Abstract
Thalidomide, lenalidomide and pomalidomide are immunomodulatory drugs (IMiDs) effective in the treatment of multiple myeloma, myelodysplastic syndrome (MDS) with deletion of chromosome 5q and other hematological malignancies. Recent studies showed that IMiDs bind to CRBN, a substrate receptor of CRL4 E3 ligase, to induce the ubiquitination and degradation of IKZF1 and IKZF3 in multiple myeloma cells, contributing to their anti-myeloma activity. Similarly, lenalidomide exerts therapeutic efficacy via inducing ubiquitination and degradation of CK1α in MDS with deletion of chromosome 5q. Recently, novel thalidomide analogs have been designed for better clinical efficacy, including CC-122, CC-220 and CC-885. Moreover, a number of neo-substrates of IMiDs have been discovered. Proteolysis-targeting chimeras (PROTACs) as a class of bi-functional molecules are increasingly used as a strategy to target otherwise intractable cellular protein. PROTACs appear to have broad implications for novel therapeutics. In this review, we summarized new generation of immunomodulatory compounds, their potential neo-substrates, and new strategies for the design of novel PROTAC drugs.
Keywords: Immunomodulatory drugs, CRL4CRBN E3 ligase, CC-122, CC-220, PROTACs
Background
Thalidomide was notorious for the teratogenic effects leading to congenital malformations in phocomelia infants [1–5]. Thalidomide and its derivatives can modulate functions of T cells and NK cells by inducing the production of cytokines, including IL-2 (interleukin-2) and interferon γ [6–9]. Thus, thalidomide and its analogs, including lenalidomide and pomalidomide, are called immunomodulatory drugs (IMiDs). In addition, IMiDs have antiangiogenic activity [10, 11]. IMiDs are widely used in combination with proteasome inhibitors, steroids, and monoclonal antibodies and playing a pivotal role in the treatment of multiple myeloma (MM) [12–18]. Lenalidomide also showed activities in a number of hematological malignancies, including myelodysplastic syndrome (MDS) with deletion of chromosome 5q (del(5q)) [19–21], mantle cell lymphoma (MCL) [22–27] and chronic lymphocytic leukemia (CLL) [28–31].
Although IMiDs have been approved for the treatment of several hematological malignancies, the molecular mechanism remained unclear at that time. Lately, the primary cellular target of thalidomide was identified to be CRBN, a substrate receptor of Cullin-RING Ligase 4 (CRL4) [32]. IMiDs target CUL4-RBX1-DDB1-CRBN (CRL4CRBN) E3 ligase to induce the ubiquitination and proteasomal degradation of Ikaros family zinc finger proteins, Ikaros (IKZF1) and Aiolos (IKZF3) which are the lymphoid transcription factors essential for myeloma cell survival [33–35]. Similarly, lenalidomide induces the ubiquitination and degradation of CK1α, leading to the death of del(5q) MDS cells [36].
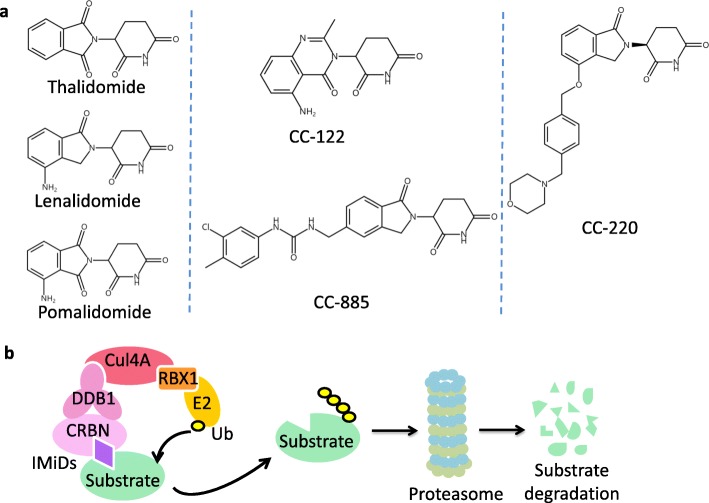
Recently, novel thalidomide analogs have been developed, including CC-122 (avadomide), CC-220 (iberdomide) and CC-885 [36–39] (Fig. 1). These novel CRBN modulators are in active clinical trials. Moreover, studies have shown that IMiDs repurpose CRL4CRBN E3 ligase to ubiquitinate and degrade a series of cellular proteins [40, 41].
Fig. 1.
Chemical structure and mechanism of action of IMiDs. a Chemical structure of thalidomide, lenalidomide, pomalidomide, CC-122, CC-885 and CC-220. b IMiDs (purple rhombuses) bind to CRBN, a substrate receptor of CRL4 E3 ligase, to recruit substrates for ubiquitination and proteasomal degradation. Ub, ubiquitin
In this review, we summarized recent advances on new generation of IMiDs, their neo-substrates and the implication for novel therapeutics.
New generation of immunomodulatory drugs
IMiDs contain a conserved glutarimide ring and a variable phthaloyl ring. The glutarimide ring interacts with a conserved hydrophobic pocket of CRBN. The phthaloyl ring, together with CRBN, forms binding interface for neo-substrates [42–44]. Hence, modifications on the variable phthaloyl ring may lead to new generation of IMiDs.
CC-122 (avadomide)
CC-122 is a novel immunomodulatory compound containing the conserved glutarimide for CRBN binding. As a derivative of thalidomide, CC-122 has pluripotent activities, including antitumor and modulation of immune cells [37, 45]. CC-122 binds CRL4CRBN E3 ligase to induce the degradation of IKZF1 and IKZF3 in MM cells, diffuse large B-cell lymphoma (DLBCL) cells and xenograft mouse models established from DLBCL cells [36, 37]. The degradation of IKZF1 and IKZF3 results in derepression of IFN-regulated genes, leading to apoptosis of several DLBCL cell lines and inhibition of tumor growth in xenograft mouse models [37]. In addition, CC-122 costimulates T cells and induces the production of IL-2 [37].
Based on the above notable antitumor activities and modulations on immune cells, CC-122 has entered clinical trials for a number of diseases, including Non-Hodgkin’s lymphoma (NHL), MM and CLL/SLL (Table 1). Recently, a multicenter, open-label, and dose-escalation phase 1 clinical trial (NCT01421524) has been conducted to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of CC-122 in patients with MM, NHL and advanced solid tumors. In the latest report, 34 patients with NHL, MM or advanced solid tumors were enrolled [46]. These patients had a median age of 57 years and had received a median of 3.5 prior anticancer therapies. In the part A portion of this trial, patients received CC-122 at an increasing dose of 0.5 to 3.5 mg orally once daily on a 28-day schedule. The median duration of CC-122 treatment was 58 days. Fatigue (44%), neutropenia (29%) and diarrhea (15%) were the most common treatment-emergent adverse events (TEAEs). The non-tolerated dose (NTD) was 3.5 mg and maximum tolerated dose (MTD) was 3.0 mg. One of five patients with NHL had a complete response (CR) and two of them achieved partial responses. This study confirmed that CC-122 induces the degradation of IKZF3 protein in a dose-dependent manner in B and T cells from peripheral blood. Moreover, B-cell numbers in peripheral blood were reduced after 15-day administration of CC-122. To summarize, CC-122 monotherapy showed acceptable safety and encouraging pharmacokinetics in patients with MM, NHL and solid tumors [46].
Table 1.
Clinical trials of CC-122 in cancer
| Phase | Conditions | Interventions | NCT ID |
|---|---|---|---|
| 1 | MM, NHL, solid tumors | CC-122 | NCT01421524 |
| 1 | DLBCL, iNHL | CC-122, obinutuzumab | NCT02417285 |
| 1 | DLBCL, FL | CC-122, rituximab, CC-223, CC-292 | NCT02031419 |
| 1, 2 | DLBCL | CC-122, R-CHOP | NCT03283202 |
| 1 | NHL | CC-122 | NCT02509039 |
| 1, 2 | CLL, SLL | CC-122, ibrutinib, obinutuzumab | NCT02406742 |
| 1, 2 | HCC | CC-122, nivolumab | NCT02859324 |
| 2 | Melanoma | CC-122, nivolumab | NCT03834623 |
Abbreviation: CLL Chronic lymphocytic leukemia, DLBCL Diffuse large B-cell lymphoma, FL Follicular lymphoma, HCC Hepatocellular carcinoma, MM Multiple myeloma, NHL Non-Hodgkin’s lymphoma, iNHL Indolent NHL, R-CHOP (Rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone), SLL Small lymphocytic lymphoma
A multi-center, open-label, and dose escalation/expansion phase 1 clinical trial (NCT02417285) is ongoing to test the safety, tolerability and preliminary efficacy of CC-122 in combination with obinutuzumab in NHL. According to the interim result, 58 patients were enrolled, including 38 with relapsed or refractory (R/R) follicular lymphoma (FL), 19 with R/R DLBCL and 1 with R/R marginal zone lymphoma [47]. These patients received increasing doses of CC-122 for 5 days per week (5/7 days) in each 28-day cycle in combination with obinutuzumab at a dose of 1000 mg on days 2, 8, and 15 of cycle 1, and day 1 of cycles 2 to 8. Among the 38 patients with R/R FL, the most common TEAEs were neutropenia (66%), pyrexia (29%) and thrombocytopenia (29%). The overall response rate (ORR) was 68% and 16 out of these 38 patients (42%) achieved a CR. CC-122 in combination with obinutuzumab was well-tolerated and showed promising efficacy in patients with R/R FL [47].
In another ongoing multi-center and open-label phase 1 clinical trial (NCT02031419), combinations of CC-122, CC-223, CC-292 and rituximab was given in patients with R/R DLBCL or FL. From the interim result of the arm D of this study, 37 patients with R/R FL received CC-122 at a dose of 2 mg or 3 mg for 5/7 days and intravenous rituximab at a dose of 375 mg/m2 in each 28-day cycle [48]. Neutropenia (46%) and anemia (24%) were the most common TEAEs. The ORR was 65% and 8 patients (22%) achieved a CR. Thus, CC-122 in combination with rituximab was well-tolerated and showed promising clinical activity in patients with R/R FL [48].
A phase 1/2 clinical trial (NCT03283202) will evaluate the safety and preliminary efficacy of CC-122 combined with R-CHOP regimen for newly-diagnosed DLBCL patients with poor risk factor (Table 1). Therefore, CC-122 has shown clinical potential for the treatment of MM and NHL.
CC-220 (iberdomide)
CC-220 is a new analog of thalidomide developed for the treatment of relapsed/refractory MM (RRMM) and systemic lupus erythematosus (SLE). CC-220 has improved efficacy to degrade IKZF1 and IKZF3 through tighter binding to the CRL4CRBN E3 ligase [38].
Recently, a double-blinded, placebo-controlled, single dose-escalation phase 1 study (NCT01733875) has been carried out in healthy volunteers to evaluate safety, pharmacokinetics and pharmacodynamics of CC-220. In the latest report, 56 healthy volunteers were enrolled and randomized into 7 cohorts [49]. In each cohort, six subjects took a single dose of 0.03 to 6 mg CC-220 and two subjects received placebo orally. In this study, no severe TEAEs were reported. CC-220 was well tolerated when taken at a single dose of 6 mg orally in these healthy volunteers. Consistently, CC-220 administration causes the degradation of IKZF1 and IKZF3 in B cells, T cells and monocytes. In addition, CC-220 inhibited the production of anti-dsDNA and anti-phospholipid autoantibodies in cultured peripheral blood mononuclear cells (PBMCs) from SLE patients [49]. Thus, this study demonstrated the tolerated safety and pharmacodynamic activity of CC-220, indicating its promising clinical development for SLE. Soon afterwards, two randomized, placebo-controlled, double-blinded, phase 2 studies (NCT02185040, NCT03161483) in SLE patients were designed to study the safety, tolerability, pharmacokinetics and pharmacodynamics of CC-220 in SLE.
At this time, a multicenter, open-label, and dose-escalation phase 1/2 study (NCT02773030) in RRMM is ongoing to evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of CC-220 when administered as monotherapy, and in combination with dexamethasone, with or without daratumumab or bortezomib. According to the preclinical studies, CC-220 combined with bortezomib induced deep IKZF1 and IKZF3 degradation at clinically relevant concentrations and showed synergistically antiproliferative effects in MM cell lines, which could be further enhanced by dexamethasone. In addition, CC-220 in combination with bortezomib induced deeper apoptosis than combinations of any other clinically approved IMiDs with bortezomib in MM cell lines. CC-220 also synergistically enhanced the anti-myeloma activity of daratumumab in complement-dependent cytotoxicity assays [50]. From the interim results, 69 patients with RRMM received CC-220 at an increasing dose of 0.3 to 1.3 mg on days 1–21 plus dexamethasone at a dose of 20 mg or 40 mg on days 1, 8,15 and 22 in each 28-day cycle [51]. The ORR was 29% and clinical benefit rate was 45%. The MTD has not been reached. Combination of CC-220 and dexamethasone showed favorable tolerability with grade 3–4 neutropenia (29%), infections (25%), and thrombocytopenia (12%) [51]. These preclinical and clinical data suggest the promising clinical application of CC-220 in combination with bortezomib, dexamethasone and daratumumab for MM treatment.
In general, current clinical trials on CC-220 mostly focus on its potential to treat SLE and MM (Table 2).
Table 2.
Clinical trials of CC-220
| Phase | Conditions | Interventions | NCT ID |
|---|---|---|---|
| 1 | Healthy volunteers | CC-220, placebo | NCT01733875 |
| 2 | SLE | CC-220, placebo | NCT03161483 |
| 2 | SLE | CC-220, placebo | NCT02185040 |
| 1,2 | MM | CC-220, DEX, Dara, BTZ | NCT02773030 |
| 1 | Healthy volunteers | CC-220 | NCT03135509 |
| 1 | Healthy volunteers | CC-220, radiation | NCT03294603 |
| 1 | Hepatic impairment, Healthy volunteers | CC-220 | NCT03824678 |
Abbreviation: SLE Systemic lupus erythematosus, DEX Dexamethasone, Dara Daratumumab, BTZ Bortezomib
CC-885
CC-885 is a new CRBN modulator with a strong anti-proliferation activity in a broad set of tumor cell lines [39]. CC-885 can induce CRL4CRBN-dependent degradation of IKZF1 and the translation termination factor GSPT1, while neither lenalidomide nor pomalidomide can trigger the depletion of GSPT1, suggesting different substrate spectrum of CC-885 from lenalidomide or pomalidomide. Moreover, CC-885 showed sub-nanomolar potency against patient-derived acute myeloid leukemia (AML) cells, though lenalidomide and pomalidomide do not have significant activity in AML [39]. CC-885 may thus have a potential for AML therapy different from other IMiDs. As CC-885 can induce the degradation of GSPT1 while other IMiDs do not, it may have extra toxicity from other IMiDs. More studies on the activity and toxicity effects of CC-885 are required.
Potential neo-substrates of immunomodulatory drugs
Structural studies have revealed that neo-substrates of IMiDs-CRL4CRBN complex, including IKZF1, IKZF3, CK1α, ZFP91, share a common structural motif containing a key glycine [42–44, 52]. Hence, cellular proteins which contain this structural feature may be targeted and degraded by IMiDs.
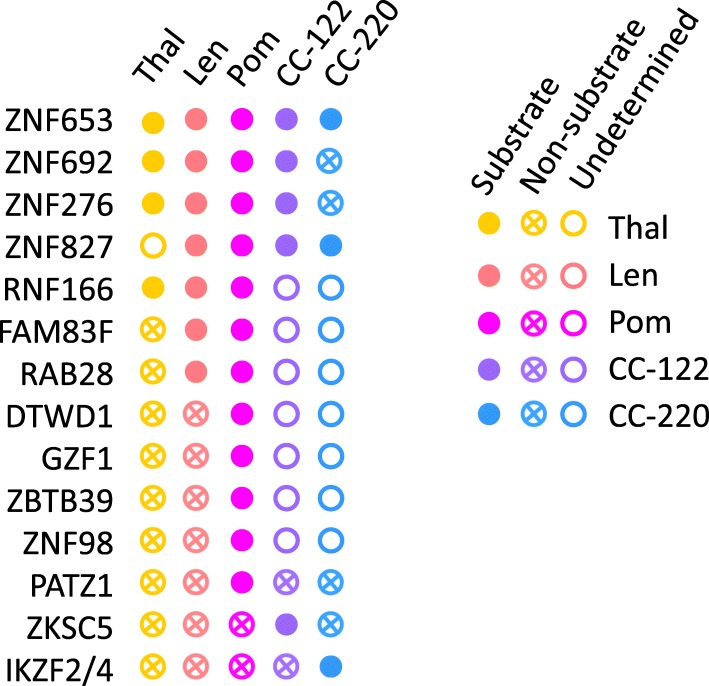
In a recent study, a mass spectrometry-based workflow was established to detect IMiDs-induced target degradation in Kelly, SK-N-DZ, human embryonic stem cells and MM1S cells [40]. These cells were treated with pomalidomide, lenalidomide, thalidomide or DMSO as a control respectively, and protein abundance was measured by multiplexed mass spectrometry. Comprehensive proteomics analysis identified several potential neo-substrates of IMiDs, including ZNF653, ZNF827, ZNF692, RNF166, FAM83F, RAB28, DTWD1, GZF1, ZBTB39, and ZNF98 [40].
Another study screening for C2H2 zinc finger (ZF) domains which might be targeted by IMiDs-CRL4CRBN complex also discovered several neo-substrates [41]. In this study, cDNAs of 6572 C2H2 ZFs were cloned into a degradation reporter vector to generate a C2H2 ZF library. This library was transduced into HEK293T cells which were then treated with pomalidomide, lenalidomide, thalidomide or DMSO, respectively. These cells were analyzed by fluorescence-activated cell sorting (FACS) and high-throughput sequencing. The results showed that 11 ZFs were degraded by IMiDs and 6 of the 11 full length proteins can be degraded, including IKZF1/IKZF3, ZFP91, ZNF692, ZNF276, ZNF653, and ZNF827 [41]. The degradation of these transcription factors was also tested in the presence of CC-122 and CC-220. These potential neo-substrates have been summarized (Fig. 2).
Fig. 2.
Potential neo-substrates of thalidomide, lenalidomide, pomalidomide, CC-122 and CC-220. Solid spheres represent potential neo-substrates. Spheres with crosses inside represent proteins that were not degraded by the corresponding compound, at least under the condition described in the references. Hollow spheres represent undetermined proteins. The five compounds were shown in different colors, as indicated. Thal, thalidomide. Len, lenalidomide. Pom, pomalidomide
IMiDs-induced substrate degradation can be affected by a series of factors, including drug concentrations, exposure time and cell types. Thus, these potential neo-substrates should be further validated under physiological or pathological conditions. IMiDs-induced degradation of target proteins, especially transcription factors previously perceived to be undruggable, provides a strategy to degrade cellular targets, which may have huge potential in the development of novel therapeutics.
PROTAC: the ubiquitin-proteasome system-mediated degradation of target proteins
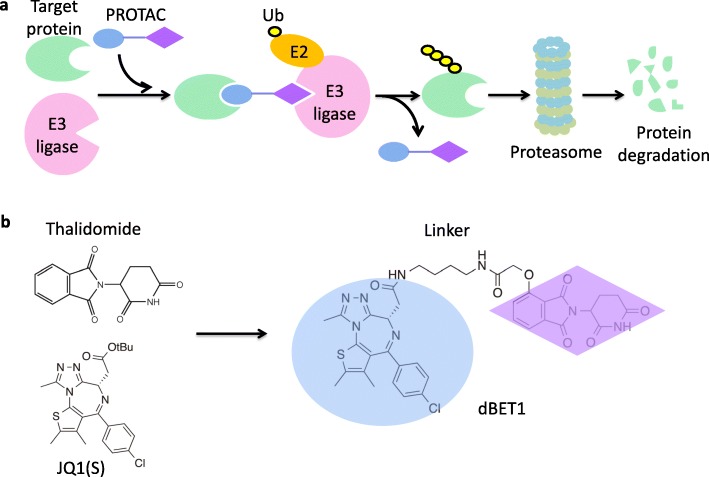
Proteolysis-targeting chimeras (PROTACs) are a class of bi-functional molecules designed to selectively degrade target proteins via cellular quality control machinery, such as the ubiquitin-proteasome system. Typically, these molecules contain an E3 ligase binding moiety, such as thalidomide analogs or a ligand to von Hippel-Lindau (VHL) E3 ligase, attached to another small molecule binding to a protein of interest through a linker (Fig. 3a) [53–55].
Fig. 3.
Targeting protein for degradation by Proteolysis-targeting chimeras (PROTACs). a PROTACs contain an E3 ligase binding moiety (purple rhombus), attached to another small molecule (blue oval) binding to the target protein through a linker. PROTACs can bring target protein to the E3 ligase for ubiquitination and subsequent degradation. b Chemical structure of thalidomide, JQ1(S) and one published PROTAC, dBET1. (adapted from Winter, GE, et al., Science 2015)
Based on this design principle, thalidomide was linked to JQ1 [56], a small molecule binding to Bromodomain-Containing Protein 4 (BRD4), to generate a bi-functional molecule called dBET1 (Fig. 3b). This PROTAC induces CRBN-dependent degradation of BRD4 and subsequent down-regulation of MYC, leading to cytotoxicity of AML cells [57]. Similar design of bi-functional degraders induce degradation of BCR-Abl [58] and Bruton’s tyrosine kinase (BTK) [59], showing therapeutic potentials in the treatment of chronic myeloid leukemia (CML) and B-cell lymphoma, respectively.
Since 2015, more and more PROTACs have been synthesized to target a broad number of cellular proteins and most of them were studied in cultured cells or animal models [57–66]. ARV-110 is an oral PROTAC that can target androgen receptor (AR) and induce AR degradation. ARV-110 is currently being tested in a phase 1 clinical trial (NCT03888612). This open-label, dose-escalation phase 1 study will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of ARV-110 in patients with metastatic castration-resistant prostate cancer who have progressed on at least two prior systemic therapies.
Unlike tyrosine kinase inhibitors (TKI), PROTACs do not occupy the binding site and can be recycled. Hence, in theory relatively fewer compounds can achieve expectant activities, which will make them more efficient with less off-target effects. IMiDs-based PROTACs have become a strategy of drug design for enhancing degradation of specific cellular targets. More PROTACs are expected to enter clinical development.
Conclusions and perspectives
IMiDs are widely used clinically to treat MM, MDS with del(5q) and other hematological cancers. To achieve better efficacy, new generation of IMiDs including CC-122, CC-220, and CC-885 have been developed. CC-122 and CC-220 have entered into phase 1/2 clinical trials. Further studies are required to evaluate their efficacy in the treatment of several blood malignancies, including MM, DLBCL and NHL. CC-885 has shown potential efficacy for AML, which is not achieved by lenalidomide or pomalidomide. A number of cellular proteins have been identified to be potential neo-substrates of IMiDs, which may facilitate development of novel IMiDs. Small molecule-based PROTACs are undergoing active clinical development and are increasingly used as a new strategy for drug design. More PROTACs will be synthesized for targeted therapy. Since immunotherapy with chimeric antigen receptor (CAR)-engineered T cells becomes a highly promising therapeutic modality for cancer therapy [67–75], it is possible to combine novel IMiDs with CAR-T cells for more potent therapies of RRMM.
Acknowledgements
Not applicable.
Abbreviations
- AML
Acute myeloid leukemia
- AR
Androgen receptor
- BRD4
Bromodomain-Containing Protein 4
- BTK
Bruton’s tyrosine kinase
- CLL
Chronic lymphocytic leukemia
- CML
Chronic myeloid leukemia
- CR
Complete response
- CRL4
Cullin-RING Ligase 4
- DLBCL
Diffuse large B-cell lymphoma
- FACS
Fluorescence-activated cell sorting
- IL-2
Interleukin-2
- IMiDs
Immunomodulatory drugs
- MCL
Mantle cell lymphoma
- MDS
Myelodysplastic syndrome
- MM
Multiple myeloma
- MTD
Maximum tolerated dose
- NHL
Non-Hodgkin’s lymphoma
- NTD
Non-tolerated dose
- ORR
Overall response rate
- PBMCs
Peripheral blood mononuclear cells
- PROTACs
Proteolysis-targeting chimeras
- RRMM
Relapsed/refractory MM
- SLE
Systemic lupus erythematosus
- TEAEs
Treatment-emergent adverse events
- TKI
Tyrosine kinase inhibitors
- TMT
Tandem mass tag
- VHL
Von Hippel-Lindau
Authors’ contributions
SG and SW drafted the manuscript. SW collected a part of references and prepared figures. YS and SG designed this review and revised the manuscript. All authors read and approved the final manuscript.
Funding
The study is partly supported by the National Natural Science Foundation of China (NSFC grant no. 81800204, SBG; NSFC grant no. 81470287, YPS), and by the Affiliated Cancer Hospital of Zhengzhou University.
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaobing Gao and Shichao Wang contributed equally to this work.
References
- 1.Speirs AL. Thalidomide and congenital abnormalities. Lancet. 1962;1(7224):303–305. doi: 10.1016/S0140-6736(62)91248-5. [DOI] [PubMed] [Google Scholar]
- 2.Somers GS. Thalidomide and congenital abnormalities. Lancet. 1962;1(7235):912–913. doi: 10.1016/S0140-6736(62)91943-8. [DOI] [PubMed] [Google Scholar]
- 3.Yang TJ, Yang TS, Liang HM. Thalidomide and congenital abnormalities. Lancet. 1963;1(7280):552–553. doi: 10.1016/S0140-6736(63)91347-3. [DOI] [PubMed] [Google Scholar]
- 4.Lenz W. A short history of thalidomide embryopathy. Teratology. 1988;38(3):203–215. doi: 10.1002/tera.1420380303. [DOI] [PubMed] [Google Scholar]
- 5.Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today. 2015;105(2):140–156. doi: 10.1002/bdrc.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haslett PA, Corral LG, Albert M, Kaplan G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J Exp Med. 1998;187(11):1885–1892. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 8.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. doi: 10.1182/blood.V98.1.210. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, Kumar S, Chauhan D, Treon SP, Richardson P, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 10.D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercurio A, Adriani G, Catalano A, Carocci A, Rao L, Lentini G, Cavalluzzi MM, Franchini C, Vacca A, Corbo F. A mini-review on thalidomide: chemistry, mechanisms of action, therapeutic potential and anti-Angiogenic properties in multiple myeloma. Curr Med Chem. 2017;24(25):2736–2744. doi: 10.2174/0929867324666170601074646. [DOI] [PubMed] [Google Scholar]
- 12.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T, Foa R, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 14.Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, Streetly M, Dalgleish AG. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22(16):3269–3276. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 15.Caers J, Paiva B, Zamagni E, Leleu X, Blade J, Kristinsson SY, Touzeau C, Abildgaard N, Terpos E, Heusschen R, et al. Diagnosis, treatment, and response assessment in solitary plasmacytoma: updated recommendations from a European expert panel. J Hematol Oncol. 2018;11(1):10. doi: 10.1186/s13045-017-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimopoulos M, Wang M, Maisnar V, Minarik J, Bensinger W, Mateos MV, Obreja M, Blaedel J, Moreau P. Response and progression-free survival according to planned treatment duration in patients with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J Hematol Oncol. 2018;11(1):49. doi: 10.1186/s13045-018-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, et al. Daratumumab, Lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, Tai YT, Ho MZG, Qiu L, Anderson KC. Interferon-alpha-based immunotherapies in the treatment of B cell-derived hematologic neoplasms in today's treat-to-target era. Exp Hematol Oncol. 2017;6:20. doi: 10.1186/s40164-017-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 20.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 21.Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M, Muus P, Te Boekhorst P, Sanz G, Del Canizo C, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low−/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118(14):3765–3776. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 22.Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K, Wride K, Ervin-Haynes A, Takeshita K, Pietronigro D, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145(3):344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 23.Zinzani PL, Vose JM, Czuczman MS, Reeder CB, Haioun C, Polikoff J, Tilly H, Zhang L, Prandi K, Li J, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892–2897. doi: 10.1093/annonc/mdt366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witzig TE, Luigi Zinzani P, Habermann TM, Tuscano JM, Drach J, Ramchandren R, Kalayoglu Besisik S, Takeshita K, Casadebaig Bravo ML, Zhang L, et al. Long-term analysis of phase II studies of single-agent lenalidomide in relapsed/refractory mantle cell lymphoma. Am J Hematol. 2017;92(10):E575–E583. doi: 10.1002/ajh.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruan J, Martin P, Christos P, Cerchietti L, Tam W, Shah B, Schuster SJ, Rodriguez A, Hyman D, Calvo-Vidal MN, et al. Five-year follow-up of lenalidomide plus rituximab as initial treatment of mantle cell lymphoma. Blood. 2018;132(19):2016–2025. doi: 10.1182/blood-2018-07-859769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balaji S, Ahmed M, Lorence E, Yan F, Nomie K, Wang M. NF-kappaB signaling and its relevance to the treatment of mantle cell lymphoma. J Hematol Oncol. 2018;11(1):83. doi: 10.1186/s13045-018-0621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladha A, Zhao J, Epner EM, Pu JJ. Mantle cell lymphoma and its management: where are we now? Exp Hematol Oncol. 2019;8(1):2. doi: 10.1186/s40164-019-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 29.Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111(11):5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badoux XC, Keating MJ, Wen S, Wierda WG, O'Brien SM, Faderl S, Sargent R, Burger JA, Ferrajoli A. Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2013;31(5):584–591. doi: 10.1200/JCO.2012.42.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vitale C, Falchi L, Ten Hacken E, Gao H, Shaim H, Van Roosbroeck K, Calin G, O'Brien S, Faderl S, Wang X, et al. Ofatumumab and Lenalidomide for patients with relapsed or refractory chronic lymphocytic leukemia: correlation between responses and immune characteristics. Clin Cancer Res. 2016;22(10):2359–2367. doi: 10.1158/1078-0432.CCR-15-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 33.Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong KK, Bradner JE, Kaelin WG., Jr The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart AK. Medicine. How thalidomide works against cancer. Science. 2014;343(6168):256–257. doi: 10.1126/science.1249543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, Chamberlain PP, Mani DR, Man HW, Gandhi AK, et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature. 2015;523(7559):183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagner PR, Man HW, Fontanillo C, Wang M, Couto S, Breider M, Bjorklund C, Havens CG, Lu G, Rychak E, et al. CC-122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood. 2015;126(6):779–789. doi: 10.1182/blood-2015-02-628669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matyskiela ME, Zhang W, Man HW, Muller G, Khambatta G, Baculi F, Hickman M, LeBrun L, Pagarigan B, Carmel G, et al. A Cereblon modulator (CC-220) with improved degradation of Ikaros and Aiolos. J Med Chem. 2018;61(2):535–542. doi: 10.1021/acs.jmedchem.6b01921. [DOI] [PubMed] [Google Scholar]
- 39.Matyskiela ME, Lu G, Ito T, Pagarigan B, Lu CC, Miller K, Fang W, Wang NY, Nguyen D, Houston J, et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature. 2016;535(7611):252–257. doi: 10.1038/nature18611. [DOI] [PubMed] [Google Scholar]
- 40.Donovan KA, An J, Nowak RP, Yuan JC, Fink EC, Berry BC, Ebert BL, Fischer ES. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane radial ray syndrome. Elife. 2018;7. 10.7554/eLife.38430. [DOI] [PMC free article] [PubMed]
- 41.Sievers Quinlan L., Petzold Georg, Bunker Richard D., Renneville Aline, Słabicki Mikołaj, Liddicoat Brian J., Abdulrahman Wassim, Mikkelsen Tarjei, Ebert Benjamin L., Thomä Nicolas H. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science. 2018;362(6414):eaat0572. doi: 10.1126/science.aat0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer ES, Bohm K, Lydeard JR, Yang H, Stadler MB, Cavadini S, Nagel J, Serluca F, Acker V, Lingaraju GM, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512(7512):49–53. doi: 10.1038/nature13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, Rychak E, Corral LG, Ren YJ, Wang M, et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014;21(9):803–809. doi: 10.1038/nsmb.2874. [DOI] [PubMed] [Google Scholar]
- 44.Petzold G, Fischer ES, Thoma NH. Structural basis of lenalidomide-induced CK1alpha degradation by the CRL4 ubiquitin ligase. Nature. 2016;532(7597):127–130. doi: 10.1038/nature16979. [DOI] [PubMed] [Google Scholar]
- 45.Cubillos-Zapata C, Cordoba R, Avendano-Ortiz J, Arribas-Jimenez C, Hernandez-Jimenez E, Toledano V, Villaescusa T, Moreno V, Lopez-Collazo E. CC-122 immunomodulatory effects in refractory patients with diffuse large B-cell lymphoma. Oncoimmunology. 2016;5(12):e1231290. doi: 10.1080/2162402X.2016.1231290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasco DW, Papadopoulos KP, Pourdehnad M, Gandhi AK, Hagner PR, Li Y, Wei X, Chopra R, Hege K, DiMartino J, et al. A first-in-human study of novel Cereblon modulator Avadomide (CC-122) in advanced malignancies. Clin Cancer Res. 2019;25(1):90–98. doi: 10.1158/1078-0432.CCR-18-1203. [DOI] [PubMed] [Google Scholar]
- 47.Michot JM, Bouabdallah R, Doorduijn JK, Vitolo U, Kersten MJ, Chiappella A, Zinzani PL, Salles G, Bijou F, Sarmiento R, et al. Avadomide (CC-122), a Novel Cereblon Modulating Agent, in Combination with Obinutuzumab (GA101) in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2018;132(Supplement 1):449. doi: 10.1182/blood-2018-99-110213. [DOI] [Google Scholar]
- 48.Nastoupil LJ, Bijou F, Ribrag V, Chavez JC, Witzig TE, Andorsky DJ, Klein LM, Greenwald D, Santoro A, Corradini P, et al. Avadomide (CC-122), a Novel Cereblon Modulating Agent, Plus Rituximab in Patients with Relapsed or Refractory Follicular Lymphoma. Blood. 2018;132(Supplement 1):1602. doi: 10.1182/blood-2018-99-114409. [DOI] [Google Scholar]
- 49.Schafer PH, Ye Y, Wu L, Kosek J, Ringheim G, Yang Z, Liu L, Thomas M, Palmisano M, Chopra R. Cereblon modulator iberdomide induces degradation of the transcription factors Ikaros and Aiolos: immunomodulation in healthy volunteers and relevance to systemic lupus erythematosus. Ann Rheum Dis. 2018;77(10):1516–1523. doi: 10.1136/annrheumdis-2017-212916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amatangelo M, Bjorklund CC, Kang J, Polonskaia A, Viswanatha S, Thakurta A. Iberdomide (CC-220) Has Synergistic Anti-Tumor and Immunostimulatory Activity Against Multiple Myeloma in Combination with Both Bortezomib and Dexamethasone, or in Combination with Daratumumab in Vitro. Blood. 2018;132(Supplement 1):1935. doi: 10.1182/blood-2018-99-113383. [DOI] [Google Scholar]
- 51.Lonial S, Amatangelo M, Popat R, Minnema MC, Zonder JA, Larsen J, Oriol Rocafiguera A, Campagnaro EL, Rodriguez Otero P, Badros AZ, et al. Translational and Clinical Evidence of a Differentiated Profile for the Novel CELMoD, Iberdomide (CC-220) Blood. 2019;134(Supplement 1):3119. doi: 10.1182/blood-2019-124298. [DOI] [Google Scholar]
- 52.An J, Ponthier CM, Sack R, Seebacher J, Stadler MB, Donovan KA, Fischer ES. pSILAC mass spectrometry reveals ZFP91 as IMiD-dependent substrate of the CRL4(CRBN) ubiquitin ligase. Nat Commun. 2017;8:15398. doi: 10.1038/ncomms15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai AC, Crews CM. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov. 2017;16(2):101–114. doi: 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coleman KG, Crews CM. Proteolysis-targeting chimeras: harnessing the ubiquitin-proteasome system to induce degradation of specific target proteins. Annu Rev Cancer Biol. 2018;2:41–58. doi: 10.1146/annurev-cancerbio-030617-050430. [DOI] [Google Scholar]
- 55.An S, Fu L. Small-molecule PROTACs: An emerging and promising approach for the development of targeted therapy drugs. EBioMedicine. 2018;36:553–562. doi: 10.1016/j.ebiom.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winter GE, Buckley DL, Paulk J, Roberts JM, Souza A, Dhe-Paganon S, Bradner JE, Drug development Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348(6241):1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai AC, Toure M, Hellerschmied D, Salami J, Jaime-Figueroa S, Ko E, Hines J, Crews CM. Modular PROTAC Design for the Degradation of oncogenic BCR-ABL. Angew Chem Int Ed Eng. 2016;55(2):807–810. doi: 10.1002/anie.201507634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zorba A, Nguyen C, Xu Y, Starr J, Borzilleri K, Smith J, Zhu H, Farley KA, Ding W, Schiemer J, et al. Delineating the role of cooperativity in the design of potent PROTACs for BTK. Proc Natl Acad Sci U S A. 2018;115(31):E7285–E7292. doi: 10.1073/pnas.1803662115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robb CM, Contreras JI, Kour S, Taylor MA, Abid M, Sonawane YA, Zahid M, Murry DJ, Natarajan A, Rana S. Chemically induced degradation of CDK9 by a proteolysis targeting chimera (PROTAC) Chem Commun (Camb) 2017;53(54):7577–7580. doi: 10.1039/C7CC03879H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olson CM, Jiang B, Erb MA, Liang Y, Doctor ZM, Zhang Z, Zhang T, Kwiatkowski N, Boukhali M, Green JL, et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol. 2018;14(2):163–170. doi: 10.1038/nchembio.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiedel M, Herp D, Hammelmann S, Swyter S, Lehotzky A, Robaa D, Olah J, Ovadi J, Sippl W, Jung M. Chemically induced degradation of Sirtuin 2 (Sirt2) by a proteolysis targeting chimera (PROTAC) based on Sirtuin rearranging ligands (SirReals) J Med Chem. 2018;61(2):482–491. doi: 10.1021/acs.jmedchem.6b01872. [DOI] [PubMed] [Google Scholar]
- 63.Steinebach C, Lindner S, Udeshi ND, Mani DC, Kehm H, Kopff S, Carr SA, Gutschow M, Kronke J. Homo-PROTACs for the chemical knockdown of Cereblon. ACS Chem Biol. 2018;13(9):2771–2782. doi: 10.1021/acschembio.8b00693. [DOI] [PubMed] [Google Scholar]
- 64.Shibata N, Nagai K, Morita Y, Ujikawa O, Ohoka N, Hattori T, Koyama R, Sano O, Imaeda Y, Nara H, et al. Development of protein degradation inducers of androgen receptor by conjugation of androgen receptor ligands and inhibitor of apoptosis protein ligands. J Med Chem. 2018;61(2):543–575. doi: 10.1021/acs.jmedchem.7b00168. [DOI] [PubMed] [Google Scholar]
- 65.Zhang C, Han XR, Yang X, Jiang B, Liu J, Xiong Y, Jin J. Proteolysis targeting chimeras (PROTACs) of anaplastic lymphoma kinase (ALK) Eur J Med Chem. 2018;151:304–314. doi: 10.1016/j.ejmech.2018.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zengerle M, Chan KH, Ciulli A. Selective small molecule induced degradation of the BET Bromodomain protein BRD4. ACS Chem Biol. 2015;10(8):1770–1777. doi: 10.1021/acschembio.5b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5(1):22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z, Song W, Rubinstein M, Liu D. Recent updates in cancer immunotherapy: a comprehensive review and perspective of the 2018 China Cancer immunotherapy workshop in Beijing. J Hematol Oncol. 2018;11(1):142. doi: 10.1186/s13045-018-0684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu J, Wang Q, Xu H, Gu C, Jiang L, Wang J, Wang D, Xu B, Mao X, Wang J, et al. Anti-BCMA CAR-T cells for treatment of plasma cell dyscrasia: case report on POEMS syndrome and multiple myeloma. J Hematol Oncol. 2018;11(1):128. doi: 10.1186/s13045-018-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, Zhang YL, Wang FX, Zhang PY, Lei B, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11(1):141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Hu Y, Huang H. Current development of chimeric antigen receptor T-cell therapy. Stem Cell Investig. 2018;5:44. doi: 10.21037/sci.2018.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu D, Zhao J, Song Y. Engineering switchable and programmable universal CARs for CAR T therapy. J Hematol Oncol. 2019;12(1):69. doi: 10.1186/s13045-019-0763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J, Song Y, Liu D. Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia. J Hematol Oncol. 2019;12(1):17. doi: 10.1186/s13045-019-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guedan S, Posey AD Jr, Shaw C, Wing A, Da T, Patel PR, SE MG, Casado-Medrano V, Kawalekar OU, Uribe-Herranz M, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3(1). 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed]
- 75.Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, Carroll MP, Matsui WH, Wang Q, Dhodapkar MV, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. 2018;3(8):1–14. doi: 10.1172/jci.insight.120505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material supporting the conclusion of this review has been included within the article.