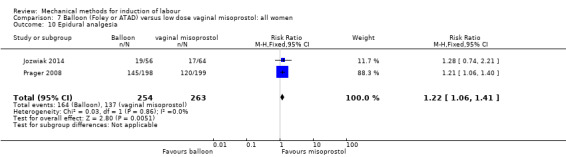
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 10 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.72, 95% CI 0.50 to 1.05; 721 women; 4 studies; Analysis 7.11).
7.11. Analysis.
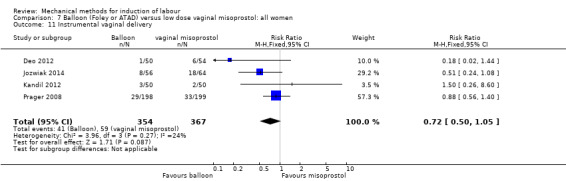
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 11 Instrumental vaginal delivery.
Meconium‐stained liquor
A balloon catheter probably reduces the risk of meconium‐stained liquor when compared to vaginal misoprostol (RR 0.64, 95% CI 0.48 to 0.87; 1268 women; 7 studies; Analysis 7.12).
7.12. Analysis.
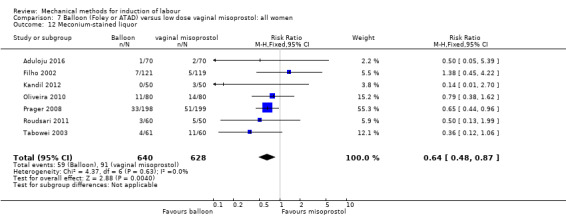
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 12 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 1.00, 95% CI 0.50 to 1.97; 941 women; 7 studies; low‐quality evidence; Analysis 7.13).
7.13. Analysis.
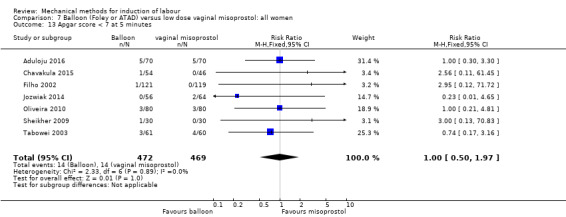
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 13 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both induction methods (RR 1.00, 95% CI 0.61 to 1.63; 1302 women; 9 studies; low‐quality evidence; Analysis 7.14).
7.14. Analysis.
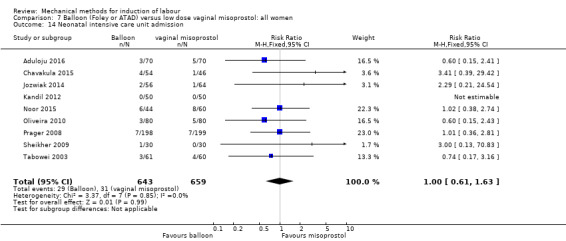
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 14 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is difference in perinatal death between both induction methods (Analysis 7.15). Of the 13 studies included for this comparison, only one study (121 women) pre‐specified this outcome. No cases of perinatal death were reported in this study.
7.15. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 15 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
It is uncertain whether there is difference in maternal vomiting between both induction methods (Analysis 7.16). Of all the 13 studies included for this comparison, only one study (60 women) pre‐specified this outcome. No cases of maternal vomiting were reported in this study.
7.16. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 16 Maternal vomiting.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is difference in postpartum haemorrhage between both induction methods (RR 1.14, 95% CI 0.24 to 5.44; 120 women; 1 study; (Analysis 7.17).
7.17. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 17 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
One study (Chavakula 2015) reported on patient satisfaction, but could not be included in the meta‐analysis. In this study, satisfaction was assessed by a visual analogue score ranging from zero to five (0 = very poor; 5 = very good), in which no difference between both induction methods was seen (100 women; 4.5 [4‐5] versus 4.45 [3‐5], respectively; P = 0.488).
Caregiver not satisfied
Not reported.
Not pre‐specified outcomes
Maternal fever during labour
It is uncertain whether there is a difference in maternal fever during labour between both methods (average RR 1.84, 95% CI 0.22 to 15.62; 617 women; 3 studies; Analysis 7.18). Also, there was substantial heterogeneity for this outcome (Tau² = 1.86; Chi² = 3.95, df = 1 (P = 0.05); I² = 75%). No sensitivity analysis was performed as no potential high‐risk studies were included for this outcome.
7.18. Analysis.
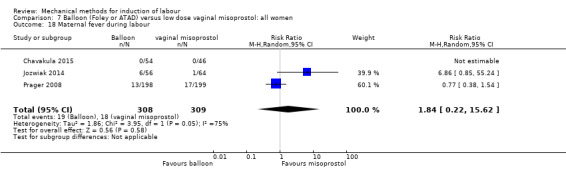
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 18 Maternal fever during labour.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 1.24, 95% CI 0.31 to 4.88; 200 women; 2 studies; Analysis 7.19).
7.19. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 19 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 2.95, 95% CI 0.12 to 71.72; 240 women; 1 study; Analysis 7.20).
7.20. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 20 Endometritis.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated (RR 0.84, 95% CI 0.67 to 1.05; 1127 women; 7 studies; Analysis 7.21).
7.21. Analysis.
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 21 Fetal distress.
Umbilical artery pH < 7.10
It is uncertain whether there is a difference in umbilical artery pH less than 7.10 directly postpartum between both induction methods (RR 1.14, 95% CI 0.35 to 3.74; 120 women; 1 study; Analysis 7.22).
7.22. Analysis.
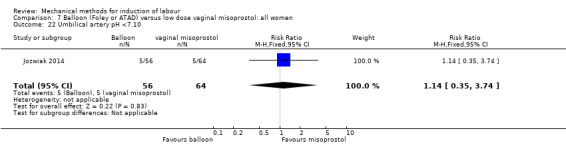
Comparison 7 Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women, Outcome 22 Umbilical artery pH <7.10.
Balloon (single or double) versus low‐dose oral misoprostol (seven trials involving 3178 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
A balloon catheter probably increases the risk of a vaginal delivery not achieved within 24 hours when compared to oral misoprostol (RR 1.28, 95% CI 1.13 to 1.46; 782 women, 2 studies. moderate‐quality evidence, Analysis 9.1), the absolute effect being 133 more per 1000 deliveries.
9.1. Analysis.
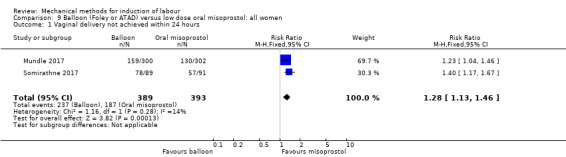
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
The same results were seen on parity subgroup comparisons for primiparous women (RR 1.19, 95% CI 1.04 to 1.37; 573 women; 2 studies; Analysis 10.1) and multiparous women (RR 1.55, 95% CI 1.17 to 2.06; 209 women; 2 studies; Analysis 11.1).
10.1. Analysis.
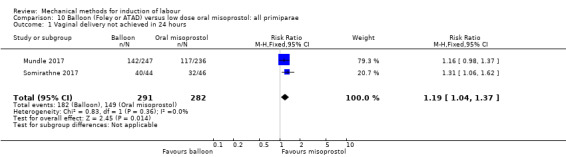
Comparison 10 Balloon (Foley or ATAD) versus low dose oral misoprostol: all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
11.1. Analysis.
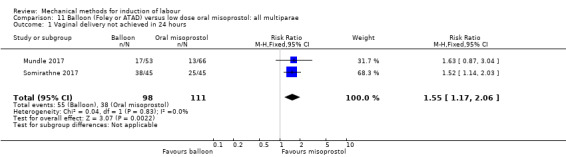
Comparison 11 Balloon (Foley or ATAD) versus low dose oral misoprostol: all multiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (RR 0.81, 95% CI 0.48 to 1.38; 2033 women; 2 studies; low‐quality evidence; Analysis 9.2).
9.2. Analysis.
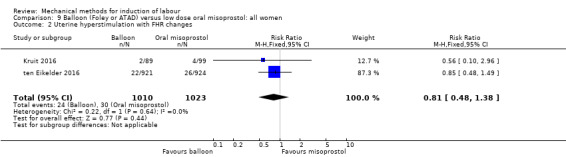
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
The same results were seen on parity subgroup comparisons for primiparous women (RR 0.81, 95% CI 0.45 to 1.46; 1206 women; 1 study; Analysis 10.2 and multiparous women (RR 1.45, 95% CI 0.24 to 8.61; 639 women; 1 study; Analysis 11.2).
10.2. Analysis.
Comparison 10 Balloon (Foley or ATAD) versus low dose oral misoprostol: all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
11.2. Analysis.
Comparison 11 Balloon (Foley or ATAD) versus low dose oral misoprostol: all multiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
Caesarean section
A balloon catheter probably slightly increases the risk of a caesarean section when compared to oral misoprostol (RR 1.17, 95% CI 1.04 to 1.32; 3178 women; 7 studies; moderate‐quality evidence; Analysis 9.3), the absolute effect being 37 more caesarean sections per 1000 deliveries.
9.3. Analysis.
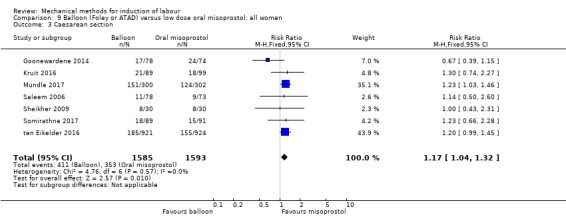
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 3 Caesarean section.
The same result was seen on the subgroup of primiparous women (RR 1.21, 95% CI 1.06 to 1.38; 1778 women; 3 studies; Analysis 10.3). For multiparous women, it is uncertain whether there is a difference in caesarean sections between both methods (RR 1.22, 95% CI 0.79 to 1.87; 848 women; 3 studies; Analysis 11.3).
10.3. Analysis.
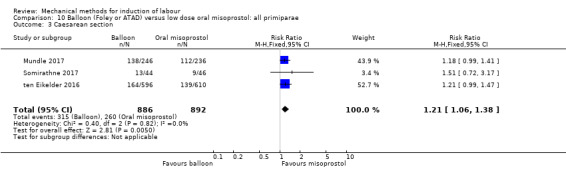
Comparison 10 Balloon (Foley or ATAD) versus low dose oral misoprostol: all primiparae, Outcome 3 Caesarean section.
11.3. Analysis.
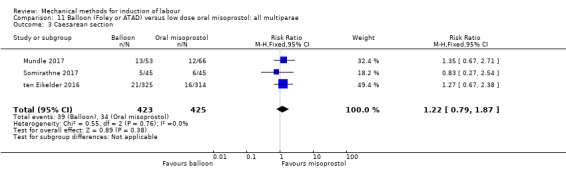
Comparison 11 Balloon (Foley or ATAD) versus low dose oral misoprostol: all multiparae, Outcome 3 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 1.11, 95% CI 0.60 to 2.06; 2627 women; 3 studies; low‐quality evidence; Analysis 9.4).
9.4. Analysis.
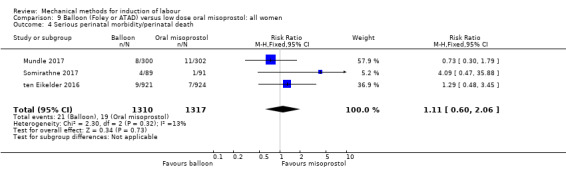
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 4 Serious perinatal morbidity/perinatal death.
The same results were seen on parity subgroup comparisons for primiparous women (RR 4.49, 95% CI 0.77 to 26.14; 1296 women; 2 studies; Analysis 10.4) and multiparous women (RR 0.98, 95% CI 0.14 to 6.86; 729 women; 2 studies; Analysis 11.4).
10.4. Analysis.
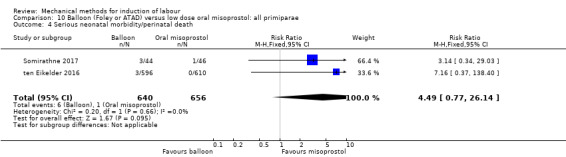
Comparison 10 Balloon (Foley or ATAD) versus low dose oral misoprostol: all primiparae, Outcome 4 Serious neonatal morbidity/perinatal death.
11.4. Analysis.
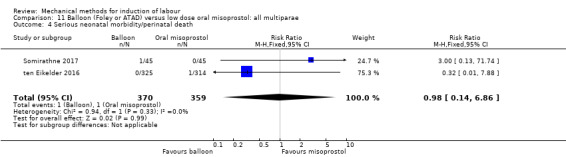
Comparison 11 Balloon (Foley or ATAD) versus low dose oral misoprostol: all multiparae, Outcome 4 Serious neonatal morbidity/perinatal death.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (RR 0.50, 95% CI 0.05 to 5.52; 2627 women; 3 studies; very low‐quality evidence; Analysis 9.5).
9.5. Analysis.
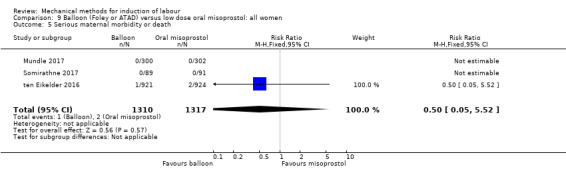
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 5 Serious maternal morbidity or death.
The same results were seen on parity subgroup comparisons for primiparous women (RR 0.51, 95% CI 0.05 to 5.63; 1296 women; 2 studies; Analysis 10.5) and multiparous women (Analysis 11.5). In the latter group, no events of maternal morbidity or death were reported (2 studies; 729 women).
10.5. Analysis.
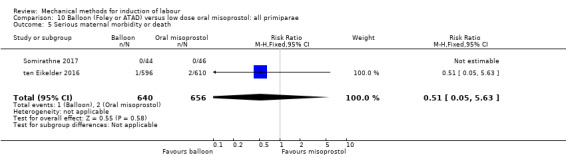
Comparison 10 Balloon (Foley or ATAD) versus low dose oral misoprostol: all primiparae, Outcome 5 Serious maternal morbidity or death.
11.5. Analysis.
Comparison 11 Balloon (Foley or ATAD) versus low dose oral misoprostol: all multiparae, Outcome 5 Serious maternal morbidity or death.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
It is uncertain whether there is a difference in an unfavourable cervix after 24 hours between both induction methods (average RR 0.98, 95% CI 0.61 to 1.56; 994 women; 4 studies; Analysis 9.6). Also, there was moderate heterogeneity for this outcome (Tau² = 0.06; Chi² = 2.96, df = 2 (P = 0.23); I² = 33%).
9.6. Analysis.
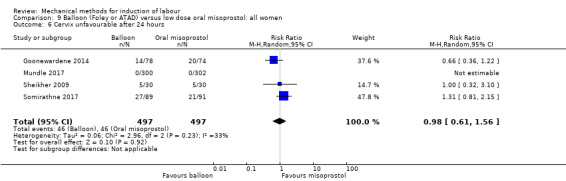
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 6 Cervix unfavourable after 24 hours.
A sensitivity analysis, after eliminating the two trials assessed as having a potentially higher risk of allocation or attrition bias (Goonewardene 2014; Sheikher 2009), did not change the result, although heterogeneity was lost (RR 1.31, 95% CI 0.81 to 2.15; 782 women; 2 studies; I² = 0%).
Oxytocin augmentation
A balloon catheter may increase the risk of oxytocin augmentation when compared to oral misoprostol (average RR 1.28, 95% CI 1.09 to 1.49; 2847 women; 5 studies; Analysis 9.7) although there was substantial heterogeneity for this outcome (Tau² = 0.03; Chi² = 31.32, df = 4 (P < 0.000001); I² = 87%).
9.7. Analysis.
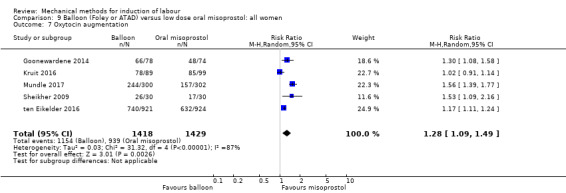
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 7 Oxytocin augmentation.
A sensitivity analysis, after eliminating the three trials assessed as having a potentially higher risk of allocation or attrition bias (Goonewardene 2014; Kruit 2016; Sheikher 2009), did not change this result, nor did it lower heterogeneity (average RR 1.35, 95% CI 1.02 to 1.79; 2447 women; 2 studies; I² = 95%).
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (average RR 0.50, 95% CI 0.12 to 2.07; 2838 women; 5 studies; Analysis 9.8). Also, there was substantial heterogeneity for this outcome (Tau² = 1.26; Chi² = 8.12, df = 4 (P = 0.09); I² = 51%).
9.8. Analysis.
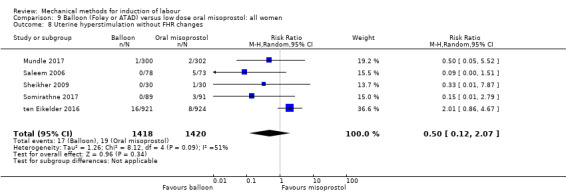
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Sheikher 2009), did not change the effect observed, nor did it lower heterogeneity (average RR 0.49, 95% CI 0.09 to 2.64; 2778 women; 4 studies; I² = 60%).
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 9.9). Of the seven studies included for this comparison, three studies (2627 women) pre‐specified this outcome. No events of uterine rupture occurred in any of these studies.
9.9. Analysis.
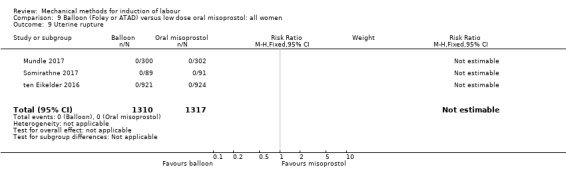
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 9 Uterine rupture.
Epidural analgesia
A balloon catheter may slightly increase the risk for epidural analgesia when compared to oral misoprostol (average RR 1.08, 95% CI 0.96 to 1.22; 2635 women; 3 studies; Analysis 9.10). However, the result is still too imprecise to make a valid judgement on this outcome. Also, there was substantial heterogeneity for this outcome (Chi² = 4.73, df = 2 (P = 0.09); I² = 58%).
9.10. Analysis.
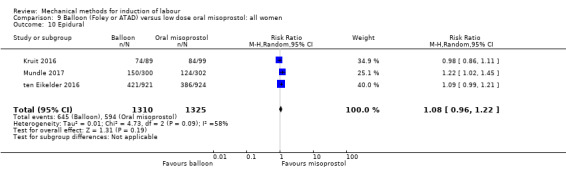
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 10 Epidural.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Kruit 2016), did not change this result, but did lower heterogeneity for this outcome (RR 1.13, 95% CI 1.03 to 1.24; 2447; 2 studies; I² = 5%).
Instrumental vaginal delivery
A balloon catheter probably reduces the risk of an instrumental vaginal delivery when compared to oral misoprostol (RR 0.71, 95% CI 0.55 to 0.92; 2627 women; 3 studies; Analysis 9.11).
9.11. Analysis.
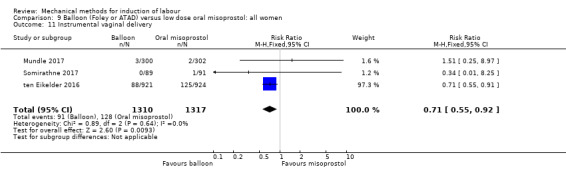
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 11 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (average RR 0.77, 95% CI 0.44 to 1.35; 2627 women; 3 studies; Analysis 9.12). Also, there was moderate heterogeneity for this outcome (Tau² = 0.11; Chi² = 3.09, df = 2 (P = 0.21); I² = 35%). No sensitivity analysis was conducted as no potential high‐risk studies were included for this outcome.
9.12. Analysis.
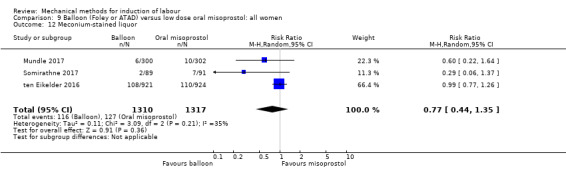
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 12 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 0.71, 95% CI 0.38 to 1.32; 2693 women; 4 studies; low‐quality evidence; Analysis 9.13).
9.13. Analysis.
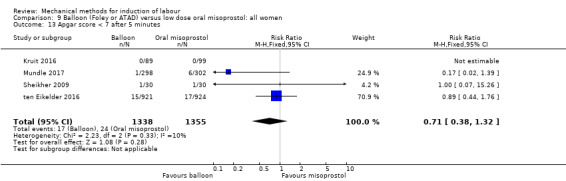
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 13 Apgar score < 7 after 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both induction methods (RR 0.82, 95% CI 0.58 to 1.17; 2873 women; 5 studies; low‐quality evidence; Analysis 9.14).
9.14. Analysis.
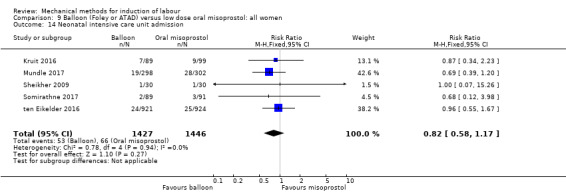
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 14 Neonatal intensive care unit admission.
Neonatal encephalopathy
It is uncertain whether there is a difference in neonatal encephalopathy between both induction methods (RR 0.81, 95% CI 0.32 to 2.03; 600 women; 1 study; Analysis 9.15).
9.15. Analysis.
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 15 Neonatal encephalopathy.
Perinatal death
It is uncertain whether there is difference in perinatal death between both induction methods (RR 1.28, 95% CI 0.49 to 3.30; 2627 women; 3 studies; Analysis 9.16) as the result was imprecise and events occurred infrequently (9/1310 versus 7/1317, respectively). In the balloon group, two cases of perinatal death were related to asphyxia, compared to one case in the misoprostol group.
9.16. Analysis.
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 16 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
It is uncertain whether there is a difference in maternal side effects between both induction methods (RR 0.61, 95% CI 0.33 to 1.13; 662 women; 2 studies; Analysis 9.17).
9.17. Analysis.
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 17 Maternal side effects (all).
Maternal nausea
Not reported.
Maternal vomiting
It is uncertain whether there is a difference in maternal vomiting between both induction methods (RR 0.73, 95% CI 0.37 to 1.46; 662 women; 2 studies; Analysis 9.18).
9.18. Analysis.
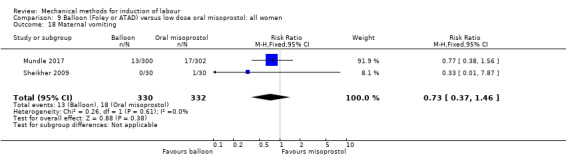
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 18 Maternal vomiting.
Maternal diarrhoea
It is uncertain whether there is a difference in maternal diarrhoea between both induction methods (RR 0.29, 95% CI 0.06 to 1.37; 602 women; 1 study; Analysis 9.19).
9.19. Analysis.
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 19 Maternal diarrhoea.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is difference in postpartum haemorrhage between both induction methods (RR 1.03, 95% CI 0.79 to 1.34; 2966 women; 5 studies; Analysis 9.20).
9.20. Analysis.
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 20 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
It is uncertain whether there is a difference in maternal death between both induction methods (Analysis 9.21). Of the 13 studies included for this comparison, three studies (2627 women) pre‐specified this outcome. No events of maternal death occurred in one of these studies.
9.21. Analysis.
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 21 Maternal death.
Woman not satisfied
A balloon catheter may increase the risk of women not being satisfied when compared to oral misoprostol (RR 1.70, 95% CI 1.15 to 2.50; 602 women; 1 study; Analysis 9.22), the absolute effect being 80 more women not satisfied per 1000 deliveries. In the one study included for this outcome, women were asked if they would choose the same induction method again in a future induction of labour.
9.22. Analysis.
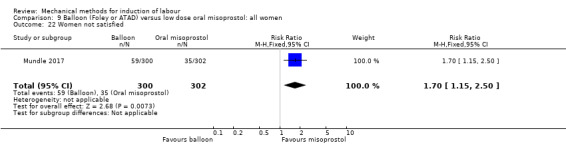
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 22 Women not satisfied.
Caregiver not satisfied
Not reported.
Not pre‐specified outcomes
Maternal fever during labour
There probably is little or no difference in maternal fever during labour between both induction methods (RR 0.98, 95% CI 0.78 to 1.24; 2033 women; 2 studies; Analysis 9.23).
9.23. Analysis.
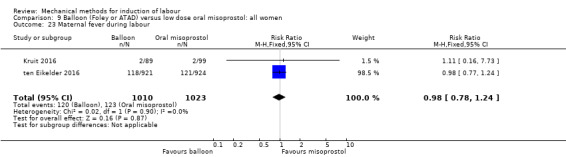
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 23 Maternal fever during labour.
Antibiotics during labour
It is uncertain whether there is a difference in antibiotics during labour between both induction methods (RR 1.22, 95% CI 0.75 to 2.00; 2033 women; 2 studies; Analysis 9.24).
9.24. Analysis.
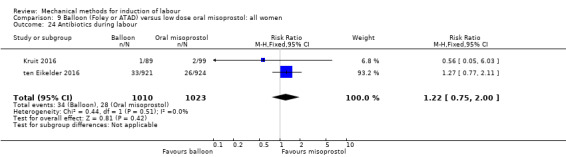
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 24 Antibiotics during labour.
Chorioamnionitis
Not reported.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 0.56, 95% CI 0.05 to 6.03; 188 women; 1 study; Analysis 9.25).
9.25. Analysis.
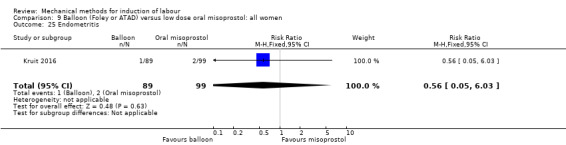
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 25 Endometritis.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 0.82, 95% CI 0.61 to 1.09; 2966 women; 5 studies; Analysis 9.26).
9.26. Analysis.
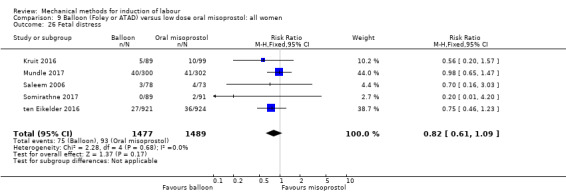
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 26 Fetal distress.
Umbilical artery pH < 7.10
It is uncertain whether there is a difference in umbilical artery pH less than 7.10 directly postpartum between both induction methods (RR 0.77, 95% CI 0.53 to 1.12; 1535 women; 2 studies; Analysis 9.27).
9.27. Analysis.
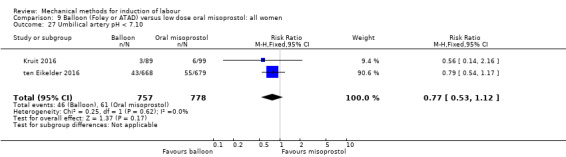
Comparison 9 Balloon (Foley or ATAD) versus low dose oral misoprostol: all women, Outcome 27 Umbilical artery pH < 7.10.
Balloon (single or double) versus oxytocin (eight trials involving 781 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
Not reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between induction of labour with a balloon and oxytocin (RR 0.20, 95% CI 0.01 to 4.11; 200 women; 1 study; Analysis 12.1).
12.1. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 1 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
A balloon catheter probably reduces the risk of a caesarean section when compared to oxytocin (RR 0.68, 95% CI 0.56 to 0.83; 781 women; 8 studies; Analysis 12.2), the absolute effect being 126 fewer caesarean sections per 1000 deliveries.
12.2. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 2 Caesarean section.
For women with a previous caesarean section, a balloon catheter may slightly reduce the risk of a caesarean section when compared to oxytocin (RR 0.80, 95% CI 0.64 to 1.00; 364 women; 3 studies; Analysis 13.1). However, the result is still too imprecise to make a valid judgement on this outcome.
13.1. Analysis.
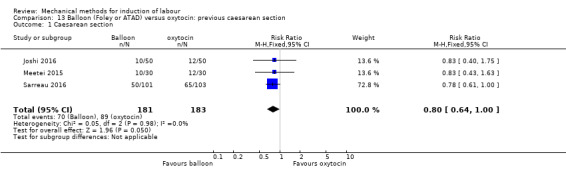
Comparison 13 Balloon (Foley or ATAD) versus oxytocin: previous caesarean section, Outcome 1 Caesarean section.
For primiparous women, it is uncertain whether there is a difference in effect as the result of this outcome was imprecise (RR 0.43, 95% CI 0.12 to 1.50; 60 women; 1 study; Analysis 14.1). For multiparous women, no outcomes were reported.
14.1. Analysis.
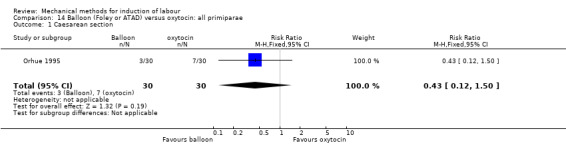
Comparison 14 Balloon (Foley or ATAD) versus oxytocin: all primiparae, Outcome 1 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (Analysis 12.3). Of the eight studies included for this comparison, one study (100 women) reported on this composite outcome. No events of neonatal morbidity or perinatal death occurred in this study.
12.3. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 3 Serious neonatal morbidity/perinatal death.
The same result was seen on a subgroup of women with a previous caesarean section. One study (100 women) reported on this outcome, in which no events of serious neonatal morbidity of perinatal death occurred (Analysis 13.2).
13.2. Analysis.
Comparison 13 Balloon (Foley or ATAD) versus oxytocin: previous caesarean section, Outcome 2 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 12.4). Of the eight studies included for this comparison, two studies (160 women) reported on this composite outcome. No events of serious maternal morbidity or death occurred in these studies.
12.4. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 4 Serious maternal morbidity or death.
The same result was seen on a subgroup of women with a previous caesarean section. One study (100 women) reported on this outcome, in which no events of serious maternal morbidity of death occurred (Analysis 13.3).
13.3. Analysis.
Comparison 13 Balloon (Foley or ATAD) versus oxytocin: previous caesarean section, Outcome 3 Serious maternal morbidity or death.
On parity subgroup comparisons, one study (60 women) reported on this outcome in primiparous women, in which no events were seen (Analysis 14.2). For multiparous women, no outcomes were reported.
14.2. Analysis.
Comparison 14 Balloon (Foley or ATAD) versus oxytocin: all primiparae, Outcome 2 Serious maternal morbidity or death.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
It is uncertain whether there is a difference in an unfavourable cervix after 24 hours between both induction methods (RR 0.56, 95% CI 0.20 to 1.54; 100 women; 1 study; Analysis 12.5).
12.5. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 5 Cervix unfavourable after 24 hours.
Oxytocin augmentation
Not a relevant outcome because all women in the comparison group received oxytocin.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (RR 1.00, 95% CI 0.23 to 4.29; 192 women; 3 studies; Analysis 12.6).
12.6. Analysis.
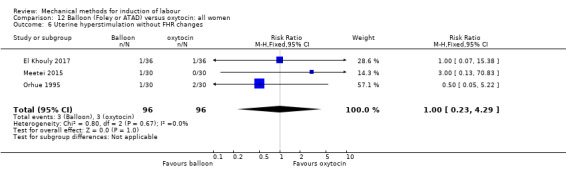
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 6 Uterine hyperstimulation without FHR changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 12.7). Of the eight studies included for this comparison, one study (100 women) pre‐specified this outcome. No events of uterine rupture occurred in this study.
12.7. Analysis.
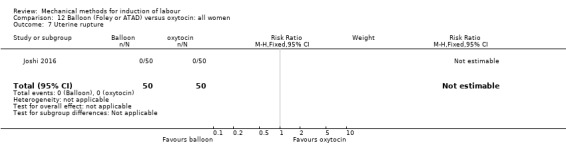
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 7 Uterine rupture.
Epidural analgesia
Not reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 1.19, 95% CI 0.55 to 2.57; 220 women; 3 studies; Analysis 12.8).
12.8. Analysis.
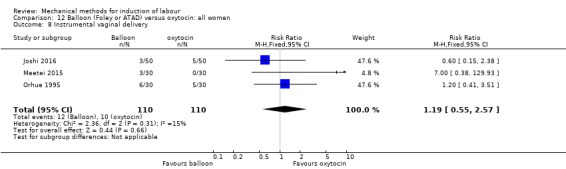
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 8 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.53, 95% CI 0.23 to 1.21; 272 women; 2 studies; Analysis 12.9).
12.9. Analysis.
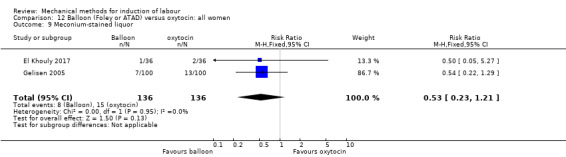
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 9 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 0.71, 95% CI 0.14 to 3.53; 300 women; 2 studies; Analysis 12.10).
12.10. Analysis.
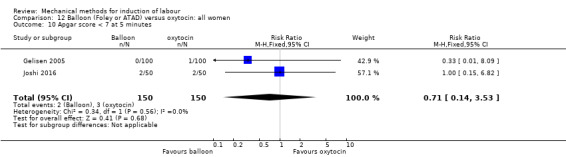
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 10 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is difference in NICU admissions between both induction methods (RR 0.80, 95% CI 0.32 to 1.98; 372 women; 3 studies; Analysis 12.11).
12.11. Analysis.
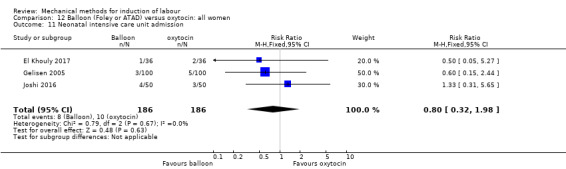
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 11 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both induction methods (Analysis 12.12). Of the eight studies included for this comparison, one study (100 women) pre‐specified this outcome. No cases of perinatal death occurred in this study.
12.12. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 12 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is difference in postpartum haemorrhage between both induction methods (RR 1.26, 95% CI 0.51 to 3.11; 396 women; 4 studies; Analysis 12.13).
12.13. Analysis.
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 13 Hemorrhagia postpartum.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
It is uncertain whether there is difference in maternal fever during labour between both induction methods (RR 0.20, 95% CI 0.01 to 4.00; 60 women; 1 study; Analysis 12.14).
12.14. Analysis.
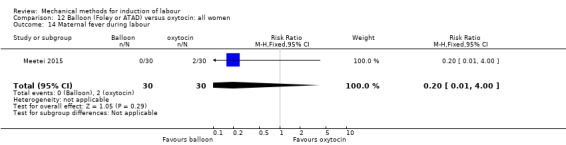
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 14 Maternal fever during labour.
Antibiotics during labour
Not reported.
Chorioamnionitis
Not reported.
Endometritis
Not reported.
Fetal distress
A balloon catheter probably reduces the risk of fetal distress for which a caesarean section is indicated when compared to oxytocin (RR 0.43, 95% CI 0.19 to 0.98; 332 women; 3 studies; Analysis 12.15).
12.15. Analysis.
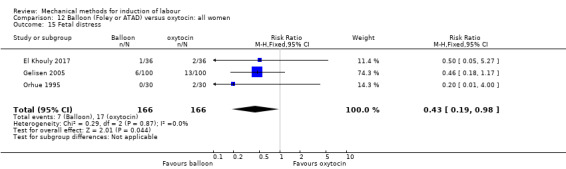
Comparison 12 Balloon (Foley or ATAD) versus oxytocin: all women, Outcome 15 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Balloon (single or double) versus amniotomy (one trial involving 20 women)
The only outcome of interest reported for this comparison was caesarean section. Other outcomes were not reported.
Caesarean section
It is uncertain whether there is difference in caesarean sections between induction of labour with a balloon and amniotomy (RR 0.25, 95% CI 0.03 to 1.86; 20 women; 1 study; Analysis 15.1).
15.1. Analysis.
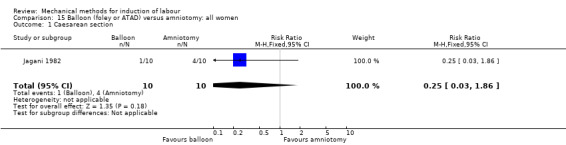
Comparison 15 Balloon (foley or ATAD) versus amniotomy: all women, Outcome 1 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Singe balloon (Foley) versus double balloon (ATAD/Cook) (five trials involving 826 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
There may be little or no difference in vaginal deliveries not achieved within 24 hours between induction of labour with a single balloon and a double balloon (average RR 0.97, 95% CI 0.75 to 1.25; 608 women; 3 studies; Analysis 16.1), although there was substantial heterogeneity for this outcome (Chi² = 5.64, df = 2 (P = 0.06); I² = 65%). No sensitivity analysis was performed as no high‐risk studies were included for this outcome.
16.1. Analysis.
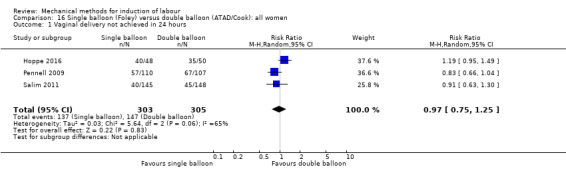
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
It is uncertain whether there is a difference in vaginal deliveries not achieved within 24 hours between both induction methods on subgroups for both primiparous women (RR 1.14, 95% CI 0.95 to 1.38; 50 women; 1 study; Analysis 17.1) and multiparous women (RR 1.24, 95% CI 0.80 to 1.93; 48 women; 1 study; Analysis 18.1) as the results for these outcomes were imprecise.
17.1. Analysis.
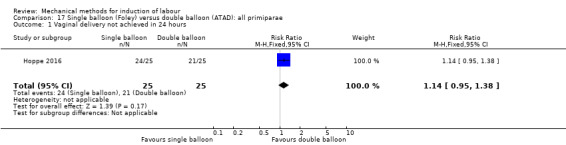
Comparison 17 Single balloon (Foley) versus double balloon (ATAD): all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
18.1. Analysis.
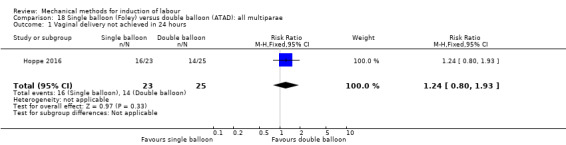
Comparison 18 Single balloon (Foley) versus double balloon (ATAD): all multiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes (Analysis 16.2), as events seem to occur infrequently after the use of both induction methods. Of the five studies included for this comparison, one study (217 women) reported on this outcome. No events of uterine hyperstimulation with FHR occurred in this study.
16.2. Analysis.
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (average RR 0.97, 95% CI 0.71 to 1.33; 862 women; 5 studies; Analysis 16.3). Also, there was moderate heterogeneity for this outcome (Chi² = 6.99, df = 4 (P = 0.14); I² = 43%).
16.3. Analysis.
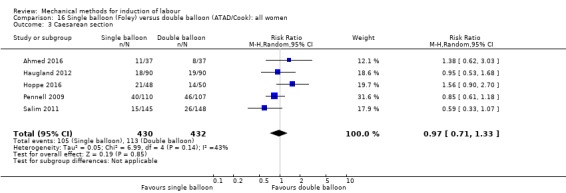
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 3 Caesarean section.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of concealment or attrition bias (Ahmed 2016), did not change the effect observed, nor did it lower heterogeneity (average RR 0.92, 95% CI 0.65 to 1.32; 788 women; 5 studies; I² = 50%).
The same result was seen on parity subgroup comparisons for primiparous women (average RR 1.30, 95% CI 0.76 to 2.22; 374 women; 4 studies; Analysis 17.2) and multiparous women (RR 0.74, 95% CI 0.30 to 1.84; 186 women; 2 studies; Analysis 18.2). Furthermore, for the primiparous group, there was also substantial heterogeneity (Tau² = 0.18; Chi² = 7.96, df = 3 (P = 0.05); I² = 62%).
17.2. Analysis.
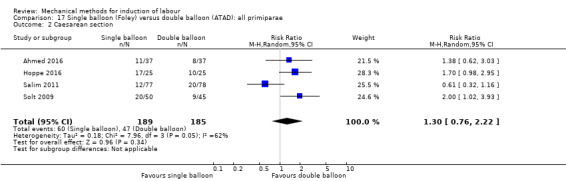
Comparison 17 Single balloon (Foley) versus double balloon (ATAD): all primiparae, Outcome 2 Caesarean section.
18.2. Analysis.
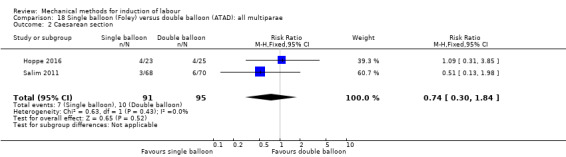
Comparison 18 Single balloon (Foley) versus double balloon (ATAD): all multiparae, Outcome 2 Caesarean section.
Serious neonatal morbidity or perinatal death
Not reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 16.4). Of the five studies included for this comparison, one study (217 women) reported on this composite outcome. No events of serious maternal morbidity or death occurred in this study.
16.4. Analysis.
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 4 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
There probably is little or no difference in oxytocin augmentation between both induction methods (RR 0.94, 95% CI 0.82 to 1.08; 278 women; 2 studies; Analysis 16.5).
16.5. Analysis.
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 5 Oxytcocin augmentation.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes (Analysis 16.6), although events seem to occur infrequently after the use of both induction methods. Of the five studies included for this comparison, one study (217 women) reported on this outcome. No events of uterine hyperstimulation without FHR occurred in this study.
16.6. Analysis.
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 6 Uterine hyperstimulation without FHR changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 16.7). Of the five studies included for this comparison, one study (217 women) reported on this outcome. No events of uterine rupture occurred in this study.
16.7. Analysis.
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 7 Uterine rupture.
Epidural analgesia
There probably is little or no difference in epidural analgesia between both induction methods (RR 0.93, 95% CI 0.83 to 1.03; 608 women; 3 studies; Analysis 16.8).
16.8. Analysis.
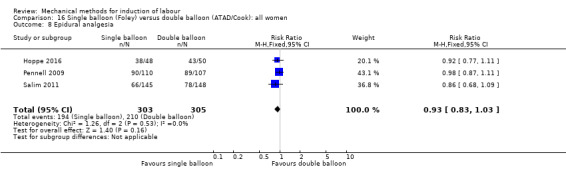
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 8 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.86, 95% CI 0.61 to 1.20; 690 women; 3 studies; Analysis 16.9).
16.9. Analysis.
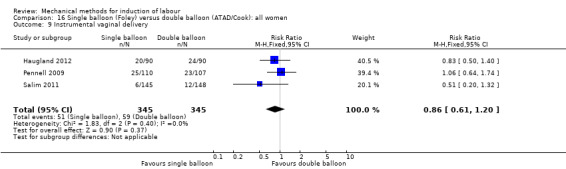
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 9 Instrumental vaginal delivery.
Meconium‐stained liquor
A single balloon may reduce the risk of meconium‐stained liquor when compared to a double balloon (RR 0.40, 95% CI 0.15 to 1.04; 98 women; 1 study; Analysis 16.10). However, the result is still too imprecise to make a valid judgement on this outcome.
16.10. Analysis.
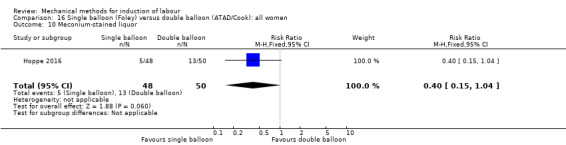
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 10 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 0.84, 95% CI 0.25 to 2.79; 608 women; 3 studies; Analysis 16.11).
16.11. Analysis.
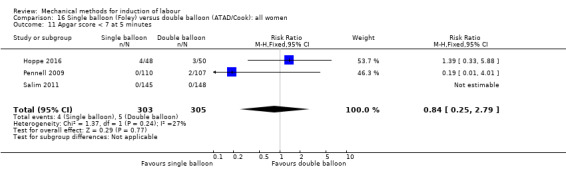
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 11 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both induction methods (RR 1.67, 95% CI 0.71 to 3.93; 391 women; 2 studies; Analysis 16.12).
16.12. Analysis.
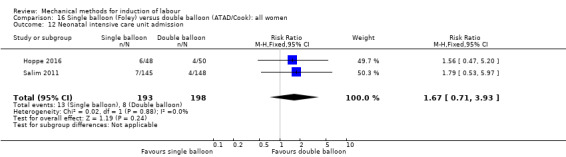
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 12 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
Not reported.
Disability in childhood
Not reported.
Maternal side effects (all)
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects: pain after insertion
It is uncertain whether there is a difference in pain after insertion of the catheter between both induction methods (RR 0.67, 95% CI 0.20 to 2.17; 74 women; 1 study; Analysis 16.13).
16.13. Analysis.
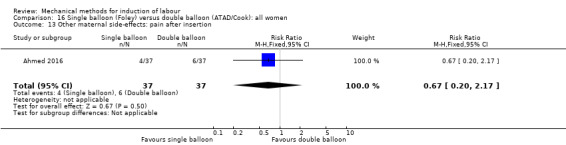
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 13 Other maternal side‐effects: pain after insertion.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 0.83, 95% CI 0.27 to 2.52; 291 women; 2 studies; Analysis 16.14).
16.14. Analysis.
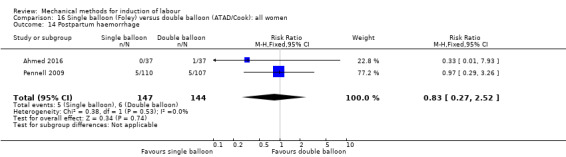
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 14 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
It is uncertain whether there is a difference in maternal fever during labour between both induction methods (average RR 0.61, 95% CI 0.16 to 2.34; 584 women; 3 studies; Analysis 16.15). Also, there was substantial heterogeneity for this outcome (Chi² = 2.85, df = 1 (P = 0.09); I² = 65%).
16.15. Analysis.
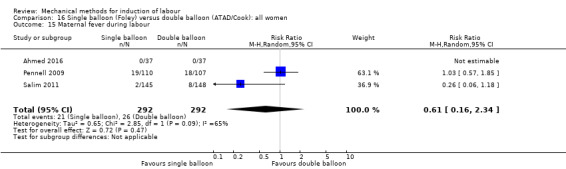
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 15 Maternal fever during labour.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Ahmed 2016), did not alter the result, nor did it lower heterogeneity (average RR 0.61, 95% CI 0.16 to 2.34; 510 women; 2 studies; I² = 65%).
Antibiotics during labour
It is uncertain whether there is a difference in antibiotics during labour between both induction methods (RR 0.97, 95% CI 0.61 to 1.56; 217 women; 1 study; Analysis 16.16).
16.16. Analysis.
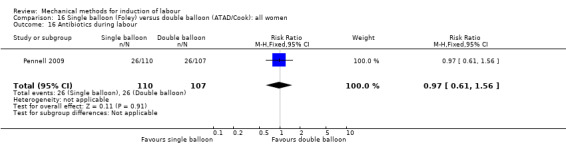
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 16 Antibiotics during labour.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 1.56, 95% CI 0.47 to 5.20; 98 women;1 study; Analysis 16.17).
16.17. Analysis.
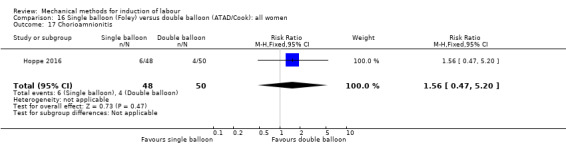
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 17 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 1.95, 95% CI 0.18 to 21.14; 217 women; 1 study; Analysis 16.18).
16.18. Analysis.
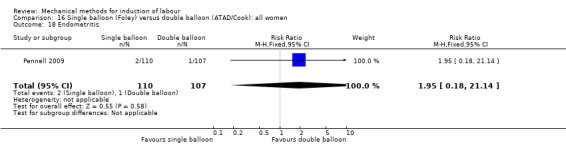
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 18 Endometritis.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated (RR 0.98, 95% CI 0.70 to 1.36; 682 women; 4 studies; Analysis 16.19).
16.19. Analysis.
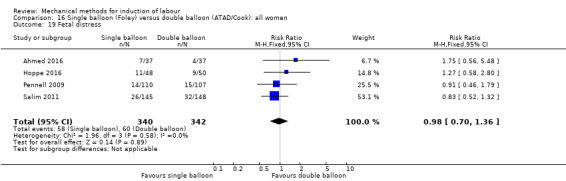
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 19 Fetal distress.
Umbilical artery pH < 7.10
It is uncertain whether there is a difference in umbilical artery pH less than 7.10 directly postpartum between both induction methods (RR 0.42, 95% CI 0.11 to 1.57; 217 women; 1 study; Analysis 16.20).
16.20. Analysis.
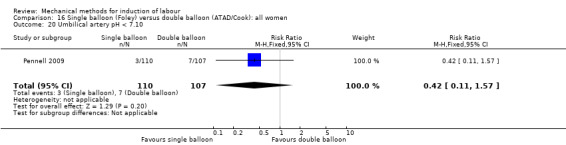
Comparison 16 Single balloon (Foley) versus double balloon (ATAD/Cook): all women, Outcome 20 Umbilical artery pH < 7.10.
Laminaria tent versus vaginal prostaglandin E2 (five trials involving 263 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
Not reported.
Uterine hyperstimulation with FHR changes
A laminaria tent probably reduces the risk of uterine hyperstimulation with FHR changes when compared to vaginal prostaglandin E2 (RR 0.11, 95% CI 0.02 to 0.60; 188 women; 3 studies; Analysis 19.1), the absolute effect being 118 fewer per 1000 deliveries.
19.1. Analysis.
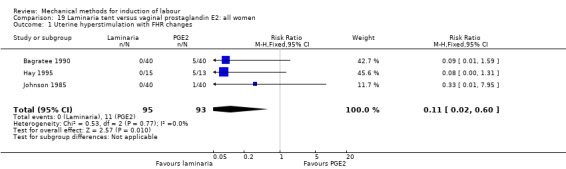
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 1 Uterine hyperstimulation with FHR changes.
For primiparous women, it is uncertain whether there is a difference in effect as the result of this outcome was imprecise (RR 0.33, 95% CI 0.01 to 7.95; 80 women; 1 study; Analysis 20.1). For multiparous women, this outcome was not reported.
20.1. Analysis.
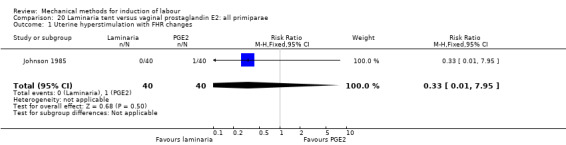
Comparison 20 Laminaria tent versus vaginal prostaglandin E2: all primiparae, Outcome 1 Uterine hyperstimulation with FHR changes.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (RR 0.91, 95% CI 0.56 to 1.48; 263 women; 5 studies; Analysis 19.2).
19.2. Analysis.
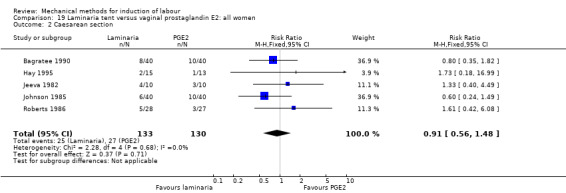
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 2 Caesarean section.
The same result was seen on parity subgroup comparisons for primiparous women (average RR 1.07, 95% CI 0.24 to 4.89; 90 women; 2 studies; Analysis 20.2) and multiparous women (RR 0.50, 95% CI 0.06 to 3.91; 10 women; 1 study; Analysis 21.1). Furthermore, for the primiparous group, there was also substantial heterogeneity (Chi² = 2.25, df = 1 (P = 0.13); I² = 56%).
20.2. Analysis.
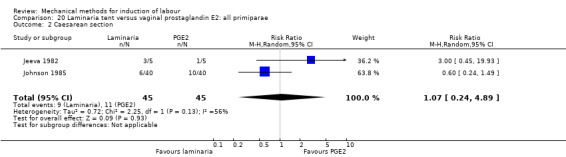
Comparison 20 Laminaria tent versus vaginal prostaglandin E2: all primiparae, Outcome 2 Caesarean section.
21.1. Analysis.
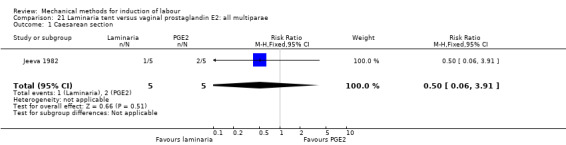
Comparison 21 Laminaria tent versus vaginal prostaglandin E2: all multiparae, Outcome 1 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (Analysis 19.3). Of the five studies included for this comparison, one study (80 women) reported on this composite outcome. No events of neonatal morbidity or perinatal death occurred in this study.
19.3. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 3 Serious perinatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 19.4). Of the five studies included for this comparison, one study (28 women) reported on this composite outcome. No events of serious maternal morbidity or death occurred in this study.
19.4. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 4 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
Not reported.
Uterine hyperstimulation without FHR changes
A laminaria tent may reduce the risk of uterine hyperstimulation without FHR changes when compared to vaginal PGE2 (RR 0.22, 95% CI 0.09 to 0.49; 180 women; 3 studies; Analysis 19.5).
19.5. Analysis.
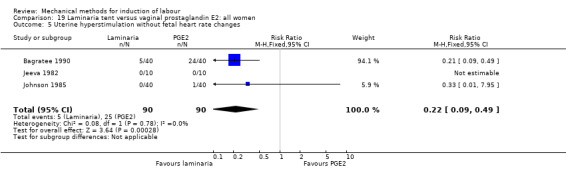
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 5 Uterine hyperstimulation without fetal heart rate changes.
Uterine rupture
Not reported.
Epidural analgesia
It is uncertain whether there is a difference in epidural analgesia between both induction methods (RR 0.91, 95% CI 0.74 to 1.13; 80 women; 1 study; Analysis 19.6).
19.6. Analysis.
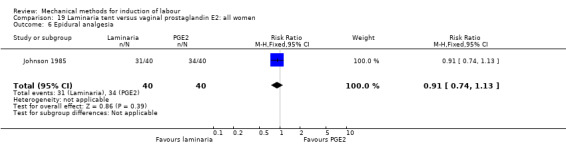
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 6 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.71, 95% CI 0.43 to 1.17; 80 women; 1 study; Analysis 19.7).
19.7. Analysis.
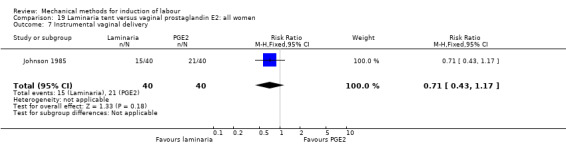
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 7 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.14, 95% CI 0.01 to 2.68; 80 women; 1 study; Analysis 19.8).
19.8. Analysis.
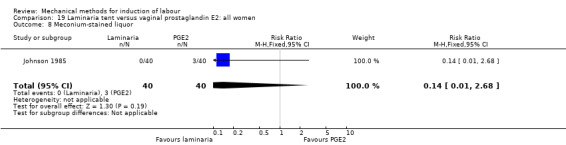
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 8 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (Analysis 19.9). Of the five studies included for this comparison, two studies (160 women) reported on this outcome. No events of Apgar scores less than seven at five minutes occurred in these studies.
19.9. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 9 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
Not reported.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both induction methods (Analysis 19.10). Of the five studies included for this comparison, one study (80 women) reported on this outcome. No events of perinatal deaths occurred in this study.
19.10. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 10 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
It is uncertain whether there is a difference in maternal side effects between both induction methods (RR 0.29, 95% CI 0.01 to 6.60; 28 women; 1 study; Analysis 19.11).
19.11. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 11 Maternal side effects: all.
Maternal nausea
It is uncertain whether there is a difference in maternal nausea between both induction methods (RR 0.29, 95% CI 0.01 to 6.60; 28 women; 1 study; Analysis 19.12).
19.12. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 12 Maternal nausea.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
Not reported.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
Not reported.
Endometritis
Not reported.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 0.62, 95% CI 0.34 to 1.15; 188 women; 3 studies; Analysis 19.13).
19.13. Analysis.
Comparison 19 Laminaria tent versus vaginal prostaglandin E2: all women, Outcome 13 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Laminaria tent versus cervical prostaglandin E2 (five trials involving 920 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
Not reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between induction of labour with a laminaria tent and cervical PGE2 (RR 0.17, 95% CI 0.02 to 1.42; 350 women; 2 studies; Analysis 22.1).
22.1. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 1 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (RR 1.16, 95% CI 0.93 to 1.45; 920 women; 5 studies; Analysis 22.2).
22.2. Analysis.
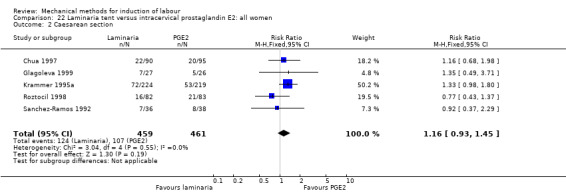
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 2 Caesarean section.
The same results were seen on parity subgroup comparisons for primiparous women (RR 1.15, 95% CI 0.62 to 2.13; 116 women; 1 study; Analysis 23.1) and multiparous women (RR 1.28, 95% CI 0.45 to 3.65; 69 women; 1 study; Analysis 24.1).
23.1. Analysis.
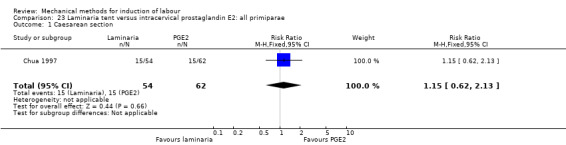
Comparison 23 Laminaria tent versus intracervical prostaglandin E2: all primiparae, Outcome 1 Caesarean section.
24.1. Analysis.
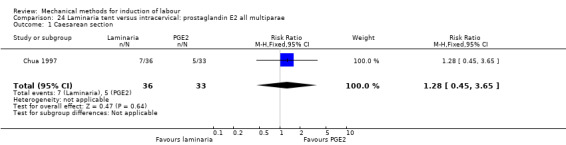
Comparison 24 Laminaria tent versus intracervical: prostaglandin E2 all multiparae, Outcome 1 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 3.16, 95% CI 0.13 to 76.70; 185 women; 1 study; Analysis 22.3). One event, a case of perinatal death, was reported in the laminaria group. No events occurred in the cervical PGE2 group.
22.3. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 3 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (RR 0.35, 95% CI 0.01 to 8.52; 185 women; 1 study; Analysis 22.4). No events occurred in the laminaria group. One event, a uterine rupture, was reported in the cervical PGE2 group.
22.4. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 4 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
It is uncertain whether there is a difference in an unfavourable cervix after 24 hours between both induction methods (average RR 0.46, 95% CI 0.11 to 1.96; 218 women; 2 studies; Analysis 22.5). Also, there was substantial heterogeneity for this outcome (Tau² = 0.62; Chi² = 1.98, df = 1 (P = 0.16); I² = 50%).
22.5. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 5 Cervix unfavourable/unchanged after 12‐24 hours.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Roztocil 1998), did not alter the result (RR 0.16, 95% CI 0.02 to 1.24; 53 women; 1 study; I² = 0%).
Oxytocin augmentation
A laminaria tent probably increases the risk of oxytocin augmentation when compared to cervical PGE2 (RR 1.41, 95% CI 1.21 to 1.64; 185 women; 1 study; Analysis 22.6).
22.6. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 6 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (RR 0.17, 95% CI 0.02 to 1.36; 601 women; 2 studies; Analysis 22.7).
22.7. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 7 Uterine hyperstimulation without FHR changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (RR 0.35, 95% CI 0.01 to 8.52; 185 women; 1 study; Analysis 22.8). One study reported on this outcome in which one uterine rupture occurred in the PGE2 group. No uterine ruptures were seen in the laminaria group.
22.8. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 8 Uterine rupture.
Epidural analgesia
Not reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 1.05, 95% CI 0.65 to 1.69; 424 women; 3 studies; Analysis 22.9).
22.9. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 9 Instrumental vaginal delivery.
Meconium‐stained liquor
Not reported.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 5.28, 95% CI 0.63 to 44.30; 185 women; 1 study; Analysis 22.10).
22.10. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 10 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both induction methods (RR 1.58, 95% CI 0.58 to 4.33; 259 women; 2 studies; Analysis 22.11).
22.11. Analysis.
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 11 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both induction methods (RR 3.16, 95% CI 0.13 to 76.70; 185 women; 1 study; Analysis 22.12). One study reported on this outcome, in which one perinatal death occurred in the laminaria group. No perinatal death were seen in the cervical PGE2 group.
22.12. Analysis.
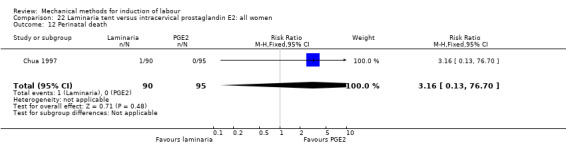
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 12 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
It is uncertain whether there is a difference in maternal side effects between both induction methods (RR 0.20, 95% CI 0.01 to 4.15; 165 women; 1 study; Analysis 22.13). The one study included for this outcome reported on gastro‐intestinal symptoms without specifying what the symptoms were.
22.13. Analysis.
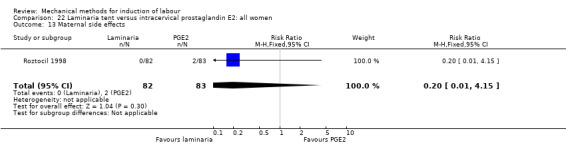
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 13 Maternal side effects.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 1.14, 95% CI 0.46 to 2.81; 239 women; 2 studies; Analysis 22.14).
22.14. Analysis.
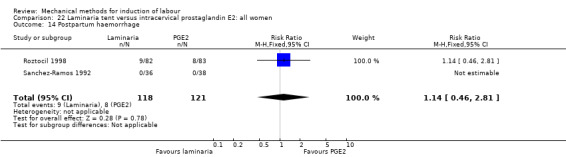
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 14 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 3.17, 95% CI 0.35 to 29.06; 74 women; 1 study; Analysis 22.15).
22.15. Analysis.
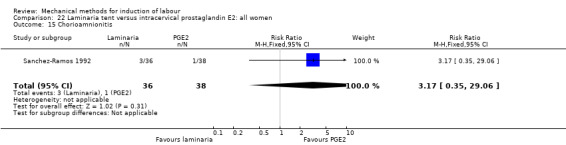
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 15 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (average RR 0.30, 95% CI 0.08 to 1.09; 490 women; 2 studies; Analysis 22.16). Also, there was substantial heterogeneity for this outcome (Tau² = 0.54; Chi² = 2.54, df = 1 (P = 0.11); I² = 61%).
22.16. Analysis.
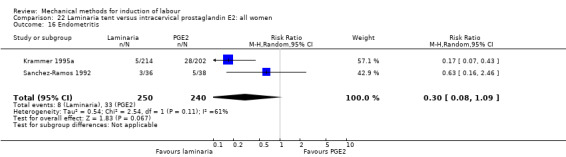
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 16 Endometritis.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Krammer 1995a), did not alter the result (RR 0.63, 95% CI 0.16 to 2.46; 74 women; 1 study; I² = 0%).
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 0.44, 95% CI 0.07 to 2.90; 128 women; 2 studies; Analysis 22.17).
22.17. Analysis.
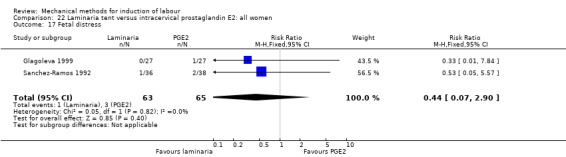
Comparison 22 Laminaria tent versus intracervical prostaglandin E2: all women, Outcome 17 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Laminaria tent versus oxytocin (two trials involving 73 women)
The only outcomes of interest reported for this comparison were caesarean section and fetal distress. Other outcomes were not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between induction of labour with a laminaria tent and oxytocin (RR 0.83, 95% CI 0.36 to 1.89; 73 women; 2 studies; Analysis 25.1).
25.1. Analysis.
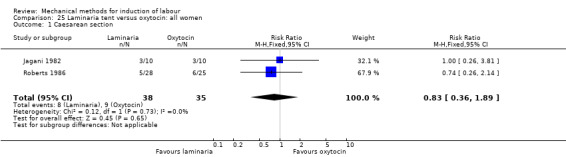
Comparison 25 Laminaria tent versus oxytocin: all women, Outcome 1 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 2.69, 95% CI 0.11 to 63.18; 53 women; 1 study; Analysis 25.2).
25.2. Analysis.
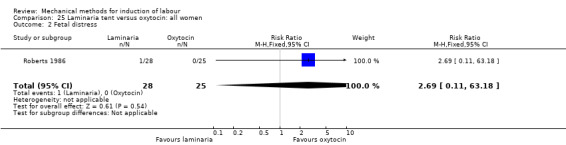
Comparison 25 Laminaria tent versus oxytocin: all women, Outcome 2 Fetal distress.
Laminaria tent versus amniotomy (one trial involving 20 women)
The only outcome of interest reported for this comparison was caesarean section. Other outcomes were not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between induction of labour with a laminaria tent compared to amniotomy (RR 0.75, 95% CI 0.22 to 2.52; 20 women; 1 study; Analysis 26.1).
26.1. Analysis.
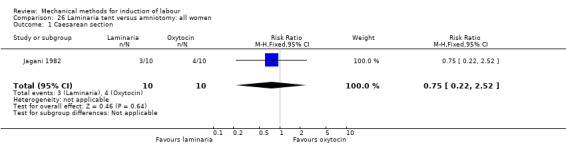
Comparison 26 Laminaria tent versus amniotomy: all women, Outcome 1 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Laminaria tent versus other hygroscopic dilators (one trial involving 41 women)
The only outcome of interest reported for this comparison was caesarean section. Other outcomes were not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between induction of labour with a laminaria tent and other hygroscopic dilators (RR 1.70, 95% CI 0.44 to 6.66; 41 women; 1 study; Analysis 27.1).
27.1. Analysis.
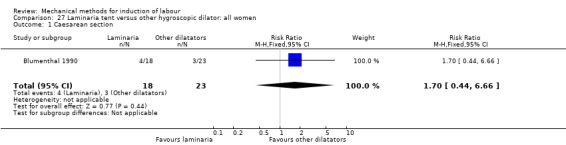
Comparison 27 Laminaria tent versus other hygroscopic dilator: all women, Outcome 1 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Extra amniotic saline infusion (EASI) versus vaginal prostaglandin E2 (two trials involving 221 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
EASI probably increases the risk of a vaginal delivery not achieved within 24 hours when compared to vaginal PGE2 (RR 1.74, 95% CI 1.21 to 2.49; 109 women; 1 study; Analysis 28.1).
28.1. Analysis.
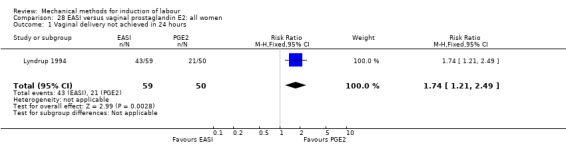
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (RR 0.23, 95% CI 0.03 to 2.07; 221 women; 2 studies; Analysis 28.2).
28.2. Analysis.
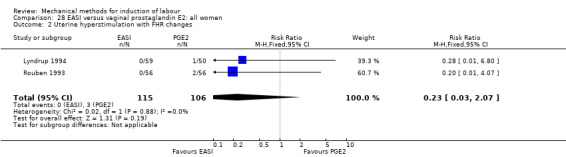
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (average RR 1.35, 95% CI 0.94 to 1.96; 221 women; 2 studies; Analysis 28.3). Also, there was substantial heterogeneity for this outcome (Chi² = 5.24, df = 1 (P = 0.02); I² = 81%). No sensitivity analysis could be done as both included studies were assessed as having a potentially higher risk of allocation or attrition bias.
28.3. Analysis.
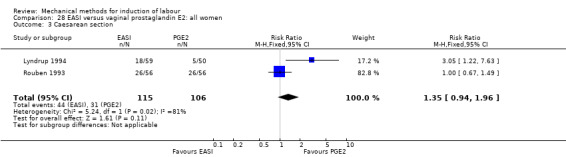
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 3 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious neonatal morbidity or perinatal death
Not reported.
Serious maternal morbidity or death
Not reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
EASI may increase the risk of oxytocin augmentation when compared to vaginal PGE2 (RR 12.71, 95% CI 3.20 to 50.57; 109 women; 1 study; Analysis 28.4).
28.4. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 4 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (RR 0.23, 95% CI 0.03 to 2.07; 221 women; 2 studies; Analysis 28.5).
28.5. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 5 Uterine hyperstimulation without fetal heart rate changes.
Uterine rupture
Not reported.
Epidural analgesia
There may be little or no difference in epidural analgesia between both induction methods (RR 1.00, 95% CI 0.97 to 1.04; 112 women; 1 study; Analysis 28.6).
28.6. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 6 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.58, 95% CI 0.30 to 1.14; 109 women; 1 study; Analysis 28.7).
28.7. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 7 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 3.00, 95% CI 0.12 to 72.10; 112 women; 1 study; Analysis 28.8).
28.8. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 8 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 4.25, 95% CI 0.21 to 86.51; 109 women; 1 study; Analysis 28.9).
28.9. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 9 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both induction methods (RR 1.50, 95% CI 0.45 to 5.03; 112 women; 1 study; Analysis 28.10).
28.10. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 10 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
Not reported.
Disability in childhood
Not reported.
Maternal side effects (all)
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
Not reported.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
It is uncertain whether there is a difference in women not being satisfied between both induction methods (RR 0.56, 95% CI 0.10 to 3.25; 109 women; 1 study; Analysis 28.11). For this outcome, women in the included study were asked to comment on the induction method, for which they could choose between recommendable, satisfactory and unsatisfactory.
28.11. Analysis.
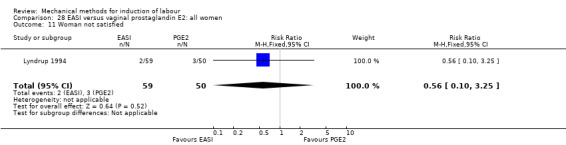
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 11 Woman not satisfied.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
Not reported.
Endometritis
Not reported.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 1.20, 95% CI 0.39 to 3.71; 112 women; 1 study; Analysis 28.12).
28.12. Analysis.
Comparison 28 EASI versus vaginal prostaglandin E2: all women, Outcome 12 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Extra amniotic saline infusion versus cervical prostaglandin E2 (two trials involving 155 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
Not reported.
Uterine hyperstimulation with FHR changes
Not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (average RR 0.73, 95% CI 0.10 to 5.12; 155 women; 2 studies; Analysis 29.1). Also, there was substantial heterogeneity for this outcome (Tau² = 1.60; Chi² = 5.11, df = 1 (P = 0.02); I² = 80%). As the results for both included studies show no overlap of CI, this makes the pooled result for this outcome less meaningful. No sensitivity analysis was performed as no potential high‐risk studies were included for this outcome.
29.1. Analysis.
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 1 Caesarean section.
The same result was seen on a subgroup comparison for primiparous women (RR 0.25, 95% CI 0.06 to 1.09; 70 women; 1 study; Analysis 30.1). For multiparous women, no outcomes were reported.
30.1. Analysis.
Comparison 30 EASI versus intracervical prostaglandin E2: all primiparae, Outcome 1 Caesarean section.
Serious neonatal morbidity or perinatal death
Not reported.
Serious maternal morbidity or death
Not reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
EASI may reduce the risk of an unfavourable cervix after 24 hours when compared to cervical PGE2 (RR 0.06, 95% CI 0.00 to 0.97; 85 women; 1 study; Analysis 29.2).
29.2. Analysis.
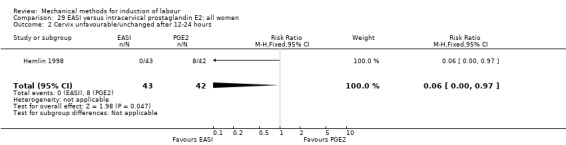
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 2 Cervix unfavourable/unchanged after 12‐24 hours.
Oxytocin augmentation
It is uncertain whether there is a difference in oxytocin augmentation between both induction methods (RR 1.10, 95% CI 0.54 to 2.25; 70 women; 1 study; Analysis 29.3).
29.3. Analysis.
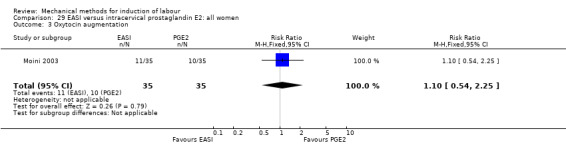
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 3 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
Not reported.
Uterine rupture
Not reported.
Epidural analgesia
Not reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.33, 95% CI 0.04 to 3.01; 85 women; 1 study; Analysis 29.4).
29.4. Analysis.
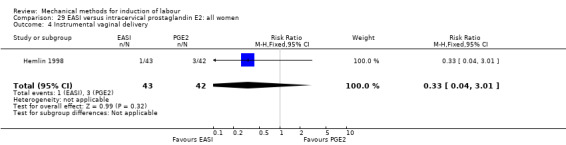
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 4 Instrumental vaginal delivery.
Meconium‐stained liquor
Not reported.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (Analysis 29.5). One study (85 women) pre‐specified this outcome in which no Apgar scores less than seven after five minutes were reported.
29.5. Analysis.
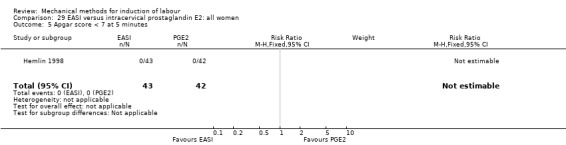
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 5 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
Not reported.
Neonatal encephalopathy
Not reported.
Perinatal death
Not reported.
Disability in childhood
Not reported.
Maternal side effects (all)
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
Not reported.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
Not reported.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (Analysis 29.6). One study (85 women) pre‐specified this outcome in which no cases of endometritis were reported.
29.6. Analysis.
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 6 Endometritis.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 0.29, 95% CI 0.06 to 1.28; 70 women; 1 study; Analysis 29.7).
29.7. Analysis.
Comparison 29 EASI versus intracervical prostaglandin E2: all women, Outcome 7 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone (eight trials involving 639 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
It is uncertain whether there is a difference in vaginal deliveries not achieved within 24 hours between induction of labour with a mechanical method combined with PGE2 and PGE2 alone (RR 0.84, 95% CI 0.53 to 1.33; 39 women; 1 study; Analysis 31.1).
31.1. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (RR 0.26, 95% CI 0.01 to 5.12; 122 women; 2 studies; Analysis 31.2).
31.2. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (average RR 0.96, 95% CI 0.66 to 1.40; 517 women; 7 studies; Analysis 31.3). Also, there was moderate heterogeneity for this outcome (Tau² = 0.11; Chi² = 11.16, df = 6 (P = 0.08); I² = 46%).
31.3. Analysis.
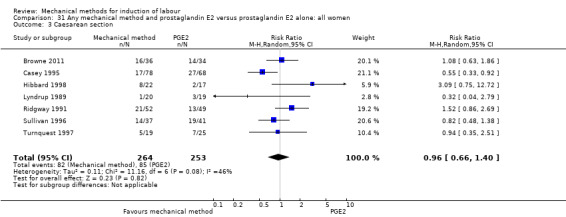
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 3 Caesarean section.
A sensitivity analysis, after eliminating three trials assessed as having a potentially higher risk of allocation or attrition bias (Browne 2011; Lyndrup 1989; Turnquest 1997), did not alter the result nor did it lower heterogeneity (average RR 1.02, 95% CI 0.56 to 1.84; 364 women; 4 studies; I² = 70%).
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious neonatal morbidity or perinatal death
Not reported.
Serious maternal morbidity or death
Not reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
A mechanical method combined with PGE2 may reduce the risk of an unfavourable cervix after 24 hours when compared to PGE2 alone (RR 0.52, 95% CI 0.31 to 0.85; 122 women; 1 study; Analysis 31.4).
31.4. Analysis.
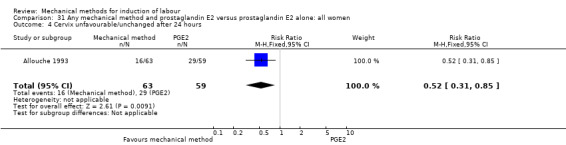
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 4 Cervix unfavourable/unchanged after 24 hours.
Oxytocin augmentation
It is uncertain whether there is a difference in oxytocin augmentation between both induction methods (RR 0.95, 95% CI 0.64 to 1.41; 44 women; 1 study; Analysis 31.5).
31.5. Analysis.
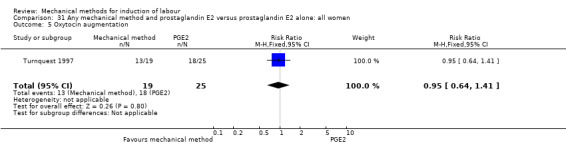
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 5 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (Analysis 31.6). Of the eight studies included for this comparison, three studies (239 women) pre‐specified this outcome. No events of uterine hyperstimulation without FHR changes occurred in these studies.
31.6. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 6 Uterine hyperstimulation without FHR changes.
Uterine rupture
Not reported.
Epidural analgesia
There may be little or no difference in epidural analgesia during labour between both induction methods (RR 0.98, 95% CI 0.77 to 1.24; 39 women; 1 study; Analysis 31.7).
31.7. Analysis.
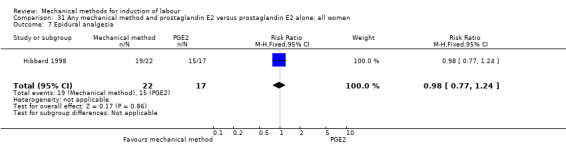
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 7 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.56, 95% CI 0.22 to 1.45; 78 women; 2 studies; Analysis 31.8).
31.8. Analysis.
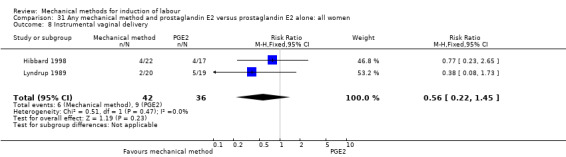
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 8 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.97, 95% CI 0.33 to 2.83; 120 women; 1 study; Analysis 31.9).
31.9. Analysis.
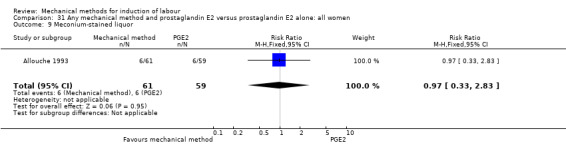
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 9 Meconium‐stained liquor.
Apgar score less than seven at five minutes
Not reported.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both methods (RR 0.26, 95% CI 0.01 to 5.12; 44 women; 1 study; Analysis 31.10).
31.10. Analysis.
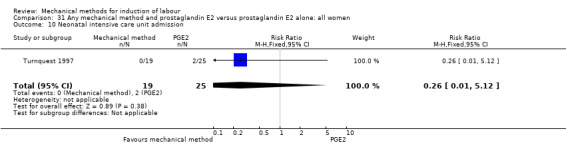
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 10 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
Not reported.
Disability in childhood
Not reported.
Maternal side effects
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (Analysis 31.11). Of the eight studies included for this comparison, one study (39 women) pre‐specified this outcome. No events of postpartum haemorrhage occurred in this study.
31.11. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 11 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 1.56, 95% CI 0.45 to 5.45; 122 women; 2 studies; Analysis 31.12).
31.12. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 12 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 1.07, 95% CI 0.41 to 2.78; 237 women; 3 studies; Analysis 31.13).
31.13. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 13 Endometritis.
Fetal distress
It is uncertain whether there is a difference fetal distress for which a caesarean section is indicated between both induction methods (RR 2.28, 95% CI 0.54 to 9.69; 140 women; 2 studies; Analysis 31.14).
31.14. Analysis.
Comparison 31 Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women, Outcome 14 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and prostaglandin E2 versus low‐dose misoprostol alone (one trial involving 127 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
A mechanical method combined with PGE2 probably reduces the risk of a vaginal delivery not achieved within 24 hours when compared to misoprostol (RR 0.32, 95% CI 0.12 to 0.82; 127 women; 1 study; Analysis 32.1). the absolute effect being 165 less per 1000 deliveries.
32.1. Analysis.
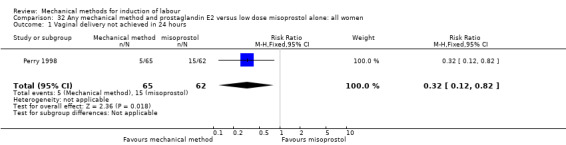
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
Not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (RR 1.09, 95% CI 0.58 to 2.04; 127 women; 1 study; Analysis 32.2).
32.2. Analysis.
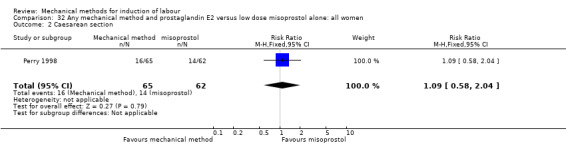
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 2 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 0.19, 95% CI 0.01 to 3.90; 127 women; 1 study; Analysis 32.3). Two events occurred in the misoprostol group, both being cases of perinatal death.
32.3. Analysis.
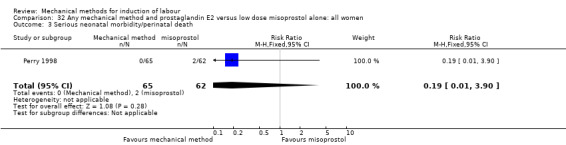
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 3 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
Not reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
A mechanical method combined with PGE2 probably reduces the risk of an unfavourable cervix after 24 hours when compared to misoprostol (RR 0.41, 95% CI 0.25 to 0.67; 127 women; 1 study; Analysis 32.4).
32.4. Analysis.
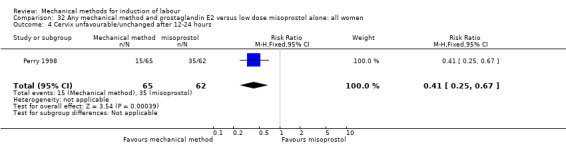
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
Oxytocin augmentation
A mechanical method combined with PGE2 probably slightly increases the risk of oxytocin augmentation when compared to misoprostol (RR 1.21, 95% CI 1.01 to 1.46; 127; 1 study; Analysis 32.5).
32.5. Analysis.
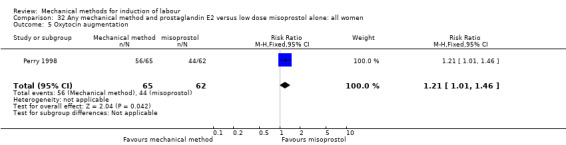
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 5 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
A mechanical method combined with PGE2 probably increases the risk of uterine hyperstimulation without FHR changes when compared to misoprostol (RR 4.05, 95% CI 1.44 to 11.38; 127; 1 study; Analysis 32.6).
32.6. Analysis.
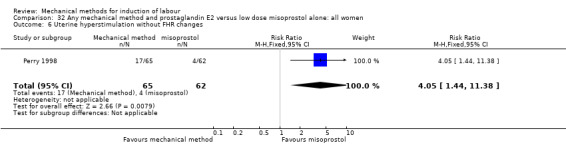
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 6 Uterine hyperstimulation without FHR changes.
Uterine rupture
Not reported.
Epidural analgesia
Not reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 1.26, 95% CI 0.77 to 2.04; 127 women; 1 study; Analysis 32.7).
32.7. Analysis.
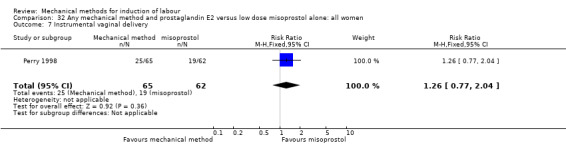
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 7 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.56, 95% CI 0.23 to 1.32; 127 women; 1 study; Analysis 32.8).
32.8. Analysis.
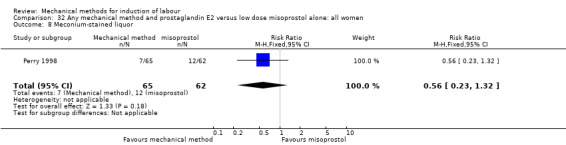
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 8 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 1.91, 95% CI 0.18 to 20.51; 127 women; 1 study; Analysis 32.9).
32.9. Analysis.
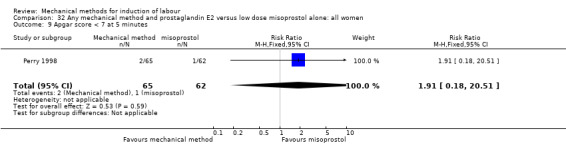
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 9 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both methods (RR 0.64, 95% CI 0.31 to 1.31; 127 women; 1 study; Analysis 32.10).
32.10. Analysis.
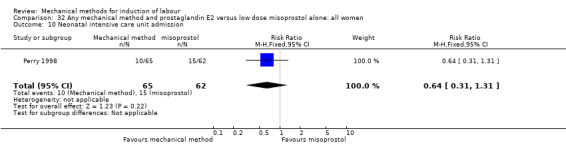
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 10 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both methods (RR 0.19, 95% CI 0.01 to 3.90; 127 women; 1 study; Analysis 32.11). Two cases of neonatal death were reported by Perry 1998, both were born to women randomised to misoprostol. The authors describe that in both cases the neonates died as a result of complications of congenital malformations and were unrelated to the induction method.
32.11. Analysis.
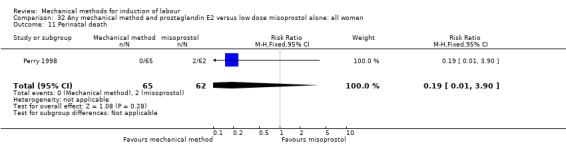
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 11 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
Not reported.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 1.91, 95% CI 0.18 to 20.51; 127 women; 1 study; Analysis 32.12).
32.12. Analysis.
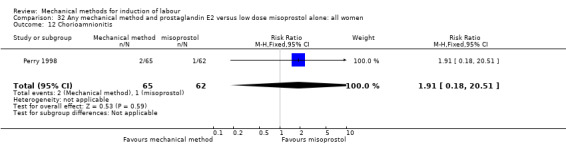
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 12 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 1.91, 95% CI 0.36 to 10.05; 127 women; 1 study; Analysis 32.13).
32.13. Analysis.
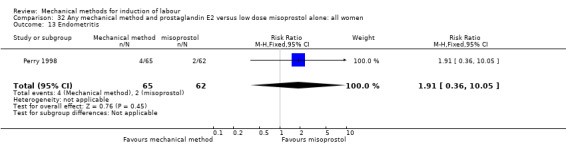
Comparison 32 Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women, Outcome 13 Endometritis.
Fetal distress
Not reported.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and prostaglandin E2 versus oxytocin alone (one trial involving 44 women)
The only outcomes of interest reported for this comparison were caesarean section, instrumental vaginal delivery and endometritis. Other outcomes were not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between induction of labour with a mechanical method combined with PGE2 versus oxytocin (RR 0.30, 95% CI 0.04 to 2.47; 44 women; 1 study; Analysis 33.1).
33.1. Analysis.
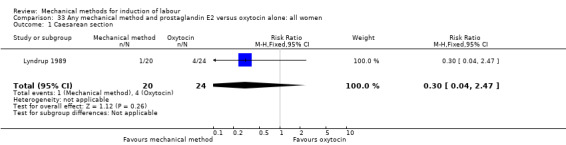
Comparison 33 Any mechanical method and prostaglandin E2 versus oxytocin alone: all women, Outcome 1 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.60, 95% CI 0.12 to 2.94; 44 women; 1 study; Analysis 33.2).
33.2. Analysis.
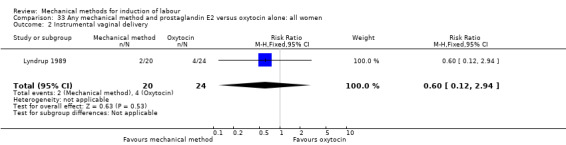
Comparison 33 Any mechanical method and prostaglandin E2 versus oxytocin alone: all women, Outcome 2 Instrumental vaginal delivery.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 3.57, 95% CI 0.15 to 83.14; 44 women; 1 study; Analysis 33.3).
33.3. Analysis.
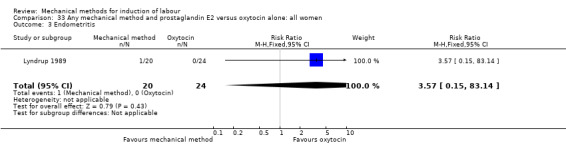
Comparison 33 Any mechanical method and prostaglandin E2 versus oxytocin alone: all women, Outcome 3 Endometritis.
Any mechanical method and low‐dose misoprostol versus prostaglandin E2 alone (one trial involving 350 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
It is uncertain whether there is a difference in vaginal deliveries not achieved within 24 hours between induction of labour with a mechanical method combined with misoprostol and prostaglandin E2 (RR 1.14, 95% CI 0.89 to 1.46; 350 women; 1 study; Analysis 34.1).
34.1. Analysis.
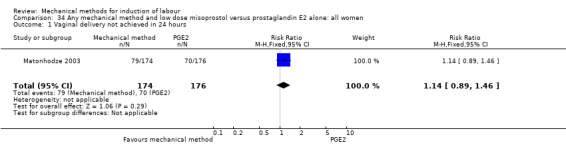
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (RR 0.75, 95% CI 0.27 to 2.13; 327 women; 1 study; Analysis 34.2).
34.2. Analysis.
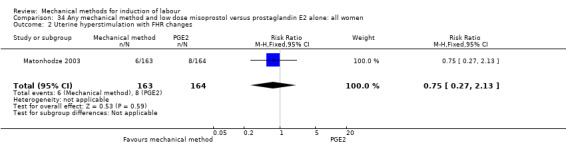
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (RR 0.85, 95% CI 0.57 to 1.25; 350 women; 1 study; Analysis 34.3).
34.3. Analysis.
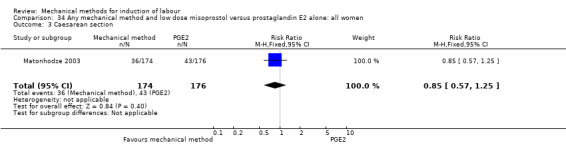
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 3 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 2.04, 95% CI 0.19 to 22.24; 345 women; 1 study; Analysis 34.4).
34.4. Analysis.
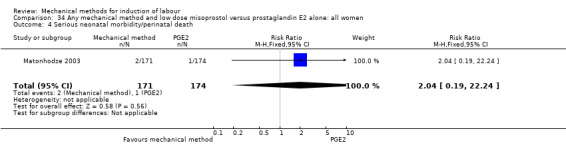
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 4 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 34.5). No events of maternal morbidity or death occurred in the one included study (350 women).
34.5. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 5 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
A mechanical method combined with misoprostol probably reduces the risk of oxytocin augmentation when compared to PGE2 (RR 0.54, 95% CI 0.34 to 0.86; 350 women; 1 study; Analysis 34.6).
34.6. Analysis.
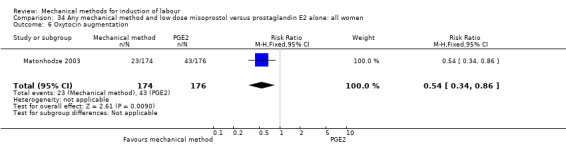
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 6 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (RR 0.54, 95% CI 0.22 to 1.32; 327 women; 1 study; Analysis 34.7).
34.7. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 7 Uterine hyperstimulation without fetal heart rate changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 34.8). No events of uterine rupture occurred in the one included study (350 women).
34.8. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 8 Uterine rupture.
Epidural analgesia
Not reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 1.01, 95% CI 0.26 to 3.98; 350 women; 1 study; Analysis 34.9).
34.9. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 9 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 1.15, 95% CI 0.60 to 2.23; 339 women; 1 study; Analysis 34.10).
34.10. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 10 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 0.68, 95% CI 0.25 to 1.88; 346 women; 1 study; Analysis 34.11).
34.11. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 11 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both methods (RR 0.68, 95% CI 0.12 to 4.03; 346 women; 1 study; Analysis 34.12).
34.12. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 12 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both induction methods (RR 1.02, 95% CI 0.06 to 16.14; 345 women; 1 study; Analysis 34.13). Two cases of perinatal death were reported by Matonhodze 2003, one in each group. No further information was given on timing or cause of the demise.
34.13. Analysis.
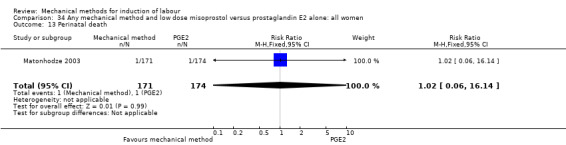
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 13 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects
It is uncertain whether there is a difference in maternal side effects between both induction methods (RR 1.16, 95% CI 0.95 to 1.43; 314 women; 1 study; Analysis 34.14).
34.14. Analysis.
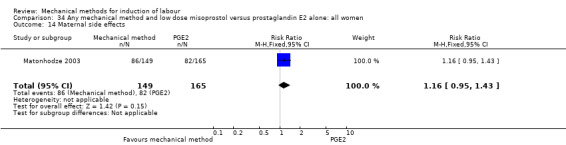
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 14 Maternal side effects.
Maternal nausea
A mechanical method combined with misoprostol may increase the risk of maternal nausea when compared to PGE2 (RR 1.65, 95% CI 0.98 to 2.79; 300 women; 1 study; Analysis 34.15). However, the result is still too imprecise to make a valid judgement on this outcome.
34.15. Analysis.
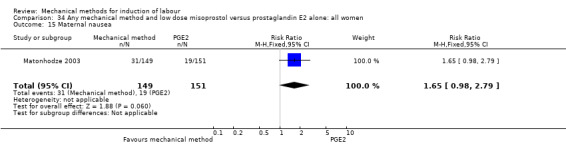
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 15 Maternal nausea.
Maternal vomiting
Not reported.
Maternal diarrhoea
A mechanical method combined with misoprostol probably increases the risk of maternal diarrhoea when compared to PGE2 (RR 3.72, 95% CI 1.53 to 9.00; 313 women; 1 study; Analysis 34.16).
34.16. Analysis.
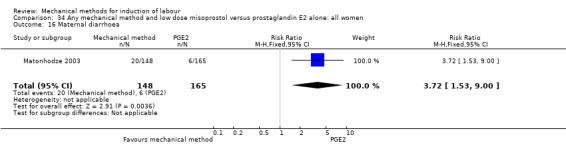
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 16 Maternal diarrhoea.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 0.98, 95% CI 0.67 to 1.41; 348 women; 1 study; Analysis 34.17).
34.17. Analysis.
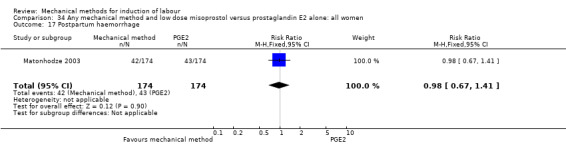
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 17 Postpartum haemorrhage.
Serious maternal complications
It is uncertain whether there is a difference in serious maternal complications between both induction methods (Analysis 34.18). One study (350 women) was included for this outcome in which no cases of septicaemia or intensive care unit admission were reported.
34.18. Analysis.
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 18 Serious maternal complications.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
It is uncertain whether there is a difference in maternal fever during labour between both induction methods (RR 1.53, 95% CI 0.26 to 9.02; 347 women; 1 study; Analysis 34.19).
34.19. Analysis.
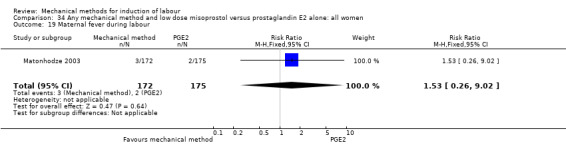
Comparison 34 Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women, Outcome 19 Maternal fever during labour.
Antibiotics during labour
Not reported.
Chorioamnionitis
Not reported.
Endometritis
Not reported.
Fetal distress
Not reported.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and low dose misoprostol versus misoprostol alone (seven trials involving 1422 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
It is uncertain whether there is a difference in vaginal deliveries not achieved within 24 hours between any mechanical method combined with misoprostol and misoprostol alone (average RR 0.70, 95% CI 0.25 to 1.95; 668 women; 2 studies; Analysis 35.1). Also, there was substantial heterogeneity for this outcome (Tau² = 0.51; Chi² = 14.00, df = 1 (P = 0.0002); I² = 93%).
35.1. Analysis.
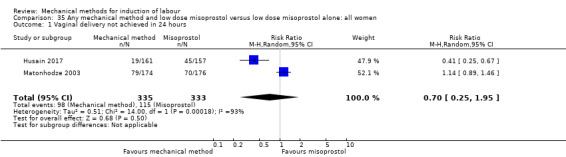
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
A sensitivity analysis, after eliminating one trial assessed as having a potentially higher risk of allocation or attrition bias (Husain 2017), did not alter the result (average RR 1.14, 95% CI 0.89 to 1.46; 350 women; 1 study).
The same results were seen on a subgroup comparison for primiparous women (RR 0.83, 95% CI 0.23 to 2.96; 53 women; 1 study; Analysis 36.1). For multiparous women, a mechanical method combined with misoprostol may reduce the risk of a vaginal delivery not achieved within 24 hours (RR 0.37, 95% CI 0.21 to 0.63; 265 women; 1 study; Analysis 37.1).
36.1. Analysis.
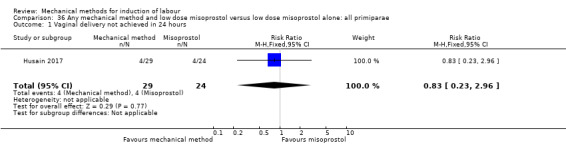
Comparison 36 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
37.1. Analysis.
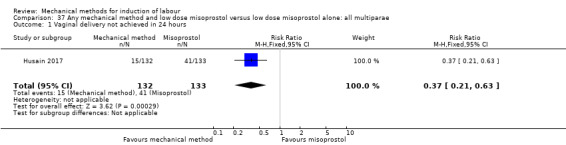
Comparison 37 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all multiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (average RR 0.54, 95% CI 0.20 to 1.45; 707 women; 4 studies; Analysis 35.2). Also, there was substantial heterogeneity for this outcome (Tau² = 0.57; Chi² = 7.40, df = 2 (P = 0.02); I² = 73%). No sensitivity analysis was performed as no potential high‐risk studies were included for this outcome.
35.2. Analysis.
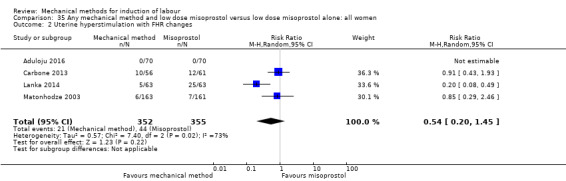
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 2 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (average RR 0.87, 95% CI 0.66 to 1.15; 1422 women; 7 studies; Analysis 35.3). Also, there was substantial heterogeneity for this outcome (Tau² = 0.07; Chi² = 13.33, df = 6 (P = 0.04); I² = 55%).
35.3. Analysis.
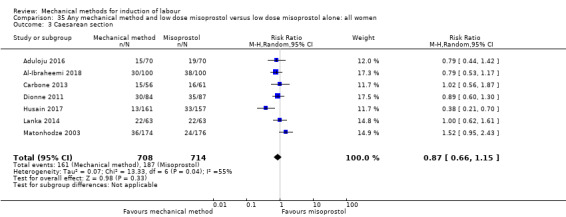
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 3 Caesarean section.
A sensitivity analysis, after eliminating one trial assessed as having a potentially higher risk of allocation or attrition bias (Husain 2017), showed there probably is little or no difference in caesarean sections between both induction methods (RR 0.96, 95% CI 0.79 to 1.17; 1104 women; 6 studies; I² = 5%).
The same results were seen on a subgroup comparison for primiparous women (RR 0.62, 95% CI 0.15 to 2.51; 53 women; 1 study; Analysis 36.2). For multiparous women, a mechanical method combined with misoprostol may reduce the risk a caesarean section (RR 0.35, 95% CI 0.18 to 0.68; 265 women; 1 study; Analysis 37.2).
36.2. Analysis.
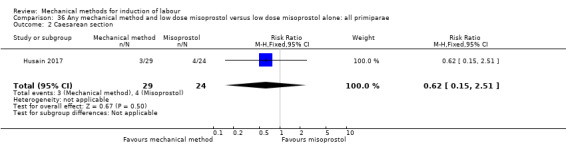
Comparison 36 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all primiparae, Outcome 2 Caesarean section.
37.2. Analysis.
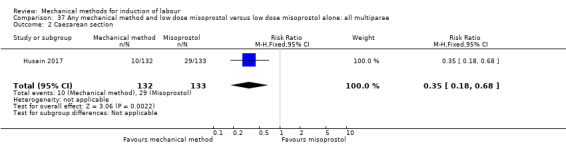
Comparison 37 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all multiparae, Outcome 2 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 1.25, 95% CI 0.34 to 4.55; 487 women; 2 studies; Analysis 35.4).
35.4. Analysis.
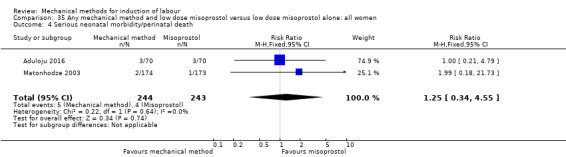
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 4 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 35.5). Of the seven studies included for this comparison, two studies (490 women) reported on this composite outcome. No events of serious maternal morbidity or death occurred in these studies.
35.5. Analysis.
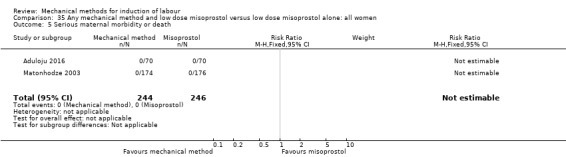
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 5 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
A mechanical method combined with misoprostol probably reduces the risk of an unfavourable cervix after 24 hours when compared to misoprostol alone (RR 0.27, 95% CI 0.08 to 0.94; 140 women; 1 study; Analysis 35.6).
35.6. Analysis.
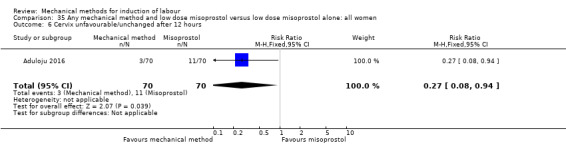
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 6 Cervix unfavourable/unchanged after 12 hours.
Oxytocin augmentation
It is uncertain whether there is a difference in oxytocin augmentation between both induction methods (average RR 0.94, 95% CI 0.70 to 1.25; 1051 women; 5 studies; Analysis 35.7). Also, there was substantial heterogeneity for this outcome (Tau² = 0.07; Chi² = 16.91, df = 4 (P = 0.002); I² = 76%).
35.7. Analysis.
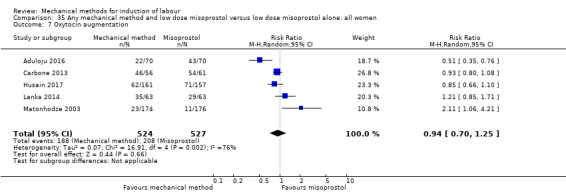
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 7 Oxytocin augmentation.
A sensitivity analysis, after eliminating one trial assessed as having a potentially higher risk of allocation or attrition bias (Husain 2017), did not alter the result nor did it lower heterogeneity (average RR 0.98, 95% CI 0.66 to 1.48; 733 women; 4 studies; I² = 82%).
Uterine hyperstimulation without FHR changes
A mechanical method combined with misoprostol probably reduces the risk of uterine hyperstimulation without FHR changes when compared to misoprostol alone (RR 0.53, 95% CI 0.32 to 0.90; 982 women; 4 studies; Analysis 35.8).
35.8. Analysis.
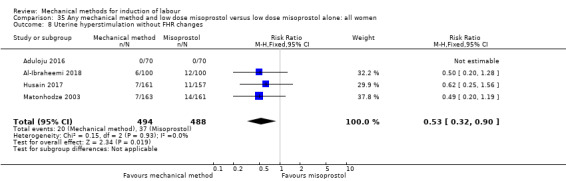
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 8 Uterine hyperstimulation without FHR changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 35.9). Of the seven studies included for this comparison, two studies (490 women) reported on this outcome. No events of uterine rupture occurred in one of these studies.
35.9. Analysis.
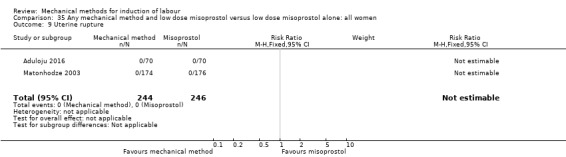
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 9 Uterine rupture.
Epidural analgesia
There may be little or no difference in epidural analgesia between both induction methods (average RR 1.00, 95% CI 0.91 to 1.10; 443 women; 3 studies; Analysis 35.10), although there was moderate heterogeneity for this outcome (Tau² = 0.00; Chi² = 3.52, df = 2 (P = 0.17); 43%).
35.10. Analysis.
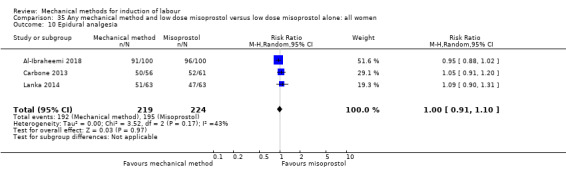
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 10 Epidural analgesia.
No sensitivity analysis was performed as no potential high‐risk studies were included for this outcome.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.93, 95% CI 0.58 to 1.51; 676 women; 3 studies; Analysis 35.11).
35.11. Analysis.
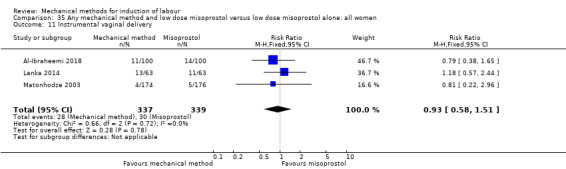
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 11 Instrumental vaginal delivery.
Meconium‐stained liquor
A mechanical method combined with misoprostol may reduce the risk of meconium‐stained liquor when compared to misoprostol alone (average RR 0.61, 95% CI 0.35 to 1.04; 1243 women; 6 studies; Analysis 35.12). However, the result is still too imprecise to make a valid judgement on this outcome. Also, there was substantial heterogeneity for this outcome (Tau² = 0.24; Chi² = 11.55, df = 5 (P = 0.04); I² = 57%).
35.12. Analysis.
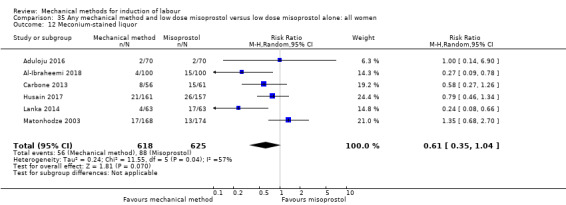
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 12 Meconium‐stained liquor.
A sensitivity analysis, after eliminating one trial assessed as having a potentially higher risk of allocation or attrition bias (Husain 2017), did not alter the result nor did it lower heterogeneity (average RR 0.55, 95% CI 0.26 to 1.14; 925 women; 5 studies; I² = 64%).
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar score less than seven at five minutes between both induction methods (average RR 0.71, 95% CI 0.37 to 1.36; 802 women; 3 studies; Analysis 35.13). Also, there was substantial heterogeneity for this outcome (Tau² = 0.11; Chi² = 2.89, df = 2 (P = 0.24); I² = 31%).
35.13. Analysis.
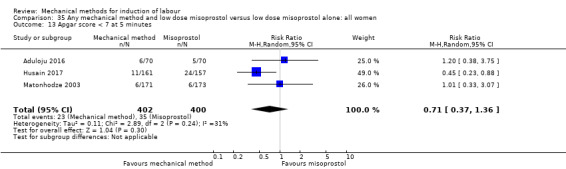
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 13 Apgar score < 7 at 5 minutes.
A sensitivity analysis, after eliminating one trial assessed as having a potentially higher risk of allocation or attrition bias (Husain 2017), did not alter the result, although heterogeneity was lost (RR 1.10, 95% CI 0.50 to 2.44; 484 women; 2 studies; I² = 0%).
Neonatal intensive care unit admission
A mechanical method combined with misoprostol may reduce the risk of NICU admission when compared to misoprostol alone (RR 0.57, 95% CI 0.36 to 0.91; 1246 women; 6 studies; Analysis 35.14), the absolute effect being 30 fewer NICU admissions per 1000 deliveries.
35.14. Analysis.
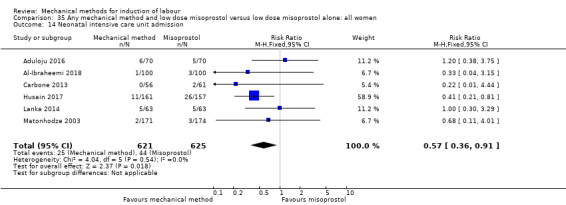
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 14 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is difference in perinatal death between both induction methods (RR 3.09, 95% CI 0.13 to 75.26; 347 women; 1 study; Analysis 35.15). One case of perinatal death was reported by Matonhodze 2003, which occurred in the combined method group. No further information was given on timing or cause of the demise.
35.15. Analysis.
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 15 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects (all)
It is uncertain whether there is a difference in maternal side effects between both induction methods (RR 1.06, 95% CI 0.87 to 1.30; 300 women; 1 study; Analysis 35.16).
35.16. Analysis.
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 16 Maternal side effects.
Maternal nausea
It is uncertain whether there is a difference in maternal nausea between both induction methods (RR 1.37, 95% CI 0.84 to 2.23; 300 women; study; Analysis 35.17).
35.17. Analysis.
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 17 Maternal nausea.
Maternal vomiting
Not reported.
Maternal diarrhoea
A mechanical method combined with misoprostol probably increases the risk of maternal diarrhoea when compared to misoprostol alone (RR 3.38, 95% CI 1.40 to 8.17; 298 women; 1 study; Analysis 35.18).
35.18. Analysis.
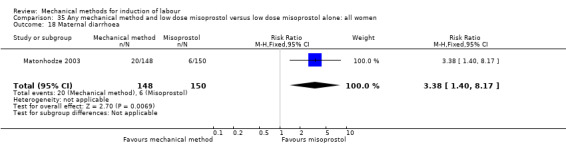
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 18 Maternal diarrhoea.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is difference in postpartum haemorrhage between both induction methods (RR 0.93, 95% CI 0.65 to 1.33; 466 women; 2 studies; Analysis 35.19).
35.19. Analysis.
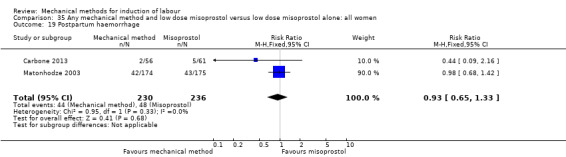
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 19 Postpartum haemorrhage.
Serious maternal complications
It is uncertain whether there is a difference in serious maternal complications between both induction methods (Analysis 35.20). One study (350 women) was included for this outcome in which no cases of septicaemia or intensive care unit admissions were seen.
35.20. Analysis.
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 20 Serious maternal complications.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 0.63, 95% CI 0.28 to 1.38; 443 women; 3 studies; Analysis 35.21).
35.21. Analysis.
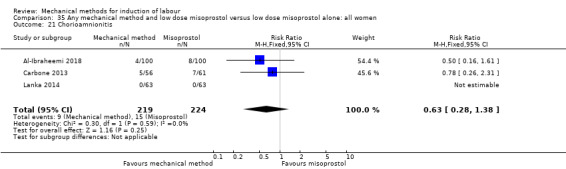
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 21 Chorioamnionitis.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 0.41, 95% CI 0.08 to 2.08; 435 women; 2 studies; Analysis 35.22).
35.22. Analysis.
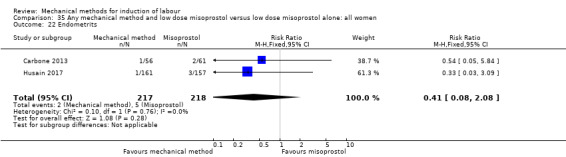
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 22 Endometrits.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 0.78, 95% CI 0.53 to 1.14; 784 women; 4 studies; Analysis 35.23).
35.23. Analysis.
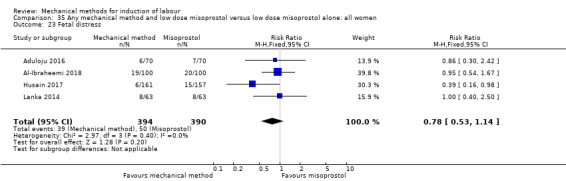
Comparison 35 Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women, Outcome 23 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and oxytocin versus prostaglandin E2 alone (four trials involving 713 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
Not reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between a mechanical method combined with oxytocin and PGE2 (RR 1.48, 95% CI 0.55 to 3.95; 151 women; 1 study; Analysis 38.1).
38.1. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 1 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (RR 0.93, 95% CI 0.72 to 1.20; 713 women; 4 studies; Analysis 38.2).
38.2. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 2 Caesarean section.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious neonatal morbidity or perinatal death
Not reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 38.3). One study (200 women) was included for this composite outcome in which no events of maternal morbidity or death occurred.
38.3. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 3 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
A mechanical method combined with oxytocin probably increases the risk of oxytocin augmentation when compared to PGE2 (RR 2.48, 95% CI 1.95 to 3.15; 200 women; 1 study; Analysis 38.4).
38.4. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 4 Oxytocin augmentation.
Uterine hyperstimulation without FHR changes
A mechanical method combined with oxytocin probably increases the risk of uterine hyperstimulation without FHR changes when compared to PGE2 (RR 2.19, 95% CI 1.39 to 3.46; 151 women; 1 study; Analysis 38.5).
38.5. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 5 Uterine hyperstimulation without FHR changes.
Uterine rupture
Not reported.
Epidural analgesia
Not reported.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.35, 95% CI 0.08 to 1.58; 41 women; 1 study; Analysis 38.6).
38.6. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 6 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 1.13, 95% CI 0.43 to 2.95; 151 women; 1 study; Analysis 38.7).
38.7. Analysis.
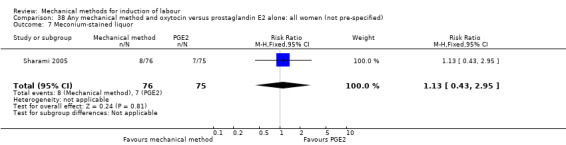
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 7 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 2.96, 95% CI 0.12 to 71.55; 151 women; 1 study; Analysis 38.8).
38.8. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 8 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both methods (RR 0.85, 95% CI 0.30 to 2.40; 151 women; 1 study; Analysis 38.9).
38.9. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 9 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
Not reported.
Disability in childhood
Not reported.
Maternal side effects
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 0.14, 95% CI 0.01 to 2.68; 151 women; 1 study; Analysis 38.10).
38.10. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 10 Postpartum haemorrhage.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
Not reported.
Chorioamnionitis
Not reported.
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (Analysis 38.11). One study (41 women) reported on this outcome. No events of endometritis occurred in this study.
38.11. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 11 Endometritis.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (average RR 0.97, 95% CI 0.61 to 1.56; 498 women; 3 studies; Analysis 38.12). Also, there was moderate heterogeneity for this outcome (Tau² = 0.06; Chi² = 2.93, df = 2 (P = 0.23); I² = 32%).
38.12. Analysis.
Comparison 38 Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified), Outcome 12 Fetal distress.
No sensitivity analysis was performed as no potential high‐risk studies were included for this outcome.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and oxytocin versus misoprostol alone (six trials involving 1779 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
A mechanical method combined with oxytocin probably reduces the risk of a vaginal delivery not being achieved within 24 hours when compared to misoprostol (RR 0.48, 95% CI 0.37 to 0.63; 362 women; 2 studies; Analysis 39.1), the absolute effect being 285 fewer per 1000 deliveries.
39.1. Analysis.
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 1 Vaginal delivery not achieved in 24 hours.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation with FHR changes between both induction methods (RR 0.43, 95% CI 0.17 to 1.11; 1463 women; 3 studies; Analysis 39.2).
39.2. Analysis.
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 2 Uterine hyperstimulation with FHR changes.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Caesarean section
There probably is little or difference in caesarean sections between both induction methods (RR 0.95, 95% CI 0.80 to 1.12; 1779 women; 5 studies; Analysis 39.3).
39.3. Analysis.
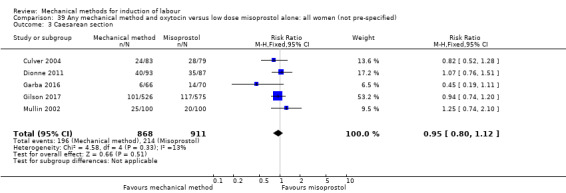
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 3 Caesarean section.
For the subgroup of primiparous women, no outcomes were reported. For multiparous women, it is uncertain whether there is a difference in caesarean sections between both induction methods (RR 0.45, 95% CI 0.19 to 1.11; 136 women; 1 study; Analysis 40.1).
40.1. Analysis.
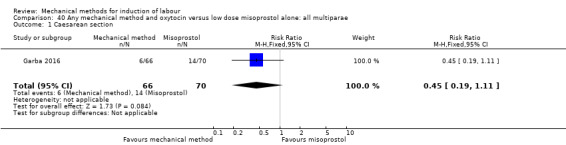
Comparison 40 Any mechanical method and oxytocin versus low dose misoprostol alone: all multiparae, Outcome 1 Caesarean section.
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 0.82, 95% CI 0.18 to 3.65;1263 women; 2 studies; Analysis 39.4). All the events included for this composite outcome were cases of neonatal death.
39.4. Analysis.
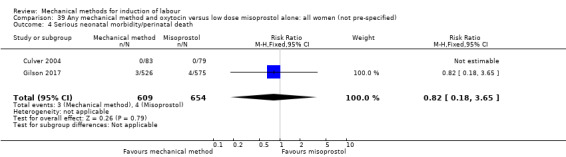
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 4 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
Not reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
It is uncertain whether there is a difference in oxytocin augmentation between both induction methods (average RR 3.89, 95% CI 0.70 to 21.72; 336 women; 2 studies; Analysis 39.5). Also, there was substantial heterogeneity for this outcome (Tau² = 1.46; Chi² = 18.47, df = 1 (P < 0.0001); I² = 95%).
39.5. Analysis.
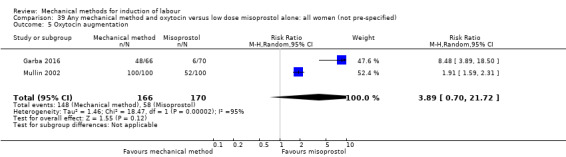
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 5 Oxytocin augmentation.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Garba 2016), changed the result in favour of misoprostol as it showed a mechanical method combined with oxytocin may increase the risk of oxytocin augmentation (RR 1.91, 95% CI 1.59 to 2.31; 200 women; 1 study).
Uterine hyperstimulation without FHR changes
A mechanical method combined with oxytocin probably reduces the risk of uterine hyperstimulation without FHR changes when compared to misoprostol (RR 0.52, 95% CI 0.30 to 0.92; 498 women; 3 studies; Analysis 39.6).
39.6. Analysis.
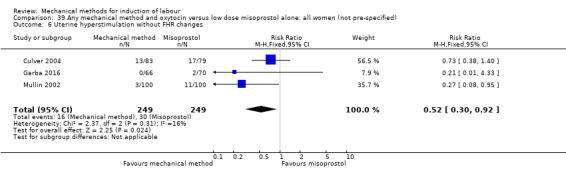
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 6 Uterine hyperstimulation without FHR changes.
Uterine rupture
Not reported.
Epidural analgesia
It is uncertain whether there is a difference in epidural analgesia between both induction methods (RR 1.07, 95% CI 0.90 to 1.27; 162 women; 1 study; Analysis 39.7).
39.7. Analysis.
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 7 Epidural analgesia.
Instrumental vaginal delivery
Not reported.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.72, 95% CI 0.43 to 1.19; 362 women; 2 studies; Analysis 39.8).
39.8. Analysis.
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 8 Meconium‐stained liquor.
Apgar score less than seven at five minutes
It is uncertain whether there is a difference in Apgar scores less than seven at five minutes between both induction methods (RR 0.95, 95% CI 0.20 to 4.58; 162 women; 1 study; Analysis 39.9).
39.9. Analysis.
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 9 Apgar score < 7 at 5 minutes.
Neonatal intensive care unit admission
A mechanical method combined with oxytocin probably reduces the risk of a NICU admission when compared to misoprostol (RR 0.66, 95% CI 0.49 to 0.90; 1599 women; 4 studies; Analysis 39.10), the absolute effect being 37 fewer NICU admissions per 1000 deliveries.
39.10. Analysis.
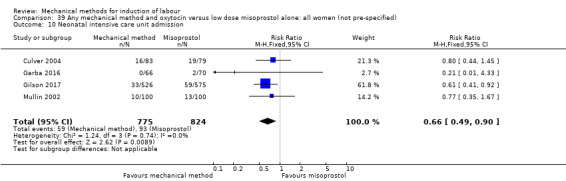
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 10 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
It is uncertain whether there is a difference in perinatal death between both induction methods (RR 0.82, 95% CI 0.18 to 3.65; 1263 women; 2 studies; Analysis 39.11). Perinatal death occurred in one of the included studies (Gilson 2017). All were cases of neonatal death. No further information was given on cause of the demise.
39.11. Analysis.
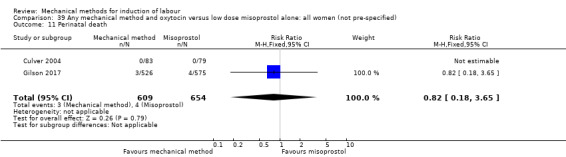
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 11 Perinatal death.
Disability in childhood
Not reported.
Maternal side effects
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
Not reported.
Serious maternal complications
Not reported.
Maternal death
Not reported.
Woman not satisfied
A mechanical method combined with oxytocin may increase the risk of women not being satisfied when compared to misoprostol (RR 1.68, 95% CI 1.47 to 1.93; 866 women; 1 study; Analysis 39.12), the absolute effect being 260 more women not satisfied per 1000 deliveries. For this outcome, women in the study of Gilson 2017 were asked if they would choose the same method again if induction of labour was needed in a future pregnancy.
39.12. Analysis.
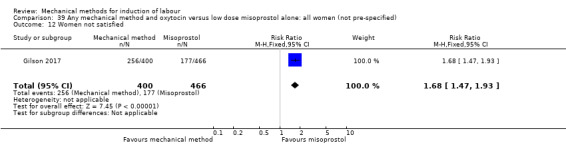
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 12 Women not satisfied.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
A mechanical method combined with oxytocin may reduce the risk of maternal fever during labour when compared to misoprostol (RR 0.13, 95% CI 0.04 to 0.50; 298 women; 2 studies; Analysis 39.13).
39.13. Analysis.
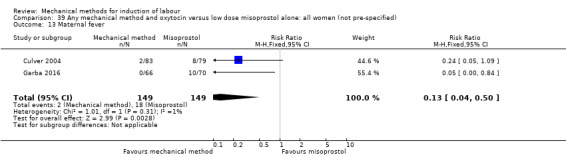
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 13 Maternal fever.
Antibiotics during labour
Not reported.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (RR 0.65, 95% CI 0.32 to 1.31; 200 women; 1 study; Analysis 39.14).
39.14. Analysis.
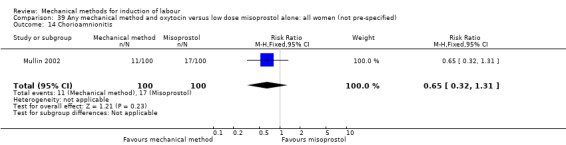
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 14 Chorioamnionitis.
Endometritis
Not reported.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 0.55, 95% CI 0.25 to 1.21; 362 women; 2 studies; Analysis 39.15).
39.15. Analysis.
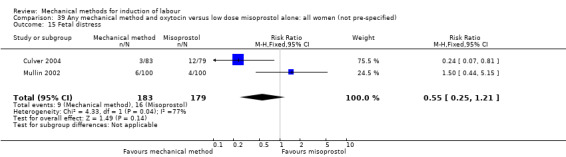
Comparison 39 Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified), Outcome 15 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Any mechanical method and oxytocin versus oxytocin alone (six trials involving 718 women)
Primary outcomes
Vaginal delivery not achieved within 24 hours
It is uncertain whether there is a difference in a vaginal delivery not being achieved within 24 hours between induction of labour with a mechanical method combined with oxytocin and oxytocin alone (average RR 0.71, 95% CI 0.21 to 2.40; 321 women; 2 studies; Analysis 41.1). Also, there was substantial heterogeneity for this outcome (Tau² = 0.72; Chi² = 19.17, df = 1 (P,0.0001); I² = 95%).
41.1. Analysis.
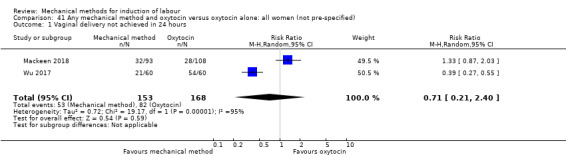
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 1 Vaginal delivery not achieved in 24 hours.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Mackeen 2018), changed the result in favour of a mechanical method combined with oxytocin as it showed it may reduce the risk of vaginal delivery not being achieved within 24 hours (RR 0.39, 95% CI 0.27 to 0.55; 120 women; 1 study), the absolute effect being 550 fewer per 1000 deliveries.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Uterine hyperstimulation with FHR changes
Not reported.
Caesarean section
It is uncertain whether there is a difference in caesarean sections between both induction methods (average RR 0.68, 95% CI 0.39 to 1.20; 718 women; 6 studies; Analysis 41.2). Also, there was substantial heterogeneity for this outcome (Tau² = 0.32; Chi² = 17.15, df = 5 (P = 0.004); I² = 71%).
41.2. Analysis.
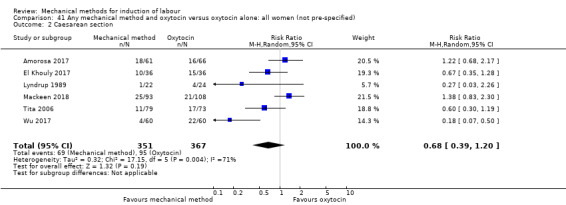
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 2 Caesarean section.
A sensitivity analysis, after eliminating the three trials assessed as having a potentially higher risk of allocation or attrition bias (Lyndrup 1989; Mackeen 2018; Tita 2006), did not alter the result nor did it lower heterogeneity (average RR 0.57, 95% CI 0.21 to 1.52; 319 women; 3 studies; I² = 82%).
Serious neonatal morbidity or perinatal death
It is uncertain whether there is a difference in serious neonatal morbidity or perinatal death between both induction methods (RR 0.71, 95% CI 0.12 to 4.13; 321 women; 2 studies; Analysis 41.3). All the events included for this composite outcome were cases of asphyxia.
41.3. Analysis.
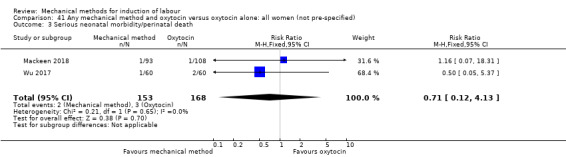
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 3 Serious neonatal morbidity/perinatal death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Serious maternal morbidity or death
It is uncertain whether there is a difference in serious maternal morbidity or death between both induction methods (Analysis 41.4). Of the six included studies for this comparison, two studies (321 women) reported on this composite outcome. No events of maternal morbidity or death occurred in these studies.
41.4. Analysis.
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 4 Serious maternal morbidity or death.
For the subgroups of primiparous and multiparous women, no outcomes were reported.
Secondary outcomes
Cervix unfavourable/unchanged after 24 hours
Not reported.
Oxytocin augmentation
Not reported.
Uterine hyperstimulation without FHR changes
It is uncertain whether there is a difference in uterine hyperstimulation without FHR changes between both induction methods (RR 0.85, 95% CI 0.34 to 2.09; 199 women; 2 studies; Analysis 41.5).
41.5. Analysis.
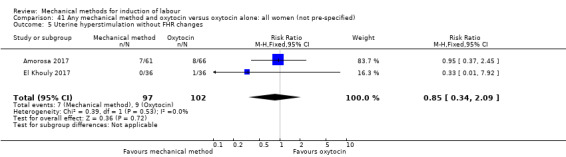
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 5 Uterine hyperstimulation without FHR changes.
Uterine rupture
It is uncertain whether there is a difference in uterine rupture between both induction methods (Analysis 41.6). Of the six included studies for this comparison, one study (120 women) reported on this outcome. No events of uterine rupture occurred in this study.
41.6. Analysis.
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 6 Uterine rupture.
Epidural analgesia
There probably is little or no difference in epidural analgesia between both induction methods (RR 1.03, 95% CI 0.98 to 1.09; 127 women; 1 study; Analysis 41.7).
41.7. Analysis.
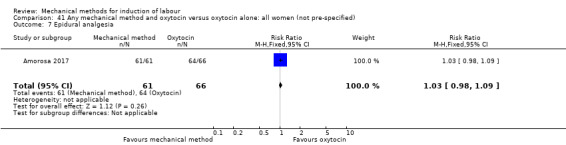
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 7 Epidural analgesia.
Instrumental vaginal delivery
It is uncertain whether there is a difference in instrumental vaginal deliveries between both induction methods (RR 0.99, 95% CI 0.48 to 2.02; 293 women; 3 studies; Analysis 41.8).
41.8. Analysis.
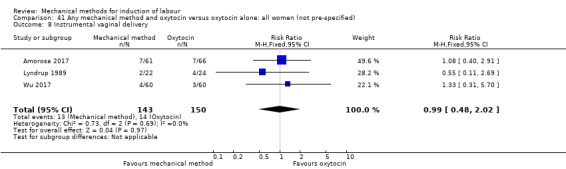
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 8 Instrumental vaginal delivery.
Meconium‐stained liquor
It is uncertain whether there is a difference in meconium‐stained liquor between both induction methods (RR 0.72, 95% CI 0.32 to 1.63; 319 women; 3 studies; Analysis 41.9).
41.9. Analysis.
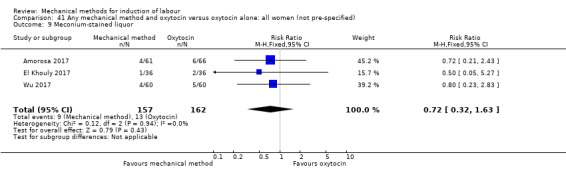
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 9 Meconium‐stained liquor.
Apgar score less than seven at five minutes
Not reported.
Neonatal intensive care unit admission
It is uncertain whether there is a difference in NICU admissions between both induction methods (RR 0.98, 95% CI 0.61 to 1.58; 400 women; 3 studies; Analysis 41.10).
41.10. Analysis.
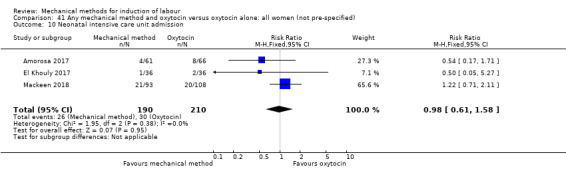
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 10 Neonatal intensive care unit admission.
Neonatal encephalopathy
Not reported.
Perinatal death
Not reported.
Disability in childhood
Not reported.
Maternal side effects
Not reported.
Maternal nausea
Not reported.
Maternal vomiting
Not reported.
Maternal diarrhoea
Not reported.
Other maternal side effects
Not reported.
Postpartum haemorrhage
It is uncertain whether there is a difference in postpartum haemorrhage between both induction methods (RR 1.18, 95% CI 0.44 to 3.18; 319 women; 3 studies; Analysis 41.11).
41.11. Analysis.
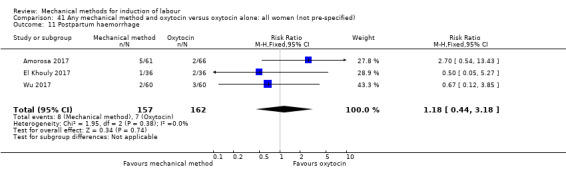
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 11 Postpartum haemorrhage.
Serious maternal complications
It is uncertain whether there is a difference in serious maternal complications between both induction methods (Analysis 41.12). Of the six included studies for this comparison, one study (201 women) reported on maternal sepsis. No events occurred in this study.
41.12. Analysis.
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 12 Serious maternal complications.
Maternal death
Not reported.
Woman not satisfied
Not reported.
Caregiver not satisfied
Not reported.
Other outcomes (not pre‐specified)
Maternal fever during labour
Not reported.
Antibiotics during labour
It is uncertain whether there is a difference in antibiotics during labour between both induction methods (RR 2.32, 95% CI 0.82 to 6.55; 201 women; 1 study; Analysis 41.13).
41.13. Analysis.
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 13 Antibiotics during labour.
Chorioamnionitis
It is uncertain whether there is a difference in chorioamnionitis between both induction methods (average RR 4.34, 95% CI 0.55 to 34.01; 328 women; 2 studies; Analysis 41.14). Also, there was moderate heterogeneity for this outcome (Tau² = 1.19; Chi² = 1.92, df = 1 (P = 0.17); I² = 48%).
41.14. Analysis.
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 14 Chorionamnionitis.
A sensitivity analysis, after eliminating the one trial assessed as having a potentially higher risk of allocation or attrition bias (Mackeen 2018), did not alter the result (RR 2.16, 95% CI 0.57 to 8.28; 127 women; 1 study).
Endometritis
It is uncertain whether there is a difference in endometritis between both induction methods (RR 1.08, 95% CI 0.16 to 7.45; 374 women; 3 studies; Analysis 41.15).
41.15. Analysis.
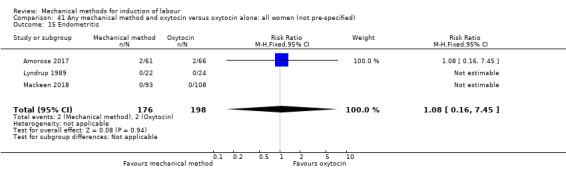
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 15 Endometritis.
Fetal distress
It is uncertain whether there is a difference in fetal distress for which a caesarean section is indicated between both induction methods (RR 1.37, 95% CI 0.68 to 2.77; 400 women; 3 studies; Analysis 41.16).
41.16. Analysis.
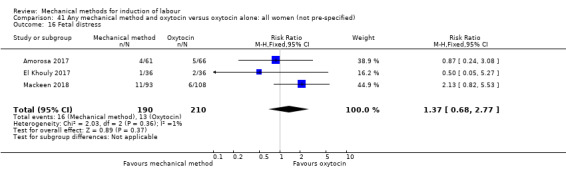
Comparison 41 Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified), Outcome 16 Fetal distress.
Umbilical artery pH < 7.10
Not reported.
Discussion
We set out to explore the effectiveness of mechanical methods for labour induction and their adverse effects for women and their babies in comparison to different pharmacological methods. We included a total of 113 studies, with 105 studies contributing data involving 22,373 women. This updated review now consists of 21 different comparisons (and 20 subgroup comparisons), where in most of the comparisons a mechanical method (balloon, laminaria or extra‐amniotic space infusion (EASI)) was compared with prostaglandin E2 (PGE2), misoprostol or oxytocin. We explored the combination of a mechanical method combined with a pharmacological method, as well as a single versus a double balloon.
Summary of main results
Balloon
Balloon versus PGE2
A balloon catheter is probably as effective for inducing labour as vaginal PGE2, as there was little or no difference in a vaginal delivery not achieved within 24 hours (low‐quality evidence) and caesarean sections (moderate‐quality evidence) between both induction methods. However, oxytocin augmentation is probably more often required when labour is induced with a balloon catheter. As for perinatal outcomes, a balloon catheter appears to have a more favourable safety profile compared to vaginal PGE2, as it probably reduces the risk of uterine hyperstimulation with and without fetal heart rate (FHR) changes (moderate‐quality evidence), fetal distress for which a caesarean section is required and an umbilical artery pH less than 7.10. Also, a balloon catheter may slightly reduce the risk of a neonatal intensive care unit (NICU) admission (low‐quality evidence), although conventional statistical significance was not reached as the result was still too imprecise to make a valid judgement. Of note, a balloon catheter probably reduces the risk of serious neonatal morbidity or perinatal death (moderate‐risk evidence). However, this outcome should be interpreted with caution as only a few studies (eight out of 28 studies), reported on this composite outcome and therefore a bias for this result could exist. Most of the serious perinatal adverse events in this composite outcome were cases of perinatal asphyxia. Regarding our other main outcomes for this comparison, it was unclear if there is a difference in five‐minute Apgar score less than seven (low‐quality evidence) or serious maternal morbidity or death (very low‐quality evidence).
There was no evidence of a difference in outcomes between induction of labour with a balloon compared to cervical PGE2, although the risk of fetal distress for which a caesarean section is indicated is probably reduced when a balloon is used.
Balloon versus misoprostol
A balloon catheter may be less effective for induction of labour when compared to low‐dose oral misoprostol, as a balloon probably increases the risk of a vaginal delivery not achieved within 24 hours (moderate‐quality evidence), oxytocin augmentation and probably slightly increases the risk of a caesarean section (moderate‐quality evidence). Regarding safety outcomes for the neonate, which are hyperstimulation with (low‐quality evidence) and without FHR changes, serious neonatal morbidity or perinatal death (low‐quality evidence), NICU admission (low‐quality evidence), five‐minute Apgar score less than seven (low‐quality evidence), fetal distress and umbilical artery pH less than 7.10, it is unclear if there is a difference between both methods as results were too imprecise to make a valid judgement. This was also the case for the composite outcome serious maternal morbidity or death (very low‐quality evidence).
When compared to low‐dose vaginal misoprostol, a balloon catheter may increase the risk of a caesarean section and oxytocin augmentation (low‐quality evidence). However, there was substantial heterogeneity for both outcomes. For the outcome caesarean section, heterogeneity was not reduced after sensitivity analysis. The risk of hyperstimulation, with and without FHR changes, is probably reduced when a balloon catheter is used, as well as the risk of meconium‐stained liquor (moderate‐quality evidence). Regarding our other main outcomes for this comparison, it was unclear if there was a difference between serious neonatal morbidity or perinatal death (very low‐quality evidence), serious maternal morbidity or death (very low‐quality evidence), NICU admission (low‐quality evidence) and five‐minute Apgar score less than seven (low‐quality evidence) as these results were too imprecise to make a valid judgement.
Epidural analgesia is probably used slightly more after induction of labour with a balloon compared to low‐dose oral misoprostol, as well as vaginal misoprostol.
Balloon versus oxytocin
In women with an unfavourable cervix, cervical ripening with a balloon seems to be more effective than induction with oxytocin as it probably reduces the risk of caesarean section and the risk of fetal distress for which a caesarean section is indicated. For women with a previous caesarean section, a balloon catheter may slightly reduce the risk of a caesarean section when compared to oxytocin. However, the result is too imprecise to make a valid judgement on this outcome.
Single balloon versus double balloon
There is no evidence of benefit of a double balloon over a single balloon. There is little or no difference in vaginal deliveries not achieved within 24 hours and in oxytocin augmentation. No clear difference in caesarean section rate was seen between these induction methods. However, the result was still too imprecise to make a valid judgement. Hyperstimulation seems to occur infrequently with either balloons, as no events of uterine hyperstimulation with or without FHR changes were reported in the one study (217 women) which reported on these outcomes.
Laminaria tent
There was no evidence of a difference in outcomes between a laminaria tent compared to vaginal PGE2. However, results were too imprecise to make a valid judgement. Compared to cervical PGE2, a laminaria tent probably reduces the risk uterine hyperstimulation both with and without FHR changes.
EASI
Only a few small studies compared EASI with other methods. When compared to vaginal PGE2, EASI may increase the risk of a vaginal delivery not achieved within 24 hours and oxytocin augmentation.
Mechanical method combined with a pharmacological method
There was no evidence of clear benefit for a mechanical method combined with PGE2 compared to PGE2 alone or to oxytocin. When compared to low‐dose misoprostol, a mechanical method combined with PGE2 may reduce the risk of a vaginal delivery not achieved within 24 hours. However, only one study (127 women) reported on this comparison. When a mechanical method is combined with misoprostol or with oxytocin, it may reduce the risk of a NICU admission when compared to misoprostol alone. However, regarding other perinatal outcomes for both comparisons, there was no evidence for a difference in serious neonatal morbidity or perinatal death, Apgar scores less than seven at five minutes or fetal distress.
Infection
Risk of infection may theoretically be associated with the insertion of foreign material in the cervix. Most studies did not report on this outcome, resulting in limited data, reported as various outcomes (maternal fever during labour, antibiotic use during labour, chorioamnionitis and endometritis). According to the limited data available, there is no evidence of an increased risk of infectious morbidity with mechanical methods. These data should however be cautiously interpreted as results were imprecise.
Women's view
Data on patient satisfaction or patient preferences are sparse and not all data could be included in the meta‐analyses. When a balloon catheter was compared to vaginal PGE2, more women who were randomised to a balloon would choose the allocated induction method again in a subsequent pregnancy, as compared to women who were randomised to PGE2. However, when a balloon catheter was compared to oral misoprostol, more women would choose misoprostol in a subsequent pregnancy. For both outcomes, only one study was included.
Overall completeness and applicability of evidence
This review was previously one of a series of Cochrane Reviews examining various methods for induction of labour and now serves as a stand‐alone review. Other reviews have examined pharmacological and non‐pharmacological methods including vaginal prostaglandins (Thomas 2014); intracervical prostaglandins (Boulvain 2008); intravenous oxytocin (Alferivic 2009); amniotomy (Bricker 2000); intravenous oxytocin with amniotomy (Howarth 2001); vaginal misoprostol (Hofmeyr 2010); oral misoprostol (Alfirevic 2014), and other methods.
Despite including 113 studies and including data from 105 studies, there were relatively few clear results. Only for the comparison of a balloon versus vaginal prostaglandin E2, including 28 studies involving 6619 women, were there enough data to make a valid judgement on effectiveness and adverse events between these methods.
Most of the outcomes of interest were poorly reported in the included studies, especially serious maternal or perinatal morbidity or death. Also, for some outcomes such as duration from start of induction to vaginal delivery, Apgar score or umbilical cord pH, only continuous data were reported and therefore were not included in this review. Outcomes should therefore be interpreted with caution. Caesarean section on the other hand, was reported in almost every study. Therefore, caesarean section may be the most reliable outcome by which to assess the effectiveness of mechanical methods for cervical ripening and induction of labour.
The external validity of our results can be questioned as the policy of labour induction varies across the different settings in which trials took place. There was a difference seen in maximum ripening time (e.g. the maximum time cervical ripening was awaited, ranging from six hours to 96 hours) and for when induction of labour was declared as failed. As it may take longer to achieve successful cervical ripening when a balloon is used, this could influence the outcome measures of effectiveness used, such as caesarean section. Also, the caesarean rate differs according to the setting in which trials took place, ranging from 9% (Deshmukh 2011) to 70% (Hudon 1999).
Studies ranged in date of publication from 1982 to 2018. While we did not consider the potential influence of date on our results, it is possible that changes in management of labour can mean that for some comparisons, in which relatively older studies were included, may not be generalisable to the current clinical context.
Quality of the evidence
Risk of bias varied throughout the included trials (see Figure 2 and Figure 3). A great proportion of the trial methods were not well reported and were assessed to be at unclear risk of bias in many domains. Three trials were assessed as using inadequate random sequence generation, and in five trials no measures were taken to conceal allocation. In almost all studies, no blinding was done due to the nature of the intervention. However, blinding of the research personnel would have been possible, but was only described in four studies. Two studies reported to have performed a double‐blind study, but did not describe how this was achieved. We rated many trials at unclear risk of attrition bias, mainly because it was not clear if intention‐to‐treat was performed. Although we did attempt to assess reporting bias, lack of trial protocols for most of the older studies, meant this assessment relied on information available in the published trial report.
The outcomes were assessed using the GRADE approach. We determined the evidence to be moderate‐quality, low‐quality or very low‐quality. All evidence was downgraded for lack of blinding. Other reasons for downgrading were predominately for imprecision (uncertain effect estimates, small sample sizes and low event rates) and inconsistencies (heterogeneity). For our three main comparisons (balloon versus vaginal PGE2; balloon versus vaginal misoprostol; balloon versus oral misoprostol), a 'Summary of findings' table was produced (Table 1; Table 2; Table 3).
Although no publication bias was detected for our main outcomes, there is still a possibility of publication bias. Most comparisons had less than 10 studies included and therefore, a funnel plot could not be produced. Also, for 11 trial registrations the anticipated end date was overdue by two years and it was not clear if the trials had started, were ongoing or finished recruiting (Baacke 2006; Behrashi 2013; Cullimore 2009; Dias 2008; EUCTR 2012; Kamilya 2011; Park 2011; Pathiraja 2014; Reif 2012; Yazdani 2011; Zhang 2014). Therefore, a potential risk exists as results from these studies were not published.
We acknowledge that with so many comparisons within the review, there is also a risk of statistical type 1 error, meaning a false‐positive result. The results where there are very few studies included, moderate or substantial heterogeneity, or those where the meta‐analysis result is of borderline statistical significance must therefore be treated with caution.
Potential biases in the review process
We are aware that the possibility of introducing bias was present at every stage of the reviewing process. We attempted to minimise bias in a number of ways; two review authors assessed studies for eligibility, assessed risk of bias and carried out data extraction. Each review author worked independently. We resolved discrepancies through discussion, or if required we consulted a third review author. Nevertheless, the process of assessing risk of bias, for example, is not an exact science and includes many personal judgements. Four review authors, Mieke ten Eikelder, Marta Jozwiak , Kitty Bloemenkamp and Ben Willem Mol are also trial authors for the following included studies: Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016. Data extraction and risk of bias assessments were conducted by other review authors for these studies (Marieke de Vaan; Kirsten Palmer).
Agreements and disagreements with other studies or reviews
This review is one the most extensive reviews on mechanical methods of labour induction as most reviews on this subject only contain one or two of the comparisons included in this review. We found eight recent systematic reviews covering one or more of our main comparisons, being balloon versus vaginal PGE2, balloon versus vaginal misoprostol or balloon versus oral misoprostol.
Our review was in line with other systematic reviews on induction of labour with a balloon versus vaginal PGE2. Liu 2018 compared a double balloon with a vaginal PGE2 insert and they found no difference in vaginal deliveries achieved within 24 hours or caesarean section rate. They also found a reduction in uterine hyperstimulation and umbilical artery pH < 7.10 when a balloon was used. All of the five studies included in the review of Liu 2018, were also included in our review. Du 2017 compared a double balloon with PGE2 (vaginal as well as cervical) and produced the same results as described in our review and the review of Liu 2018. However, they found no difference in fetal distress for which a caesarean section was indicated. All eight studies were also included in this review. Zhu 2018 compared a Foley catheter with a vaginal PGE2 and included eight studies of which one (Ghanaie 2013) was excluded in our review because oxytocin was administered concurrent to both induction methods. Just as the other reviews, Zhu 2018 found no difference in caesarean section rate. They also looked at the induction to delivery interval on a continuous level and found no difference between both induction methods. Wang 2016 however, found a longer induction to delivery interval when a Foley catheter was used in comparison to PGE2 vaginal insert. The authors did not compare vaginal delivery rates within 24 hours.
Chen 2016 performed a network meta‐analysis in which direct and indirect comparisons between different induction agents, including Foley catheter, vaginal PGE2, vaginal misoprostol and oral misoprostol were made. Studies with high‐dose misoprostol were included in the review of Chen 2016 as opposed to our review and only indirect comparisons could be made between a Foley catheter and oral misoprostol in the review of Chen 2016. The outcomes of interest were vaginal delivery not achieved within 24 hours, uterine hyperstimulation with FHR changes and caesarean section. Not all results were in line with our results. In the network meta‐analysis, a Foley catheter increased the risk of vaginal delivery not achieved within 24 hours compared to vaginal misoprostol, where in our review the outcome was uncertain. When compared to oral misoprostol, no clear difference in vaginal deliveries within 24 hours was seen by Chen 2016 compared to an increased risk in our review. In our review no clear difference was seen in uterine hyperstimulation with FHR changes, but in the network analysis of Chen 2016, a reduced risk was seen when a Foley catheter was used compared to oral misoprostol. For the outcome of caesarean section, the network meta‐analyses of Chen 2016 showed the same results as our review. They found that a Foley catheter may slightly increase the risk of a caesarean section when compared to vaginal or oral misoprostol, with moderate heterogeneity for the comparison with vaginal misoprostol.
Alfirevic 2016 performed a extensive systematic review on induction of labour. The authors included 34 active treatment types/regimens including different dose regimens and routes of administration, and performed a network meta‐analysis in which all different treatments were ranked in relation to each other, including direct as well as indirect comparisons. Ranking was done on absolute risks for all pre specified outcomes. Mechanical induction with a balloon was divided in a single or double balloon. Alfirevic 2016 used other cut‐off points in dividing oral and vaginal tablets in dose regimens. In our review low dose was defined as ≤ 50 mcg every ≥ four hours, opposed to the cut‐of point of ≥ 50 mcg in the review of Alfirevic 2016. Vaginal PGE2 was divided into tablets, gel, slow‐release and normal‐release inserts. For the outcome of a vaginal delivery not achieved within 24 hours, low‐dose vaginal misoprostol scored better, as well as all different regimens of vaginal PGE2 compared to induction with a balloon (single as well as double). For the outcome caesarean section, a single balloon and vaginal PGE2 gel had a similar mean ranking in the mid regions. Noteworthy is that low‐dose titrated oral misoprostol had one of the lowest mean rankings, as compared to oral misoprostol < 50 mcg, which was ranked relatively high. The same high ranking for this outcome was seen for a double balloon. In line with our review, all mechanical methods had a low ranking regarding uterine hyperstimulation with FHR changes. Alfirevic 2016 also looked at neonatal and maternal mortality and severe morbidity, but for these composite outcomes no network meta‐analysis was possible as events were rare and poorly reported in studies. For the outcomes of NICU‐admission as well as five‐minute Apgar score less than seven, there was considerable uncertainty on the probability of the mean ranking as the 95% confidence intervals (CIs) for these rankings were relatively broad.
Ten Eikelder 2016 looked at safety outcomes between induction of labour with a Foley catheter and misoprostol (any route, any dose) and found less uterine hyperstimulation with FHR changes and less fetal distress for which a caesarean section was indicated when a Foley was used. They found that a Foley catheter may slightly increase the caesarean section rate, although conventional statistical significance was not reached and there was moderate heterogeneity for this outcome. Studies with high‐dose misoprostol were not excluded in the review of Ten Eikelder 2016. In subgroup analyses for 25 mcg and 50 mcg vaginal misoprostol, no evidence for a difference in safety outcomes were found.
In our review, there was no evidence for a difference in outcomes related to infection between mechanical induction and other methods for induction of labour. However, the results of outcomes covering infection were still too imprecise to make a valid judgement. McMaster 2015 addressed this question by comparing induction of labour with a balloon versus locally‐applied prostaglandin and included 26 trials. Their results were in line with our results and found no evidence for a difference in chorioamnionitis, endometritis and neonatal infection. When infection outcomes were pooled, little or no difference was seen, suggesting a Foley catheter does not increase the risk of infection compared to locally‐applied prostaglandin.
Authors' conclusions
Implications for practice.
Mechanical induction with a balloon is probably as effective as induction of labour with vaginal PGE2 with little or no difference in vaginal deliveries not achieved within 24 hours and caesarean section rate between the two methods. However, a balloon seems to have a more favourable safety profile compared to vaginal PGE2, as it probably reduces the risk of uterine hyperstimulation with and without fetal heart rate (FHR) changes, fetal distress for which a caesarean section is indicated and serious neonatal morbidity or perinatal death.
A balloon catheter may be less effective for induction of labour when compared to low‐dose oral misoprostol as a balloon probably increases the risk of a vaginal delivery not achieved within 24 hours and probably slightly increases the risk of a caesarean section. It is unclear if there is a difference in hyperstimulation with FHR changes. When compared to low‐dose vaginal misoprostol, a balloon catheter may increase the risk of a caesarean section but probably reduces the risk of hyperstimulation, with and without FHR change as well as the risk of meconium‐stained liquor.
Cervical ripening with a balloon seems to be more effective than induction with oxytocin as it probably reduces the risk of caesarean section and the risk of fetal distress. For women with a previous caesarean section, a balloon catheter may slightly reduce the risk of a caesarean section when compared to oxytocin.
There is no evidence of a benefit of a double balloon over a single balloon. For the comparisons of a laminaria tent or extra‐amniotic space infusion (EASI) with other induction methods, results were mostly too imprecise to make a valid judgement.
There was no evidence of clear benefit for a mechanical method combined with PGE2 to PGE2 alone or to oxytocin. When a mechanical method is combined with misoprostol or with oxytocin, it may reduce the risk of neonatal intensive care unit (NICU) admissions when compared to misoprostol alone. However, regarding other perinatal outcomes for both comparisons, there was no evidence for a difference in serious neonatal morbidity or perinatal death, Apgar scores less than seven at five minutes or fetal distress.
The advantages of mechanical methods are their wide availability and the low cost of the devices, especially Foley catheters. Storage and preservation of mechanical devices is less problematic than PGE2, which should be kept refrigerated. However, special attention should be paid to contraindications (e.g. low‐lying placenta) when inserting these devices.
Implications for research.
There seems to be sufficient data to make a valid judgement on the safety and effectiveness of balloon in comparison to vaginal PGE2. More research on this comparison does not seem warranted as moderate‐quality evidence suggests a balloon is equally effective, but has a better safety profile. GRADE assessment for important outcomes for this comparison can never be assessed as 'high quality' because blinding is not possible and this is the reason the evidence being downgraded from high‐quality evidence to moderate‐quality evidence for key outcomes. Future research could focus on comparing a balloon with low‐dose misoprostol or a combination of mechanical methods with low‐dose misoprostol. More studies evaluating mechanical methods for induction of labour in women with a history of prior caesarean section could be of benefit.
To facilitate future meta‐analyses of labour induction, we recommend the standardisation of outcomes through core outcome sets. This would minimise the reporting challenges experienced in this review, where many included studies reported outcomes in a highly varied manner, resulting in many being excluded from analyses. Also, while there were many large randomised trials included in this review, only a few reported on rare but serious adverse events or included women's views regarding induction methods. As safety aspects and maternal satisfaction become more and more important with rising induction rates, large multicentre studies focusing on safety aspects for the neonate and maternal satisfaction, could help clinicians make a more carefully balanced choice when arranging an induction of labour.
What's new
| Date | Event | Description |
|---|---|---|
| 9 January 2018 | New citation required and conclusions have changed | In this updated review, there is now evidence that mechanical induction of labour with a balloon probably is as effective as vaginal prostaglandin E2 (PGE2), but safer for the neonate. A balloon catheter may be slightly less effective as oral misoprostol, but It remains unclear if there is a difference in safety outcomes for the neonate. When compared to low‐dose vaginal misoprostol, a balloon catheter may be less effective, but probably has a better safety profile. |
| 9 January 2018 | New search has been performed | Search updated. We included 60 new studies and excluded 74 new studies. Eighteen studies (previous included) are now excluded as they are no longer eligible. Also, 21 ongoing studies were identified (Ongoing studies) and two studies are awaiting further classification (Agboghoroma 2015; Mallah 2011). We updated the search on 19 March 2019 and added a further 38 trial reports to Studies awaiting classification for the next update. The references have been assessed but not incorporated into the review. Only seven of these trials are likely to contribute data for this review and are mainly small trials (Khatib 2019; Lim 2018; Osoti 2018; Souizi 2018; ten Eikelder 2017; Tulek 2018; Viteri 2019). We imputed the data for these trials and there is no change in results (not in direction or strength of the evidence). We will incorporate these trials fully at the next update. For this review, studies with high‐dose misoprostol were excluded. Balloons, laminaria tents and extra‐amniotic space infusion (EASI) were compared separately with other pharmacological methods and new comparisons were included. Comparisons with no intervention or placebo were excluded. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 30 September 2011 | New citation required and conclusions have changed | New trials were added and the review was edited accordingly: the conclusion on primary outcome delivery before 24 hours has changed, partly due to change in statistical method (random‐effects model, due to substantial heterogeneity). Also, some conclusions on secondary outcome measures have changed. We updated the search on 16 January 2012 and added the results to Studies awaiting classification for consideration in the next update. |
| 30 April 2011 | New search has been performed | Search updated. We have included 27 new studies and excluded 28 new studies. One trial (previously included) has now been reclassified as excluded (Abramovici 1999). Four new ongoing studies have also been identified (Hallak 2008a; Jozwiak 2009a; Lin 2006a; Manyonda 2007a). |
| 18 September 2008 | Amended | Converted to new review format. |
Acknowledgements
This review was originally conducted by Prof MJNC Keirse. We would like to thank Cornelia Lohse and Catalin Stan for their work on the Boulvain 2001 version of this review; and Benjamin Lamy, Pernille Lau and Aidan Tan for the translation of articles.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewer for her time and comments: Elaine Finucane, National University of Ireland Galway, Galway, Ireland.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIIHR, NHS or the Department of Health.
Appendices
Appendix 1. ICTRP and ClinicalTrials.gov ‐ search methods
ICTRP
Each line was run separately
foley AND induction
foley AND ripening
catheter AND induction
catheter AND ripening
balloon AND induction
balloon AND ripening
laminaria AND induction
laminaria AND ripening
lamicel AND induction
lamicel AND ripening
extraamniotic AND induction
extraamniotic AND ripening
dilapan AND induction
dilapan AND ripening
ClinicalTrials.gov
Advanced search
Interventional studies | cervical ripening | catheter
Interventional studies | induction of labor | catheter
Interventional studies | cervical ripening | balloon
Interventional studies | induction of labor | balloon
Interventional studies | cervical ripening | foley
Interventional studies | induction of labor | foley
Interventional studies | cervical ripening | mechanical
Interventional studies | induction of labor | mechanical
Interventional studies | cervical ripening | laminaria
Interventional studies | induction of labor | laminaria
Interventional studies | cervical ripening | lamicel
Interventional studies | induction of labor | lamicel
Interventional studies | cervical ripening | dilapan
Interventional studies | induction of labor | dilapan
Interventional studies | cervical ripening | extraamniotic
Interventional studies | induction of labor | extraamniotic
Data and analyses
Comparison 1. Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 7 | 1685 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.82, 1.26] |
| 2 Uterine hyperstimulation with FHR changes | 6 | 1966 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.67] |
| 3 Caesarean section | 28 | 6619 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 4 Serious neonatal morbidity/perinatal death | 8 | 2757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.25, 0.93] |
| 5 Serious maternal morbidity or death | 4 | 1481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.12] |
| 6 Oxytocin augmentation | 16 | 4828 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.35, 1.76] |
| 7 Uterine hyperstimulation without fetal heart rate changes | 15 | 2444 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.66] |
| 8 Uterine rupture | 2 | 1045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.12] |
| 9 Epidural analgesia | 8 | 2828 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.00, 1.29] |
| 10 Instrumental vaginal delivery | 16 | 4514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 11 Meconium‐stained liquor | 4 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.19] |
| 12 Apgar score < 7 at 5 minutes | 14 | 4271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.14] |
| 13 Neonatal intensive care unit admission | 12 | 3647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.04] |
| 14 Perinatal death | 5 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.27] |
| 15 Postpartum haemorrhage | 8 | 2215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.63, 1.06] |
| 16 Women not satisfied | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.97] |
| 17 Maternal fever during labour | 7 | 2362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.65, 1.17] |
| 18 Antibiotics during labour | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.89, 2.29] |
| 19 Chorioamnionitis | 1 | 376 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.49] |
| 20 Endometritis | 2 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.19, 1.27] |
| 21 Fetal distress | 20 | 4753 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.60, 0.83] |
| 22 Umbilical artery pH < 7.10 | 8 | 2675 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.44, 0.94] |
Comparison 2. Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.23] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.85] |
| 3 Caesarean section | 5 | 828 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.33] |
| 4 Serious neonatal morbidity/perinatal death | 1 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 4.24] |
| 5 Serious maternal morbidity or death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Balloon (Foley or ATAD) versus vaginal prostaglandin E2: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.38 [1.74, 10.98] |
| 2 Caesarean section | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.65, 2.63] |
Comparison 4. Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.35, 2.91] |
| 2 Uterine hyperstimulation with FHR changes | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.90] |
| 3 Caesarean section | 9 | 1309 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.15] |
| 4 Serious neonatal morbidity/perinatal death | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.05] |
| 5 Cervix unfavourable/unchanged after 24 hours | 2 | 219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.70, 1.34] |
| 6 Oxytocin augmentation | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.26] |
| 7 Uterine hyperstimulation without FHR changes | 5 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.09, 10.38] |
| 8 Epidural analgesia | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 9 Instrumental vaginal delivery | 3 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.68, 2.05] |
| 10 Meconium‐stained liquor | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.42, 3.26] |
| 11 Apgar score < 7 at 5 minutes | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.41, 1.53] |
| 12 Neonatal intensive care unit admission | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.60, 1.31] |
| 13 Perinatal death | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.29, 2.05] |
| 14 Maternal side effects | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.24] |
| 15 Postpartum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 16 Chorioamnionitis | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.75] |
| 17 Endometritis | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.61] |
| 18 Fetal distress | 6 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.89] |
Comparison 5. Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section | 3 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.86, 1.95] |
Comparison 6. Balloon (Foley or ATAD) versus intracervical prostaglandin E2: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.02] |
| 2 Caesarean section | 3 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.16, 2.78] |
Comparison 7. Balloon (Foley or ATAD) versus low dose vaginal misoprostol: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 340 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.39] |
| 2 Uterine hyperstimulation with FHR changes | 8 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.18, 0.85] |
| 3 Caesarean section | 12 | 1756 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 4 Serious neonatal morbidity/perinatal death | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.12, 2.66] |
| 5 Serious maternal morbidity or death | 4 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 hours | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [0.60, 11.89] |
| 7 Oxytocin augmentation | 9 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.38, 1.90] |
| 8 Uterine hyperstimulation without FHR changes | 9 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.14, 0.44] |
| 9 Uterine rupture | 3 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 2 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.06, 1.41] |
| 11 Instrumental vaginal delivery | 4 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 12 Meconium‐stained liquor | 7 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.48, 0.87] |
| 13 Apgar score < 7 at 5 minutes | 7 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.50, 1.97] |
| 14 Neonatal intensive care unit admission | 9 | 1302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.61, 1.63] |
| 15 Perinatal death | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal vomiting | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Postpartum haemorrhage | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.24, 5.44] |
| 18 Maternal fever during labour | 3 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 1.84 [0.22, 15.62] |
| 19 Chorioamnionitis | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.31, 4.88] |
| 20 Endometritis | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.72] |
| 21 Fetal distress | 7 | 1127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.05] |
| 22 Umbilical artery pH <7.10 | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.35, 3.74] |
Comparison 8. Balloon (Foley or ATAD versus low dose vaginal misoprostol: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
Comparison 9. Balloon (Foley or ATAD) versus low dose oral misoprostol: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.13, 1.46] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 2033 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.38] |
| 3 Caesarean section | 7 | 3178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.04, 1.32] |
| 4 Serious perinatal morbidity/perinatal death | 3 | 2627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.60, 2.06] |
| 5 Serious maternal morbidity or death | 3 | 2627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.52] |
| 6 Cervix unfavourable after 24 hours | 4 | 994 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.61, 1.56] |
| 7 Oxytocin augmentation | 5 | 2847 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.09, 1.49] |
| 8 Uterine hyperstimulation without FHR changes | 5 | 2838 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.12, 2.07] |
| 9 Uterine rupture | 3 | 2627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural | 3 | 2635 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.96, 1.22] |
| 11 Instrumental vaginal delivery | 3 | 2627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.55, 0.92] |
| 12 Meconium‐stained liquor | 3 | 2627 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.44, 1.35] |
| 13 Apgar score < 7 after 5 minutes | 4 | 2693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.32] |
| 14 Neonatal intensive care unit admission | 5 | 2873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.58, 1.17] |
| 15 Neonatal encephalopathy | 1 | 600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.32, 2.03] |
| 16 Perinatal death | 3 | 2627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.49, 3.30] |
| 17 Maternal side effects (all) | 2 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.33, 1.13] |
| 18 Maternal vomiting | 2 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.46] |
| 19 Maternal diarrhoea | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.37] |
| 20 Postpartum haemorrhage | 5 | 2966 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.34] |
| 21 Maternal death | 3 | 2627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Women not satisfied | 1 | 602 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.15, 2.50] |
| 23 Maternal fever during labour | 2 | 2033 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.24] |
| 24 Antibiotics during labour | 2 | 2033 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.75, 2.00] |
| 25 Endometritis | 1 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 6.03] |
| 26 Fetal distress | 5 | 2966 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.61, 1.09] |
| 27 Umbilical artery pH < 7.10 | 2 | 1535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.12] |
Comparison 10. Balloon (Foley or ATAD) versus low dose oral misoprostol: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.04, 1.37] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 1206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.45, 1.46] |
| 3 Caesarean section | 3 | 1778 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.06, 1.38] |
| 4 Serious neonatal morbidity/perinatal death | 2 | 1296 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.77, 26.14] |
| 5 Serious maternal morbidity or death | 2 | 1296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.63] |
Comparison 11. Balloon (Foley or ATAD) versus low dose oral misoprostol: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.17, 2.06] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 639 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.24, 8.61] |
| 3 Caesarean section | 3 | 848 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.79, 1.87] |
| 4 Serious neonatal morbidity/perinatal death | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.86] |
| 5 Serious maternal morbidity or death | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Balloon (Foley or ATAD) versus oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.11] |
| 2 Caesarean section | 8 | 781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.83] |
| 3 Serious neonatal morbidity/perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious maternal morbidity or death | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cervix unfavourable after 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.20, 1.54] |
| 6 Uterine hyperstimulation without FHR changes | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.23, 4.29] |
| 7 Uterine rupture | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Instrumental vaginal delivery | 3 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.55, 2.57] |
| 9 Meconium‐stained liquor | 2 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.23, 1.21] |
| 10 Apgar score < 7 at 5 minutes | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.53] |
| 11 Neonatal intensive care unit admission | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.32, 1.98] |
| 12 Perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Hemorrhagia postpartum | 4 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.51, 3.11] |
| 14 Maternal fever during labour | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.00] |
| 15 Fetal distress | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
Comparison 13. Balloon (Foley or ATAD) versus oxytocin: previous caesarean section.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 3 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 1.00] |
| 2 Serious neonatal morbidity/perinatal death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious maternal morbidity or death | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Balloon (Foley or ATAD) versus oxytocin: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.50] |
| 2 Serious maternal morbidity or death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Balloon (foley or ATAD) versus amniotomy: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
Comparison 16. Single balloon (Foley) versus double balloon (ATAD/Cook): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 3 | 608 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.25] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 5 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.33] |
| 4 Serious maternal morbidity or death | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Oxytcocin augmentation | 2 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.08] |
| 6 Uterine hyperstimulation without FHR changes | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Uterine rupture | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Epidural analgesia | 3 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.83, 1.03] |
| 9 Instrumental vaginal delivery | 3 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.61, 1.20] |
| 10 Meconium‐stained liquor | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.15, 1.04] |
| 11 Apgar score < 7 at 5 minutes | 3 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.25, 2.79] |
| 12 Neonatal intensive care unit admission | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.71, 3.93] |
| 13 Other maternal side‐effects: pain after insertion | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.17] |
| 14 Postpartum haemorrhage | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.27, 2.52] |
| 15 Maternal fever during labour | 3 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.16, 2.34] |
| 16 Antibiotics during labour | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.61, 1.56] |
| 17 Chorioamnionitis | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.47, 5.20] |
| 18 Endometritis | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 21.14] |
| 19 Fetal distress | 4 | 682 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.36] |
| 20 Umbilical artery pH < 7.10 | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.57] |
Comparison 17. Single balloon (Foley) versus double balloon (ATAD): all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.95, 1.38] |
| 2 Caesarean section | 4 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.76, 2.22] |
Comparison 18. Single balloon (Foley) versus double balloon (ATAD): all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.80, 1.93] |
| 2 Caesarean section | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.30, 1.84] |
Comparison 19. Laminaria tent versus vaginal prostaglandin E2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 3 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.60] |
| 2 Caesarean section | 5 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.56, 1.48] |
| 3 Serious perinatal morbidity/perinatal death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious maternal morbidity or death | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Uterine hyperstimulation without fetal heart rate changes | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.09, 0.49] |
| 6 Epidural analgesia | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.74, 1.13] |
| 7 Instrumental vaginal delivery | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.17] |
| 8 Meconium‐stained liquor | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 9 Apgar score < 7 at 5 minutes | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Perinatal death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects: all | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| 12 Maternal nausea | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.60] |
| 13 Fetal distress | 3 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.15] |
Comparison 20. Laminaria tent versus vaginal prostaglandin E2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 2 Caesarean section | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.24, 4.89] |
Comparison 21. Laminaria tent versus vaginal prostaglandin E2: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.06, 3.91] |
Comparison 22. Laminaria tent versus intracervical prostaglandin E2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.42] |
| 2 Caesarean section | 5 | 920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.93, 1.45] |
| 3 Serious neonatal morbidity/perinatal death | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.13, 76.70] |
| 4 Serious maternal morbidity or death | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.52] |
| 5 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.11, 1.96] |
| 6 Oxytocin augmentation | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.21, 1.64] |
| 7 Uterine hyperstimulation without FHR changes | 2 | 601 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.36] |
| 8 Uterine rupture | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.52] |
| 9 Instrumental vaginal delivery | 3 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.69] |
| 10 Apgar score < 7 at 5 minutes | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.28 [0.63, 44.30] |
| 11 Neonatal intensive care unit admission | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.58, 4.33] |
| 12 Perinatal death | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.13, 76.70] |
| 13 Maternal side effects | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.15] |
| 14 Postpartum haemorrhage | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.46, 2.81] |
| 15 Chorioamnionitis | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.17 [0.35, 29.06] |
| 16 Endometritis | 2 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.08, 1.09] |
| 17 Fetal distress | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.07, 2.90] |
Comparison 23. Laminaria tent versus intracervical prostaglandin E2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.62, 2.13] |
Comparison 24. Laminaria tent versus intracervical: prostaglandin E2 all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.45, 3.65] |
Comparison 25. Laminaria tent versus oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.89] |
| 2 Fetal distress | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.11, 63.18] |
Comparison 26. Laminaria tent versus amniotomy: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.52] |
Comparison 27. Laminaria tent versus other hygroscopic dilator: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.44, 6.66] |
Comparison 28. EASI versus vaginal prostaglandin E2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.21, 2.49] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 2.07] |
| 3 Caesarean section | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.94, 1.96] |
| 4 Oxytocin augmentation | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.71 [3.20, 50.57] |
| 5 Uterine hyperstimulation without fetal heart rate changes | 2 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 2.07] |
| 6 Epidural analgesia | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.04] |
| 7 Instrumental vaginal delivery | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.14] |
| 8 Meconium‐stained liquor | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.10] |
| 9 Apgar score < 7 at 5 minutes | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [0.21, 86.51] |
| 10 Neonatal intensive care unit admission | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.45, 5.03] |
| 11 Woman not satisfied | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.10, 3.25] |
| 12 Fetal distress | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.39, 3.71] |
Comparison 29. EASI versus intracervical prostaglandin E2: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.10, 5.12] |
| 2 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.97] |
| 3 Oxytocin augmentation | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.54, 2.25] |
| 4 Instrumental vaginal delivery | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.01] |
| 5 Apgar score < 7 at 5 minutes | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Endometritis | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Fetal distress | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.28] |
Comparison 30. EASI versus intracervical prostaglandin E2: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.09] |
Comparison 31. Any mechanical method and prostaglandin E2 versus prostaglandin E2 alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 5.12] |
| 3 Caesarean section | 7 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.66, 1.40] |
| 4 Cervix unfavourable/unchanged after 24 hours | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.31, 0.85] |
| 5 Oxytocin augmentation | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.64, 1.41] |
| 6 Uterine hyperstimulation without FHR changes | 3 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Epidural analgesia | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| 8 Instrumental vaginal delivery | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.45] |
| 9 Meconium‐stained liquor | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.33, 2.83] |
| 10 Neonatal intensive care unit admission | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.01, 5.12] |
| 11 Postpartum haemorrhage | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Chorioamnionitis | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.45, 5.45] |
| 13 Endometritis | 3 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.41, 2.78] |
| 14 Fetal distress | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.28 [0.54, 9.69] |
Comparison 32. Any mechanical method and prostaglandin E2 versus low dose misoprostol alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.82] |
| 2 Caesarean section | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.58, 2.04] |
| 3 Serious neonatal morbidity/perinatal death | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.90] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.25, 0.67] |
| 5 Oxytocin augmentation | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.01, 1.46] |
| 6 Uterine hyperstimulation without FHR changes | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.05 [1.44, 11.38] |
| 7 Instrumental vaginal delivery | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.77, 2.04] |
| 8 Meconium‐stained liquor | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.23, 1.32] |
| 9 Apgar score < 7 at 5 minutes | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.18, 20.51] |
| 10 Neonatal intensive care unit admission | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.31, 1.31] |
| 11 Perinatal death | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.90] |
| 12 Chorioamnionitis | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.18, 20.51] |
| 13 Endometritis | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.36, 10.05] |
Comparison 33. Any mechanical method and prostaglandin E2 versus oxytocin alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.04, 2.47] |
| 2 Instrumental vaginal delivery | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.12, 2.94] |
| 3 Endometritis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.15, 83.14] |
Comparison 34. Any mechanical method and low dose misoprostol versus prostaglandin E2 alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.89, 1.46] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.27, 2.13] |
| 3 Caesarean section | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.57, 1.25] |
| 4 Serious neonatal morbidity/perinatal death | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.19, 22.24] |
| 5 Serious maternal morbidity or death | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Oxytocin augmentation | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.34, 0.86] |
| 7 Uterine hyperstimulation without fetal heart rate changes | 1 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.32] |
| 8 Uterine rupture | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Instrumental vaginal delivery | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.98] |
| 10 Meconium‐stained liquor | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.60, 2.23] |
| 11 Apgar score < 7 at 5 minutes | 1 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.25, 1.88] |
| 12 Neonatal intensive care unit admission | 1 | 346 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 4.03] |
| 13 Perinatal death | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.06, 16.14] |
| 14 Maternal side effects | 1 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.95, 1.43] |
| 15 Maternal nausea | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.98, 2.79] |
| 16 Maternal diarrhoea | 1 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.72 [1.53, 9.00] |
| 17 Postpartum haemorrhage | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.41] |
| 18 Serious maternal complications | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Maternal fever during labour | 1 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.26, 9.02] |
Comparison 35. Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.25, 1.95] |
| 2 Uterine hyperstimulation with FHR changes | 4 | 707 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.20, 1.45] |
| 3 Caesarean section | 7 | 1422 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.66, 1.15] |
| 4 Serious neonatal morbidity/perinatal death | 2 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.55] |
| 5 Serious maternal morbidity or death | 2 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 hours | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.08, 0.94] |
| 7 Oxytocin augmentation | 5 | 1051 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.70, 1.25] |
| 8 Uterine hyperstimulation without FHR changes | 4 | 982 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.32, 0.90] |
| 9 Uterine rupture | 2 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.10] |
| 11 Instrumental vaginal delivery | 3 | 676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.51] |
| 12 Meconium‐stained liquor | 6 | 1243 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 13 Apgar score < 7 at 5 minutes | 3 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.36] |
| 14 Neonatal intensive care unit admission | 6 | 1246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.36, 0.91] |
| 15 Perinatal death | 1 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 75.26] |
| 16 Maternal side effects | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.30] |
| 17 Maternal nausea | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.84, 2.23] |
| 18 Maternal diarrhoea | 1 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [1.40, 8.17] |
| 19 Postpartum haemorrhage | 2 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.33] |
| 20 Serious maternal complications | 1 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Chorioamnionitis | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.38] |
| 22 Endometrits | 2 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.08] |
| 23 Fetal distress | 4 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.53, 1.14] |
Comparison 36. Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.23, 2.96] |
| 2 Caesarean section | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.51] |
Comparison 37. Any mechanical method and low dose misoprostol versus low dose misoprostol alone: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.63] |
| 2 Caesarean section | 1 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.18, 0.68] |
Comparison 38. Any mechanical method and oxytocin versus prostaglandin E2 alone: all women (not pre‐specified).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.55, 3.95] |
| 2 Caesarean section | 4 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.20] |
| 3 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Oxytocin augmentation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.95, 3.15] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.39, 3.46] |
| 6 Instrumental vaginal delivery | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.08, 1.58] |
| 7 Meconium‐stained liquor | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 2.95] |
| 8 Apgar score < 7 at 5 minutes | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.12, 71.55] |
| 9 Neonatal intensive care unit admission | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.30, 2.40] |
| 10 Postpartum haemorrhage | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.68] |
| 11 Endometritis | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Fetal distress | 3 | 498 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.61, 1.56] |
Comparison 39. Any mechanical method and oxytocin versus low dose misoprostol alone: all women (not pre‐specified).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.37, 0.63] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.11] |
| 3 Caesarean section | 5 | 1779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.12] |
| 4 Serious neonatal morbidity/perinatal death | 2 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.18, 3.65] |
| 5 Oxytocin augmentation | 2 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 3.89 [0.70, 21.72] |
| 6 Uterine hyperstimulation without FHR changes | 3 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.92] |
| 7 Epidural analgesia | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.27] |
| 8 Meconium‐stained liquor | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.43, 1.19] |
| 9 Apgar score < 7 at 5 minutes | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.20, 4.58] |
| 10 Neonatal intensive care unit admission | 4 | 1599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.49, 0.90] |
| 11 Perinatal death | 2 | 1263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.18, 3.65] |
| 12 Women not satisfied | 1 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.47, 1.93] |
| 13 Maternal fever | 2 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.50] |
| 14 Chorioamnionitis | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.31] |
| 15 Fetal distress | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.21] |
Comparison 40. Any mechanical method and oxytocin versus low dose misoprostol alone: all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.19, 1.11] |
Comparison 41. Any mechanical method and oxytocin versus oxytocin alone: all women (not pre‐specified).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 2 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.21, 2.40] |
| 2 Caesarean section | 6 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.39, 1.20] |
| 3 Serious neonatal morbidity/perinatal death | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.12, 4.13] |
| 4 Serious maternal morbidity or death | 2 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Uterine hyperstimulation without FHR changes | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.34, 2.09] |
| 6 Uterine rupture | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Epidural analgesia | 1 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.98, 1.09] |
| 8 Instrumental vaginal delivery | 3 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.48, 2.02] |
| 9 Meconium‐stained liquor | 3 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.32, 1.63] |
| 10 Neonatal intensive care unit admission | 3 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.61, 1.58] |
| 11 Postpartum haemorrhage | 3 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.44, 3.18] |
| 12 Serious maternal complications | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Antibiotics during labour | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.82, 6.55] |
| 14 Chorionamnionitis | 2 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 4.34 [0.55, 34.01] |
| 15 Endometritis | 3 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.16, 7.45] |
| 16 Fetal distress | 3 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.68, 2.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aduloju 2016.
| Methods | RCT | |
| Participants | Inclusion: life singleton pregnancy, cephalic presentation, intact membranes, > 37 weeks, indication for IOL, reactive non stress test. unfavourable cervix (BS < 6) Exclusion: IFD, no prenatal care in study centre, contraindication for vaginal delivery |
|
| Interventions | A: Foley catheter: 16F, 30 mL (n = 70), max 12 hours, if necessary another Foley for 12 hours (n = 70) B: Foley catheter (16F, 30 cc) + vaginal misoprostol 25 ug every 6 hours(n = 70), max dose 100 ug (4 gifts) (n = 70) C: Vaginal misoprostol alone 25 ug every 6 hours (n = 70) max dose 100 ug (4 gifts) (n = 70) Max induction time all groups: 24 hours |
|
| Outcomes | Vaginal delivery rate, time interval to achieve favourable cervix, induction delivery interval, oxytocin use, AS at 1 and 5 minutes, asphyxia, NICU admission, uterine tachysystole, uterine hypertonus, hyperstimulation, uterine rupture, FHR abnormalities | |
| Notes | Setting: Ekiti State University Teaching tertiary healthcare institution; referral centre for primary and secondary healthcare facilities, 2400 deliveries annually, Nigeria Study period: 1 September, 2014 and 31 August, 2015. Funding: no grant or fund was received Declaration of interest: no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation using random table computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not mentioned, but is probably the case looking at Figure 1. no missing data or cases. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures were reported, |
| Other bias | Low risk | No other bias detected |
Ahmed 2016.
| Methods | RCT | |
| Participants | Inclusion: postdate pregnancy (> 40 weeks) singleton gestation, intact membranes, cephalic fetal BS ≤ 4 Exclusion: previous caesarean deliveries, EFW > 4000 g, non‐reassuring fetal conditions, ruptured membranes, placenta previa, malpresentation |
|
| Interventions | Foley catheter (n = 39), 18 F, filled with 50 mL Cook balloon (n = 39), filled with 80/80 mL Max of 12 hours of priming |
|
| Outcomes | Cervical ripening and BS after 12 hours, VAS for catheter insertion, catheter insertion (easy, moderate or difficult), VAS for patient satisfaction after birth, insertion expulsion time, insertion amniotomy time, insertion delivery time and mode of delivery. Abnormal fetal presentation, cord prolapse, bleeding related to catheter insertion that required removal of the catheter and AS | |
| Notes | Setting: Gynaecology, Suez Canal University
Hospital, Egypt Study period: March 2013 to April 2014 Funding: not mentioned Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling 78 envelopes, 1:1 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, opaque? |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not mentioned, 2 women excluded because of failed placement. no missing data mentioned |
| Selective reporting (reporting bias) | High risk | Pre‐specified outcomes bleeding after insertion, cord prolapse and abnormal fetal presentation not described in results |
| Other bias | Low risk | No other bias detected |
Al‐Ibraheemi 2018.
| Methods | RCT | |
| Participants | Inclusion: > 37 weeks, singleton fetus, cephalic, BS ≤ 6 Exclusion: rupture of membranes, regular uterine contractions (3 or more contractions per 10 minutes), prior uterine surgery, multiple gestations, malpresentation, contraindication to PGs, non‐reassuring FHR tracing, vaginal bleeding, fetal demise, anomalous fetus, or any contraindication to vaginal delivery |
|
| Interventions | Foley catheter + misoprostol: (n = 100) 30 mL balloon, filled with 60 mL, gentle traction, max 24 hours and misoprostol vaginal 4‐hourly with a max of 6 doses, Misoprostol (n = 100) 25 ug vaginally, 4‐hourly with a max of 6 doses |
|
| Outcomes | Time from placement of the first misoprostol dose to delivery, time to active phase (6 cm or greater), time from active phase to delivery, caesarean delivery rate, uterine tachysystole, estimated blood loss, chorioamnionitis, cord blood pH, 5‐minute AS, NICU admission. | |
| Notes | Setting: from the Department of Obstetrics and Gynecology, Mount Sinai West Hospital, New York Study period: September 2015 to July 2016 Funding: not described Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Al‐Taani 2004.
| Methods | Randomised trial, random number table, blinding unclear. | |
| Participants | Grand multiparous women, BS 5 or less, singleton term pregnancy, intact membranes, cephalic presentation, good fetal condition. Exclusion: previous CS, contraindications for vaginal birth, suspected cephalopelvic disproportion, unexplained antenatal haemorrhage. |
|
| Interventions | Foley catheter 50 mL (72). (PGE2) tablet 3 mg (75), 6‐hourly. |
|
| Outcomes | Route of delivery, change in BS, intrapartum complications, need for augmentation. | |
| Notes | Setting: Queen Alia military hospital, Amman, Jordan Dates of study:September 2001 ‐ August 2003 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT nor reported, bu seems reasonable as the numbers in both groups are equal to randomised numbers. missing outcome data mentioned. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes are reported as pre‐specified. |
| Other bias | Low risk | No other bias detected |
Allouche 1993.
| Methods | RCT. No details were given on the method for concealment of the allocation. | |
| Participants | BS < 6, vertex, singleton, no previous CS. | |
| Interventions | PGE2 (Prepidil 0.5 mg) intracervical (59 women); Foley 50 mL (60 women); PGE2 (Prepidil 0.5 mg) and Foley 50 mL extra‐amniotic (63 women). | |
| Outcomes | Uterine hyperstimulation, discomfort during the procedure, maternal and neonatal infection. | |
| Notes | Setting: not reported Dates of study:between April and December 1992 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated women were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, no missing data or cases reported |
| Selective reporting (reporting bias) | Unclear risk | No outcomes pre‐specified |
| Other bias | Low risk | No other bias detected |
Amorosa 2017.
| Methods | RCT | |
| Participants | Inclusion: PROM, age ≥ 18 years, viable fetus, cephalic, singleton, GA > 34 weeks, < 3 cm dilation Exclusion: multifetal gestation, a known anomalous fetus, malpresentation, latex allergy, unexplained vaginal bleeding or contraindication to vaginal delivery (such as a placenta previa), antibiotics, previous uterine surgery, spontaneous labour |
|
| Interventions | Foley catheter (n = 61) 16F, 30 mL balloon filled with 60 mL saline, traction applied (no max time described), oxytocin started after 1 hour Oxytocin alone directly (n = 68) Oxytocin in both groups, started 2 mU/minute, max 30 mU/minute |
|
| Outcomes | The primary outcome measure was time from start of induction to delivery. Secondary outcomes included mode of delivery, tachysystole, chorioamnionitis, postpartum haemorrhage, neonatal AS, and admission to the NICU. | |
| Notes | Setting: Mount Sinai Hospital, New York, USA Study period: August 2014 to September 2016 Funding: not reported Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Sequential numbered sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Modified ITT, (1 woman excluded who afterwards did not met the inclusion criteria), no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures were reported in results |
| Other bias | Low risk | No other bias detected |
Atad 1996.
| Methods | RCT. Computer‐generated sequence. No details were given on the method for concealment of the allocation. | |
| Participants | Singleton vertex term pregnancies with intact membranes, BS < 5 without previous CS. | |
| Interventions | Atad ripening device (35 women); PGE2 intravaginal tablets 3 mg (30 women); oxytocin (30 women). | |
| Outcomes | Need for another method, CS, change in BS. | |
| Notes | Also reported as abstract (Abramovici 1994). Setting: Israel Study period: not reported Funding: not reported Declarations of interest: J Atad has a patent licensing arrangement for Atad ripening device and thus has the potential gain from its sales |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random‐generated allocation list |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT reported, but seems reasonable as numbers in tables are equal to randomised numbers, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Bagratee 1990.
| Methods | RCT. Random number tables, stratified for parity. No details were given on the method for concealment of the allocation. | |
| Participants | Women with unfavourable cervix (BS < 7). | |
| Interventions | Lamicel (40 women); (PGE2) (2 mg tablet) (40 women). After 6 hours, oxytocin was started in both groups. | |
| Outcomes | CS, hyperstimulation, fetal distress, perinatal death. | |
| Notes | No outcome reported in subgroups. setting: king Edward VIII hospital, Durban, South Africa Study period: for 6 months, no exact dates reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables |
| Allocation concealment (selection bias) | Unclear risk | Unclear, not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT mentioned, in table 4 cases missing, not clear why. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Barda 2018.
| Methods | RCT | |
| Participants | Inclusion criteria: GA ≥ 37 weeks, parity 1 to 3, singleton pregnancy with a vertex presentation, BS less than 5) and intact membranes. Exclusion criteria: previous CS, lack of prenatal care, contraindication for vaginal delivery |
|
| Interventions | Foley catheter (n = 150): 22 F, 80 mL balloon (max 18 hours) Dinoproston (n = 150): 3 mg tablets (every 8 hours, max 2 gifts) |
|
| Outcomes | Start induction to active labour (4 cm dilatation and 80% effacement), labour within 24 hours, CS rate, excessive haemorrhage, chorioamnionitis, non‐reassuring FHR, fetal pH, NICU admission, early neonatal sepsis | |
| Notes | Setting: Edith Wolfson Medical Centre, Holon, Israel Study period: June 2015 ‐ July 2016 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assigned; not described how this was done. In trial registration => parallel assignment. |
| Allocation concealment (selection bias) | Unclear risk | Random assigned; not described how this was done. In trial registration => parallel assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT, no tables available, so incomplete data can not be judged, no missing data described |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Unclear risk | Full text is an accepted manuscript without tables, therefore risk of bias cannot properly be determined |
Benzineb 1996.
| Methods | RCT. Blocks of 10 women. No other details were given on the method for randomisation and on concealment of the allocation. | |
| Participants | Singleton vertex term pregnancies with intact membranes, BS < 6. | |
| Interventions | Foley catheter inflated with 40 mL of water (50 women); PGE2 intracervical gel 0.5 mg every 24 hours. (n = 50?) | |
| Outcomes | Vaginal delivery not achieved within 24 hours, CS, perinatal deaths, cervix unchanged after 24 hours, postpartum haemorrhage. | |
| Notes | Setting: Charles Nicolle Hospital, Tunis Dates of study: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were random allocated in blocks of 10 |
| Allocation concealment (selection bias) | Unclear risk | Unclear. not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, no missing data or cases reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not pre‐specified |
| Other bias | Low risk | No other bias detected |
Biron‐Shental 2004.
| Methods | Randomised trial. | |
| Participants | Term, singleton pregnancy BS 4 or less, medical indication for labour induction. | |
| Interventions | PGE 2 gel 2 mg (27). Double balloon catheter (26) combined (24) |
|
| Outcomes | Change in BS, need for oxytocin augmentation. | |
| Notes | Outcomes of interest not reported. Setting: Israel Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Brief communication, for the reported data no missing. Incomplete outcome data not further mentioned in this report. |
| Selective reporting (reporting bias) | Unclear risk | Our outcomes of interest are not reported here, main outcome is change in BS. |
| Other bias | Unclear risk | Hard to say, very short report, our outcomes of interest not mentioned, not clear how sample size was calculated. |
Blumenthal 1990.
| Methods | RCT. Randomisation by drawing a blank envelope from a stack of 50 identical envelopes containing the group allocation. | |
| Participants | Vertex, intact membranes, BS < 5, no previous CS. | |
| Interventions | Dilapan (polyacrilonitrile hydrogel) inserted in the cervix, up to 6 sticks (23 women); Laminaria inserted in the cervix, as many as possible (18 women). | |
| Outcomes | CS. | |
| Notes | Results for a 3rd group of women with favourable cervix treated with oxytocin are presented. These women are not be included in the analysis, as they were not randomly allocated to the intervention. Setting: Michael Reese hospital, Chicago, USA Study period:January 1987 to January 1988 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Women choose from stack of all blank, identical envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not reported. no explanation what happened to the rest of the randomised women |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Browne 2011.
| Methods | RCT | |
| Participants | Inclusion criteria: single, live fetus, cephalic, presentation,reassuring fetal health assessment, GA between 26 and 42 weeks, Maternal age 18 and above, BS less than 5 Exclusion criteria: multiple gestation (twins, triplets, quadruplets), fetal demise Fetal malpresentation, EFW less than 500 g or more than 4000 g, placenta previa, non‐reassuring fetal health assessment Maternal asthma, Latex allergy, spontaneous labour, other contraindication to vaginal delivery |
|
| Interventions | Balloon: (n = 34): 40 mL, under traction, max 6 hours. PGE2 vaginal (36): prepidil gel in fornix posterior, no oxytocin in 6 hours after gel is applied balloon and PGE2 (31): 40 mL, under traction. prepidil gel inserted through catheter. |
|
| Outcomes | CS | |
| Notes | Grey literature: not published. primary outcomes reported in trial registration Setting: USA Study period: July 2010 ‐ February 2013 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation envelopes prepared by statisticians at the University of South Carolina Arnold School of Public Health. The investigator was given the next sequentially‐numbered study envelope by the patient's nurse. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not reported. case missing for relevant outcome (CS) |
| Selective reporting (reporting bias) | High risk | Only primary outcome en adverse events reported |
| Other bias | Unclear risk | Study not published, not clear why. |
Carbone 2013.
| Methods | RCT | |
| Participants | Inclusion: singleton, viable gestation (≥ 24 weeks), cephalic, intact membranes, BS < 7 Exclusion: malpresentation, multifetal gestation, spontaneous labour, contraindication to PGs, fetal growth restriction, anomalous fetus, fetal demise, previous CS, or other uterine surgery. |
|
| Interventions | Foley + misoprostol (n = 56): 25 mcg vaginal misoprostol every 4 hours AND Foley catheter filled with 60 mL saline; taped to the inner thigh under gentle traction. misoprostol alone (n = 61): 25 mcg vaginal misoprostol every 4 hours Not mentioned for how long misoprostol and/or Foley was given in total. |
|
| Outcomes | Induction to delivery time, mode of delivery, tachysystole, postpartum haemorrhage (> 500 cc), chorioamnionitis, neonatal AS and NICU admission. | |
| Notes | Setting: USA Study period: January 2011 to April 2012 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes, not stated if these were sequential numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Casey 1995.
| Methods | RCT. No details were given on the method for concealment of the allocation. | |
| Participants | Singleton term pregnancies, BS < 6. | |
| Interventions | PGE2 intracervical gel and intracervical Foley catheter inflated with 50 cc (78 women); PGE2 intracervical gel (68 women). | |
| Outcomes | CS. | |
| Notes | Abstract only. Setting: USA Study period: not reported, 11‐month period Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported it was a RCT |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Too little information to judge |
| Selective reporting (reporting bias) | Unclear risk | Too little information to judge |
| Other bias | Unclear risk | Abstract only |
Chavakula 2015.
| Methods | RCT | |
| Participants | Inclusion: singleton, cephalic, fetal growth restriction, ≥ 34 weeks. Exclusion: previous caesarean deliveries, uterine surgery, a multiple pregnancy, ruptured membranes, a BS > 6, severe fetal growth restriction, abnormal FHR prior to induction, pre‐partum haemorrhage. |
|
| Interventions | 1. Foley catheter (n = 54) 16F, 30 mL, catheter was removed after 12 hours. 2. Vaginal misoprostol (n = 46) every 6 hours, 25 mcg, max 3 doses |
|
| Outcomes | Hyperstimulation with FHR changes, BS at AROM, duration of induction to delivery, vaginal delivery within 12 hours and 24 hours, CS, oxytocin, chorioamnionitis, antibiotics, NICU admission, AS < 7 at 5 minutes, patients and caregiver satisfaction (VAS score) | |
| Notes | Setting: tertiary care teaching hospital in South India with approximately 13,000 deliveries per year. Study period:December 2011 to June 2012. Funding: not stated Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence, block randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Chua 1997.
| Methods | RCT. Random number table, no details on the method of concealment of the allocation. | |
| Participants | Singleton vertex presentation, unfavourable cervix (BS < 6). 185 women recruited (90 in Dilapan group, 95 in PGE2 group). | |
| Interventions | Dilapan group: 4 dilators. PGE2 Gel (Prepidil): 0.5 mg. In both groups, ripening was followed by rupture of membranes and oxytocin after 12 hours. | |
| Outcomes | Need for oxytocin, CS, instrumental delivery, uterine rupture, uterine hyperstimulation, admission to NICU, perinatal death. | |
| Notes | Setting: National University hospital, Singapore Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not mentioned but seems reasonable as numbers in tables are equal to randomised numbers, no reporting of missing cases or outcomes |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Cromi 2011.
| Methods | RCT | |
| Participants | Inclusion: singleton gestation, vertex presentation, BS ≤ 6, intact membranes, GA ≥ 34 weeks, reassuring FHR tracing Exclusion: Women with antepartum bleeding, intrauterine fetal death, previous uterine scars, known allergy to latex, placenta previa, contraindication to vaginal delivery |
|
| Interventions | 24‐hour Foley (n = 133): 18F, 50cc, 24 hours 12‐hour Foley (n = 132): 18F, 50cc, 12 hours PGE2 vaginal insert 10 mg (n = 132): vaginal fornix, 24 hours |
|
| Outcomes | vaginal delivery within 24 hours, improvement in BS after ripening, caesarean delivery, ripening‐to‐delivery interval, oxytocin administration, epidural request, neonatal outcomes. | |
| Notes | Setting:Obstetrics Department of University of Insubria, Varese, Italy. Study period: July 2008 to June 2010 Funding: none reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Allocation not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing data or cases. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Cromi 2012.
| Methods | RCT | |
| Participants | 210 patients. Inclusion: singleton gestation, vertex presentation, BS ≤ 6, intact membranes, GA > 34 weeks, and reassuring fetal heart tracing on admission. Exclusion:antepartum bleeding, intrauterine fetal death, prior uterine scars, positive vaginal or rectal group B streptococcus screening cultures, placenta previa, other contraindication to vaginal delivery. | |
| Interventions | Double‐balloon catheter (n = 105): inflated with 50 mL in either balloon. The double‐balloon device was left in place for approximately 12 hours. Dinoprostone vaginal insert 10‐mg controlled‐release (n = 105): in the vaginal fornix, max 24 hours | |
| Outcomes | Vaginal delivery within 24 hours, improvement in the BS after ripening, caesarean delivery rates, ripening‐to‐delivery interval, oxytocin administration, epidural request, NICU admission, AS < 7 at 5 minutes, umbilical artery pH < 7.00. | |
| Notes | Setting: University of In‐subria, Varese, Italy Study period: October 2010 to October 2011 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Concealment by keeping random allocation sequence in a file cabinet with access restricted to research staff? |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not an ITT analysis, women excluded after failed placement balloon or need for PGE2 gel after suppository expulsion. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in result |
| Other bias | Low risk | No other bias detected |
Culver 2004.
| Methods | RCT, computer‐generated block randomisation 4 and 6, consecutively numbered envelopes. | |
| Participants | Nulliparous women GA 28 or more weeks, BS < 6, intact membranes, singleton, cephalic presentation, Exclusion: previous uterine surgery, non‐reassuring FHR, latex allergy, contraindication to vaginal birth. |
|
| Interventions | Foley 30 cc + concurrent oxytocin (83 patients analysed). Misoprostol 25 mcg intravaginally 4‐hourly, oxytocin augmentation after ripening. (79 patients analysed.) |
|
| Outcomes | Primary: CS Secondary: tachysystole, hyperstimulation, abnormal FHR tracing, intrapartum and postpartum fever, use of antibiotics, estimated blood loss, blood transfusions, AS, neonatal resuscitation, admission to ICU, meconium aspiration, sepsis, death. |
|
| Notes | Power analysis showed 266 patients were to be included, 173 were randomised. Study was stopped because principle investigator moved to other hospital. 11 patients were excluded from analysis, either received other treatment, or incomplete data. Setting: North Caroline Women's hospital and WakeMed hospital, North Carolina, USA Study period: June 1999 to April 2001 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 patients had incomplete records (4 and 5 in both groups), they were excluded. 2 patients (1 in every group) who did not receive the treatment were excluded, but otherwise ITT. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures are reported. |
| Other bias | High risk | Recruitment goal was not reached, because PI moved to another institution. |
Dalui 2005.
| Methods | Randomised prospective study, randomisation method unclear. | |
| Participants | Singleton live fetus in cephalic presentation, 33‐42 weeks GA, intact membranes, BS < 4 Exclusion: APH, scarred uterus, low‐located placenta, cervicovaginal infection, history of cardiac disease, glaucoma, convulsive disorder, asthma, jaundice. |
|
| Interventions | Foley catheter 30 mL 12 hours, followed by oxytocin (n = 50). PGE2 gel 0.5 mg endocervically oxytocin augmentation after 12 hours (n = 50). |
|
| Outcomes | Bischop score after 12 hours, percentage of subjects entering spontaneous labour, insertion‐expulsion interval Foley, induction‐delivery interval, amount of oxytocin used, mode of delivery, side effects. | |
| Notes | No power calculation, BS lower than most studies, no notes on method of randomisation Setting: Nehru Hospital, PGIMER, Chandigarh, India Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised' method not described. |
| Allocation concealment (selection bias) | Unclear risk | 'Randomised' method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although ITT not mentioned, it seems ITT was used. no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre=specified data are reported, except for expulsion interval of Foley catheter (this is not an outcome of interest for this review). |
| Other bias | Low risk | No other bias detected |
Deo 2012.
| Methods | RCT | |
| Participants | Inclusion: full‐term singleton gestation, cephalic presentation, indication for IOL. BS < 6. Exclusion: rupture of membranes, antepartum bleeding, placenta praevia, previous induction or pre‐induction agent during the pregnancy. | |
| Interventions | Foley catheter (n = 50): 16 F with 30 mL balloon, traction applied. no max hours described.
Misoprostol vaginal (n = 54): 25 ug post fornix, every 4 hours max 8 doses. Dinoprostone vaginal gel (n = 52), 2 mg, once every 6 hours, max of 3 doses |
|
| Outcomes | Change in BS, total time for induction, delivery route, uterine tachysystole (defined as 6 contractions in 10 minutes, in 2 consecutive 10 minutes periods), uterine hypertonus (contraction lasting longer than 3 minutes), subject comfort as women were asked to evaluate their discomfort on a visual scale from 0 to 10. | |
| Notes | Setting: CSM Medical University (India). Study period: not reported, 1 year duration Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation numbers |
| Allocation concealment (selection bias) | Low risk | Consecutive, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As treated analysis (2 or 4 exclusions? (158 cases analysed, but total of included patients makes 156) Primary outcome: unclear how many women in Foley were analysed, in comparison groups 4 cases missing without explanation |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Deo 2013.
| Methods | RCT | |
| Participants | Inclusion: full‐term singleton gestation, cephalic presentation, indication for IOL. BS < 6. Exclusion: rupture of membranes, antepartum bleeding, placenta praevia, previous induction or pre‐induction agent during the pregnancy. | |
| Interventions | Foley catheter (n = 100): 16 F with 30 mL balloon, traction applied. no max hours described. dinoprostone vagina gel (n = 104), 2 mg, once every 6 hours, max of 3 doses |
|
| Outcomes | post induction BS at 6 and 13 hours, | |
| Notes | Abstract only, no outcomes of interested reported Setting: KGMU Lucknow India Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described women were randomly allocated, no more information available |
| Allocation concealment (selection bias) | Unclear risk | Only described women were randomly allocated, no more information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, too little information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Abstract only, too little information to judge risk of bias |
Deshmukh 2011.
| Methods | RCT | |
| Participants | Inclusion: primigravida > 37 weeks of gestation, singleton pregnancy, cephalic presentation BS ≤ 3, Intact membranes Exclusion: multiple pregnancy, malpresentation, absent membranes, APH, medical disease, e.g. heart disease, renal disease | |
| Interventions | Intracervical Foley (n = 200): if BS < 7 after 6 hours, PGE2 was given PGE2 gel vaginal (n = 200), dose repeated after 6 hours Failure of induction was declared if patient failed to go in active phase of labour within 24 hours of induction |
|
| Outcomes | Improvement of BS, induction‐delivery interval, mode of delivery and feto‐maternal outcomes | |
| Notes | Setting: India Study period:July 2005 to January 2008 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT. no missing cases or data. no women excluded |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Dionne 2011.
| Methods | RCT, stratified for parity | |
| Participants | Inclusion: indication for induction, BS < 6 | |
| Interventions | Foley catheter with oxytocin (n = 93) Foley catheter with intravaginal misoprostol (n = 84) Intravaginal misoprostol (n = 87) |
|
| Outcomes | Delivery within 24 hours, CS rate, maternal or fetal complications | |
| Notes | Abstract only, no information about dosage misoprostol Setting: UK Study period: October 2001 to October 2004 Funding: no information Declaration of interest: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, too little information to judge |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcome CS reported, too little information to judge |
| Other bias | Unclear risk | Abstract only, too little information to judge risk of bias |
Edwards 2014c.
| Methods | RCT | |
| Participants | Inclusion criteria: GA > 36 weeks, live singleton fetus in cephalic presentation, unfavourable cervix (less than 3 cm dilated; if 2 cm dilated, less than 80% effaced). Exclusion criteria: < 18 years, no informed consent in English, > 1 contraction/5 minutes, ruptured membranes, a prior caesarean delivery or any other prior uterine incision, a temperature of 38°C or higher, lethal fetal anomalies, placenta previa, other contraindication to vaginal delivery, suspected placental abruption or undiagnosed bleeding, a category II or III FHR pattern, HIV infection or any other immune dysfunction, an allergy to latex or dinoprostone, previous attempt of cervical ripening. |
|
| Interventions | Foley catheter (n = 185): 16F, 30 mL, minimal tension, removed after 12 hours Dinoprostone vaginal insert (n = 191): removed after 12 hours |
|
| Outcomes | Induction to delivery time, (vaginal) delivery within 12 hours, (vaginal) delivery within 24 hours, tachysystole, clinical chorioamnionitis, endometritis, other postpartum complications, caesarean delivery, early neonatal outcomes | |
| Notes | Setting: multicentre, USA period: July 2010 to February 2013 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated by an online randomisation system 1 to 1 |
| Allocation concealment (selection bias) | Low risk | Allocation web‐based |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses reported, but women who did not deliver and went home were excluded (5 to 77. Patients with missing values for arterial cord pH level. properly described how this was dealt with |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
El Khouly 2017.
| Methods | RCT | |
| Participants | Inclusion criteria: singleton pregnancy, cephalic presentation, GA > 28 weeks and the BS < 6. Exclusion criteria: contraindication vaginal delivery, EFW > 4500 g, a previous uterine scar, clinically significant cervical or vaginal infection, chorioamnionitis, unexplained vaginal bleeding, low‐lying placenta, abnormal cervical anatomy or cervical cerclage. |
|
| Interventions | Foley catheter (n = 36): 18F, 30 mL, max of 12 hours Foley catheter + oxytocin (n = 36): 18F, 30 mL balloon, oxytocin, Foley max of 12 hours) Oxytocin alone (n = 36): increased by 2 mU/minute at 30‐minute intervals until adequate uterine activity was maintained, max dose 32 mU/minute, AROM at 3 cm |
|
| Outcomes | Duration and dose of required oxytocin, induction to delivery interval, mode of delivery and reason (in case of CS), maternal and neonatal complications | |
| Notes | Setting: Menoufia University Hospital, Egypt Study period: between January 2015 and February 2016. Funding: no funding Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although ITT is not mentioned, figure 1 and results are plausible for ITT, all cases analysed, no missing data described |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Filho 2002.
| Methods | RCT. | |
| Participants | Term or post‐term, live, singleton fetus in cephalic presentation, intact membranes, BS < 6, not in labour, medically indicated for labour induction. Exclusion criteria: multiple gestations, non‐cephalic presentation, previous caesarean delivery or uterine scar, rupture of membranes, antepartum bleeding, genital herpes infection, fetal death, placenta previa or previous attempts to induce labour. |
|
| Interventions | Misoprostol (n = 119): 25 mcg 6‐hourly, max 4 doses. Foley (n = 121): 30cc traction applied 24 hours followed by oxytocin. |
|
| Outcomes | Induction‐to‐vaginal delivery time, deliveries within 24 hours, mode of delivery, uterine contraction abnormalities, puerperal infection or neonatal outcomes. | |
| Notes | Setting: Maternidade Monteiro de Morais, Recife, Brazil Dates of study: between September 2000 and December 2001 Funding sources: financial support from CAPES Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow up, analysis ITT. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified data reported. |
| Other bias | Low risk | No other bias detected |
Garba 2016.
| Methods | RCT | |
| Participants | Inclusion: multiparae, postdate (41+3), singleton, unfavourable cervix Exclusion: not mentioned |
|
| Interventions | Foley catheter + oxytocin (n = 66) (no more info available) Vaginal misoprostol (n = 70), (no more information available |
|
| Outcomes | Mode of delivery, maternal and perinatal outcomes, induction to delivery interval, AS, maternal vital signs, estimated blood loss. | |
| Notes | Setting: antenatal clinic at Aminu Kano Teaching Hospital, Nigeria Study period: February to May, 2015 Funding: no funding Declaration of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, in table 4 there are cases missing, not reported why. no missing data reported |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Unclear risk | All eligible patients where randomised. Inclusion rate of 100% of all eligible patients is doubtful |
Gelisen 2005.
| Methods | RCT, randomisation by sealed opaque envelopes, no mentioning of sequence. | |
| Participants | Singleton live pregnancy, GA 41 completed weeks, BS < 5, no contractions, AFI > 5, estimated fetal body weight < 4500 g. Exclusion: known hypersensitivity to PG, previous caesarean delivery or other uterine surgery, MBI > 30, parity > 4, low‐lying placenta. |
|
| Interventions | Foley catheter 50 mL (n = 100). Vaginal misoprostol 50 mcg 6‐hourly, max 24 hours (n = 100). (group excluded because of high dose) Oxytocin low dose protocol (n = 100). Spontaneous follow‐up (n = 300). (not in analyses) |
|
| Outcomes | CS rate, neonatal outcomes: meconium, arterial pH, acidaemia, admissions to NICU secondary, tachysystole, hyperstimulation, fetal distress. |
|
| Notes | Primary goals of study is to compare induction versus expectant management. Setting: tertiary training centre in Turkey Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 600 opaque envelopes, 1 was drawn every time. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded assessment of fetal monitor strips. (to assess hyperstimulation). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not addressed, seems like all data complete. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures are reported. |
| Other bias | Low risk | No other bias detected |
Gilson 2017.
| Methods | RCT | |
| Participants | Patients eligible for medical induction | |
| Interventions | Foley catheter + oxytocin (n = 526) Low dose titrated oral misoprostol (n = 575) dose not mentioned in abstract |
|
| Outcomes | Effectiveness, safety | |
| Notes | Abstract only Setting: Rwanda Study period: not reported Funding: not reported Declaration of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT mentioned, not clear why groups are different in size. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Abstract only, to little information to judge risk of bias |
Glagoleva 1999.
| Methods | RCT. No details were given on the method for concealment of the allocation. | |
| Participants | BS < 5. Women with a past history of CS were excluded. | |
| Interventions | Dilapan (4 tents) removed after 12 hours (27 women). PGE2 intracervical gel 0.5 mg (1‐2 doses) (26 women). | |
| Outcomes | CS. | |
| Notes | Abstract only Setting: Russia Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Abstract only, insufficient information to judge risk of bias |
Goonewardene 2014.
| Methods | RCT | |
| Participants | Inclusion: singleton pregnancy, cephalic, cervix unfavourable (MBS < 6), 40 weeks and 6 days Exclusion: multiple pregnancies, malpresentation, previous CS or any contraindication for normal delivery or misoprostol, prior intervention for ripening of the cervix, non‐reactive CTG after fetal acoustic stimulation test. |
|
| Interventions | Oral misoprostol (n = 74) 25 ug, every 4 hours, max of 2 gifts Foley catheter (n = 78) max 24 hours |
|
| Outcomes | Modified BS ≥ 6 day 2 after the intervention; Induction to delivery interval, mode of delivery, side effects of misoprostol (only reported in trial register) | |
| Notes | Setting: Academic Obstetric Unit, Teaching Hospital, Mahamodara, Galle, India Study period: January 2011 to March 2012 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence, block randomisation, stratified for parity |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not mentioned, no FIgure 1, cases excluded for some selective outcomes because of spontaneous labour after intervention, no missing data reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results, except secondary outcome 'side effects misoprostol' not reported. |
| Other bias | Low risk | No other bias detected |
Guinn 2000.
| Methods | RCT. Computer‐generated sequence. Opaque, sealed, sequentially numbered envelopes. | |
| Participants | Singleton, vertex presentation, intact membranes. Unfavourable cervix (< 2 cm dilated and effacement < 75%). Exclusion: bleeding, labour, asthma. prior vertical uterine incision, acute fetal compromise | |
| Interventions | Laminaria and IV oxytocin: as many laminaria as possible were kept for 12 hours, unless expelled or membranes ruptured. IV oxytocin was simultaneously given (165 women); EASI + IV oxytocin: Foley catheter balloon filled with 30 mL of water followed by saline infusion 30 mL/hour. IV oxytocin was simultaneously given (169 women); PGE2 intracervical gel 0.5 mg/6H, max 2 doses. IV oxytocin was started if not in labour after 2 doses of PGE2 (110 women). | |
| Outcomes | CS, delay to delivery, delivery within 24 hours, infections, haemorrhage. | |
| Notes | After interim analysis, the authors stopped recruiting in the PGE2 group. 68 protocol violations, but ITT analysis was conducted. Setting: University of Alabama and Cooper Green Hospitals Birmingham, Alabama Dates of study: January 1994 to August 1997 Funding sources: UpJohn Pharmaceuticals provided funds to purchase study drugs Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes reported for laminaria and EASI |
| Other bias | Unclear risk | After interim analysis, the authors stopped recruiting in the PGE2 group. |
Gunawardena 2012.
| Methods | RCT | |
| Participants | Uncomplicated primipara with singleton pregnancies who underwent IOL at 40 weeks + 5 days | |
| Interventions | PGE2 intracervical (72) Foley (73) |
|
| Outcomes | Change in mean MBS, uterine hyper‐stimulation, Broncho‐constriction, nausea and vomiting, postpartum haemorrhage and maternal fever, meconium at membrane rupture, AS at 5 minutes and PBU admission | |
| Notes | Abstract only Setting: Ward 5, Teaching Hospital, Kandy, India Study period: not reported Funding: not reported Declaration of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, no missing data or cases reported |
| Selective reporting (reporting bias) | Unclear risk | Maternal and neonatal adverse outcomes were not given in numbers |
| Other bias | Low risk | No other bias detected |
Haugland 2012.
| Methods | RCT | |
| Participants | Inclusion: singleton pregnancy, > 37 weeks, indication to induce birth, BS < 2 cm, term date set by US before week 21 Exclusion: IUFD, fetal malformations, low lying placenta, rupture of membranes, no understanding of Norwegian language |
|
| Interventions | Foley catheter (n = 90), 16‐19 hours Cook double balloon (n = 88), 16‐19 hours |
|
| Outcomes | Cervix dilatation ≥ 3 cm after removal or active labour | |
| Notes | Abstract only Setting: Haukeland university hospital, Bergen, Norway Study period: March 2010 ‐ January 2011 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, says in the protocol that participants and outcome assessor will be blind to allocated treatment, but not clinicians |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, in the study protocol says participant and outcome assessor will be blind to allocated treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not described mentioned, to little information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | Unclear risk | Abstract only, too little information to judge risk of bias |
Hay 1995.
| Methods | RCT. No details were given on the method for concealment of the allocation. | |
| Participants | 28 women in the comparison between Dilapan and PGE2, with a total of 39 women recruited (15 Dilapan group, 13 PGE2 group, 11 amniotomy). | |
| Interventions | Dilapan versus PGE2, no details on dosage provided. | |
| Outcomes | CS, hyperstimulation, nausea. | |
| Notes | Abstract only. Setting: UK Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Abstract only |
Hemlin 1998.
| Methods | RCT | |
| Participants | Singleton vertex term pregnancies, BS < 5 | |
| Interventions | EASI 30 to 60 mL/hour infusion (43 women) PGE2 0.5 mg intracervical (42 women) | |
| Outcomes | CS, instrumental delivery, painful contractions, vaginal delivery achieved within 12 to 24 h0urs. | |
| Notes | Setting: County hospital of Ekinstuna, Sweden Study period: November 1990 to November 1995 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were randomised using sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, women delivered by CS and women with quote: “ unsuccessful” treatment were excluded for some of the outcome measures. no missing cases of missing data for outcomes of interest. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Henry 2013.
| Methods | RCT | |
| Participants | Inclusion: women ≥ 18 years gestational > 37 weeks requiring IOL with a cervical preparation procedure Exclusion: unsuitable for outpatient management, unsuitable for randomisation to either PGE2 (e.g. previous CS) or catheter use (e.g. latex allergy), or prior attempted IOL in this pregnancy, ruptured membranes, regular uterine contractions, multiple pregnancy or non‐vertex presentation, unable to give informed consent |
|
| Interventions | Foley catheter (n = 50): 30 mL, slight traction, spigot inserted to occlude the lumen, PCM 1 g/60 mg codeine, 20 mg temazepam., went home. Next morning AROM or priming by choice of clinician (n = 50) PG gel (n = 51): (2 mg nulliparous – 1 mg multi parous), fornix posterior, repeated if necessary after 6 hours (1 mg), PCM 1 g/60 codeine, temazepam 20 mg, next morning AROM or priming by choice of clinician |
|
| Outcomes | Delivering vaginally within 12 hours of admission to Delivery Unit; total inpatient hours from induction to delivery, syntocinon for induction or augmentation of labour, mode of delivery, vaginal delivery within 24 hours of insertion of Foley catheter or first dose PGE2 gel, Induction to delivery interval, i.e. time from commencement of cervical ripening to delivery, delivery within 24 hours of insertion of Foley catheter or first dose PGE2 gel, requirement for second method of cervical ripening or (in Prostin group) 3rd dose of PG, patient satisfaction using questionnaire created for purposes of this study, return to hospital (Foleys group) prior to planned readmission and not in labour, maternal febrile morbidity, non‐reassuring FHR trace, CS or instrumental delivery for fetal distress, Admission to newborn care, AS 1 and 5 minutes, epidural. | |
| Notes | Setting: Australian metropolitan tertiary teaching hospital, Australia Time period: June 2009 to December 2010 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was performed prior to trial commencement. |
| Allocation concealment (selection bias) | Low risk | Sealed in sequentially‐numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing data reported, no missing cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results, except secondary outcome 'epidural' (pre specified in trial registration) |
| Other bias | Low risk | No other bias detected |
Hibbard 1998.
| Methods | RCT. Computer‐generated sequence. Opaque, sequentially‐numbered envelopes. | |
| Participants | Vertex, > 34 weeks, intact membranes, BS < 5. Exclusion of previous CS, cervicitis, macrosomia. | |
| Interventions | PGE2 (Prepidil) gel (17 women); PGE2 (Prepidil) and Dilapan (22 women). | |
| Outcomes | CS, instrumental delivery, painful contractions, vaginal delivery not achieved in 24 hours, uterine hyperstimulation, infection. | |
| Notes | Setting: University of Chicago, USA Study period: August 1994 ‐ May 1995 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation chart |
| Allocation concealment (selection bias) | Low risk | Sequential opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | High risk | Study ended prematurely (before power was reached) as Dilapan was removed of the USA market |
Hoppe 2016.
| Methods | RCT | |
| Participants | Inclusion: ≥ 18 years, singleton, vertex, BS of ≤ 5, < 4 contractions in 10 minutes, category I fetal monitoring. Exclusion: contraindication for vaginal delivery, planned or received exogenous PG administration, unexplained vaginal bleeding, active herpes simplex, previous caesarean delivery, previous attempt at IOL, non‐English speaking |
|
| Interventions | Single balloon 18F Foley, 30 mL, traction applied, max 12 hours Double balloon, Cook 80 mL/80 mL, max 12 hours |
|
| Outcomes | BS of > 6 at time of catheter removal, change in BS, time from catheter insertion to spontaneous expulsion or removal, mean time from catheter insertion to vaginal delivery, vaginal delivery in 24 hours, the use of pharmacologic methods for further cervical ripening or augmentation of labour, AROM, epidural use, mode of delivery, indications for CS, chorioamnionitis, AS at 5 minutes < 7, meconium, NICU admissions |
|
| Notes | Setting: University of Washington Medical Center Labor and Delivery, USA Study period: January 2010 and November 2013 Funding: no funding by Cook Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation, stratified for parity, not clear how this was done. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT reported, most likely per protocol, missing data in baseline characteristic, not in outcomes, no cases missing |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome data reported |
| Other bias | Low risk | No other bias detected |
Hudon 1999.
| Methods | RCT. No details on the method of randomisation or on concealment of the allocation. | |
| Participants | Term, unfavourable cervix (BS < 5). | |
| Interventions | Foley catheter placed above the internal os and inflated with 40 mL left in place for a max of 16 hours (56 women); intracervical PGE2 (0.5 mg), repeated if BS unfavourable (55 women). Oxytocin was given after achievement of cervical ripening. | |
| Outcomes | CS. | |
| Notes | Setting: USA Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were randomised |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Abstract only |
Hughes 2002.
| Methods | Randomised trial. | |
| Participants | Singleton gestation, cephalic presentation, intact membranes, GA 36‐42, indicated labour induction. | |
| Interventions | PGE 2 pessary (n = 34). Foley + EASI + oxytocin (n = 33). |
|
| Outcomes | Change in BS, induction‐delivery time. | |
| Notes | Outcomes of interest not reported in this abstract. Setting: USA Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no further details |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned, no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 patients excluded after randomisation in Foley group, unclear |
| Selective reporting (reporting bias) | Unclear risk | Study protocol/predefined outcomes not available |
| Other bias | Unclear risk | Only reported as abstract |
Husain 2017.
| Methods | RCT | |
| Participants | Inclusion: age 20 to 40 years, singleton pregnancy, cephalic presentation ≥ 37 weeks Exclusion: BS of > 4, cephalopelvic disproportion on examination, history of placenta previa or unexplained vaginal bleeding, history of previous CS or other uterine surgery, active herpes simplex infection, chorioamnionitis, contraindication to use of PGs, acute pelvic inflammatory disease, contraindication to vaginal delivery, a non reassuring FHR pattern prior to induction. |
|
| Interventions | Oral misoprostol (n = 157): 50 mcg, every 4 hours, max 4 gifts Foley catheter + oral misoprostol (n = 161): 16 or 18F, filled with 30 mL + oral misoprostol (50 mcg) every 4 hours, max 4 gifts both groups: if labour was not established within 4 hours of the 4th dose of misoprostol, induction was considered to have failed and such cases were then delivered by CS. |
|
| Outcomes | Failure to achieve vaginal delivery after 24 hours, induction‐to‐delivery interval, mode of delivery, reason for CS maternal complications, NICU admissions | |
| Notes | Setting: Abbasi Shaheed Hospital in Karachi, Pakistan, tertiary care centre Study period: May 2016 to October 2016. Funding: no funding reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence, block randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis not reported, cases excluded because of protocol violation. no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Jagani 1982.
| Methods | RCT. Randomisation based on case number. No measure taken to conceal the allocation. | |
| Participants | Intact membranes. | |
| Interventions | 5 groups: no treatment (n = 10; exclude); laminaria n = 10 (as many as possible); Foley catheter n = 10 (inflated with 70 ‐ 80 mL water) under traction; amniotomy (n = 10); oxytocin (n = 10) increased by 5 mU/minute every 10 ‐15 minutes. In all groups, each of 10 women, an extraovular catheter with a 5 mL balloon was used to record uterine activity. | |
| Outcomes | CS. | |
| Notes | Setting: USA Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method selected by last digit of the chart number |
| Allocation concealment (selection bias) | High risk | Inadequate.No measure taken to conceal the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, not clear how many women were actually included. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported |
| Other bias | Low risk | No other bias detected |
Jalilian 2011.
| Methods | RCT | |
| Participants | Inclusion criteria: singleton gestation, cephalic presentation, reactive FHR pattern, intact membranes and GA between 37‐41 weeks Exclusion criteria: BS at least 7 or cervical dilatation greater than 3 cm, EFW > 4500 g or < 2000 g, evidence of cephalopelvic disproportion, placenta previa or unexplained vaginal bleeding, previous section caesarean or uterine surgery and contraindications to PG |
|
| Interventions | Intravaginal dinoprostone (n = 20), 3 mg every 6 hours, max 4 doses Foley catheter, 16F, 30 mL (n = 20), removed after 12 hours |
|
| Outcomes | Not mentioned in method section | |
| Notes | Article is submitted as letter to the editor. no relevant outcomes reported Setting: Iran Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described women were randomly allocated, no more info available |
| Allocation concealment (selection bias) | Unclear risk | Only described women were randomly allocated, no more info available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, too little information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Abstract only (letter to editor), too little information to judge risk of bias |
Jeeva 1982.
| Methods | RCT. No details on the method of randomisation or on concealment of the allocation. | |
| Participants | No description of inclusion/exclusion criteria. 10 primigravidas and 10 multigravidas. | |
| Interventions | Laminaria tents (2 ‐ 3 tents) (10 women); (PGE2) 4 mg tablets vaginally. | |
| Outcomes | Change in BS after 16 hours, CS. | |
| Notes | Setting: South Africa Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were randomised |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not mentioned, but seems reasonable as numbers in tables are equal to randomised numbers, no cases missing |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information as no outcome measures were pre‐specified in report |
| Other bias | Low risk | No other bias detected |
Johnson 1985.
| Methods | RCT. Sequence based on a random number table. No details were given on the method for concealment of the allocation. | |
| Participants | Term, primiparas, BS < 6. | |
| Interventions | Lamicel (40 women); PGE2 vaginal gel (4 mg) (40 women). | |
| Outcomes | Epidural analgesia, CS, instrumental delivery, uterine hyperstimulation, fetal distress. | |
| Notes | Setting: UK Dates of study: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT unclear, no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Joshi 2016.
| Methods | RCT | |
| Participants | Inclusion criteria: 1 previous low transverse CS, singleton live pregnancy with cephalic presentation, reassuring fetal status, > 37 weeks and BS < 6 Exclusion criteria: placenta praevia, CPD, various mal presentations, short interconceptional period of 18 months, previous 2 caesareans, in a case of previous myomectomy or hysterectomy, patient demands repeat elective CS |
|
| Interventions | Foley catheter (n = 100), 16F, 30 mL, max 24 hours in situ Oxytocin (n = 100): starting from 1 mU/minute, increased to 2 mU/minute and max up to 32 mU/minute |
|
| Outcomes | Induction delivery interval, indications for CS, mode of delivery, neonatal outcome and NICU admissions were studied in both groups | |
| Notes | Setting: Swami Dayanand Hospital Dilshad Garden New Delhi, India Study period: January 2015 ‐ June 2015 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated that women were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Only stated that women were randomly allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, no figure 1 to check allocation, all cases analysed, no missing data described |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Jozwiak 2012.
| Methods | RCT | |
| Participants | Inclusion criteria: pregnant women scheduled for IOL beyond 37 weeks of gestation with a vital singleton pregnancy in cephalic presentation, intact membranes, and an unfavourable cervix (BS < 6). Exclusion criteria: women younger than 18 years, with a previous CS, placenta praevia, lethal fetal congenital anomaly, or known hypersensitivity for one of the products used for induction were ineligible | |
| Interventions | Foley catheter (n = 411): 18F, 30 cc sterile saline. PG E2 gel (408): 1 mg, followed by 1 mg after 6 hours, with a max of 2 doses per 24 hours inserted into the posterior vaginal fornix. An initial dose of 2 mg was allowed in nulliparous women. 2 days of induction, 1 day of " rest" followed by 2 more days of induction in case of BS < 6 |
|
| Outcomes | CS, maternal and neonatal morbidity and time from start induction to birth. | |
| Notes | Setting: multicentre, the Netherlands Study period: Feb 2009 ‐ May 2010 Funding: none Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Web‐based |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, missing outcome data (pH and BMI) balanced in numbers across intervention groups, with similar reasons for missing data across groups. no missing cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Jozwiak 2013.
| Methods | RCT | |
| Participants | Women > 18 years with term pregnancy and unfavourable cervix, requiring IOL. Exclusion criteria were previous CS, non‐vertex presentation of the fetus, ruptured membranes, hypersensitivity for one of the products used for induction, or a lethal congenital anomaly of the fetus | |
| Interventions | Foley catheter (107),18F 30 cc sterile saline. 10 mg slow release PG vaginal insert (n = 119). Removed after 12 hours, if BS < 6, after 24 hours new PG vaginal insert was used 2 days of induction, 1 day of " rest" followed by 2 more days of induction in case of BS < 6 |
|
| Outcomes | CS, maternal and neonatal morbidity and time from start induction to birth. | |
| Notes | Setting: multicentre, the Netherlands Study period: February 2009 ‐ May 2010 Funding: none Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Web‐based |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, missing outcome data (pH and BMI) balanced in numbers across intervention groups, with similar reasons for missing data across groups. no missing cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Jozwiak 2014.
| Methods | Pilot study within RCT | |
| Participants | Women > 18 years, ≥ 37 weeks, BS < 6, planned for IOL. Exclusion:previous CS, non vertex presentation, ruptured membranes, hypersensitivity for one of the products used for induction, lethal congenital anomaly |
|
| Interventions | Foley catheter (n = 56), 16 or 18F, 30 mL Vaginal misoprostol (n = 64) 25 mcg tablets every 4 hours, max 3 doses in 24 hours. In both groups, if the cervix was still unfavourable for amniotomy after 48 hours of treatment, women were generally assigned a day of rest followed by another 48 hours of induction |
|
| Outcomes | CS, instrumental vaginal delivery, reasons for operative delivery, time from induction to delivery, uterine hyperstimulation, uterine rupture, analgesics, antibiotics, maternal suspected intrapartum infection, maternal postpartum infection, postpartum haemorrhage (> 1000 cc) postpartum blood transfusion, AS of < 7 at 1 minute and 5 minutes, arterial cord blood pH < 7·10, neonatal admissions neonatal ward/NICU | |
| Notes | Setting:multicenter, the Netherlands Study period: February 2009 and May 2010 Funding: no funding reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence, block randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, missing data reported, but even distributed over groups and likely for the same reasons. no missing cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results, except secondary outcome maternal postpartum infection |
| Other bias | Low risk | No other bias detected |
Kandil 2012.
| Methods | Prospective quasi‐RCT | |
| Participants | Inclusion criteria: 41 weeks or more, primigravida, BS < 4, singleton living fetus, vertex presentation, no evidence of active labour, a reassuring FHR pattern, no evidence of intrauterine infection. Exclusion criteria: contra‐indication for vaginal delivery, previous uterine surgery, non‐reassuring FHR, IUFD, ruptured membranes, vaginal infection, malpresentation, macrosomic fetus, cephalopelvic disproportion, history of APH, contra‐indication to PGs | |
| Interventions | 1. 18F Foley catheter, 30 cc sterile saline. Taped to the inner thigh. Each patient received 1 g of ampicillin/6 hours. Removed after 12 hours. (N = 50) 2. 25 ug misoprostol vaginally every 4 hours (N = 50) |
|
| Outcomes | Induction to delivery time, oxytocin use, route of delivery, occurrence of chorioamnionitis, AS, admission to NICU, tachysystole, hypertonus, hyperstimulation | |
| Notes | 9 patients were insertion of Foley was not possible were replaced by 9 other patients! Setting: Menofyia University Hospital, Egypt Study period: from January 2010 to October 2010 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by odd or even admission date |
| Allocation concealment (selection bias) | High risk | Randomisation by odd or even admission date |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT was not mentioned. 9 patients in Foley group were replaced by 9 others because insertion of Foley was not possible. No flow chart, no description of lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pre‐described outcome measures were mentioned. |
| Other bias | Low risk | No other bias detected |
Khamaiseh 2012.
| Methods | RCT | |
| Participants | Inclusion criteria: age 15 years or more, term, singleton, live fetus in vertex presentation, intact membranes and BS < 6. Exclusion criteria: previous CS or history of other uterine surgery, history of ante partum haemorrhage, cephalopelvic disproportion, acute fetal distress revealed by a non stress test prior to induction, signs of infection, ruptured membranes, EFW > 4300, or known allergy to PG |
|
| Interventions | PG E2 (n = 204) tablets 3 mg, max 2 dose. AROM performed if labour did not commence after 2 doses Foley catheter (n = 210): 22/24F, 50‐60 mL in balloon.Removed after 24 hours and AROM if possible |
|
| Outcomes | Mode of delivery, time interval between the start of induction and delivery, oxytocin requirement, the indications for CS and adverse neonatal and maternal reactions to the cervical ripening agent. Hyperstimulation with and without FHR changes, failed induction. | |
| Notes | Setting: King Hussein Medical Centre and Prince Ali Bin Al‐Hussein hospital, Jordan Study period: July 2009 ‐ July 2010 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by computer |
| Allocation concealment (selection bias) | Unclear risk | Not described, only description: Randomisation was done by a computer‐generated list of random numbers |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis not reported and not clear if used, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Krammer 1995a.
| Methods | RCT. Computer‐generated sequence. No details were given on the method for concealment of the allocation. | |
| Participants | Term women with a BS < 9, absence of contraindication for labour and fetal distress. 441 women (224 in the Dilapan group and 217 in the PGE2 group). | |
| Interventions | Dilapan, as many dilators as possible (224 randomised, 214 analysed); intracervical PGE2 0.5 mg (217 women randomised, 202 analysed). In both groups, ripening was followed 6 hours later by oxytocin. | |
| Outcomes | CS, uterine hyperstimulation, fetal and neonatal infection. | |
| Notes | 25 women excluded: 10 in the Dilapan group (8 protocol violations, 2 entered spontaneous labour before insertion) and 15 in the PG group (10 protocol violations, 3 entered labour before ripening and 2 delivered before the 6‐hour interval). Authors stated that including these excluded women do not alter the results. Numbers of CS derived from Williams 1997. Setting: Tampa general hospital, USA Dates of study: June 1991 ‐ December 1993 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT was performed, but 25 women excluded: 10 in the Dilapan group (8 protocol violations, 2 entered spontaneous labour before insertion) and 15 in the PG group (10 protocol violations, 3 entered labour before ripening and 2 delivered before the 6‐hour interval) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Kruit 2016.
| Methods | RCT | |
| Participants | Inclusion: PROM > 18 hours, ≥ 37 weeks, BS < 6, vital singleton pregnancy, cephalic Exclusion: previous CS, placenta previa, vaginal bleeding, HIV, hepatitis B or C, maternal infection, fetal anomaly |
|
| Interventions | Foley (n = 89): 22ch Rush balloon, traction, 40‐50 cc, max 8 hours. if unripe, further management by discretion of clinician oral misoprostol (n = 99): 50 mcg misoprostol every 4 hours, after 3 gifts dosis increased to 100 mcg or 25 to 50 mcg vaginal every 3‐4 hours |
|
| Outcomes | CS rate, maternal and neonatal infections. Reason for CS (fetal distress, suspected infection, prolonged labour, failed induction, postpartum infection, postpartum haemorrhage, uterine hyperstimulation, fetal tachycardia, use of analgesics, AS, umbilical arterial pH, admission to neonatal care, induction to delivery interval. | |
| Notes | Trial stopped prematurely due to insufficient patient enrolment Setting: multicentre, Finland Study period: March 2012 to September 2014 Funding: grant from the Finnish medical society duodecim and Helsinky University Central Hospital research grant Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how this was done (only that they used sealed envelopes) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Seems to be per protocol analysis. Patients excluded after enrolment for cross‐over during analysing data, 3rd arm formed |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | Trial stopped prematurely due to insufficient patient enrolment |
Kuppulakshmi 2016.
| Methods | RCT | |
| Participants | Not mentioned | |
| Interventions | intracervical dinoprostone (n = 100): 0.5 mg, 6‐hourly, max 2 doses Foley catheter (n = 100), 30 mL, max 12 hours in place. |
|
| Outcomes | Vaginal delivery within 24 hours, improvement in BS, induction to onset of active labour and induction to delivery interval, mode of delivery, occurrence of maternal complications and fetal outcome, cost‐effectiveness | |
| Notes | Setting: India Study period: June 2015 ‐ July 2016 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only described that patients were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Only described that patients were randomly allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data described, difficult to judge how selection of patients was done, if there was ITT, if patients were excluded. no numbers of analysed patient in result section |
| Selective reporting (reporting bias) | Low risk | Pre specified outcomes reported in method section were all reported |
| Other bias | Low risk | No other bias detected |
Laddad 2013.
| Methods | RCT | |
| Participants | Inclusion criteria: primigravida, > 37 weeks of gestation, singleton pregnancy, cephalic presentation, BS < 4, Intact membranes Exclusion criteria: multiple pregnancy, mal‐presentation, absent membranes, APH, medical disease e.g. heart disease, renal disease, previous LSCS |
|
| Interventions | Foley catheter (n = 200): PGE2 gel intracervical (n = 200), max 2 doses, failed induction declared if patient was not in active labour after 48 hours |
|
| Outcomes | Not mentioned in method section | |
| Notes | PGE2 dose not described Setting: KIMSDU; India, Study period: January 2011 ‐ December 2012 Funding: none Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only stated that women were randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Only stated that women were randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if analysis was ITT, (no figure 1) all cases analysed, no missing data described |
| Selective reporting (reporting bias) | Unclear risk | No pre ‐specified outcome measures reported, so cannot be determined |
| Other bias | Low risk | No other bias detected |
Lanka 2014.
| Methods | RCT | |
| Participants | Inclusion: GA > 28 weeks, singletons, intact membranes, absence of labour, cephalic presentation, BS < 5. Exclusion: multifetal gestations, congenital malformations, Gravidity > 4, non‐reassuring FHR trace, ruptured membranes, active genital infection, previous uterine surgery (including CS), low‐lying placenta, chorioamnionitis, EFW > 4000 g, IUFD, known allergies to latex or PGs |
|
| Interventions | Foley + misoprostol (n = 63): 16F Foley catheter, 30 cc, traction applied, max 12 hours ‐ 25 mcg vaginal misoprostol, every 4 hours up to a max of 8 doses. vaginal misoprostol (n = 63): 25 mcg vaginal misoprostol every 4 hours, with a max of 8 doses |
|
| Outcomes | induction to delivery interval, rate of vaginal deliveries, hyperstimulation, CS rate, neonatal outcome, chorioamnionitis, oxytocin use | |
| Notes | Setting: tertiary care centre, India Study period: 2‐year period Funding: no funding Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence, block randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Lemyre 2006.
| Methods | RCT. | |
| Participants | Term pregnancy requiring cervical ripening. | |
| Interventions | Foley catheter for 12 hours (n = 31). Vaginal misoprostol 25 mcg 4‐hourly (n = 31). |
|
| Outcomes | Induction‐active labour, induction‐delivery, delivery within 12 and 20 hours, oxytocin, obstetric outcome, maternal and neonatal morbidity | |
| Notes | Reported as abstract, only outcome of interest reported is oxytocin infusion. Setting: Canada Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no details on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation concealment given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported in this abstract. |
| Selective reporting (reporting bias) | Unclear risk | In this abstract from the outcomes of interest only oxytocin infusion reported, other outcomes not reported. |
| Other bias | Unclear risk | Only abstract available. |
Lewis 1983.
| Methods | RCT. No details were given on the method for concealment of the allocation. | |
| Participants | Singleton vertex term pregnancy, unfavourable cervix. | |
| Interventions | Vaginal pessary 3 mg PGE2 (22 women); Foley catheter in the extra‐amniotic space 30 mL (22 women); Control group with no treatment (22 women; exclude) | |
| Outcomes | Change in BS, CS, uterine hyperstimulation, AS. | |
| Notes | Data on induction‐delivery intervals not interpretable to derive proportion of women with vaginal delivery not achieved in 24 hours. Setting: Manchester, UK Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation, no more details reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if ITT was performed. no missing cases or data reported |
| Selective reporting (reporting bias) | Unclear risk | No specific pre specified outcomes reported in method section. |
| Other bias | Low risk | No other bias detected |
Lokkegaard 2015.
| Methods | RCT | |
| Participants | Inclusion criteria: intact membranes, cephalic position, BS ≤ 6, indication of IOL. Exclusion criteria: ruptured membranes, spontaneous labour, placenta praevia, acute fetal distress, asthma, glaucoma, latex allergy, infections (acute herpes, GBS, condylomata), previous CS |
|
| Interventions | 1. Double balloon catheter (n = 412); 80 mL, max 12 hours, thereafter either AROM or start of oxytocin. 2. PGE2 3 mg tablet (n = 413), 2 dose a day (4‐5 hourly), max 2 days |
|
| Outcomes | Failed inductions, time interval from induction to delivery, mode of delivery, neonatal outcome as assessed by the AS after 5 minutes and referral to a neonatal care unit, subgroups by parity and indication for induction. Post hoc analyses included the percentage of women who gave birth within 24 hours and the need for additional oxytocin stimulation | |
| Notes | Setting: multicentre, Denmark Study period: September 2002 to December 2005 Funding: the randomisation procedure was funded by ‘ Snedkermester Sophus Jacobsen & Astrid Jacobsens fond and the Danish Toyota Foundation. Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated and was stratified for parity and department. |
| Allocation concealment (selection bias) | Low risk | Central allocation by telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, missing data reported, but evenly distributed. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome measures were reported |
| Other bias | Low risk | No other bias detected |
Lyndrup 1989.
| Methods | RCT. | |
| Participants | Woman with unfavourable cervix. | |
| Interventions | 4 groups:
|
|
| Outcomes | CS, forceps or vacuum extraction, endometritis. | |
| Notes | Setting: Denmark Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported women were randomised by sealed envelope method |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 women excluded for protocol violation. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre specified in method section. |
| Other bias | Low risk | No other bias detected |
Lyndrup 1994.
| Methods | RCT | |
| Participants | Singleton vertex pregnancy, intact membranes with unfavourable cervix. | |
| Interventions | Foley extra‐amniotic 30 mL (59 women) PGE2 2.5 mg pessaries (50 women) | |
| Outcomes | Vaginal delivery not achieved within 24 hours, CS, instrumental delivery, women not satisfied, caregiver not satisfied, pH, AS. | |
| Notes | Setting: Denmark Study:period: June 1990 ‐ March 1993 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on the method of generation of the sequence. |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT reported, but 4 women were lost to follow‐up. Women were excluded if delivered after 48 hours |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Mackeen 2018.
| Methods | RCT | |
| Participants | Inclusion criteria: women with a live, singleton gestation in cephalic presentation at 34 weeks of gestation or greater with PROM (at least 60 minutes prior to randomisation), an unfavourable cervical examination (less than 2 cm or 80% effaced), and no contraindication to labour. English speaking with a plan for vaginal delivery. Exclusion criteria: active labour and those with suspected intra‐amniotic infection, abruption or significant haemorrhage, latex allergy, greater than 1 prior caesarean delivery, any contraindication to vaginal delivery, or human immunodeficiency virus or acquired immunodeficiency syndrome, multifetal gestations, lethal fetal anomalies, intrauterine fetal demise, and category II or III FHR tracings. |
|
| Interventions | Oxytocin only (n = 108): start 2 mUh/minute, increase 2 mUh/minute every 30 minute, max 30 mUh/minute Foley catheter + oxytocin (n = 93): 16F, 30 mL, traction applied, max 12 hours, oxytocin concurrent (as above) If not in labour after 24 hours, management per discretion of clinician |
|
| Outcomes | Interval from induction to delivery, interval from induction to vaginal delivery, induction to delivery excluding patients with PPROM before 34 weeks of GA, CS rate, rate of vaginal delivery within 12 or 24 hours, indication for CS, infection complications, maternal LOS, 5‐minute AS < 5, neonatal infectious evaluation and diagnosis of sepsis, maternal and neonatal length of stay, NICU admission, chorioamnionitis, fetal tachycardia, endomyometritis, | |
| Notes | Setting: multicentre, USA Study period: March 2014 to July 2016 Funding: small internal grants to assist with the conduct and statistical analyses for the entire study. Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schema in random‐sized blocks stratified by multiparity or primiparity, preterm or term gestation, and hospital site. |
| Allocation concealment (selection bias) | High risk | Not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Matonhodze 2003.
| Methods | RCT: Computer‐generated random sequence, sealed opaque envelopes. | |
| Participants | Inclusion: completed 34 weeks GA, intact membranes Exclusion: uterine scar, uncontrolled medical complication, non‐vertex presentation, multiple pregnancy, fetal distress, APH. |
|
| Interventions | Foley catheter + misoprostol (n = 174): 50 mL, traction, max 24 hours, followed by oral misoprostol solution 20 mcg every 2 hours, 40 mcg 2‐hourly after 3 doses, until active labour had started. If after established labour contractions became inadequate: augmentation with misoprostol solution 5‐20 mcg hourly. If ineffective: oxytocin. Titrated oral misoprostol (n = 176): as described above Dinoprostone vaginal (n = 176). 2 mg in posterior fornix, repeated after 6 hours. If no active labour after 12 hours: oxytocin |
|
| Outcomes | Failed vaginal delivery within 24 hours, augmentation, tachysystole, hypersystole, hyperstimulation syndrome, tocolysis, analgesia, meconium, CS, instrumental delivery, maternal side effects, AS < 7, NICU admission, perinatal death, neonatal sepsis. | |
| Notes | It is not clear if all patients had an unfavourable cervix (not mentioned in baseline characteristics. Data reported for different numbers of subjects depending on outcome (selective reporting or missing outcome data?). Setting: Pakistan Study period: October 2000 ‐ December 2001 Funding: not reported Declaration of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence, |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque, sealed envelopes out of a dispenser. intact membranes/unfavourable cervix, intact membranes/favourable cervix |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis, data reported for different numbers of subjects depending on outcome. not clear why |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Mazhar 2003.
| Methods | RCT randomisation: computer‐generated random numbers, concealed. | |
| Participants | Inclusion: singleton pregnancy, cephalic presentation, reassuring fetal status, GA 37 completed weeks, BS 6 or lower. Exclusion: placenta praevia, chorioamnionitis, polyhydramnios, parity > 5, SROM, previous CS, contraindication to labour induction. |
|
| Interventions | (PGE2) vaginal pessary max 2 x 6 hours (dose not mentioned) followed by ARM and oxytocin infusion (n = 100). Foley catheter 45 mL + EASI for 12 hours followed by ARM and oxytocin infusion (n = 100). |
|
| Outcomes | Primary: time from insertion to delivery, mode of delivery. secondary: change is BS after 6 hours, neonatal AS. |
|
| Notes | No sample size calculation. 4 patients were excluded (1 left against medical advice, 1 had SROM, 2 failed inductions were induced at later stage). Setting: Pakistan Dates of study: October 2000 to December 2001 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Randomised numbers concealed in the delivery suite? |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of incomplete data, however unlikely due to nature of outcome measures. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Other bias | Low risk | No other bias detected |
Meetei 2015.
| Methods | RCT | |
| Participants | Inclusion: 1 previous low transverse CS, singleton live pregnancy, cephalic presentation, > 28 weeks and BS < 5. Exclusion: previous classical or T‐shaped incision, unknown scar, transfundal uterine surgery, medical or obstetric complications that preclude vaginal delivery, placenta previa, low‐lying placenta undiagnosed vaginal bleeding, maternal heart disease, rupture of membranes, interval between previous CS and present pregnancy/conception < 6 months, cervico‐vaginal infection, unclean vaginal examination, infection in previous CS |
|
| Interventions | 1. Foley catheter (n = 30): 16F, 30 mL balloon, max 12 hours, thereafter start of oxytocin augmentation 2. Oxytocin (n = 30): 1 mUh/minute, after 1 hour 2/mUh/minute, after 1 hour 4 mUh/minute (max 12 hours). oxytocin augmentation as above |
|
| Outcomes | Change in BS before and after 12 hours of ripening, percentage and time interval of spontaneous labour, insertion and expulsion interval of Foley catheter, route of delivery/outcome of delivery, time required from the beginning of cervical ripening to delivery, hyperstimulation, fetal distress, scar dehiscence, uterine rupture | |
| Notes | Setting: Department of Obstetrics and Gynecology, PGIMER, Chandigarh, India Study period: July 2004 and November 2005, Funding: no funding Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by Tippet's table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysing method not reported and not clear, no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Moini 2003.
| Methods | RCT: quote: "randomly assigned". | |
| Participants | Singleton gestation, cephalic presentation, GA between 37 and 42 weeks, BS < 6. Exclusion: malpresentation, ruptured membranes, active genial herpes, antepartum bleeding, fetal death, cephalopelvic disproportion, indication for emergency termination of pregnancy, history of infertility or CS, women who had undergone induction before presenting. |
|
| Interventions | Dinoprostone intracervical 0.5 mg, oxytocin infusion after 6 hours (n = 35). Foley catheter 30 mL + EASI (n = 35). |
|
| Outcomes | Change in BS (after 6 hours), induction‐delivery interval, need for oxytocin, mode of delivery, fetal complications, maternal complications. | |
| Notes | No sample size calculation. Setting: Roointan‐Arash Maternity Hospital, Iran Dates of study: April 2000 ‐ April 2001 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process quote: "randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes are reported, it is however unclear what is meant by 'fetal complications', and it is likely that there were more outcomes noted. |
| Other bias | Low risk | No other bias detected |
Mullin 2002.
| Methods | RCT: randomisation by computerised random number generator, consecutively‐numbered sealed envelopes. | |
| Participants | Singleton gestation, cephalic presentation intact membranes, BS 4 or less, < 8 contractions per hour, reactive FHR tracing. EFW > 4500 g or < 1800 g, low‐lying placenta, placenta praevia, unexplained vaginal bleeding, active genital herpes, vasa praevia, chorioamnionitis, contraindication for PGs, previous uterine surgical procedure, parity > 5. |
|
| Interventions | Foley 30 mL+ EASI (max 12 hours) + IV oxytocin infusion (n = 100). Vaginal misoprostol 25 mcg every 4 hours max 24 hours (n = 100). |
|
| Outcomes | Primary: mean time from start induction to delivery. Secondary: route of delivery, success of induction (vaginal delivery within 24 hours), uterine contraction abnormalities, chorioamnionitis, route of delivery, AS < 7, NICU admission, neonatal resuscitation. |
|
| Notes | Power calculation states that 140 patients were necessary, yet 200 were included Setting: Los Angeles County–University of Southern California Medical Center, USA Dates of study: February 1999 to July 2001 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from protocol, no mention of incomplete data. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes are reported. |
| Other bias | Unclear risk | Unclear why 200 patients were included, while 140 were calculated in the power calculation. |
Mundle 2017.
| Methods | RCT | |
| Participants | Inclusion: age ≥ 18 years, ≥ 20 weeks’ gestation or later with a live fetus, decision made to induce vaginal birth because of pre‐eclampsia or hypertension Exclusion: unable to give informed consent, previous CS, multiple pregnancy, ruptured membranes, chorioamnionitis, allergy to misoprostol. |
|
| Interventions | 1. Foley catheter (n = 300): 18F, 30 mL balloon, traction applied, max 12 hours, afterward start oxytocin or AROM 2. Oral misoprostol (n = 302) 25 mcg, 2‐hourly, max of 12 dose (24 hours). In primigravid women the dose could be increased to 50 mcg 2‐hourly after the first 2 doses oxytocin administered through gravity infusion set |
|
| Outcomes | Vaginal birth within 24 hours, induction to birth interval (vaginal births, CSs, and all births), vaginal births within 12 hours, cervix unchanged at 12 hours and 24 hours, need for oxytocin augmentation, time from randomisation to start of induction and birth, total dose of misoprostol used and the number of participants given a 50 μg dose. Maternal complications, satisfaction, fetal/neonatal complications | |
| Notes | Fetal surveillance with doptone Setting: 2 public hospitals in Nagpur, India Study period: December 2013 to June 2015 Funding: Department for International Development, Medical Research Council, and Wellcome Trust Joint Global Health Trials Scheme. The funder of the study had no role in data collection Declarations of interest: ADW is a scientific adviser to Azanta, a Danish pharmaceutical company, MAT has provided consultancy services to Chiesi, Bristol–Myers Squibb, Novartis, Shire, Janssen, and Grunenthal. both authors received no personal payment, |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated pseudo‐random numbers, block randomisation, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, missing data reported, but small numbers and not in outcomes of interest for this review, no cases missing |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Niromanesh 2003.
| Methods | Quote:"randomly assigned". | |
| Participants | BS < 5, maternal age 20 to 30 years, gravidity 1 ‐ 3, parity 1 ‐ 2, < 6 contractions per hour, singleton pregnancy. Exclusion: history of preterm labour, antepartum bleeding, low‐lying placenta, history of caesarean deliveries, active herpes infection, acute poly or oligohydramnios, high blood pressure, IUFD, GA < 40 weeks, chronic condition or contraindication for use of PGs. |
|
| Interventions | Foley catheter 30 mL max 8 hours (n = 45). (PGE2) tablet 6‐hourly, max 6 doses (n = 44). |
|
| Outcomes | Primary: BS (after ripening). Secondary: ripening time, induction time, total time, delivery route, uterine hyperstimulation, adverse side effects, non‐reassuring FHR tracing, AS. |
|
| Notes | No sample size calculation. 1 patient withdrew due to 'complications'. Time of ripening in Foley group 8 hours, PG group 12 hours Setting: Mirza Kochkhan Hospital, Tehran, Iran. Study period: March 2000 to May 2001 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of incomplete data, insufficient information to permit judgment. |
| Selective reporting (reporting bias) | Unclear risk | 1 patient was withdrawn due to 'complications', data for this patient not reported, other than this, data reported for all patients on the prespecified outcomes. |
| Other bias | Low risk | No other bias detected |
Noor 2015.
| Methods | RCT | |
| Participants | Singleton pregnancies, cephalic presentation, > 37 weeks, intact membranes, BS ≤ 4 Exclusion: rupture of the membranes, chorioamnionitis, APH, cervical dilatation > 2.5 cm, temperature > 38 degrees Celsius, contracted pelvis, fetal distress, polyhydramnios, indication for immediate delivery previous CS or other uterine surgeries. |
|
| Interventions | 1. Vaginal misoprostol (n = 60): 25 mcg, 4‐hourly, with a max of 6 doses. no effective uterine contractions after the 6th dose, then it was considered as failure of induction. 2. Foley catheter (n = 44): 18F Foley 50 mL, traction applied, no max time period reported |
|
| Outcomes | induction to delivery interval, uterine tachysystole, uterine hypertonus, uterine hyperstimulation (tachysystole + FHR changes), meconium‐stained liquor, mode of delivery, maternal and neonatal outcome, AS. | |
| Notes | Setting: Department of Obstetrics and Gynaecology in collaboration with the Department of Paediatrics, JNMCH,AMU, Aligarh (UP), India Study period: May 2013–August 2014. Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported they were randomly assigned, unequal numbers in groups? (60 vs 44) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not reported, although this is likely as numbers are equal to randomised numbers. no missing data or cases |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Ntsaluba 1997.
| Methods | RCT | |
| Participants | Singleton vertex presentation with intact membranes, BS < 6, no previous CS. | |
| Interventions | Intracervical PGE2 (0.5 mg) (59 women) Foley catheter with a 30 mL balloon extra‐amniotic (53 women) | |
| Outcomes | Change in BS, CS, uterine hyperstimulation, fever, neonatal sepsis, fetal distress. | |
| Notes | Setting: KIng Henry 8th hospital, Durban, South Africa Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No other details on the randomisation process. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT reported (although it's likely as numbers are equal as randomised numbers), no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Other bias | Low risk | No other bias detected |
Oliveira 2010.
| Methods | RCT | |
| Participants | Inclusion: singletons in cephalic presentation, GA > 37 weeks, live fetus, BS ≤ 4. Exclusion: ruptured membranes, uterine scar, placenta praevia, chorioamnionitis, EFW > 4000 g, hypersensitivity for products used in intervention |
|
| Interventions | 1. Foley catheter (n = 80): 14 or 16F, 30 cc, max 48 hours 2. Misoprostol (n = 80) 25 mcg a 6 hours, with a max dose of 200 mcg max 48 hours of induction |
|
| Outcomes | Oxytocin use, tachysystole, hypertonus of the uterus, BS > 6, total time until cervical modification, delivery route, FHR abnormalities, meconium stained liquor, AS | |
| Notes | In Portuguese, but translated Setting: Maternidade Escola de Vila Nova Cachoeirinha, public institution which is administrated by the Secretaria Municipal da Saúde de São Paulo, Brazil Study period:January 2006 to January 2008. Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing data or cases reported |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Unclear risk | Based on translated article |
Ophir 1992.
| Methods | RCT | |
| Participants | Singleton vertex presentation, BS 0‐4. | |
| Interventions | PGE2 (6 tablets 0.5 mg) intravaginally (27 women); Foley catheter with a balloon filled with 40 mL water (27 women). | |
| Outcomes | CS | |
| Notes | Setting: Israel Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence from a random numbers table |
| Allocation concealment (selection bias) | High risk | Allocation by odd and even number |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not reported, 8 women missing in Foley group and 7 in PGE2 group. not clear why. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Orhue 1995.
| Methods | RCT | |
| Participants | Singleton, vertex, term fetus, adequate pelvis, maternal height > 155 cm, BS < 5. Exclusion if previous uterine scar, placenta praevia or abruptio, age > 35 years. | |
| Interventions | (PGE2) 3 mg every 6 hours, max 3 doses (34 women randomised, 30 women analysed) oxytocin (2 mU/minute, doubled every 30 minutes, max 32 mU/minute) and ARM (30 women) Foley 30 mL (30 women) | |
| Outcomes | CS, instrumental delivery, uterine hyperstimulation, fetal distress, postpartum haemorrhage. | |
| Notes | 4 women excluded in PG group were re‐included for CS results only. Setting: University of Benin Teaching Hospital, Benin City, Nigeria Study period: April 1990 ‐ October 1991 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence from a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not reported, 4 women excluded in PG group were because of unripe cervix after 12 hours |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Peedicayil 1998.
| Methods | Randomised equivalence trial | |
| Participants | Primigravid, requiring IOL, BS < 5 | |
| Interventions | n = 60 'randomised into 2 groups' Foley 12 hours PGE2 intracervically |
|
| Outcomes | Change in BS, ripening to delivery interval | |
| Notes | None of our outcomes of interest were reported Setting: tertiary level teaching hospital, India Dates of study: not reported Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Person assessing BS was blinded to what agent was used (after 12 hours and removal of agent I presume). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported how many women were randomised to which group, thus unclear. |
| Selective reporting (reporting bias) | Unclear risk | The methods section states that only BS and ripening to delivery interval were the outcomes of interest, which is questionable. |
| Other bias | High risk | Retrospective power calculation, unclear how many women in which group. |
Pennell 2009.
| Methods | RCT: generation of sequence unclear, sealed opaque envelopes, patient chose from a selection of 12. | |
| Participants | Inclusion: primipara, GA > 36 weeks, intact membranes, BS < 4.singleton fetus, cephalic presentation, intact membranes. Exclusion criteria were age < 16 years, previous uterine surgery, low‐lying placenta, any active or purulent infection of the lower vaginal tract, or an abnormal pre‐induction FHR tracing |
|
| Interventions | Foley catheter 30cc. (110). Atad catheter 80 cc. (107). PGE 2 gel 2 mg, 6‐hourly. (113). |
|
| Outcomes | Vaginal delivery within 24 hours, uterine hyperstimulation with/without FHR changes, CS, epidural analgesia, instrumental vaginal delivery, antibiotics during labour, postpartum haemorrhage, maternal fever during labour, pH < 7.10, placental abruption, endometritis, wound infection. | |
| Notes | Data for Foley catheter and double balloon catheter were entered in 1 comparison (any mechanical method versus PG). Setting: King Edward Memorial Hospital (KEMH) in Perth, Western Australia Dates of study: July 2001 to December 2003 Funding sources: supported by a grant from the Women and Infants Research Foundation, King Edward Memorial Hospital, Perth, Australia. Adeza Biomedical Corporation contributed support for the fetal fibronectin test kits. Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process in the paper. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (but why selection of 12??). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research midwives were blinded to treatment allocation, especially important for satisfaction questionnaires. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, loss to follow‐up is described, incomplete data not mentioned. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in methods were reported, report includes all expected outcomes. |
| Other bias | Low risk | No other bias detected. |
Perry 1998.
| Methods | RCT. | |
| Participants | Inclusion: singleton gestation, cephalic presentation, BS of ≤ 4. Exclusion: spontaneous uterine contractions, rupture of membranes, placenta previa, unexplained vaginal bleeding, a non‐reactive nonstress test, an EFW > 4500 g, a prior vertical uterine incision, parity of > 5, active genital herpes infection, or a contraindication to receiving PGs |
|
| Interventions | Vaginal misoprostol 25 mcg every 4 hours (65 women) intracervical Foley of 50cc and PGE2 (4 mg) every 4 hours (62 women) | |
| Outcomes | CS, instrumental delivery, uterine hyperstimulation, AS, NICU admission, chorioamnionitis, perinatal death. | |
| Notes | Setting: University of Mississippi Medical Center Labor and Delivery Unit, Jackson, USA Study period: August 1996 ‐ April 1997 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random schedule. |
| Allocation concealment (selection bias) | Low risk | The allocation of assignment was concealed by placement in a numbered, opaque, sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not reported, although this is likely as numbers are equal to randomised numbers. No missing cases or data in outcomes of interest |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias detected |
Pineda Rivas 2016.
| Methods | RCT | |
| Participants | Inclusion criteria: obese (BMI > 30 before 20 weeks' GA). Singleton pregnancy. Vertex presentation, BS < 6, Intact membranes,GA 37 + 0 to 42 + 0. Normal fetal heart tracing on admission for ripening Exclusion: IOL for intrauterine fetal demise, Intrauterine growth restriction, Suspected abruption at the start of induction, contraindication for a vaginal delivery |
|
| Interventions | Foley catheter (n = 20): PGE2 (n = 21): dinoprostone 10 mg slow release for 24 hours |
|
| Outcomes | Time from initiation of IOL to vaginal delivery, number of vaginal deliveries within 24 hours in each group, CS operative vaginal deliveries chorioamnionitis oxytocin administration, epidural, ICU (NICU) admission, arterial pH < 7, AS < 7 at 5 minutes | |
| Notes | Abstract only Setting: Canada Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | insufficient information to judge |
| Other bias | Unclear risk | Abstract only |
Prager 2008.
| Methods | RCT, not blinded. | |
| Participants | Term pregnancy, BS 6 or less, different indication for IOL, including PROM Exclusion criteria: previous CS, malpresentation, immediate delivery indicated, contraindication to vaginal delivery, contraindication to PGs. |
|
| Interventions | Foley catheter 30 cc (199). Dinoproston 2 mg 6‐hourly (191). Misoprostol 25 mcg vaginally 4‐hourly (199). |
|
| Outcomes | Uterine hyperstimulation, CS, epidural analgesia, instrumental delivery, meconium, AS, NICU admissions, fever during delivery. | |
| Notes | Hyperstimulation is not further specified (with of without FHR changes). Patients who did not meet inclusion criteria were not excluded retrospectively Setting: Karolinska university Hospital, Sweden Dates of study: December 2004 to March 2008 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data described: 3 dinoprostone and 1 catheter. these were excluded. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in methods were reported, report includes all expected outcomes. |
| Other bias | Unclear risk | Patients who did not meet inclusion criteria were not excluded retrospectively (n = 32). |
Qamar 2012.
| Methods | Quasi‐experimental | |
| Participants | Inclusion: singleton alive fetus, cephalic presentation, gestation at or beyond 37 weeks, para 4 or less, BS less than 5, obstetric and medical indication for induction Exclusion: congenital anomalies, multiple pregnancies, mal‐presentation, CPD, placenta praevia or APH, previous CS, and PROM |
|
| Interventions | PGE2 pessary (80) dosage not known, failure of improvement of modified BS after 6 hours (< 5), a second PG E2 gel/pessary was applied and patient reassessed again after 6 hours. If still there was no improvement in BS (< 5) a 3rd PG E2 gel/pessary was applied. PGE2 intracervical (80): as above EASI with oxytocin IV (80) Foleys catheter of 24 or 26 Fr, inflated with 45 mL of distilled water. Traction applied and then saline infusion was started extra‐amniotically at 30 mL per hour for 12 hours. oxytocin infusion was started at 2 mU/minute and the dose was doubled at half‐hourly interval up to the max dose of 40 mU/minute |
|
| Outcomes | induction labour interval, induction delivery interval, mode of delivery, AS at 1 and 5 minutes, and neonatal morbidity and mortality including ICU admission. | |
| Notes | No relevant outcomes reported in article Setting: Pakistan Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation in order of admission |
| Allocation concealment (selection bias) | High risk | Method of induction could be foreseen as a rotation was used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if ITT was used, missing cases or outcomes not reported |
| Selective reporting (reporting bias) | High risk | Neonatal mortality not reported in numbers, only reported they dit not differ. neonatal morbidity not reported in results |
| Other bias | Low risk | No other bias detected |
Ridgway 1991.
| Methods | RCT. No details were given on the method for generating the allocation sequence or for the concealment of the allocation. | |
| Participants | BS < 5. | |
| Interventions | Intracervical PGE2 (0.5 mg) and Lamicel (52 women); intracervical PGE2 (0.5 mg) alone (49 women). |
|
| Outcomes | CS. | |
| Notes | Setting: San Antonio, USA Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not mentioned, insufficient information to judge |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Unclear risk | Abstract only, insufficient information to judge risk of bias |
Roberts 1986.
| Methods | RCT | |
| Participants | Unfavourable cervix (BS < 5). Women with previous history of uterine surgery, fetal malpresentation or multiple gestation were excluded. | |
| Interventions | 4 groups: PGE1 in Tylose gel 3 mg (27 women) (exclude); laminaria tents (28 women); oxytocin 1 mU/minute (25 women); no treatment (24 women exclude). Then oxytocin was given in all groups. |
|
| Outcomes | CS. | |
| Notes | Successful IOL and fetal distress not defined. Setting: Jackson, USA Dates of study: not reported Funding: supported by the Vicksburg hospital medical foundation: Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly ordered envelopes, not clear how |
| Allocation concealment (selection bias) | Low risk | Drawing a sealed envelope by a third party |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not reported, although this is likely as numbers are equal to randomised numbers. no missing cases or data |
| Selective reporting (reporting bias) | Unclear risk | No outcomes pre specified in method section |
| Other bias | Low risk | No other bias detected |
Rouben 1993.
| Methods | RCT. Allocation sequence from a table of random numbers. Blocks of 6 women. Concealment of allocation by sequentially‐numbered, sealed, opaque envelopes. | |
| Participants | Singleton vertex term pregnancies, intact membranes, BS < 6. Excluded if non‐reassuring FHR, placenta praevia. | |
| Interventions | Foley catheter inflated with 30 mL water and extra‐amniotic infusion of 1 mL/minute saline during up to 8 hours (56 women); PGE2 vaginal gel 2.85 mg (56 women). | |
| Outcomes | BS change, CS, uterine hyperstimulation, NICU admission, chorioamnionitis, spontaneous labour, failure of induction, endometritis. | |
| Notes | Also reported as abstract (Arias 1993). Setting: St. Louis, USA Dates of study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not reported, women with failed induction excluded from further analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Roudsari 2011.
| Methods | RCT | |
| Participants | Indication for IOL, GA > 37 weeks, BS < 7, singleton, gestational diabetes mellitus, reassuring FHR tracing, cephalic presentation, intact membranes, low‐located placenta (no definition), and mild pre‐eclampsia. Excluded hypersensitivity to PG, temp > 38, previous CS delivery or other uterine surgery, placenta previa, chorioamnionitis, vaginal bleeding, fetal distress, macrosomia and polyhydramnios. | |
| Interventions | Low‐dose vaginal misoprostol: 25 mcg, repeated up to 6 doses every 4 hours. If no BS > 7 after 24 hours, oxytocin was started Foley catheter (n = 59) 18 F, 50 mL. After 12 hours oxytocin was started. |
|
| Outcomes | interval time from the first intervention to the time of delivery. Uterine tachysystole, uterine hyperstimulation | |
| Notes | Setting: Department of Obstetrics, teaching hospitals, Mashhad University of Medical Sciences, Iran Study period: September 2007 to March 2008 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how |
| Allocation concealment (selection bias) | Unclear risk | Not reported how |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No ITT mentioned, no missing data, 1 woman excluded because of bad participation? |
| Selective reporting (reporting bias) | Unclear risk | Time to delivery: not clear from when till delivery, most likely from active phase. primary outcome was for first intervention to delivery. other pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Roztocil 1998.
| Methods | Allocation according to the week of admission. No concealment of allocation. | |
| Participants | Singleton vertex term pregnancies, BS < 5. Excluded if labour, fetal hypoxia, previous CS. | |
| Interventions | Dilapan S 4 units, removed after 14 hours (82 women); PGE2 intracervical gel 0.5 mg, 1 dose (83 women). In both groups, PGE2 vaginal tablets were administered after 14 hours for labour induction. | |
| Outcomes | CS, hyperstimulation with FHR changes, instrumental vaginal delivery, AS < 7, GI side effects, haemorrhage. | |
| Notes | Inadequate method of random allocation and of concealment of allocation. Setting: obstetrics department Brno, Chech Republic Study period: January 1994 to December 1996 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation according to the week of admission. |
| Allocation concealment (selection bias) | High risk | No concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not reported, but seems reasonable as numbers are equal to randomised numbers. no missing cases or data |
| Selective reporting (reporting bias) | Low risk | Pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Rudra 2012.
| Methods | RCT | |
| Participants | Inclusion criteria: low BS, AROM not possible Exclusion criteria: grande multiparas, preterm induction |
|
| Interventions | 1. Foley catheter (n = 200), 40 mL, 24 hours 2. PGE2 vaginal; 2 mg |
|
| Outcomes | Duration of labour, mode of delivery, postpartum infection and haemorrhage and perinatal, AS | |
| Notes | Abstract only Setting: Batticaloa General Hospital, Sri Lanka Study period: 18 months from 2004 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind? Not clear how this is possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind? Not clear how this is possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Too little information to judge |
| Selective reporting (reporting bias) | Unclear risk | Too little information to judge |
| Other bias | Unclear risk | Abstract only |
Saleem 2006.
| Methods | Patients randomly selected, randomisation method not described. | |
| Participants | Singleton live pregnancy BS 5 or lower, requiring induction between 37 and 42 weeks of gestation. | |
| Interventions | Foley catheter 40‐45 mL, after 8‐10 hours oxytocin infusion was started (n = 78). Dinoprostone pessary 3 mg 6‐hourly max 2 doses, followed by oxytocin infusion (n = 75). Oral misoprostol 50 mcg 4‐hourly, max 4 doses, followed by oxytocin infusion (n = 73). |
|
| Outcomes | Vaginal delivery rate, Induction to delivery interval < 12 hours, postpartum haemorrhage, tachysystole. | |
| Notes | Methods describe random selection of patients, not randomisation. No neonatal outcomes. Setting: Hamdard University Hospital and Patel Hospital, Pakistan Dates of study: July 2005 ‐ June 2005 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random selection' of patients, insufficient information for judgement. |
| Allocation concealment (selection bias) | Unclear risk | 'Random selection' of patients, insufficient information for judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear how many patients were assessed for randomisation or randomised, therefore it is also unclear if incomplete data were reported. There is no mention of this in the paper. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods section are reported, it is however interesting why they did not report any neonatal data. |
| Other bias | Unclear risk | No sample size calculated |
Salim 2011.
| Methods | RCT | |
| Participants | Inclusion: viable singleton pregnancy, cephalic presentation, intact membranes, BS of ≤ 6. Exclusion: contraindication for vaginal delivery, previous caesarean delivery, a low‐lying placenta, fetal malformations that were incompatible with postpartum life, intrauterine fetal death, clinical amnionitis, carriers of hepatitis B/C, HIV, allergy to latex. |
|
| Interventions | 1. Foley: (n = 145) 24 F, 60 mL, max 12 hours 2. Double balloon (n = 148), 80/80 mL, max 12 hours |
|
| Outcomes | Time from insertion of the catheter to delivery, mode of delivery, vaginal deliveries within 24 hours, abnormal fetal presentation, cord prolapse, intrapartum fever more than 38°C, bleeding related to catheter insertion, AS. | |
| Notes | Setting: Emek medical centre, Afula, Israel. (teaching medical centre) Study period: June 2008 and December 2010 Funding: Department of obstetrics, Emek medical centre Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers, block randomisation |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered allocation, stored in a box. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported and not clear if done, no missing data or cases. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Sanchez‐Ramos 1992.
| Methods | RCT. Computer‐generated allocation sequence. No details on concealment of allocation. | |
| Participants | Singleton vertex term pregnancies, intact membranes, BS < 6. Excluded if non‐reassuring FHR, placenta praevia, previous uterine scar, cervicitis. | |
| Interventions | Hygroscopic cervical dilators (as many as possible) (36 women); (PGE2) 4 mg gel applied to the cervical os (n = 38). After 8‐12 hours, repeat in both groups if cervix unfavourable. Followed by oxytocin and amniotomy. | |
| Outcomes | CS, instrumental vaginal delivery, haemorrhage, admission to NICU, infection. | |
| Notes | Unclear whether PG was intracervical or intravaginal. Setting: Univerity medical Center of Jacksonville, USA. largely high risk, low income obstetric population Study period: June 1988 to July 1989 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported and not clear if done, no missing data or cases reported |
| Selective reporting (reporting bias) | Unclear risk | No outcomes pre specified in method section |
| Other bias | Low risk | No other bias detected |
Sarreau 2016.
| Methods | RCT | |
| Participants | Inclusion: indication for IOL, vertex, singleton, > 37 weeks of GA, previous CS (transverse incision), BS < 5, no premature rupture of membranes, singleton in vertex presentation. Exclusion: < 18 years, placenta praevia, cervical infection, malpresentation, latex allergy, induction for CS |
|
| Interventions | 1. Foley catheter (n = 101): 50 mL, max 12 hours (N = 101) 2. oxytocin (N = 103), low‐dose perfusion |
|
| Outcomes | Vaginal birth rate, maternal and neonatal complications | |
| Notes | Abstract only (awaiting publication) Setting: France, multicentre Study period: December 2010 tot December 2013 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT, too little information to judge incomplete outcome data |
| Selective reporting (reporting bias) | Unclear risk | Too little information to judge |
| Other bias | Unclear risk | Abstract only |
Sciscione 1999.
| Methods | RCT. Computer‐generated allocation sequence. Concealment of allocation by opaque, sealed, consecutively‐numbered envelopes. | |
| Participants | Singleton vertex pregnancies with intact membranes, BS < 6. Term > 28 weeks. Inclusion of women with previous CS. | |
| Interventions | Intracervical PGE2 (0.5 mg) every 6 hours (72 women). Intracervical Foley catheter inflated with 30 mL (77 women). | |
| Outcomes | Spontaneous onset of labour, nausea, maternal discomfort measured with an analogue scale 0‐10, non‐reassuring FHR, hyperstimulation, use of epidural, use of oxytocin, shoulder dystocia, vaginal delivery. | |
| Notes | 12 women excluded (6 women in PGE2 group because of use of Foley catheter, 2 removed consent, 1 pre‐eclampsia, 1 BS of 7 and 2 breech). Setting: Medical centre of Delaware, USA. tertiary referral centre, Study period: July 1995 to July 1996 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT, women excluded because of protocol violation. no missing data. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Sharami 2005.
| Methods | Randomised trial: computer‐generated random number table, blocks of 4, sequentially‐numbered opaque sealed envelopes. | |
| Participants | Nulliparous women, admitted for induction, between 34 and 42 weeks GA, singleton in cephalic presentation, BS < 4, intact membranes, reassuring FHR tracing, < 6 contractions per hour Exclusion: significant vaginal bleeding, fetal chorioamnionitis, any contraindication to vaginal delivery, previous uterine scar, FHR abnormalities, severe pre‐eclampsia, contraindication to PG. |
|
| Interventions | Foley 30 mL + EASI + concurrent IV oxytocin for 12 hours (n = 76). PGE2 gel 0.5 mg intracervical 6‐hourly, max 3 doses (n = 75). |
|
| Outcomes | Interval from start induction to active phase, abnormal FHR tracing, tachysystole, hyperstimulation, meconium passage, caesarean delivery, chorioamnionitis, endometritis, AS < 7, admission NICU Secondary: start induction to delivery, change in dilation at 1, 6, 12 hours, CS for failed induction. |
|
| Notes | No sample size calculation. Prophylactic antibiotics after 12 hours of start induction Setting: Prenatal Clinic in Al‐Zahra Maternity Hospital, Iran Dates of study: March 2002 ‐ September 2003 Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Good description of excluded patients, quite evenly spread over the groups. There is no information about missing/incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | It seems that all outcomes prespecified in methods were reported, report includes all expected outcomes. |
| Other bias | Low risk | No other bias detected |
Shechter‐Maor 2015.
| Methods | RCT | |
| Participants | Inclusion criteria: singleton, GA 37 weeks or more, cephalic presentation, intact membranes, unfavourable cervix (BS =/< 6), oligohydramnios (AFI =/< 5) Exclusion criteria: multifetal gestation, fetal malpresentation, spontaneous labour, contraindication to PGs or a vaginal delivery (e.g. placenta previa), non‐reassuring FHR tracing, a fetus with major anomalies or previous CS |
|
| Interventions | 1. Propess (10 mg slow release PGE), n = 26 2. Double balloon (Cook), n = 26 |
|
| Outcomes | Time from induction to active labour (defined as cervical dilation of at least 5 cm), induction to delivery time, CS and operative delivery rates, oxytocin augmentation, uterine tachysystole (defined as greater than 5 uterine contractions in 5 minutes), meconium passage, FHR changes, AS and maternal satisfaction | |
| Notes | Setting: Israël Study period: not reported Funding: none received Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer‐generated, random sequences |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, no missing data described. No figure 1, all women analysed |
| Selective reporting (reporting bias) | High risk | All pre‐specified outcomes reported but secondary outcome 'AS' |
| Other bias | Unclear risk | Not mentioned in method section how long balloon/dinoprostone was given and what happened after ripening process. |
Sheikher 2009.
| Methods | RCT | |
| Participants | Inclusion: 1st or 2nd gravida, single, live fetus, cephalic, indication for IOL, GA 37‐42 weeks, BS ≤ 5, absence of uterine contractions. Exclusion: previous uterine surgery, non reassuring FHR tracing, IUGR, oligohydramnios, placenta praevia, multifetal pregnancy, chorioamnionitis, active herpes, EFW > 4 kg, renal or hepatic disease |
|
| Interventions | 1. Oral misoprostol (n = 30):, 50 mcg, repeated every 4 hours to a max of 5 doses. 2. Vaginal misoprostol (n = 30): 25 mcg, repeated every 4 hours to a max of 5 dosis. n = 30 3. Foley catheter (n = 30): 16 or 18 F, 35 mL. max of 16 hours In all 3 groups after 16 hours oxytocin was started. |
|
| Outcomes | interval from induction to birth, mode of delivery, maternal complication, neonatal outcome, failed induction | |
| Notes | Setting: SMGS hospital, Jammu, India Study period: over 1 year Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assigned, no more information reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT reported, cases missing in table 2, not clear why |
| Selective reporting (reporting bias) | High risk | No outcome measures were mentioned in the method section, induction to delivery interval is given but no SDs, |
| Other bias | Low risk | No other bias detected |
Solt 2009.
| Methods | RCT | |
| Participants | 100 primiparae and 100 multiparae women with an unfavourable BS | |
| Interventions | Foley catheter:(nulliparae n = 50) Double balloon:(nulliparae n = 45) |
|
| Outcomes | Primary outcomes were BS increment, time from catheter withdrawal to delivery, CS rate and post caesarean febrile morbidity. | |
| Notes | Abstract only, numbers only given for nulliparae Setting: Israel Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported it was a single‐blinded study, not how blinding was performed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 women excluded from analyses, not clear why |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Unclear risk | Abstract only |
Somirathne 2017.
| Methods | RCT | |
| Participants | Inclusion criteria: not delivered by 40 weeks + 5 days gestation, having uncomplicated pregnancies with a singleton fetus, longitudinal lie and cephalic presentation. Exclusion criteria were pregnancy‐induced hypertension, gestational diabetes mellitus, multiple pregnancies, planned CS, fetal growth restriction and scarred uterus |
|
| Interventions | 1. Foley catheter, (n = 89), 60 mL, max 24 hours 2. Low dose oral misoprostol (n = 91), 50 mcg, 3 gifts, 4 hourly (N = 91) In both groups, if cervix is unfavourable after 24 hours Foley group PGE2, oral misoprostol group Foley catheter) |
|
| Outcomes | The induction delivery interval following IOL, the mode of delivery, the reasons for operative delivery, maternal morbidity, hyperstimulation, uterine rupture, peripartum hysterectomy, postpartum blood transfusion or crystalloid transfusion, IV antibiotics, maternal pyrexia of > 38°C, fetal and neonatal outcome and morbidity, suspicious or pathological CTG according NICE guidelines, meconium‐stained liquor, birthweight, 1 minute AS, NICU and reason for admission | |
| Notes | Setting: University Unit of the THMG, Sri Lanka Study period: September 2014 to April 2015. Funding: none Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers, block randomisation,stratified by parity |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, no missing data or cases. referred to Figure 1, but not available |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
St Onge 1995.
| Methods | RCT. Computer‐generated allocation sequence. Concealment of allocation by sealed envelopes. | |
| Participants | Singleton vertex term pregnancies with intact membranes, BS < 5. Exclusion of women with previous CS, low‐lying placenta. | |
| Interventions | Intracervical PGE2 (0.5 mg) (30 women). Intracervical Foley catheter inflated with 30 mL (36 women). | |
| Outcomes | CS, instrumental delivery, maternal side effects, maternal pyrexia, fetal distress. | |
| Notes | 2 women excluded in each group. Also reported as abstract (Lange 1994). Setting: Foothills Hospital in Calgary, Alberta, Canada Study period: October 1991 to November 1993 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 women in each group excluded (not meeting inclusion criteria or in labour directly after randomisation). no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Suffecool 2014.
| Methods | RCT | |
| Participants | Inclusion criteria: nulliparous, 18 years or older, GA 37 weeks or more, singleton, cephalic presentation, intact membranes, BS < 6, admission for IOL. Exclusion criteria: contraindication for vaginal delivery (placenta praevia, non vertex presentation), ruptured membranes, severe pre‐eclampsia, suspected fetal growth restriction with abnormal dopplers, presence of a uterine scar, non reassuring FHR trace requiring medical intervention. |
|
| Interventions | 1. 10 mg dinoprostone vaginal insert (n = 31), max 12 hours, if after 12 hours unfavourable cervix start with oxytocin 2. Double balloon (Cook) (n = 31), 80 mL, oxytocin started 6 hours after placement. Balloon removed after max 12 hours. |
|
| Outcomes | Time from insertion of ripening method until delivery, delivery rate < 24 hours, CS rate, time to active labour, rate of operative vaginal delivery, maternal or fetal adverse events | |
| Notes | Setting: USA Study period: February 2011 ‐ September 2012 Funding: not reported Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | The allocation assignment was sealed in sequentially‐numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing data or cases. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Sullivan 1996.
| Methods | RCT. Concealment of allocation by opaque, sealed envelopes. | |
| Participants | BS < 6 and indication/no contraindication for IOL. | |
| Interventions | Intracervical PGE2 (0.5 mg) and Foley catheter inflated with 50 mL of water (41 women); intracervical PGE2 (0.5 mg) repeated after 4 to 6 hours if needed (37 women). | |
| Outcomes | CS, uterine hyperstimulation with and without FHR changes, infection. | |
| Notes | Setting: Jackson, USA Study period: October 1993 ‐ May 1994 Funding: supported by the Vicksburg hospital medical foundation Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not reported, but is reasonable as numbers are equal to randomised numbers, no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome reported. |
| Other bias | Low risk | No other bias detected |
Tabowei 2003.
| Methods | Random number table, opaque sealed envelopes. | |
| Participants | Term pregnancy, singleton fetus in cephalic presentation, BS < 4. Exclusion: ruptured membranes, placenta praevia, non‐reactive non‐stress test, EFW > 4000 g, prior uterine incision, parity > 4, contraindication to PGs. |
|
| Interventions | Foley 50 mL max 12 hours (n = 61). Vaginal misoprostol 25 mcg every 4 hours, max 6 doses (n = 60). |
|
| Outcomes | Failure to achieve ripening within 12 hours, vaginal delivery within 24 hours, need for oxytocin augmentation, CS rate, tachysystole, hypertonus, meconium, maternal and neonatal complications, AS < 7, NICU admissions, febrile morbidity. | |
| Notes | Prior uterine incision is exclusion criterion, but 18 women with previous CS included. Setting: Zonal general hospital, Nigeria Study period: June 1998 to May 2001 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data were not mentioned in the report. |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in methods were reported, report includes all expected outcomes. |
| Other bias | Unclear risk | Prior uterine incision is exclusion criterion, but 18 women with previous CS included, they were evenly divided between the groups. |
Tan 2015.
| Methods | RCT | |
| Participants | Inclusion criteria: pregnant women aged 21 to 40 years old with a singleton pregnancy with no major fetal anomaly who were suitable for vaginal delivery and scheduled for a planned IOL at 37‐41 + 6 weeks of gestation. Exclusion criteria: spontaneous labour, had a cervical dilatation of 3 cm or more, confirmed rupture of membrane, had abnormal cardiotocogram, a scarred uterus such as previous CS, malpresentation in labour, or if CS delivery was indicated |
|
| Interventions | 1. Double balloon (80 mL, balloon started with 40 mL, every following hour 20 mL inserted until 80 mL total), max 12 hours (N = 31) 2. PG 3 mg tablet, repeat once after 6 hours (N = 54) If not in labour or AROM possible after 12 hours, further management by local physician. |
|
| Outcomes | Not clearly mentioned in method section | |
| Notes | Setting: tertiary referral maternity unit in Singapore. Study period: not reported Funding: double balloons provided by Cook Medica Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of 150 envelopes with equal numbers of chance for intervention or control, labelled sequentially |
| Allocation concealment (selection bias) | Low risk | Sealed envelope, next allocated number of envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not described, seems per protocol as woman in pain and breech during labour were excluded; no missing data described |
| Selective reporting (reporting bias) | Unclear risk | No pre specified outcomes reported, so cannot be judged |
| Other bias | Low risk | No other bias detected |
ten Eikelder 2016.
| Methods | RCT | |
| Participants | Inclusion: singleton, scheduled for labour induction, GA ≥ 37 wk; BS < 6, vertex presentation, intact membranes. Exclusion: placenta previa, previous uterine scar. contraindication to receive or known allergy to latex or PG. |
|
| Interventions | 1. Foley catheter (n = 921): 30 mL, no traction, replaced after 48 hours, max 4 days 2. Low dose oral misoprostol (n = 924): 50 mg every 4 hours, max 3 times a day, max 4 days |
|
| Outcomes | Primary outcome for safety was composite of fluxus postpartum and asphyxia, and for effectiveness CS rate. Secondary outcomes included maternal and neonatal outcomes, total induction time, interval between randomisation and active phase | |
| Notes | Setting: multicentre, 6 tertiary‐care and 23 secondary‐care hospitals, the Netherlands Study period: July 2012 to October 2013, Funding: FondsNutsOhra, no role in study design, data collection, data analysis, data interpretation, writing of the report or publication Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers, block randomisation, stratified by parity and centre |
| Allocation concealment (selection bias) | Low risk | Web‐based allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no missing cases. missing data in primary outcome (pH in umbilical artery). similar reasons for missing data across groups, pre‐specified in protocol, anticipated on as followed: data missing for umbilical artery pH and a 5‐minute AS of less than 7, the outcome was classified as abnormal; for patients with missing data for umbilical artery pH and a 5‐minute AS of 7 or more, the neonatal outcome was classified as normal. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported in results |
| Other bias | Low risk | No other bias detected |
Thiery 1981.
| Methods | RCT. Concealment of allocation by envelopes. | |
| Participants | Singleton vertex term pregnancies. Favourable cervix (BS > 5). | |
| Interventions | Foley catheter with a balloon inflated with 30 mL saline and PGE2 0.5 mg extra‐amniotic (48 women); PGE2 0.5 mg extra‐amniotic (43 women); amniotomy and oxytocin if needed (52 women). | |
| Outcomes | No outcomes reported. | |
| Notes | No relevant outcomes reported. Setting: Belgium Study period: not reported Funding: received free PGE2. not clear if this gift is related to a pharmaceutical company Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prepared envelopes with numbers |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Unclear if ITT was performed, but is reasonable as numbers are equal to randomised numbers), no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | No other bias detected |
Tita 2006.
| Methods | RCT | |
| Participants | Inclusion: preterm rupture of membranes, cervix ≤ 2 cm GA ≥ 34 weeks, singleton gestation, cephalic, Exclusion: regular uterine contractions (contractions more frequent than every 5 minutes), 2 prior transverse uterine incisions/vertical uterine incision/transmural myomectomy or any obstetric contraindication to labour, evidence of chorioamnionitis, lethal fetal anomalies, intrauterine fetal demise, placenta previa, suspected abruption/significant haemorrhage, non‐reassuring FHR pattern |
|
| Interventions | Foley + oxytocin (n = 87) oxytocin only (n = 82) |
|
| Outcomes | Reported outcomes: hours from placement of Foley or initiation of oxytocin to delivery, rate of delivery (vaginal or caesarean) within 24 hours caesarean rate, induction to vaginal delivery interval. | |
| Notes | Grey literature: study terminated, primary outcomes reported in trial registration. Setting; USA Study period: December 2005 ‐ May 2008 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear if ITT analyses was used. for CS there are missing cases |
| Selective reporting (reporting bias) | High risk | Only primary outcomes were reported in trial registration |
| Other bias | Unclear risk | Study was terminated (not clear why) |
Turnquest 1997.
| Methods | RCT | |
| Participants | Term women with unfavourable cervix (BS < 5), intact membranes. | |
| Interventions | Laminaria (as many as possible) and (PGE2) vaginal gel (4 mg) (21 women); (PGE2) vaginal gel (4 mg) alone (27 women). | |
| Outcomes | Need for oxytocin, CS, uterine hyperstimulation, admission to NICU, chorioamnionitis. | |
| Notes | Setting: memorial hospital Indianapolis, USA Study period: October 1994 to May 1995 Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Concealment of allocation by consecutively‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 women excludes because of protocol violation, no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias detected |
Wang 2012.
| Methods | RCT | |
| Participants | Inclusion criteria: primiparae, full‐term, singleton with cephalic presentation; indication for labour induction; intact membranes; BS < 6; no contra indication for vaginal delivery | |
| Interventions | Foley catheter (n = 138); 16F Foley, 80cc fluid, max 24 hours Propess: (n = 124), 10 mg slow release dinoprostone, fornix posterior, max 24 hours afterwards started with oxytocin. if after 3 days labour did not started, IOL was declared failed |
|
| Outcomes | The duration of placement (of Propess or catheter, mode of delivery and time from IOL to delivery; usage of oxytocin, postpartum haemorrhage; meconium‐stained amnion fluid, AS, post‐delivery temperature monitoring (for a total of 10 days); 42 days after delivery follow‐up interview to check for lochia appearance or signs of infection. | |
| Notes | Article in Chinese => translated by native speaker Setting: China Study period; not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT, women were excluded for different reasons during the trial (n = 8) |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Unclear risk | Judged from a translated article |
Wang 2014.
| Methods | RCT | |
| Participants | Inclusion: oligohydramnios (AFI < 5 cm). GA beyond 37 0/7 weeks’, singleton pregnancy, vertex presentation, BS ≤ 6, intact membranes, the absence of documented uterine contractions, the absence of prior CS delivery, reassuring antenatal fetal testing (non‐stress test) active, and oxytocin challenge test negative upon study entry. Exclusion: antepartum bleeding, chorioamnionitis, placenta previa, or any other contraindication to vaginal delivery, women with documented PG allergy, maternal asthma history, vaginitis or cervicitis at presentation, and/or glaucoma history were not eligible for the pharmacological treatment arm |
|
| Interventions | Double balloon (n = 67): 80/80cc, no traction, max 12 hours. After 24 hours unsuccessful ripening start oxytocin 10 mg dinoprostone insert (n = 59), fornix posterior, max 24 hours, After 24 hours unsuccessful ripening start oxytocin |
|
| Outcomes | Pregnancy outcomes and success of induction | |
| Notes | Setting: The People’s Liberation Army 174th Hospital, Xiamen, China, Study period: April 2010 ‐ February 2011 Funding: financial support of The People’s Liberation Army Nanjing Military Area Command Medicine Health Department in China. Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope randomisation, opaque? |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT => 5 woman reassigned after randomisation (non re‐assuring FHR, failed placement) no missing data or cases |
| Selective reporting (reporting bias) | Unclear risk | No pre specified outcomes reported, so can't be judged |
| Other bias | Low risk | No other bias detected |
Wu 2017.
| Methods | RCT | |
| Participants | Inclusion: 18–40 years old; 37 + 0‐41 + 6 gestational weeks; BS ≤ 6; single alive fetus with cephalic presentation; in cephalopelvic proportion; without premature rupture of membrane; NST reaction type before labour induction. The indications of labour induction included delayed pregnancy, oligohydramnios (AFI = 3.0–8.0 cm), gestational diabetes mellitus, intrahepatic cholestasis of pregnancy, good control of gestational hypertension, with vaginal trial production condition and required pregnancy termination. Exclusion: placenta previa, vasa previa and APH; invasive cervical carcinoma; untreated HIV infection; allergic to induction drugs. |
|
| Interventions | Double‐balloon combined with IV drip of oxytocin (n = 60) AROM after 12 hours. IV drip of oxytocin at a concentration of 0.5% (n = 60); AROM after 12 hours If the patients did not enter the stage of active labour within 48 hours, the labour induction was regarded as failing, and other methods for pregnancy termination were used |
|
| Outcomes | Postpartum haemorrhage, cervical laceration, uterine rupture, puerperal infection, neonatal asphyxia, neonatal infection and meconium aspiration syndrome | |
| Notes | Setting: China Study period: January 2014 ‐ June 2015 Funding: grants received from the Nature Science Foundation of China, the Science and Technology Project of Special Funds of Guangzhou, Guangdong Science and Technology Project, the Natural Science Foundation of Guangdong Province and Guangzhou Science and Technology Project Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women randomly divided, no information given |
| Allocation concealment (selection bias) | Unclear risk | No information reported on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not mentioned, but is reasonable as numbers are equal to randomised numbers. no missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias detected |
Yuen 1996.
| Methods | RCT | |
| Participants | Singleton vertex presentation, intact membranes, BS < 5, no previous CS. | |
| Interventions | Atad device (100 mL) (36 women); intracervical PGE2 (0.5 mg) (39 women); vaginal pessary 0.5 mg PGE2 (39 women). | |
| Outcomes | Change in BS, vaginal delivery achieved within 12 and 24 hours, CS, instrumental delivery, vaginal bleeding, uterine hyperstimulation, AS. | |
| Notes | 5 women were excluded (2, 2 and 1, respectively). Setting: Prince of Wales Hospital, Honkong teaching hospital, China Dates of study: period of 18 months Funding sources: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 women excluded because of protocol violation, no other missing cases or data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcome reported |
| Other bias | Low risk | No other bias detected |
Zahoor 2014.
| Methods | RCT | |
| Participants | Women requiring IOL for common indications, no previous CS | |
| Interventions | PGE2 tablets (n = 100) dosage of 2 mg, every 6 hours, max of 4 doses transcervical balloon catheter(n = 100) filled with 60 mL of saline. |
|
| Outcomes | Induction to delivery interval, mode of delivery, meconium staining, CTG abnormalities, admission in NICU, low AS | |
| Notes | No relevant outcomes were reported in the abstract Setting: Pakistan Study period: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not feasible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT not reported, insufficient information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Unclear risk | Abstract only, too little information to judge risk of bias |
AFI: amniotic fluid index APH: antepartum haemorrhage ARM/AROM: artificial rupture of membranes AS: Apgar score BMI: body mass index BS: Bishop score CPD: cephalopelvic disproportion CS: caesarean section CTG: Cardiotocography EASI: extra‐amniotic saline infusion EFW: estimated fetal weight FHR: fetal heart rate GA: gestational age GBS: group B Streptococcus GI: gastrointestinal ICU: intensive care unit IOL: induction of labour IFD/IUFD intrauterine fetal death ITT: intention‐to‐treat IV: intravenous LSCS: lower segment caesarian section max: maximum Mbs: modified Bishop Score mcg: microgram mL: millilitre mg: milligram mU: milliunits NICU: neonatal intensive care unit NST: non‐stress test PBU: premature baby unit PCM: PG: prostaglandin PGE2: prostaglandin E2 PROM: pre labour rupture of membranes RCT: randomised controlled trial SROM: spontaneous rupture of membranes US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramovici 1999 | It is unclear whether all women had Foley catheter (included as 'intention to ripe the cervix' with Foley catheter). Women only received a Foley catheter when they had no dilation at the start of induction (for this study this was the control group), and concurrent oxytocin was started. It is unclear how many women received a Foley catheter. |
| Adeniji 2005a | Primary outcome fibronectin, other outcomes not mentioned. |
| Adeniji 2005b | High‐dose misoprostol |
| Adeniji 2006 | Outcome cervical scores, other outcomes not mentioned. |
| Afolabi 2005 | Only reports outcomes for the successfully induced, thus not useful. |
| Ahmad 2015 | laminaria vs Foley => not within scope of review |
| Anabosy 2014 | Trial stopped before start patient inclusion because of technical issues |
| Arsenijevic 2012 | No dilatator vs hegar vs continues, controlled, balloon dilator => not within scope of review |
| Arshad 2016 | Laminaria prior to PGE2 vs nothing prior to PGE2 => not within scope of review |
| Atad 1991 | No randomised comparison of mechanical methods. A subgroup of women were randomised to receive PGE2 or placebo. |
| Atad 1999 | Compares 2 mechanical regimens. |
| Baacke 2006 | Trial registration, expected end date expired > 2 years. no information could be obtained (authors were contacted) |
| Barrilleaux 2002a | High‐dose misoprostol |
| Behrashi 2013 | Trial registration with no publication. anticipated end date 2013 => no information could be obtained (authors were contacted) |
| Ben‐Aroya 2001 | There is no mention of randomisation in the abstract. Retrospective cohort study. |
| Buccellato 2000 | High‐dose misoprostol |
| Cahill 1988 | Alternate randomisation. |
| Caughey 2007 | Balloon high vs low volume => not within scope of review |
| Chipato 1997 | 2 regimens of extra‐amniotic infusion compared. |
| Chung 2003 | High‐dose misoprostol |
| Connolly 2016 | Foley+ oxytocin vs Foley => not within scope of review |
| Connolly 2017 | Foley + oxytocin vs Foley (multiparae) => not within scope of review |
| Cross 1978 | Randomisation based on the last digit of the hospital chart number. 6 women were excluded in the laminaria group, and 1 in the control group. No clinical outcomes were reported. |
| Cullimore 2009 | Trial registration. study terminated after n = 5).no information could be obtained (authors were contacted) |
| De Oliveira 2003 | Foley vs no ripening => not in scope |
| Delaney 2010 | Comparison of 2 mechanical methods. |
| Demirel 2015 | Nipple stimulation, no mechanical method included |
| Dias 2008 | Trial registration, expected end date expired > 2 years. no information could be obtained (authors were contacted) |
| Du 2015 | Not randomised. women could choose induction method |
| Edwards 2017 | Foley + PGE2 vs Foley => not within scope of review |
| El Sharkwy 2017 | Foley + miso vs Foley (and miso after 12 hours) => not within scope of review |
| El‐Khayat 2016 | Foley + isorbide mononitrate vs misoprostol => not within scope of review |
| El‐Torkey 1995 | Foley + EASI vs Foley => not in scope |
| Emery 1988 | No information. |
| EUCTR 2012 | Trial registration, expected end date expired > 2 years. no information obtained (authors were contacted) |
| Filshie 1992 | Insufficient information. |
| Forgie 2016 | Placement stylette vs no stylette => not within scope of review |
| Forooshani 2011 | Foley vs laminaria => not within scope of review |
| Fruhman 2017 | Tension vs no tension => not within scope of review |
| Gadel 2015 | Cervical ripening in case of stillbirth |
| Garebedian 2016 | Foley vs expectative management |
| Ghanaei 2009 | Foley + oxytocin vs EASI + oxytocin |
| Ghanaie 2013 | Foley +oxytocin vs EASI + oxytocin vs PGE2 + oxytocin => not within scope of review |
| Gibson 2013 | different kind of traction applied => not within scope of review |
| Gilson 1996 | Dilapan vs no treatment => not in scope |
| Gonsoulin 1989 | No clinical outcome reported. |
| Gower 1982 | Laminaria vs placebo => not in scope |
| Greybush 2001 | High‐dose misoprostol |
| Gu 2015 | Low‐ vs high‐volume balloon => not within scope of review |
| Guinn 2004 | Compares 2 mechanical regimens. |
| Haghighi 2015 | EASI vs isoniazide => not within scope of review |
| Hallak 2008 | Foley vs Foley + EASI vs ATAD + EASI => not in scope |
| He 2000 | Air vesicle odinopoeia => not within scope of review |
| Hill 2009 | High‐dose misoprostol |
| Hill 2013 | Balloon + miso vs balloon + placebo => not within scope of review |
| Hussein 2012 | Induction for fetal demise or early PE (begin third trimester), so no viable fetus |
| Ifnan 2006 | Hydrostatic membrane sweeping vs Foley => not within scope of review |
| Jagani 1984 | An extra‐amniotic catheter is used in all groups to record the uterine activity. This catheter uses a 5 mL balloon, which is much lower than the volume used by the other authors (30 mL to 40 mL). Thus, this study is a comparison between oxytocin and PG, with a control group without intervention. |
| Jasper 2000 | No clinical outcome reported (reported as abstract). |
| Jindal 2007 | Methods are interchanged after 24 hours, outcomes are given for the totals. |
| Jonsson 2011 | Digital vs manual placement Foley => not within scope of review |
| Kamilya 2011 | Trial registration, expected end date expired > 2 years. no information could be obtained (authors were contacted) |
| Karjane 2006 | Compares 2 mechanical regimens. |
| Kasdaglis 2007 | The randomisation scheme is unclear and the numbers in both groups are very different (32 and 24). |
| Kashanian 2006 | High‐dose misoprostol |
| Kashanian 2009a | Comparison of 2 mechanical regimens. |
| Kehl 2012 | 2 hours cook balloon before vaginal miso vs no balloon before vaginal miso => not within scope of review |
| Kehl 2015 | Balloon before oral misoprostol vs no balloon before oral misoprostol => not within scope of review |
| Keirse 1983 | No clinical outcome reported. |
| Lackritz 1979 | Laminaria vs no treatment => not in scope |
| Lam 2006 | Foley +oxytocin vs EASI + oxytocin => not within scope of review |
| Leiberman 1977 | Alternate inclusion in each group. Imbalance between groups in numbers and prognostic factors. |
| Leong 2017 | Menbrane sweeping vs Foley => not within scope of review |
| Levine 2016 | High‐dose misoprostol |
| Levy 2000 | Comparison between early and late amniotomy. |
| Levy 2004 | Comparison between 2 mechanical regimens. |
| Lin 1995 | Laminaria vs EASI => not in scope |
| Lin 2006 | Trial registration only, study terminated. |
| Lin 2007 | Compares 2 mechanical regimens. |
| Lutgendorf 2012 | Traction vs no traction => not within scope of review |
| Macpherson 1983 | No clinical outcomes mentioned. |
| Mahomed 1988 | Foley catheter under traction compared with Foley catheter with extra‐amniotic PGE2. |
| Manabe 1985 | No clinical outcomes. |
| Manish 2016 | High‐ vs low‐volume balloon |
| Manyonda 2007 | Balloon vs expectant management => not in scope |
| Martin 1989 | Comparison of induction of labour vs surveillance in post‐term pregnancy. |
| Mattingly 2015 | Double balloon 12 hours vs double balloon 24 hours |
| Mawire 1999 | EASI vs PGE f2 alpha => not in scope |
| McGee 2016 | Foley silicone vs Foley latex |
| Mei‐Dan 2009 | Comparison of 2 mechanical regimens. |
| Mei‐Dan 2012 | Trial terminated before start. |
| Mei‐Dan 2012a | Foley +EASI vs Cook balloon |
| Mei‐Dan 2014 | Single balloon + EASI vs double balloon + EASI |
| Miller 2015 | Induction vs expectant management. (choice of induction method was up to clinician.) |
| Moise 1991 | Duplicate information, already included. |
| Morrison 1993 | Insufficient information. |
| Movahed 2016 | Foley vs laminaria vs isorbide mononitrate |
| Mullin 2014 | Direct removal of Foley or not |
| Naseem 2007 | Quasi‐experimental, every second patient gets Foley |
| Nasir 2012 | Quasi‐experimental |
| Neethurani 2013 | Foley + EASI followed by miso vs miso |
| Owolabi 2005 | High‐dose misoprostol |
| Park 2011 | Trial registration, expected end date expired > 2 years. No information could be obtained (authors were contacted) |
| Pathiraja 2014 | Trial registration, anticipated end date (2014) has expired > 2 years. No information could be obtained (authors were contacted) |
| Pedersen 1981 | Comparison of the addition or not of estradiol to Foley catheter. |
| Pettker 2008 | Comparison of 2 mechanical regimens. |
| Rameez 2007 | Nitric oxide vs vitamin C |
| Reif 2012 | Trial registration, anticipated end date (2015) has expired > 2 years. No information could be obtained (authors were contacted) |
| Rezk 2014 | Foley vs isorbide mononitrate |
| Rust 2001 | High‐dose misoprostol |
| Saad 2016 | Foley vs laminaria |
| Saito 1999 | Comparison of 2 mechanical regimens. |
| Salmeen 2012 | Outpatient, pre‐induction Foley before pharmacological hospital induction |
| Sanchez‐Ramos 1990 | Insufficient information. |
| Sandberg 2017 | High vs low volume |
| Schoen 2017 | Foley + oxytocin vs Foley |
| Schreyer 1989 | Allocation of women was performed according to alternate weeks. |
| Sciscione 2001 | High‐dose misoprostol |
| Sharma 2015a | Foley: direct removal or not. |
| Sharma 2017 | Foley vs mifepristone => not in scope |
| Sherman 2001 | Comparison of PGE2 infusion vs saline infusion extra‐amniotically. This comparison is not included in this review. |
| Siddiqui 2013 | Placement Foley: stylette vs no stylette |
| Suri 2000 | No clinical outcome reported (reported only as abstract). |
| Thigpen 2004 | Compares a mechanical method with very high dose misoprostol (250 mcg). |
| Thomas 1986 | Randomisation by odd and even numbers of hospital charts |
| Torbenson 2015 | Outpatient Foley vs inpatient miso or Foley. Choice of inpatient method by clinician, so no RCT |
| Ugwu 2013 | Balloon vs misoprostol, crossover after 24 hours |
| Vengalil 1998 | High‐dose misoprostol |
| Walfisch 2014 | Foley vs expectative management |
| Walfisch 2015 | Balloon + EASI vs balloon |
| Welt 1987 | Insufficient information. |
| Wickramasinghe 2014 | Foley 24 hours vs Foley 48 hours |
| Wilkinson 2015 | Inpatient vs outpatient double balloon |
| Yaddehige 2015 | Membrane sweeping vs massage => not in scope |
| Yazdani 2011 | Trial registration of which anticipated end date (2008) has expired > 2 years. Trial was registered in retrospect. not clear why there is no publication. no information could be obtained (author contacted) |
| Zakaria 2017 | Different charriere Foley catheter |
| Zhang 2014 | Trial registration, anticipated end date (2015) has expired > 2 years. No information could be obtained (authors contacted) |
| Zimmer 1996 | No outcomes reported. The authors focused on breathing movements of the fetus. |
EASI: extra‐amniotic space infusion PG: prostaglandin PGE2: prostaglandin E2 RCT: randomised controlled trial vs: versus
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12618000510246 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Agboghoroma 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Publication could not be obtained. To try again in next update |
Amorosa 2017a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Bauer 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Chai 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Cherian 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
CTRI/2018/01/011574.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
de Vaan 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
DeCesare 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Diguisto 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
EUCTR2017‐001914‐27‐GB 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
IRCT20170326033142N2 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
IRCT20170513033941N39 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
IRCT20181123041731N1 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Khatib 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Leigh 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Lim 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Mallah 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Authors contacted. outcomes reported in Iranian magazine, asked authors for reference |
McGee 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Mohamad 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03172858 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03399266 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03435458 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03588585 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03629548.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03629548 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03670836 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03682718 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03744078 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03752073 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
NCT03866772 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Oskei 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Osoti 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Saad 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Sanmugam 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Souizi 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
ten Eikelder 2017.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Tulek 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Viteri 2019.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Found in search update of March 2019 => classification will be done in next update |
Characteristics of ongoing studies [ordered by study ID]
Argilagos 2016.
| Trial name or title | Comparison of vaginal misoprostol plus supracervical balloon versus vaginal misoprostol alone for induction of labor |
| Methods | RCT |
| Participants | |
| Interventions | Foley + vaginal misoprostol Vaginal misoprostol |
| Outcomes | |
| Starting date | Unknown |
| Contact information | |
| Notes | Author contacted: still recruiting |
Beckmann 2013.
| Trial name or title | Prostaglandin Inpatient iNduction of labour Compared with BALLOon Outpatient iNduction of labour: a randomised controlled trial ‐ The PINC BALLOON Study |
| Methods | RCT |
| Participants | |
| Interventions | Foley Vaginal PGE2 |
| Outcomes | |
| Starting date | Unknown |
| Contact information | |
| Notes | Author contacted: recruiting |
Bekele 2017.
| Trial name or title | A randomised controlled trial of sequential versus simultaneous use of Foley balloon and oxytocin for induction of labour in nulliparous pregnant women |
| Methods | |
| Participants | Foley + oxytocin Foley |
| Interventions | |
| Outcomes | |
| Starting date | 9 August 2017 |
| Contact information | |
| Notes | Status unknown. author contacted |
Berndl 2016.
| Trial name or title | High volume Foleys increasing vaginal birth (high 5 birth) pilot trial |
| Methods | |
| Participants | |
| Interventions | Balloon Prostaglandin |
| Outcomes | |
| Starting date | December 2016 |
| Contact information | |
| Notes | Recruiting (estimated end date: September 2019) |
Bhide 2017.
| Trial name or title | Prostaglandin insert (propess) versus trans‐cervical balloon catheter for out‐patient labour induction: a randomised controlled trial of feasibility |
| Methods | |
| Participants | |
| Interventions | Foley Vaginal PGE2 |
| Outcomes | |
| Starting date | September 2017 |
| Contact information | |
| Notes | Recruiting (anticipated end date: August 2018) |
Eser 2016.
| Trial name or title | Compare prostaglandin e2 against to combined transcervical Foley catheter balloon and vaginal prostaglandin e2 for induction of labour at term: a randomised study |
| Methods | |
| Participants | |
| Interventions | Foley + vaginal PGE2 Vaginal PGE2 |
| Outcomes | January 2016 |
| Starting date | |
| Contact information | |
| Notes | Recruitment completed in January 2018 |
Goli 2017.
| Trial name or title | Comparison the results of induction of vaginal misoprostol with Foley catheter in prolonged pregnancy with unripe cervix |
| Methods | |
| Participants | |
| Interventions | Foley Vaginal misoprostol |
| Outcomes | |
| Starting date | March 2017 |
| Contact information | |
| Notes | Estimated end date: June 2017 => author contacted. status unknown |
Goonewardene 2016.
| Trial name or title | Oral misoprostol for 48 hours versus an intracervical Foley catheter for 48 hours for induction of labour in post dated pregnancies: a randomised control trial |
| Methods | |
| Participants | |
| Interventions | Foley catheter Oral misoprostol |
| Outcomes | |
| Starting date | October 2016 |
| Contact information | |
| Notes | Recruitment completed |
Gupta 2016.
| Trial name or title | A randomised controlled trial of a synthetic osmotic cervical dilator for induction of labour in comparison to dinoprostone vaginal insErt: the SOLVE Trial |
| Methods | |
| Participants | |
| Interventions | Laminaria Vagina PGE2 |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Not yet recruiting |
Hassanzadeh 2017.
| Trial name or title | Misoprostol versus Foley catheter for cervical ripening in women with pre‐eclampsia or gestational hypertension |
| Methods | |
| Participants | |
| Interventions | Foley Misoprostol |
| Outcomes | |
| Starting date | February 2017 |
| Contact information | |
| Notes | Authors contacted. Still recruiting? |
Igwe 2017.
| Trial name or title | Comparison between intravaginal misoprostol tablet and intracervical Foley's catheter in a low resource setting |
| Methods | |
| Participants | |
| Interventions | Foley Vaginal misoprostol (dosage unclear) |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Recruitment completed in April 2018 |
Lacarin 2017.
| Trial name or title | Comparison between two strategies of induction in case of unfavourable cervix after 12 hours of premature rupture of membranes (prom) at term: cook cervical ripening + oxytocine from 6 hours versus dinoprostone vaginal insert |
| Methods | |
| Participants | |
| Interventions | Foley Vaginal PGE2 |
| Outcomes | |
| Starting date | October 2017 |
| Contact information | |
| Notes | Expected end date: July 2020 |
Lauterbach 2017.
| Trial name or title | A comparison between labour induction with dinoprostone and a cervical ripening balloon in women with a BMI > 30 as oppose with a BMI < 30 |
| Methods | |
| Participants | |
| Interventions | Balloon PGE2 |
| Outcomes | |
| Starting date | January 2017 |
| Contact information | |
| Notes | Expected end date: January 2019 |
Levy 2016.
| Trial name or title | A randomised controlled study comparing cervical Foley catheter, vaginal dinoprostone and a combination of the two methods for induction of labor |
| Methods | |
| Participants | |
| Interventions | Foley + PGE2 PGE2 |
| Outcomes | |
| Starting date | February 2016 |
| Contact information | |
| Notes | Not yet recruiting |
Osoti 2016.
| Trial name or title | A combination of Foley balloon and misoprostol versus misoprostol alone for induction of labour at Kenyatta national hospital, a randomised controlled trial |
| Methods | |
| Participants | |
| Interventions | Foley + misoprostol Misoprostol |
| Outcomes | |
| Starting date | March 2016 |
| Contact information | |
| Notes | Recruitment completed |
Park 2012.
| Trial name or title | Foley catheter versus dinoprostone vaginal insert for induction of labour in parous women at term: a randomised trial |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | May 2012 |
| Contact information | |
| Notes | Trial status unclear. expected end date 2017 => authors contacted |
Perrotin 2016.
| Trial name or title | Propess® versus double balloon for cervical ripening of prolonged pregnancies: a randomised controlled trial |
| Methods | |
| Participants | |
| Interventions | Double balloon Vaginal PGE2 |
| Outcomes | |
| Starting date | September 2016 |
| Contact information | |
| Notes | Expected end date: January 2020 |
Tagore 2015.
| Trial name or title | Cervical ripening balloon in induction of labour at term (crbii) ‐ a prospective randomised controlled trial |
| Methods | |
| Participants | |
| Interventions | PGE2 Balloon |
| Outcomes | |
| Starting date | December 2015 |
| Contact information | |
| Notes | Expected end date: March 2018 => recruiting |
Viteri 2015.
| Trial name or title | The efficacy of transcervical Foley balloon plus vaginal misoprostol versus vaginal misoprostol alone for cervical ripening in nulliparous obese women: a randomised, comparative effectiveness trial |
| Methods | |
| Participants | |
| Interventions | Foley + misoprostol Misoprostol |
| Outcomes | |
| Starting date | December 2015 |
| Contact information | |
| Notes | Recruiting |
Wise 2016.
| Trial name or title | Comparison of low‐risk pregnant women undergoing induction of labour at term by outpatient balloon or inpatient prostaglandin in order to assess vaginal birth rate; a randomised controlled trial |
| Methods | |
| Participants | |
| Interventions | Balloon PGE2 |
| Outcomes | |
| Starting date | March 2016 |
| Contact information | |
| Notes | Not yet recruiting |
Yildirim 2017.
| Trial name or title | Dinoprostone vaginal insert versus double balloon catheter for preinduction cervical ripening |
| Methods | |
| Participants | |
| Interventions | Double balloon PGE2 |
| Outcomes | |
| Starting date | January 2017 |
| Contact information | |
| Notes | Recruitment completed |
BMI: body, mass index PGE2: prostaglandin E2 RCT: randomised controlled trial
Differences between protocol and review
Comparisons of a balloon catheter with concurrent oxytocin or prostaglandins versus prostaglandins or oxytocin alone were added. Also, comparisons of a single versus a double balloon and different forms of laminaria tents were added. The comparisons with placebo/no treatment were excluded. Regarding studies where a comparison was made with misoprostol, we chose only to include studies in which low‐dose misoprostol was used. A number of non pre‐specified outcomes relevant to the comparisons made in this review were added (maternal fever, antibiotics during labour, endometritis, chorioamnionitis. fetal distress, umbilical artery pH < 7.10).
For this update, we have also searched ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP).
Contributions of authors
For this update, M de Vaan, M ten Eikelder, KR Palmer and BW Mol performed data extraction. Risk of bias was assessed by M de Vaan, M ten Eikelder, KR Palmer and M Jozwiak. Marieke de Vaan and Marta Jozwiak entered the data and the review was drafted M de Vaan, which was finalised after feedback from M. ten Eikelder, M Jozwiak, KR Palmer, M Davies, K Bloemenkamp, BW Mol and M Boulvain. M. ten Eikelder, M. Jozwiak and BW Mol were not involved in data extraction nor in the 'Risk of bias' assessment of Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016, due to their authorship.
Sources of support
Internal sources
University of Geneva, Switzerland.
External sources
-
Marieke de Vaan received a grant from The Netherlands Organisation for Scientific Research (NWO), Netherlands.
grant number: 023.011.051
Declarations of interest
Marieke de Vaan received a grant from The Netherlands Organisation for Scientific Research (NWO) (023.011.051).
Mieke ten Eikelder is co‐author of three included trials (Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016). She has not been involved in the 'Risk of bias' assessment and data extraction of these studies.
Marta Jozwiak is co‐author of four included trials (Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016). She has not been involved in 'Risk of bias' assessment and data extraction of these studies.
Kirsten Palmer: none known.
Miranda Davies‐Tuck: none known.
Kitty Bloemenkamp is co‐author of four included trials (Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016). She has not been involved in 'Risk of bias' assessment and data extraction of these studies.
Ben Willem Mol is co‐author of four included trials (Jozwiak 2012; Jozwiak 2013; Jozwiak 2014; ten Eikelder 2016). He has not been involved in 'Risk of bias' assessment and data extraction of these studies. Ben Willem Mol also reports receiving grants from NHMRC Australia, personal fees from ObsEva, grants and personal fees from Merck and Guerbet, outside the submitted work.
Michel Boulvain: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aduloju 2016 {published data only}
- Aduloju OP, Akintayo AA, Adanikin AI, Ade‐Ojo IP. Combined Foley's catheter with vaginal misoprostol for pre‐induction cervical ripening: A randomised controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology 2016;56:578‐84. [DOI] [PubMed] [Google Scholar]
Ahmed 2016 {published data only}
- Ahmed WA, Ibrahim ZM, Ashor OE, Mohamed ML, Ahmed MR, Elshahat AM. Use of the Foley catheter versus a double balloon cervical ripening catheter in pre‐induction cervical ripening in postdate primigravidae. Journal of Obstetrics and Gynaecology Research 2016;42(11):1489‐94. [DOI] [PubMed] [Google Scholar]
Al‐Ibraheemi 2018 {published data only}
- Al‐Ibraheemi Z, Brustman L, Bimson B, Porat N, Rosenn B. Misoprostol with foley bulb vs. misoprostol alone for cervical ripening: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2017;216(1):S473, Abstract no: 825. [DOI] [PubMed] [Google Scholar]
- Al‐Ibraheemi Z, Brustman L, Bimson BE, Porat N, Rosenn B. Misoprostol with foley bulb compared with misoprostol alone for cervical ripening: a randomized controlled trial. Obstetrics and Gynecology 2018;131(1):23‐9. [DOI] [PubMed] [Google Scholar]
- Al‐Ibraheemi Z, NCT02566005. A randomized comparison of transcervical foley bulb with vaginal misoprostol to vaginal misoprostol alone for induction of labor. clinicaltrials.gov/ct2/show/record/NCT02566005 (first received 1 October 2015).
Allouche 1993 {published data only}
- Allouche C, Dommesent D, Barjot P, Levy G. Cervical ripening: comparison of three methods. Preliminary results of a randomized prospective study. Revue Francaise de Gynecologie et d'Obstetrique 1993;88:492‐7. [PubMed] [Google Scholar]
Al‐Taani 2004 {published data only}
- Al‐Taani MI. Comparison of prostaglandin E2 tablets or foley catheter for labour induction in grand multiparas. Eastern Mediterranean Health Journal 2004;10(4/5):547‐53. [PubMed] [Google Scholar]
Amorosa 2017 {published data only}
- Amorosa J, Booker W, Miller M, Factor S, Stone J, Bianco A. A randomized trial of foley bulb for labor induction in premature rupture of membranes in nulliparas (flip). American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S31‐S32, Abstract no: 44. [DOI] [PubMed] [Google Scholar]
- Amorosa JM, Stone J, Factor SH, Booker W, Newland M, Bianco A. A randomized trial of foley bulb for labor induction in premature rupture of membranes in nulliparas (flip). American Journal of Obstetrics and Gynecology 2017;217(3):360.e1‐7. [DOI] [PubMed] [Google Scholar]
Atad 1996 {published data only}
- Abramovici H, Hallak M, Zarfati D, Packer T, Calderon I, Auslender R, et al. Induction of labor in patients with unfavorable cervices: a randomized comparison among intravaginal prostaglandin E2 (PGE2), intravenous oxytocin, and the double‐balloon ripener device. International Journal of Gynecology & Obstetrics 1994;46:7. [Google Scholar]
- Atad J, Hallak M, Auslender R, Porat‐Packer T, Zarfati D, Abramovici H. A randomized comparison of prostaglandin E2, oxytocin, and the double‐balloon device in inducing labor. Obstetrics & Gynecology 1996;87:223‐7. [DOI] [PubMed] [Google Scholar]
- Atad J, Porat‐Pecker T. A randomized comparison of PGE2 vaginal tablets, oxytocin and the double balloon device for labor induction. 1st World Congress on Controversies in Obstetrics Gynecology and Infertility; 1999 Oct 28‐31; Prague, Czech Republic. 1999.
- Hallak M. Mechanical ripening of the unfavorable cervix for induction of labor. Contemporary Reviews in Obstetrics and Gynaecology 1997;9:99‐105. [Google Scholar]
Bagratee 1990 {published data only}
- Bagratee JS, Moodley J. Synthetic laminaria tent for cervical ripening. South African Medical Journal 1990;78:738‐41. [PubMed] [Google Scholar]
Barda 2018 {published data only}
- Barda G, Ganer H, Sagiv R, Bar J. Foley catheter versus intravaginal prostaglandins E2 for cervical ripening in women at term with an unfavorable cervix: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2018;31(20):2777‐1. [DOI] [PubMed] [Google Scholar]
- Herman HG, NCT02486679. Cervical ripening at term with prostaglandin e2 tablets versus foley catheter: a randomized controlled trial. clinicaltrials.gov/show/NCT02486679 (first received 1 July 2015).
Benzineb 1996 {published data only}
- Benzineb N, Bouhaouala S, Sfar R. Prostaglandin E2 versus Foley catheter for cervical maturation at term [Prostaglandines E2 versus sonde de Foley dans les maturations cervicales à terme]. Revue Francaise de Gynecologie et d'Obstetrique 1996;91:173‐6. [Google Scholar]
Biron‐Shental 2004 {published data only}
- Biron‐Shental T, Fishman A, Fejgin MD. Medical and mechanical methods for cervical ripening. International Journal of Gynecology & Obstetrics 2004;85:159‐60. [DOI] [PubMed] [Google Scholar]
Blumenthal 1990 {published data only}
- Blumenthal PD, Ramanauskas R. Randomized trial of dilapan and laminaria as cervical ripening agents before induction of labor. Obstetrics & Gynecology 1990;75:365‐8. [PubMed] [Google Scholar]
Browne 2011 {published data only}
- Browne PC. Comparison of pre‐induction cervical ripening using prepidil gel administered through a urinary balloon catheter. clinicaltrials.gov/ct2/show/results/NCT01390233 (first received 8 July 2011).
Carbone 2013 {published data only}
- Carbone JF, NCT01279343. Cervical foley plus vaginal misoprostol versus vaginal misoprostol for cervical ripening and labor induction: a randomized trial. clinicaltrials.gov/ct2/show/record/NCT01279343 (first received6 January 2011).
- Carbone JF, Tuuli MG, Fogertey PJ, Roehl KA, Macones GA. Combination of foley bulb and vaginal misoprostol compared with vaginal misoprostol alone for cervical ripening and labor induction: a randomized controlled trial. Obstetrics and Gynecology 2013;121(2 Pt 1):247‐52. [DOI] [PubMed] [Google Scholar]
Casey 1995 {published data only}
- Casey BM, Smith LG, Wolf EJ. Combined therapy for preinduction cervical ripening is more effective than PGE2 alone. American Journal of Obstetrics and Gynecology 1995;172:424. [Google Scholar]
Chavakula 2015 {published data only}
- Chavakula PR, Benjamin SJ, Abraham A, Londhe V, Jeyaseelan V, Mathews JE. Misoprostol versus foley catheter insertion for induction of labor in pregnancies affected by fetal growth restriction. International Journal of Gynaecology and Obstetrics 2015;129(2):152‐5. [DOI] [PubMed] [Google Scholar]
- Mathews J, CTRI/2014/02/004411. Intra‐vaginal misoprostal versus Foley catheter for induction of labour in fetus with suspected fetal compromise. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/02/004411 (first received 17 February 2014).
Chua 1997 {published data only}
- Chua S, Arulkumaran S, Vanaja K, Ratnam SS. Preinduction cervical ripening: prostaglandin E2 gel vs hygroscopic mechanical dilator. Journal of Obstetrics and Gynaecology Research 1997;23:171‐7. [DOI] [PubMed] [Google Scholar]
Cromi 2011 {published data only}
- Cromi A, Ghezzi F, Agosti M, Serati M, Uccella S, Arlant V, et al. Is transcervical Foley catheter actually slower than prostaglandins in ripening the cervix? A randomized study. American Journal of Obstetrics and Gynecology 2011;204(4):338.e1‐7. [DOI] [PubMed] [Google Scholar]
Cromi 2012 {published data only}
- Cromi A, Ghezzi F, Uccella S, Agosti M, Serati M, Marchitelli G, et al. A randomized trial of preinduction cervical ripening: Dinoprostone vaginal insert versus double‐balloon catheter. American Journal of Obstetrics and Gynecology 2012;207(2):125.e1‐7. [DOI] [PubMed] [Google Scholar]
- Cromi A, NCT01170819. Double balloon catheter versus vaginal pge2 for pre‐induction cervical ripening: a randomized study. https://clinicaltrials.gov/ct2/show/record/NCT01170819 (first received 27 July 2010).
Culver 2004 {published data only}
- Culver J, Strauss R, Brody S, Dorman K, Timlin S, McMahon M. A randomized trial of intracervical foley catheter with concurrent oxytocin compared to vaginal misoprostol for labor induction in nulliparous women. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S203. [Google Scholar]
- Culver J, Strauss RA, Brody S, Dorman K, Timlin S, McMahon MJ. A randomized trial comparing vaginal misoprostol versus foley catheter with concurrent oxytocin for labor induction in nulliparous women. American Journal of Perinatology 2004;21(3):139‐46. [DOI] [PubMed] [Google Scholar]
Dalui 2005 {published data only}
- Dalui R, Suri V, Ray P, Gupta I. Comparison of extraamniotic foley catheter and intracervical prostaglandin E2 gel for preinduction cervical ripening. Acta Obstetricia et Gynecologica Scandinavica 2005;84(4):362‐7. [DOI] [PubMed] [Google Scholar]
Deo 2012 {published data only}
- Deo S, Iqbal B, Das V, Agarwal A, Singh R. Evaluation of non‐pharmacological method‐transcervical foley catheter to intravaginal misoprostol and prostaglandin E2 gel for preinduction cervical ripening. Biomedical Research 2012;23(2):247‐52. [Google Scholar]
Deo 2013 {published data only}
- Deo S. Preinduction cervical ripening: a prospective randomised comparison of intracervical foley catheter versus PGE2 gel. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E113. [Google Scholar]
- Deo S, Iqbal B, Das V, Agarwal A, Singh R. Preinduction cervical ripening: a prospective randomised comparison of intracervical foley catheter versus PGE2 gel. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):85. [Google Scholar]
Deshmukh 2011 {published data only}
- Deshmukh VL, Yelikar KA, Deshmukh AB. Comparative study of intra‐cervical Foley's catheter and PGE2 gel for pre‐induction ripening (Cervical). Journal of Obstetrics and Gynecology of India 2011;61(4):418‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dionne 2011 {published data only}
- Dionne MD, Dube J, Chaillet N. Randomized study comparing Foley catheter and intravaginal misoprostol as cervical ripening. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S48. [Google Scholar]
Edwards 2014c {published data only}
- Edwards R, Szychowski J, Berger J, Petersen M, Ingersoll M, Bodea Braescu A, et al. Effect of obesity on duration and outcome of labor inductions with either the Foley catheter or the prostaglandin E2 vaginal insert. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S278. [Google Scholar]
- Edwards R, Szychowski J, Berger J, Petersen M, Ingersoll M, Bodea Braescu A, et al. Effect of parity on duration of labor inductions with either Foley catheter or the prostaglandin E2 vaginal insert. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S292. [Google Scholar]
- Edwards R, Szychowski J, Berger J, Petersen M, Ingersoll M, Bodea Braescu A, et al. Randomized trial comparing Foley catheter to the prostaglandin E2 vaginal insert for induction of labor. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S39‐40. [Google Scholar]
- Edwards R, Szychowski J, Braescu AB, Biggio J, Lin M. Potential barriers to adopting foley catheter for induction of labor in women with an unfavorable cervix: does the labor curve differ?. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S413‐4. [Google Scholar]
- Edwards RK, Szychowski JM, Berger JL, Petersen M, Ingersoll M, Bodea‐Braescu AV. Foley catheter compared with the controlled‐release dinoprostone insert. Obstetrics & Gynecology 2014;123:1280‐7. [DOI] [PubMed] [Google Scholar]
- Edwards RK, Szychowski JM, Bodea‐Braescu AV, Biggio JR, Lin MG. Foley catheter for induction of labor: potential barriers to adopting the technique. Journal of Perinatology 2015;35(12):996‐9. [DOI] [PubMed] [Google Scholar]
- Lin MG, NCT01402050. A randomized controlled trial of transcervical foley balloon compared to controlled release prostaglandin (cervidil) for labor induction and cervical ripening in term and near term women. clinicaltrials.gov/show/NCT01402050 (first received 8 April 2011).
El Khouly 2017 {published data only}
- Khouly NI. A prospective randomized trial comparing Foley catheter, oxytocin, and combination Foley catheter‐oxytocin for labour induction with unfavourable cervix. Journal of Obstetrics and Gynaecology 2017;37(3):309‐14. [DOI] [PubMed] [Google Scholar]
- Elkhouly N, PACTR201601001428921. A randomized trial comparing foley catheter, oxytocin and combination foley catheter‐oxytocin for induction of labor with unfavourable cervix. http://www.pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201601001428921 2016; Vol. (first received 17 January 2016).
Filho 2002 {published data only}
- Filho OBM. Misoprostol versus foley catheter and oxytocin for induction of labour [Misoprostol versus sonda foley e ocitocina para inducao do parto]. Revista Brasileira de Ginecologia e Obstetricia 2002;24(10):685. [Google Scholar]
- Moraes Filho OB, Albuquerque RM, Cecatti JG. A randomized controlled trial comparing vaginal misoprostol versus Foley catheter plus oxytocin for labor induction. Acta Obstetricia et Gynecologica Scandinavica 2010;89(8):1045‐52. [DOI] [PubMed] [Google Scholar]
Garba 2016 {published data only}
- Garba I, Muhammed AS, Muhammad Z, Galadanci HS, Ayyuba R, Abubakar IS. Induction to delivery interval using transcervical Foley catheter plus oxytocin and vaginal misoprostol: A comparative study at Aminu Kano Teaching Hospital, Kano, Nigeria. Annals of African Medicine 2016;15(3):114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gelisen 2005 {published data only}
- Gelisen O, Caliskan E, Dilbaz S, Ozdas E, Dilbaz B, Ozdas E, et al. Induction of labor with three different techniques at 41 weeks of gestation or spontaneous follow‐up until 42 weeks in women with definitely unfavorable cervical scores. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;120(2):164‐9. [DOI] [PubMed] [Google Scholar]
Gilson 2017 {published data only}
- Gilson GJ. A randomized control trial of low dose oral liquid misoprostol versus foley balloon‐oxytocin for induction of labor. American Journal of Obstetrics and Gynecology 2017;216(1):S511, Abstract no: 895. [Google Scholar]
Glagoleva 1999 {published data only}
- Glagoleva EA, Nikonov AP. Preinduction cervical ripening: a comparison of intracervical prostaglandin E2 versus the hygroscopic cervical dilator dilapan. European Journal of Obstetrics & Gynecology and Reproductive Biology 1999;86:S67. [Google Scholar]
Goonewardene 2014 {published data only}
- Goonewardene M, Kumara DM, Ziard MH, Bhabu B. Intra cervical foley catheter vs oral misoprostol for pre induction cervical ripening of postdated pregnancies. Sri Lanka Journal of Obstetrics and Gynaecology 2014;36(3):66‐70. [Google Scholar]
- Goonewardene M, SLCTR/2011/002. Intra cervical foley catheter versus oral misoprostol for pre induction cervical ripening of post dated pregnancies. a randomized controlled trial. http://slctr.lk/trials/28 (first received 7 January 2011). [DOI] [PubMed]
- Kumara DM, Ziard MH, Bhabu B, Goonewardene M. Intra cervical foley catheter vs oral misoprostol for pre induction cervical ripening of post dated pregnancies. Sri Lanka Journal of Obstetrics and Gynaecology 2014;36(Suppl 1):5‐6, Abstract no:FC 1.3. [Google Scholar]
Guinn 2000 {published data only}
- Guinn DA, Goepfert AR, Christine M, Owen J, Hauth JC. Extra‐amniotic saline, laminaria, or prostaglandin E2 gel for labor induction with unfavorable cervix: a randomized controlled trial. Obstetrics & Gynecology 2000;96:106‐12. [DOI] [PubMed] [Google Scholar]
- Guinn DA, Goepfert AR, Owen J, Christine M, Hauth JC. Laminaria, extra‐amniotic saline induction (EASI) or prepidil for cervical ripening prior to labor induction. American Journal of Obstetrics and Gynecology 1997;176:S143. [Google Scholar]
Gunawardena 2012 {published data only}
- Gunawardena LD, Gunawardana GH. Intracervical foley catheter insertion versus intracervical PGE2 gel application for cervical ripening in primi gravid – A randomized controlled trial. Sri Lanka Journal of Obstetrics and Gynaecology 2012;34(Suppl 1):111‐2, Abstract no: OP 40. [Google Scholar]
- Wasalthilaka CD, Gunawardana GH. Comparison of peripartum maternal and fetal outcomes in cervical ripening using foley catheter and prostaglandin E2. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E44‐5. [Google Scholar]
- Wasalthilaka CD, Gunawardana GH. Comparison of peripartum maternal and fetal outcomes in cervical ripening using foley catheter and prostaglandin E2 gel. Sri Lanka Journal of Obstetrics and Gynaecology 2014;36(Suppl 1):20, Abstract no: FC 7.4. [Google Scholar]
Haugland 2012 {published data only}
- Haugland B, Albrechtsen S, Lamark E, Rasmussen S, Kessler J. Induction of labor with single‐ versus double‐balloon catheter ‐ a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2012;91(Suppl 159):84‐5. [Google Scholar]
- Haugland B, NCT01091285. Induction of labor with single and double balloon catheters, a randomized controlled study. clinicaltrials.gov/show/NCT01091285 (first received 20 March 2010).
Hay 1995 {published data only}
- Hay D, Robinson G, Filshie M, James D. Cervical ripening with prostaglandin E2 gel and hygroscopic cervical dilators. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:Abstract no: 480.
Hemlin 1998 {published data only}
- Hemlin J, Möller B. Extraamniotic saline infusion is promising in preparing the cervix for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 1998;77:45‐9. [PubMed] [Google Scholar]
Henry 2013 {published data only}
- Austin K, Chambers GM, Abreu RL, Madan A, Susic D, Henry A. Cost‐effectiveness of term induction of labour using inpatient prostaglandin gel versus outpatient Foley catheter. Australian & New Zealand Journal of Obstetrics & Gynaecology 2015;55(5):440‐5. [DOI] [PubMed] [Google Scholar]
- Henry A, ACTRN12609000420246. An evaluation of outpatient foley (intracervical) catheter versus inpatient prostaglandin vaginal gel (PGE2) on the induction of labour at term. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12609000420246 (first received 10 May 2009).
- Henry A, Madan A, Reid R, Tracy S, Sharpe V, Austin K, et al. Outpatient Foley catheter versus inpatient Prostin gel for cervical ripening: the FOG (Foley or Gel) trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:473‐4. [Google Scholar]
- Henry A, Madan A, Reid R, Tracy SK, Austin K, Welsh A, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy and Childbirth 2013;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry A, Reid R, Madan A, Tracy S, Sharpe V, Welsh A, et al. Satisfaction survey: outpatient Foley catheter versus inpatient Prostin gel for cervical ripening. Australian and New Zealand Journal of Obstetrics and Gynaecology 2011;51:474. [Google Scholar]
Hibbard 1998 {published data only}
- Hibbard JU, Shashoua A, Adamczyk C, Ismail M. Cervical ripening with prostaglandin gel and hygroscopic dilators. Infectious Diseases in Obstetrics and Gynecology 1998;6:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoppe 2016 {published data only}
- Hoppe K, Schiff M, Peterson S, Gravett M. Randomized controlled trial: comparing 80mL double versus 30mL single balloon catheters for pre‐induction cervical ripening. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S326. [Google Scholar]
- Hoppe KK, Schiff MA, Peterson SE, Gravett MG. 30ml single‐ versus 80 ml double‐balloon catheter for pre‐induction cervical ripening: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(12):1919‐25. [DOI] [PubMed] [Google Scholar]
Hudon 1999 {published data only}
- Hudon L, Belfort MA, Dorman K, Wilkins IA, Moise KJ. Comparison between intracervical PGE2 and supracervical Foley catheter for cervical ripening. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S126. [Google Scholar]
Hughes 2002 {published data only}
- Hughes L, El‐Azeem S. Induction of labor: a randomized comparison between the intracervical balloon catheter and slow release dinoprostone. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S166. [Google Scholar]
Husain 2017 {published data only}
- Husain S, Husain S, Izhar R. Oral misoprostol alone versus oral misoprostol and foley's catheter for induction of labor: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2017;43(8):1270‐7. [DOI] [PubMed] [Google Scholar]
- Husain S, NCT02758340. Comparison of maternal outcome between patients undergoing induction of labor with oral misoprostol alone and oral misoprostol and foley's catheter both at a tertiary care hospital. clinicaltrials.gov/show/NCT02758340 (first received 2 May 2016).
Jagani 1982 {published data only}
- Jagani N, Schulman H, Fleischer A, Mitchell J, Randolph G. Role of the cervix in the induction of labor. Obstetrics & Gynecology 1982;59:21‐6. [PubMed] [Google Scholar]
Jalilian 2011 {published data only}
- Jalilian N, Fakheri T, Ghadami MR. Intravaginal dinoprostone versus intra cervical foley catheter for induction of labor. Acta Medica Iranica 2011;49(12):831. [PubMed] [Google Scholar]
Jeeva 1982 {published data only}
- Jeeva MA, Dommisse J. Laminaria tents or vaginal prostaglandins for cervical ripening. A comparative trial. South African Medical Journal 1982;61:402‐3. [PubMed] [Google Scholar]
Johnson 1985 {published data only}
- Johnson IR, Macpherson MB, Welch CC, Filshie GM. A comparison of Lamicel and prostaglandin E2 vaginal gel for cervical ripening before induction of labor. American Journal of Obstetrics and Gynecology 1985;151:604‐7. [DOI] [PubMed] [Google Scholar]
- MacPherson M. Comparison of Lamicel with prostaglandin E2 gel as a cervical ripening agent before the induction of labour. Journal of Obstetrics and Gynaecology 1984;4:205‐6. [Google Scholar]
Joshi 2016 {published data only}
- Joshi S, Dheeraj S, Fotedar S. Induction with transcervical foleys versus iv oxytocin for trial of labor after cesarean (TOLAC). Indian Journal of Obstetrics and Gynecology Research 2016;3(3):257‐63. [Google Scholar]
Jozwiak 2012 {published data only}
- Jozwiak M, Benthem M, Oude RK, Dijksterhuis M, Graaf I, Pampus M, et al. Randomized clinical trial for the comparison of Foley catheter and prostaglandin inserts in induction of labor at term (trial registration NTR 1646). American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S40. [Google Scholar]
- Jozwiak M, NTR1646. Evaluation of chemical (Prostaglandins) versus mechanical (transcervical balloon) methods for induction of labour at term. trialregister.nl/trialreg/admin/rctview.asp?TC=1646 (first received 30 January 2009).
- Jozwiak M, Oude Rengerink K, Benthem M, Beek E, Dijksterhuis MG, Graaf IM, et al. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open‐label, randomised controlled trial. Lancet 2012;378(9809):2095‐103. [DOI] [PubMed] [Google Scholar]
- Jozwiak M, Rengerink KO, Doornbos H, Drogtrop A, Groot C, Huisjes A, et al. Prediction of cesarean section in women with an unfavorable cervix at term. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S146. [Google Scholar]
- Jozwiak M. PROBAAT study. Prostaglandin or Balloon for Induction of labour at Term. http://www.studies‐obsgyn.nl/home/page.asp?page_id=600.
- Mol BW, Post J, Rengerink KO, Papatsonis D, Jozwiak M, Huizen M, et al. Induction of labor at term: a comparison of Foley catheter and prostaglandins (trial registration NTR 1646). American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S3‐4. [Google Scholar]
- Baaren GJ, Jozwiak M, Opmeer BC, Oude Rengerink K, Benthem M, Dijksterhuis MG, et al. Cost‐effectiveness of induction of labour at term with a foley catheter compared to vaginal prostaglandin E2 gel (PROBAAT trial). BJOG: an international journal of obstetrics and gynaecology 2013;120:987‐95. [DOI] [PubMed] [Google Scholar]
- Baaren GJ, Jozwiak M, Rengerink KO, Benthem M, Dijksterhuis MG, Huizen ME, et al. Cost‐effectiveness of induction of labor at term with a Foley catheter compared to prostaglandin E2 gel (based on the PROBAAT trial; registration NTR 1646). American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S139‐40. [Google Scholar]
Jozwiak 2013 {published data only}
- Jozwiak M, Oude Rengerink K, Eikelder ML, Pampus MG, Dijksterhuis MG, Graaf IM, et al. Foley catheter or prostaglandin E2 inserts for induction of labour at term: an open‐label randomized controlled trial (PROBAAT‐P trial) and systematic review of literature. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;170(1):137‐45. [DOI] [PubMed] [Google Scholar]
Jozwiak 2014 {published data only}
- Jozwiak M, Eikelder M, Oude Rengerink K, Groot C, Feitsma H, Spaanderman M, et al. Foley catheter versus vaginal misoprostol: randomized controlled trial (PROBAAT‐M study) and systematic review and meta‐analysis of literature. American Journal of Perinatology 2014;31(2):145‐56. [DOI] [PubMed] [Google Scholar]
Kandil 2012 {published data only}
- Kandil M, Emarh M, Sayyed T, Masood A. Foley catheter versus intra‐vaginal misoprostol for induction of labor in post‐term gestations. Archives of Gynecology and Obstetrics 2012;286(2):303‐7. [DOI] [PubMed] [Google Scholar]
Khamaiseh 2012 {published data only}
- Khamaiseh K, Al‐Ma'ani W, Abdalla I. Prostaglandin E2 versus foley catheter balloon for induction of labor at term: A randomized controlled study. Journal of the Royal Medical Services 2012;19(4):42‐7. [Google Scholar]
Krammer 1995a {published data only}
- Krammer J, O'Brien W, Williams M, Sawai S. A prospective randomized comparison of Dilapan vs PGE2 for preinduction cervical ripening and their effects on labor kinetics. American Journal of Obstetrics and Gynecology 1993;170:408. [Google Scholar]
- Krammer J, O'Brien W, Williams M, Sawai S. Success of labor induction by post‐ripening cervical dilatation and agent used. American Journal of Obstetrics and Gynecology 1993;170:408. [Google Scholar]
- Krammer J, Williams MC, Sawai SK, O'Brien WF. Pre‐induction cervical ripening: a randomized comparison of two methods. Obstetrics & Gynecology 1995;85:614‐8. [DOI] [PubMed] [Google Scholar]
- Williams MC, Krammer J, O'Brien WF. The value of the cervical score in predicting successful outcome of labor induction. Obstetrics & Gynecology 1997;90:784‐9. [DOI] [PubMed] [Google Scholar]
Kruit 2016 {published data only}
- Kruit H, Tihtonen K, Raudaskoski T, Ulander VM, Aitokallio‐Tallberg A, Heikinheimo O, et al. Foley catheter or oral misoprostol for induction of labor in women with term premature rupture of membranes: a randomized multicenter trial. American Journal of Perinatology 2016;33(9):866‐72. [DOI] [PubMed] [Google Scholar]
Kuppulakshmi 2016 {published data only}
- Kuppulakshmi G, Vani K. Randomized controlled trial of preinduction cervical ripening ‐ dinoprostone versus Foley’s catheter. Indian Journal of Research 2016;5(9):41‐2. [Google Scholar]
Laddad 2013 {published data only}
- Laddad ML, Kshirsagar NS, Karale AV. A prospective randomized comparative study of intra‐cervical foley's catheter insertion versus PGE2 gel for pre‐induction cervical ripening. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(2):217‐20. [Google Scholar]
Lanka 2014 {published data only}
- Lanka S, CTRI/2012/12/003265. A clinical study to compare the combined efficacy of mechanical and pharmacological methods versus pharmacological method alone when used for induction of labor. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1301 (first received 27 December 2012).
- Lanka S, Surapaneni T, Nirmalan PK. Concurrent use of Foley catheter and misoprostol for induction of labor: A randomized clinical trial of efficacy and safety. Journal of Obstetrics and Gynaecology Research 2014;40(6):1527‐33. [DOI] [PubMed] [Google Scholar]
Lemyre 2006 {published data only}
- Lemyre M, Verret N, Turcot‐Lemay L, Brassard N, Morin V. Foley catheter or vaginal misoprostol for cervical ripening: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S105. [Google Scholar]
Lewis 1983 {published data only}
- Lewis GJ. Cervical ripening before induction of labour with prostaglandin E2 pessaries or a Foley's catheter. Journal of Obstetrics and Gynaecology 1983;3:173‐6. [Google Scholar]
Lokkegaard 2015 {published data only}
- Lokkegaard E, Lundstrom M, Kjaer MM, Christensen IJ, Pedersen HB, Nyholm H. Prospective multi‐centre randomised trial comparing induction of labour with a double‐balloon catheter versus dinoprostone. Journal of Obstetrics and Gynaecology 2015;35(8):797‐802. [DOI] [PubMed] [Google Scholar]
- Nyholm H, NCT01255839. A prospective multi‐centre randomised comparison on induction of labour with double‐balloon installation device versus prostaglandin e2 minprostin. clinicaltrials.gov/show/NCT01255839 (first received 27 December 20128 December 2010).
Lyndrup 1989 {published data only}
- Lyndrup J, Legarth J, Dahl C, Philipsen T, Eriksen PS. Induction of labor: the effect of prostaglandin pessary, IV oxytocin and lamicel. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:117.
- Lyndrup J, Legarth J, Dahl C, Philipsen T, Eriksen PS. Lamicel does not promote induction of labor. A randomized controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1989;30:205‐8. [DOI] [PubMed] [Google Scholar]
Lyndrup 1994 {published data only}
- Lyndrup J, Nickelsen C, Weber T, Molnitz E, Guldbaek E. Induction of labour by balloon catheter with extra‐amniotic saline infusion (BCEAS): a randomised comparison with PGE2 vaginal pessaries. European Journal of Obstetrics & Gynecology and Reproductive Biology 1994;53:189‐97. [DOI] [PubMed] [Google Scholar]
Mackeen 2018 {published data only}
- Mackeen AD, Durie D, Lin M, Huls C, Packard R, Sciscione A. Effect of obesity on labor inductions with foley plus oxytocin versus oxytocin alone. Obstetrics and Gynecology 2017;129(5 Suppl):142S. [Google Scholar]
- Mackeen AD, Durie DE, Lin M, Huls CK, Qureshey E, Paglia MJ, et al. Foley plus oxytocin compared with oxytocin for induction after membrane rupture: a randomized controlled trial. Obstetrics and Gynecology 2018;131(1):4‐11. [DOI] [PubMed] [Google Scholar]
- Mackeen AD, NCT01973036. Foley catheter versus oxytocin for labor induction in women with term and near term premature rupture of membranes: a randomized clinical trial (FOLCROM trial). clinicaltrials.gov/ct2/show/record/NCT01973036 (first received 17 September 2013).
- Mackeen AD, Paglia MJ, Durie DE, Lin M, Huls CK, Sun H, et al. Foley plus oxytocin versus oxytocin alone for labor induction > 34 weeks after premature rupture of membranes (PROM): a randomized controlled trial. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S72‐S73, Abstract no: 103. [Google Scholar]
Matonhodze 2003 {published data only}
- Matonhodze BB, Hofmeyr GJ, Levin J. Labour induction at term‐‐a randomised trial comparing Foley catheter plus titrated oral misoprostol solution, titrated oral misoprostol solution alone, and dinoprostone. South African Medical Journal 2003;93(5):375‐9. [PubMed] [Google Scholar]
Mazhar 2003 {published data only}
- Mazhar SB, Imran R, Alam K. Trial of extra amniotic saline infusion with oxytocin versus prostaglandin E2 pessary for induction of labor. Journal of the College of Physicians & Surgeons Pakistan 2003;13(6):317‐20. [PubMed] [Google Scholar]
Meetei 2015 {published data only}
- Meetei LT, Suri V, Aggarwal N. Induction of labor in patients with previous cesarean section with unfavorable cervix. JMS ‐ Journal of Medical Society 2015;28(1):29‐33. [Google Scholar]
Moini 2003 {published data only}
- Moini A, Riazi K, Honar H, Hasanzadeh Z. Preinduction cervical ripening with the foley catheter and saline infusion vs. cervical dinoprostone. International Journal of Gynecology & Obstetrics 2003;83:211‐3. [DOI] [PubMed] [Google Scholar]
Mullin 2002 {published data only}
- Mullin P, House M, Paul R, Wing D. A comparison of vaginally administered misoprostol with extraamniotic saline infusion for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S203. [DOI] [PubMed] [Google Scholar]
- Mullin PM, House M, Paul RH, Wing DA. A comparison of vaginally administered misoprostol with extra‐amniotic saline infusion for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 2002;187:847‐52. [DOI] [PubMed] [Google Scholar]
Mundle 2017 {published data only}
- Bracken H, Mundle S, Faragher B, Easterling T, Haycox A, Turner M, et al. Induction of labour in pre‐eclamptic women: a randomised trial comparing the Foley balloon catheter with oral misoprostol. BMC Pregnancy and Childbirth 2014;14(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundle S, Bracken H, Faragher B, Alfirevic Z, Winikoff B, Weeks A. Induction of labour in hypertensive women in India: a randomised trial comparing the foley catheter with oral misoprostol. BJOG: an international journal of obstetrics and gynaecology 2016;123(Suppl 1):8‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundle S, Bracken H, Faragher B, Easterling T, Haycox A, Turner M, et al. Induction of labour in pre‐eclamptic women: a randomised trial comparing the foley balloon catheter with oral misoprostol. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundle S, Bracken H, Faragher B, Easterling T, Winikoff B, Weeks A. Induction of labor in preeclamptic women in India: A randomized trial comparing Foley catheter with oral misoprostol. Obstetrics & Gynecology 2016;127(Suppl 5):75S. [Google Scholar]
- Mundle S, Bracken H, Khedikar V, Mulik J, Faragher B, Easterling T, et al. Foley catheterisation versus oral misoprostol for induction of labour in hypertensive women in india (inform): a multicentre, open‐label, randomised controlled trial. Lancet 2017;390(10095):669‐80. [DOI] [PubMed] [Google Scholar]
- Mundle S, CTRI/2013/07/003859. Induction of labor in preeclamptic women: a randomised trial comparing the Foley balloon catheter with oral misoprostol. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=6670 (first received 31 July 2013).
- Weeks AD. Induction of labour in pre‐eclamptic women: a randomised trial comparing the foley balloon catheter with oral misoprostol. clinicaltrials.gov/ct2/show/NCT01801410 (first received 28 February 2013). [DOI] [PMC free article] [PubMed]
Niromanesh 2003 {published data only}
- Niromanesh S, Mosavi‐Jarrahi A, Samkhaniani F. Intracervical foley catheter balloon vs. prostaglandin in preinduction cervical ripening. International Journal of Gynecology & Obstetrics 2003;81:23‐7. [DOI] [PubMed] [Google Scholar]
Noor 2015 {published data only}
- Noor N, Ansari M, Ali SM, Parveen SF. Foley catheter versus vaginal misoprostol for labour induction. International Journal of Reproductive Medicine 2015;2015:845735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ntsaluba 1997 {published data only}
- Ntsaluba A, Bagratee J, Moodley J. The use of an indwelling catheter compared to intracervical prostaglandin gel for cervical ripening prior to induction of labour. O&G Forum 1997;July:17‐21. [Google Scholar]
Oliveira 2010 {published data only}
- Oliveira MV, Oberst P, Leite GK, Aguemi A, Kenj G, Leme VD, et al. Cervical Foley catheter versus vaginal misoprostol for cervical ripening and induction of labor: a randomized clinical trial [Sonda de Foley cervical versus misoprostol vaginal para o preparo cervical e inducao do parto: um ensaio clinico randomizado]. Revista Brasileira de Ginecologia e Obstetricia 2010;32(7):346‐51. [DOI] [PubMed] [Google Scholar]
- Sass N, NCT01140971. Transcervical foley catheter (foley) versus intravaginal misoprostol for cervical ripening and induction of labor: a randomized clinical trial. clinicaltrials.gov/show/NCT01140971 (first received 8 June 2010).
Ophir 1992 {published data only}
- Ophir E, Haj N, Korenblum R, Oettinger M. Cervical ripening before induction of labor: comparison of an intracervical Foley catheter and prostaglandin E2 tablets. International Journal of Feto‐Maternal Medicine 1992;5:101‐6. [Google Scholar]
Orhue 1995 {published data only}
- Orhue AA. Induction of labour at term in primigravidae with low Bishop's score: a comparison of three methods. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:119‐25. [DOI] [PubMed] [Google Scholar]
Peedicayil 1998 {published data only}
- Peedicayil A, Jasper P, Francis S, Jayakrishnan K, Mathai M, Regi A. A randomized trial of extra‐amniotic Foley catheter and intra‐cervical prostaglandin E2 for cervical ripening. Journal of Clinical Epidemiology 1998;51 Suppl 1:21S. [Google Scholar]
Pennell 2009 {published data only}
- Pennell CE, Henderson JJ, O'Neill MJ, McCleery S, Doherty DA, Dickinson JE. Induction of labour in nulliparous women with an unfavourable cervix: a randomised controlled trial comparing double and single balloon catheters and PGE2 gel. BJOG: an international journal of obstetrics and gynaecology 2009;116(11):1143‐52. [DOI] [PubMed] [Google Scholar]
- Pennell CE, Jewell M, Doherty D, Dickinson JE. Induction of labor with an unfavorable cervix. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S207. [DOI] [PubMed] [Google Scholar]
Perry 1998 {published data only}
- Perry KG Jr, Larmon JE, May WL, Robinette LG, Martin RW. Cervical ripening: a randomized comparison between intravaginal misoprostol and an intracervical balloon catheter combined with intravaginal dinoprostone. American Journal of Obstetrics and Gynecology 1998;178:1333‐40. [DOI] [PubMed] [Google Scholar]
Pineda Rivas 2016 {published data only}
- Lett C, NCT01962831. Randomized controlled trial: induction of labour of obese women with dinoprostone or single balloon catheter. clinicaltrials.gov/ct2/show/record/NCT01962831 (first received 19 September 2013).
- Pineda Rivas M, Hilton J, Karreman E, Lett C. Single balloon catheter versus dinoprostone vaginal insert for induction of labour of obese women. Journal of Obstetrics and Gynaecology Canada 2016;38(5):497‐8. [Google Scholar]
Prager 2008 {published data only}
- Marions L, NCT00602095. A randomised comparison between intravaginal dinoprostone intravaginal misoprostol and transcervical balloon catheter for labour induction. https://clinicaltrials.gov/ct2/show/record/NCT00602095 (first received 28 January 2008). [DOI] [PubMed]
- Prager M, Eneroth‐Grimfors E, Edlund M, Marions L. A randomised controlled trial of intravaginal dinoprostone intravaginal misoprostol and transcervical balloon catheter for labour induction. BJOG: an international journal of obstetrics and gynaecology 2008;115(11):1143‐50. [DOI] [PubMed] [Google Scholar]
Qamar 2012 {published data only}
- Qamar S, Bashir A, Ibrar F. Comparison of prostaglandin E2 gel, prostaglandin E2 pessary and extra‐amniotic saline infusion with oxytocin for induction of labour. Journal of Ayub Medical College, Abbottabad: JAMC 2012;24(2):22‐5. [PubMed] [Google Scholar]
Ridgway 1991 {published data only}
- Ridgway L, Berkus M, Wright J. A randomized comparison of intracervical PGE2 versus intracervical prostin and Lamicel cervical dilator for ripening of the unfavorable cervix. American Journal of Obstetrics and Gynecology 1991;164:307. [Google Scholar]
Roberts 1986 {published data only}
- Roberts WE, North DH, Speed JE, Martin JN, Palmer SM, Morrison JC. Comparative study of prostaglandin, laminaria, and minidose oxytocin for ripening of the unfavorable cervix prior to induction of labor. Journal of Perinatology 1986;6:16‐9. [Google Scholar]
Rouben 1993 {published data only}
- Arias F, Rouben D. Extraamniotic saline infusion with foley catheter is better than 2.9mg prostaglandin E2 gel in ripening the cervix but does not result in vaginal delivery. American Journal of Obstetrics and Gynecology 1993;168:429. [PubMed] [Google Scholar]
- Rouben D, Arias F. A randomized trial of extra‐amniotic saline infusion plus intracervical Foley catheter balloon versus prostaglandin E2 vaginal gel for ripening the cervix and inducing labor in patients with unfavorable cervices. Obstetrics & Gynecology 1993;82:290‐4. [PubMed] [Google Scholar]
Roudsari 2011 {published data only}
- Roudsari FV, Ayati S, Ghasemi M, Shakeri MT, Farshidi F, Shahabian M. Comparison of vaginal misoprostol with foley catheter for cervical ripening and induction of labor. Iranian Journal of Pharmaceutical Research 2011;10(1):149‐54. [PMC free article] [PubMed] [Google Scholar]
- Roudsari FV, Ghasemi M, Ayati S, Shakeri MT, Farshidi F, Shahabian M. [Comparison of vaginal misoprostol with foley catheter for cervical ripening and induction of labor]. Journal of Isfahan Medical School 2010;28(106):177‐85. [PMC free article] [PubMed] [Google Scholar]
Roztocil 1998 {published data only}
- Roztocil A. A comparison of three preinduction cervical priming methods: prostaglandin E2 gel, dilapan s rods, and estradiol gel. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:557. [PubMed] [Google Scholar]
- Roztocil A, Pilka L, Jelinek J, Koudelka M, Miklica J. A comparison of three preinduction cervical priming methods: prostaglandin E2 gel, dilapan S rods and estradiol gel. Ceska Gynekologie 1998;63:3‐9. [PubMed] [Google Scholar]
Rudra 2012 {published data only}
- Rudra T. Is Foley's catheter a safe and cost effective way of iol in low resource countries?. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S468. [Google Scholar]
Saleem 2006 {published data only}
- Saleem S. Efficacy of dinoprostone, intracervical foleys and misoprostol in labor induction. Journal of the College of Physicians & Surgeons Pakistan 2006;16(4):276‐9. [PubMed] [Google Scholar]
Salim 2011 {published data only}
- Salim R, NCT00690040. Single balloon catheter compared with double balloon catheter for ripening of the unfavorable cervix. https://clinicaltrials.gov/ct2/show/record/NCT00690040 (31 May 2008).
- Salim R, Zafran N, Nachum Z, Garmi G, Kraiem N, Shalev E. Single‐balloon compared with double‐balloon catheters for induction of labor: a randomized controlled trial. Obstetrics and Gynecology 2011;118(1):79‐86. [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 1992 {published data only}
- Sanchez‐Ramos L, Kaunitz AM, Connor PM. Hygroscopic cervical dilators and prostaglandin E2 gel for preinduction cervical ripening. A randomized, prospective comparison. Journal of Reproductive Medicine 1992;37:355‐9. [PubMed] [Google Scholar]
Sarreau 2016 {published data only}
- Sarreau M, Ragot S, Poulain P, Fontaine B, Morel O, Villemonteix P, et al. Balloon catheter vs. ocytocin for cervical ripening in patient with previous caesarean section: open‐label multicenter randomised controlled trial. European Journal of Obstetrics & Gynecology 2016;206:e104. [Google Scholar]
Sciscione 1999 {published data only}
- Sciscione A, McCullough H, Manley P, Shlossman P, Pollock M, Colmorgen G. A prospective, randomized comparison of Foley catheter insertion versus intracervical prostaglandin E2 gel for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1999;180:55‐60. [DOI] [PubMed] [Google Scholar]
- Sciscione A, McCullough H, Shlossman P, Manley P, Pollock M, Colmorgen G. A randomized prospective comparison of intracervical PGE2 gel (Prepidil) versus Foley bulb for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1997;176:S142. [DOI] [PubMed] [Google Scholar]
Sharami 2005 {published data only}
- Sharami SH, Milani F, Zahiri Z, Mansour‐Ghanaei F. A randomized trial of prostaglandin E2 gel and extra‐amniotic saline infusion with high dose oxytocin for cervical ripening. Medical Science Monitor 2005;11(8):CR381‐CR386. [PubMed] [Google Scholar]
Shechter‐Maor 2015 {published data only}
- Biron‐Shental T, NCT00815542. Induction of labor in oligohydramnios ‐ a comparison between two modes of cervical ripening for patients with oligohydramnios at term. clinicaltrials.gov/show/NCT00815542 (first received 30 December 2008).
- Shechter‐Maor G, Biron‐Shental T, Haran G, Ganor‐Paz Y, Fejgin M. Intravaginal prostaglandin E2 versus double balloon catheter for labor induction in term isolated oligohydramnios. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S78‐9. [Google Scholar]
- Shechter‐Maor G, Haran G, Sadeh‐Mestechkin D, Ganor‐Paz Y, Fejgin MD, Biron‐Shental T. Intra‐vaginal prostaglandin E2 versus double‐balloon catheter for labor induction in term oligohydramnios. Journal of Perinatology 2015;35:95‐8. [DOI] [PubMed] [Google Scholar]
Sheikher 2009 {published data only}
- Sheikher C, Suri N, Kholi U. Comparative evaluation of oral misoprostol, vaginal misoprostol and intracervical Foley's catheter for induction of labour at term. JK Science 2009;11(2):75‐7. [Google Scholar]
Solt 2009 {published data only}
- Solt I, Ben‐Harush S, Kaminskey S, Sosnovsky V, Ophir E, Bornstein J. A prospective randomized study comparing induction of labor with a foley catheter and the cervical ripening double balloon catheter in nulliparous and multiparous women. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S124. [Google Scholar]
- Solt NCT00501033. A prospective comparative study of induction of labor with a cervical ripening double balloon vs foley. clinicaltrials.gov/show/NCT00501033 (first received 12 July 2007).
Somirathne 2017 {published data only}
- Goonewardene M, SLCTR/2014/030. A randomized control trial to compare the effectiveness of intracervical Foley catheter for 24 hours vs three doses of oral misoprostol for preinduction cervical ripening in post dated pregnancies. http://slctr.lk/trials/257 (first received 21 November 2014).
- Somirathne D, Goonewardene M. Intracervical foley catheter for 24 hours vs three doses of oral misoprostol for preinduction cervical ripening in post dated pregnancies: a randomised controlled trial. Sri Lanka Journal of Obstetrics and Gynaecology 2015;37(Suppl 1):4‐5, Abstract no: OP 7. [DOI] [PubMed] [Google Scholar]
- Somirathne D, Goonewardene M, Dahanayake L. Three doses of oral misoprostol versus an intra‐cervical foley catheter for 24 hours for pre‐induction cervical ripening in post‐ dated pregnancies: a randomized controlled trial. Ceylon Medical Journal 2017;62(2):77‐82. [DOI] [PubMed] [Google Scholar]
St Onge 1995 {published data only}
- Lange I, Onge G, Connors G, Ingelson B. A comparison of PGE2 gel versus the Foley catheter for pre‐induction cervical ripening. International Journal of Gynecology & Obstetrics 1994;46:FC005.3. [Google Scholar]
- Onge RD, Connors GT. Preinduction cervical ripening: a comparison of intracervical prostaglandin E2 gel versus the Foley catheter. American Journal of Obstetrics and Gynecology 1995;172(2):687‐90. [DOI] [PubMed] [Google Scholar]
Suffecool 2014 {published data only}
- Suffecool K, Rosenn B, Forutan J, Herrera K. Labor induction in women with an unfavorable cervix: Randomized controlled trial of double balloon catheter versus dinoprostone. Reproductive Sciences (Thousand Oaks, Calif.) 2013;20(3 Suppl):333A. [Google Scholar]
- Suffecool K, Rosenn BM, Kam S, Mushi J, Foroutan J, Herrera K. Labor induction in nulliparous women with an unfavorable cervix: Double balloon catheter versus dinoprostone. Journal of Perinatal Medicine 2014;42(2):213‐8. [DOI] [PubMed] [Google Scholar]
Sullivan 1996 {published data only}
- Sullivan CA, Benton LW, Roach H, Smith LG Jr, Martin RW, Morrison JC. Combining medical and mechanical methods of cervical ripening. Does it increase the likelihood of successful induction of labor?. Journal of Reproductive Medicine 1996;41:823‐8. [PubMed] [Google Scholar]
Tabowei 2003 {published data only}
- Tabowei TO, Oboro VO. Low dose intravaginal misoprostol versus intracervical balloon catheter for pre‐induction cervical ripening. East African Medical Journal 2003;80(2):91‐4. [DOI] [PubMed] [Google Scholar]
Tan 2015 {published data only}
- Tan TL, Ng GY, Lim SE, Tagore S, Kyaw EE, Yeo GS. Cervical ripening balloon as an alternative for induction of labour: A randomized controlled trial. British Journal of Medical Practitioners 2015;8(1):a806. [DOI] [PMC free article] [PubMed] [Google Scholar]
ten Eikelder 2016 {published data only}
- Eikelder ML, Baaren GJ, Rengerink KO, Jozwiak M, Leeuw JW, Kleiverda G, et al. Comparing induction of labour with oral misoprostol or foley catheter at term: cost effectiveness analysis of a randomised controlled multi‐centre non‐inferiority trial. BJOG: an International Journal of Obstetrics and Gynaecology 2018;125(3):375‐83. [DOI] [PubMed] [Google Scholar]
- Eikelder ML, NTR3466. Induction of labour with oral misoprostol or Foley catheter at term. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3466 (7 June 2012).
- Eikelder ML, Neervoort F, Rengerink KO, Baaren GJ, Jozwiak M, Leeuw J, et al. Induction of labour with a Foley catheter or oral misoprostol at term: the PROBAAT‐II study, a multicentre randomised controlled trial. BMC Pregnancy and Childbirth 2013;13(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelder ML, Oude Rengerink K, Jozwiak M, Leeuw JW, Graaf IM, Pampus MG, et al. Induction of labour at term with oral misoprostol versus a foley catheter (PROBAAT‐II): a multicentre randomised controlled non‐inferiority trial. Lancet 2016;387(10028):1619‐28. [DOI] [PubMed] [Google Scholar]
- Eikelder ML, Rengerink KO, Jozwiak M, Leeuw JW, Graaf I, Pampus MG, et al. Induction of labor at term with oral misoprostol or Foley catheter, the PROBAAT‐II trial (NTR3466). American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S14. [Google Scholar]
- Eikelder ML, Rengerink KO, Jozwiak M, Leeuw JW, Graaf IM, Pampus MG, et al. Induction of labor at term with oral misoprostol or foley catheter, the PROBAAT‐II trial (NTR3466). Journal of Paediatrics and Child Health 2015;51(Suppl 1):55. [Google Scholar]
Thiery 1981 {published data only}
- Thiery M, Parewijck W, Martens G, Derom R, Kets H. Extra‐amniotic prostaglandin E2 gel vs amniotomy for elective induction of labour. Zeitschrift fur Geburtshilfe und Perinatologie 1981;185:323‐6. [PubMed] [Google Scholar]
Tita 2006 {published data only}
- Tita A, NCT00290199. A randomized controlled trial of foley catheter for labor induction in women with term and near term prelabor rupture of membranes (prom). clinicaltrials.gov/ct2/show/record/NCT00290199 (first received 9 February 2006).
Turnquest 1997 {published data only}
- Lemke M, Turnquest M. Laminaria tents plus vaginal prostaglandin versus vaginal prostaglandin alone for cervical ripening. American Journal of Obstetrics and Gynecology 1996;174:482. [Google Scholar]
- Turnquest MA, Lemke MD, Brown HL. Cervical ripening: randomized comparison of intravaginal prostaglandin E2 gel with prostaglandin E2 gel plus Laminaria tents. Journal of Maternal‐Fetal Medicine 1997;6:260‐3. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang ZM, Wang L, Han LL. Propess suppository and trans‐cervical foley catheter balloon for cervical ripening and induction of labor: A prospective randomized controlled trial. Journal of Chinese General Practice 2012;15(10A):3264‐7. [Google Scholar]
- Zheng MM, Hu YL, Zhang SM, Ling JX, Wang ZQ. Trans‐cervical foley catheter balloon versus vaginal prostaglandin E2 suppository for cervical ripening and induction of labor: a prospective randomized controlled trial. Chinese Journal of Perinatal Medicine 2011;14(11):648‐52. [Google Scholar]
Wang 2014 {published data only}
- Wang W, Zheng J, Fu J, Zhang X, Ma Q, Yu S, et al. Which is the safer method of labor induction for oligohydramnios women? Transcervical double balloon catheter or dinoprostone vaginal insert?. Journal of Maternal‐Fetal and Neonatal Medicine 2014;27(17):1805‐8. [DOI] [PubMed] [Google Scholar]
Wu 2017 {published data only}
- Wu X, Li Y, Ouyang C, Liao J, Wang C, Cai W, et al. Cervical dilation balloon combined with intravenous drip of oxytocin for induction of term labor: a multicenter clinical trial. Archives of Gynecology and Obstetrics 2018;297(1):77‐83. [DOI] [PubMed] [Google Scholar]
Yuen 1996 {published data only}
- Yuen PM, Pang HY, Chung T, Chang A. Cervical ripening before induction of labour in patients with an unfavourable cervix: a comparative randomized study of the atad ripener device, prostaglandin E2 vaginal pessary, and prostaglandin E2 intracervical gel. Australian and New Zealand Journal of Obstetrics and Gynecology 1996;36(3):291‐5. [DOI] [PubMed] [Google Scholar]
- Yuen PM, Pang YY. A randomized study of two different methods for cervical ripening. 2nd International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1993 Sept 7‐10; Hong Kong. 1993:154.
Zahoor 2014 {published data only}
- Zahoor S. Prostaglandin E2, intravaginal misoprostol and intracervical balloon catheter for induction of labour at term, a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):147. [Google Scholar]
References to studies excluded from this review
Abramovici 1999 {published data only}
- Abramovici D, Goldwasser S, Mabie B, Mercer B, Sibai B. Cervical ripening and labor induction, with oral misoprostol vs mechanical methods of cervical ripening and oxytocin. American Journal of Obstetrics and Gynecology 1999;180 (1 Pt 2):S126. [DOI] [PubMed] [Google Scholar]
- Abramovici D, Goldwasser S, Mabie BC, Mercer BM, Goldwasser R, Sibai BM. A randomized comparison of oral misoprostol versus Foley catheter and oxytocin for induction of labor at term. American Journal of Obstetrics and Gynecology 1999;181:1108‐12. [DOI] [PubMed] [Google Scholar]
Adeniji 2005a {published data only}
- Adeniji AO, Olayemi O, Odukogbe AA, Oladokun A, Adeniji OI, Egbewale BE, et al. Cervico‐vaginal foetal fibronectin: a predictor of cervical response at pre‐induction cervical ripening. West African Journal of Medicine 2005;24(4):334‐7. [DOI] [PubMed] [Google Scholar]
Adeniji 2005b {published data only}
- Adeniji OA, Oladokun A, Olayemi O, Adeniji OI, Odukogbe AA, Ogunbode O, et al. Pre‐induction cervical ripening: transcervical foley catheter versus intravaginal misoprostol. Journal of Obstetrics and Gynaecology 2005;25(2):134‐9. [DOI] [PubMed] [Google Scholar]
Adeniji 2006 {published data only}
- Adeniji AO, Olayemi O, Odukogbe AA. Intravaginal misoprostol versus transcervical foley catheter in pre‐induction cervical ripening. International Journal of Gynecology & Obstetrics 2006;92(2):130‐2. [DOI] [PubMed] [Google Scholar]
- Adeniji AO, Olayemi O, Odukogbe AA, Aimakhu CO, Oladokun A, Akindele FO, et al. Comparison of changes in pre‐induction cervical factors' scores following ripening with transcervical foley catheter and intravaginal misoprostol. African Journal of Medicine & Medical Sciences 2005;34(4):377‐82. [PubMed] [Google Scholar]
Afolabi 2005 {published data only}
- Afolabi BB, Oyeneyin OL, Ogedengbe OK. Intravaginal misoprostol versus foley catheter for cervical ripening and induction of labor. International Journal of Gynecology & Obstetrics 2005;89:263‐7. [DOI] [PubMed] [Google Scholar]
Ahmad 2015 {published data only}
- Ahmad MF, Ruey S, Vijayarani S, Hussin N, Ahmad S. Evaluation of cervical ripening between transcervical foley catheter versus hygroscopic cervical dilator (laminaria tent) for induction of labour in women with previous caesarean delivery: prospective randomized study. Journal of Obstetrics and Gynaecology Research 2015;41(Suppl S1):20‐1, Abstract no: FC 5.02. [Google Scholar]
Anabosy 2014 {published data only}
- Anabosy SM, NCT02223949. Labor induction and maternal bmi: comparison of different pre‐induction cervical ripening methods: the cook double balloon catheter vs pge1 tablets in lean, overweight, and obese women. a prospective randomized study. clinicaltrials.gov/show/NCT02223949 (first recevied 22 August 2014).
Arsenijevic 2012 {published data only}
- Arsenijevic S, Vukcevic‐Globarevic G, Volarevic V, Macuzic I, Todorovic P, Tanaskovic I, et al. Continuous controllable balloon dilation: a novel approach for cervix dilation. Trials 2012;13:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arshad 2016 {published data only}
- Arshad AH, Zainuddin AA, Ghani NA, Ali A. The efficiency of laminaria as an adjunct to induction of labour with prostin: A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2016;123(Suppl 2):156. [Google Scholar]
Atad 1991 {published data only}
- Atad J, Bornstein J, Calderon I, Petrikovsky BM, Sorokin Y, Abramovici H. Nonpharmaceutical ripening of the unfavorable cervix and induction of labor by a novel double balloon device. Obstetrics & Gynecology 1991;77:146‐52. [PubMed] [Google Scholar]
Atad 1999 {published data only}
- Atad J, Calderon I, Hallah M, Peer G, Abramovici H. Labour induction ‐ a new approach. Royal Australian and New Zealand College of Obstetrics and Gynaecology, New Zealand Committee Meeting; 2000 April 8‐11; Queenstown, New Zealand. 2000:Abstract no: 8.
- Atad J, Peer G. Combination of the double balloon device (ARD) and half doses of PGE2 vaginal gel for labor induction. 1st World Congress on Controversies in Obstetrics Gynecology and Infertility; 1999 Oct 28‐31; Prague, Czech Republic. 1999.
Baacke 2006 {published data only}
- Baacke K, NCT00325026. Randomized trial comparing misoprostol and foley bulb for labor induction in the preterm gestation. clinicaltrials.gov/ct2/show/record/NCT00325026 (first received 10 May 2006).
Barrilleaux 2002a {published data only}
- Barrilleaux P, Bofill J, Rodts‐Palenik S, Moore L, May W, Martin J Jr. A randomized clinical trial comparing three methods of cervical ripening to efficiently effect delivery [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S174. [Google Scholar]
- Barrilleaux PS, Bofill JA, Terrone DA, Magann EF, May WL, Morrison JC. Cervical ripening and induction of labor with misoprostol, dinoprostone gel, and a foley catheter: a randomized trial of 3 techniques. American Journal of Obstetrics and Gynecology 2002;186:1124‐9. [DOI] [PubMed] [Google Scholar]
Behrashi 2013 {published data only}
- Behrashi M, IRCT2013010712037N1. Vaginal misoprostol versus laminaria for cervical ripening in full term pregnants. a comparative randomized trial. http://en.irct.ir/trial/12185 (first received 23 January 2013).
Ben‐Aroya 2001 {published data only}
- Ben‐Aroya Z, Hallak M, Segal D, Friger M, Katz M, Mazor M. Ripening of uterine cervix in a post cesarean parturient: PGE2 vs. intracervical Foley catheter. American Journal of Obstetrics and Gynecology 2001;184:S117. [Google Scholar]
Buccellato 2000 {published data only}
- Buccellato CA, Stika CS, Frederiksen MC. A randomized trial of misoprostol versus extra‐amniotic sodium chloride infusion with oxytocin for induction of labor. American Journal of Obstetrics and Gynecology 2000;182:1039‐44. [DOI] [PubMed] [Google Scholar]
Cahill 1988 {published data only}
- Cahill DJ, Clark HS, Martin DH. Cervical ripening: the comparative effectiveness of Lamicel and prostaglandin E2 tablets. Irish Journal of Medical Science 1988;157(4):113‐4. [DOI] [PubMed] [Google Scholar]
Caughey 2007 {published data only}
- Caughey A, NCT00451308. Induction of labor with a foley catheter balloon: a randomized trial comparing inflation with 30ml and 60ml. clinicaltrials.gov/ct2/show/record/NCT00451308 (first received 22 March 2007).
- Sparks T, Caughey AB, Shaffer B, Cheng YW, Vargas J, Delaney S, et al. Predictors of cesarean delivery in women undergoing labor induction with a Foley balloon. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S78. [DOI] [PubMed] [Google Scholar]
Chipato 1997 {published data only}
- Chipato T, Mawire CJ. RCT of extra‐amniotic saline infusion versus extra‐amniotic PGF2alpha for cervical ripening and induction of labor. Journal of Clinical Epidemiology 1997;50 Suppl 1:21S. [DOI] [PubMed] [Google Scholar]
Chung 2003 {published data only}
- Chung JH, Huang WH, Rumney PJ, Garite TJ, Nageotte MP. A prospective randomized controlled trial that compared misoprostol, foley catheter, and combination misoprostol‐foley catheter for labor induction. American Journal of Obstetrics and Gynecology 2003;189:1031‐5. [DOI] [PubMed] [Google Scholar]
- Huang W, Chung J, Rumney P, Pattillo C, Garite T, Nageotte M. A prospective, randomized controlled trial comparing misoprostol, foley catheter, and combination misoprostol‐foley for labor induction. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S57. [DOI] [PubMed] [Google Scholar]
- Huang W, Chung J, Rumney P, Pattillo C, Garite T, Nageotte M. A prospective, randomized controlled trial comparing misoprostol, foley catheter, and combination misoprostol‐foley for labor induction. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S57. [DOI] [PubMed] [Google Scholar]
Connolly 2016 {published data only}
- Connolly KA, Kohari KS, Rekawek P, Smilen B, Miller MR, Moshier E, et al. A randomized trial of Foley bulb induction of labor trial in nulliparas (FIAT). American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S30‐S31, Abstract no: 43. [DOI] [PubMed] [Google Scholar]
- Connolly KA, Kohari KS, Rekawek P, Smilen BS, Miller MR, Moshier E, et al. A randomized trial of foley balloon induction of labor trial in nulliparas (fiat‐n). American Journal of Obstetrics and Gynecology 2016; Vol. 215, issue 3:392.e1‐6. [DOI] [PubMed]
Connolly 2017 {published data only}
- Connolly KA, Factor SH, Rekawek P, Smilen BS, Stone JL, Bianco AT, et al. A randomized trial of foley balloon induction of labor trial in multiparas (FIAT‐M). American Journal of Obstetrics and Gynecology 2017;216(1):S433‐S434, Abstract no: 746. [DOI] [PubMed] [Google Scholar]
- Connolly KA, Kohari KS, Factor SH, Rekawek P, Miller MR, Smilen BS, et al. A randomized trial of foley balloon induction of labor trial in multiparas (fiat‐m). American Journal of Perinatology 2017;34(11):1108‐14. [DOI] [PubMed] [Google Scholar]
Cross 1978 {published data only}
- Cross WG, Pitkin RM. Laminaria as an adjunct in induction of labor. Obstetrics & Gynecology 1978;51:606‐8. [DOI] [PubMed] [Google Scholar]
Cullimore 2009 {published data only}
- Cullimore A, NCT00890630. Intracervical catheters for induction of labour in women with prelabour rupture of membranes at term: a pilot study. clinicaltrials.gov/show/NCT00890630 (first received 30 April 2009).
Delaney 2010 {published data only}
- Delaney S, Shaffer B, Cheng Y, Vargas J, Sparks T, Paul K, et al. Labor induction with a foley balloon trial (LIFT) ‐ a randomized controlled trial of 30mL versus 60mL foley balloon inflation. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S23‐4. [DOI] [PubMed] [Google Scholar]
- Delaney S, Shaffer BL, Cheng YW, Vargas J, Sparks TN, Paul K, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL: a randomized controlled trial. Obstetrics & Gynecology 2010;115(6):1239‐45. [DOI] [PubMed] [Google Scholar]
Demirel 2015 {published data only}
- Demirel G, Guler H. The effect of uterine and nipple stimulation on induction with oxytocin and the labor process. Worldviews on Evidence‐Based Nursing / Sigma Theta Tau International, Honor Society of Nursing 2015;12(5):273‐80. [DOI] [PubMed] [Google Scholar]
De Oliveira 2003 {published data only}
- Oliveira MG. A prospective randomized study of the foley catheter for ripening of the unfavourable cervix before induction of labour [Estudo prospectivo e randomizado da sonda foley na preparacao do colo uterino desfavoravel a inducao do parto]. Revista Brasileira de Ginecologia e Obstetricia 2003;25(5):375. [Google Scholar]
Dias 2008 {published data only}
- Dias TD, SLCTR/2008/002. A randomised controlled trial comparing intra‐vaginal Misoprostol with trans‐cervical Foley catheter for the pre‐induction cervical ripening. http://slctr.lk/trials/44 (first received 28 March 2008).
Du 2015 {published data only}
- Du C, Liu Y, Liu Y, Ding H, Zhang R, Tan J. Double‐balloon catheter vs. dinoprostone vaginal insert for induction of labor with an unfavorable cervix. Archives of Gynecology and Obstetrics 2015;291:1221‐7. [DOI] [PubMed] [Google Scholar]
Edwards 2017 {published data only}
- Edwards RK, NCT03111316. Combined use of the controlled release dinoprostone insert and foley catheter compared to the foley catheter alone for cervical ripening and labor induction in term women: a randomized controlled trial. clinicaltrials.gov/ct2/show/record/NCT03111316 (first received 13 March 2017).
El‐Khayat 2016 {published data only}
- El‐Khayat W, Alelaiw H, El‐Kateb A, Elsemary A. Comparing vaginal misoprostol versus foley catheter plus vaginal isosorbide mononitrate for labor induction. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(3):487‐92. [DOI] [PubMed] [Google Scholar]
- El‐khayat W, NCT01506388. Foley catheter plus vaginal isosorbide mononitrate versus vaginal misoprostol for induction of labour: a randomised controlled trial. clinicaltrials.gov/ct2/show/record/NCT01506388 (first received 4 January 2012).
El Sharkwy 2017 {published data only}
- Sharkwy IA, Noureldin EH, Mohamed EA, Shazly SA. Sequential versus concurrent use of vaginal misoprostol plus foley catheter for induction of labor: a randomized clinical trial. Journal of Obstetrics and Gynecology of India 2018;68(5):408‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sharkwy IA, NCT02952807. Sequential versus concurrent use of vaginal misoprostol plus foley catheter for induction of labor. https://clinicaltrials.gov/show/NCT02952807 (31 October 2016). [DOI] [PMC free article] [PubMed]
El‐Torkey 1995 {published data only}
- El‐Torkey M, Grant JM. Hydrostatic sweeping of the membranes is an effective method of preparing the unripe cervix for induction of labour. A random allocation prospective trial. Journal of Obstetrics and Gynaecology 1995;15:100‐3. [Google Scholar]
- Grant JM. Comparison of hydrostatic sweeping of the membranes (extra‐amniotic foley catheter plus extra‐amniotic water injection) and vaginal prostaglandin gel in women with an unfavourable cervix who require induction of labour [personal communication]. Letter to : Cochrane Pregnancy and Childbirth Group 1993.
Emery 1988 {published data only}
- Emery S, Neal E, Ward S, Morrison R, Filshie M. Prospective controlled trial of three methods for ripening the unfavourable cervix prior to induction of term labour. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988.
EUCTR 2012 {published data only}
- EUCTR2012‐004880‐36‐AT. Efficacy of induction of labor on term using a double balloon catheter compared to Dinoprostone vaginal‐insert – a multicenter randomized controlled trial. clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2012‐004880‐36 (first received 29 May 2013).
Filshie 1992 {published data only}
- Filshie GM. Trial to determine the relative efficacy of prostaglandins vs dilapan in ripening the unripe cervix prior to induction of labour [personal communication]. Letter to: Cochrane Pregnancy and Childbirth Group 1992.
Forgie 2016 {published data only}
- Forgie MM, Greer DM, Kram JJF, Vander KB, Salvo NP, Siddiqui DS. Foley catheter placement for induction of labor with or without stylette: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2016;214(3):397.e1‐397.e10. [DOI] [PubMed] [Google Scholar]
Forooshani 2011 {published data only}
- Forooshani M, IRCT201105016355N1. Comparison of transcervical catheter and laminaria efficacy on induction of labor in post term pregnancy. http://en.irct.ir/trial/6798 (first received 7 September 2011).
Fruhman 2017 {published data only}
- Fruhman G, Gavard J, Amon E, Flick K, Gross G. Parity and foley catheter using tension or no tension: a randomized controlled trial. Obstetrics and Gynecology 2017;129(5 Suppl):125S. [DOI] [PubMed] [Google Scholar]
- Fruhman G, Gavard JA, Amon E, Flick KV, Miller C, Gross GA. Balloon catheter for induction of labor with or without tension applied: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S253‐S254, Abstract no: 462. [DOI] [PubMed] [Google Scholar]
- Fruhman G, Gavard JA, Amon E, Flick KV, Miller C, Gross GA. Tension compared to no tension on a foley transcervical catheter for cervical ripening: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2017;216(1):67.e1‐9. [DOI] [PubMed] [Google Scholar]
- Fruhman G, NCT02606643. Balloon catheter for cervical ripening with or without traction: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02606643 (first received 17 November 2015).
Gadel 2015 {published data only}
- Gadel Rab MT, Mohammed AB, Zahran KA, Hassan MM, M Eldeen AR, Ibrahim EM, et al. Transcervical Foley's catheter versus Cook balloon for cervical ripening in stillbirth with a scarred uterus: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(10):1181‐5. [DOI] [PubMed] [Google Scholar]
Garebedian 2016 {published data only}
- Garebedian C, NCT02932319. Outpatient foley catheter for induction of labor in nulliparous for prolonged pregnancy. clinicaltrials.gov/ct2/show/record/NCT02932319 (first received 4 October 2016).
Ghanaei 2009 {published data only}
- Ghanaei MM, Sharami H, Asgari A. Labor induction in nulliparous women: a randomized controlled trial of foley catheter with extra‐amniotic saline infusion. Journal of the Turkish German Gynecology Association Artemis 2009;10(2):71‐5. [Google Scholar]
Ghanaie 2013 {published data only}
- Ghanaie MM, Jafarabadi M, Milani F, Asgary SA, Karkan MZ. A randomized controlled trial of foley catheter, extra‐amniotic saline infusion and prostaglandin E2 suppository for labor induction. Journal of Family and Reproductive Health 2013;7(2):49‐55. [PMC free article] [PubMed] [Google Scholar]
Gibson 2013 {published data only}
- Gibson K, Mercer B, Louis J. A randomized control trial of inner thigh taping versus traction for cervical ripening with a Foley catheter. American Journal of Obstetrics and Gynecology 2013;208(1 Suppl):S145‐6. [DOI] [PubMed] [Google Scholar]
- Gibson KS, Mercer BM, Louis JM. Inner thigh taping vs traction for cervical ripening with a Foley catheter: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2013;209(3):272.e1‐7. [DOI] [PubMed] [Google Scholar]
- Gibson KS, NCT00976703. Weighted bag versus inner thigh taping for cervical ripening with a foley catheter prior to an induction of labor. clinicaltrials.gov/ct2/show/record/NCT00976703 (first received 11 September 2009).
Gilson 1996 {published data only}
- Gilson GJ, Russell DJ, Izquierdo LA, Qualls CR, Curet LB. A prospective randomized evaluation of a hygroscopic cervical dilator, dilapan, in the preinduction ripening of patients undergoing induction of labor. American Journal of Obstetrics and Gynecology 1996;175:145‐9. [DOI] [PubMed] [Google Scholar]
- Gilson GJ, Smith JF, Curet LB, Izquierdo LA, Chatterjee MS, Joffe GM, et al. Efficacy of preinduction dilapan on lowering the cesarean section rate. American Journal of Obstetrics and Gynecology 1992;166:423. [Google Scholar]
- Gilson GJ, Smith JF, Curet LB, Izquierdo LA, Chatterjee MS, Joffe GM, et al. Efficacy of preinduction dilapan on lowering the cesarean section rate. American Journal of Obstetrics and Gynecology 1992;166:423. [Google Scholar]
Gonsoulin 1989 {published data only}
- Gonsoulin W, Moise KJ, Cano L. Efficacy of dilapan laminaria to intracervical prostaglandin E2 gel in cervical ripening. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians;1989 February 1‐4; New Orleans, Louisiana, USA. New Orleans, 1989:94.
Gower 1982 {published data only}
- Gower RH, Toraya J, Miller JM, Jr. Laminaria for preinduction cervical ripening. Obstetrics & Gynecology 1982;60:617‐9. [PubMed] [Google Scholar]
Greybush 2001 {published data only}
- Greybush M, Singleton C, Atlas RO, Balducci J, Rust OA. Preinduction cervical ripening techniques compared. Journal of Reproductive Medicine 2001;46(1):11‐7. [PubMed] [Google Scholar]
- Rust OA, Greybush M, Singleton C, Atlas RO, Balducci J. A comparison of preinduction cervical ripening techniques. American Journal of Obstetrics and Gynecology 1999;180:S126. [Google Scholar]
Gu 2015 {published data only}
- Gu N, Ru T, Wang Z, Dai Y, Zheng M, Xu B, et al. Foley catheter for induction of labor at term: An open‐label, randomized controlled trial. PLOS One 2015;10(8):e0136856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. Foley catheter balloon for cervical ripening in term pregnancy: a multicenter randomized controlled trial. http://www.chictr.org.cn/hvshowproject.aspx?id=5218 (first received 17 January 2013).
Guinn 2004 {published data only}
- Guinn D, Davies J, Jones RO, Wolf D. Foley catheter with extraamniotic saline infusion (easi) versus foley catheter alone for induction of labor in gravidas with an unfavorable cervix. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S169. [Google Scholar]
- Guinn DA, Davies JK, Jones RO, Sullivan L, Wolf D. Labor induction in women with an unfavorable bishop score: randomized controlled trial of intrauterine foley catheter with concurrent oxytocin infusion versus foley catheter with extra‐amniotic saline infusion with concurrent oxytocin infusion. American Journal of Obstetrics and Gynecology 2004;191:225‐9. [DOI] [PubMed] [Google Scholar]
Haghighi 2015 {published data only}
- Haghighi L, IRCT2015040721506N2. Comparison extra amniotic salin infusion and vaginal isoniazide for cervical ripening before induction and labour duration in term and post term pregnancy. http://en.irct.ir/trial/18839 (first received 28 April 2015).
Hallak 2008 {published data only}
- Hallak M, NCT00604487. Induction of labor in patients with unfavorable cervical conditions. clinicaltrials.gov/ct2/show/record/NCT00604487 (first received 30 Jan 2008).
He 2000 {published data only}
- He HY. Discussion on the nursing care of air‐vesicle odinopoeia in post‐term pregnancy. Nursing Journal of Chinese People's Liberation Army 2000;17(6):7‐8. [Google Scholar]
Hill 2009 {published data only}
- Hill JB, Thigpen BD, Bofill JA, Magann E, Moore LE, Martin JN Jr. A randomized clinical trial comparing vaginal misoprostol versus cervical Foley plus oral misoprostol for cervical ripening and labor induction. American Journal of Perinatology 2009;26(1):33‐8. [DOI] [PubMed] [Google Scholar]
Hill 2013 {published data only}
- Hill M, NCT01866488. The obstetric cook double balloon catheter in combination with oral misoprostol for induction of labor: a double‐blinded, randomized controlled trial. clinicaltrials.gov/show/NCT01866488 (first received 31 May 2013).
Hussein 2012 {published data only}
- Hussein M. A comparison between vaginal misoprostol and a combination of misoprostol and Foley catheter for cervical ripening and labour induction in early third trimester pregnancy. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S147. [Google Scholar]
Ifnan 2006 {published data only}
- Ifnan F, Jameel MB. Ripening of cervix for induction of labour by hydrostatic sweeping of membrane versus foley's catheter ballooning alone. Journal of the College of Physicians and Surgeons Pakistan 2006;16(5):347‐50. [PubMed] [Google Scholar]
Jagani 1984 {published data only}
- Jagani N, Schulman H, Fleischer A, Mitchell J, Blattner P. Role of prostaglandin‐induced cervical changes in labor induction. Obstetrics & Gynecology 1984;63:225‐9. [PubMed] [Google Scholar]
Jasper 2000 {published data only}
- Jasper MP, Blossom S, Peedicayil A. A randomised controlled trial of extra amniotic saline infusion and intracervical Foley catheter for cervical ripening. XVI FIGO World Congress of Obstetrics & Gynecology (Book 4) ; 2000 Sept 3‐8; Washington DC, USA. 2000:69‐70.
Jindal 2007 {published data only}
- Jindal P, Gill BK, Tirath B. A comparison of vaginal misoprostol versus Foley's catheter with oxytocin for induction of labor. Journal of Obstetrics and Gynaecology of India 2007;57(1):42‐7. [Google Scholar]
Jonsson 2011 {published data only}
- Jonsson M, Hellgren C, Wiberg‐Itzel E, Akerud H. Assessment of pain in women randomly allocated to speculum or digital insertion of the Foley catheter for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 2011;90(9):997‐1004. [DOI] [PubMed] [Google Scholar]
Kamilya 2011 {published data only}
- Kamilya G, CTRI/2011/08/001969. Randomized controlled trial of induction of labour comparing Foley balloon inflation to 60 ml with sublingual misoprostol. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2999 (first received 26 August 2011).
Karjane 2006 {published data only}
- Karjane NW, Brock EL, Walsh SW. Induction of labor using a foley balloon, with and without extra‐amniotic saline infusion. Obstetrics & Gynecology 2006;107(2 Pt 1):234‐9. [DOI] [PubMed] [Google Scholar]
Kasdaglis 2007 {published data only}
- Kasdaglis T, Adamczak J, Rinehart B, Antebi Y, Mendise T, Terrone D. A randomized controlled trial of cervical ripening in patients with PROM using an intracervical balloon catheter and oxytocin versus dinoprostone. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S104. [Google Scholar]
Kashanian 2006 {published data only}
- Kashanian M, Akbarian AR, Fekrat M. Cervical ripening and induction of labor with intravaginal misoprostol and foley catheter cervical traction. International Journal of Gynecology & Obstetrics 2006;92(1):79‐80. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Fekrat M. The cervical ripening and induction of labor with intravaginal misoprostol, traction on the cervix with intracervical Foley catheter, and a combination of the two methods: a randomized trial of 3 techniques. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S481. [Google Scholar]
Kashanian 2009a {published data only}
- Kashanian M, Nazemi M, Malakzadegan A. Comparison of 30‐mL and 80‐mL Foley catheter balloons and oxytocin for preinduction cervical ripening. International Journal of Gynecology & Obstetrics 2009;105(2):174‐5. [DOI] [PubMed] [Google Scholar]
Kehl 2012 {published data only}
- Kehl S, Welzel G, Ehard A, Berlit S, Spaich S, Siemer J, et al. Women's acceptance of a double‐balloon device as an additional method for inducing labour. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;168(1):30‐5. [DOI] [PubMed] [Google Scholar]
- Kehl S, Ziegler J, Schleussner E, Tuschy B, Berlit S, Mayer J, et al. Induction of labour with a balloon catheter and misoprostol ‐ a randomised controlled multi centre study [Geburtseinleitung mit einem ballonkatheter und misoprostol ‐ eine randomisierte kontrollierte multicenter‐studie]. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S145‐6. [Google Scholar]
Kehl 2015 {published data only}
- Kehl S, Ziegler J, Schleussner E, Tuschy B, Berlit S, Kirscht J, et al. Sequential use of double‐balloon catheter and oral misoprostol versus oral misoprostol alone for induction of labour at term (CRBplus trial): a multicentre, open‐label randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122:129‐36. [DOI] [PubMed] [Google Scholar]
- Kehl S/ACTRN12611000537954. Randomized multicenter study of mechanical ripening of the cervix by double balloon device (cook crb [cervical ripening balloon]) before oral misoprostol (om) versus om alone to improve efficacy in inducing labor. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000537954 (first received 10 May 2011).
Keirse 1983 {published data only}
- Keirse MJ, Thiery M, Parewijck W, Mitchell MD. Chronic stimulation of uterine prostaglandin synthesis during cervical ripening before the onset of labor. Prostaglandins 1983;25:671‐82. [DOI] [PubMed] [Google Scholar]
Lackritz 1979 {published data only}
- Lackritz R, Gibson M, Frigoletto FD, Jr. Preinduction use of laminaria for the unripe cervix. American Journal of Obstetrics and Gynecology 1979;134:349‐50. [DOI] [PubMed] [Google Scholar]
Lam 2006 {published data only}
- Lam YR, NCT00366951. A randomized clinical trial comparing the efficacy and safety of foley catheter balloon with oxytocin and extraamniotic saline infusion (easi) with oxytocin for induction of labor requiring cervical ripening. clinicaltrials.gov/show/NCT00366951 (first received 18 August 2006).
Leiberman 1977 {published data only}
- Leiberman JR, Piura B, Chaim W, Cohen A. The cervical balloon method for induction of labor. Acta Obstetricia et Gynecologie Scandinavica 1977;56:499‐503. [DOI] [PubMed] [Google Scholar]
Leong 2017 {published data only}
- Leong YS, NCT03326557. Membrane sweeping versus transcervical foley catheter for induction of labour in women with previous caesarean delivery. clinicaltrials.gov/show/NCT03326557 (first received 22 October 2017).
Levine 2016 {published data only}
- Levine LD, Downes KL, Elovitz MA, Parry S, Sammel MD, Srinivas SK. Mechanical and pharmacologic methods of labor induction: a randomized controlled trial. Obstetrics and Gynecology 2016;128(6):1357‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine LD, Sammel MD, Parry S, Williams CT, Elovitz MA, Srinivas SK. Foley or Misoprostol for the Management of Induction (The ‘FOR MOMI’ trial): A four‐arm randomized clinical trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S4, Abstract no: 5. [Google Scholar]
- NCT01916681. Foley OR MisO for the Management of Induction (FOR MOMI) Trial. clinicaltrials.gov/show/NCT01916681 (first received 30 July 2013).
Levy 2000 {published data only}
- Levy R, Ben‐Arie A, Paz B, Hazen I, Blickstein I, Hagay Z. Randomized clinical trial of early vs late amniotomy following cervical ripening with a Foley catheter. American Journal of Obstetrics and Gynecology 2000;182:S136. [DOI] [PubMed] [Google Scholar]
Levy 2004 {published data only}
- Levy R, Kanengiser B, Furman B, Ben‐Arie A, Brown D, Hagay ZJ. A randomized trial comparing a 30‐ml and an 80‐ml foley catheter balloon for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 2004;191:1632‐6. [DOI] [PubMed] [Google Scholar]
Lin 1995 {published data only}
- Lin A, Kupferminc M, Dooley SL. A randomized trial of extra‐amniotic saline infusion versus laminaria for cervical ripening. Obstetrics & Gynecology 1995;86:545‐9. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin MG, Ramsey PS. Foley catheter for labor induction in women with term or near term membrane rupture. clinicaltrials.gov/ct2/show/NCT00290199 (first received 10 February 2006).
Lin 2007 {published data only}
- Lin M, Ramsey P, Reid K, Treaster M, Nuthalapaty F, Lu G. The impact of maternal BMI, parity and GA on the comparative efficacy of transcervical foley catheter with or without an extraamniotic saline infusion for cervical ripening and labor induction in women with an unfavorable cervix. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S109. [Google Scholar]
- Lin M, Treaster M, Reid K, Nuthalapaty F, Ramsey P, Lu G. A randomized controlled trial of transcervical foley catheter with and without extra‐amniotic saline infusion (EASI) for labor induction. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S30. [DOI] [PubMed] [Google Scholar]
- Lin MG, Lu G, Ramsey PS, NCT00442663. Randomized trial of transcervical foley catheter with and without extra‐amniotic saline infusion for labor induction. clinicaltrials.gov/ct2/show/record/NCT00442663 (first received 28 February 2007).
- Lin MG, Reid KJ, Treaster MR, Nuthalapaty FS, Ramsey PS, Lu GC. Transcervical foley catheter with and without extraamniotic saline infusion for labor induction: a randomized controlled trial. Obstetrics & Gynecology 2007;110(3):558‐65. [DOI] [PubMed] [Google Scholar]
Lutgendorf 2012 {published data only}
- Lutgendorf MA, Johnson A, Terpstra ER, Snider TC, Magann EF. Extra‐amniotic balloon for preinduction cervical ripening: A randomized comparison of weighted traction versus unweighted. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(6):581‐6. [DOI] [PubMed] [Google Scholar]
Macpherson 1983 {published data only}
- Macpherson M, Welch C, Powell M, Filshie M. A trial to compare lamicel, a new induction agent with prostaglandin E2 gel to ripen the cervix prior to induction of labour. Proceedings of 23rd British Congress of Obstetrics and Gynaecology; 1983 July 12‐15; Birmingham, UK. 1983:79.
Mahomed 1988 {published data only}
- Mahomed K. Foley catheter under traction versus extra‐amniotic prostaglandin gel in pre‐treatment of unripe cervix ‐ a randomised controlled trial. Central African Journal of Medicine 1988;34:98‐102. [PubMed] [Google Scholar]
Manabe 1985 {published data only}
- Manabe Y, Yoshimura S, Mori T, Aso T. Plasma levels of 13,14‐dihydro‐15‐keto prostaglandin F2‐alpha, estrogens and progesterone during stretch‐induced labor at term. Prostaglandins 1985;30(1):141‐51. [DOI] [PubMed] [Google Scholar]
Manish 2016 {published data only}
- Manish P, Rathore S, Benjamin SJ, Abraham A, Jeyaseelan V, Mathews JE. A randomised controlled trial comparing 30 ml and 80 ml in foley catheter for induction of labour after previous caesarean section. Tropical Doctor 2016;46(4):205‐11. [DOI] [PubMed] [Google Scholar]
- Mathews J, CTRI/2014/02/004412. Randomised trial comparing intrauterine balloon catheter with 30ml fluid with intrauterine balloon catheter with 80ml of fluid to start labor in women with one previous caesarean section. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4199 (first received 17 February 2014).
Manyonda 2007 {published data only}
- Manyonda IT. A randomised controlled trial of the use of the Foley catheter balloon for induction of labour to reduce the incidence of caesarean section in diabetic pregnancies: a prospective clinical, economic and psychological evaluation. isrctn.com/ISRCTN39708525 (first received 28 September 2007).
Martin 1989 {published data only}
- Martin JN Jr, Sessums JK, Howard P, Martin RW, Morrison JC. Alternative approaches to the management of gravidas with prolonged‐postterm‐postdate pregnancies. Journal of the Mississippi State Medical Association 1989;30:105‐11. [PubMed] [Google Scholar]
Mattingly 2015 {published data only}
- Mattingly P, Temming L, Bliss S. Cervical ripening with a double‐lumen balloon catheter for six versus twelve hours: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S264. [Google Scholar]
- Mattingly PJ, Temming LA, Bliss SA. Cervical ripening with a double‐lumen balloon catheter for 6 compared with 12 hours. A randomized controlled trial. Obstetrics & Gynecology 2015;125(5 Suppl):71S. [Google Scholar]
Mawire 1999 {published data only}
- Mawire CJ, Chipato T, Rusakaniko S. Extra‐amniotic saline infusion versus extra‐amniotic prostaglandin F2alpha for cervical ripening and induction of labor. International Journal of Gynecology & Obstetrics 1999;64:35‐41. [DOI] [PubMed] [Google Scholar]
McGee 2016 {published data only}
- McGee T, ACTRN12615000795594. Foley catheter latex versus silicone for cervical ripening prior to term induction of labour: a randomized controlled trial. anzctr.org.au/ACTRN12615000795594.aspx (first received 18 June 2016).
Mei‐Dan 2009 {published data only}
- Mei‐Dan E, Walfisch A, Easton SS, Hallak M. Foley's catheter with extra‐amniotic saline infusion ‐ a faster and sheaper ripener device: prospective randomized trial. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S125. [Google Scholar]
Mei‐Dan 2012 {published data only}
- Mei‐Dan E, NCT01615107. Comparison between the use of standard oxytocin induction protocol and the double‐balloon catheter device with concurrent oxytocin. clinicaltrials.gov/ct2/show/record/NCT01615107 (first received 8 June 2012).
Mei‐Dan 2012a {published data only}
- Mei‐Dan E, Walfisch A, Suarez‐Easton S, Hallak M. Comparison of two mechanical devices for cervical ripening: A prospective quasi‐randomized trial. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(6):723‐7. [DOI] [PubMed] [Google Scholar]
- Mei‐Dan E, Walfisch A, Valencia C, Hallak M. Cervical ripening with extra amniotic saline infusion: a randomized comparison of two mechanical devices. Reproductive Sciences 2012;19(3Suppl):229A. [Google Scholar]
Mei‐Dan 2014 {published data only}
- Mei‐Dan E, Walfisch A, Valencia C, Hallak M. Making cervical ripening EASI: A prospective controlled comparison of single versus double balloon catheters. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(17):1765‐70. [DOI] [PubMed] [Google Scholar]
Miller 2015 {published data only}
- Miller NR, Cypher RL, Foglia LM, Pates JA, Nielsen PE. Elective induction of labor compared with expectant management of nulliparous women at 39 weeks of gestation: a randomized controlled trial. Obstetrics and Gynecology 2015;126(6):1258‐64. [DOI] [PubMed] [Google Scholar]
- Miller NR, NCT01076062. Elective induction of nulliparous labor: a randomized clinical trial elective induction of nulliparous labor: a randomized clinical trial. clinicaltrials.gov/ct2/show/NCT01076062 (first received 25 February 2010).
Moise 1991 {published data only}
- Moise KJ, Cano LE, Hesketh DE. A prospective, randomized comparison of a new synthetic laminaria, intracervical prostaglandin E2 gel, and oxytocin for preinduction ripening of the term cervix. Proceedings of 39th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1991; USA. 1991:24.
Morrison 1993 {published data only}
- Morrison JC. Cervical ripening techniques [personal communication]. Letter to: Cochrane Pregnancy and Childbirth Group 1993.
Movahed 2016 {published data only}
- Movahed F, Seyed E, Pakniat H, Iranipour M, Yazdi Z. Comparison of the effects of transcervical catheter, laminaria and isosorbide mononitrate on cervical ripening. Journal of Babol University of Medical Sciences 2016;18(3):19‐24. [Google Scholar]
Mullin 2014 {published data only}
- Mullin PM, NCT02210598. Outpatient labor induction with the transcervical foley balloon: a randomized trial comparing outpatient immediate removal foley versus standard inpatient foley induction. clinicaltrials.gov/ct2/show/record/NCT02210598 (first received 19 March 2014).
Naseem 2007 {published data only}
- Naseem A, Nouman D, Iqbal J, Majeed MA, Khan MM. Intracervical foley`s catheter balloon versus prostaglandin e2 vaginal pessary for induction of labor. Journal Rawalpindi Medical College 2007; Vol. 12, issue 2:94‐9.
Nasir 2012 {published data only}
- Nasir S, Chaudhry R. Comparison of intracervical foley catheter plus oral misoprostol with oral misoprostol alone for cervical ripening in primigravidas at term. BJOG: an international journal of obstetrics and gynaecology 2012;119(Suppl 1):11‐2. [Google Scholar]
Neethurani 2013 {published data only}
- Neethurani VK, CTRI/2013/10/004106. The efficacy of transcervical Foley catheter with extra amniotic saline infusion in cervical ripening before the induction of labour with intravaginal Prostaglandin E1‐ a randomized controlled trial. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5865 (first received 28 October 2013).
Owolabi 2005 {published data only}
- Owolabi AT, Kuti O, Ogunlola IO. Randomised trial of intravaginal misoprostol and intracervical foley catheter for cervical ripening and induction of labour. Journal of Obstetrics and Gynaecology 2005;25(6):565‐8. [DOI] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park KH, NCT01317862. A comparison of transcervical foley catheter and prostaglandins for induction of labor at term. clinicaltrials.gov/show/NCT01317862 (first received 15 March 2011).
Pathiraja 2014 {published data only}
- Pathiraja PD, SLCTR/2014/025. Induction of multiparous women at term using different methods: Prostaglandin E2 (dinopristone) vaginal gel, intracervical foley catheter insertion and sweeping of membrane: an open‐label, randomised controlled trial. http://slctr.lk/trials/244 (first received 9 October 2014).
Pedersen 1981 {published data only}
- Pedersen S, Moller‐Petersen J, Aegidius J. The effect on induction of labour of endocervical balloon catheter with and without oestradiol therapy. Ugeskrift for Laeger 1981;143:3379‐81. [PubMed] [Google Scholar]
Pettker 2008 {published data only}
- Pettker CM, Pocock SB, Smok DP, Devine PC. A prospective, randomized trial of transcervical foley catheter with or without oxytocin for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S27. [DOI] [PubMed] [Google Scholar]
- Pettker CM, Pocock SB, Smok DP, Lee SM, Devine PC. Transcervical foley catheter with and without oxytocin for cervical ripening: a randomized controlled trial. Obstetrics & Gynecology 2008;111(6):1320‐6. [DOI] [PubMed] [Google Scholar]
Rameez 2007 {published data only}
- Rameez MF, Goonewardene IM. Nitric oxide donor isosorbide mononitrate for pre‐induction cervical ripening at 41 weeks' gestation: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2007;33(4):452‐6. [DOI] [PubMed] [Google Scholar]
Reif 2012 {published data only}
- Reif P, NCT01720394. Efficacy of induction of labor on term using a double balloon catheter compared to dinoprostone vaginal‐insert ‐ a multicenter randomized controlled trial. https://clinicaltrials.gov/show/NCT01720394 (first received 2 November 2012).
Rezk 2014 {published data only}
- Rezk M, Sanad Z, Dawood R, Masood A, Emarh M, Halaby AA. Intracervical foley catheter versus vaginal isosorbid mononitrate for induction of labor in women with previous one cesarean section. Journal of Clinical Gynecology and Obstetrics 2014;3(2):55‐61. [Google Scholar]
Rust 2001 {published data only}
- Rust O, Greybush M, Atlas R, Balducci J, Jones K. Does combination pharmacologic and mechanical preinduction cervical ripening improve ripening to delivery interval?. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S136. [Google Scholar]
- Rust OA, Greybush M, Atlas RO, Jones KJ, Balducci J. Preinduction cervical ripening A randomized trial of intravaginal misoprostol alone vs a combination of transcervical foley balloon and intravaginal misoprostol. Journal of Reproductive Medicine 2001;46:899‐904. [PubMed] [Google Scholar]
Saad 2016 {published data only}
- Saad A, NCT02899689. Induction of labor in women with unfavorable cervix: randomized control study comparing dilapan to foley bulb. clinicaltrials.gov/ct2/show/record/NCT02899689 (first received 31 August 2016).
Saito 1999 {published data only}
- Saito K, Shoda T, Tani A, Yoshihara H, Amano K, Shimada N, et al. Pre‐induction priming method for unripe cervix ‐ comparative study with laminaria tents and metreurynter. Acta Obstetrica et Gynaecologica Japonica 1999;51(7):474‐8. [Google Scholar]
Salmeen 2012 {published data only}
- Salmeen K, NCT01641601. Randomized controlled trial of prehospital cervical ripening with an outpatient transcervical foley balloon and the duration of induction and maternal satisfaction. clinicaltrials.gov/show/NCT01641601 (first received 3 July 2012).
Sanchez‐Ramos 1990 {published data only}
- Sanchez‐Ramos L, Conner PM, Kaunitz AM. Prostaglandin E2 gel vs hypan in cervical ripening before induction of labor. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:481.
Sandberg 2017 {published data only}
- Sandberg EM, Schepers EM, Sitter RL, Huisman CM, Wijngaarden WJ. Foley catheter for induction of labour filled with 30ml or 60ml: a randomized controlled trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2017;211:150‐5. [DOI] [PubMed] [Google Scholar]
- Wijngaarden WJ, NTR5578. Foley catheter for induction of labour filled with 30mL or 60mL ‐ FILL study. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=5578 (first received 9 December 2015). [DOI] [PubMed]
Schoen 2017 {published data only}
- Schoen C, Berghella V, Grant G, Hoffmann M, Sciscione A. The intracervical foley catheter with and without oxytocin for labor induction: a randomized trial. American Journal of Obstetrics and Gynecology 2017;216(1 Suupl):S30‐S31, Abstract no: 43. [DOI] [PubMed] [Google Scholar]
- Schoen C, NCT02273115. Foley with oxytocin versus foley no oxytocin for induction of labor (NOFOX): a randomized control trial. clinicaltrials.gov/ct2/show/record/NCT02273115 (first received 20 October 2014).
- Schoen CN, Grant G, Berghella V, Hoffman MK, Sciscione A. Intracervical foley catheter with and without oxytocin for labor induction: a randomized controlled trial. Obstetrics and Gynecology 2017;129(6):1046‐53. [DOI] [PubMed] [Google Scholar]
Schreyer 1989 {published data only}
- Schreyer P, Sherman DJ, Ariely S, Herman A, Caspi E. Ripening the highly unfavorable cervix with extra‐amniotic saline instillation or vaginal prostaglandin E2 application. Obstetrics & Gynecology 1989;73:938‐42. [DOI] [PubMed] [Google Scholar]
Sciscione 2001 {published data only}
- Manley J, Nguyen L, Shlossman P, Colmorgen G, Sciscione A. A randomized prospective comparison of the intracervical Foley bulb to intravaginal misoprostol (cytotec) for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1999;180:S76. [DOI] [PubMed] [Google Scholar]
- Sciscione AC, Muench M, Pollock M, Jenkins TM, Tildon‐Burton J, Colmorgen GH. Transcervical foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstetrics & Gynecology 2001;98:751‐6. [DOI] [PubMed] [Google Scholar]
- Sciscione AC, Nguyen L, Manley J, Pollock M, Maas B, Colmorgen G. A randomized comparison of transcervical Foley catheter to intravaginal Misoprostol for preinduction cervical ripening. Obstetrics & Gynecology 2001;97(4):603‐7. [DOI] [PubMed] [Google Scholar]
- Sciscione AC, Nguyen L, Manley JS, Shlossman PA, Colmorgen GH. Uterine rupture during preinduction cervical ripening with misoprostol in a patient with a previous Caesarean delivery. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38:96‐7. [PubMed] [Google Scholar]
Sharma 2015a {published data only}
- Sharma K, Grubbs B, Mullin P, Opper N, Lee R. Labor induction utilizing the Foley balloon: a randomized trial comparing delayed verus immediate removal. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S326. [Google Scholar]
- Sharma KJ, Grubbs BH, Mullin PM, Opper N, Lee RH. Labor induction utilizing the foley balloon: a randomized trial comparing standard placement versus immediate removal. Journal of Perinatology 2015;35(6):390‐5. [DOI] [PubMed] [Google Scholar]
Sharma 2017 {published data only}
- Sharma C, Soni A, Gupta A, Verma A, Verma S. Mifepristone vs balloon catheter for labor induction in previous cesarean: a randomized controlled trial. Archives of Gynecology and Obstetrics 2017;296(2):241‐8. [DOI] [PubMed] [Google Scholar]
- Sharma C, Soni A, Thakur S, Verma S. Induction of labour in women with previous one caesarean section; mifepristone versus transcervical Folley's catheter. A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):303. [Google Scholar]
Sherman 2001 {published data only}
- Sherman DJ, Frenkel E, Pansky M, Caspi E, Bukovsky I, Langer R. Balloon cervical ripening with extra‐amniotic infusion of saline or prostaglandin E2: a double blind, randomized controlled study. Obstetrics & Gynecology 2001;97(3):375‐80. [DOI] [PubMed] [Google Scholar]
Siddiqui 2013 {published data only}
- Siddiqui DS, NCT02044458. A randomized control trial of foley catheter placement for induction of labor: stylette versus no stylette. clinicaltrials.gov/ct2/show/record/NCT02044458 (first received 9 July 2013).
Suri 2000 {published data only}
- Suri V, Dalui R, Gupta I, Ray P. Preinduction cervical ripening: a comparison of extraamniotic Foley catheter balloon and intracervical prostaglandin E2 gel. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA. Washington DC, 2000; Vol. 4:69.
Thigpen 2004 {published data only}
- Thigpen B, Bofill J, Bufkin L, Woodring T, Moore L, Morrison J. A randomized controlled trial comparing vaginal misoprostol to cervical foley plus oral misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S18. [Google Scholar]
Thomas 1986 {published data only}
- Thomas IL, Chenoweth JN, Tronc GN, Johnson IR. Preparation for induction of labour of the unfavourable cervix with Foley catheter compared with vaginal prostaglandin. Australian and New Zealand Journal of Obstetrics and Gynaecology 1986;26:30‐5. [DOI] [PubMed] [Google Scholar]
Torbenson 2015 {published data only}
- Torbenson V, NCT02546193. Outpatient foley catheter compared to usual inpatient care for cervical ripening: a randomized controlled trial. clinicaltrials.gov/show/NCT02546193 (first received 10 September 2015).
Ugwu 2013 {published data only}
- Ugwu EO, Onah HE, Obi SN, Dim CC, Okezie OA, Chigbu CO, et al. Effect of the Foley catheter and synchronous low dose misoprostol administration on cervical ripening: a randomised controlled trial. Journal of Obstetrics and Gynaecology 2013;33(6):572‐7. [DOI] [PubMed] [Google Scholar]
Vengalil 1998 {published data only}
- Vengalil SR, Guinn DA, Olabi NF, Burd LI, Owen J. A randomized trial of misoprostol and extra‐amniotic saline infusion for cervical ripening and labor induction. Obstetrics & Gynecology 1998;91:774‐9. [DOI] [PubMed] [Google Scholar]
Walfisch 2014 {published data only}
- Walfisch A. Management of labor in patients with previous cesarian section and premature rupture of membranes who desire TOLAC: comparison between the use of standard expectant management and the double‐balloon catheter device. a prospective randomized study. clinicaltrials.gov/ct2/show/NCT02196103 (first received 21 April 2014).
Walfisch 2015 {published data only}
- Anabusi S, Mei‐Dan E, Hallak M, Walfisch A. Mechanical labor induction in the obese population: a secondary analysis of a prospective randomized trial. Archives of Gynecology and Obstetrics 2016;293(1):75‐80. [DOI] [PubMed] [Google Scholar]
- Walfisch A, Mei‐Dan E, Hallak M. Trans‐cervical double balloon catheter with and without extra‐amniotic saline infusion for cervical ripening: A prospective quasi‐randomized trial. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(7):848‐53. [DOI] [PubMed] [Google Scholar]
Welt 1987 {published data only}
- Welt SI. Comparison of mechanical and pharmacologic means for induction of labor [personal communication]. Letter to: Oxford Database of Perinatal Trials 1987.
Wickramasinghe 2014 {published data only}
- Wickramasinghe W, SLCTR/2014/006. Effectiveness and safety in keeping the intra uterine Foley catheter for 24 hours versus 48 hours for induction of labour: a randomized controlled trial. http://slctr.lk/trials/209 (first received 25 March 2014).
Wilkinson 2015 {published data only}
- Wilkinson C, ACTRN12612001184864. A pilot randomised controlled trial of outpatient balloon catheter priming for induction of labour. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001184864 (first received 8 November 2012).
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy and Childbirth 2015;15(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaddehige 2015 {published data only}
- Yaddehige SS, Kalansooriya HD, Rameez MF. Comparison of cervical massage with membrane sweeping for pre‐induction cervical ripening at term ‐ A randomized control trial. Sri Lanka Journal of Obstetrics and Gynaecology 2015;37(Suppl 1):5‐6, Abstract no: OP 10. [Google Scholar]
Yazdani 2011 {published data only}
- Yazdani S, IRCT201012071760N10. Efficacy of prostaglandine e2 and intra‐cervical foley balloon in labor induction. http://en.irct.ir/trial/1274 (first received 2 February 2011).
Zakaria 2017 {published data only}
- Zakaria RB, ISRCTN21224268. A randomized trial of labour induction using the Foley catheter of different bores (French sizes 16, 22 and 28: 1 French size equals 0.33 mm). isrctn.com/ISRCTN21224268 (first received 29 October 2017).
Zhang 2014 {published data only}
- Zhang L, NCT02202083. The comparison of oxytocin induced labor and cook balloon induced labor. clinicaltrials.gov/show/NCT02202083 (first received 28 July 2014).
Zimmer 1996 {published data only}
- Zimmer EZ, Jakobi P, Weissman A. The effect of ripening the cervix with PGE2 or trancervical catheter on breathing and body movements. Journal of Maternal‐Fetal Investigation 1996;6:104‐6. [Google Scholar]
References to studies awaiting assessment
ACTRN12618000510246 2018 {published data only}
- ACTRN12618000510246. Amongst women undergoing induction of labour using a balloon catheter, is leaving the balloon in for 6 hours, compared to 12 hours, associated with similar changes in the cervix to prepare for labour, similar clinical outcomes, and a similar healthcare experience?. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000510246. (2 April 2018) 2018.
Agboghoroma 2015 {published data only}
- Agboghoroma CO, Ngonadi N. A randomized controlled study comparing prostaglandin e2 vaginal suppository with intra‐cervical foleys catheter balloon for preinduction cervical ripening at term. West African Journal of Medicine 2015; Vol. 34, issue 2:77‐82. [PubMed]
Amorosa 2017a {published data only}
- Amorosa JM, Stone J, Factor SH, Booker W, Newland M, Bianco A. A randomized trial of foley bulb for labor induction in premature rupture of membranes in nulliparas (flip). American Journal of Obstetrics and Gynecology 2017;217(3):360. [DOI] [PubMed] [Google Scholar]
Bauer 2018 {published data only}
- Bauer AM, Lappen JR, Gecsi KS, Hackney DN. Cervical ripening balloon with and without oxytocin in multiparas: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2018;219(3):294.e1‐294.e6. [DOI] [PubMed] [Google Scholar]
Chai 2018 {published data only}
- Chai Y. Application effect of single balloon catheters in labor induction of pregnant women in late‐term pregnancy and their influences on stress and inflammatory responses. Experimental and Therapeutic Medicine 2018;15(3):2968‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherian 2018 {published data only}
- Cherian AG, CTRI/2018/10/016154. A randomized controlled trial comparing a 30‐ml Foley catheter balloon without weight and a 30‐ml Foley catheter balloon with 500gm weight [500ml of 5% DEXTROSE ] for preinduction cervical ripening for women with past dates requiring Induction of labour. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=28074. (first received 25 October 2018) 2018.
CTRI/2018/01/011574 {published data only}
- CTRI/2018/01/011574. Comparative evaluation of intravaginal slow release dinoprostone insert vs transcervical foleys catheter for induction of labour, in patients with poor bishops score ‐ a randomized control study. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=21188 (first received 25 January 2018).
DeCesare 2018 {published data only}
- DeCesare A, Decesare J, Manek K. Transcervical balloon catheter for cervical ripening: weighted traction or tension. Obstetrics and Gynecology 2018;131:47S. [Google Scholar]
de Vaan 2019 {published data only}
- Vaan M, Blel D, Bloemenkamp K, Heus R, Willem de Leeuw J, Oudijk M, et al. 30: does mechanical induction of labor increase the risk of preterm birth in a subsequent pregnancy?. American Journal of Obstetrics and Gynecology 2019;220(1):S24. [Google Scholar]
Diguisto 2017 {published data only}
- Diguisto C, Gouge A, Giraudeau B, Perrotin F. Mechanical cervicAl ripeninG for women with PrOlongedPregnancies (MAGPOP): protocol for a randomised controlled trial of a silicone double balloon catheter versus the Propess system for the slow release of dinoprostone for cervical ripening of prolonged pregnancies. BMJ Open 2017;7(9):e016069. [DOI] [PMC free article] [PubMed] [Google Scholar]
EUCTR2017‐001914‐27‐GB 2018 {published data only}
- EUCTR2017‐001914‐27‐GB. Prostaglandin insert (Propess) versus tran‐scervical balloon catheter for out‐patient labour induction: A randomised controlled trial of feasibility (PROBIT‐F) ‐ Trans‐cervical balloon catheter and prostaglandin for labour induction. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2017‐001914‐27 (14 May 2018). [DOI] [PMC free article] [PubMed]
IRCT20170326033142N2 2018 {published data only}
- IRCT20170326033142N2. Comparison of vaginal misoprostol with Foley catheter for cervical ripening and labor induction. https://en.irct.ir/trial/25642 (28 July 2018). [PMC free article] [PubMed]
IRCT20170513033941N39 2018 {published data only}
- IRCT20170513033941N39. Comparison of intravaginal misoprostol, seaweed Laminaria and Foley catheter for cervical ripening and induction of labor in term pregnant women. https://en.irct.ir/trial/33983 (21 October 2018).
IRCT20181123041731N1 2019 {published data only}
- IRCT20181123041731N1. Investigation of the effect of misoprostol alone in comparison with misoprostol with Foley catheter on cervical ripening for labor induction in women with preterm premature rupture of the membrane. https://en.irct.ir/trial/35515. IRCT20181123041731N1 (27 January 2019).
Khatib 2019 {published data only}
- Khatib N, Dabaja H, Lauterbach R, Beloosesky R, Ginsberg Y, Weiner Z, et al. 790: outcomes following medical induction compared to mechanical induction of labor in obese pregnant women. American Journal of Obstetrics and Gynecology 2019;220(1):S516. [Google Scholar]
Leigh 2018 {published data only}
- Leigh S, Granby P, Haycox A, Mundle S, Bracken H, Khedikar V, et al. Foley catheter vs. Oral misoprostol to induce labour among hypertensive women in india: a cost‐consequence analysis alongside a clinical trial. BJOG : an International Journal of Obstetrics and Gynaecology 2018;125(13):1734‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2018 {published data only}
- Lim SE, Tan TL, Ng GY, Tagore S, Kyaw EE, Yeo GS. Patient satisfaction with the cervical ripening balloon as a method for induction of labour: a randomised controlled trial. Singapore Medical Journal 2018;59(8):419‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallah 2011 {published data only}
- Mallah F, IRCT201012225448N1. Efficacy and side effects of transcervical catheter and vaginal misoprostol on cervical ripening. http://en.irct.ir/trial/5860 (first received 4 May 2011).
McGee 2018 {published data only}
Mohamad 2018 {published data only}
- Mohamad A, Ismail NA, Rahman RA, Kalok AH, Ahmad S. A comparison between in‐patient and out‐patient balloon catheter cervical ripening: A prospective randomised controlled trial in PPUKM. Medical Journal of Malaysia 2018;73:22. [Google Scholar]
NCT03172858 2017 {published data only}
- NCT03172858. A randomized trial of intracervical balloon placement versus intravenous oxytocin in women with premature rupture of membranes and unripe cervices. https://clinicaltrials.gov/ct2/show/NCT03172858 (1 June 2017).
NCT03399266 2018 {published data only}
- NCT03399266. Mechanical induction of labor in women with previous cesarean section and premature rupture of membranes who desire TOLAC: a prospective randomized study. https://clinicaltrials.gov/ct2/show/NCT03399266 (16 January 2018).
NCT03435458 2018 {published data only}
- NCT03435458. Balloon to induce labor in generous women. https://clinicaltrials.gov/ct2/show/NCT03435458 (16 February 2018).
NCT03588585 2018 {published data only}
- NCT03588585. A prospective, randomized comparison of tension versus no tension with foley transcervical catheters for pre‐induction cervical ripening. https://clinicaltrials.gov/ct2/show/NCT03588585 (17 July 2018).
NCT03629548 {published data only}
- NCT03629548. Comparing combined foley catheter balloon and pge2 vaginal ovule with early amniotomy and pge2 for induction of labor at term: a randomized study. https://clinicaltrials.gov/ct2/show/NCT03629548 (14 August 2018).
NCT03629548 2018 {published data only}
- NCT03629548. Comparing foley catheter balloon with early amniotomy for induction of labor at term. Https://clinicaltrials.gov/show/nct03629548 (14 August 2018).
NCT03670836 2018 {published data only}
- NCT03670836. Comparison of misoprostol ripening efficacy with Dilapan. Https://clinicaltrials.gov/show/nct03670836 (14 September 2018).
NCT03682718 2018 {published data only}
- NCT03682718. Vaginal misoprostol with intracervical foley catheter in induction of labor. Https://clinicaltrials.gov/show/nct03682718 (25 September 2018).
NCT03744078 2018 {published data only}
- NCT03744078. A randomized trial of foley bulb and pge2 for labor induction in premature rupture of membranes. https://clinicaltrials.gov/ct2/show/NCT03744078 (16 November 2018).
NCT03752073 2018 {published data only}
- NCT03752073. Comparison of two mechanical methods of outpatient ripening of the cervix. https://clinicaltrials.gov/ct2/show/NCT03752073 (22 November 2018).
NCT03866772 2019 {published data only}
- NCT03866772. Labor induction with double balloon device, oral misoprostol and concomitant use of both. multicenter randomized controlled trial‐ idom trial. https://clinicaltrials.gov/ct2/show/NCT03866772 (7 March 2019).
Oskei 2018 {published data only}
- Oskei AD, Bayat F, Haji ZM, Kolifarhood G. Individual and combined administration of intravaginal misoprostol and transcervical foley catheter in cervical ripening in nulliparous women. Iranian Journal of Obstetrics, Gynecology and Infertility 2018;21(2):16‐22. [Google Scholar]
Osoti 2018 {published data only}
- Osoti A, Kibii DK, Tong TM, Maranga I. Effect of extra‐amniotic Foley's catheter and vaginal misoprostol versus vaginal misoprostol alone on cervical ripening and induction of labor in Kenya, a randomized controlled trial. BMC Pregnancy and Childbirth 2018;18(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saad 2019 {published data only}
- Saad A, Villareal J, Eid J, Spencer N, Ellis V, Hankins GD, et al. 21: a randomized controlled trial of pre‐induction cervical ripening comparing dilapan‐s versus foley balloon (dilafol trial). American Journal of Obstetrics and Gynecology 2019; Vol. 220, issue 1. [DOI] [PubMed]
- Saad AF, Villarreal J, Eid J, Spencer N, Ellis V, Hankins GD, et al. A randomized controlled trial of dilapan‐s vs foley balloon for preinduction cervical ripening (dilafol trial). American Journal of Obstetrics and Gynecology 2019; Vol. 220, issue 3:275.e1‐9. [DOI] [PubMed]
Sanmugam 2018 {published data only}
- Sanmugam S, ISRCTN16957529. Comparing two methods of stimulating the cervix (neck of the womb) to become ready for childbirth in women who have had one previous Caesarean and are at term in their pregnancy. http://www.isrctn.com/ISRCTN16957529. ISRCTN16957529 (14 November 2018) 2018.
Souizi 2018 {published data only}
- Souizi B, Mortazavi F, Haeri S, Borzoee F. Comparison of vaginal misoprostol, laminaria, and isosorbide dinitrate on cervical preparation and labor duration of term parturient: a randomized double‐blind clinical trial. Electronic Physician 2018;10(5):6756‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
ten Eikelder 2017 {published data only}
- Eikelder ML, Meent MM, Mast K, Rengerink KO, Jozwiak M, Graaf IM, et al. Women's experiences with and preference for induction of labor with oral misoprostol or foley catheter at term. American Journal of Perinatology 2017;34(2):138‐46. [DOI] [PubMed] [Google Scholar]
Tulek 2018 {published data only}
- Tulek F, Gemici A, Soylemez F. Double balloon catheters: a promising tool for induction of labor in multiparous women with unfavourable cervices. Journal of the Turkish German Gynecological Association 2018 [epub ahead of print]. [DOI] [PMC free article] [PubMed]
Viteri 2019 {published data only}
- Viteri OA, Tabsh KK, Lopez J, Fok R, Salazar XC, Alrais MA, et al. 22: transcervical ballon+vaginal misoprostol versus misoprostol for cervical ripening in nulliparous‐obese women: a multicenter randomized trial. American Journal of Obstetrics and Gynecology 2019;220(1):S19‐S20. [Google Scholar]
References to ongoing studies
Argilagos 2016 {published data only}
- Argilagos AV, NCT02762942. Prospective randomized clinical trial comparing the effect of vaginal misoprostol synchronously with supracervical balloon versus vaginal misoprostol alone for induction of labor. clinicaltrials.gov/ct2/show/NCT02762942 (first received 5 May 2016).
Beckmann 2013 {published data only}
- Beckmann M, ACTRN12614000039684. Prostaglandin inpatient induction of labour compared with balloon outpatient induction of labour: a randomised controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000039684 (first received 9 December 2013).
Bekele 2017 {published data only}
- Bekele D, PACTR201709002509200. A randomized controlled trial of sequential versus simultaneous use of foley balloon and oxytocin for induction of labor in nulliparous pregnant women. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201709002509200 (first received 9 August 2017).
Berndl 2016 {published data only}
- Berndl A, NCT02993432. High volume foleys increasing vaginal birth (high five birth) pilot trial. clinicaltrials.gov/ct2/show/record/NCT02993432 (first received 5 December 2016).
Bhide 2017 {published data only}
- Bhide A, NCT03199820. Prostaglandin insert (propess) versus trans‐cervical balloon catheter for out‐patient labour induction: a randomised controlled trial of feasibility. clinicaltrials.gov/ct2/show/record/NCT03199820 (first received 27 June 2017).
Eser 2016 {published data only}
- Eser A, NCT02861079. Compare prostaglandin e2 against to combined transcervical foley catheter balloon and vaginal prostaglandin e2 for induction of labor at term: a randomized study. clinicaltrials.gov/ct2/show/record/NCT02861079 (first received 1 August 2016).
Goli 2017 {published data only}
- Goli G, IRCT2017052710340N13. Comparison the results of induction of vaginal misoprostol with Foley catheter in prolonged pregnancy with unripe cervix. http://en.irct.ir/trial/10863 (first received 26 June 2017).
Goonewardene 2016 {published data only}
Gupta 2016 {published data only}
- Gupta J, NCT03001661. A randomised controlled trial of a synthetic osmotic cervical dilator for induction of labour in comparison to dinoprostone vaginal insErt: the SOLVE Trial. clinicaltrials.gov/ct2/show/record/NCT03001661 (first received 11 November 2016).
Hassanzadeh 2017 {published data only}
- Hassanzadeh E, IRCT2017010731725N1. Misoprostol versus foley catheter for cervical ripening in women with preeclampsia or gestational hypertension. http://en.irct.ir/trial/24897http://en.irct.ir/trial/24897 (first received 20 February 2017). [DOI] [PMC free article] [PubMed]
Igwe 2017 {published data only}
- Igwe M, NCT02574338. Cervical ripening: a comparison between intravaginal misoprostol tablet and intracervical foley's catheter in a low resource setting. clinicaltrials.gov/ct2/show/record/NCT02574338 (first received 20 February 2017).
Lacarin 2017 {published data only}
- Lacarin P, NCT03310333. Comparison between two strategies of induction in case of unfavourable cervix after 12 hours of premature rupture of membranes (prom) at term: cook cervical ripening + oxytocine from 6 hours versus dinoprostone vaginal insert. clinicaltrials.gov/show/NCT03310333 (first received16 October 2017).
Lauterbach 2017 {published data only}
- Lauterbach R, NCT03033264. A comparison between labor induction with dinoprostone and a cervical ripening balloon in women with a BMI>30 as oppose with a BMI<30. clinicaltrials.gov/ct2/show/record/NCT03033264 (first received 26 January 2017).
Levy 2016 {published data only}
- Levy R, NCT02815865. A randomized controlled study comparing cervical foley catheter, vaginal dinoprostone and a combination of the two methods for induction of labor. clinicaltrials.gov/ct2/show/record/NCT02815865 (first received26 February 2016).
Osoti 2016 {published data only}
- Osoti A, PACTR201604001535825. A combination of foley balloon and misoprostol versus misoprostol alone for induction of labour at Kenyatta national hospital, a randomized controlled trial. http://www.pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201604001535825 (first received 14 March 2016).
Park 2012 {published data only}
- Park KH, NCT01596296. Foley catheter versus dinoprostone vaginal insert for induction of labor in parous women at term: a randomized trial. clinicaltrials.gov/ct2/show/record/NCT01596296 (first received 9 May 2012).
Perrotin 2016 {published data only}
- Perrotin F, NCT02907060. Propess® versus double balloon for cervical ripening of prolonged pregnancies: a randomised controlled trial. clinicaltrials.gov/ct2/show/record/NCT02907060 (first received 6 September 2016).
Tagore 2015 {published data only}
- Tagore S, NCT02620215. Cervical ripening balloon in induction of labour at term (crbii) ‐ a prospective randomized controlled trial. clinicaltrials.gov/show/NCT02620215 (first received 2 December 2015).
Viteri 2015 {published data only}
- Viteri OA, NCT02639429. The efficacy of transcervical foley balloon plus vaginal misoprostol versus vaginal misoprostol alone for cervical ripening in nulliparous obese women: a randomized, comparative effectiveness trial. clinicaltrials.gov/show/NCT02639429 (first received 15 December 2015).
Wise 2016 {published data only}
- Wise M, ACTRN12616000739415. Comparison of low‐risk pregnant women undergoing induction of labour at term by outpatient balloon or inpatient prostaglandin in order to assess vaginal birth rate; a randomised controlled trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000739415 (first received 15 March 2016).
Yildirim 2017 {published data only}
- Yildirim GY/ NCT03016442. Dinoprostone vaginal insert versus double balloon catheter for preinduction cervical ripening. clinicaltrials.gov/ct2/show/record/NCT03016442 (first received 10 January 2017).
Additional references
Abramovici 1994
- Abramovici H, Hallak M, Zarfati D, Packer T, Calderon I, Auslender R, et al. Induction of labor in patients with unfavorable cervices: a randomized comparison among intravaginal prostaglandin E2 (PGE2), intravenous oxytocin, and the double‐balloon ripener device. International Journal of Gynecology & Obstetrics 1994;46:7. [Google Scholar]
Alferivic 2009
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2014
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001338.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2016
- Alfirevic Z, Keeney E, Dowswell T, Welton NJ, Medley N, Dias S, et al. Which method is best for the induction of labour? A systematic review, network meta‐analysis and cost‐effectiveness analysis. Health Technology Assessment 2016;20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2016
- Chen W, Xue J, Peprah MK, Wen SW, Walker M, Gao Y, et al. A systematic review and network meta‐analysis comparing the use of Foley catheters, misoprostol, and dinoprostone for cervical ripening in the induction of labour. BJOG: an international journal of obstetrics and gynaecology 2016;123(3):346‐54. [DOI] [PubMed] [Google Scholar]
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Du 2017
- Du YM, Zhu LY, Cui LN, Jin BH, Ou JL. Double‐balloon catheter versus prostaglandin E2 for cervical ripening and labour induction: a systematic review and meta‐analysis of randomised controlled trials. BJOG: an international journal of obstetrics and gynaecology 2017;124:891‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, Dowswell T. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD000941] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howarth 2001
- Howarth G, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krammer 1995b
- Krammer J, O'Brien WF. Mechanical methods of cervical ripening. Clinical Obstetrics and Gynecology 1995;38(3):280‐6. [DOI] [PubMed] [Google Scholar]
Liu 2018
- Liu YR, Pu CX, Wang XY, Wang XY. Double‑balloon catheter versus dinoprostone insert for labour induction: a meta‑analysis. Archives of Gynecology and Obstetrics 2018;299:7‐12. [DOI] [PubMed] [Google Scholar]
McMaster 2015
- McMaster K, Sanchez‐Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection: a systematic review and meta‐analysis. Obstetrics and Gynecology 2015;126(3):539‐51. [DOI] [PubMed] [Google Scholar]
NHS 2017
- NHS Digital. NHS Maternity Statistics 2016‐2017. https://files.digital.nhs.uk/pdf/l/1/hosp‐epis‐stat‐mat‐repo‐2016‐17.pdf.
NICE 2008
- NICE. Induction of Labour. Clinical Guideline CG70. https://www.nice.org.uk/guidance/CG70.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ten Eikelder 2016
- Eikelder ML, Mast K, Velden A, Bloemenkamp KW, Mol BW. Induction of labor using a Foley catheter or misoprostol: a systematic review and meta‐analysis. Obstetrical and Gynecological Survey 2016;71(10):620‐30. [DOI] [PubMed] [Google Scholar]
Thiery 1989
- Thiery M, Baines CJ, Keirse MJ. The development of methods for inducing labour. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:971. [Google Scholar]
Thomas 2014
- Thomas J, Fairclough A, Kavanagh J, Kelly AJ. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003101.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016
- Wang H, Hong S, Liu Y, Duan Y, Yin H. Controlled‐release dinoprostone insert versusFoley catheter for labor induction: a meta‐analysis. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(14):2382‐8. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO recommendations for Induction of labour. http://apps.who.int/iris/bitstream/handle/10665/44531/9789241501156_eng.pdf?sequence=1 2011. [PubMed]
Zhu 2018
- Zhu L, Zhang C, Cao F, Liu Q, Gu X, Xu J, et al. Intracervical Foley catheter balloon versus dinoprostone insert for induction cervical ripening: a systematic review and meta‐analysis of randomized controlled trials. Medicine 2018;97(48):e13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Boulvain 2001
- Boulvain M, Kelly AJ, Lohse C, Stan CM, Irion O. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001233] [DOI] [PubMed] [Google Scholar]
Jozwiak 2012
- Jozwiak M, Bloemenkamp KW, Kelly AJ, Mol BW, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD001233.pub2] [DOI] [PubMed] [Google Scholar]
Keirse 1995
- Keirse MJNC. Mechanical methods for cervical ripening. [revised 03 April 1992] In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software:Update Software; 1995.


