Abstract
Background
About 10% of reproductive‐aged women suffer from endometriosis, which is a costly, chronic disease that causes pelvic pain and subfertility. Laparoscopy is the gold standard diagnostic test for endometriosis, but it is expensive and carries surgical risks. Currently, there are no non‐invasive tests available in clinical practice that accurately diagnose endometriosis. This is the first diagnostic test accuracy review of endometrial biomarkers for endometriosis that utilises Cochrane methodologies, providing an update on the rapidly expanding literature in this field.
Objectives
To determine the diagnostic accuracy of the endometrial biomarkers for pelvic endometriosis, using a surgical diagnosis as the reference standard. We evaluated the tests as replacement tests for diagnostic surgery and as triage tests to inform decisions to undertake surgery for endometriosis.
Search methods
We did not restrict the searches to particular study designs, language or publication dates. To identify trials, we searched the following databases: CENTRAL (2015, July), MEDLINE (inception to May 2015), EMBASE (inception to May 2015), CINAHL (inception to April 2015), PsycINFO (inception to April 2015), Web of Science (inception to April 2015), LILACS (inception to April 2015), OAIster (inception to April 2015), TRIP (inception to April 2015) and ClinicalTrials.gov (inception to April 2015). We searched DARE and PubMed databases up to April 2015 to identify reviews and guidelines as sources of references to potentially relevant studies. We also performed searches for papers recently published and not yet indexed in the major databases. The search strategies incorporated words in the title, abstract, text words across the record and the medical subject headings (MeSH).
Selection criteria
We considered published peer‐reviewed, randomised controlled or cross‐sectional studies of any size that included prospectively collected samples from any population of reproductive‐aged women suspected of having one or more of the following target conditions: ovarian, peritoneal or deep infiltrating endometriosis (DIE).
Data collection and analysis
Two authors independently extracted data from each study and performed a quality assessment. For each endometrial diagnostic test, we classified the data as positive or negative for the surgical detection of endometriosis and calculated the estimates of sensitivity and specificity. We considered two or more tests evaluated in the same cohort as separate data sets. We used the bivariate model to obtain pooled estimates of sensitivity and specificity whenever sufficient data were available. The predetermined criteria for a clinically useful test to replace diagnostic surgery was one with a sensitivity of 94% and a specificity of 79%. The criteria for triage tests were set at sensitivity at or above 95% and specificity at or above 50%, which in case of negative results rules out the diagnosis (SnOUT test) or sensitivity at or above 50% with specificity at or above 95%, which in case of positive result rules in the diagnosis (SpIN test).
Main results
We included 54 studies involving 2729 participants, most of which were of poor methodological quality. The studies evaluated endometrial biomarkers either in specific phases of the menstrual cycle or outside of it, and the studies tested the biomarkers either in menstrual fluid, in whole endometrial tissue or in separate endometrial components. Twenty‐seven studies evaluated the diagnostic performance of 22 endometrial biomarkers for endometriosis. These were angiogenesis and growth factors (PROK‐1), cell‐adhesion molecules (integrins α3β1, α4β1, β1 and α6), DNA‐repair molecules (hTERT), endometrial and mitochondrial proteome, hormonal markers (CYP19, 17βHSD2, ER‐α, ER‐β), inflammatory markers (IL‐1R2), myogenic markers (caldesmon, CALD‐1), neural markers (PGP 9.5, VIP, CGRP, SP, NPY, NF) and tumour markers (CA‐125). Most of these biomarkers were assessed in single studies, whilst only data for PGP 9.5 and CYP19 were available for meta‐analysis. These two biomarkers demonstrated significant diversity for the diagnostic estimates between the studies; however, the data were too limited to reliably determine the sources of heterogeneity. The mean sensitivities and specificities of PGP 9.5 (7 studies, 361 women) were 0.96 (95% confidence interval (CI) 0.91 to 1.00) and 0.86 (95% CI 0.70 to 1.00), after excluding one outlier study, and for CYP19 (8 studies, 444 women), they were were 0.77 (95% CI 0.70 to 0.85) and 0.74 (95% CI 0.65 to 84), respectively. We could not statistically evaluate other biomarkers in a meaningful way. An additional 31 studies evaluated 77 biomarkers that showed no evidence of differences in expression levels between the groups of women with and without endometriosis.
Authors' conclusions
We could not statistically evaluate most of the biomarkers assessed in this review in a meaningful way. In view of the low quality of most of the included studies, the findings of this review should be interpreted with caution. Although PGP 9.5 met the criteria for a replacement test, it demonstrated considerable inter study heterogeneity in diagnostic estimates, the source of which could not be determined. Several endometrial biomarkers, such as endometrial proteome, 17βHSD2, IL‐1R2, caldesmon and other neural markers (VIP, CGRP, SP, NPY and combination of VIP, PGP 9.5 and SP) showed promising evidence of diagnostic accuracy, but there was insufficient or poor quality evidence for any clinical recommendations. Laparoscopy remains the gold standard for the diagnosis of endometriosis, and using any non‐invasive tests should only be undertaken in a research setting. We have also identified a number of biomarkers that demonstrated no diagnostic value for endometriosis. We recommend that researchers direct future studies towards biomarkers with high diagnostic potential in good quality diagnostic studies.
Keywords: Female, Humans, Biomarkers, Biomarkers/analysis, Endometriosis, Endometriosis/diagnosis, Endometrium, Endometrium/chemistry, Menstrual Cycle, Menstruation, Menstruation/metabolism
Plain language summary
Endometrial biomarkers for the non‐invasive diagnosis of endometriosis
Review question
Can physicians use biomarkers (distinctive molecules, genes or other characteristics that appear in certain conditions) to reduce the need to surgically diagnose endometriosis?
Background
The endometrium refers to the tissue that lines the womb and is shed during menstruation. Women with endometriosis have endometrial tissue growing outside the womb, within the pelvic cavity. This tissue responds to reproductive hormones causing painful periods, chronic lower abdominal pain and difficulty conceiving. Currently the only reliable way of diagnosing endometriosis is to perform keyhole surgery and visualise the endometriotic deposits inside the abdomen. Because surgery is risky and expensive, various tests within the endometrium that can be obtained during an in‐office womb sampling procedure have been assessed for their ability to detect endometriosis non‐invasively or with minimal invasion. An accurate test could lead to the diagnosis of endometriosis without the need for surgery, or it could reduce the need for diagnostic surgery so only women who were most likely to have endometriosis would require it. Review teams have also evaluated other non‐invasive ways of diagnosing endometriosis using blood, urine and imaging tests as well as a combination of several testing methods in separate Cochrane reviews within this series.
Study characteristics
The evidence in this review is current to April 2015. We included 54 studies involving 2729 participants. All studies evaluated reproductive‐aged women who were undertaking diagnostic surgery to investigate symptoms of endometriosis or for other indications. Twenty‐six studies evaluated the role of 22 different biomarkers in diagnosing endometriosis, and 31 studies identified 77 additional biomarkers that had no value in differentiating between women with and without the disease.
Key results and quality of evidence
Only two of the assessed biomarkers, a neural fibre marker PGP 9.5 and hormonal marker CYP19, were assessed in sufficient number of studies to obtain meaningful results. PGP 9.5 identified endometriosis with enough accuracy to replace surgical diagnosis. Several additional biomarkers (endometrial proteome, 17βHSD2, IL‐1R2, caldesmon and other neural markers) show promise in detecting endometriosis, but there are too few studies to be sure of their diagnostic value.
The studies differed in how they were conducted, which groups of women were studied and how the surgery was undertaken. The reports were of low methodological quality, which is why readers cannot consider these results to be reliable unless confirmed in large, high quality studies. Overall, there is not enough evidence to recommend any endometrial test for use in clinical practice for the diagnosis of endometriosis.
Future research
Further high quality research is necessary to accurately evaluate the diagnostic potential of the endometrial biomarkers for the diagnosis of endometriosis.
Summary of findings
Summary of findings'. 'Biomarkers evaluated as a diagnostic test for endometriosis.
| Review question | What is the diagnostic accuracy of the endometrial biomarkers in detecting endometriosis? | Pelvic endometriosis (any site and depth of invasion) | |||||
| Ovarian endometriosis | |||||||
| Importance | A simple and reliable non‐invasive test for endometriosis with the potential to either replace laparoscopy or to triage patients in order to reduce surgery, would minimise surgical risk and reduce diagnostic delay | ||||||
| Patients | Reproductive‐aged women with suspected endometriosis or persistent ovarian mass, or women undergoing infertility work‐up | ||||||
| Settings | Hospitals (public or private of any level), outpatient clinics (general gynaecology, reproductive medicine, pelvic pain) or radiology departments | ||||||
| Reference standard | Visualisation of endometriosis at surgery (laparoscopy or laparotomy) with or without histological confirmation | ||||||
| Study design | Cross sectional of single gate design (N = 16) or two‐gate design (N = 11); prospective enrolment; one study could assess more than one test or more than one type of endometriosis | ||||||
| Risk of bias | Overall judgement | Poor quality of most of the studies (only 2 studies had 'low risk' assessment in all 4 domains) | |||||
| Patient selection bias | High risk: 20 studies; unclear risk: 4 studies; low risk ‐ 3 studies | ||||||
| Index test interpretation bias | High risk: 18 studies; unclear risk: 5 studies; low risk ‐ 4 studies | ||||||
| Reference standard interpretation bias | High risk: 0 studies; unclear risk: 10 studies; low risk ‐ 17 studies | ||||||
| Flow and timing selection bias | High risk: 7 studies; unclear risk: 0 studies; low risk ‐ 20 studies | ||||||
| Applicability concerns | Concerns regarding patient selection | High concern ‐ 13 studies; unclear concern ‐ 4 studies; low concern ‐ 10 studies | |||||
| Concerns regarding index test | High concern ‐ 0 studies; unclear concern ‐ 1 study; low concern ‐ 26 studies | ||||||
| Concerns regarding reference standard | High concern ‐ 0 studies; unclear concern ‐ 0 studies; low concern ‐ 27 studies | ||||||
| Diagnostic criteria | Replacement test: sensitivity ≥ 0.94 and specificity ≥ 0.79 SnOUT triage test: sensitivity ≥ 0.95 and specificity ≥ 0.50 SpIN triage test: sensitivity ≥ 0.50 and specificity ≥ 0.95 |
||||||
| Test | N participants (studies) | Outcomes | Diagnostic estimates (95% CI) | Implications | |||
| True positives (endometriosis) | False positives (incorrectly classified as endometriosis) | False negatives (incorrectly classified as disease‐free) | True negatives (disease‐free) | ||||
| 1. Angiogenesis and growth factors and their receptors | |||||||
| PROK‐1 mRNA (glandular) (prokineticin 1 gene evaluated in glandular epithelium) |
24 (1) | 8 | 2 | 4 | 10 | Sens = 0.67 (0.35 to 0.90); spec = 0.83 (0.52 to 0.98) |
Insufficient evidence to draw meaningful conclusions |
| 2. Cell adhesion molecules and other matrix‐related proteins | |||||||
| Depolarised α‐6 integrin (glandular) (depolarised alpha‐6 integrin expression assessed in glandular epithelium) |
49 (1) | 20 | 3 | 10 | 16 | Sens = 0.67 (0.47 to 0.83); spec = 0.84 (0.60 to 0.97) |
Insufficient evidence to draw meaningful conclusions |
| α3β1 integrin (glandular) (alpha‐3 beta‐1 integrin chain evaluated in glandular epithelium) |
32 (1) | 17 | 11 | 0 | 4 | Sens = 1.00 (0.08 to 1.00); spec = 0.27 (0.08 to 0.55) |
Insufficient evidence to draw meaningful conclusions |
| α3β1 integrin (stroma) (alpha‐3 beta‐1 integrin chain evaluated in stroma cells) |
32 (1) | 9 | 11 | 8 | 4 | Sens = 0.53 (0.28 to 0.77); spec = 0.27 (0.08 to 0.55) |
Insufficient evidence to draw meaningful conclusions |
| α4β1 integrin (glandular) (alpha‐4 beta‐1 integrin chain evaluated in glandular epithelium) |
32 (1) | 11 | 9 | 6 | 6 | Sens = 0.65 (0.38 to 0.86); spec = 0.40 (0.16 to 0.68) |
Insufficient evidence to draw meaningful conclusions |
| α4β1 integrin (stroma) (alpha‐4 beta‐1 integrin chain evaluated in stroma cells) |
32 (1) | 10 | 12 | 7 | 3 | Sens = 0.59 (0.33 to 0.82); spec = 0.20 (0.04 to 0.48) |
Insufficient evidence to draw meaningful conclusions |
| β1 integrin (glandular) (beta‐1 integrin chain evaluated in glandular epithelium) |
32 (1) | 3 | 2 | 14 | 13 | Sens = 0.18 (0.04 to 0.43); spec = 0.87 (0.60 to 0.98) |
Insufficient evidence to draw meaningful conclusions |
| β1 integrin (stroma) (beta‐1 integrin chain evaluated in stroma cells) |
32 (1) | 13 | 15 | 4 | 0 | Sens = 0.76 (0.50 to 0.93); spec = 0.00 (0.00 to 0.22) |
Insufficient evidence to draw meaningful conclusions |
| 3. DNA‐repair and telomer maintenance molecules | |||||||
| hTERT mRNA (human telomerase reverse transcriptase gene) |
69 (1) | 7 | 9 | 18 | 35 | Sens = 0.28 (0.12 to 0.49); spec = 0.80 (0.65 to 0.90) |
Insufficient evidence to draw meaningful conclusions |
| 4. High throughput markers | |||||||
| Endometrial proteome by SELDI‐TOF‐MSa (high throughput surface enhanced laser desorption/ionisation time‐of‐flight mass spectrometry) |
(5 peptide peaks of 2072 m/z; 2973 m/z; 3623 m/z; 3680 m/z and 21,133 m/z) | Insufficient evidence to draw meaningful conclusions | |||||
| 27 (1) | 15 | 2 | 2 | 8 | Sens = 0.88 (0.64 to 0.99); spec = 0.80 (0.44 to 0.97) |
||
| (5 protein peaks of 5385 m/z, 5425 m/z, 6898 m/z, 5891 m/z, 6448 m/z) | Approaches the criteria for a replacement, SnOUT and SpIN triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
||||||
| 26 (1) | 12 | 1 | 1 | 12 | Sens = 0.92 (0.64 to 1.00); spec = 0.92 (0.64 to 1.00) |
||
| Mitichondrial proteome by SELDI‐TOF‐MS (high throughput surface enhanced laser desorption/ionisation time‐of‐flight mass spectrometry) |
53 (1) | 21 | 4 | 3 | 25 | Sens = 0.88 (0.68 to 0.97); spec = 0.86 (0.68 to 0.96) |
Insufficient evidence to draw meaningful conclusions |
| 5. Hormonal markers | |||||||
| CYP19 (aromatase cytochrome P450) |
444 (8) | 171 | 60 | 50 | 163 | Mean estimates: sens = 0.77 (0.70 to 0.85); spec = 0.74 (0.65 to 0.84) |
Summary estimates do not meet the predetermined criteria for a replacement or triage test. |
| 17βHSD2 mRNA (17‐beta hydroxysteroid dehydrogenase type 2 gene) The biomarker was evaluated for DIE only |
53 (1) | 16 | 2 | 14 | 24 | Sens = 0.53 (0.34 to 0.72); spec = 0.91 (0.72 to 0.99) | Approaches the criteria for a SpIN triage test; Insufficient evidence to draw meaningful conclusions; Promising marker for further investigation |
| ER‐α (glandular) (oestrogen receptor ‐ alpha evaluated in glandular epithelium irrespective of menstrual cycle phase) |
90 (1) | 44 | 17 | 16 | 13 | Sens = 0.73 (0.60 to 0.84); spec = 0.43 (0.25 to 0.63) |
Insufficient evidence to draw meaningful conclusions |
| ER‐α (stroma) (oestrogen receptor ‐ alpha evaluated in stromal cells irrespective of menstrual cycle phase) |
90 (1) | 46 | 15 | 14 | 15 | Sens = 0.77 (0.64 to 0.87); spec = 0.50 (0.31 to 0.69) |
Insufficient evidence to draw meaningful conclusions |
| ER‐β (glandular) (oestrogen receptor ‐ beta evaluated in glandular epithelium irrespective of menstrual cycle phase) |
90 (1) | 40 | 11 | 20 | 19 | Sens = 0.67 (0.53 to 0.78); spec = 0.63 (0.44 to 0.80) | Insufficient evidence to draw meaningful conclusions |
| ER‐β (stroma) (oestrogen receptor ‐ beta evaluated in stromal cells irrespective of menstrual cycle phase) |
90 (1) | 38 | 8 | 22 | 22 | Sens = 0.63 (0.50 to 0.75) spec = 0.73 (0.54 to 0.88) |
Insufficient evidence to draw meaningful conclusions |
| 6. Immune system and inflammatory markers | |||||||
| IL‐1R2 mRNA (glandular) (interleukin‐1 receptor type II gene evaluated in glandular epithelium irrespective of menstrual cycle phase) |
31 (1) | 16 | 7 | 0 | 8 | Sens = 1.00 (0.79 to 1.00); spec = 0.53 (0.27 to 0.79) |
Meets the criteria for a SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| IL‐1R2 mRNA (stroma) (Interleukin‐1 receptor type II gene evaluated in stromal endometrial cells irrespective of menstrual cycle phase) |
32 (1) | 14 | 4 | 1 | 13 | Sens = 0.93 (0.68 to 1.00); spec = 0.76 (0.50 to 0.93) |
Approaches the criteria for a replacement and SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| IL‐1R2 mRNA (glandular secretory) (interleukin‐1 receptor type II gene assessed in glandular epithelium in secretory phase of menstrual cycle) |
19 (1) | 10 | 3 | 0 | 6 | Sens = 1.00 (0.69 to 1.00); spec = 0.67 (0.30 to 0.93) |
Meets the criteria for a SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| IL‐1R2 mRNA (stroma secretory) (interleukin‐1 receptor type II gene assessed in stromal endometrial cells in secretory phase of menstrual cycle) |
20 (1) | 9 | 1 | 1 | 9 | Sens = 0.90 (0.55 to 1.00); spec = 0.90 (0.55 to 1.00) |
Approaches the criteria for a replacement, SnOUT and SpIN triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| 7. Myogenic markers (markers of smooth muscle differentiation) | |||||||
| Caldesmon (proliferative) (calmodulin binding protein evaluated in proliferative phase of menstrual cycle) |
35 (1) | 19 | 0 | 1 | 15 | Sens = 0.95 (0.75 to 1.00); spec = 1.00 (0.78 to 1.00) | Meets the criteria for a replacement, SnOUT and SpIN triage test; Insufficient evidence to draw meaningful conclusions; Promising marker for further investigation |
| Caldesmon (secretory) (calmodulin binding protein evaluated in secretory phase of menstrual cycle) |
35 (1) | 18 | 1 | 2 | 14 | Sens = 0.90 (0.68 to 0.99); spec = 0.93 (0.68 to 1.00) |
Approaches the criteria for a replacement, SnOUT and SpIN triage test; Insufficient evidence to draw meaningful conclusions; Promising marker for further investigation |
| CALD1 mRNA (proliferative) (gene encoding for caldesmon evaluated in proliferative phase of menstrual cycle) |
35 (1) | 12 | 2 | 8 | 13 | Sens = 0.60 (0.36 to 0.81); spec = 0.87 (0.60 to 0.98) |
Insufficient evidence to draw meaningful conclusions |
| CALD1 mRNA (secretory) (gene encoding for caldesmon evaluated in secretory phase of menstrual cycle) |
35 (1) | 15 | 5 | 5 | 10 | Sens = 0.75 (0.51 to 0.91); spec = 0.67 (0.38 to 0.88) |
Insufficient evidence to draw meaningful conclusions |
| 8. Nerve sheath and nerve growth markers | |||||||
| PGP 9.5 (protein gene product 9.5) | 429 (8) 361 (7)b |
192 | 33 | 49 | 155 | Mean estimatesb: Sens = 0.96 (0.91 to 1.00); spec = 0.86 (0.70 to 1.00) |
Summary estimates meet the predetermined criteria for a replacement and SnOUT triage test |
| VIP (vasoactive intestinal polypeptide) | 40 (1) | 19 | 4 | 1 | 16 | Sens = 0.95 (0.75 to 1.00); spec = 0.80 (0.56 to 0.94) |
Meets the criteria for a replacement and SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| CGRP (calcitonin gene‐related protein) | 40 (1) | 18 | 3 | 2 | 17 | Sens = 0.90 (0.68 to 0.99); spec = 0.85 (0.62 to 0.97) | Approaches the criteria for a replacement and SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| SP (substance P) | 40 (1) | 19 | 4 | 1 | 16 | Sens = 0.95 (0.75 to 1.00); spec = 0.80 (0.56 to 0.94) |
Meets the criteria for a replacement and SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| NPY (neuropeptide Y) | 40 (1) | 19 | 7 | 1 | 13 | Sens = 0.95 (0.75 to 1.00); spec = 0.65 (0.41 to 0.85) |
Meets the criteria for a SnOUT triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| NF (neurofilament) | 40 (1) | 19 | 18 | 1 | 2 | Sens = 0.95 (0.75 to 1.00); spec = 0.10 (0.01 to 0.32) |
Insufficient evidence to draw meaningful conclusions |
| Combined test (VIP, PGP 9.5, SP) (Combination of 3 neural markers (vasoactive intestinal polypeptide, protein gene product 9.5 and substance P) | 40 (1) | 19 | 0 | 1 | 20 | Sens = 0.95 (0.75 to 1.00); spec = 1.00 (0.83 to 1.00) |
Meets the criteria for a replacement, SnOUT and SpIN triage test; insufficient evidence to draw meaningful conclusions; promising marker for further investigation |
| 9. Tumour markers | |||||||
| CA‐125 (menstrual fluid) (cancer antigen 125 evaluated in menstrual fluid) |
104 (1) | 27 | 7 | 13 | 59 | Sens = 0.66 (0.49 to 0.80); spec = 0.89 (0.79 to 0.96) |
Insufficient evidence to draw meaningful conclusions |
aDifferent groups of proteins were discovered and evaluated in each study, hence the data were not combined in meta‐analysis. bMean estimates of 7 studies in 361 women, excluding the outlier study Leslie 2013.
Background
Target condition being diagnosed
Endometriosis
Endometriosis is an inflammatory condition associated with pelvic pain and infertility, characterised by lesions of endometrial‐like tissue outside of the uterus (Johnson 2013). Endometriotic lesions can occur at different locations, including the pelvic peritoneum and the ovary, or penetrate pelvic structures below the surface of the peritoneum as deeply infiltrating endometriosis. Each of these types of endometriosis is thought to represent a separate clinical entity but can also coexist in the same patient. Rarely, endometriotic implants can be found at more distant sites, including lung, liver, pancreas and operative scars, with consequent variations in presenting symptoms.
Endometriosis afflicts 10% of reproductive‐aged women, causing dysmenorrhoea (painful periods), dyspareunia (painful intercourse), chronic pelvic pain and infertility (Vigano 2004). The clinical presentations may vary from asymptomatic and unexplained infertility to severe dysmenorrhoea and chronic pain. These symptoms can occur with bowel or urinary symptoms, an abnormal pelvic examination or the presence of a pelvic mass; however, no symptom is specific to endometriosis. The prevalence of endometriosis in the symptomatic population is reported as 50% to 60% in women and teenage girls with pelvic pain, and in up to 50% of women with infertility (Eskenazi 1997; Goldstein 1980).
Women with endometriosis are also at an increased risk of developing several cancers and autoimmune disorders (Sinaii 2002; Somigliana 2006). The presence of disease is associated with changes in the immune response, vascularisation, neural function, the peritoneal environment and the eutopic endometrium (tissue lining the uterine cavity), suggesting that endometriosis is a systemic rather than localised condition (Giudice 2004). Endometriosis has a profound effect on psychological and social well‐being and imposes a substantial economic burden on society. Women with endometriosis may incur significant direct medical expenses from diagnostic and therapeutic surgeries, hospital admissions and fertility treatments, while indirect costs, including absenteeism and loss of productivity, compound the economic impact (Gao 2006;Simoens 2012). In the United States, the financial burden of endometriosis is about USD 12,419 per woman (Simoens 2012).
Although research has not been able to fully elucidate the pathogenesis of endometriosis, specialists commonly believe that it occurs when endometrial tissue contained within the menstrual fluid implants at an ectopic site within the pelvic cavity through retrograde flow (Sampson 1927). However, this theory does not explain the fact that only 10% of women develop endometriosis while retrograde menstruation occurs in up to 90% of women (Halme 1984). There is evidence that a variety of environmental, immunological and hormonal factors are associated with endometriosis and genetic loci that confer a risk of endometriosis, but the relative contribution of these and other causal factors is still unclear (Nyholt 2012; Vigano 2004).
Although it is impossible to time the onset of disease, on average, women have a 6‐ to 12‐year history of symptoms before obtaining a surgical diagnosis, indicative of considerable diagnostic delay (Matsuzaki 2006). Untreated endometriosis is associated with reduced quality of life and contributes to outcomes such as depression, inability to work, sexual dysfunction and missed opportunity for motherhood (Gao 2006).
Treatment of endometriosis
There is no cure for endometriosis. Treatment options include expectant management, pharmacological (hormonal) therapy and surgery (Johnson 2013). Treatment is individualised, taking into consideration a therapeutic goal (pain relief or conception) and the location of the disease. Current pharmacological therapies such as the combined oral contraceptive pill, progestogens, weak androgens and GnRH agonists and antagonists act to reduce the effect of oestrogen on endometrial tissues and suppress menstruation. These drugs can ameliorate the symptoms of dysmenorrhoea and chronic pelvic pain but are associated with side effects such as breast discomfort, irritability, androgenic symptoms and bone loss. Surgical excision of endometriotic lesions can reduce pain symptoms, but it is associated with high recurrence rates of 40% to 50% at five years postsurgery (Guo 2009). Early treatment of endometriosis improves pain levels as well as physical and psychological functioning. Furthermore, improvements in menstrual management (the use of the intrauterine system (hormonal coil) and the continuous use of the combined contraceptive pill) and fertility preservation (oocyte vitrification) raise the possibility of suppressing the progression of endometriosis and prospectively managing subfertility in endometriosis sufferers. The potential success of these preventive strategies depends on an accurate and early diagnosis. A major impediment to earlier and more efficacious treatment of this disease is diagnostic delay, due to the invasive nature of standard diagnostic tests (Dmowski 1997).
Diagnosis of endometriosis
Clinical history and pelvic examination can raise the possibility of a diagnosis of endometriosis, but the heterogeneity in clinical presentation, the high prevalence of asymptomatic endometriosis (2% to 50%) and the poor association between presenting symptoms and severity of the disease contribute to the difficulty in obtaining a reliable diagnosis based solely on presenting symptoms (Ballard 2008; Fauconnier 2005; Spaczynski 2003). Although an abnormal pelvic examination correlates with the presence of endometriosis on laparoscopy in 70% to 90% of cases (Ling 1999), there is a wide differential diagnosis for most positive physical findings. Furthermore, a normal clinical examination does not exclude endometriosis, as laparoscopically proven disease has been diagnosed in more than 50% of women with a clinically normal pelvic examination (Eskenazi 2001). A variety of tests utilising pelvic imaging, blood markers, eutopic endometrium characteristics, urinary markers or peritoneal fluid components have been suggested as diagnostic measures for endometriosis. Although large numbers of the reported markers distinguish women with and without endometriosis in small pilot studies, many do not show convincing potential as a diagnostic test when they are evaluated in larger studies by different research groups. The diagnostic value of these tests has not previously been fully systematically evaluated and summarised using Cochrane methods. Currently, there is no simple non‐invasive test for the diagnosis of endometriosis that is routinely implemented in clinical practice.
Surgical diagnostic procedures for endometriosis include laparoscopy (minimal access, or keyhole surgery) or laparotomy (open surgery via an abdominal incision). In the last several decades, laparoscopy has become an increasingly common procedure and has largely replaced traditional open surgery in patients suspected of having endometriosis (Yeung 2009). Laparoscopy has significant advantages over laparotomy, including fewer complications and shorter recovery times. Furthermore, a magnified view at laparoscopy allows better visualisation of the peritoneal cavity. Despite continuing controversy in the literature with regard to the superiority of one surgical modality over another in treating pelvic pathology, laparoscopy is the preferred technique to evaluate the pelvis and abdomen and to treat benign conditions such as ovarian endometriomas (Medeiros 2009). Surgery is currently also the only acceptable method of determining the extent and severity of endometriosis. There are several different classification systems for endometriosis (Adamson 2008; Batt 2003; Chapron 2003a; Martin 2006), but most researchers and clinicians use the revised American Society for Reproductive Medicine (rASRM) classification, which is internationally accepted as a respected tool for the objective assessment of the disease (ASRM 1997). The rASRM classification system considers the appearance, size and depth of peritoneal or ovarian implants and adhesions that are visualised during laparoscopy (Table 2) and allows uniform documentation of the extent of disease. Unfortunately, this classification system has little value in clinical practice due to the lack of correlation between laparoscopic staging, the severity of symptoms and response to treatment (Chapron 2003b; Guzick 1997; Vercellini 1996). The World Endometriosis Society has recently undertaken an endeavour to attain consensus around the optimal classification for endometriosis (Johnson 2015).
1. Staging of endometriosis, rASRM classification.
| Location of endometriosis | Extent | Depth | ||
| < 1 cm | 1‐3 cm | > 3 cm | ||
| Peritoneum | Superficial | 1 | 2 | 4 |
| Deep | 2 | 4 | 6 | |
| Ovary | R Superficial | 1 | 2 | 4 |
| Deep | 4 | 16 | 20 | |
| L Superficial | 1 | 2 | 4 | |
| Deep | 4 | 16 | 20 | |
| Posterior cul‐de‐sac obliteration | Partial | Complete | ||
| 4 | 40 | |||
| Adhesions | < 1/3 Enclosure | 1/3‐2/3 Enclosure | > 2/3 Enclosure | |
| Ovary | R Filmy | 1 | 2 | 4 |
| Dense | 4 | 8 | 16 | |
| L Filmy | 1 | 2 | 4 | |
| Dense | 4 | 8 | 16 | |
| Tube | R Filmy | 1 | 2 | 4 |
| Dense | 4a | 8a | 16 | |
| L Filmy | 1 | 2 | 4 | |
| Dense | 4a | 8a | 16 | |
Stage ·1 (Minimal) ‐ score 1‐5; Stage II (Mild) ‐ score 6‐15; Stage III (Moderate) ‐ score 16‐40; Stage IV (Severe) ‐ score >40
aIf the fimbriated end of the fallopian tube is completely enclosed, change the point assignment to 16 (ASRM 1997)
The European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group for Endometriosis stated in their diagnostic and treatment guidelines that for most forms of endometriosis, women presenting with symptoms cannot obtain a definitive diagnosis without visual inspection of the pelvis at laparoscopy as the gold standard investigation (Kennedy 2005). Currently the visual or histological identification of endometriotic tissue in the pelvic cavity during surgery is not just the best available but the only diagnostic test for endometriosis that is used routinely in clinical practice.
The disadvantages of laparoscopic surgery include (but are not limited to) the high cost, the need for general anaesthesia and the potential for adhesion formation post procedure. Laparoscopy has been associated with a 2% risk of injury to pelvic organs, a 0.001% risk of damaging a major blood vessel and a mortality rate of 0.0001% (Chapron 2003c). Only a third of women who undertake a laparoscopic procedure will receive a diagnosis of endometriosis; therefore many disease‐free women are unnecessarily exposed to surgical risk (Frishman 2006).
The validity of laparoscopy as a reference test for endometriosis has is highly dependent on the skills of the surgeon. The diagnostic accuracy of laparoscopic visualisation has been compared with histological confirmation in a sole systematic review, and it was estimated as having a 94% sensitivity and 79% specificity (Wykes 2004). Subsequent studies suggested that incorporating histological verification in the diagnosis of endometriosis may improve diagnostic accuracy (Almeida Filho 2008; Marchino 2005; Stegmann 2008), but these papers have not been systematically reviewed. The clinical significance of histological verification remains debatable, and a diagnosis based on visual findings is generally reliable as long as properly trained and experienced surgeons perform an appropriate inspection of the abdominal cavity (Redwine 2003). Furthermore, excised potential endometriotic tissues are rarely serially sectioned in clinical practice, and pathologists can miss small lesions in mild disease. Thus sampling inconsistencies are also likely to influence the accuracy of histological reporting.
Summary
A diagnostic test without the need for surgery would reduce the associated surgical risks, increase accessibility to a diagnostic test and improve treatment outcomes. The need for an accurate non‐invasive diagnostic test for endometriosis continues to encourage extensive research in the field and was endorsed at the international consensus workshop at the 10th World Congress of Endometriosis in 2008 (Rogers 2009). Although multiple markers and imaging techniques have been explored as diagnostic tests for endometriosis, none of them have been implemented routinely in clinical practice, and many have not been subject to a systematic review.
Index test(s)
This review is part of the review series on the non‐invasive diagnostic tests for endometriosis and looks at endometrial biomarkers that have been proposed for the diagnosis of endometriosis. The other reviews from this series are 'Blood biomarkers for the non‐invasive diagnosis of endometriosis', 'Urinary biomarkers for the non‐invasive diagnosis of endometriosis', 'Imaging modalities for the non‐invasive diagnosis of endometriosis' and 'Combination of the non‐invasive tests for the diagnosis of endometriosis'.
The definition of 'non‐invasive' varies between medical dictionaries but refers to a procedure that does not involve penetration of the skin or physical entrance to the body (McGraw‐Hill Dictionary of Medicine 2006; The Gale Encyclopedia of Medicine 2011). Although the endometrial tests are associated with intrauterine tissue sampling and therefore are invasive by this definition, these tests are generally considered to be non‐invasive or minimally invasive compared with surgery. For the purposes of our series, we define all tests that do not involve anaesthesia and surgery as non‐invasive. This review concentrated on studies that investigated eutopic endometrial and menstrual fluid biomarkers.
The potential advantages of using endometrial tissue/menstrual fluid samples for the diagnosis of endometriosis include their non‐ or minimally invasive nature, lower cost and increased availability when compared to surgery. These tests are more acceptable to patients and usually provide a rapid result. However, the testing is dependent on the skills of the surgeon performing the biopsy, the type of instrument used for the procedure, the time of the menstrual cycle, the time to process the sample, the reliability of laboratory techniques and the quality control protocols in place.
Researchers have identified the cellular and molecular processes to characterise ectopic endometrium and peritoneal fluid in human and animal models (D'Hooghe 2001; Hull 2008; Kao 2003). Animal (baboon) and human studies demonstrate clear differences in the eutopic endometrium in subsets of individuals with endometriosis versus normal controls, suggesting that endometriosis induces characteristic changes in eutopic endometrial tissues (Akoum 1995; Fedele 1990; Jones 2006) or that the eutopic endometrium of women who develop endometriosis is basically different from the endometrium of women who do not develop the condition (Al Jefout 2009b). The identification of the proteins and transcripts that probably account for these changes could form the basis of a diagnostic test utilising endometrial biopsy tissue. A growing body of literature on aberrant expression of genes in the endometrium of women with endometriosis supports this assumption (Brosens 2003). Proteolytic enzymes and immune cell populations have displayed a differential expression in eutopic endometrium of women with and without endometriosis (Chung 2001; Cox 2001; Klentzeris 1995). Additional studies have evaluated and eliminated glycodelin A, CYR61, annexin 1, osteopontin and aromatase P450 as potential endometrial biomarkers (Absenger 2004; Dheenadayalu 2002; Kao 2003; Kitawaki 1999b; Wei 2009). A promising biomarker for endometriosis is the immunohistochemical identification of small nerve fibres in the functional layer of the endometrium using an antibody against PGP 9.5 (protein gene product 9.5) (Al Jefout 2009a). Endometrial fluid, aspirated from the uterine cavity, may be another possible diagnostic biomarker for endometriosis (Ametzazurra 2009).
To date, a limited number of small studies with varying methodologies, laboratory techniques and types of assays have assessed endometrial tests. One large systematic review from 2011 studied endometrial differences in women with endometriosis (May 2011). The review included 32 eligible papers, 9 of which were of high quality. Six papers of high quality examined nerve fibre growth or cell cycle control in endometrial biopsies. These biomarkers showed promise as a minimally invasive form of diagnosis for endometriosis. An updated review in 2015 reviewed the literature on plasma, urine and endometrial biomarkers (Fassbender 2015). They were unable to confidently identify any modality of testing with high sensitivity or specificity. There is a current need to re‐evaluate the diagnotic test accuracy of the endometrial tests using Cochrane methods.
Clinical pathway
Women presenting with symptoms of endometriosis (dysmenorrhoea, dyspareunia, chronic pelvic pain or difficulty conceiving) are generally investigated with a pelvic ultrasound scan to exclude other pathologies, which is in line with international guidelines (ACOG 2010; Dunselman 2014; SOGC 2010). There are no other standard investigative tests, and MRI is used conservatively because of its cost. If patients seek pain management rather than conception, physicians generally initiate empirical treatment with progestogens or the combined oral contraceptive pill. Diagnostic laparoscopy is considered if empirical treatment fails or if women decline or do not tolerate empirical treatment. In women who have difficulty conceiving, laparoscopy can be undertaken before fertility treatment (particularly if severe pelvic pain or endometrioma are present) or after failed assisted reproductive technology (ART) treatments. Physicians may also diagnosis endometriosis during fertility investigations in women who have minimal or no pain symptomatology.
On average there is a delay of 6 to 12 years from onset of symptoms to definitive diagnosis at surgery. Early referral to a gynaecologist with the capability to perform diagnostic surgery is associated with a shorter time to diagnosis. Collectively, young women, women in remote and rural locations and women of lower socioeconomic status have reduced access to surgery and are less likely to obtain a prompt diagnosis of endometriosis.
Prior test(s)
Most women presenting with symptoms suggestive of endometriosis have a full history, examination and a routine gynaecological ultrasound before a decision is made to have diagnostic surgery. However, there is no consensus on whether or not a routine ultrasound or any other test should be part of a standardised approach.
Role of index test(s)
A new diagnostic test can fulfil one of three roles:
1. Replacement: replacing an existing test due to better accuracy or a similar accuracy with other advantages.
2. Triage: used as an initial step in a diagnostic pathway to identify the group of patients who need further testing with an existing test. Although ideally a triage test has a high sensitivity and specificity, it may have a lower sensitivity but higher specificity than the current test or vice versa. The triage test does not aim to improve the diagnostic accuracy of the existing test but rather to reduce the number of individuals having an unnecessary diagnostic test.
3. Add‐on: used in addition to existing testing to improve diagnostic performance (Bossuyt 2008).
Ideally a diagnostic test is expected to correctly identify all patients with a disease and to exclude all patients without that disease; in other words it should have a sensitivity and specificity of 100%. A high sensitivity indicates that there are a low number of patients who have a negative test and do have the disease (i.e. a low number of false negative results). High specificity corresponds to a low number of patients who have a positive test but do not have the disease (i.e. low false positive results). In practice, however, it is extremely rare to find a test with equally high sensitivity and specificity. An acceptable replacement test would need to have a similar or higher sensitivity and specificity than the current gold standard. In the case of laparoscopy for diagnosis of endometriosis, the only systematic review reported a sensitivity of 94% and a specificity of 79%, and we have taken this as a cut‐off for a replacement test (Wykes 2004).
The purpose of triage tests can vary depending on the clinical context and patients' priorities. One reasonable approach is to exclude the diagnosis to avoid further unnecessary and expensive diagnostic investigations. High sensitivity tests have few false negative results and act to rule conditions out (SnOUT). A negative result from a test with high sensitivity will exclude the disease with high certainty independent of the specificity. As women without disease would be assured of having a negative test, unnecessary invasive interventions can be avoided. However, a positive result has less diagnostic value, particularly when the specificity is low. We predetermined that a clinically useful 'SnOUT' triage test should have a sensitivity of 95% or more and a specificity of 50% and above. The sensitivity cut‐off for a 'SnOUT' triage test was set at 95% and above, assuming that a 5% false negative rate is statistically and clinically acceptable. The specificity cut‐off was set at 50% and above, to avoid diagnostic uncertainty in more than 50% of the population with a positive result.
An alternative approach would be to avoid a missed diagnosis. High specificity tests have few false positive results and act to rule conditions 'in' (SpIN). A positive result for a highly specific triage test indicates a high likelihood of having endometriosis. This information could be used to prioritise these patients for surgical treatment. A positive 'SpIN' test could also provide a clinical rationale to start targeted disease‐specific medical management in a patient without a surgical diagnosis, under the assumption that disease is present. Surgical management could then be reserved for cases when conservative treatment fails. This is particularly relevant in some populations where the therapeutic benefits of surgery for endometriosis have to be carefully balanced with the disadvantages (e.g. young women, women with medical conditions or pain‐free patients with a history of infertility). In this scenario we considered a sensitivity of 50% and above and a specificity of 95% and higher as suitable cut‐offs for a 'SpIN' triage test.
We evaluated combinations of tests for their potential to replace surgery (replacement test) or to improve the selection of patients for surgery (triage test) that can either rule out (SnOUT) or rule in (SpIN) the disease. Both types of triage test are clinically useful, minimising the number of unnecessary interventions. Sequential implementation of SnOUT and SpIN tests can also optimise a diagnostic algorithm (Figure 1). We did not assess any test as an add‐on test, as we sought tests that reduce the need for surgery and not tests that improve the accuracy of the currently available surgical diagnosis.
1.
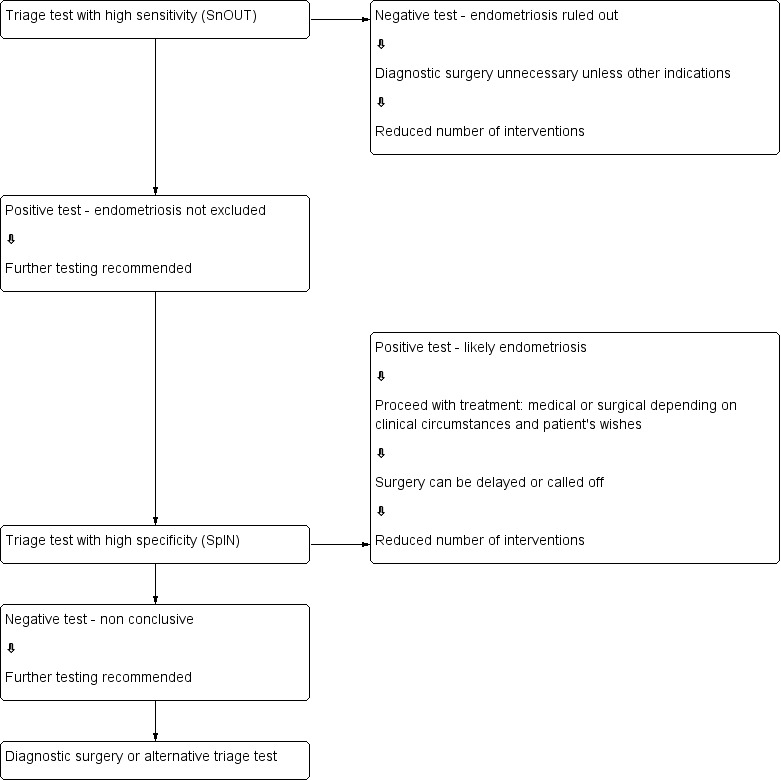
Sequential approach to non‐invasive testing of endometriosis
Alternative test(s)
There are no alternative tests for the diagnosis of endometriosis that are available in routine clinical practice.
Rationale
Many women with endometriosis suffer longstanding pelvic pain and infertility prior to a diagnosis. Surgery is the only current method of diagnosing endometriosis, but it is associated with high costs and surgical risks. A simple and reliable non‐invasive test for endometriosis with the potential to either replace laparoscopy or to triage patients in order to reduce surgery, would minimise surgical risk and reduce diagnostic delay. Endometriosis could then be detected at a less advanced stage and earlier interventions instituted. This would provide the opportunity for a preventive approach for this debilitating disease. Healthcare‐related costs of endometriosis could drop with early diagnosis and more cost‐effective and efficient treatments. Furthermore, identifying endometrial biomarkers that do not pertain to endometriotic disease would help clinicians and researchers focus on clinically relevant biomarker detection.
Objectives
Primary objectives
To determine the diagnostic accuracy of the endometrial biomarkers for pelvic endometriosis, using a surgical diagnosis as the reference standard. We evaluated the tests as replacement tests for diagnostic surgery and as triage tests to inform decisions to undertake surgery for endometriosis.
Secondary objectives
-
To investigate the influence of heterogeneity on the diagnostic accuracy of endometrial biomarkers for endometriosis. Potential sources of heterogeneity include:
participant characteristics: age (adolescents versus later reproductive years), clinical presentation (subfertility, pelvic pain, ovarian mass, asymptomatic women), stage of disease (rASRM classification system), geographic location of study;
histological confirmation in conjunction with laparoscopic visualisation compared to laparoscopic visualisation alone;
changes in technology over time: year of publication, modifications applied to conventional laboratory techniques;
methodological quality: differences in the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) evaluation (Table 3), including low versus unclear or high risk; consecutive versus non‐consecutive enrolment; and blinding of surgeons to the results of index tests;
study design (single‐gate design versus two‐gate design studies).
To assess the biomarkers that were not affected by endometriosis and hence are unlikely to discriminate between patients with and without the disease.
2. QUADAS 2‐ risk of bias assessment tool: methodological items and applicability judgement.
| Domain 1 ‐ Patient selection | |
| Description | Describe methods of patient selection; describe included patients (previous testing, presentation, intended use of index test, and setting) |
| Type of bias assessed | Selection bias, spectrum bias |
| Review question | Women of reproductive age with clinically suspected endometriosis (symptoms, clinical examination ± presence of pelvic mass), scheduled for surgical exploration of pelvic or abdominal cavity for confirmation of the diagnosis ± treatment |
| Informaton collected | Study objectives, study population, selection (inclusion and exclusion criteria), study design, clinical presentation, age, number of enrolled and number of available for analysis, setting, place and period of the study |
| Signalling question 1 | Was a consecutive or random sample of patients enrolled? |
| Yes | If a consecutive sample or a random sample of the eligible patients was included in the study |
| No | If a consecutive sample or a random sample of the eligible patients was not included in the study |
| Unclear | All the studies that did not specify enrolment as consecutive or random sample of patients were classified as 'no'; therefore none of the included studies were classified as 'unclear' |
| Signalling question 2 | Did the study avoid inappropriate exclusions? |
| Yes | If inclusion and exclusion criteria were presented and all patients with suspected endometriosis were included, with an exception for those who either had a history of medical conditions or were on medical therapy that would have potentially interfered with interpretation of index test (e.g. malignancy, pregnancy, autoimmune disorders, infectious diseases, treatment with hormonal or immunomodulator substances); refused to participate in the study; or were unfit for surgery |
| No | If the study excluded the patients based on education level, psychosocial factors, genetic testing or phenotype or excluded patients with any comorbidities commonly present in general population, including a population that could have undergone a testing for endometriosis in clinical setting (hypertension, asthma, obesity, benign gastrointestinal or renal disease, etc.) |
| Unclear | If the study did not provide clear definition of the selection (inclusion/exclusion) criteria and 'no' judgement was not applicable |
| Signalling question 3 | Was a 'two‐gate' design avoided? |
| Yes | If the study had a single set of inclusion criteria, defined by the clinical presentation (i.e. only participants in whom the target condition is suspected) ‐ a single‐gate design |
| No | If the study had more than one set of inclusion criteria in respect to clinical presentation (i.e. participants suspected of target condition and participants with alternative diagnosis in whom the target condition would not be suspected in clinical practice) ‐ a 'two‐gate' study design |
| Unclear | If it was unclear whether a 'two‐gate deign' was avoided or not |
| Risk of bias | Could the selection of patients have introduced bias? |
| Low | If 'yes' classification for all the above 3 questions |
| High | If 'no' classification for any of the above 3 questions |
| Unclear | If 'unclear' classification for any of the above 3 questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the included patients do not match the review question? |
| Low | If the study includes only clinically relevant population that would have undergone index test in real practice and includes representative form of target condition |
| High | If the study population differed from the population defined in the review question in terms of demographic features and comorbidity (e.g. studies with multiple sets of inclusion criteria with respect to clinical presentation including either healthy controls or alternative diagnosis controls that would not have undergone index test in real practice). Further, if target condition diagnosed in the study population was not representative of the entire spectrum of disease, such as limited spectrum of severity (e.g. only mild forms) or limited type of endometriosis (e.g. only DIE) |
| Unclear | If this information was unclear (e.g. severity of endometriosis was not reported) |
| Domain 2 ‐ Index test | |
| Description | Describe the index test, how it was conducted and interpreted |
| Type of bias assessed | Test review bias, clinical review bias, interobserver variation bias |
| Review question | Any test of endometrial tissue or menstrual fluid sample |
| Informaton collected | Index test name, description of positive case definition by index test as reported, threshold for positive result, examiners (number, level of expertise, blinding), interobserver variability |
| Signalling question 1 | Were the index test results interpreted without knowledge of the results of the reference standard? |
| Yes | If the operators performing/interpreting index test were unaware of the results of reference standard |
| No | If the operators performing/interpreting index test were not blinded to the results of reference standard |
| Unclear | If this information was unclear |
| Signalling question 2 | If a threshold was used, was it pre‐specified? |
| Yes | If study clearly provided a threshold for positive result, which was defined before execution/interpretation of index test |
| No | If a threshold for positive result was not provided or not defined prior to test execution |
| Unclear | If it was unclear whether a threshold was pre‐specified or not |
| Signalling question 3 | Was a menstrual cycle phase considered in interpreting the index test? |
| Yes | If all the included participants were in the same phase of menstrual cycle, if the study reported subgroup analyses per cycle phase, or if study reported the pooled estimates after impact of the cycle phase on biomarker expression was not detected |
| No | If study included participants in different phases of menstrual cycle, but effect of cycle phase on index test was not assessed |
| Unclear | If the cycle phase was not reported |
| Risk of bias | Could the conduct or interpretation of the index test have introduced bias? |
| Low | If 'yes' classification for all the above 3 questions |
| High | If 'no' classification for any of the above 3 questions |
| Unclear | If 'unclear' classification for any of the above 3 questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the index test, its conduct, or interpretation differ from the review question? |
| Low | We considered all types of eutopic endometrial biomarkers as eligible, therefore all the included studies were classified as 'low concern', unless 'unclear' judgement was applicable |
| High | We did not consider the studies where index tests other than eutopic endometrial biomarkers were included (or excluded information on other index tests reported in addition to endometrial tests) or where index test looked at other target conditions not specified in the review (e.g. studies aimed at classifying pelvic masses as benign and malignant); therefore none of the included studies was classified as 'high concern' |
| Unclear | If study did not present sufficient information on at least one of the following: laboratory method, sample handling, reagents used or experience of the test operators |
| Domain 3 ‐ Reference standard | |
| Description | Describe the reference standard, how it was conducted and interpreted |
| Type of bias assessed | Verification bias, bias in estimation of diagnostic accuracy due to inadequate reference standard |
| Review question | Target condition ‐ pelvic endometriosis, ovarian endometriosis, DIE. Reference standard ‐ visualisation of endometriosis at surgery (laparoscopy or laparotomy) with or without histological confirmation |
| Informaton collected | Target condition, prevalence of target condition in the sample, reference standard, description of positive case definition by reference test as reported, examiners (number, level of expertise, blinding) |
| Signalling question 1 | Is the reference standard likely to correctly classify the target condition? |
| Yes | If the study reported at least one of the following: surgical procedure was described in sufficient detail; criteria for positive reference standard were stated; diagnosis was confirmed by histopathology; or the procedure was performed by the team with high level of expertise in diagnosis/surgical treatment of target condition, including tertiary referral centres for endometriosis |
| No | If reference standard did not classify target condition correctly; considering the inclusion criteria, none of the studies were classified as 'no' for this item |
| Unclear | If information on execution of the reference standard, its interpretation or operators was unclear |
| Signalling question 2 | Were the reference standard results interpreted without knowledge of the results of the index tests? |
| Yes | If operators performing the reference test were unaware of the results of index test |
| No | If operators performing the reference test were aware of the results of index test |
| Unclear | If this information was unclear |
| Risk of bias | Could the reference standard, its conduct, or its interpretation have introduced bias? |
| Low | If 'yes' classification for all the above 2 questions |
| High | If 'no' classification for any of the above 2 questions |
| Unclear | If 'unclear' classification for any of the above 2 questions and 'high risk' judgement was not applicable |
| Concerns about applicability | Are there concerns that the target condition as defined by the reference standard does not match the question? |
| Low | Considering the inclusion criteria, all the studies were classified as 'low concern', as anticipated |
| High | We excluded the studies where participants did not undergo surgery for diagnosis of endometriosis, therefore none of the included studies were classified as 'high concern' |
| Unclear | Only studies were laparoscopy/laparotomy served as a reference test were included; therefore none of the included studies was classified as 'unclear concern' |
| Domain 4 ‐ Flow and timing | |
| Description | Describe any patients who did not receive the index tests or reference standard or who were excluded from the 2 × 2 table; describe the interval and any interventions between index tests (sample collection) and the reference standard |
| Type of bias assessed | Disease progression bias, bias of diagnostic performance due to missing data |
| Review question | Less than 12‐month interval between index test (sample collection) and reference standard ‐ endometriosis may progress over the time, so we had chosen an arbitrary time interval of 12 months as an acceptable time interval between the sample collection and surgical confirmation of diagnosis |
| Informaton collected | Time interval between index test (sample collection) and reference standard, withdrawals (overall number of reported and if were explained) |
| Signalling question 1 | Was there an appropriate interval between index test (sample collection) and reference standard? |
| Yes | If time interval was reported and was less than 12 months |
| No | We excluded all the studies where time interval was longer than 12 months; therefore none of the included studies were classified as 'no' for this item |
| Unclear | If time interval was not stated clearly, but authors description allowed to assume that the interval was reasonably short |
| Signalling question 2 | Did all patients receive the same reference standard? |
| Yes | If all participants underwent laparoscopy/laparotomy as a reference standard. Considering the inclusion criteria, all the studies were classified as 'yes' for this item, as anticipated |
| No | If all participants did not undergo surgery or had alternative reference standard or if only a subset of participants had surgery as reference standard, but the information on this population was not available in isolation. Considering the inclusion criteria, none of the included studies were classified as 'no' for this item |
| Unclear | If this information was unclear. Considering the inclusion criteria, none of the included studies were classified as 'unclear' for this item |
| Signalling question 3 | Were all patients included in the analysis? |
| Yes | If all the patients were included in the analysis or if the patients were excluded because they did not meet inclusion criteria prior to execution of index test or if the withdrawals were less than 5% of the enrolled population (arbitrary selected cut‐off) |
| No | If any patients were excluded from the analysis because of un interpretable results, inability to undergo either index test or reference standard, or if withdrawals were more than 5% of the enrolled population |
| Unclear | If this information was unclear |
| Risk of bias | Could the patient flow have introduced bias? |
| Low | If 'yes' classification for all the above 3 questions |
| High | If 'no' classification for any of the above 3 questions |
| Unclear | If 'unclear' classification for any of the above 3 questions and 'high risk' judgement was not applicable |
Methods
Criteria for considering studies for this review
Types of studies
Published peer‐reviewed studies that compared the results of one or several types of eutopic endometrial biomarker tests with the results obtained by surgical visualisation of lesions for the diagnosis of endometriosis.
We included the following types of studies.
Randomised controlled trials.
-
Observational studies with the following designs.
'Single‐gate design' (studies with a single set of inclusion criteria defined by clinical presentation). All participants had clinically suspected endometriosis.
'Two‐gate design' (studies where participants are sampled from distinct populations with respect to clinical presentation). The same study includes participants with a clinical suspicion of having the target condition (e.g. women with pelvic pain) and also participants in whom the target condition is not suspected (e.g. women admitted for tubal ligation). Two‐gate studies were eligible only where all cases and controls belonged to the same population with respect to the reference standard (i.e. all the participants were scheduled for laparoscopy) (Rutjes 2005).
Studies performed on prospectively collected samples, irrespective of the actual time of the test assay. The timing of sample collection relative to surgery is important because the surgical excision of endometriotic lesions could influence endometrial biomarker expression and hence bias the results. Therefore, we only included studies where the biological sample was collected before the surgical procedure, i.e. 'prospectively collected'. We considered to be eligible the studies performed on tissue bank samples collected from prospectively recruited, well‐defined populations, which prevented the omission of valuable data from adequately designed studies. The time interval between sample collection and laboratory testing may influence test outcomes, which could be dependent on sample storage conditions and the stability of each individual biomarker during storage and freeze‐thawing. This information was not readily available for most molecules, and we did not address it in this review, but we will consider it in future updates if more evidence emerges.
We did not impose limits on eligibility related to the healthcare settings where the study took place, the language of publication, the number of participants in the included studies or the number of studies that evaluated each index test.
We excluded the following types of studies.
Narrative or systematic reviews.
Studies of retrospective design where investigators collected samples after execution of the reference test.
Studies of retrospective design where investigators selected participants from retrospective review of the case notes/archived samples and where information on recruitment methods or study population was not available.
Case reports or case series.
Studies reported only in abstract form or in conference proceedings where the full text was not available. We applied this limitation after facing substantial difficulty in obtaining the information from the abstracts, which precluded a reliable assessment of eligibility and methodological quality.
Participants
Study participants included reproductive‐aged women (puberty to menopause) with suspected endometriosis based on clinical symptoms, pelvic examination or both, who undertook the index test as well as the reference standard.
Participants came from populations of women undergoing abdominal surgery for the following indications.
Clinically suspected endometriosis (pelvic pain, infertility, abnormal pelvic examination, or a combination of the above).
Ovarian mass, regardless of symptoms.
A mixed group consisting of women with suspected endometriosis/ovarian mass or women with other benign gynaecological conditions (e.g. surgical sterilisation, fibroid uterus, etc).
Asymptomatic women who have an incidental finding of endometriosis at surgery performed for another indication.
Articles that included participants of postmenopausal age were eligible when the data for the reproductive age group was available in isolation. We excluded studies with participants that clearly would not undergo the index test in the relevant clinical situation or would not benefit from the test (e.g. women with ectopic pregnancies or acute pelvic inflammatory disease). We also excluded publications that only analysed participants with a positive index test or reference standard and did not provide data for the whole cohort.
Index tests
Any type of eutopic endometrial biomarker (including biomarkers in menstrual fluid), which were assessed either separately or in combination with other endometrial tests. We have classified the assessed index tests according to the type of biomarker, presented these categories in Table 4. To assist readers in the search for a specific biomarker, we include an index of all biomarkers with biological annotation in Appendix 1.
3. Endometrial biomarkers evaluated in this review.
| Biomarker | Diagnostic potential for endometriosis | |
| 1 | Angiogenesis and growth factors and their receptors | |
| A | EGF (epidermal growth factor) | Expression not altered in endometriosis |
| B | FGF‐2 (fibroblast growth factor‐2) | Expression not altered in endometriosis |
| C | Glycodelin A (PP14 or PAEP) (placental protein 14 or progestogen‐associated endometrial protein) | Expression not altered in endometriosis |
| D | PDGF (platelet derived growth factor) | Expression not altered in endometriosis |
| E | PIGF (placental growth factor) | Expression not altered in endometriosis |
| F | PKR1 (prokineticin receptor 1), EG‐VEGF receptor | Expression not altered in endometriosis |
| G | PKR2 (prokineticin receptor 2), EG‐VEGF receptor | Expression not altered in endometriosis |
| H | PROK‐1 (prokineticin 1) | Diagnostic accuracy assessed |
| I | TSP‐1 (thrombospondin‐1) | Expression not altered in endometriosis |
| J | TYMP (thymidine phosphorylase) | Expression not altered in endometriosis |
| K | VEGF (vascular endothelial growth factor) | Expression not altered in endometriosis |
| 2 | Apoptosis markers and regulators | |
| A | Bax (BCL2‐associated X protein) | Expression not altered in endometriosis |
| B | Bcl‐xL (B‐cell lymphoma‐extra large, or BCL2‐like 1 isoform 1) | Expression not altered in endometriosis |
| C | Bcl‐xL:Bcl‐xS ratio (ratio B‐cell lymphoma‐extra large/B‐cell lymphoma‐extra small) | Expression not altered in endometriosis |
| 3 | Cell adhesion molecules and other matrix‐related proteins | |
| A | α2β1 integrin | Expression not altered in endometriosis |
| B | α3β1 integrin | Diagnostic accuracy assessed; expression not altered in endometriosis in some studies |
| C | α4β1 integrin | Diagnostic accuracy assessed; expression not altered in endometriosis in some studies |
| D | α5β1 integrin | Expression not altered in endometriosis |
| E | α6β1 integrin | Expression not altered in endometriosis |
| F | αVβ3 integrin | Expression not altered in endometriosis |
| G | αVβ5 integrins | Expression not altered in endometriosis |
| H | αVβ6 integrins | Expression not altered in endometriosis |
| I | β1 integrin | Diagnostic accuracy assessed |
| J | Depolarised α6 integrin | Diagnostic accuracy assessed |
| K | ICAM‐1 (intercellular adhesion molecule‐1) or sICAM‐1 (soluble form of intercellular adhesion molecule‐1) | Expression not altered in endometriosis |
| L | E‐cadherin | Expression not altered in endometriosis |
| M | LAMA5 (laminin subunit alpha‐5) | Expression not altered in endometriosis |
| N | LFA‐3 (CD58) (leukocyte function associated molecule‐3) | Expression not altered in endometriosis |
| O | MMP‐1 (matrix metalloproteinase‐1) | Expression not altered in endometriosis |
| P | MMP‐9 (matrix metalloproteinase‐9) | Expression not altered in endometriosis |
| Q | OPN (osteopontin) | Expression not altered in endometriosis |
| R | PAI‐1/‐2/‐3 (plasminogen activator inhibitors 1/2/3) | Expression not altered in endometriosis |
| S | PAs: tPA, uPA (plasminogen activators: tissue‐type PA, urokinase‐type PA) | Expression not altered in endometriosis |
| T | TIMP‐1 (tissue inhibitor of metalloproteinases) | Expression not altered in endometriosis |
| U | VCAM‐1 (CD106) (vascular cell adhesion molecule‐1) | Expression not altered in endometriosis |
| 4 | Cell cycle regulatory molecules | |
| A | Cyclin B1 | Expression not altered in endometriosis |
| B | Cdc2 (cyclin dependent kinase‐2) | Expression not altered in endometriosis |
| 3 | CPlk1 (polo‐like kinase‐1) | Expression not altered in endometriosis |
| 5 | Cell proliferation markers | |
| A | Ki‐67 (antigen KI‐67 or MKI67, marker of cellular proliferation) | Expression not altered in endometriosis |
| B | BW 495/36, endometrial epithelial marker | Expression not altered in endometriosis |
| 6 | Cytoskeleton molecules | |
| A | Cytokeratin 18 | Expression not altered in endometriosis |
| B | CK19 or CYFRA 21‐1 (cytokeratin 19) | Expression not altered in endometriosis |
| C | Vimentin | Expression not altered in endometriosis |
| 7 | DNA‐repair and telomer maintenance molecules | |
| A | hTERT (human telomerase reverse transcriptase) | Diagnostic accuracy assessed |
| B | Telomerase activity | Expression not altered in endometriosis |
| 8 | High throughput markers | |
| A | Endometrial proteome | Diagnostic accuracy assessed |
| B | Mitochondrial proteome | Diagnostic accuracy assessed |
| C | mRNAome (mRNA micro‐array) | Expression not altered in endometriosis |
| 9 | Hormonal markers | |
| A | 17βHSD2 (17‐β hydroxysteroid dehydrogenase type 2) | Diagnostic accuracy assessed |
| B | CYP19 (aromatase cytochrome P450) | Diagnostic accuracy assessed |
| C | ER‐α (oestrogen receptor‐alpha) | Diagnostic accuracy assessed |
| D | ER‐β (oestrogen receptor‐beta) | Diagnostic accuracy assessed |
| E | EST (oestrogen sulphotransferase) | Expression not altered in endometriosis |
| F | LGR7 (leucine‐rich G protein‐coupled receptor 7), relaxin receptor | Expression not altered in endometriosis |
| G | Relaxin | Expression not altered in endometriosis |
| 10 | Immune system and inflammatory markers | |
| A | Cytokines | |
| i | LIF (leukaemia‐inhibitory factor) | Expression not altered in endometriosis |
| ii | TNF‐α (tumour necrosis factor alpha) | Expression not altered in endometriosis |
| B | Immune cells: peripheral blood mononuclear cells (PBMC) | |
| i | Lymphocytes | Expression not altered in endometriosis |
| ii | B‐lymphocytes | Expression not altered in endometriosis |
| iii | Monocytes/macrophages | Expression not altered in endometriosis |
| iv | NK (natural killer cells) | Expression not altered in endometriosis |
| v | T‐lymphocytes | Expression not altered in endometriosis |
| C | Interleukins | |
| i | IL‐1β | Expression not altered in endometriosis |
| ii | IL‐11 | Expression not altered in endometriosis |
| iii | IL‐1R1 (interleukin‐1 receptor type II) | Expression not altered in endometriosis |
| iv | IL‐1R2 (interleukin‐1 receptor type II) | Diagnostic accuracy assessed |
| D | Other immune/inflammatory markers | |
| i | MPO (myeloperoxidase) | Expression not altered in endometriosis |
| ii | NAG (N‐acetyl‐b‐D‐Glucosaminidase) | Expression not altered in endometriosis |
| 11 | Mediators of prostaglandin biosynthesis | |
| A | Akr1B1 mRNA (aldoketoreductase ‐1B1, PGF2a synthase) | Expression not altered in endometriosis |
| B | Akr1C3 mRNA (aldoketoreductase ‐1C3, PGF2a synthase) | Expression not altered in endometriosis |
| C | Cox‐1 mRNA (cyclo‐oxygenase‐1) | Expression not altered in endometriosis |
| D | 15‐PGDH mRNA (15‐hydroxyprostaglandin dehydrogenase) | Expression not altered in endometriosis |
| E | cPGES mRNA (cytosolic PGE2 synthase) | Expression not altered in endometriosis |
| 12 | Myogenic markers (markers of smooth muscle differentiation) | |
| A | Caldesmon (calmodulin binding protein) | Diagnostic accuracy assessed |
| B | CALD1 (gene encoding for caldesmon) | Diagnostic accuracy assessed |
| 13 | Nerve sheath and nerve growth markers | |
| A | CGRP (calcitonin gene‐related protein) | Diagnostic accuracy assessed |
| B | NF (neurofilament) | Diagnostic accuracy assessed, but expression not altered in endometriosis |
| C | NPY (neuropeptide Y) | Diagnostic accuracy assessed |
| D | PGP 9.5 (protein gene product 9.5) | Diagnostic accuracy assessed, expression not altered in endometriosis in some studies |
| E | SP (substance P) | Diagnostic accuracy assessed |
| F | VIP (vasoactive intestinal polypeptide) | Diagnostic accuracy assessed |
| 14 | Other peptides and proteins | |
| A | hBD‐2 (human b‐defensin‐2) | Expression not altered in endometriosis |
| 15 | Transcription factors and signalling molecules | |
| A | AKT1 (RAC‐alpha serine/threonine‐protein kinase) | Expression not altered in endometriosis |
| B | JAG1 (jagged‐1 protein) | Expression not altered in endometriosis |
| 16 | Tumour markers | |
| A | CA‐125 (cancer antigen 125) in menstrual fluid | Diagnostic accuracy assessed |
We included index tests performed on the whole tissue sample or separate endometrial compartments and reported them the same way as presented by the authors (e.g. separate testing of glandular epithelium, stromal cells or mixed cell sample). We included tests performed in one or several phases of the menstrual cycle.
The combined evaluations of endometrial biomarkers with other methods for diagnosing endometriosis (e.g. pelvic examination or blood tests) are beyond the scope of this review and are presented separately in another review: 'Combined tests for the non‐invasive diagnosis of endometriosis'. We excluded the studies that solely assessed specific technical aspects, qualitative descriptions of lesion appearance or interobserver variability of the index tests without reporting the data on diagnostic performance. We only considered studies in which the evaluated biomarker(s) showed differential expression between the groups of women with and without endometriosis if the data were reported in sufficient detail for the construction of 2 × 2 contingency tables. We included studies in which the expression levels of the index test did not significantly differ between the groups and where contingency tables were not available, as long as the inclusion criteria were met otherwise. We considered these to be studies reporting unchanged biomarker expression in the presence of endometriosis, and we presented them in the descriptive portion of the review. Thus, we evaluated the adequately designed studies that identified biomarkers without diagnostic value, as they provide information that is likely to guide future research towards other more clinically useful biomarkers. This methodology also identified biomarkers that presented conflicting findings associated with endometriosis in some but not other publications.
We considered the diagnostic performance of an index test to be high when the test reached the criteria for a replacement test (sensitivity at or above 94% with specificity at or above 79%) or triage test (sensitivity at or above 95% with specificity at or above 50% or vice versa), or approached these criteria (diagnostic estimates within 5% of the set thresholds). We considered all other diagnostic estimates to be low.
Target conditions
Pelvic endometriosis, defined as endometrial tissue located in the pelvic cavity: involving any of the pelvic organs, peritoneum and pouch of Douglas.
We assessed three types of pelvic endometriosis.
Peritoneal endometriosis, defined as endometrial deposits detected on peritoneum covering pelvic organs, pelvic side walls or pouch of Douglas.
Ovarian endometriosis (endometrioma), defined as an ovarian cyst lined by endometrial tissue, appearing as an ovarian mass of varying size.
Deep infiltrating endometriosis (DIE), defined as subperitoneal infiltration of endometrial implants, i.e. when the endometriotic implants penetrate the retroperitoneal space at a distance of 5 mm or more (Koninckx 1991). DIE may be present in multiple locations, involving either the anterior or posterior pelvic compartments, or both.
We did not include certain rare types of endometriosis such as extrapelvic, bladder and ureteric endometriosis because the majority were reported in case reports or case series, and laparoscopy or laparotomy are not reliable reference standards for these conditions.
We excluded the studies where diagnosis of endometriosis was not the primary outcome (e.g. malignant versus benign masses or normal versus abnormal pelvis) and separate data for endometriosis was not available.
We did include studies that recruited selected populations of women with endometriosis (i.e. those with specific rASRM stages), because there is a poor correlation between the rASRM classification and infertility or pain symptoms. Exclusion of these studies could result in the loss of potentially important diagnostic information from otherwise eligible publications. Where possible, we addressed the impact of these studies in the assessment of heterogeneity. When a study analysed a large population with a wide spectrum of endometriosis and additionally reported a subgroup analysis of the different stages of disease severity, we only considered estimates for the entire population. This is because a subgroup analysis would not directly address the review question regarding the clinical utility of the biomarker in disease detection.
Reference standards
The reference standard was visualisation of endometriosis at surgery (laparoscopy or laparotomy) with or without histological confirmation, as this is currently the best available test for endometriosis. If reported, we reviewed information regarding the inter‐ and intraobserver correlation of the reference standard.
We only included studies in which the reference test was performed within 12 months of the sample collection, on the assumption that disease status could change within a period of one year or longer, either naturally or as a result of treatment. We excluded studies in which the participants did not undergo the reference standard or where the findings of the index test formed the basis of selection for undertaking the reference standard, as this was likely to distort an assessment of the diagnostic value of the index test.
Summary of inclusion and exclusion criteria
Inclusion criteria
-
Types of studies
Published and peer‐reviewed
RCTs
-
Observational designs, including:
single‐gate design (single set of inclusion criteria defined by clinical presentation): all the participants had clinically suspected endometriosis;
two‐gate design (two sets of inclusion criteria with respect to clinical presentation and one set of inclusion criteria with respect to reference standard): the participants with or without a clinical suspicion of endometriosis scheduled for abdominal surgery;
Published in any language
Performed in any healthcare setting
Any sample size
Prospectively recruited participants (consecutive or non‐consecutive enrolment)
-
Participants
Reproductive‐aged women
-
Clinically suspected endometriosis, including:
women who underwent abdominal surgery for other benign gynaecological conditions and had a surgical assessment for presence/absence of endometriosis;
asymptomatic women who have an incidental finding of endometriosis at surgery performed for another indication;
Undertook both the index test and reference standard
-
Index tests
One or several types of eutopic endometrial biomarkers (including biomarkers in menstrual fluid)
Data reported in sufficient detail for the construction of 2 × 2 tables for the tests that showed differential expression between the groups
-
Target condition
Pelvic endometriosis: peritoneal endometriosis, ovarian endometrioma, DIE or combinations of the above
-
Reference standard
Surgical visualisation of lesions for the diagnosis of endometriosis (laparoscopy or laparotomy) with or without histological verification
Performed within 12 months of the endometrial sample collection
Exclusion criteria
-
Types of studies
Narrative or systematic reviews
Retrospective design where the index test was performed after execution of reference test or participants were selected from retrospective review of the case notes
Prospectively collected samples that were selected from the archived material, but where information on the study population or the selection process was unclear
Case reports or case series
Conference proceedings
-
Participants
Included cohort was not representative of the target population that would benefit from the test (e.g. women with known genital tract malignancy, ectopic pregnancies or acute pelvic inflammatory disease)
Study included participants of postmenopausal age, where data for the reproductive age group were not available in isolation
Only participants with positive index test or positive reference standard were included in the analysis;
-
Index tests
Endometrial biomarkers presented in combination with other diagnostic tests for endometriosis and where separate information for endometrial biomarkers was not available
Study presented only specific technical aspects of an index test or data on interobserver variability
Study presented only qualitative description of the tissue samples or focused on the biological events, rather than diagnostic performance of the test
-
Target condition
Endometriosis was not the primary outcome of the trial (e.g. malignant versus benign masses or normal versus abnormal pelvis)
Atypical, rare sites of endometriosis
-
Reference standard
Endometriosis was not the primary outcome of the trial (e.g. malignant versus benign masses or normal versus abnormal pelvis)
Reference standard performed only in a subset of the study or control group
Findings of the index test formed the basis of selection for the reference standard
Search methods for identification of studies
We developed the search strategy in collaboration with the Trials Search Coordinator of the Gynaecology and Fertility Review Group, following recommendations of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (De Vet 2008). We did not limit the searches to particular types of study design or impose language or publication date restrictions. The search strategy incorporated words in the title, abstract, text words across the record and the medical subject headings (MeSH). We initially created the search for one broad review looking at all diagnostic markers for endometriosis, but due to complexity, the review team split the originally planned review into five separate reviews. We designed two separate search strategies: one for all the biomarkers‐based tests, and another for the imaging tests; we used the former in this review. We performed all searches from database inception to April ‐ July 2015. We present the search strategies for each database and the number of hits per search in Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6. The summary of the results is presented in Results of the search.
Electronic searches
We searched the following databases to identify the published articles that assessed the diagnostic value of endometrial biomarkers for endometriosis.
CENTRAL (2015, July).
MEDLINE (inception to May 2015).
EMBASE (inception to May 2015).
CINAHL (inception to April 2015).
PsycINFO (inception to April 2015).
Web of Science (inception to April 2015).
LILACS (inception to April 2015).
OAIster (inception to April 2015).
TRIP (inception to April 2015).
-
Databases of the trial registers.
ClinicalTrials.gov (inception to April 2015).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (inception to April 2015).
-
Databases to identify reviews and guidelines as sources of references to potentially relevant studies.
MEDION (inception to January 2014, the last available date).
DARE (inception to April 2015).
PubMed, a 'Systematic Review' search under the 'Clinical Queries' link (inception to April 2015).
-
Searches for papers recently published and not yet indexed in the major databases.
PubMed (simple search for the 6 months to April 2015).
Searching other resources
We handsearched the reference list of all relevant publications (retrieved full texts of the key articles and identified reviews).
We abandoned an initial attempt to locate the grey literature (unpublished studies and conference proceedings), as we faced substantial difficulty in obtaining full‐text publications or further details of studies reported in an abstract form.
Data collection and analysis
Selection of studies
Two authors of this review (DG, VN) and four other authors from the other reviews in this series (Deepika Arora, Emily Liu, Lucy Prentice and Rabia Shaikh) scanned the titles of studies identified by our search to remove any clearly irrelevant articles. We reviewed the titles and abstracts of the remaining studies to select potentially relevant publications. We then divided the relevant articles into four categories of endometriosis biomarkers: serum, endometrial, urinary and combined tests. Two out of three review authors (DG, LM or VN) independently reviewed the full‐text versions of each article selected by title and abstract and assessed them for eligibility based on the considerations listed in Criteria for considering studies for this review. A single failed eligibility criterion was sufficient to exclude a study from the review.
The review authors who assessed the relevance of the studies and eligibility for inclusion were not blind to the information about each article, including the publishing journal, the names of authors, the institution and the results. We resolved any disagreements by discussion and, if necessary, with an additional reviewer (the late Professor Ali Akoum), an expert in the field and in methodological aspects of systematic reviews.
When papers updated previous publications and were performed on the same study population at different recruitment points, we used the most complete data set that superseded previous publications to avoid double counting participants or studies. We directly contacted authors to retrieve missing data needed to clarify study eligibility. When we found potentially relevant studies in languages other than English, we had it translated. For excluded studies, we documented the reasons for exclusion and details of which criteria were not met. We present the characteristics of included and excluded studies in Characteristics of included studies and Characteristics of excluded studies, respectively.
Data extraction and management
Two out of three review authors (DG, LM or VN) independently extracted data from each eligible study, resolving disagreements through an additional reviewer (the late Professor Ali Akoum). If required, we contacted the study investigators to resolve questions regarding the data.
To collect details from included studies, we designed a specific data extraction form for this review and piloted it on three studies of diagnostic accuracy tests for endometriosis. The following information was recorded for each study.
General information and study design: first author, year of publication, country, language, setting, objectives, inclusion/exclusion criteria, type of enrolment.
Characteristics of the study participants: age, symptoms/history/previous tests, type of target condition and its prevalence in the study population, number of participants enrolled and available for analysis, reasons for withdrawal.
Features of the index test and reference standard: type; diagnostic criteria; number and experience of the operators; blinding of the operators to other tests, clinical data or both; interobserver variability; time interval between index test and reference standard.
The reported number of true positives (TP), false negatives (FN), true negatives (TN) and false positives (FP) was used to construct a 2 × 2 table for each index test. If studies did not report these values, we attempted to reconstruct the 2 × 2 tables from the diagnostic estimates presented in the article.
We extracted data into Review Manager (RevMan) software, which was used to graphically display the quality assessment, the diagnostic estimates data and the descriptive analyses (RevMan 2014).
Assessment of methodological quality
We used QUADAS‐2, a modified version of the QUADAS tool, to assess the quality of each included study (Whiting 2011).
We present the review‐specific QUADAS‐2 tool and explanatory document in Table 3. For each paper, we assigned a 'low', 'high' or 'unclear' risk of bias for four domains and assessed concerns about applicability in three domains. We considered studies as having low methodological quality when classified as having high or unclear risk of bias and raising high concerns on applicability at least in one domain. Two of the three reviewers (DG, LM or VN) independently assessed each included study for quality, settling disagreements by consensus. Two review authors (DG, LM) independently piloted the topic‐specific tool to rate four of the included studies with a high level of agreement. Modifications specific to the endometrial biomarkers review were made to the signalling questions of the original QUADAS‐2 tool.
Domain 1: We rephrased the original signalling question 'Was a case‐control design avoided?' as 'Was a two‐gate design avoided?'. The diagnostic studies are cross‐sectional in nature, aiming to compare the result of an index test with the result of the reference standard in the same group of participants. In these studies the parameters are measured at a single point of time and the groups are classified by the outcome of the reference standard, albeit the analysis is performed retrospectively. Therefore, the terminology 'cohort' and 'case‐control' is less informative for diagnostic test trials than for epidemiological studies, and we substituted it for 'single‐gate' and 'two‐gate' designs. We included this question because a two‐gate design has more potential to introduce selection bias.
Domain 2: We introduced an additional signalling question, 'Was the phase of the menstrual cycle considered in interpreting the index test?' to assess bias in the interpretation of the test results. Endometrium is a menstrual cycle‐dependent tissue and is a sensitive target for steroid sex hormones, which can result in differential expression of biomarkers at different cycle phases.
The assessment of methodological quality was undertaken for each domain, but we did not calculate a summary score to estimate the overall quality of studies (Whiting 2005).
Statistical analysis and data synthesis
We entered the extracted data into RevMan 2014 to produce forest plots of sensitivity and specificity, and to plot study‐specific estimates of sensitivity and specificity in the receiver operating characteristic (ROC) space for each index test. We investigated the diagnostic performance of each test and visually explored between‐study variation in performance of each index test and its relationship to patient characteristics, study design and study quality factors. In case of two or more tests evaluated in the same cohort, we included them as separate data sets, since the unit of analysis was the test result, not the patient.
For studies that reported the subgroup analyses per phase of menstrual cycle, we applied judgement to what was relevant to present. For instance, we presented only pooled estimates where available when there was no statistically significant difference in biomarker expression between the cycle phases. Alternatively, where putative biomarkers demonstrated cycle‐dependent expression or were noted to be modulated by ovarian hormones, we reported the test performance either for several time points across the menstrual cycle or for the most distinctive phase.
We estimated the expected operating point (mean sensitivity and specificity) and corresponding 95% confidence region using the bivariate logistic normal random‐effects model for all meta‐analyses with four studies or more. When the number of studies was fewer than five, we did not attempt to estimate the covariance and reported a zero. To estimate the performance of the other tests in meta‐analyses with a small number of studies (two or three), we performed fixed effect meta‐analysis of sensitivity and specificity (summary sensitivity and specificity), in the absence of substantial heterogeneity. We performed the meta‐analyses using SAS NLMIXED software (Cary, NC: SAS Institute Inc). We entered results from SAS into RevMan to provide plots of the mean or summary point(s) and confidence region(s), superimposed on the study specific estimates of sensitivity and specificity.
We assessed the comparative accuracy of index tests in two ways. In direct, fully paired comparisons where all the study participants received more than one index test as well as the reference standard, we plotted the estimates in RevMan. If meta‐analysis was possible, we used test‐level covariates in the bivariate logit normal model to identify statistically significant differences. Otherwise we reported the available comparative data in a narrative way and illustrated it using forest and ROC plots.
When judging test performance against the predetermined diagnostic criteria, we considered the point estimates of sensitivity and specificity as the most informative presentation of test performance. We acknowledge that tests with point estimates that did not reach the predetermined criteria, but with confidence intervals (CIs) that contained values above the threshold, could have diagnostic value. Furthermore, tests with point estimates that reached the criteria but with CIs containing values below the threshold could have an overestimated diagnostic value. If we use the range of the CIs rather than the point estimates of the data, the predetermined cut‐off becomes meaningless. Therefore we did not consider CIs in qualifying the test performance but utilised this information in interpreting the reliability of the obtained data.
Dealing with missing data
We defined missing data as any information on the study population, index tests or reference standard that were not available from the publication and that were required to determine the eligibility of the study for inclusion, assess the methodological quality, or construct the results table. If we identified missing data, we contacted the authors in an attempt to obtain them. If missing data prevented a clear judgment regarding applicability for inclusion or the construction of accurate 2 × 2 tables and the data were unavailable from the primary investigators (for example we were unable to locate the contact details of the authors, there was no reply from the authors or the authors replied that the requested information was unavailable), we excluded the study from the review.
Investigations of heterogeneity
We initially assessed heterogeneity by visually examining the forest plots of sensitivities and specificities and the ROC plots for each index test. We describe the potential sources of heterogeneity in the Secondary objectives. For diagnostic tests where there were more than 10 eligible studies, we initially planned to formally explore heterogeneity by using study level covariates, but we were unable to do so because of the small numbers of studies in each group.
Sensitivity analyses
We planned to conduct sensitivity analyses to assess the impact of methodological quality of included studies on the results of meta‐analysis if sufficient data were available. We defined low quality studies as having a high risk of bias for one or more QUADAS‐2 domains. We also planned to assess the sensitivity of results to the inclusion and exclusion of outlying studies in all analyses and planned to use the 'leave‐one‐out' procedure (Higgins 2008) to assess the impact of each study on the meta‐analysis results (leading study effect). However, we refrained from doing so because of the small number of studies for most analyses. It is important to use caution when interpreting small meta‐analyses (few studies) with a limited total sample size.
Assessment of reporting bias
A comprehensive search of multiple sources for eligible studies, a search of trial registers and no language restrictions minimised the risk of reporting bias. However, publication bias generally arises when studies have a higher chance of being published if their results are positive. Therefore, we initially searched and evaluated unpublished and published study databases and conference proceedings. During the process of qualifying the studies for inclusion in this review, we faced substantial difficulty in obtaining full‐text publications or further details of studies published in an abstract form. This precluded a reliable assessment of eligibility and methodological quality, and we decided not to include these publication sources in the review.
Results
Results of the search
The literature search identified 33,438 references from: CENTRAL (N = 226), MEDLINE (n = 10,328), EMBASE (n = 10,313), CINAHL (n = 1,131), PsycINFO (n = 174), Web of Science (n = 7,425), LILACS (n = 420), OAIster (n = 446), Trip (n = 1,648), Trial registers for ongoing and registered trials (n = 523), MEDION (n = 2), DARE (n = 99), PubMed, a ‘Systematic Review’ search (n = 418) and simple search PubMed (n = 267). These databases were searched from inception to 20 April ‐ 31 July 2015. We present the flow of the selection process in Figure 2. We screened titles to exclude duplicates (N = 9312) and clearly irrelevant studies (N = 21,534), and we eliminated another 2224 references after reading the abstracts because they did not address the research question or clearly did not meet the inclusion criteria. We retrieved the full texts of the remaining 368 references and assessed them for eligibility. We needed author clarification on data from 35 studies, and we had 33 non‐English publications translated. Ultimately, 54 studies were eligible according to the inclusion criteria and provided data for the review, while 314 studies were ineligible and excluded.
2.
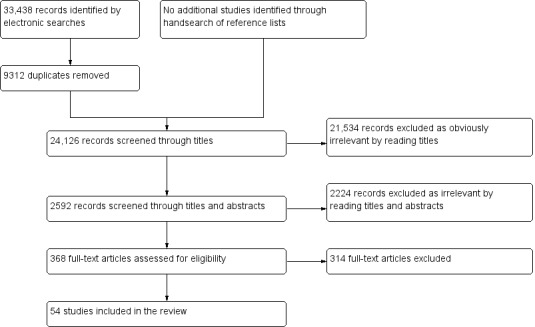
Flow of the studies identified in literature search for systematic review on endometrial biomarkers for a non‐invasive diagnosis of endometriosis.
Basic features of included studies
We present the details of the included studies in Characteristics of included studies. The 54 eligible studies included 2729 participants, with a median of 49 women per study (range 16 to 104). Twenty‐six studies took place in Europe, 15 in Asia, 6 in South America, 3 in North America, 3 in Australia, and 1 in Africa. Fifty‐three of the included studies took place at university hospitals, of which nine were tertiary referral centres for endometriosis. The earliest article was published in 1990; 50 articles were published after 2000, and 17 studies were published after 2010. All the included studies evaluated women of reproductive age.
While a number of research groups explored more than one marker, some authors reported several estimates for the same biomarker at different menstrual cycle phases or in different endometrial compartments, with each estimation considered a separate test. Twenty‐seven studies evaluated the diagnostic accuracy of endometrial biomarkers. An additional 31 methodologically eligible studies examined biomarkers that demonstrated no differential expression in endometriosis, so we did not evaluate the diagnostic test accuracy of these biomarkers. Four studies assessed diagnostic accuracy in only some of the investigated biomarkers.
Twenty‐five studies had a 'single‐gate design', 27 studies had a 'two‐gate design', and 2 studies presented insufficient information to determine which study design they used. Laparoscopy was the predominant surgical modality, whereas laparotomy was co‐utilised in 52% (28/54) of the studies. Fifty‐four per cent (29/54) of the included studies used histopathology to confirm the surgical diagnosis. The reported prevalence of endometriosis varied, ranging from 30% to 77%. Seven studies included only participants with minimal‐mild endometriosis (rASRM stage I‐II), eight studies included participants with moderate‐severe endometriosis (rASRM stage III‐IV), and in nine studies the information on the severity of the disease was not available.
Basic features of excluded studies
We describe the excluded studies in Characteristics of excluded studies. After a full‐text assessment, we excluded 314 publications. Of these 314, 165 studies contained insufficient diagnostic accuracy information to construct 2 × 2 contingency tables. We excluded an additional 84 studies that not adequately describe methods or population; 10 of these studies used archived samples with poor population definition. In 32 studies, the index test was outside the inclusion criteria. Many articles in this excluded group were comparisons between ectopic endometrium in cases versus endometrium in controls (N = 16), or they presented data on physiological events of endometrium (N = 22) without direct measurement or comparison of biomarker expression between the groups. We excluded seven papers because they enrolled a wide age group, pregnant women or women with genital tract malignancies, and we could not independently assess reproductive‐aged women without these conditions. Another eight studies failed to meet eligibility criteria because they had an overlapping cohort with another updated included paper. Fourteen excluded studies used a reference standard other than abdominal surgery or did not provide data on surgical diagnosis. In four of the excluded studies, the target condition was outside inclusion criteria, reporting data for benign versus malignant masses or normal versus abnormal pelvis, with no separate data for endometriosis. We excluded one study because the time interval between index test and surgery exceeded 12 months and another because we were not able to locate the full text. One publication was retracted, one paper was a review article and one was a case report.
Methodological quality of included studies
We show the quality of the included studies in the QUADAS‐2 results summary (Figure 3; Figure 4). Overall, the studies were of poor methodological quality, and there were only two studies (Al‐Jefout 2009 and Leslie 2013) with a low risk of bias in every domain assessed.
3.
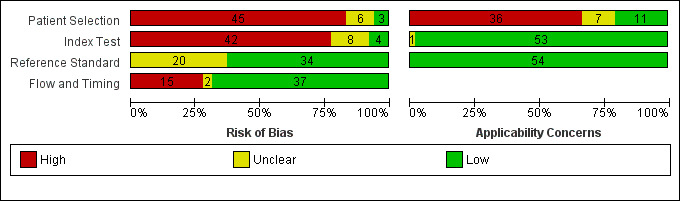
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
4.
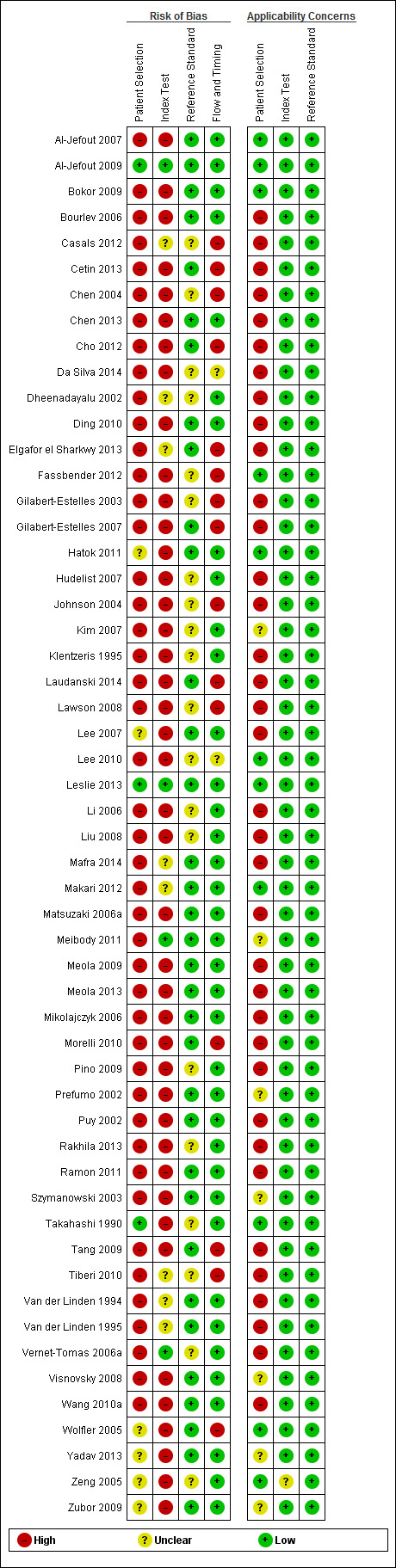
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Methodological quality assessment for the patient selection
Forty‐five studies presented a high risk of patient selection bias (Al‐Jefout 2007; Bokor 2009; Bourlev 2006; Casals 2012; Cetin 2013; Chen 2004; Chen 2013; Cho 2012; Da Silva 2014;Dheenadayalu 2002; Ding 2010; Elgafor el Sharkwy 2013; Fassbender 2012; Gilabert‐Estelles 2003; Gilabert‐Estelles 2007; Hudelist 2007; Johnson 2004; Kim 2007; Klentzeris 1995; Laudanski 2014; Lawson 2008; Lee 2010; Li 2006; Liu 2008; Mafra 2014; Makari 2012; Matsuzaki 2006a; Meibody 2011; Meola 2009; Meola 2013; Mikolajczyk 2006; Morelli 2010; Pino 2009; Prefumo 2002; Puy 2002; Rakhila 2013; Ramon 2011; Szymanowski 2003; Tang 2009; Tiberi 2010; Van der Linden 1994; Van der Linden 1995; Vernet‐Tomas 2006a; Visnovsky 2008; Wang 2010a), and six studies demonstrated an unclear risk (Hatok 2011; Lee 2007; Wolfler 2005; Yadav 2013; Zeng 2005; Zubor 2009). Non‐consecutive or non‐random patient selection, utilisation of a two‐gate design for patient selection, the absence of a clear definition of inclusion/exclusion criteria and use of a highly selected group of women were the main reasons for a high risk assessment of bias. Three studies demonstrated a low risk of bias (Al‐Jefout 2009; Leslie 2013; Takahashi 1990), as they enrolled a consecutive group of women, had clearly defined selection criteria, utilised a single‐gate design and included a wide spectrum of endometriosis.
Thirty‐six studies presented a high concern for patient selection applicability (Bourlev 2006; Casals 2012; Cetin 2013; Chen 2004; Chen 2013; Cho 2012; Da Silva 2014; Dheenadayalu 2002; Ding 2010; Elgafor el Sharkwy 2013; Gilabert‐Estelles 2003; Gilabert‐Estelles 2007; Hudelist 2007; Johnson 2004; Klentzeris 1995; Laudanski 2014; Lawson 2008; Lee 2007; Li 2006; Liu 2008; Mafra 2014; Matsuzaki 2006a; Meola 2009; Meola 2013; Mikolajczyk 2006; Morelli 2010; Pino 2009; Puy 2002; Rakhila 2013; Ramon 2011; Tang 2009; Tiberi 2010; Van der Linden 1994; Van der Linden 1995; Vernet‐Tomas 2006a; Wang 2010a), and seven demonstrated an unclear concern (Kim 2007; Meibody 2011; Prefumo 2002; Szymanowski 2003; Visnovsky 2008; Yadav 2013; Zubor 2009). We assigned a high concern in patient selection applicability if the study utilised a two‐gate selection of women as cases and controls or if the study only evaluated a limited spectrum of disease. These types of study design introduce additional uncertainty to the accuracy of the index test, which usually varies across patient subgroups. In our view, any sampling deviation from a representative group of the entire clinically relevant population could skew the estimates of diagnostic accuracy in any direction, which raises concerns for applicability of the test in practice. The remained 11 studies were of low concern (Al‐Jefout 2007; Al‐Jefout 2009; Bokor 2009; Fassbender 2012; Hatok 2011; Lee 2010; Leslie 2013; Makari 2012; Takahashi 1990; Wolfler 2005; Zeng 2005). These studies included only a clinically relevant population that would have undergone the index test in clinical practice and displayed a representative form of the target condition.
Methodological quality assessment for the index test
Forty‐two studies carried a high risk of index test interpretation bias (Al‐Jefout 2007; Bokor 2009; Bourlev 2006; Cetin 2013; Chen 2004; Chen 2013; Da Silva 2014; Ding 2010; Fassbender 2012;Gilabert‐Estelles 2003; Gilabert‐Estelles 2007; Hatok 2011; Hudelist 2007; Johnson 2004; Kim 2007; Klentzeris 1995; Laudanski 2014; Lawson 2008; Lee 2007; Lee 2010; Li 2006; Liu 2008; Matsuzaki 2006a; Meola 2009; Meola 2013; Mikolajczyk 2006; Morelli 2010; Pino 2009; Prefumo 2002; Puy 2002; Rakhila 2013; Ramon 2011; Szymanowski 2003; Takahashi 1990; Tang 2009; Visnovsky 2008; Wang 2010a; Wolfler 2005; Yadav 2013; Zeng 2005; Zubor 2009), and eight studies demonstrated an unclear risk (Casals 2012; Dheenadayalu 2002; Elgafor el Sharkwy 2013; Mafra 2014; .Makari 2012; Tiberi 2010; Van der Linden 1994; Van der Linden 1995). A lack of clear pre‐specified criteria for a positive diagnosis and failure to blind index test operators to the results of the reference standard were the main reasons for a high risk assessment. Few studies reported the skill level of a test operator or interobserver variability, both of which directly relate to the performance of tests. Four studies presented a low risk of bias and clearly mentioned the use of a predetermined cut‐off threshold, an impact of the cycle phase on biomarker expression and blinding of the test operators to the results of the reference standard (Al‐Jefout 2009; Leslie 2013; Meibody 2011; Vernet‐Tomas 2006a). Overall, the interobserver variability was rarely mentioned across the included studies.
We did not consider the studies including index tests other than eutopic endometrial biomarkers (or we excluded information on other index tests reported in addition to endometrial tests). Likewise, we did not consider studies where the index test assessed other target conditions not specified in the review (e.g. studies aimed at classifying pelvic masses as benign and malignant); therefore we did not classify any of the included studies as 'high concern'. One study presented an unclear concern of index test applicability (Zeng 2005). We assigned an unclear concern when the study did not present sufficient information regarding the conduct of the tests, such as the laboratory methods or reagents used or the level of expertise of the test operators. The remaining 53 studies were of low concern and presented adequate information to conclude that performance or interpretation of the test did not differ from the review question.
Methodological quality assessment for the reference standard
We addressed the risk of bias and concerns about applicability related to the reference standard via some key questions. These included whether the reference standard was interpreted without the knowledge of the index test results and whether this was an appropriate investigation for the classification of the target condition (endometriosis).
There were no studies that demonstrated a high risk of bias for the reference standard domain, while twenty studies demonstrated an unclear risk (Casals 2012; Chen 2004; Da Silva 2014; Dheenadayalu 2002; Fassbender 2012; Gilabert‐Estelles 2007; Hudelist 2007; Johnson 2004; Kim 2007; Klentzeris 1995; Lawson 2008; Lee 2010; Li 2006; Liu 2008; Pino 2009; Rakhila 2013; Takahashi 1990; Tiberi 2010; Vernet‐Tomas 2006a; Zeng 2005). We assigned an unclear risk of bias if there was not enough information to determine how likely the reference standard was to have correctly classified the target condition. Specifically, this might occur if studies did not adequately describe surgical procedures or the criteria for a positive reference standard, or if it was unclear if histology was used to confirm surgical diagnosis or there was no information regarding the experience of the surgeons or the pathologists involved. Thirty‐four studies were at low risk of bias in the 'reference standard' domain and reported at least one of the following adequately: surgical procedure, the criteria for positive reference standard, histopathological confirmation of diagnosis, and high level of expertise in team performing the procedure to diagnose the target condition (Al‐Jefout 2007; Al‐Jefout 2009; Bokor 2009; Bourlev 2006; Cetin 2013; Chen 2013; Cho 2012; Ding 2010; Elgafor el Sharkwy 2013; Gilabert‐Estelles 2003; Hatok 2011; Laudanski 2014; Lee 2007; Leslie 2013; Mafra 2014; Makari 2012; Matsuzaki 2006a; Meibody 2011; Meola 2009; Meola 2013; Mikolajczyk 2006; Morelli 2010; Prefumo 2002; Puy 2002; Ramon 2011; Szymanowski 2003; Tang 2009; Van der Linden 1994; Van der Linden 1995; Visnovsky 2008; Wang 2010a; Wolfler 2005; Yadav 2013; Zubor 2009).
None of the studies had high or unclear concerns for applicability in regards to the reference standard. All 54 studies were of low concern and implemented pelvic surgery (laparoscopy or laparotomy) as a reference standard, which could be relied upon to match the review question.
Methodological quality assessment for flow and timing
Fifteen studies presented a high risk of bias in the flow and timing domain (Casals 2012; Cetin 2013; Chen 2004; Cho 2012; Elgafor el Sharkwy 2013; Fassbender 2012; Gilabert‐Estelles 2003; Gilabert‐Estelles 2007; Johnson 2004; Laudanski 2014; Lawson 2008; Morelli 2010; Tang 2009; Tiberi 2010; Wolfler 2005), and two studies demonstrated an unclear risk (Da Silva 2014; Lee 2010). All participants in every study received the same reference standard. The time interval between the index test (sample collection) and the reference standard was established as 12 months or less, and the most commonly reported time interval was immediately before surgery. We assigned a high risk of bias if any patients were excluded from the analysis because of uninterpretable results, inability to undergo either index test or reference standard or if withdrawals were more than 5% of the enrolled population (arbitrary selected cut‐off). Where the time interval was not stated clearly, but the authors' description suggested that the interval was reasonably short, we classified this study as having unclear risk. Thirty‐seven studies presented a low risk of bias in the 'flow and timing' domain (Al‐Jefout 2007; Al‐Jefout 2009; Bokor 2009; Bourlev 2006; Chen 2013; Dheenadayalu 2002; Ding 2010; Hatok 2011; Hudelist 2007; Kim 2007; Klentzeris 1995; Lee 2007; Leslie 2013; Li 2006; Liu 2008; Mafra 2014; Makari 2012; Matsuzaki 2006a; Meibody 2011; Meola 2009; Meola 2013; Mikolajczyk 2006; Pino 2009; Prefumo 2002; Puy 2002; Rakhila 2013; Ramon 2011; Szymanowski 2003; Takahashi 1990; Van der Linden 1994; Van der Linden 1995; Vernet‐Tomas 2006a; Visnovsky 2008; Wang 2010a; Yadav 2013; Zeng 2005; Zubor 2009). We assigned a low risk of bias in this domain where all the patients were included in the analysis, were excluded because they did not meet inclusion criteria prior to execution of index test, or if the withdrawals were less than 5% of the enrolled population and the time interval between the sample collection and reference standard was clearly stated.
Findings
We evaluated a total of 95 endometrial biomarkers in the 54 included studies. Of these, 22 biomarkers had a diagnostic evaluation (Table 1; Figure 5), 77 biomarkers were not altered by the presence of endometriosis (Appendix 7), and four biomarkers exhibited conflicting results (α3β1 and α4β1 integrins, PGP 9.5 and NF). Endometrial biomarkers were evaluated in either whole endometrial tissue or in separate endometrial compartments (stromal cells or glandular epithelium), or in menstrual fluid. The tests were performed in specific phases of the menstrual cycle or irrespective of the cycle phase. Most studies assessed any type of pelvic endometriosis, one study solely focused on DIE (17βHSD2 in Matsuzaki 2006a) and another study compared ovarian endometriosis with other types of non‐malignant ovarian cysts (relaxin and its receptor LGR7 in Morelli 2010).
5.
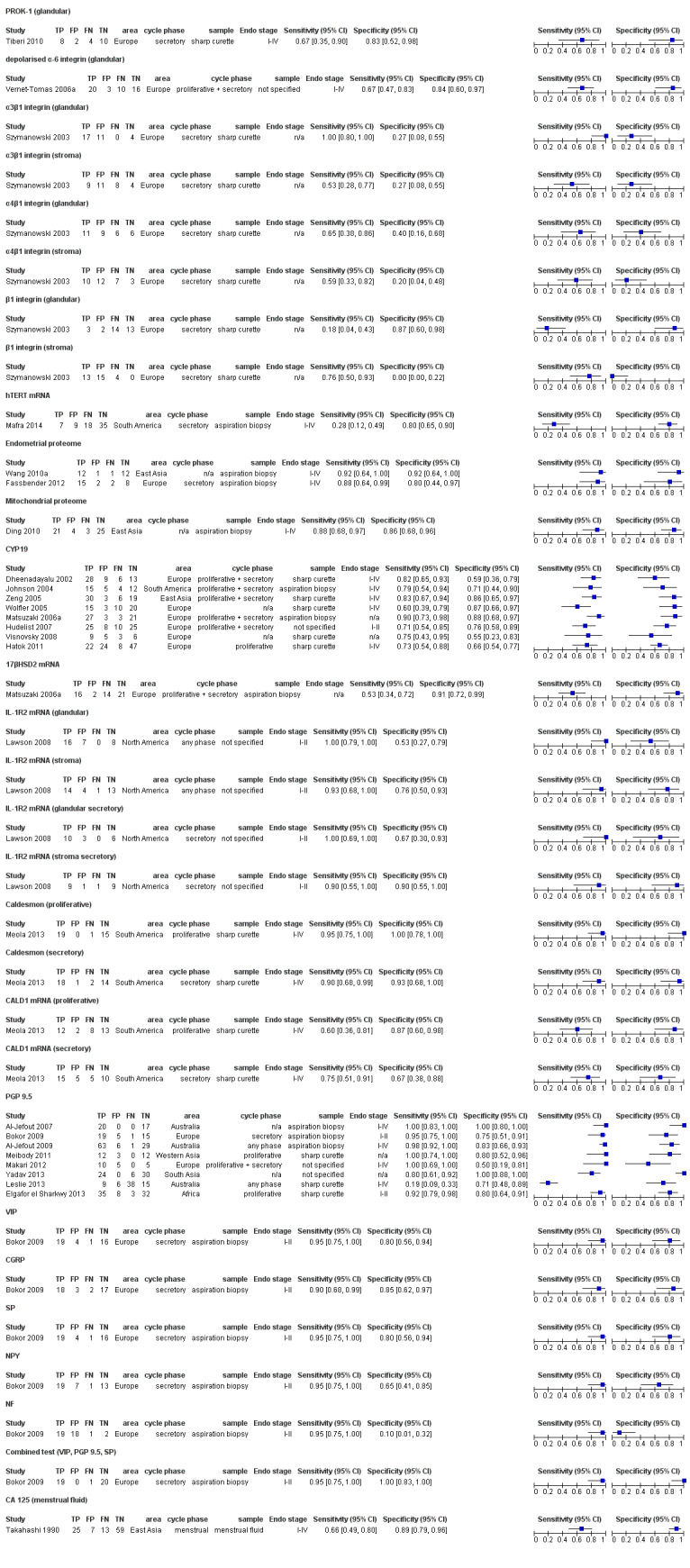
Forest plot of all endometrial biomarkers evaluated for detection of pelvic endometriosis. Plot shows study‐specific estimates of sensitivity and specificity (squares) with 95% CI (black line) along with country in which the study was conducted, stage of menstrual cycle when tissue was collected, method of sample collection and stage of endometriosis assessed. The studies for each test are ordered according to the year of publication. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
The endometrial biomarkers that were evaluated as a diagnostic test for endometriosis comprise the main findings of this review. The endometrial biomarkers that do not distinguish between women with and without endometriosis represent biomarkers with limited diagnostic potential and have been reported to add to the completeness of the presented topic.
Biomarkers evaluated as a diagnostic test for endometriosis
1. Angiogenesis and growth factors
1.1 PROK‐1 (prokineticin 1)
One European study involving 24 women assessed the value of PROK‐1 in detecting pelvic endometriosis (rASRM I‐IV) (Tiberi 2010). Endometrial samples were collected via the sharp curette technique in the secretory phase of the menstrual cycle. The authors utilised RT‐PCR to assess PROK‐1 levels and demonstrated reduced expression in women affected by endometriosis as compared to disease‐free controls. Using a negative PROK‐1 expression to define endometriosis, the sensitivity was 0.67 (95% CI 0.35 to 0.90), and the specificity was 0.83 (95% CI 0.52 to 0.98), which did not meet the criteria for either replacement or triage tests. Further testing in larger studies including participants at different phases of the menstrual cycle is needed to confirm the role of PROK‐1 in detecting endometriosis.
2. Cell adhesion molecules
Two European studies assessed the diagnostic performance of different integrins, focusing on different sets of molecules (Szymanowski 2003; Vernet‐Tomas 2006a).
2.1 Depolarised α6 (alpha‐6) integrin
One study involving 49 women evaluated the accuracy of depolarised α6 integrin within the glandular epithelium in detecting endometriosis (rASRM I‐IV) (Vernet‐Tomas 2006a). The authors performed the biopsies in the proliferative cycle phase; however, they did not specify the biopsy type. Using immunohistochemistry (IHC), investigators evaluated the percentage of positive glandular cells and the localisation of expression in each section, considering α6 to be polarised when expression was exhibited only on the basal side of the cell and depolarised when expression was observed at any side of the cell. Using depolarised expression as a positive test result, the sensitivity was 0.67 (95 CI 0.47 to 0.83) and specificity was 0.84 (95% CI 0.60 to 0.97). It did not meet the criteria for either replacement or triage test (Figure 6).
6.
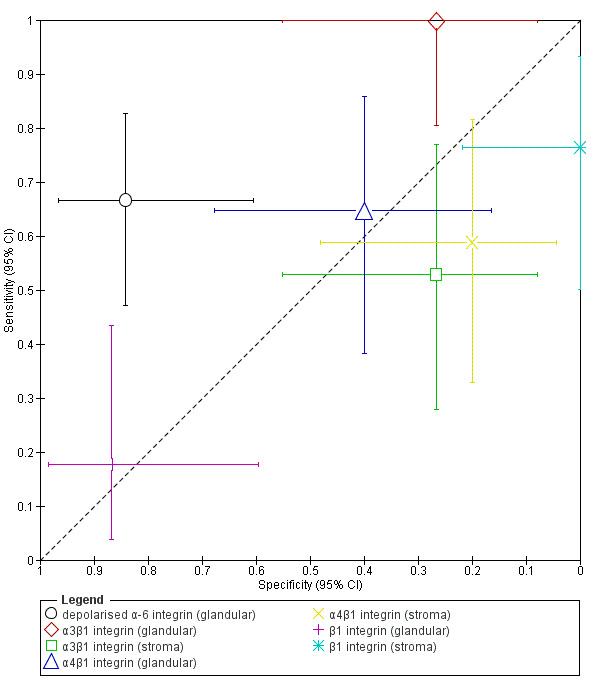
Summary ROC plot of tests depolarised α‐6 Integrin and of α3β1, α4β1 and β1 Integrins in glandular and stromal compartments of endometrium for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study, the bars correspond to 95% CIs of each individual study. The size of each point is proportional to the study sample size, each shape corresponds to different type of test.
2.2 Integrins α3β1 (alpha‐3 beta‐1), α4β1 (alpha‐4 beta‐1) and β1 (beta‐1)
Another study (32 women, rASRM stage not reported) evaluated the role of three integrins in diagnosing endometriosis (α3β1, α4β1 and β1) (Szymanowski 2003). The investigators performed a sharp curette in the secretory cycle phase and subdivided the specimens into glandular and stromal epithelium. They used IHC to assess integrin expression and defined a positive test as positive staining of any intensity. In glandular epithelium, α3β1 integrin had a sensitivity of 1.00 (95% CI 0.80 to 1.00) and a specificity of 0.27 (95% CI 0.08 to 0.55), and in stromal epithelium, it had a sensitivity of 0.53 (95% CI 0.28 to 0.77) and a specificity of 0.27 (95% CI 0.08 to 0.55). For its part, α4β1 integrin in glandular epithelium demonstrated a sensitivity of 0.65 (95% CI 0.38 to 0.86) and specificity of 0.40 (95% CI 0.16 to 0.68), and in stromal epithelium it exhibited a sensitivity of 0.59 (95% CI 0.33 to 0.82) and a specificity of 0.20 (95% CI 0.4 to 0.48). In glandular epithelium, β1 integrin demonstrated a sensitivity of 0.18 (95% CI 0.04 to 0.43) and a specificity of 0.87 (95% CI 0.60 to 0.98) and in stromal epithelium, it had a sensitivity of 0.76 (95% CI 0.50 to 0.93) and a specificity of 0.00 (95% CI 0.00 to 0.22) (Figure 6). One additional study (16 women, rASRM I, early proliferative cycle phase) showed that α3β1 and α4β1 integrins remained unchanged in endometriosis (Van der Linden 1994). Collectively, the results for all the above integrins were discouraging, but not sufficient to draw conclusions regarding the role of these biomarkers in the detection of endometriosis. These findings require further validation in larger studies with well‐defined populations at different cycle phases across a wide spectrum of the disease.
3. DNA repair and telomere maintenance molecules
3.1 hTERT (human telomerase reverse transcriptase)
One study conducted in South America with a total of 69 women assessed the performance of endometrial hTERT in diagnosing endometriosis (rASRM I‐IV) (Mafra 2014). The authors performed aspiration endometrial biopsies in the secretory phase of the menstrual cycle, using qRT‐PCR to assess hTERT mRNA, which was found to have a higher expression in endometriosis compared to controls. Using positive hTERT mRNA expression as the definition of a positive test, hTERT had a sensitivity of 0.28 (95% CI 0.12 to 0.49) and specificity of 0.80 (95% CI 0.65 to 0.90). The test did not meet the predefined criteria for a replacement or triage test, but further work is required to support this observation.
4. High‐throughput molecular markers
4.1 Endometrial proteome
Two small studies assessed the accuracy of endometrial proteome in detection of endometriosis. Fassbender 2012 took place in Europe (27 women, rASRM I‐IV, secretory cycle phase), and Wang 2010a was in East Asia (26 women, rASRM I‐IV, cycle phase not reported). Both studies performed aspiration biopsies for endometrial sampling and utilised SELDI‐TOF‐MS (surface enhanced laser desorption ionisation‐time of flight mass spectrometry) protein chip array technology for biomarker detection. Each group implemented varying approaches to the data analysis and the construction of a diagnostic model. Each study identified different sets of proteins that were used in a diagnostic model to discriminate the women with and without endometriosis, therefore these two studies were not combined into a meta‐analysis.
Fassbender 2012 identified five peptide peaks of 2072 m/z (mass over charge); 2973 m/z; 3623 m/z; 3680 m/z and 21,133 m/z, reporting a sensitivity of 0.88 (95% CI 0.64 to 0.99) and a specificity of 0.80 (95% CI 0.44 to 0.97), which did not meet the criteria for a replacement or triage test (Figure 7). Wang 2010a observed five protein peaks with different spectral intensities of 5385 m/z, 5425 m/z, 6898 m/z, 5891 m/z and 6448 m/z., with a sensitivity of 0.92 (95% CI 0.64 to 1.00) and a specificity of 0.92 (95% CI 0.64 to 1.00) (Figure 7). The test approached the criteria for a replacement as well as SnOUT and SpIN triage tests. Although promising, this observation provides too few data to draw conclusions regarding the diagnostic role of these endometrial peptides in endometriosis. These results require further validation in large, independent, well‐defined cohorts, using standardised and reproducible methodologies.
7.
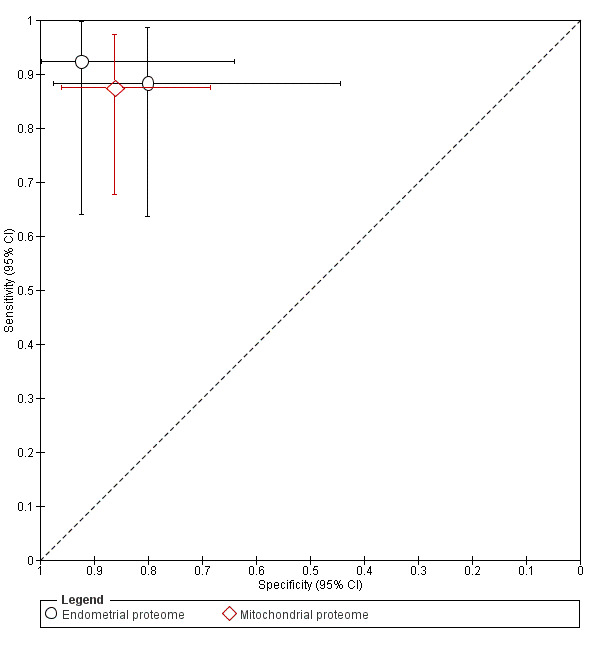
Summary ROC plot of tests endometrial proteome and mitochondrial proteome for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study, the bars correspond to 95% CIs of each individual study. The size of each point is proportional to the study sample size, each shape corresponds to the type of tested proteome.
4.2 Mitochondrial proteome
One study conducted in East Asia comprising 53 women evaluated diagnostic performance of mitochondrial proteome for pelvic endometriosis (rASRM I‐IV) (Ding 2010). The authors performed aspiration biopsies in unspecified phases of the menstrual cycle and isolated three potential biomarkers for endometriosis within the endometrium. Authors reported mitochondrial proteome to have a sensitivity of 0.88 (95% CI 0.68 to 0.97) and a specificity of 0.86 (95% CI 0.68 to 0.96) (Figure 7) and did not qualify as a replacement or a triage test, although these data are not sufficient to draw a meaningful conclusion.
5. Hormonal markers
5.1 CYP19 (aromatase cytochrome P450)
Eight studies with a total of 444 women determined the accuracy of CYP19 in detecting pelvic endometriosis (Dheenadayalu 2002; Hatok 2011; Hudelist 2007; Johnson 2004; Matsuzaki 2006a; Visnovsky 2008; Wolfler 2005; Zeng 2005). Six studies took place in Europe, one in South America and one in East Asia. Five studies performed the biopsy using a sharp curette, two studies utilised aspiration techniques, and one study did not report the method of sample collection. Five studies assessed endometrium in the proliferative or secretory cycle phase; one study, only in proliferative phase; the menstrual cycle phase was not reported in two studies. Five studies included women with stages I‐IV endometriosis, one study evaluated only stages I‐II, and two studies did not specify severity of the disease. Four studies were of a single‐gate design, of which three studies included women presenting either with pelvic pain or infertility (Visnovsky 2008; Wolfler 2005; Zeng 2005), and one study included only women with pelvic pain (Hatok 2011). The remaining four studies had a two‐gate design and included a symptomatic group as well as symptom‐free women undergoing tubal ligation or gynaecological surgery for other indications (Dheenadayalu 2002; Hudelist 2007; Johnson 2004; Matsuzaki 2006a). Five studies utilised RT‐PCR method for CYP19 detection, and three studies utilised IHC, all demonstrating increased expression of CYP19 in endometriosis. The estimates for sensitivity ranged from 0.60 to 0.90 and for specificity from 0.55 to 0.86. The mean sensitivity and specificity of all these evaluations were 0.78 (95% CI 0.70 to 0.85) and 0.74 (95% CI 0.65 to 0.84), which did not meet the criteria for either a replacement or triage test. Forest plots (Figure 8) and the ROC plot (Figure 9) showed a high degree of heterogeneity for estimates of both sensitivity and specificity, which could be attributed to the differences in patient selection, cycle phase, spectrum of the disease and laboratory methods. None of the individual studies presented diagnostic estimates that met the predetermined diagnostic criteria for a replacement or triage test. These data suggest that CYP19 is either not sensitive or specific enough to be clinically useful in diagnosing endometriosis.
8.

Forest plot of CYP19 for detection of pelvic endometriosis. Plot shows study‐specific estimates of sensitivity and specificity (squares) with 95% CI (black line) along with country in which the study was conducted, stage of menstrual cycle when tissue was collected, method of sample collection and stage of endometriosis assessed. The studies are ordered according to the year of publication. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
9.
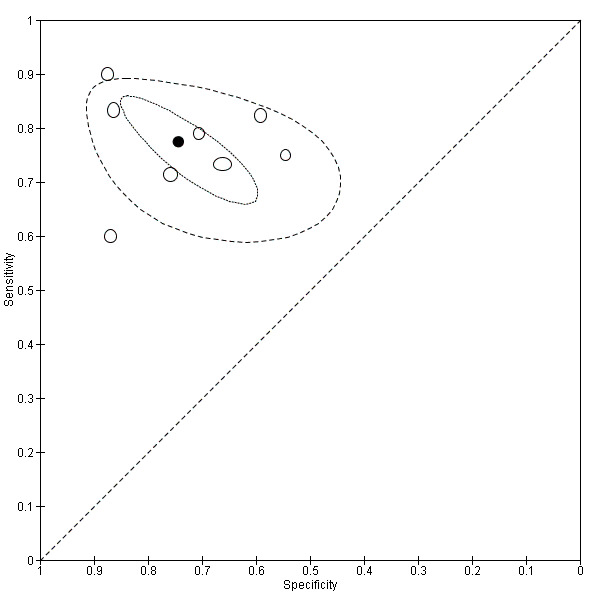
Summary ROC plot of CYP19 for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study. The size of each point is proportional to the study sample size. The solid black circle represents the mean sensitivity and specificity which is surrounded by a 95% confidence region (dotted line) and 95% prediction region (dashed line).
5.2 17bßHSD2 (17‐β hydroxysteroid dehydrogenase type 2)
One study conducted in Europe (53 women, rASRM stage not reported) evaluated the diagnostic role of 17bßHSD2 in DIE (Matsuzaki 2006a). Aspiration technique was utilised for endometrial biopsy, which was performed in varying stages of the menstrual cycle (proliferative, early‐ mid‐ and late‐secretory). By using qRT‐PCR, the authors demonstrated higher expressions of 17bßHSD2 mRNA in endometriosis in stromal endometrial cells, and there was no statistically significant difference between the study and control groups for glandular epithelium. Interestingly, there was a differential expression of 17bßHSD2 only in secretory cycle phase, but no statistically significant difference in the proliferative phase. Considering both cycle phases, 17HSD2 mRNA in stromal cells had a sensitivity of 0.53 (95% CI 0.34 to 0.72) with a specificity of 0.91 (95% CI 0.72 to 0.99). The test approached the criteria for a SpIN triage test, and possibly could perform better if testing were limited to the secretory cycle phase. However, separate data for only the secretory phase was not available from this paper. Further data from additional studies that include a broad spectrum of disease, clearly defined populations and sub‐analysis per cycle phase is necessary to draw meaningful conclusions.
5.3 ER‐α (oestrogen receptor alpha) and 5.4 ER‐β (oestrogen receptor ‐ beta)
One study conducted in Asia (90 women, rASRM stage not reported) examined endometrial expression of ER‐α and ER‐β in endometriosis. The authors did not specify a method of endometrial sampling and performed the test in either the proliferative or secretory cycle phase in glandular epithelium and in stromal cells. Considering IHC staining of any intensity as a positive test, the diagnostic estimates for ER‐α in either glandular cells (sensitivity 0.73, 95% CI 0.60 to 0.84; specificity 0.43, 95% CI 0.25 to 0.63) or in stromal component (sensitivity 0.77, 95% CI 0.64 to 0.87; specificity 0.50, 95% CI 0.31 to 0.69) did not meet the predetermined criteria for either replacement or triage test and displayed wide confidence intervals (Figure 10). Likewise, testing for ER‐β exhibited unsatisfactory diagnostic performance in glandular cells (sensitivity 0.67, 95% CI 0.53 to 0.78; specificity 0.63, 95% CI 0.44 to 0.80) and in stromal cells (sensitivity 0.63, 95% CI 0.50 to 0.75; specificity 0.73, 95% CI 0.54, 0.88) (Figure 10). The authors also presented separate data for each phase of the menstrual cycle, but the cycle‐specific evaluations did not meet or approach the threshold for a replacement or triage test, and none were superior to the estimates of the tests performed irrespective of the cycle phase (data not shown).
10.
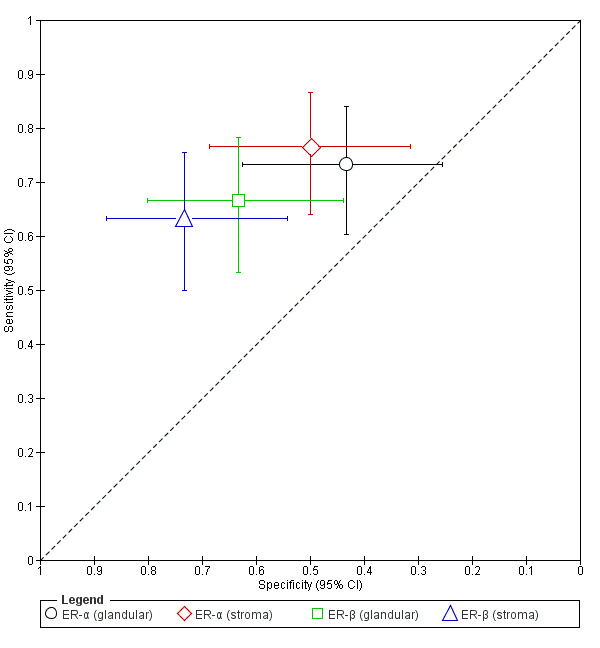
Summary ROC plot of ER‐α and ER‐β in glandular and stromal compartments of endometrium for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study, the bars correspond to 95% CIs of each individual study. The size of each point is proportional to the study sample size, each shape corresponds to different type of test.
6. Immune system and inflammatory markers
6.1 IL‐1R2 (interleukin‐1 receptor type II)
One study conducted in North America (71 women, rASRM I‐II) examined the value of IL‐1R2 in detecting endometriosis (Lawson 2008). The authors reported levels of IL‐1R2 in glandular and stromal epithelium in both proliferative and secretory phases of the menstrual cycle. Authors did not specify the method of endometrial sampling. They evaluated IL‐1R2 expression by using fluorescence in situ hybridisation (FISH) and noted a decrease in endometriosis in both types of cells. The cut‐off value of hybridisation score (HS) > 3 demonstrated a comparable diagnostic estimate in glandular epithelium at any cycle stage (sensitivity 1.00, 95% CI 0.79 to 1.00; specificity 0.53, 95% CI 0.27 to 0.79) and when only considering the secretory stage (sensitivity 1.00, 95% CI 0.69 to 1.00; specificity 0.67, 95% CI 0.30 to 0.93). Both tests met the criteria for a SnOUT triage test, although there were wide confidence intervals, especially for specificity values (Figure 11). The cut‐off value of HS > 2 for stromal cells demonstrated a sensitivity of 0.93 (95% CI 0.68 to 1.00) and a specificity of 0.76 (95% CI 0.76 to 0.93) for testing irrespective of cycle phase, which approached the criteria for both replacement and SnOUT triage tests (Figure 11). When considering only the secretory cycle phase and using the same threshold, testing for IL‐1R2 in stromal cells revealed a sensitivity of 0.90 (95% CI 0.55 to 1.00) and a specificity of 0.90 (95% CI 0.55 to 1.00), which approached the criteria for replacement, SnOUT and SpIN triage tests, albeit with wide confidence intervals that undermine the reliability of the observed estimates. Larger diagnostic accuracy studies need to validate these data in a well‐characterised population.
11.
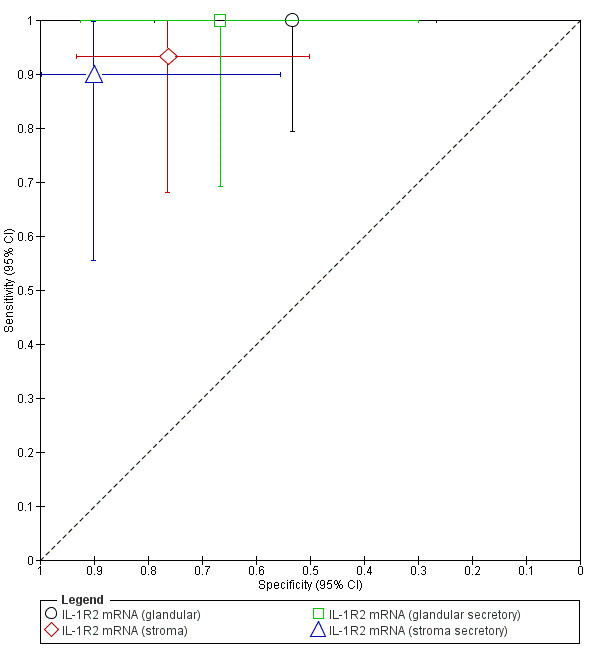
Summary ROC plot of tests IL‐1R2 mRNA (glandular) and IL‐1R2 mRNA (stroma) for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study, the bars correspond to 95% CIs of each individual study, different shapes represent different endometrial compartments tested. The size of each point is proportional to the study sample size.
7. Myogenic markers
7.1 Caldesmon (calmodulin binding protein) and 7.2 CALD1 (caldesmon gene)
One study conducted in South America (35 women, rASRM I‐IV) assessed the diagnostic accuracy of caldesmon protein and gene in detecting endometriosis (Meola 2013). The authors sampled the endometrium via a sharp curette in both the proliferative and secretory phases of the menstrual cycle. CALD1 gene was detected by RT‐PCR and caldesmon protein by IHC stain intensity. The phase of menstrual cycle did not influence the gene expression or the protein levels in endometrial tissue. The CALD1 in the proliferative phase had a sensitivity of 0.60 (95 CI 0.36 to 0.81) and a specificity of 0.87 (95% CI 0.60 to 0.98), and in the secretory phase a sensitivity of 0.75 (95% CI 0.51 to 0.91) and a specificity of 0.67 (95% CI 0.38 to 0.88). The test did not meet the diagnostic criteria for a replacement or triage test (Figure 12). Caldesmon protein in the proliferative phase had a sensitivity of 0.95 (95% CI 0.75 to 1.00) with a specificity of 1.00 (95% CI 0.78 to 1.00), meeting the criteria for replacement, SnOUT and SpIN triage tests. In the secretory phase the sensitivity and specificity of caldesmon protein were slightly lower at 0.90 (95% CI 0.68 to 0.99) and 0.93 (95% CI 0.68 to 1.00), respectively, approaching the criteria for replacement, SnOUT and SpIN triage tests (Figure 12). Further work to confirm or refute this observation and determine the value of caldesmon endometrial testing to diagnose endometriosis is warranted.
12.
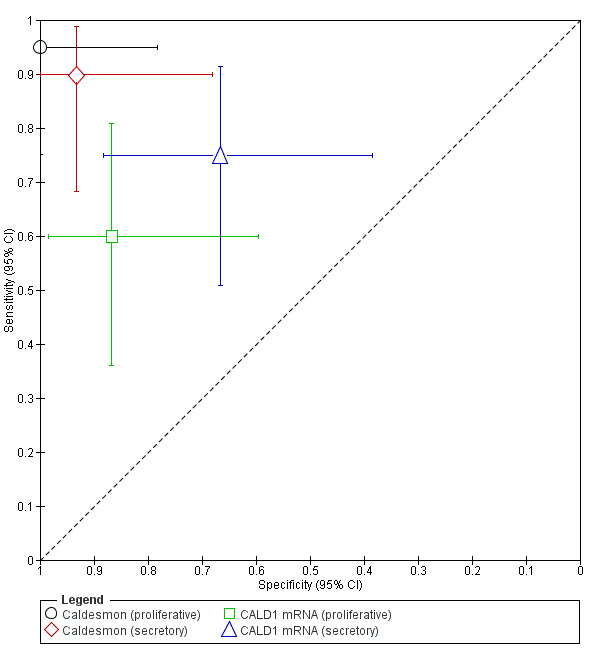
Summary ROC plot of tests Caldesmon (proliferative), caldesmon (secretory), CALD1 mRNA (proliferative) and CALD1 mRNA (secretory) for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study, the bars correspond to 95% CIs of each individual study, different shapes represent different test (protein or mRNA in different endometrial compartments). The size of each point is proportional to the study sample size.
8. Nerve sheath and nerve growth markers
8.1 PGP 9.5 (protein gene product 9.5)
Eight studies involving 429 women evaluated PGP 9.5 for the diagnosis of pelvic endometriosis (Al‐Jefout 2007; Al‐Jefout 2009; Bokor 2009; Elgafor el Sharkwy 2013; Leslie 2013; Makari 2012; Meibody 2011; Yadav 2013). Three studies took place in Australia, two in Europe and one each in Africa, South Asia and Western Asia. Three studies performed endometrial biopsy using different aspiration techniques, and three studies utilised sharp curette. The other two studies did not report the method of endometrial sampling. Three studies assessed endometrium in the proliferative phase of the menstrual cycle; one study, in secretory; and three studies, in any cycle phase. One study did not report the cycle phase. Two studies evaluated only women with minimal to mild endometriosis (rASRM I‐II), four studies assessed a wide range of disease (rASRM I‐IV), and two studies did not report the severity of the disease. All the included studies had a single‐gate design and included women with suspected endometriosis who presented with pelvic pain, infertility or an adnexal mass.
The biopsy specimen underwent IHC staining for PGP 9.5 followed by nerve fibre identification under a digital camera (photomicrography) in all but Leslie 2013, which used conventional light microscopy. The number of small nerve fibres present for the diagnosis varied between 2 and 50 nerve fibres per mm2. The estimates for sensitivity ranged from 0.19 to 1.00 and for specificity from 0.50 to 1.00, with substantial heterogeneity between the studies (Figure 13; Figure 14).
13.
Forest plot of PGP 9.5 for detection of pelvic endometriosis. Plot shows study‐specific estimates of sensitivity and specificity (squares) with 95% CI (black line) along with country in which the study was conducted, stage of menstrual cycle when tissue was collected, method of sample collection and stage of endometriosis assessed. The studies are ordered according to the year of publication. FN: false negative; FP: false positive; TN: true negative; TP: true positive.
14.
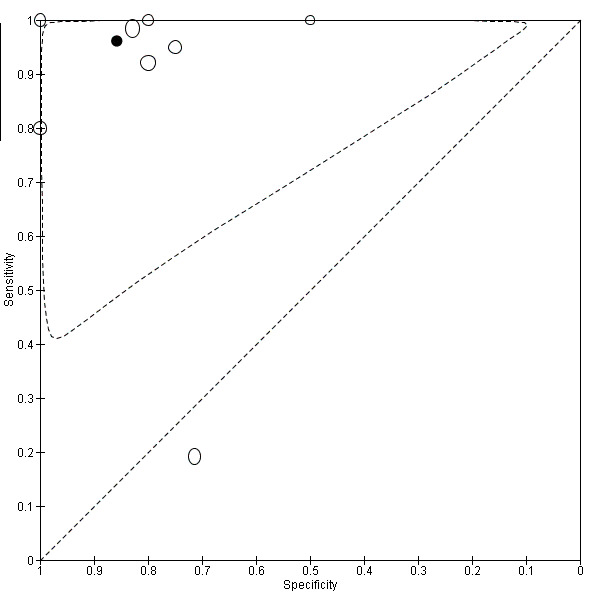
Summary ROC plot of PGP 9.5 for the diagnosis of endometriosis ( the data is presented for 7 studies, excluding one outlier study (Leslie 2013)). Each point represents the pair of sensitivity and specificity from a study. The size of each point is proportional to the study sample size. The solid black circle represents the mean sensitivity and specificity which is surrounded by a 95% prediction region (dashed line). The outlier study is presented on the graph for descriptive purposes, but not included in the meta‐analysis.
Leslie 2013 (68 women, rASRM I‐IV, proliferative or secretory cycle phase, sample collected with sharp curettage or Pipelle aspiration biopsy) found a markedly lower sensitivity of 0.19 (95% CI 0.09 to 0.33) with a specificity of 0.71 (95% CI 0.48 to 0.89). There are a couple of reasons as to why these authors may have reported a lower sensitivity in comparison to the other studies. The included patients had been and were on hormonal remedies, and 7 of the 68 patients had other pathologies present at the time of laparoscopy. Importantly, the method of endometrial biopsy was not full thickness nor as adequate as reported by other studies. The authors obtained biopsies with a Pipelle and a metal curette, each of which provided only small tissue fragments that could not be specifically orientated for microscopic examination. This study also utilised conventional light microscopy compared to photomicrography, which investigators used in the studies that demonstrated a higher sensitivity. When we excluded this outlier study, the mean sensitivity and specificity (7 studies, 361 women) were 0.96 (95% CI 0.91 to 1.00) and 0.86 (95% CI 0.70 to 1.00), which met the criteria for replacement and SnOUT triage tests.
Alternatively, Al‐Jefout 2007 (37 women, rASRM I‐IV, cycle phase not specified) demonstrated the highest diagnostic estimates (sensitivity 1.00, 95% CI 0.83 to 1.00; specificity 1.00, 95% CI 0.80 to 1.00). A subsequent larger study from the same group (99 women, rASRM I‐IV, any cycle phase) demonstrated a high diagnostic performance (sensitivity 0.98, 95% CI 0.92 to 1.00; specificity 0.83, 95% CI 0.66 to 0.93) (Al‐Jefout 2009). Possible reasons for this high diagnostic performance include meticulous attention to endometrial sampling using the endo sampler, which ensured that operators obtained a narrow but deep sample of endometrium, with adequate amounts of stroma for assessment. This technique appears to be a necessary prerequisite for a successful diagnosis, as nerve fibres are only found in stroma, especially in the deeper portions of the functional layer. The Pipelle biopsy is good for demonstrating structures in the epithelium and superficial stroma but may not be as good for deeper stroma and nerve fibres.
Notably, Cetin 2013 (60 women, rASRM I‐IV, any cycle phase, sample collected with aspiration biopsy) detected no expression of PGP 9.5 in endometrium in either study (N = 31) or control (N = 29) groups, including those presenting with pelvic pain. The main point of difference from all the other studies assessing PGP 9.5 is that Cetin 2013 utilised ready‐to‐use immunohistochemical markers, which can explain the difference in PGP 9.5 expression in this study. The authors observed positive PGP 9.5 staining in the muscle layers of intestinal tissue, which they used as a positive control on each slide, suggesting that the markers used could not be applicable to endometrial tissue. Adequate discrimination of PGP 9.5 seems to be very dependent on good optimisation of only one or two of the commercially available markers. Fine nerve fibres may be difficult to distinguish, so using a bright coloured chromogen, such as 'Fast Red' may help them to stand out. Therefore, in addition to the required technical precision, laboratory technique may hinder/influence the applicability of the test to routine clinical practice. Overall the diagnostic studies for PGP 9.5 are encouraging, but we recommend more comprehensive assessment of this diagnostic tool in large high quality studies performing standardised endometrial sampling and laboratory methods.
8.1 Other neural markers
One European study evaluated the role of other endometrial neural markers, including VIP (vasoactive intestinal polypeptide), CGRP (calcitonin gene related protein), SP (substance P), NPY (neuropeptide Y), NF (neurofilament) and combinations of the above (40 women, rASRM I‐II) (Bokor 2009). While PGP 9.5 stains all nerve fibres, these nerve markers stain for specific nerve fibres and hence can improve the diagnostic accuracy of the endometrial test. The investigators performed aspiration biopsies in the secretory phase of menstrual cycle, evaluating nerve fibre density in the biopsy specimen following IHC. The reported diagnostic estimates for either VIP or SP (sensitivity 0.95, 95% CI 0.75 to 1.00; specificity 0.80, 95% CI 0.56 to 0.94) met the criteria for replacement and SnOUT triage tests. For CGRP, they approached these criteria (sensitivity 0.90, 95% CI 0.68 to 0.99; specificity 0.85, 95% CI 0.62 to 0.97), and for NPY, they met the criteria for a SnOUT triage test (sensitivity 0.95, 95% CI 0.75 to 1.00; specificity 0.65, 95% CI 0.41 to 0.85). The combined test for the panel of neural markers (VIP + PGP 9.5 + SP) showed the highest diagnostic estimates (sensitivity 0.95, 95% CI 0.75 to 1.00; specificity 1.00, 95% CI 0.83 to 1.00) and could qualify for either replacement, SnOUT or SpIN triage tests (Figure 15). In this study, the levels of NF were not significantly different in women with and without endometriosis. Investigators calculated the diagnostic test estimates and observed poor test performance, with a good sensitivity of 0.95 (95% CI 0.75 to 1.00) but unacceptably low specificity of 0.10 (95% CI 0.01 to 0.32). Another study demonstrated no staining with NF in endometrium from women with and without endometriosis (Cetin 2013). The authors reported similar findings for PGP 9.5 and, as presented above, it is quite possible that the assay selected in this study is not sensitive enough for endometrial tissue, hence these findings cannot confirm or refute the previous observations on endometrial expression of PGP 9.5 and NF. The reported alterations in VIP, CGRP, SP and NPY expression in endometriosis indicate that these biomarkers are suitable for detection of the disease, whereas endometrial NF is of little diagnostic value; however, there is insufficient data to draw meaningful conclusions on the findings from a single small study. These results require further validation in large, well‐defined populations across a wide spectrum of disease utilising a standardised, reproducible methodology.
15.
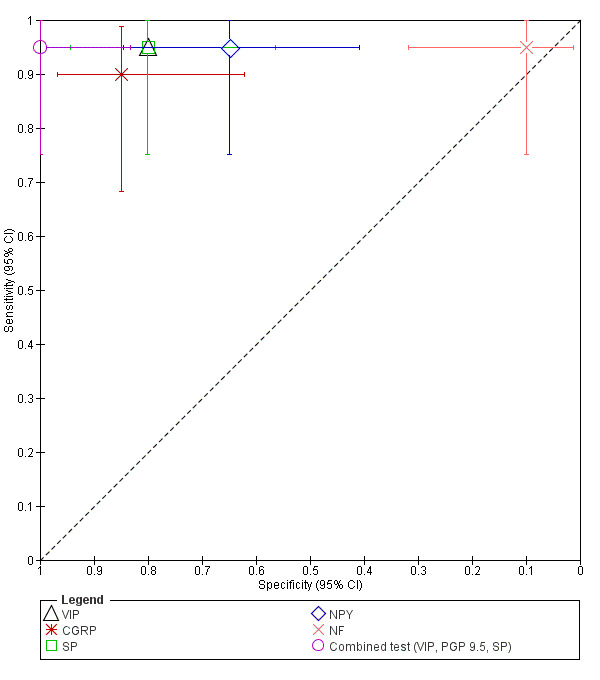
Summary ROC plot of tests VIP, CGRP, SP, NPY, NF and combined test (VIP, PGP9.5, SP) for the diagnosis of endometriosis. Each point represents the pair of sensitivity and specificity from a study, the bars correspond to 95% CIs of each individual study. The size of each point is proportional to the study sample size.
9. Tumour markers
9.1 CA‐125 (cancer antigen‐125) in menstrual fluid
One study conducted in East Asia involving 104 women explored the role of CA‐125 in pelvic endometriosis (rASRM I‐IV) (Takahashi 1990). Investigators assessed the biomarker within the menstrual fluid using radioimmunoassay with commercial kits, demonstrating a sensitivity of 0.66 (95% CI 0.49 to 0.80) and a specificity of 0.89 (95% CI 0.79 to 0.96). Further data is required before commenting on its diagnostic role.
The eligible studies did not assess any other biomarkers in menstrual fluid.
Biomarkers that did not distinguish between women with and without endometriosis
Seventy‐seven biomarkers showed no difference in expression levels between the groups of women with and without endometriosis, as presented in Appendix 7. Most of these biomarkers were assessed in small individual studies, and their association with endometriosis remains unclear. Six biomarkers were assessed in two studies or more and included:
CD68 + cells (macrophages) (Cetin 2013;Klentzeris 1995);
PAI‐1 (plasminogen activator inhibitor‐1) protein or mRNA (Gilabert‐Estelles 2003; Gilabert‐Estelles 2007);
TIMP‐1 (tissue inhibitor of metalloproteinases‐1) protein or mRNA (Chen 2004; Gilabert‐Estelles 2003; Gilabert‐Estelles 2007; Laudanski 2014; Li 2006);
TNF‐α (tumour necrosis factor‐alpha) protein or mRNA (Chen 2013; Da Silva 2014);
TSP‐1 (thrombospondin‐1) protein or mRNA (Gilabert‐Estelles 2007; Ramon 2011); and
VEGF (vascular endothelial growth factor) protein or mRNA (Cho 2012; Da Silva 2014; Lee 2010).
All six biomarkers showed a similar trend of no differential expression between the groups, which indicates their limited value in detection of endometriosis.
Investigations of heterogeneity and sensitivity analysis
We outline potential sources of heterogeneity in Secondary objectives. Although we attempted to assess these sources of heterogeneity, there were not enough studies evaluating each test to make this a meaningful analysis. Furthermore, the sensitivity analyses were not possible due to the small number of studies.
Discussion
Summary of main results
The review presents data from 2729 reproductive‐aged women who had presented with symptoms of endometriosis and underwent eutopic endometrial biopsies during or prior to diagnostic surgery. We evaluated diagnostic performance for 22 endometrial biomarkers and identified 71 biomarkers that were not altered by endometriosis. Most of the biomarkers were assessed in a limited number studies, and only two biomarkers (PGP 9.5 and CYP19) provided sufficient data for a meta‐analysis.
PGP 9.5, the marker of small unmyelinated sensory C nerve fibres, was the most studied biomarker. The meta‐analysis revealed that evaluation of nerve fibre density with PGP 9.5 staining could qualify as a replacement test (mean sensitivity of 0.96, 95% CI 0.91 to 1.00; specificity 0.86, 95% CI 0.70 to 1.00), after exclusion of one outlier study. However, there was a notable variability in reporting of the diagnostic estimates, which could be attributed to a difference in the endometrial sampler used, the method of curettage and the laboratory technique.
Estimates of aromatase cytochrome P450 (CYP19), the key enzyme in conversion of C19 steroids into oestrogen, did not meet the diagnostic criteria to qualify for either a replacement or triage test (mean sensitivity 0.77, 95% CI 0.70 to 0.85; specificity 0.74, 95% CI 0.65 to 0.84).
The findings of the meta‐analyses presented in this review need to be interpreted with caution. Considering both the level of heterogeneity and the high or unclear risk of bias of the included studies, the results do not seem to have sufficient reliability to direct clinical practice.
The remaining biomarkers were classified as follows.
-
Tests to be validated for their diagnostic potential. This group included:
those with an adequate diagnostic performance, but insufficient data to confidently comment on their diagnostic role (fewer than three studies with the diagnostic estimates meeting the criteria for either a replacement or triage test); and
tests where the diagnostic estimates approached the criteria for replacement or triage tests in a small number of studies, with a possibility that they would reach these criteria if further studies were performed (fewer than three studies with the diagnostic estimates within 5% of the criteria for either replacement or triage tests). We present these tests in Table 5.
-
Tests of limited diagnostic value (at least three studies demonstrating low diagnostic estimates that do not meet or approach the criteria for either replacement or triage test, or demonstrating no differential expression in endometriosis). These tests are presented in Appendix 8 and include:
TIMP‐1 (tissue inhibitor of metalloproteinases‐1) protein or mRNA (Chen 2004; Gilabert‐Estelles 2003; Gilabert‐Estelles 2007; Laudanski 2014; Li 2006);
VEGF (vascular endothelial growth factor) protein or mRNA (Cho 2012; Da Silva 2014; Lee 2010). We advise against further evaluation of these biomarkers for the diagnosis of endometriosis;
aromatase cytochrome P450 (CYP19) as suggested by the meta‐analysis presented above.
Tests that possibly have limited diagnostic value, but where there are insufficient data to confidently comment on their diagnostic role (fewer than three studies with low diagnostic estimates or demonstrating no differential expression in endometriosis). The full list of tests from this group is presented in Appendix 9. We advise considering further investigation of these biomarkers with a focus on specific phases of the menstrual cycle, specific phenotypes of endometriosis, by implementation of different cut‐off values or by utilising different laboratory methods.
4. Endometrial biomarkers to be validated for their diagnostic potential in endometriosis.
| Endometrial biomarkers | Replacement test | SnOUT triage test | SpIN triage test |
| 1. High throughput molecular markers | |||
| Endometrial proteome by SELDI‐TOF‐MS (5 protein peaks with molecular weights of 5,385 m/z, 5,425 m/z, 6,898 m/z, 5,891 m/z, 6,448 m/z) |
± | ± | ± |
| 2. Hormonal markers | |||
| 17βHSD2 mRNA (17‐β hydroxysteroid dehydrogenase type 2 gene) | — | — | ± |
| 3. Immune system and inflammatory markers | |||
| IL‐1R2 mRNA (interleukin‐1 receptor type II gene) (glandular epithelium) | — | + | — |
| IL‐1R2 mRNA (interleukin‐1 receptor type II gene) (stromal endometrial cells) | ± | ± | — |
| IL‐1R2 mRNA (interleukin‐1 receptor type II gene) (glandular epithelium, secretory phase of menstrual cycle) | + | — | |
| IL‐1R2 mRNA (stromal endometrial cells, secretory phase of menstrual cycle) | ± | ± | ± |
| 4. Myogenic markers (markers of smooth muscle differentiation) | |||
| Caldesmon (proliferative phase of menstrual cycle) | + | + | + |
| Caldesmon (secretory phase of menstrual cycle) | ± | ± | ± |
| 5. Neural and nerve sheath markers | |||
| PGP 9.5 (protein gene product 9.5) | + | + | — |
| VIP (vasoactive intestinal polypeptide) | + | + | — |
| CGRP (calcitonin gene‐related protein) | ± | ± | — |
| SP (substance P) | + | + | — |
| NPY (neuropeptide Y) | — | + | — |
| Combined test (VIP + PGP 9.5 + SP) | + | + | + |
| Notes: + meets the criteria
± approaches the criteria (within 5% of the pre‐defined criteria) — does not meet criteria | |||
Strengths and weaknesses of the review
This review is part of a comprehensive review series of minimally invasive biomarkers for the diagnosis of endometriosis. The main strength of this review is its attempt to systematically review the vast number of studies with an aim to present the most accurate picture of diagnostic test accuracy of endometrial tests for endometriosis.
The main strengths of the review are the following.
We carried out a thorough search of the current literature, including for studies written in languages other than English.
Two or three independent reviewers extracted data and used the modified QUADAS‐2 tool to perform quality assessments.
Stringent selection criteria ensured that eligible studies utilised prospectively collected samples and only included women of reproductive age, which minimised the risk of bias in interpreting the reference standard and index test.
We approached the authors of the studies in an attempt to obtain any missing information that was required to assess eligibility and critically appraise the studies.
We included studies that demonstrated biomarker levels that did not significantly differ in endometriosis, providing a more comprehensive evaluation of the diagnostic role of the biomarkers and identifying the tests of limited value in diagnosing the disease. These biomarkers are evaluated in adequately designed studies and provide information that is likely to focus future research on other more clinically useful tests. Although this information has little influence on the conclusions of this review due to the paucity of the available data, the relevance of these studies will increase in future updates with a growing body of evidence.
The limitations of this review are the following.
There were only individual small studies for most evaluated index tests, and a meta‐analysis was possible only for two endometrial biomarkers.
The low number of studies for the most studied biomarkers, most of which presented a high or unclear risk of bias, contributed to the low quality of evidence presented in this review and undermined the reliability of the estimates from the meta‐analyses. There was variation between studies with respect to the included populations, the severity of endometriosis, the menstrual cycle phase at testing, endometrial sampling and laboratory methods. We were unable to formally explore sources of heterogeneity due to the low number of studies for each biomarker. There was a loss of patients from the analyses due to poor quality of eutopic endometrium samples. This excluded potentially viable patients from the analysis and further reduced the population size. We now have a standardised methodology available for tissue biospecimen collection, processing and storage, and we recommend adhering to these standards in future diagnostic studies (Fassbender 2014).
Thresholds for a positive diagnostic test were not always pre‐specified, and often the definition of a positive test varied for the same test between the studies. Threshold definition was less likely when the primary design of the study was to determine differential regulation in endometriosis rather than diagnostic accuracy.
We cannot rule out the presence of publication bias, since we were unable to include unpublished studies due to difficulties in obtaining the data.
We cannot rule out the risk of patient selection bias, since most of the studies took place in academic institutions and some in referral centres for endometriosis. The reported prevalence of endometriosis in this review (30% to 77%) was generally higher than previously reported prevalence for endometriosis (6% to 10% in the general female population and 35% to 50% in symptomatic women) (Giudice 2004). This may reflect a high level of surgical diagnostic expertise but could be due to a tertiary referral pattern with possible pre‐selection of more challenging cases. Selection bias appeared to be reduced but not completely eliminated by consecutively enrolling participants. However, the information on method of enrolment was missing in most of the included studies.
There was variation in the selection of the case and control groups with inclusion of participants that may not reflect a clinically representative population. In this review 27 studies (50%) had a two‐gate design that included a wide group of participants who underwent surgery for various indications, including either healthy asymptomatic individuals or participants with other pathological conditions within the control group. This could have biased the test outcomes, since such a control group is broader than that normally seen in clinical practice and includes the women that would not be tested in the relevant clinical situation. In this review 15 studies (28%) included women with a limited spectrum of endometriosis, and 9 studies did not report the spectrum of the evaluated condition. We included these studies to avoid omission of potentially valuable diagnostic information, but considering only mild or only severe forms of the disease could skew the diagnostic estimates in either direction and subsequently interfere with the interpretation of the index test results. It was not possible to evaluate population and disease spectrum effects on the data because there were too few reports for most of the evaluated tests.
We could not rule out inappropriate assignment to the endometriosis and control groups. Surgical misdiagnosis is a potential cause of bias, as the number and experience of the surgical team, the surgical diagnostic criteria and the surgical methods were poorly described in most of the included studies. We now have a standardised technique for performing laparoscopy, and we recommend that any future studies use this standardised method for undertaking laparoscopy (Becker 2014). Additionally, we did not confine the studies included in this review to those that reported histological confirmation of endometriotic lesions. Although a recent ESHRE guideline stated that evidence is lacking to support laparoscopy without histology to confirm endometriosis (Dunselman 2014), the clinical significance of histological verification remains debatable. Diagnosis by surgical visualisation alone remains a common clinical practice and can be considered reliable when an accurate inspection of the abdominal cavity is performed by experienced surgeons. We chose to include the 25 studies (46%) that reported surgical visualisation as the only reference standard, as we did not wish to lose potentially valuable information by excluding these studies that did not confirm the diagnosis histologically; however, this could impact on the accuracy of assignment to the case and control groups.
There are no well‐established criteria for replacement or triage diagnostic tests, therefore we chose the criteria that were both realistic and clinically applicable to assist in the interpretation of the complex results. For a replacement test, we considered the threshold reported by the only systematic review on accuracy of the reference standard (laparoscopy) in the detection of endometriosis to be the most objective (Wykes 2004). The meta‐analysis was published in 2004 and included four eligible studies comprising 433 women. We acknowledge the limitations associated with emphasising a single review, particularly if it does not present the latest and possibly most accurate data that reflect advances in surgical expertise and technology. Several studies on accuracy of laparoscopy in detecting endometriosis have been published in the last decade; however, no reviews have assessed their results in a systematic way. A further systematic analysis to determine the accuracy of laparoscopy was beyond the scope of this review. The criteria for triage tests were based on the common concepts of SnOUT and SpIN in medical statistics, and the cut‐offs were set at levels we considered to be clinically relevant (see Role of index test(s)). We encourage readers to apply independent interpretations of the presented diagnostic estimates using thresholds that may be more applicable to specific populations and clinical circumstances.
Applicability of findings to the review question
In terms of applicability, there was a high concern regarding patient selection in 36 reports, unclear concern in 7 articles and low concern in only 11 studies (20%). We assigned high or unclear concern when the set of participants in the study was broader than that seen in clinical practice or when the spectrum of the target condition was limited, possibly making the findings inapplicable to the review question and clinical practice. Only one study presented an unclear concern with respect to index test applicability. The remaining 53 studies were of low concern regarding implementation of the index test and presented adequate information to conclude that conduct or interpretation of the test did not differ from the review question. We judged applicability of the reference standard to be satisfactory for all studies. However, the majority of included studies took place in academic institutions with a high level of expertise in laboratory techniques, and the index test outcome measures may not be reproducible in all institutions or extrapolated to general practice.
We excluded some potentially relevant, well‐designed studies, as they did not directly address the review question. For example, we excluded studies that reported on biomarkers with differential expression in endometriosis, but without providing enough information to assess the diagnostic performance of the biomarker. We also excluded some forms of endometriosis, such as bladder, ureteric or the forms involving extra‐pelvic sites (e.g. umbilicus, hernia sacs, abdominal wall, lung, kidney, etc.), as they were predominantly reported in case reports or small case series, and diagnostic laparoscopy is not an applicable reference test for these conditions. Although these target conditions are rare, from a clinical perspective the diagnostic options for these forms of endometriosis remain unclear.
Authors' conclusions
Implications for practice.
The ability to diagnose endometriosis with minimally invasive or non‐invasive tests would avoid invasive diagnostic surgery and reduce the potential delay in diagnosis. The ability to perform an endometrial biopsy in a clinical setting would allow earlier diagnosis, with fewer follow‐up visits, speedier institution of effective treatment and expected cost savings benefits for the patient and health service.
The review demonstrated that PGP 9.5, a marker of small nerve fibres, met the criteria for a replacement diagnostic test, but the included studies displayed substantial heterogeneity of diagnostic estimates. The expression of PGP 9.5 appears to be highly dependent on the type of sample collection and laboratory assays. The test requires further validation via utilisation of reproducible universal methodology before meriting a recommendation for its use in clinical practice.
Endometrial proteome (of which five peaks emerged as potential biomarkers for endometriosis) also remains a long‐term promising diagnostic test. Of note, separate research groups identified different sets of biomarkers by utilising a similar method of proteome detection, implying that a standardised analytical approach needs to be established along with further testing in several independent cohorts before making any conclusions.
Additional biomarkers identified in this review, such as 17βHSD2, IL‐1R2, caldesmon, and neural markers VIP, CGRP, SP, NPY displayed diagnostic estimates that qualified for either replacement or triage test, but these results came from individual studies, and hence further research is necessary.
In light of the above, currently there is not enough evidence to recommend any endometrial biomarker for use in clinical practice as a substitute for laparoscopy.
In this review we identified a list of biomarkers that have no value in detection of endometriosis and hence are not recommended for evaluation in future diagnostic studies. This is important for appropriate allocation of research resources and to guide clinically relevant experimental work in the field. These biomarkers include: CYP19, TIMP‐1 and VEGF.
As there is an absence of well‐established criteria for an adequate diagnostic test, we determined the diagnostic criteria for replacement and triage tests in a way that we believe will aid the interpretation for clinically active readers. However, we encourage readers to apply a different and relevant criteria according to each clinical population and situation.
Implications for research.
Currently randomised controlled treatment trials require women with and without endometriosis to have had diagnostic surgery for accurate group allocation. For ethical reasons, therapeutic surgery is usually performed at the same time, potentially biasing treatment trial outcomes. Thus, our current inability to diagnose and assess the progression of endometriosis in a non‐invasive way is a significant limitation in the advancement of clinical research in endometriosis.
Critical appraisal of the studies included in this review has identified several weaknesses in study design that can affect an objective evaluation of the presented findings, and in general, we rated the available evidence as low quality. Although several biomarkers appeared to have high diagnostic estimates, further well‐designed diagnostic studies are required to establish the diagnostic test accuracy and utility of these endometrial tests. We recommend that future authors consider:
recruiting a larger pool of participants following power calculations for sample size determination (Liu 2005b);
focusing on a single‐gate design that only includes a clinically relevant population (Rutjes 2005);
using a diagnostic test accuracy study design that adheres to the STARD reporting standards and includes the 25 items on the checklist and the QUADAS checklist (Bossuyt 2003; Whiting 2011);
working on improving techniques of endometrial biopsy, including endocervical analgesia, making further improvements on the biopsy cannulas and possibly developing endometrial brushes for superficial sampling of the endometrium (although this might not be suited for markers that are only expressed within the stroma);
developing a simplified and improved detection technique for neuronal immunohistochemical biomarkers, such as PGP 9.5, by utilising digitally enhanced assessment of differences in IHC appearances;
establishing universally acceptable laboratory methodologies and diagnostic criteria for a positive test, and formally assessing the inter‐ and intraobserver variability of the laboratory methods (Fassbender 2014);
utilising universally acceptable methods of performing laparoscopy as the reference standard test (Becker 2014);
undertaking direct comparisons of promising tests in conjunction with a cost‐effectiveness analysis;
applying testing to different clinical phenotypes rather than to women classified according to rASRM staging (Vitonis 2014);
assessing the long‐term outcomes and lifetime healthcare costs of women that have participated in diagnostic test accuracy trials of specific diagnostic tests.
Notes
The Cochrane review group split the initially planned single review on the non‐invasive tests for diagnosis of endometriosis into several smaller reviews in order to facilitate data handling and interpretation, due to abundance and diversity of the suggested tests. All of the reviews in the series drew from a common protocol designed for the purpose. The other reviews from the series include 'Blood biomarkers for the non‐invasive diagnosis of endometriosis', 'Urinary biomarkers for the non‐invasive diagnosis of endometriosis', 'Imaging modalities for the non‐invasive diagnosis of endometriosis' and 'Combined biomarkers for the non‐invasive diagnosis of endometriosis', which summarises the review findings of the series.
Acknowledgements
We would like to thank Associate Professor Petra Macaskill for her valuable comments and substantial contribution to the development of the statistical methods for the review. Sincere thanks to the late Professor Ali Akoum and Professor Cindy Farquhar for their intellectual input and help with drafting of the protocol. We are grateful to Marian Showell, the Trials Search Co‐ordinator of the Cochrane Gynaecology and Fertility Group, for her help in designing and conducting the literature search and in locating the full texts of relevant studies. We gratefully acknowledge the help of Wai Sun Lam, who provided comprehensive Chinese to English translation of numerous papers. We also thank the authors of the review series Deepika Arora, Emily Liu, Lucy Prentice and Rabia Shaikh for their dedicated assistance in the study selection process. Finally, we thank all contacted authors who contributed information to this review.
Appendices
Appendix 1. Alphabetical index of endometrial biomarkers
| Biomarker | Biological group | Biological sub‐group | |
| 1 | 15‐PGDH mRNA (15‐hydroxyprostaglandin dehydrogenase) | Mediators of prostaglandin biosynthesis | — |
| 2 | 17βHSD2 (17‐β hydroxysteroid dehydrogenase type 2) | Hormonal markers | — |
| 3 | Akr1B1 mRNA (aldoketoreductase ‐1B1, PGF2a synthase) | Mediators of prostaglandin biosynthesis | — |
| 4 | Akr1C3 mRNA (aldoketoreductase ‐1C3, PGF2a synthase) | Mediators of prostaglandin biosynthesis | — |
| 5 | AKT1 (RAC‐alpha serine/threonine‐protein kinase) | Transcription factors and signalling molecules | — |
| 6 | Bax (BCL2‐associated X protein) | Apoptosis markers and regulators | — |
| 7 | Bcl‐xL (B‐cell lymphoma‐extra large, or BCL2‐like 1 isoform 1) | Apoptosis markers and regulators | — |
| 8 | Bcl‐xL:Bcl‐xS ratio (ratio B‐cell lymphoma‐extra large/B‐cell lymphoma‐extra small) | Apoptosis markers and regulators | — |
| 9 | BW 495/36, endometrial epithelial marker | Cell proliferation markers | — |
| 10 | CD 4+ (T helper cells) | Immune system and inflammatory markers | Immune cells: peripheral blood mononuclear cells (PBMC) |
| 11 | CD 8+ (T suppressor cells) | Immune system and inflammatory markers | Immune cells: PBMC |
| 12 | CD 16+ (natural killer cells) | Immune system and inflammatory markers | Immune cells: PBMC |
| 13 | CD 22+ (B‐lymphocytes) | Immune system and inflammatory markers | Immune cells: PBMC |
| 14 | CD 38+ (granulated lymphocytes) | Immune system and inflammatory markers | Immune cells: PBMC |
| 15 | CD 56+ (granulated lymphocytes) | Immune system and inflammatory markers | Immune cells: PBMC |
| 16 | CD 68+ (macrophages) | Immune system and inflammatory markers | Immune cells: PBMC |
| 17 | CA‐125 (cancer antigen‐125) in menstrual fluid | Tumour markers | — |
| 18 | CALD1 (gene encoding for caldesmon) | Myogenic markers | — |
| 19 | Caldesmon (calmodulin binding protein) | Myogenic markers | — |
| 20 | Cdc2 (cyclin dependent kinase‐2) | Cell cycle regulatory molecules | — |
| 21 | CGRP (calcitonin gene‐related protein) | Nerve sheath and nerve growth markers | — |
| 22 | CK19 or CYFRA 21‐1 (cytokeratin 19) | Cytoskeleton molecules | — |
| 23 | Cox‐1 mRNA (cyclo‐oxygenase‐1) | Mediators of prostaglandin biosynthesis | — |
| 24 | cPGES mRNA (cytosolic PGE2 synthase) | Mediators of prostaglandin biosynthesis | — |
| 25 | Cyclin B1 | Cell cycle regulatory molecules | — |
| 26 | CYP19 (aromatase cytochrome P450) | Hormonal markers | — |
| 27 | Cytokeratin 18 | Cytoskeleton molecules | |
| 28 | Depolarised α6 Integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 29 | E‐cadherin | Cell adhesion molecules and other matrix‐related proteins | — |
| 30 | EGF (epidermal growth factor) | Angiogenesis and growth factors and their receptors | — |
| 31 | Endometrial proteome | High throughput markers | — |
| 32 | EST (oestrogen sulphotransferase) | Hormonal markers | — |
| 33 | ER ‐ α (oestrogen receptor ‐ alpha) | — | |
| 34 | ER ‐ β (oestrogen receptor ‐ beta) | — | |
| 35 | FGF‐2 (fibroblast growth factor‐2) | Angiogenesis and growth factors and their receptors | — |
| 36 | glycodelin A (PP14 or PAEP) ((placental protein 14 or progestogen‐associated endometrial protein)) | Angiogenesis and growth factors and their receptors | — |
| 37 | hBD‐2 (human b‐defensin‐2) | Other peptides and proteins | — |
| 38 | hTERT (human telomerase reverse transcriptase) | DNA‐repair and Telomer maintenance molecules | — |
| 39 | ICAM‐1 (intercellular adhesion molecule‐1) or sICAM‐1 (soluble form of intercellular adhesion molecule‐1) | Cell adhesion molecules and other matrix‐related proteins | — |
| 40 | IL‐11 | Immune system and inflammatory markers | Interleukins |
| 41 | IL‐1R1 (interleukin‐1 receptor type II) | Immune system and inflammatory markers | Interleukins |
| 42 | IL‐1R2 (interleukin‐1 receptor type II) | Immune system and inflammatory markers | Interleukins |
| 43 | IL‐1β | Immune system and inflammatory markers | Interleukins |
| 44 | JAG1 (jagged‐1 protein) | Transcription factors and signalling molecules | — |
| 45 | Ki‐67 (antigen KI‐67 or MKI67, marker of cellular proliferation) | Cell proliferation markers | — |
| 46 | LAMA5 (laminin subunit alpha‐5) | Cell adhesion molecules and other matrix‐related proteins | — |
| 47 | LFA‐3 (CD58) (leukocyte function associated molecule‐3) | Cell adhesion molecules and other matrix‐related proteins | — |
| 48 | LGR7 (leucine‐rich G protein‐coupled receptor 7), relaxin receptor | Hormonal markers | — |
| 49 | LIF (leukaemia‐inhibitory factor) | Immune system and inflammatory markers | Cytokines |
| 50 | Mitochondrial proteome | High throughput markers | — |
| 51 | MMP‐1 (matrix metalloproteinase‐1) | Cell adhesion molecules and other matrix‐related proteins | — |
| 52 | MMP‐9 (matrix metalloproteinase‐9) | Cell adhesion molecules and other matrix‐related proteins | — |
| 53 | MPO (myeloperoxidase) | Immune system and inflammatory markers | other immune/inflammatory markers |
| 54 | mRNAome (mRNA micro‐array) | High throughput markers | — |
| 55 | NAG (N‐acetyl‐b‐D‐Glucosaminidase) | Immune system and inflammatory markers | other immune/inflammatory markers |
| 56 | NF (neurofilament) | Nerve sheath and nerve growth markers | — |
| 57 | NPY (neuropeptide Y) | Nerve sheath and nerve growth markers | — |
| 58 | OPN (osteopontin) | Cell adhesion molecules and other matrix‐related proteins | — |
| 59‐61 | PAI‐1/‐2/‐3 (plasminogen activator inhibitors 1/2/3) | Cell adhesion molecules and other matrix‐related proteins | — |
| 62 | PDGF (platelet derived growth factor) | Angiogenesis and growth factors and their receptors | — |
| 63 | PGP 9.5 (protein gene product 9.5) | Nerve sheath and nerve growth markers | — |
| 64 | PIGF (Placental growth factor) | Angiogenesis and growth factors and their receptors | — |
| 65 | PKR1 (prokineticin receptor 1), EG‐VEGF receptor | Angiogenesis and growth factors and their receptors | — |
| 66 | PKR2 (prokineticin receptor 2), EG‐VEGF receptor | Angiogenesis and growth factors and their receptors | — |
| 67 | Plk1 (polo‐like kinase‐1) | Cell cycle regulatory molecules | — |
| 68 | PROK‐1 (prokineticin 1) | Angiogenesis and growth factors and their receptors | — |
| 69 | Relaxin | Hormonal markers | — |
| 70 | SP (substance P) | Nerve sheath and nerve growth markers | — |
| 71 | Telomerase activity | DNA‐repair and Telomer maintenance molecules | — |
| 72 | TIMP‐1 (tissue inhibitor of metalloproteinases) | Cell adhesion molecules and other matrix‐related proteins | — |
| 73 | TNF‐α (tumour necrosis factor alpha) | Immune system and inflammatory markers | Cytokines |
| 74 | tPA (tissue‐type plasminogen activator) | Cell adhesion molecules and other matrix‐related proteins | — |
| 75 | TSP‐1 (thrombospondin‐1) | Angiogenesis and growth factors and their receptors | — |
| 76 | TYMP (thymidine phosphorylase) | Angiogenesis and growth factors and their receptors | — |
| 77 | uPA (urokinase‐type plasminogen activator) | Cell adhesion molecules and other matrix‐related proteins | — |
| 78 | VCAM‐1 (CD106) (vascular cell adhesion molecule‐1) | Cell adhesion molecules and other matrix‐related proteins | — |
| 79 | VEGF (vascular endothelial growth factor) | Angiogenesis and growth factors and their receptors | — |
| 80 | Vimentin | Cytoskeleton molecules | — |
| 81 | VIP (vasoactive intestinal polypeptide) | Nerve sheath and nerve growth markers | — |
| 82 | α2β1 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 83 | α3β1 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 84 | α4β1 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 85 | α5β1 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 86 | α6 β1 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 87 | αVβ3 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
| 88 | αVβ5 integrins | Cell adhesion molecules and other matrix‐related proteins | — |
| 89 | αVβ6 integrins | Cell adhesion molecules and other matrix‐related proteins | — |
| 90 | β1 integrin | Cell adhesion molecules and other matrix‐related proteins | — |
Appendix 2. Search strategy for CENTRAL (OVID platform)
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <July 2015 (3.09.2015)>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (biomarker$ or marker$).tw. (23692)
2 Laboratory Test$.tw. (2793)
3 growth factor$.tw. (5448)
4 scatter factor$.tw. (8)
5 cytokine$.tw. (6264)
6 hepatocyte growth factor.tw. (111)
7 (FGF or fibroblast growth factor$).tw. (433)
8 (PDGF or platelet derived growth factor$).tw. (250)
9 (EGF or epidermal growth factor$).tw. (1077)
10 (IGF‐I or insulin‐like growth factor$ or IGF1).tw. (2132)
11 (TGF‐a or transforming growth factor alfa or TGFa).tw. (519)
12 (TGF‐b or transforming growth factor beta or TGFb).tw. (236)
13 (EGFR or epidermal growth factor receptor$).tw. (1905)
14 (VEGF or vascular endothelial growth factor$).tw. (1532)
15 exp Luteinizing Hormone/bl [Blood] (151)
16 leptin$.tw. (1399)
17 exp Progesterone/bl [Blood] (58)
18 Proteolytic enzyme$.tw. (136)
19 exp matrix metalloproteinase 1/ or exp matrix metalloproteinase 2/ or exp matrix metalloproteinase 3/ or exp matrix metalloproteinase 9/ (292)
20 matrix metalloproteinase$.tw. (676)
21 MMP$.tw. (905)
22 TIMP$.tw. (229)
23 exp "tissue inhibitor of metalloproteinase‐1"/ or exp "tissue inhibitor of metalloproteinase‐2"/ (101)
24 exp Glycoproteins/ (10108)
25 (Ca‐125 or Ca125 or cancer antigen 125).tw. (305)
26 (Ca‐19‐9 or Ca19‐9 or cancer antigen 19‐9).tw. (71)
27 (PP 14 or PP14).tw. (23)
28 serum placental protein$.tw. (6)
29 exp Follistatin/ (13)
30 Osteopontin$.tw. (80)
31 exp intercellular adhesion molecule‐1/ or exp selectins/ (929)
32 soluble intercellular adhesion.tw. (256)
33 Soluble adhesion molecule$.tw. (89)
34 sICAM.tw. (319)
35 sVCAM$.tw. (223)
36 (sEcadherin or soluble E‐cadherin).tw. (4)
37 (sEselectin or soluble E‐selectin).tw. (99)
38 exp t‐lymphocytes/ or exp natural killer t‐cells/ (2645)
39 Immune cells alteration$.tw. (1)
40 (T helper$ or T supressor$ or T helper$ T supressor$ ratio).tw. (445)
41 Total complement level$.tw. (0)
42 Autoantibodies.tw. (428)
43 exp Antibodies, Antiphospholipid/ (85)
44 Anti‐endometrial.tw. (0)
45 Antiphospholipid$.tw. (152)
46 exp hla antigens/ or exp hla‐a1 antigen/ or exp hla‐a2 antigen/ (563)
47 (HLA or human leucocyte antigen$).tw. (1724)
48 Anti‐laminin‐1.tw. (0)
49 Anti‐thyroid.tw. (49)
50 Anti‐Thomsen Friedenreich antigen$.tw. (0)
51 Anti‐transferrin.tw. (0)
52 Anti‐LDL.tw. (3)
53 (Anti‐2HSG or Heremans‐Schmidt glycoprotein).tw. (0)
54 interleukin$.tw. (7276)
55 (MCP‐I or monocyte chemoattractant protein‐I).tw. (0)
56 (MIF or migration inhibitory factor$).tw. (75)
57 (TNF‐a or tumour necrosis factor$ alfa).tw. (3923)
58 Fas ligand$.tw. (47)
59 Endometrial marker$.tw. (2)
60 CAMs.tw. (53)
61 cell adhesion molecule$.tw. (568)
62 exp Integrins/ (781)
63 Integrin$.tw. (248)
64 Selectin$.tw. (2183)
65 Cadherin$.tw. (71)
66 Aromatase P450.tw. (3)
67 estrogen receptor$.tw. (1252)
68 progesterone receptor$.tw. (531)
69 MTMMP$.tw. (0)
70 cyr61.tw. (1)
71 exp Cysteine‐Rich Protein 61/ (1)
72 cysteine‐rich heparin‐binding protein$.tw. (0)
73 (ANXA 1 or ANXA1).tw. (3)
74 (Annexin 1 or Annexin1).tw. (2)
75 (PGP 9?5 or PGP9?5 or protein gene product$).tw. (18)
76 serum marker$.tw. (411)
77 neural marker$.tw. (9)
78 cell surface marker$.tw. (46)
79 inflammatory marker$.tw. (1739)
80 microarray$.tw. (501)
81 microRNA$.tw. (103)
82 proteomic$.tw. (176)
83 genomic$.tw. (526)
84 (endometri$ adj2 biops$).tw. (464)
85 Follistatin$.tw. (26)
86 Vascular Endothelial Growth Factor A/ (560)
87 Vitamin D‐Binding Protein/ (18)
88 exp Cytokines/ (13960)
89 exp interleukins/ or exp interleukin‐1/ or exp interleukin‐6/ or exp interleukin‐8/ or exp interleukin‐12/ or exp interleukin‐13/ (4413)
90 exp Epidermal Growth Factor/ (91)
91 exp Fibroblast Growth Factors/ (197)
92 Platelet‐Derived Growth Factor/ (99)
93 Keratin‐19/ (19)
94 exp Clinical Laboratory Techniques/ (35164)
95 (Luteinizing Hormone$ or LH).tw. (2935)
96 cytokeratin‐19.tw. (25)
97 (VDBP or vitamin D‐binding protein$).tw. (44)
98 urinary peptide$.tw. (8)
99 VDBP‐Cr.tw. (0)
100 urinary VDBP corrected for creatinine expression.tw. (0)
101 urinary marker$.tw. (67)
102 or/1‐101 (90390)
103 Endometriosis/di [Diagnosis] (6)
104 102 or 103 (90394)
105 exp Endometriosis/ (469)
106 Endometrio$.tw. (1026)
107 105 or 106 (1067)
108 104 and 107 (226)
109 (animals not (humans and animals)).sh. (1)
110 108 not 109 (226)
Appendix 3. Search strategy for MEDLINE (OVID platform)
Database: MEDLINE (Ovid) <1946 to February, week 2 2015 (16.2.2015)> >
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
Search Strategy:
1 (biomarker$ or marker$).tw. (605002)
2 Laboratory Test$.tw. (29839)
3 growth factor$.tw. (272049)
4 scatter factor$.tw. (1287)
5 cytokine$.tw. (250618)
6 hepatocyte growth factor.tw. (8053)
7 (FGF or fibroblast growth factor$).tw. (31798)
8 (PDGF or platelet derived growth factor$).tw. (19864)
9 (EGF or epidermal growth factor$).tw. (58069)
10 (IGF‐I or insulin‐like growth factor$ or IGF1).tw. (43539)
11 (TGF‐a or transforming growth factor alfa or TGFa).tw. (281)
12 (TGF‐b or transforming growth factor beta or TGFb).tw. (28842)
13 (EGFR or epidermal growth factor receptor$).tw. (41719)
14 (VEGF or vascular endothelial growth factor$).tw. (53588)
15 exp Luteinizing Hormone/bl (Blood) (24587)
16 leptin$.tw. (24994)
17 exp Progesterone/bl (Blood) (18412)
18 Proteolytic enzyme$.tw. (9768)
19 exp matrix metalloproteinase 1/ or exp matrix metalloproteinase 2/ or exp matrix metalloproteinase 3/ or exp matrix metalloproteinase 9/ (22968)
20 matrix metalloproteinase$.tw. (34522)
21 MMP$.tw. (44439)
22 TIMP$.tw. (10777)
23 exp "tissue inhibitor of metalloproteinase‐1"/ or exp "tissue inhibitor of metalloproteinase‐2"/ (6146)
24 exp Glycoproteins/ (637149)
25 (Ca‐125 or Ca125 or cancer antigen 125).tw. (6761)
26 (Ca‐19‐9 or Ca19‐9 or cancer antigen 19‐9).tw. (4194)
27 (PP 14 or PP14).tw. (229)
28 serum placental protein$.tw. (33)
29 exp Follistatin/ (1134)
30 Osteopontin$.tw. (6769)
31 exp intercellular adhesion molecule‐1/ or exp selectins/ (25302)
32 soluble intercellular adhesion.tw. (1588)
33 Soluble adhesion molecule$.tw. (779)
34 sICAM.tw. (2258)
35 sVCAM$.tw. (1277)
36 (sEcadherin or soluble E‐cadherin).tw. (95)
37 (sEselectin or soluble E‐selectin).tw. (689)
38 exp t‐lymphocytes/ or exp natural killer t‐cells/ (272580)
39 Immune cells alteration$.tw. (1)
40 (T helper$ or T supressor$ or T helper$ T supressor$ ratio).tw. (21275)
41 Total complement level$.tw. (23)
42 Autoantibodies.tw. (33457)
43 exp Antibodies, Antiphospholipid/ (7522)
44 Anti‐endometrial.tw. (23)
45 Antiphospholipid$.tw. (9974)
46 exp hla antigens/ or exp hla‐a1 antigen/ or exp hla‐a2 antigen/ (64462)
47 (HLA or human leucocyte antigen$).tw. (80501)
48 Anti‐laminin‐1.tw. (33)
49 Anti‐thyroid.tw. (1414)
50 Anti‐Thomsen Friedenreich antigen$.tw. (6)
51 Anti‐transferrin.tw. (275)
52 Anti‐LDL.tw. (181)
53 (Anti‐2HSG or Heremans‐Schmidt glycoprotein).tw. (3)
54 interleukin$.tw. (175195)
55 (MCP‐I or monocyte chemoattractant protein‐I).tw. (44)
56 (MIF or migration inhibitory factor$).tw. (4479)
57 (TNF‐a or tumour necrosis factor$ alfa).tw. (1344)
58 Fas ligand$.tw. (6032)
59 Endometrial marker$.tw. (11)
60 CAMs.tw. (1756)
61 cell adhesion molecule$.tw. (20903)
62 exp Integrins/ (44414)
63 Integrin$.tw. (39960)
64 Selectin$.tw. (55426)
65 Cadherin$.tw. (20780)
66 Aromatase P450.tw. (180)
67 estrogen receptor$.tw. (38819)
68 progesterone receptor$.tw. (16623)
69 MTMMP$.tw. (7)
70 cyr61.tw. (559)
71 exp Cysteine‐Rich Protein 61/ (386)
72 cysteine‐rich heparin‐binding protein$.tw. (9)
73 (ANXA 1 or ANXA1).tw. (313)
74 (Annexin 1 or Annexin1).tw. (339)
75 (PGP 9?5 or PGP9?5 or protein gene product$).tw. (2096)
76 serum marker$.tw. (5429)
77 neural marker$.tw. (925)
78 cell surface marker$.tw. (4456)
79 inflammatory marker$.tw. (10916)
80 microarray$.tw. (75404)
81 microRNA$.tw. (29731)
82 proteomic$.tw. (45292)
83 genomic$.tw. (190985)
84 (endometri$ adj2 biops$).tw. (3411)
85 Follistatin$.tw. (1663)
86 Vascular Endothelial Growth Factor A/ (35738)
87 Vitamin D‐Binding Protein/ (1282)
88 exp Cytokines/ (547522)
89 exp interleukins/ or exp interleukin‐1/ or exp interleukin‐6/ or exp interleukin‐8/ or exp interleukin‐12/ or exp interleukin‐13/ (188479)
90 exp Epidermal Growth Factor/ (21298)
91 exp Fibroblast Growth Factors/ (25075)
92 Platelet‐Derived Growth Factor/ (11030)
93 Keratin‐19/ (1090)
94 exp Clinical Laboratory Techniques/ (2132820)
95 (Luteinizing Hormone$ or LH).tw. (56679)
96 cytokeratin‐19.tw. (1469)
97 (VDBP or vitamin D‐binding protein$).tw. (1158)
98 urinary peptide$.tw. (137)
99 VDBP‐Cr.tw. (1)
100 urinary VDBP corrected for creatinine expression.tw. (1)
101 urinary marker$.tw. (638)
102 or/1‐101 (4086291)
103 Endometriosis/di (Diagnosis) (3354)
104 102 or 103 (4088946)
105 exp Endometriosis/ (17244)
106 Endometrio$.tw. (21492)
107 105 or 106 (24940)
108 104 and 107 (10490)
109 (animals not (humans and animals)).sh. (3892900)
110 108 not 109 (10113)
Additional search February 2015 ‐ May 2015
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present (3.9.2015)>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (biomarker$ or marker$).tw. (652345)
2 Laboratory Test$.tw. (31389)
3 growth factor$.tw. (287701)
4 scatter factor$.tw. (1326)
5 cytokine$.tw. (267766)
6 hepatocyte growth factor.tw. (8585)
7 (FGF or fibroblast growth factor$).tw. (33674)
8 (PDGF or platelet derived growth factor$).tw. (20842)
9 (EGF or epidermal growth factor$).tw. (61625)
10 (IGF‐I or insulin‐like growth factor$ or IGF1).tw. (45386)
11 (TGF‐a or transforming growth factor alfa or TGFa).tw. (306)
12 (TGF‐b or transforming growth factor beta or TGFb).tw. (30559)
13 (EGFR or epidermal growth factor receptor$).tw. (46446)
14 (VEGF or vascular endothelial growth factor$).tw. (58203)
15 exp Luteinizing Hormone/bl [Blood] (24870)
16 leptin$.tw. (26783)
17 exp Progesterone/bl [Blood] (18699)
18 Proteolytic enzyme$.tw. (9992)
19 exp matrix metalloproteinase 1/ or exp matrix metalloproteinase 2/ or exp matrix metalloproteinase 3/ or exp matrix metalloproteinase 9/ (24504)
20 matrix metalloproteinase$.tw. (37055)
21 MMP$.tw. (47849)
22 TIMP$.tw. (11419)
23 exp "tissue inhibitor of metalloproteinase‐1"/ or exp "tissue inhibitor of metalloproteinase‐2"/ (6447)
24 exp Glycoproteins/ (662211)
25 (Ca‐125 or Ca125 or cancer antigen 125).tw. (7058)
26 (Ca‐19‐9 or Ca19‐9 or cancer antigen 19‐9).tw. (4399)
27 (PP 14 or PP14).tw. (232)
28 serum placental protein$.tw. (34)
29 exp Follistatin/ (1180)
30 Osteopontin$.tw. (7267)
31 exp intercellular adhesion molecule‐1/ or exp selectins/ (26225)
32 soluble intercellular adhesion.tw. (1663)
33 Soluble adhesion molecule$.tw. (795)
34 sICAM.tw. (2374)
35 sVCAM$.tw. (1360)
36 (sEcadherin or soluble E‐cadherin).tw. (97)
37 (sEselectin or soluble E‐selectin).tw. (713)
38 exp t‐lymphocytes/ or exp natural killer t‐cells/ (284378)
39 Immune cells alteration$.tw. (1)
40 (T helper$ or T supressor$ or T helper$ T supressor$ ratio).tw. (22494)
41 Total complement level$.tw. (24)
42 Autoantibodies.tw. (35161)
43 exp Antibodies, Antiphospholipid/ (7759)
44 Anti‐endometrial.tw. (22)
45 Antiphospholipid$.tw. (10351)
46 exp hla antigens/ or exp hla‐a1 antigen/ or exp hla‐a2 antigen/ (66724)
47 (HLA or human leucocyte antigen$).tw. (83856)
48 Anti‐laminin‐1.tw. (33)
49 Anti‐thyroid.tw. (1478)
50 Anti‐Thomsen Friedenreich antigen$.tw. (8)
51 Anti‐transferrin.tw. (284)
52 Anti‐LDL.tw. (183)
53 (Anti‐2HSG or Heremans‐Schmidt glycoprotein).tw. (3)
54 interleukin$.tw. (184697)
55 (MCP‐I or monocyte chemoattractant protein‐I).tw. (46)
56 (MIF or migration inhibitory factor$).tw. (4718)
57 (TNF‐a or tumour necrosis factor$ alfa).tw. (1428)
58 Fas ligand$.tw. (6204)
59 Endometrial marker$.tw. (11)
60 CAMs.tw. (1823)
61 cell adhesion molecule$.tw. (22033)
62 exp Integrins/ (46487)
63 Integrin$.tw. (42447)
64 Selectin$.tw. (58540)
65 Cadherin$.tw. (22688)
66 Aromatase P450.tw. (182)
67 estrogen receptor$.tw. (41210)
68 progesterone receptor$.tw. (17437)
69 MTMMP$.tw. (7)
70 cyr61.tw. (620)
71 exp Cysteine‐Rich Protein 61/ (425)
72 cysteine‐rich heparin‐binding protein$.tw. (9)
73 (ANXA 1 or ANXA1).tw. (355)
74 (Annexin 1 or Annexin1).tw. (358)
75 (PGP 9?5 or PGP9?5 or protein gene product$).tw. (2190)
76 serum marker$.tw. (5721)
77 neural marker$.tw. (1026)
78 cell surface marker$.tw. (4751)
79 inflammatory marker$.tw. (12244)
80 microarray$.tw. (81764)
81 microRNA$.tw. (35967)
82 proteomic$.tw. (49911)
83 genomic$.tw. (205064)
84 (endometri$ adj2 biops$).tw. (3518)
85 Follistatin$.tw. (1762)
86 Vascular Endothelial Growth Factor A/ (38477)
87 Vitamin D‐Binding Protein/ (1356)
88 exp Cytokines/ (575020)
89 exp interleukins/ or exp interleukin‐1/ or exp interleukin‐6/ or exp interleukin‐8/ or exp interleukin‐12/ or exp interleukin‐13/ (197567)
90 exp Epidermal Growth Factor/ (21875)
91 exp Fibroblast Growth Factors/ (26259)
92 Platelet‐Derived Growth Factor/ (11355)
93 Keratin‐19/ (1179)
94 exp Clinical Laboratory Techniques/ (2203416)
95 (Luteinizing Hormone$ or LH).tw. (57796)
96 cytokeratin‐19.tw. (1538)
97 (VDBP or vitamin D‐binding protein$).tw. (1262)
98 urinary peptide$.tw. (148)
99 VDBP‐Cr.tw. (1)
100 urinary VDBP corrected for creatinine expression.tw. (1)
101 urinary marker$.tw. (679)
102 or/1‐101 (4283825)
103 Endometriosis/di [Diagnosis] (3449)
104 102 or 103 (4286552)
105 exp Endometriosis/ (17833)
106 Endometrio$.tw. (22478)
107 105 or 106 (26003)
108 104 and 107 (10936)
109 (animals not (humans and animals)).sh. (4004321)
110 108 not 109 (10539)
111 (201501$ or 201502$ or 201503$ or 201504$).ed. (322721)
112 110 and 111 (215)
Appendix 4. Search strategy for EMBASE (OVID platform)
Database: EMBASE (Ovid) <1980 to 2015 Week 07 (16.02.2015)>
Search strategy:
1 Laboratory Test$.tw. (41662)
2 growth factor$.tw. (318593)
3 scatter factor$.tw. (1388)
4 cytokine$.tw. (322134)
5 hepatocyte growth factor.tw. (9594)
6 (FGF or fibroblast growth factor$).tw. (37191)
7 (PDGF or platelet derived growth factor$).tw. (23530)
8 (EGF or epidermal growth factor$).tw. (69553)
9 (IGF‐I or insulin‐like growth factor$ or IGF1).tw. (49806)
10 (TGF‐a or transforming growth factor alfa or TGFa).tw. (542)
11 (TGF‐b or transforming growth factor beta or TGFb).tw. (30820)
12 (EGFR or epidermal growth factor receptor$).tw. (64664)
13 (VEGF or vascular endothelial growth factor$).tw. (73191)
14 exp luteinizing hormone/ec (Endogenous Compound) (21924)
15 leptin$.tw. (32576)
16 exp progesterone blood level/ or exp progesterone urine level/ (6285)
17 Proteolytic enzyme$.tw. (9643)
18 exp matrix metalloproteinase/ (19364)
19 matrix metalloproteinase$.tw. (41445)
20 MMP$.tw. (58466)
21 TIMP$.tw. (14174)
22 exp "tissue inhibitor of metalloproteinase 2"/ (4824)
23 exp "tissue inhibitor of metalloproteinase 1"/ (8779)
24 exp glycoprotein/ec (Endogenous Compound) (246077)
25 (Ca‐125 or Ca125 or cancer antigen 125).tw. (9536)
26 (Ca‐19‐9 or Ca19‐9 or cancer antigen 19‐9).tw. (6054)
27 (PP 14 or PP14).tw. (244)
28 serum placental protein$.tw. (43)
29 exp follistatin/ (2148)
30 Osteopontin$.tw. (8475)
31 exp intercellular adhesion molecule 1/ (32066)
32 exp selectin/ (3082)
33 soluble intercellular adhesion.tw. (1788)
34 Soluble adhesion molecule$.tw. (919)
35 sICAM.tw. (2888)
36 sVCAM$.tw. (1793)
37 (sEcadherin or soluble E‐cadherin).tw. (120)
38 (sEselectin or soluble E‐selectin).tw. (822)
39 exp T lymphocyte/ (374675)
40 exp natural killer T cell/ (5800)
41 Immune cells alteration$.tw. (6)
42 (T helper$ or T supressor$ or T helper$ T supressor$ ratio).tw. (24786)
43 Total complement level$.tw. (20)
44 Autoantibodies.tw. (42037)
45 exp phospholipid antibody/ (9920)
46 Anti‐endometrial.tw. (23)
47 Antiphospholipid$.tw. (13777)
48 exp HLA antigen/ (81011)
49 exp HLA A1 antigen/ (597)
50 exp HLA A2 antigen/ (3288)
51 (HLA or human leucocyte antigen$).tw. (104497)
52 Anti‐laminin‐1.tw. (43)
53 Anti‐thyroid.tw. (1873)
54 Anti‐Thomsen Friedenreich antigen$.tw. (5)
55 Anti‐transferrin.tw. (290)
56 Anti‐LDL.tw. (186)
57 (Anti‐2HSG or Heremans‐Schmidt glycoprotein).tw. (4)
58 interleukin$.tw. (199692)
59 (MCP‐I or monocyte chemoattractant protein‐I).tw. (112)
60 (MIF or migration inhibitory factor$).tw. (5063)
61 (TNF‐a or tumour necrosis factor$ alfa).tw. (5998)
62 Fas ligand$.tw. (6708)
63 Endometrial marker$.tw. (18)
64 CAMs.tw. (2100)
65 cell adhesion molecule$.tw. (24039)
66 exp integrin/ (29036)
67 Integrin$.tw. (48293)
68 Selectin$.tw. (67300)
69 Cadherin$.tw. (27150)
70 Aromatase P450.tw. (202)
71 estrogen receptor$.tw. (46656)
72 progesterone receptor$.tw. (19861)
73 MTMMP$.tw. (15)
74 cyr61.tw. (755)
75 exp cysteine rich protein 61/ (753)
76 cysteine‐rich heparin‐binding protein$.tw. (12)
77 (ANXA 1 or ANXA1).tw. (452)
78 (Annexin 1 or Annexin1).tw. (425)
79 (PGP 9?5 or PGP9?5 or protein gene product$).tw. (2620)
80 serum marker$.tw. (7720)
81 neural marker$.tw. (1119)
82 cell surface marker$.tw. (5851)
83 inflammatory marker$.tw. (17339)
84 microarray$.tw. (101846)
85 microRNA$.tw. (40082)
86 proteomic$.tw. (55191)
87 genomic$.tw. (217184)
88 (endometri$ adj2 biops$).tw. (4369)
89 Follistatin$.tw. (1945)
90 exp vasculotropin/ (69810)
91 Vascular Endothelial Growth Factor A.tw. (2275)
92 exp vitamin D binding protein/ (2064)
93 exp cytokine/ (1034772)
94 exp interleukin derivative/ (2790)
95 exp interleukin 1/ (48499)
96 exp interleukin 6/ (136328)
97 exp interleukin 8/ (48884)
98 exp interleukin 12/ (31842)
99 exp interleukin 13/ (13584)
100 exp epidermal growth factor/ (32130)
101 exp fibroblast growth factor/ (13858)
102 cytokeratin 19/ (3601)
103 platelet derived growth factor/ (18930)
104 cytokeratin‐19.tw. (1918)
105 (VDBP or vitamin D‐binding protein$).tw. (1413)
106 urinary peptide$.tw. (174)
107 VDBP‐Cr.tw. (1)
108 urinary VDBP corrected for creatinine expression.tw. (1)
109 urinary marker$.tw. (830)
110 exp blood analysis/ (118854)
111 exp endometrium biopsy/ (4988)
112 exp urinalysis/ or exp biological marker/ (210153)
113 (biomarker or biomarkers).tw. (159748)
114 or/1‐113 (2734501)
115 endometriosis/di (Diagnosis) (4979)
116 114 or 115 (2738583)
117 exp endometriosis/ (25923)
118 Endometriosis.tw. (22110)
119 117 or 118 (27911)
120 116 and 119 (10326)
121 Animal/ not Human/ (1204497)
122 120 not 121 (10279)
Additional search February 2015 ‐ May 2015
Embase <1980 to 2015 Week 35 (3.09.2015)>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Laboratory Test$.tw. (44290)
2 growth factor$.tw. (335543)
3 scatter factor$.tw. (1407)
4 cytokine$.tw. (343623)
5 hepatocyte growth factor.tw. (10104)
6 (FGF or fibroblast growth factor$).tw. (39159)
7 (PDGF or platelet derived growth factor$).tw. (24591)
8 (EGF or epidermal growth factor$).tw. (73599)
9 (IGF‐I or insulin‐like growth factor$ or IGF1).tw. (51838)
10 (TGF‐a or transforming growth factor alfa or TGFa).tw. (583)
11 (TGF‐b or transforming growth factor beta or TGFb).tw. (32580)
12 (EGFR or epidermal growth factor receptor$).tw. (71526)
13 (VEGF or vascular endothelial growth factor$).tw. (79087)
14 exp luteinizing hormone/ec [Endogenous Compound] (22767)
15 leptin$.tw. (34921)
16 exp progesterone blood level/ or exp progesterone urine level/ (6534)
17 Proteolytic enzyme$.tw. (9903)
18 exp matrix metalloproteinase/ (20462)
19 matrix metalloproteinase$.tw. (44380)
20 MMP$.tw. (63208)
21 TIMP$.tw. (15146)
22 exp "tissue inhibitor of metalloproteinase 2"/ (5136)
23 exp "tissue inhibitor of metalloproteinase 1"/ (9381)
24 exp glycoprotein/ec [Endogenous Compound] (260024)
25 (Ca‐125 or Ca125 or cancer antigen 125).tw. (10051)
26 (Ca‐19‐9 or Ca19‐9 or cancer antigen 19‐9).tw. (6446)
27 (PP 14 or PP14).tw. (243)
28 serum placental protein$.tw. (44)
29 exp follistatin/ (2283)
30 Osteopontin$.tw. (9173)
31 exp intercellular adhesion molecule 1/ (33492)
32 exp selectin/ (3217)
33 soluble intercellular adhesion.tw. (1865)
34 Soluble adhesion molecule$.tw. (944)
35 sICAM.tw. (3049)
36 sVCAM$.tw. (1924)
37 (sEcadherin or soluble E‐cadherin).tw. (125)
38 (sEselectin or soluble E‐selectin).tw. (861)
39 exp T lymphocyte/ (394405)
40 exp natural killer T cell/ (6310)
41 Immune cells alteration$.tw. (6)
42 (T helper$ or T supressor$ or T helper$ T supressor$ ratio).tw. (26082)
43 Total complement level$.tw. (20)
44 Autoantibodies.tw. (44153)
45 exp phospholipid antibody/ (10362)
46 Anti‐endometrial.tw. (25)
47 Antiphospholipid$.tw. (14399)
48 exp HLA antigen/ (83748)
49 exp HLA A1 antigen/ (622)
50 exp HLA A2 antigen/ (3409)
51 (HLA or human leucocyte antigen$).tw. (109332)
52 Anti‐laminin‐1.tw. (43)
53 Anti‐thyroid.tw. (2059)
54 Anti‐Thomsen Friedenreich antigen$.tw. (7)
55 Anti‐transferrin.tw. (297)
56 Anti‐LDL.tw. (191)
57 (Anti‐2HSG or Heremans‐Schmidt glycoprotein).tw. (4)
58 interleukin$.tw. (210083)
59 (MCP‐I or monocyte chemoattractant protein‐I).tw. (114)
60 (MIF or migration inhibitory factor$).tw. (5342)
61 (TNF‐a or tumour necrosis factor$ alfa).tw. (6488)
62 Fas ligand$.tw. (6895)
63 Endometrial marker$.tw. (18)
64 CAMs.tw. (2198)
65 cell adhesion molecule$.tw. (25207)
66 exp integrin/ (30330)
67 Integrin$.tw. (50938)
68 Selectin$.tw. (71624)
69 Cadherin$.tw. (29496)
70 Aromatase P450.tw. (207)
71 estrogen receptor$.tw. (49530)
72 progesterone receptor$.tw. (21068)
73 MTMMP$.tw. (16)
74 cyr61.tw. (822)
75 exp cysteine rich protein 61/ (829)
76 cysteine‐rich heparin‐binding protein$.tw. (12)
77 (ANXA 1 or ANXA1).tw. (500)
78 (Annexin 1 or Annexin1).tw. (440)
79 (PGP 9?5 or PGP9?5 or protein gene product$).tw. (2760)
80 serum marker$.tw. (8158)
81 neural marker$.tw. (1234)
82 cell surface marker$.tw. (6222)
83 inflammatory marker$.tw. (19492)
84 microarray$.tw. (110181)
85 microRNA$.tw. (47554)
86 proteomic$.tw. (60599)
87 genomic$.tw. (233444)
88 (endometri$ adj2 biops$).tw. (4589)
89 Follistatin$.tw. (2081)
90 exp vasculotropin/ (74115)
91 Vascular Endothelial Growth Factor A.tw. (2526)
92 exp vitamin D binding protein/ (2196)
93 exp cytokine/ (1094317)
94 exp interleukin derivative/ (3281)
95 exp interleukin 1/ (50850)
96 exp interleukin 6/ (147379)
97 exp interleukin 8/ (52281)
98 exp interleukin 12/ (33479)
99 exp interleukin 13/ (14685)
100 exp epidermal growth factor/ (33057)
101 exp fibroblast growth factor/ (14499)
102 cytokeratin 19/ (3886)
103 platelet derived growth factor/ (19655)
104 cytokeratin‐19.tw. (2030)
105 (VDBP or vitamin D‐binding protein$).tw. (1520)
106 urinary peptide$.tw. (189)
107 VDBP‐Cr.tw. (1)
108 urinary VDBP corrected for creatinine expression.tw. (1)
109 urinary marker$.tw. (883)
110 exp blood analysis/ (124468)
111 exp endometrium biopsy/ (5197)
112 exp urinalysis/ or exp biological marker/ (232619)
113 (biomarker or biomarkers).tw. (182609)
114 or/1‐113 (2911073)
115 endometriosis/di [Diagnosis] (5173)
116 114 or 115 (2915302)
117 exp endometriosis/ (27433)
118 Endometriosis.tw. (23449)
119 117 or 118 (29532)
120 116 and 119 (10922)
121 Animal/ not Human/ (1261620)
122 120 not 121 (10862)
123 (201501$ or 201502$ or 201503$ or 201504$).em. (49200)
124 122 and 123 (34)
Appendix 5. Search strategy for CINAHL (EBSCO platform)
Database: CINAHL Plus with Full Text (EBSCOhost) <1980 to 20.04.2015>
Search strategy:
| # | Query | Results |
| S97 | S3 AND S96 | 1131 |
| S96 | S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81 OR S82 OR S83 OR S84 OR S85 OR S86 OR S87 OR S88 OR S89 OR S90 OR S91 OR S92 OR S93 OR S94 OR S95 | 341775 |
| S95 | TX urinary peptide* | 1598 |
| S94 | TX (VDBP or vitamin D‐binding protein*) | 134 |
| S93 | TX cytokeratin‐19 | 109 |
| S92 | TX (Luteinizing Hormone* or LH) | 18041 |
| S91 | (MH "Diagnosis, Laboratory+") | 101773 |
| S90 | "Keratin‐19" | 2 |
| S89 | (MH "Platelet‐Derived Growth Factor") | 394 |
| S88 | (MH "Epidermal Growth Factors") | 1264 |
| S87 | (MH "Interleukins") | 6584 |
| S86 | (MH "Cytokines") | 6860 |
| S85 | TX Vitamin D‐Binding Protein | 131 |
| S84 | (MH "Vascular Endothelial Growth Factor A") | 194 |
| S83 | TX (endometri* N2 biops*) | 432 |
| S82 | TX (endometri* adj2 biops*) | 0 |
| S81 | TX genomic$ | 7487 |
| S80 | TX proteomic* | 2434 |
| S79 | TX microRNA | 824 |
| S78 | TX microarray | 3123 |
| S77 | TX (PGP 95 or PGP95 or protein gene product*) | 9925 |
| S76 | TX (Annexin 1 or Annexin1) | 472 |
| S75 | TX (ANXA 1 or ANXA1) | 41 |
| S74 | TX cysteine‐rich heparin‐binding protein* | 12 |
| S73 | (MH "Protein Array Analysis") | 73 |
| S72 | TX cyr61 | 34 |
| S71 | TX MTMMP* | 0 |
| S70 | TX progesterone receptor* | 1927 |
| S69 | TX estrogen receptor* | 5193 |
| S68 | TX Aromatase P450 | 38 |
| S67 | TX Cadherin* | 900 |
| S66 | TX Selectin* | 28411 |
| S65 | TX Integrin* | 1587 |
| S64 | TX cell adhesion molecule* | 1578 |
| S63 | TX CAMs | 550 |
| S62 | TX Endometrial marker* | 54 |
| S61 | TX Fas ligand | 338 |
| S60 | TX (TNF‐a or tumour necrosis factor* alfa) | 1489 |
| S59 | TX (MIF or migration inhibitory factor*) | 399 |
| S58 | TX (MCP‐I or monocyte chemoattractant protein‐I) | 13 |
| S57 | TX interleukin | 13809 |
| S56 | TX (Anti‐2HSG or Heremans‐Schmidt glycoprotein) | 7 |
| S55 | TX Anti‐LDL | 9 |
| S54 | TX Anti‐transferrin | 3 |
| S53 | TX Anti‐Thomsen Friedenreich antigen* | 1 |
| S52 | TX Anti‐thyroid | 109 |
| S51 | TX Anti‐laminin‐1 | 15 |
| S50 | TX (HLA or human leucocyte antigen*) | 4202 |
| S49 | (MM "HLA Antigens") | 638 |
| S48 | TX Antiphospholipid* | 1249 |
| S47 | TX Anti‐endometrial | 34 |
| S46 | (MH "Antibodies/BL/DU") | 1294 |
| S45 | TX Autoantibodies | 4385 |
| S43 | TX Total complement level | 3 |
| S42 | TX (T helper* or T supressor*) | 2341 |
| S41 | TX Immune cells alteration* | 24 |
| S40 | TX natural killer t‐cells | 669 |
| S39 | (MM "T Lymphocytes") | 2404 |
| S38 | TX (sEselectin or soluble E‐selectin) | 91 |
| S37 | TX (sEcadherin or soluble E‐cadherin) | 8 |
| S36 | TX sVCAM | 100 |
| S35 | TX sICAM | 173 |
| S34 | TX Soluble adhesion molecule | 368 |
| S33 | TX soluble intercellular adhesion | 237 |
| S32 | (MM "Cell Adhesion Molecules") | 52 |
| S31 | TX Osteopontin* | 416 |
| S30 | TX Follistatin | 74 |
| S29 | TX serum placental protein* | 11 |
| S28 | TX (Ca‐19‐9 or Ca19‐9 or cancer antigen 19‐9) | 262 |
| S27 | TX (Ca‐125 or Ca125 or cancer antigen 125) | 831 |
| S26 | (MM "Glycoproteins/BL/DU") | 224 |
| S25 | TX tissue inhibitor of metalloproteinase | 423 |
| S24 | TX TIMP* | 1845 |
| S23 | TX MMP* | 4244 |
| S22 | TX matrix metalloproteinase* | 3325 |
| S21 | TX Proteolytic enzyme* | 1461 |
| S20 | (MM "Progesterone/BL/DU") | 51 |
| S19 | TX leptin* | 3258 |
| S18 | (MM "Luteinizing Hormone/BL/DU") | 38 |
| S17 | TX (VEGF or vascular endothelial growth factor*) | 7166 |
| S16 | TX (EGFR or epidermal growth factor receptor*) | 6188 |
| S15 | TX (TGF‐b or transforming growth factor beta or TGFb) | 2972 |
| S14 | TX (TGF‐a or transforming growth factor alfa or TGFa) | 464 |
| S13 | TX (IGF‐I or insulin‐like growth factor* or IGF1) | 3588 |
| S12 | TX (EGF or epidermal growth factor*) | 6250 |
| S11 | TX (PDGF or platelet derived growth factor*) | 3195 |
| S10 | TX (FGF or fibroblast growth factor*) | 3395 |
| S9 | TX hepatocyte growth factor* | 880 |
| S8 | TX cytokine* | 20821 |
| S7 | TX scatter factor* | 1864 |
| S6 | TX growth factor* | 76163 |
| S5 | TX Laboratory Test* | 82732 |
| S4 | TX (biomarker* or marker*) | 84857 |
| S3 | S1 OR S2 | 2841 |
| S2 | TX Endometrio* | 2841 |
| S1 | (MM "Endometriosis") | 889 |
| S4 | TX (biomarker* or marker*) | 61,794 |
| S3 | S1 OR S2 | 2,174 |
| S2 | TX Endometrio* | 2,174 |
| S1 | (MM "Endometriosis") | 1,306 |
Appendix 6. The search strategy for other databases
Searches for clinical studies
Database: PsycINFO (Ovid) <1806 to April Week 2 2015 (20.04.2015)>
Search strategy:
1. endometriosis.tw. (174)
Database: Web of Science Core Collection (Thomson Reuters) <1900 to Present (20.04.2015)>
Search strategy:
1. Topic=(endometrio*) AND Topic=(diagnos* OR test*); Timespan=All Years (2518)
Database: LILACS <20.04.2015>
Search strategy:
1. (tw:(endometriosis)) AND (tw:(diagnos*)) (420)
Database: OAIster (WorldCat.org) <20.04.2015>
Search strategy:
1. endometriosis and (marker* or biomarker*) (11)
2. endometriosis and diagnos* (446)
Database: TRIP <20.04.2015>
Search strategy:
1. (endometriosis and diagnos*) (1648)
Searches of trial registers for ongoing and registered trials
Database: ClinicalTrials.gov (US NIH) <20.04.2015>
Search strategy:
1. endometriosis (220)
2. endometriosis AND diagnosis (22)
Database: WHO International Clinical Trials Registry Platform (ICTRP) <20.04.2015>
Search strategy:
1. endometriosis (523)
Searches for the reviews as source of references to potentially relevant studies
Database: MEDION <10.01.2014> (the last available date; database is not updated further)
Search strategy:
ICP Code female genital system (including breast), Signssymp medical imaging, laboratory tests, histology and cytology, endoscopy and laparoscopy. Filter: systematic reviews of diagnostic studies. (2)
Database: DARE (CRD) <20.04.2015>
Search strategy:
1. endometriosis (99)
PubMed, a 'Systematic Review' search under the 'Clinical Queries' link <20.04.2015>
Search strategy:
1. (endometriosis) AND systematic(sb) (418)
Category: Diagnosis; Scope: Broad
Searches for papers recently published and not yet indexed in the major databases
Search engine: PubMed <20.10.2014 to 20.04.2015>
Search strategy:
| 1. marker (14979) 2. test (61151) 3. diagnos* (69743) 4. biomarker (10806) 5. or/1‐4 (7943) Filters: Publication date from 2014/10/20 to 2015/04/20 |
Index test(s) set |
| 6. Endometriosis (584) Filters: Publication date from 2014/10/20 to 2015/04/20 |
Target condition set |
| 7. 5 and 6 (267) Filters: Publication date from 2014/10/20 to 2015/04/20 |
Combined sets |
Appendix 7. Summary of findings table 2 ‐ Endometrial biomarkers that do not distinguish between women with and without endometriosis
| Review question | Which endometrial biomarkers are unlikely to serve as a basis for a diagnostic test for endometriosis? | ||||
| Importance | Biomarkers that did not show differential expression in women with and without endometriosis are unlikely to be helpful in a diagnostic sense and hence are not worth pursuing. These findings are based on adequately designed studies that met inclusion criteria for this review. | ||||
| Patients | Reproductive‐aged women with suspected endometriosis or persistent ovarian mass, or women undergoing infertility work‐up | ||||
| Settings | Hospitals (public or private of any level), outpatient clinics (general gynaecology, reproductive medicine, pelvic pain) or radiology departments | ||||
| Reference standard | Visualisation of endometriosis at surgery (laparoscopy or laparotomy) with or without histological confirmation | ||||
| Study design | Cross‐sectional, single gate design (N = 11) or two‐gate design (N = 18); unable to determine if single‐ or two‐gate design for 2 studies. Prospective enrolment; a study could assess more than 1 test or more than 1 type of endometriosis | ||||
| Risk of bias | Overall judgement | Poor quality (no studies had 'low risk' assessment in all 4 domains) | |||
| Patient selection bias | High risk: 29 studies; unclear risk: 2 studies; low risk: 0 studies | ||||
| Index test interpretation bias | High risk: 28 studies; unclear risk: 3 studies; low risk: 0 studies | ||||
| Reference standard interpretation bias | High risk: 0 studies; unclear risk: 13 studies; low risk: 18 studies | ||||
| Flow and timing selection bias | High risk: 12 studies; unclear risk: 2 studies; low risk: 17 studies | ||||
| Applicability concerns | Concerns regarding patient selection | High concern ‐ 25 studies; unclear concern ‐ 3 studies; low concern ‐ 3 studies | |||
| Concerns regarding index test | High concern ‐ 0 studies; unclear concern ‐ 0 studies; low concern ‐ 31 studies | ||||
| Concerns regarding reference standard | High concern ‐ 0 studies; unclear concern ‐ 0 studies; low concern ‐ 31 studies | ||||
| Biomarker | Expression levels |
rASRM stage |
Menstrual cycle phase |
Sample collection method |
Reference |
| 1. Angiogenesis and growth factors and their receptors | |||||
| EGF mRNA (epidermal growth factor) | Endometriosis (N = 35)a: 0.13 ± 0.01 Controls (N = 31): 0.24 ± 0.02; P = 0.120 # sub‐analysis per cycle phase ‐ similar findings |
III‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ likely sharp | Lee 2007 |
| FGF‐2 mRNA (fibroblast growth factor‐2) | Endometriosis (N = 35)a: 17.38 ± 1.73 Controls (N = 31): 20.0 ± 2.53; P = 0.572 # sub‐analysis per cycle phase ‐ similar findings |
III‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ likely sharp | Lee 2007 |
| Glycodelin mRNA (PP14 or PAEP) (placental protein 14 or progestogen‐associated endometrial protein) | Endometriosis (N = 17)a: 2.5 (0.6‐4.5) Controls (N = 10): 0.936 (0.1‐1.9); P = NS | II‐IV | Proliferative | Sharp curettage | Meola 2009 |
| PDGF‐A mRNA (platelet‐derived growth factor A) | Endometriosis (N = 35)a: 6.52 ± 0.58 Controls (N = 31): 13.02 ± 1.60; P = 0.572 # sub‐analysis per cycle phase ‐ similar findings |
III‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ likely sharp | Lee 2007 |
| PIGF (placental growth factor) | Endometriosis (N = 18)a: 120 ± 31 Controls (N = 23): 87 ± 16; P = NS Endometriosis (N = 22)a: 87 ± 30 Controls (N = 27): 109 ± 15; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| PIGF mRNA (placental growth factor) | Endometriosis (N = 18)a: 0.050 ± 0.017 Controls (N = 23): 0.032 ± 0.009 Endometriosis (N = 22)a: 0.037 ± 0.016 Controls (N = 27): 0.024 ± 0.004; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| PKR1 mRNA (prokineticin receptor 1), EG‐VEGF receptor | Endometriosis (N = 9)b: 1.89 (0.1–5.5) Controls (N = 14): 3.92 (0.0–23.4); P = NS Endometriosis (N = 6)b: 0.43 (0–2.1) Controls (N = 19): 0.64 (0.0–6.7); P = NS |
— | Proliferative Secretory |
Aspiration curettage | Lee 2010 |
| PKR2 mRNA (prokineticin receptor 2), EG‐VEGF receptor | Endometriosis (N = 9)b: 0.93 (0.0–10.7) Controls (N = 14): 0.75 (0.0–6.6); P = NS Endometriosis (N = 6)b: 0.24 (0.0–1.2) Controls (N = 19): 0 (0.0–1.0); P = NS |
— | Proliferative Secretory |
Aspiration curettage | Lee 2010 |
| TSP‐1 (thrombospondin‐1) | Endometriosis (N = 18)a: 313 ± 46 ng/mg Controls (N = 23): 210 ± 27 ng/mg; P = NS Endometriosis (N = 22)a: 413 ± 63 ng/mg Controls (N = 27): 317 ± 42 ng/mg; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| TSP‐1 (thrombospondin‐1) | Endometriosis (N = 58)a: 140 ± 28 ng/mg Controls (N = 38): 81 ± 16 ng/mg; P = NS Endometriosis (N = 58)a: 69 ± 16 ng/mg Controls (N = 38): 69 ± 13 ng/mg; P = NS |
— | Proliferative Secretory |
Aspiration curettage | Ramon 2011 |
| TSP‐1 mRNA (thrombospondin‐1) | Endometriosis (N = 18)a: 2.621 ± 0.735 Controls (N = 23): 1.510 ± 0.270; P = NS Endometriosis (N = 22)a: 3.093 ± 0.7002 Controls (N = 27): 2.370 ± 0.910; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| TSP‐1 mRNA (thrombospondin‐1) | Endometriosis (N = 58)a: 1.79 ± 0.36 Controls (N = 38): 2.36 ± 0.49; P = NS Endometriosis (N = 58)a: 2.65 ± 0.87 Controls (N = 38): 1.52 ± 0.39; P = NS |
— | Proliferative Secretory |
Aspiration curettage | Ramon 2011 |
| TYMP (thymidine phosphorylase) | Endometriosis (N = 15)b: 11.8 (2.7–38.6) ng/ml Controls (N = 14): 16.8 (1.1–37.6) ng/ml; P = 0.68 |
III‐IV | Proliferative | Aspiration curettage | Laudanski 2014 |
| VEGF (vascular endothelial growth factor) | Endometriosis (N = 10)b: 0.142 (0.087‐0.943) Controls (N = 7): 0.129 (0.097‐0.837); P = 0.943 |
II‐IV | Menstrual | Menstrual fluid | Da Silva 2014 |
| VEGF mRNA (vascular endothelial growth factor) | Endometriosis (N = 24)b: 5.83 (2.5‐8.75) Controls (N = 23): 5.01 (1.7‐10.83); P = NS # sub‐analysis per cycle phase ‐ similar findings |
III‐IV | Proliferative/secretory | 'Endometrial biopsy curette' ‐ not specified | Cho 2012 |
| EG‐VEGF mRNA (endocrine gland‐derived VEGF) | Endometriosis (N = 9)b: 0.04 (2.9 × 10‐4– 0.6) Controls (N = 14): 0.03 (5.2 × 10‐6–1.0); P = NS Endometriosis (N = 6)b: 0.28 (2.0 × 10‐4–5.6) Controls (N = 19): 1.50 (0.0–27.9); P = NS |
— | Proliferative Secretory |
Aspiration curettage | Lee 2010 |
| VEGF/sFlt (VEGF and soluble fms‐like tyrosine kinase ratio) | Endometriosis (N = 24)b: 1.06 (0.71‐2.59) Controls (N = 23): 0.71 (0.35‐1.06); P = 0.064 # sub‐analysis per cycle phase ‐ similar findings |
III‐IV | Proliferative/secretory | 'Endometrial biopsy curette' ‐ not specified | Cho 2012 |
| 2. Apoptosis markers and regulators | |||||
| Bax mRNA (BCL2‐associated X protein) | Endometriosis (15)b: 1.22 Controls (N = 30): 1.15; P = NS |
I‐IV | Proliferative | Sharp curettage | Zubor 2009 |
| Bcl‐xL (B‐cell lymphoma‐extra large, or BCL2‐like‐1 isoform 1) | Endometriosis (15)b: 1.08 Controls (N = 30): 1.07; P = NS |
I‐IV | Proliferative | Sharp curettage | Zubor 2009 |
| Bcl‐xL:Bcl‐xS ratio (ratio B‐cell lymphoma‐extra large/B‐cell lymphoma‐extra small)E | Endometriosis (15)b: 2.63 Controls (N = 30): 5.63; P = 0.2965 |
I‐IV | Proliferative | Sharp curettage | Zubor 2009 |
| 3. Cell adhesion molecules and other matrix‐related proteins | |||||
| α2β1 integrin | Positively stained menstrual effluent: endometriosis (N = 8)c: 7/8 controls (N = 8): 6/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1994 |
| α3β1 integrin | Positively stained menstrual effluent: endometriosis (N = 8)c: 7/8 controls (N = 8): 6/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 7/8 controls (N = 8): 7/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1994 |
| α4β1 integrin | Positively stained menstrual effluent: endometriosis (N = 8)c: 2/8 controls (N = 8): 3/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 7/8 controls (N = 8): 5/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1994 |
| α5β1 integrin | Positively stained menstrual effluent: endometriosis (N = 8)c: 7/8 controls (N = 8): 4/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1994 |
| α6β1 integrin | Positively stained menstrual effluent: endometriosis (N = 8)c: 2/8 controls (N = 8): 3/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1994 |
| αVβ3 integrin | Positive samples: endometriosis (N = 20)c: 10 (50) infertile controls (N = 20): 8 (40) fertile controls (N = 20): 7 (35); P = NS endometriosis (N = 16)c: 16 (100) infertile controls (N = 16): 16 (100) fertile controls (N = 19): 18 (94.7); P = NS |
l‐II | Mid‐secretory Late‐secretory |
— | Casals 2012 |
| αVβ5 integrins | Glandular cells H Score: endometriosis (N = 40)b: 0.47 (0.0‐1.0) controls (N = 12): 0.6 (0.3‐0.8); P = 0.460 endometriosis (N = 40)b: 1.2 (0.5‐1.9) controls (N = 12): 1.3 (0.9‐1.7); P = 0.507 |
I‐IV | Proliferative Secretory |
— | Puy 2002 |
| Stromal cells H Score: endometriosis (N = 40)b: 2.0 (1.0‐2.7) controls (N = 12): 1.9 (1.4‐2.5); P = 0.920 endometriosis (N = 40)b: 1.7 (0.8‐2.7) controls (N = 12): 1.5 (1.1‐2.4); P = 0.752 |
I‐IV | Proliferative Secretory |
— | Puy 2002 | |
| αVβ6 integrins | Glandular cells H Score : endometriosis (N = 40)b: 0.5 (0.1‐1.3) controls (N = 12): 0.75 (0.3‐1.2); P = 0.856 endometriosis (N = 40)b: 0.7 (0.3‐0.9) controls (N = 12): 1.0 (0.8‐1.2); P = 0.975 |
I‐IV | Proliferative Secretory |
— | Puy 2002 |
| Stromal cells H Score: endometriosis (N = 40)b: 1.4 (0.6‐2.2) controls (N = 12): 1.2 (0.3‐2.5); P = 0.651 endometriosis (N = 40)b: 1.8 (0.8‐2.6) controls (N = 12): 1.7 (1.0‐2.2); P = 0.635 |
I‐IV | Proliferative Secretory |
— | Puy 2002 | |
| ICAM1 (intercellular adhesion molecule1) | Endometriosis (N = 8)a: 0.56 ± 0.25 Control (N = 8): 0.69 ± 0.19; P = NS # sub‐analysis per cycle phase ‐ similar findings |
I‐IV | Proliferative/secretory | Aspiration curettage | Pino 2009 |
| ICAM1 (CD54) (intercellular adhesion molecule1) | (Small/medium/large cells): endometriosis (N = 10)b: 96 (25–193)/92 (13–106)/106 (18–143) controls (12): 51 (4–453)/48 (5–129)/49 (4–103); P = NS # significant difference between the groups in secretory phase ‐ no data for 2 × 2 table |
— | Proliferative | Aspiration curettage | Prefumo 2002 |
| sICAM1 (soluble ICAM1) | Endometriosis (N = 8)a: 0.31 ± 0.16 Control (N = 8): 0.13 ± 0.13; P = NS # sub‐analysis per cycle phase ‐ similar findings |
I‐IV | Proliferative/secretory | Aspiration curettage | Pino 2009 |
| ICAM1 mRNA (intercellular adhesion molecule1) | Endometriosis (N = 6)a: 0.44 ± 0.11 Control (N = 6): 0.76 ± 0.11; P = NS |
I‐IV | Proliferative/secretory | Aspiration curettage | Pino 2009 |
| E‐cadherin | Positively stained menstrual effluent: endometriosis (N = 8)c: 3/8 controls (N = 8): 3/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1994 |
| LAMA5 (laminin subunit alpha‐5) | Endometriosis (N = 15)b: 1500 (1060–1840) pg/ml controls (N = 14):1400 (1340–1780) pg/ml; P = 0.95 |
III‐IV | Proliferative | Aspiration curettage | Laudanski 2014 |
| LFA‐3 (CD58) (leukocyte function associated molecule‐3) | (Small/medium/large cells): endometriosis (N = 10)b: 101 (64 – 302)/108 (28–155)/65 (35–197) controls (12): 315 (65 – 824)/150 (33–264)/124 (67–164); P = NS endometriosis (N = 10)b: 70 (34–252)/49 (26–184)/48 (37–118) controls (12): 171 (145–643)/134 (94–223)/162 (56–228); P = NS |
— | Proliferative Secretory |
Aspiration curettage | Prefumo 2002 |
| MMP‐1 (matrix metalloproteinase‐1) | Endometriosis (N = 8)a: just above detection limit Control (N = 8): just above detection limit; P = NS |
I‐IV | Proliferative/secretory | Aspiration curettage | Pino 2009 |
| MMP‐9 (matrix metalloproteinase‐9) | Endometriosis (N = 8)a: 2.75 ± 1.5 Control (N = 8): 0.75 ± 0.13; P = NS # sub‐analysis per cycle phase ‐ similar findings |
I‐IV | Proliferative/secretory | Aspiration curettage | Pino 2009 |
| MMP‐9 mRNA (matrix metalloproteinase‐9) | Endometriosis (N = 6)a: 0.21 ± 0.06 Control (N = 6): 0.33 ± 0.07; P = NS |
I‐IV | Proliferative/secretory | Aspiration curettage | Pino 2009 |
| OPN (osteopontin) | Positive samples: endometriosis (N = 20)c: 14 (70) infertile controls (N = 20): 13 (65) fertile controls (N = 20): 14 (70); P = NS endometriosis (N = 16)c: 16 (100) infertile controls (N = 16): 16 (100) fertile controls (N = 19): 19 (100); P = NS |
I‐II | Mid‐secretory Late‐secretory |
— | Casals 2012 |
| OPN (+)/αVβ3 (+) | Positive samples: endometriosis (N = 20)c: 8 (40) infertile controls (N = 20): 7 (35) fertile controls (N = 20): 6 (30); P = NS endometriosis (N = 16)c: 16 (100) infertile controls (N = 16): 16 (100) fertile controls (N = 19): 18 (94.7); P = NS |
I‐II | Mid‐secretory Late‐secretory |
— | Casals 2012 |
| PAI‐1 (plasminogen activator inhibitor ‐ 1) | Antigenic levels: endometriosis (N = 21)a: 0.46 ± 0.18 ng/mg controls (N = 35): 0.73 ± 0.19 ng/mg; P = NS Functional levels: endometriosis (N = 21)a: 1.64 ± 0.59 U/mg controls (N = 35): 1.12 ± 0.35 U/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| PAI‐1 (plasminogen activator inhibitor ‐ 1) | Endometriosis (N = 18)a: 1.73 ± 0.56 ng/mg Controls (N = 23): 1.48 ± 0.40 ng/mg Endometriosis (N = 22)a: 2.51 ± 0.65 ng/mg Controls (N = 27): 1.44 ± 0.44 ng/mg; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| PAI‐1 mRNA (gene encoding for (plasminogen activator inhibitor ‐ 1) | Endometriosis (N = 18)a: 0.197 ± 0.068 Controls (N = 23): 0.117 ± 0.030 Endometriosis (N = 22)a: 0.496 ± 0.158 Controls (N = 27): 0.131 ± 0.041; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| PAI‐2 (plasminogen activator inhibitor ‐ 2) | Antigenic levels: endometriosis (N = 21)a: 10.69 ± 3.33 ng/mg controls (N = 35): 4.53 ± 1.75 ng/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| PAI‐3 (plasminogen activator inhibitor ‐ 3) | Antigenic levels: endometriosis (N = 21)a: 233 ± 50 ng/mg controls (N = 35): 340 ± 45 ng/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| tPA (tissue‐type plasminogen activator) | Antigenic levels: endometriosis (N = 21)a: 12.69 ± 1.52 ng/mg controls (N = 35): 9.14 ± 1.33 ng/mg; P = NS Functional levels: endometriosis (N = 21)a: 0.41 ± 0.09 U/mg controls (N = 35): 0.58 ± 0.10 U/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| uPA (urokinase‐type plasminogen activator) | Functional levels: endometriosis (N = 21)a: 0.11 ± 0.02 ng/mg controls (N = 35): 0.06 ± 0.01 ng/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| uPA‐R (urokinase‐type plasminogen activator receptor) | Antigenic levels: endometriosis (N = 21)a: 2.51 ± 0.55 ng/mg controls (N = 35): 2.90 ± 0.32 ng/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| tPA‐PAI‐3 (tissue plasminogen activator and plasminogen activator inhibitor ‐ 3 complex) | Antigenic levels: endometriosis (N = 21)a: 0.20 ± 0.10 ng/mg controls (N = 35): 0.06 ± 0.02 ng/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| uPA‐PAI‐3 (urokinase plasminogen activator and plasminogen activator inhibitor ‐ 3 complex) | Antigenic levels: endometriosis (N = 21)a: 0.27 ± 0.13 ng/mg controls (N = 35): 0.36 ± 0.18 ng/mg; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 |
| TIMP‐1 protein (tissue inhibitor of metalloproteinases‐1) | Endometriosis (N = 45)b: 0.253 ± 0.018 controls (N = 15): 0.267 ± 0.010; P = NS |
II‐IV | Proliferative/secretory | — | Chen 2004 |
| Endometriosis (N = 21)a: 31± 6 ng/ml controls (N = 35): 30 ± 5 ng/ml; P = NS |
III‐IV | Menstrual/proliferative/secretory | — | Gilabert‐Estelles 2003 | |
| Endometriosis (N = 15)b: 56.7 (13.5–213.5) ng/ml Controls (N = 14): 58 (36.2–187.8) ng/ml; P = 0.45 |
III‐IV | Proliferative | Aspiration curettage | Laudanski 2014 | |
| TIMP‐1 mRNA (tissue inhibitor of metalloproteinases‐1) | Endometriosis (N = 18)a: 1.190 ± 0.228 Controls (N = 23): 1.589 ± 0.335 Endometriosis (N = 22)a: 2.028 ± 0.392 Controls (N = 27): 1.506 ± 0.363; P = NS |
III‐IV | Proliferative Secretory |
Aspiration curettage | Gilabert‐Estelles 2007 |
| Endometriosis (N = 35)b: 2.31 ± 1.21 Controls (N = 20): 2.40 ± 0.89; P = NS |
I‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ not specified | Li 2006 | |
| VCAM‐1 (CD106) (vascular cell adhesion molecule‐1) | (Small/medium/large cells): endometriosis (N = 10)b: 8 (0–42)/3 (2–22)/43 (0–57) controls (12): 34 (0–60)/14 (0–24)/21 (0–64); P = NS endometriosis (N = 10)b: 28 (5–64)/22 (6–60)/18 (10–126) controls (12): 27 (5–130)/18 (3–62)/33 (13–238); P = NS |
— | Proliferative Secretory |
Aspiration curettage | Prefumo 2002 |
| 4. Cell cycle regulatory molecules | |||||
| Cyclin B1 mRNA | Endometriosis (N = 20)b: 0.79 ± 1.08 Controls: (N = 30): 0.51 ± 1.04; P = NS Endometriosis (N = 20)b: 0.98 ± 1.24 Controls (N = 30): 0.50 ± 0.49; P = NS # similar findings were observed for protein expression |
II‐III | Proliferative Secretory |
— | Tang 2009 |
| Cdc2 mRNA (cyclin dependent kinase‐2) | Endometriosis (N = 20)b: 5.0 ± 5.0 Controls: (N = 30): 5.0 ± 5.0; P = NS Endometriosis (N = 20)b: 4.5 ± 5.5 Controls (N = 30): 4.5 ± 5.5; P = NS # similar findings were observed for protein expression |
II‐III | Proliferative Secretory |
— | Tang 2009 |
| Plk1 mRNA (polo‐like kinase‐1) | Endometriosis (N = 20)b: 1.64 ± 1.18 Controls (N = 30): 1.35 ± 0.91; P = NS Endometriosis (N = 20)b: 0.84 ± 1.04 Controls (N = 30): 0.52 ± 0.44; P = NS # similar findings were observed for protein expression |
II‐III | Proliferative Secretory |
— | Tang 2009 |
| 5. Cell proliferation markers | |||||
| Ki‐67 (Antigen KI‐67 or MKI67, marker of cellular proliferation) | Stromal cells: endometriosis (N = 13)b: 8.4 ± 1.4 controls (N = 6): 10.9 ± 4.8; P = NS endometriosis (N = 12)b: 9.6 ± 1.8 controls (N = 8): 10.5 ± 4.8; P = NS # data for glandular epithelium and blood vessels are also reported ‐ no difference between the groups |
II‐III | Proliferative Secretory |
Sharp curettage | Bourlev 2006 |
| BW 495/36, endometrial epithelial marker | Positively stained menstrual effluent: endometriosis (N = 8)c: 8/8 controls (N = 8): 6/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 7/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1995 |
| 6. Cytoskeleton molecules | |||||
| cytokeratin 18 | Positively stained menstrual effluent: endometriosis (N = 8)c: 8/8 controls (N = 8): 7/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1995 |
| CK19 or CYFRA 21‐1 (cytokeratin 19) | Positively stained menstrual effluent: endometriosis (N = 8)c: 8/8 controls (N = 8): 7/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1995 |
| Vimentin | Positively stained menstrual effluent: endometriosis (N = 8)c: 8/8 controls (N = 8): 7/8; P = NS Positively stained endometrium: endometriosis (N = 8)c: 8/8 controls (N = 8): 8/8; P = NS |
I | Early proliferative (day 2‐5) | Aspiration curettage | Van der Linden 1995 |
| 7. DNA‐repair and telomer maintenance molecules | |||||
| Telomerase activity (relative telomerase activity (RTA)) | Endometriosis (N = 30)b: 17.8 ± 30.8 Controls (N = 30): 7.6 ± 13.2; P = NS |
I‐IV | Proliferative/secretory | — | Kim 2007 |
| 8. High throughput markers | |||||
| mRNAome (mRNA microarray) | Endometriosis (N = 31)d: 0 Controls (N = 18): 0 |
I‐IV | Menstrual/secretory | Aspiration curettage | Fassbender 2012 |
| 9. Hormonal markers | |||||
| EST (oestrogen sulphotransferase) | Glandular cells staining: endometriosis (N = 35)e: strong 2, medium 12, weak 8, absent 25 controls (N = 33): strong 9, medium 8, weak 6, absent 10; P = NS Stromal cells staining: endometriosis (N = 35)e: absent 35 controls (N = 33): absent 33; P = NS |
I‐II | Proliferative/secretory | 'Endometrial curettage' ‐ likely sharp | Hudelist 2007 |
| LGR7 relaxin receptor mRNA (leucine‐rich G protein‐coupled receptor 7) evaluated to distinguish endometrioma from other non‐malignant ovarian masses |
Endometriosis (N = 4)f: 0.95 Controls (N = 7): 1; P = NS Endometriosis (N = 9)f: 1.27 Controls (N = 12): 1; P = NS |
III‐IV | Proliferative Secretory |
— | Morelli 2010 |
| Relaxin mRNA evaluated to distinguish endometrioma from other non‐malignant ovarian masses |
Endometriosis (N = 13)c: 9 (69.2) Control (N = 19): 14 (73.7); P = NS # sub‐analysis per cycle phase ‐ similar findings |
III‐IV | Proliferative/secretory | — | Morelli 2010 |
| 10. Immune system and inflammatory markers | |||||
| A Cytokines | |||||
| LIF (leukaemia‐inhibitory factor) | Endometriosis (N = 14)b: 25.53 (12.63–43.32) pg/ml Controls (N = 21): 36.26 (14.45–59.32); P = NS |
I‐II | Secretory | Uterine flushing | Mikolajczyk 2006 |
| LIF mRNA (leukaemia‐inhibitory factor gene) | Endometriosis (N = 14)b: 0.90 (0.73–1.09) Controls (N = 21): 0.92 (0.74–1.56); P = NS |
I‐II | Secretory | Aspiration curettage | Mikolajczyk 2006 |
| TNF‐a (tumour necrosis factor‐alpha) | Endometriosis (N = 10)b: 386.4 (48.31‐3636.06) Controls (N = 7): 56.02 (10.42‐619.205); P = 0.219 |
II‐IV | Menstrual | Menstrual fluid | Da Silva 2014 |
| TNF‐a mRNA (tumour necrosis factor‐alpha) | Endometriosis (N = 25)b: 0.0005 (0.0003–0.0020) Controls (N = 25): 0.0002 (0.0001–0.0007); P = NS |
I‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ not specified | Chen 2013 |
| B Immune cells: peripheral blood mononuclear cells (PBMC) | |||||
| CD56+ cells (endometrial granulated lymphocytes) | Early/mid/late secretory phase: endometriosis (N = 16)a: 1120 ± 160/1320 ± 160/2200 ± 240 controls (N = 17): 1200 ± 140/1360 ± 200/2440 ± 200; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| CD38+ cells (endometrial granulated lymphocytes) | Early/mid/late secretory phase: endometriosis (N = 16)a: 950 ± 200/1650 ± 300/3000 ± 300 controls (N = 17): 1550 ± 300/1750 ± 350/3000 ± 250; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| CD22+ cells (B lymphocytes) | early/mid/late secretory phase: endometriosis (N = 16)a: 87 ± 28/92 ± 31/120 ± 24 controls (N = 17): 90±31/104 ± 39/115 ± 28; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| CD68+ cells (macrophages) | Endometriosis (N = 31)b: 216.10 ± 104.41 Controls (N = 29): 175.93 ± 43.05; p = 0.06 # sub‐analysis per cycle phase ‐ significant difference between the groups only in proliferative phase ‐ no data for 2 × 2 table |
I‐IV | Any phase | Aspiration curettage | Cetin 2013 |
| CD68+ cells (macrophages) | Early/mid/late secretory phase: endometriosis (N = 16)a: 1178 ± 186/1271 ± 287/1643 ± 248 controls (N = 17): 1054 ± 155/1116 ± 155/1519 ± 287; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| CD16+ cells (natural killer cells) | Early/mid/late secretory phase: endometriosis (N = 16)a: 1276 ± 377/1305 ± 290/1421 ± 348 controls (N = 17): 957 ± 348/1044 ± 377/1189 ± 290; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| CD8+ cells (T suppressor/cytotoxic lymphocytes) | Early/mid/late secretory phase: endometriosis (N = 16)a: 691 ± 109/855 ± 127/891 ± 91 controls (N = 17): 782 ± 73/891 ± 91/964 ± 91; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| CD4+ cells (T helper/inducer lymphocytes) | Early/mid/late secretory phase: endometriosis (N = 16)a: 321 ± 57/357 ± 50/400 ± 64 controls (N = 17): 293 ± 57/307 ± 57/343 ± 57; P = NS |
I‐IV | Secretory | — | Klentzeris 1995 |
| C Interleukins | |||||
| IL‐1β mRNA (interleukin ‐1β) | Endometriosis (N = 25)b: 0.0295 (0.0056–0.1039) Controls (N = 25): 0.008 (0.0040–0.0251); P = NS |
I‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ not specified | Chen 2013 |
| IL‐11 (interleukin ‐ 11) | Endometriosis (N = 14)b: not detected Controls (N = 21): not detected; P = NS |
I‐II | Secretory | Uterine flushing | Mikolajczyk 2006 |
| IL‐11 mRNA (interleukin ‐ 11) | Endometriosis (N = 14)b: 0.48 (0.39–0.59) Controls (N = 21): 0.52 (0.42–0.61); P = NS |
I‐II | Secretory | Aspiration curettage | Mikolajczyk 2006 |
| IL‐1R1 mRNA (interleukin ‐1 receptor type II) | Stroma positive samples: endometriosis (N = 17)c: 9 (53) controls (N = 17): 14 (82); P = 0.299 Glandular cells positive samples: endometriosis (N = 16)c: 13 (81) controls (N = 17): 14 (82); P = 0.411 # sub‐analysis per cycle phase ‐ similar findings |
I‐II | Proliferative/secretory | — | Lawson 2008 |
| D other immune/inflammatory markers | |||||
| MPO (myeloperoxidase) | Endometriosis (N = 10)b: 1.229 (0.778‐2.094) Controls (N = 7): 1.69 (0.95‐1.84); P = 0.669 |
II‐IV | Menstrual | Menstrual fluid | Da Silva 2014 |
| NAG (N‐acetyl‐β‐D‐Glucosaminidase) | Endometriosis (N = 10)b: 723.26 (536.7‐768.73) Controls (N = 7): 663.62 (357.5‐1214.1); P = 1.0 |
II‐IV | Menstrual | Menstrual fluid | Da Silva 2014 |
| 11. Mediators of prostaglandin biosynthesis | |||||
| Akr1B1 mRNA (aldoketoreductase ‐1B1, PGF2a synthase) | Endometriosis (N = 22)b: 216.9 ± 25.9 Controls (N = 12): 217.6 ± 15.6; P = NS Endometriosis (N = 23)b: 121.2 ± 18.7 Controls (N = 17): 188.3 ± 31.9; P = NS |
I‐IV | Proliferative Secretory |
— | Rakhila 2013 |
| Akr1C3 mRNA (aldoketoreductase ‐1C3, PGF2a synthase) | Endometriosis (N = 22)b: 132.8 ± 21.1 Controls (N = 12): 127.9 ± 26.5; P = NS Endometriosis (N = 23)b: 185.9 ± 31.1 Controls (N = 17): 196.0 ± 45.9; P = NS |
I‐IV | Proliferative Secretory |
— | Rakhila 2013 |
| Cox‐1 mRNA (cyclo‐oxygenase‐1) | Endometriosis (N = 22)b: 263.6 ± 103.1 Controls (N = 12): 237.9 ± 78.3; P = NS Endometriosis (N = 23)b: 1045.0 ± 313.8 Controls (N = 17): 564.6 ± 151.2; P = NS |
I‐IV | Proliferative Secretory |
— | Rakhila 2013 |
| 15‐PGDH mRNA (15‐hydroxyprostaglandin dehydrogenase) | Endometriosis (N = 22)b: 41.3 ± 11.4 Controls (N = 12): 18.4 ± 3.2; P = NS Endometriosis (N = 23)b: 77.2 ± 51.4 Controls (N = 17): 48.1 ± 26.8; P = NS |
I‐IV | Proliferative Secretory |
— | Rakhila 2013 |
| cPGES mRNA (cytosolic PGE2 synthase) | Endometriosis (N = 22)b: 63.6 ± 4.6 Controls (N = 12): 68.3 ± 4.8; P = NS Endometriosis (N = 23)b: 55.2 ± 4.3 Controls (N = 17): 61.4 ± 3.9; P = NS |
I‐IV | Proliferative Secretory |
— | Rakhila 2013 |
| 12. Nerve sheath and nerve growth markers | |||||
| NF (neurofilament) | Endometriosis (N = 20)b: 0.02 ± 0.10 Controls (N = 20): 0.025 ± 1.04; P = NS |
I‐II | Secretory | Aspiration curettage | Bokor 2009 |
| NF (neurofilament) | Endometriosis (N = 31): no staining Controls (N = 29): no staining |
I‐IV | Any phase | Aspiration curettage | Cetin 2013 |
| PGP 9.5 (protein gene product 9.5) | Endometriosis (N = 31): no staining Controls (N = 29): no staining |
I‐IV | Any phase | Aspiration curettage | Cetin 2013 |
| 13. Other peptides and proteins | |||||
| hBD‐2 mRNA (human b‐defensin‐2) | Endometriosis (N = 25)b: 0.0343 (0.0025–2.0326) Controls (N = 25): 0.0034 (0.0025–0.0424); P = NS |
I‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ not specified | Chen 2013 |
| hBD‐2 (human b‐defensin‐2) | Negative samples: endometriosis (N = 25)c: 8 (32) controls (N = 25): 10 (40); P = NS |
I‐IV | Proliferative/secretory | 'Endometrial curettage' ‐ not specified | Chen 2013 |
| 14. Transcription factors and signalling molecules | |||||
| AKT1 (RAC‐alpha serine/threonine‐protein kinase) | Endometriosis (N = 15)b: 7.4 (1.4–15.1) ng/ml Controls (N = 14): 5.6 (1.4–15.4) ng/ml; P = 0.9 |
III‐IV | Proliferative | Aspiration curettage | Laudanski 2014 |
| JAG1 (jagged 1 protein) | Endometriosis (N = 15)b: 1.5 (0.3–6.5) ng/ml Controls (N = 14): 1.5 (0.4–2.8) ng/ml; P = 0.82 |
III‐IV | Proliferative | Aspiration curettage | Laudanski 2014 |
| NS = Not stated | |||||
Appendix 8. Endometrial biomarkers that have limited diagnostic vaue in endometriosis
| Testa |
| Angiogenesis and growth factors and their receptors |
| VEGF (vascular endothelial growth factor) protein or mRNA |
| Cell adhesion molecules and other matrix‐related proteins |
| TIMP‐1 (tissue inhibitor of metalloproteinases‐1) protein or mRNA |
| Hormonal markers |
| CYP19 (aromatase cytochrome P450) |
| Notes:a Limited diagnostic value was assigned when there were at least three studies demonstrating low diagnostic estimates that do not meet or approach the criteria for either replacement or triage test, or demonstrating no differential expression in endometriosis. We advise against further evaluation of these biomarkers for the diagnosis of endometriosis. |
Appendix 9. Endometrial biomarkers that possibly have limited diagnostic value in endometriosis
| Testa |
| 1. Angiogenesis and growth markers and their receptors |
| EGF (epidermal growth factor) |
| FGF‐2 (fibroblast growth factor‐2) |
| glycodelin A (PP14 or PAEP) (placental protein 14 or progestogen‐associated endometrial protein) |
| PDGF (platelet derived growth factor) |
| PIGF (placental growth factor) |
| PKR1 (prokineticin receptor 1), EG‐VEGF receptor |
| PKR2 (prokineticin receptor 2), EG‐VEGF receptor |
| PROK‐1 (prokineticin 1) |
| TSP‐1 (thrombospondin‐1) |
| TYMP (thymidine phosphorylase) |
| 2. Apoptosis markers and regulators |
| Bax (BCL2‐associated X protein) |
| Bcl‐xL (B‐cell lymphoma‐extra large, or BCL2‐like 1 isoform 1) |
| Bcl‐xL:Bcl‐xS ratio (ratio B‐cell lymphoma‐extra large/B‐cell lymphoma‐extra small) |
| 3. Cell adhesion molecules and other matrix‐related proteins |
| α2β1 integrin |
| α3β1 integrin |
| α4β1 integrin |
| α5β1 integrin |
| α6 β1 integrin |
| αVβ3 integrin |
| αVβ5 integrins |
| αVβ6 integrins |
| β1 integrin |
| depolarised α6 integrin |
| ICAM‐1 (intercellular adhesion molecule‐1) or sICAM‐1 (soluble form of intercellular adhesion molecule‐1) |
| E‐cadherin |
| LAMA5 (laminin subunit alpha‐5) |
| LFA‐3 (CD58) (leukocyte function associated molecule‐3) |
| MMP‐1 (matrix metalloproteinase‐1) |
| MMP‐9 (matrix metalloproteinase‐9) |
| OPN (osteopontin) |
| PAI‐1/‐2/‐3 (plasminogen activator inhibitors 1/2/3) |
| PAs: tPA, uPA (plasminogen activators: tissue‐type PA, urokinase‐type PA) |
| VCAM‐1 (CD106) (vascular cell adhesion molecule‐1) |
| 4. Cell cycle regulatory molecules |
| cyclin B1 |
| cdc2 (cyclin dependent kinase‐2) |
| Plk1 (polo‐like kinase‐1) |
| 5. Cell proliferation markers |
| Ki‐67 (antigen KI‐67 or MKI67, marker of cellular proliferation) |
| BW 495/36, endometrial epithelial marker |
| 6. Cytoskeleton molecules |
| cytokeratin 18 |
| CK19 or CYFRA 21‐1 (cytokeratin 19) |
| vimentin |
| 7. DNA‐repair and telomer maintenance molecules |
| hTERT (human telomerase reverse transcriptase) |
| telomerase activity |
| 8. High throughput markers |
| mitochondrial proteome |
| mRNAome (mRNA micro‐array) |
| 9. Hormonal markers |
| EST (oestrogen sulphotransferase) |
| ER ‐ α (oestrogen receptor ‐ alpha) |
| ER ‐ β (oestrogen receptor ‐ beta) |
| LGR7 (leucine‐rich G protein‐coupled receptor 7), relaxin receptor |
| relaxin |
| 10. Immune system and inflammatory markers |
| Cytokines |
| LIF (leukaemia‐inhibitory factor) |
| TNF‐α (tumour necrosis factor alpha) |
| Immune cells: peripheral blood mononuclear cells (PBMC) |
| lymphocytes (all, B‐, T‐cells) |
| monocytes/ macrophages |
| NK (natural killer cells) |
| Interleukins |
| IL‐1β |
| IL‐11 |
| IL‐1R1 (interleukin‐1 receptor type II) |
| other Immune/ inflammatory markers |
| MPO (myeloperoxidase) |
| NAG (N‐acetyl‐b‐D‐Glucosaminidase) |
| 11. Mediators of prostaglandin biosynthesis |
| Akr1B1 mRNA (aldoketoreductase ‐1B1, PGF2a synthase) |
| Akr1C3 mRNA (aldoketoreductase ‐1C3, PGF2a synthase) |
| Cox‐1 mRNA (cyclo‐oxygenase‐1) |
| 15‐PGDH mRNA (15‐hydroxyprostaglandin dehydrogenase) |
| cPGES mRNA (cytosolic PGE2 synthase) |
| 12. Myogenic markers (markers of smooth muscle differentiation ) |
| CALD1 (gene encoding for caldesmon) |
| 13. Nerve sheath and nerve growth markers |
| NF (neurofilament) |
| 14. Other peptides and proteins |
| hBD‐2 (human b‐defensin‐2) |
| 15. Transcription factors and signalling molecules |
| AKT1 (RAC‐alpha serine/threonine‐protein kinase) |
| JAG1 (jagged‐1 protein) |
| 16. Tumour markers |
| Ca‐125 (cancer antigen‐125) in menstrual fluid |
| Notes:a This group comprises the tests that likely to have limited diagnostic value, but where there are insufficient data to confidently comment on their diagnostic role (fewer than three studies with low diagnostic estimates or no differential expression in endometriosis). Further investigation of these biomarkers is possible with a focus on specific phases of the menstrual cycle, specific phenotypes of endometriosis, by implementation of different cut‐off values or by utilising different laboratory methods. |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 PROK‐1 (glandular) | 1 | 24 |
| 2 depolarised α‐6 integrin (glandular) | 1 | 49 |
| 3 α3β1 integrin (glandular) | 1 | 32 |
| 4 α3β1 integrin (stroma) | 1 | 32 |
| 5 α4β1 integrin (glandular) | 1 | 32 |
| 6 α4β1 integrin (stroma) | 1 | 32 |
| 7 β1 integrin (glandular) | 1 | 32 |
| 8 β1 integrin (stroma) | 1 | 32 |
| 9 hTERT mRNA | 1 | 69 |
| 10 Endometrial proteome | 2 | 53 |
| 11 Mitochondrial proteome | 1 | 53 |
| 12 CYP19 | 8 | 444 |
| 13 17βHSD2 mRNA | 1 | 53 |
| 14 ER‐α (glandular) | 1 | 90 |
| 15 ER‐α (stroma) | 1 | 90 |
| 16 ER‐β (glandular) | 1 | 90 |
| 17 ER‐β (stroma) | 1 | 90 |
| 18 IL‐1R2 mRNA (glandular) | 1 | 31 |
| 19 IL‐1R2 mRNA (stroma) | 1 | 32 |
| 20 IL‐1R2 mRNA (glandular secretory) | 1 | 19 |
| 21 IL‐1R2 mRNA (stroma secretory) | 1 | 20 |
| 22 Caldesmon (proliferative) | 1 | 35 |
| 23 Caldesmon (secretory) | 1 | 35 |
| 24 CALD1 mRNA (proliferative) | 1 | 35 |
| 25 CALD1 mRNA (secretory) | 1 | 35 |
| 26 PGP 9.5 | 8 | 429 |
| 27 VIP | 1 | 40 |
| 28 CGRP | 1 | 40 |
| 29 SP | 1 | 40 |
| 30 NPY | 1 | 40 |
| 31 NF | 1 | 40 |
| 32 Combined test (VIP, PGP 9.5, SP) | 1 | 40 |
| 33 CA 125 (menstrual fluid) | 1 | 104 |
1. Test.

PROK‐1 (glandular).
2. Test.

depolarised α‐6 integrin (glandular).
3. Test.

α3β1 integrin (glandular).
4. Test.

α3β1 integrin (stroma).
5. Test.

α4β1 integrin (glandular).
6. Test.

α4β1 integrin (stroma).
7. Test.
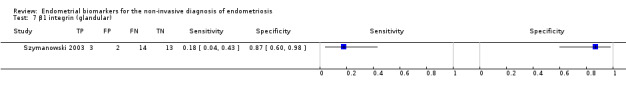
β1 integrin (glandular).
8. Test.

β1 integrin (stroma).
9. Test.
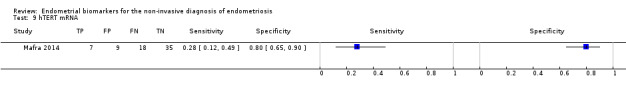
hTERT mRNA.
10. Test.
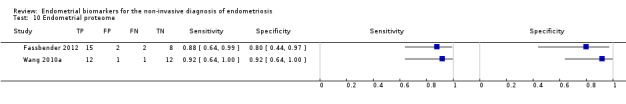
Endometrial proteome.
11. Test.

Mitochondrial proteome.
12. Test.
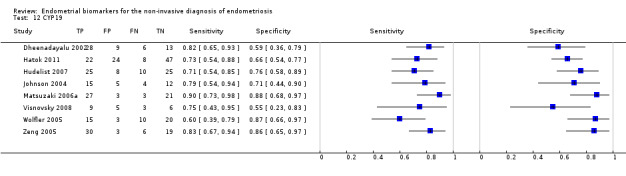
CYP19.
13. Test.

17βHSD2 mRNA.
14. Test.

ER‐α (glandular).
15. Test.

ER‐α (stroma).
16. Test.

ER‐β (glandular).
17. Test.

ER‐β (stroma).
18. Test.

IL‐1R2 mRNA (glandular).
19. Test.

IL‐1R2 mRNA (stroma).
20. Test.

IL‐1R2 mRNA (glandular secretory).
21. Test.

IL‐1R2 mRNA (stroma secretory).
22. Test.

Caldesmon (proliferative).
23. Test.

Caldesmon (secretory).
24. Test.

CALD1 mRNA (proliferative).
25. Test.

CALD1 mRNA (secretory).
26. Test.
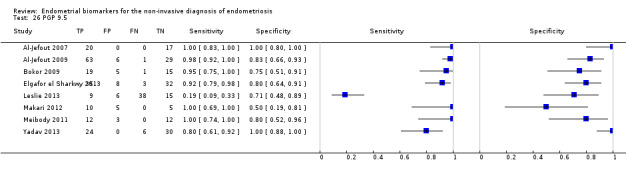
PGP 9.5.
27. Test.

VIP.
28. Test.

CGRP.
29. Test.

SP.
30. Test.

NPY.
31. Test.

NF.
32. Test.

Combined test (VIP, PGP 9.5, SP).
33. Test.

CA 125 (menstrual fluid).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Jefout 2007.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate endometrial biopsy and curettage in detecting small nerve fibres in eutopic endometrium for diagnosis of endometriosis Participants: reproductive‐aged women undergoing laparoscopy for suspected endometriosis or infertility Selection criteria: exclusion criteria: current hormonal treatment for endometriosis, pregnancy and unwillingness to participate Study design: observational single‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: chronic pelvic pain, infertility or both Age: reproductive age, not specified Number enrolled: 37 women Number available for analysis: 37 women (menstrual cycle phase not specified) Setting: Royal Prince Alfred Hospital, a tertiary referral centre Place of study: Sydney, Australia Period of study: 1 January 2006 to 1 December 2006 Language: English |
||
| Index tests | Index test: endometrial nerve fibres: PGP 9.5 Description of positive case definition by index test as reported: presence of nerve fibres in the functional layer of endometrium, measured by IHC staining for PGP 9.5 (immunostaining was carried out on a Dako Autostainer Model S3400 (Dako Cytomation, Inc, CA); images analysed by using an Olympus BX51 digital camera (Olympus, Japan)); laboratory technique described Examiners: 3 pathologists, 2 of whom had good experience in nerve fibre counting; 'blinded counting' Interobserver variability: close correlation between the 3 reviewers |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample n/N = 20/37 (54%); stage I‐II 15, stage III‐IV 5 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visualisation of endometriotic lesions with surgical staging according to rASRM system and peritoneal biopsy confirmation in most cases Examiners: 3 gynaecologists with extensive experience in endometriosis |
||
| Flow and timing | TIme interval between index test and reference standard: "prior to laparoscopy" Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Carefully taken endometrial biopsy maybe a reliable means of making a diagnosis of endometriosis. Nerve fibres may be present in other pathologies (adenomyosis, fibroids, endometritis) Comments: The reported estimates for index test performed on specimens obtained by curettage were similar to those obtained by Pipelle endometrial biopsy; not presented in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Al‐Jefout 2009.
| Study characteristics | |||
| Patient sampling | Primary objective: to detect small unmyelinated nerve fibres immunohistochemically (using the pan‐neuronal marker protein gene product 9.5 (PGP 9.5)) in the functional layer of endometrium in women undergoing diagnostic laparoscopy for pelvic pain or infertility Participants: reproductive‐aged women undergoing laparoscopy for infertility, pelvic pain or both Selection criteria: exclusion criteria: hormonal treatment for 3/12 months prior to surgery, pregnancy, unwillingness to participate Study design: observational single‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: pelvic pain symptoms alone (n = 52), infertility alone (n = 24), pelvic pain + infertility (n = 20), no pain and no infertility (n = 3) Age: mean age 33.9 years (range 20‐50 years) Number enrolled: 103 women Number available for analysis: 99 women (menstrual cycle phase n = 15; proliferative n = 39; mid‐cycle n = 14; secretory n = 31) Setting: Royal Prince Alfred Hospital, a tertiary referral centre Place of study: Sydney, Australia Period of study: 12 December 2007 to 10 December 2008 Language: English |
||
| Index tests | Index test: endometrial nerve fibres: PGP 9.5 Description of positive case definition by index test as reported: presence of endometrial nerve fibres in functional layer by IHC staining for PGP 9.5 (Immunostaining on a Dako Autostainer Model S3400 (Dako, Australia); image analysis by using an Olympus microscope BX51 and digital camera DP70 (Olympus, Japan)); laboratory technique described Examiners: 2 people with experience in nerve fibre counting, blinded to the patients' data and each others' results Interobserver variability: close (98%) correlations between the 2 operators |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample (n/N) = 64/99 (64%): stage I‐II 33, stage III‐IV 31; controls n/N = 35/99 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visualisation of endometriotic lesions with surgical staging of the disease according to rAFS system; biopsy confirmation of lesions was available in almost all cases. Examiners: 5 gynaecologists with extensive experience in endometriosis |
||
| Flow and timing | TIme interval between index test and reference standard: prior to laparoscopy Withdrawals: 4 participants were excluded due to poor sample quality (assessed prior to nerve fibre counting) |
||
| Comparative | |||
| Notes | Conclusion: Endometrial biopsy, with detection of nerve fibres, provided a reliable diagnosis of endometriosis that is close to the accuracy of laparoscopic assessment by experienced gynaecological laparoscopists | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bokor 2009.
| Study characteristics | |||
| Patient sampling | Primary objective: to assess density of sensory small‐diameter nerve fibres in the functional layer of endometrium in women with minimal to mild endometriosis and in women with a normal pelvis in order to develop a possible semi‐invasive diagnostic tool for minimal to mild endometriosis Participants: endometrial samples selected from tissue bank, which were collected from women undergoing laparoscopies for infertility, pain or both Selection criteria: inclusion criteria: no medical treatment for 3/12 months preceding surgery, secretory phase of menstrual cycle Study design: observational single‐gate design, prospective sample collection, retrospective selection of cases |
||
| Patient characteristics and setting | Clinical presentation: infertility, 100%; dysmenorrhoea, 25% Age: mean age 33 ± 10 years, endometriosis; 32 ± 5 years, controls Number enrolled: 40 women (retrospective selection) Number available for analysis: 40 women (all in secretory phase of menstrual cycle) Setting: University Hospital Gasthuisberg Place of study: Leuven, Belgium Period of study: not provided Language: English |
||
| Index tests | Index test: endometrial neural markers: PGP 9.5, VIP, CGRP, SP, NPY, NF Description of positive case definition by index test as reported: nerve fibre density was defined as total number of nerve fibres divided by the total surface area of the examined endometrium; nerve fibres were evaluated by IHC for each marker and counted in HPF areas for the slide section (antibody detection with REAL Detection System, Alkaline Phosphatase/RED, Rabbit/Mouse (Dako); analysis by image analysis software KS400 3.0 (Zeiss, Germany) linked to a Zeiss microscope); the whole surface of each section was evaluated on high‐power images; procedure described; thresholds not pre‐specified; reported cut‐off values: PGP 9.5 − 0.49, VIP − 0.08, CGRP − 0.23, SP − 0.2, NPY − 0.13, NF − 0.19 Examiners: 1 examiner who was blinded to the diagnosis Interobserver variability: NA |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 20/40 (50%), all stage I‐II Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: laparoscopically and histologically confirmed endometriosis, staged according to rASRM Examiners: none mentioned |
||
| Flow and timing | TIme interval between index test and reference standard: prior to laparoscopy Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The combined analysis of neural markers PGP 9.5, VIP and SP could predict the presence of minimal to mild endometriosis with 95% sensitivity, 100% specificity and 97.5% accuracy. Prospective studies are required to confirm our findings. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bourlev 2006.
| Study characteristics | |||
| Patient sampling | Primary objective: to examine the relationship between microvessel density, mitotic activity in blood vessels and expression of VEGF‐A and its receptors VEGFR‐1 and VEGFR‐2 in eutopic endometrium from women with and without endometriosis and in peritoneal endometriotic lesions Participants: women with laparoscopically confirmed endometriosis and women who underwent laparoscopic sterilisation with confirmed normal pelvis Selection criteria: exclusion criteria: patients with irregular menstrual cycles or gynaecological disorders other than endometriosis and those who had received hormonal treatment within the last 3 months Study design: observational two‐gate design, prospective sample collection, retrospective selection of cases |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: mean age 32.7 years (range 22‐44), endometriosis; 29.0 years (range 20–37); controls Number enrolled: 39 women (retrospective selection) Number available for analysis: 39 women (19 in proliferative and 20 in secretory phase of menstrual cycle) Setting: Research Centre of Obstetrics, Gynaecology and Perinatology, Russian Academy of the Medical Sciences Place of study: Moscow, Russia Period of study: not provided Language: English |
||
| Index tests | Index test: MVD, Ki‐67, VEGF‐A, VEGFR‐1, VEGFR‐2 Description of positive case definition by index test as reported: MVD was determined by IHC staining as number of microvessels per mm2, calculated as mean value of 5 randomly chosen fields, 0.109 mm2 each; proliferative index (PI) was calculated as the percentage of cells that were Ki‐67 positive (IHC method); VEGF‐A, VEGFR‐1 and VEGFR‐2 levels defined by IC staining intensity and classified 0 when comparable to the negative control, 1 when weak (clearly visible but no more), 2 when between weak and strong, 3 when strong; laboratory technique described in details Examiners: examination by 2 independent observers, unclear if were blinded to diagnosis Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 25/39 (64%), all stage II‐III Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: laparoscopically and histologically confirmed endometriosis, staged according to rASRM Examiners: none mentioned |
||
| Flow and timing | TIme interval between index test and reference standard: prior to laparoscopy Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: There seems to be a dysregulation of angiogenic activity in the eutopic endometrium of women with endometriosis, and endometriotic lesions with high proliferative activity were accompanied by higher local angiogenic activity and higher levels of VEGF in serum and peritoneal fluid. Comments: For Ki‐67 there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For MVD, VEGF‐A, VEGFR‐1 and VEGFR‐2 there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables ‐ not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Casals 2012.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate the endometrial expression and co‐expression of αvβ3 integrin and OPN in women with early stages of endometriosis Participants: women undergoing investigations for infertility and women undergoing tubal ligation Selection criteria: inclusion criteria: regular menstruation and not taking any medications Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis and infertile controls ‐ infertility, normal fertility work‐up (other than endometriosis); fertile controls ‐ healthy asymptomatic women with no history of infertility or miscarriage, requesting tubal ligation Age: mean age 31.5 ± 0.7 years, endometriosis; 32.1 ± 0.5 years, unexplained infertility controls; 34.9 ± 0.9 years, fertile controls Number enrolled: 60 women Number available for analysis: 60 women (all in early and 51 in late luteal phase of the cycle) Setting: University hospital ‐ Hospital Clınic, IDIBAPS, University of Barcelona Place of study: Barcelona, Spain Period of study: not provided Language: English |
||
| Index tests | Index test: osteopontin (OPN) and αvβ3 integrin expression Description of positive case definition by index test as reported: For all biomarkers positive staining was defined when IHC staining of any intensity was detected in both endometrial glands and luminal surface epithelium (detected by the automated immunohistochemical system TechMate 500 TM (Dako Co., Carpinteria, USA)); in addition, intensity of staining was evaluated by mean staining score and by H‐score method (defined); laboratory technique described Examiners: no information provided; unclear if were blinded to the result of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 20/60 (33%): all stage I‐II; controls = 40 Reference standard: laparoscopy Description of positive case definition by reference test as reported: none given Examiners: no information provided |
||
| Flow and timing | TIme interval between index test and reference standard: "Endometrial evaluation was performed in all women as a part of a routine infertility work‐up and always before laparoscopyE Withdrawals: In 9 patients late luteal biopsy could not be performed |
||
| Comparative | |||
| Notes | Conclusions: Endometrial OPN and αvβ3 integrin expression or co‐expression is not impaired during the window of implantation in patients with Stage I–II endometriosis. Comment: For OPN and αvβ3 integrin there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Cetin 2013.
| Study characteristics | |||
| Patient sampling | Primary objective: to determine whether the number of macrophage cells in the endometrium, the detection of nerve fibres or both can be used in the diagnosis of endometriosis Participants: patients undergoing and having undergone laparoscopy/laparotomy Selection criteria: exclusion criteria: adenomyosis, malignancy, use of hormonal treatment for 3/12 months before surgery Study design: cross‐sectional two‐gate, prospective collection of samples (prospective recruitment, n = 31 and retrospective random selection, n = 34) |
||
| Patient characteristics and setting | Clinical presentation: indications for surgery: ovarian cysts, pelvic pain, infertility, tubal ligation, endometrial hyperplasia, AUB, myoma uteri Age: mean age 38.29 ± 6.24 years, endometriosis group; 37.24 ± 5.40 years, controls Number enrolled: 65 women Number available for analysis: 60 women (any stage of menstrual cycle) Setting: university hospital: Istanbul University Istanbul School of Medicine Hospital Place of study: Istanbul, Turkey Period of study: 2006‐2011 Language: English |
||
| Index tests | Index test: endometrial nerve fibres ‐ PGP 9.5 and NF; macrophages ‐ CD68 + Description of positive case definition by index test as reported: neural marker results were noted as present or absent via IHC staining (evaluation by using Olympus BX‐51 microscope); macrophages markers reported as sum of IHC stained macrophage cells (evaluation under 400 × magnification in 10 fields); threshold was not reported Examiners: All samples were evaluated by a single pathologist who was blinded to patient data and who is highly experienced in gynaeco‐pathology Interobserver variability: NA |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 31/60 (52%): stage I‐II 6, stage III‐IV 25; controls 29 Reference standard: laparoscopy/laparotomy + histology Description of positive case definition by reference test as reported: visual inspection confirmed by biopsy; staging according to the rAFS Examiners: no information provided |
||
| Flow and timing | TIme interval between index test and reference standard: samples were taken preoperatively Withdrawals: 5 women were excluded due to insufficient tissue sample |
||
| Comparative | |||
| Notes | Conclusions: The detection of nerve fibres in the eutopic endometrium with the markers of PGP 9.5 and NF is not found to be helpful in the diagnosis of endometriosis. Macrophage cells may be helpful in the diagnosis only in the proliferative phase Comments: Nerve fibres markers were not detected in either group For macrophages there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7); subanalysis revealed differential expression only in proliferative phase of the cycle, but there was no data for construction of 2 × 2 tables |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Chen 2004.
| Study characteristics | |||
| Patient sampling | Primary objective: to study the expression of matrix metalloproteinase‐9 (MMP‐9) and Tissue Inhibitor of Metalloproteinase‐1 (TIMP‐1) in ectopic and eutopic endometrium in patients with endometriosis Participants: patients who underwent surgery for ovarian endometriosis (study group) and for fibroid uterus (controls) Selection criteria: inclusion criteria: no history of hormone therapy and endometriosis drug treatment for 3/12 months before surgery Study design: cross‐sectional two‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: mean age 39 ± 3 years, endometriosis group; 41 ± 3 years, controls Number enrolled: 77 women (54 in proliferative and 23 in secretory cycle phase) Number available for analysis: 60 women Setting: university hospital: Xiamen First Hospital of Xiamen University Place of study: Fujian Xiamen, China Period of study: May 1999 to February 2003 Language: Chinese |
||
| Index tests | Index test: MMP‐9, TIMP‐1 Description of positive case definition by index test as reported: the expression of MMP‐9 and TIMP‐1 evaluated by IHC; cells with yellow granules present in the cytoplasm were called positive cells, and the scanning areas for each positive cell were calculated (4 visual fields were selected for each sliced sample, and each visual field was measured 3 times and then their average taken); threshold was not reported Examiners: all samples were evaluated by the same person by using the same equipment Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: ovarian endometriosis Prevalence of target condition in the sample: n/N = 45/77 (58%): stage II 5, stage III‐IV 40; controls 32 (15 included in analysis) Reference standard: laparoscopy/laparotomy, not specified Description of positive case definition by reference test as reported: staging according to rAFS Examiners: no information provided |
||
| Flow and timing | TIme interval between index test and reference standard: samples taken at surgery Withdrawals: 17 women from control group were not included in the analysis; reason not specified |
||
| Comparative | |||
| Notes | Conclusions: The change of expression of MMP‐9 and TIMP‐1 in ectopic endometrium may be related to the pathogenesis of endometriosis. Comments: For TIMP‐1 there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) For MMP‐9 there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Chen 2013.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate the expression of human b‐defensin‐2 (hBD‐2) in the endometrium of patients with endometriosis and explore the potential role of hBD‐2 in pathogenesis of endometriosis Participants: women who were hospitalised at the authors' institution and underwent laparoscopy for ovarian mass (endometriosis group) and hysterectomy for fibroids (controls) Selection criteria: inclusion criteria: regular menstruation, no other endocrine, immune, metabolic or serious medical disorders; exclusion criteria: history of long‐term hormone use or hormone treatment 3/12 months preceding surgery, endometrial hyperplasia or endometrial inflammatory diseases Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: mean age 35.8 ±5.6 years (endometriosis), 37.5 ± 6.8 years (controls) Number enrolled: 50 women Number available for analysis: 50 women (29 in proliferative and 21 in secretory cycle phase) Setting: university teaching hospital ‐ The First Affiliated Hospital, Sun Yat‐sen University Place of study: Guangzhou, China Period of study: February 2008 to April 2010 Language: English |
||
| Index tests | Index test: hBD‐2 mRNA and protein, IL‐1β mRNA, TNF‐α mRNA Description of positive case definition by index test as reported: hBD‐2, IL‐1β, and TNF‐α mRNA expression levels defined as the ratio of the target gene to the reference gene (gene expression assessed by using qRT‐PCR; automatically analysed; quantified by a standard curve method). hBD‐2 protein expression according to the percentage of positively IHC stained cells: negative (−),< 5%; positive (+), 5%‐24%; positive (++), 25%‐49%; positive (+++), ≥50% (qualitative positioning method); laboratory techniques described; threshold not provided Examiners: IHC ‐ double‐blinded by two experienced pathologists; PCR ‐ no information; unclear if were blinded to the results of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 25/50 (50%): stage I‐II 9, stage III‐IV 16; controls 25 Reference standard: laparoscopy/laparotomy + histology Description of positive case definition by reference test as reported: visual inspection and pathological examination; staging according rAFS system Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: endometrial sample was collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: High levels of hBD‐2 gene and protein expressions in the ectopic endometrium of endometriosis patients may be an important contributor in the pathogenesis of endometriosis. TNF‐α and IL‐1β may promote the up regulation of hBD‐2 expression. Comment: #For hBD‐2, IL‐1β, and TNF‐α there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Cho 2012.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate the expression of VEGF and its soluble receptor (sFlt‐1) in peritoneal fluid, peritoneal endometriotic lesions and eutopic endometrial tissues of patients with endometriosis. Participants: women undergoing laparoscopy for different indications (pelvic masses, pelvic pain, suspicious endometriosis, infertility, and diagnostic evaluation) Selection criteria: exclusion criteria: postmenopausal women, previous hormone or GnRH agonist users, patients who had adenomyosis, endometrial pathology, infectious diseases, malignancy, autoimmune diseases, or cardiovascular diseases Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: pelvic masses, pelvic pain, infertility Age: mean age 31.57 ± 1.04 years, endometriosis group; 34.60 ± 1.48 years, controls Number enrolled: 82 women Number available for analysis: 47 women (22 in proliferative and 25 in secretory phase of menstrual cycle) Setting: Gangnam Severance Hospital, Yonsei University, College of Medicine Place of study: Seoul, Republic of Korea Period of study: not provided Language: English |
||
| Index tests | Index test: VEGF mRNA, sFlt‐1 mRNA Description of positive case definition by index test as reported: The amount of target normalised to the endogenous reference (GAPDH), compared to the calibrator and quantified by the 2‐ΔΔCt method (expression levels were measured by SYBR green real‐time PCR using the ABI 7300 instrument (Applied Biosystems, CA)); laboratory technique described; threshold not provided Examiners: no information provided; unclear if blinded to the result of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 24/47 (51%): all stage III‐IV Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visual inspection and pathological confirmation; staging according to rASRM Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples obtained prior to surgery Withdrawals: for 35 women, endometrial samples were not collected ‐ excluded from analysis |
||
| Comparative | |||
| Notes | Conclusion: No cyclic differences in VEGF mRNA expressions between and within the 2 groups were noted, whereas sFlt‐1 mRNA expressions were significantly lower in patients with endometriosis than in the controls during the proliferative phase. Comments: For VEGF there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For sFlt‐1 there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables ‐ not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Da Silva 2014.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate evaluate the presence of MPO, NAG, TNF‐α and VEGF in peripheral and menstrual blood in women with and without endometriosis Participants: women undergoing laparoscopy for chronic pelvic pain, infertility or tubal sterilisation Selection criteria: inclusion criteria: regular menstrual cycles in the 6 months preceding sample collection, no use of hormonal nor anti‐inflammatory agents in the previous 3 months and surgical confirmation or exclusion of endometriosis in agreement with the ESHRE guidelines Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ pelvic pain, infertility; controls ‐ infertility or request for sterilisation; none of the women had a significant past medical history Age: median age 36 years, range 31‐48 years Number enrolled: 17 women Number available for analysis: 17 women (all in early proliferative cycle phase, day 1‐4) Setting: university hospital ‐ Hospital das Clýnicas at Universidade Federal de Minas Gerais (HC‐UFMG) Place of study: Belo Horizonte, Brazil Period of study: February 2011 to December 2012 Language: English |
||
| Index tests | Index test: NAG, MPO, TNF‐α, VEGF in menstrual fluid Description of positive case definition by index test as reported: NAG and MPO activity (expressed as change in absorbance (OD) at 400 and 450 nm, respectively); TNF‐α and VEGF levels measured by using commercial specific ELISA kits; laboratory technique described; threshold not provided Examiners: no information provided; unclear if blinded to the result of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: unable to estimate as two‐gate design (endometriosis n = 10: stage II 5, stage IV 2, undetermined stage 3; controls 7) Reference standard: laparoscopy Description of positive case definition by reference test as reported: diagnosis according to ESHRE guidelines; staging according to rASRM Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: not specified, from the context ‐ perioperative sample collection Withdrawals: none |
||
| Comparative | |||
| Notes | Conclusion: These findings point to the existence of an increased local inflammatory activity in women with endometriosis. Comments: For NAG, MPO, TNF‐α and VEGF there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Dheenadayalu 2002.
| Study characteristics | |||
| Patient sampling | Primary objective: to determine whether expression of aromatase P450 mRNA in eutopic endometrium is predictive of the presence of pelvic endometriosis Participants: women undergoing laparoscopy for infertility, pelvic pain or tubal sterilisation Selection criteria: not provided Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility (n = 38), pelvic pain (n = 13) and tubal sterilisation (n = 9); 56 (93%) women had regular spontaneous menstrual cycles; none of the patients had been treated surgically for endometriosis; 4 (7%) women were taking the combined oral contraceptive pill Age: mean age 33.2 ± 5.2 years (range 21–46) Number enrolled: 60 women Number available for analysis: 56 women (25 in proliferative and 27 in secretory phase of menstrual cycle) Setting: 4 tertiary centres for reproductive medicine Place: London, UK Period of study: not provided Language: English |
||
| Index tests | Index test: aromatase P450 mRNA Description of positive case definition by index test as reported: presence of aromatase P450 mRNA (detected by RT‐PCR with amplification by using Access RT‐PCR System (Promega) positive staining for P450; southern blot for sequence detection); laboratory technique described Examiners: no information provided, unclear if blinded to the results of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 34/56 (61%): stage I‐II 28, stage III‐IV 6; controls 22 Reference standard: laparoscopy Description of positive case definition by reference test as reported: visual inspection, rAFS classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: Endometrial biopsies were taken at the time of laparoscopy Withdrawals: 4 women were excluded (2 of the biopsies were insufficient for RNA extraction and in 2 specimens RT‐PCR failed to amplify GAPDH mRNA) |
||
| Comparative | |||
| Notes | Conclusion: Most women with pelvic endometriosis or uterine proliferative disorders express aromatase P450 in the endometrium, regardless of the phase of the menstrual cycle. However, the potential of endometrial aromatase P450 mRNA detection as a clinically useful diagnostic marker of pelvic disease is limited by the observation that approximately 30% of aromatase P450‐negative women were found to have visual evidence of endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ding 2010.
| Study characteristics | |||
| Patient sampling | Primary objective: to detect specific mitochondrial proteins in eutopic endometrial samples from women with and without endometriosis and to build diagnostic models Participants: women who underwent laparoscopy for benign indications, without clinical symptoms of endometriosis (controls) and women who had endometriosis with laparoscopy Selection criteria: exclusion criteria: hormonal therapy or antiinflammatory medications for 6/12 months prior to surgery; adenomyosis detected at surgery Study design: observational two‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: not specified; control group with "no symptoms of endometriosis" Age: mean age 35.2 ± 6.4 years (controls), 38.4 ± 5.8 years (endometriosis) Number enrolled: 53 women Number available for analysis: 53 women (menstrual cycle phase not provided) Setting: university hospital ‐ Second Affiliated Hospital, Medical College, Zhejiang University Place: Hangzhou, Zhejiang, China Period of study: not stated Language: English |
||
| Index tests | Index test: mitochondrial proteome by SELDI‐TOF‐MS (3 potential biomarkers with mass‐to‐charge ratio (m/z) of 15,334, 15,128, and 16,069) Description of positive case definition by index test as reported: SVM model with the highest Youden index (proteins with different physicochemical properties were detected by using Ciphergen SELDI‐TOF‐MS (PBS‐IIplus; Ciphergen Biosystems Inc., CA) and CM10 protein chip); analysis by using SVM classifier and validated by leave‐one cross‐validation); technique described; diagnostic classifiers not pre‐specified Examiners: not stated; unclear if blinded to the results of reference standard Interobserver variability: CV of the selected normalised peaks = 15.2%, CV of the selected peaks' mass = 0.03%; little variation with day‐to‐day sampling, instrumentation or chip variation |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 24/53 (45%): stage I‐II 19, stage III‐IV 5; controls 29 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visible evidence of endometriosis confirmed by histopathology ‐ 'pelvic organs were examined carefully for the presence and extent of endometriosis; all of the patients were confirmed by pathologic examination of biopsied tissue'; staging according to the rAFS system Examiners: no information provided |
||
| Flow and timing | TIme interval between index test and reference standard: endometrial tissues were obtained during the operation Withdrawals: none |
||
| Comparative | |||
| Notes | Conclusion: The mitochondrial protein fingerprint models identified by protein chip technology had a relative feasibility. 3 biomarkers could be used for early detection and screening of endometriosis | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Elgafor el Sharkwy 2013.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate the diagnostic value of serum measurement of IL‐6 combined with the presence of nerve fibres in the functional layer of endometrium for diagnosis of minimal‐mild endometriosis Participants: women undergoing laparoscopy for infertility, pelvic pain or both Selection criteria: inclusion criteria: reproductive age, follicular phase of the cycle and regular menstrual cycle; exclusion criteria: any current infection, any medication within 1 month prior to laparoscopy, previous surgery for endometriosis and smoking or drinking alcohol Study design: observational, single‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation (n/N): infertility ‐ 91/114; dysmenorrhoea ‐ 64/114; dyspareunia ‐ 17/114; dyschezia ‐ 6/114; other pelvic pain ‐ 35/114 Age: mean age 29 ± 0.6 years, controls; 31 ± 1.1 years, endometriosis Number enrolled: 114 women Number available for analysis: 78 women (all in follicular cycle phase; only control and endometriosis stage I‐II were analysed) Setting: University hospital ‐ Zagazig University Hospital Place of study: Zagazig, Egypt Period of study: December 2010 to April 2012 Language: English |
||
| Index tests | Index test: endometrial nerve fibres ‐ PGP 9.5 Description of positive case definition by index test as reported: presence of nerve fibres in the functional layer of endometrium, assessed by IHC staining for PGP 9.5 (an average of 4–5 sections per specimen were examined by using an Olympus microscope) Examiners: 2 pathologists, both of whom have good experience in nerve fibre identification Interobserver variability: close (96%) correlation between the 2 pathologists |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 74/114 (65%): stage I‐II 38, stage III‐IV 36; controls 40 Reference standard: laparoscopy Description of positive case definition by reference test as reported: visualisation of the endometriotic lesions with surgical staging according to rASRM classification Examiners: 3 experienced gynaecologists in endometriosis |
||
| Flow and timing | Time interval between index test and reference standard: endometrial biopsy was obtained prior to laparoscopy Withdrawals: data were not available for all women with advanced endometriosis (stage III‐IV), n = 36 |
||
| Comparative | |||
| Notes | Conclusion: Serum IL‐6 and nerve fibres in the functional layer of endometrium will both allow more accurate detection of women who are at risk of having early stages of endometriosis. Comment: The reported data on the serum marker or combination of serum‐endometrial marker are not presented in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Fassbender 2012.
| Study characteristics | |||
| Patient sampling | Primary objective: to perform a combined mRNA microarray and proteomic analysis on the same eutopic endometrium sample obtained from patients with and without endometriosis Participants: biobank samples collected from women undergoing laparoscopy for infertility, pain or both Selection criteria: exclusion criteria: patients using the oral contraceptive pill or being on chronic medication; surgery within 6 months prior to the time of sample collection Study design: observational single‐gate, retrospective recruitment, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation (n/N): infertility + pain ‐ 27/49; infertility only ‐ 19/49, pain only ‐ 3/49; regular cycle ‐ 35/49 Age: mean age 31.96 ± 4.54 years, median 32 years, range 24‐43 years Number enrolled: 49 women Number available for analysis: 49 women (22 in menstrual and in 27 in early luteal phase of menstrual cycle) Setting: University Hospital Gasthuisberg Place of study: Leuven, Belgium Period of study: Samples were collected between 2005 and 2009 Language: English |
||
| Index tests | Index test: endometrial mRNAome by microarray and endometrial proteome by SELDI‐TOF MS (5 peptide peaks of 2,072 m/z; 2,973 m/z; 3,623 m/z; 3,680 m/z and 21,133 m/z) Description of positive case definition by index test as reported: mRNAome ‐ probe sets with an absolute fold change > 2 and a corrected P‐value < 0.05 (detection by using hybridisation microarray (Affymetrix GeneChip Human Gene 1.0 ST Arrays); analysis based on the RMA expression values through xps package 1.7.2 of Bioconductor) Proteome ‐ mass spectrometry peaks, which best discern between endometriosis and controls detected by LS‐SVM model in training and validated in tests set; the average performance of the model was calculated over the 100 splits (detection by using ProteinChip System, Series 4000 SELDI‐TOF‐MS instrument (Bio‐Rad); analysis by using repeated random sub‐sampling cross‐validation); laboratory techniques and analyses described in details; diagnostic classifiers were not pre‐defined Examiners: no information provided; the study was not a blinded study Interobserver variability: proteome ‐ intra‐assay CV of 9% and 10%; interassay CV of 8% and 11% using the reference sample spotted on the CM10 SPA or IMAC CHCA SELDI surface, respectively |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 31/49 (63%): stage I‐II 16, stage III‐IV 15; controls 18 Reference standard: laparoscopy Description of positive case definition by reference test as reported: rAFS classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were collected "from women undergoing laparoscopies"; implies prior to surgery Withdrawals: the proteome data was not available for all women in menstrual cycle phase, n = 22 |
||
| Comparative | |||
| Notes | Conclusions: mRNA expression of eutopic endometrium was comparable in women with and without endometriosis. Proteomic analysis of luteal phase endometrium allowed the diagnosis of endometriosis with high sensitivity and specificity in training and test sets. Comments: For endometrial mRNAome there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) The reported data on proteome per different stages of endometriosis is not presented The data for proteome in diagnosing endometriosis was available only for secretory phase |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Gilabert‐Estelles 2003.
| Study characteristics | |||
| Patient sampling | Primary objective: to analyse several components of the plasminogen activator (PA) pathway and the matrix metalloproteinase (MMP) system in endometriotic tissue, endometrium and peritoneal fluid from women with and without endometriosis Participants: women undergoing laparoscopy/laparotomy at the authors' institution for various indications Selection criteria: exclusion criteria: no hormonal treatment for 3/12 months preceding or pregnant/breastfeeding for 6/12 months before surgery Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ pelvic pain (n = 16) and infertility (n = 23); controls ‐ pelvic pain (n = 4), infertility (n = 3), pelvic floor defect (n = 3), asymptomatic (n = 25) Age: mean age 35 years (range 23‐47), endometriosis; 40 years (range 29‐47), controls Number enrolled: 74 women (19 in proliferative, 51 in secretory and 4 in menstrual cycle phase) Number available for analysis: 56 women Setting: university hospital ‐ Hospital Universitario La Fe Place of study: Valencia, Spain Period of study: not provided Language: English |
||
| Index tests | Index test: plasminogen activators: tPA, uPA, uPAR, PAI‐1, PAI‐2, PAI‐3, tPA‐PAI3, uPA‐PAI3 and MMPs: TIMP‐1, MMP‐3 Description of positive case definition by index test as reported: antigen levels of each of the tested markers assessed with a commercially available ELISAs (in cytosolic and membrane extracts); functional levels of tPA, PAI‐1 by chromogenic assay and functional level of uPA by immunosorbent activity assay (in cytosolic extract); laboratory techniques described Examiners: no information provided; unclear if blinded to the result of reference standard Interobserver variability: the intra‐ and interassay variabilities for tPA were 8% and 12%; uPA: 4% and 10%; uPAR: 5% and 7%; PAI‐1: 3% and 7%, PAI‐2: 5% and 8%, PAI‐3: 4‐8% and 6‐9%; tPA‐PAI3, uPA‐PAI3: 5‐9% and 7‐12%, MMP‐3: 5% and 9%, TIMP‐1: 4% and 7% |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 39/74 (53%): all stage III‐IV; controls 35 Reference standard: laparoscopy, n = 35/ laparotomy, n = 39 Description of positive case definition by reference test as reported: visual inspection with systematic examination of the abdominal cavity and attention for early and atypical lesions; staging according to the rASRM classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: not specified, but from the context appears that the samples were obtained at surgery Withdrawals: 18 endometriosis patients were not included because endometrial tissue was not collected |
||
| Comparative | |||
| Notes | Conclusion: The increase in antigenic levels of uPA and MMP‐3 in endometrium of women with endometriosis might contribute to the invasive potential of endometrial cells. Once the ovarian endometriotic cyst is developed, an increase in PAI‐1 and TIMP‐1 is detected and significant proteolytic activity is no longer observed, which could explain the frequent clinical finding of isolated endometriotic cyst without invasion of the surrounding ovarian tissue Comments: For tPA, uPAfc, PAI‐1, PAI‐2, PAI‐3, tPA‐PAI3, uPA‐PAI3, TIMP‐1 there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) For uPAag and MMP‐3 there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Gilabert‐Estelles 2007.
| Study characteristics | |||
| Patient sampling | Primary objective: to analyse mRNA expression and protein levels of VEGF, PlGF and TSP‐1 in endometrium and peritoneal fluid of women with and without endometriosis and in ovarian endometrioma patients Participants: women of reproductive age undergoing laparoscopy for various indications Selection criteria: exclusion criteria: menstrual phase, irregular menstruation, pregnancy or breastfeeding in the previous 6/12 months, hormonal treatment for 3/12 months before surgery Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: indications for surgery ‐ clinical or sonographic suspicion of endometriosis (endometriosis group) and pelvic pain (12%), sterility (18%) or tubal sterilisation (70%) in controls Age: mean age: 32.7 years (range 19–46), endometriosis; 36.5 years (range 20–52 years), controls Number enrolled: 121 women (56 in proliferative and 65 in secretory cycle phase) Number available for analysis: 90 women Setting: University hospital ‐ Hospital Universitario La Fe Place of study: Valencia, Spain Period of study: not provided Language: English |
||
| Index tests | Index test: VEGF, PlGF, TSP‐1, uPA, PAI‐1, MMP‐3, TIMP‐1 Description of positive case definition by index test as reported: mRNA expression by RT‐PCR (analysis in a LightCycler apparatus v 3.5 (Roche Molecular Biochemicals, Germany), normalised to b‐actin); antigen protein levels of VEGF, PIGF, uPA, PAI‐1, MMP‐3 and TIMP‐1 by commercially available ELISAs and TSP‐1 ‐ by indirect ELISA; laboratory technique and sample handling described Examiners: no information provided; unclear if blinded to the result of reference standard Interobserver variability: CV for TSP‐1 protein assay was 4.8%, not reported for other tests |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 71/121 (59%): all stage III‐IV; controls 50 Reference standard: laparoscopy + histopathology Description of positive case definition by reference test as reported: visual inspection of abdominal cavity confirmed by histology; staging according to rASRM classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: not specified, but from the context appears that the samples were obtained at surgery Withdrawals: 31 endometriosis patients were excluded due to insufficient sample quantity |
||
| Comparative | |||
| Notes | Conclusion: Endometrium and peritoneal fluid from women with endometriosis have increased levels of VEGF, uPA and MMP‐3. Therefore, the development of endometriotic implants at ectopic sites may be facilitated, promoting the progress of endometriosis Comments: For PIGF, TSP‐1 and PAI‐1, TIMP‐1 mRNA there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For VEGF, uPA, MMP‐3 and TIMP‐1 protein there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Hatok 2011.
| Study characteristics | |||
| Patient sampling | Primary objective: to prospectively evaluate whether detection of CYP19 mRNA in eutopic endometrium could be used as specific screening tool for endometriosis as well as reflect its severity Participants: women with regular menstrual period undergoing laparoscopic surgery for pelvic pain Selection criteria: exclusion criteria: irregular menstrual cycle, secretory phase, menopause, history of hormonal treatment before surgery, previous abdominal surgery, pregnancy, history of IVF prior to laparoscopy Study design: observational single‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: pelvic pain Age: mean age 43.02 ± 6.8 years (range 25‐55) Number enrolled: 105 women Number available for analysis: 101 women (all in early proliferative phase) Setting: University hospital ‐ Jessenius Faculty of Medicine, Comenius University Place of study: Bratislava, Slovak Republic Period of study: not provided Language: English |
||
| Index tests | Index test: Aromatase P450 (CYP19) mRNA expression Description of positive case definition by index test as reported: "[i]ncreased aromatase mRNA expression" ‐ not specified (detection by RT‐PCR; analysis by gel electrophoresis with GeneTools software; quantified by relative quantification: expression of target relative to housekeeping gene (β‐actin); laboratory technique described; diagnostic threshold not provided Examiners: no information provided Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 30/101 (30%): stage I‐II 15, stage III‐IV 15; adenomyosis ‐ 25, healthy controls ‐ 46, total non‐endometriosis controls 71 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: surgical inspection confirmed by histological examination; rAFS classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: tissue samples were obtained at surgery Withdrawals: 4 participants were excluded for low sample quality |
||
| Comparative | |||
| Notes | Conclusion: Significantly increased level of CYP19 mRNA was detected in patients, and this expression was significantly dependent on disease severity. These findings provide direct evidence that screening for endometrial aromatase could be beneficial in the prediction of oestrogen dependent disease. Comment: For the purpose of this review diagnostic estimates were calculated for endometriosis versus non‐endometriosis (controls included women with adenomyosis) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hudelist 2007.
| Study characteristics | |||
| Patient sampling | Primary objective: to analyse the expression of both aromatase and EST in the uterine and ectopic endometrium of patients with endometriosis Participants: women undergoing surgery for suspected endometriosis or non‐malignant conditions (e.g. fibroids) Selection criteria: exclusion criteria: history of PID, malignancy, adenomyosis uteri, intake of GnRH agonists, or exposure to steroids within the 6/12 months prior to surgery Study design: observational two‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: pain ‐ 26/35 and infertility ‐ 9/35, endometriosis; controls ‐ not specified Age: median age 39.6 years, endometriosis; 37.8 years, controls Number enrolled: 68 women Number available for analysis: 68 women (38 in proliferative and 27 in secretory cycle phase) Setting: Division of Special Gynecology, University of Vienna and LKH Villach hospital Place of study: Villach and Vienna, Austria Period of study: 2002‐2005 Language: English |
||
| Index tests | Index test: aromatase and EST Description of positive case definition by index test as reported: the expression was quantified by immunoreactive score (IRS), defined by IHC stain intensity: IRS > 8 ‐ strong immunoreactivity; > 4 and ≤ 8 ‐ moderate; > 0 and ≤ 4 ‐ weak, 0 ‐ negative; laboratory techniques described; no threshold provided Examiners: independent blinded assessment by 2 pathologists Interobserver variability: not reported; decision by consensus |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 35/68 (40%) (all stage I‐II); controls 33 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: visual inspection, AFS classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: eutopic tissue specimens were obtained at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The elevated expression of aromatase in eutopic and ectopic endometrium from patients with endometriosis in the presence of comparable EST provides further evidence for unopposed local biosynthesis of estrogens in endometriosis. Comments: For aromatase the data is presented only for glandular cells, diagnostic threshold defined by the review authors as presence or absence of expression; there was no statistically significant difference between the groups in the overall uterine endometrium (not presented) For EST there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Johnson 2004.
| Study characteristics | |||
| Patient sampling | Primary objective: to study the estrogenic microenvironment in eutopic endometria of women with and without endometriosis. Participants: women undergoing laparoscopy for suspected endometriosis or sterilisation Selection criteria: exclusion criteria: endocrine therapy, such as GnRH agonists, danazol or oral contraceptives 6/12 months prior to surgery Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ CPP, severe dysmenorrhoea, infertility or both; controls ‐ eumenorrhoeic fertile women requesting sterilisation Age: mean age 32.6 ± 5.8 years, endometriosis; 36.2 ± 4.8 years, controls Number enrolled: 43 women Number available for analysis: 36 women (8 in proliferative and 28 in secretory cycle phase) Setting: University hospital ‐ Hospital Clínico San Borja Arriarán, Universidad de Chile Place of study: Santiago, Chile Period of study: not specified Language: Spanish |
||
| Index tests | Index test: aromatase cytochrome P450 mRNA, E2, Aromatase activity Description of positive case definition by index test as reported: presence of aromatase mRNA expression as detected by RT‐PCR with amplification (semi‐quantification by using the System Analysis and Kodak Electrophoresis Documentation (EDAS 290)/Kodak 1D Image Analysis (Rochester, NY)); aromatase activity was determined by conversion of testosterone to E2 (assessed as E2 level after incubation with testosterone); baseline and stimulated E2 was measured by RIA (Diagnostic Sys Lab, Webster, Texas) with sensitivity 5.0 pg/ml; laboratory techniques described; no thresholds provided Examiners: no information provided; unclear if were blinded to the result of reference standard Interobserver variability: For E2 intra‐/interassay CV = 4.1%/6.7%; for aromatase ‐ none reported |
||
| Target condition and reference standard(s) | Target condition: Endometriosis Prevalence of target condition in the sample: n/N = 23/43 (53%): stage I‐II 11, stage III‐IV 12; controls 20 Reference standard: laparoscopy Description of positive case definition by reference test as reported: classification by rASRM Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were obtained at laparoscopy Withdrawals: 7 patients were not included in the experiments, reasons not provided |
||
| Comparative | |||
| Notes | Conclusion: In women with endometriosis, the microenvironment in the endometria is estrogenic as a consequence of an increased expression and activity of the P450 aromatase. Comment: For aromatase activity and baseline Estradiol there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Kim 2007.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate the replication potential of the endometrium in patients with endometriosis by examining hTERT mRNA expression and telomerase activity. Participants: women undergoing laparotomy or operative laparoscopy at the authors institution Selection criteria: exclusion criteria: postmenopausal women, previous hormone or GnRH agonist users, and patients with adenomyosis or endometrial cancer/hyperplasia/polyps Study design: observational, unclear if single‐ or two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: mean age 32.7 ± 6.8 years, endometriosis; 34.6 ± 6.7 years, controls Number enrolled: 60 women Number available for analysis: 60 women (26 in proliferative and 34 in secretory cycle phase) Setting: University hospital ‐ Yongdong Severance Hospital, Yonsei University College of Medicine Place of study: Seoul, South Korea Period of study: September 2005 to March 2006 Language: English |
||
| Index tests | Index test: hTERT mRNA, telomerase activity Description of positive case definition by index test as reported: hTERT mRNA expression level as measured by RT–PCR (TaqMan methodology, normalised to the endogenous control GAPDH and compared to the calibrator as per 2‐ΔΔCt method); telomerase ‐ relative telomerase activity (RTA), defined as the activity equivalent to that in 100 molecules of TSR (determined by the telomerase repeat amplification protocol assay, referenced to primary source (Kim et al.,1994); laboratory techniques described; no thresholds provided Examiners: no information provided Interobserver variability: no information provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 30/60 (50%): stage I‐II 8, stage III‐IV 22; controls 30 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: disease stages established by rASRM Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: biopsies were performed at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The demonstrated overexpression of hTERT mRNA and telomerase activity in the endometrium of endometriosis patients suggests that replication potential of endometrial cells may have an important role in the pathogenesis of endometriosis. Comments: For hTERT there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review For telomerase activity there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Klentzeris 1995.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate whether the endometrium of women with endometriosis differs immunologically from the endometrium of normal fertile women Participants: women undergoing laparoscopy for infertility or requesting tubal sterilisation/reversal Selection criteria: inclusion criteria: reproductive age (20‐40), regular cycles, no hormonal treatment at biopsy, no IUD for 3/12 months preceding surgery Study design: observational, two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ infertility; controls ‐ fertile women requesting tubal sterilisation or reversal Age: mean age 33.2 ± 1.6 years (range 22‐41), endometriosis; 35.4 ± 2.8 years (range 24‐40), controls Number enrolled: 36 women Number available for analysis: 33 women (all in secretory cycle phase) Setting: unclear; the authors affiliations are academic departments: Queens Medical Centre and Nottingham City Hospital Place of study: Nottingham, UK Period of study: not reported Language: English |
||
| Index tests | Index test: endometrial leukocytes: T lymphocytes (CD8+, CD4+ cells), endometrial granulated lymphocytes (CD56+ and CD38+ cells), macrophages (CD68+ cells), natural killers (CD16+ cells) and B lymphocytes (CD22+ cells) Description of positive case definition by index test as reported: positive IHC staining ‐ the area of precipitate within the endometrial stroma for each monoclonal antibody, which was proportional to the number of positive cells (detected by using Quantimet 970 image analyser (Cambridge instruments Ltd, UK); 5 non‐overlapping fields of view were examined per biopsy in a systematic random sampling pattern; magnification × 250); laboratory techniques described; no thresholds provided Examiners: no information provided Interobserver variability: no information provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 16/33 (59%): 7 stage 1, 4 stage 2, 2 stage 3, 3 stage 4; controls 17 Reference standard: laparoscopy Description of positive case definition by reference test as reported: disease stages established by rASRM Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: endometrial biopsies were performed at surgery Withdrawals: 3 women were excluded because of "retarded endometrium" |
||
| Comparative | |||
| Notes | Conclusion: This study has shown that the endometrial lymphoid tissue of women with endometriosis does not differ qualitatively or qualitatively from that of normal fertile controls. However, functional differences of endometrial leukocytes between the two groups cannot be excluded. Comments: For all the endometrial leukocytes there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Laudanski 2014.
| Study characteristics | |||
| Patient sampling | Primary objective: to compare the expression level of the most relevant angiogenesis‐related genes in the eutopic endometrium of women with and without endometriosis Participants: regularly menstruating premenopausal women, undergoing diagnostic or surgical laparoscopic surgery for non‐malignant ovarian lesions Selection criteria: Exclusion criteria: patients with autoimmune disease, PID, adenomyosis, myomas, uterine adhesion and anomalies, DUB and those who took NSAIDs, GnRH agonists and steroids for the past 3/12 months Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility ‐ 19/32, ovarian cysts ‐ 22/32 Age: mean age 31.85 ± 0.93 years, endometriosis; 29.73 ± 0.94 years, controls Number enrolled: 32 women Number available for analysis: 29 women (all in proliferative cycle phase) Setting: University hospital ‐ Medical University of Bialystok Place of study: Bialystok, Poland Period of study: not provided Language: English |
||
| Index tests | Index test: AKT1, TYMP, JAG1, LAMA5 and TIMP‐1 mRNAs and proteins Description of positive case definition by index test as reported: gene expression values measured by PCR array and calculated by 2‐ΔΔCt method (experiments on ABI 7900HT instrument (Life Technologies, Applied Biosystems, Poland); protein levels assessed by Western Blot and ELISA; laboratory techniques described; no thresholds provided Examiners: no information provided, unclear if blinded to the results of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 18/32 (56%): all stage III‐IV; controls 14 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: laparoscopic findings according to the rAFS classification; each case was confirmed by histopathology Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were collected in the operating room before the laparoscopic procedure Withdrawals: In 3 women from endometriosis group protein analysis was not performed (insufficient sample) |
||
| Comparative | |||
| Notes | Conclusion: Changes in the expression of selected genes might lead to or be a consequence of an early defect in the physiological activity of proliferative endometrium ultimately resulting in its overgrowth outside the uterine cavity. Comments: The reported data for mRNA levels are not included in the review as data of high throughput method was not corrected for multiplicity and has not verified in singleplex experiments For AKT1, TYMP, JAG1, LAMA5, TIMP‐1 proteins there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Lawson 2008.
| Study characteristics | |||
| Patient sampling | Primary objective: to elucidate the endometriosis associated changes in expression of IL‐1 receptors and to investigate further at the mRNA level the expression of IL‐1R1 and IL‐1R2 in ectopic endometrial tissue in comparison with endometrial tissue from matched women with endometriosis and normal controls Participants: women undergoing laparoscopy for infertility, pelvic pain or tubal ligation Selection criteria: inclusion criteria: no signs of hyperplasia or neoplasia, no use of hormonal or anti‐inflammatory medications for 3/12 months before surgery Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility, pelvic pain or tubal ligation Age: mean age 35 ± 4 years Number enrolled: unclear Number available for analysis: 34 women (13 in proliferative and 21 in secretory phase of menstrual cycle) Setting: University hospital ‐ Hopital Saint‐Francois d’Assise, Centre Hospitalier Universitaire de Québec Place of study: Quebec, Canada Period of study: not stated Language: English |
||
| Index tests | Index test: IL‐1R1 and IL‐1R2 mRNA Description of positive case definition by index test as reported: mRNA hybridisation score defined as intensity of hybridisation signal green to yellow from 3 evaluations in 3 randomly selected areas, scored as following: 0 = absent, 1 = light, 2 = moderate and 3 = intense signal (detection by FISH, viewing using a Leica microscope (Leica Corp. Germany) with image analysis system (ISIS, Metasystems, Germany)); no threshold cut‐off given; personal communication with the authors: for epithelial cells positive test defined as HS < 3, for stromal cells ‐ HS < 2 Examiners: 2 observers who had no knowledge of clinical status and diagnosis of the patients Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N =17/34 (50%), all stage I‐II; controls 17 Reference standard: laparoscopy Description of positive case definition by reference test as reported: staged according to rAFS classification Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: tissues obtained during laparoscopy Withdrawals explained: unclear number of recruited, different number for stroma and glandular cells experiments; withdrawals not explained |
||
| Comparative | |||
| Notes | Conclusion: The present study has shown a combined defect in IL‐1R1 and IL‐1R2 gene expression in endometriosis. Comments: For mRNA levels by RT‐PCR there was no data available to construct 2 × 2 tables; not presented in the review; demonstrated similar trends to the presented FISH data For IL‐1R2 there was statistically significant difference in secretory and no difference in proliferative cycle phase, hence the diagnostic estimates reported for the pooled phases and for sub‐analysis in secretory phase For IL‐1R1 there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Lee 2007.
| Study characteristics | |||
| Patient sampling | Primary objective: to compare the levels of expression of EGF, FGF‐2 and PDGF‐A mRNA in the eutopic endometrium of women with and without endometriosis Participants: patients of reproductive age who underwent laparoscopic surgery, exploratory laparotomy, or transabdominal hysterectomy Selection criteria: inclusion criteria: regular menstrual cycles, no hormonal therapy in the past 6/12 months; exclusion criteria: PID, DUB or any endometrial pathology; patients with visually suspected endometriotic lesions without histological confirmation Study design: observational, unclear if single‐ or two‐gate design, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: endometriosis ‐ not specified; controls ‐ fertile women undergoing surgery for ovarian cysts Age: mean 35.2 ± 6.3 years (endometriosis), 37.5 ± 5.1 years (controls) Number enrolled: 66 women Number available for analysis: 66 women (29 in proliferative and 37 in secretory cycle phase) Setting: College of Medicine, University of Ulsan, Asan Medical Centre Place of study: Seoul, South Korea Period of study: not provided Language: English |
||
| Index tests | Index test: PDGF‐A, EGF, FGF‐2 mRNA Description of positive case definition by index test as reported: levels of mRNAs assessed by RT‐PCR (cycle threshold was defined as the cycle number at which the fluorescent signal passed a fixed value; the expression of each target gene was normalised to the expression of the control gene (h‐GAPDH)); laboratory technique described; no threshold provided Examiners: none stated, unclear if were blinded to the results of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 35/66 (53%), all stages III‐IV; controls 31 Reference standard: laparoscopy/ laparotomy + histology Description of positive case definition by reference test as reported: endometriosis was staged according to rASRM Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: tissue samples were obtained at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The expression level of PDGF‐A, but not EGF and FGF‐2, might be decreased during the secretory phase in the eutopic endometrium of women with advanced stage endometriosis. Comment: For PDGF‐A, EGF, FGF‐2 mRNA there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Lee 2010.
| Study characteristics | |||
| Patient sampling | Primary objective: to study the expression of vascular endothelial growth factor (VEGF), endocrine gland‐derived VEGF (EG‐VEGF/PK1), and its receptors (PKR1 and PKR2) in eutopic and ectopic endometrial tissues Participants: infertile women undergoing diagnostic laparoscopy for tubal patency Selection criteria: inclusion criteria: regular cycles, no inflammatory or hormonal medication for at least 3/12 months before surgery Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility Age: mean age 34 years (range 29–38), endometriosis; 33 years (range 24–39), controls Number enrolled: 48 women Number available for analysis:48 women (23 in proliferative and 25 in secretory phase of the cycle) Setting: University reproduction unit, LKS Faculty of Medicine, the University of Hong Kong Place of study: Hong Kong, China Period of study: not stated Language: English |
||
| Index tests | Index test: EG‐VEGF, PKR 1 and PKR2 mRNA Description of positive case definition by index test as reported: mRNA levels detected by RTPCR following laser‐captured microdissection (detection by ABI 7500 Sequence Detector (Applied Biosystems, CA); relative gene expression values were calculated by the 2‐ΔΔCt method, normalised to endogenous control 18S); laboratory technique described; no threshold provided Examiners: none stated; unclear if were blinded to the results of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 15/48 (31%); stages not specified; controls 33 Reference standard: laparoscopy Description of positive case definition by reference test as reported: rAFS scoring was used to classify the stages of endometriosis Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: not specified, but from context appears that tissue were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Expression of VEGF mRNA and protein was higher in red peritoneal endometriotic lesion than in other types of peritoneal lesions. There was no difference in the mRNA expression of VEGF between eutopic endometrium and ovarian endometriotic tissues. Comment: For EG‐VEGF, PKR 1 and PKR2 mRNA there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
Leslie 2013.
| Study characteristics | |||
| Patient sampling | Primary objective: to assess presence of nerve fibres in endometrial functional layer as a specific and sensitive marker of concurrent peritoneal endometriosis Participants: patients undergoing laparoscopy for suspected endometriosis Selection criteria: exclusion criteria: histological diagnosis not available (ablated lesions). Hormonal pretreatment was not an exclusion Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clincial presentation: pain ‐ 45/68, infertility ‐ 14/68; adnexal mass/ menorrhagia ‐ 7/68; hormonal therapy ‐ 11/68; information was not available in 1 control and 11 cases Age: mean age 35 years (range 21–53) Number enrolled: 68 women Number available for analysis: 68 women (25 in proliferative, 19 in secretory cycle phase; 24 ‐ unclear/hormonal treatment) Setting: university hospital ‐ King Edward Memorial Hospital and private hospital ‐ Hollywood Hospital Place of study: Perth, Australia Period of study: 2006‐2011 Language: English |
||
| Index tests | Index test: endometrial functional layer nerve fibres ‐ PGP 9.5 Description of positive case definition by index test as reported: presence of functional layer nerve fibres as detected by PGP 9.5 IHC staining (lower uterine, cervical and basal layer staining was not considered; magnification using a Leica DM2500 light microscope); laboratory technique described Examiners: Single pathologist unaware of the results for the reference standard; positive and equivocal biopsies were blindly reviewed by the 2nd pathologist, disagreement resolved by consensus Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition: n/N = 47/68 (69%); stage I‐IV, number of women in each subgroup of severity not reported; controls 21 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: not stated (all laparoscopic procedures and endometrial biopsies were performed according to the individual clinician’s standard practice) Examiners: not stated |
||
| Flow and timing | Time interval between reference standard and index test: "Laparoscopy and concurrent endometrial biopsy" ‐ implies at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Endometrial nerve fibre assessment in a general patient population undergoing laparoscopy and using routine endometrial sampling and immunohistochemical analysis proved neither sensitive nor specific in the diagnosis of endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Li 2006.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate mRNA expression of matrix metalloproteinase (MMP‐9) and tissue inhibitor of metalloproteinase (TIMP‐1) in ectopic endometriosis tissue and uterine endometrium from women with and without endometriosis Participants: endometriosis patients who underwent surgical treatment (study group) and women who underwent surgery for ovarian cyst or for fibroid uterus (controls) Selection criteria: inclusion criteria: normal menstrual regularity, no other internal or surgical diseases, and no history of hormonal therapy 3 months prior to surgery Study design: cross‐sectional two‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: mean age 33 ± 5 years Number enrolled: 58 women Number available for analysis: 55 women (23 in proliferative and 32 in secretory cycle phase) Setting: university hospital: Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Science Place of study: Peking, China Period of study: January 2000 to December 2000 Language: Chinese |
||
| Index tests | Index test: MMP‐9, TIMP‐1 Description of positive case definition by index test as reported: The expression of MMP‐9 and TIMP‐1 evaluated by RT‐PCR (normalised to β‐actin; detection and semi‐quantification of PCR products by using gel electrophoresis; a gel scanner was used to determine the presence of the target band and the stripe absorbance (A) value); laboratory technique described; threshold was not reported Examiners: no information provided Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 38/58 (66%): stage I‐II 12, stage III‐IV 26; controls 20 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: staging according to rAFS Examiners: no information provided |
||
| Flow and timing | TIme interval between index test and reference standard: samples were taken at surgery Withdrawals: 3 women from endometriosis group were not included (failure to obtain endometrial samples) |
||
| Comparative | |||
| Notes | Conclusions: An increase of MMP‐9 mRNA expression of eutopic endometrium with endometriosis might enhance the endometrial implantation ability, thus facilitating the ectopic implantation of endometrium. Ectopic lesions express significantly less TIMP‐1 mRNA, indicating they have increased invasive ability, which might facilitate the development of endometriosis. Comments: For TIMP‐1 there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For MMP‐9 there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Liu 2008.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate the distribution of ER isoforms in endometriosis and eutopic endometrium Participants: patients who underwent surgical treatment of ovarian endometrioma (study group) and total hysterectomy for cervical lesions in the authors' institution (controls) Selection criteria: inclusion criteria: regular menstrual cycle and no history of hormonal therapy 6 months prior to surgery Study design: cross‐sectional two‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: mean age 36 years (range 24‐49), endometriosis; 40 years (range 27‐46), controls Number enrolled: 90 women Number available for analysis: 90 women (45 in proliferative and 45 in secretory cycle phase) Setting: university hospital: Chinese People's Liberation Army General Hospital, affiliated to Peking Union Medical College Place of study: Beijing, China Period of study: January 2004 to December 2006 Language: Chinese |
||
| Index tests | Index test: ER‐α and ER‐β Description of positive case definition by index test as reported: positive expression of ER‐α and ER‐β was evaluated by IHC and classified by using Sinicrope Improved Semi‐quantitative Method (the percentage of stained cells and the intensity of staining was scored each by a 4 tier point system in each sliced section; both scores were added up and interpreted as: < 0.5 points means negative; 0.5‐1.5 points means weakly positive (+); 1.6‐2.5 points means averagely positive (++); > 2.5 points means strongly positive (+++).; laboratory technique described; threshold was not pre‐specified Examiners: no information provided Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 60/90 (67%): stage not reported; controls 30 Reference standard: laparotomy/laparoscopy Description of positive case definition by reference test as reported: no information provided Examiners: no information provided |
||
| Flow and timing | TIme interval between index test and reference standard: samples were taken at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusions: Both ER‐α and ER‐β have higher expression levels in eutopic endometrium of patients with ovarian endometriotic cysts. ER‐β is predominantly expressed in endometriotic cysts, whereas the expression of ER‐α is limited. The different distribution of ER‐α and ER‐β may play an important role in the development of ovarian endometriosis. Comments: The authors also report findings for endometriotic cysts; not included in this review The authors also report findings for each cycle phase; not presented in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mafra 2014.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate the expression of telomerase in the endometrium and peritoneal endometriotic lesions from women with endometriosis and controls Participants: women who underwent laparoscopy for infertility ± pain (recruited from endometriosis clinic) and women undergoing sterilisation (recruited from family planning clinic) Selection criteria: inclusion criteria: regular cycle, no use of hormonal therapy for 3/12 months prior to surgery; exclusion criteria: personal/family history of autoimmune or chronic disorders Study design: observational, two‐gate design, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis: infertility ‐ 25/25, pelvic pain ‐ 15/25; controls: fertile women requesting tubal ligation Age: mean age 33.9 ± 3.3, endometriosis; 35.3 ± 4.6 years, controls Number enrolled: 69 women Number available for analysis: 69 women (all in luteal phase of menstrual cycle) Setting: Human Reproduction and Genetics Center of the Faculdade de Medicina do ABC Place of study: Santo Andre, Brazil Period of study: not stated Language: English |
||
| Index tests | Index test: hTERT mRNA Description of positive case definition by index test as reported: Positive hTERT mRNA expression by qRT‐PCR (detection by the TaqMan methodology using StepOne Real‐Time PCR System (Applied Biosystems, CA); target was normalised to the endogenous reference (GAPDH) and compared to calibrator by the 2‐ΔΔCt method); laboratory technique described Examiners: not stated; unclear if were blinded to reference standard Interobserver variability: not stated |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 25/69 (36%): stage I‐II 15, stage III‐IV 10; controls 44 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visual inspection with obligatory histological confirmation of the disease; staging according ASRM classification Examiners: not stated |
||
| Flow and timing | Time interval between index test and reference standard: < 12 months Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: There was an association between the expression of telomerase (hTERT mRNA) and the genesis and progression of endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Makari 2012.
| Study characteristics | |||
| Patient sampling | Primary objective: to test the hypothesis that nerve fibres are present in a higher density in endometrium of patients with endometriosis when compared to women with a normal pelvis Participants: patients that presented for laparoscopy for infertility, pelvic pain or both Selection criteria: inclusion criteria: reproductive age (18‐45 years); exclusion criteria: hormonal treatment 3/12 months before surgery, pregnancy or oncology cases Study design: observational single‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: dysmenorrhoea ‐ 10/20, chronic pelvic pain ‐ 11/20, infertility, dyspareunia, dysuria, dyschezia Age: mean age 36.1 ± 6.10, endometriosis; 30 13 ± 6.38 years, controls Number enrolled: 20 women Number available for analysis: 20 women (15 in proliferative and 5 in secretory cycle phase) Setting: university hospital ‐ Hospital of Lithuanian University of Health Sciences Kaunas Clinics Place of study: Kaunas, Lithuiania Period of study: 2009‐2011 Language: Lithuianian |
||
| Index tests | Index test: endometrial nerve fibres ‐ PGP 9.5 Description of positive case definition by index test as reported: presence of nerve fibres as detected by IHC staining for PGP 9.5 (evaluatIoin under × 400 magnification, microscope Olympus BX51; the number of immunoreactive nerve fibres was also calculated for each cross‐sectional area to assess nerve fibre density) Examiners: not stated, unclear if were blinded to the reference standard Interobserver variability: not stated |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 10/20 (50%): stage I‐II 4, stage III‐IV 6; controls 10 Reference standard: laparoscopy + histology Description of positive case definition by reference standard as reported: staging according to rASRM classification Examiners: not stated |
||
| Flow and timing | Time interval between index test and reference standard: tissue samples were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Endometrial biopsy for detecting density of nerve fibres by usage of PGP 9.5 provided a reliable marker for diagnosing endometriosis. The density of small nerve fibres was significantly higher in endometrium of patients with endometriosis when compared to women with a normal pelvis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Matsuzaki 2006a.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate mRNA expression of aromatase and βHSD2 in eutopic and ectopic endometrium from patients with endometriosis Participants: patients undergoing laparoscopy for deep endometriosis (cases) and women undergoing tubal reversal or sterilisation (controls) Selection criteria: inclusion criteria ‐ regular cycle, exclusion criteria: no hormonal remedies or IUCD for 6/12 months prior to surgery Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis ‐ not specified; controls ‐ fertile women requesting tubal sterilisation or reversal Age: reproductive age Number enrolled: 54 women Number available for analysis: 54 women (15 in late proliferative, 12 in early secretory, 14 in mid secretory, 13 in late secretory phase of the cycle) Setting: university hospital ‐ Polyclinique de l'Hotel Dieu, CHU Clermont‐Ferrand Place of study: Clermont‐Ferrand, France Period of study: beginning May 2001 ‐ concluding date not reported Language: English |
||
| Index tests | Index test: aromatase mRNA and 17HSD2 mRNA Description of positive case definition by index test as reported: SYBR Green I kit (Roche, Germany); target concentration was normalised to a housekeeping gene GAPDH; melting curve was used to verify specificity of reaction); laboratory technique described; threshold not pre‐specified Examiners: none stated; unclear if blinded to the result of reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: DIE Prevalence of target condition in the sample: n/N = 30/54 (55%): stages not specified; controls 24 Reference standard: laparoscopy Description of positive case definition by reference test as reported: DIE lesion deeper than 5 mm from peritoneal surface Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: samples were collected at surgery Withdrawals: 1 patient was not included in analysis of 17HSD2 mRNA |
||
| Comparative | |||
| Notes | Conclusion: Local oestrogen concentration may be much higher in epithelial cells than in stromal cells in deep endometriotic tissue. Comments: For 17HSD2 mRNA the diagnostic estimates are reported only for stromal cells; there was no statistically significant difference between the group for glandular epithelium For 17HSD2 mRNA in stromal cells there was no statistically significant difference between the groups in proliferative phase, but separate accuracy estimates for secretory phase were not available ‐ pooled data presented For aromatase the diagnostic estimates are presented for pooled cycle phases and pooled cell types |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Meibody 2011.
| Study characteristics | |||
| Patient sampling | Primary objective: to test the hypothesis that small demyelinated nerve fibres in functional layer of endometrium are present in higher density in women with endometriosis, which is more significant in secretory phase. Participants: women undergoing laparoscopy/laparotomy for infertility or pelvic pain Selection criteria: inclusion criteria: reproductive age, regular menstrual cycle; exclusion criteria: unwillingness to participate and use of hormonal medications for the past 3/12 months Study design: observational single‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: chronic pelvic pain ‐ 23/27, dyspareunia ‐ 5/27, dysmenorrhoea ‐ 7/27, infertility ‐ 5/27 Age: mean age 39.5 ± 5.9 years, endometriosis; 41.6 ± 5.7 years, controls Number enrolled: 27 women Number available for analysis: 27 women (all in proliferative cycle phase) Setting: university hospital ‐ Minimally Invasive Surgery Research Center, Rassoul Akram Hospital, Iran University of Medical Sciences Place of study: Tehran, Iran Period of study: 2007‐2009 Language: English |
||
| Index tests | Index test: endometrial small nerve fibres in eutopic endometrium ‐ PGP 9.5 Description of positive case definition by index test as reported: Presence of nerve fibres detected by IHC staining for PGP 9.5 seen in 10 HPF (IHC by using Dako Denmark A/S Produktionsej42 DK‐2600, Denmark and Olympus microscope; assessment of 3‐4 sections per slide; density of NF was also calculated by intensity of staining); laboratory technique described Examiners: pathologist was blinded to reference standard result Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition: n/N = 12/27 (44%): stage not reported; controls 15 Reference standard: Laparoscopy/laparotomy + histology Description of positive case definition by index test as reported: visualisation of the endometriotic lesions with surgical staging of the disease and histological confirmation Examiners: 3 experienced gynaecologists in endometriosis |
||
| Flow and timing | Time interval between index test and reference standard: endometrial biopsy was obtained prior to surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Assessment of neural fibre density in endometrial biopsy by PGP 9.5 staining is as accurate as surgical assessment by experienced gynaecologists. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Meola 2009.
| Study characteristics | |||
| Patient sampling | Primary objective: to analyse the expression of the glycodelin gene to better understand the molecular environment of endometriotic lesions and to elucidate the potential mechanisms that underlie the complex physiopathology of endometriosis Participants: women undergoing laparoscopy for pelvic pain, infertility or sterilisation Selection criteria: inclusion criteria: reproductive age (18‐40 years), early proliferative phase of the menstrual cycle (days 5‐8), regular cycles, and no history of any hormone therapy during the 6/12 months prior to surgery Study design: observational two‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: endometriosis: pelvic pain, infertility or both; controls referred for tubal ligation Age: range 18‐40 Number enrolled: 28 women Number available for analysis: 28 women (all in early proliferative phase) Setting: tertiary referral centre, School of Medicine of Ribeirao Preto, University of Sao Paulo Place of study: Ribeirao Preto, Brazil Period of study: not provided Language: English |
||
| Index tests | Index test: glycodelin mRNA Description of positive case definition by index test as reported: mRNA expression by RT‐PCR (TaqMan methodology, target level normalised to endogenous control (GAPDH) and the calibrator sample, calculated by 2‐ΔΔCt method); laboratory technique described; threshold not pre‐specified Examiners: none stated Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 17/28 (61%): stage II 4, stage III‐IV 13; controls 11 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: staging according to rASRM classification, diagnosis was confirmed through histopathologic analysis Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: before procedure Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Glycodelin may be one of the molecules that contributes to the loss of cellular homeostasis in endometriotic lesions. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Meola 2013.
| Study characteristics | |||
| Patient sampling | Primnary objective: to investigate whether average CALD1 expression and caldesmon protein levels were altered in the endometrium and endometriotic lesions and to evaluate the performance of CALD1 gene and caldesmon protein as potential biomarker for endometriosis Participants: women who underwent laparoscopy for suspected endometriosis and for tubal ligation Selection criteria: inclusion criteria: reproductive age, no hormone therapy for at least 6/12 months and no history of reproductive tract disorders or tumours Study design: observational two‐gate, prospective collection of the samples |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group: infertility ‐ 21/40, pelvic pain ‐ 19/40; controls ‐ healthy fertile women requesting tubal ligation Age: mean age 33.5 ± 0.72 years (endometriosis), 33.87 ± 0.69 years (controls) Number enrolled: 55 women Number available for analysis: 55 women (35 in proliferative and 35 in secretory phase of menstrual cycle; controls samples × 2 in each cycle phase) Setting: tertiary hospital of the School of Medicine of Ribeirao Preto (University of Sao Paulo) Place of study: Ribeirao Preto, Brazil Period of study: not stated Language: English |
||
| Index tests | Index test: CALD1 mRNA and caldesmon protein Description of positive case definition by index test as reported: relative expression of CALD 1 gene detected by RT‐PCR (Taqman methodology, relative quantification by the 2‐ΔΔCt method, calibrated and normalised to GAPDH and ACTB genes); caldesmon protein level by IHC stain intensity; laboratory techniques described; thresholds not pre‐specified Examiners: examiners blinded to the clinical data Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition: n/N = 40/55 (72%): stage I‐II 17, stage III‐IV 23; controls 15 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: laparoscopic and histological diagnosis of endometriosis, staging according to rASRM classification Examiners: not stated |
||
| Flow and timing | Time interval between reference test and index test: during laparoscopy Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Caldesmon is a possible predictor of endometrial dysregulation in patients with endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Mikolajczyk 2006.
| Study characteristics | |||
| Patient sampling | Primary objective: to test whether there is an altered secretion of IL‐11 and LIF, which could explain receptivity defect in patients with minimal endometriosis Participants: women undergoing laparoscopy for infertility, pelvic pain or both Selection criteria: exclusion criteria: any abnormality in infertility workup, hormonal medications for 3/12 months preceding surgery, advanced endometriosis diagnosed at the index surgery Study design: observational singe‐gate, prospective collection of samples |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ Infertility; controls ‐ pelvic pain, fertile women Age: mean age 26 years (range 22‐34), endometriosis; 24 years (range 22‐34), controls Number enrolled: 84 women Number available for analysis: 35 women (all in luteal phase) Setting: Division of Reproduction, University of Medical Sciences Place of study: Poznan, Poland Period of study: not provided Language: English |
||
| Index tests | Index test: LIF and IL‐11 protein in uterine flushing; LIF and IL‐11 mRNA in endometrium Description of positive case definition by index test as reported: concentration of IL‐11 and LIF in uterine flushing detected by ELISA; mRNA expression levels in endometrium detected by RT‐PCR (Qiagen OneStep RT–PCR kit with amplification, normalised to GAPDH); experiments described; thresholds not pre‐specified Examiners: no information provided, unclear if blinded to the result of index test Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 14/35 (40%): all stage I‐II; controls 21 Reference standard: laparoscopy ± histology Description of positive case definition by reference test as reported: diagnosis of endometriosis was based on visualisation of endometriotic lesions found during laparoscopy; in 70% of patients, the diagnosis was also confirmed by histopathologist; staging according to rAFS Examiners: All laparoscopies were performed by a skilled surgeon with over 15 years of experience in detection and treatment of endometriosis |
||
| Flow and timing | Time interval between index test and reference standard: uterine flushing and tissue biopsy were performed at surgery Withdrawals: 48 women were excluded because did not meet inclusion criteria ‐ before sample processing |
||
| Comparative | |||
| Notes | Conclusion: There is no receptivity defect with regard to LIF and IL‐11 secretions by eutopic endometrium in infertile women with endometriosis. Comment: For LIF and IL‐11 in uterine flushing and in endometrium there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Morelli 2010.
| Study characteristics | |||
| Patient sampling | Primary objective: to determine whether relaxin and or its LGR7 receptor are expressed In human endometriotic tissues and whether their expression differs from that in endometrium of normal controls Participants: women undergoing surgery for findings of an ovarian cyst on USS Selection criteria: exclusion criteria: laparoscopic evidence and histologic confirmation of extra ovarian endometriosis at index surgery Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: ovarian cyst, not specified Age: range 24‐37 years Number enrolled: 40 women Number available for analysis: 40 women (11 in proliferative and 29 in secretory phase of the cycle) Setting: Department of Paediatrics, Obstetrics and Reproductive Medicine, University of Siena Place of study: Siena, Italy Period of study: October 2006 to May 2007 Language: English |
||
| Index tests | Index test: Relaxin mRNA, LGR7 receptor mRNA Description of positive case definition by index test as reported: LGR7 ‐ mRNA expression detected by RT‐PCR; Relaxin ‐ proportion of tissues expressing relaxin (detection by using the Rotor‐Gene 3000 PCR system (Corbett Research, CA) with using Platinum SYBR Green qPCR SuperMix (Invitrogen); normalised to β‐actin mRNA expression via 2‐ΔΔCt method; experiments described; thresholds not pre‐specified Examiners: none stated; unclear if blinded to the results of reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: ovarian endometriosis Prevalence of target condition in the sample: n/N = 21/40 (52%): all stage III‐IV; controls 19 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: laparoscopic evidence and histologic confirmation of extra ovarian endometriosis; staging according to the rASRM classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: samples were collected at surgery (appears from the context) Withdrawals: 8 patients with endometriosis were excluded as no tissue was available |
||
| Comparative | |||
| Notes | Conclusion: Significantly decreased expression of relaxin mRNA and LGR7 relaxin receptor mRNA in ectopic endometriotic tissues relative to their expression in eutopic endometrium and in endometrium from normal controls suggests that relaxin exerts a protective effect against endometriosis. Comment: For LGR7 relaxin receptor and relaxin mRNA there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Pino 2009.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate the association between MMP‐1 and MMP‐9 activities and ICAM1 cleavage mediated by TNF in eutopic endometrial stromal cells from women with and without endometriosis during culture Participants: women without endometriosis who underwent laparoscopic tubal sterilisation or hysterectomy for a benign non‐endometrial gynaecologic condition (control group), and women undergoing laparoscopy for diagnosis of endometriosis, which was later surgically confirmed Selection criteria: exclusion criteria: hormonal treatment or contraceptives during the previous 6 or 3 months, respectively; neoplastic, endocrine or infectious diseases Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ not specified, controls ‐ eumenorrhoeic reproductively normal women without endometriosis who underwent laparoscopic tubal sterilisation Age: mean age 35.2 ± 0.7, endometriosis group; 38.4 ± 0.5 years, controls Number enrolled: 20 women Number available for analysis: 20 women (7 in proliferative and 13 in secretory phase of the cycle) Setting: Institute of Maternal and Child Research, San Borja Arriarán Clinical Hospital, University of Chile Place of study: Santiago, Chile Period of study: not reported Language: English |
||
| Index tests | Index test: MMP‐1, MMP‐9, ICAM1, sICAM1 mRNA and protein Description of positive case definition by index test as reported: MMP‐1, MMP‐9, ICAM1 and sICAM1 mRNA expression by RT‐PCR (internal control 18S rRNA, semi quantification by using agarose gel electrophoresis); ICAM1 and MMP1 protein levels by Western blot; activities of MMP1 and MMP9 by using casein zymography in cell culture (results are expressed as arbitrary units regarding the total µg of proteins loaded (MMP1) or as values normalised to the internal standard (MMP9)); concentration of active MMP‐1 by using enzymatic activity assay (Human Active MMP‐1 Fluorescent Assay kit (R&D System Inc., Minneapolis, USA) per manufacturer’s instructions; detection limit 0.052 ng/ml); sICAM concentration by using ELISA (Inmunoassay Human sICAM1 kit (R&D System) per manufacturer’s instructions, minimal detectable concentration of 0.096 ng/mL); sample processing and laboratory technique described in details; thresholds not provided Examiners: none stated; unclear if blinded to the results of reference standard Interobserver variability: intra‐ and interassay reproducibility for sICAM: 3.3%‐4.8% and 6.0%‐10.1%, for MMP‐1: 9.6%‐10.0% and 8.7%‐17.7% |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 10/20 (50%): stage I‐II 6, stage III‐IV 4; controls 10 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: staging according to the rASRM classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: samples were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The deregulation of MMP‐9, and the TNF participation in the MMP‐1 and proMMP‐9 secretions, in the MMP‐9 expression and in the expression and cleavage of ICAM1 may contribute to the pathophysiology of this disease Comment: The reported estimates for the assessed biomarkers are for basal conditions only, prior to incubation with TNF For ICAM mRNA, ICAM protein, sICAM protein, MMP‐9 mRNA, ‐ protein and MMP‐1 protein there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For MMP‐1 mRNA there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Prefumo 2002.
| Study characteristics | |||
| Patient sampling | Primary objective: to quantify the expression of CD54, CD58 and CD106, three adhesion molecules with a crucial role in cytotoxic mechanisms, on fresh endometrial cells by immunofluorescence and flow cytometry in women with and without endometriosis Participants: regularly cycling patients undergoing laparoscopy because of pelvic pain, infertility or both Selection criteria: inclusion criteria: no hormonal treatment in 3 months before surgery Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: pelvic pain, infertility or both Age: median age 29.5 years (range 20–44), endometriosis; 34 years (range 26‐46), controls Number enrolled: 22 women Number available for analysis: 22 women (11 in proliferative and 11 in secretory phase of menstrual cycle) Setting: university hospital ‐ UO di Ostetricia e Ginecologia, Istituto Giannina Gaslini, Università di Genova Place of study: Genoa, Italy Period of study: not reported Language: English |
||
| Index tests | Index test: adhesion molecules CD54, CD58, CD106 Description of positive case definition by index test as reported: cell size and number of molecules expressed on the cell surface by flow cytometric methods (analyses by using a flow cytometer (Coulter EPICS XL, Milan, Italy) on cells gated to exclude nonviable cells; a minimum of 1 × 104 cells were examined per sample; quantitative analysis of the expression of surface molecules on the endometrial cells were calculated using different standard beads of known diameter and number of FITC equivalent (Coulter Science, Miami, USA); experiments described; thresholds not provided Examiners: no information provided; unclear if blinded to the result of reference standard Interobserver variability: not stated |
||
| Target condition and reference standard(s) | Target condition: peritoneal endometriosis Prevalence of target condition in the sample: n/N = 10/22 (45%): stages not reported; controls 12 Reference standard: laparoscopy Description of positive case definition by reference test as reported: peritoneal endometriosis was defined as the presence of red, black or white lesions on the peritoneal surface Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were obtained at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: These findings could account for an apparently cyclic defect in the expression of CD54 that could result in poor binding of immune effectors to secretory endometrial cells in vivo. Comments: For CD58, CD106 and CD54 follicular there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For CD54 luteal there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Puy 2002.
| Study characteristics | |||
| Patient sampling | Primary objective: To compare the glandular and stromal immunoreactivity patterns of αvβ5 and αvβ6 integrins between eutopic and ectopic endometrium from women with endometriosis and in eutopic endometrium of women without endometriosis; to analyse vascular αvβ5 and αvβ6 immunoreactivity in these tissue specimens Participants: patients who underwent laparoscopy at the authors' institution, including tubal ligation Selection criteria: exclusion criteria: known prior endometrial disease or patients receiving hormonal therapy Study design: observational two‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group: not reported; controls ‐ women requesting tubal ligation Age: reproductive age group; not specified Number enrolled: 57 women Number available for analysis: 52 women (22 in proliferative and 30 in secretory cycle phase) Setting: university‐affiliated tertiary care centre ‐ Women’s College Health Sciences Centre Place of study: Toronto, Canada Period of study: not reported Language: English |
||
| Index tests | Index test: epithelial, stromal and vascular αvβ5 and αvβ6 integrins Description of positive case definition by index test as reported: staining intensity of endometrial epithelial and nonepithelial cells by IHC using HSCORE (0 ‐ no staining, 1 ‐ weak, 2 ‐ moderate, 3 ‐ strong staining) and quantification of the percentage of immunolabelled blood vessels (evaluation by using a computerised image analysis system (CIAS): endometrial cells ‐ mean of 5 fields at × 100 magnification; blood vessel cells ‐ mean of 10 fields at × 1000 magnification; percentage of immunolabelled blood vessels was evaluated as mean of 10 fields at × 400 magnification); technique described; thresholds not provided Examiners: All samples were evaluated independently by the two observers, and each was blinded to all clinical data Interobserver variability: The overall correlation coefficient for intraobserver median HSCOREs was 0.97 (P < 0.0001) and for interobserver variation was 0.96 (P < 0.0001). |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 40/52 (77%): stage I‐II 14, stage III‐IV 26; controls 12 Reference standard: laparoscopy Description of positive case definition by reference test as reported: staging according to rAFS classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were obtained at surgery Withdrawals explained: 5 endo cases excluded due to asynchronous histological dating (excluded before the experiments) |
||
| Comparative | |||
| Notes | Conclusion: The differences that we observed for αvβ5 and αvβ6 integrin immunostaining between eutopic and ectopic endometrium remain to be elucidated. Comments: For epithelial and stromal αvβ5 and αvβ6, there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) For αvβ5 and αvβ6 integrins in blood vessel walls there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Rakhila 2013.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate prostaglandin (PG) biosynthesis and catabolism pathways in eutopic and ectopic endometrium of women with endometriosis Participants: women undergoing laparoscopy for pelvic pain, infertility or both (endometriosis group) and women scheduled for tubal ligation Selection criteria: exclusion criteria: no other pelvic pathology, signs of endometrial hyperplasia, or neoplasia and any nonsteroidal anti‐inflammatory drugs (NSAIDs) or hormonal medication for 3 months before surgery Study design: observational two‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group ‐ chronic pelvic pain, infertility or both; controls – disease‐free women requesting tubal ligation Age: Mean age 34.2 ± 3.6 yeas (endometriosis group), 35.3 ± 3.8 years (controls) Number enrolled: 74 women Number available for analysis: 74 women (34 in proliferative and 40 in secretory cycle phase) Setting: Centre Hospitalier Universitaire de Québec, Hôpital Saint‐François d'Assise Place of study: Quebec, Canada Period of study: not specified Language: English |
||
| Index tests | Index test: prostaglandin synthases: Cox‐1, Cox‐2, mPGES‐1, mPGES‐2, cPGES, AKR‐1B1, AKR‐1C3; prostaglandin catabolic enzyme 15‐PGDH mRNA Description of positive case definition by index test as reported: expression levels of the biomarkers by RT‐PCR (using SYBR Green chemistry, carried out in ABI 7000 Thermal Cycler (Applied Biosystems); quantification by using a relative quantification method, normalised to GAPDH mRNA levels); sample handling and laboratory technique described Examiners: no information provided Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 45/74 (61%); stage I‐IV, number per subgroup not provided; controls 29 Reference standard: laparoscopy Description of positive case definition by reference test as reported: staging according to the rASRM system Examiners: no information provided |
||
| Flow and timing | Time interval between index test and reference standard: tissue samples were obtained at laparoscopy Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: This study reveals for the first time multiple defects in PG biosynthesis pathways, which differ between eutopic intrauterine and ectopic endometrial tissues and may, owing to the wide spectrum of PG properties, contribute to the initial steps of endometrial tissue growth and development and have an important role to play in the pathogenesis and symptoms of this disease Comments: For Cox‐1, cPGES, Akr1B1, Akr1C3 and 15‐PGDH mRNA there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) For Cox‐2, mPGES1 and mPGES2 mRNA there was a statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ramon 2011.
| Study characteristics | |||
| Patient sampling | Primary objective: to analyse several miRNAs related to angiogenesis and the angiogenic factors, VEGF‐A and TSP‐1, in endometriotic lesions and eutopic endometrium from women with endometriosis. Participants: women undergoing surgery for pelvic pain, sterilisation, infertility or endometriosis Selection criteria: exclusion criteria: menstrual phase of the cycle, irregular menstruation, pregnancy or breastfeeding in the previous 6/12 months, hormonal treatment for the last 3/12 months Study design: observational two‐gate, prospective sample selection |
||
| Patient characteristics and setting | Clinical presentation: indications for surgery ‐ endometriosis group: clinical or ultrasonographic suspicion of endometriosis; controls: pelvic pain ‐ 7.90%, sterility ‐ 21.0%, tubal sterilisation ‐ 71.1% Age: mean age 34.9 years (range 24‐47), endometriosis; 36.3 years (range 21‐47), controls Number enrolled: 96 women Number available for analysis: 96 women (46 in proliferative and 50 in secretory cycle phase) Setting: university hospital ‐ Hospital Universitario La Fe and Hospital Arnau de Vilanova Place of study: Valencia, Spain Period of study: not reported Language: English |
||
| Index tests | Index test: VEGF‐A and TSP‐1 mRNA and protein Description of positive case definition by index test as reported: relative mRNA expression by RT‐PCR (in a LightCycler apparatus, using version 3.5 software (Roche Molecular Biochemicals, Mannheim, Germany); normalised to β‐actin; protein level as quantified by ELISA; experiments described; no threshold provided Examiners: no information provided; unclear if blinded to the result of reference standard Interobserver variability: ELISA intra‐ and interassay variabilities for VEGF: 4–6% and 7–10%; for TSP‐1: 5–6% and 8–11% |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 58/96 (60%): stage not specified; controls 38 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: meticulous examination of the peritoneum, ovaries, intestine and diaphragm to detect any typical or atypical endometriotic lesion; biopsies of suspicious areas; complete excision of the endometriotic tissue Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were obtained at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: expression levels of mRNAs related to angiogenesis were different in eutopic endometrium from that observed in ovarian endometrioma. This could influence the expression of angiogenic factors and play a role in the pathogenesis of endometriosis. Comments: For VEGF A mRNA and protein there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review For TSP‐1 mRNA and protein there was no statistically significant difference between the groups; no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Szymanowski 2003.
| Study characteristics | |||
| Patient sampling | Primary objective: to describe the expression of α3β1, α4β1 and β1 integrin chain in endometrial biopsy in women suffering from endometriosis Participants: women with infertility, pelvic pain or both undergoing laparoscopy and hysteroscopy Selection criteria: inclusion criteria: regular cycles, no hormonal treatment at least 1/12 month prior surgery; exclusion criteria: pregnancy, non‐peritoneal endometriosis Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility ‐ 23/32, pelvic pain ‐ 11/32 Age: mean age 29.2 ± 4.19 years Number enrolled: 32 women Number available for analysis: 32 women (all in secretory phase) Setting: university hospital ‐ Division of Reproduction, K. Marcinkowski University of Medical Sciences Place of study: Poznan, Poland Period of study: January 1998 to June 2000 Language: English |
||
| Index tests | Index test: α3β1, α4β1 and β1 Integrin chain Description of positive case definition by index test as reported: Positive IHC staining (staining intensity was defined as weak +, moderate ++, strong +++; not defined in glandular and stromal cells); threshold not pre‐specified Examiners: blinded observer Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: peritoneal endometriosis Prevalence of target condition in the sample: n/N = 17/32 (53%): stage not specified; controls 15 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: direct visualisation of endometrial foci with histological confirmation; rAFS classification Examiners: not stated |
||
| Flow and timing | Time interval between reference test and index test: tissue sample was obtained at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The selected integrins do not yet allow identification of a pattern characteristic for endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Takahashi 1990.
| Study characteristics | |||
| Patient sampling | Primary objective: to discover clinical usefulness of levels of CA‐125 in menstrual blood in patients with endometriosis Participants: women undergoing laparoscopy or laparotomy for infertility or pelvic pain Selection criteria: not stated Study design: observational single‐gate, prospective recruitment and sample collection; consecutive patients |
||
| Patient characteristics and setting | Clinical presentation: infertility/pelvic pain Age: reproductive‐aged women Number enrolled: 104 women Number available for analysis: 104 women (all in menstrual phase of the cycle) Setting: university hospital ‐ Department of O&G, Shimane Medical University Place of study: Izumo, Japan Period of study: not stated Language: English |
||
| Index tests | Index test: CA‐125 in menstrual fluid Description of positive case definition by index test as reported: CA‐125 level by RIA (commercial kit Centocor Inc, Malvern, PA); sample handling described; threshold > 100,000 U/ml ‐ not pre‐specified Examiners: not stated; unclear if blinded to reference standard Interobserver variability: not provided |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in sample: n/N = 38/104 (36%): stage I‐II 20, stage III‐IV 14; controls 66: normal pelvis 30, other pelvic pathology 36 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: visual typical appearance of disease, staged according to rAFS classification Examiners: not stated |
||
| Flow and timing | Time interval between reference test and index test: sample was collected pre‐operatively with interval of 1‐4 weeks Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Obtaining CA‐125 concentration in menstrual discharge was found to be more sensitive than serum CA‐125 levels drawn during menses in endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tang 2009.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate the possible roles of cyclin B1, cyclin‐dependent kinase (cdc2) and Polo‐like kinase 1 (Plk1) in the pathogenesis of endometriosis Participants: women who were undergoing laparoscopic surgery or hysterectomy Selection criteria: inclusion criteria: regular menstrual cycles (21–35 days), not on hormonal treatment, not pregnant, not breastfeeding, and not using IUD in the past 6/12 months; exclusion criteria: PID, adenomyosis, DUB Study design: observational study, two‐gate design, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: not specified Age: range 24‐46 years Number enrolled: 59 women Number available for analysis: 50 women (27 in proliferative and 23 in secretory cycle phase) Setting: university hospital ‐ Women’s Hospital of Zhejiang University Medical School Place of study: Hangzhou, Zhejiang, China Period of study: 2005‐2006 Language: English |
||
| Index tests | Index test: cyclin B1, cdc2, Plk1 mRNA and protein Description of positive case definition by index test as reported: mRNA expression by RT‐PCR (using multiscribe reverse transcriptase (Invitrogen), single tube reaction with amplification; β‐actin gene used as internal reference); protein levels detected by Western Blot (Bradford assay (Bio‐Rad Laboratories, CA), normalised to β‐actin); laboratory technique described; thresholds not provided Examiners: none stated; unclear if blinded to the result of reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 20/50 (40%): all stages II‐III; controls 30 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: staging according to rAFS Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: not stated; the context suggests timing around surgery Withdrawals: 9 participants were not included in the analyses, reasons not explained |
||
| Comparative | |||
| Notes | Conclusion: the increased expression of cyclin B1, cdc2, and Plk1 in ectopic endometrium may be involved in the up‐regulated proliferation activity and neoplastic features of endometriosis. Comment: For Cyclin B1, cdc2, Plk1 mRNA and protein, there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Tiberi 2010.
| Study characteristics | |||
| Patient sampling | Primary objective: to examine prokineticin 1 (PROK1) mRNA expression in eutopic endometrial glands obtained from patients with or without endometriosis Participants: women of reproductive age undergoing laparoscopy for subfertility or suspected endometriosis Selection criteria: inclusion criteria: regular menstrual cycles, written consent; exclusion criteria: no hormonal medications for the 3 months preceding surgery, endometrium with pathology or hormonal alterations Study design: observational single‐gate, prospective enrolment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility (blocked tubes n = 10, unexplained infertility n = 1, without previous pregnancy n = 1) or suspected endometriosis (CPP n = 2, suspected endometriomas n = 18) Age: range 26‐40 years Number enrolled: 32 women Number available for analysis: 24 women (all in secretory cycle phase) Setting: university hospital ‐ Universita Cattolica del Sacro Cuore Place of study: Rome, Italy Period of study: not provided Language: English |
||
| Index tests | Index test: PROK1 mRNA in glandular epithelium Description of positive case definition by index test as reported: Positive or negative mRNA expression by RT‐PCR; negative mRNA expression was defined as no amplification detected after 40 cycles (TaqMan methodology, expression was evaluated using the i‐Cycler iQTM system (Bio‐Rad Laboratories); normalised to GAPDH gene and calibrator sample by the 2−ΔΔCt method); sample handling and laboratory technique described Examiners: none stated; unclear if blinded to the result of reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 12/24 (50%): all stage III‐IV; controls 12 Reference standard: laparoscopy Description of positive case definition by reference test as reported: staging according to rASRM classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: "All samples were collected by uterine curettage specifically during the putative window of implantation . . . from women undergoing laparoscopy" suggests that the samples were collected before surgery and the time interval sample collection‐surgery was reasonably short Withdrawals: 8 women from control group were excluded prior to analysis because of other ovarian pathology |
||
| Comparative | |||
| Notes | Conclusion: PROK1 is a newly discovered angiogenic factor implicated in the vascular function of peri‐implantation endometrium and early pregnancy. An altered expression of PROK1 could be one of the several biochemical abnormalities characterising eutopic endometrium in endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Van der Linden 1994.
| Study characteristics | |||
| Patient sampling | Primary objective: to detect the expression of integrins and E‐cadherin in cells from peritoneal fluid (PF), endometrium, menstrual effluent, peritoneum, and endometriotic lesions during the early follicular phase of the menstrual cycle Participants: women who underwent a diagnostic laparoscopy as part of a subfertility work‐up Selection criteria: inclusion criteria: regular ovulatory cycles Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertiliy Age: reproductive age, not specified Number enrolled: 16 women Number available for analysis: 16 women (all in early proliferative cycle phase) Setting: tertiary care university medical centre ‐ the University of Limburg Place of study: Maastricht, the Netherlands Period of study: not reported Language: English |
||
| Index tests | Index test: Integrins α2β1, α3β1, α4β1, α5β1, α6β1, E‐cadherin in menstrual fluid and endometrium Description of positive case definition by index test as reported: positive IHC staining; laboratory technique described Examiners: none stated; unclear if blinded to the result of reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 8/16 (50%): all stage I; controls 8 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visual inspection confirmed by histopathology, staging according to rAFS Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: samples were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Integrins α2β1, α3β1, α4β1, α5β1, α6β1 and E‐cadherin, important cell adhesion molecules, are expressed in endometriotic lesions and in cells and tissues that are potentially involved in the development of endometriosis. These cell adhesion molecules could be involved in the shedding of endometrial tissue during menstruation and the attachment of endometrial tissue fragments to the peritoneum Comment: For α2β1, α3β1, α4β1, α5β1, α6β1 and E‐cadherin there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Van der Linden 1995.
| Study characteristics | |||
| Patient sampling | Primary objective: to examine the immunohistochemical properties of epithelial cells in peritoneal fluid and to compare the staining characteristics with cells of endometrium, menstrual effluent, peritoneum, and endometriotic lesions Participants: women who underwent a diagnostic laparoscopy as part of a subfertility work‐up Selection criteria: inclusion criteria: regular ovulatory cycles Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility Age: reproductive age, not specified Number enrolled: 16 women Number available for analysis: 16 women (all in early proliferative cycle phase) Setting: tertiary care university medical centre ‐ the University of Limburg Place of study: Maastricht, the Netherlands Period of study: not reported Language: English |
||
| Index tests | Index test: vimentin, cytokeratin 18, cytokeratin 19 and endometrial epithelial marker BW 495/36 in menstrual fluid and endometrium Description of positive case definition by index test as reported: positive IHC staining; laboratory technique described Examiners: none stated; unclear if blinded to the result of reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 8/16 (50%): all stage I; controls 8 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visual inspection confirmed by histopathology, staging according to rAFS Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: samples were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: These results support the contention of transport of menstrual detritus to the peritoneal cavity in women with patent fallopian tubes. Comment: For vimentin, cytokeratin 18, cytokeratin 19 and endometrial epithelial marker BW 495/36, there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Vernet‐Tomas 2006a.
| Study characteristics | |||
| Patient sampling | Primary objective: to compare the expression and localisation of integrin subunit α6 in women with and without endometriosis Participants: women who underwent surgery for various indications (pelvic pain, infertility, menstrual disturbances, adnexal masses or tubal ligation) Selection criteria: exclusion criteria: history of immune disease, malignancy, hormone therapy, or immune therapy Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis: pelvic pain ‐ 12/30, infertility ‐ 8/30, adnexal mass ‐ 6/30, menstrual disturbances ‐ 4/30; controls: pelvic pain ‐ 5/19, infertility ‐ 3/19, tubal sterilisation ‐ 11/19 Age: mean age 32.8 ± 7.11 years (range 20‐50), endometriosis; 33.95 ± 3.36 years (range 27‐41), controls Number enrolled: 52 women Number available for analysis: 49 women (19 in proliferative and 30 in secretory cycle phase) Setting: university hospital ‐ O&G Department Hospital Universitari del Mar and Hospital Gemans Trias i Pujol Place of study: Barcelona, Spain Period of study: not reported Language: English |
||
| Index tests | Index test: depolarised α6 integrin Description of positive case definition by index test as reported: the percentage of positively stained glandular cells and the localisation of expression: polarised α6 ‐ expression exhibited only on the basal side of the cell, depolarised ‐ when expression was observed in the basal side, as well as in any other side of the cell (IHC staining; examining ten non‐overlapping fields per biopsy with a magnification of × 400, counting a total of 300‐400 cells per case); technique described Examiners: 2 independent observers who were blinded to the study group Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 30/52 (58%); stage I‐II 4, stage III‐IV 26; controls 19 Reference standard: laparoscopy n = 32/laparotomy n = 17 Description of positive case definition by reference test as reported: rAFS classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were collected at surgery Withdrawals: 3 women were excluded due to ineligible tissue samples |
||
| Comparative | |||
| Notes | Conclusion: The endometria of women with endometriosis more frequently show a depolarised expression of integrin subunit α6, a characteristic usually found in highly proliferating cells with migrating and invasive abilities. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Visnovsky 2008.
| Study characteristics | |||
| Patient sampling | Primary objective: to determine clinical benefits of mRNA aromatase expression in entopic endometrium as a diagnostic marker of endometriosis Participants: women undergoing laparoscopy or laparotomy for pelvic pain, infertility of benign pelvic tumours Selection criteria: inclusion criteria: confirmed benign pelvic condition by pre‐operative ultrasound Study design: observational single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis: pelvic pain, infertility, pelvic mass Age: mean age 39.7; SD and range not reported Number enrolled: 23 women Number available for analysis: 23 women (menstrual cycle phase not specified) Setting: university hospital ‐ O&G Department Jessenius Medical Faculty and Martin University Hospital Place of study: Martin, Czech Republic Period of study: May 2006 to August 2007 Language: English |
||
| Index tests | Index test: aromatase mRNA Description of positive case definition by index test as reported: mRNA expression by RT‐PCR, semi‐quantitative expression scored 0‐3: 0 ‐ no expression; 1 ‐ low; 2 ‐ medium; 3 ‐ high expression; technique not described; threshold ‐ low expression (score 1 and above, not pre‐specified) Examiners: not reported; unclear if were blinded to the results of reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 12/23 (52%); stage not specified; controls 11 Reference standard: laparoscopy n = 17/laparotomy n = 6 + histology Description of positive case definition by reference test as reported: visual inspection confirmed by histology, rAFS staging Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: endometrial samples were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Aromatase expression in eutopic endometrium is a good diagnostic marker for endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wang 2010a.
| Study characteristics | |||
| Patient sampling | Primary objective: to find the biomarkers of eutopic endometrium in endometriosis patients by using SELDI‐TOF‐MS protein chip array technology Participants: women who underwent laparoscopy for suspected endometriosis (cases) and for tubal ligation or hysterectomy for benign reasons (controls) Selection criteria: inclusion criteria: normal menstrual cycles, no hormonal treatment for at least 6/12 months before operation Study design: observational two‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: endometriosis group: pelvic pain ‐ 2/13, infertility ‐ 4/13, dysmenorrhoea ‐ 3/13, ovarian cyst ‐ 4/13; controls: women requesting tubal ligation, symptoms not specified Age: median age 34 years (range 20‐45), endometriosis; 36 years (range 21‐48), controls Number enrolled: 26 women Number available for analysis: 26 women Setting: university hospital ‐ the 2nd Affiliated Hospital, Zhejiang University School of Medicine Place of study: Hangzhou, China Period of study: not specified Language: English |
||
| Index tests | Index test: endometrial proteome by SELDI‐TOF‐MS array (5 protein peaks of 5385 m/z, 5425 m/z, 6898 m/z, 5891 m/z, 6448 m/z) Description of positive case definition by index test as reported: protein peaks significantly different between the groups with higher ROC (detection on the Protein Biological System II (PBSII) and mass spectrometer reader (Ciphergen Biosystems); analysed by using ProteinChip Software 3.1 (Ciphergen): protein mass‐dependent velocities (m/z) peaks were analysed using an artificial neural network); technique described; threshold not pre‐specified Examiners: no information provided; unclear if blinded to the reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 13/26 (50%): stage I‐II 8, stage III‐IV 5; controls 13 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: visual inspection (careful assessment of the pelvic organs) confirmed by histology; staged according to rAFS classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: tissue samples were collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: 5 potential biomarkers were found, and the diagnostic system separated the endometriosis from the healthy samples with a sensitivity of 91.7%, a specificity of 90.0%. This method showed great potential in screening better biomarkers for endometriosis. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Wolfler 2005.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate whether endometrial biopsy prior to laparoscopy in symptomatic women to screen for the presence of aromatase by RT‐PCR and IHC combined with select patient characteristics is of value to predict endometriosis Participants: women attending for diagnosis of unexplained infertility, dysmenorrhoea, dyspareunia or chronic pelvic pain scheduled for laparoscopy Selection criteria: exclusion criteria: oestrogen dependent disease other than endometriosis, no endocrine therapy prior to inclusion (GnRH analogues, danazol or oral contraceptives) Study design: observational single‐gate, prospective enrolment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: unexplained infertility ‐ 32/48, dysmenorrhoea ‐ 13/48, dyspareunia ‐ 1/48, chronic pelvic pain ‐ 12/48 Age: mean age 32.9 years, range 21‐48 years Number enrolled: 64 women Number available for analysis: 48 women (cycle phase not reported) Setting: tertiary centre ‐ Division of Gynaecological Endocrinology and Reproductive Medicine, Medical University of Vienna Place of study: Vienna, Austria Period of study: not stated Language: English |
||
| Index tests | Index test: aromatase mRNA and protein Description of positive case definition by index test as reported: mRNA expression levels by RT‐PCR (Taqman methodology, normalised to internal reference GAPDH gene according to the 2‐ΔΔCt method); protein level ‐ positive staining on IHC (detection by ChemMate Antibody Detection Kit (Dako); microscopical examination of 6 section per slide); laboratory techniques described; threshold pre‐defined for IHC Examiners: IHC ‐ 3 independent trained physicians who were unaware of the patients' history Interobserver variability: not stated |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of reference condition in the sample: n/N = 25/48 (52%): stages I‐IV, numbers not specified; controls 23 Reference standard: laparoscopy + histology Description of positive cased definition by index test as reported: laparoscopic visualisation followed by histopathological assessment; rAFS classification; visual diagnosis that could not be confirmed by histopathology was considered as negative Examiners: not stated |
||
| Flow and timing | TIme interval between reference standard and index test: tissue samples were collected prior to laparoscopy Withdrawals: 16 patients were excluded from the analysis: 7 patients ‐ poor quality samples, 2 patients ‐ insufficient RNA extraction, 2 patients ‐ insufficient sample for duplicates, 5 patients ‐ were using COCs |
||
| Comparative | |||
| Notes | Conclusion: Screening for eutopic endometrial aromatase in combination with clinical data could be of discriminative value in the prediction of disease. Comments: The reported diagnostic estimates of predictive model based on endometrial sample and clinical data are beyond the scope of this review. For aromatase mRNA there was statistically significant difference between the groups, but no data available to construct 2 × 2 tables; not presented in this review. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Yadav 2013.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate the diagnostic accuracy of detection of nerve fibres in eutopic endometrium and biopsy from peritoneal implants and endometriomas in cases of endometriosis Participants: patients who underwent laparoscopy for infertility/pelvic pain/suspected endometriosis Selection criteria: exclusion criteria: hormonal therapy in the preceding 3/12 months, acute PID, suspected pregnancy, suspected or diagnosed genital malignancy, undiagnosed vaginal bleeding, documented genital tuberculosis, contraindication for laparoscopy or unwillingness to undergo surgery Study design: observational study, single gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility ‐ 32/60, CPP ‐ 19/60, infertility + pain symptoms (dysmenorrhoea, dyspareunia, dyschezia) ‐ 9/60; regular menstrual cycle ‐ 57/60 Age: range 15‐45 years Number enrolled: 60 women Number available for analysis: 60 women (cycle phase not specified) Setting: university hospital ‐ O&G Department, University College of Medical Sciences and Guru Teg Bahadur Hospital Place of study: Delhi, India Period of study: November 2009 to April 2012 Language: English |
||
| Index tests | Index test: endometrial nerve fibres Description of positive case definition by index test as reported: positive IHC staining for PGP 9.5 identified as single cell positive or linear nerve fibres; technique described Examiners: senior pathologist blinded to patients' data Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of reference condition in the sample: n = 30/60 (50%): stages not reported; controls 30 Reference standard: laparoscopy + histology Description of positive cased definition by index test as reported: laparoscopic visualisation with histological confirmation of suspected lesions; staging according to rAFS classification Examiners: not stated |
||
| Flow and timing | TIme interval between reference standard and index test: endometrial biopsy was collected at surgery Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: Eutopic endometrium exhibited positivity for nerve fibres in 24 out of 30 cases of proven endometriosis, though the density of nerve fibres was low as compared to that reported in a few studies. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Zeng 2005.
| Study characteristics | |||
| Patient sampling | Primary objective: to evaluate the diagnostic value of evaluating endometrial biopsy specimens for aromatase CYP450 and CA‐125 for endometriosis Participants: women who underwent laparoscopy or laparotomy for pelvic pain, infertility or both Selection criteria: inclusion criteria: reproductive age regular menstrual cycle; exclusion criteria: hormonal treatment for 3/12 months prior reproductive age, preoperative diagnosis of uterine fibroids, adenomyosis Study design: observational study, single‐gate, prospective sample collection |
||
| Patient characteristics and setting | Clinical presentation: infertility or pelvic pain Age: Mean age 33 ± 4 years (range 26‐40), endometriosis; 32 ± 4 years (range 25‐39), controls Number enrolled: 58 women Number available for analysis: 58 women (31 women in proliferative and 27 women in secretory phase of the cycle) Setting: Department of Obstetrics and Gynecology, Third Xiangya Hospital, Central South University Place of study: Changsha, China Period of study: March 2003 to February 2004 Language: Chinese |
||
| Index tests | Index test: aromatase protein Description of positive case definition by index test as reported: positive IHC staining indicated by presence of brown particles within the cytoplasm; laboratory technique not described Examiners: not stated; unclear if blinded to the result of reference standard Interobserver variability: not stated |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in sample: n/N = 36/58 (62%): stage I‐II 20, stage III‐IV 16; controls 22 Reference standard: laparoscopy/laparotomy Description of positive case definition by reference test as reported: visual inspection; rAFS classification Examiners: not stated |
||
| Flow and timing | TIme interval between reference standard and index test: endometrial biopsy was collected intraoperatively Withdrawals: none reported |
||
| Comparative | |||
| Notes | Conclusion: The combination assay of aromatase cytochrome P450 in eutopic endometrium and CA‐125 can be used as a diagnostic test for endometriosis, especially for the early stage of endometriosis, which is superior to the assay of CA‐125. Comment: The reported diagnostic estimates for combined test, including blood and endometrial sample is beyond the scope of this review. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Was a menstrual cycle phase considered in interpreting the index test | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Zubor 2009.
| Study characteristics | |||
| Patient sampling | Primary objective: to investigate the expression of pro‐apoptotic and anti‐apoptotic genes in eutopic endometrium from women with endometriosis and healthy controls in relation to disease occurrence and severity Participants: women undergoing laparoscopy for pelvic pain Selection criteria: inclusion criteria: reproductive age, regular menstrual cycle (26‐34 days), proliferative phase of the menstrual cycle, no history of hormonal treatment, chronic pelvic pain lasting at least 6/12 months, written informed consent; exclusion criteria: previous abdominal surgery, pregnancy; history of in vitro fertilisation treatment Study design: observational single‐gate, prospective recruitment and sample collection |
||
| Patient characteristics and setting | Clinical presentation: pelvic pain Age: mean age 41.5 ± 5.6 years (range 28–50) Number enrolled: 51 women Number available for analysis: 45 women (all at proliferative cycle phase) Setting: university hospital ‐ Clinic of O&G, Jessenius Faculty of Medicine, Comenius University Place of study: Martin, Slovakia Period of study: not reported Language: English |
||
| Index tests | Index test: p53 mRNA, Bcl‐Xs mRNA, Bax mRNA, Bcl‐XL mRNA, Bcl‐XL:BclXS ratio Description of positive case definition by index test as reported: mRNA expression by RT‐PCR (Detection by using (RevertAidTM H Minus First Strand cDNA Synthesis Kit, Fermentas–EU); normalised to b‐actin mRNA; the products tested with gel electrophoresis, expression intensity analysed by Gene Tools software (Syngene, UK); technique described; thresholds not provided Examiners: none stated; unclear if blinded to the reference standard Interobserver variability: not reported |
||
| Target condition and reference standard(s) | Target condition: endometriosis Prevalence of target condition in the sample: n/N = 15/45 (33%): stages I‐II 9, stages III‐IV 6 ; controls 30 Reference standard: laparoscopy + histology Description of positive case definition by reference test as reported: endometriosis was defined as the presence of glandular epithelium and endometroid stroma in lesions outside of the uterus and disease was staged according to the rAFS classification Examiners: none stated |
||
| Flow and timing | Time interval between index test and reference standard: tissue biopsies obtained at surgery Withdrawals: 6 women did not fulfil inclusion criteria (excluded before the experiments) |
||
| Comparative | |||
| Notes | Conclusion: Results suggest that an increased transcription of pro‐apoptotic genes (p53 and Bcl‐xS) in eutopic endometrium is significantly associated with endometriosis, which indicates dysregulation of apoptotic gene transcription associated with disease. Comments: For p53, Bcl‐XS there was statistically significant difference between the groups, but there were insufficient data to construct 2 × 2 tables; not included in this review For Bax, Bcl‐XL, Bcl‐XL:BclXS ratio there was no statistically significant difference between the groups ‐ no data available for meta‐analysis (Appendix 7) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a 'two‐gate' design avoided? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Was a menstrual cycle phase considered in interpreting the index test | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
(r)AFS: (revised) American Fertility Society; (r)ASRM: (revised) American Society for Reproductive Medicine; AUB: abnormal uterine bleeding; CA‐125: cancer antigen; COC: combined oral contraceptive; CPP: chronic pelvic pain; CV: coefficient of variation; DIE: deep infiltrating endometriosis; DUB: dysfunctional uterine bleeding; EGF: epidermal growth factor; ER: oestrogen receptor; ESHRE: European Society of Human Reproduction and Embryology; FISH: fluorescence in situ hybridisation; GnRH: gonadotropin‐releasing hormone; HPF: high‐power fields; HS: hybridisation score; IC: intracellular;IHC: immunohistochemistry/immunohistochemical; IL: interleukin; IRS: immunoreactive score; IUD: intrauterine device; LS‐SVM: least squares support vector machine; MMP: matrix metalloproteinase; mRNA: messenger RNA; N: total sample size; n: number of events; NA: not applicable; NSAIDS: nonsteroidal anti‐inflammatory drugs; PCR: polymerase chain reaction; PID: pelvic inflammatory disease; qRT‐PCR: quantitative reverse transcription PCR; RIA: radioimmunoassay; SD: standard deviation; SVM: support vector machine; TNF: tumour necrosis factor; TSR: telomerase substrate oligonucleotide.
For a full list of endometrial biomarkers and their biological annotation, please see Appendix 1.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abae 1992 | Target condition outside inclusion criteria (only healthy women included) |
| Absenger 2004 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Abu Musa 1992 | Population likely overlapped with Takahashi 1990, unable to clarify with the authors |
| Acien 2007 | Index test outside inclusion criteria (comparisons between endometrium in control group and endometriotic lesions) |
| Adamyan 1993 | Insufficient description of the study methods and population (unclear patient selection); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Aghajanova 2009a | Insufficient description of study population (unclear if all controls underwent abdominal surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm the reported negative result) |
| Aghajanova 2009b | Insufficient description of study population (unclear if all controls underwent abdominal surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm the reported negative result) |
| Aghajanova 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Agic 2007 | Insufficient description of the study methods and population |
| Akoum 1999 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Akoum 2001 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Akoum 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Akoum 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table); population likely overlapped with Lawson 2008 |
| Alizadeh 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ametzazurra 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Andersson 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Anger 2007 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Antsiferova 2005 | Reference standard outside inclusion criteria (no laparoscopy in control group); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Badawy 1984 | Insufficient description of the study methods and population (unclear timing of sample collection); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Baka 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Balasch 1985 | Reference standard outside inclusion criteria (controls did not have pelvic surgery) |
| Ballester 2012 | Insufficient description of the study methods and population |
| Barrier 2006 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Bartosik 1987 | Insufficient description of study population (unclear if all the participants were of reproductive age, unable to clarify with the authors) |
| Bellehumeur 2005 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Bellelis 2013 | Index test outside inclusion criteria (comparisons between endometrium in control group and endometriotic lesions) |
| Berbic 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Berbic 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Bergqvist 2001 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Bohler 2007 | Reference standard outside inclusion criteria (no laparoscopy in control group); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Braun 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Braun 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to verify negative result) |
| Browne 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Budrys 2012 | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Bukulmez 2008 | Insufficient description of population recruitment and characteristics; insufficient diagnostic accuracy information (unable to construct 2 × 2 table or confirm negative findings) |
| Bulmer 1998 | Study design outside exclusion criteria (archived samples, poorly defined population); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Burlev 2005 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Burlev 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Burney 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Calcagno 2011 | Index test outside inclusion criteria (evaluation of ectopic endometrium in endometriosis vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Carneiro 2007 | Insufficient diagnostic accuracy information [unable to construct 2x2 table or to confirm negative result] |
| Carneiro 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative result) |
| Carvalho 2013 | Index test outside inclusion criteria (evaluation of peritoneal fluid and ectopic endometrium; no comparative data on eutopic endometrium between the groups) |
| Chand 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chang 2013 | Insufficient description of study population (unclear if all controls underwent abdominal surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chegini 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chehna‐Patel 2010 | Insufficient description of study methods and population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chen 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chen 2012a | Insufficient description of the study methods and study population |
| Chen 2012b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chen 2012c | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Cho 2009 | Reference standard outside inclusion criteria (no surgery in 15% of participants); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Cho 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Chung 2001 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table; unable to confirm the reported negative result) |
| Chung 2002a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table; unable to confirm the reported negative result) |
| Chung 2002b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Colette 2004 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Colette 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table; unable to confirm the reported negative result) |
| Cosin 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Cunha‐Filho 2001 | Index test outside inclusion criteria (luteal insufficiency measured as combined blood‐endometrium test) |
| D'Amico 2013 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Daftary 2004 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Dai 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| De Graaff 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Debrock 2004 | Review article |
| Delbandi 2013 | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Delvoux 2009 | Index test outside inclusion criteria (focus on biological events in endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Dharmaraj 2014 | Insufficient description of study population (unclear if all controls had pelvic surgery) |
| Di Carlo 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Dimitriadis 2006 | Insufficient description of study methods and population (unclear timing of sample collection) |
| Dmowski 2001 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Donnez 1998 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Donnez 2013 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Ejskjaer 2009 | Reference standard outside inclusion criteria (no laparoscopy in control group); insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm the reported negative result) |
| Fang 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Fasciani 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Fazleabas 1987 | Insufficient description of study methods and population |
| Fedele 1988 | Insufficient description of study methods and population |
| Fernandez‐Shaw 1995a | Insufficient description of study methods and population (unclear age of the participants) |
| Fernandez‐Shaw 1995b | Index test outside inclusion criteria (focus on biological events in endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Ferriani 1993 | Index test outside inclusion criteria (focus on biological events in endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Finas 2008 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Fowler 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Fujino 2006 | Insufficient description of the study methods and population; the study presents negative findings |
| Fukaya 1999 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Gaetje 2006 | Insufficient description of study methods and population (unclear if all controls had abdominal surgery and timing of sample collection) |
| Gaetje 2007a | Reference standard outside inclusion criteria (not all controls had abdominal surgery); index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Gaetje 2007b | Insufficient description of study methods and population (unclear if all controls had abdominal surgery) |
| Gagné 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Gebel 1998 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Giannelli 2007 | Index test outside inclusion criteria (focus on biological events in endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Gonzalez Ramos 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Gori 2013 | Index test outside inclusion criteria (focus on biological events in endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Guay 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Guo 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hapangama 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hapangama 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hapangama 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hassa 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm the reported negative result) |
| Hawkins 2011 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; functional study of endometrium) |
| Hey‐Cunningham 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hii 1998 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative findings) |
| Hsu 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Huang 2012 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Huang 2013 | Index test outside inclusion criteria (functional study of endometrium) |
| Hudelist 2005a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative findings) |
| Hudelist 2005b | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Hudelist 2008 | Index test outside inclusion criteria (functional study of endometrium) |
| Hur 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hurst 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Hwang 2013 | Insufficient information on study population and sample collection; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Igarashi 2005 | Reference standard outside inclusion criteria (controls did not have pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Isaacson 1990 | Insufficient description of study methods and population |
| Jana 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Johnson 2005 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Jolicoeur 1998 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Jones 1995 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Jones 1996 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Jones 1998 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Jones 2009 | Reference standard outside inclusion criteria (controls did not have pelvic surgery) |
| Juhasz‐Boss 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm the reported negative result) |
| Jurgensen 1996 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative result) |
| Kao 2003 | Insufficient description of study methods (unclear timing of sample collection); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Karalok 2014 | Reference standard outside inclusion criteria (controls did not have pelvic surgery) |
| Khan 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Khan 2005a | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Khan 2005b | Index test outside inclusion criteria (evaluation of peritoneal macrophages, not endometrial biomarker) |
| Khan 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Khan 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Khan 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kharfi 2001 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kharfi 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kharfi 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Khorram 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kim 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kim 2013a | Index test outside inclusion criteria (functional study of endometrium, focus on endometrial activity in response to TNF‐α treatment) |
| Kim 2013b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kim 2013c | Index test outside inclusion criteria (functional study of endometrium, focus on endometrial activity following manipulation of Pak4) |
| Kitawaki 1999a | Target condition outside inclusion criteria (diseased vs disease‐free tissue, no separate diagnostic data for endometriosis) |
| Kitawaki 2000a | Insufficient information on study population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table or confirm negative findings) |
| Kitawaki 2000b | Target condition outside inclusion criteria (diseased vs disease‐free tissue, no separate diagnostic data for endometriosis) |
| Klemmt 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Klemmt 2007 | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Kobayashi 2012 | Study design outside exclusion criteria (archived samples, poorly defined population); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kocbek 2014a | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Kocbek 2014b | Index test outside inclusion criteria (comparisons between endometrium in control group and endometriotic lesions) |
| Koshiba 2005 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative result) |
| Kreiner 1986 | Insufficient information on study population (unclear if all the participants were of reproductive age), unable to contact the authors |
| Kyama 2006a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative findings) |
| Kyama 2006b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Kyama 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative findings) |
| Kyama 2011 | Insufficient description of the population recruitment and demographics (unclear if all the controls had pelvic surgery; unclear timing of sample collection in relation to surgery) |
| Laudanski 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Laudanski 2013a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Laudanski 2013b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lawson 2007 | Index test outside inclusion criteria (only ectopic endometrium assessed) |
| Lebovic 2002 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Lee 2011 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Lee 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Leiva 1994 | Insufficient description of study methods and/or population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lessey 1989 | Insufficient description of study methods and/or population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lessey 1993 | Insufficient description of study population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lessey 1994 | Insufficient description of study population (unclear if all controls had pelvic surgery) |
| Li 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Li 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Li 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Li 2012a | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Li 2012b | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lima‐Couy 2004 | Insufficient description of study methods and population (unclear if all controls had pelvic surgery, if prospective sample collection and time interval sampling to surgery) |
| Lin 2005 | Reference standard outside inclusion criteria (controls did not have pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lin 2010 | Population outside inclusion criteria (women with genital tract malignancy were included); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lin 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Liu 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Liu 2005a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Lo Vasco 2012 | Case report (1 participant in each group) |
| Locci 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Luk 2005 | Index test outside inclusion criteria (functional study of endometrium, focus on endometrial activity regulated by sex steroids) |
| Luk 2010 | Index test outside inclusion criteria (functional study of endometrium) |
| Luo 2006a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Luo 2006b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Maia 2012 | Insufficient description of study methods and population |
| Makri 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Malik 2006 | Time interval outside inclusion criteria (exceeded 12 months) |
| Mathur 1990 | Insufficient description of study population and sample collection methods; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2004a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2004b | Population outside inclusion criteria (only women with endometriosis were included, comparison eutopic vs ectopic endometrium) |
| Matsuzaki 2005a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or confirm negative result) |
| Matsuzaki 2005b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2006b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2010a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2010b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Matsuzaki 2012 | Index test outside inclusion criteria (functional study of endometrium;focus on localization of ABCG2þ cells and expression across menstrual cycle) |
| Matsuzaki 2013 | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| McBean 1993 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Mei 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Mei 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Meola 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Meola 2013a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Meresman 2000 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Meresman 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Mettler 1996 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table confirm negative findings) |
| Mettler 1997 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or confirm negative findings) |
| Mihalich 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Mikolajczyk 2007 | Population overlapped with Mikolajczyk 2006 |
| Mikolajczyk 2009 | Insufficient description of study methods and/or population (unclear if controls had surgery and if prospective sample collection) |
| Minina 1989 | Insufficient description of study methods and population (unclear if all controls had abdominal surgery and timing of sample collection) |
| Morsch 2009 | Insufficient description of study methods and population |
| Mu 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Newman 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or confirm negative findings) |
| Nikoo 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Noble 1996 | Insufficient description of study methods and population |
| Nomiyama 1997 | Insufficient description of study methods and population (unclear how the groups were selected from the entire cohort); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Novella‐Maestre 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ordi 2003 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative findings) |
| Ota 1996 | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ota 1997a | Insufficient information on study population (unclear if all controls had pelvic surgery; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ota 1997b | Insufficient information on study population (unclear if all controls had pelvic surgery; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ota 2000 | Insufficient information on study population (unclear if all controls had pelvic surgery; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ota 2001a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ota 2001b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ota 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Pabona 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Pan 2007 | Publication was retracted |
| Pan 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Pan 2009 | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Park 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Pellegrini 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Penna 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Petracco 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Pillai 1996 | Population outside inclusion criteria (women post Caesarean Section were included); insufficient description of study population (unclear if all the participants were of reproductive age) |
| Plante 2012 | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ponce 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Prentice 1992 | Insufficient description of study methods and/or population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Rai 1996 | Insufficient description of study methods and/or population |
| Rai 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ramon 2005 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ramon 2008 | Population outside inclusion criteria (only women positive for certain genetic polymorphisms were tested) |
| Rey 1998 | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Rocha 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Rocha 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Rombauts 2006 | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ruan 2010 | Insufficient description of study population (unclear if all controls had pelvic surgery) |
| Ruan 2013 | Insufficient description of the study population (unclear if all controls had abdominal surgery, unclear timing of sample collection); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Saracoglu 1985 | Insufficient description of study methods and population (unclear timing of sample collection) |
| Sbracia 1997 | Insufficient description of study population (unclear if all controls had pelvic surgery) |
| Schor 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Schulke 2009 | Index test outside inclusion criteria (endometrial samples from full‐thickness uterine block, not curette); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Schutt 2015 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to confirm negative results) |
| Seo 2010a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Seo 2010b | Insufficient description of study population (unclear if all controls had pelvic surgery) |
| Sha 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Sharpe Timms 2000 | Insufficient description of study population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Sharpe‐Timms 1994 | Insufficient description of study population (unclear age group and clinical/demographic characteristics) |
| Shen 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Shen 2013 | Insufficient description of study population (unclear if all controls had pelvic surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Sherwin 2008 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Shi 2014a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Shi 2014b | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Silveira 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Stephens 2010 | Insufficient description of study population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Sun 2002 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Szczepanska 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table); population likely overlapped with Szczepanska 2010a and Szczepanska 2010b |
| Szczepanska 2010a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Szczepanska 2010b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Szymanowski 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Szymanowski 2008 | Unable to locate a full text |
| Takahashi 1988a | Population likely overlapped with Takahashi 1990, unable to clarify with the authors |
| Takahashi 1988b | Population likely overlapped with Takahashi 1990, unable to clarify with the authors |
| Takahashi 1989 | Reference standard outside inclusion criteria (no pelvic surgery in controls); insufficient description of study population) |
| Takahashi 1991 | Population likely overlapped with Takahashi 1990, unable to clarify with the authors |
| Takehara 2004 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Tan 2001 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Tan 2002 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ten Have 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Tokushige 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table, descriptive data for separate endometrial layers); ˜30% samples from full‐thickness uterine block, not curette |
| Tokushige 2007 | Index test outside inclusion criteria (test of full‐thickness uterine block of endometrium and contiguous myometrium from hysterectomy samples); insufficient diagnostic accuracy information (data for separate layers of endometrium functional and basal); population overlapped with Tokushige 2006 |
| Torres 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table; unable to confirm the reported negative result) |
| Trio 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Tshishi 2010 | Insufficient description of study population; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ulukus 2005 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Ulukus 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Uz 2011 | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Uzan 2004 | Insufficient description of population sampling and characteristics |
| Uzan 2005 | Insufficient description of population sampling and characteristics |
| Velasco 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table; negative assay in both control and study groups) |
| Vergetaki 2013 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Vernet‐Tomas 2006b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Vestergaard 2011 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table or to verify the reported negative result) |
| Wang 2005a | Insufficient description of the study population (unclear if all the participants were of reproductive age, unclear timing of sample collection) |
| Wang 2005b | Insufficient description of the study population (unclear if all controls had abdominal surgery, unclear timing of sample collection) |
| Wang 2010b | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Wang 2012 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Wei 2009 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Wei 2013 | Insufficient description of the study population (unclear if all controls had abdominal surgery); differing reference standards between cases and controls; insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Wenzl 1998 | Study design outside exclusion criteria (archived samples, poorly defined population) |
| Wolun‐Cholewa 2011 | Index test outside inclusion criteria (focus on technical aspects of the test) |
| Wu 1999 | Insufficient description of the study population (unclear if all controls had abdominal surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Wu 2003 | Index test outside inclusion criteria (evaluation of ectopic endometrium vs eutopic endometrium in controls; no data on eutopic endometrium in women with endometriosis) |
| Wu 2007 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Xu 2010 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Yi 2003 | Index test outside inclusion criteria (functional study of endometrium) |
| Yin 2006 | Insufficient description of the study population (unclear if all controls had abdominal surgery); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) for the positive tests |
| Yoo 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Zeng 2012 | Population outside inclusion criteria (postmenopausal women and women with genital tract malignancy were included); insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Zhang 2005 | Insufficient description of study design and population characteristics; insufficient diagnostic accuracy information (unable to construct 2 × 2 tables) |
| Zhang 2006 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Zhang 2009a | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Zhang 2009b | Population outside inclusion criteria (control group included only women with adenomyosis and leiomyoma) |
| Zhang 2010 | Population outside inclusion criteria (only women with endometriosis were included, comparison eutopic vs ectopic endometrium) |
| Zhang 2011a | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Zhang 2011b | Index test outside inclusion criteria (functional study of endometrium; no direct measurement or comparison of biomarker expression between the groups) |
| Zhao 2014 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
| Zong 2004 | Insufficient diagnostic accuracy information (unable to construct 2 × 2 table) |
Differences between protocol and review
General scope: this review is a part of the review series arising from the same generic protocol. We adjusted the following sections to the main topic of the review as follows.
Background. We modified the section on the index test and removed all the irrelevant information on imaging. We updated the Rationale section to include a clearer definition of triage diagnostic tests.
-
Objectives.
We updated the list of the sources of heterogeneity.
During the revision of the literature on the subject, we identified a substantial body of studies looking at the biomarkers that were not altered in the presence of endometriosis (we found no statistically significant difference between women with and without the disease). We believe that presenting this type of data, when obtained from adequately designed studies, is important for both clinicians and researchers. We explained our reasoning in the Background section under Rationale, in the Methods section under Criteria for considering studies for this review, Index tests and added a secondary objective to Objectives: "to assess the biomarkers that were not affected by endometriosis and hence are unlikely to discriminate between patients with and without the disease".
-
Methods. We updated the criteria for considering studies for this review as follows.
Types of studies: We removed the 'cohort' and 'case control' classification and introduced the concept of single‐gate design and two‐gate' design, defined as the presence of a single or multiple set of inclusion criteria with regard to the clinical condition or the reference standard. We found this classification more informative to describe diagnostic studies, all of which are cross‐sectional in nature. We limited the inclusion criteria to the studies with a single set of inclusion criteria by reference standard (i.e. all women who underwent abdominal surgery), but included single or multiple sets of inclusion criteria by clinical presentation (i.e. women with suspected endometriosis or other indications for abdominal surgery), referring to these as single‐gate and two‐gate designs, respectively.
Likewise, we removed the terminology 'prospective studies' and introduced 'studies performed on prospectively collected samples'. This decision was guided by the fact that most diagnostic studies are retrospective in nature, as they aim to compare the results of the index test with the results of the reference standard in the same group of participants, where the groups are classified by the outcome of the reference standard. Also, the analysis of the index test could have been performed retrospectively in a single batch on stored samples after the prospective collection of samples. The timing of sample collection (before or after surgical treatment of the disease) from preoperatively recruited population has more impact on the test result than the timing of the laboratory assay. Therefore, we included only studies that collected endometrial tissue before the reference surgical procedure, i.e. 'prospectively collected', irrespective of the actual timing of test performance. In this way, we avoided the confusion of labelling studies as 'prospective' or 'retrospective'. This allowed us to include the studies from well‐established high quality tissue banks using well‐characterised archived samples, as omission of these studies could result in the loss of potentially valuable data. We discuss this choice in the Methods under Criteria for considering studies for this review.
We modified index tests to include only eutopic endometrial markers, updating the table listing the tests of interest (Table 4) accordingly.
Target conditions now also include deep pelvic endometriosis in view of the growing body of literature on this condition as a separate entity and its diagnostic importance to optimise the surgical approach.
Spectrum of disease: Following an ad hoc observation, we included the studies that involved only selected populations of women with endometriosis (i.e. specific rASRM stages) in view of the emerging evidence on poor correlation of this classification with infertility and pain symptoms. Exclusion of such studies could result in the loss of potentially important diagnostic information from otherwise eligible publications. Where possible we aimed to address the impact of the inclusion of these studies in investigations of heterogeneity.
-
Search methods for identification of studies.
In the protocol, we stated that we would identify grey literature (unpublished studies including conference proceedings and reports) and also define specific search strategies. In practice, the paucity of relevant data that was available from abstracts made it impossible to apply the selection criteria and methodological quality judgement to these studies. We anticipated that identification of this type of study and attempts to obtain the necessary information directly from the study investigators would increase the already labour‐intensive work involved in preparation of this review. Therefore, by consensus among the key authors, we removed the unpublished studies we had already identified and did not complete an intended search for unpublished material.
We updated the search strings for all biomarkers excluding imaging (searched separately), applying the same principles as presented in the protocol.
Assessment of methodological quality: We tailored the QUADAS‐2 tool for the topic of the review, outlining the differences between the original QUADAS‐2 tool and the tool designed for this review in the relevant section (see Assessment of methodological quality).
-
Analysis.
We amended the section on statistical methods and tailored it to the types of tests included in the review.
We performed no sensitivity analyses and no assessment of heterogeneity due to insufficient data.
When we judged a test performance against the predetermined diagnostic criteria, we only considered the point estimates of sensitivity and specificity, as we believe that presenting these metrics of test performance is the most helpful and informative way to summarise the diagnostic data. We acknowledge that the choice of the most helpful summary is subjective. There are tests where the point estimates did not reach the predetermined criteria, but the confidence intervals (CIs) contain the values above the thresholds for replacement tests, triage tests or both. These tests could have diagnostic value if the point values underestimated their diagnostic potential. For the tests where the point estimates reached the criteria for a replacement or triage tests but the CIs contained values below the thresholds, point values could have overestimated the diagnostic performance of the test. If we use the range of the CIs rather than the point estimates of the data, the predetermined cut‐off becomes meaningless. We did not consider CIs in qualifying the test performance; however, we utilised the CIs in interpreting the reliability of the obtained data.
The authors' list and order changed to accurately reflect their contribution to the review.
Contributions of authors
Vicki Nisenblat and Louise Hull coordinated the production of the protocol, performed literature search and coordinated production of the review series. Devashana Gupta and Vicki Nisenblat undertook the review and took the primary role in writing the review. Laura Miller participated in data collection and quality appraisal of the included studies. Patrick Bossuyt provided advice on statistical methods for the review and performed the analyses. Ian Fraser and Neil Johnson contributed to the design of the review and critically appraised the discussion. All the authors contributed to the revision and drafting of the review.
Sources of support
Internal sources
-
Cochrane Menstrual Disorders and Subfertility Group, University of Auckland, New Zealand.
Technical support
-
The Robinson Institute, University of Adelaide, Australia.
Access to academic resources
External sources
No sources of support supplied
Declarations of interest
Vicki Nisenblat: none known.
Louise Hull: Dr M. L. Hull obtained a grant of $10,000 to carry out a prevalence study of ultrasonographically diagnosed endometriosis in a fertility population.
Devashana Gupta: none known.
Laura Miller: none known.
Patrick Bossuyt: none known.
Ian Fraser: Ian Fraser is a member of Advisory Boards on Contraception and/or Abnormal Uterine Bleeding for Bayer Schering Pharma, Merck/MSD and Vifor Pharma; he has also received lecture fees, consultancy fees and expenses for contraception and abnormal uterine bleeding from Bayer Schering Pharma, Merck/MSD and Daiichi Sankyo. He has received a small grant for a collaborative research study with the University of Melbourne on an animal model of endometriosis.
Neil Johnson: Professor Neil Johnson is involved in research funded by Abb‐Vie. He has received support to attend conferences from MSD, Merck‐Serono and Bayer.
New
References
References to studies included in this review
Al‐Jefout 2007 {published data only}
- Al‐Jefout M, Andreadis N, Tokushige N, Markham R, Fraser I. A pilot study to evaluate the relative efficacy of endometrial biopsy and full curettage in making a diagnosis of endometriosis by the detection of endometrial nerve fibers. American Journal of Obstetrics and Gynecology 2007;197(6):578 e1‐4. [DOI] [PubMed] [Google Scholar]
Al‐Jefout 2009 {published data only}
- Al‐Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, Markham R, et al. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Human Reproduction 2009;24(12):3019‐24. [DOI] [PubMed] [Google Scholar]
Bokor 2009 {published data only}
- Bokor A, Kyama CM, Vercruysse L, Fassbender A, Gevaert O, Vodolazkaia A, et al. Density of small diameter sensory nerve fibres in endometrium: a semi‐invasive diagnostic test for minimal to mild endometriosis. Human Reproduction 2009;24:3025‐32. [DOI] [PubMed] [Google Scholar]
Bourlev 2006 {published data only}
- Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A, Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor‐A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction 2006;132(3):501‐9. [DOI] [PubMed] [Google Scholar]
Casals 2012 {published data only}
- Casals G, Ordi J, Creus M, Fabregues F, Carmona F, Casamitjana R, Balasch J. Expression pattern of osteopontin and v3 integrin during the implantation window in infertile patients with early stages of endometriosis. Human Reproduction 2012;27(3):805‐13. [DOI] [PubMed] [Google Scholar]
Cetin 2013 {published data only}
- Cetin C, Serdaroglu H, Tuzali S. The importance of endometrial nerve fibres and macrophage cell count in the diagnosis of endometriosis. Iranian Journal of Reproductive Medicine 2013;11(5):405‐14. [PMC free article] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen QH, Qu JY, Xu YY, Qiu NX, Zhuang YZ, Zhong S, et al. Expressions of matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinase‐1 in ectopic and eutopic endometrium. Zhonghua Fuchanke Zazhi [Chinese Journal of Obstetrics and Gynecology] 2004;39(12):809‐12. Chinese. [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen SQ, Li JB, Jiang HY, Yuan L, Niu G, Yao SZ. Expression of human‐defensin‐2 in the eutopic and ectopic endometrial tissues in patients with endometriosis. Archives of Gynecology and Obstetrics 2013;287(6):1151‐7. [DOI] [PubMed] [Google Scholar]
Cho 2012 {published data only}
- Cho S, Choi YS, Jeon Y, EIm KJ, Choi YM, Yim SY, et al. Expression of vascular endothelial growth factor (VEGF) and its soluble receptor‐1 in endometriosis. Microvascular Research 2012;83(2):237‐42. [DOI] [PubMed] [Google Scholar]
Da Silva 2014 {published data only}
- Silva CM, Belo AV, Andrade SP, Campos PP, Ferreira MCF, Silva‐Filho AL, et al. Identification of local angiogenic and inflammatory markers in the menstrual blood of women with endometriosis. Biomedicine and Pharmcotherapy 2014;68(7):899‐904. [DOI] [PubMed] [Google Scholar]
Dheenadayalu 2002 {published data only}
- Dheenadayalu K, Mak I, Gordts S, Campo R, Higham J, Puttemans P, et al. Aromatase P450 messenger RNA expression in eutopic endometrium is not a specific marker for pelvic endometriosis. Fertility and Sterility 2002;78(4):825‐9. [DOI] [PubMed] [Google Scholar]
Ding 2010 {published data only}
- Ding X, Wang L, Ren Y, Zheng W. Detection of mitochondrial biomarkers in eutopic endometria of endometriosis using surface‐enhanced laser desorption/ionization time‐of‐flight mass spectrometry. Fertility and Sterility 2010;94(7):2528‐30. [DOI] [PubMed] [Google Scholar]
Elgafor el Sharkwy 2013 {published data only}
- Elgafor el Sharkwy IA. Combination of non‐invasive and semi‐invasive tests for diagnosis of minimal to mild endometriosis. Archives of Gynecology and Obstetrics 2013;288(4):793‐7. [DOI] [PubMed] [Google Scholar]
Fassbender 2012 {published data only}
- Fassbender A, Verbeeck N, Brnigen D, Kyama CM, Bokor A, Vodolazkaia A, et al. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Human Reproduction 2012;27(7):2020‐9. [DOI] [PubMed] [Google Scholar]
Gilabert‐Estelles 2003 {published data only}
- Gilabert‐Estelles J, Estelles A, Gilabert J, Castello R, Espana F, Falco C, et al. Expression of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis. Human Reproduction 2003;18(7):1516‐22. [DOI] [PubMed] [Google Scholar]
Gilabert‐Estelles 2007 {published data only}
- Gilabert‐Estelles J, Ramon LA, Espana F, Gilabert J, Vila V, Reganon E, Castello R, Chirivella M, Estelles A. Expression of angiogenic factors in endometriosis: relationship to fibrinolytic and metalloproteinase systems. Human Reproduction 2007;22(8):2120‐7. [DOI] [PubMed] [Google Scholar]
Hatok 2011 {published data only}
- Hatok J, Zubor P, Galo S, Kirschnerova R, Dobrota D, Danko J, Racay P. Endometrial aromatase mRNA as a possible screening tool for advanced endometriosis and adenomyosis. Gynecological Endocrinology 2011;27(5):331‐6. [DOI] [PubMed] [Google Scholar]
Hudelist 2007 {published data only}
- Hudelist G, Czerwenka K, Keckstein J, Haas C, Fink‐Retter A, Gschwantler‐Kaulich D, et al. Expression of aromatase and estrogen sulfotransferase in eutopic and ectopic endometrium: evidence for unbalanced estradiol production in endometriosis. Reproductive Sciences 2007;14(8):798‐805. [DOI] [PubMed] [Google Scholar]
Johnson 2004 {published data only}
- Johnson MC, Pinto C, Alves A, Palomino A, Fuentes A, Boric MA, et al. P450Arom and estrogenic microenvironment of eutopic endometria in endometriosis [P450 arom y microambiente estrogénico en endometrios eutópicos de mujeres con endometriosis]. Revista Medica de Chile 2004;132(12):1475‐82. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim CM, Oh YJ, Cho SH, Chung DJ, Hwang JY, Park KH, et al. Increased telomerase activity and human telomerase reverse transcriptase mRNA expression in the endometrium of patients with endometriosis. Human Reproduction 2007;22(3):843‐9. [DOI] [PubMed] [Google Scholar]
Klentzeris 1995 {published data only}
- Klentzeris LD, Bulmer JN, Liu DT, Morrison L. Endometrial leukocyte subpopulations in women with endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1995;63(1):41‐7. [DOI] [PubMed] [Google Scholar]
Laudanski 2014 {published data only}
- Laudanski P, Charkiewicz R, Kuzmicki M, Szamatowicz J, Swiatecka J, Mroczko B, et al. Profiling of selected angiogenesis‐related genes in proliferative eutopic endometrium of women with endometriosis. European Journal of Obstetrics, Gynecology and Reproductive Biology 2014;172(1):85‐92. [DOI] [PubMed] [Google Scholar]
Lawson 2008 {published data only}
- Lawson C, Bourcier N, Al‐Akoum M, Maheux R, Naud F, Akoum A. Abnormal interleukin 1 receptor types I and II gene expression in eutopic and ectopic endometrial tissues of women with endometriosis. Journal of Reproductive Immunology 2008;77(1):75‐84. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee SR, Kim SH, Lee YJ, Hong SH, Chae HD, Kim CH, et al. Expression of epidermal growth factor, fibroblast growth factor‐2, and platelet‐derived growth factor‐A in the eutopic endometrium of women with endometriosis. Jornal of Obstetrics and Gynaecology Research 2007;33(3):242‐7. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee KF, Lee YL, Chan RWS, Cheong AWY, Ng EHY, Ho PC. Up‐regulation of endocrine gland‐derived vascular endothelial growth factor but not vascular endothelial growth factor in human ectopic endometriotic tissue. Fertility and Sterility 2010;93(4):1052‐60. [DOI] [PubMed] [Google Scholar]
Leslie 2013 {published data only}
- Leslie C, Ma T, McElhinney B, Leake R, Stewart CJ. Is the detection of endometrial nerve fibers useful in the diagnosis of endometriosis?. International Journal of Gynecological Pathology 2013;32(2):149‐55. [DOI] [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li Y, Lang JH. Expressions of matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinase‐1 mRNA in endometriosis. Zhonghua Fuchanke Zazhi [Chinese Journal of Obstetrics and Gynecology] 2006;41(1):30‐3. Chinese. [PubMed] [Google Scholar]
Liu 2008 {published data only}
- Liu A, Guan Z, Zhang Z, Wei L, Li Y. Study on expression of estrogen receptor isoforms in eutopic and ectopic endometrium of ovarian endometriosis. Zhonghua Binglixue Zazhi [Chinese Journal of Pathology] 2008;37(9):584‐8. Chinese. [PubMed] [Google Scholar]
Mafra 2014 {published data only}
- Mafra FA, Christofolini DM, Cavalcanti V, Vilarino FL, Andre GM, Kato P, et al. Aberrant telomerase expression in the endometrium of infertile women with deep endometriosis. Archives of Medical Research 2014;45(1):31‐5. [DOI] [PubMed] [Google Scholar]
Makari 2012 {published data only}
- Makari S, Svedas E, Railaite D R, Cizauskas A, Makari J. Endometrial nerve fibers in the diagnosis of endometriosis [Endometriumo nervu skaidulu reiksme diagnozuojant endometrioze]. Lietuvos akuserija ir ginekologija [Lithuanian Obstetrics and Gynaecology] 2012;15(2):125‐30. [Google Scholar]
Matsuzaki 2006a {published data only}
- Matsuzaki S, Canis M, Pouly JL, Dechelotte PJ, Mage G. Analysis of aromatase and 17beta‐hydroxysteroid dehydrogenase type 2 messenger ribonucleic acid expression in deep endometriosis and eutopic endometrium using laser capture microdissection. Fertility and Sterility 2006;85(2):308‐13. [DOI] [PubMed] [Google Scholar]
Meibody 2011 {published data only}
- Aghaey Meibody F, Mehdizadeh Kashi A, Zare Mirzaie A, Ghajarie Bani Amam M, Shariati Behbahani A, Zolali B, et al. Diagnosis of endometrial nerve fibers in women with endometriosis. Archives of Gynecology and Obstetrics 2011;284(5):1157‐62. [DOI] [PubMed] [Google Scholar]
Meola 2009 {published data only}
- Meola J, Dentillo DB, Rosa e Silva JC, Ferriani RA, Veiga LC, Paro de Paz CC, et al. Glycodelin expression in the endometrium of healthy women and in the eutopic and ectopic endometrium of women with endometriosis. Fertility and Sterility 2009;91(5):1676‐80. [DOI] [PubMed] [Google Scholar]
Meola 2013 {published data only}
- Meola J, Hidalgo Gdos S, Silva JC, Silva LE, Paz CC, Ferriani RA. Caldesmon: new insights for diagnosing endometriosis. Bioloy of Reproduction 2013;88(5):122. [DOI] [PubMed] [Google Scholar]
Mikolajczyk 2006 {published data only}
- Mikolajczyk M, Wirstlein P, Skrzypczak J. Leukaemia inhibitory factor and interleukin 11 levels in uterine flushings of infertile patients with endometriosis. Human Reproduction 2006;21(12):3054‐8. [DOI] [PubMed] [Google Scholar]
Morelli 2010 {published data only}
- Morelli SS, Petraglia F, Weiss G, Luisi S, Florio P, Wojtczuk A, et al. Endometrial expression of relaxin and relaxin receptor in endometriosis. Fertility and Sterility 2010;94(7):2885‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pino 2009 {published data only}
- Pino M, Galleguillos C, Torres M, Sovino H, Fuentes A, Boric MA, et al. Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reproduction 2009;138(5):837‐47. [DOI] [PubMed] [Google Scholar]
Prefumo 2002 {published data only}
- Prefumo F, Semino C, Melioli G, Venturini PL. A defective expression of ICAM‐1 (CD54) on secretory endometrial cells is associated with endometriosis. Immunology Letters 2002;80(1):49‐53. [DOI] [PubMed] [Google Scholar]
Puy 2002 {published data only}
- Puy LA, Pang C, Librach CL. Immunohistochemical analysis of alphavbeta5 and alphavbeta6 integrins in the endometrium and endometriosis. International Journal of Gynecological Pathology 2002;21(2):167‐77. [DOI] [PubMed] [Google Scholar]
Rakhila 2013 {published data only}
- Rakhila H, Carli C, Daris M, Lemyre M, Leboeuf M, Akoum A. Identification of multiple and distinct defects in prostaglandin biosynthetic pathways in eutopic and ectopic endometrium of women with endometriosis. Fertility and Sterility 2013;100(6):1650‐9. [DOI] [PubMed] [Google Scholar]
Ramon 2011 {published data only}
- Ramon LA, Braza‐Boils A, Gilabert‐Estelles J, Gilabert J, Espana F, Chirivella M, et al. microRNAs expression in endometriosis and their relation to angiogenic factors. Human Reproduction 2011;26(5):1082‐90. [DOI] [PubMed] [Google Scholar]
Szymanowski 2003 {published data only}
- Szymanowski K, Skrzypczak J, Mikolajczyk M. Integrin pattern in human endometrium‐ new diagnostic tool in pelvic endometriosis?. GInekologia Polska 2003;74(4):257‐61. [PubMed] [Google Scholar]
Takahashi 1990 {published data only}
- Takahashi K, Nagata H, Abu‐Masa A, Shibukawa T, Yamasaki H, Kitao M. Clinical usefulness of Ca125 levels in the menstrual discharge in patients with endometriosis. Fertility and Sterility 1990;54(2):360‐2. [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang L, Wang TT, Wu YT, Zhou CY, Huang HF. High expression levels of cyclin B1 and Polo‐like kinase 1 in ectopic endometrial cells associated with abnormal cell cycle regulation of endometriosis. Fertility and Sterility 2009;91(4):979‐87. [DOI] [PubMed] [Google Scholar]
Tiberi 2010 {published data only}
- Tiberi F, Tropea A, Apa R, Romani F, Lanzone A, Marana R. Prokineticin 1 mRNA expression in the endometrium of healthy women and in the eutopic endometrium of women with endometriosis. Fertility and Sterility 2010;93(7):2145‐9. [DOI] [PubMed] [Google Scholar]
Van der Linden 1994 {published data only}
- Linden PJ, Goeij AF, Dunselman GA, Linden EP, Ramaekers FC, Evers JL. Expression of integrins and E‐cadherin in cells from menstrual effluent, endometrium, peritoneal fluid, peritoneum, and endometriosis. Fertility and Sterility 1994;61(1):85‐90. [DOI] [PubMed] [Google Scholar]
Van der Linden 1995 {published data only}
- Linden PJ, Dunselman GA, Goeij AF, Linden EP, Evers JL, Ramaekers FC. Epithelial cells in peritoneal fluid‐‐of endometrial origin?. American Journal of Obstetrics and Gynecology 1995;173(2):566‐70. [DOI] [PubMed] [Google Scholar]
Vernet‐Tomas 2006a {published data only}
- Vernet‐Tomas MdM, Perez‐Ares C T, Verdu N, Molinero J L, Fernandez‐Figueras M T, Carreras R. The depolarized expression of the alpha‐6 integrin subunit in the endometria of women with endometriosis. Journal of the Society of Gynecologic Investigation 2006;13:292‐6. [DOI] [PubMed] [Google Scholar]
Visnovsky 2008 {published data only}
- Visnovsky J, Galo S, Zubor P, Hatok J, Racay P, Danko J. Semiquantitative analysis of mRNA aromatase expression in eutopic endometrium as a diagnostic marker of endometriosis and estrogen dependent diseases [Semikvantitativna analyza expresie mRNA aromatazy v eutopickom endometriu ako diagnosticky marker endometriozy a estrogendependentnych ochoreni]. Ceska Gynekologie 2008;73(4):213‐17. [PubMed] [Google Scholar]
Wang 2010a {published data only}
- Wang L, Zheng W, Ding XY, Yu JK, Jiang WZ, Zhang SZ. Identification biomarkers of eutopic endometrium in endometriosis using artificial neural networks and protein fingerprinting. Fertility and Sterility 2010;93(7):2460‐2. [DOI] [PubMed] [Google Scholar]
Wolfler 2005 {published data only}
- Wolfler MM, Nagele F, Kolbus A, Seidl S, Schneider B, Huber JC, et al. A predictive model for endometriosis. Human Reproduction 2005;20(6):1702‐8. [DOI] [PubMed] [Google Scholar]
Yadav 2013 {published data only}
- Yadav G, Radhakrishnan G, Singh N, Radhika AG. Detection of endometrial nerve fibres‐ a novel technique to diagnose endometriosis. Journal of Endometriosis and Pelvic Pain Disorders 2013;5(4):144‐50. [Google Scholar]
Zeng 2005 {published data only}
- Zeng F, Xuem I, Zevallos HBV, Lai D, Arthur J, et al. Diagnostic value of the detection of aromatase cytochrome P450 and Ca 125 for endometriosis. Zhongnan Daxue Xuebao (Yixue Ban) [Journal of Central South University (Medical Sciences)] 2005;30(6):682‐85. Chinese. [PubMed] [Google Scholar]
Zubor 2009 {published data only}
- Zubor P, Hatok J, Galo S, Dokus K, Klobusiakova D, Danko J, et al. Anti‐apoptotic and pro‐apoptotic gene expression evaluated from eutopic endometrium in the proliferative phase of the menstrual cycle among women with endometriosis and healthy controls. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;145(2):172‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abae 1992 {published data only}
- Abae M, Gibson M, Chapitis J, Riddick DH, Brumsted JR. Ca125 in human uterine fluid. Fertility and Sterility 1992;57(3):531‐4. [PubMed] [Google Scholar]
Absenger 2004 {published data only}
- Absenger Y, Hess‐Stumpp H, Kreft B, Kratzschmar J, Haendler B, Schutze N, et al. Cyr61, a deregulated gene in endometriosis. Molecular Human Reproduction 2004;10(6):399‐407. [DOI] [PubMed] [Google Scholar]
Abu Musa 1992 {published data only}
- Abu‐Masa A, Takahashi K, Nagata H, Yamasaki H, Mizoguchi S, Kitao M. Ca125 in menstrual discharge in patients with chronic pelvic pain. International Journal Gynecology and Obstetrics 1992;37(2):111‐4. [DOI] [PubMed] [Google Scholar]
Acien 2007 {published data only}
- Acien P, Velasco I, Gutierrez M, Martinez‐Beltran M. Aromatase expression in endometriotic tissues and its relationship to clinical and analytical findings. Fertility and Sterility 2007;88(1):32‐8. [DOI] [PubMed] [Google Scholar]
Adamyan 1993 {published data only}
- Adamyan LV, Fanchenko ND, Alexeyeva ML, Andreyeva YeN, Novikov YeA, Jahan I. Hormonal and immunologic methods in the diagnosis and treatment of patients with benign ovarian tumors and endometriotic cysts. International Journal of Fertility 1993;38(2):92‐8. [PubMed] [Google Scholar]
Aghajanova 2009a {published data only}
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biology of Reproduction 2009;80(1):105‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aghajanova 2009b {published data only}
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic‐5 is involved in the pathophysiology of endometriosis. Endocrinology 2009;150(8):3863‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aghajanova 2011 {published data only}
- Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, et al. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biology of Reproduction 2011;84(4):801‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agic 2007 {published data only}
- Agic A, Xu H, Altgassen C, Noack F, Wolfler MM, Diedrich K, et al. Relative expression of 1,25‐dihydroxyvitamin D3 receptor, vitamin D 1 alpha‐hydroxylase, vitamin D 24‐hydroxylase, and vitamin D 25‐hydroxylase in endometriosis and gynecologic cancers. Reproductive Sciences 2007;14(15):486‐97. [DOI] [PubMed] [Google Scholar]
Akoum 1999 {published data only}
- Akoum A, Lemay A, Lajeunesse Y, Marois M, Koutsilieris M. Immunohistochemical localization of insulin‐like growth factor‐binding protein‐3 in eutopic and ectopic endometrial tissues. Fertility and Sterility 1999;72(6):1085‐92. [DOI] [PubMed] [Google Scholar]
Akoum 2001 {published data only}
- Akoum A, Jolicoeur C, Kharfi A, Aube M. Decreased expression of the decoy interleukin‐1 receptor type II in human endometriosis. American Journal of Pathology 2001;158(2):481‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akoum 2006 {published data only}
- Akoum A, Metz CN, Al‐Akoum M, Kats R. Macrophage migration inhibitory factor expression in the intrauterine endometrium of women with endometriosis varies with disease stage, infertility status, and pelvic pain. Fertility and Sterility 2006;85(5):1379‐85. [DOI] [PubMed] [Google Scholar]
Akoum 2007 {published data only}
- Akoum A, Lawson C, Herrmann‐Lavoie C, Maheux R. Imbalance in the expression of the activating type I and the inhibitory type II interleukin 1 receptors in endometriosis. Human Reproduction 2007;22(5):1464‐73. [DOI] [PubMed] [Google Scholar]
Alizadeh 2011 {published data only}
- Alizadeh Z, Shokrzadeh N, Saidijam M, Sanoee MF. Semi‐quantitative analysis of HOXA11, leukemia inhibitory factor and basic transcriptional element binding protein 1 mRNA expression in the mid‐secretory endometrium of patients with endometriosis. Iranian Biomedical Journal 2011;15(3):66‐72. [PMC free article] [PubMed] [Google Scholar]
Ametzazurra 2009 {published data only}
- Ametzazurra A, Matorras R, Garcia‐Velasco JA, Prieto B, Simon L, Martinez A, et al. Endometrial fluid is a specific and non‐invasive biological sample for protein biomarker identification in endometriosis. Human Reproduction 2009;24(4):954‐65. [DOI] [PubMed] [Google Scholar]
Andersson 2014 {published data only}
- Andersson KL, Bussani C, Fambrini M, Polverino V, Taddei GL, Gemzell‐Danielsson K, et al. DNA methylation of HOXA10 in eutopic and ectopic endometrium. Human Reproduction 2014;29(9):1906‐11. [DOI] [PubMed] [Google Scholar]
Anger 2007 {published data only}
- Anger DL, Zhang B, Boutross‐Tadross O, Foster WG. Tyrosine receptor kinase B (TrkB) protein expression in the human endometrium. Endocrine 2007;31(2):167‐73. [DOI] [PubMed] [Google Scholar]
Antsiferova 2005 {published data only}
- Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. Changes in the T‐helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertility and Sterility 2005;84(6):1705‐11. [DOI] [PubMed] [Google Scholar]
Badawy 1984 {published data only}
- Badawy SZ, Cuenca V, Stitzel A, Jacobs RD, Tomar RH. Autoimmune phenomena in infertile patients with endometriosis. Obstetrics & Gynecology 1984;63(3):271‐5. [PubMed] [Google Scholar]
Baka 2011 {published data only}
- Baka S, Frangou‐Plemenou M, Panagiotopoulou E, Makrakis E, Kaltsakas G, Hassiakos D, et al. The expression of human leukocyte antigens class I and II in women with endometriosis or adenomyosis. Gynecological Endocrinology 2011;27(6):419‐24. [DOI] [PubMed] [Google Scholar]
Balasch 1985 {published data only}
- Balasch J, Vanrell JA. Mild endometriosis and luteal function. International Journal of Fertility 1985;30(3):4‐6. [PubMed] [Google Scholar]
Ballester 2012 {published data only}
- Ballester M, Gonin J, Rodenas A, Bernaudin JF, Rouzier R, Coutant C, et al. Eutopic endometrium and peritoneal, ovarian and colorectal endometriotic tissues express a different profile of Nectin‐1,‐3,‐4 and nectin‐like molecule 2. Human Reproduction 2012;27(11):3179‐86. [DOI] [PubMed] [Google Scholar]
Barrier 2006 {published data only}
- Barrier BF, Kendall BS, Ryan CE, Sharpe‐Timms KL. HLA‐G is expressed by the glandular epithelium of peritoneal endometriosis but not in eutopic endometrium. Human Reproduction 2006;21(4):864‐9. [DOI] [PubMed] [Google Scholar]
Bartosik 1987 {published data only}
- Bartosik D, Damjanov I, Viscarello RR, Riley JA. Immunoproteins in the endometrium: clinical correlates of the presence of complement fractions C3 and C4. American Journal of Obstetrics & Gynecology 1987;156(1):11‐5. [DOI] [PubMed] [Google Scholar]
Bellehumeur 2005 {published data only}
- Bellehumeur C, Collette T, Maheux R, Mailloux J, Villeneuve M, Akoum A. Increased soluble interleukin‐1 receptor type II proteolysis in the endometrium of women with endometriosis. Human Reproduction 2005;20(5):1177‐84. [DOI] [PubMed] [Google Scholar]
Bellelis 2013 {published data only}
- Bellelis P, Barbeiro DF, Rizzo LV, Baracat EC, Abrao MS, Podgaec S. Transcriptional changes in the expression of chemokines related to natural killer and T‐regulatory cells in patients with deep infiltrative endometriosis. Fertility and Sterility 2013;99(7):1987‐93. [DOI] [PubMed] [Google Scholar]
Berbic 2009 {published data only}
- Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Human Reproduction 2009;24(2):325‐32. [DOI] [PubMed] [Google Scholar]
Berbic 2010 {published data only}
- Berbic M, Hey‐Cunningham AJ, Ng C, Tokushige N, Ganewatta S, Markham R, et al. The role of Foxp3+ regulatory T‐cells in endometriosis: a potential controlling mechanism for a complex, chronic immunological condition. Human Reproduction 2010;25(4):900‐7. [DOI] [PubMed] [Google Scholar]
Bergqvist 2001 {published data only}
- Bergqvist A, Bruse C, Carlberg M, Carlstrom K. Interleukin 1‐B, interleukin 6, and tumour necrosis factor‐a in endometriotic tissue and endometrium. Fertility and Sterility 2001;75(3):489‐95. [DOI] [PubMed] [Google Scholar]
Bohler 2007 {published data only}
- Bohler HC, Gercel‐Taylor C, Lessey BA, Taylor DD. Endometriosis markers: immunologic alterations as diagnostic indicators for endometriosis. Reproductive Sciences 2007;14(6):595‐604. [DOI] [PubMed] [Google Scholar]
Braun 2002 {published data only}
- Braun DP, Ding J, Shen J, Rana N, Fernandez BB, Dmowski WP. Relationship between apoptosis and the number of macrophages in eutopic endometrium from women with and without endometriosis. Fertility and Sterility 2002;78(4):830‐5. [DOI] [PubMed] [Google Scholar]
Braun 2007 {published data only}
- Braun DP, Ding J, Shaheen F, Willey JC, Rana N, Dmowski WP. Quantitative expression of apoptosis‐regulating genes in endometrium from women with and without endometriosis. Fertility and Sterility 2007;87(2):263‐8. [DOI] [PubMed] [Google Scholar]
Browne 2012 {published data only}
- Browne AS, Yu J, Huang RP, Francisco AM, Sidell N, Taylor RN. Proteomic identification of neurotrophins in the eutopic endometrium of women with endometriosis. Fertility and Sterility 2012;98(3):713‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Budrys 2012 {published data only}
- Budrys NM, Nair HB, Liu YG, Kirma NB, Binkley PA, Kumar S, et al. Increased expression of macrophage colony‐stimulating factor and its receptor in patients with endometriosis. Fertility and Sterility 2012;97(5):1129‐35.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bukulmez 2008 {published data only}
- Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008;149(3):1190‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bulmer 1998 {published data only}
- Bulmer JN, Jone RK, Searle RF. Intraepithelial leukocytes in endometriosis and adenomyosis:comparison of eutopic and ectopic endometrium with normal endometrium. Human Reproduction 1998;13(10):2910‐5. [DOI] [PubMed] [Google Scholar]
Burlev 2005 {published data only}
- Burlev VA, Il'yasova NA, Dubinskaya ED. Proliferative activity of microvessels and angiogenesis in eutopic endometrium in patients with peritoneal endometriosis. Bulletin of Experimental Biology and Medicine 2005;139(6):727‐31. [DOI] [PubMed] [Google Scholar]
Burlev 2006 {published data only}
- Burlev VA, Pavlovich SV, Il’yasova NA. Apoptosis and proliferative activity in endometrium during peritoneal endometriosis. Bulletin of Experimental Biology and Medicine 2006;141(2):204‐7. [DOI] [PubMed] [Google Scholar]
Burney 2009 {published data only}
- Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Molecular Human Reproduction 2009;15(10):625‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calcagno 2011 {published data only}
- Calcagno A, Grassi T, Mariuzzi L, Marzinotto S, Londero AP, Orsaria M, et al. Expression patterns of Aurora A and B kinases, Ki‐67 and the estrogen and progesterone receptors determined using an endometriosis tissue microarray model. Human Reproduction 2011;26(10):2731‐41. [DOI] [PubMed] [Google Scholar]
Carneiro 2007 {published data only}
- Carneiro MM, Morsch DM, Camargos AF, Spritzer PM, Reis FM. Expression of 17beta‐hydroxysteroid dehydrogenase type 2 in pelvic endometriosis. Gynecological Endocrinology 2007;23(4):188‐92. [DOI] [PubMed] [Google Scholar]
Carneiro 2008 {published data only}
- Carneiro MM, Morsch DM, Camargos AF, Reis FM, Spritzer PM. Androgen receptor and 5alpha‐reductase are expressed in pelvic endometriosis. BJOG: An International Journal of Obstetrics and Gynaecology 2008;115(1):113‐7. [DOI] [PubMed] [Google Scholar]
Carvalho 2013 {published data only}
- Carvalho LF, Abrao MS, Biscotti C, Sharma R, Nutter B, Falcone T. Oxidative cell injury as a predictor of endometriosis progression. Reproductive Sciences 2013;20(6):688‐98. [DOI] [PubMed] [Google Scholar]
Chand 2007 {published data only}
- Chand AL, Murray AS, Jones RL, Hannan NJ, Salamonsen LA, Rombauts L. Laser capture microdissection and cDNA array analysis of endometrium identify CCL16 and CCL21 as epithelial‐derived inflammatory mediators associated with endometriosis. Reproductive Biology and Endocrinology 2007;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2013 {published data only}
- Chang KK, Liu LB, Jin LP, Meng YH, Shao J, Wang Y, et al. NME1 suppression of endometrial stromal cells promotes angiogenesis in the endometriotic milieu via stimulating the secretion of IL‐8 and VEGF. International Journal of Clinical and Experimental Pathology 2013;6(10):2030‐8. [PMC free article] [PubMed] [Google Scholar]
Chegini 2003 {published data only}
- Chegini N, Roberts M, Ripps B. Differential expression of interleukins (IL)‐13 and IL‐15 in ectopic and eutopic endometrium of women with endometriosis and normal fertile women. American Journal of Reproductive Immunology 2003;49(2):75‐83. [DOI] [PubMed] [Google Scholar]
Chehna‐Patel 2010 {published data only}
- Chehna‐Patel N, Sachdeva G, Gajbhiye R, Warty N, Khole V. "Spot"‐ting differences between the ectopic and eutopic endometrium of endometriosis patients. Fertility and Sterility 2010;94(6):1964‐1971.e1. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen Y, Yang J, Liu Y, Luo R. Expression and significance of HGF, c‐Met and MMP‐2 in the eutopic endometrium of endometriosis. Wuhan Yike Daxue Xuebao [Medical Journal of Wuhan University] 2006;27(1):54‐6. Chinese. [Google Scholar]
Chen 2012a {published data only}
- Chen P, Zhang Z, Chen Q, Ren F, Li T, Zhang C, et al. Expression of Th1 and Th2 cytokine‐associated transcription factors, T‐bet and GATA‐3, in the eutopic endometrium of women with endometriosis. Acta Histochemica 2012;114(8):779‐84. [DOI] [PubMed] [Google Scholar]
Chen 2012b {published data only}
- Chen Q, Zhang CY, Chen YH, Lou J, Wang DB. Identification of endometriosis‐related genes by representational difference analysis of cDNA. Australian and New Zealand Journal of Obstetrics and Gynaecology 2012;52(2):140‐5. [DOI] [PubMed] [Google Scholar]
Chen 2012c {published data only}
- Chen SF, Zhang J, Huang CX, Lu W, Liang Y, Wan XP. Expression of the T regulatory cell transcription factor FoxP3 in peri‐implantation phase endometrium in infertile women with endometriosis. Reproductive Biology and Endocrinology 2012;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cho 2009 {published data only}
- Cho S, Ahn YS, Choi YS, Seo SK, Nam A, Kim HY, et al. Endometrial osteopontin mRNA expression and plasma osteopontin levels are increased in patients with endometriosis. American Journal of Reproductive Immunology 2009;61(4):286‐93. [DOI] [PubMed] [Google Scholar]
Cho 2010 {published data only}
- Cho S, Park SH, Choi YS, Seo SK, Kim HY, Park KH, et al. Expression of cyclooxygenase‐2 in eutopic endometrium and ovarian endometriotic tissue in women with severe endometriosis. Gynecologic and Obstetric Investigation 2010;69(2):93‐100. [DOI] [PubMed] [Google Scholar]
Chung 2001 {published data only}
- Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M. Matrix metalloproteinase‐9 and tissue inhibitor of metalloproteinase‐3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertility and Sterility 2001;75(1):152‐9. [DOI] [PubMed] [Google Scholar]
Chung 2002a {published data only}
- Chung HW, Wen Y, Choi EA, Hao‐Li, Moon HS, Yu HK, et al. Pleiotrophin (PTN) and midkine (MK) mRNA expression in eutopic and ectopic endometrium in advanced stage endometriosis. Molecular Human Reproduction 2002;8(4):350‐5. [DOI] [PubMed] [Google Scholar]
Chung 2002b {published data only}
- Chung HW, Lee JY, Moon HS, Hur SE, Park MH, Wen Y, et al. Matrix metalloproteinase‐2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase‐2 expression in ectopic and eutopic endometrium. Fertility and Sterility 2002;78(4):787‐95. [DOI] [PubMed] [Google Scholar]
Colette 2004 {published data only}
- Collette T, Bellehumeur C, Kats R, Maheux R, Mailloux J, Villeneuve M, et al. Evidence for an increased release of proteolytic activity by the eutopic endometrial tissue in women with endometriosis and for involvement of matrix metalloproteinase‐9. Human Reproduction 2004;19(6):1257‐64. [DOI] [PubMed] [Google Scholar]
Colette 2006 {published data only}
- Collette T, Maheux R, Mailloux J, Akoum A. Increased expression of matrix metalloproteinase‐9 in the eutopic endometrial tissue of women with endometriosis. Human Reproduction 2006;21(12):3059‐67. [DOI] [PubMed] [Google Scholar]
Cosin 2009 {published data only}
- Cosin R, Gilabert‐Estelles J, Ramon LA, Espana F, Gilabert J, Romeu A, et al. Vascular endothelial growth factor polymorphisms (‐460C/T, +405G/C, and 936C/T) and endometriosis: their influence on vascular endothelial growth factor expression. Fertility and Sterility 2009;92(4):1214‐20. [DOI] [PubMed] [Google Scholar]
Cunha‐Filho 2001 {published data only}
- Cunha‐Filho JS, Gross JL, Lemos NA, Brandelli A, Castillos M, Passos EP. Hyperprolactinemia and luteal insufficiency in infertile patients with mild and minimal endometriosis. Hormone and Metabolic Research 2001;33(4):216‐20. [DOI] [PubMed] [Google Scholar]
D'Amico 2013 {published data only}
- D'Amico F, Skarmoutsou E, Quaderno G, Malaponte G, Corte C, Scibilia G, et al. Expression and localisation of osteopontin and prominin‐1 (CD133) in patients with endometriosis. International Journal of Molecular Medicine 2013;31(5):1011‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daftary 2004 {published data only}
- Daftary GS, Taylor HS. EMX2 gene expression in the female reproductive tract and aberrant expression in the endometrium of patients with endometriosis. The Journal of Clinical Endocrinology and Metabolism 2004;89(5):2390‐6. [DOI] [PubMed] [Google Scholar]
Dai 2012 {published data only}
- Dai L, Gu L, Di W. MiR‐199a attenuates endometrial stromal cell invasiveness through suppression of the IKKbeta/nf‐b pathway and reduced interleukin‐8 expression. Molecular Human Reproduction 2012;18(3):136‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Debrock 2004 {published data only}
- Debrock S, Hill JA, D' Hooghe TM. Quantitative assessment of endometrial‐peritoneal interaction in vitro:a non invasive diagnostic test for women with endometriosis. Gynecologic and Obstetric Investigation 2004;57(1):49‐51. [PubMed] [Google Scholar]
De Graaff 2012 {published data only}
- Graaff AA, Delvoux B, Vijver KK, Kyama CM, Dhooghe TM, Dunselman GAJ, et al. Paired‐box gene 2 is down‐regulated in endometriosis and correlates with low epidermal growth factor receptor expression. Human Reproduction 2012;27(6):1676‐84. [DOI] [PubMed] [Google Scholar]
Delbandi 2013 {published data only}
- Delbandi AA, Mahmoudi M, Shervin A, Akbari E, Jeddi‐Tehrani M, Sankian M, et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertility and Sterility 2013;100(3):761‐9. [DOI] [PubMed] [Google Scholar]
Delvoux 2009 {published data only}
- Delvoux B, Groothuis P, D'Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17β‐estradiol in endometriosis lesions is the result of impaired metabolism. The Journal of Clinical Endocrinology and Metabolism 2009;94(3):876‐83. [DOI] [PubMed] [Google Scholar]
Dharmaraj 2014 {published data only}
- Dharmaraj N, Chapela PJ, Morgado M, Hawkins SM, Lessey BA, Young SL, et al. Expression of the transmembrane mucins, MUC1, MUC4 and MUC16, in normal endometrium and in endometriosis. Human Reproduction 2014;29(8):1730‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Carlo 2009 {published data only}
- Carlo C, Bonifacio M, Tommaselli GA, Bifulco G, Guerra G, Nappi C. Metalloproteinases, vascular endothelial growth factor, and angiopoietin 1 and 2 in eutopic and ectopic endometrium. Fertility and Sterility 2009;91(6):2315‐23. [DOI] [PubMed] [Google Scholar]
Dimitriadis 2006 {published data only}
- Dimitriadis E, Stoikos C, Stafford‐Bell M, Clark I, Paiva P, Kovacs G, et al. Interleukin‐11, IL‐11 receptor‐alpha and leukemia inhibitory factor are dysregulated in endometrium of infertile women with endometriosis during the implantation window. Journal of Reproductive Immunology 2006;69(1):53‐64. [DOI] [PubMed] [Google Scholar]
Dmowski 2001 {published data only}
- Dmowski WP, Ding J, Shen J, Rana N, Fernandez BB, Braun DP. Apoptosis in endometrial glandular and stromal cells in women with and without endometriosis. Human Reproduction 2001;16(9):1802‐8. [DOI] [PubMed] [Google Scholar]
Donnez 1998 {published data only}
- Donnez J, Smoes P, Gillerot S, Casanas‐Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Human Reproduction 1998;13(6):1686‐90. [DOI] [PubMed] [Google Scholar]
Donnez 2013 {published data only}
- Donnez O, Soares M, Defrere S, Kerk O, Langendonckt A, Donnez J, et al. Nerve fibers are absent in disease‐free and eutopic endometrium, but present in endometriotic (especially deep) lesions. Journal of Endometriosis 2013;5(2):68‐76. [Google Scholar]
Ejskjaer 2009 {published data only}
- Ejskjaer K, Sorensen BS, Poulsen SS, Mogensen O, Forman A, Nexo E. Expression of the epidermal growth factor system in eutopic endometrium from women with endometriosis differs from that in endometrium from healthy women. Gynecologic and Obstetric Investigation 2009;67(2):118‐26. [DOI] [PubMed] [Google Scholar]
Fang 2003 {published data only}
- Fang XL, Xia XM, Lin QH. Expression of vascular endothelial growth factor in the peritoneal fluid and eutopic endometrium of patients with endometriosis. Hunan Yike Daxue Xuebao [Bulletin of Hunan Medical University] 2003;28(3):288‐90. Chinese. [PubMed] [Google Scholar]
Fasciani 2010 {published data only}
- Fasciani A, Quilici P, Biscaldi E, Flamini M, Fioravanti A, Orlandi P, et al. Overexpression and functional relevance of somatostatin receptor‐1,‐2, and‐5 in endometrium and endometriotic lesions. Journal of Clinical Endocrinology and Metabolism 2010;95(12):5315‐9. [DOI] [PubMed] [Google Scholar]
Fazleabas 1987 {published data only}
- Fazleabas A, Khan‐Dawood F, Dawood M. Protein, progesterone, and protease inhibitors in uterine and peritoneal fluids of women with endometriosis. Fertility and Sterility 1987;47(2):218‐24. [DOI] [PubMed] [Google Scholar]
Fedele 1988 {published data only}
- Fedele L, Vercellini P, Arcaini P, Grazia da Dalt M, Candiani GB. CA 125 in serum, peritoneal fluid, active lesions, and endometrium of patients with endometriosis. American Journal of Obstetrics and Gynecology 1988;158(1):166‐9. [DOI] [PubMed] [Google Scholar]
Fernandez‐Shaw 1995a {published data only}
- Fernandez‐Shaw S, Clarke MT, Hicks B, Naish CE, Barlow DH, Starkey PM. Bone marrow‐derived cell populations in uterine and ectopic endometrium. Human Reproduction 1995;10(9):2285‐9. [DOI] [PubMed] [Google Scholar]
Fernandez‐Shaw 1995b {published data only}
- Fernandez‐Shaw S, Marshall JM, Hicks B, Barlow DH, Starkey PM. Plasminogen activators in ectopic and uterine endometrium. Fertility and Sterility 1995;63(1):45‐51. [DOI] [PubMed] [Google Scholar]
Ferriani 1993 {published data only}
- Ferriani RA, Charnock‐Jones DS, Prentice A, Thomas EJ, Smith SK. Immunohistochemical localization of acidic and basic fibroblast growth factors in normal human endometrium and endometriosis and the detection of their mRNA by polymerase chain reaction. Human Reproduction 1993;8(1):11‐6. [DOI] [PubMed] [Google Scholar]
Finas 2008 {published data only}
- Finas D, Huszar M, Agic A, Dogan S, Kiefel H, Riedle S, et al. L1 cell adhesion molecule (L1CAM) as a pathogenetic factor in endometriosis. Human Reproduction 2008;23(5):1053‐62. [DOI] [PubMed] [Google Scholar]
Fowler 2007 {published data only}
- Fowler PA, Tattum J, Bhattacharya S, Klonisch T, Hombach‐Klonisch S, Gazvani R, et al. An investigation of the effects of endometriosis on the proteome of human eutopic endometrium: a heterogeneous tissue with a complex disease. Proteomics 2007;7(1):130‐42. [DOI] [PubMed] [Google Scholar]
Fujino 2006 {published data only}
- Fujino K, Ueda M, Takehara M, Futakuchi H, Kanda K, Yamashita Y, et al. Transcriptional expression of survivin and its splice variants in endometriosis. Molecular Human Reproduction 2006;12(6):383‐8. [DOI] [PubMed] [Google Scholar]
Fukaya 1999 {published data only}
- Fukaya T, Sugawara J, Yoshida H, Murakami T, Yajima A. Intercellular adhesion molecule‐1 and hepatocyte growth factor in human endometriosis: original investigation and a review of literature. Gynecologic and Obstetric Investigation 1999;47 Suppl 1:11‐6. [DOI] [PubMed] [Google Scholar]
Gaetje 2006 {published data only}
- Gaetje R, Rody A, Kissler S, Kaufmann M, Ahr A. Integrin expression in eutopic and ectopic endometrium [Integrinexpression im eutopen und ektopen endometrium von endometriosepatientinnen]. Zentralblatt fur Gynakologie 2006;128(3):135‐7. [DOI] [PubMed] [Google Scholar]
Gaetje 2007a {published data only}
- Gaetje R, Holtrich U, Engels K, Kourtis K, Cikrit E, Kissler S, et al. Expression of membrane‐type 5 matrix metalloproteinase in human endometrium and endometriosis. Gynecological Endocrinology 2007;23(10):567‐73. [DOI] [PubMed] [Google Scholar]
Gaetje 2007b {published data only}
- Gaetje R, Holtrich U, Karn T, Cikrit E, Engels K, Rody A, Kaufmann M. Characterization of WNT7A expression in human endometrium and endometriotic lesions. Fertility and Sterility 2007;88(6):1534‐40. [DOI] [PubMed] [Google Scholar]
Gagné 2003 {published data only}
- Gagné D, Rivard M, Pagé M, Lépine M, Platon C, Shazand K, et al. Development of a nonsurgical diagnostic tool for endometriosis based on the detection of endometrial leukocyte subsets and serum CA‐125 levels. Fertility and Sterility 2003;80(4):876‐85. [DOI] [PubMed] [Google Scholar]
Gebel 1998 {published data only}
- Gebel HM, Braun DP, Tambur A, Frame D, Rana N, Dmowski WP. Spontaneous apoptosis of endometrial tissue is impaired in women with endometriosis. Fertility and Sterility 1998;69(6):1042‐7. [DOI] [PubMed] [Google Scholar]
Giannelli 2007 {published data only}
- Giannelli G, Sgarra C, Naro E, Lavopa C, Angelotti U, Tartagni M, et al. Endometriosis is characterized by an impaired localization of laminin‐5 and alpha3beta1 integrin receptor. International Journal of Gynecological Cancer 2007;17(1):242‐7. [DOI] [PubMed] [Google Scholar]
Gonzalez Ramos 2012 {published data only}
- Gonzalez‐Ramos R, Rocco J, Rojas C, Sovino H, Poch A, Kohen P, et al. Physiologic activation of nuclear factor kappa‐B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertility and Sterility 2012;97(3):645‐51. [DOI] [PubMed] [Google Scholar]
Gori 2013 {published data only}
- Gori I, Rodriguez Y, Pellegrini C, Achtari C, Hornung D, Chardonnens E, et al. Augmented epithelial multidrug resistance‐associated protein 4 expression in peritoneal endometriosis: regulation by lipoxin A(4). Fertility and Sterility 2013;99(7):1965‐73. [DOI] [PubMed] [Google Scholar]
Guay 2011 {published data only}
- Guay S, Michaud N, Bourcier N, Leboeuf M, Lemyre M, Mailloux J, et al. Distinct expression of the soluble and the membrane‐bound forms of interleukin‐1 receptor accessory protein in the endometrium of women with endometriosis. Fertility and Sterility 2011;95(4):1284‐90. [DOI] [PubMed] [Google Scholar]
Guo 2013 {published data only}
- Guo Y, Chen Y, Liu LB, Chang KK, Li H, Li MQ, et al. IL‐22 in the endometriotic milieu promotes the proliferation of endometrial stromal cells via stimulating the secretion of CCL2 and IL‐8. International Journal of Clinical and Experimental Pathology 2013;6(10):2011‐20. [PMC free article] [PubMed] [Google Scholar]
Hapangama 2008 {published data only}
- Hapangama DK, Turner MA, Drury JA, Quenby S, Saretzki G, Martin‐Ruiz C, et al. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Human Reproduction 2008;23(7):1511‐9. [DOI] [PubMed] [Google Scholar]
Hapangama 2009 {published data only}
- Hapangama DK, Turner MA, Drury JA, Quenby S, Hart A, Maddick M, et al. Sustained replication in endometrium of women with endometriosis occurs without evoking a DNA damage response. Human Reproduction 2009;24(3):687‐96. [DOI] [PubMed] [Google Scholar]
Hapangama 2012 {published data only}
- Hapangama DK, Raju RS, Valentijn AJ, Barraclough D, Hart A, Turner MA, et al. Aberrant expression of metastasis‐inducing proteins in ectopic and matched eutopic endometrium of women with endometriosis: implications for the pathogenesis of endometriosis. Human Reproduction 2012;27(2):394‐407. [DOI] [PubMed] [Google Scholar]
Hassa 2009 {published data only}
- Hassa H, Tanir HM, Tekin B, Artan S, Dundar E, Kirilmaz SD, et al. Apoptosis patterns in eutopic and ectopic endometrium, adhesions and normal‐looking peritoneum from women with or without endometriosis. Archives of Gynecology and Obstetrics 2009;280(2):195‐9. [DOI] [PubMed] [Google Scholar]
Hawkins 2011 {published data only}
- Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, et al. Functional microRNA involved in endometriosis. Molecular Endocrinology 2011;25(5):821‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hey‐Cunningham 2013 {published data only}
- Hey‐Cunningham AJ, Markham R, Fraser IS, Berbic M. Dysregulation of vascular endothelial growth factors and their neuropilin receptors in the eutopic endometrium of women with endometriosis. Reproductive Sciences 2013;20(11):1382‐9. [DOI] [PubMed] [Google Scholar]
Hii 1998 {published data only}
- Hii LLP, Rogers PAW. Endometrial vascular and glandular expression. Human Reproduction 1998;13(4):1030‐5. [DOI] [PubMed] [Google Scholar]
Hsu 2014 {published data only}
- Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen HS, Chang Y, et al. miRNA‐199a‐5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. Journal of Pathology 2014;232(3):330‐43. [DOI] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang X, Chen L, Fu G, Xu H, Zhang X. Decreased expression of pigment epithelium‐derived factor and increased microvascular density in ovarian endometriotic lesions in women with endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2012;165(1):104‐9. [DOI] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang F, Wang H, Zou Y, Liu Q, Cao J, Yin T. Effect of GnRH‐II on the ESC proliferation, apoptosis and VEGF secretion in patients with endometriosis in vitro. International Journal of Clinical and Experimental Pathology 2013;6(11):2487‐96. [PMC free article] [PubMed] [Google Scholar]
Hudelist 2005a {published data only}
- Hudelist G, Keckstein J, Czerwenka K, Lass H, Walter I, Auer M, Wieser F, Wenzl R, Kubista E, Singer CF. Estrogen receptor beta and matrix metalloproteinase 1 are coexpressed in uterine endometrium and endometriotic lesions of patients with endometriosis. Fertility and Sterility 2005;84 Suppl 2:1249‐56. [DOI] [PubMed] [Google Scholar]
Hudelist 2005b {published data only}
- Hudelist G, Lass H, Keckstein J, Walter I, Wieser F, Wenzl R, et al. Interleukin 1alpha and tissue‐lytic matrix metalloproteinase‐1 are elevated in ectopic endometrium of patients with endometriosis. Human Reproduction 2005;20(6):1695‐701. [DOI] [PubMed] [Google Scholar]
Hudelist 2008 {published data only}
- Hudelist G, Huber A, Knoefler M, Haider S, Kolbus A, Czerwenka K, Helmy S, Kubista E, Singer CF. beta‐HCG/LH receptor (beta‐HCG/LH‐R) expression in eutopic endometrium and endometriotic implants: evidence for beta‐HCG sensitivity of endometriosis. Reproductive Sciences 2008;15(6):543‐51. [DOI] [PubMed] [Google Scholar]
Hur 2006 {published data only}
- Hur SE, Lee JY, Moon H‐S, Chung HW. Angiopoietin‐1, angiopoietin‐2 and Tie‐2 expression in eutopic endometrium in advanced endometriosis. Molecular Human Reproduction 2006;12(7):421‐6. [DOI] [PubMed] [Google Scholar]
Hurst 2014 {published data only}
- Hurst BS, Shimp KE, Elliot M, Marshburn PB, Parsons J, Bahrani‐Mostafavi Z. Molecular evaluation of proliferative‐phase endometrium may provide insight about the underlying causes of infertility in women with endometriosis. Archives of Gynecology and Obstetrics 2014;289(5):1119‐24. [DOI] [PubMed] [Google Scholar]
Hwang 2013 {published data only}
- Hwang JW, Oh JJ, Wang T, Jin YC, Lee JS, Choi JR, et al. Identification of biomarkers for endometriosis in eutopic endometrial cells from patients with endoemtriosis using a proteomics approach. Molecular Medicine Reports 2013;8(1):183‐8. [DOI] [PubMed] [Google Scholar]
Igarashi 2005 {published data only}
- Igarashi TM, Bruner‐Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. Reduced expression of progesterone receptor‐B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8‐tetrachlorodibenzo‐p‐dioxin. Fertility and Sterility 2005;84(1):67‐74. [DOI] [PubMed] [Google Scholar]
Isaacson 1990 {published data only}
- Isaacson KB, Galman M, Coutifaris C, Lyttle CR. Endometrial synthesis and secretion of complement component‐3 by patients with and without endometriosis. Fertility and Sterility 1990;53(5):836‐41. [PubMed] [Google Scholar]
Jana 2013 {published data only}
- Jana SK, Banerjee P, Mukherjee R, Chakravarty B, Chaudhury K. HOXA‐11 mediated dysregulation of matrix remodeling during implantation window in women with endometriosis. Journal of Assisted Reproduction and Genetics 2013;30(11):1505‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2005 {published data only}
- Johnson MC, Torres M, Alves A, Bacallao K, Fuentes A, Vega M, et al. Augmented cell survival in eutopic endometrium from women with endometriosis: Expression of c‐myc, TGF‐beta1 and bax genes. Reproductive Biology and Endocrinology 2005;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolicoeur 1998 {published data only}
- Jolicoeur C, Boutouil M, Drouin R, Paradis I, Lemay A, Akoum A. Increased expression of monocyte chemotactic protein‐1 in the endometrium of women with endometriosis. American Journal of Pathology 1998;152(1):125‐33. [PMC free article] [PubMed] [Google Scholar]
Jones 1995 {published data only}
- Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Human Reproduction 1995;10(12):3272‐9. [DOI] [PubMed] [Google Scholar]
Jones 1996 {published data only}
- Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of stromal leukocytes in ovarian endometriosis: comparison of eutopic and ectopic endometrium with normal endometrium. Fertility and Sterility 1996;66(1):81‐9. [DOI] [PubMed] [Google Scholar]
Jones 1998 {published data only}
- Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl‐2 expression in normal human endometrium, endometriosis and adenomyosis. Human Reproduction 1998;13(12):3496‐502. [DOI] [PubMed] [Google Scholar]
Jones 2009 {published data only}
- Jones CJP, Inuwa IM, Nardo LG, Litta P, Fazleabas AT. Eutopic endometrium from women with endometriosis shows altered ultrastructure and glycosylation compared to that from healthy controls‐a pilot observational study. Reproductive Sciences 2009;16(6):559‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juhasz‐Boss 2011 {published data only}
- Juhasz‐Boss I, Fischer C, Lattrich C, Skrzypczak M, Malik E, Ortmann O, et al. Endometrial expression of estrogen receptor and its splice variants in patients with and without endometriosis. Archives of Gynecology and Obstetrics 2011;284(4):885‐91. [DOI] [PubMed] [Google Scholar]
Jurgensen 1996 {published data only}
- Jurgensen A, Mettler L, Volkov NI, Parwaresch R. Proliferative activity of the endometrium throughout the menstrual cycle in infertile women with and without endometriosis. Fertility and Sterility 1996;66(3):369‐75. [DOI] [PubMed] [Google Scholar]
Kao 2003 {published data only}
- Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease‐based implantation failure and infertility. Endocrinology 2003;144(7):2870‐81. [DOI] [PubMed] [Google Scholar]
Karalok 2014 {published data only}
- Karalok HM, Aydin E, Saglam O, Torun A, Guzeloglu‐Kayisli O, Lalioti MD, et al. MRNA‐binding protein TIA‐1 reduces cytokine expression in human endometrial stromal cells and is down‐regulated in ectopic endometrium. Journal of Clinical Endocrinology and Metabolism 2014;99(12):E2610‐E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2003 {published data only}
- Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Immunoexpression of hepatocyte growth factor and c‐Met receptor in the eutopic endometrium predicts the activity of ectopic endometrium. Fertility and Sterility 2003;79(1):173‐81. [DOI] [PubMed] [Google Scholar]
Khan 2005a {published data only}
- Khan KN, Masuzaki H, Fujishita A, Kitajima M, Hiraki K, Sekine I, et al. Interleukin‐6‐ and tumour necrosis factor alpha‐mediated expression of hepatocyte growth factor by stromal cells and its involvement in the growth of endometriosis. Human Reproduction 2005;20(10):2715‐23. [DOI] [PubMed] [Google Scholar]
Khan 2005b {published data only}
- Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Matsuyama T, et al. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Human Reproduction 2005;20(7):2004‐13. [DOI] [PubMed] [Google Scholar]
Khan 2010 {published data only}
- Khan KN, Kitajima M, Hiraki K, Yamaguchi N, Katamine S, Matsuyama T, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertility and Sterility 2010;94(7):2860‐3. [DOI] [PubMed] [Google Scholar]
Khan 2012 {published data only}
- Khan KN, Kitajima M, Yamaguchi N, Fujishita A, Nakashima M, Ishimaru T, et al. Role of prostaglandin E2 in bacterial growth in women with endometriosis. Human Reproduction 2012;27(12):3417‐24. [DOI] [PubMed] [Google Scholar]
Khan 2013 {published data only}
- Khan KN, Kitajima M, Inoue T, Tateishi S, Fujishita A, Nakashima M, et al. Additive effects of inflammation and stress reaction on Toll‐like receptor 4‐mediated growth of endometriotic stromal cells. Human Reproduction 2013;28(10):2794‐803. [DOI] [PubMed] [Google Scholar]
Kharfi 2001 {published data only}
- Kharfi A, Akoum A. Correlation between decreased type‐II interleukin‐1 receptor and increased monocyte chemotactic protein‐1 expression in the endometrium of women with endometriosis. American Journal of Reproductive Immunology 2001;45(4):193‐9. [DOI] [PubMed] [Google Scholar]
Kharfi 2002 {published data only}
- Kharfi A, Boucher A, Akoum A. Abnormal interleukin‐1 receptor type II gene expression in the endometrium of women with endometriosis. Biology of Reproduction 2002;66(2):401‐6. [DOI] [PubMed] [Google Scholar]
Kharfi 2003 {published data only}
- Kharfi A, Labelle Y, Mailloux J, Akoum A. Deficient expression of tumor necrosis factor receptor type 2 in the endometrium of women with endometriosis. American Journal of Reproductive Immunology 2003;50(1):33‐40. [DOI] [PubMed] [Google Scholar]
Khorram 2002 {published data only}
- Khorram O, Lessey BA. Alterations in expression of endometrial endothelial nitric oxide synthase and alpha(v)beta(3) integrin in women with endometriosis. Fertility and Sterility 2002;78(4):860‐4. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim SH, Lee HW, Kim YH, Koo YH, Chae HD, Kim CH, et al. Down‐regulation of p21‐activated kinase 1 by progestin and its increased expression in the eutopic endometrium of women with endometriosis. Human Reproduction 2009;24(5):1133‐41. [DOI] [PubMed] [Google Scholar]
Kim 2013a {published data only}
- Kim KH, Park JK, Choi YW, Kim YH, Lee EN, Lee JR, et al. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM‐1 and VCAM1 in TNF‐‐activated human endometrial stromal cells. International Journal of Molecular Medicine 2013;32(1):67‐78. [DOI] [PubMed] [Google Scholar]
Kim 2013b {published data only}
- Kim SH, Ihm HJ, Oh YS, Chae HD, Kim CH, Kang BM. Increased nuclear expression of nuclear factor kappa‐B p65 subunit in the eutopic endometrium and ovarian endometrioma of women with advanced stage endometriosis. American Journal of Reproductive Immunology 2013;70(6):497‐508. [DOI] [PubMed] [Google Scholar]
Kim 2013c {published data only}
- Kim SH, Kim SR, Ihm HJ, Oh YS, Chae HD, Kim CH, et al. Regulation of P21‐activated kinase‐4 by progesterone and tumor necrosis factor‐ in human endometrium and its increased expression in advanced‐stage endometriosis. Journal of Clinical Endocrinology and Metabolism 2013;98(2):E238‐48. [DOI] [PubMed] [Google Scholar]
Kitawaki 1999a {published data only}
- Kitawaki J, Kusuki I, Koshiba H, Tsukamato K, Fushiki S, Honjo H. Detection of aromatase cytochrome P450 in endometrial biopsy. Fertilty and Sterility 1999;72(6):1100‐6. [DOI] [PubMed] [Google Scholar]
Kitawaki 2000a {published data only}
- Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Expression of leptin receptor in human endometrium and fluctuation during the menstrual cycle. Journal of Clinical Endocrinology and Metabolism 2000;85(5):1946‐50. [DOI] [PubMed] [Google Scholar]
Kitawaki 2000b {published data only}
- Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Progesterone induction of 17beta‐hydroxysteroid dehydrogenase type 2 during the secretory phase occurs in the endometrium of estrogen‐dependent benign diseases but not in normal endometrium. Journal of Clinical Endocrinology and Metabolism 2000;85(9):3292‐6. [DOI] [PubMed] [Google Scholar]
Klemmt 2006 {published data only}
- Klemmt PAB, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertility and Sterility 2006;85(3):564‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klemmt 2007 {published data only}
- Klemmt PA, Carver JG, Koninckx P, McVeigh EJ, Mardon HJ. Endometrial cells from women with endometriosis have increased adhesion and proliferative capacity in response to extracellular matrix components: towards a mechanistic model for endometriosis progression. Human Reproduction 2007;22(12):3139‐47. [DOI] [PubMed] [Google Scholar]
Kobayashi 2012 {published data only}
- Kobayashi H, Yamashita Y, Iwase A, Yoshikawa Y, Yasui H, Kawai Y, et al. The ferroimmunomodulatory role of ectopic endometriotic stromal cells in ovarian endometriosis. Fertility and Sterility 2012;98(2):415‐22. [DOI] [PubMed] [Google Scholar]
Kocbek 2014a {published data only}
- Kocbek V, Bersinger NA, Brglez V, Mueller MD, Petan T, Rizner TL. Phospholipas A2 group IIA is elevated in endometriomas but not in peritoneal fluid and serum of ovarian endometriosis patients. Gynecological Endocrinology 2015;31(3):214‐8. [DOI] [PubMed] [Google Scholar]
Kocbek 2014b {published data only}
- Kocbek V, Hevir‐Kene N, Bersinger NA, Mueller MD, Rizner TL. Increased levels of biglycan in endometriomas and peritoneal fluid samples from ovarian endometriosis patients. Gynecological Endocrinology 2014;30(7):520‐4. [DOI] [PubMed] [Google Scholar]
Koshiba 2005 {published data only}
- Koshiba H, Kitawaki J, Teramoto M, Kitaoka Y, Ishihara H, Obayashi H, et al. Expression of allograft inflammatory factor‐1 in human eutopic endometrium and endometriosis: possible association with progression of endometriosis. Journal of Clinical Endocrinology and Metabolism 2005;90(1):529‐37. [DOI] [PubMed] [Google Scholar]
Kreiner 1986 {published data only}
- Kreiner D, Fromowitz FB, Richardson DA, Kenigsberg D. Endometrial immunofluorescnce associated with endometriosis and PID. Fertility and Sterility 1986;46(2):243‐6. [PubMed] [Google Scholar]
Kyama 2006a {published data only}
- Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertility and Sterility 2006;85(6):1667‐75. [DOI] [PubMed] [Google Scholar]
Kyama 2006b {published data only}
- Kyama CM, T'Jampens D, Mihalyi A, Simsa P, Debrock S, Waelkens E, et al. ProteinChip technology is a useful method in the pathogenesis and diagnosis of endometriosis: a preliminary study. Fertility and Sterility 2006;86(1):203‐9. [DOI] [PubMed] [Google Scholar]
Kyama 2008 {published data only}
- Kyama CM, Overbergh L, Mihalyi A, Meuleman C, Mwenda JM, Mathieu C, et al. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertility and Sterility 2008;89(2):301‐10. [DOI] [PubMed] [Google Scholar]
Kyama 2011 {published data only}
- Kyama CM, Mihalyi A, Gevaert O, Waelkens E, Simsa P, Plas R, Meuleman C, Moor B, D'Hooghe TM. Evaluation of endometrial biomarkers for semi‐invasive diagnosis of endometriosis. Fertility and Sterility 2011;95(4):1338‐43 e1‐3. [DOI] [PubMed] [Google Scholar]
Laudanski 2009 {published data only}
- Laudanski P, Szamatowicz J, Kowalczuk O, Kuźmicki M, Grabowicz M, Chyczewski L. Expression of selected tumor suppressor and oncogenes in endometrium of women with endometriosis. Human Reproduction 2009;24(8):1880‐90. [DOI] [PubMed] [Google Scholar]
Laudanski 2013a {published data only}
- Laudanski P, Gorodkiewicz E, Ramotowska B, Charkiewicz R, Kuzmicki M, Szamatowicz J. Determination of cathepsins B, D and G concentration in eutopic proliferative endometrium of women with endometriosis by the surface plasmon resonance imaging (SPRI) technique. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;169(1):80‐3. [DOI] [PubMed] [Google Scholar]
Laudanski 2013b {published data only}
- Laudanski P, Charkiewicz R, Kuzmicki M, Szamatowicz J, Charkiewicz A, Niklinski J. MicroRNAs expression profiling of eutopic proliferative endometrium in women with ovarian endometriosis. Reproductive Biology and Endocrinology 2013;11(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawson 2007 {published data only}
- Lawson C, Al‐Akoum M, Maheux R, Akoum A. Increased expression of interleukin‐1 receptor type 1 in active endometriotic lesions. Reproduction 2007;133(1):265‐74. [DOI] [PubMed] [Google Scholar]
Lebovic 2002 {published data only}
- Lebovic DI, Baldocchi RA, Mueller MD, Taylor RN. Altered expression of a cell cycle suppressor gene. Fertility and Sterility 2002;78(4):849‐54. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee MY, Kim SH, Ihm HJ, Chae HD, Kim CH, Kang BM. Up‐regulation of p21‐activated kinase 1 by in vitro treatment with interleukin 1‐beta and its increased expression in ovarian endometriotic cysts. Fertility and Sterility 2011;96(2):508‐11. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee YH, Tan CW, Venkatratnam A, Tan CS, Cui L, Loh SF, et al. Dysregulated sphingolipid metabolism in endometriosis. Journal of Clinical Endocrinology and Metabolism 2014;99(10):1913‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leiva 1994 {published data only}
- Leiva MC, Hasty LA, Lyttle CR. Inflammatory changes of the endometrium in endometriosis. Fertility and Sterility 1994;62(5):967‐72. [PubMed] [Google Scholar]
Lessey 1989 {published data only}
- Lessey BA, Metzgher DA, Haney AF, McCarty KS. Immunohistochemical analysis of estrogen and progesterone receptors in endometriosis: comparison with normal endometrium during the menstrual cycle and the effect of medical therapy. Fertility and Sterility 1989;51(3):409‐15. [DOI] [PubMed] [Google Scholar]
Lessey 1993 {published data only}
- Lessey BA, Pindzola JA. Tumour associated glycoprotein TAG 72 in Endometriotic Implants. Journal of Clinical Endocrinology and Metabolism 1993;76(4):1075‐9. [DOI] [PubMed] [Google Scholar]
Lessey 1994 {published data only}
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium. Journal of Clinical Endocrinology and Metabolism 1994;79(2):643‐9. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li CY, Lang JH, Liu HY, ZHou HM. Expression of annexin‐1 in patients with endometriosis. Zhonghua Yixue Zazhi [Chinese Medical Journal] 2008;121(10):927‐31. Chinese. [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li CY, Liu HY, Lang JH, Wang HQ, Fan XL. Increased expression of stathmin in eutopic endometrium of patients with endometriosis. Zhonghua Yixue Zazhi [Chinese Medical Journal] 2010;123(16):2190‐4. Chinese. [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li MQ, Hou XF, Lv SJ, Meng YH, Wang XQ, Tang CL, et al. CD82 gene suppression in endometrial stromal cells leads to increase of the cell invasiveness in the endometriotic milieu. Journal of Molecular Endocrinology 2011;47(2):195‐208. [DOI] [PubMed] [Google Scholar]
Li 2012a {published data only}
- Li MQ, Li HP, Meng YH, Wang XQ, Zhu XY, Mei J, et al. Chemokine CCL2 enhances survival and invasiveness of endometrial stromal cells in an autocrine manner by activating Akt and MAPK/Erk1/2 signal pathway. Fertility and Sterility 2012;97(4):919‐29. [DOI] [PubMed] [Google Scholar]
Li 2012b {published data only}
- Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, et al. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1‐triggered PTEN/AKT signal pathway. Human Reproduction 2012;27(7):2107‐16. [DOI] [PubMed] [Google Scholar]
Lima‐Couy 2004 {published data only}
- Lima‐Couy I, Cervero A, Bonilla‐Musoles F, Pellicer A, Simon C. Endometrial leptin and leptin receptor expression in women with severe/moderate endometriosis. Molecular Human Reproduction 2004;10(11):777‐82. [DOI] [PubMed] [Google Scholar]
Lin 2005 {published data only}
- Lin WC, Hsieh YY, Tsai FJ, Chang WC, Chang CYY, Tsai HD. Estrogen receptor‐alpha and ‐beta in endometriosis and normal endometrium in humans. Mid‐Taiwan Journal of Medicine 2005;10(2):65‐72. [Google Scholar]
Lin 2010 {published data only}
- Lin W, Chen S, Li M, Wang B, Qu X, Zhang Y. Expression of macrophage migration inhibitory factor in human endometriosis: relation to disease stage, menstrual cycle and infertility. Journal of Obstetrics and Gynaecology Research 2010;36(2):344‐51. [DOI] [PubMed] [Google Scholar]
Lin 2012 {published data only}
- Lin M, Weng H, Wang X, Zhou B, Yu P, Wang Y. The role of tissue factor and protease‐activated receptor 2 in endometriosis. American Journal of Reproductive Immunology 2012;68(3):251‐7. [DOI] [PubMed] [Google Scholar]
Liu 2002 {published data only}
- Liu Y, Luo L, Zhao H. Immunohistochemical study of HLA‐DR antigen in endometrial tissue of patients with endometriosis. Huazhong Keji Daxue Xuebao [Journal of Huazhong University of Science and Technology] 2002;22(1):60‐1. Chinese. [DOI] [PubMed] [Google Scholar]
Liu 2005a {published data only}
- Liu Y, Luo R, Chen Y. Expression and significance of HGF and MMP‐2 in the eutopic endometrium of endometriosis. Wuhan Yike Daxue Xuebao [Medical Journal of Wuhan University] 2005;26(3):402‐4. Chinese. [Google Scholar]
Locci 2013 {published data only}
- Locci R, Nisolle M, Angioni S, Foidart JM, Munaut C. Expression of the gamma 2 chain of laminin‐332 in eutopic and ectopic endometrium of patients with endometriosis. Reproductive Biology and Endcrinology 2013;11(94):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo Vasco 2012 {published data only}
- Lo Vasco VR, Leopizzi M, Chiappetta C, Businaro R, Polonia P, Della Rocca C, et al. Expression of phosphoinositide‐specific phospholipase C enzymes in normal endometrium and in endometriosis. Fertility and Sterility 2012;98(2):410‐4. [DOI] [PubMed] [Google Scholar]
Luk 2005 {published data only}
- Luk J, Seval Y, Kayisli UA, Ulukus M, Ulukus CE, Arici A. Regulation of interleukin‐8 expression in human endometrial endothelial cells: a potential mechanism for the pathogenesis of endometriosis. Journal of Clinical Endocrinology and Metabolism 2005;90(3):1805‐11. [DOI] [PubMed] [Google Scholar]
Luk 2010 {published data only}
- Luk J, Seval Y, Ulukus M, Ulukus EC, Arici A, Kayisli UA. Regulation of monocyte chemotactic protein‐1 expression in human endometrial endothelial cells by sex steroids: a potential mechanism for leukocyte recruitment in endometriosis. Reproductive Sciences 2010;17(3):278‐87. [DOI] [PubMed] [Google Scholar]
Luo 2006a {published data only}
- Luo Q, Ning W, Wu Y, Zhu X, Jin F, Sheng J, et al. Altered expression of interleukin‐18 in the ectopic and eutopic endometrium of women with endometriosis. Journal of Reproductive Immunology 2006;72(1‐2):108‐17. [DOI] [PubMed] [Google Scholar]
Luo 2006b {published data only}
- Luo Q, Ning W, Wu Y, Zhu X, Jin F, Sheng J, et al. Altered expression of interleukin‐18 in the ectopic and eutopic endometrium of women with endometriosis. Journal of Reproductive Immunology 2006;72(1‐2):108‐17. [DOI] [PubMed] [Google Scholar]
Maia 2012 {published data only}
- Maia H Jr, Haddad C, Casoy J. Correlation between aromatase expression in the eutopic endometrium of symptomatic patients and the presence of endometriosis. International Journal of Women's Health 2012;4:61‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makri 2012 {published data only}
- Makri A, Msaouel P, Petraki C, Milingos D, Protopapas A, Liapi A, et al. KISS1/KISS1R expression in eutopic and ectopic endometrium of women suffering from endometriosis. In Vivo 2012;26(1):119‐27. [PubMed] [Google Scholar]
Malik 2006 {published data only}
- Malik S, Day K, Perrault I, Charnock‐Jones DS, Smith SK. Menstrual effluent in endometriosis shows no difference in volume, VEGF‐A, MMP2 and MMP9 or sFLT. Reproductive Biomedicine Online 2006;12(2):174‐81. [DOI] [PubMed] [Google Scholar]
Mathur 1990 {published data only}
- Mathur S, Garza DE, Smith LF. Endometrial autoantigens eliciting immunoglobulin (Ig) G, IgA, and IgM responses in endometriosis. Fertility and Sterility 1990;54(1):56‐63. [PubMed] [Google Scholar]
Matsuzaki 2004a {published data only}
- Matsuzaki S, Canis M, Pouly JL, Wattiez A, Okamura K, Mage G. Cyclooxygenase‐2 expression in deep endometriosis and matched eutopic endometrium. Fertility and Sterility 2004;82(5):1309‐15. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2004b {published data only}
- Matsuzaki S, Canis M, Vaurs‐Barriere C, Pouly JL, Boespflug‐Tanguy O, Penault‐Llorca F, et al. DNA microarray analysis of gene expression profiles in deep endometriosis using laser capture microdissection. Molecular Human Reproduction 2004;10(10):719‐28. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2005a {published data only}
- Matsuzaki S, Canis M, Pouly JL, Dechelotte P, Okamura K, Mage G. The macrophage stimulating protein/RON system: a potential novel target for prevention and treatment of endometriosis. Molecular Human Reproduction 2005;11(5):345‐9. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2005b {published data only}
- Matsuzaki S, Canis M, Vaurs‐Barriere C, Boespflug‐Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertility and Sterility 2005;84(Suppl 2):1180‐90. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2006b {published data only}
- Matsuzaki S, Canis M, Darcha C, Déchelotte PJ, Pouly JL, Mage G. Expression of WT1 is down‐regulated in eutopic endometrium obtained during the midsecretory phase from patients with endometriosis. Fertility and Sterility 2006;86(3):554‐8. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2009 {published data only}
- Matsuzaki S, Canis M, Darcha C, Pouly JL, Mage G. HOXA‐10 expression in the mid‐secretory endometrium of infertile patients with either endometriosis, uterine fibromas or unexplained infertility. Human Reproduction 2009;24(12):3180‐7. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2010a {published data only}
- Matsuzaki S, Darcha C, Maleysson E, Canis M, Mage G. Impaired down‐regulation of E‐cadherin and beta‐catenin protein expression in endometrial epithelial cells in the mid‐secretory endometrium of infertile patients with endometriosis. Journal of Clinical Endocrinology and Metabolism 2010;95(7):3437‐45. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2010b {published data only}
- Matsuzaki S, Maleysson E, Darcha C. Analysis of matrix metalloproteinase‐7 expression in eutopic and ectopic endometrium samples from patients with different forms of endometriosis. Human Reproduction 2010;25(3):742‐50. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2012 {published data only}
- Matsuzaki S, Darcha C. Adenosine triphosphate‐binding cassette transporter G2 expression inendometriosis and in endometrium from patients with and without endometriosis. Fertility and Sterility 2012;98(6):1512‐20. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2013 {published data only}
- Matsuzaki S, Darcha C. In vitro effects of a small‐molecule antagonist of the Tcf/s‐catenin complex on endometrial and endometriotic cells of patients with endometriosis. PLOS ONE 2013;8(4):e61690. [DOI] [PMC free article] [PubMed] [Google Scholar]
McBean 1993 {published data only}
- McBean JH, Brumsted JR. In vitro CA‐125 secretion by endometrium from women with advanced endometriosis. Fertility and Sterility 1993;59(1):89‐92. [DOI] [PubMed] [Google Scholar]
Mei 2012 {published data only}
- Mei J, Jin LP, Ding D, Li MQ, Li DJ, Zhu XY. Inhibition of IDO1 suppresses cyclooxygenase‐2 and matrix metalloproteinase‐9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Molecular Human Reproduction 2012;18(10):467‐76. [DOI] [PubMed] [Google Scholar]
Mei 2013 {published data only}
- Mei J, Li MQ, Ding D, Li DJ, Jin LP, Hu WG, Suppl 2. Indoleamine 2,3‐dioxygenase‐1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. International Journal of Clinical and Experimental Pathology 2013;6(3):431‐44. [PMC free article] [PubMed] [Google Scholar]
Meola 2010 {published data only}
- Meola J, Rosa e Silva JC, Dentillo DB, Silva WA Jr, Veiga‐Castelli LC, Bernardes LA, et al. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertility and Sterility 2010;93(6):1750‐73. [DOI] [PubMed] [Google Scholar]
Meola 2013a {published data only}
- Meola J, Dentillo DB, Rosa e Silva JC, Hidalgo GdS, Paz CCPd, Ferriani RA. RHOC: a key gene for endometriosis. Reproductive Sciences 2013;20(8):998‐1002. [DOI] [PubMed] [Google Scholar]
Meresman 2000 {published data only}
- Meresman GF, Vighi S, Buquet RA, Contreras‐Ortiz O, Tesone M, Rumi LS. Apoptosis and expression of Bcl‐2 and Bax in eutopic endometrium from women with endometriosis. Fertility and Sterility 2000;74(4):760‐6. [DOI] [PubMed] [Google Scholar]
Meresman 2002 {published data only}
- Meresman GF, Auge L, Baraao RI, Lombardi E, Tesone M, Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertility and Sterility 2002;77(6):1141‐7. [DOI] [PubMed] [Google Scholar]
Mettler 1996 {published data only}
- Mettler L, Volkov NI, Kulakov VI, Jurgensen A, Parwaresch MR. Lymphocyte subsets in the endometrium of patients with endometriosis throughout the menstrual cycle. American Journal of Reproductive Immunology 1996;36(6):342‐8. [DOI] [PubMed] [Google Scholar]
Mettler 1997 {published data only}
- Mettler L, Jurgensen A, Volkov NI, Kulakov V, Parwaresch MR. Immune histochemical profile of endometrium in patients with genital endometriosis. Diagnostic and Therapeutic Endoscopy 1997;3(3):127‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mihalich 2003 {published data only}
- Mihalich A, Reina M, Mangioni S, Ponti E, Alberti L, Vigano P, et al. Different basic fibroblast growth factor and fibroblast growth factor‐antisense expression in eutopic endometrial stromal cells derived from women with and without endometriosis. Journal of Clinical Endocrinology and Metabolism 2003;88(6):2853‐9. [DOI] [PubMed] [Google Scholar]
Mikolajczyk 2007 {published data only}
- Mikolajczyk M, Wirstlein P, Skrzypczak J. The impact of leukemia inhibitory factor in uterine flushing on the reproductive potential of infertile women‐‐a prospective study. American Journal of Reproductive Immunology 2007;58(1):65‐74. [DOI] [PubMed] [Google Scholar]
Mikolajczyk 2009 {published data only}
- Mikolajczyk M, Wirstlein P, Skrzypczak J. Lack of varied endometrial expression of proprotein convertase 6 in infertile women with minimal grade endometriosis and idiopathic infertility [Brak ró˝nic w ekspresji konwertazy proproteinowej 6u kobiet niepΠodnych z endometriozà minimalnego stopniai u kobiet z niepΠodnoÊcià idiopatycznà]. Ginekologia Polska 2009;80(6):414‐8. [PubMed] [Google Scholar]
Minina 1989 {published data only}
- Minina LS, Volkov NI, Novikov EA, Alekseeva ML. Estrogen‐ and progesterone receptor systems in the endometrium in patients with "minor" forms of endometriosis [Estrogen‐ i progesteron‐retseptornye sistemy v endometrii bol'nykh s "malymi" formami endometrioza]. Akusherstvo i ginekologiia 1989;2:71‐3. Russian. [PubMed] [Google Scholar]
Morsch 2009 {published data only}
- Morsch DM, Carneiro MM, Lecke SB, Araújo FC, Camargos AF, Reis FM, et al. C‐fos gene and protein expression in pelvic endometriosis: a local marker of estrogen action. Journal of Molecular Histology 2009;40(1):53‐8. [DOI] [PubMed] [Google Scholar]
Mu 2008 {published data only}
- Mu L, Zheng W, Wang L, Chen XJ, Zhang X, Yang JH. Alteration of focal adhesion kinase expression in eutopic endometrium of women with endometriosis. Fertility and Sterility 2008;89(3):529‐37. [DOI] [PubMed] [Google Scholar]
Newman 2013 {published data only}
- Newman TA, Bailey JL, Stocker LJ, Woo YL, Macklon NS, Cheong YC. Expression of neuronal markers in the endometrium of women with and those without endometriosis. Human Reproduction 2013;28(9):2502‐10. [DOI] [PubMed] [Google Scholar]
Nikoo 2014 {published data only}
- Nikoo S, Ebtekar M, Jeddi‐Tehrani M, Shervin A, Bozorgmehr M, Vafaei S, et al. Menstrual blood‐derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Molecular Human Reproduction 2014;20(9):905‐18. [DOI] [PubMed] [Google Scholar]
Noble 1996 {published data only}
- Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. Journal of Clinical Endocrinology & Metabolism 1996;81(1):174‐9. [DOI] [PubMed] [Google Scholar]
Nomiyama 1997 {published data only}
- Nomiyama M, Hachisuga T, Sou H, Nakamura K, Matsumoto Y, Iwasaka T, et al. Local immune response in infertile patients with minimal endometriosis. Gynecologic & Obstetric Investigation 1997;44(1):32‐7. [DOI] [PubMed] [Google Scholar]
Novella‐Maestre 2010 {published data only}
- Novella‐Maestre E, Carda C, Ruiz‐Sauri A, Garcia‐Velasco JA, Simon C, Pellicer A. Identification and quantification of dopamine receptor 2 in human eutopic and ectopic endometrium: a novel molecular target for endometriosis therapy. Biology of Reproduction 2010;83(5):866‐73. [DOI] [PubMed] [Google Scholar]
Ordi 2003 {published data only}
- Ordi J, Creus M, Casamitjana R, Cardesa A, Vanrell JA, Balasch J. Endometrial pinopode and alphavbeta3 integrin expression is not impaired in infertile patients with endometriosis. Journal of Assisted Reproduction and Genetics 2003;20(11):465‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ota 1996 {published data only}
- Ota H, Igarashi S, Tanaka T. Expression of gamma delta T cells and adhesion molecules in endometriotic tissue in patients with endometriosis and adenomyosis. American Journal of Reproductive Immunology 1996;35(5):477‐82. [DOI] [PubMed] [Google Scholar]
Ota 1997a {published data only}
- Ota H, Igarashi S, Hatazawa J, Tanaka T. Distribution of heat shock proteins in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertility and Sterility 1997;68(1):23‐8. [DOI] [PubMed] [Google Scholar]
Ota 1997b {published data only}
- Ota H, Tanaka T. Integrin adhesion molecules in the endometrial glandular epithelium in patients with endometriosis or adenomyosis. Journal of Obstetrics and Gynaecology Research 1997;23(5):485‐91. [DOI] [PubMed] [Google Scholar]
Ota 2000 {published data only}
- Ota H, Igarashi S, Kato N, Tanaka T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertility and Sterility 2000;74(2):313‐8. [DOI] [PubMed] [Google Scholar]
Ota 2001a {published data only}
- Ota H, Igarashi S, Tanaka T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertility and Sterility 2001;75(4):785‐90. [DOI] [PubMed] [Google Scholar]
Ota 2001b {published data only}
- Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase‐2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Human Reproduction 2001;16(3):561‐6. [DOI] [PubMed] [Google Scholar]
Ota 2002 {published data only}
- Ota H, Igarashi S, Sato N, Tanaka H, Tanaka T. Involvement of catalase in the endometrium of patients with endometriosis and adenomyosis. Fertility and Sterility 2002;78(4):804‐9. [DOI] [PubMed] [Google Scholar]
Pabona 2012 {published data only}
- Pabona JMP, Simmen FA, Nikiforov MA, Zhuang DZ, Shankar K, Velarde MC, et al. Krüppel‐like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. Journal of Clinical Endocrinology and Metabolism 2012;97(3):E376–E392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2007 {published data only}
- Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro‐RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Molecular Human Reproduction 2007;13(11):797‐806. [DOI] [PubMed] [Google Scholar]
Pan 2008 {published data only}
- Pan H, Sheng JZ, Tang L, Zhu R, Zhou TH, Huang HF. Increased expression of c‐fos protein associated with increased matrix metalloproteinase‐9 protein expression in the endometrium of endometriotic patients. Fertility and Sterility 2008;90(4):1000‐7. [DOI] [PubMed] [Google Scholar]
Pan 2009 {published data only}
- Pan XY, Li X, Weng ZP, Wang B. Altered expression of claudin‐3 and claudin‐4 in ectopic endometrium of women with endometriosis. Fertility and Sterility 2009;91(5):1692‐9. [DOI] [PubMed] [Google Scholar]
Park 2009 {published data only}
- Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertility and Sterility 2009;92(4):1246‐9. [DOI] [PubMed] [Google Scholar]
Pellegrini 2012 {published data only}
- Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, et al. The expression of estrogen receptors as well as GREB1, c‐MYC, and cyclin D1, estrogen‐regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertility and Sterility 2012;98(5):1200‐8. [DOI] [PubMed] [Google Scholar]
Penna 2008 {published data only}
- Penna I, Du H, Ferriani R, Taylor HS. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Molecular Human Reproduction 2008;14(10):613‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petracco 2011 {published data only}
- Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. Journal of Clinical Endocrinology and Metabolism 2011;96(12):E1925‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pillai 1996 {published data only}
- Pillai S, Zhou GX, Arnaud P, Jiang HX, Butler WJ, Zhang HM. Antibodies to endometrial transferrin and alpha 2‐Heremans Schmidt (HS) glycoprotein in patients with endometriosis. American Journal of Reproductive Immunology 1996;35(5):483‐94. [DOI] [PubMed] [Google Scholar]
Plante 2012 {published data only}
- Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, et al. G protein‐coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reproductive Sciences 2012;19(7):684‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ponce 2009 {published data only}
- Ponce C, Torres M, Galleguillos C, Sovino H, Boric MA, Fuentes A, et al. Nuclear factor kappaB pathway and interleukin‐6 are affected in eutopic endometrium of women with endometriosis. Reproduction 2009;137(4):727‐37. [DOI] [PubMed] [Google Scholar]
Prentice 1992 {published data only}
- Prentice A, Thomas EJ, Weddell A, McGill A, Randall BJ, Horne CHW. Epidermal growth factor receptor expression in normal endometrium and endometriosis: an immunohistochemical study. British Journal of Obstetrics and Gynaecology 1992;99(5):395‐8. [DOI] [PubMed] [Google Scholar]
Rai 1996 {published data only}
- Rai V, Hopkisson J, Kennedy S, Bergqvist A, Barlow DH, Mardon HJ. Integrins alpha 3 and alpha 6 are differentially expressed in endometrium and endometriosis. Journal of Pathology 1996;180(2):181‐7. [DOI] [PubMed] [Google Scholar]
Rai 2010 {published data only}
- Rai P, Kota V, Deendayal M, Shivaji S. Differential proteome profiling of eutopic endometrium from women with endometriosis to understand etiology of endometriosis. Journal of Proteome Research 2010;9(9):4407‐19. [DOI] [PubMed] [Google Scholar]
Ramon 2005 {published data only}
- Ramon L, Gilabert‐Estelles J, Castello R, Gilabert J, España F, Romeu A, et al. mRNA analysis of several components of the plasminogen activator and matrix metalloproteinase systems in endometriosis using a real‐time quantitative RT‐PCR assay. Human Reproduction 2005;20(1):272‐8. [DOI] [PubMed] [Google Scholar]
Ramon 2008 {published data only}
- Ramón LA, Gilabert–Estellés J, Cosín R, Gilabert J, España F, Castelló R, et al. Plasminogen activator inhibitor‐1 (PAI‐1) 4G/5G polymorphism and endometriosis. Influence of PAI‐1 polymorphism on PAI‐1 antigen and mRNA expression. Thrombosis Research 2008;122(6):854‐60. [DOI] [PubMed] [Google Scholar]
Rey 1998 {published data only}
- Rey J, Pujol P, Dechaud H, Edouard E, Hedon B, Maudelonde T. Expression of oestrogen receptor‐alpha splicing variants and oestrogen receptor‐beta in endometrium of infertile patients. Molecular Human Reproduction 1998;4(7):641‐7. [DOI] [PubMed] [Google Scholar]
Rocha 2011 {published data only}
- Rocha AL, Carrarelli P, Novembri R, Sabbioni L, Luisi S, Reis FM, et al. Altered expression of activin, cripto, and follistatin in the endometrium of women with endometrioma. Fertility and Sterility 2011;95(7):2241‐6. [DOI] [PubMed] [Google Scholar]
Rocha 2012 {published data only}
- Rocha ALL, Carrarelli P, Novembri R, Pascalis F, Luisi S, Reis FM, et al. Activin A stimulates interleukin 8 and vascular endothelial growth factor release from cultured human endometrial stromal cells: possible implications for the pathogenesis of endometriosis. Reproductive Sciences 2012;19(8):832‐8. [DOI] [PubMed] [Google Scholar]
Rombauts 2006 {published data only}
- Rombauts L, Donoghue J, Cann L, Jones RL, Healy DL. Activin‐A secretion is increased in the eutopic endometrium from women with endometriosis. Australian and New Zealand Journal of Obstetrics and Gynaecology 2006;46(2):148‐53. [DOI] [PubMed] [Google Scholar]
Ruan 2010 {published data only}
- Ruan F, Lin J, Wu RJ, Xu KH, Zhang XM, Zhou CY, Huang, XF. Phosphatase of regenerating liver‐3: a novel and promising marker in human endometriosis. Fertility and Sterility 2010;94(6):1980‐4. [DOI] [PubMed] [Google Scholar]
Ruan 2013 {published data only}
- Ruan Y, Qian WP, Zhang CH, Zhou L, Hou ZH. Study on microRNA expression in endometrium of luteal phase and its relationship with infertility of endometriosis. Zhonghua Fuchanke Zazhi [Chinese Journal of Obstetrics and Gynecology] 2013;48(12):907‐10. Chinese. [PubMed] [Google Scholar]
Saracoglu 1985 {published data only}
- Saracoglu OF, Aksel S, Yeoman RR, Wiebe RH. Endometrial estradiol and progesterone receptors in patients with luteal phase defects and endometriosis. Fertility and Sterility 1985;43(6):851‐5. [DOI] [PubMed] [Google Scholar]
Sbracia 1997 {published data only}
- Sbracia M, Zupi E, Alo P, Manna C, Marconi D, Scarpellini F, et al. Differential expression of IGF‐I and IGF‐II in eutopic and ectopic endometria of women with endometriosis and in women without endometriosis. American Journal of Reproductive Immunology 1997;37(4):326‐9. [DOI] [PubMed] [Google Scholar]
Schor 2009 {published data only}
- Schor E, Silva ID, Sato H, Baracat EC, Girao MJ, Freitas V. P27Kip1 is down‐regulated in the endometrium of women with endometriosis. Fertility and Sterility 2009;91(3):682‐6. [DOI] [PubMed] [Google Scholar]
Schulke 2009 {published data only}
- Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Human Reproduction 2009;24(7):1695‐703. [DOI] [PubMed] [Google Scholar]
Schutt 2015 {published data only}
- Schutt AK, Atkins KA, Slack‐Davis JK, Stovall DW. VCAM‐1 on peritoneum and alpha4beta1 integrin in endometrium and their implications in endometriosis. International Journal of Gynecological Pathology 2015;34(1):85‐9. [DOI] [PubMed] [Google Scholar]
Seo 2010a {published data only}
- Seo SK, Nam A, Jeon YE, Cho S, Choi YS, Lee BS. Expression and possible role of non‐steroidal anti‐inflammatory drug‐activated gene‐1 (NAG‐1) in the human endometrium and endometriosis. Human Reproduction 2010;25(12):3043‐9. [DOI] [PubMed] [Google Scholar]
Seo 2010b {published data only}
- Seo SK, Yang HI, Lee KE, Kim HY, Cho S, Choi YS, et al. The roles of thioredoxin and thioredoxin‐binding protein‐2 in endometriosis. Human Reproduction 2010;25(5):1251‐8. [DOI] [PubMed] [Google Scholar]
Sha 2009 {published data only}
- Sha G, Zhang Y, Zhang C, Wan Y, Zhao Z, Li C, et al. Elevated levels of gremlin‐1 in eutopic endometrium and peripheral serum in patients with endometriosis. Fertility and Sterility 2009;91(2):350‐8. [DOI] [PubMed] [Google Scholar]
Sharpe‐Timms 1994 {published data only}
- Sharpe‐Timms KL, Bruno PL, Penney LL, Bickel JT. Immunohistochemical localization of granulocyte‐macrophage colony‐stimulating factor in matched endometriosis and endometrial tissues. American Journal of Obstetrics and Gynecology 1994;171(3):740‐5. [DOI] [PubMed] [Google Scholar]
Sharpe Timms 2000 {published data only}
- Sharpe‐Timms KL, Ricke EA, Piva M, Horowitz GM. Dfifferential expression and localization of de‐novo synthesized endometriotic haptoglobin in endometrium and endometriotic lesions. Human Reproduction 2000;15(10):2180‐5. [DOI] [PubMed] [Google Scholar]
Shen 2012 {published data only}
- Shen L, Liu P, Zhang P, Zhang X, Cui J. Characterization of periostin expression in human endometrium and endometriotic lesions. Gynecological Endocrinology 2012;28(10):815‐8. [DOI] [PubMed] [Google Scholar]
Shen 2013 {published data only}
- Shen LC, Yang SY, Huang W, Xu WM, Wang QS, Song Y, et al. MicroRNA23a and MicroRNA23b deregulation derepresses SF‐1 and upregulates estrogen signaling in ovarian endometriosis. Journal of Clinical Endocrinology and Metabolism 2013;98(4):1575‐82. [DOI] [PubMed] [Google Scholar]
Sherwin 2008 {published data only}
- Sherwin JR, Sharkey AM, Mihalyi A, Simsa P, Catalano RD, D'Hooghe TM. Global gene analysis of late secretory phase, eutopic endometrium does not provide the basis for a minimally invasive test of endometriosis. Human Reproduction 2008;23(5):1063‐8. [DOI] [PubMed] [Google Scholar]
Shi 2014a {published data only}
- Shi XY, Gu L, Chen J, Guo XR, Shi YL. Downregulation of miR‐183 inhibits apoptosis and enhances the invasive potential of endometrial stromal cells in endometriosis. International Journal of Molecular Medicine 2014;33(1):59‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2014b {published data only}
- Shi X, Xu W, Dai HH, Sun Y, Wang XL. The role of SRC1 and SRC2 in steroid‐induced SDF1 expression in normal and ectopic endometrium. Reproduction 2014;147(6):847‐53. [DOI] [PubMed] [Google Scholar]
Silveira 2012 {published data only}
- Silveira CGT, Krampe J, Ruhland B, Diedrich K, Hornung D, Agic A. Cold‐shock domain family member YB‐1 expression in endometrium and endometriosis. Human Reproduction 2012;27(1):173‐82. [DOI] [PubMed] [Google Scholar]
Stephens 2010 {published data only}
- Stephens AN, Hannan NJ, Rainczuk A, Meehan KL, Chen J, Nicholls PK, et al. Post‐translational modifications and protein‐specific isoforms in endometriosis revealed by 2D DIGE. Journal of Proteome Research 2010;9(5):2438‐49. [DOI] [PubMed] [Google Scholar]
Sun 2002 {published data only}
- Sun WS, Misao R, Iwagaki S, Fujimoto J, Tamaya T. Coexpression of growth arrest‐specific gene 6 and receptor tyrosine kinases, Axl and Sky, in human uterine endometrium and ovarian endometriosis. Molecular Human Reproduction 2002;8(6):552‐8. [DOI] [PubMed] [Google Scholar]
Szczepanska 2007 {published data only}
- Szczepanska M, Wirstlein P, Skrzypczak J. Evaluation of HOXA group gene expression in endometrium of women with endometriosis [Ocena ekspresji genow z grupy HOXA w endometrium kobiet z endometrioza]. Przeglad Menopauzalny 2007;6(5):266‐71. [Google Scholar]
Szczepanska 2010a {published data only}
- Szczepanska M, Wirstlein P, Luczak M, Jagodzinski PP, Skrzypczak J. Reduced expression of HOXA10 in the midluteal endometrium from infertile women with minimal endometriosis. Biomedicine and Pharmacotherapy 2010;64(10):697‐705. [DOI] [PubMed] [Google Scholar]
Szczepanska 2010b {published data only}
- Szczepanska M, Wirstlein P, Skrzypczak J. HOXA11 gene expression in women with and without impaired infertility [Ekspresja genu HOXA11 w endometrium kobiet bez i z zaburzeniami plodnosci]. Ginekologia Polska 2010;81(6):414‐21. [PubMed] [Google Scholar]
Szymanowski 2007 {published data only}
- Szymanowski K. Apoptosis pattern in human endometrium in women with pelvic endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;132(1):107‐10. [DOI] [PubMed] [Google Scholar]
Szymanowski 2008 {published data only}
- Szymanowski K, Mikolajczyk M, Wirstlein P, Dera A. Matrix metalloproteinase‐2 (MMP‐2), MMP‐9, tissue inhibitor of matrix metalloproteinases (TIMP‐1) and transforming growth factor‐beta2 (TGF‐beta2) expression in eutopic endometrium of women with peritoneal endometriosis. International Journal of Fertility and Women's Medicine 2008;53(6):197‐205. [DOI] [PubMed] [Google Scholar]
Takahashi 1988a {published data only}
- Takahashi K, Nagata H, Uchida A, Kusakari M, Araki Y, Nishigaki A, et al. Menstrual Ca125 as a marker for patients with endometriosis: a preliminary report. Japanese Journal of Fertility and Sterility 1988;33(3):585‐8. [Google Scholar]
Takahashi 1988b {published data only}
- Takahashi K, Nagata H, Kijima S, Kusakari M, Shirai T, Yoshino K, Kitao M. Clinical usefulness of determination of CA 125 levels in the serum and menstrual blood. Gynecologic and Obstetric Investigation 1988;26(1):63‐5. [DOI] [PubMed] [Google Scholar]
Takahashi 1989 {published data only}
- Takahashi K, Nagata H, Kitao M. Clinical usefulness of determination of estradiol level in the menstrual blood for patients with endometriosis. Nihon Sanka Fujinka Gakkai Zasshi 1989;41(11):1849‐50. Japanese. [PubMed] [Google Scholar]
Takahashi 1991 {published data only}
- Takahashi K, Nagata H, Kitao M. Ca125 in the menstrual blood is an effective marker for diagnosing early stage endometriosis: a preliminary report. Japanese Journal of Fertility and Sterility 1991;36(2):356‐9. [Google Scholar]
Takehara 2004 {published data only}
- Takehara M, Ueda M, Yamashita Y, Terai Y, Hung YC, Ueki M. Vascular endothelial growth factor A and C gene expression in endometriosis. Human Pathology 2004;35(11):1369‐75. [DOI] [PubMed] [Google Scholar]
Tan 2001 {published data only}
- Tan X, Lang J, Liu D. Expression of monocyte chemotactic protein‐1 in the eutopic endometrium of women with endometriosis. Zhonghua Fuchanke Zazhi [Chinese Journal of Obstetrics and Gynecology] 2001;36(2):89‐91. Chinese. [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan XJ, Lang JH, Liu DY, Shen K, Leng JH, Zhu L. Expression of vascular endothelial growth factor and thrombospondin‐1 mRNA in patients with endometriosis. Fertility and Sterility 2002;78(1):148‐53. [DOI] [PubMed] [Google Scholar]
Ten Have 2007 {published data only}
- Have S, Fraser I, Markham R, Lam A, Matsumoto I. Proteomic analysis of protein expression in the eutopic endometrium of women with endometriosis. Proteomics. Clinical Applications 2007;1(10):1243‐51. [DOI] [PubMed] [Google Scholar]
Tokushige 2006 {published data only}
- Tokushige N, Markham R, Russell P, Fraser IS. High density of small nerve fibres in the functional layer of the endometrium in women with endometriosis. Human Reproduction 2006;21(3):782‐7. [DOI] [PubMed] [Google Scholar]
Tokushige 2007 {published data only}
- Tokushige N, Markham R, Russell P, Fraser IS. Different types of small nerve fibers in eutopic endometrium and myometrium in women with endometriosis. Fertility and Sterility 2007;88(4):795‐803. [DOI] [PubMed] [Google Scholar]
Torres 2009 {published data only}
- Torres PB, Florio P, Galleri L, Reis FM, Borges LE, Petraglia F. Activin A, activin receptor type II, nodal, and cripto mRNA are expressed by eutopic and ectopic endometrium in women with ovarian endometriosis. Reproductive Sciences 2009;16(8):727‐33. [DOI] [PubMed] [Google Scholar]
Trio 2007 {published data only}
- Trio C, Calienno C, Monti B, Varisco E, Cortese M, Ieda N. Levels of CCL2 and VEGF in pathological tissue and eutopic endometrium of women with ovarian endometriosis [Livelli di CCL2 e VEGF nel tessuto patologico e nell'endometrio eutopico nelle donne con endometriosi ovarica]. Italian Journal of Gynaecology and Obstetrics 2007;19(4):188‐92. [Google Scholar]
Tshishi 2010 {published data only}
- Tshishi TA, Tozin R, Yanga JJ, Bamoleke S, Philomène K. Immunoradiometric assay for alpha V beta 3 integrin used as a diagnosis tool in case of endometriosis [Dosage immunoradiométrique de l’intégrine alpha V bêta 3 dans le diagnostic d’infertilité due à l’endométriose]. Immuno‐analyse & Biologie Spécialisée 2010;25(3):159‐61. [Google Scholar]
Ulukus 2005 {published data only}
- Ulukus M, Ulukus EC, Seval Y, Zheng W, Arici A. Expression of interleukin‐8 receptors in endometriosis. Human Reproduction 2005;20(3):794‐801. [DOI] [PubMed] [Google Scholar]
Ulukus 2009 {published data only}
- Ulukus M, Ulukus EC, Tavmergen Goker EN, Tavmergen E, Zheng W, Arici A. Expression of interleukin‐8 and monocyte chemotactic protein 1 in women with endometriosis. Fertility and Sterility 2009;91(3):687‐93. [DOI] [PubMed] [Google Scholar]
Uz 2011 {published data only}
- Uz YH, Murk W, Bozkurt I, Kizilay G, Arici A, Kayisli UA. Increased c‐Jun N‐terminal kinase activation in human endometriotic endothelial cells. Histochemistry and Cell Biology 2011;135(1):83‐91. [DOI] [PubMed] [Google Scholar]
Uzan 2004 {published data only}
- Uzan C, Cortez A, Dufournet C, Fauvet R, Siffroi JP, Darai E. Eutopic endometrium and peritoneal, ovarian and bowel endometriotic tissues express a different profile of matrix metalloproteinases‐2, ‐3 and ‐11, and of tissue inhibitor metalloproteinases‐1 and ‐2. Virchows Archiv 2004;445(6):603‐9. [DOI] [PubMed] [Google Scholar]
Uzan 2005 {published data only}
- Uzan C, Cortez A, Dufournet C, Fauvet R, Siffroi JP, Darai E. Endometrium from women with and without endometriosis, and peritoneal, ovarian and bowel endometriosis, show different c‐kit protein expression. Journal of Reproductive Immunology 2005;65(1):55‐63. [DOI] [PubMed] [Google Scholar]
Velasco 2006 {published data only}
- Velasco I, Rueda J, Acien P. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Molecular Human Reproduction 2006;12(6):377‐81. [DOI] [PubMed] [Google Scholar]
Vergetaki 2013 {published data only}
- Vergetaki A, Jeschke U, Vrekoussis T, Taliouri E, Sabatini L, Papakonstanti EA, et al. Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis. PLOS ONE 2013;8(4):e62313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernet‐Tomas 2006b {published data only}
- Vernet‐Tomas Mdel M, Perez‐Ares CT, Verdu N, Molinero JL, Fernandez‐Figueras MT, Carreras R. The endometria of patients with endometriosis show higher expression of class I human leukocyte antigen than the endometria of healthy women. Fertility and Sterility 2006;85(1):78‐83. [DOI] [PubMed] [Google Scholar]
Vestergaard 2011 {published data only}
- Vestergaard AL, Knudsen UB, Munk T, Rosbach H, Martensen PM. Transcriptional expression of type‐I interferon response genes and stability of housekeeping genes in the human endometrium and endometriosis. Molecular Human Reproduction 2011;17(4):243‐54. [DOI] [PubMed] [Google Scholar]
Wang 2005a {published data only}
- Wang F, He YL, Peng DX, Liu MB. Expressions of nuclear factor‐kappaB and intercellular adhesion molecule‐1 in endometriosis. Di 1 Jun Yi Da Xue Xue Bao [Academic journal of the first medical college of PLA] 2005;25(6):703‐5. Chinese. [PubMed] [Google Scholar]
Wang 2005b {published data only}
- Wang H, Lang J, Leng J, Zhu L, Liu Z, Sun D. Expression of vascular endothelial growth factor receptors in the ectopic and eutopic endometrium of women with endometriosis. Zhonghua Yixue Zazhi [Chinese Medical Journal] 2005;85(22):1555‐9. Chinese. [PubMed] [Google Scholar]
Wang 2010b {published data only}
- Wang G, Tokushige N, Russell P, Dubinovsky S, Markham R, Fraser IS. Neuroendocrine cells in eutopic endometrium of women with endometriosis. Human Reproduction 2010;25(2):387‐91. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang DB, Chen Q, Zhang CY, Ren F, Li T. DNA hypomethylation of the COX‐2 gene promoter is associated with up‐regulation of its mRNA expression in eutopic endometrium of endometriosis. European Journal of Medical Research 2012;17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2009 {published data only}
- Wei Q, Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertility and Sterility 2009;91(5):1686‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2013 {published data only}
- Wei WD, Ruan F, Tu FX, Zhou CY, Lin J. Expression of suppressor of cytokine signaling‐3 and caspase‐3 in endometriosis and their correlation. Zhonghua Binglixue Zazhi [Chinese Journal of Pathology] 2013;42(8):515‐8. Chinese. [PubMed] [Google Scholar]
Wenzl 1998 {published data only}
- Wenzl RJ, Heinzl H. Localization of matrix metalloproteinase‐2 in uterine endometrium and ectopic implants. Gynecologic and Obstetric Investigation 1998;45(4):253‐7. [DOI] [PubMed] [Google Scholar]
Wolun‐Cholewa 2011 {published data only}
- Wolun‐Cholewa M, Szymanowski K, Nowak‐Markwitz E, Warchol W. Photodiagnosis and photodynamic therapy of endometriotic epithelial cells using 5‐aminolevulinic acid and steroids. Photodiagnosis and Photodynamic Therapy 2011;8(1):58‐63. [DOI] [PubMed] [Google Scholar]
Wu 1999 {published data only}
- Wu J, Zhang J, Liu Y. Expression of integrin beta 3 and intracellular adhesion molecule in endometrium and endometriotic tissue. Zhonghua Fuchanke Zazhi [Chinese Journal of Obstetrics and Gynecology] 1999;34(4):204‐6. [PubMed] [Google Scholar]
Wu 2003 {published data only}
- Wu MY, Chao KH, Yang JH, Lee TH, Yang YS, Ho HN. Nitric oxide synthesis is increased in the endometrial tissue of women with endometriosis. Human Reproduction 2003;18(12):2668‐71. [DOI] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertility and Sterility 2007;87(1):24‐32. [DOI] [PubMed] [Google Scholar]
Xu 2010 {published data only}
- Xu YL, Wang DB, Liu QF, Chen YH, Yang Z. Silencing of cofilin‐1 gene attenuates biological behaviours of stromal cells derived from eutopic endometria of women with endometriosis. Human Reproduction 2010;25(10):2480‐8. [DOI] [PubMed] [Google Scholar]
Yi 2003 {published data only}
- Yi L, Liqun L, Guijin Z. Anginogenesis of eutopic and ectopic endometria in endometriosis. Huazhong Keji Daxue Xuebao [Journal of Huazhong University of Science and Technology] 2003;23(2):190‐1. Chinese. [DOI] [PubMed] [Google Scholar]
Yin 2006 {published data only}
- Yin L, Sun J, Ma H, Mi S, Guo S, Shi Y. Expression of interleukin1‐alpha, beta and interferon‐gamma in macrophages from endometrium of women with endometriosis. Zhonghua Fuchanke Zazhi [Chinese Journal of Obstetrics and Gynecology] 2006;41(5):295‐8. Chinese. [PubMed] [Google Scholar]
Yoo 2014 {published data only}
- Yoo JY, Shin H, Kim TH, Choi WS, Ferguson SD, Fazleabas AT, et al. CRISPLD2 is a target of progesterone receptor and its expression is decreased in women with endometriosis. PLOS ONE 2014;9(6):e100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2012 {published data only}
- Zeng B, Hu J, Yuan R, Hu L, Zhong L, Kang K. Increased expression of importin13 in endometriosis and endometrial carcinoma. Medical Science Monitor 2012;18(6):361‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2005 {published data only}
- Zhang H, Niu YD, Feng J, Guo HF, Ye X. Applciation of two dimensional electrophoresis, western blot and mass spectrum to screen markers of endometriosis. Beijing Daxue Xuebao [Journal of Peking University] 2005;37(4):366‐70. [PubMed] [Google Scholar]
Zhang 2006 {published data only}
- Zhang H, Niu Y, Feng J, Guo H, Ye X, Cui H. Use of proteomic analysis of endometriosis to identify different protein expression in patients with endometriosis versus normal controls. Fertility and Sterility 2006;86(2):274‐82. [DOI] [PubMed] [Google Scholar]
Zhang 2009a {published data only}
- Zhang H, Li M, Zheng X, Sun Y, Wen Z, Zhao X. Endometriotic stromal cells lose the ability to regulate cell‐survival signaling in endometrial epithelial cells in vitro. Molecular Human Reproduction 2009;15(10):653‐63. [DOI] [PubMed] [Google Scholar]
Zhang 2009b {published data only}
- Zhang X, Lu B, Huang X, Xu H, Zhou C, Lin J. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertility and Sterility 2009;92(5):1799‐801. [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang LH, Liu HY, Li HL, Zhang XY, Sun M. Differential proteomic analysis of endometriosis. Chemical Research in Chinese University 2010;26(1):70‐4. [Google Scholar]
Zhang 2011a {published data only}
- Zhang JJ, Xu ZM, Chang H, Zhang CM, Dai HY, Ji XQ, et al. Pyrrolidine dithiocarbamate attenuates nuclear factor‐B activation, cyclooxygenase‐2 expression and prostaglandin E2 production in human endometriotic epithelial cells. Gynecologic and Obstetric Investigation 2011;72(3):163‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2011b {published data only}
- Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ, Wang XF, et al. Pyrrolidine dithiocarbamate inhibits nuclear factor‐B pathway activation, and regulates adhesion, migration, invasion and apoptosis of endometriotic stromal cells. Molecular Human Reproduction 2011;17(3):175‐81. [DOI] [PubMed] [Google Scholar]
Zhao 2014 {published data only}
- Zhao L, Yang H, Xuan Y, Luo Z, Lin Q, Zhao J, et al. Increased expression of fibroblast growth factor receptor 1 in endometriosis and its correlation with endometriosis‐related dysmenorrhea and recurrence. European Journal of Obstetrics Gynecology and Reproductive Biology 2014;184:117‐24. [DOI] [PubMed] [Google Scholar]
Zong 2004 {published data only}
- Zong LL, Li YL, Song ST, Jiang ZF, Zhao J. Expression of hepatocyte growth factor and its receptor c‐met gene in the endometrium of women with endometriosis. Di 1 Jun Yi Da Xue Xue Bao [Academic Journal of the First Medical College of PLA] 2004;24(6):619‐22. Chinese. [PubMed] [Google Scholar]
Additional references
ACOG 2010
- The American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of Endometriosis. Obstetrics and Gynecology 2010;116(1):223‐36. [DOI] [PubMed] [Google Scholar]
Adamson 2008
- Adamson GD. Endometriosis Fertility Index (EFI): the new validated endometriosis staging system [Conference poster 33]. Art and Science of Endometriosis.World Congress on Endometriosis. 2008 Mar 11‐14; Melbourne, Australia. 2008:7.2.
Akoum 1995
- Akoum A, Lemay A, Brunet C, Hébert J. Secretion of monocyte chemotactic protein‐1 by cytokine‐stimulated endometrial cells of women with endometriosis. Le groupe d'investigation en gynécologie. Fertility and Sterility 1995;63(2):322‐8. [DOI] [PubMed] [Google Scholar]
Al Jefout 2009a
- Al Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, Markham R, et al. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Human Reproduction 2009;24(12):3019‐24. [DOI] [PubMed] [Google Scholar]
Al Jefout 2009b
- Al Jefout M, Tokushige N, Hey‐Cunningham AJ, Manconi F, Ng C, Schukle L, et al. Mircoanatomy and function of the ectopic endometrium in women with endometriosis. Expert Review of Obstetrics & Gynecology 2009;4(1):61‐79. [Google Scholar]
Almeida Filho 2008
- Almeida Filho DP, Oliveira LJ, Amaral VF. Accuracy of laparoscopy for assessing patients with endometriosis. Sao Paulo Medical Journal 2008;126(6):305‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
ASRM 1997
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and Sterility 1997;67(5):817‐21. [DOI] [PubMed] [Google Scholar]
Ballard 2008
- Ballard KD, Seaman HE, Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case‐control study ‐ part 1. BJOG: An International Journal of Obstetrics and Gynaecology 2008;115(11):1382‐91. [DOI] [PubMed] [Google Scholar]
Batt 2003
- Batt R, Mitwally MF. Endometriosis from thelarche to midteens: pathogenesis and prognosis, prevention and pedagogy. Journal of Pediatric and Adolescent Gynecology 2003;16(6):333‐47. [DOI] [PubMed] [Google Scholar]
Becker 2014
- Becker CM, Laufer MR, Stratton P, Hummelshoj l, Missmer SA, Zondervan KT, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: I. Surgical phenotype data collection in endometriosis research. Fertility and Sterility 2014;102(5):1213‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bossuyt 2003
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326(7379):41‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bossuyt 2008
- Bossuyt PM, Leeflang MM. Chapter 6: Developing criteria for including studies. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.4 [updated September 2008]. The Cochrane Collaboration, 2008. Available from: http://dta.cochrane.org/handbook‐dta‐reviews. The Cochrane Collaboration.
Brosens 2003
- Brosens J, Timmerman D, Strazinski‐Powitz A, Brosens I. Noninvasive diagnosis of endometriosis: the role of imaging and markers. Obstetrics and Gynecology Clinics of North America 2003;30(1):95‐114. [DOI] [PubMed] [Google Scholar]
Chapron 2003a
- Chapron C, Fauconnier A, Vieira M, Barakat Dousset HB, Pansini V, Vacher‐Lavenu MC, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Human Reproduction 2003;18(1):157‐61. [DOI] [PubMed] [Google Scholar]
Chapron 2003b
- Chapron C, Fauconnier A, Dubuisson JB, Barakat H, Vieira M, Bréart G. Deep infiltrating endometriosis: relation between severity of dysmenorrhea and extent of disease. Human Reproduction 2003;18(4):760‐6. [DOI] [PubMed] [Google Scholar]
Chapron 2003c
- Chapron C, Cravello L, Chopin N, Kreiker G, Blanc B, Dubuisson JB. Complications during set‐up procedures for laparoscopy in gynecology: open laparoscopy does not reduce the risk of major complications. Acta Obstetrica et Gynecologica Scandinavica 2003;82(12):1125‐9. [DOI] [PubMed] [Google Scholar]
Cox 2001
- Cox KE, Piva M, Sharpe‐Timms KL. Differential regulation of matrix metalloproteinase‐3 gene expression in endometriotic lesions compared with endometrium. Biology of Reproduction 2001;65(4):1297‐303. [DOI] [PubMed] [Google Scholar]
D'Hooghe 2001
- D'Hooghe TM, Bambra CS, Xiao L, Peixe K, Hill JA. Effect of menstruation and intrapelvic injection of endometrium on inflammatory parameters of peritoneal fluid in the baboon (Papio anubis and Papio cynocephalus). American Journal of Obstetrics & Gynecology 2001;184(5):917‐25. [DOI] [PubMed] [Google Scholar]
De Vet 2008
- Vet HCW, Eisinga A, Riphagen II, Aertgeerts B, Pewsner D. Chapter 7: Searching for studies. In: Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 0.4 [updated September 2008]. The Cochrane Collaboration, 2008. Available from: http://dta.cochrane.org/handbook‐dta‐reviews. The Cochrane Collaboration.
Dmowski 1997
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertility and Sterility 1997;67(2):238‐43. [DOI] [PubMed] [Google Scholar]
Dunselman 2014
- Dunselman GA, Vermeulen N, Becker C, Calhaz‐Jorge C, D'Hooghe T, Bie B, et al. ESHRE guideline: management of women with endometriosis. Human Reproduction 2014;29(3):400‐12. [DOI] [PubMed] [Google Scholar]
Eskenazi 1997
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstetrics & Gynecology Clinics of North America 1997;24:235‐58. [DOI] [PubMed] [Google Scholar]
Eskenazi 2001
- Eskenazi B, Warner M, Bosignore L, Olive D, Samuels S, Vercellini P. Validation study of nonsurgical diagnosis of endometriosis. Fertility and Sterility 2001;76(5):929‐35. [DOI] [PubMed] [Google Scholar]
Fassbender 2014
- Fassbender A, Rahmioglu N, Vitonis AF, Vigano P, Giudice LC, D’Hooghe TM, et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertility and Sterility 2014;102(5):1244‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fassbender 2015
- Fassbender A, Burney RO, Dorien FO, D'Hooghe T, Giudice L. Update on biomarkers for the detection of endometriosis. Biomed Research International 2015;2015:130854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fauconnier 2005
- Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Human Reproduction Update 2005;11(6):595‐606. [DOI] [PubMed] [Google Scholar]
Fedele 1990
- Fedele L, Marchini M, Bianchi S, Dorta M, Arcaini L, Fontana PE. Structural and ultrastructural defects in preovulatory endometrium of normo‐ovulating infertile women with minimal or mild endometriosis. Fertility and Sterility 1990;53(6):989‐93. [DOI] [PubMed] [Google Scholar]
Frishman 2006
- Frishman GN, Salak JR. Conservative surgical management of endometriosis in women with pelvic pain. Journal of Minimally Invasive Gynecology 2006;13(6):546‐58. [DOI] [PubMed] [Google Scholar]
Gao 2006
- Gao X, Yeh YC, Outley J, Simon J, Botteman M, Spalding J. Health‐related quality of life burden of women with endometriosis: a literature review. Current Medical Research and Opinion 2006;22(9):1787‐97. [DOI] [PubMed] [Google Scholar]
Giudice 2004
- Giudice LC, Kao LC. Endometriosis. The Lancet 2004;364(9447):1789‐99. [DOI] [PubMed] [Google Scholar]
Goldstein 1980
- Goldstein DP, DeCholnoky C, Emans SJ, Leventhal JM. Laparoscopy in the diagnosis and management of pelvic pain in adolescents. Journal of Reproductive Medicine 1980;24:251‐6. [PubMed] [Google Scholar]
Guo 2009
- Guo SW. Recurrence of endometriosis and its control. Human Reproduction Update 2009;15(4):441‐61. [DOI] [PubMed] [Google Scholar]
Guzick 1997
- Guzick DS, Silliman NP, Adamson GD, Buttram VC Jr, Canis M, Malinak LR, et al. Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicines revised classification of endometriosis. Fertility and Sterility 1997;67(5):822‐9. [DOI] [PubMed] [Google Scholar]
Halme 1984
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstetrics and Gynecology 1984;64(2):151‐4. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT. Commentary: Heterogeneity in meta‐analysis should be expected and appropriately quantified [International Journal of Epidemiology]. Oxford Journals 2008;37(7):1158‐60. [DOI] [PubMed] [Google Scholar]
Hull 2008
- Hull ML, Escareno CR, Godsland JM, Doig JR, Johnson CM, Phillips SC, et al. Endometrial‐peritoneal interactions during endometriotic lesion establishment. American Journal of Pathology 2008;173(3):700‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2013
- Johnson NP, Hummelshoj L, World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Human Reproduction 2013;28(6):155‐68. [DOI] [PubMed] [Google Scholar]
Johnson 2015
Jones 2006
- Jones CJ, Denton J, Fazleabas AT. Morphological and glycosylation changes associated with the endometrium and ectopic lesions in a baboon model of endometriosis. Human Reproduction 2006;21(12):3068‐80. [DOI] [PubMed] [Google Scholar]
Kennedy 2005
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, et al. ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Human Reproduction 2005;20(10):2698‐704. [DOI] [PubMed] [Google Scholar]
Kitawaki 1999b
- Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Expression of aromatase cytochrome P450 in eutopic endometrium and its application as a diagnostic test for endometriosis. Gynecologic and Obstetric Investigation 1999;48(Suppl 1):21‐8. [DOI] [PubMed] [Google Scholar]
Koninckx 1991
- Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertility and Sterility 1991;55(4):759‐65. [DOI] [PubMed] [Google Scholar]
Ling 1999
- Ling F. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstetrics and Gynecology 1999;93(1):51‐8. [DOI] [PubMed] [Google Scholar]
Liu 2005b
- Liu A, Schisterman EF, Mazumdar M, Hu J. Power and sample size calculation of comparative diagnostic accuracy studies with multiple correlated test results. Biometrical Journal 2005;47(2):140‐50. [DOI] [PubMed] [Google Scholar]
Marchino 2005
- Marchino GL, Gennarelli G, Enria R, Bongioanni F, Lipari G, Massobrio M. Diagnosis of pelvic endometriosis with use of macroscopic versus histologic findings. Fertility and Sterility 2005;84(1):12‐5. [DOI] [PubMed] [Google Scholar]
Martin 2006
- Martin DC. Applying STARD criteria to the laparoscopic identification of endometriosis [Abstract] . Fertility and Sterility 2006;86(Suppl 2):270. [Google Scholar]
Matsuzaki 2006
- Matsuzaki S, Canis M, Pouly JL, Rabischong B, Botchorishvili R, Mage G. Relationship between delay of surgical diagnosis and severity of disease in patients with symptomatic deep infiltrating endometriosis. Fertility and Sterility 2006;86(5):1314‐6. [DOI] [PubMed] [Google Scholar]
May 2011
- May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Human Reproduction Update 2011;17(5):637‐53. [DOI] [PubMed] [Google Scholar]
McGraw‐Hill Dictionary of Medicine 2006
- Segen JC. McGraw‐Hill Concise Dictionary of Modern Medicine. 2nd Edition. New York: The McGraw‐Hill Companies, Inc, 2006. [Google Scholar]
Medeiros 2009
- Medeiros LR, Rosa DD, Bozzetti MC, Fachel JM, Furness S, Garry R, et al. Laparoscopy versus laparotomy for benign ovarian tumour. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004751.pub3] [DOI] [PubMed] [Google Scholar]
Nyholt 2012
- Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, et al. Genome‐ wide association meta‐ analysis identifies new endometriosis risk loci. Nature Genetics 2012;44(12):1355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redwine 2003
- Redwine DB. 'Invisible' microscopic endometriosis: a review. Gynecologic and Obstetric Investigation 2003;55(2):63‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogers 2009
- Rogers PA, D'Hooghe TM, Fazleabas A, Gargett CE, Giudice LC, Montgomery GW, et al. Priorities for endometriosis research: recommendations from an international consensus workshop. Reproductive Sciences 2009;16(4):335‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutjes 2005
- Rutjes AWS, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PMM. Case–control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51(8):1335‐41. [DOI] [PubMed] [Google Scholar]
Sampson 1927
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. American Journal of Pathology 1927;3(2):93‐110. [PMC free article] [PubMed] [Google Scholar]
Simoens 2012
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis:costs and quality of life of women with endometriosis and treated in referral centres. Human Reproduction 2012;27(5):1292‐9. [DOI] [PubMed] [Google Scholar]
Sinaii 2002
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Human Reproduction 2002;17(10):2715‐24. [DOI] [PubMed] [Google Scholar]
SOGC 2010
- Society of Obstetricians Gynaecologists of Canada. Endometriosis: diagnosis and management. SOGC clinical practice guideline no. 244. Journal of Obstetrics and Gynaecology Canada 2010;32:S1‐S28. [PubMed] [Google Scholar]
Somigliana 2006
- Somigliana E, Vigano P, Parazzini F, Stoppelli S, Giambattista E, Vercellini P. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecologic Oncology 2006;101(2):331‐41. [DOI] [PubMed] [Google Scholar]
Spaczynski 2003
- Spaczynski RZ, Duleba AJ. Diagnosis of endometriosis. Seminars in Reproductive Medicine 2003;21(2):193‐208. [DOI] [PubMed] [Google Scholar]
Stegmann 2008
- Stegmann BJ, Sinaii N, Liu S, Segars J, Merino M, Nieman LK, et al. Using location, color, size, and depth to characterize and identify endometriosis lesions in a cohort of 133 women. Fertility and Sterility 2008;89(6):1632‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
The Gale Encyclopedia of Medicine 2011
- Olendorf D, Jeryan C, Boyden K (editors). The Gale Encyclopedia of Medicine (5 volume set). 4th Edition. Detroit: The Gale Group, Inc, 2011. [Google Scholar]
Vercellini 1996
- Vercellini P, Trespidi L, Giorgi O, Cortesi I, Parazzini F, Crosignani GP. Endometriosis and pelvic pain: relation to disease stage and localization. Fertility and Sterility 1996;65(2):299‐304. [PubMed] [Google Scholar]
Vigano 2004
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Practice and Research. Clinical Obstetrics and Gynaecology 2004;18(2):177‐200. [DOI] [PubMed] [Google Scholar]
Vitonis 2014
- Vitonis AF, Vincent K, Rahmioglu N, Fassbender A, Buck Louis G, Hummelshoj L, et al. World Endometriosis Research Foundation Endometriosis Phenome and biobanking harmonization project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertility and Sterility 2014;102(5):1223‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2005
- Whiting PF, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Medical Research Methodology 2005;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, the QUADAS‐2 Group. QUADAS‐2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Wykes 2004
- Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG ‐ an International Journal of Obstetrics and Gynaecology 2004;111(11):1204‐12. [DOI] [PubMed] [Google Scholar]
Yeung 2009
- Yeung PP Jr, Shwayder J, Pasic RP. Laparoscopic management of endometriosis: comprehensive review of best evidence. Journal of Minimally Invasive Gynecology 2009;16(3):269‐81. [DOI] [PubMed] [Google Scholar]


