Abstract
Objective
To assess changes in mortality rates in extended-release and long-acting (ER/LA) opioid analgesics after the implementation of the Risk Evaluation and Mitigation Strategy (REMS).
Setting
All drug poisoning deaths in three states: Florida, Oregon, and Washington. Data were obtained through state vital records offices and the Researched Abuse, Diversion and Addiction-Related Surveillance System Medical Examiner Program.
Methods
Using cause-of-death literal text from death certificates, individual opioid active pharmaceutical ingredients (APIs) involved in each death were identified using rules-based natural language processing. Population-adjusted and prescriptions dispensed–adjusted mortality rates were calculated for all ER/LA opioid analgesic and individual opioid APIs. Rates before and after implementation of the REMS were compared. Rate changes were compared with rates from two APIs with little or no inclusion in the REMS: benzodiazepines and hydrocodone.
Results
The mean ER/LA opioid analgesic population-adjusted mortality rate significantly decreased in all three states (FL: P = 0.003; OR: P = 0.003; WA: P < 0.001). Mortality rates for benzodiazepines and hydrocodone also decreased and were not statistically different. Significant heterogeneity in mortality rates of individual opioids was observed between the three states. When adjusted for prescription volume, the ER/LA opioid analgesic mortality rate decreased in all three states, but was significant only for Washington (P < 0.001).
Conclusions
The population-adjusted mortality rate of ER/LA opioid analgesics has decreased in three states. Notably, the contributions to mortality rates by individual opioid analgesics were not uniform across the three states in this study. However, these changes were not generally distinct from changes in mortality rates where comparator substances were involved.
Keywords: Prescription Drug Abuse, Mortality, Opioid Analgesics, REMS, ER/LA
Introduction
The high rates of prescription opioid overdose and overprescribing [1,2], the substantial health burden caused by abuse and misuse [3–6], and increasing mortality [7,8] in the last 15 to 20 years have been well documented. Several contributing factors to the rise in opioid-related poisoning deaths have been identified, such as increased overall consumption of opioid analgesics, increased nonmedical use of these drugs, and their potential for abuse. Opioid consumption rose during the 2000s [9,10], and accidental opioid overdose is a known medical outcome for addicted individuals [11]. In one study, substance abuse indicators, such as an unintended route of administration, were found to be present in a majority of unintentional opioid-related deaths in West Virginia [12]. Similarly, another study showed that a majority of opioid overdose deaths in Utah had relatives or friends who were concerned about the decedent’s prescription opioid misuse [13]. A dose-dependent relationship between the dose prescribed and mortality has been observed [14,15].
The Food and Drug Administration (FDA) has attempted to curb the opioid epidemic through a Risk Evaluation and Mitigation Strategy (REMS) for extended release and long-acting (ER/LA) opioid analgesics, which was approved by FDA in July 2012. This is one strategy among several efforts to reduce the risk of abuse, misuse, addiction, overdose, and death from prescription opioid drug products. The specific goal of the REMS has been to reduce serious adverse outcomes such as addiction, unintentional overdose, and death resulting from inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. The primary element of the ER/LA REMS has been to provide prescriber education and increase patient awareness about safe opioid use [16]. The ER/LA REMS consists of multiple elements, including a medication guide, prescriber training, and patient counseling on safe use, serious risks, storage, and disposal of ER/LA REMS opioids. By the end of 2015, approximately 300,000 prescribers and other health care practitioners had participated in REMS-compliant continuing education (CE) activities, and approximately 122,000 completed all components of an educational activity. The REMS educational programs were comprised of live and online activities [17]. The REMS Program Companies had awarded funding for accredited CE providers to administer approximately 750 educational activities. These accredited CE providers varied from academically affiliated institutions to professional or independent organizations. The duration of program lengths varied, but on average took three hours to complete. Following the implementation of the ER/LA REMS, a decrease was observed in rates of misuse, intentional abuse, and major medical outcomes [18].
The affected drugs are both branded and generic drug products, including:
extended-release, oral dosage forms containing hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, or tapentadol;
fentanyl and buprenorphine-containing transdermal delivery systems;
methadone tablets and solutions that are indicated for use as analgesics;
extended-release hydrocodone (introduced to the market in March 2014).
The value of the ER/LA REMS has been measured by the impact the education has on prescribing practices and patient outcomes, such as misuse, abuse, and emergency room visits [17,18]. This study is a continuation of that prior work, and here we evaluated the rates of opioid mortality in three states before and after the initiation of the ER/LA REMS program. The purpose was to assess whether implementation of the REMS program is associated with changes in mortality. Changes are evaluated for the entire group of ER/LA opioid analgesics and for individual substances comprising this group. Comparisons are made to two other drug groups.
Methods
Data Source
The Medical Examiner Program is part of the Researched Abuse, Diversion and Addiction-Related Surveillance (RADARS) System. The purpose of the RADARS System is to provide timely surveillance data to meet the needs of pharmaceutical companies, policy-makers, regulatory agencies, medical/public health officials, and the general public in addressing the concerns of prescription drug abuse, misuse, and diversion. The Medical Examiner Program monitors mortality trends from individual states. Data were obtained from death indices in the states of Florida, Oregon, and Washington directly from state vital statistics offices for the period of third quarter 2010 through fourth quarter 2015. Each state collects data using a standardized database and is required to use the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes for classification and processing of death cases; however, the data variables collected and formats used are not standardized across states. Physicians and medical examiners may list more than one disease, injury, contributing factor, or complication that caused the death. States may have a medical examiner, a coroner, or a mixed system. Florida and Oregon have medical examiner systems, and Washington has a mixed system. These states were selected for the high quality of the death records, the involvement of certified medical examiners in review of death records, and the availability of data. In alignment with Colorado Multiple Institutional Review Board’s Policies and Procedures for the Protection of Human Subjects, the Principal Investigator of the Medical Examiner Program determined that analysis of DIM data involves nonhuman subjects research, per 45 CFR 46.102(f) [2]. No data were obtained from living individuals.
Identification of Drug Involvement and Target Drug Groups
Each condition reported on the death certificate is assigned a code based on the ICD-10. Following ICD-10 rules, one of these conditions is selected as the underlying cause of death, which is defined as “(a) the disease or injury which initiated the train of morbid events leading directly to death, or (b) the circumstances of the accident or violence which produced the fatal injury.” Deaths assigned a drug poisoning code as identified by the National Center for Health Statistics (NCHS; i.e., X40-X44, X60-64, X85, and Y10-14) were included in this analysis [7].
This study’s target drugs included the following prescription opioids with ER/LA formulations: fentanyl, hydromorphone, morphine, oxycodone, oxymorphone, tapentadol, methadone, and buprenorphine. Deaths involving any of these opioids were aggregated into a single overall ER/LA opioid analgesics drug group. Subgroup analyses assessing five opioids individually (fentanyl, hydromorphone, morphine, oxycodone, and oxymorphone) were also conducted. Two comparators were used to evaluate whether changes in mortality from drug products in the ER/LA opioid analgesics group were distinct from secular trends in mortality due to other prescription drugs. Deaths involving all formulations of hydrocodone were used as a separate comparator group due to the lack of the ER/LA formulation of hydrocodone before October 2013. The group of benzodiazepines served as a nonopioid comparator drug class and included alprazolam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, estazolam, flurazepam, lorazepam, midazolam, oxazepam, quazepam, temazepam, and triazolam.
A rules-based natural language processing method was used to identify the active pharmaceutical ingredient (API) named in a death. Standard cause of death (COD) ICD-10 coding includes the identification of only two specific opioid APIs: heroin (T40.1) and methadone (T40.3). The COD literal text on the death certificate, however, typically includes specific API information and has recently been used for identification of APIs named in death certificates [19,20]. A similar approach is used here. The COD text from Box 32 and Box 43 from the US standard death certificate was preprocessed using the Google “Did You Mean” application programming interface [21] to detect misspellings of common and more technical words, such as drug APIs. Misspellings were corrected, and API mentions were identified by searching the corrected text for exact spellings of all APIs of interest. A random subset of 300 deaths (100 from each state) was given to a clinical toxicologist for manual review of the identified APIs; she confirmed that all APIs in this subset of cases were correctly identified with 100% sensitivity and specificity relative to what was printed on the death certificate. An API was considered involved in a death if a spell-corrected API was named in the COD text. Sample size tables for the target drug groups and comparators are provided in the Supplementary Data.
Statistical Analysis
The study was broken into three time periods. The first, the pre-REMS implementation period, ranged from third quarter 2010 through second quarter 2012. The second, the transition period, ranged from third quarter 2012 through second quarter 2013. The third, the active REMS period, ranged from third quarter 2013 through fourth quarter 2015—the most recent quarter with fully available data. Data from 2016 were not included due to known limitations on the timeliness of death certificate data from drug overdose deaths [22]. Deaths by drug groups were aggregated quarterly for each state.
Two separate rate adjustments for this study were considered: US population and number of prescriptions dispensed. These adjustments scale the number of deaths to a common denominator, and they are interpreted as the number of deaths per 100,000 population and number of deaths per 10,000 prescriptions dispensed. Quarterly population estimates were extrapolated from yearly total state population data obtained from the US Census website. Detailed quarterly data on the projected number of prescriptions dispensed by drug, formulation, and three-digit ZIP code were purchased from IQVIA (Durham, NC, USA) from third quarter 2010 to fourth quarter 2015. Data were aggregated to determine the total number of prescriptions dispensed separately for the study group, subgroups, and comparator groups within each state for each year-quarter during the study period.
Poisson regression was used to compare changes in mortality rates at the year-quarter level between the pre-implementation and active periods. The transition period was excluded from the analysis to allow the REMS activities to take effect. Two fixed effects were included: time period (pre-implementation and active) and drug group (ER/LA opioid analgesics, benzodiazepines, and hydrocodone). Also included was a residual-type (R-side) random component with drug group–specific variances. A single model was fit for the primary study group (ER/LA opioid analgesics) and comparator groups, and separate models were fit for each of the subgroups (fentanyl, hydromorphone, morphine, oxycodone, and oxymorphone) and the comparators. Percent changes are calculated as the rate ratio of the pre-implementation and active periods minus 1. Percent changes <0% indicate decreasing means, whereas percent changes >0% indicate increasing means. An interaction term between the drug groups and the periods was also included to estimate the difference in percent change from the pre-implementation period to the active period for the ER/LA opioid analgesics relative to other comparator groups. Rate adjustment parameters (i.e., population and number of prescriptions dispensed) enter the model as offset terms.
Three hypotheses were tested within a single model, and the population adjustment was considered the primary analysis. First, the mean rate of the ER/LA opioid analgesics in the pre-implementation period is equal to the mean rate for the active period. Second, the percent change in rate between the active period and the pre-implementation period is equal to a corresponding percent change between periods for the benzodiazepine comparator group. Finally, the percent change in rate between the active period and the pre-implementation period is equal to a corresponding percent change between periods for the hydrocodone comparator group. All statistical procedures were performed using SAS, version 9.4 or later (SAS Institute Inc., Cary, NC, USA). R, version 3.3.2 or later, was used for graphing. Statistical significance for the study was set at the α = 0.05 level. Because the subgroup analyses were considered exploratory, no corrections for multiple testing were conducted; therefore the subgroup analysis should be considered more critically than the primary analysis.
Results
Primary Drug Group: ER/LA Opioid Analgesics
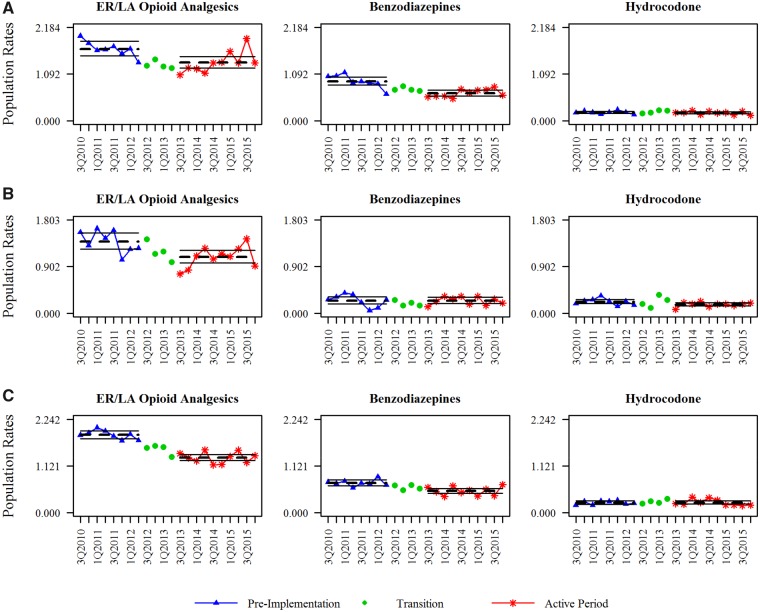
The mean ER/LA opioid analgesics mortality rate per 100,000 population (Table 1 and Figure 1) decreased across all three states from the pre-implementation period to the active period. The percent change for this group was –18.96% (95% confidence interval [CI] = –29.61% to –6.70%), –21.59% (95% CI = –33.19% to –7.97%), and –29.43% (95% CI = –34.51% to –23.95%) for Florida, Oregon, and Washington, respectively; all decreases were statistically significant. Trends across states were less uniform for the benzodiazepine and hydrocodone drug groups. The observed mortality rate for the benzodiazepine group decreased in all states; however, this change in rate ranged from –1.02% (95% CI = –31.81% to 43.68%) in Oregon, which was not statistically significant, to a significant decrease of –30.07% (95% CI = –39.47% to –19.21%) in Florida. The percent changes in mortality rate for the hydrocodone group were statistically significant in Oregon, –23.81% (95% CI = –40.92% to –1.74%), but were not significant in either Florida or Washington. When the percent changes were tested for the ER/LA opioid group vs the comparator groups, there was only one statistically significant result: hydrocodone in Washington. This indicates that the –29.43% decline in the population-adjusted death rate from ER/LA opioid analgesics was statistically different than the 1.36% increase from hydrocodone in Washington. Overall, the interaction assessments in the three states indicate that although the population-adjusted mortality rates decreased significantly for the ER/LA opioid analgesic group, these decreases were generally not statistically different from similar decreases in the comparator groups.
Table 1.
Population-adjusted mortality rates for ER/LA opioid analgesics, benzodiazepines, and hydrocodone per 100,000 population
| Drug Group | Pre-implementation Mean Mortality | Active Period Mean Mortality | Active to Pre-implementation % Change (95% CI) | P Value for % Change | P Value for Interaction* |
|---|---|---|---|---|---|
| Florida | |||||
| ER/LA opioid analgesics | 1.68 | 1.36 | –18.96% (–29.61% to –6.70%) | 0.003 | |
| Benzodiazepines | 0.92 | 0.65 | –30.07% (–39.47% to –19.21%) | <0.001 | 0.152 |
| Hydrocodone | 0.19 | 0.18 | –5.31% (–21.70% to 14.52%) | 0.574 | 0.197 |
| Oregon | |||||
| ER/LA opioid analgesics | 1.39 | 1.09 | –21.59% (–33.19% to –7.97%) | 0.003 | |
| Benzodiazepines | 0.24 | 0.24 | –1.02% (–31.81% to 43.68%) | 0.957 | 0.260 |
| Hydrocodone | 0.22 | 0.17 | –23.81% (–40.92% to –1.74%) | 0.036 | 0.851 |
| Washington | |||||
| ER/LA opioid analgesics | 1.87 | 1.27 | –29.43% (–34.51% to –23.95%) | <0.001 | |
| Benzodiazepines | 0.71 | 0.50 | –27.21% (–37.21% to –15.62%) | <0.001 | 0.714 |
| Hydrocodone | 0.24 | 0.21 | 1.36% (–21.24% to 30.43%) | 0.917 | 0.007 |
CI = confidence interval; ER = extended-release; LA = long-acting.
The P values for interaction are testing whether the percent change in mean rates for the active to pre-implementation periods for the ER/LA opioid analgesics group is different than the change in mean rates for the comparator group.
Figure 1.
Observed and predicted population-adjusted mortality rates. Deaths were identified by an ICD-10 underlying cause of death code of poisoning. Specific active pharmaceutical ingredients were identified by natural language processing techniques. Triangle points indicate the pre-implementation period, circle points indicate the transition period (not modeled), and star points indicate the active period. The dotted horizontal line is the predicted mean quarterly mortality rate for each period, with 95% confidence intervals shown as solid horizontal lines. Rates are shown as the number of deaths per 100,000 population.
Observed percent changes for prescription-dispensed adjusted rates (Table 2) were somewhat different. The only statistically significant decrease in prescription-adjusted mortality rate for the ER/LA opioid analgesics group was in Washington, which decreased –29.54% (95% CI = –34.58% to –24.11%). Changes in mortality rate for this primary target group were not statistically significant in Florida or Oregon; in fact, the estimated rates for the pre-implementation and active periods in Florida were nearly identical. Mortality for the benzodiazepine group decreased in Florida and Washington (–22.96% and –24.52%, respectively); the mortality rate for the hydrocodone group statistically increased in Florida and Washington (22.15% and 26.78%, respectively). Interactions with the comparator groups were statistically significant in Florida and Washington, though for different comparator groups. Specifically, the decrease in prescription-adjusted mortality rate for the ER/LA opioid analgesics group was statistically different than the corresponding decrease in the benzodiazepine group in Florida and statistically different than the increase in the hydrocodone group in Washington.
Table 2.
Prescription-adjusted mortality rates for ER/LA opioid analgesics, benzodiazepines, and hydrocodone per 1,000 prescriptions dispensed
| Drug Group | Pre-implementation Mean Mortality | Active Period Mean Mortality | Active to Pre-implementation % Change (95% CI) | P Value for % Change | P Value for Interaction* |
|---|---|---|---|---|---|
| Florida | |||||
| ER/LA opioid analgesics | 0.16 | 0.16 | –0.70% (–12.03% to 12.09%) | 0.910 | |
| Benzodiazepines | 0.06 | 0.05 | –22.96% (–32.47% to –12.11%) | <0.001 | 0.005 |
| Hydrocodone | 0.02 | 0.02 | 22.15% (1.31% to 47.28%) | 0.036 | 0.069 |
| Oregon | |||||
| ER/LA opioid analgesics | 0.11 | 0.09 | –14.83% (–28.25% to 1.09%) | 0.066 | |
| Benzodiazepines | 0.02 | 0.03 | 11.36% (–22.33% to 59.67%) | 0.558 | 0.188 |
| Hydrocodone | 0.01 | 0.01 | –3.44% (–25.97% to 25.95%) | 0.796 | 0.436 |
| Washington | |||||
| ER/LA opioid analgesics | 1.87 | 1.27 | –29.54% (–34.58% to –24.11%) | <0.001 | |
| Benzodiazepines | 0.71 | 0.50 | –24.52% (–35.08% to –12.25%) | <0.001 | 0.422 |
| Hydrocodone | 0.24 | 0.21 | 26.78% (0.72% to 59.59%) | 0.043 | <0.001 |
CI = confidence interval; ER/LA = extended release/long-acting.
The P values for interaction are testing whether the percent change in mean rates for the active to pre-implementation periods for the ER/LA opioid analgesics group is different than the change in mean rates for the comparator group.
Active Pharmaceutical Ingredient Subgroup Analysis
Five opioids from the ER/LA opioid analgesics group were selected for subgroup analysis to determine if mean changes differed among APIs. Patterns in population-adjusted mortality rates when stratified by API differed notably between states (Table 3). In Florida, a mixture of increases and decreases were observed. The population-adjusted mortality rate for fentanyl deaths statistically increased by 307.44% (95% CI = 102.64% to 719.25%) from the pre-implementation period to the active period; the population-adjusted mortality rate for morphine also increased by 34.11% (95% CI = 14.17% to 57.53%). These changes were counterbalanced by a significant decrease in rate for oxycodone deaths (–57.62%). The change in mortality rate for oxymorphone was significant. In Washington, a general decrease in prescription drug mortality rates was observed for most APIs (though they were either not significant or had borderline P values), with the exception of oxymorphone. The population-adjusted mortality rate from oxymorphone significantly increased, 514.13% (95% CI = 60.48% to 2,250.16%); however, with a pre-implementation rate so small (0.01 deaths per 100,000 population), any slight change would result in a substantial percent change. No change for the mortality rate of any API grouping was statistically significant in Oregon, though estimated signs and magnitudes of changes were generally similar to Washington. Population-adjusted mortality rates involving the fentanyl group did differ between these two states, though neither change was significant. Changes in mortality rates for specific API groupings had generally similar trends in direction and magnitude regardless of the adjustment method (see Table 4 for rates adjusted by prescriptions dispensed).
Table 3.
Population-adjusted mortality rates for individual active pharmaceutical ingredients per 100,000 population
| Drug Group | Pre-implementation Mean Mortality | Active Period Mean Mortality | Active to Pre-implementation % Change (95% CI) | P Value for % Change | P Value for Benzodiazepine Interaction* | P Value for Hydrocodone Interaction* |
|---|---|---|---|---|---|---|
| Florida | ||||||
| Fentanyl | 0.11 | 0.44 | 307.44% (102.64% to 719.25%) | <0.001 | <0.001 | <0.001 |
| Hydromorphone | 0.07 | 0.10 | 42.82% (–7.60% to 120.76%) | 0.109 | 0.002 | 0.090 |
| Morphine | 0.24 | 0.33 | 34.11% (14.17% to 57.53%) | <0.001 | <0.001 | 0.006 |
| Oxycodone | 0.91 | 0.39 | –57.62% (–65.07% to –48.57%) | <0.001 | <0.001 | <0.001 |
| Oxymorphone | 0.05 | 0.03 | –28.20% (–46.76% to –3.16%) | 0.030 | 0.876 | 0.126 |
| Oregon | ||||||
| Fentanyl | 0.10 | 0.13 | 20.82% (–21.02% to 84.85%) | 0.383 | 0.489 | 0.068 |
| Hydromorphone | 0.03 | 0.03 | –7.21% (–69.84% to 185.46%) | 0.896 | 0.915 | 0.737 |
| Morphine | 0.35 | 0.26 | –24.12% (–43.42% to 1.77%) | 0.065 | 0.272 | 0.984 |
| Oxycodone | 0.40 | 0.39 | –1.76% (–22.45% to 24.46%) | 0.883 | 0.974 | 0.151 |
| Oxymorphone | <0.01 | 0.02 | 518.62% (–51.66% to 7,816.37%) | 0.161 | 0.163 | 0.109 |
| Washington | ||||||
| Fentanyl | 0.15 | 0.12 | –14.49% (–37.40% to 16.80%) | 0.325 | 0.360 | 0.406 |
| Hydromorphone | 0.14 | 0.13 | –6.64% (–33.09% to 30.29%) | 0.686 | 0.181 | 0.700 |
| Morphine | 0.41 | 0.34 | –17.41% (–30.42% to –1.96%) | 0.029 | 0.274 | 0.188 |
| Oxycodone | 0.59 | 0.48 | –18.69% (–30.81% to –4.45%) | 0.012 | 0.321 | 0.149 |
| Oxymorphone | 0.01 | 0.03 | 514.13% (60.48% to 2,250.16%) | 0.008 | 0.002 | 0.010 |
CI = confidence interval; ER/LA = extended release/long-acting.
The P values for interaction are testing whether the percent change in mean rates for the active to pre-implementation periods for the ER/LA opioid analgesics group is different than the change in mean rates for the comparator group.
Table 4.
Prescription-adjusted mortality rates for individual active pharmaceutical ingredients per 1,000 prescriptions dispensed
| Drug Group | Pre-implementation Mean Mortality | Active Period Mean Mortality | Active to Pre-implementation % Change (95% CI) | P Value for % Change | P Value for Benzodiazepine Interaction* | P Value for Hydrocodone Interaction* |
|---|---|---|---|---|---|---|
| Florida | ||||||
| Fentanyl | 0.23 | 0.92 | 294.49% (100.37% to 676.67%) | <0.001 | <0.001 | 0.001 |
| Hydromorphone | 0.16 | 0.21 | 29.77% (–12.17% to 91.74%) | 0.191 | 0.013 | 0.784 |
| Morphine | 0.28 | 0.33 | 19.41% (3.51% to 37.76%) | 0.015 | <0.001 | 0.850 |
| Oxycodone | 0.13 | 0.07 | –40.18% (–47.21% to –32.21%) | <0.001 | 0.006 | <0.001 |
| Oxymorphone | 0.42 | 0.33 | –21.52% (–42.84% to 7.74%) | 0.134 | 0.916 | 0.018 |
| Oregon | ||||||
| Fentanyl | 0.12 | 0.16 | 37.01% (–15.54% to 122.24%) | 0.202 | 0.501 | 0.214 |
| Hydromorphone | 0.06 | 0.06 | –3.84% (–68.05% to 189.44%) | 0.945 | 0.804 | 0.994 |
| Morphine | 0.20 | 0.17 | –15.15% (–37.18% to 14.61%) | 0.284 | 0.256 | 0.528 |
| Oxycodone | 0.05 | 0.05 | 5.01% (–18.27% to 34.93%) | 0.702 | 0.793 | 0.653 |
| Oxymorphone | 0.06 | 0.37 | 527.50% (–53.39% to 8,348.33%) | 0.166 | 0.197 | 0.160 |
| Washington | ||||||
| Fentanyl | 0.30 | 0.27 | –10.06% (–34.01% to 22.60%) | 0.502 | 0.318 | 0.081 |
| Hydromorphone | 0.30 | 0.24 | –19.94% (–43.44% to 13.33%) | 0.210 | 0.760 | 0.031 |
| Morphine | 0.40 | 0.30 | –25.23% (–37.41% to –10.67%) | 0.001 | 0.938 | <0.001 |
| Oxycodone | 0.09 | 0.07 | –16.73% (–30.11% to –0.80%) | 0.040 | 0.404 | 0.004 |
| Oxymorphone | 0.10 | 0.55 | 441.72% (42.16% to 1,964.28%) | 0.013 | 0.004 | 0.036 |
CI = confidence interval; ER = extended-release; LA = long-acting.
The P values for interaction are testing whether the percent change in mean rates for the active to pre-implementation periods for the ER/LA opioid analgesics group is different than the change in mean rates for the comparator group.
Discussion
In the three states of this study (Florida, Oregon, and Washington), the population-adjusted mortality rate from drug poisoning that involved an ER/LA opioid analgesic decreased after the implementation of the REMS initiative; the estimated decrease in mortality rates ranged from 18.96% to 29.43%. However, these decreases in mortality rate for opioids under the ER/LA REMS initiative were not statistically different than those of another class of drugs with widespread involvement in overdose deaths, benzodiazepines [23], nor those of an opioid with limited impact from the REMS initiative, hydrocodone. When adjusted for the number of prescriptions dispensed, a significant decrease was observed only in Washington. This finding suggests that changes in number of deaths corresponded to changes in the supply of this drug, and one interpretation is that the mortality rate is tied to the general availability of the drug. In fact, some studies indicate that an increase in heroin use occurs concurrently with a decrease in prescription drug abuse [24], and it is suggested that prescription drug abuse can lead to heroin abuse in some cases [25]. Further study into individual patterns of misuse and abuse of individual pharmaceutical ingredients would elucidate the impact of drug availability on rates of overdose death.
Changes in mortality rates over time were not consistent across subsets of APIs. In each state, APIs that were primary contributors to the overall rate differed. In Florida, there was an overall decrease in mortality due to ER/LA opioid analgesics, but individual APIs examined had distinct, diverging changes in mortality. A dramatic increase in mortality rates in which fentanyl was involved was observed, as well as increasing mortality rates in which morphine was involved. This was counteracted by decreases in observed rates where oxycodone and oxymorphone were involved. Conversely, in Washington, the estimated percent changes have uniformly decreased; the only exception was the mortality rate in which oxymorphone was involved. This mortality rate rose sharply and significantly, though the absolute change in rate was small. The directions of the changes for specific API groupings in the two northwest states, Oregon and Washington, were similar; however, statistical significance was not observed for Oregon. A contributing factor was the overall low number of deaths in Oregon, which could indicate a type II error.
Understanding mortality trends related to drug overdose has often been restricted to studying all opioids of interest combined. The ICD-10 codes alone cannot identify the substances involved in the death. Including contributing codes (T40.0–T40.9) in a definition of substance-specific deaths and using more refined methods of determining case inclusion [26] both fail to fully identify all substances involved in the death. Identification of individual substances using coding rule–based natural language processing of the cause of death fields on the death certificate has recently been reported by the National Vital Statistics System [20] and the Washington vital records office [19].
There are several strengths of this study. Two distinct types of comparators were used. One comparator, the benzodiazepine class, was a different class of molecules, but still subject to abuse. Another, hydrocodone, was an opioid with limited inclusion in the ER/LA REMS. Only two hydrocodone products were included during the study period, and the first hydrocodone product was added during the active period (October 2013). The use of either substance as a comparator has limitations, however. A majority of cases where a benzodiazepine was present also had an opioid from the ER/LA group. This would affect the significance of the interaction between groups, though it would not limit the conclusion that mortality due to ER/LA opioids has decreased across study periods. Death data were obtained directly from state vital records offices and represent the most up-to-date mortality data available for individual deaths. National records cannot be publicly accessed for individual deaths, making searching for deaths associated with individual APIs impossible. States selected include both large metropolitan areas and rural populations, allowing for contributions from areas with variations in population density.
Limitations, however, are also present. Causal relationships between the decreases in mortality rates by opioids with an ER/LA formulation, excluding hydrocodone, and initiation of the ER/LA REMS are confounded by other concurrent interventions. These include but are not limited to the increasing influence of prescription drug monitoring programs, increasing preference for abuse-deterrent formulations by insurance companies (such as through pharmacy benefit management systems), state-level legislative actions, increasing availability of counteragents such as naloxone, and law enforcement actions. One example of confounding is the rescheduling of combination hydrocodone products, which caused a reduction in prescriptions dispensed [27]. This would confound any difference in percent changes between the ER/LA opioid group and hydrocodone in Table 1, and thus limit attribution of differences in trends to either the REMS or the rescheduling. Other interventions would have similar confounding effects. Thus, interpreting the decreases in mortality rates for ER/LA formulations excluding hydrocodone as only associative, and not causal, is more valid. Postmortem drug identification is limited to the API and cannot distinguish between formulations or brands within a drug class. For example, immediate-release oxycodone often cannot be distinguished from ER oxycodone. For this reason, analysis groups were defined broadly as those APIs with at least one ER/LA REMS drug product. Illicitly sourced fentanyl cannot be distinguished from prescription fentanyl, nor can some chemical analogues of fentanyl be distinguished from fentanyl using this method of processing text. The comparisons between pre-implementation and active period changes assume that both the pool of medical examiners and their diagnostic acumen were consistent across the study period. This may not be true in light of the position paper released in 2014 where recommendations were given for the practice of death investigation and autopsy [28]. This report contains data from three states and may not represent broader national trends in mortality. This study was an observational epidemiological study and relied on all available data in the three states. For some analyses, the numbers of deaths were small. Changes observed may be too small to reach statistical significance. Finally, COD literal text analysis has inherent limitations that would cause underestimates in mortality rates, including delays in processing [22], missing data [29], and under-reporting [30].
Materials from the ER/LA REMS aim at controlling inappropriate prescribing from health care providers, and therefore the number of prescriptions filled in a year-quarter changes throughout the study period. Adjusting mortality based on the number of prescriptions filled has the advantage of assessing mortality independent of the changing availability of the drug products and accounts for the bias associated with more readily available drug products. This also accounts for the entrance of new drug products and the unstable market share of different products. However, this adjustment would obscure changes in mortality due to changes in prescribing patterns, and therefore changes interpretation of the mortality rate. Aggregate rates calculated for multiple drug products using population adjustments tend to be dominated by commonly prescribed drug products. Some drug products may be frequently involved in a death, but if there is a low market share, the drug product might not influence population-adjusted rates. Rates calculated from availability adjustments increase the relevancy of drug products with low market share. Mortality rates adjusted by population and prescriptions dispensed are necessary to assess both the mortality burden within the population and the mortality burden controlling for availability of drug products.
Conclusions
The mortality rate of ER/LA opioid analgesics decreased in the time period after the implementation of the ER/LA REMS initiative compared with the time period before this intervention. Mortality rates for individual ER/LA REMS API groupings also generally decreased, though with some notable exceptions localized to individual states. However, these changes were generally not distinct from changes in mortality rates in which comparator substances were involved. This study illustrates that the ER/LA REMS initiative, in conjunction with other concurrent interventions at national and state levels, is temporally associated with a decrease in mortality where opioids are involved.
Supplementary Material
Acknowledgments
The authors would like to gratefully acknowledge Becki Bucher Bartelson for her mentoring and insight into statistical modeling strategies. The authors would like to thank Janetta Iwanicki for her review of death certificates. Vital records were obtained from the Florida Department of Health, the Oregon Health Authority, and the Washington State Department of Health.
Funding sources: The RADARS System is the property of Denver Health and Hospital Authority, a political subdivision of the State of Colorado. The RADARS System is supported by subscriptions from pharmaceutical manufacturers, as well as government and nongovernment agencies for surveillance, research, and reporting services. Denver Health retains exclusive ownership of all data, databases, and systems. Subscribers do not participate in data collection or analysis, nor do they have access to the raw data.
Disclaimer: Any published findings and conclusions are those of the authors and do not necessarily represent the official position of the Florida Department of Health, the Oregon Health Authority, or the Washington State Department of Health.
Disclosure and conflicts of interest: This study was sponsored by the REMS Program Companies, a consortium of companies that sponsor the ER/LA Opioid Analgesic REMS. MSC is an employee of Janssen Research & Development, an affiliate of Janssen Pharmaceuticals, Inc., which markets several analgesic drug products. GPW is an employee of Upsher-Smith Laboratories, which markets an opioid analgesic. At the time of the study, JCB, GEB, TR, JLG, and RCD were employees of Denver Health and Hospital Authority, which received financial support from the REMS Program Companies in connection with the study and development of this article. GPW was employed by Upsher-Smith Laboratories, LLC. MSC was employed by Janssen Research & Development.
References
- 1. Paulozzi LJ, Jones CM, Mack KA, Rudd RA.. Vital signs: Overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep 2011;60:1487–92. [PubMed] [Google Scholar]
- 2. Paulozzi LJ, Mack KA, Hockenberry JM.. Vital signs: Variation among states in prescribing of opioid pain relievers and benzodiazepines—United States, 2012. MMWR Morb Mortal Wkly Rep 2014;63:563–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Drug Abuse Warning Network. National Estimates of Drug-Related Emergency Department Visits. HHS Publication No. (SMA) 13-4760, DAWN Series D-39. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [PubMed]
- 4.Centers for Disease Control and Prevention. CDC Year in Review: Working 24/7/365. Centers for Disease Control and Prevention; 2016. Available at: https://www.cdc.gov/media/dpk/cdc-24-7/eoy-2016/dpk-eoy.html (accessed April 18, 2018).
- 5.Substance Abuse and Mental Health Services Administration. Treatment Episode Data Set (TEDS) 2004–2014. National Admissions to Substance Abuse Treatment Services. BHSIS Series S-84, HHS Publication No. (SMA) 16-4986. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016.
- 6. Patrick SW, Schumacher RE, Benneyworth BD, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. J Am Med Assoc 2012;307(18):1934–40. [DOI] [PubMed] [Google Scholar]
- 7. Rudd RA, Aleshire N, Zibbell JE, Gladden RM.. Increases in drug and opioid overdose deaths—United States 2000–2014 MMWR Morb Mort Weekly Rep 2016;4(50):1378–82. [DOI] [PubMed] [Google Scholar]
- 8. Warner M, Trinidad JP, Bastian BA, Miniño AM, Hedegaard H.. Drugs most frequently involved in drug overdose deaths—United States 2010–2014 Natl Vital Stat Rep 2016;65(10):1–15. [PubMed] [Google Scholar]
- 9. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18(12):1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER.. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med 2014;9(2):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hser Y, Hoffman V, Grella CE, Anglin MD.. A 33-year follow-up of narcotics addicts. Arch Gen Psychiatry 2001;58(5):503–8. [DOI] [PubMed] [Google Scholar]
- 12. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. J Am Med Assoc 2008;300(22):2613–20. [DOI] [PubMed] [Google Scholar]
- 13. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: Description of decedents by next of kin or best contact, Utah, 2008–2009. J Gen Intern Med 2013;28(4):522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med 2016;17(1):85–98. [DOI] [PubMed] [Google Scholar]
- 15. Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. J Am Med Assoc 2011;305(13):1315–21. [DOI] [PubMed] [Google Scholar]
- 16. Casty FE, Wieman MS, Shusterman N.. Current topics in opioid therapy for pain management: Addressing the problem of abuse. Clin Drug Invest 2013;33(7):459–68. [DOI] [PubMed] [Google Scholar]
- 17. Cepeda MS, Coplan PM, Kopper NW, et al. ER/LA opioid analgesics REMS: Overview of ongoing assessments of its progress and its impact on health outcomes. Pain Med 2017;18(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bucher Bartelson B, Le Lait MC, Green JL, et al. Changes in misuse and abuse of prescription opioids following implementation of extended-release and long-acting opioid analgesic Risk Evaluation and Mitigation Strategy. Pharmacoepidemiol Drug Saf 2017;26(9):1061–70. [DOI] [PubMed] [Google Scholar]
- 19. Ossiander EM. Using textual cause-of-death data to study drug poisoning deaths. Am J Epidemiol 2014;179(7):884–94. [DOI] [PubMed] [Google Scholar]
- 20. Trinidad JP, Warner M, Bastian BA, Miniño AM, Hedegaard H.. Using literal text from the death certificate to enhance mortality statistics: Characterizing drug involvement in deaths. Natl Vital Stat Rep 2016;65(9):1–15. [PubMed] [Google Scholar]
- 21. Brin S, Page L.. Reprint of: The anatomy of a large-scale hypertextual web search engine. Comput Netw 2012;56(18):3825–33. [Google Scholar]
- 22. Spencer MR, Ahmad F.. Timeliness of Death Certificate Data for Mortality Surveillance and Provisional Estimates. National Center for Health Statistics; 2017. Available from: https://www.cdc.gov/nchs/data/vsrr/report001.pdf. [Google Scholar]
- 23. Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL.. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health 2016;106(4):686–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Affairs 2016;35(6):1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute on Drug Abuse. Prescription Opioids and Heroin. Bethesda, MD: National Institute on Drug Abuse; 2015. Available at: https://www.drugabuse.gov/publications/research-reports/prescription-opioids-heroin (accessed April 18, 2018).
- 26. Dasgupta N, Proescholdbell S, Sandford C, et al. Defining controlled substances overdose: Should deaths from substance use disorders and pharmaceutical adverse events be included? J Clin Toxicol 2013;03(03):1–8. [Google Scholar]
- 27. Jones CM, Lurie PG, Throckmorton DC.. Effect of US drug enforcement administrations rescheduling of hydrocodone combination analgesic products on opioid analgesic prescribing. JAMA Intern Med 2016;176(3):399–402. [DOI] [PubMed] [Google Scholar]
- 28. Davis GG. Complete republication: National Association of Medical Examiners position paper: Recommendations for the investigation, diagnosis, and certification of deaths related to opioid drugs. J Med Toxicol 2014;10(1):100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buchanich JM, Balmert LC, Williams KE, Burke DS.. The effect of incomplete death certificates on estimates of unintentional opioid-related overdose deaths in the United States, 1999–2015. Public Health Rep 2018;133(4):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horon IL, Singal P, Fowler DR, Sharfstein JM.. Standard death certificates versus enhanced surveillance to identify heroin overdose-related deaths. Am J Public Health 2018;108(6):777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.