Abstract
Background
Increasing molecular evidence supports that bats and/or their ectoparasites may harbor vector-borne bacteria, such as bartonellae and borreliae. However, the simultaneous occurrence of rickettsiae in bats and bat ticks has been poorly studied.
Methods
In this study, 54 bat carcasses and their infesting soft ticks (n = 67) were collected in Shihezi City, northwestern China. The heart, liver, spleen, lung, kidney, small intestine and large intestine of bats were dissected, followed by DNA extraction. Soft ticks were identified both morphologically and molecularly. All samples were examined for the presence of rickettsiae by amplifying four genetic markers (17-kDa, gltA, ompA and ompB).
Results
All bats were identified as Pipistrellus pipistrellus, and their ticks as Argas vespertilionis. Molecular analyses showed that DNA of Rickettsia parkeri, R. lusitaniae, R. slovaca and R. raoultii was present in bat organs/tissues. In addition, nine of the 67 bat soft ticks (13.43%) were positive for R. raoultii (n = 5) and R. rickettsii (n = 4). In the phylogenetic analysis, these bat-associated rickettsiae clustered together with conspecific sequences reported from other host and tick species, confirming the above results.
Conclusions
To the best of our knowledge, DNA of R. parkeri, R. slovaca and R. raoultii was detected for the first time in bat organs/tissues. This is also the first molecular evidence for the presence of R. raoultii and R. rickettsii in bat ticks. To our knowledge, R. parkeri was not known to occur in Asia. Our results highlight the need to assess rickettsial agents in a broader range of bat species and associated tick species.
Keywords: Rickettsia, Chiroptera, Vespertilionidae, Argasidae
Background
Bats (order Chiroptera), including at least 1400 species [1], are the only mammals which actively fly [2]. Among the consequences of this trait, bats show a geographically widespread distribution and may even undergo short- to long-distance seasonal migration [2]. Bats are special in their capacity to act as reservoir hosts for intracellular pathogens [3]. Several species of bat-associated soft (Acari: Argasidae) and hard (Acari: Ixodidae) ticks, i.e. Carios kelleyi, Argas vespertilionis, A. transgariepinus, Ornithodoros sp., Ixodes vespertilionis, I. ariadnae and I. simplex, were shown to carry DNA of vector-borne bacteria and protozoans [4, 5]. Among these tick species, A. vespertilionis (which has a wide distribution in Europe, Africa and Asia) was reported to bite humans [6]. In addition, increasing molecular evidence supports that bats and/or their ticks may harbor vector-borne zoonotic bacteria, such as bartonellae and borreliae [7, 8]. However, the occurrence of rickettsiae in bats and bat ticks appears to be poorly studied, particularly in Eurasia.
Rickettsiae are Gram-negative, obligatory intracellular bacteria, which may cause disease in animals and humans, and are associated with arthropod vectors (such as ticks, lice, fleas or mites), both as transmitters and reservoirs [9]. Concerning rare reports on rickettsiae from bats and their ticks, previously Rickettsia africae was detected in the blood of bats in the Caribbean region [10] and several rickettsiae were identified in bat ticks from Central America, Europe, Africa and Asia [11]. However, to the best of our knowledge, a simultaneous analysis of bat tissues and bat ticks for the presence of rickettsiae in central Asia has not been carried out. Our hypothesis is that bats might be susceptible to infection with some rickettsial species. In our study, we aimed at evaluating the susceptibility of bats to rickettsial infection and the involvement of these flying mammals and their ticks in Rickettsia transmission cycles in central Asia.
Methods
Sample collection and identification
Fifty-four bat carcasses were collected from an idle classroom in Shihezi University, Xinjiang Uygur Autonomous Region (XUAR) in northeastern China during 2015–2019. The heart, liver, spleen, lung, kidney, small intestine and large intestine of bat carcasses were removed, similarly to what has been reported in studies of bat haemoparasites [12]. Genomic DNA was extracted from these organs, as well as from ticks collected from the bats using the 96 Flux Automatic Nucleic Acid Extraction Instrument (Bio Teke, Beijing, People’s Republic of China) with a matching commercial kit (Cell & Tissue Kit, Bio Teke) according to the manufacturer’s instructions. To confirm the morphological identification of bats, the cytochrome b (cytb) gene was analyzed [13] and a representative cytb sequence was deposited in the GenBank database under the accession number MF106222.
Simultaneously, a total of 67 tick larvae were collected from bat bodies. The ticks were morphologically identified according to the standard taxonomic keys as previously described [14]. From five ticks, the 16S rDNA gene was amplified following a previously reported protocol [15]. The corresponding 16S rDNA sequence was deposited in the GenBank database under the accession number MF106219.
Detection of rickettsiae, sequencing and phylogenetic analyses
Four genetic markers, including 17 kDa antigen (17-kDa), citrate synthase (gltA), and outer membrane proteins A and B (ompA and ompB) genes were assessed within each sample to investigate the presence of rickettsiae [15]. The primers and PCR cycling conditions in this study are shown in Additional file 1: Text S1 and Table S1. Each PCR assay included a negative control (distilled water instead of tick DNA template) and a positive control (containing sequence-verified DNA from R. raoultii obtained from the tick Dermacentor nuttalli collected in XUAR) [15]. Purification and sequencing of the PCR products were performed as described above [16, 17].
Sequences were manually edited, aligned and compared to reference GenBank sequences by nucleotide BLASTn program (https://blast.ncbi.nlm.nih.gov). A phylogenetic tree was constructed using the maximum-likelihood and neighbor-joining algorithms implemented in MEGA 6.0 software [18].
Results
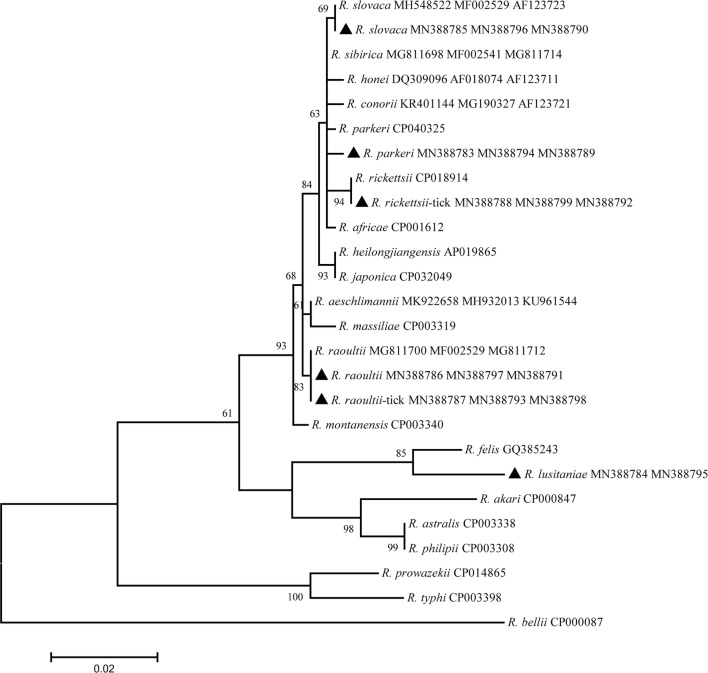
All bats were identified as Pipistrellus pipistrellus, and their ticks as A. vespertilionis. Out of 378 bat organs/tissues and 67 bat ticks, 6 bats and 9 ticks were positive for the four Rickettsia genetic markers (17-kDa, gltA, ompA and ompB). Sequencing identified R. parkeri in the heart, liver and kidney of a bat, R. lusitaniae in the heart, liver and small intestine of a bat, R. slovaca in the lung and kidney of two bats and R. raoultii was only found in the liver of two bats (Fig. 1, Table 1). Concerning bat ticks, R. raoultii was detected in five and R. rickettsii in four specimens. Interestingly, four ticks positive to R. raoultii were removed from a R. raoultii-positive bat.
Fig. 1.
Phylogenetic tree of the ompA-gltA-ompB concatenated sequences for the rickettsial agents in bats and their infesting ticks. The new sequences provided by the present study are indicated by a black triangle
Table 1.
The prevalence of Rickettsia parkeri, R. lusitaniae, R. slovaca, R. raoultii, R. rickettsii detected in the bat organs
| Species | Heart | Liver | Spleen | Lung | Kidney | Small intestine | Large intestine |
|---|---|---|---|---|---|---|---|
| R. parkeri | 1/54 (1.9%) | 1/54 (1.9%) | – | – | 1/54 (1.9%) | – | – |
| R. lusitaniae | 1/54 (1.9%) | 1/54 (1.9%) | – | – | – | 1/54 (1.9%) | – |
| R. slovaca | – | – | 2/54 (3.7%) | 2/54 (3.7%) | – | – | |
| R. raoultii | – | 2/54 (3.7%) | – | – | – | – | – |
Regarding sequence comparisons based on the four genetic markers, R. parkeri in this study had sequence identities within the range of 99.7–100% when compared with the sequence of R. parkeri from the tick Amblyomma ovale in Colombia (GenBank: CP040325); Rickettsia lusitaniae showed 98.6–100% identity compared with the sequence of R. lusitaniae from A. vespertilionis infesting P. pipistrellus in China [11]; Rickettsia slovaca had 99.8% sequence identity with a conspecific bacteria from Dermacentor marginatus in Turkey; Rickettsia raoultii showed 99.1–100% identity with R. raoultii from D. nuttalli infesting Spermophilus undulatus in northwestern China [13]; and Rickettsia rickettsii was 99.7–99.9% identical to R. rickettsii from D. variabilis in the USA [19]. The detailed similarities and divergences of the sequences in this study are shown in Table 2 and Additional file 2: Table S2.
Table 2.
All data for the newly sequenced isolates and their GenBank accession numbers for all genes
| Gene | Species | Host | Isolate | GenBank ID |
|---|---|---|---|---|
| cytb | P. pipistrellus | P. pipistrellus | P. pipistrellus | MF106222 |
| 16S | A. vespertilionis | A. vespertilionis | A. vespertilionis | MF106219 |
| OmpA | R. parkeri | P. pipistrellus | Heart, liver, kidney | MN388783 |
| OmpA | R. lusitaniae | P. pipistrellus | Heart, liver, small intestine | MN388784 |
| OmpA | R. slovaca | P. pipistrellus | Lung, kidney | MN388785 |
| OmpA | R. raoultii | P. pipistrellus | Liver | MN388786 |
| OmpA | R. raoultii | A. vespertilionis | A. vespertilionis | MN388787 |
| OmpA | R. rickettsii | A. vespertilionis | A. vespertilionis | MN388788 |
| OmpB | R. parkeri | P. pipistrellus | Heart, liver, kidney | MN388789 |
| OmpB | R. slovaca | P. pipistrellus | Lung, kidney | MN388790 |
| OmpB | R. raoultii | P. pipistrellus | Liver | MN388791 |
| OmpB | R. rickettsii | A. vespertilionis | A. vespertilionis | MN388792 |
| OmpB | R. raoultii | A. vespertilionis | A. vespertilionis | MN388793 |
| gltA | R. parkeri | P. pipistrellus | Heart, liver, kidney | MN388794 |
| gltA | R. lusitaniae | P. pipistrellus | Heart, liver, small intestine | MN388795 |
| gltA | R. slovaca | P. pipistrellus | Lung, kidney | MN388796 |
| gltA | R. raoultii | P. pipistrellus | Liver | MN388797 |
| gltA | R. raoultii | A. vespertilionis | A. vespertilionis | MN388798 |
| gltA | R. rickettsii | A. vespertilionis | A. vespertilionis | MN388799 |
| 17-kDa | Rickettsia sp. | P. pipistrellus | P. pipistrellus | MN388800 |
Discussion
Based on a recent review, R. lusitaniae, R. slovaca, R. raoultii and R. rickettsii have already been found to occur in Asia [20]. To the best of our knowledge, R. parkeri was detected for the first time on the Eurasian continent in the present study. Previously, R. conorii and R. orientalis have been identified in human urine and urine of albino Swiss mice [21, 22] and R. helvetica has been detected in bat feces [23]. Here, the kidneys from common pipistrelle bats were positive for R. parkeri and R. slovaca, while the small intestine was positive for R. lusitaniae, and the lung was positive for R. slovaca. These findings suggest that P. pipistrellus might become infected with R. parkeri, R. lusitaniae and R. slovaca. Importantly, PCR-positivity of the kidneys and the small intestine warrant further studies to investigate, if rickettsiae also pass with the faeces and urine of bats, because some of the rickettsiae (including R. rickettsii detected here in bat ticks) are known to cause infection via aerosol transmission [24].
In our previous studies we provided molecular evidence for the presence of R. raoultii in road-killed marbled polecat (Vormela peregusna) and its infesting tick Haemaphysalis erinacei [18]. Here, we detected R. raoultii in two bat livers and bat ticks for the first time. Interestingly, the gltA, ompA and ompB sequences from bat tissues were 100% identical with those from the PCR-positive bat ticks. Argas vespertilionis has a broad geographical distribution in the Old World, parasitizing several bat species, such as Eptesicus serotinus and P. pipistrellus [25, 26]. Thus, the present findings suggest that, in relevant regions of Eurasia, R. raoultii may co-circulate between the bat P. pipistrellus and the bat tick A. vespertilionis.
In 1994, Jaenson et al. [27] reported that two persons living near Stockholm were bitten by the bat tick A. vespertilionis in their bedroom, and consequently clinical signs (fever, ulceration, erythema and edema) developed. It has also been reported that certain soft tick species can be infected and are capable of transmitting human pathogenic rickettsiae, as exemplified by R. slovaca and R. rickettsii in Argas persicus and Ornithodoros spp., respectively [28, 29]. These literature data underline the significance of the present findings and justify the need to evaluate further the actual epidemiological risks associated with the presence of R. raoultii, R. slovaca, R. parkeri and R. rickettsii in bats and their ticks.
Bats are susceptible to several vector-borne disease agents, including Trypanosoma cruzi, Babesia vesperuginis and Polychromophilus murinus [30]. Furthermore, some of these microorganisms may cause pathological changes in bats, as exemplified by B. vesperuginis, inducing anemia, splenomegaly, hemoglobinuria, and elevated reticulocyte and leukocyte counts [31]. In addition, the pathogenic role of rickettsiae is documented in several mammalian species (e.g. in the pine vole, Microtus pinetorum, these bacteria elicit tremor, fur ruffling and heavy breathing [32]). However, the clinico-pathological role of rickettsial infection in bats remains to be elucidated.
Here, 11.11% of bats (6/54) were infected with R. parkeri, R. lusitaniae, R. raoultii or R. slovaca. In addition, bat soft ticks contained the DNA of R. raoultii and R. rickettsii. These results warrant future studies, investigating (i) the routes of infection for bats (i.e. whether bat soft ticks are competent vectors or not; and if so, whether rickettsiae are transmitted by them transstadially and/or transovarially); (ii) clinical and pathological aspects of rickettsial infection in bats; as well as (iii) epidemiological risks (if any) of zoonotic transmission.
Conclusions
To our knowledge, this study provides the first report of R. parkeri, R. slovaca and R. raoultii in bats. Rickettsia raoultii and R. rickettsii were detected for the first time in bat soft ticks. Our findings contribute to the knowledge on the geographical distribution, and tick and vertebrate hosts for rickettsiae.
Supplementary information
Additional file 1: Text S1. PCR protocol for the detection of Rickettsia spp. in bats and their ticks. Table S1. Primers used for the identification of Rickettsia spp.
Additional file 2: Table S2. Closest sequences to the partial 17-kDa, gltA, ompA, ompB gene sequences of Rickettsia parkeri, R. lusitaniae, R. slovaca, R. raoultii, R. rickettsii detected in bats and their ticks in the present study.
Acknowledgements
The authors thank the contributions by the staff at the School of Medicine, Shihezi University, China.
Abbreviations
- SFG
spotted fever group
- TG
typhus group
- cytb
cytochrome b
- 17-kDa
17 kDA antigen
- gltA
citrate synthase
- ompA
outer membrane proteins A
- ompB
outer membrane proteins B
- XUAR
Xinjiang Uygur Autonomous Region
- PCR
polymerase chain reaction
Authors’ contributions
SZ, MY, GL and YW conceived and designed the study. SZ, CS and WT processed the samples and performed molecular and phylogenetic analyses. SZ and SH contributed to manuscript writing. All authors read and approved the final manuscript.
Funding
This study was supported in part by the National Key Research & Development Programme of China (2018ZX10101002-007) and the National Natural Science Foundation of China (81960379).
Availability of data and materials
Data supporting the conclusions of this article are included within the article and its additional files. The newly generated sequences were submitted to the GenBank database under the accession numbers MF106222, MF106219 and MN388783-MN388800.
Ethics approval and consent to participate
This study was approved by the Animal Ethics Committee of Shihezi University (Approval no. AECSU2015-01).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuo Zhao, Meihua Yang and Gang Liu contributed equally to this work
Contributor Information
Shuo Zhao, Email: 954855721@qq.com.
Meihua Yang, Email: 1328251980@qq.com.
Gang Liu, Email: 1519816612@qq.com.
Yuanzhi Wang, Email: wangyuanzhi621@126.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-020-3885-x.
References
- 1.Burgin CJ, Colella JP, Kahn PL, Upham NS. How many species of mammals are there? J Mammal. 2018;99:1–14. doi: 10.1093/jmammal/gyx147. [DOI] [Google Scholar]
- 2.Hutterer R, Ivanova T, Meyer-Cords C, Rodrigues L. Bat migrations in Europe. A review of banding data and literature. Naturschutz und Biologische Viefalt 28. Bonn: Federal Agency for Nature Conservation; 2005. p. 162.
- 3.Brook CE, Dobson AP. Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends Microbiol. 2015;23:172–180. doi: 10.1016/j.tim.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornok S, Szőke K, Kováts D, Estók P, Görföl T, Boldogh SA, et al. DNA of piroplasms of ruminants and dogs in ixodid bat ticks. PLoS ONE. 2016;11:e0167735. doi: 10.1371/journal.pone.0167735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill JS, Ullmann AJ, Loftis AD, Schwan TG, Raffel SJ, Schrumpf ME, et al. Novel relapsing fever spirochete in bat tick. Emerg Infect Dis. 2008;14:522–523. doi: 10.3201/eid1403.070766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Socolovschi C, Kernif T, Raoult D, Parola P. Borrelia, Rickettsia, and Ehrlichia species in bat ticks, France, 2010. Emerg Infect Dis. 2012;18:1966–1975. doi: 10.3201/eid1812.111237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalik J, Wodecka B, Liberska J, Dabert M, Postawa T, Piksa K, et al. Diversity of Borrelia burgdorferi sensu lato species in Ixodes ticks (Acari: Ixodidae) associated with cave-dwelling bats from Poland and Romania. Ticks Tick-borne Dis. 2020;11:101300. doi: 10.1016/j.ttbdis.2019.101300. [DOI] [PubMed] [Google Scholar]
- 8.Veikkolainen V, Vesterinen EJ, Lilley TM, Pulliainen AT. Bats as reservoir hosts of human bacterial pathogen, Bartonella mayotimonensis. Emerg Infect Dis. 2014;20:960–967. doi: 10.3201/eid2006.130956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merhej V, Raoult D. Rickettsial evolution in the light of comparative genomics. Biol Rev Camb Philos Soc. 2011;86:379–405. doi: 10.1111/j.1469-185X.2010.00151.x. [DOI] [PubMed] [Google Scholar]
- 10.Reeves WK, Beck J, Orlova MV, Daly JL, Pippin K, Revan F, et al. Ecology of bats, their ectoparasites, and associated pathogens on Saint Kitts Island. J Med Entomol. 2016;9:78. doi: 10.1093/jme/tjw078. [DOI] [PubMed] [Google Scholar]
- 11.Hornok S, Szőke K, Meli ML, Sándor AD, Görföl T, Estók P, et al. Molecular detection of vector-borne bacteria in bat ticks (Acari: Ixodidae, Argasidae) from eight countries of the Old and New Worlds. Parasites Vectors. 2019;12:50. doi: 10.1186/s13071-019-3303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Concannon R, Wynnowen K, Simpson VR, Birtles RJ. Molecular characterization of haemoparasites infecting bats (Microchiroptera) in Cornwall, UK. Parasitology. 2005;131:489–496. doi: 10.1017/S0031182005008097. [DOI] [PubMed] [Google Scholar]
- 13.Sudman PD, Hafner BMS. Familial affinity of Tomopeas ravus (Chiroptera) based on protein electrophoretic and cytochrome b sequence data. J Mammal. 1994;75:365–377. doi: 10.2307/1382555. [DOI] [Google Scholar]
- 14.Roshdy MA. Comparative internal morphology of subgenera of Argas ticks (Ixodoidea, Argasidae). 3. Subgenus Secretargas: Argas transgariepinus White, 1846. J Parasitol. 1963;49:851–856. doi: 10.2307/3275935. [DOI] [Google Scholar]
- 15.Zhao S, Yang M, Jiang M, Yan B, Zhao S, Yuan W, et al. Rickettsia raoultii and Rickettsia sibirica in ticks from the long-tailed ground squirrel near the China-Kazakhstan border. Exp Appl Acarol. 2019;77:425–433. doi: 10.1007/s10493-019-00349-5. [DOI] [PubMed] [Google Scholar]
- 16.Anstead CA, Chilton NB. A novel Rickettsia species detected in vole ticks (Ixodes angustus) from western Canada. Appl Environ Microbiol. 2013;79:7583–7589. doi: 10.1128/AEM.02286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anstead CA, Chilton NB. Detection of a novel Rickettsia (Alphaproteobacteria: Rickettsiales) in rotund ticks (Ixodes kingi) from Saskatchewan, Canada. Ticks Tick Borne Dis. 2013;4:202–206. doi: 10.1016/j.ttbdis.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Guo LP, Mu LM, Xu J, Jiang SH, Wang AD, Chen CF, et al. Rickettsia raoultii in Haemaphysalis erinacei from marbled polecats, China-Kazakhstan border. Parasites Vectors. 2015;8:461. doi: 10.1186/s13071-015-1065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noriea NF, Clark TR, Mead D, Hackstadt T. Proteolytic cleavage of the immunodominant outer membrane protein rOmpA in Rickettsia rickettsii. J Bacteriol. 2017;199:e00826. doi: 10.1128/JB.00826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satjanadumrong J, Robinson MT, Hughes T, Blacksell SD. Distribution and ecological drivers of spotted fever group Rickettsia in Asia. EcoHealth. 2019;16:611–626. doi: 10.1007/s10393-019-01409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milon H, Pasquier J, Loire R, Guillermet F, Brun F, Descos L, et al. Acute cardiac failure with positive sero-agglutination to Rickettsia conorii. Arch Mal Coeur Vaiss. 1970;63:1635–1646. [PubMed] [Google Scholar]
- 22.Fox NJ. The long persistence of Rickettsia orientalis in the blood and tissues of infected animals. Fed Proc. 1948;7:305. [PubMed] [Google Scholar]
- 23.Hornok S, Szőke K, Estók P, Krawczyk A, Haarsma AJ, Kováts D, et al. Assessing bat droppings and predatory bird pellets for vector-borne bacteria: molecular evidence of bat-associated Neorickettsia sp. in Europe. Antonie Van Leeuwenhoek. 2018;111:1707–1717. doi: 10.1007/s10482-018-1043-7. [DOI] [PubMed] [Google Scholar]
- 24.Saslaw S, Carlisle HN. Aerosol infection of monkeys with Rickettsia rickettsii. Bacteriol Rev. 1966;30:636–645. doi: 10.1128/br.30.3.636-645.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Yan B, Wang Q, Jiang M, Tu C, Chen C, et al. Babesia vesperuginis in common pipistrelle (Pipistrellus pipistrellus) and the bat soft tick Argas vespertilionis in the People’s Republic of China. J Wildl Dis. 2018;54:419–421. doi: 10.7589/2017-08-206. [DOI] [PubMed] [Google Scholar]
- 26.Cacho ED, Estrada-Peña A, Sanchez A, Serra J. Histological response of Eptesicus serotinus (Mammalia: Chiroptera) to Argas vespertilionis (Acari: argasidae) J Wildl Dis. 1994;30:340–345. doi: 10.7589/0090-3558-30.3.340. [DOI] [PubMed] [Google Scholar]
- 27.Jaenson TG, Talleklint L, Lundqvist L, Olsen B, Chirico J, Mejlon H. Geographical distribution, host associations, and vector roles of ticks (Acari: Ixodidae, Argasidae) in Sweden. J Med Entomol. 1994;31:240–256. doi: 10.1093/jmedent/31.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogstraal H. Ticks in relation to human diseases caused by Rickettsia species. Annu Rev Entomol. 1967;12:377–420. doi: 10.1146/annurev.en.12.010167.002113. [DOI] [PubMed] [Google Scholar]
- 29.Rehácek J, Urvölgyi J, Kovácová E. Massive occurrence of rickettsiae of the spotted fever group in fowl tampan, Argas persicus, in the Armenian S.S.R. Acta Virol. 1977;21:431–438. [PubMed] [Google Scholar]
- 30.Gardner RA, Molyneux DH, Stebbings RE. Studies on the prevalence of haematozoa of British bats. Mammal Rev. 1987;17:75–80. doi: 10.1111/j.1365-2907.1987.tb00051.x. [DOI] [Google Scholar]
- 31.Gardner RA, Molyneux DH. Babesia vesperuginis: natural and experimental infections in British bats (Microchiroptera) Parasitology. 1988;95:461–469. doi: 10.1017/S0031182000057887. [DOI] [PubMed] [Google Scholar]
- 32.Eremeeva ME, Liang Z, Paddock C, Zaki S, Vandenbergh JG, Dasch GA, et al. Rickettsia rickettsia infection in the pine vole, Microtus pinetorum. Ann N Y Acad Sci. 2003;990:468–473. doi: 10.1111/j.1749-6632.2003.tb07412.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Text S1. PCR protocol for the detection of Rickettsia spp. in bats and their ticks. Table S1. Primers used for the identification of Rickettsia spp.
Additional file 2: Table S2. Closest sequences to the partial 17-kDa, gltA, ompA, ompB gene sequences of Rickettsia parkeri, R. lusitaniae, R. slovaca, R. raoultii, R. rickettsii detected in bats and their ticks in the present study.
Data Availability Statement
Data supporting the conclusions of this article are included within the article and its additional files. The newly generated sequences were submitted to the GenBank database under the accession numbers MF106222, MF106219 and MN388783-MN388800.