Abstract
Background
Chronic neck pain is a highly prevalent condition, affecting 10% to 24% of the general population. Transcutaneous electrical nerve stimulation (TENS) is the noninvasive, transcutaneous use of electrical stimulation to produce analgesia. It is a simple, low‐cost and safe intervention used in clinical practice as an adjunct treatment for painful musculoskeletal conditions that have a considerable impact on daily activities, such as chronic neck pain. This review is a split from a Cochrane Review on electrotherapy for neck pain, published in 2013, and focuses specifically on TENS for chronic neck pain.
Objectives
To evaluate the effectiveness of transcutaneous electrical nerve stimulation (TENS) (alone or in association with other interventions) compared with sham and other clinical interventions for the treatment of chronic neck pain.
Search methods
We searched Cochrane Back and Neck Trials Register, CENTRAL, MEDLINE, Embase, five other databases and two trials registers to 9 November 2018. We also screened the reference lists of relevant studies to identify additional trials. There were no language, source, or publication date restrictions.
Selection criteria
We included randomised controlled trials (RCTs) involving adults (≥ 18 years of age) with chronic neck pain (lasting > 12 weeks) that compared TENS alone or in combination with other treatments versus active or inactive treatments. The primary outcomes were pain, disability and adverse events.
Data collection and analysis
Two independent review authors selected the trials, extracted data and assessed the risk of bias of included studies. A third review author was consulted in case of disagreements. We used the Cochrane 'Risk of bias' tool (adapted by Cochrane Back and Neck), to assess the risk of bias of individual trials and GRADE to assess the certainty of evidence. We used risk ratios (RRs) to measure treatment effects for dichotomous outcomes, and mean differences (MDs) for continuous outcomes, with their respective 95% confidence intervals (CIs).
Main results
We included seven RCTs with a total of 651 participants, mean age 31.7 to 55.5 years, conducted in three different countries (Turkey, Jordan and China). The length of follow‐up ranged from one week to six months. Most RCTs used continuous TENS, with a frequency of 60 Hz to 100 Hz, pulse width of 40 μs to 250 μs and tolerable intensity, described as a tingling sensation without contraction, in daily sessions lasting 20 to 60 minutes. Due to heterogeneity in interventions and outcomes, we did not pool individual study data into meta‐analyses. Overall, we judged most studies as being at low risk for selection bias and high risk for performance and detection bias.
Based on the GRADE approach, there was very low‐certainty evidence from two trials about the effects of conventional TENS when compared to sham TENS at short‐term (up to 3 months after treatment) follow‐up, on pain (assessed by the Visual Analogue Scale (VAS)) (MD ‐0.10, 95% CI ‐0.97 to 0.77) and the percentage of participants presenting improvement of pain (RR 1.57, 95% CI 0.84 to 2.92). None of the included studies reported on disability or adverse events.
Authors' conclusions
This review found very low‐certainty evidence of a difference between TENS compared to sham TENS on reducing neck pain; therefore, we are unsure about the effect estimate. At present, there is insufficient evidence regarding the use of TENS in patients with chronic neck pain. Additional well‐designed, ‐conducted and ‐reported RCTs are needed to reach robust conclusions.
Keywords: Adult, Female, Humans, Male, Middle Aged, Chronic Pain, Chronic Pain/therapy, Neck Pain, Neck Pain/therapy, Pain Management, Pain Measurement, Randomized Controlled Trials as Topic, Transcutaneous Electric Nerve Stimulation, Transcutaneous Electric Nerve Stimulation/methods, Treatment Outcome
Plain language summary
Transcutaneous electrical nerve stimulation (TENS) for chronic neck pain
Review question
What are the benefits and harms of TENS for people with chronic (> 12 weeks) neck pain?
Background
Chronic neck pain is defined as any continuous pain in the region of the cervical spine that extends from the base of the head to the upper shoulder, lasting 12 weeks or more, usually associated with reduced neck movement. TENS is a popular treatment for chronic neck pain. It is based on the use of a device that delivers an electric current to the skin, to promote pain relief. Although TENS is widely used in clinical practice, there is a lack of evidence about its benefits and harms for people with chronic neck pain.
Search date
We included studies published up to 9 November 2018.
Study characteristics
We included seven studies that enrolled a total of 651 participants (mean age 31.7 to 55.5 years) with chronic neck pain. Each study included between 30 and 218 participants. The participants received TENS or a control intervention (placebo or another type of treatment). The studies were very different in terms of the duration of the TENS sessions (from 20 to 60 minutes), number of sessions (from 1 to 12) and total duration of the treatment programmes (from 1 to 45 days).
Key results
Because of the differences between each of the included studies, we decided that it would not be appropriate to combine their results. Out of the seven studies included, two reported that TENS was no better than inactive treatment (placebo) in reducing the participants' neck pain. None of the included studies assessed disability or adverse events.
Certainty of evidence
There was very low‐certainty evidence about the effects of TENS for treating chronic neck pain.
Summary of findings
Summary of findings for the main comparison. Transcutaneous electrical nerve stimulation (TENS) compared to sham TENS for chronic neck pain.
| TENS compared to sham TENS for chronic neck pain | ||||||
| Patient or population: adults (≥ 18 years of age) with chronic neck pain Setting: ambulatory Intervention: TENS Comparison: sham TENS | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with sham TENS | Risk with TENS | |||||
| Pain (at short‐term follow‐up) assessed with: VAS Scale from: 0 to 10 Follow‐up: mean 1 week | The mean pain (VAS) at short‐term follow‐up was 6.95 points | MD 0.10 points lower (0.97 lower to 0.77 higher) | ‐ | 38 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The evidence is uncertain about the effect of TENS on pain at short‐term follow‐up. |
| Pain (at short‐term follow‐up) assessed with: percentage of participants presenting improvement of pain Follow‐up: mean 1 week | Study population | RR 1.57 (0.84 to 2.92) | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The evidence is uncertain about the effect of TENS on pain at short‐term follow‐up. | |
| 467 per 1000 | 733 per 1000 (392 to 1000) | |||||
| Disability ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Disability was not reported in the included studies. |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Adverse events were not reported in the included studies. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; TENS: transcutaneous electrical nerve stimulation; VAS: Visual Analogue Scale | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels due to risk of bias (high risk on performance and detection bias). bDowngraded two levels for imprecision (small sample size and wide CI including null effect).
Background
Description of the condition
Chronic neck pain is a highly prevalent condition, affecting 10% to 24% of the general population (Chow 2009). It can also limit the daily activities of 11% to 14% of all workers annually (Hogg‐Johnson 2008), leading to work absenteeism and economic implications.
Neck pain is one of the main causes of work absenteeism and visits to healthcare professionals. Neck pain is defined as any specific pain located below the superior nuchal line and above the spine of the scapula line from the back, as well as above the superior border of the clavicle and the suprasternal notch (Guzman 2008; Monticone 2013). Although most cases of neck pain are generally acute and resolve spontaneously regardless of treatment (Viikari‐Juntura 2001), some patients go on to develop chronic neck pain, defined as continuous pain of 12 weeks or more usually associated with reduced range of neck movement. The exact cause of neck pain is obscure in most patients and treatment generally consists of interventions to control symptoms and prevent disability (Tsang 2001), without a specific treatment being recommended (Niemisto 2003). A glossary of terms can be found in Appendix 1.
Description of the intervention
Transcutaneous electrical nerve stimulation (TENS) is the noninvasive, transcutaneous, use of electrical stimulation to produce analgesia; it has been the subject of clinical research since it was developed in 1967 (Johnson 2016; Sluka 2003; Sluka 2013; Wall 1967). TENS is a portable and inexpensive device which generates mild pulsed electrical currents delivered across the skin surface to stimulate peripheral nerves through electrode pads (Johnson 2015). The frequency (pulses per second), intensity (pulse amplitude) and pulse duration (periods when the electrical current is deliver) settings can be adjusted, leading to different types of TENS being used in clinical practice (Johnson 2017; Khadilkar 2008; Sluka 2013). TENS frequency can be set at high, low or burst (bursts of high‐frequency stimulation applied at a much lower frequency) levels (Moran 2011; Sluka 2003). The intensity of the electrical pulse can be set at four different levels: subsensory, sensory, motor, and noxious (Allen 2006), depending on the patient's response.
It is important to obtain a positive pain response by adjusting the intensity of TENS. With sensory level (low intensity) TENS, the amplitude is increased until the patient feels a comfortable sensation without motor contraction. If the intensity is increased to produce motor contraction, it becomes motor level TENS. If motor level intensity is increased to the maximal level, it becomes noxious level (high intensity) TENS (Allen 2006; Bjordal 2003; Moran 2011; Sluka 2013). In general, high‐frequency TENS is applied at low intensities (conventional TENS). In contrast, low‐frequency TENS is typically applied at high intensities so that a motor contraction is produced (Sluka 2003). Sensory level TENS is the most widely‐used modality, although motor and noxious level TENS are recommended by some investigators for patients with chronic pain. Subsensory level TENS is the stimulation below the motor threshold (Cameron 2003).
Conventional TENS is applied at high frequency (from 50 Hz to 130 Hz), low intensity (comfortable, not painful) and small pulse duration (50 μs to 200 μs). This type of TENS is the most used in clinical practice and long‐term patients typically report that administering a higher frequency, nonpainful current at the site of pain is beneficial (Johnson 2007b; Johnson 2016; Sluka 2013). On the other hand, another TENS technique such as acupuncture‐like TENS (also called AL‐TENS), where electrodes were placed over acupuncture points, involves the application of low frequency (2 Hz to 4 Hz), higher intensity (tolerable to the patient) and longer pulse duration (100 μs to 400 μs). Low‐frequency bursts (2 Hz to 4 Hz) of high‐frequency pulses (100 Hz to 200 Hz) (burst TENS) are also used in clinical practice. Lastly, high frequencies (up to 200 Hz) with high intensities (intense TENS) are used for minor procedures and for short periods of time (Johnson 2007a). Modulated TENS applies stimulation across a range of frequencies and may help ameliorate development of tolerance to TENS (Gibson 2019; Sluka 2013).
The main contraindication to TENS use is in patients with pacemakers. Precautions include pregnant women or people with epilepsy, which requires positioning the electrodes to avoid thorax, abdomen, head and neck. Patients with active tumours also have restricted and careful use of TENS, besides those with fragility or skin disease (Johnson 2015).
How the intervention might work
The mechanism of action of TENS evolved from Shealy’s developmental work on neuromodulation techniques in the 1960s (Shealy 1967), which was underpinned by 'gate control theory of pain' (Melzack 1965), one of the theories to explain the inhibition of pain signals (Johnson 2007a; Sluka 2003). As proposed by this theory, TENS produces an activation of inhibitory interneurons in the substantia gelatinosa in the dorsal horn of the spinal cord by the electric stimulation of large diameter fibres (A‐beta‐fibres), which inhibit the transmission of nociceptive signals from small diameter fibres (A‐delta and C). The other postulated mechanism of pain relief mediated by TENS include the promotion of endorphin release, leading to a vasodilatation in injured tissue (Han 1991; Hughes 1984; Kalra 2001; Sjolund 1976; Sluka 2003).
The physiological response of TENS is dependent on the frequency and intensity of the treatment. Thus, the use of conventional TENS (high frequency and low intensity) selectively activates non‐noxious cutaneous afferents (A‐beta‐fibres), leading to a strong and comfortable sensation through the electrodes when intensity is slightly increased. The purpose of acupuncture‐like TENS is to stimulate afferent nerve fibres of small diameter in the muscles by means of current‐induced pulsate sensations in the skin, leading to the activation of the descending inhibitory pathways of pain (Johnson 2007b; Johnson 2015; Johnson 2017).
There are major controversies regarding the effectiveness of TENS, including its possible placebo effect, since it is almost impossible to blind patients during treatment. Moreover, investigators seldom specify the exact parameters of stimulation and often use different equipment configurations, and electrode placement varies considerably between studies (Sluka 2003).
Why it is important to do this review
Despite the lack of evidence to support its effectiveness, TENS is a simple, low‐cost and safe intervention with limited potential for toxicity; it is used in clinical practice as an adjunct treatment for painful musculoskeletal conditions that have a considerable impact on daily activities, such as chronic neck pain (Gibson 2019; Johnson 2016; Nnoaham 2008). Previous systematic reviews assessed the effects of TENS in a wide range of clinical conditions, and most of them showed inconclusive results due to low certainty evidence. This review is a split from another Cochrane Review on electrotherapies for neck pain (Kroeling 2013), and focuses specifically on TENS for chronic neck pain.
Objectives
To evaluate the effectiveness of transcutaneous electrical nerve stimulation (TENS) (alone or in association with other interventions) compared with sham and other clinical interventions for the treatment of chronic neck pain.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) regardless of publication status (published, unpublished or ongoing). The first phase of cross‐over trials was also eligible for inclusion.
Types of participants
We included trials that recruited adults (≥ 18 years of age) with chronic neck pain (lasting longer than 12 weeks) with the following conditions.
Neck pain without specific cause, whiplash‐associated disorder (WAD) category I and II (Guzman 2008; Spitzer 1987; Spitzer 1995), myofascial pain syndrome in the upper trapezius muscle region, and neck pain associated with degenerative changes (Schumacher 1993).
Cervicogenic headache (Olesen 1988; Olesen 1997; Sjaastad 1990).
Neck disorders with radicular findings (Rubinstein 2007), including degenerative joint or disc disease with spinal stenosis, spondylolisthesis, or discogenic radiculopathy; WAD category III (Spitzer 1987; Spitzer 1995).
We excluded studies if they included participants with:
definitive or possible long tract signs (e.g. myelopathies);
neck pain caused by other pathological entities (i.e. head and neck cancer and fibromyalgia) (Schumacher 1993);
headache not of cervical origin, but associated with the neck;
coexisting headache when either neck pain was not dominant or the headache was not provoked by neck movements or sustained neck postures; or
'mixed' headache, which includes more than one headache classification;
myofascial pain restricted to lower trapezius muscle region (shoulder pain).
We included studies that recruited patients with chronic and non‐chronic neck pain, and studies including chronic pain in different anatomical regions (i.e. back, neck, shoulder, legs), only when the results were presented separately for the subgroup of interest for this review.
Types of interventions
We included studies that used any conventional mode of transcutaneous electrical nerve stimulation (TENS) as the intervention in at least one group, alone or associated with another active therapy (this active therapy must be presented also as a control group). The TENS should be applied to the cervical region and not be used together with acupuncture needles (acupuncture TENS). The following were accepted as comparators: sham TENS, waiting list control, other active treatment (pharmacological or not) or no intervention.
The possible comparisons were:
TENS versus inactive intervention (placebo, sham TENS, no intervention or waiting list control);
TENS versus other interventions;
TENS in addition to another intervention versus the other intervention alone.
Types of outcome measures
We included and reported any study that fulfilled our inclusion criteria even if the study did not consider any of our planned outcomes. The outcomes should have been measured using a validated tool. When available, adverse events were also described. The duration of the follow‐up period was defined as:
Immediately post‐treatment: up to one day
Short‐term: more than one day and up to three months
Intermediate‐term: more than three months and up to one year
Long‐term: more than one year
Primary outcomes
Pain (e.g. Visual Analogue Scale (VAS), assessed as dichotomous or continuous data)
Disability (e.g. Neck Disability Index (NDI), assessed as dichotomous or continuous data)
Adverse events
Secondary outcomes
Quality of life (e.g. Short Form‐36 (SF‐36))
Range of motion
Global perceived effect
Use of medication for pain
Work disability
Patient satisfaction
Search methods for identification of studies
Electronic searches
We searched the following databases from inception to 9 November 2018 without language restrictions.
Cochrane Back and Neck Trials Register (Cochrane Register of Studies (CRS)).
Cochrane Central Register of Controlled Trials (CENTRAL, searched using CRS Web).
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) (OvidSP, 1946 to 9 November 2018).
Embase (OvidSP, 1980 to 2018 Week 45).
CINAHL (EBSCO, 1981 to 9 November 2018).
Latin American and Caribbean Health Sciences Literature (LILACS, 1982 to 9 November 2018).
Physiotherapy Evidence Database (PEDro, inception to 9 November 2018).
PubMed (15 December 2015).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP).
ClinicalTrials.gov (ClinicalTrials.gov).
System for Information on Grey Literature in Europe (OpenSIGLE).
In 2015 we searched PubMed for studies not in MEDLINE using the strategy by Duffy 2014. In 2017 we began searching MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) which allows multiple MEDLINE databases to be searched through one Ovid interface. In 2018, we searched CENTRAL and Cochrane Back and Neck Trials Registers in CRS Web; previously they were searched in CRS stand alone desktop database. Search strategies can be found in Appendix 2.
Searching other resources
We handsearched the reference lists of relevant studies.
Data collection and analysis
Selection of studies
Two review authors (GJMP, ALCM) independently screened all titles and abstracts retrieved by the search strategy for eligibility. Those deemed potentially relevant were retrieved for full‐text assessment by the same authors (GJMP, ALCM) who assessed whether the reports fulfilled the selection criteria. We recorded the reasons for exclusion in the 'Characteristics of excluded studies' table. When necessary, a third review author (RR) resolved any disagreements regarding study inclusion. We used a PRISMA flowchart (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) to summarize the results of the search and the study selection process (Liberati 2009).
Data extraction and management
Two review authors (GJMP, ALCM) independently extracted the data from the primary studies using a standard data extraction form to collect the following details.
Participants: number of participants, age, gender, baseline functional data; inclusion and exclusion criteria.
Methods: diagnostic criteria, number of patients randomised, number of patients analysed.
Interventions: description of interventions and controls including duration and frequency of sessions; frequency of stimulation (high, low or burst), intensity of stimulation (subsensory, sensory, motor, and noxious), pulse duration settings and presence of cointerventions.
Outcomes: as listed under Types of outcome measures.
We recorded the methods used for measuring the outcomes for a subsequent analysis.
Assessment of risk of bias in included studies
Two review authors (GJMP, ALCM) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted by Cochrane Back and Neck (Furlan 2015; Table 2; Table 3). Any disagreement was resolved by a third review author (RR). We assessed the risk of bias according to the following domains.
1. Sources of risk of bias.
| Bias domain | Source of bias | Possible answers |
| Selection | (1) Was the method of randomisation adequate? | Yes/no/unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/no/unsure |
| Performance | (3) Was the participant blinded to the intervention? | Yes/no/unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/no/unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/no/unsure |
| Attrition | (6) Was the dropout rate described and acceptable? | Yes/no/unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they were allocated? | Yes/no/unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/no/unsure |
| Selection | (9) Were the groups similar at baseline regarding the most important prognostic indicators? | Yes/no/unsure |
| Performance | (10) Were cointerventions avoided or similar? | Yes/no/unsure |
| Performance | (11) Was the compliance acceptable in all groups? | Yes/no/unsure |
| Detection | (12) Was the timing of the outcome assessment similar in all groups? | Yes/no/unsure |
| Other | (13) Are other sources of potential bias unlikely? | Yes/no/unsure |
(based on Furlan 2015)
2. Criteria for a judgement of 'yes' for the sources of risk of bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered sealed envelopes, sequentially‐ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the participants. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the participant. |
| 3 | Index and control groups are indistinguishable for the participants or if the success of blinding was tested among the participants and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored 'yes' if the success of blinding was tested among the outcome assessors and it was successful or:
|
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias a 'yes' is scored. (NB these percentages are arbitrary, not supported by literature). |
| 7 | All randomised participants are reported/analysed in the group they were allocated to by randomisation for the most important moments of effect measurement (minus missing values) irrespective of noncompliance and cointerventions. |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgement. |
| 9 | Groups have to be similar at baseline regarding demographic factors, duration and severity of complaints, percentage of participants with neurological symptoms, and value of main outcome measure(s). |
| 10 | If there were no cointerventions or they were similar between the index and control groups. |
| 11 | The reviewer determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore it is necessary to assess how many sessions each patient attended. For single session interventions (e.g. surgery), this item is irrelevant. |
| 12 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 13 | Other types of biases. Examples as follows.
|
COI: conflict of interest
(based on Furlan 2015)
Selection bias (random sequence generation and allocation concealment).
Performance bias (blinding of participants and personnel).
Detection bias (blinding of outcome assessors).
Attrition bias (incomplete outcome data).
Reporting bias (selective reporting).
We classified the risk of bias as low, high or unclear (Higgins 2011).
Measures of treatment effect
We used risk ratios (RRs) to analyse dichotomous data and mean differences (MDs) for continuous data. We calculated 95% confidence intervals (CIs), for both cases. We planned to use the standardised mean difference (SMD), with 95% CI, when different scales were used to evaluate the same outcome. We also planned to analyse the counting and rates data as a single 'pair‐wise' analysis to avoid double‐counting of subjects, however, we did not find these data.
Unit of analysis issues
We considered the individual patient to be the unit of analysis. We excluded cluster trials. In cross‐over trials, we only considered the first phase of the study (before crossing).
Dealing with missing data
We contacted authors of studies in the case of missing data regarding methods, participants, interventions and/or outcomes. In cases where no answer was obtained from the authors, we presented the information narratively. We planned to impute data when standard deviations (SDs) for outcomes were not reported, assuming the SD of the missing outcome to be the average of the SDs from those studies. However, this was not necessary. We also planned to conduct both complete case analysis and intention‐to‐treat analysis for dichotomous data of primary outcomes, but this was not necessary.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using the Chi2 and the I2 statistic and clinical heterogeneity in a subgroup analysis. This was not done because we did not conduct a meta‐analysis. We defined P < 0.10 as evidence of statistical heterogeneity and an I2 value greater than 50% as indicative of significant statistical heterogeneity (Higgins 2011).
Assessment of reporting biases
We planned to use a funnel plot to explore the likelihood of reporting bias in meta‐analyses with 10 or more trials. This was not done because we did not conduct any meta‐analyses. We also planned to perform exploratory analyses to investigate possible reasons for visual asymmetry of the funnel plot (chance, publication bias, and true heterogeneity).
Data synthesis
We planned to combine the outcome measures from individual trials through meta‐analysis using a random‐effects model as we expected clinical and methodological heterogeneity in the included trials. This was not possible because of lack of data and the data were described qualitatively. The results from clinically comparable trials were described separately.
We used the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and adapted in the Cochrane Back and Neck method guidelines (Furlan 2015), to asses the overall certainty of the evidence for all outcomes. Factors that may decrease the certainty of the evidence are: study design and risk of bias, inconsistency of results, indirectness (not generalisable), imprecision (sparse data) and other factors (e.g. reporting bias). We reduced the certainty of the evidence for a specific outcome by a level, according to the performance of the studies against the five factors, described in Appendix 3.
'Summary of findings' table
We created a 'Summary of findings' table following the methods and recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), using GRADEpro GDT (GRADEpro GDT 2015). We included all primary outcomes for the comparison TENS versus sham TENS, at short‐term follow‐up (between 1 day and 3 months after completion of treatment).
Subgroup analysis and investigation of heterogeneity
We could not perform subgroup analysis due to lack of data. Our planned analysis can be found in Table 4.
3. Planned subgroup analysis and investigation of heterogeneity.
| Planned subgroup analysis and investigation of heterogeneity |
We planned to investigate heterogeneity and perform subgroup analyses considering the following factors.
In the presence of two or more subgroups, we planned to considered the I2 statistic to assess the heterogeneity among them. This statistic describes the percentage of the variability in effect estimates from the different subgroups that is due to genuine subgroup differences rather than sampling error (chance). We considered an I2 statistic of 50% or higher as suggestive of substantial heterogeneity among subgroups. |
TENS: transcutaneous electrical nerve stimulation
Sensitivity analysis
We could not perform sensitivity analysis due to lack of data. Our planned analysis can be found in Table 5.
4. Planned sensitivity analysis.
| Planned sensitivity analysis |
We planned to perform the following sensitivity analyses to assess the impact of:
|
RCT: randomised controlled trial
Results
Description of studies
The detailed description of the included studies can be found in Characteristics of included studies. We contacted all authors for additional information and only one replied (Acedo 2015).
Results of the search
The search retrieved 2235 records. After removing duplicates, we screened the titles and abstracts of 1846 records and selected 40 records (36 studies) as potentially eligible. After reading the full texts, we excluded 17 studies (see Characteristics of excluded studies) and retained 19 studies (23 records). Ten studies (12 records) are awaiting classification (see Characteristics of studies awaiting classification), and two studies are ongoing (see Characteristics of ongoing studies). We included in the review a total of seven studies, reported in nine records (2 ancillary records of primary studies). The flow diagram of the process of study identification and selection is presented in Figure 1.
1.
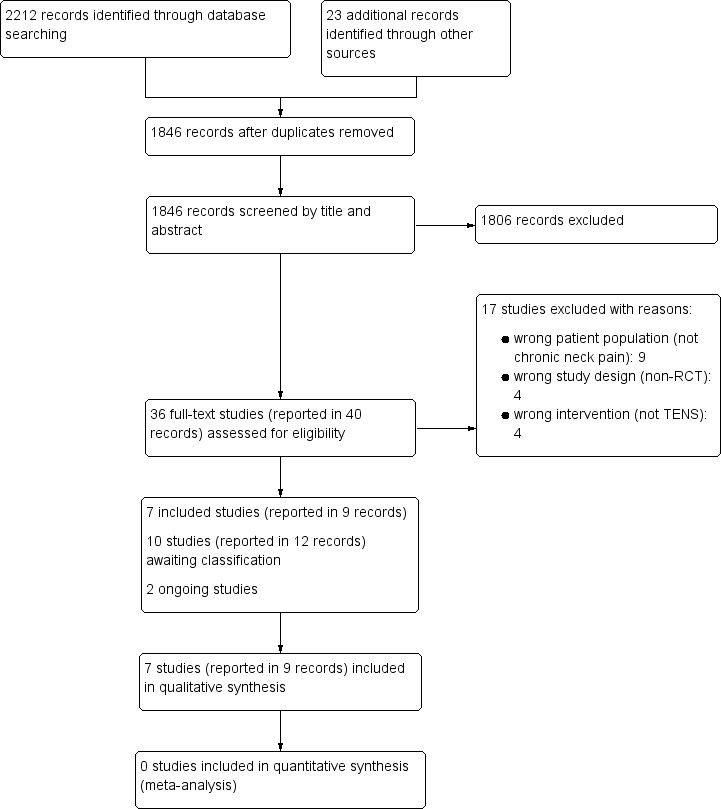
Study flow diagram.
Included studies
See: Characteristics of included studies.
Setting
Six out of seven studies were single centre trials, carried out in three different countries: Turkey (Azatcam 2016; Gul 2009; Sahin 2011), Jordan (Maayah 2010), and China (Chiu 2005; Chen 2007). One was a multicentre study conducted in Turkey (Yesil 2018).
Design of the studies
All included studies were randomised controlled trials (RCTs) with parallel design (Azatcam 2016; Chen 2007; Chiu 2005; Gul 2009; Yesil 2018; Maayah 2010; Sahin 2011).
Participants
A total of 651 participants with mean age ranging from 31.7 in Sahin 2011 to 55.5 years in Maayah 2010 were enrolled in the seven included trials. All studies included participants with more than 12 weeks of neck pain. Three studies reported the mean duration of disease: 21.82 (± 13.28) months (Azatcam 2016), 18.51 (± 8.43) months (Chen 2007), and 21.70 (± 16.69) months (Yesil 2018). Four studies included participants with non‐specific neck pain (Chiu 2005; Yesil 2018; Maayah 2010; Sahin 2011), two studies included participants with myofascial pain syndrome (Azatcam 2016; Gul 2009), and one study included participants with cervicogenic headache (Chen 2007).
Interventions
The seven trials used transcutaneous electrical nerve stimulation (TENS) with the following parameters (Table 6).
5. TENS schemes and dosages reported in the studies included.
| Study ID | TENS mode | Frequency (Hz) | Pulse waveform | Pulse width (μs) | Intensity (mA) | Duration of session | Total of sessions | Electrodes location | |
| Azatcam 2016 | Convetional | 60 | Symmetrical, biphasic rectangular |
100 | Comfortable sensation without contraction | 20 minutes daily | 10 | One negative electrode placed on upper trapezius muscle and one positive electrode placed on acromial tendon | |
| Chen 2007 | Conventional | 100 | Symmetrical, biphasic rectangular |
250 | Comfortable sensation without contraction | 20 minutes daily | 10 | Two electrodes placed on each side of upper cervical vertebra | |
| Chiu 2005 | Conventional | 80 | Symmetrical, monophasic rectangular |
150 | Comfortable sensation without contraction | 30 minutes twice a week | 10 | Four electrodes were placed on the following acupuncture points: neck, upper trapezius and elbow | |
| Gul 2009 | Conventional Burst TENS Modulated I Modulated II |
60‐100 2‐4 100 100 |
Not reported | Not reported | 60‐100 150‐250 150‐200 150‐200 |
20 minutes daily 30 minutes daily 20 minutes daily 20 minutes daily |
60 | Not reported | |
| Maayah 2010 | Burst TENS | 4‐8 | Not reported | Not reported | Comfortable sensation without contraction | 60 minutes | 1 | Two electrodes were placed on acupuncture points around the neck | |
| Sahin 2011 | Conventional Burst TENS Acupuncture‐like TENS |
100 High (100) and low (2) 4 |
Not reported | 40 40 250 |
Comfortable sensation without contraction Comfortable sensation without contraction Intensity at a level of muscle contraction |
30 minutes daily | 10 | Four electrodes on the trigger points bilaterally around the neck | |
| Yesil 2018 | Conventional | 80 | Not reported | Not reported | Comfortable sensation without contraction (10‐30 mA) | 25 minutes daily | 15 | Four electrodes on painful region in the neck |
TENS: transcutaneous electrical nerve stimulation
Mode: all studies used conventional TENS and one study used also burst TENS and acupuncture‐like TENS (TENS applied over acupuncture points) (Sahin 2011).
Duration of sessions: five studies used TENS for 15 to 30 minutes (Azatcam 2016; Chen 2007; Chiu 2005; Yesil 2018; Sahin 2011), and one study used TENS for 20 to 30 minutes (Gul 2009). Only one study used TENS for one hour (Maayah 2010).
Number of sessions: five studies had 10 to 15 sessions (Azatcam 2016; Chen 2007; Chiu 2005; Yesil 2018; Sahin 2011), one study had a single session of TENS (Maayah 2010), and one study, 60 sessions (Gul 2009).
Duration of the treatment programmes: one day (single session of TENS) (Maayah 2010), two weeks (Azatcam 2016), three weeks (Yesil 2018), four weeks (Chen 2007; Gul 2009; Sahin 2011), and six weeks (Chiu 2005).
The seven trials used different comparators, as follows.
Sham TENS: two studies (Maayah 2010; Sahin 2011).
Neck exercises: two studies (Chiu 2005; Yesil 2018).
Kinesio taping: one study (Azatcam 2016).
Manipulation treatment: one study (Chen 2007).
Low‐level laser: one study (Gul 2009).
Lidocaine injection 2 mL: one study (Gul 2009).
Botulinum toxin‐A injection 25 U: one study (Gul 2009).
Two studies tested TENS combined with another intervention versus the same intervention alone: TENS added to infrared (Chiu 2005), and TENS added to trapezius stretching exercise (Azatcam 2016).
Four studies had multiple comparison groups: Azatcam 2016 and Chiu 2005 had three groups, and Gul 2009 and Sahin 2011, four groups.
Outcomes
The following outcomes of interest were reported by the included studies.
Pain: seven studies (Azatcam 2016; Chen 2007; Chiu 2005; Gul 2009; Maayah 2010; Sahin 2011; Yesil 2018).
Disability: three studies (Azatcam 2016; Chiu 2005; Yesil 2018).
Use of medication for pain: three studies (Chiu 2005; Maayah 2010; Yesil 2018).
Range of motion: three studies (Azatcam 2016; Chen 2007; Yesil 2018).
Work disability: one study (Chiu 2005).
Quality of life: one study (Yesil 2018).
None of the included studies reported on adverse events. The length of follow‐up ranged from one week in Maayah 2010 and Sahin 2011 to six months in Chiu 2005.
Excluded studies
We excluded 17 studies because: they did not include participants with chronic neck pain (Airaksinen 1992; Bloodworth 2004; Farina 2004; Gemmell 2011; Kim 2014; Prabhakar 2011; Rodriguez‐Fernandez 2011; Salim 1996; Smania 2005), did not include TENS as an intervention (Hurwitz 2002; Jordan 1998; Lee 1997; Seo 2013), or were not randomised clinical trials (Chee 1986; Kruger 1998; Mysliwiec 2011; Simons 2006). The detailed reasons for exclusion of each study are presented in Characteristics of excluded studies.
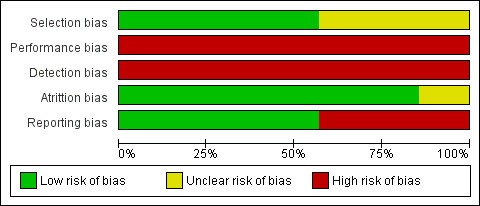
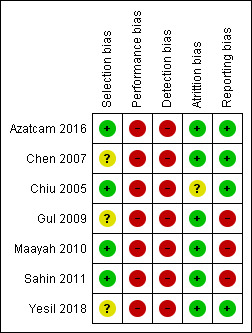
Risk of bias in included studies
Figure 2 and Figure 3 present the results of the 'Risk of bias' assessments. The overall risk of bias was low as we judged most of the studies as having unclear risk of bias for random sequence generation, allocation concealment and compliance; as well as a high risk of bias for blinding of participants, personnel and outcome assessors.
2.
3.
Allocation
Two studies reported the use of adequate methods for randomisation and allocation concealment (a computer‐generated random method and sealed and opaque envelopes prepared by a person who did not know the purpose of the study). We classified both studies as having a low risk of bias (Chiu 2005; Sahin 2011). We judged two studies as having a low risk of bias for sequence generation because they used a random numbers table (Azatcam 2016; Maayah 2010), but we judged them as having an unclear risk of bias for allocation concealment due to lack of information. The overall risk of bias for selection bias domain was low.
Blinding
Two studies compared TENS versus sham TENS (application of electrodes on the skin without delivering electrical current) (Maayah 2010; Sahin 2011). Maayah 2010 reported an adequate method to blind participants by specifying that participants were TENS‐naive (low risk of bias). Sahin 2011 did not report if the participants were naive in relation to the use of TENS; we therefore judged this study as having an unclear risk of bias for blinding participants (performance bias). We judged blinding of personnel and providers as high risk for both studies.
We classified the other studies as having a high risk of bias due to the nature of the compared interventions (e.g. manipulation therapy, Kinesio taping). Despite the impossibility of blinding the personnel in this scenario, we judged all these studies as having a high risk of bias (performance bias) considering both: (a) that the impossibility of blinding does not nullify the bias, and (b) that the outcomes can be considered subjective (Azatcam 2016; Chen 2007; Chiu 2005; Gul 2009; Yesil 2018).
Only Sahin 2011 described adequate methods to blind outcome assessors and we judged it as having a low risk of bias for this domain. Four studies reported the method for masking outcome assessors (Azatcam 2016; Chiu 2005; Maayah 2010; Yesil 2018), however, the primary outcomes (pain and disability) were patient‐dependent and there was no blinding of the participants. We therefore judged these studies as having a high risk of detection bias. Finally, two studies did not provide information (Chen 2007; Gul 2009), and we classified them as having an unclear risk of bias for blinding of outcome assessors (detection bias).
The overall risk of bias for performance and detection bias domains were high.
Incomplete outcome data
We judged all studies, apart from one, as having a low risk of attrition bias because they had few losses (< 20% for short‐term and 30% for long‐term follow‐up), the losses were balanced between the groups and the authors reported the reasons for the losses. We only considered one study as having an unclear risk of bias (Chiu 2005). It reported the loss of 16.5% of its participants, the distribution was different between groups, and it was not clear if these differences were relevant. Therefore, the overall risk of bias for attrition was low.
Selective reporting
None of the included studies presented available protocols and we judged them as having an unclear risk of bias for this domain. The overall risk for the reporting bias domain was unclear.
Other potential sources of bias
We did not identify any other potential bias and for this reason we rated this domain at low risk of bias.
Effects of interventions
See: Table 1
We could not pool the included studies data in meta‐analysis due to heterogeneity between comparisons and outcomes reported. Therefore, we described the results of the studies in a descriptive form.
Overall, we are uncertain regarding the effects of TENS for all included primary and secondary outcomes. In the three comparisons conducted by included studies and detailed below, the evidence was based on small studies and confidence intervals (CIs) (when it was possible to calculate) were wide for most analyses. The certainty of evidence was very low for all outcomes in all comparisons, downgraded due to risk of bias (performance and detection bias) and imprecision (small sample size and wide CIs).
Comparison 1: TENS versus sham TENS
Primary outcomes
Pain
Based on the results from one study (Sahin 2011), the evidence is uncertain about the effects of conventional TENS on pain reduction, when compared to sham TENS (mean difference (MD) ‐0.10, 95% CI ‐0.97 to 0.77; 38 participants; very low‐certainty evidence; Table 1). Other types of TENS also did not present a relevant difference when compared to sham TENS: burst TENS (MD ‐0.85, 95% CI ‐1.95 to 0.25) and acupuncture‐like TENS (MD ‐0.40, 95% CI ‐1.22 to 0.42) (Analysis 1.1). We downgraded the certainty of evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide CIs).
1.1. Analysis.
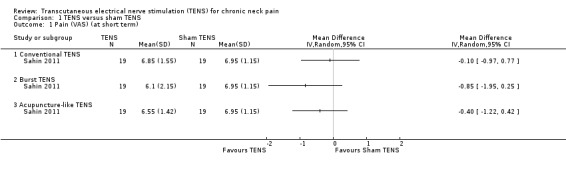
Comparison 1 TENS versus sham TENS, Outcome 1 Pain (VAS) (at short term).
Another small study also resulted in uncertain evidence on the effects of TENS (Maayah 2010), when compared to sham TENS, on pain at short‐term follow‐up (risk ratio (RR) 1.57, 95% CI 0.84 to 2.92; 30 participants; very low‐certainty evidence; Analysis 1.2; Table 1). Additionally, the authors reported that there was no difference in pain threshold measurements (myometer score) at short‐term follow‐up (MD 3.60, 95% CI ‐3.44 to 10.64; very low‐certainty evidence; Analysis 1.3), but the CI of this analysis is very wide and an important difference on effect cannot be ruled out. We downgraded the certainty of evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide CIs).
1.2. Analysis.
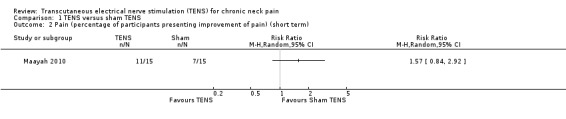
Comparison 1 TENS versus sham TENS, Outcome 2 Pain (percentage of participants presenting improvement of pain) (short term).
1.3. Analysis.
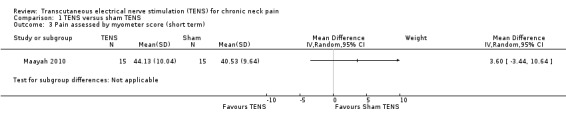
Comparison 1 TENS versus sham TENS, Outcome 3 Pain assessed by myometer score (short term).
Disability
None of the studies reported this outcome.
Adverse events
None of the studies reported this outcome.
Secondary outcomes
Quality of life
One study reported a subset of quality of life and found no differences between conventional versus sham TENS (Sahin 2011), or between Burst TENS versus sham at short‐term follow‐up (38 participants; very low‐certainty evidence; no numerical data were provided to compute CIs). We downgraded the certainty of evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
Range of motion
None of the studies reported this outcome.
Global perceived effect
None of the studies reported this outcome.
Use of medication for pain
The registry of drug intake was recorded by one study with 30 participants (Maayah 2010). The authors report no differences between the TENS and sham groups at short‐term follow‐up (very low‐certainty evidence; no numerical data were provided to compute CIs). We downgraded the certainty of evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
Work disability
None of the studies reported this outcome.
Patient satisfaction
None of the studies reported this outcome.
Comparison 2: TENS versus other interventions
Primary outcomes
Pain
TENS versus neck exercises
Results from Chiu 2005 showed improvement in Visual Analogue Scale (VAS) favouring neck exercises at short‐term follow‐up (MD 1.32, 95% CI 0.67 to 1.97; 151 participants; very low‐certainty evidence; Analysis 2.1). However, there was no important difference between groups at intermediate‐term follow‐up (MD 0.34, 95% CI ‐0.40 to 1.08; very low‐certainty evidence; Analysis 2.2). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
2.1. Analysis.
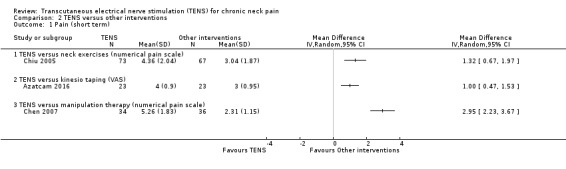
Comparison 2 TENS versus other interventions, Outcome 1 Pain (short term).
2.2. Analysis.
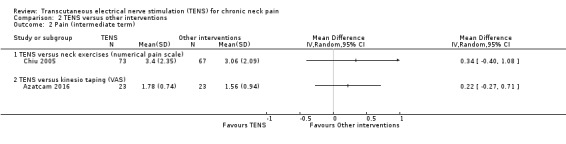
Comparison 2 TENS versus other interventions, Outcome 2 Pain (intermediate term).
TENS versus Kinesio taping
Azatcam 2016 presented improvement in numerical pain scale favouring Kinesio taping at short‐term follow‐up (MD 1.00, 95% CI 0.47 to 1.53; 46 participants; very low‐certainty evidence; Analysis 2.1), but not at intermediate‐term follow‐up (MD 0.22, 95% CI ‐0.27 to 0.71; 46 participants; very low‐certainty evidence; Analysis 2.2). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
TENS versus neck manipulation therapy
Chen 2007 presented an important improvement in numerical pain scale favouring manipulation therapy at short‐term follow‐up (MD 2.95, 95% CI 2.23 to 3.67; 70 participants; very low‐certainty evidence; Analysis 2.1). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
TENS versus botulinum toxin‐A
Gul 2009 (50 participants) reported a difference in favour of botulinum toxin at short‐term follow‐up (mean VAS = 4.6 versus 3.0; P < 0.01; very low‐certainty evidence). However, no additional numerical data (e.g. SD) were provided to compute CIs. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
TENS versus low‐level laser
Gul 2009 (50 participants) reported a difference in favour of TENS at short‐term follow‐up (mean VAS = 4.6 versus 5.4; P < 0.010; very low‐certainty evidence). However, no additional numerical data (e.g. SD) were provided to compute CIs. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
TENS versus lidocaine
Gul 2009 (50 participants) reported a difference in favour of lidocaine at short‐term follow‐up (mean VAS = 4.6 versus 3.7 points; very low‐certainty evidence). However, no additional numerical data (e.g. SD or P value) were provided to compute CIs. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
Disability
TENS versus neck exercises
Results from Chiu 2005 showed an improvement in disability in favour of TENS, assessed by the Northwick Park Neck Pain Questionnaire at short‐term follow‐up (MD 0.17, 95% CI 0.02 to 0.32; 151 participants; very low‐certainty evidence; Analysis 2.3), and a small or no improvement in favour of TENS at intermediate‐term follow‐up (MD 0.17, 95% CI ‐0.01 to 0.35; 151 participants; very low‐certainty evidence; Analysis 2.4). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
2.3. Analysis.
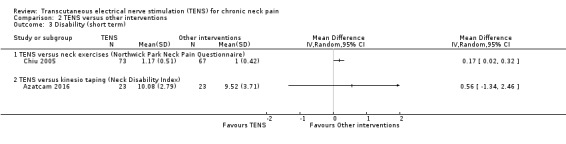
Comparison 2 TENS versus other interventions, Outcome 3 Disability (short term).
2.4. Analysis.
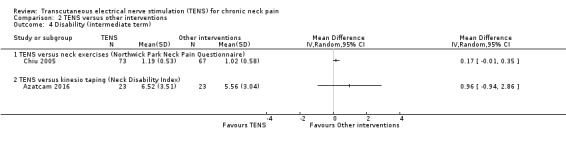
Comparison 2 TENS versus other interventions, Outcome 4 Disability (intermediate term).
TENS versus Kinesio taping
Azatcam 2016 found little to no difference between groups in the Neck Disability Index at short‐term (MD 0.56, 95% CI ‐1.34 to 2.46; 46 participants; very low‐certainty evidence; Analysis 2.3); and intermediate‐term follow‐up (MD 0.96, 95% CI ‐0.94 to 2.86; very low‐certainty evidence; Analysis 2.4). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
Adverse events
None of the studies reported this outcome.
Secondary outcomes
Quality of life
None of the studies reported this outcome.
Range of motion
TENS versus Kinesio taping
Azatcam 2016 found little to no difference between groups in cervical range of motion (lateral flexion) at short‐term follow‐up (MD ‐0.20, 95% CI ‐0.67 to 0.27; 46 participants; very low‐certainty evidence; Analysis 2.9). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
2.9. Analysis.
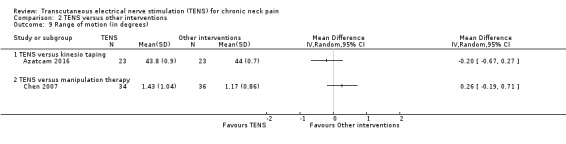
Comparison 2 TENS versus other interventions, Outcome 9 Range of motion (in degrees).
TENS versus neck manipulation therapy
Chen 2007 found little to no difference between groups in cervical range of motion at short‐term follow‐up (MD 0.26, 95% CI ‐0.19 to 0.71; 70 participants; very low‐certainty evidence; Analysis 2.9). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
Range of motion
None of the studies reported this outcome.
Global perceived effect
None of the studies reported this outcome.
Use of medication for pain
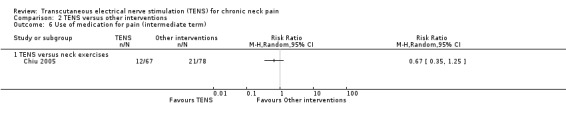
Chiu 2005 reported no difference between TENS and neck exercises in reducing the use of pain medication at short and intermediate‐term follow‐up (RR 0.71, 95% CI 0.36 to 1.40; 151 participants; very low‐certainty evidence (Analysis 2.5); RR 0.67, 95% CI 0.35 to 1.25; 151 participants; very low‐certainty evidence (Analysis 2.6), respectively). These confidence intervals are also wide and important risk reductions/increase cannot be ruled out. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
2.5. Analysis.
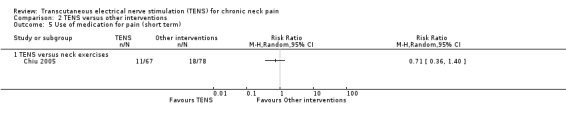
Comparison 2 TENS versus other interventions, Outcome 5 Use of medication for pain (short term).
2.6. Analysis.
Comparison 2 TENS versus other interventions, Outcome 6 Use of medication for pain (intermediate term).
Work disability
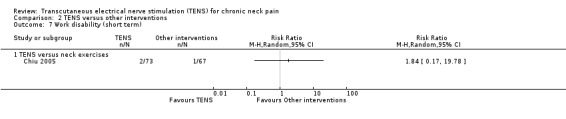
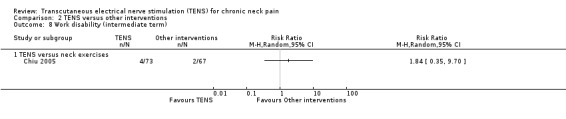
TENS versus neck exercises
Results from Chiu 2005 showed no difference in number of subjects taking sick leave because of neck pain at short‐term follow‐up (RR 1.84, 95% CI 0.17 to 19.78; 151 participants; very low‐certainty evidence; Analysis 2.7), nor in the assessment carried out at intermediate‐term follow‐up (RR 1.84, 95% CI 0.35 to 9.70; 151 participants; very low‐certainty evidence; Analysis 2.8). Thus, these estimates are imprecise and the direction of the effect (reduce or increase pain medication) is unclear. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
2.7. Analysis.
Comparison 2 TENS versus other interventions, Outcome 7 Work disability (short term).
2.8. Analysis.
Comparison 2 TENS versus other interventions, Outcome 8 Work disability (intermediate term).
Comparison 3: TENS added to an intervention versus intervention alone
Primary outcomes
Pain
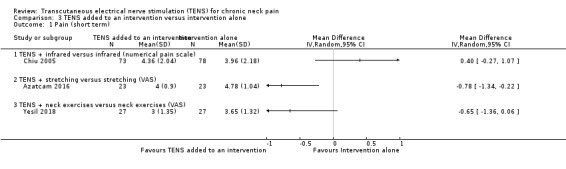
TENS added to infrared versus infrared alone
Chiu 2005 found little to no difference between groups in pain reduction assessed by a numerical pain scale at short‐term follow‐up (MD 0.40, 95% CI ‐0.27 to 1.07; 151 participants; very low‐certainty evidence; Analysis 3.1), nor in the assessment carried out at intermediate‐term follow‐up (MD ‐0.21, 95% CI ‐0.92 to 0.50; 151 participants; very low‐certainty evidence; Analysis 3.2). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
3.1. Analysis.
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 1 Pain (short term).
3.2. Analysis.
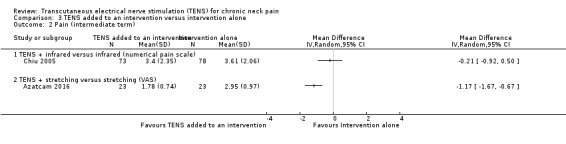
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 2 Pain (intermediate term).
TENS added to trapezius stretching versus trapezius stretching alone
Azatcam 2016 showed a small reduction in VAS favouring TENS, at short‐term follow‐up (MD ‐0.78, 95% CI ‐1.34 to ‐0.22; 46 participants; very low‐certainty evidence; Analysis 3.1), and a small reduction favouring TENS added to trapezius stretching at intermediate‐term follow‐up (MD ‐1.17, 95% CI ‐1.67 to ‐0.67; 46 participants; very low‐certainty evidence; Analysis 3.2). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
TENS added to exercise versus exercise alone
Yesil 2018 found little to no difference between groups in pain assessed by a numerical scale at short‐term follow‐up (MD ‐0.65, 95% CI ‐1.36 to 0.06; 54 participants; very low‐certainty evidence; Analysis 3.1). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
Disability
TENS added to infrared versus infrared alone
Chiu 2005 assessed disability using the Northwick Park Neck Pain Questionnaire and reported no differences between groups at short‐term follow‐up (MD 0.04, 95% CI ‐0.13 to 0.21; 151 participants; very low‐certainty evidence; Analysis 3.3), nor at intermediate‐term follow‐up (MD 0.03, 95% CI ‐0.14 to 0.20; 151 participants; very low‐certainty evidence; Analysis 3.4). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
3.3. Analysis.
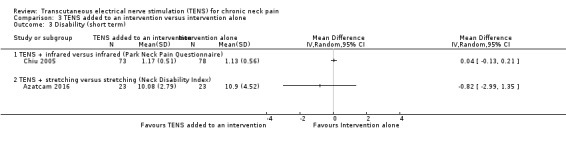
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 3 Disability (short term).
3.4. Analysis.
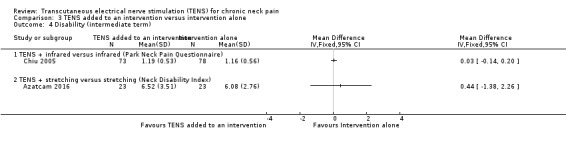
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 4 Disability (intermediate term).
TENS added to exercise versus exercise alone
Yesil 2018 (54 participants) assessed disability using the Neck Disability index (NDI). The authors reported that there was no difference between groups at short‐term follow‐up (very low‐certainty evidence). However, no numerical data were provided to compute confidence intervals. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and one level for imprecision (small sample size).
TENS added to trapezius stretching versus trapezius stretching alone
Azatcam 2016 showed no difference between groups in the Neck Disability Index at short‐ and intermediate‐term follow‐up (MD ‐0.82, 95% CI ‐2.99 to 1.35; 46 participants; very low‐certainty evidence; Analysis 3.3; MD 0.44, 95% CI ‐1.38 to 2.26; 46 participants; very low‐certainty evidence; Analysis 3.4, respectively). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
Adverse events
None of the studies reported this outcome.
Secondary outcomes
Quality of life
Yesil 2018 (54 participants) assessed quality of life by using the health‐related quality of life assessment SF‐36. One domain (vitality) showed difference in favour of exercises when compared to TENS added to exercises at short‐term follow‐up (MD 9.37, 95% CI 3.22 to 15.52). One domain (social functioning) showed difference in favour of TENS (MD ‐9.34, 95% CI ‐17.98 to ‐0.70). The other domains of the SF‐36 questionnaire showed a small or no difference between groups: physical functioning (MD 2.90, 95% CI ‐4.32 to 10.12); physical role (MD 2.75, 95% CI ‐14.74 to 20.24); pain (MD ‐4.58, 95% CI ‐12.59 to 3.43); general health (MD 4.26, 95% CI ‐1.80 to 10.32); emotional role (MD ‐5.41, 95% CI ‐20.96 to 10.14) and mental health (MD ‐0.41, 95% CI ‐6.60 to 5.78) (Analysis 3.9). The certainty of the evidence was very low, downgraded due to risk of bias (performance and detection bias) and imprecision (small sample size and wide confidence interval).
3.9. Analysis.
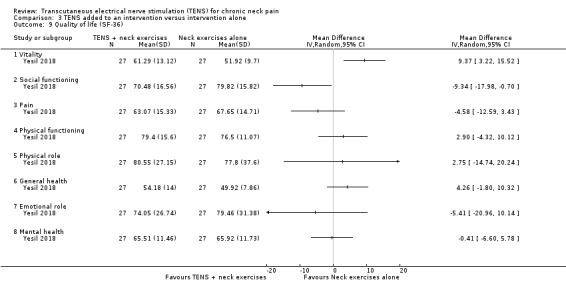
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 9 Quality of life (SF‐36).
Range of motion
TENS added to exercise versus exercise alone
Yesil 2018 (54 participants) found little to no difference between groups at short‐term follow‐up regarding neck flexion (MD ‐1.37, 95% CI ‐2.94 to 0.20), right lateral flexion (MD ‐0.07, 95% CI ‐1.79 to 1.65), left lateral flexion (MD ‐0.33, 95% CI ‐2.08 to 1.42), right rotation (MD ‐0.97, 95% CI ‐6.57 to 4.63) and left rotation (MD 3.17, 95% CI ‐2.00 to 8.34). A small improvement was observed in extension range of motion in favour of TENS (MD ‐6.06, 95% CI ‐9.69 to ‐2.43) (Analysis 3.10). The certainty of the evidence was very low, downgraded due to risk of bias (performance and detection bias) and imprecision (small sample size and wide confidence interval).
3.10. Analysis.
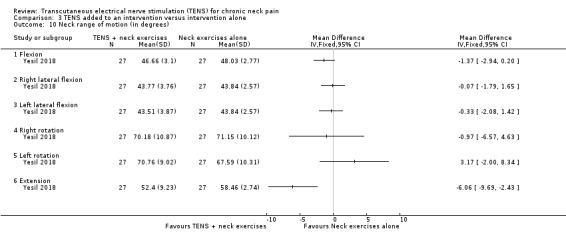
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 10 Neck range of motion (in degrees).
TENS added to trapezius stretching versus trapezius stretching alone
Results from Azatcam 2016 showed little to no difference in cervical contralateral lateral flexion at short‐term follow‐up (MD ‐0.20, 95% CI ‐0.67 to 0.27; 46 participants; very low‐certainty evidence; Analysis 3.11). We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
3.11. Analysis.
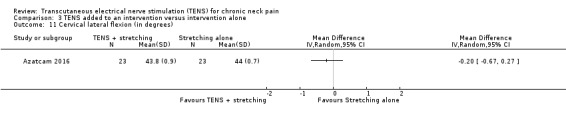
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 11 Cervical lateral flexion (in degrees).
Global perceived effect
None of the studies reported this outcome.
Use of medication for pain
TENS added to infrared versus infrared alone
Chiu 2005 showed no difference in reducing the use of pain medication at short‐term follow‐up (RR 1.01, 95% CI 0.56 to 1.80; 151 participants; very low‐certainty evidence), and in the assessment carried out at intermediate‐term follow‐up (RR 0.86, 95% CI 0.50 to 1.51; very low‐certainty evidence; Analysis 3.5). Thus, these estimates are imprecise and the direction of the effect (reduce or increase pain medication) is unclear. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
3.5. Analysis.
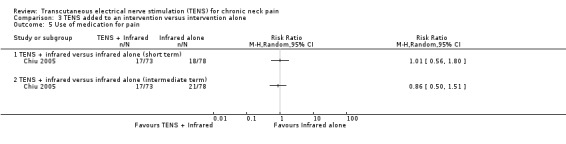
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 5 Use of medication for pain.
TENS added to exercise versus exercise alone
(Yesil 2018) showed no difference between groups regarding paracetamol dose reduction (in grams), at short‐term follow‐up (MD 5.10, 95% CI ‐6.33 to 16.53; 54 participants; very low‐certainty evidence; Analysis 3.6). Thus, this estimate is imprecise and the direction of the effect (reduce or increase pain medication) is unclear. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
3.6. Analysis.
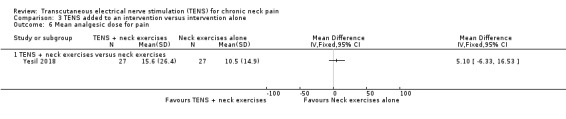
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 6 Mean analgesic dose for pain.
Work disability
There was no difference between TENS added to infrared versus infrared alone in number of subjects taking sick leave because of neck pain in Chiu 2005 at short‐term follow‐up (RR 0.43, 95% CI 0.09 to 2.13; 151 participants; very low‐certainty evidence; Analysis 3.7), nor in the assessment carried out at intermediate‐term follow‐up (RR 0.61, 95% CI 0.19 to 2.00; 151 participants; very low‐certainty evidence; Analysis 3.8). Thus, these estimates are imprecise and the direction of the effect (reduce or increase pain medication) is unclear. We downgraded the certainty of the evidence two levels for risk of bias (performance and detection bias) and two levels for imprecision (small sample size and wide confidence interval).
3.7. Analysis.
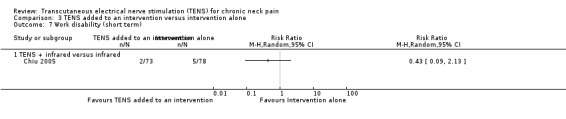
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 7 Work disability (short term).
3.8. Analysis.
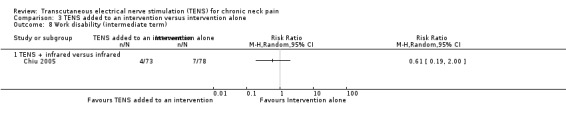
Comparison 3 TENS added to an intervention versus intervention alone, Outcome 8 Work disability (intermediate term).
Patient satisfaction
None of the studies reported this outcome.
Discussion
Summary of main results
This systematic review assessed the effects (benefits and harms) of transcutaneous electrical nerve stimulation (TENS) for the treatment of chronic neck pain. We found seven randomised clinical trials (RCTs) that could not be combined in meta‐analyses and the results of the individual studies are described in a narrative form. Based on the GRADE approach, there was very low‐certainty evidence about the effects of TENS when compared to sham TENS: uncertain difference in pain at short‐term (immediately after 10 sessions of 30 minutes or one week after a single session of 60 minutes) follow‐up. None of the included studies that assessed this comparison reported on disability or adverse events.
Overall completeness and applicability of evidence
We included seven RCTs that assessed TENS alone or combined with another intervention, in adult participants (mean age 31.7 to 55.5 years) with chronic neck pain. Most studies used conventional TENS with a frequency between 60 Hz to 100 Hz, a pulse width of 40 μs to 250 μs and comfortable intensity, followed by burst TENS and acupuncture‐like TENS. The study participants had daily TENS sessions that lasted 20 to 60 minutes and a total of one to 60 sessions. The maximum follow‐up was six months (intermediate‐term). Electrodes were placed on the most painful region of the neck, including the upper trapezius muscle (Table 6). Most studies used TENS parameters and dosages that follow current practice, i.e. with a frequency below 200 Hz, a pulse width between 50 μs to 250 μs and intensity less than 70 mA (Johnson 2007a; Sluka 2013). None of the included studies assessed the outcomes: adverse events, global perceived effect and patient satisfaction. Additionally, there was a paucity of data about the other outcomes of interest.
We should also point out that we have 10 studies awaiting classification. We were not able to decide if those studies should be included or excluded due to the lack of information regarding symptoms duration and anatomical region of TENS application. We tried to contact the authors to retrieve further information, but have received no response. Therefore, we considered that most of the effect estimates are influenced by some degree of publication bias, mainly due to poor reporting by some studies, that led us to have more studies awaiting classification than included studies.
Certainty of the evidence
As presented in Table 1, the certainty of evidence for all outcomes under each comparison was very low. The quality of individual RCTs was limited mainly due to: (a) lack of blinding of participants, personnel and outcome assessors (given that the nature of the intervention precludes masking), and problems with (b) allocation concealment and (c) selective outcome reporting. The lack of data and clinical heterogeneity between studies precluded us from performing meta‐analyses. We also downgraded the certainty of the evidence due to imprecision because of the small number of participants in each study for all outcomes and wide confidence intervals.
Potential biases in the review process
To minimise the risk of bias of the review, we followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions for searching, study selection, methodological appraisal, data collection and data analysis (Higgins 2011). We also conducted a sensitive electronic and manual literature search. Limitations of this review include: (a) the lack of meta‐analyses due to differences in study outcomes and comparison groups, (b) the lack of some outcome data in the included RCTs, and (c) we classified 10 studies as awaiting classification due to lack of information about the duration of symptoms.
Agreements and disagreements with other studies or reviews
This review is a split from a previously published Cochrane systematic review (Kroeling 2013), that assessed a broader research question (electrotherapy for neck pain). In this review, we limited the inclusion criteria to studies that tested only a modality of electrotherapy, TENS, for people with chronic (> 3 months) neck pain. As expected, due to important differences in the inclusion criteria, the number of included studies in the two reviews is different. However, in both reviews, the overall conclusions regarding TENS, are similar, with very low‐certainty evidence and no robust conclusions for practice. Another systematic review evaluated conservative treatments for adults with non‐specific neck pain and included only one small study about TENS that reported no significant results for pain or disability (Leaver 2010).
Authors' conclusions
Implications for practice.
This review found very low‐certainty evidence of a difference between TENS compared to sham TENS on reducing neck pain. Very low‐certainty evidence means that we are unsure about the effect estimate. At present, there is insufficient evidence regarding the use of TENS in people with chronic neck pain. Additional well‐designed, ‐conducted and ‐reported RCTs are needed to reach robust conclusions.
Implications for research.
Due to very low‐certainty evidence, heterogeneity between existing studies, and the lack of data on important outcomes (adverse events, global perceived effect and patient satisfaction), more research is needed on TENS for the treatment of people with chronic neck pain. Future RCTs should be well‐designed and reported (following the CONSORT statement (Schulz 2010), and compare conventional TENS versus sham. The authors of these new trials should also follow the IMMPACT (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; Turk 2008) recommendations when planning the selection and measurement of their outcomes.
Acknowledgements
We would like to thank the following.
Teresa Marin and Megan Prictor for assistance in the preparation of the protocol.
The Cochrane Brazil team for methodological support.
Praveen Kumar for providing us (via Cochrane Task Exchange) the full‐text of one eligible study.
Shireen Harbin for assistance in the editorial process.
Maurits van Tulder, Kathryn Armitstead and Bruno Saragiotto for peer review comments.
Kaitlyn Brethour for the English proof.
Clare Dooley for copy editing.
Appendices
Appendix 1. Glossary
Nuchal line: the nuchal lines are on the external surface of the occipital bone, which makes up the rear base of the skull. These lines form anatomical reference points and are also points of attachment for some of the muscles involved in the control of the head and neck.
Amplitude: all waves carry energy, including light, sound, infrared, microwaves, x‐rays and water. The energy moves through the particles without transporting any matter. Amplitude is the measurement of the energy carried by any wave. The greater the amplitude of the wave, the higher the level of energy that is carried by the wave.
Nociceptive signals: nociception (also nocioception or nociperception) is the encoding and processing of harmful stimuli in the nervous system and, therefore, the ability of a body to sense potential harm. It is the afferent activity in the peripheral and central nervous systems produced by stimulation of specialised free nerve endings called nociceptors or 'pain receptors'. Once stimulated, a nociceptor sends a signal along a chain of nerve fibres via the spinal cord to the brain.
Radicular findings: the findings related to any process that carries compression of the nerve roots (radicular). The aetiology of root compression can be traumatic and non‐traumatic, and within the latter classification is contained neoplasms, degenerative disc pathologies, infections, parasitic infections, haematoma and spontaneous genetic defects.
Cervicogenic headache: cervicogenic headache is referred pain (pain perceived as occurring in a part of the body other than its true source) perceived in the head from a source in the neck. Cervicogenic headache is a secondary headache, which means that it is caused by another illness or physical issue.
Appendix 2. Search strategies
Cochrane Back and Neck Trials register in CRS
Last searched 9 November 2018
((Transcutaneous Electric* Nerve Stimulation OR TENS OR Transcutaneous nerve stimulation OR TNS OR Transcutaneous Electric* Stimulation OR Transcutaneous electric* neurostimulation OR Analgesic Cutaneous Electrostimulation OR TENMS OR Transcutaneous Electric* Nerve and Muscle Stimulation OR Transcutaneous Muscle Stimulation OR transcutaneous electrostimulation OR Transdermal electric* stimulation OR Transdermal Electrostimulation OR Percutaneous Electric* Nerve Stimulation OR Peripheral conditioning stimulation OR Percutaneous neural stimulation OR Microamperage electrical stimulation OR electroanalgesia OR electrotherapy OR Electric Stimulation Therapy OR Electric Stimulation) AND (neck OR neck pain OR whiplash OR trapezius OR myofascial pain OR myofascial trigger point* OR cervicogenic headache OR cervical radicul* OR cervical pain OR neck injuries OR neck muscles OR neck disorders OR cervical spine OR cervicalgia OR cervicodynia OR cervicobrachial* OR cervico‐brachial*)) AND (2017 TO 2018:YR)
CENTRAL
Search performed on 9 November 2018 using CRS Web
1 MESH DESCRIPTOR Neck Pain EXPLODE ALL AND CENTRAL:TARGET
2 MESH DESCRIPTOR Neck Muscles EXPLODE ALL AND CENTRAL:TARGET
3 MESH DESCRIPTOR Neck Injuries EXPLODE ALL AND CENTRAL:TARGET
4 MESH DESCRIPTOR Whiplash Injuries EXPLODE ALL AND CENTRAL:TARGET
5 MESH DESCRIPTOR Neck EXPLODE ALL AND CENTRAL:TARGET
6 MESH DESCRIPTOR Cervical Plexus EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Cervical Vertebrae EXPLODE ALL AND CENTRAL:TARGET
8 neck pain or neckache* or neck ache* or cervicodynia or cervicalgia AND CENTRAL:TARGET
9 neck AND CENTRAL:TARGET
10 neck disorder* AND CENTRAL:TARGET
11 whiplash AND CENTRAL:TARGET
12 MESH DESCRIPTOR Myofascial Pain Syndromes EXPLODE ALL AND CENTRAL:TARGET
13 (myofascial NEAR (pain or trigger point*)) AND CENTRAL:TARGET
14 trapezius AND CENTRAL:TARGET
15 MESH DESCRIPTOR Radiculopathy EXPLODE ALL AND CENTRAL:TARGET
16 (cervical near (radiculopath* or pain)) AND CENTRAL:TARGET
17 cervical spine AND CENTRAL:TARGET
18 cervicobrachial* or cervico‐brachial* AND CENTRAL:TARGET
19 cervicogenic headache* AND CENTRAL:TARGET
20 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
21 MESH DESCRIPTOR Transcutaneous Electric Nerve Stimulation AND CENTRAL:TARGET
22 MESH DESCRIPTOR Electric Stimulation Therapy AND CENTRAL:TARGET
23 MESH DESCRIPTOR Electric Stimulation AND CENTRAL:TARGET
24 TENS AND CENTRAL:TARGET
25 Transcutaneous electric* nerve stimulation AND CENTRAL:TARGET
26 transcutaneous nerve stimulation AND CENTRAL:TARGET
27 TNS or TENMS AND CENTRAL:TARGET
28 Transcutaneous Electric* Stimulation AND CENTRAL:TARGET
29 Transcutaneous electric* neurostimulation AND CENTRAL:TARGET
30 Analgesic Cutaneous Electrostimulation AND CENTRAL:TARGET
31 Transcutaneous Electric* Nerve and Muscle Stimulation AND CENTRAL:TARGET
32 Transcutaneous Muscle Stimulation AND CENTRAL:TARGET
33 Transdermal electric* stimulation AND CENTRAL:TARGET
34 Transcutaneous electrostimulation AND CENTRAL:TARGET
35 Transdermal Electrostimulation AND CENTRAL:TARGET
36 Percutaneous Electric* Nerve Stimulation AND CENTRAL:TARGET
37 Peripheral conditioning stimulation AND CENTRAL:TARGET
38 Percutaneous neural stimulation AND CENTRAL:TARGET
39 Microamperage electric* stimulation AND CENTRAL:TARGET
40 electroanalgesia AND CENTRAL:TARGET
41 electrotherapy AND CENTRAL:TARGET
42 #41 OR #40 OR #39 OR #38 OR #37 OR #36 OR #35 OR #34 OR #33 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #26 OR #25 OR #24 OR #23 OR #21 OR #22
43 #42 AND #20
44 #43 AND (2017 TO 2018:YR
2017 search
#1 MeSH descriptor: [Neck Pain] explode all trees
#2 MeSH descriptor: [Neck Muscles] explode all trees
#3 MeSH descriptor: [Neck Injuries] explode all trees
#4 MeSH descriptor: [Whiplash Injuries] explode all trees
#5 MeSH descriptor: [Neck] explode all trees
#6 MeSH descriptor: [Cervical Plexus] explode all trees
#7 MeSH descriptor: [Cervical Vertebrae] explode all trees
#8 neck pain or neckache* or neck ache* or cervicodynia or cervicalgia
#9 neck
#10 neck disorder*
#11 whiplash
#12 MeSH descriptor: [Myofascial Pain Syndromes] explode all trees
#13 (myofascial near (pain or trigger point*))
#14 trapezius
#15 MeSH descriptor: [Radiculopathy] explode all trees
#16 (cervical near (radiculopath* or pain))
#17 cervical spine
#18 cervicobrachial* or cervico‐brachial*
#19 cervicogenic headache*
#20 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#21 MeSH descriptor: [Transcutaneous Electric Nerve Stimulation] this term only
#22 MeSH descriptor: [Electric Stimulation Therapy] this term only
#23 MeSH descriptor: [Electric Stimulation] this term only
#24 TENS
#25 Transcutaneous electric* nerve stimulation
#26 transcutaneous nerve stimulation
#27 TNS or TENMS
#28 Transcutaneous Electric* Stimulation
#29 Transcutaneous electric* neurostimulation
#30 Analgesic Cutaneous Electrostimulation
#31 Transcutaneous Electric* Nerve and Muscle Stimulation
#32 Transcutaneous Muscle Stimulation
#33 Transdermal electric* stimulation
#34 Transcutaneous electrostimulation
#35 Transdermal Electrostimulation
#36 Percutaneous Electric* Nerve Stimulation
#37 Peripheral conditioning stimulation
#38 Percutaneous neural stimulation
#39 Microamperage electric* stimulation
#40 electroanalgesia
#41 electrotherapy
#42 #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41
#43 #20 and #42
#44 #43 Publication Year from 2015 to 2017, in Trials
MEDLINE
Last searched 9 November 2018. In 2015, only MEDLINE and MEDLINE In‐Process & Other Non‐Indexed Citations databases were searched and anatomy and intervention terms were searched in the .mp. field instead of .tw,kf. fields.
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 pragmatic clinical trial.pt.
4 random$.ti,ab.
5 placebo.ab,ti.
6 drug therapy.fs.
7 trial.ab,ti.
8 groups.ab.
9 or/1‐8
10 (animals not (humans and animals)).sh.
11 9 not 10
12 neck pain/
13 (neck pain or neckache? or neck ache? or cervicodynia or cervicalgia).ti,ab.
14 neck muscles/
15 neck/
16 neck injuries/
17 cervical plexus/
18 neck.tw,kf.
19 neck disorder?.tw,kf.
20 whiplash injuries/
21 whiplash.tw,kf.
22 myofascial pain syndromes/
23 (myofascial adj3 (pain or trigger point?)).tw,kf.
24 radiculopathy/
25 (cervical adj3 (radiculopath* or pain)).tw,kf.
26 cervical spine.tw,kf.
27 cervical vertebrae/
28 trapezius.tw,kf.
29 (cervicobrachial* or cervico‐brachial*).tw,kf.
30 cervicogenic headache?.tw,kf.
31 or/12‐30
32 Transcutaneous Electric Nerve Stimulation/
33 TENS.tw,kf.
34 Transcutaneous electric* nerve stimulation.tw,kf.
35 transcutaneous nerve stimulation.tw,kf.
36 TNS.tw,kf.
37 Transcutaneous Electric* Stimulation.tw,kf.
38 transcutaneous electric* neurostimulation.tw,kf.
39 transcutaneous electrostimulation.tw,kf.
40 Analgesic Cutaneous Electrostimulation.tw,kf.
41 TENMS.tw,kf.
42 (Transcutaneous Electric* Nerve and Muscle Stimulation).tw,kf.
43 Transcutaneous Muscle Stimulation.tw,kf.
44 transdermal electric* stimulation.tw,kf.
45 Transdermal Electrostimulation.tw,kf.
46 Percutaneous Electric* Nerve Stimulation.tw,kf.
47 peripheral conditioning stimulation.tw,kf.
48 percutaneous neural stimulation.tw,kf.
49 microamperage electric* stimulation.tw,kf.
50 Electric Stimulation Therapy/
51 Electric Stimulation/
52 electroanalgesia.tw,kf.
53 electrotherapy.tw,kf.
54 or/32‐53
55 11 and 31 and 54
56 limit 55 to yr=2017‐2018
57 limit 55 to ed=20170104‐20181109
58 56 or 57
Embase
Last searched 9 November 2018. In 2015 anatomy and intervention terms were searched in the .mp. field instead of .tw,kw. fields.
1 Randomized Controlled Trial/
2 exp Controlled clinical trial/
3 Controlled Study/
4 Double Blind Procedure/
5 Single Blind Procedure/
6 crossover procedure/
7 placebo/
8 random*.ti,ab.
9 placebo?.ti,ab.
10 allocat*.ti,ab.
11 assign*.ti,ab.
12 blind*.ti,ab.
13 (cross‐over or crossover).ti,ab.
14 (compare or compared or comparing or comparison or comparative).ti,ab.
15 (controlled adj7 (study or design or trial)).ti,ab.
16 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).ti,ab.
17 trial.ti,ab.
18 or/1‐17
19 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
20 human/ or normal human/ or human cell/
21 19 and 20
22 19 not 21
23 18 not 22
24 neck/
25 neck pain/
26 neck muscle/
27 neck injury/
28 neck.tw,kw.
29 (neck pain or neckache? or neck ache? or cervicodynia or cervicalgia).tw,kw.
30 whiplash injury/
31 whiplash.tw,kw.
32 myofascial pain/
33 (myofascial adj3 (pain or trigger point?)).tw,kw.
34 secondary headache/
35 cervicogenic headache?.tw,kw.
36 (cervicobrachial* or cervico‐brachial*).tw,kw.
37 cervical spine/
38 cervical spine.tw,kw.
39 exp radiculopathy/
40 radicular pain/
41 (cervical adj3 (radiculopath* or pain)).tw,kw.
42 trapezius muscle/
43 trapezius.tw,kw.
44 neck disorder?.tw,kw.
45 or/24‐44
46 Transcutaneous Electric Nerve Stimulation/
47 TENS.tw,kw.
48 Transcutaneous electric* nerve stimulation.tw,kw.
49 transcutaneous nerve stimulation.tw,kw.
50 TNS.tw,kw.
51 Transcutaneous Electric* Stimulation.tw,kw.
52 transcutaneous electric* neurostimulation.tw,kw.
53 transcutaneous electrostimulation.tw,kw.
54 Analgesic Cutaneous Electrostimulation.tw,kw.
55 TENMS.tw,kw.
56 (Transcutaneous Electric* Nerve and Muscle Stimulation).tw,kw.
57 Transcutaneous Muscle Stimulation.tw,kw.
58 transdermal electric* stimulation.tw,kw.
59 Transdermal Electrostimulation.tw,kw.
60 Percutaneous Electric* Nerve Stimulation.tw,kw.
61 peripheral conditioning stimulation.tw,kw.
62 percutaneous neural stimulation.tw,kw.
63 microamperage electric* stimulation.tw,kw.
64 Electrostimulation Therapy/
65 Electrostimulation/
66 electroanalgesia.tw,kw.
67 electrotherapy.tw,kw.
68 or/46‐67
69 23 and 45 and 68
70 limit 69 to yr=2017‐2018
71 limit 69 to dd=20170104‐20181109
72 70 or 71
CINAHL
Last searched 9 November 2018
S70 S68 OR S69
S69 S67 AMD EM 220170104‐20181109
S68 S67 Limiters ‐ Published Date: 20170131‐20181109
S67 S24 AND S45 AND S66
S66 S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65
S65 electrotherapy
S64 (MH "Electrotherapy")
S63 "electroanalgesia"
S62 Microamperage electric* stimulation
S61 Percutaneous neural stimulation
S60 Peripheral conditioning stimulation
S59 Percutaneous Electric* Nerve Stimulation
S58 Transdermal Electrostimulation
S57 Transdermal electric* stimulation
S56 Transcutaneous Muscle Stimulation
S55 Transcutaneous Electric* Nerve and Muscle Stimulation
S54 Analgesic Cutaneous Electrostimulation
S53 Transcutaneous electric* neurostimulation
S52 Transcutaneous Electric* Stimulation
S51 TNS or TENMS
S50 transcutaneous nerve stimulation
S49 Transcutaneous electric* nerve stimulation
S48 "TENS"
S47 (MH "Electric Stimulation")
S46 (MH "Transcutaneous Electric Nerve Stimulation")
S45 S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44
S44 "cervical spine"
S43 "cervicogenic headache#"
S42 cervicobrachial* or cervico‐brachial*
S41"trapezius"
S40 (MH "Trapezius Muscles")
S39 (MH "Cervical Vertebrae")
S38 (cervical W3 (radiculopath* or pain))
S37 (MH "Radiculopathy")
S36 (myofascial W3 (pain or trigger point*))
S35 (MH "Myofascial Pain Syndromes")
S34 neck
S33 "whiplash"
S32 neck disorder*
S31 neck pain or neckache* or neck ache* or cervicodynia or cervicalgia
S30 (MH "Cervical Plexus")
S29 (MH "Whiplash Injuries")
S28 (MH "Neck Injuries+")
S27 (MH "Neck Muscles")
S26 (MH "Neck") 4,219
S25 (MH "Neck Pain")
S24 S22 not S23
S23 (MH "Animals+")
S22 S21 or S20 or S19 or S18 or S17 or S16 or S15 or S14 or S13 or S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S21 volunteer*
S20 prospectiv*
S19 control*
S18 followup stud*
S17 follow‐up stud*
S16 (MH "Prospective Studies+")
S15 (MH "Evaluation Research+")
S14 (MH "Comparative Studies")
S13 latin square
S12 (MH "Study Design+")
S11 (MH "Random Sample+")
S10 random*
S9 placebo*
S8 (MH "Placebos")
S7 (MH "Placebo Effect")
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 randomi?ed controlled trial*
S1 (MH "Clinical Trials+")
LILACS
Last searched 9 November 2018
(TENS AND (neck OR "neck pain" OR "myofascial pain" OR trapezius OR whiplash OR radiculopathy OR "cervicogenic headache" OR "myofascial trigger points"))
Limit to LILACS database
PEDro
Last searched 9 November 2018
Abstract & Title: TENS
Problem: pain
Body part: head and neck
New records added since 04/01/2017
PubMed
Last searched 15 December 2015
((Transcutaneous Electric Nerve Stimulation OR TENS OR Transcutaneous electrical nerve stimulation OR Transcutaneous nerve stimulation OR TNS OR Transcutaneous Electric Stimulation OR Transcutaneous Electrical Stimulation OR Transcutaneous electric neurostimulation OR Transcutaneous electrical neurostimulation OR Analgesic Cutaneous Electrostimulation OR TENMS OR Transcutaneous Electric Nerve and Muscle Stimulation OR Transcutaneous Electrical Nerve and Muscle Stimulation OR Transcutaneous Muscle Stimulation OR transcutaneous electrostimulation OR Transdermal electric stimulation OR Transdermal electrical stimulation OR Transdermal Electrostimulation OR Percutaneous Electric Nerve Stimulation OR Percutaneous Electrical Nerve Stimulation OR Peripheral conditioning stimulation OR Percutaneous neural stimulation OR Microamperage electrical stimulation OR electroanalgesia OR electrotherapy OR Electric Stimulation Therapy OR Electric Stimulation) AND (neck OR neck pain OR whiplash OR trapezius OR myofascial pain OR myofascial trigger points OR cervicogenic headache OR cervical radiculopathy OR cervical pain OR neck injuries OR neck muscles OR neck disorders OR cervical spine OR cervicalgia OR cervicodynia OR cervicobrachial OR cervico‐brachial) AND (pubstatusaheadofprint OR publisher[sb] or pubmednotmedline[sb]))
WHO ICTRP
Last searched 9 November 2018
TENS AND neck pain OR TENS AND whiplash OR TENS AND myofascial pain OR TENS AND myofascial trigger points OR TENS AND trapezius OR TENS AND radicul*
ClinicalTrialsgov
Last searched 9 November 2018
condition: neck pain OR whiplash OR trapezius OR myofascial pain OR myofascial trigger points OR cervicogenic headache OR radiculopathy
Intervention: TENS
OpenSigle
Last searched 9 November 2018
((((transcutaneous OR percutaneous OR peripheral OR microamperage OR peripheral OR transdermal OR analgesic ) NEAR/5 (stimulation OR electrostimulation OR neurostimulation )) OR TENS OR TENMS OR electrotherapy OR electroanalgesia OR electric stimulation) AND (neck pain OR “myofascial pain” OR “myofascial trigger points” OR trapezius OR whiplash OR radicul* OR "cervicogenic headache"))
Appendix 3. The GRADE approach to evidence synthesis
We will categorise the certainty of evidence as follows.
High (⊕⊕⊕⊕): we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate (⊕⊕⊕⊖): we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low (⊕⊕⊖⊖): our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low (⊕⊖⊖⊖): we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We will grade the evidence available to answer each subquestion on the domains in the following manner.
1. Risk of bias
Limitations in the study design and implementation may bias the estimates of the treatment effect. Our confidence in the estimate of the effect and in the following recommendation decreases if studies suffer from major limitations. We will examine all studies with regard to the following five types of biases.
Selection (random sequence generation, allocation concealment, group similarities at baseline).
Performance (blinding of participants, blinding of healthcare providers).
Attrition (dropouts and intention‐to‐treat analysis).
Measurement (blinding of the outcome assessors and timing of outcome assessment).
Reporting bias (selective reporting).
2. Inconsistency
Inconsistency refers to unexplained heterogeneity in results. Widely differing estimates of the treatment effect (i.e. heterogeneity or variability in results) across studies suggest true differences in underlying treatment effect. Inconsistency may arise from differences in: populations (e.g. drugs may have larger relative effects in sicker populations), interventions (e.g. larger effects with higher drug doses), or outcomes (e.g. diminishing treatment effect with time). We will downgrade the certainty of evidence:
by one level when the heterogeneity or variability in results is large (for example: I2 above 80%);
by two levels when the heterogeneity or variability in results is large, and there was inconsistency arising from populations, interventions or outcomes.
3. Indirectness
Indirect population, intervention, comparator, or outcome – the question being addressed in this systematic review is different from the available evidence regarding the population, intervention, comparator, or an outcome in the included randomised trial.
We will downgrade the certainty of evidence:
by one level when there is indirectness in only one area;
by two levels when there is indirectness in two or more areas.
4. Imprecision
Results are imprecise when studies include relatively few patients and few events and thus have wide confidence intervals around the estimate of the effect. In this case we judge the certainty of the evidence lower than it otherwise would be, because of consequential uncertainty in the results. Each outcome is considered separately.
For dichotomous outcomes
We will consider imprecision for either of the following two reasons.
There is only one study. When there is more than one study, the total number of events is less than 300 (a threshold rule of thumb value) (Mueller 2007).
The 95% confidence interval around the pooled or best estimate of effect includes both: a) no effect; and b) appreciable benefit or appreciable harm. The threshold for 'appreciable benefit' or 'appreciable harm' is a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%.
We will downgrade the certainty of evidence:
by one level when there is imprecision due to either of the reasons above;
by two levels when there is imprecision due to both of the reasons above.
For continuous outcomes
We will consider imprecision for either of the following two reasons.
There is only one study. When there is more than one study, total population size is less than 400 (a threshold rule of thumb value; using the usual α and β, and an effect size of 0.2 standard deviations, representing a small effect).
The 95% confidence interval includes no effect and the upper or lower confidence limit crosses an effect size (standardised mean difference) of 0.5 in either direction.
We will downgrade the certainty of evidence:
by one level when there is imprecision due to either of the reasons above;
by two levels when there is imprecision due to both of the reasons above.
5. Publication bias
Publication bias is a systematic underestimate or an overestimate of the underlying beneficial or harmful effect due to the selective publication of studies. We will downgrade the certainty of evidence:
by one level when the funnel plot suggests publication bias.
Data and analyses
Comparison 1. TENS versus sham TENS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS) (at short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Conventional TENS | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Burst TENS | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Acupuncture‐like TENS | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain (percentage of participants presenting improvement of pain) (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Pain assessed by myometer score (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 2. TENS versus other interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (short term) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 TENS versus neck exercises (numerical pain scale) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 TENS versus kinesio taping (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 TENS versus manipulation therapy (numerical pain scale) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain (intermediate term) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 TENS versus neck exercises (numerical pain scale) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 TENS versus kinesio taping (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Disability (short term) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 TENS versus neck exercises (Northwick Park Neck Pain Questionnaire) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 TENS versus kinesio taping (Neck Disability Index) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Disability (intermediate term) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 TENS versus neck exercises (Northwick Park Neck Pain Questionnaire) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 TENS versus kinesio taping (Neck Disability Index) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Use of medication for pain (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 TENS versus neck exercises | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Use of medication for pain (intermediate term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 TENS versus neck exercises | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Work disability (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 TENS versus neck exercises | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Work disability (intermediate term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 TENS versus neck exercises | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Range of motion (in degrees) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 TENS versus kinesio taping | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 TENS versus manipulation therapy | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. TENS added to an intervention versus intervention alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (short term) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 TENS + infrared versus infrared (numerical pain scale) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 TENS + stretching versus stretching (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 TENS + neck exercises versus neck exercises (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain (intermediate term) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 TENS + infrared versus infrared (numerical pain scale) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 TENS + stretching versus stretching (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Disability (short term) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 TENS + infrared versus infrared (Park Neck Pain Questionnaire) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 TENS + stretching versus stretching (Neck Disability Index) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Disability (intermediate term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 TENS + infrared versus infrared (Park Neck Pain Questionnaire) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 TENS + stretching versus stretching (Neck Disability Index) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Use of medication for pain | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 TENS + infrared versus infrared alone (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 TENS + infrared versus infrared alone (intermediate term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Mean analgesic dose for pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 TENS + neck exercises versus neck exercises | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Work disability (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1 TENS + infrared versus infrared | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Work disability (intermediate term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 TENS + infrared versus infrared | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life (SF‐36) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Vitality | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Social functioning | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Pain | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 Physical functioning | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 Physical role | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 General health | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 Emotional role | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.8 Mental health | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Neck range of motion (in degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Right lateral flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Left lateral flexion | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 Right rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.5 Left rotation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.6 Extension | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Cervical lateral flexion (in degrees) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azatcam 2016.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Turkey |
|
| Participants |
Sample size/available for analysis: 72/69 Gender: 48 females/21 males Age (years, mean and range): 38.34 (20–65) years Duration of disease (months): 21.82 (± 13.28) months Inclusion criteria:
Exclusion criteria: "Injection to the myofascial trigger point or use of physical therapy modalities in the 1 year before commencement of the study; history of acute trauma; inflammatory joint or muscle disease, infection or malignancy; diagnosed cervical radiculopathy or myelopathy; the symptom and findings fulfilling the 1990 ACR criteria for fibromyalgia; poor cooperation." |
|
| Interventions |
Group 1: TENS + trapezius stretching (n = 23) Group 2: KT + trapezius stretching (n = 23) Group 3: trapezius stretching (n = 23) Treatment duration: two weeks Follow‐up duration: three months Scheme of TENS: TENS was applied using the Enraf device (Endomed 182) in the form of symmetrical, biphasic rectangular pulses for 100 μs. Frequency was 60 Hertz; intensity was set according to the paraesthesia perception of the patient. The negative electrode was placed over the active myofascial trigger point on the upper trapezius muscle; the positive electrode was placed on the acromial tendon. The total duration for the application was 20 min. Participants had a total of 10 sessions, consisting of 1 daily session for 2 weeks. The participants went to the hospital for their TENS sessions. Scheme of KT: standard 20 cm Pino tape was used for banding on the upper trapezius muscle. Banding was performed in cervical flexion and ipsilateral rotation positions by using I‐strip muscle technique, starting on acromion with maximum stretching of the head of the band in order to benefit from the inhibitory effect. The arm was taped throughout the upper edge of the trapezius muscle up to the hairline, with no stretching, A total of 4 KT consisting of 2 sessions weekly were performed during the treatment period. Scheme of trapezius stretching: the participants received instructions on how to do trapezius stretching exercises; exercise brochures were given to all participants included in the study. They were instructed to perform stretching exercises three times a day, with 10 repetitions of the stretching sets, for 2 weeks. At the end of the first week and throughout the study period, all participants were called by phone and invited to come for a control visit. |
|
| Outcomes |
|
|
| Notes |
Funding sources: none declared Conflict of interest: none reported Full text language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Patients were divided into three groups by using random numbers table". Comment: the method used for sequence generation seems to be appropriate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information available |
| Blinding of participants | High risk | Quote: "... for this randomized, controlled, single‐blind and prospective study..." Comment: the participants were unblinded |
| Blinding of personnel/providers | High risk | Quote: "... for this randomized, controlled, single‐blind and prospective study..." Comment: there was no blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "... for this randomized, controlled, single‐blind and prospective study..." Comment: the method for masking the outcome assessors seems to be appropriate. However, the primary outcomes, pain and disability were patient‐dependent and there was no blinding of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "During the study, one patient from each group was lost and the study was completed with 69 patients". Comment: the study had an overall loss of 4% (one patient from each group). |
| ITT analysis | High risk | Comment: ITT analyses were not conducted. |
| Selective reporting (reporting bias) | Unclear risk | Comment: study protocol was not available. |
| Group similarity at baseline | Low risk | Quote: "There was no statistically significant difference between the groups in terms of demographic and clinical parameters during pretreatment evaluation (P > 0.05). Demographic characteristics of the patients are presented in Table 1". Comment: the three groups seem to be similar at baseline. |
| Cointerventions | Low risk | Comment: the cointervention (trapezius stretching exercises) was the same for all groups. |
| Compliance | Unclear risk | Comment: compliance with the intervention was not reported. |
| Timing of outcome assessments | Low risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures (pre, post‐treatment and after 12 weeks). |
| Other bias | Low risk | Comment: no other biases were identified. |
Chen 2007.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients and hospitalised patients, single centre Country: Republic of China |
|
| Participants |
Sample size/available for analysis: 70/70 Gender: 30 females/40 males Age (years, mean and SD): 42.47 (± 14.07) years Duration of disease (months, mean and SD): 18.51 (± 8.43) months Inclusion criteria: "cervicogenic headache in accordance with the diagnostic criteria of Sjaastad; and at the same time one of the following conditions: course ≥ 6 months; nearly 3 months without any medication; cervical spine X‐ray inspection shows that the cervical spine has no degenerative changes." Exclusion criteria: "neurological diagnosis of other types of headache; cervical spine fracture, dislocation or discectomy and severe osteoporosis are not suitable for manipulation or nerve stimulation therapy." |
|
| Interventions |
Group 1: TENS (n = 34) Group 2: manipulation treatment (n = 36) Treatment duration: 4 weeks Follow‐up duration: 6 weeks Schemes of TENS: two 75 mm × 115 mm electrodes were placed in the upper cervical spine on both sides, symmetrical two‐way square wave output, frequency 100 Hz, pulse width 250 μs, 20 min each time during the course of treatment (10 sessions) |
|
| Outcomes |
|
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: Chinese |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...with cervicogenic headache were randomly allocated to receive manipulation treatment and TENS treatment". Comment: information provided is not sufficient. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...with cervicogenic headache were randomly allocated to receive manipulation treatment and TENS treatment". Comment: information provided is not sufficient. |
| Blinding of participants | High risk | Comment: different interventions (TENS and manipulation therapy) were compared and blinding was not possible. |
| Blinding of personnel/providers | High risk | Comment: due to the nature of the intervention, it was not possible to blind the physician who provided the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no available information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no losses of participants. |
| ITT analysis | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. |
| Group similarity at baseline | Low risk | Comment: the three groups seem to be similar at baseline. |
| Cointerventions | Low risk | No cointerventions |
| Compliance | Unclear risk | Comment: compliance with the intervention was not reported. |
| Timing of outcome assessments | Low risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures. |
| Other bias | Low risk | Comment: no other biases were identified. |
Chiu 2005.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: China |
|
| Participants |
Sample size/available for analysis: 218/218 Gender: 149 females/69 males Age (years, mean and SD): 43.45 ± 9.72 Duration of disease (weeks, mean): more than three months Inclusion criteria: "age range 20‐70 years, with a history of more than three months of intermittent neck pain, and the ability to read Chinese." Exclusion criteria: "Presence of a previous history of injury to the neck or upper back from TI to T6; an inflammatory condition (e.g. rheumatoid arthritis); previous surgery to the neck; a history of malignancy; congenital abnormality of the spine; been receiving concurrent treatment (e.g. from a chiropractor or bone setter); other musculoskeletal problems at the same time; and acute neck pain with no freedom of movement" |
|
| Interventions |
Group 1: TENS + Infrared (n = 73) Group 2: infrared (n = 78) Group 3: neck exercises + Infrared (n = 67) Treatment duration: six weeks Follow‐up duration: six months Schemes of TENS: conventional TENS for 30 minutes twice a week, from a dual channel portable TENS unit; continuous stimulation of 150 pulse width and frequency of 80 Hz; Four electrodes 4 X 4 cm placed on acupuncture points on the neck region, upper trapezius and elbow. The intensity was adjusted to produce a tingling sensation. Schemes of neck exercise programme: a set of activation of the deep neck muscles (muscle stabilisation), followed by active and resistive exercises of flexion and extension of the neck. Two sessions per week for six weeks, supervised by a physiotherapist. |
|
| Outcomes |
|
|
| Notes |
Funding sources: Area of Strategic Development Fund of the Hong Kong Polytechnic University, and the Health Services Research fund of the Hong Kong Government (HSRF 821017) Conflict of interest: none reported Full text language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to the exercise group, TENS group or the control group using a computer‐generated minimization method" Comment: the method used for sequence generation seems to be appropriate. |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐based randomization helps to establish allocation concealment" Comment: the method for assuring allocation concealment seems to be appropriate. |
| Blinding of participants | High risk | Comment: there was no blinding of participants. |
| Blinding of personnel/providers | High risk | Comment: there was no blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Patients were assessed by an independent assessor who was blinded to the grouping at baseline, after the six week treatment and at the six‐month follow‐up." Comment: the method for masking the outcome assessors seems to be appropriate. However, the primary outcomes pain, and disability were patient‐dependent and there was no blinding for participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study had an overall loss of 16.5%. The percentage of losses and distribution of reasons were different in the three groups, but it is unclear if these differences were statistically significant. |
| ITT analysis | Low risk | Quote: "Statistical analysis was based on the intention‐to‐treat approach." Comment: ITT analyses were used. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. |
| Group similarity at baseline | Low risk | Quote: "There was no significant difference in age, pain and education between patients across the three groups. In particular, no statistically significant differences were observed between the intervention groups and the control group in pain intensity (P = 0.33), neck pain questionnaire scores (P = 0.06) and isometric neck muscle strength before the intervention." Comment: the three groups seem to be similar at baseline. |
| Cointerventions | Low risk | Comment: the cointervention (infrared) was the same for all groups. |
| Compliance | Unclear risk | Comment: compliance with the intervention was not reported. |
| Timing of outcome assessments | Unclear risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures (pre, post‐treatment and after six months). |
| Other bias | Low risk | Comment: no other biases were identified. |
Gul 2009.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Turkey |
|
| Participants |
Sample size/available for analysis: 100/100 Gender: 69 females/31 males Age (years, mean and SD): 42.52 ± 10.64 Duration of disease (weeks, mean): more than 3 months Inclusion criteria: "The diagnosis of myofascial pain syndrome; being between 18 and 60 years old; literacy; tests are at normal limits" Exclusion criteria: "Cervical disc hernia, radiculopathy or myelopathy presence; tumoral, infectious, psychiatric, systemic disease and bleeding diathesis; Stage 3‐4 osteo degeneration; criteria for 1990 American College of Rheumatology according to the diagnosis of fibromyalgia syndrome; presence of kyphoscoliosis; pregnancy; having had brain or shoulder surgery before; treated for myofascial pain syndrome within the last 6 months; onset of symptoms is shorter than 3 months; uncontrolled hypertension" |
|
| Interventions |
Group 1: TENS (n = 25) Group 2: laser therapy (n = 25) Group 3: lidocaine injection (n = 25) Group 4: botulinum toxin‐A injection (n = 25) Treatment duration: 4 weeks Follow‐up duration: 45 days Schemes of TENS: the treatment consisted of a total of 60 sessions. Conventional TENS (60‐100 Hz, at 60‐100 mA of amplitude for 20 minutes), burst TENS (2‐4 Hz, at 150‐250 mA amplitude for 30 minutes), modulation TENS I (100 Hz, 20 minutes at 150‐200 mA amplitude), modulation TENS II (100 Hz, 150‐200 mA amplitude for 20 minutes) |
|
| Outcomes |
|
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: Turkish (translated via Cochrane Task Exchange) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants | High risk | Comment: different interventions (injection, TENS and laser) were compared and blinding was not possible. |
| Blinding of personnel/providers | High risk | Comment: different interventions (injection, TENS and laser) were compared and blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no losses of participants. |
| ITT analysis | Unclear risk | No available information |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. |
| Group similarity at baseline | Low risk | Comment: the groups were similar at baseline. |
| Cointerventions | Low risk | There was no cointervention |
| Compliance | Unclear risk | Comment: compliance with the intervention was not reported. |
| Timing of outcome assessments | Low risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures (pre, 15, 30 and 45 days after treatment). |
| Other bias | Low risk | Comment: no other biases were identified. |
Maayah 2010.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Jordan |
|
| Participants |
Sample size/available for analysis: 30/30 Gender: 15 females/15 males Age (years, mean and SD): 55.5 ± 7.81 Duration of disease (weeks, mean): 48% of the subjects reported mild pain for more than 5 years, 3% of the subjects had experienced severe pain for 7 months, 20% indicated that their pain had been quite severe for 5 years and 29% had a vague history of pain Inclusion criteria: "clinically and radiologically diagnosed neck pain due to musculoskeletal disorders; aged between 20 to 75 years; neck pain existed most days in the last month; received no treatment for neck pain other than oral analgesia for the duration of one week after the end of the first session; no previous TENS treatment" Exclusion criteria: "cardiac pacemaker; history of malignancy, which could be a current cause of bone pain" |
|
| Interventions |
Group 1: TENS (n = 15) Group 2: Sham TENS (n = 15) Treatment duration: single session of one hour Follow‐up duration: one week Schemes of TENS: one hour session; two silicone polymer electrodes by a two cord lead; the local used were acupuncture points around neck; the intensity was regulated to a comfort level; frequency was set at 4‐8 Hz (burst TENS). |
|
| Outcomes |
|
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "According to a block randomized allocation table (generated by sample size 2.0 Int), the enrolled subjects were allocated to either the TENS group or the placebo group" Comment: the method used for sequence generation seems to be appropriate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no available information. The authors did not answer our email asking for additional information. |
| Blinding of participants | Low risk | Quote: "subject should have had no previous TENS treatment". Comment: the participants were blinded. |
| Blinding of personnel/providers | High risk | Comment: due to the nature of the intervention, it was not possible to blind the physician who provided the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "It was therefore impossible for the investigator to be “blind” to treatment. Every attempt was made to ensure that treatment procedures were the same for each subject." Comment: unblinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there were no losses of participants. |
| ITT analysis | Unclear risk | Comment: no available information |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. |
| Group similarity at baseline | High risk | Quote: "The range of ages in the two groups was not similar: 23 ‐ 70 years in the treatment group and 35 ‐ 72 years in the control group. The mean age of the treatment group was 53.53 years compared to 58.2 years in the control group". "The duration of symptoms of musculoskeletal disorders was found to be greater among the treatment group". Comment: we are not sure to what extent these facts could influence the results. |
| Cointerventions | Low risk | There was no cointervention |
| Compliance | Low risk | Quote: "Each subject received one session for one hour." Comment: for single session interventions this item is irrelevant. |
| Timing of outcome assessments | Low risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures (pre, post‐treatment and after one week). |
| Other bias | Low risk | Comment: no other biases were identified. |
Sahin 2011.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Turkey |
|
| Participants |
Sample size/available for analysis: 80/75 Gender: 40 females/35 males Age (years, mean and SD): 31.67 ± 6.44 Duration of disease (weeks, mean): longer than three months Inclusion criteria: "Patients with chronic soft tissue neck pain for longer than three months, visual analogue scale [VAS] score more than 3 for pain, aged between 18 and 65 years, and had no physical therapy in the last six months" Exclusion criteria: "Patients with radicular pain complaints, neurological deficit and disc herniation, sensory deficit, cervical neural foramen stenosis and facet osteoarthritis as radiologic, fracture, congenital neck deformation, cervical spondylolysis and spondylolisthesis, serious trauma history, vertebra collapse, infection, malignancy, systemic disease, thoracic outlet syndrome, temporomandibular joint dysfunction, spinal surgery history, psychotic defect diagnosis, pregnancy, pacemaker, or having manual therapy" |
|
| Interventions |
Group 1: conventional TENS (n = 20) Group 2: acupuncture‐like TENS (n = 20) Group 3: burst TENS (n = 20) Group 4: sham TENS (n = 20) Treatment duration: 4 weeks Follow‐up duration: one week after treatment Schemes of TENS: all groups received 10 sessions of 30 minutes each; Group 1 used a frequency of 100 Hz, 40 μs duration, low amplitude, intensity that does not cause the patient discomfort and creates a mild tingling without contraction at a level below the motor threshold; Group 2 used a frequency of 4 Hz, 250 μs duration, high amplitude, and a high intensity at a level of muscle contraction; Group 3 used high [100 Hz] and low [2 Hz] frequencies, 40 μs duration, high amplitude, and high intensity at a level of muscle contraction and with consecutive stimuli. |
|
| Outcomes |
|
|
| Notes |
Funding sources: none Conflict of interest: none Full text language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The names of 80 patients were sealed in opaque envelopes and they were allocated randomly into four groups in a 1:1:1:1 ratio" Comment: the method used for sequence generation seems to be appropriate. |
| Allocation concealment (selection bias) | Low risk | Quote: "After the doctor’s examination, she gave the names of the patients included in the study to the person who prepared the envelope" Comment: the method used to maintain allocation concealment seems to be appropriate. |
| Blinding of participants | Unclear risk | Quote: "The patient and evaluating physician were kept blind to the type of the therapy" Comment: the authors did not report if participants were naive for TENS application. |
| Blinding of personnel/providers | High risk | Comment: due to the nature of the intervention, it was not possible to blind the physician who provided the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The evaluating physician was different from the physician who applied the therapy". "The patient and evaluating physician were kept blind to the type of the therapy" Comment: outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in each of the conventional, burst, and placebo groups, and two patients in the acupuncture‐like group dropped out of the study, equalling five patients unable to obtain permission from work to attend" Comment: there were few and balanced losses. |
| ITT analysis | Unclear risk | Comment: no available information |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. |
| Group similarity at baseline | Low risk | Quote: "There was no significant difference in age, sex, and BMI between groups" Comment: the groups were similar at baseline. |
| Cointerventions | Low risk | Comment: there was no cointervention. |
| Compliance | Unclear risk | Comment: no information available |
| Timing of outcome assessments | Low risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures (pre, post‐treatment and after one week) |
| Other bias | Low risk | Comment: no other biases were identified. |
Yesil 2018.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, multicentre Country: Turkey |
|
| Participants |
Sample size/available for analysis: 81/81 Gender: 56 females/25 males Age (years, mean and SD): Group 1: 36.03 ± 7.86/Group 2: 38.59 ± 9.19/Group 3: 39.74 ± 8.76 Duration of disease (weeks, mean): 21.70 (± 16.69) Inclusion criteria: "Eligible patients aged from 20 to 50 years old with at least three months of neck pain were included in the study" Exclusion criteria: "history of any contraindication for electrotherapy, involvement of any disease that may interfere with treatment, a disc herniation with neurological deficits, neoplasia, neck pain secondary to neurological or vascular diseases, infection or arthritis in the cervical region, diagnosed with any psychiatric diseases and treated for it, pregnancy, history of spinal surgery, physical therapy for neck region within the past six months" |
|
| Interventions |
Group 1: TENS + neck exercises (n = 27) Group 2: neck exercises (n = 27) Group 3: neck exercises + IFC (n = 27) Treatment duration: 5 times a week for 3 weeks Follow‐up duration: three months Schemes of TENS: (ITO ES‐320, Enraf Sonopuls 692, and Chattanooga Intelect) was applied at a frequency of 80 Hz with 10 mA to 30 mA intensity for 25 minutes. Four surface electrodes (5x5 cm each) were placed over the painful region in the neck with intensity in the tactile sensation threshold. Schemes of neck exercise programme: active and resistive exercises of neck muscles, supervised by a physical therapist. Five times a week for 3 weeks. |
|
| Outcomes |
|
|
| Notes |
Funding sources: no funds were received in support of this work. Conflict of interest: no relevant financial activities outside the submitted work. Full‐text language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed by the principal center of the study into three treatment groups". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was performed by the principal center of the study into three treatment groups". |
| Blinding of participants | High risk | Comment: different interventions (exercise and TENS) and they did not use any sham technique. |
| Blinding of personnel/providers | High risk | Comment: different interventions (exercise and TENS) and they did not use any sham technique. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "investigators, and analysts were blinded, with the exception of patients" Comment: the method for masking the outcome assessors seems to be appropriate. However, the primary outcomes pain and disability were patient‐dependent and there was no blinding for participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient in group 1 could not attend the 3rd month follow‐up visit due to health problems not related with neck pain". |
| ITT analysis | High risk | Comment: ITT analyses were not conducted. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was not available. |
| Group similarity at baseline | Low risk | Quote: "There were no significant differences among the groups regarding the demographic characteristics". |
| Cointerventions | Low risk | Comment: the cointervention (neck stabilisation exercises) was the same for all groups. |
| Compliance | Unclear risk | Comment: no information available |
| Timing of outcome assessments | Low risk | Comment: timing of outcome assessment is identical for all intervention groups and for all primary outcome measures. |
| Other bias | Low risk | Comment: no other biases were identified. |
ACR: American College of Rheumatology IFC: interferential current ITT: intention‐to‐treat KT: Kinesio taping SF‐36: Short Form‐36 SD: standard deviation TENS: transcutaneous electrical nerve stimulation VAS: Visual Analogue Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Airaksinen 1992 | Wrong patient population. The study excluded participants with headache of cervical origin. |
| Bloodworth 2004 | Wrong patient population. This study included participants with back and leg pain and imaging confirming lumbosacral radiculopathy. |
| Chee 1986 | Wrong study design. Not a RCT. |
| Farina 2004 | Wrong patient population. Not chronic neck pain. |
| Gemmell 2011 | Wrong patient population. Not chronic neck pain. |
| Hurwitz 2002 | Wrong intervention. The study did not consider TENS as an intervention, only EMS. |
| Jordan 1998 | Wrong intervention. The study did not consider TENS as an intervention. |
| Kim 2014 | Wrong patient population. Not chronic neck pain. |
| Kruger 1998 | Wrong study design. Quasi‐randomised study: the first patient was randomly allocated to a group by drawing lots, thereafter participants were alternated into treatment groups. |
| Lee 1997 | Wrong intervention. The study did not consider TENS as an intervention. |
| Mysliwiec 2011 | Wrong study design. Quasi‐randomised study: participants were alternated into treatment groups. |
| Prabhakar 2011 | Wrong patient population. Not chronic neck pain. |
| Rodriguez‐Fernandez 2011 | Wrong patient population. This study included asymptomatic participants with a latent trigger point in the upper fibres of the trapezius muscle. |
| Salim 1996 | Wrong patient population. No chronic neck pain. |
| Seo 2013 | Wrong intervention. This study compared two forms of electrical stimulation (with muscle contraction). |
| Simons 2006 | Wrong study design. This study is a narrative review of the literature. |
| Smania 2005 | Wrong patient population. No chronic neck pain. |
EMS: electrical muscle stimulation RCT: randomised controlled trial TENS: transcutaneous electrical nerve stimulation
Characteristics of studies awaiting assessment [ordered by study ID]
Acedo 2015.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Brazil |
| Participants |
Sample size/available for analysis: 64/64 Gender: all females Age (years, mean and SD): Group 1: 22.0 ± 3.0/Group 2: 23.0 ± 3.0 Duration of disease (months, mean and SD): Group 1: 2.1 ± 1.7/Group 2: 2.8 ± 0.9. The study included chronic and non‐chronic disease. We contacted the authors and asked them for data only for the chronic participants (at least 3 months). Inclusion criteria: "patients with a history of chronic nonspecific neck discomfort with a visual analogue scale (VAS) score over 3/10, if they use computer for at least 14 hours per week or 2 hours daily, and if they had pain during trigger point palpation of the neck area. For this evaluation, the trigger point was considered active if the subject presented local pain during a moderate digital pressure in the middle third of the upper trapezius." Exclusion criteria: "musculoskeletal disorders, physical therapy in the last 6 months, referred or irradiated pain, body mass index over 28, previous surgery involving the upper extremities, if they were pregnant or using corticosteroids or anti‐inflammatory medication, as well as other traditional TENS or IFC contraindications. A standard cervical clinical examination was performed to rule out concomitant pathology of the upper extremities." |
| Interventions |
Group 1: TENS (n = 32) Group 2: IFC (n = 32) Treatment duration: three days Follow‐up duration: five days Schemes of TENS: the TENS equipment (Quark, TensVif 993) was used in the burst mode, with a frequency of 100 Hz and pulse duration of 150 µs. Scheme of IFT: "The IFC (KLD Biosistemas, Endophasys) equipment had a carrier frequency of 4.000 Hz, an amplitude modulated frequency (AMF) of 100 Hz, a variation frequency (ΔF) of 60 Hz, and a slope of 6/6. The intensity (mA) in both devices was set at the tactile sensation threshold. The subjects of both groups (TENS and IFC) were submitted to the current application by self‐adhesive silicone electrodes, with the bipolar technique, i.e. 2 electrodes on each side of the upper trapezius for 30 minutes. In each side, one electrode was placed laterally on the C7 spinous process, and the other on the supraspinatus fossa. The intensity (mA) was increased according to the subject’s tolerance, remaining in the sensorial level." |
| Outcomes |
Primary outcomes
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason to await classification: it is not clear if the population includes chronic neck pain. Action: we contacted the authors asking for separate data from chronic participants (by email ‐ tfukuda10@yahoo.com.br ‐ on 30 January 2019). Author's response on 30 January 2019: the authors were unable to provide additional data. |
Ardic 2002.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Turkey |
| Participants |
Sample size/available for analysis: 40/40 Gender: 36 women / 4 men Age: 41.9 ± 7.8 years Duration of disease (weeks, mean and SD): Group 1: 3.47 ± 0.99/Group 2: 3.33 ± 1.23/Group 3: 3.50 ± 1.08. The study included chronic and non‐chronic disease. We asked the authors for the data only for chronic participants (at least 3 months). Inclusion criteria: participants with active MTrP in one side of the upper trapezius muscles. Exclusion criteria: "Fibromyalgia, myofascial trigger point injection or receiving physical therapy modalities within 1 year before the study, acute trauma, history of inflammatory joint or muscle disease, infection or malignancy, neurological deficit, inadequate cooperation, diagnosis of cervical radiculopathy or myelopathy." |
| Interventions |
Group 1: TENS and trapezius stretching exercises (n = 15) Group 2: EMS and trapezius stretching exercises (n = 15) Group 3: trapezius stretching exercises (n = 10) Treatment duration: one session therapy per day for two weeks Follow‐up duration: 3 months Schemes of TENS: "TENS was applied by a portable machine (ITO CO. Ltd, Japan) that generates symmetric, bi‐phasic rectangular pulses with 100 µsec duration. The current frequency was set at 60 Hz and intensity was increased up to patient’s perception of paresthesia. The negative electrode was placed on the active MTrP of the upper trapezius muscle and the positive one was placed on acromial tendon insertional site. The total duration of application was 20 minutes." Schemes of EMS: "A functional electrical muscle stimulator (ITO CO. Ltd, Japan) was used. The negative electrode was placed on the active MTrP of the upper trapezius muscle and the positive one was placed on acromial tendon insertional site. Symmetric, bi‐phasic rectangular pulses were set with current frequency at 25 Hz, pulse width as 250 µsec, hold time as 3 sec, rest time as 6 sec and ramp up/down time as 0.6 sec. The intensity was increased up to the patient’s tolerance, producing a strong upper trapezius muscle contraction. The total duration of application was 20 minutes." Schemes of control group: "The patients were instructed to follow a home program that included self‐stretching of the trapezius muscle. All the patients in all groups were instructed to do ten repetition of the exercises 3 times each day during the two weeks." |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason to await classification: it is not clear if the population included chronic neck pain. Action: we contacted authors asking for separate data from chronic participants (by email ‐ fardic@pamukkale.edu.tr ‐ on 30 January 2019 and 22 October 2019). The authors did not reply. |
Dissanayaka 2016.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Sri Lanka |
| Participants |
Sample size/available for analysis: 105/105 Gender: 58 females/47 males Age (years, mean): 35.97 Duration of disease (weeks, mean): not reported Inclusion criteria: "patients between 18 and 65 years of age who could read and understand a daily newspaper published in the Sinhala language and had at least one unilateral active upper trapezius MTrP, diagnosed by the presence of a sensitive (tender) spot in a palpable taut band with reproduction of pain when the sensitive spot was compressed" Exclusion criteria: "patients taking analgesics within 48 hours before the first physiotherapy treatment and/or patients who had been diagnosed with fibromyalgia, sensory disorders, radiculopathy/myelopathy, disc disease, psychologic disorders, degenerative joint diseases, or fracture or dislocation of the cervical vertebrae." |
| Interventions |
Group 1: TENS + standard care (n = 35) Group 2: IFC therapy + standard care (n = 35) Group 3: standard care (n = 35) Treatment duration: four weeks Follow‐up duration: one week All participants were provided with a home programme of self‐administered treatment. Schemes of TENS: "conventional TENS (SI no. 4270; Technomed Electronics, Tamil Nadu, India) for 20 minutes two times per week to deliver asymmetrical rectangular biphasic pulsed electrical currents at a pulse repetition frequency (rate) of 100 Hz and pulse duration (width) of 250 Ksecs. TENS was administered using a single channel and two electrodes (40 mm 50 mm), with the negative electrode (cathode) placed on the MTrP of the upper trapezius muscle and the positive electrode placed on the insertion of acromial tendon." Scheme of IFT: "IFT was administered by a dual channel IFT device (SI no. 4270; Technomed Electronics), using a quadripolar technique with electrodes placed around the MTrP of the upper trapezius muscle. One channel delivered sinusoidal B carrier currents [at a frequency of 4000 Hz and the other channel delivered currents at a frequency of 4100 Hz. This generated an amplitude modulated interference wave of 100 Hz (i.e. beat frequency). Pulse amplitude was set at a level to produce a strong but nonpainful TENS sensation but without visible or noticeable muscle contraction." Standard care: "Standard care consisted of hot pack treatment followed by active range of movement (AROM) exercises and myofascial release treatment. A hot pack was placed on the patients cervical, paraspinal, and upper thoracic areas (including the upper trapezius muscle with a MTrP) for 20 minutes. This was followed by AROM exercise for cervical spine joints. Participants were asked to actively flex the neck so that the head dropped toward the nonpainful (contralateral) trapezius muscles, causing stretch of the affected side. Patients then rotated the head toward the affected (ipsilateral) side. This exercise was repeated five times. Myofascial release was performed with the patient supine and the principal investigator sitting behind the patients head. The principal investigator placed her hands on the upper shoulders of the patient and stretched the upper trapezius muscle of the affected side downward and outward. This unilateral stretching and traction of the shoulder portion involving the upper trapezius with the MTrP were applied for 90 to 300 seconds until tightness was released. Participants were then provided with advice on correct posture (standing, sitting, sleeping, working) and treatment exercises (AROM, stretching, strengthening, and scapular stability exercises for upper trapezius) to carry out at home each day until the completion of final measurement. For each exercise, the participants were asked to perform one set of 10 repetitions three times a day. The patients were trained on correct posture and how to undertake exercises by the principal investigator at the end of the first treatment session. Participants were checked for competency and any errors in techniques of performing these, and if noted, those were corrected before they were sent home. Participants were asked to maintain a diary of home exercises, and this was checked by the principal investigator at each treatment session." |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
Funding sources: none Conflict of interest: none Full text language: English Reason to await classification: it is not clear if the population included chronic neck pain. Action: we contacted the authors asking for separate data from chronic participants (by email ‐ thushfhs@yahoo.com ‐ on 30 January, 2019 and October 22, 2019). The authors did not reply. |
Escortell‐Mayor 2011.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Spain |
| Participants |
Sample size/available for analysis: 71/90 Gender: not reported Age (years, mean): not reported Duration of disease (weeks, mean): not reported Inclusion criteria: "Mechanical neck disorders patient aged between 18 and 60 to be treated in primary health care physiotherapy units. Diagnoses of subacute or chronic mechanical neck disorder without neurological damage, according to the Classification of the Quebec Task Force on Spinal Disorders; full physical and psychological capacity to follow the clinical trial’s requirements; and their consent to participate." Exclusion criteria: "Signs of neurological damage according to the Neurologic Screening Checklist, pregnant women, previous neck rachis surgery, patients who received physical therapy or an alternative treatment of the neck or shoulder 6 months prior to the beginning of the study, those who intended to receive other treatments during the study or those with important psychiatric disorders or other health problems that would contraindicate the techniques to be used (i.e. pacemaker). Patients with neck pain caused by an inflammatory, neurological or rheumatic disease, severe osteoporosis, fracture, luxation or vertebrobasilar insufficiency." |
| Interventions |
Group 1: TENS (n = 43) Group 2: manual therapy (n = 47) Treatment duration: 10 treatment sessions of 30 min of manual therapy or TENS on alternate days Follow‐up duration: six months after treatment Schemes of TENS: TENS electrode placements were: in the painful area, in the metamere or in the nerve’s pathway. The frequency was 80 Hz, with 150 μs pulse duration and adjusted amplitude. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
Funding sources: this study was funded by the Instituto de Salud Carlos III, Fondo de Investigación Sanitaria/Fondos Europeos de Desarrollo Regional (PI N: 041320), Madrid, Spain. Full text language: English and Spanish Reason to await classification: it is not clear if the population included chronic neck pain. Action: we contacted authors asking for separate data on chronic participants (duration of symptoms longer than 12 weeks) (by email ‐ eescortell.gapm03@salud.madrid.org ‐ on 23 February 2019 and 10 October 2019). The authors did not reply. |
Graff‐Radford 1989.
| Methods |
Design: randomised controlled trial Setting: outpatients, single centre Country: USA |
| Participants |
Sample size/available for analysis: 60/losses were not reported Gender: 45 males/15 females Age: Mean 43.3 years (range 20 to 84) Duration of disease (weeks, mean and SD): not reported Inclusion criteria: “For inclusion in the study subjects had to have clinically active TPs which reproduced their pain complaint when palpated.” Exclusion criteria: not reported |
| Interventions |
Group 1: TENS "rate 2 Hz, pulse width 250 psec, delivered in an asymmetrical rectangular biphasic wave form (cathode phase), with zero net DC current, and an intensity set to the strongest tolerable sensation with muscular contraction (approximately lo‐40 mA)." N = 12 Group 2: TENS "rate 100 Hz, pulse width 250 psec, delivered in an asymmetrical rectangular biphasic wave form (cathode phase), with zero net DC current, and an intensity set to the patients comfort, below the threshold of muscular contraction (less than 39 mA)." N = 12 Group 3: TENS "rate 100 Hz, pulse width 50 psec, delivered in an asymmetrical rectangular biphasic wave form (cathode phase), with zero net DC current, and an intensity set to the patients comfort, below the threshold of muscular contraction (less than 39 mA)." Group 4: TENS "also termed the Pain Suppressor unit offers a low output amperage (max 4 mA), and 15 msec bursts of high frequency pulses (120%20,000 Hz rectified to a monophasic wave) with a burst frequency of 15 Hz. The intensity set at a level just below that perceived by the subject at a low amperage of approximately 1‐4 mA." N = 12 Group 5: control "this group was divided into 2 groups, 6 received placement of the Staodynamics unit, and the remaining 6 subjects, the Pain Suppressor unit. The battery was not in place in the control group TENS devices. TENS lasted 10 min and the electrodes were placed bilaterally at the same location, with the negative electrode over the active TP." N = 12 Treatment duration: one session (10 minutes) Follow‐up duration: the assessment was made after the intervention |
| Outcomes |
Primary outcomes
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason for awaiting classification: it is not clear if the population included chronic neck pain Action: we contacted authors asking for separate data on chronic participants (duration of symptoms longer than 12 weeks) (by email ‐ graffs@cshs.org ‐ on 22 October 2019. The authors did not reply. |
Hou 2002.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Republic of China |
| Participants |
Sample size/available for analysis: 71/71 Gender: 59 women, 12 men Age (years, range): 30 to 60 years Duration of disease (weeks, mean): not reported Inclusion criteria: "patients with cervical myofascial pain, with clinically active, palpable MTrPs in a single side or both sides of the upper trapezius muscle. No neck or shoulder surgery within the past year; no clinical evidence of radiculopathy or myelopathy; no history of disk disease, degenerative joint disease, fracture, or dislocation in the cervical vertebrae; no cognitive deficits; and a willingness to participate" Exclusion criteria: not reported |
| Interventions |
Group 1: hot pack (20 minutes) plus active ROM (n = 21) Group 2: hot pack (20 minutes) plus active ROM and ischaemic compression (n = 13) Group 3: TENS plus hot pack (20 minutes) plus active ROM, ischaemic compression (n = 9) Group 4: hot pack (20 minutes) plus active ROM and stretch with spray (n = 10) Group 5: TENS plus hot pack (20 minutes) plus active ROM, stretch with spray (n = 9) Group 6: hot pack (20 minutes) plus active ROM, IFC, and myofascial release technique (n = 9) Treatment duration: 1 session Follow‐up duration: within 5 minutes of completing treatment Schemes of TENS: the negative electrode was placed on the MTrP of the upper trapezius muscle, and the positive electrode was placed on the acromial tendon insertional site of the muscle. The current, with an asymmetrical rectangular biphasic form, was applied at a pulse repetition frequency of 100Hz and duty cycle of 250s; the intensity was set at a level that each subject could feel but that was not strong enough to induce muscle contraction. The current was applied for 20 minutes. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
Funding sources: none Conflict of interest: none Full text language: English Reason to await classification: it is not clear if the population included chronic neck pain. Action: we contacted the authors asking for separate data from chronic participants (by email ‐ hcr@speech114.csie.ncku.edu.tw ‐ on 23 February 2019 and 22 October 2019). The authors did not reply. |
Hsueh 1997.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Republic of China |
| Participants |
Sample size/available for analysis: 60/58 Gender: 35 females/25 males Age (years, mean and SD): 44.4 ± 13.9 Duration of disease (weeks, mean): not reported Inclusion criteria: "Patients with MTrPs in one side of the upper trapezius muscles" Exclusion criteria: "age less than 18 yr or more than 80 yr; acute or serious illness; mental retardation; neurologic deficits involving the investigated upper limb; advanced osteopathic or arthropathic disorder of the cervical spine or the shoulder of the investigated side." |
| Interventions |
Group 1: Sham TENS (n = 18) Group 2: TENS (n = 20) Group 2: NMES (n = 20) Treatment duration: single session Follow‐up duration: no follow‐up Schemes of TENS: the negative electrode was placed on the MTrP of the upper trapezius muscle and the positive one was placed on the acromial tendon insertion site. The frequency was 60Hz, intensity was not strong enough to induce muscle contraction. The duration of the session was 20 min. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason to await classification: it is not clear if the population included chronic neck pain. Action: we did not find the authors email. We contacted the institution where the study was conducted (by email ‐ em75284@email.ncku.edu.tw ‐ on 10 December 2018 and 22 October 2019). The authors did not reply. |
Ko 2002.
| Methods | The study was found only in Korean, and we were unable to retrieve most of the information from the manuscript. We tried to contact the authors but did not receive an answer. We are waiting for the translation for this paper, but we were not able to retrieve sufficient information to include or exclude it. Country: Korea |
| Participants |
Sample size/available for analysis: 45 Gender: 26 males/19 females Age: mean 47.2 years (SD 15.8) Full‐text language: Korean |
| Interventions | We were unable to retrieve this information. |
| Outcomes | We were unable to retrieve this information. |
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason for awaiting classification: we are not sure if this study is indeed a randomised controlled trial. We also were not able to retrieve the information regarding the duration of the disease nor if data for neck pain are available separately from other muscles, as they seem to investigate other anatomical sites. Action: we contacted the authors by email ‐ mhko@moak.chonbuk.ac.kr ‐ 22 October 2019). The authors did not reply. |
Suh 2015.
| Methods |
Design: single‐blind, randomised placebo‐controlled trial Setting: outpatients, multicentre Country: Korea |
| Participants |
Sample size/available for analysis: 47 Gender: not reported Age (years, mean): not reported Duration of disease (weeks, mean): not reported Inclusion criteria: "(1) 20–50 years of age, (2) employed for at least two years as a full‐time worker, and (3) neck and shoulder pain during more than 2 months of subacute state." Exclusion criteria: "any history of cervical spinal or upper limb surgery, structural abnormality, severe musculoskeletal disability, or use of pacemaker." |
| Interventions |
Group 1: TENS (n = 24) Group 2: Sham TENS (n = 23) Treatment duration: single session lasting for 60 min Follow‐up duration: measured immediately after TENS, and at 1 hour, 3 hours, and 1 day after TENS application. Schemes of TENS: high frequency (frequency 100Hz, pulse width 100 µs, motor threshold) was applied to tender trigger points of both the levator scapulae and trapezius muscles, using a 2‐channel TENS unit. Applied stimulation usually evoked the occurrence of visual muscle contraction. While electrodes were attached at the same location, no electrical stimuli were administered in the sham TENS group. |
| Outcomes |
Primary and secondary outcomes (not specified in text)
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason to await classification: it is not clear if the population included chronic neck pain. Action: we contacted the authors asking for separate data from chronic participants (by email ‐ gshan@gachon.ac.kr ‐ on 2 February 2019 and 22 October 2019). The authors did not reply. |
Vitiello 2007.
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients, single centre Country: Australia |
| Participants |
Sample size/available for analysis: 30/24 Gender: 10 males and 14 females Age: median age of 40 years Duration of disease (weeks, mean): minimum 6 weeks duration Inclusion criteria: "Adults from the geographically local population surrounding Sydney, Australia. Aged between 18 and 50 years. Chronic neck pain of a minimum 6 weeks duration. Assessed as non‐complicated neck pain, i.e. no sign or symptom implying cervical spine discogenic disease or radiculopathy." Exclusion criteria: "Suspicion of relevant Red Flag Conditions such as Spinal fractures, Osseous and Cartilaginous infections, Inflammatory Arthritic conditions, and Malignancy. Yellow Flag Conditions such as Non‐finalised Workers Compensation or Third Party Insurance Claim, Any other non‐finalised compensatory litigation. WAD grade 1–4 whiplash injury within the last six months. Presence of significant vascular disease. Severe or acute relapse of neck pain within the last three months. Motor vehicle accident, serious falls or any other accident requiring medical/hospital treatment within the last three months. Current neurological signs, symptoms or syndromes, e.g. muscle wasting or nerve root signs, epilepsy or paraplegia. Pregnancy or likelihood of pregnancy within the trial period. Spinal or orthopaedic surgery within the past two years. Bowel, or bladder/sexual dysfunction as a result of either lumbar spine or prostate dysfunction. Currently undergoing a course of manual therapy or psychological intervention. Participants not prepared to attend 12 treatment sessions within the first six weeks and a further three assessment sessions over the next 18 weeks." |
| Interventions |
Group 1: ENAR therapy (n = 9) Group 2: TENS therapy (n = 7) Group 3: ENAR sham control (n = 8) Treatment duration: 6 weeks Follow‐up duration: weeks 1, 6, 12, 18 and 24 of the trial Schemes of TENS: electrodes were applied to the skin overlying the posterior surface of the neck and upper thorax regions. Dosage was set to comfortable tolerance level set below muscle fasciculation response. Each of the groups received 10 minutes of their respective therapy. |
| Outcomes |
Primary and secondary outcomes (not specified in text)
|
| Notes |
Funding sources: not reported Conflict of interest: not reported Full text language: English Reason to await classification: it is not clear if the population included chronic neck pain. Action: we contacted the authors asking for separate data from chronic participants (by email ‐ mychiro@iinet.net.au ‐ on 23 February 2019 and 22 October 2019). The authors did not reply. |
EMS: electrical muscle stimulation ENAR: Electro Neuro Adaptive Regulator IFC: interferential current therapy MTrP: myofascial trigger point NMES: Neuromuscular Electrical Stimulation ROM: range of motion VAS: Visual Analogue Scale
Characteristics of ongoing studies [ordered by study ID]
RBR‐3knbwp.
| Trial name or title | Effects of a program of therapeutic exercises associated or not to electrotherapy in patients with chronic neck pain: blinded randomized clinical trial |
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients Country: Universidade Federal do Maranhão, São Luís/Brazil |
| Participants |
Inclusion criteria: "Individuals of both genders; aged between 18 and 45 years; and with chronic cervicalgia (for more than 90 days)". Exclusion criteria: "Individuals who presented a history of cervical trauma; head, face or cervical surgery; cervical hernia; degenerative diseases of the spine; pain radiated to the upper limbs; have undergone physiotherapeutic treatment for the cervical region in the last three months; use of analgesic, anti‐inflammatory or muscle relaxants in the last week; presence of systemic diseases; medical diagnosis of fibromyalgia." |
| Interventions | "Subjects will be submitted to 8 treatment sessions, two weekly sessions, for four weeks and lasting 50 minutes each session. The treatment programs will be applied by a physiotherapist with experience in clinical practice with patients with chronic pain. In addition, there will be training for six months prior to the start of the study to familiarize and standardize the proposed treatment programs." All groups will receive a 45‐minute pain education session prior to the intervention programme. Subjects will be randomised into three groups:
After the TENS application, the same therapeutic exercise programme will be applied in the three groups, in the same sequence, and repetitions. |
| Outcomes |
Primary outcomes
|
| Starting date | 17 January 2018 |
| Contact information | Almir Vieira Dibai Filho ‐ dibaifilho@gmail.com (Universidade Federal do Maranhão ‐ São Luís, MA, Brazil) |
| Notes |
RBR‐6c65dw.
| Trial name or title | Evaluation of the effects of chiropractic and transcutaneous electrical nerve stimulation (tension) on pain in patients with of mechanical origin neck pain |
| Methods |
Design: randomised controlled trial, parallel design Setting: outpatients Country: Faculdade Santo Agostinho, Teresina, Brazil |
| Participants |
Inclusion criteria: "To be between 20 and 39 years of age; to be of both sexes; to have cervicalgia of mechanical origin." Exclusion criteria: "Present a positive result for the klein test; acute fracture; spinal cord tumour; acute infections; malignant spinal neoplasm; frank disc herniation with signs of progressive neurological deficit; neoplasms of muscle tissue or other soft tissues; generalized congenital hypermobility; syringomyelia; hydrocephalus of unknown etiology." |
| Interventions | 40 people were equally divided into four groups:
|
| Outcomes |
Primary outcomes
|
| Starting date | 15 October 15 2017 |
| Contact information | Gabriel Martins de Barros ‐ gabrielmarrosthe@hotmail.com (Faculdade Santo Agostinho, Teresina, Brazil) |
| Notes |
Differences between protocol and review
Background: we added more details about transcutaneous electrical nerve stimulation (TENS) parameters and mechanisms, based on more recent studies.
Type of participants (Methods section): to avoid including people who had shoulder pain, we limited the inclusion criteria to participants with myofascial pain syndrome located in the upper trapezius muscle region.
Types of outcome measures: we included studies that did not report the outcomes of interest.
'Risk of bias' assessment: we report the results by bias domain (selection, performance, detection, attrition and reporting bias).
Data synthesis (Methods section): we removed the I² threshold for choosing between models and decided to use the random‐effects model for all meta‐analyses.
Assessment of heterogeneity (Methods section): we changed the criteria for substantial heterogeneity from 75% to 50%, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
'Summary of findings' table (Methods section): we assessed only primary outcomes for the main comparison: TENS versus sham TENS.
Contributions of authors
GJMP co‐ordinated the contributions from the co‐authors and wrote the final draft of the protocol. GJMP, RR, ALCM and MRT contributed to writing the methods and statistical analysis sections of the protocol. GJMP, RR and ALCM drafted the clinical sections of the protocol. GJMP, RR, ALCM and MRT contributed to writing the final draft of the protocol.
RR and ALCM contributed to study selection, data extraction, data analysis, 'Risk of bias' assessment and the GRADE assessment, as well as reviewing and editing the review. RLP contributed to data analysis, GRADE assessment, revision and editing of the review. MRT contributed to the revision and editing of the review and English proof.
Declarations of interest
ALCM has no known conflicts of interest.
GJMP has no known conflicts of interest.
RLP has no known conflicts of interest.
MRT has no known conflicts of interest.
RR has no known conflicts of interest.
New
References
References to studies included in this review
Azatcam 2016 {published data only}
- Azatcam G, Atalay NS, Akkaya N, Sahin F, Aksoy S, Zincir O, et al. Comparison of effectiveness of transcutaneous electrical nerve stimulation and kinesio taping added to exercises in patients with myofascial pain syndrome. Journal of Back Musculoskeletal Rehabilitation 2016;30(2):291‐8. [DOI] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen L, Zhang X, Ding H, Tao Y, Zhan H. Comparative study on effects of manipulation treatment and TENS on patients with cervicogenic headache. Zhong Xi Yi Jie He Xue Bao [Journal of Chinese Integrative Medicine] 2007;5:403‐6. [PUBMED: 17631795] [DOI] [PubMed] [Google Scholar]
Chiu 2005 {published data only}
- Chiu TT, Hui‐Chan CW, Chein G. A randomized clinical trial of TENS and exercise for patients with chronic neck pain. Clinical Rehabilitation 2005;19(8):850‐60. [PUBMED: 16323384] [DOI] [PubMed] [Google Scholar]
- Chiu TT, Lam TH, Hedley AJ. A randomized controlled trial on the efficacy of exercise for patients with chronic neck pain. Spine 2005;30(1):E1‐7. [PUBMED: 15626966] [PubMed] [Google Scholar]
Gul 2009 {published data only}
- Gul K, Onal SA. Comparison of non‐invasive and invasive techniques in the treatment of patients with myofascial pain syndrome [Miyofasiyal ağrı sendromlu hastaların tedavisinde non‐invazif ve invazif tekniklerin karşılaştırılması]. Ağrı (Algoloji) Derneği'nin Yayın organıdır [The journal of the Turkish Society of Algology] 2009;21(3):104‐12. [PUBMED: 19780001] [PubMed] [Google Scholar]
Maayah 2010 {published data only}
- Maayah M, Al‐Jarrah M. Evaluation of transcutaneous electrical nerve stimulation as a treatment of neck pain due to musculoskeletal disorders. Journal of Clinical Medicine Research 2010;2(3):127‐36. [DOI: 10.4021/jocmr2010.06.370e; PUBMED: 21629525] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahin 2011 {published data only}
- Sahin N, Albayrak I, Ugurlu H. Effect of different transcutaneous electrical stimulation modalities on cervical myofascial pain syndrome. Journal of Musculoskeletal Pain 2011;19(1):18‐23. [DOI: 10.3109/10582452.2010.538825] [DOI] [Google Scholar]
Yesil 2018 {published data only}
- Yesil H, Hepguler S, Dundar U, Taravati S, Isleten B. Does the use of analgesic current therapies increase the effectiveness of neck stabilization exercises for improving pain, disability, mood, and quality of life in chronic neck pain? a randomized, controlled, single‐blind study (a pilot study). Annals of the Rheumatic Diseases. Conference: Annual European Congress of Rheumatology, EULAR 2017;76(Suppl 2):475‐6. [Google Scholar]
- Yesil H, Hepguler S, Dundar U, Taravati S, Isleten B. Does the use of electrotherapies increase the effectiveness of neck stabilization exercises for improving pain, disability, mood, and quality of life in chronic neck pain? A randomized, controlled, single blind study. Spine 2018;43(20):E1174‐83. [DOI: 10.1097/BRS.0000000000002663] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Airaksinen 1992 {published data only}
- Airaksinen O, Pontinen PJ. Effects of the electrical stimulation of myofascial trigger points with tension headache. Acupuncture & Electro‐therapeutics Research 1992;17(4):285‐90. [DOI] [PubMed] [Google Scholar]
Bloodworth 2004 {published data only}
- Bloodworth DM, Nguyen BN, Garver W, Moss F, Pedroza C, Tran T, et al. Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. American Journal of Physical Medicine & Rehabilitation 2004;83(8):584‐91. [DOI] [PubMed] [Google Scholar]
Chee 1986 {published data only}
- Chee EK, Walton H. Treatment of trigger points with microamperage transcutaneous electrical nerve stimulation (TENS)‐(the Electro‐Acuscope 80). Journal of Manipulative and Physiological Therapeutics 1986;9(2):131‐4. [PubMed] [Google Scholar]
Farina 2004 {published data only}
- Farina S, Casarotto M, Benelle M, Tinazzi M, Fiaschi A, Goldoni M, et al. A randomized controlled study on the effect of two different treatments (FREMS AND TENS) in myofascial pain syndrome. Europa Medicophysica 2004;40(4):293‐301. [PubMed] [Google Scholar]
Gemmell 2011 {published data only}
- Gemmell H, Hilland A. Immediate effect of electric point stimulation (TENS) in treating latent upper trapezius trigger points: a double blind randomised placebo‐controlled trial. Journal of Bodywork and Movement Therapies 2011;15(3):348‐54. [DOI] [PubMed] [Google Scholar]
Hurwitz 2002 {published data only}
- Hurwitz EL, Morgenstern H, Harber P, Kominski GF, Yu F, Adams AH. A randomized trial of chiropractic manipulation and mobilization for patients with neck pain: clinical outcomes from the UCLA neck‐pain study. American Journal of Public Health 2002;92(10):1634‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 1998 {published data only}
- Jordan A, Bendix T, Nielsen H, Hansen FR, Host D, Winkel A. Intensive training, physiotherapy, or manipulation for patients with chronic neck pain. A prospective, single‐blinded, randomized clinical trial. Spine 1998;23(3):311‐8; discussion 319. [DOI] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim DH, Yoon KB, Park S, Jin TE, An YJ, Schepis EA, et al. Comparison of NSAID patch given as monotherapy and NSAID patch in combination with transcutaneous electric nerve stimulation, a heating pad, or topical capsaicin in the treatment of patients with myofascial pain syndrome of the upper trapezius: a pilot study. Pain Medicine 2014;15(12):2128‐38. [DOI] [PubMed] [Google Scholar]
Kruger 1998 {published data only}
- Kruger LR, Linden WJ, Cleaton‐Jones PE. Transcutaneous electrical nerve stimulation in the treatment of myofascial pain dysfunction. South African Journal of Surgery 1998;36(1):35‐8. [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee JC, Lin DT, Hong CZ. The effectiveness of simultaneous thermotherapy with ultrasound and electrotherapy with combined AC and DC current on the immediate pain relief of myofascial trigger points. Journal of Musculoskeletal Pain 1997;1:81‐90. [Google Scholar]
Mysliwiec 2011 {published data only}
- Mysliwiec A, Saulicz E, Kuszewski M, Kokosz M, Wolny T. Assessment of the influence of Saunders traction and transcutaneous electrical nerve stimulation on hand grip force in patients with neck pain. Ortopedia, Traumatologia, Rehabilitacja 2011;13(1):37‐44. [DOI] [PubMed] [Google Scholar]
Prabhakar 2011 {published data only}
- Prabhakar R, Ramteke GJ. Cervical spinal mobilization versus TENS in the management of cervical radiculopathy: a comparative, experimental and randomized controlled trial. Indian Journal of Physiotherapy & Occupational Therapy 2011;5(1):95‐9. [Google Scholar]
Rodriguez‐Fernandez 2011 {published data only}
- Rodriguez‐Fernandez AL, Garrido‐Santofimia V, Gueita‐Rodriguez J, Fernandez‐de‐Las‐Penas C. Effects of burst‐type transcutaneous electrical nerve stimulation on cervical range of motion and latent myofascial trigger point pain sensitivity. Archives of Physical Medicine and Rehabilitation 2011;92(9):1353‐8. [DOI] [PubMed] [Google Scholar]
Salim 1996 {published data only}
- Salim M. Transcutaneous electrical nerve stimulation (TENS) in chronic pain. Alternative Therapies in Clinical Practice 1996;3(4):33‐5. [Google Scholar]
Seo 2013 {published data only}
- Seo HG, Bang MS, Chung SG, Jung SH, Lee SU. Effect of electrical stimulation on botulinum toxin a therapy in patients with chronic myofascial pain syndrome: a 16‐week randomized double‐blinded study. Archives of Physical Medicine and Rehabilitation. 2013;94(3):412‐8. [DOI] [PubMed] [Google Scholar]
Simons 2006 {published data only}
- Simons DG, Dommerholt J. Myofascial Pain Syndromes ‐ Trigger Points. Journal of Musculoskeletal Pain 2006;14(2):59‐64. [Google Scholar]
Smania 2005 {published data only}
- Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM, Tinazzi M. Repetitive magnetic stimulation a novel therapeutic approach for myofascial pain syndrome. Journal of Neurology 2005;252(3):307‐14. [PUBMED: 15726272] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Acedo 2015 {published data only}
- Acedo AA, Antunes AC, dos Santos Ab, Olveira CB, dos Santos CT, Colonezi GL, et al. Upper trapezius relaxation induced by TENS and interferential current in computer users with chronic nonspecific neck discomfort: An electromyographic analysis. Journal of Back and Musculoskeletal Rehabilitation 2015;28(1):19‐24. [DOI] [PubMed] [Google Scholar]
Ardic 2002 {published data only}
- Ardic F, Sarhus M, Topuz O. Comparison of two different techniques of electrotherapy on myofascial pain. Journal of Back and Musculoskeletal Rehabilitation 2002;16(1):11‐6. [DOI] [PubMed] [Google Scholar]
Dissanayaka 2016 {published data only}
- Dissanayaka TD, Pallegama RW, Suraweera HJ, Johnson MI, Kariyawasam AP. Comparison of the effectiveness of transcutaneous electrical nerve stimulation and interferential therapy on the upper trapezius in myofascial pain syndrome: A randomized controlled study. American Journal of Physical Medicine and Rehabilitation 2016;95(9):663‐72. [DOI] [PubMed] [Google Scholar]
Escortell‐Mayor 2011 {published data only}
- Escortell‐Mayor E, Riesgo‐Fuertes R, Garrido‐Elustondo S, Asunsolo‐Del Barco A, Diaz‐Pulido B, Blanco‐Diaz M, et al. Primary care randomized clinical trial: manual therapy effectiveness in comparison with TENS in patients with neck pain. Manual Therapy 2011;16(1):66‐73. [PUBMED: 20691631] [DOI] [PubMed] [Google Scholar]
- Mayor EE, Perez GL, Martin YP, Barco AA, Fuertes RR, Requejo CS. Randomised clinical trial for primary care patients with neck pain: Manual therapy versus electrical stimulation. Atencion Primaria 2008;40(7):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido BD, Asunsolo A, Ortiz MF, Escortell E, Pecos D, Perez Y. Randomized clinical trial: Manual therapy effectiveness in comparison with tens in cervical mobility in patients with mechanical neck disorders. World Physical Therapy; 2011 Jun 20‐23; Amsterdam, Netherlands. 2011:eS1026‐7.
Graff‐Radford 1989 {published data only}
- Graff‐Radford SB, Reeves JL, Baker RL, Chiu D. Effects of transcutaneous electrical nerve stimulation on myofascial pain and trigger point sensitivity. Pain 1989;37(1):1‐5. [DOI] [PubMed] [Google Scholar]
Hou 2002 {published data only}
- Hou CR, Tsai LC, Cheng KF, Chung KC, Hong CZ. Immediate effects of various physical therapeutic modalities on cervical myofascial pain and trigger‐point sensitivity. Archives of Physical Medicine and Rehabilitation 2002;83:1406‐14. [DOI] [PubMed] [Google Scholar]
Hsueh 1997 {published data only}
- Hsueh TC, Cheng PT, Kuan TS, Hong CZ. The immediate effectiveness of electrical nerve stimulation and electrical muscle stimulation on myofascial trigger points. American Journal of Physical Medicine and Rehabilitation 1997;76(6):471‐6. [PUBMED: 9431265] [DOI] [PubMed] [Google Scholar]
Ko 2002 {published data only}
- Ko MH, Byeon HT, Seo JH, Kim YH. The effect of intramuscular electrical stimulation in myofascial pain syndrome. Journal of Korean Academy of Rehabilitation Medicine 2002;26(5):562‐6. [Google Scholar]
Suh 2015 {published data only}
- Suh HR, Kim TH, Han GS. The effects of high‐frequency transcutaneous electrical nerve stimulation for dental professionals with work‐related musculoskeletal disorders: a single‐blind randomized placebo‐controlled trial. Evidence‐based Complementary and Alternative Medicine 2015 Nov 17 [Epub ahead of print]. [DOI: 10.1155/2015/327486] [DOI] [PMC free article] [PubMed]
Vitiello 2007 {published data only}
- Vitiello AL, Bonello R, Pollard H. The effectiveness of ENAR for the treatment of chronic neck pain in Australian adults: a preliminary single‐blind, randomised controlled trial. Chiropractic & Osteopathy 2007;15:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
RBR‐3knbwp {published data only}
- RBR‐3knbwp. Effects of a therapeutic exercise program associated or not with electrical currents in patients with chronic neck pain. apps.who.int/trialsearch/Trial2.aspx?TrialID=RBR‐3knbwp (first received 17 January 2018).
RBR‐6c65dw {published data only}
- RBR‐6c65dw. Joint manipulation and electrotherapy in cervical pain. www.braziliantrials.com/index/view/ensaios/2069?_language=pt‐br&keywords=&order=ensaios.patrocinador_primario (first received 15 October 2017).
Additional references
Allen 2006
- Allen RJ. Physical agents used in the management of chronic pain by physical therapists. Physical Medicine and Rehabilitation Clinics of North America 2006;17(2):315‐45. [DOI] [PubMed] [Google Scholar]
Bjordal 2003
- Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption. A meta‐analysis with assessment of optimal treatment parameters for postoperative pain. European Journal of Pain 2003;7(2):181‐8. [DOI] [PubMed] [Google Scholar]
Cameron 2003
- Cameron MH. Physical Agents in Rehabilitation: From Research to Practice. Philadelphia: WB Saunders, 2003. [Google Scholar]
Chow 2009
- Chow RT, Johnson MI, Lopes‐Martins RA, Bjordal JM. Efficacy of low‐level laser therapy in the management of neck pain: a systematic review and meta‐analysis of randomised placebo or active‐treatment controlled trials. Lancet 2009;374(9705):1897‐908. [DOI: 10.1016/S0140-6736(09)61522-1] [DOI] [PubMed] [Google Scholar]
Duffy 2014
- Duffy S, Misso K, Noake C, Ross J, Stirk L. Supplementary searches of PubMed to improve currency of MEDLINE and MEDLINE In‐Process searches via OvidSP. Kleijnen Systematic Reviews Ltd, York. Poster presented at the UK InterTASC Information Specialists' Sub‐Group (ISSG) Workshop; 9 July 2014; Exeter: UK (2014): medicine.exeter.ac.uk/media/universityofexeter/medicalschool/research/pentag/documents/Steven_Duffy_ISSG_Exeter_2014_poster_1.pdf.
Furlan 2015
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, et al. Editorial Board of the Cochrane Back, Neck Group. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660‐73. [DOI] [PubMed] [Google Scholar]
Gibson 2019
- Gibson W, Wand BM, Meads C, Catley MJ, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain ‐ an overview of Cochrane Reviews. Cochrane Database of Systematic Reviews 2019, Issue 2. [DOI: 10.1002/14651858.CD011890.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 27 November 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guzman 2008
- Guzman J, Hurwitz EL, Carroll LJ, Haldeman S, Côté P, Carragee EJ, et al. A new conceptual model of neck pain: linking onset, course, and care: the Bone and Joint Decade 2000‐2010 Task Force on Neck Pain and its Associated Disorders. Spine 2008;33:S14‐23. [DOI: 10.1097/BRS.0b013e3181643efb] [DOI] [PubMed] [Google Scholar]
Han 1991
- Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, et al. Effect of low and high frequency TENS on Met‐enkephalin‐Arg‐Phe and dynorphin A immunoreactivity inhuman lumbar CSF. Pain 1991;47(3):295‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hogg‐Johnson 2008
- Hogg‐Johnson S, Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, et al. The burden and determinants of neck pain in the general population: results of the Bone and Joint Decade 2000‐2010 Task Force on Neck Pain and its Associated Disorders. Spine 2008;33(4 Suppl):S39‐51. [DOI: 10.1097/BRS.0b013e31816454c8] [DOI] [PubMed] [Google Scholar]
Hughes 1984
- Hughes GS, Lichstein PR, Whitlock D, Harker C. Response of plasma beta‐endorphins to transcutaneous electrical nerve stimulation in healthy subjects. Physical Therapy 1984;64:1062‐6. [DOI] [PubMed] [Google Scholar]
Johnson 2007a
- Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: A meta‐analysis of randomized controlled trials. Pain 2007;130:157‐65. [DOI] [PubMed] [Google Scholar]
Johnson 2007b
- Johnson M. Transcutaneous Electrical Nerve Stimulation: Mechanisms, Clinical Application and Evidence. Reviews in Pain 2007;1(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2015
- Johnson MI, Paley CA, Howe TE, Sluka KA. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD006142.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2016
Johnson 2017
- Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD012172.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalra 2001
- Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation(TENS). Journal of Pharmacology and Experimental Therapeutics 2001;298:257‐63. [PubMed] [Google Scholar]
Khadilkar 2008
- Khadilkar A, Odebiyi DO, Brosseau L, Wells GA. Transcutaneous electrical nerve stimulation (TENS) versus placebo for chronic low‐back pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003008.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroeling 2013
- Kroeling P, Gross A, Graham N, Burnie SJ, Szeto G, Goldsmith CH, et al. Electrotherapy for neck pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004251.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leaver 2010
- Leaver AM, Refshauge KM, Maher CG, McAuley JH. Conservative interventions provide short‐term relief for non‐specific neck pain: a systematic review. Journal of Physiotherapy 2010;56(2):73‐85. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;21(6(7)):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971‐8. [DOI] [PubMed] [Google Scholar]
Monticone 2013
- Monticone M, Cedraschi C, Rocca B, Fiorentini R, Restelli M, Gianola SE, et al. Cognitive‐behavioural treatment for subacute and chronic neck pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moran 2011
- Moran F, Leonard T, Hawthorne S, Hughes CM, McCrum‐Gardner E, Johnson MI, et al. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. Journal of Pain 2011;12(8):929‐35. [DOI] [PubMed] [Google Scholar]
Mueller 2007
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Ideas and Opinions 2007;146:878‐82. [DOI] [PubMed] [Google Scholar]
Niemisto 2003
- Niemisto L, Kalso EA, Malmivaara A, Seitsalo S, Hurri H. Radiofrequency denervation for neck and back pain. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004058] [DOI] [PubMed] [Google Scholar]
Nnoaham 2008
- Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003222.pub2] [DOI] [PubMed] [Google Scholar]
Olesen 1988
- Olesen J. Classification and diagnostic criteria for headache disorders, cranial neuroalgias and facial pain. Cephalgia 1988;8(Suppl 7):61‐2. [PubMed] [Google Scholar]
Olesen 1997
- Olesen J, Gobel H. ICD‐10 Guide for Headaches. Guide to the classification, diagnosis and assessment of headaches in accordance with the tenth revision of the International Classification of Diseases and Related Health Problems and its application to neurology. Cephalagia 1997;17(Suppl 19):29‐30. [Google Scholar]
Rubinstein 2007
- Rubinstein SM, Pool JJ, Tulder MW, Riphagen II, Vet HC. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. European Spine Journal 2007;16(3):307‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine 2010;8(18):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 1993
- Schumacher HR, Klippel JH, Koopman WJ, editor(s). Primer on the Rheumatic Diseases. 10th Edition. Atlanta: Arthritis Foundation, 1993. [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shealy 1967
- Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns. Anesthesia and Analgesia: Current Researches 1967;46:299‐304. [PubMed] [Google Scholar]
Sjaastad 1990
- Sjaastad O, Fredriksen TA, Pfaffenrath V. Cervicogenic headache: diagnostic criteria. Headache 1990;30:725‐6. [DOI] [PubMed] [Google Scholar]
Sjolund 1976
- Sjolund B, Eriksson M. Electro‐acupuncture and endogenous morphines. Lancet 1976;308(7994):1085. [DOI] [PubMed] [Google Scholar]
Sluka 2003
- Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. Journal of Pain 2003;4(3):109‐21. [DOI] [PubMed] [Google Scholar]
Sluka 2013
- Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Physical Therapy 2013;93(10):1397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spitzer 1987
- Spitzer WO, Leblanc FE, Dupuis M. Scientific approach to the assessment and management of activity related spinal disorders. Spine 1987;Suppl 7:S1‐S59. [PubMed] [Google Scholar]
Spitzer 1995
- Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, et al. Scientific monograph of the Quebec Task Force on Whiplash‐Associated Disorders: redefining "whiplash" and its management. Spine 1995;20(8 Suppl):1S‐73S. [PubMed] [Google Scholar]
Tsang 2001
- Tsang I. Rheumatology: 12. Pain in the neck. Canadian Medical Association Journal 2001;164(8):1182–7. [PMC free article] [PubMed] [Google Scholar]
Turk 2008
- Turk DC, Dworkin RH, McDermott MP, Bellamy N, Burke LB, Chandler JM, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008;137(2):276‐85. [DOI] [PubMed] [Google Scholar]
Viikari‐Juntura 2001
- Viikari‐Juntura E, Martikainen R, Luukkonen R, Mutanen P, Takala EP, Riihimäki H. Longitudinal study of work‐related and individual risk factors of radiating neck pain. Occupational and Environmental Medicine 2001;58:345‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wall 1967
- Wall PD, Sweet WH. Temporary abolition of pain in man. Science 1967;155(3758):108‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Porfirio 2015
- Porfírio GJM, Martimbianco ALC, Brønfort G, Torloni MR, Riera R. Transcutaneous electrical nerve stimulation (TENS) for chronic neck pain. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011927] [DOI] [PMC free article] [PubMed] [Google Scholar]


