Abstract
After decades of research, immunotherapies for cancer are demonstrating increasing success. These agents can amplify existent antitumour immunity or induce durable antitumour immune responses in a wide array of cancers. The spectrum of immunotherapeutics is broad, spanning monoclonal antibodies and their derivatives, tumour vaccines, and adoptive therapies using T cells and natural killer cells. Only a small number of immunotherapies have been tested in paediatric cancers, but impressive antitumour effects have already been observed. Mononclonal antibodies targeting GD2 that induce antibody-dependent cell-mediated cytotoxicity improve survival in high-risk neuroblastoma. Bi-specific monoclonal antibodies that simultaneously target CD19 and activate T cells can induce remission in acute B-cell lymphoblastic leukaemia (B-ALL) and adoptive immunotherapy using T cells genetically engineered to express chimeric antigen receptors targeting CD19 induce impressive responses in B-ALL. Efforts are underway to generate and test new immunotherapies in a wider array of paediatric cancers. Major challenges include a need to identify immunotherapy targets on the most lethal childhood cancers, to expand availability of technology-intense platforms, such as adoptive cell therapy, to optimize management of novel toxicities associated with this new class of cancer therapies and to determine how best to incorporate these therapies into standard treatment paradigms.
Introduction
Impressive survival gains for childhood cancer occurred from 1975 to 1995, but survival rates have changed little in the past two decades.1 Cytotoxic chemotherapy mediates high response rates in most paediatric cancers and multiagent cytotoxic regimens improved survival in many settings; however, chemoresistance and toxicities associated with increasing dose intensity are barriers to continued progress. The molecular revolution in oncology has spawned development of an impressive array of targeted therapeutics for adult cancers, but genomic analyses of paediatric tumours has thus far identified few targetable mutations. Immunotherapies have shown increasing promise in the treatment of adult cancer and a small but increasing body of evidence suggests that immunotherapy could improve outcomes for childhood cancers as well.2-4 Emerging evidence demonstrating that immune-based therapies are effective against chemoresistant cancer cells, leads to the prediction that immunotherapies could prove effective for diseases not currently curable with cytotoxics and could be administered in combination with cytotoxics to improve outcomes. Furthermore, given the prevalence and extent of long-term morbidity associated with cytotoxic regimens required for cure of childhood cancer, the prospect that immunotherapy could enable reduction in dose intensity of toxic agents, and thus diminish long-term morbidity for cancer survivors, is compelling. We review immunotherapies with established efficacy in paediatric cancers as well as immunotherapies currently under study or likely to be studied in the near future. We also discuss the emerging science driving this rapidly moving field and highlight the most pressing challenges that must be overcome for continued progress in this arena.
Graft-versus-tumour effects after transplantation
Allogeneic haematopoietic stem-cell transplantation (alloHSCT) can cure paediatric patients with leukaemia who do not respond to standard frontline regimens or who experience recurrence. Although initially intended as a rescue therapy following myeloablative chemotherapy or radiation, it is now well established that immunoreactivity—termed graft-versus-leukaemia (GVL)—contributes to therapeutic benefit. Infusion of donor lymphocytes (DLI) to patients with leukaemia recurrence after alloHSCT can induce remissions as a single therapeutic manoeuvre, although DLI therapy is often associated with off-tumour toxic effects in the form of graft-versus-host disease (GVHD).5 T cells mediate a major component of the GVL effect, as demonstrated by higher leukaemia relapse rates in recipients of T-cell depleted grafts and reduced relapse rates in patients with GVHD. Ongoing research seeks to enhance GVL without GVHD. One area of focus includes adoptive transfer of donor-derived T cells with greater tumour specificity and less specificity to non-malignant tissues, which is discussed later.
Another approach is to augment the potency of GVL mediated by natural killer (NK) cells. Seminal studies in murine models have demonstrated that NK cells do not induce, and may actually diminish, GVHD.6 Because NK cells are regulated by inhibitory signals delivered following engagement of killer immunoglobulin receptors (KIR) to specific HLA alleles (Figure 1), donor recipient pairs can be selected with more or less NK inhibitory signalling.7 Early observations showed that in AML, 20 donor recipient pairs with HLA-C or HLA-Bw4 mismatch (such as KIR ligand mismatch) had a 0% probability of relapse at 5 years, compared with a 75% relapse rate in the 37 patients with KIR ligand compatible donors.6 KIR ligand mismatch posed no greater risk of GVHD following T-cell depleted haploidentical HSCT.6 Some subsequent studies, however, did not confirm these initial observations, and further scrutiny ultimately revealed the complexity in this system.8 For instance, alloreactive T cells diminish NK reactivity after alloHSCT, and thus KIR ligand mismatch has a less-potent effect in T-cell replete HSCT.9 Furthermore, knowledge of specific inhibitory KIR–KIR ligand interactions for some alleles has continued to evolve resulting in modifications to the original classification scheme. Furthermore, other inhibitory and activating receptors including CD94/NKGD2A, KIR2DS1 and activating B/x haplotypes are now believed to significantly modulate NK cell activity.7,8,10 Thus optimal donor selection requires more than evaluation for KIR ligand mismatch. Despite these complexities, several studies that have included paediatric patients have demonstrated improved outcomes using donors bearing a genotype that predicts NK activation following T-cell depleted alloHSCT,7,8 and studies are underway to further improve outcomes by optimizing donor selection algorithms.11
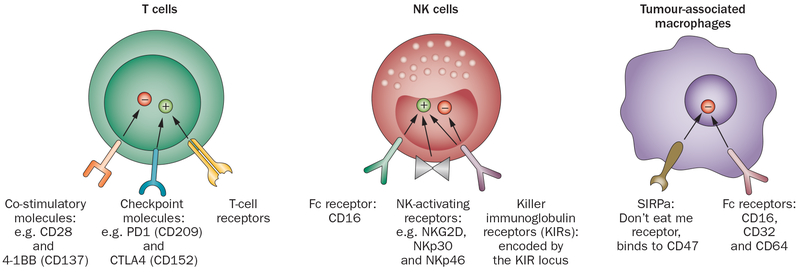
Figure 1 ∣.
Selected cell surface molecules with important roles in immune-based therapies. T-cell receptors, endow T cells with an exquisite range of specificities, but are restricted to peptides presented by self-MHC on a target cell. Co-stimulatory molecules such as CD28 and 41BB transmit essential ‘second signals’ needed for maximal T-cell activation. Checkpoint molecules such as CTLA4 and PD-1 transmit inhibitory signals limiting T cell activation and allowing tumours to escape T cell recognition. KIRs, present on NK cell surface, can transmit inhibitory signals after engaging MHC molecules or activating signals. NK activating receptors transmit activating signals after engaging ligands on “stressed” tissues. The full range of NK activating receptors and their ligands are still being identified. Expression of activating receptors is increased on activated NK cells. Fc receptor, CD16 is the only FcR on NK cells and is the most potent NK activating receptor. CD16 engagement by IgG1 and IgG2a antibodies induces ADCC. Tumour-associated macrophages. Fc Receptors, CD16, CD32 and CD64 expressed on macrophages may transmit activating or inhibitory signals. The tumour microenvironment induces immunosuppressive M2 macrophages, which predominantly express inhibitory FcRs and do not mediate ADCC upon binding of mAbs. SIRPa binds CD47, which is expressed on a wide array of tissues, and most tumours. SIRPa signalling transmits an inhibitory signal that prevents macrophage-mediated killing of CD47+ cells. Abbreviations: ADCC, antibody-dependent cell-mediated cytotoxicity; KIRs, killer immunoglobulin receptors; MHC, major histocompatibility complex; NK, natural killer.
Some groups are attempting to improve NK cell immune reconstitution following HSCT by depleting the graft of T cells and CD19+ B cells, but leaving NK progenitors intact.12-14 Others are employing adoptive transfer of autologous and allogeneic NK cells to amplify the antitumour effects of this lymphocyte subset. In the first report, remission was observed in 5 of 19 adult patients treated with haploidentical NK cells plus recombinant human interleukin-2 (rhIL-2) without HSCT.15 A study in children wherein 10 patients with AML in first remission received haploidentical NK cells plus rhIL-2 reported a 100% estimated 2-year event-free survival (EFS).16 Cytokines and/or artificial antigen-presenting cells have also been used to enhance killing potency of adoptively transferred NK cells, and thus potentially eliminate the need for KIR or KIR ligand mismatch.17,18 Several studies that employ adoptive transfer of resting or activated autologous or allogeneic NK cells for children with cancer are underway.19-22 Increasing insights regarding the potential of these approaches will be available in the coming years.
Monoclonal antibody-based immunotherapy
Naked antibodies can mediate antitumour effects through a myriad of mechanisms (Figure 2) including growth factor blockade (such as trastuzumab in HER2-expressing tumours), death receptor agonism (such as lexatumumab targeting TRAIL-R2), induction of antibody-dependent cell-mediated cytotoxicity (ADCC, for example, rituximab, anti-GD2) or blockade of immune inhibitory) signals (anti-CTLA4, anti-PD1). Monoclonal antibodies targeting death agonists have not proceeded along the pharmaceutical development pipeline, despite signals of activity in paediatric solid tumours.23 Therefore, most naked monoclonal antibody-based therapeutics currently under study in paediatric cancer augment host immune responses.24
Figure 2 ∣.
Tumour cell death induced by monoclonal antibody based therapeutics. Naked monoclonal antibodies (red) can work through activation of innate immune effectors bearing the Fc receptors, most often NK cells or macrophages. The NK cell or macrophage becomes activated as a result of signalling via its Fc receptor, and then induces apoptosis of the cancer cell. Bispecific mAbs activate a T cell or an NK cell through binding to CD3ζ or an NK cell activating receptor, and simultaneously, bind to the tumour surface. Recruitment and activation of the T cells or NK cells at the tumour site induces antitumour effects, either via direct cytolysis and/or cytokine production. Antibodies that bind to growth factor receptor or death ligand receptors (purple) can directly induce tumour cell death without activation of immune effectors. Antibodies can be conjugated to toxins, chemotherapeutics or radioisotopes and induce cell death with lower systemic exposure to the toxic agents. Abbreviations: mAb, monoclonal antibody; NK, natural killer.
Monoclonal antibodies that target GD2 disialoganglioside have been tested in numerous clinical trials in neuroblastoma during the past 25 years. GD2 is highly expressed on all neuroblastomas, and is also expressed on normal tissues in the central nervous system (CNS) and peripheral nerves, albeit at lower levels.25 In patients with refractory neuroblastoma, early clinical studies with two murine anti-GD2 monoclonal antibodies, 3F8 and 14.G2a, showed safety and some limited antitumour activity.26,27 To diminish immunogenicity, a chimeric monoclonal antibody derived from 14.G2a (dinutuximab, ch14.18) was developed. This agent showed a similar activity and safety profile in refractory neuroblastoma when combined with rhIL-2 or GM-CSF—agents co-administered to augment ADCC.28 Ch14.18 also had promising effects on overall survival when administered as an adjuvant single agent following standard therapy for high-risk neuroblastoma.29 In a pivotal randomized phase III study, patients with neuroblastoma who received autoHSCT plus isotretinoin plus ch14.18, GM-CSF and rhIL-2 demonstrated a significantly increased EFS and overall survival compared to patients receiving autoHSCT plus isotretinoin alone (EFS: 66% versus 46% P = 0.01; overall survival 86% versus 75% at 2 years, P = 0.02, respectively).2 Sequential single-arm studies in with patients high-risk neuroblastoma that were treated with the 3F8 anti-GD2 monoclonal antibody following autologous HSCT also demonstrated promising effects.30 Together, this literature provides conclusive evidence that anti-GD2 monoclonal antibody therapy should be considered an essential element in frontline therapy for patients with high-risk neuroblastoma.
Several challenges remain if we are to maximize the benefits and minimize the toxic effects of anti-GD2 therapies. In patients with bulk disease, anti-GD2 monoclonal antibodies show limited activity and many children with high-risk neuroblastoma cannot enter a minimal residual disease state. Hence, there is keen interest in augmenting the potency of these agents, largely by modifying the function of effector cells that mediate ADCC (Figure 2). One approach involves generation of a fusion protein linking ch14.18 to rhIL-2 (hu14.18-IL2), which activates NK cells through the IL-2 receptor. Hu14.18-IL2 did not induce tumour shrinkage in children with bulk disease, but a 22% complete response rate was observed in children with disease burdens detectable only by MIBG (meta-iodobenzylguanidine) therapy.31 Interestingly, 29% of patients predicted to have increased NK cell reactivity based upon KIR–KIR–ligand mismatch showed objective responses or clinical improvement whereas no responses were observed in those without KIR–KIR-ligand mismatch (P = 0.03), and there was a trend toward improved response rates in patients with an Fc receptor (FcR) polymorphism, which is predicted to mediate a more-potent ADCC effect.32 Improved outcomes have also been observed in children treated with 3F8 who had KIR-ligand mismatch.33 Given this emerging evidence suggesting that NK cells are important mediators of antitumour effects of anti-GD2 monoclonal antibodies, several studies are investigating adoptive NK cell therapy to further augment anti-GD2 potency.34
Preclinical work has demonstrated that blockade of CD47—the ligand for the inhibitory signalling receptor SIRPα on macrophages—augments ADCC and can improve the efficacy of antibody therapies (Figure 1).35 CD47 is expressed in many cancers and trials of anti-CD47 monoclonal antibodies are planned, both as a single agent and in combination with tumour-specific antibodies aimed at inducing ADCC. Given the recent data demonstrating GD2 expression on osteosarcomas,36 several groups are enrolling non-neuroblastoma GD2-positive paediatric patients with solid tumours onto trials incorporating anti-GD2, including osteosarcoma and melanoma.24
Strategies are also underway to diminish the toxicity associated with anti-GD2 therapy, in particular pain associated with the monoclonal antibody infusion, which is presumably due to complement activation and inflammation targeting GD2+ peripheral nerves. A humanized anti-GD2 (Hu14.18K322A) with a point mutation that prevents complement activation was shown to be safe with modest clinical activity in a phase I study; however, definitive results regarding whether this agent induces less pain than other anti-GD2 agents will require further study.37 Alternatively, an anti-O-acetyl GD2 monoclonal antibody has been developed that binds to a form of GD2 that is absent from peripheral nerves, but expressed at high levels on neuroblastoma cells.25
Several other naked monoclonal antibodies targeting antigens expressed on paediatric leukaemias are approved for use in adults, but there is a paucity of controlled clinical trial data to define their role in paediatric malignancies.38 For example, the anti-CD20 monoclonal antibody rituximab mediates impressive improvements in outcomes when combined with chemotherapy for adults with B-cell lymphoma.39 CD20 is expressed on mature B-cell leukaemias and lymphomas in children (including Burkitt lymphoma/leukaemia and some cases of diffuse, large B-cell lymphoma), but only limited studies have been conducted with rituximab in childhood cancer.40 Rituximab has single agent activity in newly diagnosed Burkitt lymphoma and is safe when administered in combination with standard chemotherapies, but definitive evidence that adding rituximab to frontline regimens improves outcome is lacking.40 Importantly rituximab has an established role in the treatment of children and adults with post-transplant lymphoproliferative disorder,40 and several benign diseases including immune thrombocytopenic purpura,41 which are beyond the scope of this Review. Newer anti-CD20 agents optimized for ADCC activity are likely to undergo study in children in the coming years. Similarly, the anti-CD22 monoclonal antibody epratuzumab has been tested in children with B-ALL, and is safe and shows some single agent activity, but studies have not yet defined a clear role for this agent in the treatment of B-ALL.35
Although we have emphasized agents that work by modulating the host immune system, it is important to note that monoclonal antibodies conjugated to toxins, drugs or radionuclides are also an important emerging class of therapeutics in paediatric cancer (Figure 2). Antibody conjugates that have demonstrated activity in paediatric cancers include ibritumomab tiuextan (targeting CD20 in B cell lymphoma), inotuzumab ozogamycin and moxetumomab (targeting CD22 in B-ALL and B-cell lymphoma), gemtuzumab ozogamicin (targeting CD33 in AML) and brentuximab vendotin (targeting CD30 in Hodgkin lymphoma and anaplastic large-cell lymphoma).42-44
Bispecific antibodies
Bispecific antibodies link a tumour-targeting domain to a domain that activates a receptor on an immune effector cell (Figure 2). An anti-CD19/anti-CD3 bispecific antibody (blinatumomab), which mediates an interaction between CD19+ on leukaemic blasts and CD3+ on T cells, is currently under study in B-ALL.45 Clinical trials first tested this agent in adults with minimal residual B-ALL where a 78% complete response rate was reported.46 Of note, essentially all patients on this study had chemorefractory disease. In a subsequent small study that enrolled children (n = 9) with relapse following allogeneic HSCT, 67% of patients entered remission following blinatumomab therapy.47 Response rates from a large phase I study of blinatumomab in children with relapsed B-ALL are awaited and a phase III trial testing blinatumomab in relapsed paediatric B-ALL is planned.45 The dose limiting toxicity of this agent is cytokine-release syndrome, which can be associated with neurological toxicity.48,49 Furthermore, recurrence following blinatumomab therapy has been associated with the loss of CD19 expression in some cases.46 Other bi-specific reagents are currently in clinical development in adults and are likely to be tested in children in the near future, including agents that augment NK cells to mediate antitumour effects. Tri-specific reagents that incorporate both T cell and NK cell engaging components with a tumour-targeting domain or two tumour-targeting domains with a single immune effector cell engager are soon to be tested in clinical trials.50
Checkpoint inhibitors
Some antibody subclasses, such as IgG4, do not engage Fc receptors and can block access of the receptors to their natural ligands, which inhibits receptor signalling and does not induce ADCC. Checkpoint inhibitors are a new class of immunotherapeutics designed to block cell surface molecules expressed on T cells that transmit inhibitory signals (Figure 1). Ipilimumab (anti-CTLA4) is the prototype and has been demonstrated to improve survival in patients with metastatic melanoma, albeit with significant autoimmune toxicity.51 Ipilimumab has been tested in a phase I trial in children with cancer, but preliminary results did not report objective responses.52 Interestingly, ipilimumab induced significant immune activation and autoimmune toxicity, providing proof-of-principle that, similar to the situation in adults, children harbour autoreactive T cells that can be ‘turned on’ by checkpoint inhibition. A second class of checkpoint inhibitors includes those that target PD-1. Adult studies of anti-PD1 monoclonal antibodies have demonstrated efficacy in a wide array of cancers with less toxicity than ipilimumab.53 Clinical trials of anti-PD1 have not yet been initiated in children with cancer, but are anticipated to open within the year.
Tumour vaccines
Seminal studies in the early 1990s elucidated the molecular targets of T-cell recognition on cancer cells and ushered in the modern era of immunotherapy for cancer.54 Tumour vaccines targeting defined tumour antigens were among the first molecularly based immunotherapies tested. Animal models consistently demonstrated that tumour vaccines prevented tumour growth when administered before tumour inoculation, but were less effective in the setting of established tumours. Clinical studies have largely confirmed this paradigm, concluding that tumour vaccines are safe, but not adequate to mediate regression of established tumours.55 This situation likely relates to the multitude of factors contributing to immune escape that are not impacted by the vaccine per se (Box 1). Despite these challenges, some randomized studies have shown improvements in survival in patients with bulky tumours treated with tumour vaccines, leading to the first FDA approved tumour vaccine for men with metastatic hormone-refractory prostate cancer.56 Although tumour shrinkage was not observed, improved survival was attributed to diminished tumour growth rate.57,58
Box 1 ∣. Major mechanisms of tumour immune evasion.
T regulatory cells (Tregs) can be CD4+ or CD8+ and suppress immune responses in an antigen-specific or antigen nonspecific manner. The most common Treg implicated in tumour evasion are CD4+CD25+FOXP3+ T cells, which are essential for preventing autoimmunity
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of immature myeloid cells that accumulate in tumour-bearing hosts. They suppress T-cell responses via antigen nonspecific effects, largely by depleting nutrients in the microenvironment through the production of arginase and inducible nitric oxide synthase
Downregulation of major histocompatibility complex (MHC) antigens on tumour cells prevents them from presenting peptides to CD8+ killer T cells and, therefore, enables escape from tumour-specific cytolytic T cells. MHC downregulation is predicted to make tumour cells more responsive to NK-cell-mediated killing
Mutations in IFNγ signalling on tumour cells prevents the tumours from upregulating MHC in response to IFNγ production by activated T cells or NK cells and is a common component of immune escape
Production of immunosuppressive cytokines (such as IL-10, TGFβ, IL-6) by tumour and non-malignant cells within the microenvironment leads to a general state of local immunosuppression
Beyond the inability of tumour vaccines to mediate cancer regression, other challenges have hampered rapid progress in this arena. Investigators have used a multitude of approaches to deliver vaccine therapies, from peptides administered with adjuvant to viral based vectors to dendritic cells. It remains unclear whether any approach is superior, and the array of therapeutics tested has made comparisons across platforms, diseases and tumour antigens difficult. Furthermore, to improve potency, investigators are administering tumour vaccines with other cancer therapies and immunotherapies, compounding the complexities of comparing results across platforms. Finally, biological end points, such as T-cell responses, have not correlated closely with clinical outcomes in tumour vaccine trials, making it difficult to define surrogate end points for vaccine efficacy.59 Thus, although some single arm and randomized phase II studies of therapeutic tumour vaccines have demonstrated promising results,60,61 definitive trials are lacking for most tumour vaccines.
Numerous single arm clinical trials of tumour vaccines have been conducted in children with cancer and several ongoing studies are underway (Table 1), the results of which are awaited. Similar to the results in adults, paediatric cancer tumour vaccines are safe to administer, but tumour regression has been rarely observed. Because many paediatric cancers are aggressive by nature, slowing tumour growth rate might not have meaningful clinical benefits. Despite these challenges, it remains plausible that tumour vaccines could provide an approach to diminish recurrence in high-risk paediatric tumours, particularly given the minimal toxicity. Preliminary results were reported from one trial using tumour lysate pulsed dendritic cells plus autologous lymphocyte infusions with or without recombinant IL-7 following standard upfront therapy in patients with metastatic or recurrent Ewing sarcoma and rhabdomyosarcoma.62 Immune responses to autologous tumour lysates were induced in approximately one third of patients, and intent-to-treat analysis of the subset of patients enrolled at the time of a new diagnosis of metastatic Ewing sarcoma or rhabdomyosarcoma showed 83% 5-year overall survival and 54% 5-year EFS, which is superior to previous outcomes reported for this population.
Table 1 ∣.
Tumour vaccine trials for paediatric cancer*
| Trial number | Regimen | Diseases | Status |
|---|---|---|---|
| HLA-A2 restricted tumour-associated peptides plus poly I:C | Paediatric gliomas | Open | |
| EGFRvIII peptide: KLH | Diffuse intrinsic pontine glioma | Terminated | |
| HLA-A2 restricted tumour-associated peptides plus montanide-ISA51VG | CNS tumours | Ongoing, not recruiting | |
| Autologous dendritic cells (cancer testis antigen) plus imiquimod and decitabine | Neuroblastoma Ewing sarcoma Osteosarcoma Rhabdomyosarcoma Synovial sarcoma |
Recruiting | |
| Tumour lysate pulsed autologous dendritic cells | Gliomas | Completed | |
| Autologous tumour cells expressing GM-CSF (GVAX) | Clear-cell sarcoma Alveolar soft part sarcoma Renal cell carcinoma |
Ongoing, not recruiting | |
| Tumour lysate pulsed autologous dendritic cells plus imiquimod | Gliomas | Recruiting | |
| Tumour lysate pulsed autologous dendritic cells plus imiquimod | Brain tumours | Recruiting | |
| Tumour lysate pulsed autologous dendritic cells | High-risk solid tumours following stem-cell transplantation | Ongoing, not recruiting | |
| Tumour lysate pulsed autologous dendritic cells with autologous lymphocytes with or without recombinant IL-7 | Ewing sarcoma Rhabdomyosarcoma Neuroblastoma following standard therapy |
Ongoing, not recruiting | |
| Gene (IL2, lymphotactin) modified allogeneic neuroblastoma cells | Neuroblastoma following metronomic cyclophosphamide | Ongoing, not recruiting |
Data from Clinicaltrials.gov as of May 2014. Abbreviations: CNS, central nervous system; IL, interleukin; KLH, keyhole limpet hemocyanin.
Adoptive T-cell therapy
Tumour-infiltrating lymphocytes
Adoptive T-cell immunotherapy has shown activity in a small subset of cancers, with increasing evidence for efficacy and increasing experience in paediatrics.3,4 Fundamental factors driving development of adoptive T-cell immunotherapy for cancer is the evidence that T cells administered to receptive hosts can undergo multi-log expansion, eradicate large tumour burdens and persist for months to years.63,64 Adoptive T-cell immunotherapy seeks to administer tumour reactive T cells with enhanced number and/or functionality. In most cases, cells are administered after lymphodepleting preparative regimens, since lymphopenia enhances expansion of adoptively transferred T cells.65,66 Adoptive T-cell immunotherapy products can be generated using a variety of different approaches, which we discuss on this basis of the method used to enrich for tumour specificity (Table 2).
Table 2 ∣.
Adoptive T-cell therapy approaches for paediatric cancer
| Cell product | Method used to enhance tumour specificity |
Paediatric cancers tested | Caveats |
|---|---|---|---|
| Donor leukocyte infusions | None | Relapsed ALL, AML and CML post-HSCT | Efficacy in CML>AML>ALL; can also cause GVHD |
| TILs | Surgical harvest and ex vivo expansion of T cells in the tumour microenvironment | Malignant melanoma | Limited study in paediatrics; TILs not reliably generated from most paediatric cancers |
| Viral specific CTL | Repetitive stimulation with antigen expressing APCs | EBV post-transplant lymphoproliferative disorder; EBV-associated lymphoma |
CTL from third-party donors have also been effective, raising the prospect of off-the-shelf cell banks available on demand |
| Viral specific CTL | CTL selection based upon cytokine production | EBV post-transplant lymphoproliferative disorder | Short production time (1–2 days); Limited cell numbers; Difficult to obtain from naïve donors; Could be adapted to tumour-specific antigens |
| Transgenic TCRs | Engineered expression of an MHC-restricted tumour-specific TCR | Synovial sarcoma: NY-ESO-1+HLA-A2+ | MHC restriction currently limits application to HLA-A2+ patients |
| Chimeric antigen receptors | Engineered expression of a non-MHC restricted, T-cell signalling receptor | Neuroblastoma, B-acute lymphoblastic leukaemia, glioma, osteosarcoma, GD2+ sarcoma | MHC non-restricted, therefore applicable across populations; Co-stimulatory domains integrated into the receptor enhance both efficacy and toxicity |
Abbreviations: ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; APC, antigen-presenting cells; CML, chronic myeloid leukaemia; CTL, cytotoxic T lymphocyte; EBV, Epstein-Barr virus; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; HSCT, haematopoietic stem-cell transplantation; MHC, major histocompatibility complex; TCR, T-cell receptor; TIL, tumour-infiltrating lymphocyte.
Adoptive transfer of tumour infiltrating lymphocytes (TILs) transfers T cells harvested from the tumour bed, which are then expanded ex vivo. TILs have shown impressive results in metastatic malignant melanoma,67 but cannot be reliably generated from most tumour histologies and have not been developed to target paediatric cancers. Nonetheless, it is important to note that TILs were the first immunotherapy to mediate regression of large tumour burdens and some patients have remained disease free for many years after such therapy.67 These encouraging results raise the prospect that bulky tumours and metastatic disease could be cured with T-cell-based immunotherapy. Furthermore, TILs can mediate significant antitumour effects in patients with CNS metastases,68 indicating that systemic immunotherapies could also be used for immunotherapy of primary brain tumours. Studies of TILs have also provided insights into the molecules driving natural immune responses to cancer. These can be categorized into tissue differentiation antigens, cancer-testis antigens and more recently, mutated genes. One recent study demonstrated impressive results in one patient where next-generation sequencing identified a mutant tumour antigen driving expansion of tumour infiltrating lymphocytes, which mediated tumour regression upon adoptive transfer.69 Recent studies emphasizing mutated genes as immunodominant tumour antigens raise fundamental questions regarding whether the low mutation rate observed in paediatric tumours70 translates into diminished immunogenicity for paediatric cancers compared to their adult counterparts, and this issue is likely to be addressed in greater depth in the coming years.
Expanded viral and tumour antigen reactive T cells
A second approach to adoptive T-cell immunotherapy enriches antigen reactive T cells through ex vivo expansion and/or selection of antigen-specific cells. The efficacy of this approach has been demonstrated through the use of antigen-specific cytolytic T cells (CTLs) to treat viral infections in immunocompromised hosts and Epstein–Barr virus (EBV)-associated post-transplantation lymphoproliferative disease (PTLD).71-73 Most patients treated on these trials have been adults, but a sizable number of children have received these therapies and efficacy has not varied based upon age. Investigators have recently extended this approach to EBV-associated lymphomas, which are less immunogenic than PTLD-associated neoplasms. Approximately 40% of classic Hodgkin lymphoma patients expresses EBV antigens LMP1 and/or LMP2, and this is more common in children under 10 years (especially boys), and in paediatric Hodgkin lymphoma patients based in developing countries.74,75 A smaller fraction of paediatric diffuse large B-cell lymphomas are EBV associated. Outcomes recently reported for 50 children and adults with EBV-associated lymphoma who received LMP1 and/or LMP2 targeted CTLs, demonstrated no relapses among 29 patients treated in first or later remission and approximately 60% of patients treated with evident disease experienced an antitumour response.76 Thus, viral specific CTL are an effective treatment modality for viral infections occurring in immunocompromised hosts, and for PTLD occurring in states of severe immunosuppression. Recent data demonstrates that they may also have activity in children and adults with EBV-associated lymphoma.76 Given that a large fraction of patients treated with these cells received them following alloHSCT without complications related to GVHD, some groups are now attempting to use antigen selection and/or expansion to generate adoptive T-cell products targeting tumour antigens following alloHSCT to prevent leukaemic relapse.77
Genetically engineered TCR and CARs
Experience using adoptive immunotherapy and genetically engineered T-cell products with specificity for tumour-associated antigens is increasing rapidly. Transgenic T-cell receptors (TCRs) are structurally identical to native TCRs, and are major histocompatibility complex (MHC) restricted and their applicability is limited to patients who share their restricting HLA allele. Most transgenic TCRs assessed in clinical trials were derived from naturally occurring tumour reactive TCRs that have been manipulated to enhance avidity before use in clinical studies.78 While transgenic TCRs are not available to target the vast majority of paediatric cancers, there is some emerging experience using this approach in NY-ESO-1 expressing synovial sarcomas.79 In the initial report, four of six patients treated for NY-ESO-1+ synovial sarcoma had objective responses, and studies in children and adults are ongoing.80 Although RNA expression of NY-ESO-1 has been reported in a wide array of paediatric sarcomas, NY-ESO-1 protein expression is limited to synovial sarcomas; therefore, these studies are currently limited to patients with synovial sarcoma.81
Chimeric antigen receptors (CARs) are non-native receptors that incorporate an MHC non-restricted antigen-binding domain with signalling domains designed to ‘imitate’ the downstream signalling of natural T cells. Early CAR trials incorporated the TCR zeta signal to activate T cells (first-generation CARs) whereas subsequent studies have used two downstream activation signals (second-generation CARs, Figure 3) or three signals (third-generation CARs). In 2007, Park et al.82 reported treatment of six children with CARs targeting L1CAM (CD171) in patients with neuroblastoma. No toxicity was observed, however, the cells did not persist for prolonged periods and no objective responses were reported. In 2008, Pule et al.83 reported results of a study of a first generation GD2-CAR derived from the anti-GD2 mAb, 14.G2a. Objective responses occurred in two out of eight patients with evaluable disease and long-term follow-up of 19 patients treated in this manner demonstrated continued complete responses in two out of 11 patients with evaluable disease and continued remission in four out of eight patients with no evident disease at the time of GD2-CAR infusion.84 GD2-CAR therapy had no evident short-term or long-term toxicity and molecular evidence for low-level persistence of GD2-CAR T cells was found in essentially all surviving patients, with the longest duration noted to be 192 weeks. Clinical trials are currently underway using a third-generation GD2-CAR, designed to enhance potency, as discussed below.85,86
Figure 3 ∣.
Chimeric antigen receptors. The first-generation chimeric antigen receptor contains an antigen-binding domain (blue), usually comprised of an scFv from a mAb. This is genetically engineered into a receptor that contains a transmembrane domain (black), as well as a signalling domain, typically from the CD3 chain. All second-generation chimeric antigen receptors incorporate a co-stimulatory endodomain in addition to the CD3ζ domain. Co-stimulatory domains have been shown to lead to enhanced expansion and persistence compared to CARs lacking a co-stimulatory endodomain. In clinical trials of CD19-CAR for B-ALL, T cells bearing CARs expressing the 41BB endodomain can show more prolonged persistence compared to T cells bearing CARs expressing the CD28 endodomain. Third-generation CARs containing two co-stimulatory domains, in addition to the CD3ζ domain, are also being tested, but thus far, have not demonstrated greater potency than second-generation CARs. Abbreviations: CAR, chimeric antigen receptor; mAb, monoclonal antibody.
In an effort to enhance expansion and persistence of CAR T cells, several groups have incorporated additional co-stimulatory endodomains, and a clinical study using CD19-CAR T cells for treatment of lymphoma in adults confirmed enhanced expansion and persistence with these constructs.87 Thus, modern CAR T cells that deliver at least two signals upon recognition of the tumour antigen are believed to be more potent on a per cell basis than native T cells or first-generation CAR-expressing T cells. The first clinical results of CD19-CAR therapy came from studies in adults where antitumour effects were reported in CD19-expressing lymphomas and chronic lymphocytic leukaemia.88-91 More recent reports demonstrated that CD19-CAR therapy mediates very high response rates in B-ALL, both in children4,92,93 and adults.94 Two large paediatric series of CD19-CAR were reported. A phase I trial reported the use of CD19-CAR T cells co-expressing TCR zeta plus CD28 (Figure 3) to 21 patients within 11 days of enrolment on study following a consistent preparative regimen incorporating cyclophosphamide and fludarabine.4,95 The study demonstrated feasibility of generating CD19-CAR T cells meeting protocol prescribed doses in 90% of patients, and intent-to-treat analysis of all patients enrolled revealed a 66.7% complete response rate with 70% of all patients with B-ALL rendered into a complete remission and in 60% of patients, there was no evidence of minimal residual neoplastic disease. Using a non-intent-to-treat analysis and variable post-cell harvest chemotherapy regimens, Maude et al.93 reported a 90% complete remission rate in 30 patients with B-ALL treated with CD19-CAR therapy. In this study, T cells were transduced with a lentiviral vector encoding a second-generation CD19-CAR co-expressing TCR zeta plus 4-1BB (Figure 3). In both studies, complete remissions were observed in patients with disease that was refractory to standard chemotherapy, including a total of nine patients with primary chemorefractory disease. Both studies also observed trafficking of CD19-CAR cells into the cerebrospinal fluid and clearance of CNS leukaemia; toxicity in both studies was primarily related to cytokine-release syndrome.96 Despite the similarities, differential persistence of the genetically engineered T cells was noted. CD19-CAR cells expressing the CD28 endodomain showed peak expansion around day 14 and then disappeared with recovery of normal B cells within 60 days.4 By contrast, CD19-CAR cells expressing the 41BB endodomain persisted for several months with persistent B-cell aplasia.93 It remains to be seen whether CD19-CAR therapy can cure refractory B-ALL and whether this varies depending upon the co-stimulatory domain and persistence of the genetically engineered cells. This important issue will be addressed in future studies. In another study, Cruz et al.97 administered donor-derived virus-specific T cells expressing CD19 CAR after alloHSCT. GVHD was not observed and a complete response was reported in one of two patients treated with measurable B-ALL. In summary, the literature demonstrates high response rates using second-generation CD19-CAR therapy for chemorefractory B-ALL and future studies will focus on improving the safety of the therapy, improving exportability and determining how best to integrate this novel therapeutic into standard regimens for B-ALL. Several studies using CD19-CAR therapies are currently open and enrolling children with refractory B-cell malignancies.95,96,98-100
Challenges
Immune escape
It is becoming increasingly clear that immune pressure can select antigen-loss variants as a means of immune escape. This has been most notable in the context of CD19-directed treatments for B-ALL. Although B-ALL is universally CD19-positive at presentation, and CD19 apparently contributes to the survival of malignant B lymphoblasts,101 CD19-negative B-ALL emerges in a significant number of patients following blinatumomab or CD19-CAR cell therapy.3,4,46,93 Investigators are attempting to tackle this problem by targeting other molecules, and in the context of CARs for B-ALL, a CD22-directed CAR is soon to enter the clinic that demonstrates similar potency as the CD19-CARs in preclinical models.102 Bivalent therapeutics are also likely to be tested (termed tandem CARs) that seem effective in preclinical models at mitigating selection of antigen-loss variants.103 For therapies using MHC-restricted T cells, immune escape can also occur via loss of antigen expression, or downregulation of MHC, which is commonly observed.9 For this reason, simultaneous targeting using T cells and NK cells has been considered, because MHC downregulation as a means of escape from T-cell mediated responses would enhance susceptibility to NK-mediated killing.
Toxicity
Toxic effects associated with immune-based therapies are distinct from toxic effects resulting from cytotoxic regimens, and can be divided into antigen-specific and antigen nonspecific toxicities. Antigen-specific toxicities occur when immune responses are directed toward non-cancer tissues expressing the targeted antigen. Pain associated with anti-GD2 therapy, B-cell depletion associated with CD19 directed therapies, and immune mediated events occurring in patients treated with checkpoint inhibitors fall into this class. Autoimmune toxicity is especially common following treatment with ipilimumab, and occurs in over 50% of children.52 The severity of autoimmune toxicity associated with immunotherapy varies greatly depending upon the tissue targeted and the efficacy of clinical intervention. For example, autoimmune colitis in early adult studies of ipilimumab was an important cause of morbidity, but with improved clinical surveillance and management, this adverse effect is now largely controlled. By contrast, hypophysitis associated with ipilimumab usually leads to lifelong panhypopituitarism.104
In the context of adoptive T-cell therapies, autoimmune antigen-specific toxicity can be severe and even fatal. Two separate clinical trials using T cells targeting the cancer testis antigen MAGE-A3 in adults resulted in fatal toxicity owing to previously unappreciated expression of the target or a crossreactive peptide on cardiac and neural tissues.105,106 For this reason, first-in-human adoptive T-cell therapies must carefully consider the potential for unexpected on-target and off-tumour toxicity because the severity of the effect is predicted to increase as the immunotherapeutics acquires enhanced potency. One approach used when the risk of such toxicities is deemed to be high, is to incorporate a suicide domain into the receptors, which allows one to rapidly eradicate the genetically engineered cells. Proof-of-principle for this approach has been demonstrated in the context of allogeneic DLI for GVHD, and such a vector is currently in use with chimeric antigen receptors targeting GD2 on paediatric cancers.107 Alternatively, platforms that transiently express the targeted receptor as a means to minimize the risk of toxicity are in early phase trials.
The second major class of toxicities associated with immunotherapies for treating cancer is the cytokine-release syndrome. Here, supraphysiologic levels of immune cell activation lead to high levels of inflammatory cytokines, which mediate a variety of toxic effects and can result in multisystem failure and death. Cytokine-release syndrome was first observed following treatment with monoclonal antibodies for cancer and has been observed in the context of adoptive cell-based therapies, especially those using second-generation and third-generation CARs.108 The syndrome is characterized by fever and constitutional symptoms that can progress to cardiac, renal, hepatic or neurological dysfunction. In non-controlled trials, treatment with anti-IL6R monoclonal antibody and/or corticosteroids effectively reverses the syndrome.108 Given the potential severity of this syndrome as well as its eminent treatability, it is imperative that clear guidelines for grading and treatment of cytokine-release syndrome are incorporated into early phase immunotherapy trials where this adverse effect is likely to occur.
New targets
Recent advances in genetic engineering have allowed the generation of monoclonal antibodies and/or T cells bearing an almost infinite array of specificities, and improved understanding of immune cell biology renders optimism that potent immune responses can be induced in most cancer patients. However, a fundamental barrier to progress is a paucity of immune targets for cancer in general and paediatric cancers in particular. Optimal immune targets should be highly expressed on tumour tissues and not expressed on normal tissues, and a role for the target in oncogenesis is preferred, albeit not required. Beyond mutated tumour antigens, which are individual-specific, tumour-specific targets are rare. Alternatives include targeting molecules expressed on tumour cells and non-vital tissues, such as CD19. Although CD19 targeted therapies induce profound B-cell depletion, this is manageable in the short-term and even potentially in the long-term.89 For T-cell malignancies, myeloid neoplasms and solid tumours defining targets restricted to tumour and non-vital tissues has proven difficult. Studies are underway using novel genomic approaches to identify targets for immunotherapy of paediatric cancers and results of these studies are anxiously awaited.109 Another possibility is to use combinatorial targeting, especially using CARs, which could allow one to target pairs of antigens on tumours when such pairs are not expressed on normal tissues.110 Tumours can also express aberrant glycosylation patterns that could be exploited with some monoclonal antibodies or their derivatives, but would not be identified using genomic approaches. An important consideration is that targeting of tumours using antibody-based approaches including CAR T cells requires cell surface expression, thus excluding a large group of intracellular proteins that are differentially expressed in tumours. For these antigens, T-cell receptor-based approaches are required. Continued success in the field of immunotherapy for paediatric cancer will require a concerted and creative effort by the paediatric oncology community to identify optimal targets for this exciting new class of therapeutics.
Technical challenges
Other challenges involve the export of immunotherapies out of major academic centres. This is particularly relevant to cell-based immunotherapies for which specialized facilities for production of cells is needed. Owing to the need for these specialized facilities, it is anticipated that such therapies could incur substantial cost. For this reason, ‘off-the-shelf’ immunotherapies, such as bispecific antibodies that can engage host immune cells without the need for ex vivo cell generation, might be preferred if response rates and toxicities are similar. It remains to be seen whether such off-the-shelf reagents will be a potent as cell-based immunotherapy, and whether a lack of persistent immune pressure induced with antibody versus cellular therapies will impact overall outcomes.
Conclusions
Immunotherapy is one of the most rapidly moving fields in cancer therapy. The momentum is driven by evidence that immune-based killing can eradicate chemoresistant tumour cells combined with dramatic technological advances that allow efficient engineering of complex monoclonal antibodies and T-cell-based therapeutics and effective ex vivo and in vivo expansion of T-cell and NK effector populations. Current trials tend to focus on one modality, but it is anticipated that combinations of immune-based therapies will be additive or even synergistic and, therefore, combinatorial regimens are likely to emerge during the next decade. The paediatric oncology community will play a vital role in bringing these powerful therapeutics to help treat childhood cancer, by identifying novel targets, developing algorithms for diagnosis and management of immunotherapy-related toxicities and through identifying optimal approaches for incorporating this new class of therapeutics into standard treatment regimens to maximize cure and minimize toxicities.
Key points.
Graft-versus-leukaemic effects improve survival following allogeneic stem cell transplantation for childhood leukaemia, and provide proof-of-principle that immune mediated effects can eradicate chemoresistant cancer cells
Anti-GD2 monoclonal antibodies can be curative when administered in the setting of minimal residual disease for neuroblastoma, and efficacy requires optimal induction of antibody-dependent cell-mediated cytotoxicity (ADCC)
In general, tumour vaccines do not regress established tumours, but can diminish tumour growth or recurrence; randomized studies will be needed to define their efficacy in paediatric cancer
Adoptive T-cell immunotherapies have demonstrated efficacy against Epstein–Barr virus-associated lymphoproliferative neoplasms and impressive results have been reported using genetically engineered T cells to treat paediatric leukemia
Expansion of the immunotherapeutic armamentarium for paediatric cancers hinges on identification of new immune targets
Toxicity associated with immunotherapies is distinct from toxicity associated with cytotoxic agents and small-molecule kinase inhibitors; most common toxic effects include cytokine-release syndrome and autoimmune reactions
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health.
Footnotes
Competing interests
C.L.M. is a co-inventor on a patent for a CD22-CAR that will soon enter clinical trials. M.S.M. and T.J.F. declare no competing interests.
References
- 1.Smith MA, Altekruse SF, Adamson PC, Reaman GH & Seibel NL Declining childhood and adolescent cancer mortality. Cancer 120, 2497–2506 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu AL et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med 363, 1324–1334 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grupp SA et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med 368, 1509–1518 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DW et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet 10.1016/S0140-6736(14)61403-3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankester AC et al. Will post-transplantation cell therapies for paediatric patients become standard of care? Biol Blood Marrow Transplant 10.1016/j.bbmt.2014.07.018 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri L et al. Effectiveness of donor natural killer cell alloreactivity in mismatched haematopoietic transplants. Science 295, 2097–2100 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Bari R et al. Effect of donor KIR2DL1 allelic polymorphism on the outcome of paediatric allogeneic haematopoietic stem-cell transplantation. J. Clin. Oncol 31, 3782–3790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung W Use of NK cell activity in cure by transplant. Br. J. Haematol 155, 14–29 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Leone P et al. MHC class I antigen processing and presenting machinery: organization, function, and defects in tumour cells. J. Natl Cancer Inst 105, 1172–1187 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Moretta F, Brescia L & Merli P Natural killer cells in the treatment of high-risk acute leukaemia. Semin. Immunol 26, 173–179 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Zhou H et al. Donor selection for killer immunoglobulin-like receptors B haplotype of the centromeric motifs can improve the outcome after HLA-identical sibling haematopoietic stem cell transplantation. Biol. Blood Marrow Transplant 20, 98–105 (2014). [PubMed] [Google Scholar]
- 12.Schumm M et al. Depletion of T-cell receptor alpha/beta and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy 15, 1253–1258 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Michaelis SU et al. KIR haplotype B donors but not KIR-ligand mismatch result in a reduced incidence of relapse after haploidentical transplantation using reduced intensity conditioning and CD3/CD19-depleted grafts. Ann. Haematol 10.1007/s00277-014-2084-2 (2014). [DOI] [PubMed] [Google Scholar]
- 14.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01919866 (2013).
- 15.Miller JS et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Rubnitz JE et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol 28, 955–959 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho D et al. Cytotoxicity of activated natural killer cells against paediatric solid tumours. Clin. Cancer Res 16, 3901–3909 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H et al. Activating signals dominate inhibitory signals in CD137L/IL-15 activated natural killer cells. J. Immunother 34, 187–195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01287104 (2014).
- 20.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01944982 (2014).
- 21.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01807468 (2014).
- 22.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01974479NLM (2014).
- 23.Merchant MS et al. Phase I trial and pharmacokinetic study of lexatumumab in paediatric patients with solid tumours. J. Clin. Oncol 30, 4141–4147 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT00743496 (2014).
- 25.Alvarez-Rueda N et al. A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumour activity without peripheral nervous system crossreactivity. PLoS ONE 6, e25220 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung NK, Burch L, Kushner BH & Munn DH Monoclonal antibody 3F8 can effect durable remissions in neuroblastoma patients refractory to chemotherapy: a phase II trial. Prog. Clin. Biol. Res 366, 395–400 (1991). [PubMed] [Google Scholar]
- 27.Frost JD et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14.G2a plus interleukin-2 in children with refractory neuroblastoma: a report of the Children’s Cancer Group. Cancer 80, 317–333 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Ozkaynak MF et al. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after haematopoietic stem-cell transplantation: a Children’s Cancer Group Study. J. Clin. Oncol 18, 4077–085 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Simon T et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J. Clin. Oncol 22, 3549–3557 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Cheung NK et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol 30, 3264–3270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shusterman S et al. Antitumour activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J. Clin. Oncol 28, 4969–4975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delgado DC et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 70, 9554–9561 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venstrom JM et al. KIR and HLA genotypes are associated with disease progression and survival following autologous haematopoietic stem cell transplantation for high-risk neuroblastoma. Clin. Cancer Res 15, 7330–7334 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT00877110 (2014).
- 35.Chao MP et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142, 699–713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roth M et al. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 120, 548–554 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Navid F et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol 32, 1445–1452 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barth M, Raetz E & Cairo MS The future role of monoclonal antibody therapy in childhood acute leukaemias. Br. J. Haematol 159, 3–17 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Dunleavy K et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med 368, 1408–1416 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samochatova EV et al. Therapy of advanced-stage mature B-cell lymphoma and leukemia in children and adolescents with rituximab and reduced intensity induction chemotherapy (B-NHL 2004M Protocol): the results of a multicentre study. J. Paediatr. Haematol. Oncol 36, 395–401 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Wayne AS et al. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive haematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin. Cancer Res 16, 1894–1903 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wayne AS, Fitzgerald DJ, Kreitman RJ & Pastan I Immunotoxins for leukemia. Blood 123, 2470–2477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palanca-Wessels MC & Press OW Advances in the treatment of haematologic malignancies using immunoconjugates. Blood 123, 2293–2301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sievers EL & Senter PD Antibody-drug conjugates in cancer therapy. Annu. Rev. Med 64, 15–29 (2013). [DOI] [PubMed] [Google Scholar]
- 45.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT02101853 (2014).
- 46.Topp MS et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol 29, 2493–2498 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Schlegel P et al. Paediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab. Haematologica 99, 1212–1219 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Stackelberg A et al. A Phase 1/2 Study of Blinatumomab in Paediatric Patients with Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia in American Society of Haematology [abstract], ASH Annual Meeting a70 (2013). [Google Scholar]
- 49.Teachey DT et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121, 5154–5157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vyas M, Koehl U, Hallek M & von Strandmann EP Natural ligands and antibody-based fusion proteins: harnessing the immune system against cancer. Trends Mol. Med 20, 72–82 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merchant MS, Baird K, Wexler LH, Rodriguez-Galindo R & Mackall CL Ipilimumab: First results of a phase I trial in paediatric patients with advanced solid tumours [abstract]. J. Clin. Oncol 30 (Suppl.), a9545 (2012). [Google Scholar]
- 53.Topalian SL et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Bruggen P et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991). [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg SA, Yang JC & Restifo NP Cancer immunotherapy: moving beyond current vaccines. Nat. Med 10, 909–915 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kantoff PW et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med 363, 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Madan RA, Gulley JL, Fojo T & Dahut WL Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist 15, 969–975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein WD et al. Tumour regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin. Cancer Res 17, 907–917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robert-Tissot C, Nguyen LT, Ohashi PS & Speiser DE Mobilizing and evaluating anticancer T cells: pitfalls and solutions. Expert Rev. Vaccines 12, 1325–1340 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Kantoff PW et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol 28, 1099–1105 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sampson JH et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol 28, 4722–4729 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mackall CL et al. Survival in metastatic Ewing sarcoma (EWS) and rhabdomyosarcoma (RMS) following consolidation immunotherapy with autologous lymphocyte infusion, dendritic cell vaccines ± CYT107 (rhIL-7) [abstract]. J. Clin. Oncol 31 (Suppl.), a10013 (2013). [Google Scholar]
- 63.Scholler J et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med 4, 132ra53 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gattinoni L, Powell DJ Jr, Rosenberg SA & Restifo NP Adoptive immunotherapy for cancer: building on success. Nat. Rev. Immunol 6, 383–393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui Y et al. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood 114, 3831–3840 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dudley ME et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol 23, 2346–2357 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosenberg SA et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res 17, 550–557 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong JJ et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin. Cancer Res 16, 4892–898 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran E et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawrence MS et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leen AM et al. Multicentre study of banked third-party virus-specific T cells to treat severe viral infections after haematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Doubrovina E et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic haematopoietic cell transplantation. Blood 119, 2644–2656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Icheva V et al. Adoptive transfer of Epstein-Barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J. Clin. Oncol 31, 39–48 (2013). [DOI] [PubMed] [Google Scholar]
- 74.Kapatai G & Murray P Contribution of the Epstein Barr virus to the molecular pathogenesis of Hodgkin lymphoma. J. Clin. Pathol 60, 1342–1349 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuppers R, Engert A & Hansmann ML Hodgkin lymphoma. J. Clin. invest 122, 3439–3447 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bollard CM et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol 32, 798–808 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01430390 (2014).
- 78.Robbins PF et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. immunol 180, 6116–6131 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robbins PF et al. Tumour regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol 29, 917–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01343043 (2014).
- 81.Lai JP et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumours: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod. Pathol 25, 854–858 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park JR et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther 15, 825–833 (2007). [DOI] [PubMed] [Google Scholar]
- 83.Pule MA et al. Virus-specific T cells engineered to coexpress tumour-specific receptors: persistence and antitumour activity in individuals with neuroblastoma. Nat. Med 14, 1264–1270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Louis CU et al. Antitumour activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01822652 (2014).
- 86.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT02107963 (2014).
- 87.Savoldo B et al. CD28 co-stimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. invest 121, 1822–1826 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kochenderfer JN et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brentjens RJ et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porter DL, Levine BL, Kalos M, Bagg A & June CH Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med 365, 725–733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kochenderfer JN et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grupp SA et al. 67 T cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) produce significant in vivo proliferation, complete responses and long-term persistence without GVHD in children and adults with relapsed, refractory ALL [abstract]. ASH Annual Meeting a67 (2013). [Google Scholar]
- 93.Maude SL et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med 10.1056/NEJMoa1407222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davila ML et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med 6, 224ra25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01593696 (2014).
- 96.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01626495 (2014).
- 97.Cruz CR et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 122, 2965–2973 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT00586391 (2014).
- 99.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT02028455 (2014).
- 100.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/show/NCT01860937 (2014).
- 101.Chung EY et al. CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J. Clin. invest 122, 2257–2266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haso W et al. Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hegde M et al. Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol. Ther 21, 2087–2101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fecher LA, Agarwala SS, Hodi FS & Weber JS Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist 18, 733–743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morgan RA et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. immunother 36, 133–151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cameron BJ et al. Identification of a Titin-derived HLA-A1-presented peptide as a crossreactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med 5, 197ra103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Di Stasi A et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med 365, 1673–1683 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee DW et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orentas RJ et al. Identification of cell surface proteins as potential immunotherapy targets in 12 paediatric cancers. Front. Oncol 2, 194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kloss CC, Condomines M, Cartellieri M, Bachmann M & Sadelain M Combinatorial antigen recognition with balanced signalling promotes selective tumour eradication by engineered T cells. Nat. Biotechnol 31, 71–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]