Abstract
The canonical wingless (Wnt) and fibroblast growth factor (FGF) signaling pathways involving CTNNB1 and TBX4, respectively, are crucial for the regulation of human development. Perturbations of these pathways and disruptions from biological homeostasis have been associated with abnormal morphogenesis of multiple organs, including the lung. The aim of this study was to identify the underlying genetic cause of abnormal lung growth, pulmonary hypertension (PAH), severe microcephaly, and muscle spasticity in a full term newborn, who died at four months of age due to progressively worsening PAH and respiratory failure. Family trio exome sequencing revealed a de novo heterozygous nonsense c.1603C>T (p.Arg535*) variant in CTNNB1 and a paternally inherited heterozygous missense c.1198G>A (p.Glu400Lys) variant in TBX4, both predicted to be likely deleterious. We expand the phenotypic spectrum associated with CTNNB1 and TBX4 variants and indicate that they could act synergistically to produce a distinct more severe phenotype. Our findings further support a recently proposed complex compound inheritance model in lethal lung developmental diseases and the contention that dual molecular diagnoses can parsimoniously explain blended phenotypes.
Keywords: beta-catenin, T-box transcription factor 4, neonatal lung disease, epistatic interactions
Graphical Abstract

In a patient with abnormal lung growth, pulmonary hypertension, microcephaly, and spasticity, who died at four months of age due to progressively worsening pulmonary hypertension and respiratory failure, we identified a de novo heterozygous nonsense c.1603C>T (p.Arg535*) variant in CTNNB1 and a paternally inherited heterozygous missense c.1198G>A (p.Glu400Lys) variant in TBX4. We expand the phenotypic spectrum associated with CTNNB1 and TBX4 variants and indicate that they could act synergistically to produce a distinct severe phenotype.
INTRODUCTION
Beta-catenin, encoded by CTNNB1 (MIM#116806) and T-box transcription factor 4, encoded by TBX4 (MIM#601719) are crucial proteins in Wnt and FGF-TBX4 signaling, respectively.1,2
While germline CTNNB1 variants have been identified in patients with intellectual disability and neurodevelopmental abnormalities or exudative vitreoretinopathy 7 (MIM#617572),3,4 somatic CTNNB1 variants have been identified in different types of neoplasms, including colorectal (MIM#114500) or ovarian (MIM#167000) cancer. Deletion of Ctnnb1 in embryonic development in mice led to inhibition of specification of lung progenitors and tracheal budding5, or to respiratory failure associated with restriction of formation and differentiation of the peripheral lung.6
Point mutations and copy-number variant (CNV) deletions involving TBX4 have been associated with pulmonary hypertension (PAH) and ischiocoxopodopatellar syndrome (MIM#147891).7–9 Most recently, TBX4 abnormalities have been identified in individuals with lethal lung developmental disease, i.e. acinar dysplasia, congenital alveolar dysplasia, pulmonary hypoplasia, and alveolar growth abnormality.10–12 TBX4 null or Tbx4+/− mice exhibit reduced number of lung branching tips.13
Here, we present a de novo nonsense variant in CTNNB1 coexisting with an inherited missense variant in TBX4 in an infant with lung growth abnormality, PAH, severe microcephaly, and spasticity, who died due to a respiratory failure during the neonatal period.
MATERIALS AND METHODS
Human subjects.
Specimens were collected from proband (lung tissue) and his parents (blood) after obtaining informed consent. The study protocol was approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine (H-8712).
Histopathological evaluation.
Histopathological evaluation was performed using formalin-fixed paraffin-embedded lung tissue specimens from lung biopsy and autopsy stained with hematoxylin and eosin or D2-40.
Exome sequencing (ES).
ES and family based genomics for the family trio was performed according to previously described protocols14 (Supplementary Materials and Methods).
RESULTS
Clinical findings.
The male proband was the second child of non-consanguineous Caucasian parents aged 30 years with no reported history of disease. He was born at term following an uneventful pregnancy. Apgar scores were 9/1 and 9/5. Growth parameters showed birth weight 3,395 g (32nd centile), head circumference 34 cm (10th centile), and length 54 cm (98th centile). At 26 hours, he developed respiratory distress with grunting. He required intubation and ventilation. He was treated for presumed sepsis, necrotising enterocolitis, and persistent PAH of the newborn. He was ventilated for six days and required nitric oxide for three days. Echocardiogram showed a structurally normal heart as well as PAH with supra-systemic pressures and a dilated right ventricle. He was established on full oral feeds and weaned off all respiratory support by day 18 when he was discharged home.
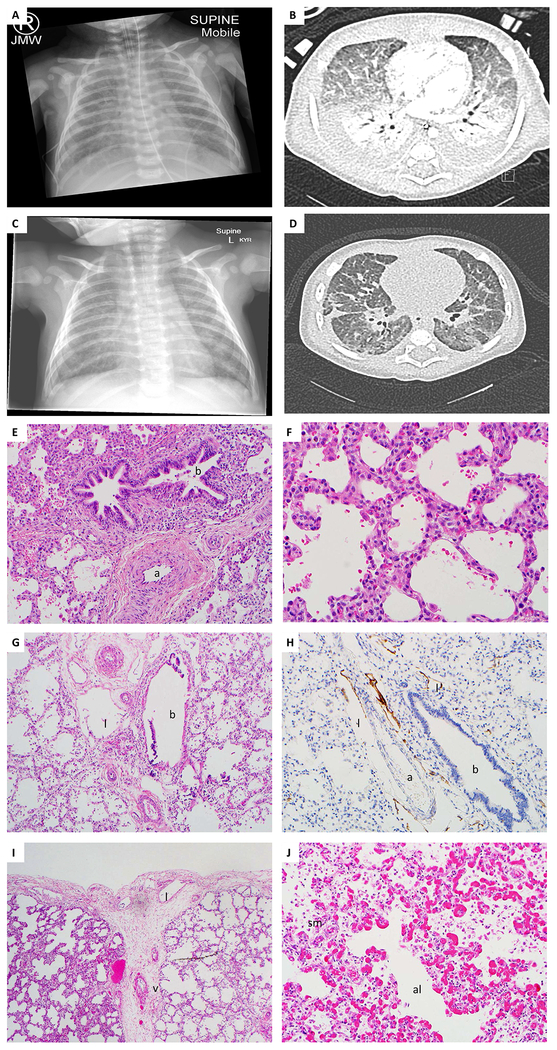
At 7 weeks, echocardiogram revealed supra-systemic PAH with a crescent-shaped and a slit like left ventricle. A CXR and a chest HRCT scan noted bilateral pleural effusions with background of ground glass changes. On HRCT areas of collapse and consolidation and interlobular septal thickening were also presented (Figure 1A,B). He was discharged home on oxygen 27 days after his initial presentation.
Figure 1. Clinical and histopathological characterization of the patient’s lungs.
A) A CXR noting bilateral pleural effusions with a reticular pattern on a background of ground glass change; B) A chest HRCT scan showing dependant areas of collapse and consolidation, interlobular septal thickening and ground glass opacities. Bilateral pleural effusions are present; C) A CXR showing a small pleural effusion on the right and bilateral ground glass appearance; D) A chest HRCT showing significant reduction in size of the bilateral pleural effusions and improved aeration in the dependent lung; The degree of diffuse ground glass opacity throughout both lungs is reduced; Lung biopsy with E) thickened arterioles (a), adjacent to bronchus (b) and F) enlarged simplified alveoli resulting in immature appearing lung. Increased cells in interstitium, suggestive of pulmonary interstitial glycogenosis; Autopsy lung showing G) dilated lymphatics (l), thickened arterioles (a) and bronchus (b); H) lymphatic staining multiple dilated lymphatics (l) around the artery (a); bronchus (b) (stained with D2–40); I) thickened pleura with increased numbers of thin walled lymphatics (l). Interlobular septa with thickened vein (v), and enlarged simplified alveoli; J) thickened alveolar septa with prominent capillaries with enlarged simplified alveoli (al). There is peripheral extension of the pulmonary arteriolar smooth muscle (sm).
Five days later, he was re-admitted with increased work of breathing and tachypnoea. There was ongoing evidence of PAH on echocardiogram. CXR showed a small pleural effusion on the right and bilateral ground glass appearance (Figure 1C). A chest HRCT showed significant reduction in size of the bilateral pleural effusions and reduced degree of diffuse ground glass opacity. There was worsening of diffuse inter- and intralobular septal thickening (Figure 1D). He deteriorated after the chest CT with recurrent episodes of tachypnoea, hypotension, cyanosis and desaturations. A repeat echocardiogram showed supra-systemic PAH with pulmonary regurgitation. After he developed increasing bouts of severe irritability and agitation palliative care approach was adopted. He died peacefully three days later at four months of age (Supplementary file).
Histopathological findings.
The microscopic evaluation of lung biopsy performed at six weeks of life showed a mildly immature appearance with slightly thickened pleura, increased lymphatics in the interlobular septa, and pulmonary marked thick-walled pulmonary arterioles. The alveolar septa were cellular with clear cells, suggestive of pulmonary interstitial glycogenosis (Figure 1E–F). The gross examination of the autopsy lungs at four months of life revealed no abnormalities. The microscopic evaluation of the lungs showed enlarged and simplified alveoli with no features of hypoplasia (Figure 1G–J, Supplementary file).
Molecular and computational analyses
ES trio analysis in the proband revealed a de novo heterozygous nonsense c.1603C>T variant (p.Arg535*; rs886039332) in CTNNB1 (Vr/Tr=77/181) by DNM-Finder and a heterozygous missense variant c.1198G>A (Vr/Tr=74/168) substitution (p.Glu400Lys; rs765739860) in TBX4 inherited from the apparently healthy father. Both heterozygous variant alleles were independently confirmed by trio Sanger sequencing (Supplementary Figure 1A).
The CTNNB1 variant is absent in the ExAc (v1.0) and gnomAD (v2.1.1) databases, while the TBX4 variant has been observed in one individual in the ExAC (MAF=0.00000824) and in six individuals in gnomAD (MAF=0.00001122) database; de novo nature or parent of origin of variant alleles are unknown. Neither of the variant identified in the proband have been reported in our internal BHCMG ES cohort consisting of more than 8,800 individuals of different ethnicities and phenotypes nor in ~10,000 ARIC population controls. The nonsense c.1603C>T variant (p.Arg535*) in CTNNB1 results in a premature termination codon and is predicted by NMDEscPredictor15 to be degraded by nonsense-mediated decay with reduction of CTNNB1 expression below sufficient level. The missense c.1198G>A variant in TBX4 is predicted to replace a negatively charged glutamic acid residue for positively charged lysine (p.Glu400Lys), which can lead to destabilization of the protein structure, and it maps outside the T-box protein domain (Supplementary Figure 1B). According to multiple sequence alignment, Glu400 in TBX4 is highly conserved across phylogeny, suggesting that this amino acid residue has an important structural and functional role (Supplementary Figure 1C). This notion has been supported by the in silico tools, including MutationTaster and PolyPhen-2, which predicted a deleterious effect of the identified variants in the TBX4 protein. Moreover, the combined annotation dependent depletion score16 is 23.9 for the variant in CTNNB1 and 38 for the variant in TBX4, indicating that they are in the top (1% and 0.1%, respectively) of the most deleterious substitutions in the human genome.
DISCUSSION
The most common phenotype associated with germline CTNNB1 mutations includes intellectual disability, behavioral anomalies, craniofacial abnormalities, microcephaly, and progressive spasticity.3,17 Interestingly, the c.1603C>T variant (p.Arg535*) in CTNNB1, described here, was reported in two unrelated patients of 4 and 14.5 years of age who in addition to clinical characteristics mentioned above presented with low oxygen saturation or persistent PAH during the neonatal period.3
While severe microcephaly and spasticity detected in our patient overlap the clinical features of these two cases with c.1603C>T variant in CTNNB1,3 the lung phenotype observed here is distinct and more severe. Patient #2 reported by Kharbanda et al. had a normal echo at that time and this low oxygen saturation was thought to be secondary to persistent PAH of the newborn and has had no further respiratory problems since.3 Patient #4 also had the persistent PAH of the newborn and required incubator oxygen for two days and has not had any ongoing respiratory problems since the neonatal period.3
The lung malformations in our patient include abnormal lung growth with enlarged simplified alveoli, prominent lymphangiectasias, and PAH with mild venous hypertensive changes. We propose that these findings can be partially explained by the contribution of the paternally inherited c.1198G>A TBX4 variant.
A significant involvement of both de novo or inherited TBX4 variants has been described in patients with early-onset idiopathic PAH.8 The observed inheritance of TBX4 variants from an healthy parent was explained by incomplete penetrance, suggesting that the effect of risk variant on phenotype is likely dependent on genetic and/or environmental background.8 Recent studies performed in large group of patients with TBX4 variants and rare interstitial lung diseases also denoted that heterozygous variation of TBX4 alone is not sufficient to cause abnormal lung phenotype, indicating complex compound inheritance.12 This could explain the absence of lung disease in the father with the same heterozygous TBX4 variant as observed in his affected child.
During respiratory system formation, Wnt and FGF signaling are integrated into a complex regulatory network, which modulate continuous epithelial-mesenchymal interactions required for lung development.18 Perturbation of these mechanisms during prenatal development can result in complete or partial inhibition of lung morphogenesis.6,18 Therefore, we suggest that variants in the CTNNB1 and TBX4 genes, crucial members of Wnt and FGF signaling,1,2 could act synergistically to produce a distinct phenotype observed in our patient, resulting in respiratory failure during the neonatal period. Our findings expand the mutational burden of the previously proposed genetic model of lethal lung diseases, in which both rare non-coding SNVs and heterozygous TBX4 or FGF10 SNVs or deletion CNVs, as well as double heterozygous TBX4 and TBX5 variants, contribute to the lung phenotype and suggest epistatic interactions of genes from the same signaling pathway.12
In summary, we present the clinical, histopathological, and molecular findings in a deceased patient with a lethal respiratory condition, including abnormal lung development, PAH, microcephaly, and muscle spasticity. Our data expand the known phenotypic spectrum of CTNNB1 and TBX4 variants, add to the growing body of evidence suggesting dual molecular diagnoses are important to blended phenotypes19 and further implicate a complex genetic cause of a mutational burden underlying the identified clinical features.12
Supplementary Material
Acknowledgements
This work was supported by grant awarded by the US National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI) R01HL137203 (P.St.), the US National Human Genome Research Institute (NHGRI)/NHLBI grant number UM1HG006542 to the Baylor-Hopkins Center for Mendelian Genomics (BHCMG), and the National Institute of Neurological Disorders and Stroke (NINDS) R35 NS105078 (J.R.L.).
Footnotes
Conflict of interest
The authors declare no conflicts of interest
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson J, Francavilla C. Regulation of FGF10 Signaling in Development and Disease. Front Genet. 2018;9:500. doi: 10.3389/fgene.2018.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharbanda M, Pilz DT, Tomkins S, et al. Clinical features associated with CTNNB1 de novo loss of function mutations in ten individuals. Eur J Med Genet. 2017;60(2):130–135. doi: 10.1016/j.ejmg.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuechler A, Willemsen MH, Albrecht B, et al. De novo mutations in beta-catenin (CTNNB1) appear to be a frequent cause of intellectual disability: expanding the mutational and clinical spectrum. Hum Genet. 2015;134(1):97–109. doi: 10.1007/s00439-014-1498-1 [DOI] [PubMed] [Google Scholar]
- 5.Goss AM, Tian Y, Tsukiyama T, et al. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17(2):290–298. doi: 10.1016/j.devcel.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucenski ML, Wert SE, Nation JM, et al. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278(41):40231–40238. doi: 10.1074/jbc.M305892200 [DOI] [PubMed] [Google Scholar]
- 7.Bongers EMHF, Duijf PHG, van Beersum SEM, et al. Mutations in the human TBX4 gene cause small patella syndrome. Am J Hum Genet. 2004;74(6):1239–1248. doi: 10.1086/421331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N, Gonzaga-Jauregui C, Welch CL, et al. Exome Sequencing in Children With Pulmonary Arterial Hypertension Demonstrates Differences Compared With Adults. Circ Genom Precis Med. 2018;11(4):e001887. doi: 10.1161/CIRCGEN.117.001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerstjens-Frederikse WS, Bongers EMHF, Roofthooft MTR, et al. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet. 2013;50(8):500–506. doi: 10.1136/jmedgenet-2012-101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szafranski P, Coban-Akdemir ZH, Rupps R, et al. Phenotypic expansion of TBX4 mutations to include acinar dysplasia of the lungs. Am J Med Genet A. 2016;170(9):2440–2444. doi: 10.1002/ajmg.a.37822 [DOI] [PubMed] [Google Scholar]
- 11.Suhrie K, Pajor NM, Ahlfeld SK, et al. Neonatal Lung Disease Associated with TBX4 Mutations. J Pediatr. 2019;206:286–292.e1. doi: 10.1016/j.jpeds.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karolak JA, Vincent M, Deutsch G, et al. Complex Compound Inheritance of Lethal Lung Developmental Disorders Due to Disruption of the TBX-FGF Pathway. Am J Hum Genet. 2019;104(2):213–228. doi: 10.1016/j.ajhg.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet. 2012;8(8):e1002866. doi: 10.1371/journal.pgen.1002866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lupski JR, Gonzaga-Jauregui C, Yang Y, et al. Exome sequencing resolves apparent incidental findings and reveals further complexity of SH3TC2 variant alleles causing Charcot-Marie-Tooth neuropathy. Genome Med. 2013;5(6):57. doi: 10.1186/gm461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coban-Akdemir Z, White JJ, Song X, et al. Identifying Genes Whose Mutant Transcripts Cause Dominant Disease Traits by Potential Gain-of-Function Alleles. Am J Hum Genet. 2018;103(2):171–187. doi: 10.1016/j.ajhg.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rentzsch P, Witten D, Cooper GM, Shendure J, Kircher M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 2019;47(D1):D886–D894. doi: 10.1093/nar/gky1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tucci V, Kleefstra T, Hardy A, et al. Dominant β-catenin mutations cause intellectual disability with recognizable syndromic features. J Clin Invest. 2014;124(4):1468–1482. doi: 10.1172/JCI70372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volckaert T, De Langhe SP. Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development. Dev Dyn. 2015;244(3):342–366. doi: 10.1002/dvdy.24234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posey JE, Harel T, Liu P, et al. Resolution of Disease Phenotypes Resulting from Multilocus Genomic Variation. N Engl J Med. 2017;376(1):21–31. doi: 10.1056/NEJMoa1516767 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.