Abstract
Background
Penetrating abdominal trauma (PAT) is a common type of trauma leading to admission to hospital, which often progresses to septic complications. Antibiotics are commonly administered as prophylaxis prior to laparotomy for PAT. However, an earlier Cochrane Review intending to compare antibiotics with placebo identified no relevant randomised controlled trials (RCTs). Despite this, many RCTs have been carried out that compare different agents and durations of antibiotic therapy. To date, no systematic review of these trials has been performed.
Objectives
To assess the effects of antibiotics in penetrating abdominal trauma, with respect to the type of agent administered and the duration of therapy.
Search methods
We searched the following electronic databases for relevant randomised controlled trials, from database inception to 23 July 2019; Cochrane Injuries Group's Specialised Register, CENTRAL, MEDLINE Ovid, MEDLINE Ovid In‐Process & Other Non‐Indexed Citations, MEDLINE Ovid Daily and Ovid OLDMEDLINE, Embase Classic + Embase Ovid, ISI Web of Science (SCI‐EXPANDED, SSCI, CPCI‐S & CPSI‐SSH), and two clinical trials registers. We also searched reference lists from included studies. We applied no restrictions on language or date of publication.
Selection criteria
We included RCTs only. We included studies involving participants of all ages, which were conducted in secondary care hospitals only. We included studies of participants who had an isolated penetrating abdominal wound that breached the peritoneum, who were not already taking antibiotics.
Data collection and analysis
Two study authors independently extracted data and assessed risk of bias. We used standard Cochrane methods. We aggregated study results using a random‐effects model. We also conducted trial sequential analysis (TSA) to help reduce type I and II errors in our analyses.
Main results
We included 29 RCTs, involving a total of 4458 participants. We deemed 23 trials to be at high risk of bias in at least one domain.
We are uncertain of the effect of a long course of antibiotic prophylaxis (> 24 hours) compared to a short course (≤ 24 hours) on abdominal surgical site infection (RR 1.00, 95% CI 0.81 to 1.23; I² = 0%; 7 studies, 1261 participants; very low‐quality evidence), mortality (Peto OR 1.67, 95% CI 0.73 to 3.82; I² = 8%; 7 studies, 1261 participants; very low‐quality evidence), or intra‐abdominal infection (RR 1.23, 95% CI 0.84 to 1.80; I² = 0%; 6 studies, 111 participants; very‐low quality evidence).
Based on very low‐quality evidence from fifteen studies, involving 2020 participants, which compared different drug regimens with activity against three classes of gastrointestinal flora (gram positive, gram negative, anaerobic), we are uncertain whether there is a benefit of one regimen over another.
TSA showed the majority of comparisons did not cross the alpha adjusted boundary for benefit or harm, or reached the required information size, indicating that further studies are required for these analyses. However, in the three analyses which crossed the boundary for futility, further studies are unlikely to show benefit or harm.
Authors' conclusions
Very low‐quality evidence means that we are uncertain about the effect of either the duration of antibiotic prophylaxis, or the superiority of one drug regimen over another for penetrating abdominal trauma on abdominal surgical site infection rates, mortality, or intra‐abdominal infections.
Future RCTs should be adequately powered, test currently used antibiotics, known to be effective against gut flora, use methodology to minimise the risk of bias, and adequately report the level of peritoneal contamination encountered at laparotomy.
Plain language summary
Antibiotics for injuries to the abdomen that break through the skin: which antibiotics are effective, and for how long should they be taken?
Review Question
We reviewed the evidence regarding the use of antibiotics to prevent infections or death in people undergoing an operation for penetrating abdominal injuries.
Background
Penetrating abdominal trauma (gunshot or stab wounds to the abdomen) are a major cause of admission to hospital and often require an operation. If a person survives the initial injury, they may subsequently develop infections, which can lead to death. Before antibiotics were available, the majority of people with these injuries died from infections.
Study Characteristics
We searched for trials involving participants of any age or sex, who underwent an emergency operation to treat penetrating abdominal trauma. The evidence is current to 23 July 2019. We included 29 studies that included 4458 participants. There were problems with the design and conduct of all of these studies, which means that we were uncertain about the results. Most of these studies were carried out over 20 years ago, using antibiotics that are not often used today. Surgical techniques and practice have also evolved substantially during this time. Seven out of the 29 studies received funding from pharmaceutical companies, whilst the other studies did not state their funding sources.
Key Results
Because of the very low‐quality of the evidence, we are uncertain whether giving longer courses of antibiotics after penetrating injury reduces the rate of infections after an operation. We are also uncertain if one antibiotic treatment is better than any other that was tested in the trials.
Quality of the evidence
The quality of evidence for all outcomes was very low, mainly due to problems with the way the studies were run. These problems were not using placebos (medications that look identical to the study drug but do not contain the active ingredient), a lack of blinding of both participants or the investigators or inadequate methods of randomly allocating treatments to the participants. There were also key differences in the methods used between the studies. New, better quality studies are required in order to answer questions about the use of antibiotics to reduce infections following penetrating abdominal injury.
Summary of findings
Background
Description of the condition
Penetrating abdominal trauma (PAT) is a major cause of trauma admissions in the United States; the most common mechanisms of injury are stabbing and gunshot wounds (Butt 2009). After surviving the initial insults, subsequent mortality and morbidity are usually due to sepsis. Common septic complications include wound infections, intra‐abdominal abscesses, necrotising fasciitis, and diffuse suppurative peritonitis (Fabian 1992). The presence of hollow viscus injury is one of the most important factors in the development of infection, with colonic injury associated with the highest incidence of intra‐abdominal infection (Fabian 1992). In the pre‐antibiotics era, PAT was associated with a high mortality rate of up to 70%, mainly due to sepsis (Wallace 1916). Sepsis also places a significant burden on the healthcare system. An epidemiological study of 30,303 participants identified that trauma associated with sepsis significantly increased the length of stay in intensive care units (22 versus 5 days), and increased the overall inpatient length of stay (34 versus 7 days) compared with non‐septic trauma (Osborn 2004).
Description of the intervention
Early studies have shown that antibiotics are most effective when administered prior to bacterial contamination (Burke 1961; Miles 1957), whilst the administration of prophylactic preoperative antibiotics is already established as standard clinical practice to prevent surgical site infection in abdominal surgery (Nelson 2014). The use of antibiotics in PAT is less clear, as contamination may have occurred before the administration of antibiotics.
How the intervention might work
In the early 1960s, Burke established that there is a short duration of time during which the tissues are free from infection after contamination (Burke 1961). A decade later, Fullen 1972 showed that antibiotics were effective in reducing infection in PAT. The use of antibiotics was significantly more effective when given preoperatively (resulting in 7% infection in the group), compared to intraoperative (33%) and postoperative administration (30%).
Why it is important to do this review
Single‐dose, preoperative antibiotic therapy has been shown to be effective in elective abdominal surgery for reducing surgical site infection (Nelson 2014). However, the evidence is less clear about the role of antibiotics and surgical site infection, and other septic complications in PAT. Unlike elective surgery, contamination may have already taken place before the administration of antibiotics in PAT. Therefore, the use of antibiotics in this situation may be therapeutic rather than prophylactic. Our aim was to investigate the effectiveness of antibiotic use in preventing septic complications from PAT. In order to compare septic complications between studies, we used abdominal surgical site infection, as it has the greatest risk of infection during surgical procedures (Pollock 1987), and is a good quality indicator (Biscione 2009). The Cochrane review 'Prophylactic antibiotics for penetrating abdominal trauma' examined the outcomes of participants who were treated with antibiotics versus placebo or no antibiotics; however, the review identified no eligible studies (Brand 2013; Brand 2019). Our review aimed to compare various antibiotic regimens and durations of use.
Objectives
To assess the effects of antibiotics in penetrating abdominal trauma (PAT), with respect to the type of agent administered and the duration of therapy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only. We included studies that compared either the same regimen for a single dose, short (≤ 24 hours) or long (> 24 hours) durations, and studies that compared different antibiotic regimens for the same duration.
Types of participants
We included studies with participants that had an isolated, penetrating abdominal wound that had breached the peritoneum (a membrane that forms the lining of the abdominal cavity), and who were not already taking antibiotics. We included studies involving participants of all ages, which were conducted in secondary care only.
Types of interventions
We included studies that used antibiotics that targeted gram‐positive, gram‐negative, and anaerobic bacteria. The first comparison was based on antibiotic duration for a particular antibiotic regimen (single dose, ≤ 24 hours, and > 24 hours). The second set of comparisons compared different antibiotic regimens, based on antimicrobial coverage (gram‐positive, gram‐negative, or anaerobic bacteria, or a combination).
Types of outcome measures
Primary outcomes
Abdominal surgical site infection
Secondary outcomes
Mortality
Intra‐abdominal infection
Search methods for identification of studies
In order to reduce publication and retrieval bias, we did not restrict our search by language, date, or publication status.
Electronic searches
The Cochrane Injuries Group Information Specialist searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 7) in the Cochrane Library (searched 23 July 2019);
MEDLINE Ovid and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily (1946 to 23 July 2019);
Embase Classic + Embase Ovid (1947 to 23 July 2019);
-
Clarivate Analytics Web of Science databases:
Science Citation Index Expanded (1970 to 23 July 2019);
Conference Proceedings Citation Index ‐ Science (1990 to 23 July 2019);
Emerging Sources Citation Index (2015 to 23 July 2019).
All search strategies are listed in Appendix 1.
Searching other resources
We screened the reference lists of published articles for potential studies. We also searched the following clinical trials registries:
ClinicalTrials.gov (http://www.clinicaltrials.gov/; searched 23 July 2019);
Current Controlled Trials (http://www.controlled‐trials.com/; searched 23 July 2019).
Data collection and analysis
We conducted data analysis using Review Manager 5 according to the methods stipulated in the published review protocol (RevMan 2014; Tou 2013).
Selection of studies
We reviewed the titles of all articles from the first search, and excluded studies that did not fit our inclusion and exclusion criteria. ST and AB independently considered the remaining abstracts for inclusion. We obtained the full text of eligible studies, and ST and AB independently assessed whether each trial met the inclusion criteria. We documented the excluded studies and the reasons for exclusion in the 'Characteristics of excluded studies' table. We excluded studies which did not report any of our outcomes of interest. We resolved disagreement in selection of these studies by discussion between all review authors.
Data extraction and management
Two authors (HBC and JB) independently extracted the data, using a computer‐based data extraction form. Where possible, we extracted the following data: year of publication, study design, age and gender of participants, inclusion and exclusion criteria, severity of injuries, presence or absence of hollow viscus injuries, timing of antibiotics administered, types and duration of antibiotics given, incidence of septic complications, other complications, mortality, and attrition rates.
Assessment of risk of bias in included studies
We used two authors (PH and BD) to independently assess the risk of bias in the included studies, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). They resolved disagreements through discussion with a third review author (HBC). We used six domains to assess the risk of bias in the included studies: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. For each study, we described each domain as being at high risk, low risk or unclear risk of bias.
Measures of treatment effect
We used risk ratios (RR) and 95% confidence intervals (CI) for most dichotomous outcomes (surgical site and intra‐abdominal infections). For mortality, due to the low number of events, we used the Peto odds ratio (OR).
Unit of analysis issues
The unit of allocation was an individual participant, so we did not anticipate unit of allocation issues. We did not include cluster‐RCTs, due to the inherent bias from different surgeons, hospitals, and surgical teams.
Dealing with missing data
We had complete data available for all of the papers included. If they had not been, we would have contacted study authors for further details. We did not plan to use any methods of imputation for missing data.
Assessment of heterogeneity
For clinical heterogeneity, we examined the trial methods and characteristics in the table of included studies. We did not perform meta‐analyses if there was important clinical heterogeneity. For statistical heterogeneity, we used the Chi² test and the I² statistic. We regarded an I² > 50%, or a P < 0.1 as evidence of statistical heterogeneity.
Assessment of reporting biases
We assessed reporting bias as part of the Cochrane 'Risk of bias' tool. If more than ten studies were included in an analysis, we had planned to assess publication bias using funnel plots and Egger's linear regression test.
Data synthesis
We analysed data using Review Manager 5 software (RevMan 2014). We pooled data using the random‐effects model, apart from calculating a Peto OR, where we aggregated data using a fixed‐effect model. We performed our analyses on an intention‐to‐treat basis where possible.
We compared antibiotics according to their anti‐bacterial activity. We achieved this by identifying the three most common organisms isolated in all the included studies: gram positive (Enterococcus spp, Staphlococcus spp, and Streptococcous spp), gram negative (Escherichia coli spp, Klebsiella spp, Enterobacter spp), and anaerobic bacteria (Bacteroides fragilis spp, Bacteroides spp (non fragilis), and Clostridia spp. We then graded the antibiotics used in the study papers as having low, moderate, or strong activity against these bacteria, using the Mayo Clinic Antimicrobial Therapy Guide, Second Edition (Mayo Clinic 2012). The antibiotic regimen in each study was then categorised in a binary fashion, as to whether the regimen had at least a mean activity score of 1 (moderate activity) against the three organisms in each of the categories, or less than 1 (less than moderate activity). This allowed us to formulate comparable groups in relation to a spectrum of activity, in order to undertake meta‐analyses.
We also compared durations of therapy. They were categorised as single dose, 24 hours, or more than 24 hours.
Subgroup analysis and investigation of heterogeneity
We reported subgroup analyses of the primary and secondary outcomes for the following, where reported: presence or absence of hollow viscus injury; presence or absence of colonic injury.
Sensitivity analysis
We undertook sensitivity analyses to assess trial methodological quality. This was achieved by excluding trials with inadequate or unclear allocation concealment.
Trial sequential analysis
When two or more studies were included in an analysis, we conducted trial sequential analysis (TSA) in order to help exclude type I and II errors in analysis (Doleman 2019; Imberger 2013). We used alpha adjusted monitoring boundaries using the O'Brien‐Fleming method (analogous to an adjustment for multiple comparisons). We conducted all calculations assuming a power of 80%, and we calculated an information size (number of participants to achieve adequate power) for each analysis. We substituted zero events with a constant of 0.5. We used a DerSimonian‐Laird random‐effects model for our analyses, calculating risk ratios for all outcomes except mortality, which we expressed as a Peto OR.
Incidences in the control group were estimated from those studies included in the analysis. For incidences that were greater than 10%, we regarded a relative risk reduction of 20% as clinically significant. For incidences < 10%, we regarded a relative risk reduction of 50% as clinically significant. We also constructed boundaries of futility, which indicate when the conduct of further studies is unlikely to change the conclusions of the review. We based adjustments for diversity on the studies included in the analysis. We conducted all analyses with TSA software from the Copenhagen Trial Unit (Version 0.9.5.5 beta) (Thorland 2018).
Results
Description of studies
Results of the search
We identified 8610 studies from searches of the electronic databases. We did not identify any relevant studies from clinical trials databases or reference lists. One study identified from the database search was in abstract form only. However, we found the full published manuscript, detailing the same study, in a search on Google Scholar, and therefore included it. We did not require any further information from any authors. We considered 29 randomised controlled trials (RCT) eligible for inclusion in the final review. Figure 1 displays a PRISMA flow diagram.
1.
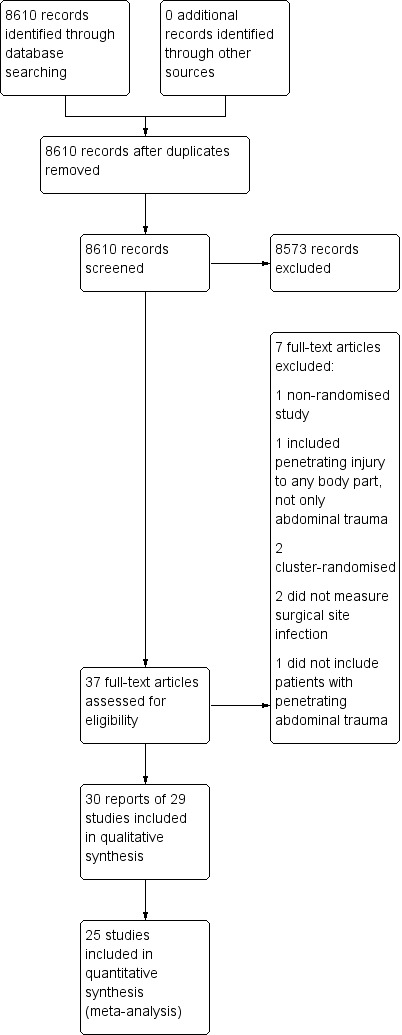
Study flow diagram
Included studies
We included 29 RCTs, comprising 4458 participants. All studies were published as full manuscripts, conducted in secondary care in the USA and South Africa, and published in English. The first study was published in 1982, and the latest in 2000. Twenty‐one studies compared different antibiotic regimens for the same duration of time, and seven studies compared the same antibiotic regimen given for different durations of time. One study compared different antibiotic regimens given over different time durations. Twenty‐five studies reported postoperative surgical site infections, whilst four studies did not report this adequately (Bivins 1989; Crenshaw 1983; Fabian 1994; Nelson 1986). Of these 25 studies, 21 sufficiently discriminated between wound and deep intra‐abdominal infections in their reporting, whilst four did not specify this (Ericsson 1989; Hesseltine 1986; Okamoto 1993; Schmidt‐Matthiesen 1999). Twenty‐five studies reported mortality as an outcome, whilst four did not (Bivins 1989; Crenshaw 1983; Nelson 1986; Rowlands 1984).
The studies used a range of antibiotic regimens with differing spectrums of activity against gram‐positive, gram‐negative, and anaerobic bacteria. Antibiotics included second and third generation cephalosporins, aminoglycosides, penicillins, tetracyclines, clindamycin, and metronidazole, used as monotherapy or in combination (See 'Characteristics of included studies' table). One study compared single‐dose prophylaxis with 24‐hour prophylaxis, whilst six studies compared 24 hours of therapy with longer durations of therapy.
Excluded studies
We excluded seven studies (see Characteristics of excluded studies). O'Donnell 1978 was not an RCT; Schmidt‐Matthiesen 1999 included participants with penetrating injury to any part of the body; Felliciano 1986 and Gentry 1984 were cluster‐RCTs; Thadepalli 1972 and Thadepalli 1973 reported on microbiology cultures and did not measure our primary outcome of surgical site infection; and Tornqvist 1985 did not include participants with penetrating abdominal trauma.
Risk of bias in included studies
We performed "Risk of bias" assessments using the Cochrane tool for assessing risk of bias (Higgins 2011). We assessed twenty‐three studies at high risk of bias in at least one domain, predominantly due to issues with blinding of participants, whilst we assessed six studies at either low risk or unclear risk in all domains. The assessments for each study can be found in Figure 2 and Figure 3.
2.
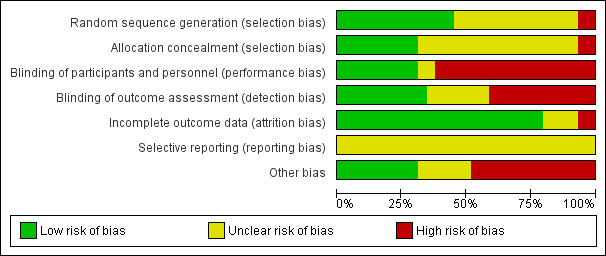
Risk of bias graph: review authors' judgements about each risk of bias item, presented as percentages across all included studies. Twenty‐nine studies are included in this review.
3.
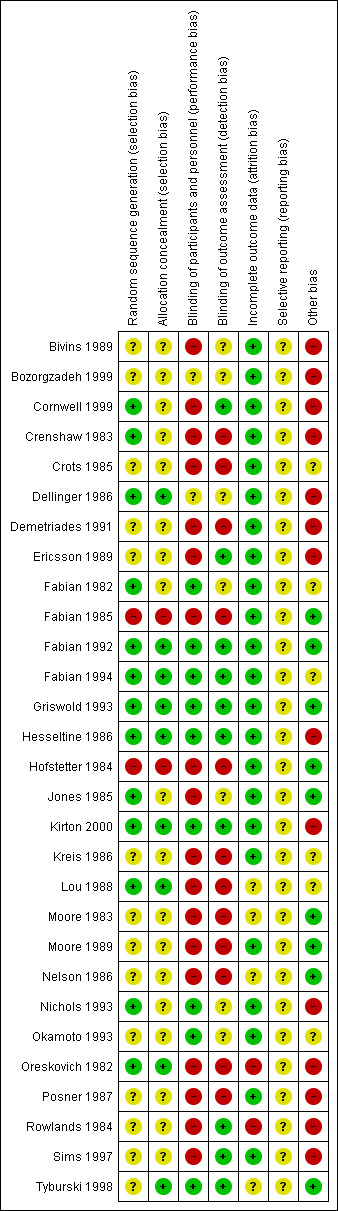
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Thirteen studies did not provide enough details about randomisation or allocation concealment for us to judge risk of selection bias. We labelled them as unclear risk for both domains (Bivins 1989; Bozorgzadeh 1999; Crots 1985; Demetriades 1991; Ericsson 1989; Kreis 1986; Moore 1983; Moore 1989; Nelson 1986; Okamoto 1993; Posner 1987; Rowlands 1984; Sims 1997). We judged two studies at high risk of bias in both domains because they randomised by either hospital number or day of admission (Fabian 1985; Hofstetter 1984). We judged five studies at low risk for randomisation; however, they did not provide sufficient information about allocation concealment, so we labelled them as unclear risk (Cornwell 1999; Crenshaw 1983; Fabian 1982; Jones 1985; Nichols 1993). One study did not provide enough information regarding randomisation, but had a low risk of bias for allocation concealment (Tyburski 1998). We judged eight studies at low risk of bias in both domains (Dellinger 1986; Fabian 1992; Fabian 1994; Griswold 1993; Hesseltine 1986; Kirton 2000; Lou 1988; Oreskovich 1982).
Blinding
Fourteen of the trials did not use a placebo in the comparison group; they also used different antibiotics with different dosing schedules. Due to this, we deemed them all at high risk of performance bias (Bivins 1989; Cornwell 1999; Demetriades 1991; Ericsson 1989; Fabian 1985; Hofstetter 1984; Jones 1985; Kreis 1986; Lou 1988; Moore 1983; Moore 1989; Nelson 1986; Oreskovich 1982; Posner 1987). Six of the studies provided insufficient information to enable us to assess performance bias, and we labelled them as unclear risk (Bozorgzadeh 1999; Cornwell 1999; Crenshaw 1983; Crots 1985; Rowlands 1984; Sims 1997). We assessed nine of the studies at low risk of performance and detection bias because they used a placebo, and likely blinded outcome assessment (Fabian 1982; Fabian 1992; Fabian 1994; Griswold 1993; Hesseltine 1986; Kirton 2000; Nichols 1993; Okamoto 1993; Tyburski 1998). One study was double‐blinded (Fabian 1994).
We deemed 10 studies at low risk of detection bias, since they adequately described blinding of the outcome assessors (Cornwell 1999; Ericsson 1989; Fabian 1992; Fabian 1994; Griswold 1993; Hesseltine 1986; Kirton 2000; Rowlands 1984; Sims 1997; Tyburski 1998). We could not assess detection bias in seven studies, as they made no mention of assessors (Bivins 1989; Bozorgzadeh 1999; Dellinger 1986; Fabian 1982; Jones 1985; Nichols 1993; Okamoto 1993). We considered 12 studies at high risk of detection bias, because they neither used a placebo, nor blinded assessors (Crenshaw 1983; Crots 1985; Demetriades 1991; Fabian 1985; Hofstetter 1984; Jones 1985; Kreis 1986; Lou 1988; Moore 1983; Moore 1989; Nelson 1986; Oreskovich 1982; Posner 1987).
Incomplete outcome data
We deemed three of the studies as unclear risk of attrition bias, as it was not apparent from which arm of the study participants dropped out (Lou 1988; Moore 1983; Nelson 1986; Rowlands 1984). We classified one study at high risk after participants were excluded from the analysis for reasons not stipulated in their methods (Oreskovich 1982), and another due to a disproportionate number of dropouts in one group due to protocol violations (Rowlands 1984). We regarded the remaining studies at low risk, as either all participants were analysed, or only very small numbers were classed as dropouts.
Selective reporting
We could not identify any published clinical trial registrations or protocols. Therefore, we regarded all of the included studies as being at unclear risk of bias for this domain. All of the studies included in this review were published prior to ICJME statement requiring registration as a prerequisite for publication (ICMJE 2019).
Other potential sources of bias
Nine of the studies had participants with similar baseline characteristics and no other risk of bias; therefore, we classified them at low risk (Fabian 1985; Fabian 1992; Griswold 1993; Hofstetter 1984; Jones 1985; Moore 1983; Moore 1989; Nelson 1986; Tyburski 1998). Six studies lacked clarity regarding the role of industry funding, therefore, we regarded them as unclear risk of bias (Crots 1985; Fabian 1982; Fabian 1994; Kreis 1986; Lou 1988; Okamoto 1993). We classed the remaining 14 studies at high risk of other sources of bias, due to the variation in baseline characteristics between groups (which could affect outcome), and other interventions they received as therapy (e.g. number of units of blood transfused).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. More than 24 hours of antibiotics compared to 24 hours of antibiotics for penetrating abdominal trauma.
| More than 24 hours of antibiotics compared to 24 hours of antibiotics | |||||
| Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA Intervention: antibiotics administered for > 24 h Comparison: antibiotics administered for 24 h only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antibiotics administered for 24 h | Antibiotics administered for > 24 h | ||||
| Abdominal surgical site infection | 183 per 1000 | 183 per 1000 (148 to 225) | RR 1.00 (0.81 to 1.23) | 1261 (7 RCTs) | ⊕⊝⊝⊝ very lowa,b,c |
| Mortality | 13 per 1000 | 22 per 1000 (9 to 50) | OR 1.67 (0.73 to 3.82) | 1261 (7 RCTs) | ⊕⊝⊝⊝ very lowa,b,c |
| Intra‐abdominal infection | 79 per 1000 | 97 per 1000 (66 to 142) | RR 1.23 (0.84 to 1.80) | 1111 (6 RCTs) | ⊕⊝⊝⊝ very lowa,b,c |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; OR: Odds Ratio; h: hours | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 1 level for risk of bias: lack of blinding in most studies, lack of allocation concealment in majority of studies bDowngraded 2 levels for inconsistency: large amount of both clinical and methodological heterogeneity cDowngraded 1 level for indirectness: majority of antibiotics given in the included studies are not in routine clinical use today
Summary of findings 2. Single dose of antibiotics compared to 24 hours of antibiotics for penetrating abdominal trauma.
| Single dose of antibiotics compared to 24 hours of antibiotics | |||||
|
Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA Intervention: single dose of antibiotics Comparison: 24 h of antibiotics | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antibiotics administered for 24 h | Single dose of antibiotics | ||||
| Abdominal surgical site infection | 115 per 1000 | 170 per 1000 (100 to 290) | RR 1.48 (0.87 to 2.52) | 360 (1 RCT) | ⊕⊝⊝⊝ very lowa,b,c |
| Mortality | No deaths reported in either arm | 360 (1 RCT) |
⊕⊝⊝⊝ very lowa,b,c | ||
| Intra‐abdominal infection | 45 per 1000 | 34 per 1000 (11 to 105) | RR 0.76 (0.25 to 2.32) | 360 (1 RCT) |
⊕⊝⊝⊝ very lowa,b,c |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; h: hours | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 1 level for risk of bias: lack of blinding in most studies, lack of allocation concealment in majority of studies bDowngraded 1 level for indirectness: majority of antibiotics given in the included studies are not in routine clinical use today cDowngraded 1 level for imprecision: low event rate and wide confidence intervals
Summary of findings 3. Anaerobic coverage only compared to gram‐positive, gram‐negative, and anaerobic coverage for penetrating abdominal trauma.
| Anaerobic coverage compared to gram‐positive, gram‐negative, and anaerobic coverage | |||||
| Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA and South Africa Intervention: antibiotics providing only anaerobic coverage Comparison: antibiotics providing gram‐positive, gram‐negative, and anaerobic coverage | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antibiotics providing gram‐positive, gram‐negative, and anaerobic cover | Antibiotics providing anaerobic coverage | ||||
| Abdominal surgical site infection | 79 per 1000 | 116 per 1000 (62 to 215) | RR 1.46 (0.79 to 2.71) | 395 (4 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| Mortality | No deaths reported in either arm | 309 (3 RCTs) |
⊕⊝⊝⊝ very lowa,b,c,d | ||
| Intra‐abdominal infection | 21 per 1000 | 51 per 1000 (14 to 188) | RR 2.46 (0.66 to 9.14) | 309 (3 RCTs) |
⊕⊝⊝⊝ very lowa,b,c,d |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 2 levels for risk of bias: high risk of bias from inadequate randomisation and allocation concealment bDowngraded 2 levels for inconsistency: substantial clinical and methodological heterogeneity cDowngraded 1 level for indirectness: many antibiotics are not in routine clinical use today dDowngraded 1 level for imprecision: low event rate and wide confidence intervals
Summary of findings 4. Anaerobic coverage only compared to gram‐positive and gram‐negative coverage for penetrating abdominal trauma.
| Anaerobic coverage compared to gram‐positive plus gram‐negative coverage | |||||
| Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA Intervention: antibiotics providing anaerobic coverage Comparison: antibiotics providing gram‐positive and gram‐negative coverage | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antibiotics providing gram‐positive and gram‐negative coverage | Antibiotics providing anaerobic coverage | ||||
| Abdominal surgical site infection | 175 per 1000 | 119 per 1000 (56 to 254) | RR 0.68 (0.32 to 1.45) | 160 (2 RCTs) | ⊕⊝⊝⊝ very lowa,b,c |
| Mortality | One death reported in the gram‐positive and gram‐negative coverage arm; no deaths reported in the anaerobic coverage arm | 160 (2 RCTs) | ⊕⊝⊝⊝ very lowa,b,c | ||
| Intra‐abdominal infection | 171 per 1000 | 96 per 1000 (25 to 354) | RR 0.56 (0.15 to 2.07) | 66 (1 RCT) | ⊕⊝⊝⊝ very lowa,b,c |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 1 level for risk of bias: one study was at high risk of bias due to inadequate randomisation, whilst one study was at low risk bDowngraded 1 level for indirectness: majority of antibiotics given in the included studies are not in routine clinical use today cDowngraded 1 level for imprecision: low event rate
Summary of findings 5. Anaerobic coverage only compared to gram‐negative and anaerobic coverage for penetrating abdominal trauma.
| Anaerobic coverage compared to gram‐negative and anaerobic coverage | |||||
| Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA Intervention: antibiotics providing only anaerobic coverage Comparison: antibiotics providing gram‐negative and anaerobic coverage | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antibiotics providing gram‐negative and anaerobic coverage | Antibiotics providing anaerobic coverage | ||||
| Abdominal surgical site infection | 148 per 1000 | 151 per 1000 (101 to 225) | RR 1.02 (0.68 to 1.52) | 523 (4 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| Mortality | No deaths reported in either arm | 417 (2 RCTs) |
⊕⊝⊝⊝ very lowa,b,c,d | ||
| Intra‐abdominal infection | 67 per 1000 | 78 per 1000 (40 to 155) | RR 1.17 (0.59 to 2.32) | 437 (3 RCTs ) | ⊕⊝⊝⊝ very lowa,b,c,d |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 2 levels for risk of bias: many studies at high risk of bias due to lack of blinding and inadequate allocation concealment bDowngraded 2 levels for inconsistency: large amount of clinical and methodological heterogeneity; different estimates of effect across studies cDowngraded 1 level for indirectness: most antibiotics used in the studies are not in routine clinical use today dDowngraded 1 level for imprecision: Wide confidence intervals
Summary of findings 6. Gram‐negative and anaerobic coverage compared to gram‐positive, gram‐negative, and anaerobic coverage for penetrating abdominal trauma.
| Gram‐negative and anaerobic coverage compared to gram‐positive, gram‐negative, and anaerobic coverage | |||||
| Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA Intervention: antibiotics providing gram‐negative and anaerobic coverage Comparison: antibiotics providing gram‐positive, gram‐negative, and anaerobic coverage | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| antibiotics providing gram‐positive, gram‐negative, and anaerobic coverage | antibiotics providing gram‐negativeand anaerobic coverage | ||||
| Abdominal surgical site infection | 57 per 1000 | 80 per 1000 (31 to 202) | RR 1.40 (0.55 to 3.56) | 516 (6 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| Mortality | No deaths reported in either arm | 177 (3 RCTs) |
⊕⊝⊝⊝ very lowa,b,c,d | ||
| Intra‐abdominal infection | 50 per 1000 | 59 per 1000 (22 to 163) | RR 1.19 (0.43 to 3.28) | 430 (5 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 2 levels for risk of bias: many studies at high risk of bias due to lack of blinding and inadequate allocation concealment bDowngraded 2 levels for inconsistency: large amount of clinical and methodological heterogeneity cDowngraded 1 level for indirectness: most antibiotics used in the studies are not in routine clinical use today dDowngraded 1 level for imprecision: wide confidence intervals
Summary of findings 7. Gram‐positive and gram‐negative coverage compared to gram‐negative and anaerobic coverage for penetrating abdominal trauma.
| Gram‐positive and gram‐negative coverage compared to gram‐negative and anaerobic coverage | |||||
| Participant or population: people undergoing laparotomy for penetrating abdominal trauma Setting: secondary care hospitals in the USA Intervention: antibiotics providing gram‐positive and gram‐negative coverage Comparison: antibiotics providing gram‐negative and anaerobic coverage | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Antibiotics providing gram‐negative and anaerobic coverage | Antibiotics providing gram‐positive and gram‐negative coverage | ||||
| Abdominal surgical site infection | 85 per 1000 | 93 per 1000 (55 to 159) | RR 1.10 (0.65 to 1.88) | 555 (3 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| Mortality | 14 per 1000 | 8 per 1000 (1 to 38) | OR 0.55 (0.11 to 2.77) | 555 (3 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| Intra‐abdominal infection | 67 per 1000 | 72 per 1000 (40 to 133) | RR 1.08 (0.59 to 1.98) | 555 (3 RCTs) | ⊕⊝⊝⊝ very lowa,b,c,d |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; OR: Odds Ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded 2 levels for risk of bias: all studies at risk of bias due to Inadequate randomisation and allocation concealment bDowngraded 2 levels for inconsistency: large amount of clinical and methodological heterogeneity cDowngraded 1 level for indirectness: many of the antibiotics used in the studies are no longer in routine clinical use dDowngraded 1 level for imprecision: wide confidence intervals
1. Duration of antibiotic therapy: 24 hours versus > 24 hours
(seven RCTs, 1261 participants; Table 1)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether antibiotic prophylaxis lasting longer than 24 hours reduced the risk of abdominal surgical site infection (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.81 to 1.23; I² = 0%; 7 RCTs, 1261 participants; very low‐quality evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Duration of antibiotic therapy (24 h vs > 24 h), Outcome 1 Abdominal surgical site infection.
Results from a subgroup analysis of participants who suffered a hollow viscus injury were also inconclusive (RR 1.02, 95% CI 0.75 to 1.39; 3 RCTs, 495 participants; Analysis 1.1). Results remained inconclusive when we excluded trials with inadequate or unclear allocation concealment (sensitivity analysis). The results did not cross the conventional or alpha adjusted boundary for statistical significance with trial sequential analysis (TSA), nor did they achieve the required information size (3289 participants).
Secondary outcomes
Mortality
The results were inconclusive as to whether antibiotic prophylaxis lasting longer than 24 hours reduced the risk of mortality (RR 1.67, 95% CI 0.73 to 3.82; I² = 8%; 7 RCTs, 1261 participants; very low‐quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 Duration of antibiotic therapy (24 h vs > 24 h), Outcome 2 Mortality.
Results from a subgroup analysis of participants who suffered a hollow viscus injury showed decreased risk of mortality when antibiotic prophylaxis lasted longer than 24 hours (RR 3.74, 95% CI 1.11 to 12.56; 3 RCTs, 495 participants; Analysis 1.2). Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (9349 participants).
Intra‐abdominal infection
The results were inconclusive as to whether antibiotic prophylaxis lasting longer than 24 hours reduced the risk of intra‐abdominal infection (RR 1.23, 95% CI 0.84 to 1.80; I² = 0%; 6 RCTs, 1111 participants; very low‐quality evidence; Analysis 1.3).
1.3. Analysis.
Comparison 1 Duration of antibiotic therapy (24 h vs > 24 h), Outcome 3 Intra‐abdominal infection.
Results from a subgroup analysis of participants who suffered a hollow viscus injury were also inconclusive (RR 1.38, 95% CI 0.84 to 2.29; 3 RCTs, 495 participants; Analysis 1.3). Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1107 participants), but they did cross the boundary for futility.
2. Duration of antibiotic therapy: single dose versus 24 hours
(one RCT, 360 participants; Table 2)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether reducing antibiotic prophylaxis from 24 hours to a single dose increased the risk of abdominal surgical site infection (RR 1.48, 95% CI 0.87 to 2.52; 1 RCT, 360 participants; very low‐quality evidence; Analysis 2.1). There were too few data to perform TSA.
2.1. Analysis.
Comparison 2 Duration of antibiotic therapy (single dose vs 24 h), Outcome 1 Abdominal surgical site infection.
Secondary outcomes
Mortality
There were no deaths reported in either treatment arm.
Intra‐abdominal infection
The results were inconclusive as to whether reducing antibiotic prophylaxis from 24 hours to a single dose increased the risk of intra‐abdominal infection (RR 0.76, 95% CI 0.25 to 2.32; 1 RCT, 360 participants; very low‐quality evidence; Analysis 2.2). There were too few data to perform TSA.
2.2. Analysis.
Comparison 2 Duration of antibiotic therapy (single dose vs 24 h), Outcome 2 Intra‐abdominal infection.
3. Antimicrobial coverage: antibiotics with activity against anaerobic bacteria only versus antibiotics with activity against gram‐positive, gram‐negative, and anaerobic bacteria (complete coverage)
(four RCTs, 395 participants; Table 3)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether antibiotic prophylaxis against anaerobic bacteria alone increased the risk of abdominal surgical site infection when compared to antibiotic prophylaxis that provided complete coverage (RR 1.46, 95% CI 0.79 to 2.71; I² = 0%; 4 RCTs, 395 participants; very low‐quality evidence; Analysis 3.1). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1107 participants).
3.1. Analysis.
Comparison 3 Anaerobic vs gram‐positive, gram‐negative, and anaerobic coverage, Outcome 1 Abdominal surgical site infection.
Secondary outcomes
Mortality
None of the trials reported any deaths.
Intra‐abdominal infection
The results were inconclusive as to whether antibiotic prophylaxis against anaerobic bacteria alone increased the risk of intra‐abdominal infection when compared to antibiotic prophylaxis that provided complete coverage (RR 2.46, 95% CI 0.66 to 9.14; I² = 0%; 4 RCTs, 395 participants; very low‐quality evidence; Analysis 3.2). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (4639 participants).
3.2. Analysis.
Comparison 3 Anaerobic vs gram‐positive, gram‐negative, and anaerobic coverage, Outcome 2 Intra‐abdominal infection.
4. Antimicrobial coverage: antibiotics with activity against anaerobic bacteria only versus antibiotics with activity against gram‐positive and gram‐negative bacteria
(two RCTs, 160 participants; Table 4)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether antibiotic prophylaxis against anaerobic bacteria alone increased the risk of abdominal surgical site infection when compared to antibiotic prophylaxis against gram‐positive and gram‐negative bacteria (RR 0.68, 95% CI 0.32 to 1.45; I² = 0%; 2 RCTs, 160 participants; very low‐quality evidence; Analysis 4.1). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (453 participants).
4.1. Analysis.
Comparison 4 Anaerobic vs gram‐positive + gram‐negative coverage, Outcome 1 Abdominal surgical site infection.
Secondary outcomes
Mortality
One trial reported one death in the gram‐positive and gram‐negative bacteria coverage arm. There were too few data to perform TSA.
Intra‐abdominal infection
The results were inconclusive as to whether antibiotic prophylaxis against anaerobic bacteria alone increased the risk of intra‐abdominal infection when compared to antibiotic prophylaxis against gram‐positive and gram‐negative bacteria (RR 0.56, 95% CI 0.15 to 2.07; 1 RCT, 66 participants; very low‐quality evidence; Analysis 4.2). The study did not provide data for hollow viscus injuries. There were too few data to perform TSA.
4.2. Analysis.
Comparison 4 Anaerobic vs gram‐positive + gram‐negative coverage, Outcome 2 Intra‐abdominal infection.
5. Antimicrobial coverage: antibiotics with activity against anaerobic bacteria only versus antibiotics with activity against gram‐negative and anaerobic bacteria
(four RCTs, 523 participants; Table 5)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether antibiotic prophylaxis against anaerobic bacteria alone increased the risk of abdominal surgical site infection when compared to antibiotic prophylaxis against gram‐negative and anaerobic bacteria (RR 1.02, 95% CI 0.68 to 1.52; I² = 0%; 4 RCTs, 523 participants; very low‐quality evidence; Analysis 5.1). None of the studies provided data on hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (4074 participants).
5.1. Analysis.
Comparison 5 Anaerobic vs gram‐negative + anaerobic coverage, Outcome 1 Abdominal surgical site infection.
Secondary outcomes
Mortality
Two RCTs with 417 participants reported this outcome. There were no deaths in either trial, therefore, no data with which to perform TSA.
Intra‐abdominal infection
The results were inconclusive as to whether antibiotic prophylaxis against anaerobic bacteria alone increased the risk of intra‐abdominal infection when compared to antibiotic prophylaxis against gram‐negative and anaerobic bacteria (RR 1.17, 95% CI 0.59 to 2.32; I² = 0%; 3 RCTs, 437 participants; very low‐quality evidence; Analysis 5.2). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1275 participants).
5.2. Analysis.
Comparison 5 Anaerobic vs gram‐negative + anaerobic coverage, Outcome 2 Intra‐abdominal infection.
6. Antimicrobial coverage: antibiotics with activity against gram‐negative and anaerobic bacteria versus antibiotics with activity against gram‐positive, gram‐negative, and anaerobic bacteria (complete coverage)
(six RCTs, 516 participants; Table 6)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether antibiotic prophylaxis against gram‐negative and anaerobic bacteria increased the risk of abdominal surgical site infection when compared to antibiotic prophylaxis that provided complete coverage (RR 1.40, 95% CI 0.55 to 3.56; I² = 29%; 6 RCTs, 516 participants; very low‐quality evidence; Analysis 6.1). None of the studies provided data for hollow viscus injuries. All trials were either at high or unclear risk of bias for allocation concealment, therefore, we did not complete a sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1712 participants).
6.1. Analysis.
Comparison 6 Gram‐negative + anaerobic vs gram‐positive, gram‐negative, + anaerobic (complete) coverage, Outcome 1 Abdominal surgical site infection.
Secondary outcomes
Mortality
Three RCTs with 177 participants reported this outcome. There were no deaths in any of the trials, therefore, no data with which to perform TSA.
Intra‐abdominal infection
The results were inconclusive as to whether antibiotic prophylaxis against gram‐negative and anaerobic bacteria increased the risk of intra‐abdominal infection when compared to antibiotic prophylaxis that provided complete coverage (RR 1.19, 95% CI 0.43 to 3.28; I² = 14%; 5 RCTs, 430 participants; very low‐quality evidence; Analysis 6.2). None of the studies provided data for hollow viscus injuries. All trials were either at high or unclear risk of bias for allocation concealment, therefore, we did not complete a sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1814 participants).
6.2. Analysis.
Comparison 6 Gram‐negative + anaerobic vs gram‐positive, gram‐negative, + anaerobic (complete) coverage, Outcome 2 Intra‐abdominal infection.
7. Antimicrobial coverage: antibiotics with activity against gram‐positive and gram‐negative bacteria versus antibiotics with activity against gram‐negative and anaerobic bacteria
(three RCTs, 555 participants; Table 7)
Primary outcome
Abdominal surgical site infection
The results were inconclusive as to whether antibiotic prophylaxis against gram‐positive and gram‐negative bacteria increased the risk of abdominal surgical site infection when compared to antibiotic prophylaxis against gram‐negative and anaerobic bacteria (RR 1.10, 95% CI 0.65 to 1.88; I² = 0%; 3 RCTs, 555 participants; very low‐quality evidence; Analysis 7.1). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1107 participants), but they did cross the boundary for futility.
7.1. Analysis.
Comparison 7 Gram‐positive + gram‐negative vs gram‐negative + anaerobic coverage, Outcome 1 Abdominal surgical site infection.
Secondary outcomes
Mortality
The results were inconclusive as to whether antibiotic prophylaxis against gram‐positive and gram‐negative bacteria increased the risk of mortality when compared to antibiotic prophylaxis against gram‐negative and anaerobic bacteria (RR 0.55, 95% CI 0.11 to 2.77; I² = 0%; 3 RCTs, 555 participants; very low‐quality evidence; Analysis 7.2). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (9349 participants).
7.2. Analysis.
Comparison 7 Gram‐positive + gram‐negative vs gram‐negative + anaerobic coverage, Outcome 2 Mortality.
Intra‐abdominal infection
The results were inconclusive as to whether antibiotic prophylaxis against gram‐positive and gram‐negative bacteria increased the risk of intra‐abdominal infection when compared to antibiotic prophylaxis against gram‐negative and anaerobic bacteria (RR 1.08, 95% CI 0.59 to 1.98; I² = 0%; 3 RCTs, 555 participants; very low‐quality evidence; Analysis 7.3 ). None of the studies provided data for hollow viscus injuries. Results remained inconclusive with sensitivity analysis. The results did not cross the conventional or alpha adjusted boundary for statistical significance with TSA, nor did they achieve the required information size (1275 participants), but they did cross the boundary for futility.
7.3. Analysis.
Comparison 7 Gram‐positive + gram‐negative vs gram‐negative + anaerobic coverage, Outcome 3 Intra‐abdominal infection.
Discussion
Summary of main results
Our review included 29 randomised controlled trials (RCTs; 4458 participants) comparing prophylactic antibiotic regimens for people undergoing laparotomy for penetrating abdominal trauma. Eight studies compared antibiotic prophylaxis given for different lengths of time, whilst 21 studies evaluated different antibiotic regimens. All but six of the studies were at a high risk of bias in at least one domain. We assessed the evidence for all outcomes as very low quality, due to concerns about study bias, inconsistency due to clinical and methodological heterogeneity, imprecision (low participant numbers and wide confidence intervals), and indirectness, since all of the studies were conducted using techniques and antibiotics that are no longer comparable to current practice.
Due to very low‐quality evidence from eight trials, we are uncertain of the effect of the duration of antibiotic prophylaxis on abdominal surgical site infection, mortality, or intra‐abdominal infection.
Twenty‐one studies compared different antibiotic regimens, which we classified into groups, based on whether the antibiotics tested had at least moderate activity (on average) against the three most common gram‐positive, gram‐negative, and anaerobic bacteria isolated in the studies (Mayo Clinic 2012). Due to very low‐quality evidence, we are uncertain as to whether there is a benefit to any regimen over another on abdominal surgical site infection, mortality, or intra‐abdominal infection.
Our prespecified sensitivity analysis, accounting for trials at high risk of bias due to unclear or inadequate allocation concealment, did not change the certainty of our results.
Trial sequential analysis (TSA) also failed to demonstrate any significance for any of our comparisons, after adjusting for multiple comparisons. TSA also demonstrated that given the low event rate in the majority of our included trials, all of our comparisons lacked power to find a true difference. Finally, the boundary of futility was crossed in several of our comparisons suggesting further trials aimed at answering these questions may be unwise (Analysis 1.3; Analysis 7.1; Analysis 7.3).
Overall completeness and applicability of evidence
During our database searches, we identified 29 RCTs, and were able to obtain all the information we required for these studies from the published papers. We are unaware of any applicable studies not included in this review.
All of the studies included were carried out in either the USA or South Africa, countries with a large case volume of penetrating abdominal trauma, therefore, it is unclear how the results of these studies apply to other countries with fewer cases of penetrating abdominal trauma. Similarly, the most recent trial we identified was published in 2000, with the earliest published nearly 40 years prior to this. Management of penetrating abdominal trauma has progressed in all aspects over the last 40 years. Pre‐, intra‐, peri‐ and postoperative care have all changed considerably over the last four decades. Furthermore, there have been nine editions of the Advanced Trauma Life Support Manual advocating the early use of cross‐sectional imaging and the conservative management of trauma patients without visceral injury. Therefore, it is unclear how the results of these trials may apply to modern surgical practice. Similarly, many of the antibiotics used in the trials we included are rarely used in today's practice, whilst antimicrobial resistance has emerged as a threat that requires careful consideration in prescribing antibiotics for ineffective indications.
Quality of the evidence
Two authors (HBC and PH) independently assessed the quality of evidence for each outcome using the GRADE system (Ryan 2016). They assessed the quality of the evidence to be very low for all outcomes and comparisons (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
They downgraded the quality of evidence throughout most domains due to high risk of bias in the studies, inconsistency from both clinical and methodological heterogeneity, applicability to modern clinical practice (as all of the studies were conducted using techniques and antibiotics that are no longer comparable to current surgical practice), and serious levels of imprecision, as indicated by TSA.
Potential biases in the review process
Two authors independently conducted abstract screening, full‐text reviews, assessments for risk of bias and GRADE, and resolved disagreements by discussion with a third author, as per our protocol, to which we strictly adhered. Allowing for this, some decisions for judging risk of bias may remain subjective, as some of the study methodology was poorly described in the trials.
Due to the vast clinical heterogeneity of antibiotic regimens used throughout the studies, we made the post‐hoc decision to group the antibiotics by their antimicrobial coverage, according to the The Mayo Clinic Antimicrobial Therapy guide, in order to permit useful data synthesis of the effect of antibiotic class. We did not include data from five studies in any quantitative analysis, as the antibiotics they compared provided the same coverage (Fabian 1994; Moore 1983; Nichols 1993; Sims 1997; Tyburski 1998).
Agreements and disagreements with other studies or reviews
We are unaware of any other systematic reviews comparing the use of prophylactic antibiotics for people with penetrating abdominal trauma. However, a Cochrane Review attempting to compare the use of prophylactic antibiotics with placebo identified no studies that met its inclusion criteria Brand 2019). Another Cochrane Review, which compared antibiotic prophylaxis for elective colorectal surgery, determined there was no significant benefit to long‐term compared to short‐term antibiotic prophylaxis (Nelson 2014).
Authors' conclusions
Implications for practice.
At present, we are uncertain if there is a benefit to extending the duration of antibiotic prophylaxis for people undergoing laparotomy for penetrating abdominal trauma beyond 24 hours on abdominal surgical site infection rates, mortality, or intra‐abdominal infections. Likewise, we are uncertain whether some drug regimens are superior to others. All of the studies included in this review were at high risk of bias, and the majority were carried out several decades ago, making application of this evidence to current surgical practice difficult.
Implications for research.
Future randomised controlled trials (RCT) should be adequately powered, using currently utilised antibiotics known to be effective against gut flora, and employ effective methodology to minimise the risk of bias. Future trials should adequately report the level of peritoneal contamination encountered at laparotomy. Due to the very low‐quality evidence across all outcomes, further RCTs are required to answer our review objective.
Notes
In future updates of this review we will apply the inclusion criteria required by the Cochrane Injuries Group (Roberts 2015); in particular, any new studies must have been prospectively registered.
Acknowledgements
We would like to thank the Cochrane Injuries Group, particularly Emma Sydenham, Co‐ordinating Editor, and Helen Wakeford, Managing Editor, for their support with this review. We would also like to thank Jane Falconer, the Information Specialist who carried out our electronic searches, and Marialena Trivella, the Statistical Editor who helped with our methodology. We are grateful for the comments of the external peer referees, Zsolt Balogh, Massimo Sartelli and Stephanie Goldberg, which enhanced the quality of this review, and for the efforts of Vicki Pennick who copy‐edited the manuscript.
This project was supported by the UK National Institute for Health Research (NIHR), through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies
Cochrane Injuries Group Specialised Register
((((abdominal or abdomen or thorax or thoracic) AND (injur* or trauma* or perforat* or penetrat*))) OR (((splenic or spleen) AND (rupture*)) or ((stomach or gastric) AND (rupture or perforation or injur* or burst*)) or ((stab* or gunshot or shot or "penetrat* wound*") AND (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic))) AND (((antibiotic*) AND (prophylaxis or prophylactic* or premedication*)) or ((antibacterial or anti‐bacterial) AND (agent*)))) AND ( INREGISTER)
Cochrane Central Register of Controlled Trials (CENTRAL, in the Cochrane Library)
#1 MeSH descriptor: [Abdominal Injuries] explode all trees (140)
#2 MeSH descriptor: [Thoracic Injuries] explode all trees (382)
#3 ((abdominal or abdomen or thorax or thoracic) near/3 (injur* or trauma* or perforat* or penetrat*)):ti,ab,kw (936)
#4 ((splenic or spleen) near/3 rupture*):ti,ab,kw (16)
#5 ((stomach or gastric) near/3 (rupture or perforation or injur* or burst*)):ti,ab,kw (406)
#6 ((stab* or gunshot or shot or penetrat* wound* or bullet?) near/3 (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic)):ti,ab,kw (818)
#7 MeSH descriptor: [Wounds, Stab] explode all trees (106)
#8 MeSH descriptor: [Wounds, Gunshot] this term only (49)
#9 MeSH descriptor: [Wounds, Penetrating] this term only (170)
#10 MeSH descriptor: [Rupture] explode all trees (910)
#11 #7 or #8 or #9 or #10 (1219)
#12 (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic):ti,ab,kw (74440)
#13 #11 and #12 (175)
#14 #1 or #2 or #3 or #4 or #5 or #6 or #13 (2373)
#15 MeSH descriptor: [Antibiotic Prophylaxis] this term only (1224)
#16 (antibiotic* near/5 (prophylaxis or prophylactic* or premedication*)):ti,ab,kw (5052)
#17 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees (11257)
#18 ((antibacterial or anti‐bacterial) near/3 agent*):ti,ab,kw (10428)
#19 MeSH descriptor: [Amoxicillin] explode all trees (2628)
#20 MeSH descriptor: [Ampicillin] this term only (990)
#21 (amox*):ti,ab,kw (5894)
#22 (clavulan*):ti,ab,kw (1775)
#23 MeSH descriptor: [Cefotaxime] explode all trees (1384)
#24 MeSH descriptor: [Cephalosporins] this term only (1381)
#25 (cefotaxim*):ti,ab,kw (1041)
#26 (ceftriaxone):ti,ab,kw (1571)
#27 MeSH descriptor: [Piperacillin] explode all trees (403)
#28 (piperac*):ti,ab,kw (957)
#29 (tazobactam):ti,ab,kw (620)
#30 MeSH descriptor: [Thienamycins] explode all trees (480)
#31 (meropenem):ti,ab,kw (608)
#32 (imipenem):ti,ab,kw (709)
#33 (cilastatin):ti,ab,kw (402)
#34 MeSH descriptor: [Ciprofloxacin] explode all trees (1139)
#35 (ciprofloxacin):ti,ab,kw (2635)
#36 MeSH descriptor: [Metronidazole] explode all trees (2161)
#37 (metronidazole):ti,ab,kw (4482)
#38 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 (27206)
#39 #14 and #38 (170)
MEDLINE(R) Ovid, MEDLINE(R) Ovid In‐Process & Other Non‐Indexed Citations, MEDLINE(R) Daily Ovid, and OLDMEDLINE(R) Ovid
1. exp Abdominal Injuries/ (20049)
2. exp Thoracic Injuries/ (26101)
3. ((abdominal or abdomen or thorax or thoracic) adj3 (injur* or trauma* or perforat* or penetrat*)).ti,ab. (20348)
4. ((splenic or spleen) adj3 rupture*).ti,ab. (3700)
5. ((stomach or gastric) adj3 (rupture or perforation or injur* or burst*)).ti,ab. (5678)
6. ((stab* or gunshot or shot or penetrat* wound* or bullet?) adj3 (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic)).ti,ab. (1865)
7. exp Wounds, Stab/ (7770)
8. Wounds, Gunshot/ (14887)
9. Wounds, Penetrating/ (11421)
10. exp Rupture/ (46781)
11. or/7‐10 (78662)
12. (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic).ti,ab. (687822)
13. 11 and 12 (16336)
14. or/1‐6,13 [TOTAL ABDOMINAL INJURIES] (68171)
15. Antibiotic Prophylaxis/ (13160)
16. (antibiotic* adj5 (prophylaxis or prophylactic* or premedication*)).ti,ab. (16055)
17. exp Anti‐Bacterial Agents/ (701868)
18. ((antibacterial or anti‐bacterial) adj3 agent*).ti,ab. (8916)
19. exp Amoxicillin/ (10885)
20. Ampicillin/ (13297)
21. amox*.ti,ab. (17859)
22. clavulan*.ti,ab. (8078)
23. exp cefotaxime/ (11547)
24. cephalosporins/ (18937)
25. cefotaxim*.ti,ab. (8308)
26. ceftriaxone.ti,ab. (10054)
27. exp Piperacillin/ (2708)
28. piperac*.ti,ab. (7084)
29. tazobactam.ti,ab. (4391)
30. exp Thienamycins/ (6188)
31. meropenem.ti,ab. (5988)
32. imipenem.ti,ab. (9936)
33. cilastatin.ti,ab. (1307)
34. exp Ciprofloxacin/ (12859)
35. ciprofloxacin.ti,ab. (24424)
36. Metronidazole/ (12429)
37. metronidazole.ti,ab. (14893)
38. or/15‐37 [ANTIBIOTICS] (736907)
39. randomi?ed.ab,ti. (578306)
40. randomized controlled trial.pt. (485792)
41. controlled clinical trial.pt. (93170)
42. placebo.ab. (199345)
43. exp Clinical Trials as Topic/ (328145)
44. randomly.ab. (314813)
45. trial.ti. (201916)
46. comparative study/ (1835236)
47. or/39‐46 [CLINICAL TRIALS] (2931723)
48. animals/ not (humans/ and animals/) (4567683)
49. 47 not 48 (2434514)
50. 14 and 38 and 49 (150)
51. remove duplicates from 50 (149)
Embase Classic + Embase Ovid
1 exp abdominal injury/ (165983)
2 exp thorax injury/ (82196)
3 ((abdominal or abdomen or thorax or thoracic) adj3 (injur* or trauma* or perforat* or penetrat*)).ti,ab. (26160)
4 ((splenic or spleen) adj3 rupture*).ti,ab. (4797)
5 ((stomach or gastric) adj3 (rupture or perforation or injur* or burst*)).ti,ab. (7735)
6 ((stab* or gunshot or shot or penetrat* wound* or bullet?) adj3 (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic)).ti,ab. (2543)
7 stab wound/ (5413)
8 gunshot injury/ (19004)
9 penetrating trauma/ (12804)
10 exp rupture/ (114147)
11 or/7‐10 (147285)
12 (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic).ti,ab. (1043589)
13 11 and 12 (24937)
14 or/1‐6,13 [TOTAL ABDOMINAL INJURIES] (266153)
15 antibiotic prophylaxis/ (29984)
16 (antibiotic* adj5 (prophylaxis or prophylactic* or premedication*)).ti,ab. (23805)
17 exp antiinfective agent/ (3538995)
18 ((antibacterial or anti‐bacterial) adj3 agent*).ti,ab. (12155)
19 amoxicillin/ (60295)
20 amoxicillin plus clavulanic acid/ (36320)
21 amoxicillin derivative/ (72)
22 ampicillin/ (86369)
23 ampicillin derivative/ (127)
24 amox*.ti,ab. (26820)
25 clavulan*.ti,ab. (11561)
26 cefotaxime/ (40281)
27 cephalosporin derivative/ (28049)
28 cefotaxim*.ti,ab. (11095)
29 ceftriaxone.ti,ab. (15527)
30 piperacillin/ (18896)
31 piperacillin plus tazobactam/ (24599)
32 piperacillin derivative/ (31)
33 piperac*.ti,ab. (11463)
34 tazobactam.ti,ab. (7730)
35 thienamycin derivative/ (447)
36 meropenem/ (29527)
37 meropenem.ti,ab. (10032)
38 imipenem/ (36081)
39 cilastatin plus imipenem/ (4655)
40 cilastatin/ (2644)
41 imipenem.ti,ab. (14420)
42 cilastatin.ti,ab. (1937)
43 ciprofloxacin/ (93985)
44 ciprofloxacin.ti,ab. (33573)
45 metronidazole/ (66235)
46 metronidazole.ti,ab. (21342)
47 or/15‐46 [ANTIBIOTICS] (3553657)
48 exp controlled study/ (6968492)
49 comparative study/ (851033)
50 randomi?ed.ab,ti. (829337)
51 placebo.ab. (287657)
52 *Clinical Trial/ (19134)
53 major clinical study/ (3507971)
54 randomly.ab. (418211)
55 (trial or study).ti. (1936952)
56 or/48‐55 [CLINICAL TRIALS] (10822904)
57 exp animal/ not (exp human/ and exp animal/) (5267381)
58 56 not 57 (8819418)
59 14 and 47 and 58 (8194)
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) & Conference Proceedings Citation Index‐Science (CPCI‐S)
# 1 TOPIC: ((abdominal or abdomen or thorax or thoracic) near/3 (injur* or trauma* or perforat* or penetrat*)) (18,098)
# 2 TOPIC: ((splenic or spleen) near/3 rupture*) (2,350)
# 3 TOPIC: ((stomach or gastric) near/3 (rupture or perforation or injur* or burst*)) (5,711)
# 4 TOPIC: ((stab* or gunshot or shot or "penetrat* wound*" or bullet$) near/3 (abdomen* or abdominal or stomach or splenic or spleen or thorax or thoracic)) (2,295)
# 5 #1 OR #2 OR #3 OR #4 (27,411)
# 6 TOPIC: (antibiotic* near/5 (prophylaxis or prophylactic* or premedication*)) (15,631)
# 7 TOPIC: ((antibacterial or anti‐bacterial) near/3 agent*) (14,397)
# 8 TOPIC: (amox*) (19,583)
# 9 TOPIC: (clavulan*) (8,640)
# 10 TOPIC: (cefotaxim*) (7,653)
# 11 TOPIC: (ceftriaxone) (9,783)
# 12 TOPIC: (piperac*) (7,464)
# 13 TOPIC: (tazobactam) (4,669)
# 14 TOPIC: (meropenem) (6,082)
# 15 TOPIC: (imipenem) (9,687)
# 16 TOPIC: (cilastatin) (1,474)
# 17 TOPIC: (ciprofloxacin) (30,841)
# 18 TOPIC: (metronidazole) (16,452)
# 19 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 (114,359)
# 20 TOPIC: (randomi?ed OR randomly OR "random order" OR "random sequence" OR "random allocation" OR "randomly allocated" OR "at random" OR "randomi?ed controlled trial") (1.092,871)
# 21 TOPIC: ("controlled clinical trial" OR "controlled trial" OR "clinical trial" OR placebo) (592,530)
# 22 TOPIC: ((singl* OR doubl* OR trebl* OR tripl*) NEAR/5 (blind* OR mask*)) (269.973)
# 23 #20 OR #21 OR #22 (1,376,075)
# 24 #5 AND #19 AND #23 (68)
Data and analyses
Comparison 1. Duration of antibiotic therapy (24 h vs > 24 h).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 7 | 1261 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
| 1.1 Any penetrating abdominal trauma | 4 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.31] |
| 1.2 Hollow viscus injuries only | 3 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.75, 1.39] |
| 2 Mortality | 7 | 1261 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.73, 3.82] |
| 2.1 Any penetrating abdominal trauma | 4 | 766 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.26, 2.55] |
| 2.2 Hollow viscus injuries only | 3 | 495 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.74 [1.11, 12.56] |
| 3 Intra‐abdominal infection | 6 | 1111 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.80] |
| 3.1 Any penetrating abdominal trauma | 3 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.59, 1.88] |
| 3.2 Hollow viscus injuries only | 3 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.84, 2.29] |
Comparison 2. Duration of antibiotic therapy (single dose vs 24 h).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Intra‐abdominal infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Anaerobic vs gram‐positive, gram‐negative, and anaerobic coverage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 4 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.79, 2.71] |
| 2 Intra‐abdominal infection | 3 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.66, 9.14] |
Comparison 4. Anaerobic vs gram‐positive + gram‐negative coverage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.32, 1.45] |
| 2 Intra‐abdominal infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Anaerobic vs gram‐negative + anaerobic coverage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 4 | 523 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.52] |
| 2 Intra‐abdominal infection | 3 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.59, 2.32] |
Comparison 6. Gram‐negative + anaerobic vs gram‐positive, gram‐negative, + anaerobic (complete) coverage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 6 | 516 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.55, 3.56] |
| 2 Intra‐abdominal infection | 5 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.43, 3.28] |
Comparison 7. Gram‐positive + gram‐negative vs gram‐negative + anaerobic coverage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abdominal surgical site infection | 3 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.65, 1.88] |
| 2 Mortality | 3 | 555 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.11, 2.77] |
| 3 Intra‐abdominal infection | 3 | 555 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.59, 1.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bivins 1989.
| Methods | Parallell, randomised study, conducted in a secondary care hospital in the USA | |
| Participants | People who sustained penetrating abdomina trauma, excluding those who had received antibiotics in the preceding three days. 129 participants (43 in each group); mean age 31 years (group 1), 28 years (group 2) and 27.3 years (group 3) | |
| Interventions | Group 1: Cefotaxime 2 g IV, 8 hourly
Group 2: Cefoxitine, 2 g IV, 6 hourly Group 3: Clindamycin 900 mg IV, 8 hourly, plus Gentamycin 3 mg/kg to 5mg/kg, IV, 8 hourly Antibiotics were continued for 3 days minimum in participants without hollow viscus injury, and 5 days minimum in participants with a hollow viscus injury. |
|
| Outcomes | Failure of therapy, defined by presence of clinical evidence of continued or new infection, accompanied by:
Success of therapy, defined by recovery without evidence of infection |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | states "randomised list", but does not provide details |
| Allocation concealment (selection bias) | Unclear risk | states kept blind, but unclear details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different intervention regimens, therefore not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no mention of who performs outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants analysed, no dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | variation between groups in proportion of colonic and small bowel injuries |
Bozorgzadeh 1999.
| Methods | Parallell, randomised trial, conducted in secondary care hospital in the USA | |
| Participants | Consecutive participants with penetrating abdominal trauma, excluding participants with allergy to study antibiotics, and death within 24 hours. 300 participants, (148 in group 1, mean age 27.5 years; and 152 in group 2, mean age 26.4 years) | |
| Interventions | Group 1: Cefoxitin 1 g IV, 6 hourly for 24 hours Group 2: Cefoxitin 1 g IV, 6 hourly for 5 days |
|
| Outcomes | Development of deep infection or nosocomial infection Hospital length of stay |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention of randomisation |
| Allocation concealment (selection bias) | Unclear risk | no mention of blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | no mention of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | eight participants excluded for death, without specifying which group; however, low number and unlikely to bias |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | significant differences between groups at baseline in blood loss |
Cornwell 1999.
| Methods | Parallell, randomised trial, conduced in secondary care trauma centre in the USA | |
| Participants | Participants aged > 16 years, with penetrating abdominal trauma at high risk for postoperative infections (full thickness injuries to colon). Excluded patients; allergy to study antibiotics, renal failure, or injures requiring non‐protocol antibiotics. (63 participants: 31 in group 1, and 32 in group 2) |
|
| Interventions | Group 1: Cefoxitin 2 g IV, 24 hours duration Group 2: Cefoxitin 2 g IV, 5 days duration |
|
| Outcomes | Development of complications: septic or otherwise Number of days on the ventilator, and in the intensive care unit Lenght of stay Survival |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | card shuffling |
| Allocation concealment (selection bias) | Unclear risk | cards in envelopes, numbered, however, no mention of opacity |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no blinding, vastly different lengths of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients designated by blinded surgical investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all data analysed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | unequal groups with respect to ISS >15, and > 6 units of blood transfused |
Crenshaw 1983.
| Methods | Parallell, randomised study, undertaken in a secondary care hospital in the USA | |
| Participants | Participants > 18 years, with penetrating abdominal trauma, excluded if allergy to protocol antibiotics, pregnancy, or hepatic or renal disease were present 94 participants: 49 in group 1 (mean age 24.9 years), and 45 in group 2 (mean age 32.8 years) |
|
| Interventions | Group 1: Cefamandole 2 g IV, 6 hourly Group 2: Tobramycin 3 mg/kg IV, plus Cephalothin 2 g IV, 6 hourly Both groups received therapy for minimum of 72 hours |
|
| Outcomes | No clear outcomes stipulated | |
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens, and doctors could extend treatment if necessary |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | outcome assessment was carried out by unblinded investigators, who could choose to extend the duration of treatment if they felt the individual needed further antibiotics |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | dropouts unlikely to bias |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | more gunshot wounds in group 2 |
Crots 1985.
| Methods | Parallell, randomised trial in a secondary care hospital in the USA | |
| Participants | Participants sustaining penetrating abdominal trauma undergoing laparotomy, excluding those who had received antibiotics in preceding 3 days. 50 total participants, 25 in each group with a mean age of 27 years in each |
|
| Interventions | Group 1: Clindamycin 600 mg IV, 6 hourly, plus Gentamycin 3 mg/kg to 5 mg/kg IV, 8 hourly Group 2: Moxalactam 2 g IV, 12 hourly In both groups, antibiotics continued for a minimum of 3 days in participants without evidence of hollow viscus injury, and 5 days in participants with hollow viscus injury. |
|
| Outcomes | Failure of therapy, defined by presence of clinical evidence of continued or new infection accompanied by:
Success of therapy, defined by recovery without evidence of infection |
|
| Notes | No funding source reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no details on how randomisation list generated |
| Allocation concealment (selection bias) | Unclear risk | states investigators blinded, but no details on envelopes, etc |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no placebo used, different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | outcome assessment carried out by unblinded investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Unclear risk | industry funded; however, unclear what role the sponsor had in the conduct of the study |
Dellinger 1986.
| Methods | Parallell, randomised trial in a secondary care hospital in the USA | |
| Participants | Participants with penetrating abdominal trauma, with injury into the lumen of the bowel, confirmed at laparotomy 114 participants: 31 in group 1, mean age 30 years; 25 in group 2, mean age 35 years; 30 in group 3, mean age 30 years, 28 in group 4, mean age 35 years |
|
| Interventions | Group 1: Penicillin 1 million units IV, 4 hourly for 24 hours, plus a one‐off dose of Doxycycline 300 mg IV Group 2: Penicillin 1 million units IV, 4 hourly, plus Doxycycline 300 mg IV, once daily for 5 days Group 3: Cefoxitin 2 g IV, 4 hourly for 1 day Group 4: Cefoxitin 2 g IV, 4 hourly for 5 days |
|
| Outcomes | Incidence of:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random numbers table" |
| Allocation concealment (selection bias) | Low risk | pharmacy‐controlled randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | placebo only mentioned for second part of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no mention of blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | only 2 dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | large variation between groups in the amount of blood transfused |
Demetriades 1991.
| Methods | Parallell, randomised study in a secondary care hospital in South Africa | |
| Participants | Participants with penetrating abdominal trauma who underwent laparotomy Excluded from study if allergy to study antibiotic, delay to theatre of > 12 hours, or injuries requiring antibiotic not in study protocol 123 participants in total: 60 in group 1, mean age 28 years; 63 in group 2, mean age 30 years |
|
| Interventions | Group 1: Ceftriaxone 1 g IV, once daily Group 2: Cefoxitin 1 g IV, 6 hourly In both groups, antibiotics continued for 24 hours if no colonic injury, and for 48 hours if colonic injury present |
|
| Outcomes | Developement of:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised" ‐ no details |
| Allocation concealment (selection bias) | Unclear risk | no mention of allocation concealment made in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens, with no placebo mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | outcome assessors not blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | Unequal numbers of participants with bullet wounds or > 3 organs injured |
Ericsson 1989.
| Methods | Parallell, randomised study, undertaken in a secondary care setting in the USA | |
| Participants | Participants with both penetrating and blunt abdominal trauma, who were undergoing laparotomy Excluded those with no hollow viscus injury at surgery, or who died within 72 hours of presentation 149 eligible participants: 47 in group 1, 52 in group 2, 51 in group 3. Mean age was 31 years in all three groups |
|
| Interventions | Group 1: Single dose Clindamycin 1.2 g IV, plus Amikacin 7.5 mg/kg IV Group 2: Clindamycin 1.2 g IV, plus Amikacin 7.5 mg/kg IV, 12 hourly for 3 days Group 3: Clindamycin 600 mg IV, 6 hourly, plus Amikacin 7.5 mg/kg, 12 hourly for 3 days |
|
| Outcomes | Development of wound or intra‐abdominal infection | |
| Notes | Partly funded by grant from the UpJohn Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomised" ‐ no details |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens with no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded evaluator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants analysed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | baseline imbalances in gunshot wounds; industry funded |
Fabian 1982.
| Methods | Parallel, randomised, multicenter study, undertaken in a secondary care setting the USA | |
| Participants | Participants > 17 years of age, with penetrating abdominal trauma. Excluded: pregnancy, allergy, and recent antibiotic therapy 360 participants included: 116 in group 1 (mean age 31 years), 127 in group 2 (mean age 30 years), 117 in group 3 (mean age 29 years) |
|
| Interventions | Group 1: one‐off dose of 1 g of Cefotaxime IV Group 2: 1 g Cefotaxime IV, 6 hourly for 1 day Group 3: Cefazolin 1 g IV for 1 day |
|
| Outcomes | Developement of infection:
|
|
| Notes | Partially funded by Hoechst Roussel Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomisation" |
| Allocation concealment (selection bias) | Unclear risk | sealed envelopes, but not numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants analysed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Unclear risk | industry sponsored; however, role of sponsor in conduct of the trial is unclear |
Fabian 1985.
| Methods | Parallell, randomised study, undertaken in secondary care hospital in the USA | |
| Participants | Participants with penetrating abdominal trauma, excluding those with allergies to protocol antibiotics or significant medical comorbidity (renal, pulmonary, cardiac or hepatic.) 85 participants, randomised to two groups: 32 in group 1, mean age 29 years; 53 in group 2, mean age 27 years |
|
| Interventions | Group 1: Gentamycin 1.5 mg/kg IV plus Clindamycin 600 mg IV, 8 hourly for 1 day Group 2: Ticarillin 3 g IV plus Clavulanic acid 100 mg IV, 4 hourly for 1 day |
|
| Outcomes | Development of wound or intra‐abdominal infection | |
| Notes | Partially funded by Beecham Laboratories | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | randomised based on hospital number |
| Allocation concealment (selection bias) | High risk | randomisation based on hospital number; thus, allocation concealment not possible |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens with no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | investigators not blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | similar reasons for dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Fabian 1992.
| Methods | Parallel, randomised study undertaken in a regional trauma centre in the USA | |
| Participants | Participants with penetrating abdominal trauma, excluding those who were pregnant, had allergies to protocol antibiotics, or significant medical comorbidity (renal, pulmonary, cardiac, or hepatic), or ongoing active infection 515 participants randomised to four groups; if found to have hollow viscus injury at laparotomy, antibiotics were continued. Therefore, analysed: 53 in group 1, mean age 32 years; 65 in group 2, mean age 30 years; 55 in group 3, mean age 30 years; 62 in group 4, mean age 31 years |
|
| Interventions | Group 1: Cefoxitin 2 g IV, 6 hourly for 1 day Group 2: Cefotetan 2 g IV, 12 hourly for 1 day Group 3: Cefoxitin 2 g IV, 6 hourly for 5 days Group 4: Cefotetan 2 g IV, 12 hourly for 5 days |
|
| Outcomes | Major infections: intra‐abdominal, peritonitis, necrotising fascitis Minor infections: wound |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated sheet" |
| Allocation concealment (selection bias) | Low risk | pharmacy‐controlled randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | likely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants analysed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | comparable groups |
Fabian 1994.
| Methods | Parallel, randomised multi‐centre study undertaken in secondary care hospitals in the USA | |
| Participants | Participants > 18 years, with penetrating abdominal trauma. Excluded: pregnancy, allergy, or recent antibiotic therapy 119 participants: 58 in group 1 (mean age 31 years), and 61 in group 2 (mean age 28 years) |
|
| Interventions | Group 1: Gentamycin 5 mg/kg IV plus Clindamycin 900 mg IV, 8 hourly Group 2: Aztreonam 2 g IV, 8 hourly In both groups, antibiotics were continued for 24 hours if there was no evidence of hollow viscus injury, and for 5 days if evidence of hollow viscus injury was present. |
|
| Outcomes | Antibiotic failure in the form of wound, intra‐abdominal, or necrotising fascitis infections. | |
| Notes | Partially funded by the Grant Bristol Myers Squibb Company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated sheet" |
| Allocation concealment (selection bias) | Low risk | pharmacy‐controlled randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | dropouts equally distributed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Unclear risk | industry funded, but role of sponsor unclear |
Griswold 1993.
| Methods | Parallel, randomised study, undertaken in a secondary care hospital in the USA | |
| Participants | Participants > 15 years old, with penetrating abdominal trauma requiring a laparotomy. Participants were excluded if they had allergies to protocol antibiotics, were pregnant, or had a bleeding disorder. 102 participants in total: 31 in group 1, 36 in group 2, and 35 in group 3. Demographics of study participants were not apparent. |
|
| Interventions | Group 1: Cefoxitin 1 g IV, 6 hourly Group 2: Ceftriaxone 1 g IV, 12 hourly Group 3: Mezlocillin 4 g IV, 12 hourly All participants continued antibiotics for 1 day |
|
| Outcomes | Development of infection:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer randomisation |
| Allocation concealment (selection bias) | Low risk | pharmacy allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | likely blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Hesseltine 1986.
| Methods | Parallel, randomised study undertaken in secondary care setting in the USA | |
| Participants | Participants aged 18 to 65 years, with penetrating abdominal trauma and symptoms suggestive of enteric injury. Excluded: physiologically shocked patients, and those with chest injuries Study included 75 participants; 34 in group 1 (mean age 25.4 years), and 41 in group 2 (mean age 26.9 years) |
|
| Interventions | Group 1: Cefoxitin 2 g IV, 6 hourly Group 2: Clindamycin 600 mg IV, 6 hourly plus Gentamycin 1.5 mg/kg IV, 8 hourly |
|
| Outcomes | Cure, defined as recovery without signs of infection Failure, defined by intra‐abdominal, wound, or sepsis of unknown origin, or readmission due to infection within 6 months |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer generated list |
| Allocation concealment (selection bias) | Low risk | pharmacy allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | low |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | higher number of colonic injuries in one group |
Hofstetter 1984.
| Methods | Parallel, randomised trial undertaken in secondary care hospital in the USA | |
| Participants | All participants undergoing laparotomy for penetrating abdominal trauma. 119 participants included: 69 in group 1 (mean age 33 years), and 50 in group 2 (mean age 32 years) |
|
| Interventions | Group 1: Cefoxitin 2 g IV, 6 hourly for 1 day Group 2: Gentamycin or Tobramycin 80 mg IV, 8 hourly plus Clindamycin 600 mg IV, 6 hourly, plus Ampicillin 2 g IV, 8 hourly Both groups had antibiotics continued for 1 day |
|
| Outcomes | Development of infection:
|
|
| Notes | no funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "divided by date of admission" |
| Allocation concealment (selection bias) | High risk | allocated according to date of admission; thus, allocation concealment not possible |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | no mention of blinding of outcome assessors, participants given different dosing regimes, thus, not possible to blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants analysed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Jones 1985.
| Methods | Parallel, randomised trial undertaken in a secondary care hospital in USA | |
| Participants | Study included participants with penetrating abdominal trauma, who had an organ injury, without evidence of delayed presentation, significant systemic disease, or injury requiring non‐protocol antibiotics. 257 participants in total: 85 in group 1 (mean age 31.9 years), 78 in group 2 (mean age 30 years), and 94 in group 3 (mean age 32.1 years) |
|
| Interventions | Group 1: Clindamycin 600 mg IV plus Tobramycin 3 mg/kg to 5 mg/kg IV, 6 hourly Group 2: Cefamandole 1 g IV, 4 hourly Group 3: Cefoxitin 1 g IV, 4 hourly All antibiotics continued for 2 days |
|
| Outcomes | Infection:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "blinded, numbered card generated by computer resources centre" |
| Allocation concealment (selection bias) | Unclear risk | envelopes, but unclear of safeguards |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different regimens |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | dropouts even between groups |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Kirton 2000.
| Methods | Parallell, randomised control multi‐centred study undertaken in a secondary care setting in the USA | |
| Participants | All participants 18 years to 65 years of age, who sustained a colon or hollow viscus injury following penetrating abdominal. Patients excluded if they had an allergy to protocol antibiotics, received or required non‐protocol antibiotics within 72 hours of operation. 317 participants in total; 159 in group 1, and 158 in group 2. Mean age in both groups was 31 years. |
|
| Interventions | All participants received 24 hours of unblinded Ampicillin/Sulbactam, then were randomised into one of two groups. Group 1: Ampicillin/Sulbactam 3 g IV, 6 hourly for 4 days Group 2: Placebo saline IV, 6 hourly for 4 days |
|
| Outcomes | Development of infection:
|
|
| Notes | Partially funded by Pfizer Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | pharmacy‐controlled randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | different numbers of hollow viscus injuries in each group; industry funding, but unclear of sponsor role in conduct of trial |
Kreis 1986.
| Methods | Parallel, randomised study undertaken in a secondary care hospital in the USA | |
| Participants | Participants > 16 years old, with penetrating abdominal trauma, undergoing laparotomy 42 participants were included: 20 in group 1, mean age 31 years; 22 in group 2, mean age 35.4 years |
|
| Interventions | Group 1: Moxalactam 2 g IV, 12 hourly Group 2: Gentamycin 80 mg IV, 8 hourly plus Clindamycin 600 mg IV, 6 hourly In both groups, participants without hollow viscus injury received a minimum of 3 days, and those with a hollow viscus injury received 5 days. |
|
| Outcomes | Definitive outcomes unclear | |
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Unclear risk | industry funding, but unclear of sponsor role in conduct of trial |
Lou 1988.
| Methods | Parallell, randomised study undertaken in a secondary care hospital in the USA | |
| Participants | Participants with penetrating abdominal trauma, attending the emergency department; excluded if history of allergy, recent antibiotic therapy of significant renal, cardiovascular, or hepatic disease. 147 participants evaluated: 74 in group 1, mean age 29 years; and 73 in group 2, mean age 30 years |
|
| Interventions | Group 1: Mezlocillin 4 g IV, 6 hourly Group 2: Gentamycin 80 mg IV, 8 hourly plus Clindamycin 600 mg IV, 8 hourly Antibiotics continued for a minimum of 48 hours in participants without hollow viscus injury, and for 5 days minimum in those with hollow viscus injury. Total duration was at discretion of investigator. |
|
| Outcomes | Development of postoperative complication:
|
|
| Notes | Partially funded by Miles Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | pharmacy‐based randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | not specified from which groups dropouts occurred |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Unclear risk | industry funding, but unclear of sponsor role in conduct of trial |
Moore 1983.
| Methods | Parallel, randomised study undertaken in secondary care hospital in the USA | |
| Participants | Participants with penetrating abdominal trauma undergoing laparotomy. 86 participants included: 30 in group 1, mean age 30.8 years; 26 in group 2, mean age 27.9 years; 30 in group 3, mean age 31.4 years |
|
| Interventions | Group 1: Ampicillin 2 g IV, 6 hourly plus Amikacin 7.5 mg/kg IV loading, followed by 5 mg/kg total IV plus Clindamycin 600 mg IV, 6 hourly Group 2: Penicillin 1,000,000 units IV, 4 hourly plus Doxycycline 200 mg IV loading and Doxycycline 100 mg IV, 12 hourly Antibiotics in both groups continued for 5 days in participants with colonic injury, 2 days for other hollow viscus injury, and 1 day for all others. |
|
| Outcomes | Major septic complications:
Minor infections:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no menton |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unclear which groups had exclusions |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Moore 1989.
| Methods | Parallel, randomised study undertaken in secondary care hospital in the USA | |
| Participants | Participants with penetrating abdominal trauma 278 participants included in analysis: 136 in group 1 (mean age 31 years) and 142 in group 2 (mean age 30 years) |
|
| Interventions | Group 1: Mezlocillin 4 g IV, 6 hourly Group 2: Gentamycin 1.5 mg/kg IV plus Clindamycin 600 mg IV, 6 hourly Antibiotics in both groups were continued for 5 days in those with colon injury, and for 2 days in those with other hollow viscus injuries |
|
| Outcomes | Septic complications:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens, no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all who met inclusion criteria were analysed |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Nelson 1986.
| Methods | Parallel, randomised study undertaken at a secondary care hospital in the USA | |
| Participants | Participants under going laparotomy for penetrating abdominal trauma Total included 163: 78 in group 1, 85 in group 2 Demographics were not stated |
|
| Interventions | Group 1: Moxalactam 2 g IV, 8 hourly Group 2: Clindamycin 800 mg IV, 8 hourly plus Tobramycin 1 mg/kg IV, 8 hourly Antibiotics in both groups continued for 5 days in presence of hollow viscus injury, and for 3 days in those without evidence of hollow viscus injury |
|
| Outcomes | intra‐abdominal infection | |
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens and no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | unclear which groups had dropouts; participants were excluded if they died before they completed the course of antibiotics (up to 5 days) |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | similar groups |
Nichols 1993.
| Methods | Parallell, randomised study undertaken in a secondary care hospital in the USA | |
| Participants | Participants > 18 years of age, with penetrating abdominal trauma requiring a laparotomy Exclusions included allergy to protocol antibiotics, recent antibiotic therapy of infections/injuries, or requiring non‐protocol antibiotics. 170 included in analysis: 83 in group 1, and 87 in group 2; no age demographics reported |
|
| Interventions | Group 1: Cefotetan 2 g IV, 12 hourly Group 2: Cefoxitin 2 g IV, 6 hourly In both groups, antibiotics were continued for 2 or 5 days, depending on the probability of an infection, calculated by the operating surgeon at the time of fascial closure |
|
| Outcomes | Major infections:
Minor infections:
|
|
| Notes | Partially funded by grant from ICI Americas Inc | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | equal dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | unequal groups; differences in gender, blood product usage, and colonic injuries |
Okamoto 1993.
| Methods | Parallell, randomised study undertaken in secondary care hospital in the USA | |
| Participants | Participants aged 17 years to 56 years, with intestinal perforation secondary to penetrating abdominal trauma. Patients excluded if pregnant, allergic to protocol antibiotics, or had recent antibiotic therapy. 67 participants analysed: 36 in group 1, mean age 26.8 years; 31 in group 2, mean age 26.8 years |
|
| Interventions | Group 1: Cefmetazole 2 g IV, 6 hourly Group 2: Cefoxitin 2 g IV, 6 hourly Antibiotics in both groups continued until participants were afebrile (a minimum of 3 days) |
|
| Outcomes | Clinical cure: recovery without intra‐abdominal or wound infection, and were afebrile for 48 hours Clinical failure: continued fever and chills, subfascial collection, wound cellulitis, or start of non‐protocol antibiotics |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no mention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | exclusions based on exclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Unclear risk | industry funding, but unclear of sponsor role in conduct of trial; similar groups |
Oreskovich 1982.
| Methods | Parallel, randomised study undertaken in a secondary care setting in the USA | |
| Participants | 82 participants with penetrating abdominal trauma with suspected visceral injury: 42 in group 1, mean age 29 years; 40 in group 2, mean age 33 years | |
| Interventions | Group 1: Penicillin G 1 million units IV, 4 hourly for 1 day Group 2: Penicillin G 1 million units IV, 4 hourly plus Doxycycline 300 mg IV, once daily; both continued for 5 days |
|
| Outcomes | Development of infection:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | random number table |
| Allocation concealment (selection bias) | Low risk | pharmacy‐based randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | one participant excluded due to death from sepsis from a missed duodenal injury, which was not a prespecified exclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | unequal groups: more major shock in one group |
Posner 1987.
| Methods | Parallell, randomised study, undertaken in secondary care hospital in the USA | |
| Participants | 130 participants included in analysis, all undergoing laparotomy following penetrating abdominal trauma: 61 in group 1, mean age 32.7 years; 69 in group 2, mean age 32.2 years | |
| Interventions | Group 1: Mezlocilllin 4 g IV, 6 hourly Group 2: Clindamycin 600 mg IV, 6 hourly plus Gentamycin 1.5 mg/kg IV, 8 hourly Antibioitics continued for 5 days in those with colonic injury, and for 3 days in those with hollow viscus injuries. |
|
| Outcomes | Postoperative infections:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | as above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | low number of dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | more gunshot wounds in Clindamycin group |
Rowlands 1984.
| Methods | 2 separate, parallel, randomised studies, undertaken in a trauma centre in the USA | |
| Participants | Participants > 18 years old, with penetrating abdominal trauma requiring a laparotomy. Participants excluded if they received non‐protocol antibiotics. Separated into two individual RCTs. Study 1 had 151 participants in three groups: 51 in group 1; 54 in group 2; 46 in group 3 Study 2 had 92 participants: 47 in group 1; 45 in group 2 Demographics not clearly stipulated |
|
| Interventions |
Study 1 Group 1: Cefamandole 2 g IV, 4 hourly Group 2: Cefoxitin 2 g IV, 4 hourly Group 3: Clindamycin 600 mg IV, 6 hourly plus Tobramycin 1.5 mg/kg IV, 6 hourly Antibiotics in all groups continued for 3 days Study 2 Group 1: Moxlactam 2 g IV, 4 hourly Group 2: Clindamycin 600 mg IV plus Tobramycin 1.5 mg/kg IV, 8 hourly Antibiotics in both groups continued for 5 days. |
|
| Outcomes | Infection:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens, no placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients were followed by one investigator who was unaware of the allocation of antibiotic regimes" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | large number of protocol violations noted |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | significant differences in baseline injury severity scores |
Sims 1997.
| Methods | Parallel, randomised study undertaken in secondary care hospital in the USA | |
| Participants | Participants > 14 years of age, with penetrating abdominal trauma. Excluded those with allergies to protocol antibiotics, were pregnant, or had a history of recent antibiotic use 291 participants included in analysis: 101 in group 1, mean age 28.5 years; 95 in group 2, mean age 28.5 years; 95 in group 3, mean age 27 years |
|
| Interventions | Group 1: Cefoperazone 2 g IV, 12 hourly Group 2: Metronidazole 500 mg IV, 6 hourly plus Ceftriaxone 2 g IV, 12 hourly Group 3: Metronidazole 500 mg IV, 6 hourly plus Gentmycin 120 mg IV, 6 hourly plus Ampicillin 1g IV, 6 hourly In all groups, antibiotics were discontinued postoperatively if there was no evidence of hollow viscus injury, and continued for 5 days if injury was confirmed. |
|
| Outcomes | Cure Failure Recurrence |
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | Unclear risk | no mention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | different dosing regimens |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "examiner blinded study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | no dropouts |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | High risk | large imbalance in colonic injuries and negative laparotomies |
Tyburski 1998.
| Methods | Parallell, randomised study undertaken in a secondary care setting in the USA | |
| Participants | Participants > 18 years of age, with penetrating abdominal trauma requiring laparotomy. Excluded those with allergies to protocol antibiotics, those who were pregnant, or those who showed evidence of viscus injury on surgery. Total participants: 68: 35 in group 1, mean age 29 years; 33 in group 2, mean age 31 years |
|
| Interventions | Group 1: Ciprofloxacin 400 mg IV, 12 hourly plus Metronidazole 500 mg IV, 6 hourly Group 2: Gentamycin 2.5 mg/kg IV, 12 hourly plus Metronidazole 500 mg IV, 6 hourly In both groups, antibiotics given for 96 hours to those with colonic injury, and for 24 hours to those with hollow viscus injuries. |
|
| Outcomes | Development of infection:
|
|
| Notes | No funding source reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "previously prepared randomisation schedule", but no details provided on how this was produced |
| Allocation concealment (selection bias) | Low risk | pharmacy controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | low number of dropouts; unlikely to bias results |
| Selective reporting (reporting bias) | Unclear risk | no protocol or trial registration available |
| Other bias | Low risk | groups similar in demographics and clinical characteristics; no other sources of bias identified |
intravenous ‐ IV; intention‐to‐treat ‐ ITT
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Felliciano 1986 | Cluster‐randomised trial. |
| Gentry 1984 | Cluster‐randomised trial. |
| O'Donnell 1978 | This was not a randomised controlled trial, but a comparison between two separate case series, before and after the introduction of a protocol governing the use of prophylactic antibiotics. |
| Schmidt‐Matthiesen 1999 | This paper was not specifically investigating the management of penetrating abdominal trauma, rather it dealt with penetrating trauma to any part of the body, including limb trauma. Outcome data for abdominal trauma were not displayed separately from other trauma, which in any case, only comprised a small number of the cases included. |
| Thadepalli 1972 | Reports the results of microbiology cultures and did not measure our primary outcome of surgical site infection. |
| Thadepalli 1973 | Reports the results of microbiology cultures and did not measure our primary outcome of surgical site infection. Appears to be a duplicate publication of the same study reported in Thadepalli 1972. |
| Tornqvist 1985 | Did not include participants with penetrating abdominal trauma. Instead, this study included participants with primary infectious abdominal surgical disease, such as perforated peptic ulcer, appendicitis, and perforated colon cancer. |
Differences between protocol and review
We have now excluded cluster‐RCTs due to the inherent bias from different surgeons, hospitals, and surgical teams. We also chose to exclude studies which did not measure any of our primary or secondary outcomes. Due to the vast clinical heterogeneity of antibiotic regimens used throughout the studies, we decided to group the antibiotics by their antimicrobial coverage according to The Mayo Clinic Antimicrobial Therapy guide in order to permit useful data synthesis of the effect of antibiotic classes.
Contributions of authors
ST, AB, JNL, and RLN wrote the protocol for the review. ST and AB undertook the screening of abstracts and assessment of full texts for eligibility. HBC and JB extracted the study data, and PH and BD assessed risk of bias. BD, HBC, and PJH performed the statistical analysis. PJH and HBC completed the GRADE assessment. PJH, HBC, and BD prepared the manuscript, which all authors critically appraised.
Sources of support
Internal sources
-
University of Nottingham, UK.
Use of the IT facilities and the library
-
Royal Derby Hospital, UK.
Use of the hospital library
External sources
No sources of support supplied
Declarations of interest
None of the authors have conflicts of interest in relation to this review.
PJH: PJH was the recipient of a two year research training fellowship jointly awarded by the Royal College of Surgeons of England and the Dunhill Medical Trust from 2016‐2018.
HBC: none known
BD: Received grants from National Institute of Academic Anaesthesia for studies not related to this Cochrane Review. Previously won Drager oral presentation prize which included payment from the AAGBI in a presentation competition.
JB: none known
JPW: none known
AB: none known
RLN: Invited speaker with travel and accomodation paid at United European Gastroenterology Week, Cochrane symposium (10/2011).
ST: none known
JNL: none known
We did not seek funding for this review.
New
References
References to studies included in this review
Bivins 1989 {published data only}
- Bivins BA, Crots L, Obeid FN, Sorensen VJ, Horst HM, Fath JJ. Antibiotics for penetrating abdominal trauma: a prospective comparative trial of single agent cephalosporin therapy versus combination therapy. Diagnostic Microbiology and Infectious Disease 1989;12(1):113‐8. [DOI] [PubMed] [Google Scholar]
- Bivins BA, Crots L, Sorensen VJ, Obeid FN, Horst HM. Preventative antibiotics for penetrating abdominal trauma ‐ single agent or combination therapy. Drugs 1988;35(Supplement 2):100‐2. [DOI] [PubMed] [Google Scholar]
Bozorgzadeh 1999 {published data only}
- Bozorgzadeh A, Pizzi WF, Barie PS, Khaneja SC, LaMaute HR, Mandava N, et al. The duration of antibiotic administration in penetrating abdominal trauma. American Journal of Surgery 1999;177(2):125‐31. [DOI] [PubMed] [Google Scholar]
Cornwell 1999 {published data only}
- Cornwell EE 3rd, Dougherty WR, Berne TV, Velmahos G, Murray JA, Chahwan S, et al. Duration of antibiotic prophylaxis in high‐risk patients with penetrating abdominal trauma: a prospective randomized trial. Journal of Gastrointestinal Surgery 1999;3(6):648‐53. [DOI] [PubMed] [Google Scholar]
Crenshaw 1983 {published data only}
- Crenshaw C, Glanges E, Webber C, McReynolds DB. A prospective random study of a single agent versus combination antibiotics as therapy in penetrating injuries of the abdomen. Surgery, Gynecology & Obstetrics 1983;156(3):289‐94. [PubMed] [Google Scholar]
Crots 1985 {published data only}
- Crots LD, Obeid FN, Horst HM, Bivins BA. Twice‐daily moxalactam versus gentamicin and clindamycin in patients with penetrating abdominal trauma. Clinical Pharmacy 1985;4(3):316‐20. [PubMed] [Google Scholar]
Dellinger 1986 {published data only}
- Dellinger EP, Wertz MJ, Lennard ES, Oreskovich MR. Efficacy of short‐course antibiotic prophylaxis after penetrating intestinal injury. A prospective randomized trial. Archives of Surgery 1986;121(1):23‐30. [DOI] [PubMed] [Google Scholar]
Demetriades 1991 {published data only}
- Demetriades D, Lakhoo M, Pezikis A, Charalambides D, Pantanowitz D, Sofianos C. Short‐course antibiotic prophylaxis in penetrating abdominal injuries: ceftriaxone versus cefoxitin. Injury 1991;22(1):20‐4. [DOI] [PubMed] [Google Scholar]
Ericsson 1989 {published data only}
- Ericsson CD, Fischer RP, Rowlands BJ, Hunt C, Miller‐Crotchett P, Reed L 2nd. Prophylactic antibiotics in trauma: the hazards of underdosing. The Journal of Trauma 1989;29(10):1356‐61. [PubMed] [Google Scholar]
Fabian 1982 {published data only}
- Fabian TC, Hoefling SJ, Strom PR, Stone HH. Use of antibiotic prophylaxis in penetrating abdominal trauma. Clinical Therapeutics 1982;5(Supplement A):38‐47. [PubMed] [Google Scholar]
Fabian 1985 {published data only}
- Fabian TC, Boldreghini SJ. Antibiotics in penetrating abdominal trauma. Comparison of ticarcillin plus clavulanic acid with gentamicin plus clindamycin. American Journal of Medicine 1985;79(Supplement 5B):157‐60. [DOI] [PubMed] [Google Scholar]
Fabian 1992 {published data only}
- Fabian TC, Croce MA, Payne LW, Minard G, Pritchard FE, Kudsk KA. Duration of antibiotic therapy for penetrating abdominal trauma: a prospective trial. Surgery 1992;112(4):788‐94. [PubMed] [Google Scholar]
Fabian 1994 {published data only}
- Fabian TC, Hess MM, Croce MA, Wilson RS, Wilson SE, Charland SL, et al. Superiority of aztreonam/clindamycin compared with gentamicin/clindamycin in patients with penetrating abdominal trauma. American Journal of Surgery 1994;167(3):291‐6. [DOI] [PubMed] [Google Scholar]
Griswold 1993 {published data only}
- Griswold JA, Muakkassa FF, Betcher E, Poole GV. Injury severity dictates individualized antibiotic therapy in penetrating abdominal trauma. The American Surgeon 1993;59(1):34‐9. [PubMed] [Google Scholar]
Hesseltine 1986 {published data only}
- Heseltine PN, Berne TV, Yellin AE, Gill MA, Appleman MD. The efficacy of cefoxitin vs. clindamycin/gentamicin in surgically treated stab wounds of the bowel. The Journal of Trauma 1986;26(3):241‐5. [DOI] [PubMed] [Google Scholar]
Hofstetter 1984 {published data only}
- Hofstetter SR, Pachter HL, Bailey AA, Coppa GF. A prospective comparison of two regimens of prophylactic antibiotics in abdominal trauma: cefoxitin versus triple drug. Journal of Trauma 1984;24(4):307‐10. [DOI] [PubMed] [Google Scholar]
Jones 1985 {published data only}
- Jones RC, Thal ER, Johnson NA, Gollihar LN. Evaluation of antibiotic therapy following penetrating abdominal trauma. Annals of Surgery 1985;201(5):576‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirton 2000 {published data only}
- Kirton OC, O'Neill PA, Kestner M, Tortella BJ. Perioperative antibiotic use in high‐risk penetrating hollow viscus injury: a prospective randomized, double‐blind, placebo‐control trial of 24 hours versus 5 days. Journal of Trauma 2000;49(5):822‐32. [DOI] [PubMed] [Google Scholar]
Kreis 1986 {published data only}
- Kreis D, Augenstein D, Martinez O, Echenique M, Plasencia G, Vopal JJ, et al. A prospective randomized study of moxalactam versus gentamicin and clindamycin in penetrating abdominal trauma. Surgery, Gynecology & Obstetrics 1986;163(1):1‐4. [PubMed] [Google Scholar]
Lou 1988 {published data only}
- Lou MA, Thadepalli H, Mandal AK. Safety and efficacy of mezlocillin: a single‐drug therapy for penetrating abdominal trauma. Journal of Trauma 1988;28(11):1541‐7. [DOI] [PubMed] [Google Scholar]
Moore 1983 {published data only}
- Moore FA, Moore EE, Mill MR. Preoperative antibiotics for abdominal gunshot wounds. A prospective, randomized study. American Journal of Surgery 1983;146(6):762‐5. [DOI] [PubMed] [Google Scholar]
Moore 1989 {published data only}
- Moore FA, Moore EE, Ammons LA, McCroskey BL. Presumptive antibiotics for penetrating abdominal wounds. Surgery, Gynecology & Obstetrics 1989;169(2):99‐103. [PubMed] [Google Scholar]
Nelson 1986 {published data only}
- Nelson RM, Benitez PR, Newell MA, Wilson RF. Single‐antibiotic use for penetrating abdominal trauma. Archives of Surgery 1986;121(2):153‐6. [DOI] [PubMed] [Google Scholar]
Nichols 1993 {published data only}
- Nichols RL, Smith JW, Robertson GD, Muzik AC, Pearce P, Ozmen V, et al. Prospective alterations in therapy for penetrating abdominal trauma. Archives of Surgery 1993;128(1):55‐64. [DOI] [PubMed] [Google Scholar]
Okamoto 1993 {published data only}
- Okamoto MP, Gill MA, Nakahiro RK, Chin A, Yellin AE, Berne TV, et al. Cost analysis of cefmetazole versus cefoxitin in the treatment of penetrating abdominal trauma. Current Therapeutic Research 1993;53(2):159‐66. [Google Scholar]
Oreskovich 1982 {published data only}
- Oreskovich MR, Dellinger EP, Lennard ES, Wertz M, Carrico CJ, Minshew BH. Duration of preventive antibiotic administration for penetrating abdominal trauma. Archives of Surgery 1982;117(2):200‐5. [DOI] [PubMed] [Google Scholar]
Posner 1987 {published data only}
- Posner MC, Moore EE, Harris LA, Allo MD. Presumptive antibiotics for penetrating abdominal wounds. Surgery, Gynecology & Obstetrics 1987;165(1):29‐32. [PubMed] [Google Scholar]
Rowlands 1984 {published data only}
- Rowlands BJ, Ericsson CD. Comparative studies of antibiotic therapy after penetrating abdominal trauma. The American Journal of Surgery 1984;148(7):791‐5. [DOI] [PubMed] [Google Scholar]
Sims 1997 {published data only}
- Sims EH, Thadepalli H, Ganesan K, Mandal AK. How many antibiotics are necessary to treat abdominal trauma victims?. The American Surgeon 1997;63(6):525‐35. [PubMed] [Google Scholar]
Tyburski 1998 {published data only}
- Tyburski JG, Wilson RF, Warsow KM, McCreadie S. A trial of ciprofloxacin and metronidazole vs gentamicin and metronidazole for penetrating abdominal trauma. Archives of Surgery 1998;133(12):1289‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Felliciano 1986 {published data only}
- Feliciano DV, Gentry LO, Bitondo CG, Burch JM, Mattox KL, Cruse PA, et al. Single agent cephalosporin prophylaxis for penetrating abdominal trauma. Results and comment on the emergence of the enterococcus. American Journal of Surgery 1986;152(6):674‐81. [DOI] [PubMed] [Google Scholar]
Gentry 1984 {published data only}
- Gentry LO, Feliciano DV, Lea AS, Short HD, Mattox KL, Jordan G. Perioperative antibiotic therapy for penetrating injuries of the abdomen. Annals of Surgery 1984;200(5):561‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 1978 {published data only}
- O'Donnell VA, Lou S, Alexander JL, Mandal AK. Role of antibiotics in penetrating abdominal trauma. The American Surgeon 1978;44(9):574‐7. [PubMed] [Google Scholar]
Schmidt‐Matthiesen 1999 {published data only}
- Schmidt‐Matthiesen A, Roding H, Windolf J, Sommerfeldt D, Gutt C, Pannike A, et al. A prospective, randomised comparison of single‐ vs. multiple‐dose antibiotic prophylaxis in penetrating trauma. Chemotherapy 1999;45(5):380‐91. [DOI] [PubMed] [Google Scholar]
Thadepalli 1972 {published data only}
- Thadepalli, H, Gorbach SL, Broido P, Norsen J. A prospective study of infections in penetrating abdominal trauma. The American Journal of Clinical Nutrition 1972;25(12):1405‐8. [DOI] [PubMed] [Google Scholar]
Thadepalli 1973 {published data only}
- Thadepalli H, Gorbach SL, Broido PW, Norsen J, Nyhus L. Abdominal trauma, anaerobes, and antibiotics. Surgery, Gynecology & Obstetrics 1973;137(2):270‐6. [PubMed] [Google Scholar]
Tornqvist 1985 {published data only}
- Tornqvist A, Forsgren A, Leandoer L, Ursing J. Antibiotic treatment during surgery for diffuse peritonitis: a prospective randomized study comparing the effects of cefuroxime and of a cefuroxime and metronidazole combination. British Journal of Surgery 1985;72(4):261‐4. [DOI] [PubMed] [Google Scholar]
Additional references
Biscione 2009
- Biscione FM. Rates of surgical site infection as a performance measure: are we ready?. World Journal of Gastrointestinal Surgery 2009;30:11‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brand 2013
- Brand M, Grieve A. Prophylactic antibiotics for penetrating abdominal trauma. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD007370.pub3] [DOI] [PubMed] [Google Scholar]
Brand 2019
- Brand M, Grieve A. Prophylactic antibiotics for penetrating abdominal trauma. Cochrane Database of Systematic Reviews 2019, Issue 12. [DOI: 10.1002/14651858.CD007370.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 1961
- Burke JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery 1961;50:161‐8. [PubMed] [Google Scholar]
Butt 2009
- Butt MU, Zacharias N, Velmahos GC. Penetrating abdominal injuries: management controversies. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2009;17:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doleman 2019
- Doleman B, Williams JP, Lund J. Why most published meta‐analysis findings are false. Techniques in Coloproctology 2019;23:925‐8. [DOI] [PubMed] [Google Scholar]
Fullen 1972
- Fullen WD, Hunt J, Altemeier WA. Prophylactic antibiotics in penetrating wounds of the abdomen. Journal of Trauma 1972;12:282‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Versions 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICMJE 2019
- International Committee of Medical Journal Editors. Clinical Trials Registration. www.icmje.org/about‐icmje/faqs/clinical‐trials‐registration Accessed 22 November 2019.
Imberger 2013
- Imberger G, Wetterslev J, Gluud C. Trial sequential analysis has the potential to improve the reliability of conclusions in meta‐analysis. Contemporary Clinical Trials 2013;36:254‐5. [DOI] [PubMed] [Google Scholar]
Mayo Clinic 2012
- Wilson JW, Estes LL. The Mayo Clinic Antimicrobial Therapy Second Edition. Mayo Clinic Scientific Press, Oxford University Press 2012.
Miles 1957
- Miles AA, Miles EM, Burke J. The value and duration of defence reactions of the skin to the primary lodgement of bacteria. British Journal of Experimental Pathology 1957;38:79‐96. [PMC free article] [PubMed] [Google Scholar]
Nelson 2014
- Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD001181.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2004
- Osborn TM, Tracy K, Dunne JR, Pasquale M, Napolitano LM. Epidemiology of sepsis in patients with traumatic injury. Critical Care Medicine 2004;32:2234‐40. [DOI] [PubMed] [Google Scholar]
Pollock 1987
- Pollock A. Surgical Infections. London: Edward Arnold, 1987. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2015
- Roberts I, Ker K, Edwards P, Beecher D, Manno D, Sydenham E. The knowledge system underpinning healthcare is not fit for purpose and must change. BMJ 2015;350:h2463. [DOI: 10.1136/bmj.h2463] [DOI] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. CCCG Version 3.0. Available at cccrg.cochrane.org/author‐resources December 2016.
Thorland 2018 [Computer program]
- Thorlund K, Engstrom J, Wetterslev J, Brok J, Imberger G, Gluud C. Trial sequential analysis. Copenhagen Trial Unit, 2018.
Tou 2013
- Tou S, Bhalla A, Lund J, Nelson RL. Prophylactic antibiotics for penetrating abdominal trauma: duration and antibiotic choice. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010808] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 1916
- Wallace C. A study of 1200 cases of gunshot wounds of the abdomen. British Medical Journal 1916;4:679‐743. [Google Scholar]


