Abstract
Background
This is an update of a Cochrane review first published in The Cochrane Library (2010, Issue 7).
To increase the success rate of assisted reproductive technologies (ART), adherence compounds such as hyaluronic acid (HA) and fibrin sealant have been introduced into subfertility management. Adherence compounds are added to the embryo transfer medium to increase the likelihood of embryo implantation, with the potential for higher clinical pregnancy and live birth rates.
Objectives
To determine whether embryo transfer media containing adherence compounds improved live birth and pregnancy rates in ART cycles.
Search methods
The Menstrual Disorders and Subfertility Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) and MEDLINE, EMBASE and PsycINFO electronic databases were searched (up to 13 November 2013) to look for publications that described randomised controlled trials on the addition of adherence compounds to embryo transfer media. Furthermore, reference lists of all obtained studies were checked, and conference abstracts were handsearched.
Selection criteria
Only truly randomised controlled trials comparing embryo transfer media containing functional (e.g. 0.5 mg/ml HA) concentrations of adherence compounds versus transfer media containing low or no concentrations of adherence compounds were included. The adherence compounds that were identified for evaluation were HA and fibrin sealant.
Data collection and analysis
Two review authors selected trials for inclusion according to the above criteria, after which two review authors independently extracted the data for subsequent analysis. Statistical analysis was performed in accordance with the guidelines developed by The Cochrane Collaboration.
Main results
Seventeen studies with a total of 3898 participants were analysed. One studied fibrin sealant, and the other 16 studied HA. No evidence was found of a treatment effect of fibrin sealant as an adherence compound. For HA, evidence of a positive treatment effect was identified in the six trials that reported live birth rates (odds ratio (OR) 1.41, 95% confidence interval (CI) 1.17 to 1.69; six RCTs, N = 1950, I2 = 0%, moderate‐quality evidence). Furthermore, the 14 trials reporting clinical pregnancy rates showed evidence of treatment benefit when embryos were transferred in media containing functional concentrations of HA (OR 1.39, 95% CI 1.21 to 1.60; 14 RCTs, N = 3452, I2 = 46%, moderate‐quality evidence) as compared with low or no use of HA. The multiple pregnancy rate (OR 1.86, 95% CI 1.49 to 2.31; five RCTs, N = 1951, I2 = 0%, moderate‐quality evidence) was significantly increased in the high HA group, but no significant differences in adverse event rates were found (OR 0.74, 95% CI 0.49 to 1.12; four RCTs, N = 1525, I2 = 0%, moderate‐quality evidence).
Authors' conclusions
Evidence suggests improved clinical pregnancy and live birth rates with the use of functional concentrations of HA as an adherence compound in ART cycles. However, the evidence obtained is of moderate quality. The increase in multiple pregnancy rate may be the result of use of a combination of an adherence compound and a policy of transferring more than one embryo. Further studies of adherence compounds with single embryo transfer need to be undertaken.
Plain language summary
Adherence compounds in embryo transfer media for assisted reproductive technologies
Review question
Cochrane review authors assessed the effect of the addition of adherence compounds in embryo transfer media on fertility outcomes.
Background
Couples who have trouble getting pregnant are able to make use of fertility treatments such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI). Over the years, much research has been performed to determine whether there are ways to increase the success rate of such treatments. One area of research has focused on the medium in which embryos are transferred back into the uterus. Adherence compounds have been added to the embryo transfer medium in attempts to increase the chance of the embryo adhering to the uterus, with a greater chance of pregnancy and the birth of a healthy newborn as a result. Many studies of these adherence compounds have been undertaken with some positive and negative results.
Study characteristics
Seventeen randomised controlled trials (3898 participants) were included in the review. One studied fibrin sealant, and the other 16 studied HA. Investigators compared embryo transfer in a medium containing high versus low or no hyaluronic acid and in a medium containing fibrin sealant versus transfer in a medium with no fibrin sealant. Outcomes reported included live birth rates, clinical pregnancy rates, implantation rates, multiple pregnancy rates and other adverse events. The mean age of the women ranged from 27.5 to 35.7 years. The evidence gathered is current to November 2013.
Key results
Analysis of the 16 studies that were identified using functional concentrations of HA showed an increase in the chances of pregnancy and live birth (450 vs 367 per 1000) but also an increase in the chance of the more risky outcome of multiple pregnancy (282 vs 175 per 1000). This increase in multiple pregnancy rate may be the result of improved pregnancy outcomes due to the addition of the adherence compound and the policy of transferring more than one embryo back into the uterus.
Quality of the evidence
Evidence obtained for these comparisons was of moderate quality. It is important to note that evidence of a higher delivery rate was not found in all analyses; however, it was found in the overall meta‐analysis. Based on the single identified study that used fibrin sealant, no evidence indicates that the addition of this compound to an embryo transfer medium improved pregnancy outcomes.
Summary of findings
Background
Description of the condition
The first IVF (in vitro fertilisation) baby was born in 1978. Much progress has been made in the intervening years in assisted reproductive technology (ART) to improve live birth outcomes for couples. Embryo implantation into the lining of the endometrium is one of the major determining factors in successful human IVF (Gardner 2003). Much research has therefore focused on the interaction between the embryo and the endometrium at the time of implantation. The composition of the medium that surrounds the embryo at the time of IVF transfer is now considered to be important at this crucial stage of development.
Much research has therefore focused on the effect on implantation and pregnancy rate of adding a specific adherence compound to the medium in which the embryo is transferred into the womb.
Description of the intervention
One reported beneficial component that has been introduced into transfer media is hyaluronic acid (HA). HA is a naturally existing molecule and is one of the major macromolecules present in the female reproductive tract. It is present in the human endometrium (Salamonsen 2001), and its levels have been shown to increase dramatically on the day of implantation in mice (Carson 1987). Initial studies in mouse embryo transfers showed that inclusion of HA in transfer medium significantly increased implantation rates and enhanced fetal development when compared with no HA in the transfer medium (Gardner 1999).
Although the mechanism by which HA promotes implantation has yet to be elucidated, HA does have several properties that make it a potential candidate as an implantation‐enhancing molecule. Hyaluronic acid has been demonstrated to increase cell‐to‐cell adhesion and cell‐to‐matrix adhesion (Turley 1984). It produces a viscous solution that might enhance the embryo transfer process and prohibit expulsion, or it may facilitate diffusion and integration of the embryos in the viscous solution that characterises intrauterine secreted fluid (Simon 2003). The viscosity alone, however, does not explain its involvement in implantation, as not all highly viscous solutions (such as human placental collagen) can improve implantation (Menezo 1989). The action of HA during implantation could also be receptor mediated, as the primary receptor for HA is CD44, which is expressed both on the preimplantation embryo (Campbell 1995) and in the stroma (supporting framework) of the human endometrium (Behzad 1994). HA is available to be added to embryo transfer media as a product named EmbryoGlue (Vitrolife AB, Göteborg, Sweden).
Albumin traditionally has been used as the main macromolecule in most embryo culture media, as it is abundant in the female reproductive tract. However, serum albumin, which is derived from blood, is not a pure substance and carries a risk of contamination from viruses. In a human trial, Simon et al showed that HA can successfully replace albumin as the sole macromolecule in an embryo transfer medium, resulting in high pregnancy rates (Simon 2003). Although the risks associated with a biologically derived product have been overcome in part by the development of recombinant human serum albumin, HA is preferable to albumin because it is a polysaccharide and can be synthesised and isolated in a pure form (Gardner 1999).
Another implantation‐enhancing molecule that has been introduced into transfer media is fibrin in the form of a two‐component fibrin sealant, which consists of fibrinogen and thrombin, together with a fibrinolysis inhibitor (aprotinin). It was introduced into IVF to improve the pregnancy rate and to avoid ectopic pregnancy (Feichtinger 1992). Fibrin sealant had already been used in many surgical procedures to promote haemostasis, for example, in coating and sealing vascular prostheses, gluing parenchyma in surgery, supporting wound healing and treating premature rupture of membranes in pregnancy. Fibrin sealant is a viscous solution that quickly and firmly adheres to the tissue (Ben‐Rafael 1995). After experience was gained with mouse embryos, fibrin sealant was introduced for human embryo transfer. It was added to the transfer medium to create a fibrin plug in the uterine cavity at the time of embryo transfer, to decrease the possibility of embryo expulsion and ectopic pregnancy (Feichtinger 1990). Fibrin sealant seemed to have an effect on pregnancy rate only in older women (39 to 42 years of age). It has been suggested that fibrinolysis provoked by the presence of fibrin in utero may cause chemical absorption of the membrane of the zona pellucida, which is thickened in older women, resulting in hatching of the embryo (Ben‐Rafael 1995). Other possible explanations for the beneficial effect of fibrin sealant have been postulated. First, embryos that are surrounded by the sealant are compelled to stay in place for at least a few days, until the clot dissolves, and therefore cannot be expelled. Second, the enhanced adhesive quality of the embryo surface facilitates the initial implantation process. Finally, the increase in size of the embryo and the medium complex that is achieved by the addition of the sealant may increase the chances of the embryo remaining within the uterine cavity (Bar‐Hava 1999).
Other macromolecules that have been investigated include bovine serum albumin (BSA), polyvinyl alcohol (PVA) and dextran, but none of these has been shown to improve implantation rates compared with no macromolecule (Gardner 1999). Heparanase has been shown to increase implantation rates in mice (Revel 2005) but has never been studied in human embryo transfers.
How the intervention might work
Trials conducted to assess the effect of HA in transfer media use a standard concentration of 0.5 mg/ml, which is considered a functional level for an adherence compound. It is important to note that some commercial culture and transfer media contain a much lower concentration of HA (0.125 mg/ml), which is intended to support embryo growth as opposed to embryo adherence. For clarification, HA groups are labelled as high (0.5 mg/ml), low (0.125 mg/ml) or no HA (0.0 mg/ml).
In humans, transfer of the embryo back into the uterus can be performed after two, three, four, five or six days of in vitro culture. The day of transfer could be important, as it is not clear whether the small volume of adherence compound in media transferred on days two to four would still be present and would have a potential effect on the day of implantation (day six to seven) (Simon 2003). However, adherence compounds in the media may play an important role at this early stage because of their physical properties and may prohibit expulsion, as has been mentioned. Therefore, in this review, the influence of day of embryo transfer is analysed as a subgroup. It also was not known whether inclusion of HA in transfer media provided any added benefit in frozen embryos compared with fresh embryos, or vice versa. Therefore, fresh and frozen‐thawed embryos are analysed as subgroups.
A third subgroup analysis is included to assess the influence of oocyte donation. It was interesting to see whether the presence of adherence compounds led to different effects in studies in which couples participated with their own oocytes compared with studies that also included donor oocytes.
The effect of exposure time of the embryo to adherence compounds before embryo transfer is analysed in a fourth subgroup. It is possible that length of exposure to adherence compounds before the day of implantation (day six to seven) may have an impact on the outcome. Many included studies are expected to use EmbryoGlue, which contains HA, as the adherence compound; therefore, it was decided that exposure time of 10 minutes should be used as the cutoff point for this subgroup analysis. This is the time recommended by the manufacturer (Vitrolife). The outcomes of studies in which embryos were exposed to adherence compounds for up to 10 minutes are compared with the outcomes of studies in which embryos were exposed for a longer period.
The fifth subgroup analysis includes a comparison of participant groups with different prognoses. The outcomes of studies that actively selected poor prognosis participants on the basis of age, number of previous treatment failures and, in some trials, embryo quality are compared with the outcomes of studies that selected good prognosis participants and studies with unselected participants.
It is very important to determine whether the combination of adherence compounds and an embryo transfer policy of transferring multiple embryos per treatment cycle affects outcome measures, especially multiple pregnancy and adverse event rates; therefore, a sixth and final subgroup analysis compares different embryo transfer policies. Trials on single embryo transfer are also compared with trials in which a mean of two or more embryos were transferred.
Why it is important to do this review
Because the rate of human implantation (and consequent pregnancy and delivery) is innately low—between 10% and 30% (Gardner 2004)—it is often difficult to establish small but significant improvement, particularly with the relatively low volume of women seen in many clinics over a year. Systematic meta‐analysis of all randomised controlled trials (RCTs) is, therefore, an important tool for assessing whether an innovation offers a true advancement in technology. The available literature has been reviewed in an attempt to identify whether inclusion of adherence compounds in embryo transfer media benefits couples when compared with use of transfer media that do not include adherence compounds. Any improvement in the implantation rate may lead to a reduction in the need to replace multiple embryos, with subsequent multiple pregnancies, and may maximise the chance that subfertile couples can have a normal, healthy baby.
Objectives
To determine whether embryo transfer media containing adherence compounds improved live birth and pregnancy rates in ART cycles.
Methods
Criteria for considering studies for this review
Types of studies
We included all truly randomised controlled trials (RCTs) comparing embryo transfer media containing high concentrations of adherence compounds versus embryo transfer media with no or low concentration of adherence compounds. Quasi‐randomised trials have not been included. Cross‐over trials would be included in the review only for completeness; because the cross‐over design is not valid in the context of subfertility trials (Vail 2003), only data from the first phase were to be included.
Types of participants
Couples undergoing embryo transfer after in vitro fertilisation (IVF), intracytoplasmic sperm injection (ICSI) or an embryo thaw cycle for therapeutic reasons, or after oocyte donation.
Types of interventions
All known culture methods for IVF and/or ICSI comparing embryo transfer media containing high concentrations of adherence compounds versus embryo transfer media with no or low concentrations of such adherence compounds. Embryos were grown before transfer for two to six days in vitro or were frozen‐thawed, or both.
Types of outcome measures
Primary outcomes
Primary outcome measures
Live birth rate per randomly assigned couple: defined as number of live births per randomly assigned couple.
Secondary outcomes
Secondary outcome measures
Clinical pregnancy rate per randomly assigned couple: defined as number of clinical pregnancies (demonstrated by the presence of a gestational sac on ultrasound scan) per randomly assigned couple.
Multiple pregnancies per randomly assigned couple.
Adverse events such as ectopic pregnancy, miscarriage, fetal or congenital defects and pelvic inflammation or other adverse events per randomly assigned couple.
Additional outcome measures
Implantation rate: defined as number of gestational sacs divided by number of embryos transferred.
Data on implantation rate cannot be pooled in a meta‐analysis together with other outcome measures because of the difference in denominators (Vail 2003). Implantation rate is defined per number of embryos transferred, and other outcome measures are defined per randomly assigned couple. However, because of the frequency with which implantation rate is reported in the literature, it was decided to analyse these data separately for completeness.
Search methods for identification of studies
All published and unpublished RCTs on the addition of an adherence compound to the embryo transfer medium versus transfer medium devoid of an adherence compound have been sought using the following search strategy, with no language restrictions and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator. The search terms used are given in Appendix 1, Appendix 2, Appendix 3 and Appendix 4.
Electronic searches
The following electronic databases, trial registers and websites have been searched to 13 November 2013, using the search terms provided in the appendices.
Menstrual Disorders and Subfertility Group (MDSG) Trials Register.
Cochrane Central Register of Controlled Trials (CENTRAL) (current issue).
MEDLINE, EMBASE and PsycINFO.
Other electronic sources of trials that were searched were as follows.
CINAHL database.
The Cochrane Library (www.cochrane.org/index.htm).
Trial registers for ongoing and registered trials: Current Controlled Trials (www.controlled‐trials.com/); ClinicalTrials.gov, a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home); the World Health Organisation International Trials Registry Platform search portal (www.who.int/trialsearch/Default.aspx).
Citation indexes (http://scientific.thomson.com/products/sci/).
Conference abstracts on the Web of Knowledge (http://wokinfo.com/).
LILACS database, a source of trials from the Portuguese and Spanish speaking world (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F).
ClinicalStudyResults, clinical trial results on marketed pharmaceuticals (www.clinicalstudyresults.org/).
PubMed (www.ncbi.nlm.nih.gov/pubmed/), with the randomised controlled trial filters for PubMed that can be found in Chapter 6 of The Cochrane Handbook for Systematic.Reviews of Interventions.
OpenSIGLE database for grey literature in Europe (http://opensigle.inist.fr/).
We handsearched appropriate journals. The journals searched are listed in the MDSG Module, which can be found in The Cochrane Library under BROWSE—'By Review Group'—'Cochrane Menstrual Disorders and Subfertility Group'—then 'about this group'.
Searching other resources
Reference lists of trial reports retrieved by the search were handsearched. Furthermore, European Society of Human Reproduction & Embryology (ESHRE) and American Society for Reproductive Medicine (ASRM) supplements were handsearched and contact made with experts and manufacturers of transfer media including adherence compounds to obtain additional relevant data.
Data collection and analysis
Selection of studies
Two review authors (DB and SB) performed a selection of trials by scanning titles and abstracts retrieved from the search and removing those that were clearly irrelevant. The full text of all trials considered to be potentially eligible was retrieved. Two review authors (MJH and SB) independently examined the full‐text articles for compliance with the inclusion criteria and selected eligible studies for inclusion in the review. When required, the review authors corresponded with study investigators to clarify study eligibility. Disagreements on eligibility were resolved by consensus or with the help of a third review author (DB). Excluded articles are detailed in the table Characteristics of excluded studies. Included trials were assessed against the risk of bias criteria and for methodological details. This information is presented in the table Characteristics of included studies and provides a context for assessing the reliability of results.
Timeline
A search for new trials will be conducted every two years, and the review will be updated as and when new trials are found. The original search was performed on 26 May 2009; the first search update was performed on 28 March 2012, a second search update was performed on 23 January 2013 and a third on 13 November 2013.
Data extraction and management
Data were independently extracted by two review authors (MJH and SB), who used a data extraction form designed and pilot tested by the review authors (see Appendix 5). If disagreements could not be resolved by consensus, a third review author (DB) was available to resolve any discrepancies. Additional information on trial methodology or actual original trial data were requested from the authors of trials that appeared to meet eligibility criteria to clarify any aspects of methodology or to obtain data in a suitable form. Reminder correspondence was sent when a reply was not received within three weeks. When studies had multiple publications, the main trial report was used as the reference and was supplemented by additional details from secondary papers.
Assessment of risk of bias in included studies
The included studies have been assessed for risk of bias in the following domains.
Sequence generation.
A low risk of bias was allocated if investigators described a random component in the sequence generation process such as using:
a computerised random number generator; or
a random numbers table.
Allocation concealment.
A low risk of bias was allocated if participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation.
Central computer randomisation.
Serially numbered, sealed, opaque envelopes.
Blinding.
A low risk of bias was allocated if blinding of participants, scientists and clinicians or nurses had been ensured.
Completeness of outcome data.
A low risk of bias was allocated if no data were missing, which meant that live birth rate and length of follow‐up were stated, losses to follow‐up accounted for and an intention‐to‐treat (ITT) analysis carried out.
Selective outcome reporting.
A low risk of bias was allocated if all of the study's primary, secondary and additional outcomes of interest in the review were reported in a prespecified way.
Other sources of bias.
A low risk of bias was allocated if:
the trial was free of any commercial source of funding;
the culture and transfer media were comparable between treatment and control groups with the exception of the addition of the adherence compound to the medium in the treatment group; or
investigators reported multiple pregnancy rates when multiple embryos were transferred per treatment cycle.
All three of these aspects had to be correct for a low risk of other sources of bias to be allocated.
Similarity between treatment and control groups in culture and transfer media was assessed by checking the manufacturers of the media and that all parameters up to the moment of embryo transfer were comparable between groups.
-
With the addition of an adherence compound to the embryo transfer medium, it was important to report multiple pregnancies when the embryo transfer policy consisted of transferring multiple embryos per treatment cycle. It can be considered to be a risk of bias when the authors failed to report the multiple pregnancy rate in these cases, as they had ignored a higher risk of the adverse event of a multiple pregnancy.
These domains have been assessed by two authors (MJH and SB) with any disagreements resolved by consensus or by contacting a third review author (DB). All judgements have been fully described. The conclusions are presented in the risk of bias figures and are incorporated into the interpretation of review findings.
Measures of treatment effect
Dichotomous data (e.g. clinical pregnancy rate) outcomes from each study were expressed as odds ratios (ORs) with 95% confidence intervals (CIs) and, when possible, were combined for meta‐analysis with RevMan software using the Mantel‐Haenszel method. All measured outcomes yielded dichotomous data, so continuous and ordinal data were not assessed.
Unit of analysis issues
The primary analysis of the review was expressed as per randomly assigned couple. Reported data that did not allow valid analysis (e.g. per embryo transfer) were presented in meta‐view but were not pooled. Most included trials reported their results per randomly assigned woman or participant.
When possible, reported multiple live births were counted as a single live birth event.
Only first‐phase data from cross‐over trials would have been included. However, all included trials were parallel‐group RCTs.
When possible, the data have been analysed using ITT analysis. The number of couples randomly assigned was used as the denominator.
Dealing with missing data
The data have been analysed on an ITT basis as far as possible, and original investigators have been contacted regarding missing data. If unavailable, imputation of individual values has been undertaken for the primary outcome only. Live births were regarded not to have occurred if not reported.
Only available data were analysed; any imputation undertaken has been subjected to sensitivity analysis.
Success rates of subfertility treatments decline as the number of treatment cycles and women's age increase (Schröder 2004). Study outcomes can be affected by participants enrolling in studies with multiple treatment cycles, as this increases the number of cycles and creates uncertainty about the number of cycles per participant. The number of cycles per participant generally was not stated in the articles. All original investigators have therefore been contacted to ask for information on the number of cycles undertaken by participants in the trial in an attempt to resolve this matter.
Assessment of heterogeneity
Heterogeneity has been considered by the review authors when clinical and methodological characteristics of included studies were similar enough that a meta‐analysis could provide a meaningful summary. Statistical analyses have been performed in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration (Higgins 2011). Heterogeneity between results of different studies was assessed by the I2 statistic, which can be interpreted in the following broad terms.
0% to 40%: might not be important.
30% to 60%: represents moderate heterogeneity.
50% to 90%: represents substantial heterogeneity.
75% to 100%: represents considerable heterogeneity (Higgins 2011).
In case of substantial or considerable heterogeneity, explanations have been sought, including those involving the sensitivity analyses performed for the primary outcome measures. It was planned to look at the possible contribution of differences in trials, for example, transfer of embryos on different days. When possible, the outcomes were pooled.
Assessment of reporting biases
The review authors aimed to minimise the potential impact of publication and reporting biases by performing a comprehensive search for eligible studies and looking for duplication of data. If 10 or more studies were included in an analysis, a funnel plot was used to investigate the possibility of small‐study effects (a tendency for estimates of the intervention effect to have a bigger impact in smaller studies).
When included studies did not report the primary outcome measure of live birth or interim outcomes such as clinical pregnancy, informal assessment was undertaken to check whether those studies reporting primary outcome measures reflected typical findings for the interim outcomes.
Assessment of reporting biases was addressed in the Included studies portion of the Main results section. See Other potential sources of bias.
Data synthesis
Data from primary studies were combined using a fixed‐effect model in the following comparison.
Embryo transfer medium with inclusion of adherence compounds versus embryo transfer medium without such adherence compounds added, or with a lower concentration, stratified as follows.
High concentration versus low concentration or no hyaluronic acid.
Fibrin sealant versus no fibrin sealant.
As described in the Background section under How the intervention might work, the clinical trials include control groups that may be completely devoid of HA or may have low levels of HA (often also present in culture media). Based on the results of the previous Cochrane meta‐analysis, consideration was given to combining these trials in the current review as a primary analysis for overall treatment effect.
No trials were found that compared fibrin sealant with a lower concentration of fibrin.
An increase in the odds of a particular outcome—either a beneficial effect or a detrimental effect—has been displayed graphically in the meta‐analyses to the right of the centre line, and a decrease in the odds of an outcome has been displayed to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
The following six subgroup analyses were performed.
Subgroup A: studies in which the day of embryo transfer was early stage (up to and including day four) versus studies in which the day of embryo transfer was late stage (days five and six).
Subgroup B: studies in which the embryos were frozen‐thawed versus studies in which fresh embryo transfers were performed.
Subgroup C: studies in which oocyte donations were included versus studies in which oocytes were strictly the participants' own.
Subgroup D: as length of exposure to adherence compounds before the day of implantation (day six to seven) may have an impact on outcomes, studies with exposure time up to 10 minutes versus studies with longer exposure time.
Subgroup E: studies that actively selected for good prognosis participants (by limiting the number of previous treatment cycles and the participant's age, or by applying other strict inclusion criteria) versus studies that selected poor prognosis participants versus studies with unselected participants.
Subgroup F: studies using different embryo transfer policies (i.e. transferring single embryos per cycle vs transferring a mean of two or more embryos per cycle).
Sensitivity analysis
Sensitivity analyses were performed to verify whether conclusions made about the primary outcome measure are robust to arbitrary decisions made regarding eligibility of studies and analysis of data. In this way, it was checked whether conclusions would have differed if the following decisions had been made.
Eligibility was restricted to studies without high risk of bias. When a study was assessed as Unclear or No in one of the following domains—adequate sequence generation, allocation concealment or blinding—it no longer had a low risk of bias.
Studies with outlying results were excluded. Outlying results were those that caused heterogeneity because they differed too much from the other results included in the meta‐analysis.
Alternative imputation strategies were adopted.
A random‐effects model was adopted.
Studies using a functional adherence compound concentration different from 0.5 mg/ml in the treatment group were excluded.
When sensitivity analyses identified particular data that greatly influenced the findings of the review, we tried to resolve uncertainties; this led the review authors to conclude that further research is mandated.
Overall quality of the body of evidence: Summary of findings table
We generated Summary of findings tables using GRADE Profiler software. These tables evaluated the overall quality of the body of evidence for main review outcomes using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) were justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
A total of 180 studies were located using the search strategies; 54 of these were found during a search update in 2012 (see Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 6). These included 32 studies from MEDLINE, 43 from CENTRAL, 54 from EMBASE, 39 from the MDSG Specialised Register and 10 from handsearching, with many duplicates. No potentially eligible trials were identified during the search update of January 2013. However, the search update of November 2013 revealed two new potentially eligible trials in the MDSG Specialised Register. In total, 40 studies appeared to meet the basic inclusion criteria.
After further in‐depth eligibility assessment, data examination and contacting of principal investigators, 17 of the potentially eligible studies were excluded, resulting in 21 included studies. Two studies (Hazlett 2004; Hazlett 2005) were found to be conference abstracts for the same trial published in Hazlett 2008. All three studies remain listed as included studies, but the data were incorporated once in this review. By contacting the authors, it was established that Walker 2005 was an interim analysis of the trial that was published in a bigger study (Morbeck 2007); therefore only data from the study of Morbeck 2007 were analysed. Of note, the trial of Morbeck et al was suspended before the completion date because of negative results; Walker 2005 remains listed as an included study.
Twenty studies were included in the original systematic review (published in 2010). The search update from 2012 resulted in the inclusion of two new studies (Balaban 2011; Fancsovits 2011), which now are incorporated within the current review. The Balaban 2011 study reported live birth rate data resulting from clinical pregnancies reported in another study that had already been included in the previous version of this systematic review (Urman 2008) but did not report on the live birth rate itself. Both studies are included in this systematic review, but the data were extracted only once for meta‐analysis and are reported under Urman 2008.
The search update of November 2013 resulted in two new potentially eligible trials (Nakagawa 2012; Nakagawa 2012‐II); however, further in‐depth analysis showed that these trials had a quasi‐randomised study design.
See Figure 1 for details of the screening and selection process.
1.
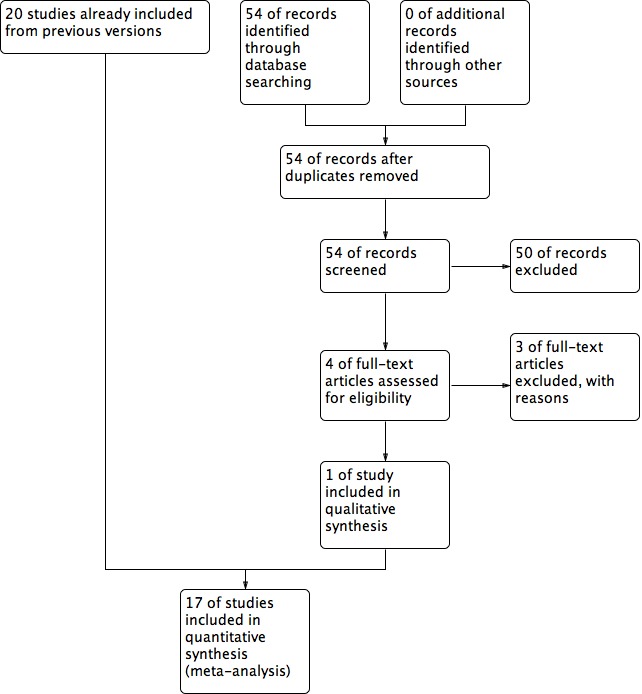
Study flow diagram.
Included studies
Twenty‐one studies have been included; data were extracted from 17 studies with a total of 3898 participants (see Characteristics of included studies) because of duplication of the data. Not all published data could be used for analysis (see Appendix 7). Three studies reported outcomes as percentages alone (Friedler 2005; Khan 2004; Walker 2005). See the table Characteristics of included studies for further information. Morbeck et al did not publish actual data because the study was suspended prematurely; these data were retrieved by contacting the principal author. Chen et al reported only the biochemical pregnancy rate, which is not an outcome measure for this review. Thirteen of the included studies reported implantation rate as well. However, the data on this outcome measure could not be used in a meta‐analysis because this review requires that the number of embryos transferred should be used as the denominator instead of the number of embryos transferred per number of randomly assigned couples.
Study characteristics
All included studies were RCTs that compared the results of an intervention group versus those of a control group. Participant recruitment was performed in a prospective manner. Methods of participant sampling varied between studies. Nine studies recruited participants consecutively (Balaban 2004; Ben‐Rafael 1995; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Korošec 2007; Morbeck 2007; Urman 2008)—one study in a non‐consecutive order (Simon 2003) and the rest using an unclear method. Hazlett et al reported both consecutive and non‐consecutive sampling in different publications of the same trial.
Thirteen were single‐centre studies (Balaban 2004; Chen 2001; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008; Khan 2004; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Urman 2008; Yakin 2004), and four were multi‐centre trials. Seven of the included studies were performed in part at academic medical centres (Ben‐Rafael 1995; Dittmann‐Műller 2009; Friedler 2005; Friedler 2007; Korošec 2007; Mahani 2007; Simon 2003). Five studies were performed in Israel (Ben‐Rafael 1995; Friedler 2005; Friedler 2007; Ravhon 2005; Simon 2003), four in the United States of America (Hazlett 2008; Khan 2004; Morbeck 2007; Schoolcraft 2002), three in Turkey (Balaban 2004; Urman 2008; Yakin 2004), one in Taiwan (Chen 2001), one in Germany and Switzerland (Dittmann‐Műller 2009), one in Iran (Mahani 2007), one in Slovenia and Austria (Korošec 2007) and one in Hungary (Fancsovits 2011).
Eight studies used strict inclusion and exclusion criteria for participant selection (Ben‐Rafael 1995; Friedler 2005; Friedler 2007; Hazlett 2008; Korošec 2007; Mahani 2007; Morbeck 2007; Simon 2003) (see Characteristics of included studies). These focused mainly on the woman's age and the number of previous treatment cycles. For example, Simon et al included only women up to 35 years of age with a maximum of three previous treatment failures. Five studies performed an a priori power calculation to determine sample size (Friedler 2007; Hazlett 2008; Korošec 2007; Morbeck 2007; Urman 2008) (see Characteristics of included studies).
Participants
The data from Hazlett et al were divided into two subgroups for analysis: HA in day three and day five embryo transfers. The day three subgroup compared HA in the transfer medium versus no HA in the medium; the day five subgroup compared high (0.5 mg/ml) versus low concentrations of HA (0.125 mg/ml).
Eight studies with a total of 1121 participants compared transfer medium to which 0.5 mg/ml HA (high concentration) had been added versus transfer medium without HA (Chen 2001; Friedler 2005; Friedler 2007; Hazlett 2008 (day three); Khan 2004; Korošec 2007; Mahani 2007; Simon 2003). The data from Chen et al could not be analysed because the study used an outcome that was not included in this review. Khan et al did not report numbers of participants in the study groups while reporting outcomes as percentages. Therefore, these two trials could not be incorporated into the meta‐analysis, resulting in actual analysis of six studies with a total of 886 participants. After contact was made with the original authors regarding the number of treatment cycles per participant, it appeared that Hazlett et al and Korošec et al allowed participants to enrol for multiple cycles; three studies allowed only a single cycle per participant (Friedler 2005; Friedler 2007; Simon 2003); and the policy of the other studies remains unclear. Information on the number of embryos transferred can be found under Characteristics of included studies.
Nine studies with a total of 2566 participants compared high HA (0.5 mg/ml) versus low HA (0.125 mg/ml) (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Hazlett 2008 (day five); Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Urman 2008; Yakin 2004). Contact with the original study authors regarding the number of treatment cycles per participant revealed that Balaban et al and Hazlett et al appeared to allow participants to enrol for multiple cycles; four studies allowed only a single cycle per participant (Dittmann‐Műller 2009; Morbeck 2007; Urman 2008; Yakin 2004); and the policy of the other studies remains unclear. Information on the number of embryos transferred can be found under Characteristics of included studies.
One study involving 211 participants compared the effect of fibrin sealant as transfer medium versus the effect of a medium without fibrin sealant (Ben‐Rafael 1995). Participants could enrol in the trial for one treatment cycle. A total of 759 embryos were transferred.
The age of participants was reported as a mean with standard deviation or as a range. Mean age ranged from 27.5 to 35.7 years. Two studies (Dittmann‐Műller 2009; Schoolcraft 2002) did not report participants' ages.
Six studies (Balaban 2004; Ben‐Rafael 1995; Dittmann‐Műller 2009; Friedler 2007; Korošec 2007; Urman 2008) reported the primary cause of subfertility of study participants (see Characteristics of included studies). Six studies (Balaban 2004; Ben‐Rafael 1995; Dittmann‐Műller 2009; Mahani 2007; Ravhon 2005; Urman 2008) reported the mean duration of subfertility for participants before the start of the study (see Characteristics of included studies).
Eleven studies (Balaban 2004; Ben‐Rafael 1995; Dittmann‐Műller 2009; Friedler 2005; Friedler 2007; Korošec 2007; Mahani 2007; Morbeck 2007; Ravhon 2005; Simon 2003; Urman 2008) reported the (mean) number of previous subfertility treatments that participants had received as an inclusion criterion or as a study measure (see Characteristics of included studies).
Age analysis was performed in four studies (Ben‐Rafael 1995; Fancsovits 2011; Morbeck 2007; Urman 2008). Ben‐Rafael et al divided participants into subgroups of < 31 years of age, 31 to 38 years of age and 39 to 42 years of age. Morbeck et al and Urman et al compared outcomes in women < 35 years versus those in women ≥ 35 years, and Fancsovits et al compared participants up to 40 years of age versus older participants (see Characteristics of included studies).
Interventions
Embryo transfer in medium containing high versus low or no hyaluronic acid
Sixteen studies (Balaban 2004; Chen 2001; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008 (day three and day five); Khan 2004; Korošec 2007; Mahani 2007; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Urman 2008; Yakin 2004) were included in this comparison. However, the results of two studies (Chen 2001; Khan 2004) could not be pooled (see Characteristics of included studies), resulting in 14 studies analysed with a total of 3252 participants.
Nine of the 16 studies compared high (0.5 mg/ml) versus low concentrations of HA (0.125 mg/ml) (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Hazlett 2008 (day five); Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Urman 2008; Yakin 2004). Transfer media of treatment and control groups in all these studies were obtained from the same manufacturer. Culture media were comparable between treatment and control groups in all nine studies.
Eight of the 16 trials compared a high concentration of HA (0.5 mg/ml) versus no HA in the comparison group (Chen 2001; Friedler 2005; Friedler 2007; Hazlett 2008 (day three); Khan 2004; Korošec 2007; Mahani 2007; Simon 2003). Six trials used EmbryoGlue, containing 0.5 mg/ml HA, as the transfer medium for the treatment group (Friedler 2005; Friedler 2007; Hazlett 2008 (day three); Khan 2004; Korošec 2007; Mahani 2007). One study (Simon 2003) used culture medium supplemented with 0.5 mg/ml HA as the transfer medium for the treatment group, and one study (Chen 2001) used the culture medium supplemented with 0.125 mg/ml HA as the treatment transfer medium.
One of the eight studies (Chen 2001) used transfer media from the same manufacturer in both treatment and control groups, whilst the others used media from different manufacturers. Of the eight studies, five (Chen 2001; Hazlett 2008 (day three); Khan 2004; Korošec 2007; Simon 2003) in this comparison used comparable embryo culture medium in both arms of the studies up to the time of embryo transfer; for three studies (Friedler 2005; Friedler 2007; Mahani 2007), it remained unclear whether embryo culture media were comparable.
Embryo transfer in medium containing fibrin sealant versus transfer in medium with no fibrin sealant
One study was included in this comparison (Ben‐Rafael 1995). The transfer media used in the treatment and control groups of the study were obtained from different manufacturers, and it was unclear whether the embryo culture medium was similar in the two groups (see Characteristics of included studies).
Further overall intervention details
Eight studies (Balaban 2004; Friedler 2007; Korošec 2007; Mahani 2007; Ravhon 2005; Simon 2003; Urman 2008; Yakin 2004) performed randomisation of participants to treatment or control arms on the day of embryo transfer. One study (Morbeck 2007) performed randomisation before commencement of the treatment cycle, another (Dittmann‐Műller 2009) between commencement of treatment and before a fertilisation check. Two studies (Ben‐Rafael 1995; Fancsovits 2011) randomly assigned participants between fertilisation check and day of embryo transfer. Timing of randomisation remained unclear in five studies (Chen 2001; Friedler 2005; Hazlett 2008; Khan 2004; Schoolcraft 2002). Hazlett et al was inconsistent in describing the timing of randomisation in different publications of the same trial.
Six studies (Fancsovits 2011; Friedler 2007; Khan 2004; Mahani 2007; Schoolcraft 2002; Simon 2003) exposed embryos in the treatment group to the adherence compound for up to 10 minutes before the transfer was made. Six studies (Balaban 2004; Dittmann‐Műller 2009; Hazlett 2008 (days three and five); Korošec 2007; Morbeck 2007; Urman 2008) exposed embryos in the treatment group to the adherence compounds for longer than 10 minutes. Exposure time remained unclear in the other five studies (Ben‐Rafael 1995; Chen 2001; Friedler 2005; Ravhon 2005; Yakin 2004).
Twelve studies (Ben‐Rafael 1995; Chen 2001; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Khan 2004; Mahani 2007; Morbeck 2007; Schoolcraft 2002; Simon 2003; Yakin 2004) performed the transfer early in embryo development (day two to three). Two studies (Balaban 2004; Korošec 2007) performed the transfer late in embryo development (day five and later). Two studies (Hazlett 2008; Urman 2008) performed transfers both early and late. The data from these trials have been analysed separately for the subgroup analysis on timing of the intervention.
Three studies (Morbeck 2007; Simon 2003; Yakin 2004) transferred embryos only after following a frozen‐thaw protocol. One study (Korošec 2007) included both fresh and frozen‐thawed embryos. Data from this trial were analysed separately for the subgroup analysis on frozen‐thawed versus fresh embryos. Eight studies (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2007; Hazlett 2008 (days three and five); Mahani 2007; Ravhon 2005; Urman 2008) transferred only fresh embryos. The other studies remain unclear in their procedures.
Two studies (Morbeck 2007; Schoolcraft 2002) reported on both donor and non‐donor oocytes, and the data from these trials were analysed separately for the subgroup analysis on oocyte donation. The policy on oocyte donation remained unclear for two studies (Balaban 2004; Yakin 2004). The other studies transferred only non‐donor oocytes.
One study (Korošec 2007) followed the procedure of transferring only singleton embryos per treatment cycle. All other studies transferred multiple embryos per treatment cycle, with a mean range of 2.1 to 3.9 embryos per treatment cycle.
Pregnancy was determined by the presence of a fetal heartbeat on ultrasound scan in six studies (Hazlett 2008 (days three and five); Korošec 2007; Mahani 2007; Morbeck 2007; Schoolcraft 2002; Simon 2003). Eleven studies (Balaban 2004; Ben‐Rafael 1995; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2007; Hazlett 2008 (days three and five); Korošec 2007; Mahani 2007, Morbeck 2007, Simon 2003, Urman 2008) used ultrasound scanning to determine pregnancy by demonstrating gestational sacs. Eight studies (Chen 2001; Fancsovits 2011; Friedler 2007; Hazlett 2008 (days three and five); Korošec 2007; Mahani 2007; Simon 2003; Urman 2008) used biochemical pregnancy tests to determine pregnancy. The method of pregnancy determination remained unclear in the remaining studies (Friedler 2005; Khan 2004; Ravhon 2005; Yakin 2004).
Outcomes
Six studies (Hazlett 2008; Fancsovits 2011; Korošec 2007; Morbeck 2007; Simon 2003, Urman 2008) reported live birth rates (see Characteristics of included studies). Two of these studies (Simon 2003; Urman 2008) published the results in the article, although Urman et al published them not in the original article but in a second publication deriving from the same trial (Balaban 2011). The other studies reported data on live birth rates after contact was made with the principal investigators.
All but two studies (Chen 2001; Khan 2004) reported clinical pregnancy rates (see Characteristics of included studies).
Five studies (Balaban 2004; Dittmann‐Műller 2009; Friedler 2007; Simon 2003; Urman 2008) reported the multiple pregnancy rate. All these studies reported the multiple pregnancy rate as a percentage of the number of pregnancies.
Six studies (Ben‐Rafael 1995; Friedler 2005; Friedler 2007; Korošec 2007; Mahani 2007; Urman 2008) reported adverse events. Four of these (Friedler 2005, Korošec 2007; Mahani 2007; Urman 2008) reported miscarriages; Ben‐Rafael et al reported ectopic pregnancies; Friedler et al reported both miscarriages and ectopic pregnancies (Friedler 2007). These data were combined for analysis in the review. The data from Friedler 2005 could not be used, as this study reported miscarriages as a percentage without clarifying group size.
Fourteen studies (Balaban 2004; Ben‐Rafael 1995; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008; Khan 2004; Mahani 2007; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Urman 2008; Yakin 2004) reported implantation rates. Data from five studies (Friedler 2005; Khan 2004; Ravhon 2005; Schoolcraft 2002; Yakin 2004) could not be used (see Characteristics of included studies).
Eight studies (Balaban 2011; Chen 2001; Fancsovits 2011; Hazlett 2008; Korošec 2007; Simon 2003; Urman 2008; Yakin 2004) reported outcome measures that were not included in this review. Chen 2001 reported pregnancy rate, as determined by a biochemical pregnancy test, which could not be used. Along with live birth and clinical pregnancy rates, Hazlett et al reported ongoing pregnancy rate as pregnancy demonstrated by fetal cardiac activity at seven weeks of gestation, assessed as viable pregnancy. Korošec 2007 reported clinical pregnancy rates in cycles after a previous implantation failure. Simon 2003 reported deliveries, ongoing pregnancy rate per embryo transfer, singleton pregnancy rate and clinical pregnancy rate per embryo transfer. Urman 2008 reported clinical pregnancy and implantation rates stratified by age, previous treatment failures and quality of the embryos (see Characteristics of included studies). Balaban 2011 reported the live birth rate resulting from the Urman 2008 trial per embryo transfer. Yakin 2004 reported on the cryosurvival rate. Fancsovits 2011 reported the fertilisation rate and the rate of positive human chorionic gonadotrophin (hCG) tests.
Studies that reported outcome measures in such a way that they could not be incorporated into this review have been summarised in Appendix 7. The original investigators who responded to our additional data queries and the data they provided are summarised in Appendix 8.
Excluded studies
Eighteen studies were excluded (see Characteristics of excluded studies), 10 because they failed to use a truly randomised design (Balaban 2005; Chao 2008; Feichtinger 1990; Feichtinger 1992; Hambiliki 2010; Karimian 2004; Nakagawa 2012; Nakagawa 2012‐II; Sun 2010; Valojerdi 2006). Data from two other reviews could not be incorporated into this systematic review (Loutradi 2008; Sallam 2010). Loutradi et al wrote a review on the effect of HA on embryo implantation, but not all included studies were randomised controlled trials. Sallam et al wrote a systematic review on the effects of assisted reproductive technologies, including EmbryoGlue, but did not report the actual data in the conference abstract in which the review was published. Five studies (Bungum 2003; Chatziioannou 2010; Romano 2004; Sieren 2006; Venetis 2009) were excluded because they did not consider the comparison of interest. One study was excluded because oocytes instead of participants were randomly assigned (Sifer 2009).
Risk of bias in included studies
Based on descriptions provided within the original publications, the potential risks of bias seemed high. However, upon contact with the original authors, many concerns about sources of bias were resolved. See Appendix 8 for information on which ambiguities were resolved in this way.
Allocation
Seven studies (Balaban 2004; Fancsovits 2011; Friedler 2007; Hazlett 2008; Korošec 2007; Schoolcraft 2002; Urman 2008) used a computerised random number generator for allocation of participants into different arms of the study. One study (Morbeck 2007) used a random number table for participant randomisation. Another study (Simon 2003) reported that participants were allocated to an arm of the study using information in a random sealed envelope. This study does not clearly state the actual method of randomisation, but the method appears to be adequate. One study (Dittmann‐Műller 2009) reported the use of a cube as a method of randomisation, allocating even numbers to the treatment arm and odd numbers to the control arm of the trial. The remaining seven studies (Ben‐Rafael 1995; Chen 2001; Friedler 2005; Khan 2004; Mahani 2007; Ravhon 2005; Yakin 2004) reported only that participants were randomly divided into treatment and control groups, without explaining the actual method of randomisation.
Allocation concealment was reported in six studies, either in the published version or by study authors contacted for further information. Two of those studies (Balaban 2004; Friedler 2007) used a third party or central computer randomisation for their allocation concealment. The other four studies (Hazlett 2008; Morbeck 2007; Simon 2003; Urman 2008) used serially numbered, sealed, opaque envelopes. The remaining studies did not clearly report allocation concealment (see Characteristics of included studies and Figure 2 and Figure 3).
2.
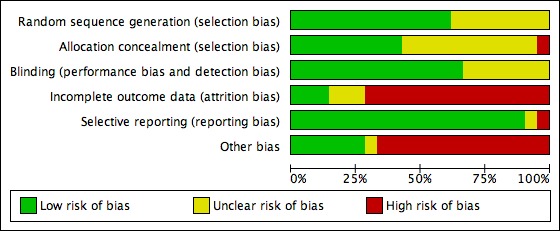
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
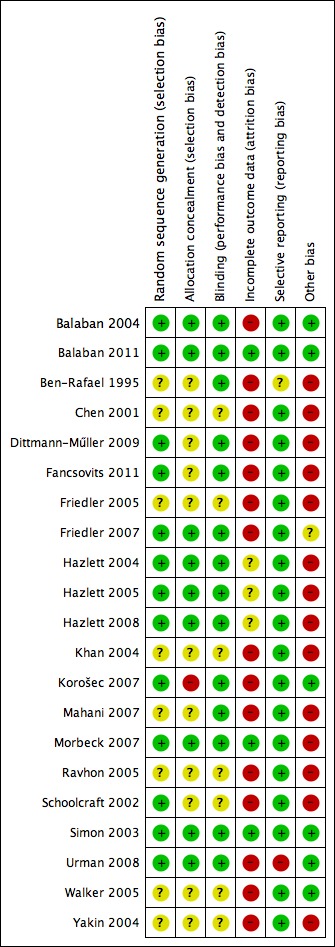
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Blinding
Blinding was performed in 11 (Balaban 2004; Ben‐Rafael 1995; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2007; Hazlett 2008; Korošec 2007; Mahani 2007; Morbeck 2007; Simon 2003; Urman 2008) of the 16 studies. Neither participants nor treating physicians and/or nurses knew to which arm of the study participants had been allocated. None of the studies described the process of analysis used for blinded results.
Incomplete outcome data
Six studies (Fancsovits 2011; Hazlett 2008; Korošec 2007; Morbeck 2007; Simon 2003; Urman 2008) reported live births. However, the live birth rate was reported not in the Urman et al study but in a second publication following from the same trial (Balaban 2011). Korošec et al recorded live births only in the subgroup for fresh embryo transfers.
Eleven studies (Balaban 2004; Ben‐Rafael 1995; Chen 2001; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2007; Hazlett 2008; Korošec 2007; Morbeck 2007; Simon 2003; Urman 2008) reported length of follow‐up per participant. In one study (Mahani 2007), length of follow‐up could be determined indirectly from the text but was not clearly stated in the article.
Loss to follow‐up was described in five studies (Balaban 2004; Dittmann‐Műller 2009 (no loss); Hazlett 2008; Korošec 2007; Morbeck 2007). Korosec et al accurately reported loss to follow‐up but did not publish the results of all participants in the results table (see Characteristics of included studies).
An ITT analysis was performed in two studies (Balaban 2004; Urman 2008).
Therefore, three studies (Morbeck 2007; Simon 2003; Urman 2008) have been classified as complete in reporting of outcome data, and one study remains classified as unclear (Hazlett 2008). All five studies reported live births and length of follow‐up. However, not all studies performed an ITT analysis. In terms of risk of bias, Hazlett et al was assessed as unclear because of loss of participants. Simon et al had no loss of participants, and Morbeck et al excluded 38 participants before randomisation.
Selective reporting
Fifteen studies (Balaban 2004; Chen 2001; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008; Khan 2004; Korošec 2007; Mahani 2007; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Yakin 2004) reported outcome measures in a prespecified manner. Some studies reported more outcome measures than planned; this was not considered to be a source of bias. However, when fewer outcome measures were reported than planned, this was considered to be a source of bias (see Characteristics of included studies). One study (Urman 2008) reported fewer outcomes than planned, but in a second publication following from the same trial (Balaban 2011), the live birth rate was reported in a prespecified manner; one study (Ben‐Rafael 1995) did not specify the outcome measures beforehand and therefore was assessed as unclear.
Other potential sources of bias
See Assessment of risk of bias in included studies for information on how the risk of other sources of bias was assessed.
Ten studies (Balaban 2004; Ben‐Rafael 1995; Fancsovits 2011; Friedler 2007; Hazlett 2008; Korošec 2007; Morbeck 2007; Ravhon 2005; Simon 2003; Urman 2008) reported that the study was free of commercial funding. Two studies (Dittmann‐Műller 2009; Schoolcraft 2002) received commercial funding. The other studies did not report on funding.
Twelve studies (Balaban 2004; Chen 2001; Dittmann‐Műller 2009; Fancsovits 2011; Khan 2004; Korošec 2007; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Urman 2008; Yakin 2004) used similar embryo culture media and media brands for treatment and control groups, so all parameters could be considered similar until the moment of embryo transfer.
Five studies (Balaban 2004; Dittmann‐Műller 2009; Friedler 2007; Simon 2003; Urman 2008) reported multiple pregnancies while transferring multiple embryos per treatment cycle.
Four studies (Balaban 2004; Korošec 2007; Simon 2003; Urman 2008) were regarded as free of other sources of bias. In one study (Friedler 2007), we could not determine with certainty whether culture media were similar between treatment and control groups; therefore the risk of other bias was rated as unclear.
Assessment of reporting biases
Fourteen studies were included in the analysis of clinical pregnancy rates for the overall comparison of transfer medium with HA added versus transfer medium with no HA or with a low concentration of HA. Therefore, a funnel plot was used to investigate the possibility of small‐study effects (see Figure 4). The funnel plot showed most of the studies around the pooled estimate, creating an inverted funnel, which indicated low risk of small‐study effects and reporting biases. One must keep in mind that many investigators had to be contacted to ask for additional data and for clarification of details (see Characteristics of included studies).
4.
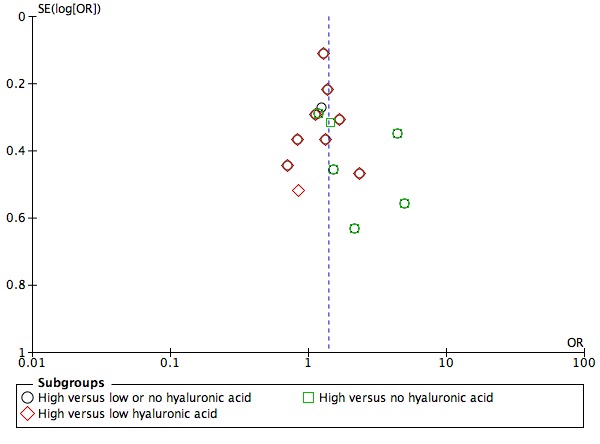
Funnel plot of comparison: 3 Embryo transfer in medium enriched with hyaluronic acid versus medium devoid of, or with a lower concentration of, hyaluronic acid, outcome 3.14 Clinical pregnancy rate.
One study (Chen 2001) did not report the primary outcome measure of live birth nor interim outcomes such as clinical pregnancy. Instead, Chen et al reported on the biochemical pregnancy rate alone. This study showed a trend in favour of the addition of HA acid to embryo transfer medium over the control medium. These findings are plausible when compared with findings of the other included studies.
Effects of interventions
for the main comparison.
High versus low or no hyaluronic acid for assisted reproductive technologies
| High versus low or no hyaluronic acid for assisted reproductive technologies | ||||||
| Population: couples undergoing embryo transfer Settings: assisted reproduction Intervention: high hyaluronic acid Comparison: low or no hyaluronic acid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low or no hyaluronic acid | High hyaluronic acid | |||||
| Live birth rate—high versus low or no hyaluronic acid | 374 per 1000 |
458 per 1000 (412 to 503) |
OR 1.41 (1.17 to 1.69) | 1950 (six studies) | ⊕⊕⊕⊝ moderate1 | |
| Live birth rate—high versus low hyaluronic acid | 347 per 1000 |
430 per 1000 (382 to 479) |
OR 1.42 (1.16 to 1.73) | 1626 (four studies) | ⊕⊕⊕⊝ moderate2 | |
| Live birth rate—high versus no hyaluronic acid | 385 per 1000 |
458 per 1000 (350 to 570) |
OR 1.35 (0.86 to 2.12) | 324 (three studies) | ⊕⊕⊕⊝ moderate1 | |
| Clinical pregnancy rate—high versus low or no hyaluronic acid | 350 per 1000 |
428 per 1000 (394 to 462) |
OR 1.39 (1.21 to 1.6) | 3452 (14 studies) | ⊕⊕⊕⊝ moderate1,3 | |
| Clinical pregnancy rate—high versus low hyaluronic acid | 448 per 1000 |
506 per 1000 (467 to 546) |
OR 1.26 (1.08 to 1.48) | 2566 (nine studies) | ⊕⊕⊕⊝ moderate2 | |
| Clinical pregnancy rate—high versus no hyaluronic acid | 178 per 1000 |
299 per 1000 (241 to 367) |
OR 1.97 (1.46 to 2.67) | 886 (six studies) | ⊕⊕⊕⊝ moderate1,4 | |
| Multiple pregnancy rate | 20 per 1000 |
37 per 1000 (30 to 45) |
OR 1.86 (1.49 to 2.31) | 1951 (five studies) | ⊕⊕⊕⊝ moderate1 | |
| Adverse event rate | 63 per 1000 |
48 per 1000 (32 to 70) |
OR 0.74 (0.49 to 1.12) | 1525 (four studies) | ⊕⊕⊕⊝ moderate2 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1All studies except one at high risk of bias in one or more domains. 2All studies at high risk of bias in one or more domains. 3Moderate heterogeneity: I2 = 46%. 4Moderate heterogeneity: I2 = 60%.
2.
Fibrin sealant versus no fibrin sealant for assisted reproductive technologies
| Fibrin sealant versus no fibrin sealant for assisted reproductive technologies | ||||||
| Population: couples undergoing embryo transfer Settings: assisted reproduction Intervention: fibrin sealant versus no fibrin sealant | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No fibrin sealant | Fibrin sealant | |||||
| Clinical pregnancy rate (per randomly assigned couple) | 291 per 1000 | 287 per 1000 (181 to 422) | OR 0.98 (0.54 to 1.78) | 211 (one study) | ⊕⊝⊝⊝ very low1,2 | |
| Adverse event rate (per randomly assigned couple) | Zero per 1000 | Zero per 1000 (zero to zero) | OR 5.55 (0.26 to 117.06) | 211 (one study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk is the control group risk. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1High risk of attrition bias. 2Single study, wide confidence intervals compatible with appreciable benefit or harm, or no effect.
1. Embryo transfer in medium containing high versus no or low concentration of hyaluronic acid (HA)
Live birth rate—high versus no or low HA
Six of the 16 included studies reported on live birth (Fancsovits 2011; Hazlett 2008; Korošec 2007; Morbeck 2007; Simon 2003; Urman 2008). The combined results of these studies with a total of 1950 participants were pooled, and evidence showed an increased number of live births with transfer media containing high concentrations of HA (OR 1.41, 95% CI 1.17 to 1.69; six studies, 1950 participants, I2 = 0%, moderate‐quality evidence) (Analysis 1.1) (see Figure 5 and Table 1).
1.1.
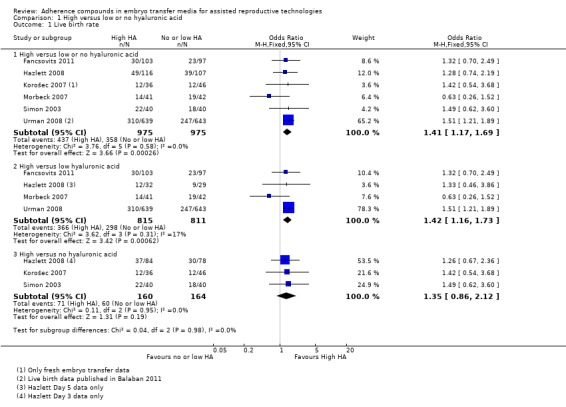
Comparison 1 High versus low or no hyaluronic acid, Outcome 1 Live birth rate.
5.
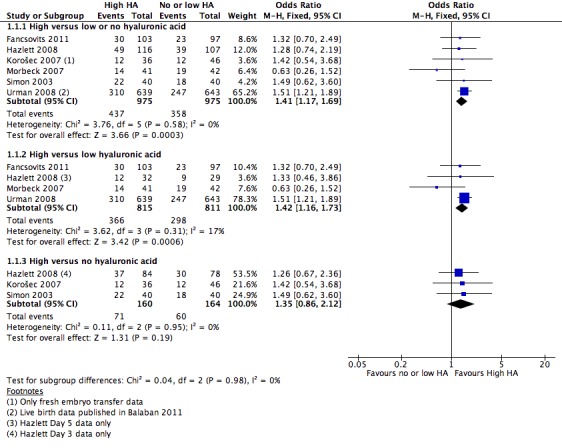
Forest plot of comparison: 1 High hyaluronic acid versus low/no hyaluronic acid, outcome: 1.1 Live birth rate.
Sensitivity analyses
Sensitivity analyses were performed, but none changed the outcome of the analysis in such a way that the 95% confidence interval crossed the line of no effect.
Subgroup analysis, live birth rate (grouped by timing of intervention) (Analysis 1.2)
1.2.
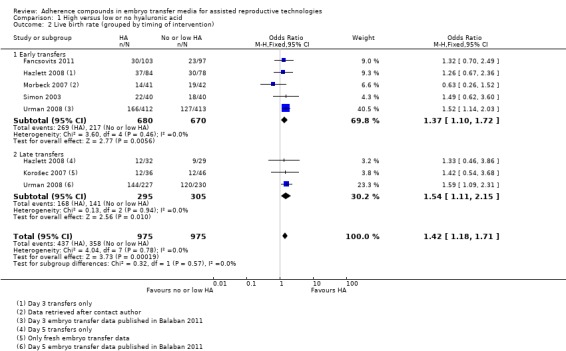
Comparison 1 High versus low or no hyaluronic acid, Outcome 2 Live birth rate (grouped by timing of intervention).
Two studies (Hazlett 2008; Urman 2008) reported live birth data resulting from both early and late embryo transfers; these data have been extracted separately for this subgroup analysis.
Five combined studies (Fancsovits 2011; Hazlett 2008; Morbeck 2007; Simon 2003; Urman 2008) with a total of 1350 participants performed the transfers early in embryo development (day three). Evidence of a beneficial treatment effect was noted (OR 1.37, 95% CI 1.10 to 1.72; three studies, 1350 participants, I2 = 0%, moderate‐quality evidence). Three combined studies (Hazlett 2008; Korošec 2007; Urman 2008) with a total of 600 participants performed transfers late in embryo development (day five)and also showed evidence of a beneficial treatment effect (OR 1.54, 95% CI 1.11 to 2.15; three studies, 600 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, live birth rate (grouped by frozen‐thawed or fresh embryos) (Analysis 1.3)
1.3.
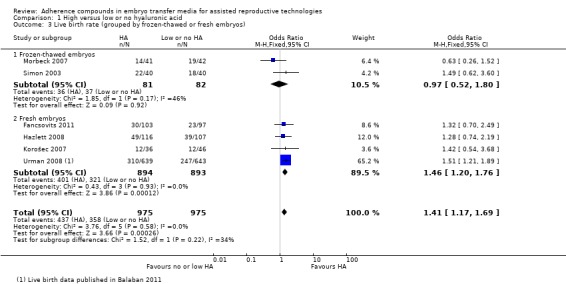
Comparison 1 High versus low or no hyaluronic acid, Outcome 3 Live birth rate (grouped by frozen‐thawed or fresh embryos).
Two combined studies (Morbeck 2007; Simon 2003) with a total of 163 participants transferred frozen‐thawed embryos. No evidence of a treatment effect was found (OR 0.97, 95% CI 0.52 to 1.80; two studies, 163 participants, I2 = 46%, moderate‐quality evidence). Four combined studies (Fancsovits 2011; Hazlett 2008; Korošec 2007; Urman 2008) with a total of 1787 participants transferred fresh embryos and showed evidence of a beneficial treatment effect from transfer media containing high concentrations of HA (OR 1.46, 95% CI 1.20 to 1.76; four studies, 1787 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, live birth rate (grouped by oocyte donation) (Analysis 1.4)
1.4.
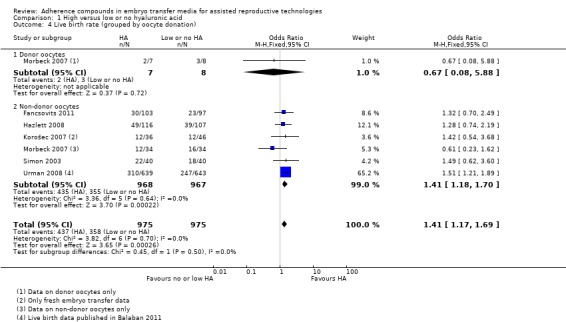
Comparison 1 High versus low or no hyaluronic acid, Outcome 4 Live birth rate (grouped by oocyte donation).
One study (Morbeck 2007) reported on live birth rates resulting from both donor oocytes and non‐donor oocytes; the data have been extracted separately for this subgroup analysis.
Donor oocyte data from Morbeck 2007 (15 participants) provided no evidence of a treatment effect (OR 0.67, 95% CI 0.08 to 5.88; one study, 15 participants, moderate‐quality evidence). Six combined studies (Fancsovits 2011; Hazlett 2008; Korošec 2007; Morbeck 2007; Simon 2003; Urman 2008) with a total of 1935 participants reported on non‐donor oocytes. Evidence showed a beneficial treatment effect (OR 1.41, 95% CI 1.18 to 1.70; six studies, 1935 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, live birth rate (grouped by exposure time to high‐concentration HA) (Analysis 1.5)
1.5.
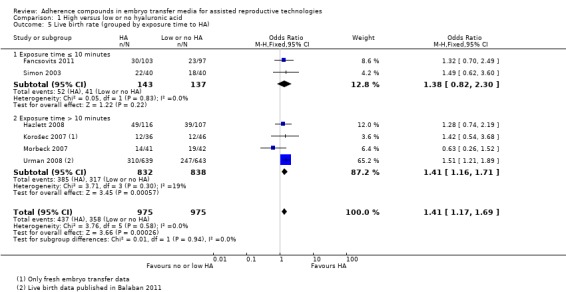
Comparison 1 High versus low or no hyaluronic acid, Outcome 5 Live birth rate (grouped by exposure time to HA).
Two studies (Fancsovits 2011; Simon 2003) with 280 participants exposed the embryos to HA for up to 10 minutes before transfer, and the combined data showed no evidence of a treatment effect (OR 1.38, 95% CI 0.82 to 2.30; two studies, 280 participants, I2 = 0%, moderate‐quality evidence). Four combined studies (Hazlett 2008; Korošec 2007; Morbeck 2007; Urman 2008) with a total of 1670 participants exposed the embryos to HA for longer than 10 minutes before transfer. Evidence of a beneficial treatment effect was found (OR 1.41, 95% CI 1.16 to 1.71; four studies, 1670 participants, I2 = 19%, moderate‐quality evidence).
Subgroup analysis, live birth rate (grouped by embryo transfer policy) (Analysis 1.6)
1.6.
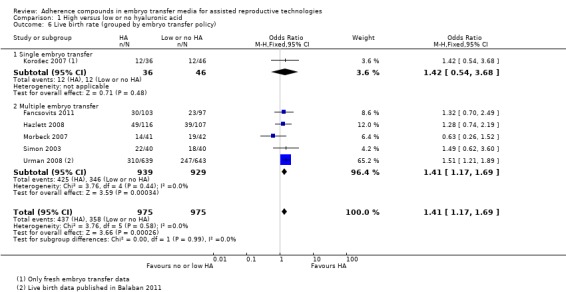
Comparison 1 High versus low or no hyaluronic acid, Outcome 6 Live birth rate (grouped by embryo transfer policy).
One study (Korošec 2007) with 82 participants transferred only one embryo per treatment cycle and found no evidence of a treatment effect (OR 1.42, 95% CI 0.54 to 3.68; one study, 82 participants, moderate‐quality evidence). Five combined studies (Fancsovits 2011; Hazlett 2008; Morbeck 2007; Simon 2003; Urman 2008) with a total of 1868 participants transferred multiple embryos per treatment cycle. Evidence showed a beneficial treatment effect (OR 1.41, 95% CI 1.17 to 1.69; five studies, 1868 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, live birth rate (grouped by participant selection) (Analysis 1.7)
1.7.
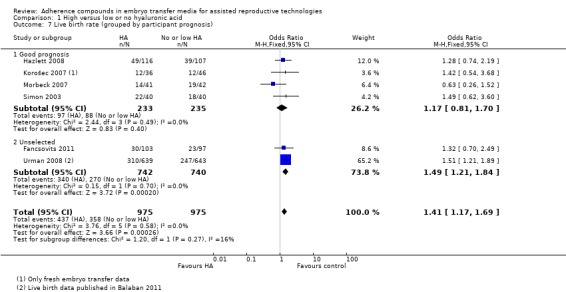
Comparison 1 High versus low or no hyaluronic acid, Outcome 7 Live birth rate (grouped by participant prognosis).
Four combined studies (Hazlett 2008; Korošec 2007; Morbeck 2007; Simon 2003) with a total of 468 participants included only good prognosis participants and showed a P value of 0.40 (OR 1.17, 95% CI 0.81 to 1.70; four studies, 468 participants, I2 = 0%, moderate‐quality evidence). Two studies (Fancsovits 2011; Urman 2008) with a total of 1482 participants did not use strict inclusion criteria for participant selection, and the combined data provide evidence of an increased live birth rate (OR 1.49, 95% CI 1.21 to 1.84; two studies, 1482 participants, I2 = 0%, moderate‐quality evidence).
Live birth rate—high versus low HA
Four studies (Fancsovits 2011; Hazlett 2008 (day five); Morbeck 2007; Urman 2008) in this comparison group reported on live births. Live birth data from Urman 2008 were published in an updated article (Balaban 2011). The combined results of these studies with a total of 1626 participants were pooled, and evidence showed an increased live birth rate with HA‐enriched transfer media (OR 1.42, 95% CI 1.16 to 1.73; four studies, 1626 participants, I2 = 17%, moderate‐quality evidence) (Analysis 1.1) (see Figure 5 and Table 1).
Sensitivity analyses
The following sensitivity analyses could be performed and showed that evidence of a treatment effect is robust. None of the planned sensitivity analyses showed any relevant differences.
| Sensitivity analysis | Results |
| Exclusion of trials with high risk of bias | Fancsovits 2011 excluded (OR 1.43, 95% CI 1.16 to 1.76; I2 = 44%, N = 1426) |
| Random‐effects model instead of fixed‐effect model | OR 1.34, 95% CI 1.01 to 1.79; I2 = 17%, N = 1626 |
Live birth rate—high versus no HA
Three studies (Hazlett 2008 (day three); Korošec 2007; Simon 2003) reported live births for this comparison. The combined results of these three studies with a total of 324 participants showed no evidence of a treatment effect (OR 1.35, 95% CI 0.86 to 2.12; three studies, 324 participants, I2 = 0%, moderate‐quality evidence) (Analysis 1.1) (see Figure 5 and Table 1).
Sensitivity analyses
The sensitivity analyses that could be performed for this outcome were the exclusion of studies with a higher risk of bias (Korošec 2007) and use of a random‐effects model instead of a fixed‐effect model. They showed no relevant differences in results.
Clinical pregnancy rate
Fourteen studies (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008; Korošec 2007; Mahani 2007; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Urman 2008; Yakin 2004) reported on clinical pregnancy rate. The combined results of these 14 studies with a total of 3452 participants were pooled, and evidence showed an increased clinical pregnancy rate with HA‐enriched transfer media (OR 1.39, 95% CI 1.21 to 1.60; 14 studies, 3452 participants, I2 = 46%, moderate‐quality evidence) (Analysis 1.8) (see Table 1). Because more than 10 studies were included in this analysis, a funnel plot was constructed to assess the risk of small‐study effects (see Figure 4). The funnel plot showed low risk of small‐study effect or reporting biases.
1.8.
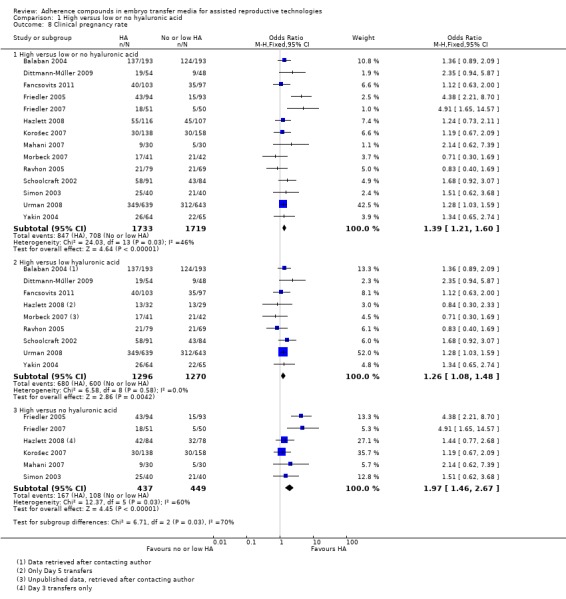
Comparison 1 High versus low or no hyaluronic acid, Outcome 8 Clinical pregnancy rate.
Subgroup analysis, clinical pregnancy rate (grouped by timing of intervention) (Analysis 1.9)
1.9.
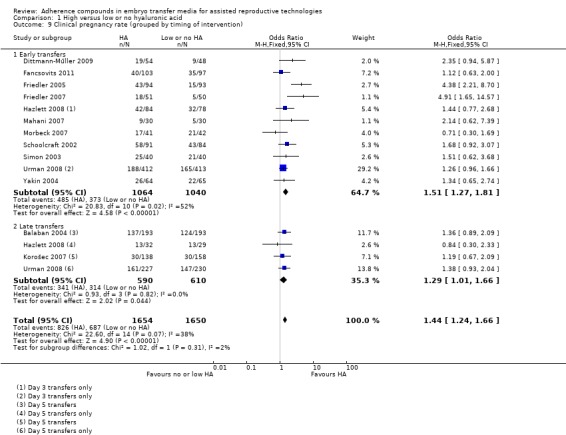
Comparison 1 High versus low or no hyaluronic acid, Outcome 9 Clinical pregnancy rate (grouped by timing of intervention).
Eleven combined studies (Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008 (day three); Mahani 2007; Morbeck 2007; Schoolcraft 2002; Simon 2003; Urman 2008 (day three) Yakin 2004) with a total of 2104 participants transferred embryos early in their development (day two to three), and evidence showed an increased clinical pregnancy rate (OR 1.51, 95% CI 1.27 to 1.81; 11 studies, 2104 participants, moderate‐quality evidence). Heterogeneity was substantial with an I2 statistic of 52%. Four combined studies (Balaban 2004; Hazlett 2008; Korošec 2007; Urman 2008) with a total of 1200 participants transferred embryos late in their development (day five). Evidence showed an increased clinical pregnancy rate (OR 1.29, 95% CI 1.01 to 1.66; five studies, 1200 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, clinical pregnancy rate (grouped by frozen‐thawed or fresh embryos) (Analysis 1.10)
1.10.
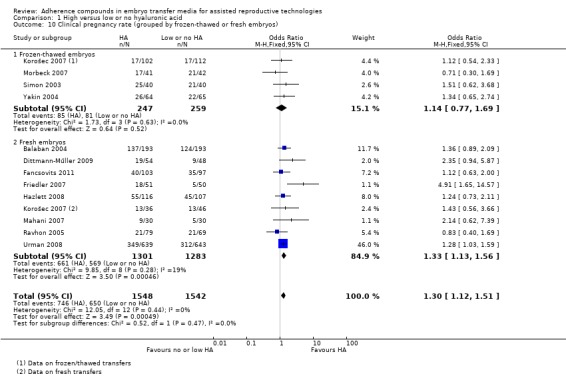
Comparison 1 High versus low or no hyaluronic acid, Outcome 10 Clinical pregnancy rate (grouped by frozen‐thawed or fresh embryos).
Four studies (Korošec 2007 (frozen‐thawed transfers); Morbeck 2007; Simon 2003; Yakin 2004) with a total of 506 participants transferred frozen‐thawed embryos. No evidence of a treatment effect was found (OR 1.14, 95% CI 0.77 to 1.69; four studies, 506 participants, I2 = 0%, moderate‐quality evidence). Nine studies (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2007; Hazlett 2008; Korošec 2007 (fresh transfers); Mahani 2007; Ravhon 2005; Urman 2008) with a total of 2584 participants transferred fresh embryos and showed evidence of an increased clinical pregnancy rate (OR 1.33, 95% CI 1.13 to 1.56; nine studies, 2584 participants, I2 = 19%, moderate‐quality evidence).
Subgroup analysis, clinical pregnancy rate (grouped by oocyte donation) (Analysis 1.11)
1.11.
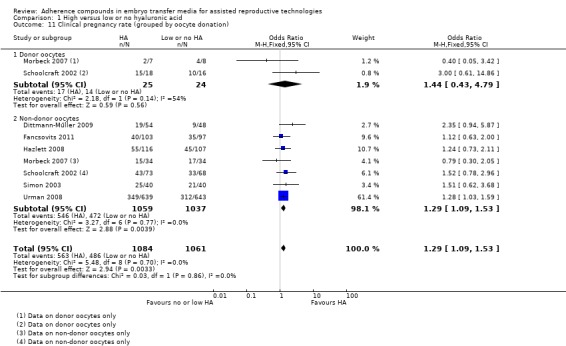
Comparison 1 High versus low or no hyaluronic acid, Outcome 11 Clinical pregnancy rate (grouped by oocyte donation).
Two studies (Morbeck 2007; Schoolcraft 2002) reported on both donor and non‐donor oocytes. Their combined results on donor oocytes with a total of 49 participants showed no evidence of a treatment effect (OR 1.44, 95% CI 0.43 to 4.79; two studies, 49 participants, moderate‐quality evidence). Heterogeneity was substantial with an I2 statistic of 54%. Seven combined studies (Dittmann‐Műller 2009; Fancsovits 2011; Hazlett 2008; Morbeck 2007; Schoolcraft 2002; Simon 2003; Urman 2008) with a total of 2096 participants used only non‐donor oocytes and found evidence of an increased clinical pregnancy rate (OR 1.29, 95% CI 1.09 to 1.53; seven studies, 2096 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, clinical pregnancy rate (grouped by exposure time to HA) (Analysis 1.12)
1.12.
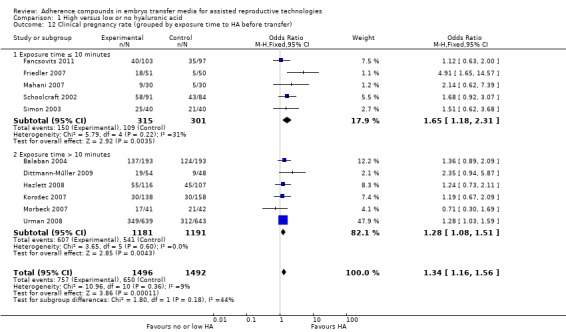
Comparison 1 High versus low or no hyaluronic acid, Outcome 12 Clinical pregnancy rate (grouped by exposure time to HA before transfer).
Five combined studies (Fancsovits 2011; Friedler 2007; Mahani 2007; Schoolcraft 2002; Simon 2003) with a total of 616 participants exposed the embryos to HA for up to 10 minutes before transfer. Evidence showed an increased clinical pregnancy rate (OR 1.65, 95% CI 1.18 to 2.31; five studies, 616 participants, I2 = 31%, moderate‐quality evidence). Six combined studies (Balaban 2004; Dittmann‐Műller 2009; Hazlett 2008; Korošec 2007; Morbeck 2007; Urman 2008) with a total of 2372 participants exposed the embryos to HA for longer than 10 minutes before transfer and also found evidence of an increased clinical pregnancy rate (OR 1.28, 95% CI 1.08 to 1.51; six trials, 2372 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis, clinical pregnancy rate (grouped by participant prognosis) (Analysis 1.13)
1.13.
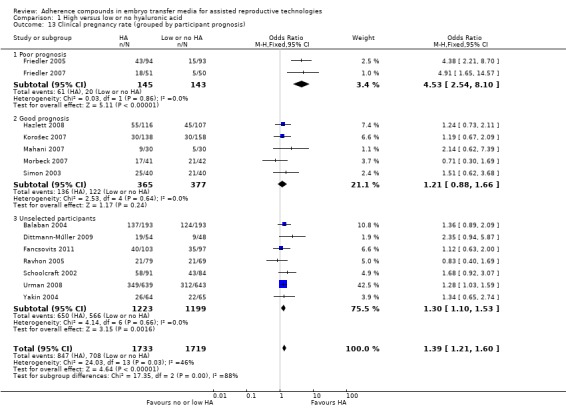
Comparison 1 High versus low or no hyaluronic acid, Outcome 13 Clinical pregnancy rate (grouped by participant prognosis).
Two combined studies (Friedler 2005; Friedler 2007) with a total of 288 participants included only poor prognosis participants and found evidence of an increased clinical pregnancy rate (OR 4.53, 95% CI 2.54 to 8.10; two studies, 288 participants, I2 = 0%, moderate‐quality evidence). Five combined studies (Hazlett 2008; Korošec 2007; Mahani 2007; Morbeck 2007; Simon 2003) with a total of 742 participants included only good prognosis participants. No evidence of a treatment effect was found (OR 1.21, 95% CI 0.88 to 1.66; five trials, 742 participants, I2 = 0%, moderate‐quality evidence). Seven combined studies (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Ravhon 2005; Schoolcraft 2002; Urman 2008; Yakin 2004) with a total of 2422 participants did not select participants on the basis of prognosis and showed evidence of an increased clinical pregnancy rate (OR 1.30, 95% CI 1.10 to 1.53; seven studies, 2422 participants, I2 = 0%, moderate‐quality evidence).
Subgroup analysis clinical pregnancy rate (grouped by embryo transfer policy) (Analysis 1.14)
1.14.
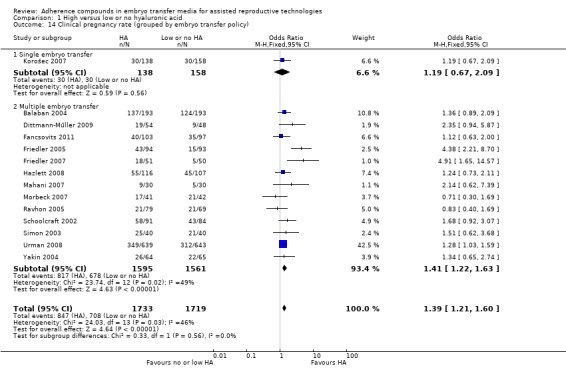
Comparison 1 High versus low or no hyaluronic acid, Outcome 14 Clinical pregnancy rate (grouped by embryo transfer policy).
One study (Korošec 2007) with 296 participants transferred only one embryo per treatment cycle and found no evidence of a treatment effect (OR 1.19, 95% CI 0.67 to 2.09; one trial, 296 participants, moderate‐quality evidence). Thirteen combined studies (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Friedler 2005; Friedler 2007; Hazlett 2008; Mahani 2007; Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Simon 2003; Urman 2008; Yakin 2004) with a total of 3156 participants transferred multiple embryos per treatment cycle and found evidence of an increased clinical pregnancy rate (OR 1.41, 95% CI 1.22 to 1.63; 13 studies, 3156 participants, moderate‐quality evidence). Heterogeneity was moderate with an I2 statistic of 49%.
Clinical pregnancy rate—high versus low HA
All nine studies (Balaban 2004; Dittmann‐Műller 2009; Fancsovits 2011; Hazlett 2008 (day five); Morbeck 2007; Ravhon 2005; Schoolcraft 2002; Urman 2008; Yakin 2004) in this comparison reported on clinical pregnancies. The combined results of these studies with a total of 2566 participants were pooled, and evidence showed an increased clinical pregnancy rate with HA‐enriched transfer media (OR 1.26, 95% CI 1.08 to 1.48; nine studies, 2566 participants, I2 = 0%, moderate‐quality evidence) (Analysis 1.8) (see Table 1).
Clinical pregnancy rate—high versus no HA
Six studies (Friedler 2005; Friedler 2007; Hazlett 2008 (day three); Korošec 2007; Mahani 2007; Simon 2003) in this comparison group reported clinical pregnancies. The combined results of the six studies with a total of 886 participants were pooled, and evidence showed an increased clinical pregnancy rate (OR 1.97, 95% CI 1.46 to 2.67; six studies, 886 participants, moderate‐quality evidence) (Analysis 1.8). Heterogeneity was substantial with an I2 statistic of 60% (see Table 1).
Multiple pregnancy rate
Five studies (Balaban 2004; Dittmann‐Műller 2009; Friedler 2007; Simon 2003; Urman 2008) reported on multiple pregnancy rates. The combined results of these five studies with a total of 1951 participants were pooled, and evidence showed an increased multiple pregnancy rate with HA‐enriched transfer media (OR 1.86, 95% CI 1.49 to 2.31; five studies, 1951 participants, I2 = 0%, moderate‐quality evidence) (Analysis 1.15) (see Table 1).
1.15.
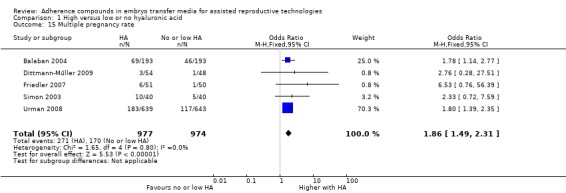
Comparison 1 High versus low or no hyaluronic acid, Outcome 15 Multiple pregnancy rate.
Adverse events rate
Five studies (Friedler 2005; Friedler 2007; Korošec 2007; Mahani 2007; Urman 2008) reported on adverse event rates. However, the data from one study (Friedler 2005) could not be analysed. The combined results of the remaining four studies with a total of 1525 participants showed no evidence of a treatment effect from HA‐enriched transfer media (OR 0.74, 95% CI 0.49 to 1.12; five studies, 1525 participants, I2 = 0%, moderate‐quality evidence) (Analysis 1.16) (see Figure 6 and Table 1). Adverse events concerned the number of miscarriages in all four studies, although one study (Friedler 2007) included ectopic pregnancies as well.
1.16.
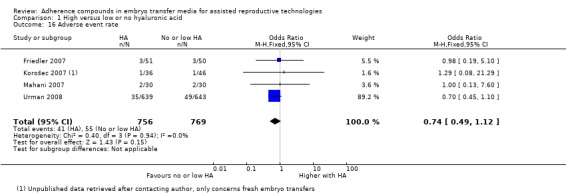
Comparison 1 High versus low or no hyaluronic acid, Outcome 16 Adverse event rate.
6.
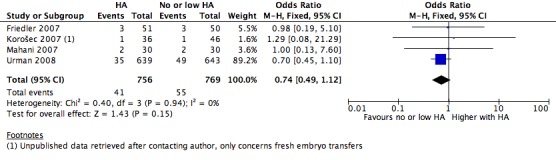
Forest plot of comparison: 1 High versus low or no hyaluronic acid, outcome: 1.16 Adverse event rate.
Implantation rate
Implantation rate was also recorded but could not be part of the meta‐analysis because it uses as the denominator the number of embryos transferred instead of the number of couples or participants. However, the data have been presented in a meta‐view without pooling. Results of the eight studies (Balaban 2004; Fancsovits 2011; Friedler 2007; Hazlett 2008; Mahani 2007; Morbeck 2007; Simon 2003; Urman 2008) that reported implantation rates were analysed (Analysis 1.17).
1.17.
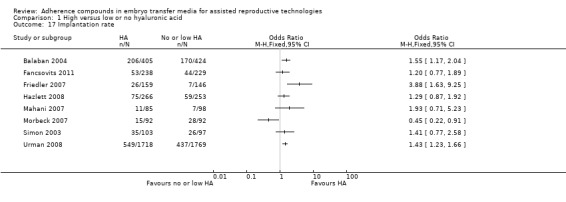
Comparison 1 High versus low or no hyaluronic acid, Outcome 17 Implantation rate.
2. Embryo transfer in medium containing fibrin sealant versus transfer in medium with no fibrin sealant
Clinical pregnancy rate
One study (Ben‐Rafael 1995) with a total of 211 participants reported on clinical pregnancies in this comparison. No evidence was found of a treatment effect for transfer media with fibrin sealant (OR 0.98, 95% CI 0.54 to 1.78; one study, 211 participants, very low‐quality evidence) (Analysis 2.1) (see Figure 7 and Table 2).
2.1.
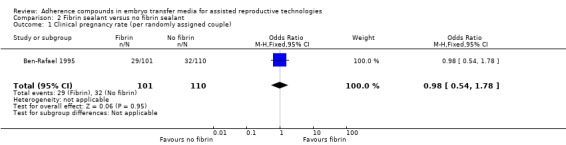
Comparison 2 Fibrin sealant versus no fibrin sealant, Outcome 1 Clinical pregnancy rate (per randomly assigned couple).
7.
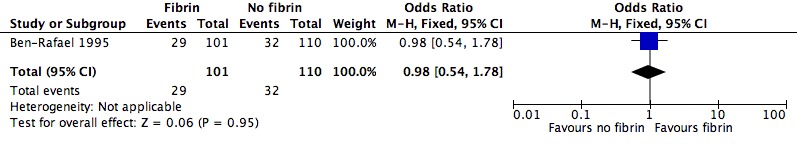
Forest plot of comparison: 2 Fibrin sealant versus no fibrin sealant, outcome: 2.1 Clinical pregnancy rate (per randomly assigned couple).
Adverse events rate
One study (Ben‐Rafael 1995) with a total of 211 participants reported on adverse events (ectopic pregnancies) in this comparison, and no evidence was found of a treatment effect for transfer media with fibrin sealant (OR 5.55, 95% CI 0.26 to 117.06; one study, 211 participants, very low‐quality evidence) (Analysis 2.2) (see Figure 8 and Table 2).
2.2.
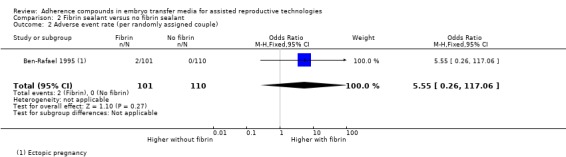
Comparison 2 Fibrin sealant versus no fibrin sealant, Outcome 2 Adverse event rate (per randomly assigned couple).
8.

Forest plot of comparison: 2 Fibrin sealant versus no fibrin sealant, outcome: 2.2 Adverse event rate (per randomly assigned couple).
Implantation rate
Implantation rate was also recorded but could not be part of the meta‐analysis because it uses as the denominator the number of embryos transferred instead of the number of couples or participants. However, the data have been presented in a meta‐view. Results of the study (Ben‐Rafael 1995) are presented in Analysis 2.3.
2.3.
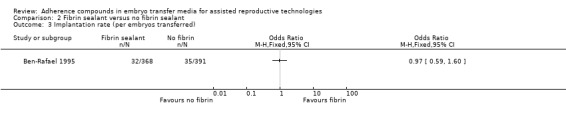
Comparison 2 Fibrin sealant versus no fibrin sealant, Outcome 3 Implantation rate (per embryos transferred).
Discussion
Summary of main results
This series of meta‐analyses of the best available evidence indicates that the addition of hyaluronic acid as an adherence compound to embryo transfer medium has a clinical benefit. Widespread use of hyaluronic acid was introduced into clinical practice in the late 1990s, and HA was initially marketed as an embryo glue. Since that time, a large number of journal papers and proceedings have been published, demonstrating a mixture of positive treatment effects and indifferent results; one high‐quality study notably showed a strong negative effect (Morbeck 2007). Funnel plot analysis of results of these trials revealed no evidence of publication bias. Careful consideration of the baseline characteristics resulted in a total of 21 trials that were acceptable for inclusion in this systematic review. Of these trials, 14, involving 3452 participants, could be included in a meta‐analysis on the addition of hyaluronic acid. The systematic analysis of these data has provided a level of confidence in supporting continued use of hyaluronic acid and offers insight into its underlying mechanism of action, given that both cleavage and blastocyst transfers appear to benefit from the addition of HA.
The objective of this review was to consider trials on all reported forms of adherence compounds. In addition to hyaluronic acid, a compound known as fibrin sealant was identified. With only one trial meeting the inclusion criteria, we could not perform a meta‐analysis. Although fibrin sealant has not appeared in the literature for over 10 years, the objective of this Cochrane analysis enables other novel compounds to be reviewed in the future.
The presence of low levels of hyaluronic acid in the culture medium before embryo transfer was a confounding factor that was not anticipated at the onset of this review. For this reason, a postprotocol amendment was initially made to split the trials into two comparisons, whereby one control group consisted of media containing a low concentration of HA (0.125 mg/ml) for both culture and control transfer media, and another control group had no HA in the transfer media. During the current review update, this amendment was revised, and we returned to the comparison of combined data of high HA versus no or low HA for the primary outcomes. Logic suggested that a low concentration of HA in the control group media might reduce the power of differences from the treatment group. However, this has now been adequately disproved for both live birth and clinical pregnancy rates, thus supporting the revised protocol. The resulting analysis has provided improved clarity and is less cumbersome to follow.
For the primary outcome of this review, live birth rate, evidence of a beneficial treatment effect was identified in the overall comparison group, even when compared with embryos that had been grown in hyaluronic acid–containing media until the time of transfer (Analysis 1.1). Evidence of this treatment effect was based mainly on the data of one large randomised controlled trial of good quality (Urman 2008), although a total of six studies reported on this outcome measure. In the comparison between embryo transfer media containing hyaluronic acid and those containing no HA, only a trend towards a beneficial treatment effect could be found (Analysis 1.1) based on just three trials and small numbers of participants (160 women). This trend could not be specified through subgroup or sensitivity analyses.
The most robust outcome of this review lies with the clinical pregnancy rate. A clear positive effect of hyaluronic acid in the embryo transfer medium was identified in all three comparison groups (Analysis 1.8).
A raised multiple pregnancy rate is the expected natural consequence of increased implantation and pregnancy rates when more than one embryo is replaced, and indeed the results of this comparison reflect this (Analysis 1.15). Multiple pregnancies have likely increased as a result of the combination of an adherence compound and a policy of transferring more than one embryo. The multiple pregnancy rate was markedly higher in all three comparison groups, but with only five studies reporting this outcome measure, the impact of this result is reduced.
The number of studies reporting on the live birth rate is limited for reasons that deserve consideration. The most obvious assumption is that a large proportion of pregnancies ended with miscarriage before birth, yet this is difficult to confirm without more reported data on this event. A more probable explanation can be found in the frequent practice of reporting study findings before the last participant has given birth. Many publications fail to report this important outcome measure, reflecting either inadequate reporting capabilities for deliveries or an eagerness to publish. This limitation poses a considerable burden on investigators who intend to maintain the golden standard of 'live birth' as a primary outcome in Cochrane meta‐analyses. Nevertheless, any new intervention, such as the addition of adherence compounds, could potentially have an effect on the ultimate outcome of a live baby and remains of paramount importance. This point was demonstrated in this meta‐analysis, together with a clear positive effect of treatment with adherence compounds on ongoing clinical and multiple pregnancy rates.
No treatment effect on adverse events could be identified. A disappointing finding of this review is that few studies have reported on miscarriage and ectopic pregnancies. The possibility that an adherence compound could facilitate the implantation of a low‐quality embryo, resulting in an increased miscarriage rate, remains unconfirmed. However, current data suggest that this is not a major concern.
Finally, one of the more interesting and perhaps clinically relevant aspects of this analysis was the bigger treatment effect on live birth and clinical pregnancy rates seen after the addition of hyaluronic acid, regardless of the stage of embryo development at the time of transfer (Analysis 1.2; Analysis 1.9). If the primary mechanism of hyaluronic acid action was indeed as an adhesive during implantation, one might expect this to be beneficial only for embryos that were transferred close to the time of embryo attachment (day five to six). The fact that it is equally beneficial for early‐stage embryos transferred on day two to three supports an alternative or facilitating action of hyaluronic acid during implantation.
Overall completeness and applicability of evidence
Although we were able to analyse 17 studies across four comparisons, the primary outcome measure, live birth rate, was reported in only six studies. Of these studies, only one (Simon 2003) actually published the results on live births and the number of deliveries; one study (Urman 2008) reported the live birth rate only in a second publication a few years later (Balaban 2011), after reporting only the clinical pregnancy rate in the original publication. The authors of the other four studies reported live births only after they were contacted by the review authors. Data on secondary outcomes and adverse events are also limited. Little is known about the effects of adherence compounds on the incidence of such adverse events as late miscarriages because most of the included studies do not follow up past the stage of clinical pregnancy. Also of note is the lack of reporting on multiple pregnancy rate, as all but one of the included studies added adherence compounds to an embryo transfer policy of multiple embryos per cycle. With only five studies reporting on multiple pregnancies while studying such a combination, important data have not been reported.
All original investigators of the 17 analysed studies have been contacted regarding data queries; however, the authoring team of one study (Chen 2001) could not be found. In total, we received responses from nine study authors, which helped to resolve queries regarding data and study characteristics. Ambiguities remain regarding the other eight studies.
Quality of the evidence
We included 21 studies in this review. However, the data could be analysed from only 17 of them with a total of 3698 participants. These 17 studies were distributed over four comparisons, from which data on the five outcome measures were extracted. A meta‐analysis on the treatment effect of hyaluronic acid eventually could be performed with data from only 14 of these trials with a total of 3452 participants. A total of 58 a priori identified subgroup analyses have been performed. It was not possible to perform all six planned subgroup analyses for each outcome measure.
A beneficial treatment effect on live birth rate due to the use of embryo transfer media containing hyaluronic acid has been identified in this systematic review. It should be noted that this result is due mainly to the outcomes of one large randomised controlled trial of good quality (Urman 2008), which reported on the actual birth rate of its original cohort of participants only in an updated article (Balaban 2011) in response to the first publication of this systematic review. The newly published live birth data have been included in the updated version of this meta‐analysis.
Included and pooled studies contained methodological limitations and differences in baseline characteristics of participants. Some studies allowed participants to enrol and undertake multiple treatment cycles in the trial; this has created ambiguities regarding the actual outcomes. Most studies transferred multiple embryos per treatment cycle, which limits the possibility of finding the actual treatment effects of adding adherence compounds to embryo transfer media. Only one study (Korošec 2007) adhered to a single embryo transfer policy, but investigators allowed participants to enrol into the study with multiple treatment cycles. Some studies sampled participants consecutively; others sampled participants non‐consecutively or did not describe their methods. Few included trials performed a power calculation a priori to determine sample size. Causes and durations of subfertility differed between studies but mainly remain unreported. The same goes for the number of previous treatment cycles—a factor that has a big influence on the success rate of ART. Most studies performed randomisation on the day of embryo transfer, which limits the loss of participants but creates a study population that is not truly representative of the subfertile population, although it is representative of the population undergoing embryo transfer. Regarding methods of randomisation and allocation concealment, most studies were not clear in their published articles. A lot of these ambiguities could be resolved by contacting the original investigators, although the concept of allocation concealment in particular remains unclear for many of the studies. Most studies performed trials in a double‐blind fashion (which means that both participant and physician or embryologists did not know to which treatment arm the participant was allocated) and reported outcomes in a prespecified way. However, few studies reported live birth rates or performed an intention‐to‐treat analysis, resulting in an overall high risk of incomplete outcome data. Furthermore, the overall risk for other sources of bias was high because some studies used very different transfer media in the treatment and control arms, and many studies did not report on multiple pregnancy rates whilst transferring multiple embryos per treatment cycle.
Even though all included studies reported their results in different units of analysis, for example, pregnancies per participant or pregnancies per couple, it was considered possible to use the number of randomised couples as the denominator for the overall data analysis. Implantation rate could be assessed only per embryo transferred and therefore could not be part of the meta‐analysis.
The overall quality of the evidence was rated using GRADE methods, which consider not only study limitations (i.e. risk of bias) but also consistency of effect, imprecision, indirectness and publication bias. Evidence was rated of moderate quality for all comparisons of high versus low or no hyaluronic acid (Table 1 and of very low quality for the comparison of fibrin sealant versus no fibrin sealant (Table 2).
Potential biases in the review process
During the review process, it appeared that hyaluronic acid was present in standard embryo culture and transfer media of the control groups in many studies. Therefore, in the first review, the hyaluronic acid trials were divided over two comparison groups—those comparing transfer medium with hyaluronic acid versus medium without hyaluronic acid and those comparing medium with hyaluronic acid versus medium with a lower concentration of hyaluronic acid. As a result, the data on the same adherence compound had to be divided, which led to less significant results. As discussed previously, whilst performing the current meta‐analysis, review authors decided to pool the data together to get an overall view of the treatment effect. Even though the included studies are not completely similar in their intervention and control groups, all do compare an embryo transfer medium to which hyaluronic acid has been added as an adherence compound versus a control transfer medium.
Other potential adherence compounds such as heparinase have been identified; however, no randomised controlled trials of their applicability in human assisted reproductive technologies could be found.
As stated in the protocol, when data were not reported, it was planned to impute data on the primary outcome as if live births had not occurred. In the process of data analysis, it appeared that imputation of these data had no influence on the overall treatment effect. The planned sensitivity analyses on this matter therefore were not performed. The same goes for other planned sensitivity analyses that could not be performed with the included data, such as excluding studies that used a different concentration of adherence compounds.
As stated in the protocol, the aim was to count multiple live births as a single live birth event. We were able to do this for two studies (Simon 2003; Urman 2008); however, some ambiguities arose regarding these data because the results published in the text were not the same as those reported in the table of results.
Agreements and disagreements with other studies or reviews
To our knowledge, one other systematic review has examined the addition of adherence compounds to embryo transfer media (Kolibianakis 2008), and this was published as a conference abstract. This review investigated whether the addition of HA to human embryo culture could increase pregnancy rates after in vitro fertilisation; it included 13 randomised controlled trials with a total of 4476 participants, which, it is interesting to note, accounted for more participants from fewer trials than in our review. Kolibianakis et al might have used different inclusion criteria. Their analysis showed a positive treatment effect on the clinical pregnancy rate with the addition of HA. This finding is comparable with results of the HA comparison groups in our review, even though participant numbers differed and Kolibianakis et al used a random‐effects model for data analysis rather than a fixed‐effect model, which we had used. It is unclear whether a full article has been published on this systematic review and which studies were considered eligible for inclusion. The review authors have been contacted, but no response has been received to date.
Authors' conclusions
Implications for practice.
Moderate‐quality evidence suggests that adherence compounds such as hyaluronic acid are valuable in improving the success rate of assisted reproductive technologies such as in vitro fertilisation and intracytoplasmic sperm injection, with resultant evidence of an increase in the number of live births. An overall increase of 8% in the live birth rate was found with the addition of adherence compounds (0.5 mg/ml HA) to the embryo transfer medium (44.8% in the HA group and 36.7% in the control group), with the number needed to treat for beneficial outcome (NNTB) of 12.5. A treatment effect was noted for both early (day two to three) and late (day five) embryo transfers; this implies that the actual working mechanism of adherence compounds may not involve enhancement of adhesion per se. An increase in the multiple pregnancy rate was noted but is likely to be a consequence of both the effect of the adherence compound and a policy of transferring multiple embryos per treatment cycle. The addition of adherence compounds to a single embryo transfer policy might yield the best combination with higher clinical pregnancy rates and higher live birth rates as a result, and without increasing the chance of multiple pregnancies. With each incremental improvement in the in vitro fertilisation technique, a compounding effect on multiple pregnancy rates will further entrench the drive towards single embryo transfer.
Implications for research.
The most important outcome measure that should be addressed is the live birth rate. Only six of the 16 studies included in this systematic review reported on this outcome measure. The lack of studies reporting on the number of live births may be a result of the large proportion of pregnancies that fail to progress to birth, or it may reflect the frequent practice of reporting studies before the last study participant has given birth, suggesting either inadequate reporting capabilities or an eagerness to publish. Other important outcome measures that have not been reported fully are multiple pregnancies and other adverse events such as miscarriages.
Further research on the actual working mechanism of adherence compounds might be useful. Additional studies of adherence compounds with single embryo transfer need to be undertaken. Also, randomised controlled trials on other potential adherence compounds should be performed in the future.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2015 | Amended | Minor corrections to Summary of Findings table and to references. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 10 November 2014 | Amended | Correction: in the updated review published in issue 2, 2014, there was a change to the conclusion for the primary outcome live birth. There was evidence of an increased number of live births with transfer media containing high concentrations of hyaluronic acid. |
| 13 November 2013 | New search has been performed | Meta‐analyses on hyaluronic acid have been grouped together. Instead of division into three different comparison groups, now only one group with a subgroup analysis of HA versus low HA and HA versus no HA for the live birth rate and the clinical pregnancy rate. Furthermore, ongoing pregnancy rate has been removed as a secondary outcome measure, and subgroup analyses have been removed from the secondary outcome measures of multiple pregnancy rate and adverse events rate |
| 13 November 2013 | New citation required but conclusions have not changed | Two studies added; no change made to conclusions |
| 12 May 2010 | Amended | Post‐protocol change: originally implantation rate was not planned for analysis but was to be presented as an additional table. However, we decided to present this outcome measure without pooling. |
| 8 July 2009 | Amended | Changed title and authoring team. Changes to protocol: inclusion of all types of adherence compounds, different outcome measures, multiple comparison groups, additional subgroup analyses. |
Acknowledgements
The authors of this systematic review would like to thank Dr P Fancsovits, Dr B Balaban, Dr B Ata, Dr B Urman, Prof Z Ben‐Rafael, Prof A Simon, Dr E Schenkman, Dr S Korošec, Dr S Friedler, Dr D Hazlett, Dr KP Zollner, Dr U Zollner, Dr AR Thornhill and Dr D Morbeck for sending us additional data and information on their studies.
Furthermore, we would like to thank Marian Showell, Vanessa Jordan, Julie Brown and Professor Cindy Farquhar of the Cochrane Menstral Disorders and Subfertility Group (MDSG). Without their help and advice, the writing of this systematic review would not have been possible. Eleanor Williams wrote the original protocol.
We acknowledge the contribution of Professor Maas Jan Heineman to previous versions of this review.
Appendices
Appendix 1. CENTRAL search
Searched up to 26‐05‐09
1 exp embryo transfer/ or exp fertilization in vitro/ (1315) 2 exp Intracytoplasmic Sperm Injection/ (250) 3 (embryo$ adj2 transfer$).tw. (930) 4 in vitro fertilization.tw. (1067) 5 (intracytoplas$ adj5 sperm).tw. (339) 6 (ivf or icsi).tw. (1860) 7 or/1‐6 (2679) 8 exp Hyaluronic Acid/ (456) 9 hyalur$.tw. (897) 10 HA.tw. (552) 11 embryo glue$.tw. (1) 12 embryoglue$.tw. (6) 13 G5.tw. (36) 14 GIII.tw. (25) 15 ver$ 5.tw. (558) 16 exp Fibrin Tissue Adhesive/ (199) 17 Fibrin.tw. (947) 18 or/8‐17 (2967) 19 18 and 7 (36) 20 from 19 keep 1‐36 (36)
This search was updated on 28 March 2012, 23 January 2013 and 13 November 2013.
Appendix 2. MEDLINE search
Searched from 1950 to May Week 3 2009 (26‐05‐2009)
1 exp embryo transfer/ or exp fertilization in vitro/ (25747) 2 exp Intracytoplasmic Sperm Injection/ (3007) 3 (embryo$ adj2 transfer$).tw. (8162) 4 in vitro fertilization.tw. (11898) 5 (intracytoplas$ adj5 sperm).tw. (3485) 6 (ivf or icsi).tw. (13092) 7 or/1‐6 (31552) 8 exp Hyaluronic Acid/ (11473) 9 hyalur$.tw. (17717) 10 HA.tw. (24335) 11 embryo glue$.tw. (1) 12 embryoglue$.tw. (4) 13 G5.tw. (931) 14 GIII.tw. (425) 15 ver$ 5.tw. (2262) 16 exp Fibrin Tissue Adhesive/ (3014) 17 Fibrin.tw. (23875) 18 randomized controlled trial.pt. (270902) 19 controlled clinical trial.pt. (79205) 20 randomized.ab. (180817) 21 placebo.tw. (115355) 22 clinical trials as topic.sh. (143208) 23 randomly.ab. (131272) 24 trial.ti. (78897) 25 cross over.ab. (12985) 26 or/18‐25 (634041) 27 (animals not (humans and animals)).sh. (3281171) 28 26 not 27 (588169) 29 or/8‐17 (68842) 30 28 and 7 and 29 (24) 31 from 30 keep 1‐24 (24)
This search was updated on 28 March 2012, 23 January 2013 and 13 November 2013.
Appendix 3. EMBASE search
Searched from 1980 to 2009 Week 21 (26‐05‐09)
1 exp embryo transfer/ or exp fertilization in vitro/ or exp Intracytoplasmic Sperm Injection/ (26078) 2 (embryo$ adj2 transfer$).tw. (6916) 3 in vitro fertilization.tw. (10706) 4 (intracytoplas$ adj5 sperm).tw. (3446) 5 (ivf or icsi).tw. (13051) 6 or/1‐5 (29168) 7 exp Hyaluronic Acid/ (12770) 8 hyalur$.tw. (13984) 9 embryo glue$.tw. (1) 10 embryoglue$.tw. (4) 11 exp Fibrin Glue/ (3649) 12 Fibrin.tw. (19102) 13 HA.tw. (23456) 14 (G5 or ver$ 5).tw. (2927) 15 GIII.tw. (309) 16 or/7‐15 (60778) 17 Clinical Trial/ (541751) 18 Randomized Controlled Trial/ (169143) 19 exp randomization/ (26816) 20 Single Blind Procedure/ (8180) 21 Double Blind Procedure/ (72527) 22 Crossover Procedure/ (21330) 23 Placebo/ (126930) 24 Randomi?ed controlled trial$.tw. (33445) 25 Rct.tw. (2768) 26 random allocation.tw. (640) 27 randomly allocated.tw. (10275) 28 allocated randomly.tw. (1357) 29 (allocated adj2 random).tw. (562) 30 Single blind$.tw. (7527) 31 Double blind$.tw. (85312) 32 ((treble or triple) adj blind$).tw. (140) 33 placebo$.tw. (110907) 34 prospective study/ (82325) 35 or/17‐34 (711785) 36 case study/ (6079) 37 case report.tw. (120253) 38 abstract report/ or letter/ (499476) 39 or/36‐38 (623463) 40 35 not 39 (686987) 41 6 and 16 and 40 (24) 42 from 41 keep 1‐24 (24)
Search update 28‐03‐2012:
1 exp embryo transfer/ or exp fertilization in vitro/ or exp Intracytoplasmic Sperm Injection/ (45274) 2 (embryo$ adj2 transfer$).tw. (12958) 3 in vitro fertilization.tw. (16488) 4 (intracytoplas$ adj5 sperm).tw. (5601) 5 (ivf or icsi).tw. (23250) 6 or/1‐5 (52160) 7 exp Hyaluronic Acid/ (21322) 8 hyalur$.tw. (23836) 9 embryo glue$.tw. (4) 10 embryoglue$.tw. (11) 11 exp Fibrin Glue/ (5896) 12 Fibrin.tw. (30654) 13 HA.tw. (40172) 14 (G5 or ver$ 5).tw. (5104) 15 GIII.tw. (691) 16 adherence compound$.tw. (6) 17 or/7‐16 (102185) 18 Clinical Trial/ (862803) 19 Randomized Controlled Trial/ (318508) 20 exp randomization/ (57568) 21 Single Blind Procedure/ (15595) 22 Double Blind Procedure/ (107813) 23 Crossover Procedure/ (33346) 24 Placebo/ (194847) 25 Randomi?ed controlled trial$.tw. (72598) 26 Rct.tw. (8838) 27 random allocation.tw. (1124) 28 randomly allocated.tw. (16791) 29 allocated randomly.tw. (1783) 30 (allocated adj2 random).tw. (703) 31 Single blind$.tw. (11911) 32 Double blind$.tw. (125667) 33 ((treble or triple) adj blind$).tw. (263) 34 placebo$.tw. (171422) 35 prospective study/ (199004) 36 or/18‐35 (1231050) 37 case study/ (14928) 38 case report.tw. (221515) 39 abstract report/ or letter/ (824756) 40 or/37‐39 (1056765) 41 36 not 40 (1196494) 42 6 and 17 and 41 (53) 43 (2009$ or 2010$ or 2011$ or 2012$).em. (3730038) 44 42 and 43 (30)
This search was updated on 28 March 2012, 23 January 2013 and 13 November 2013.
Appendix 4. PsycINFO search
Searched up to 26‐05‐09
1 exp embryo transfer/ or exp fertilization in vitro/ (1315) 2 exp Intracytoplasmic Sperm Injection/ (250) 3 (embryo$ adj2 transfer$).tw. (930) 4 in vitro fertilization.tw. (1067) 5 (intracytoplas$ adj5 sperm).tw. (339) 6 (ivf or icsi).tw. (1860) 7 or/1‐6 (2679) 8 exp Hyaluronic Acid/ (456) 9 hyalur$.tw. (897) 10 HA.tw. (552) 11 embryo glue$.tw. (1) 12 embryoglue$.tw. (6) 13 G5.tw. (36) 14 GIII.tw. (25) 15 ver$ 5.tw. (558) 16 exp Fibrin Tissue Adhesive/ (199) 17 Fibrin.tw. (947) 18 or/8‐17 (2967) 19 18 and 7 (36) 20 from 19 keep 1‐36 (36)
This search was updated on 28 March 2012, 23 January 2013 and 13 November 2013.
Appendix 5. Data extraction form
| Assessment | Final | |||||||||||
| Assessor | MJH SB NJ |
Inclusion | ||||||||||
| Date | Exclusion; because: ............................................................ | |||||||||||
| Awaiting; because: ............................................................. | ||||||||||||
| Study information | ||||||||||||
| 1. Ref ID | ||||||||||||
| 2. First author | ||||||||||||
| 3. Year | ||||||||||||
| 4. Published | Yes No | |||||||||||
| 5. Language | ||||||||||||
| 6. Retrieval | Electronic search Handsearched |
After citation tracking After contacting author in the field |
||||||||||
| Notes: | ||||||||||||
| Criteria for eligibility | ||||||||||||
| Participants | Couples undergoing embryo transfer after IVF, ICSI and/or an embryo thaw cycle | Yes No |
||||||||||
| Intervention | Embryo transfer with media containing hyaluronic acid or fibrin sealant for embryos · Grown in vitro for two to four days
· Both fresh and frozen‐thawed |
Yes No |
||||||||||
| Comparison | Embryo transfer with standard media for embryos · Grown in vitro for two to four days
· Both fresh and frozen‐thawed |
Yes No |
||||||||||
| Outcome | Primary | |||||||||||
| Live birth rate (per randomly assigned couple) | Yes No |
|||||||||||
| Secondary | ||||||||||||
| Ongoing pregnancy rate (per randomly assigned couple) (12+ weeks viable, fetal heartbeat positive, pregnancy) | Yes No |
|||||||||||
| Clinical pregnancy rate (per randomly assigned couple) (positive pregnancy test, gestational sac on ultrasound) | Yes No |
|||||||||||
| Multiple pregnancy rate (per randomly assigned couple) | Yes No |
|||||||||||
| Additional | ||||||||||||
| Implantation rate (per randomly assigned couple) (gestational sac per embryo transfer) | Yes No |
|||||||||||
| Adverse events (ectopic pregnancies, miscarriage, fetal/congenital defects, pelvic inflammation or other) (per randomly assigned couple) | Yes No |
|||||||||||
| Notes: | ||||||||||||
| Study characteristics | ||||||||||||
| Design | ||||||||||||
| 1. Study design | RCT Parallel (intervention vs control) Cross‐over (participants used as intervention and control groups) .................................. Quotes: ........................................................................................ |
|||||||||||
| 2. Participant recruitment | Prospective Retrospective Unclear Quotes: ............................................................................................ |
|||||||||||
| 3. Sampling (How was the sampling group formed?) |
Consecutive Non‐consecutive Unclear Quotes: ................................................................................................................ |
|||||||||||
| 4. Setting | Single‐centre Multi‐centre Country .................................................................................... |
|||||||||||
| Participants: included and excluded | ||||||||||||
| 5. Study criteria for participant inclusion | ||||||||||||
| 6. Study criteria for participant exclusion | ||||||||||||
| 7. Description control/ comparison treatment | ||||||||||||
| 8. Power calculation was performed and followed | Yes No Unclear Quotes: ................................................................................................................. |
|||||||||||
| Notes: | ||||||||||||
| Participants | ||||||||||||
| Baseline characteristics | ||||||||||||
| Age (of female): Not reported |
Mean: | SD: | ||||||||||
| Intervention: | ||||||||||||
| Control: | ||||||||||||
| Subfertility | Primary Secondary Both Not reported |
|||||||||||
| Cause and duration of subfertility | Reported Not reported |
|||||||||||
| Previous IVF and/or ICSI treatment | Reported Not reported |
|||||||||||
| Undergoing IVF or ICSI, or both | IVF ICSI Both |
|||||||||||
| Age group analysis | Yes, define: No |
|||||||||||
| Notes: | ||||||||||||
| Flow chart of participants | ||||||||||||
| Remarks: | ||||||||||||
| Intervention | ||||||||||||
| Embryo transfer after IVF, ICSI and/or frozen‐thaw cycle | ||||||||||||
| 1. Time of randomisation during cycle | Before commencement of treatment cycle After commencement of treatment and before fertilisation check From fertilisation check to day of embryo transfer On day of embryo transfer |
|||||||||||
| 2. Nature of intervention | Addition of hyaluronic acid to embryo transfer medium, concentration was ....... Addition of fibrin sealant to embryo transfer medium, concentration was ................. |
|||||||||||
| 3. Exposure time to hyaluronic acid or fibrin sealant before ET | .......................................................................... | |||||||||||
| 4. Timing of intervention | Early in embryo development (day two to and including day four) Late in embryo development (days five and six) Both early and late in embryo development |
|||||||||||
| 5. Frozen‐thaw protocol | Yes No Unclear |
|||||||||||
| 6. Including oocyte donations | Yes No Unclear |
|||||||||||
| 7. Culture and transfer (with and without adherence compound) medium brand | ............................................................................ | |||||||||||
| 8. Mean number of embryos transferred | ............................................. | Not reported | ||||||||||
| 9. Pregnancy determination | Fetal heartbeat | |||||||||||
| Demonstration of gestational sac on ultrasound scan | ||||||||||||
| Pregnancy test | ||||||||||||
| Not reported | ||||||||||||
| Notes: | ||||||||||||
| Primary outcomes | ||||||||||||
| Total occurrence N = Total non‐occurrence N = | ||||||||||||
| Notes: | ||||||||||||
| Secondary outcomes | ||||||||||||
| Total occurrence N = Total non‐occurrence N = | ||||||||||||
| Notes: | ||||||||||||
| Total occurrence N = Total non‐occurrence N = | ||||||||||||
| Notes: | ||||||||||||
| Total occurrence N = Total non‐occurrence N = | ||||||||||||
| Notes: | ||||||||||||
| Additional outcomes | ||||||||||||
| Implantation rate (gestational sacs per embryos transferred) | Occurrence of outcome | Non‐occurrence of outcome | ||||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Adverse events | ||||||||||||
| Ectopic pregnancy | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Miscarriage | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Fetal/congenital defects | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Pelvic inflammation | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Other adverse events | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Other outcomes studied | ||||||||||||
| Miscarriage | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Miscarriage | Occurrence of outcome | Non‐occurrence of outcome | Total (by group) | |||||||||
| Treatment | ||||||||||||
| Control | ||||||||||||
| Total (by event) | ||||||||||||
| Notes: | ||||||||||||
| Risk of bias assessment | ||||||||||||
|
Selection bias |
Was the allocation sequence adequately generated? Explain the method used by the authors to assess whether it should produce comparable groups. |
Yes No Unclear | ||||||||||
| Was participant allocation concealment adequate? Explain. (adequate: Central computer randomisation, on‐site assignment can be determined only after participant data are entered; serially numbered, sealed opaque envelopes) | Yes No Unclear | |||||||||||
| How was randomisation performed? | Computer generated Random numbers table Not stated |
|||||||||||
| Selective outcome reporting | Are reports of the study free of the suggestion of selective outcome reporting? Explain (compare Methods with Results). ............................................................ | Yes No Unclear | ||||||||||
|
Detection bias |
Length of follow‐up was long enough? | Yes No Unclear |
||||||||||
| Was the clinician or nurse blinded? | Yes No Unclear | |||||||||||
| Was the scientist blinded? | Yes No Unclear | |||||||||||
| Was the participant blinded? | Yes No Unclear | |||||||||||
| Attrition bias | Was loss to follow‐up accounted for? (Is it stated in the study?) | Yes No Unclear | ||||||||||
| Was an intention‐to‐treat analysis performed? | Yes No Unclear | |||||||||||
| Source of funding | Was the source of funding stated? | Yes No Unclear | ||||||||||
| Other remarks on quality | ||||||||||||
Appendix 6. Menstrual Disorders and Subfertility Group Specialised Register search
Searched up to 26‐05‐09
Keywords CONTAINS "ivf" or "icsi" or "Embryo" or "IVF‐ET" or "in‐vitro fertilisation " or "in vitro fertilization" or "intracytoplasmic sperm injection" or "Sperm Injections, Intracytoplasmic" or "ART" or "assisted reproduction" or Title CONTAINS "ivf" or "icsi" or "Embryo" or "IVF‐ET" or "in‐vitro fertilisation " or "in vitro fertilization" or "intracytoplasmic sperm injection" or "Sperm Injections, Intracytoplasmic" or "ART" or "assisted reproduction"
AND
Keywords CONTAINS "hyaluronan" or "hyaluronic acid" or "EmbryoGlue" or "fibrin sealant" or "G3 culture media" or Title CONTAINS "hyaluronan" or "hyaluronic acid" or "EmbryoGlue" or "fibrin sealant" or "G3 culture media"
Search updates 28‐03‐2012, 23‐01‐2013 and 13‐11‐13:
Keywords CONTAINS "ivf" or "icsi" or "Embryo" or "IVF‐ET" or "in‐vitro fertilisation " or "in vitro fertilization" or "intracytoplasmic sperm injection" or "Sperm Injections, Intracytoplasmic" or "ART" or "assisted reproduction" or Title CONTAINS "ivf" or "icsi" or "Embryo" or "IVF‐ET" or "in‐vitro fertilisation " or "in vitro fertilization" or "intracytoplasmic sperm injection" or "Sperm Injections, Intracytoplasmic" or "ART" or "assisted reproduction"
AND
Keywords CONTAINS "hyaluronan" or "hyaluronic acid" or "EmbryoGlue" or "fibrin sealant" or "G3 culture media" or "adherence" or "adhesion" or Title CONTAINS "hyaluronan" or "hyaluronic acid" or "EmbryoGlue" or "fibrin sealant" or "G3 culture media" or "adherence" or "adhesion"
Appendix 7. Trials with non‐useable data
| Trial | Non‐useable data |
| Chen 2001 | Only biochemical pregnancy rate reported |
| Friedler 2005 | Adverse event rate, implantation rate |
| Khan 2004 | Ongoing pregnancy rate, implantation rate |
| Ravhon 2005 | Implantation rate |
| Schoolcraft 2002 | Implantation rate |
| Yakin 2004 | Implantation rate |
Appendix 8. Responses to data queries
| Trial | Additional data supplied by original investigator |
| Balaban 2004 | No power calculation performed, causes of subfertility, types of treatments, number of previous treatment cycles, exposure time to HA before embryo transfer, only fresh embryo transfers, method of pregnancy determination, raw data on clinical pregnancy, multiple pregnancy and implantation rates, number of embryos transferred, allocation concealment, blinding, length of follow‐up per participant, loss of participants, intention‐to‐treat analysis, no funding, number of treatment cycles per participant |
| Ben‐Rafael 1995 | Method of pregnancy determination, participant enrolment, number of previous treatment cycles, timing of randomisation, methods of randomisation and allocation concealment, no intention‐to‐treat analysis, no power calculation, length of follow‐up, no funding, one treatment cycle per participant |
| Dittmann‐Műller 2009 | Participant enrolment, no power calculation, participant age, subfertility causes, subfertility duration and number of previous treatment cycles, timing of randomisation, only fresh embryos transferred, no donor oocytes, method of pregnancy determination, number of embryos transferred, method of randomisation, length of follow‐up, blinding, no loss of participants, no intention‐to‐treat analysis, funding source, one treatment cycle per participant |
| Fancsovits 2011 | Method and frequency of participant enrolment, no power calculation, method of pregnancy demonstration, oocyte donation, raw data live birth rate, clinical pregnancy rate, implantation rate, number of transferred embryos, method of randomisation, method of blinding, length of follow‐up, lack of intention‐to‐treat analysis, no commercial funding |
| Friedler 2007 | Participant enrolment, ongoing pregnancy rate determination, length of follow‐up, no overlap in data with Friedler 2005, no intention‐to‐treat analysis, no funding, one treatment cycle per participant |
| Hazlett 2004 | Overlap in data with Hazlett 2005 and Hazlett 2008, participant enrolment, power calculation, number of embryos transferred, exposure time to HA before transfer, timing of randomisation, methods of allocation concealment and blinding, no intention‐to‐treat analysis performed |
| Hazlett 2005 | Overlap in data with Hazlett 2004 and Hazlett 2008, participant enrolment, power calculation, raw data live birth, clinical pregnancy, multiple pregnancy and implantation rate, number of participants, participant age, method of embryo selection for day three or five transfers, method of randomisation, allocation concealment and blinding, no intention to treat, number of treatment cycles per participant |
| Hazlett 2008 | Overlap in data with Hazlett 2004 and Hazlett 2005, participant enrolment, live birth rate data, length of follow‐up, blinding, number of treatment cycles, no intention‐to‐treat analysis, no funding |
| Korošec 2007 | Participant enrolment, timing of randomisation, methods of allocation concealment and blinding, raw data divided for fresh and frozen‐thawed embryo transfers, live birth rate in fresh embryo transfer group, number of treatment cycles per participant, no intention‐to‐treat analysis, no funding |
| Morbeck 2007 | Participant enrolment, power calculation performed, participant age, number of participants, number of embryos transferred, number of participant exclusions, number of donor oocytes, timing of randomisation, exposure time to HA before transfer, method of pregnancy determination, raw data on live birth, clinical pregnancy and implantation rates, methods of randomisation and allocation concealment, blinding, length of follow‐up, number of treatment cycles per participant |
| Simon 2003 | Participant enrolment, no power calculation performed, methods of allocation concealment and blinding, definition of ongoing pregnancy rate, data implantation rate, length of follow‐up, no intention‐to‐treat analysis, no funding, one treatment cycle per participant |
Data and analyses
1.
High versus low or no hyaluronic acid
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 High versus low or no hyaluronic acid | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 1.2 High versus low hyaluronic acid | 4 | 1626 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.16, 1.73] |
| 1.3 High versus no hyaluronic acid | 3 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.86, 2.12] |
| 2 Live birth rate (grouped by timing of intervention) | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.18, 1.71] |
| 2.1 Early transfers | 5 | 1350 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.10, 1.72] |
| 2.2 Late transfers | 3 | 600 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.11, 2.15] |
| 3 Live birth rate (grouped by frozen‐thawed or fresh embryos) | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 3.1 Frozen‐thawed embryos | 2 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.52, 1.80] |
| 3.2 Fresh embryos | 4 | 1787 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.20, 1.76] |
| 4 Live birth rate (grouped by oocyte donation) | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 4.1 Donor oocytes | 1 | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.08, 5.88] |
| 4.2 Non‐donor oocytes | 6 | 1935 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.18, 1.70] |
| 5 Live birth rate (grouped by exposure time to HA) | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 5.1 Exposure time ≤ 10 minutes | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.82, 2.30] |
| 5.2 Exposure time > 10 minutes | 4 | 1670 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.16, 1.71] |
| 6 Live birth rate (grouped by embryo transfer policy) | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 6.1 Single embryo transfer | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.54, 3.68] |
| 6.2 Multiple embryo transfer | 5 | 1868 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 7 Live birth rate (grouped by participant prognosis) | 6 | 1950 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.17, 1.69] |
| 7.1 Good prognosis | 4 | 468 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.81, 1.70] |
| 7.2 Unselected | 2 | 1482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.21, 1.84] |
| 8 Clinical pregnancy rate | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 High versus low or no hyaluronic acid | 14 | 3452 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.21, 1.60] |
| 8.2 High versus low hyaluronic acid | 9 | 2566 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.08, 1.48] |
| 8.3 High versus no hyaluronic acid | 6 | 886 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.46, 2.67] |
| 9 Clinical pregnancy rate (grouped by timing of intervention) | 13 | 3304 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.24, 1.66] |
| 9.1 Early transfers | 11 | 2104 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.27, 1.81] |
| 9.2 Late transfers | 4 | 1200 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.01, 1.66] |
| 10 Clinical pregnancy rate (grouped by frozen‐thawed or fresh embryos) | 12 | 3090 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.12, 1.51] |
| 10.1 Frozen‐thawed embryos | 4 | 506 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.69] |
| 10.2 Fresh embryos | 9 | 2584 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.13, 1.56] |
| 11 Clinical pregnancy rate (grouped by oocyte donation) | 7 | 2145 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.09, 1.53] |
| 11.1 Donor oocytes | 2 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.43, 4.79] |
| 11.2 Non‐donor oocytes | 7 | 2096 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.09, 1.53] |
| 12 Clinical pregnancy rate (grouped by exposure time to HA before transfer) | 11 | 2988 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.16, 1.56] |
| 12.1 Exposure time ≤ 10 minutes | 5 | 616 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.18, 2.31] |
| 12.2 Exposure time > 10 minutes | 6 | 2372 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.08, 1.51] |
| 13 Clinical pregnancy rate (grouped by participant prognosis) | 14 | 3452 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.21, 1.60] |
| 13.1 Poor prognosis | 2 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.53 [2.54, 8.10] |
| 13.2 Good prognosis | 5 | 742 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.88, 1.66] |
| 13.3 Unselected participants | 7 | 2422 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.10, 1.53] |
| 14 Clinical pregnancy rate (grouped by embryo transfer policy) | 14 | 3452 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.21, 1.60] |
| 14.1 Single embryo transfer | 1 | 296 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.67, 2.09] |
| 14.2 Multiple embryo transfer | 13 | 3156 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.22, 1.63] |
| 15 Multiple pregnancy rate | 5 | 1951 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.49, 2.31] |
| 16 Adverse event rate | 4 | 1525 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.12] |
| 17 Implantation rate | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
2.
Fibrin sealant versus no fibrin sealant
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical pregnancy rate (per randomly assigned couple) | 1 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.54, 1.78] |
| 2 Adverse event rate (per randomly assigned couple) | 1 | 211 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.55 [0.26, 117.06] |
| 3 Implantation rate (per embryos transferred) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel randomised controlled trial Prospective recruitment of participants Consecutive participant sampling Single‐centre trial at the VKV American Hospital in Istanbul, Turkey Inclusion criterion: blastocyst‐stage embryos No exclusion criteria No power calculation Participants were allowed in the trial with multiple treatment cycles Participants were enrolled for a period of four months, actual length of follow‐up per participant was four weeks. It was intentionally kept short to reduce the chance of loss of participants, of which none occurred An intention‐to‐treat analysis was performed |
|
| Participants | Age (years): treatment group 31.2, control group 31.6. No SD given Primary or secondary subfertility: not reported Causes of subfertility: 1. Male factor: treatment group 129, control group 121; 2. Unexplained subfertility: 35 treatments, 30 controls; 3. Endometriosis: three treatments, five controls, More than one factor: 26 treatments, 37 controls Mean duration subfertility (years): treatment group 2.9, control group 3.2 Previous IVF and/or ICSI treatment (mean): treatment group 2.9, control group 3.2 All participants underwent ICSI. No IVF No age analysis 386 blastocyst‐stage transfers were recruited and randomly assigned: 193 to treatment group and 193 to control group. 405 embryos were transferred in treatment group and 424 in control group, resulting in a total of 829 transferred embryos. The total number of treatment cycles is unclear because participants were able to enrol multiple times. No loss, so the results of 386 participants were analysed |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus embryo transfer in G2.3 (0.125 mg/ml HA). All embryos were cultured in G‐III series culture medium. Both culture and transfer media were manufactured by Vitrolife. EmbryoGlue was provided by the American Hospital of Istanbul Randomisation on day of embryo transfer Embryos in treatment group were exposed to EmbryoGlue for 30 minutes before transfer Timing of embryo transfer: late in embryo development (day five) Oocyte donation was unclear All transferred embryos were fresh, no frozen‐thaw protocol followed Mean number of embryos transferred: treatment group 2.1, control group 2.1 Pregnancy determination: demonstration of gestational sac on ultrasound scan |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Abstract of ASRM conference presentation; no full article has been published regarding this trial Additional data retrieved after study authors were contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation into treatment or control group was performed using a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Randomisation list was maintained by the chief embryologist, who did not participate in daily laboratory work. The embryologist preparing the transfer was given allocation information immediately before actual transfer |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinician and participant were blinded, the scientist was not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Live birth rate was not reported, actual length of follow‐up was four weeks, which was done intentionally. Intention‐to‐treat analysis was performed, yet no loss of participants was reported |
| Selective reporting (reporting bias) | Low risk | Outcomes were prespecified in Materials and Methods section |
| Other bias | Low risk | EmbryoGlue was provided by the American Hospital. Transfer media used in both arms of the trial were comparable except for the addition of EmbryoGlue in the treatment arm. Multiple pregnancy rate was reported |
| Methods | Same as Urman 2008 | |
| Participants | Same as Urman 2008 | |
| Interventions | Same as Urman 2008 | |
| Outcomes | Live birth rate: reported as the take‐home baby rate and defined as the number of live births divided by the number of participants | |
| Notes | Update of live birth rate data resulting from clinical pregnancy rate was reported in Urman 2008, which did not use the live birth rate as an endpoint itself. No new inclusion of participants. Live births reported in the publication of Balaban 2011 will be extracted in this meta‐analysis under Urman 2008 Conference proceeding at 27th Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE) in Stockholm, Sweden |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Same as Urman 2008. Participants were randomly assigned to treatment or control group using a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Same as Urman 2008. Allocation to study arm was provided after opening of consecutively numbered, sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Same as Urman 2008. Both clinician and participant were blinded to the group to which the participant was allocated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Live birth rate was reported; follow‐up was long enough, and data were analysed according to the intention‐to‐treat principle |
| Selective reporting (reporting bias) | Low risk | Live birth rate was reported in a prespecified manner |
| Other bias | Low risk | Same as Urman 2008 |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Consecutive participant sampling Multi‐centre trial in the Hasharon Hospital of the Rabin Medical Center and in the Sackler School of Medicine of Tel Aviv University in Israel Inclusion criteria: at least three embryos ready for transfer and no more than three previous treatment cycles Exclusion criteria: fewer than three embryos ready for transfer No power calculation was performed Participants were included in the trial with only one treatment cycle Participants were recruited over a period of six months Length of follow‐up per participant was until delivery No intention‐to‐treat analysis was performed Study was not supported by any commercial funding sources |
|
| Participants | Age (years): mean 34.2, SD 4.9 Patients were admitted for oocyte retrieval if two or more follicles of at least 18 mm mean diameter were present and the hormone profile was satisfactory Not reported whether primary or secondary subfertility Causes of subfertility were mechanical, male subfertility and combined causes. Duration of subfertility ranged from three to 21 years (mean 8.3 ± 6.9) Previous IVF or ICSI was not reported, although participants could have had no more than three previous cycles Participants underwent IVF Age analysis: subgroups of < 31 years, 31 to 38 years and 39 to 42 years 211 patients who were admitted to the IVF unit were recruited for the trial, and all were randomly assigned: 101 to the treatment group and 110 to the control group. In total, 759 embryos were transferred: 368 in the treatment group and 391 in the control group. No participants were lost to follow‐up, so 211 participants were analysed |
|
| Interventions | Embryo transfer using a two‐component fibrin sealant versus transfer in regular medium, consisting of EBSS‐P‐SR2 with 10 mg/ml human serum albumin, manufactured by MediCult. The fibrin sealant was made of two components. The first consisted of fibrinogen, fibronectin and an aprotinin solution. The second component consisted of thrombin and a calcium chloride solution The sealant was manufactured by Immuno AG Randomisation was performed between fertilisation check and day of embryo transfer Exposure time to fibrin sealant was not stated Timing of transfer was early in embryo development, 48 to 50 hours after oocyte retrieval Inclusion of oocyte donations was unclear Unclear whether embryos were frozen‐thawed or fresh Two different culture and transfer medium brands: MediCult (culture medium and transfer medium control group) and Immuno AG (treatment group) Mean number of embryos transferred: treatment group 3.64, control group 3.55 Method of pregnancy determination: demonstration of gestational sac on ultrasound scan |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Additional data were retrieved after contact with the original investigators, although raw data such as numbers of multiple pregnancies and live births could no longer be retraced | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into treatment and control groups, method of randomisation was not stated |
| Allocation concealment (selection bias) | Unclear risk | Centralised randomisation by lab technician, who decided who would go into treatment or control group, unclear how this decision was made |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both participant and doctor were blinded. Embryologist was not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported, even though length of follow‐up was until delivery. No intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | Proposed results were not prespecified in Materials and Methods section |
| Other bias | High risk | No commercial funding. Different transfer media brands were used in both arms of the trial. No multiple pregnancy rate was reported, although multiple embryos have been replaced in each treatment cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Participant sampling unclear Single‐centre trial performed at the IVF‐Unit of Dr Tsai and Dr Chen's Women Hospital in Chang‐Hua, Taiwan Inclusion criterion: patients undergoing IVF/ET who were having a day three embryo transfer No exclusion criteria Unclear whether a power calculation was performed Participants were followed for 14 days after embryo transfer Unclear whether participants were able to participate in multiple treatment cycles Unclear whether intention‐to‐treat analysis was performed, but no mention was made of loss to follow‐up. Length of follow‐up was 14 days |
|
| Participants | Mean age (range): treatment group 31.35 (22 to 43), control group 32.47 (23 to 40) years Primary or secondary subfertility not reported Cause and duration of subfertility not reported Participants underwent IVF. Whether they had been through previous IVF treatments was not reported No age analysis 70 participants were recruited and were randomly assigned to two groups: 35 to the treatment group and 35 to the control group. The exact number of embryos transferred is unclear. No loss of participants occurred, so the number of participants analysed was 70 |
|
| Interventions | Embryo transfer in basal XI HTF(=transfer medium) with 10% human serum albumin (HSA) and 0.125 mg/ml HA versus transfer in basal XI HTF with 10% HSA Exposure time to HA before transfer was not stated Timing of randomisation was unclear, but it most likely occurred on day of embryo transfer because of the inclusion criterion of day three transfers Embryo transfer was performed early in embryo development (day three) Frozen‐thaw protocol unclear Oocyte donation unclear Culture and transfer medium brands not stated; however, medium appears to be similar between treatment and control groups, except for the addition of HA to treatment group Mean number of embryos transferred (range): treatment group 2.71 (2 to 5), control group 3 (1 to 5) Pregnancy was determined via a pregnancy test |
|
| Outcomes | Other outcomes
|
|
| Notes | Abstract of ESHRE conference presentation. Authors cannot be found to provide additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to study or control group, but method of randomisation was unclear |
| Allocation concealment (selection bias) | Unclear risk | Concealment of participant's allocation was not clear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated in text |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live birth rate reported, length of follow‐up was 14 days. Loss to follow‐up is unclear, just as whether an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Outcome of biochemical pregnancy test was announced in Methods section |
| Other bias | High risk | Commercial funding source unclear. Transfer media used in both arms of the trial were comparable except for the addition of HA to the treatment arm. No multiple pregnancy rate was reported, and multiple embryos were replaced per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Consecutive participant sampling Multi‐centre study performed at the IVF Unit of the Women's Hospital in Chemnitz, Germany, the IVF/ICSI Centre in Basel, Switzerland, and the Department of Obstetrics and Gynecology of the University Hospital in Würzburg, Germany Inclusion criterion: undergoing IVF or ICSI between January 2006 and March 2007. No further inclusion criteria No exclusion criteria No power calculation was performed Actual length of follow‐up per participant was four weeks after embryo transfer. Participants were enrolled in the trial between January 2006 and March 2007 and could participate in only one treatment cycle Study was commercially funded by Vitrolife No intention‐to‐treat analysis was performed |
|
| Participants | Mean age (SD): treatment group 33.4 (4.0), control group 33.6 (4.4) years Primary or secondary subfertility not reported Mean duration of subfertility was 4 ± 2.4 years. Indications for subfertility treatment were tubal factors (21.6%), andrologic factors (69.6%), cycle abnormalities (1%), others (12.7%) and idiopathic causes (17.6%). Some participants had multiple causes Participants underwent both IVF and ICSI; most participants participated for the first time, but some were already in their third (or above) treatment cycle No age analysis was performed 102 participants were recruited and were randomly assigned to a treatment group of 54 or a control group of 48. No loss of participants was reported, so the data on 102 participants were analysed. The exact number of embryos transferred is unclear |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus transfer in G‐2 (0.125 mg/ml HA). Embryos in both groups were cultured in G‐1 and G‐2 version 3 plus, supplemented with 10% recombinant human albumin Randomisation was performed on the day before oocyte pick‐up, which is between commencement of treatment and fertilisation check Exposure time to EmbryoGlue (=higher concentration of HA) before transfer was 30 minutes Transfer was performed early in embryo development, on day three All transferred embryos were fresh No oocyte donations were included in the trial Both culture and transfer media were manufactured by Vitrolife Mean number of embryos transferred (of both treatment and control groups) was 2.7 Pregnancy was determined by demonstration of gestational sac on ultrasound scan |
|
| Outcomes | Secondary outcomes
|
|
| Notes | Trial presented at ESHRE Conference. A full article is planned. Additional unpublished data received after contact with original investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment or control group with the use of a cube. Even numbers formed the treatment arm, odd numbers the control arm |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both participants and clinician were blinded to treatment. Scientist was not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported. Length of follow‐up per participant was four weeks post ET. No intention‐to‐treat analysis was performed, yet no loss of participants was reported |
| Selective reporting (reporting bias) | Low risk | In Materials and Methods section, it was announced that pregnancy rates will be recorded, and they are accounted for in the Results section |
| Other bias | High risk | Trial was commercially funded. Transfer media used in both arms of the trial were comparable except for the addition of EmbryoGlue to the treatment arm. Multiple pregnancy rate was reported |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Consecutive participant sampling Single‐centre study performed at the Semmelweis University School of Medicine in Budapest, Hungary No strict inclusion or exclusion criteria No power calculation was performed Actual length of follow‐up per participant was four weeks after embryo transfer. Participants were enrolled in the trial between January 2006 and March 2007 and could participate in only one treatment cycle Study was commercially funded by Vitrolife No intention‐to‐treat analysis was performed |
|
| Participants | Mean age (SD): treatment group 35.7 (4.1), control group: 34.2 (4.6) years Type, cause and duration of subfertility not reported Number of previous IVF or ICSI treatments not reported Included participants could undergo IVF or ICSI for the trial An age analysis was performed; outcome data from participants 40 years of age or younger were compared with those from participants over 40 years of age 200 cycles were randomly assigned to a treatment group of 103 and a control group of 97. The total number of transferred embryos was 467: 238 in the treatment group and 229 in the control group |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus transfer in G‐2 (0.125 mg/ml HA). Embryos were incubated in EmbryoGlue or control medium for five to 10 minutes before transfer. Embryos in both groups were cultured in G‐1 and G‐2 until two to three days after fertilisation. The timing of the intervention was therefore early in embryo development. Randomisation was performed one day before embryo transfer. All culture and transfer media were manufactured by the same company—Vitrolife. All embryos were fresh, and oocyte donations were not included. The mean number of transferred embryos was 2.3 (± 0.8) in the treatment group and 2.4 (± 0.7) in the control group. Pregnancy was demonstrated via hCG pregnancy test and demonstration of a gestational sac on ultrasound | |
| Outcomes | Primary outcomes
Secondary outcomes
Additional outcomes
|
|
| Notes | Conference abstract of a trial presented at ESHRE meeting in 2011. Additional data and study information were provided by the original investigator after contact was made with the authors of this review. See Appendix 8 In this trial, cycles instead of participants were randomly assigned; this is not compatible with data analysis that is part of this systematic review because of the possibility of participants enrolling with multiple cycles. However, after contact was made with the original investigator, it appeared that the number of multiple entries was less than 10% of the total number (seven in the treatment group and 12 in the control group), which was deemed acceptable by the review authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation into treatment or control group, with randomisation achieved by a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participant and clinician/nurse |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up was long enough Unclear whether any loss to follow‐up occurred No intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Outcome measures were reported in a prespecified fashion. Of note, certain outcomes such as the live birth rate were not reported in the original publication but only after contact was made with the trial authors |
| Other bias | High risk | No commercial funding Culture media/environment in treatment and control groups comparable Multiple pregnancy rate not reported whilst multiple embryo transfer policy was followed |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Consecutive participant sampling Single‐centre trial performed at the IVF and Infertility Unit of the Assaf Harofeh Medical Center of the University of Tel Aviv in Israel Inclusion criteria: Participants had to be younger than 43 years, undergoing IVF/ICSI and failed to achieve pregnancy after four previous embryo transfers Exclusion criteria: 43 years or older, more or fewer than four previous treatment cycles Unclear whether a power calculation was performed Actual length of follow‐up was unclear, yet it appeared to be long enough for outcome measures to be reported Participants were enrolled for only one treatment cycle (because of inclusion criterion of four previous attempts) Unclear whether an intention‐to‐treat analysis was performed, neither whether was any loss to follow‐up reported |
|
| Participants | Mean age (SD): treatment group 33.8 (4.97), control 33.8 (4.97) years Primary and/or secondary subfertility not reported Cause and duration of subfertility not reported Four previous IVF or ICSI treatments Trials in which participants were undergoing both IVF and ICSI were included No age analysis was performed 187 participants were recruited and randomly assigned to treatment group of 94 or control group of 93. No loss of participants was reported; therefore the data on 187 participants were analysed. Exact number of embryos transferred was unclear |
|
| Interventions | Embryo transfer with EmbryoGlue (0.5 mg/ml HA) versus transfer with HTF medium enriched with 20% serum substitute supplement (SSS) Timing of randomisation was unclear Exposure time to HA before transfer was not stated Transfer was performed early in embryo development (day two to four) Unclear whether a frozen‐thaw protocol was followed Unclear whether oocyte donations were included Transfer medium in treatment group was manufactured by Vitrolife; transfer medium for the control group was manufactured by Irvine Scientific Mean number of embryos transferred: treatment group 3.4 ± 1.05, control group 3.2 ± 1.05 Method of pregnancy determination was not reported |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Abstract of an ESHRE conference presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to treatment or control group, but method of randomisation was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported, actual length of follow‐up per participant was unclear, participant loss and intention‐to‐treat analysis were unclear |
| Selective reporting (reporting bias) | Low risk | Outcome measures were reported in a prespecified way |
| Other bias | High risk | Commercial funding source was unclear. Transfer media of treatment and control groups were made by different manufacturers. No multiple pregnancy rate was reported, and multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Consecutive participant sampling Single‐centre trial performed at the IVF and Infertility Unit of the Assaf Harofeh Medical Center, Sackler School of Medicine, University of Tel Aviv, Israel Inclusion criteria: patients who failed to achieve an ongoing pregnancy after more than four previous embryo transfers, during which two to four embryos were transferred each time, including at least one optimal embryo. Had to be younger than 43 years of age and had to have given informed consent. Undergoing ICSI at the IVF Unit Exclusion criteria: patients older than 43 years of age, suffering from a systemic disease, BMI > 29 kg/m2, uterine malformation, evidence of low ovarian response, elevated baseline FSH (> 12 IU/L), hydrosalpinx or participation in any other clinical study Power calculation performed but not followed. Group size of 112 in each arm of the study was proposed, but after an interim analysis of 101 participants, the trial was stopped. This was done for ethical reasons, and the study is therefore eligible Participants were enrolled in the trial from June 2005 to November 2006. Actual length of follow‐up per participant was up to nine months. However, one of the outcome measures of the study was delivered or ongoing pregnancy rate Participants were able to enrol in the study with only one single cycle An intention‐to‐treat analysis was not performed Study was free from commercial funding |
|
| Participants | Mean age (SD): treatment group 33.1 (5.1), control group 31.7 (5.6) years Not reported whether study concerned primary or secondary subfertility Causes of subfertility included male factor, tubal factor, endometriosis, unexplained subfertility and combination of female and male factors Duration of subfertility was not reported Participants had to have undergone at least four previous treatment cycles. Average was 5.5 previous unsuccessful embryo transfers All participants underwent ICSI No age analysis was performed 101 participants were recruited and randomly assigned to a treatment group of 51 or a control group of 50. 159 embryos were transferred in the treatment group and 146 in the control group, resulting in a total of 305 embryos transferred. No participants were excluded, withdrawn or lost to follow‐up, so data on 101 participants were analysed |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA and 2.5 mg/ml recombinant human albumin) versus transfer in human tubal fluid (HTF) with gentamycin, enriched with 20% serum substitute supplement Randomisation on day of embryo transfer Exposure time to HA before embryo transfer was 10 minutes Transfer was performed early in embryo development (days two and three) No frozen‐thaw protocol was followed Unclear whether oocyte donations were included Transfer media of treatment and control groups were manufactured by two different companies: treatment medium by Vitrolife and control medium by Irvine Scientific Mean number of embryos transferred: treatment group 3.1 ± 0.73, control group 2.9 ± 0.63 Pregnancy was determined by demonstration of gestational sac on ultrasound scan and pregnancy test |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | No overlap in participants with Friedler 2005 Additional data retrieved after contact was made with study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment or control group based on computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Participant allocation was performed by the chief embryologist just before embryo transfer, according to a computer‐generated random number sequence |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both participant and clinician were blinded. The embryologist was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported, even though the actual length of follow‐up per participant was up to nine months. No loss of participants, therefore not accounted for. No intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate was announced in the Methods section. However, many other outcomes were reported in the Results section |
| Other bias | Unclear risk | Free from commercial funding. Embryos in treatment and control groups were transferred in media from different manufacturers, and similarity between culture media was unclear. Multiple pregnancy rate was reported |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Participant sampling in a non‐consecutive order Single‐centre trial performed at the Department of Embryology of Karande and Associates in Hoffman Estates, Illinois, USA No inclusion or exclusion criteria Power calculation was performed Length of follow‐up per participant was four to six weeks post–positive hCG test. No intention‐to‐treat analysis was performed It is unclear whether participants were able to enrol in multiple treatment cycles in this trial, but because they were included in the bigger trial that followed this trial, it can be assumed that this also occurred in the current trial |
|
| Participants | Mean age (SD): treatment group 34.0 (4.5), control group 33.1 (5.1) years Causes, duration and whether primary or secondary subfertility not reported Whether participants have undergone previous IVF/ICSI treatments was unclear Included participants underwent both IVF and ICSI 94 participants were recruited for this trial and were randomly assigned to a treatment group of 46 or a control group of 48. No loss of participants occurred, so the data on 94 participants have been analysed 266 embryos were transferred in the treatment group and 253 in the control group, resulting in a total of 519 transferred embryos. These data were retrieved after the original authors were contacted. However, these numbers are not comparable with the mean numbers given in the abstract, which states that a mean number of 2.4 ± 0.8 embryos were transferred in the treatment group. These numbers reflect the number of embryos transferred in the study that followed from this trial (Hazlett 2008) |
|
| Interventions | Embryo transfer on day three and day five of embryo development in EmbryoGlue (0.5 mg/ml HA) versus transfer in IVC‐1 on day three and transfer in G2.3 (0.125 mg/ml HA) on day five Embryos were cultured in IVC‐1 up to day three and in G2.3 from day three to day five Randomisation was performed from fertilisation check to embryo transfer, but it is unclear whether it occurred on the day of embryo transfer itself Exposure time to EmbryoGlue before transfer was 10 to 60 minutes No frozen‐thaw protocol was followed Only the participants' own oocytes were fertilised, so no donor oocytes were included The manufacturers of the culture and transfer media were In Vitro Care and Vitrolife Mean number of embryos transferred: treatment group 2.4 ± 0.8, control group 2.4 ± 0.9 Methods of pregnancy determination: hCG pregnancy test and sonogram to determine gestational sac and fetal heartbeat |
|
| Outcomes | Secondary outcomes
Additional outcomes
Other outcomes
|
|
| Notes | Abstract from ESHRE conference presentation Data from this trial have been incorporated in a bigger trial (Hazlett 2008); therefore outcome data will be extracted only from Hazlett 2008 Additional data retrieved after study authors were contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to treatment or control group based on computerised allocation system, information retrieved after contact with authors |
| Allocation concealment (selection bias) | Low risk | Computerised allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant, clinician/nurse and embryologist were all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No live births were reported, no intention‐to‐treat analysis was performed. However, no loss of participants was reported. Live births were retrieved after contact with study author regarding Hazlett 2008 |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported in a prespecified way |
| Other bias | High risk | Trial was free of commercial funding. Data for the day three and day five control groups are not comparable because transfers were performed in media of different media brands. No multiple pregnancy rates were reported, and multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Participant sampling non‐consecutive Single‐centre study performed at Karande and Associates in Hoffman Estates, Illinois, USA Non‐donor, normal and low‐responding IVF patients were included in the study Power calculation was performed, which estimated that a 20% difference in clinical pregnancy rate would be found, with 5% significance and 80% power if at least 107 participants were included in each arm of the study Length of follow‐up appears to be nine weeks of gestation Participants were able to partake in multiple treatment cycles in case of treatment failure Intention‐to‐treat analysis was not performed |
|
| Participants | Mean age: treatment group 32.4, control group 33.3 years Cause, duration and primary or secondary subfertility not reported Included participants were undergoing IVF or ICSI. Unclear whether participants had undergone previous treatments No age analysis was reported. Participants were divided into groups of normal and low responders, based on concentration of gonadotrophin received daily by participants 223 participants were randomly assigned to a treatment group of 116 and a control group of 107. The treatment group was divided into 84 participants who had a day three transfer and 32 participants who had a day five transfer. The control group comprised 78 day three transfers and 29 day five transfers The total number of transferred embryos was 519: 266 in the treatment group and 253 in the control group Participant data were clarified by contacting study author |
|
| Interventions | Embryo transfers on day three or day five of development in EmbryoGlue (0.5 mg/ml HA) versus transfer in IVC‐1 or IVC‐2 plus 0.5% HSA (human serum albumin) on day three and G2.3 plus 0.5% HSA on day 5 Embryos were cultured in IVC‐1 or IVC‐2 until day three, and in G2.3 plus 0.5% HSA until day five Randomisation took place on the day before transfer Embryos in the treatment group were exposed to EmbryoGlue for 10 to 60 minutes before transfer Unclear whether all embryos were fresh or frozen‐thawed Oocyte donations were not included Culture and transfer media were manufactured by different companies: In Vitro Care and Vitrolife Mean number of embryos transferred not provided in printed text Pregnancy determination by hCG pregnancy test and demonstration of gestational sac and fetal heartbeat on ultrasound scan |
|
| Outcomes | Primary outcomes
Secondary outcomes
Additional outcomes
Other outcomes
|
|
| Notes | Abstract of ASRM conference presentation Data from this trial were also published in the study Hazlett 2008; therefore, outcome data will be extracted only from Hazlett 2008 Additional data were retrieved by contacting study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment and control groups by computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computerised allocation with sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and clinicians were blinded. Scientist were not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Length of follow‐up appears to be nine weeks, and no intention‐to‐treat analysis was stated. No loss was accounted for. Live births were retrieved by contacting study author regarding Hazlett 2008 |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported in a prespecified way |
| Other bias | High risk | No commercial funding source. Embryos in treatment and control groups were transferred in media from different brands, even within the control group. Embryo transfer policy was unreadable; therefore, no conclusions can be made about the multiple pregnancy rate |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Participant sampling in consecutive order but stated as non‐consecutive in Hazlett 2005 Single‐centre trial performed at the Department of Embryology of Karande and Associates in Hoffman Estates, Illinois, USA Patients who were excluded were diagnosed as having a low success rate, which meant having a diminished ovarian reserve (FSH of at least 10 IU/ml), being older than 40 years of age or having a hydrosalpinx Participants were selected for a day five embryo transfer if a minimum of five embryos with little fragmentation were present on day three and/or if they had eight or more fertilised zygotes A power calculation was performed, which estimated that a 20% difference in clinical pregnancy rate would be found, with 5% significance and 80% power if at least 107 participants were included in each arm of the study Length of follow‐up per participant was 11 weeks No intention‐to‐treat analysis was performed Participants were able to enrol in the study with multiple treatment cycles in case of treatment failure |
|
| Participants | Mean age (SD): Participants were divided into day three and day five transfer subgroups. Treatment groups: day three, 33.4 (4.4), day five, 31.4 (4.2) years; control groups: day three, 33.4 (5.0), day five, 33.1 (4.8) years Causes and duration of subfertility not reported. Nor was it reported whether study concerned primary or secondary subfertility. Yet text states that there were no differences between treatment and control groups regarding cause and duration Included participants underwent IVF or ICSI. Unclear whether they underwent previous treatments No age analysis 233 participants appear to be recruited, even though only 224 were stated in the text. Of 233 participants, 223 were randomly assigned to a treatment group of 116 or a control group of 107 Five participants were part of a preliminary study and were not randomly assigned. Five others were withdrawn for protocol violations. The treatment group was divided into 84 participants who had a day three transfer and 32 who had a day five transfer. The control group comprised 78 day three transfers and 29 day five transfers The total number of transferred embryos was 519: 266 in the treatment group and 253 in the control group |
|
| Interventions | Embryo transfer on day three or day five of development in EmbryoGlue (0.5 mg/ml HA) versus transfer in IVC‐1 or IVC‐2 + 0.5% HSA (human serum albumin) on day three or G2.3 (0.125 mg/ml HA) + 0.5% HSA on day five. Therefore, for data analysis, this trial is divided into day three transfers, which compare HA versus no HA, and day five transfers, which compare HA versus low concentrations of HA Embryos were cultured in IVC‐1 or IVC‐2 until day three and in G2.3 for the additional two days Timing of randomisation was unclear. It appears that it occurred on the day before embryo transfer, for it is stated this was in the trial Hazlett 2005, which comprises data on the same participants Exposure time to EmbryoGlue in the treatment group was 10 to 60 minutes before embryo transfer No frozen‐thaw protocol was followed No donor oocytes were included Transfer and culture media were manufactured by two different manufacturers: In Vitro Care and Vitrolife Mean number of transferred embryos: treatment group day three: 2.5 ± 0.9, day five: 2.1 ± 0.5; control group day three: 2.4 ± 0.8, day five: 2.1 ± 0.9 Pregnancy was determined via pregnancy tests and demonstration of gestational sac and fetal heartbeat on ultrasound scan |
|
| Outcomes | Primary outcomes
Secondary outcomes
Additional outcomes
Other outcomes
|
|
| Notes | This study comprises data from previous publications (Hazlett 2004 and Hazlett 2005), but only outcome data have been extracted from it Additional data were retrieved by contacting study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment or control groups by a computer‐generated random numbers sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by computer‐generated random numbers sequence using sealed envelopes to allocate to the treatment arm |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participant and clinician/nurse were blinded. Embryologist was not |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Live births were reported after contact with study author. No intention‐to‐treat analysis was performed. Loss to follow‐up was accounted for. Actual length of follow‐up per participant in published study was 11 weeks; live birth data were retrieved later on |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate and implantation rate were reported in a prespecified way |
| Other bias | High risk | Free from commercial funding. Different media brands were used, therefore not comparable. No multiple pregnancy rate was reported, although multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Sampling unclear Single‐centre trial performed at IVF Michigan in Rochester Hills in the USA Participants with all types of subfertility diagnosis were included, but they had to be younger than 39 years of age Unclear whether a power calculation was performed Length of follow‐up per participant is unclear Unclear whether an intention‐to‐treat analysis was performed, or whether any loss to follow‐up occurred Unclear whether participants were able to enrol in the trial on multiple treatment cycles |
|
| Participants | Mean age (range): treatment group 32.7 (24 to 39), control group 33.8 (23 to 39) years Causes and duration of subfertility not specified. Nor was it stated whether study concerned primary or secondary subfertility Unclear whether participants underwent IVF or ICSI or both, and whether they had received previous treatments No age analysis was performed 165 participants were recruited for this trial and were randomly assigned to treatment and control groups. No mention in text of group sizes, nor of any loss to follow‐up or numbers of embryos transferred. Study authors were contacted, but no response has been received yet |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5% mg/ml HA) versus transfer in P1 Complete Medium (contains gentamycin, taurine and 10% protein supplement; no HA) All embryos were cultured in P1 Complete Medium Timing of randomisation was unclear Embryos in treatment group were exposed to EmbryoGlue for approximately 10 minutes before transfer Transfers were performed early in embryo development (day three) Unclear whether frozen‐thawed embryos were included in the trial Unclear whether donor oocytes were included All embryos were cultured in medium manufactured by Irvine Scientific, and control group was transferred in same medium; treatment group was transferred in EmbryoGlue manufactured by Vitrolife Mean number of embryos transferred: treatment group 3.3, control group 3.1 Method of pregnancy determination was not reported |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Abstract of an ESHRE conference presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment or control group, but method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear whether participants, clinicians and/or embryologists were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Length of follow‐up unclear. No live births reported. No information on actual participant numbers or loss. No intention‐to‐treat analysis reported |
| Selective reporting (reporting bias) | Low risk | Ongoing pregnancy and implantation rate were reported in a prespecified way |
| Other bias | High risk | Commercial funding source was unclear. Different transfer media brands were used. However, culture and transfer media for both arms were comparable, with the exception of EmbryoGlue added to the medium in the treatment arm. No multiple pregnancy rate was reported, although multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment and consecutive sampling Multi‐centre trial performed at the Department of Obstetrics and Gynecology of the University Medical Centre of Ljubljana, Slovenia, and at the Institute for Reproductive Medicine and Endocrinology in Bregenz, Austria Inclusion criteria: Women had to be younger than 37 years of age and within their first three treatment cycles, resulting in selection of twin‐prone women A power calculation that estimated 80% power was performed according to preliminary results before the entire study population was randomly assigned Length of follow‐up per participant was 30 days. However, a subgroup of participants undergoing a fresh embryo transfer was checked for live births a year later. These data have not been published No intention‐to‐treat analysis was performed Participants were able to enrol in the study with multiple cycles |
|
| Participants | 328 women were recruited for this study; it included a group of women who had received a single fresh embryo transfer and a group who had received a single frozen‐thawed embryo transfer. Participants were randomly assigned to a treatment group of 138 and a control group of 158. 32 women declined participation after randomisation was carried out. Because only a single embryo was transferred per woman, 296 (138 + 158) transfers were performed and analysed. The tables in the Results section of the article present only the data on 279 transfers, but in the text, the data on 17 women who had received a compulsory single transfer were reported Mean age (SD): treatment group fresh 31.3 (3.7), frozen‐thawed 32.1 (3.5); control group fresh 31.9 (3.7), frozen‐thawed 32.7 (3.2) Causes of subfertility included tubal factor, endometriosis, endocrine disorders, idiopathic causes, male factors, combined female factors and combined female and male factors Duration of subfertility was not reported, nor whether primary and/or secondary subfertility was present Included women underwent IVF or ICSI and could have received up to two previous treatments No age analysis was performed |
|
| Interventions | Fresh and frozen‐thawed embryo transfers in EmbryoGlue (0.5 mg/ml HA) versus fresh and frozen‐thawed transfers in M2 medium Embryos were cultured to the blastocyst stage sequentially in M1 and M2 culture medium, which contains no HA Randomisation was performed on the day of embryo transfer Embryos in the treatment group were exposed to HA for at least four hours Transfers were performed in the blastocyst stage of embryo development, which occurs on day five Donor oocyte inclusion was unclear Culture and transfer media were manufactured by MediCult and Vitrolife Only one embryo was transferred per cycle Pregnancy was determined by hCG pregnancy test, and gestational sacs and fetal heartbeat were demonstrated on ultrasound scan |
|
| Outcomes | Primary outcomes
Secondary outcomes
Other outcomes
|
|
| Notes | Additional data were retrieved by contacting study authors. Important note: Only single embryos were transferred, and no multiple pregnancies occurred | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to a treatment or control group by a computerised randomisation table |
| Allocation concealment (selection bias) | High risk | Allocation was performed at the site by the investigator just before the intervention was provided, according to the computerised randomisation table. Information was retrieved by contacting the study author |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinician and participants were blinded, the scientist was not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Live birth rate was reported when original investigators were contacted, but it was not recorded for the entire study group. Loss to follow‐up was accounted for, but no intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rates were reported in a prespecified way. Live birth rate was retrieved by study author contact |
| Other bias | Low risk | The trial was free from commercial funding. Different transfer media brands were used in treatment and control groups, but culture media were comparable up to the moment of embryo transfer. No multiple pregnancy rate was reported, but only singleton embryo transfers |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Participant sampling unclear Multi‐centre trial performed at the Department of Obstetrics and Gynaecology of the Afzallipour Hospital, Kerman University of Medical Sciences, in Kerman, Iran, and the Research and Clinical Centre for Infertility of the Sadoughi University of Medical Sciences in Yazd, Iran Inclusion criteria: 35 years of age or younger, at least three embryos suitable for transfer and no previous IVF/ICSI cycles Unclear whether a power calculation was performed Participants were included from September 2003 to January 2004, length of follow‐up per participant appears to be 10 weeks Unclear whether participants could enrol with multiple treatment cycles Unclear whether an intention‐to‐treat analysis was performed; no mention of loss to follow‐up |
|
| Participants | 60 women were recruited and randomly assigned to a treatment group of 30 and a control group of 30. No loss of participants was reported, so data on all 60 women were analysed. 183 embryos were transferred: 85 in the treatment group and 98 in the control group Mean age (SD): treatment group 27.5 (4.26), control group 28.6 (3.68) years Causes of subfertility and whether it concerned primary or secondary subfertility not reported Mean duration of subfertility was 7.24 (3.68) years in treatment group and 6.93 (3.6) years in control group Both IVF and ICSI participants were included, but they could not have received previous treatment No age analysis was performed |
|
| Interventions | Embryo transfers in EmbryoGlue (0.5 mg/ml HA) versus transfers in standard medium containing 20% albumin Culture medium was not stated Randomisation occurred on day of embryo transfer Embryos in treatment group were exposed to HA for 10 minutes before transfer Transfer was performed on day three of embryo development All embryos were fresh, no frozen‐thaw protocol was followed Donor oocyte inclusion was unclear Transfer medium from treatment group was manufactured by Vitrolife, transfer medium from control group by Bayer Corporation Mean number of embryos transferred (SD): treatment group 2.68 (0.66), control group 2.7 (0.79) Pregnancy demonstrated by hCG pregnancy test 14 days after transfer; gestational sac and fetal viability were demonstrated on ultrasound scan |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Article was translated by Interlibrary Loans and Document Delivery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment or control group. However, method of randomisation was unclear |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both clinician and participant were blinded to treatment. The scientist was not |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported. No intention‐to‐treat analysis nor loss of participants was reported. Length of follow‐up appears to be 10 weeks |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy and implantation rates were reported in a prespecified way. Miscarriage was not announced but was added to the Results section |
| Other bias | High risk | Commercial funding source was unclear. Different transfer media brands were used for both study groups. However, it was unclear whether two different transfer media were used, or if EmbryoGlue was added to the same transfer medium used in the control group. No multiple pregnancy rate was reported, although multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Consecutive participant group sampling Single‐centre trial performed at the Mayo Clinic in Rochester, Minnesota, USA Inclusion criteria: frozen‐thawed embryo transfers; men over the age of 18 years and women from 18 to 42 years (if using their own oocytes and embryos frozen before 39 completed years) or from 18 to 50 years (if using donor oocytes) Exclusion criteria: participation in prior study. Blastocyst transfers. Single embryo transfers for medical reasons. Prior embryo transfer with large amount of blood on outside of the catheter. Three or more previous treatment failures A power calculation was performed; no further information Participants were enrolled in the study from May 2003 to June 2005. Length of follow‐up per participant was up to time of delivery Unclear whether an intention‐to‐treat analysis was performed Participants were allowed in the study with only one treatment cycle |
|
| Participants | 150 participants were scheduled to enrol in the trial, but only 121 were recruited. Of these, 38 were excluded (reasons unknown), resulting in 83 participants randomly assigned to a treatment group of 41 and a control group of 42. 92 embryos were transferred per group, resulting in a total of 184 embryos transferred Participant mean age (SD): treatment group 31.4 (3.8), control group 30.5 (4.2) years Causes, duration and kind of subfertility not reported Participants could not have received more than two previous treatment failures, but no further information on previous IVF/ICSI treatments was provided All participants appeared to undergo IVF; ICSI was not stated Age analysis: two sets: participants < 35 years versus ≥ 35 years, both in blocks of four |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus transfer in G2 culture medium (0.125 mg/ml HA). All embryos were cultured in G2 culture medium Randomisation was performed before commencement of the treatment cycle Treatment group was exposed to EmbryoGlue for an average of 15 minutes before transfer, with a range of 6 to 44 minutes (one transfer 62 minutes and another 131 minutes) Transfer was performed early in embryo development (day three) All embryos were frozen‐thawed Donor oocytes were included, but outcomes were not reported as a comparison between donor oocytes and non‐donor oocytes Culture and transfer media for both groups were manufactured by Vitrolife Mean number of transferred embryos per participant was 2.2 in both treatment and control groups (numbers calculated from unpublished data provided by the study author) Pregnancy was determined by fetal heartbeat monitoring and demonstration of gestational sac on ultrasound scan |
|
| Outcomes | Primary outcomes
Secondary outcomes
Additional outcomes
|
|
| Notes | From ClinicalTrial.gov This study was suspended because the implantation rate was significantly lower in the treatment group than in the control group. Outcome data originally were not published but were received by contacting the principal investigator |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment or control groups by a random numbers table |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was performed with sealed, opaque, numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both participant and clinician were blinded to treatment. The scientist was not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Live births were reported. Length of follow‐up was up to time of delivery. Outcomes originally were not reported because the study was suspended, but data were retrieved by contacting the original investigators. Unclear whether an intention‐to‐treat analysis was performed. No loss to follow‐up was apparent, apart from the 38 participants who were excluded, so loss of participants is accounted for |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy and implantation rates were prespecified. Ongoing pregnancy rate was reported when the original study author was contacted |
| Other bias | High risk | Trial was funded by and was performed at the Mayo Clinic, but this is not considered to be commercial funding. Transfer media in both study groups were comparable, with the exception of EmbryoGlue added to the medium in the treatment group. Multiple pregnancy was not reported, although multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Participant sampling unclear Single‐centre trial performed at the Edith Wolfson Medical Center in Holon, Israel Only fresh embryo transfers were included in the study Unclear whether a power calculation was performed Participants were enrolled in the study between July 2004 and November 2004, but the actual length of follow‐up was not reported An intention‐to‐treat analysis was not mentioned in the text, so unclear whether it was performed Unclear whether multiple treatment cycles per participant were included in the study |
|
| Participants | 148 participants were recruited and randomly assigned to a treatment group of 79 or a control group of 69. No loss to follow‐up was apparent, so the data on all 148 participants were analysed The number of embryos transferred was unclear, for only mean numbers per participant were given Mean age (SD): treatment group 34.8 (5.8), control group 34.3 (5.9) years Causes of subfertility and whether it concerned primary or secondary subfertility not reported Subfertility duration (SD): treatment group 3.9 (4.9) years, control group 3.6 (2.8) years All participants underwent IVF Number of previous cycles (SD): treatment group 4.5 (4.1), control group 5.4 (4.9) No age analysis was performed |
|
| Interventions | Fresh embryo transfers in EmbryoGlue (0.5 mg/ml HA) versus fresh transfers in G1 medium (0.125 mg/ml HA) All embryos were cultured in G1 medium Randomisation was performed on day of embryo transfer Exposure time to EmbryoGlue before transfer was not reported Timing of transfer during embryo development was not reported All embryos were fresh, no frozen‐thaw protocol was followed Inclusion of donor oocytes was unclear Culture and transfer media were manufactured by Vitrolife Mean number of embryos transferred per participant: treatment group 2.3 ± 0.8, control group 2.2 ± 0.8 Method of pregnancy determination was not reported |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Abstract of a ASRM conference presentation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to a treatment or a control group, but method of randomisation was unclear |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unclear whether participants, clinicians and/or scientists were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Actual length of follow‐up was unclear. No live births were reported. Loss to follow‐up was not accounted for, and it was unclear whether an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy and implantation rates were reported in a prespecified way |
| Other bias | High risk | No commercial funding. Same transfer media brand in treatment and control groups. Transfer media were comparable, with the addition of EmbryoGlue to the treatment group. No multiple pregnancy rate was reported, although multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Method of sampling of participants was unclear Single‐centre trial performed at the Colorado Center for Reproductive Medicine in Englewood, Colorado, USA Both IVF patients with their own oocytes and oocyte donors were included Unclear whether a power calculation was performed Participants were enrolled in this trial from January 2001 to February 2002. The exact length of follow‐up per participant was not stated, but it was long enough to permit measurement of the trial's proposed outcomes Unclear whether an intention‐to‐treat analysis was performed Unclear whether multiple treatment cycles or only one treatment cycle per participant were included in the trial Trial was supported by Vitrolife |
|
| Participants | A total of 175 IVF participants and oocyte donors were recruited for this trial. 141 of them were IVF patients, and 34 were oocyte donors. 91 participants were randomly assigned to the treatment group and 84 to the control group. No loss was reported, so the data on all 175 participants were analysed. Number of transferred embryos was unclear; only the mean number per group was published Age: not reported Not reported whether study concerned primary and/or secondary subfertility Cause and duration of subfertility not reported Trial studied only IVF participants, no participants receiving ICSI. Not reported whether participants had received previous IVF treatment No age analysis was reported |
|
| Interventions | Embryo transfer of participants' own or donated fertilised oocytes in G2.3 medium supplemented with EmbryoGlue (0.5 mg/ml HA) versus transfer in G2.3 medium (0.125 mg/ml HA) All embryos were cultured in G1.3 medium Timing of randomisation was unclear Embryos were exposed to the higher concentration of HA just before transfer Transfer was performed on day three of embryo development Donor oocytes were included Unclear whether embryos had to be fresh or if frozen‐thawed embryos were also included All culture and transfer media were manufactured by Vitrolife Mean number of embryos transferred: treatment IVF group 3.9, treatment donor oocytes 3.9; control IVF group 3.3, control donor oocytes 3.2 Pregnancy and implantation rates were determined by demonstration of fetal heartbeat |
|
| Outcomes | Secondary outcomes
Additional outcomes
|
|
| Notes | Abstract of ASRM conference presentation The primary author was contacted regarding unclear details in published abstract, but further participation was declined. So some uncertainty cannot be resolved |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation into treatment or control group by a computer‐generated randomisation sheet |
| Allocation concealment (selection bias) | Unclear risk | Allocation was correctly performed, but concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported. Length of follow‐up per participant was unclear. No intention‐to‐treat analysis was reported |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy and implantation rates were reported in a prespecified way |
| Other bias | High risk | Trial was commercially funded by Vitrolife. Same transfer media brand was used in treatment and control groups. Transfer media were comparable, with the addition of EmbryoGlue to the treatment group. No multiple pregnancy rate was reported, although multiple embryos were transferred per cycle |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Non‐consecutive group sampling Single‐centre trial performed at the IVF Unit of the Department of Obstetrics and Gynecology of the Hadassah University Hospital Ein Kerem in Jerusalem, Israel Inclusion criteria: women had to be 35 years of age or younger with at least three embryos suitable for transfer and three or fewer previous treatment failures No power calculation was performed, information was received by contacting the study author Participants were followed up until the pregnancy had ended No intention‐to‐treat analysis was performed Participants were enrolled in the study with only a single cycle The trial received no commercial funding |
|
| Participants | 80 participants were recruited and were randomly assigned to a treatment group of 40 or a control group of 40. No loss of participants was reported, so all were analysed A total of 200 embryos were transferred: 103 in the treatment group, 97 in the control group Mean age (SD): treatment group 28.7 (3.3), control group 29.7 (3.8) years Primary or secondary subfertility not reported Cause and duration of subfertility not reported Both IVF and ICSI cases were included. Participants could not have received more than three previous treatments No age analysis was performed |
|
| Interventions | Embryo transfers in culture medium were supplemented with 0.5 mg/ml HA versus transfer in culture medium Embryos were cultured in P1 medium containing 10% synthetic serum substitute (SSS) Embryos in treatment group were exposed to HA for five to 10 minutes before transfer Randomisation was performed on the day of embryo transfer Transfer was performed on day three of embryo development Contact with the study authors indicated that a frozen‐thaw protocol was followed No donor oocytes were included in the trial The P1 culture/transfer medium was manufactured by Irvine Scientific. The HA was manufactured by Biolon, Bio‐Technology Ltd Mean number of embryos transferred (SD): treatment group 2.6 (0.6), control group 2.4 (0.5) Methods of pregnancy determination: hCG pregnancy test, demonstration of a gestational sac on transvaginal ultrasound scan and determination of fetal viability (fetal heartbeat) on serial ultrasounds |
|
| Outcomes | Primary outcomes
Secondary outcomes
Additional outcomes
Other outcomes
|
|
| Notes | Additional data retrieved by contacting study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to treatment or control arm of the trial based on what was stated in a random sealed envelope. Actual method of randomisation was not clarified, but it appears to be correct |
| Allocation concealment (selection bias) | Low risk | A sealed envelope was drawn at the laboratory when a suitable participant arrived for transfer. According to what was stated on the envelope, the participant was allocated to either arm of the trial |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both clinician and participant were blinded to treatment received by participant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Live births were reported. Length of follow‐up was until pregnancy ended. No intention‐to‐treat analysis was performed. However, no loss of participants was reported, so there was no reason for such an analysis |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy and implantation rates were reported in a prespecified way. On top of this, live birth, ongoing pregnancy and multiple pregnancy were reported |
| Other bias | Low risk | Study received no commercial funding. Transfer media in both arms of the trial were comparable, with the exception of EmbryoGlue added to the medium in the treatment group. Multiple pregnancy rate was reported |
| Methods | Parallel randomised controlled trial Prospective patient recruitment Consecutive group sampling Single‐centre trial performed at the Assisted Reproduction Unit of the American Hospital of Istanbul, Turkey Participants with treatment cycles reaching embryo transfer were included in this study. The IVF/ICSI cycles had to be fresh and had to use the participant's own oocytes An a priori power calculation revealed that 537 participants would be necessary in each study group to detect a 15% increase in clinical pregnancy rate The maximum length of follow‐up per participant was 16 weeks, but the average length of follow‐up per participant was unclear All analyses were done according to the intention‐to‐treat principle Only one treatment cycle per participant was included in the trial |
|
| Participants | A total of 1282 couples undergoing IVF/ICSI were recruited and randomly assigned to a treatment group of 639 and a control group of 643. 825 of the 1282 received an embryo transfer on day three of embryo development and 457 on day five. No loss of participants was reported, so the data on 1282 couples were analysed. A total of 3487 embryos were transferred: 1718 in the treatment group and 1769 in the control group Mean age: treatment group 32.8, control group 32.9 years Primary and/or secondary subfertility not reported Causes of subfertility included male factor, ovarian, endometriosis, tubal factor and unexplained causes Mean duration of subfertility: treatment group 6.9 years, control group 7.2 years Participants underwent both IVF and ICSI Mean number of previous treatment cycles: treatment group 2.0, control group 2.1 Age analysis: women < 35 years versus women ≥ 35 years of age |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus transfer in G2 version 3 (0.125 mg/ml HA) supplemented with HSA (human serum albumin) All embryos were cultured in G1 version three until day three and in G2 version three from day three onwards The EmbryoGlue used for the trial was provided by the American Hospital of Istanbul Randomisation was performed on day of embryo transfer Embryos in treatment group were exposed to EmbryoGlue for 30 minutes before transfer Transfer was performed on day three or day five of embryo development All embryos were fresh, no frozen‐thaw protocol was followed No donor oocytes were included All culture and transfer media were manufactured by Vitrolife Mean number of embryos transferred: treatment group 2.69, control group 2.75 Method of pregnancy determination: hCG pregnancy test and demonstration of gestational sac on transvaginal ultrasound |
|
| Outcomes | Secondary outcomes
Additional outcomes
Other outcomes studied
|
|
| Notes | Embryo transfer was performed on day three or day five of embryo development. Outcomes were reported for all embryo transfers and for day three and day five transfers separately. When necessary (for instance for subgroup analyses), data were analysed separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment or control group by a computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Allocation to study arm was performed after consecutively numbered, sealed opaque envelopes were opened |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both clinician and participant were blinded to the group to which the participant was allocated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live birth data were available. Length of follow‐up was 16 weeks. Intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | High risk | Clinical pregnancy, implantation, adverse events and multiple pregnancy rates were reported in a prespecified way. However, ongoing pregnancy was also announced but was not reported on |
| Other bias | Low risk | The EmbryoGlue was provided by the American Hospital, where the trial was performed. All media were manufactured by the same company (Vitrolife) and were therefore comparable, with the exception of EmbryoGlue added to the medium in the treatment group. Multiple pregnancy rate was reported |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Group sampling unclear Single‐centre trial performed at the Mayo Clinic College of Medicine in Rochester, Minnesota, USA. However, one of the authors was based at the London Bridge Fertility Clinic in London, UK Exclusion criteria: prior participation in this study, blastocyst‐stage embryos, single embryo transfer for medical reasons, prior embryo transfer with a large amount of blood on the catheter, three or more consecutive failed embryo transfers. Participants appear to have a maximum age of 39 years Unclear whether a power calculation was performed or if the number of included participants was planned prior to trial commencement Trial received no commercial funding Actual length of follow‐up per participant was unclear Unclear whether an intention‐to‐treat analysis was performed Unclear whether participants could partake in multiple treatment cycles |
|
| Participants | Of a planned total of 250 participants, 98 were recruited and randomly assigned. By the time of publication of this interim analysis, only 68 of these 98 completed treatment: 34 in the treatment group and 34 in the control group. The text was not clear on whether all 98 participants were randomly assigned, or just the 68. It appears that 30 participants were lost, so data on the 68 participants were analysed. For the data analysis of the review, the group size of 34 was used as the denominator Total number of embryos transferred was unclear Mean age of women (SD): treatment group 31.1 (4.0), control group 30.6 (4.4) years Not reported whether study concerned primary and/or secondary subfertility, although no difference between treatment and control groups was reported regarding previous live births Causes and duration of subfertility were not reported The trial appears to focus only on IVF participants, not on participants given ICSI. Participants could not have had more than two consecutive previous treatment failures, but actual data per study group were not reported Age analysis: Participants were stratified by age (< 35 and 35 to < 39 years) |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus transfer in G1 version 3 (0.125 mg/ml HA) All embryos were cultured in G1 version three Timing of randomisation was unclear Timing of transfer during embryo development was unclear Embryos in treatment group were exposed to EmbryoGlue just before transfer All transferred embryos were frozen‐thawed Unclear whether donor oocytes were included All transfer and culture media were manufactured by Vitrolife Mean number of embryos transferred (SD): treatment group 2.2 (0.7), control group 2.2 (0.6) Method of pregnancy demonstration was not reported |
|
| Outcomes | Secondary outcomes
Additional outcomes
Other outcomes
|
|
| Notes | Abstract of ASRM conference presentation of an interim analysis of a bigger study. Contact with the study authors of Morbeck 2007 revealed that the bigger study appeared to be theirs. Therefore, the data from this study of Walker et al were not analysed, although the study remains included for additional information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment or control group, but method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported. Length of follow‐up was unclear. Unclear what happened to 30 of the 98 included participants; whether they were randomly assigned, and if so, whether an intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in a prespecified way |
| Other bias | Low risk | No commercial funding. All media used were obtained from the same manufacturer and therefore are comparable, with the exception of EmbryoGlue added to the medium in the treatment group. Multiple pregnancy rate was reported |
| Methods | Parallel randomised controlled trial Prospective participant recruitment Method of participant sampling was unclear Single‐centre trial performed at the Assisted Reproduction Unit of the VKV American Hospital in Istanbul, Turkey Included embryos had to be frozen‐thawed Unclear whether a power calculation was performed Length of follow‐up was not reported. Not clear whether an intention‐to‐treat analysis was performed, nor whether any loss to follow‐up occurred Only one treatment cycle per participant was included in the trial |
|
| Participants | Supernumerary embryos were cryopreserved in 204 cycles; only one cycle was included per patient, so this means that 204 participants were recruited. Only 129 embryos were thawed and randomly assigned to a treatment group of 64 or a control group of 65. Some confusion exists regarding the group size (see Notes). No further loss was reported, so the data on 129 participants were analysed Total number of embryos transferred was unclear Mean age: treatment group 31.6, control group 32.1 years Not reported whether study concerned primary or secondary subfertility Cause and duration of subfertility not reported Not reported whether study concerned IVF or ICSI participants, or both, nor whether participants had received previous subfertility treatments No age analysis was performed |
|
| Interventions | Embryo transfer in EmbryoGlue (0.5 mg/ml HA) versus transfer in G2 version three culture medium (0.125 mg/ml HA) All embryos were cultured in G1 version three medium, followed by G2 version three on day three of embryo development Randomisation was performed on day of embryo transfer Exposure time to EmbryoGlue before transfer was not reported All embryos were frozen on day three of development and were transferred after thawing, which means that transfer was also performed on day three of development Unclear whether donor oocytes were included in trial All culture and transfer media were manufactured by Vitrolife Mean number of embryos transferred: treatment group 3.1, control group 3.2 Method of pregnancy determination was not reported |
|
| Outcomes | Secondary outcomes
Additional outcomes
Other outcomes
|
|
| Notes | Abstract of ESHRE conference presentation. In the text of the abstract, it is stated that the treatment group consisted of 65 participants and the control group of 64 participants, although data are presented the other way around in the Results table | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment or control group, but it is unclear in what way |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No live births were reported. 204 cycles were frozen, but only 129 were thawed. It remains unclear why. Length of follow‐up was unclear. No intention‐to‐treat analysis was reported |
| Selective reporting (reporting bias) | Low risk | Implantation and clinical pregnancy rates were reported in a prespecified way |
| Other bias | High risk | Unclear whether trial received any commercial funding. All media were manufactured by Vitrolife; study groups were therefore comparable, with the exception of EmbryoGlue added to the medium in the treatment group. No multiple pregnancy rate was reported, although multiple embryos were transferred per cycle |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Balaban 2005 | Quasi‐randomised trial. Randomisation was undertaken according to alternating weekdays |
| Bungum 2003 | Randomised controlled trial comparing implantation and pregnancy rates between two different culture media, both containing HA. Not suitable for this systematic review because this RCT did not compare a treatment group with addition of an adherence compound versus a control group devoid of, or with a lower concentration of, such a compound |
| Chao 2008 | Quasi‐randomised trial. Allocation to treatment or control group was based on consecutive participant list. Every other participant was placed in the treatment group. Information was retrieved by contacting study authors |
| Chatziioannou 2010 | RCT comparing different embryo culture media. Not suitable for this systematic review because this RCT did not compare a treatment group with addition of an adherence compound versus a control group devoid of, or with a lower concentration of, such a compound |
| Feichtinger 1990 | Preliminary trial, not an RCT |
| Feichtinger 1992 | Quasi‐randomised trial. Randomisation to treatment or control arm of the trial was based on the week in which embryo transfer took place |
| Hambiliki 2010 | Quasi‐randomised trial. Randomisation to treatment or control arm of the trial according to alternating weeks |
| Karimian 2004 | Duplication of data from Valojerdi 2006 trial, which was quasi‐randomised as well |
| Loutradi 2008 | Review on trials studying the effect of hyaluronic acid on embryo implantation rates. However, not all reviewed trials were RCTs |
| Nakagawa 2012 | Quasi‐randomised trial. Allocation to different treatment groups was based on odd or even identification numbers |
| Nakagawa 2012‐II | Conference abstract of quasi‐randomised trial (Nakagawa 2012) |
| Romano 2004 | Randomised controlled trial comparing implantation and pregnancy rates between three different culture media. Not suitable for this systematic review because this RCT did not compare a treatment group with addition of an adherence compound versus a control group devoid of, or with a lower concentration of, such a compound |
| Sallam 2010 | Meta‐analysis of different methods of assisted reproductive technologies, including the use of EmbryoGlue. No data were reported, only lack of evidence of a beneficial treatment effect |
| Sieren 2006 | Randomised controlled trial comparing implantation and pregnancy rates between two different culture media but without studying the specific effect of the addition of HA. This RCT did not compare a treatment group with addition of an adherence compound versus a control group devoid of, or with a lower concentration of, such a compound |
| Sifer 2009 | Randomisation of oocytes instead of participants |
| Sun 2010 | Retrospective analysis |
| Valojerdi 2006 | Quasi‐randomisation. Randomisation to treatment or control group was based on consecutive weekdays |
| Venetis 2009 | RCT comparing different embryo culture media, with similar levels of hyaluronic acid at time of embryo transfer |
Differences between protocol and review
The authoring team changed at full review stage. Two review authors (MJH and SB) joined, and one review author (EW) resigned.
The title has changed from "Hyaluronic acid inclusion in embryo transfer media for assisted reproductive technologies" to "Adherence compounds in embryo transfer media for assisted reproductive technologies" to permit inclusion of all kinds of 'embryo glues' in the review. We acknowledge the Cochrane review entitled "Post‐embryo transfer interventions for in vitro fertilisation and intracytoplasmic sperm injection patients" (Abou‐Setta 2014), which covers one of the interventions included in this review.
The secondary outcome measures of ongoing pregnancy rate and adverse event rate have been added. Additional outcome measures include live birth rate per oocyte pickup (OPU) and embryo transfer (ET), clinical pregnancy rate per OPU and ET and the proportion of women in whom at least one embryo has implanted has been replaced by the outcome measure of 'implantation rate'. Originally, implantation rate was planned not to be analysed but only to be presented as an additional table. However, it has been decided to analyse this outcome measure for completeness. This has been done separately from analysis of the other outcome measures because of the difference in denominators.
Certain baseline characteristics have changed, for example, 'over the age of 37 years and undergoing IVF or ICSI, or both' has changed into 'age group analysis', and the interventions of ovarian stimulation and luteal support have been removed.
The search has been adapted to include all kinds of 'embryo glues', instead of only hyaluronic acid.
The subgroup analyses of oocyte donation, exposure time to adherence compounds, different prognosis groups and different embryo transfer policies have been added.
After the meta‐analysis was performed on the hyaluronic acid per concentration comparison, it was decided to pool the data together to get an overall view of the treatment effect. Even though the included studies are not completely similar in their intervention and control groups, all do compare the addition of hyaluronic acid as an adherence compound to the embryo transfer medium versus a control transfer medium.
Contributions of authors
Stephan Bontekoe (SB) and Maas Jan Heineman (MJH) performed searches, selected studies and extracted and analysed data. Stephan Bontekoe wrote the review, together with Debbie Blake (DB).
Debbie Blake (DB) was an independent advisor, reviewed included studies, resolved discrepancies and wrote the original protocol. Furthermore, she rewrote much of the main text of the review for the updated 2013 version.
Neil Johnson (NJ) was an independent advisor and reviewed included studies.
Sources of support
Internal sources
-
University of Amsterdam, Netherlands.
Stephan Bontekoe (SB) applied for a grant from the University of Amsterdam to perform scientific research outside of the Netherlands.
External sources
No sources of support supplied
Declarations of interest
The Biotechnology Research Institute has an affiliation with KODE Biotech Ltd, which has licensed to ORIGIO/MediCult the rights for modifying human embryos with KODE constructs, including those that may contain hyaluronic acid.
Neil Johnson is involved in research into lipiodol and its possible properties for enhancement of endometrial receptivity to implantation.
Edited (no change to conclusions)
References
References to studies included in this review
Balaban 2004 {published data only}
- Balaban B, Urman B, Yakin K, Isiklar A, Kilic Y, Aksoy S. High pregnancy and implantation rates can be achieved in blastocyst transfers using hyaluronan enriched culture and transfer medium. Fertility and Sterility 2004;82 Suppl 2:221. [Google Scholar]
Balaban 2011 {published data only}
- Balaban B, Yakin K, Ata B, Isiklar A, Urman B. Effect of hyaluronan‐enriched transfer medium on take home baby rate after day 3 and day 5 embryo transfers: a prospective randomized study. Human Reproduction 2011;26:i24. [DOI] [PubMed] [Google Scholar]
Ben‐Rafael 1995 {published data only}
- Ben‐Rafael Z, Ashkenazi J, Shelef M, Farhi J, Voliovitch I, Feldberg D, Orvieto R. The use of fibrin sealant in in vitro fertilization and embryo transfer. International Journal of Fertility and Menopausal Studies 1995;40(6):303‐6. [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen C, Tsai F, Lin J. The effect of combining human serum albumin and hyaluronic acid in the transfer medium on the pregnancy rate in IVF cycles: a prospective randomized study. Human Reproduction 2001;16 Suppl 1:116. [Google Scholar]
Dittmann‐Műller 2009 {published data only}
- Dittmann‐Muller X, Zollner KP, Zollner U. Prospective randomised clinical trial about the efficacy of a human embryo transfer medium (EmbryoGlue(R)). Human Reproduction 2009;24 Suppl 1:167. [Google Scholar]
Fancsovits 2011 {published data only}
- Fancsovits P, Murber A, Tothne Gilan Z, Rigo J Jr, Urbancsek J. Effect of hyaluronan containing transfer media on pregnancy and implantation rates in human IVF‐ET cycles. A prospective randomized study. Human Reproduction 2011;26:i24. [Google Scholar]
Friedler 2005 {published data only}
- Friedler S, Raziel A, Schachter M, Strassburger D, Kasterstein E, Komarovsky D, et al. Efficacy of hyaluronan‐enriched embryo transfer medium in patients with repeated IVF‐ET failures. Human Reproduction 2005;20 Suppl 1:159. [Google Scholar]
Friedler 2007 {published data only}
- Friedler S, Schachter M, Strassburger D, Esther K, Ron El R, Raziel A. A randomized clinical trial comparing recombinant hyaluronan/recombinant albumin versus human tubal fluid for cleavage stage embryo transfer in patients with multiple IVF‐embryo transfer failure. Human Reproduction 2007;22(9):2444‐8. [DOI] [PubMed] [Google Scholar]
Hazlett 2004 {published data only}
- Hazlett WD, Meyer LR, Karande VC, Nasta TE, Mangan PA. Prospective, randomized evaluation of the impact of using EmbryoGlue for the transfer medium in a non‐selected group of patients. Human Reproduction 2004;19 Suppl 1:147. [Google Scholar]
Hazlett 2005 {published data only}
- Hazlett WD, Meyer L, Nasta T, Mangan P, Karande VC. EmbryoGlue(R) and its impact on pregnancy and implantation rates in normal and low responding IVF patients. Fertility and Sterility 2005;84 Suppl 1:375‐6. [Google Scholar]
Hazlett 2008 {published data only}
- Hazlett WD, Meyer LR, Nasta TE, Mangan PA, Karande VC. Impact of EmbryoGlue as the embryo transfer medium. Fertility and Sterility 2008;90(1):214‐6. [DOI] [PubMed] [Google Scholar]
Khan 2004 {published data only}
- Khan I, Urich M, Sasy M, Shmoury M, Abuzeid M, Ayers J, et al. A prospective, randomized study comparing EmbryoGlue and P1 Complete as embryo transfer medium. Human Reproduction 2004;19 Suppl 1:126. [Google Scholar]
Korošec 2007 {published data only}
- Korosec S, Virant‐Klun I, Tomazevic T, Zech NH, Meden‐Vrtovec H. Single fresh and frozen‐thawed blastocyst transfer using hyaluronan‐rich transfer medium. Reproductive Biomedicine Online 2007;15(6):701‐7. [DOI] [PubMed] [Google Scholar]
Mahani 2007 {published data only}
- Mahani IM, Davar R. Hyaluronic acid versus albumin in human embryo transfer medium. Eastern Mediterranean Health Journal 2007;13(4):876‐80. [PubMed] [Google Scholar]
Morbeck 2007 {published data only}
- Morbeck DE. Prospective randomized clinical trial of novel implantation promoting medium (EmbryoGlue) to improve IVF success rates [NTC00588250]. ClinicalTrials.gov 2007.
Ravhon 2005 {published data only}
- Ravhon A, Nahum H, Weissman A, Biran G, Umansky N, Levran D. Embryo transfer in hyaluronan enriched transfer medium does not improve pregnancy rate in IVF treatment. Fertility and Sterility 2005;84 Suppl 1:376‐7. [Google Scholar]
Schoolcraft 2002 {published data only}
- Schoolcraft W, Lane M, Stevens J, Gardner DK. Increased hyaluronan concentration in the embryo transfer medium results in a significant increase in human embryo implantation rate. Fertility and Sterility 2002;78 Suppl 1:5. [Google Scholar]
Simon 2003 {published data only}
- Simon A, Safran A, Revel A, Aizenman E, Reubinoff B, Porat‐Katz A, et al. Hyaluronic acid can successfully replace albumin as the sole macromolecule in a human embryo transfer medium. Fertility and Sterility 2003;79(6):1434‐8. [DOI] [PubMed] [Google Scholar]
Urman 2008 {published data only}
- Urman B, Yakin K, Ata B, Isiklar A, Balaban B. Effect of hyaluronan‐enriched transfer medium on implantation and pregnancy rates after day 3 and day 5 embryo transfers: a prospective randomized study. Fertility and Sterility 2008;90(3):604‐12. [DOI] [PubMed] [Google Scholar]
Walker 2005 {published data only}
- Walker DL, Thornhill AR, Allemand MC, Tatpati LL, Wentworth MA, Tummon IS. A randomized, controlled, double blinded trial of EmbryoGlue® in frozen embryo transfer cycles: an interim analysis. Fertility and Sterility 2005;84 Suppl 1:415‐6. [Google Scholar]
Yakin 2004 {published data only}
- Yakin K, Balaban B, Isiklar A, Bozdag H, Urman B. Improved clinical outcome in frozen‐thawed embryo transfers with the use of hyaluronan‐enriched medium. Human Reproduction 2004;19 Suppl 1:89. [Google Scholar]
References to studies excluded from this review
Balaban 2005 {published data only}
- Balaban B, Urman B. Comparison of two sequential media for culturing cleavage‐stage embryos and blastocysts: embryo characteristics and clinical outcome. Reproductive Biomedicine Online 2005;10(4):485‐91. [DOI] [PubMed] [Google Scholar]
Bungum 2003 {published data only}
- Bungum M, Povlsen B, Madsen K, Poulsen AL, Elliman P, Humaidan P. Comparison of G1.2/G2.2 and the GIII series, sequential media for culture of human embryos. A prospective, randomized study. Fertility and Sterility 2003;80 Suppl 3:198‐9. [Google Scholar]
Chao 2008 {published data only}
- Chao S, Schenkman E, Kim S, Kenigsberg D, Brenner S, Moodie G. The effect of embryo glue on clinical pregnancy rate in frozen embryo transfers. Fertility and Sterility 2008;90 Suppl 1:434. [Google Scholar]
Chatziioannou 2010 {published data only}
- Chatziioannou E, Kolibianakis EM, Venetis CA, Paggou T, Tokmakidou A, Sanopoulou T, et al. The effect of 2 different embryo culture systems on the probability of pregnancy: a randomized controlled trial. Journal fur Reproduktionsmedizin und Endokrinologie 2010;7(4):267. [Google Scholar]
Feichtinger 1990 {published data only}
- Feichtinger W, Barad D, Feinman M, Barg P. The use of two‐component fibrin sealant for embryo transfer. Fertility and Sterility 1990;54(4):733‐4. [DOI] [PubMed] [Google Scholar]
Feichtinger 1992 {published data only}
- Feichtinger W, Strohmer H, Radner KM, Goldin M. The use of fibrin sealant for embryo transfer: development and clinical studies. Human Reproduction 1992;7(6):890‐3. [DOI] [PubMed] [Google Scholar]
Hambiliki 2010 {published data only}
- Hambiliki F, Ljunger E, Karlstrom PO, Stavreus‐Evers A. Hyaluronan‐enriched transfer medium in cleavage‐stage frozen‐thawed embryo transfers increases implantation rate without improvement of delivery rate. Fertility & Sterility October 2010;94(5):1669‐73. [DOI] [PubMed] [Google Scholar]
Karimian 2004 {published data only}
- Karimian L, Valojerdi MR, Baghestani AR, Moeini A. A prospective randomized comparison of two commercial embryo transfer medium in IVF/ICSI cycles. Human Reproduction 2004;19 Suppl 1:52. [Google Scholar]
Loutradi 2008 {published data only}
- Loutradi KE, Tarlatzi TB, Kolibianakis EM, Tarlatzis BC. Does hyaluronan improve embryo implantation?. Current Opinion in Obstetrics and Gynecology 2008;20(3):305‐7. [DOI] [PubMed] [Google Scholar]
Nakagawa 2012 {published data only}
- Nakagawa K, Takahashi C, Nishi Y, Jyuen H, Sugiyama R, Kuribayashi Y, Sugiyama R. Hyaluronan‐enriched transfer medium improves outcome in patients with multiple embryo transfer failures. Journal of Assisted Reproduction and Genetics 12 April 2012;29:679‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakagawa 2012‐II {published data only}
- Nakagawa K, Ojiro Y, Takahashi C, Sugiyama R, Juen H, Nishi Y, et al. Hyalurona‐ enriched transfer medium improved implantation in vitrified‐ warmed embryo transfer cycles regardless of preparation method of the uterine endometrium. Human Reproduction July 2012;27 Suppl 2:ii59‐ii61: Abstract number: O‐155. [Google Scholar]
Romano 2004 {published data only}
- Romano S, Scarselli F, Minasi MG, Martinez F, Iacobelli M, Sapienza F, et al. Effect of different culture systems on embryo developmental potential. Human Reproduction 2004;19 Suppl 1:6‐7. [Google Scholar]
Sallam 2010 {published data only}
- Sallam HN, Ezzeldin F, Sallam NH. The evidence‐based management of repeated implantation failure in ART. Reproductive BioMedicine Online. October 2010;20:S18. [Google Scholar]
Sieren 2006 {published data only}
- Sieren KR, Grainger DA, Tjaden BL, Ball GD. In vitro outcome of a prospective, randomized trial comparing the GIII series(TM) and Quinn's Advantage(R) sequential media systems for embryo development. Fertility and Sterility 2006;86 Suppl 2:233‐4. [Google Scholar]
Sifer 2009 {published data only}
- Sifer C, Handelsman D, Grange E, Porcher R, Poncelet C, Martin‐Pont B, et al. An auto‐controlled prospective comparison of two embryos culture media (G III series versus ISM) for IVF and ICSI treatments. Journal of Assisted Reproduction & Genetics 2009 Nov‐Dec;26(11‐12):575‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2010 {published data only}
- Sun HX, Hu YL, Zhang NY, Wang B. Quality improvement research in a large IVF clinic and the effects of hyaluronan‐containing transfer medium on delivery rate. Journal fur Reproduktionsmedizin und Endokrinologie 2010;7(4):311. [Google Scholar]
Valojerdi 2006 {published data only}
- Valojerdi MR, Karimian L, Yazdi PE, Gilani MA, Madani T, Baghestani AR. Efficacy of a human embryo transfer medium: a prospective, randomized clinical trial study. Journal of Assisted Reproduction and Genetics 2006;23(5):207‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Venetis 2009 {published data only}
- Venetis CA, Kolibianakis EM, Papanikolaou EG, Karakosta C, Pagou E, Tarlatzis BC. The effect of embryo culture system on pregnancy rates: a randomized controlled trial. Fertility and Sterility September 2009;92(3 Suppl 1):s232. [Google Scholar]
Additional references
Abou‐Setta 2014
- Abou‐Setta AM, D'Angelo A, Sallam HN, Hart RJ, Al‐Inany HG. Post‐embryo transfer interventions for assisted reproduction technology cycles. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD006567.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bar‐Hava 1999
- Bar‐Hava I, Krissi H, Ashkenazi J, Orvieto R, Shelef M, Ben‐Rafael Z. Fibrin glue improves pregnancy rates in women of advanced reproductive age and in patients in whom in vitro fertilization attempts repeatedly fail. Fertility and Sterility 1999;71(5):821‐4. [DOI] [PubMed] [Google Scholar]
Behzad 1994
- Behzad F, Seif MW, Campbell S, Aplin JD. Expression of two isoforms of CD44 in human endometrium. Biology of Reproduction 1994;51:739‐47. [DOI] [PubMed] [Google Scholar]
Campbell 1995
- Campbell S, Swann H, Aplin JD, Seif MW, Kimber SJ, Elstein M. CD44 is expressed throughout pre‐implantation human embryo development. Human Reproduction 1995;10(2):425‐30. [DOI] [PubMed] [Google Scholar]
Carson 1987
- Carson DD, Dutt A, Tang J. Glycoconjugate synthesis during early pregnancy: hyaluronate synthesis and function. Developmental Biology 1987;120:228‐35. [DOI] [PubMed] [Google Scholar]
Gardner 1999
- Gardner DK, Rodriguez‐Martinez H, Lane M. Fetal development after transfer is increased by replacing protein with the glycosaminoglycan hyaluronan for mouse embryo culture and transfer. Human Reproduction 1999;14(10):2575‐80. [DOI] [PubMed] [Google Scholar]
Gardner 2003
- Gardner DK, Lane M. Blastocyst transfer. Clinical Obstetrics and Gynecology 2003;46:231‐8. [DOI] [PubMed] [Google Scholar]
Gardner 2004
- Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertility and Sterility 2004;81:551‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Kolibianakis 2008
- Kolibianakis EM, Loutradi K, Venetis CA, Papanikolaou EG, Tarlatzi TB, Tarlatzis BC. Increased clinical pregnancy rates by using hyaluronan in in‐vitro fertilization: a systematic review and meta‐analysis. Fertility and Sterility 2008;90 Suppl 1:349. [Google Scholar]
Menezo 1989
- Menezo Y, Arnal F, Humeau C. Increased viscosity in transfer medium does not improve the pregnancy rates after embryo replacement. Fertility and Sterility 1989;52(4):680‐2. [DOI] [PubMed] [Google Scholar]
Revel 2005
- Revel A, Helman A, Koler M, Shushan A, Goldshmidt O, Zcharia E, et al. Heparanase improves mouse embryo implantation. Fertility and Sterility March 2005;83(3):580‐6. [DOI] [PubMed] [Google Scholar]
Salamonsen 2001
- Salamonsen LA, Shuster S, Stern R. Distribution of hyaluronan in human endometrium across the menstrual cycle: implications for implantation and menstruation. Cell Tissue Research 2001;306:335‐40. [DOI] [PubMed] [Google Scholar]
Schröder 2004
- Schröder AK, Katalinic A, Diedrich K, Ludwig M. Cumulative pregnancy rates and drop‐out rates in a German IVF programme: 4102 cycles in 2130 patients. Reproductive BioMedicine Online 2004;8(5):600‐6. [DOI] [PubMed] [Google Scholar]
Turley 1984
- Turley E, Moore D. Hyaluronate binding proteins also bind to fibronectin, laminin and collagen. Biochemical and Biophysical Research Communications 1984;121(3):808‐14. [DOI] [PubMed] [Google Scholar]
Vail 2003
- Vail A, Gardener E. Common statistical errors in the design and analysis of subfertility trials. Human Reproduction 2003;18(5):1000‐4. [DOI] [PubMed] [Google Scholar]


