Abstract
Objective
To characterize neuropathic-like pain among individuals with or at risk for knee osteoarthritis.
Subjects
One hundred eighty-four individuals who self-identified as non-Hispanic black or non-Hispanic white and presented with unilateral or bilateral knee pain.
Design
Neuropathic-like pain was assessed using the painDETECT, and those with high vs low neuropathic-like pain were compared on clinical pain, psychological symptoms, physical function, and quantitative sensory testing. Analyses were unadjusted, partially and fully adjusted for relevant covariates.
Results
Thirty-two (17.4%) participants reported experiencing neuropathic-like pain features above the painDETECT clinical cut-score. The neuropathic-like pain group reported significantly greater pain severity on all measures of clinical pain and higher levels of psychological symptoms when fully adjusted for covariates, but no differences emerged for disability and lower extremity function. The neuropathic-like pain group also reported greater overall heat pain ratings during the heat pain threshold and increased temporal summation of heat pain in the fully adjusted model. Additionally, those with neuropathic-like pain symptoms reported greater painful after-sensations following heat pain temporal summation in all analyses. No significant group differences in pressure pain threshold emerged at any of the testing sites. In contrast, temporal summation of mechanical pain was significantly greater at both the index knee and the ipsilateral hand for the neuropathic-like pain group in all analyses.
Conclusions
Participants with or at risk for knee osteoarthritis who reported high neuropathic-like pain experienced significantly greater clinical pain and increased heat and mechanical temporal summation at the index knee and other body sites tested, suggesting central sensitization.
Keywords: Neuropathic-Like Pain, Knee Osteoarthritis, painDETECT, Pain Modulation
Introduction
Osteoarthritis (OA) affects 27 million adults in the United States, making OA a leading cause of chronic pain, functional limitations, and disability [1,2]. OA of the knee is most prevalent [3] and has doubled since the mid-20th century, due in part to increased age and obesity [4,5]. However, knee OA is typically considered a localized joint disease driven primarily by peripheral nociceptive input, which has not explained the widespread discordance between radiographic evidence of joint changes and knee pain severity, disability, or the suboptimal treatment response of OA [6].
Efforts to improve treatment outcomes involve elucidating the peripheral and central mechanisms contributing to the pain experience, as well as phenotyping pain in individuals with OA [7]. Thus, mechanism-based pain assessment using quantitative sensory testing (QST) and measures of pain characteristics, such as the painDETECT [8], are being employed to identify mechanisms contributing to knee OA pain and disability [9,10]. Indeed, approximately 19–28% of individuals with knee OA experience neuropathic-like pain symptoms, based on the painDETECT, a validated tool to assess neuropathic-like pain features in individuals with chronic pain conditions [9,11–13]. Neuropathic-like pain symptoms in individuals with knee OA are associated with increased pain levels, decreased function, and QST findings, suggesting central sensitization [9,10]. However, the extent of central sensitization is unclear, as prior findings have been focused on testing of the knee. Moreover, whether neuropathic-like symptoms are associated with multiple aspects of psychosocial functioning has not been determined.
To better understand neuropathic-like pain qualities, a multimodal assessment approach is needed, including dynamic measures of QST (e.g., temporal summation measures, conditioned pain modulation) and measurement of key psychosocial, functional, and disability factors. Therefore, the purpose of the present study is to comprehensively investigate neuropathic-like pain symptoms in a community-based sample of non-Hispanic black and non-Hispanic white individuals with or at risk for knee OA. This study is the first to incorporate a comprehensive battery of measures to compare clinical pain, physical function, psychological symptoms, and QST profiles among those who scored high vs low on the painDETECT. Further, the study adds to the literature by adjusting for influential covariates that are related to neuropathic-like pain symptoms [4]. In particular, because pain severity is higher among patients with neuropathic-like pain [9,10,14–16], any differences between groups with neuopathic-like vs non-neuropathic-like pain could be attributed to the greater pain severity in the former. Therefore, we include analyses that adjust for pain severity in order to determine whether differences associated with neuropathic-like pain persist even after the greater pain severity of these individuals is controlled. This would provide at least indirect evidence that features of neuropathic-like pain above and beyond pain intensity are associated with the outcome measures. We hypothesized that neuropathic-like pain would be associated with a QST profile reflecting central sensitization and with higher levels of clinical pain, disability, and psychological symptoms.
Methods
Design
The current study is a substudy of a larger ongoing observational cohort study that aims to elucidate the mechanisms underlying racial/ethnic group differences in knee pain being conducted at the University of Florida (UF) and the University of Alabama at Birmingham (UAB). All procedures were reviewed and approved by the Institutional Review Boards at UF and UAB.
Study Participants
Participants were 184 community-dwelling adults between 45 and 85 years of age who self-identified as non-Hispanic black or non-Hispanic white, presented with unilateral or bilateral knee pain, and screened positive for clinical knee OA [17]. This screening questionnaire showed 87% sensitivity and 92% specificity for radiographically confirmed symptomatic knee OA [18]. All participants were negative for other rheumatologic conditions (e.g., rheumatoid arthritis, fibromyalgia) that could explain knee pain. Given widespread variability in definitions of OA [19], we adopted this approach to be as inclusive as possible in recruitment, as our primary focus is on understanding factors associated with knee pain rather than OA pathophysiology itself. Moreover, because this prospective observational study is designed to evaluate progression of OA-related symptoms, we sought to enroll a cohort with a broad range of OA characteristics, from very early signs to advanced disease.
Participants were recruited through the community via multiple advertisement methods (e.g., posted fliers) and clinic-based methods. Participants were excluded for the following self-reported conditions: 1) prosthetic knee replacement or other clinically significant surgery to the arthritic knee; 2) uncontrolled hypertension; 3) heart disease; 4) peripheral neuropathy in which pain testing was contraindicated; 5) systemic rheumatic disorders including rheumatoid arthritis, systemic lupus erythematosus, gout, and fibromyalgia; 6) neurological diseases such as Parkinson’s, multiple sclerosis, stroke with loss of sensory or motor function, or uncontrolled seizures; 7) significantly greater pain in body sites other than the knee; 8) daily opioid use; 9) hospitalization within the preceding year for psychiatric illness; or 10) pregnant or nursing.
Procedures
Participants completed a health assessment session (HAS) followed by a QST session approximately nine days apart (Figure 1).
Figure 1.
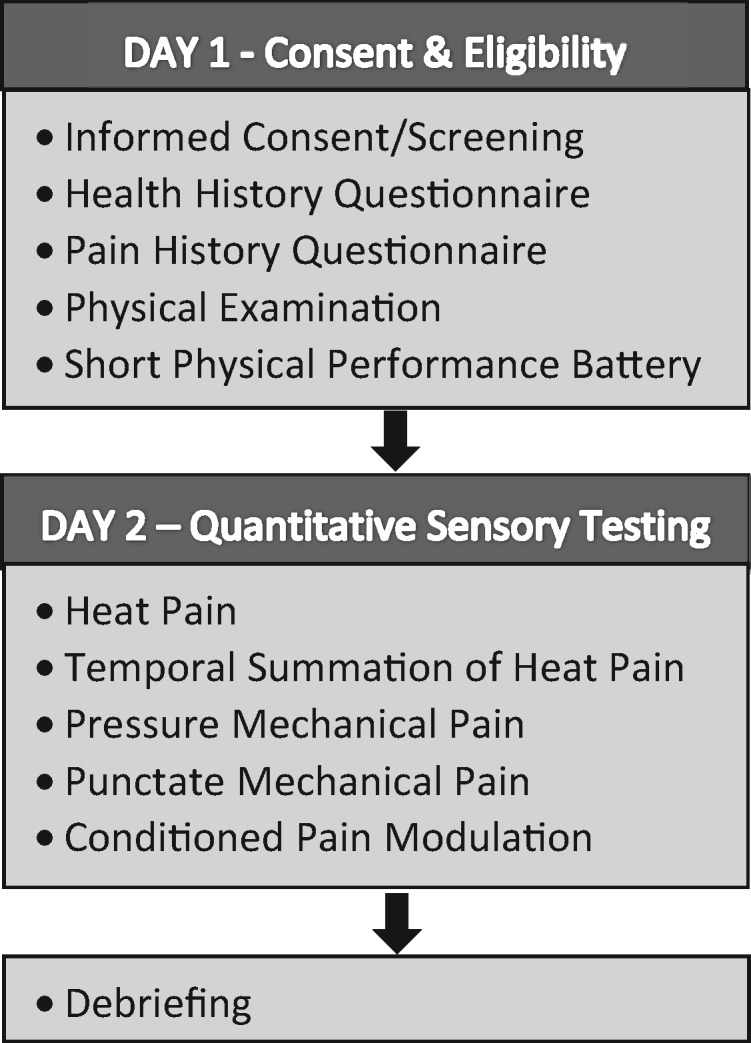
Study procedures.
Health Assessment Session
Individuals participated in an HAS where they provided informed consent, completed health and pain history questionnaires, and their height and weight were obtained and body mass index (BMI) calculated. Participants were included if they reported knee pain and fulfilled at least one additional criterion for knee OA using the American College of Rheumatology clinical criteria for symptomatic knee OA [17]. The participants’ most painful knee was designated as the index knee for QST procedures. Participants completed the validated Short Physical Performance Battery (SPPB) [20], a measure of lower extremity function.
Quantitative Sensory Testing
Laboratory sessions lasted two to two and a half hours, and trained experimenters performed all procedures. Stimulus parameters for QST were based on procedures described previously [21].
Heat Pain Testing
Heat stimuli were delivered to the ipsilateral ventral forearm and the medial joint line of the index knee using a 16×16-mm thermode attached to a Medoc Pathway Thermal Sensory Analyzer (Ramat Yishai, Israel). Heat testing assessed the first sensations of warmth (i.e., warmth threshold), first sensation of pain (i.e., heat pain threshold), and the point at which pain could no longer be tolerated (i.e., heat pain tolerance). Each trial began at the baseline temperature (32°C), and the temperature gradually increased (0.5°C/sec) until the participant pressed a button to indicate their response. Participants also provided a pain rating (0–100 numeric rating scale [NRS]; 0 = no pain and 100 = most intense pain imaginable) for each trial of heat pain threshold and tolerance. The mean or trimmed mean temperature from the three trials within 3°C was used for analysis.
Temporal Summation of Heat Pain
Participants provided pain ratings after each of five heat pulses at each of three separate temperatures (i.e., 44°C, 46°C, 48°C) delivered using a contact heat-evoked potential stimulator thermode. Each trial started at baseline temperature (35°C), and the temperature increased rapidly (20°C/sec) to the target temperature (i.e., 44°C, 46°C, 48°C). Participants were asked to rate any pain after-sensations 15 seconds following the fifth stimulus for each temperature. The procedure was terminated if the participant provided a rating of 100 or requested to stop. Temporal summation of heat pain was calculated by subtracting the first trial rating from the maximum rating provided during the series of five trials.
Pressure Pain
Pressure pain threshold was assessed at the medial and lateral joint lines of the index knee, ipsilateral quadriceps, and trapezius muscle, with site order randomly counterbalanced. Three pressure pain threshold trials were conducted at each site and averaged for data analysis. Using a digital, handheld, clinical-grade pressure algometer (Algomed, Medoc, Ramat Yishai, Israel), the examiner applied a constant rate (30 kPa/sec) of pressure, and the participant pressed a button when the sensation first became painful. A trial terminated when the participant reported pain or reached the maximum pressure level (600 kPa for knee sites and 1,000 kPa for all other sites).
Punctate Mechanical Pain
A nylon monofilament (Touchtest Sensory Evaluator 6.65) calibrated to bend at 300 grams of pressure was applied at the patella of the index knee and the dorsal aspect of the ipsilateral hand. Order of testing sites was randomized. Participants provided a pain rating (0–100) following a single contact of the monofilament, after which they provided another pain rating following a series of 10 contacts at a rate of one contact per second. The mean difference between the pain ratings for the single vs series of 10 contacts reflected temporal summation.
Conditioned Pain Modulation
For the test stimulus, pressure pain threshold was assessed on the left trapezius as described above. For the conditioning stimulus, participants immersed the right hand for 60 seconds in a cold water bath maintained at 12°C by a refrigeration unit (Neslab, Portsmouth, NH, USA). Pressure pain threshold was assessed before immersion and after 30 seconds of hand immersion. To operationalize conditioned pain modulation (CPM), the pre-immersion pressure pain threshold was subtracted from the pressure pain threshold obtained during cold water immersion, such that higher scores reflect greater pain inhibition.
Questionnaires
Questionnaires assessing clinical pain, disability, and psychological functioning were administered at various times throughout the multisession protocol to reduce participant burden.
Measures of Clinical Pain and Function
painDETECT
The painDETECT consists of nine items that evaluate pain quality, pattern, and radiation and was developed and validated for the purpose of identifying neuropathic-like elements of pain [8]. Scores range from –1 to 38, with higher scores being suggestive of neuropathic-like pain. The painDETECT was originally developed for use in persons with low back pain, but it has been used in a number of clinical populations, including knee OA [22]. The developers determined values for sensitivity, specificity, and predictive accuracy of 80–84% in a group of patients with mixed pain conditions using the following cutoff points relative to pain health care providers’ clinical assessments: Scores ≥19 indicate likely neuropathic-like pain. In the current study, participants were asked to think about their knee pain on the side that bothers them the most (i.e., index knee) when completing the painDETECT.
Pain Sites
Participants identified body areas, in addition to the knee, where they experienced pain on more days than not over the past three months. The areas were summed to determine the total number of pain sites.
Western Ontario and McMaster Universities Osteoarthritis Index
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is a reliable (Cronbach’s alpha ≥ 0.80) and well-validated measure of lower extremity pain and function in persons with OA [23,24]. The WOMAC assesses symptoms of knee OA in the past 48 hours.
Graded Chronic Pain Scale
The Graded Chronic Pain Scale (GCPS) is a seven-item scale that evaluates global pain severity and pain-related interference over the past six months. The GCPS yields a “characteristic pain intensity” score and an overall “disability” score [25]. With a 0–10 NRS, participants rated the intensity of their current knee pain and their worst and average pain during the past six months. These three items were averaged and multiplied by 10 to generate a characteristic pain intensity score. Using the same scale, participants rated the degree to which their knee pain interfered with daily activities during the past six months, which was averaged and multiplied by 10 to generate a disability score.
Revised Short-Form McGill Pain Questionnaire
Participants completed the Revised Short-Form McGill Pain Questionnaire (SF-MPQ-2) by rating the extent to which their pain was consistent with 22 pain descriptors in the past week [26].
Short Physical Performance Battery
The SPPB is a standardized measure of lower extremity function [20]. Its measures include standing balance, four-meter gait speed, and chair-rising tasks. Subscale scores and the summary performance scores are calculated with lower scores indicating poorer function. A 0–100 knee pain rating was obtained during each task [27].
Psychological Questionnaires
Coping Strategies Questionnaire–Revised
The Coping Strategies Questionnaire–Revised (CSQ-R) Pain Catastrophizing subscale assesses the helplessness dimension of catastrophizing. The reliability and validity of the CSQ-R subscales have previously been shown to be acceptable [28,29].
Patient Health Questionnaire 15-Item Somatic Symptom Severity Scale
The Patient Health Questionnaire 15-Item Somatic Symptom Severity Scale (PHQ-15) is a somatic symptom subscale derived from the full PHQ; each symptom is scored from 0 (“not bothered at all”) to 2 (“bothered a lot”) [30].
Patient-Reported Outcomes Measurement Information System Anxiety and Depression
Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety and Depression [31] measures self-reported symptoms of anxiety and depression over the past seven days using a five-category numerical scale, ranging from 1 (never) to 5 (always).
Data Analysis
As in previous studies [9,22], participants with painDETECT scores ≥19/38 were included in the neuropathic-like pain group, whereas participants with painDETECT scores ≤18 comprised the non-neuropathic-like pain group. Using SPSS software, version 25 (IBM, Chicago, IL), the Student t test and chi-square test for independence analyses were conducted to determine group differences in participant characteristics (e.g., demographic factors), one-way analysis of variance (ANOVA), and analysis of covariance (ANCOVA) for analysis of outcome variables. Group differences on all continuous variables were examined first with unadjusted analyses, followed by partially adjusting for demographic and anthropometric covariates (site, age, education, race, and BMI), then by fully adjusting for these same covariates plus clinical pain severity (site, age, education, race, BMI, and Graded Chronic Pain Scale pain intensity) in order to demonstrate whether group differences were robust to adjustment for these covariates, including the impact of covarying for pain severity. Few studies adjust for influential covariates [32], with many studies not adjusting for any covariates [9, 16] or minimally adjusted [14]. Data were evaluated to determine if they met the assumption of normality using the Shapiro-Wilk test. Non-normal data were identified and were log-transformed before being analyzed. The results were similar for all outcome variables, except for three variables that went from frankly to marginally statistically significant and one variable that went from nonsignificant to significant. However, the analyses conducted with the log-transformed variables did not change the interpretation of the data; therefore, we choose to retain the original data format. Statistical significance for all tests was set at a P value <0.05. Data on heat pain tolerance and heat pain tolerance ratings in both the knee and arm were excluded for one participant in the neuropathic-like pain group and two participants in the non-neuropathic-like pain group due to invalid data resulting from not following instructions.
Results
Participant Characteristics
Thirty-two (17.4%) participants were classified as having neuropathic-like pain features. Table 1 presents group comparisons on demographic and other relevant characteristics. Participants in the neuropathic-like pain group were significantly younger, had a higher BMI, and reported greater pain over the past six months than participants in the non-neuropathic-like pain group. Although the neuropathic-like group had a higher BMI, this is consistent with previous research showing higher prevalence of neuropathic-like knee pain in women who are obese [33]. Although not statistically significant, Table 1 indicates that the proportion of individuals with neuropathic-like pain was over twice as high among non-Hispanic blacks, with 23% of non-Hispanic blacks (22 out of 96), but only 11% of whites (10 out of 88), falling in the neuropathic-like pain group.
Table 1.
Comparison of study participants with and without neuropathic-like pain symptoms by demographic characteristics and OA-related factors
| Characteristics | NP Group (N = 32) |
Non-NP Group (N = 152) |
|||||
|---|---|---|---|---|---|---|---|
| No. | M (or %) | SD | No. | M (or %) | SD | P | |
| Demographic factors | |||||||
| Age, y | 32 | 54.6 | 7.0 | 152 | 58.6 | 7.7 | 0.01 |
| Sex | 0.89 | ||||||
| Male | 12 | 37.5 | 55 | 36.2 | |||
| Female | 20 | 62.5 | 97 | 63.8 | |||
| Race | 0.06 | ||||||
| Non-Hispanic black | 22 | 68.8 | 74 | 48.7 | |||
| Non-Hispanic white | 10 | 31.3 | 78 | 51.3 | |||
| Education (% ≤ high school) | 22 | 68.8 | 66 | 43.4 | 0.06 | ||
| Body mass index | 32 | 35.3 | 10.8 | 152 | 31.2 | 6.6 | 0.05 |
| Normal | 4 | 13.3 | 27 | 18.1 | |||
| Overweight | 7 | 23.3 | 40 | 26.8 | |||
| Obese | 19 | 63.3 | 82 | 55.0 | |||
| Pain length | 32 | 151 | 0.53 | ||||
| <6 mo | 1 | 3.1 | 9 | 6.0 | |||
| 6 mo–1 y | 5 | 15.6 | 10 | 6.6 | |||
| 1–3 y | 8 | 25.0 | 38 | 25.2 | |||
| 3–5 y | 4 | 12.5 | 22 | 14.6 | |||
| ≥5 y | 14 | 43.8 | 72 | 47.7 | |||
| Pain variables | |||||||
| No. of painful joints | 32 | 8.2 | 7.6 | 152 | 5.5 | 5.5 | 0.07 |
| Right knee pain | 5 | 16.7 | 40 | 29.6 | |||
| Left knee pain | 5 | 16.7 | 28 | 20.7 | |||
| Bilateral knee pain | 20 | 66.7 | 67 | 49.6 | |||
| GCPS pain intensity | 32 | 78.4 | 14.0 | 152 | 51.2 | 22.0 | <0.01 |
| Concomitant conditions | |||||||
| Chronic hip pain | 21 | 65.6 | 82 | 54.3 | 0.33 | ||
| Diabetes mellitus | 5 | 15.6 | 18 | 11.8 | 0.77 | ||
| No. of painful sites | 32 | 4.7 | 2.7 | 152 | 3.9 | 2.2 | 0.08 |
No covariates were included in these analyses. Bold formatting indicates significant P values, at P < 0.05.
GCPS = Grade Chronic Pain Scale; NP = neuropathic-like pain; OA = osteoarthritis.
painDETECT Questionnaire Results
Median painDETECT scores were higher for participants in the neuropathic-like pain group (21.5) compared with the non-neuropathic-like pain group (8.0). Frequencies of scores for individual items on the painDETECT are shown in Table 2. The neuropathic-like pain group most frequently endorsed burning and pain attacks/electric shocks.
Table 2.
Frequency of response for individual items on the painDETECT questionnaire
| Questionnaire Items (Score) | NP Group (N = 32) |
Non-NP Group (N = 152) |
||||
|---|---|---|---|---|---|---|
| painDETECT (–1 to 38) | No. | (%) | No. | (%) | ||
| Knee pain pattern* (–1, 0, 1) | ||||||
| Persistent pain with slight variations (0) | 3/32 | 9.4 | 34/151 | 22.5 | ||
| Persistent pain with pain attacks (–1) | 10/32 | 31.3 | 33/151 | 21.9 | ||
| Pain attacks without pain between them (1) | 3/32 | 9.4 | 48/151 | 31.8 | ||
| Pain attacks with pain between them (1) | 15/32 | 46.9 | 32/151 | 21.2 | ||
| Knee pain radiation† (% yes) (0, 2) | 28/31 | 90.3 | 57/152 | 37.5 | ||
| (% moderately or more, score ≥3/5) | No. | (%) | Median | No. | (%) | Median |
| Knee pain quality (0–5)‡ | ||||||
| Pain attacks/electric shocks | 29/31 | 90.7 | 4 | 36/151 | 23.9 | 2 |
| Burning | 29/31 | 93.6 | 3 | 26/151 | 17.2 | 1 |
| Sensitivity to pressure | 24/32 | 75 | 3.5 | 32/151 | 21.4 | 1 |
| Tingling or prickling | 24/32 | 77.4 | 3 | 16/151 | 10.6 | 1 |
| Numbness | 23/32 | 72 | 3 | 13/152 | 8.6 | 0 |
| Sensitivity to cold or heat | 14/32 | 43.8 | 2 | 9/151 | 6 | 0 |
| Sensitivity to light touch | 11/32 | 34.3 | 2 | 4/150 | 2.7 | 0 |
NP = neuropathic-like pain.
Responders selected one of four possible response options to indicate the knee pain pattern; No. shows the number of participants who selected the specific item to describe their knee pain pattern, and % indicates the percentage of participants per group.
Percentage of responders who reported yes to the question about pain radiating from the knee.
Min–max score range was 0–5 (0 = never, 1 = hardly noticed, 2 = slightly, 3 = moderately, 4 = strongly, 5 = very strongly) for all pain quality questions; the median score for each knee pain quality was recordedm and (% moderately or more, score ≥3/5) is the cumulative percentage for responses reported to be moderate, strong, and very strong when describing the knee pain quality.
Zero-Order Correlation
Zero-order correlations of painDETECT with standard measures of clinical pain, disability, and functional pain are presented in Table 3. Statistically significant relationships were found among painDETECT and all measures of clinical pain, disability, and functional pain (r = 0.48–0.76).
Table 3.
Zero-order correlations among painDETECT and clinical pain, disability, and functional pain characteristics
| Characteristics | No. | Total Sample (N = 184) |
|---|---|---|
| painDETECT | ||
| painDETECT | – | |
| WOMAC | ||
| Pain | 183 | 0.68** |
| Stiffness | 183 | 0.60** |
| Physical function | 184 | 0.66** |
| GCPS | ||
| Pain intensity | 184 | 0.63** |
| Disability score (0–100) | 184 | 0.55** |
| SF-MPQ-2 | ||
| Continuous | 178 | 0.71** |
| Intermittent | 176 | 0.73** |
| Neuropathic | 176 | 0.74** |
| Affective | 179 | 0.68** |
| Total score | 173 | 0.76** |
| SPPB pain ratings | ||
| Balance pain rating | 181 | 0.49** |
| Chair stand pain rating | 176 | 0.48** |
| Walking pain rating | 183 | 0.52** |
GCPS = Graded Chronic Pain Scale; SPPB = Short Physical Performance Battery; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index; SF-MPQ-2 = short-Form McGill Pain Questionnaire-2.
P < 0.01.
Clinical Pain and Disability
Uncontrolled analyses and both partially and fully controlled analyses for covariates yielded a statistically significant group difference in clinical pain, such that the neuropathic-like pain group reported significantly greater pain severity, stiffness, and functional impairments related to knee OA on the WOMAC and higher scores on all the SF-MPQ-2 subscales, compared with the non-neuropathic-like pain group (Table 4). Group differences in disability emerged when analyses were uncontrolled or partially controlled, but not fully controlled, such that the neuropathic-like pain group reported greater interference on the GCPS, compared with the non-neuropathic-like pain group.
Table 4.
Comparison between participants with and without neuropathic-like pain symptoms on clinical variables
| Unadjusted |
Partially Adjusted |
Fully Adjusted |
|||||
|---|---|---|---|---|---|---|---|
| NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | ||
| Characteristics | n/N | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| WOMAC | |||||||
| Pain | 32/151 | 12.6 (3.3) | 6.9 (3.8)** | 11.7 (3.3) | 7.1 (3.8)** | 10.0 (3.3) | 7.4 (3.8)** |
| Stiffness | 32/151 | 5.5 (1.7) | 2.9 (1.7)** | 5.1 (1.7) | 3.0 (1.7)** | 4.5 (1.7) | 3.1 (1.7)** |
| Physical function | 32/152 | 40.1 (11.4) | 22.0 (13.3)** | 36.7 (11.4) | 22.7 (13.3)** | 30.2 (11.4) | 24.1 (13.3)** |
| GCPS | |||||||
| Disability score (0–100) | 32/152 | 75.3 (23.2) | 42.5 (28.3)** | 68.7 (23.2) | 43.8 (28.3)** | 55.0 (23.2) | 46.7 (28.3) |
| Chronic pain grade | 31/151 | ||||||
| Grade I: low disability–low intensity | 0 | 57 | |||||
| Grade II: low disability–high intensity | 4 | 35 | |||||
| Grade III: high disability–moderately limiting | 14 | 41 | |||||
| Grade IV: high disability–severely limiting | 13 | 18 | |||||
| Short-Form McGill Pain Questionnaire–2 | |||||||
| Continuous | 31/147 | 6.5 (1.6) | 2.9 (2.3)** | 6.0 (1.6) | 3.1 (2.3)** | 4.8 (1.6) | 3.3 (2.3)** |
| Intermittent | 31/145 | 6.3 (1.7) | 2.2 (2.1)** | 5.8 (1.7) | 2.3 (2.1)** | 5.0 (1.7) | 2.5 (2.1)** |
| Neuropathic | 28/148 | 4.7 (2.0) | 1.2 (1.6)** | 4.3 (2.0) | 1.3 (1.6)** | 3.8 (2.0) | 1.4 (1.6)** |
| Affective | 31/148 | 5.6 (2.3) | 1.7 (2.2)** | 5.1 (2.3) | 1.8 (2.2)** | 4.3 (2.3) | 1.9 (2.2)** |
| Total score | 28/145 | 5.7 (1.5) | 2.0 (1.8)** | 5.2 (1.5) | 2.1 (1.8)** | 4.4 (1.5) | 2.3 (1.8)** |
Fully adjusted = included all partially adjusted covariates (site, age, education, race group, body mass index), in addition to the Graded Chronic Pain Scale pain intensity; GCPS = Graded Chronic Pain Scale; NP = neuropathic-like pain; partially adjusted = covariates were site, age, education, race group, and body mass index; unadjusted = no covariates were included in analyses; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
P < 0.01.
Psychological Variables
Participants in the neuropathic-like pain group engaged in significantly higher rates of pain catastrophizing. This group also reported significantly more somatic symptoms. Further, these participants reported higher levels of anxiety and depression over the past seven days, compared with the non-neuropathic-like pain group (Table 5).
Table 5.
Comparison between participants with and without neuropathic-like pain symptoms on psychological variables
| Unadjusted |
Partially Adjusted |
Fully Adjusted |
|||||
|---|---|---|---|---|---|---|---|
| NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | ||
| Characteristics | n/N | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| Psychological variables | |||||||
| Pain catastrophizing (0–6) | 32/151 | 2.5 (1.5) | 1.1 (1.1)** | 2.1 (1.5) | 1.2 (1.1)** | 1.8 (1.5) | 1.3 (1.1)* |
| Somatic Symptom Severity Scale | 32/152 | 11.0 (4.5) | 7.2 (3.5)** | 10.5 (4.5) | 7.3 (3.5)** | 10.0 (4.5) | 7.4 (3.5)** |
| PROMIS Anxiety | 30/149 | 56.5 (9.8) | 49.8 (8.9)** | 55.5 (9.8) | 50.0 (8.9)** | 54.8 (9.8) | 50.2 (8.9)* |
| PROMIS Depression | 30/150 | 55.3 (10.2) | 46.8 (8.6)** | 54.2 (10.2) | 47.0 (8.6)** | 53.1 (10.2) | 47.2 (8.6)** |
Fully adjusted = included all partially adjusted covariates (site, age, education, race group, body mass index), in addition to the Graded Chronic Pain Scale pain intensity; NP = neuropathic-like pain; partially adjusted = covariates were site, age, education, race group, and body mass index; PROMIS = Patient-Reported Outcomes Measurement Information System; unadjusted = no covariates were included in analyses.
P < 0.05; **P < 0.01.
Physical Function Variables
Unadjusted analyses revealed significant group differences for performance on the chair stand, walking, and the overall score, as well as movement-evoked knee pain after all three tasks in the neuropathic-like pain group. In the partially adjusted analyses, the neuropathic-like pain group had a significantly lower overall score on SPPB and reported significantly more movement-evoked knee pain following all three tasks. In contrast, after controlling for all covariates in the fully adjusted model, no group differences emerged for performance on the SPPB tasks, the overall score, or the movement-evoked knee pain following the tasks (Table 6).
Table 6.
Comparison between participants with and without neuropathic-like pain symptoms on SPPB functional test and pain
| Characteristics | Unadjusted |
Partially Adjusted |
Fully Adjusted |
||||
|---|---|---|---|---|---|---|---|
| NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | ||
| n/N | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
| Functional and pain test | |||||||
| SPPB balance | 32/152 | 3.6 (0.8) | 3.8 (0.6) | 3.6 (0.8) | 3.7 (0.6) | 3.7 (0.8) | 3.7 (0.6) |
| SPPB balance pain | 30/151 | 37.8 (32.5) | 16.0 (21.4)** | 34.6 (32.5) | 16.6 (21.4)** | 25.6 (32.5) | 18.4 (21.4) |
| SPPB chair stand | 31/150 | 1.6 (1.2) | 2.1 (1.2)* | 1.7 (1.2) | 2.1 (1.2) | 1.8 (1.2) | 2.0 (1.2) |
| SPPB chair stand pain | 31/145 | 44.9 (35.1) | 22.3 (25.1)** | 41.1 (35.1) | 23.1 (25.1)** | 28.0 (35.1) | 25.9 (25.1) |
| SPPB walking | 32/152 | 3.4 (0.7) | 3.7 (0.6)* | 3.5 (0.7) | 3.7 (0.6) | 3.7 (0.7) | 3.5 (0.6) |
| SPPB walking pain | 31/152 | 43.6 (34.8) | 17.3 (24.4)** | 39.4 (34.8) | 18.2 (24.4)** | 29.0 (34.8) | 20.3 (24.4) |
| SPPB total | 32/152 | 8.6 (1.7) | 9.6 (1.7)** | 8.8 (1.7) | 9.5 (1.7)* | 9.0 (1.7) | 9.5 (1.7) |
Fully adjusted = included all partially adjusted covariates (site, age, education, race group, body mass index), in addition to the Graded Chronic Pain Scale pain intensity; NP = neuropathic-like pain; partially adjusted = covariates were site, age, education, race group, and body mass index; SPPB = Short Physical Performance Battery; unadjusted = no covariates were included in analyses.
P < 0.05; **P < 0.01.
Heat Pain
No group differences emerged for warmth threshold or heat pain threshold in unadjusted or adjusted analyses in either the index knee or forearm (Table 7). However, in unadjusted analyses, the neuropathic-like pain group exhibited lower heat pain tolerance compared with the non-neuropathic-like pain group. Further, uncontrolled and partially adjusted analyses revealed that the neuropathic-like pain group reported greater pain during the heat pain threshold in the index knee, whereas all analyses were statistically significant for greater pain during the heat pain threshold in the forearm. In partially adjusted analyses, the neuropathic-like pain group reported greater pain during heat pain tolerance both in the index knee and in the forearm. Regarding heat pain temporal summation, the average pain ratings for each heat temperature during temporal summation were higher in the neuropathic-like pain group but were statistically significantly different only at 44°C in uncontrolled analyses. The neuropathic-like pain group showed greater temporal summation at 48°C applied to the index knee and the forearm in all analyses. In unadjusted and partially adjusted analyses, the neuropathic-like pain group evidenced greater temporal summation for temperature at 44°C and 46°C applied to the forearm. Additionally, the neuropathic-like pain group reported greater painful after-sensations at 15 seconds for all three temperatures at both body sites tested.
Table 7.
Comparison between participants with and without neuropathic-like pain symptoms on heat pain testing, pain, and temporal summation of heat at the index knee and forearm
| Unadjusted |
Partially Adjusted |
Fully Adjusted |
|||||
|---|---|---|---|---|---|---|---|
| NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | ||
| Characteristics | n/N | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| Heat pain sensitivity at index knee | |||||||
| Warm threshold | 32/151 | 37.6 (3.6) | 37.3 (3.6) | 37.7 (3.6) | 37.3 (3.6) | 37.3 (3.6) | 37.4 (3.6) |
| Heat pain threshold | 32/151 | 42.1 (3.1) | 42.1 (3.5) | 42.6 (3.1) | 42.0 (3.5) | 42.6 (3.1) | 42.0 (3.5) |
| Heat pain threshold rating | 32/151 | 43.8 (26.0) | 28.4 (22.1)** | 43.5 (26.0) | 28.4 (22.1)** | 36.7 (26.0) | 29.8 (22.1) |
| Heat pain tolerance | 31/148 | 45.3 (1.8) | 45.7 (2.6) | 45.9 (1.8) | 45.6 (2.6) | 46.0 (1.8) | 45.6 (2.6) |
| Heat pain tolerance rating | 31/148 | 70.7 (24.8) | 61.6 (24.7) | 74.4 (24.8) | 60.8 (24.7)* | 68.0 (24.8) | 62.1 (24.7) |
| Heat pain sensitivity at forearm | |||||||
| Warm threshold | 32/151 | 35.2 (1.5) | 35.2 (2.2) | 35.3 (1.5) | 35.2 (2.2) | 35.1 (1.5) | 35.2 (2.2) |
| Heat threshold | 32/151 | 40.7 (3.8) | 41.3 (3.8) | 41.2 (3.8) | 41.2 (3.8) | 41.1 (3.8) | 41.2 (3.8) |
| Heat pain threshold rating | 32/151 | 38.4 (24.0) | 22.7 (19.1)** | 38.2 (24.0) | 22.8 (19.1)** | 34.0 (24.0) | 23.7 (19.1)* |
| Heat pain tolerance | 31/149 | 44.6 (2.6) | 45.7 (2.6)* | 45.1 (2.6) | 45.6 (2.6) | 45.1 (2.6) | 45.6 (2.6) |
| Heat pain tolerance rating | 31/149 | 65.6 (22.1) | 58.3 (24.9) | 68.5 (22.1) | 57.7 (24.9)* | 62.0 (22.1) | 59.0 (24.9) |
| Temporal summation of heat at index knee | |||||||
| 44°C | 23/137 | 9.7 (13.8) | 5.5 (12.2) | 9.9 (13.8) | 5.5 (12.2) | 9.9 (13.8) | 5.5 (12.2) |
| 44°C 15-sec rating | 32/149 | 15.4 (24.6) | 4.0 (10.2)** | 15.0 (24.6) | 4.1 (10.2)** | 13.5 (24.6) | 4.4 (10.2)** |
| 46°C | 24/134 | 11.5 (17.6) | 9.6 (15.2) | 11.7 (17.6) | 9.5 (15.2) | 11.1 (17.6) | 9.6 (15.2) |
| 46°C 15-sec rating | 32/150 | 20.1 (28.6) | 6.4 (13.5)** | 20.2 (28.6) | 6.4 (13.5)** | 17.3 (28.6) | 7.0 (13.5)* |
| 48°C | 23/131 | 25.3 (23.6) | 14.5 (18.4)* | 26.4 (23.6) | 14.3 (18.4)* | 25.3 (23.6) | 14.5 (18.4)* |
| 48°C 15-sec rating | 32/146 | 24.6 (29.3) | 10.2 (16.1)** | 24.3 (29.3) | 10.2 (16.1)** | 21.4 (29.3) | 10.9 (16.1)* |
| Average pain ratings during temporal summation of heat at index knee | |||||||
| 44°C | 32/150 | 41.0 (32.8) | 29.3 (27.6)* | 39.1 (32.8) | 29.7 (27.6) | 35.6 (32.8) | 30.5 (27.6) |
| 46°C | 32/150 | 46.9 (32.1) | 42.4 (30.7) | 44.5 (32.1) | 42.9 (30.7) | 39.9 (32.1) | 43.9 (30.7) |
| 48°C | 32/149 | 60.7 (28.1) | 51.6 (31.5) | 58.8 (28.1) | 52.0 (31.5) | 54.5 (28.1) | 53.0 (31.5) |
| Temporal summation of heat at forearm | |||||||
| 44°C | 25/139 | 14.9 (18.8) | 7.2 (13.7)* | 14.5 (18.8) | 7.3 (13.7)* | 13.9 (18.8) | 7.4 (13.7) |
| 44°C 15-sec rating | 32/150 | 14.7 (23.8) | 4.8 (11.4)* | 14.3 (23.8) | 4.8 (11.4)** | 12.1 (23.8) | 5.3 (11.4)* |
| 46°C | 26/136 | 20.2 (20.2) | 10.2 (15.2)** | 19.7 (20.2) | 10.3 (15.2)* | 17.4 (20.2) | 10.8 (15.2) |
| 46°C 15-sec rating | 32/150 | 19.6 (28.4) | 7.5 (13.8)** | 19.4 (28.4) | 7.5 (13.8)** | 16.9 (28.4) | 8.1 (13.8)* |
| 48°C | 24/134 | 24.3 (20.3) | 14.0 (16.1)* | 25.9 (20.3) | 13.7 (16.1)** | 23.7 (20.3) | 14.1 (16.1)* |
| 48°C 15-sec rating | 31/148 | 23.7 (30.7) | 9.9 (15.5)** | 23.3 (30.7) | 10.0 (15.5)** | 19.4 (30.7) | 10.8 (15.5)* |
| Average pain ratings during temporal summation of heat at forearm | |||||||
| 44°C | 32/150 | 47.1 (28.0) | 35.8 (27.4)* | 45.2 (28.0) | 36.2 (27.4) | 40.4 (28.0) | 37.2 (27.4) |
| 46°C | 32/150 | 49.7 (30.7) | 42.8 (29.2) | 46.6 (30.7) | 43.5 (29.2) | 41.5 (30.7) | 44.6 (29.2) |
| 48°C | 32/150 | 62.7 (28.8) | 53.0 (30.4) | 60.2 (28.8) | 53.5 (30.4) | 54.9 (28.8) | 54.6 (30.4) |
Fully adjusted = included all partially adjusted covariates (site, age, education, race group, body mass index), in addition to the Graded Chronic Pain Scale pain intensity; NP = neuropathic-like pain; partially adjusted = covariates were site, age, education, race group, and body mass index; unadjusted = no covariates were included in analyses.
P < 0.05; **P < 0.01.
Mechanical Pain
Both unadjusted and adjusted analyses revealed no group differences in pressure pain threshold at any testing site (Table 8). In contrast, temporal summation of mechanical pain was significantly greater at both the index knee and the dorsal aspect of the ipsilateral hand for the neuropathic-like pain group compared with the non-neuropathic-like pain group in all analyses.
Table 8.
Comparison between participants with and without neuropathic-like pain symptoms on mechanical testing: Pressure and punctate pain at the most painful knee and ipsilateral nonknee sites
| Unadjusted |
Partially Adjusted |
Fully Adjusted |
|||||
|---|---|---|---|---|---|---|---|
| NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | NP (N = 32) | Non-NP (N = 152) | ||
| Characteristics | n/N | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) |
| Pressure, kPa | |||||||
| Medial joint line index knee | 32/150 | 257.1 (114.3) | 277.0 (142.4) | 281.2 (114.3) | 271.9 (142.4) | 279.7 (114.3) | 272.2 (142.4) |
| Lateral joint line index knee | 32/149 | 310.2 (154.4) | 299.0 (150.1) | 333.2 (154.4) | 294.0 (150.1) | 340.6 (154.4) | 292.5 (150.1) |
| Quadriceps | 32/150 | 344.4 (155.3) | 361.9 (186.8) | 358.2 (155.3) | 361.8 (186.8) | 358.3 (155.3) | 361.0 (186.8) |
| Trapezius | 32/150 | 218.5 (93.4) | 252.4 (149.8) | 244.9 (93.4) | 246.8 (149.8) | 247.7 (93.4) | 246.2 (149.8) |
| Punctate | |||||||
| Punctate temporal summation at patella | 32/151 | 26.0 (20.5) | 16.1 (16.7)** | 26.9 (20.5) | 15.9 (16.7)** | 23.8 (20.5) | 16.6 (16.7)* |
| Punctate temporal summation dorsal hand | 32/151 | 20.6 (16.5) | 11.0 (12.9)** | 19.8 (16.5) | 11.2 (12.9)** | 18.0 (16.5) | 11.6 (12.9)* |
| Conditioned pain modulation | |||||||
| Prepressure pain threshold | 31/148 | 237.1 (127.2) | 255.5 (143.9) | 256.2 (127.2) | 251.5 (143.9) | 251.6 (127.2) | 252.4 (143.9) |
| During-pressure pain threshold | 27/129 | 312.8 (175.6) | 351.0 (188.5) | 338.7 (175.6) | 345.5 (188.5) | 346.0 (175.6) | 344.0 (188.5) |
| Conditioned pain modulation | 27/128 | 80.1 (95.1) | 91.1 (109.2) | 90.4 (95.1) | 88.9 (109.2) | 98.7 (95.1) | 87.2 (109.2) |
Fully adjusted = included all partially adjusted covariates (site, age, education, race group, body mass index), in addition to the Graded Chronic Pain Scale pain intensity; NP = neuropathic-like pain; partially adjusted = covariates were site, age, education, race group, and body mass index; unadjusted = no covariates were included in analyses.
P < 0.05; **P < 0.01.
Conditioned Pain Modulation
No group differences emerged in CPM in any analyses with a P value >0.05 (Table 8).
Discussion
This investigation compared clinical pain, psychological symptoms, physical function, and a comprehensive array of QST measures among adults with or at risk for knee OA who experienced high vs low neuropathic-like pain symptoms assessed with the painDETECT. Further, group differences were examined using unadjusted analyses and were both partially and fully adjusted for relevant covariates. Our findings provide evidence that a small yet distinct subgroup of individuals with symptoms of knee OA experience high neuropathic-like pain features. Our results are consistent with other recent studies, which show that a subset of adults with knee OA screen positive for neuropathic-like pain [9,12,13,32]. More importantly, our a priori hypothesis that neuropathic-like pain would be associated with evidence of central sensitization, higher levels of clinical pain, and psychological symptoms was confirmed; however, we were surprised at the absence of group differences in disability, physical function, and CPM in the fully adjusted model, suggesting that neuropathic-like features are not associated with these outcomes net of pain intensity. Because the groups did not differ on length of time with knee pain, any group differences in outcomes cannot be attributed to this potential confound.
Static measures of pain thresholds and tolerances were not significantly different in neuropathic-like vs non-neuropathic-like groups, at either the index knee or other body regions. This contrasts with previous work comparing pressure, cold, and heat pain thresholds in OA participants who scored “positive neuropathic-like” (painDETECT scored ≥19), “unclear group” (painDETECT scored 13–18), and “negative neuropathic-like” (painDETECT scored ≤12) [9]. These investigators reported that the “positive neuropathic-like” group had higher pain sensitivity compared with the “negative neuropathic-like” group but did not differ from the “unclear group.” Consistent with other studies [10,12], we included all participants with painDETECT scores ≤19 in the non-neuropathic-like pain group, which may explain our lack of observed differences in pain thresholds. Further, the previous study did not adjust for relevant covariates [9], and this difference in analytic approach may have contributed to differences in results. Our study extends the literature by demonstrating that adults with knee OA and neuropathic-like pain show evidence of central sensitization (e.g., enhanced heat and mechanical temporal summation) but not impaired pain inhibition (e.g., CPM). Moreover, temporal summation of heat and mechanical pain were higher at all body sites (i.e., index knee and non–index knee sites), which points toward generalized rather than localized central sensitization, further implicating central pathophysiological mechanisms in the neuropathic-like pain group. This could be a function of central brain changes, such as descending modulation, which has been shown to be impaired in individuals with OA [10,34]. Furthermore, in a previous cohort of individuals with knee OA, we found that mechanical temporal summation was associated with current and future clinical pain severity [35]. This aligns with our present findings, in which the neuropathic-like pain groups exhibited not only increased mechanical temporal summation, but also higher levels of clinical pain across all measures, including movement-evoked pain. Additionally, those with neuropathic-like pain symptoms reported greater painful after-sensations following heat pain temporal summation. Interestingly, CPM was not significantly different between the groups, suggesting that pain inhibitory mechanisms may be less impacted. A recent study found that longer durations of neuropathic pain (more than two years) were associated with more efficient CPM compared with patients with short pain duration [36]. More studies are needed to fully assess any impact on pain inhibition in the neuropathic-like pain subgroup.
Several additional patterns in our findings deserve consideration. First, non-Hispanic blacks were slightly over-represented in the neuropathic-like pain group. However, it is difficult to determine whether this reflects racial/ethnic differences in symptom trajectory of knee OA or a more general predisposition toward central sensitization given prior evidence documenting greater pain sensitivity and pain facilitation among non-Hispanic blacks [37,38]. More research is needed to determine whether there are racial/ethnic differences in neuropathic-like pain and what mechanisms, including disease-specific and more general pain-related biological and psychosocial risk factors, might drive these differences. Second, the neuropathic-like pain group experienced significantly higher levels of pain catastrophizing, somatic symptom severity, anxiety, and depression. It is unclear whether these psychosocial factors represent consequences or causes of the more severe clinical symptoms and concurrent changes in central pain processing observed in the neuropathic-like pain group. Regardless, this highlights the potential importance of psychosocial factors as treatment targets, as past research provides compelling evidence that psychosocial factors can modulate the experience of knee pain [21], suggesting that psychological treatments will likely enhance outcomes. Finally, neuropathic-like symptoms may represent a unique phenotype observed in only a subset of OA patients, or neuropathic-like features may reflect a common sign of OA progression. However, duration of symptoms was similar in our two groups, which argues against the progression hypothesis. Moreover, a recent study of end-stage knee and hip OA reported lower rates of neuropathic-like pain than we observed [39]. Thus, it seems likely that neuropathic-like pain emerges only in a subset of people with knee OA.
Implications and Future Directions
Given the complexity of interacting biological, psychological, and sociocultural factors that contribute to chronic pain [40], mechanistic pain assessment can provide valuable information to guide treatment. Identifying the characteristic phenotype of neuropathic-like pain in individuals with knee OA is a first step in improving assessment and tailoring treatments for pain management and reversal of disability in this subgroup. Our findings highlight the potential importance of multimodal treatment strategies that target central pain mechanisms, as well as psychosocial factors. Indeed, well-chosen behavioral and/or pharmacological therapies could synergistically address both central sensitization and psychological functioning in patients with knee OA pain. For example, Racine et al. [41] note that decreasing catastrophizing reduces pain intensity and interference in patients with neuropathic-like pain. Understanding the relationship between neuropathic-like pain and knee OA has great relevance not only for diagnosis and treatment but for improving patient outcomes. It is possible that individuals with neuropathic-like pain who show signs of central sensitization and high pain intensity may demonstrate poorer outcomes for traditional pharmacological and surgical approaches [42,43].
What remains unclear is the critical point(s) in the OA trajectory when peripheral sensitization transitions into central sensitization. For example, some of those scoring below the painDETECT cut-point also show some emerging signs of neuropathic-like pain. Whether early intervention could prevent or reverse this progression is an important mechanistic and clinical question. Future longitudinal studies may help answer this question.
It is important to note that knee pain severity was significantly greater in the neuropathic-like pain group. Interestingly, painDETECT was indeed correlated with measures of OA pain severity, which justifies controlling for pain intensity. Therefore, we adjusted for pain severity (i.e., clinical GCPS pain intensity) in the fully adjusted model, demonstrating that the observed associations are likely driven by neuropathic-like mechanisms rather than pain severity. However, neuropathic-like pain mechanisms may produce more severe pain, in which case adjusting for pain severity may be inappropriate. The fact that a subgroup of patients with knee OA who experience neuropathic-like pain report more intense pain creates a logical conundrum. Does more severe OA pain artificially inflate painDetect scores, or is there a link between possible neuropathic-like pain mechanisms and the severity of OA pain [11]? Indeed, previous data addressing this issue provide evidence that pain severity is higher in patients with neuropathic-like pain symptoms [9,10,14–16]. If neuropathic-like knee OA pain is indeed more severe due to the underlying mechanisms, then controlling for pain severity would underrepresent the constellation of abnormal pain perception reported in this and other studies [32]. Interestingly, Table 2 provides evidence that a small proportion of non-neuropathic-like pain participants report experiencing pain with neuropathic-like features, and they report lower median scores on individual items of pain quality on the painDETECT.
Strengths and Limitations
Several study strengths and limitations should be noted. First, although our data are cross-sectional, our analysis was strengthened by a large, racially diverse sample size. In contrast to other studies of QST and knee OA, our sample included a comparable proportion of non-Hispanic blacks, who have historically been absent from experimental pain studies [44]. However, controlling for multiple demographic factors limits the inferences we can make about socioeconomic contributions to neuropathic-like pain and chronic pain in the population. Additional strengths include incorporating measures of functional limitations and multiple measures of clinical and experimental pain. We also present the data in unadjusted, partially adjusted, and fully adjusted models to provide information regarding the contributions of demographic and anthropometric variables, as well as pain severity. Our fully adjusted model runs the risk of overcontrolling for pain intensity, which could impact conclusions drawn about the phenotype of patients with neuropathic-like pain. For example, in several cases when conducting analyses on disability and pain scores (Tables 4, 6, and 7), findings that were significant in partially adjusted analyzes became nonsignificant in the fully adjusted model, suggesting that the association between neuropathic-like pain and these outcomes (i.e., disability, functional pain, and QST measures) operates largely through the higher pain intensity associated with neuropathic-like pain. Additional research is needed to better characterize the relationship between neuropathic pain mechanisms and general measures of pain severity in patients with OA. Greater neuropathic-like pain could be due to factors not measured such as vitamin B deficiency. However, our classification of neuropathic-like pain is based on screening questionnaires with high sensitivity and specificity, which decreased the probability of false-positive screens. We did not confirm damage to the nervous system/nerves arising from knee OA through electromyography or nerve conduction velocity, nor did we examine loss of sensory function (e.g., vibratory or tactile detection). We did not control for medications that could impact neuropathic-like symptoms, as few people were taking medications typically prescribed for neuropathy. The current study could have benefitted from using elements of the German Research Network on Neuropathic Pain QST protocol [45], with which we could have compared our QST findings to other large studies of patients with well-defined neuropathic pain conditions. These comparisons could have enhanced interpretation of our QST findings, particularly from the static QST measures (i.e., threshold and tolerance). Lastly, we reported that CPM was not significantly different between the groups; however, CPM should be replicated with other racially diverse samples to ensure that findings are generalizable.
Overall, we found support for differences in central sensitization, clinical pain, and poorer psychological function in adults with or at risk for knee OA who have neuropathic-like pain symptoms. Nearly half of individuals in the neuropathic-like pain group are classified as having high disability and moderate to severe limitations on the GCPS, but unlike Moss et al. [9], our study did not find differences in functional measures between the neuropathic-like group and the non-neuropathic-like group in the fully adjusted model, which controlled for pain intensity. Future research is needed to determine the mechanistic underpinnings and clinical relevance of these findings.
Authors’ Contributions
All authors were involved in drafting the article or revising it critically, and all authors approved the final version to be published. 1) study conception and design (RBF, KTS, RS, LBH, JCE, DTR, LAB, BRG, TLG), 2) acquisition of data (ELT, SQB, JSC, EJB, TLG, IAV, KAT, HWB, ASA), 3) analysis and interpretation of data (ELT, SQB, RBF), and 4) administrative, technical, or logistic support (JSC).
Acknowledgments
The authors would like to thank the research team that made this work possible: Ralisa Pop and Eric Weber.
Funding sources: Funding and support provided by National Institutes of Health (NIH) grants R37AG033906 (RBF), T32AG049673 and K22NS102334 (ELT), and R01AG054370 and K23AR062099 (KTS); and Clinical and Translational Science Awards grants UL1TR001427 (UF) and UL1TR001417 (UAB) from the NIH Center for Advancing Translational Sciences. Support was also provided by NIH grants K99AG052642 (EJB), TL1TR001418 (KAT), and R25CA090314 (HWB) and by the UF McKnight Brain Institute Career Enhancement Award (ELT).
Conflicts of interest: All authors declare that there are no conflicts of interest related to this study.
References
- 1. Ma VY, Chan L, Carruthers KJ.. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 2014;95(5):986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Rheumatol Arthritis 2008;58:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cushnaghan J, Dieppe P.. Study of 500 patients with limb joint osteoarthritis. I. Analysis by age, sex, and distribution of symptomatic joint sites. Ann Rheum Dis 1991;50(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neogi T, Zhang Y.. Epidemiology of OA. Rheum Dis Clin North Am 2013;39(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallace IJ, Worthington S, Felson DT, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci U S A 2017;114(35):9332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedson J, Croft PR.. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet Disord 2008;9:116.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu K, Robbins SR, McDougall JJ.. Osteoarthritis: The genesis of pain. Rheumatology (Oxford) 2018;57(suppl_4):iv43–50. [DOI] [PubMed] [Google Scholar]
- 8. Freynhagen R, Baron R, Gockel U, Tölle TR.. Pain DETECT: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22(10):1911–20. [DOI] [PubMed] [Google Scholar]
- 9. Moss P, Benson HA, Will R, Wright A.. Patients with knee osteoarthritis who score highly on the painDETECT questionnaire present with multimodality hyperalgesia, increased pain, and impaired physical function. Clin J Pain 2018;34:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hochman J, Davis A, Elkayam J, Gagliese L, Hawker G.. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage 2013;21(9):1236–42. [DOI] [PubMed] [Google Scholar]
- 11. Thakur M, Dickenson AH, Baron R.. Osteoarthritis pain: Nociceptive or neuropathic? Nat Rev Rheumatol 2014;10(6):374–80. [DOI] [PubMed] [Google Scholar]
- 12. Hochman J, Gagliese L, Davis A, Hawker G.. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage 2011;19(6):647–54. [DOI] [PubMed] [Google Scholar]
- 13. French HP, Smart KM, Doyle F.. Prevalence of neuropathic pain in knee or hip osteoarthritis: A systematic review and meta-analysis. Semin Arthritis Rheum 2017;47(1):1–8. [DOI] [PubMed] [Google Scholar]
- 14. Valdes AM, Suokas AK, Doherty SA, Jenkins W, Doherty M.. History of knee surgery is associated with higher prevalence of neuropathic pain-like symptoms in patients with severe osteoarthritis of the knee. Semin Arthritis Rheum 2014;43(5):588–92. [DOI] [PubMed] [Google Scholar]
- 15. Ohtori S, Orita S, Yamashita M, et al. Existence of a neuropathic pain component in patients with osteoarthritis of the knee. Yonsei Med J 2012;53(4):801–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roubille C, Raynauld J-P, Abram F, et al. The presence of meniscal lesions is a strong predictor of neuropathic pain in symptomatic knee osteoarthritis: a cross-sectional pilot study. Arthritis research & therapy 2014;16:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheumatol 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 18. Roux CH, Saraux A, Mazieres B, et al. Screening for hip and knee osteoarthritis in the general population: Predictive value of a questionnaire and prevalence estimates. Ann Rheum Dis 2008;67(10):1406–11. [DOI] [PubMed] [Google Scholar]
- 19. Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS.. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis Cartilage 2015;23(8):1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 21. Cruz‐Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis care & research 2013;65:1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Care Res 2009;61:1226–34. [DOI] [PubMed] [Google Scholar]
- 23. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW.. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 24. Theiler R, Spielberger J, Bischoff H, et al. Clinical evaluation of the WOMAC 3.0 OA Index in numeric rating scale format using a computerized touch screen version. Osteoarthritis Cartilage 2002;10(6):479–81. [DOI] [PubMed] [Google Scholar]
- 25. Von Korff M, Ormel J, Keefe FJ, Dworkin SF.. Grading the severity of chronic pain. Pain 1992;50(2):133–49. [DOI] [PubMed] [Google Scholar]
- 26. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-Form McGill Pain Questionnaire (SF-MPQ-2). Pain 2009;144(1–2):35–42. [DOI] [PubMed] [Google Scholar]
- 27. Cruz-Almeida Y, Cardoso J, Riley IIJ, et al. Physical performance and movement-evoked pain profiles in community-dwelling individuals at risk for knee osteoarthritis. Exp Gerontol 2017;98:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenstiel AK, Keefe FJ.. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain 1983;17(1):33–44. [DOI] [PubMed] [Google Scholar]
- 29. Jensen MP, Keefe FJ, Lefebvre JC, Romano JM, Turner JA.. Item measures of pain beliefs and coping strategies. Pain 2003;104(3):453–69. [DOI] [PubMed] [Google Scholar]
- 30. Kroenke K, Spitzer RL, Williams JB.. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64(2):258–66. [DOI] [PubMed] [Google Scholar]
- 31. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150(1):173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blikman T, Rienstra W, van Raay JJ, et al. Neuropathic-like symptoms and the association with joint-specific function and quality of life in patients with hip and knee osteoarthritis. PLoS One 2018;13(6):e0199165.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fernandes GS, Valdes AM, Walsh DA, Zhang W, Doherty M.. Neuropathic-like knee pain and associated risk factors: A cross-sectional study in a UK community sample. Arthritis Res Ther 2018;20:215.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soni A, Wanigasekera V, Mezue M, et al. Central sensitisation in knee osteoarthritis: Relating pre‐surgical brainstem neuroimaging and painDETECT based patient stratification to arthroplasty outcome. Arthritis Rheumatol 2019;71(4):550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: ethnic differences. Psychosomatic medicine 2014;76:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Granovsky Y, Nahman-Averbuch H, Khamaisi M, Granot M.. Efficient conditioned pain modulation despite pain persistence in painful diabetic neuropathy. Pain reports 2017;2:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cruz‐Almeida Y, Sibille KT, Goodin BR, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol 2014;66:1800–10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vina E, Ran D, Ashbeck E, Kwoh C.. Natural history of pain and disability among African-Americans and whites with or at risk for knee osteoarthritis: A longitudinal study. Osteoarthritis Cartilage 2018;26(4):471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Power J, Perruccio A, Gandhi R, et al. Neuropathic pain in end-stage hip and knee osteoarthritis: Differential associations with patient-reported pain at rest and pain on activity. Osteoarthritis Cartilage 2018;26(3):363–9. [DOI] [PubMed] [Google Scholar]
- 40. Fillingim RB. Individual differences in pain: Understanding the mosaic that makes pain personal. Pain 2017;158:S11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Racine M, Moulin DE, Nielson WR, et al. The reciprocal associations between catastrophizing and pain outcomes in patients being treated for neuropathic pain: A cross-lagged panel analysis study. Pain 2016;157(9):1946–53. [DOI] [PubMed] [Google Scholar]
- 42. Forsythe ME, Dunbar MJ, Hennigar AW, Sullivan MJ, Gross M.. Prospective relation between catastrophizing and residual pain following knee arthroplasty: Two-year follow-up. Pain Res Manag 2008;13(4):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riddle DL, Wade JB, Jiranek WA, Kong X.. Preoperative pain catastrophizing predicts pain outcome after knee arthroplasty. Clin Orthop Relat Res 2010;468(3):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cottler LB, McCloskey DJ, Aguilar-Gaxiola S, et al. Community needs, concerns, and perceptions about health research: Findings from the clinical and translational science award sentinel network. Am J Public Health 2013;103(9):1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rolke R, Baron R, Maier Ca, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006;123(3):231–43. [DOI] [PubMed] [Google Scholar]


