Abstract
Background
Colorectal resection through a midline laparotomy is a commonly performed surgical procedure to treat various bowel conditions. The typical postoperative hospital stay after this operation is 6 to 10 days. The main factors hindering early recovery and discharge are thought to include postoperative pain and delayed return of bowel function.
Continuous infusion of a local anaesthetic into tissues surrounding the surgical incision via a multi‐lumen indwelling wound catheter placed by the surgeon prior to wound closure may reduce postoperative pain, opioid consumption, the time to return of bowel function, and the length of hospital stay.
Objectives
To evaluate the efficacy and adverse events of continuous local anaesthetic wound infusion for postoperative pain after midline laparotomy for colorectal resection in adults.
Search methods
We searched the CENTRAL, MEDLINE and Embase databases to January 2019 to identify trials relevant to this review. We also searched reference lists of relevant trials and reviews for eligible trials. Additionally, we searched two clinical trials registers for ongoing trials.
Selection criteria
We considered randomised controlled trials (including non‐standard designs) or quasi‐randomised controlled trials comparing continuous wound infusion of a local anaesthetic versus a placebo or a sham after midline laparotomy for colorectal resection in adults. We did not compare continuous local anaesthetic wound infusion to other techniques, such as transverse abdominis plane block or thoracic epidural analgesia. We allowed non‐randomised analgesic co‐interventions carried out equally in the intervention and control groups.
Data collection and analysis
Two review authors independently identified trials for inclusion and assessed their quality using the Cochrane 'Risk of bias' tool. We extracted data using standardised forms, including pain at rest and on movement (10‐point scale), opioid consumption via a patient‐controlled analgesia (PCA) system (mg morphine equivalent), postoperative opioid‐related adverse events, the time to rescue analgesia, the time to first flatus and to first bowel movement, the time to ambulation, the length of hospital stay, serious postoperative adverse events, and patient satisfaction. We quantitatively synthesised the data by meta‐analysis. We summarised and graded the certainty of the evidence for critical outcomes using the GRADEpro tool and created a 'Summary of findings' table.
Main results
This review included six randomised controlled trials that enrolled a total of 564 adults undergoing elective midline laparotomy for colorectal resection comparing continuous wound infusion of a local anaesthetic to a normal saline placebo. Due to 23 post‐randomisation exclusions, a total of 541 participants contributed data to the analysis of at least one outcome (local anaesthetic 268; control 273). Most participants were aged 55 to 65 years, with normal body mass index and low to moderate anaesthetic risk (American Society of Anesthesiologists class I‐III). Random sequence generation, allocation concealment, and blinding were appropriately carried out in most trials. However, we had to downgrade the certainty of the evidence for most outcomes due to serious study limitations (risk of bias), inconsistency, indirectness, imprecision and reporting bias.
Primary outcomes
On postoperative day 1, pain at rest (mean difference (MD) −0.59 (from 3.1), 95% confidence interval (CI) −1.12 to −0.07; 5 studies, 511 participants; high‐certainty evidence), pain on movement (MD −1.1 (from 6.1), 95% CI −2.3 to −0.01; 3 studies, 407 participants; low‐certainty evidence) and opioid consumption via PCA (MD −12 mg (from 41 mg), 95% CI −20 to −4; 6 studies, 528 participants; moderate‐certainty evidence) were reduced in the local anaesthetic group compared to the control group.
Secondary outcomes
There was a reduction in the time to first bowel movement (MD −0.67 from 4.4 days, 95% CI −1.17 to −0.17; 4 studies, 197 participants; moderate‐certainty evidence) and the length of hospital stay (MD −1.2 from 7.4 days, 95% CI −2.0 to −0.3; 4 studies, 456 participants; high‐certainty evidence) in the local anaesthetic group compared to the control group.
There was no evidence of a difference in any serious postoperative adverse events until hospital discharge (RR 1.04, 95% CI 0.68 to 1.58; 6 studies, 541 participants; low‐certainty evidence) between the two study groups.
Authors' conclusions
After elective midline laparotomy for colorectal resection, continuous wound infusion of a local anaesthetic compared to a normal saline placebo reduces postoperative pain at rest and the length of hospital stay, on the basis of high‐certainty evidence. This means we are very confident that the effect estimates for these outcomes lie close to the true effects. There is moderate‐certainty evidence to indicate that the intervention probably reduces opioid consumption via PCA and the time to first bowel movement. This means we are moderately confident that effect estimates for these outcomes are likely to be close to the true effects, but there is a possibility that they are substantially different. The intervention may reduce postoperative pain on movement, however, this conclusion is based on low‐certainty evidence. This means our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. There is low‐certainty evidence to indicate that the intervention may have little or no effect on the rates of any serious postoperative adverse events until hospital discharge. High‐quality randomised controlled trials to evaluate the intervention with a focus on important clinical and patient‐centred outcomes are needed.
Plain language summary
Continuous delivery of a local anaesthetic around the wound to treat pain after bowel surgery through a vertical cut in the abdomen
Background
People with bowel disease can be treated with surgery to remove a part of the bowel (colorectal resection). A long vertical cut in the abdomen (midline laparotomy) is often required. Recovery after this type of surgery can be slow and painful. Continuous injection of a local anaesthetic (numbing a specific area of the body, e.g. around the wound) may reduce pain after this type of surgery. The local anaesthetic may also reduce the amount of morphine‐like pain killers required and side effects related to these medications. This could mean a shorter recovery time for the patient and earlier discharge from the hospital.
Study characteristics
We searched for clinical trials to January 2019 looking at the benefits and harms of continuous injection of a local anaesthetic after surgery to remove the bowel through a vertical cut in the abdomen. We looked for trials comparing local anaesthetic to an inactive substance (placebo) such as salty water (normal saline). We found six clinical trials including 541 participants. Most participants were aged 55 to 65 years, of varying health status from fit and healthy to having a severe systemic disease (a disease that affects the whole body).
Key findings
In people who received a local anaesthetic, pain at rest, pain on movement, and requirement for morphine‐like pain killers were reduced on the first day after surgery compared to people who received an inactive substance.
People who received a local anaesthetic also opened their bowels about half a day earlier and were discharged from hospital about a day earlier compared to people who received an inactive substance.
We did not find a difference between people who received a local anaesthetic and those who received an inactive substance in the rates of any serious complications after surgery until hospital discharge.
Certainty of evidence
We rated the certainty of the evidence from studies using four levels: high, moderate, low, very low. Reasons for downgrading the certainty of the evidence included limitations problems with the design of the studies, missing data, differences between trials and how the outcomes were measured, and the small number of participants. We need more high‐quality trials to evaluate this treatment, especially its effects on recovery after surgery, side effects and complications.
We rated the certainty of the evidence for pain after surgery at rest and the length of hospital stay as high, meaning that we are very confident in the findings about the effects of the treatment on these outcomes. We rated the certainty of the evidence for the requirement for morphine‐like pain killers and the time until the first bowel movement as moderate. This means that we are moderately confident in the findings about the effects of the treatment on these outcomes. We rated the certainty of the evidence for pain after surgery on movement and the rates of any serious complications after surgery until hospital discharge as low, meaning that we have limited confidence in the findings about the effects of the treatment on these outcomes.
Summary of findings
Summary of findings for the main comparison. Continuous local anaesthetic wound infusion compared to placebo for postoperative pain after midline laparotomy for colorectal resection in adults.
| Continuous local anaesthetic wound infusion compared to placebo for postoperative pain after midline laparotomy for colorectal resection in adults | |||||
| Patient or population: adults undergoing elective midline laparotomy for colorectal resection Setting: tertiary hospitals in resource‐rich countries Intervention: continuous local anaesthetic wound infusion Comparison: continuous wound infusion of a placebo (normal saline) | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | |
| Response or risk with placebo | Response or risk with local anaesthetic | ||||
| Pain score at rest postoperative day 1 Assessed with: numerical rating scale or equivalent Scale from: 0 to 10 (0 = no pain) | Weighted mean 3.1 points | MD 0.59 points lower (1.1 points lower to 0.07 points lower) | ‐ | 511 (5 RCTs) | ⊕⊕⊕⊕ High |
| Pain score on movement postoperative day 1 Assessed with: numerical rating scale or equivalent Scale from: 0 to 10 (0 = no pain) | Weighted mean 6.1 points | MD 1.1 points lower (2.3 points lower to 0.01 points lower) | ‐ | 407 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b |
| Opioid consumption via patient controlled analgesia postoperative day 1 Assessed with: milligrams of morphine equivalent | Weighted mean 41 mg | MD 12 mg lower (20 mg lower to 4.2 mg lower) | ‐ | 528 (6 RCTs) | ⊕⊕⊕⊝ Moderatec |
| Time to first bowel movement (days) | Weighted mean 4.4 days | MD 0.67 days lower (1.17 days lower to 0.17 days lower) | ‐ | 197 (4 RCTs) | ⊕⊕⊕⊝ Moderated |
| Length of hospital stay (days) | Weighted mean 7.4 days | MD 1.2 days lower (2.0 days lower to 0.33 days lower) | ‐ | 456 (4 RCTs) | ⊕⊕⊕⊕ High |
|
Any serious postoperative adverse event (until hospital discharge) |
Study population | RR 1.04 (0.68 to 1.58) | 541 (6 RCTs) | ⊕⊕⊝⊝ Lowb,e | |
| 139 per 1000 | 141 per 1000 (92 to 212) | ||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; mg: milligram; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High: we are very confident that the true effect lies close to that of the estimate of the effect; further research is very unlikely to change our confidence in the estimate of effect. Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect; any estimate of effect is very uncertain. | |||||
aDowngraded by one level for inconsistency: high level of heterogeneity (I2 > 75%). bDowngraded by one level for reporting bias: selective outcome reporting in trials where this outcome was predefined but the results were not published. cDowngraded by one level for serious study limitations (risk of bias): attrition bias arising from large numbers of post‐randomisation exclusions and missing data for this outcome. dDowngraded by one level for imprecision: small total number of participants (< 400). eDowngraded by one level for some indirectness: unclear or different definitions or methods of assessment for this outcome.
Background
Description of the condition
Open colorectal resection is associated with substantial postoperative pain. It is the definitive treatment for a broad range of benign and malignant conditions of the large bowel, including colorectal cancer, inflammatory bowel disease, diverticular disease, and large bowel obstruction. In 2012, the worldwide annual incidence of colorectal cancer was 1.4 million. It was the second most common cancer in women after breast cancer, and the third most common cancer in men after lung and prostate cancer (WCR 2014). In the UK, approximately 60% of people with colorectal cancer will undergo major colorectal resection (NBOCA 2015). Since advancement of laparoscopic surgical techniques, the number of cases of elective, open resections for colorectal cancer has decreased in high‐income countries, such as the UK and Australia (BCCA 2015; NBOCA 2015). In Australia, the rate of open resection decreased from approximately 70% in 2009 to approximately 40% in 2014 (BCCA 2015). Nevertheless, open resection remains necessary in many settings, such as in people with locally advanced disease or unfavourable anatomy, for unplanned emergency cases, or in poorly resourced communities (Amin 2015; Plummer 2011; Ray‐Offor 2014; SAGES 2012).
Open colorectal resection is commonly performed through a midline incision in the abdominal wall. Midline incisions provide easy, quick and excellent exposure of the abdominal cavity, and are particularly useful for complex, exploratory or urgent procedures. However, midline incisions transect nerve fibres crossing the abdominal wall in a mediocaudal direction, which results in more postoperative pain compared to other incisions (Brown 2005; Burger 2002; Grantcharov 2001). Standard elective open colorectal resection typically requires a postoperative hospital stay of 6 to 10 days on average (Walter 2009; Wind 2006). The main factors hindering early recovery and discharge are thought to include postoperative pain and delayed return of bowel function (Kehlet 2008).
Multimodal analgesia aims to achieve more effective pain relief and reduce adverse events through the additive or synergistic effects of different analgesic agents or routes of administration (Buvanendran 2009; Jin 2001; Kehlet 1999). However, up to 70% of people undergoing major abdominal surgery still experience moderate to severe postoperative pain and opioid‐related adverse events, such as nausea, vomiting and ileus, despite a multimodal analgesia protocol involving patient‐controlled analgesia (PCA) with opioids combined with opioid‐sparing agents (Apfelbaum 2003; Gan 2014; Sommer 2008).
Description of the intervention
New pump or balloon devices allow local anaesthetics, such as bupivacaine, levobupivacaine and ropivacaine, to be continuously infused into tissues surrounding an incisional wound via a multi‐lumen, indwelling catheter, placed by the surgeon prior to wound closure. For abdominal surgery, the wound catheter may be positioned within the subcutaneous (suprafascial), musculofascial or preperitoneal (subfascial) layers of the anterior abdominal wall.
How the intervention might work
Local anaesthetics produce analgesic effects by decreasing the excitability of peripheral nociceptive nerve fibres by inhibiting voltage‐gated sodium channels (Butterworth 1990). Local anaesthetics also possess anti‐inflammatory and antimicrobial properties (Hollmann 2000; Johnson 2008). Continuous wound infusion allows the direct and sustained action of a local anaesthetic within tissues surrounding an incisional wound, inhibiting parietal nociception. Use of other drugs in multimodal analgesia is needed for complete analgesia by coverage of visceral nociception.
When used within a multimodal analgesia protocol, continuous local anaesthetic wound infusion may reduce postoperative pain, reduce opioid consumption and postoperative opioid‐related adverse events, and reduce the length of hospital stay. Furthermore, continuous local anaesthetic wound infusion may be an effective alternative to analgesic modalities such as epidural analgesia or peripheral nerve blocks, especially in situations where these techniques are impractical, difficult, poorly tolerated, or contraindicated (Rawal 2012).
Why it is important to do this review
Previous systematic reviews and meta‐analyses included a broad range of surgeries, including gastrointestinal surgery, obstetric and gynaecological surgery, urological surgery, cardiothoracic surgery and orthopaedic surgery (Gupta 2011; Liu 2006), and abdominal incisions, including midline, paramedian, oblique and laparoscopic (Karthikesalingam 2008; Ventham 2014). The validity and relevance of pooling outcomes from different surgical procedures have been questioned, since the mechanisms and intensity of pain, the placebo response and the treatment effects differ between different surgical procedures and surgical incisions (Beaussier 2012; Espitalier 2013; Gerbershagen 2013; Gerbershagen 2014; Gray 2005). There is increasing recognition of the need for evidence‐based guidelines for procedure‐specific pain management (Joshi 2013; Kehlet 2007). At present, there is no systematic review and meta‐analysis examining the procedure‐specific outcomes of continuous local anaesthetic wound infusion after midline laparotomy for colorectal resection.
Objectives
To evaluate the efficacy and adverse events of continuous local anaesthetic wound infusion for postoperative pain after midline laparotomy for colorectal resection in adults.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all randomised placebo‐ or sham‐controlled trials, including non‐standard designs (such as cluster‐ or cross‐over randomised controlled trials) and quasi‐randomised trials. We required full‐text journal publications, with the exception of abstracts with sufficient information to be assessed for eligibility and quality, and appropriate data for analysis.
Types of participants
Adults aged 18 years and above undergoing elective or emergency colorectal resection through a midline vertical incision on the abdominal wall. We planned to include studies involving other types of abdominal incisions, provided that more than 90% of participants had midline vertical incisions. We planned to exclude studies involving procedures that require more than one abdominal incision and those that require extension of the abdominal incision to thoracotomy, sternotomy, or symphysiotomy.
Types of interventions
Any local anaesthetic at any dose that is continuously infused for at least 24 hours via a multi‐lumen catheter embedded within or adjacent to the incisional wound by the surgeon. The comparator must be continuous wound infusion of a placebo or a sham continuous wound infusion. We allowed non‐randomised analgesic co‐interventions if carried out equally in all study groups.
We excluded studies assessing the following interventions.
Continuous wound infusion of agents other than a local anaesthetic or a placebo
Single or intermittent wound infiltration of any agent
Single, intermittent or continuous intraperitoneal injection or infusion of any agent
Single, intermittent or continuous peripheral nerve block in the anterior abdominal wall, such as transverse abdominis plane block or rectus sheath block
Single, intermittent or continuous epidural injection or infusion of any agent
Types of outcome measures
We excluded studies that did not assess postoperative pain.
Primary outcomes
Postoperative pain at rest and on movement on postoperative day 1, measured on or converted to a 10‐point numerical rating scale (NRS) or equivalent
Postoperative opioid consumption via patient‐controlled analgesia (PCA) on postoperative day 1, measured in or converted to milligrams (mg) of morphine‐equivalent opioid dose
Secondary outcomes
Postoperative pain at rest and on movement after postoperative day 1, measured as above
Postoperative opioid consumption via PCA after postoperative day 1, measured as above
Postoperative opioid‐related adverse events: for example, nausea or vomiting, ileus, urinary retention, pruritus, sedation, respiratory depression, sleep disturbance, or other opioid‐related adverse events reported by trial authors
Time to rescue analgesia
Time to first flatus and time to first bowel movement
Time to ambulation
Length of hospital stay
Serious postoperative adverse events: death by any cause after surgery, or adverse events after surgery that result in death, are life‐threatening, require prolongation of hospitalisation, result in a persistent or severe disability, for example: pulmonary complications (atelectasis, pneumonia, respiratory failure), venous thromboembolic complications (deep vein thrombosis, pulmonary embolism), wound catheter‐related complications (visceral or vascular injury, laparotomy wound breakdown, laparotomy wound infection, intra‐abdominal infection), local anaesthetic systemic toxicity (severe hyper‐ or hypotension, cardiac arrhythmias, loss of consciousness, seizures), or other serious postoperative adverse events reported by trial authors. We combined all reported data on serious postoperative adverse events from included trials into a composite 'any serious postoperative adverse events' outcome, under the assumption that such events are rare, independent and pose a similar health burden.
Patient satisfaction
Search methods for identification of studies
Electronic searches
We searched the following databases without language restrictions on 7 January 2019:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1) via Cochrane Central Register of Studies Online (CRSO);
MEDLINE & MEDLINE in Process (OVID) 1946 to 7 January 2019;
Embase (OVID) searched 1974 to 7 January 2019.
Appendix 1 shows the search strategies.
Searching other resources
We searched the metaRegister of Controlled Trials (mRCT; controlled‐trials.com/mrct), clinicaltrials.gov (clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch/) for ongoing trials. In addition, we checked reference lists of relevant reviews and primary studies identified through the search for additional studies. Where necessary, we contacted trial authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (SL and BK) independently determined eligibility of records from database searches. The review authors screened titles and abstracts of all identified citations, and eliminated citations that were clearly ineligible. The review authors then obtained and assessed the full‐text journal publications of the remaining citations for eligibility. Two review authors (SL and EA) independently determined eligibility of records from trial registry searches. In the event of disagreement, a third review author (ZY) independently adjudicated the decision. We included a PRISMA flow chart to show the status of all identified citations (Moher 2009).
Data extraction and management
Two review authors (SL and one of AY, BK and EA) independently extracted the following data using a standard form and checked for agreement.
Publication year
Study year, location and number of centres
Study inclusion and exclusion criteria
Sample size (including sample size allocated and analysed) in each study group
Indication and urgency of the midline laparotomy for colorectal resection
Location and size of the incision wound
Location and number of wound catheters
Rate and duration of the wound infusion
Type and strength of the local anaesthetic in the intervention group
Details of the placebo or sham used in the control group
Perioperative analgesic co‐interventions and adjuncts, including rescue analgesia
Outcomes (see Types of outcome measures)
Risk of bias (see Assessment of risk of bias in included studies)
Funding sources
In the event of disagreement, a third review author (ZY) independently adjudicated the decision.
We collated multiple reports of the same study.
Assessment of risk of bias in included studies
Two review authors (SL and one of AY, BK and EA) independently assessed risk of bias in each study arising from the following sources using the Cochrane 'Risk of bias' assessment tool (Higgins 2017).
Sequence generation (selection bias): low risk of bias (any truly random process, such as random number table or computer random number generator); unclear risk of bias (method not clearly stated). We excluded studies that used a non‐random process.
Allocation concealment (selection bias): low risk of bias (any adequate concealment, such as use of centralised randomisation or consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation.
Blinding of participants and personnel (performance bias): low risk of bias (any adequate method to achieve blinding, such as use of identical study solutions prepared by personnel external to the study); unclear risk of bias (method not clearly stated). We excluded studies without blinding.
Blinding of outcome assessment (detection bias): low risk of bias (study had a clear statement that outcome assessors were unaware of treatment allocation, and ideally described how this was achieved); unclear risk of bias (method not clearly stated). We excluded studies without blinding.
Incomplete outcome data (attrition bias): low risk of bias (< 10% of data missing, accounted for the nature of the missing data, used mean imputation or multiple imputation); unclear risk of bias (used last observation carried forward analysis); high risk of bias (used complete case analysis).
Selective outcome reporting (reporting bias): low risk of bias (reported all pre‐specified outcomes in sufficient detail, including measures of effect size and variance); unclear risk of bias (reported all pre‐specified outcomes with some details missing); high risk of bias (did not report all pre‐specified outcomes).
We considered additional sources of bias as follows.
Size of trial (small study bias): small studies tend to be imprecise and tend to overestimate the effect size. This is partly explained by publication bias, although association between small trial size and other forms of bias is inconsistent (Chaimani 2013; Dechartres 2013; Nuesch 2010). Furthermore, small trials may be more prone to the effects of random chance (Moore 1998). We assessed trials as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Industry funding or sponsorship (industry bias). Industry‐sponsored trials tend to be more favourable to the sponsors’ products, compared with non‐industry‐sponsored trials, which suggests industry sponsorship should be treated as a form of bias (Lundh 2012). We assessed trials as being at low risk of bias (trials with no industry funding or sponsorship, or trials with declared industry funding with a clear statement about how the authors ensured no sponsor involvement in the trial); unclear risk of bias (trials with no declaration of funding sources, or trials with declared industry funding with no statement of sponsor involvement in the trial); high risk of bias (trials with declared industry funding with sponsor involvement in the trial).
Inclusion and exclusion criteria (selection bias). We assessed trials as being at low risk of bias (selection criteria include all relevant clinical characteristics of the trial population); unclear risk of bias (selection criteria not stated clearly); high risk of bias (selection criteria ignore important clinical characteristics of the trial population or exclude important subsets of the trial population).
Methods of outcome assessment (information bias). We assessed trials as being at low risk of bias (used reliable and accurate methods to assess outcomes); unclear risk of bias (methods of outcome assessment not stated clearly); high risk of bias (used unreliable or inaccurate methods to assess outcomes).
Methods of statistical analysis (analytical bias). We assessed trials as being at low risk of bias (used intention‐to‐treat analysis or used sensitivity analysis to assess protocol violation; used appropriate statistical tests to compare continuous and categorical outcome variables between treatment arms); unclear risk of bias (methods of statistical analysis not stated clearly); high risk of bias (did not use intention‐to‐treat analysis and did not use sensitivity analysis to assess to assess protocol violation; did not use appropriate statistical tests).
In the event of disagreement, a third review author (ZY) independently adjudicated the decision.
Measures of treatment effect
We expressed the treatment effects for continuous outcomes as the mean difference (MD) or the standardised mean difference (SMD) with 95% confidence interval (CI). We expressed the treatment effects for dichotomous outcomes as risk ratio (RR) with 95% CI.
Unit of analysis issues
We used the person undergoing midline laparotomy for colorectal resection as the unit of analysis.
Dealing with missing data
We contacted the trial authors about any missing, unclear or 'unusable' data. We derived or estimated data required for meta‐analysis, where necessary and possible, based on reported data according to established methods (Higgins 2011). We estimated the mean for continuous outcomes using the reported median and derived the standard deviation from the reported confidence interval, interquartile range or P value. We derived the number of events for dichotomous outcomes from the reported percentage of events. We excluded the trial from the meta‐analysis of the particular outcome affected if none of these methods were successful.
Assessment of heterogeneity
We quantified heterogeneity using the I2 statistic (Higgins 2003), and assessed the statistical significance using the Chi2 test with significance level α = 0.10 (Higgins 2002). We interpreted I2 values of less than 50%, 50% to 75% and greater than 75% as low, moderate and high levels of heterogeneity, respectively (Deeks 2017).
Assessment of reporting biases
We planned to undertake regression analysis for asymmetry on funnel plots to assess reporting bias if at least 10 studies were included (Egger 1997).
Data synthesis
We performed meta‐analysis of outcomes in the software package Review Manager 5 (RevMan 5; Review Manager 2014), using the inverse‐variance method with random‐effects models (Demets 1987; DerSimonian 1986).
Certainty of the evidence
Two review authors (SL and EA) independently rated the certainty of the body of evidence for each outcome using the GRADEprofiler Guideline Development Tool software (GRADEpro GDT), and the guidelines provided in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017).
The GRADE approach considers study limitations (risk of bias), inconsistency, imprecision, indirectness, publication bias, magnitude of effect, confounding, and dose‐response to assess the certainty of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect; further research is very unlikely to change our confidence in the estimate of effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect; any estimate of effect is very uncertain.
We decreased the grade rating up to a maximum of −3 to 'very low' if we identified the following issues.
Risk of bias: serious (−1) or very serious (−2) study limitations, where the proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results.
Inconsistency: important inconsistency (−1) as indicated by the presence of wide variance of point estimates, and/or minimal or no overlap of confidence intervals, and/or a high level of heterogeneity (I2 > 75%) across studies.
Indirectness: some (−1) or major (−2) uncertainty about directness due to differences in populations, interventions and/or outcome assessment across studies. Please note that we considered issues relating to methods of outcome assessment at the study level identified in the 'Risk of bias' assessment for their impact on 'indirectness' at the outcome level here, and not under the 'Risk of bias' portion of the GRADE assessment.
Imprecision: imprecision of estimates of effect (−1) if the total number of participants was small (< 400). We considered issues relating to sample size at the study level identified in the 'Risk of bias' assessment for their impact on 'imprecision' at the outcome level here, rather than under the 'Risk of bias' portion of the GRADE assessment.
Reporting bias: high risk of reporting bias (−1) related to publication bias and/or selective outcome reporting. Please note that we considered issues relating to selective outcome reporting at the study level identified in the 'Risk of bias' assessment for their impact on 'reporting bias' at the outcome level here, and not under the 'Risk of bias' portion of the GRADE assessment.
'Summary of findings' table
We included a 'Summary of findings' table to present the main findings for clinically and functionally important outcomes in a transparent and simple tabular format, including pain at rest and with movement on postoperative day 1, opioid consumption via PCA on postoperative day 1, the time to first bowel movement, the length of hospital stay, and the composite outcome of any serious postoperative adverse events. We included key information concerning the certainty of the body of evidence for each outcome, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes. We did not include individual postoperative opioid‐related adverse events in a 'Summary of findings' table as there were too many to enumerate and would exceed the recommended number of outcomes.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analyses for pain at rest, pain on movement and opioid consumption on postoperative day 1 to explore potential sources of clinical or methodological heterogeneity.
Elective versus emergency surgery
Location of wound catheter
Local anaesthetic agent
Local anaesthetic dose
Wound infusion programme
Co‐analgesic agents
We planned to use the test for interaction to identify differences between subgroups, and to use meta‐regression to determine the influence of the above factors on the treatment effect if we included at least 10 studies.
Sensitivity analysis
We planned to perform the following sensitivity analyses to establish the robustness of the primary meta‐analysis.
Excluding unpublished data supplied by study authors
Excluding estimated or derived data
Excluding trials assessed to be at high risk of bias
Results
Description of studies
Results of the search
We identified a total of 4180 records through searches in the following databases: CENTRAL (1213 records), MEDLINE (1140 records) and Embase (1827 records). We removed 1504 duplicates and a further 2656 records deemed irrelevant based on screening of titles and abstracts. We attempted to retrieve full‐text articles of the remaining 20 records to review their eligibility. Additionally, we identified two potentially relevant ongoing studies by searching trial registries and also reviewed them for eligibility. Of the 22 records reviewed in detail for eligibility, 11 records met the inclusion criteria (six full‐text articles plus five conference abstracts reporting six randomised controlled trials). We excluded seven records (five full‐text articles and two trial registry records), and four records are awaiting classification (two conference abstracts and two trial registry records). Results of the search and reasons for exclusions are shown in Figure 1.
1.
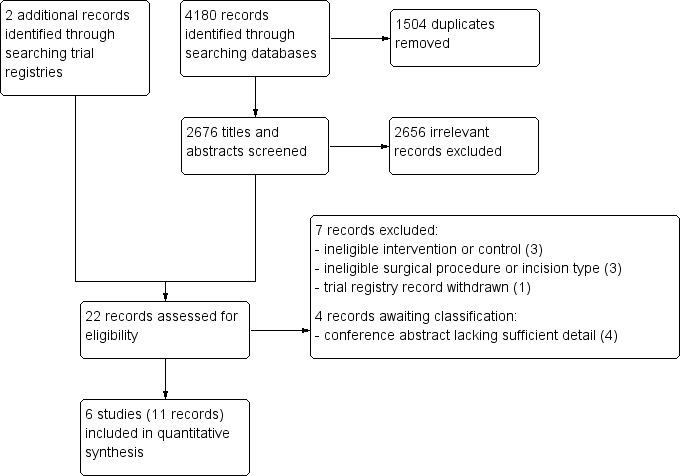
Study flow diagram
Included studies
Full details about study design, sample size, participant characteristics, inclusion and exclusion criteria, post‐randomisation exclusions, intervention and control, and outcomes reported in the included studies are shown in the Characteristics of included studies tables.
We included six randomised controlled trials that enrolled a total of 564 participants undergoing midline laparotomy for colorectal resection to continuous wound infusion with a local anaesthetic or a normal saline placebo (Baig 2006; Beaussier 2007; Fustran 2015; Krishnan 2014; Polglase 2007; Wang 2010). We also included five additional publications (conference abstracts) of the same trials as secondary publications of the primary included study (Krishnan 2014; Polglase 2007; Wang 2010). There were a total of 23 post‐randomisation exclusions across these trials. Hence, we included a total of 541 participants in the analysis of at least one outcome (268 in local anaesthetic group; 273 in control group).
All six trials were conducted in the setting of elective colorectal resection. Two trials included participants undergoing the procedure via either midline laparotomy or laparoscopic approaches, who were stratified into subgroups by the approach (Fustran 2015; Krishnan 2014). We included only the open laparotomy subgroup in this review. The remaining four trials included participants undergoing colorectal resection via midline laparotomy only. The indication for surgery was restricted to colorectal cancer in Beaussier 2007 and Fustran 2015, which excluded individuals with other bowel conditions such as inflammatory bowel disease. Two trials excluded individuals with intra‐abdominal sepsis or abscess (Baig 2006; Fustran 2015). Wang 2010 included participants regardless of the underlying pathology. The indication for surgery was unspecified in the remaining three trials. The reported mean or median age of the participants were similar at around 55 to 65 years across all six included trials. Three trials included only participants with American Society of Anesthesiologists (ASA) class I to III (Baig 2006; Fustran 2015; Krishnan 2014). Beaussier 2007 included only ASA class I or II participants. Polglase 2007 included participants regardless of ASA status, thus included a small number of participants with ASA class up to V. Wang 2010 did not specify any selection criteria based on ASA status.
There were considerable differences between trials in the implementation details of continuous local anaesthetic wound infusion. Two trials positioned the wound catheter in the subcutaneous (suprafascial) layer of the abdominal wall (Baig 2006; Polglase 2007), one trial in the musculofascial layer (Wang 2010), and three trials in the preperitoneal layer (Beaussier 2007; Fustran 2015; Krishnan 2014). The trials used various types, strengths, doses, and volumes of local anaesthetics: Baig 2006 used bupivacaine 0.5% at 4 mL/hour (20 mg/hour); Krishnan 2014 used levobupivacaine 0.25% at 10 mL/hour (25 mg/hour), and Beaussier 2007; Fustran 2015; Polglase 2007; and Wang 2010 used ropivacaine 0.2% to 0.54% at 4 to 10 mL/hour (16 to 21.6 mg/hour). Two trials gave a 10 mL bolus prior to commencing continuous infusion (Beaussier 2007; Fustran 2015). All six included trials used normal saline as the placebo in the control group. The infusion was continued for two days in Fustran 2015 and Wang 2010, three days in Baig 2006 and Polglase 2007, and four days in Beaussier 2007 and Krishnan 2014. All six trials used continuous wound infusion of local anaesthetic or placebo as a part of a multimodal analgesic regimen, with other non‐randomised analgesic co‐interventions carried out equally in the two study groups. These co‐interventions universally included an intravenous opioid patient‐controlled analgesia (PCA) system. Baig 2006 did not use any other additional analgesic agents. Wang 2010 and Beaussier 2007 added a single agent only (paracetamol or a non‐steroidal anti‐inflammatory drug). Fustran 2015; Krishnan 2014 and Polglase 2007 used two to three additional analgesic agents (paracetamol plus a non‐steroidal anti‐inflammatory drug, with another opioid such as oxycodone or tramadol). See Characteristics of included studies for details of co‐analgesic regimens.
Excluded studies
See the Characteristics of excluded studies tables.
We excluded three studies due to ineligible intervention or control. The control group did not receive a placebo or sham in Abadir 2009. The intervention was epidural infusion of bupivacaine in Nalda 1977. We excluded a trial registry record because the control was thoracic epidural analgesia (this trial was terminated due to low recruitment rate; NCT01062919).
We excluded three studies due to ineligible type of incision (paramedian in Gibbs 1988), or surgical procedure (cholecystectomy in Fry 1984, gastrectomy or gastrojejunostomy in Dhanapal 2017).
We excluded one trial registry record as it was withdrawn before enrolling any participants (NCT00557843).
Studies awaiting classification
See the Characteristics of studies awaiting classification tables.
We were only able to find conference abstracts for four studies with insufficient details to allow classification (Araujo 2014; Arino 2012; Cano 2012; Maric 2009).
Risk of bias in included studies
We assessed that random sequence generation, allocation concealment, and blinding were appropriately carried out in nearly all of the included trials. However, all trials had high risk of bias in at least one other domain, relating mainly to the assessment and reporting of specific outcomes, high rates of attrition and missing data, small sample size, and industry bias. The risk of bias in the included trials is summarised in Figure 2 and Figure 3.
2.
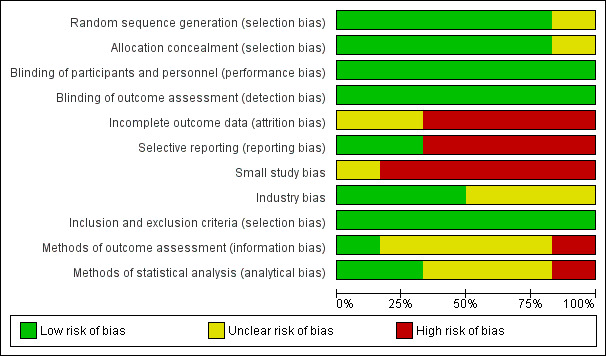
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
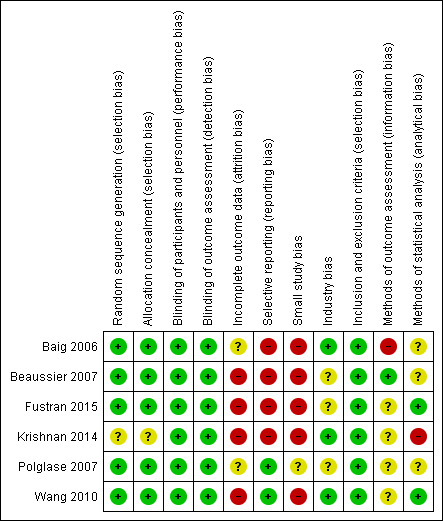
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Five trials described random sequence generation using a computer‐generated randomisation schedule as well as adequate allocation concealment using sealed envelopes and visually identical unlabelled study solutions (Baig 2006; Beaussier 2007; Fustran 2015; Polglase 2007; Wang 2010). We considered these trials to have low risk of selection bias. The remaining trial did not describe these processes in sufficient detail for a judgment to be made (Krishnan 2014). We considered Krishnan 2014 to have an unclear risk of selection bias.
Blinding
All six included trials described adequate blinding of participants and outcome assessors (Baig 2006; Beaussier 2007; Fustran 2015; Krishnan 2014, Polglase 2007; Wang 2010). In all trials, only the pharmacist who prepared the study solution but was otherwise unconnected to the study was not blinded. We considered all six trials to have low risk of performance and detection bias.
Incomplete outcome data
In one trial, missing data for opioid consumption from 12 out of 310 participants (3.9%) were handled by the last observation carried forward method (Polglase 2007). This method may distort the estimated means and standard deviations, as opioid consumption would be expected to reduce over time. In the same trial, data for pain on movement were missing from 5 out of 310 participants (1.6%), and complete case analysis was performed. However, the effect of handling the missing data in this manner may be negligible due to the small proportion of participants affected. Polglase 2007 had an additional post‐randomisation dropout rate of 4.9%. Overall, we judged this trial to have unclear risk of attrition bias.
Another trial did not report the number of participants who underwent randomisation or the number of losses and exclusions post‐randomisation (Baig 2006). Furthermore, Baig 2006 assessed adverse events and complications by retrospective chart review, which is prone to incomplete and missing data. However, they did not describe the prevalence and handling of missing data. For these reasons, we judged this trial to have unclear risk of attrition bias.
We considered the remaining four trials (67%), with an attrition rate of 10% or higher, and complete case analysis, to have high risk of attrition bias (Beaussier 2007; Fustran 2015; Krishnan 2014; Wang 2010).
Selective reporting
Two trials reported all predefined, clinically relevant, and reasonably expected outcomes, including pain, opioid consumption, adverse outcomes and complications (Polglase 2007; Wang 2010). Hence, we considered these studies to be at low risk of reporting bias.
In Baig 2006, pain at rest and on movement were both predefined outcomes, but they only reported results for pain at rest. In addition, the trial authors reported statistically significant results about the number of PCA doses, although it was not a predefined outcome. Beaussier 2007 omitted to report results for sedation, a predefined outcome. Furthermore, the trial authors reported that, “no major adverse event occurred”, but did not describe the definitions or methods of assessing these events. Fustran 2015 omitted to report results regarding pruritus, sedation and respiratory depression predefined in the methods. Krishnan 2014 omitted to report a number of clinically important predefined outcomes, including pain at rest, pain on movement, and opioid consumption. We considered these studies to be at high risk of reporting bias for the reasons described.
Other potential sources of bias
Small study bias
We considered Polglase 2007 (local anaesthetic = 143, control = 167) to be at unclear risk of small study bias. We considered the remaining five trials to be at high risk of small study bias (Baig 2006, local anaesthetic = 35, control = 35; Beaussier 2007, local anaesthetic = 21, control = 21; Fustran 2015, local anaesthetic = 17, control = 17 in the laparotomy subgroup; Krishnan 2014, local anaesthetic = 24, control = 6 in the laparotomy subgroup; Wang 2010, local anaesthetic = 28, control = 27). Small trials tend to produce imprecise overestimates of the true effect, may be associated with higher risk of bias, and may be more prone to the effects of random chance (Chaimani 2013; Dechartres 2013; Moore 1998; Nuesch 2010).
Industry bias
We considered risk of industry bias to be unclear in three trials. Beaussier 2007 received a grant from the manufacturer of ropivacaine, the local anaesthetic used in the study. Fustran 2015 received equipment and expenses from the device manufacturer. Polglase 2007 received equipment from the device manufacturer and grants from a hospital research foundation and a non‐profit organisation. However, these trials did not state the involvement of the industry sponsor in the design, conduct, analysis and reporting of the trials.
We considered the remaining three trials to have low risk of industry bias. Baig 2006 was funded by a private donation. Krishnan 2014 and Wang 2010 received equipment from the device manufacturer, but explicitly stated that the industry sponsor had no involvement in any aspects of the study. Additionally, Krishnan 2014 received a grant from a hospital research foundation.
Inclusion and exclusion criteria (selection bias)
We had no concerns regarding the selection criteria used in any of the six included trials (Baig 2006; Beaussier 2007; Fustran 2015; Krishnan 2014; Polglase 2007; Wang 2010), and judged them to have low risk of bias.
Methods of outcome assessment (information bias)
We considered Beaussier 2007 to be at low risk of bias.
We considered Fustran 2015; Krishnan 2014; Polglase 2007 and Wang 2010 to have unclear risk of bias, as the definitions and methods of assessing adverse events were not described at all or in sufficient detail.
In addition to unclear definitions, Baig 2006 identified adverse events by retrospective review of charts, which is prone to be unreliable and inaccurate. Hence, we considered this study to be at high risk of bias.
Methods of statistical analysis (analytical bias)
We considered Fustran 2015 and Wang 2010 to be at low risk of bias.
Baig 2006; Beaussier 2007 and Polglase 2007 did not clearly state if they had performed intention‐to‐treat analysis, so we considered these studies to have unclear risk of bias.
We considered Krishnan 2014 to be at high risk of bias due to making inappropriate comparisons or not performing appropriate statistical tests for some outcomes. It was also not clear if analyses in this trial were performed by intention‐to‐treat.
Effects of interventions
See: Table 1
We included six randomised controlled trials that enrolled a total of 564 participants undergoing midline laparotomy for colorectal resection comparing continuous wound infusion with a local anaesthetic to a normal saline placebo (Baig 2006; Beaussier 2007; Fustran 2015; Krishnan 2014; Polglase 2007; Wang 2010). There were a total of 23 post‐randomisation exclusions across these trials. Hence, we included a total of 541 participants in the analysis of at least one outcome (268 in local anaesthetic group; 273 in control group).
The results for the key outcomes are summarised in the Table 1.
Postoperative pain at rest and on movement
(See Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6)
1.1. Analysis.
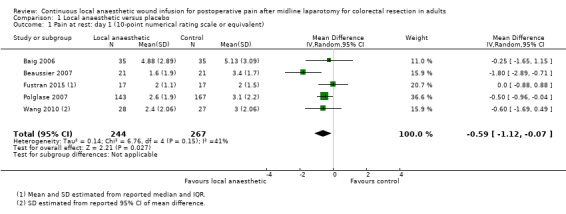
Comparison 1 Local anaesthetic versus placebo, Outcome 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent).
1.2. Analysis.
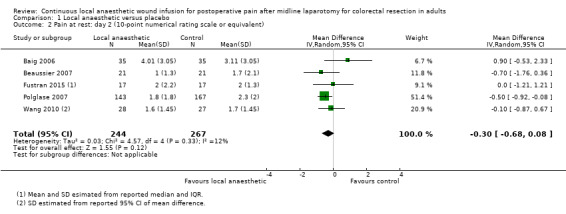
Comparison 1 Local anaesthetic versus placebo, Outcome 2 Pain at rest: day 2 (10‐point numerical rating scale or equivalent).
1.3. Analysis.
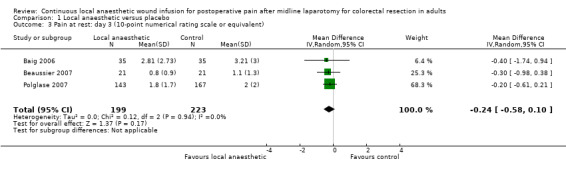
Comparison 1 Local anaesthetic versus placebo, Outcome 3 Pain at rest: day 3 (10‐point numerical rating scale or equivalent).
1.4. Analysis.
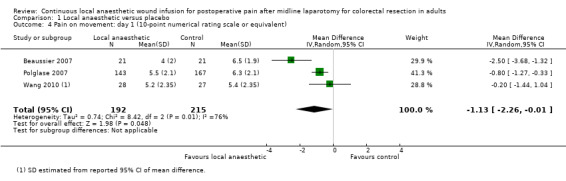
Comparison 1 Local anaesthetic versus placebo, Outcome 4 Pain on movement: day 1 (10‐point numerical rating scale or equivalent).
1.5. Analysis.
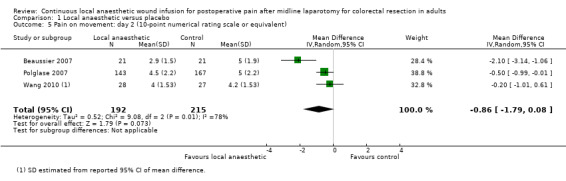
Comparison 1 Local anaesthetic versus placebo, Outcome 5 Pain on movement: day 2 (10‐point numerical rating scale or equivalent).
1.6. Analysis.
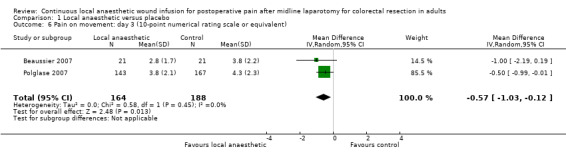
Comparison 1 Local anaesthetic versus placebo, Outcome 6 Pain on movement: day 3 (10‐point numerical rating scale or equivalent).
All six included trials assessed postoperative pain at rest using a 10‐point numerical rating scale (0 = no pain; 1 to 3 = mild pain; 4 to 6 = moderate pain; 7 to 10 = severe pain) or equivalent. All but one trial (Fustran 2015), assessed postoperative pain on movement. Fustran 2015 and Wang 2010 monitored postoperative pain for two days, Baig 2006 and Polglase 2007 for three days, and Beaussier 2007 and Krishnan 2014 for four days.
Although Baig 2006 described assessment of pain on movement in the methods, they did not publish the results. We were unable to obtain unpublished data for this outcome. Furthermore, this trial referred to the day of the operation as "Day 1", whereas conventionally postoperative day 1 refers to the following day. Baig 2006 assessed pain twice per day. We used the results from this trial labelled as "Day 2 AM" for the analysis of pain score on postoperative day 1, results labelled as "Day 3 AM " for the analysis of pain score on postoperative day 2, and so on, to achieve consistency with convention and with other included trials.
We estimated mean and standard deviation of pain scores in Fustran 2015 from the reported median and interquartile range. We estimated standard deviation of pain scores in Wang 2010 from the reported 95% confidence interval of the mean difference.
Krishnan 2014 described assessing pain at rest and on movement, but did not publish the results. We obtained unpublished data from the lead author's PhD thesis stored in the university archive. However, we did not find the standard deviation of the pain scores or any other parameters that we could use for estimation of the standard deviation reported. Hence, we could not include this trial in our analysis.
Compared to the control group, pain at rest was reduced in the local anaesthetic group on postoperative day 1 (MD −0.59, 95% CI −1.12 to −0.07; I2 = 41%; 5 studies, 511 participants; high‐certainty evidence). There was no evidence of a difference in pain at rest in the local anaesthetic group on postoperative day 2 (MD −0.30, 95% CI −0.68 to 0.08; I2 = 12%; 5 studies, 511 participants; high‐certainty evidence) or day 3 (MD −0.24, 95% CI −0.58 to 0.10; I2 = 0%; 3 studies, 422 participants; high‐certainty evidence). Weighted mean pain score at rest in the control group was 3.1 on postoperative day 1 (SD = 1.0; 5 studies), 2.1 on day 2 (SD 1.1; 5 studies), and 1.9 on day 3 (SD 1.4; 3 studies).
Compared to the control group, pain on movement was reduced in the local anaesthetic group on postoperative day 1 (MD −1.13, 95% CI −2.26 to −0.01; I2 = 76%; 3 studies, 407 participants; low‐certainty evidence). There was no evidence of a difference in pain on movement in the local anaesthetic group on postoperative day 2 (MD −0.86, 95% CI −1.79 to 0.08; I2 = 78%; 3 studies, 407 participants; low‐certainty evidence). There was a reduction in pain on movement in the local anaesthetic group on postoperative day 3 (MD −0.57, 95% CI −1.03 to −0.12; I2 = 0%; 2 studies, 352 participants; low‐certainty evidence). Weighted mean pain score on movement in the control group was 6.1 on postoperative day 1 (SD 1.2; 3 studies), 4.7 on day 2 (SD 1.1; 3 studies), and 4.2 on day 3 (SD 2.0; 2 studies).
Only one trial contributed data on pain at rest and on movement on postoperative day 4 (Beaussier 2007), showing no evidence of a difference at rest (MD 0.10, 95% CI −0.75 to 0.95; 42 participants; moderate‐certainty evidence) or on movement (MD −0.50, 95% CI −1.7 to 0.68; 42 participants; moderate‐certainty evidence) in the local anaesthetic group. Mean pain score on postoperative day 4 in the control group in this study was 0.9 (SD 1.3) at rest and 3 (SD 2) on movement.
We did not downgrade the certainty of the evidence for postoperative pain at rest on days 1, 2 and 3. We downgraded the certainty of the evidence for pain at rest on day 4 by one level to moderate for imprecision due to the small total number of participants.
For pain on movement on day 1 and day 2, we downgraded the certainty of the evidence by two levels to low for inconsistency, indicated by a high level of heterogeneity and reporting bias arising from selective outcome reporting. We downgraded the certainty of the evidence for pain on movement on day 3 by two levels to low for imprecision, due to the small total number of participants and reporting bias arising from selective outcome reporting. We downgraded the certainty of the evidence for pain on movement on day 4 by one level to moderate for imprecision due to the small total number of participants.
Postoperative opioid consumption via patient‐controlled analgesia (PCA)
(See Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10)
1.7. Analysis.
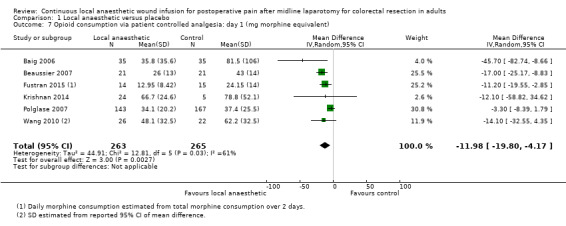
Comparison 1 Local anaesthetic versus placebo, Outcome 7 Opioid consumption via patient controlled analgesia: day 1 (mg morphine equivalent).
1.8. Analysis.
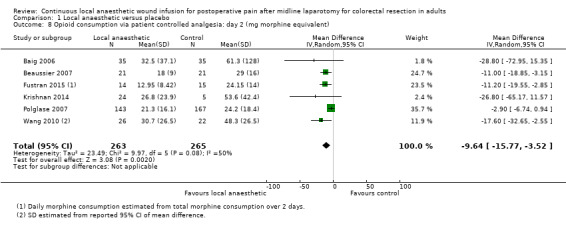
Comparison 1 Local anaesthetic versus placebo, Outcome 8 Opioid consumption via patient controlled analgesia: day 2 (mg morphine equivalent).
1.9. Analysis.
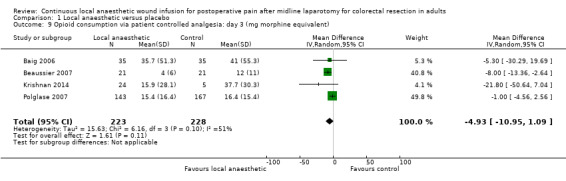
Comparison 1 Local anaesthetic versus placebo, Outcome 9 Opioid consumption via patient controlled analgesia: day 3 (mg morphine equivalent).
1.10. Analysis.
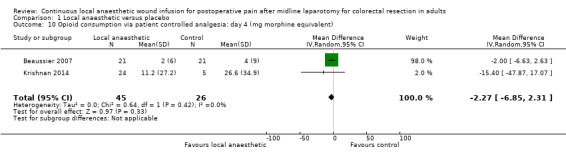
Comparison 1 Local anaesthetic versus placebo, Outcome 10 Opioid consumption via patient controlled analgesia: day 4 (mg morphine equivalent).
All six included trials compared postoperative opioid consumption via PCA between the two study groups. Fustran 2015 and Wang 2010 monitored postoperative opioid consumption for two days, Baig 2006 and Polglase 2007 for three days, and Beaussier 2007 and Krishnan 2014 for four days.
Four of these trials used morphine PCA (Baig 2006; Beaussier 2007; Fustran 2015; Polglase 2007). Fustran 2015 only reported the total consumption over two days. We estimated the mean and standard deviation of daily consumption assuming equal daily consumption, although we recognise that consumption on day 2 is likely to be less than day 1. Krishnan 2014 used PCA with fentanyl or oxycodone, and reported opioid consumption in milligrams of oxycodone equivalent. As intravenous morphine and oxycodone have 1:1 dose equivalence, we used the reported values directly in our analysis. Wang 2010 used morphine PCA in all participants except four (local anaesthetic 2/28; control 2/27), who received fentanyl PCA due to renal impairment or previous history of morphine‐induced hallucinations. This study only reported on morphine consumption in those participants who received morphine PCA. We estimated standard deviation of opioid consumption via PCA in Wang 2010 from the reported 95% confidence interval of the mean difference.
Compared to the control group, opioid consumption via PCA was reduced among participants who received continuous local anaesthetic wound infusion on postoperative day 1 (MD −12 mg, 95% CI −20 to −4; I2 = 61%; 6 studies, 528 participants; moderate‐certainty evidence) and day 2 (MD −10 mg, 95% CI −16 to −3.5; I2 = 50%; 6 studies, 528 participants; moderate‐certainty evidence). There was no evidence of a difference in opioid consumption on postoperative day 3 (MD −5 mg, 95% CI −11 to 1; I2 = 51%; 4 studies, 451 participants; moderate‐certainty evidence) or day 4 (MD −2 mg, 95% CI −7 to 2; I2 = 0%; 2 studies, 71 participants; low‐certainty evidence). Weighted mean opioid consumption via PCA (mg morphine equivalent) in the control group on postoperative day 1 was 41 (SD 11; 6 studies), 30 (SD 9.3; 6 studies) on day 2, and 17 (SD 9.4; 4 studies) on day 3.
We downgraded the certainty of the evidence for PCA opioid consumption on day 1 and day 2 by one level to moderate for attrition bias (serious study limitations), arising from large numbers of post‐randomisation exclusions and missing data. We downgraded the certainty of the evidence for PCA opioid consumption on day 3 by one level to moderate for attrition bias (serious study limitations). We downgraded the certainty of the evidence for PCA opioid consumption on day 4 by two levels to low for attrition bias (serious study limitations) and imprecision due to the small total number of participants.
Postoperative opioid‐related adverse events
Nausea or vomiting
(See Analysis 1.11)
1.11. Analysis.
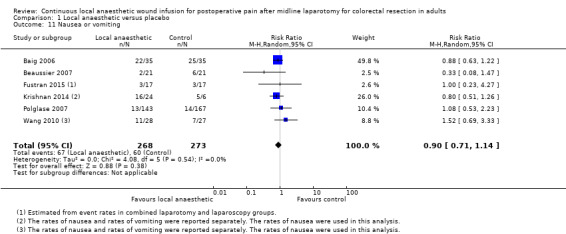
Comparison 1 Local anaesthetic versus placebo, Outcome 11 Nausea or vomiting.
Four trials reported the rates of postoperative nausea or vomiting (Baig 2006; Beaussier 2007; Fustran 2015; Polglase 2007). Fustran 2015 estimated the rates in the laparotomy subgroup based on reported rates in the overall study population, which additionally included participants undergoing laparoscopic colorectal resection. Two trials reported the rates of nausea and rates of vomiting separately (Krishnan 2014; Wang 2010). We used the reported rates of nausea in our analysis, based on the assumption that participants with vomiting must have also had nausea and therefore were a subset of those participants with nausea. Beaussier 2007 defined postoperative nausea or vomiting as requiring specific treatment with intravenous ondansetron. Polglase 2007 described evaluating nausea on a 10‐point numerical rating scale (0 = no nausea). The remaining trials did not describe how they defined and assessed this outcome, so it was not possible know if they had used acceptable approaches.
These six trials reported 127 cases of nausea or vomiting among their 541 included participants, and there was no evidence of a difference between the two study groups (67/268 (25%) in local anaesthetic group; 60/273 (22%) in control group; RR 0.90, 95% CI 0.71 to 1.14; I2 = 0%; 6 studies, 541 participants; moderate‐certainty evidence).
We downgraded the certainty of the evidence for this outcome by one level to moderate for indirectness relating to outcome assessment.
Ileus
(See Analysis 1.12)
1.12. Analysis.
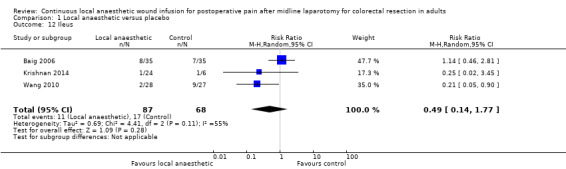
Comparison 1 Local anaesthetic versus placebo, Outcome 12 Ileus.
Varying definitions of postoperative ileus exist, but it is typically defined as a functional inhibition of bowel motility for more than three days after surgery (Luckey 2003), although some propose that delayed return of bowel motility is only clinically significant if it persists for more than six days (Artinyan 2008). Three trials reported the rates of postoperative ileus (Baig 2006; Krishnan 2014; Wang 2010). None of the included trials described how they defined and assessed this outcome, so it was not possible know if they had used acceptable and consistent approaches.
Among 155 participants included in three trials (Baig 2006; Krishnan 2014; Wang 2010), there were 28 cases of postoperative ileus in total (11/87 (13%) in local anaesthetic group; 17/68 (25%) in control group). There was no evidence of a difference between the study groups (RR 0.49, 95% CI 0.14 to 1.77; I2 = 55%; low‐certainty evidence).
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Urinary retention
In Krishnan 2014, there was no evidence of a difference in rates of postoperative urinary retention between the two study groups (1/24 (4%) in local anaesthetic group; 0/6 (0%) in control group; RR 0.84, 95% CI 0.04 to 18.44; very low‐certainty evidence). Urinary retention was a predefined outcome in Baig 2006, but they did not publish the results. There are numerous definitions of postoperative urinary retention based on history and physical examination, the need for bladder catheterisation and ultrasonographic assessment (Baldini 2009). However, how this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by three levels (maximum reached) to very low for risk of bias (serious study limitations) because all data for this outcome were contributed by a single trial with unclear random sequence generation and allocation concealment, indirectness relating to outcome assessment, imprecision due to the small number of participants, and reporting bias arising from selective outcome reporting.
Pruritus
(See Analysis 1.13)
1.13. Analysis.
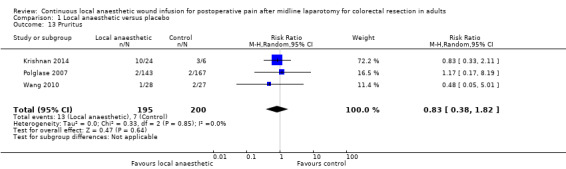
Comparison 1 Local anaesthetic versus placebo, Outcome 13 Pruritus.
In total, three trials reported 20 cases of pruritus in 395 participants (Krishnan 2014; Polglase 2007; Wang 2010), with no evidence of a difference between the study groups (13/195 (7%) in local anaesthetic group; 7/200 (4%) in control group; (RR 0.83, 95% CI 0.38 to 1.82; I2 = 0%; 3 studies, 395 participants; very low‐certainty evidence). Fustran 2015 predefined pruritus as an outcome but did not publish the results. There lacks a uniform approach to identify and assess postoperative pruritus across studies in the literature (Waxler 2005). However, how this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by three levels to very low for indirectness relating to outcome assessment, imprecision due to the small number of participants, and reporting bias arising from selective outcome reporting.
Sedation
Wang 2010 reported eight cases of sedation, with no evidence of a difference between the two study groups (4/28 (1%) in local anaesthetic group; 4/27 (1%) in control group; RR 0.96, 95% CI 0.27 to 3.47; very low‐certainty evidence). Three other trials predefined sedation as an outcome, but did not publish the results (Baig 2006; Beaussier 2007; Fustran 2015). Beaussier 2007 described evaluating sedation on a three‐point scale (0 = fully alert). The remaining trials did not describe how they defined and assessed this outcome, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by three levels to very low for indirectness relating to outcome assessment, imprecision due to the small number of participants, and reporting bias arising from selective outcome reporting.
Sleep disturbance
None of the included trials evaluated the outcome of sleep disturbance as objectively assessed by polysomnography. Pain and opioids are important contributors to postoperative sleep disturbance, characterised by reduction in total sleep time, elimination of rapid eye movement (REM) sleep, reduction in amounts of slow wave sleep, and increase in stage 2 non‐REM sleep (Rosenberg‐Adamsen 1996). Polysomnography is required to objectively assess postoperative sleep disturbance.
Beaussier 2007 assessed subjective sleep quality as an outcome. Participants were asked to rate their sleep quality on postoperative day 1 and day 2 on a 10‐point numerical rating scale (0 = very poor quality of sleep, 10 = excellent quality of sleep). Compared to the control group, participants in the local anaesthetic group reported improved sleep quality scores on day 1 (MD 2.9, 95% CI 1.4 to 4.4; 42 participants; low‐certainty evidence) and day 2 (MD 1.7, 95% CI 0.55 to 2.9; 42 participants; low‐certainty evidence). Mean sleep quality scores in the control group in this study were 5 (SD 3.2) on day 1 and 6.9 (SD 2.4) on day 2.
We downgraded the certainty of the evidence for this outcome by two levels to low due to indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Respiratory depression
(See Analysis 1.14)
1.14. Analysis.
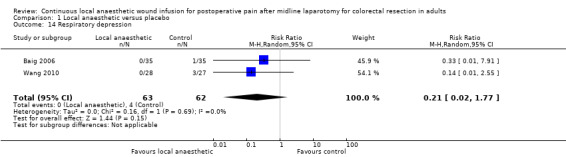
Comparison 1 Local anaesthetic versus placebo, Outcome 14 Respiratory depression.
Two trials with 125 participants (Baig 2006; Wang 2010), reported a total of four cases of respiratory depression (0/63 (0%) in local anaesthetic group; 4/62 (6%) in control group). There was no evidence of a difference between the two study groups (RR 0.21, 95% CI 0.02 to 1.77; I2 = 0%; 2 studies, 125 participants; very low‐certainty evidence). Respiratory depression was predefined as an outcome in two other trials (Beaussier 2007; Fustran 2015), but they did not publish the results. How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence by three levels to very low for indirectness relating to outcome assessment, imprecision due to the small number of participants, and reporting bias arising from selective outcome reporting.
Time to rescue analgesia
None of the included trials evaluated the outcome of time to rescue analgesia.
Time to first flatus and time to first bowel movement
(See Analysis 1.15)
1.15. Analysis.
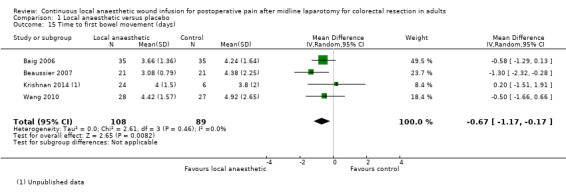
Comparison 1 Local anaesthetic versus placebo, Outcome 15 Time to first bowel movement (days).
Two trials evaluated the time to first flatus (Beaussier 2007; Polglase 2007). Polglase 2007 reported that there was "no difference" between the two study groups in the time to first flatus, but did not report any statistical parameters that we could include in our analysis for this outcome. Beaussier 2007 found no evidence of a difference in the time to first flatus between the two study groups (2.25 days (SD 0.67) in local anaesthetic group; 3 days (SD 1.71) in control group; MD −0.75 days, 95% CI −1.54 to 0.04; 42 participants; moderate‐certainty evidence).
Five trials evaluated time to first bowel movement (Baig 2006; Beaussier 2007; Krishnan 2014; Polglase 2007; Wang 2010). Again, Polglase 2007 only reported that there was "no difference" between the two study groups, but did not report any statistical parameters that we could include in our analysis for this outcome. Based on the remaining four trials, time to first bowel movement was reduced in the local anaesthetic group (MD −0.67 days, 95% CI −1.17 to −0.17; I2 = 0%; 4 studies, 197 participants; moderate‐certainty evidence), compared to the weighted mean time of 4.4 days (SD 1.1) in the control group.
We downgraded the certainty of the evidence for the time to first flatus and the time to first bowel movement by one level to moderate for imprecision due to the small total number of participants.
Time to ambulation
(See Analysis 1.16.)
1.16. Analysis.
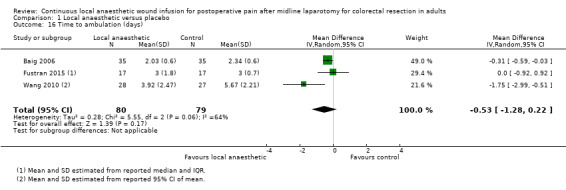
Comparison 1 Local anaesthetic versus placebo, Outcome 16 Time to ambulation (days).
Four trials evaluated the time to ambulation (Baig 2006; Fustran 2015; Krishnan 2014; Wang 2010). Krishnan 2014 reported the mean time to ambulation within each study group, but did not report the standard deviation or other statistical parameters that we could use to estimate the standard deviation, so we could not include this trial in our analysis for this outcome. We estimated the mean and standard deviation of the time to ambulation from the reported median and interquartile range in Fustran 2015, and from the reported 95% confidence interval of the mean in Wang 2010.
There was no evidence of a difference in the time to ambulation in the local anaesthetic group (MD −0.53 days, 95% CI −1.28 to 0.22; I2 = 64%; 3 studies, 159 participants; moderate‐certainty evidence), compared to the weighted mean time of 3.3 days (SD 0.6) in the control group.
We downgraded the certainty of the evidence for this outcome by one level to moderate for imprecision due to the small total number of participants.
Length of hospital stay
(See Analysis 1.17)
1.17. Analysis.
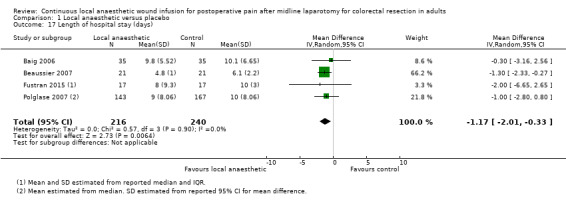
Comparison 1 Local anaesthetic versus placebo, Outcome 17 Length of hospital stay (days).
All six included trials evaluated the length of hospital stay (Baig 2006; Beaussier 2007; Fustran 2015; Krishnan 2014; Polglase 2007; Wang 2010). Krishnan 2014 reported the mean length of hospital stay within each study group, but did not report the standard deviation or other statistical parameters that we could use to estimate the standard deviation, so we could not include this trial in our analysis for this outcome. Wang 2010 reported that there was "no significant effect" on the length of hospital stay, but did not report any statistical parameters that we could include in our analysis for this outcome. We estimated mean and standard deviation of the length of hospital stay from the reported median and interquartile range in Fustran 2015, and from the reported median and 95% confidence interval for the mean difference in Polglase 2007.
The length of hospital stay was reduced in the local anaesthetic group (MD −1.2 days, 95% CI −2.0 to −0.3; I2 = 0%; 4 studies, 456 participants; high‐certainty evidence), compared to the weighted mean length of hospital stay of 7.4 days (SD 2.4) in the control group. We did not downgrade the certainty of the body of evidence for this outcome.
Serious postoperative adverse events
(See Analysis 1.18; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22.)
1.18. Analysis.
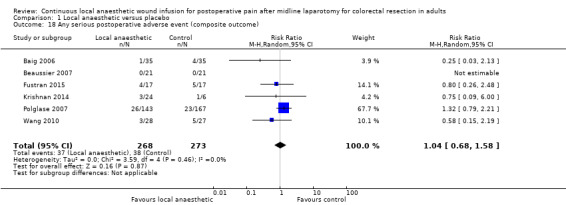
Comparison 1 Local anaesthetic versus placebo, Outcome 18 Any serious postoperative adverse event (composite outcome).
1.19. Analysis.
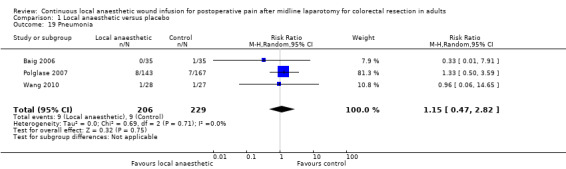
Comparison 1 Local anaesthetic versus placebo, Outcome 19 Pneumonia.
1.20. Analysis.
Comparison 1 Local anaesthetic versus placebo, Outcome 20 Laparotomy wound breakdown.
1.21. Analysis.
Comparison 1 Local anaesthetic versus placebo, Outcome 21 Laparotomy wound infection.
1.22. Analysis.
Comparison 1 Local anaesthetic versus placebo, Outcome 22 Local anaesthetic systemic toxicity.
Any serious postoperative adverse event (composite outcome)
Six trials reported a total of 75 serious postoperative adverse events among 541 participants (37/268 (14%) in local anaesthetic group; 38/273 (14%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 1.04, 95% CI 0.68 to 1.58; I2 = 0%; low‐certainty evidence).
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and reporting bias arising from selective outcome reporting.
Death by any cause
Only Krishnan 2014 reported this outcome until hospital discharge. Among 30 participants in the laparotomy subgroup, there was one death in the control group (1/6 (17%)) due to aspiration pneumonia, and none in the local anaesthetic group (0/24 (0%)). There was no evidence of a difference (RR 0.09, 95% CI 0.00 to 2.05; low‐certainty evidence).
We downgraded the certainty of the evidence for this outcome by two levels to low for risk of bias (serious study limitations) because all data for this outcome were contributed by a single trial with unclear random sequence generation and allocation concealment, and imprecision due to the small total number of participants.
Pulmonary complications
Pneumonia
Three trials (Baig 2006; Polglase 2007; Wang 2010) reported a total of 18 cases of pneumonia among 435 participants (9/206 (4%) in local anaesthetic group; 9/229 (4%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 1.15, 95% CI 0.47 to 2.82; I2 = 0%; moderate‐certainty evidence). The trials did not report how they defined or assessed this outcome, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by one level to moderate for indirectness relating to outcome assessment.
Respiratory failure
Baig 2006 reported one case of respiratory failure among 70 participants (0/35 (0%) in local anaesthetic group; 1/35 (3%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 0.33, 95% CI 0.01 to 7.91; low‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Pulmonary oedema
Baig 2006 reported one case of pulmonary oedema among 70 participants (0/35 (0%) in local anaesthetic group; 1/35 (3%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 0.33, 95% CI 0.01 to 7.91; low‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Venous thromboembolic complications
Deep vein thrombosis
Polglase 2007 reported two cases of deep vein thrombosis in the control group (2/167 (1%)), and none in the local anaesthetic group (0/143 (0%)) until hospital discharge. There was no evidence of a difference between the two study groups (RR 0.23, 95% CI 0.01 to 4.82; very low‐certainty evidence). Deep vein thrombosis was predefined as an outcome in Baig 2006, but the results were not published. How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by three levels to very low for indirectness relating to outcome assessment, imprecision due to the small total number of participants, and reporting bias arising from selective outcome reporting.
Pulmonary embolism
Polglase 2007 reported one case of pulmonary embolism in the local anaesthetic group (1/143 (1%)), and none in the control group (0/167 (0%)) until hospital discharge. There was no evidence of a difference between the two study groups (RR 3.50, 95% CI 0.14 to 85.25; low‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Wound catheter‐related complications
Visceral or vascular injury
None of the included trials evaluated the outcome of wound catheter‐related visceral or vascular injury.
Laparotomy wound breakdown
Two trials (Krishnan 2014; Polglase 2007) reported a total of 14 cases of laparotomy wound breakdown or dehiscence among 340 participants (3/167 (2%) in local anaesthetic group; 11/173 (6%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 0.32, 95% CI 0.09 to 1.12; I2 = 0%; low‐certainty evidence). All cases occurred in the same trial (Polglase 2007). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Laparotomy wound infection
Five trials evaluated the rates of laparotomy wound infection (Baig 2006; Fustran 2015; Krishnan 2014; Polglase 2007; Wang 2010). In Fustran 2015, rates in the laparotomy subgroup were estimated based on reported rates in the overall study population, which additionally included participants undergoing laparoscopic colorectal resection. A total of 32 cases of laparotomy wound infection were reported among 499 participants (18/247 (7%) in local anaesthetic group; 14/252 (6%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 1.18, 95% CI 0.58 to 2.37; I2 = 0%; moderate‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by one level to moderate for indirectness relating to outcome assessment.
Intra‐abdominal infection
Fustran 2015 evaluated the rates of intra‐abdominal infection until hospital discharge. Rates in the laparotomy subgroup (2/17 (12%) in local anaesthetic group; 1/17 (6%) in control group) were estimated based on reported rates in the overall study population, which additionally included participants undergoing laparoscopic colorectal resection. There was no evidence of a difference between the study groups (RR 2.00, 95% CI 0.20 to 20.04; low‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment, and imprecision due to the small total number of participants.
Local anaesthetic systemic toxicity
Local anaesthetic systemic toxicity is a potentially life‐threatening adverse event that may occur after administration of local anaesthetics. It is characterised by progressive cardiovascular and neurological depression, with a constellation of symptoms including severe hyper‐ or hypotension, cardiac arrhythmias, loss of consciousness, and seizures (El‐Boghdadly 2018).
Beaussier 2007 and Krishnan 2014 evaluated the rates of local anaesthetic systemic toxicity among trial participants until hospital discharge. There were no cases of local anaesthetic systemic toxicity in these trials (0/45 (0%) in local anaesthetic group; 0/27 (0%) in control group; RR not available; 95% CI not available; very low‐certainty evidence). The trial authors did not report how they had defined or assessed this outcome so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by three levels to very low for risk of bias (serious study limitations) because nearly half of all data for this outcome were contributed by a trial with unclear random sequence generation and allocation concealment, indirectness relating to outcome assessment, and imprecision due to the small total number of participants.
Other
Myocardial infarction
Wang 2010 reported one case of myocardial infarction among 55 participants (0/28 (0%) in local anaesthetic group; 1/27 (4%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 0.32, 95% CI 0.01 to 7.57; low‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Renal impairment
Polglase 2007 reported eight cases of renal impairment among 310 participants (4/143 (3%) in local anaesthetic group; 4/167 (2%) in control group) until hospital discharge. There was no evidence of a difference between the two study groups (RR 1.12, 95% CI 0.29 to 4.39; low‐certainty evidence). How this outcome was defined or assessed in the included trials was not reported, so it was not possible know if they had used acceptable approaches.
We downgraded the certainty of the evidence for this outcome by two levels to low for indirectness relating to outcome assessment and imprecision due to the small total number of participants.
Patient satisfaction
None of the included trials evaluated patient satisfaction.
Unavailable or unusable data
A number of studies did not report or only partially reported the results of certain predefined outcomes, and we could not included them in the meta‐analysis. We have summarised studies and outcomes with unavailable or unusable data in Appendix 2.
Subgroup analyses
See Appendix 3.
Sensitivity analyses
See Appendix 4.
Discussion
Summary of main results
This review compared the effectiveness of continuous wound infusion of local anaesthetic versus placebo for controlling postoperative pain after midline laparotomy for colorectal resection. We identified six trials, contributing a total of 541 participants to our meta‐analysis (local anaesthetic 268; control 273; Baig 2006; Beaussier 2007; Fustran 2015; Krishnan 2014; Polglase 2007; Wang 2010).
All six included trials randomised participants to continuous wound infusion of a local anaesthetic or a placebo as a component of a multimodal analgesic regimen in which all participants were given additional simple non‐opioid analgesics as well as opioids via a patient‐controlled analgesia (PCA) system as required to achieve adequate pain relief. All trials used opioid consumption via PCA in addition to pain at rest and on movement, scored on a 10‐point numerical rating scale or equivalent (0 = no pain; 1 to 3 = mild pain; 4 to 6 = moderate pain; 7 to 10 = severe pain) to evaluate the overall analgesic efficacy of continuous wound infusion of local anaesthetic versus placebo.
On postoperative day 1, our analysis found a reduction in pain at rest by approximately 1 point from the control group benchmark of approximately of 3 points. We have high confidence in this result. Pain on movement was reduced by approximately 1 point from the control group benchmark of 6 points, but we have limited confidence in this result. At the same time, we have moderate confidence that opioid consumption via PCA was reduced by approximately 10 mg morphine‐equivalent in the local anaesthetic group from the control group benchmark of approximately 40 mg. The simultaneous reduction in opioid consumption via PCA in addition to the reduction in pain scores indicate that the analgesic efficacy of the intervention is more substantial than is reflected by the reduction in pain scores alone. Overall, continuous local anaesthetic wound infusion versus placebo acutely improves postoperative pain control after midline laparotomy for colorectal resection in adults.
However, the analgesic effect of continuous local anaesthetic wound infusion versus placebo is probably not sustained, with little or no effect on pain scores after postoperative day 1 or opioid consumption via PCA after postoperative day 2. Our analysis found no evidence of a difference in pain at rest on postoperative day 2, day 3 or day 4, or pain on movement on postoperative day 2 or day 4, or opioid consumption via PCA on postoperative day 3 or day 4. We have moderate to low confidence in these results, except high confidence in the result for pain at rest on postoperative day 2 and day 3. There was a reduction in pain on movement by approximately 1 point in the local anaesthetic group on postoperative day 3 from the control group benchmark approximately 4 points, but we have low confidence in this result. We have moderate confidence that there was a reduction in opioid consumption via PCA by approximately 10 mg morphine‐equivalent in the local anaesthetic group on postoperative day 2 from the control group benchmark of approximately 30 mg.
Recognised postoperative adverse events related to opioids include nausea or vomiting, ileus, urinary retention, pruritus, sedation, sleep disturbance and respiratory depression, although many other contributing factors are usually also present in the postoperative period. All six included trials monitored and reported rates of nausea or vomiting, but only a few trials assessed other postoperative opioid‐related adverse events. None of the trials evaluated sleep disturbance objectively. Based on subjective assessment in one trial, continuous local anaesthetic wound infusion versus placebo may improve sleep quality. Our analysis found no evidence of a difference between the local anaesthetic group and the control group in the rates of postoperative opioid‐related adverse events other than sleep quality. This may be due to a lack of power to detect small effect sizes. We have very low confidence in the available results for most outcomes, except moderate confidence in the result for nausea or vomiting and low confidence in the results for ileus and sleep disturbance. Although continuous local anaesthetic wound infusion versus placebo probably reduces opioid consumption via PCA in the early postoperative period, it likely has little or no effect on the opoid‐related adverse events of nausea or vomiting and ileus. This is not surprising as opioid use is only one of a multitude of perioperative risk factors that contribute to these adverse events (Apfel 2012; Artinyan 2008; Gan 2006; Rybakov 2017). The effects of the intervention on the rates of other postoperative opioid‐related adverse events are uncertain.
Recovery of bowel function can be assessed by the time to first flatus and the time to first bowel movement, as well as rates of ileus. Overall functional recovery after surgery is reflected by the time to ambulation and the length of hospital stay. Moreover, the length of hospital stay is a particularly important outcome for patients and the healthcare system. Our analysis found no evidence of a difference between the local anaesthetic group and the control group in ileus, time to first flatus or time to ambulation, although this could be due to insufficient power to detect small effect sizes. The time to first bowel movement was reduced by approximately one day in the local anaesthetic group from the control group benchmark of approximately four days. We are moderately confident in this result. The length of hospital stay was reduced by approximately one day in the local anaesthetic group compared to the control group benchmark of seven days, with high certainty. Overall, continuous local anaesthetic wound infusion compared to placebo improves functional recovery after midline laparotomy for colorectal resection in adults.
The intervention may have little or no effect on the rates of any serious postoperative adverse events. Our analysis found no evidence of a difference between the two study groups in any serious postoperative adverse events, including death by any cause, pulmonary complications, venous thromboembolic complications, wound catheter‐related complications (including laparotomy wound infection), local anaesthetic systemic toxicity, myocardial infarction, or renal impairment. We have moderate confidence in the results for pneumonia and laparotomy wound infection. However, due to the small number of trials contributing data on the other serious postoperative adverse events considered, we have low to very low confidence in these results and we caution against drawing conclusions.
The implementation of continuous wound infusion varied across the included trials in factors such as the number and location of the wound catheter, the local anaesthetic agent and dose, the infusion programme (rate of infusion, bolus prior infusion), and the non‐randomised co‐analgesic regimen used in the trials. This may account for the moderate to high levels of heterogeneity in a small number of analyses in this review. Subgroup analyses suggested that preperitoneal placement of the wound catheter and delivery of a bolus prior to commencing continuous wound infusion may potentially increase the efficacy of the intervention. This warrants further investigation through high‐quality, randomised controlled trials.
Overall completeness and applicability of evidence
All six trials included in this review involved adults undergoing elective midline laparotomy for colorectal resection. This review was dominated by a single medium‐sized trial (310 participants) with broad inclusion criteria irrespective of anaesthetic risk, underlying pathology or co‐morbidities (Polglase 2007). The remaining five trials all had small sample sizes (30 to 70 participants). Small trials tend to produce imprecise overestimates of the true effect, may be associated with higher risk of bias, and may be more prone to the effects of random chance (Chaimani 2013; Dechartres 2013; Moore 1998; Nuesch 2010). Most trials excluded individuals with high anaesthetic risk (ASA class IV or greater), renal or hepatic failure, obesity, chronic pain, psychiatric disorders, or drug or alcohol abuse. In two trials, the indication for surgery was limited to bowel cancer, and individuals with other bowel conditions such as inflammatory bowel disease were excluded (Beaussier 2007; Fustran 2015). These two trials accounted for under 15% of the total number of participants. The indication for surgery was unrestricted in Wang 2010 and unspecified in the remaining trials (Baig 2006; Krishnan 2014; Polglase 2007). All six trials were conducted in the elective setting, so the findings may not be applicable to patients with pathologies such as bowel perforation, acute bowel obstruction or intra‐abdominal infection that typically necessitate emergency surgery. Individuals with intra‐abdominal sepsis or abscess were excluded from two trials (Baig 2006; Fustran 2015).
Most trials measured and reported data on pain at rest and on movement, opioid consumption, opioid‐related adverse event of nausea or vomiting, the time to first bowel movement, the length of hospital stay, and the serious postoperative adverse event of laparotomy wound infection. However, few trials reported data on the postoperative opioid‐related adverse events of ileus, urinary retention, pruritus, sedation, respiratory depression, or the functional recovery outcomes of the time to first flatus and the time to ambulation. There was also a paucity of data on serious postoperative adverse events, including death by any cause, pulmonary complications, venous thromboembolic complications, wound catheter‐related complications (other than laparotomy wound infection), local anaesthetic systemic toxicity, myocardial infarction, and renal impairment. There were no available data on the time to rescue analgesia, patient satisfaction, or the serious postoperative adverse event of wound catheter‐related visceral or vascular injury. There were no objective data on the opioid‐related adverse event of sleep disturbance, but subjective data were available from one trial. Incomplete reporting of predefined outcomes was present in all six included trials. Most of our attempts to obtain the unpublished results were unsuccessful. We were required to estimate the mean and standard deviation of several continuous outcomes and impute the event rates of several dichotomous outcomes from other reported parameters. All six included trials in our review compared continuous wound infusion of a local anaesthetic against a normal saline placebo. This required a wound catheter to be inserted in participants in both intervention and control groups, which may confound any potential complications related to the physical placement of a wound catheter, such as visceral and vascular injury, laparotomy wound breakdown, laparotomy wound infection, and intra‐abdominal infection.
Quality of the evidence
We assessed all six included trials to be at high or unclear risk of bias for at least one of the examined domains (Characteristics of included studies). We assessed random sequence generation and allocation concealment to be adequate in all trials except one (Krishnan 2014), where insufficient details were provided. We assessed blinding to be adequate in all trials. The main study limitations included attrition bias in trials with large numbers of post‐randomisation exclusions or missing data for some outcomes, reporting bias related to selective reporting of outcomes in trials where the results of some predefined outcomes were not published, and information bias in trials using unclear or different definitions or methods of assessment for some outcomes.
We downgraded the certainty of the evidence for serious study limitations, inconsistency between trials, indirectness of outcome assessment, and imprecision of estimates of effect. We rated the certainty of the evidence for a small number of clinically and functionally important outcomes, including pain at rest on postoperative day 1, opioid consumption via PCA on postoperative day 1, the time to first bowel movement, and the length of hospital stay, as moderate or high (Table 1). We rated the certainty of the evidence for other key outcomes as low (pain on movement on postoperative day 1, any serious postoperative adverse event; Table 1), suggesting that the true effects of the intervention on these outcomes may be substantially different from the effect estimates, and further research is very likely to change the effect estimates and have an important impact on our confidence in the results.
Potential biases in the review process
We conducted a thorough literature search using a large number of synonymous search terms within each domain of the search strategy in order to capture as many relevant results as possible. We included pain as a domain because we expected pain reduction to be a primary endpoint in all trials evaluating continuous local anaesthetic wound infusion, and therefore pain or words related to pain would be mentioned in the title or abstract of the publication. Similarly, we included randomised controlled trial as a domain because we only wanted to include high‐quality randomised controlled trials in this review. Although unlikely, it is possible that potentially relevant trials that did not mention these two domains in the title or abstract may have been missed by this search strategy. We minimised human error in study selection and data extraction by having two review authors independently complete this process. To minimise publication bias, we sought unpublished data from authors of trials published in full text as well as trials published as conference abstracts only. We needed to estimate or impute meta‐analysis parameters for a large number of outcomes due to incomplete outcome reporting. Sensitivity analyses showed that our primary analysis was robust despite the use of estimated or imputed data.
Agreements and disagreements with other studies or reviews
There is increasing recognition of the need for evidence‐based guidelines for procedure‐specific pain management (Joshi 2013; Kehlet 2007), since the mechanisms and intensity of pain, the placebo response, and the treatment effects may differ between different surgical procedures and surgical incisions (Beaussier 2012; Espitalier 2013; Gerbershagen 2013; Gerbershagen 2014; Gray 2005). We are not aware of any published systematic review and meta‐analysis that adequately evaluated the efficacy of continuous local anaesthetic wound infusion after midline laparotomy for colorectal resection.
Liu 2006 included 44 trials of continuous wound infusion of a local anaesthetic versus a placebo for the management of postoperative pain following various obstetric, gynaecological, urological, orthopaedic, cardiothoracic, and general surgical procedures. The 'general surgery' subgroup contained a total of 12 trials, mainly consisting of trials in inguinal hernia repair (5 trials) and cholecystectomy (3 trials), with a small number of trials in colorectal surgery (2 trials), abdominal aortic aneurysm repair (1 trial), and unspecified major abdominal surgery (1 trial). Compared to the control group, there was a small reduction in mean daily pain score on a 0 to 100 mm visual analogue scale at rest (MD −11 mm, 95% CI −17 to −1; P = 0.02) in the local anaesthetic group, but there was no difference in pain on movement. Reductions in mean daily opioid consumption (MD −12 mg, 95% CI −19 to −6; P < 0.001) and postoperative nausea or vomiting (odds ratio 0.47, 95% CI 0.24 to 0.89; P = 0.02) were also found. There was no difference in the length of hospital stay. Liu 2006 did not examine other relevant clinical outcomes, including adverse events. They did not examine heterogeneity. They excluded trials that did not directly report means and standard deviations and made no attempts to impute these parameters. Only one of the six trials included in our Cochrane Review (Baig 2006), was also included by Liu 2006. In contrast to our assessment (Characteristics of included studies), Liu 2006 gave this trial a Jadad score of 4 (rigorous) using the Oxford quality scoring system (Jadad 1996), which has been criticised as an over‐simplistic, flawed, and unreliable method of assessing quality and risk of bias (Higgins 2017).
Another heterogeneous review included 32 trials of continuous infusion or intermittent injection of a local anaesthetic through a wound catheter versus a placebo or no wound catheter for the management of postoperative pain following various major and minor gastrointestinal, obstetric, gynaecological, urological, plastic, thoracic, vascular surgical procedures (Gupta 2011). Subgroup analyses for "major abdominal surgery", consisting of a heterogeneous mix of gastrointestinal, urological and vascular procedures, showed no difference in pain at rest, pain on movement or opioid consumption with moderate or high levels of heterogeneity present in all end points. Gupta 2011 conducted all other analyses by pooling data from all trials. They examined a small number of postoperative opioid‐related adverse events and found no difference in postoperative nausea or vomiting, ileus or pruritus. There was no difference in laparotomy wound infection, but there was a lower risk of wound breakdown in the local anaesthetic group (3/163 in local anaesthetic group, 12/191 in control group; P = 0.048). The length of hospital stay was shorter in the local anaesthetic group (MD −0.6 days, 95% CI −1.2 to 0.0; P = 0.04). Gupta 2011 included three of the six trials included in this Cochrane Review (Baig 2006; Beaussier 2007; Polglase 2007), and all three trials received a Jadad score of 5 (most rigorous) using the Oxford quality scoring system, in contrast to our assessment (Characteristics of included studies).
Reviews by Karthikesalingam 2008 and Ventham 2014 specifically examined trials in colorectal resection. We included in our Cochrane Review, three of the five trials included in Karthikesalingam 2008 (Baig 2006; Beaussier 2007; Polglase 2007), however, Karthikesalingam 2008 also pooled in the analysis two additional trials with different surgical incisions and different comparators (Cheong 2001; Fredman 2001). Pooled analysis in Karthikesalingam 2008 showed reduced postoperative opioid consumption on day 1 in the local anaesthetic group compared to the control group (MD −8.34 mg, 95% CI −16.38 to −0.31; P = 0.04), but not on day 2 and day 3. The local anaesthetic group also had small reductions in postoperative pain at rest on day 3, but not on day 1 and day 2, and in pain on movement on day 1 to day 3. There was no difference in the time to first bowel movement or the length of hospital stay. However, statistically significant heterogeneity was present in all end points. Karthikesalingam 2008 did not examine other clinically important outcomes, including adverse events. They reportedly used the Oxford quality scoring system to assess the quality of all included trials but did not publish the scores. Ventham 2014 included a total of 12 trials, appropriately separated into subgroups by surgical technique (laparoscopic and open) and intervention (wound infiltration, intraperitoneal instillation and peripheral nerve block). They further divided six trials involving open surgery and wound infiltration into two subgroups, based on the plane of wound infiltration (subfascial and suprafascial). We included four of these trials in our Cochrane Review (Baig 2006; Beaussier 2007; Polglase 2007; Wang 2010). However, Ventham 2014 also included two additional trials with different surgical incisions and different comparators in these subgroup analyses (Cheong 2001; Ozturk 2011). Local anaesthetic administered via a subfascial wound catheter in open surgery was associated with reduced postoperative opioid consumption on day 1 (MD −16.6 mg, 95% CI −23.9 to −9.2; P < 0.001) and day 2 (MD −12.18 mg, 95% CI −19.05 to −5.30; P = 0.0005), reduced the time to first bowel movement (MD −0.8 days, 95% CI −1.5 to 0.03; P = 0.04), but there was no difference in pain on movement, nausea or vomiting, or the length of hospital stay. Local anaesthetic administered via a suprafascial wound catheter in open surgery was associated with reduced postoperative pain on movement on day 1 (MD −0.71, 95% CI −1.14 to −0.28; P = 0.001) and day 2 (MD −0.50, 95% CI −0.93 to −0.07; P = 0.02), but there was no difference in opioid consumption, nausea or vomiting, the time to first bowel movement, or the length of hospital stay. They found no difference in rates of ileus, wound complications or venous thromboembolic complications in the pooled analysis combining all subgroups. They did not examine other postoperative opioid‐related adverse events and other clinically important adverse events. Ventham 2014 used the Cochrane 'Risk of bias' assessment tool and the results were similar to our assessment (Characteristics of included studies).
Authors' conclusions
Implications for practice.
For clinicians
In adults undergoing elective midline laparotomy for colorectal resection, continuous wound infusion of a local anaesthetic compared to a normal saline placebo probably has beneficial effects on pain and recovery when used as a component of multimodal management of postoperative pain.
The intervention reduces postoperative pain at rest and the length of hospital stay (high‐certainty evidence), probably reduces postoperative opioid consumption via PCA and the time to first bowel movement (moderate‐certainty evidence), and may reduce pain on movement (low‐certainty evidence). The intervention may have little or no effect on the rates of any serious postoperative adverse events, including death by any cause, pulmonary complications, venous thromboembolic complications, wound catheter‐related complications, local anaesthetic systemic toxicity, myocardial infarction, or renal impairment (low‐certainty evidence). The reasons for downgrading the certainty of the evidence included risk of bias relating to study design or missing data, inconsistency between trials, indirectness of outcome assessment, and imprecision of the estimates of effect.
The findings of our review are most applicable to adults undergoing elective midline laparotomy for resection of colorectal cancer, as those undergoing the surgery for other bowel conditions such as inflammatory bowel disease were excluded from two of six trials, although the remaining four trials did not restrict the indication for surgery. The findings may not be applicable to adults undergoing emergency surgery for treatment of acute bowel pathologies.
For patients
Continuous injection of a local anaesthetic into the wound, compared to an inactive substance such as salty water (normal saline), reduces pain at rest and leads to earlier hospital discharge after an elective surgery to remove a part of the bowel through a long vertical cut in the abdomen. It probably reduces the requirement for morphine‐like pain killers and leads to earlier bowel movement, and may reduce pain on movement. It does not appear to affect the rates of any serious complications, although we have limited confidence in this result. The reasons for downgrading the certainty of the evidence included limitations related to study design or missing data, inconsistency between trials, indirectness in the way the outcomes were measured in some trials, and imprecise results due to the small number of participants.
The findings of our review are most relevant to adults electively having this type of surgery to treat bowel cancer, as those having the surgery to treat other bowel conditions such as inflammatory bowel disease were excluded from two of six trials, although the remaining four trials did not restrict the reason for needing surgery. The findings may not be relevant to adults needing to have this type of surgery in an emergency.
For policy makers and funders of the intervention
Overall, our review supports the use of continuous local anaesthetic wound infusion as a component of a multimodal management strategy for postoperative pain after elective midline laparotomy for colorectal resection, based on high‐certainty evidence that the intervention reduces pain at rest and the length of hospital stay, and moderate‐certainty evidence that the intervention probably reduces opioid consumption via PCA and the time to first bowel movement. In addition, our review found low‐certainty evidence that the intervention may reduce pain on movement. The intervention does not appear to affect the rates of any serious postoperative adverse events, although we have limited confidence in this result. Ongoing attention and vigilance must be applied to monitor serious postoperative adverse events.
Implications for research.
General
There are a number of areas to be addressed by further research. High‐quality randomised controlled trials are necessary to evaluate the effectiveness of continuous local anaesthetic wound infusion after midline laparotomy for colorectal resection compared to a placebo or sham, or to an alternative method, such as transverse abdominis plane block or thoracic epidural analgesia. In addition, the optimal implementation of continuous wound infusion in terms of number and location of wound catheter(s), choice and dose of analgesic agent(s), and infusion programme needs to be established. Subgroup analyses suggested that preperitoneal placement of the wound catheter and delivery of a bolus prior to commencing continuous wound infusion may potentially increase the efficacy of the intervention. This warrants further investigation through high‐quality randomised controlled trials. Health economic evaluations of continuous local anaesthetic wound infusion are warranted to balance the current high cost of wound infusion devices against the economic benefits of potentially fewer adverse events and faster recovery.
Study design
Follow guidance on study design provided by the CONSORT statement (Schulz 2010).
Consider a stratified design with predefined subgroup analyses to evaluate the intervention in different settings (e.g. emergency versus elective surgery, open versus laparoscopic‐assisted approach) and participants (e.g. by age, body mass index, risk factors for postoperative opioid‐related adverse events).
Clearly define the non‐randomised multimodal analgesic regimen provided. The regimen should be based on current best practice.
Consider novel study designs, such as a platform trial design, that can enable efficient comparison of multiple adaptive treatment groups in a heterogenous population (Berry 2015).
Adopt strategies to mitigate the study limitations affecting previous trials as extensively outlined in this review, including: small sample size, attrition bias, reporting bias, information bias, industry bias.
Outcome measurement and reporting
Include the following outcomes: daily pain score at rest and with movement, daily opioid consumption, rates of postoperative opioid‐related adverse events (e.g. nausea or vomiting, ileus, urinary retention, pruritus, sedation, respiratory depression, sleep disturbance), the time to first flatus, the time to first bowel movement, the time to ambulation, the length of hospital stay, patient satisfaction.
If other forms of opioids are used concurrently with an opioid PCA system as a part of the multimodal analgesia regimen, total opioid consumption should be included as an outcome measure in addition to PCA opioid consumption.
Closely monitor and record rates of adverse events and complications, including: potential treatment complications (e.g. local anaesthetic toxicity, wound catheter injury), wound complications (e.g. laparotomy wound infection, laparotomy wound breakdown), surgical complications (e.g. anastomotic leak, intra‐abdominal infection), other serious postoperative adverse events (e.g. pulmonary complication, myocardial infarction, renal failure, thromboembolic complications), mortality.
Consider inclusion of health economic outcome measures, such as cost of hospitalisation and interventions.
Consider medium‐ to long‐term outcomes such as return to work, functional recovery and development of chronic pain.
Clearly state the definitions and methods of assessment for all outcomes.
Report all specified outcomes for all treatment groups at baseline and all assessment intervals with appropriate summary statistics, including estimates of effect (e.g. mean, median) and precision (e.g. standard deviation, range, interquartile ranges, confidence interval). Consider including this information in an appendix if unable to be included in the primary publication or depositing data sets in an appropriate public data repository.
Avoid multiple publications of the same study.
Acknowledgements
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
The review authors would like to thank the following peer reviewers for the review: Dario Bugada, Katie LeBlanc, and Andrew Moore.
The authors acknowledge Prof Michel B. Edye (Professor of Surgery, School of Medicine, Western Sydney University, NSW, Australia) for reviewing the protocol, and Dr Caroline Banh (Resident Medical Officer, Blacktown Hospital, NSW, Australia) for helping translate a trial published in French to enable the assessment of its eligibility for inclusion.
Appendices
Appendix 1. Search strategies
CENTRAL (CRSO)
#1MESH DESCRIPTOR Anesthesia, Local #2MESH DESCRIPTOR Anesthetics, Local EXPLODE ALL TREES #3MESH DESCRIPTOR Analgesia EXPLODE ALL TREES #4MESH DESCRIPTOR Bupivacaine EXPLODE ALL TREES #5MESH DESCRIPTOR Lidocaine EXPLODE ALL TREES #6MESH DESCRIPTOR Mepivacaine EXPLODE ALL TREES #7(((local or regional or peripheral or intraabdominal or intra‐abdominal or preperitoneal or pre‐peritoneal or subfascial or suprafascial or "transversus abdominis plane*" or TAP or rectus sheath* or subcutaneous) adj5 (an?esthe* or analgesi* or block*))):TI,AB,KY #8((bupivacaine or levobupivacaine or ropivacaine or lignocaine or lidocaine or mepivacaine)):TI,AB,KY #9#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10MESH DESCRIPTOR Infusion Pumps EXPLODE ALL TREES #11MESH DESCRIPTOR Catheters EXPLODE ALL TREES #12((continuous or catheter or pump or infusion or infiltration or perfusion or diffusion or instillation)):TI,AB,KY #13#10 OR #11 OR #12 #14MESH DESCRIPTOR Pain, Postoperative #15pain*:TI,AB,KY #16#14 OR #15 #17#9 AND #13 AND #16 #18MESH DESCRIPTOR Colorectal Surgery #19MESH DESCRIPTOR General Surgery #20MESH DESCRIPTOR Laparotomy #21MESH DESCRIPTOR Digestive System Surgical Procedures EXPLODE ALL TREES #22MESH DESCRIPTOR Traumatology #23(((abdom* or general or gastrointestinal or GI or colorectal or open or trauma or emergency or fast track) adj5 (surger* or operation* or procedure*))):TI,AB,KY #24laparotom*:TI,AB,KY #25#18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 #26#17 AND #25
MEDLINE (OVID)
1. Anesthesia, Local/ 2. exp Anesthetics, Local/ 3. exp Analgesia/ 4. Bupivacaine/ or Lidocaine/ or Mepivacaine/ 5. ((local or regional or peripheral or intraabdominal or intra‐abdominal or preperitoneal or pre‐peritoneal or subfascial or suprafascial or "transversus abdominis plane*" or TAP or rectus sheath* or subcutaneous) adj5 (an?esthe* or analgesi* or block*)).tw. 6. (bupivacaine or levobupivacaine or ropivacaine or lignocaine or lidocaine or mepivacaine).tw. 7. or/1‐6 8. exp Infusion Pumps/ or exp Catheters/ 9. (continuous or catheter or pump or infusion or infiltration or perfusion or diffusion or instillation).tw. 10. 8 or 9 11. Pain, Postoperative/ 12. pain*.tw. 13. 11 or 12 14. 7 and 10 and 13 15. Colorectal Surgery/ 16. General Surgery/ 17. Laparotomy/ 18. exp Digestive System Surgical Procedures/ 19. Traumatology/ 20. ((abdom* or general or gastrointestinal or GI or colorectal or open or trauma or emergency or fast track) adj5 (surger* or operation* or procedure*)).tw. 21. laparotom*.tw. 22. or/15‐21 23. 14 and 22 24. randomized controlled trial.pt. 25. controlled clinical trial.pt. 26. randomized.ab. 27. placebo.ab. 28. drug therapy.fs. 29. randomly.ab. 30. trial.ab. 31. groups.ab. 32. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. exp animals/ not humans.sh. 34. 32 not 33 35. 23 and 34
Embase (OVID)
1. Anesthesia, Local/ 2. exp local anesthetic agent/ 3. exp Analgesia/ 4. Bupivacaine/ or Lidocaine/ or Mepivacaine/ 5. ((local or regional or peripheral or intraabdominal or intra‐abdominal or preperitoneal or pre‐peritoneal or subfascial or suprafascial or "transversus abdominis plane*" or TAP or rectus sheath* or subcutaneous) adj5 (an?esthe* or analgesi* or block*)).tw. 6. (bupivacaine or levobupivacaine or ropivacaine or lignocaine or lidocaine or mepivacaine).tw. 7. or/1‐6 8. exp Infusion Pump/ or exp Catheter/ 9. (continuous or catheter or pump or infusion or infiltration or perfusion or diffusion or instillation).tw. 10. 8 or 9 11. Postoperative pain/ 12. pain*.tw. 13. 11 or 12 14. 7 and 10 and 13 15. Colorectal Surgery/ 16. General Surgery/ 17. Laparotomy/ 18. exp abdominal surgery/ 19. Traumatology/ 20. ((abdom* or general or gastrointestinal or GI or colorectal or open or trauma or emergency or fast track) adj5 (surger* or operation* or procedure*)).tw. 21. laparotom*.tw. 22. or/15‐21 23. 14 and 22 24. random$.tw. 25. factorial$.tw. 26. crossover$.tw. 27. cross over$.tw. 28. cross‐over$.tw. 29. placebo$.tw. 30. (doubl$ adj blind$).tw. 31. (singl$ adj blind$).tw. 32. assign$.tw. 33. allocat$.tw. 34. volunteer$.tw. 35. Crossover Procedure/ 36. double‐blind procedure.tw. 37. Randomized Controlled Trial/ 38. Single Blind Procedure/ 39. or/24‐38 40. (animal/ or nonhuman/) not human/ 41. 39 not 40 42. 23 and 41
Appendix 2. Unavailable and unusable data
| Outcome | Study | Local anaesthetic | Control | Explanation | ||||||
| Mean | SD | Events | Total | Mean | SD | Events | Total | |||
| Pain score at rest:day 1 | Krishnan 2014 | 2.4 | ‐ | ‐ | 24 | 4.3 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Pain score at rest:day 2 | Krishnan 2014 | 2.0 | ‐ | ‐ | 24 | 3.2 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Pain score at rest:day 3 | Krishnan 2014 | 1.8 | ‐ | ‐ | 24 | 2.4 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Pain score at rest:day 4 | Krishnan 2014 | 1.6 | ‐ | ‐ | 24 | 2.4 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Pain score on movement:day 1 | Baig 2006 | ‐ | ‐ | ‐ | 35 | ‐ | ‐ | ‐ | 35 | Outcome predefined in methods but results not published |
| Krishnan 2014 | 4.5 | ‐ | ‐ | 24 | 5.6 | ‐ | ‐ | 6 | SD not reported and not estimable | |
| Pain score on movement:day 2 | Baig 2006 | ‐ | ‐ | ‐ | 35 | ‐ | ‐ | ‐ | 35 | Outcome predefined in methods but results not published |
| Krishnan 2014 | 3.9 | ‐ | ‐ | 24 | 4.2 | ‐ | ‐ | 6 | SD not reported and not estimable | |
| Pain score on movement:day 3 | Baig 2006 | ‐ | ‐ | ‐ | 35 | ‐ | ‐ | ‐ | 35 | Outcome predefined in methods but results not published |
| Krishnan 2014 | 3.7 | ‐ | ‐ | 24 | 4.2 | ‐ | ‐ | 6 | SD not reported and not estimable | |
| Pain score on movement:day 4 | Krishnan 2014 | 3.3 | ‐ | ‐ | 24 | 3.6 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Urinary retention | Baig 2006 | ‐ | ‐ | ‐ | 35 | ‐ | ‐ | ‐ | 35 | Outcome predefined in methods but results not published |
| Pruritus | Fustran 2015 | ‐ | ‐ | ‐ | 17 | ‐ | ‐ | ‐ | 17 | Outcome predefined in methods but results not published |
| Sedation | Baig 2006 | ‐ | ‐ | ‐ | 35 | ‐ | ‐ | ‐ | 35 | Outcome predefined in methods but results not published |
| Beaussier 2007 | ‐ | ‐ | ‐ | 21 | ‐ | ‐ | ‐ | 21 | Outcome predefined in methods but results not published | |
| Fustran 2015 | ‐ | ‐ | ‐ | 17 | ‐ | ‐ | ‐ | 17 | Outcome predefined in methods but results not published | |
| Respiratory depression | Beaussier 2007 | ‐ | ‐ | ‐ | 21 | ‐ | ‐ | ‐ | 21 | Outcome predefined in methods but results not published |
| Fustran 2015 | ‐ | ‐ | ‐ | 17 | ‐ | ‐ | ‐ | 17 | Outcome predefined in methods but results not published | |
| Time to first flatus | Polglase 2007 | ‐ | ‐ | ‐ | 143 | ‐ | ‐ | ‐ | 167 | Results reported as "no significant difference" only. Mean and SD not reported and not estimable |
| Time to first bowel movement | Polglase 2007 | ‐ | ‐ | ‐ | 143 | ‐ | ‐ | ‐ | 167 | Results reported as "no significant difference" only. Mean and SD not reported and not estimable |
| Time to ambulation | Krishnan 2014 | 9.7 | ‐ | ‐ | 24 | 10.4 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Length of hospital stay | Krishnan 2014 | 10.4 | ‐ | ‐ | 24 | 9.7 | ‐ | ‐ | 6 | SD not reported and not estimable |
| Wang 2010 | ‐ | ‐ | ‐ | 28 | ‐ | ‐ | ‐ | 27 | Results reported as "no significant effect" only. Mean and SD not reported and not estimable | |
| Deep vein thrombosis | Baig 2006 | ‐ | ‐ | ‐ | 35 | ‐ | ‐ | ‐ | 35 | Outcome predefined in methods but results not published |
Appendix 3. Subgroup analyses
By urgency: elective versus emergency
We did not conduct this subgroup analysis because participants in all six included trials underwent elective surgery.
By location of wound catheter: subcutaneous (suprafascial) versus musculofascial versus preperitoneal
(See Analysis 7.1 to Analysis 7.3)
7.1. Analysis.
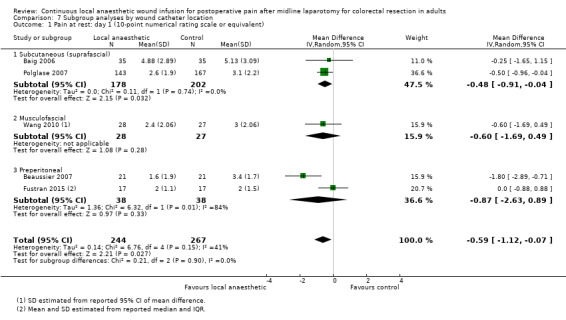
Comparison 7 Subgroup analyses by wound catheter location, Outcome 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent).
7.3. Analysis.
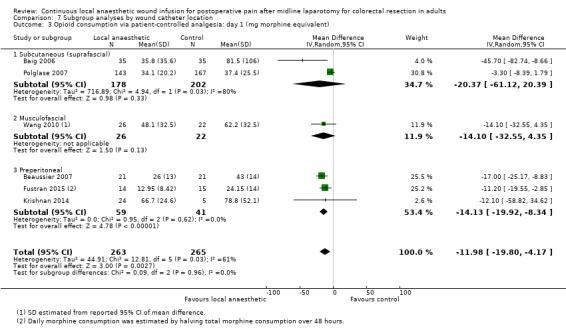
Comparison 7 Subgroup analyses by wound catheter location, Outcome 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent).
The test for subgroup differences by the location of the wound catheter was significant for pain on movement (P = 0.01) on postoperative day 1, where preperitoneal placement appeared to be associated with the largest effect, although each of the three subgroups only had one trial that contributed data. There was no significant subgroup difference for pain at rest (P = 0.90) or opioid consumption (P = 0.96) on postoperative day 1.
By local anaesthetic agent: bupivacaine/levobupivacaine versus ropivacaine
(See Analysis 2.1 to Analysis 2.3)
2.1. Analysis.
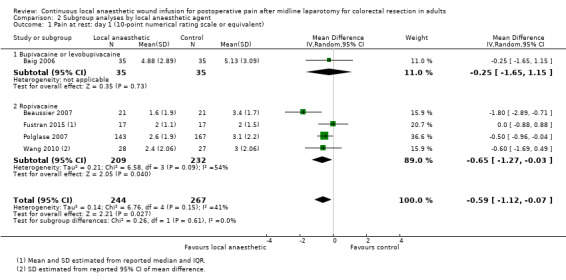
Comparison 2 Subgroup analyses by local anaesthetic agent, Outcome 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent).
2.3. Analysis.
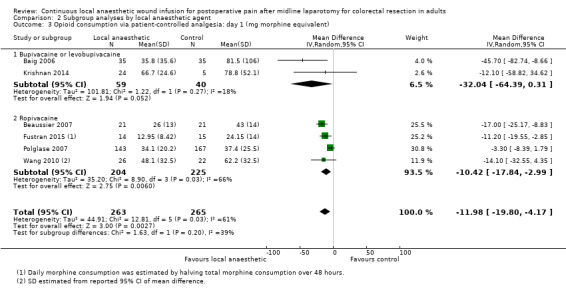
Comparison 2 Subgroup analyses by local anaesthetic agent, Outcome 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent).
There was no significant subgroup difference for pain at rest (P = 0.61) or opioid consumption (P = 0.20) on postoperative day 1. We could not conduct subgroup analysis for pain on movement on postoperative day 1 due to paucity of data.
By local anaesthetic dose: maximum safe dose versus submaximal dose
Both trials of bupivacaine or levobupivacaine used doses above the recommended maximum dose of 400 mg in 24 hours (20 mg/hour = 480 mg/24 hours in Baig 2006; 25 mg/hour = 600 mg/24 hours in Krishnan 2014), while all four trials of ropivacaine used doses below the recommended maximum dose of 770 mg in 24 hours (16 to 21.6 mg/hour = 384 to 518.4 mg/24 hours). As such, we did not conduct subgroup analysis by local anaesthetic dose as it produced identical subgroups as the subgroup analysis by local anaesthetic agent.
By continuous wound infusion programme: bolus versus no bolus prior to infusion
(See Analysis 3.1 to Analysis 3.3)
3.1. Analysis.
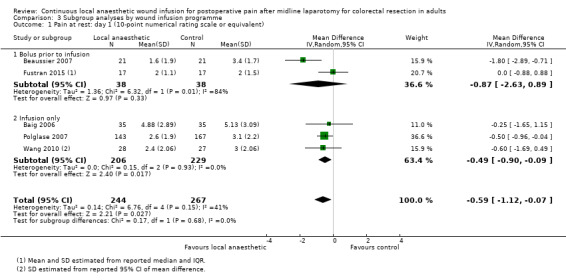
Comparison 3 Subgroup analyses by wound infusion programme, Outcome 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent).
3.3. Analysis.
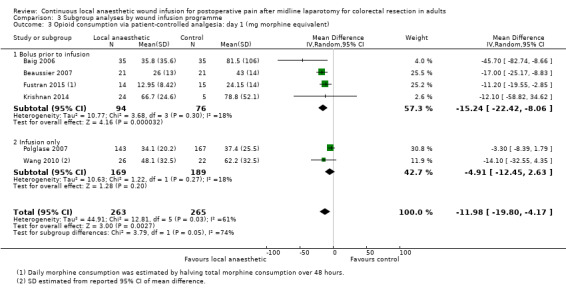
Comparison 3 Subgroup analyses by wound infusion programme, Outcome 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent).
The test for subgroup differences by the infusion programme was significant for pain on movement (P = 0.006) and opioid consumption (P = 0.05) on postoperative day 1, where administration of a bolus prior to infusion appeared to be associated with the largest effect, although only a small number of trials were available in each subgroup. There was no significant subgroup difference for pain at rest (P = 0.68) on postoperative day 1.
By co‐analgesic agents in addition to opioid patient‐controlled analgesia: none versus at least one agent
(See Analysis 4.1 to Analysis 4.3)
4.1. Analysis.
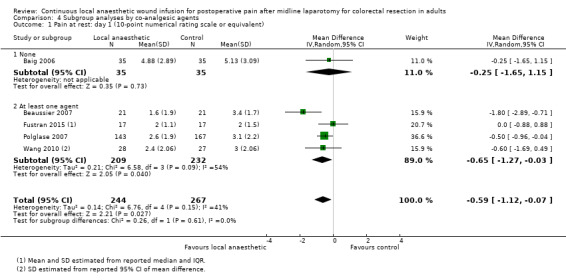
Comparison 4 Subgroup analyses by co‐analgesic agents, Outcome 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent).
4.3. Analysis.
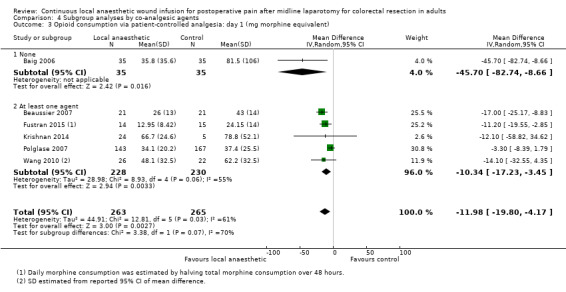
Comparison 4 Subgroup analyses by co‐analgesic agents, Outcome 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent).
There was no significant subgroup difference for pain at rest on postoperative day 1 (P = 0.61). We could not conduct subgroup analysis for pain on movement and opioid consumption on postoperative day 1 due to paucity of data.
Appendix 4. Sensitivity analyses
Excluding unpublished data
(See Analysis 5.1)
5.1. Analysis.
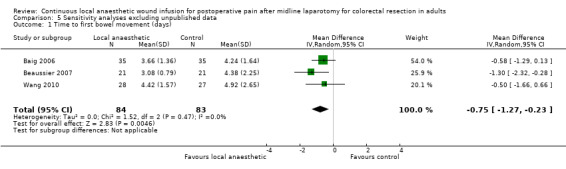
Comparison 5 Sensitivity analyses excluding unpublished data, Outcome 1 Time to first bowel movement (days).
We used unpublished data from Krishnan 2014 in our analysis of time to first bowel movement. The effects of continuous local anaesthetic wound infusion versus placebo on the time to first bowel movement were robust to the exclusion of unpublished data.
Excluding estimated or derived data
(See Analysis 6.1 to Analysis 6.10)
6.1. Analysis.
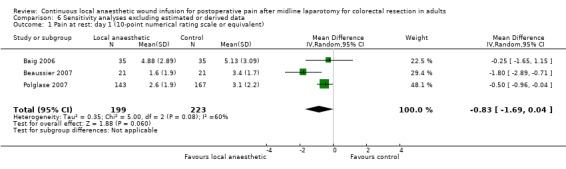
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent).
6.10. Analysis.
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 10 Laparotomy wound infection.
Incomplete reporting of predefined outcomes was present in all six included trials. We were required to estimate the mean and standard deviation of several continuous outcomes and impute the event rates of several dichotomous outcomes from other reported parameters. The effects of continuous local anaesthetic wound infusion versus placebo were robust to the exclusion of estimated or imputed data. The magnitude of reductions in pain on movement on postoperative day 1 and opioid consumption on postoperative day 2 were robust, but the effects were no longer statistically significant.
Excluding trials at high risk of bias
We did not conduct this sensitivity analysis because all six included trials were at high risk of bias in at least one of the assessed domains.
Data and analyses
Comparison 1. Local anaesthetic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent) | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.12, ‐0.07] |
| 2 Pain at rest: day 2 (10‐point numerical rating scale or equivalent) | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.68, 0.08] |
| 3 Pain at rest: day 3 (10‐point numerical rating scale or equivalent) | 3 | 422 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.58, 0.10] |
| 4 Pain on movement: day 1 (10‐point numerical rating scale or equivalent) | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 5 Pain on movement: day 2 (10‐point numerical rating scale or equivalent) | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.79, 0.08] |
| 6 Pain on movement: day 3 (10‐point numerical rating scale or equivalent) | 2 | 352 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.03, ‐0.12] |
| 7 Opioid consumption via patient controlled analgesia: day 1 (mg morphine equivalent) | 6 | 528 | Mean Difference (IV, Random, 95% CI) | ‐11.98 [‐19.80, ‐4.17] |
| 8 Opioid consumption via patient controlled analgesia: day 2 (mg morphine equivalent) | 6 | 528 | Mean Difference (IV, Random, 95% CI) | ‐9.64 [‐15.77, ‐3.52] |
| 9 Opioid consumption via patient controlled analgesia: day 3 (mg morphine equivalent) | 4 | 451 | Mean Difference (IV, Random, 95% CI) | ‐4.93 [‐10.95, 1.09] |
| 10 Opioid consumption via patient controlled analgesia: day 4 (mg morphine equivalent) | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐2.27 [‐6.85, 2.31] |
| 11 Nausea or vomiting | 6 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.71, 1.14] |
| 12 Ileus | 3 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.14, 1.77] |
| 13 Pruritus | 3 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.38, 1.82] |
| 14 Respiratory depression | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.02, 1.77] |
| 15 Time to first bowel movement (days) | 4 | 197 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.17, ‐0.17] |
| 16 Time to ambulation (days) | 3 | 159 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.28, 0.22] |
| 17 Length of hospital stay (days) | 4 | 456 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐2.01, ‐0.33] |
| 18 Any serious postoperative adverse event (composite outcome) | 6 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.68, 1.58] |
| 19 Pneumonia | 3 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.47, 2.82] |
| 20 Laparotomy wound breakdown | 2 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.09, 1.12] |
| 21 Laparotomy wound infection | 5 | 499 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.58, 2.37] |
| 22 Local anaesthetic systemic toxicity | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Subgroup analyses by local anaesthetic agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent) | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.12, ‐0.07] |
| 1.1 Bupivacaine or levobupivacaine | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.65, 1.15] |
| 1.2 Ropivacaine | 4 | 441 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.27, ‐0.03] |
| 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent) | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 2.1 Bupivacaine or levobupivacaine | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Ropivacaine | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent) | 6 | 528 | Mean Difference (IV, Random, 95% CI) | ‐11.98 [‐19.80, ‐4.17] |
| 3.1 Bupivacaine or levobupivacaine | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐32.04 [‐64.39, 0.31] |
| 3.2 Ropivacaine | 4 | 429 | Mean Difference (IV, Random, 95% CI) | ‐10.42 [‐17.84, ‐2.99] |
2.2. Analysis.
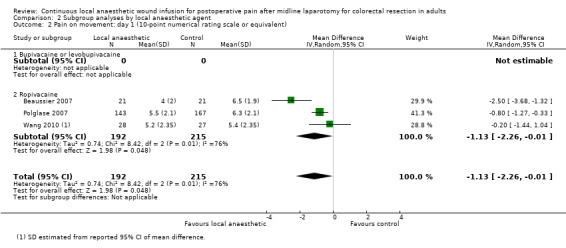
Comparison 2 Subgroup analyses by local anaesthetic agent, Outcome 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent).
Comparison 3. Subgroup analyses by wound infusion programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent) | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.12, ‐0.07] |
| 1.1 Bolus prior to infusion | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐2.63, 0.89] |
| 1.2 Infusion only | 3 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.90, ‐0.09] |
| 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent) | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 2.1 Bolus prior to infusion | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐3.68, ‐1.32] |
| 2.2 Infusion only | 2 | 365 | Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.16, ‐0.29] |
| 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent) | 6 | 528 | Mean Difference (IV, Random, 95% CI) | ‐11.98 [‐19.80, ‐4.17] |
| 3.1 Bolus prior to infusion | 4 | 170 | Mean Difference (IV, Random, 95% CI) | ‐15.24 [‐22.42, ‐8.06] |
| 3.2 Infusion only | 2 | 358 | Mean Difference (IV, Random, 95% CI) | ‐4.91 [‐12.45, 2.63] |
3.2. Analysis.
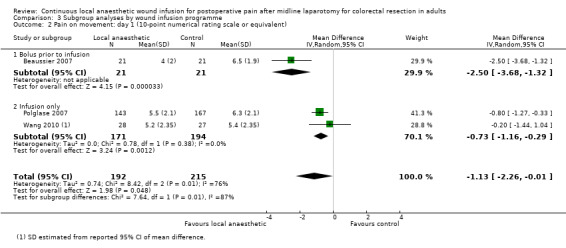
Comparison 3 Subgroup analyses by wound infusion programme, Outcome 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent).
Comparison 4. Subgroup analyses by co‐analgesic agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent) | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.12, ‐0.07] |
| 1.1 None | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.65, 1.15] |
| 1.2 At least one agent | 4 | 441 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.27, ‐0.03] |
| 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent) | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 2.1 None | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 At least one agent | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent) | 6 | 528 | Mean Difference (IV, Random, 95% CI) | ‐11.98 [‐19.80, ‐4.17] |
| 3.1 None | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐45.70 [‐82.74, ‐8.66] |
| 3.2 At least one agent | 5 | 458 | Mean Difference (IV, Random, 95% CI) | ‐10.34 [‐17.23, ‐3.45] |
4.2. Analysis.
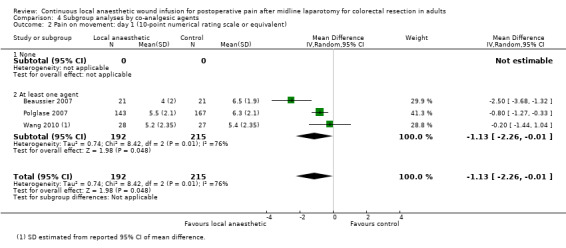
Comparison 4 Subgroup analyses by co‐analgesic agents, Outcome 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent).
Comparison 5. Sensitivity analyses excluding unpublished data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to first bowel movement (days) | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.27, ‐0.23] |
Comparison 6. Sensitivity analyses excluding estimated or derived data.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent) | 3 | 422 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.69, 0.04] |
| 2 Pain at rest: day 2 (10‐point numerical rating scale or equivalent) | 3 | 422 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.01, 0.40] |
| 3 Pain on movement: day 1 (10‐point numerical rating scale or equivalent) | 2 | 352 | Mean Difference (IV, Random, 95% CI) | ‐1.56 [‐3.22, 0.10] |
| 4 Pain on movement: day 2 (10‐point numerical rating scale or equivalent) | 2 | 352 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.79, 0.33] |
| 5 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent) | 4 | 451 | Mean Difference (IV, Random, 95% CI) | ‐13.60 [‐26.89, ‐0.30] |
| 6 Opioid consumption via patient‐controlled analgesia: day 2 (mg morphine equivalent) | 4 | 451 | Mean Difference (IV, Random, 95% CI) | ‐7.72 [‐15.53, 0.09] |
| 7 Nausea or vomiting | 3 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.18] |
| 8 Time to ambulation (days) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Length of hospital stay (days) | 2 | 112 | Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐2.16, ‐0.21] |
| 10 Laparotomy wound infection | 4 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.67, 3.20] |
6.2. Analysis.
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 2 Pain at rest: day 2 (10‐point numerical rating scale or equivalent).
6.3. Analysis.
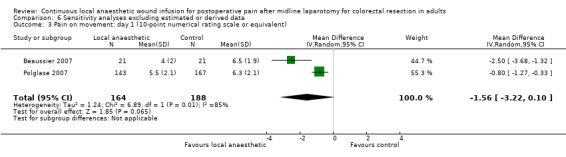
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 3 Pain on movement: day 1 (10‐point numerical rating scale or equivalent).
6.4. Analysis.
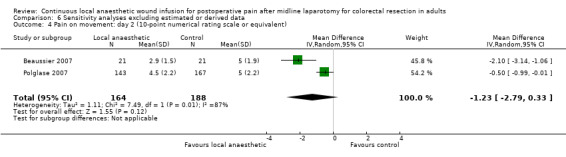
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 4 Pain on movement: day 2 (10‐point numerical rating scale or equivalent).
6.5. Analysis.
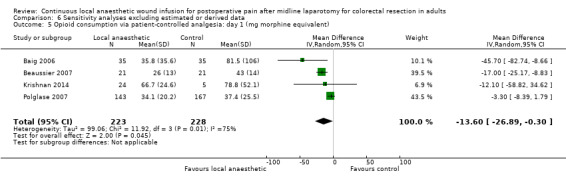
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 5 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent).
6.6. Analysis.
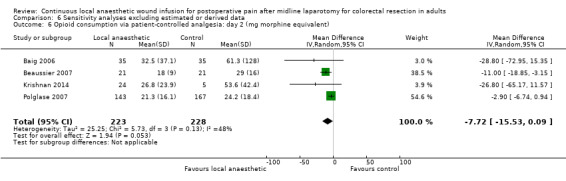
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 6 Opioid consumption via patient‐controlled analgesia: day 2 (mg morphine equivalent).
6.7. Analysis.
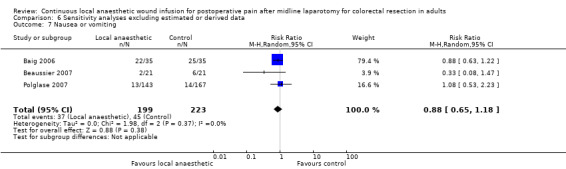
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 7 Nausea or vomiting.
6.8. Analysis.
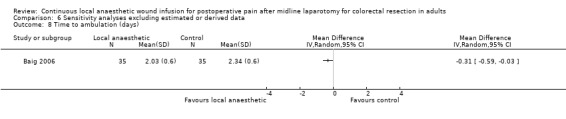
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 8 Time to ambulation (days).
6.9. Analysis.
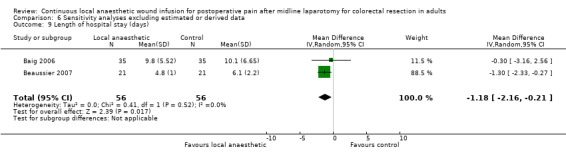
Comparison 6 Sensitivity analyses excluding estimated or derived data, Outcome 9 Length of hospital stay (days).
Comparison 7. Subgroup analyses by wound catheter location.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain at rest: day 1 (10‐point numerical rating scale or equivalent) | 5 | 511 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.12, ‐0.07] |
| 1.1 Subcutaneous (suprafascial) | 2 | 380 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.91, ‐0.04] |
| 1.2 Musculofascial | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.69, 0.49] |
| 1.3 Preperitoneal | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐2.63, 0.89] |
| 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent) | 3 | 407 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐2.26, ‐0.01] |
| 2.1 Subcutaneous (suprafascial) | 1 | 310 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.27, ‐0.33] |
| 2.2 Musculofascial | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.44, 1.04] |
| 2.3 Preperitoneal | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.5 [‐3.68, ‐1.32] |
| 3 Opioid consumption via patient‐controlled analgesia: day 1 (mg morphine equivalent) | 6 | 528 | Mean Difference (IV, Random, 95% CI) | ‐11.98 [‐19.80, ‐4.17] |
| 3.1 Subcutaneous (suprafascial) | 2 | 380 | Mean Difference (IV, Random, 95% CI) | ‐20.37 [‐61.12, 20.39] |
| 3.2 Musculofascial | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐14.10 [‐32.55, 4.35] |
| 3.3 Preperitoneal | 3 | 100 | Mean Difference (IV, Random, 95% CI) | ‐14.13 [‐19.92, ‐8.34] |
7.2. Analysis.
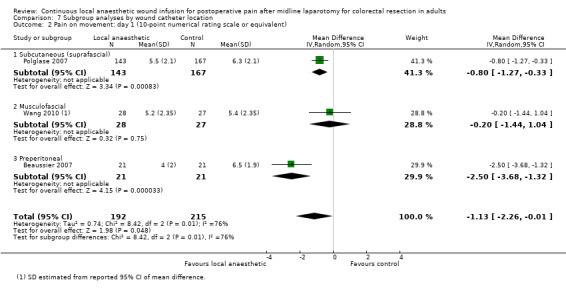
Comparison 7 Subgroup analyses by wound catheter location, Outcome 2 Pain on movement: day 1 (10‐point numerical rating scale or equivalent).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baig 2006.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: not reported |
|
| Participants | Country: USA Centres: 1 Recruitment period: March 2001‐May 2002 Follow‐up period: 4 weeks after discharge from hospital Surgery: open segmental or total colectomy with primary anastomosis Indication: not reported Urgency: elective Incision: midline Length of incision: not reported Exclusion criteria
Number randomised: not reported Post‐randomisation exclusion: not reported Number analysed = 70 (local anaesthetic n = 35; control n = 35) Demographics
|
|
| Interventions | Continuous wound infusion with:
Number of wound catheters: 2 Length of wound catheter: 6‐10 cm Wound catheter position: subcutaneous (suprafascial); 1 catheter placed along on each wound edge Infusion rate: 4 mL/h total (2 mL/h per catheter) Infusion duration: 72 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions:
|
|
| Outcomes | Outcomes relevant to current review
Other outcomes reported
|
|
| Notes |
aOutcomes predefined in methods but results not published. bOutcomes published in paper but not predefined in methods. Funding source: donation from the Caporella family |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Allocation contained in sealed envelopes that were opened by the pharmacist. Infusion pump filled with allocated study drug by the pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the pharmacist had the code that defined the type of solution. The surgeon who placed the pump, the staff who subsequently recorded various parameters and the participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the pharmacist had the code that defined the type of solution. The surgeon who placed the pump, the staff who subsequently recorded various parameters and the participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Identification of adverse outcomes by retrospective review of charts may be at risk of missing or incomplete outcome data. Number of enrolments, number of post‐randomisation exclusions, and number with missing data were not reported. Unclear if ITT analysis was performed. |
| Selective reporting (reporting bias) | High risk | Predefined both pain at rest and on movement as outcomes, but only published pain at rest. Did not predefine number of PCA doses as an outcome, but published statistically significant results |
| Small study bias | High risk | < 50 participants per treatment arm |
| Industry bias | Low risk | Funded by donation. No industry funding or sponsorship |
| Inclusion and exclusion criteria (selection bias) | Low risk | No obvious concerns |
| Methods of outcome assessment (information bias) | High risk | Definitions of adverse events not described in sufficient detail. Identification of adverse events by retrospective review of charts |
| Methods of statistical analysis (analytical bias) | Unclear risk | Unclear if analysed by ITT. No sensitivity tests for protocol violation |
Beaussier 2007.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: not reported |
|
| Participants | Country: France and Switzerland Centres: 3 Recruitment period: July 2005‐May 2006 Follow‐up period: 8‐12 weeks after discharge from hospital Surgery: open colonic resection with primary anastomosis Indication: bowel cancer Urgency: elective Incision: midline Length of incision (mean): local anaesthetic 22 cm; control 19 cm Exclusion criteria
Number randomised = 49 Post‐randomisation exclusion
Number analysed = 42 (local anaesthetic n = 21; control n = 21) Demographics
|
|
| Interventions | Continuous wound infusion with:
Number of wound catheters: 1 Length of wound catheter: not reported Wound catheter position: preperitoneal; catheter placed along full length of wound Infusion rate: 10 mL bolus followed by 10 mL/h Infusion duration: 48 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
|
| Outcomes | Outcomes relevant to current review
Other outcomes reported
|
|
| Notes |
aInformation regarding outcomes presented graphically in paper without numerical results obtained through private correspondence with study authors on 30 May 2015. bOutcomes predefined in methods but results not published in results. Funding source: grant from AstraZeneca, a manufacturer of ropivacaine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Allocation contained in sealed envelopes that were opened by the pharmacist. Infusion pump filled with allocated study drug by the pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the pharmacist had the code that defined the type of solution. The participant and the physicians in charge of the participant were blinded during both intra‐operative and postoperative periods. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the pharmacist had the code that defined the type of solution. The participant and the physicians in charge of the participant were blinded during both intra‐operative and postoperative periods. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/49 (14%) participants were excluded from the study post‐randomisation. Reasons for exclusion were outlined above. ITT analysis was not performed. |
| Selective reporting (reporting bias) | High risk | Predefined sedation as an outcome but did not publish results. Trial authors reported “no major adverse event occurred”, but did not report on the definitions or methods of assessing major adverse events used. |
| Small study bias | High risk | < 50 participants per treatment arm |
| Industry bias | Unclear risk | Funded by grant from AstraZeneca, a manufacturer of ropivacaine. Involvement of the sponsor in the design, conduct, analysis and reporting of the trial was not stated. |
| Inclusion and exclusion criteria (selection bias) | Low risk | No obvious concerns |
| Methods of outcome assessment (information bias) | Low risk | No obvious concerns |
| Methods of statistical analysis (analytical bias) | Unclear risk | Unclear if analysed by ITT |
Fustran 2015.
| Methods | Study design: parallel, stratified, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: yes |
|
| Participants | Country: Spain
Centres: 1
Recruitment period: April 2010‐April 2012
Follow‐up period: 48 h postoperatively
Surgery: open or laparoscopic colorectal resection (stratified)
Indication: malignant colorectal tumour
Urgency: elective Incision (laparotomy subgroup): midlinea Length of incision (median; all participants): local anaesthetic 16.5 cm; control 17.5 cm Exclusion criteria
Number randomised (total) = 67
Number analysed (total) = 67
Missing data
Demographics (all participants)
|
|
| Interventions | Interventions in the laparotomy subgroup are described here only Continuous wound infusion with:
Number of wound catheters: 1 Length of wound catheter: 15 cm Wound catheter position: preperitoneal; catheter placed along full length of wound Infusion rate: 10 mL bolus followed by 5 mL/h Infusion duration: 48 h Intra‐operative analgesic co‐interventions
Postoperative analgesic co‐interventions
|
|
| Outcomes | Outcomes relevant to current review
Other outcomes reported
|
|
| Notes |
aInformation obtained through private correspondence with trial author (8 January 2017). bOutcomes predefined in methods but results not published. cOutcomes reported in all participants without stratification into laparotomy and laparoscopy subgroups. Funding source: equipment supplied by Baxter, a manufacturer of continuous wound infusion devices. Baxter also provided expenses of an independent external monitor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Study drug prepared by central pharmacy department and placed in numbered sealed boxes according to the random number sequence. Infusion pump filled with allocated study drug by an independent nurse. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants, the researchers and the statistician were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants, the researchers and the statistician were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/34 (15%) participants in the laparotomy subgroup (local anaesthetic 3; control 2) were excluded from analysis of opioid consumption due to missing data as a result of inability to use PCA or intolerance. Complete case analysis was performed for opioid consumption. ITT analysis was performed for all other outcomes. |
| Selective reporting (reporting bias) | High risk | Pruritus, sedation and respiratory depression predefined in methods but results not published |
| Small study bias | High risk | < 50 participants per treatment arm in the laparotomy subgroup |
| Industry bias | Unclear risk | Quote: "Multi‐holed catheters and elastomeric pumps supplied by Baxter, manufacturer of the devices. Baxter provided expenses of an independent external monitor." Comment: involvement of the sponsor in the design, conduct, analysis and reporting of the trial was not stated. |
| Inclusion and exclusion criteria (selection bias) | Low risk | No obvious concerns |
| Methods of outcome assessment (information bias) | Unclear risk | Definitions and methods of assessing adverse events not described in sufficient detail |
| Methods of statistical analysis (analytical bias) | Low risk | No obvious concerns |
Krishnan 2014.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial with 2:1 allocation ratio Analysis by ITT: not reported | |
| Participants | Country: Australia Centres: 1 Recruitment period: September 2007‐October 2009a Follow‐up period: 30 days after surgery Surgery: open or laparoscopic colorectal resection Indication: not reported Urgency: elective Incision (laparotomy subgroup): 97% midline, 3% transverse Length of incision (mean) (laparotomy subgroup): 24.9 cma Exclusion criteria
Number randomised (total): n = 90
Post‐randomisation exclusion (total)
Number analysed (total) = 81
Demographics (laparotomy subgroup)
|
|
| Interventions | Only the laparotomy subgroup is summarised here Continuous wound infusion with:
Number of wound catheters: 2 Length of wound catheter: not reported Wound catheter position: preperitoneal; 1 catheter placed along on each wound edge Infusion rate: 10 mL/h total (5 mL/h per catheter) for first 48 h, 5 mL/h total (2.5 mL/h per catheter) thereafter Infusion duration: 96 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
|
| Outcomes | Outcomes relevant to current review
Other outcomes reported
|
|
| Notes |
aAdditional information for incompletely published outcomes obtained from lead author's PhD thesis.
bSD not reported and not estimable. In addition to the primary reference, we identified 3 conference abstracts describing this study during literature search (see Results of the search). We included these conference abstracts and the lead author's PhD thesis as secondary references. Funding source: grant from The Hospital Research Foundation. Equipment supplied by I‐Flow Corp, a manufacturer of continuous wound infusion devices. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Pharmacist … applied randomisation schedule” Comment: generation of randomisation sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Allocation was controlled by the … pharmacist” Comment: concealment of the allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants, medical staff, nursing staff and assessors were blinded to the treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants, medical staff, nursing staff and assessors were blinded to the treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall, 9/90 (10%) participants were excluded from the study post‐randomisation. Number of participants excluded from the laparotomy subgroup was not reported. Reasons for exclusion were outlined above. ITT analysis was not performed. |
| Selective reporting (reporting bias) | High risk | Predefined pain at rest and on movement and opioid consumption as outcomes but did not publish complete results in paper. Predefined local anaesthetic toxicity as outcome but did not publish results in paper. |
| Small study bias | High risk | < 50 participants per treatment arm in the laparotomy subgroup |
| Industry bias | Low risk | Quote: "The project received commercial untied support by way of gratis PainBuster devices from the I‐Flow Corp (United States) via Surgical Specialties (Australia). These sources had no input into any aspects of the study from design through to manuscript. Financial support was also provided by The Hospital Research Foundation via a program grant to the Discipline of Surgery, The University of Adelaide." |
| Inclusion and exclusion criteria (selection bias) | Low risk | No obvious concerns |
| Methods of outcome assessment (information bias) | Unclear risk | Definitions and methods of assessing adverse events not described in sufficient detail |
| Methods of statistical analysis (analytical bias) | High risk | Unclear if analysed by ITT. Compared outcomes between laparotomy to laparoscopic subgroups rather than comparing treatment versus control within each subgroup. Some conclusions were drawn by comparing mean estimates without performing any statistical test. |
Polglase 2007.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: not reported | |
| Participants | Country: Australia Centres: 1 Recruitment period: December 2003‐February 2006 Follow‐up period: until discharge from hospital Surgery: open colorectal resection Indication: not reported Urgency: elective Incision: midline Length of incision (mean): local anaesthetic 20.9 cm; control 22.5 cm Exclusion criteria:
Number randomised = 326 (local anaesthetic n = 153; control n = 173) Post‐randomisation exclusion
Number analysed = 310 (local anaesthetic n = 143, control n = 167) Missing data
Demographics
|
|
| Interventions | Continuous wound infusion with:
Number of wound catheters: 2 Length of wound catheter: 6.5 cm Wound catheter position: subcutaneous (suprafascial); 1 catheter placed along the upper part and 1 catheter placed along the lower part of the wound Infusion rate: 4 mL/h (2 mL/h per catheter) Infusion duration: 60 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
|
| Outcomes | Outcomes relevant to current review
Other outcomes reported
|
|
| Notes |
aOutcomes reported only as "no significant difference". We identified 1 additional record of a conference abstract describing this study during literature search (see Results of the search). Funding source: grant from Tackling Bowel Cancer, Cabrini Hospital Clinical Education and Research Institute. Grant from Mazda Foundation. Equipment supplied by I‐Flow Corporation, a manufacturer of continuous wound infusion devices. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | The syringes containing the study drugs appeared identical and were labelled with numbers corresponding to the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study drugs were prepared by an external third party and were delivered by a trial nurse not involved with participant care. All participants, medical and nursing staff were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were assessed postoperatively by blinded outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 participants (4.9%) were excluded post‐randomisation. ITT analysis was not performed. In addition, 12 participants (3.9%) did not complete 72 h of data collection for opioid consumption due to various reasons outlined above. Missing data were handled by the last observation carried forward method, which may distort means and standard deviations as opioid consumption would be expected to reduce over time. 5 participants (1.6%) were missing data for pain on movement due to set‐up difficulties preventing recording of this outcome. Complete case analysis was performed for this outcome. |
| Selective reporting (reporting bias) | Low risk | Predefined and reported clinically relevant and reasonable outcomes, including adverse outcomes |
| Small study bias | Unclear risk | 50‐199 participants per treatment arm |
| Industry bias | Unclear risk | Quote: "Supported by Tackling Bowel Cancer – Cabrini Hospital Clinical Education and Research Institute Melbourne, Victoria, Australia; Mazda Foundation – Private Bag 40 Mount Waverly, Victoria 3149; and I‐Flow Corporation, Pleasant Plain, Ohio: Supplied Painbuster Soakeri devices for this trial." Comment: involvement of the sponsor in the design, conduct, analysis and reporting of the trial was not stated. |
| Inclusion and exclusion criteria (selection bias) | Low risk | No obvious concerns |
| Methods of outcome assessment (information bias) | Unclear risk | Definitions and methods of assessing adverse outcomes not described |
| Methods of statistical analysis (analytical bias) | Unclear risk | Unclear if analysed by ITT |
Wang 2010.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: yes | |
| Participants | Country: Australia Centres: 1 Recruitment period: September 2005‐March 2008 Follow‐up period: until discharge from hospital Surgery: open colorectal surgery Indications: irrespective of underlying pathology Urgency: elective Incision: midline Length of incision (mean): local anaesthetic 25.4 cm; control 25.6 cm Exclusion criteria
Number randomised = 55 (local anaesthetic n = 28; control n = 27) Number analysed = 55 (local anaesthetic n = 28; control n = 27) Missing data
Demographics
|
|
| Interventions | Study groups
Number of wound catheters: 2 Length of wound catheters: ? Wound catheter position: musculofascial, 1 catheter placed along each wound edge Infusion rate: 8 mL/h (4 mL/h per catheter) Infusion duration: 67.5 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
|
| Outcomes | Outcomes relevant to current review
Other outcomes reported
|
|
| Notes |
aOutcomes reported only as "no significant effect". We identified 1 additional record of a conference abstract describing this study during literature search (see Results of the search). Funding source: funding from I‐Flow Corporation, a manufacturer of continuous wound infusion devices |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Study drug was loaded by pharmacist into identical pumps with identical sticker label. Randomisation code was kept locked in pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The intervention assignment schedule was ... blinded from participants, all staff administering the treatment (i.e. surgeon, surgical assistants and scrub nurses), as well as from those who monitored outcomes (i.e. doctors, nurses, allied health staff and members of the Acute Pain Service)." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The intervention assignment schedule was ... blinded from participants, all staff administering the treatment (i.e. surgeon, surgical assistants and scrub nurses), as well as from those who monitored outcomes (i.e. doctors, nurses, allied health staff and members of the Acute Pain Service)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/55 (13%) participants were missing data for morphine consumption. Complete case analysis was performed for morphine consumption. All other outcomes were analysed by ITT. |
| Selective reporting (reporting bias) | Low risk | Predefined and reported clinically relevant and reasonable outcomes, including adverse outcomes |
| Small study bias | High risk | < 50 participants per treatment arm |
| Industry bias | Low risk | Quote: "I‐Flow Corporation and its Australian distributor, Surgical Synergies, provided funding for the study but were not involved in the design, implementation, analysis and reporting of the study." |
| Inclusion and exclusion criteria (selection bias) | Low risk | No obvious concerns |
| Methods of outcome assessment (information bias) | Unclear risk | Definitions and methods of assessing adverse outcomes not described |
| Methods of statistical analysis (analytical bias) | Low risk | No obvious concerns. Appropriate use of logrank statistic to analyse time to events |
ASA: American Association of Anesthesiologists physical status classification (I = healthy, II = mild systemic disease, III = severe systemic disease, IV = severe systemic disease that is a constant threat to life, V = not expected to survive without the operation, VI = brain‐dead); BMI: body mass index; cm: centimetre; dL: decilitre; g: gram; ITT: intention‐to‐treat; IV: intravenous; kg: kilogram; m: metre; mg: milligram; mmol: millimole; n: number of participants; NSAID: non‐steroidal anti‐inflammatory drug; PCA: patient‐controlled analgesia; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abadir 2009 | No placebo or sham control group for comparison |
| Dhanapal 2017 | Surgical procedure was predominantly gastrectomy or gastrojejunostomy. Only 13 of 94 participants (14%) in the study underwent right hemicolectomy. No subgroup data available |
| Fry 1984 | Surgical procedure was cholecystectomy, not colorectal resection |
| Gibbs 1988 | Surgical incision was paramedian, not midline laparotomy |
| Nalda 1977 | Intervention was epidural infusion of bupivacaine |
| NCT00557843 | Trial withdrawn before enrolling any participants |
| NCT01062919 | Control was epidural infusion of bupivacaine. Trial terminated due to low recruitment rate |
Characteristics of studies awaiting assessment [ordered by study ID]
Araujo 2014.
| Methods | Study design: parallel, randomised clinical trial Blinding: not reported Control: not reported Analysis by ITT: not reported |
| Participants | Country: Portugal Centres: not reported Recruitment period: not reported Follow‐up period: not reported Surgery: open major abdominal surgery Indication: not reported Urgency: not reported Incision: not reported Length of incision: not reported Exclusion criteria: not reported Number randomised = 22 Post‐randomisation exclusion: not reported Number analysed: not reported Demographics: not reported |
| Interventions | Continuous wound infusion at 10 mL/h for 48 h Treatment group: not reported Control group: not reported |
| Outcomes | Outcomes relevant to current review
|
| Notes | Currently only available as conference abstract. No contact information available |
Arino 2012.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: not reported |
| Participants | Country: Spain Centres: 1 Recruitment period: not reported Follow‐up period: not reported Surgery: open colorectal resection Indication: not reported Urgency: elective Incision: midline Length of incision: not reported Exclusion criteria: not reported Number randomised = 33 (local anaesthetic n = 15; control n = 18) Post‐randomisation exclusion: 4 (12%), reasons not reported Number analysed = 29 (local anaesthetic n = 13; control n = 16) Demographics: not reported |
| Interventions | Continuous wound infusion with:
Number of wound catheters: 1 Length of wound catheter: 15 cm Wound catheter position: preperitoneal Infusion rate: 5 mL bolus followed by 5 mL/h Infusion duration: 48 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
| Outcomes | Outcomes relevant to current review
|
| Notes | Currently only available as conference abstract. Contacted trial author on 15 February 2017. Did not receive response aResults reported qualitatively only ("pain scores were not reduced during 48 h after surgery... no side effects were observed") |
Cano 2012.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: not reported |
| Participants | Country: Spain Centres: not reported Recruitment period: not reported Follow‐up period: not reported Surgery: colorectal surgery Indication: colorectal cancer Urgency: not reported Length of incision: not reported Exclusion criteria: not reported Number randomised = 60 Post‐randomisation exclusion: not reported Number analysed: not reported Demographics: not reported |
| Interventions | Continuous wound infusion with:
Number of wound catheters: 1 Length of wound catheter: not reported Wound catheter position: preperitoneal Infusion rate: 7 mL/h Infusion duration: 72 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
| Outcomes | Outcomes relevant to current review
|
| Notes | Study presently only available in conference abstract form. No contact information available |
Maric 2009.
| Methods | Study design: parallel, randomised, double‐blind, placebo‐controlled clinical trial Analysis by ITT: not reported |
| Participants | Country: Croatia
Centres: not reported
Recruitment period: not reported
Follow‐up period: not reported
Surgery: open abdominal colorectal surgery
Indication: not reported
Urgency: elective
Incision: midline
Length of incision: not reported
Exclusion criteria: not reported Number randomised = 50 (local anaesthetic n = 25; control n = 25) Post‐randomisation exclusion: not reported Number analysed: not reported Demographics: not reported |
| Interventions | Continuous wound infusion with:
Number of wound catheters: 2 Length of wound catheter: not reported Wound catheter position: suprafascial; 1 catheter placed along on each wound edge Infusion rate: 8 mL bolus followed by 8 mL/h total (4 mL bolus followed by 4 mL/h per catheter) Infusion duration: 48 h Intra‐operative analgesic co‐interventions: not reported Postoperative analgesic co‐interventions
|
| Outcomes | Outcomes relevant to current review
|
| Notes | Currently only available as conference abstract. Contacted trial author on 22 February 2017. Did not receive response. aResults reported qualitatively only ("Satisfactory analgesia was achieved in both groups... Nausea and vomiting were less present in Group L. No side effects were observed.”). |
ITT: intention‐to‐treat; IV: intravenous; PCA: patient‐controlled analgesia; VAS: visual analogue scale
Differences between protocol and review
Changes to the previously published protocol adopted prior to conducting data analysis (Liang 2016).
Removed limited timeframe of three days for postoperative pain score and opioid consumption in order to evaluate longer‐term effects of the intervention. Downgraded results after postoperative day 1 to secondary outcomes to avoid excessive number of primary outcomes.
Added respiratory depression and urinary retention as examples of postoperative opioid‐related adverse events.
Added the time to first flatus as an outcome as it reflects delayed bowel movement and is a clinically relevant opioid‐related adverse effect.
Removed duration of surgery as an outcome because both intervention and control groups require wound catheter insertion so this would equally impact both study groups.
Removed cost of hospital stay as an outcome as this review is primarily focused on evaluating the clinical effectiveness of the intervention. A separate, comprehensive health economic evaluation of continuous local anaesthetic wound infusion is certainly warranted.
Changed from reporting both fixed‐ and random‐effects models to reporting either fixed‐effect model or random‐effects model based on level of heterogeneity, as this would enable clearer presentation and interpretation of the results, as using random‐effects models is mainly beneficial when substantial heterogeneity is present.
Removed planned subgroup analyses of pain score and opioid consumption on postoperative day 1 by the duration of wound infusion, comparing 24 hours versus 48 hours versus 72 hours. Upon further consideration, it is not clinically plausible for differences in the duration of wound infusion to impact outcomes on postoperative day 1.
Changed planned subgroup analyses for co‐analgesic agents from comparing paracetamol versus non‐steroidal anti‐inflammatory agent to comparing number of additional co‐analgesic agents (none versus single agent versus two or more agents) to encompass the possibility of other agents being used as a part of a multimodal analgesic regimen.
Added subgroup analyses by whether or not a bolus was administered prior to continuous wound infusion, as this may plausibly impact the primary outcomes.
Changes in the previously published protocol adopted after conducting data analysis based on input from editorial team and external peer reviewers (Liang 2016).
Removed visual inspection of funnel plots for assessing reporting biases as this is too subjective. Retained regression analysis for funnel plot asymmetry if more than 10 studies are available.
Clarified that types of studies included non‐standard designs (such as cluster‐ or cross‐over randomised controlled trials).
Renamed outcomes 'opioid‐related adverse effects' to 'postoperative opioid‐related adverse events' to be consistent with Cochrane language and to clarify the time of interest of these events. Also downgraded opioid‐related adverse effects to secondary outcomes to avoid excessive number of primary outcomes. Clarified that in addition to the examples of postoperative opioid‐related adverse events given, we would also include other postoperative opioid‐related adverse events reported by trial authors.
Separated outcomes 'time to flatus, bowel movement and ambulation' into 'time to first flatus and time to first bowel movement' and 'time to ambulation' as these are different aspects of functional recovery.
Renamed outcomes 'serious adverse events' to 'serious postoperative adverse events' to more clearly convey the scope of these outcomes. Explicitly stated that 'death by any cause' was considered as a part of this outcome. Clarified that in addition to the examples of serious postoperative adverse events given, we would also include other serious postoperative adverse events reported by trial authors. Clarified that we combined all reported serious operative adverse events into a composite outcome.
Renamed outcome 'wound catheter complications' to 'wound catheter‐related complications' as wound catheter may not be directly responsible for these complications.
Renamed outcome 'systemic local anaesthetic toxicity' to 'local anaesthetic systemic toxicity' to be consistent with literature.
Funding sources for each study was recorded as part of the 'Risk of bias' assessment. Added this to data extraction and management for clarification.
Separated and clarified criteria for assessment of performance bias and detection bias.
Clarified description, rationale and criteria for assessment of small study bias, industry bias (previously named 'funding bias' in the protocol), selection bias, information bias, and analytical bias.
Clarified description and criteria for GRADE assessment of the certainty of the evidence. We added further details about our methods for applying GRADE and assessing the quality of the evidence.
We added further details about our methods for applying GRADE and assessing the quality of the evidence.
Changed wording from 'quality' to 'certainty' of evidence to be consistent with GRADE language. Clarified criteria for downgrading evidence.
Added justification for choice of outcomes in 'Summary of findings' table.
Applied random‐effects model to all outcomes. Firstly, there was reasonable expectation of at least moderate levels of clinical heterogeneity among included studies. Secondly, presenting results of both fixed‐ and random‐effects models was noted to be confusing without additional value.
Contributions of authors
ZY and SL conceived, designed and co‐ordinated the review.
ZY was the primary supervisor for the review.
GS supervised and contributed to aspects of the review pertaining to systematic review methodology.
SL and EA developed the search strategy.
SL and BK assessed eligibility of records from database searches.
SL and EA assessed eligibility of records from trial registry searches.
SL, AY, BK and EA completed the data extraction.
SL, AY, BK and EA completed the 'Risk of bias' assessments.
SL and EA completed the GRADE assessments.
SL completed the data analysis.
SL drafted the final manuscript.
All review authors reviewed, revised and contributed important intellectual content to the final manuscript.
ZY and SL are guarantors of the review.
ZY, SL, AY, BK and EA will be responsible for updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Avant Doctor in Training Research Scholarship, Australia.
The Avant Doctor in Training Research Scholarship Program is a scholarship program offered by Avant Mutual Group Limited to provide financial assistance to support research in advancement of medicine and safety, quality and risk management in healthcare.
Declarations of interest
SL received the Doctor in Training Research Scholarship for this project from Avant Mutual Group in 2015 (USD 15,000 over 1 year). Avant Mutual Group is a medical defence organisation and provider of medical indemnity insurance, with no affiliation to any manufacturer or distributor of devices or drugs used for continuous local anaesthetic wound infusion. SL uses this intervention in clinical practice.
AY: none known
EA: none known
BK: none known
GS: none known
AB: none known. AB uses this intervention in clinical practice.
HC: none known
DD: none known
ZY: none known. ZY uses this intervention in clinical practice.
New
References
References to studies included in this review
Baig 2006 {published data only}
- Baig MK, Zmora O, Derdemezi J, Weiss EG, Nogueras JJ, Wexner SD. Use of the ON‐Q pain management system is associated with decreased postoperative analgesic requirement: double blind randomized placebo pilot study. Journal of the American College of Surgeons 2006;202(2):297‐305. [DOI] [PubMed] [Google Scholar]
Beaussier 2007 {published data only}
- Beaussier M, El'Ayoubi H, Schiffer E, Rollin M, Parc Y, Mazoit JX, et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double‐blind, placebo‐controlled study. Anesthesiology 2007;107(3):461‐8. [DOI] [PubMed] [Google Scholar]
Fustran 2015 {published data only}
- Fustran N, Dalmau A, Ferreres E, Camprubi I, Sanzol R, Redondo S, et al. Postoperative analgesia with continuous wound infusion of local anaesthesia vs saline: a double‐blind randomized, controlled trial in colorectal surgery. Colorectal Disease 2015;17(4):342‐50. [DOI] [PubMed] [Google Scholar]
Krishnan 2014 {published and unpublished data}
- Krishnan S. The efficacy of local anaesthetic infiltrated at the incision site for post‐operative pain management following abdominal surgery: an application to fast‐track surgery. digital.library.adelaide.edu.au/dspace/bitstream/2440/82083/8/02whole.pdf (accessed 10 March 2019).
- Krishnan S, Hewett PJ, Tou S, Field J, Karatassas A, Tonkin J, et al. Local anaesthetic infused at the incision site for post‐operative pain management following abdominal surgery: a double blinded randomised study. Colorectal Disease. 2011; Vol. 13:10‐11.
- Krishnan S, Morris R, Hewett P, Karatassas A, Tonkin J, Field J. Focused Conference Group: PW12 ‐ Experimental pain methods in development and validation of analgesics local anaesthetic infused at the incision site for post‐operative pain management following abdominal surgery. Basic and Clinical Pharmacology and Toxicology. 2010; Vol. 107:390‐1.
- Krishnan S, Morris R, Hewett P, Karatassas A, Tonkin J, Field J. The efficacy of 96 hour duration local anaesthesia infused at the incision site for post‐operative pain management. An interim analysis. Basic and Clinical Pharmacology and Toxicology. 2009; Vol. 105:61.
- Krishnan S, Morris RG, Hewett PJ, Field J, Karatassas A, Tou S, et al. A randomized double‐blind clinical trial of a continuous 96‐hour levobupivacaine infiltration after open or laparoscopic colorectal surgery for postoperative pain management‐including clinically important changes in protein binding. Therapeutic Drug Monitoring 2014;36(2):202‐10. [DOI] [PubMed] [Google Scholar]
Polglase 2007 {published data only}
- Polglase A L, McMurrick P J, Simpson P J, Wale R J, Carne P W, Johnson W, et al. Continuous wound infusion of local anesthetic for the control of pain after elective abdominal colorectal surgery. Diseases of the Colon and Rectum 2007;50(12):2158‐67. [DOI] [PubMed] [Google Scholar]
- Polglase AL, McMurrick PJ, Simpson PJ, Chee J, Ooi CW, Kindsland SR. Continuous wound infusion of local anaesthetic in the control of pain following elective abdominal colorectal surgery. ANZ Journal of Surgery. 2007; Vol. 77, issue Supplement 1:A16. [DOI] [PubMed]
Wang 2010 {published data only}
- Wang L W, Wong S W, Crowe P J, Khor K E, Jastrzab G, Parasyn A D, et al. Wound infusion with local anaesthesia after laparotomy: a randomized controlled trial. ANZ Journal of Surgery 2010;80(11):794‐801. [DOI] [PubMed] [Google Scholar]
- Wang LW, Wong SW, Crowe PJ, Khor KE, Jastrzab G, Parasyn AD, et al. Continuous local anaesthesia wound infusion after laparotomy: a double‐blinded randomised placebo‐controlled trial. Journal of Gastroenterology and Hepatology. 2009; Vol. 24, issue Supplement s2:A248.
References to studies excluded from this review
Abadir 2009 {published data only}
- Abadir AR, Nicolas F, Gharabawy R, Shah T, Michael R. Efficacy of postoperative continuous wound infiltration with local anesthetic after major abdominal surgery. Proceedings of the Western Pharmacology Society 2009;52:35‐8. [PubMed] [Google Scholar]
Dhanapal 2017 {published data only}
- Dhanapal B, Sistla SC, Badhe AS, Ali SM, Ravichandran NT, Galidevara I. Effectiveness of continuous wound infusion of local anesthetics after abdominal surgeries. Journal of Surgical Research 2017;212:94‐100. [DOI] [PubMed] [Google Scholar]
Fry 1984 {published data only}
- Fry EN. The direct perfusion of surgical wounds with local anaesthetic solution: an approach to postoperative pain. Annals of the Royal College of Surgeons of England 1984;66(3):220. [PMC free article] [PubMed] [Google Scholar]
Gibbs 1988 {published data only}
- Gibbs P, Purushotham A, Auld C, Cuschieri RJ. Continuous wound perfusion with bupivacaine for postoperative wound pain. British Journal of Surgery 1988;75(9):923‐4. [DOI] [PubMed] [Google Scholar]
Nalda 1977 {published data only}
- Nalda Felipe MA, Muriel Villoria C, las Mulas Bejar M. Continuous perfusion of bupivacaine in the treatment of postoperative pain following abdominal surgery [La perfusion continue de bupivacaine dans le traitement de la douleur postoperatoire en chirurgie abdominale]. Anesthesie, Analgesie, Reanimation 1977;34(6):1167‐80. [PubMed] [Google Scholar]
NCT00557843 {unpublished data only}
- Kurz, A. Continuous bupivacaine infusion following colonic surgery. clinicaltrials.gov/ct2/show/NCT00557843 (accessed 6 March 2019).
NCT01062919 {unpublished data only}
- Carli, F. Local anesthetic wound infusion and functional recovery after colon surgery. clinicaltrials.gov/ct2/show/NCT01062919 (accessed 6 March 2019).
References to studies awaiting assessment
Araujo 2014 {published data only}
- Araujo R, Marques C, Fernandes D, Freitas S, Rodrigues M, Bernardo R, et al. Post‐operative pain management of major abdominal surgery with continuous wound infusion: the (R) evolution?. Regional Anesthesia and Pain Medicine 2014;39(Supplement 1):E207. [Google Scholar]
Arino 2012 {published and unpublished data}
- Arino JJ, Tejerina JG, Mayol J, Velasco JM, Moral P, Lopez Timoneda F. Analgesic efficacy of L‐bupivacaine delivered by wound catheter after abdominal surgery. European Journal of Anaesthesiology. 2012; Vol. 29, issue Supplement 50:205.
Cano 2012 {published data only}
- Cano Serrano ME, Adame Reyes M, Macias Mellado A, Bravo F, Martinez Telleria A. Postoperative analgesia in colorectal surgery with levobupivacaine infusion administrated by subaponeurotic multiperforated catheter. European Journal of Anaesthesiology 2012;29:207. [Google Scholar]
Maric 2009 {published data only}
- Maric S, Stefancic L, Supic DK, Vrdoljak D, Sakic KZ. Continuous wound infusion of levobupivacaine provides effective analgesia after colorectal surgery. Pain Practice. 2009; Vol. 9:148.
Additional references
Amin 2015
- Amin AT, Ahmed BM, Khallaf SM. Safety and feasibility of laparoscopic colo‐rectal surgery for cancer at a tertiary center in a developing country: Egypt as an example. Journal of the Egyptian National Cancer Institute 2015;27:91‐5. [DOI] [PubMed] [Google Scholar]
Apfel 2012
- Apfel CC, Heidrich FM, Jukar‐Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence‐based analysis of risk factors for postoperative nausea and vomiting. British Journal of Anaesthesia 2012;109(5):742‐53. [PUBMED: 23035051] [DOI] [PubMed] [Google Scholar]
Apfelbaum 2003
- Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesthesia Analgesia 2003;97:534‐40. [DOI] [PubMed] [Google Scholar]
Artinyan 2008
- Artinyan A, Nunoo‐Mensah JW, Balasubramaniam S, Gauderman J, Essani R, Gonzalez‐Ruiz C, et al. Prolonged postoperative ileus‐definition, risk factors, and predictors after surgery. World Journal of Surgery 2008;32(7):1495‐500. [PUBMED: 18305994] [DOI] [PubMed] [Google Scholar]
Baldini 2009
- Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology 2009;110(5):1139‐57. [PUBMED: 19352147] [DOI] [PubMed] [Google Scholar]
BCCA 2015
- Colorectal Surgical Society of Australia and New Zealand. The Bi‐National Colorectal Cancer Audit Report 2015. Colorectal Surgical Society of Australia and New Zealand, 2015. [Google Scholar]
Beaussier 2012
- Beaussier M, White PF, Raeder J. Is a negative meta‐analyses consisting of heterogenic studies on wound catheters sufficient to conclude that no additional studies are needed?. Acta Anaesthesiologica Scandinavica 2012;56:396‐7; author reply 397‐8. [DOI] [PubMed] [Google Scholar]
Berry 2015
- Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 2015;313(16):1619‐20. [PUBMED: 25799162] [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown SR, Tiernan J. Transverse verses midline incisions for abdominal surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005199.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burger 2002
- Burger JW, Van't Riet M, Jeekel J. Abdominal incisions: techniques and postoperative complications. Scandinavian Journal of Surgery 2002;91:315‐21. [DOI] [PubMed] [Google Scholar]
Butterworth 1990
- Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia: a review. Anesthesiology 1990;72:711‐34. [DOI] [PubMed] [Google Scholar]
Buvanendran 2009
- Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Current Opinion in Anaesthesiology 2009;22:588‐93. [DOI: 10.1097/ACO.0b013e328330373a] [DOI] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G. Effects of study precision and risk of bias in networks of interventions: a network meta‐epidemiological study. International Journal of Epidemiology 2013;42(4):1120‐31. [PUBMED: 23811232] [DOI] [PubMed] [Google Scholar]
Cheong 2001
- Cheong WK, Seow‐Choen F, Eu KW, Tang CL, Heah SM. Randomized clinical trial of local bupivacaine perfusion versus parenteral morphine infusion for pain relief after laparotomy. British Journal of Surgery 2001;88(3):357‐9. [PUBMED: 11260098] [DOI] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [PUBMED: 23616031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Boghdadly 2018
- El‐Boghdadly K, Pawa A, Chin KJ. Local anesthetic systemic toxicity: current perspectives. Local and Regional Anesthesia 2018;11:35‐44. [PUBMED: 30122981] [DOI] [PMC free article] [PubMed] [Google Scholar]
Espitalier 2013
- Espitalier F, Tavernier E, Remerand F, Laffon M, Fusciardi J, Giraudeau B. Heterogeneity in meta‐analyses of treatment of acute postoperative pain: a review. British Journal of Anaesthesia 2013;111:897‐906. [DOI] [PubMed] [Google Scholar]
Fredman 2001
- Fredman B, Zohar E, Tarabykin A, Shapiro A, Mayo A, Klein E, et al. Bupivacaine wound instillation via an electronic patient‐controlled analgesia device and a double‐catheter system does not decrease postoperative pain or opioid requirements after major abdominal surgery. Anesthesia and Analgesia 2001;92(1):189‐93. [PUBMED: 11133625] [DOI] [PubMed] [Google Scholar]
Gan 2006
- Gan TJ. Risk factors for postoperative nausea and vomiting. Anesthesia and Analgesia 2006;102(6):1884‐98. [PUBMED: 16717343] [DOI] [PubMed] [Google Scholar]
Gan 2014
- Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post‐surgical pain: results from a US national survey. Current Medical Research and Opinion 2014;30(1):149‐60. [PUBMED: 24237004] [DOI] [PubMed] [Google Scholar]
Gerbershagen 2013
- Gerbershagen HJ, Aduckathil S, Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013;118:934‐44. [DOI] [PubMed] [Google Scholar]
Gerbershagen 2014
- Gerbershagen HJ, Pogatzki‐Zahn E, Aduckathil S, Peelen LM, Kappen TH, Wijck AJ, et al. Procedure‐specific risk factor analysis for the development of severe postoperative pain. Anesthesiology 2014;120:1237‐45. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (last accessed on 16 August 2019).
Grantcharov 2001
- Grantcharov TP, Rosenberg J. Vertical compared with transverse incisions in abdominal surgery. European Journal of Surgery 2001;167:260‐7. [DOI] [PubMed] [Google Scholar]
Gray 2005
- Gray A, Kehlet H, Bonnet F, Rawal N. Predicting postoperative analgesia outcomes: NNT league tables or procedure‐specific evidence?. British Journal of Anaesthesia 2005;94:710‐14. [DOI] [PubMed] [Google Scholar]
Gupta 2011
- Gupta A, Favaios S, Perniola A, Magnuson A, Berggren L. A meta‐analysis of the efficacy of wound catheters for post‐operative pain management. Acta Anaesthesiologica Scandinavica 2011;55:785‐96. [DOI: 10.1111/j.1399-6576.2011.02463] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011.. Available from www.handbook.cochrane.org..
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hollmann 2000
- Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication?. Anesthesiology 2000;93:858‐75. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [PUBMED: 8721797] [DOI] [PubMed] [Google Scholar]
Jin 2001
- Jin F, Chung F. Multimodal analgesia for postoperative pain control. Journal of Clinical Anesthesia 2001;13:524‐39. [DOI] [PubMed] [Google Scholar]
Johnson 2008
- Johnson SM, Saint John BE, Dine AP. Local anesthetics as antimicrobial agents: a review. Surgical Infections 2008;9:205‐13. [DOI] [PubMed] [Google Scholar]
Joshi 2013
- Joshi GP, Kehlet H. Procedure‐specific pain management: the road to improve postsurgical pain management?. Anesthesiology 2013;118:780‐2. [DOI] [PubMed] [Google Scholar]
Karthikesalingam 2008
- Karthikesalingam A, Walsh SR, Markar SR, Sadat U, Tang TY, Malata CM. Continuous wound infusion of local anaesthetic agents following colorectal surgery: systematic review and meta‐analysis. World Journal of Gastroenterology 2008;14:5301‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kehlet 1999
- Kehlet H, Werner M, Perkins F. Balanced analgesia: what is it and what are its advantages in postoperative pain?. Drugs 1999;58:793‐7. [DOI] [PubMed] [Google Scholar]
Kehlet 2007
- Kehlet H, Wilkinson RC, Fischer HB, Camu F. PROSPECT: evidence‐based, procedure‐specific postoperative pain management. Best Practice & Research: Clinical Anaesthesiology 2007;21:149‐59. [DOI] [PubMed] [Google Scholar]
Kehlet 2008
- Kehlet H. Fast‐track colorectal surgery. Lancet 2008;371:791‐3. [DOI] [PubMed] [Google Scholar]
Liu 2006
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. Journal of the American College of Surgeons 2006;203:914‐32. [DOI] [PubMed] [Google Scholar]
Luckey 2003
- Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Archives of Surgery 2003;138(2):206‐14. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [PUBMED: 9870574] [DOI] [PubMed] [Google Scholar]
NBOCA 2015
- Association of Coloproctology of Great Britain and Ireland. National Bowel Cancer Audit Annual Report 2015. UK: Association of Coloproctology of Great Britain and Ireland, 2015. [DOI] [PubMed] [Google Scholar]
Nuesch 2010
- Nuesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [PUBMED: 20639294] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozturk 2011
- Ozturk E, Yilmazlar A, Coskun F, Isik O, Yilmazlar T. The beneficial effects of preperitoneal catheter analgesia following colon and rectal resections: a prospective, randomized, double‐blind, placebo‐controlled study. Techniques in Coloproctology 2011;15(3):331‐6. [PUBMED: 21769617] [DOI] [PubMed] [Google Scholar]
Plummer 2011
Rawal 2012
- Rawal N. Epidural technique for postoperative pain: gold standard no more?. Regional Anesthesia and Pain Medicine 2012;37:310‐7. [DOI] [PubMed] [Google Scholar]
Ray‐Offor 2014
- Ray‐Offor E, Okoro P, Gbobo I, Allison A. Pilot study on laparoscopic surgery in Port‐Harcourt, Nigeria. Nigerian Journal of Surgery 2014;20:23‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenberg‐Adamsen 1996
- Rosenberg‐Adamsen S, Kehlet H, Dodds C, Rosenberg J. Postoperative sleep disturbances: mechanisms and clinical implications. British Journal of Anaesthesia 1996;76(4):552‐9. [PUBMED: 8652329] [DOI] [PubMed] [Google Scholar]
Rybakov 2017
- Rybakov EG, Shelygin YA, Khomyakov EA, Zarodniuk IV. Risk factors for postoperative ileus after colorectal cancer surgery. Colorectal Disease 2017;20(3):189‐94. [PUBMED: 28921903] [DOI] [PubMed] [Google Scholar]
SAGES 2012
- Society of American Gastrointestinal and Endoscopic Surgeons. Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer. Society of American Gastrointestinal and Endoscopic Surgeons, 2012. [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sommer 2008
- Sommer M, Rijke JM, Kleef M, Kessels AG, Peters ML, Geurts JW, et al. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. European Journal of Anaesthesiolology 2008;25(4):267‐74. [DOI] [PubMed] [Google Scholar]
Ventham 2014
- Ventham NT, O'Neill S, Johns N, Brady RR, Fearon KC. Evaluation of novel local anesthetic wound infiltration techniques for postoperative pain following colorectal resection surgery: a meta‐analysis. Diseases of the Colon and Rectum 2014;57:237‐50. [DOI] [PubMed] [Google Scholar]
Walter 2009
- Walter CJ, Collin J, Dumville JC, Drew PJ, Monson JR. Enhanced recovery in colorectal resections: a systematic review and meta‐analysis. Colorectal Disease 2009;11:344‐53. [DOI] [PubMed] [Google Scholar]
Waxler 2005
- Waxler B, Dadabhoy ZP, Stojiljkovic L, Rabito SF. Primer of postoperative pruritus for anesthesiologists. Anesthesiology 2005;103(1):168‐78. [PUBMED: 15983470] [DOI] [PubMed] [Google Scholar]
WCR 2014
- Stewart BW, Wild CP (ed). World Cancer Report. International Agency for Research on Cancer, 2014. [Google Scholar]
Wind 2006
- Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. British Journal of Surgery 2006;93:800‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liang 2016
- Liang SS, Ying AJ, Affan ET, Kakala BF, Strippoli GF, Bullingham A, et al. Continuous local anaesthetic wound infusion for postoperative pain after midline laparotomy for colorectal resection in adults. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012310] [DOI] [PMC free article] [PubMed] [Google Scholar]


