Abstract
Background
Asymptomatic bacteriuria is a bacterial infection of the urine without any of the typical symptoms that are associated with a urinary infection, and occurs in 2% to 15% of pregnancies. If left untreated, up to 30% of mothers will develop acute pyelonephritis. Asymptomatic bacteriuria has been associated with low birthweight and preterm birth. This is an update of a review last published in 2015.
Objectives
To assess the effect of antibiotic treatment for asymptomatic bacteriuria on the development of pyelonephritis and the risk of low birthweight and preterm birth.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) on 4 November 2018, and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCT) comparing antibiotic treatment with placebo or no treatment in pregnant women with asymptomatic bacteriuria found on antenatal screening. Trials using a cluster‐RCT design and quasi‐RCTs were eligible for inclusion, as were trials published in abstract or letter form, but cross‐over studies were not.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked for accuracy. We assessed the quality of the evidence using the GRADE approach.
Main results
We included 15 studies, involving over 2000 women. Antibiotic treatment compared with placebo or no treatment may reduce the incidence of pyelonephritis (average risk ratio (RR) 0.24, 95% confidence interval (CI) 0.13 to 0.41; 12 studies, 2017 women; low‐certainty evidence). Antibiotic treatment may be associated with a reduction in the incidence of preterm birth (RR 0.34, 95% CI 0.13 to 0.88; 3 studies, 327 women; low‐certainty evidence), and low birthweight babies (average RR 0.64, 95% CI 0.45 to 0.93; 6 studies, 1437 babies; low‐certainty evidence). There may be a reduction in persistent bacteriuria at the time of delivery (average RR 0.30, 95% CI 0.18 to 0.53; 4 studies; 596 women), but the results were inconclusive for serious adverse neonatal outcomes (average RR 0.64, 95% CI 0.23 to 1.79, 3 studies; 549 babies). There were very limited data on which to estimate the effect of antibiotics on other infant outcomes, and maternal adverse effects were rarely described.
Overall, we judged only one trial at low risk of bias across all domains; the other 14 studies were assessed as high or unclear risk of bias. Many studies lacked an adequate description of methods, and we could only judge the risk of bias as unclear, but in most studies, we assessed at least one domain at high risk of bias. We assessed the quality of the evidence for the three primary outcomes with GRADE software, and found low‐certainty evidence for pyelonephritis, preterm birth, and birthweight less than 2500 g.
Authors' conclusions
Antibiotic treatment may be effective in reducing the risk of pyelonephritis in pregnancy, but our confidence in the effect estimate is limited given the low certainty of the evidence. There may be a reduction in preterm birth and low birthweight with antibiotic treatment, consistent with theories about the role of infection in adverse pregnancy outcomes, but again, the confidence in the effect is limited given the low certainty of the evidence.
Research implications identified in this review include the need for an up‐to‐date cost‐effectiveness evaluation of diagnostic algorithms, and more evidence to learn whether there is a low‐risk group of women who are unlikely to benefit from treatment of asymptomatic bacteriuria.
Plain language summary
Antibiotics for bacterial infection in the urine in pregnancy when there are no symptoms
What is the issue?
Can giving antibiotics to pregnant women who have a urinary infection but no symptoms improve the outcomes for women and their babies?
Why is this important?
A bacterial infection of the urine without any of the typical symptoms that are associated with a urinary infection (asymptomatic bacteriuria) occurs in a number (2% to 15%) of pregnancies. Because of the changes happening in their body, pregnant women are more likely to develop a kidney infection (pyelonephritis) if they have a urinary infection. The infection may also contribute to a baby who is born preterm (before 37 weeks), or at a low birthweight (weighs less than 2500 g (5.5 pounds)).
What evidence did we find?
We found 15 randomised controlled studies involving over 2000 pregnant women with urinary infections, but no symptoms. Antibiotics may be effective in reducing the incidence of kidney infection in the mother (12 studies, 2017 women) and clearing the infection from the urine (four studies, 596 women). They may also reduce the incidence of preterm births (three studies, 327 women) and low birthweight babies (six studies, 1437 babies). None of the studies adequately assessed any adverse effects of antibiotic treatment for the mother or her baby, and often the way the study was done was not well described.
We assessed the three main outcomes with the GRADE approach, and found low‐certainty evidence that antibiotic treatment may prevent pyelonephritis, preterm birth, and birthweight less than 2500 g.
What does this mean?
Antibiotic treatment may reduce the risk of kidney infections in pregnant women who have a urine infection but show no symptoms of infection. Antibiotics may also reduce the chance a baby will be born too early or have a low birthweight. However, because of the low certainty of the evidence, it is difficult to draw conclusions; more research is needed.
Summary of findings
Summary of findings for the main comparison. Antibiotics compared to no treatment for asymptomatic bacteriuria in pregnancy.
| Antibiotics compared to no treatment for asymptomatic bacteriuria in pregnancy | ||||||
|
Patient or population: pregnant women with asymptomatic bacteriuria Setting: hospital‐based clinics in North America, UK and Ireland, Australia; hospital and community midwifery practices in the Netherlands Intervention: antibiotics Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with antibiotics | |||||
| Development of pyelonephritis | Study population | RR 0.24 (0.13 to 0.41) | 2017 (12 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 199 per 1000 | 48 per 1000 (26 to 82) | |||||
| Preterm birth < 37 weeks | Study population | RR 0.34 (0.13 to 0.88) | 327 (3 RCTs) | ⊕⊕⊝⊝ Lowc,d | ||
| 174 per 1000 | 59 per 1000 (23 to 153) | |||||
| Birthweight < 2500 g | Study population | RR 0.64 (0.45 to 0.93) | 1437 (6 RCTs) | ⊕⊕⊝⊝ Lowe,f | ||
| 136 per 1000 | 87 per 1000 (61 to 126) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded 1 level for serious limitations in study design: most of the trials contributing outcome data either had important design limitations related to lack of allocation concealment and lack of blinding, or there were insufficient details provided in the report to assess risk of bias. Many of the studies were performed in the 1960s and 1970s, prior to more rigorous study designs and reporting standards. bWe downgraded 1 level for serious limitations in inconsistency: the rate of pyelonephritis in the control groups ranged from 2.2% to 36%; there was significant heterogeneity, which was not explained by the duration of treatment (I² = 60%). cWe downgraded 1 level for serious limitations in study design: only one trial was judged at low risk of bias across all domains; for the other two, the risk of bias was either unclear because details were not provided, or judged high risk. dWe downgraded 1 level for serious limitations in indirectness: the rate of preterm birth in the control group ranged from 4.4% to 37.5%. There have been substantial changes in obstetric practices over the four decades from the earliest to the latest study. In one study, only women with group B streptococcus bacteriuria were enrolled, and treatment was with penicillin. eWe downgraded 1 level for serious limitations in study design: all of the trials contributing outcome data either had important design limitations related to lack of allocation concealment and lack of blinding, or there were insufficient details provided in the report to assess risk of bias. The studies contributing data to this outcome were performed in the 1960s and 1970s, prior to more rigorous study designs and reporting standards. fWe downgraded 1 level for serious limitations in indirectness: 5 of the 6 studies included in this outcome continued antibiotic treatment for 6 weeks (1 study), or until term (4 studies). In one study, women received a single dose of antibiotics, and in no study did women receive what is now considered a standard course of antibiotics for 3 to 7 days.
Background
Description of the condition
Asymptomatic bacteriuria, generally defined as true bacteriuria in the absence of specific symptoms of acute urinary tract infection, is a common finding, and occurs in 2% to 15% of all pregnancies (Ipe 2013).
While rates from recent observational studies fall within this range (Abdel‐Aziz 2017; Bandyopadhyay 2005; Celen 2011; Fatima 2006; Kazemier 2015; McIsaac 2005; McNair 2000; Mohammad 2002; Olamijulo 2016; Tugrul 2005), rates of over 20% are reported in studies from some low‐income countries (Ajayi 2012; Rizvi 2011; Tadesse 2014). The prevalence of asymptomatic bacteriuria was reported to be as high as 86.6% in a population from Nigeria that included Staphylococcus aureus, a possible contaminant, as a uropathogen (Akerele 2001). Rates were reported to be higher in HIV positive women in Nigeria (Awolude 2010; Ezechi 2013), but not in a study from South Africa (Widmer 2010). In a large retrospective study, the strongest predictor of bacteriuria was an antepartum urinary tract infection (Pastore 1999). A study from an electronics factory in China found an association between urinary tract infections in pregnancy and frequency of voiding; voiding three or more times a shift was protective (Su 2009). In a study from Iran, there was an association between infection, frequency of sexual intercourse, and genital hygiene practices (Amiri 2009). The prevalence of infection is related to socioeconomic status (Haider 2010; Turck 1962; Whalley 1967), although this may not always apply (Awoleke 2015; Kovavisarach 2009). Other contributing factors, recognised as associated with an increased risk for bacteriuria, include diabetes and anatomical abnormalities of the urinary tract.
The original criterion for diagnosing asymptomatic bacteriuria was a count of more than 100,000 bacteria/mL on two consecutive clean‐catch samples (Kass 1960a). The detection of more than 100,000 bacterial/mL in a single voided midstream urine sample is accepted as an adequate and more practical alternative, although there is only an 80% probability the woman has true bacteriuria; this increases to 95% if two or more consecutive cultures are positive for the same organism (Kass 1960a). Because the performance of rapid urine screening tests in pregnancy is poor, quantitative culture remains the gold standard for diagnosis (Bachman 1993; Garingalo‐Molina 2000; McNair 2000; Mignini 2009; Rogozińska 2016).
Escherichia coli is the most common pathogen associated with asymptomatic bacteriuria, representing up to 80% of isolates (Ipe 2013). Other organisms include other gram‐negative bacteria, e.g. Klebsiella spp., Proteus mirabilis, and group B streptococci. These bacteria colonise the vaginal introitus and periurethral area. Uropathogenic gram‐negative bacteria possess specific virulence factors that enhance both colonisation and invasion of the urinary tract, for example, the P‐fimbriae of certain strains of E. coli that allow for adherence to uroepithelial cells (Eisenstein 1988; Stenqvist 1987). Some strains of E. coli isolated from pregnant women with asymptomatic bacteriuria have a similar virulence pattern to strains from women with symptomatic infections (Lavigne 2011), but this does not always hold true (Stenqvist 1987). While Staphylococcus saprophyticus is recognised as a urinary pathogen, other species of Staphylococci, including Staphylococcus aureus, may reflect contamination rather than a true infection, and prevalence data where the number of Staphylococcus spp. is high, are difficult to interpret. Maternal urinary tract infection with group B streptococci is associated with vaginal colonisation with the organism, and antibiotic treatment during labour is recommended to prevent early onset neonatal group B streptococcal disease (Allen 2012).
While asymptomatic bacteriuria in non‐pregnant women is generally benign, obstruction to the flow of urine in pregnancy leads to stasis, and increases the likelihood that pyelonephritis will complicate asymptomatic bacteriuria (Nicolle 2014). Mechanical compression from the enlarging uterus is the principal cause of hydroureter and hydronephrosis, but smooth muscle relaxation induced by progesterone may also play a role (Sobel 1995). Differences in urine pH and osmolality and pregnancy‐induced glycosuria and aminoaciduria may facilitate bacterial growth. If asymptomatic bacteriuria in pregnancy is untreated, it has generally been accepted that up to 20% to 30% of mothers will develop acute pyelonephritis (Nicolle 2015). Current estimates are difficult to identify because there is almost universal implementation of screening and treatment, however, a recent study from a low‐risk population in the Netherlands, where screening never became standard, reported a rate of pyelonephritis of 2.4% (Kazemier 2015). Clinical signs of pyelonephritis include fever, chills, costovertebral tenderness, dysuria, and frequency. Nausea and vomiting are common, and if infection is associated with bacteraemia, women may present with high fever, shaking chills, and low blood pressure. Maternal complications include maternal respiratory insufficiency, septicaemia, renal dysfunction, and anaemia (Hill 2005; Wing 2014). In the pre‐antibiotic era, acute pyelonephritis was associated with a 20% to 50% incidence of preterm birth. A prospective longitudinal study, in the era of routine screening, over a two‐year period from 2000 to 2001 in Texas, reported an incidence of acute pyelonephritis in pregnancy of 1.4% (Hill 2005). From an 18‐year retrospective review, in an era of routine screening and treatment for asymptomatic bacteriuria, the incidence of acute pyelonephritis in pregnancy was 0.5%, and pyelonephritis was associated with preterm birth (odds ratio (OR) 1.3, 95% confidence interval (CI) 1.2 to 1.5); women with pyelonephritis were more likely to be black or Hispanic, young, less educated, initiate prenatal care late, and smoke (Wing 2014). An association between acute pyelonephritis and preterm birth was described in a retrospective study of 219,612 deliveries from Israel (OR 2.6, 95% CI 1.7 to 3.9; Farkash 2012).
An association between asymptomatic bacteriuria, low birthweight, and preterm birth has been described since the earliest studies of Kass (Kass 1960a), but population‐based studies have produced conflicting results. A retrospective study from Israel, which controlled for confounders, showed an association between asymptomatic bacteriuria and preterm birth (OR 1.9, 95% CI 1.7 to 2.0; Sheiner 2009); in contrast, findings from the Cardiff Birth Survey reported that asymptomatic bacteriuria, adjusted for demographic and social factors, was not associated with preterm birth (OR 1.2, 95% CI 0.9 to 1.5; Meis 1995). However, when preterm deliveries were categorised into those medically indicated because of complications of pregnancy (e.g. antepartum haemorrhage, eclampsia, or renal disease) and spontaneous preterm births, there was a significant association between bacteriuria and medically‐indicated preterm deliveries (OR 2.03, 95% CI 1.5 to 2.8), but not for spontaneous preterm births (OR 1.07, 95% CI 0.78 to 1.46). The authors concluded that if asymptomatic bacteruria does not progress to pyelonephritis, it is not associated with preterm birth (Meis 1995a).
Description of the intervention
The goal of treatment for asymptomatic bacteriuria is to treat and clear the infection. The urinary bacterial isolate should be susceptible to the antibiotic chosen, the length of treatment should be adequate, adherence should be assured, and the drug should have favourable pharmacokinetic parameters. The treatment should be safe in pregnancy, for both the mother and developing fetus. Many antibiotics have been used to treat bacteriuria, including sulphonamides or sulphonamide‐containing combinations, penicillins, cephalosporins, fosfomycin, and nitrofurantoin. However, not all the antibiotics previously evaluated are currently available, e.g. certain sulphonamides and methenamine, or recommended during pregnancy, e.g. tetracycline. Increasing bacterial resistance of urinary pathogens can make it difficult to select an appropriate regimen, especially in under‐resourced settings, where facilities for urine culture and antimicrobial susceptibility testing are limited (Assefa 2008; Enayat 2008; Hernandez Blas 2007; Rizvi 2011; Tadesse 2014). There is no evidence that non‐pharmacological interventions, e.g. cranberry juice, are effective (Wing 2008), although no data exist to suggest the use of cranberry has any harmful effects on pregnancy (Heitmann 2013).
How the intervention might work
Urinary pathogens causing asymptomatic bacteriuria are similar to those causing pyelonephritis; antibiotic treatment and eradication of bacteriuria is expected to prevent ascending urinary tract infection and the development of clinical pyelonephritis.
The relationship between asymptomatic bacteriuria, low birthweight, and preterm birth is controversial, since a biological mechanism for an association between preterm labor and asymptomatic bacteriuria has not been established. Microbial‐induced preterm labor is mediated by an inflammatory process (Goldenberg 2000; Romero 2014). Microorganisms and their products are sensed by pattern‐recognition receptors, such as toll‐like receptors (TLRs), which induce the production of chemokines, prostaglandins, and proteases, leading to the onset of labour. While this mechanism has been well defined for ascending intra‐amniotic infection, there has been no recent research to explore the mechanisms through which asymptomatic bacteriuria might exert adverse pregnancy outcomes.
Why it is important to do this review
Screening for, and treating asymptomatic bacteriuria in pregnancy, has become a standard of obstetric care. While most antenatal guidelines include routine screening for asymptomatic bacteriuria, questions have been raised about the quality of the evidence on which these guidelines are based, and the lack of data on the harms of screening (Angelescu 2016; Moore 2018). Using a decision analysis, screening for, and treating asymptomatic bacteriuria to prevent pyelonephritis, has been shown to be cost‐effective over a wide range of estimates, although the cost‐benefit is diminished if the rate of asymptomatic bacteriuria is less than 2% (Rouse 1995; Wadland 1989). The low prevalence of infection in certain populations, the cost of different screening tests, and uncertainty about the benefits of treatment in decreasing adverse outcomes of pregnancy have been used to argue against screening and treatment as universal recommendations; preventing unnecessary antibiotic use has become an important aspect of programmes to decrease the development of antimicrobial resistance. A rigorous evaluation of studies of the effect of treatment of asymptomatic bacteriuria could provide clarity around these issues. This is an update of a review last published in 2015 (Smaill 2015).
Objectives
To assess the effect of antibiotic treatment for asymptomatic bacteriuria on the development of pyelonephritis and the risk of low birthweight and preterm birth.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials and quasi‐randomised trials (e.g. alternation). Cluster‐randomised trials were eligible for inclusion. Cross‐over trials were not eligible for inclusion. Trials published in abstract form only, or as a letter, were eligible for inclusion.
Types of participants
Pregnant women found, on antenatal screening, to have asymptomatic bacteriuria, as defined by the study authors, at any stage of pregnancy.
Types of interventions
We included studies if any antibiotic regimen was compared with placebo or no treatment for asymptomatic bacteriuria.
Types of outcome measures
Primary outcomes
Development of pyelonephritis
Preterm birth less than 37 weeks
Birthweight less than 2500 g
Secondary outcomes
Persistent bacteriuria
Neonatal mortality or other serious adverse neonatal outcome
Maternal side effects
Costs, as defined by trial authors
Birthweight
Gestational age
Women's satisfaction, as measured by trial authors
Persistent bacteriuria was defined as bacteriuria persisting at the time of delivery.
We used the World Health Organization's definition of prematurity: a baby born before 37 completed weeks of gestation (Blencowe 2012).
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register, by contacting their Information Specialist (4 November 2018).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full, current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service; please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist, and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE Ovid;
weekly searches of Embase Ovid;
monthly searches of CINAHL EBSCO;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Search results are independently screened by two people, and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned, and ongoing trial reports on 4 November 2018, using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous versions of this review, seeSmaill 1993, Smaill 2007 and Smaill 2015.
For this update, we used the following methods to assess the reports that were identified as a result of the updated search, based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. If any new studies were included, both review authors independently extracted the data, using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager 5 software, and checked for accuracy (Review Manager 2014).
If information regarding any of the above had been unclear, we had planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups, or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias, and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias
Where identified, we described bias due to problems not covered elsewhere.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias, and whether we considered it was likely to impact the findings. We planned to explore the impact of the level of bias by undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence, using GRADE
For this update, we assessed the quality of the evidence using the GRADE approach (Schünemann 2009). We assessed the quality of the body of evidence relating to the following outcomes, for the main comparison of antibiotic versus no treatment.
Development of pyelonephritis
Preterm birth less than 37 weeks
Birthweight less than 2500 g
We used GRADEpro GDT software to import data from Review Manager 5 software, to create a ’Summary of findings’ table (GRADEpro GDT; Review Manager 2014). We produced a summary of the intervention effect and a measure of quality for each of the above outcomes, using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. In future updates, we plan to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
If any were identified, we planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. In future updates of this review, if any cluster‐randomised trials are included, we will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, Section 16.3.4 or 16.3.6, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs, and the interaction between the effect of the intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit, and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect, by completing a sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, and where reasonable, we attempted to include all participants randomised to each group in the analyses.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the I² and Chi² statistics, and Tau². We regarded heterogeneity as substantial, if an I² was greater than 30%, and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we explored it by prespecified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually, and at any suggestion of asymmetry, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (Review Manager 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects, and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not have combined trials. Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup analysis and sensitivity analysis. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analysis to determine whether there was an effect of the duration of antibiotic therapy on the outcomes.
Single dose versus no treatment
Short course (three to seven days) versus no treatment
Intermediate course (three to six weeks) versus no treatment
Continuous antibiotic therapy until delivery versus no treatment
We conducted subgroup analyses for the following outcomes.
Development of pyelonephritis
Preterm birth less than 37 weeks
Birthweight less than 2500 g
We assessed subgroup differences by interaction tests available within RevMan 5 (Review Manager 2014). We reported the results of the subgroup analysis quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We had planned to carry out sensitivity analysis to explore the effect of risk of bias on the overall results, by excluding studies where the overall risk of bias was high or there was insufficient detail provided to judge risk of bias from the analysis, for the primary outcomes.
Results
Description of studies
Results of the search
See: Figure 1.
1.
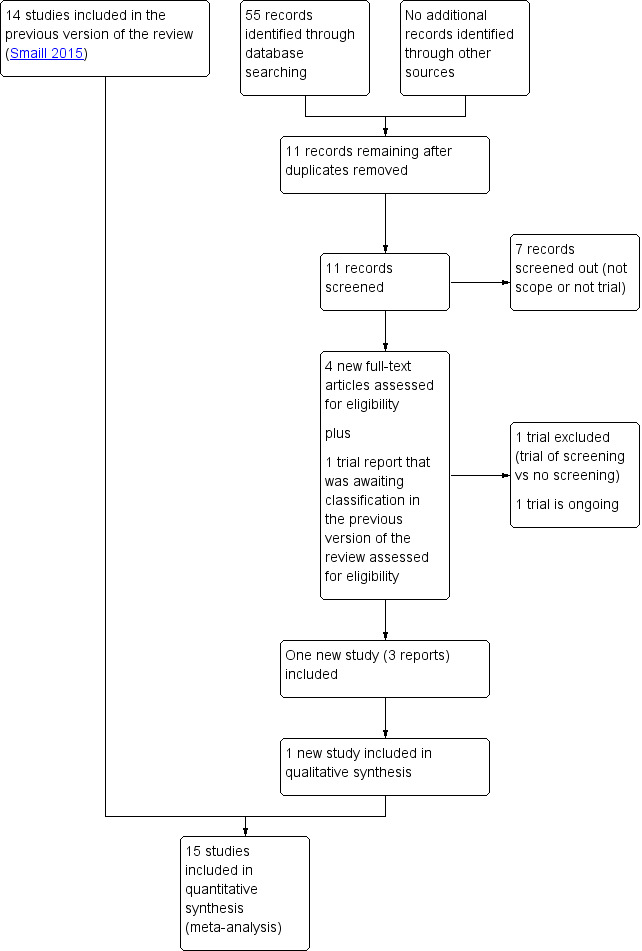
Study flow diagram
We assessed four new trial reports (Kazemier 2015; NCT03274960;NCT03275623;NL2921), and reassessed one trial report that was awaiting classification from the previous version of the review (Kazemier 2012). We excluded NCT03274960 because the intervention was screening for asymptomatic bacteriuria versus no screening. NCT03275623 is a study of treatment versus no treatment for low level bacteriuria and is ongoing. We included one new study (Kazemier 2015). Kazemier 2012 and NL2921 are additional reports of that study.
Included studies
We included 15 studies, in 22 reports, involving over 2000 women. For details, see Characteristics of included studies. One study enrolled only women with group B streptococci in the urine (Thomsen 1987). Where there was more than one published report that in the opinion of the review authors referred to the same study, we abstracted information from whichever report provided the most detailed information. The earliest published report was from 1960 (Kass 1960), and the most recent from 2015 (Kazemier 2015). Most studies (N = 11) enrolled women prior to 1970. Only Kazemier 2015 reported any potential conflicts of interest. Two studies did not report a funding source (Foley 1987; Mulla 1960). Local or national research funding sources were reported for the majority of the other studies, and several studies described in‐kind support, where the antibiotic was provided by a pharmaceutical company. We did not find any cluster‐randomised trials.
Participants
Most women were enrolled from hospital‐based clinics in North America (Elder 1966; Elder 1971; Gold 1966; Kass 1960; Mulla 1960), the UK and Ireland (Brumfitt 1975; Foley 1987; Little 1966; Williams 1969), and Australia (Furness 1975; Kincaid‐Smith 1965; Wren 1969); the recent study from the Netherlands was set in the Dutch Obstetric Consortium, and enrolled women from university, teaching and non‐teaching hospitals, ultrasound centres, and midwifery practices (Kazemier 2015).
The majority of studies enrolled women at the first antenatal visit (Brumfitt 1975; Elder 1971; Foley 1987; Kass 1960; Kincaid‐Smith 1965; Little 1966; Williams 1969; Wren 1969). Some studies enrolled women at the second antenatal visit (Furness 1975), between 16 and 22 weeks' gestation (Kazemier 2015), before 24 weeks (Pathak 1969), between 27 and 31 weeks (Thomsen 1987), between 30 and 32 weeks (Mulla 1960), any gestational age < 32 weeks (Elder 1966), or at any prenatal visit (Gold 1966).
Two studies did not specify microbiological criteria for enrolment (Brumfitt 1975; Mulla 1960). Where there were microbiological criteria, bacteriuria was usually defined as at least one clean‐catch, midstream, or catheterised urine specimen with more than 100,000 bacteria/mL on culture. Some studies required one positive culture of > 100,000 bacteria/mL (Foley 1987; Furness 1975; Kazemier 2015); several studies required confirmation with a second culture (Furness 1975; Gold 1966; Kincaid‐Smith 1965; Little 1966; Pathak 1969; Williams 1969; Wren 1969), and some required a third culture (Elder 1966; Elder 1971; Kass 1960). One study included women with a lower colony count of more than 10,000 bacteria/mL on two occasions (Furness 1975); one enrolled women with any growth of group B streptococcus on a mid‐stream urine culture (Thomsen 1987).
Interventions
Several different antibiotic regimens were used for treatment (seeCharacteristics of included studies for details), including the study of group B streptococci, which compared penicillin to placebo (Thomsen 1987). Treatment varied as well; antibiotics were given in a single dose (Brumfitt 1975), for three to seven days (Foley 1987; Kazemier 2015; Mulla 1960; Thomsen 1987; Williams 1969), for three weeks (Pathak 1969), for six weeks (Elder 1971), continued until delivery (Elder 1966; Furness 1975; Gold 1966; Kass 1960; Kincaid‐Smith 1965), or up to six weeks after delivery (Little 1966; Wren 1969). In four studies, a repeat antibiotic course with the same drug was administered if the infection persisted (Kazemier 2015; Mulla 1960; Pathak 1969; Thomsen 1987). In several studies, an alternative agent was used for persisting or resistant organisms (Foley 1987; Kass 1960; Kincaid‐Smith 1965; Little 1966; Williams 1969). Most studies used antibiotics that are no longer routinely used for treating bacteriuria, including certain sulphonamides (Brumfitt 1975; Elder 1966; Foley 1987; Gold 1966; Kass 1960; Kincaid‐Smith 1965; Little 1966; Mulla 1960), tetracycline (Elder 1971), and methenamine (Furness 1975). Some studies used nitrofurantoin, as either first line treatment (Kazemier 2015; Little 1966; Pathak 1969), or for failures (Elder 1971; Kass 1960; Kincaid‐Smith 1965; Little 1966; Williams 1969). In other studies, ampicillin was used for failures (Kincaid‐Smith 1965; Little 1966; Williams 1969). In one study, women received a fixed rotation of nitrofurantoin, ampicillin, sulphurazole, and nalidixic acid(Wren 1969). In only one study were data on antimicrobial susceptibility used to select the antibiotic (Foley 1987).
Outcomes
Most studies (N = 12) included the outcome of pyelonephritis (Brumfitt 1975; Elder 1971; Foley 1987; Furness 1975; Gold 1966; Kass 1960; Kazemier 2015; Kincaid‐Smith 1965; Little 1966; Mulla 1960; Pathak 1969; Williams 1969).
Six studies reported the outcome of birthweight < 2500 g (Brumfitt 1975; Elder 1971; Kass 1960; Kincaid‐Smith 1965; Little 1966; Wren 1969). In many of the studies conducted during the 1960s, the standard definition of preterm birth was low birthweight, defined as birthweight less than 2500 g, rather than a gestational age less than 37 weeks. Two studies defined preterm birth as a gestational age of less than 37 weeks (Thomsen 1987; Wren 1969); Kazemier 2015 included results for gestational ages < 37 weeks, < 34 weeks, and < 28 weeks. One study did not provide a definition of preterm birth (Gold 1966), and Furness 1975 used a definition of less than 38 weeks. While mean birthweight in the two groups were reported by Brumfitt 1975, Elder 1971, Furness 1975, and Kazemier 2015, and mean gestational age by Kass 1960, Kazemier 2015, and Thomsen 1987, not all authors reported the standard deviation of the mean, and we could not include these data in the analyses.
Four studies defined persistent bacteriuria as a positive culture at delivery or the last prenatal visit (Elder 1966; Elder 1971; Gold 1966; Pathak 1969); two defined it as a positive culture at six weeks to three months postpartum (Furness 1975; Kincaid‐Smith 1965); one did not define it (Foley 1987). Three studies also measured long‐term rates of bacteriuria: one between three and nine months postpartum (Pathak 1969), one at six months (Kincaid‐Smith 1965), and one at 10 to 14 years (Kass 1960).
Only two studies specifically commented on maternal side effects: Mulla 1960 stated there were no side effects necessitating discontinuation of treatment, and Gold 1966 reported "no toxic manifestations in women in the treatment group".
Neonatal mortality or other serious adverse neonatal outcomes were incompletely reported, and there were no standard definitions. Neonatal deaths were reported by Elder 1971 and Kass 1960, and Kazemier 2015 defined and reported on severe neonatal morbidity (presence of one or more of the following: severe respiratory distress syndrome, bronchopulmonary dysplasia, periventricular leukomalacia > grade 1, intracerebral haemorrhage > grade 2, necrotizing enterocolitis > grade 1, or proven sepsis).
No studies reported on women's satisfaction with the intervention.
Excluded studies
Six studies, in seven reports, were excluded because they did not meet the inclusion criteria. In one study, we could not ascertain whether the women had been randomly allocated to treatment or no treatment (Calderon‐Jaimes 1989), and Mohammad 2002 was an observational study, with no treatment intervention. In Sanderson 1984, only women who had been successfully treated initially were randomised to continue treatment. LeBlanc 1964 included symptomatic women in the outcomes; the asymptomatic group was not reported separately. The intervention in NCT03274960 was screening versus no screening, rather than treatment versus no treatment, and the women in Rafalskiy 2013 were randomised to one of two treatment groups, without a no treatment group. SeeCharacteristics of excluded studies table for details.
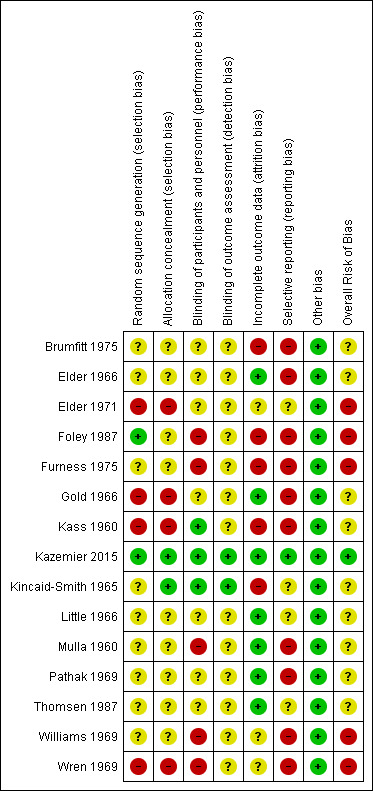
Risk of bias in included studies
For most studies, there was only a brief and incomplete description of the research methods, which made it difficult to assess the risk of bias in the studies. Kazemier 2015 described the methods in detail, and published the study protocol.
See Figure 2. The description of the characteristics of the study groups was generally poor. In only one study, were the similarities in age, parity, and socioeconomic status between the treatment and no treatment groups adequately described (Thomsen 1987); Kass 1960a described the racial distribution of the two groups, which was comparable; in four other studies, the urinary bacterial isolates for the two groups were listed (Elder 1966; Elder 1971; Gold 1966; Mulla 1960), but otherwise, there was no attempt to demonstrate the comparability of the study groups. Kazemier 2015 described the baseline characteristics of the study populations, including age, body mass index, education level, smoking status, alcohol use, parity, pre‐existing hypertension, gestational age at screening, and pregnancy occurring after fertility treatment, but included the untreated group (N = 163) with the women given placebo (N = 45) in the comparison with the asymptomatic bacteriuria‐positive women who received nitrofurantoin. No other study included the rates of maternal smoking, a recognised risk for low birthweight. There was no description of the presence of co‐existing genital infections, although one study excluded women with positive serology for syphilis (Pathak 1969). Details on the management of recurrent urinary tract infection or persistent infection, the treatment of symptomatic lower urinary tract infection (cystitis), and concurrent antibiotic administration were generally incomplete. Some studies included twin deliveries, while other studies excluded these.
2.
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
There was no consistent application of standard definitions for the measured outcomes. Pyelonephritis usually referred to symptoms of loin pain, fever, dysuria, or frequency, with or without a significant urine culture. While rates of low birthweight were usually reported, most studies described this as prematurity. For studies that reported rates of preterm births, the definition of preterm birth was inconsistent, and there were insufficient data presented in most of the studies to compare gestational ages and birthweight between treatment and control groups.
Allocation
In only one study was group assignment based on a web‐based application with a computer‐generated list (Kazemier 2015); in another study, the random component in the sequence generation was described as a coin toss (Foley 1987). For the other studies, there was either no description of the method of randomisation, or the method was clearly inadequate: in four studies, women were allocated to treatment by alternation, so we assessed them at high risk of bias (Elder 1971; Gold 1966; Kass 1960; Wren 1969).
In the majority of studies, there was inadequate description of concealment of allocation to judge selection bias. One study referred to the use of sealed envelopes, but the envelope was drawn from a pool of sealed envelopes rather than a consecutively numbered pile, so we assessed this study as unclear risk of bias (Little 1966). One study described allocation as centrally controlled (Kincaid‐Smith 1965); the remaining studies made no such statement.
Blinding
In 10 of the 15 studies, the control group received a placebo (Brumfitt 1975; Elder 1966; Elder 1971; Gold 1966; Kass 1960; Kazemier 2015; Kincaid‐Smith 1965; Little 1966; Pathak 1969; Thomsen 1987). Two of these studies were described as placebo‐controlled, without any further details (Gold 1966; Little 1966); four of the studies were described as double‐blinded, again, without further details (Brumfitt 1975; Elder 1966; Pathak 1969; Thomsen 1987); and Elder 1971 reported the placebo was 'identical appearing'. However there was insufficient information provided in the reports of these seven studies to know whether the blinding could have been broken, and therefore, we classified them at unclear risk of bias. In three studies, there was a specific mention that neither the women nor treating physician was aware of allocation to treatment group, and we judged them at low risk of performance bias (Kass 1960; Kazemier 2015; Kincaid‐Smith 1965). No treatment was given to the control group in the other five studies, so we judged them at high risk of bias, given the outcomes may have been influenced by lack of blinding (Foley 1987; Furness 1975; Mulla 1960; Williams 1969; Wren 1969).
Only Kazemier 2015 specifically commented that the researchers remained blind to group allocation, although in Kincaid‐Smith 1965, it was reported that "a code of instructions to the pharmacist ensured that the trial remained double‐blind..." We judged these two studies at low risk of detection bias. Although four of the studies were described as double‐blinded, there were insufficient details provided to know whether indeed the outcome assessment was blinded (Brumfitt 1975; Elder 1966; Pathak 1969; Thomsen 1987). We judged these four studies, along with the other nine studies where no information was provided, at unclear risk of bias. However, it is likely that in the five studies where there was no use of placebo, the risk of detection bias was high.
Incomplete outcome data
We judged seven studies as low risk for attrition bias: in four studies, there was no loss to follow‐up and outcomes were reported for all enrolled women (Gold 1966; Little 1966; Mulla 1960; Thomsen 1987); in two studies, information was provided on women lost to follow‐up, and missing outcome data were reasonably balanced across groups (Elder 1966; Pathak 1969), and in Kazemier 2015, five women, enrolled in error, were included in the intention‐to‐treat analysis. We judged five studies to have high risk of attrition bias, given that the missing data may have introduced a clinically important effect on the estimate of treatment. There was no explanation provided for missing outcome data in four studies (Brumfitt 1975; Foley 1987; Furness 1975; Kass 1960), while in Kincaid‐Smith 1965, outcomes were not reported for women excluded because of poor compliance. In three studies, we were unclear about the risk of attrition bias: there was no explanation for the differences in group sizes in Williams 1969, and while the reasons for excluding women were provided in two studies, details on the allocation group were not (Elder 1971; Wren 1969).
Selective reporting
In only one study was a study protocol published and the study's prespecified outcomes reported; we judged this study at low risk of selective reporting bias (Kazemier 2015). For several studies, pregnancy outcomes of interest were either not reported (Elder 1966; Foley 1987; Mulla 1960; Pathak 1969; Williams 1969), or there was no definition of prematurity (Gold 1966); we judged these studies at high risk of reporting bias. We judged studies that failed to include the primary outcome of pyelonephritis (Brumfitt 1975; Elder 1966; Wren 1969), studies where the outcome of pyelonephritis in pregnancy was not reported for all women allocated to treatment (Brumfitt 1975; Furness 1975), or those in which there was no clear definition of pyelonephritis (Foley 1987; Kass 1960; Mulla 1960), at high risk of reporting bias. For the remaining four studies, there was insufficient information to permit judgement; we classified these as unclear.
Other potential sources of bias
We did not identify any other obvious sources of bias, and so we judged this category at low risk, for each of the studies.
Overall risk of bias
We judged five studies at high overall risk of overall bias (Elder 1971; Foley 1987; Furness 1975; Williams 1969; Wren 1969), and one study at low overall risk (Kazemier 2015). While we assessed the overall risk of bias as unclear for the other nine studies, we assessed at least one domain at high risk of bias in each of these studies.
Effects of interventions
See: Table 1
1. Antibiotics versus no treatment for asymptomatic bacteriuria
Primary outcomes
Antibiotic treatment may reduce the incidence of pyelonephritis in women with asymptomatic bacteriuria (average risk ratio (RR) 0.24, 95% confidence interval (CI) 0.13 to 0.41; 12 studies, 2017 women; I² = 60%; low‐certainty evidence; Analysis 1.1). There was significant heterogeneity among the studies, and we used a random‐effects analysis. For most studies, a beneficial effect was seen with treatment. We assessed reporting biases (such as publication bias) using a funnel plot. There was no strong evidence of funnel plot asymmetry by visual assessment (Figure 3).
1.1. Analysis.
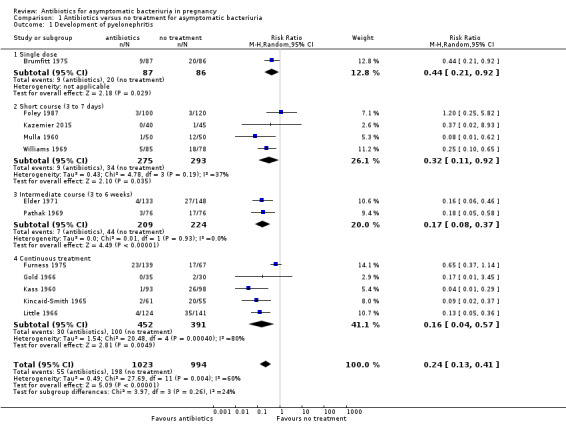
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 1 Development of pyelonephritis.
3.
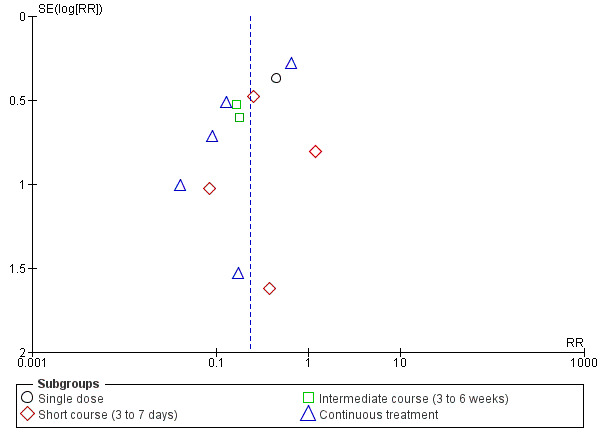
Funnel plot of comparison: 1 Antibiotic versus no treatment for asymptomatic bacteriuria, outcome: 1.1 Development of pyelonephritis
Antibiotic treatment may reduce the incidence of preterm birth, when this was defined as a gestational age of less than 37 weeks (RR 0.34, 95% CI 0.13 to 0.88; 3 studies, 327 women; I² = 32%; low‐certainty evidence; Analysis 1.2; Kazemier 2015; Thomsen 1987; Wren 1969). Thomsen 1987 only enrolled women with group B streptococcal bacteriuria.
1.2. Analysis.
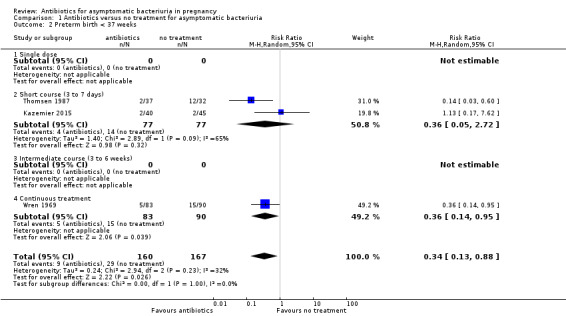
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 2 Preterm birth < 37 weeks.
Antibiotic treatment may reduce the incidence of birthweight less than 2500 g (average RR 0.64, 95% CI 0.45 to 0.93; 6 studies, 1437 babies; I² = 28%; low‐certainty evidence; Analysis 1.3).
1.3. Analysis.
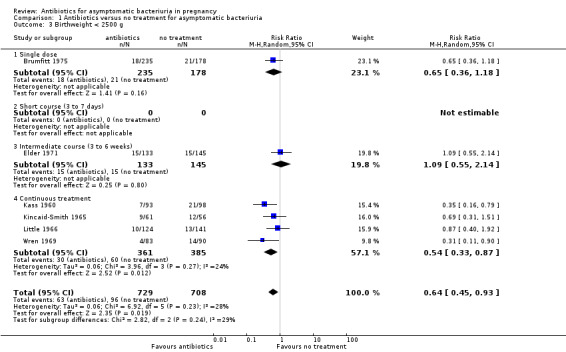
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 3 Birthweight < 2500 g.
We used a random‐effects meta‐analysis for preterm birth and low birthweight because we predicted there would be real differences in treatment effect from study to study given the clinically important differences in study populations and interventions.
Secondary outcomes
Antibiotic treatment is probably effective in clearing asymptomatic bacteriuria (average RR 0.30, 95% CI 0.18 to 0.53; 4 studies, 596 women; I² = 76%; Analysis 1.4). Without treatment, bacteriuria was present at the time of delivery in 66% of women. Although there was significant statistical heterogeneity among trials, likely explained by differences in study design and intervention, the direction of the effect was consistent. Treatment with antibiotics probably has no effect on the long‐term incidence of bacteriuria (one study reported results at between three and nine months postpartum, one reported at six months, and one at 10 to 14 years).
1.4. Analysis.
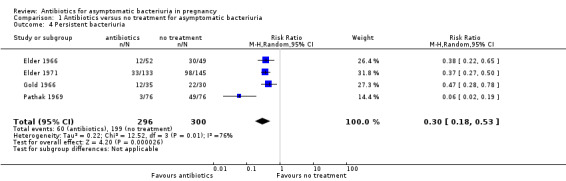
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 4 Persistent bacteriuria.
It is uncertain whether antibiotic treatment has any effect on serious adverse neonatal outcomes, because there was insufficient information from a small number of trials with few events (RR 0.64, 95% CI 0.23 to 1.79; 3 studies, 549 babies; Analysis 1.5).
1.5. Analysis.
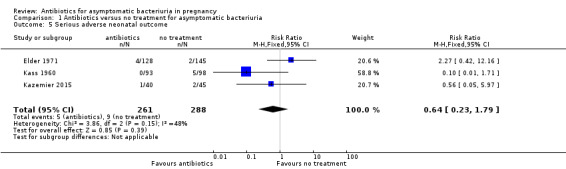
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 5 Serious adverse neonatal outcome.
Information on maternal adverse events was incompletely reported and we could not analyse the results.
There were too few studies that reported on mean birthweight and gestational age for us to draw any conclusions. The results were inconclusive for mean birthweight (mean difference (MD) 21.03, 95% CI ‐83.65 to 125.70; 2 studies, 495 babies; Analysis 1.6), and mean gestational age (MD 1.00, 95% CI 0.01 to 1.99; 1 study, 203 babies; Analysis 1.7)
1.6. Analysis.
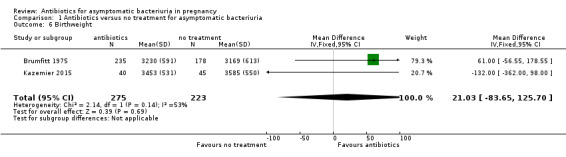
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 6 Birthweight.
1.7. Analysis.
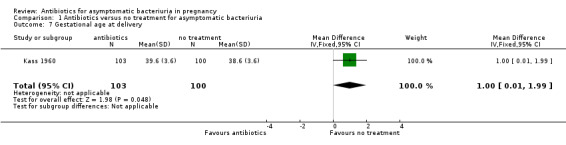
Comparison 1 Antibiotics versus no treatment for asymptomatic bacteriuria, Outcome 7 Gestational age at delivery.
None of the studies measured cost or women's satisfaction with the treatment.
2. Antibiotic treatment versus no treatment: subgroups by duration of treatment
There was a reduction in the incidence of pyelonephritis for all subgroups, regardless of duration of treatment (Analysis 1.1)
There were too few studies to make a meaningful interpretation of the effect of duration of treatment on the risk of preterm birth (Analysis 1.2).
On visual inspection of the graphs, it appeared that there was a difference in the incidence of low birthweight with duration of treatment although the interaction test did not suggest a difference (test for subgroup differences: Chi² = 2.82, df = 2 (P = 0.24), I² = 29.1%; Analysis 1.3), and there were too few studies to be confident that a longer duration of therapy was associated with a better outcome.
We judged that in all but one of the studies the overall risk of bias was low; therefore, we did not perform a sensitivity analysis based on risk of bias.
Discussion
Summary of main results
While the results of these studies are consistent, suggesting there may be a reduction in the incidence of pyelonephritis, low birthweight, and preterm birth with treatment of asymptomatic bacteriuria, these conclusions are based on low‐certainty evidence. There was significant heterogeneity observed among the studies, which may be explained by study design or quality, type of antibiotic used, and the changes in obstetrical practice in the past five decades between the earliest and the latest study. Duration of antibiotic treatment did not appear to explain any heterogeneity.
The overall incidence of pyelonephritis in the untreated group was 20%, but ranged from 2.2% to 36%. While different definitions of pyelonephritis could explain some of this variation, there may be other factors, for example, type of infecting organism, socioeconomic status, other care given in pregnancy, which if defined, could identify groups of women with asymptomatic bacteriuria with different risks of developing pyelonephritis. However, in the absence of this type of information, the presence of asymptomatic bacteriuria itself defines a population at risk of pyelonephritis.
Overall completeness and applicability of evidence
The studies reported here (with only three exceptions) date from the 1960s and 1970s; microbiological methodology for the diagnosis of bacteriuria has not significantly changed over this interval. Although not all of the antibiotics used in these studies remain available currently, and the use of tetracycline is now contraindicated in pregnancy, it is valid to assume that the results are applicable to other antibiotics active against urinary pathogens that are safe in pregnancy. A Cochrane Review of treatments for symptomatic urinary tract infections during pregnancy concluded that although antibiotic treatment is probably effective for the cure of urinary tract infections, there are insufficient data to recommend any specific regimen (Vazquez 2011); there were similar conclusions for the treatment of asymptomatic bacteriuria (Guinto 2010). The choice of a sulphonamide or sulphonamide‐containing combination, a penicillin, cephalosporin, fosfomycin, or nitrofurantoin, based on the results of susceptibility testing, may be appropriate regimens for the management of asymptomatic bacteriuria. However, increasing antibiotic resistance complicates the choice of empiric regimens, and can make it difficult to select an appropriate regimen (Assefa 2008; Enayat 2008; Hernandez Blas 2007; Tadesse 2014). In India, the presence of extended‐spectrum ß‐lactamases (ESBL), making the strain resistant to all penicillins and cephalosporins, was described in 47% of isolates of E coli, and 36.9% of isolates of Klebsiella pneumoniae (Rizvi 2011). However, a recent case‐control study from Israel reported no difference in obstetric outcomes between women with bacteriuria caused by ESBL versus non‐ESBL isolates of Enterobacteriaceae (Yagel 2018). Surveys of antibiotic susceptibility in pathogens causing community‐acquired uncomplicated urinary tract infections demonstrate considerable regional variation: resistance to ampicillin in E. coli in a survey of European countries and Canada averaged 29.8%, but was as high as 53.9% in Spain (Kahlmeter 2003). In the most recent study, 99% of the strains of E. coli from a low‐risk population of pregnant women in the Netherlands were sensitive to nitrofurantoin (Kazemier 2015),
Both continuous treatment and short‐course therapy strategies may show a benefit in the reduction of pyelonephritis. A small randomised study that compared intermittent therapy with continuous treatment suggested that both strategies may be equally effective (Whalley 1977). While short‐course therapy of asymptomatic bacteriuria has become accepted practice, the optimal duration of treatment is unknown; a three to seven day treatment regimen is currently recommended (Widmer 2011).
There may be an association between antibiotic treatment and preterm birth (low‐certainty evidence), but only three studies reported this outcome, one of which only included women with group B streptococcus bacteriuria. Although we chose to combine data from the three studies, given the very different populations and interventions, the effect of treatment on preterm birth is very uncertain. While preterm births are associated with low birthweight, some low birthweight infants are small‐for‐gestational age as a consequence of intrauterine growth retardation, for which there are many possible etiologies. The reduction in the incidence of low birthweight with antibiotic treatment of asymptomatic bacteriuria is consistent with current theories about the role of infection as a cause of adverse pregnancy outcomes, but a greater understanding of the basic mechanisms by which the treatment of bacteriuria could lead to a reduction in low birthweight is required. Prevention of pyelonephritis, which in studies conducted prior to the availability of effective antimicrobial therapy was associated with preterm birth, may be a factor, but treatment of bacteriuria with antibiotics may also eradicate organisms colonizing the cervix and vagina that are associated with adverse pregnancy outcomes. The relationship between genital infections, such as bacterial vaginosis, and preterm labor was not recognised when most of these studies on the treatment of asymptomatic bacteriuria were originally designed.
Quality of the evidence
We assessed and rated the quality of the evidence for the three primary outcomes using GRADEpro GDT software and the GRADE approach. See Table 1. We rated the evidence for pyelonephritis as low certainty; the certainty of the evidence was downgraded by important design limitations leading to a high risk of bias (including lack of allocation concealment and blinding) and inconsistency (heterogeneity in the results and important differences in the population and intervention). We rated the evidence for preterm birth and for birthweight less than 2500 g as low certainty. For preterm birth, there were important differences in the population and intervention, and in one of the studies contributing data, important design limitations. For birthweight less than 2500 g, there were important design limitations (including lack of allocation concealment and blinding) and differences in the intervention.
Many of the studies only contributed data to one or two of the outcomes; data are missing for most of the outcomes. Most studies did include the outcome of pyelonephritis. When these studies were being designed, there was already an awareness of a possible association between asymptomatic bacteriuria and low birthweight and preterm birth (Kass 1960a), but we found no explanation for why these outcomes were not systematically collected and reported in most of the trials. None of the studies systematically collected or reported the adverse effects of antibiotics. They neither systematically collected the incidence of allergic reactions, vaginal yeast infections, gastrointestinal side effects, or the development of bacterial resistance, nor did they systematically collect neonatal outcomes. While it is not possible to compare the benefits versus the disadvantages of antibiotic therapy from these studies, it is unlikely that the expected side effects from a short course of antibiotics would be significant, although increasingly, there are concerns about the effect of antibiotics on the human microbiome and the developing immune system.
Potential biases in the review process
The inclusion criteria for this review were broad, and included any antibiotic regimen, with the aim of being able to include all of the possible evidence, but this did lead to trials that differed in important ways, in respect to the treatment intervention, which could not be resolved by subgroup analysis.
We acknowledge that there was the potential for bias in the reviewing process. For the earliest iterations of this review, there was no predefined protocol, and the methods for including trials, extracting data, and assessing bias were not well described. However, we did address this in later versions of the review, by ensuring two authors independently assessed all the studies for inclusion, extracted data, and assessed risk of bias. But updates to the review cannot be done blinded to the knowledge of previous outcomes, and although the review has become more methodologically robust, there remains the potential to introduce subjective and unconscious biases.
Agreements and disagreements with other studies or reviews
Results of a meta‐analysis of 17 cohort studies showed an association between asymptomatic bacteriuria and low birthweight and preterm birth, but failed to resolve the question of whether or not asymptomatic bacteriuria was merely a marker for low socioeconomic status, which is associated with low birthweight (Romero 1989). The authors of a recent systematic review concluded there was no reliable evidence to support routine screening for asymptomatic bacteriuria, given the serious methodological shortcomings of the studies identified, and the low number of outcomes reported from the more recent, high‐quality study (Angelescu 2016). The recent publication from the Canadian Task Force on Preventive Health Care gave screening for asymptomatic bacteriuria in pregnancy a weak recommendation, based on very low‐quality evidence, because of the small but uncertain benefit, variation in women's values and preferences, and the judgment that harms were likely minimal (Moore 2018).
Authors' conclusions
Implications for practice.
Antibiotic treatment of asymptomatic bacteriuria may be indicated to reduce the risk of pyelonephritis in pregnancy, but the evidence is of low certainty. Both short course therapy and continuous treatment may reduce the incidence of pyelomephritis, but the evidence is of low certainty.
The optimal time to perform the urine culture is unknown; in these studies, the urine culture was performed at the first prenatal visit, but a single culture before 20 weeks may miss more than half of women with asymptomatic bacteriuria (McIsaac 2005).
In the studies included in this review, insufficient data were presented to determine the effectiveness of treatment to prevent recurrent bacteriuria; the studies did not specifically evaluate the effectiveness of a strategy of repeating a culture following treatment, and re‐treating as necessary.
Implications for research.
This review has identified several implications for research.
Incorporating risk factors for pyelonephritis in a screening algorithm
In an era when routine prenatal screening for asymptomatic bacteriuria was standard, women with pyelonephritis were more likely to be black or Hispanic, young, less educated, nulliparous, initiate prenatal care late, and smoke during pregnancy (Wing 2014). However, while some of these factors and other risk factors that are associated with asymptomatic bacteriuria may be amenable to interventions, or used to identify women at greater risk of an adverse outcome, there has been no evaluation of a screening algorithm that incorporates risk factors.
Understanding the pathogenesis of infection
A better understanding of the basic mechanisms by which treatment of asymptomatic bacteriuria could prevent low birthweight is required. Any study of the relationship between other infections and adverse outcomes of pregnancy needs to control for asymptomatic bacteriuria and its treatment, but it is unlikely that the particular contribution of asymptomatic bacteriuria to preterm birth and low birthweight will ever be conclusively determined.
The significance of lower colony counts and different urinary pathogens
The studies included in this review generally used a urine colony count of more than 100,000 bacteria/mL to identify participants. Although lower colony counts have been shown to be associated with active infection in other populations, their significance in pregnancy has not been established (Stamm 1982). Treatment of asymptomatic pregnant women with lower colony counts is not currently recommended, but further study of appropriate strategies to manage these women is warranted, and there is an ongoing trial studying this question (NCT03275623). Staphylococcus saprophyticus is a recognised cause of symptomatic infection in non‐pregnant women; however, the importance of this organism in asymptomatic pregnant women has not been established. While E. coli remains the predominant organism in most studies, the increasing prevalence of Proteus mirabilis and other Enterobacteriaceae, along with other Staphylococcus spp., suggests different variables may be influencing the epidemiology of bacteriuria in developing countries (Nicolle 2014).
Studies now show that the urinary tract is not sterile; the role of the maternal urinary microbiome and organisms not detected by traditional culture methods in the outcomes of pregnancy is an interesting area of new research (Kalinderi 2018).
Urine screening tests: methods, timing and frequency
Quantitative urine culture of a midstream or clean‐catch urine is the gold standard for detecting asymptomatic bacteriuria in pregnancy, but this test is expensive, and may not always be available in all clinical settings. Although rapid urine screening tests, for example, urine microscopy and urine dipstick, have not been shown to perform satisfactorily in this population, their use may be cost‐beneficial (Rouse 1995). Any new urine screening test that is developed needs to be evaluated in the context of screening for asymptomatic bacteriuria of pregnancy.
None of these studies adequately addressed the most appropriate time to perform the initial screening culture, how often to repeat a negative culture, or how best to monitor women initially treated for asymptomatic bacteriuria. There is a need to define the appropriate frequency of follow‐up cultures and re‐treatment strategies.
Adherence to guidelines
Despite almost uniform national guidelines, there is little evidence of adherence to screening recommendations. In Australia, poor adherence with screening for asymptomatic bacteriuria in indigenous communities has been proposed as one explanation for worse pregnancy outcomes in this population; a structural problem related to provision of care in remote communities was identified as the cause (Bookallil 2005). Screening rates from 1% to 96% were reported in a pilot survey of quality indicators of antenatal care in the UK (Vause 1999). There is an opportunity to evaluate screening for asymptomatic bacteriuria as a measure of quality of care, and gain a better understanding of the implementation of screening policies for asymptomatic bacteriuria in low‐income countries.
Cost‐effectiveness
While there are no new data to indicate that women should not be screened for asymptomatic bacteriuria, it is difficult to estimate accurately the cost‐effectiveness of screening without up‐to‐date information on the prevalence of asymptomatic bacteriuria, and a more accurate estimate of the reduction in pyelonephritis, low birthweight, and preterm births with treatment. A Health Technology Assessment report from the UK on screening to prevent preterm birth estimated that antibiotic treatment for all women without any testing was the most cost‐effective option for preventing birth before 37 weeks; however, they did not take into account the potential side effects of antibiotics or issues, such as resistance, and the conclusions were based on low‐quality evidence associating treatment with a reduction in preterm births (Honest 2009). There needs to be prospective evaluation of cost‐effective diagnostic algorithms, that include risk factors and up‐to‐date outcomes, in different populations.
Research in low‐risk populations
Despite the demonstrated association between antibiotic treatment and the prevention of pyelonephritis, there is an opportunity for research to provide better quality data to inform the management of asymptomatic bacteriuria. Kazemier 2015 performed a carefully designed randomised, placebo‐controlled trial in a low risk group of pregnant women, and although they enrolled 248 women with asymptomatic bacteriuria in their cohort, only 95 (33%) were enrolled in the randomised controlled trial of treatment, limiting the generalisability of the results, and compromising the power of the study (Nicolle 2015). Further, well‐designed clinical trials could provide useful information on alternative management strategies and adverse events of treatment. The majority (66%) of women with asymptomatic bacteriuria in Kazemier 2015 declined to participate in the randomised trial because they did not want to receive antibiotics for an asymptomatic condition; further research should explore these low‐risk women's values and preferences regarding treatment of asymptomatic bacteriuria. Preventing inappropriate and unnecessary antibiotic use has become an important community‐wide goal, giving researchers the impetus to produce high‐quality evidence that could identify women with asymptomatic bacteriuria in whom antibiotic treatment may not be necessary.
Feedback
Fenton, September 2015, 28 September 2015
Summary
Hi, I was just entering search uncertainties with this review in UK DUETs, and was looking at the ongoing studies. Should this one study now be in awaiting assessment as it has completed and has been submitted for publication?
Kind regards,
Mark Fenton
NICE, UK DUETs
Reply
Cochrane Editorial Office, 29 September 2015
Many thanks for your feedback. Yes we agree that this study should have been assigned to the 'studies awaiting classification' section of the review rather than the 'ongoing studies' section. We amended the review and moved the study to the 'studies awaiting classification' section. This study, Kazemier 2012, which was published in full in August 2015, will be assessed as part of the next update.
Contributors
Mark Fenton, NICE, UK DUETS
Cochrane Pregnancy and Childbirth Editorial Office
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2018 | New search has been performed | Review updated. One new study added (Kazemier 2015). |
| 4 November 2018 | New citation required but conclusions have not changed | Overall conclusions are unchanged. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 29 September 2015 | Amended | In response to feedback, we have moved the study Kazemier 2012a, from the ongoing section to studies awaiting classification. |
| 19 March 2015 | New citation required but conclusions have not changed | Overall conclusions unchanged, but quality of the evidence in support of an effect of antibiotics for the primary outcomes rated as low to very low. |
| 19 March 2015 | New search has been performed | We updated the search and identified four new studies; two references to a single study were excluded because they did not meet the inclusion criteria (Rafalskiy 2013), one was another reference to a previously included study (Elder 1971), and one was a reference to an ongoing study (Kazemier 2012). Methods and 'Risk of bias' table updated. A 'Summary of findings' table was incorporated. The World Health Organization's definition of prematurity of less than 37 weeks has been used. |
| 1 September 2008 | Amended | Converted to new review format. |
| 31 January 2007 | New search has been performed | We updated the search and identified two new studies. One additional study (Elder 1966) has been included and another excluded (Mohammad 2002). We have moved the LeBlanc 1964 study to the excluded studies because this study did not meet the inclusion criteria. |
| 31 January 2007 | New citation required but conclusions have not changed | This review has been extensively rewritten. Low birthweight has been separated from preterm birth as outcomes; subgroup and sensitivity analyses are described and heterogeneity of studies discussed. |
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
Each line was run separately
ICTRP
bacteriuria AND pregnancy
bacteriuria AND pregnant
ClinicalTrials.gov
Advanced search
Interventional Studies | Bacteriuria in Pregnancy
pregnant | Interventional Studies | Asymptomatic Bacteriuria
pregnancy | Interventional Studies | Asymptomatic Bacteriuria
pregnant | Interventional Studies | Bacteriuria
pregnancy | Interventional Studies | Bacteriuria
Data and analyses
Comparison 1. Antibiotics versus no treatment for asymptomatic bacteriuria.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Development of pyelonephritis | 12 | 2017 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.13, 0.41] |
| 1.1 Single dose | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.21, 0.92] |
| 1.2 Short course (3 to 7 days) | 4 | 568 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.11, 0.92] |
| 1.3 Intermediate course (3 to 6 weeks) | 2 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.08, 0.37] |
| 1.4 Continuous treatment | 5 | 843 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.04, 0.57] |
| 2 Preterm birth < 37 weeks | 3 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.13, 0.88] |
| 2.1 Single dose | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Short course (3 to 7 days) | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.05, 2.72] |
| 2.3 Intermediate course (3 to 6 weeks) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Continuous treatment | 1 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.14, 0.95] |
| 3 Birthweight < 2500 g | 6 | 1437 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.93] |
| 3.1 Single dose | 1 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 3.2 Short course (3 to 7 days) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Intermediate course (3 to 6 weeks) | 1 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.55, 2.14] |
| 3.4 Continuous treatment | 4 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.87] |
| 4 Persistent bacteriuria | 4 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.18, 0.53] |
| 5 Serious adverse neonatal outcome | 3 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.23, 1.79] |
| 6 Birthweight | 2 | 498 | Mean Difference (IV, Fixed, 95% CI) | 21.03 [‐83.65, 125.70] |
| 7 Gestational age at delivery | 1 | 203 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.01, 1.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brumfitt 1975.
| Methods | Placebo‐controlled; 2 parallel groups | |
| Participants | Inclusion criteria: 'significant' bacteriuria (clean‐catch urine) at first antenatal visit and 7 to 10 days later; microbiological criteria not stated Setting: London and Birmingham, UK | |
| Interventions | Sulphonamide (sulphormethoxine 2 g single dose) vs placebo (see Williams 1968 for description of treatment regimen) | |
| Outcomes | Low birthweight (< 2500 g); mean birthweight, mean gestational age Pyelonephritis (loin pain, fever or rigours; fever of at least 100 °F; > 100,000 bacteria/mL) | |
| Notes | Outcome of low birthweight (N = 425) Outcome of pyelonephritis in placebo group (55/179) Outcome of pyelonephritis reported for subset of treated women (N = 87): 0/45 successful treatment after 1 course, 4/22 successful after 2 courses; failed (persistent infection) 5/20. Data on persistent bacteriuria provided for treatment group only Outcome reported for women who developed anaemia during pregnancy (haemoglobin 10.2 g/100 mL or less at 32 weeks): 16.8% treated vs 25.9% placebo, P < 0.01 There is no explanation for the difference in numbers in the placebo group (reported as 179 for the outcome of pyelonephritis and 178 for other outcomes), nor the total number of participants, in any reports of this study. Dates of study: 1967 to 1968 (estimated) Funding sources: Board of Governors of the United Birmingham Hospitals and the Birmingham Regional Hospital Board Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The investigators do not describe the sequence generation process: "... were originally assigned to the placebo group ..." There is no description of how women were assigned to treatment or placebo. There is no explanation for the unequal numbers in the treatment and placebo groups. |
| Allocation concealment (selection bias) | Unclear risk | No information provided to judge |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "... were given placebo under double‐blind conditions". Method not described in sufficient detail. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "... were given placebo under double‐blind conditions". Method not described in sufficient detail. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Inconsistencies in total number of women not explained (number of < 2500 g babies provided for 413/426 bacteriuric women); results not provided for outcome of pyelonephritis for all women in treated group |
| Selective reporting (reporting bias) | High risk | Results not provided for outcome of pyelonephritis for all women allocated to treatment. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Unclear overall |
Elder 1966.
| Methods | Placebo‐controlled; 2 parallel groups | |
| Participants | Inclusion criteria: bacteriuria (same bacterial species in first 3 uncontaminated clean‐voided urine specimens, with 2 samples > 100,000 bacteria/mL and 1 sample > 10,000 bacteria/mL) Exclusion criteria: > 32 weeks' gestation Setting: Boston City Hospital, USA |
|
| Interventions | Sulfasymazine 0.5 g daily until delivery (N = 54) or placebo (N = 52) | |
| Outcomes | Persistent bacteriuria, after 3 weeks of treatment (13/52 treatment vs 48/50 in placebo group) and at last clinic visit before delivery (12/52 vs 30/49) | |
| Notes | 2 women were lost to follow‐up in the treatment group and 3 women lost to follow‐up in the placebo group and have not been included in the analysis 7/52 women in the placebo group developed asymptomatic pyelonephritis (not further defined and not included as an outcome) 1 adverse event reported in treatment group (vomiting); no rash, pruritus or photosensitivity; no newborn kernicterus diagnosed Dates of study: June 1965 to March 1966 Funding sources: Public Health Service grants HD‐01288 from the National Institutes of Health, and FR‐76 from the Division of Research Facilities and Resources, National Institutes of Health. Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... a random sequence"; insufficient information provided to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "... double‐blind trial"; no information provided to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "... double‐blind trial"; no information provided to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information provided on women lost to follow‐up, reasonably balanced between groups |
| Selective reporting (reporting bias) | High risk | Results not provided for outcome of pyelonephritis for all participants; no pregnancy outcomes (gestational age, birthweight) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Unclear overall |
Elder 1971.
| Methods | Placebo‐controlled; 2 parallel groups. Quasi‐RCT | |
| Participants | Inclusion criteria: bacteriuria (in clean‐voided specimen with 2 samples > 100,000 bacteria/mL and 1 sample > 10,000 bacteria/mL) at first prenatal visit Exclusion: > 32 weeks' gestation; previously treated for a urinary tract infection during the current pregnancy prior to their first obstetrical visit, delivered or aborted after registering but before first obstetric visit, went elsewhere for prenatal care after registering; did not deliver a singleton pregnancy Setting: Boston City Hospital, USA Number of participants: N = 281 |
|
| Interventions | Tetracycline 250 mg 4 times a day x 6 weeks (N = 133) vs identically appearing placebo taken similarly (N = 145). If infection did not clear, an alternative drug (usually nitrofurantoin) was given. | |
| Outcomes | Persistent bacteriuria (bacteriuria was said to have cleared if the colony count was less than 1000/mL on 2 successive cultures) up to the time of delivery; includes recurrences Pyelonephritis (fever with signs and symptoms localised to the urinary tract, without other explanation) Low birthweight (< 2500 g) Neonatal outcomes (neonatal deaths (respiratory distress syndrome, other respiratory causes, perforated ulcer), congenital malformations, birth trauma, infection, anaemia) Mean gestational age (38.46 weeks in treated group N = 107 vs 38.25 weeks in placebo group N = 122 (calculated from numbers in paper)) |
|
| Notes | Tetracycline associated with staining of teeth in one‐third of children. No women lost to follow‐up for outcome of pyelonephritis: 3 women (1%) lost to follow‐up for outcome of persistent bacteriuria and low birthweight. Outcome of persistent bacteriuria in placebo group does not include women who developed pyelonephritis. 7 women moved out of Boston and the outcome of their pregnancies is not known. 4 bacteriuric women delivered twins and are not included. Only live births included in outcome of low birthweight. Prematurity was defined as birthweight of < 2500 g regardless of gestational length. Dates of study: January 1963 to July 1965 Funding sources: National Institute of Child Health and Human Development (HD‐01288), National Institute of Allergy and Infectious Diseases (TO1 AI‐00068) and Division of Research Facilities and Resources (FR‐76‐02), National Institutes of Health. Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "...alternate bacteriuric ... were assigned". |
| Allocation concealment (selection bias) | High risk | Participants were allocated by alternation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "identical‐appearing placebo"; insufficient information provided to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "identical‐appearing placebo"; insufficient information provided to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided to judge |
| Selective reporting (reporting bias) | Unclear risk | Unable to judge; twin deliveries were excluded |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | High risk | Judged at overall high risk of bias. |
Foley 1987.
| Methods | Randomised trial, 2 parallel groups. | |
| Participants | Inclusion: bacteriuric (> 100,000 bacteria/mL x 1; midstream urine) at first prenatal visit.
Setting: Dublin, Ireland Number of participants: N = 220 |
|
| Interventions | Sulphamethizole 300 mg or nitrofurantoin 150 mg daily x 3 days (based on susceptibility of the organism); re‐treatment or maintenance treatment as necessary (N = 100). Control group received no treatment (N = 120) | |
| Outcomes | Persistent bacteriuria (at follow‐up, not defined further) Pyelonephritis, only reported as 'admitted with pyelonephritis', no definition provided | |
| Notes | Description of study provided in letter to editor; no publication Dates of study: 1985 Funding sources: not stated Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to treatment or no treatment by "toss of a coin". |
| Allocation concealment (selection bias) | Unclear risk | No information was provided to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No description of any attempt at blinding; not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of any attempt at blinding; not placebo‐controlled. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: 19%; no reasons provided for missing outcome data on these women |
| Selective reporting (reporting bias) | High risk | No pregnancy outcomes (gestational age, birthweight) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | High risk | Judged at overall high risk of bias |
Furness 1975.
| Methods | Randomised trial, 3 parallel groups | |
| Participants | Inclusion: bacteriuric (> 100,000 bacteria/mL x 1 or > 10,000 bacteria/mL x 2; midstream urine) at second antenatal visit Setting: South Australia Enrollment period: not stated Number of participants: N = 206 | |
| Interventions | Methenamine mandelate or methenamine hippurate 1 g, 4 times a day vs no treatment Treatment continued until delivery | |
| Outcomes | Pyelonephritis (frequency and burning on micturition, pyrexia, or loin tenderness and significant bacteriuria) Preterm birth (defined as less than or equal to 38 weeks' gestation); treatment 24/139 (17%) vs control 10/67 (15%) Mean birthweight: methenamine hippurate 3273 g SE ± 70.7; methenamine mandelate 3303 SE ± 68.2; control 3353 g SE ± 73.9; no difference Postnatal bacteriuria at 6 weeks: 26/73 treatment vs 10/27 no treatment |
|
| Notes | Women randomised to either methenamine mandelate (N = 69), methenamine hippurate (N = 70), or no treatment (N = 67); for analyses, treatment groups combined. Unable to separate incidence of pyelonephritis during pregnancy and puerperium; results combined. Dates of study: 1971 to 1972 (estimated) Funding sources: Queen Victoria Research Foundation, South Australia Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | " by random allocation"; no additional details provided to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | No information provided to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20/226 women withdrawn from trial; no details provided. All women included in outcome of pyelonephritis; 17% loss to follow‐up for outcome of low birthweight and gestational age at delivery. |
| Selective reporting (reporting bias) | High risk | Unable to separate incidence of pyelonephritis during pregnancy and puerperium; results combined. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | High risk | Judged at overall high risk of bias. |
Gold 1966.
| Methods | Placebo‐controlled, randomised trial; 2 parallel groups. Quasi‐RCT | |
| Participants | Inclusion criteria: bacteriuria (> 100,000 bacteria/mL x 2: midstream urine) at any prenatal visit Setting: New York, NY (85% non‐white) Number of participants: N = 65 |
|
| Interventions | Sulfadimethoxine 500 mg daily; sulphadiazine 1 g, 3 times a day after 36 weeks vs placebo Treatment continued until delivery |
|
| Outcomes | Persistent bacteriuria at delivery Pyelonephritis Preterm birth (not defined further): treatment group 2/35; placebo 0/30 No infants developed jaundice; no toxic manifestations in women in treatment group |
|
| Notes | Only antepartum episodes of pyelonephritis included in analysis. There were 2 postpartum episodes of pyelonephritis in the placebo group, none in treatment group. Dates of study: February 1962 to December 1964 Funding sources: Health Research Council of the City of New York (U‐1177); in‐kind support (antibiotic and placebo tablets) Hoffman‐La Roche Inc. Nutley, NJ Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women allocated to treatment based on study number: odd number treatment, even number control |
| Allocation concealment (selection bias) | High risk | Allocated to treatment based on study number |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled; no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It does not appear that there was any loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | No definition provided for prematurity. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Overall unclear |
Kass 1960.
| Methods | Placebo‐controlled trial, 2 parallel groups. Quasi‐RCT | |
| Participants | Inclusion: bacteriuric (> 100,000 bacteria/mL at first prenatal visit, confirmed x 2). Women were randomised after the second positive sample, but only included if the third sample was positive. Exclusion: > 32 weeks' gestation; chronic renal insufficiency Setting: Boston City Hospital, US (approximately 50% black) Number of participants: N = 214 (includes 11 women identified through Renal Clinic) |
|
| Interventions | Sulfamethoxypyridazine 500 mg daily with nitrofurantoin for failures (N = 103) or placebo tablet (N = 100) supplied by same manufacturer Treatment continued until term. |
|
| Outcomes | Pyelonephritis (dysuria, frequency, and flank pain, fever or chills); however, it was not clear that women were indeed febrile Low birthweight (< 2500 g); prematurity was defined as birthweight < 2500 g Long‐term persistence of bacteriuria (10 to 14 years): 18/63 treatment vs 18/71 placebo Mean gestational age: 39.6 ±3.6 SD for treated bacteriurics; 38.6 ± 3.6 SD for placebo bacteriurics There were 2 stillbirths, both in the placebo group; there were 5 neonatal deaths in the placebo group, and no neonatal deaths in the treatment group. |
|
| Notes | For outcome of low birthweight, results are given for total number of deliveries (3 twin deliveries in placebo group vs none in treated group). There are several publications related to this study; where there is a discrepancy in methodology, the most detailed description was used. Dates of study: October 1956 to April 1960 Funding sources: National Institute of Child Health and Human Development, National Institutes of Health, United States Public Health Service (HD‐01288); National Institute of Allergy and Infectious Diseases (TOI AI‐00068); Massachusetts Heart Association Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Alternate women received a placebo". |
| Allocation concealment (selection bias) | High risk | Allocation was based on alternation: "Alternate women received a placebo". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used, and "the nature of the treatment was not know to the patient or to the attending obstetrical staff". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although a placebo was used, there are no further details provided to know whether the outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 women, initially identified, were not enrolled, either because they were > 32 weeks before treatment could be started (N = 30), or they had already received treatment for symptomatic infection (N = 10). Loss to follow‐up: 23 (11%) for outcomes of pyelonephritis and low birthweight; no details provided. 69 (34%) for long‐term persistence of bacteriuria |
| Selective reporting (reporting bias) | High risk | 3 women had a subsequent pregnancy in the study period and were reassigned to their original treatment group and included in the analysis. In 5 patients in the placebo group, it was assumed they had symptomatic disease but no symptoms were documented. Not all women in the symptomatic group were confirmed to have fever and women treated for infections other that in the urinary tract were also included in the symptomatic group if they were found to have cleared their bacteriuria. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Overall unclear |
Kazemier 2015.
| Methods | Randomised, placebo‐controlled trial, 2 parallel groups, embedded within multicentre prospective cohort study | |
| Participants | Inclusion: women 18 years or older, singleton pregnancy, between 16 and 22 weeks of pregnancy, without symptoms of urinary tract infection Positive urine dipslide (≥ 1 x 10⁵ CFU/mL of a single organism or when 2 organisms were present, 1 had concentration of ≥ 1 x 10⁵ CFU/mL) Exclusion: women with history of preterm delivery before 34 weeks, warning signs of an imminent preterm delivery, fetal congenital malformations, antibiotic use within 2 weeks of screening, known G6PD deficiency, hypersensitivity to nitrofurantoin, risk factors for complicated urinary tract infection (e.g. diabetes, immunosuppression, abnormalities of the urinary tract) Setting: 8 hospitals and 5 ultrasound centres, The Netherlands Number of participants: 85 |
|
| Interventions | Nitrofurantoin 100 mg twice daily for 5 consecutive days (N = 40) or identical placebo capsules (N = 45) Women whose follow‐up dipslide 1 week after the end of treatment was persistently positive were given a further course of nitrofurantoin or matching placebo at the same dose and schedule according to their original allocation group, repeated for a maximum of 2 rounds of treatment. |
|
| Outcomes | Primary outcomes: pyelonephritis, defined as hospital admission with at least 2 of the following: fever (≥ 38.0 °C), symptoms of pyelonephritis (nausea, vomiting, chills, and costovertebral tenderness) and a positive urine culture. Delivery before 34 weeks' gestation (treatment 1, placebo 0) Secondary outcomes: adverse neonatal outcome, neonatal death before discharge, or severe neonatal morbidity (presence of 1 or more of the following: severe respiratory distress syndrome, bronchopulmonary dysplasia, periventricular leukomalacia > grade 1, intracerebral haemorrhage > grade 2, necrotising enterocolitis > grade 1, or proven sepsis) Other outcomes: neonatal birthweight (treatment mean (SE) 3453 g (84), placebo 3585 g (82)), time to delivery, spontaneous preterm birth between 32 and 37 weeks, admission to the neonatal intensive care unit, and maternal morbidity including urinary tract infection (clinical report of a urinary tract infection treated with antibiotics); treatment 4, placebo 8 |
|
| Notes | This study was a multicentre prospective cohort study with an embedded randomised trial. In the final cohort of 4283 women screened for asymptomatic bacteriuria, 248 were asymptomatic bacteriuria‐positive: 40 were randomly assigned to nitrofurantoin, 45 to placebo, and 163 women refused to be enrolled into the randomised component of the study and were followed without treatment. Only the women randomised to treatment or placebo have contributed data to this review, and only outcomes that were reported separately for the treatment and placebo groups have been included. Details of the uropathogen were provided for the ASB‐positive women who received nitrofurantoin: 29/36 were E. coli, 5 Staphylococcus spp, 1 Acinetobacter, and 1 'other' Dates of study: October 2011 to June 2013 Funding sources: ZonMw (the Netherlands Organisation for Health Research and Development) Declarations of interest: Ben WJ Mol received fees from ObsEva, Ferring and Merck; other authors declared no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was based on a web‐based application with a computer generated list with random block sizes of 2, 4, or 6 participants rendered by an independent data manager" |
| Allocation concealment (selection bias) | Low risk | Allocation performed by an independent data manager |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women received 100 mg capsules of nitrofurantoin or identical appearing capsules of placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Women, treating physicians, and researchers remained unaware of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 randomised patients communicated by laboratory as ASB‐positive were strictly ASB‐negative (1 yeast, 3 uropathogens at low colony count, 1 contaminated dipslide) included in intention‐to‐treat analysis (4 nitrofurantoin, 1 placebo) |
| Selective reporting (reporting bias) | Low risk | The study protocol was published and the study's prespecified comes were reported as specified. |
| Other bias | Low risk | This has been judged as low risk. Of 248 women with asymptomatic bacteriuria, 163 refused to be randomised to treatment or no treatment and may have introduced an unknown bias. |
| Overall Risk of Bias | Low risk | Overall low |
Kincaid‐Smith 1965.
| Methods | Randomised, 'double‐blind' placebo‐controlled; 2 parallel groups | |
| Participants | Inclusion criteria: bacteriuria (> 100,000 bacteria/mL x 2, mid‐stream urine) at first antenatal visit (< 26 weeks). Women with bacteriuria on the first sample that was not confirmed on the second sample were enrolled and results analysed separately. Setting: Melbourne, Australia Number of participants: N = 145 |
|
| Interventions | Sulphamethoxydiazine 500 mg daily or sulphadimidine 1 g, 3 times a day (after 30 weeks) (N = 61)) vs placebo (N = 55) Treatment continued until delivery Ampicillin or nitrofurantoin given if organism known to be resistant |
|
| Outcomes | Pyelonephritis (loin pain or tenderness, with or without pyrexia and rigours, with or without dysuria and frequency) Preterm birth (birthweight < 2500 g) Fetal loss: after 28 weeks 4/61 (6.6%) in treatment group and 4/56 (7.2%) in placebo group Bacteriuria long term: (6 weeks to 3 months after delivery (N = 101) 9/51 treatment vs 18/50 placebo; 6 months after delivery (N = 43) 6/26 treatment vs 6/17 placebo |
|
| Notes | 29/145 women randomised to treatment but bacteriuria not confirmed on second culture; not included in outcomes reported for this analysis Results for incidence of pyelonephritis and prematurity also provided for women who had bacteriuria at first visit, which was not confirmed on second sample (11/72 in treatment group, 18/73 in placebo group) Dates of study: 1964 to 1965 Funding sources: Felton Bequest Committee; Schering A.G. (Berlin), also provided antibiotic tablets and matching placebo; Beecham Research Laboratories (antibiotic capsules and matching placebo); National Heatlh and Medical Research Council of Australia Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process. |
| Allocation concealment (selection bias) | Low risk | "a code of instructions to the pharmacist ensured that the trial remained double‐blind despite .... alterations in therapeutic regimen." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "a code of instructions to the pharmacist ensured that the trial remained double‐blind despite .... alterations in therapeutic regimen." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "a code of instructions to the pharmacist ensured that the trial remained double‐blind despite .... alterations in therapeutic regimen." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 240 women initially identified as bacteriuric; no information available on 55 women randomised to treatment (treatment allocation not provided) but not included in the analysis because of poor compliance (attended infrequently or failed to take tablets continuously). For outcome of long‐term persistence of bacteriuria (at 6 months), only 43 women were available for follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided to judge |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Overall unclear |
Little 1966.
| Methods | Placebo‐controlled, randomised; 2 parallel groups | |
| Participants | Inclusion criteria: bacteriuria (> 100,000 bacteria/mL x 2, midstream urine) at first prenatal visit Setting: London, England Number of participants: N = 265 |
|
| Interventions | Sulphamethoxypyridazine 500 mg or (later) nitrofurantoin 100 mg daily continued until 6 weeks after delivery; ampicillin or nitrofurantoin were alternatives for failures (N = 124) or placebo (N = 141) | |
| Outcomes | Pyelonephritis (loin pain and tenderness, fever and > 100,000 bacteria/mL) Low birthweight (< 2500 g) |
|
| Notes | Dates of study: 1962 to 1965 Funding sources: Dan Mason Research Foundation of the West London Medical Trust; Smith Kline and French Laboratories, Eaton Laboratories, Park Davis and Co, Beecham Research Laboratories, Cerebos Ltd and the Ockley Brick Works Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about sequence generation to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Allocation to treatment or control was drawn from "a pool of sealed envelopes containing a slip of paper", but there was no information provided to ensure appropriate safeguards to prevent investigators being aware of treatment group. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Women in the control group "were given placebo"; no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided to judge whether outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Overall unclear |
Mulla 1960.
| Methods | Randomised trial, 2 parallel groups | |
| Participants | Inclusion: bacteriuria at 30 to 32 weeks; microbiological criteria not stated Setting: Ohio, USA Number of participants: N = 100 |
|
| Interventions | Sulfadimethoxine 250 mg twice a day x 7 days, repeated if bacteriuria persisted (N = 50) vs no treatment (N = 50) | |
| Outcomes | Pyelonephritis (criteria for diagnosis not given; described as "acute symptoms of cystopyelitis"). | |
| Notes | Half (13/26) infections developed postpartum; only antepartum infections included in analysis No side effects necessitating discontinuation of treatment Dates of study: not stated Funding sources: not stated Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Women were "randomly divided into two groups"; no other details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not placebo‐controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided to judge whether outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | No definition for outcome of "cystopyelitis"; no pregnancy outcomes (gestational age, birthweight) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Judged at overall high risk of bias. |
Pathak 1969.
| Methods | Placebo‐controlled; 2 parallel groups | |
| Participants | Inclusion: bacteriuria (> 100,000 bacteria/mL x 2) Exclusion: > 24 weeks' gestation; BP > 130/90 mmHg Setting: Kingston, Jamaica |
|
| Interventions | Nitrofurantoin 100 mg twice a day x 3 weeks; 400 mg in 4 doses for further 4 days for those who did not respond (6 women), (N = 76) vs identical appearing placebo (N = 76) | |
| Outcomes | Persistence of bacteriuria (at end of pregnancy); pyelonephritis (criteria not described) Postpartum bacteriuria (3 to 9 months): 6/24 treatment vs 16/45 placebo |
|
| Notes | 12/88 women in treatment group and 14/90 in control group not included in analysis (treated for positive treponemal serology N = 21; defaulted from clinic N = 5) Rates for preterm birth/fetal loss only presented by bacteriuric status, not treatment group Rates for postpartum bacteriuria available for 69 women Dates of study: not stated Funding sources: Norwich Pharmacal Company, Norwich, New York; National Institutes of Health, U.S. Public Health service (R 01‐HD 02139‐05) Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "on a random basis". Insufficient information provided to permit further judgement. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided to permit judgement. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced; reasons similar and unlikely to have introduced bias. |
| Selective reporting (reporting bias) | High risk | No pregnancy outcomes (gestational age, birthweight) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Unclear overall |
Thomsen 1987.
| Methods | Randomised, placebo‐controlled trial; 2 parallel groups | |
| Participants | Inclusion: positive midstream urine culture for group B streptococcus at 27 to 31 weeks' gestation Setting: University Hospital, Denmark Number of participants: N = 69 |
|
| Interventions | Penicillin 10 million IU 3 times a day x 6 days, retreated if repeat cultures positive (N = 37) or placebo tablets (N = 32) | |
| Outcomes | Preterm birth (< 37 weeks' gestation) Mean gestational age (39.6 weeks in treatment group (N = 37) vs 36.2 weeks in placebo group (N = 32)) |
|
| Notes | All mothers positive for group B streptococcus at delivery and their babies were treated with antibiotics Dates of study: October 1984 to October 1986 Funding sources: Leo Pharmaceutical Products (tablets); Nunc, Denmark (equipment) Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly allocated" but no description of sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled, described as "double‐blind" but no additional details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as "double blinded" but no specific information provided to ensure outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | Unclear risk | Overall unclear |
Williams 1969.
| Methods | Randomised trial; 2 parallel groups | |
| Participants | Inclusion: bacteriuria (> 100,000 bacteria/mL x 2, midstream urine) at first antenatal visit Setting: University Hospital, Cardiff, Wales Number of subjects: N = 163 |
|
| Interventions | Sulphadimidine 1 g, 3 times a day x 7 days, nitrofurantoin 100 mg twice a day, or ampicillin 250 mg, 3 times a day x 7 days for failures (N = 85), or no treatment. For no treatment group, sulphadimidine 1 g, 3 times a day x 7 days if symptoms presented (frequency, dysuria, fever, or loin pain), (N = 78) |
|
| Outcomes | Pyelonephritis (loin pain with tenderness or fever, or both); includes postpartum infection (n = 6) | |
| Notes | No loss to follow‐up Dates of study: 1967 Funding sources: United Cardiff Hospitals Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated at random"; no additional information provided to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | No information provided to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; outcome may have been influenced by lack of blinding. No treatment group was given antibiotics to take if symptoms of infection developed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding; assessment of outcome (pyelonephritis) may have been influenced by knowledge of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No explanation for unequal group sizes; no information provided on any missing data. An unknown number of women in the control group (no treatment) were given antibiotic treatment if they developed symptoms of urinary tract infection. |
| Selective reporting (reporting bias) | High risk | No pregnancy outcomes (gestational age, birthweight) |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | High risk | Judged at high risk of bias |
Wren 1969.
| Methods | 2 parallel groups. Quasi‐RCT | |
| Participants | Inclusion: bacteriuria (midstream urine) x 2 at initial antenatal visits; microbiological criteria not stated Setting: University Hospital, New South Wales, Australia Number of participants: N = 183 |
|
| Interventions | Nitrofurantoin 100 mg twice a day x 2 weeks, then ampicillin 250 mg every 6 hours x 1 week, then sulphurazole 500 mg every 6 hours x 4 weeks, then nalidixic acid 500 mg every 6 hours x 2 weeks; repeat until 1 to 6 weeks after delivery (N = 83), or no treatment (N = 90) | |
| Outcomes | Preterm birth (< 37 weeks) or low birthweight (< 2500 g) | |
| Notes | There were no stillbirths or neonatal deaths in the treated group; 6 in the no treatment group Dates of study: November 1965 to December 1968 Funding sources: Smith Kline and French Laboratories, Beecham Laboratoies, Roche Products, Winthrop Laboratories Declarations of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Women "were divided into two groups, alternate patients being treated". |
| Allocation concealment (selection bias) | High risk | Women "were divided into two groups, alternate patients being treated". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; knowledge of treatment group may have influenced outcome; women in untreated group who developed clinical urinary tract infection (33/90) were given antibiotics at the choice of the obstetrician, continued to delivery in over 50% of cases. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding; however, outcome of birthweight unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 women not included in outcomes: 2 sets of twins excluded, 6 women moved and only 2 could be traced, 3 women delivered before antibiotics could be started, 1 woman refused treatment |
| Selective reporting (reporting bias) | High risk | Outcome of pyelonephritis not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
| Overall Risk of Bias | High risk | Judged as high risk of bias |
Please attend closely to the study period for patient enrolment (found under 'Method'); in several instances there were significant delays between the enrolment period and the published report.
ASB: asymptomatic bacteriuria BP: blood pressure CFU: colony forming units G6PD: glucose‐6‐phosphate‐dehydrogenase IU: international unit RCT: randomised controlled trial SD: standard deviation SE standard error vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Calderon‐Jaimes 1989 | This was a prospective study where 986 pregnant women with asymptomatic bacteriuria were "divided in 2 groups", a treatment group that received nitrofurantoin 100 mg daily for 10 days and a control group. There is no further description of how the women were allocated to treatment or no treatment. The authors could not be contacted to provide clarification of the method of allocation and whether the women had been allocated to treatment group by a random or quasi‐random method. |
| LeBlanc 1964 | In this study, all pregnant women with bacteriuria (N = 129), including those with urinary symptoms at the time of presentation, were randomised to 1 of 4 groups: no drug or 1 of 3 different antibiotic regimens that was continued until term at a prophylactic dose, once the culture had become negative. A group of patients with a history of urinary tract infection but negative cultures were also placed on randomised drug therapy. Results for the outcomes of pyelonephritis and prematurity for the group of women with asymptomatic bacteriuric (as compared to symptomatic infection or history of infection) were not provided separately. For the outcome of pyelonephritis, in the no treatment group, the outcome for women who were not treated and women who discontinued treatment were combined. |
| Mohammad 2002 | This was an observational study. 1661 pregnant women were screened for bacteriuria by urine culture, for an overall prevalence of significant growth of 1.9%. There was no treatment intervention reported. |
| NCT03274960 | The intervention in this study of pregnant women is screening for asymptomatic bacteriuria versus no screening, rather than randomising the women to treatment or no treatment for asymptomatic bacteriuria. In this study, set in a low‐resource setting in Zimbabwe, pregnant women presenting before 22 weeks' gestation will be randomised either to the control group and receive usual current antenatal services, which do not include any screening for asymptomatic bacteriuria, or to the intervention group. The intervention group will be screened for asymptomatic bacteriuria each trimester, using the Griess test (for the detection of nitrites in the urine), confirmed with culture, and symptomatic women will be treated. The number of preterm births between the 2 groups will be compared. |
| Rafalskiy 2013 | In this study, pregnant women with asymptomatic bacteriuria (N = 112) were randomised to treatment with cefixime or amoxicillin/clavulanate. There was no "no treatment" group. |
| Sanderson 1984 | In this study, 44 pregnant women with bacteriuria were treated with pivmecillinam, subsequently, 30 out of 33 women in whom treatment was successful were randomised to low dose pivmecillinam for 3 months, or no treatment. The group randomised to treatment, or no treatment did not represent the whole population of pregnant women with asymptomatic bacteriuria, but a subset of women in whom treatment had been successful. |
Characteristics of ongoing studies [ordered by study ID]
NCT03275623.
| Trial name or title | Management of sub‐clinical bacteriuria in pregnancy: a feasibility trial |
| Methods | Randomised, open label; 2 parallel groups |
| Participants | Pregnant women, without symptoms of urinary tract infection, with a low level of bacteria (less than 100,000 colony forming units/mL). Exclusion criteria include risk factors for complicated urinary tract infection, use of immunosuppressive drugs, abnormalities of the urinary tract, history of renal disease, and urine culture with > 100,000 CFU/mL of any organism. |
| Interventions | Standard prenatal care plus either nitrofurantoin, cephalexin, or amoxicillin (choice determined by the physician) or no treatment |
| Outcomes | Number of women with cystitis (defined as urine culture with > 100,000 CFU/mL); number of women with pyelonephritis (urine culture with > 100,000 CFU/mL with fever) |
| Starting date | September 2017 |
| Contact information | Akwugo A Eziefule, MD University of Texas Health Center at Houston Houston, Texas, USA 77030 Telephone 713‐500‐6421 Akwugo.A.Eziefule@uth.tmc.edu |
| Notes | Follow‐up for the last patient enrolled is planned for February 2019 |
Differences between protocol and review
Review substantially rewritten to incorporate current methodology. Primary and secondary outcomes reclassified; definition of prematurity changed to less than 37 weeks; adverse outcomes systematically collected. Discussion rewritten; GRADE tool used to produce a 'Summary of findings' table.
We added an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports.
Contributions of authors
Fiona Smaill had the major responsibility for the preparation of this review. Dr Vazquez reviewed drafts of the review and provided suggestions for revisions. Both review authors assessed studies for inclusion, carried out data extraction, and undertook the GRADE assessments for the 'Summary of findings' table.
Sources of support
Internal sources
McMaster University, Canada.
Hamilton Health Sciences, Canada.
Instituto Nacional de Endocrinologia (INEN), Cuba.
External sources
No sources of support supplied
Declarations of interest
Fiona M Smaill: none known
Juan C Vazquez: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Brumfitt 1975 {published data only}
- Brumfitt W. The effects of bacteriuria in pregnancy on maternal and fetal health. Kidney International 1975;8:S113‐9. [PubMed] [Google Scholar]
- Condie AP, Williams JD, Reeves DS, Brumfitt W. Complications of bacteriuria in pregnancy. Urinary Tract Infection, Proceedings of the First National Symposium. London: Oxford University Press, 1968:148‐59.
- Williams JD, Reeves DS, Condie AP, Franklin ISN, Leigh DA, Brumfitt W. The treatment of bacteriuria in pregnancy. In: O'Grady F, Brumfitt W editor(s). Urinary Tract Infection, Proceedings of the First National Symposium. London: Oxford University Press, 1968:160‐9. [Google Scholar]
Elder 1966 {published data only}
- Elder HA, Santamarina BAG, Smith SA, Kass EH. Use of sulfasymazine in the treatment of bacteriuria of pregnancy. Antimicrobial Agents and Chemotherapy 1966;6:142‐8. [PubMed] [Google Scholar]
Elder 1971 {published data only}
- Elder HA, Santamarina BA, Smith SA, Kass EH. Excess prematurity in tetracycline‐treated bacteriuric patients whose infection persisted or returned. Antimicrobial Agents and Chemotherapy 1967;7:101‐9. [PubMed] [Google Scholar]
- Elder HA, Santamarina BAG, Smith S, Kass EH. The natural history of asymptomatic bacteriuria during pregnancy: the effect of tetracycline on the clinical course and the outcome of pregnancy. American Journal of Obstetrics and Gynecology 1971;111:441‐62. [DOI] [PubMed] [Google Scholar]
Foley 1987 {published data only}
- Foley ME, Farquharson R, Stronge JM. Is screening for bacteriuria in pregnancy worthwhile?. British Medical Journal 1987;295:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furness 1975 {published data only}
- Furness ET, McDonald PJ, Beasley NV. Urinary antiseptics in asymptomatic bacteriuria of pregnancy. New Zealand Medical Journal 1975;81:417‐9. [PubMed] [Google Scholar]
Gold 1966 {published data only}
- Gold EM, Traub FB, Daichman I, Terris M. Asymptomatic bacteriuria during pregnancy. Obstetrics & Gynecology 1966;27:206‐9. [PubMed] [Google Scholar]
Kass 1960 {published data only}
- Kass EH. Pyelonephritis and bacteriuria. A major problem in preventive medicine. Annals of Internal Medicine 1962;56:46‐53. [DOI] [PubMed] [Google Scholar]
- Kass EH. The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn EL, Kass EH editor(s). Biology of Pyelonephritis. Boston: Little Brown and Company, 1960:399‐412. [Google Scholar]
- Savage WE, Hajj SN, Kass EH. Demographic and prognostic characteristics of bacteriuria in pregnancy. Medicine (Baltimore) 1967;46:385‐407. [DOI] [PubMed] [Google Scholar]
- Zinner SH, Kass EH. Long term (10 to 14 years) follow‐up of bacteriuria of pregnancy. New England Journal of Medicine 1971;285:820‐4. [DOI] [PubMed] [Google Scholar]
Kazemier 2015 {published data only}
- Kazemier BM, Koningstein FN, Schneeberger C, Ott A, Bossuyt PM, Miranda E, et al. Maternal and neonatal consequences of treated and untreated asymptomatic bacteriuria in pregnancy: a prospective cohort study with an embedded randomised controlled trial. Lancet Infectious Diseases 2015;15(11):1324‐33. [DOI] [PubMed] [Google Scholar]
- Kazemier BM, Schneeberger C, Miranda E, Wassenaer A, Bossuyt PM, Vogelvang TE, et al. Costs and effects of screening and treating low risk women with a singleton pregnancy for asymptomatic bacteriuria, the ASB study. BMC Pregnancy and Childbirth 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NL2921. ASB treat study: costs and effects of screening and treating healthy women for asymptomatic bacteriuria. www.trialregister.nl/trial/2921 (first received 5 September 2011). [NTR3068]
Kincaid‐Smith 1965 {published data only}
- Kincaid‐Smith P, Bullen M. Bacteriuria in pregnancy. Lancet 1965;1:1382‐7. [DOI] [PubMed] [Google Scholar]
Little 1966 {published data only}
- Little PJ. The incidence of urinary infection in 5000 pregnant women. Lancet 1966;2:925‐8. [DOI] [PubMed] [Google Scholar]
Mulla 1960 {published data only}
- Mulla N. Bacteriuria in pregnancy. Obstetrics and Gynecology 1960;16:89‐92. [PubMed] [Google Scholar]
Pathak 1969 {published data only}
- Pathak UN, Tang K, Williams LL, Stuart KL. Bacteriuria of pregnancy: results of treatment. Journal of Infectious Diseases 1969;120(1):91‐5. [DOI] [PubMed] [Google Scholar]
Thomsen 1987 {published data only}
- Thomsen AC, Morup L, Brogaard Hansen K. Antibiotic elimination of group‐B streptococci in urine in prevention of preterm labour. Lancet 1987;1:591‐3. [DOI] [PubMed] [Google Scholar]
Williams 1969 {published data only}
- Williams GL, Campbell H, Davies KJ. Urinary concentrating ability in women with asymptomatic bacteriuria in pregnancy. British Medical Journal 1969;3:212‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wren 1969 {published data only}
- Wren BG. Subclinical renal infection and prematurity. Medical Journal of Australia 1969;2:596‐600. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Calderon‐Jaimes 1989 {published data only}
- Calderon‐Jaimes EC, Arredondo‐Garcia JLA, Olvera‐Salinas JO, Echaniz‐Aviles GE, Conde‐Gonzalez CC, Hernandez‐Nevarez PH. Urinary infection prevention during pregnancy in patients with asymptomatic bacteriuria. Ginecologia y Obstetricia de Mexico 1989;57:90‐7. [PubMed] [Google Scholar]
LeBlanc 1964 {published data only}
- LeBlanc AL, McGanity WJ. The impact of bacteriuria in pregnancy ‐ a survey of 1300 pregnant patients. Biologie Medicale 1964;22:336‐47. [PubMed] [Google Scholar]
Mohammad 2002 {published data only}
- Mohammad M, Mahdy ZA, Omar J, Maan N, Jamil MA. Laboratory aspects of asymptomatic bacteriuria in pregnancy. Southeast Asian Journal of Tropical Medicine and Public Health 2002;33(3):575‐80. [PubMed] [Google Scholar]
NCT03274960 {published data only}
- NCT03274960. The effect of screening and treatment of asymptomatic bacteriuria every trimester during pregnancy on incidence of preterm birth in Harare, Zimbabwe. clinicaltrials.gov/ct2/show/NCT03274960 (first received 7 September 2017). [NCT03274960]
Rafalskiy 2013 {published data only}
- Rafal'skii VV, Dovgan' EV, Kozyrev IuV, Gustovarova TA, Khlybova SV, Novoselova AV, et al. The efficacy and safety of cefixime and amoxicillin/clavulanate in the treatment of asymptomatic bacteriuria in pregnant women: a randomized, prospective, multicenter study. Urologiia 2013;5(24):26‐8. [PubMed] [Google Scholar]
- Rafalskiy V, Dovgan E, Kozyrev Y, Gustovarova T, Khlybova S, Novoselova A, et al. Cefixime vs. amoxicillin/clavulanate in pregnant women with asymptomatic bacteriuria: Multicentre randomised study. Clinical Microbiology and Infection 2012;18(Suppl s3):425‐6. [Google Scholar]
Sanderson 1984 {published data only}
- Sanderson P, Menday P. Pivmecillinam for bacteriuria in pregnancy. Journal of Antimicrobial Chemotherapy 1984;13:383‐8. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03275623 {published data only}
- NCT03275623. Management of sub‐clinical bacteriuria in pregnancy: a feasibility trial. clinicaltrials.gov/ct2/show/NCT03275623 (first received 7 September 2017). [NCT03275623]
Additional references
Abdel‐Aziz 2017
- Abdel‐Aziz EM, Barnett‐vanes A, Dabour MF, Cheng F. Prevalence of undiagnosed asymptomatic bacteriuria and associated risk factors during pregnancy: a cross‐sectional study at two tertiary centres in Cairo, Egypt. BMJ Open 2017;7(3):e013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ajayi 2012
- Ajayi AB, Nwabuisi C, Aboyeji AP, Ajayi NS, Fowotade A, Fakeye OO. Asymptomatic bacteriuria in antenatal patients in Ilorin, Nigeria. Oman Medical Journal 2012;27(1):31‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Akerele 2001
- Akerele J, Abhulimen P, Okonofua F. Prevalance of asymptomatic bacteriuria among pregnant women in Benin City, Nigeria. Journal of Obstetrics and Gynaecology 2001;21(2):141‐4. [DOI] [PubMed] [Google Scholar]
Allen 2012
- Allen VM, Yudin MH, Bouchard C, Boucher M, Caddy S, Castillo E, et al. Management of group B streptococcal bacteriuria in pregnancy. Journal of Obstetrics and Gynaecology Canada: JOGC 2012;34(5):482‐6. [DOI] [PubMed] [Google Scholar]
Amiri 2009
- Amiri FN, Rooshan MH, Ahmady MH, Soliamani MJ. Hygiene practices and sexual activity associated with urinary tract infection in pregnant women. East Mediterrean Health Journal 2009;15(1):104‐10. [PubMed] [Google Scholar]
Angelescu 2016
- Angelescu K, Nussbaumer‐Streit B, Sieben W, Scheibier F, Gartlehner G. Benefits and harms of screening for and treatment of asymptomatic bacteriuria in pregnancy: a systematic review. BMC Pregnancy Childbirth 2016;16(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Assefa 2008
- Assefa A, Asrat D, Woldeamanuel Y, G/Hiwot Y, Abdella A, Melesse T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia. Ethiopian Medical Journal 2008;46(3):227‐35. [PubMed] [Google Scholar]
Awoleke 2015
- Awoleke JO, Adanikin AI, Ajayi DD, Ayosanmi OS. Predictors of asymptomatic bacteriuria among pregnant women in a low‐resource setting. Journal of Obstetrics and Gynaecology 2015;35(1):25‐9. [DOI] [PubMed] [Google Scholar]
Awolude 2010
- Awolude OA, Adesina OA, Oladokun A, Mutiu WB, Adewole IF. Asymptomatic bacteriuria among HIV positive pregnant women. Virulence 2010;1(3):130‐3. [DOI] [PubMed] [Google Scholar]
Bachman 1993
- Bachman JW, Heise RH, Naessens JM, Timmerman MG. A study of various tests to detect asymptomatic urinary tract infections in an obstetric population. JAMA 1993;270:1971‐4. [PubMed] [Google Scholar]
Bandyopadhyay 2005
- Bandyopadhyay S, Thakur JS, Ray P, Kumar R. High prevalence of bacteriuria in pregnancy and its screening methods in north India. Journal of the Indian Medical Association 2005;103(5):259‐62, 266. [PubMed] [Google Scholar]
Blencowe 2012
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A‐B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012;379:2161‐72. [DOI] [PubMed] [Google Scholar]
Bookallil 2005
- Bookallil M, Chalmers E, Andrew B. Challenges in preventing pyelonephritis in pregnant women in Indigenous communities. Rural and Remote Health 2005;5(3):395. [PubMed] [Google Scholar]
Celen 2011
- Celen S, Orus AS, Karavalcin R, Saygan S, Unlu S, Polat B, et al. Asymptomatic bacteriuria and antibacterial susceptibility patterns in an obstetric population. ISRN Obstetrics and Gynecology 2011;2011:721872. [DOI: 10.5402/2011/721872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenstein 1988
- Eisenstein BI, Jones GW. The spectrum of infections and pathogenic mechanisms of Escherichia coli. Advances in Internal Medicine 1988;33:231‐52. [PubMed] [Google Scholar]
Enayat 2008
- Enayat K, Fariba F, Bahram N. Asymptomatic bacteriuria among pregnant women referred to outpatient clinics in Sanandaj, Iran. International Brazilian Journal of Urology 2008;34(6):699‐704. [DOI] [PubMed] [Google Scholar]
Ezechi 2013
- Ezechi OC, Gab‐Okafor CV, Oladele DA, Kalejaiye OO, Oke BO, Ekama SO, et al. Prevalence and risk factors of asymptomatic bacteriuria among pregnant Nigerians infected with HIV. Journal of Maternal‐fetal & Neonatal Medicine 2014;26(4):402‐6. [DOI] [PubMed] [Google Scholar]
Farkash 2012
- Farkash E, Weintraub AY, Sergienko R, Wiznitzer A, Zlotnik A, Sheiner E. Acute antepartum pyelonephritis in pregnancy: a critical analysis of risk factors and outcomes. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2012;162(1):24‐7. [DOI] [PubMed] [Google Scholar]
Fatima 2006
- Fatima N, Ishrat S. Frequency and risk factors of asymptomatic bacteriuria during pregnancy. Journal of the College of Physicians and Surgeons Pakistan 2006;16(4):273‐5. [PubMed] [Google Scholar]
Garingalo‐Molina 2000
- Garingalo‐Molina FD. Asymptomatic bacteriuria among pregnant women: overview of diagnostic approaches. Philippine Journal of Microbiology and Infectious Diseases 2000;29:177‐86. [Google Scholar]
Goldenberg 2000
- Goldberg RL, Hauth JC, Andrews WW. Mechanisms of disease: intrauterine infection and preterm delivery. New England Journal of Medicine 2000;342(20):1500‐7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed Jan 27, 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guinto 2010
- Guinto VT, Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007855.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haider 2010
- Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. Journal of Pakistan Medical Association 2010;60(3):213‐6. [PubMed] [Google Scholar]
Heitmann 2013
- Heitmann K, Nordeng H, Holst L. Pregnancy outcome after use of cranberry in pregnancy ‐ the Norwegian Mother and Child cohort study. BMC Complementary and Alternative Medicine 2013;13:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez Blas 2007
- Hernandez Blas F, Lopez Carmona JM, Rodriguez Moctezuma JR, Peralta Pedrero ML, Rodriguez Gulierrez RS, Ortiz Aguirre AR. Asymptomatic bacteriuria frequency in pregnant women and uropathogen in vitro antimicrobial sensitivity. Ginecologia y Obstetricia de Mexico 2007;75(6):325‐31. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2005
- Hill JB, Sheffield JS, McIntire DD, Wendel GD. Acute pyelonephritis in pregnancy. Obstetrics and Gynecology 2005;105(1):18‐23. [DOI] [PubMed] [Google Scholar]
Honest 2009
- Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technology Assessment 2009;13(43):1‐627. [DOI: 10.3310/hta13430] [DOI] [PubMed] [Google Scholar]
Ipe 2013
- Ipe DS, Sundas L, Benjamin WH, Moore KH, Ulett GC. Asymptomatic bacteriuria: prevalence rates of causal microorganisms, etiology of infection in different patient populations, and recent advances in molecular detection. FEMS Microbiology Letters 2013;346:1‐10. [DOI] [PubMed] [Google Scholar]
Kahlmeter 2003
- Kahlmeter G. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. Journal of Antimicrobial Chemotherapy 2003;51:69‐76. [DOI] [PubMed] [Google Scholar]
Kalinderi 2018
- Kalinderi K, Delkos D, Kalinderis M, Athanasiadid A, Kalogiannidis I. Urinary tract infection during pregnancy: current concepts on a common multifaceted problem. Journal of Obstetrics and Gynaecology 2018;38(4):448‐53. [DOI] [PubMed] [Google Scholar]
Kass 1960a
- Kass EH. The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn EL, Kass EH editor(s). Biology of Pyelonephritis. Boston: Little, Brown and Co, 1960:399‐412. [Google Scholar]
Kazemier 2012
- Kazemier BM, Schneeberger C, Miranda E, Wassenaer A, Bossuyt PM, Vogelvang TE, et al. Costs and effects of screening and treating low risk women with a singleton pregnancy for asymptomatic bacteriuria, the ASB study. BMC Pregnancy and Childbirth 2012;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovavisarach 2009
- Kovavisarach E, Vichaipruck M, Kanjarahareutai S. Risk factors related to asymptomatic bacteriuria in pregnant women. Journal of Medical Association of Thailand 2009;92(5):606‐10. [PubMed] [Google Scholar]
Lavigne 2011
- Lavigne J‐P, Boutet‐Dubois A, Laouini D, Combescure C, Bouziges N, Mares P, et al. Virulence potential of Escherichia coli strains causing asymptomatic bacteriuria during pregnancy. Journal of Clinical Microbiology 2011;49(11):3950‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIsaac 2005
- McIsaac W, Carroll JC, Biringer A, Bernstein P, Lyons E, Low DE, et al. Screening for asymptomatic bacteriuria in pregnancy. Journal of Obstetrics and Gynaecology Canada: JOGC 2005;27(1):20‐4. [DOI] [PubMed] [Google Scholar]
McNair 2000
- McNair RD, MacDonald SR, Dooley SL, Peterson LR. Evaluation of the centrifuged and Gram‐stained smear, urinalysis, and reagent strip testing to detect asymptomatic bacteriuria in obstetric patients. American Journal of Obstetrics and Gynecology 2000;182(5):1076‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meis 1995
- Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, et al. Factors associated with preterm birth in Cardiff, Wales. I. Univariable and multivariable analysis. American Journal of Obstetrics and Gynecology 1995;173(2):590‐6. [DOI] [PubMed] [Google Scholar]
Meis 1995a
- Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, et al. Factors associated with preterm birth in Cardiff, Wales. II. Indicated and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 1995;173(2):597‐602. [DOI] [PubMed] [Google Scholar]
Mignini 2009
- Mignini L, Carroli G, Abalos E, Widmer M, Amigot S, Nardin JM, et al. Accuracy of diagnostic tests to detect symptomatic bacteriuria during pregnancy. Obstetrics and Gynecology 2009;113(2 (Pt 1)):346‐52. [DOI] [PubMed] [Google Scholar]
Moore 2018
- Moore A, Doull M, Grad R, Groulx S, Pottie K, Tonelli M, et al. Recommendations on screening for asymptomatic bacteriuria in pregnancy. CMAJ 2018;190(27):E823‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolle 2014
- Nicolle LE. Asymptomatic bacteriuria. Current Opinion in Infectious Diseases 2014;27(1):90‐6. [DOI] [PubMed] [Google Scholar]
Nicolle 2015
- Nicolle LE. Management of asymptomatic bacteriuria in pregnant women. Lancet Infectious Diseases 2015;15:1252‐4. [DOI] [PubMed] [Google Scholar]
NL2921
- NL2921. ASB treat study: costs and effects of screening and treating healthy women for asymptomatic bacteriuria. www.trialregister.nl/trial/2921 (first received 5 September 2011). [NTR3068]
Olamijulo 2016
- Olamijulo JA, Adewale CO, Olaleye O. Asymptomatic bacteriuria among antenatal women in Lagos. Journal of Obstetrics and Gynaecology 2016;36(6):722‐5. [DOI] [PubMed] [Google Scholar]
Pastore 1999
- Pastore LM, Savitz DA, Thorp JM. Predictors of urinary tract infection at the first prenatal visit. Epidemiology 1999;10(3):282‐7. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rizvi 2011
- Rizvi M, Khan F, Shukla I, Malik A, Shaheen MA. Rising prevalence of antimicrobial resistance in urinary tract infections during pregnancy: necessity for exploring newer treatment options. Journal of Laboratory Physicians 2011;3(2):98‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogozińska 2016
- Rogozińska E, Formina S, Zamora J, Mignini L, Khan KS. Accuracy of onsite tests to detect asymptomatic bacteriuria in pregnancy: a systematic review and meta‐analysis. Obstetrics and Gynecology 2016;128:495‐502. [DOI] [PubMed] [Google Scholar]
Romero 1989
- Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC. Meta‐analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstetrics and Gynecology 1989;73:577‐82. [PubMed] [Google Scholar]
Romero 2014
- Romero A, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345(6198):760‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rouse 1995
- Rouse DJ, Andrews WW, Goldenberg RL, Owen J. Screening and treatment of asymptomatic bacteriuria of pregnancy to prevent pyelonephritis: a cost‐effectiveness and cost‐beneficial analysis. Obstetrics and Gynecology 1995;86:119‐23. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Sheiner 2009
- Sheiner E, Mazor‐Drey E, Levy A. Asymptomatic bacteriuria during pregnancy. Journal of Maternal‐fetal & Neonatal Medicine 2009;22(5):423‐7. [DOI] [PubMed] [Google Scholar]
Sobel 1995
- Sobel JD, Kaye D. Urinary tract infections. In: Mandell GL, Bennett JE, Dolin R editor(s). Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 4th Edition. New York: Churchill Livingstone, 1995:662‐90. [Google Scholar]
Stamm 1982
- Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. New England Journal of Medicine 1982;307:463‐8. [DOI] [PubMed] [Google Scholar]
Stenqvist 1987
- Stenqvist K, Sandberg T, Lidin‐Janson G, Orskov F, Orskov L, Svanborg‐Eden C. Virulence factors of Escherichia coli in urinary isolates from pregnant women. Journal of Infectious Diseases 1987;156(6):870‐7. [DOI] [PubMed] [Google Scholar]
Su 2009
- Su SB, Wang JN, Lu CW, Wang HY, Guo HR. Prevalence of urinary tract infections and associated factors among pregnant workers in the electronics industry. International Urogynecology Journal and Pelvic Floor Dysfunction 2009;20(8):939‐45. [DOI] [PubMed] [Google Scholar]
Tadesse 2014
- Tadess E, Teshome M, Merid Y, Kibret B, Shimelis T. Asymptomatic urinary tract infection among pregnant women attending the antenatal clinic of Hawassa Referral Hospital, Southern Ethiopia. BMC Research Notes 2014;17(7):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tugrul 2005
- Tugrul S, Oral O, Kumru P, Kose D, Alkan A, Yildirim G. Evaluation and importance of asymptomatic bacteriuria in pregnancy. Clinical and Experimental Obstetrics and Gynecology 2005;32(4):237‐40. [PubMed] [Google Scholar]
Turck 1962
- Turck M, Goff BS, Petersdorf RG. Bacteriuria in pregnancy; relationship to socioeconomic factors. New England Journal of Medicine 1962;266:857‐60. [DOI] [PubMed] [Google Scholar]
Vause 1999
- Vause S, Maresh M. Indicators of quality of antenatal care: a pilot study. British Journal of Obstetrics and Gynaecology 1999;106:197‐205. [DOI] [PubMed] [Google Scholar]
Vazquez 2011
- Vazquez JC, Abalos E. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD002256.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wadland 1989
- Wadland WC, Plante DA. Screening for asymptomatic bacteriuria in pregnancy. A decision and cost analysis. Journal of Family Practice 1989;29:372‐6. [PubMed] [Google Scholar]
Whalley 1967
- Whalley P. Bacteriuria of pregnancy. American Journal of Obstetrics and Gynecology 1967;97:723‐38. [DOI] [PubMed] [Google Scholar]
Whalley 1977
- Whalley PJ, Cunningham FG. Short‐term versus continuous antimicrobial therapy for asymptomatic bacteriuria in pregnancy. Obstetrics and Gynecology 1977;49:262‐5. [PubMed] [Google Scholar]
Widmer 2010
- Widmer TA, Theron G, Grove D. Prevalence and risk factors of asymptomatic bacteriuria among HIV positive women. South African Journal of Epidemiology and Infection 2010;25(1):28‐32. [Google Scholar]
Widmer 2011
- Widmer M, Gulmezoglu AM, Mignini L, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000491.pub2] [DOI] [PubMed] [Google Scholar]
Williams 1968
- Williams JD, Reeves DS, Condie AP, Franklin ISN, Leigh DA, Brumfitt W. The treatment of bacteriuria in pregnancy. In: O'Grady F, Brumfitt W editor(s). Urinary Tract Infection, Proceedings of the First National Symposium. London: Oxford University Press, 1968:160‐9. [Google Scholar]
Wing 2008
- Wing DA, Rumney PJ, Preslicka CW, Chung JH. Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. Journal of Urology 2008;180(4):1367‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 2014
- Wing DA, Fassett MJ, Getahun D. Acute pyelonephritis in pregnancy: an 18 year retrospective analysis. American Journal of Obstetrics and Gynecology 2014;210(3):219. [DOI] [PubMed] [Google Scholar]
Yagel 2018
- Yagel Y, Natiy H, Riesenberg K, Nesher L, Saidel‐Odes L, Smolyakov R. Outcomes of UTI and bacteriuria caused by ESBL vs. non‐ESBL enterobacteriaceae isolates in pregnancy: a matched case‐control study. Epidemiology and Infection 2018;146(6):771‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Smaill 1993
- Smaill F. Antibiotic vs no treatment for asymptomatic bacteriuria. [revised 22 April 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C editor(s). Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Smaill 2001
- Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD000490] [DOI] [PubMed] [Google Scholar]
Smaill 2007
- Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000490.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smaill 2015
- Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD000490.pub3] [DOI] [PubMed] [Google Scholar]


