1. Introduction
Ovarian cancer is the most lethal gynecologic cancer, accounting for over 16,000 deaths. In the US annually (1). The poor prognosis of this disease is due to the lack of reliable screening tools, the late stage of disease at the time of diagnosis, the high rate of recurrence of the disease, and the poor response to chemotherapy in the recurrent setting. Patients who present with stage I disease have over 90% 5-year survival, while those diagnosed with stage III disease have less than 20% 5-year survival (2). However, only 25% of patients with ovarian cancer are diagnosed with stage I disease (1). Despite new chemotherapeutic regimens and radical surgical debulking procedures, only minimal improvement in overall survival has been appreciated in the last several decades.
Epithelial ovarian cancer encompasses four major histotypes: papillary serous, endometrioid, mucinous, and clear cell. These histotypes resemble various müllerian cell types, with serous tumors resembling Fallopian tube, endometrioid tumors resembling uterine endometrium, mucinous tumors resembling the endocervix, and clear cell tumors resembling endometrial glands during pregnancy (2). Tumors are graded from 1 to 3, with grade 3 being the most poorly differentiated. Tumors of low malignant potential (LMP) display the atypical cellular features of cancer, but do not invade into the ovarian stroma (2).
The clinical characteristics of ovarian tumors vary according to histology and grade. Although clear cell ovarian cancer usually presents with earlier stage of the disease, there is a higher rate of recurrence among these patients. In fact, the 5-year survival for patients with stage I of clear cell ovarian cancer is only 60% (2). Late stage clear cell cancer also carries a worse prognosis when compared with papillary serous cancer, with an overall median survival of 12 months compared with 22 months (3). Mucinous cancers have a poorer response to chemotherapy and worse survival than the other epithelial ovarian histotypes (4). Patients with endometrioid ovarian cancers tend to have a better prognosis. Higher tumor grade correlates with poorer prognosis. Patients with LMP tumors have a 5-year survival over 95%. Among patients with early stage of disease, the survival drops down from 97% for patients with grade I tumors to 50% for those with grade III tumors (5).
These clear clinical differences among ovarian tumors likely reflect different underlying molecular mechanisms. Elvcidating how these tumors vary from a molecular biology standpoint can help us understand the pathogenesis and clinicopathologic characteristics of these tumors. Since gene expression is a critical determinate for many molecular features, gene expression profiling is a powerful approach to determine the underlying mechanism for these biologic and clinical differences.
Recent advances in molecular technology have provided the ability to perform whole genome expression profiling. This technique provides a global analysis of the transcriptional activity in ovarian tumors, which can then be correlated to pathologic and clinical determinates. Microarray determines the expression of genes by measuring mRNA levels using the ability of mRNA to hybridize to the DNA template. Over a dozen commercial platforms are currently available, each utilizing different technologies to fabricate their microarray chip. Much progress has been achieved in the microarray field since it was first introduced over a decade ago, and whole genome expression profiling, analyzing over 37,000 genes, is now possible on a single microarray chip.
2. Choice of Normal Ovarian Control
Identifying genes whose expression is altered during the transformation process relies on comparing malignant cells with their normal counterpart. Expression profiling of normal ovarian epithelium has utilized several sources of “normal” cells, including whole ovary samples (WO), ovarian surface epithelium exposed to short-term culture (NOSE), and immortalized ovarian surface epithelium cell lines (IOSE). WO has the advantage of providing a large amount of RNA; however, a large stromal component may mask true genomic expression differences within the epithelial component. Short-term cultures of ovarian surface epithelium scrapings provide a robust sample of ovarian surface epithelia, but are exposed to tissue culture conditions. Cultured media may select a subset of cells that are not representative of the original culture, altering overall gene expression. Immortalization methods of ovarian surface epithelia have utilized SV40 large T-antigen (6) and telomerase immortalization techniques (TIOSE) (7). These immortalized cells are exposed to tissue culture conditions, and they can demonstrate a large increase in chromosomal imbalance that could cause gene expression differences due to the immortalization process (8).
Another option for obtaining normal ovarian cells involves the preservation of ovarian surface epithelium brushings (OSE) without culturing (9). Brushings are obtained at the time of surgery followed by direct immersion in solution to preserve RNA quality. These cells are not immortalized and are not exposed to culture.
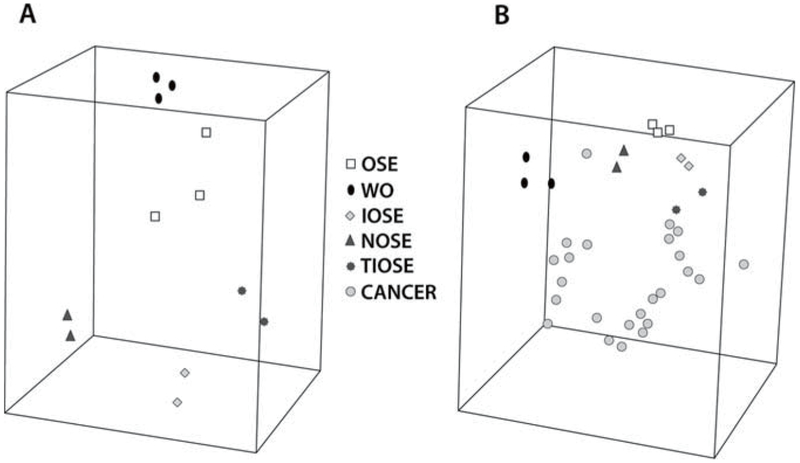
Zorn et al. (10) analyzed these five types of ovarian surface epithelium by comparing each group’s expression profile with that of a set of 24 serous ovarian carcinomas. Hierarchical clustering and multidimensional scaling of the expression profiles of these groups demonstrated very distinct clusters (Fig. 1). In fact, the differences between “normal” samples were larger than those among the cancer specimens. When any two “normal” groups were compared with one another, the Pearson correlation coefficient for all combinations ranged from 0.04 to 0.54 (Table 1). When the individual gene lists were compared with the gene expression profile for the set of serous ovarian cancer samples, there was a majority of genes that were unique to each list. No gene appeared on all five lists. From their analysis, WO, NOSE, IOSE, TIOSE, and OSE had distinct expression profiles, and they concluded that OSE brushings seemed to be the most reasonable control as this sample was not affected by culture conditions or immortalization techniques.
Fig. 1.
Multidimensional scaling (MDS) of the expression profiles of ovarian specimens. MDS allows assessment of the likeness of the samples’ expression patterns by compressing their gene expression profiles into a three-dimensional space. Samples with similar profiles cluster relatively close. (a) Unsupervised MDS of the normal groups. (b) Unsupervised MDS of the normal groups and the serous ovarian carcinoma samples
Table 1.
Pearson correlation coefficient (r) of expression profiles between different normal ovarian epithelium samples in a group and between groups
| Group | Within group | With OSE | With WO | With IOSE | With TIOSE | With NOSE |
|---|---|---|---|---|---|---|
| OSE | 0.78 | 1 | 0.47 | 0.23 | 0.04 | 0.36 |
| WO | 0.86 | 0.47 | 1 | 0.10 | 0.22 | 0.39 |
| IOSE | 0.73 | 0.23 | 0.10 | 1 | 0.28 | 0.54 |
| TIOSE | 0.93 | 0.04 | 0.22 | 0.28 | 1 | 0.27 |
| NOSE | 0.90 | 0.36 | 0.39 | 0.54 | 0.27 | 1 |
3. Genes Expression Profiling of Different Tumor Grades
Serous ovarian tumors represent about 50% of all ovarian cancer. A small proportion of these tumors are classified as those of low malignant potential (LMP). LMP tumors display an atypical nuclear structure and can be metastatic, but because they lack stromal invasion, they are not characterized as “cancer.” There is a debate as to how LMP tumors relate to the other frankly invasive tumors. One hypothesis states that LMP tumors are a distinct disease from invasive carcinoma, while another hypothesis argues that these tumors represent an early precursor lesion that eventually develops into malignant disease. Given that LMP tumors with micropapillary features have a lower overall survival when compared with LMPs without these features (11), it has been hypothesized that this small subset of LMP tumors with micropapillary histology can develop into low-grade invasive ovarian cancer (12).
Bonome et al. addressed this debate by evaluating the biological relationship among serous LMP, low-grade, and high-grade invasive ovarian carcinomas (13). They generated global gene expression profiles for 66 microdissected serous tumors. Unsupervised hierarchical clustering demonstrated distinct clusters that differentiated LMP and high-grade tumors. Of note, the majority of low-grade tumors clustered with LMP tumors. This strongly supports that LMP tumors are a unique disease entity from high-grade invasive cancer. Low-grade invasive cancers are essentially indistinguishable from LMP tumors.
Gene ontological analysis between high-grade tumors and LMP tumors found statistically significant differences (p < 0.001) between the number of genes involved in mitotic cell cycle, M phase, mitosis, G2-M transition, and cytokinesis. The majority of genes were upregulated in the high-grade specimens when compared with normal ovarian surface epithelium (Table 2). Specifically, genes linked to cell proliferation that were upregulated on the microarray analysis in high-grade tumors but not in LMP tumors included PDC4, CCNDBP1, E2F3, CDC2, CCNB1, CCNB2, ASK, STMN1, CCNE1, MCM4, MCM5, MCM7, RFC4, FEN1, STK6, CENP-A, CDC20, EIF4G1, PTTG, and PCNA.
Table 2.
GO categories associated with cell cycle progression in high-grade, low-grade, and LMP tumors
| Late-stage high-grade | Early-stage high-grade | LMP/low-grade | ||||
|---|---|---|---|---|---|---|
| Gene ontology category | Present | Number of genes | Present | Number of genes | Present | Number of genes ® |
| Mitotic cell cycle | Yes | 70 | Yes | 58 | No | 0 |
| M phase | Yes | 66 | Yes | 55 | No | 0 |
| Mitosis | Yes | 51 | Yes | 43 | No | 0 |
| G2/M transition | Yes | 14 | Yes | 14 | No | 0 |
| Cytokinesis | Yes | 28 | No | 0 | No | 0 |
There was a statistically significant difference (p < 0.05) in number of genes associated with cell cycle progression that were over-expressed in high-grade tumors that were not differentially expressed in low-grade and LMP tumors
Major differences between p53 and its associated genes were noted between LMP tumors and high-grade tumors. LMP tumors displayed elevated levels of p53 RNA and its principal effector CDKNIA, while high-grade tumors did not. Dysregulated genes that were unique to LMP tumors included UBE2D1 and ADNP. Both are negative regulators of p53 (14), and they were down-regulated in LMP tumors. PPM1A, which has been shown to increase the overall level of p53, was over-expressed in LMP tumors (15). LMP tumors also demonstrated an overexpression of important targets of p53. PML, which modulates apoptosis (16), and GDF15, which mediates growth arrest (17) are both over-expressed in LMP tumors but not in high-grade tumors. These results demonstrated clear differences between LMP tumors and high-grade tumors among p53-modulated genes, suggesting this pathway may play an important role in the distinct phenotypic differences between these two tumor grades.
4. Gene Expression Profiling of Different Tumor Histotypes
Gene expression profiling has also been used to characterize differences among the four main histotypes of ovarian cancer. Marquez et al. (18) compared whole genome expression profiles of serous, endometrioid, mucinous, and clear cell ovarian tumors with each other and to mucosal scrapings of normal fallopian tube, endometrium, and colon. Hierarchical clustering displayed grouping by the individual histotypes. They found a statistically significant correlation between serous tumors and normal fallopian tube, mucinous tumors with normal colonic epithelium, and endometrioid and clear cell tumors with normal endometrium. Their analysis utilized whole genome expression profiling, and when comparing the individual histotypes, mucinous cancers displayed a greater number of dysregulated genes than the other histotypes.
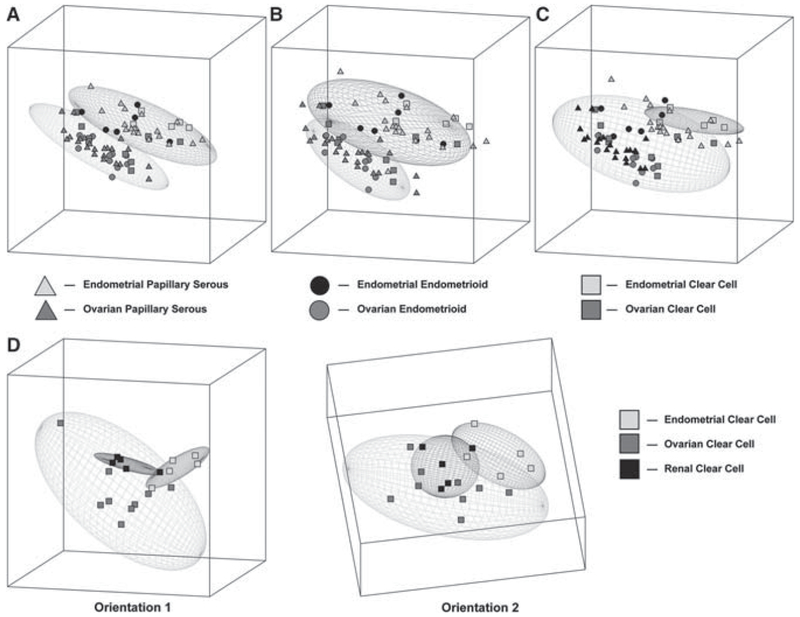
A comparison of tumors of similar histotypes across different organs has also been analyzed (19). Serous, endometrioid, and clear cell cancer histotypes of ovarian and endometrial origin were compared using a cDNA microarray. Although distinct expression patterns were appreciated among serous and endometrial tumors with respect to their organs of origin, clear cell tumors demonstrated a similar gene expression pattern for tumors originating in the ovary and endometrium (Fig. 2). This unique gene signature for clear cell tumors is consistent with an earlier study (20). Furthermore, in Zorn et al.’s analysis, expression profiling of renal clear cell cancers demonstrated that they were unable to be distinguished from clear cell tumors from the ovary or endometrium. This common pattern of clear cell tumor gene expression among these organs may represent a common precursor cell or similar processes of transformation.
Fig. 2.
Graphic depiction of the principal component analysis of ovarian and endometrial cancers categorized by histology. The ellipses represent a region where an additional sample of particular groups would fall with a 95% confidence interval. a. Analysis of serous tumors demonstrates nonoverlapping ellipses separating endometrial (top) and ovarian (bottom) specimens. b. Analysis of endometrioid tumors demonstrates nonoverlapping ellipses separating endometrial (top) and ovarian (bottom) specimens. c. Analysis of clear cell tumors showing overlapping of endometrial (top) and ovarian (bottom) specimens. d. Analysis of clear cell tumors of ovarian, endometrial, and renal origin demonstrate three overlapping elliptical regions, with two different orientations (1 and 2)
Clear cell ovarian cancer is a rare histotype, and its clinical course presents a poorer prognosis. Patients with early stage disease have a higher recurrence rate, with 37% of stage IC patients recurring (21). Clear cell tumors also have a lower response rate to standard platinum and taxane chemotherapy, which is the usual first line therapy for ovarian cancer. Clear cell ovarian tumors have chemotherapy response rates as low as 15%, when compared with over 70% in serous tumors (22). In the analysis by Zorn et al. (19), genes that were common in clear cell tumors of the ovary and endometrium included ANXA4 and UGT1A1. Both genes have been associated with chemoresistence, wherein ANXA4 has been associated with paclitaxel resistance and UGT1A1 has been shown to detoxify the active metabolite of irinotecan, SN-38 (23, 24).
The conclusions from these expression profiling studies of ovarian cancers of different histologies suggest that clear cell tumors represent a unique disease. Clear cell ovarian tumors are clinically and biologically distinct tumors from the other ovarian histotypes. As such, clinical trials addressing the optimal treatment of these tumors are needed. Research is needed that will hopefully identify molecular pathways that are unique to clear cell tumors, exposing targets for chemotherapeutic intervention.
Mucinous tumors also represent a rare ovarian cancer subtype, with the majority of tumors being benign (2). Although advanced stage disease represents the minority of mucinous tumors, this group has been found to have a worse prognosis and poorer response to chemotherapy when compared with other epithelial ovarian cancers, with chemotherapy response rates as low as 26% (4). Invasive mucinous ovarian tumors frequently have coexisting cells of varying malignancy, transitioning between benign and malignant cells on the same tumor (Fig. 3). Furthermore, identical K-ras mutations are frequently found in coexisting LMP and invasive epithelia within the same mucinous tumor (25). This suggests a progression model for mucinous ovarian tumors.
Fig. 3.
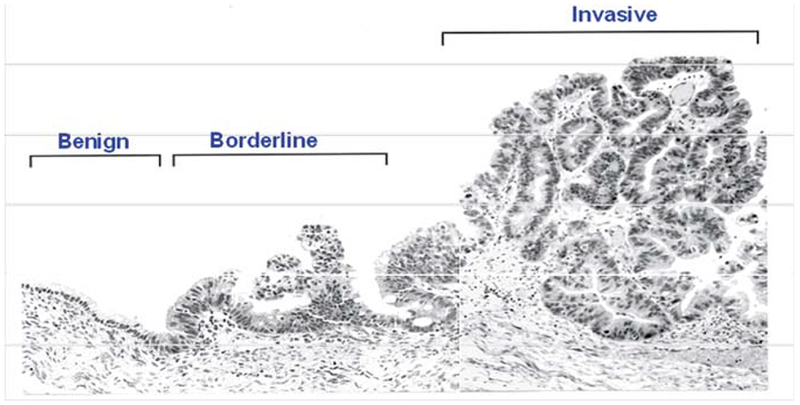
This mucinous tumor specimen demonstrates close regions that display cells that are invasive as well as those with low malignant potential
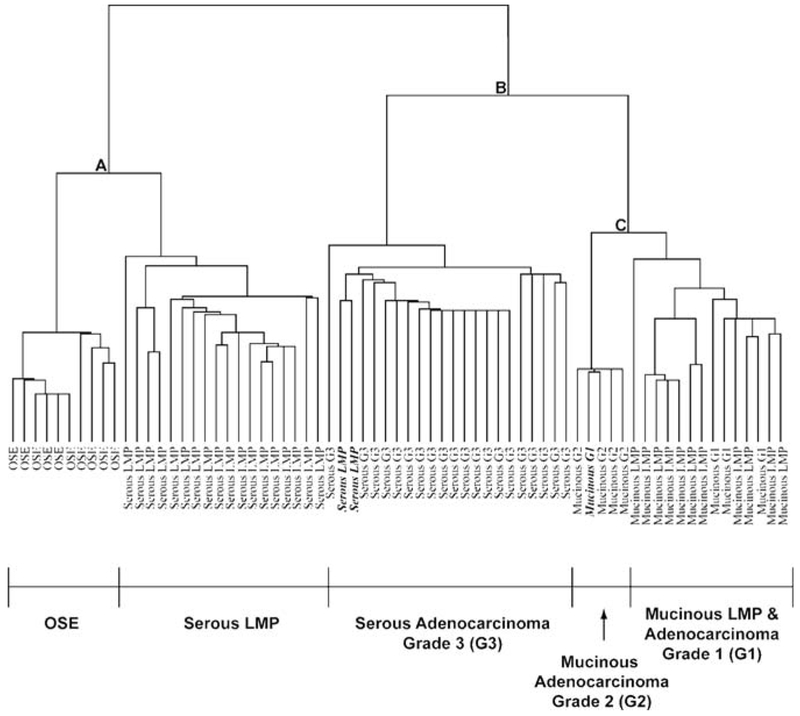
To evaluate the potential reasons behind the biological and clinical differences between mucinous tumors and other epithelial ovarian tumors, Wamunyokoli et al. performed global gene expression of mucinous cystadenomas, tumors of low malignant potential, and cystadenocarcinomas (26). The expression profiles of the mucinous tumors were compared with OSE and serous tumors. Unsupervised hierarchical clustering and binary tree analysis showed clustering of OSE with serous LMP tumors, and clustering of grade III serous tumors with invasive and LMP mucinous tumors (Fig. 4). Serous tumors had distinct clustering between LMP tumors and advanced stage tumors, while mucinous tumors of all grades had a high misclassification rate among the grades. Furthermore, the clustering of mucinous tumors with advanced stage serous tumors suggests the existence of a set of genes that may account for the poorer prognosis of mucinous ovarian cancer.
Fig. 4.
Unsupervised hierarchical clustering of OSE, mucinous cystadenomas, mucinous LMP tumors, mucinous adenocarcinomas, and serous tumors. This dendogram illustrates OSE specimens grouping independently of serous LMP tumors, while high-grade serous tumors were closely associated with mucinous LMP and invasive tumors
To analyze what genes may be associated with the development of the mucinous phenotype, gene lists for mucinous cystadenomas and cystadenocarcinomas were evaluated to identify coregulated pathways. Genes common and unique to the different grades of tumors were also identified. Genes that were found to be upregulated in LMP tumors and cystadenocarimomas, but not OSE and cystadenomas, included NET1 and ERBB3, suggesting the involvement of these genes in transformation. These genes have been found to increase tumorigenicity and promote invasiveness (27, 28). Genes involved in multidrug resistance, such as ABCC3 and ABCC6 (29) were upregulated in mucinous LMP tumors and cystadenocarimomas, but not cystadenomas. This is consistent with the known lower response rate of mucinous tumors to chemotherapy. Genes that modulate cell morpholophy, such as CDC42, ECT2, IQGAP2, and Cortactin (30, 31), were found to be upregulated in mucinous cystadenocarcinomas, but not in mucinous LMP tumors. The differential regulation of these genes by tumor grade suggests a role of these genes in tumor progression.
5. Conclusion
Gene expression profiling can be used to evaluate ovarian cancer and identify genes and pathways important in tumor transformation and progression. Serous LMP tumors and low-grade serous cancer appear to have pathogenetic pathways that differ from high-grade serous cancer, implying these tumors are separate entities. Recent studies evaluating clear cell, serous, and mucinous epithelial ovarian cancer have found molecular differences that could explain their different clinical and biologic phenotypes. Clear cell ovarian tumors have an expression profile that is distinct from the other ovarian histologies, and similar to clear cell tumors originating in other organs. Mucinous ovarian cancer pathogenesis, unlike serous tumors, represents a continuum. Mucinous benign tumors may develop from ovarian inclusion cysts, acquire further KRAS mutations and other molecular changes to become mucinous borderline tumors, and then progress to low-grade and subsequently high-grade tumors. With gene expression profiling, novel pathways for these tumors will eventually be identified, exposing new and more specific targets for chemotherapy.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ (2005). “Cancer statistics, 2005.” CA Cancer J Clin 55(1): 10–30. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins WJ (2005). Principles and practice of gynecologic oncology. Philadelphia, Lippincott. [Google Scholar]
- 3.Goff BA, de la Cuesta R. Sainz , Muntz HG, Fleischhacker D, Ek M, Rice LW, Nikrui N, Tamimi HK, Cain JM, Greer BE and Fuller AF Jr. (1996). “Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease.” Gynecol Oncol 60(3): 412–7. [DOI] [PubMed] [Google Scholar]
- 4.Hess V, A’Hern R, Nasiri N, King DM, Blake PR, Barton DP, Shepherd JH, Ind T, Bridges J, Harrington K, Kaye SB and Gore ME (2004). “Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment.” J Clin Oncol 22(6): 1040–4. [DOI] [PubMed] [Google Scholar]
- 5.Shimada M, Kigawa J, Kanamori Y, Itamochi H, Oishi T, Minagawa Y, Ishihara K, Takeuchi Y, Okada M and Terakawa N (2005). “Outcome of patients with early ovarian cancer undergoing three courses of adjuvant chemotherapy following complete surgical staging.” Int J Gynecol Cancer 15(4): 601–5. [DOI] [PubMed] [Google Scholar]
- 6.Jazaeri AA, Yee CJ, Sotiriou C, Brantley KR, Boyd J and Liu ET (2002). “Gene expression profiles of BRCA1-linked, BRCA2-linked, and sporadic ovarian cancers.” J Natl Cancer Inst 94(13): 990–1000. [DOI] [PubMed] [Google Scholar]
- 7.Wernert N, Locherbach C, Wellmann A, Behrens P and Hugel A (2001). “Presence of genetic alterations in microdissected stroma of human colon and breast cancers.” Anticancer Res 21(4A): 2259–64. [PubMed] [Google Scholar]
- 8.Tsao SW, Wong N, Wang X, Liu Y, Wan TS, Fung LF, Lancaster WD, Gregoire L and Wong YC (2001). “Nonrandom chromosomal imbalances in human ovarian surface epithelial cells immortalized by HPV16-E6E7 viral oncogenes.” Cancer Genet Cytogenet 130(2): 141–9. [DOI] [PubMed] [Google Scholar]
- 9.Shridhar V, Lee J, Pandita A, Iturria S, Avula R, Staub J, Morrissey M, Calhoun E, Sen A, Kalli K, Keeney G, Roche P, Cliby W, Lu K, Schmandt R, Mills GB, Bast RC Jr., James CD, Couch FJ, Hartmann LC, Lillie J and Smith DI (2001). “Genetic analysis of early- versus late-stage ovarian tumors.” Cancer Res 61(15): 5895–904. [PubMed] [Google Scholar]
- 10.Zorn KK, Jazaeri AA, Awtrey CS, Gardner GJ, Mok SC, Boyd J and Birrer MJ (2003). “Choice of normal ovarian control influences determination of differentially expressed genes in ovarian cancer expression profiling studies.” Clin Cancer Res 9(13): 4811–8. [PubMed] [Google Scholar]
- 11.Longacre TA, McKenney JK, Tazelaar HD, Kempson RL and Hendrickson MR (2005). “Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or = 5-year) follow-up.” Am J Surg Pathol 29(6): 707–23. [DOI] [PubMed] [Google Scholar]
- 12.Shih Ie M and Kurman RJ (2004). “Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis.” Am J Pathol 164(5): 1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, Lu KH, Sood AK, Gershenson DM, Mok SC and Birrer MJ (2005). “Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary.” Cancer Res 65(22): 10602–12. [DOI] [PubMed] [Google Scholar]
- 14.Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, Bassan M, Wollman Y, Eyre HJ, Mulley JC, Brenneman DE and Gozes I (2001). “Cloning and characterization of the human activity-dependent neuroprotective protein.” J Biol Chem 276(1): 708–14. [DOI] [PubMed] [Google Scholar]
- 15.Ofek P, Ben-Meir D, Kariv-Inbal Z, Oren M and Lavi S (2003). “Cell cycle regulation and p53 activation by protein phosphatase 2C alpha.” J Biol Chem 278(16): 14299–305. [DOI] [PubMed] [Google Scholar]
- 16.Pearson M and Pelicci PG (2001). “PML interaction with p53 and its role in apoptosis and replicative senescence.” Oncogene 20(49): 7250–6. [DOI] [PubMed] [Google Scholar]
- 17.Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, Austin RC and Klamut HJ (2000). “Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression.” J Biol Chem 275(26): 20127–35. [DOI] [PubMed] [Google Scholar]
- 18.Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, Atkinson EN, Smith DI, Hartmann L, Fishman D, Berchuck A, Whitaker R, Gershenson DM, Mills GB, Bast RC Jr. and Lu KH (2005). “Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon.” Clin Cancer Res 11(17): 6116–26. [DOI] [PubMed] [Google Scholar]
- 19.Zorn KK, Bonome T, Gangi L, Chandramouli GV, Awtrey CS, Gardner GJ, Barrett JC, Boyd J and Birrer MJ (2005). “Gene expression profiles of serous, endometrioid, and clear cell subtypes of ovarian and endometrial cancer.” Clin Cancer Res 11(18): 6422–30. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz DR, Kardia SL, Shedden KA, Kuick R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, Reed H, Ellenson LH, Giordano TJ, Fearon ER, Hanash SM and Cho KR (2002). “Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas.” Cancer Res 62(16): 4722–9. [PubMed] [Google Scholar]
- 21.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K (2000). “Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy.” Cancer 88(11): 2584–9. [PubMed] [Google Scholar]
- 22.Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M and Terakawa N (2002). “Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary.” Obstet Gynecol 100(2): 281–7. [DOI] [PubMed] [Google Scholar]
- 23.Han EK, Tahir SK, Cherian SP, Collins N and Ng SC (2000). “Modulation of paclitaxel resistance by annexin IV in human cancer cell lines.” Br J Cancer 83(1): 83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagne JF, Montminy V, Belanger P, Journault K, Gaucher G and Guillemette C (2002). “Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38).” Mol Pharmacol 62(3): 608–17. [DOI] [PubMed] [Google Scholar]
- 25.Garrett AP, Lee KR, Colitti CR, Muto MG, Berkowitz RS and Mok SC (2001). “k-ras mutation may be an early event in mucinous ovarian tumorigenesis.” Int J Gynecol Pathol 20(3): 244–51. [DOI] [PubMed] [Google Scholar]
- 26.Wamunyokoli FW, Bonome T, Lee JY, Feltmate CM, Welch WR, Radonovich M, Pise-Masison C, Brady J, Hao K, Berkowitz RS, Mok S and Birrer MJ (2006). “Expression profiling of mucinous tumors of the ovary identifies genes of clinicopathologic importance.” Clin Cancer Res 12(3 Pt 1): 690–700. [DOI] [PubMed] [Google Scholar]
- 27.Chan AM, Takai S, Yamada K and Miki T (1996). “Isolation of a novel oncogene, NET1, from neuroepithelioma cells by expression cDNA cloning.” Oncogene 12(6): 1259–66. [PubMed] [Google Scholar]
- 28.Sithanandam G, Fornwald LW, Fields J and Anderson LM (2005). “Inactivation of ErbB3 by siRNA promotes apoptosis and attenuates growth and invasiveness of human lung adenocarcinoma cell line A549.” Oncogene 24(11): 1847–59. [DOI] [PubMed] [Google Scholar]
- 29.Ohishi Y, Oda Y, Uchiumi T, Kobayashi H, Hirakawa T, Miyamoto S, Kinukawa N, Nakano H, Kuwano M and Tsuneyoshi M (2002). “ATP-binding cassette superfamily transporter gene expression in human primary ovarian carcinoma.” Clin Cancer Res 8(12): 3767–75. [PubMed] [Google Scholar]
- 30.Hall A (1998). “Rho GTPases and the actin cytoskeleton.” Science 279(5350): 509–14. [DOI] [PubMed] [Google Scholar]
- 31.Sahai E and Marshall CJ (2002). “RHO-GTPases and cancer.” Nat Rev Cancer 2(2): 133–42. [DOI] [PubMed] [Google Scholar]