Abstract
Background
Endometrial cancer is one of the most common gynaecological cancers in developed countries. Treatment of advanced endometrial cancer usually involves radiotherapy, chemotherapy, endocrine therapy or a combination of these. However, survival outcomes are poor in advanced or metastatic disease. Better systemic treatment options are needed to improve survival and safety outcomes for these women. The PI3K/AKT/mTOR pathway is a frequently altered signalling pathway in endometrial cancer. Single‐arm studies have reported some encouraging results of the PI3K/AKT/mTOR inhibition in advanced or recurrent endometrial cancer.
Objectives
To assess the efficacy and safety of PI3K/AKT/mTOR inhibitor‐containing regimens in women with locally‐advanced, metastatic or recurrent endometrial cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE and Embase to 16 January 2019; and the World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov in July 2018. We also reviewed reference lists from included studies and endometrial cancer guidelines.
Selection criteria
We included randomised controlled trials (RCTs) comparing a regimen with a PI3K/AKT/mTOR inhibitor (either alone or in combination with other treatments, such as chemotherapy or hormonal therapy) versus a comparator regimen without a PI3K/AKT/mTOR inhibitor. There were no restrictions on which comparator(s) were included.
Data collection and analysis
We extracted data independently, and assessed risks of bias and the certainty of the evidence. The primary outcome measures were progression‐free survival and toxicity (grade 3/4 where available). We derived hazard ratios (HRs) for time‐to‐event outcomes and risk ratios (RRs) for dichotomous outcomes. Secondary outcomes included overall survival, objective tumour response rate, quality of life and treatment‐related death. We used GRADEproGDT to assess the certainty of the evidence for the most important outcomes (by first‐line and second/third‐line therapy for progression‐free survival and overall survival).
Main results
We included two RCTs involving 361 women. One study assessed the effects of the mTOR inhibitor temsirolimus, in combination with carboplatin/paclitaxel versus carboplatin/paclitaxel and bevacizumab in treatment‐naïve women with advanced or recurrent endometrial cancer. The second study compared the mTOR inhibitor ridaforolimus alone versus progestin or investigator choice of chemotherapy in women who had received prior treatment for metastatic or recurrent endometrial cancer. We identified five ongoing studies on the effects of PI3K and AKT inhibitors, metformin and dual mTOR inhibitors.
For first‐line therapy, an mTOR inhibitor‐containing regimen may worsen progression‐free survival (HR 1.43, 95% CI 1.06 to 1.93; 1 study, 231 participants; low‐certainty evidence), while for second/third‐line therapy, an mTOR inhibitor probably improves progression‐free survival compared to chemotherapy or endocrine therapy (HR 0.53, 95% CI 0.31 to 0.91; 1 study, 95 participants; moderate‐certainty evidence). Data on toxicity were available from both studies: administering an mTOR inhibitor regimen may increase the risk of grade 3/4 mucositis (RR 10.42, 95% CI 1.34 to 80.74; 2 studies, 357 participants; low‐certainty evidence), but may result in little to no difference in risk of anaemia or interstitial pneumonitis (low‐certainty evidence for both toxicities). Overall, event rates were low. For first‐line therapy, an mTOR inhibitor‐containing regimen may result in little to no difference in overall survival compared to chemotherapy (HR 1.32, 95% CI 0.98 to 1.781 study, 231 participants; low‐certainty evidence). The finding was similar for second/third‐line therapy (HR 1.06, 95% CI 0.70 to 1.61; 1 study, 130 participants; low‐certainty evidence). Administering mTOR inhibitor‐containing regimens may result in little to no difference in tumour response compared to chemotherapy or hormonal therapy in first‐line or second/third‐line therapy (first line: RR 0.93, 95% CI 0.75 to 1.17; 1 study, 231 participants; second/third line: RR 0.22, 95% CI 0.01 to 4.40; 1 study, 61 participants; low‐certainty evidence).
Neither study collected or reported quality‐of‐life data.
Authors' conclusions
Two RCTs have been reported to date, with low certainty of evidence. In a recurrent disease setting, mTOR inhibitors may result in improved progression‐free survival, but we found no clear benefit in overall survival or tumour response rate. We await the publication of at least five ongoing studies investigating the role of PI3K/AKT/mTOR inhibitors in advanced or recurrent endometrial cancer before any conclusions can be drawn on their use.
Plain language summary
Drugs targeting PI3K/AKT/mTOR pathway for locally‐advanced, metastatic or recurrent endometrial cancer
What is the aim of this review? To find out whether drugs that inhibit the PI3K/AKT/mTOR pathway (known as PI3K, AKT and mTOR inhibitors) can improve survival of women diagnosed with locally‐advanced (cancer that has spread beyond the uterus/womb), metastatic or recurrent endometrial cancer.
Key messages There is a low certainty of evidence from two clinical trials about the use of drugs targeting PI3K/AKT/mTOR pathway in women with locally‐advanced, metastatic or recurrent endometrial cancer. Based on the small number of completed studies, women who have received prior treatment for advanced or recurrent endometrial cancer and received an mTOR inhibitor may have a lower risk of their cancer progressing compared to those who received chemotherapy/hormonal therapy alone. However, in women who received mTOR inhibitor‐containing chemotherapy as part of their treatment when first diagnosed with advanced disease, mTOR inhibitor‐containing treatment may result in their disease progressing more quickly and probably with increased complications compared to chemotherapy or hormonal therapy alone. Although mTOR inhibitors may change how long it takes for their cancer to progress, there may be little or no difference in how long women lived after treatment (known as overall survival). We await the publication of at least five studies examining the role of PI3K, AKT and mTOR inhibitors in advanced or recurrent endometrial cancer.
What was studied in the review? Treatment for women with metastatic or recurrent endometrial cancer usually involves radiotherapy, chemotherapy, endocrine therapy or a combination of these to try to shrink or slow the growth of the cancer. The response of the cancer to these treatments is variable, but mostly modest. New treatments are needed to improve outcomes. The PI3K/AKT/mTOR pathway within an endometrial cancer cell is involved in the growth of endometrial cancer, and various drugs have been developed to target this pathway with the aim of reducing the growth of endometrial cancer cells. These are known as PI3K, AKT and mTOR inhibitors. We found relevant studies looking at mTOR inhibitors. mTOR inhibitors can be given alone or in combination with other cancer treatment drugs. They may be given along with chemotherapy or endocrine therapy. mTOR inhibitors act by blocking cancer cells from dividing and reproducing. Their adverse events can include ulcers along the digestive tract (known as mucositis), inflammation of lung tissues (known as pneumonitis) and low red blood cell counts (anaemia).
We include two studies that randomised 361 women. In one study, women received either an mTOR inhibitor (temsirolimus) in combination with other chemotherapy drugs, or the same chemotherapy drugs without the mTOR inhibitor and with a different targeted therapy (bevacizumab). This was given as part of their 'first‐line' treatment after their initial diagnosis of advanced endometrial cancer. In the second study, women with recurrent disease or who had been treated with chemotherapy at least once before received an mTOR inhibitor (ridaforolimus) on its own, compared with a chemotherapy or hormonal therapy and no mTOR inhibitor.
What are the main results of the review? For women who received mTOR inhibitor drugs as part of their first treatment, there may be a higher risk of disease worsening with an mTOR inhibitor than with conventional treatment and bevacizumab. However, for women with recurrent disease who had received chemotherapy previously, receiving an mTOR inhibitor drug may reduce the risk of the disease worsening compared to further chemotherapy or hormonal treatments. These results are based on one study only in each treatment setting.
There were side effects from mTOR inhibitors. Women may be more likely to experience ulcers within the digestive tract with mTOR inhibitors than women who received treatments without these drugs. There was probably little or no difference in the rates of inflammation of the lungs or anaemia between those who received mTOR inhibitors and those who did not, although we have only low certainty evidence about the result. None of the studies reported quality‐of‐life information.
There are five clinical trials currently recruiting women. We hope to have a clearer answer in the next update of this review, once data from these studies are available.
How up‐to‐date is this review? We searched for studies that had been published up to January 2019.
Summary of findings
Summary of findings for the main comparison. mTOR inhibitors compared to chemotherapy/hormone therapy for advanced or recurrent endometrial cancer.
| mTOR inhibitors compared to chemotherapy/hormone therapy for advanced or recurrent endometrial cancer | |||||
| Participant or population: women with advanced or recurrent endometrial cancer Setting: hospital Intervention: mTOR inhibitor with or without chemotherapy Comparison: chemotherapy or hormone therapy | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with chemotherapy/hormone therapy | Risk with mTOR | ||||
| Progression‐free survival: first‐line trials
(follow‐up range: 4 to 25 months) (*the baseline risk in the control arm was estimated at 12 months) |
Moderate risk of disease progression | HR 1.43 (1.06 to 1.93) | 231 (1 RCT) | ⊕⊝⊝⊝ LOWa,b | |
| 320 per 1000* | 424 per 1000 (336 to 525) | ||||
| Progression‐free survival: second/third‐line trials (follow‐up range: 1 to 14 months) (*the baseline risk in the control arm was estimated at 12 months) | High risk of disease progression | HR 0.53 (0.31 to 0.91) | 95 (1 RCT) | ⊕⊕⊕⊝ MODERATEb | |
| 970 per 1000* | 844 per 1000 (663 to 959) | ||||
| Haematological toxicities ‐ Anaemia | 173 per 1000 | 246 per 1000 (144 to 423) | RR 1.42 (0.83 to 2.44) | 357 (2 RCTs) | ⊕⊝⊝⊝ LOWc |
| Skin toxicities ‐ Mucositis | 6 per 1000# | 58 per 1000 (7 to 451) | RR 10.42 (1.34 to 80.74) | 357 (2 RCTs) | ⊕⊝⊝⊝ LOWd |
| Respiratory toxicity ‐ Interstitial pneumonitis | 11 per 1000# | 82 per 1000 (10 to 687) | RR 7.36 (0.88 to 61.52) | 357 (2 RCTs) | ⊕⊝⊝⊝ LOWe |
| Overall survival: first‐line trials
(follow‐up range: 1 to 36 months) (*the baseline risk in the control arm was estimated at 24 months) |
Moderate risk of death | HR 1.32 (0.98 to 1.78) | 231 (1 RCT) | ⊕⊝⊝⊝ LOWf | |
| 360 per 1000* | 445 per 1000 (354 to 548) | ||||
| Overall survival: second/third‐line trials
(follow‐up range: 1 to 26 months) (*the baseline risk in the control arm was estimated at 24 months) |
High risk of death | HR 1.06 (0.70 to 1.61) | 130 (1 RCT) | ⊕⊝⊝⊝ LOWf | |
| 910 per 1000* | 922 per 1000 (815 to 979) | ||||
| Objective response rate: first‐line trial | 586 per 1000 | 545 per 1000 (440 to 686) | RR 0.93 (0.75 to 1.17) | 231 (1 RCT) | ⊕⊝⊝⊝ LOWg |
| Objective response rate: second/third‐line trials | 63 per 1000 | 14 per 1000 (1 to 275) | RR 0.22 (0.01 to 4.40) | 61 (1 RCT) | ⊕⊝⊝⊝ LOWh |
| Quality of life ‐ not reported | Not reported | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard ratio; RCT: randomised controlled trial; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
#Due to zero events in the comparator arm in Aghajanian 2018 and Oza 2015, we derived an estimate of the control risk from studies that described the incidence of grade 3/4 mucositis following doxorubicin, carboplatin and paclitaxel in women with stage III/IV endometrial cancer (Fleming 2004; incidence being less than 1%) and the incidence of interstitial pneumonitis after liposomal doxorubicin (Nevadunsky 2013; incidence being less than 1%). aThe impact of unblinding on the assessment of progression‐free survival in Aghajanian 2018 was unclear. We therefore downgraded by one level for risk of bias. bThe optimal information size was not met (as per GRADE guidance; Guyatt 2011).We therefore downgraded by one level for imprecision. cThe confidence interval was wide, indicating no effect and appreciable harm and benefit from mTOR inhibitors, and the optimal information size was not met. We therefore downgraded by two levels for imprecision. dThe confidence interval was very wide, although the effect of mTOR inhibitors showed appreciable harm only, and the optimal information size was not met. We therefore downgraded by two levels for imprecision. eThe confidence interval was very wide, indicating no effect, and appreciable harm from mTOR inhibitors, and the optimal information size was not met. We therefore downgraded by two levels for imprecision. fThe optimal information size was not met (as per GRADE guidance; Guyatt 2011) and the confidence interval was wide. We therefore downgraded by two levels for imprecision. gThe optimal information size was not met and the study did not use an independent adjudication committee to assess tumour response rate. We therefore downgraded by one level each for imprecision and risk of bias. hThe optimal information size was not met (only two events (i.e. tumour responded to treatment)) and 75% or fewer participants were available for the ORR analysis. We therefore downgraded by one level each for imprecision and risk of bias (attrition bias).
Background
Description of the condition
Endometrial cancer (EC) arises from cells lining the inside of the womb. EC is the fourth most common cancer in women (Ferlay 2015). Worldwide, the incidence is increasing, with 320,000 new cases in 2012 (Ferlay 2015) compared to 287,000 new cases in 2008 (Ferlay 2010). The condition is consistently associated with obesity (Schmandt 2011), and it is more common in developed countries where the age standardised rate is 14.7/100,000 compared to 5.5/100,000 in less developed regions (Ferlay 2015).
ECs can be classified as type 1 or type 2, according to clinical and molecular pathology criteria. Type 1 ECs account for 80% to 90% of all ECs diagnoses, are generally oestrogen‐dependent, of endometrioid histology and have a better prognosis. Type 2 EC are non‐oestrogen‐dependent and generally have more aggressive histological types, such as clear cell or serous, which are associated with a poorer prognosis (Hecht 2006). Seventy to ninety per cent of women with type 1 EC are obese and have obesity‐related health issues, such as diabetes, hypertension and cardiac disease (Fader 2009; Von Gruenigen 2005). In women with Type 1 EC the mainstay of treatment is with endocrine treatment. More recently, molecular analysis classified endometrial tumours in the Cancer Genome Atlas Research project into four groups: POLE ultramutated, MSI hypermutated, copy‐number (CN) low, and CN high (Cancer Genome Atlas Research Network 2013). These subtypes were strongly associated with progression‐free survival, with POLE tumours showing the best prognosis (Cancer Genome Atlas Research Network 2013).
Most women with EC are diagnosed at an early stage when they are likely to be cured by surgery alone. The optimal treatment after surgery for those with risk factors for recurrence continues to be debated, but women may be offered radiotherapy, chemotherapy or a combination of these depending on stage of disease and risk factors. Approximately 17% of women have regional spread of tumour at the time of diagnosis (International Federation of Gynecology and Obstetrics (FIGO) stage III) and 9% have distant metastases (FIGO stage IV), and survival outcomes for these women are poor (Jemal 2008). The five‐year survival for women presenting with metastatic EC is less than 20% (SEER 2019). Treatment of advanced disease (FIGO stage III or greater or those with recurrent disease) is individualised and usually involves radiotherapy, chemotherapy, endocrine therapy or a combination of these. The response rates to chemotherapy are generally higher in treatment regimens using combinations of drugs compared with a single drug, but the response rates remain modest (34% to 66%) (Fleming 2004; Randall 2006; Sorbe 2008). Responses to chemotherapy are usually of short duration, with typical survival of approximately 12 months (Dellinger 2009). Chemotherapy may have significant side effects and toxicity, which can be worse in women with EC who have multiple medical co‐morbidities, such as obesity, hypertension and diabetes mellitus (Nicholas 2014).
In participants with advanced EC where chemotherapy is not thought to be needed initially, especially if Type 1 EC, hormonal therapy, such as a progesterone or oestrogen‐receptor antagonist, is often considered. Responses to hormonal therapy of 15% to 30% (DeCruze 2007) have been reported, predominantly in lower‐grade, endometrioid histology, oestrogen/progesterone‐dependent EC. These responses are usually of short duration, but responses can be up to years (Markman 2005). A recent review indicated higher response rates to tamoxifen or a combination of tamoxifen and progestin compared to aromatase inhibitors, and emphasised the importance of testing for hormonal receptor status for optimal treatment selection (Van Weelden 2019). Hormonal therapy is mostly well tolerated and lacks the toxicities associated with chemotherapy. Even though a survival benefit needs to be proven (Kokka 2010), hormonal therapy is often a good option for the individualised treatment of participants.
To improve on the outcomes of women with Type 1 EC, combining treatment with endocrine treatment and potentially blockade of a pathway known as the PI3K/AKT/mTOR pathway (described below) or cyclin kinase inhibitors may be beneficial (Colon‐Otero 2019). Given the poorer prognosis of those with Type 2 EC, who have more aggressive histological subtypes, chemotherapy is more likely to be administered upfront, since the risk of recurrence is higher, even with early stage disease. To improve on outcomes of women with Type 2 EC, future treatment options include the combination of chemotherapy with inhibition of the PI3K/AKT/mTOR pathway. Treatment of recurrent disease is guided by the site of the metastases and the associated symptoms. Treatment options include systemic treatment and best supportive care. Better systemic treatment options are needed to improve survival and safety outcomes for these women.
Description of the intervention
The PI3K/AKT/mTOR pathway regulates cell survival, proliferation and growth. In many cancers, including EC, this pathway is activated. Increased activity of this pathway is often associated with tumour progression and resistance to many cancer therapies (Shaw 2006; Slomovitz 2012). Treating participants with drugs that target this pathway aims to slow cancer growth. The drugs that target this pathway are known as PI3K/AKT/mTOR inhibitors, and there are four main types, each targeting one or multiple parts of the pathway:
PI3K inhibitors, e.g. BKM120;
AKT inhibitors e.g. AZD5363, perfosine;
mTOR complex 1/2 inhibitors, e.g. everolimus, ridaforolimus, metformin, AZD8055;
dual mTOR/PI3K inhibitors, e.g. XL765.
These drugs have been tested in the laboratory and in women with endometrial cancer. They can be taken as tablets or given as intravenous infusions. They can be taken alone or in combination with chemotherapy. Current research indicates that some women with EC may respond to treatment with such drugs, but currently it is not possible to tell from a blood or tumour sample test which participants are most likely to benefit, as responses that have been seen have not clearly correlated with molecular abnormalities in the pathway in individual tumours (Mackay 2014).
Participants who are treated with PI3K/AKT/mTOR inhibitors often develop resistance to treatment and then relapse (Burris 2013). Researchers are trying to understand the molecular mechanisms of resistance to PI3K/AKT/mTOR‐targeted therapy and how to counter them. One of the most promising strategies to overcome resistance has been trialing a combination of these targeted drugs.
The typical toxicity profile of these drugs includes the following side effects:
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, diarrhoea);
genitourinary;
skin (rash, stomatitis, mucositis);
vascular disorders (venous thrombosis, pulmonary embolism);
neurological (peripheral, central);
metabolic abnormalities (hyperglycaemia, hyperlipidaemia);
respiratory (interstitial pneumonitis).
How the intervention might work
The PI3K/AKT/mTOR pathway is involved in the development of EC (Shaw 2006). The tumour suppressor PTEN gene regulates this pathway and mutations in this gene are present in up to 70% of type 1 and 35% of type 2 ECs (Slomovitz 2012). Mutations in PIK3CA lead to increased activation of the PI3K/AKT/mTOR pathway, occurring in 41% to 52% of type 1 and 33% to 38% of type 2 ECs (Slomovitz 2012). Activation of this pathway, especially if associated with loss of PTEN function, is associated with poor survival in solid tumours (Ocana 2014). The observation that these genetic alterations are so widespread in both type 1 and type 2 ECs has led to interest in inhibition of this pathway to potentially improve clinical outcomes (Slomovitz 2012).
Metformin is an anti‐diabetes medication that has been shown to slow cancer growth in women with EC (Schuler 2015). This is thought to be through inhibition of mTOR (Dowling 2007; Schuler 2015) and through reducing AKT activity through inhibition of insulin receptor substrate 1 (Zakikhani 2010; Schuler 2015). Therapeutic trials investigating the effects of metformin on women with EC are ongoing.
Why it is important to do this review
The incidence of EC is increasing and yet survival has not improved substantially over the past 30 years (SEER 2019). Although most cases are detected and treated at an early stage, a significant number of women present at an advanced stage and have a poor prognosis (Jemal 2008). Treatment outcomes in advanced or recurrent EC remain modest and are even poorer with subsequent therapies. Response rates for second‐line chemotherapy are generally less than 20% (Dellinger 2009). Combination treatments with chemotherapy have significant side effects and toxicities, which may be exacerbated in women with multiple co morbidities. Treatment with drugs that inhibit the PI3K/AKT/mTOR pathway, either alone or in combination with other treatments such as endocrine therapy, has the potential to improve outcomes for participants with a range of solid tumours, including EC, and the toxicity profile associated with these drugs is generally reasonable. There are several recently‐published phase II studies and some ongoing trials, but to date we have found no systematic reviews of the literature. This systematic review will form a preliminary basis for an assessment of the safety and efficacy of these new drugs. As data mature from clinical trials in progress, it is likely that an update of this review will be required relatively quickly.
Objectives
To assess the efficacy and safety of PI3K/AKT/mTOR inhibitor‐containing regimens in women with locally‐advanced, metastatic or recurrent endometrial cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with locally‐advanced (FIGO Stage III) or metastatic (FIGO Stage IV) endometrial cancer (EC), either newly diagnosed or recurrent disease of any stage.
We include studies in which women were randomised to receive PI3K/AKT/mTOR inhibitors as first‐line treatment (i.e. no previous therapy except as adjuvant therapy) or subsequent line therapy.
Types of interventions
We include all studies that compared a regimen including a PI3K/AKT/mTOR inhibitor (either alone or in combination with other treatments such as chemotherapy or hormonal therapy) versus any comparator regimen which did not include a PI3K/AKT/mTOR inhibitor. There were no restrictions on which comparator(s) were included. Studies involving co‐interventions such as radiotherapy and surgery were expected to occur in the same way to both treatment arms. If there were some differences in the co‐interventions applied, these studies would still be included and any heterogeneity would be explored.
Types of outcome measures
Primary outcomes
Progression‐free survival (PFS), defined as time from randomisation to documented disease progression or death.
The proportion of participants experiencing toxicity. We grouped grades of toxicity according to Common Terminology Criteria for Adverse Events (CTCAE 2017):
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, diarrhoea);
genitourinary;
skin (stomatitis, mucositis);
vascular disorders (venous thrombosis, pulmonary embolism);
neurological (peripheral, central);
metabolic abnormalities (hyperglycaemia, hyperlipidaemia);
respiratory (interstitial pneumonitis).
Secondary outcomes
Overall survival (OS), defined as time from randomisation to time of death from any cause.
Objective response rate (ORR), defined as complete response plus partial response, with treatment response assessed according to Response Evaluation Criteria in Solid Tumours (RECIST) guidelines.
Quality of life (QoL), assessed using validated questionnaires, noting the type of questionnaire used (e.g. Hospital Anxiety Depression Score, European Organization for Research and Treatment of Cancer quality of life questionnaire).
Treatment‐related death, as defined as due to the toxicity of the drug and not to disease progression.
Search methods for identification of studies
There were no language restrictions. We searched for papers in all languages and if required, would have had them translated as necessary. We restricted the literature search from 1995 to the present.
Electronic searches
We searched the following electronic databases on 16 January 2019:
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1), in the Cochrane Library;
MEDLINE via Ovid (1995 to January week 2 2019);
Embase via Ovid (1995 to 2019 week 2);
WHO International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials in July 2018;
Clinicaltrials.gov (ClinicalTrials.gov/) in July 2018.
The search strategy is provided for MEDLINE in Appendix 1, the WHO International Clinical Trials Registry Platform (ICTRP) search portal in Appendix 2, ClinicalTrials.gov in Appendix 3, Embase in Appendix 4, and CENTRAL in Appendix 5.
For databases other than MEDLINE, we adapted the search strategy accordingly.
Searching other resources
We screened studies from reference lists of the identified relevant trial or reviews.
Handsearching
We handsearched the citation lists of the included studies. The conference proceedings listed below are incorporated in the Embase database and we therefore did not handsearch these separately. The conference years for each major cancer conference included in Embase are specified.
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist; 2009 to 2017);
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society; 2011 to 2016);
Annual Meeting of European Society of Medical Oncology (ESMO; 2008 and 2016);
Annual Meeting of the American Society of Clinical Oncology (ASCO; 2009 to 2017).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (Endnote) and removed duplicate references. Two review authors (FR and KL) examined the remaining references independently. We excluded those studies which clearly did not meet the inclusion criteria. We obtained copies of the full text of potentially relevant references. Two review authors (FR and KL) independently assessed the eligibility of the retrieved reports/publications. We resolved any disagreement through discussion or, if required, we consulted a third person (LM). We identified and excluded duplicates. We recorded the selection process in the PRISMA flow diagram (Liberati 2009). In future updates, we will record any excluded studies in the Characteristics of excluded studies table.
We included studies reported in full text and published as abstracts only.
Data extraction and management
Two review authors (FR and KL) independently extracted study characteristics and outcome data from the included studies on to a pre‐piloted data collection form. We note in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by consensus or by involving a third person (LM). Two review authors (FR and MW) transferred data into the Review Manager 5 (RevMan 2014) file. We double‐checked that data were entered correctly by comparing the data presented in the review with the study report.
For the included studies, we extracted the following data:
Author, year of publication, accrual period, and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology and accrual period
Study population: total number enrolled, participants' baseline characteristics ‐ age, co morbidities such as diabetes, hypertension, obesity; European Cooperative Oncology Group (ECOG) performance status, prior lines of treatment and type of agent, type 1 or type 2 endometrial cancer (EC), recurrent or advanced disease, stage at diagnosis, first‐line/second‐line
Intervention details (dose, cycles of treatment, route of administration, additional information as appropriate)
Comparison (dose, cycles of treatment, route of administration, additional information as appropriate)
Risk of bias in study (see below)
Duration of follow‐up
Outcomes: For each outcome, we extracted the outcome definition and unit of measurement (if relevant). For adjusted estimates, we would record variables adjusted for in analyses
Results: We extracted the number of participants allocated to each intervention group, the total number analysed for each outcome, and the missing participants
Notes: Funding for trial, and notable conflicts of interest of trial authors
We extracted results as follows:
For time‐to‐event data (PFS, OS), we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports (Tierney 2007). In the case of one included study (Aghajanian 2018), we estimated the HR by indirectly using the methods described by Tierney 2007, using other available summary statistics
For dichotomous outcomes (e.g. adverse events and ORR), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio
In this review, none of the reported outcomes were continuous outcomes.
If reported, we extracted both unadjusted and adjusted statistics.
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in the groups to which they were assigned.
We noted the time points at which outcomes were collected and reported.
Assessment of risk of bias in included studies
We assessed and reported the methodological quality and risks of bias of included studies, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), which recommends the explicit reporting of the following individual elements for RCTs:
Selection bias: random sequence generation and allocation concealment;
Performance bias: blinding of participants and personnel (participants and treatment providers) (This may only be applicable to outcome assessors);
Detection bias: blinding of outcome assessment;
Attrition bias: incomplete outcome data. We assessed whether outcome data are missing in both treatment arms in the study, if the numbers were different across treatment arms and if the proportion of missing data were large enough to have a clinically relevant impact on the effect estimate, and if missing data had been imputed using appropriate methods (Chapter 8, Cochrane Handbook for Systematic Reviews of Interventions);
Reporting bias: selective reporting of outcomes;
Other possible sources of bias.
Two review authors (FR and KL) applied the 'Risk of bias' tool independently, and where required resolved differences by discussion or by appeal to a third review author (LM). We judged each item as being at high, low or unclear risk of bias, as set out in the criteria provided by Higgins 2017, and provided a quote from the study report or a statement or both as justification for the judgement for each item in the 'Risk of bias' table. We summarise results in a 'Risk of bias' summary.
For phase II or III oncology studies, open‐label studies are common owing to difficulty in concealing different chemotherapy schedules, toxicities, etc. The blinding of the outcome assessment domain was therefore grouped into those outcome measures most unlikely or most likely to be influenced by a lack of blinding. The outcomes were segregated into: (a) overall survival; (b) progression‐free survival, response rates, and toxicity; and (c) quality of life.
Measures of treatment effect
We used the following measures of the effect of treatment:
Time‐to‐event outcomes (PFS, OS) were expressed as an HR with its associated variance and was extracted directly from the trial publication(s) where possible. An HR less than 1.0 favoured regimens containing a PI3K/AKT/mTOR inhibitor;
Dichotomous outcomes (response rate and toxicity) were expressed as a risk ratio (RR) and 95% confidence interval (CI).
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
Not applicable.
Assessment of heterogeneity
Where we considered studies to be similar enough (based on participants and interventions) to allow pooling of data using meta‐analysis, we assessed the degree of heterogeneity by visual inspection of forest plots, by estimation of the percentage of heterogeneity (I2 measurement) between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Chi2) (Deeks 2001) and, if possible, by subgroup analyses. We used the I2 statistic as a rough guide to assess heterogeneity:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We evaluated the value of the I2 statistic alongside the magnitude and direction of effects, and the P value for the Chi2 test (Higgins 2011).
Assessment of reporting biases
In this review, only two studies were available for inclusion and we therefore did not assess reporting bias.
Data synthesis
We pooled the results in meta‐analyses using the random‐effects model with the inverse variance for meta‐analysis in Cochrane Review Manager 5 software (RevMan 2014).
For time‐to‐event data (OS and PFS), we pooled hazard ratios using the generic inverse variance function of RevMan 2014.
For dichotomous outcomes, we calculated the risk ratio (RR) for each study and pooled them using the inverse variance for random‐effects analysis.
We conducted meta‐analyses only where this is meaningful, i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
Main outcomes of 'Summary of findings' table for assessing the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2011). We have presented a 'Summary of findings' table reporting the following outcomes listed according to priority:
progression‐free survival;
toxicity (haematological: anaemia; respiratory: interstitial pneumonitis; skin: mucositis)
overall survival;
objective response rate;
quality of life.
Two authors (FR and MW) graded the evidence and developed the 'Summary of findings' table in GRADEproGDT.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was not possible due to the limited number of studies available on this topic.
Sensitivity analysis
Sensitivity analysis was not possible due to the limited number of studies available on this topic.
Results
Description of studies
We include two studies:
Aghajanian 2018 is a phase II clinical trial in chemotherapy‐naïve women with EC, randomised to paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer, using historical controls for comparison. These women had FIGO stage III or IVA/IVB EC and a good baseline performance status (0, 1 or 2). The primary endpoint was PFS, with secondary endpoints of overall survival and tumour response.
Oza 2015 is a randomised phase II trial of oral ridaforolimus compared with progestin or investigator choice of chemotherapy in women with metastatic or recurrent EC who have had progressive disease following one or two lines of chemotherapy and no hormonal therapy. The primary endpoint was PFS, with secondary endpoints including PFS at 16 and 26 weeks, OS, best response rate, and assessment of the safety and tolerability of oral ridaforolimus.
Results of the search
We identified 970 references through medical database searches, and removed 17 duplicates. We screened the title and abstract of 953 references, which resulted in five full texts or abstracts potentially fulfilling our eligibility criteria. Following examination of the full‐text articles, we included these five references relating to two studies (Aghajanian 2018: three references; Oza 2015: two references). In addition, through searching the WHO ICTRP search portal and ClinicalTrials.gov, we retrieved 366 references and removed 204 duplicates. We screened 162 references against title and trial information, and retained seven references; five references were related to five ongoing studies (NCT01935973; NCT02065687; NCT02228681; NCT02725268; NCT02730923) and two references were the clinical trial registry records for the two aforementioned included studies. Refer to the PRISMA flowchart: Figure 1.
1.
Study flow diagram.
Included studies
We include two studies that examined the effect of an mTOR inhibitor‐containing regimen compared with either hormonal therapy or chemotherapy. The mTOR inhibitors were temsirolimus (Aghajanian 2018) and ridaforolimus (Oza 2015).
Aghajanian 2018 was a randomised phase II, three‐arm study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus or ixabepilone/carboplatin/bevacizumab as primary therapy for measurable stage III or IVA, stage IVB (with or without measurable disease) or recurrent endometrial cancer. A fourth group was added to the trial publication that was referred to as the historical reference from GOG 0209. This historical reference arm included women enrolled to the paclitaxel and carboplatin treatment arm of GOG 0209 with similar disease characteristics to the experimental arms. Data from only the intervention and most appropriate comparator treatment group (carboplatin, paclitaxel and bevacizumab, arm 1 in the trial publication) were used in the analysis despite arm 1 not being standard care. The vast majority of participants in all groups had endometrioid histologies; only 15% (carboplatin/taxane/bevacizumab) to 26% (ixabepilone/carboplatin/bevacizumab) had serous tumours at baseline. Only 3.6% of the participants had been exposed to endocrine treatment prior to inclusion. The study enrolled 115 women in the paclitaxel/carboplatin/temsirolimus arm and 116 women in the paclitaxel/carboplatin/bevacizumab arm. The primary outcome of this study was progression‐free survival (PFS).
Oza 2015 was an open‐label, multicentre, randomised phase II study of the oral mTOR inhibitor ridaforolimus, given as second‐ or third‐line treatment, where women had not had prior endocrine therapy. Treatment with mTOR inhibitor alone was compared to progestin or chemotherapy where the investigator could choose the chemotherapy from the options of carboplatin, paclitaxel, topotecan, doxorubicin or liposomal doxorubicin. More than half of the participants (53.8%) had endometrioid histology. The same proportion had grade 3 tumours; 26.2% had a diagnosis of serous endometrial cancer. Most women had stage IIIc and IVb disease (75%). The study enrolled 130 women; of the 65 participants in the comparator arm, 13 women received chemotherapy and 52 women received progestin. The primary outcome of this study was progression‐free survival. Refer to Characteristics of included studies.
The five ongoing studies identified are assessing a range of interventions, including the safety and effectiveness of AKT inhibitors (NCT01935973), metformin (NCT02065687), mTOR inhibitors (everolimus: NCT02228681), dual mTOR inhibitors (NCT02725268; NCT02730923) and PI3K inhibitors (NCT02725268), compared to chemotherapy or hormonal therapy. Refer to Characteristics of ongoing studies.
Excluded studies
We did not exclude any studies.
Risk of bias in included studies
Refer to Figure 2 for a summary of the 'Risk of bias' judgements for each 'Risk of bias' domain of the included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
The method of random sequence generation was described adequately (that is, with low risk of bias) in both studies. These studies reported either the use of a dynamic randomisation allocation procedure across strata (Aghajanian 2018) or a stratified randomisation process (Oza 2015).
Allocation concealment
The two included studies were at low risk of bias for allocation concealment. Both studies described central randomisation systems (computer or interactive voice response system).
Blinding
Blinding of participants and personnel
The two studies were described as "open‐label". We could not rule out performance bias owing to the lack of blinding of participants and personnel; we judged these two studies to be at high risk for this domain.
Blinding of outcome assessment
We assessed detection bias by grouping outcomes with similar risks of bias: (a) overall survival (b) progression‐free survival and objective response rate, and (c) toxicity. As quality of life was not measured in the two studies, we have excluded it from this 'Risk of bias' assessment,but we will include it if quality of life is reported in future trials.
For overall survival, we perceived a lack of blinding as being unlikely to have an impact on this outcome assessment, so we rated all studies at low risk of bias. For outcome measures that were more likely to be influenced by a lack of blinding, i.e. progression‐free survival, objective tumour response rate, and toxicity, we judged whether outcome assessments were confirmed through imaging and biochemical tests and reviewed by independent panels/adjudication committees (especially for tumour response rates) in each study. We rated Oza 2015 at low risk of bias for assessment of progression‐free survival and objective tumour response rate. Both studies were at unclear risk of bias for the remaining outcomes assessed because there were either no details of an independent adjudication committee or no information provided.
Incomplete outcome data
Aghajanian 2018 reported that data analyses were conducted according to intention‐to‐treat principles (ITT) or provided information, or both, for participant exclusions (if these occurred) in their analyses. All participants were included in the efficacy outcomes and we judged the study to be at low risk of bias. Oza 2015 reported that the final PFS and RR analyses were conducted on the full analysis set (FAS) population, which resulted in 75% of participants in the ridaforolimus group and 71% of participants in the progestin/chemotherapy group being included in the analysis of these outcomes. We therefore judged this part of the study results to be at high risk of attrition bias. For the analysis of overall survival (OS), however, the ITT population was used and included all participants enrolled up to the time of the database lock in August 2012. For OS, we therefore consider the risk of attrition bias to be low.
Selective reporting
Both included studies reported the outcomes listed in the trial registration record in the Results section of the main trial publications. We therefore rated both studies at low risk of bias for this domain.
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
See: Table 1
One included study (Aghajanian 2018) assessed the effects of an mTOR inhibitor‐containing regimen in women who were treatment naïve, whereas the second included study (Oza 2015) examined the effect of an mTOR inhibitor in women who had previously received one or two lines of chemotherapy treatment. In the analysis, we presented the treatment effects separately for each treatment setting.
Progression‐free survival (PFS)
First‐line trials
Based on one study (Aghajanian 2018), administering an mTOR inhibitor‐containing regimen may worsen progression‐free survival compared to chemotherapy with bevacizumab (HR 1.43, 95% CI 1.06 to 1.93; 231 participants; low‐certainty evidence; Analysis 1.1; Figure 3). Participants were followed up to 24 months; 182 of 231 women progressed following treatment.
1.1. Analysis.

Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 1 Progression‐free survival.
3.

Forest plot of comparison: 1 mTOR versus chemotherapy/hormone therapy, outcome: 1.1 Progression‐free survival.
Second/third‐line trials
Based on one study (Oza 2015), single agent mTOR inhibitor probably improves progression‐free survival compared to chemotherapy or hormonal therapy (HR 0.53, 95% CI 0.31 to 0.91; 95 participants; moderate‐certainty evidence; Analysis 1.1; Figure 3). Participants were followed up to 14 months.
Toxicity
Both studies reported on a range of toxicity outcomes, detailing grade 3 or 4 events unless otherwise stated below or in the forest plots.
Haematological
Leucopenia
Data were not reported for this outcome.
Anaemia
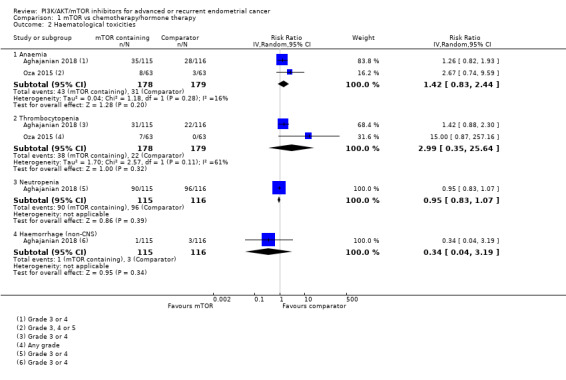
Data were available from both studies. Administering an mTOR inhibitor‐containing regimen may result in little to no difference in risk of anaemia (RR 1.42, 95% CI 0.83 to 2.44; 357 participants; low‐certainty evidence; Analysis 1.2; Figure 4). Seventy‐four participants had grade 3 or 4 anaemia in 357 participants.
1.2. Analysis.
Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 2 Haematological toxicities.
4.

Forest plot of comparison: 1 mTOR vs chemotherapy/hormone therapy, outcome: 1.2 Haematological toxicities.
Thrombocytopenia
Data were available from both studies. However, Oza 2015 reported any grade of thrombocytopenia only, while Aghajanian 2018 reported grade 3 or 4 events. Administering mTOR inhibitor‐containing regimens may result in little to no difference in risk of thrombocytopenia compared to the non‐mTOR inhibitor‐containing regimen (RR 2.99, 95% CI 0.35 to 25.64; 357 participants; low‐certainty evidence; Analysis 1.2; Figure 4). Sixty participants had thrombocytopenia in 357 participants.
Neutropenia
Data were available from one study (Aghajanian 2018). Administering an mTOR inhibitor‐containing regimen may result in little to no difference in grade 3 or 4 neutropenia (RR 0.95, 9% CI 0.83 to 1.07; 231 participants, 186 events; low‐certainty evidence; Analysis 1.2; Figure 4).
Haemorrhage
Data were available from one study (Aghajanian 2018). Administering an mTOR inhibitor‐containing regimen may result in little to no difference in grade 3 or greater non‐CNS haemorrhage (RR 0.34, 95% CI 0.04 to 3.19; 231 participants, 4 events; low‐certainty evidence; Analysis 1.2; Figure 4). There was no bleeding in the CNS reported in either the mTOR‐containing or comparator groups.
Gastrointestinal
Nausea
Data were reported in one study (Oza 2015) for this outcome. Administering an mTOR inhibitor may result in little to no difference in nausea (grade ≥ 3) (RR 0.50, 95% CI 0.05 to 5.38; 126 participants, 3 events; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 3 Gastrointestinal toxicities.
Vomiting
Data were reported in one study (Oza 2015) for this outcome. Administering an mTOR inhibitor may result in little to no difference in vomiting (grade ≥ 3) (RR 5.00, 95% CI 0.24 to 102.10; 126 participants, 2 events; low‐certainty evidence; Analysis 1.3).
Anorexia
Data were reported in one study (Oza 2015) for this outcome. Administering an mTOR inhibitor may result in little to no difference in anorexia (RR 3.00, 95% CI 0.32 to 28.07; 126 participants; 4 events; low‐certainty evidence; Analysis 1.3).
Diarrhoea
Data were reported in one study (Oza 2015) for this outcome. Administering an mTOR inhibitor may result in little to no difference in diarrhoea (grade ≥ 3) (RR 7.00, 95% CI 0.89 to 55.25; 126 participants, 8 events; low‐certainty evidence; Analysis 1.3).
Genitourinary
Data were not reported on genitourinary toxicity outcomes.
Skin
Stomatitis
Data were reported in one study (Oza 2015). Administering an mTOR inhibitor may result in little to no difference in stomatitis (grade ≥ 3) (RR 9.00, 95% CI 0.49 to 163.75; 126 participants, 4 events; low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 4 Skin toxicities.
Mucositis
Data were available from both studies. Administering mTOR inhibitor‐containing regimens may result in an increase in mucositis (RR 10.42, 95% CI 1.34 to 80.74; 357 participants; low‐certainty evidence; Analysis 1.4). Ten participants had grade 3 or 4 mucositis in 357 participants.
Vascular disorders
Venous thrombosis
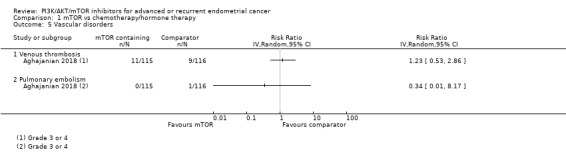
Data were available from one study (Aghajanian 2018). Administering an mTOR inhibitor‐containing regimen may result in little to no difference in grade 3 or greater events of venous thrombosis (RR 1.23, 95% CI 0.53 to 2.86; 231 participants, 20 events; low‐certainty evidence; Analysis 1.5).
1.5. Analysis.
Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 5 Vascular disorders.
Pulmonary embolism
Data were available from one study (Aghajanian 2018). Administering an mTOR inhibitor‐containing regimen may result in little to no difference in grade 3 or greater events of pulmonary embolism (RR 0.34, 95% CI 0.01 to 8.17; 231 participants, 1 event; low‐certainty evidence; Analysis 1.5).
Neurological
Peripheral
Neuropathy was reported in one study (Aghajanian 2018) with four events occurring in the mTOR inhibitor treatment group and five events occurring in the comparator group.
Central
Data were not reported on central nervous system toxicities.
Metabolic abnormalities
Hyperglycaemia
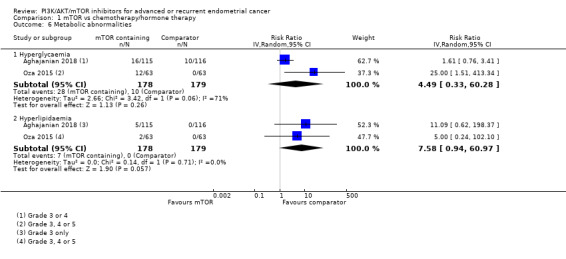
Data were available from both studies. mTOR inhibitor‐containing regimens may result in little to no difference in hyperglycaemia (RR 4.49, 95% CI 0.33 to 60.28; 357 participants; low‐certainty evidence; Analysis 1.6). Thirty‐eight participants had grade 3 or 4 hyperglycaemia in 357 participants.
1.6. Analysis.
Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 6 Metabolic abnormalities.
Hyperlipidaemia
Data were available from both studies. mTOR inhibitor‐containing regimens may result in little to no difference in hyperlipidaemia (RR 7.58, 95% CI 0.94 to 60.97; 357 participants; low‐certainty evidence; Analysis 1.6). Seven participants had grade 3 or 4 hyperlipidaemia in 357 participants.
Respiratory
Interstitial pneumonitis
Data were available from both studies. mTOR inhibitor‐containing regimens appear to result in little to no difference in pneumonitis (any grade) (RR 7.36, 95% CI 0.88 to 61.52; 357 participants; low‐certainty evidence Analysis 1.7). Eight participants had any grade of pneumonitis in 357 participants.
1.7. Analysis.

Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 7 Respiratory toxicity.
Treatment‐related death
Both studies reported on deaths thought to be attributable to the study treatment. In Oza 2015 no deaths were reported in either treatment arm, while in Aghajanian 2018 nine deaths were reportedly related to the study treatment. Aghajanian 2018 specified the possible cause of treatment‐related death (i.e. sepsis: three participants; pulmonary embolism: one participant; subventricular tachycardia, febrile neutropenia, nausea and vomiting: one participant; dyspnoea with infection: one participant; death not otherwise specified possibly due to sepsis, pneumonia or cardiac collapse: one participant; intestinal perforation: one participant; and possibly treatment or other but not specified: one participant), but not which treatment group the participants were in.
Overall survival (OS)
First‐line trials
Based on one study (Aghajanian 2018), administering an mTOR inhibitor‐containing regimen may result in little to no difference in overall survival compared to the chemotherapy (HR 1.32, 95% CI 0.98 to 1.78; 231 participants; low‐certainty evidence; Analysis 1.8; Figure 5). In Aghajanian 2018, 68 deaths in 115 women and 58 deaths in 116 women were reported in the temsirolimus‐containing regimen and comparator groups, respectively. Participants were monitored up to 36 months.
1.8. Analysis.

Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 8 Overall survival.
5.

Forest plot of comparison: 1 mTOR versus chemotherapy/hormone therapy, outcome: 1.8 Overall survival.
Second/third‐line trials
Based on one study (Oza 2015), a single‐agent mTOR inhibitor may result in little to no difference in overall survival compared to chemotherapy or hormonal therapy (HR 1.06, 95% CI 0.70 to 1.61; 130 participants; low‐certainty evidence; Analysis 1.8; Figure 5). In Oza 2015, 93 deaths in 130 women were reported overall. Participants were monitored up to 26 months.
Objective response rate (ORR)
First‐line trials
Based on data from Aghajanian 2018, administering mTOR inhibitor‐containing regimens may result in little to no difference in tumour response rate (RR 0.93, 95% CI 0.75 to 1.17; 231 participants; low‐certainty evidence; Analysis 1.9; Figure 6).
1.9. Analysis.

Comparison 1 mTOR vs chemotherapy/hormone therapy, Outcome 9 Objective response rate.
6.

Forest plot of comparison: 1 mTOR versus chemotherapy/hormone therapy, outcome: 1.9 Objective response rate.
Second/third‐line trials
Based on data from Oza 2015, administering mTOR inhibitor‐containing regimens may result in little to no difference in tumour response rate (RR 0.22, 95% CI 0.01 to 4.40; 61 participants; low‐certainty evidence; Analysis 1.9; Figure 6).
Quality of life (QoL)
Neither study collected or reported data on this outcome.
Discussion
Summary of main results
Overall, based on low‐certainty evidence, in women with advanced (stage III or IVA with measurable disease, or metastatic IVB) or recurrent endometrial cancer who received a first‐line mTOR inhibitor‐containing regimen (i.e. temsirolimus, carboplatin and paclitaxel), there may be an increased risk of relapse compared to those without an mTOR inhibitor (i.e. carboplatin, paclitaxel and bevacizumab; Aghajanian 2018). In contrast, women with advanced and recurrent endometrial cancer who received second/third‐line treatment with the mTOR inhibitor ridaforolimus may have a reduced risk of relapse compared to those in the chemotherapy or hormonal therapy arms (Oza 2015). However, ridaforolimus made little to no difference in survival or tumour response rate when compared to the chemotherapy/hormone therapy group, although this study was not powered to detect a difference in survival, so we have low certainty in this outcome. Although there appeared to be a modest clinical benefit of mTOR on risk of relapse in the second/third‐line setting from one study, there appeared to be an increased risk of mucositis from mTOR inhibitors in both first and second/third‐line settings. The low event rates of selected toxicities preclude any definite conclusions about the toxicity of mTOR inhibitors in comparison with other treatment regimens, but mTOR inhibitors may increase the risk of mucositis.The nine deaths reported to be related to the study treatment were not reported by treatment arm.
Overall completeness and applicability of evidence
There were two relevant published clinical trials with results that could be included in this systematic review. Although Oza 2015 and Aghajanian 2018 are relevant to the review question and also judged to be high‐quality randomised phase II clinical trials, it is difficult to establish external validity for this review as there are data awaiting publication from ongoing clinical trials involving PI3K/AKT/mTOR inhibitors in advanced or recurrent endometrial cancer. There are insufficient published studies to address all of the objectives of this review. For example, data on quality of life were not assessed or reported. PI3K and AKT inhibitors, as well as a combination PI3K/AKT/mTOR inhibitors that would inhibit this pathway (instead of one type of mTOR inhibitor) could not be examined in this review. Moreover, neither clinical trial differentiated between women who had Type 1 or 2 EC, with both trials enrolling women with either type of EC. Some participants may have already been exposed and had their disease worsen whilst on endocrine treatment, while others in the treatment‐naïve group may not have been exposed to any endocrine treatment at all.
Oza 2015 showed that the mTOR inhibitor, ridaforolimus, administered during second/third‐line treatment may provide some clinical benefit (in terms of progression‐free survival) in this population with advanced or recurrent endometrial cancer compared to standard care, which is usually chemotherapy or progestin therapy. Currently, this drug is not widely available for treatment of endometrial cancer, and there are no other PI3K/AKT/mTOR inhibitors that are widely available for treatment of women with advanced or recurrent endometrial cancer. The study did, however, provide preliminary evidence that targeting mTOR could be an effective option for this population of women, at the cost of fairly significant toxicity. Further studies are needed to confirm this improved progression‐free survival and reports of significant toxicity. Aghajanian 2018 showed that temsirolimus, carboplatin and paclitaxel, when compared to carboplatin, paclitaxel and bevacizumab, is unlikely to be beneficial in this population with advanced or recurrent endometrial cancer. This study confirmed some significant toxicity.
Quality of the evidence
The overall risk of bias for both studies was generally low. There were 361 women randomised in total, but one study examined the effect of mTOR inhibitors in women who were treatment‐naïve while the other study included women who were receiving second‐ or third‐line treatment. The study populations were therefore not directly comparable, despite both studies being judged to be high‐quality randomised phase II trials. There was imprecision due to single‐trial analysis for first‐ or second/third‐line therapy, and low event rates for toxicity. There is currently not enough evidence to draw a robust conclusion about the efficacy and safety of inhibitors of the PI3K/AKT/mTOR pathway in women with recurrent or advanced endometrial cancer.
Potential biases in the review process
We were not able to obtain any unpublished data for any other clinical trials, as all of these studies were identified as ongoing, based on the most recent search of the clinical trial registries in July 2018. Oza 2015 and Aghajanian 2018 were generally judged to be at low risk of bias, although they were conducted as open‐label trials. It is highly likely that we have identified all relevant studies and have obtained all published relevant data for this review. Two review authors independently searched databases and conducted online handsearches, study selection and data collection. There was complete agreement on the included studies.
Agreements and disagreements with other studies or reviews
One other systematic review was identified (Kassem 2016) although this review included mostly single‐arm studies and did not include the study by Aghajanian 2018. The review reported that the complete tumour response ranged from 21% to 60% and median PFS ranged from 2.8 months to 7.3 months in the mTOR inhibitor group however there was no comparator provided. The Cochrane review included randomised phase II studies with the finding that further research is needed to investigate the role of PI3K/AKT/mTOR inhibitors in advanced or recurrent endometrial cancer, which concurs with the latest recommendation from the ESMO 2016 consensus‐based recommendations. In these recommendations, ESMO states that the PI3K/AKT/mTOR pathway is known to be altered in endometrial cancer and "their relevance should be studied in clinical trials with targeted agents" (p.34). We await the completion of ongoing studies.
Authors' conclusions
Implications for practice.
Based on only one clinical trial, there is preliminary evidence that administering mTOR‐inhibitors as second/third‐line treatment for women with advanced or recurrent endometrial cancer may improve progression‐free survival, but there was little or no benefit in overall survival or tumour response rate. Also, based on only one clinical trial, the use of mTOR‐containing regimens in treatment‐naïve women probably does not result in improvements in progression‐free survival, overall survival or tumour response. In women who were treatment‐naïve or had prior treatment, there may be worsened toxicity in those who received mTOR‐inhibitors compared to those who did not, although the toxicity event rate remained low. Based on these two studies, there is insufficient evidence to justify mainstream use of an mTOR inhibitor in either setting. We await the publication of at least five ongoing studies investigating the role of PI3K/AKT/mTOR inhibitors in advanced or recurrent endometrial cancer.
Implications for research.
We await the completion of ongoing trials relevant to the aims of this systematic review. These studies are assessing the efficacy and safety of PI3K/AKT/mTOR inhibitors, alone and in combination with other chemotherapies in endometrial cancer. Future directions should consider selecting participants based on their histological subtype and try to avoid combining several histological diagnoses with probably very different responses to treatment. Studies may also consider whether women have had previous exposure and progression on endocrine therapy, as well as their receptor expression profile, if they have not been exposed yet. Assessing molecular profiles on a recent biopsy, instead of archival tumour tissue, would be important. From these two clinical trials, all that can be concluded so far is that there has not been a clear benefit, but both trials appeared to enrol a mixed population of women with EC. Future trial design for women with EC may include an umbrella trial design, with enrolment based on molecular histopathological subtype and a range of interventions targeting molecular histopathological subtypes. At this stage, based on the currently available evidence, data do not support the use of PI3K/AKT/mTOR inhibitors outside of clinical trial settings. In this population of women with advanced endometrial cancer, which in many cases will be a palliative setting, it is vitally important that further studies should include participant‐reported outcomes, including health‐related quality of life data.
What's new
| Date | Event | Description |
|---|---|---|
| 24 September 2019 | Amended | Edits to figure captions. |
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jo Platt for designing the search strategy, and Gail Quinn, Clare Jess and Tracey Harrison for their contribution to the editorial process.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health, United Kingdom.
The authors and Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Team are grateful to the following peer reviewers for their time and comments: Andrew Bryant, Daloni Carlisle and Marcia Hall.
Appendices
Appendix 1. MEDLINE search strategy
1. exp Endometrial Neoplasms/ 2. (endom* adj5 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*)).mp. 3. 1 or 2 4. exp Phosphatidylinositol 3‐Kinases/ 5. (PI3k* or PI‐3k* or phosphatidylinositol 3 kinas* or kinases pi3* or ptdins3‐kinas* or 3‐kinases* or pi3‐kinas* or phosphoinositide 3‐hydroxykinas*).mp. 6. exp TOR Serine‐Threonine Kinases/ 7. (rapamycin* or serine‐threonine* or mTOR* or TOR* FKBP12* or FK506‐Binding* or FKBP‐Rapamycin*).mp. 8. exp Proto‐Oncogene Proteins c‐akt/ 9. (akt* or protein‐serine‐threonine* or proto‐oncogene* or akt‐beta* or c‐akt* or akt‐gamma* or akt‐alpha* or protein‐kinase‐b* or PKB* or RAC*).mp. 10. Metformin/ 11. (metformin* or dimethylbiguanidine* or dimethylbiguanidium* or dimethylguanylguanidine* or glucophage* or glucovance* or glumetza* or fortamet* or riomet*).mp. 12. Sirolimus/ 13. (sirolimus* or everolimus* or RAD001* or rapamycin* or temsirolimus* or rapamune* or cci779* or AY22989* or ay 22‐989* or SDZRAD*).mp. 14. (mTOR inhibitor* or afinitor* or torisel* or certican* or zortress* or sila9268a* or wy‐090217* or ridaforolimus* or mk‐8669* or ap23573* or deforolimus* or temsirolimus*).mp. 15. (bay80‐6946* or copanlisib* or gdc‐0980* or xl765* or sar245409* or nvb‐brz235* or dactolisib* or gdc‐0941* or pictilisib* or nvp‐bkm120* or bkm120* or buparlisib* or xl‐147* or sar245408* or azd‐8055* or ink‐128* or osi‐027* or nvp‐byl719* or mln0128* or byl719* or cal‐101* or gs‐1101* or zydelig* or gdc‐0032* or gdc‐0068* or azd‐5363* or perifosine* or d‐21266* or octadecylphosphopiperidine*).mp. 16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. 3 and 16 18. randomized controlled trial.pt. 19. controlled clinical trial.pt. 20. randomized.ab. 21. placebo.ab. 22. drug therapy.fs. 23. randomly.ab. 24. trial.ti. 25. groups.ab. 26. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. (animals not (humans and animals)).sh. 28. 26 not 27 29. 17 and 28
Key:
mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract sh=subject heading ti=title
Appendix 2. WHO ICTRP search portal
Basic searches:
endometrial neoplasms and PI3k
endometrial cancer and PI3k
endometrial tumor and PI3k
endometrial neoplasms and AKT
endometrial cancer and AKT
endometrial tumor and AKT
endometrial neoplasms and mTOR
endometrial cancer and mTOR
endometrial tumor and mTOR
endometrial neoplasms and metformin
endometrial cancer and metformin
endometrial tumor and metformin
Advanced searches: Search 1: Title: PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer Search 2: Condition: metastatic endometrial cancer or locally advanced endometrial cancer Intervention: PI3K or PI3‐kinase or AKT or mTOR or TOR or metformin or rapamycin
Appendix 3. ClinicalTrials.gov
Basic searches:
endometrial neoplasms and PI3k
endometrial cancer and PI3k
endometrial tumor and PI3k
endometrial neoplasms and AKT
endometrial cancer and AKT
endometrial tumor and AKT
endometrial neoplasms and mTOR
endometrial cancer and mTOR
endometrial tumor and mTOR
endometrial neoplasms and metformin
endometrial cancer and metformin
endometrial tumor and metformin
Advanced searches: Search 1: Title: PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer Search 2: Condition: endometrial neoplasms or endometrial cancer or endometrial tumor Intervention: PI3K or PI3‐kinase or AKT or mTOR or TOR or metformin or rapamycin
Appendix 4. Embase Search Strategy
1. exp endometrium cancer/ 2. (endom* adj5 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*)).mp. 3. 1 or 2 4. exp phosphatidylinositol 3 kinase/ 5. (PI3k* or PI‐3k* or phosphatidylinositol 3 kinas* or kinases pi3* or ptdins3‐kinas* or 3‐kinases* or pi3‐kinas* or phosphoinositide 3‐hydroxykinas*).mp. 6. exp "target of rapamycin kinase"/ 7. (rapamycin* or serine‐threonine* or mTOR* or TOR* FKBP12* or FK506‐Binding* or FKBP‐Rapamycin*).mp. 8. exp protein kinase B/ 9. (akt* or protein‐serine‐threonine* or proto‐oncogene* or akt‐beta* or c‐akt* or akt‐gamma* or akt‐alpha* or protein‐kinase‐b* or PKB* or RAC*).mp. 10. exp metformin/ 11. (metformin* or dimethylbiguanidine* or dimethylbiguanidium* or dimethylguanylguanidine* or glucophage* or glucovance* or glumetza* or fortamet* or riomet*).mp. 12. exp rapamycin/ 13. (sirolimus* or everolimus* or RAD001* or rapamycin* or temsirolimus* or rapamune* or cci779* or AY22989* or ay 22‐989* or SDZRAD*).mp. 14. (mTOR inhibitor* or afinitor* or torisel* or certican* or zortress* or sila9268a* or wy‐090217* or ridaforolimus* or mk‐8669* or ap23573* or deforolimus* or temsirolimus*).mp. 15. (bay80‐6946* or copanlisib* or gdc‐0980* or xl765* or sar245409* or nvb‐brz235* or dactolisib* or gdc‐0941* or pictilisib* or nvp‐bkm120* or bkm120* or buparlisib* or xl‐147* or sar245408* or azd‐8055* or ink‐128* or osi‐027* or nvp‐byl719* or mln0128* or byl719* or cal‐101* or gs‐1101* or zydelig* or gdc‐0032* or gdc‐0068* or azd‐5363* or perifosine* or d‐21266* or octadecylphosphopiperidine*).mp. 16. 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 17. 3 and 16 18. crossover procedure/ 19. double‐blind procedure/ 20. randomized controlled trial/ 21. single‐blind procedure/ 22. random*.mp. 23. factorial*.mp. 24. (crossover* or cross over* or cross‐over*).mp. 25. placebo*.mp. 26. (double* adj blind*).mp. 27. (singl* adj blind*).mp. 28. assign*.mp. 29. allocat*.mp. 30. volunteer*.mp. 31. 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 32. 17 and 31
Key:
mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract sh=subject heading ti=title
Appendix 5. CENTRAL search strategy
1. MeSH descriptor: [Endometrial Neoplasms] explode all trees 2. endom* near/5 (cancer* or neoplas* or carcinom* or adenocarcinom* or malignan* or tumor* or tumour*) 3. #1 or #2 4. MeSH descriptor: [Phosphatidylinositol 3‐Kinase] explode all trees 5. PI3k* or “PI‐3k*” or “phosphatidylinositol 3 kinas*” or “kinases pi3*” or “ptdins3‐kinas*” or “3‐kinases*” or “pi3‐kinas*” or “phosphoinositide 3‐hydroxykinas*” 6. MeSH descriptor: [TOR Serine‐Threonine Kinases] explode all trees 7. rapamycin* or “serine‐threonine*” or mTOR* or “TOR* FKBP12*” or “FK506‐Binding*” or “FKBP‐Rapamycin*” 8. MeSH descriptor: [Proto‐Oncogene Proteins c‐akt] explode all trees 9. akt* or “protein‐serine‐threonine*” or “proto‐oncogene*” or “akt‐beta*” or “c‐akt*” or “akt‐gamma*” or “akt‐alpha*” or “protein‐kinase‐b*” or PKB* or RAC* 10. MeSH descriptor: [Metformin] explode all trees 11. metformin* or dimethylbiguanidine* or dimethylbiguanidium* or dimethylguanylguanidine* or glucophage* or glucovance* or glumetza* or fortamet* or riomet* 12. MeSH descriptor: [Sirolimus] explode all trees 13. sirolimus* or everolimus* or RAD001* or rapamycin* or temsirolimus* or rapamune* or cci779* or AY22989* or “ay 22‐989*” or SDZRAD* 14. mTOR inhibitor* or afinitor* or torisel* or certican* or zortress* or sila9268a* or “wy‐090217*” or ridaforolimus* or “mk‐8669*” or ap23573* or deforolimus* or temsirolimus* 15. “bay80‐6946*” or copanlisib* or “gdc‐0980*” or xl765* or sar245409* or “nvb‐brz235*” or dactolisib* or “gdc‐0941*” or pictilisib* or “nvp‐bkm120*” or bkm120* or buparlisib* or “xl‐147*” or sar245408* or “azd‐8055*” or “ink‐128*” or “osi‐027*” or “nvp‐byl719*” or mln0128* or byl719* or “cal‐101*” or “gs‐1101*” or zydelig* or “gdc‐0032*” or “gdc‐0068*” or “azd‐5363*” or perifosine* or “d‐21266*” or octadecylphosphopiperidine* 16. #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 17. #3 and #16
Data and analyses
Comparison 1. mTOR vs chemotherapy/hormone therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 1.1 First‐line treatment | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Second/third‐line treatment | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Haematological toxicities | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Anaemia | 2 | 357 | Risk Ratio (IV, Random, 95% CI) | 1.42 [0.83, 2.44] |
| 2.2 Thrombocytopenia | 2 | 357 | Risk Ratio (IV, Random, 95% CI) | 2.99 [0.35, 25.64] |
| 2.3 Neutropenia | 1 | 231 | Risk Ratio (IV, Random, 95% CI) | 0.95 [0.83, 1.07] |
| 2.4 Haemorrhage (non‐CNS) | 1 | 231 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.04, 3.19] |
| 3 Gastrointestinal toxicities | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Nausea | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vomiting | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Anorexia | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Diarrhoea | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Skin toxicities | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Stomatitis | 1 | 126 | Risk Ratio (IV, Random, 95% CI) | 9.00 [0.49, 163.75] |
| 4.2 Mucositis | 2 | 357 | Risk Ratio (IV, Random, 95% CI) | 10.42 [1.34, 80.74] |
| 5 Vascular disorders | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Venous thrombosis | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Pulmonary embolism | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Metabolic abnormalities | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Hyperglycaemia | 2 | 357 | Risk Ratio (IV, Random, 95% CI) | 4.49 [0.33, 60.28] |
| 6.2 Hyperlipidaemia | 2 | 357 | Risk Ratio (IV, Random, 95% CI) | 7.58 [0.94, 60.97] |
| 7 Respiratory toxicity | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Interstitial pneumonitis | 2 | 357 | Risk Ratio (IV, Random, 95% CI) | 7.36 [0.88, 61.52] |
| 8 Overall survival | 2 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 8.1 First‐line treatment | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Second/third‐line treatment | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Objective response rate | 2 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 9.1 First‐line treatment | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Second/third‐line treatment | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aghajanian 2018.
| Methods | Accrual: September 2009 to January 2012
Multicentre phase II trial RCT, conducted in the USA 349 women randomised |
|
| Participants | Median age: intervention ‐ 63 (range 38 to 82 years); comparator ‐ 62 (range 36 to 87 years) Most participants had either endometrioid (grade 1 to 3) or serous histology. There was an imbalance of histologic type by treatment arm ‐ fewer participants with serous histology in comparator arm (14% versus 23% to 26%) and more with grade 2 endometrioid (31% versus 21% to 23%) FIGO 2009 stage: intervention ‐ III (11%), IVb (51%), recurrent (38%); comparator ‐ III (10%), IVa (1%), IVb (49%) and recurrent (40%) Most had not received prior hormonal therapy |
|
| Interventions | Intervention (labelled as arm 2 in the trial publication): paclitaxel 175 mg/m2 iv over 3 hours, carboplatin area under the curve (AUC) 5 iv over 30 minutes on day 1, and temsirolimus 25 mg iv on days 1 and 8 (concurrent with chemotherapy) and days 1, 8 and 15 (during maintenance), every 3 weeks for 6 cycles. Cycle 7 and on repeated temsirolimus every 3 weeks Comparator (labelled as arm 1 in the trial publication): paclitaxel 175 mg/m2 iv over 3 hours, carboplatin AUC 6 iv over 30 minutes followed by bevacizumab 15 mg/kg iv on day 1, every 3 weeks for 6 cycles. Cycle 7 and on repeated bevacizumab every 3 weeks There was a second comparator arm (labelled as arm 3 in the trial publication)‐ ixabepilone 30 mg/m2 iv over 1 hour, carboplatin AUC 6 iv over 30 minutes, followed by bevacizumab 15 mg/kg iv. Data only from the Intervention and Comparator arms (listed above) were used for this review because the 3rd arm was seen as the least appropriate comparison. The trial also included analyses using a historical control arm from GOG209 trial | |
| Outcomes | Primary outcome: ‐ PFS, defined as the time alive, progression‐free from date of study entry Secondary outcomes: ‐ OS, defined as the duration of time from date of study entry until date of death ‐ Objective tumour response, using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Clinical trial record states that complete and partial responses are included in the objective tumour response rate, and confirmation of response was not required ‐ Frequency and severity of acute adverse effects, graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI‐CTCAE) version 3.0 |
|
| Notes | NCT00977574
Censoring at 25 months for PFS analysis and 36 months for OS analysis. We calculated the hazard ratios using the numbers at risk in the PFS and OS plots (a method outlined by Tierney 2007) Funding considerations: National Cancer Institute (USA) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A dynamic randomisation allocation procedure was used that tends to balance the arms across strata (1:1:1)" (page 3) |
| Allocation concealment (selection bias) | Low risk | Registration took place centrally at the GOG statistical and Data Center |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) Progression‐free survival & tumour response rate | Unclear risk | Computed tomography scan conducted within 4 weeks of start of treatment then every 9 weeks thereafter for 2 years of therapy or follow‐up, then every 3 months until disease progression. No details of independent adjudication committee, so outcome assessment may have been influenced by known treatment allocation |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | The study used a formalised toxicity criterion (NCI CTCAE version 3) and measured a range of toxicity outcomes where some may be affected by unblinding (e.g. neuropathy) in borderline cases |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in intervention and comparator arms included in intention‐to‐treat analysis for efficacy outcomes; 15% and 13% discontinued treatment in intervention and comparator arms (due to participant refusal) which was similar across groups. All participants were included for OS, PFS and ORR assessments |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes in ClinicalTrials.gov record (NCT00977574) and the Methods section of the trial publication are the same. All outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
Oza 2015.
| Methods | Accrual: August 2008 to August 2012
Multicentre phase II trial RCT, conducted across 36 sites worldwide (including USA) 130 women randomised |
|
| Participants | Median age: 66.0 (range 37 to 81 years) 80% of participants had either endometrioid or papillary serous histology 75% had stage IIIc and IVb disease, 54% had grade 3 tumours 95% had at least 1 prior treatment regimen for endometrial cancer |
|
| Interventions | Intervention: oral ridaforolimus 40 mg/day for 5 consecutive days by 2‐day dosing holiday Comparator: progestin (i.e. oral medroxyprogesterone 200 mg/day or megestrol 160 mg/day) or chemotherapy. Chemotherapy options that investigators could chose from included carboplatin, paclitaxel, topotecan, doxorubicin or liposomal doxorubicin Treatment cycle consisted of a 4‐week period; participants expected to receive 2 or more cycles of treatment Additional cycles permitted if participants continued to have at least stable disease and tolerated therapy |
|
| Outcomes | Primary outcome: ‐ PFS, defined as the time from random assignment to documented disease progression or death, whichever occurred first; PFS rates at 16 and 26 weeks also calculated Secondary outcomes: ‐ OS ‐ Response rate, according to RECIST (Response Evaluation Criteria in Solid Tumors) ‐ Adverse events, graded according to the US National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI‐CTC) version 3.0 |
|
| Notes | ClinicalTrials.gov record: NCT00739830 Final PFS and response rate analyses were based on full analysis set population (protocol prespecified interim analysis) OS analysis was based on intention‐to‐treat population (n = 130) Safety analyses based on all participants being treated as the population (n = 128) Funding considerations: Merck Sharp & Dohme Corp; Merck also funded writing assistance |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation in a multicentre trial |
| Allocation concealment (selection bias) | Low risk | Randomisation was implemented with an interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) Overall survival | Low risk | Assessment of overall survival is unlikely to be influenced by no or incomplete blinding |
| Blinding of outcome assessment (detection bias) Progression‐free survival & tumour response rate | Low risk | Disease progression and objective tumour responses were evaluated every 8 weeks by site investigators and an independent review committee who used computed tomography scans, even though independent radiology review and investigator‐assessed results showed the same trend in terms of differences between the treatment arms, absolute response rate (ARR) in each group varied between the 2 assessments. PFS was assessed in full analysis set, not in ITT prespecified analysis |
| Blinding of outcome assessment (detection bias) Toxicity | Unclear risk | Information on assessments of toxicity not provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The final PFS and RR analyses were conducted on the full analysis set (FAS) population at the time of the interim analysis in September 2010, which was scheduled after 58 events had been recorded. This resulted in 75% of participants and 71% of participants in the ridaforolimus and progestin/chemotherapy group, respectively, being included in the PFS and ORR results. The ITT population was used to determine OS and included participants enrolled up to the time of the database lock in August 2012 |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes in ClinicalTrials.gov record (NCT0073983) and the Methods section of the trial publication are the same. All outcomes were reported in the Results section |
| Other bias | Low risk | None identified |
AUC: area under the curve FAS: full analysis set ITT: intention‐to‐treat iv: intravenous OS: overall survival PFS: progression‐free survival RR: response rate
Characteristics of ongoing studies [ordered by study ID]
NCT01935973.
| Trial name or title | Trametinib with or without GSK2141795 in treating patients with recurrent or persistent endometrial cancer |
| Methods | Accrual: September 2013 to February 2017 Accrual target: 148 Multicentre, phase 2 RCT conducted in the USA |
| Participants | Endometrial cancer (clear cell, mixed, serous, undifferentiated, recurrent)
Measurable disease by RECIST Must have had 1 prior chemotherapeutic regimen, allowed to receive but are not required to receive 1 additional cytotoxic regimen for recurrent or persistent disease |
| Interventions | Intervention: trametinib by mouth each day and Akt inhibitor GSK2141795 by mouth each day on days 1 to 28 Comparator: trametinib by mouth each day on days 1 to 28 |
| Outcomes | Primary outcomes: ‐ Progression‐free survival ‐ Frequency of adverse events ‐ Severity of adverse events ‐ Incidence of dose‐limiting toxicity (DLT) Secondary outcomes: ‐ KRAS status ‐ Tumour response by regimen, assessed using RECIST ‐ PFS by regimen ‐ OS by regimen ‐ Response duration by KRAS mutation and regimen ‐ Proportion of responding participants ‐ Baseline genomic biomarkers |
| Starting date | Study start date: September 2013 Primary completion date: February 2017 |
| Contact information | Shannon Westin NRG oncology |
| Notes | NCT01935973 Sponsor: National Cancer Institute (USA) |
NCT02065687.
| Trial name or title | Paclitaxel and carboplatin with or without metformin hydrochloride in treating participants with stage III, IV or recurrent endometrial cancer |
| Methods | Accural: March 2014 to September 2019 Accrual target: 540 Multicentre, phase 2/3 RCT conducted in the USA |
| Participants | Measurable stage III, IVA, IVB or recurrent endometrial carcinoma
Histologic confirmation of endometrioid, serous adenoma, undifferentiated, clear cell, mixed cell epithelial, adenoca NOS Measurable disease by RECIST First line ‐ must not have received prior chemotherapy or targeted therapy; may have received prior RT and prior hormonal therapy |
| Interventions | Intervention: paclitaxel iv day 1, carboplatin iv day 1, metformin hydrochloride by mouth twice a day on days 1 to 21 (once a day in course 1). Treatment every 21 days for 6 cycles in absence of disease progression or unacceptable toxicity. Participants then receive maintenance therapy ‐ metformin hydrochloride by mouth twice a day on days 1 to 21 Comparator: paclitaxel iv and carboplatin iv as per Intervention arm and placebo by mouth twice a day on days 1 to 21 (once a day in course 1) Treatment every 21 days for 6 cycles in absence of disease progression or unacceptable toxicity |
| Outcomes | Primary outcomes: ‐ OS (phase II and III) ‐ PFS (phase II) Secondary outcomes: ‐ Proportion of participants responding to therapy ‐ Duration of response by treatment ‐ Adverse events ‐ Level of obesity ‐ OS in phase II ‐ PFS in phase III Other outcomes: ‐ Expression of MATE2 ‐ Incidence of PIK3 mutations/amplifications ‐ Levels of key targets of the metformin/mTOR signalling pathway ‐ Metabolic factor levels |
| Starting date | Study start date: March 2014 Estimated completion date: September 2019 |
| Contact information | Victoria Bae‐Jump NRG Oncology |
| Notes | NCT02065687 Sponsor: Gynecologic Oncology Group |
NCT02228681.
| Trial name or title | Everolimus and letrozole or hormonal therapy to treat endometrial cancer |
| Methods | Accrual: February 2015 to June 2017 Accrual target: 74 Multicentre, phase 2 RCT, conducted in the USA |
| Participants | FIGO III or IV persistent or recurrent endometrial cancer, no histological confirmation required
Measurable disease
1 target lesion at least Prior chemo‐ and radiotherapy for pelvic recurrence is permitted, adjuvant chemotherapy is permitted PS 0‐1 No prior chemotherapy in stage 4 setting permitted Exclude: previous everolimus, any other mTOR or agent targeting the pathway, previous hormonal therapy, uncontrolled diabetes, CTCAEG2 hypoxia, class II heart failure |
| Interventions | Intervention: everolimus 10 mg daily and letrozole 2.5 mg by mouth daily Comparator: tamoxifen 20 mg by mouth twice a day; on alternating weeks, medroxyprogesterone acetate 200 mg by mouth daily with tamoxifen 20 mg by mouth twice a day |
| Outcomes | Primary outcome: ‐ Response rate Secondary outcomes: ‐ Time to disease progression ‐ Toxicity Other outcome measures: ‐ Hormone receptor immunohistochemistry ‐ mTOR pathway immunohistochemistry ‐ mutation analysis |
| Starting date | Study start date: February 2015 Estimated completion date: January 2018 |
| Contact information | Brian Slomovitz |
| Notes | NCT02228681 Sponsor: Gynecologic Oncology Group and Novartis pharmaceuticals |
NCT02725268.
| Trial name or title | Phase 2 study of MLN0128 (a dual TORC1/2 inhibitor), combination of MLN0128 with MLN1117 (a PI3Kα inhibitor), paclitaxel and combination of MLN0128 with paclitaxel in women with endometrial cancer |
| Methods | Accrual: April 2016 to September 2019
Accrual target: 242 Multicentre, phase 2 RCT, conducted in Europe |
| Participants | Advanced, recurrent, or persistent endometrial cancer and has relapsed or is refractory to curative therapy or established treatments Prior platinum‐based chemotherapy permitted (but not more than 2 prior chemotherapy regimens). Prior chemotherapy/radiation therapy and/or consolidation/maintenance therapy allowed Measurable disease by RECIST 1.1 ECOG performance status of 0 to 2 Exclude: previous treatment with any weekly taxane or PI3K, AKT, dual PI3K/mTOR inhibitors, TORC1/2 or TORC1 inhibitors |
| Interventions | Intervention I: paclitaxel 80 mg/m2 weekly (days 1, 8 and 15) of 28‐week cycle and MLN0128 4 mg (days 2 ‐ 4, 9 ‐ 11, 16 ‐ 18, 23 to 25) of 28‐day cycle Intervention II: MLN0128 30 mg once weekly (days 1, 8, 15, 22) of 28‐week cycle Intervention III: MLN0128 4 mg and MLN1117 200 mg (days 1 ‐ 3, 8 ‐ 10, 15 ‐ 17 and 22 ‐ 24) of 28‐week cycle Comparator: paclitaxel 80 mg/m2 weekly (days 1, 8 and 15) of 28‐week cycle |
| Outcomes | Primary outcome:
‐ Progression‐free survival
Secondary outcomes: ‐ Percentage of participants who experience at least 1 treatment‐emergent adverse event ‐Overall survival ‐ Time to progression ‐ Overall response rate ‐ Clinical benefit rate ‐ Clinical benefit rate at week 16 |
| Starting date | Study start date: March 2016 Estimated completion date: 30 September 2019 |
| Contact information | Takeda study registration call centre |
| Notes | NCT02725268 Sponsors: Millennium Pharmaceuticals Inc, European Network of Translational Research in Ovarian Cancer, European Network of Individualized Treatment in Endometrial Cancer |
NCT02730923.
| Trial name or title | Hormone receptor‐positive endometrial carcinoma treated by dual mTORC1/mTORC2 inhibitor and anastrozole (VICTORIA) |
| Methods | Accrual: April 2016 to May 2019 Accrual target: 72 Multicentre, phase 2 (safety run‐in phase to evaluate safety of AZD2014 and anastrozole) RCT, conducted in France |
| Participants | Postmenopausal, histologically‐confirmed advanced or recurrent endometrial cancer, not amenable to curative treatment Carcinosarcoma ineligible ER/PR‐positive Disease progression after no more than 1 prior first‐line chemotherapy and/or more than 2 lines endocrine therapy in metastatic setting ECOG 0‐1 Measurable disease |
| Interventions | Intervention: AZD2014 plus anastrozole Comparator: anastrozole |
| Outcomes | Primary outcomes: ‐ Toxicities ‐ Non‐progression rate Secondary outcomes: ‐ Adverse events ‐ PFS ‐ OS ‐ Best response rate ‐ Duration of objective response ‐ Area under the curve of AZD2014 ‐ Apparent clearance of AZD2014 ‐ Accumulation of the 47S precursor rRNA ‐ Expression of rRNA methylation ‐ Levels of anti‐fibrillarin, nucleolin, protein B23, upstream binding factor and phosphorylated upstream binding factor |
| Starting date | Study start date: April 2016 Estimated completion date: May 2019 |
| Contact information | Pierre‐Etienne Heudel, pierre‐etienne.heudel@lyon.unicancer.fr |
| Notes | NCT02730923 Sponsor: Centre Leon Berard |
DLT: dose‐limiting toxicity ECOG: Eastern Cooperative Oncology Group ER: oestrogen receptor iv: intravenous NOS: not otherwise stated PFS: progression‐free survival PR: progesterone receptor OS: overall survival r: ribosomal RCT: randomised controlled trial RECIST: response evaluation criteria in solid tumours RNA: ribonucleic acid RT: radiotherapy
Differences between protocol and review
In the 'Secondary outcomes' section, we added treatment‐related death. This was defined as death due to the toxicity of the drug and not to disease progression.
In future updates of this review:
we will obtain a copy of the full article for each reference reporting a potentially eligible trial. Where this is not possible, we will try to contact authors to obtain additional information;
if there are multiple reports of the same study, we will collate the reports so that each study rather than each report is the unit of interest in the review;
one review author will 'spot‐check' study characteristics, data extraction and entry for accuracy against the trial report. In cases where an included study has multiple reports, we will maximise yield of information by collating all available data and will use the most complete data set aggregated across all known publications. We will give priority to the publication reporting the longest follow‐up associated with our review's primary or secondary outcomes;
if HRs are not reported, we will obtain them indirectly, using the methods described by Tierney 2007, using either other available summary statistics, or data extracted from published Kaplan‐Meier curves (Parmar 1998). In studies that do not report the relevant effect estimates and required curve extraction, we will adjust the numbers at risk based on estimated minimum and maximum follow‐up times. If these are not reported, we will estimate minimum follow‐up using the estimated time taken to complete treatment, and estimate maximum follow‐up using the last event reported in the relevant time‐to‐event curve (as given in Tierney 2007). We will record these follow‐up estimates in the 'Characteristics of included studies' table under 'Notes';
for continuous outcomes (e.g. quality‐of‐life measures), we will extract the final value and standard deviation of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms, and its standard error;
when interpreting treatment effects and meta‐analyses, we will take into account the risk of bias for the studies that contribute to that outcome. In addition, where information on risk of bias relates to unpublished data or correspondence with a trialist, we will note this in the 'Risk of bias' table;
we will express continuous outcomes (quality‐of‐life assessments) as the mean difference (MD) between treatment arms with a 95% CI. If the MD is reported without individual group data, we will use this to report the study results. If more than one study measures the same outcome using different tools, we will calculate the standardised mean difference (SMD) and 95% CI;
we will attempt to contact study authors to obtain missing data (participant, outcome, or summary data). For participant data, we will, where possible, conduct the analysis on an intention‐to‐treat basis; otherwise we will analyse data as reported. We will not impute missing outcome data;
if there is evidence of substantial clinical, methodological or statistical heterogeneity across included studies, we will not report pooled results from meta‐analysis but will instead use a narrative approach to synthesise the data. In this event, we will investigate and report the possible clinical or methodological reasons;
we will try to minimise potential reporting biases, including publication bias, multiple (duplicate) publication bias and language bias in this review, by conducting a sensitive search of multiple sources with no restriction by language. We will also search for ongoing trials and unpublished trials. If we include 10 studies or more investigating a particular outcome, we will examine funnel plots corresponding to meta‐analysis of the outcome to assess the potential for small‐study effects. We plan to assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform an exploratory analysis to investigate it;
for continuous outcomes, we will pool the mean differences (MDs) between the treatment arms at the end of follow‐up, if all trials measure the outcome on the same scale; otherwise we will pool standardised mean differences (SMDs). We will use the inverse variance method in random‐effects analysis;
if we are unable to pool the data statistically using meta‐analysis, we will conduct a narrative synthesis of results. We will present the major outcomes and results, organised by intervention categories according to the major types and/or aims of the identified interventions. Depending on the assembled research, we may also explore the possibility of organising the data by population. Within the data categories, we will explore the main comparisons of the review;
if sufficient data are available, we will consider undertaking the following subgroup analyses: advanced versus recurrent disease; different categories of PI3K/AKT/mTOR inhibitors; type 1 versus type 2 EC;
if adequate data are available, we will perform a sensitivity analysis by comparing studies at high or unclear risk of bias and at low risk of bias for allocation concealment for each outcome.
Contributions of authors
Draft the protocol: JM, MW, LM, FR and KL Study selection: FR and KL Extract data from studies: FR, MW, KL Enter data into RevMan: FR, MW Carry out the analysis: FR, MW, KL Interpret the analysis: FR, KL, MW, LM Draft the final review: FR, MW, KL, LM Disagreement resolution: LM Update the review: FR and KL
Sources of support
Internal sources
In‐kind support from the NHMRC Clinical Trials Centre, The University of Sydney, Other.
External sources
No sources of support supplied
Declarations of interest
Felicia Roncolato: none known Kristina Lindemann: none known Melina L Willson: none known Julie Martyn: none known Linda Mileshkin: none known
New
References
References to studies included in this review
Aghajanian 2018 {published data only}
- Aghajanian C, Filiaci V, Dizon DS, Carlson JW, Powell MA, Secord AA, et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecologic Oncology 2018;150(2):274‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian C, Filiaci VL, Dizon DS, Carlson J, Powell MA, Secord AA, et al. A randomized phase II study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus and ixabepilone/carboplatin/bevacizumab as initial therapy for measurable stage III or IVA, stage IVB or recurrent endometrial cancer, GOG‐86P. Journal of Clinical Oncology 2015;20:33 (Suppl 1). [Google Scholar]
- NCT00977574. Paclitaxel, carboplatin, and bevacizumab or paclitaxel, carboplatin, and temsirolimus or ixabepilone, carboplatin, and bevacizumab in treating patients with stage III, stage IV, or recurrent endometrial cancer. clinicaltrials.gov/show/NCT00977574 (first received 15 September 2009).
Oza 2015 {published data only}
- Mackay H, Welch S, Tsao MS, Biagi JJ, Elit L, Ghatage P, et al. Phase II study of oral ridaforolimus in patients with metastatic and/or locally advanced recurrent endometrial cancer: NCIC CTG IND 192. Journal of Clinical Oncology. 2011; Vol. 29:(15 Suppl), 5013.
- NCT00739830. Clinical trial of ridaforolimus compared to progestin or chemotherapy for advanced endometrial carcinoma (MK‐8669‐007 AM6). clinicaltrials.gov/show/NCT00739830 (first received 22 August 2008).
- Oza AM, Pignata S, Poveda A, McCormack M, Clamp A, Schwartz B, et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. Journal of Clinical Oncology 2015;33(3):3576‐81. [DOI] [PubMed] [Google Scholar]
- Oza AM, Poveda A, Clamp AR, Pignata S, Scambia G, Campo JM, et al. A randomized phase II (RP2) trial of ridaforolimus (R) compared with progestin (P) or chemotherapy (C) in female adult patients with advanced endometrial carcinoma. Journal of Clinical Oncology 2011;29:15 Suppl 1. [Google Scholar]
References to ongoing studies
NCT01935973 {published data only}
- NCT01935973. Trametinib with or without GSK2141795 in treating patients with recurrent or persistent endometrial cancer. clinicaltrials.gov/show/NCT01935973 (first received 5 September 2013).
NCT02065687 {published data only}
- NCT02065687. Paclitaxel and carboplatin with or without metformin hydrochloride in treating patients with stage III, IV or recurrent endometrial cancer. clinicaltrials.gov/show/NCT02065687 (first received 19 February 2014).
NCT02228681 {published data only}
- NCT02228681. Everolimus and letrozole or hormonal therapy to treat endometrial cancer. clinicaltrials.gov/show/NCT02228681 (first received 29 August 2014).
NCT02725268 {published data only}
- NCT020725268. Phase 2 study of MLN0128, combination of MLN0128 with MLN1117, paclitaxel and combination of MLN0128 with paclitaxel in women with endometrial cancer. clinicaltrials.gov/show/NCT02725268 (first received 31 March 2016).
NCT02730923 {published data only}
- NCT02730923. Hormone receptor positive endometrial carcina treated by dual mTORC1/mTORC2 inhibitor and anastrozole (VICTORIA). clinicaltrials.gov/show/NCT02730923 (first received 7 April 2016).
Additional references
Burris 2013
- Burris HA 3rd. Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemotherapy and Pharmacology 2013;71(4):829‐42. [DOI] [PubMed] [Google Scholar]
Cancer Genome Atlas Research Network 2013
- Cancer Genome Atlas Research Network, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497(7447):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colon‐Otero 2019
- Colon‐Otero G, Weroha J, Zanfagnin V, Foster NR, Asmus E, Hendrickson AE, et al. Results of a phase 2 trial of ribociclib and letrozole in patients with either relapsed estrogen receptor (ER)‐positive ovarian cancers or relapsed ER‐positive endometrial cancers. Journal of Clinical Oncology 2019;37(Suppl):Abstract no. 5510. [Google Scholar]
DeCruze 2007
- DeCruze SB, Green JA. Hormonal therapy in advanced and recurrent endometrial cancer: a systematic review. International Journal of Gynecological Cancer 2007;17(5):964‐78. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [DOI: 10.1002/9780470693926.ch15] [DOI] [Google Scholar]
Dellinger 2009
- Dellinger TH, Monk BJ. Systemic therapy for recurrent endometrial cancer: a review of North American trials. Expert Review of Anticancer Therapy 2009;9(7):905‐16. [DOI] [PubMed] [Google Scholar]
Dowling 2007
- Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin‐dependent translation initiation in breast cancer cells. Cancer Research 2007;67(22):10804‐12. [DOI] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. Endnote version X7. Thomson Reuters, 2013.
ESMO 2016
- Colombo N, Creutzberg C, Amant F, Bosse T, González‐Martín A, Ledermann J, et al. ESMO‐ESGO‐ESTRO Consensus Conference on endometrial cancer: diagnosis, treatment and follow‐up. Annals of Oncology 2016;27(1):16‐41. [DOI] [PubMed] [Google Scholar]
Fader 2009
- Fader AN, Nieves Arriba L, Frasure HE, Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecologic Oncology July 2009;114(1):121‐7. [DOI] [PubMed] [Google Scholar]
Ferlay 2010
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer 2010;127(12):2893‐917. [DOI] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. [DOI] [PubMed] [Google Scholar]
Fleming 2004
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2004;22(11):2159‐66. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org. GRADEproGDT: GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc). Available from www.gradepro.org, 2015.
Hecht 2006
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. Journal of Clinical Oncology 2006;24(29):4783‐91. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer Journal for Clinicians 2008;58(2):71‐96. [DOI] [PubMed] [Google Scholar]
Kassem 2016
- Kassem L, Abdel‐Rahman O. Targeting mTOR pathway in gynecological malignancies: biological rationale and systematic review of published data. Critical Reviews in Oncology/Hematology 2016;108:1‐12. [DOI] [PubMed] [Google Scholar]
Kokka 2010
- Kokka F, Brockbank E, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD007926.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mackay 2014
- Mackay HJ, Eisenhauer EA, Kamel‐Reid S, Tsao M, Clarke B, Karakasis K, et al. Molecular determinants of outcome with mammalian target of rapamycin inhibition in endometrial cancer. Cancer 2014;120(4):603‐10. [DOI] [PubMed] [Google Scholar]
Markman 2005
- Markman M. Hormonal therapy of endometrial cancer. European Journal of Cancer 2005;41(5):673‐5. [DOI] [PubMed] [Google Scholar]
Nevadunsky 2013
- Nevadunsky NS, Mbagwu C, Mizrahi N, Burton E, Goldberg GL. Pulmonary fibrosis after pegylated liposomal doxorubicin in a patient with uterine papillary serous carcinoma. Journal of Clinical Oncology 2013;31(10):e167‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicholas 2014
- Nicholas Z, Hu N, Ying J, Soisson P, Dodson M, Gaffney DK. Impact of comorbid conditions on survival in endometrial cancer. American Journal of Clinical Oncology 2014;37(2):131‐4. [DOI] [PubMed] [Google Scholar]
Ocana 2014
- Ocana A, Vera‐Badillo F, Al‐Mubarak M, Templeton AJ, Corrales‐Sanchez V, Diez‐Gonzalez L, et al. Activation of the PI3K/mTOR/AKT pathway and survival in solid tumors: systematic review and meta‐analysis. PLoS One 2014;9(4):e95219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Randall 2006
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole‐abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2006 Jan;24(1):36‐44. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schmandt 2011
- Schmandt RE, Iglesias DA, Co NN, Lu KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. American Journal of Obstetrics and Gynecology 2011;205(6):518‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schuler 2015
- Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, et al. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Medicine 2015;4(2):161‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SEER 2019
- Surveillance, Epidemiology and End Results Program (SEER). Cancer Stat Facts: Uterine cancer. seer.cancer.gov/statfacts/html/corp.html (accessed 12 September 2019).
Shaw 2006
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006;441(7092):424‐30. [DOI] [PubMed] [Google Scholar]
Slomovitz 2012
- Slomovitz BM, Coleman RL. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clinical Cancer Research 2012;18(21):5856‐64. [DOI] [PubMed] [Google Scholar]
Sorbe 2008
- Sorbe B, Andersson H, Boman K, Rosenberg P, Kalling M. Treatment of primary advanced and recurrent endometrial carcinoma with a combination of carboplatin and paclitaxel ‐ long‐term follow up. International Journal of Gynecological Cancer 2008 July/August;18(4):803‐8. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Weelden 2019
- Weelden WJ, Massuger LF, ENITEC, Pijnenborg JM, Romano A. Anti‐estrogen treatment in endometrial cancer: a systematic review. Frontiers in Oncology 2019;9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Gruenigen 2005
- Gruenigen VE, Gil KM, Frasure HE, Jenison EL, Hopkins MP. The impact of obesity and age on quality of life in gynecologic surgery. American Journal of Obstetrics and Gynecology 2005;193(4):1369‐75. [DOI] [PubMed] [Google Scholar]
Zakikhani 2010
- Zakikhani M, Blouin MJ, Piura E, Pollak MN. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Research and Treatment 2010;123(1):271‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Martyn 2016
- Martyn J, Roncolato F, Willson ML, Lindemann K, Mileshkin L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD012160] [DOI] [PMC free article] [PubMed] [Google Scholar]


