Abstract
Objectives:
To determine the percentage of patients with dermatitis herpetiformis (DH) who experience at least 2 years of remission and to identify factors associated with DH remission.
Design:
Retrospective cohort study.
Setting:
National Institutes of Health (NIH).
Patients:
Patients seen at the NIH during the 1972–2010 period who had clinical findings consistent with DH, whose normal skin showed the presence of granular IgA deposits at the dermoepidermal junction on direct immunofluorescence (DIF) examination, whose age of disease onset was known, who had DH for at least 2 years, and who were followed up for at least 3 years after the initial NIH visit.
Main Outcome Measure:
Remission, defined as absence of skin lesions and symptoms of DH for more than 2 years while not taking sulfones (dapsone or sulfoxone), sulfapyridine, anti–tumor necrosis factor agents, or oral steroids and not adhering to a gluten-free diet.
Results:
Among 86 patients, in 10 (12%) the disease underwent remission (95% confidence interval, 6%−20%). Factors associated with DH remission included DH age of onset at 39 years or older vs onset at ages 8 to 38 years (unadjusted P=.02; adjusted P=.07) and DH onset year between 1960 and 1972 vs onset between 1935 and 1959 or after 1972 (P=.02 for global comparison of 4 onset-year groups).
Conclusions:
Dermatitis herpetiformis can go into remission. Clinicians should attempt to wean patients with well-controlled DH from a gluten-free diet and/or use of sulfones or other therapies to determine if the DH might have remitted. Our findings provide insight into the pathogenesis and course of this disease and may serve to guide long-term management of patients with DH.
Dermatitis herpetiformis (DH), also known as Duhring-Brocq disease, is an autoimmune blistering skin disease that manifests as pruritic papules and vesicles and is associated with celiac disease.1 Dermatitis herpetiformis is diagnosed by characteristic clinical and histopathologic findings and the presence of granular IgA deposits at the dermoepidermal junction of uninvolved skin. Histopathologic characteristics include microabscesses or subepidermal blisters with neutrophilic infiltrates at dermal papillary tips.2 The skin lesions respond to sulfone or sulfapyridine therapy and to strict restriction of gluten from the diet.3,4 Diseases associated with DH include celiac disease, autoimmune thyroid disease, and pernicious anemia.5
Although other autoimmune blistering diseases, most notably bullous pemphigoid and pemphigus vulgaris, may go into remission without continued dependence on pharmacotherapeutic interventions,6,7 the long-term prognosis for patients with DH is unclear. Some dermatology textbooks describe the occurrence of persistent eruptions with occasional, short-lived, lesion-free periods in an unspecified proportion of affected patients with DH.8–10 Only a few studies report remission of DH in as many as 10%11,12 or 15% of patients.13 However, these studies did not rigorously define remission, including the duration of the lesion-free period. In this study, our objectives were to determine the percentage of patients with DH whose disease goes into remission and to identify factors associated with DH remission.
METHODS
STUDY DESIGN
We conducted a retrospective cohort study at the National Institutes of Health (NIH) in Bethesda, Maryland, covering 38 years and 1 month (March 23, 1972, through April 14, 2010). All patients were already enrolled in a research protocol approved by the institutional review board at the time of initial evaluation.
Participants were patients evaluated for possible DH by one of us (S.I.K.) during this time period and were identified by a search of dermatology clinic records and the NIH Clinical Research Information System database. As part of the standard of care practiced by the treating physician (S.I.K.) for patients with DH, all patients tapered their dose of sulfones to the lowest dose possible to control their disease, including, if possible, no medication at all. Patients evaluated and diagnosed as having DH at the NIH met with an in-house nutritionist, who educated them on a gluten-free diet. Patients who were strictly adhering to a gluten-free diet were encouraged to introduce gluten-containing foods to their diet if they were no longer dependent on dapsone or sulfapyridine therapy. In our analysis, we included only patients who had clinical findings consistent with DH, whose normal skin showed the presence of granular IgA deposits at the dermoepidermal junction on direct immunofluorescence (DIF) examination, whose age of disease onset was known, who had DH for at least 2 years, and who were followed up for at least 3 years after the initial NIH visit. Patients were included regardless of whether they were alive at the time of medical chart review.
For this study, data were retrospectively collected and recorded from medical charts by one of us (S.Y.P.). We ascertained current disease status by interviews during in-person clinic visits at the NIH or via standardized telephone interviews with patients or with relatives if patients were deceased. Patients were asked about the presence of active skin lesions consistent with DH, date of last DH-like lesion, and current management, including medications and adherence to a gluten-free diet. The following variables were collected from the medical chart or by interview for each study participant: sex; date of birth; year of remission, year of death, or date of last follow-up (as applicable); age at onset of DH symptoms; history of the following at any time since DH onset: pernicious anemia, hyperthyroidism or hypothyroidism, celiac disease or presence of gastrointestinal tract symptoms consistent with celiac disease (eg, combination of abdominal bloating, flatulence, loose stools reported at least once during the course of disease), and malignant neoplasm; family history of any DH-like skin or gastrointestinal tract disease in first-degree biological relatives; and disease management history, including use of medications and adherence to a gluten-free diet.
PRIMARY OUTCOME
The long-term primary outcome was defined as absence of skin lesions and symptoms of DH for more than 2 years while not taking sulfones (dapsone or sulfoxone), sulfapyridine, anti–tumor necrosis factor (TNF) agents, or oral steroids and not adhering to a gluten-free diet. Only those who were no longer taking these drugs and no longer adhering to a gluten-free diet were considered to be in remission.
STATISTICAL ANALYSIS
Time to remission was calculated as the number of whole calendar years between the year of onset and the year of remission or last available year of follow-up, after which data were censored. The Kaplan-Meier method was used to calculate the probability or likelihood that a patient’s DH would remit as a function of time. Statistical significance of the difference between Kaplan-Meier curves created on the basis of a variety of patient characteristics was determined by log-rank tests. Adjusted P values comparing 2 Kaplan-Meier curves were equal to 3 times the unadjusted values to account for comparison of 3 possible adjacent age categories. Differences in age between patients who experienced remission and those who did not were analyzed by an exact Wilcoxon rank sum test. Differences in all categorical variables between those groups were determined by the Fisher exact test. Except as noted, all P values are 2-sided and have not been corrected for multiple comparisons. Analyses were performed using SAS software, version 8.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
CHARACTERISTICS OF THE STUDY POPULATION
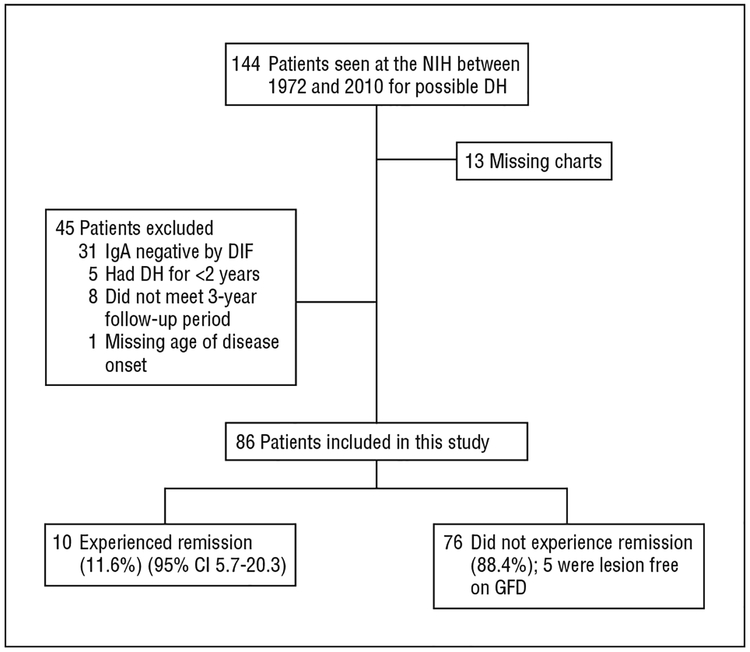
Among 144 patients evaluated at the NIH between March 23, 1972, and April 14, 2010, for possible DH (Figure 1), 58 were excluded (40%). Of these 58, 13 patients did not have a medical chart available (22%), and 45 did not meet inclusion criteria (78%) (31 had negative IgA DIF findings; 5 had DH for <2 years; 8 had follow-up for <3 years; and 1 patient’s age of onset was unavailable). Among the 86 patients included in the study sample, 26 patients had died but were included in the study (30%). Fourteen of the 26 deceased patients had DH at the time of their death, as noted in their medical charts, and the disease status of the remaining 12 was obtained from a living relative by telephone interview. Of the 60 living patients, 12 were seen in person at the NIH, and 48 were contacted by telephone follow-up. Most patients had taken sulfones (dapsone, 25–300 mg/d, or sulfoxone, 165–825 mg/d before 1990) or sulfapyridine (500 mg/d to 3 g/d) and were prescribed varying doses of these drugs during their disease course. Three patients had been treated with corticosteroids or anti-TNF therapy (etanercept) for concomitant problems. Characteristics of all study participants are listed in Table 1.
Figure 1.
Flowchart of the study. CI indicates confidence interval; DH, dermatitis herpetiformis; DIF, direct immunofluorescence; GFD, gluten-free diet; NIH, National Institutes of Health.
Table 1.
Characteristics of All Study Participantsa
| Characteristic (Patients Included in Analysis, No.) | All Patients | Remission (n-10) | Not in Remission (n=76) | P Value |
|---|---|---|---|---|
| Male sex (n=86) | 43 (50) | 5(50) | 38 (50) | >.99b |
| Age at DH onset, mean (SD) [range], y (n=86) | 29.9 (16.3) [9–71] | 29.9 (11.5) [17–46] | 29.9 (16.9) [9–71] | .51c |
| Year of DH onset, median (Q1, Q3) [range] (n=86) | 1968 (1961, 1972) [1936–1990] | 1966(1963, 1970) [1958–1972] | 1969 (1961, 1973) [1936–1990] | >.99b |
| DH onset 1935–1967 | 40 (47) | 5(50) | 35 (46) | NA |
| DH onset ≥1968 | 46 (53) | 5(50) | 41 (54) | NA |
| Family history of skin disease (n=81) | 12(15) | 3(30) | 9(12) | .16b |
| Family history of Gl disease (n=86) | 3(4) | 1(10) | 2(3) | .31b |
| History of thyroid disease (n=85) | 8(9) | 3(30) | 5(7) | .06b |
| History of pernicious anemia (n=85) | 7(8) | 2(20) | 5(7) | .19b |
| Symptoms of celiac disease (n=84)d | 29 (35) | 3(30) | 26 (34) | >.99b |
Abbreviations: DH, dermatitis herpetiformis, GI, gastrointestinal tract; NA, not applicable; Q1, first quartile; Q3, third quartile.
Unless otherwise indicated, data are reported as number (percentage) of patients.
By Fisher exact test.
By Wilcoxon rank sum test.
Symptoms (eg, combination of abdominal bloating, flatulence, and loose stools) at any time during their years of follow-up.
REMISSION
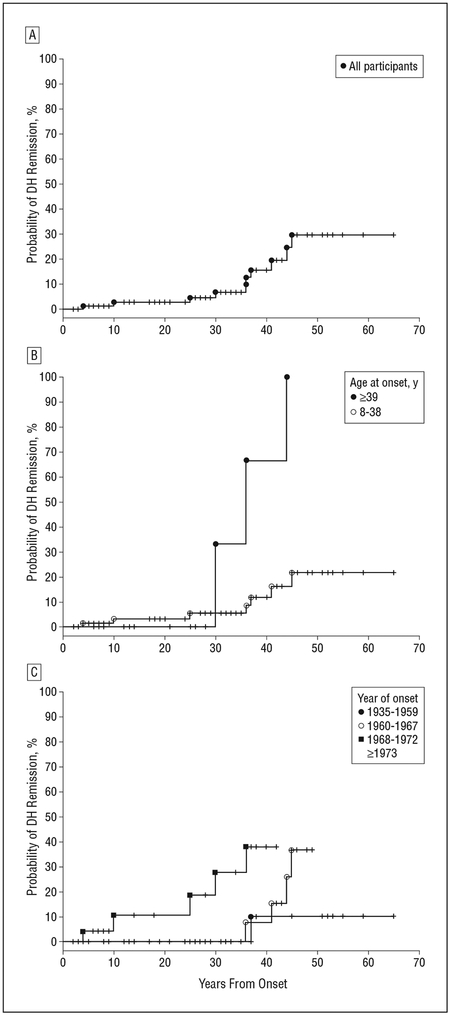
Among 86 patients, DH went into remission in 10 (12%) (95% confidence interval [CI], 6%−20%) (Figure 2A). Although initially quite similar, DH in patients who were 39 years or older compared with those aged 8 to 38 years at disease onset eventually exhibited a greater probability of going into remission (unadjusted P=.02; adjusted P=.07, accounting for evaluation of 3 possible age cutoffs) (Figure 2B). This difference is based on limited numbers of remissions and should be interpreted carefully. The disease in 7 of 64 patients in the 8- to 38-year-old age group and 3 of 22 patients in the group 39 years or older went into remission. Nine of 45 patients with onset between 1960 and 1972 had DH remission (20%), while only 1 of 20 patients with onset between 1935 and 1959 and none of 21 patients with onset after 1972 had disease remission (P=.02 for global comparison of 4 onset-year groups) (Figure 2C). eTable 1 and eTable 2 (http://www.archdermatol.com) list the 95% CIs for probability of DH remission by age at onset and year of onset.
Figure 2.
Kaplan-Meier estimates of the probability of dermatitis herpetiformis (DH) remission as a function of years from onset of disease. Each tick represents a patient who died or was no longer followed up. For 95% confidence intervals, see eTable 1 and eTable 2. A, Probability of DH remission in all study participants (n=86). B, Probability of DH remission in patients with disease onset between ages 8 and 38 years and at 39 years or older (unadjusted P=.02; adjusted P=.07). C, Probability of DH remission in patients with year of disease onset between 1935 and 1959; 1960 and 1967; 1968 and 1972; and 1973 or later (no graph) (global P=.02).
Of the 9 remissions in patients with disease onset between 1960 and 1972, DH in 4 of 20 patients in the 1960–1967 disease-onset group and 5 of 25 patients in the 1968–1972 disease-onset group underwent remission. We did not find a significant association of sex (P>.99), pernicious anemia (P=.19), family history of skin disease (P=.16), gastrointestinal tract disease (P=.31), or symptoms of celiac disease (P>.99) with DH remission. Thyroid disease showed some association with disease remission in this cohort, but the association did not reach significance (P=.06). Only 1 patient was diagnosed as having cutaneous T-cell lymphoma. Of the 34 patients who reported attempting to adhere closely to a gluten-free diet, 5 were lesion-free while adhering to the diet (15%). The probability of disease remission in patients as assessed by 5-year intervals from onset of disease is summarized in Table 2. Forty years after DH onset, the probability of disease remission was 15.5% (95% CI, 7.4%−29.6%). The longer the disease persists, the more likely it is that the disease will remit.
Table 2.
Probability of DH Remission by 5-Year Intervals After Disease Onset
| Years After DH Onset | Probability, % (95% CI) (n=86) |
|---|---|
| 5 | 1.2(0.2–6.4) |
| 10 | 2.6(0.7–9.1) |
| 15 | 2.6(0.7–9.1) |
| 20 | 2.6(0.7–9.1) |
| 25 | 4.5(1.5–12.6) |
| 30 | 6.8(2.6–16.6) |
| 35 | 6.8(2.6–16.6) |
| 40 | 15.5(7.4–29.6) |
| 45 | 29.6(15.7–49.6) |
| 50 | 29.6(15.7–49.6) |
Abbreviations: CI, confidence interval; DH, dermatitis herpetiformis.
COMMENT
In this retrospective cohort study, DH remitted in 12% of patients (10 of 86). To our knowledge, this study is the first rigorous evaluation of DH remission, and results of our analysis align with previous observations of DH remission (10%−15%). Over the years of follow-up, all patients were asked to taper their sulfone or sulfapyridine medication to be certain that they were taking the lowest dose possible to control their disease. This is, in part, how we learned that some patients no longer required therapy. The results of this study emphasize the importance of reducing sulfone or sulfapyridine therapy and attempting to wean patients with well-controlled DH from a gluten-free diet. However, before changing their diets, patients with symptomatic celiac disease should discuss options with the physician managing their gastrointestinal tract condition because stopping a gluten-free diet might exacerbate the gluten-sensitive enteropathy.
A critical yet unanswered question is what are the factors involved in DH remission in these patients? Patients in the present study whose disease was manifest when they were older had disease remission more commonly than those with earlier disease onset. One may postulate that those with earlier DH onset had more severe disease, but there is no evidence to support this; in fact, these patients did not require greater drug therapy to control their disease. There did not seem to be an association between DH remission and drug dosage:some patients who had DH remission were taking as much as 200 to 300 mg/d of dapsone at some time during their course of disease. We can offer no good reason why year of onset of DH (between 1960 and 1972) also seemed to correlate with DH remission.
Several autoimmune diseases are seen with increased incidence in patients with DH. Thyroid disease14 and pernicious anemia5 are 2 such diseases evaluated in this study. Our results indicate a potential clinical association of thyroid disease with DH remission (P=.06). While the statistical association was not significant, clinical association cannot be excluded. Among all the disease history parameters, thyroid disease was the only variable modestly associated with DH remission.
Previous reports have also suggested an increased risk of lymphoma with DH, particularly in those patients with concurrent celiac disease. Hervonen et al15 reported the occurrence of B-cell–type lymphomas and enteropathy-associated T-cell lymphoma in 1% of study participants. Viljamaa et al16 also noted a significantly increased risk of non-Hodgkin lymphoma in their patients with DH. However, a recent cohort study by Lewis et al17 presented no increased risk of gastrointestinal tract or lymphoproliferative cancers. In our cohort of 86 patients, 1 patient was diagnosed as having cutaneous T-cell lymphoma.
This study is subject to at least 3 limitations. First, the sample size was small, limiting the power of the study to detect differences in DH remission probabilities between groups. A small percentage of the cohort was also lost to follow-up. Considering the rarity of the disease, however, our cohort is one of the largest in the literature. Second, information collected from patients and family members was self-reported and subject to recall bias. In addition to the inherent restrictions of a retrospective study, including working within the scope of available medical chart data, studies involving self-reported data are susceptible to recall errors that can affect internal validity. We attempted to minimize recall bias by strictly defining the research question, standardizing patient interviews with appropriately detailed questions, and standardizing data collection. Third, we did not assess the duration of DH remission following the initial 2 lesion-free years required to meet the study definition of remission.
Results of this study show that DH can go into remission. Therefore, clinicians should continually reevaluate the need for medical therapy and a gluten-free diet for their patients with well-controlled DH, with the idea that DH might actually be in remission in some patients. Our findings provide insight into the pathogenesis and course of this disease and may serve to guide long-term management of patients with DH. We also provide valuable information that could be shared with patients at the time of diagnosis to establish better understanding of their disease.
Funding/Support:
This study was supported in part by the Clinical Research Training Program (Ms Paek), a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc), and by the Center for Cancer Research, National Cancer Institute (Ms Paek and Drs Steinbergand Katz).
Financial Disclosure: None reported.
Footnotes
Online-Only Material: eTable 1 and eTable 2 are avail-able at http://www.archdermatol.com.
REFERENCES
- 1.Hall RP III. Dermatitis herpetiformis. J Invest Dermatol. 1992;99(6):873–881. [DOI] [PubMed] [Google Scholar]
- 2.Rose C, Bröcker EB, Zillikens D. Clinical, histological and immunopathological findings in 32 patients with dermatitis herpetiformis Duhring. J Dtsch Dermatol Ges. 2010;8(4):265–270, 265–271. [DOI] [PubMed] [Google Scholar]
- 3.Kárpáti S. Dermatitis herpetiformis: close to unravelling a disease. J Dermatol Sci. 2004;34(2):83–90. [DOI] [PubMed] [Google Scholar]
- 4.Hardman CM, Garioch JJ, Leonard JN, et al. Absence of toxicity of oats in patients with dermatitis herpetiformis. N Engl J Med. 1997;337(26):1884–1887. [DOI] [PubMed] [Google Scholar]
- 5.Reunala T, Collin P. Diseases associated with dermatitis herpetiformis. Br J Dermatol. 1997;136(3):315–318. [PubMed] [Google Scholar]
- 6.Bernard P, Reguiai Z, Tancrède-Bohin E, et al. Risk factors for relapse in patients with bullous pemphigoid in clinical remission: a multicenter, prospective, cohort study. Arch Dermatol. 2009;145(5):537–542. [DOI] [PubMed] [Google Scholar]
- 7.Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000;42(3):422–427. [DOI] [PubMed] [Google Scholar]
- 8.Wojnarowska F, Eady R, Burge S. Dermatitis herpetiformis In: Champion R, Burton J, Burns D, Breathnach S, eds. Rook/Wilkinson/Ebling Textbook of Dermatology. Vol 3 6th ed Malden, MA: Blackwell Science; 1998:1888–1892. [Google Scholar]
- 9.Hall R III, Katz S. Dermatitis herpetiformis In: Wolff G, Katz G, Paller L, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1 7th ed New York, NY: McGraw-Hill Professional; 2007:500–504. [Google Scholar]
- 10.Fine J. Dermatitis herpetiformis In: Moschella S, Hurley H, eds. Dermatology. Vol 1 3rd ed Philadelphia, PA: WB Saunders; 1992:674–676. [Google Scholar]
- 11.Hull C, Zone J. Dermatitis herpetiformis and linear IgA bullous dermatosis In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 1 2nd ed Philadelphia, PA: Mosby; 2008:447–452. [Google Scholar]
- 12.Garioch JJ, Lewis HM, Sargent SA, Leonard JN, Fry L. 25 years’ experience of a gluten-free diet in the treatment of dermatitis herpetiformis. Br J Dermatol. 1994; 131(4):541–545. [DOI] [PubMed] [Google Scholar]
- 13.Fry L. Dermatitis herpetiformis. Baillieres Clin Gastroenterol. 1995;9(2):371–393. [DOI] [PubMed] [Google Scholar]
- 14.Gaspari AA, Huang CM, Davey RJ, Bondy C, Lawley TJ, Katz SI. Prevalence of thyroid abnormalities in patients with dermatitis herpetiformis and in control subjects with HLA-B8/-DR3. Am J Med. 1990;88(2):145–150. [DOI] [PubMed] [Google Scholar]
- 15.Hervonen K, Vornanen M, Kautiainen H, Collin P, Reunala T. Lymphoma in patients with dermatitis herpetiformis and their first-degree relatives. Br J Dermatol. 2005;152(1):82–86. [DOI] [PubMed] [Google Scholar]
- 16.Viljamaa M, Kaukinen K, Pukkala E, Hervonen K, Reunala T, Collin P. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-year population-based study. Dig Liver Dis. 2006;38(6):374–380. [DOI] [PubMed] [Google Scholar]
- 17.Lewis NR, Logan RF, Hubbard RB, West J. No increase in risk of fracture, malignancy or mortality in dermatitis herpetiformis: a cohort study. Aliment Pharmacol Ther. 2008;27(11):1140–1147. [DOI] [PubMed] [Google Scholar]