Abstract
Background
Dementia is a chronic condition which progressively affects memory and other cognitive functions, social behaviour, and ability to carry out daily activities. To date, no treatment is clearly effective in preventing progression of the disease, and most treatments are symptomatic, often aiming to improve people's psychological symptoms or behaviours which are challenging for carers. A range of new therapeutic strategies has been evaluated in research, and the use of trained animals in therapy sessions, termed animal‐assisted therapy (AAT), is receiving increasing attention.
Objectives
To evaluate the efficacy and safety of animal‐assisted therapy for people with dementia.
Search methods
We searched ALOIS: the Cochrane Dementia and Cognitive Improvement Group's Specialised Register on 5 September 2019. ALOIS contains records of clinical trials identified from monthly searches of major healthcare databases, trial registries, and grey literature sources. We also searched MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), CINAHL (EBSCOhost), ISI Web of Science, ClinicalTrials.gov, and the WHO's trial registry portal.
Selection criteria
We included randomised controlled trials (RCTs), cluster‐randomised trials, and randomised cross‐over trials that compared AAT versus no AAT, AAT using live animals versus alternatives such as robots or toys, or AAT versus any other active intervention.
Data collection and analysis
We extracted data using the standard methods of Cochrane Dementia. Two review authors independently assessed the eligibility and risk of bias of the retrieved records. We expressed our results using mean difference (MD), standardised mean difference (SMD), and risk ratio (RR) with their 95% confidence intervals (CIs) where appropriate.
Main results
We included nine RCTs from 10 reports. All nine studies were conducted in Europe and the US. Six studies were parallel‐group, individually randomised RCTs; one was a randomised cross‐over trial; and two were cluster‐RCTs that were possibly related where randomisation took place at the level of the day care and nursing home. We identified two ongoing trials from trial registries.
There were three comparisons: AAT versus no AAT (standard care or various non‐animal‐related activities), AAT using live animals versus robotic animals, and AAT using live animals versus the use of a soft animal toy. The studies evaluated 305 participants with dementia. One study used horses and the remainder used dogs as the therapy animal. The duration of the intervention ranged from six weeks to six months, and the therapy sessions lasted between 10 and 90 minutes each, with a frequency ranging from one session every two weeks to two sessions per week. There was a wide variety of instruments used to measure the outcomes. All studies were at high risk of performance bias and unclear risk of selection bias. Our certainty about the results for all major outcomes was very low to moderate.
Comparing AAT versus no AAT, participants who received AAT may be slightly less depressed after the intervention (MD –2.87, 95% CI –5.24 to –0.50; 2 studies, 83 participants; low‐certainty evidence), but they did not appear to have improved quality of life (MD 0.45, 95% CI –1.28 to 2.18; 3 studies, 164 participants; moderate‐certainty evidence). There were no clear differences in all other major outcomes, including social functioning (MD –0.40, 95% CI –3.41 to 2.61; 1 study, 58 participants; low‐certainty evidence), problematic behaviour (SMD –0.34, 95% CI –0.98 to 0.30; 3 studies, 142 participants; very‐low‐certainty evidence), agitation (SMD –0.39, 95% CI –0.89 to 0.10; 3 studies, 143 participants; very‐low‐certainty evidence), activities of daily living (MD 4.65, 95% CI –16.05 to 25.35; 1 study, 37 participants; low‐certainty evidence), and self‐care ability (MD 2.20, 95% CI –1.23 to 5.63; 1 study, 58 participants; low‐certainty evidence). There were no data on adverse events.
Comparing AAT using live animals versus robotic animals, one study (68 participants) found mixed effects on social function, with longer duration of physical contact but shorter duration of talking in participants who received AAT using live animals versus robotic animals (median: 93 seconds with live versus 28 seconds with robotic for physical contact; 164 seconds with live versus 206 seconds with robotic for talk directed at a person; 263 seconds with live versus 307 seconds with robotic for talk in total). Another study showed no clear differences between groups in behaviour measured using the Neuropsychiatric Inventory (MD –6.96, 95% CI –14.58 to 0.66; 78 participants; low‐certainty evidence) or quality of life (MD –2.42, 95% CI –5.71 to 0.87; 78 participants; low‐certainty evidence). There were no data on the other outcomes.
Comparing AAT using live animals versus a soft toy cat, one study (64 participants) evaluated only social functioning, in the form of duration of contact and talking. The data were expressed as median and interquartile ranges. Duration of contact was slightly longer in participants in the AAT group and duration of talking slightly longer in those exposed to the toy cat. This was low‐certainty evidence.
Authors' conclusions
We found low‐certainty evidence that AAT may slightly reduce depressive symptoms in people with dementia. We found no clear evidence that AAT affects other outcomes in this population, with our certainty in the evidence ranging from very‐low to moderate depending on the outcome. We found no evidence on safety or effects on the animals. Therefore, clear conclusions cannot yet be drawn about the overall benefits and risks of AAT in people with dementia. Further well‐conducted RCTs are needed to improve the certainty of the evidence. In view of the difficulty in achieving blinding of participants and personnel in such trials, future RCTs should work on blinding outcome assessors, document allocation methods clearly, and include major patient‐important outcomes such as affect, emotional and social functioning, quality of life, adverse events, and outcomes for animals.
Plain language summary
Animal‐assisted therapy for people with dementia
Review question
Do therapy sessions that involve live animals help people with dementia?
Background
Dementia is an increasingly common condition across the world. People with dementia have progressive loss of the ability to think, remember, and communicate; to manage their daily activities; and to mix successfully with other people. Many people with dementia also develop depression and related problems. To date, no treatment has proven able to cure the disease or stop it from getting worse. However, many treatments are in use which aim to improve the well‐being of people with dementia and the people who look after them. Animal‐assisted therapy (AAT) is one of the types of treatment that has been studied. It is thought that animals could help people with dementia by providing companionship and support in daily activities and that this might lead to improvements in physical and mental health, including better mood and fewer problematic behaviours.
Search date
We searched medical databases to September 2019.
Key characteristics of included studies
We included nine randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups), involving 305 people with dementia, which compared AAT to a control treatment (either usual care or an alternative treatment). All studies took place in Europe or the US. Seven studies compared AAT to usual care or to another activity which had nothing to do with animals. Two studies compared AAT (using live animals) to the use of robotic animals. One study compared AAT to the use of a soft toy cat. There were some features of the studies which could have biased the results. Study participants and care staff knew what treatment a person was receiving and this might have affected some results. Also, it was not always clear that the randomisation to treatments had been done as well as possible.
Funding sources
The studies received funding from various sources, including research grants (four studies), personal donation (one study), and support from an institute that promotes AAT (two studies). Two studies did not describe how they were funded.
Key results
We found evidence from two studies with 83 participants that people with dementia who had AAT were possibly slightly less depressed at the end of treatment than people who had standard care or other interventions not related to animals. We also found evidence from three studies with 164 participants that people who received AAT had no clear difference in their quality of life compared to those who did not. However, we found no evidence of an effect on social functioning (interactions with their environment and families), behaviour, agitation, activities of daily living, self‐care ability or balance. There were no clear differences when AAT was compared with the use of a robotic animal in two studies with 156 participants (in social functioning, behaviour, and quality of life), or with the use of a soft toy cat in one study with 64 participants (in social functioning). There were no data on harmful effects of the treatment on the participants and nothing was reported about the effect on the animals in any study.
Certainty of the evidence
We took several factors into account when deciding how certain we could be of our results. In this review, two main factors reduced our level of certainty. First, for all the outcomes we looked at, there was only a small number of studies and participants. Second, we thought there was a significant risk that all of the results could have been biased by the way the studies were designed or conducted. For a few outcomes, our confidence was also reduced by inconsistent results between studies. Overall, our certainty about the results ranged from very low to moderate.
Conclusions
AAT may slightly reduce depressive symptoms. Otherwise, no conclusions can yet be drawn on whether AAT is beneficial or safe for people with dementia. The small size of the included studies, and the diversity of outcomes and outcome measures, were major issues. We recommend further well‐conducted studies with the inclusion of important outcomes such as emotional and social well‐being, quality of life, side effects, and effects on the animals.
Summary of findings
Background
Description of the condition
The term dementia describes a collection of symptoms caused by disorders affecting the brain. Dementia is a chronic and progressive condition characterised by a deterioration in memory, cognitive, social, and daily functional abilities beyond what might be expected from normal ageing. According to a World Health Organization (WHO) report in 2017, five to eight per 100 people worldwide have dementia, with around 50 million people affected globally (WHO 2017a). It is estimated that the number of people with dementia worldwide will increase at a rate of 10 million per year, and that the total population with dementia will reach 82 million by 2030 and 152 million by 2050 (WHO 2017b), with most of the affected population from low‐ and middle‐income countries (WHO 2015). Dementia represents a major cause of disability and dependency among older adults, with the total global societal cost of dementia estimated to be around USD 820 billion, equivalent to 1.1% of the global gross domestic product (Prince 2015).
The most common cause of dementia is Alzheimer's disease, which affects 60% to 80% of people with dementia, followed by vascular dementia, mixed dementia, and dementia of Lewy bodies (ALZ 2018). Some types of dementia or dementia‐like symptoms are reversible, for example, alcohol and medication related dementia, dementia induced by depression, structural and surgically removable brain lesion such as tumours or haematoma, or metabolic disorders such as hypothyroidism (Tripathi 2009), but most are not. People who have dementia experience progressive worsening of symptoms, from occasional forgetfulness and disorientation in place and time, deterioration in self‐care and communication skills, to a total loss of mobility and the ability to recognise family members. Most people with dementia demonstrate behavioural changes characterised by repeated questioning, wandering, and aggressiveness. In the early stages, these changes may not be obvious as the symptoms tend to develop slowly. However, as the disease progresses, the symptoms become more evident as the decline in cognition and functional ability begins to interfere with the person's normal day‐to‐day activities. To date, no treatment has been identified that is clearly and consistently effective in preventing or halting progression of the disease (Chau 2016; Schwarz 2012). The major goals of currently available treatments are symptomatic, targeting challenging behaviour and psychological symptoms of patients, as well as their quality of life and that of their carers (NHS 2015). Animal‐assisted therapy (AAT) is one intervention that has been proposed to improve symptoms and possibly functional abilities in people who have dementia.
Description of the intervention
AAT refers to the use of an animal that is considered suitable to work with human care recipients in the treatment of human physical or psychological disorders, co‐ordinated by a human professional with indepth knowledge of the animal(s) involved and who has been formally certified (IAHAIO 2014). AAT is designed to promote improvements in human physical, social, emotional, or cognitive functions, and can be provided in individualised or group settings, with documentation and evaluation of the process and outcomes (AVMA 2018; Lefebvre 2008; Marino 2012). The use of animals in human therapy was first described in 1792 (McCulloch 1986). AAT as a treatment mode was formally introduced in 1969 by Dr Boris Levinson (Levinson 1969), a psychiatrist, who observed the interaction between a dog and a child with autism (Jacobs 2013). AAT for dementia has been documented since the 1990s (Behling 2011; Walsh 1995). Animals used in AAT for dementia include dogs and cats (Filan 2006; Motomura 2004), as well as aquatic animals (Filan 2006).
How the intervention might work
AAT has been reported to help in people with dementia by initiating social interaction in a controlled manner, which may lead to a decreased sense of loneliness and agitation (Banks 2002; LeRoux 2009; Sellers 2006). Increased levels of neurochemicals associated with relaxation and bonding have been reported in human recipients of AAT after treatment (Filan 2006). In terms of socio‐emotional aspects, AAT may benefit the care recipients by offering companionship to reduce boredom and the sense of isolation; providing pleasure, relaxation, and a source of motivation (Ohtani 2015); and by addressing unmet physical and emotional needs through joint participation in goal‐related activities (Ebener 2017). In one pilot survey, AAT appeared to be associated with increased muscle strength and range of movement; improved pain management; reduced blood pressure and heart rate; and increased responsibility, self‐esteem, and patient independence in nursing home residents (Darrah 1996). In some cohort studies, AAT has been reported to improve nutritional intake (Edwards 2002), reduce depression (Travers 2013), and reduce medication usage in older people with dementia (Lust 2007). It is unclear over what time frame AAT works best in people with dementia, although one study on AAT for institutionalised elderly people showed that it appeared to have different overall effects on the physical, cognitive, and emotional functions of the care recipients in the first six months and thereafter (Kawamura 2007). One Cochrane protocol on the use of AAT in people with serious mental illness uses a cut‐off of six months to define a short‐term outcome assessment period (Downes 2013).
We have constructed a logic framework that delineates the condition, its clinical symptoms and progression, possible or hypothesised consequences, and possible points of intervention where AAT may work, following the guidance by Kneale 2015 and the Cochrane Infectious Diseases Group (CIDG 2016). The logic framework is depicted in a flow diagram (Figure 1).
1.
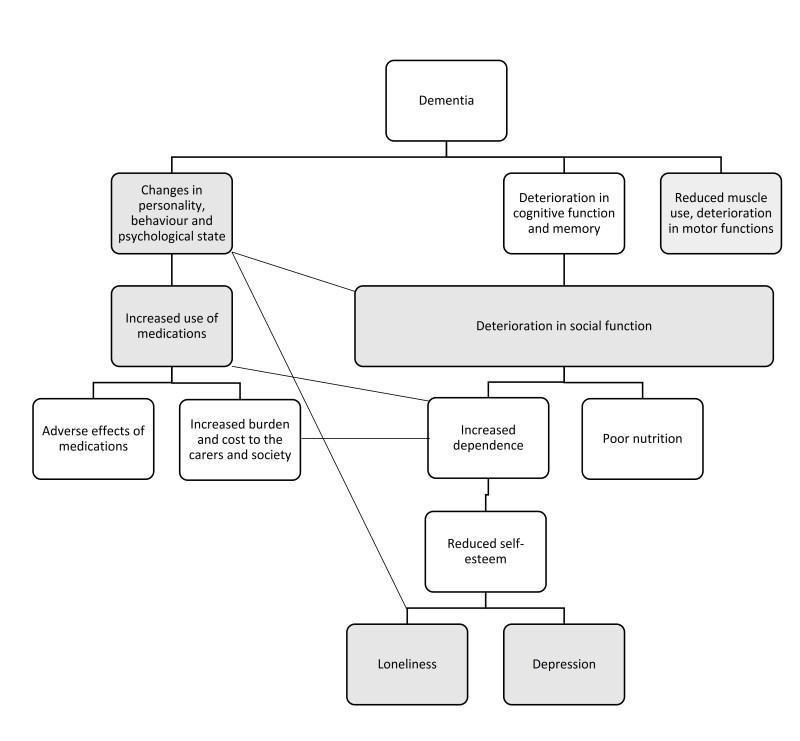
A logic framework model that depicts the progressive clinical manifestations and possible consequences of dementia, as well as possible points where animal‐assisted therapy may act, as shaded in grey.
Possible adverse effects of AAT include transmission of zootopic diseases, animal aggression, and compromised animal welfare. One report from Japan found that no zootopic diseases occurred among children with sickle cell disease and healthcare workers in a children's hospital where AAT was regularly used (Yamauchi 2008). To address the issues of animal welfare and aggression, policies and guidelines have been published by established institutions such as the American Veterinary Medical Association (AVMA) (AVMA 2018), and the International Association of Human–Animal Interaction Organizations (IAHAIO) (IAHAIO 2014). One study that measured the salivary cortisol level of therapy dogs as an indication of their stress level showed no major difference in levels between their working days and off days (Glenk 2014). The animal welfare and ethical issues associated with AAT have been studied and commented on (Glenk 2017; Hatch 2007).
Why it is important to do this review
The increasing number of people with dementia worldwide has been accompanied by an increased volume of dementia‐related research, including high‐quality research such as randomised controlled trials (RCTs) on various interventions to alleviate symptoms or to slow progression of the disease. Among the non‐pharmacological interventions studied, RCTs on AAT have been published since the 1990s and include published studies evaluating robotic animals (Sakairi 2004; Tamura 2004; Wada 2008). However, to date, there has been no systematic review of RCTs that has synthesised data specifically on AAT in people with dementia. The closest is a Cochrane protocol on AAT for people with serious mental illness, and the population will not include people with dementia (Downes 2013). It is important that relevant individual studies on AAT in people with dementia are synthesised in a rigorous manner with regular updates, as we plan to undertake here, to provide reliable and up‐to‐date guidance on practice, guideline and policy development, and future research.
Objectives
To evaluate the efficacy and safety of animal‐assisted therapy for people with dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, cluster‐RCTs (e.g. trials in which randomisation was performed at the level of nursing care home/assisted living facilities or at subunit level within these institutions), and randomised cross‐over studies.
Types of participants
We included studies that recruited participants with dementia, as defined by the study authors. The dementia could have been of any severity.
We performed sensitivity analyses to assess the impact on the pooled results of different methods used to identify dementia in participants, or of the inclusion of studies in which some participants may not have had dementia (e.g. mixed care home populations) (see Sensitivity analysis).
We included studies that enrolled participants living in the community or in any type of institution.
Types of interventions
Intervention
Any form of AAT, in which a live animal that was considered suitable to serve as companion to human care recipients was introduced with a specific therapeutic aim of improving symptoms and signs of dementia, with or without a concurrent role in providing assistance in daily activities (e.g. the use of guide dogs in facilitating memory training or physical activities as well as helping to retrieve daily items or crossing the road).
There needed to be a clear documentation of the intervention being co‐ordinated by a human healthcare provider with the appropriate expertise, as stated in the definition of AAT (see Description of the intervention). However, anticipating that the information would not be available in all potentially eligible studies, we accepted studies that provided any relevant description of animal involvement in therapeutic activities as mentioned above, with or without documentation on human co‐ordination. The intervention could have involved any species of animal, and could have been conducted in an individual or group setting.
We excluded studies that examined animal‐assisted activities alone (e.g. the use of guide dogs only for retrieving daily items or crossing the road), or pet ownership/companion animals, or the use of surrogates such as toys, robotic animals, or animals in digital applications as the main intervention of interest (although we accepted studies that compared the use of live animals with these surrogates).
We accepted any length and frequency (number of sessions per week) of therapy.
Comparison
Standard care only, or therapy intended to achieve the same goals in physical or mental functions without the involvement of animals, or another form of therapy being compared head‐to‐head with AAT, such as standardised physical or occupational therapy, or both.
We also planned to include trials that compared different forms of AAT, for example, using different species of animals.
Any concurrent interventions, such as the use of medication, non‐pharmacological treatment, and lifestyle changes, needed to be clearly stated and identical between the two groups.
Types of outcome measures
Among our predefined outcomes, 11 (including all six primary outcomes) related directly to the person with dementia, one to carers, and one to the therapy animal.
Primary outcomes
Affect and emotional well‐being, in particular, depression, as measured by suitable scales such as the Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos 1988) or Geriatric Depression Scale (GDS) (Yesavage 1982).
Social functioning, measured by suitable scales such as the Social Functioning in Dementia Scale (SF‐DEM); De Jong Gierveld Loneliness Scale (de Jong Gierveld 2006); Communication Observation Scale; and Multidimensional Observation Scale for Elderly Subjects (MOSES) Withdrawal subscale (Helmes 1987).
Overall behavioural and psychological symptoms of dementia (BPSD), measured with any validated instrument, for example, the Neuropsychiatric Inventory (NPI) (Cummings 1994).
Agitation and irritability, measured with any validated instrument, for example, Cohen‐Mansfield Agitation Inventory (CMAI) (Cohen‐Mansfield 1989), MOSES Irritability subscale (Helmes 1987).
Health‐related quality of life (HRQOL), using validated condition‐specific quality‐of‐life scales.
Adverse effects, including injuries or trauma.
Secondary outcomes
Physical functioning, such as activities of daily living (ADL), measured by validated tools such as: the Lawton Physical Self‐Maintenance Scale (PSMS) (Lawton 1969), Alzheimer's Disease Activities of Daily Living International Scale (ADCS‐ADL) (Galasko 1997); Gottries‐Brane‐Steen‐Skala, ADL subscale (GBS‐ADL) (Bråne 2001).
Cognitive functioning in different domains measured by validated scales, for example, global cognitive function, assessed with Alzheimer's Disease Assessment Scale – Cognitive subscale (ADAS‐cog) (Rosen 1984) or Mini‐Mental State Examination (MMSE) (Folstein 1975), or other global measures of cognition.
Overall dementia severity measured by validated tools such as: Clinical Dementia Rating Scale – Sum of Boxes (CDR‐SOB) (O'Bryant 2008) or Alzheimer's Disease Cooperative Study – Clinical Global Impression of Change (CIBIC‐Plus) (Schneider 1997).
Mortality.
Rates of institutionalisation.
Carer satisfaction and stress.
Animal outcomes: physical, emotional, and other outcomes assessed for the animals involved, including animal injuries or trauma, or other adverse effects.
We accepted all outcomes assessed at variable time points throughout the conduct of the study, including short‐term (less than six months) and long‐term (six months or longer) periods. We recorded the period of outcome assessment in the Characteristics of included studies table and classified it as short‐ or long‐term. If there was substantial heterogeneity in our results, as detailed under the Assessment of heterogeneity section, we considered the dose of intervention (including session frequency, length of sessions, and duration of intervention) as part of our assessment for possible causes of heterogeneity, to decide whether or not to pool data.
Search methods for identification of studies
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group's Specialised Register, on 5 September 2019. ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia (prevention and treatment), mild cognitive impairment, and cognitive improvement. The studies were identified from the following databases from their inception date to 5 September 2019.
Monthly searches of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO, and LILACS.
Monthly searches of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others).
Quarterly search of the Cochrane Library's Central Register of Controlled Trials (CENTRAL).
Six‐monthly searches of grey literature sources from ISI Web of Science Core Collection.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website (www.medicine.ox.ac.uk/alois).
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL, and conference proceedings can be viewed on the Dementia and Cognitive Improvement Group's website (dementia.cochrane.org/our‐trials‐register).
We performed additional searches in many of the sources listed above, to cover the time frame from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible.
We describe the search strategies used in Appendix 1. We carried out the most recent search on 5 September 2019.
Additionally, we searched animal‐based journals, including Anthrozoos, Animals, Animal Behaviour, Applied Animal Behaviour Science, Journal of Animal Science and Technology, and Journal of Animal Health and Behavioural Science using the terms 'animal‐assisted', 'animal‐facilitated', 'pet‐assisted', and 'pet‐facilitated'.
We did not limit the language of the studies included in our review. For non‐English studies, we enlisted the help of a translator via the Cochrane Task Exchange platform to translate the essential information of the studies into English (taskexchange.cochrane.org/).
Searching other resources
We contacted the authors of relevant trials to request details of any additional unpublished or ongoing studies that might meet the inclusion criteria for this review. We also reviewed the reference lists and citations of retrieved articles to look for additional trials for inclusion.
Data collection and analysis
Selection of studies
We used standard Cochrane methods, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Two review authors (NML and SMWC) independently screened for potentially eligible studies by inspecting the titles and abstracts to generate a shortlist. Two review authors (NML and SSN) then independently inspected the abstracts or full texts, or both, of these short‐listed studies further to determine final eligibility, using the predefined inclusion and exclusion criteria. We resolved any disagreement with the help of a third review author (FS) who acted as an arbiter. We delineated the study selection process in a PRISMA diagram.
We included published and unpublished studies available in full‐text article or abstract form, and contacted the authors of unpublished studies and studies available only as abstracts to request additional information not provided in the available reports, including details such as: methods of sequence generation, allocation and blinding, participant withdrawal and prespecified outcomes, and full outcome data. If we found multiple reports of the same study, we grouped them under a single study ID, and assigned the report with the most amount of relevant information as the primary publication. We summarised any studies excluded after full‐text assessment and their reason for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors (SMWC and NML) independently extracted and coded all data from each included study using a dedicated data collection form, after an assessment of its usability via a round of piloting on five included studies. We collected study characteristics, including study design, setting, country, methods of allocation, participants, interventions, comparators, outcomes, sponsorship details, declaration of interests of the primary investigators, methods used to control possible conflicts of interests, and other information considered relevant according to Section 7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We resolved potential discrepancies through discussion and involved a third review author if necessary. In case of language ambiguity that remained after translation, we contacted researchers in the field familiar with the language in question, or the study authors for clarification if necessary.
We extracted the outcome data using an electronic data collection form. For continuous data, we extracted the mean value of the outcome measurement in each group at each time point (or, if this was unavailable, the mean change from baseline), the standard deviation (SD) values, and the number of participants used to measure the outcome for each group. For dichotomous outcomes, we extracted the number of participants in each outcome group at each time point. We contacted the study authors to obtain important missing data. If the study report only provided the summary effect sizes (e.g. risk ratio (RR) for dichotomous data and mean difference (MD) or standardised mean difference (SMD) for continuous data), we extracted those measures as well as the accompanying standard errors (SE) or 95% confidence intervals (CI) to prepare the data for combination via the generic inverse variance method. Had there been studies that provided the outcome data in figures or graphs without accompanying annotation or numerical report, we would have attempted to estimate the data from the figures using Plot Digitizer software (Jelicic Kadic 2016; Vucic 2015). Once the data was collected, one review author (SMWC) transferred the data to Review Manager 5 software (Review Manager 2014), and a second review author (NML) checked the accuracy of the data entry.
Assessment of risk of bias in included studies
Two review authors (NML and FS) independently assessed each included study for risk of bias according to the following six criteria, in accordance with the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Sequence generation.
Allocation concealment.
Blinding.
Incomplete outcome data.
Selective outcome reporting.
Other issues (e.g. extreme baseline imbalance).
For cluster‐RCTs and cross‐over trials, we included additional 'Risk of bias' domains under 'other bias', as follows (Higgins 2011c).
Cluster‐randomised controlled trials
Was there evidence of further recruitment of participants into the clusters after randomisation ('recruitment bias')?
Was there clear evidence of baseline imbalance between randomised clusters?
Was there evidence of loss of clusters in addition to the loss of participants after trial commencement?
Was there a unit of analysis error (i.e. failure to adjust for the clustering effect)? (for details, see the Unit of analysis issues section)
Cross‐over trials
Was the use of cross‐over design appropriate?
Can it be assumed that the trial was not biased from carry‐over effects?
Are unbiased data (e.g. data from both periods of the trial, data with removal of dropout from any one period) presented?
We made a judgement of low, high, or unclear risk of bias, with justifications based on the information obtained from the papers. We completed a 'Risk of bias' table for each eligible study and presented our overall 'Risk of bias' assessment using a 'Risk of bias' graph and 'Risk of bias' summary. We resolved any disagreements by discussion to achieve a consensus.
Measures of treatment effect
We reported the pooled outcome estimates for categorical data in relative terms using RRs, and also in absolute terms using risk differences (RDs). For continuous data, we reported MDs with their respective 95% CIs, if all data were of the same measurement scale. For continuous outcome data in different measurement scales that measured the same construct, we combined them using the SMD with their respective 95% CIs. If pooled analyses were not possible due to reasons such as major discrepancies in study characteristics or outcome reporting, as detailed under the Assessment of heterogeneity section, we reported the results of the studies individually.
Unit of analysis issues
For cluster‐RCTs (e.g. trials in which the assignment to intervention or control group was made at the level of the institution), we assessed whether adjustment had been made for the effects of clustering in order to account for non‐independence among the participants in a cluster via the use of an appropriate analysis model such as a Generalised Estimating Equation (GEE) model. If the study authors did not state the unit of analysis, we inspected the width of the SE or 95% CI of the estimated treatment effects. If we found inappropriately small SEs or narrow 95% CIs, we contacted the study authors to request information on the unit of analysis.
If no adjustment had been made for the effects of clustering, we performed adjustment by multiplying the SEs of the final effect estimates by the square root of the 'design effect', represented by the formula 1 + (M – 1) × ICC, where M was the mean cluster size (number of participants per cluster) and ICC was the intracluster correlation. We determined the mean cluster size (M) from each trial by dividing the total number of participants by the total number of clusters. We used a relatively large assumed ICC of 0.10, which is a commonly used and considered a realistic general estimate (Campbell 2001). We combined the adjusted final effect estimates from each trial with their SEs in meta‐analysis using generic inverse‐variance methods, as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
If determination of the unit of analysis or adjustment was not possible, we planned to include the studies concerned in a meta‐analysis using the effect estimates reported by the study authors. We would then have performed a sensitivity analysis to assess how the overall results were affected by these studies.
The review includes two cluster‐RCTs (Olsen 2016a; Olsen 2016b). In both studies, adjustment of the final estimate was impossible, as the estimates were reported as changes within each group, rather than differences between groups. We have therefore extracted the outcome data as reported by the authors. We separated the included studies into subgroups of individually randomised trials and cluster‐RCTs, in accordance with our strategy as detailed in the Subgroup analysis and investigation of heterogeneity section. We were then able to assess the pooled estimates with and without the inclusion of the cluster‐RCTs, without the need to perform sensitivity analysis as stated originally in our protocol. In case of substantial heterogeneity in the pooled estimates that involved a cluster‐RCT, we explored the possibility of the cluster‐RCT design being a plausible contributor to heterogeneity, as detailed in our results under the Effects of interventions (comparison 1: animal‐assisted therapy versus no animal‐assisted therapy, primary outcome number 4: agitation and irritability, analysis 1.6).
For cross‐over studies, our strategy for data analysis depended on the risk of bias judgement of the included study. If we considered the included study to have low risk of bias across all three additional domains specific for cross‐over trials, as detailed under the Assessment of risk of bias in included studies section, we included data from both phases of the trial, namely, before and after the cross‐over. In such cases, we attempted to extract paired data from each participant if available. If we judged the trial to have unclear or high risk of bias in any of the additional risk of bias domain, we only used data from the first phase before the cross‐over took place. If the results were not reported separately for each phase, we still pooled the overall result but evaluated the impact of excluding such studies via sensitivity analyses. Additionally, if data from both phases were reported separately but no paired data were extractable, we also pooled the overall results and conducted sensitivity analysis to assess the impact of such studies.
Dealing with missing data
We followed the recommendations in Section 8.13.2 in the Cochrane Handbook for Systematic Reviews of Intervention in assessing the risk of bias from incomplete outcome data (Higgins 2011e).
We performed our analyses for all outcomes, where possible, using intention‐to‐treat (ITT) data (analysed according to randomisation, irrespective of subsequent discontinuation of the study or deviation from the protocol, if the outcome data of these participants were available or were imputed by the study authors). If there were missing outcome data that were not imputed, we would have performed a modified ITT analysis (analysed according to randomisation with only available outcome data and without the missing data) (Higgins 2019). If ITT data were not provided, we included outcome data of the participants either in a 'per protocol' or 'as treated' manner, as provided by the study authors, but made a corresponding note in the Characteristics of included studies table.
If sufficient studies were available, we performed sensitivity analyses to assess how the overall results were affected by the inclusion of studies with a high risk of attrition bias from incomplete outcome data, and studies that did not provide ITT data.
Assessment of heterogeneity
We used the I2 statistic to quantify the degree of inconsistency in the results (Higgins 2011d), with a cut‐off of 50% and above considered as the level at which the degree of heterogeneity was of sufficient concern to justify an exploration of possible explanations. In such a situation, we evaluated studies in terms of their clinical and methodological characteristics using the following criteria to determine whether the degree of heterogeneity may be explained by differences in those characteristics, and whether a meta‐analysis was appropriate.
We assessed the following criteria.
Characteristics of the participants (e.g. age, type, and severity of dementia).
Settings of the studies (e.g. community or institution).
Interventions (type of animal, dosage (intensity or duration of therapy)).
Risk of bias (as detailed in the Assessment of risk of bias in included studies section).
If we identified any of the above‐mentioned factors during our exploration that we considered to be a plausible explanation of the observed heterogeneity, we separated the studies into subgroups according to the factors concerned if there were sufficient studies in each subgroup. In the case of risk of bias, we conducted sensitivity analyses excluding the studies at higher risk of bias.
Assessment of reporting biases
We planned to use a funnel plot and Egger's test to screen for publication bias if there were at least 10 studies included in the analysis of the relevant outcomes (Egger 1997). If publication bias was suggested by significant asymmetry of the funnel plot, we included a statement in our results with a corresponding note of caution in our discussion, bearing in mind that funnel plot asymmetry does not necessarily equate to the presence of publication bias. If possible, we compared conference abstracts and available trial protocols of included studies with published data.
Data synthesis
We performed meta‐analyses if there were at least two studies with broadly similar population, intervention, comparison, and outcome (PICO) measures, using a random‐effects model in Review Manager 5 (Review Manager 2014). Our primary data analyses followed the ITT principle; namely, we analysed all participants in whom relevant outcome data were available in the group originally allocated. We expressed our results as RRs, RDs, number needed to treat for an additional beneficial outcome (NNTB), number needed to treat for an additional harmful outcome (NNTH), and MDs with their respective 95% CIs, as detailed in the Measures of treatment effect section. For cluster‐RCTs, our proposed methods of analysis are detailed in the Unit of analysis issues section.
If there were substantial differences between the characteristics of the PICO measures that precluded a meta‐analysis, we summarised the results of the studies narratively.
Subgroup analysis and investigation of heterogeneity
Apart from the assessment of heterogeneity and subgroup analysis as detailed in the Assessment of heterogeneity section, we conducted the following subgroup analyses, if data are available.
Type of studies
Individually randomised versus cluster‐randomised trials.
Population
Setting: community versus institution (such as care home).
Stage of dementia, differentiating very mild, mild, moderate, and severe dementia, as defined by validated tools such as the CDR‐SOB (O'Bryant 2008).
Type of dementia.
Intervention
Individual versus group therapy.
Use of different animals, each species forming an individual subgroup.
Intensity ('dosage') of intervention: three or more versus fewer sessions per week.
'Summary of findings' table
We developed a 'Summary of findings' table highlighting the certainty of the evidence using the GRADE approach for our major outcomes as listed below. We used the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the evidence for each of these outcomes based on the body of evidence generated by the studies that contributed data to the meta‐analyses.
Specifically, for the criterion of study limitations, we made the decision on the overall risk of bias across the pool of relevant studies that contributed to each specific outcome rated on two levels: 1. determining the overall risk of bias of any single study, and 2. determining the risk of bias across the pool of relevant studies (namely, overall study limitation). To determine the overall risk of bias of any single study, we assigned the overall risk of bias status of the single study according to the worst risk of bias domain that was relevant to the specific outcome, apart from the domain of selective outcome reporting. To determine the risk of bias across the pool of relevant studies, we referred to the guideline as detailed in Table 42.2.d of the Cochrane Handbook for Systematic Reviews of Intervention (Schünemann 2011).
1. Outcome data for Thodberg 2016.
| Group | Duration (seconds; median (IQR)) | ||
| AAT using dog | Robot seal | Toy cat | |
| Number of participants | 34 | 34 | 30 |
| Outcome: physical contact | 93 (1–213) | 28 (0–309) | 0 (0–48) |
| Outcome: talk directed at a person | 164 (41–265) | 206 (123–403) | 297 (128–338) |
| Outcome: talk in total | 263 (41–428) | 307 (162–474) | 298 (128–338) |
AAT: animal‐assisted therapy; IQR: interquartile range.
If we identified an issue in any of the five GRADE criteria that we considered to pose a serious enough risk to influence the outcome estimate, we downgraded the certainty of evidence by one level, and when we considered the issue to be very serious, we downgraded the certainty of evidence by two levels (Schünemann 2011). Whenever we decided to downgrade the certainty of evidence from the default high certainty, we justified our decision and described the level of downgrading in the footnotes of the table. We constructed the 'Summary of findings' table using an Internet‐based version of GRADEpro software (GRADEpro GDT 2015), according to the methods and recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011f).
We included the following outcomes in the 'Summary of findings' table, regardless of the availability of data.
Depression, measured by validated scales such as CSDD or GDS.
Social functioning, measured by validated scales such as the SF‐DEM or De Jong Gierveld Loneliness Scale.
Overall BPSD, measured with any validated instrument, for example, the NPI (Cummings 1994).
Agitation and irritability, measured with any validated instrument, for example, CMAI (Cohen‐Mansfield 1989) and MOSES Irritability subscale (Helmes 1987).
HRQOL, measured using validated condition‐specific quality of life scales.
ADL measured by suitable scales such as the Lawton PSMS.
Adverse events.
Sensitivity analysis
If a sufficient number of studies were available, we planned to perform sensitivity analyses for the primary outcomes and secondary outcomes to assess the impact on pooled results of excluding studies based on the following characteristics.
-
High risk of bias:
high risk of selection bias (for either criterion or both criteria of random sequence generation and allocation concealment);
high risk of attrition bias (incomplete outcome data);
studies reporting non‐ITT data only.
-
Participant factors:
studies that did not use recognised criteria to identify dementia (e.g. the Diagnostic and Statistical Manual of Mental Disorders V (DSM‐V) or previous editions of DSM (APA 2013), the International Classification of Diseases 10 (ICD‐10) or previous editions of ICD (WHO 2016), National Institute of Neurological Disorders and Stroke/Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS‐AIREN) Criteria for the Diagnosis of Vascular Dementia (Román 1993), or National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) Alzheimer's criteria (McKhann 2011);
studies which may have included some participants without dementia (e.g. mixed care home populations).
-
Intervention factors:
studies that did not clearly document involvement of an appropriately trained human facilitator.
Results
Description of studies
Results of the search
The initial search through the ALOIS repository performed by the Cochrane Dementia and Cognitive Improvement group Information Specialist, identified 3010 records with 2355 records remaining after removing duplicates. Of these, 80 articles appeared to be relevant after we inspected the titles. We further evaluated these 80 articles by reading the abstracts, excluding 45 records in the process. We assessed the full‐texts of the remaining 35 articles to determine final eligibility, and included nine articles in our analyses. Two included studies, one in abstract form (Holthoff 2013) and one in full text (Quibel 2017) did not contribute quantitative data in our meta‐analysis, leaving seven studies available for quantitative analysis. We identified two relevant on‐going studies with no results posted in the trial registry website (ISRCTN93568533; NCT02829801). The flow diagram of the studies from the initial search to the meta‐analysis is shown in Figure 2. We describe all the included studies in the Characteristics of included studies table and provide a brief description of the studies excluded after inspection of their full‐texts, with the reason for exclusion, in the Characteristics of excluded studies table.
2.
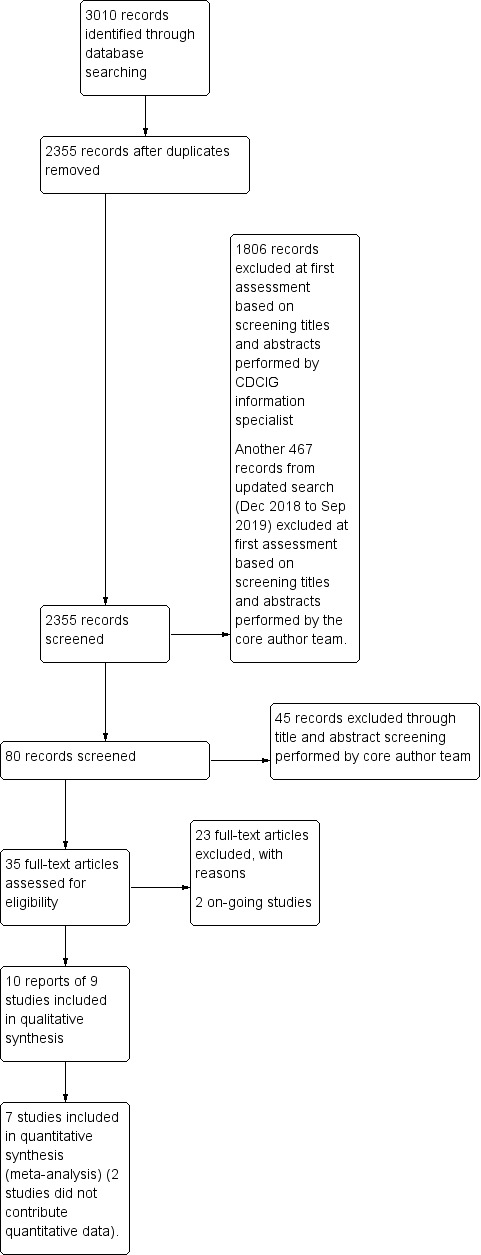
Study flow diagram. CDCIG: Cochrane Dementia and Cognitive Improvement Group.
Included studies
We included nine eligible studies: six parallel‐group, individually‐randomised RCTs (four two‐arm (Friedmann 2015; Holthoff 2013; Quibel 2017; Zisselman 1996) and two three‐arm (Thodberg 2016; Valenti‐Soler 2015)), one randomised cross‐over trial (Dabelko‐Schoeny 2014), and two separate but possibly related cluster‐RCTs (Olsen 2016a; Olsen 2016b). The studies were conducted in six countries, including USA (three studies); Norway (two studies); and Germany, France, Spain, and Denmark (one study each). The number of participants recruited ranged from 12 (Quibel 2017) to 124 (Thodberg 2016).
Setting and population
All studies included participants of both sexes, except Quibel 2017 in which participants were all women. The mean age of the participants ranged from around 76 years (Zisselman 1996) to 88.5 years (Holthoff 2013).
Participants were recruited from adult day services (two studies), assisted living facilities or nursing homes (four studies), a unit specifically catering for people with dementia (two studies) or a psychiatry unit in a hospital (one study). All studies recruited participants with dementia either exclusively (six studies), or among older adults with other psychological conditions (three studies). Three of the six studies that reported including only participants with dementia specified the criteria used to identify dementia: MMSE score below 25 (Olsen 2016a; Olsen 2016b) or below 23 (Friedmann 2015), or diagnosis of Alzheimer's disease (Dabelko‐Schoeny 2014).
Two studies were conducted by the same principal author over a similar period (Olsen 2016a; Olsen 2016b). However, the participants differed (home dwelling people in one and nursing home residents in another) and the studies were registered as separate studies under ClinicalTrial.gov. Although they appeared to be two separate studies, we could not exclude the possibility of overlapping participants, hence double‐counting in the outcome data, as we have not received a reply from the authors. However, we considered the possibility of double‐counting to be small and only one outcome of quality of life (measured using the Quality of Life in Late‐Stage Dementia scale (QUALID)) could have been affected. Therefore, we reported the outcome data of the participants in these two trials as if they were non‐overlapping, but have incorporated a corresponding explanation under the heading of the outcome and in Table 1.
Summary of findings for the main comparison. Animal‐assisted therapy (AAT) compared to no AAT for dementia.
| AAT compared to no AAT for dementia | ||||||
| Patient or population: dementia Setting: nursing home or assisted‐living facilities Intervention: AAT Comparison: no AAT (standard care, reminiscing activities, cooking, or exercise therapy) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no AAT | Risk with AAT | |||||
| Affect and emotional well‐being: depression measured using CSDD (0–19, higher score indicates more severe depressive symptoms) | The mean score for the control group ranged from 8.76 to 9.58 | MD 2.87 lower (5.24 lower to 0.50 lower) | — | 83 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Social functioning measured using MOSES, Withdrawn Behaviour subscale (8‐ 32, higher score indicates more severe withdrawn behaviour) | The mean score MOSES, Withdrawn Behaviour subscale was 20 | MD 0.4 lower (3.41 lower to 2.61 higher) | — | 58 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — |
| Behaviour measured using NHBPS (0–116, higher score indicates more severe behaviour problem), NPI (0–144, higher score indicates more severe neuropsychiatric symptoms) or MOSES Disoriented Behaviour subscale (8–32, higher score indicates more severe disoriented behaviour) | The mean scores for the control group, expressed using different instruments, were: NHBPS: 3.75; NPI: 28.66; MOSES Disoriented Behaviour subscale: 15.4 | SMD 0.34 lower (0.98 lower to 0.30 higher) | — | 142 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | As each of the 3 studies used different instrument to measure the outcome, the pooled estimates were expressed using SMD. |
| Agitation or irritability measured using CMAI (14–70, higher score indicates more severe agitation or irritability), BARS (10–70, higher score indicates more severe agitation or irritability) or MOSES Irritability subscale (9–36, higher score indicates more severe irritability) | The mean scores for the control group, expressed using different instruments, were: CMAI: 20; BARS: 24; MOSES Irritability subscale: 13.7 | SMD 0.39 lower (0.89 lower to 0.1 higher) | — | 143 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | As each of the 3 studies used different instrument to measure the outcome, the pooled estimates were expressed using SMD. |
| Health‐related quality of life measured using QUALID (12–45, higher score indicates poorer quality of life) | The mean quality of life (QUALID) was 15.23 to 26.48 | MD 0.45 higher (1.28 lower to 2.18 higher) | — | 164 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | 2/3 included studies were conducted by the same principal author over a similar period. However, the participants differed (home dwelling people in 1 and nursing home residents in 1) and the studies were registered as separate studies under ClinicalTrials.gov. Although they appeared to be 2 separate studies, we could not exclude the possibility of overlapping participants, hence double‐counting in the outcome data, as we have not heard back from the authors. However, we considered the possibility of double‐counting to be small. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| Physical functioning, measured using Barthel Index for ADL (0–100, higher score indicates better abilities) | The mean score from Barthel Index for ADL was 71.83 | MD 4.65 higher (16.05 lower to 25.35 higher) | — | 37 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — |
| Physical functioning: self‐care ability measured using MOSES Self‐Care Functioning subscale (8–32, higher score indicates poorer function) | The mean score on self‐care ability measured using MOSES Self‐Care Functioning subscale was 14.1. | MD 2.2 higher (1.23 lower to 5.63 higher) | — | 58 (1 RCT) | ⊕⊕⊝⊝ Lowa,e | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is taken from the total event rate (dichotomous outcome) or the range of mean scores (continuous outcome) in the comparison group of the included studies. AAT: animal‐assisted therapy; ADL: activities of daily living; BARS: Brief Agitation Rating Scale; CI: confidence interval; CMAI: Cohen‐Mansfield Agitation Inventory; CSDD: Cornell Scale for Depression in Dementia; MD: mean difference; MOSES: Multidimensional Observation Scale for Elderly Subjects; NHBPS: Nursing Home Behaviour Problem Scale; NPI: Neuropsychiatric Inventory; QUALID: Quality of Life in Dementia; RCT: randomised controlled trial; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level. The included studies had unclear risk of selection bias and high risk of performance bias. bDowngraded one level. The 95% CI ranged from a moderate reduction in depressive symptoms to virtually no difference with a small sample size from a single study, which is likely to translate into different decisions if either was the true effect. cDowngraded one level. The 95% CI ranged from substantially lower (reflecting meaningful benefit) to substantially higher (reflecting meaningful harm) scores, which is likely to translate into different decisions if either was the true effect. dDowngraded one level. Substantial degree of heterogeneity present as suggested by an I2 greater than 50%. eDowngraded one level. The 95% CI ranged from a moderately lower (reflecting meaningful benefit) to substantially higher (reflecting meaningful harm) score, which is likely to translate into different decisions if either was the true effect.
Intervention and comparison
We organised the included studies into three comparisons.
Comparison 1: AAT versus no AAT (standard care or an alternative non‐animal‐related intervention) (Dabelko‐Schoeny 2014; Friedmann 2015; Holthoff 2013; Olsen 2016a; Olsen 2016b; Quibel 2017; Zisselman 1996).
Comparison 2: AAT (live animals) versus robotic animals (Thodberg 2016; Valenti‐Soler 2015).
Comparison 3: AAT (live animals) versus a soft toy (Thodberg 2016).
Eight studies used dogs as the therapy animals, and one used horses (Dabelko‐Schoeny 2014). Three studies mentioned the type of dog used as Cardigan Welsh Corgi (Friedmann 2015), retriever of a retriever mix (Thodberg 2016), and black Labrador retrievers (Valenti‐Soler 2015). In studies that used dog‐assisted therapy, the therapy sessions lasted between 10 and 90 minutes each, with a frequency ranging from one session every two weeks to twice per week. The total duration of the intervention ranged from six weeks to six months. In the single study that used horses, the sessions took place one day per week (duration not specified) for four weeks. Five studies stated the number of participants per group per session, ranging from three to 10 participants per group.
The type of activities in the therapy sessions varied. Interventions involved a brief visit to the participants with simple interaction with the animal (Thodberg 2016; Zisselman 1996); more elaborate activities, such as feeding, grooming, and dressing the animals (Dabelko‐Schoeny 2014; Friedmann 2015); a predesigned but flexible set of activities that aimed to promote motor and social activities assisted by the therapy dogs in the presence of human facilitator/s (Friedmann 2015; Holthoff 2013; Olsen 2016a; Olsen 2016b); or a more strictly structured therapeutic programme with different sets of activities at different difficulty levels (Valenti‐Soler 2015).
In terms of facilitator training and certification, there were clear statements in two studies that the facilitators received specific training and were certified to work with the animals used in the studies (Dabelko‐Schoeny 2014; Valenti‐Soler 2015). Two studies mentioned that the facilitators were "qualified dog‐handlers" (Olsen 2016a; Olsen 2016b), and one study mentioned that the dog owners were certified to work as volunteers with dog‐assisted interventions in nursing homes (Thodberg 2016). There was no specific mention of the training status or experience of the human facilitators in the remaining four studies (Friedmann 2015; Holthoff 2013; Quibel 2017; Zisselman 1996).
In studies that compared AAT with no AAT, the activities of the control group, where documented, included "reminiscing" activities with training on social and motor skills (Friedmann 2015), cooking workshops (Quibel 2017), exercise therapy (Zisselman 1996), "standard care" or "treatment as usual" (Dabelko‐Schoeny 2014; Holthoff 2013; Olsen 2016b). Two studies did not clearly state the activities of the control group (Olsen 2016a; Valenti‐Soler 2015).
Outcomes
In the comparison of AAT with no AAT, one or more studies evaluated all our prespecified primary outcomes, except for adverse effects. Two studies assessed depression (affect and emotional well‐being) (Friedmann 2015; Olsen 2016b) using the CSDD. A single study evaluated apathy (Friedmann 2015), depressed or anxious mood (affect and emotional well‐being) and withdrawn behaviour (social functioning) (both measured as different MOSES subscales) (Zisselman 1996). Three studies measured overall behaviours, each using different instruments, including the Nursing Home Behaviour Problem Scale (NHBPS; Dabelko‐Schoeny 2014), NPI (Valenti‐Soler 2015), and MOSES Disoriented Behaviour subscale (Zisselman 1996). Similarly, three different studies assessed agitation and irritability using three different instruments, including the CMAI (Friedmann 2015), Brief Agitation Rating Scale (BARS) (Olsen 2016b), and MOSES Irritability subscale (Zisselman 1996). Three studies evaluated quality of life using the quality of life score tailored for people with dementia (QUALID) (Olsen 2016a; Olsen 2016b; Valenti‐Soler 2015).
Of our prespecified secondary outcomes, the studies comparing AAT to no AAT assessed only physical functioning. However, each of the three studies assessed different aspects of physical functioning, including ADL (Barthel Index) (Friedmann 2015), balance (Berg's Balance Scale) (Olsen 2016a), and self‐care ability (MOSES Self‐Care Functioning subscale) (Zisselman 1996). No included studies assessed the other prespecified secondary outcomes, including cognitive function, overall dementia severity, mortality, rates of institutionalisation, carer satisfaction and stress, and animal‐level outcomes.
In the comparison of AAT using live animals with the use of robotic animals, the first study evaluated two outcomes, behaviour measured using the NPI and quality of life measured using the QUALID (Valenti‐Soler 2015). The second study evaluated social function in the form of duration of contact and talking, although the outcome data from this study are presented separately in Table 4 as they were reported as median and interquartile ranges (Thodberg 2016).
In the comparison of AAT using live animals with use of a soft toy animal, the single study measured only social functioning in the form of duration of contact and talking (Thodberg 2016). These results were reported as median and interquartile ranges and are presented in Table 4.
All outcomes were assessed mostly in the short‐term, immediately after the intervention period, ranging from day five (Zisselman 1996) to four weeks (Dabelko‐Schoeny 2014), six weeks (Thodberg 2016), or 12 weeks (Friedmann 2015; Olsen 2016a; Olsen 2016b; Valenti‐Soler 2015). The only study that assessed outcomes beyond our prespecified short‐term period of six months was Holthoff 2013, which assessed outcomes at six, 12, and 15 months after the commencement of the intervention. There was no clear statement on the time point of the final measurement in Quibel 2017, although it was stated that the outcomes were assessed every two weeks. Two studies did not contribute to our meta‐analysis due to the lack of extractable quantitative outcome data (Holthoff 2013; Quibel 2017).
Sources of funding
The studies reported various sources of funding, including a university research grant (Olsen 2016a; Olsen 2016b), national research grant (Valenti‐Soler 2015), external research grant (Friedmann 2015), personal donation (Dabelko‐Schoeny 2014), and external funding from a company that promoted the use of animals to help improve human well‐being, which might pose a concern for potential conflict of interest (Thodberg 2016; Zisselman 1996). The remaining two studies did not state funding sources (Holthoff 2013; Quibel 2017).
Excluded studies
We excluded 23 articles based on one or more of the following reasons.
Relevance of topic (two articles): although the titles were suggestive, the topics examined were unrelated to AAT.
Study design or article type (17 articles): the studies were single‐group, pre‐and‐post, repeated measure, or observational studies (five studies); or non‐randomised comparative studies including matched case control studies (five studies); or 1 commentary on the roles of animals in entertainment.
Population (four articles): the participants in the studies were either not cognitively impaired or with conditions unrelated to dementia.
Intervention (two articles): the studies assessed personalised engagement time of different forms, without the use of animals.
A description of each study is available in the Characteristics of excluded studies table.
Risk of bias in included studies
The proportions of studies with low, high, and unclear risks of bias in each domain is illustrated in Figure 3, and the risk of bias judgement of each included study in each domain is depicted in Figure 4. Overall, there was a wide variation in the risks of bias of the studies across six domains, but there were serious concerns in the major domains of allocation concealment and blinding of participants and personnel, as all studies had unclear risks of bias in the former and high risk of bias in the latter. We provided a detailed description of the risk of bias of each study in the Characteristics of included studies table. We summarised our risk of bias assessments for each domain below.
3.
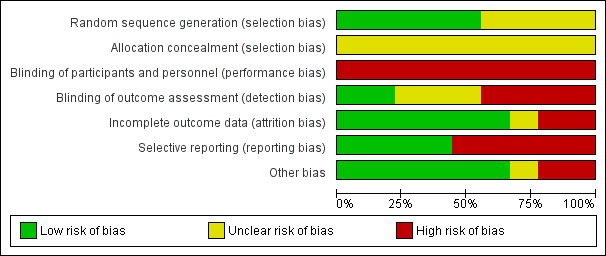
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
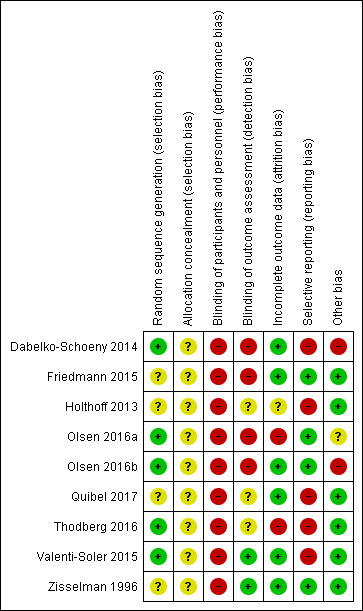
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Half of the included studies had low risks of bias in random sequence generation, while the other half had unclear risks of bias. All studies had unclear risks of bias in allocation concealment. Two studies showed some clear differences in baseline characteristics between the groups (Dabelko‐Schoeny 2014; Olsen 2016a); however, we were unclear whether the differences in baseline characteristics would translate into important differences in the outcome, for various reasons as detailed in the 'Risk of bias' table under the domain of allocation concealment. One study, published in abstract, did not provide a list of baseline characteristics between the groups (Holthoff 2013), and in the remaining studies, there were no marked differences in the baseline characteristics between the groups. Overall, we considered the risk of selection bias in this review as unclear.
Blinding
All included studies had high risks of performance bias, while risk of assessment bias varied: low (Valenti‐Soler 2015; Zisselman 1996), unclear (Holthoff 2013; Quibel 2017; Thodberg 2016), and high (Dabelko‐Schoeny 2014; Friedmann 2015; Olsen 2016a; Olsen 2016b). Overall, we considered the risk of bias from the domain of blinding in this review as high.
Incomplete outcome data
Six studies had low risk of bias in incomplete outcome data, while one study had unclear risk (Holthoff 2013), and two studies had high risk of attrition bias (Olsen 2016a; Thodberg 2016). Overall, we considered the risk of attrition bias in this review as low.
Selective reporting
Four studies had low risk of reporting bias (Friedmann 2015; Olsen 2016a; Olsen 2016b; Zisselman 1996), while the remaining five had high risk, giving us an overall high risk of reporting bias in this review.
Other potential sources of bias
Under the domain of 'other bias,' one study was at high risk of bias, as it was a randomised cross‐over trial in which the authors did not present complete and unbiased data of both phases separately (Dabelko‐Schoeny 2014). Two related cluster‐RCTs were at unclear risk, as they were at unclear risk in two (Olsen 2016a) and one (Olsen 2016b) out of five risk of bias items specific to cluster‐RCT. We did not identify concerns under 'other bias' for the remaining studies and therefore rated all other studies as having low risks of bias under this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 2. Animal‐assisted therapy (AAT (live animal)) compared to robotic animals for dementia.
| AAT (live animal) compared to robotic animals for dementia | ||||||
| Patient or population: dementia Setting: nursing home or assisted‐living facilities Intervention: AAT (live animal) Comparison: robotic animals | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with robotic animals | Risk with AAT (live animal) | |||||
| Affect and emotional well‐being: depression | — | — | — | — | — | No studies assessed this outcome. |
| Social functioning: duration of physical contact | The median duration of physical contact was 28 seconds | The difference in median duration of physical contact was 65 seconds longer | — | 68 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Study presented the outcome data in median and interquartile ranges. |
| Social functioning: duration of talk directed at a person | The median duration of talk directed at a person was 206 seconds | The difference in median duration of talk directed at a person was 42 seconds shorter | — | 68 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Study presented the outcome data in median and interquartile ranges. |
| Social functioning: duration of talk in total | The median duration of talk in total was 307 seconds | The difference in median duration of talk in total was 44 seconds shorter | — | 68 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Study presented the outcome data in median and interquartile ranges. |
| Behaviour measured using NPI (0–144, higher score indicates more severe neuropsychiatric symptoms) | The mean score using NPI was 29.29 | MD 6.96 lower (14.58 lower to 0.66 higher) | — | 78 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — |
| Agitation and irritability | — | — | — | — | — | No studies assessed this outcome. |
| Health‐related quality of life measured using QUALID (12–45, higher score indicates poorer quality of life) | The mean quality of life score, measured using QUALID was 26.75 | MD 2.42 lower (5.71 lower to 0.87 higher) | — | 78 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| Physical functioning: activities of daily living | — | — | — | — | — | No studies assessed this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is taken from the total event rate (dichotomous outcome) or the range of mean scores (continuous outcome) in the comparison group of the included studies. AAT: animal‐assisted therapy; CI: confidence interval; MD: mean difference; NPI: Neuropsychiatric Inventory; QUALID: Quality of Life in Dementia; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level. The single included study had unclear risk of bias in allocation concealment and high risk of bias in blinding of participants and personnel. bDowngraded one level. The study had a small sample with an imprecise estimate as reflected by wide interquartile ranges for all outcomes reported. cDowngraded one level. The 95% CI ranged from a substantial lower score (reflecting meaningful benefit) to moderately higher (reflecting meaningful harm), which is very likely to translate into different decisions should either of them have been shown as the true effect.
Summary of findings 3. Animal‐assisted therapy (AAT (live animal)) compared to soft toy cat for dementia.
| AAT (live animal) compared to soft toy cat for dementia | ||||||
| Patient or population: dementia Setting: nursing home or assisted‐living facilities Intervention: AAT (live animal) Comparison: soft toy cat | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with soft toy cat | Risk with AAT (live animal) | |||||
| Affect and emotional well‐being: depression | — | — | — | — | — | No studies assessed this outcome. |
| Social functioning: duration of physical contact | The median duration of physical contact was 0 seconds | The difference in median duration of physical contact was 93 seconds longer | — | 64 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Study presented the outcome data in median and interquartile ranges. |
| Social functioning: duration of talk directed at a person | The median duration of talk directed at a person was 297 seconds | The difference in median duration of talk directed at a person was 133 seconds shorter | — | 64 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Study presented the outcome data in median and interquartile ranges. |
| Social functioning: duration of talk in total | The median duration of talk in total was 298 seconds | The difference in median duration of talk in total was 35 seconds shorter | — | 64 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Study presented the outcome data in median and interquartile ranges. |
| Behaviour | — | — | — | — | — | No studies assessed this outcome. |
| Agitation and irritability | — | — | — | — | — | No studies assessed this outcome. |
| Health‐related quality of life | — | — | — | — | — | No studies assessed this outcome. |
| Adverse events | — | — | — | — | — | No studies assessed this outcome. |
| Physical functioning: activities of daily living | — | — | — | — | — | No studies assessed this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The assumed risk in the comparison group is taken from the total event rate (dichotomous outcome) or the range of mean scores (continuous outcome) in the comparison group of the included studies. AAT: animal‐assisted therapy; CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level. The single included study has unclear risk of bias in allocation concealment and high risk of bias in blinding of participants and personnel. bDowngraded one level. The study has a small sample with an imprecise estimate as reflected by wide interquartile ranges for all outcomes reported.
In total, seven studies with 305 participants contributed to the data. Two studies did not contribute quantitative outcome data (Holthoff 2013; Quibel 2017). Three comparisons were evaluated, namely, AAT versus no AAT; AAT using live animals versus the use of robot animals or devices; and AAT versus other alternative to live animals, such as soft toys.
Animal‐assisted therapy versus no animal‐assisted therapy (comparison 1)
See Table 1 for the major outcomes under this comparison that we planned to highlight in our main summary of findings table as specified in our protocol (Lai 2019), with their corresponding certainty of evidence where data were available. Additionally, we rated and reported the certainty of evidence for all outcome estimates along with the reasons for downgrading, regardless whether or not they were our prespecified major outcomes
Primary outcomes
1. Affect and emotional well‐being
Two studies assessed depressive symptoms using the CSDD (0–19, higher score indicates more severe depressive symptoms) (Friedmann 2015; Olsen 2016b). Participants who received AAT were slightly less depressed at the time of the final assessment (from six to 12 weeks after commencement of the intervention) compared to participants in the control group, although the certainty of the evidence was low ((MD –2.87, 95% CI –5.24 to –0.50; studies = 2, participants = 83; I2 = 0%; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.1, Figure 5). There was no substantial difference between the findings of the individually randomised trial (Friedmann 2015) and the cluster‐RCT (Olsen 2016b).
1.1. Analysis.
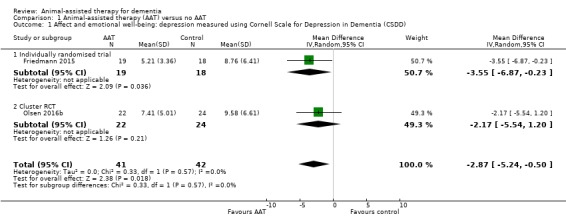
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 1 Affect and emotional well‐being: depression measured using Cornell Scale for Depression in Dementia (CSDD).
5.
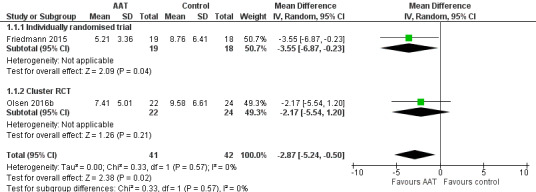
Forest plot of comparison: 1 Animal‐assisted therapy (AAT) versus no AAT, outcome: 1.1 Affect and emotional well‐being: depression measured using Cornell Scale for Depression in Dementia (CSDD).
One study assessed apathy using the Apathy Evaluation Scale (AES; 7–28, higher score indicates more severe apathy) (Friedmann 2015). The estimate of the effect was imprecise and compatible with a possible effect in either direction (MD 1.81, 95% CI –0.58 to 4.20; participants = 37; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.2).
1.2. Analysis.
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 2 Affect and emotional well‐being: apathy, measured using Apathy Evaluation Scale (AES).
One study measured depressed or anxious mood using the MOSES Depressed or Anxious Mood subscale (7–28, higher score indicates more severe depressive or anxiety symptoms) (Friedmann 2015). The estimate of the effect was imprecise and compatible with a possible effect in either direction, and the overall certainty of the evidence was low (MD –0.30, 95% CI –3.52 to 2.92; participants = 58; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.3).
1.3. Analysis.
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 3 Affect and emotional well‐being: depressed or anxious mood, measured using Multidimensional Observation Scale for Elderly Subjects (MOSES) Depressed or Anxious Mood subscale.
2. Social functioning
One study measured social functioning using the MOSES Withdrawn Behaviour subscale (8–32, higher score indicates more severe withdrawn behaviour) (Zisselman 1996). The estimate of the effect was imprecise and compatible with a possible effect in either direction, and the overall certainty of the evidence was low (MD –0.40, 95% CI –3.41 to 2.61; participants = 58; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.4).
1.4. Analysis.
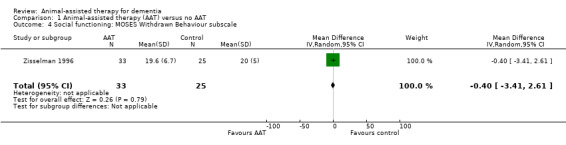
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 4 Social functioning: MOSES Withdrawn Behaviour subscale.
3. Overall behavioural and psychological symptoms of dementia
Three studies measured aspects of behaviour, but each used a different instrument, namely, the NHBPS (0–116, higher score indicates more severe behavioural problems), NPI (0–144, higher score indicates more severe neuropsychiatric problems), and MOSES Disoriented Behaviour subscale (8–32, higher score indicates more severe disoriented behaviour) (Dabelko‐Schoeny 2014; Valenti‐Soler 2015; Zisselman 1996). As they evaluated the same broad outcome using instruments with the same direction of effect, we pooled their findings using the SMD. The effect estimate was very imprecise and the overall certainty of the evidence was very low, so we were unable to draw any conclusion about the effect on behaviour (SMD –0.34, 95% CI –0.98 to 0.30; participants = 142; studies = 3; I2 = 67%; very low‐certainty evidence, downgraded one level each due to concerns about the risk of bias of the included studies, inconsistency due to substantial level of heterogeneity present, and imprecision) (Analysis 1.5; Figure 6). There was no evidence of a difference between our prespecified subgroups by type of therapy animal (horse: Dabelko‐Schoeny 2014; dog: Valenti‐Soler 2015; Zisselman 1996; P = 0.07).
1.5. Analysis.
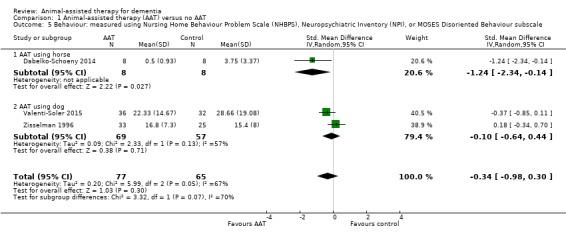
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 5 Behaviour: measured using Nursing Home Behaviour Problem Scale (NHBPS), Neuropsychiatric Inventory (NPI), or MOSES Disoriented Behaviour subscale.
6.
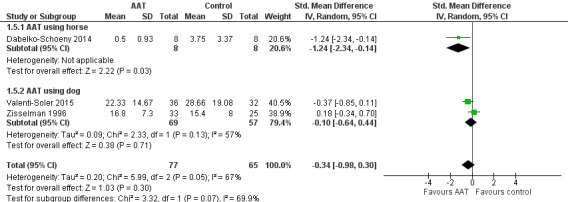
Forest plot of comparison: 1 Animal‐assisted therapy (AAT) versus no AAT, outcome: 1.5 Behaviour: measured using Nursing Home Behaviour Problem Scale (NHBPS), Neuropsychiatric Inventory (NPI), or MOSES Disoriented Behaviour subscale.
There was substantial heterogeneity in the pooled estimate for this outcome, as shown by an I2 statistic of 67%. We explored possible explanations for the substantial heterogeneity present, from study design to characteristics of the population, intervention/comparison, outcome measurement, and risk of bias profile. In terms of study design, all three studies were RCTs, with only Dabelko‐Schoeny 2014 being a randomised cross‐over trial. However, we only extracted data from the first phase from Dabelko‐Schoeny 2014, and excluding this study from the pooled analysis only reduced the I2 statistic from 67% to 57%. This makes study design an unlikely explanation for the observed degree of heterogeneity.
In terms of population, two studies recruited participants with Alzheimer's disease (Dabelko‐Schoeny 2014; Valenti‐Soler 2015), and the third study recruited a mixed population, and only some of the participants had dementia, with an unknown proportion having Alzheimer's disease (Zisselman 1996). However, excluding Zisselman 1996 from the pooled analysis only modestly reduced the I2 statistic from 67% to 51%. This makes population an unlikely major explanation for the observed degree of heterogeneity.
In terms of intervention, Dabelko‐Schoeny 2014 used horses as the therapy animal and the other two studies used dogs. However, excluding Dabelko‐Schoeny 2014 from the analysis only reduced the I2 statistic from 67% to 57%, making the type of therapy animal an unlikely explanation for the observed heterogeneity.
In terms of comparison, Dabelko‐Schoeny 2014 and Valenti‐Soler 2015 compared AAT with "standard care," while Zisselman 1996 compared AAT specifically with exercise therapy. However, excluding Zisselman 1996 from the pooled analysis only modestly reduced the I2 statistic from 67% to 51%. This makes the nature of comparison an unlikely major explanation for the observed degree of heterogeneity.
In terms of outcome measurement, each of the three studies used different instruments, which were measuring slightly different constructs, and excluding a single study from the remaining two in the pooled analysis did not reduce the I2 statistic substantially. We considered it unlikely that the difference in instruments used to measure the outcome was a plausible major contributor to the observed heterogeneity.
All three studies had a similar risk of bias profile with unclear or high risks of selection bias and low risks of attrition bias, so risk of bias was also an unlikely explanation for the degree of heterogeneity.
Despite the substantial degree of heterogeneity observed, which may relate to the different outcome measurement instruments, we considered the studies to be evaluating the sufficiently similar broad outcome of behaviour, and decided to keep the pooled analysis of the three studies, and accept the pooled findings with lower certainty due to inconsistency, as shown in our rating of certainty of evidence.
4. Agitation and irritability
Three studies measured level of agitation, but each used a different instrument, namely, CMAI (14–70, higher score indicates more severe agitation or irritability), BARS (9–36, higher score indicates more severe agitation or irritability), and MOSES Irritability subscale (9–36, higher score indicates more severe irritability) (Friedmann 2015; Olsen 2016b; Zisselman 1996). As they evaluated the same outcome of agitation, we decided to pool their estimates using the SMD. The effect estimate was very imprecise and the overall certainty of the evidence was very low, so we were unable to draw any conclusion about the effect on agitation (SMD –0.39, 95% CI –0.89 to 0.10; participants = 143; studies = 3; I2 = 53%; evidence downgraded one level each due to concerns about risk of bias, inconsistency due to substantial level of heterogeneity present, and imprecision; Analysis 1.6; Figure 7).
1.6. Analysis.
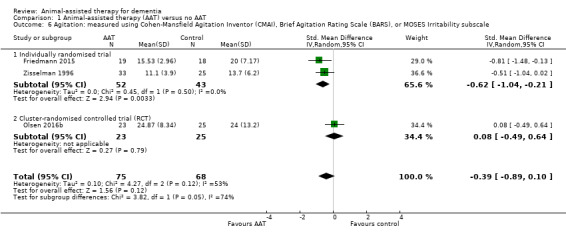
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 6 Agitation: measured using Cohen‐Mansfield Agitation Inventor (CMAI), Brief Agitation Rating Scale (BARS), or MOSES Irritability subscale.
7.
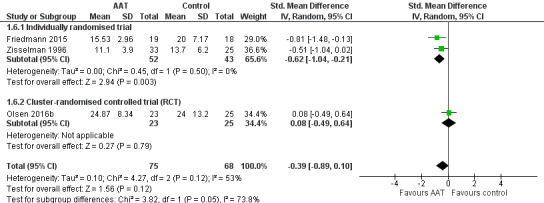
Forest plot of comparison: 1 Animal‐assisted therapy (AAT) versus no AAT, outcome: 1.6 Agitation: measured using Cohen‐Mansfield Agitation Inventor (CMAI), Brief Agitation Rating Scale (BARS), or MOSES Irritability subscale.
There was substantial heterogeneity in the pooled estimate for this outcome, as shown by an I2 statistic of 53%. We explored possible explanations for the substantial heterogeneity present, from study design to characteristics of the population, intervention/comparison, outcome measurement, and risk of bias profile. In terms of study design, both Friedmann 2015 and Zisselman 1996 were RCTs, while Olsen 2016b was a cluster‐RCT. The findings of Olsen 2016b were clearly different to those of other two studies, as Olsen 2016b showed a point estimate with a higher agitation level for AAT group while others showed the reverse. Excluding Olsen 2016b from the pooled analysis substantially reduced the I2 statistic from 53% to 0%. Based on this, we considered that the cluster‐RCT study design is a plausible explanation for the observed degree of heterogeneity. However, we are unclear whether the cluster‐RCT design contributed to the findings which were at odds with that of individual RCTs due to some differences in the effects of the intervention when administered in clusters or some other explanation.
There were differences in the population among the three studies, as Friedmann 2015 and Olsen 2016b recruited participants with dementia, while Zisselman 1996 recruited a mixture of participants, with only a proportion having dementia. However, excluding Zisselman 1996 from the analysis increased the I2 statistic from 53% to 74%, making the population characteristics an unlikely explanation for the observed degree of heterogeneity.
In terms of intervention, all three studies employed AAT as the intervention using therapy dogs with a similar range and duration of activities. However, both Friedmann 2015 and Zisselman 1996 had active comparators ("reminiscing" with training of social and motor skills for Friedmann 2015, and exercise therapy for Zisselman 1996) while the control group in Olsen 2016b received "treatment as usual," which included diverse group activities such as reminiscence, music therapy, "sensory garden," singing, exercise, cooking, and handicrafts. Although this could have partially explained the observed degree of heterogeneity, we considered it less likely to be the major explanation as opposed to the study design, as the range of activities in the control group of Olsen 2016b comprised reminiscence and exercise, which were the comparators in Friedmann 2015 (reminiscence) and Zisselman 1996 (exercise).
In terms of outcome measures, each of these three studies used different instruments, but the instruments used by Friedmann 2015 (CMAI) and Olsen 2016b (BARS) were related, as BARS was derived from CMAI as an abbreviated version. Zisselman 1996 used the MOSES Irritability subscale, an instrument unrelated to the CMAI and BARS, but it was Olsen 2016b and not this study that contributed to the observed heterogeneity. Therefore, we considered that variation in outcome measurement instruments to be an unlikely contributor to the observed heterogeneity.
Additionally, we did not consider risk of bias to be a likely contributor either, as all three studies had similar risk of bias profiles, with mostly unclear selection bias, high risk of performance bias, and low risk of attrition bias.
Overall, we postulated that the cluster‐RCT design of Olsen 2016b was most likely a major factor contributing to the observed degree of heterogeneity, with the control group activities being a possible additional contributor, although a minor one. In accordance with our strategy in conducting subgroup analysis, we divided the included studies for this outcome into two subgroups according to study design (individually randomised versus cluster‐randomised trial). Our results according to subgroups are reported as follows. From the findings of two studies In the subgroup of individually randomised trials (Friedmann 2015; Zisselman 1996), participants who received AAT appeared to be slightly less agitated compared to those in the control group (SMD –0.62, 95% CI –1.04 to –0.21; participants = 95; studies = 2; I2 = 0%; moderate‐certainty evidence, downgraded one level due to concerns on the risks of bias of the included studies). From the finding of a single study in the subgroup of cluster‐RCT (Olsen 2016b), there were no clear differences between the participants who received AAT versus those in the control group in the level of agitation (SMD 0.08, 95% CI –0.49 to 0.64; participants = 48; low‐certainty evidence, downgraded one level each due to concerns on the risk of bias of the included study and imprecision). There was a marginal significant difference in the estimates between these two subgroups, as indicated by a P value of 0.05 in the test of subgroup differences.
5. Health‐related quality of life
From the findings of three studies, there were no clear differences in the quality of life of participants in both groups, as measured using the QUALID scale (12–45, higher score indicates poorer quality of life) (MD 0.45, 95% CI –1.28 to 2.18; participants = 164; studies = 3; I2 = 0%; moderate‐certainty evidence, downgraded one level due to concerns about risk of bias; Analysis 1.7) (Olsen 2016a; Olsen 2016b; Valenti‐Soler 2015). The estimates were similar between the single study in the subgroup of individually randomised trial (Valenti‐Soler 2015), and the two studies in the subgroup of cluster‐RCT (Olsen 2016a; Olsen 2016b).
1.7. Analysis.
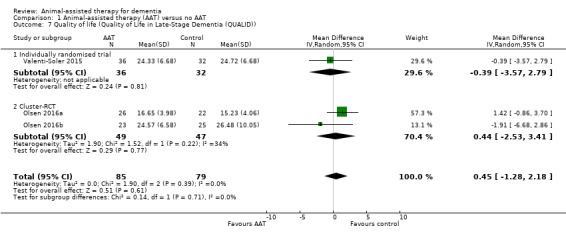
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 7 Quality of life (Quality of Life in Late‐Stage Dementia (QUALID)).
Two of the three included studies were conducted by the same principal author over a similar period (Olsen 2016a; Olsen 2016b). However, the participants differed (home dwelling people in one and nursing home residents in another) and the studies were registered as separate studies under ClinicalTrial.gov. Although they appeared to be two separate studies, we could not exclude the possibility of overlapping participants, hence double‐counting in the outcome data, as we have not received a reply from the authors. However, we considered the possibility of double‐counting to be small, and have combined the outcome data as if the participants were non‐overlapping. We have included a similar note of explanation in the Table 1.
6. Adverse effects
No studies assessed adverse effects.
Secondary outcomes
1. Physical functioning
One study measured ADL using the Barthel Index (0–100, higher score indicates better abilities) (Friedmann 2015). The estimate of the effect was imprecise and compatible with a possible effect in either direction, and the overall certainty of the evidence was low (MD 4.65, 95% CI –16.05 to 25.35; participants = 37; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.8).
1.8. Analysis.
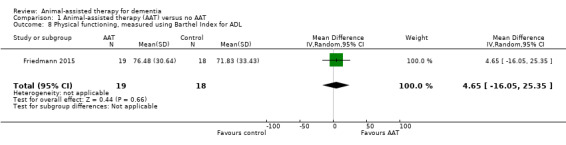
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 8 Physical functioning, measured using Barthel Index for ADL.
One study measured balance using the Berg's Balance Scale (0–56, higher score indicates better balance) (Olsen 2016a). The estimate of the effect was imprecise and compatible with a possible effect in either direction, and the overall certainty of the evidence was low (MD –2.29, 95% CI –5.66 to 1.08; participants = 52; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.9).
1.9. Analysis.
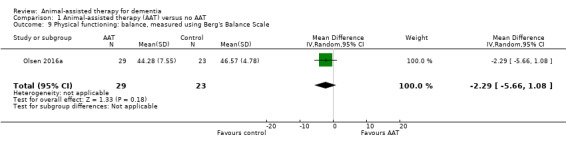
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 9 Physical functioning: balance, measured using Berg's Balance Scale.
One study measured self‐care ability using the MOSES Self‐Care Functioning subscale (8–32, higher score indicates better functioning) (Zisselman 1996). The estimate of the effect was imprecise and compatible with a possible effect in either direction, and the overall certainty of the evidence was low (MD 2.20, 95% CI –1.23 to 5.63; participants = 58; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 1.10).
1.10. Analysis.
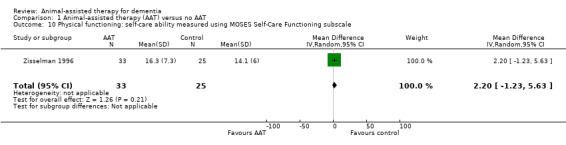
Comparison 1 Animal‐assisted therapy (AAT) versus no AAT, Outcome 10 Physical functioning: self‐care ability measured using MOSES Self‐Care Functioning subscale.
2. Cognitive functioning
No studies assessed cognitive functioning.
3. Overall dementia severity
No studies assessed overall dementia severity.
4. Mortality
No studies assessed mortality.
5. Rates of institutionalisation
No studies assessed rates of institutionalisation.
6. Carer satisfaction and stress
No studies assessed carer satisfaction and stress.
7. Animal outcomes
No studies assessed animal outcomes.
Animal‐assisted therapy versus the use of robotic animal or devices (comparison 2)
See Table 2 for the major outcomes under this comparison that we planned to highlight in our main summary findings table as specified in our protocol (Lai 2019), with their corresponding certainty of evidence where data were available. Additionally, we rated and reported the certainty of evidence for all outcomes estimates along with the reasons for downgrading, regardless whether or not they were our prespecified major outcomes.
Primary outcomes
1. Affect and emotional well‐being
No studies assessed affect and emotional well‐being.
2. Social functioning
One study reported social functioning as duration of contact and talking using medians and interquartile ranges (Thodberg 2016). The duration of contact with other people appeared longer in participants who received AAT compared to those interacting with a robot seal, although we are uncertain of the estimate due to the low‐certainty evidence presented for this outcome (median at final visit: 93 seconds with dog versus 28 seconds with seal; participants = 68; Table 4).
There appeared to be no substantial differences in the duration of talking between the two groups, with a possibly slightly longer duration of talking in the group who received the robot seal, although we are uncertain of the estimates due to the low‐certainty evidence presented for this outcome. We downgraded the evidence one level each due to concerns about the risk of bias and imprecision due to small sample size.
3. Overall behavioural and psychological symptoms of dementia
One study reported BPSD using the NPI (0–144, higher score indicates more severe neuropsychiatric symptoms) (Valenti‐Soler 2015). The estimate of the effect on overall behaviour was imprecise and compatible with a possible effect in either direction, and the overall certainty of the evidence was low (MD –6.96, 95% CI –14.58 to 0.66; participants = 78; evidence downgraded one level each due to concerns about the risk of bias and imprecision; Analysis 2.1).
2.1. Analysis.
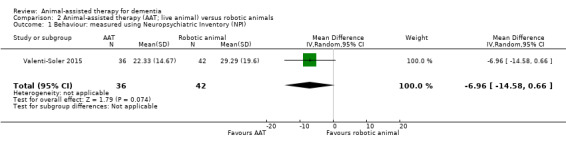
Comparison 2 Animal‐assisted therapy (AAT; live animal) versus robotic animals, Outcome 1 Behaviour: measured using Neuropsychiatric Inventory (NPI).
4. Agitation and irritability
No studies assessed agitation and irritability.
5. Health‐related quality of life
One study reported HRQOL using the QUALID scale (12–45, higher score indicates poorer quality of life) (Valenti‐Soler 2015). The estimate of the effect on quality of life was imprecise and compatible with a possible effect in either direction, with uncertainties due to the low‐certainty evidence (MD –2.42, 95% CI –5.71 to 0.87; participants = 78; evidence downgraded one level each due to concerns about risk of bias and imprecision; Analysis 2.2).
2.2. Analysis.
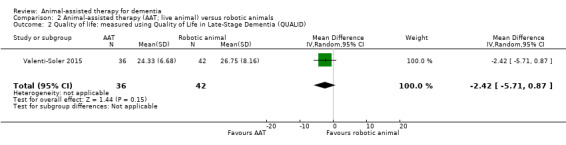
Comparison 2 Animal‐assisted therapy (AAT; live animal) versus robotic animals, Outcome 2 Quality of life: measured using Quality of Life in Late‐Stage Dementia (QUALID).
6. Adverse effects
No studies assessed adverse effects.
Secondary outcomes
1. Physical functioning
No studies assessed physical functioning.
2. Cognitive functioning
No studies assessed cognitive functioning.
3. Overall dementia severity
No studies assessed overall dementia severity.
4. Mortality
No studies assessed mortality.
5. Rates of institutionalisation
No studies assessed rates of institutionalisation.
6. Carer satisfaction and stress
No studies assessed carer satisfaction and stress.
7. Animal outcomes
No studies assessed animal outcomes.
Animal‐assisted therapy versus the use of other alternative, such as soft toys (comparison 3)
Primary outcomes
1. Affect and emotional well‐being
No studies assessed affect and emotional well‐being.
2. Social functioning
One study reported social functioning as duration of contact and talking using medians and interquartile ranges (Thodberg 2016). The duration of contact with other people appeared substantially longer in participants who received AAT compared to those who received a toy cat (median at final visit: 93 seconds with dog versus 0 seconds with toy cat; participants = 64; Table 4). The duration of talking appeared to be longer in the group who received a toy cat compared to the group who received AAT with a dog (median duration of talking directed at a person: 164 seconds with dog versus 297 seconds with toy cat; median duration of talking in total: 263 seconds with dog versus 298 seconds with toy cat; participants = 64; Table 4). However, we are uncertain of the estimates due to the low‐certainty evidence (evidence downgraded one level each due to concerns about risk of bias and imprecision due to small sample size).
3. Overall behavioural and psychological symptoms of dementia
No studies assessed BPSD.
4. Agitation and irritability
No studies assessed agitation and irritability.
5. Health‐related quality of life
No studies assessed HRQOL.
6. Adverse effects
No studies assessed adverse effects.
Secondary outcomes
1. Physical functioning
No studies assessed physical functioning.
2. Cognitive functioning
No studies assessed cognitive functioning.
3. Overall dementia severity
No studies assessed overall dementia severity.
4. Mortality
No studies assessed mortality.
5. Rates of institutionalisation
No studies assessed rates of institutionalisation.
6. Carer satisfaction and stress
No studies assessed carer satisfaction and stress.
7. Animal outcomes
No studies assessed animal outcomes.
Subgroup analysis
Apart from the subgroup analyses conducted based on study design and intervention (type of animal used) as mentioned above under the respective comparison and outcome, there were insufficient data to enable subgroup analyses according to the other criteria, such as the setting of the study, type of dementia, and delivery of the intervention (individual versus group, intensity or dosage of the therapy).
Sensitivity analysis
One study recruited a mixture of participants, some of whom did not have dementia (Zisselman 1996). In accordance with our protocol (Lai 2019), we performed sensitivity analysis to assess the impact of excluding this study where there are multiple studies in two outcomes where this is applicable: behaviour: measured using NHBPS, NPI, or MOSES Disoriented Behaviour subscale (outcome 1.5) and agitation: measured using CMAI, BARS, or MOSES Irritability subscale (outcome 1.6).
Behaviour: measured using the NHBPS, NPI, or MOSES Disoriented Behaviour subscale, the exclusion of Zisselman 1996 did not result in a substantial change in the overall estimates (from SMD –0.34, 95% CI –0.98 to 0.30 to SMD –0.66, 95% CI –1.47 to 0.14 with Zisselman 1996 excluded).
Agitation: measured using CMAI, BARS, or MOSES Irritability subscale, the exclusion of Zisselman 1996 similarly did not result in a substantial change in the overall estimates (from SMD –0.39, 95% CI –0.89 to 0.10 to SMD –0.34, 95% CI –1.21 to 0.52 with Zisselman 1996 excluded).
Otherwise, there were insufficient data to enable our predefined sensitivity analyses based on risk of bias, participant factors (definition of dementia), and intervention factor (with the presence of clearly documented trained human facilitator versus without).
Discussion
Summary of main results
This review shows with some uncertainty, that people with dementia who received AAT appeared to be modestly less depressed at the end of the AAT intervention period compared with those who did not. However, there appeared to be no clear difference in the quality of life of participants who received AAT compared to those who did not. There were no clear benefits or risks from AAT in terms of any other outcomes, including other aspects of affect and emotional well‐being, social functioning, behaviour, physical and cognitive functioning, other dementia‐related morbidities, mortality, and adverse effects, based on the evidence that we gathered in this systematic review.
In the comparisons between AAT using live animals versus robotic animal and soft toys, evidence from a single study did not provide clear evidence of important benefits or harms. All included studies focused on human outcomes and none assessed animal‐level outcomes. On the basis of the current evidence, a sound postulation could not be made to explain why AAT might reduce depression, as the available evidence is very limited, with no clear evidence yet showing the effects of AAT on cognitive and motor function, which have been postulated to affect social function and self‐esteem, with consequent effects on loneliness and depression, as depicted in our proposed logic framework (Figure 1).
Overall completeness and applicability of evidence
Through a comprehensive search strategy, we identified nine eligible studies and two on‐going studies. We believe through our search strategy and the range of databases that was covered by the Cochrane Dementia Information Specialist, we captured all relevant literature that are specific to this review. Studies recruited 305 older adults of both sexes from assisted living facilities, nursing homes, or a dedicated unit for dementia in a hospital, which represents the typical settings in the countries where the studies were conducted. However, as all studies were conducted in Europe or the USA, it is unclear whether our findings are generalisable to other parts of the world. The criteria for diagnosing dementia from the studies with documentation were part of a common set of diagnostic criteria that should be applicable generally.
One notable issue in applicability is the type of animal used in AAT, as most studies used dogs as therapy animals. While dogs are commonly accepted around the world, they may be unsuitable in settings where there are people of certain religious groups, such as Muslims, for whom dogs are usually considered as ritually unclean (El Fadl 2004).
Due to insufficient data, we were unable to undertake most of our prespecified subgroup analyses to further determine applicability of the findings to older adults with different prognostic factors as laid out in the Subgroup analysis and investigation of heterogeneity section.
Quality of the evidence
There was overall very low‐ to moderate‐certainty evidence in the major outcomes which were reported by a small number of studies with a small number of participants. The clearest risk of bias issue was the lack of blinding of participants and care personnel in all studies, as blinding was impossible because one group used live animals and the others did not. The lack of blinding of participants and care personnel posed serious concerns on the overall certainty of evidence, as all outcomes evaluated, such as affect and emotional and social well‐being, behaviour, and quality of life involved a subjective component. The certainty of the estimates of many outcomes were also affected by imprecision, due to the small sample sizes in single studies which were underpowered to detect important differences in the effects of the intervention and control groups. For a detailed list of the certainty of evidence, see Table 1 for the major prespecified outcomes to be highlighted in our review for the comparison between AAT and no AAT, Table 2 for the major prespecified outcomes to be highlighted in our review for the comparison between AAT using live animals and the use of robotic animals.
Potential biases in the review process
The evidence gathered in this review was the result of a comprehensive search from multiple databases with independent screening, selection, and assessment of eligible studies. However, there are two on‐going studies that are yet to be included in our analyses, and with the small number of studies and participants in most outcomes, the inclusion of these studies might change the overall findings.
Agreements and disagreements with other studies or reviews
Through a MEDLINE (PubMed) search in June 2019 using a combination of "animal‐assisted" (TI/AB) and "animal assisted therapies" (MeSH term), and limited to "meta‐analysis" under publication type, we retrieved seven systematic reviews related to AAT, one involving people with cognitive impairment (Hu 2018a), three mainly focusing on psychological or psychiatric outcomes for all adults (Berget 2011; Ein 2018; Waite 2018), two specifically on the roles of hippotherapy and horse riding on motor outcomes for children with cerebral palsy (Tseng 2013; Zadnikar 2011), and one on horseback riding in general (Stergiou 2017). Through the references of the other relevant articles, we further retrieved five relevant systematic reviews, including three that focused on psychosocial outcomes including depression in all adults (Maber‐Aleksandrowicz 2016; Maujean 2015; Souter 2007), one involving participants in long‐term care facilities (Ebener 2017), and one general review on AAT (Nimer 2007).
The closest systematic reviews to the current review are Hu 2018a and Ebener 2017. Hu 2018a included 10 studies, including five quasi‐RCTs, on the roles of AAT in people with cognitive impairment from all causes. The review reported that AAT significantly reduced behavioural and psychological symptoms of dementia, especially depression and agitation, with no significant improvements in daily activities, quality of life, or cognitive scores. The findings of this review are in line with the findings of our review, although the certainty of evidence, hence confidence on the estimates, were not included in the review, and as a result, the conclusions regarding the potential benefits of AAT on behavioural and psychological symptoms appear to be overstated. Ebener 2017 performed a broad systematic review of all types of studies that evaluated the various roles of animals in residents in long‐term care facilities and gathered mostly non‐randomised trials that used various animals such as birds, cats, dogs, and fish, with a narrative synthesis of their findings. The authors reported that the participants as a whole experienced to variable degrees behavioural, social, physical, and mental benefits from different types of animals. As the synthesis of the findings were mainly done qualitatively, and without a rating of the certainty of evidence (which would possibly be low to very low, given that most included studies were non‐randomised studies), we are unable to judge the extent of the possible benefits and the confidence in the estimates.
The other systematic reviews on the roles of AAT included populations that were different to that in this review. They showed that in general, AAT appeared to improve psychosocial and behavioural outcomes (Berget 2011; Ein 2018; Maber‐Aleksandrowicz 2016; Maujean 2015; Nimer 2007; Souter 2007; Waite 2018), but most reviews advocated further studies with rigorous methodologies to increase confidence on the outcome estimates. One review reports that horse riding may improve balance and gait in general (Stergiou 2017), although there were conflicting results in the reviews that assessed the benefits of hippotherapy and horse riding on motor outcomes of children with cerebral palsy (Tseng 2013; Zadnikar 2011).
Authors' conclusions
Implications for practice.
There is some evidence from randomised controlled trials (RCTs) that animal‐assisted therapy (AAT) appears to modestly reduce depressive symptoms in people with dementia, but there is so far no clear evidence that AAT affects other outcomes such as social functioning, quality of life, or physical or cognitive functioning in this population, with no data on adverse effects and animal‐level outcomes. However, the certainty of the estimates for all outcomes (or the quality of evidence) was very low to moderate, which means there is a clear possibility that the overall findings may change with further research.
Implications for research.
In view of the very low‐ to moderate‐certainty evidence presented in this review, more well‐conducted RCTs are needed. Future RCTs should have clear documentation of methods of sequence generation and provide sufficient details on the process of random sequence generation and its relationship to allocation to enable a clear assessment of selection bias. Given the difficulties in achieving blinding of participants and personnel, it is essential that future research adheres to rigorous standards with clear documentation, to offer any improvement in the overall certainty of evidence to answer the review questions. In particular, blinding of outcome assessors can usually be achieved regardless of the status of blinding of participants and personnel. Additionally, outcome data should be presented clearly as event rates and the total number of participants for dichotomous outcomes, and mean and SDs for continuous outcomes to enable extraction and meaningful synthesis of evidence. Future research should also be adequately powered by enrolling sufficient numbers of participants to increase certainty in the overall estimates by increasing precision, as this was another major aspect that led to downgrading of evidence in the current review. Further, more well‐conducted cluster‐RCTs should be undertaken to further evaluate the seemingly discrepant findings of cluster‐RCTs compared to individually randomised trials in one of the outcomes reported. Future studies should include major relevant outcomes such as affect, emotional and social functioning, quality of life, and animal‐level outcomes if possible, with clear documentation on the presence or absence of adverse events associated with AAT including trauma and infections. Researchers planning for future trials should consider evaluating the outcomes in dementia in accordance with an organised framework that depicts the relationship between various effects of dementia, such as the logic framework that we proposed in our review (Figure 1), and employs common and validated tools to assess each outcome, as suggested under Types of outcome measures in our review.
Acknowledgements
Our thanks to Sue Marcus, Managing Editor in processing the draft protocol and review, and Dr Jenny McCleery, Co‐ordinating Editor of Cochrane Dementia along with the editorial team for their support in providing important and helpful feedback to the draft, and to Candida Fenton, Information Specialist, for developing the search strategy and running the searches.
We are grateful to Dr Elena Antonelli, principal author of one of the short‐listed studies for providing additional information on the study participants in a timely manner to enable us to finalise the study selection (Antonelli 2012).
Our grateful thanks to Martin Vuillěme, who generously provided translation of an article in French (Quibel 2017).
Our thanks for Dr Sandra Min San Chong, who assisted in extracting data for the included studies.
We would like to thank peer reviewers Edwin Chan and Catherine Travers for their comments and feedback.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| ALOIS (Cochrane Dementia and Cognitive Improvement Group's Specialised Register search via CRS) (Date of most recent search: 5 September 2019) |
"animal assisted" AND INREGISTER "animal facilitated" AND INREGISTER cat AND INREGISTER cats AND INREGISTER dog AND INREGISTER dogs AND INREGISTER equine AND INREGISTER "fish tank*" AND INREGISTER hippotherapy AND INREGISTER horse AND INREGISTER pets AND INREGISTER "pet therapy" AND INREGISTER seal AND INREGISTER #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 |
Sep 2018: 92 Sep 2019: 13 |
| CENTRAL (the Cochrane Library) crso.cochrane.org/SearchSimple.php (Date of most recent search: 5 September 2019) |
#1 MESH DESCRIPTOR Dementia EXPLODE ALL TREES #2 MESH DESCRIPTOR Delirium #3 MESH DESCRIPTOR Wernicke Encephalopathy #4 MESH DESCRIPTOR Neurocognitive Disorders #5 dement*:TI,AB,KY #6 alzheimer*:TI,AB,KY #7 (lewy* adj2 bod*):TI,AB,KY #8 (chronic adj2 cerebrovascular):TI,AB,KY #9 ("organic brain disease" or "organic brain syndrome"):TI,AB,KY #10 ("benign senescent forgetfulness"):TI,AB,KY #11 (cerebr* adj2 deteriorat*):TI,AB,KY #12 (cerebral* adj2 insufficient*):TI,AB,KY #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 14917 #14 MESH DESCRIPTOR Animal Assisted Therapy EXPLODE ALL TREES #15 MESH DESCRIPTOR Animals, Domestic EXPLODE ALL TREES #16 MESH DESCRIPTOR Bonding, Human‐Pet EXPLODE ALL TREES #17 MESH DESCRIPTOR Equine‐Assisted Therapy EXPLODE ALL TREES #18 MESH DESCRIPTOR Pets EXPLODE ALL TREES #19 (animal assisted):TI,AB,KY #20 (animal facilitated):TI,AB,KY #21 animal‐assisted:TI,AB,KY #22 animal‐facilitated:TI,AB,KY #23 AAA:TI,AB,KY #24 AAI:TI,AB,KY #25 AAT:TI,AB,KY #26 (Animal Human Bond*):TI,AB,KY #27 (animal visit*):TI,AB,KY #28 (Animal‐Human Bond*):TI,AB,KY #29 aquaria:TI,AB,KY #30 aquarium*:TI,AB,KY #31 cat:TI,AB,KY #32 cats:TI,AB,KY #33 (companion animal*):TI,AB,KY #34 dog:TI,AB,KY #35 dogs:TI,AB,KY #36 equine:TI,AB,KY #37 (Fish tank*):TI,AB,KY #38 hippotherapy:TI,AB,KY #39 horse*:TI,AB,KY #40 (horseback riding therap*):TI,AB,KY #41 (human animal teams):TI,AB,KY #42 (human‐animal teams):TI,AB,KY #43 (pet facilitated therap*):TI,AB,KY #44 (Pet Human Bond*):TI,AB,KY 0 #45 pets:TI,AB,KY #46 pet‐therap*:TI,AB,KY #47 (pet adj5 (therap* or visit* or assist* or robot* or resident* or companion*)):TI,AB,KY #48 (recreational horseback riding therapy):TI,AB,KY #49 (resident cat*):TI,AB,KY #50 dog‐assisted:TI,AB,KY #51 (service animal program*):TI,AB,KY #52 (therapeutic adj3 animal*):TI,AB,KY #53 (visiting animal*):TI,AB,KY #54 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 #55 #13 AND #54 |
Dec 2018: 101 Sep 2019: 27 |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) (Date of most recent search: 5 September 2019) |
1 exp Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 dement*.mp. 6 alzheimer*.mp. 7 (lewy* adj2 bod*).mp. 8 (chronic adj2 cerebrovascular).mp. 9 ("organic brain disease" or "organic brain syndrome").mp. 10 "benign senescent forgetfulness".mp. 11 (cerebr* adj2 deteriorat*).mp. 12 (cerebral* adj2 insufficient*).mp. 13 or/1‐12 14 exp Animal Assisted Therapy/ 15 exp Animals, Domestic/ 16 exp Bonding, Human‐Pet/ 17 exp Equine‐Assisted Therapy/ 18 Pets/ 19 "animal assisted".ti,ab. 20 "animal facilitated".ti,ab. 21 "animal‐assisted".ti,ab. 22 "animal‐facilitated".ti,ab. 23 AAA.ti,ab. 24 AAI.ti,ab. 25 AAT.ti,ab. 26 Animal Human Bond*.ti,ab. 27 animal visit*.ti,ab. 28 Animal‐Human Bond*.ti,ab. 29 aquaria.ti,ab. 30 aquarium*.ti,ab. 31 cat.ti,ab. 32 cats.ti,ab. 33 companion animal*.ti,ab. 34 dog.ti,ab. 35 dogs.ti,ab. 36 equine.ti,ab. 37 Fish tank*.ti,ab. 38 hippotherapy.ti,ab. 39 horse*.ti,ab. 40 horseback riding therap*.ti,ab. 41 human animal teams.ti,ab. 42 human‐animal teams.ti,ab. 43 pet facilitated therap*.ti,ab. 44 Pet Human Bond*.ti,ab. 45 pets.ti,ab. 46 pet‐therap*.ti,ab. 47 (pet adj5 (therap* or visit* or assist* or robot* or resident* or companion*)).ti,ab. 48 recreational horseback riding therapy.ti,ab. 49 resident cat*.ti,ab. 50 dog‐assisted.ti,ab. 51 service animal program*.ti,ab. 52 (therapeutic adj3 animal*).ti,ab. 53 visiting animal*.ti,ab. 54 or/14‐53 55 13 and 54 56 randomized controlled trial.pt. 57 controlled clinical trial.pt. 58 randomized.ab. 59 placebo.ab. 60 drug therapy.fs. 61 randomly.ab. 62 trial.ab. 63 groups.ab. 64 or/56‐63 65 exp animals/ not humans.sh. 66 64 not 65 67 55 and 66 |
Dec 2018: 249 Sep 2019: 114 |
| EMBASE (OvidSP) 1974 to present (Date of most recent search: 5 September 2019) |
1 Dementia/ 2 Delirium/ 3 Wernicke Encephalopathy/ 4 Delirium, Dementia, Amnestic, Cognitive Disorders/ 5 ("benign senescent forgetfulness" or ("normal pressure hydrocephalus" and "shunt*") or ("organic brain disease" or "organic brain syndrome") or ((cerebral* or cerebrovascular or cerebro‐vascular) adj2 insufficien*) or (cerebr* adj2 deteriorat*) or (chronic adj2 (cerebrovascular or cerebro‐vascular)) or (creutzfeldt or jcd or cjd) or (lewy* adj2 bod*) or (pick* adj2 disease) or alzheimer* or binswanger* or deliri* or dement* or huntington* or korsako*).tw. 6 "major neurocognitive disorder".ti,ab. 7 or/1‐6 8 exp animal assisted therapy/ 9 exp domestic animal/ 10 exp human‐animal bond/ 11 exp hippotherapy/ 12 exp pet animal/ 13 animal assisted.ti,ab. 14 animal facilitated.ti,ab. 15 animal‐assisted.ti,ab. 16 animal‐facilitated.ti,ab. 17 AAA.ti,ab. 18 AAI.ti,ab. 19 AAT.ti,ab. 20 Animal Human Bond*.ti,ab. 21 animal visit*.ti,ab. 22 Animal‐Human Bond*.ti,ab. 23 aquaria.ti,ab. 24 aquarium*.ti,ab. 25 cat.ti,ab. 26 cats.ti,ab. 27 companion animal*.ti,ab. 28 dog.ti,ab. 29 dogs.ti,ab. 30 equine.ti,ab. 31 Fish tank*.ti,ab. 32 hippotherapy.ti,ab. 33 horse*.ti,ab. 34 horseback riding therap*.ti,ab. 35 human animal teams.ti,ab. 36 human‐animal teams.ti,ab. 37 pet facilitated therap*.ti,ab. 38 Pet Human Bond*.ti,ab. 39 pets.ti,ab. 40 pet‐therap*.ti,ab. 41 (pet adj5 (therap* or visit* or assist* or robot* or resident* or companion*)).ti,ab. 42 resident cat*.ti,ab. 43 recreational horseback riding therapy.ti,ab. 44 dog‐assisted.ti,ab. 45 service animal program*.ti,ab. 46 (therapeutic adj3 animal*).ti,ab. 47 visiting animal*.ti,ab. 48 or/8‐47 49 7 and 48 50 randomized controlled trial/ 51 controlled clinical trial/ 52 random$.ti,ab. 53 randomization/ 54 intermethod comparison/ 55 placebo.ti,ab. 56 (compare or compared or comparison).ti. 57 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 58 (open adj label).ti,ab. 59 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 60 double blind procedure/ 61 parallel group$1.ti,ab. 62 (crossover or cross over).ti,ab. 63 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 64 (assigned or allocated).ti,ab. 65 (controlled adj7 (study or design or trial)).ti,ab. 66 (volunteer or volunteers).ti,ab. 67 trial.ti. 68 or/50‐67 69 49 and 68 70 from 69 keep 1‐649 |
Dec 2018: 649 Sep 2019: 117 |
| PSYCINFO (OvidSP) (Date of most recent search: 5 September 2019) |
1 exp Dementia/ 2 exp Delirium/ 3 exp Huntingtons Disease/ 4 exp Kluver Bucy Syndrome/ 5 exp Wernickes Syndrome/ 6 exp Cognitive Impairment/ 7 dement*.mp. 8 alzheimer*.mp. 9 (lewy* adj2 bod*).mp. 10 deliri*.mp. 11 (chronic adj2 cerebrovascular).mp. 12 ("organic brain disease" or "organic brain syndrome").mp. 13 "supranuclear palsy".mp. 14 ("normal pressure hydrocephalus" and "shunt*").mp. 15 "benign senescent forgetfulness".mp. 16 (cerebr* adj2 deteriorat*).mp. 17 (cerebral* adj2 insufficient*).mp. 18 (pick* adj2 disease).mp. 19 (creutzfeldt or jcd or cjd).mp. 20 huntington*.mp. 21 binswanger*.mp. 22 korsako*.mp. 23 ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24 "major neurocognitive disorder".ti,ab. 25 or/1‐24 26 exp Animal Assisted Therapy/ 27 exp Interspecies Interaction/ 28 exp Pets/ 29 exp Horses/ 30 animal assisted.ti,ab. 31 animal facilitated.ti,ab. 32 animal‐assisted.ti,ab. 33 animal‐facilitated.ti,ab. 34 AAA.ti,ab. 35 AAI.ti,ab. 36 AAT.ti,ab. 37 Animal Human Bond*.ti,ab. 38 animal visit*.ti,ab. 39 Animal‐Human Bond*.ti,ab. 40 aquaria.ti,ab. 41 aquarium*.ti,ab. 42 cat.ti,ab. 43 cats.ti,ab. 44 companion animal*.ti,ab. 45 dog.ti,ab. 46 dogs.ti,ab. 47 equine.ti,ab. 48 Fish tank*.ti,ab. 49 hippotherapy.ti,ab. 50 horse*.ti,ab. 51 horseback riding therap*.ti,ab. 52 human animal teams.ti,ab. 53 human‐animal teams.ti,ab. 54 pet facilitated therap*.ti,ab. 55 Pet Human Bond*.ti,ab. 56 pets.ti,ab. 57 pet‐therap*.ti,ab. 58 (pet adj5 (therap* or visit* or assist* or robot* or resident* or companion*)).ti,ab. 59 recreational horseback riding therapy.ti,ab. 60 resident cat*.ti,ab. 61 dog‐assisted.ti,ab. 62 service animal program*.ti,ab. 63 (therapeutic adj3 animal*).ti,ab. 64 visiting animal*.ti,ab. 65 or/26‐64 66 25 and 65 67 exp Clinical Trials/ 68 randomly.ab. 69 randomi?ed.ti,ab. 70 placebo.ti,ab. 71 groups.ab. 72 "double‐blind*".ti,ab. 73 "single‐blind*".ti,ab. 74 RCT.ti,ab. 75 or/67‐74 76 66 and 75 77 from 76 keep 1‐101 |
Dec 2018: 101 Sep 2019: 7 |
| CINAHL (EBSCOhost) (Date of most recent search: 5 September 2019) |
S74 S60 AND S73 S73 S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 S72 MH "Random Assignment" S71 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S70 MH "Crossover Design" S69 MH "Factorial Design" S68 MH "Placebos" S67 MH "Clinical Trials" S66 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S65 TX crossover OR "cross‐over" S64 AB placebo* S63 TX random* S62 TX trial* S61 TX "latin square" S60 S20 AND S59 S59 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 S58 TX visiting animal* S57 TX therapeutic N3 animal* S56 TX service animal program* S55 TX dog‐assisted S54 TX resident cat* S53 TX recreational horseback riding therapy S52 TX pet N5 (therap* or visit* or assist* or robot* or resident* or companion*) S51 TX pet‐therap* S50 TX pets S49 TX Pet Human Bond* S48 TX pet facilitated therap* S47 TX human‐animal teams S46 TX human animal teams S45 TX horseback riding therap* S44 TX horse* S43 TX hippotherapy S42 TX Fish tank* S41 TX equine S40 TX dogs S39 TX dog S38 TX companion animal* S37 TX cats S36 TX cat S35 TX aquarium* S34 TX aquaria S33 TX Animal Human Bond* S32 TX AAT S31 TX AAI S30 TX AAA S29 TX animal‐facilitated S28 TX animal‐assisted S27 TX animal facilitated S26 TX animal assisted S25 (MH "Pets") S24 (MH "Equine‐Assisted Therapy") S23 (MH "Human‐Pet Bonding") S22 (MH "Animal Assisted Therapy (Iowa NIC)") S21 (MH "Pet Therapy+") S20 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S19 TX "major neurocognitive disorder" S18 TX korsako* S17 TX binswanger* S16 TX huntington* S15 TX creutzfeldt or jcd or cjd S14 TX pick* N2 disease S13 TX cerebral* N2 insufficient* S12 TX cerebr* N2 deteriorat* S11 TX "benign senescent forgetfulness" S10 TX "normal pressure hydrocephalus" and "shunt*" S9 TX "organic brain disease" or "organic brain syndrome" S8 TX chronic N2 cerebrovascular S7 TX deliri* S6 TX lewy* N2 bod* S5 TX alzheimer* S4 TX dement* S3 MH "Wernicke's Encephalopathy" S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S1 (MH "Dementia+") |
Dec 2018: 388 Sep 2019: 76 |
| Web of Science – core collection (ISI Web of Science) (Date of most recent search: 5 September 2019) |
TOPIC: (dement* OR alzheimer* OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL OR "cognit* impair*" OR FTD OF FTLD OR "cerebrovascular insufficienc*" OR AD OR VCI) ANDTOPIC: ("Animal Assisted" OR "animal facilitated" OR "animal visit" OR "visiting animal" OR CAT OR DOG OR HORSE OR "companion animal*" OR "service animal *") AND TOPIC:(randomly OR randomised OR randomized OR "random allocat*" OR RCT OR CCT OR "double blind*" OR "single blind*" OR "double blind*" OR "single blind*" OR trial) | Dec 2018: 450 Sep 2019: 78 |
| LILACS (BIREME) (Date of most recent search: 5 September 2019) |
"Animal Assisted" OR "animal facilitated" OR "animal visit" OR "visiting animal" OR "companion animal$" OR "service animal$" [Words] and alzheimer OR alzheimers OR alzheimer's OR dementia OR demenc$ [Words] and randomly OR randomised OR randomized OR RCT OR "controlled trial" OR "double blind$" OR placebo [Words] | Dec 2018: 0 Sep 2019: 0 |
| ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 5 September 2019] |
"Animal Assisted" OR "animal facilitated" OR "animal visit" OR "visiting animal" OR CAT OR DOG OR HORSE OR "companion animal*" OR "service animal*" | dementia OR alzheimers OR cognition OR cognitive | Dec 2018: 417 Sep 2019: 16 |
| ICTRP [Date of most recent search: 5 September 2019] |
"Animal Assisted" OR "animal facilitated" OR "animal visit" OR "visiting animal" OR "companion animal*" OR "service animal*" | dementia OR alzheimers OR cognition OR cognitive | Dec 2018: 144 Sep 2019: 58 |
| TOTAL before de‐duplication | Dec 2018:2517 Sep 2019:493 TOTAL: 3010 |
|
| TOTAL after deduplication | Dec 2018: 1879 Sep 2019: 476 TOTAL: 2355 |
|
| TOTAL after first assessment of titles and abstract by CDCIG information specialists (performed for the first search but not the top‐up search) | Dec 2018:73 Sep 2019: 476 TOTAL: 549 |
|
Data and analyses
Comparison 1. Animal‐assisted therapy (AAT) versus no AAT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Affect and emotional well‐being: depression measured using Cornell Scale for Depression in Dementia (CSDD) | 2 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐5.24, ‐0.50] |
| 1.1 Individually randomised trial | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐3.55 [‐6.87, ‐0.23] |
| 1.2 Cluster RCT | 1 | 46 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐5.54, 1.20] |
| 2 Affect and emotional well‐being: apathy, measured using Apathy Evaluation Scale (AES) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐0.58, 4.20] |
| 3 Affect and emotional well‐being: depressed or anxious mood, measured using Multidimensional Observation Scale for Elderly Subjects (MOSES) Depressed or Anxious Mood subscale | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐3.52, 2.92] |
| 4 Social functioning: MOSES Withdrawn Behaviour subscale | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐3.41, 2.61] |
| 5 Behaviour: measured using Nursing Home Behaviour Problem Scale (NHBPS), Neuropsychiatric Inventory (NPI), or MOSES Disoriented Behaviour subscale | 3 | 142 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.98, 0.30] |
| 5.1 AAT using horse | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.34, ‐0.14] |
| 5.2 AAT using dog | 2 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.64, 0.44] |
| 6 Agitation: measured using Cohen‐Mansfield Agitation Inventor (CMAI), Brief Agitation Rating Scale (BARS), or MOSES Irritability subscale | 3 | 143 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.89, 0.10] |
| 6.1 Individually randomised trial | 2 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.04, ‐0.21] |
| 6.2 Cluster‐randomised controlled trial (RCT) | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.49, 0.64] |
| 7 Quality of life (Quality of Life in Late‐Stage Dementia (QUALID)) | 3 | 164 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.28, 2.18] |
| 7.1 Individually randomised trial | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐3.57, 2.79] |
| 7.2 Cluster‐RCT | 2 | 96 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐2.53, 3.41] |
| 8 Physical functioning, measured using Barthel Index for ADL | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 4.65 [‐16.05, 25.35] |
| 9 Physical functioning: balance, measured using Berg's Balance Scale | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐2.29 [‐5.66, 1.08] |
| 10 Physical functioning: self‐care ability measured using MOSES Self‐Care Functioning subscale | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.23, 5.63] |
Comparison 2. Animal‐assisted therapy (AAT; live animal) versus robotic animals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Behaviour: measured using Neuropsychiatric Inventory (NPI) | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐14.58, 0.66] |
| 2 Quality of life: measured using Quality of Life in Late‐Stage Dementia (QUALID) | 1 | 78 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐5.71, 0.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dabelko‐Schoeny 2014.
| Methods | Randomised cross‐over trial Setting: ADS centre in Mid‐West metropolitan area of the US |
|
| Participants | Number of participants: 16 People with Alzheimer's disease. About 1/3 participants relied on wheelchairs for mobility. |
|
| Interventions | Intervention: equine‐assisted therapy, which included grooming and interaction with the horses, followed by painting and washing the horses, and ended by feeding the horses. 4 therapy horses aged 12–22 years were used. The intervention took place 1 day per week for 4 weeks. Control: standard care, which included crafts, rest periods, exercise, or discussion groups. After 4 weeks, the control group received equine‐assisted therapy while the group that received this intervention earlier served as controls with standard care as mentioned. Sessions facilitated by equine‐assisted learning certified and horse‐handler staff from an equine education centre. |
|
| Outcomes | Behaviour and Affect (modified Philadelphia Geriatrics Centre Affect Rating Scale), disruptive behaviours (Modified NHBPS; 0–88, higher score indicates worse behaviour), and salivary cortisol concentrations. Outcomes assessed 4 weeks after commencement of intervention or control. |
|
| Notes | Among the outcomes reported, only disruptive behaviours (Modified NHBPS) were reported separately for each phase, although no paired data were extractable. We evaluated the impact of excluding this study via sensitivity analyses, as reported in the 'Effects of intervention' under the outcome of 'Behaviour'. Study funded by a personal donation, as stated in the acknowledgement: "This work was supported by a generous donation to The Ohio State University College of Veterinary Medicine by Mr. Duncan Alexander." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods, design: quote: "Participants: once the pool of participants were identified, each individual was randomly assigned to either the first or second intervention period group using a computer‐generated list of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Methods, design: quote: "In this design, one randomly selected group received the intervention while the second group served as the comparison group." There was no further information on sequence generation and allocation to enable a meaningful assessment on the relationship between sequence generation and allocation. There were some imbalance in certain baseline characteristics of the allocated group, including a higher proportion of females, a higher score in MMSE and a lower proportion of those with instrumental assistance in daily living in the group that received equine‐assisted therapy first. However, it is unclear whether such differences would affect the outcome and in which direction, especially in view of the cross‐over nature of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but blinding was highly unlikely as 1 group received equine‐assisted therapy while another group did not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not clearly stated, but the day centre staff and researchers who obtained the outcome information were highly unlikely to have been blinded, as the timing of the outcome assessment included the period when participants were receiving either equine‐assisted therapy or standard care. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appeared that all 16 participants initially recruited completed the study. |
| Selective reporting (reporting bias) | High risk | It appeared that the prespecified outcomes of behaviours and affect, disruptive behaviours, and salivary cortisol were reported in the results (although there was no study protocol available for confirmation), but only the Modified NHBPS was reported in sufficient detail for meta‐analysis. |
| Other bias | High risk | Additional domains for cross‐over trials
Based on the risk of bias judgement given in the domains above, we accorded the study an overall high risk of bias in the additional domains specific to cross‐over trials. In accordance with our predefined strategy in handling the unit of analyses issues in cross‐over trials, as detailed under Unit of analysis issues, we would only have used data from the first phase before cross‐over took place if available. However, only NHBPS data, reported in separate phases, were available for meta‐analysis. |
Friedmann 2015.
| Methods | RCT Setting: 7 AL facilities in USA Period of study: December 2010 to December 2012 |
|
| Participants | Number of participants: 40; 22 in intervention group; 18 in control group Member of AL staff identified residents who might meet the inclusion/exclusion criteria. Inclusion criteria: mild‐to‐moderate cognitive impairment (MMSE > 8 and < 23), aged ≥ 55 years, anticipated length of stay in AL facility ≥ 6 months, English speaking, and with either prior experience with or interest in interacting with a dog. |
|
| Interventions | Intervention: 60‐ to 90‐minute PAL sessions with a therapy dog twice per week for 12 weeks. No specific mention on formal training of the facilitators on the use of therapy animal. Unclear whether delivered in individual or group setting. Control: reminiscing sessions twice per week for 12 weeks conducted in a group setting. Reminiscing activities with training on social and motor skills. |
|
| Outcomes | Physical function (Barthel Index 0–100 (best) and ActiGraph); emotional function (CSDD, 0–38 (most severe)), and 7‐Item AES (7 (highest apathy)–28); and behavioural function (CMAI, 14–70 (highest agitation level)). Outcomes assessed 12 weeks after commencement of intervention. |
|
| Notes | Study funded by a research award, as stated in the acknowledgment: "This research was supported by an ISAZ/WALTHAM Collaborative Research Award." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information to enable an assessment on the relationship between sequence generation and allocation. No marked baseline imbalance in major prognostic factors between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, binding appeared highly unlikely, as 1 group received a AAT and the other group did not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Methods, assessments: quote: "Assessments were completed by an independent nurse observer, who was not involved with the intervention. The residence staff member served as the informant for rating the resident's emotional function with the 7‐Item Apathy Evaluation Scale (AES) Cornell Scale for Depression in Dementia (CSDD), the resident's behavioral function with the Cohen‐Mansfield Agitation Inventory (CMAI), and the nurse rated the resident's physical function with the Barthel Index. The same staff member evaluated each resident on all occasions." Although it was not clearly stated whether the assessors of the outcomes were blinded to the allocation status of the participants, this appeared highly unlikely, as the assessors of most outcomes were the nurses or carers of the residents. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 40 participants randomised, 2 residents, both in the PAL group, died due to pre‐existing conditions; heart failure and lung disease in 1 case and pneumonia in 1 case, over the course of the study; and 1 PAL participant moved from the residence prior to the end of the study. Although all 3 participants who withdrew were from the PAL group, we considered the study as having low risk of bias in this domain due to the small number of withdrawals (3/60, 5%). |
| Selective reporting (reporting bias) | Low risk | The major prespecified outcomes of physical, emotional, and behavioural functions were reported in sufficient details in the results. |
| Other bias | Low risk | None identified. |
Holthoff 2013.
| Methods | 2‐arm RCT (Germany) Setting: 2 nursing homes |
|
| Participants | Number of participants: 60 People with dementia |
|
| Interventions | Intervention: 1 weekly session of standardised dog‐assisted therapy session. Performed by specifically trained dog owners using a standardised programme adapted for people with dementia from the Pet Encounters programme. There was no specific mention on formal training of the facilitators on the use of therapy animal. Control: TAU including routine activation in the lounge of the nursing homes (e.g. games, storytelling). Intervention lasted 6 months. |
|
| Outcomes | Social interaction and affective arousal (video‐analyses), and clinical measures using scales such as MMSE, CERAD, ADCS‐ADL, NPI, and Qual‐AD. All measurements were performed at baseline, 6, 12, and 15 months after study commencement. However, extractable quantitative outcome data were not provided in the published abstract. |
|
| Notes | Study published in abstract form. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | No information to enable meaningful assessment on the relationship between sequence generation and allocation. Baseline characteristics of participants according to the allocated groups were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not explicitly stated but blinding appeared highly unlikely, as intervention group received interventions with an animal while control group did not. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on whether the outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of missing data was unclear as there was no information on the number of participants who were loss to follow‐up vs number initially allocated. |
| Selective reporting (reporting bias) | High risk | No specific outcome data presented in the results of the abstract to be included in meta‐analysis, only the following statements: "Analysis for the intervention effect revealed a significant increase in pro‐social behaviour and reduction in behavioural disturbances during the dog interaction when compared to the TAU group. The clinical measures did not differ significantly between both groups at any time point." |
| Other bias | Low risk | No other bias identified. |
Olsen 2016a.
| Methods | Cluster‐RCT Setting: 16 adapted day‐care centres for home‐dwelling people with dementia in the Norwegian counties of Østfold, Vestfold, Oslo, and Akershus, Norway |
|
| Participants | Mean number of people per cluster: 5 (each cluster asked to recruit 5–8 initially)) Number of participants: 80 in total; 42 in intervention group; 38 in control group Inclusion criteria: aged ≥ 65 years, diagnosis of dementia or a cognitive deficit measured as an MMSE score < 25. Exclusion criteria: people with a fear of dogs or with a dog allergy. |
|
| Interventions | Intervention: 30‐minute sessions of AAT in groups of 3–7 participants twice per week for 12 weeks led by a qualified dog handler. Protocol was followed to ensure consistency between the intervention sessions held in the day‐care centres. Intervention had a standardised, strict design, despite 1 study objective being to see whether it was possible to measure effects when AAT occurred in a realistic setting with a representative sample of participants and different dog teams. Since the main aim of the study was to see whether interventions with a dog would have an impact on participants' balance, the protocol was designed with that in mind. For each session, the participants were randomly seated in a semi‐circle, and the dog handler moved around the group so that each participant was able to greet the dog and feed it treats. Next, the handler organised different activities such as petting, brushing, or feeding the dog a treat, or throwing a toy for the dog to fetch. No specific mention on formal training of the facilitators on the use of therapy animal. Duration of intervention: 12 weeks. Control: no AAT |
|
| Outcomes | Balance (BBS; 0–56, higher score indicates better balance) and quality of life (QUALID; 12–45, higher score indicates poorer quality of life) Measurements at baseline (T0), end of intervention period (T1), and 3 months after the completion of the 12‐week intervention (T2). |
|
| Notes | Authors also performed change measure from T0 to T1 and from T1 to T2, and stated that there was statistically significant improvement in BBS from T0 to T1 but not from T1 to T2. We have decided to only report end scores at T1 and T2 as we considered the difference in score at baseline between AAT and control, although statistically significant, was modest (mean difference of 3.76 out of a total score of 56) and not clinically important, with emphasis on end score at T2 after 3 months as the main outcome. The study was supported by university research grant and institute's internal funding, as stated in the finance disclosure: "The project is funded by grant nr. 217516 from the Oslofjordfondet and RFF Hovedstaden, NMBU and Cooperating partners (The Norwegian Centre of Anthrozoology, Buskerud and Vestfold University College, Centre for Development of Institutional and Home Care Services in Vestfold, Nøtterøy municipality). Cooperating partners supported the project with internal financing." Although this study appeared similar to Olsen 2016b, we did not have sufficient evidence to suggest that they were the same study with the same, or overlapping, participants, as the setting of the studies differed (this study enrolled home‐dwelling people attending day‐care centres, while Olsen 2016b enrolled nursing home residents), and they were registered as two separate studies in ClinicalTrial.gov. We have written to the main author for confirmation with no reply. Therefore, we decided to consider these as 2 separate, but possibly related, studies, and made corresponding notes as appropriate in our report of the results and Table 1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Materials and methods: quote: "After recruitment, each day‐care center was randomized, by computerized random numbers at Uni Helse in Bergen, to either animal‐assisted activity with a dog (AAA) or to a control group." |
| Allocation concealment (selection bias) | Unclear risk | Although the statement above suggests that randomisation was probably performed centrally, there was no clear statement to confirm the independence of allocation from randomisation. In terms of baseline characteristics, other than a higher proportion of participants in the AAT group who used rollator (14 in AAT group vs 5 in control group) among other types of walking aids, there were no marked differences in the baseline characteristics between groups. We are uncertain on the overall impact of the higher proportion of rollator users in the AAT group on the outcome. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding was highly unlikely as 1 group received AAT while the other group did not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Methods, authors stated that the outcomes were assessed by "pre‐trained health care workers working at the day‐care centres" who most likely knew the allocation status of the participants. This is confirmed in the discussion: "The assessments were not blind, which with QUALID is impossible because of the required profound knowledge of the person." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from 21/80 (26.3%) participants were missing for BBS, while 2/80 (2.5%) were missing for QUALID score. The number of participants with missing data per group was not stated. Missing data were imputed. Based on the high absolute proportion of missing data for BBS, we accorded the study high risk for this domain. |
| Selective reporting (reporting bias) | Low risk | The 2 major outcomes prespecified in the trial registration record of this study (NCT02008630), namely balance and quality of life, were reported in sufficient details in the results. |
| Other bias | Unclear risk | Additional domains for cluster‐RCT.
Overall, due to the unclear risks in 2 domains in the risk of bias area of cluster‐RCT, the study was rated at unclear risk in the domains specific to cluster‐RCT. |
Olsen 2016b.
| Methods | Cluster‐RCT Settings: 90 nursing homes in 3 Norwegian counties, 10 adapted units for residents with dementia agreed to participate. Nursing homes had to provide the facilities required to carry out the interventions. |
|
| Participants | Participants had to abstain from any dog‐visiting activities for 3 months prior to the intervention, and during the whole intervention period. The health personnel in the nursing homes were asked to recruit 5–8 participants each. Inclusion criteria: aged ≥ 65 years with dementia or a cognitive deficit MMSE score < 25, excluded nursing home residents with fear of dogs or with a dog allergy. Of 130 eligible people in the 10 units, 58 (45%) agreed to participate; 7 (12%) died during the study period and were subsequently excluded. Thus, the study population consisted of 51 participants (24 in intervention group, 27 in control group). |
|
| Interventions | Intervention: 30‐min session with AAT twice weekly for 12 weeks in groups of 3–6 participants led by a qualified dog handler. For each session, the participants were randomly seated in a semi‐circle. Each session started with a greeting round, when each participant had the opportunity to pet the dog and feed it treats. Thereafter, the handler started the different activities, which included any of the following: petting the dog, feeding the dog a treat and throwing a toy for the dog to fetch. All activities were supposed to follow the protocol but should have been individually tailored to each participant based on the health personnel's knowledge of the participant. However, no activities were mandatory, and the sessions therefore included activities that occurred between the participants and between each participant and the dog. No specific mention on formal training of the facilitators on the use of therapy animal. Control: no new activities, and TAU, including diverse group activities such as reminiscence, music therapy, sensory garden, singing, exercise, cooking, and handicrafts. |
|
| Outcomes | Depression (CSDD; 0–19, higher score indicates more severe depressive symptoms); agitation and restlessness (BARS; 10–70, higher score indicates more severe agitation); quality of life (QUALID; 12–45, higher score indicates poorer quality of life) Measurements at baseline, end of intervention period, and 3 months after the completion of the 12‐week intervention. |
|
| Notes | Study was supported by a university research grant and institute's internal funding, as stated in the financial disclosure: "The project is funded by grant nr. 217516 from the Oslofjordfondet and RFF Hovedstaden, NMBU and Cooperating partners (The Norwegian Centre of Anthrozoology, Buskerud and Vestfold University College, Centre for Development of Institutional and Home Care Services in Vestfold, Nøtterøy municipality). Cooperating partners supported the project with internal financing." Although this study appeared similar to Olsen 2016a, we did not have sufficient evidence to suggest that they were the same study with the same, or overlapping, participants, as the setting of the studies differed (this study enrolled nursing home residents, while Olsen 2016a enrolled home‐dwelling people attending day‐care centres), and they were registered as 2 separate studies in ClinicalTrial.gov. We have written to the main author for confirmation with no reply. Therefore, we decided to consider these as 2 separate, but possibly related, studies, and made corresponding notes as appropriate in our report of the results and Table 1. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods: quote: "Computer‐generated random numbers were used to randomize nursing home units to either an AAA group with a dog or to a control group with treatment as usual." |
| Allocation concealment (selection bias) | Unclear risk | Although the statement above suggests that randomisation was probably performed centrally, there was no clear statement to confirm the independence of allocation from randomisation. The was no marked imbalance in the baseline characteristics between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding was highly unlikely as 1 group received AAT while the other group did not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessments were performed by health professionals who worked in the nursing homes which most likely would be aware of the interventions assigned to the participants. Quote: "Two health professionals from each nursing home unit attended lectures with instructions on how to use the instruments. They later scored all assessments at all three time points," |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/51 (5.8%) participants (2 from AAT group and 1 from control group) lost to follow‐up. The participants who were lost to follow‐up were included in the analysis with their outcome data imputed by "person mean substitution method" where applicable. We considered the study as having low risk of bias in this domain due to the small number lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes (depression, agitation and restlessness, and quality of life) were reported in sufficient details in the results. |
| Other bias | High risk | Additional domains for cluster‐RCT.
Overall, due to the unclear risks in 1 domain in the risk of bias area of cluster‐RCT, the study was rated at unclear risk in the domains specific to cluster‐RCT. |
Quibel 2017.
| Methods | 2‐arm RCT Setting: protected unit "with a dementia‐related syndrome" in France |
|
| Participants | People with a dementia syndrome with behavioural problems living in assisted protected unit of a nursing home. At time of study, protected unit only accommodated women; the groups are therefore composed of women only. Number of participants: 12 in total; 6 per group |
|
| Interventions | Intervention: AAT, in the form of 5 AAT sessions with a dog and a female facilitator. No specific mention on formal training of the facilitators on the use of AAT. Control: received cooking workshops with female facilitators. 30‐ to 45‐minute sessions held every 2 weeks at the same time (end of morning) and in the same room. |
|
| Outcomes | Behavioural disorders in daily life and care. Participants' behaviours recorded before, during, and after each AAT session and cooking workshop session. NPI and MMSE, supplemented by semi‐directive interviews conducted with participants who were able to respond to questions before and after the study. However, the results were only presented descriptively, with no quantitative outcome data available to be extracted for meta‐analysis, and no relevant tables or appendices. We summarised the results of this study narratively in our discussion. Time point of outcome assessment not stated. |
|
| Notes | Article translated from French from a translator who responded via Cochrane Task Exchange, as stated in our Acknowledgements. Funding sources not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although authors stated this was a "randomised, comparative study," there was no mention of the methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No information to enable an assessment on the relationship between sequence generation and allocation concealment. Overall number of participants was small, and all were women, and there was no marked imbalance in the other baseline characteristics between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding was highly unlikely, as 1 group received AAT and the other group cooking demonstration, while all participants were living in the same premise. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether outcome assessors were blinded to allocation of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | While not directly stated, it appeared that the outcome data for all participants were obtained. |
| Selective reporting (reporting bias) | High risk | While it appeared that all outcomes data were collected, they were only summarised narratively in the results on the difference between groups, with no extractable figures in the texts, table, or appendices to be included in the meta‐analysis. Consequently, this article did not contribute data to our quantitative analysis. |
| Other bias | Low risk | None identified. |
Thodberg 2016.
| Methods | 3‐arm RCT Setting: 4 nursing homes in Denmark. Residents from a broad spectrum of the elderly population, but with a high prevalence of people with dementia at different stages. |
|
| Participants | 124 nursing home residents | |
| Interventions | Following are 3 types of interventions evaluated, all of which consisted of twice‐weekly visits for 6 weeks, totalling 12 visits: Intervention 1: visits with a dog (either a retriever or a retriever mix) (n = 36) Intervention 2: visits with a robot seal (n = 41) Intervention 3: visits with a soft toy cat (n = 34) Of 124 participants enrolled, 111 were initially randomised, as above. During the visits, the visitors interacted with the participants making use of the animals to enhance the experience by encouraging interaction between the participants and the animals. Other than stating that the dog owners (the facilitators) were certified to work as volunteers with dog‐assisted interventions in nursing homes, there was no specific mention on formal training of the facilitators on the use of AAT. |
|
| Outcomes | Behavioural observation, using a composite sum of the frequencies in "physical contact" and "talk to the visiting animal." However, only the duration of engagement (contact and talking) were reported in numerical figures. The data were in the form of median and interquartile range, hence unsuitable for meta‐analysis and were reported narratively in our results. The other outcome were only reported in terms of P values or in graphical forms, which were not extractable. The authors performed certain measures of affective and cognitive functions, such as the MMSE, Gottfries‐Brane‐Steen scale (which evaluates disabilities, language, psychiatric symptoms, mean daily living function, and behaviour of the participants), and Geriatric Depression Scale, but these were measured only at baseline and not as an outcome measure. Outcomes assessed 6 weeks after the commencement of the intervention. |
|
| Notes | Financed by TrygFonden, Denmark, who also mediated the contact to dog owners. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods, Design: quote: "The design of the study was a randomized complete block design. Each nursing home was a block, and after informed consent had been given, the participants were assigned randomly to one of three visit types, using a program for blocked randomization in R software (R CoreTeam 2013)." |
| Allocation concealment (selection bias) | Unclear risk | Although the statement above suggests that randomisation was probably performed centrally, there was no clear statement to confirm the independence of allocation from randomisation. The authors stated that there were no significant difference among the 3 groups on the baseline psychiatric scores. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding was highly unlikely as 1 group received AAT while the other groups received robots or soft toys. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "One person, the visitor, accompanied the animal, and the other person, the observer, made direct observations and a video recording of the visit." The study mentioned that, "The visitors were part of the project staff and were not the owners of the dogs," but there was no further information clarifying the background of the observer, or whether they were from the nursing homes or were project staff. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 124 enrolled participants, the authors stated that 24 dropped out during the experimental period either due to illness or because they did not want to receive the visit. However, we found that 111 participants were randomised initially, suggesting that some of the 24 who dropped out did so before randomisation and some after, with no clarification provided by the authors. In the last analysis, only 98 participants remained. The attrition rate per group from the first to the last analysis was 2 (5.6%) in the AAT group, 7 (17.1%) in the seal group, and 4 (11.8%) in the toy cat group. However, it was unclear how many of the 24 participants who were stated to have dropped out by the author were from each group. We rated this domain as high‐risk due to the overall high dropout rate and the unclear information regarding specific attrition rate from each group. |
| Selective reporting (reporting bias) | High risk | Among the outcome data, only the duration of engagement (contact and talking) were reported numerically, although as the data were in the form of medians and interquartile ranges, it was unsuitable for meta‐analysis and was reported narratively in our results. The other outcome were only reported in terms of P value or in graphical forms, which were not extractable. |
| Other bias | Low risk | None identified |
Valenti‐Soler 2015.
| Methods | 3‐arm RCT (Spain), with 2‐arm comparison in phase 2 where live animals were used. Setting: Alzheimer Center Reina Sofia Foundation, a public nursing home and day‐care centre in Spain. Period of study: 2012–2013 |
|
| Participants | People with dementia. Study consisted of 2 phases, with 101 participants in phase 1 and 110 in phase 2. The review focuses on phase 2 as phase 1 did not involve live animals. | |
| Interventions | Structured therapeutic sessions of 30–40 minutes using either pet robot (group 1, n = 42), live animals (dogs) (group 2, n = 36) and control (group 3, n = 32) (phase 2 of the study, as phase 1 did not involve the use of live animals), run by certified occupational therapists and physiotherapists. All sessions had the same overall structure: greeting the group, introduction, therapeutic exercises (cognitive or physical therapy) and ending. It was not stated how the sessions of the control group were run, except that they did not receive AAT. The dogs used in the therapy were black Labrador retrievers. The authors stated the therapists specifically trained to work with animals attended all the sessions with the dogs, and the animals received training prior to the sessions. The intervention was administered for 12 weeks, 2 days a week. |
|
| Outcomes | GDS scores (1–7, higher score indicates more severe cognitive decline), sMMSE and MMSE scores (0–30 for both scales, higher score indicates better cognitive function), NPI scores (0–144, higher scores indicate more severe neuropsychiatric symptoms), APADEM‐NH scores (0–78; 26 items, 3 points each, higher scores indicate more severe apathy) and Apathy Inventory scores (3–36; 3 questions, each ranges from 1 to 12), higher scores indicate more severe apathy), QUALID scores (12–45, higher score indicates poorer quality of life). Outcomes assessed 12 weeks after the commencement of the interventions or control. |
|
| Notes | Only data from phase 2 of the study were extracted as phase 1 did not involve the use of live animals. Out of these, only QUALID and NPI data were sufficient for meta‐analysis, as the other outcome data were expressed in graphical form as mean change without accompanying measures of dispersion such as standard errors or standard deviations. The study was funded by a national research grant from the Spanish Ministry of Science and Innovation and the Spanish Ministry of Health, Social Policy and Equality. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods, design: quote: "Randomization was performed before the baseline evaluations using a six‐sided dice." |
| Allocation concealment (selection bias) | Unclear risk | There was no clear statement to confirm the independence of allocation from randomisation. There were no marked differences in baseline characteristics between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding was highly unlikely as 1 group received AAT while the other groups did not. The therapist who conducted the sessions was employed by the nursing home. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluation was carried out by blinded raters. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all 101 participants (Phase 1) and 110 participants (Phase 2) were reported. |
| Selective reporting (reporting bias) | High risk | All outcomes were reported in the results (GDS, QUALID, sMMSE, and NPI). However, GDS and sMMSEs were expressed in graphical forms as mean change without accompanying measurement of dispersion such as standard errors or standard deviations, and hence were insufficient for meta‐analysis. Only QUALID and NPI scores were available. |
| Other bias | Low risk | None identified. |
Zisselman 1996.
| Methods | 2‐arm RCT (USA) Setting: Wills Eye Hospital Geriatric Psychiatry Unit, Philadelphia, PA Period of study: February to May 1994 |
|
| Participants | Elderly people with chronic age‐related psychiatric, medical, and neurological conditions such as depression, dementia, Parkinson's disease, stroke, and accompanying medical disorders Number of participants: 33 in intervention group; 25 in control group |
|
| Interventions | Intervention: participants had contact with and fed the visiting dogs, and were encouraged to reminisce about their own experiences with pets and other animals; and heard a brief talk about the dogs. 1 hour per day for 5 consecutive days. No specific mention on formal training of the facilitators on the use of therapy animal. Control: exercise therapy. 1 hour per day for 5 consecutive days. |
|
| Outcomes | MOSES (8–32, higher score indicates increased impairment). Outcomes assessed at day 5 after the commencement of intervention. |
|
| Notes | Study funded in part by a grant from Sandoz/Jeff's Companion Animal Centre. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention on the methods of sequence generation. Methods, settings, and subjects: quote: "All patients hospitalised on the unit between February 1994 and May 1994 (N = 58) were randomly assigned to receive the pet therapy intervention (n = 33) or an exercise intervention (n=25), which is the unit's usual activity programming." |
| Allocation concealment (selection bias) | Unclear risk | No clarification on the methods and independence of random allocation. Methods, settings, and subjects: quote: "All patients hospitalised on the unit between February 1994 and May 1994 (N = 58) were randomly assigned to receive the pet therapy intervention (n = 33) or an exercise intervention (n 25), which is the unit's usual activity programming." There were no major differences in the demographic characteristics between groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not clearly stated, blinding was highly unlikely as 1 group received AAT while the other group did not (exercise therapy). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurement and procedure: quote: "A research assistant who was blinded to the group assignments completed the MOSES for each subject by interviewing the nursing staff member most familiar with that subject. The nursing staff member was also blind to group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes for all 58 participants were reported in the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes (MOSES‐self‐care functioning, disoriented behaviour, depressed or anxious mood, irritable behaviour, withdrawn behaviour) were reported in sufficient details in the results. |
| Other bias | Low risk | None identified |
AAT: animal‐assisted therapy; ADCS‐ADL: Alzheimer's Disease Activities of Daily Living International Scale; AES: Apathy Evaluation Scale; AL: assisted living; APADEM‐NH: Apathy Scale for Institutionalised Patients with Dementia Nursing Home Version; ADS: adult day service; BARS: Brief Agitation Rating Scale; BBS: Berg's Balance Score; CMAI: Cohen‐Mansfield Agitation Inventory; CSDD: Cornell Scale for Depression in Dementia; GDS: Global Deterioration Scale; MMSE: Mini‐Mental State Examination; MOSES: Multidimensional Observation Scale for Elderly Subjects; NHBPS: Nursing Home Behavior Problem Scale; NPI: Neuropsychiatric Inventory; PAL: pet‐assisted living; QUALID: Quality of Life in Late‐Stage Dementia; Qual‐AD: Quality of Life in Alzheimer's Disease; RCT: randomised controlled trial; sMMSE: Severe Mini‐Mental State Examination; TAU: treatment as usual.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12609000564257 | Cross‐over study on the effect of personalised Montessori‐type intervention in managing difficult behaviours for people with dementia. Study did not examine AAT. Basis of exclusion: intervention. |
| Antonelli 2012 | RCT evaluating effects of AAT on older adults with no or mild cognitive impairment, with a cut‐off MMSE score of 18/30, who visit a day‐care service. None of the participants were diagnosed with dementia (communication with the contact author). Although the cut‐off for MMSE was below the conventional cut‐off of 24 for dementia, diagnosing dementia requires more tests than MMSE, and on that consideration with additional information provided by the author, we decided to exclude this study. Basis of exclusion: population. |
| Boehmer 2016 | Non‐randomised, single‐group, 2‐phase study that examined the effects of having a residential dog on quality of life and loneliness in people with dementia who were living in a community living centre. Basis of exclusion: study design. |
| Borges 2019 | Case series that described the effects of EAT on balance, functional capacity, and cognition in older adults diagnosed with Alzheimer's disease. Basis of exclusion: study design. |
| Cesari 2014 | Review that described the phenomenon of "horse‐racing effect" in clinical trials in older people, utilising the horse racing analogy with some of the observed difference in certain physiological parameters (e.g. blood pressure) that occur with ageing. Basis of exclusion: relevance of topic. |
| Churchill 1999 | Within‐subject study with repeated measure design that examined the effects of a therapy dog on agitation and desocialisation in people with Alzheimer's disease. Basis of exclusion: study design. |
| Colombo 2006 | Non‐randomised study on the effects of AAT on a group of non‐cognitively impaired institutionalised older adults. Basis of exclusion: study design and population. |
| Farid 2019 | Review of AAT with dogs in people with dementia. Basis of exclusion: study design. |
| Gocheva 2018 | RCT that examined the effects of AAT on people with acquired brain injuries. The causes of acquired brain injuries included traumatic and non‐traumatic (mainly stroke). Basis of exclusion: population. |
| Hu 2018b | Systematic review and meta‐analysis on AAT for people with cognitive impairment. Basis of exclusion: study design. |
| ISRCTN31919196 | Non‐randomised observational study on the role of Protected Engagement Time for people with dementia. Basis of exclusion: study design and intervention. |
| Kanamori 2001 | Non‐randomised comparative study that assessed the effects of AAT on participants with senile dementia. The experimental group consisted of participants who liked animals, and the control group of participants who did not like animals. Basis of exclusion: study design. |
| Ko 2016 | RCT that assessed the effects of pet insects on psychological outcomes of relatively healthy community‐dwelling older adults. Basis of exclusion: population. |
| Majic 2013 | "Matched case control study" that assessed the effects of AAT using therapy dogs (border collies) in nursing home residents with dementia. As the study was matched case control, the allocation of the participants to the intervention and control group was not performed randomly. However, there was no mention on the basis of allocation of participants to AAT and control groups. Basis of exclusion: study design. |
| Menna 2016 | Non‐randomised, controlled study that assessed the use of AAT using dogs on people with Alzheimer's disease in a day‐care centre. The allocation was non‐random as (quote) "The AAT group included 20 patients (16 women, 4 men) selected according to the following criteria: (i) absence of fear or aversion towards the dog; (ii) willingness to interact with the dog; and (iii) patient's personal history with animals (i.e. patient had a dog in the past)." Basis of exclusion: study design. |
| Moretti 2011 | Non‐randomised, 2‐arm comparative study of AAT and control in Italy, where cases and controls were selected on the basis of sociodemographic and clinical features. Basis of exclusion: study design. |
| Nordgren 2014 | Non‐randomised, comparative study by clusters (nursing home) that examined the effects of dog‐assisted therapy on behavioural and psychological symptoms of dementia. Basis of exclusion: study design. |
| NTR5026 | Assessed the diagnostic utility of amyloid measured by positron‐emission tomography scan as a diagnostic marker for dementia. AAT was not examined. Basis of exclusion: relevance of study topic. |
| Peluso 2018 | Review on AAT for elderly people with dementia and psychiatric disorders. Basis of exclusion: study design. |
| Richeson 2003 | Pre‐and‐post study on the effects of AAT on agitated behaviours and social interactions of older adults with dementia. Basis of exclusion: study design. |
| Scanes 2018 | Commentary on the roles of animals in entertainment. Basis of exclusion: article type (not an original study). |
| Spattini 2018 | Systematic review and meta‐analysis on the effects of AAT for people with mental disorders. Basis of exclusion: study design. |
| Zafra‐Tanaka 2019 | Systematic review and meta‐analysis on the effects of dog‐assisted therapy for adults with dementia. Basis of exclusion: study design. |
AAT: animal‐assisted therapy; EAT: equine‐assisted therapy; MMSE: Mini‐Mental State Examination; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
ISRCTN93568533.
| Trial name or title | A study into the effects of activities with animals on the well‐being of Dutch nursing home patients with dementia |
| Methods | Single‐centre RCT (Netherlands) |
| Participants | People with dementia living in De Zorgboog, a large Dutch nursing home |
| Interventions | Nursing home clients randomised via computer‐generated random numbers to 3 groups. Group 1: dog‐assisted activity group sessions with handler. Group 2: robot‐assisted activity group sessions with handler (FurReal Friend robot). Group 3: control: group sessions with a visiting student Each participant will participate in 1 intervention session per week. All sessions will be videotaped for the entire duration of the study (8 weeks). Videos will be analysed after the intervention period using video‐coding to calculate the amount of social interactions and neuropsychiatric symptoms displayed during the sessions. Total duration: 8 weeks Follow‐up: 4 weeks |
| Outcomes | Primary outcomes: Social interaction during sessions Neuropsychiatric symptoms during sessions Secondary outcomes: Questionnaires will be used to measure several secondary outcomes at all or a subset of the following time points: baseline (t0), after 4 weeks (t1, halfway), after 8 weeks (t2, at the end of the intervention period), after 12 weeks (t3, 4 weeks postintervention follow‐up): Quality of life, measured using QUALIDEM at t0, t1, t2, t3 Depression, measured using CSDD at t0, t1, t2, t3 Neuropsychiatric symptoms, measured using NPI‐Q at t0, t1, t2, t3 Agitation, measured using CMAI at t0, t1, t2, t3 Medication usage, measured using medical records at t0, t2 Intercurrent diseases, measured using medical records at t0, t2 Dementia stage, measured using CDR at t0, t2 Functional state, measured using IDDD at t0, t2 Dementia, assessed using GIP, general dementia assessment tool, Dutch at t0, t2 |
| Starting date | 1 September 2014 |
| Contact information | Ms Lonneke Schuurmans, Postalnr: 5330, Postbus 16, Bakel 5760 AA, Netherlands |
| Notes | No results posted in the study registry record. |
NCT02829801.
| Trial name or title | Evaluation of the animal intervention used as therapy (ELIAUT) |
| Methods | Single‐centre RCT (France) |
| Participants | 40 people > 65 years old who were residents in Charpennes Day Hospital, France who fulfilled the criteria of 'Alzheimer's disease at the major neuro cognitive disorder (NCD) stage with or without vascular disorders. |
| Interventions | Intervention: AAT in addition to cognitive stimulation and rehabilitation of social tie. The participants underwent 8 workshops, 1 per week. The workshop begins with 15‐minute introductory activities that allowed the establishment of the relationship with the therapy dog and the participants, followed by AAT which included games, caresses, and brushing. Control: cognitive stimulation and rehabilitation of social tie. |
| Outcomes | Primary outcome: well‐being (EVIBE scale) Secondary outcomes: psycho‐behavioural symptoms of dementia (NPI, Carer version), depressive symptoms (Geriatric Depression Scale 30 items), anxiety (State‐Trait Anxiety Inventory), cognitive performance (Alzheimer's Disease Assessment Scale – Cognitive subscale, GRECO version) All measured at baseline and 3 months. |
| Starting date | 12 July 2016 |
| Contact information | Pierre Krolak‐Salmon, pierre.krolak‐salmon@chu‐lyon.fr |
| Notes | No results posted in the study registry record. |
AAT: animal‐assisted therapy; CDR: Clinical Dementia Rating; CMAI: Cohen‐Mansfield Agitation Inventory; CSDD: Cornell Scale for Depression in Dementia; GIP: Gedragsobservatie Intramurale Psychogeriatrie; IDDD: Interview for Deteriorating in Daily living activities in Dementia; NPI: Neuropsychiatric Inventory; NPI‐Q: Neuropsychiatric Inventory Questionnaire; RCT: randomised controlled trial.
Differences between protocol and review
In the protocol (Lai 2019), we stated that we would use Covidence for screening, selection, data collection, and risk of bias assessment. In the review, we did not use Covidence, as the search results were retrieved by the review group Trial Search Co‐ordinators and sent to the authors team as a text file (which was not compatible with the format required by Covidence), from which screening, selection, and data extraction were performed.
Contributions of authors
NML: screened and selected studies, inspected the abstracts or full texts, extracted data, checked accuracy of data entry in RevMan, assessed risk of bias, and wrote the review.
SMWC: screened and selected studies, extracted data, transferred data to Review Manager 5, and wrote the review.
SSN: inspected the abstracts or full texts for selection, and wrote the review.
SLT: wrote the review.
NC: wrote the review.
FS: assessed risk of bias, acted as arbiter, and wrote the review.
Sources of support
Internal sources
Taylor's University Flagship Research Grant: evidence synthesis projects on interventions to improve physical, mental, and social well‐being of older adults, Malaysia.
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the National Health Service (NHS), or the Department of Health
Declarations of interest
NML: none.
SMWC: none.
SSN: none.
SLT: none.
NC: none.
FS: none.
New
References
References to studies included in this review
Dabelko‐Schoeny 2014 {published data only}
- Dabelko‐Schoeny H, Phillips G, Darrough E, DeAnna S, Jarden M, Johnson D, et al. Equine‐assisted intervention for people with dementia. Anthrozoos 2014;27(1):141‐55. [Google Scholar]
Friedmann 2015 {published data only}
- Friedmann E, Galik E, Thomas SA, Hall PS, Chung SY, McCune S. Evaluation of a pet‐assisted living intervention for improving functional status in assisted living residents with mild to moderate cognitive impairment: a pilot study. American Journal of Alzheimer's Disease and Other Dementias 2015;30(3):276‐89. [PUBMED: 25118333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holthoff 2013 {published data only}
- Holthoff V, Beckmann A, Gerner A, Wesenberg S, Werner J, Marschner K, et al. Dog‐assisted therapy for people with dementia: a randomized, controlled trial. Alzheimer's & Dementia 2013;9(4):P297. [Google Scholar]
Olsen 2016a {published data only}
- NCT02008630. Animal‐assisted interventions in health promotion for elderly with dementia. clinicaltrials.gov/show/nct02008630 (first received 11 December 2013). [NCT02008630]
- Olsen C, Pedersen I, Bergland A, Enders‐Slegers MJ, Ihlebaek C. Effect of animal‐assisted activity on balance and quality of life in home‐dwelling persons with dementia. Geriatric Nursing 2016;37(4):284‐91. [PUBMED: 27155968] [DOI] [PubMed] [Google Scholar]
Olsen 2016b {published data only}
- NCT01998490. Animal‐assisted or robot‐assisted interventions in health promotion for elderly with dementia. clinicaltrials.gov/show/nct01998490 (first received 29 November 2013). [NCT01998490]
- Olsen C, Pedersen I, Bergland A, Enders‐Slegers MJ, Patil G, Ihlebaek C. Effect of animal‐assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. International Journal of Geriatric Psychiatry 2016;31(12):1312‐21. [PUBMED: 26807956] [DOI] [PubMed] [Google Scholar]
Quibel 2017 {published data only}
- Quibel C, Bonin M, Bonnet M, Gaimard M, Mourey F, Moesch I, et al. Evaluation of the animal‐assisted therapy in Alzheimer's disease [Evaluation de l'effet therapeutique de la mediation animale dans la maladie d'Alzheimer]. Soins Gerontologie 2017;22(125):35‐8. [PUBMED: 28533045] [DOI] [PubMed] [Google Scholar]
Thodberg 2016 {published data only}
- Thodberg K, Sorensen LU, Videbech PB, Poulsen PH, Houbak B, Damgaard V, et al. Behavioral responses of nursing home residents to visits from a person with a dog, a robot seal or a toy cat. Anthrozoos 2016;29(1):107‐21. [Google Scholar]
Valenti‐Soler 2015 {published data only}
- Valenti Soler M, Aguera‐Ortiz L, Olazaran RJ, Mendoza RC, Perez MA, Rodriguez PI, et al. Social robots in advanced dementia. Frontiers in Aging Neuroscience 2015;7:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zisselman 1996 {published data only}
- Zisselman MH, Rovner BW, Shmuely Y, Ferrie P. A pet therapy intervention with geriatric psychiatry inpatients. American Journal of Occupational Therapy 1996;50(1):47‐51. [PUBMED: 8644836] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12609000564257 {published data only}
- ACTRN12609000564257. Personalised, one‐to‐one interaction using Montessori‐type activities as a treatment of challenging behaviours in people with dementia: a pilot controlled trial. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=308163 (first received 9 July 2009). [ACTRN12609000564257]
Antonelli 2012 {published data only}
- Antonelli E, Cusinato E. Animal‐assisted activities: effects on subjective well‐being of elderly women attending a day care service. Giornale di Gerontologia 2012;60(4):215‐23. [Google Scholar]
Boehmer 2016 {published data only}
- Boehmer EE. Effect of a residential dog on quality of life and loneliness in veterans living at the North Florida/South Georgia VAMC, Community Living Center Lake City Division; CAPC National Seminar; 2016 Oct 26‐29; Orlando (US);A24‐A25. 2016.
Borges 2019 {published data only}
- Borges de Araujo T, Martins WR, Freitas MP, Camargos E, Mota J, Safons MP. An exploration of equine‐assisted therapy to improve balance, functional capacity, and cognition in older adults with Alzheimer disease. Journal of Geriatric Physical Therapy (2001) 2019;42(3):E155‐60. [DOI] [PubMed] [Google Scholar]
Cesari 2014 {published data only}
- Cesari M, Canevelli M. Horse‐racing effect and clinical trials in older persons. Frontiers in Aging Neuroscience 2014;6:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Churchill 1999 {published data only}
- Churchill M, Safaoui J, McCabe BW, Baun MM. Using a therapy dog to alleviate the agitation and desocialization of people with Alzheimer's disease. Journal of Psychosocial Nursing and Mental Health Services 1999;37(4):16‐22. [PUBMED: 10218187] [DOI] [PubMed] [Google Scholar]
Colombo 2006 {published data only}
- Colombo G, Buono MD, Smania K, Raviola R, Leo D. Pet therapy and institutionalized elderly: a study on 144 cognitively unimpaired subjects. Archives of Gerontology and Geriatrics 2006;42(2):207‐16. [PUBMED: 16191447] [DOI] [PubMed] [Google Scholar]
Farid 2019 {published data only}
- Farid A. Review of animal assisted therapy with visiting dogs in dementia. Age and Ageing 2019;48 (Suppl 1):i32. [Google Scholar]
Gocheva 2018 {published data only}
- Gocheva V, Hund‐Georgiadis M, Hediger K. Effects of animal‐assisted therapy on concentration and attention span in patients with acquired brain injury: a randomized controlled trial. Neuropsychology 2018;32(1):54‐64. [PUBMED: 29035068] [DOI] [PubMed] [Google Scholar]
Hu 2018b {published data only}
- Hu M, Zhang P, Leng M, Li C, Chen L. Animal‐assisted intervention for individuals with cognitive impairment: a meta‐analysis of randomized controlled trials and quasi‐randomized controlled trials. Psychiatry Research 2018;260:418‐27. [DOI] [PubMed] [Google Scholar]
ISRCTN31919196 {published data only}
- ISRCTN31919196. Protected Engagement Time (PET) for people with dementia. www.isrctn.com/ISRCTN31919196 (first received 16 October 2013). [ISRCTN31919196]
Kanamori 2001 {published data only}
- Kanamori M, Suzuki M, Yamamoto K, Kanda M, Matsui Y, Kojima E, et al. A day care program and evaluation of animal‐assisted therapy (AAT) for the elderly with senile dementia. American Journal of Alzheimer's Disease and Other Dementias 2001;16(4):234‐9. [PUBMED: 11501346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2016 {published data only}
- Ko HJ, Youn CH, Kim SH, Kim SY. Effect of pet insects on the psychological health of community‐dwelling elderly people: a single‐blinded, randomized, controlled trial. Gerontology 2016;62(2):200‐9. [PUBMED: 26383099] [DOI] [PubMed] [Google Scholar]
Majic 2013 {published data only}
- Majic T, Gutzmann H, Heinz A, Lang UE, Rapp MA. Animal‐assisted therapy and agitation and depression in nursing home residents with dementia: a matched case‐control trial. American Journal of Geriatric Psychiatry 2013;21(11):1052‐9. [PUBMED: 23831177] [DOI] [PubMed] [Google Scholar]
Menna 2016 {published data only}
- Menna LF, Santaniello A, Gerardi F, Maggio A, Milan G. Evaluation of the efficacy of animal‐assisted therapy based on the reality orientation therapy protocol in Alzheimer's disease patients: a pilot study. Psychogeriatrics 2016;16(4):240‐6. [PUBMED: 26370064] [DOI] [PubMed] [Google Scholar]
Moretti 2011 {published data only}
- Moretti F, Bernabei V, Marchetti L, Bonafede R, Forlani C, Ronchi D, et al. P01‐364 – a pet therapy intervention on elderly inpatients: an epidemiological study. 18th European Congress of Psychiatry; 2010 Feb 27‐Mar 2; Munich (Germany);577. 2010.
- Moretti F, Ronchi D, Bernabei V, Marchetti L, Ferrari B, Forlani C, et al. Pet therapy in elderly patients with mental illness. Psychogeriatrics 2011;11(2):125‐9. [PUBMED: 21707862] [DOI] [PubMed] [Google Scholar]
Nordgren 2014 {published data only}
- Nordgren L, Engstrom G. Effects of dog‐assisted intervention on behavioural and psychological symptoms of dementia. Nursing Older People 2014;26(3):31‐8. [PUBMED: 24673326] [DOI] [PubMed] [Google Scholar]
NTR5026 {published data only}
- NTR5026. The ABIDE‐PET study. www.trialregister.nl/trial/4924 (first received 5 January 2015).
Peluso 2018 {published data only}
- Peluso S, Rosa A, Lucia N, Antenora A, Illario M, Esposito M, et al. Animal‐assisted therapy in elderly patients: evidence and controversies in dementia and psychiatric disorders and future perspectives in other neurological diseases. Journal of Geriatric Psychiatry and Neurology 2018;31(3):149‐57. [DOI] [PubMed] [Google Scholar]
Richeson 2003 {published data only}
- Richeson NE. Effects of animal‐assisted therapy on agitated behaviors and social interactions of older adults with dementia. American Journal of Alzheimer's Disease and Other Dementias 2003;18(6):353‐8. [PUBMED: 14682084] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scanes 2018 {published data only}
- Scanes Colin G. Chapter 10 – animals in entertainment. In: Scanes CG, Toukhsati SR editor(s). Animals and Human Society. Cambridge (MA): Academic Press, 2018:225‐55. [Google Scholar]
Spattini 2018 {published data only}
- Spattini L, Mattei G, Raisi F, Ferrari S, Pingani L, Galeazzi GM. Efficacy of animal assisted therapy on people with mental disorders: an update on the evidence. Minerva Psichiatrica 2018;59(1):54‐66. [Google Scholar]
Zafra‐Tanaka 2019 {published data only}
- Zafra‐Tanaka JH, Pacheco‐Barrios K, Tellez WA, Taype‐Rondan A. Effects of dog‐assisted therapy in adults with dementia: a systematic review and meta‐analysis. BMC Psychiatry 2019;19:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN93568533 {published data only}
- ISRCTN93568533. A study into the effects of activities with animals on the well‐being of Dutch nursing home patients with dementia. www.isrctn.com/ISRCTN93568533 (first received 23 August 2017). [ISRCTN93568533]
NCT02829801 {published data only}
- NCT02829801. Evaluation of the animal intervention used as therapy. clinicaltrials.gov/show/nct02829801. Clinicaltrial.gov.org, (first received 12 July 2016). [ NCT02829801]
Additional references
Alexopoulos 1988
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biological Psychiatry 1988;23(3):271‐84. [PUBMED: 3337862] [DOI] [PubMed] [Google Scholar]
ALZ 2018
- Alzheimer's Association. Alzheimer's and dementia: types of Alzheimer's. 2018. alz.org/ (accessed 18 January 2018).
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington (DC): American Psychiatric Publishing, 2013. [Google Scholar]
AVMA 2018
- American Veterinary Medical Association. Animal‐assisted interventions: guidelines. 2018. AVMA.org (accessed 18 January 2018).
Banks 2002
- Banks MR, Banks WA. The effects of animal‐assisted therapy on loneliness in an elderly population in long‐term care facilities. Journal of Gerontology 2002;57A(7):M428‐32. [DOI] [PubMed] [Google Scholar]
Behling 2011
- Behling RJ, Haefner J, Stowe M. Animal programs and animal assisted therapy in Illinois long‐term care facilities twenty years later (1990–2010). Academy of Health Care Management Journal 2011;7(2):109‐17. [Google Scholar]
Berget 2011
- Berget B, Braastad BO. Animal‐assisted therapy with farm animals for persons with psychiatric disorders. Annali dell'Istituto Superiore di Sanità 2011;47(4):384‐90. [DOI] [PubMed] [Google Scholar]
Bråne 2001
- Bråne G, Gottfries CG, Winblad B. The Gottfries‐Bråne‐Steen scale: validity, reliability and application in anti‐dementia drug trials. Dementia and Geriatric Cognitive Disorders 2001;12(1):1‐14. [DOI] [PubMed] [Google Scholar]
Campbell 2001
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20(3):391‐9. [DOI] [PubMed] [Google Scholar]
Chau 2016
- Chau SA, Liu CS, Ruthirakuhan M, Lanctôt KL, Herrmann N. Pharmacotherapy of dementia. In: Chiu H, Shulman K editor(s). Mental Health and Illness of the Elderly. Singapore: Springer, 2016. [DOI: 10.1007/978-981-10-0370-7_20-1] [DOI] [Google Scholar]
CIDG 2016
- Cochrane Infectious Diseases Group. Developing logic models: an introduction to using logic models in CIDG reviews. September 2016. cidg.cochrane.org/sites/cidg.cochrane.org/files/public/uploads/cidg_logic_models_sep16.pdf (accessed 1 March 2018).
Cohen‐Mansfield 1989
- Cohen‐Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. Journal of Gerontology 1989;44(3):M77‐84. [PUBMED: 2715584] [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308‐14. [DOI] [PubMed] [Google Scholar]
Darrah 1996
- Darrah JP. A pilot survey of animal‐facilitated therapy in Southern California and South Dakota nursing homes. Occupational Therapy International 1996;3(2):105‐21. [DOI: 10.1002/oti.31] [DOI] [Google Scholar]
de Jong Gierveld 2006
- Jong Gierveld J, Tilburg T. A 6‐item scale for overall, emotional, and social loneliness: confirmatory tests on survey data. Research on Aging 2006;28(5):582‐98. [Google Scholar]
Downes 2013
- Downes MJ, Dean R, Bath‐Hextall FJ. Animal‐assisted therapy for people with serious mental illness. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010818] [DOI] [Google Scholar]
Ebener 2017
- Ebener J, Oh H. A review of animal‐assisted interventions in long‐term care facilities. Activities, Adaptation & Aging 2017;41(2):107‐28. [Google Scholar]
Edwards 2002
- Edwards NE, Beck AM. Animal‐assisted therapy and nutrition in Alzheimer's disease. Western Journal of Nursing Research 2002;24(6):697‐712. [DOI: 10.1177/019394502320555430] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ein 2018
- Ein N, Li L, Vickers K. The effect of pet therapy on the physiological and subjective stress response: a meta‐analysis. Stress Health 2018;34(4):477‐89. [DOI] [PubMed] [Google Scholar]
El Fadl 2004
- Fadl KA. Dogs in the Islamic tradition and nature. Encyclopedia of Religion and Nature. New York (NY): Continuum International, 2004. [Google Scholar]
Filan 2006
- Filan SL, Llewellyn‐Jones RH. Animal‐assisted therapy for dementia: a review of the literature. International Psychogeriatrics 2006;18(4):597‐611. [DOI: 10.1017/S1041610206003322] [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [DOI] [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1977;11(Suppl 2):S33‐9. [PubMed] [Google Scholar]
Glenk 2014
- Glenk LM, Kothgassner OD, Stetina BU, Palme R, Kepplinger B, Baran H. Salivary cortisol and behavior in therapy dogs during animal‐assisted interventions: a pilot study. Journal of Veterinary Behavior 2014;9(3):98‐106. [DOI: 10.1016/j.jveb.2014.02.005] [DOI] [Google Scholar]
Glenk 2017
- Glenk LM. Current perspectives on therapy dog welfare in animal‐assisted interventions. Animals 2017;7(2):7. [DOI: 10.3390/ani7020007] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 March 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hatch 2007
- Hatch A. The view from all fours: a look at an animal‐assisted activity program from the animals' perspective. Anthrozoos 2007;20(1):37‐50. [Google Scholar]
Helmes 1987
- Helmes E, Csapo KG, Short JA. Standardization and validation of the Multidimensional Observation Scale for Elderly Subjects (MOSES). Journal of Gerontology 1987;42(4):395‐405. [PUBMED: 3598087] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA. Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks J, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011d
- Higgins JP, Altman DG, Sterne JA. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011e
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011f
- Higgins JP, Altman DG, Sterne JA. Chapter 11: Presenting results and "Summary of findings" table. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
- Higgins JP, Savovic J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomised trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hu 2018a
- Hu M, Zhang P, Leng M, Li C, Chen L. Animal‐assisted intervention for individuals with cognitive impairment: a meta‐analysis of randomized controlled trials and quasi‐randomized controlled trials. Psychiatry Research 2018;260:418‐27. [DOI] [PubMed] [Google Scholar]
IAHAIO 2014
- Jegatheesan B, Beetz A, Ormerod E, Johnson R, Fine A, Yamazaki K, et al. The IAHAIO definitions for animal‐assisted intervention and guidelines for wellness of animals involved. 2014. iahaio.org/wp/wp‐content/uploads/2017/05/iahaio‐white‐paper‐final‐nov‐24‐2014.pdf (accessed 15 January 2018).
Jacobs 2013
- Jacobs CI. Animal‐assisted therapy and the child‐animal bond: children's well‐being and behavior. Research Papers 2013;427:opensiuc.lib.siu.edu/gs_rp/427. [Google Scholar]
Jelicic Kadic 2016
- Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. Journal of Clinical Epidemiology 2016;74:119‐23. [DOI] [PubMed] [Google Scholar]
Kawamura 2007
- Kawamura N, Niiyama M, Niiyama H. Long‐term evaluation of animal‐assisted therapy for institutionalized elderly people: a preliminary result. Psychogeriatrics 2007;7(1):8‐13. [Google Scholar]
Kneale 2015
- Kneale D, Thomas J, Harris K. Developing and optimising the use of logic models in systematic reviews: exploring practice and good practice in the use of programme theory in reviews. PloS One 2015;10(11):e0142187. [DOI: 10.1371/journal.pone.0142187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawton 1969
- Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9(3):179‐86. [PUBMED: 5349366] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre SL, Golab GC, Christensen E, Castrodale L, Aureden K, Bialachowski A, et al. Guidelines for animal‐assisted interventions in health care facilities. American Journal of Infection Control 2008;36(2):78‐85. [DOI: 10.1016/j.ajic.2007.09.005] [DOI] [PubMed] [Google Scholar]
LeRoux 2009
- LeRoux MC, Kemp R. Effects of a companion dog on depression and anxiety levels of elderly residents in a long‐term care facility. Psychogeriatrics 2009;9(1):23‐6. [Google Scholar]
Levinson 1969
- Levinson BM. Pet‐Oriented Child Psychotherapy. 1st Edition. Michigan (MI): Thomas CC, 1969. [Google Scholar]
Lust 2007
- Lust E, Ryan‐Haddad A, Coover K, Snell J. Measuring clinical outcomes of animal‐assisted therapy: impact on resident medication usage. Consultant Pharmacist 2007;22(7):580‐5. [DOI: 10.4140/TCP.n.2007.580] [DOI] [PubMed] [Google Scholar]
Maber‐Aleksandrowicz 2016
- Maber‐Aleksandrowicz S, Avent C, Hassiotis A. A systematic review of animal‐assisted therapy on psychosocial outcomes in people with intellectual disability. Research in Developmental Disabilities 2016;49‐50:322‐38. [DOI] [PubMed] [Google Scholar]
Marino 2012
- Marino L. Construct validity of animal‐assisted therapy and activities: how important is the animal in AAT?. Anthrozoös 2012;25(Suppl 1):s139‐51. [DOI: 10.2752/175303712X13353430377219] [DOI] [Google Scholar]
Maujean 2015
- Maujean A, Pepping CA, Kendall E. A systematic review of randomized controlled trials of animal‐assisted therapy on psychosocial outcomes. Anthrozoös 2015;28(1):23‐36. [Google Scholar]
McCulloch 1986
- McCulloch MJ. Animal‐facilitated therapy. Overview and future direction. National Forum 1986;66(1):19. [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia 2011;7(3):263‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Motomura 2004
- Motomura N, Yagi T, Ohyama H. Animal assisted therapy for people with dementia. Psychogeriatrics 2004;4(2):40‐2. [DOI: 10.1111/j.1479-8301.2004.00062.x] [DOI] [Google Scholar]
NHS 2015
- National Health Service UK. Dementia guide: is there a cure for dementia? 17 June 2015. www.nhs.uk/conditions/dementia/cure/ (accessed 28 January 2018).
Nimer 2007
- Nimer J, Lundahl B. Animal‐assisted therapy: a meta‐analysis. Anthrozoös 2007;20(3):225‐38. [Google Scholar]
O'Bryant 2008
- O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Archives of Neurology 2008;65(8):1091‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohtani 2015
- Ohtani N, Narita S, Yoshihara E, Ohta M, Iwahashi K. Psychological evaluation of animal‐assisted intervention (AAI) programs involving visiting dogs and cats for alcohol dependents: a pilot study. Nihon Arukoru Yakubutsu Igakkai Zasshi [Japanese Journal of Alcohol Studies & Drug Dependence] 2015;50(6):289‐95. [PUBMED: 26964290] [PubMed] [Google Scholar]
Prince 2015
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Y, Prina M. World Alzheimer Report 2015. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. www.alz.co.uk/research/world‐report‐2015. London: Alzheimer’s Disease International;, (accessed 15 January 2018).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [DOI] [PubMed] [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating Scale for Alzheimer’s disease. American Journal of Psychiatry 1984;141:1356‐64. [DOI] [PubMed] [Google Scholar]
Sakairi 2004
- Sakairi K. Research of robot‐assisted activity for the elderly with senile dementia in a group home. SICE 2004 Annual Conference; 2004 Aug 4‐6; Sapporo (Japan). 2005.
Schneider 1997
- Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11(Suppl 2):S22‐32. [DOI] [PubMed] [Google Scholar]
Schwarz 2012
- Schwarz S, Froelich L, Burns A. Pharmacological treatment of dementia. Current Opinion in Psychiatry 2012;25(6):542‐50. [DOI: 10.1097/YCO.0b013e328358e4f2] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AJ, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sellers 2006
- Sellers DM. The evaluation of an animal assisted therapy intervention for elders with dementia in long‐term care. Activities, Adaptation & Aging 2006;30(1):61‐77. [DOI: 10.1300/J016v30n01_04] [DOI] [Google Scholar]
Souter 2007
- Souter MA, Miller MD. Do animal‐assisted activities effectively treat depression? A meta‐analysis. Anthrozoös 2007;20(2):167‐80. [Google Scholar]
Stergiou 2017
- Stergiou A, Tzoufi M, Ntzani E, Varvarousis D, Beris A, Ploumis A. Therapeutic effects of horseback riding interventions: a systematic review and meta‐analysis. American Journal of Physical Medicine and Rehabilitation 2017;96(10):717‐25. [DOI] [PubMed] [Google Scholar]
Tamura 2004
- Tamura T, Yonemitsu S, Itoh A, Oikawa D, Kawakami A, Higashi Y, et al. Is an entertainment robot useful in the care of elderly people with severe dementia?. Journal of Gerontology 2004;59(1):83‐5. [DOI: 10.1093/gerona/59.1.M83] [DOI] [PubMed] [Google Scholar]
Travers 2013
- Travers C, Perkins J, Rand J, Barlett H, Morton J. An evaluation of dog‐assisted therapy for residents of aged care facilities with dementia. Anthrozoös 2013;26(2):213‐25. [DOI: 10.2752/175303713X13636846944169] [DOI] [Google Scholar]
Tripathi 2009
- Tripathi Manjari, Vibha Deepti. Reversible dementias. Indian journal of psychiatry 2009;51 Suppl 1(Suppl1):S52‐5. [PMC free article] [PubMed] [Google Scholar]
Tseng 2013
- Tseng SH, Chen HC, Tam KW. Systematic review and meta‐analysis of the effect of equine assisted activities and therapies on gross motor outcome in children with cerebral palsy. Disability and Rehabilitation 2013;35(2):89‐99. [DOI] [PubMed] [Google Scholar]
Vucic 2015
- Vucic K, Jelicic Kadic A, Puljak L. Survey of Cochrane protocols found methods for data extraction from figures not mentioned or unclear. Journal of Clinical Epidemiology 2015;68(10):1161‐4. [DOI] [PubMed] [Google Scholar]
Wada 2008
- Wada K, Shibata T, Musha T, Kimura S. Robot therapy for elders affected by dementia. IEEE Engineering in Medicine and Biology Magazine 2008;27(4):53‐60. [DOI: 10.1109/MEMB.2008.919496] [DOI] [Google Scholar]
Waite 2018
- Waite TC, Hamilton L, O'Brien W. A meta‐analysis of animal assisted interventions targeting pain, anxiety and distress in medical settings. Complementary Therapies in Clinical Practice 2018;33:49‐55. [DOI] [PubMed] [Google Scholar]
Walsh 1995
- Walsh PG, Mertin PG, Verlander DF, Pollard CF. The effects of a 'pets as therapy' dog on persons with dementia in a psychiatric ward. Australian Occupational Therapy Journal 1995;42(4):161‐6. [DOI: 10.1111/j.1440-1630.1995.tb01331.x] [DOI] [Google Scholar]
WHO 2015
- World Health Organization. The epidemiology and impact of dementia: current state and future trends. 2015. www.who.int/mental_health/neurology/dementia/dementia_thematicbrief_epidemiology.pdf (accessed 15 January 2018). [WHO/MSD/MER/15.3]
WHO 2016
- World Health Organisation. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10)‐WHO Version for 2016. https://icd.who.int/browse10/2016/en#/XX (accessed 19 November 2019).
WHO 2017a
- World Health Organization. Dementia. Factsheet updated December 2017. www.who.int/news‐room/fact‐sheets/detail/dementia. Geneva: World Health Organization, (accessed 18 January 2018).
WHO 2017b
- World Health Organization. Overview of the global situation. Global Action Plan on the Public Health Response to Dementia 2017–2025. Geneva: World Health Organization, 2017:2. [Google Scholar]
Yamauchi 2008
- Yamauchi T, Pipkin E. Six years experience with animal‐assisted therapy in a children's hospital: is there patient risk?. American Journal of Infection Control 2008;36(5):E117. [DOI: 10.1016/j.ajic.2008.04.132] [DOI] [Google Scholar]
Yesavage 1982
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 1982;17(1):37‐49. [PUBMED: 7183759] [DOI] [PubMed] [Google Scholar]
Zadnikar 2011
- Zadnikar M, Kastrin A. Effects of hippotherapy and therapeutic horseback riding on postural control or balance in children with cerebral palsy: a meta‐analysis. Developmental Medicine and Child Neurology 2011;53(8):684‐91. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lai 2019
- Lai NM, Chang SM, Ng SS, Stanaway F, Tan SL, Chaiyakunapruk N. Animal‐assisted therapy for dementia. Cochrane Database of Systematic Reviews 2019, Issue 1. [DOI: 10.1002/14651858.CD013243] [DOI] [PMC free article] [PubMed] [Google Scholar]


