Abstract
Introduction
Rheumatoid arthritis (RA) and periodontitis share risk factors and inflammatory pathways that could be related to cytokines, such as interleukin (IL)-6, IL-8, IL-17A, and tumour necrosis factor-α (TNF-α). The aim of this study was to compare periodontal status and salivary levels of selected cytokines between patients diagnosed with RA and periodontitis. RA patients were assessed for the potential influence of anti-rheumatic therapy.
Material and methods
One hundred and six patients were enrolled in a cross-sectional study. Medical assessment and periodontal examination were performed in 35 patients with chronic periodontitis, in 35 patients with RA and chronic periodontitis, and in 36 controls. Unstimulated whole saliva samples were analysed for IL-6, IL-8, IL-17A, and TNF-α.
Results
Significant differences in biomarkers and periodontal parameters were found among groups. Study groups exhibited higher mean pocket depth (PD), number of PD > 4 mm, and mean clinical attachment loss, when compared with controls. The RA group had lower bleeding on probing index and PD, but higher values of plaque indices than the periodontitis group. Concentration of evaluated cytokines were higher in the RA and periodontitis groups, compared with controls. The periodontitis group showed also higher levels of IL-6, IL-17A, and TNF-α in comparison to RA. RA patients were treated with disease-modifying anti-rheumatic drugs (DMARDs) and glucocorticosteroids.
Conclusions
Salivary levels of IL-6, IL-8, IL-17A, and TNF-α can be affected by periodontitis, RA, and presumably DMARDs. DMARD therapy appears to reduce destructive and inflammatory processes in periodontal tissues because lower values of PD, BOP, and salivary levels of IL-6, IL-17A, and TNF-α were found in RA.
Keywords: periodontitis, rheumatoid arthritis, salivary diagnostics, interleukin 6, interleukin 8, interleukin 17A, tumour necrosis factor α
Introduction
Periodontitis is a chronic destructive inflammatory condition that affects soft and hard tissues surrounding and supporting the teeth. According to recent epidemiological studies, periodontitis is a social disease with high occurrence among the middle-aged and elderly population [1]. Prevalence of periodontitis has been associated with other systemic conditions such as type II diabetes, cardiovascular diseases, osteoporosis, premature birth, and low birth weight [2]. Recent studies indicate a possible link between periodontitis and rheumatoid arthritis (RA), which is supported by case-control [3, 4], prospective cohort [5, 6], and cross-sectional studies [7, 8]. Individuals with RA were found to be at increased risk of developing periodontitis in two independent meta-analyses [9, 10]. Even though this association might be based on shared environmental and genetic risk factors, such as smoking [11, 12] and genetic factors [13-15], a possible causal relation was advocated by interventional studies [16-19].
The diagnosis of periodontitis is currently based on clinical and radiological findings. This methodology is relatively time-consuming and may be subject to error due to lack of inter- and intra-examiner reproducibility [20]. This led clinicians to search for additional chair side measures to aid in early diagnosis and monitoring. Studies showed that oral fluids, such as gingival crevicular fluid (GCF) and saliva, can be used for this purpose [21-23]. The use of saliva is appealing because it has the advantage of being non-invasive and easy to collect, with potential use for point-of-care analysis [24].
Systemic inflammatory conditions may influence levels of certain salivary biomarkers because saliva contains serum-derived components, which may tamper with periodontal diagnostics. The imbalance between pro-inflammatory and anti-inflammatory cytokines is a common feature of both RA and periodontitis, which might result in the inflammatory destruction of specific tissues. Studies have shown that certain salivary cytokines levels or biomarkers might be elevated in individuals with both periodontitis and RA [25-27].
Interleukin (IL)-6 is the most abundantly expressed cytokine in synovium in individuals with RA, and together with IL-8 might play a key role in development of the disease [28, 29]. It has been demonstrated that by inducing endothelial cells to produce IL-8 and to activate expression of adhesion molecules and recruit leukocytes to involved joints, IL-6 was involved in local joint inflammation [30]. Both IL-6 and IL-8 significantly contributed to periodontitis; IL-6 has shown several biological activities such as B-lymphocyte differentiation, T-lymphocyte proliferation, and the stimulation of immunoglobulin secretion [31].
IL-8 is a potent chemoattractant cytokine and activator of neutrophils in inflammatory regions, which can be released from endothelial cells, gingival fibroblasts, neutrophils, monocytes, and phagocytes in the gingival crevice [32]. IL-6 and IL-8 levels seem to correlate with the status of periodontium and react to periodontal treatment [33, 34].
Tumour necrosis factor α (TNF-α) is a potent macrophage-derived cytokine that presents in inflamed synovium and gingiva during the course of RA and periodontitis [35, 36]. TNF-α induces expression of matrix-degrading proteases, prostanoids, IL-1, IL-6, IL-8, and granulocyte-macrophage colony stimulating factor [37]. Concentration of TNF-α in saliva correlated with periodontal status [38, 39].
IL-17A secreting helper CD4 T cells (Th17 cells) acts on neutrophils, macrophages, fibroblasts, and osteoclasts to induce an inflammatory reaction in bone and cartilage. IL-17A provokes an inflammatory reaction by itself, but it is equally capable of synergising with TNF-α in the expression of IL-1, IL-6, and IL-8 [40]. It was suggested that Th17 cells and IL-17A could play a crucial role in the progression of both RA and periodontitis [41, 42].
As found in previous reports, both periodontitis and RA might be associated with the elevation of certain biomarkers in saliva, the aim of this study was to evaluate the influence of rheumatoid arthritis and periodontitis on salivary concentrations of selected cytokines: IL-6, IL-8, IL-17A, and TNF-α. Furthermore, all cytokines as well as periodontal and rheumatic parameters were analysed to determine significant correlations. RA patients were assessed for any potential influence of anti-rheumatic therapy on salivary cytokines or periodontal status.
Material and methods
Qualification of patients
One hundred and six patients were enrolled in this cross-sectional study performed at the Department of Periodontology and Oral Mucosa Diseases, Medical University of Warsaw and the Department of Rheumatology, National Institute of Geriatrics, Rheumatology and Rehabilitation. Thirty-five randomly selected patients with diagnosed severe chronic periodontitis (periodontitis group – PG) based on the criteria defined by the American Academy of Periodontology [43], 35 randomly selected individuals with diagnosis of severe chronic periodontitis and seropositive RA (rheumatoid arthritis group – RAG) according to the ACR/EULAR criteria [44], and 36 randomly selected non-periodontitis systemically healthy control patients (control group – CG) were introduced to the study. However, it should be underlined that taking into consideration the new classification system of periodontal diseases, all of the periodontitis cases involved in this study would be categorised as periodontitis Stage III or IV Grade B [45]. The study received the positive approval by the institutional review board. All clinical procedures were carried out in accordance with the Helsinki Declaration of 1975, as revised in Tokyo in 2004. Written, informed consent forms were signed by every patient.
The inclusion criteria for the study were: 1) the presence of at least 15 teeth (excluding third molars) and 2) age between 18 and 75 years. Additional criteria for PG were: 1) the presence of at least two teeth with pocket depth (PD) ≥ 5 mm and interproximal clinical attachment loss (CAL) ≥ 5 mm in each quadrant, 2) bleeding on probing (BOP) ≥ 30%, 3) evidence of radiographic alveolar bone loss ≥ 5 mm at ≥ 30%; and for RAG: 1) mild or severe disease activity according to disease activity score (DAS28) [46], 2) treatment with classic disease-modifying anti-rheumatic drugs (DMARDs). CG patients had: 1) no sites with PD ≥ 5 mm, 2) no sites with CAL ≥ 2 mm, and 3) no evident radiographic alveolar bone loss. The exclusion criteria were as follows: 1) no periodontal treatment during the 12 months prior to the study, 2) taking local or systemic antibiotics in the previous three months, 3) active smoking or smoking within the previous five years, 4) diagnosis of any systemic disease that may modulate the course of periodontal disease or affect the systemic inflammatory response (excluding RA), and 5) pregnancy and lactation.
Clinical evaluation
The clinical examination included measurements at six points within each tooth (three lingual/palatal: mesial, central, and distal and three buccal: mesial, central, and distal). The clinical examination was performed by one calibrated examiner after collection of saliva and included evaluation of bleeding on probing (BOP) according to Ainamo and Bay protocol [47], plaque index (PI) according to O’Leary et al. [48], approximal plaque index (API) according to Lange et al. [49], PD, and CAL. Measurements were carried out with a manual periodontal probe (PCPUNC 15, Hu-Friedy, USA), and the reference point for evaluation of clinical attachment loss was the cementoenamel junction (CEJ). If it was not possible to locate the CEJ, the apical margin of the restoration or prosthetic crown was assumed as the reference point.
The examination of the joints in patients in the RAG was carried out by one rheumatologist and included an assessment of the number of painful and swollen joints, as well as a global assessment of the patient’s disease. The patient assessed global disease activity on the visual analogue scale. Data on current treatment with DMARDs and steroids (their type and dose) were collected. In addition, the levels of C-reactive protein, erythrocyte sedimentation rate, rheumatic factor, and cyclic citrullinated peptide antibodies were measured.
Saliva collection
Five millilitres of whole unstimulated saliva was collected from all patients. The material was collected in the morning (between 08.00 and 12.00), at least two hours after the last meal, according to the technique described by Navazesh [50]. The collected samples were frozen at –80°C, and their analysis was performed within a period not exceeding six months from the moment of freezing.
Cytokine analysis
Luminex xMAP technology for multiplexed quantification of cytokines in the saliva samples was used. The multiplexing analysis was performed using the Bio-Plex Luminex™ 200 system (Bio-Rad Laboratories, Hercules, CA, USA). Cytokines were simultaneously measured using a Bio-Plex Pro Human Cytokine Assay (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed using Statistica 13.1 software (StatSoft Inc., Tulsa, USA). Compatibility with the normal distribution of data obtained was assessed with the Shapiro-Wilk test. For normally distributed groups one-way analysis of variance (ANOVA) was used to compare differences among groups. The Tukey test was used in conjunction with ANOVA to find which means were significantly different from one another. Kruskal-Wallis test was used to compare differences among groups that were not normally distributed, with the Bonferroni test used to find which ranks were significantly different from one another. Correlations were based on Spearman’s and Pearson’s rank correlation coefficient. Values of p < 0.05 were considered statistically significant.
Results
A total of 106 patients (35 in the PG, 35 in the RAG, and 36 in the CG) were included in the study. The demographics and clinical parameters of all groups are shown in Table 1. The mean ages for the three groups were similar, but they differed in terms of clinical parameters. In a comparison between PG and RAG significantly higher values of BOP and PD were observed in the former, but PI and API values were higher in RAG. The mean CAL values were similar. The RAG in contrast to the CG had significantly higher values of PI and API as well as PD, PD ≥ 4 mm and CAL. When compared with controls, PG had similar values of PI and API, but significantly higher values of BOP, PD, PD ≥ 4 mm and CAL. The CG had a significantly higher mean number of teeth than the other groups.
Table 1.
Comparison of study groups
| Characteristic | PG (n = 35) | RAG (n = 35) | CG (n = 36) | p-value |
|---|---|---|---|---|
| Age (years, mean ±SD) | 59.31 ±9.75 | 55.66 ±10.56 | 53.92 ±10.96 | NS |
| Females (%) | 48.57 | 85.71 | 75.00 | 0.00023* |
| Number of teeth (mean ±SD) | 21.94 ±4.07 | 20.69 ±5.93 | 25.51 ±2.20 | 0.0001** |
| BOP (%, mean ±SD) | 63.59 ±19.52 | 53.55 ±17.32 | 50.29 ±15.65 | 0.0054* |
| PI (%, mean ±SD) | 50.00 ±20.50 | 74.28 ±18.69 | 48.49 ±16.74 | < 0.0001*** |
| API (%, mean ±SD) | 64.84 ±25.07 | 90.03 ±15.33 | 52.15 ±19.97 | < 0.0001*** |
| PD (mm, mean ±SD) | 3.87 ±0.80 | 3.14 ±0.54 | 2.46 ±0.35 | < 0.0001**** |
| PD ≥ 4 (n, mean ±SD) | 45.65 ±32.50 | 31.37 ±19.35 | 4.25 ±2.52 | < 0.0001** |
| CAL (mm, mean ±SD) | 3.68 ±1.15 | 3.12 ±1.25 | 1.81 ±0.61 | < 0.0001** |
API – approximal plaque index, BOP – bleeding on probing, CAL – clinical attachment loss, CG – control group, NS – not statistically significant, PD – pocket depth, PG – periodontitis group, PI – plaque index, RAG – rheumatoid arthritis group
applies to comparisons of periodontitis vs. RA or control groups
applies to comparisons of periodontitis or RA vs. control group
applies to comparisons of RA vs. periodontitis or control groups
applies to comparisons between all groups
The characteristics of RAG patients are presented in Table 2. The most commonly used DMARD was methotrexate (MTX), followed by sulfasalazine, leflunomide, and hydroxychloroquine. Seventeen of the RA patients, in addition to DMARDs, were on glucocorticoids with a mean dose of 6.35 ±4.14 mg of methylprednisolone.
Table 2.
Characteristics of rheumatoid arthritis patients
| Characteristic | Value |
|---|---|
| DAS28 (mean ±SD) SDAI (mean ±SD) severe disease activity (n, %) |
5.74 ±1.42 32.52 ±15.88 23 (71.87) |
| Positive for RF (n, %) Positive for ACPA (n, %) |
28 (80) 32 (91.43) |
| DMARDs (mean, n ±SD) methotrexate (n, %) sulfasalazine (n, %) leflunomide (n, %) hydroxychloroquine (n, %) |
1.19 ±0.60 22 (62.86) 5 (17.5) 5 (17.5) 5 (17.5) |
| Glucocorticoids (n, %) dose (mg of methylprednisolone, mean ±SD) |
17 (48.57) 6.35 ±4.14 |
ACPA – antibodies to citrullinated peptides, DAS28 – Disease Activity Score, DMARDs – disease-modifying antirheumatic drugs, RF – rheumatoid factor, SDAI – Simple Disease Activity Index
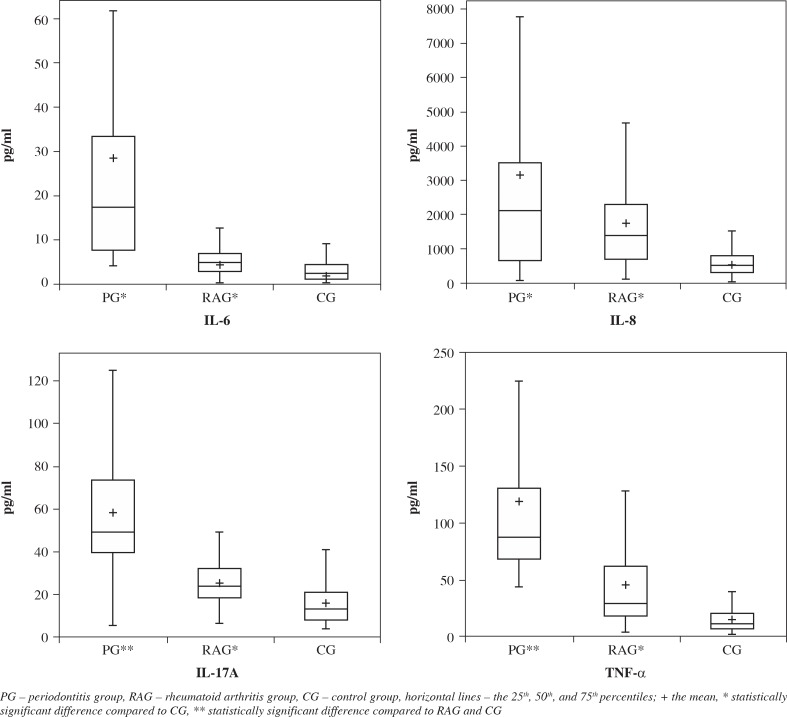
Salivary levels of IL-6, IL-8, IL-17A, and TNF-α in the three groups are presented as box plots in Figure 1. Mean levels of all cytokines were significantly elevated in periodontitis and RA when compared with controls (p < 0.0001). Concentrations of IL-6, IL-17A, and TNF-α were statistically higher in PG than in RAG (p < 0.0001). In RAG no differences between patients receiving different treatment modalities were found.
Fig. 1.
Box plots of salivary concentrations of IL-6, IL-8, IL-17A, and TNF-α in all three groups
In RAG the levels of IL-6 significantly correlated with DAS28 and SDAI (simple disease activity index) scores (r = 0.40 and 0.36, respectively; p < 0.05). In the same group BOP and PD ≥ 4 mm positively correlated with IL-17A (r = 0.38 and 0.36, respectively; p < 0.05). Serum RF levels correlated significantly with PI (r = 0.34, p < 0.05) and the number of teeth (r = –0.39, p < 0.05). The number of swollen joints positively correlated with the number of teeth (r = 0.39, p < 0.05). There were no correlations that were significant in PG.
Discussion
This study found that salivary levels of IL-6, IL-8, IL-17A, and TNF-α were elevated in both study groups (PG and RAG) when compared with controls (CG). However, lower values were found in RAG patients than in PG. There are only a few studies that have dealt with this topic. Mirrielees et al. [25] evaluated the influence of RA and periodontitis on salivary biomarkers and observed elevated TNF-α levels in the PG. This is in agreement with our findings of salivary TNF-α levels. The same study found significantly lower salivary TNF-α levels in RA patients treated with anti-TNF therapy. In our report patients were treated with classical DMARDs, but no differences between patients treated with various DMARDs were noted. Another research conducted by Gamel et al. [26] assessed salivary TNF-α levels and reported no differences between periodontitis and RA. Our result demonstrates that RAG had elevated salivary IL-6 levels when compared with the non-periodontitis CG, which is in accordance with results obtained by Silvestre-Rangil et al. [51]. To the best of our knowledge, no other studies evaluated salivary cytokines between periodontitis and RA. Our study examined the influence of RA on salivary IL-8 and IL-17A levels for the first time. Another report that assessed IL-6 levels in GCF found no differences in IL-6 levels between periodontitis and RA, despite significantly higher PI values in the latter group [52]. Cetinkaya et al. [53] investigated differences in clinical status and GCF levels of IL-1b, IL-4, IL-10, and TNF-α among periodontitis, RA, and controls, quite similarly to our research. The authors recorded no significant differences in terms of TNF-α levels, but RA subjects exhibited significantly higher PI despite lower BOP, PD, and CAL. This is in agreement with our findings. We demonstrated higher PD and BOP in PG, even though RAG showed worse oral hygiene as assessed with API and PI. The similar attachment loss found in both groups could indicate a comparable rate of progression of periodontitis in the past.
We can only speculate that less severe periodontal disease and lower levels of IL-6, IL-8, IL-17A, and TNF-α in RAG might be related to DMARD or glucocorticosteroid therapy. This opinion is supported by results obtained by Ziebolz et al. [54], which suggest that RA therapy with different DMARDs can affect periodontal inflammation and levels of periodontal pathogenic bacteria. MTX, which was the most commonly used DMARD, inhibits the enzyme dihydrofolate reductase and hence thymidine synthesis, transmethylation of RNA, DNA, proteins, phospholipids, and de novo purine synthesis. As a result, MTX is able to suppress secretion of IL-6, IL-8, IL-17A, and TNF-α [55-58]. The use of DMARDs combined with glucocorticoids have also been found to reduce levels of IL-6 [59]. Leflunomide is a DMARD that inhibits dihydroorotase dehydrogenase and thereby pyrimidine synthesis and has been reported to potentially reduce levels of IL-6, IL-8, IL-17A, and TNF-α [60-62]. Another DMARD, sulfasalazine, has several mechanisms of action that include inhibition of chemotaxis of inflammatory cells, inhibition of cytokine expression in mononuclear cells, inhibition of lymphocyte proliferation and activation, as well as inhibition of angiogenesis. Various studies have reported that it has the potential to inhibit expression of IL-8 and TNF-α [63, 64]. Hydroxychloroquine is an antimalarial agent that has been used in RA treatment for many years. Despite the lack of complete understanding of its anti-inflammatory mechanism, it is proposed that the inhibition of Toll-like receptor signalling was responsible for this phenomenon [65]. Evidence from an in vitro study suggests that hydroxychloroquine might inhibit the production of IL-6 and IL-17A [66].
Salivary IL-6 correlations with disease activity displayed by DAS28 and SDAI scores are consistent with observations found in other fluids. Several studies exhibited elevated IL-6 concentration in serum and synovium in RA individuals, which was associated with disease activity [28, 67]. Kobayashi et al. [68] found that serum IL-6 and TNF-α significantly correlated with DAS28 in RA patients.
Current knowledge about salivary cytokine levels in patients with both RA and PD is limited. Our study expands knowledge on this subject. The strength of our study is the clinical evaluation of both the PD and RA status of all individuals recruited. The biggest limitation is the cross-sectional character of the study and the relatively small sample size. Large, prospective studies would be the most appropriate, especially for studying the impact of RA therapy on salivary cytokines levels. Furthermore, the studied group were not sex matched; in the RA group there were significantly more females compared to the PD and CG, which reflects the higher prevalence of females in the RA population [69]. However, this could affect our results only to a small extent because several previous studies showed that salivary levels of evaluated cytokines are not gender dependent [70-75].
Conclusions
The present study underscores evidence that salivary levels of IL-6, IL-8, IL-17A, and TNF-α can be influenced by local and systemic inflammatory diseases, as well as by anti-inflammatory drugs like DMARD and glucocorticoids. Our findings provide further support for the use of salivary diagnostics in monitoring periodontal status, especially in otherwise healthy individuals.
Footnotes
The authors declare no conflict of interest.
References
- 1.Konopka T, Dembowska E, Pietruska M, et al. Periodontal status and selected parameters of oral condition of Poles aged 65 to 74 years. Przegl Epidemiol. 2015;69:537–542. [PubMed] [Google Scholar]
- 2.Kuo LC, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122:417–433. doi: 10.1016/j.puhe.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Ayravainen L, Leirisalo-Repo M, Kuuliala A, et al. Periodontitis in early and chronic rheumatoid arthritis: a prospective follow-up study in Finnish population. BMJ Open. 2017;7:e011916. doi: 10.1136/bmjopen-2016-011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi IA, Kim JH, Kim YM, et al. Periodontitis is associated with rheumatoid arthritis: a study with longstanding rheumatoid arthritis patients in Korea. Korean J Intern Med. 2016;31:977–986. doi: 10.3904/kjim.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasso MA, Comer AC, DiRenzo DD, et al. Using Big Data to Evaluate the Association between Periodontal Disease and Rheumatoid Arthritis. AMIA Annu Symp Proc. 2016;2015:589–593. [PMC free article] [PubMed] [Google Scholar]
- 6.Chou YY, Lai KL, Chen DY, et al. Rheumatoid Arthritis Risk Associated with Periodontitis Exposure: A Nationwide, Population-Based Cohort Study. PLoS One. 2015;10:e0139693. doi: 10.1371/journal.pone.0139693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmickler J, Rupprecht A, Patschan S, et al. Cross-Sectional Evaluation of Periodontal Status and Microbiologic and Rheumatoid Parameters in a Large Cohort of Patients With Rheumatoid Arthritis. J Periodontol. 2017;88:368–379. doi: 10.1902/jop.2016.160355. [DOI] [PubMed] [Google Scholar]
- 8.Khantisopon N, Louthrenoo W, Kasitanon N, et al. Periodontal disease in Thai patients with rheumatoid arthritis. Int J Rheum Dis. 2014;17:511–518. doi: 10.1111/1756-185X.12315. [DOI] [PubMed] [Google Scholar]
- 9.Tang Q, Fu H, Qin B, et al. A Possible Link Between Rheumatoid Arthritis and Periodontitis: A Systematic Review and Meta-analysis. Int J Periodontics Restorative Dent. 2017;37:79–86. doi: 10.11607/prd.2656. [DOI] [PubMed] [Google Scholar]
- 10.Fuggle NR, Smith TO, Kaul A, et al. Hand to Mouth: A Systematic Review and Meta-Analysis of the Association between Rheumatoid Arthritis and Periodontitis. Front Immunol. 2016;7:80. doi: 10.3389/fimmu.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62:835–841. doi: 10.1136/ard.62.9.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 13.Bonfil JJ, Dillier FL, Mercier P, et al. A “case control” study on the role of HLA DR4 in severe periodontitis and rapidly progressive periodontitis. Identification of types and subtypes using molecular biology (PCR.SSO) J Clin Periodontol. 1999;26:77–84. doi: 10.1034/j.1600-051x.1999.260203.x. [DOI] [PubMed] [Google Scholar]
- 14.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 15.de Almeida DE, Ling S, Holoshitz J. New insights into the functional role of the rheumatoid arthritis shared epitope. FEBS Lett. 2011;585:3619–3626. doi: 10.1016/j.febslet.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz P, Bissada NF, Palomo L, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80:535–540. doi: 10.1902/jop.2009.080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Katma MK, Bissada NF, Bordeaux JM, et al. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol. 2007;13:134–137. doi: 10.1097/RHU.0b013e3180690616. [DOI] [PubMed] [Google Scholar]
- 18.Khare N, Vanza B, Sagar D, et al. Nonsurgical Periodontal Therapy decreases the Severity of Rheumatoid Arthritis: A Case-control Study. J Contemp Dent Pract. 2016;17:484–488. doi: 10.5005/jp-journals-10024-1877. [DOI] [PubMed] [Google Scholar]
- 19.Erciyas K, Sezer U, Ustun K, et al. Effects of periodontal therapy on disease activity and systemic inflammation in rheumatoid arthritis patients. Oral Dis. 2013;19:394–400. doi: 10.1111/odi.12017. [DOI] [PubMed] [Google Scholar]
- 20.Andrade R, Espinoza M, Gomez EM, et al. Intra- and inter-examiner reproducibility of manual probing depth. Braz Oral Res. 2012;26:57–63. doi: 10.1590/s1806-83242012000100010. [DOI] [PubMed] [Google Scholar]
- 21.Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–229. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Kim CJ, Camargo PM. Salivary biomarkers in the diagnosis of periodontal diseases. J Calif Dent Assoc. 2013;41:119–124. [PMC free article] [PubMed] [Google Scholar]
- 23.Giannobile WV, Beikler T, Kinney JS, et al. Saliva as a diagnostic tool for periodontal disease: current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci. 2007;1098:411–428. doi: 10.1196/annals.1384.035. [DOI] [PubMed] [Google Scholar]
- 25.Mirrielees J, Crofford LJ, Lin Y, et al. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol. 2010;37:1068–1074. doi: 10.1111/j.1600-051X.2010.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gamel EB, Hashim NT, Satti A, Gismalla BG. Salivary TNF-alpha levels in groups of subjects with rheumatoid arthritis and chronic periodontitis. BMC Res Notes. 2017;10:34. doi: 10.1186/s13104-016-2341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathnayake N, Akerman S, Klinge B, et al. Salivary biomarkers of oral health: a cross-sectional study. J Clin Periodontol. 2013;40:140–147. doi: 10.1111/jcpe.12038. [DOI] [PubMed] [Google Scholar]
- 28.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Georganas C, Liu H, Perlman H, et al. Regulation of IL-6 and IL-8 expression in rheumatoid arthritis synovial fibroblasts: the dominant role for NF-kappa B but not C/EBP beta or c-Jun. J Immunol. 2000;165:7199–7206. doi: 10.4049/jimmunol.165.12.7199. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki M, Hashizume M, Yoshida H, Mihara M. Anti-inflammatory mechanism of tocilizumab, a humanized anti-IL-6R antibody: effect on the expression of chemokine and adhesion molecule. Rheumatol Int. 2010;30:309–315. doi: 10.1007/s00296-009-0953-0. [DOI] [PubMed] [Google Scholar]
- 31.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–449. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 32.Finoti LS, Nepomuceno R, Pigossi SC, et al. Association between interleukin-8 levels and chronic periodontal disease: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6932. doi: 10.1097/MD.0000000000006932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada Y, Komatsu Y, Ikezawa-Suzuki I, et al. The effect of periodontal treatment on serum leptin, interleukin-6, and C-reactive protein. J Periodontol. 2010;81:1118–1123. doi: 10.1902/jop.2010.090741. [DOI] [PubMed] [Google Scholar]
- 34.Lagdive SS, Marawar PP, Byakod G, Lagdive SB. Evaluation and comparison of interleukin-8 (IL-8) level in gingival crevicular fluid in health and severity of periodontal disease: a clinico-biochemical study. Indian J Dent Res. 2013;24:188–192. doi: 10.4103/0970-9290.116675. [DOI] [PubMed] [Google Scholar]
- 35.Husby G, Williams RC. Synovial localization of tumor necrosis factor in patients with rheumatoid arthritis. J Autoimmun. 1988;1:363–371. doi: 10.1016/0896-8411(88)90006-6. [DOI] [PubMed] [Google Scholar]
- 36.Jiang ZL, Cui YQ, Gao R, et al. Study of TNF-alpha, IL-1beta and LPS levels in the gingival crevicular fluid of a rat model of diabetes mellitus and periodontitis. Dis Markers. 2013;34:295–304. doi: 10.3233/DMA-130974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 38.Gumus P, Nizam N, Lappin DF, Buduneli N. Saliva and serum levels of B-cell activating factors and tumor necrosis factor-alpha in patients with periodontitis. J Periodontol. 2014;85:270–280. doi: 10.1902/jop.2013.130117. [DOI] [PubMed] [Google Scholar]
- 39.Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44:411–417. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lubberts E, Joosten LA, Oppers B, et al. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 41.Azizi G, Jadidi-Niaragh F, Mirshafiey A. Th17 Cells in Immunopathogenesis and treatment of rheumatoid arthritis. Int J Rheum Dis. 2013;16:243–253. doi: 10.1111/1756-185X.12132. [DOI] [PubMed] [Google Scholar]
- 42.Cheng WC, Hughes FJ, Taams LS. The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol. 2014;4:541–549. doi: 10.1111/jcpe.12238. [DOI] [PubMed] [Google Scholar]
- 43.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 45.Caton J, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45:S1–S8. doi: 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 46.Prevoo ML, van ‘t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 47.Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229–235. [PubMed] [Google Scholar]
- 48.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 49.Lange DE, Plagmann HC, Eenboom A, Promesberger A. Clinical methods for the objective evaluation of oral hygiene. Dtsch Zahnarztl Z. 1977;32:44–47. [PubMed] [Google Scholar]
- 50.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 51.Silvestre-Rangil J, Bagán L, Silvestre FJ, et al. Periodontal, salivary and IL-6 status in rheumatoid arthritis patients. A cross-sectional study. Med Oral Patol Oral Cir Bucal. 2017;22:e595–e600. doi: 10.4317/medoral.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bozkurt FY, Berker E, Akkus S, Bulut S. Relationship between interleukin-6 levels in gingival crevicular fluid and periodontal status in patients with rheumatoid arthritis and adult periodontitis. J Periodontol. 2000;71:1756–1760. doi: 10.1902/jop.2000.71.11.1756. [DOI] [PubMed] [Google Scholar]
- 53.Cetinkaya B, Guzeldemir E, Ogus E, Bulut S. Proinflammatory and anti-inflammatory cytokines in gingival crevicular fluid and serum of patients with rheumatoid arthritis and patients with chronic periodontitis. J Periodontol. 2013;84:84–93. doi: 10.1902/jop.2012.110467. [DOI] [PubMed] [Google Scholar]
- 54.Ziebolz D, Rupprecht A, Schmickler J, et al. Association of different immunosuppressive medications with periodontal condition in patients with rheumatoid arthritis: Results from a cross-sectional study. J Periodontol. 2018;89:1310–1317. doi: 10.1002/JPER.17-0616. [DOI] [PubMed] [Google Scholar]
- 55.Seitz M. Molecular and cellular effects of methotrexate. Curr Opin Rheumatol. 1999;11:226–232. doi: 10.1097/00002281-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwal A, Misra R. Methotrexate inhibits interleukin-6 production in patients with juvenile rheumatoid arthritis. Rheumatol Int. 2003;23:134–137. doi: 10.1007/s00296-002-0267-y. [DOI] [PubMed] [Google Scholar]
- 57.Gao IK, Leins C, Bohlen H, et al. Inhibition of interleukin-8 synthesis by intraarticular methotrexate therapy in patients with rheumatoid arthritis. Z Rheumatol. 1998;57:95–100. doi: 10.1007/s003930050066. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Jiang L, Zhang S, et al. Methotrexate attenuates the Th17/IL-17 levels in peripheral blood mononuclear cells from healthy individuals and RA patients. Rheumatol Int. 2012;32:2415–2422. doi: 10.1007/s00296-011-1867-1. [DOI] [PubMed] [Google Scholar]
- 59.Gerards AH, de Lathouder S, de Groot ER, et al. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology (Oxford) 2003;42:1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 60.Dessein PH, Joffe BI. Suppression of circulating interleukin-6 concentrations is associated with decreased endothelial activation in rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:161–167. [PubMed] [Google Scholar]
- 61.Zhu M, Xu Q, Li XL, et al. Modulating effects of leflunomide on the balance of Th17/Treg cells in collageninduced arthritis DBA/1 mice. Cent Eur J Immunol. 2014;39:152–158. doi: 10.5114/ceji.2014.43714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li WD, Ran GX, Teng HL, Lin ZB. Dynamic effects of leflunomide on IL-1, IL-6, and TNF-alpha activity produced from peritoneal macrophages in adjuvant arthritis rats. Acta Pharmacol Sin. 2002;23:752–756. [PubMed] [Google Scholar]
- 63.Klimiuk PA, Kita J, Chwiecko J, Sierakowski S. The changes in serum chemokines following leflunomide therapy in patients with rheumatoid arthritis. Clin Rheumatol. 2009;28:17–21. doi: 10.1007/s10067-008-0974-1. [DOI] [PubMed] [Google Scholar]
- 64.Rodenburg RJ, Ganga A, van Lent PL, et al. The antiinflammatory drug sulfasalazine inhibits tumor necrosis factor alpha expression in macrophages by inducing apoptosis. Arthritis Rheum. 2000;43:1941–1950. doi: 10.1002/1529-0131(200009)43:9<1941::AID-ANR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 65.Lodowska J, Gruchlik A, Wolny D, et al. The effect of sulfasalazine and 5-aminosalicylic acid on the secretion of interleukin 8 by human colon myofibroblast. Acta Pol Pharm. 2015;72:917–921. [PubMed] [Google Scholar]
- 66.Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol. 2006;2:458–459. doi: 10.1038/ncprheum0292. [DOI] [PubMed] [Google Scholar]
- 67.Silva JC, Mariz HA, Rocha LF, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 2013;68:766–771. doi: 10.6061/clinics/2013(06)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dasgupta B, Corkill M, Kirkham B, et al. Serial estimation of interleukin 6 as a measure of systemic disease in rheumatoid arthritis. J Rheumatol. 1992;19:22–25. [PubMed] [Google Scholar]
- 69.Kobayashi T, Murasawa A, Komatsu Y, et al. Serum cytokine and periodontal profiles in relation to disease activity of rheumatoid arthritis in Japanese adults. J Periodontol. 2010;81:650–657. doi: 10.1902/jop.2010.090688. [DOI] [PubMed] [Google Scholar]
- 70.van Vollenhoven RF. Sex differences in rheumatoid arthritis: more than meets the eye. BMC Med. 2009;7:12. doi: 10.1186/1741-7015-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dineshkumar T, Ashwini BK, Rameshkumar A, et al. Salivary and Serum Interleukin-6 Levels in Oral Premalignant Disorders and Squamous Cell Carcinoma: Diagnostic Value and Clinicopathologic Correlations. Asian Pac J Cancer Prev. 2016;17:4899–4906. doi: 10.22034/APJCP.2016.17.11.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.St John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:929–935. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- 73.Wu M, Chen SW, Su WL, et al. Sex Hormones Enhance Gingival Inflammation without Affecting IL-1beta and TNF-alpha in Periodontally Healthy Women during Pregnancy. Mediators Inflamm. 2016;2016:4897890. doi: 10.1155/2016/4897890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan A. Detection and quantitation of forty eight cytokines, chemokines, growth factors and nine acute phase proteins in healthy human plasma, saliva and urine. J Proteomics. 2012;75:4802–4819. doi: 10.1016/j.jprot.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 75.Szczeklik K, Owczarek D, Pytko-Polończyk J, et al. Proinflammatory cytokines in the saliva of patients with active and non-active Crohn’s disease. Pol Arch Med Wewn. 2012;122:200–208. doi: 10.20452/pamw.1256. [DOI] [PubMed] [Google Scholar]