Abstract
Background
Chest physiotherapy is widely prescribed to assist the clearance of airway secretions in people with cystic fibrosis (CF). Positive expiratory pressure (PEP) devices provide back pressure to the airways during expiration. This may improve clearance by building up gas behind mucus via collateral ventilation and by temporarily increasing functional residual capacity. The developers of the PEP technique recommend using PEP with a mask in order to avoid air leaks via the upper airways and mouth. In addition, increasing forced residual capacity (FRC) has not been demonstrated using mouthpiece PEP. Given the widespread use of PEP devices, there is a need to determine the evidence for their effect. This is an update of a previously published review.
Objectives
To determine the effectiveness and acceptability of PEP devices compared to other forms of physiotherapy as a means of improving mucus clearance and other outcomes in people with CF.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising of references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. The electronic database CINAHL was also searched from 1982 to 2017.
Most recent search of the Group's CF Trials Register: 20 February 2019.
Selection criteria
Randomised controlled studies in which PEP was compared with any other form of physiotherapy in people with CF. This included, postural drainage and percussion (PDPV), active cycle of breathing techniques (ACBT), oscillating PEP devices, thoracic oscillating devices, bilevel positive airway pressure (BiPaP) and exercise.
Data collection and analysis
Three authors independently applied the inclusion and exclusion criteria to publications, assessed the risk of bias of the included studies and assessed the quality of the evidence using the GRADE recommendations.
Main results
A total of 28 studies (involving 788 children and adults) were included in the review; 18 studies involving 296 participants were cross‐over in design. Data were not published in sufficient detail in most of these studies to perform any meta‐analysis. In 22 of the 28 studies the PEP technique was performed using a mask, in three of the studies a mouthpiece was used with nose clips and in three studies it was unclear whether a mask or mouthpiece was used. These studies compared PEP to ACBT, autogenic drainage (AD), oral oscillating PEP devices, high‐frequency chest wall oscillation (HFCWO) and BiPaP and exercise.
Forced expiratory volume in one second was the review's primary outcome and the most frequently reported outcome in the studies (24 studies, 716 participants). Single interventions or series of treatments that continued for up to three months demonstrated little or no difference in effect between PEP and other methods of airway clearance on this outcome (low‐ to moderate‐quality evidence). However, long‐term studies had equivocal or conflicting results regarding the effect on this outcome (low‐ to moderate‐quality evidence).
A second primary outcome was the number of respiratory exacerbations. There was a lower exacerbation rate in participants using PEP compared to other techniques when used with a mask for at least one year (five studies, 232 participants; moderate‐ to high‐quality evidence). In one of the included studies which used PEP with a mouthpiece, it was reported (personal communication) that there was no difference in the number of respiratory exacerbations (66 participants, low‐quality evidence).
Participant preference was reported in 10 studies; and in all studies with an intervention period of at least one month, this was in favour of PEP. The results for the remaining outcome measures (including our third primary outcome of mucus clearance) were not examined or reported in sufficient detail to provide any high‐quality evidence; only very low‐ to moderate‐quality evidence was available for other outcomes. There was limited evidence reported on adverse events; these were measured in five studies, two of which found no events. In a study where infants performing either PEP or PDPV experienced some gastro‐oesophageal reflux , this was more severe in the PDPV group (26 infants, low‐quality evidence). In PEP versus oscillating PEP, adverse events were only reported in the flutter group (five participants complained of dizziness, which improved after further instructions on device use was provided) (22 participants, low‐quality evidence). In PEP versus HFCWO, from one long‐term high‐quality study (107 participants) there was little or no difference in terms of number of adverse events; however, those in the PEP group had fewer adverse events related to the lower airways when compared to HFCWO (high‐certainty evidence).
Many studies had a risk of bias as they did not report how the randomisation sequence was either generated or concealed. Most studies reported the number of dropouts and also reported on all planned outcome measures.
Authors' conclusions
The evidence provided by this review is of variable quality, but suggests that all techniques and devices described may have a place in the clinical treatment of people with CF.
Following meta‐analyses of the effects of PEP versus other airway clearance techniques on lung function and patient preference, this Cochrane Review demonstrated that there was high‐quality evidence that showed a significant reduction in pulmonary exacerbations when PEP using a mask was compared with HFCWO. It is important to note that airway clearance techniques should be individualised throughout life according to developmental stages, patient preferences, pulmonary symptoms and lung function. This also applies as conditions vary between baseline function and pulmonary exacerbations.
Plain language summary
Using positive expiratory pressure physiotherapy to clear the airways of people with cystic fibrosis
Review question
We reviewed the evidence on the effect of positive expiratory pressure (PEP) physiotherapy to clear the airways of people with cystic fibrosis (CF).
Background
CF affects approximately one in 3000 live births in white populations and causes frequent lung infection, due to mucus blocking the airways. Chest physiotherapy is often used to try to clear the mucus from the lungs. We wanted to discover whether using a PEP device (a form of chest physiotherapy) was better or worse than other other forms of chest physiotherapy for clearing the mucus from the lungs in people with CF. A PEP device provides positive pressure behind the mucus to try to push it out of the lungs. This is an update of a previously published review.
Search date
The evidence is current to 20 February 2019.
Study characteristics
The review includes 28 studies with 788 people (from infants to adults) with CF with mild to severe lung disease. The studies compared PEP to other methods of chest physiotherapy; the length of treatment ranged from a single session to two years of treatment.
Key results
Generally, the efficacy of PEP is similar to other methods of chest physiotherapy such as postural drainage with percussion, active cycle of breathing techniques, autogenic drainage, oscillatory PEP devices such as the flutter and acapella, thoracic oscillating devices such as the 'Vest', and bilevel positive airway pressure (BiPaP) (typically used for ventilatory support, but by changing the inspiratory and expiratory pressures on the device and combining it with huffing, BiPaP has been used for airway clearance). We found no difference between PEP and other forms of chest physiotherapy in lung function, the amount of mucus cleared from the airways or its related effects on the health of people with CF. However, the rate of flare ups of respiratory symptoms decreased in people using PEP compared to other forms of physiotherapy such as a vibrating PEP device or a vibrating vest. There was some evidence that people with CF may prefer PEP to other chest physiotherapy methods. There was no evidence of PEP causing harm, except in one study where infants performing either PEP or percussion in various positions which use gravity to help drain secretions, experienced some gastro‐oesophageal reflux (regurgitation of food) in head‐down positions; this was more severe in the group using postural drainage with percussion. In all the other trials PEP was performed in a sitting position.
In 10 of the 28 studies studied single PEP treatment sessions. The results from these studies are very limited as they could not report on the number of respiratory infections and lung function did not change with just one treatment. Two one‐year studies compared PEP to postural drainage and percussion; in the study with children, PEP improved their lung function, while in the adult study, lung function declined slightly with both PEP and postural drainage and percussion. Also, the method of performing PEP was different in the two age groups.
Although PEP seems to have an advantage in reducing flare ups (based on the combined results of a few studies), different physiotherapy techniques and devices may be more or less effective at varying times and in different individuals during baseline function and chest flare ups. Each person should talk to their clinician to help choose which method of airway clearance is best for them and which they will adhere to, so as to provide the best quality of life and long‐term outcomes.
Quality of the evidence
Some studies were of low quality. These studies highlight the difficulty in comparing studies using PEP compared to other forms of chest physiotherapy. Factors such as age and severity of lung disease in the participants may affect the results as well as the method of performing each treatment. Overall, the evidence provided by this review for whether PEP reduces flare ups compared to other forms of chest physiotherapy was moderate to high quality, but evidence for other outcomes was of very low to moderate quality, as results were limited.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is a relatively common, inherited, life‐limiting disorder. The genetic defect causes abnormal mucus secretion in the airways, potentially leading to airway obstruction and mucus plugging (Zach 1990). This predisposes the airways to infection and inflammation, which in turn promote further mucus secretion. Persistent infection and inflammation within the lungs are the major contributory factors to airway damage and the progressive loss of respiratory function (Cantin 1995; Konstan 1997).
Description of the intervention
Treatment methods which improve mucus clearance are considered essential in optimising respiratory status and reducing the progression of lung disease. A variety of methods are used, some physical, e.g. airway clearance techniques, and some chemical, e.g. inhaled medications.
Airway clearance techniques (also referred to as chest physiotherapy) are widely prescribed to assist the clearance of airway mucus and usually commenced as soon as the diagnosis of CF is made. Traditionally, airway clearance consisted of postural drainage (gravity‐assisted drainage positions) combined with percussion and vibration (performed by an assistant such as a physiotherapist or relative), and forced expirations (huffing and coughing). Some protocols included deep breathing exercises. This form of airway clearance is time‐consuming and sometimes uncomfortable. It also requires assistance, which may have an adverse effect on adherence. Recently, several self‐administered alternatives that are able to be used in upright sitting positions have been developed. Among these are a range of positive expiratory pressure (PEP) devices, which provide a back pressure to the airways during expiration.The most common method of using PEP was defined by the Danes and is known as the, 'PEP technique' (Falk 1984). It consists of breathing through a flow‐dependant PEP device attached to a face mask with a closed system creating a PEP of between 10 to 20 cm H₂0 for 12 to 15 breaths. The PEP mask is then removed from the individual's face and he or she then performs two to three huffing manoeuvres (also known as a forced expiration). For the purposes of this paper we have included studies using pressures between 8 to 20 cm H₂O. Usually the PEP device is attached to a mask, but occasionally a mouthpiece with nose clips are used instead. However, there have only been a few studies performed using PEP with a mouthpiece. Another method of using PEP devices is defined by Oberwaldner in Austria and is known as, 'high‐pressure PEP' (Hi‐PEP). In Hi‐PEP, the expiratory pressure may be reach 40 to 100 cm H₂0. Hi‐PEP also incorporates forced expiratory manoeuvres through the PEP device, which generates higher pressures and may stimulate coughing through the mask (Oberwaldner 1986).
How the intervention might work
A theory is that PEP devices are able to improve clearance by increasing gas pressure behind mucus via collateral ventilation and a temporary increase in functional residual capacity (FRC). The FRC level is gradually increased over the 12 to 15 breaths. The forced expiratory manoeuvres then assist the movement of mucus from the peripheral airways centrally to where they can be expectorated (Andersen 1979; Groth 1985). It has also been hypothesised that Hi‐PEP may stabilise airways by splinting them open during expiration, which may facilitate airway clearance (Oberwaldner 1986).
Why it is important to do this review
A Cochrane Review comparing any form of chest physiotherapy with no chest physiotherapy found evidence to demonstrate the benefit of chest physiotherapy for increasing mucus transport, but did not find evidence for any long‐term outcomes (Warnock 2013). Several narrative reviews have compared different types of chest physiotherapy, including PEP, with conflicting conclusions (McIlwaine 1996; Prasad 1993; Prasad 2000; Williams 1994; Zach 1987). This review will examine the effect and acceptability of PEP compared to other techniques used for secretion clearance.
The most effective technique for secretion clearance during an infective exacerbation of CF may differ from that which is most effective for maintenance therapy. The PEP technique is also used in combination with various other interventions, e.g. pharmacological therapies, other physical therapy techniques, or a modification to the PEP technique. It is therefore important to establish the effect of PEP in each stage of CF lung disease with and without co‐interventions. This review is an update of previously published versions of the Cochrane Review (Elkins 2004; Elkins 2006; McIlwaine 2015).
Objectives
To determine the effect of PEP on the clearance of airway secretions compared to other airway clearance techniques in people with CF and test the following hypotheses:
PEP improves outcomes for people with CF more than other airway clearance techniques;
PEP is more acceptable to people with CF than other airway clearance techniques.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies.This included both parallel and cross‐over designs. Blinding was not a determinate for inclusion since participants could not be blinded as to which technique they were performing. Eligible studies included both individual and cluster randomised designs.
Types of participants
People with CF, of any age, diagnosed on the basis of clinical criteria and sweat testing or genotype analysis, with any degree of disease severity. People with CF, post‐lung transplant, were excluded.
Types of interventions
In the existing literature, variation occurs in the application of each individual airway clearance technique. For example; when using PEP as an airway clearance technique, some people perform 12 breaths through the device, whereas others perform 15 breaths. This may be followed by two or three forced expiratory manoeuvres. As separate analyses of variations within each technique would render this review unmanageable, it has been necessary to group these variations within broad definitions of the established treatment modalities.
One of the interventions used in the studies will be required to meet one of the two following descriptions.
PEP mask, or mouthpiece as described by the authors, with or without additional techniques. Originally, PEP was defined as breathing with a positive expiratory pressure of 10 to 20 cm H₂O (Falk 1984).
High‐pressure PEP (Hi‐PEP) mask therapy as described by the authors, with or without additional techniques. Hi‐PEP includes a full forced expiration against a fixed mechanical resistance which usually generates pressures ranging from 40 to 100 cm H₂O (Oberwaldner 1986).
At least one comparator intervention used in the studies will be required to meet one of the following descriptions.
Postural drainage with percussion and vibration (PDPV) ‐ in other reviews this has been described as conventional chest physiotherapy (CCPT).
Active cycle of breathing techniques (ACBT) ‐ this comprises relaxation or breathing control, forced expiration technique (FET), thoracic expansion exercises and may include postural drainage or percussion.
Autogenic drainage (AD) ‐ this breathing technique uses high expiratory flow rates at varying lung volumes to enhance mucous clearance while avoiding airway closure.
Oral oscillatory devices ‐ include flutter, cornet, acapella and intrapulmonary percussive ventilation. The flutter, cornet and acapella devices produce an oral oscillatory PEP effect within the airways. Intrapulmonary percussive ventilation provides continuous oscillation of the air pressure in the airways via the mouth.
Thoracic oscillating devices ‐ these include Thairapy Vest®, InCourage system, Smart vest, and the Hiyak Oscillator which provide external chest wall oscillation.
BiPaP ‐ a bilevel PEP system which delivers both inspiratory and expiratory positive pressure.
Exercise ‐ prescribed for the purpose of airway clearance either independently or as an adjunct to other techniques.
Types of outcome measures
Primary outcomes
Forced expiratory volume at one second (FEV1) (change in FEV1 between baseline and post‐intervention; L and per cent (%) predicted values are both stated wherever possible)
Number of respiratory exacerbations between baseline and post‐intervention (respiratory exacerbations must have been defined either by symptoms or by initiation of antibiotics for respiratory symptoms after medical assessment)
Direct measures of mucus clearance (mucus transport rate or mucociliary clearance rate as assessed by radioactive tracer)
Secondary outcomes
Expectorated secretions, dry or wet weight, or volume (an increase in the amount of expectorated secretions as a short‐term (less than seven days) effect of the intervention is considered as beneficial; in long‐term studies this outcome variable will not be included)
-
Other pulmonary parameters (post‐intervention change from baseline)
forced vital capacity (FVC)
forced expiratory flow 25% ‐ 75% (FEF25‐75)
total lung capacity (TLC)
residual volume (RV)
functional residual capacity (FRC)
Exercise tolerance (subjective exercise tolerance, or objective measures such as six‐minute walk test)
Well‐being (quality of life scales such as the CF Quality of Life scale, or ability to participate in activities of daily living using instruments such as the HAES (Habitual Activity Estimation Scale)
Blood oxygen levels (measured by arterial blood gas, pulse oximetry or transcutaneous oximetry)
Lung clearance index (LCI)
Ventilation scanning (radiological or nuclear medicine imaging)
Cost of intervention (equipment and duration)
Adherence to treatment or participant preference (may be determined either as the nominated technique of choice by the participant at the conclusion of the study, or by a comparison of technique acceptability (e.g. visual analogue scales))
Adverse effects (such as pneumothorax, haemoptysis, deaths or other adverse changes in condition from baseline)
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
The Cochrane Cystic Fibrosis and Genetic Disorders Group's Information Specialist conducted a search of the Group's Cystic Fibrosis Trials Register for relevant trials using the term: positive expiratory pressure*.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register: 20 February 2019.
We also searched the following trials registries:
US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov (www.clinicaltrials.gov; searched 20 February 2019);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch; searched 20 February 2019).
For details of our search strategies, please see Appendix 1.
Searching other resources
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
The review authors contacted manufacturers of PEP devices regarding any additional studies. The authors contacted other centres where studies on PEP were being undertaken. Authors of included studies were contacted to see if they knew of any unpublished studies.
The review authors also handsearched the proceedings of the North American Cystic Fibrosis Conference (2008 to 2018) and the European Cystic Fibrosis Conference (2008 to 2019).
Data collection and analysis
Selection of studies
Three authors independently reviewed all citations and abstracts identified by the search to determine which papers should be included. The authors resolved disagreements by discussion and consensus.
Data extraction and management
Three authors independently extracted data for each of the outcome measures listed above. Where studies were published in insufficient detail, the review authors contacted the study authors with a request to provide the required data. The authors used the Cochrane software (Review Manager) to compile and analyse the data (Review Manager 2014).
For all included studies, the authors recorded the following details where possible: criteria for diagnosis of CF; methods of participant selection; and baseline characteristics of the active and placebo groups including age, sex, genotype and lung function.
Assessment of risk of bias in included studies
Three authors independently assessed the risk of bias using text from study reports to make judgements of high, low or unclear risk for six features of a study (Higgins 2003). These include: random sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other types of bias. The authors resolved any disagreements through discussion. The review authors used both published data and additional data obtained from study authors in determining whether criteria were met.
Measures of treatment effect
For continuous outcomes, the review authors recorded either the mean change from baseline for each group or mean post‐treatment or intervention values and the standard deviation (SD) for each group. The authors combined data using the mean difference (MD) and 95% confidence intervals (CIs).
In the case of binary outcomes, the authors combined the data from the studies using risk ratios (RR) and 95% CIs.
Unit of analysis issues
None of the included studies were cluster randomised.
Elbourne discusses methods for meta‐analysing cross‐over studies (Elbourne 2002). These methods rely on the data that are reported within the primary paper. The authors have adopted a method within this review which uses the data from the first period only, ignoring any data from the second period that was available if a carryover effect was identified. If the authors did not identify a carryover effect and the papers reported data sufficiently, then the review authors planned to use the methods stated by Elbourne (Elbourne 2002).
When a study included multiple interventions, the review authors included each in the relevant comparison, as specified in the data synthesis section below.
Dealing with missing data
In order to allow an intention‐to‐treat analysis, the authors collected data on the number of participants with each outcome event by allocated treated group irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded for treatment or follow‐up.
The authors contacted the primary investigators of the included studies for any additional data they thought were missing. Unpublished data were provided and reported on in the review for four studies (Darbee 1990; Gaskin 1998; Tyrrell 1986; van Asperen 1987).
Assessment of heterogeneity
If the authors had been able to include adequate numbers of studies, they would have looked for heterogeneity between studies. The authors planned to assess this visually in the forest plots and using the I² statistic which describes the percentage of total variation across studies that are due to heterogeneity rather than chance (Higgins 2003). The values of I² lie between 0% and 100%, and a simplified categorization of heterogeneity that the authors planned to use is of low (I² value of 25%), moderate (I² value of 50%), and high (I² value of 75%) (Higgins 2003). They will also consider the Chi² test with a P value less than 0.10 indicating significant heterogeneity, although the authors will be cautious with interpretation due to the low power of this test.
Assessment of reporting biases
The authors planned to construct a funnel plot if there were sufficient studies (i.e. 10) to assess publication bias. If the funnel plot is asymmetrical then they will consider other reasons as well as publication bias, i.e. heterogeneity, small study effects and outcome reporting bias.
The authors planned to assess outcome reporting bias in the risk of bias section, by comparing protocols, if available, to the study reports, or comparing the methods section to the results section with knowledge of the clinical area.
Data synthesis
The authors have analysed the data using a fixed‐effect model. If, in the future, they are able to include more studies and they identify a moderate or high degree of heterogeneity, as defined above, they will use a random‐effects model in the data analysis.
Different interventions were analysed separately. In this update, the comparisons include:
PEP compared with PDPV;
PEP compared with oscillating PEP;
PEP compared with HFCWO.
The authors analysed studies in which the intervention consists of a single treatment separately from those studies in which a course of treatments is used. Within the latter group, the authors analysed studies of up to seven days treatment separately from studies of longer duration. The authors grouped outcome data from longer‐term studies (more than seven days) into those measured at one, three, six, 12 months, and annually thereafter. If studies recorded outcome data at other time periods, then the authors considered examining these as well.
Subgroup analysis and investigation of heterogeneity
To investigate any heterogeneity identified, the authors planned to perform separate subgroup analyses based on the following factors: a PEP level of 8 to 20 cm H₂O; a PEP level of over 20 cm H₂O as used in Hi‐PEP; disease state (exacerbation versus stable); use of co‐interventions (positioning, other airway clearance techniques); age (paediatric, adolescent, adult); gender; and disease severity (FEV1% predicted > 90%, 70% to 90%, 40% to 69%, < 40%).
Sensitivity analysis
The authors planned to test the robustness of their results by performing sensitivity analyses such as excluding studies that were at high risk of bias for blinding and using the random‐effects model if they detected a moderate or high degree of heterogeneity.
Summary of findings tables and quality of the evidence (GRADE)
In a post hoc change in line with current Cochrane guidance, at the 2017 update we added a summary of findings table for each comparison presented in the review (Table 1; Table 2; Table 3; Table 4; Table 5). We selected the following seven outcomes to report (chosen based on relevance to clinicians and consumers):
Summary of findings for the main comparison. Summary of findings ‐ PEP compared with PDPV for cystic fibrosis.
| PEP compared with PDPV for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: PEP Comparison: PDPV | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PDPV | PEP | |||||
|
FEV1: change from baseline (% predicted) Follow‐up: 2 days to 2 years |
There was significant advantage to the PEP group compared to the PDPV group in the change in FEV1 from baseline in 1 study (1 year duration) The other 4 studies showed no significant difference between groups in FEV1 |
NA | 155 (5 studies including 3 cross‐over studies) |
⊕⊕⊝⊝ lowa | ||
|
Number of respiratory exacerbations Follow‐up: NA |
1 study which used PEP with a mouthpiece, it was reported (personal communication) that there was no difference in the number of respiratory exacerbations | NA | 66 (1 study) |
⊕⊕⊝⊝ lowc,d | ||
|
Direct measures of mucus clearance: radio‐labelled aerosol clearance Follow‐up: 3 months |
There was no significant difference in clearance between treatment groups | NA | 20 (1 cross‐over study) | ⊕⊕⊝⊝ lowb,c | ||
|
Exercise tolerance Follow‐up: 2 years |
1 study conducted exercise testing using cycle ergometry, but reported no data for this outcome measure. | NA | 66 (1 study) |
⊕⊕⊝⊝ lowc,d | ||
|
Well being: QWB scale Follow‐up: 2 years |
Neither group demonstrated a significant change in QWB scores, which was similar at baseline and no further data were available. | NA | 66 (1 study) |
⊕⊕⊝⊝ lowc,d | ||
|
LCI Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Adverse events Follow‐up: 1 year |
No adverse events were reported in 1 study. In the other study, gastro‐oesophageal reflux was reported more commonly in the PEP group than the PDPV group, but more participants withdrew due to severe gastro‐oesophageal reflux in the PDPV group than the PEP group. |
NA | 66 (2 studies) | ⊕⊕⊝⊝ lowd,e | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CF: cystic fibrosis; FEV1: forced expiratory volume at 1 second; LCI: lung clearance index; NA: not applicable; PDPV: postural drainage, percussion and ventilation; PEP: positive expiratory pressure; quality of well‐being; QWB: quality of well‐being. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a. Downgraded twice due to serious risk of bias: cross‐over studies did not have washout periods so were at high risk of bias due to carryover effects. Methodological details of the studies relating to randomisation and allocation concealment were unclear and some studies were at high risk of selective reporting bias.
b. Downgraded once due to risk of bias: Methodological details of the study relating to randomisation and allocation concealment were unclear and the cross‐over study did not have washout periods so were at high risk of bias due to carryover effects.
c. Downgraded once due to imprecision: no numerical data available to enter into analysis.
d. Downgraded once due to risk of bias: Methodological details of the studies relating to randomisation and allocation concealment were unclear and some of the studies were at high risk of selective reporting bias.
e. Downgraded once due to applicability: the two studies recruited children only, so results are not applicable to adults
Summary of findings 2. Summary of findings ‐ PEP compared to oscillating PEP for cystic fibrosis.
| PEP compared with oscillating PEP for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: PEP Comparison: oscillating PEP (Acapella, Flutter and Cornet) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oscillating PEP | PEP | |||||
|
FEV1: change from baseline (% predicted) Follow‐up: single treatment to 13 months |
There was no significant difference in the change from baseline in FEV1 (% predicted) between treatment groups in any study. | NA | 217 (7 studies including 2 cross‐over studies) |
⊕⊕⊕⊝ moderatea | ||
|
Number of respiratory exacerbations: requiring either IV antibiotics or hospitalisation Follow‐up: 1 year to 13 months |
One study of 1 year duration showed that significantly fewer hospitalisations occurred in the PEP group compared to the oscillating PEP group. The other two studies showed no significant differences in the number of respiratory exacerbations between groups |
NA | 112 (3 studies) | ⊕⊕⊕⊝ moderatea | ||
|
Direct measures of mucus clearance Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Exercise tolerance: modified shuttle test Follow up: 10 days to 1 year |
There was no significant difference in exercise tolerance between treatment groups in either study. | NA | 68 (2 studies) |
⊕⊕⊕⊝ moderatea | ||
|
Well being: QWB scale. CF Short Form ‐36 and Chronic Respiratory Questionnaire Follow up: 1 year |
There was no significant change from baseline between groups in the QWB scale, Short Form ‐36 domains or Chronic Respiratory Questionnaire domains. | NA | 75 (2 studies) |
⊕⊕⊝⊝ lowa,b | ||
|
LCI Follow up: 12 months |
The mean (SD) LCI in the oscillating PEP group was 0.2 (2.47). | The mean LCI in the PEP group was 0.80 higher (1.36 lower to 2.96 higher). |
NA | 30 (1 study) |
⊕⊕⊝⊝ lowa,c | Outcome measured by multiple, breath inert gas washout |
|
Adverse events Follow‐up: 2 weeks |
Five participants complained of dizziness when using the Flutter device which improved after further instructions on breathing techniques when using the device was provided. No adverse events were reported in the PEP group. |
NA | 22 (1 cross‐over study) | ⊕⊕⊝⊝ lowa,d | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CF: cystic fibrosis; IV: intravenous; LCI: lung clearance index; NA: not applicable; PEP: positive expiratory pressure; QWB: quality of well‐being; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a.Downgraded once due to risk of bias: Methodological details of the studies relating to randomisation and allocation concealment were unclear and some of the studies were at high risk of bias due to incomplete outcome data.
b. Downgraded once due to imprecision: no numerical data available to enter into analysis.
c. Downgraded once due to applicability: the study recruited children only, so results are not applicable to adults
d. Downgraded once due to potential risk of bias: six out of seven studies did not clearly state whether any adverse events occurred or not during the study
Summary of findings 3. Summary of findings ‐ PEP compared to HFCWO for cystic fibrosis.
| PEP compared with HFCWO for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: PEP Comparison: HFCWO | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| HFCWO | PEP | |||||
|
FEV1: change from baseline (% predicted) Follow‐up: single treatment to 1 year |
There was no significant difference in the change from baseline in FEV1 (% predicted) between treatment groups in any study. | NA | 174 (4 studies including 3 cross‐over studies) | ⊕⊕⊝⊝ lowa | ||
|
Number of respiratory exacerbations Follow‐up: 1 year |
833 per 1000 |
609 per 1000 (458 to 791 per 1000). |
RR:0.73 (0.55 to 0.95) |
107 (1 study) |
⊕⊕⊕⊕ high | |
|
Direct measures of mucus clearance Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Exercise tolerance Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Well being Follow‐up: NA |
Outcome not reported. | NA | ||||
|
LCI Follow‐up: single treatment |
1 study showed an improvement in ventilation distribution and gas mixing with both treatments which was not significantly different between both techniques. | NA | 15 (1 cross‐over study) |
⊕⊝⊝⊝ very lowa,b | Outcome measured using a single‐breath inert gas test which examines distribution of ventilation | |
|
Adverse events Follow‐up: 1 year |
200 adverse events were reported. A mean of 2.46 events related to the lower airway were reported. |
163 adverse events were reported. A mean of 1.72 events related to the lower airway were reported. |
NA | 107 (1 study) |
⊕⊕⊕⊕ high | The total number of adverse events was not significantly different between the groups. However, there were significantly more adverse events related to the lower airways (increased cough, chest infection, haemoptysis, decreased lung function and chest pain) in the HFCWO group compared to the PEP group (P = 0.023). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CF: cystic fibrosis; CI: confidence interval; FEV1: forced expiratory volume at 1 second; HFCWO: high frequency chest wall oscillation; LCI: lung clearance index; NA: not applicable; PEP: positive expiratory pressure;RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a. Downgraded twice due to serious risk of bias: cross‐over studies did not have washout periods so were at high risk of bias due to carryover effects. Methodological details of the studies relating to randomisation and allocation concealment were unclear and some studies were at high risk of selective reporting bias.
b. Downgraded once due to imprecision: no numerical data available to enter into analysis.
Summary of findings 4. Summary of findings ‐ PEP compared to BiPAP for cystic fibrosis.
| PEP compared with BiPAP for CF | ||||||
|
Patient or population: adults and children with CF Settings: outpatients Intervention: PEP Comparison: BiPAP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| BiPAP | PEP | |||||
|
FEV1 Follow‐up: single treatment to 3 months |
There was no significance difference in FEV1 between treatment groups in either study. | NA | 52 (2 studies including 1 cross‐over study) | ⊕⊕⊝⊝ lowa,b | One study reported FEV1 in litres and % predicted. In the other study, it was unclear how FEV1 was measured |
|
|
Number of respiratory exacerbations Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Direct measures of mucus clearance Follow‐up: NA |
Outcome not reported. | NA | ||||
|
Exercise tolerance: 6‐minute walk test Follow‐up: 3 months |
There was no significant difference between treatment groups in terms of exercise tolerance. | NA | 32 (1 study) | ⊕⊕⊝⊝ lowa,b | ||
|
Well being Follow up: NA |
Outcome not reported. | NA | ||||
|
LCI Follow up: 3 months |
One study reported a significant improvement in the distribution of ventilation following BiPaP compared to PEP (P = 0.01). | NA | 32 (1 study) |
⊕⊕⊝⊝ lowa,b | Outcome measured using single‐breath inert gas test which examines distribution of ventilation. | |
|
Adverse events Follow‐up: single treatment |
One study reported than no untoward effects were observed in any participant. | NA | 20 (1 cross‐over study) |
⊕⊕⊝⊝ lowa,c | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CF: cystic fibrosis; BiPAP: bilevel positive airway pressure; CI: Confidence interval;LCI: lung clearance index; NA: Not applicable; PEP: positive expiratory pressure. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a.Downgraded once due to risk of bias: Methodological details of the studies relating to randomisation and allocation concealment were unclear and some of the studies
b. Downgraded once due to imprecision: no numerical data available to enter into analysis.
c. Downgraded once due to potential risk of bias: one of the studies did not clearly state whether any adverse events occurred or not during the study
Summary of findings 5. Summary of findings ‐ PEP compared to airway clearance techniques for cystic fibrosis.
| PEP compared with airway clearance techniques for CF | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: PEP Comparison: airway clearance techniquesa | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Airway clearance techniques | PEP | |||||
|
FEV1: change from baseline (% predicted) Follow‐up: single treatment to 3 months |
One study found that FEV1 (% predicted) was significantly lower after a treatment of AD followed by Hi‐PEP, compared to AD alone. All other studies found no significant difference between treatment groups in FEV1 (% predicted). |
NA | 95 (6 cross‐over studies) | ⊕⊕⊝⊝ lowb | ||
|
Number of respiratory exacerbations Follow‐up: single treatment to 1 month |
The two studies reported participants being withdrawn due to exacerbations, although these are not well‐defined and it is unclear which treatments the participants were randomised to at the time of departure from the studies. | NA | 43 (2 cross‐over studies) |
⊕⊝⊝⊝ very lowb,c | ||
|
Direct measures of mucus clearance: radioisotope retention Follow‐up: single treatment to 2 weeks |
One study showed that radioisotope retention 2 hours after a 20‐minute treatment of PEP and FET was significantly less than for FET alone. No significant difference in clearance was identified between PEP plus FET and PD plus FET or exercise plus FET. |
NA | 31 (3 cross‐over studies) | ⊕⊕⊝⊝ lowb | ||
|
Exercise tolerance: modified shuttle test Follow up: 1 year |
There was no significant difference in exercise tolerance between treatment groups | NA | 45 (1 study) |
⊕⊕⊝⊝ lowc,d | ||
|
Well being: Short Form ‐36 Follow up: 1 year |
There was no significant change from baseline between groups in the Short Form ‐36 domains. | NA | 45 (1 study) |
⊕⊕⊝⊝ lowc,d | ||
|
LCI Follow‐up: 3 months |
One study reported worsening of the distribution of ventilation following PEP and high pressure PEP compared to control. However, in this study gas mixing improved suggesting that PEP opened up previously closed partially obstructed airways. | NA | 6 (1 cross‐over study) |
⊕⊝⊝⊝ very lowb,c | Outcome measured using single‐breath inert gas test which examines distribution of ventilation | |
|
Adverse events Follow‐up: NA |
Outcome not reported. | NA | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: Active cycle of breathing techniques; AD: autogenic drainage; CF: cystic fibrosis; CI: confidence interval;FET: forced expiration technique; FEV1: forced expiratory volume at 1 second; Hi‐PEP: high‐pressure positive expiratory pressure; LCI: lung clearance index; NA: not applicable; PD: postural drainage; PDP: percussion and drainage therapy; PEP: positive expiratory pressure. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a. Three studies compared PEP to ACBT, one study Darbee compared two types of PEP, one study compared PEP and autogenic drainage (AD), one study compared PEP alone, PDPV and FET, or five minutes of PEP followed by PDPV and FET, one study compared PEP, PDP and AD and one study compared PEP, PD and physical exercise.
b. Downgraded twice due to serious risk of bias: cross‐over studies did not have washout periods so were at high risk of bias due to carryover effects. Methodological details of the studies relating to randomisation and allocation concealment were unclear and some studies were at high risk of selective reporting bias.
c. Downgraded once due to imprecision: no numerical data available to enter into analysis and unclear results.
d.Downgraded once due to risk of bias: Methodological details of the study relating to allocation concealment, blinding and selective reporting were unclear
FEV1
Number of respiratory exacerbations
Direct measures of mucus clearance
Exercise tolerance
Well being
LCI
Adverse events
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if they considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Studies awaiting classification.
Results of the search
The search retrieved 116 citations which represented 70 studies. No extra studies were identified with the CINAHL search or through contacting manufacturers of PEP devices.
A total of 28 studies involving 788 participants met the inclusion criteria; 21 were published as full articles (Braggion 1995; Darbee 2004; Darbee 2005; Fainardi 2011; Falk 1984; Hofmeyr 1986; Lagerkvist 2006; Lannefors 1992; McIlwaine 1997; McIlwaine 2001; McIlwaine 2013; Mortensen 1991; Newbold 2005; Pfleger 1992; Pryor 2010; Rodriguez 2016; Steen 1991; Tyrrell 1986; van Asperen 1987; van Winden 1998; West 2010). Seven studies were published in abstract form only (Costantini 2001; Darbee 1990; Falk 1993; Gaskin 1998; Kofler 1998; McIlwaine 1991; Tannenbaum 2005).
A total of 33 studies are listed as excluded.
Six studies are awaiting assessment as they have been published in abstract form only; the study design or outcome data have been reported in insufficient detail to determine whether the inclusion criteria have been met (Elkins 2005; Grzincich 2008; Parreira 2008; Kofler 1994; Parreira 2008; Wong 2000).
Included studies
Additional data were obtained from the authors of nine of the studies (Costantini 2001; Darbee 1990; Gaskin 1998; Kofler 1998; McIlwaine 1991; McIlwaine 1997; McIlwaine 2001; Tyrrell 1986; van Asperen 1987).
Trial design
The 28 included studies (788 participants) had sample sizes in individual studies ranging from six (Darbee 2004) to 107 participants (McIlwaine 2013). 10 studies had more than two treatment arms (i.e. more than one comparator to PEP).
With regards to duration, 10 studies examined single treatments (Darbee 2005; Darbee 2004; Fainardi 2011; Falk 1984; Falk 1993; Kofler 1998; Lagerkvist 2006; Lannefors 1992; Mortensen 1991; Pfleger 1992). In two studies the duration of each treatment arm was less than seven days (Braggion 1995; Hofmeyr 1986). In the remaining 16 studies, the duration of each treatment arm ranged from 10 days to two years (Costantini 2001; Darbee 1990; Gaskin 1998; McIlwaine 1997; McIlwaine 1991; McIlwaine 2001; McIlwaine 2013; Newbold 2005; Pryor 2010; Rodriguez 2016; Tannenbaum 2005; Steen 1991; Tyrrell 1986; van Asperen 1987; van Winden 1998; West 2010).
There were 18 cross‐over studies, involving 296 participants. The four studies conducted in participants experiencing a respiratory exacerbation all used a cross‐over design with a duration of one or two days in each arm (Braggion 1995; Darbee 2005; Fainardi 2011; Hofmeyr 1986). Data from the end of the first period were obtained for three of the 18 cross‐over studies (Darbee 1990; Tyrrell 1986; van Asperen 1987), but could not be obtained for the remaining 15 studies, although analysis was undertaken on all available data (Braggion 1995; Darbee 2004; Darbee 2005; Fainardi 2011; Falk 1984; Falk 1993; Hofmeyr 1986; Kofler 1998; Lagerkvist 2006; Lannefors 1992; McIlwaine 1991; Mortensen 1991; Pfleger 1992; Steen 1991; van Winden 1998). Six studies had a washout period between techniques which ranged from two days to eight weeks (Darbee 2004; Lagerkvist 2006; Lannefors 1992; McIlwaine 1991; Mortensen 1991; van Winden 1998).
One study was a multicentre study involving 12 CF centres in Canada (McIlwaine 2013).
Participants
One study was conducted exclusively with infants under four months of age (Costantini 2001). Nine studies were conducted exclusively with children between the ages of six years and 18 years of age (Lagerkvist 2006; McIlwaine 1991; McIlwaine 1997; McIlwaine 2001; Tannenbaum 2005; Tyrrell 1986; van Asperen 1987; van Winden 1998; West 2010). Five studies were conducted exclusively in adults (Darbee 1990; Fainardi 2011; Newbold 2005; Pryor 2010; Rodriguez 2016). Both paediatric and adult participants were recruited to 12 studies; only one of these provided data for any age subgroup independently (Gaskin 1998). One study did not report the age of the participants (Falk 1993).
The gender of the participants was reported in 22 of the included studies involving 692 participants (Braggion 1995; Costantini 2001; Darbee 1990; Darbee 2004; Fainardi 2011; Falk 1984; Gaskin 1998; Hofmeyr 1986; Kofler 1998; Lagerkvist 2006; Lannefors 1992; McIlwaine 1997; McIlwaine 2001; McIlwaine 2013; Mortensen 1991; Newbold 2005; Pfleger 1992; Rodriguez 2016; Tannenbaum 2005; Tyrrell 1986; Pryor 2010; van Winden 1998). Two had an even gender ratio (Braggion 1995; Rodriguez 2016), but most had more male than female participants, resulting in an overall male: female ratio of 3:2.
Four cross‐over studies were conducted in participants experiencing a respiratory exacerbation with a duration of one or two days in each arm; hence they provide limited evidence for the effect of PEP for treatment of an exacerbation (Braggion 1995; Darbee 2005; Fainardi 2011; Hofmeyr 1986). Three studies did not report any measure of disease severity of the included participants. A total of 25 studies reported the FEV1% predicted values of participants at baseline. In three of these studies, FEV1 values were only in the moderate to severe range (less than 70% predicted) (Darbee 2004; Falk 1984; Rodriguez 2016). The remaining 21 studies included participants with a wide range of lung function impairment, most commonly from severe to normal (less than 40% to greater than 90% predicted). Those studies reporting Shwachman scores as a measure of disease severity also included participants with a wide range of scores.
Interventions
In two of the included studies, the intervention included a full forced expiration against a fixed mechanical resistance at pressures greater than 20 cm H₂O and thus met the definition of Hi‐PEP (Darbee 2004; Pfleger 1992).
Eight studies (207 participants) compared PEP with PDPV (Braggion 1995; Costantini 2001; Darbee 1990; Falk 1984; Gaskin 1998; McIlwaine 1997; Tyrrell 1986; van Asperen 1987). Seven studies (247 participants) compared PEP with oscillating PEP (acapella, flutter and cornet) ( Darbee 2005; Lagerkvist 2006; McIlwaine 2001; Newbold 2005; Pryor 2010; van Winden 1998; West 2010). Four studies (174 participants) compared PEP with HFCWO (Braggion 1995; Darbee 2005; Fainardi 2011; McIlwaine 2013). Two studies compared PEP with BiPAP (Kofler 1998; Rodriguez 2016). In eight cross‐over studies, PEP was compared to a variety of different airway clearance techniques.
In 22 of the 28 included studies the PEP technique was performed using a mask (Costantini 2001; Darbee 1990; Darbee 2004; Darbee 2005; Fainardi 2011; Falk 1984; Falk 1993; Kofler 1998; Lannefors 1992; McIlwaine 1991; McIlwaine 1997; McIlwaine 2001; McIlwaine 2013; Mortensen 1991; Newbold 2005; Pfleger 1992; Rodriguez 2016; Steen 1991; Tyrrell 1986; van Asperen 1987; van Winden 1998; West 2010). Three studies reported using a mouthpiece (Gaskin 1998; Hofmeyr 1986; Lagerkvist 2006) and in three studies it was unclear whether the PEP technique was performed using a mask or a mouthpiece (Braggion 1995; Pryor 2010; Tannenbaum 2005).
Outcome measures
Individual outcomes are reported for each PEP comparison, with FEV1 being reported in 26 studies. One of the two studies who did not report FEV1 used FEV0.75 (Tyrrell 1986) and the second study was in infants (Costantini 2001). LCI was used in three studies (Darbee 2005; Rodriguez 2016; Tannenbaum 2005). More details on the reported outcomes can be found in the characteristics tables (Characteristics of included studies).
Excluded studies
A total of 33 studies were excluded from the review.
Eight studies did not compare PEP to a physical airway clearance technique (Aquino 2006; Aubriot 2016; Falk 1988; Laube 2000; Orlik 2015; Reychler 2015; Wettstein 2014; Wilson 2015) and in a further six studies, neither of the interventions was PEP (Aquino 2012; Kraemer 1996; Liedtke 1996; Oermann 2001; Patel 2013; Roos 1987). Eight studies were excluded as the PEP technique used did not meet the definition of PEP for this review; two used underwater tubing (Balestri 2004; Battistini 2001), two used a flow‐independant PEP device (Padman 1999; van der Schans 1991), three did not include huffing (McCarren 2006; Placidi 2001; Sanchez Riera 1999) and one was positive end‐expiratory pressure and not PEP (Dosman 2003). In six studies, PEP versus other airway clearance techniques was not the randomised intervention (Bishop 2011; Borka 2012; Fitzgerald 2001; Marks 1998; Orlik 2000; Znotina 2000). Two studies did not report any data in their published papers for outcomes of interest in this review (Castle 1994; Gotz 1995). Two studies recruited participants not eligible for this review; one study was performed on post‐transplant individuals (Munro 2007) and one on people with chronic bronchitis (van Hengstum 1987). Another study compared reported adherence against objectively measured adherence (Richmond 2016).
Studies awaiting classification
Nine studies are currently awaiting classification and will be fully assessed at the next update of the review (Elkins 2005; Grzincich 2008; Kofler 1994; Parreira 2008; Radtke 2018; Tonnesen 1982; Vendrusculo 2019; Ward 2018; Wong 2000).
Risk of bias in included studies
Allocation
Sequence generation
Five studies described the randomisation procedure (Fainardi 2011; McIlwaine 2013; Pryor 2010; Rodriguez 2016; West 2010) and were considered to have a low risk of bias. In the remaining 23 studies, the participants were described as being randomly allocated to groups (in those that were cross‐over in design, to treatment order), but no further details were provided; these studies were therefore at an unclear risk of bias.
Allocation concealment
In five studies the allocation was concealed (i.e., the person who determined if a participant was eligible for inclusion in the study was unaware, when this decision was made, to which group the participant would be allocated) and these five studies were therefore deemed at low risk of bias (McIlwaine 2001; McIlwaine 2013; Newbold 2005; Rodriguez 2016; West 2010). None of the remaining 23 studies discussed the method of allocation concealment and thus were deemed to have an unclear risk of bias.
Blinding
Due to the nature of the therapy, the participants in each of the studies were aware of which group they had been allocated to. All studies were therefore at a high risk of bias. Also, after randomisation occurred, the person applying the therapy knew which group the participants were allocated to.
In 10 of the studies the person assessing at least one outcome measure did not know which group the participants had been allocated to and they were therefore deemed to be at a low risk of bias (Fainardi 2011; Falk 1984; McIlwaine 1997; McIlwaine 2001; McIlwaine 2013; Mortensen 1991; Newbold 2005; Pryor 2010; Rodriguez 2016; West 2010). For self‐reported outcomes (e.g. visual analogue scale, pain diary), the assessor is only considered to be blinded if the participant was blinded. No other study reported on who was blinded and are judged to have an unclear risk of bias.
Incomplete outcome data
In 21 studies, the measures of at least one key outcome at one time point were obtained from more than 85% of the participants initially allocated to groups (Darbee 1990; Darbee 2005; Fainardi 2011; Falk 1984; Gaskin 1998; Hofmeyr 1986; Kofler 1998; Lagerkvist 2006; Lannefors 1992; McIlwaine 1991; McIlwaine 1997; McIlwaine 2013; Mortensen 1991; Newbold 2005; Pfleger 1992; Steen 1991; Pryor 2010; Rodriguez 2016; Tannenbaum 2005; van Winden 1998; West 2010).
In seven studies, all participants, for whom outcome measures were available, received the treatment or control condition as allocated or, where this was not the case, data for at least one key outcome was analysed by 'intention‐to‐treat' (Falk 1984; Gaskin 1998; Lagerkvist 2006; McIlwaine 2013; Pryor 2010; Rodriguez 2016; West 2010). This criterion is satisfied (even if there is no mention of analysis by intention‐to‐treat) if the report explicitly states that all participants received treatment or control conditions as allocated. Intention‐to‐treat analysis was explicitly mentioned in two studies (McIlwaine 2013; Pryor 2010); however, 13 participants in the Pryor study withdrew as they did not like the intervention they had been randomised to; it is unclear if these participants were included in the intention‐to‐treat group (Pryor 2010). In the 2013 McIlwaine study, 16 participants withdrew after randomisation but prior to initiation of study therapy regimens, these participants were not included in the intention‐to‐treat analysis (McIlwaine 2013). In the 2001 McIlwaine study, three participants dropped out from the PEP group due to non‐compliance while five participants dropped out from the flutter group as they believed that the flutter was ineffective in clearing their secretions (McIlwaine 2001). It is unclear whether these participants were included in the analysis. In the Tyrrell study, three out of 19 participants were excluded due to non‐adherence (Tyrrell 1986). The risk of bias is low in the first two studies (McIlwaine 2013; Pryor 2010) and unclear in the last two studies (McIlwaine 2001; Tyrrell 1986).
Selective reporting
The results of between‐group statistical comparisons are reported for all outcomes in 13 studies (Darbee 2004; Darbee 2005; Fainardi 2011; Falk 1984; Falk 1993; Lagerkvist 2006; McIlwaine 2001; McIlwaine 2013; Mortensen 1991; Newbold 2005; Rodriguez 2016; van Winden 1998; West 2010). The results of between‐group statistical comparisons are reported for at least one key outcome in 13 studies (Darbee 1990; Gaskin 1998; Hofmeyr 1986; Kofler 1998; Lannefors 1992; McIlwaine 1991; McIlwaine 1997; Pfleger 1992; Pryor 2010; Tannenbaum 2005; Steen 1991; Tyrrell 1986; van Asperen 1987). Two studies did not report any between group statistical comparisons (Braggion 1995; Costantini 2001).
In 27 studies the following are provided, either:
(a) point measures and measures of variability for at least one continuous outcome; or
(b) the number of participants in each category for at least one categorical outcome; or
both (a) and (b) (Costantini 2001; Darbee 1990; Darbee 2004; Darbee 2005; Fainardi 2011; Falk 1984; Falk 1993; Gaskin 1998; Hofmeyr 1986; Kofler 1998; Lagerkvist 2006; Lannefors 1992; McIlwaine 1991; McIlwaine 1997; McIlwaine 2001; McIlwaine 2013; Mortensen 1991; Newbold 2005; Pfleger 1992; Pryor 2010; Rodriguez 2016; Steen 1991; Tannenbaum 2005; Tyrrell 1986; van Asperen 1987;van Winden 1998; West 2010). The risk of bias for these studies is low. In one study neither (a) or (b) were provided (Braggion 1995). In this study the risk of bias was assessed as high.
Other potential sources of bias
In one study, no information was provided regarding similarities at baseline (unclear risk of bias) (Costantini 2001). In the remaining 27 studies, the groups were similar at baseline regarding the most important prognostic indicators (i.e. based on at least one measure of the severity of CF and one outcome measure at baseline, the groups' outcomes would not be expected to differ by a clinically significant amount), which indicates a low risk of bias.
There were 18 studies of cross‐over design which has the potential to increase the risk of bias. Eight studies were of one or two days in duration, with no washout period, which may be a potential source of bias (Braggion 1995; Darbee 2005; Fainardi 2011; Falk 1984; Falk 1993; Hofmeyr 1986; Kofler 1998; Pfleger 1992). Cross‐over studies of longer duration have a higher risk of bias and in these 10 studies, there were three which lasted one month and had no washout period between techniques (Steen 1991; Tyrrell 1986; van Asperen 1987).
There are relatively few meetings for reporting research about CF; however, it is possible that some studies may have been published or presented as abstracts at physiotherapy conferences that do not appear on online searches.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
1. PEP compared with PDPV
Eight studies included in this review (207 participants) make comparisons of PEP versus conventional physiotherapy (PDPV) for CF (Braggion 1995; Costantini 2001; Darbee 1990; Falk 1984; Gaskin 1998; McIlwaine 1997; Tyrrell 1986; van Asperen 1987). These comparisons are also made by the Cochrane Review 'Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis' (Main 2005).
Primary outcomes
1. FEV1
Five studies (148 participants) measured FEV1 (Braggion 1995; Darbee 1990; Gaskin 1998; McIlwaine 1997; van Asperen 1987) (Analysis 1.1). Unpublished data were provided for three studies allowing these studies to be included in analysis (Darbee 1990; Gaskin 1998; van Asperen 1987). First‐period data only from two studies were included in analysis due to concerns over risk of bias as these cross‐over studies did not have a washout period (Darbee 1990; van Asperen 1987).
1.1. Analysis.
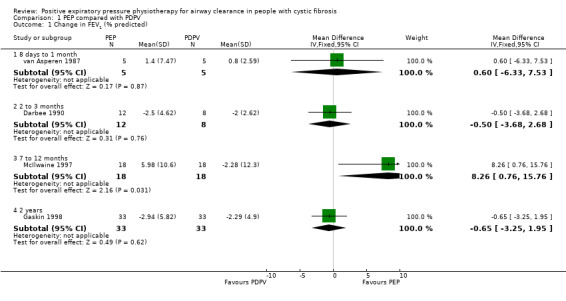
Comparison 1 PEP compared with PDPV, Outcome 1 Change in FEV1 (% predicted).
a. Short‐term (up to seven days)
One study (16 participants) measured FEV1 after four treatments of PEP, postural drainage with undefined chest physiotherapy techniques, or flutter over two days and found no significant differences in FEV1 (% predicted) (low‐quality evidence) (Braggion 1995). This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained.
b. Long‐term studies (more than seven days)
Four studies (119 participants) measured FEV1 after a series of treatments over more than seven days (low‐quality evidence) (Darbee 1990; Gaskin 1998; McIlwaine 1997; van Asperen 1987) (Analysis 1.1). Results are reported as change in % predicted for FEV1.
No significant differences in FEV1 were demonstrated after one month of PEP or PDPV, MD 0.60% predicted (95% CI ‐6.33 to 7.53) (13 participants) (van Asperen 1987), or after three months of PEP or PDPV, MD ‐0.50% predicted (95% CI ‐3.68 to 2.68) (13 participants) (Darbee 1990).
Two studies lasted at least one year (106 participants) (Gaskin 1998; McIlwaine 1997). In the one year study of children and adolescents, FEV1 improved by a mean of 5.98% predicted for the PEP group, while in the PDPV group it deteriorated by 2.28% predicted, this was a significant difference favouring PEP, MD 8.26 (95% CI 0.76 to 15.76) (40 participants) (McIlwaine 1997). However, in a two‐year study, predominately in adults, no significant difference in the rates of decline in FEV1 were reported between the PEP group and the PDPV group, with mean annual declines of 2.76% predicted and 2.11% predicted, respectively, MD ‐0.65 (95% CI ‐3.25 to 1.95) (66 participants) (Gaskin 1998).
One cross‐over study (13 participants) reported participants being withdrawn due to exacerbations, although these are not well‐defined (van Asperen 1987). It is also unclear which treatments the participants were randomised to at the time of departure.
2. Number of respiratory exacerbations between baseline and post‐intervention
In the Gaskin study (which used PEP with a mouthpiece), it was reported (personal communication) that there was no difference in the number of respiratory exacerbations (low‐quality evidence) (Gaskin 1998).
3. Direct measures of mucus clearance
One study (20 participants) measured radio‐labelled aerosol clearance after a single treatment of PEP and found no significant difference in clearance between PEP and PDPV (low‐quality evidence) (Darbee 1990). This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained so no data have been entered into the review.
Secondary outcomes
1. Expectorated secretions, dry or wet weight, or volume
Three cross‐over studies reported measures of expectorated sputum (43 participants) (Braggion 1995; Falk 1984; van Asperen 1987).
a. Single treatment
One study (14 participants) found that wet weight of sputum during and for 50 minutes after PEP (whether in sitting or PD positions) was greater than that induced by PDPV or pursed lip breathing (Falk 1984). These results were not available by treatment period, so are presented in an additional table (Table 6).
1. Wet weight of sputum during and 50 minutes after treatment (Falk 1984).
| Treatment |
Number of participants |
Mean (range) weight |
P value |
| PEP in sitting | 14 | 1421.6 (12.5 ‐ 53.5) g | P < 0.01 |
| PEP in PD positions | 14 | 17.4 (5.8 ‐ 50.7) g | P < 0.01 |
| Pursed Lip Breathing | 14 | 15.0 (5.4 ‐ 44.9) g | P < 0.01 |
| PDPV | 14 | 10.0 (1.9 ‐ 51.1) g | P < 0.01 |
PD: postural drainage PDPV: postural drainage, percussion and vibration PEP: positive expiratory pressure
b. Short‐term (up to seven days)
In one study (13 participants), no significant difference in expectorated secretions was identified between the treatment groups, measured by sputum volume (van Asperen 1987). In a further study (16 participants), no significant difference in wet or dry weight of sputum was identified between PEP and PDPV or HFCC and no further data were available (Braggion 1995).
c. Long‐term (more than seven days)
As outlined in the Types of outcome measures section, this review does not examine this outcome where it is measured after more than seven days of treatment.
2. Other pulmonary parameters
a. FVC
Six studies (165 participants) measured FVC (Darbee 1990; Falk 1984; Gaskin 1998; McIlwaine 1997; Tyrrell 1986; van Asperen 1987) (Analysis 1.2). Unpublished data were provided for four of these, allowing these studies to be included in analysis (Darbee 1990; Gaskin 1998; Tyrrell 1986; van Asperen 1987). First‐period data for only three studies were included in the analysis due to concerns over risk of bias as these cross‐over studies did not have a washout period (Darbee 1990; Tyrrell 1986; van Asperen 1987). Results are reported as change in % predicted for FVC.
1.2. Analysis.
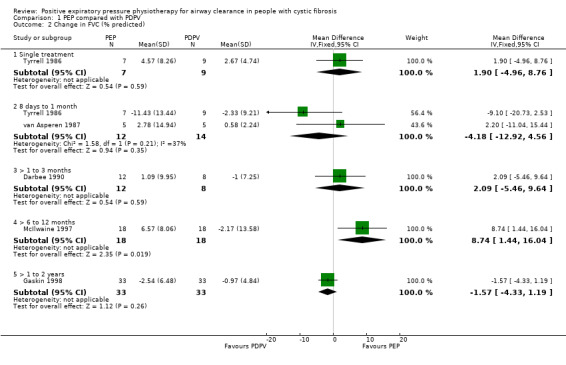
Comparison 1 PEP compared with PDPV, Outcome 2 Change in FVC (% predicted).
i. Single treatment
One study (19 participants) measured FVC after a single treatment (Tyrrell 1986). After a single treatment with PEP or PDPV, no significant difference in FVC was demonstrated, MD 1.90 (95% CI ‐4.96 to 8.76) (Tyrrell 1986) (Analysis 1.2).
ii. Short term (up to seven days)
In one study (14 participants), after two days of twice‐daily treatment, FVC significantly increased in a group performing PEP in sitting and significantly decreased in a group performing PDPV (Falk 1984). These results were not available by treatment period, so are presented in an additional table (Table 7).
2. FVC change after four treatments (Falk 1984).
| Treatment |
Number of participants |
Mean (range) change | P value |
| PEP in sitting | 14 | +6.6 (0 ‐ 11) % | P < 0.01 |
| PDPV | 14 | ‐ 4.7 (0 ‐ 7.9) % | P < 0.01 |
| PEP in PD positions | NA | not stated | NA |
| Pursed Lip Breathing | NA | not stated | NA |
Note: It is unclear whether these percentages refer to absolute percentage change or change in % predicted
FVC: forced vital capacity
NA: Not applicable
PD: postural drainage PDPV: postural drainage, percussion and vibration PEP: positive expiratory pressure
iii. Long term (more than seven days)
Five studies (151 participants) measured FVC after a series of treatments over more than seven days (Darbee 1990; Gaskin 1998; McIlwaine 1997; Tyrrell 1986; van Asperen 1987). After one month of twice‐daily treatments with PEP or PDPV, no significant difference in FVC was found in adolescents (19 participants) (Tyrrell 1986), or in children and adolescents (13 participants) (van Asperen 1987). Meta‐analysis of these two studies indicated a significant difference in favour of PDPV, MD ‐4.18 (95% CI ‐12.92 to 4.56) (Tyrrell 1986; van Asperen 1987) (Analysis 1.2). Results are reported as change in % predicted for FVC. In a further trial (13 participants), no significant difference in FVC was demonstrated after three months of PEP or PDPV, MD 2.09 (95% CI ‐5.46 to 9.64) (Darbee 1990).
At the end of a one‐year study (40 participants), mean FVC for the PEP group increased by 6.57% predicted, and mean FVC for the PDPV group decreased by approximately 2.17% predicted; this was a significant difference favouring PEP, MD 8.74 (95% CI 1.44 to 16.04) (McIlwaine 1997). In a two‐year study (66 participants), no significant difference in the rates of decline in FVC were reported between the PEP group and the PDPV group, with mean annual declines of 2.54% predicted and 0.97% predicted, respectively, MD ‐1.57 (95% CI ‐4.33 to 1.19) (Gaskin 1998).
b. FEF25‐75
Three studies (66 participants) reported results for FEF25‐75 (Darbee 1990; McIlwaine 1997; van Asperen 1987). Unpublished data were provided for the Darbee and van Asperen studies, allowing these studies to be included in analysis (Darbee 1990; van Asperen 1987). First‐period data only of two studies were included in analysis due to concerns over risk of bias as these cross‐over studies did not have a washout period (Darbee 1990; van Asperen 1987). Results are reported as change in % predicted for FEF25‐75.
i. Single treatment
No studies reported at this time point.
ii. Short‐term (up to seven days)
No studies reported at this time point.
iii. Long‐term (more than seven days)
Three (66 participants) studies measured FEF25‐75 after a series of treatments over more than seven days (Darbee 1990; McIlwaine 1997; van Asperen 1987) (Analysis 1.3).
1.3. Analysis.
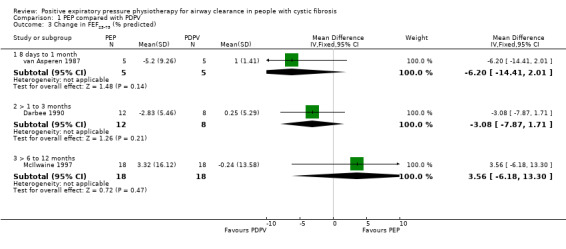
Comparison 1 PEP compared with PDPV, Outcome 3 Change in FEF25‐75 (% predicted).
No significant differences in FEF25‐75 were demonstrated after one month of PEP or PDPV, MD ‐6.20 (95% CI ‐14.41 to 2.01) (13 participants) (van Asperen 1987). No significant difference in FEF25‐75 was demonstrated after three months of PEP or PDPV, MD ‐3.08 (95% CI ‐7.87 to 1.71) (13 participants) (Darbee 1990).
At the end of a one‐year study (40 participants), mean FEF25‐75 for the PEP group increased by 3.32% predicted; mean FEF25‐75 for the PDPV group decreased by approximately 0.24% predicted. This equates to a MD for this study of 3.56 (95% CI ‐6.18 to 13.30) (McIlwaine 1997).
c. TLC
One study (13 participants) measured TLC (Darbee 1990), unpublished data were provided for the first period. No statistically significant difference in TLC was demonstrated after three months of PEP or PDPV, MD ‐3.38 (95% CI ‐13.67 to 6.91) (Darbee 1990) (Analysis 1.4).
1.4. Analysis.
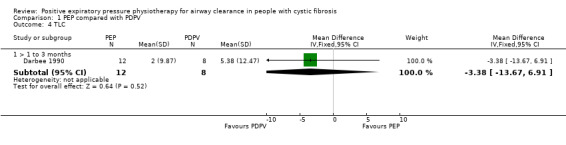
Comparison 1 PEP compared with PDPV, Outcome 4 TLC.
d. RV
No studies reported on this outcome.
3. Exercise tolerance
One study (66 participants) conducted exercise testing using cycle ergometry, but reported no data for this outcome measure (low‐quality evidence) (Gaskin 1998).
4. Well‐being
One study (66 participants) reported well‐being as an outcome measure and used the quality of well‐being (QWB) scale (Gaskin 1998). In the two‐year, parallel study of PEP versus PDPV, neither group demonstrated a significant change in QWB scores, which was similar at baseline and no further data were available (low‐quality evidence) (Gaskin 1998).
5. Blood oxygen levels
Two studies (40 participants) measured blood oxygen levels (Costantini 2001; Falk 1984).
a. Single treatment
No studies reported at this time point.
b. Short term (up to seven days)
In a study (14 participants) comparing four treatments once each over two days, the mean gain in SpO₂ 35 minutes after treatment was significantly higher for PEP in sitting than for PEP in postural drainage (PD) positions, for pursed lip breathing, or for PDPV (Falk 1984). These results were not available by treatment period, so are presented in an additional table (Table 8). It should be noted that the treatment durations were unequal in this study.
3. Oxygenation change by 35 minutes after treatment (Falk 1984).
| Treatment |
Number of participants |
Median (1‐3 quartile) oxygenation change |
Median (1‐3 quartile) treatment duration |
P value |
| PEP in sitting | 14 | 14.4 (4.6 ‐ 27.4) % | 20 (18‐32) min | P < 0.01 |
| PEP in pd | 14 | 3.2 (0 ‐ 15.4) % | 39 (28‐45) min | |
| PLB | 14 | 2.4 (‐8.0 ‐ 11.3) % | 21 (16‐32) min | |
| PDPV | 14 | 4.3 (‐9.4 ‐ 12.1) % | 37 (33‐43) min |
Note: Treatment durations were unequal
PD: postural drainage PDPV: postural drainage, percussion and ventilation PEP: positive expiratory pressure PLB: pursed lip breathing Rx: treatment
c. Long‐term (more than seven days)
One study (26 participants) measured SpO₂ after a series of treatments over more than seven days (Costantini 2001). In a one‐year study of infants, oxygen saturation values in the PEP group are described as "higher than the PDPV group in every evaluation (98.1% versus 96.7%, P = 0.049)". Participants were evaluated at 0, 6 and 12 months in this study, so it is unclear to which evaluation the data refer. The data have not been entered in Data and analyses as no measures of variability were available (Costantini 2001).
6. LCI
No studies reported on this outcome.
7. Ventilation scanning
Three studies (132 participants) reported on this outcome (Costantini 2001; Gaskin 1998; McIlwaine 1997) (Analysis 1.5; Analysis 1.6)
1.5. Analysis.
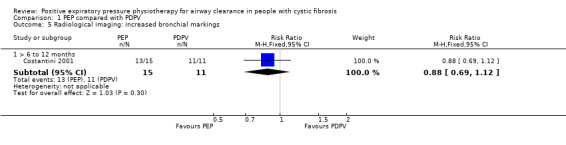
Comparison 1 PEP compared with PDPV, Outcome 5 Radiological imaging: increased bronchial markings.
1.6. Analysis.
Comparison 1 PEP compared with PDPV, Outcome 6 Radiological imaging: change in Brasfield score.
In a year‐long study of 26 infants, an increase in radiologic bronchial markings was less common in the PEP group than the PDPV group, RR 0.88 (95% CI 0.69 to 1.12) (Costantini 2001) (Analysis 1.5). In the same study, hyperinflation was assessed, but only data for the PDPV group are reported for this outcome.
A one‐year study of PEP versus PDPV (40 participants) measured Brasfield chest radiograph score, and reported identical results for the two groups, MD 0.00 (95% CI ‐1.20 to 1.20) (McIlwaine 1997) (Analysis 1.6). A two‐year study of PEP versus PDPV (66 participants) also measured Brasfield chest radiograph score, but reported no data for this outcome measure (Gaskin 1998).
8. Cost of intervention
No studies reported on this outcome.
9. Adherence to treatment and participant preference
One study (40 participants) monitored adherence with PEP versus PDPV over one year (McIlwaine 1997). Levels of compliance were recorded as 96% in the PEP group and 92% in the PDPV group (McIlwaine 1997), this is presented in an additional table and no SDs were reported (Table 9).
4. Adherence at one year (McIlwaine 1997).
| Treatment |
Number of participants |
Adherence |
| PEP | 18 | 92% (SD not stated) |
| PDPV | 18 | 96% (SD not stated) |
PDPV: postural drainage, percussion and ventilation PEP: positive expiratory pressure SD: standard deviation
Five studies (109 participants) reported on technique acceptability or participant preference (Braggion 1995; Costantini 2001; Darbee 1990; Falk 1984; McIlwaine 1997).
a. Single treatment
One study (14 participants) measured participant preference after a single treatment (Falk 1984). The preferred treatment for 11 of 14 participants was PEP in sitting when compared to PEP in PD positions, PDPV, or pursed lip breathing. It was reported that "even though all participants had received postural drainage and percussion as an integral part of treatment, they did not hesitate to accept (PEP in sitting), which was easier, less time‐consuming and could be used when needed" (Falk 1984).
b. Short‐term (up to seven days)
One study involving 16 participants measured participant preference after a short‐term treatment course. A two‐day course of four treatments with PEP was compared with the same regimen of PD with undefined chest physiotherapy techniques, or flutter. Three‐point rating scales (criteria unspecified) of effectiveness and tolerance were recorded after each arm, with no significant differences between interventions and no further data were available (Braggion 1995).
c. Long‐term (more than seven days)
Three studies (79 participants) measured participant preference after a treatment course of greater than seven days duration (Costantini 2001; Darbee 1990; McIlwaine 1997). In the long‐term, cross‐over study (13 participants), PEP was the treatment of choice when compared to PDPV, "patients... preferred PEP mask for convenience, independence and ease of use, as determined by a standardized written questionnaire" (not described) (Darbee 1990).
Participant preference also favoured PEP in two one‐year parallel studies (66 participants) (Costantini 2001; McIlwaine 1997). In the study of PEP versus PDPV (40 participants), participant preference was only recorded in the PEP group, as all participants were PEP‐naive prior to starting the study; and all 18 participants in the PEP group nominated the PEP intervention as their preferred airway clearance modality (McIlwaine 1997). Although it was not stated how participant preference was determined, the conclusion from the study of PEP versus PDPV in 26 infants was that the parents and infants 'greatly' preferred PEP (Costantini 2001). Data from these studies could not be obtained in sufficient detail for inclusion in analysis.
10. Adverse effects
Two studies with 66 participants reported adverse events as an outcome measure (low‐quality evidence) (Costantini 2001; McIlwaine 1997). In a year‐long study of PEP versus conventional PDPV in 40 children and adolescents, no adverse events were reported by either group (McIlwaine 1997).
In a one‐year study of 26 infants, side effects were described as rare. Although gastro‐oesophageal reflux was reported more commonly in the PEP group than the PDPV group, risk ratio (RR) 1.25 (95% CI 0.43 to 3.63), those in the PEP group described their reflux as mild. Reflux severe enough to cause withdrawal from the study was also examined, with all three cases occurring in the PDPV group, RR 0.14 (95% CI 0.01 to 2.52) (Costantini 2001) (Analysis 1.7).
1.7. Analysis.
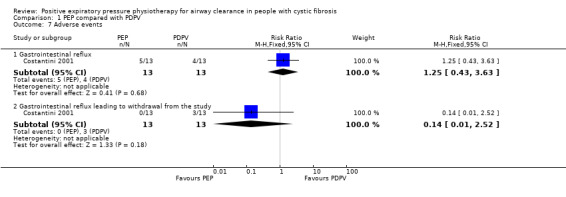
Comparison 1 PEP compared with PDPV, Outcome 7 Adverse events.
2. PEP compared with oscillating PEP (acapella, flutter and cornet)
This comparison was made in seven studies (247 participants) (Lagerkvist 2006; McIlwaine 2001; Newbold 2005; Pryor 2010; Tannenbaum 2005; van Winden 1998; West 2010). However, the Pryor study (75 participants) had five treatment arms and three treatment arms with 15 participants randomised to each arm (i.e. 45 participants in total) are included in this comparison (PEP, and two types of oscillating PEP (flutter and cornet)) (Pryor 2010).
Primary outcomes
1. FEV1
All seven studies measured FEV1 (moderate‐quality evidence) (Lagerkvist 2006; McIlwaine 2001; Pryor 2010; Newbold 2005; Tannenbaum 2005; van Winden 1998; West 2010) (Analysis 2.1).
2.1. Analysis.
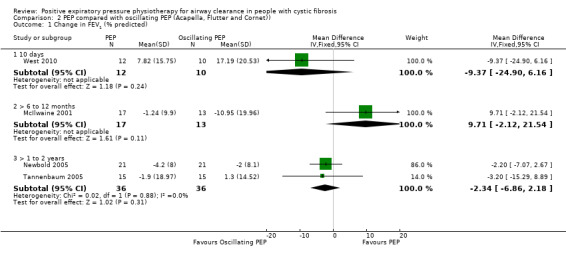
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 1 Change in FEV1 (% predicted).
a. Single treatment
Two studies (37 participants) found no significant difference in FEV1 (% predicted) after PEP compared to the flutter after a single treatment (Lagerkvist 2006; van Winden 1998). The two studies were cross‐over studies from which data from the end of the first randomisation arm could not be obtained.
b. Short term (up to seven days)
No studies considered this outcome in the short term.
c. Long term (more than seven days)
Six studies (202 participants) measured FEV1 after a series of treatments over more than seven days (McIlwaine 2001; Newbold 2005; Pryor 2010; Tannenbaum 2005; van Winden 1998; West 2010). All data entered into analysis is reported as the change from baseline in FEV1 (% predicted) (Analysis 2.1).
In one study (22 participants), no significant differences in FEV1 (% predicted) were demonstrated after two weeks of treatment with either PEP or flutter (van Winden 1998). This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained.
In a further study (23 participants), no significant differences in FEV1 were demonstrated after 10 days of treatment with either PEP or acapella, MD ‐9.37 (95% CI ‐24.90 to 6.16) (Analysis 2.1) (West 2010). However, enrolment did not meet the necessary threshold to detect a difference in FEV1.
Four studies lasted at least one year. In a one‐year study in 40 children and adolescents, FEV1 declined by a mean of 1.24% predicted in the PEP group, while in the flutter group it deteriorated by 10.95%, MD 9.71 (95% CI ‐2.12 to 21.54) (McIlwaine 2001) (Analysis 2.1). However, in a 13‐month study in 42 adults, the annual decline in FEV1 was 4.2% predicted in the PEP group and 2% predicted in the flutter group, MD ‐2.20 (95% CI ‐7.07 to 2.67) (Newbold 2005) (Analysis 2.1). Another one‐year study (30 participants) reported a decrease in FEV1 of 1.9% in the PEP group and an increase of 1.3% in the cornet group, MD ‐3.20 (95% CI ‐15.29 to 8.89) (Tannenbaum 2005). There was no significant difference between groups when the two studies were combined, MD ‐2.34 (95% CI ‐6.86 to 2.18) (Analysis 2.1).
In the Pryor study (45 participants), no reported significant difference was found in FEV1 between PEP, cornet and flutter with a decline over a one‐year period of 0.15 L with PEP, 0.03 L with cornet and 0.03 L with flutter (between‐group change data were not reported) (Pryor 2010).
2. Number of respiratory exacerbations between baseline and post‐intervention
Two parallel studies (82 participants) reported the number of respiratory exacerbations severe enough to require either intravenous (IV) antibiotics or hospitalisation (moderate‐quality evidence) (McIlwaine 2001; Newbold 2005). In a one‐year study with 20 participants per group, respiratory exacerbations severe enough to require hospitalisation occurred five times in the PEP group and 18 times in the flutter group. A Wilcoxon rank sum test indicated this difference was statistically significant favouring PEP (P = 0.03) but the number of hospitalisations per individual is not reported, so these data do not appear in our analysis (McIlwaine 2001). Similarly, a 13‐month study with 21 participants per group found respiratory exacerbations severe enough to require hospitalisation occurred six times in the PEP group and 14 times in the flutter group (moderate‐quality evidence) (Newbold 2005). This represented a mean of 0.3 hospitalisations per participant in the PEP group and 0.7 hospitalisations per participant in the flutter group, MD ‐0.40 (95% CI ‐0.92 to 0.12) (Analysis 2.2).
2.2. Analysis.
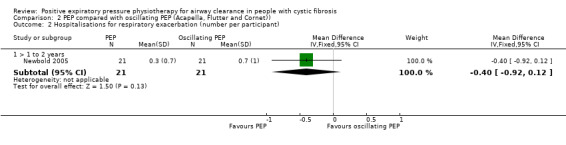
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 2 Hospitalisations for respiratory exacerbation (number per participant).
A further parallel study of one year (30 participants), reported the number of respiratory exacerbations requiring either oral or IV antibiotics as the primary outcome (Tannenbaum 2005) but no standard deviations were reported so this data could not be entered into analysis. This study reported that there were eight respiratory exacerbations reported in 14 participants in the PEP group (mean 0.57) and six respiratory exacerbations in 10 participants in the cornet group (mean 0.6) which was not significantly different (moderate‐quality evidence). It is unclear if these participants required IV only or IV or oral antibiotics.
3. Direct measures of mucus clearance
No studies reported on this outcome.
Secondary outcomes
1. Expectorated secretions, dry or wet weight, or volume
No studies reported this outcome.
2. Other pulmonary parameters
a. FVC
Six studies (217 participants) measured FVC (Lagerkvist 2006; McIlwaine 2001; Newbold 2005; Pryor 2010; van Winden 1998; West 2010) (Analysis 2.3)
2.3. Analysis.
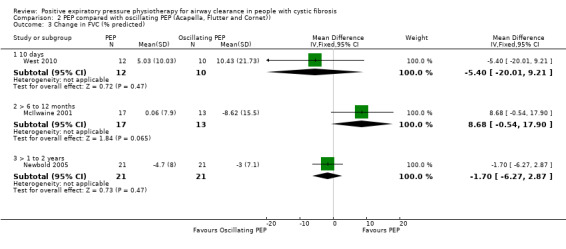
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 3 Change in FVC (% predicted).
i. Single treatment
Two cross‐over studies (37 participants) measured FVC after a single treatment, but data from the end of the first randomisation arm could not be obtained (Lagerkvist 2006; van Winden 1998). No significant difference in FVC (% predicted) was demonstrated after one treatment with either PEP or flutter (Lagerkvist 2006; van Winden 1998).
ii. Short term (up to seven days)
No studies reported at this time point.
iii. Long term (more than seven days)
Five studies (172 participants) measured FVC after a series of treatments over more than seven days (McIlwaine 2001; Newbold 2005; Pryor 2010; van Winden 1998; West 2010). All data entered into analysis is reported as the change from baseline in FVC (% predicted) (Analysis 2.3).
In one study (22 participants), no significant difference in FVC (% predicted) was demonstrated after two weeks of treatment with PEP or flutter (as above, no data available from first arm) (van Winden 1998). In a further study (23 participants), no significant difference in FVC was demonstrated after 10 days of treatment with PEP compared to acapella, MD ‐5.40 (95% CI ‐20.01 to 9.21) (West 2010) (Analysis 2.3).
In a one‐year study (40 participants), a decrease in mean FVC was reported with flutter of 8.62% predicted, while in the PEP group mean FVC increased 0.06% predicted, but this was not significant, MD 8.68 (95% CI ‐0.54 to 17.90) (McIlwaine 2001). In a 13‐month study (42 participants), the annual decline in FVC was 4.7% predicted in the PEP group and 3% predicted in the flutter group, MD ‐1.70 (95% CI ‐6.27 to 2.87) (Newbold 2005) (Analysis 2.3).
In another one‐year study (45 participants) comparing PEP to flutter and cornet, no significant difference between techniques were found although the study did not report the actual data (Pryor 2010).
b. FEF25‐75
Four studies (127 participants) reported results for FEF25‐75 (McIlwaine 2001; Newbold 2005; van Winden 1998; West 2010) (Analysis 2.4).
2.4. Analysis.
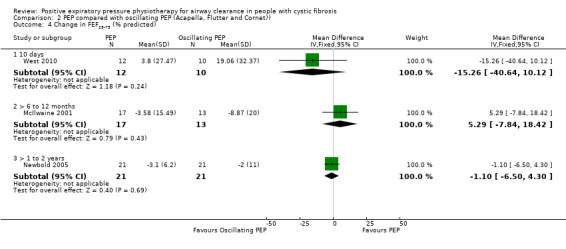
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 4 Change in FEF25‐75 (% predicted).
i. Single treatment
In one study (22 participants), no significant difference in FEF25‐75 (% predicted) was noted after one treatment with PEP or flutter (van Winden 1998). This study was a cross‐over study from which data from the first randomisation arm could not be obtained.
ii. Short term (up to seven days)
No studies reported at this time point.
iii. Long term (more than seven days)
Four studies (127 participants) measured FEF25‐75 after a series of treatments over more than seven days (McIlwaine 2001; Newbold 2005; van Winden 1998; West 2010). All data entered into analysis is reported as the change from baseline in FEF25‐75 (% predicted) (Analysis 2.4).
In one study (22 participants), no significant differences in FEF25‐75 were demonstrated after two weeks of treatment with either PEP or flutter (as above, no data available from first arm) (van Winden 1998). In a further study (23 participants), no significant differences in FEF25‐75 were demonstrated after 10 days of treatment with PEP compared to acapella, MD ‐15.26 (95% CI ‐40.64 to 10.12) (West 2010) (Analysis 2.4).
In a one‐year study in 40 children and adolescents, FEF25‐75 declined by a mean of 3.58% predicted in the PEP group, while in the flutter group it deteriorated by 8.87% predicted; however, the difference was not significant, MD 5.29 (95% CI ‐7.84 to 18.42) (McIlwaine 2001). In a 13‐month study (42 participants), annual decline in FEF25‐75 was 3.1% predicted in the PEP group and 2% predicted in the flutter group, MD ‐1.10 (95% CI ‐6.50 to 4.30) (Newbold 2005) (Analysis 2.4).
c. TLC
One cross‐over study (22 participants) measured TLC (as above, no data available from first arm) (van Winden 1998). No significant changes in TLC occurred both after one treatment and after two weeks of treatment with PEP and flutter (van Winden 1998).
d. RV
No studies reported this.
3. Exercise tolerance
Two studies (68 participants) conducted exercise testing using the modified shuttle test and reported no significant difference in outcome between PEP, AD, ACBT, cornet and flutter over a one‐year period (Pryor 2010) or between PEP and acapella over 10 day treatment, MD 6.32 (95% CI ‐15.46 to 28.10) (West 2010) (Analysis 2.5).
2.5. Analysis.
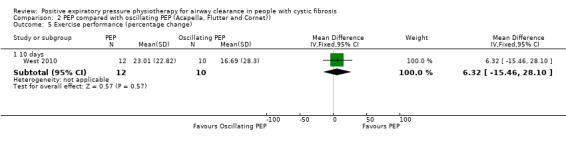
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 5 Exercise performance (percentage change).
4. Well‐being
Two studies (75 participants) reported well‐being as an outcome measure (low‐quality evidence) (Pryor 2010; Tannenbaum 2005). In the Tannenbaum study (30 participants), there was no significant change from baseline in either group on the QWB scale; no data are available as this study were only published in abstract form (Tannenbaum 2005).
Pryor (45 participants) found no significant differences between the five groups (PEP, cornet and flutter as well as AD and ACBT) in the physical domain (P = 0.99) or the mental domain (P = 0.27) using Short Form ‐36; and no significant differences among the five groups in the dyspnoea domain (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39) or mastery (P = 0.82) using the Chronic Respiratory Questionnaire (Pryor 2010). No data were reported that could be entered into analysis.
5. Blood oxygen levels
Two studies (37 participants) measured blood oxygen levels (Lagerkvist 2006; van Winden 1998).
a. Single treatment
In one cross‐over study (15 participants), with no data available for the first arm, both transcutaneous oxygen levels (Pto2) and carbon dioxide levels (Ptco2) were measured. The immediate results after a single treatment of flutter showed higher Pto2 (P = 0.05) and lower Ptco2 (P < 0.0001) compared to PEP, but at steady state after treatment all differences had disappeared (Lagerkvist 2006).
b. Short term (up to seven days)
No studies reported at this time point.
c. Long term (more than seven days)
One cross‐over study (22 participants), with no data available for the first arm, measured SpO₂ after a series of treatments over more than seven days (van Winden 1998). In this two‐week study, there was no difference in SPO₂ between flutter and PEP measured before, during and after treatment.
6. LCI
One study (30 participants) used multiple, breath inert gas washout to examine lung clearance (Tannenbaum 2005). They reported no significant difference in LCI between PEP and cornet over a one‐year period, MD 0.80 (95% CI ‐1.36 to 2.96) (low‐quality evidence) (Analysis 2.6).
2.6. Analysis.
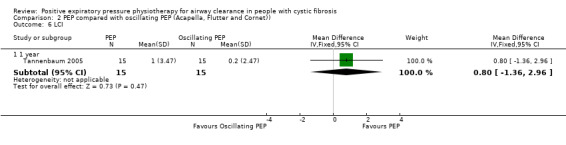
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 6 LCI.
7. Ventilation scanning
In a one‐year study of PEP versus flutter (40 participants), a blinded radiologist evaluated changes in chest radiographs. The groups were not significantly different, although no data were published to support this (McIlwaine 2001).
8. Cost of intervention
No studies reported on this outcome.
9. Adherence to treatment and participant preference
Three studies monitored adherence or patient satisfaction (McIlwaine 2001; Tannenbaum 2005; West 2010).
a. Single treatment
No studies reported at this time point.
b. Short term (up to seven days)
No studies reported at this time point.
c. Long term (more than seven days)
The West study used a patient satisfaction survey and found no significant difference between PEP and acapella after 10 days, MD ‐0.36 (95% CI ‐0.85 to 0.13) (West 2010) (Analysis 2.7).
2.7. Analysis.
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 7 User satisfaction (patient satisfaction survey).
In the one‐year study of PEP versus flutter (40 participants), two participants were withdrawn from the PEP group due to non‐compliance, RR 5.00 (95% CI 0.26 to 98.00) (McIlwaine 2001) (Analysis 2.8). While no participants were withdrawn from the flutter group for non‐compliance, five dropped out due to a perceived lack of treatment efficacy with flutter, and a further two were withdrawn due to clinical deterioration. Overall, adherence was reported as 95.6% for the PEP group and 93.8% for the flutter group (McIlwaine 2001). This is presented in an additional table (Table 10).
2.8. Analysis.
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 8 Adherence: at least 85% of prescribed treatments performed.
5. Adherence at one year (McIlwaine 2001).
| Treatment | Adherence |
| PEP | 95.6% (SD not stated) |
| Flutter | 93.8% (SD not stated) |
PEP: positive expiratory pressure SD: standard deviation
Participants preferred PEP in the one‐year parallel study of PEP versus flutter which reported "discontinuation due to lack of perceived effectiveness in clearing their secretions" (McIlwaine 2001). Of 40 participants, five discontinued for this reason, all from the flutter group; however, there was no significant difference between treatment groups, RR 0.09 (95% CI 0.01 to 1.54) (McIlwaine 2001) (Analysis 2.9).
2.9. Analysis.
Comparison 2 PEP compared with oscillating PEP (Acapella, Flutter and Cornet)), Outcome 9 Participant preference: self‐withdrawal due to lack of perceived effectiveness.
A one‐year study of PEP versus cornet (30 participants) reported that one child in each group stopped using the device as they found it 'ineffective and fiddly to clean' (Cornet group) or they preferred a previously used device (PEP group) (Tannenbaum 2005). No data were available to enter into analysis.
10. Adverse effects
One cross‐over study (22 participants) reported that five participants complained of dizziness when using the Flutter device which improved after further instructions on breathing techniques when using the device was provided (low‐quality evidence) (van Winden 1998).
No other studies reported than any adverse events occurred
3. PEP compared with HFCWO
This comparison was made in four studies (174 participants) (Braggion 1995; Darbee 2005; Fainardi 2011; McIlwaine 2013).
Primary outcomes
1. FEV1
Four studies measured FEV1 (Braggion 1995; Darbee 2005; Fainardi 2011; McIlwaine 2013) (Analysis 3.1; Analysis 3.2).
3.1. Analysis.
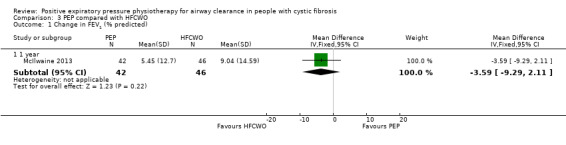
Comparison 3 PEP compared with HFCWO, Outcome 1 Change in FEV1 (% predicted).
3.2. Analysis.
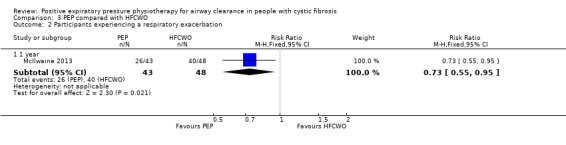
Comparison 3 PEP compared with HFCWO, Outcome 2 Participants experiencing a respiratory exacerbation.
a. Single treatment
Two studies (51 participants) measured FEV1 after a single treatment (Darbee 2005; Fainardi 2011). There was no significant difference in FEV1 (% predicted) after PEP compared to HFCWO (low‐quality evidence) (Darbee 2005; Fainardi 2011). Both were cross‐over studies from which data from the end of the first randomisation arm could not be obtained.
b. Short term (up to seven days)
One cross‐over study (16 participants), with no data available for the first arm, measured FEV1 after four treatments of PEP, postural drainage with undefined airway clearance techniques, or flutter over two days and found no significant differences in FEV1 (% predicted) (low‐quality evidence) (Braggion 1995).
c. Long term (more than seven days)
One study (107 participants) measured FEV1 after a series of treatments over more than seven days (McIlwaine 2013). In the only one‐year study comparing PEP to HFCWO, FEV1 increased by a mean of 0.22 L with PEP and 0.23 L with HFCWO (McIlwaine 2013). Data were provided at each study visit and the change in FEV1 (% predicted) over one year was not significantly different between the two groups, MD ‐3.59 (95% CI ‐9.29 to 2.11) (low‐quality evidence) (Analysis 3.1).
2. Number of respiratory exacerbations between baseline and post‐intervention
One parallel study with 107 participants which ran for one year, reported the number of respiratory exacerbations requiring either oral or IV antibiotics as the primary outcome (McIlwaine 2013). In 43 participants performing PEP, 26 had 49 respiratory exacerbations compared with 96 respiratory exacerbations in 40 of the 48 participants performing HFCWO, which showed a significant difference in favour of PEP, RR 0.73 (95% CI 0.55 to 0.95) (high‐quality evidence) (Analysis 3.2). This study by McIlwaine also reported that respiratory exacerbations, which were severe enough to require either IV antibiotics or hospitalisation, occurred six times in the PEP group (six participants) and 19 times in the HFCWO group (13 participants). This represented a mean of 0.12 respiratory exacerbations requiring IV antibiotics per participant in the PEP group and a mean of 0.4 respiratory exacerbations requiring IV antibiotics per participant in the HFCWO group (McIlwaine 2013).
3. Direct measures of mucus clearance
No study reported on this outcome.
Secondary outcomes
1. Expectorated secretions, dry or wet weight, or volume
Two cross‐over studies (52 participants), with no data available for the first arms, reported measures of expectorated sputum (Braggion 1995; Fainardi 2011).
a. Single treatment
One study (36 participants) measured expectorated secretions after a single treatment (Fainardi 2011). Wet weight of sputum was not significantly different after single treatments of PEP and HFCWO (Fainardi 2011).
b. Short term (up to seven days)
In one study (16 participants), no significant difference in wet or dry weight of sputum was identified between PEP and PDPV or HFCWO (Braggion 1995).
c. Long term (more than seven days)
As outlined in the Data extraction and management section, this review does not examine this outcome where it is measured after more than seven days of treatment.
2. Other pulmonary parameters
a. FVC
Three studies measured FVC (158 participants) (Darbee 2005; Fainardi 2011; McIlwaine 2013) (Analysis 3.3).
3.3. Analysis.
Comparison 3 PEP compared with HFCWO, Outcome 3 Change in FVC (% predicted).
i. Single treatment
Two cross‐over studies (51 participants), with no data available for the first arms, measured FVC (% predicted) after a single treatment (Darbee 2005; Fainardi 2011). In one study (15 participants), both PEP and HFCWO significantly improved FVC during an acute exacerbation, but there was no significant difference between either treatment (Darbee 2005). A second study (36 participants) comparing PEP to HFCWO over a single treatment also showed no significant difference (Fainardi 2011).
ii. Short term (up to seven days)
No study reported at this time point.
iii. Long term (more than seven days)
One study (107 participants) measured FVC after a series of treatments over more than seven days (McIlwaine 2013). No significant difference in FVC was demonstrated at the end of a one‐year study comparing PEP to HFCWO (McIlwaine 2013). The PEP group increased by mean 0.3 L and HFCWO by mean 0.35 L. Data were provided at each study visit and the change in FVC (% predicted) over one year was not significantly different between the two groups, MD ‐5.00 (95% CI ‐10.30 to 0.30) (Analysis 3.3).
b. Forced expiratory flow 25‐75%
Three studies measured FEF25‐75 (158 participants) (Darbee 2005; Fainardi 2011; McIlwaine 2013) (Analysis 3.4).
3.4. Analysis.
Comparison 3 PEP compared with HFCWO, Outcome 4 Change in FEF25‐75 (% predicted).
i. Single treatment
Two cross‐over studies (41 participants), with no data available for the first arms, measured FEF25‐75 (% predicted) after a single treatment (Darbee 2005; Fainardi 2011). In one study (15 participants), there was no significant difference between treatments (Darbee 2005). A second study (36 participants) comparing PEP to HFCWO over a single treatment also showed no significant difference (Fainardi 2011).
ii. Short term (up to seven days)
No study reported at this time point.
iii. Long term (more than seven days)
One study (107 participants) measured FEF25‐75 after a series of treatments over more than seven days (McIlwaine 2013). No significant difference in FEF25‐75 was demonstrated at the end of a one year study comparing PEP to HFCWO (McIlwaine 2013). The PEP group increased by mean 0.27 L and HFCWO by mean 0.19 L. Data were provided at each study visit and the change in FEF25‐75 (% predicted) over one year was not significantly different between the two groups, MD ‐0.34 (95% CI ‐12.54 to 11.86) (Analysis 3.4).
c. TLC
No study reported this outcome.
d. RV
No study reported this outcome.
3. Exercise tolerance
No study reported this outcome.
4. Well‐being
No study reported this outcome.
5. Blood oxygen levels
Two studies (51 participants) measured blood oxygen levels (Darbee 2005; Fainardi 2011). HFCWO was associated with a significant decrease in SpO₂ during treatment whereas the PEP technique produced significant increases in SpO₂ during treatment and is presented as a figure in the trial report.
a. Single treatment
One cross‐over study (36 participants), for which data were not available for the first arm, reported that during a single treatment of HFCC compared to HFCC, there was a small but significant decrease in SpO₂ values after PEP treatment (98% ± 1% versus 97% ± 1.2%; P < 0.001), but not after HFCC (97% ± 1.6% versus 97% ± 1.2%) (Fainardi 2011).
b. Short term (up to seven days)
No study reported at this time point.
c. Long term (more than seven days)
No study reported at this time point.
6. LCI
One cross‐over study (15 participants), for which data were not available for the first arm, reported the results of lung clearance using a single‐breath inert gas test which examines distribution of ventilation (Darbee 2005). This study compared PEP to HFCWO and showed an improvement in ventilation distribution and gas mixing with both treatments which was not significantly different between both techniques (very low‐quality evidence) (Darbee 2005).
7. Ventilation scanning
No study reported this outcome.
8. Cost of intervention
One study (107 participants) discussed costs of devices (PEP device at GBP 50 versus a HFCWO device at GBP 7000), but no further information was provided (McIlwaine 2013).
9. Adherence to treatment and participant preference
In a one‐year study (107 participants) comparing PEP to HFCWO, adherence was measured by participants keeping a daily diary and reported on monthly phone calls, adherence was recorded at 96% in both groups (McIlwaine 2013).
Two studies (52 participants) reported on technique acceptability or participant preference (Braggion 1995; Fainardi 2011).Due to the cross‐over design of these studies, data cannot be entered into analysis.
a. Single treatment
One study measured participant preference after a single treatment (Fainardi 2011). In this group of 36 participants comparing PEP to HFCWO,18 preferred PEP, three preferred HFCWO and 13 had no preference.
b. Short term (up to seven days)
One study involving 16 participants measured participant preference after a short‐term treatment course. A two‐day course of four treatments with PEP was compared with the same regimen of PD with undefined chest physiotherapy techniques, or flutter. Three‐point rating scales (criteria unspecified) of effectiveness and tolerance were recorded after each arm, with no significant differences between interventions (Braggion 1995) and no further data were available.
c. Long term (more than seven days)
No studies reported at this time point.
10. Adverse events
One parallel study with 107 participants which ran for one year, reported the number of adverse events (McIlwaine 2013). In total, 163 events were reported in the PEP group and 200 in the HFCWO group; this was not significantly different between the groups. However, there were significantly more adverse events related to the lower airways (increased cough, chest infection, haemoptysis, decreased lung function and chest pain) in the HFCWO group compared to the PEP group (mean 2.46 versus mean 1.72, P = 0.023) (high‐quality evidence).
4. PEP compared with BiLevel positive airway pressure (BiPAP)
Two studies compared PEP with BiPAP. BiPAP is typically used for ventilatory support, however, by changing the inspiratory and expiratory pressures on the device and combining it with huffing, it has been used as an airway clearance device (52 participants) (Kofler 1998; Rodriguez 2016). One was a short‐term cross‐over study (20 participants) (Kofler 1998) while the other was a long‐term comparative study (32 participants) (Rodriguez 2016).
Rodriguez reported data before and after treatment, analysing within group and between group differences with a generalised linear model (Rodriguez 2016). The data presented could not be entered into analysis so is presented in an additional table (Table 11).
6. Measures of lung function and exercise tolerance before and after treatment (Rodriguez 2016).
| Measure | PEP | NIV | P value | ||
| Pre | Post | Pre | Post | ||
| Forced Expiratory Volume in 1 second (litres) | 2.10 (0.6) | 2.03 (0.6) | 1.60 (0.5) | 1.54 (0.36) | 0.98 |
| Forced Expiratory Volume in 1 second (% predicted) | 55 (15) | 54 913) | 43 (12) | 41 (12) | 0.52 |
| Forced Vital Capacity (litres) | 3.6 (0.9) | 3.61 (0.87) | 2.82 (0.77) | 2.77 (0.84) | 0.54 |
| Forced Vital Capacity (% predicted) | 78 (13) | 78 (12) | 64 (12) | 61 (16) | 0.25 |
| Lung Clearance Index | 9.69 (2.5) | 9.76 (2.5) | 10.2 (2.37) | 9.2 (2.55) | 0.01 |
| 6 minute walk test (metres) | 539 (55) | 553 (77) | 553 (69) | 559 (95) | 0.76 |
All reported values are mean and standard deviation
NIV: Non invasive ventilation, PEP; positive expiratory pressure
Primary outcomes
1. FEV1
Both studies measured FEV1 (Kofler 1998; Rodriguez 2016).
a. Single treatment
One study (20 participants) measured FEV1 after a single treatment (Kofler 1998). There was no significant difference in FEV1 after PEP compared to non‐invasive bilevel ventilatory support (BiPAP) (low‐quality evidence) (Kofler 1998). This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained. It was unclear whether FEV1 was measured in litres or % predicted.
b. Short term (up to seven days)
No studies reported at this time point.
c. Long term (more than seven days)
One study (32 participants) measured FEV1 after a series of treatments over more than seven days (Rodriguez 2016). No significant differences in FEV1 (litres or % predicted) were demonstrated after three months of PEP compared to BiPAP (P = 0.98 and P = 0.52 respectively) (low‐quality evidence) (Rodriguez 2016) (Table 11)
2. Number of respiratory exacerbations between baseline and post‐intervention
No studies reported at this time point.
3. Direct measures of mucus clearance
No studies reported at this time point.
Secondary outcomes
1. Expectorated secretions, dry or wet weight, or volume
a. Single treatment
No studies reported at this time point.
b. Short term (up to seven days)
No short‐term studies were reported.
c. Long term (more than seven days)
As outlined above in the Types of outcome measures section, this review does not examine this outcome where it is measured after more than seven days of treatment.
2. Other pulmonary parameters
a. FVC
FVC was measured in the two studies (52 participants) (Kofler 1998; Rodriguez 2016).
i. Single treatment
One study (20 participants) measured FVC after a single treatment (Kofler 1998). No significant difference in FVC was noted after one treatment with PEP or BiPaP (Kofler 1998). This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained. It was unclear whether FVC was measured in litres or % predicted.
ii. Short term (up to seven days)
No studies reported at this time point.
iii. Long term (more than seven days)
One study (32 participants) measured FVC over a three‐month period (Rodriguez 2016). This study reported no significant differences in FVC (litres or % predicted) with either PEP or BiPAP (P = 0.54 and P = 0.25 respectively) (Rodriguez 2016) (Table 11).
b. FEF25‐75
One study (20 participants) reported results for FEF25‐75 (Kofler 1998).
i. Single treatment
No significant difference in FEF25‐75 was noted after one treatment with PEP or BiPAP (Kofler 1998) This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained.It was unclear whether FEF25‐75 was measured in litres or % predicted.
ii. Short term (up to seven days)
No studies reported at this time point.
iii. Long term (more than seven days)
No studies reported at this time point.
c. TLC
No studies reported on this outcome.
d. RV
No studies reported on this outcome.
3. Exercise tolerance
One study (32 participants) conducted exercise testing using the six‐minute walk test and reported no significant difference in outcome (P = 0.76) between PEP and BiPAP over a three‐month period (low‐quality evidence) (Rodriguez 2016) (Table 11).
4. Well‐being
No studies reported on this outcome.
5. Blood oxygen levels
Two studies (52 participants) measured blood oxygen levels (Kofler 1998; Rodriguez 2016).
a. Single treatment
One study (20 participants) was a single treatment. The improvement in SpO₂ during a single BiPAP treatment was statistically significantly greater than with one treatment with PEP (Kofler 1998), this is presented in an additional table (Table 12).
7. Oxygenation change during treatment (Kofler 1998).
| Treatment | Mean (SD) Chg SpO2 | P value |
| PEP | 0.04 (1.28) % | P = 0.036 |
| nBVS | 1.2 (2.12) % | P = 0.036 |
PEP: positive expiratory pressure nBVS: non‐invasive bilevel ventilatory support SD: standard deviation SpO2: percentage of oxygen saturation in blood
b. Short term (up to seven days)
There were no short‐term studies
c. Long term (more than seven days)
One study (32 participants) measured SpO₂ after a series of treatments over more than seven days (Rodriguez 2016). No significant differences in SpO₂ was demonstrated after three months of PEP or BiPaP (numerical results not presented) (Rodriguez 2016).
6. LCI
One study (32 participants) reported the results of lung clearance using a single‐breath inert gas test which examines distribution of ventilation (Rodriguez 2016). This study reported a significant improvement in the distribution of ventilation following BiPAP compared to PEP (P = 0.01) (low‐quality evidence) (Rodriguez 2016) (Table 11).
7. Ventilation scanning
No studies reported on this outcome.
8. Cost of intervention
No studies reported on this outcome.
9. Adherence to treatment and participant preference
One study (20 participants) measured participant preference after a single treatment (Kofler 1998). When compared to BiPAP, 60% of participants preferred BiPAP, 25% preferred PEP, and 15% had no preference (Kofler 1998).
10. Adverse events
Kofler reported that no untoward effects were found in any of the 20 participants (low‐quality evidence) (Kofler 1998). Rodriguez (32 participants) did not report whether any adverse events occurred (Rodriguez 2016).
5. PEP compared with a variety of different airway clearance techniques
Eight cross‐over studies compared PEP with different airway clearance techniques (116 participants) (Darbee 2004; Falk 1993; Hofmeyr 1986; Lannefors 1992; McIlwaine 1991; Mortensen 1991; Pfleger 1992; Steen 1991). Darbee (six participants) compared two types of PEP (Darbee 2004). Three studies (40 participants) compared PEP to ACBT (Falk 1993; Hofmeyr 1986; Mortensen 1991); Pfleger (15 participants) compared PEP and autogenic drainage (AD) (Pfleger 1992); Steen (28 participants) compared PEP alone, PDPV and FET, or five minutes of PEP followed by PDPV and FET (Steen 1991); McIlwaine (18 participants) compared PEP, PDP and AD (McIlwaine 1991); and Lannefors (nine participants) compared PEP, PD and physical exercise (Lannefors 1992).
As these studies were all cross‐over in design, limited data are available as data from the end of the first randomisation arm could not be obtained so no data are entered into analysis for this comparison.
An additional parallel group study, the Pryor study (75 participants) had five treatment arms and three treatment arms with 15 participants randomised to each arm (i.e. 45 participants in total) are included in this comparison (PEP, ACBT and AD) (Pryor 2010).
Primary outcomes
1. FEV1
Six studies (95 participants) measured FEV1 (low‐quality evidence) (Darbee 2004; Hofmeyr 1986; McIlwaine 1991; Mortensen 1991; Pfleger 1992; Steen 1991).
a. Single treatment
Two studies (21 participants) measured FEV1 after a single treatment (Darbee 2004; Pfleger 1992). In the Darbee study (six participants), there was no significant difference in FEV1 (% predicted) after PEP compared > 20 cm H₂O PEP (Darbee 2004). The Pfleger study (15 participants) found that FEV1 (% predicted) was significantly lower after a treatment of AD followed by Hi‐PEP, compared to AD alone (Pfleger 1992). This is presented in an additional table (Table 13).
8. FEV1 after single treatment (Pfleger 1992).
| Treatment | Mean (SD) FEV1 | P value |
| Hi‐PEP | 54 (20) % predicted | P < 0.05 |
| AD | 56 (19) % predicted | NS |
| Hi‐PEP then AD | 55 (18) % predicted | P < 0.02 |
| AD then Hi‐PEP | 54 (19) % predicted | NS |
AD: autogenic drainage NS: non significant PEP: positive expiratory pressure
b. Short term (up to seven days)
One study (18 participants) measured FEV1 after four treatments of PEP in sitting, PEP in postural drainage positions, or breathing exercises in postural drainage positions on a single day and found no significant differences in FEV1 (% predicted) (Hofmeyr 1986).
c. Long term (more than seven days)
Three studies (56 participants) measured FEV1 after a series of treatments over more than seven days (McIlwaine 1991; Mortensen 1991; Steen 1991). In one study (28 participants), no significant differences in FEV1 (% predicted) were demonstrated after one month of PEP, PEP followed by PDPV, PDPV, or PEP + FET (Steen 1991). Similarly, after two months of PEP, AD, or PDP (18 participants) there were no significant differences (McIlwaine 1991), nor in a further study (10 participants) after two months of PEP or PD plus FET (Mortensen 1991).
In the Pryor study (45 participants), no reported significant difference was found in FEV1 between PEP, cornet and flutter with a decline over a one‐year period of 0.15 L with PEP, 0.07 L with ACBT and 0.02 L with AD (between‐group change data were not reported) (Pryor 2010).
2. Number of respiratory exacerbations between baseline and post‐intervention
Two cross‐over studies (43 participants) reported participants being withdrawn due to exacerbations, although these are not well‐defined (very low‐quality evidence) (Pfleger 1992; Steen 1991). It is also unclear which treatments the participants were randomised to at the time of departure from any of these studies.
3. Direct measures of mucus clearance
Three studies (31 participants) measured radio‐labelled aerosol clearance after a single treatment of PEP (low‐quality evidence) (Falk 1993; Lannefors 1992; Mortensen 1991). All were cross‐over studies from which data from the end of the first randomisation arm could not be obtained so no data have been entered in the Data and analyses.
In one study (12 participants), radioisotope retention two hours after a 20‐minute treatment of PEP and FET was significantly less than for FET alone (Falk 1993), this is presented in an additional table (Table 14). For two studies (19 participants) no significant difference in clearance was identified between PEP plus FET and PD plus FET (Lannefors 1992; Mortensen 1991) or exercise plus FET (nine participants) (Lannefors 1992).
9. Percentage of radioaerosol retention (Falk 1993).
| Treatment | Mean (SD) at 0.5 hr | Mean (SD) at 1.0 hr | Mean (SD) at 2.0 hr | Mean (SD) at 24 hr |
| PEP + FET | 92.4 (5.0) % | 90.1 (4.8) % | 86.9 (5.1) % | 59.2 (8.0)% |
| FET | 92.7 (5.3) % | 90.8 (5.4) % | 89.9 (6.4) % | 61.3 (9.1%) |
FET: forced expiration technique PEP: positive expiratory pressure SD: standard deviation
Secondary outcomes
1. Expectorated secretions, dry or wet weight, or volume
Four cross‐over studies (49 participants) reported measures of expectorated sputum (Darbee 2004; Hofmeyr 1986; Mortensen 1991; Pfleger 1992).
a. Single treatment
Four studies measured expectorated secretions after a single treatment (Darbee 2004; Hofmeyr 1986; Mortensen 1991; Pfleger 1992). One study (18 participants) found wet weight of sputum was significantly greater during PD with FET and breathing exercises compared to PEP and FET in sitting (P < 0.001) or PEP and FET in PD positions (P < 0.025) (Hofmeyr 1986). This is presented in an additional table (Table 15). However, in one study (10 participants), wet weight of sputum expectorated during and for 120 minutes after treatment demonstrated no significant difference between PEP and PD (Mortensen 1991). In one study (six participants), dry weight of sputum was not significantly different after single treatments of PEP and > 20 cm H₂O PEP (Darbee 2004). One study (15 participants) demonstrated Hi‐PEP produced significantly more sputum than either AD (P < 0.001) or AD then Hi‐PEP (P < 0.001) (Pfleger 1992). This is presented in an additional table (Table 16).
10. Wet weight of sputum during and for 30 minutes after treatment (Hofmeyer 1986).
| Treatment | Mean (range) weight |
| BE in PD positions | 79.8 (30.7 ‐ 219.8) g |
| PEP in PD positions | 70.6 (24.7 ‐ 256.8) g |
| PEP in sitting | 66.1 (15.3 ‐ 189.4) g |
BE: breathing exercises PD: postural drainage PEP: positive expiratory pressure
11. Wet weight of sputum during treatment (Pfleger 1992).
| Treatment | Mean (SD) weight |
| Hi‐PEP | 50 (29) g |
| AD | 35 (25) g |
| Hi‐PEP then AD | 44 (29) g |
| AD then Hi‐PEP | 39 (23) g |
| NB Data measured from graph |
AD: autogenic drainage PEP: positive expiratory pressure SD: standard deviation
b. Short term (up to seven days)
When wet weight of sputum was measured during and for 30 minutes after treatment, breathing exercises in postural drainage positions induced significantly greater sputum expectoration than PEP in postural drainage positions. The latter in turn produced significantly more expectorate than PEP in sitting (18 participants) (Hofmeyr 1986). This is presented in an additional table (Table 15).
c. Long term (more than seven days)
As outlined above in the Types of outcome measures section, this review does not examine this outcome where it is measured after more than seven days of treatment.
2. Other pulmonary parameters
a. FVC
FVC was measured in four studies (67 participants) (Darbee 2004; McIlwaine 1991; Pfleger 1992; Steen 1991).
i. Single treatment
Two studies (21 participants) measured FVC after a single treatment (Darbee 2004; Pfleger 1992). In one study (six participants), no significant difference in FVC was demonstrated with a single treatment of PEP versus > 20 cm H₂O PEP (Darbee 2004). The second study (15 participants) found that FVC was significantly lower after a treatment of AD followed by Hi‐PEP, compared to AD alone (Pfleger 1992). This is presented in an additional table (Table 17).
12. FVC after single treatment (Pfleger 1992).
| Treatment | Mean (SD) FVC | P value |
| Hi‐PEP | 73 (20) % predicted | P < 0.01 |
| AD | 74 (19) % predicted | P < 0.05 |
| Hi‐PEP then AD | 73 (20) % predicted | P < 0.01 |
| AD then Hi‐PEP | 71 (21) % predicted | NS |
AD: autogenic drainage FVC: forced vital capacity NS: non significant PEP: positive expiratory pressure SD: standard deviation
ii. Short term (up to seven days)
No studies reported at this time point.
iii. Long term (more than seven days)
Two studies (46 participants) measured FVC after a series of treatments over more than seven days (McIlwaine 1991; Steen 1991). One study (28 participants) reported no significant differences in FVC (% predicted) were demonstrated after one month of PEP, PEP followed by PDPV, PDPV, and PEP + FET (Steen 1991). A study (18 participants) comparing two months of PEP, PDPV or AD also showed no significant differences in FVC (% predicted) (McIlwaine 1991).
In another one‐year study (45 participants) comparing PEP to ACBT and AD, no significant difference between techniques were found although the study did not report the actual data (Pryor 2010).
b. FEF25‐75
Four studies (62 participants) reported results for FEF25‐75 (Darbee 2004; McIlwaine 1991; Mortensen 1991; Steen 1991).
i. Single treatment
No significant difference in FEF25‐75 (% predicted) was noted after one treatment with PEP or > 20 cm H₂O PEP (six participants) (Darbee 2004). This was a cross‐over study from which data from the end of the first randomisation arm could not be obtained.
ii. Short term (up to seven days)
No studies reported at this time point.
iii. Long term (more than seven days)
Two studies (46 participants) measured FEF25‐75 after a series of treatments over more than seven days (McIlwaine 1991; Steen 1991). In one study (28 participants) there were no significant differences in FEF25‐75 (% predicted) were demonstrated after one month of PEP, PEP followed by PDPV, PDPV, and PEP + FET (Steen 1991). A study (18 participants) comparing two months of PEP, PDP or AD also showed no significant differences in FEF25‐75 (% predicted) (McIlwaine 1991).
c. TLC
No studies reported on this outcome.
d. RV
In one study (six participants), it was reported that the change in RV from baseline was not significantly different after a single treatment of PEP or > 20 cm H₂O PEP (Darbee 2004).
3. Exercise tolerance
One study (45 participants) conducted exercise testing using the modified shuttle test and reported no significant difference in outcome between the five groups (PEP, AD and ACBT, as well as cornet and flutter) over a one‐year period (Pryor 2010)
4. Well‐being
The Pryor study (45 participants) found no significant differences between the five groups (PEP, AD and ACBT, as well as cornet and flutter) in the physical domain (P = 0.99) or the mental domain (P = 0.27) using Short Form ‐36; and no significant differences among the five groups in the dyspnoea domain (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39) or mastery (P = 0.82) using the Chronic Respiratory Questionnaire (Pryor 2010). No data were reported that could be entered into analysis.
5. Blood oxygen levels
Two studies (24 participants) measured blood oxygen levels (Darbee 2004; Hofmeyr 1986).
a. Single treatment
One study (six participants) was a single treatment; the change in SpO₂ was not significantly different after a single treatment of PEP versus > 20 cm H₂O PEP (Darbee 2004).
b. Short term (up to seven days)
In one study (18 participants), there were no significant mid‐ or post‐treatment differences between four treatments of breathing exercises with forced expirations in postural drainage positions, PEP in postural drainage positions, and PEP in sitting in a single day (Hofmeyr 1986).
c. Long term (more than seven days)
No studies reported on this outcome.
6. LCI
One study (six participants) reported the results of lung clearance using a single‐breath inert gas test which examines distribution of ventilation (Darbee 2004). This study reported worsening of the distribution of ventilation following PEP and high‐pressure PEP compared to control (Darbee 2004); however, in this study gas mixing improved suggesting that PEP opened up previously closed partially obstructed airways.
7. Ventilation scanning
No studies reported on this outcome.
8. Cost of intervention
No studies reported on this outcome.
9. Adherence to treatment and participant preference
Two studies (46 participants) reported on technique acceptability or participant preference (McIlwaine 1991; Steen 1991).
a. Single treatment
No studies reported at this time point.
b. Short term (up to seven days)
No studies reported at this time point.
c. Long term (more than seven days)
Two studies measured participant preference after a treatment course of greater than seven days duration (McIlwaine 1991; Steen 1991).
The cross‐over study (18 participants) comparing two months of PEP, conventional PDPV, and AD recorded five subjective measures which may influence participant preference: treatment duration; treatment comfort; flexibility of treatment times; control in performing own treatment; and how interruptive treatment was to daily living (McIlwaine 1991). It was reported that PEP had a significantly shorter treatment time than PDPV or AD; also, PEP was rated significantly better than PDPV and not significantly different to AD on each of the other four measures (McIlwaine 1991). Standard deviations were not available for these outcomes and mean data are presented in an additional table (Table 18).
13. Measures of technique acceptability (McIlwaine 1991).
| Treatment | Mean Duration of Rx | Comfort Score | Flexibility Score | In Control of Own Rx | Disruption Score |
| PEP | 21 | 75 | 73 | 89 | 33 |
| AD | 25 | 84 | 73 | 87 | 35 |
| PDPV | 27 | 49 | 42 | 62 | 63 |
| Scales | 0 = very uncomfortable 100 = very comfortable |
0 = very rigid 100 = very flexible |
0 = no control 100 = full control |
0 = Rx not interruptive 100 = Rx very interruptive |
|
AD: autogenic drainage PDPV: postural drainage, percussion and vibration PEP: positive expiratory pressure Rx: treatment
In another long‐term, cross‐over study (28 participants), PEP was the treatment of choice (Steen 1991). A total of 23 of 24 participants chose PEP in combination with FET in preference to PEP alone, PDPV and FET, or five minutes of PEP followed by PDPV and FET as their long‐term airway clearance physiotherapy (Steen 1991).
10. Adverse events
None of the studies reported whether adverse events occurred or not.
Subgroup analyses
None of the intended subgroup analyses were possible due to small numbers of studies or insufficient detail to allow the separation of subgroup data within any study. One study provided subgroup analysis based on age, which did not conform to the age groups for subgroup analysis defined in the protocol for this review. The data for the subgroup used are presented as an additional table (Table 19).
14. FEV1 change over two years in participants under 19 years of age (Gaskin 1998).
| Treatment | FEV1 change |
| PEP | ‐1.58% predicted per year (SD not stated) |
| PDPV | ‐1.65% predicted per year (SD not stated) |
PDPV: postural drainage, percussion and ventilation PEP: positive expiratory pressure SD: standard deviation
Sensitivity analyses
None of the intended sensitivity analyses were possible due to small numbers of studies included in each comparison.
Discussion
Summary of main results
A total of 28 studies involving 788 participants with CF (infants, children, adolescents and adults) met the review's inclusion criteria. Sample sizes in individual studies ranged from five to 107 participants; 21 studies were reported in full articles and seven were published in abstract form only.
There was a wide range of therapies to which PEP was compared. The duration of the intervention period varied from single treatments to two years. These factors together with the frequent use and poor reporting of cross‐over design, the small number of studies, and the limited information provided by some authors restricted the number of meta‐analyses that could be performed.
A one‐year study in children and adolescents showed a reduced rate of hospital admission due to respiratory exacerbations with PEP as opposed to flutter (McIlwaine 2001). A similar study in adults showed similar results (Newbold 2005). A large (107 participants) well‐designed one‐year multicentre randomised controlled trial comparing PEP with HFCWO found that PEP reduces the number of pulmonary exacerbations requiring oral, inhaled or IV antibiotics during the 12 months and results in a longer time to the first pulmonary exacerbation compared to the HFCWO group (McIlwaine 2013).
The FEV1 measurement of lung function is important in CF because of its correlation with survival and quality of life (Liou 2001). Six studies did not report FEV1 as an outcome measure. Four of these studies examined mucus clearance using a radio‐labelled isotope (Darbee 1990; Falk 1993; Lannefors 1992; Mortensen 1991). One study measured FEV0.75 (Tyrrell 1986), and another study was on infants where FEV1 could not be measured (Costantini 2001). Five studies measured FEV1 and compared PEP with PDPV; one was a study of less than seven days and four studies lasted more than seven days. Two studies lasted at least one year. In a 12‐month study in children with CF, FEV1 improved in the PEP group; with an increase in the PEP group and decreased in the PDPV group (McIlwaine 1997). However, in a two‐year study (predominantly in adults) comparing PEP with PDPV, there was no difference in the rates of decline in FEV1. Both groups experienced rates of annual decline greater than 2% (Gaskin 1998). When PEP was compared with oscillating PEP using the flutter or acapella there were seven studies that measured FEV1. Three short‐term studies (less than seven days) found that there was probably little or no difference between techniques. Five studies measured FEV1 after a series of treatments over more than seven days. Again, in relation to these techniques in a two‐week study, it was shown that there is probably little or no difference between the techniques and there were mixed results in the four studies lasting one year. In one study of children and adolescents, FEV1 declined more in the flutter group than the PEP group (McIlwaine 2001); while in a 13‐month study in adults both groups declined similarly (Newbold 2005). No difference was found in two further one‐year studies comparing these and other techniques including ACBT, AD, oscillating PEP using the cornet device and BiPAP (Tannenbaum 2005; Pryor 2010; Rodriguez 2016).
A small number of studies found significant differences in expectorated sputum measures when other types of chest physiotherapy were compared to PEP in single treatment and short‐term studies up to seven days (Falk 1984; Hofmeyr 1986) or Hi‐PEP (Pfleger 1992). However, these measures may be confounded by expectorated saliva, swallowed secretions and regurgitated contents and are generally regarded as less useful outcomes than measurement of mucociliary clearance. One cross‐over study measured radio‐labelled aerosol clearance after a single treatment of PEP versus PDPV; no significant difference in clearance was found (Darbee 1990).
LCI is becoming a popular outcome measure for use in CF studies. LCI was reported in four studies. In one low‐quality study it was shown that PEP probably makes little or no difference when compared to the Cornet over a one year period (Tannenbaum 2005). Darbee in a single treatment demonstrated an improvement in LCI with both PEP and HFCWO, but we are uncertain whether PEP, as compared to HFCWO, improves LCI as the certainty of the evidence has been assessed as very low (Darbee 2005). In a three‐month study BiPAP demonstrated an improvement in LCI when compared to PEP (Rodriguez 2016).
Many other outcomes did not show a difference between PEP as compared to other therapies. In the year‐long study in infants, blinded examination of chest radiographs showed no difference in the incidence of increased bronchial markings between the PEP and PDPV groups (Costantini 2001). When compared to flutter in children and adolescents, similar results occurred in FEF25‐75 and TLC (McIlwaine 2001).
There was limited evidence reported on adverse events; these were measured in five studies, two of which found no events. It was recorded that there were no adverse events in the PEP group or in the PDPV group in the year long study of 40 children (McIlwaine 1997). Nor were adverse events recorded in the PEP or the flutter groups in the year‐long study of 40 children (McIlwaine 2001). In a year‐long study of 26 infants, which was published in abstract form only, there was no statistically significant difference in the incidence of reflux between the PEP and PDPV groups; gastro‐oesophageal reflux severe enough to cause withdrawal from the study occurred in three participants in the PDPV group and in no participants in the PEP group (low‐quality evidence) (Costantini 2001). In PEP versus oscillating PEP, adverse events were only reported in the flutter group (five participants complained of dizziness, which improved after further instructions on device use was provided) (22 participants, low‐quality evidence) (van Winden 1998). In PEP versus HFCWO, from one long‐term high‐quality study (107 participants) there was little or no difference in terms of number of adverse events; however, those in the PEP group had fewer adverse events related to the lower airways when compared to HFCWO (high‐certainty evidence) (McIlwaine 2013).
In the majority of studies included in this Cochrane Review, there were no significant differences in outcomes when PEP was compared to other techniques. However, three long‐term trials undertaken in Canada using PEP have found that there was an improvement in outcomes in the PEP group as compared to PDPV in children and adolescents (McIlwaine 1997), oscillating PEP using the flutter (McIlwaine 2001) and HFCWO in a multicentre trial across all age groups (McIlwaine 2013). These studies provide evidence that PEP may be more effective in limiting exacerbations and potentially preserving lung function in the longer term. Of note, these three studies all employed PEP via the mask set‐up.
In the study comparing PEP with HFCWO, treatment time was significantly shorter in the PEP group (McIlwaine 2013). Limited evidence was identified in support of the hypothesis that PEP is more acceptable to people with CF than other forms of airway clearance therapy. In studies with an intervention period of at least one month, any measures of participant preference were in favour of PEP. The tools used to record patient preference were generally not well‐described or validated.
There were a large number of cross‐over studies in the sample included in the review. Elbourne discusses methods for meta‐analysing cross‐over studies which rely on the data that are reported within the primary papers (Elbourne 2002). The method that has been adopted within this review uses the data from the first period only, ignoring any data from the second period that was available if a carryover effect was identified. In this review three studies of one month duration were identified as having no washout period and thus may have had a carryover effect (Steen 1991; Tyrrell 1986; van Asperen 1987). A limitation of this study was that data from the first period of cross‐over studies was only available in three out of the 18 cross‐over studies.
Overall completeness and applicability of evidence
There is a large body of evidence to support the use of PEP as a stand‐alone airway clearance technique in CF. Numerous studies found PEP to be equal to other compared techniques, however, a few long‐term studies suggest that PEP may be superior to other techniques. In the majority of these studies PEP was administered using a facemask in order to obtain a seal during the cycles of PEP which resulted in a temporary increase in FRC during the cycles; this in turn allowed air to accumulate behind secretions and move them proximally. Where PEP was found to be superior to other techniques, the way in which it was applied may have contributed to these superior findings. Thus the applicability of the evidence is highly based on these studies which have demonstrated fewer exacerbations and decreased need for treatment with antibiotics.
In 22 out of the 28 studies PEP was administered using a face mask in order to obtain a seal during the cycles of PEP which resulted in a temporary increase in FRC during the cycles; this in turn allowed air to accumulate behind secretions and move them proximally. Where PEP was found to be superior to other techniques, the way in which it was applied may have contributed to these superior findings. In two similar studies where PEP was compared to PD&P over a one year period, in the one study where PEP was performed using a mask, there was a significant increase in FEV1, while in the other study where a mouthpiece was used, there was no significant difference in FEV1 (low‐quality evidence) (Gaskin 1998; McIlwaine 1997).
Quality of the evidence
The quality of the evidence varied between studies carried out over a few days to well‐designed year‐long randomised controlled trials. Small sample sizes and dropouts in some studies were likely to have impacted on the precision of the results and the quality of the evidence. As stated below, cross‐over designs are not really appropriate for CF studies and results from studies of this design are likely to be of low quality. Blinding of participants and researchers in these studies is not possible, however, blinded assessors have been utilised in a number of the studies increases the quality of the evidence and reduced the risk of biased results. Some unclear information regarding methodology (randomisation sequences and allocation concealments) increased the risk of bias.
Evidence provided for by this review for primary outcome number of respiratory exacerbations was judged to be vary between very low to high quality, although this outcome was not reported for all comparisons. Evidence for other outcomes, including the primary outcome FEV1 was more limited, inconsistent and imprecise and ranged in quality from very low to moderate quality.
Potential biases in the review process
The search process was rigorous and undertaken according to the Cochrane Collaboration's recommendations. It is possible that studies have been undertaken that have not been identified with this online review process or that omissions have inadvertently occurred in the search of conference publications because they have not emerged using the recommended search process. Three authors were involved in the selection of studies to be included in the review and disagreements were resolved by discussion and consensus. When a study that included one of the authors of this report was being considered for inclusion, the author recused themselves from the selection process in relation to that article.
Authors' conclusions
Implications for practice.
The quality of the evidence varied between studies carried out over a few days to well‐designed year‐long randomised controlled trials; however, the evidence from the studies described in this updated Cochrane Review suggests that all techniques and devices may have a place in the clinical treatment of patients with cystic fibrosis (CF). In relation to respiratory exacerbations, high‐quality evidence (a long‐term multicentre study across all ages) showed that, when compared to high‐frequency chest wall oscillation (HFCWO), positive expiratory pressure (PEP) using a mask reduced respiratory exacerbations.. There was some evidence to recommend PEP as a more acceptable intervention long term than other forms of physiotherapy for people with CF. However, the evidence that PEP was preferred over other techniques came from studies which were generally of low quality. Airway clearance techniques should be individualised throughout life according to developmental stages, patient choice, pulmonary symptoms and lung function. They may need to be varied during pulmonary exacerbations versus baseline function. It should be noted that although PEP via a mouthpiece system is commonly used, most of the literature pertains to PEP using a mask. The mouthpiece has not shown to increase FRC in the manner achieved by mask PEP and there is ambiguity around opportunity for upper airway air leaks when using a mouthpiece.
Implications for research.
Short‐term interventions on stable participants may be of little value given the nature of CF lung disease which frequently follows a chronic course with acute exacerbations. Future studies should be planned to reflect clinical practice by focusing on short‐term interventions during exacerbations or long term studies on initially stable patients.
Blinding of researchers and participants in studies comparing different airway clearance techniques is not possible. However, blinded assessors have been utilised in a number of the reviewed studies which increases the quality of the evidence and reduces the risk of bias. Blinded assessors should be involved in all future studies which may have resource and cost implications which should be considered when planning studies.
Cross‐over studies are not the best design for clinical studies in CF due to the unstable nature of the disease (Southern 2003). They may be influenced by carry‐over effects. Future parallel, randomised clinical studies comparing PEP with other airway clearance modalities are needed. These studies should be adequately powered and a multicentre approach may facilitate this. Such studies should in particular, examine the influence of PEP and other therapies on lung function parameters, quality of life, exercise tolerance, cost and survival.
The abstract format often prevents evaluation of the scientific methodology of the study. Abstracts should be structured to contain essential information about method and results. The large proportion of studies which were published only as abstracts highlights the need for full publication in peer reviewed journals.
The studies in this review frequently found no significant difference in efficacy between treatments. Expectorated sputum measures may be confounded by expectorated saliva, swallowed secretions and regurgitated contents and are generally regarded as less useful outcomes than measurement of mucociliary clearance. Sputum rheology differs between individuals with CF and between different organisms. Research to determine the best technique for situations where sputum is thin and liquid versus thick and viscous may be a new area for more targeted research and application of different techniques in different patients.
Exacerbation rate and time to first exacerbation in longer term trials (at least 12 months) between compared airway clearance techniques may be of greater use and relevance in CF, a long‐term disease. Future studies should include validated measures of patient preference including treatment time (a potential barrier to adherence) as this may assist in determining a suitable treatment when measures of efficacy are equivocal. Similarly, cost and adverse effect outcome data would assist consumers in decision‐making. Studies that broaden the knowledge of the effects of different techniques on different aspects of the pathophysiology of CF will progress the aspiration for evidence based physiotherapy in CF.
What's new
| Date | Event | Description |
|---|---|---|
| 25 November 2019 | New citation required but conclusions have not changed | The addition of two new studies (55 participants) to this review update have not resulted in significant changes to the conclusions. |
| 25 November 2019 | New search has been performed | Two new studies (55 participants) have been included in the review (Rodriguez 2016; West 2010). Six studies have been added to 'Excluded studies' (Aubriot 2016; Orlik 2015; Reychler 2015; Richmond 2016; Wettstein 2014; Wilson 2015). A further three trials have been added to 'Studies awaiting classification' and will be further assessed at the next update (Radtke 2018; Vendrusculo 2019; Ward 2018). |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 20 May 2015 | New search has been performed | A search of the Group's Cystic Fibrosis Trials register identified potentially eligible trials. Six newly identified studies met the inclusion criteria (Darbee 2005; Fainardi 2011; Lagerkvist 2006; McIlwaine 2013; Pryor 2010; Tannenbaum 2005). A further eight were excluded after assessment (Aquino 2006; Borka 2012; Bishop 2011; McCarren 2006; Munro 2007; Patel 2013; Placidi 2001; Sanchez Riera 1999). A total of six studies are awaiting assessment (Elkins 2005; Kofler 1994; Parreira 2008; Rodriguez 2013a; West 2010a; Wong 2000). Five studies that were included in the previous publication of this paper have been excluded (Balestri 2004; Battistini 2001; Padman 1999; Placidi 2001; van der Schans 1991). Two used an underwater positive expiratory pressure (PEP) technique, two used a flow‐independent PEP system and the fifth one, which had been published previously only as an abstract, has now been published as a full paper and revealed that the technique studied was not the PEP technique. A new review team has updated the review which was originally Pubilshed by Mark Elkins, Alice Jones and Cees van der Schans. |
| 20 May 2015 | New citation required and conclusions have changed | The conclusions of this review have been amended due to the inclusion of new studies and the exclusion of previously included studies. |
| 12 November 2008 | Amended | Converted to new review format. |
| 22 February 2006 | New search has been performed | Five studies have been added to the list of included studies in this update (Balestri 2004; Battistini 2001; Darbee 2004; Newbold 2005; Placidi 2001). Five studies have been added to the list of excluded studies (Castle 1994; Dosman 2003; Fitzgerald 2001; Oermann 2001; Orlik 2000). |
| 22 February 2006 | Amended | Cees van der Schans has stepped down as co‐author on this review as from February 2006. |
Acknowledgements
The review authors would like to acknowledge the patient assistance of our Managing Editors, Tracey Remmington and Nikki Jahnke, and previous editor, Dr Gerard Ryan.
The review authors would also like to acknowledge Mark Elkins, Cees van der Schans and Alice Jones for their contribution to the original protocol and review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search Methods – Electronic Searches
| Database/ Resource | Strategy |
| Clinicaltrials.gov | [Advanced Search] OTHER TERMS: positive expiratory pressure OR PEP OR chest physiotherapy STUDY TYPE: Interventional Studies CONDITION/ DISEASE: cystic fibrosis OR cf OR mucoviscidosis |
| WHO ICTRP | [Advanced Search] CONDITION: cystic fibrosis OR cf OR mucoviscidosis AND INTERVENTION: positive expiratory pressure OR PEP OR chest physiotherapy RECRUITMENT STATUS: All |
Data and analyses
Comparison 1. PEP compared with PDPV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (% predicted) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 8 days to 1 month | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐6.33, 7.53] |
| 1.2 2 to 3 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.68, 2.68] |
| 1.3 7 to 12 months | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 8.26 [0.76, 15.76] |
| 1.4 2 years | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐3.25, 1.95] |
| 2 Change in FVC (% predicted) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Single treatment | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐4.96, 8.76] |
| 2.2 8 days to 1 month | 2 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐4.18 [‐12.92, 4.56] |
| 2.3 > 1 to 3 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 2.09 [‐5.46, 9.64] |
| 2.4 > 6 to 12 months | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 8.74 [1.44, 16.04] |
| 2.5 > 1 to 2 years | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐4.33, 1.19] |
| 3 Change in FEF25‐75 (% predicted) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 8 days to 1 month | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐6.2 [‐14.41, 2.01] |
| 3.2 > 1 to 3 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.08 [‐7.87, 1.71] |
| 3.3 > 6 to 12 months | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 3.56 [‐6.18, 13.30] |
| 4 TLC | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 > 1 to 3 months | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐3.38 [‐13.67, 6.91] |
| 5 Radiological imaging: increased bronchial markings | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 > 6 to 12 months | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 6 Radiological imaging: change in Brasfield score | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 > 6 to 12 months | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.20, 1.20] |
| 7 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Gastrointestinal reflux | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.43, 3.63] |
| 7.2 Gastrointestinal reflux leading to withdrawal from the study | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.52] |
Comparison 2. PEP compared with oscillating PEP (Acapella, Flutter and Cornet)).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (% predicted) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 10 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐9.37 [‐24.90, 6.16] |
| 1.2 > 6 to 12 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 9.71 [‐2.12, 21.54] |
| 1.3 > 1 to 2 years | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐2.34 [‐6.86, 2.18] |
| 2 Hospitalisations for respiratory exacerbation (number per participant) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 > 1 to 2 years | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.92, 0.12] |
| 3 Change in FVC (% predicted) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 10 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐5.40 [‐20.01, 9.21] |
| 3.2 > 6 to 12 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 8.68 [‐0.54, 17.90] |
| 3.3 > 1 to 2 years | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.27, 2.87] |
| 4 Change in FEF25‐75 (% predicted) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 10 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐15.26 [‐40.64, 10.12] |
| 4.2 > 6 to 12 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 5.29 [‐7.84, 18.42] |
| 4.3 > 1 to 2 years | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐1.1 [‐6.50, 4.30] |
| 5 Exercise performance (percentage change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 10 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 6.32 [‐15.46, 28.10] |
| 6 LCI | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 1 year | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [‐1.36, 2.96] |
| 7 User satisfaction (patient satisfaction survey) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 10 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.85, 0.13] |
| 8 Adherence: at least 85% of prescribed treatments performed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 > 6 to 12 months | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| 9 Participant preference: self‐withdrawal due to lack of perceived effectiveness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 > 6 to 12 months | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.54] |
Comparison 3. PEP compared with HFCWO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 1 year | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐3.59 [‐9.29, 2.11] |
| 2 Participants experiencing a respiratory exacerbation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 1 year | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.95] |
| 3 Change in FVC (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 1 year | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐10.30, 0.30] |
| 4 Change in FEF25‐75 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 1 year | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐12.54, 11.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Braggion 1995.
| Methods | RCT. Cross‐over design. Each treatment given twice daily for 2 days. | |
| Participants | CF confirmed by sweat test. 16 participants (8 male); mean age 20.3 years, range 15 ‐ 27 years; mean FEV1 52.5, range 32% ‐ 98% predicted; mean Schwachman score 65.1, range 45 ‐ 87 points. Entry to study at time of hospital treatment of an acute pulmonary exacerbation. | |
| Interventions | 4 interventions: No washout period between interventions
1. PEP treatment ‐ participants breathed through a Medipep, (Nuova Tecnomedica) mask with a steady PEP of 10 ‐ 20 cm H₂O;
2. PDPV ‐ 6 positions based on recent chest radiography for each participant;
3. HFCC ‐ using ThAIRapy Bronchial Drainage System, chest compression in sitting at frequencies of 6, 8, 14, 15, 18 and 19 Hz were performed for 6 treatment sessions;
4. Control ‐ resting in sitting, spontaneous coughing allowed. Each treatment lasted 50 minutes: six 5‐minute periods of the specific treatment, each followed by a 3‐minute period of the FET. |
|
| Outcomes | FEV1, FVC, and FEF25‐75 were measured before and 30 minutes after each treatment. Expectorated sputum wet and dry weights during and for 30 minutes after each treatment were also measured. Technique acceptability was assessed using a 3‐point rating of effectiveness completed by the participant, and a 3‐point rating of tolerance, completed by the participant and also by the physiotherapist. | |
| Notes | No statement on withdrawals or dropouts.
Participants were familiar with PDPV and PEP interventions. All were introduced to HFCC on the day before their first use. The participant's usual airway clearance regimen was used for 2 days between the 2nd and 3rd treatment periods. This was a published paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant and the physiotherapist providing the therapy were not blinded, no information provided regarding other assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on withdrawals or dropouts. Outcome data were recorded. |
| Selective reporting (reporting bias) | High risk | All outcome data were only reported as no significant difference. |
| Other bias | High risk | No washout period between interventions, potential risk for carry‐over effect. |
Costantini 2001.
| Methods | RCT. Parallel design. Treatment for 1 year. | |
| Participants | CF identified by newborn screening within the 2nd month of life and confirmed on sweat test and genotyping 26 participants (14 male); aged under 4 months. | |
| Interventions | 2 interventions: 13 participants each group
1. PEP treatment ‐ applied via a mask;
2. PDPV. Each treatment was performed for 30 minutes, twice daily. The airway clearance intervention was applied by the carer(s), who received a 2‐week training period in either PEP or PDPV. Participants were followed as outpatients for 1 year. |
|
| Outcomes | The number of courses of total and intravenous antibiotic treatment were recorded, although it is not stated whether these were prescribed in response to a respiratory exacerbation. Possible adverse effects were monitored. Oxygen saturation, chest radiographs, and growth were assessed. No method of radiograph assessment is mentioned. Measurements were conducted at 0, 6 and 12 months. | |
| Notes | 3 participants in the PDPV group withdrew from the study. These were among 4 participants in this group who developed gastro‐oesophageal reflux. The 3 who withdrew did so "for the severity of their symptoms and were not evaluated". Published as abstract only, no further information obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant and the person providing the therapy were not blinded, no information provided regarding other assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants in the PDPV group withdrew from the study. These were among 4 participants in this group who developed gastro‐oesophageal reflux. The 3 who withdrew did so "for the severity of their symptoms and were not evaluated". All outcome measures were reported. |
| Selective reporting (reporting bias) | High risk | Not all outcome measures were reported in full. No statistics were provided for any outcomes, only percentages and mean measurements were reported. |
| Other bias | Unclear risk | Unclear whether groups were similar at baseline. |
Darbee 1990.
| Methods | RCT. Cross‐over design. Each treatment given 2 ‐ 3 times daily for 3 months. | |
| Participants | CF confirmed by sweat test. 13 participants (7 male); mean age 25.7 years, range 18 ‐ 34 years are reported in the abstract. Results from 20 participants were presented at conference. Data on 20 participants was shared with authors. Outcome data for 20 participants were used in the outcome analysis. | |
| Interventions | 2 interventions: No washout period between interventions
1. PEP treatment ‐ participants exhaled through a mask for 8 ‐ 10 breaths, then exhaled to a low lung volume through the mask which usually stimulated a cough; this was repeated 5 ‐ 6 times;
2. PDPV ‐ percussion was applied for 3 minutes over all segments, participants breathed deeply several times at each minute, 3 vibrations followed with exhalation through an open mouth, without force, until productive coughing occurred. Participants were instructed to treat until clear, 2 ‐ 3 times per day. |
|
| Outcomes | 2 measures of mucociliary clearance were repeated after each 3‐month treatment arm: the time taken for half the radiolabeled sputum in the whole lung to clear (T1/2‐W) and the same in the peripheral region (T1/2‐P). Convenience, independence and ease of use was determined with a standardised written questionnaire (not described). | |
| Notes | No statement on withdrawals or dropouts.
Participants reported that they got clearer faster with PEP. Published as an abstract only, further information obtained from authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant and the person providing the therapy were not blinded, no information provided regarding other assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on withdrawals or dropouts. All outcome measure were reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported in full. |
| Other bias | High risk | No washout period between each 3 month period of interventions, potential risk for carry‐over effect. Groups similar at baseline regarding the most important prognostic indicators. |
Darbee 2004.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CFconfirmed by sweat test; stable (not defined) and not hospitalised during the previous month for management of an exacerbation. 6 participants (3 female), mean age 18 years, range 13 years to 22 years; mean FEV1 52, range 35 ‐ 68% predicted. | |
| Interventions | 2 interventions: each interventions was separated by a 5 day washout period
1. PEP treatment ‐ pressure 10 ‐ 20 cm H₂O, participants breathed through a mask with an expiratory resistor between 10 ‐ 20 cm H₂O for 8 ‐ 10 breaths, followed by coughing; this was repeated 6 times;
2. High‐pressure PEP using a resistor at which FVC with PEP exceeded FVC with no PEP ‐ participants were instructed to perform 8 ‐ 10 slightly lager tidal volume breaths through the PEP device followed by an inspiration to TLC and a forced expiration into the mask; 6 cycles were performed. Each intervention was applied on a different day (order randomised). A 3rd intervention was a "control", Participants sat for 20 minutes and outcome measures were performed pre and post this time period. This was performed on Day 1 by all participants and was not randomised |
|
| Outcomes | FEV1, FEF25‐75, RV, SVC, dry weight of sputum, and SpO₂ were recorded before, after, and 45 minutes after each intervention session. Distribution of ventilation and gas mixing were also measured by lung clearance examining the phase 111 slope. | |
| Notes | 1 participant's data were excluded when it was determined that there was a pulmonary exacerbation. Published paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant and the person providing the therapy were not blinded, no information provided regarding other assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant's data were excluded when it was determined that there was a pulmonary exacerbation. All outcome measures were reported under results. |
| Selective reporting (reporting bias) | Low risk | Outcome measures are reported in full. |
| Other bias | Low risk | 5 day washout period between each intervention thus low risk of carry over effect. Groups similar at baseline regarding the most important prognostic indicators. |
Darbee 2005.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF confirmed by sweat test; hospitalised with a pulmonary exacerbation. 15 participants 8 males, 7 females, mean (SD) age 17.5 (4.2) years, BMI 18.3 (2). | |
| Interventions | 2 Interventions: No washout period between interventions PEP using pressures between 10 ‐ 20 cm H₂O via facemask for 8 breaths followed by huffing and coughing; this was repeated for 8 ‐ 10 cycles. HFCWO, pressure 5, frequency 10 Hz for 15 minutes, then 15 Hz for next 15 minutes; 6 cycles of 5 minutes with a pause for huffing and coughing in between each cycle. |
|
| Outcomes | Lung clearance using nitrogen washout measuring Phase III N₂ slope, index values. PFT measurements of FVC and FEV1, and pulse oximetry. | |
| Notes | Published paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were assigned treatment order by numbering 1 ‐ 15 at study entrance. Coin toss was used to allocate whether odd numbered participants received treatment first, thus all odd numbered participants received the same treatment allocation. |
| Allocation concealment (selection bias) | High risk | All odd numbered participant were pre‐allocated order of treatment intervention. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Study does not mention blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no reported dropouts. All outcomes tested are reported in the results. |
| Selective reporting (reporting bias) | Low risk | The outcomes are reported fully reported for all participants pre‐ and post‐treatment. |
| Other bias | High risk | No washout period between interventions, thus potential risk for carryover effect. Groups are similar at baseline based on one prognostic factor. |
Fainardi 2011.
| Methods | RCT. Cross‐over, single treatment. |
|
| Participants | 36 participants, confirmed diagnosis of CF.
Age > 18 years, mean (SD) 26 (6.5) years. 14 males, 20 females. Mild to moderate lung function impairment. Incusion criteria included in hospital for a pulmonary exacerbation. |
|
| Interventions | 2 Interventions: One day washout period between interventions. PEP using pressures between 10 ‐ 20 cm H₂O, consisted of cycles of 15 breathes through PEP mask interspersed with huffing, number of cycles was individualised. HFCC also known as HFCWO used Hillrom Vest Model 4, with pressure 6 ‐ 10 and frequency between 15 ‐ 20 Hz. Huffing and coughing was interspersed throughout HFCC. |
|
| Outcomes | PFTs measuring FVC, FEV1 and FEF25‐75 % predicted measured pre and post, pulse oxygen saturation (SpO₂%), sputum weight. | |
| Notes | 34 participants completed the study, 2 participants withdrew due to discomfort of HFCC device. Published paper |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were assigned to either intervention by numbering consecutively: odd numbers got control intervention first. Even numbers got PEP intervention first. There was no random allocation to intervention. |
| Allocation concealment (selection bias) | High risk | Thee was no apparent concealment as it was a consecutive numbering sequence. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participant and the person providing the therapy were not blinded ‐ Unclear risk. Outcome assessors were blinded ‐ low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes in study design are reported. |
| Selective reporting (reporting bias) | Low risk | 2 participants withdrew due to discomfort of HFCC device. All outcomes are reported in full. |
| Other bias | Unclear risk | Only one day washout period between interventions. More outcomes were reported than documented in study design. |
Falk 1984.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF diagnosis, chronic pseudomonas infection, and expectoration of greater than 1.5 g/hr of sputum. 14 participants (10 male); mean age 18 years, range 14 ‐ 30 years; mean FEV1 34, range 15% ‐ 55% predicted. Participants were excluded during or immediately after anti‐pseudomonas treatment or a change in routine medication. | |
| Interventions | 4 interventions: 1 day washout period between interventions
1. PEP treatment in sitting (PEPs) ‐ pressure 17 cm H₂O using an Astra Meditec PEP mask; seated participants exhaled 6 ‐ 12 times, followed by forced expirations with the glottis open and coughing as needed, this was repeated for 20 minutes;
2. PEP treatment in PD positions (PEPpd) ‐ participants performed the same breathing regimen for 4 ‐ 5 minutes in each of 7 PD positions, this intervention lasted 35 minutes;
3. PDPV ‐ during the same PD regimen, participants received manual percussion, followed by 3 deep breaths with vibration, and FET. This intervention lasted 35 minutes;
4. PLBs ‐ seated participants inhaled slowly and exhaled through pursed lips 5 ‐ 8 times, followed by FET, this intervention lasted 20 minutes. The 4 interventions were randomised over 2 days: 1 each morning and 1 each afternoon, with an interval of at least 5 hours. |
|
| Outcomes | FEV1 and FVC were measured before and 50 minutes after each intervention session. Wet weight of expectorated sputum during and until 50 minutes after each intervention session. Transcutaneous pO₂ was measured during the intervention and for 50 minutes after each intervention. Technique efficiency and acceptability were assessed using a questionnaire completed by the participant, although details of the questionnaire are not provided. | |
| Notes | No withdrawals nor dropouts.
The authors state that 7 participants were studied during admission for their usual anti‐pseudomonas treatment and the other 7 at least 1 month after treatment. This appears inconsistent with the exclusion criteria; see 'Participants'. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and person providing the therapy were not blinded ‐ unclear risk Outcome assessors were blinded ‐ low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals nor dropouts. Data for at least 1 key outcome analysed by 'intention‐to‐treat'. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were reported in full. |
| Other bias | Unclear risk | Only one day washout period between interventions. Groups similar at baseline regarding the most important prognostic indicators. |
Falk 1993.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF diagnosis. 12 participants. | |
| Interventions | 3 interventions: One day washout period between interventions
1. FET;
2. Combined intervention of PEP with FET (PEP + FET). This is a combined intervention;
3. Control (not defined). Each intervention was applied for 20 minutes on 1 of 3 consecutive days. |
|
| Outcomes | Retention of radiolabeled secretions in the lung was recorded at 0.5, 1, 2, and 24 hours after the start of the intervention. (The 24‐hour value was used as a measure of the radioaerosol deposition.) Wet weight of sputum expectorated for the half hour during which the intervention was applied, and for the subsequent 1.5 hours was measured. The number of huffs and coughs during the half hour during which the intervention was applied, and for the subsequent 1.5 hours were counted. | |
| Notes | No statement on withdrawals or dropouts. Published as abstract only, no further information obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on withdrawals or dropouts.All outcome measure were reported. |
| Selective reporting (reporting bias) | Low risk | Outcome measures were reported in full. |
| Other bias | Unclear risk | Only one day washout period between interventions. Groups similar at baseline regarding the most important prognostic indicators. |
Gaskin 1998.
| Methods | RCT. Parallel design. Treatment for 2 years. | |
| Participants | CF diagnosed by Toronto CF Clinics (criteria not stated); FEV1 > 40% predicted. 66 participants (34 males); mean age 21.6 years, range 11 ‐ 45 years; mean FEV1 70.2% predicted (PEP group) and 65.3% predicted (PDPV group). | |
| Interventions | 2 interventions: 33 participants were randomised to each intervention.
1. PEP treatment ‐ participants exhaled through the Astra Meditec PEP mask;
2. PDPV ‐ not described beyond "conventional postural drainage and percussion". The daily regimen for use of the devices is not described. |
|
| Outcomes | FEV1, FVC, QWBS, a cycle ergometer exercise test, and the Brasfield chest radiograph score. All were recorded at 3‐monthly intervals. The participants also kept adherence and exercise diaries. | |
| Notes | 5 participants withdrew from the study, but none were lost to follow up. 4 withdrew from the PDPV group withdrew soon after randomisation and 1 from the PEP group moved away, but returned to the clinic. No reason is provided for the withdrawals. Published as an abstract only, further information obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants withdrew from the study, but none were lost to follow up. 4 from the PDPV group withdrew soon after randomisation and 1 from the PEP group moved away, but returned to the clinic. No reason is provided for the withdrawals. Data for at least 1 key outcome analysed by 'intention‐to‐treat'. |
| Selective reporting (reporting bias) | Unclear risk | Statistical analysis was only reported for 1 outcome measure (FEV1). |
| Other bias | Low risk | Groups similar at baseline regarding the most important prognostic indicators. |
Hofmeyr 1986.
| Methods | RCT. Cross‐over design. Each treatment given 4 times daily for 1 day. | |
| Participants | CF confirmed by positive sweat test, malabsorption, and chronic lung infection. 18 participants (12 male); mean age 22.5 years, range 13 ‐ 37 years; mean FEV1 1.3, range 0.45 ‐ 3.25 L; and FVC was 2.5, range 1.1 ‐ 5.1 L. All participants were studied close to the end of an admission to hospital with an exacerbation of their lung infection. | |
| Interventions | 3 interventions: No washout period between interventions
1. PEP treatment in sitting (PEP) ‐ pressure 12 ‐ 17 cm H₂O using an Astra Meditec PEP mouthpiece; seated participants exhaled 6 times through the mouthpiece, followed by relaxed breathing, 1 ‐ 2 forced expirations (huffs) from mid to low lung volume, relaxed breathing, and a huff or cough from high lung volume if secretions reached the upper airways;
2. PEPpd ‐ the same breathing regimen was performed in (usually) 2 PD positions chosen before the start of the study as the most appropriate from (undescribed) clinical assessment;
3. BEpd ‐ consisting of 4 deep inspirations with relaxed expiration, breathing control and the FET which included 1 ‐ 2 forced expirations (huffing) from mid to low lung volume, followed by breathing control then forced expirations or a cough from high lung volumes. In each intervention, the respiratory manoeuvres described above were continued in cycles until the participant and physiotherapist felt that forced expiration and coughing no longer resulted in expectoration. 4 treatment sessions were performed per day. |
|
| Outcomes | FEV1 and FVC were measured before and 30 minutes after each intervention session. Wet weight of sputum expectorated during and for 30 minutes after the intervention session was measured. SpO₂ was recorded before, during, and for 30 minutes after each intervention session. | |
| Notes | There were no withdrawals or dropouts. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals or dropouts. All outcome measures are reported. |
| Selective reporting (reporting bias) | High risk | Statistiicial outcome measures are provided for wet weight of sputum expectorated during and for 30 minutes and SpO₂ but not for FEV1 and FVC. |
| Other bias | High risk | No washout period between interventions, thus there is a potential for a carryover effect. Groups similar at baseline regarding the most important prognostic indicators. |
Kofler 1998.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF diagnosed by CF Clinic at Children's Hospital, Rome. 20 participants (11 males); mean age 15.25, range 6 ‐ 23 years; mean (SD) Schwachman score 80.8 (15.3) points. | |
| Interventions | 2 interventions: One day washout period between interventions
1. PEP treatment ‐ followed the Danish protocol of breathing through a PEP mask against a PEP of 10 ‐ 20 cm H₂O pressure, followed by a pause, 2 ‐ 3 huffs and coughing; no further information was provided;
2. BiPAP ‐ while sitting participants breathed against 11cm H₂O inspiratory positive pressure and 9 cm H₂O expiratory positive pressure applied via a mask attached to a Puritan Bennett 335, followed by a pause, 2 ‐ 3 huffs and coughing. Single treatments of 15 minutes were applied on 2 consecutive days. All participants were using PEP as their airway clearance therapy prior to the study. This was stopped the day before the study commenced. |
|
| Outcomes | FEV1, FVC and FEF25‐75 were measured at the beginning, at the end, 15 minutes after and 30 minutes after each session. Oxygen saturation and heart rate were continuously monitored throughout this time via pulse‐oximetry. Following the 2 sessions, participant preference was recorded. | |
| Notes | No statement on withdrawals or dropouts.
All participants were performing PEP prior to the study. PEP was applied "according to the Danish protocol" (not defined). Published as an abstract only, no further information obtained. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on withdrawals or dropouts. All outcome measures are reported. |
| Selective reporting (reporting bias) | Unclear risk | FEV1, FVC and FEF25‐75 were only reported as no significant difference between groups. |
| Other bias | Unclear risk | Only one day washout period between interventions. Groups similar at baseline regarding the most important prognostic indicators. |
Lagerkvist 2006.
| Methods | RCT. Cross‐over design. Single treatment. |
|
| Participants | 15 people with CF, 6 females, 9 males, age 6.9 to 21.5 years. CF confirmed by sweat test. |
|
| Interventions | 2 interventions: 8‐week washout period between interventions 1. PEPusing Astra Tech system with mouthpiece pressures 10 ‐ 20cm H₂O ‐ participant breathed through the device for 2 minutes followed by huffing; this was repeated 4 times; 2. Oscillating PEP using the flutter ‐ participants were instructed to inhale deeply then exhale quickly through the device and repeat for 1 minute followed by huffing; this was repeated 4 times. |
|
| Outcomes | PFTs consisting of FVC, FEV1, MEF50 and MEF 25 all of 5 predicted. Pt02, Ptco2. |
|
| Notes | Published paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of how the participants were randomised. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how the allocation was concealed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes in study design are reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in full. |
| Other bias | Low risk | Good washout period, thus low risk of carry over effect. Groups similar at baseline regarding the most important prognostic indicators. No dropouts. |
Lannefors 1992.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF with daily sputum production. 9 participants (6 male); mean age 25, range 12 ‐ 36 years; mean FEV1 51, range 20 ‐ 78% predicted; mean Schwachman score 66, range 39 ‐ 94 points. | |
| Interventions | 3 interventions: 1 to 5 day washout period between interventions.
1. PEP treatment ‐ performed in a sitting position using a PEP mask and positive expiratory pressures 15 ‐ 20 cm H₂O;
2. PD ‐ participants alternated between deep and relaxed breaths while lying on the left side, rotated slightly backward towards supine, 15 degrees head down tilt, (PD position for PD from right middle lobe) and sat up to cough; no percussion or vibrations were performed;
3. Physical exercise ‐ physical exercise was performed on a cycle ergometer at 80% of the participant's peak work capacity (as assessed on their most recent annual maximal exercise test). Each 20‐minute intervention session consisted of three 3‐minute periods of performing the intervention, each followed by a 3‐minute pause, during which a standard number of forced expirations from mid‐lung volume and relaxed breaths were performed. |
|
| Outcomes | Mucus clearance was measured by delivering a radioaerosol (99mTc‐labelled colloidal albumin) to the airways and measuring the distribution of radiolabeled secretions in the lung fields. Anterior and posterior planar gamma camera images of the thorax were collected for 2 minutes at baseline, after 15 minutes rest in sitting, after the 20‐minute intervention, and after another 15 minutes rest in sitting. Clearance was calculated as a reduction in count rate between successive images. Whole lung clearance was calculated. In addition, the planar images were divided into a central 'hilar' region and peripheral region, and clearance from these regions was calculated. | |
| Notes | No withdrawals or dropouts. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts. |
| Selective reporting (reporting bias) | High risk | Not all outcome measures were reported with statistical analysis. |
| Other bias | Low risk | 1 ‐ 5 day washout period between interventions, thus low risk of carry over effect. Groups similar at baseline regarding the most important prognostic indicators. |
McIlwaine 1991.
| Methods | RCT. Cross‐over design. Treatment for 2 months. | |
| Participants | CF diagnosis. 18 participants, aged 6 ‐ 18 years of age. | |
| Interventions | 3 interventions each performed twice a day: One month washout period between interventions 1. PEP treatment using the Astra Meditech mask ‐ participants performed 15 breaths through the mask with a PEP between 10 ‐ 20 cm H₂0; this was followed by 2 ‐ 3 huffs from a mid to high lung volume followed by a cough and cycle was repeated 6 times; 2. PDP ‐ participants performed PD using 6 positions in the morning and 5 positions in the evening; in each position their chest wall was percussed for 3 minutes, this was followed by huffing and coughing; 3. AD ‐ performed in a sitting position and participants were instructed to exhale to RV then perform TV manoeuvres at this level while adjusting the velocity of their expiratory airflow until they felt secretions moving; they then progressed to TV breathing at mid‐lung volumes and then to high lung volumes where they would cough up any secretions. Number of cycles was individualised to each participant, but each treatment session lasted between 20 ‐ 30 minutes. | |
| Outcomes | FEV1, FVC, and FEF25‐75 were measured at the start and end of each 2‐month treatment period. Sputum weight weighed after one treatment session per week. Other measures included reported treatment duration, treatment comfort, requirement for assistance with treatment, flexibility of treatment times, control in performing own treatment, and how interruptive treatment was to daily living. | |
| Notes | Published as an abstract only, further information obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and person providing the therapy were not blinded ‐ unclear risk. Outcome assessors were blinded low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Results reported for all outcome measures but not in full. |
| Other bias | Low risk | one month washout period between each intervention, thus low risk of carry over effect. Groups similar at baseline regarding the most important prognostic indicators Author of the study is one of this review's authors, thus to eliminate bias, the study was assessed by the other two independent authors of this paper and by the previous authors. |
McIlwaine 1997.
| Methods | RCT. Parallel design. Treatment for 1 year. | |
| Participants | CF confirmed by sweat test. 40 participants (22 male); age range 6 ‐ 17 years; mean age 10.40 years (PEP group) and 9.75 years (PDPV group); mean FEV1 80.47, range 37 ‐ 115% predicted. Participants were excluded if their condition was not stable as judged by clinical evaluation, chest radiograph and pulmonary function. Also, no participant entered the study within 1 month of discharge from hospital or use of IV antibiotics or other intensive therapy for an exacerbation. | |
| Interventions | 2 interventions: 20 participants were randomised to each group.
1. PEP treatment ‐ pressure 10 ‐ 20 cm H₂O using an Astra Meditec PEP mask; seated participants breathed 15 times through the mask, followed by 2 ‐ 3 forced expirations, cough and relaxed breathing, this was repeated 6 times over a 20‐minute session;
2. PDP ‐ performed forced expirations and vigorous coughing in 5 ‐ 6 positions, 3 ‐ 5 minutes of percussion, 2 ‐ 4 minutes of expiratory vibrations; these sessions lasted 30 minutes. Both interventions were performed twice daily. |
|
| Outcomes | FEV1, FVC, and FEF25‐75 were measured at 3‐month intervals. Clinical assessments using Shwachman and Huang scores. Chest radiographs were performed at the start and end of the 1 year period and measured using the Brasfield scoring system. Compliance was measured via daily record keeping, with those compliant with less than 85% of the twice‐daily sessions over a 1‐month period being withdrawn from the study. Adverse events and participant preference were assessed via questionnaire. | |
| Notes | 2 dropouts from each arm, due to non‐compliance (< 85% of twice‐daily sessions performed) or non‐attendance at clinic. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised. Participants were first matched as pairs based on age, sex and FEV1. Participants within each pair were randomly assigned by computer to either group. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and person providing the therapy were not blinded ‐ unclear risk. Outcome assessors were blinded ‐ low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts from each arm, due to non‐compliance (< 85% of twice‐daily sessions performed) or non‐attendance at clinic. Intention to treat approach used. |
| Selective reporting (reporting bias) | Unclear risk | Not all outcome measures are reported with full statistical analysis. |
| Other bias | Low risk | Participants were matched as pairs at baseline, thus groups were similar at baseline regarding the most important prognostic indicators. Author of the study is one of this review's author's, thus to eliminate bias, the study was assessed by the other two independent authors of this paper and by the previous authors. |
McIlwaine 2001.
| Methods | RCT. Parallel design. Treatment for 1 year. | |
| Participants | CF confirmed by sweat test. 40 participants (24 male); age range 7 ‐ 17 years; FEV1 range 47 ‐ 107% predicted; Schwachman score range 54 ‐ 98 points. Participants were excluded if they had been hospitalised within the past month for a pulmonary exacerbation, or if they were not stable on clinical evaluation, chest radiograph or pulmonary function. | |
| Interventions | 2 interventions: 20 participants were randomised to each group.
1. PEP treatment ‐ participants inhaled and exhaled through the Astra Meditec PEP mask in sitting; the resistor which produced 10 to 20 cm H₂O pressure during mid‐expiration was used. Over approximately 2 minutes, 15 tidal breaths with slightly active expiration were performed. Participants then removed the mask, performed 2 or 3 forced expirations, and coughed, followed by 1 ‐ 2 minutes of relaxed breathing. This sequence was repeated 6 times and these 20‐minute sessions were repeated twice daily;
2. Oscillating PEP ‐ participants exhaled through the flutter device which was angled to maximise the sensation of vibration in the chest. In sitting, participants inhaled deeply through the nose, followed by a breath hold for 2 ‐ 3 seconds, and exhaled through the device slightly into the expiratory reserve volume. After 10 ‐ 15 breaths, participants huffed through the device, increasing the TV and speed of exhalation to precipitate coughing and expectoration. This sequence was repeated "until clear" and not for less than 15 minutes per session, twice daily. The daily regimen for use of the devices is not described. |
|
| Outcomes | FEV1, FVC, and FEF25‐75 and clinical assessment using Shwachman and Huang scores were measured at the beginning and at 3‐monthly intervals throughout the study. Number of hospitalizations for pulmonary exacerbations were recorded throughout the study Compliance with the interventions was recorded daily by the participants. A monthly questionnaire recorded physical activity, general well‐being, cough, sputum production, subjective impression of the therapy, and adverse events. Chest radiographs were evaluated by a blinded radiologist at the beginning and end of the study. |
|
| Notes | 3 participants were withdrawn due to non‐compliance (< 85% of twice‐daily sessions performed over 1 month) in the PEP group. 5 participants dropped out from the flutter group stating that subjectively the flutter did not appear to clear their secretions. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised. Randomised using a computer‐generated block of numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and person providing the therapy were not blinded ‐ unclear risk. Outcome assessors were blinded ‐ low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were withdrawn due to non‐compliance (< 85% of twice‐daily sessions performed over 1 month) in the PEP group. 5 participants dropped out from the flutter group stating that subjectively the flutter did not appear to clear their secretions, they also had a clinically significant deterioration in pulmonary function. Intention‐to‐treat approach used. |
| Selective reporting (reporting bias) | Low risk | All outcome measures are reported in full. |
| Other bias | Low risk | Both groups were reported to be similar at baseline regarding the most important prognostic indicators. Author of the study is one of the authors on this review, thus to eliminate bias, the study was assessed by the other two independent authors of this paper and by the previous authors. |
McIlwaine 2013.
| Methods | Multi‐centre RCT. Parallel design. Treatment for 1 year. | |
| Participants | CF confirmed by sweat test or genotyping. 107 participants from 12 CF centres (57 males); age range 6 ‐ 47 years; FEV1 over 40% predicted. Participants were excluded if they had been hospitalised within the past month for a pulmonary exacerbation, or if they were not stable on clinical evaluation, chest radiograph or pulmonary function. On entering the study, participants performed a 2‐month washout period before being allocated to an intervention. | |
| Interventions | 2 interventions: 51 participants were randomised to PEP and 56 to HFCWO. 1. PEP ‐ using a mask with pressures 10 ‐ 20 cm H₂0, participants breathed through the device for 15 breaths followed by 2 ‐3 huffs and a cough; this was repeated for 6 cycles; 2. HFCWO ‐ using the InCourageTM system; 6 sets of 5‐minute cycles were performed with frequencies between 6 ‐ 15 Hz, this was interspersed with huffing and coughing. |
|
| Outcomes | Number of pulmonary exacerbations and time to first exacerbation. PFTs measuring FVC, FEV1 and FEF 25‐75% in absolute change. Quality of life using the Cystic Fibrosis Questionnaire and patient satisfaction visual analogue scale. |
|
| Notes | There were 16 dropouts during the washout period before participants commenced one of the two interventions being studied. These were not included in the results. A further 3 dropped out during the intervention period. These were included in analysis with intention‐to‐treat analysis. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised was by an independent statistician using a computer‐generated randomisation table. Participants were matched for age, sex and pseudomonas status. The statistician also attempted to block patients within each centre to control for any treatment differences between centres. |
| Allocation concealment (selection bias) | Low risk | The randomisation was performed by an independent statistician who provided the randomisation to the centre after the participant had enrolled in the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and person providing the therapy were not blinded ‐ unclear risk. Outcome assessors were blinded ‐ low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes in study design are reported.Dropouts prior to commencement of interventions being studies were not included in analysis. 3 dropouts during the intervention period were included in analysis with intention‐to‐treat approach. |
| Selective reporting (reporting bias) | Low risk | All outcomes in study design are reported. PFTs results were provided by the author. |
| Other bias | Low risk | Author of the study is one of this review's authors, thus to eliminate bias, the study was assessed by the other two independent authors of this paper. |
Mortensen 1991.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF diagnosis and chronic pseudomonas infection. 10 participants (6 male); mean age was 20 years, range 15 ‐ 26 years; mean FEV1 38.5, range 26 ‐ 101% predicted. Participants entered the study in the last 2 weeks of regular hospital admission for intravenous anti‐pseudomonas treatment. | |
| Interventions | 3 interventions: 2 day washout period between interventions 1. PEP treatment ‐ pressure 10 ‐ 20 cm H₂O using a mask; seated participants breathed TV breathing with slightly active expirations through the system for 1 minute, followed by 1 ‐ 2 forced expirations from mid to low lung volume, relaxed breathing and cough; this breathing regimen was repeated for 20 minutes; 2. BEpd ‐ participants breathed deeply 4 times followed by relaxed breathing for 10 minutes in each of right and left side lying with 20 degrees head down tilt; this was again followed by 1 ‐ 2 forced expirations from mid to low lung volume, relaxed breathing and cough. The same number of huffs and coughs performed with the first treatment were matched with the subsequent active intervention. 3. Control ‐ 20 minutes of resting in sitting with spontaneous coughing allowed. | |
| Outcomes | Mucus clearance was measured directly by delivering a radioaerosol (99mTc‐labelled albumin colloid) to the airways and then measuring the distribution of radiolabeled secretions within the lung fields. Posterior planar gamma camera images of the thorax were collected as single 5‐minute exposures every 30 minutes for 3 hours. Clearance was calculated as a reduction in count rate between successive images. Whole lung clearance was calculated. In addition, the planar images were divided into central, mid and peripheral regions, and upper, mid and basal regions. Clearance from these regions was calculated. Wet weight of sputum expectorated during the initial 30‐minute (intervention) period and for the remainder of the 3‐hour clearance measurement period was measured. Sputum weight pre and post and 3 hours post and 99mTc‐labelled sputum measured post. |
|
| Notes | No statement on withdrawals or dropouts. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information was provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement on withdrawals or dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcome measures are fully reported. |
| Other bias | Low risk | Two day washout period between each intervention, thus low risk of carry over effect. Groups similar at baseline using FEV1 as the most important prognostic indicators. |
Newbold 2005.
| Methods | RCT. Parallel design. Treatment for 13 months. | |
| Participants | CF diagnosed by St Michael's Hospital CF Clinic, Toronto. 42 participants (24 male). PEP Group: 21 participants (15 male); mean age 28, SD 8.1 years; mean FEV1 2.5, SD 1.2 litres; mean FEV1 66, SD 19.9% predicted. Flutter Group: 21 participants (9 male); mean (SD) age 31 (8.7) years; mean (SD) FEV1 2.2 (0.7) litres; mean (SD) FEV1 69(18.5) % predicted. Participants were excluded if they had been hospitalised within the past month for a pulmonary exacerbation, had changed their medication within the past month, or did not have a daily cough or daily sputum. | |
| Interventions | 2 interventions: 21 participants were randomised to each group. 1. PEP treatment ‐ pressure 10 ‐ 20 cm H₂O using an Astra Meditec PEP mask; seated participants breathed 10 ‐ 15 times through the mask, followed by huffing, coughing and relaxed breathing, this was repeated 5 ‐ 6 times, over a 20‐minute session, twice daily; 2. Oscillating PEP ‐ participants exhaled through the flutter device (Axcan Scandipharm) which was angled to maximise the sensation of vibration in the chest. In sitting, participants inhaled deeply through the nose, followed by a breath hold for 2 ‐ 3 seconds, and exhalation through the device. After 5 ‐ 10 breaths, participants increased the TV and speed of exhalation through the device, to precipitate coughing and expectoration. This sequence was repeated "until clear" or for approximately 20 minutes, twice daily. | |
| Outcomes | Slope of change in FEV1 , FVC, and FEF25‐75 (absolute and % predicted). Number of hospitalisations. Adherence. | |
| Notes | 1 participant was withdrawn when he stopped attending the CF clinic. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table and block randomisation were used to ensure that groups would be of equal size. |
| Allocation concealment (selection bias) | Low risk | Allocation was sealed in opaque envelopes by an independent assistant. The envelopes were open in sequence after a participant was enrolled. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and person providing the therapy were not blinded ‐ unclear risk. Outcome assessors were blinded ‐ low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was withdrawn when he stopped attending the CF clinic. |
| Selective reporting (reporting bias) | Low risk | Results are reported for all outcome measures. |
| Other bias | Low risk | Groups similar at baseline regarding the most important prognostic indicators. |
Pfleger 1992.
| Methods | RCT. Cross‐over design. Each treatment given once. | |
| Participants | CF confirmed by repeat sweat tests, and sputum production of > 20 ml per day. 15 participants (9 female, 1 unspecified); mean age 16 years, range 9.8 ‐ 22.4 years; mean Schwachman score 62.2, range 26 ‐ 90 points. Participants were excluded if unstable at the time of investigation (criteria unspecified). 6 months before the study, each participant was trained in the 2 self‐administered techniques (PEP and AD). | |
| Interventions | 5 interventions: 1 day washout period between each intervention.
1. Hi‐PEP intervention ‐ expiratory resistance chosen to increase the FVC to the greatest extent when performed through the PEP mask; participants inhale and exhale 8 ‐ 10 times followed by a forced expiratory manoeuvre, all through the mask;
2. AD ‐ participants breathed at low lung volumes with progressive increases in the lung volume at which breathing was performed in response to evidence of secretion transport; coughing and forced expiratory manoeuvres were avoided;
3. Hi‐PEP for the first half of the session, followed by AD;
4. AD for the first half of the session, followed by Hi‐PEP;
5. Control ‐ spontaneous coughing only. Each intervention session was equal to the time taken for the individual participant to clear their lungs using AD, as judged from pre‐study experience. |
|
| Outcomes | FEV1 , FVC, RV, and TLC were measured at all PFT measurement points. Wet weight of expectorated sputum during the complete (both halves) intervention period was also measured. | |
| Notes | 1 withdrawal due to development of an acute respiratory viral infection during the study. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 withdrawal due to development of an acute respiratory viral infection during the study. |
| Selective reporting (reporting bias) | High risk | Not all outcome measure results are reported in full. |
| Other bias | Unclear risk | Only one day washout period between each intervention. Groups similar at baseline regarding the most important prognostic indicators. |
Pryor 2010.
| Methods | RCT. Parallel design. Treatment period: 12 months. |
|
| Participants | 75 participants were enrolled into the study. Age range 17 ‐ 63 years, 47 males, 28 females CF Diagnosed by sweat test. Inclusion criteria FEV1 over 25% predicted. Enrolled when stable. |
|
| Interventions | 5 interventions all performed in a sitting position. Number of treatments per day and length of treatment was individualised to each participant. 15 participants randomised to each group. 1. Active cycle of breathing techniques 2. AD 3. PEP 4. Oscillating PEP using the flutter 5. Oscillating PEP using the RC cornet |
|
| Outcomes | Primary outcome was FEV1. Other PFT outcomes were FVC, MEF 25 and RV BMI, modified shuttle test, chronic respiratory questionnaire, short form 36 and number of course of IV antibiotics were also measured. |
|
| Notes | 10 participants lost to follow‐up. Data reported on 65 using intention‐to‐treat. 53 participants completed the study. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computerised and used a random number sequence, stratified by FEV1 and sputum volume. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and person providing the therapy were not blinded ‐ unclear risk. Outcome assessors were blinded ‐ low risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants requiring IV antibiotics is listed under outcome measures but was not reported as could not be analysed due to small numbers and the scattered nature of the data. |
| Selective reporting (reporting bias) | Unclear risk | Only the results of the primary outcome FEV1 were reported in full. All the other outcome measures were reported only as no significant difference. |
| Other bias | Unclear risk | 13 participants withdrew as they did not like the regimen they had been randomised to. Unsure if these participants were included in intention‐to‐treat or lost to follow‐up. |
Rodriguez 2016.
| Methods | RCT. Parallel design Treatment length 3 months. |
|
| Participants | 32 participants with CF. Age > 18 years of age FEV1 20% ‐ 69% predicted |
|
| Interventions | Two interventions both of which were proceeded by inhalation of a bronchodilator and 7% hypertonic saline. During this inhalation, participants performed autogenic drainage for 15 minutes. 1. PEP mask therapy was performed using a Astra‐Tech mask, The participant breathed through the PEP mask for 10 breaths creating an expiratory pressure of between 10 ‐ 20 cm H₂O. This was followed by huffing. Cycle consisting of inhalation, AD, PEP and was repeated for one hour twice a day. 2. NIV with a Bilevel‐PAP device. A minimum inspiratory pressure of 20 cm H₂O and an expiratory pressure of 10 cm H₂O was given. Participants were instructed to breathe in and out for 2 minutes followed by huffing and coughing. Cycle consisting of inhalation, AD, NIV and was repeated for one hour twice a day. |
|
| Outcomes | Lung function tests measuring FVC, FEV1, in L and % predicted. were performed at baseline and at week 12. LCI performed at baseline and at week 12. Sputum samples were obtained monthly to measure inflammatory markers. 6‐minute walk test was performed at baseline and week 12. Blood gases, pulse oximetry and respiratory rate were measured monthly. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly selected an envelope which indicated which intervention they were randomised to. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in an envelope |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants were not blinded to which intervention they were performing, but outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study. Outcome data were reported for all outcome measures |
| Selective reporting (reporting bias) | Unclear risk | Data results on blood gases, inflammatory markers and FRC were reported as no significant change but results were not shown. |
Steen 1991.
| Methods | RCT. Cross‐over design. Each treatment given for 1 month. | |
| Participants | CF confirmed by sweat tests. 28 participants (gender unspecified); mean age 14 years, range 8 ‐ 21 years; mean FEV1 68, range 15 ‐ 114% predicted; mean Schwachman score 65, range 33 ‐ 91 points. | |
| Interventions | 4 interventions: No washout period between each intervention
1. PEP treatment ‐ pressure 10 ‐ 15 cm H₂O; seated participants exhaled 10 ‐ 15 times through an Astra or Vitapep mask, followed by forced expiration and cough, if required. This cycle was then repeated;
2. PEP & FET intervention ‐ the following was added to the above technique, 1 or 2 forced expirations with an open glottis from mid‐lung volume to low‐lung volume followed by a period of relaxed diaphragmatic breathing (FET);
3. PDPV & FET intervention ‐ participants received percussion in PD positions, with FET;
4. PEP‐PDPV & FET intervention ‐ participants performed PEP (position not defined) for 5 minutes, followed by PDPV & FET. Frequency and duration of treatment sessions was not specified. There was no washout period between months. |
|
| Outcomes | FEV1 and FVC were measured at the start and finish of each month. At the end of each month, the wet weight of expectorated sputum over a 2‐hour period which included a treatment with that month's intervention was measured. At the end of the study period, participants nominated which intervention they would use as ongoing airway clearance physiotherapy. | |
| Notes | 2 withdrawals (1 death, 1 non‐compliance) and 2 dropouts (1 pneumothorax, 1 subjective lack of effect).
A fifth intervention, FET alone, was undertaken by a subset of 5 participants. This treatment was performed after the 4 randomly‐assigned interventions and therefore does not form part of the randomised study. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 withdrawals (1 death, 1 non‐compliance) and 2 dropouts (1 pneumothorax, 1 subjective lack of effect). |
| Selective reporting (reporting bias) | High risk | Not all outcomes were reported in full. |
| Other bias | High risk | There was no washout period between months, thus there is a potential for a carryover effect. |
Tannenbaum 2005.
| Methods | RCT. Parallel design. Treatment period of 12 months. |
|
| Participants | 30 children with CF, age range 6 ‐ 15 years, 20 females. | |
| Interventions | 2 interventions: 15 participants were randomised to each group. 1. PEP (no further data provided); 2. Oscillating PEP provided by the RC cornet (no further data provided). |
|
| Outcomes | QWBS FEV1, pulmonary exacerbations, LCI. |
|
| Notes | Information was provided from 3 abstracts, no further information obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified by age, sex and FEV1. How randomisation was generated was not recorded. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts are reported, one from each group. One found the cornet ineffective and difficult to clean. The other preferred a previously used device. Used Intention‐to‐treat approach. |
| Selective reporting (reporting bias) | Unclear risk | Not all outcome measures results were provided in full. QWBS was reported as no significant changes over the year. |
| Other bias | Low risk | Groups were similar at baseline regarding the most important prognostic indicators. |
Tyrrell 1986.
| Methods | RCT. Cross‐over design. Each treatment given for 1 month. | |
| Participants | CF diagnosed by the Nottingham City Hospital Cystic Fibrosis Clinic. 19 participants (after withdrawals, 9 females and 7 males); mean age 13 years, range 10 ‐ 18 years; mean Schwachman score 62, range 47 ‐ 85 points. | |
| Interventions | 2 interventions:There was no washout period between each treatment period. 1. PEP treatment ‐ pressure 10 ‐ 15 cm H₂O; seated participants exhaled 10 times through an Astra mask, followed by "forced expiratory coughing"; 2. PDP ‐ participants received percussion and performed coughing in PD positions. Treatment was performed for 20 minutes, twice daily. |
|
| Outcomes | FEV 0.75, FVC, PEFR were recorded before, 20 minutes after, and 90 minutes after a single supervised treatment at the beginning of the randomisation month. Wet weight of sputum expectorated during the therapy was also measured. The same measures were repeated over a single treatment at the end of the randomisation month. In addition, during each treatment month, diary card records were kept regarding the following symptoms: sleep, cough, wheeze, activity, sputum production. (Details of the scoring system for these symptoms are not provided.) Although not listed as a formal outcome measures, antibiotic use and participant preference are also discussed in the results section. | |
| Notes | 3 withdrawals due to non‐adherence.
Those children who showed airway reversibility with salbutamol were asked to use it before treatment throughout the whole study. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 withdrawals due to non‐adherence. |
| Selective reporting (reporting bias) | High risk | Not all outcomes were reported in full. |
| Other bias | High risk | There was no washout period between each treatment period, thus there is a potential for a carryover effect. |
van Asperen 1987.
| Methods | RCT. Cross‐over design. Each treatment given for 4 weeks. | |
| Participants | CF diagnosed by Camperdown or Westmead Hospitals, and daily sputum production. 13 participants (gender unspecified); age range 7 ‐ 18 years. No change in treatment in the 2 months prior to commencing the study. | |
| Interventions | 2 interventions:There was no washout period between each treatment period. 1. PEP treatment ‐ pressure 10 ‐ 15 cm H₂O; participants inhaled and exhaled 10 ‐ 15 times through an Astra mask (position unspecified), followed by forced expiration and coughing; 2. PDP ‐ participants received manual percussion to all areas in PD positions, followed by forced expiration and coughing. The PEP intervention was continued for 20 minutes. PDP lasted "at least 20 minutes". Each intervention treatment was administered twice daily for 4 weeks. There was no washout period. |
|
| Outcomes | FEV1 and FVC were measured before and 1 hour after the first treatment of each randomisation period. Volume of expectorated sputum was measured over 1 hour which commenced with the first treatment of the randomisation period. | |
| Notes | 2 withdrawals due to infective exacerbations and 1 dropout. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals due to infective exacerbations and one dropout. |
| Selective reporting (reporting bias) | High risk | Not all outcomes were reported in full. |
| Other bias | High risk | Groups similar at baseline regarding the most important prognostic indicators. There was no washout period between each treatment period, thus there is a potential for a carryover effect. |
van Winden 1998.
| Methods | RCT. Cross‐over design. Each treatment given for 2 weeks. | |
| Participants | CF confirmed by sweat tests or DNA mutation analysis. 22 participants (12 male); median age 12 years, range 7 ‐ 17 years; median FEV1 82, range 55% ‐ 129% predicted. Participants were excluded if they had been clinically unstable during the 2 weeks prior to entering the study, according to PEFR and symptoms scores (criteria not specified). | |
| Interventions | 2 interventions:There was a one week washout period between each treatment period.
1. PEP treatment ‐ pressure 8 ‐ 12 cm H₂O; seated participants breathed through an Astra Meditec PEP mask 15 times, followed by 3 huffs and coughing, this sequence was repeated 5 times;
2. Oscillating PEP (flutter) ‐ participants inhaled deeply, held their breath for 2 ‐ 3 seconds, then exhaled through the VarioRaw flutter device 15 times, following which the participant again huffed 3 times and coughed. This sequence was also repeated 5 times. The flutter was tilted upwards or downwards a few degrees from horizontal until the maximum vibration sensation was obtained. Each intervention was performed twice per day for 2 weeks, preceded by a 1‐week washout period. During the washout weeks, all participants performed "routine physiotherapy" with huff and cough manoeuvres. |
|
| Outcomes | FEV1, FVC, and TLC were measured before the initial, 1‐week washout period. These measures were repeated on the first day of each of the 2 treatment periods, before and 30 minutes after the first session of therapy. At the end of the 2‐week treatment periods, these measures were again taken 30 minutes after physiotherapy. Oxygen saturation via pulse oximetry was measured before during and after the first and last treatments of each 2‐week period. | |
| Notes | No withdrawals or dropouts. One week washout period between treatments. Published paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported in full. |
| Other bias | Low risk | There was a one week washout period between each treatment period, thus there is low risk of any carry over effect. Groups similar at baseline regarding the most important prognostic indicators.There was a one week washout period between treatments. |
West 2010.
| Methods | RCT. Parallel design Treatment for 10 days |
|
| Participants | 23 participants with CF admitted to hospital with an acute exacerbation of CF. 12 randomised to PEP and 11 to acapella. Aged between 5 ‐ 18 years of age. FEV1 range 29 to 114% predicted |
|
| Interventions | 1. PEP mask 10 breaths followed by one to two huffs and a cough, repeated for 10 sets, performed twice daily for 10 days. 2. Acapella was performed same as the PEP for consistency. 10 breaths followed by one to two huffs and a cough repeated for 10 sets, performed twice daily for 10 days. |
|
| Outcomes | Change in lung function. FEV1, FVC, FEF25‐75%and PEFR were measured at baseline and after 10 days. Exercise performance was measured using the 10 metre shuttle test at baseline and after 10 days. Total sputum expectorated during each treatment was weighed. Patient satisfaction was measured using a patient satisfaction survey completed at the end of 10 days. |
|
| Notes | Published as paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was achieved by placing equal number of papers for each treatment in double‐sealed envelopes |
| Allocation concealment (selection bias) | Low risk | A research coordinator not involved in the recruitment, assessment or treatment withdrew randomly one of the double sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and person providing the therapy were not blinded‐ unclear risk outcome assessors were blinded‐ low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was one dropout from the acapella group (reason given) and the study did not meet the sample size required for the power analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes were fully reported |
| Other bias | Unclear risk | Demographics at baseline were not matched. |
AD: autogenic drainage BEpd: breathing exercises in postural drainage positions BMI: body mass index CE: cycle ergometry CF: cystic fibrosis FEV 0.75: forced expiratory volume in 0.75 sec FEF25‐75: forced expiratory flow 25‐75% FET: forced expiratory technique FEV1: forced expiratory volume at 1 second FRC: functional residual capacity FVC: forced vital capacity HFCC: high‐frequency chest compression HFCWO: high‐frequency chest wall oscillation Hi‐PEP: high‐pressure PEP IV: intravenous LCI: lung clearance index PD: postural drainage PDP: postural drainage with percussion PDPV: postural drainage with percussion and vibration PEP: positive expiratory pressure PEFR: peak expiratory flow rate PEPpd: PEP in postural drainage PFT: pulmonary function test PLBs: pursed lip breathing in sitting pO2: blood test measuring oxygen in the blood Ptco2: transcutaneously measured carbon dioxide tension Pt02: transcutaneous oxygen tension QWBS: quality of life using the quality of life well‐being scale RCT: randomised controlled trial RV: residual volume SD: standard deviation SpO₂: saturation of haemoglobin with oxygen using pulse oximetry TLC: total lung capacity TV: tidal volume nBVS: non‐invasive bilevel ventilatory support SVC: slow vital capacity W/kg: watt per kilogram
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aquino 2006 | The intervention, to which PEP was compared, was not a physical airway clearance therapy. |
| Aquino 2012 | Neither of the interventions were PEP. |
| Aubriot 2016 | The delivery of an inhaled antibiotic was evaluated using a PEP system compared to a jet nebulizer. This is not study of the efficacy of PEP compared to another airway clearance technique. |
| Balestri 2004 | Used a different type of PEP system (underwater tubing) which did not meet the PEP criteria for this review. |
| Battistini 2001 | Used a different type of PEP system (underwater tubing) which did not meet the PEP criteria for this review. |
| Bishop 2011 | PEP versus other airway clearance was not the randomised comparison made in this study. |
| Borka 2012 | Study was not randomised. |
| Castle 1994 | No outcome data were reported. |
| Dosman 2003 | Used a different type of PEP system (positive end expiratory pressure) which did not meet the PEP criteria for this review. |
| Falk 1988 | The intervention, to which PEP was compared, was not a physical airway clearance therapy. |
| Fitzgerald 2001 | PEP versus other airway clearance was not the randomised comparison made in this study. |
| Gotz 1995 | No data were reported for the outcomes of interest. |
| Kraemer 1996 | Neither of the interventions were PEP. |
| Laube 2000 | The intervention to which PEP was compared was not a physical airway clearance therapy. |
| Liedtke 1996 | Neither of the interventions were PEP. |
| Marks 1998 | The use of PEP versus the other physical airway clearance therapy (flutter) was not the randomised comparison made in this study. |
| McCarren 2006 | Study used PEP breathing, but not the PEP technique as defined in this review. |
| Munro 2007 | Study was performed on post‐transplant patients. |
| Oermann 2001 | Neither of the interventions being compared was PEP. |
| Orlik 2000 | The use of PEP versus the other physical airway clearance therapies was not the factor which was random in this study. |
| Orlik 2015 | This was a retrospective study on the use of a PEP system to deliver mucolytics. This is not study of the efficacy of PEP compared to another airway clearance technique. |
| Padman 1999 | Used a different type of PEP system (Vitalsigns flow independent system) which did not meet the PEP criteria for this review. |
| Patel 2013 | Neither of the interventions being compared was PEP. |
| Placidi 2001 | Study used PEP breathing, but not the PEP technique as defined in this review. |
| Reychler 2015 | The manoeuvre being compared to PEP was not a recognized airway clearance technique. |
| Richmond 2016 | This was a study examining adherence to PEP therapy. It was not randomised and there was no control. |
| Roos 1987 | Niether of the interventions were PEP. |
| Sanchez Riera 1999 | PEP technique did not include huffing and was performed in a postural drainage position. |
| van der Schans 1991 | Used a different type of PEP system (Vitalsigns flow independent system) which did not meet the PEP criteria for this review. |
| van Hengstum 1987 | The study was performed in participants with chronic bronchitis. |
| Wettstein 2014 | The intervention, to which PEP was compared, was not a physical airway clearance therapy. |
| Wilson 2015 | The delivery of hypertonic saline was evaluated using a PEP system compared to an eFlow nebulizer. This is not study of the efficacy of PEP compared to another airway clearance technique. |
| Znotina 2000 | The use of PEP versus the other physical airway clearance therapy (oscillating PEP) was not the factor which was randomised in this study. |
PEP: positive expiratory pressure
Characteristics of studies awaiting assessment [ordered by study ID]
Elkins 2005.
| Methods | Cross‐over RCT. |
| Participants | 12 participants with CF aged 16 to 34 years. |
| Interventions | PEP compared to oscillating PEP, PDPV and ACBT. |
| Outcomes | Mucociliary clearance. |
| Notes | Results have not been published. |
Grzincich 2008.
| Methods | RCT, participants randomised to receive either HFCWO or PEP during the first 3 days of hospitalisation for an exacerbation. |
| Participants | 23 participants. 12 female, mean age 25 years. |
| Interventions | Use of HFCWO at setting of 20 Hz for 30 minutes compared with 30 minutes of PEP; this occurred during the first 3 days of treatment. |
| Outcomes | FEV1, FVC and FEF25‐75 were assessed pre and 30 minutes post intervention. Sputum volume was collected after each intervention. |
| Notes | Abstract only, full paper not published as yet. |
Kofler 1994.
| Methods | Cross‐over RCT. |
| Participants | 33 children (19 boys, 14 girls) with CF, mean (SD) age 11 (3.9) years. 23 completed. |
| Interventions | ACBT compared to PEP mask in addition to conventional CF therapy. 4 months treatment A then changed to other treatment for a further 4 months. |
| Outcomes | FVC, FEV1, MEEF, questionnaire re acceptability of techniques. Outcomes measured before and after each treatment period. |
| Notes | Abstract only. |
Parreira 2008.
| Methods | RCT. |
| Participants | 13 participants with CF mean (SD) age 18 (3) years. |
| Interventions | Flutter® compared to EPAP. |
| Outcomes | Short‐term lung function changes. |
| Notes | Abstract only. |
Radtke 2018.
| Methods | Cross‐over RCT. |
| Participants | Individuals with CF were recruited from the Adult CF Center at the University Hospital Zurich, Switzerland, between June 2016 and January 2017. Patients aged 18 years and older with a confirmed diagnosis of CF able to provide sputum samples were included. Exclusion criteria were as follows: i) listing for lung transplantation or status post lung transplantation, ii) chronic pulmonary infection with BBC, iii) unstable clinical condition (i.e., major hemoptysis or pneumothorax within the last 3 months, acute pulmonary exacerbation [13], intravenous antibiotic treatment during the last 4 weeks, change in pulmonary medication during the study period); iv) cardiac arrhythmias with exercise ;and v) requirement of additional oxygen with exercise. |
| Interventions | A single bout of continuous cycling exercise at moderate intensity (experiment A, control condition) vs a combination of interval cycling exercise plus Flutter® (experiment B). |
| Outcomes | Sputum properties (viscoelasticity, yield stress, solids content, spinnability, and ease of sputum expectoration), pulmonary diffusing capacity for nitric oxide (DLNO) and carbon monoxide (DLCO) were assessed at rest, directly and 45 minutes post‐exercise (recovery) at 2 consecutive visits. Primary outcome was change in in sputum viscoelasticity (G’, storage modulus; G”, loss modulus) over a broad frequency range (0.1–100 rad.s− 1). |
| Notes | NCT02750722 |
Tonnesen 1982.
| Methods | Cross‐over RCT. |
| Participants | 14 participants with CF, age range 12 ‐ 29 years, mean age 15.9 years, chronic infection with Pseudomonas aeruginosa. |
| Interventions | PEP compared to conventional physiotherapy, 4 days |
| Outcomes | FVC, FEV1, RV, FRC, TLC, PF, bacteriology (P aeruginosa, S aureus, E coli) |
| Notes | Paper in Danish, needs full translation. |
Vendrusculo 2019.
| Methods | Pilot cross‐over RCT. |
| Participants | 12 children, mean (SD) age 12.83 years (1.85 years). Those who had chronic infection and with BCC and non‐tuberculous M Abscessus were excluded. |
| Interventions | ACT prior to CPET vs CPET alone. |
| Outcomes | Spirometry, plethysmography and 2 CPET scans were performed 1 month apart ‐ 1 with ACT immediately prior to CPET and the other without. |
| Notes |
Ward 2018.
| Methods | RCT. |
| Participants | 13 adults (FEV1 ≥ 70% predicted). |
| Interventions | Daily PEP plus exercise or exercise‐only for 3 months. |
| Outcomes | FEV1 L, respiratory function tests, respiratory exacerbation rate and health‐related quality of life. |
| Notes |
Wong 2000.
| Methods | RCT. |
| Participants | 17 participants with CF and suspected GER, mean age 12.6 years, 4 did not complete study. |
| Interventions | Oesophageal pH monitoring for 48 hours, in this time 2 sessions of PDP and 2 sessions of PEP in an upright position. |
| Outcomes | Reflux episodes per hour, fractional reflux time, cough. |
| Notes | Abstract only, waiting for further information. |
ACBT: active cycle of breathing technique ACT: airway clearance therapy BBC: Burkholderia cepacia complex CF: cystic fibrosis CPET: cardiopulmonary exercise testing EPAP: expiratory positive airway pressure E coli: Escherichia coli FEV1: forced expiratory volume at 1 second FRC: functional residual capacity FVC: forced vital capacity GER: gastroesophageal reflux M Abscessus:Mycobacterium Abscessus NIV: non‐invasive ventilation PAP: positive airway pressure PEP: positive expiratory pressure PDP: postural drainage and percussion PF: pulmonary function P aeruginosa:Pseudomonas aeruginosa RCT: randomised controlled trial RV: residual volume S aureus: Staphylococcal aureus TLC: total lung capacity
Differences between protocol and review
The protocol for this review was based on the previous review of 2006. A new team undertook the 2014 review and we amended the primary outcomes to include direct measures of mucus clearance. The number of respiratory exacerbations was kept as a primary outcome, however the number of days of intravenous antibiotic use was removed. This was because, between 2006 and 2014, respiratory exacerbations had further been defined to include both oral and IV antibiotics. Well‐being, adverse effects, exercise tolerance and patient preference were moved to secondary outcomes as none of the studies reviewed had reported any of them as a primary outcome.
Contributions of authors
Maggie McIlwaine, Brenda Button and Sarah Nevitt updated the review which was originally drafted by Mark Elkins, Alice Jones and Cees van der Schans.
Maggie McIlwaine and Brenda Button independently assessed studies for inclusion in the updates of this review from 2014; Kerry Dwan and Brenda Button independently assessed the studies which were authored by McIlwaine. All review authors contributed to data extraction and updated the text and analyses in this review. Maggie McIlwaine wrote the text of this version with contributions from Brenda Button; the original text was written by Mark Elkins, with contributions from Alice Jones.
Maggie McIlwaine acts as guarantor of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Maggie McIlwaine is the Principal Investigator for four of the included studies. These studies were independently assessed by the other review authors.
Brenda Button declares no potential conflict of interest.
Sarah Nevitt declares no potential conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Braggion 1995 {published data only}
- Braggion C, Cappelletti LM, Cornacchia M, Zanolla L, Mastella G. Short‐term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: a cross‐over study. Pediatric Pulmonology 1995;19(1):16‐22. [CFGD Register: PE26b] [DOI] [PubMed] [Google Scholar]
- Cappelletti LM, Cornacchia M, Braggion C, Zanolla L, Mastella G. Short‐term effects of 3 physiotherapy (CPT) regimens in cystic fibrosis (CF) patients hospitalized for a pulmonary exacerbation: a cross‐over randomized trial. 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid. 1993:W9.3. [CFGD Register: PE26a] [DOI] [PubMed]
- Cappelletti LM, Cornacchia M, Braggion C, Zanolla L, Mastella G. Short‐term effects of three chest physiotherapy regimens on patients with cystic fibrosis hospitalized for pulmonary exacerbation: a crossover randomized study. Excerpta Medica International Congress Series 1993;1034:239‐46. [CFGD Register: PE26c] [Google Scholar]
Costantini 2001 {published data only (unpublished sought but not used)}
- Costantini D, Brivio A, Brusa D, Delfino R, Fredella C, Russo, et al. PEP‐mask versus postural drainage in CF infants a long‐term comparative trial. 24th European Cystic Fibrosis Conference; 2001. Vienna, 2001:100. [CFGD Register: PE94b]
- Costantini D, Brivio A, Brusa D, Delfino R, Fredella C, Russo, et al. PEP‐mask versus postural drainage in CF infants a long‐term comparative trial. Pediatric Pulmonology 2001;Suppl 22:308. [CFGD Register: PE94c] [Google Scholar]
- Costantini D, Brivio A, Delfino R, Sguera A, Brusa D, Padoan R, et al. PEP mask versus postural drainage in CF infants. Pediatric Pulmonology 1998;Suppl 17:342. [CFGD Register: PE94a] [Google Scholar]
Darbee 1990 {published and unpublished data}
- Dadparvar S, Darbee J, Jehan A, Bensel K, Slizofski WJ, Holsclaw D. Tc‐DTPA aerosol ventilation evaluates the effectiveness of PEP mask in the treatment of cystic fibrosis. European Respiratory Journal 1995;8 Suppl 19:177s. [Google Scholar]
- Darbee J, Dadparvar S, Bensel K, Jehan A, Watkins M, Holsclaw D. Radionuclide assessment of the comparative effects of chest physical therapy and positive expiratory pressure mask in cystic fibrosis. Pediatric Pulmonology 1990;Suppl 5:251. [Google Scholar]
Darbee 2004 {published data only}
- Darbee JC, Grant BJ, Ohtake PJ, Cerny FC. Positive expiratory pressure breathing and gas distribution in cystic fibrosis. Pediatric Pulmonology 2000;Suppl 20:304. [Google Scholar]
- Darbee JC, Ohtake PJ, Grant BJB, Cerny FJ. Physiologic evidence for the efficacy of positive expiratory pressure as an airway clearance technique in patients with cystic fibrosis. Physical Therapy 2004;84(6):524‐37. [PubMed] [Google Scholar]
Darbee 2005 {published data only}
- Darbee JC, Kanga JF, Ohtake PJ. Physiological evidence for high‐frequency chest wall oscillation and positive expiratory breathing in hospitalized subjects with cystic fibrosis. Physical Therapy 2005;85(12):1278‐89. [PubMed] [Google Scholar]
- Darbee JC, KangaJF, Ohtake PJ. Physiological evidence for high frequency chest wall oscillation and positive expiratory breathing in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 2005;40 Suppl 21:322. [PubMed] [Google Scholar]
Fainardi 2011 {published data only}
- Fainardi V, Longo F, Faverzani S, Tripodi MC, Chetta A, Pisi G. Short‐term effects of high‐frequency chest compressions and positive expiratory pressure in patients with cystic fibrosis. Journal of Clinical Medicine Research 2011;3(6):279‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falk 1984 {published data only}
- Falk M, Kelstrup M, Andersen JB, Kinoshita T, Falk P, Stovring S, et al. Improving the ketchup bottle method with positive expiratory pressure, PEP, in cystic fibrosis. European Journal of Respiratory Diseases 1984;65(6):423‐32. [PubMed] [Google Scholar]
Falk 1993 {published data only}
- Falk M, Mortensen J, Kelstrup M, Lanng S, Larsen L, Ulrik CS. Short‐term effects of positive expiratory pressure and the forced expiration technique on mucus clearance and lung function in CF. Pediatric Pulmonology 1993;Suppl 9:241. [Google Scholar]
- Larsen L, Mortensen J, Falk M, Kelstrup M, Lanng S, Ulrik CS. Radiolabelled mucus clearance in patients with cystic fibrosis is improved by physiotherapy with positive expiratory pressure and the forced expiration technique. Clinical Physiology 1994;14:365. [Google Scholar]
- Mortensen J, Falk M, Kelstrup M, Lanng S, Ulrik CS. Effect of positive expiratory pressure and the forced expiration technique on mucus clearance in patients with cystic fibrosis. European Respiratory Journal 1993;6(Suppl 17):409S. [Google Scholar]
Gaskin 1998 {published and unpublished data}
- Gaskin L. [personal communication]. Conversation with: M McIlwaine before 11 November 2019.
- Gaskin L, Corey M, Shin J, Reisman JJ, Thomas J, Tullis DE. Long term trial of conventional postural drainage and percussion vs. positive expiratory pressure. Pediatric Pulmonology 1998;Suppl 17:345. [Google Scholar]
Hofmeyr 1986 {published data only}
- Hofmeyr JL, Webber BA, Hodson ME. Evaluation of positive expiratory pressure as an adjunct to chest physiotherapy in the treatment of cystic fibrosis. Thorax 1986;41(12):951‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber BA, Hofmeyer JL, Hodson ME, Batten JC. Evaluation of positive expiratory pressure as an adjunct to postural drainage. 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov 3‐8; Jerusalem. 1985:95.
Kofler 1998 {published data only}
- Kofler AM, Carlesi A, Cutrera R, Leone P, Lucidi V, Rosati S, et al. BiPAP versus PEP as chest physiotherapy in patients with cystic fibrosis. Pediatric Pulmonology 1998;Suppl 17:344. [Google Scholar]
Lagerkvist 2006 {published data only}
- Lagerkvist AL, Sten G, Lindblad A, Redfors S. Chest physiotherapy with positive expiratory pressure (PEP) and oscillating positive expiratory pressure (flutter) in patients with cystic fibrosis ‐ a comparative study. 21st European Cystic Fibrosis Conference 1997 June 1‐6. 1997:132.
- Lagerkvist AL, Sten GM, Redfors SB, Lindblad AG, Hjalmarson O. Immediate changes in blood‐gas tensions during chest physiotherapy with positive expiratory pressure and oscillating positive expiratory pressure in patients with cystic fibrosis. Respiratory Care 2006;51(10):1154‐61. [PubMed] [Google Scholar]
Lannefors 1992 {published data only}
- Lannefors L, Wollmer P. Mucus clearance in cystic fibrosis (CF) ‐ a comparison between postural drainage, PEP‐mask and physical exercise. 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:AHP31. [PubMed]
- Lannefors L, Wollmer P. Mucus clearance with three chest physiotherapy regimes in cystic fibrosis: a comparison between postural drainage, PEP and physical exercise. European Respiratory Journal 1992;5(6):748‐53. [PubMed] [Google Scholar]
McIlwaine 1991 {published and unpublished data}
- Davidson AGF, McIlwaine PM, Wong TK, Nakielna EM, Pirie GE. A comparative trial of positive expiratory pressure, autogenic drainage and conventional percussion and drainage techniques. Pediatric Pulmonology 1988;Suppl 2:137. [CFGD Register: PE44a] [Google Scholar]
- McIlwaine PM, Davidson AGF. Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of CF. 17th European Cystic Fibrosis Conference; 1991 Jun 18‐21; Copenhagen. 1991:S8.4. [CFGD Register: PE44c]
- McIlwaine PM, Davidson QGF, Wong LTK, Pirie GE, Nakielna EM. Comparison of positive expiratory pressure and autogenic drainage with conventional percussion and drainage therapy in the treatment of CF. 10th International Cystic Fibrosis Congress; Mar 5‐10; Sydney. 1988; Vol. 74:3. [CFGD Register: PE44b]
- McIlwaine PM, Wong LT, Pirie GE, Davidson AG. Comparison of positive expiratory pressure and autogenic drainage therapy in the treatment of cystic fibrosis (as supplied 21 July 2003). Data on file. [CFGD Register: PE44d]
McIlwaine 1997 {published and unpublished data}
- Button B, Herbert R, Maher C. Positive expiratory pressure therapy better maintains pulmonary function than postural drainage and percussion in patients with cystic fibrosis. Australian Journal of Physiotherapy 1998;44(4):285‐6. [Google Scholar]
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long‐term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. 12th International Cystic Fibrosis Conference; 1996 Jun 16‐21; Jerusalem. 1996:S193. [DOI] [PubMed]
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long‐term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. Journal of Pediatrics 1997;131(4):570‐4. [DOI] [PubMed] [Google Scholar]
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long‐term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. Pediatric Pulmonology 1995;Suppl 12:268. [DOI] [PubMed] [Google Scholar]
McIlwaine 2001 {published data only}
- Davidson AG, McIlwaine PM, Wong LT, Peacock D. "Flutter versus PEP": A long‐term comparative trial of positive expiratory pressure (PEP) versus oscillating positive pressure (Flutter) physiotherapy techniques. 22nd European Cystic Fibrosis Conference; 1998 Jun 13‐19; Berlin. 1998:71.
- McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long‐term comparative trial of positive expiratory pressure versus oscillating positive expiratory pressure (flutter) physiotherapy in the treatment of cystic fibrosis. Journal of Pediatrics 2001;138(6):845‐50. [DOI] [PubMed] [Google Scholar]
- McIlwaine PM, Wong LTK, Peacock D, Davidson AGF. "Flutter versus PEP": A long‐term comparative trial of positive expiratory pressure (PEP) versus oscillating positive expiratory pressure (Flutter) physiotherapy techniques. Pediatric Pulmonology 1997;Suppl 14:299. [Google Scholar]
McIlwaine 2013 {published data only}
- Alarie N, Agnew JL, McIlwaine M, Ratjen F, Davidson G, Lands LC. Canadian National Airway Clearance Study: how physically active are CF patients?. Pediatric Pulmonology 2012;47(S35):367. [CENTRAL: 875004; CFGD Regsiter: PE187e; CRS: 5500125000000032] [Google Scholar]
- Alarie N, Agnew LL, McIlwaine MP, Ratjen F, Davidson GF, Milner R, et al. Evaluation of physical activity using the habitual activity estimation scale (HAES) questionnaire in a multicenter study. Journal of Cystic Fibrosis 2013;12 Suppl 1:S28. [CENTRAL: 875001; CFGD Register: PE187f; CRS: 5500100000011657] [Google Scholar]
- McIlwaine M, Agnew J, Alarie N, Ratjen F, Lands L, Milner R, et al. Canadian national airway clearance study: patient satisfaction with positive expiratory pressure versus high frequency chest wall oscillation. Pediatric Pulmonology 2012;47 Suppl 35:367. [CFGD Register: PE187b] [Google Scholar]
- McIlwaine M, Agnew JL, Alarie N, Lands L, Ratjen F, Milner R, et al. Canadian national airway clearance study: positive expiratory pressure mask versus high frequency chest wall oscillation. Journal of Cystic Fibrosis 2012;11 Suppl 1:S23. [CFGD Register: PE187a] [Google Scholar]
- McIlwaine MP, Alarie N, Davidson GF, Lands LC, Ratjen F, Milner R, et al. Long‐term multicentre randomised controlled study of high frequency chest wall. Thorax 2013;68(8):746‐51. [CFGD Register: PE187c; ] [DOI] [PubMed] [Google Scholar]
- McIlwaine MP, Alarie N, Davidson GF, Lands LC, Ratjen F, Milner R, et al. Long‐term multicentre randomised controlled study of high frequency chest wall. Thorax 2013;68(8):746‐51. Online supplement. [CFGD Register: PE187d; ] [DOI] [PubMed] [Google Scholar]
- McIlwaine MP, Richmond M, Agnew JL, Alarie N, Lands L, Chivers M, et al. Cost‐effectiveness of preforming positive expiratory pressure versus high frequency chest wall oscillation. Journal of Cystic Fibrosis 2014;13 Suppl 2:S11. [Abstract no.: WS5.6; CFGD Register: PE187g] [Google Scholar]
Mortensen 1991 {published data only}
- Falk M, Mortensen J, Jensen C, Groth S, Jensen T. Postural drainage or PEP: effects on tracheobronchial clearance in cystic fibrosis. Pediatric Pulmonology 1990;Suppl 5:226. [Google Scholar]
- Mortensen J, Falk M, Groth S, Jensen C. The effects of postural drainage and positive expiratory pressure physiotherapy on tracheobronchial clearance in cystic fibrosis. Chest 1991;100(5):1350‐7. [DOI] [PubMed] [Google Scholar]
- Mortensen J, Groth S, Falk M, Jensen C, Jensen T. Assessment of tracheobronchial clearance by sputum expectorated during chest physiotherapy in cystic fibrosis. European Respiratory Journal 1990;3 Suppl 10:260s‐61s. [Google Scholar]
Newbold 2005 {published data only}
- Newbold ME, Brooks D, Tullis DE, Ross BG. Effectiveness of the flutter device versus the PEP mask in the treatment of adult cystic fibrosis. Pediatric Pulmonology 2000;Suppl 20:304. [Google Scholar]
- Newbold ME, Tullis E, Corey M, Ross B, Brooks D. The flutter device versus the PEP mask in the treatment of adults with cystic fibrosis. Physiotherapy Canada 2005;57(3):199‐207. [Google Scholar]
Pfleger 1992 {published data only}
- Pfleger A, Theissl B, Oberwaldner B, Zach MS. Self‐administered chest physiotherapy in cystic fibrosis: a comparative study of high‐pressure PEP and autogenic drainage. Lung 1992;170(6):323‐30. [DOI] [PubMed] [Google Scholar]
- Theissl B, Pfleger A, Oberwaldner B, Zach M. Chest physiotherapy (PT) in cystic fibrosis (CF) ‐ a comparative study of high‐pressure PEP and autogenic drainage. Pediatric Pulmonology 1990;Suppl 5:256. [DOI] [PubMed] [Google Scholar]
Pryor 2010 {published data only}
- Pryor JA, Tannenbaum E, Cramer D, Scott SF, Burgess J, Gyi K, et al. A comparison of five airway clearance techniques in the treatment of people with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl 1:S76. [DOI] [PubMed] [Google Scholar]
- Pryor JA, Tannenbaum E, Scott SF, Burgess J, Cramer D, Gyi K, et al. Beyond postural drainage and percussion: Airway clearance in people with cystic fibrosis. Journal of Cystic Fibrosis 2010;9(3):187‐92. [DOI] [PubMed] [Google Scholar]
Rodriguez 2016 {published data only}
- Rodriguez Hortal MC, Hjelte L. Non invasive ventilation as airway clearance technique compared to PEP in adult patients with cystic fibrosis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S18. [Abstract no: WS9.4; CENTRAL: 875000; CRS: 5500100000011654] [Google Scholar]
- Rodriguez Hortal MC, Nygen‐Bonnier M, Hjelte L. Non‐invasive ventilation as airway clearance technique in cystic fibrosis. Phyiotherapy Research International 2016;22(3):1‐9. [DOI] [PubMed] [Google Scholar]
Steen 1991 {published data only}
- Steen HJ, Redmond AO, O'Neill D, Beattie F. Evaluation of the PEP mask in cystic fibrosis. Acta Paediatrica Scandinavica 1991;80(1):51‐6. [DOI] [PubMed] [Google Scholar]
- Steen HJ, Redmond AO, O'Neill D, Beattie F. Has the PEP mask a role in the management of teenage patients?. 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov. 3‐8; Jerusalem. 1985:94.
Tannenbaum 2005 {published data only}
- Main E, Tannenbaum E, Stanojevic S, Scrase E, Prasad A. The effects of positive expiratory pressure (PEP) or oscillatory positive pressure (RC Cornet®) on FEV1 and lung clearance index over a twelve month period in children with CF. Pediatric Pulmonology 2006;41 Suppl 29:351. [Google Scholar]
- Prasad A, Tannenbaum E, Bryon M, Main E. One year trial of two airway clearance techniques in children with cystic fibrosis: Limitations of the quality of well‐being scale. Pediatric Pulmonology 2005;Suppl 28:323. [Google Scholar]
- Tannenbaum E, Prasad SA, Main. E, Scrase E. Long term effects of positive expiratory pressure (PEP) or oscillatory positive pressure (RC Cornet®) on FEV1 and perceived health in children with CF. 28th European Cystic Fibrosis Conference; 2005 Jun 22‐25; Crete, Greece. 2005:S100.
Tyrrell 1986 {published and unpublished data}
- Tyrrell JC, Hiller EJ, Martin J. Face mask physiotherapy in cystic fibrosis. Archives of Disease in Childhood 1986;61(6):598‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell JC, Martin J, Hiller EJ. 'PEP' mask physiotherapy in cystic fibrosis. 13th Annual Meeting of the European Working Group for Cystic FIbrosis; 1985 Nov 3‐8; Jerusalem. 1985:23.
van Asperen 1987 {published and unpublished data}
- Asperen PP, Jackson L, Hennessy P, Brown J. Comparison of a positive expiratory pressure (PEP) mask with postural drainage in patients with cystic fibrosis. Australian Journal of Paediatrics 1987;23(5):283‐4. [DOI] [PubMed] [Google Scholar]
van Winden 1998 {published data only}
- Winden CM, Visser A, Hop W, Sterk PJ, Beckers S, Jongste JC. Effects of Flutter and PEP‐MASK on expectoration and lung function in cystic fibrosis. 12th International Cystic Fibrosis Conference; 1996 Jun 16‐21; Jerusalem, Israel. 1996:S275.
- Winden CM, Visser A, Hop W, Sterk PJ, Beckers S, Jongste JC. Effects of flutter and PEP mask physiotherapy on symptoms and lung function in children with cystic fibrosis. European Respiratory Journal 1998;12(1):143‐7. [DOI] [PubMed] [Google Scholar]
West 2010 {published data only}
- West K, Wallen M, Follett J. Acapella vs. PEP mask therapy: a randomised trial in children with cystic fibrosis during respiratory exacerbation. Physiotherapy Theory and Practice 2010;26(3):143‐9. [CENTRAL: 753270; CRS: 5500050000000049; PUBMED: 20331370] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aquino 2006 {published data only}
- Aquino A, Balestri E, Dall 'Ara S, Lami I, Gobbi F, Ambroni M, et al. Efficacy of physical exercise playing a video game for mucus clearance in patients with Cystic Fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl:S83. [Google Scholar]
Aquino 2012 {published data only}
- Aquino ES, Shimura F, Santos AS, Goto DM, Coelho CC, Fucciiio MB, et al. CPAP has no effect on clearance, sputum properties or expectorated volume in cystic fibrosis. Respiratory Care 2012;57(11):1914‐9. [DOI] [PubMed] [Google Scholar]
Aubriot 2016 {unpublished data only}
- Aubriot A, Lebecque P, Teresinha L, Liistro G, Reychler G. Evaluation of the combination of positive expiratory pressure and nebulization with cystic fibrosis patients. Paediatric Pulmonology 2016;51 Suppl 45:371. [Abstract no.: 468] [Google Scholar]
Balestri 2004 {published data only}
- Balestri E, Ambroni M, Dall'Ara S, Miano A. Efficacy of physical exercise for mucus clearance in patients with cystic fibrosis (CF). Pediatric Pulmonology 2004;Suppl 27:316. [Google Scholar]
Battistini 2001 {published data only}
- Battistini R, Balestri E, Ambroni M, Miano A. Efficacy of underwater positive expiratory pressure therapy (UPEP) for mucus clearance in patients with cystic fibrosis. 24th European Cystic Fibrosis Conference; 2001 Jun 6‐9; Vienna. 2001:P104.
Bishop 2011 {published data only}
- Middleton PG, Bishop J. Dornase alpha and physiotherapy ‐ which should be first? A randomised, double‐blind, placebo‐controlled trial in CF adults. Pediatric Pulmonology 2001;32 Suppl 22:310. [CFGD Register: BD102c] [Google Scholar]
Borka 2012 {published data only}
Castle 1994 {published data only}
- Castle T, Metcalfe C, Knox A. A comparison between the active cycle of breathing technique (A.C.B.T.) and positive expiratory pressure (PEP) mask plus A.C.B.T. on sputum production and lung volumes in adults with Cystic Fibrosis. 19th European Cystic Fibrosis Conference; 1994 May 29‐Jun 3. 1994:O17.
Dosman 2003 {published data only}
- Dosman CF, Zuberbuhler PC, Tabak JI, Jones RL. Effects of positive end‐expiratory pressure on oscillated volume during high frequency chest compression in children with cystic fibrosis. Canadian Respiratory Journal 2003;10(2):94‐8. [DOI] [PubMed] [Google Scholar]
Falk 1988 {published data only}
- Falk M, Kelstrup M, Andersen JB, Pedersen SS, Rossing I, Dirksen H. PEP treatment or physical exercise. Effects on secretions expectorated and indices of central and peripheral airway function. A controlled study. 10th International Cystic Fibrosis Conference; 1988 Mar 5‐10; Sydney. 1988.
Fitzgerald 2001 {published data only}
- Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics 2005;116(4):e549. [CFGD Register: BD102b] [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Hilton J, Smith L, Jepson B. Is dornase alfa (Pulmozyme) more effective before or after physiotherapy? A cross‐over, randomised, placebo‐controlled trial. Pediatric Pulmonology 2001; Vol. 32 Suppl 22:309‐10. [CFGD Register: BD102a; ]
Gotz 1995 {published data only}
- Gotz M, Wolkerstorfer A. Physiotherapy in cystic fibrosis: intrapulmonary percussive ventilation (IPV) versus positive expiratory pressure (PEP). Pediatric Pulmonology 1995;Suppl 12:267. [Google Scholar]
Kraemer 1996 {published data only}
- Kraemer D, Liedtke C, Casaulta AC. Bronchodilator inhalation (BD) treatment in sequence with flutter VRP1 chest physiotherapy (CPT) in patients with cystic fibrosis. Israel Journal of Medical Sciences 1996;32 Suppl:S192. [Google Scholar]
Laube 2000 {published data only}
- Laube BL, Geller DE, Lin TC, Dalby RN, Diener‐West M, Zeitlin PL. Positive expiratory pressure changes aerosol distribution in patients with cystic fibrosis. Respiratory Care 2005;50(11):1438‐44. [PubMed] [Google Scholar]
- Laube BL, Lin T, Geller D, Dalby R, Zeitlin P. Positive expiratory pressure alters aerosol distribution in CF. Pediatric Pulmonology 2000;Suppl 20:247. [Google Scholar]
Liedtke 1996 {published data only}
- Liedtke D, Castaulta AC, Martin N, Schibler A, Kraemer R. Mucociliar clearance (MCC) in patients with cystic fibrosis (CF)‐Efficacy of beta 2‐inhalation therapy ( beta 2) in combination with respiratory physiotherapy. Schweizerische Medizinische Wochenschrift 1996;126 Suppl 78:29s. [Google Scholar]
Marks 1998 {published data only}
- Marks JH, Fooy C, Anderson K, Homnick DN. Nebulized albuterol delivered with positive expiratory pressure (PEP) and the flutter device in patients with cystic fibrosis: an assessment of bronchodilator response compared to standard nebulizer therapy. American Journal of Respiratory and Critical Care Medicine 1998; Vol. 157, issue 3 Suppl:A130. []
McCarren 2006 {published data only}
- McCarren B, Alison JA. Comparison of vibration to other physiotherapy interventions for secretion clearance. European Respiratory Journal 2005;26 Suppl 49:497s. [] [Google Scholar]
- McCarren B, Alison JA. Physiological effects of vibration in subjects with cystic fibrosis. European Respiratory Journal 2006; Vol. 27, issue 6:1204‐9. [] [DOI] [PubMed]
Munro 2007 {published data only}
- Munro P, Button B, Bailey M, Ellis S, Whitford H, Snell G. A prospective randomized trial comparing airway clearance strategies following lung transplant. Journal of Cystic Fibrosis 2007;6 Suppl 1:S62. [Google Scholar]
- Munro PE, Button B, Bailey M, Whitford H, Ellis S, Snell G. A prospective randomized trial comparing airway clearance strategies following lung transplantation. Pediatric Pulmonology 2007;42 Suppl 30:352. [Google Scholar]
Oermann 2001 {published data only}
- Oermann CM, Sockrider MM, Giles D, Sontag MK, Accurso FJ, Castile RG. Comparison of high‐frequency chest wall oscillation and oscillating positive expiratory pressure in the home management of cystic fibrosis: a pilot study. Pediatric Pulmonology 2001;32(5):372‐7. [DOI] [PubMed] [Google Scholar]
Orlik 2000 {published data only}
- Orlik T. Evaluation of autodrainage methods in a selected group of cystic fibrosis patients with home environment factors taken into consideration. Medycyna Wieku Rozwojowego 2000;4(3):247‐59. [PubMed] [Google Scholar]
Orlik 2015 {published data only}
- Orlik T, Sands D. Application of positive expiratory pressure PEP in cystic fibrosis patient inhalations. Developmenyal Period Medicine 2015;19(1):50‐9. [PubMed] [Google Scholar]
Padman 1999 {published data only}
- Padman R, Geouque DM, Engelhardt MT. Effects of the flutter device on pulmonary function studies among pediatric cystic fibrosis patients. Delaware Medical Journal 1999;71(1):13‐8. [PubMed] [Google Scholar]
Patel 2013 {published data only}
- Patel P, Fukushima L, Balekian A, Chou W, Lu A, Gali V, et al. Is Metaneb comparable to high frequency chest compression in the setting of a severe pulmonary exacerbation in adults with cystic fibrosis. Pediatric Pulmonology 2013;48 Suppl 36:359. [Abstract No.: 420; CENTRAL: 887112; CRS: 5500125000000289] [Google Scholar]
Placidi 2001 {published data only}
- Placidi G, Cornacchia M, Cappelletti LM, Mastella G, Assael BM, Braggion C. Short‐term effects of positive airway pressure on sputum clearance by directed coughing: A cross‐over, randomized study. Pediatric Pulmonology 2001;32 Suppl 22:313‐4. [Google Scholar]
- Placidi G, Cornacchia M, Polese G, Zanolla L, Assael BM, Braggion C. Chest physiotherapy with positive airway pressure: a pilot study of short‐term effects on sputum clearance in patients with cystic fibrosis and severe airway obstruction. Respiratory Care 2006;51(10):1145‐53. [PubMed] [Google Scholar]
Reychler 2015 {published data only}
- Reychler G, Jacques L, Arnold D, Scheers I, Smets F, Sokal E, et al. Influence of chest physiotherapy on gastro‐oesophagael reflux in children. Revue des Maladies Respiratories 2015;32(5):493‐9. [DOI] [PubMed] [Google Scholar]
Richmond 2016 {unpublished data only}
- Richmond M, Lee Son N, Chilvers M, McIlwaine M. Use of technology to measure adherence to and quality of airway clearance techniques. Paediatric Pulmonology 2016;51 Suppl 45:371. [Abstract no.: 467; CFGD Register: MH47] [Google Scholar]
Roos 1987 {published data only}
- Roos S, Birrer P, Rudeberg A, Kraemer R. First experience with intrapulmonary percussive ventilation (IPV) in the treatment of patients with cystic fibrosis. 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo. 1987.
Sanchez Riera 1999 {published data only}
- Sanchez Riera H, Dapena Fernandez FJ, Gomez Dominguez F, Ortega Ruiz F, Elias Hernandez T, Montemayor Rubio T, et al. Comparative study of the efficacy of 2 respiratory physiotherapy protocols for patients with cystic fibrosis [Estudio comparativo de la eficacia de dos protocolos de fisioterapia respiratoria en pacientes con fibrosis quistica.]. Archivos de Bronconeumologia 1999;35(6):275‐9. [DOI] [PubMed] [Google Scholar]
- Toral J, Sanchez H, Ortega F, Elfas T, Castillo D, Montemayor T. Comparative study of two treatments of respiratory physiotherapy for cystic fibrosis [Estudio comparativo de dos tratamientos de fisioterapia respiratoria en la fibrosis qufstica]. Archivos de Bronconeumologia 1997;33:39. [Google Scholar]
van der Schans 1991 {published data only}
- Schans CP, Mark TW, Vries G, Piers DA, Beekhuis H, Dankert‐Roelse JE, et al. Effect of positive expiratory pressure breathing in patients with cystic fibrosis. Thorax 1991;46(4):252‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Hengstum 1987 {published data only}
- Hengstum M, Festen J, Beurskens C, Hankel M, Broek W, Buijs W, et al. The effect of positive expiratory pressure (PEP) versus forced expiration technique (FET) on tracheobronchial clearance in chronic bronchitics. 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo. 1987.
Wettstein 2014 {published data only}
- Wettstein M, Radlinger L, Riedel T. Effect of different breathing aids on ventilation distribution in adults with cystic fibrosis. PLoS One 2014;9(9):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 2015 {unpublished data only}
- Wilson P, Lambert C, Nwokoro C. Safety of nebulised hypertonic saline via Pari eFlow with Pari positive expiratory pressure (PEP) device in circuit for paediatric cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14 Suppl 1. [Abstract no.: 170] [Google Scholar]
Znotina 2000 {published data only}
- Znotina I, Svabe V. The effectiveness of physiotherapy for children with cystic fibrosis. 13th International Cystic Fibrosis Congress; 2000 Jun 4‐8; Stockholm. 2000:152.
References to studies awaiting assessment
Elkins 2005 {published data only}
- Elkins MR, Eberl S, Constable C, White J, Robinson M, Daviskas E, et al. The effect of manual chest physiotherapy, positive expiratory pressure (PEP), and oscillating PEP on mucociliary clearance in subjects with cystic fibrosis. Pediatric Pulmonology 2005; Vol. 40 Suppl 28:321. []
Grzincich 2008 {published data only}
- Grzincich GL, Longon F, Faverzani S, Chetta A, Spaggiari C, Pisi G. Short‐term effects of high‐frequency chest compression (HFCC) and positive expiratory pressure (PEP) in adults with cystic fibrosis. European Respiratory Society Annual Congress; 2008 Oct 4‐8; Berlin, Germany. 2008:502s. []
Kofler 1994 {published data only}
- Kofler AM, Belluscio M, Bressan T, Carlesi A, Leone P, Lucidi V, et al. PEP‐mask and active cycle of breathing techniques. What is better in children with cystic fibrosis. 19th European Cystic Fibrosis Conference; 1994 May 29‐Jun 3; Paris. 1994:066.
Parreira 2008 {published data only}
- Parreira V, Pires S, Sulmonett N, Camargos P, Haddad J, Britto R. Positive expiratory pressure and lung function in cystic fibrosis patients. European Respiratory Society Annual Congress; 2008 Oct 4‐8; Berlin, Germany. 2008:E1779. [CENTRAL: 679902; CRS: 5500050000000051]
Radtke 2018 {published data only}
- Radtke T, Boni L, Bohnacker P, Fischer P, Benden C, Dressel H. The many ways sputum flows ‐ Dealing with high within‐subject variability in cystic fibrosis sputum rheology. Respiratory Physiology & Neurobiology 2018;254:36‐9. [CFGD Register: PE255c] [DOI] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Maggi‐Beba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. BMC Pulmonary Medicine 2018;18(1):99. [CFGD Register: PE255a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Maggi‐Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomised, controlled, crossover trial. Journal of Cystic Fibrosis 2018;17 Suppl 3:S15. [CFGD Register: PE255b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tonnesen 1982 {published data only}
- Tonnesen P, Kelstrup M. Self‐administered positive end expiratory pressure (PEEP) using a face mask as an alternative to conventional lung physiotherapy [Selvadministeret positivt sluteksspiratorisk tryk (PEEP) pa maske som alternativ til konventionel lungefysioterapi.]. Ugeskr Laeger 1982;144(21):1532‐6. [PubMed] [Google Scholar]
Vendrusculo 2019 {published data only}
- Vendrusculo FM, Johnstone Z, Dhouieb E, Donadio MVF, Cunningham S, Urquhart DS. Airway clearance physiotherapy can reduce dead space and lead to improvements in ventilatory inhomogeniety during exercise in patients with cystic fibrosis: a pilot study. Journal of Cystic Fibrosis 2017;16 Suppl 1:S8. [Abstract no.: WS04.6; CFGD Register: PE250a] [Google Scholar]
- Vendrusculo FM, Johnstone Z, Dhouieb E, Donadio MVF, Cunningham S, Urquhart DS. Airway clearance physiotherapy improves ventilatory dynamics during exercise in patients with cystic fibrosis: a pilot study. Archives of Disease in Childhood 2019;104(1):37‐42. [CFGD Register: PE250b] [DOI] [PubMed] [Google Scholar]
Ward 2018 {published data only}
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis (ACE‐CF): a feasibility study. Respiratory Medicine 2018;142:23‐8. [CFGD Register: PE257b] [DOI] [PubMed] [Google Scholar]
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis: clinical outcomes. Respirology 2018;23(Suppl 1):140. [CFGD Register: PE257a] [DOI] [PubMed] [Google Scholar]
Wong 2000 {published data only}
- Wong LT, McIlwaine PM, Davidson AG. Gastroesophageal reflux during chest physiotherapy: a comparison of positive expiratory pressure and postural drainage with percussion. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm. 2000:130.
- Wong LT, McIlwaine PM, Davidson AG. Gastroesophageal reflux during chest physiotherapy: a comparison of positive expiratory pressure and postural drainage with percussion. Pediatric Pulmonology 1999;Suppl 19:288. [Google Scholar]
Additional references
Andersen 1979
- Andersen JB, Qvist H, Kann T. Recruiting collapsed lung through collateral channels with positive end‐expiratory pressure. Scandinavian Journal of Respiratory Disease 1979;60:260‐266. [PubMed] [Google Scholar]
Cantin 1995
- Cantin A. Cystic fibrosis lung inflammation: Early, sustained, and severe. American Journal of Respiratory and Critical Care Medicine 1995;151(4):939‐41. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Groth 1985
- Groth S, Stafanger G, Dirksen H, Andersen JB, Falk M, Kelstrup M. Positive expiratory pressure (PEP‐Mask) physiotherapy improves ventilation and reduces volume of trapped gas in cystic fibrosis. Bulletin Europeen de Physiopathologie Respiratoire 1985;21(4):339‐43. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstan 1997
- Konstan MW, Berger M. Curent understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatric Pulmonology 1997;24(2):137‐42. [DOI] [PubMed] [Google Scholar]
Liou 2001
- Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5‐year survivorship model of cystic fibrosis. American Journal of Epidemiology 2001;153(4):345‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Main 2005
- Main E, Prasad A, Schans C. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue Issue 1. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIlwaine 1996
- McIlwaine MP, Davidson AG. Airway clearance techniques in the treatment of cystic fibrosis. Current Opinion in Pulmonary Medicine 1996;2(6):447‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Oberwaldner 1986
- Oberwaldner B, Evans JC, Zach MS. Forced expirations against a variable resistance: a new chest physiotherapy method in cystic fibrosis. Pediatric Pulmonology 1986;2:358‐267. [DOI] [PubMed] [Google Scholar]
Prasad 1993
- Prasad SA. Current concepts in physiotherapy. Journal of the Royal Society of Medicine 1993;86(Suppl 20):23‐9. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Prasad 2000
- Prasad SA, Tannenbaum EL, Mikelsons C. Physiotherapy in cystic fibrosis. Journal of the Royal Society of Medicine 2000;93(Suppl 38):27‐36. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Southern 2003
- Southern KW, Smyth RL. Design of clinical trials in cystic fibrosis. Lancet 2003;361(9354):349‐50. [DOI] [PubMed] [Google Scholar]
Warnock 2013
- Warnock L, Gates A, Schans CP. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD001401.pub2] [DOI] [PubMed] [Google Scholar]
Williams 1994
- Williams MT. Chest physiotherapy and cystic fibrosis. Why is the most effective form of treatment still unclear?. Chest 1994;106(6):1872‐82. [DOI] [PubMed] [Google Scholar]
Zach 1987
- Zach MS, Oberwaldner B. Chest physiotherapy: the mechanical approach to antiinfective therapy in cystic fibrosis. Infection 1987;15(5):381‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zach 1990
- Zach MS. Lung disease in cystic fibrosis ‐ an updated concept. Pediatric Pulmonology 1990;8(3):188‐202. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Elkins 2004
- Elkins MR, Jones A, Schans C. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003147.pub2] [DOI] [PubMed] [Google Scholar]
Elkins 2006
- Elkins M, Jones A, Schans CP. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003147.pub3] [DOI] [PubMed] [Google Scholar]
McIlwaine 2015
- McIlwaine M, Button B, Dwan K. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD003147.pub4] [DOI] [PubMed] [Google Scholar]


