Abstract
Background
Chronic hepatitis B is associated with high morbidity and mortality. Chronic hepatitis B requires long‐term management aiming at reduction of the risks of hepatocellular inflammatory necrosis, liver fibrosis, decompensated liver cirrhosis, liver failure, and liver cancer, and improving health‐related quality of life. The Chinese herbal medicine formula Xiao Chai Hu Tang has been used to decrease discomfort and replication of the virus in people with chronic hepatitis B. However, the benefits and harms of Xiao Chai Hu Tang formula have never been established with rigorous review methodology.
Objectives
To assess the benefits and harms of Xiao Chai Hu Tang formula versus placebo or no intervention in people with chronic hepatitis B.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE Ovid, Embase Ovid, and seven other databases to 1 March 2019. We also searched the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp), ClinicalTrials.gov (www.clinicaltrials.gov/), and the Chinese Clinical Trial Registry for ongoing or unpublished trials to 1 March 2019.
Selection criteria
We included randomised clinical trials, irrespective of publication status, language, and blinding, comparing Xiao Chai Hu Tang formula versus no intervention or placebo in people with chronic hepatitis B. We included participants of any sex and age, diagnosed with chronic hepatitis B according to guidelines or as defined by the trialists. We allowed co‐interventions when the co‐interventions were administered equally to all the intervention groups.
Data collection and analysis
Review authors independently retrieved data from reports and after correspondence with investigators. Our primary outcomes were all‐cause mortality, serious adverse events, and health‐related quality of life. Our secondary outcomes were hepatitis B‐related mortality, hepatitis B‐related morbidity, and adverse events considered 'not to be serious'. We presented the meta‐analysed results as risk ratios (RR) with 95% confidence intervals (CI). We assessed the risks of bias using risk of bias domains with predefined definitions. We used GRADE methodology to evaluate our certainty in the evidence.
Main results
We included 10 randomised clinical trials with 934 participants, but only five trials with 490 participants provided data for analysis. All the trials compared Xiao Chai Hu Tang formula with no intervention. All trials appeared to have been conducted and published only in China. The included trials assessed heterogeneous forms of Xiao Chai Hu Tang formula, administered for three to eight months. One trial included participants with hepatitis B and comorbid tuberculosis, and one trial included participants with hepatitis B and liver cirrhosis. The remaining trials included participants with hepatitis B only. All the trials were at high risk of bias, and the certainty of evidence for all outcomes that provided data for analyses was very low. We downgraded the evidence by one or two levels because of outcome risk of bias, inconsistency or heterogeneity of results (opposite direction of effect), indirectness of evidence (use of surrogate outcomes instead of clinically relevant outcomes), imprecision of results (the CIs were wide), and publication bias (small sample size of the trials). Additionally, 47 trials lacked the necessary methodological information needed to ensure the inclusion of these trials in our review.
None of the included trials aimed to assess clinically relevant outcomes such as all‐cause mortality, serious adverse events, health‐related quality of life, hepatitis B‐related mortality, or hepatitis B‐related morbidity. The effects of Xiao Chai Hu Tang formula on the proportion of participants with adverse events considered 'not to be serious' is uncertain (RR 0.43, 95% CI 0.02 to 11.98; I2 = 69%; very low‐certainty evidence). Only three trials with 222 participants reported the proportion of people with detectable hepatitis B virus DNA (HBV‐DNA), but the evidence that Xiao Chai Hu Tang formula reduces the presence of HBV‐DNA in the blood (a surrogate outcome) is uncertain (RR 0.62, 95% CI 0.45 to 0.85; I2 = 0%; very low‐certainty evidence). Only two trials with 160 participants reported the proportion of people with detectable hepatitis B virus e‐antigen (HBeAg; a surrogate outcome) (RR 0.72, 95% CI 0.50 to 1.02; I2 = 38%; very low‐certainty evidence) and the evidence is uncertain. The evidence is also uncertain for separately reported adverse events considered 'not to be serious'.
Funding: two of the 10 included trials received academic funding from government or hospital. None of the remaining eight trials reported information on funding.
Authors' conclusions
The clinical effects of Xiao Chai Hu Tang formula for chronic hepatitis B remain unclear. The included trials were small and of low methodological quality. Despite the wide use of Xiao Chai Hu Tang formula, we lack data on all‐cause mortality, serious adverse events, health‐related quality of life, hepatitis B‐related mortality, and hepatitis B‐related morbidity. The evidence in this systematic review comes from data obtained from a maximum three trials. We graded the certainty of evidence as very low for adverse events considered not to be serious and the surrogate outcomes HBeAg and HBV‐DNA. We found a large number of trials which lacked clear description of their design and conduct, and hence, these trials are not included in the present review. As all identified trials were conducted in China, there might be a concern about the applicability of this review outside China. Large‐sized, high‐quality randomised sham‐controlled trials with homogeneous groups of participants and transparent funding are lacking.
Plain language summary
Xiao Chai Hu Tang, a Chinese herbal medicine formula, for chronic hepatitis B
Review question
To assess the benefits and harms of Xiao Chai Hu Tang formula versus placebo or no intervention in people with chronic hepatitis B virus infection.
Background
Chronic hepatitis B virus infection is a common liver disease, associated with high morbidity (illness) and death. It causes psychological stress and is a burden to people with chronic hepatitis B and their families. Xiao Chai Hu Tang formula has been used for treating people with chronic hepatitis B as it is believed that it decreases discomfort and prevents the replication of the virus in people with chronic hepatitis B. However, the benefits and harms of Xiao Chai Hu Tang formula have never been established in reviews with rigorous review methodology.
Search date
The review includes trials published up to 1 March 2019.
Study characteristics
We included 10 randomised clinical trials (studies where people are randomly put into one of two or more treatment groups) with 934 participants. All trials compared Xiao Chai Hu Tang formula with no treatment. The trials assessed different formulas and doses for three to eight months. One trial included participants with tuberculosis (a disease of the lungs that can make you cough mucous), and one trial included participants with liver cirrhosis (scarring). Only five trials with 490 participants provided data for analysis
Study funding sources
Two of the 10 included trials reported receiving academic funding. None of the remaining eight trials reported information of support or funding.
Key results
None of the 10 included trials reported data on all‐cause mortality (death from any cause), serious side effects (untoward medical occurrences that result in serious outcomes such as death or disability), health‐related quality of life (a measure of physical, mental, emotional, and social functioning a measure of a person's satisfaction with their life and health), hepatitis B‐related death, and hepatitis B‐related morbidity. We are uncertain whether Xiao Chai Hu Tang formula versus no intervention has a positive or negative effect regarding side effects considered 'not to be serious', the proportion of people with detectable HBeAg (a hepatitis B viral protein that indicates active viral replication), and separately reported side effects considered 'not to be serious'. Xiao Chai Hu Tang formula compared with no intervention seems to reduce the proportion of people with detectable HBV‐DNA (which is used to indicate how much hepatitis B virus is in the blood) but the reliability of this finding is low. Surrogate outcomes are markers that are used in research as a substitute for a clinically meaningful measure that directly measures patient outcomes. We cannot always be certain that such surrogate outcomes are reliable substitutes for important outcomes as they need to be officially examined. Caution is needed with this beneficial finding as the trials are at high risk of bias, and this outcome has not yet been proven relevant to patients. We identified an additional 47 studies as potential randomised clinical trials, but the data they reported were of no use. Accordingly, properly designed randomised clinical trials are needed before the benefits and harms of Xiao Chai Hu Tang formula for chronic hepatitis B can be determined.
Reliability of the evidence
The reliability of the evidence on the use of Xiao Chai Hu Tang formula in people with chronic hepatitis B virus in terms of its beneficial or harmful effects on death, health‐related quality of life, risk of dying due to hepatitis B virus infection, and serious side effects cannot be determined as no trials aimed to explore these. The reliability of the evidence that Xiao Chai Hu Tang formula, when compared with no intervention, in terms of side effects considered 'not to be serious', the proportion of people with detectable HBV‐DNA, and the proportion of people with detectable HBeAg is very low. These assessments of the reliability of the evidence are due to the poor design and reporting of the included trials.
Summary of findings
Summary of findings for the main comparison. Xiao Chai Hu Tang formula compared with no intervention for chronic hepatitis B.
| Xiao Chai Hu Tang formula compared with no intervention for chronic hepatitis B | ||||||
| Patient or population: chronic hepatitis B Setting: outpatient or hospital Intervention: Xiao Chai Hu Tang formula Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with Xiao Chai Hu Tang formula | |||||
| All‐cause mortality | No data | |||||
| Proportion of participants with ≥ 1 serious adverse events | No data | |||||
| Health‐related quality of life | No data | |||||
| Hepatitis B‐related mortality | No data | |||||
| Hepatitis B‐related morbidity | No data | |||||
|
Proportion of participants with ≥ 1 adverse events considered 'not to be serious' (at maximum follow‐up: 3–4 months; median 3.5 months) |
Study population | RR 0.43 (0.02 to 11.98) | 240 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | The review authors did not search Japanese and Korean medical databases. | |
| 58 per 1000 | 25 per 1000 (1 to 699) | |||||
|
Proportion of participants with detectable HBV‐DNA in serum or plasma (at maximum follow‐up: 4–12 months; median 8 months) |
Study population |
RR 0.62 (0.45 to 0.85) |
222 (3 RCTs) |
⊕⊝⊝⊝ Very lowa,e,f,g | The review authors did not search Japanese and Korean medical databases. | |
| 471 per 1000 | 292 per 1000 (212 to 400) |
|||||
|
Proportion of participants with detectable HBeAg in serum or plasma (at maximum follow‐up: 8–12 months; median 10 months) |
Study population | RR 0.72 (0.50 to 1.02) | 160 (2 RCTs) |
⊕⊝⊝⊝ Very lowa,e,h,i | The review authors did not search Japanese and Korean medical databases. | |
| 688 per 1000 | 495 per 1000 (344 to 701) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level. Concerns with allocation concealment, blinding, and selective outcome reporting. bDowngraded one level. Opposite direction of effect: P value of heterogeneity < 0.1, and I2 > 50%. cDowngraded two levels. Number of events fewer than 300 and the CI included both no effect and potential for important harm. dDowngraded one level. All included studies were small. Trials conducted in another country than China may not have been identified. eDowngraded one level. Use of surrogate outcomes instead of clinically relevant outcomes. hepatitis B virus DNA and hepatitis B virus e‐antigen were related to chronic hepatitis B‐related mortality and morbidity (Su 2016; Osawa 2017; Kouamé 2018; Hung 2019). fDowngraded one level. Even though the CI did not cross the threshold of 1, the optimal information size criteria were not met and the sample size was not very large (fewer than 2000 participants). The number of events was small. gDowngraded one level. The two included studies were small and showed positive effect. Trials conducted in countries other than China may not have been identified. hDowngraded one level. The optimal information size criteria were not met and the sample size was not very large (fewer than 2000 participants). The number of events was small. iDowngraded one level. The two included studies were small. The fixed‐effect analysis showed positive effect. Trials conducted in countries other than China may not have been identified.
Background
Description of the condition
Approximately 257 million people worldwide, or 3.5% of the world's population, are infected with the hepatitis B virus (WHO 2017). The estimated prevalence of hepatitis B virus infection is highest in the Western Pacific region (6.2%) and Africa (6.1%) (WHO 2017). In 2015, 880,000 people may have died because of chronic hepatitis B virus infection (WHO 2017). In 2006, about 93 million people in China were carriers of hepatitis B virus, accounting for 8% to 10% of the total population in China (Qi 2011). Chronic hepatitis B virus infection has a substantial economic, psychological, and life impact on people with chronic hepatitis B and their families (Alizadeh 2008; Lu 2013; Keshavarz 2015; Ezbarami 2017).
Hepatitis B virus is commonly spread through blood, body fluids, mother‐to‐child transmission, sexual contact, or induced unintentionally through medical procedures (WHO 2017). Hepatitis B infection can either be acute or chronic, ranging in severity from asymptomatic to a symptomatic progressive disease (WHO 2015). Hepatitis B virus DNA (HBV‐DNA), the core of the hepatitis B virus particle, is the most sensitive marker for replication of hepatitis B virus. Covalently closed circular DNA (cccDNA) acts as a template for new viral ribonucleic acids (RNAs) (Peng 2000; Nassal 2008), and is responsible for the persistence of hepatitis B virus infection and reactivation (Moraleda 1997; Delmas 2002; Gripon 2002; Zoulim 2005). The initial evaluation of people with chronic hepatitis B virus infection includes a thorough history, physical examination, assessment of liver disease activity and severity, and markers of hepatitis B virus infection (AASLD 2016; EASL 2017). Reducing the risk of hepatocellular inflammatory necrosis, liver fibrosis, decompensated liver cirrhosis, liver failure, and liver cancer, improving quality of life, and prolonging survival is the aim of the long‐term treatment of chronic hepatitis B (WHO 2015; EASL 2017).
Description of the intervention
Xiao Chai Hu Tang (also called XCHT, Sho‐sai‐ko‐to, or minor Bupleurum decoction), a herbal formula, was first recorded in the Treatise on Febrile Disease (Shang Han Lun) in about 280 AD (Zhang 2005a). The ingredients of Xiao Chai Hu Tang formula are Chai Hu (Bupleuri Radix, Bupleurum falcatum Linne; approximately 26%), Ban Xia (Pinelliae Tuber,Pinellia ternata breitenbach; approximately 26%), Sheng Jiang (Zingiberis Rhizoma, Zingiber officinale roscoe; approximately 10%), Da Zao (Zizyphi Fructus, Zizyphus jujuba Miller var. inermis Rehder; approximately 10%), Ren Shen (Ginseng Radix Rubra, Panax ginseng Carl Anton von Meyer; approximately 10%), Huang Qin (Scutellariae Radix, Scutellaria baicalensis Georgi; approximately 10%), and Gan Cao (Glycyrrhizae Radix, Glycyrrhiza uralensis Fisher, or Glycyrrhiza glabra Linneá; approximately 10%) (Zhang 2005a; MHLW 2016) (note: percentages are review authors' calculations). Xiao Chai Hu Tang is effective against some generic symptoms which may be present in people diagnosed with chronic hepatitis B (Li 1999; Yuan 2002; Zhang 2008). In ancient times, this formula was used to treat people with symptoms, such as loss of appetite, nausea, and mild right upper quadrant discomfort, which are similar to the symptoms that characterise chronic hepatitis B. Nowadays, this formula, with either traditional ingredients (Li 2001a; Xiong 2003) or modified ingredients (Yu 2000), is administered in China (Zhang 1998; Yu 2000; Li 2001a; Xiong 2003; Wu 2009a), and Japan (Tajiri 1991; Yamashiki 1992), especially when people are unable to take antiviral therapies because of adverse events (Shu 2015), or due to high cost (WHO 2000; Zheng 2014). This formula is administered by different formulations, such as water decoction, tablets, capsules, granules, and injections (Zhang 1998; Li 2001a; Xiong 2003; Wu 2009a). Treatment duration ranges from one month to 13 months (Li 2001a; Chen 2008).
Adverse events, such as pneumonia (Takada 1993; Hatakeyama 1997; Sato 1997), pseudoaldosteronism (Tsumura 2014), acute liver damage (Itoh 1995; Stickel 2000), acute hepatitis (Hsu 2006), acute thrombocytopenic purpura (Kiguchi 2000), and acute respiratory distress syndrome (Sakamoto 2003) were reported to be associated with Xiao Chai Hu Tang formula.
How the intervention might work
According to traditional Chinese medicine, the Xiao Chai Hu Tang formula can complement the healthy qi (a vital force or energy that can control the human body), dispel the unhealthy qi, and mediate qi and blood circulation in and around the liver and gallbladder. Possible mechanisms of action of Xiao Chai Hu Tang have been studied in animals (ducks, mice, and rats) and animal or human cells (dendritic cells, hepatic stellate cells, and hepatoma cells), and include: inhibition of hepatitis B virus replication (Wen 2000), improvement of the immune function (Gai 2007; Liu 2010), inhibition of the hepatic inflammatory response and amelioration of hepatic fibrosis (Bachem 1993; Ma 1997a; Ono 2000; Zhang 2005b; Liu 2010; Chen 2017), protection of hepatocytes (Zhang 2006a), and an antitumour effect (Yano 1994; Cao 2003; Wang 2004; Mao 2005).
Why it is important to do this review
We found three meta‐analyses on the Xiao Chai Hu Tang formula for chronic hepatitis B. Qin 2010 assessed Xiao Chai Hu Tang formula alone or in combination with antiviral drugs versus placebo, a non‐specific treatment (e.g. vitamin C), or antiviral drugs. Qin 2010 showed that the combination therapy compared with the antiviral drugs (interferon‐α‐2b, adefovir dipivoxil, lamivudine, and ribavirin) reduced the surface antigen of the hepatitis B virus (HBsAg), the hepatitis B e‐antigen (HBeAg), HBV‐DNA, and alanine aminotransferase (ALT) levels. Hu 2011 compared Xiao Chai Hu Tang formula plus pegylated interferon‐α (peg‐IFNα) versus peg‐IFNα alone; the combination therapy had higher rates of ALT levels improvement, HBeAg seroconversion, and reduction of influenza‐like symptoms caused by peg‐IFNα. However, this meta‐analysis included only seven randomised clinical trials with 668 participants. Yang 2015 assessed Xiao Chai Hu Tang formula plus lamivudine versus lamivudine alone. The combination therapy reduced ALT levels and HBeAg seroconversion rate. All three meta‐analyses assessed surrogate outcomes (Qin 2010; Hu 2011; Yang 2015). Whether surrogate outcome results do indeed lead to improvement in clinically important outcomes is still questionable (Gluud 2007; Flemming 2012; Ciani 2017; Jakobsen 2017; Kemp 2017; Jakobsen 2018). Furthermore, none of these meta‐analyses took account of random errors, neither did they grade the evidence (Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Mustafa 2013; Guyatt 2017). Therefore, we still need to answer the question of the benefits and harms of Xiao Chai Hu Tang formula for people with chronic hepatitis B, in terms of patient‐relevant outcomes. The current review, aimed to assess the benefits and harms of Xiao Chai Hu Tang formula versus placebo or no intervention in people with chronic hepatitis B. Only when we succeed in determining the benefits and harms of Xiao Chai Hu Tang formula versus other interventions will a review comparing Xiao Chai Hu Tang formula versus other interventions be needed.
Objectives
To assess the benefits and harms of Xiao Chai Hu Tang formula versus placebo or no intervention in people with chronic hepatitis B.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials irrespective of blinding, language, year, publication format, or publication status.
Types of participants
Inclusion criteria
Participants of any sex and age, diagnosed with chronic hepatitis B, as defined by the trialists or according to guidelines (HBsAg positivity for more than six months, serum HBV‐DNA positivity more than 2000 IU/mL (i.e. 104 copies/mL), persistent or intermittent elevation in levels of aspartate aminotransferase (AST) or ALT, and liver biopsy findings that showed chronic hepatitis B with moderate or severe necro‐inflammation) (AASLD 2016; EASL 2017).
In addition to chronic hepatitis B, participants could also have had cirrhosis, hepatocellular carcinoma, concomitant HIV infection or AIDS, hepatitis C, hepatitis D, or other concomitant diseases.
Exclusion criteria
None.
Types of interventions
Xiao Chai Hu Tang formula in any dose, formulation, and regimen compared with placebo or no intervention.
We also allowed inclusion of trials assessing the Xiao Chai Hu Tang formula if the herbal components of the formula had been obtained from different sources, or if the content of the formula was modified but still contained the following four main herbs: Chai Hu, Ban Xia, Ren Shen, and Huang Qin.
We allowed co‐interventions in the experimental and control groups, provided that the co‐interventions were administered equally to all the groups of a trial.
Types of outcome measures
Primary outcomes
All‐cause mortality: death from any cause.
Proportion of participants with one or more serious adverse events; that is, any untoward medical occurrence that resulted in death, was life threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was a congenital anomaly or birth defect (ICH‐E2A 1994; ICH‐GCP E6(R2) 2016).
Health‐related quality of life: any scale used by trialists to assess the participants' reporting of their quality of life.
Secondary outcomes
Hepatitis B‐related mortality.
Hepatitis B‐related morbidity (proportion of participants with one or more of the following events: cirrhosis, ascites, variceal bleeding, hepatorenal syndrome, hepatocellular carcinoma, hepatic encephalopathy, or liver transplantation, and who had not died).
Proportion of participants with one or more adverse events considered 'not to be serious': any untoward medical occurrence in a participant that did not meet the above criteria for a serious adverse event was defined as a non‐serious adverse event (ICH‐E2A 1994; ICH‐GCP E6(R2) 2016).
Exploratory outcomes
Proportion of participants with detectable HBV‐DNA in serum or plasma.
Proportion of participants with detectable hepatitis B e‐antigen (HBeAg) in serum or plasma.
Separately reported serious adverse events.
Separately reported adverse events considered 'not to be serious'.
Separately reported hepatitis B‐related morbidity.
We assessed all outcomes at maximum follow‐up.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Hepato‐Biliary Group (CHBG) Controlled Trials Register (maintained and searched internally by the CHBG Information Specialist via the Cochrane Register of Studies Web), Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE Ovid, Embase Ovid, LILACS (Latin American and Caribbean Health Science Information database; Bireme), Science Citation Index Expanded (Web of Science), and Conference Proceedings Citation Index – Science (Web of Science) (Royle 2003). We also searched the China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), Wanfang Data, and SinoMed.
Appendix 1 provides the search strategies with the time spans for the searches.
Searching other resources
We searched reference lists of systematic reviews and meta‐analyses on this topic, and retrieved studies. We searched the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp), ClinicalTrial.gov (www.clinicaltrials.gov/), and the Chinese Clinical Trial Registry (ChiCTR) for ongoing or unpublished trials.
Data collection and analysis
We conducted our review following the recommendations in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2019) and the Methodological Expectations of Cochrane Intervention Reviews (MECIR) guidelines (MECIR 2019).
We performed analyses using Review Manager 5 (Review Manager 2014) and Trial Sequential Analysis version 0.9.5.10 Beta software (Thorlund 2011a; TSA 2011).
Selection of studies
Review authors (DZK, ZZ, YL, JL, XHL, SBL) working in pairs independently screened titles and abstracts to identify potentially eligible trials. We listed multiple reports of the same trial under their main reference, and ineligible studies with reasons for exclusion in the Characteristics of excluded studies table. We resolved any disagreements through discussion, or we asked JPL to arbitrate. We recorded the selection process in a PRISMA flow diagram (PRISMA 2009; Figure 1).
1.
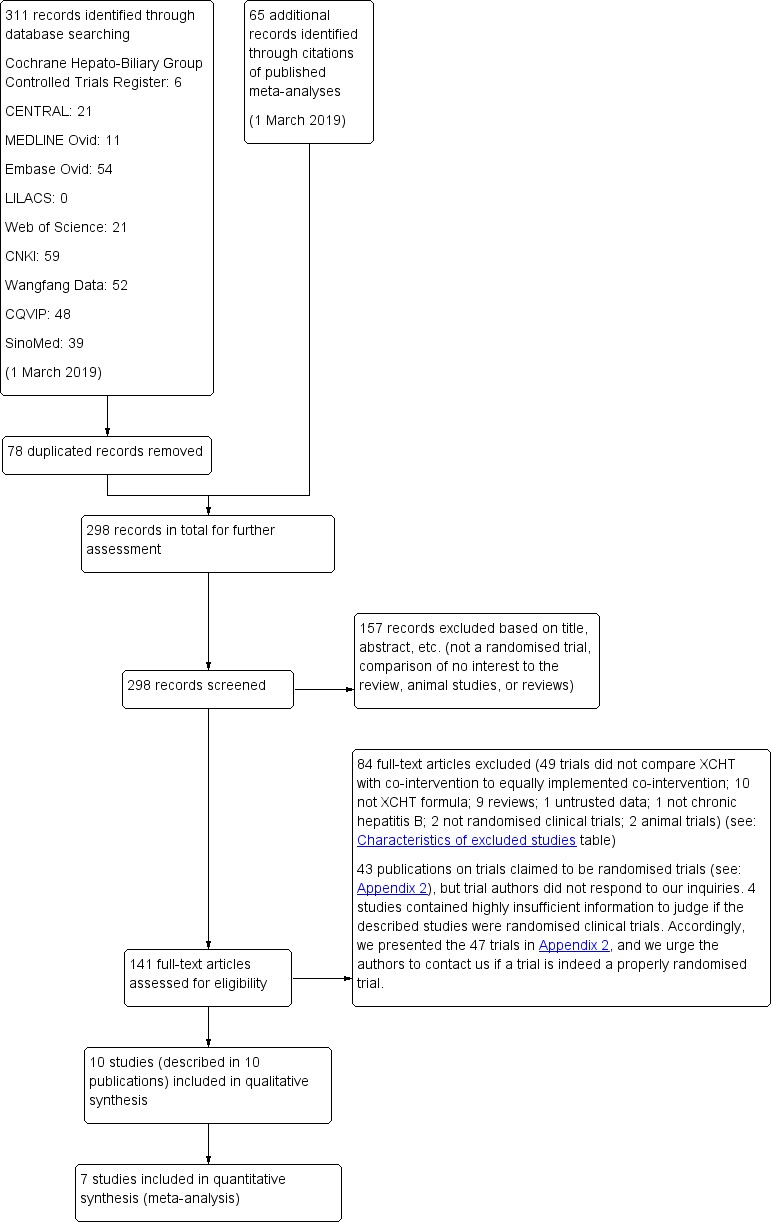
Study flow diagram. Date of last search 1 March 2019. CNKI: China National Knowledge Infrastructure; CQVIP: Chongqing VIP; XCHT: Xiao Chai Hu Tang.
We also considered quasi‐randomised studies, controlled clinical studies, and other observational studies for data on harms if retrieved with our searches for randomised clinical trials. This is because adverse events are rarely reported in randomised clinical trials. Moreover, observational studies may provide information on rare or late‐occurring adverse events (Storebø 2018). We are aware that the decision not to search for all observational studies may have biased our review towards assessment of benefits and may have overlooked certain harms such as very late or very rare harms.
Data extraction and management
Review authors (DZK, JL, XHL, SBL) working in pairs independently extracted data using a prepiloted electronic data collection form created in Microsoft Excel. In case of discrepancies, we rechecked the extracted data. When disagreements persisted, we tried to resolve any disagreements through discussion. We contacted JPL to arbitrate when disagreements still existed, before proceeding with the analyses.
Review authors working in pairs independently extracted the following information: publication data (i.e. year, country, authors); study characteristics and design; characteristics of trial participants; trial inclusion and exclusion criteria; interventions; outcomes; follow‐up; types of data analyses (i.e. intention‐to‐treat, modified intention‐to‐treat, per protocol). We contacted trial authors for the missing information. We extracted data at maximum follow‐up.
Assessment of risk of bias in included studies
Review authors (NL, JL, XHL, SBL) working in pairs independently assessed the risk of bias in the included trials. We assessed risk of bias according to the Cochrane 'Risk of bias' tool (Higgins 2019) and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Savović 2018), using the following definitions.
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random numbers table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We planned to include such studies only for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation, so the intervention allocations may have been foreseen before, or during, enrolment.
High risk of bias: it is likely that the investigators who assigned the participants knew the allocation sequence. We planned to only include such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: either of the following: blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken; or rarely, no blinding or incomplete blinding, but the review authors judged that the outcome was not likely to be influenced by lack of blinding, such as mortality.
Unclear risk of bias: either of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the study did not address this outcome.
High risk of bias: either of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Blinding of outcome assessment
Low risk of bias: either of the following: blinding of outcome assessment ensured, and unlikely that the blinding could have been broken; or rarely, no blinding of outcome assessment, but the review authors judged that the outcome measurement was not likely to be influenced by lack of blinding such as mortality.
Unclear risk of bias: either of the following: insufficient information to permit judgement of 'low risk' or 'high risk'; or the study did not address this outcome.
High risk of bias: either of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding; or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputation, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data, in combination with the method used to handle missing data, were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality; serious adverse events; and health‐related quality of life. If the original trial protocol was available, the outcomes should have been those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g. www.clinicaltrials.gov), the outcomes sought were those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all predefined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more predefined outcomes were not reported.
Other bias
Low risk of bias: the study appeared free of other factors that could have put it at risk of bias.
Unclear risk of bias: the study may or may not have been free of other factors that could have put it at risk of bias.
High risk of bias: there were other factors in the study that could have put it at risk of bias.
Overall risk of bias
Low risk of bias: the outcome result was classified at overall low risk of bias only if all of the risk of bias sources described above were classified at low risk of bias.
High risk of bias: the outcome result was classified at high risk of bias if any of the risk of bias sources described above were classified at unclear risk of bias or high risk of bias.
We tried to reach consensus through discussion. We planned that JPL would arbitrate in cases of disagreement.
We planned to base our primary conclusions on the results of all our primary and secondary outcome results at low overall risk of bias; however, we found no trials at low overall risk of bias.
Measures of treatment effect
We used the risk ratio (RR) for measuring dichotomous outcomes, and we intended to use the mean difference (MD) for continuous data, with 95% confidence intervals (CIs) for head‐to‐head comparison meta‐analysis. If studies used different instruments to measure the same continuous outcome, we planned to calculate the standardised mean difference (SMD), with 95% CI.
Unit of analysis issues
We followed the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
The unit of analysis was the participants randomised to the trial intervention groups. For trials with multiple intervention groups, we intended to include the groups that compared our experimental and control interventions. We intended to divide the control group into two to avoid double‐counting where this was a common comparator.
For cluster‐randomised trials, we intended to directly extract data from the analysis that properly account for the cluster design. We intended to determine the inflated standard errors that accounted for clustering if there was no control of the clustering. We intended to use the inverse‐variance method in Review Manager 5 (Review Manager 2014).
For cross‐over trials, we intended to extract only data from the first period to avoid residual treatment effects (Higgins 2019).
Dealing with missing data
We attempted to contact trial authors for missing data or information that was not clearly presented.
We performed our analysis using the intention‐to‐treat method whenever possible. If this was not possible, we used the data that were available to us. We planned to include participants with incomplete or missing data, for all outcomes, in sensitivity analyses by imputing them as follows.
For dichotomous outcomes:
best‐worst‐case scenario: we planned to assume that all participants lost to follow‐up in the experimental group had survived, had improvement in clinical symptoms, had no serious adverse event, and had no morbidity (for all dichotomous variables); and that all participants lost to follow‐up in the control group had not survived, had no improvement in clinical symptoms, had a serious adverse event, and had morbidities (for all dichotomous variables);
worst‐best‐case scenario: we planned to assume that all participants lost to follow‐up in the experimental group had not survived, had no improvement in clinical symptoms, had a serious adverse event, and had morbidities (for all dichotomous variables); and that all participants lost to follow‐up in the control group had survived, had improvement in clinical symptoms, had no serious adverse event, and had no morbidity (for all dichotomous variables).
For continuous outcomes:
we planned to base the 'beneficial' outcome for the group mean plus two standard deviations (SDs), or one SD, and the 'harmful' outcome for the group mean minus two SDs, or one SD (Jakobsen 2014).
We intended to request the information from trial authors or calculate SDs using data from the trial, if not reported.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by carefully examining trial participant characteristics and the design of the included trials. We assessed the presence of clinical heterogeneity by comparing effect estimates in trial reports in terms of participants with different diagnostic criteria, participants diagnosed with only chronic hepatitis B, participants diagnosed with concomitant diseases, formula types, formula forms, different duration and dosages of the intervention, co‐interventions, different control interventions, and follow‐up (see Subgroup analysis and investigation of heterogeneity). Different study designs and risk of bias can contribute to methodological heterogeneity. We assessed statistical heterogeneity by comparing the results of the fixed‐effect model meta‐analysis and the random‐effects model meta‐analysis. We started by looking at the forest plots for signs of statistical heterogeneity. Next, we used the Chi2 test with significance threshold set as P < 0.10 and measured the amount of heterogeneity using the I2 statistic to assess to what extent heterogeneity is present (Higgins 2002; Higgins 2003; Higgins 2019). We interpreted the I2 statistic as suggested in Higgins 2019: 0% to 40%: might not be important; 30% to 60%: might represent moderate heterogeneity; 50% to 90%: might represent substantial heterogeneity; 75% to 100%: considerable heterogeneity.
For the heterogeneity adjustment of the diversity‐adjusted required information size (DARIS) in the Trial Sequential Analysis, we used diversity (D2) because the I2 statistic used for this purpose might underestimate the required information size (Wetterslev 2009).
Assessment of reporting biases
We planned to assess reporting bias using funnel plots, provided that we had obtained data from at least 10 trials per comparison. To assess risk of bias, we intended to look for symmetry or asymmetry of each funnel plot. For dichotomous outcomes, we intended to assess asymmetry using the Harbord test (Harbord 2006). For continuous outcomes, we intended to apply the regression asymmetry test (Egger 1997).
Data synthesis
Meta‐analysis
We performed the analyses according to the instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We analysed data using Review Manager 5 (Review Manager 2014).
We assessed our intervention effects with both fixed‐effect model and random‐effects model meta‐analyses, and we reported results of both when results differed (e.g. one giving a significant intervention effect, the other no significant intervention effect). We put greater weight on the estimate closest to the zero effect (the highest P value) (Jakobsen 2014).
We assessed three primary outcomes with a P value of 0.025 or less as statistically significant and three secondary outcomes with a P value of 0.025 or less as statistically significant in order to secure a family‐wise error rate below 0.05 (Jakobsen 2014). For exploratory outcomes, we considered a P value less than 0.05 as statistically significant because we viewed these outcomes as only hypothesis‐generating outcomes. Whether we presented our data synthesis as a meta‐analysis or in a narrative way depended on our assessment of the statistical and clinical heterogeneity of the meta‐analysed trial data per comparison.
We did not impute any missing data in our primary analysis; however, we planned to impute missing values in our sensitivity analysis of continuous and dichotomous data (see Sensitivity analysis; Jakobsen 2014).
We planned to use Fisher's exact test for dichotomous data (Fisher 1922), as well as Student's t‐test for continuous data when data from only one trial were available (Student 1908).
Subgroup analysis and investigation of heterogeneity
In cases of available data, we planned to perform the following subgroup analyses.
Trials at low risk of bias compared to trials at high risk of bias (because trials at high risk of bias tend to overestimate or underestimate intervention effects) (Guyatt 2011b).
Different control interventions: no intervention compared to placebo intervention (because placebo is shown to have a possible effect on a patient) (Vrhovac 1977).
Traditional Xiao Chai Hu Tang formula compared to modified Xiao Chai Hu Tang formula (because Chinese medicine formula is a complex mixture of herbs and we do not know how individual herbs may interact and influence absorption of the herbs) (Li 2008).
Different forms of Xiao Chai Hu Tang formula (because it may change formula effects) (Li 2008; Zhang 2017).
Different duration and dosages of the intervention, stratified according to the medians observed (because different treatment durations may influence absorption of the herbs) (Wang 2014a).
Participants defined as having chronic hepatitis B according to guidelines compared to participants defined as having chronic hepatitis B by trialists (because different diagnostic criteria may lead to recruiting participants with different levels of disease severity, which may influence formula effects) (Guyatt 2011f).
Participants diagnosed with only chronic hepatitis B, compared to participants diagnosed with concomitant diseases (cirrhosis, hepatocellular carcinoma, HIV infection, AIDS, hepatitis C, hepatitis D, or a combination of these) (because concomitant diseases may influence formula effects). We planned to analyse each concomitant disease separately (because each concomitant disease may have influenced formula effects to a different extent) (Guyatt 2011f).
Sensitivity analysis
In addition to the sensitivity analysis described under Dealing with missing data, we compared our GRADE imprecision assessments for proportion of participants with one or more adverse events considered 'not to be serious'; the proportion of participants with detectable HBV‐DNA outcome; and the proportion of participants with detectable HBV‐DNA versus those conducted via Trial Sequential Analysis (Jakobsen 2014; Castellini 2018; Gartlehner 2019).
Trial Sequential Analysis
As cumulative meta‐analysis involves risk of producing random errors due to sparse data and repetitive testing, we performed Trial Sequential Analysis. To control random errors, we calculated the required information size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Wetterslev 2008; Thorlund 2011b; TSA 2011). The required information size calculation should also account for the diversity present in the meta‐analysis (Wetterslev 2008; Wetterslev 2009; Wetterslev 2017). A more detailed description of Trial Sequential Analysis can be found at www.ctu.dk/tsa (Thorlund 2011a; TSA 2011).
We controlled the risks of type I errors and type II errors for both dichotomous and continuous outcomes (Brok 2008; Wetterslev 2008; Brok 2009; Wetterslev 2009; Thorlund 2010; Casetllini 2017; Wetterslev 2017). For dichotomous outcomes, we estimated the diversity‐adjusted required information size (DARIS) based on the event proportion in the control group, a relative risk reduction of 15%, an alpha of 2.5% for primary outcomes, 2.5% for secondary outcomes, 5.0% for exploratory outcomes, a beta of 10% (Casetllini 2017), and diversity suggested by the trials in the meta‐analysis (Wetterslev 2009; Jakobsen 2014). We intended to include participants with different severity of chronic hepatitis B, but no participants had died in the control group. Therefore, we conducted three posthoc Trial Sequential Analyses based on assumed proportions of participants dying being low (4%, young participants with mild diseases), moderate (20%, middle‐aged participants with mild diseases), and high (40%, middle‐aged or old‐aged participants with severe diseases) within one year in the control group. For continuous outcomes, we intended to estimate the DARIS, based on the SD observed in the control group, a minimal relevant difference of 50% of this SD, an alpha of 2.5%, a beta of 10% (Casetllini 2017), and diversity suggested by the trials in the meta‐analysis (Wetterslev 2009; Jakobsen 2014).
We tested statistical significance using statistical monitoring boundaries for benefit and harm, and futility using futility boundaries (Thorlund 2011a). If the Z‐curve crosses the statistical monitoring boundaries for benefit or harm before reaching DARIS, the effect of the intervention is considered superior or inferior to the control intervention. If the Z‐curve crosses the futility monitory boundaries before reaching the DARIS, it would mean that the intervention does not possess the postulated effect, and further randomised trials might be futile. Furthermore, if the trial sequential monitoring boundaries are not surpassed, and the trial monitoring boundaries for futility are not crossed, it is probably necessary to continue doing trials in order to detect or reject a certain intervention effect (Wetterslev 2008; Thorlund 2011b). In our cases where the monitoring boundaries are not reached, we also displayed the Trial Sequential Analysis‐adjusted CI.
Summary of findings
We constructed a 'Summary of findings' table to show our results and confidence in the evidence for all Primary outcomes and Secondary outcomes. We displayed information on assumed control group risk, corresponding intervention group risk, relative effect, MD, CI, statistical significance of relative effect, number of participants, and certainty of the evidence. We calculated the corresponding risk (and its 95% CI) using the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Using GRADEpro GDT software (GRADEpro GDT), we assessed five factors of the evidence referring to limitations in the study design and implementation that suggest the certainty of the evidence: within‐study risk of bias, indirectness of the evidence (population, intervention, control, outcomes), unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results, and risk of publication bias (GRADEpro GDT; Balshem 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Mustafa 2013; Guyatt 2017).
We classified the evidence as follows.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
Through our electronic searches, we identified 311 records (Figure 1). We found 65 additional references through searching the references of the publications retrieved with the searches. After excluding 235 duplicated or clearly irrelevant references, we read the full text of 141 publications. Eighty‐four studies failed to fulfil the inclusion criteria. Forty‐three studies failed to provide clear description of the random sequence generation method even after asking investigators for the missing information, and four studies contained highly insufficient information to judge if the described studies were randomised clinical trials or not (Shiraki 1991; Heydtmann 2000; Ye 2011; Feng 2016). We prepared a table with summary information on these studies, not included in our meta‐analysis (Appendix 2). We found no ongoing studies and no unpublished studies.
Included studies
Ten randomised clinical trials fulfilled the inclusion criteria (Sun 2004; Wang 2013; Chen 2014; Mao 2014; Zhao 2014; Wu 2015; Kang 2016; Wang 2016; Chen 2017; Liu 2017), but only five of them, with 490 participants, provided data for analysis (Wang 2013; Zhao 2014; Wang 2016; Kang 2016; Liu 2017). The remaining five trials did not study the outcomes of interest for our review, and hence, we used the provided information only in a narrative way. All 10 trials were conducted in China and were published as full paper articles in Mandarin. The 10 trials seemed to have been published only once as we found no other publications describing the same trials. For details on the included trials, see the Characteristics of included studies table.
Funding: two included trials received academic funding from government or hospital (Chen 2014; Mao 2014). None of the remaining eight trials reported information on funding.
Participants
The 10 trials randomised 934 participants diagnosed with chronic hepatitis B (Sun 2004; Wang 2013; Chen 2014; Mao 2014; Zhao 2014; Wu 2015; Kang 2016; Wang 2016; Chen 2017; Liu 2017). The number of participants in the trials ranged from 79 to 160. The age of participants in the trials ranged from 33 to 64 years. Nine trials reported sex of the participants, and the ratio of males to females was 512:335 (Sun 2004; Wang 2013; Chen 2014; Mao 2014; Wu 2015; Kang 2016; Wang 2016; Chen 2017; Liu 2017).
All trials included participants with chronic hepatitis B. Six trials used diagnostic criteria described in guidelines (Sun 2004; Chen 2014; Mao 2014; Zhao 2014; Wang 2016; Chen 2017), and three trials followed diagnosis by trial investigators (Wang 2013; Kang 2016; Liu 2017). The remaining trial did not report on the criteria for establishing the diagnosis of chronic hepatitis B (Wu 2015). In addition to the diagnosis of chronic hepatitis B, one trial included participants with tuberculosis (Chen 2014), and one trial included participants with liver cirrhosis (Chen 2017).
Two trials excluded participants with liver cirrhosis (Mao 2014; Wang 2016), three trials excluded participants with other types of hepatitis (Chen 2014; Wang 2016; Chen 2017), four trials excluded participants with cardiovascular, cerebrovascular, lung, kidney, endocrine, and haematopoietic diseases (Mao 2014; Zhao 2014; Kang 2016; Liu 2017), four trials excluded pregnant or breastfeeding women (Chen 2014; Mao 2014; Zhao 2014; Wang 2016), two trials excluded participants with cancer (Chen 2014; Zhao 2014); one trial excluded participants with hepatic encephalopathy (Zhao 2014); and four trials excluded participants who had received antiviral drugs, immunomodulator, or anti‐fibrosis drugs, before the randomised trials were initiated (Chen 2014; Zhao 2014; Wang 2016; Chen 2017). One trial did not state the exclusion criteria (Wu 2015).
Interventions and comparisons
All 10 trials compared Xiao Chai Hu Tang formula plus co‐interventions with equal co‐interventions. One trial evaluated the oral granules of Xiao Chai Hu Tang formula (Zhao 2014), and nine trials evaluated the oral water extraction of Xiao Chai Hu Tang formula (Sun 2004; Wang 2013; Chen 2014; Mao 2014; Wu 2015; Kang 2016; Wang 2016; Chen 2017; Liu 2017). Three trials evaluated the modified Xiao Chai Hu Tang formula (Zhao 2014; Wang 2016; Chen 2017); five trials evaluated the traditional Xiao Chai Hu Tang formula (Sun 2004; Wang 2013; Chen 2014; Kang 2016; Liu 2017); three trials evaluated modified Xiao Chai Hu Tang formula (Zhao 2014; Wang 2016; Chen 2017); and two trials did not report the detailed composition of Xiao Chai Hu Tang formula (Mao 2014; Wu 2015).
The dosage of Xiao Chai Hu Tang formula when assessed by the amount of the king herb (i.e. the herb with a major pharmacological activity in a traditional Chinese herb formula), Chai Hu, ranged from 6 g to 25 g daily, and the duration of treatment ranged from three to eight months (Yi 2004). The follow‐up of the trial participants in all 10 trials ended with the end of treatment.
Participants in nine trials received co‐interventions such as adefovir (Zhao 2014); lamivudine (Mao 2014; Wu 2015); entecavir (Kang 2016); diammonium glycyrrhizinate (Sun 2004); adefovir plus entecavir (Liu 2017) and lamivudine plus antituberculosis treatment (Chen 2014); entecavir and diammonium glycyrrhizinate (Wang 2016); and hepatoprotective enzymes and immunomodulatory drugs (Chen 2017).
Outcomes
None of the included randomised clinical trials reported results on mortality, serious adverse events, health‐related quality of life, hepatitis B‐related mortality, and hepatitis B‐related morbidity. Two trials reported adverse events considered 'not to be serious' (Wang 2013; Liu 2017), two trials reported the proportion of participants with detectable HBeAg in serum (Zhao 2014; Kang 2016), and three trials reported the proportion of participants with detectable HBV‐DNA in serum (Zhao 2014; Kang 2016; Wang 2016).
Included trials also reported other biomarkers such as AST, ALT, and total bilirubin (TBIL) (Sun 2004; Wang 2013; Chen 2014; Zhao 2014; Wu 2015; Wang 2016; Chen 2017), and a composite outcome consisting of multiple surrogate outcome measures (Liu 2017).
Excluded studies
We excluded 84 studies after reading the full texts of the articles. We explained the reasons for their exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We carried out the risk of bias assessment based on the information retrieved from the publications and some additional information received from the author of one of the trials (Figure 2; Figure 3; Sun 2004).
2.
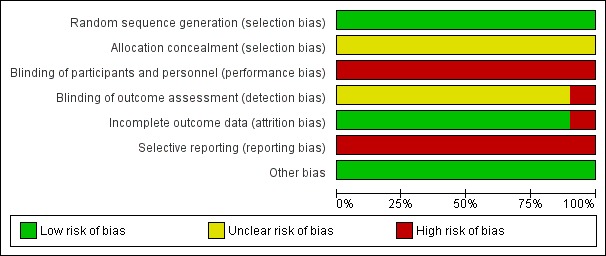
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
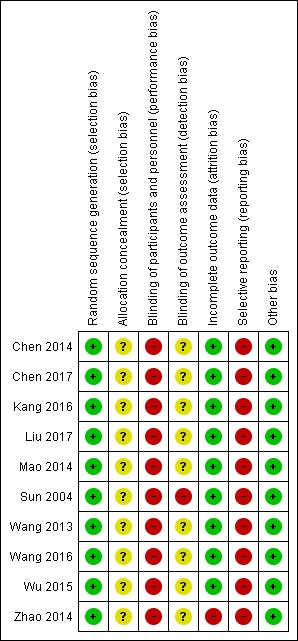
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All 10 included trials reported use of a computer or a random number table to generate allocation sequence (low risk of bias). None of the investigators reported how the allocation sequence was performed. Accordingly, all trials were at unclear risk of selection bias.
Blinding
None of the included randomised clinical trials reported blinding of participants or researchers, or both. We assessed the 10 included trials at high risk of performance bias. One of the included trials reported no blinding of outcome assessment (Sun 2004), and we classified this trial at high risk of detection bias. None of the remaining nine included trials reported blinding of outcome assessment, and therefore the trials were assessed at unclear risk of detection bias.
Incomplete outcome data
Nine of the 10 included randomised clinical trials reported having no missing outcome data and included all participants in data analyses (Sun 2004; Wang 2013; Chen 2014; Mao 2014; Wu 2015; Kang 2016; Wang 2016; Chen 2017; Liu 2017). Therefore, we assessed these nine trials at low risk of bias. Zhao 2014 excluded from the analysis 8/79 trial participants (i.e. 10% proportion of participants). We classified the trial at high risk of attrition bias.
Selective reporting
All included trials may have high risk of reporting bias because of lack of published trial protocols and lack of data on mortality, serious adverse events, and health‐related quality of life outcomes.
Other potential sources of bias
All the included randomised clinical trials appeared free of other factors that could put them at risk of bias. We classified the included randomised clinical trials at low risk of other biases.
Overall risk of bias
We assessed the included randomised clinical trials at high overall risk of bias.
Effects of interventions
See: Table 1
All the 10 randomised clinical trials compared the effects of adding Xiao Chai Hu Tang formula to a co‐intervention compared with a similar co‐intervention. We found no trials comparing Xiao Chai Hu Tang formula versus placebo or no intervention in the control group alone.
Below, in accordance with our protocol (Kong 2018), all outcomes are presented with the random‐effects model meta‐analysis.
Primary outcomes
All‐cause mortality
None of the included randomised clinical trials reported data on all‐cause mortality.
Trial Sequential Analysis
Assuming that the proportion of participants dying within one year in the control group is low (4%), the diversity adjusted required information size (DARIS) obtained with Trial Sequential Analysis for all‐cause mortality is 49,136 trial participants. For the calculation of DARIS, we used event proportion in the control group 4%, relative risk reduction 15%, alpha 2.5%, power 90%, and diversity 0% (Chen 2014; Chen 2017). By looking at the accrued information size (180 participants) and the DARIS of 49,136 participants, we calculated the accrued proportion of participants to be 0.37%. The monitoring boundaries were ignored because only 0.37% (180/49136) of the information size was accrued.
Assuming that the proportion of participants dying within one year in the control group is moderate (20%), the DARIS obtained with Trial Sequential Analysis for all‐cause mortality is 8317 trial participants. For the calculation of DARIS, we used event proportion in the control group 20%, relative risk reduction 15%, alpha 2.5%, power 90%, and diversity 0% (Sun 2004; Wang 2013; Mao 2014; Zhao 2014; Wu 2015; Kang 2016; Wang 2016). By looking at the accrued information size (594 participants) and the DARIS of 8317 participants, we calculated the accrued proportion of participants to be 7.1%. The monitoring boundaries were ignored because only 7.1% (594/8317) of the information size was accrued.
Assuming that the proportion of participants dying within one year in the control group is high (40%), the DARIS obtained with Trial Sequential Analysis for all‐cause mortality was 3215 trial participants. For the calculation of DARIS, we used event proportion in the control group 40%, relative risk reduction 15%, alpha 2.5%, power 90%, and diversity 0% (Liu 2017). By looking at the accrued information size (80 participants) and the DARIS of 3215 participants, we calculated the accrued proportion of participants to be 2.49%. The monitoring boundaries were ignored because only 2.49% (80/3215) of the information size was accrued.
Proportion of participants with one or more serious adverse events
None of the included randomised clinical trials reported data on proportion of participants with one or more serious adverse events.
Health‐related quality of life
None of the included randomised clinical trials reported data on health‐related quality of life.
Secondary outcomes
Hepatitis B‐related mortality
None of the included randomised clinical trials reported data on hepatitis B‐related mortality.
Hepatitis B‐related morbidity
None of the included randomised clinical trials reported data on hepatitis B‐related morbidity.
Proportion of participants with one or more adverse events considered 'not to be serious'
Only two randomised clinical trials with 240 participants randomised provided data on proportion of participants with one or more adverse events considered 'not to be serious' (Wang 2013; Liu 2017). There was no evidence of a difference between Xiao Chai Hu Tang formula and no intervention in the proportion of participants with one or more adverse events considered 'not to be serious' (RR 0.43, 95% CI 0.02 to 11.98, P = 0.62; I2 = 69%; Analysis 1.1).
1.1. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 1 Proportion of participants with ≥ 1 adverse events considered 'not to be serious' – overall.
Trial Sequential Analysis
The DARIS obtained with Trial Sequential Analysis for the proportion of participants with one or more adverse events considered 'not to be serious' outcome was 110,984 trial participants. For the calculation of DARIS, we used event proportion in the control group 5.8%, relative risk reduction 15%, alpha 2.5%, power 90%, and diversity 70%. The monitoring boundaries were ignored because only 0.22% (240/110,984) of the information size was accrued (Wang 2013; Liu 2017). Thus, the Trial Sequential Analysis found insufficient evidence to support or refute a 15% risk reduction of Xiao Chai Hu Tang formula on proportion of participants with one or more adverse events considered 'not to be serious'.
Subgroup analysis
We performed a subgroup analysis on participants diagnosed with only chronic hepatitis B compared to participants diagnosed with concomitant diseases. We found no statistically significant subgroup differences (test for subgroup differences: Chi2 = 2.97, P = 0.09, I2 = 66.3%; Analysis 1.2).
1.2. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 2 Proportion of participants with ≥ 1 adverse events considered 'not to be serious' – concomitant disease.
Sensitivity analysis
The two trials that reported on proportion of participants with one or more adverse events considered 'not to be serious' included all trial participants and described the events per intervention group as randomised. Hence, the conductance of 'best‐worst' and 'worst‐best' scenario analyses became irrelevant.
Our GRADE and Trial Sequential Analysis assessments on imprecision for the proportion of participants with one or more adverse events considered 'not to be serious' outcome did not differ. We downgraded the evidence for imprecision by two levels with GRADE because the number of events was fewer than 300 and the CI overlapped no effect, failing to exclude important benefit (RR less than 0.75) and important harm (RR greater than 1.25) (GRADE 2013; Schünemann 2016). We downloaded the evidence for imprecision by two levels with the Trial Sequential Analysis because none of the sequential boundaries for benefit, harm, or futility were crossed and less than 50% of the required information size was reached (Jakobsen 2014).
Exploratory outcomes
Proportion of participants with detectable HBV‐DNA in serum or plasma
Three randomised clinical trials with 222 participants provided data on the proportion of participants with detectable HBV‐DNA (Wang 2013; Zhao 2014; Kang 2016). Xiao Chai Hu Tang formula was associated with a lower proportion of participants with detectable HBV‐DNA (RR 0.62, 95% CI 0.45 to 0.85; I2 = 0%; Analysis 1.3).
1.3. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 3 Proportion of participants with detectable HBV‐DNA in serum or plasma – overall.
Trial Sequential Analysis
The Trial Sequential Analysis of the three trials (event proportion in the control group 47.1%, relative risk reduction 15%, alpha 5.0%, power 90%, and diversity 0%) showed that the Z‐curve did not reach the DARIS (2068 trial participants) neither did it cross the statistical monitoring boundaries for benefit, harm, and futility. The Trial Sequential Analysis found insufficient evidence to support or refute a 15% risk reduction of Xiao Chai Hu Tang formula on proportion of participants with detectable HBV‐DNA (Trial Sequential Analysis‐adjusted RR 0.62, 95% CI 0.17 to 2.80; Figure 4).
4.
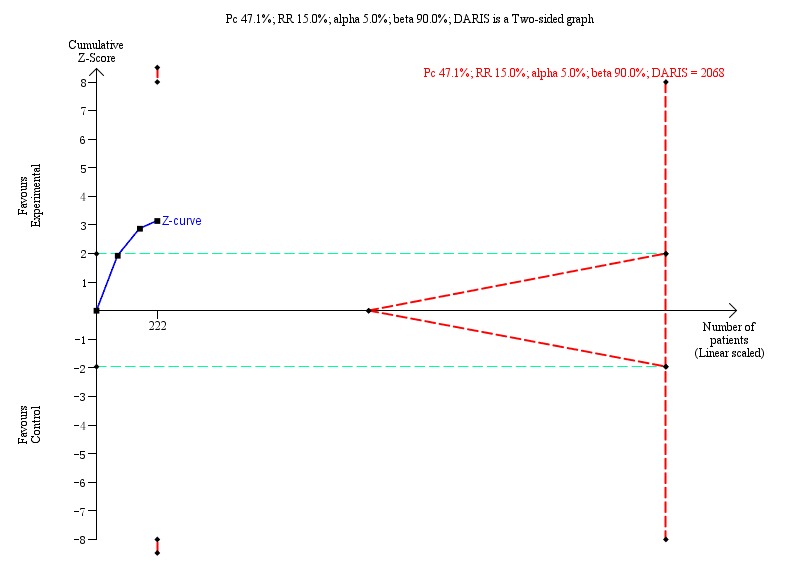
Proportion of participants with detectable hepatitis B virus DNA (HBV‐DNA): Trial Sequential Analysis (risk ratio (RR) random‐effects model) including randomised clinical trials comparing Xiao Chai Hu Tang formula versus no intervention for people with chronic hepatitis B. The pair‐wise meta‐analysis included three trials with 222 participants and found an RR of 0.62 (95% CI 0.17 to 2.80). The Trial Sequential Analysis was made with event proportion in the control group 47.1%, relative risk reduction 15%, alpha 5.0%, power 90%, and model‐based diversity 0%. The Trial Sequential Analysis‐adjusted confidence interval was 0.17 to 2.80.
Subgroup analysis
We could not perform all of the prespecified subgroup analyses because of insufficient data (see Subgroup analysis and investigation of heterogeneity).
We found no statistically significant subgroup difference regarding:
risk of bias (test for subgroup differences: Chi2 = 0.91, P = 0.34, I2 = 0%; Analysis 1.4);
when comparing trials with traditional Xiao Chai Hu Tang formula to trials with modified Xiao Chai Hu Tang formula (test for subgroup differences: Chi2 = 0.01, P = 0.92, I2 = 0%; Analysis 1.5);
when comparing trials with water extraction of Xiao Chai Hu Tang formula to trials with granule of Xiao Chai Hu Tang formula (test for subgroup differences: Chi2 = 0.91, P = 0.34, I2 = 0%; Analysis 1.6);
when comparing trials with treatment duration less than six months to trials with treatment duration more than six months (test for subgroup differences: Chi2 = 0.42, P = 0.52, I2 = 0%; Analysis 1.7);
when comparing trials with dosage of the king herb, Chai Hu, more than 15 g to trials with dosage of Chai Hu less than 15 g (test for subgroup differences: Chi2 = 0.47, P = 0.49, I2 = 0%; Analysis 1.8); and
when comparing participants diagnosed with chronic hepatitis B by trialists to trials where participants were diagnosed according to guidelines (test for subgroup differences: Chi2 = 0.04, P = 0.84, I2 = 0%; Analysis 1.9).
1.4. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 4 Proportion of participants with detectable HBV‐DNA in serum or plasma – incomplete data.
1.5. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 5 Proportion of participants with detectable HBV‐DNA in serum or plasma – formula type.
1.6. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 6 Proportion of participants with detectable HBV‐DNA in serum or plasma – forms.
1.7. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 7 Proportion of participants with detectable HBV‐DNA in serum or plasma – duration.
1.8. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 8 Proportion of participants with detectable HBV‐DNA in serum or plasma – dosage.
1.9. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 9 Proportion of participants with detectable HBV‐DNA in serum or plasma – diagnostic criteria.
Sensitivity analysis
We did not perform the planned sensitivity analysis as all three trials reported on proportion of participants with detectable HBV‐DNA (Wang 2013; Zhao 2014; Kang 2016).
Our GRADE and Trial Sequential Analysis assessments on imprecision for the proportion of participants with detectable HBV‐DNA outcome differed. We downgraded the evidence for imprecision by one level with GRADE because the optimal information size criteria was not met and the sample size was not very large (fewer than 2000 participants) (GRADE 2013; Schünemann 2016), and by two levels with Trial Sequential Analysis because none of the trial sequential boundaries for benefit, harm, or futility were crossed and less than 50% of the required information size was reached (Jakobsen 2014).
Proportion of participants with detectable hepatitis B e‐antigen in serum or plasma
Two randomised clinical trials with 160 participants provided data on proportion of participants with detectable HBeAg (Zhao 2014; Kang 2016). Fixed‐effect meta‐analysis showed there was reduction of Xiao Chai Hu Tang formula on proportion of participants with detectable HBeAg (RR 0.70, 95% CI 0.55 to 0.91, P = 0.007; I2 = 38%; Analysis 1.10), but the random‐effects meta‐analysis showed no evidence of a difference of Xiao Chai Hu Tang formula on proportion of participants with detectable HBeAg (RR 0.72, 95% CI 0.50 to 1.02, P = 0.06; I2 = 38%; Analysis 1.11). We put more weight on the estimate closest to zero effect (i.e. RR 0.72, 95% CI 0.50 to 1.02, P = 0.06), which in this case came from the random‐effects meta‐analysis considering high heterogeneity, in accordance with our protocol. We were uncertain whether Xiao Chai Hu Tang formula had an effect on proportion of participants with detectable HBeAg.
1.10. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 10 Proportion of participants with detectable HBeAg in serum or plasma in fixed‐effect model – overall.
1.11. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 11 Proportion of participants with detectable HBeAg in serum or plasma in random‐effects model – overall.
Trial Sequential Analysis
The Trial Sequential Analysis of the two trials (event proportion in the control group 68.75%, relative risk reduction 15%, alpha 5.0%, power 90%, and diversity 57%) showed that the Z‐curve did not reach the DARIS (2143 trial participants) neither did it cross the statistical monitoring boundaries for benefit, harm, or futility, or the conventional boundaries. The Trial Sequential Analysis found insufficient evidence to support or refute a 15% risk reduction of Xiao Chai Hu Tang formula on proportion of participants with detectable HBeAg (Trial Sequential Analysis‐adjusted RR 0.72, 95% CI 0.17 to 3.02; Figure 5).
5.
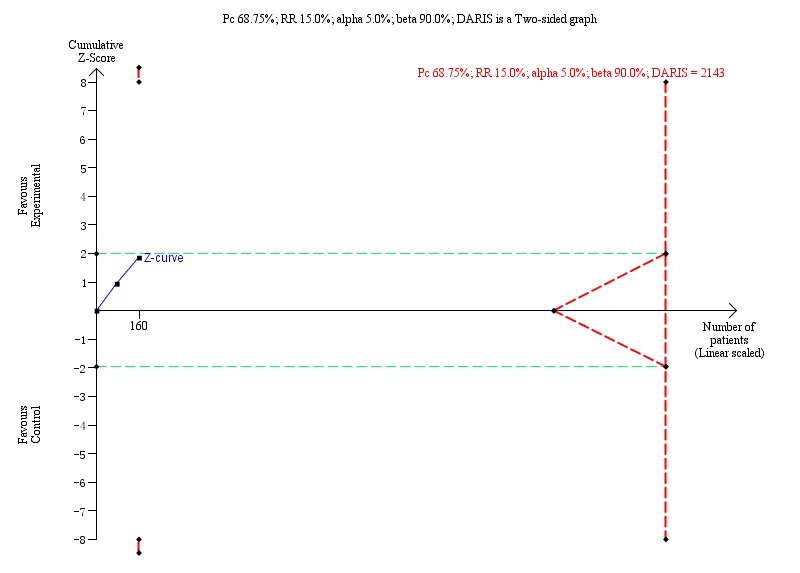
Proportion of participants with detectable HBeAg: Trial Sequential Analysis (risk ratio (RR) random‐effects model) including randomised clinical trials comparing Xiao Chai Hu Tang formula versus no intervention for people with chronic hepatitis B. The pair‐wise meta‐analysis includes 2 trials with 160 participants and found a RR of 0.72 (95% CI 0.17 to 3.02). The Trial Sequential Analysis was made with event proportion in the control group 68.75%, relative risk reduction 15%, alpha 5.0%, power 90%, and model‐based diversity 57%. The Trial Sequential Analysis‐adjusted CI was 0.17 to 3.02.
Subgroup analysis
We could not perform all of the prespecified subgroup analyses because of insufficient information data (See Subgroup analysis and investigation of heterogeneity). We found no statistically significant subgroup difference when comparing trials:
at low risk of bias with trials at high risk of bias (test for subgroup differences: Chi2 = 1.31, P = 0.25, I2 = 23.9%; Analysis 1.12);
with traditional Xiao Chai Hu Tang formula to trials with modified Xiao Chai Hu Tang formula (test for subgroup differences: Chi2 = 1.31, P = 0.25, I2 = 23.9%; Analysis 1.13);
with water extraction of Xiao Chai Hu Tang formula to trials with granule of Xiao Chai Hu Tang formula (test for subgroup differences: Chi2 = 1.31, P = 0.25, I2 = 23.9%; Analysis 1.14);
with treatment duration less than six months to trials with treatment duration more than six months (test for subgroup differences: Chi2 = 1.31, P = 0.25, I2 = 23.9%; Analysis 1.15); and
with dosage of the king herb, Chai Hu, more than 15 g to trials with dosage of the king herb, Chai Hu, less than 15 g (test for subgroup differences: Chi2 = 1.31, P = 0.25, I2 = 23.9%; Analysis 1.16).
1.12. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 12 Proportion of participants with detectable HBeAg in serum or plasma – incomplete data.
1.13. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 13 Proportion of participants with detectable HBeAg in serum or plasma – formula type.
1.14. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 14 Proportion of participants with detectable HBeAg in serum or plasma – forms.
1.15. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 15 Proportion of participants with detectable HBeAg in serum or plasma – duration.
1.16. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 16 Proportion of participants with detectable HBeAg in serum or plasma – dosage.
Sensitivity analysis
We did not perform the planned sensitivity analysis as both trials reported on proportion of participants with detectable HBeAg (Zhao 2014; Kang 2016).
Our GRADE and Trial Sequential Analysis assessments on imprecision for the proportion of participants with detectable HBV‐DNA outcome differed. We downgraded the evidence for imprecision by one level with GRADE because the optimal information size criteria were not met and the sample size was not very large (fewer than 2000 participants) (GRADE 2013; Schünemann 2016), and by two levels with Trial Sequential Analysis because none of the trial sequential boundaries for benefit, harm, or futility were crossed and less than 50% of the required information size was reached (Jakobsen 2014).
Separately reported serious adverse events
We found no trials reporting serious adverse events separately.
Separately reported adverse events considered 'not to be serious'
Following our protocol, we presented the results of dichotomous outcomes from one trial, using Fisher's exact test. As the results between Fisher's exact test (not shown) and those obtained with Review Manager 5 analysis did not differ, and in view of future updates of the review, we presented the analysis result obtained with Review Manager 5 only.
Nausea: we were uncertain whether Xiao Chai Hu Tang formula had an effect on proportion of participants with nausea (RR 5.00, 95% CI 0.24 to 102.53; 1 trial, 160 participants; Analysis 1.17; Wang 2013).
Nausea and vomiting: we are uncertain whether Xiao Chai Hu Tang formula has an effect on proportion of participants with nausea and vomiting (RR 2.00, 95% CI 0.19 to 21.18; 1 trial, 80 participants; Analysis 1.18; Liu 2017).
Dizziness and sleep disorders: we are uncertain whether Xiao Chai Hu Tang formula has an effect on proportion of participants with dizziness and sleep disorders (RR 0.11, 95% CI 0.01 to 2.03; 1 trial, 160 participants; Analysis 1.19; Wang 2013).
Dizziness and fatigue: we are uncertain whether Xiao Chai Hu Tang formula has an effect on proportion of participants with dizziness and fatigue (RR 1.00, 95% CI 0.06 to 15.44; 1 trial, 80 participants; Analysis 1.20; Liu 2017).
Dry feeling or bitter taste in the mouth: we are uncertain whether Xiao Chai Hu Tang formula has an effect on proportion of participants with a dry feeling or bitter taste in the mouth (RR 1.00, 95% CI 0.06 to 15.44; 1 trial, 80 participants; Analysis 1.21; Liu 2017).
Bloating and belching: we are uncertain whether Xiao Chai Hu Tang formula has an effect on proportion of participants with bloating and belching (RR 1.00, 95% CI 0.06 to 15.44; 1 trial, 80 participants; Analysis 1.22; Liu 2017).
Loss of appetite: we are uncertain whether Xiao Chai Hu Tang formula has an effect on proportion of participants with loss of appetite (RR 1.00, 95% CI 0.06 to 15.44; 1 trial, 80 participants; Analysis 1.23; Liu 2017).
1.17. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 17 Proportion of participants with nausea.
1.18. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 18 Proportion of participants with nausea and vomiting.
1.19. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 19 Proportion of participants with dizziness and sleep disorders.
1.20. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 20 Proportion of participants with dizziness and fatigue.
1.21. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 21 Proportion of participants with a dry feeling or bitter taste in the mouth.
1.22. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 22 Proportion of participants with bloating and belching.
1.23. Analysis.
Comparison 1 Xiao Chai Hu Tang (XCHT) formula versus no intervention, Outcome 23 Proportion of participants with loss of appetite.
Separately reported hepatitis B‐related morbidity
We found no trials reporting hepatitis B‐related morbidity separately.
'Summary of findings' tables
We constructed a 'Summary of findings' table for the comparison 'Xiao Chai Hu Tang formula versus no intervention' with an intention to present GRADE assessments of all primary and secondary outcomes (Primary outcomes; Secondary outcomes), using the GRADE factors: risk of bias, unexplained heterogeneity or inconsistency of results, indirectness of the evidence, imprecision, and publication bias. Two trials provided data for 'proportion of participants with one or more adverse events considered 'not to be serious' (Wang 2013; Liu 2017; Table 1). The certainty of evidence for this outcome was very low. Posthoc, we decided to present the results of our exploratory outcomes: proportion of participants with detectable HBV‐DNA and proportion of participants with detectable HBeAg because these outcomes, defined also as surrogate, are related to chronic hepatitis B‐related mortality and morbidity (Su 2016; Osawa 2017; Kouamé 2018; Hung 2019). It is believed that Xiao Chai Hu Tang formula helps in preventing the replication of the virus in people with chronic hepatitis B. We assessed the certainty of evidence for these two outcomes as very low (Table 1).
Discussion
Summary of main results
We included 10 randomised clinical trials with 934 participants. We assessed all 10 trials at overall high risk of bias. For our meta‐analyses, we could gather quantitative data from five of the trials with 490 participants. None of the included randomised clinical trials reported data on our three primary outcomes and the two of the secondary outcomes (i.e. all‐cause mortality, proportion of participants with one or more serious adverse events, health‐related quality of life, hepatitis B‐related mortality, and hepatitis B‐related morbidity). We found no trials comparing Xiao Chai Hu Tang formula with placebo or no intervention in the control group alone. All 10 included randomised clinical trials compared the effects of adding Xiao Chai Hu Tang formula with co‐interventions compared with equal co‐interventions. The evidence on the proportion of participants with one or more adverse events considered 'not to be serious', based on only two randomised trials, of very low quality, showed no evidence of a difference. The result was also observed in a subgroup analysis (Analysis 1.2). The Trial Sequential Analysis suggested that more information was needed. There was no evidence of a subgroup difference in trials including participants with concomitant diseases and participants without concomitant diseases. A sensitivity analysis on imprecision showed no evidence of a difference between the GRADE imprecision assessments and the Trial Sequential Analysis imprecision assessments. Regarding our surrogate outcome on proportion of participants with detectable HBV‐DNA, we are uncertain whether Xiao Chai Hu Tang formula has an effect on the proportion of participants with detectable HBV‐DNA. The Trial Sequential Analysis result showed insufficient evidence to support or reject any effects of Xiao Chai Hu Tang formula on this outcome. None of the remaining subgroup analyses found evidence of a difference in the beneficial or harmful effects of Xiao Chai Hu Tang formula on proportion of participants with detectable HBV‐DNA, between trials at low risk of bias and at high risk of bias regarding incomplete outcome data, trials with traditional Xiao Chai Hu Tang formula compared to trials with modified Xiao Chai Hu Tang formula, trials with water extraction of Xiao Chai Hu Tang formula compared to granule of Xiao Chai Hu Tang formula, trials with treatment duration less than six months compared to trials with treatment duration more than six months, and trials including participants with diagnostic criteria according to guidelines compared to trials including participants with diagnostic criteria defined by trialists. Regarding our surrogate outcome on proportion of participants with detectable HBeAg, we are uncertain whether there is a difference in the effect of Xiao Chai Hu Tang formula compared with no intervention. The Trial Sequential Analysis result showed insufficient evidence to support or reject any effects of Xiao Chai Hu Tang formula on this outcome. None of the remaining subgroup analyses found evidence of a difference in the beneficial or harmful effects of Xiao Chai Hu Tang formula on proportion of participants with detectable HBeAg, between trials assessed as at low risk of bias and at high risk of bias regarding incomplete outcome data, trials with traditional Xiao Chai Hu Tang formula compared to trials with modified Xiao Chai Hu Tang formula, trials with water extraction of Xiao Chai Hu Tang formula compared to granule of Xiao Chai Hu Tang formula, trials with treatment duration less than six months compared to trials with treatment duration more than six months, trials including participants with diagnostic criteria defined by trialists compared to trials including participants with diagnostic criteria according to guidelines. Regarding our surrogate outcomes on proportion of participants with adverse events considered 'not to be serious', analysed separately (i.e. nausea, nausea and vomiting, dizziness and fatigue, dizziness and sleep disorders, a dry feeling or bitter taste in mouth, bloating and belching, and loss of appetite), we are uncertain whether there is a difference in the effect of Xiao Chai Hu Tang formula compared with no intervention.
Overall completeness and applicability of evidence
We contacted authors of 55 studies, of possible interest to our review, for more information related to the trial design and methodology. However, only one author provided some information related to the trial methodology of one of the 10 trials that we included. We found no unpublished or ongoing trials comparing Xiao Chai Hu Tang formula with no intervention or placebo, randomising people with chronic hepatitis B. We identified 43 studies which failed to describe whether a random generation method was used. Another four studies contained information highly insufficient to judge their relevance to our review protocol. These 47 studies in total, not included in the analysis of our review, lacked information on patient‐centred outcomes, and these studies were at high risks of systematic errors and design errors. The problem of misused randomisation in Chinese herbal medicine trials is still significantly influencing the quality of clinical evidence (Liu 2002a; Wu 2009b). To assess further the methodological deficiencies of the identified studies that we could not include in this review, an article, currently in editorial, is focusing on errors in clinical trials on Xiao Chai Hu Tang formula for people with chronic hepatitis B (Kong 2019).
The participants included in each of the 10 trials did not fully reflect the characteristics of the general chronic hepatitis B population. None of the trials included children. The included trials covered granules and water extraction of Xiao Chai Hu Tang formula, modified and traditional Xiao Chai Hu Tang formula, commonly used daily dose of Xiao Chai Hu Tang formula, and a variety of treatment durations of Xiao Chai Hu Tang formula. We could perform meta‐analyses on only one of our predefined outcomes, namely adverse events considered 'not to be serious', and only two of 10 trials provided data. We could find no report on the patient‐centred outcomes such as all‐cause mortality, health‐related quality of life, hepatitis B‐related mortality, and hepatitis B‐related morbidity. In contrast, we found data on surrogate outcomes such as detectable HBV‐DNA and detectable HBeAg in the blood. Two trials also specified the adverse events considered 'not to be serious' that had occurred during the treatment.
All 10 trials were conducted in China and were published as full paper articles in Mandarin, which may threaten the applicability of our review results outside China.
We also conducted subgroup analyses and sensitive analyses in an attempt to identify differences in treatment effects depending on the predefined clinical factors.
Quality of the evidence
The lack or insufficiency of clinically relevant data are a serious limitation of our review and findings. Below, we describe our assessments of each of the five GRADE factors.
Within‐study risk of bias
Risk of bias is known to be responsible for overestimation and underestimation of intervention benefits and harms in randomised clinical trials with inadequate methodological quality (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Savović 2012a; Savović 2012b; Savović 2018). Of the 10 included trials, 10 (100%) reported adequate generation of the randomisation sequence, none (0%) reported adequate allocation concealment, 10 (100%) were conducted without blinding of participants and hence were at high risk of performance bias, nine did not report blinding of outcome assessment and one reported no blinding of outcome assessment and hence were at high risk of detection bias, eight (80%) appeared to be uninfluenced by incomplete outcome data, none (0%) appeared to be free from selective reporting.
We assessed the included trials as at high risks of systematic errors and design errors.
Indirectness of the evidence
As all the included trials in our review assessed Xiao Chai Hu Tang formula in people with chronic hepatitis B, our results are applicable for this group of people. However, our results came from data of surrogate outcomes such as HBV‐DNA and HBeAg, and not from data of clinically relevant outcomes. This is why we downgraded the evidence of each of these two outcomes by one level for indirectness.
Heterogeneity (inconsistency) of results
We explored statistical heterogeneity with the Chi2 test and quantified heterogeneity using the I2 statistic (Higgins 2002). We considered the outcome, proportion of participants with one or more adverse events considered 'not to be serious', to have substantial level of heterogeneity (I2 = 69%). Only two trials reported data on this outcome (Wang 2013; Liu 2017). Analysis of clinical characteristics of the two trials showed that Wang 2013 included participants with concomitant diseases while Liu 2017 did not report whether the trial participants had or did not have concomitant diseases. This could be a reason for the heterogeneity.
We considered the proportion of participants with detectable HBeAg outcome to have a moderate level of heterogeneity (I2 = 38%). We analysed clinical characteristics of the two included trials, and found out that Zhao 2014 reported using 6 g of the king herb, Chai Hu, in the Xiao Chai Hu Tang formula while Kang 2016 reported using 25 g of the king herb, Chai Hu, in the Xiao Chai Hu Tang formula, and this could be a reason for the heterogeneity.
We considered the outcome 'proportion of participants with detectable HBV‐DNA' to have no significant heterogeneity (I2 = 0%).
We applied both fixed‐effect and random‐effects meta‐analysis models, and we reported both models when we found differences. In our review, both the fixed‐effect model and the random‐effects model identified statistically significant differences in the proportion of participants with detectable HBV‐DNA. However, the fixed‐effect model identified statistically significant differences in the proportion of participants with detectable HBeAg, which were not identified by the random‐effects model.
Imprecision of results
In keeping with the GRADE criteria for assessing imprecision, we downgraded the evidence by two levels for the outcome 'proportion of participants with one or more adverse events considered 'not to be serious' '. This was because the number of events was fewer than 300 and the CIs overlapped no effect, failing to exclude important benefit (RR less than 0.75) and important harm (RR greater than 1.25).
Regarding the outcomes 'proportion of participants with detectable HBV‐DNA' and 'proportion of participants with detectable HBeAg', we downgraded the evidence by one level. This was because the optimal information size criteria were not met and the sample size was not very large (fewer than 2000 participants). The number of events was also small (GRADE 2013; Schünemann 2016).
We also performed Trial Sequential Analysis to assess imprecision, and the results were consistent with the GRADE assessment for the 'proportion of participants with one or more adverse events considered 'not to be serious' ' outcome. Our assessments differed when the GRADE assessment of imprecision was compared to that conducted via Trial Sequential Analysis for the 'proportion of participants with detectable HBV‐DNA' outcome, and 'proportion of participants with detectable HBeAg' outcome. We downgraded the evidence for imprecision by two levels with Trial sequential Analysis because none of the sequential boundaries for benefit, harm, or futility were crossed and less than 50% of the required information size was reached (Jakobsen 2014). The trial sequential analysis‐adjusted CI included both significant benefit and harm,
Risk of publication bias
We could not construct funnel plots because data were derived from a maximum of three trials per outcome. We suspected publication bias because the included trials had small sample sizes and two studies showed positive effects in surrogate outcomes (GRADEpro GDT; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2013a; Guyatt 2013b; Guyatt 2013c; Guyatt 2013d; Guyatt 2017).
Potential biases in the review process
We performed our systematic review based on recommended methodology (Higgins 2019). We followed our prepublished and peer‐reviewed protocol with predefined participants, interventions, comparisons, and outcomes to avoid biases in the review process (Kong 2018). We performed comprehensive search strategies which covered published studies and registered study protocols. We searched the reference lists of the identified studies manually. We combined the electronic searches with the manual data searches. We extracted all available data to perform our predefined analyses, including subgroup and sensitivity analyses.
Unpublished trials with negative results, which we did not identify during the preparation of this review, are a possible source of bias. We contacted the authors of the 10 included trials and the 47 potential randomised clinical trials listed in Appendix 2 to obtain missing data of relevance to our review methodology and outcome data; however, only one author replied. The lack of reporting of serious adverse events poses another limitation and lack of conclusions in our review on patient‐centred outcomes. For trials reporting adverse events considered 'not to be serious', we selected the highest number of events among the separately reported number of events that had occurred in the experimental or control groups to calculate the proportion of participants with one or more adverse events considered 'not to be serious'. This may have been problematic as it might have underestimated the proportion of participants with one or more adverse events considered 'not to be serious'.
All the trials we found were carried out in China, possibly because Xiao Chai Hu Tang formula is most often used in Asian countries. Although we have made a broad strategy to identify all available trials, studies published in languages other than Chinese and English may not have been found. If possible, Japanese and Korean medical databases should be added for the update of this review, and experts should be asked to find out if there are more studies on this topic.
Observational studies may provide information on rare late‐occurring adverse events and quality of life, which are outcomes of interest to this review. We planned to consider quasi‐randomised studies, controlled clinical studies, and other observational studies for data on harms of Xiao Chai Hu Tang formula if retrieved through our searches for randomised clinical trials. The 47 studies, listed in Appendix 2 because of missing information on the study design, could be a valuable source of data on adverse events. Our decision not to perform specific searches for observational studies and our posthoc decision not to assess potential data on adverse events in the Characteristics of excluded studies table, as well as the studies in Appendix 2, might have biased our review, causing us to overlook potential harms (late or rare harms) (Storebø 2018). The defect may become less problematic as we find no beneficial effect of Xiao Chai Hu Tang formula on patient‐centred outcomes in this review. However, a systematic review of observational studies on harms of Xiao Chai Hu Tang formula may be launched in future.
A possible limitation of our review could also be our choice of a cut‐off value for duration of treatment for subgroup analyses. We used six months as a cut‐off value instead of the observed median treatment duration of 3.5 months because the two included trials reporting on the proportion of participants with detectable HBV‐DNA and proportion of participants with detectable HBeAg had treatment duration of more than 3.5 months. However, our cut‐off could be arbitrary, and thus the results could be different, had we used another cut‐off value.
We included several subgroup analyses and numerous surrogate outcomes. There could have been problems with multiplicity in terms of the seven subgroup analyses for each outcome (Imberger 2011). We did not adjust the threshold for subgroups analyses, as we consider subgroup analyses results as exploratory and hypothesis‐generating.
We conducted Trial Sequential Analyses for the outcomes proportion of participants with adverse events considered 'not to be serious' (secondary outcome); with detectable HBV‐DNA (exploratory outcome); and with detectable HBeAg (exploratory outcome) (Wetterslev 2008; Thorlund 2011b; TSA 2011; Wetterslev 2017). We calculated the DARIS on the basis of type I error of 2.5% for secondary outcomes, 5.0% for exploratory outcomes, type II error of 10%, and risk reduction of 15%, and events proportion in the control group (Wetterslev 2009). None of the cumulative Z‐curve of the two Trial Sequential Analyses crossed trial sequential monitoring boundaries or the futility boundaries, and the DARIS was not reached. Therefore, we cannot exclude the risk of random errors regarding our results on the aforementioned outcomes.
Evaluation of the intervention efficacy of a traditional Chinese medicine is a complex task. Perhaps the approach in this review is a reductionist one because the definition of a disease in China can differ from the same disease in a Western country. This is why it could be problematic to integrate western medicine and traditional Chinese medicine.
Our search was conducted on 1 March 2019. It is possible that further studies of relevance to our review could have been published since then. This will be dealt with in future updates.
Agreements and disagreements with other studies or reviews
Three non‐Cochrane meta‐analyses on Xiao Chai Hu Tang formula for people with chronic hepatitis B have been published (Qin 2010; Hu 2011; Yang 2015). None of the three meta‐analyses assessed the effects of Xiao Chai Hu Tang formula on patient‐centred outcomes such as mortality, adverse events, or health‐related quality of life. Compared with the risk of bias tool used in the identified three meta‐analyses, we found that our bias risk assessment tool was much more rigorous. We did not use scores to assess risk of bias as the Jadad scoring system does (Jadad 1996), and we used seven predefined domains to assess the possible risk of reporting bias (Higgins 2019). We used Trial Sequential Analyses to control random errors and GRADE assessments to define the certainty of the evidence.
Qin 2010 showed that Xiao Chai Hu Tang plus antiviral drugs compared with the antiviral drugs alone (interferon‐α‐2b, adefovir dipivoxil, lamivudine, and ribavirin) reduced HBsAg, HBeAg, HBV‐DNA, and ALT levels. Hu 2011 showed that Xiao Chai Hu Tang plus peg‐IFNα had higher rates of alanine (amino)transaminase (ALT) improvement, HBeAg seroconversion, and reduction of influenza‐like symptoms. Yang 2015 showed that Xiao Chai Hu Tang plus antiviral drugs reduced ALT levels and HBeAg seroconversion rate compared to antiviral drugs alone. All the three meta‐analyses only assessed surrogate outcomes. In our review, we found no beneficial or harmful effect of Xiao Chai Hu Tang formula on adverse events considered 'not to be serious', proportion of participants with HBV‐DNA, or proportion of participants with HBeAg when compared with no intervention, and the quality of the evidence was very low.
Authors' conclusions
Implications for practice.
The clinical effects of Xiao Chai Hu Tang formula for chronic hepatitis B remain unclear. The included trials are small and of low methodological quality. Despite the wide use of Xiao Chai Hu Tang formula in China, we lack data on all‐cause mortality, health‐related quality of life, serious adverse events, hepatitis B‐related mortality, and hepatitis B‐related morbidity. The evidence in this systematic review came from data obtained from a maximum three trials. We graded the certainty of evidence as very low for the outcome 'adverse events considered 'not to be serious' ' as well as for the surrogate outcomes hepatitis B e‐antigen (HBeAg) and hepatitis B virus DNA (HBV‐DNA). We found a large number of trials which lacked clear description of their design and conduct, and hence, these trials are not included in the present review. As all identified trials were conducted in China, there might be a concern about the applicability of this review results outside China.
Implications for research.
In view of the wide usage of Xiao Chai Hu Tang formula, we need large, high‐quality randomised placebo‐controlled trials with proper design and homogeneous groups of participants, in which patient‐related outcomes are assessed. We suggest the following implications for research (Brown 2006).
Evidence (what is the current state of the evidence)?
This review includes 10 randomised clinical trials with 934 participants. These trials did not provide data on patient‐centred outcomes including all‐cause mortality, proportion of participants with one or more serious adverse events, health‐related quality of life, hepatitis B‐related mortality, and hepatitis B‐related morbidity. The evidence showed beneficial effect of Xiao Chai Hu Tang formula on the proportion of participants with detectable HBV‐DNA outcome. However, this surrogate outcome is not validated. Our certainty in the evidence was very low. The diversity‐adjusted required information size (DARIS) was not reached for any of the assessed outcomes. Therefore, we are very uncertain about these findings. Further randomised clinical trials are needed and they ought to be designed according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) standards (Chan 2013), and reported according to the CONSORT standards (Moher 2001).
Participants (what is the population of interest)?
We focused on people with chronic hepatitis B. We could only obtain some information about concomitant diseases from two trials (Chen 2014; Chen 2017). Since there were only a very few trials providing data for a very few outcomes, further trials with detailed information on concomitant diseases and strictly defined diagnostic criteria are needed. When concomitant disease are present, stratified randomisation should be employed.
Interventions (what are the interventions of interest)?
We assessed Xiao Chai Hu Tang formula administered orally. However, because of the few identified trials and very low certainty of the evidence, future randomised clinical trials should be designed to look for differences in formula compositions and dosages, formula formats, and treatment durations of Xiao Chai Hu Tang formula.
Comparisons (what are the comparisons of interest)?
We aimed to assess the benefits and harms of Xiao Chai Hu Tang formula compared with placebo or no intervention in patient‐relevant outcomes for people with chronic hepatitis B. The effects of Xiao Chai Hu Tang formula on adverse events considered 'not to be serious' could have been influenced by the lack of placebo‐controlled trials and blinding. Future randomised clinical trials should compare Xiao Chai Hu Tang formula with placebo with or without co‐interventions.
Outcomes (what are the outcomes of interest)?
The primary outcomes planned to be assessed in this review (all‐cause mortality, proportion of participants with one or more serious adverse events, and health‐related quality of life) should be included as primary outcomes in all future trials. Future randomised clinical trials also need to validate the relationship between surrogate outcomes and patient‐centred outcomes.
Notes
Cochrane Reviews can be expected to have a high percentage of overlap in the methods section because of standardised methods. In addition, overlap may be observed across several of our protocols and reviews, as they share at least three common authors.
Acknowledgements
We acknowledge the great help of Sarah Klingenberg, the Information Specialist in the Cochrane Hepato‐Biliary Group in designing the search strategies. We also acknowledge Dr L Susan Wieland of the Cochrane Complementary Medicine Field (USA) who co‐ordinated the peer review process.
Peer reviewers: Vanja Giljaca, UK; Yasemin Balaban, Turkey.
Contact editor: Goran Poropat, Croatia; Vanja Giljaca, UK.
Sign‐off editor: Agostino Colli, Italy.
Cochrane Abdomen and Endocrine Network Associate Editor: Rachel Richardson, UK; Liz Bickerdike, UK.
Cochrane Review Group funding acknowledgement: the Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State, the Copenhagen Trial Unit, National Center for Complementary and Integrative Health (NCCIH), and the National Institutes of Health.
This work was partially funded by Grant Number R24 AT001293 from the NCCIH.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | March 2019 | (Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai*u or bupleur* or sho‐sai‐ko* or shosaiko* or minor bupleurum decoction*) AND ((hepatitis B or hep B or hbv) and chronic) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library | March 2019 | #1 (Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai*u or bupleur* or sho‐sai‐ko* or shosaiko* or minor bupleurum decoction*) #2 MeSH descriptor: [Hepatitis B, Chronic] explode all trees #3 ((hepatitis B or hep B or hbv) and chronic) #4 #2 or #3 #5 #1 and #4 |
| MEDLINE Ovid | 1946 to March 2019 | 1. (Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai*u or bupleur* or sho‐sai‐ko* or shosaiko* or minor bupleurum decoction*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 2. exp Hepatitis B, Chronic/ 3. ((hepatitis B or hep B or hbv) and chronic).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 4. 2 or 3 5. 1 and 4 |
| Embase Ovid | 1974 to March 2019 | 1. (Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai*u or bupleur* or sho‐sai‐ko* or shosaiko* or minor bupleurum decoction*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] 2. exp chronic hepatitis B/ 3. ((hepatitis B or hep B or hbv) and chronic).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] 4. 2 or 3 5. 1 and 4 |
| LILACS (Latin American and Caribbean Health Science Information database) (Bireme) | 1982 to March 2019 | (Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai$u or bupleur$ or sho‐sai‐ko$ or shosaiko$ or minor bupleurum decoction) [Words] and ((hepatitis B or hep B or hbv) and chronic) [Words] |
| Science Citation Index Expanded (Web of Science) | 1900 to March 2019 | #3 #2 AND #1 #2 TS=((hepatitis B or hep B or hbv) and chronic) #1 TS=(Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai*u or bupleur* or sho‐sai‐ko* or shosaiko* or minor bupleurum decoction*) |
| Conference Proceedings Citation Index – Science (Web of Science) | 1990 to March 2019 | #3 #2 AND #1 #2 TS=((hepatitis B or hep B or hbv) and chronic) #1 TS=(Xiao‐Chai‐Hu or xiaochaihu or XCHT or chai*u or bupleur* or sho‐sai‐ko* or shosaiko* or minor bupleurum decoction*) |
| China National Knowledge Infrastructure (CNKI) | 1979 to March 2019 | #1 hepatitis B in abstract #2 'Xiao‐Chai‐Hu Tang' or 'Xiao‐Chai‐Hu granule' or 'Xiao‐Chai‐Hu capsule' or 'Xiao‐Chai‐Hu tablet' in abstract #3 random in abstract #4 randomly grouped in abstract #5 #3 OR #4 #6 #1 AND #2 AND #5 |
| Chongqing VIP (CQVIP) (VIP) | 1989 to March 2019 | #1 hepatitis B in abstract #2 'Xiao‐Chai‐Hu Tang' or 'Xiao‐Chai‐Hu granule' or 'Xiao‐Chai‐Hu capsule' or 'Xiao‐Chai‐Hu tablet' in abstract #3 random in abstract #4 randomly grouped in abstract #5 #3 OR #4 #6 #1 AND #2 AND #5 |
| Wanfang Data | 1990 to March 2019 | #1 hepatitis B in abstract #2 'Xiao‐Chai‐Hu Tang' or 'Xiao‐Chai‐Hu granule' or 'Xiao‐Chai‐Hu capsule' or 'Xiao‐Chai‐Hu tablet' in abstract #3 random in abstract #4 randomly grouped in abstract #5 #3 OR #4 #6 #1 AND #2 AND #5 |
| SinoMed | 1860 to March 2019 | #1 hepatitis B in abstract #2 'Xiao‐Chai‐Hu Tang' or 'Xiao‐Chai‐Hu granule' or 'Xiao‐Chai‐Hu capsule' or 'Xiao‐Chai‐Hu tablet' in abstract #3 random in abstract #4 randomly grouped in abstract #5 #3 OR #4 #6 #1 AND #2 AND #5 |
Appendix 2. Potential randomised clinical trials
|
Trial ID (volume;issue:pages) |
First author | English title | Journal – English name | Contact information | Treatment duration | Number randomised | Interventiona | Control | |
| Interventiona | Control | ||||||||
| Yang 2009 (28;11:38–9) |
Yang YN | 60 people with chronic hepatitis B treated with Xiao Chai Hu decoction | Nei Mongol Journal of Traditional Chinese Medicine | Contacted authors on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 2 months | 60 | 60 | Xiao Chai Hu Tang | No intervention |
| Li 2001 (17;3:187) |
Li XH | 56 people with chronic hepatitis B treated with q‐1b interferon and Bupleurum Chinese decoction | Journal of Clinical Hepatobiliary Diseases | Contacted authors on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 12 weeks | 56 | 42 | Xiao Chai Hu Tang | No intervention |
| Wang 2014 (12;13:32–3) |
Wang SM | Clinical effect of Chinese medicine on chronic hepatitis B | Modern Chinese Medicine Education | Contacted authors on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 3 months | 39 | 39 | Xiao Cha Hu Tang | No intervention |
| He 2008 (16;6:384–5) |
He BF | Clinical study on the treatment of chronic hepatitis B liver fibrosis with minor Bupleurum tablet and interferon | Chinese Journal of Integrated Chinese and Western Medicine Digestion | Contacted authors on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6 months | 73 | 64 | Xiao Chai Hu Tang | No intervention |
| Xiong 2003 (25;10:10–1) |
Xiong F | Clinical observation on treatment of liver fibrosis with interferon and Xiao Chai Hu decoction | Hubei Journal of Traditional Chinese Medicine | Contacted authors on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 3 months | 48 | 38 | Xiao Chai Hu Tang | No intervention |
| Sun 2005 (NR;NR:34) |
Sun Y | 32 people with chronic hepatitis B treated with Xiao Chai Hu decoction | Heilongjiang Traditional Chinese Medicine | Contacted authors on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6–8 weeks | 32 | 30 | Xiao Chai Hu Tang | No intervention |
| Zeng 2015 (35;6:1284–6) |
Zeng B | 60 people with chronic hepatitis B cirrhosis treated with Xiao Chai Hu Tang decoction | Henan Traditional Chinese Medicine | Contacted authors on 19 March 2019 by e‐mail: received no reply. | NR | 60 | 60 | Xiao Chai Hu Tang | No intervention |
| Zhou 2015b (34;8:41+145) |
Zhou WF | People with chronic hepatitis B treated with Xiao Chai Hu decoction | Nei Mongol Journal of Traditional Chinese Medicine | Contacted authors on 23 March 2019 by telephone: received no reply. Could not find e‐mail address. | 3 months | 41 | 41 | Xiao Chai Hu Tang | No intervention |
| Wang 2014b (12;7:30–1) |
Wang MH | People with chronic hepatitis B treated with modified Xiao Chai Hu decoction combined with adefovir dipivoxil tablets | Journal of Community Medicine | Contacted authors on 27 March 2018 by telephone: the telephone number was invalid. Could not find e‐mail address. | 48 weeks | 90 | 90 | Xiao Chai Hu Tang | No intervention |
| Tian 2010 (10;9:49–50) |
Tian GJ | The interference of minor radix bupleuri decoction plus or minus on common adverse reaction in interferon treatment for chronic hepatitis B | Modern Hospital | Contacted authors on 31 March 2018 by e‐mail: received no reply. | 6 months | 20 | 20 | Xiao Chai Hu Tang | No intervention |
| Li 2015 (9;6:325) |
Li JX | People with chronic hepatitis B treated with minor Bupleurum decoction plus adefovir dipivoxil | For All Health | Contacted authors on 31 March 2018 by telephone: the author could provide no more effective information about the study. | 10 months | 50 | 50 | Xiao Chai Hu Tang | No intervention |
| Wang 2013 (13;7:11–3) |
Wang CD | People with hepatitis B treated with minor Bupleurum decoction plus antiviral drugs | Practical Combination of Traditional Chinese and Western Medicine | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 12 months | 48 | 48 | Xiao Chai Hu Tang | No intervention |
| Chen 2013 (11;23:79) |
Chen L | 35 people with chronic hepatitis B treated with Xiao Chai Hu decoction plus lamivudine | Chinese Medicine Modern Distance Education of China | Contacted author on 26 March 2018 by telephone: the author did not work there any longer. Could not find e‐mail address. | 6 months | 35 | 35 | Xiao Chai Hu Tang | No intervention |
| Qiu 2010 (17;1:38–9) |
Qiu B | People with chronic hepatitis B with liver fibrosis treated with Xiao Chai Hu decoction plus silymarin | Chinese Journal of Primary Medicine and Pharmacy | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6 months | 50 | 40 | Xiao Chai Hu Tang | No intervention |
| Chen 2011 (20;31:3928–30) |
Chen DD | People with liver fibrosis and chronic hepatitis B treated with Xiao Chai Hu decoction | Modern Journal of Integrated Traditional Chinese and Western Medicine | Contacted author on 31 March 2018 by e‐mail: the author could provide no more effective information about the study. | 4 months | 40 | 40 | Xiao Chai Hu Tang | No intervention |
| Wu 1994 (4;4:31) |
Wu W | Treatment of chronic hepatitis B with autologous LAK cell transfusion plus Xiao Chai Hu decoction: a report of 32 people | Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases | Could find no contact information. | 6 weeks | 12 | 20 | Xiao Chai Hu Tang | No intervention |
| Shi 2006 (28;12:35) |
Shi WY | People with chronic hepatitis B treated with interferon plus Xiao Chai Hu decoction | Hubei Journal of Traditional Chinese Medicine | Contacted author on 27 March 2018 by telephone: received no reply. Could not find e‐mail address. | 16 weeks | 46 | 30 | Xiao Chai Hu Tang | No intervention |
| Li 2009 (31;5:709–10) |
Li ZQ | People with chronic hepatitis B treated with minor Bupleurum decoction plus lamivudine | Hebei Journal of Traditional Chinese Medicine | Could not find any contact information. | 6 months | 34 | 34 | Xiao Chai Hu Tang | No intervention |
| Liu 2011 (19;4:218–20) |
liu ZJ | Effects of spiced Bupleurum decoction on the variation rate of YMDD of hepatitis B virus | Chinese Journal of Integrated Chinese and Western Medicine Digestion | Contacted author on 27 March 2018 by telephone: received no reply. Could not find e‐mail address. | NR | 59 | 61 | Xiao Chai Hu Tang | No intervention |
| Wang 2012 (NR;NR:157–61) |
Wang M | Efficacy of Xiao Chai Hu decoction combined with lamivudine in the treatment of HBeAg positive chronic hepatitis B and its effect on the variation of hepatitis B virus YMDD | NR | Contacted author on 27 March 2018 by telephone: received no reply. Could not find e‐mail address. | 48 weeks | 40 | 58 | Xiao Chai Hu Tang | No intervention |
| Wang 1992 (8;4:191–4) |
Wang CG | Curative effect analysis of 173 people with chronic hepatitis B treated by autologous LAK cell transfusion plus Xiao Chai Hu decoction or interleukin‐2 | Journal of Clinical Hepatobiliary Diseases | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6 weeks | NR | NR | Xiao Chai Hu Tang | No intervention |
| Jia 1990 (15;2:95–7) |
Jia KM | People with chronic active hepatitis treated with Bupleurum decoction | Medical Journal of Chinese People's Liberation Army | We found no contact information | 3 months | NR | NR | Xiao Chai Hu Tang | No intervention |
| Yu 2000 (10;3:133–5) |
Yu AQ | People with chronic hepatitis B treated with minor Bupleurum decoction plus viral azole | Zhejiang Journal of Integrated Traditional Chinese and Western Medicine | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 3 months | 57 | 55 | Xiao Chai Hu Tang | No intervention |
| Zhang 2001 (17;8:778–9) |
Zhang YP | 44 people with chronic hepatitis B treated with Bupleurum plus Gan Li Xin | Journal of Applied Medicine | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 1 month | 44 | 40 | Xiao Chai Hu Chong Ji | No intervention |
| Li 2001b (11;S1:95) |
Li Z | People with hepatic fibrosis treated with interferon plus Xiao Chai Hu decoction | Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases | Could not find any contact information | 3 months | 60 | 50 | Xiao Chai Hu Tang | No intervention |
| Bo 2006 (46;8:82–3) |
Bo QL | 50 people with chronic hepatitis B treated with interferon and Xiao Chai Hu decoction | Shandong Medicine | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | NR | 50 | 46 | Xiao Chai Hu Tang | No intervention |
| Hong 2005 (27;6:23–4) |
Hong XT | People with chronic hepatitis B treated with Xiao Chai Hu decoction plus Qing Kai Ling injection | Hubei Journal of Traditional Chinese Medicine | Contacted author on 26 March 2018 by telephone: received no reply. Could not find e‐mail address. | 3 months | 100 | 98 | Xiao Chai Hu Tang | No intervention |
| Cheng 2015 (34;6:57) |
Cheng L | People with hepatitis B liver fibrosis treated with adefovir plus Xiao Chai Hu Tang | Nei Mongol Journal of Traditional Chinese Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 48 weeks | 40 | 40 | Xiao Chai Hu Tang | No intervention |
| Zhou 2015 (21;4:659–62) |
Zhou XH | People with chronic hepatitis B treated with Xiao Chai Hu decoction plus Wu Ling Gan Fu capsule – effects and safety | Hebei Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6 months | 40 | 40 | Xiao Chai Hu Tang | No intervention |
| Lin 2017 (15;20:204–5) |
Lin JB | People with chronic hepatitis B treated with Xiao Chai Hu decoction plus entecavir | Guide of China Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 32 weeks | 49 | 49 | Xiao Chai Hu Tang | No intervention |
| Zou 2016 (8;24:23–5) |
Zou ZC | People with liver stagnation and spleen deficiency type of chronic hepatitis B treated with Xiao Chai Hu decoction plus entecavir | Clinical Journal of Chinese Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | Unclear | 48 | 48 | Xiao Chai Hu Tang | No intervention |
| Jiang 2015 (4;NR:209–10) |
Jiang FX | People with chronic hepatitis B treated with Xiao Chai Hu decoction plus Sheng Jiang powder | Yi Xue Mei Xue Mei Rong | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 4 weeks | 31 | 31 | Xiao Chai Hu Tang plus Sheng Jiang powder | No intervention |
| Shan 2006 (8;NR:528–9) |
Shan XM | Effect of Xiao Chai Hu decoction on interferon influenza‐like response | Shandong Journal of Traditional Chinese Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 4 weeks | 50 | 46 | Xiao Chai Hu Tang | No intervention |
| Huang 2001 (5;NR:561–2) |
Huang HC | 40 people with chronic hepatitis B treated with interferon plus Xiao Chai Hu granule | Suzhou University Journal of Medical Science | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6 months | 40 | 40 | Xiao Chai Hu granule | No intervention |
| Wang 2009 (2;NR:NR) |
Wang LF | People with chronic hepatitis B treated with lamivudine plus Xiao Chai Hu | Zhong Jing Yi Xue Qiu Zhen | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 24 weeks | 15 | 13 | Xiao Chai Hu Tang | No intervention |
| Hu 2017 (36;20:65–6) |
Hu DQ | Study on the Xiao Chai Hu decoction for chronic hepatitis B and its histological mechanism | Nei Mongol Journal of Traditional Chinese Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 3 months | 60 | 60 | Xiao Chai Hu Tang | No intervention |
| Zhang 2004 (3;NR:170–1) |
Zhang XD | People with hepatitis B virus and immune regulation problems treated with lamivudine plus Xiao Chai Hu decoction | Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 12 months | 49 | 71 | Xiao Chai Hu Tang | No intervention |
| Wei 2007 (3;NR;294–5) |
Wei KX | People with chronic hepatitis B treated with Xiao Chai Hu decoction | Chinese Journal of Experimental and Clinical Virology | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | NR | NR | NR | Xiao Chai Hu Tang | No intervention |
| Chen 2016 (6;6:25–7) |
Chen XT | People with chronic hepatitis B liver fibrosis treated with Chinese plus western medicine | Integrated Traditional Chinese and Western Medicine | Contacted author on 23 March 2018 by telephone: received no reply. Could not find e‐mail address. | 6 months | 41 | 40 | Xiao Chai Hu Tang | No intervention |
| Chen 2008 (12;NR:786–8) |
Chen XT | People with hepatitis B liver fibrosis treated with adefovir dipivoxil plus Xiao Chai Hu decoction | Public Medical Forum Magazine | Contacted authors on 27 March 2018 by telephone: the first author has retired and the second author said relevant documents had been lost, therefore they could not provide more information. | 12 months | 60 | 60 | Xiao Chai Hu Tang | No intervention |
| Hirayama 1989 (24;NR:715–9) |
Hirayama C | Multicentre randomised controlled clinical trial of people with chronic active hepatitis treated with Sho‐sai‐ko‐to | Gastroenterologia Japonica | Could not obtain any contact information of the authors | 12 weeks | 116 | 106 | EK 9 (Kanebo Pharmaceutical Co., Tokyo), which contained 0.9 g of SST per gram | A placebo of similar appearance and smell, which contained 0.09 g of SST per gram |
| Tajiri 1991 (NR;2:121–9) |
Tajiri HK | Children with chronic hepatitis B virus infection and with sustained liver disease treated with Xiao Chai Hu Tan. Measured HBeAg clearance | American Journal of Chinese Medicine | Could not obtain any contact information of the authors | 16.8 (SD 5.0) months | 23 | 20 | Xiao Chai Hu granule | No intervention |
| Sata 1994 (71;NR:814–20) |
Sata M | People with chronic active hepatitis B treated with interferon‐α (Feron) plus Sho‐sai‐ko‐to | Rinsho to Kenkyu (Japanese Journal of Clinical and Experimental Medicine) | Could not obtain any contact information of the authors | 6 months | 28 | 34 | Xiao Chai Hu Tang | No intervention |
| Shiraki 1991 (NA;44:2146–51) |
Shiraki K | Children with HBe antigen‐positive chronic hepatitis B treated with Sho‐sai‐ko‐to | Shonika Rinsho (Japanese Journal) | NA | NA | NA | NA | NA | NA |
| Heydtmann 2000 (NA;16:61–2) |
Heydtmann M | New findings in hepatology | Therapiewoche Schweiz | NA | NA | NA | NA | NA | NA |
| Feng 2016 (22;6:A54–5) |
Feng S | Herbal preparations for chronic hepatitis B virus carriers: the analysis from 31 randomised clinical trials | Journal of Alternative and Complementary Medicine | NA | NA | NA | NA | NA | NA |
| Ye 2011 (15;1:S76) |
Ye YA | People with long‐term anti‐HBV infection treated with Chinese herbal medicine | International Journal of Infectious Diseases | NA | NA | NA | NA | NA | NA |
|
aXiao Chai Hu Tang is also called XCHT, Sho‐sai‐ko‐to, or minor Bupleurum decoction. HBeAg: hepatitis B virus e‐antigen; LAK: lymphokine‐activated killer; NA: not applicable/available; NR: not reported; SD: standard deviation. | |||||||||
Data and analyses
Comparison 1. Xiao Chai Hu Tang (XCHT) formula versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with ≥ 1 adverse events considered 'not to be serious' – overall | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.02, 11.98] |
| 2 Proportion of participants with ≥ 1 adverse events considered 'not to be serious' – concomitant disease | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.02, 11.98] |
| 2.1 Participants diagnosed with only chronic hepatitis B | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.34] |
| 2.2 Participants diagnosed with concomitant diseases | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.19, 21.18] |
| 3 Proportion of participants with detectable HBV‐DNA in serum or plasma – overall | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 4 Proportion of participants with detectable HBV‐DNA in serum or plasma – incomplete data | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 4.1 Trials at low risk of bias | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.87] |
| 4.2 Trials at high risk of bias | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.01] |
| 5 Proportion of participants with detectable HBV‐DNA in serum or plasma – formula type | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 5.1 Experimental intervention with traditional XCHT formula | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.95] |
| 5.2 Experimental intervention with modified XCHT formula | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.96] |
| 6 Proportion of participants with detectable HBV‐DNA in serum or plasma – forms | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 6.1 Water extraction of XCHT formula | 2 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.87] |
| 6.2 Granule of XCHT | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.47, 1.01] |
| 7 Proportion of participants with detectable HBV‐DNA in serum or plasma – duration | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 7.1 Duration < 6 months | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.09, 1.62] |
| 7.2 Duration > 6 months | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.46, 0.88] |
| 8 Proportion of participants with detectable HBV‐DNA in serum or plasma – dosage | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 8.1 XCHT < 15 g | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.96] |
| 8.2 XCHT > 15 g | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.95] |
| 9 Proportion of participants with detectable HBV‐DNA in serum or plasma – diagnostic criteria | 3 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.45, 0.85] |
| 9.1 Participants with diagnostic criteria according to guidelines | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.96] |
| 9.2 Participants with diagnosis by trialists | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.95] |
| 10 Proportion of participants with detectable HBeAg in serum or plasma in fixed‐effect model – overall | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.91] |
| 11 Proportion of participants with detectable HBeAg in serum or plasma in random‐effects model – overall | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.02] |
| 12 Proportion of participants with detectable HBeAg in serum or plasma – incomplete data | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.02] |
| 12.1 Trials at low risk of bias | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.97] |
| 12.2 Trials at high risk of bias | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 13 Proportion of participants with detectable HBeAg in serum or plasma – formula type | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.02] |
| 13.1 Experimental intervention with traditional XCHT formula | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.97] |
| 13.2 Experimental intervention with modified XCHT formula | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 14 Proportion of participants with detectable HBeAg in serum or plasma – forms | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.02] |
| 14.1 Water extraction of XCHT formula | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.97] |
| 14.2 Granule of XCHT | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 15 Proportion of participants with detectable HBeAg in serum or plasma – duration | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.02] |
| 15.1 Duration < 6 months | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 15.2 Duration > 6 months | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.97] |
| 16 Proportion of participants with detectable HBeAg in serum or plasma – dosage | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.50, 1.02] |
| 16.1 XCHT < 15 g | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.03] |
| 16.2 XCHT > 15 g | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.97] |
| 17 Proportion of participants with nausea | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 18 Proportion of participants with nausea and vomiting | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 19 Proportion of participants with dizziness and sleep disorders | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20 Proportion of participants with dizziness and fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21 Proportion of participants with a dry feeling or bitter taste in the mouth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 22 Proportion of participants with bloating and belching | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23 Proportion of participants with loss of appetite | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2014.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 120 participants diagnosed with chronic hepatitis B and tuberculosis according to the 'The Guideline of Prevention and Treatment for Tuberculosis' (no reference provided), and the 'The Guideline of Diagnosis and Treatment for Chronic Hepatitis B' (no reference provided) Male:female: 73:47 Mean age: 34.32 years Exclusion criteria: obvious liver dysfunction; used antiviral drugs and immunomodulators before participating the trial; pregnant or women preparing for pregnancy; autoimmune hepatitis, fatty liver, or other types of hepatitis; malignant tumour; severe heart, liver, lung, kidney or other organ dysfunction; history of drug allergy |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (Chai Hu 10 g, Huang Qin 6 g, Ren Shen 6 g, Qing Ban Xia 6 g, Zhi Gan Cao 6 g, Sheng Jiang 3 g, Da Zao 10 pieces), 1 dose a day divided into 3 doses, water decoction, 3 months (n = 60) Control intervention: no intervention (n = 60) Co‐intervention: conventional antituberculosis treatment, i.e. isoniazid, rifampicin, beta‐ammonium, streptomycin or ethambutol; lamivudine 100 mg once daily, 3 months |
|
| Outcomes | Chest X‐ray, HBV‐DNA levels in serum, changes in liver function bio‐markers (ALT, AST, and TBIL), and incidence of liver injury | |
| Notes |
Study dates: January 2011 to January 2013 Authors used intention‐to‐treat We contacted the authors' hospital by telephone on 26 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Chen 2017.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 64 participants diagnosed with chronic hepatitis B and liver fibrosis according to the Chronic Hepatitis B Prevention and Treatment Guideline (CMA‐CSH and CMA‐SID 2006), the Diagnosis and Treatment Guidelines for Liver Fibrosis with Integration of Chinese and Western Medicine (CAIMH 2007), and the Guiding Principles of Clinical Research on New Drugs of Chinese Medicines (Zheng 2002), who gave informed consent. Male:female: 39:25 Mean age: experimental group 32.5 years, control group 35.2 years Exclusion criteria: did not meet the inclusion criteria; used antiviral therapy or antifibrotic drugs within 3 months before the trial; hepatitis C virus or hepatitis D virus infection; congenital, alcoholic, immunological, and drug‐induced hepatitis; severe cardiovascular, cerebrovascular, renal, blood, and other system diseases; pregnant or lactating women |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (Chai Hu 15 g, Huang Qin 12 g, Zhi Ban Xia 10 g, Sheng Jiang 6 g, Zhi Gan Cao 6 g, Da Zao 6 g, Dang Shen 15 g, Huang Qi 15 g, Dan Shen 12 g, Nv Zhen Zi 15 g, Mo Han Lian 15 g, Yin Chen Hao 15 g), 1 dose a day divided into 2 doses, water decoction, 12 weeks (n = 32) Control intervention: no intervention (n = 32) Co‐intervention: hepatoprotective enzymes, immunomodulatory and antiviral drugs such as vitamins, reductive glutathione, and inosine (without detailed regimen), 12 weeks |
|
| Outcomes | Liver function biomarkers (ALT, AST, and TBIL) and liver fibrosis biomarker (HA, LN, PCIII, IV‐C). | |
| Notes |
Study dates: January 2015 to November 2016 Authors used intention‐to‐treat We contacted the authors' hospital by telephone on 23 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Kang 2016.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 81 participants diagnosed with chronic hepatitis B (without detailed diagnostic criteria or inclusion criteria). Male:female: 52:29 Mean age: experimental group: 41.89 years, control group: 42.33 years Exclusion criteria: other acute or chronic diseases, or serious organic diseases |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (Chai Hu 25 g, Huang Qin 10 g, Gan Cao 8 g, Ban Xia 10 g, Sheng Jiang 5 g, Ren Shen 8g, Da Zao 20 g), 1 dose (300 mL) a day divided into 3 doses, water decoction, 8 months (n = 40) Control intervention: no intervention (n = 41) Co‐intervention: entecavir tablets 0.5 g once daily, 8 months |
|
| Outcomes | A compound outcome: clinical efficiency, ALT recovery rate, HBeAg negative rate and response rate, HBV‐DNA negative rate, and response rate | |
| Notes |
Study dates: February 2015 to September 2016 Authors used intention‐to‐treat We contacted the authors by telephone on 23 March 2018, but authors refused to provide any further information or their e‐mail addresses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Liu 2017.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 80 participants diagnosed with chronic hepatitis B (without detailed diagnostic criteria or inclusion criteria) Male:female: 47:33 Mean age: experimental group: 63.5 years, control group: 4.7 years Exclusion criteria: did not fulfil the diagnostic criteria; mental diseases; multiple system dysfunction such as heart failure, lung failure, or kidney failure; did not sign the informed consent; organic lesions; uncompleted clinical information |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (Chai Hu 10 g, Huang Qin 6 g, Zhi Gan Cao 6 g, Ban Xia 6 g, Sheng Jiang 3 g, Dang Shen 6 g, Da Zao 10 pieces), 1 dose a day divided into 3 doses, water decoction, 3 months (n = 40) Control intervention: no intervention (n = 40) Co‐intervention: adefovir dipivoxil tablets 10 mg once daily, entecavir tablets 0.5 mg once daily, and conventional interventions (details not reported), 3 months |
|
| Outcomes | Compound outcome: overall efficiency and adverse events considered 'not to be serious' | |
| Notes |
Study dates: February 2015 to September 2016 Authors used intention‐to‐treat We contacted the authors' hospital by telephone on 27 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Mao 2014.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 68 participants diagnosed with chronic hepatitis B according to the 'The Guideline of Prevention and Treatment for Chronic Hepatitis B: A 2010 Update' (CMA‐CSH and CMA‐SID 2011), and the 'Guiding Principles of Clinical Research on New Drugs of Chinese Medicines (Zheng 2002) Male:female: 47:21 Mean age: experimental group: 49.89 years, control group: 50.10 years Exclusion criteria: severe complications such as cirrhosis, ascites; pregnant, preparing for pregnancy, or breastfeeding women; concomitant diseases that significantly affect the identification of the Chinese medicine syndrome; dropout or do not co‐operate with the interventions; taking part in other clinical research, receiving interventions for other diseases |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula, 1 dose a day divided into 2 doses, water decoction, 3 months (n = 47) Control intervention: no intervention (n = 21) Co‐intervention: lamivudine 0.1 g once daily, capsule, 3 months |
|
| Outcomes | Traditional Chinese Medicine Syndrome Scale | |
| Notes |
Study dates: January 2013 to December 2013 Authors used intention‐to‐treat We contacted the authors' hospital by telephone on 27 and 30 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Sun 2004.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 100 participants diagnosed with chronic hepatitis B according to the 'Diagnostic criteria of viral hepatitis' (IPBCMA 1995) Male:female: 66:34 Mean age: experimental group: 43.2 years, control group: 42.1 years Exclusion criteria: not reported |
|
| Interventions |
Experimental intervention: Xiao Chai‐Hu Tang formula (Chai Hu 20 g, Huang Qin 12 g, Ban Xia 12 g, Ren Shen 9 g, Gan Jiang 9 g, Da Zao 4 pieces, Zhi Gan Cao 5 g), 1 dose a day divided into 2 doses, water decoction, 3 months (n = 60) Control intervention: no intervention (n = 40) Co‐intervention: diammonium glycyrrhizinate for injection 30 mL once daily, 3 months |
|
| Outcomes | Improvement of liver function biomarkers (ALT, AST, ALB, TBIL level in serum) and improvement of liver pathological lesions biomarkers (HA, PCIII, LN, IV‐C level in serum) | |
| Notes |
Study dates: January 2001 to July 2002 Authors used intention‐to‐treat. We contacted the authors' hospital by telephone on 27 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | According to the author, the study did not blind the outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Wang 2013.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 160 participants diagnosed with chronic hepatitis B with liver cirrhosis (without detailed diagnostic criteria or inclusion criteria). Male:female: 78:82 Mean age: experimental group: 40.2 years, control group: 42.1 years Exclusion criteria: not reported. |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (Chai Hu 15 g, Huang Qin 9 g, Zhi Gan Cao 9 g, Ban Xia 9 g, Sheng Jiang 9 g, Ren Shen 9 g, Da Zao 9 g), 1 dose a day divided into 2 doses, water decoction, 120 days (n = 80). Control intervention: no intervention (n = 80) Co‐intervention: conventional liver protect interventions (details not reported), 120 days |
|
| Outcomes | Adverse events considered 'not to be serious', biomarkers: ALT, AST, ALB, and GLB level in serum, liver fibrosis improvement (without report of detailed measurement or data), HBV‐DNA negative conversion rate (without report of detailed data) | |
| Notes |
Study dates: September 2010 to September 2012 Authors used intention‐to‐treat We contacted the authors' hospital by telephone on 26 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Wang 2016.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 82 participants diagnosed with chronic hepatitis B according to the 'Chronic Hepatitis B Prevention and Treatment Guideline' (CMA‐CSH and CMA‐SID 2011), and the 'Guidelines for the Diagnosis and Treatment of Liver Fibrosis in Integrative Medicine Practice' (CAIMH 2010), with HBV‐DNA copy number ≥ 105 copies/mL in serum biomarkers such as HBsAg, HBeAg, HBeAb, and HBcAb positive, with no history of antiviral therapy, and who signed the informed consent and were approved by hospital medical ethics committee. Male:female: 55:27 Mean age: experimental group: 35.1 years, control group: 35.8 years Exclusion criteria: other types of hepatitis, liver cirrhosis, abnormal liver function metabolism, etc.; pregnant or preparing for pregnancy women. |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (Dang Shen 30 g, Fu Ling 30 g, Chi Shao 15 g, Dan Shen 15 g, Chai Hu 15 g, Bai Zhu 15 g, Dang Gui 12 g, Huang Qin 10 g, Fa Ban Xia 10 g, Yu Jin 10 g, Yin Chen 10 g, Zhi Gan Cao 6 g, Zhi Qiao 10 g, with Chuan Qiu 10 g added for participants with flank pain, Xi Huang Cao 15 g added for participants with jaundice, Tao Ren 15 g added for participants with blood stasis syndrome), 1 dose a day divided into 3 doses, water decoction, 12 weeks, and then reduced to ≥ 15 doses a month (n = 41). Control intervention: no intervention (n = 41) Co‐intervention: entecavir tablets 0.5 mg once daily and diammonium glycyrrhizinate for injection 30 mL once daily, 6 months |
|
| Outcomes | Improvement of liver function (ALT, AST, TBIL level in serum), changes in HBV‐DNA copy number, and response rate of the intervention: complete response: clinical symptoms completely relieved, biochemical markers recovered in the blood, HBV antigen turned negative, HBV‐DNA copy number < 5 × 102 copies/mL, and improvement of liver pathological lesions; partial response: biochemical markers recovered in the blood, HBeAg turned negative or began serological conversion but not negative, HBV‐DNA turned negative, and improvement of liver pathological lesions; no response: could not meet the above criteria (the overall response rate was calculated as complete response and partial response). | |
| Notes |
Study dates: June 2014 to June 2015 Authors used intention‐to‐treat. We contacted the authors' hospital by telephone on 23 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Random number table |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Wu 2015.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 92 participants diagnosed with chronic hepatitis B (not reported any diagnosis criteria or inclusion criteria). Male:female: 55:37 Mean age: experimental group: 44.1 years, control group: 44.5 years. Exclusion criteria: not reported |
|
| Interventions |
Experimental intervention: Xiao Chai Hu Tang formula (detailed constitution of formula not reported), 100 mL twice a day, water decoction, 24 weeks (n = 46) Control intervention: no intervention (n = 46) Co‐intervention: lamivudine 100 mg once daily, 24 weeks |
|
| Outcomes | Improvement of liver function biomarkers (ALT, AST, ALB, TBIL level in serum) and portal vein dynamics (comparison of portal vein width and portal vein velocity) | |
| Notes |
Study dates: November 2013 to December 2014
Authors used intention‐to‐treat We contacted the authors by telephone on 23 March 2018, but authors did not want to provide any information. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received no nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of participants included in the analysis was equal to the number of participants randomised. |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
Zhao 2014.
| Methods | Randomised clinical trial, China Parallel group design |
|
| Participants | 87 hospitalised participants diagnosed with chronic hepatitis B according to the 'Chronic Hepatitis B Prevention and Treatment Guideline' (CMA‐CSH and CMA‐SID 2006), with HBV DNA ≥ 105 copies/mL and ALT ≥ 2 × ULN in serum, had not had antiviral drugs and immunomodulators for 4 months before the trial were included. 8 participants were excluded from analysis because they had changed their job and residence place, and therefore, could not continue participating in the trial. As the trial authors did not report the number of withdrawals per group, we did not use these 8 participants in our analysis. Male:female: not reported Mean age: experimental group: 38.1 years, control group: 40.0 years Exclusion criteria: other viral hepatitis; HBeAg‐negative hepatitis B; pneumonia caused by drug poisoning, ethanol poisoning and other factors; autoimmune hepatitis; liver cancer; hepatic encephalopathy; history of severe hepatitis; decompensated liver cirrhosis; severe cardiovascular, lung, kidney, endocrine, and hematopoietic system diseases; serious primary mental illnesses; pregnant or preparing for pregnancy women, lactating women; with allergies or allergic to multiple drugs |
|
| Interventions |
Experimental intervention: modified Xiao Chai Hu Tang formula (Chai Hu 6 g, Huang Qin 10 g, Dang Shen 10 g, Huang Qi 24 g, Zhu Ling 15 g, Fu Ling 15 g, Yin Chen 15 g, Hu Zhang 15 g, She She Cao 20 g, Pu Gong Ying 15 g, Xia Ku Cao 10 g, Yu Jin 10 g, Zhi Gan Cao 6 g), 1 dose a day divided by into 2 doses, water decoction, 48 weeks (40 participants who were included in analysis) Control intervention: no intervention (39 participants who were included in analysis) Co‐intervention: adefovir 10 mg once daily, capsule, 48 weeks |
|
| Outcomes | Liver function biomarkers (ALT, AST, TBIL); hepatitis B virus biomarkers: HBsAg negative conversion rate and HBV‐DNA negative conversion rate; improvement rate of signs and symptoms | |
| Notes |
Study dates: October 2014 Per‐protocol analysis We contacted the authors' hospital by telephone on 26 March 2018, but authors were not available. No further contact details. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Except the equally implemented co‐interventions, the experimental group received Xiao Chai Hu Tang formula and the control group received nothing. This could have unblinded the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants who were not included in the analysis was > 5% of the total participants (8 participants were lost after randomisation, but the time when this happened was not specified). |
| Selective reporting (reporting bias) | High risk | This study did not report any of our predefined primary outcomes, and we could not find the study protocol. |
| Other bias | Low risk | The study appeared free of other factors that could have put it at risk of bias. |
ALB: albumin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GLB; globulin; HA: hyaluronic acid; HBcAb: hepatitis B core antibody; HBeAg: hepatitis B virus e‐antigen; HBsAg: hepatitis B virus S‐antigen; HBV‐DNA: hepatitis B virus DNA; IV‐C: type Ⅳ collagen; LN: laminin; n: number of participants; PCIII: type III procollagen; TBIL: total bilirubin; ULN: upper limit of normal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbar 1999 | Animal study |
| Chen 1990 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Chen 1996 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Chen 2005 | XCHT formula not used. |
| Chen 2012 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Chen 2016 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Cheng 1997 | XCHT formula not used. |
| Deng 1996 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Dong 2001 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Dong 2002 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Dong 2015 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Forns 2002 | Review article |
| Gao 1999 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Guo 1994 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Guo 2016a | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Guo 2016b | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Hong 2015 | Review article |
| Hsu 2006 | Case report |
| Hu 1995 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Hu 2003a | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Hu 2003b | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Hu 2003c | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Hu 2011 | Review article |
| Huang 2005 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Jiang 2007 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Jin 2002 | Not XCHT formula |
| Kang 2015 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Lee 2011a | Review article |
| Lee 2011b | Review article |
| Li 1997 | Not XCHT formula |
| Li 1999 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Li 2001b | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Li 2012 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Li 2016 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Liu 1999 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Liu 2000 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Liu 2000a | Animal study |
| Liu 2002b | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Liu 2005 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Liu 2008 | Review article |
| Liu 2013 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Luk 2007 | Review article |
| Ma 1997b | Not XCHT formula |
| Miu 2001 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Modi 2007 | Review article |
| Ohtake 2004 | Review article |
| Qi 2013 | Review article |
| Qin 2010 | Review article |
| Ren 2001 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Seeff 2007 | Review article |
| Shen 2005 | Not XCHT formula |
| Shi 2012 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Stickel 2007 | Review article |
| Stickel 2015 | Review article |
| Sun 1997 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Sun 2003 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Tacke 2010 | Review article |
| Tang 2009 | Not XCHT formula |
| Tian 2012 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Tsai 2016 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Tu 1996 | Did not fulfil the intervention inclusion criteria. |
| Verma 2007 | Review article |
| Wang 2002 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Wang 2003 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Wang 2006 | Not XCHT formula |
| Wang 2014b | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Wang 2015 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Wong 2005 | Survey research |
| Wu 1995 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Wu 2009a | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Xie 1995 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Yang 2005 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Yang 2008 | Review article |
| Yang 2015 | Review article |
| Ye 2002 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Yuan 2002 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Zhang 1998 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Zhang 2002 | Not XCHT formula |
| Zhang 2004 | Not XCHT formula |
| Zhang 2006b | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Zhang 2008 | Untrusted data |
| Zhang 2011 | People with liver cancer |
| Zhao 2015 | Did not compare XCHT with co‐intervention to equally implemented co‐intervention. |
| Zheng 2013 | Review article |
| Zheng 2015 | Review article |
XCHT: Xiao Chai Hu Tang.
Differences between protocol and review
We changed the title of our protocol "Xiao Chai Hu Tang, a Chinese herbal medicine formula, for chronic hepatitis B" into "Xiao Chai Hu Tang, a herbal medicine, for chronic hepatitis B" as the latter reflects better the content of the review.
From the planned subgroup analyses in the protocol on risk of bias factors, we left only the subgroup analysis on trials at overall low risk of bias compared to trials at overall high risk of bias. Following Cochrane guidance, large numbers of undirected subgroup analyses can lead to spurious explanations of heterogeneity.
We performed subgroup analysis comparing trials with treatment duration more than six months to less than six months instead of the observed median treatment duration of 3.5 months, because all the included trials which reported the two outcomes (i.e. proportion of participants with detectable HBV‐DNA and proportion of participants with detectable HBeAg), had treatment durations more than the observed median treatment duration of 3.5 months.
In case of one trial providing outcome data, we posthoc compared the results with Fisher's exact test and Review Manager 5 (there was no difference). Therefore, in view of future updates of the review, we will keep and present only the results obtained with the Review Manager 5 analysis.
The belief, that Xiao Chai Hu Tang formula helps in decreasing discomfort and prevents the replication of the virus in people with chronic hepatitis B, is widespread in China. As data for our primary and secondary outcomes were lacking (except for one secondary outcome to which two studies provided data) and we had few data for our exploratory outcomes, posthoc, we decided to present the results of the surrogate 'proportion of participants with detectable HBV‐DNA' and 'the proportion of participants with detectable HBeAg' outcomes in the Abstract, Plain Language Summary, and in the 'Summary of findings' table. Following the GRADE Handbook, "Guideline developers should consider surrogate outcomes only when evidence about population‐important outcomes is lacking." As this is also the case in our review, we found it helpful to report the results of the two surrogate outcomes.
We did not assess the data on harmful effects of Xiao Chai Hu Tang reported in studies, listed in the Characteristics of excluded studies table and Appendix 2. This is because we intend to conduct a separate systematic review on harms of Xiao Chai Hu Tang reported in observational studies.
In keeping with Cochrane CENTRAL requirements, we have now excluded the risk of bias domain on 'for‐profit funding' from the risk of bias domains listed in the protocol part of the review, and instead, we reported in a narrative way the information provided in the trial publications.
In addition, consistent with Cochrane CENTRAL requirements, we assessed imprecision via Trial Sequential Analysis in a separate assessment of imprecision with GRADE in a sensitivity analysis.
Contributions of authors
DZK: developed and drafted the protocol; selected trials for inclusion; extracted data from trials; contacted the authors for missing data; assessed the risk of bias assessment; interpretation of data; drafted the final review.
NL: developed and co‐ordinated the protocol; interpreted data; commented on the final review.
GLY: selected trials for inclusion; commented on the final review.
ZZ: selected trials to include; commented on the final review.
YL: selected trials to include; commented on the final review.
JL: selected trials to include; extracted data from trials; contacted study authors for missing data; performed the risk of bias assessment; commented on the final review.
XHL: selected trials to include; extracted data from trials; contacted study authors for missing data; performed the risk of bias assessment; commented on the final review.
SBL: selected trials to include; extracted data from trials; contacted study authors for missing data; performed the risk of bias assessment; commented on the final review.
DN: developed, co‐ordinated, and advised on the protocol; advised on the interpretation of data; commented on the final review.
JCJ: developed, co‐ordinated, and advised on the protocol; advised on the interpretation of data; commented on the final review.
CG: developed, co‐ordinated, and advised on the protocol; advised on the interpretation of data; commented on the final review.
JPL: initiated the review; advised and commented on the protocol and the final review.
All authors approved the final review for publication.
Sources of support
Internal sources
No sources of support supplied
External sources
2017 Open‐end Fund of Education Ministry Key Laboratory for Research and Application of 'Zang Xiang' Theory in Liaoning University of Traditional Chinese Medicine (zyzx1702), China.
Science and Technology Research Project of Department of Education of Liaoning Province (L201713), China.
-
National Center for Complementary and Integrative Health (NCCIH) (Grant Number R24 AT001293), USA.
- This work was partially funded by Grant Number R24 AT001293 from the National Center for Complementary and Integrative Health (NCCIH). The contents of this systematic review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCIH or the National Institutes of Health.
Declarations of interest
DZK: none.
NL: none.
GLY: none.
ZZ: none.
YL: none.
JL: none.
XHL: none.
SBL: none.
DN: none.
JCJ: none.
CG: none.
JPL: none.
New
References
References to studies included in this review
Chen 2014 {published data only}
- Chen Y, Zhou XD. Treatment of 60 cases of chronic hepatitis B complicated with pulmonary tuberculosis with Xiao Chaihu decoction combined with lamivudine [小柴胡汤联合拉米夫定治疗慢性乙型肝炎合并肺结核60例]. Henan Traditional Chinese Medicine 2014;34(7):1291‐2. [Google Scholar]
Chen 2017 {published data only}
- Chen XZ. Xiaochaihu decoction for 32 cases of chronic hepatitis B liver fibrosis [小柴胡汤加味治疗慢性乙型肝炎肝纤维化32例]. Hunan Journal of Traditional Chinese Medicine 2017;33(5):57‐8. [Google Scholar]
Kang 2016 {published data only}
- Kang ZH. A randomized controlled study of xiaochaihu decoction combined with entecavir for chronic hepatitis B [小柴胡汤联合恩替卡韦治疗慢性乙型肝炎的随机对照研究]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine 2016;16(2):71‐2. [Google Scholar]
Liu 2017 {published data only}
- Liu L. Clinical effect of xiaochaihu decoction on chronic hepatitis B [小柴胡汤治疗慢性乙肝临床治疗的效果探讨]. Medical Frontier 2017;7(23):351‐2. [Google Scholar]
Mao 2014 {published data only}
- Mao G, Cai GG, Chen B. Study on the best prescription for chronic hepatitis B with liver depression and spleen deficiency based on TCM syndrome efficacy evaluation [基于中医证候疗效评价的慢性乙型肝炎肝郁脾虚证最佳方药的探究]. Hunan Journal of Traditional Chinese Medicine 2014;30(8):12‐4. [Google Scholar]
Sun 2004 {published data only}
- Sun XN, Sun JB. Treatment of 60 cases of hepatitis liver fibrosis with Xiao Chai Hu decoction combined with diammonium glycyrrhizinate injection [小柴胡汤联合甘利欣注射液治疗肝炎肝纤维化60例]. Chinese Journal of Chinese Medicine Information 2004;11(6):534‐5. [Google Scholar]
Wang 2013 {published data only}
- Wang MH. Clinical observation of Xiao Chaihu decoction in treating chronic hepatitis B with liver fibrosis [小柴胡汤治疗慢性乙型肝炎肝纤维化的临床观察]. Medical Frontier 2013;33:357. [Google Scholar]
Wang 2016 {published data only}
- Wang Y. Effects of xiaochaihu decoction combined with western medicine on liver function and HBV‐DNA copy number of chronic hepatitis B patients [小柴胡汤联合西药对慢性乙型肝炎患者肝功能及HBV‐DNA拷贝数的影响]. Journal of New Chinese Medicine 2016;48(7):74‐6. [Google Scholar]
Wu 2015 {published data only}
- Wu HX. Clinical observation of Xiao Chaihu decoction combined with lamivudine in the treatment of hepatitis B cirrhosis [小柴胡汤联合拉米夫定治疗乙型肝炎肝硬化疗效观察]. World Clinical Medicine 2015;9(6):114. [Google Scholar]
Zhao 2014 {published data only}
- Zhao CH. Treatment of 40 cases of HBeAg positive chronic hepatitis B with modified Xiao Chaihu decoction combined with adefovir dipivoxil [加味小柴胡汤联合阿德福韦酯治疗HBeAg阳性慢性乙型肝炎40例]. Henan Traditional Chinese Medicine 2014;34(10):1924‐5. [Google Scholar]
References to studies excluded from this review
Akbar 1999 {published data only}
- Akbar S‐M, Yamamoto K, Abe M, Ninomiya T, Tanimoto K, Masumoto T, et al. Potent synergistic effect of sho‐saiko‐to, a herbal medicine, during vaccine therapy in a murine model of hepatitis B virus carrier. European Journal of Clinical Investigation 1999;29(9):786‐92. [DOI] [PubMed] [Google Scholar]
Chen 1990 {published data only}
- Chen NL, Gu F, Jia KM, Peng XJ, Deng YL, Wang GL. Discussion on Xiaochaihu decoction in the treatment of 26 cases of hepatitis B with hepatitis D [小柴胡汤对26例乙型、丁型肝炎重叠感染治疗的探讨]. Chinese Journal of Internal Medicine 1990;29(3):144‐6. [Google Scholar]
Chen 1996 {published data only}
- Chen ZX, Luo ZQ, Ma CY, Zhang SJ, Ma ZK, Yang HL, et al. Observation on Xiaochaihu decoction for anti‐chronic hepatitis B immunity and liver damage [小柴胡汤加减抗慢性活动性乙型肝炎免疫异常及肝损害的观察]. China Journal of Traditional Chinese Medicine and Pharmacy 1996;11(4):25‐6. [Google Scholar]
Chen 2005 {published data only}
- Chen ZX, Zhang SJ, Lao SX, Hu HT, Zhang CY, Guan SH, et al. He Jie Tang in the treatment of chronic hepatitis B patients. World Journal of Gastroenterology 2005;11(42):6638‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen GW. Clinical observation of Xiaochaihu decoction combined with Baisinuo in treating people with chronic hepatitis B with pulmonary tuberculosis [小柴胡汤联合百赛诺治疗慢乙肝合并肺结核的临床观察]. Journal of Liaoning Medical University 2012;33(3):248‐50. [Google Scholar]
Chen 2016 {published data only}
- Chen XT. Clinical observation on combined treatment of traditional Chinese medicine and western medicine for liver fibrosis of chronic hepatitis B [中西医联合治疗慢性乙型肝炎肝纤维化的临床观察]. China Medicine and Pharmacy 2016;6(6):25‐7. [Google Scholar]
Cheng 1997 {published data only}
- Cheng ML, Zhou XQ, Mi ZB, Luo YF, Fang YS, Ye XM, et al. Clinical and experimental study of Yigan Powder in treating chronic hepatitis B [乙肝散治疗慢性乙肝的临床和实验研究]. China Journal of Traditional Chinese Medicine and Pharmacy 1997;12(3):23‐5. [Google Scholar]
Deng 1996 {published data only}
- Deng ZD, Xiao JS, Yu HL. Effect of modified Xiaochaihu decoction on hepatic fibrosis markers in patients with chronic liver disease [改良小柴胡汤对慢性肝病患者肝纤维化标志物的作用]. Chinese Journal of Integrated Traditional and Western Medicine on Digestion 1996;4:205‐7. [Google Scholar]
Dong 2001 {published data only}
- Dong GF. Treatment of 65 cases of chronic hepatitis B with Xiaochaihu granule [小柴胡冲剂治疗慢性乙型肝炎65例]. Shaanxi Journal of Traditional Chinese Medicine 2001;22:413. [Google Scholar]
Dong 2002 {published data only}
- Dong L, Zhao JX. Clinical observation on treatment of people with chronic hepatitis B and alpha fetoprotein positive by Xiaochaihu decoction and bifen diester [小柴胡汤、联苯双酯联合治疗慢性乙型肝炎及甲胎蛋白阳性者的临床观察]. Ningxia Medicine Journal 2002;24(8):484‐5. [Google Scholar]
Dong 2015 {published data only}
- Dong SX. Clinical observation of entecavir combined with Xiaochaihu decoction in the treatment of hepatitis B cirrhosis [恩替卡韦联合小柴胡汤治疗乙型肝炎肝硬化临床疗效观察]. Medical Information 2015;28:226. [Google Scholar]
Forns 2002 {published data only}
- Forns X, Sanchez Tapias JM, Pares A, Llovet JM, Bruix J, Rodes J. Expected developments in hepatology. Bailliere's Best Practice and Research in Clinical Gastroenterology 2002;16(6):957‐70. [DOI] [PubMed] [Google Scholar]
Gao 1999 {published data only}
- Gao ZG, Lin J. Treatment of 50 cases of chronic hepatitis B with Xiaochaihu decoction [小柴胡汤加减治疗慢性乙型肝炎50例]. Journal of Zhejiang College of Traditional Chinese Medicine 1996;23:36. [Google Scholar]
Guo 1994 {published data only}
- Guo HY, Zhang KL, Qi ZY, Wu JZ, Shi YB. Treatment of 30 cases of viral hepatitis with Xiaochaihu decoction [小柴胡汤治疗病毒性肝炎30例探讨]. Chinese Journal of Integrated Traditional and Western Medicine 1994;11:960‐1. [Google Scholar]
Guo 2016a {published data only}
- Guo TS, Wang H. Clinical pharmacological study of Kushensu in treating hepatic fibrosis caused by hepatitis B [苦参素治疗乙肝所致肝纤维化的临床药理研究]. Chinese Journal of Ethnomedicine and Ethnopharmacy 2016;25(1):114‐5. [Google Scholar]
Guo 2016b {published data only}
- Guo BJ. A randomised parallel controlled trial of Xiaochaihu decoction combined with Zhuyu Lidan recipe in the treatment of chronic hepatitis B with cholestasis [小柴胡汤联合逐瘀利胆方治疗胆汁淤积型慢性乙型病毒性肝炎的随机平行对照研究]. Journal of Aerospace Medicine 2016;27(5):608‐10. [Google Scholar]
Hong 2015 {published data only}
- Hong M, Li S, Tan HY, Wang N, Tsao S‐W, Feng Y. Current status of herbal medicines in chronic liver disease therapy: the biological effects, molecular targets and future prospects. International Journal of Molecular Sciences 2015;16(12):28705‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2006 {published data only}
- Hsu L‐M, Huang Y‐S, Tsay S‐H, Chang F‐Y, Lee S‐D. Acute hepatitis induced by Chinese hepatoprotective herb, xiao‐chai‐hu‐tang. Journal of the Chinese Medical Association 2006;69(2):86‐8. [DOI] [PubMed] [Google Scholar]
Hu 1995 {published data only}
- Hu JT, Pu YG. Treatment of 56 cases of chronic active hepatitis B with Xiaochaihu decoction [小柴胡汤加减治疗慢性活动性乙型病毒性肝炎56例]. Journal of Sichuan of Traditional Chinese Medicine 1995;06:22‐3. [Google Scholar]
Hu 2003a {published data only}
- Hu JX, Zheng Q, Sun WF. Short‐term efficacy of multiple drugs in the treatment of chronic hepatitis B [多种药物联合治疗慢性乙型肝炎的近期疗效观察]. Practical Clinical Medicine 2003;4(1):83‐4. [Google Scholar]
Hu 2003b {published data only}
- Hu NK. Clinical study on treatment of chronic active hepatitis B with Yi Gan Zhuan Yin tablet [乙肝转阴片治疗慢性乙型活动性肝炎临床研究]. Journal of Practical Traditional Chinese Internal Medicine 2003;17(4):264‐5. [Google Scholar]
Hu 2003c {published data only}
- Hu SP. Treatment of 68 cases of chronic hepatitis B with Chai Huo He Fang [柴藿合方治疗慢性乙型肝炎68例]. Journal of Practical Traditional Chinese Internal Medicine 2003;17(4):283. [Google Scholar]
Hu 2011 {published data only}
- Hu XY, Zhong S, Chen J, Li H. Effectiveness and safety of Xiao Chai Hu Tang plus interferon for chronic hepatitis B: a systematic review. Chinese Journal of Evidence Based Medicine 2011;11(3):308‐13. [Google Scholar]
Huang 2005 {published data only}
- Huang MQ, Xu L. Effect of Xiaochaihu decoction on IL‐10 and TNF‐α in patients with hepatitis [小柴胡汤对肝炎患者IL‐10及TNF‐α的影响]. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine 2005;15(4):240‐1. [Google Scholar]
Jiang 2007 {published data only}
- Jiang S, You SG, Zhan GQ, Zhu L. Treatment of 40 cases of chronic hepatitis B with liver fibrosis by Xiaochaihu decoction [小柴胡汤治疗慢性乙型肝炎肝纤维化40例]. Journal of Practical Medicine 2007;23(19):3105‐6. [Google Scholar]
Jin 2002 {published data only}
- Jin WJ, Ye XG, Tang ZY, Huang ZX, Zhang CY, Li YB, et al. Clinical study of Qiling Chaihu solution for chronic hepatitis B patients. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 2002;12(5):268‐9. [Google Scholar]
Kang 2015 {published data only}
- Kang GJ. Clinical observation of adefovir dipivoxil combined with traditional Chinese medicine in the treatment of hepatitis B cirrhosis [阿德福韦酯联合中药治疗乙型肝炎肝硬化临床观察]. Medical Information 2015;28(10):185. [Google Scholar]
Lee 2011a {published data only}
- Lee J‐K, Kim J‐H, Shin H‐K. Therapeutic effects of the oriental herbal medicine Sho‐saiko‐to on liver cirrhosis and carcinoma. Hepatology Research 2011;41(9):825‐37. [DOI] [PubMed] [Google Scholar]
Lee 2011b {published data only}
- Lee CH, Wang JD, Chen PC. Risk of liver injury associated with Chinese herbal products containing radix bupleuri in 639,779 patients with hepatitis B virus infection. PloS One 2011;6(1):e16064. [DOI: 10.1371/journal.pone.0016064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 1997 {published data only}
- Li KQ, Hu CY, Fu J. Clinical observation on 126 cases of chronic hepatitis B treated by TCM based on the differential diagnosis [中医辨证治疗慢性乙型肝炎126例]. Forum on Traditional Chinese Medicine 1997;12:28‐30. [Google Scholar]
Li 1999 {published data only}
- Li P, Huang QX. Treatment of 44 cases of chronic hepatitis B with Xiaochaihu decoction [小柴胡汤加味治疗慢性乙型肝炎44例]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1999;9(6):39. [Google Scholar]
Li 2001b {published data only}
- Li RF. Therapeutic effect of Xiaochaihu tablet and nifedipine on liver cirrhosis [小柴胡片、硝苯地平联合治疗肝硬化疗效观察]. Hainan Medical Journal 2001;12(8):34. [Google Scholar]
Li 2012 {published data only}
- Li KJ, Liu YQ, Li BJ, Wang QL, Qi YX. Observation on the effect of Xiao Chaihu decoction combined with Shengjiang powder in the treatment of chronic hepatitis B. China Medical Herald 2012;9(3):109‐10. [Google Scholar]
Li 2016 {published data only}
- Li FY. Study on the Application of Xiaochaihu Decoction in Treating Chronic Hepatitis B Based on the Theory of Professor Lan Qingqiang [Masters thesis]. Nanning (China): Guangxi University of Chinese Medicine, 2016. [Google Scholar]
Liu 1999 {published data only}
- Liu ZW, Wang P. Therapeutic effect of Xiaochaihu decoction combined with polymyocytes on 70 patients with hepatitis B virus carriers [小柴胡汤联合聚肌胞治疗乙肝病毒携带者70例疗效分析]. Chinese Journal of School Doctor 1999;13(3):198‐9. [Google Scholar]
Liu 2000 {published data only}
- Liu ZJ. Study on the Treatment and Mechanism of Modified Xiao Chai Hu Decoction on Chronic Hepatitis B [加味小柴胡汤治疗慢性乙型肝炎及其组方机理的研究] [PhD thesis]. Guangzhou (China): Guanzhou University of Chinese Medicine, 2000. [Google Scholar]
Liu 2000a {published data only}
- Liu Z, Xiong M, Zhang H. Experimental study on inhibitory effect of Xiaochaihu decoction on duck hepatitis B virus. Chinese Journal of Integrated Traditional and Western Medicine 2000;20(11):853‐5. [PubMed] [Google Scholar]
Liu 2002b {published data only}
- Liu ZJ, Liu S, Yuan YB. Immunomodulatory effects of modified Xiaochaihu decoction on patients with chronic hepatitis B [加味小柴胡汤对慢性乙型肝炎患者的免疫调节作用]. Chinese Journal of Traditional Medical Science and Technology 2002;9(4):234. [Google Scholar]
Liu 2005 {published data only}
- Liu AL, Wei M, Zeng ZG, Sun JX, Yang M. 50 cases of anti‐liver fibrosis treated by Xiaochaihu decoction and Casey Lai [小柴胡汤与凯西莱联用抗肝纤维化50例]. Shaanxi Journal of Traditional Chinese Medicine 2005;26(9):873‐4. [Google Scholar]
Liu 2008 {published data only}
- Liu JY, Song JS, Sun Y. Quality evaluation of randomized controlled trials on Xiaochaihu decoction for chronic hepatitis B. Journal of Jiangxi University of TCM 2008;20(2):38‐44. [Google Scholar]
Liu 2013 {published data only}
- Liu AJ. Clinical analysis of Xiaochaihu decoction and Shengjiang powder in treating chronic hepatitis B [小柴胡汤合升降散治疗慢性乙型肝炎的临床分析]. World Health Digest Medical Periodieal 2013;10(23):395‐6. [Google Scholar]
Luk 2007 {published data only}
- Luk JM, Wang X, Liu P, Wong K‐F, Chan KL, Tong Y, et al. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver International 2007;27(7):879‐90. [DOI] [PubMed] [Google Scholar]
Ma 1997b {published data only}
- Ma YF, Li GP, Tang BZ, Li M. Clinical study on traditional Chinese medicine 9250 in treating post‐hepatitis cirrhosis [中药'9250'治疗肝炎后肝硬化的临床研究]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 1997;7(1):12‐4. [Google Scholar]
Miu 2001 {published data only}
- Miu WF, Li HY. Observation on 30 cases of chronic hepatitis B treated by modified Xiaochaihu decoction and Danshen injection [加减小柴胡汤合丹参注射液治疗慢性乙型病毒性肝炎30例观察]. Journal of Practical Traditional Chinese Medicine 2001;17(6):3‐4. [Google Scholar]
Modi 2007 {published data only}
- Modi AA, Wright EC, Seeff LB. Complementary and alternative medicine (CAM) for the treatment of chronic hepatitis B and C: a review. Antiviral Therapy 2007;12(3):285‐95. [PubMed] [Google Scholar]
Ohtake 2004 {published data only}
- Ohtake N, Nakai Y, Yamamoto M, Sakakibara I, Takeda S, Amagaya S, et al. Separation and isolation methods for analysis of the active principles of sho‐saiko‐to (SST) oriental medicine. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 2004;812:135‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qi 2013 {published data only}
- Qi FH, Wang ZX, Cai PP, Zhao L, Gao JJ, Kokudo N, et al. Traditional Chinese medicine and related active compounds: a review of their role on hepatitis B virus infection. Drug Discoveries and Therapeutics 2013;7(6):212‐24. [DOI] [PubMed] [Google Scholar]
Qin 2010 {published data only}
- Qin XK, Li P, Han M, Liu JP. Xiaochaihu Tang for treatment of chronic hepatitis B: a systematic review of randomized trials. Journal of Chinese Integrate Medicine 2010;8(4):312‐20. [DOI] [PubMed] [Google Scholar]
Ren 2001 {published data only}
- Ren GR, Zhu M. Clinical study on the therapeutic effect of Rougan mixture on liver fibrosis [柔肝合剂对肝纤维化治疗作用的临床研究任光荣朱牧87]. Suzhou Yi Xue Za Zhi 2001;24(2):87‐8. [Google Scholar]
Seeff 2007 {published data only}
- Seeff LB. Herbal hepatotoxicity. Clinics in Liver Disease 2007;11(3):577‐96. [DOI] [PubMed] [Google Scholar]
Shen 2005 {published data only}
- Shen WS, Yang HZ, Hong Q, Zhang YQ, Xie HP, Bian Z. Two‐year observation of the clinical efficacy in treating chronic hepatitis B Patients with Ganxian recipe and lamivudine. Chinese Journal of Integrative Medicine 2005;11(1):5‐10. [DOI] [PubMed] [Google Scholar]
Shi 2012 {published data only}
- Shi XF. Clinical analysis of Xiaochaihu decoction combined with entecavir in the treatment of chronic hepatitis B [小柴胡汤联合恩替卡韦治疗慢性乙型肝炎的临床疗效分析]. Chinese Community Doctors 2012;14:203‐4. [Google Scholar]
Stickel 2007 {published data only}
- Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Digestive and Liver Disease 2007;39(4):293‐304. [DOI] [PubMed] [Google Scholar]
Stickel 2015 {published data only}
- Stickel F, Hellerbrand C. Herbs to treat liver diseases: more than placebo?. Clinical Liver Disease 2015;6(6):136‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 1997 {published data only}
- Sun QC, Zhao XD. Observation on 6 cases of chronic hepatitis treated by Ganyanling and Xiaochaihu decoction [肝炎灵合小柴胡汤治疗慢性肝炎6例观察]. Journal of Practical Traditional Chinese Medicine 1997;06:4‐5. [Google Scholar]
Sun 2003 {published data only}
- Sun WH, Song MQ, Liu ZJ. Treatment of 64 cases of hepatitis liver fibrosis with Xiaochaihu decoction combined with oxymatrine injection [小柴胡汤联合苦参素注射液治疗肝炎肝纤维化64例]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 2003;13:41‐2. [Google Scholar]
Tacke 2010 {published data only}
- Tacke F, Weiskirchen R. Liver fibrosis – pathogenesis and novel therapeutic approaches. Internist 2010;51(1):21‐9. [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang LL, Sheng JF, Xu CH, Liu KZ. Clinical and experimental effectiveness of Astragali compound in the treatment of chronic viral hepatitis B. Journal of International Medical Research 2009;37:662‐7. [DOI] [PubMed] [Google Scholar]
Tian 2012 {published data only}
- Tain YH. Clinical observation on 98 cases of chronic hepatitis B treated by Xiaochaihu decoction and Shengjiang powder [小柴胡汤合升降散治疗慢性乙型肝炎98例疗效观察]. Journal of Bethune Military Medical College 2012;10(5):438‐9. [Google Scholar]
Tsai 2016 {published data only}
- Tsai TY, Livneh H, Hung TH, Lin IH, Lu MC, Yeh CC. Associations between prescribed Chinese herbal medicine and risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide population‐based cohort study. BMJ Open 2017;7:e014571. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tu 1996 {published data only}
- Tu YS. Treating 66 cases of chronic active hepatitis B with modified Xiaochaihu decoction [加味小柴胡汤治疗慢性活动性乙肝66例]. Hunan Guiding Journal of Traditional Chinese Medicine and Pharmacology 1996;2:11‐2. [Google Scholar]
Verma 2007 {published data only}
- Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clinical Gastroenterology and Hepatology 2007;5(4):408‐16. [DOI] [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang TX, Li LQ, Bin JP. Treating 80 cases of chronic hepatitis B with Kang Ao Zhuan Yin decoction combined with interferon [抗澳转阴汤干扰素并用治疗慢性乙型肝炎80例]. Study Journal of Traditional Chinese Medicine 2002;20(4):533,543. [Google Scholar]
Wang 2003 {published data only}
- Wang YF. Effect of Ganlixin combined with Xiaochaihu decoction on liver fibrosis of chronic hepatitis (with 93 clinical reports) [甘利欣联合小柴胡汤对慢性肝炎肝纤维化的影响(附93例临床报告)]. Journal of Chinese Physician 2003;5(5):709‐10. [Google Scholar]
Wang 2006 {published data only}
- Wang J, Pei L, Zhou Y, Wang EJ, Lang XY, Li D. Effect of compound huoxiang and baishao on the reversion of hepatic fibrosis in patients with chronic hepatitis B based on the turbid‐poison theory. Journal of Clinical Rehabilitative Tissue Engineering Research 2006;10:108‐11. [Google Scholar]
Wang 2014b {published data only}
- Wang XH. Clinical Observation of Modified XiaoChaihu Tang Chronic Hepatitis (Syndrome of Stagnation of Liver Qi and Spleen Deficiency) [Masters thesis]. Changchun (China): Changchun University of Chinese Medicine, 2014. [Google Scholar]
Wang 2015 {published data only}
- Wang ZB. A randomized controlled trial of Xiaochaihu decoction and Zhuyu Lidan recipe in the treatment of chronic hepatitis B with cholestatic [小柴胡汤加逐瘀利胆方治疗胆汁淤积型慢性乙型病毒性肝炎随机平行对照研究]. Journal of Practical Traditional Chinese Internal Medicine 2015;29(3):27‐9. [Google Scholar]
Wong 2005 {published data only}
- Wong VW, Law MY, Hui AY, Lo AO, Li CY, Soo MT, et al. A hospital clinic‐based survey on traditional Chinese medicine usage among chronic hepatitis B patients. Complementary Therapies in Medicine 2005;13(3):175‐82. [DOI] [PubMed] [Google Scholar]
Wu 1995 {published data only}
- Wu SM, Jiang J. Comparison of Xiaochaihu granule with sedum granule in treating chronic hepatitis B [小柴胡冲剂治疗慢性乙型肝炎与垂盆草冲剂疗效对比观察]. Shanghai Journal of Preventive Medicine 1995;7:124‐6. [Google Scholar]
Wu 2009a {published data only}
- Wu QQ. Revised small Bupleurum decoction treat hepatocirrhosis of hepatitis B 69 cases Wu Qiaoqing TCM hospital of Wuyi county, Zhejiang province. Journal of Zhejiang Chinese Medical University 2009;33(4):517‐8. [Google Scholar]
Xie 1995 {published data only}
- Xie SD. Treatment of 71 cases of chronic hepatitis B with Xiaochaihu decoction or hepatitis B vaccine [小柴胡汤或合用乙肝疫苗治疗慢性乙型肝炎71例]. Jiangsu Journal of Traditional Chinese Medicine 1996;16:7. [Google Scholar]
Yang 2005 {published data only}
- Yang M. Research report on Xiaochaihu decoction for eliminating HBV‐DNA in human serum [小柴胡汤清除人体血清HBV‐DNA的研究报告]. Occupation and Health 2005;21(6):896‐7. [Google Scholar]
Yang 2008 {published data only}
- Yang H. Evidence‐Based Medicine Evaluation of 112 Cases of Modern Clinical Research in 'Treatise on Cold Pathogenic Diseases' [伤寒论112方现代临床研究的循证医学评价]. Beijing (China): Beijing University of Chinese Medicine, 2008. [Google Scholar]
Yang 2015 {published data only}
- Yang YW, Liu HT, Li FF, Deng X. Meta‐analysis of randomized controlled trial of Xiaochaihu decoction combined with lamivudine in the treatment of chronic hepatitis B [小柴胡汤联合拉米夫定治疗慢性乙型肝炎随机对照试验的Meta分析]. Journal of Guangxi University of Traditional Chinese Medicine 2015;18(4):140‐2. [Google Scholar]
Ye 2002 {published data only}
- Ye HQ, Liang RQ, Jiang YS. Treatment of 37 cases of chronic hepatitis B with interferon combined with Chinese patent medicine [干扰素联合中成药治疗慢性乙型肝炎37例]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 2002;12(2):108‐9. [Google Scholar]
Yuan 2002 {published data only}
- Yuan YB, Liu S, Liu ZJ. Treatment of 35 cases of chronic hepatitis B with Xiaochaihu decoction combined with lamivudine [小柴胡汤联合拉米夫定治疗慢性乙型肝炎35例]. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 2002;12(4):239‐40. [Google Scholar]
Zhang 1998 {published data only}
- Zhang XH, Guo XZ. Effect of Xiaochaihu decoction on HBV and ALT in people with chronic hepatitis B [小柴胡汤对慢乙肝HBVM及ALT的影响]. Chinese Journal of Information on Traditional Chinese Medicine 1998;5(8):36. [Google Scholar]
Zhang 2002 {published data only}
- Zhang SJ, Chen ZX, Li JB, Huang BJ. The effect of Hejie decoction on the TCRV beta 7 of chronic hepatitis B patients [和解汤对慢性乙型肝炎TCRVβ7基因表达的影响]. Journal of Chinese Medicinal Materials 2002;25(6):451‐3. [PubMed] [Google Scholar]
Zhang 2004 {published data only}
- Zhang SJ, Chen ZX, Lao SX, Huang BJ. Effect of Hejie decoction on T cell immune state of chronic hepatitis B patients. World Journal of Gastroenterology 2004;10(10):1436‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2006b {published data only}
- Zhang B. Observation on the treatment of chronic hepatitis B by modified Xiaochaihu decoction and Matrine acupoint injection [加味小柴胡汤合苦参碱穴位注射治疗慢性乙型肝炎观察]. Journal of Practical Traditional Chinese Medicine 2006;22(4):205. [Google Scholar]
Zhang 2008 {published data only}
- Zhang YC, Liu ZJ. Preliminary clinical observation on the efficacy of modified Xiaochaihu decoction combined with interferon in the treatment of HBeAg‐positive chronic hepatitis B [加味小柴胡汤联合干扰素治疗HbeAg阳性慢性乙型肝炎疗效初步临床观察]. China Practical Medicine 2008;3(33):50‐1. [Google Scholar]
Zhang 2011 {published data only}
- Zhang TS, Shan GZ. Clinical observation on 46 cases of primary liver cancer treated by integrative Chinese and Western medicine [中西医结合治疗原发性肝癌46 例临床观察]. Jiangsu Journal of Traditional Chinese Medicine 2011;43(6):39‐40. [Google Scholar]
Zhao 2015 {published data only}
- Zhao X. Clinical observation of lamivudine combined with traditional Chinese medicine in the treatment of hepatitis B cirrhosis [拉米夫定联合中药治疗乙型肝炎肝硬化的临床观察]. Journal of Clinical Medicine 2015;2(10):1814‐5. [Google Scholar]
Zheng 2013 {published data only}
- Zheng N, Dai J, Cao H, Sun S, Fang J, Li Q, et al. Current understanding on anti hepatocarcinoma effects of Xiao Chai Hu Tang and its constituents. Evidence‐based Complementary and Alternative Medicine 2013;2013:529458. [DOI: 10.1155/2013/529458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2015 {published data only}
- Zheng X, Wang J, Yang D. Antiviral therapy for chronic hepatitis B in China. Medical Microbiology and Immunology 2015;204(1):115‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AASLD 2016
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology (Baltimore, Md.) 2016;63(1):261‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alizadeh 2008
- Alizadeh AH, Ranjbar M, Yadollahzadeh M. Patient concerns regarding chronic hepatitis B and C infection. La Revue de Santé de la Méditerranée Orientale 2008;14(5):1142‐7. [PubMed] [Google Scholar]
Bachem 1993
- Bachem MG, Meyer D, Schäfer W, Riess U, Melchior R, Sell KM, et al. The response of rat liver perisinusoidal lipocytes to polypeptide growth regulator changes with their transdifferentiation into myofibroblast‐like cells in culture. Journal of Hepatology 1993;18:40‐52. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ (Clinical Research Ed.) 2006;333:804‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
CAIMH 2007
- Chinese Association of Integrated Medicine on Hepatology. Diagnosis and treatment guidelines for liver fibrosis with the integration of Chinese and Western medicine. Drug Evaluation 2007;4:260‐5. [Google Scholar]
CAIMH 2010
- Chinese Association of Integrative Medicine on Hepatology. Guidelines for the diagnosis and treatment of liver fibrosis in integrative medicine practice. Chinese Journal of Liver Diseases (Electronic Version) 2010;2(4):54‐9. [Google Scholar]
Cao 2003
- Cao HY. The new mechanism of Xiaochaihu decoction in inhibiting cancer. Foreign Medical Sciences 2003;24:232‐3. [Google Scholar]
Casetllini 2017
- Castellini G, Nielsen EE, Gluud C. Comment on: 'Cell therapy for heart disease: trial sequential analyses of two Cochrane reviews'. Clinical Pharmacology and Therapeutics 2017;102:21‐4. [DOI] [PubMed] [Google Scholar]
Castellini 2018
- Castellini G, Bruschettini M, Gianola S, Gluud C, Moja L. Assessing imprecision in Cochrane systematic reviews: a comparison of GRADE and Trial Sequential Analysis. Systematic Reviews 2018;7(110):1‐10. [doi.org/10.1186/s13643‐018‐0770‐1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krle A‐Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2008
- Chen FP, Kung YY, Chen YC, Jong MS, Chen TJ, Chen FJ, et al. Frequency and pattern of Chinese herbal medicine prescriptions for chronic hepatitis in Taiwan. Journal of Ethnopharmacology 2008;117:84‐91. [DOI] [PubMed] [Google Scholar]
Ciani 2017
- Ciani O, Buyse M, Drummond M, Rasi G, Saad ED, Taylor RS. Time to review the role of surrogate end points in health policy: state of the art and the way forward. Value Health 2017;20(3):487‐95. [DOI] [PubMed] [Google Scholar]
CMA‐CSH and CMA‐SID 2006
- Chinese Society of Hepatology, CMA and Society of Infectious Disease, CMA. The guideline of prevention and treatment for chronic hepatitis B. World Clinic Drugs 2006;27(2):36‐41. [Google Scholar]
CMA‐CSH and CMA‐SID 2011
- Chinese Society of Hepatology, CMA, Society of Infectious Disease, CMA. Chronic hepatitis B prevention and treatment guideline 2010 update. Chinese Journal of Gastroenterology 2011;16:351‐67. [Google Scholar]
Delmas 2002
- Delmas J, Schorr O, Jamard C, Gibbs C, Trépo C, Hantz O, et al. Inhibitory effect of adefovir on viral DNA synthesis and covalently closed circular DNA formation in duck hepatitis B virus‐infected hepatocytes in vivo and in vitro. Antimicrobial Agents and Chemotherapy 2002;46(2):425‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
EASL 2017
- European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. Journal of Hepatology 2017;67(2):370‐98. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ezbarami 2017
- Ezbarami ZT, Hassani P, Tafreshi MZ, Majd HA. A qualitative study on individual experiences of chronic hepatitis B patients. Nursing Open 2017;4(4):310‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2016
- Feng S, Liu W, Liu JP, Wang LQ. Herbal preparations for chronic hepatitis B virus carriers: the analysis from 31 randomized clinical trials. Journal of Alternative and Complementary Medicine 2016;22(6):A54‐5. [Google Scholar]
Fisher 1922
- Fisher RA. On the interpretation of X2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society 1922;85(1):87‐94. [Google Scholar]
Flemming 2012
- Fleming TR, Powers JH. Biomarkers and surrogate endpoints in clinical trials. Statistics in Medicine 2012;31(25):2973‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gai 2007
- Gai LL. The Effect of Minor Bupleurum Tablets on Antigen Presentation of Peripheral Blood Dendritic Cells from Patients with Chronic Hepatitis B Infection. Guangzhou (China): Guangzhou University of Chinese Medicine, 2007. [Google Scholar]
Gartlehner 2019
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. [DOI] [PubMed] [Google Scholar]
GRADE 2013
- GRADE Working Group 2013. GRADE handbook for grading quality of evidence and strength of recommendations. gdt.gradepro.org/app/handbook/handbook.html (accessed 1 November 2018).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 17 October 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gripon 2002
- Gripon P, Rumin S, Urban S, Seyec J, Glaise D, Cannie I, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proceedings of the National Academy of Sciences of the United States of America 2002;99(24):15655‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence – risk of bias. Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence – publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt G, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence – inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence – indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables – binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] [DOI] [PubMed] [Google Scholar]
Guyatt 2013c
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing Summary of Findings tables – continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] [DOI] [PubMed] [Google Scholar]
Guyatt 2013d
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. [DOI] [PubMed] [Google Scholar]
Guyatt 2017
- Guyatt GH, Ebrahim S, Alonso‐Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14‐22. [PUBMED: 28529188] [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Hatakeyama 1997
- Hatakeyama S, Tachibana A, Morita M, Suzuki K, Okano H. Five cases of pneumonitis induced by Sho‐Saiko‐To. Nihon Kyobu Shikkan Gakkai Zasshi 1997;35(5):505‐10. [PubMed] [Google Scholar]
Heydtmann 2000
- Heydtmann M. New findings in hepatology. Therapiewoche Schweiz 2000;16:61‐2. [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Hung 2019
- Hung TH, Tsai CC, Lee HF. Population‐based study of entecavir and long‐term mortality in chronic hepatitis B‐related decompensated liver cirrhosis. Clinics and Research in Hepatology and Gastroenterology 2019;02:1‐6. [DOI] [PubMed] [Google Scholar]
ICH‐E2A 1994
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonised tripartite guideline. Clinical safety data management: definitions and standards for expedited reporting E2A, 1994. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2A/Step4/E2A_Guideline.pdf (accessed 2 November 2018).
ICH‐GCP E6(R2) 2016
- International Council on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH harmonised guideline. Integrated addendum to ICH E6(R1): guideline for good clinical practice E6(R2), 2016. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf (accessed 02 October 2018).
Imberger 2011
- Imberger G, Vejlby AD, Hansen SB, Møller AM, Wetterslev J. Statistical multiplicity in systematic reviews of anaesthesia interventions: a quantification and comparison between Cochrane and non‐Cochrane reviews. Plos One 2011;6(12):e28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
IPBCMA 1995
- Infectious Parasitology Branch of Chinese Medical Association. Diagnostic criteria of viral hepatitis. Chinese Journal of Infectious Diseases 1995;13(4):242. [Google Scholar]
Itoh 1995
- Itoh S, Marutani K, Nishijima T, Matsuo S, Itabashi M. Liver injuries induced by herbal medicine, syo‐saiko‐to (xiao‐chai‐hu‐tang). Digestive Diseases and Sciences 1995;40(8):1845‐8. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Dawn C, Crispin J, Reynolds D, John M, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen J, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2017
- Jakobsen JC, Nielsen EE, Feinberg J, Katakam KK, Fobian K, Hauser G, et al. Direct‐acting antivirals for chronic hepatitis C. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD012143.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2018
- Jakobsen JC, Nielsen EE, Koretz RL, Gluud C. Do direct acting antivirals cure chronic hepatitis C?. BMJ (Clinical Research Ed.) 2018;10(361):k1382. [DOI] [PubMed] [Google Scholar]
Kemp 2017
- Kemp R, Prasad V. Surrogate endpoints in oncology: when are they acceptable for regulatory and clinical decisions, and are they currently overused?. BMC Medicine 2017;15(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keshavarz 2015
- Keshavarz K, Kebriaeezadeh A, Alavian SM, Sari AA, Dorkoosh FA, Keshvari M, et al. Economic burden of hepatitis B virus‐related diseases: evidence from Iran. Hepatitis Monthly 2015;15(4):e25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiguchi 2000
- Kiguchi T, Kimura F, Niiya K, Katayama Y, Harada M. Acute thrombocytopenic purpura after ingestion of Sho‐saiko‐to for hepatitis. Liver 2000;20(6):491. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Kong 2019
- Kong DZ, Liang N, Yang GL, Zhang Z, Jiang JW, Liu Y, et al. A qualitative and visual assessment approach of evidence – exemplified by Xiao Chai Hu Tang formula for chronic hepatitis B. BMC Complementary and Alternative Medicine 2019;6:S61. [Google Scholar]
Kouamé 2018
- Kouamé GM, Boyd A, Moh R, Badje A, Gabillard D, Ouattara E, et al. Higher mortality despite early antiretroviral therapy in human immunodeficiency virus and hepatitis B virus (HBV)‐coinfected patients with high HBV replication. Clinical Infectious Diseases 2018;66(1):112‐20. [DOI] [PubMed] [Google Scholar]
Li 2001a
- Li Z, Liao HH, Wu MJ, Lin ZH. Study of γ interferon combined with Xiaochaihu Tang for treatment of liver fibrosis. Zhong Xi Yi Jie He Gan Bing Za Zhi 2001;11(S1):95. [Google Scholar]
Li 2008
- Li J, Xie M, Gan Y. Effect of Xiaochaihu decoction and different herbal formulation of component on inhibiting H22 liver cancer in mice and enhancing immune function. Zhongguo Zhong Yao Za Zhi 2008;33(9):1039‐44. [PubMed] [Google Scholar]
Liu 2002a
- Liu JP, Kjaergard LL, Gluud C. Misuse of randomization: a review of Chinese randomized trials of herbal medicines for chronic hepatitis B. American Journal of Chinese Medicine 2002;30(1):173‐6. [DOI] [PubMed] [Google Scholar]
Liu 2010
- Liu ZJ, Wang ZL, Sun XH, Liu S, Gu YH. Effect of modified Xiaochaihu decoction on expression of IL‐12 , IL‐4, and IFN‐γ mRNA in HBV transgenic mice. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 2010;20:289‐90. [Google Scholar]
Lu 2013
- Lu JJ, Xu AQ, Wang J, Zhang L, Song LZ, Li RP, et al. Direct economic burden of hepatitis B virus related diseases: evidence from Shandong, China. BMC Health Services Research 2013;31(13):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 1997a
- Ma YR, Shimizu I, Ito S, Mizobuchi Y, Yasuda M, Escobar E. Effect of Xiaochaihu decoction on experimental hepatic fibrosis in rats. Journal of Luzhou Medical College 1997;20:81‐6. [Google Scholar]
Mao 2005
- Mao M, Fu H, Huang X‐S, Jia B. Small radix bupleuri decoction inducing bearing cancer mice S180 cell apoptosis and influence of it on cell cycle. Modern Journal of Integrated Traditional Chinese and Western Medicine 2005;14:2646‐8. [Google Scholar]
MECIR 2019
- Higgins JP, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, et al. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London. Version October 2019. [community.cochrane.org/mecir‐manual]
MHLW 2016
- Ministry of Health, Labour, Welfare. The Japanese Pharmacopoeia, seventeenth edition, 2016. www.mhlw.go.jp/file/06‐Seisakujouhou‐11120000‐Iyakushokuhinkyoku/JP17_REV_1.pdf (accessed 20 July 2018).
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D, for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Journal of the American Medical Association 2001;285:1987‐91. [DOI] [PubMed] [Google Scholar]
Moraleda 1997
- Moraleda G, Saputelli J, Aldrich CE, Averett D, Condreay L, Mason WS. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. Journal of Virology 1997;71:9392‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mustafa 2013
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42. [PUBMED: 23623694] [DOI] [PubMed] [Google Scholar]
Nassal 2008
- Nassal M. Hepatitis B viruses: reverse transcription a different way. Virus Research 2008;134:235‐49. [DOI] [PubMed] [Google Scholar]
Ono 2000
- Ono M, Miyamura M, Kyotani S, Saibara T, Ohnishi S, Nishioka Y. Effect of Sho‐saiko‐to extract on HGF and TGF‐beta levels of intra organs in liver‐injured rats after partial hepatectomy. Journal of Pharmacy and Pharmacology 2000;52:111‐8. [DOI] [PubMed] [Google Scholar]
Osawa 2017
- Osawa M, Akuta N, Suzuki F, Fujiyama S, Kawamura Y, Sezaki H, et al. Prognosis and predictors of hepatocellular carcinoma in elderly patients infected with hepatitis B virus. Journal of Medical Virology 2017;89(12):2144‐8. [DOI] [PubMed] [Google Scholar]
Peng 2000
- Peng WW, editor(s). Infectious Diseases – Hepatitis B. 5th Edition. Beijing (China): People's Health Publishing House, 2000. [Google Scholar]
PRISMA 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research Ed.) 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qi 2011
- Qi XQ, Wang Y. Sero‐Epidemiological Investigation Report on Hepatitis B in the National Population. Beijing (China): People's Medical Publishing House (PMPH), 2011. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Sakamoto 2003
- Sakamoto O, Ichikado K, Kohrogi H, Suga M. Clinical and CT characteristics of Chinese medicine‐induced acute respiratory distress syndrome. Respirology 2003;8(3):344‐50. [DOI] [PubMed] [Google Scholar]
Sato 1997
- Sato A, Toyoshima M, Kondo A, Ohta K, Sato H, Ohsumi A. Pneumonitis induced by the herbal medicine Sho‐saiko‐to in Japan. Nihon Kyobu Shikkan Gakkai Zasshi 1997;35(4):391‐5. [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2018
- Savović J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JP, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane reviews: the ROBES meta‐epidemiologic study. American Journal of Epidemiology 2018;187(5):1113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2016
- Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision?. Journal of Clinical Epidemiology 2016;75:6‐15. [DOI] [PubMed] [Google Scholar]
Shiraki 1991
- Shiraki K, Tanimoto K, Togashi T. A study of the efficacy of shosaikoto in children with HBe antigen‐positive chronic hepatitis B. Shonika Rinsho (Japanese Journal of Pediatrics) 1991;44:2146‐51. [Google Scholar]
Shu 2015
- Shu FM, Gong SM, Li HB, Feng WS, Jiang C. Research progress on treatment of chronic hepatitis B with famous Chinese medicine formula [古之名方治疗慢性乙型肝炎研究进展]. Journal of Liaoning University of Traditional Chinese Medicine 2015;17(2):191‐3. [Google Scholar]
Stickel 2000
- Stickel F, Egerer G, Seitz HK. Hepatotoxicity of botanicals. Public Health Nutrition 2000;3(2):111. [DOI] [PubMed] [Google Scholar]
Storebø 2018
- Storebø OJ, Pedersen N, Ramstad E, Kielsholm ML, Nielsen SS, Krogh HB, et al. Methylphenidate for attention deficit hyperactivity disorder (ADHD) in children and adolescents – assessment of adverse events in non‐randomised studies. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD012069.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Student 1908
- Student. The probable error of a mean. Biometrika 1908;6(1):1‐25. [Google Scholar]
Su 2016
- Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, et al. Four‐year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver International 2016;36(12):1755‐64. [DOI] [PubMed] [Google Scholar]
Tajiri 1991
- Tajiri H, Kozaiwa K, Ozaki Y, Miki K, Shimuzu K, Okada S. Effect of sho‐saiko‐to (xiao‐chai‐hu‐tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. American Journal of Chinese Medicine 1991;19(2):121‐9. [DOI] [PubMed] [Google Scholar]
Takada 1993
- Takada N, Arai S, Kusuhara N, Katagiri M, Yanase N, Abe T, et al. A case of sho‐saiko‐to‐induced pneumonitis, diagnosed by lymphocyte stimulation test using bronchoalveolar lavage fluid. Nihon Kyobu Shikkan Gakkai Zasshi 1993;31(9):1163‐9. [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011a
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 16 January 2017).
Thorlund 2011b
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis – a simulation study. PloS One 2011;6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA – Trial Sequential Analysis. Version 0.9.5.10 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Tsumura 2014
- Tsumura, Co. TSUMURA shosaikoto extract granules for ethical use, 2014. www.tsumura.co.jp/english/products/pi/JPR_T009.pdf (accessed 20 July 2018).
Vrhovac 1977
- Vrhovac B. Placebo and its importance in medicine. International Journal of Clinical Pharmacology and Biopharmacy 1977;15(4):161‐5. [PubMed] [Google Scholar]
Wang 2004
- Wang SJ, Ai Q. Effect of Xiaochaihu decoction on hepatic fibrosis and liver cancer. Progress in Japanese Medicine 2004;25:42‐4. [Google Scholar]
Wang 2014a
- Wang H, Zheng ZM, Gao BL. Guanxinkang decoction exerts its antiatherosclerotic effect partly through inhibiting the endoplasmic reticulum stress. Evidence‐based Complementary and Alternative Medicine : ECAM 2014;2014:465640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2000
- Wen ZJ, Cao Y, Peng LL. Study on anti‐HBV activity of Xiaochaihu decoction. Chinese Journal of Integrated Traditional and Western Medicine on Liver Diseases 2000;10:29‐30. [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Hepatitis B, 2000. www.who.int/news‐room/fact‐sheets/detail/hepatitis‐b (accessed 20 July 2018).
WHO 2015
- World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection, 2015. www.who.int/hiv/pub/hepatitis/hepatitis‐b‐guidelines/en/ (accessed 20 July 2018). [PubMed]
WHO 2017
- World Health Organization. Global hepatitis report, 2017. www.who.int/hepatitis/publications/global‐hepatitis‐report2017/en/ (accessed 20 July 2018).
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2009b
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;10:46. [DOI: 10.1186/1745-6215-10-46] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiong 2003
- Xiong F, Sun J, Xiong W. Clinical observation of interferon combined with Xiaochaihu Tang for treatment of liver fibrosis. Hubei Zhong Yi Za Zhi 2003;25(10):10‐1. [Google Scholar]
Yamashiki 1992
- Yamashiki M, Kosaka Y, Nishimura A, Takase K, Ichida F. Efficacy of a herbal medicine 'sho‐saiko‐to' on the improvement of impaired cytokine production of peripheral blood mononuclear cells in patients with chronic viral hepatitis. Journal of Clinical & Laboratory Immunology 1992;37(3):111‐21. [PubMed] [Google Scholar]
Yano 1994
- Yano H, Mizoguchi A, Fukuda K, Haramaki M, Ogasawara S, Momosaki S, et al. The herbal medicine sho‐saiko‐to inhibits proliferation of cancer cell lines by inducing apoptosis and arrest at the G0/G1 phase. Cancer Research 1994;54:448‐54. [PubMed] [Google Scholar]
Ye 2011
- Ye YA, Min LQ. Chinese herbal medicine long‐term anti‐HBV infection personalized treatments. International Journal of Infectious Diseases 2011;15(1):S76. [Google Scholar]
Yi 2004
- Yi YD, Chang IM. An overview of traditional Chinese herbal formulae and a proposal of a new code system for expressing the formula titles. Evidence‐based Complementary and Alternative Medicine : ECAM 2004;1(2):125‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2000
- Yu AQ, Wang KQ, Zhang WD, Li CY, Shen LM, Zhang W. Modified Xiaochaihu Tang combined with ribavirin for treatment of chronic hepatitis B. Zhejiang Zhong Xi Yi Jie He Za Zhi 2000;10(3):133‐5. [Google Scholar]
Zhang 2005a
- Zhang ZJ. Treatise on Febrile Disease (Shang Han Lun). Beijing (China): People's Medical Publishing House, 2005. [Google Scholar]
Zhang 2005b
- Zhang Q, Tian ZB. Interfere study on Xiaochaihu decoction on expression of collagen I and III of hepatic fibrosis in experimental rats. Journal of Shandong University of TCM 2005;29:316‐8. [Google Scholar]
Zhang 2006a
- Zhang XM, Xie B. The protective effect of modified radix bupleuri decoction on liver injured by D‐galactosamine. China Tropical Medicine 2006;6:1134‐5. [Google Scholar]
Zhang 2017
- Zhang SK, Cui NQ, Zhuo YZ, Hu JG, Liu JH, Li DH, et al. Modified Xiaochaihu decoction (加味小柴胡汤) promotes collagen degradation and inhibits pancreatic fibrosis in chronic pancreatitis rats. Chinese Journal of Integrative Medicine 2017 Nov 28 [Epub ahead of print]. [DOI: 10.1007/s11655-017-2413-0] [DOI] [PubMed]
Zheng 2002
- Zheng XY. Guiding Principles of Clinical Research on New Drugs of Chinese Medicines. Beijing (China): China Medical Science Press, 2002. [Google Scholar]
Zheng 2014
- Zheng X, Wang J, Yang D. Antiviral therapy for chronic hepatitis B in China. Medical Microbiology and Immunology 2015;204:115‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zoulim 2005
- Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. Journal of Hepatology 2005;42(3):302‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kong 2018
- Kong DZ, Liang N, Liu JP, Nikolova D, Jakobsen JC, Gluud C. Xiao Chai Hu Tang, a Chinese herbal medicine formula, for chronic hepatitis B. Cochrane Database of Systematic Reviews 2018, Issue 8. [DOI: 10.1002/14651858.CD013090] [DOI] [PMC free article] [PubMed] [Google Scholar]


