Abstract
Background
Malaria diagnostics by rapid diagnostic test (RDT) relies primarily on the qualitative detection of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and Plasmodium spp lactate dehydrogenase (pLDH). As novel RDTs with increased sensitivity are being developed and implemented as point of care diagnostics, highly sensitive laboratory-based assays are needed for evaluating RDT performance. Here, a quantitative suspension array technology (qSAT) was developed, validated and applied for the simultaneous detection of PfHRP2 and pLDH in a variety of biological samples (whole blood, plasma and dried blood spots) from individuals living in different endemic countries.
Results
The qSAT was specific for the target antigens, with analytical ranges of 6.8 to 762.8 pg/ml for PfHRP2 and 78.1 to 17076.6 pg/ml for P. falciparum LDH (Pf-LDH). The assay detected Plasmodium vivax LDH (Pv-LDH) at a lower sensitivity than Pf-LDH (analytical range of 1093.20 to 187288.5 pg/ml). Both PfHRP2 and pLDH levels determined using the qSAT showed to positively correlate with parasite densities determined by quantitative PCR (Spearman r = 0.59 and 0.75, respectively) as well as microscopy (Spearman r = 0.40 and 0.75, respectively), suggesting the assay to be a good predictor of parasite density.
Conclusion
This immunoassay can be used as a reference test for the detection and quantification of PfHRP2 and pLDH, and could serve for external validation of RDT performance, to determine antigen persistence after parasite clearance, as well as a complementary tool to assess malaria burden in endemic settings.
Keywords: Malaria, Rapid diagnostic test, Histidine-rich protein 2, Parasite lactate dehydrogenase, Luminex, Quantitative suspension array technology
Background
The availability of field-deployable malaria rapid diagnostic tests (RDTs) in recent years has markedly facilitated access to malaria diagnostics. Since World Health Organization (WHO) recommendations in 2010 to test all suspected malaria cases [1]. RDTs have gained a crucial role in the management of clinical episodes, as well as for malaria surveillance. Malaria RDTs have supplanted conventional light microscopy in many endemic areas as standard practice, accounting in 2017 for 75% of all diagnostic tests done in sub-Saharan Africa, where most RDTs are distributed (66%) [2]. The vast majority of RDTs used worldwide are based on the detection of parasite bioproduct histidine-rich protein 2 (PfHRP2), expressed only in Plasmodium falciparum, and the parasite metabolic enzyme lactate dehydrogenase (pLDH), present in all human-infecting Plasmodium species.
PfHRP2 is a water-soluble glycoprotein produced by P. falciparum throughout its asexual lifecycle and early sexual stages; it is expressed on the surface of infected erythrocytes and released into the peripheral blood circulation during schizogony [3, 4]. Given the ability of mature P. falciparum parasites to sequester in vascular beds during the last half of their asexual life-cycle, where they are not accessible for microscopic diagnosis, it has been proposed that the quantitative detection of PfHRP2 can provide a more accurate measurement of parasite biomass and potentially assist in determining the prognosis of severe malaria [5–7]. During pregnancy, P. falciparum infections can remain undetectable in peripheral blood as the parasites sequester in the intervillous spaces of the placenta by specific adhesion to chondroitin sulfate A [8, 9]. In such scenario, PfHRP2-detecting RDTs have been shown to have higher sensitivity on peripheral blood compared to conventional light microscopy [10], although still lower than PCR [11].
RDTs detecting PfHRP2 only are the most widely used products [12], accounting for 66% of the 276 million RDTs sold worldwide in 2017 [2]. Nonetheless it has been suggested that PfHRP2-detecting RDTs have limited clinical specificity for diagnosis of current malaria infection in areas of high transmission [13] and following treatment [14, 15] due to the persistence of the protein in the blood circulation after parasite clearance. The time span of a positive test result following parasite clearance is mainly dependent on the duration and density of parasitaemia prior to treatment, with values ranging from 26 days in Ugandan children with parasitaemia less than 1000 parasites per microlitre (p/μl) up to 37 days for parasite density > 50,000 p/μl [16].
The parasite LDH is a metabolic enzyme required for survival and is produced by all five Plasmodium species infective to humans [17, 18]. In contrast to PfHRP2, pLDH does not persist in blood after clearance of malaria infections and is therefore a better marker of acute and current infection [19]. Upon treatment, pLDH clearance in blood has been shown to closely track with that of parasites, suggesting pLDH to be a suitable predictor for treatment failure [20]. However, sensitivity of RDTs based on this antigen is generally lower than that of PfHRP2-based RDTs [21].
Currently, enzyme-linked immunoabsorbent assay (ELISA) is the standard practice immunoassay for the detection and quantification of PfHRP2 and pLDH, and is used as an external validation tool for RDTs performance. ELISAs are however costly, time and sample consuming, and generally only allow for the detection of one analyte at the time. The recent release of a highly-sensitive RDT for PfHRP2 (Alere™ Malaria Ag P.f), with two to ten-fold higher sensitivity than other currently available RDTs [22, 23], as well as the work in progress to develop new generation pLDH-based RDTs, underpins the need for new highly-sensitive, laboratory-based, reference immunoassays than can provide lower limit of detection than classical ELISAs [24–27]. Highly sensitive quantitative assays should not only be a more suitable tool for validation of new-generation RDTs, but could also be used to better understand antigen kinetics, particularly that of PfHRP2, and to support malaria surveillance. In this work, a high-throughput quantitative suspension array approach based on the Luminex technology that allows for the simultaneous and highly sensitive detection and quantification of PfHRP2 and pLDH antigens in different biological samples (whole blood, plasma and dried blood spots) collected from individuals living in malaria-endemic regions is presented. This assay provides an additional tool to externally evaluate the performance of new generation antigen-detecting malaria RDTs, and can be used for research purposes to address biological questions such as PfHRP2 persistence and the relationship between antigen levels and disease severity.
Methods
Development and optimization of the bead suspension array
Biotinylation of detection monoclonal antibodies (mAbs)
Detection monoclonal mouse IgG α-PfHRP2 (MyBioSource, San Diego, CA, USA) and monoclonal mouse IgG α-PAN-pLDH (AccessBio, Somerset, NJ, USA) were biotinylated using the EZ-Link Sulfo-NHS-Biotin Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions with minor modifications (see Additional file 1: Text S1).
Coupling of mAbs to magnetic beads
Coupling of magnetic microspheres was performed similarly as described elsewhere [28]. Briefly, two MagPlex® microspheres (Luminex Corp., Austin, TX, USA) with different spectral signatures selected for the detection of PfHRP2 and PAN-pLDH were washed with distilled water and activated with Sulfo-NHS (N-hydroxysulfosuccinimide) and EDC (1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) (Pierce, Thermo Fisher Scientific Inc., Rockford, IL, USA), both at 50 mg/mL, in activation buffer (100 mM Monobasic Sodium Phosphate, pH = 6.2). Microspheres were washed with 50 mM MES potassium salt (4-morpholineethane sulfonic acid, Sigma Aldrich, St Louis, MO, USA) pH 5.0 to a 10,000 beads/µl concentration, and covalently coupled with capture antibodies against PfHRP2 (MyBiSource, San Diego, CA, USA) and PAN-pLDH (PA-12, AccessBio, Somerset, NJ, USA), both at a concentration of 25 µg/ml. Beads were incubated on a rotatory shaker overnight at 4 °C and protected from light. Microspheres were blocked with PBS-BN (PBS with 1% BSA and 0.05% sodium azide (Sigma, Tres Cantos, Madrid, Spain), and resuspended in PBS-BN (from now on named assay buffer) to be quantified on a Guava Personal Cell Analysis desktop cytometer (Guava, Hayward, CA, USA) to determine the percentage recovery after the coupling procedure. Coupling validation was performed by incubating 50 µl of each bead suspension (2000 beads/well) with 50 µl α-mouse IgG-Biotin (goat anti-Mouse IgG-Biotin, Sigma Aldrich, St Louis, MO, USA) at 1:1000 dilution in a 96-well flat bottom plate for 2 h in gentle agitation. The plate was washed by pelleting microspheres using a magnetic separator (EMDMillipore, Burlington, MA, USA) and resuspended with wash buffer (0.05% Tween 20/PBS). Beads were incubated with 100 µl streptavidin-phycoerythrin (Sigma Aldrich, St. Louis, MO, USA) diluted 1:1000 in assay buffer for 30-min with gentle agitation in the dark. Finally, the beads were washed and resuspended in assay buffer, and the plate was read using the Luminex xMAP® 100/200 analyser (Luminex Corp., Austin, TX, USA). A reading higher than 25,000 median fluorescence intensity (MFI) implied a successful coupling reaction. Coupled beads were stored multiplexed at a concentration of 1000 beads/µl/region at 4 °C and protected from light.
To optimize the coupling concentration of detection antibodies, a concentration range from 10 to 100 µg/ml of α-PfHRP2 and α-PAN-pLDH mAbs was conjugated to magnetic beads, and assayed against serially diluted recombinant PfHRP2 and pLDH and a selection of plasma samples from P. falciparum-positive individuals. The mAb concentration that provided the highest MFI values was selected as the optimal concentration.
PfHRP2 and pLDH reference materials
Recombinant PfHRP2 protein type A from FCQ79 P. falciparum strain expressed in Escherichia coli (890015, Microcoat GmbH, Germany) was selected as PfHRP2 reference material. Antigen concentration after reconstitution was determined by ELISA (Malaria Ag CELISA, CeLLabs, Australia). Purified recombinant P. falciparum and P. vivax pLDH proteins expressed in insect cells (3001, ReliaTech GmbH, Germany) were used as reference material. The pLDH concentrations were measured in a previous study using a commercially available ELISA (QUALISA Malaria kit, Qualpro Diagnostics, India) [21]. Reference materials were used to prepare the standard curves for the bead suspension array, starting at concentrations of 50 ng/ml for PfHRP2 type A and at 1000 ng/ml for P. falciparum and P. vivax pLDH. WHO International Standard for P. falciparum antigens was provided by the National Institute for Biological Standards and Control (Ridge, UK) (NIBSC code: 16/376). WHO International Standard for P. falciparum antigens was quantified, and the obtained antigen concentrations in pg/ml were used to calculate the number of antigen picograms corresponding to 1 International Unit (IU).
Optimization of assay standard curves
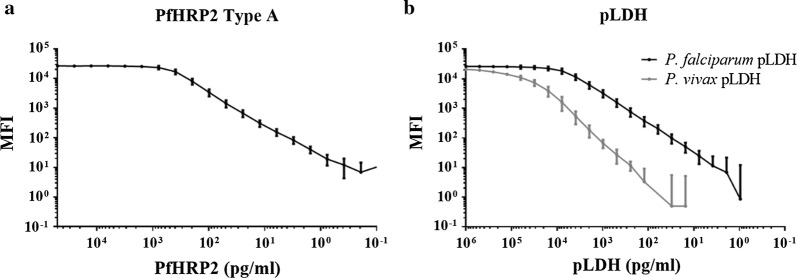
Standard curves were prepared for the detection of PfHRP2 and pLDH. The conjugated beads were incubated with serial dilutions of recombinant PfHRP2 types A, B and C and recombinant P. falciparum and P. vivax pLDH in assay buffer to produce standard curves ranging from 50,000 to 0.024 pg/ml for PfHRP2, and from 1000,000 to 0.48 for both P. falciparum pLDH and P. vivax pLDH (Fig. 1b) (for a more detailed assay procedure, see Additional file 1: Text S2).
Fig. 1.
Calibration curves to detect PfHRP2, Plasmodium falciparum pLDH and Plasmodium vivax pLDH. Recombinant P. falciparum HRP2 type A (a) and P. falciparum (b, back line) and P. vivax (B, grey line) pLDH were serially diluted to investigate the assay analytical range. Error bars show the standard deviation of the mean from 66 independent reads for PfHRP2 type A and P. falciparum pLDH, and 12 reads for P. vivax pLDH. X axis: MFI value after subtraction of the background
Assay parameters
Limit of detection, limits of quantification and range
A calibration curve prepared with serially diluted reference PfHRP2 and P. falciparum pLDH was assayed in 66 runs on the Luminex xMAP® 100/200 analyser, along with 2 blank samples (consisting of assay buffer alone) per run. For P. vivax pLDH, serial dilutions of reference antigen were assayed in 6 independent runs. The lower limits of detection (LLOD), defined as lowest amount of analyte that can be detected, and of quantification (LLOQ), defined as the lowest concentration of an analyte in a sample that can be quantified, were determined by measuring the MFI of 132 wells containing blank samples. The upper limit of quantification (ULOQ), corresponding to the highest concentration that can be quantitatively determined, was defined as the maximum value of the fitted mean standard curve minus its 10% to avoid quantifying samples falling close to the saturation plateau. The analytical range was set within the lower and the upper limits of quantification.
To quantify the LLOD and the LLOQ, 3 and 6 standard deviations (SD) were added to the mean MFI of blanks (n = 132), respectively. Each calibration or standard curve was fitted using a 5 parameters logistic (5PL) regression, and the mean curve was calculated. To present the LLOD and the LLOQ as concentration values, the calculated MFI values were interpolated to the mean calibration curve.
Dilution linearity and accuracy
Dilution linearity and accuracy were evaluated on the same serial dilutions of recombinant PfHRP2 type A and P. falciparum pLDH read over 66 independent runs. Dilution linearity was calculated as the mean per cent change in dilution-corrected concentration from one dilution to the previous one within the assay range. Dilution linearity was considered acceptable if the per cent change in concentration did not exceed the recovery range of 80–120% [29]. Accuracy was determined as the mean per cent deviation (% DEV) from the expected concentration, calculated by dividing the difference between the experimental value and the expected value and then multiplying by 100. Acceptable accuracy was defined as the %DEV not surpassing by 20% the expected concentration (by 25% for samples with concentrations falling at the LLOQ and ULOQ).
Precision
Intra-assay and inter-assay precision were evaluated by assaying cultured P. falciparum W2 strain spiked in assay buffer at five dilutions spanning a wide range of antigen concentration in triplicate over four runs. Intra-assay precision over the four runs was defined as the average coefficient of variation (% CV) of individual samples. The % CV for each sample was calculated by determining the SD of the three replicate results, dividing it by the mean of the triplicate results, and multiplying by 100. Inter-assay precision was defined as the overall % CV, calculated by dividing the SD of plate means by the mean of plate means and then multiplying by 100. Calculations were performed on non-transformed MFI values. Precision was considered acceptable when % CV did not exceed 10% for intra-assay variation and 20% for inter-assay variation [30].
Selectivity
To investigate the selectivity of the assay for the target antigens, 75 plasma samples from 25 Spanish pregnant women never exposed to malaria were assayed to demonstrate that the bead suspension array does not detect plasma components other than the target antigens (PfHRP2 and pLDH).
Study samples
To test against samples collected in endemic areas, different sample sets were assayed (characteristics of clinical samples used are summarized in Table 1).
Table 1.
Clinical samples tested on the qSAT assay
| Samples (n) | Type of sample | Origin | Sampling period | Population | Reference assay | P. falciparum positive (n) | GM parasite density (p/µl) (95% CI) | Others |
|---|---|---|---|---|---|---|---|---|
| 765 | Plasma | Mozambique | 2010–2012 | Pregnant women | qPCR on DBS | 59 | 127 (58.4–276.3) | |
| 103 | Serum | Colombia | 2005–2007 | Pregnant women | Microscopy | 32 | 3901 (2059–7391) | |
| 110 | EDTA whole blood | Senegal | NA | Adults | Nested PCR and qPCR on WBa | 55 | 4.9 (2.6–9.4) | |
| 16 | EDTA whole blood | Peru | April–Aug 2015 | Adults | Nested PCR and qPCR on WBa | 16 | 52.7 (19.1–146.0) | pfhrp2 deleted |
| 639 | EDTA whole blood | Nigeria | April–Aug 2017 | Age ≥ 5 years | Microscopy and PCRb | 639 | 4713 (3530–6292) | |
| 323 | EDTA whole blood | Peru | March–Oct 2017 | Age ≥ 5 years | Microscopy and PCRb | 323 | 1719 (1328–2225) | |
| 75 | Plasma | Spain | 2010 | Pregnant women | NA | NA | NA |
GM Geometric mean
aNested PCR was used for species determination and qPCR for parasite density quantification. Plasmodium falciparum samples were positive for both methods
bPCR was used for species determination and microscopy for parasite density quantification. Geometric mean parasite density is calculated on the basis of microscopy positive samples (n = 247/639 in Nigeria, and 191/323 in Peru)
Plasmodium falciparum culture samples and Plasmodium vivax clinical samples
W2, Benin I, Borneo and Santa Lucia P. falciparum strains were cultured under standard hypoxic conditions. Culture in exponential growth phase was harvested, infected red blood cells were spun down, aliquoted, and frozen at − 80 °C as previously described [21]. Plasmodium vivax isolates were collected from symptomatic adult volunteers with a P. vivax mono-species infection as confirmed by microscopy during a specimen collection campaign organized in April 2016 in the area of Iquitos (Peru).
Plasma and serum samples
PfHRP2 and pLDH were measured in 765 plasma samples collected at three time points during pregnancy from 255 pregnant women residing in Manhiça (Southern Mozambique) who participated in a clinical trial of intermittent preventive treatment during pregnancy (IPTp) from 2010 to 2012 [31, 32], and in 103 serum samples from 77 pregnant women in the Urabá-Antioquia region (Colombia) collected between 2005 and 2007 [33]. Additionally, 75 plasma samples collected at three time points from 25 pregnant women never exposed to malaria, who attended the Hospital Clínic of Barcelona during pregnancy and delivery in 2010, were included in the assay as negative controls. Plasma and serum samples were stored at − 80 °C. Infection status and parasite densities were previously determined by qPCR on dried blood spots (DBS) for samples from Mozambique [34], and by light microscopy (LM) in Colombian samples.
Whole blood samples
EDTA-anticoagulated whole blood samples were collected from consenting asymptomatic adults with no recent clinical episode of malaria (in previous 4 weeks) during cross-sectional surveys in Peru [35], and Senegal. Samples were assessed and categorized as P. falciparum mono-species infection or Plasmodium negative samples using nested PCR, and parasitaemia was quantified using quantitative PCR as described previously at the Hospital for Tropical Diseases (UK) [36]. The pfhrp2 gene status of P. falciparum PCR positive samples was investigated by PCR as previously described [36]. Whole blood samples from asymptomatic adults were used to prepare DBS (see Additional file 1: Text S4). EDTA-anticoagulated whole blood samples were collected between March and October 2017 in Peru Amazon region and Nigeria Lagos state from consenting symptomatic (with fever within the previous 3 days) and asymptomatic (no fever history in previous 3 days) patients enrolled during a clinical trial of a new multiplex fever diagnostic test. Antigens were quantified in those samples that were positive for P. falciparum by PCR (n = 323 in Peru and 629 in Nigeria). Individuals participating in this clinical trial had been previously tested by on-site microscopy (final result based on reading from 2 independent microscopists), and by SD BIOLINE Malaria Ag P.f (HRP2/pLDH) (05FK90, Abbott, Chicago, IL, USA) in Nigeria and by CareStart™ Malaria Pf/PAN (HRP2/pLDH) (RMRM-02571) and Carestart Pf/PAN (pLDH) Ag (RMLM-02571) (AccessBio, Somerset, NJ, USA) RDTs in Peru.
Statistical analysis
The relationship between the MFIs in singleplex and multiplex assays and the correlation between parasite densities and antigen levels were assessed by the non-parametric Spearman’s rank correlation method. Statistical analyses were performed with GraphPad Prism (version 6, Graphpad, Inc). The 95% confidence intervals (95% CI) for sensitivity and specificity were calculated by Wilson score method in Microsoft Excel (2013).
Results
Development of the bead suspension array for PfHRP2 and pLDH detection
Optimization of standard curves for the detection of PfHRP2 and pLDH
The coupling conditions were optimized based on a concentration range of 10 to 100 ug/mL of coupled HRP2 and pan-pLDH antibodies and testing with recombinant antigens as well as plasma samples from P. falciparum-infected pregnant women, showing slightly higher MFI values at 25 ug/mL. A range of in-house biotinylated detection mAbs was tested, and the optimal concentration was found to be 1 μg/ml for the detection of both antigens.
Slightly higher MFI values were obtained for PfHRP2 type A compared to types B and C (see Additional file 2: Fig. S1A), similarly to previously reported data [24, 25]. PfHRP2 type A was selected as reference material. Recombinant P. falciparum pLDH was detected down to lower concentrations compared to P. vivax pLDH, indicating higher assay sensitivity for the detection of recombinant P. falciparum pLDH (Fig. 1b). Similarly, the assay was able to detect lower concentrations of native P. falciparum pLDH compared to P. vivax pLDH (see Additional file 2: Fig. S1B). Additionally, the detection of PfHRP2 and pLDH in assay buffer spiked with recombinant proteins, cultured parasites or plasma samples yielded similar MFI values in singleplex and multiplex (see Additional file 2: Figure S1C), with a clear correlation for both PfHRP2 (n = 25, r = 0.995; p < 0.001) and pLDH (n = 31, r = 0.992; p < 0.001), indicating no cross-reactivity between PfHRP2 and pLDH components.
Correspondence to international units
In the qSAT assay presented here, 1 IU PfHRP2 corresponds to 23.5 pg PfHRP2, whereas 1 IU pLDH corresponds to 160 pg/ml pLDH.
Assay parameters
Limit of detection, limits of quantification and range
The lower limit of detection (LLOD) of the assay was determined to be 6.0, 56.1 and 1093.20 pg/ml for recombinant PfHRP2 type A, P. falciparum pLDH and P. vivax pLDH respectively; and the lower limit of the quantification (LLOQ) was estimated at 6.8 pg/ml for PfHRP2, 78.1 pg/ml for P. falciparum pLDH and 1343.5 pg/ml for P. vivax pLDH. The ULOQ was found to be 762.8 pg/ml, 17,076.6 pg/ml and 187,288.5 pg/ml for PfHRP2, P. falciparum pLDH and P. vivax pLDH, respectively. The limits of detection for PfHRP2 types B and C were 17.2 pg/ml and 15.8 pg/ml, respectively.
Dilution linearity and accuracy
The mean per cent change in dilution-corrected concentration between contiguous dilutions was 13.6 and 11.1% for PfHRP2 and P. falciparum pLDH, respectively, as determined over 66 independent runs. These data are within the acceptance criteria of ± 20% [29]. However, at concentrations close to the ULOQ, the per cent change showed an overestimation greater than 20% for both PfHRP2 and P. falciparum pLDH (Table 2). The overall per cent deviation between the experimental concentration and the expected concentration for each serial dilution point falling within or close to the analytical range was 19.6 and 16.4% for PfHRP2 and pLDH, respectively. At concentrations falling at the LLOQ and the ULOQ, accuracy decreased both for PfHRP2 and P. falciparum pLDH detection as shown in Table 2.
Table 2.
Dilution linearity and accuracy of qSAT assay
| Sample | Pf pLDH | PfHRP2 | ||||
|---|---|---|---|---|---|---|
| Expected concentration (pg/ml) | % change | % deviation | Expected concentration (pg/ml) | % change | % deviation | |
| 1 | 15,625 | 41.6 | 24.2 | 781.3 | 32.8 | 23.4 |
| 2 | 78,12.5 | 18.3 | 17.4 | 390.6 | 18.5 | 10.8 |
| 3 | 3906.3 | 0.9 | 17.1 | 195.3 | 6.8 | 10.2 |
| 4 | 1953.1 | 2 | 10.7 | 97.7 | 0 | 8.5 |
| 5 | 976.6 | 5.6 | 10.4 | 48.8 | 5.2 | 10.1 |
| 6 | 488.3 | 4.2 | 14.2 | 24.4 | 14.7 | 19 |
| 7 | 244.1 | 5.5 | 14.9 | 12.2 | 12.7 | 27.9 |
| 8 | 122.1 | 10.4 | 22.2 | 6.1 | 18.5 | 47 |
| Overall | – | 11.1 | 16.4 | – | 13.6 | 19.6 |
Precision
Intra-assay variation was 8.3 and 9.8% for PfHRP2 and pLDH, respectively. The inter-assay % CV was 8.4% for the detection of PfHRP2 and 11.2% for the detection of pLDH. For both antigens, intra-assay and inter-assay variation fell within the acceptance criteria of 10 and of 20% variation, respectively [30].
PfHRP2 and pLDH recovery from dried blood spots
To determine the loss of antigen when recovering PfHRP2 and pLDH from filter papers as compared to same volumes of whole blood samples, DBS were prepared with whole blood samples from Senegalese and Peruvian asymptomatic individuals (see Table 1). Blood was eluted from DBS in assay buffer (see Additional file 1: Text S4) and eluted samples were assayed on the bead-suspension array along with the original whole blood samples used to prepare the DBS. The geometric mean antigen concentration obtained from DBS-eluted product was 0.04 ng/ml (95% CI 0.03–0.07 ng/ml) for pfHRP2 and 0.10 ng/ml (95% CI 0.06–0.16 ng/ml) for pLDH. These concentrations are 22.8 (n = 38, 95% CI 15.6–33.5) and 59.7 (n = 18, 95% CI 35.4–100.6) times lower than the concentrations obtained in whole blood for PfHRP2 and pLDH, respectively (0.77 ng/ml (95% CI 0.37–1.61 ng/ml) for PfHRP2 and 5.77 ng/ml (95% CI 2.45–13.57 ng/ml) for pLDH), for identical blood volumes.
Assay selectivity for the target antigens
An important step in the development of the assay was to investigate whether it was selective for the target antigens. Significant MFI signal for PfHRP2 and pLDH was observed in P. falciparum-positive samples (PfHRP2: Mean = 10,195, SD = 12,545; pLDH: Mean = 9634, SD = 11,765), whereas positive P. vivax samples (n = 12) showed only fluorescence signal for pLDH (Mean = 12,960; SD = 3735), and not for PfHRP2 (Mean = 75.0, SD = 39.3) as expected (Fig. 2). Five out of 71 and 7 out of 738 negative samples by microscopy and PCR, respectively, showed MFI values above the LLOQ for both PfHRP2 and pLDH, and 4 other P. falciparum-positive samples by microscopy and 4 P. falciparum-positive samples by qPCR yielded greater MFI values than the LLOQ for pLDH and PfHRP2, respectively. In addition, 2 P. falciparum-positive samples with pfhrp2 deletion showed MFI values above the LLOQ. Finally, all plasma samples (n = 75) from Spanish malaria-naïve pregnant women yielded negligible fluorescence signals for both antigens (Fig. 2).
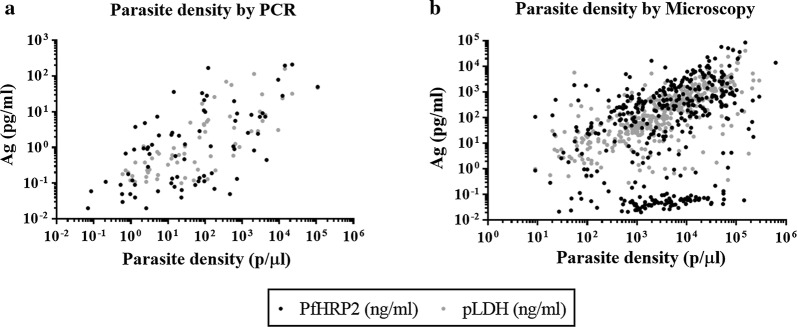
Fig. 2.

The quantitative bead suspension array is selective for PfHRP2 and pLDH. Median fluorescence intensity with blank subtracted for PfHRP2 (a) and pLDH (b) for P. falciparum-positive samples (n = 1098), P. falciparum with hrp2 gene deletions (n = 16), P. vivax-positive samples (n = 12), Plasmodium-negative samples, and samples from naïve individuals (n = 75). pfhrp2 -: Plasmodium falciparum with hrp2 gene deletion
Correlation between antigen levels and parasite densities
In samples positive for one or two antigens, the correlation between antigen concentrations and parasite densities was investigated. Overall, a significant correlation between PfHRP2 and parasite densities was found regardless of whether parasite densities were quantified by qPCR (Spearman r = 0.59; p < 0.0001) or microscopy (Spearman r = 0.40; p < 0.0001) (Fig. 3). pLDH levels showed a higher correlation with parasite densities compared to PfHRP2, both in samples for which densities were determined by qPCR (Spearman r = 0.75; p < 0.0001) and by microscopy (Spearman r = 0.75; p < 0.0001) (Fig. 3). The correlation between parasite densities and antigen levels differed across the different sample sets analysed (see Additional file 3: Table S1). Interestingly, the correlation between pLDH levels with parasite densities in whole blood samples from Peru (Spearman r = 0.76; p < 0.0001) and Nigeria (Spearman r = 0.78; p < 0.0001) was very similar, whereas for PfHRP2, a better correlation with parasite densities was found in samples from Nigeria (Spearman r = 0.47; p < 0.0001) compared to samples from Peru (Spearman r = 0.20; p = 0.0308).
Fig. 3.
Antigen levels correlate with parasite densities. Correlation of parasite densities (p/µl) with PfHRP2 and pLDH concentration (pg/ml) in P. falciparum positive samples by PCR (a), and by microscopy (b)
Discussion
In the present study, a quantitative suspension array, based on Luminex technology, for the simultaneous detection and quantification of P. falciparum HRP2 and P. falciparum and P. vivax pLDH is described. The qSAT allows the determination of protein concentrations as low as 6.0, 56.1 and 1042.7 pg/ml, respectively. Hence, the assay provides increased sensitivity compared to commercially available ELISA kits, which have LODs of approximately 400 pg/ml and 1000 pg/ml for PfHRP2 and pLDH, respectively [27, 37]. The assay shows good levels of dilution linearity, accuracy and precision, and can be used to effectively and rapidly quantify malaria antigens in large quantities of different biosamples.
The performance of the bead suspension array to quantify PfHRP2 and pLDH was evaluated using reference recombinant proteins as well as cultured parasites, and in different biofluids from malaria-exposed and malaria-naïve individuals. The assay is selective for the target antigens and has an analytical range of 6.8–762.8 and of 78.1–17,076.6 pg/ml for PfHRP2 and P. falciparum pLDH, respectively. Additionally, the assay can also quantify P. vivax pLDH down to 1211.6 pg/ml. The assay analytical sensitivity to detect PfHRP2 is comparable to that of a recently developed bead suspension assay based on Luminex technology [25], as well as to other immunoassays that use different technologies [20, 27]. This suggests that with the current technology available for the quantification of PfHRP2 using antibodies, the lowest limit of detection achievable is in the range of 0.5–10 pg/ml. The limit of detection for pLDH is more divergent across assays, ranging from approximately 10 pg/ml [27] up to 4000 pg/ml [25], but in all assays it is always higher than that for PfHRP2. This underpins the need to further improve the sensitivity of pLDH-based diagnostics.
The bead suspension array described here can successfully be used as for detection and quantification of PfHRP2 and pLDH in whole blood, eluted DBS and plasma or serum samples. The concentration of eluted PfHRP2 from DBS was equivalent to approximately a 1:20 dilution from whole blood, similarly to previously reported data [38]. Differently, for pLDH it was found that antigen concentration in eluted DBS corresponds to a 1:60 whole blood dilution, which differs from previously published data showing no differences in antigen recovery between PfHRP2 and pLDH [20]. However, such differences could be explained by the different extraction methodologies and storage conditions used.
The quantification of PfHRP2 and pDLH is performed by interpolating MFI values to a regression curve fitted from a calibration curve consisting of recombinant proteins PfHRP2 type A and P. falciparum pLDH. Particularly for PfHRP2, the use of a single recombinant protein as a reference material to quantify antigen levels in field samples may provide an approximate estimate of the true concentration. PfHRP2 contains sequences rich in histidine that form the epitopes targeted by the mAbs in RDTs [39], which have been shown to be highly polymorphic in sequence composition of the repeated motifs, as well as in overall length and number of repeated motifs between different parasite strains [39]. Baker et al. classified PfHRP2 as types A, B, or C depending on the frequency of two epitope repeats (named type 2 and type 7) which confer increased reactivity to mAbs in RDTs [39, 40]. According to this classification, PfHRP2 Type A comprises the higher number of repeat types 2 and 7, followed by PfHRP2 Type B, and finally PfHRP2 Type C. These results on the detection of different PfHRP2 types (see Additional file 2: Fig. S1A) align with these data and resemble recently published results [24, 25].
An overall positive, significant correlation between antigen levels and parasite densities similar to that found in previous studies was observed [24], although the correlation was different among the groups of samples analysed (see Additional file 3: Table S1), probably because of the type of sample used for antigen quantification, operational variations and sample storage. Of note, pLDH better correlated with parasite densities compared to PfHRP2. This finding can be explained by the fact that PfHRP2, differently from pLDH, is secreted to the blood stream and persists in circulation for several days. In addition, it was observed that the correlation between PfHRP2 and parasite densities was lower in samples from Peru compared to samples from Nigeria, whereas pLDH levels correlated very similarly to parasite densities in both groups of samples. The high number of suspected P. falciparum-positive samples with pfhrp2 gene deletions within the group of samples from Peru most probably explains this finding.
A potential limitation of the current assay is that it was not evaluated for possible cross-reactivity of anti-PfHRP2 mAbs with PfHRP3, a P. falciparum protein homologous to PfHRP2 [41], which is thought to contribute to the detection sensitivity of PfHRP2-based RDTs [42, 43]. Another limitation of PfHRP2-detecting immunoassays, including RDTs, is the recent global spread of P. falciparum populations lacking the pfhrp2 or pfhrp3 or both genes, which lead to PfHRP2-based RDT false-negative results [44, 45]. In this regard, the assay presented here could be used to estimate the prevalence of P. falciparum parasites with pfhrp2/3 deletions, although mixed infections with wild type and mutant parasites would still produce PfHRP2.
Conclusions
The quantitative suspension array technology presented here allows for a simultaneous, highly sensitive detection of the most commonly used target antigens in malaria RDTs. The assay could be used as a tool to validate next generation RDTs, as well as to estimate malaria burden in endemic areas and to evaluate the impact of malaria control activities. Finally, this assay has the potential to be further upgraded by multiplexing the detection and quantification of antibodies against parasite antigens that could serve as a supplementary tool to quantify malaria transmission intensity, as well as the detection of other infectious diseases antigens.
Supplementary information
Additional file 1: Text S1. Biotinylation of detection mAbs. Text S2. Bead suspension array procedure. Text S3. Singleplex versus Multiplex testing. Text S4. Preparation and extraction of proteins from dried blood spots.
Additional file 2: Figure S1. Assay optimization. (A) Serial dilutions of recombinant PfHRP2 types A, B and C were assayed to determine the lowest concentration at which each antigen is detected; (B) P. falciparum Benin I and Borneo and P. vivax field isolates were assayed in a serial dilution fashion to assess differences between the analytical sensitivity for P. falciparum and P. vivax pLDH; (C) PfHRP2 and pLDH positive samples (plasma, cultured field isolates and recombinant proteins) were assayed in singleplex (X axes) and multiplex (Y axes).
Additional file 3: Table S1. Correlation between antigen levels and parasite densities for each group of clinical samples analysed.
Acknowledgements
We thank the study participants; the staff of the Hospitals, clinical officers, field supervisors and data managers. We acknowledge the teams at CISM in Mozambique, at University of Antioquia in Colombia, at University Cheikh Anta Diop in Senegal, at Universidad Peruana Cayetano Heredia in Peru, and at University of Lagos in Nigeria, who conducted the recruitment of participants and sample processing. We would also like to thank Iveth González, Aida Valmaseda, Marta Vidal and Himanshu Gupta for providing important inputs for optimization of the quantitative bead suspension array; and Laura Puyol, Diana Barrios and Pau Cisteró for providing logistic support. The CISM is supported by the Government of Mozambique and the Spanish Agency for International Development (AECID). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.
Abbreviations
- α
anti
- Ag
antigen
- CI
confidence intervals
- DBS
dried blood spot
- EDC
1-ethyl-3-[3-dimethylaminopropyl] carbodimide hydrochloride
- ELISA
enzyme-linked immunoabsorbent assay
- GM
geometric mean
- IgG
immunoglobulin G
- LLOD
lower limit of detection
- LLOQ
lower limit of quantification
- LM
light microscopy
- mAbs
monoclonal antibodies
- MFI
median fluorescence intensity
- p/μl
parasites per microliter
- PCR
polymerase chain reaction
- pg/ml
picograms per millilitre
- PfHRP2
Plasmodium falciparum histidine-rich protein 2
- PfHRP3
Plasmodium falciparum histidine-rich protein 3
- pLDH
parasite lactate dehydrogenase
- qPCR
quantitative polymerase chain reaction
- qSAT
quantitative suspension array technology
- RDT
rapid diagnostic test
- RT
room temperature
- SD
standard deviation
- Sulfo-NHS
N-hydroxysulfosuccinimide
- ULOQ
upper limit of quantification
- µl
microliter
- 5PL
five parameters logistic
- %CV
percent coefficient of variation
- %DEV
percent deviation
Authors’ contributions
AM and AC conceived and designed the study. CM, EM, ES and RG obtained the plasma samples from Mozambican pregnant women. AV obtained the serum samples from Colombian pregnant women. XD and SD were in charge of studies that allowed collecting the whole blood samples from Senegalese and Peruvian asymptomatic patients, and from Nigerian and Peruvian febrile patients, respectively. AJ and XMV performed all laboratory experiments. AJ, AM and XMV performed the statistical analyses and manuscript preparation. AC, AM, IG and XD provided overall study supervision. All authors read and approved the final manuscript.
Funding
This research was supported by FIND using funds from the Australian Government; the European and Developing Countries Clinical Trials Partnership (EDCTP); the Malaria in Pregnancy (MiP) Consortium and the Department d’Universitats i Recerca de la Generalitat de Catalunya (AGAUR; 2017SGR664). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya (http://cerca.cat/en/suma/). This research is part of ISGlobal’s Program on the Molecular Mechanisms of Malaria, which is partially supported by the Fundación Ramón Areces.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The Mozambican National Health and Bioethics Committee, the Medical Research Center Ethics Committee at the Medicine Faculty of Universidad de Antioquia and the Hospital Clinic of Barcelona Ethics Committee approved the use of non-identifiable plasma and serum samples in the current study. Written informed consent was obtained from all participants. The Senegal National Ethics Committee (Comité National d’Ethique pour la Recherche en Santé) reviewed and approved on 15 January 2015 the study protocol associated with the collection of whole blood specimens from consenting asymptomatic adults in Senegal to support the development and evaluation of new assays for the detection of malaria infections (Protocol SEN14/74). Written informed consent was obtained from all participants. The Unversidad Peruana Cayetano Heredia Institutioanl Review Board (Comité Institucional de Ética) reviewed and approved on 10 March 2015 the study protocol associated with the collection of whole blood specimens from consenting asymptomatic adults in Peru to support the development and evaluation of new assays for the detection of malaria infections (Protocol 100-02-15). Written informed consent was obtained from all participants. The study protocol for the evaluation of a multiplex fever diagnostic test was submitted for ethics approval in October 2016 and December 2016 in Peru and Nigeria, respectively, and approvals were obtained in November 2016 in Peru, and January 2017 in Nigeria. Informed consent was obtained from all participants or by legal guardians in cases of underage participants. The institutional review board at the Universidad Peruana Cayetano Heredia (Lima, Peru) approved the study protocol the specimen collection campaign organized in April 2016 in the area of Iquitos (Peru).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-019-3083-5.
References
- 1.WHO . Guidelines for the treatment of malaria. 2. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 2.WHO . World malaria report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 3.Biswas S, Tomar D, Rao DN. Investigation of the kinetics of histidine-rich protein 2 and of the antibody responses to this antigen, in a group of malaria patients from India. Ann Trop Med Parasitol. 2005;99:553–562. doi: 10.1179/136485905X51463. [DOI] [PubMed] [Google Scholar]
- 4.Desakorn V, Silamut K, Angus B, Sahassananda D, Chotivanich K, Suntharasamai P, et al. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg. 1997;91:479–483. doi: 10.1016/S0035-9203(97)90292-3. [DOI] [PubMed] [Google Scholar]
- 5.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubach MP, Mukemba J, Florence S, John B, Crookston B, Lopansri BK, et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian Children. PLoS ONE. 2012;7:e35985. doi: 10.1371/journal.pone.0035985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox LL, Taylor TE, Pensulo P, Liomba A, Mpakiza A, Varela A, et al. Histidine-rich protein 2 plasma levels predict progression to cerebral malaria in Malawian children with Plasmodium falciparum infection. J Infect Dis. 2013;208:500–503. doi: 10.1093/infdis/jit176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 9.Uneke CJ. Diagnosis of Plasmodium falciparum malaria in pregnancy in sub-Saharan Africa: the challenges and public health implications. Parasitol Res. 2008;102:333–342. doi: 10.1007/s00436-007-0782-6. [DOI] [PubMed] [Google Scholar]
- 10.Kattenberg JH, Tahita CM, Versteeg IAJ, Tinto H, Traoré-Coulibaly M, Schallig HDFH, et al. Antigen persistence of rapid diagnostic tests in pregnant women in Nanoro, Burkina Faso, and the implications for the diagnosis of malaria in pregnancy. Trop Med Int Health. 2012;17:550–557. doi: 10.1111/j.1365-3156.2012.02975.x. [DOI] [PubMed] [Google Scholar]
- 11.Mayor A, Moro L, Aguilar R, Bardají A, Cisteró P, Serra-Casas E, et al. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis. 2012;54:1561–1568. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
- 12.WHO, Special Programme for Research and Training in Tropical Diseases, Foundation for Innovative New Diagnostics, Centers for Disease Control. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 4 (2012). World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases; 2012. p. 126.
- 13.Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, et al. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malar J. 2008;7:202. doi: 10.1186/1475-2875-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houzé S, Boly MD, Le Bras J, Deloron P, Faucher J-F. PfHRP2 and PfLDH antigen detection for monitoring the efficacy of artemisinin-based combination therapy (ACT) in the treatment of uncomplicated falciparum malaria. Malar J. 2009;8:211. doi: 10.1186/1475-2875-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayxay M, Pukrittayakamee S, Chotivanich K, Looareesuwan S, White NJ. Persistence of Plasmodium falciparum HRP-2 in successfully treated acute falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95:179–182. doi: 10.1016/S0035-9203(01)90156-7. [DOI] [PubMed] [Google Scholar]
- 16.Kyabayinze DJ, Tibenderana JK, Odong GW, Rwakimari JB, Counihan H. Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221. doi: 10.1186/1475-2875-7-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown WM, Yowell CA, Hoard A, Vander Jagt TA, Hunsaker LA, Deck LM, et al. Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of human malarial parasites. Biochemistry. 2004;43:6219–6229. doi: 10.1021/bi049892w. [DOI] [PubMed] [Google Scholar]
- 18.Mccutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis. 2008;14:1750–1752. doi: 10.3201/eid1411.080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iqbal J, Siddique A, Jameel M, Hira PR. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol. 2004;42:4237–4241. doi: 10.1128/JCM.42.9.4237-4241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markwalter CF, Gibson LE, Mudenda L, Kimmel DW, Mbambara S, Thuma PE, et al. Characterization of plasmodium lactate dehydrogenase and histidine-rich protein 2 clearance patterns via rapid on-bead detection from a single dried blood spot. Am J Trop Med Hyg. 2018;98:1389–1396. doi: 10.4269/ajtmh.17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez A, Rees-Channer RR, Perera R, Gamboa D, Chiodini PL, González IJ, et al. Analytical sensitivity of current best-in-class malaria rapid diagnostic tests. Malar J. 2017;16:128. doi: 10.1186/s12936-017-1780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landier J, Haohankhunnatham W, Das S, Konghahong K, Raksuansak J, Phattharakokoedbun P, et al. Operational performance of a Plasmodium falciparum ultrasensitive rapid diagnostic test for the 1 detection of asymptomatic infections in Eastern Myanmar. J Clin Microbiol. 2018;56:e00565-18. doi: 10.1128/JCM.00565-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das S, Jang IK, Barney B, Peck R, Rek JC, Arinaitwe E, et al. Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg. 2017;97:1540–1550. doi: 10.4269/ajtmh.17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogier E, Plucinski M, Lucchi N, Mace K, Chang M, Lemoine JF, et al. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS ONE. 2017;12:e0172139. doi: 10.1371/journal.pone.0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plucinski MM, Herman C, Jones S, Dimbu R, Fortes F, Ljolje D, et al. Screening for Pfhrp2/3-deleted Plasmodium falciparum, non-falciparum, and low-density malaria infections by a multiplex antigen assay. J Infect Dis. 2019;219:437–447. doi: 10.1093/infdis/jiy525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang IK, Das S, Barney RS, Peck RB, Rashid A, Proux S, et al. A new highly sensitive enzyme-linked immunosorbent assay for the detection of Plasmodium falciparum histidine-rich protein 2 in whole blood. Malar J. 2018;17:403. doi: 10.1186/s12936-018-2545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang IK, Tyler A, Lyman C, Kahn M, Kalnoky M, Rek JC, et al. Simultaneous quantification of Plasmodium antigens and host factor CRP in asymptomatic individuals with confirmed malaria using a novel multiplex immunoassay. J Clin Microbiol. 2019;57:e00948-18. doi: 10.1128/JCM.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fonseca AM, Quinto L, Jiménez A, González R, Bardají A, Maculuve S, et al. Multiplexing detection of IgG against Plasmodium falciparum pregnancy-specific antigens. PLoS ONE. 2017;12:e0181150. doi: 10.1371/journal.pone.0181150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plikaytis BD, Holder PF, Pais LB, Maslanka SE, Gheesling LL, Carlone GM. Determination of parallelism and nonparallelism in bioassay dilution curves. J Clin Microbiol. 1994;32:2441–2447. doi: 10.1128/JCM.32.10.2441-2447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamandis EP, Christopoulos TK. Immunoassay. San Diego: Academic Press; 1996. p. 579. [Google Scholar]
- 31.González R, Desai M, Macete E, Ouma P, Kakolwa MA, Abdulla S, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial. PLoS Med. 2014;11:e1001735. doi: 10.1371/journal.pmed.1001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.González R, Mombo-Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med. 2014;11:e1001733. doi: 10.1371/journal.pmed.1001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmona-Fonseca J, Maestre BA. Incidencia de la malaria gestacional, congénita y placentaria en Urabá (Antioquia, Colombia), 2005–2007. Rev Colomb Obstet Ginecol. 2009;60:19–33. [Google Scholar]
- 34.Mayor A, Bardají A, Macete E, Nhampossa T, Fonseca AM, González R, et al. Changing trends in Pfalciparum burden, immunity, and disease in pregnancy. N Engl J Med. 2015;373:1607–1617. doi: 10.1056/NEJMoa1406459. [DOI] [PubMed] [Google Scholar]
- 35.Serra-Casas E, Manrique P, Ding XC, Carrasco-Escobar G, Alava F, Gave A, et al. Loop-mediated isothermal DNA amplification for asymptomatic malaria detection in challenging field settings: technical performance and pilot implementation in the Peruvian Amazon. PLoS ONE. 2017;12:e0185742. doi: 10.1371/journal.pone.0185742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berhane A, Anderson K, Mihreteab S, Gresty K, Rogier E, Mohamed S, et al. Major threat to malaria control programs by Plasmodium falciparum lacking histidine-rich protein 2. Eritrea. Emerg Infect Dis. 2018;24:462–470. doi: 10.3201/eid2403.171723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waitumbi J, Awinda G, Rajasekariah G-H, Kifude C, Martin SK. Unified parasite lactate dehydrogenase and histidine-rich protein ELISA for quantification of Plasmodium falciparum. Am J Trop Med Hyg. 2018;80:516–522. [PubMed] [Google Scholar]
- 38.Plucinski MM, Dimbu PR, Fortes F, Abdulla S, Ahmed S, Gutman J, et al. Posttreatment HRP2 clearance in patients with uncomplicated Plasmodium falciparum malaria. J Infect Dis. 2018;217:685–692. doi: 10.1093/infdis/jix622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker J, Ho M-F, Pelecanos A, Gatton M, Chen N, Abdullah S, et al. Global sequence variation in the histidine-rich proteins 2 and 3 of Plasmodium falciparum: implications for the performance of malaria rapid diagnostic tests. Malar J. 2010;9:129. doi: 10.1186/1475-2875-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker J, McCarthy J, Gatton M, Kyle DE, Belizario V, Luchavez J, et al. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J Infect Dis. 2005;192:870–877. doi: 10.1086/432010. [DOI] [PubMed] [Google Scholar]
- 41.Wellems TE, Howard RJ. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci USA. 1986;83:6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee N, Gatton ML, Pelecanos A, Bubb M, Gonzalez I, Bell D, et al. Identification of optimal epitopes for Plasmodium falciparum rapid diagnostic tests that target histidine-rich proteins 2 and 3. J Clin Microbiol. 2012;50:1397–1405. doi: 10.1128/JCM.06533-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker J, Gatton ML, Peters J, Ho MF, McCarthy JS, Cheng Q. Transcription and expression of Plasmodium falciparum histidine-rich proteins in different stages and strains: implications for rapid diagnostic tests. PLoS ONE. 2011;6:e22593. doi: 10.1371/journal.pone.0022593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, Bell D, et al. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J. 2014;13:283. doi: 10.1186/1475-2875-13-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson OJ, Slater HC, Verity R, Parr JB, Mwandagalirwa MK, Tshefu A, et al. Modelling the drivers of the spread of Plasmodium falciparum hrp2 gene deletions in sub-Saharan Africa. Elife. 2017;6:e25008. doi: 10.7554/eLife.25008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Text S1. Biotinylation of detection mAbs. Text S2. Bead suspension array procedure. Text S3. Singleplex versus Multiplex testing. Text S4. Preparation and extraction of proteins from dried blood spots.
Additional file 2: Figure S1. Assay optimization. (A) Serial dilutions of recombinant PfHRP2 types A, B and C were assayed to determine the lowest concentration at which each antigen is detected; (B) P. falciparum Benin I and Borneo and P. vivax field isolates were assayed in a serial dilution fashion to assess differences between the analytical sensitivity for P. falciparum and P. vivax pLDH; (C) PfHRP2 and pLDH positive samples (plasma, cultured field isolates and recombinant proteins) were assayed in singleplex (X axes) and multiplex (Y axes).
Additional file 3: Table S1. Correlation between antigen levels and parasite densities for each group of clinical samples analysed.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.