Abstract
Background
Perioperative fluid management is a crucial element of perioperative care and has been studied extensively recently; however, 'the right amount' remains uncertain. One concept in perioperative fluid handling is goal‐directed fluid therapy (GDFT), wherein fluid administration targets various continuously measured haemodynamic variables with the aim of optimizing oxygen delivery. Another recently raised concept is that perioperative restrictive fluid therapy (RFT) may be beneficial and at least as effective as GDFT, with lower cost and less resource utilization.
Objectives
To investigate whether RFT may be more beneficial than GDFT for adults undergoing major non‐cardiac surgery.
Search methods
We searched the following electronic databases on 11 October 2019: Cochrane Central Register of Controlled Trials, in the Cochrane Libary; MEDLINE; and Embase. Additionally, we performed a targeted search in Google Scholar and searched trial registries (World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov) for ongoing and unpublished trials. We scanned the reference lists and citations of included trials and any relevant systematic reviews identified.
Selection criteria
We included randomized controlled trials (RCTs) comparing perioperative RFT versus GDFT for adults (aged ≥ 18 years) undergoing major non‐cardiac surgery.
Data collection and analysis
Two review authors independently screened references for eligibility, extracted data, and assessed risk of bias. We resolved discrepancies by discussion and consulted a third review author if necessary. When necessary, we contacted trial authors to request additional information. We presented pooled estimates for dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs), and for continuous outcomes as mean differences (MDs) with standard deviations (SDs). We used Review Manager 5 software to perform the meta‐analyses. We used a fixed‐effect model if we considered heterogeneity as not important; otherwise, we used a random‐effects model. We used Poisson regression models to compare the average number of complications per person.
Main results
From 6396 citations, we included six studies with a total of 562 participants. Five studies were performed in participants undergoing abdominal surgery (including one study in participants undergoing cytoreductive abdominal surgery with hyperthermic intraperitoneal chemotherapy (HIPEC)), and one study was performed in participants undergoing orthopaedic surgery. In all studies, surgeries were elective. In five studies, crystalloids were used for basal infusion and colloids for boluses, and in one study, colloid was used for both basal infusion and boluses. Five studies reported the ASA (American Society of Anesthesiologists) status of participants. Most participants were ASA II (60.4%), 22.7% were ASA I, and only 16.9% were ASA III. No study participants were ASA IV. For the GDFT group, oesophageal doppler monitoring was used in three studies, uncalibrated invasive arterial pressure analysis systems in two studies, and a non‐invasive arterial pressure monitoring system in one study. In all studies, GDFT optimization was conducted only intraoperatively. Only one study was at low risk of bias in all domains. The other five studies were at unclear or high risk of bias in one to three domains.
RFT may have no effect on the rate of major complications compared to GDFT, but the evidence is very uncertain (RR 1.61, 95% CI 0.78 to 3.34; 484 participants; 5 studies; very low‐certainty evidence). RFT may increase the risk of all‐cause mortality compared to GDFT, but the evidence on this is also very uncertain (RD 0.03, 95% CI 0.00 to 0.06; 544 participants; 6 studies; very low‐certainty evidence). In a post‐hoc analysis using a Peto odds ratio (OR) or a Poisson regression model, the odds of all‐cause mortality were 4.81 times greater with the use of RFT compared to GDFT, but the evidence again is very uncertain (Peto OR 4.81, 95% CI 1.38 to 16.84; 544 participants; 6 studies; very low‐certainty evidence). Nevertheless, sensitivity analysis shows that exclusion of a study in which the final volume of fluid received intraoperatively was higher in the RFT group than in the GDFT group revealed no differences in mortality. Based on analysis of secondary outcomes, such as length of hospital stay (464 participants; 5 studies; very low‐certainty evidence), surgery‐related complications (364 participants; 4 studies; very low‐certainty evidence), non‐surgery‐related complications (74 participants; 1 study; very low‐certainty evidence), renal failure (410 participants; 4 studies; very low‐certainty evidence), and quality of surgical recovery (74 participants; 1 study; very low‐certainty evidence), GDFT may have no effect on the risk of these outcomes compared to RFT, but the evidence is very uncertain. Included studies provided no data on administration of vasopressors or inotropes to correct haemodynamic instability nor on cost of treatment.
Authors' conclusions
Based on very low‐certainty evidence, we are uncertain whether RFT is inferior to GDFT in selected populations of adults undergoing major non‐cardiac surgery. The evidence is based mainly on data from studies on abdominal surgery in a low‐risk population. The evidence does not address higher‐risk populations or other surgery types. Larger, higher‐quality RCTs including a wider spectrum of surgery types and a wider spectrum of patient groups, including high‐risk populations, are needed to determine effects of the intervention.
Plain language summary
Is limiting the amount of fluid given to adults during surgery as good as using haemodynamic monitoring, which continuously measures changes in blood pressure or speed of blood flow inside the arteries, to guide fluid administration?
Review question
Our objective was to review evidence from randomized controlled trials (RCTs) on whether limiting the amount of fluid given to adults during surgery is as good as using haemodynamic monitoring to guide fluid administration. RCTs are clinical studies in which people are randomly put into one of two or more treatment groups. Haemodynamic monitoring is continuous, beat‐to‐beat measurement of changes in blood pressure or speed of blood flow inside the arteries.
Background
During operations, adults receive additional fluids into their veins (intravenously) to cover their normal needs for fluid and to supplement any fluids lost during surgery because of bleeding, or for other reasons, for example, increased perspiration. It still is not clearly understood how much fluid should be given to adults during surgery. In the past, a lot of fluid was given during operations because it was thought that a large amount of fluid vaporizes during surgery from open cavities, lungs, and skin, and that a lot of fluid is accumulated in operated tissues, and because people require a long fasting time before surgery. Many new studies have disputed these findings, and recently, techniques that use haemodynamic monitoring have been developed to guide doctors on how much fluid is actually necessary during surgery. This technique is called goal‐directed fluid therapy (GDFT). Another concept is that simply giving less fluid than was recommended in the past may confer the same benefit. This technique is called restrictive fluid therapy (RFT). RFT is cheaper and easier to use because it does not require additional equipment.
Study characteristics
The evidence is current to 11 October 2019. We included studies that randomly assigned adults to intervention groups comparing the two techniques described above. We found six studies including a total of 562 participants. Five studies involved abdominal surgery, and one involved orthopaedic surgery. No studies involved emergency surgery nor patients suffering from serious medical conditions before surgery.
Key results
The number of deaths was slightly lower in the GDFT group compared with the RFT group, but this may be due to chance. No difference in the frequency of major complications was observed between the two groups. In addition, no differences were observed between RFT and GDFT groups in the following outcomes: length of hospital stay, surgery‐related complications (related directly to the operation site, e.g. problems with wound healing), non‐surgery‐related complications (related to problems with other organs, e.g. heart or lungs), renal failure, and quality of surgical recovery.
Certainty of evidence
We judged the certainty of evidence obtained for this review as very low because conclusions are based on very small numbers of participants in included studies, the quality of included studies is low, and studies were performed only on selected groups of patients that did not reflect the real population of people undergoing surgery. This means that new studies are very likely to change the results of this review. The review does not answer the question of whether results would be the same for adults who have other serious health problems before surgery, or for adults undergoing other types of surgery besides abdominal surgery and orthopaedic surgery.
Summary of findings
Summary of findings for the main comparison. Perioperative restrictive fluid therapy compared with goal‐directed fluid therapy for adults undergoing major non‐cardiac surgery.
| Perioperative restrictive fluid therapy compared with goal‐directed fluid therapy for adults undergoing major non‐cardiac surgery | ||||||
|
Population: adults receiving intravenous fluids while undergoing major non‐cardiac surgery Settings: major non‐cardiac surgery in hospitals in Europe, Australia, New Zealand, or China Intervention: restrictive fluid therapy Comparison: goal‐directed fluid therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| GDFT | RFT | |||||
|
Major complications (during longest follow‐up period ‐ 30 days after surgery) |
Lower‐risk population | RR 1.61 (0.78 to 3.34) | 484 (5) | ⊕⊝⊝⊝ Very lowa | ||
| 20 per 1000 | 32 per 1000 (16 to 67) | |||||
| Medium‐risk population | ||||||
| 105 per 1000 | 169 per 1000 (82 to 351) | |||||
| Higher‐risk population | ||||||
| 189 per 1000 | 304 per 1000 (147 to 631) | |||||
|
All‐cause mortality (during longest follow‐up period ‐ 30 days after surgery or until discharge) |
14 per 1000 | 68 per 1000 (20 to 238) | RD 0.03 (0.00 to 0.06) | 544 (6) | ⊕⊝⊝⊝ Very lowb | Peto OR 4.81 (1.38 to 16.84) |
|
Length of hospital stay (days) |
Mean length of stay ranged across control groups from 6.67 to 10.7 days | Mean length of stay in the intervention groups was 0.02 days less (0.55 days lower to 0.5 days higher) | MD ‐0.02 (‐0.55 to 0.50) | 464 (5) | ⊕⊝⊝⊝ Very lowc | |
|
Surgery‐related complications
(during longest follow‐up period ‐ 30 days after surgery or until discharge) |
Lower‐risk population | RR 1.54 (0.87 to 2.72) | 364 (4) | ⊕⊝⊝⊝ Very lowd | Surgery‐related complications were defined as tissue‐healing complications in one study; major abdominal complications in one study; surgical complications in one study (including intra‐abdominal collections, anastomotic leak, wound infection, and ileus); and surgical site infection or bowel obstruction in one study | |
| 50 per 1000 | 77 per 1000 (44 to 136) | |||||
| Medium‐risk population | ||||||
| 113 per 1000 | 174 per 1000 (99 to 307) | |||||
| Higher‐risk population | ||||||
| 378 per 1000 | 582 per 1000 (329 to 1028) | |||||
|
Non‐surgery‐related complications (during longest follow‐up period ‐ 30 days after surgery) |
324 per 1000 | 324 per 1000 (169 to 625) | RR 1.00 (0.52 to 1.93) | 74 (1) | ⊕⊝⊝⊝ Very lowe | Non‐surgery‐related complications included cardiorespiratory, urinary, haemorrhage, and other complications |
|
Renal failure (during longest follow‐up period ‐ 30 days after surgery) |
Lower‐risk population | RR 1.38 (0.57 to 3.36) | 410 (4) | ⊕⊝⊝⊝ Very lowf | ||
| 13 per 1000 | 18 per 1000 (7 to 44) | |||||
| Higher‐risk population | ||||||
| 125 per 1000 | 173 per 1000 (71 to 420) | |||||
|
Quality of surgical recovery assessed in any way (e.g. as a surgical recovery score) (during longest follow‐up period ‐ 30 days after surgery) |
Data presented only on a graph in the study | 74 (1) |
⊕⊝⊝⊝ Very lowg | Study authors reported no difference in SRS between RFT and GDFT groups at any point (day 1, 3, 7, 14, or 30) | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GDFT: goal‐directed fluid therapy; MD: mean difference; OR: odds ratio; RD: risk difference; RFT: restrictive fluid therapy; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded one level for study limitations (two studies were judged at high risk of bias in the incomplete outcome data domain; 'worst‐case scenario' analysis for missing data influenced the results), one level for imprecision of results (optimal information size not met, small number of events, wide confidence intervals), and one level for indirectness of evidence (most studies were performed on abdominal surgery, most included participants were ASA II, RFT protocols were imprecise).
bDowngraded one level for study limitations (two studies were judged at high risk of bias in the incomplete outcome data domain; 'worst‐case scenario' analysis for missing data influenced the results), one level for indirectness of evidence (most studies were performed on abdominal surgery, most included participants were ASA II, RFT protocols were imprecise), and one level for imprecision of results (optimal information size not met, small number of events, wide confidence intervals).
cDowngraded one level for study limitations (one study was judged at high risk of bias in the blinding of participants and personnel domain, one study at unclear risk of bias in the blinding of participants and personnel domain, and one study at high risk of bias in incomplete outcome data) and one level for indirectness of evidence (most studies were on abdominal surgery, most included participants were ASA II, RFT protocols were imprecise), and one level forimprecision of results (optimal information size not met, confidence intervals crossing the line of no effect and including both benefit and harm).
dDowngraded one level for study limitations (two studies were judged at high risk of bias in blinding of participants and personnel domain, one study at unclear risk of bias in blinding of participants and personnel domain, and two studies at high risk of bias in Incomplete outcome data domain); one level for imprecision of results (optimal information size not met, small number of events, wide confidence intervals crossing the line of no effect and including both benefit and harm); and one level for indirectness of evidence (most studies were on abdominal surgery, most included participants were ASA II, RFT protocols were imprecise).
eDowngraded one level forstudy limitations in the included study (judged at high risk of bias in Incomplete outcome data domain) and one level for indirectness of evidence (included study was performed on abdominal surgery, most included participants were ASA II, RFT protocol was imprecise), and one level for imprecision of results (optimal information size not met ‐ single study with small number of participants and few events, wide confidence intervals crossing the line of no effect, and including both benefit and harm).
fDowngraded one level for study limitations (one study was judged at high risk of bias in blinding of participants and personnel domain, and one study at unclear risk of bias in blinding of participants and personnel domain); one level for indirectness of evidence (most studies were on abdominal surgery, most included participants were ASA II, RFT protocols were imprecise); and one level for imprecision of results (optimal information size not met, small number of events, wide confidence intervals crossing the line of no effect and including both benefit and harm).
gDowngraded one level for study limitations in the included study (one study judged at high risk of bias in incomplete outcome data domain); one level for indirectness of evidence (included study was performed on abdominal surgery, most included participants were ASA II, RFT protocol was imprecise); and one level for imprecision of results (optimal information size not met ‐ single study with small number of participants and few events).1Based on population risk in the included studies.
Background
Description of the condition
Major surgery may be associated with a high rate of complications, many of which may be avoidable (Jhanji 2008). Perioperative complications strongly correlate with long‐term mortality and morbidity and generate increased healthcare costs (Khuri 2005). Depending on the type of procedure performed, the average complication rate may vary from 5% to 64%. Colorectal surgery undertaken in accordance with procedures of 'traditional' perioperative care may involve complication rates of approximately 15% to 35%, as reported in meta‐analyses of clinical trials (Nygren 2012; Varadhan 2010; Zhuang 2013); evidence concerning orthopaedic surgery suggests complication rates of 5% to 16% (Barbieri 2009; Molina 2015); major vascular surgery is associated with complication rates of 16% to 44% (Garcia 2009; Lange 2009); and in major urological surgery, meta‐analyses report complication rates of 20% to 64% (Shabsigh 2009; Svatek 2010). Implementation of enhanced recovery after surgery (ERAS) programmes leads to a decrease in overall perioperative complication rates in major surgery. ERAS programmes postulate that multiple, relatively minor interventions, when combined, result in a significant cumulative beneficial effect. These interventions include adjustment of long‐term medication (Lewis 2018), alteration of lifestyle factors (Egholm 2018), use of intraoperative anaesthetic measures (Guay 2018), and good pain relief after surgery (Salicath 2018). A recent meta‐analysis estimated that implementation of elements of the ERAS protocol leads to a 40% reduction in overall morbidity in colorectal surgery (Greco 2014). One of the major elements of ERAS programmes is perioperative fluid restriction (Awad 2013; Cao 2012; Feldheiser 2016; Güenaga 2011; Smith 2011).
Perioperative fluid management is a crucial element of perioperative care and is currently one of the most frequently discussed issues of perioperative medicine. The goals are to restore and maintain fluid and electrolyte physiological balance in situations where patients are unable to control their own fluid intake, and to ensure adequate circulating volume, which will, in turn, secure adequate tissue perfusion and oxygenation (Nygren 2012; Varadhan 2010). Intravenous fluids can also provide other benefits such as reducing nausea and vomiting (Jewer 2019). Fluid management in the perioperative period has been extensively studied (Odor 2018), but despite this, understanding of 'the right amount' remains uncertain (Corcoran 2012).
One of the concepts of perioperative fluid handling is goal‐directed fluid therapy (GDFT). This is a perioperative strategy, wherein fluid administration targets continuously measured haemodynamic variables, such as cardiac output, stroke volume, stroke volume variation, pulse pressure variation, and other factors, with the aim of optimizing tissue perfusion and oxygen delivery (Corcoran 2012; Hahn 2017; Joosten 2015). Some approaches to GDFT can be based on assessment of non‐haemodynamic variables, such as lactate levels or superior vena cava oxygen saturation (ScvO₂). It has been shown in clinical trials and meta‐analyses that GDFT leads to a reduction in perioperative complications and mortality (Cecconi 2013; Hamilton 2011), especially in people at high perioperative risk (Hamilton 2011; Pearse 2014), and in situations where there is large intravascular fluid loss (Miller 2015; Mythen 2012). Such an approach has been recommended in many guidelines (Cecconi 2013; Feldheiser 2012; Gan 2002; Gustafsson 2013; Mythen 2012; Soni 2009; Vallet 2013).
Recently, another concept has been raised, suggesting that perioperative restrictive fluid therapy (RFT), also referred to as a near‐zero perioperative fluid balance or a zero‐balance approach, may also be beneficial and at least as effective as GDFT. Moreover, it may not involve additional costs and resource utilization, as are incurred with GDFT (Brandstrup 2012).
Description of the intervention
Restrictive fluid therapy, also called a zero‐fluid balance, is distinct from 'traditional' fluid management (also referred to as standard or liberal), which is still recommended in medical textbooks and articles and is a common clinical practice (Brandstrup 2006; Chappell 2008). The standard fluid approach is based on high fluid requirements. Commonly, a 4‐2‐1 rule is used to calculate basal fasting requirements (mL/h = 4 × first 10 kg + 2 × 10 kg + 1 × every kg bodyweight after), and additional amounts of fluid are given to cover blood loss, vaporization, and losses to the so‐called 'third space'. This approach, however, has recently been questioned, with some suggestion that the amounts of fluid proposed might be overestimated (Chappell 2008; Feldheiser 2016; Woodcock 2012).
RFT is not clearly defined in the medical literature. Generally, this approach proposes much smaller perioperative fluid infusion volumes than are used in the 'traditional' approach. The amount of fluid infused should cover basal fluid requirements and fluid losses associated directly with surgery, mainly due to surgical bleeding. These losses should be covered, usually in a 1:1 ratio, to avoid tissue cumulation. No additional fluid should be infused to cover losses to the so‐called 'third space' postulated in the past, since its existence has not been confirmed in more recent studies using sounder methods of measurement (Brandstrup 2006; Jacob 2009). Insensible perspiration from the skin is negligible and has been shown to be 0.3 mL/kg/h in an awake adult and during surgery (Lamke 1977b; Reithner 1980). Insensible perspiration from the airways is absent because during surgery, people are ventilated with moist air. Perspiration from the abdominal cavity during large abdominal surgery is also negligible, since it is estimated to vary between 2 and 32 grams/h depending on incision size and time of possible bowel exteriorization (Lamke 1977a). Additionally, preoperative fasting probably does not significantly influence blood volume (Chappell 2008; Jacob 2008). RFT should aim for unchanged postoperative body weight, while not impairing circulation, tissue perfusion, or oxygenation (Della Rocca 2014; Voldby 2016).
Restrictive fluid therapy has shown advantages over standard fluid therapy in some clinical trials and meta‐analyses of abdominal surgery (Brandstrup 2003; Nisanevich 2005; Rahbari 2009). It has been widely incorporated and recommended in ERAS programmes and constitutes a crucial element of them (Feldheiser 2016).
How the intervention might work
The rationale for perioperative fluid therapy is based on an assumption of keeping normal volaemic status and efficient peripheral tissue perfusion, while reducing the risk of fluid and electrolyte overdose. Fluid excess may lead to shifting of intravascular volume into interstitial space and accumulation of fluid in this area. This may be reflected by postoperative weight gain up to 10 kg, which directly correlates with mortality (Lowell 1990). Such findings may suggest that the 'traditional' fluid requirement calculations are overestimated.
Hypervolaemia has been shown to cause damage to the glycocalyx, an endovascular structure responsible for the integrity of the endothelium. Damage to the glycocalyx leads to fluid shift into interstitial space. Atrial natriuretic peptide (ANP) also plays an important role in this mechanism, and ANP is secreted during hypervolaemia (Bruegger 2005). In situations where the glycocalyx is damaged, such as ischaemia, inflammation, surgery, and acute hypervolaemia, colloids as well as crystalloids leak through the vascular barrier into the interstitial space and collect there (Bruegger 2005; Chappell 2008).
These preclinical findings may suggest that a reduction in the dose of fluid may have beneficial effects in a clinical setting, and that the benefit of GDFT may be due to fluid dose reduction in comparison with standard abundant fluid therapy. Based on this assumption, fluid restriction may potentially lead to the same benefit as is observed with GDFT.
Why it is important to do this review
RFT may offer benefits comparable with GDFT to people undergoing major surgery. New RCTs have been conducted recently to address this issue (Brandstrup 2012; Phan 2014; Srinivasa 2013; Zhang 2012); however, no systematic review has so far evaluated this new evidence. In this review, we try to determine the role of RFT in modern perioperative care.
Objectives
To investigate whether perioperative restrictive fluid therapy (RFT) may be more beneficial than goal‐directed fluid therapy (GDFT) for adults undergoing major non‐cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We excluded observational studies and quasi‐randomized trials.
Types of participants
We included studies in adults (aged ≥ 18 years) undergoing major non‐cardiac surgery.
Major surgery was defined as grade II or grade III surgery according to Johns Hopkins criteria (Donati 2004; Appendix 1), which grade surgical procedures depending on surgical risk. If we noted variability within a study, we considered that study to fulfil the criteria if at least 80% of participants met the requirements.
We considered studies including patients undergoing elective or emergency surgery, or both.
Types of interventions
We based the definition of RFT on study authors' classification, provided that it fit within the general criteria of RFT described in the Background section of this review (Description of the intervention), and that no additional haemodynamic monitoring was used to guide fluid infusion rates.
We defined GDFT as any fluid administration targeting continuously measured haemodynamic variables designed to maximize tissue perfusion and oxygen delivery. These variables included assessment of haemodynamic variables such as cardiac output, stroke volume, stroke volume variation, pulse pressure variation, or other factors, as measured by any device. We did not include studies for which GDFT protocols were based not on haemodynamic variables but on other variables, such as lactate levels or superior vena cava oxygen saturation (ScvO₂).
Types of outcome measures
Primary outcomes
Major complications (as defined by the authors of included studies) during longest follow‐up period, analysed as dichotomous outcomes (number of participants with at least one major complication) (We accepted the study authors' definition provided that it referred to life‐threatening conditions including the need for reoperation or transfer to an intensive care unit, fitting into Grade III or IV of the Clavien‐Dindo Classification of Surgical Complications (Appendix 2; Dindo 2004))
All‐cause mortality during longest follow‐up period
Secondary outcomes
Length of hospital stay (hospital LOS) in days
Surgery‐related complications, including tissue‐healing complications such as wound infection, rupture, dehiscence, breakdown, or haematoma during longest follow‐up period
Non‐surgery‐related complications, including cardiovascular events, pneumonia, sepsis, ileus, or organ failure during longest follow‐up period
Renal failure, including acute kidney injury or renal replacement therapy during longest follow‐up period
Vasopressor or inotrope administration during longest follow‐up period to correct haemodynamic instability. We excluded vasopressors or inotropes given as a predefined element of the RFT or GDFT protocol, and not associated with correction of haemodynamic instability
Quality of surgical recovery, assessed in any way (e.g. as a surgical recovery score)
Cost of treatment
We considered the follow‐up period to run from the time of surgery to the longest postoperative observation period for every outcome. Studies were eligible for inclusion if they reported data on either primary or secondary outcomes, or both.
Search methods for identification of studies
We identified RCTs through literature searching designed to identify relevant trials without restrictions by language or publication status.
Electronic searches
We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL) (11 October 2019).
MEDLINE (Ovid SP, 1946 to 11 October 2019).
Embase (Ovid SP, 1974 to 11 October 2019).
We developed a draft search strategy for MEDLINE. This can be found in Appendix 3 and was used as the basis for the search strategies listed for other databases (Appendix 3).
We scanned the following trials registries for ongoing and unpublished trials on 11 October 2019.
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch/).
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We performed a targeted search in Google Scholar (11 October 2019). We scanned the reference lists and citations of included trials, and of any relevant systematic reviews identified, for further references to potentially relevant trials (October 2019).
When necessary, we contacted trial authors to request additional information.
Data collection and analysis
Selection of studies
We identified and excluded duplicates, and we collated multiple reports of the same study, so that each study rather than each report was the unit of interest in the review. We eliminated duplicate records of the same study using reference management software (EndNote). Two review authors (from AW, WS, JJW, RZ, MJ, MMB) independently screened titles and abstracts for inclusion of all studies identified as a result of the search, and coded them as 'retrieve' (eligible or potentially eligible or unclear) or 'do not retrieve'. We resolved discrepancies by discussion, with recourse to a third review author if necessary (MMB or AW or JW or MJ). We retrieved the full‐text study reports/publications, and two review authors (from AW, WS, JJW, RZ, MJ) independently screened the full texts and identified studies for inclusion. We identified and recorded the reasons for exclusion of ineligible studies. We resolved disagreements through discussion, or, if required, we consulted a third review author (MMB or AW or JW or MJ). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1), as well as a Characteristics of excluded studies table (Moher 2009).
1.
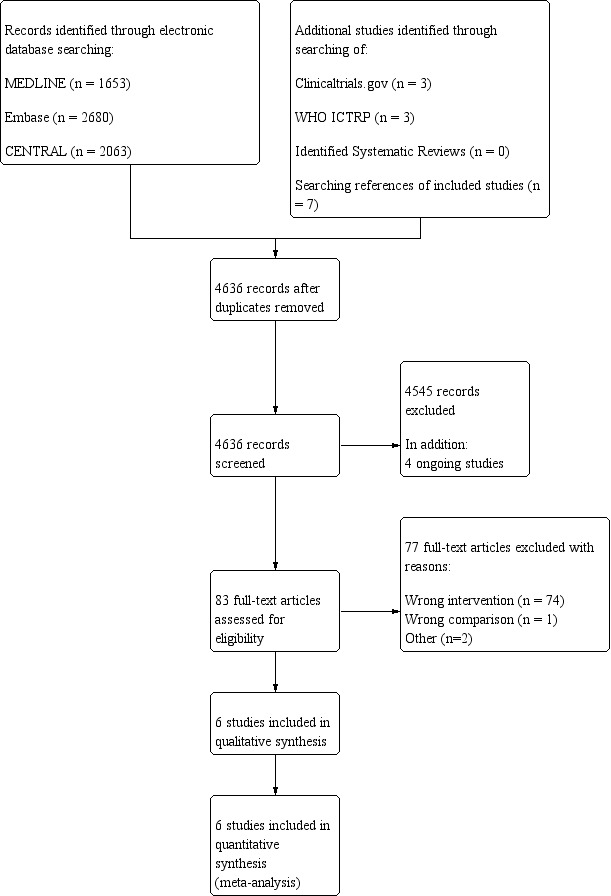
Study flow diagram.
Data extraction and management
We used a data collection form for study design, methods, population, intervention, outcomes, and results. We used a Microsoft Excel spreadsheet for data extraction. We pretested a data collection form in case this needed further adjustment. Two review authors (from AW, MJS, WS, JJW, RZ, MJ) independently extracted data from the included studies, with recourse to a third review author (MMB or AW or JW or MJ), if necessary. We extracted the following study characteristics.
General information: date of study, publication status, number of study centres and locations (country), and types of participating hospitals (general, narrow specialty, e.g. surgical, academic, number of beds if available).
Methods: study design, randomization method, blinding method, total duration of study, length of follow‐up, and withdrawals.
Participants: N, mean age, age range, gender, types of surgery, comorbidities, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications or interventions, medications or interventions excluded.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for study, and notable conflicts of interest of study authors.
Assessment of risk of bias in included studies
Two review authors (of AW, MJS, WS, JJW, RZ, MJ) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (MMB, AW, JW, MJ). We attempted to contact study authors directly for clarification, when details were not available in the study report.
We assessed risks of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other potential bias.
We graded each potential source of bias as high, low, or unclear, and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarized risk of bias judgements across different studies for each of the domains listed. When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to this outcome.
Because it was not feasible to blind personnel to the study intervention, we acknowledge that this introduces an unavoidable risk of performance bias. We judged that mortality and major complications are not likely to be influenced by lack of blinding, whereas lack of blinding may influence other outcomes. We acknowledged this when considering treatment effects.
We classified risks of bias for each study by outcomes. For mortality and major complications, we classified studies as low risk of bias if they were at low risk of bias in all domains except blinding of participants and personnel. For other outcomes, we classified studies as low risk of bias if they were at low risk of bias in all domains. Otherwise, we rated studies at high risk of bias.
In the main analyses, we included all studies meeting the inclusion criteria, but we performed sensitivity analyses according to risk of bias of the study for random sequence generation and allocation concealment. (See Sensitivity analysis.)
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section (Wrzosek 2017).
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) and the number needed to treat for an additional beneficial outcome (NNTB) with 95% confidence intervals (CIs) to establish statistical differences. We calculated NNTBs as the reciprocal of absolute risk reduction (ARR). For unwanted effects, the NNTB becomes the number needed to treat for an additional harmful outcome (NNTH), which we calculated in the same manner. For mortality, because of a low event rate in the GDFT group, we conducted an additional post‐hoc Peto odds ratio analysis and used a Poisson regression model to compare groups, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For low event rate comparisons, we presented the results as risk differences (RDs) because this better reflects magnitude of treatment effect for low event rates. For continuous measures, such as hospital length of stay (LOS), we calculated mean differences (MDs) when means and standard deviations (SDs) were available. When the distribution of these variables was presented as median and range or interquartile range or both, we converted the values to means and standard deviations using algorithms described by Wan 2014. We estimated the average number of complications per person with 95% CIs and compared these using a Poisson regression model. We presented all results with 95% CIs. We considered P values equal to or less than 0.05 (two‐sided alpha) as statistically significant.
Unit of analysis issues
The unit of analysis for this review was an individual participant. In the case of multi‐arm studies, which included multiple restrictive or goal‐directed fluid therapy groups, we combined groups to enable a single pair‐wise comparison.
Dealing with missing data
We tried to contact authors of included trials to obtain missing data. When we could not obtain the missing information, we analysed only available data in the main analysis. In sensitivity analyses, we performed the 'worst‐case scenario', where we replaced the missing data with the worst possible outcomes in the treatment group and the best possible outcomes in the control group.
Assessment of heterogeneity
As a first step, we determined whether clinical heterogeneity was significant between studies. We assessed clinical heterogeneity by comparing participants, interventions, and outcomes among studies. If we found significant discrepancies between studies, we did not report the pooled effect.
If we found no clear evidence of clinical heterogeneity, we assessed heterogeneity between trials by visually inspecting forest plots and quantified statistical heterogeneity by calculating the I² statistic, which describes the percentage of total variation across studies that was due to heterogeneity rather than chance (Higgins 2011). We regarded statistical heterogeneity as low if the I² statistic was less than 30%, moderate if between 30% and 50%, substantial if between 50% and 75%, and considerable if above 75% (Higgins 2011). We planned that if we found evidence of heterogeneity, we would investigate and report the possible reasons for this. In cases of considerable heterogeneity, we planned to not report the pooled effect.
Assessment of reporting biases
We searched multiple sources to minimize reporting bias. We planned to create and examine funnel plots to explore possible small‐study and publication biases if we were able to pool more than 10 studies. In our review, we found only six studies and thus we did not create funnel plots.
Data synthesis
When at least two studies performed similar comparisons and reported the same outcome measures, with heterogeneity indicating that reporting the pooled effect was appropriate, we performed meta‐analyses using Review Manager 5 software (Review Manager 2014). We used a fixed‐effect model for meta‐analysis when we considered that heterogeneity was not important. If we found moderate or greater heterogeneity among studies, we used a random‐effects model (Higgins 2011). We calculated 95% CIs and considered corresponding P values equal to or less than 0.05 (two‐sided alpha) as statistically significant.
Subgroup analysis and investigation of heterogeneity
We had planned to perform the following subgroup analyses if we had enough data, to determine whether study results differ by:
type of surgery (i.e. open, laparoscopic, abdominal, urological, orthopaedic, trauma, burns, other);
type of anaesthesia (general vs spinal vs epidural vs a combination of these);
American Society of Anesthesiologists (ASA) status of participants;
type of surgery according to surgical risk, based on Johns Hopkins criteria (Appendix 1) (grade II or grade III procedures) (Donati 2004);
-
various fluid regimens used in the RFT group;
volume of fluid allowed (basal infusion rate and boluses);
type of fluid given (colloids vs crystalloids); or
presence or absence of postoperative fluid restriction;
-
various protocols of GDFT. Depending on available evidence, we planned to distinguish subgroups based on:
type of haemodynamic monitor (pulmonary artery catheter, calibrated and uncalibrated arterial pressure analysis systems, oesophageal doppler, or other techniques);
type of therapeutic goal (cardiac output, stroke volume, stroke volume variation, pulse pressure variation on other or combinations of the above variables);
type of intervention (fluids only or fluids and inotropes or vasopressors);
type of fluid given (colloids vs crystalloids); or
time frame of intravascular fluid optimization with GDFT (before surgery and/or intraoperatively and/or postoperatively); and
presence or absence of preoperative fluid deficit. We considered lack of fluid deficit (zero‐fluid balance at the beginning of surgery) if participants were allowed to drink up to two hours before surgery to cover their basal water requirements and did not have mechanical bowel preparation (MBP), or had preoperative MBP but received minimum 1000 mL of fluid preoperatively to cover the deficit associated with MBP.
If we noted variability within a study for variables analysed in points 1 to 6, we planned to consider the study to fulfil the criteria if at least 80% of participants met the requirements.
We planned to restrict subgroup analyses to primary outcome measures. We planned to assess differences in outcomes between subgroups using the Q‐test for heterogeneity.
However, due to the small number of studies included in the review (fewer than 10), and the small number of studies per possible subgroups (in every possible analysis, there was a subgroup with only one study), we were not able to perform meaningful subgroup analyses.
Sensitivity analysis
For primary outcome measures, we performed sensitivity analyses for:
risk of bias (studies with low risk of bias vs studies with high risk of bias for random sequence generation, allocation concealment, and blinding of outcome assessors in the case of subjective outcomes);
missing data (we applied 'worst‐case scenario' as described in the Dealing with missing data section); and
exclusion of the Colantonio 2015 study.
Summary of findings and assessment of the certainty of the evidence
We used the principles of the GRADE system to assess the certainty of the body of evidence associated with specific outcomes in our review (Guyatt 2008). We constructed a 'Summary of findings' (SoF) table by using GRADE software (GRADEpro GDT). The GRADE approach appraises the certainty of a body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item assessed. The certainty of a body of evidence was based on within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
In Table 1, we included the following outcomes.
Major complications (as defined by the authors of included studies) during longest follow‐up period, analysed as dichotomous outcomes (number of participants with at least one major complication).
All‐cause mortality during longest follow‐up period.
Length of hospital stay (hospital LOS) in days.
Surgery‐related complications, including tissue‐healing complications such as wound infection, rupture, dehiscence, breakdown, or haematoma during longest follow‐up period.
Non‐surgery‐related complications, including cardiovascular events, pneumonia, sepsis, ileus, or organ failure during longest follow‐up period.
Renal failure, including acute kidney injury or renal replacement therapy during longest follow‐up period.
Quality of surgical recovery, assessed in any way (e.g. as a surgical recovery score).
Results
Description of studies
See Characteristics of included studies,Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies tables.
Results of the search
Our search of electronic databases on 11 October 2019 yielded 6396 references, which after de‐duplication provided 4623 unique references to screen. Additionally, we searched the references of the included studies and of any relevant systematic reviews identified during screening; we identified seven additional references. Of these, we checked 83 references in full text. We excluded 77 references for the following reasons: wrong intervention (74 studies); wrong comparison (1 study); and other reasons (2 studies). We primarily classified Martini 2009 as meeting the inclusion criteria based on the published abstract. However, additional information from study authors revealed that randomization was started without ethical committee full approval (some missing papers), and the study was finally completed as a retrospective analysis. Based on information from study authors, we excluded the study due to ineligible study design.
Finally, we included six studies (Benes 2015; Brandstrup 2012; Colantonio 2015; Phan 2014; Srinivasa 2013; Zhang 2012). Additionally, through searches of trials registries on 11 October 2019, we identified four ongoing studies.
See Figure 1 for the flow chart on study selection.
Included studies
All six included studies were published in medical journals and were RCTs published between 2012 and 2015. Three studies had their protocols registered prospectively in trials registries (Benes 2015; Phan 2014; Srinivasa 2013).
We have presented detailed information about the included studies in the Characteristics of included studies table.
In the six published studies, study sample size varied from 60 to 151 participants. A total of 562 participants were randomized and analysed for the outcomes relevant to this review.
Five of the included studies were performed on participants undergoing abdominal surgery.
Brandstrup 2012: colorectal surgery.
Colantonio 2015: cytoreductive abdominal surgery with hyperthermic intraperitoneal chemotherapy (HIPEC).
Phan 2014: major colorectal surgery (laparoscopic or open).
Srinivasa 2013: colectomy (laparoscopic or open).
Zhang 2012: gastrectomy or colectomy.
The sixth study was performed in orthopaedic participants undergoing total knee or hip replacement (Benes 2015).
None of the studies were performed in patients undergoing emergency surgery.
All surgeries were classified as Grade II surgeries according to modified Johns Hopkins surgical criteria (Appendix 1).
Five studies reported the ASA status of included participants: 101 (22.7%) ASA I, 268 (60.4%) ASA II, and 75 (16.9%) ASA III participants. One study did not report ASA status; however, trial authors included only participants with ASA status I to III, and the rate of comorbidities in the included population was low (Phan 2014).
Five of the six included studies were two‐arm studies comparing RFT versus GDFT. The remaining study had three arms: one RFT arm and two arms comparing GDFT with different fluid regimens (Ringer’s lactate vs hydroxyethyl starch) (Zhang 2012).
Preoperative fluid restrictions
Four studies reported the absence of fluid deficit preoperatively. Colantonio 2015 did not report any information on preoperative fluid deficit, and in Zhang 2012, preoperative fluid deficit was present ‐ the surgery was preceded by an eight‐hour fasting period.
Restrictive fluid therapy (RFT) description
In five of the six included studies, crystalloid solutions were used for the basal infusion; colloid solutions were used in Brandstrup 2012. Additional boluses of colloids were allowed in Benes 2015, Brandstrup 2012, Colantonio 2015, Phan 2014, and Srinivasa 2013; additional boluses of crystalloid were allowed in Zhang 2012. Infusion of additional boluses was based on traditional clinical parameters such as mean arterial pressure, heart rate, and clinical signs in all studies, and additionally on CVP in Brandstrup 2012, Colantonio 2015, and Zhang 2012, and diuresis in Colantonio 2015, Srinivasa 2013, and Zhang 2012.
In Colantonio 2015, study authors declare that the fluid protocol in the intervention group was 'mainly restrictive' and participants received basal infusion of crystalloid ranging from 4 to 10 mL/kg/h. This overlaps with infusion rates set in other included studies; however, the upper limit is higher, which could result in less rigorous fluid restriction in this study compared with other included studies.
Goal‐directed fluid therapy (GDFT) description
To guide GDFT, three included studies used oesophageal doppler to measure:
stroke volume (SV) (Brandstrup 2012);
stroke volume index (SVI) and flow time corrected (FTc) (Phan 2014); and
FTc and SV (Srinivasa 2013).
Two studies used uncalibrated arterial pressure analysis systems.
Flowtrac/Vigileo System (Edwards Lifesciences) measuring SVI, cardiac index (CI), and stroke volume variation (SVV) (Colantonio 2015).
Datex Ohmeda S5 measuring pulse pressure variation (PPV) (Zhang 2012).
In the remaining study, a completely non‐invasive arterial pressure monitoring device that measured PPV was applied (CNAP) (Benes 2015).
Total volume of fluid received by participants intraoperatively
Detailed information on the total intraoperative volume of fluid given in each study per group per patient is provided in the Characteristics of included studies table. In four studies, the final volume of fluid given was smaller in the RFT group compared with the GDFT group (Brandstrup 2012; Phan 2014; Srinivasa 2013, Zhang 2012). In one study, volumes were comparable in both groups (Benes 2015). In another study, the final volume of fluid given was higher in the RFT group compared with the GDFT group (Colantonio 2015).
Postoperative fluid restrictions
In three studies, no restrictions were imposed on fluid uptake after surgery (Benes 2015; Brandstrup 2012; Srinivasa 2013). Similarly, in Phan 2014, oral fluids were encouraged four hours post surgery and oral diet commenced from day 1. Zhang 2012 reported that the accelerated surgical recovery programme was not adopted, and Colantonio 2015 did not provide any information on postoperative fluid restrictions.
Outcomes
Primary outcomes differed among the included studies and comprised the number of participants with any postoperative organ or infectious complication (Benes 2015); a combined endpoint of postoperative complications and mortality (Brandstrup 2012); the incidence of major abdominal complications (Colantonio 2015); postoperative hospital LOS (Phan 2014; Zhang 2012); and the surgical recovery score (Srinivasa 2013).
Secondary endpoints were multiple and included, for example, hospital LOS (Benes 2015; Brandstrup 2012; Colantonio 2015; Srinivasa 2013); all‐cause mortality (Benes 2015; Colantonio 2015); the incidence of complications (Colantonio 2015; Phan 2014; Srinivasa 2013; Zhang 2012); intravenous fluid volumes administered to participants (Phan 2014; Srinivasa 2013; Zhang 2012); change in participants’ haemodynamic parameters (Benes 2015; Phan 2014; Zhang 2012); and urine output (Srinivasa 2013; Zhang 2012).
Excluded studies
In total, we excluded 77 references from this review. The reasons for exclusions were as follows: wrong intervention (74 studies); wrong comparison (1 study); and other reasons (2 studies).
For details, see Characteristics of excluded studies.
Studies awaiting classification
We identified no studies that are awaiting classification.
Ongoing studies
We searched trials registries on 11 October 2019 and identified four ongoing studies (ChiCTR1800014777; NCT02625701; NCT03039946; NCT03519165).
For details, see Characteristics of ongoing studies.
Risk of bias in included studies
Details of the risk of bias evaluation are presented in the Characteristics of included studies table. Figure 2 shows the overall risk of bias in each domain for all studies in this review; Figure 3 shows the risk of bias by trial.
2.
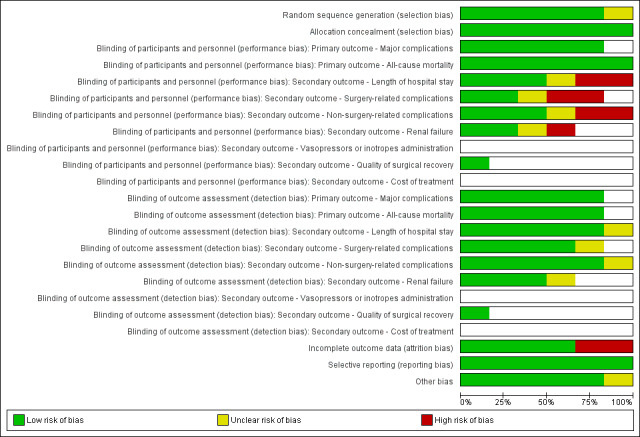
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
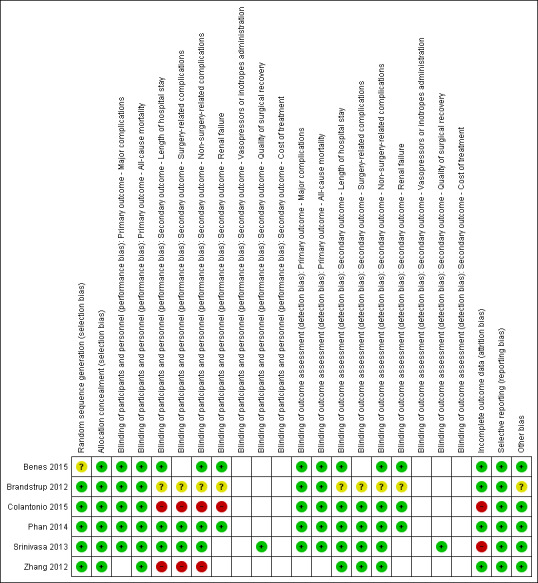
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Only one study was at low risk of bias in all domains (Phan 2014). Two studies were judged as having high risk of bias in the blinding of participants and personnel domain (Colantonio 2015; Zhang 2012). Two studies were judged as having high risk of bias in the incomplete outcome data domain (Colantonio 2015; Srinivasa 2013). Benes 2015 was judged as having unclear risk of bias in random sequence generation, and Brandstrup 2012 was judged as having unclear risk of bias in blinding of participants and personnel, blinding of outcome assessment, and other bias.
Allocation
In five of the included studies, the risk of bias for random sequence generation and allocation concealment domains was low. One study provided insufficient information for review authors to judge risk of bias in the random sequence generation domain (Benes 2015).
Blinding
We judged that mortality and major complications are not likely to be influenced by lack of blinding, whereas lack of blinding may influence other outcomes. Two studies were judged as having high risk of bias in the blinding of participants and personnel domain. The remaining four studies were judged as having low risk of bias in the blinding of participants and personnel domain (Benes 2015; Brandstrup 2012; Phan 2014; Srinivasa 2013).
Incomplete outcome data
Four studies performed an Intention‐to‐treat analysis (Benes 2015; Brandstrup 2012; Phan 2014; Zhang 2012). In three of these studies, no participants were lost to follow‐up and none were excluded. In the fourth study (Brandstrup 2012), one participant was excluded from the analysis because the planned surgery was cancelled. These studies were judged as having low risk of bias in this domain.
In two other studies, per‐protocol analysis was performed (Colantonio 2015; Srinivasa 2013). Reasons for exclusion of participants were provided; however, in Colantonio 2015, more participants (four) were excluded in the GDFT group than in the RFT group (two) due to cancellation of surgery or appearance of intraoperative anaesthesiological complications. In Srinivasa 2013, five participants in the RFT group and six in the GDFT group did not receive the allocated intervention; additionally, there were three protocol violations in the intraoperative period (two RFT; one GDFT). 'Worst‐case scenario' analysis showed that excluding these participants may have influenced the results for both major complications (Analysis 3.1) and mortality (Analysis 3.2). Both studies were judged as having high risk of bias.
3.1. Analysis.
Comparison 3 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis for missing data, worst‐case scenario, Outcome 1 Major complications.
3.2. Analysis.
Comparison 3 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis for missing data, worst‐case scenario, Outcome 2 All‐cause mortality.
Selective reporting
Only three studies were registered in trials registries. In these cases, adherence to the protocol could be assessed (Benes 2015; Phan 2014; Srinivasa 2013). All three studies were judged as having low risk of bias in this domain as all outcomes were reported as described in the protocol.
The remaining three studies did not have protocols registered. However, no concerns were raised, as methods sections were described systematically, and both primary and secondary outcomes were reported in sufficient detail. Therefore, these studies were judged as having low risk of bias in this domain.
Other potential sources of bias
For five studies, we did not identify any source of potential bias. Only in Brandstrup 2012 was the presence of both the anaesthetist and the surgeon mandatory for inclusion of participants, and hence strictly consecutive participant inclusion was not preserved.
Effects of interventions
See: Table 1
See Table 1 for further information.
Primary outcomes
Major complications (as defined by authors of included studies) during longest follow‐up period, analysed as dichotomous outcomes (number of participants with at least one major complication)
Five studies (484 participants) reported data on the number of participants with at least one major complication (Benes 2015; Brandstrup 2012; Colantonio 2015; Phan 2014; Srinivasa 2013).
Major complications were defined as:
Calvien‐Dindo grade 3 and 4 in both Phan 2014 and Srinivasa 2013;
life‐threatening complications, including re‐operation or transfer to the intensive care unit (ICU) in Brandstrup 2012; and
anastomotic leakage, enteric fistula, perforation, and abdominal abscess in Colantonio 2015 (this study presented only major abdominal complications. No information on non‐abdominal major complications was provided by the study authors).
Benes 2015 did not provide a precise definition of major complications.
Meta‐analysis of trial results showed no statistically significant differences between restrictive fluid therapy (RFT) and goal‐directed fluid therapy (GDFT) (risk ratio (RR) 1.61, 95% confidence interval (CI) 0.78 to 3.34; I² = 47%; random‐effects model; Analysis 1.1; Figure 4). We judged the certainty of evidence to be very low for this outcome.
1.1. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 1 Major complications.
4.
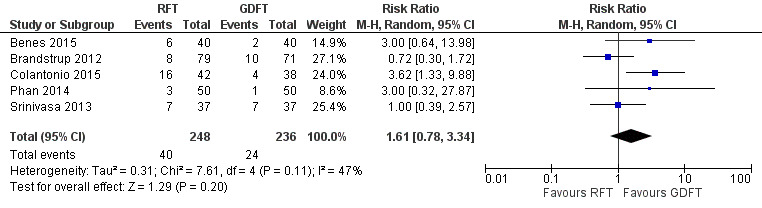
Forest plot of comparison: 1 Restrictive versus goal‐directed fluid therapy, outcome: 1.1 Major complications.
All‐cause mortality during longest follow‐up period
All six included studies with 544 participants reported data on mortality. Meta‐analysis of trial results showed that there was a difference between groups at borderline significance (risk difference (RD) 0.03, 95% CI 0.00 to 0.06; number needed to treat for an additional beneficial outcome (NNTB) 33.33, 95% CI ∞ to 16.66; I² = 7%; fixed‐effect model), favouring the GDFT group (Analysis 1.2; Figure 5).
1.2. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 2 All‐cause mortality.
5.
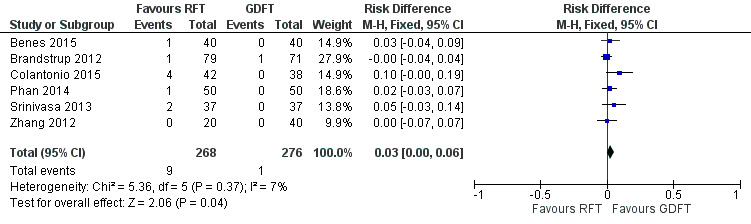
Forest plot of comparison: 1 Restrictive versus goal‐directed fluid therapy, outcome: 1.2 All‐cause mortality.
Because of the low event rate in study groups, post‐hoc Peto odds ratio (OR) analysis of mortality was conducted as suggested by the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). This analysis showed increased odds of mortality in the RFT group compared to the GDFT group (Peto OR 4.81, 95% CI 1.38 to 16.84; Analysis 1.3; Figure 6). Also, the post‐hoc Poisson regression model showed that the rate of mortality may be reduced in the GDFR group (P = 0.035; Table 2). We judged the certainty of evidence to be very low for this outcome, so the evidence is very uncertain. It has to be mentioned that in Peto OR analysis, one study (Colantonio 2015) performed cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) was assigned the greatest weight, and its exclusion resulted in no difference in the odds of mortality between the RFT group and the GDFT group. Moreover, this is the only study in which the final volume of fluid received by participants in the RFT group was higher than the volume received by participants in the GDFT group. This indicates that Colantonio 2015 has a great influence on the results of analysis.
1.3. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 3 Peto OR all‐cause mortality.
6.
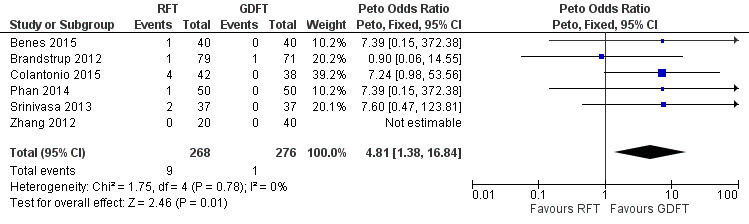
Forest plot of comparison: 1 Restrictive versus goal‐directed fluid therapy, outcome: 1.3 Peto OR all‐cause mortality.
1. All‐cause mortality ‐ Poisson regression analysis.
| Outcome | RFT (95% CI) | GDFT (95% CI) | P value |
| All‐cause mortality | 0.034 (0.017 to 0.065) |
0.004 (0.001 to 0.026) |
0.035 |
CI: confidence interval.
GDFT: goal‐directed fluid therapy.
RFT: restrictive fluid therapy.
Secondary outcomes
Length of hospital stay (hospital LOS) in days
Five of the six studies (464 participants) reported data on hospital LOS (Benes 2015; Brandstrup 2012; Phan 2014; Srinivasa 2013; Zhang 2012). Three studies defined this outcome as readiness for discharge (Benes 2015; Brandstrup 2012; Phan 2014). The other two studies did not provide clarification. Results were presented as means or medians with various measures of dispersion (SD, 95% CI, range, interquartile range). When possible, means and SD values were calculated from results presented in the studies to be combined in meta‐analysis (Benes 2015; Brandstrup 2012; Phan 2014; Srinivasa 2013; Zhang 2012). Pooled results showed that there is no statistically significant difference between RFT and GDFT in hospital LOS (MD ‐0.02, 95% CI ‐0.55 to 0.50; I² = 0%; fixed‐effect model; Analysis 1.4). We judged the certainty of evidence to be very low for this outcome.
1.4. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 4 Length of hospital stay.
Surgery‐related complications, including tissue‐healing complications such as wound infection, rupture, dehiscence, breakdown, or haematoma during longest follow‐up period
Four studies (364 participants) reported data on the number of participants with surgery‐related complications (Brandstrup 2012; Colantonio 2015; Srinivasa 2013; Zhang 2012).
Surgery‐related complications were defined as:
tissue‐healing complications in Brandstrup 2012;
major abdominal complications in Colantonio 2015;
surgical complications in Srinivasa 2013; and
surgical site infection or bowel obstruction in Zhang 2012.
Major abdominal complications reported by Colantonio 2015 were additionally included in the analysis of major complications. Meta‐analysis of trial results showed no statistically significant difference between RFT and GDFT (RR 1.54, 95% CI 0.87 to 2.72; I² = 34%; random‐effects model; Analysis 1.5). We judged the certainty of evidence to be very low for this outcome.
1.5. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 5 Surgery‐related complications.
Non‐surgery‐related complications, including cardiovascular events, pneumonia, sepsis, ileus, or organ failure during longest follow‐up period
Only one study (74 participants) reported data on the number of participants with at least one non‐surgery‐related complication (Srinivasa 2013). No statistical difference was observed between groups (RR 1.00, 95% CI 0.52 to 1.93; Analysis 1.6). We judged the certainty of evidence to be very low for this outcome.
1.6. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 6 Non‐surgery‐related complications.
Data on the number of participants with at least one non‐surgery‐related complication were very limited (reported only by Srinivasa 2013). As most studies reported the numbers of complications but not the numbers of participants with complications, we decided to perform a post‐hoc analysis of the average number of non‐surgery‐related complications per person. We additionally compared the average number of complications per person divided by the following groups by type of complication.
Cardiovascular (including cardiorespiratory) complications.
Respiratory complications.
Thrombotic or coagulation disorders or bleeding.
Renal or urinary complications.
Gastrointestinal complications.
Neurological or cerebrovascular complications.
Infection, sepsis, and multi‐organ failure.
All six included studies (544 participants) provided data on various non‐surgery‐related complications (presented as the number of particular complications). The total number of non‐surgery‐related complications was calculated as a sum of complications in particular groups. The Poisson regression model showed a significantly higher average number of non‐surgery‐related complications per person in the RFT group (0.5 vs 0.36; P = 0.01). Analysis for particular complication types showed a significantly higher average number of gastrointestinal complications per person in the RFT group (0.17 vs 0.10; P = 0.049) compared with the GDFT group. Differences were not observed for other groups of complications (see Table 3). We judged the certainty of evidence to be very low for this outcome.
2. Average number of complications per person ‐ Poisson regression analysis.
| Outcome |
RFT (95% CI) |
GDFT (95% CI) |
P value |
| Total number of complications | 0.69 (0.6 to 0.8) |
0.54 (0.46 to 0.63) |
0.02 |
| Non‐surgery‐related complications | 0.50 (0.44 to 0.55) |
0.36 (0.29 to 0.43) |
0.01 |
| Cardiovascular (including cardiorespiratory) | 0.9 (‐0.06 to 0.13) |
0.67 (‐0.04 to 0.11) |
0.39 |
| Respiratory | 0.05 (‐0.03 to 0.09) |
0.04 (‐0.02 to 0.07) |
0.5 |
| Thrombotic, coagulation disorders, or bleeding | 0.05 (0.03 to 0.08) |
0.02 (0.01 to 0.05) |
0.1 |
| Renal or urinary | 0.06 (0.04 to 0.10) |
0.05 (0.03 to 0.08) |
0.49 |
| Gastrointestinal | 0.17 (0.13 to 0.23) |
0.10 (0.73 to 0.15) |
0.049 |
| Neurological or cerebrovascular | 0.02 (0.01 to 0.04) |
0.03 (0.01 to 0.05) |
0.40 |
| Infection, sepsis, multi‐organ failure | 0.02 (0.01 to 0.05) |
0.01 (0.01 to 0.03) |
0.3 |
CI: confidence interval.
GDFT: goal‐directed fluid therapy.
RFT: restrictive fluid therapy.
Total number of complications per person
We decided to perform a post‐hoc analysis of the average total number of complications per person. The total number of complications was calculated as a sum of non‐surgery‐ and surgery‐related complications. All six included studies (544 participants) provided data on this outcome.
The Poisson regression model showed a significantly higher average number of complications per person in the RFT group (0.69 vs 0.54; P = 0.02) compared with the GDFT group (see Table 3). We judged the certainty of evidence to be very low for this outcome.
Renal failure, including acute kidney injury or renal replacement therapy during longest follow‐up period
Four studies (410 participants) reported data on the number of participants with renal failure (Benes 2015; Brandstrup 2012; Colantonio 2015; Phan 2014). Diagnostic criteria for renal failure varied between studies. Benes 2015 reported participants in stage 1 or in stage 2 or 3 according to the Acute Kidney Injury Network (AKIN) classification (Cruz 2010); Brandstrup 2012 provided only the number of participants on renal replacement therapy; Colantonio 2015 defined renal failure as oliguria < 0.5 mL/kg/h for longer than four hours, or an increase in the creatinine level of minimum 30%, or the need for dialysis. Phan 2014 reported participants with acute kidney injury (AKI) without providing a precise definition. Meta‐analysis of the results showed no statistically significant difference between RFT and GDFT (RR 1.38, 95% CI 0.57 to 3.36; I² = 0%; fixed‐effect model; Analysis 1.7). We judged the certainty of evidence to be very low for this outcome.
1.7. Analysis.
Comparison 1 Restrictive versus goal‐directed fluid therapy, Outcome 7 Renal failure.
Vasopressor or inotrope administration to correct haemodynamic instability
None of the included studies reported on administration of vasopressors or inotropes to correct haemodynamic instability.
We excluded vasopressors or inotropes given as a predefined element of the RFT or GDFT protocol, and not associated with correction of haemodynamic instability (see Secondary outcomes).
Quality of surgical recovery, assessed in any way (e.g. as a surgical recovery score)
This outcome was reported in only one study with 74 participants through the use of surgical recovery score (SRS), which assessed fatigue, vigour, mental function, and impact on participant activity and activities of daily living (Srinivasa 2013). There was no difference in SRS between RFT and GDFT groups at any point (day 1, 3, 7, 14, or 30). We judged the certainty of evidence to be very low for this outcome.
Cost of treatment
None of the six included studies reported on the costs of treatment.
Subgroup analysis and investigation of heterogeneity
We were not able to perform a meaningful subgroup analysis because the total number of studies included in the review was low (fewer than 10), and the number of studies per possible subgroup was very low (in every possible analysis, there was a subgroup with only one study).
Sensitivity analysis
We performed sensitivity analysis for risk of bias, for missing data, and as per exclusion of the Colantonio 2015 study.
Sensitivity analysis for risk of bias
For the primary outcome measures, we performed sensitivity analysis for risk of bias (studies at low risk of bias vs studies at high risk of bias for random sequence generation and allocation concealment). All of the included studies except Benes 2015 were judged as having low risk of bias in the above‐mentioned domains. Sensitivity analysis did not show any differences between effects of the intervention in low risk of bias versus high risk of bias studies with respect to major complications and mortality (Analysis 2.1; Analysis 2.2).
2.1. Analysis.
Comparison 2 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis for risk of bias, Outcome 1 Major complications.
2.2. Analysis.
Comparison 2 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis for risk of bias, Outcome 2 All‐cause mortality.
Sensitivity analysis for missing data
For the primary outcome measures, we performed sensitivity analysis for missing data. We applied 'worst‐case scenario', where we replaced the missing data in two studies with the worst possible outcomes in the treatment group and the best possible outcomes in the control group (Colantonio 2015; Srinivasa 2013). Results showed that excluding these participants may have influenced results of the main analysis. For major complications, the pooled result was similar to the main analysis, and for mortality, pooled results showed a significantly lower mortality rate in the GDFT group compared with the RFT group.
Sensitivity analysis as per exclusion of the Colantonio 2015 study
In most of the included studies, the total volume of fluid finally received by participants intraoperatively was smaller in the RFT group compared with the GDFT group, except for the Benes 2015 study, in which volumes were comparable, and for the Colantonio 2015 study, in which participants in the RFT group received more fluid than participants in the GDFT group. Moreover, in Colantonio 2015, study authors declare that the fluid protocol in the intervention group was 'mainly restrictive', and that participants received basal infusion of crystalloid at a rate ranging from 4 to 10 mL/kg/h. This overlaps with infusion rates set in other included studies; however, the upper limit is higher, which could result in less rigorous fluid restriction in this study compared with other included studies. Additionally, Colantonio 2015 was performed in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC). This procedure may have a great impact on fluid balance due to long duration and instillation of chemotherapeutic agent in the peritoneal cavity at high temperature (41°C to 43°C) with its possible vasodilatory effect. For these reasons, we decided to perform sensitivity analysis to test how exclusion of Colantonio 2015 influenced the results of this review.
Exclusion of Colantonio 2015 did not change results for the analysis of major complications, and in the analysis of all‐cause mortality, the results became not significant for both risk difference and Peto odds ratio (Analysis 4.1; Analysis 4.2; Analysis 4.3).
4.1. Analysis.
Comparison 4 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis as per exclusion of Colantonio 2015 study, Outcome 1 Major complications.
4.2. Analysis.
Comparison 4 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis as per exclusion of Colantonio 2015 study, Outcome 2 All‐cause mortality.
4.3. Analysis.
Comparison 4 Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis as per exclusion of Colantonio 2015 study, Outcome 3 Peto OR all‐cause mortality.
This indicates that Colantonio 2015 has had a significant impact on the results of this analysis.
Discussion
Summary of main results
This review included six trials with a total of 562 participants. It included five studies on participants undergoing elective abdominal surgery (including one on participants undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy) and one study on participants undergoing elective orthopaedic surgery, with the majority of participants having American Society of Anesthesiologists (ASA) II status. Evidence on the effects of restrictive fluid therapy (RFT) on all‐cause mortality is very uncertain. Based on very low‐certainty evidence, restrictive fluid therapy (RFT) may increase the risk of all‐cause mortality compared to GDFT, but the evidence is very uncertain (six studies with 544 participants). These results are based on a small number of events and borderline significance. However, in an unplanned analysis using the Peto odds ratio (OR) method or the Poisson regression model (performed to avoid bias due to low event rates), a significant increase was seen in the risk of all‐cause mortality with RFT as compared with GDFT, but this evidence is very uncertain (six studies with 544 participants). This result was not significant after the exclusion of Colantonio 2015, in which the final volume of fluid received intraoperatively was higher in the RFT group than in the GDFT group. Based on very low‐certainty evidence, RFT may have no effect on major complication rates, but the evidence on this is also very uncertain (five studies with 484 participants). In the analysis of secondary outcomes, such as hospital length of stay (LOS) (five studies with 465 participants), surgery‐related complications (four studies with 364 participants), non‐surgery‐related complications (one study with 74 participants), renal failure (four studies with 410 participants), and quality of surgical recovery (one study with 74 participants), no differences between RFT and GDFT were found. We graded the evidence as very low certainty for all these outcomes; therefore, evidence on the effects of RFT on these outcomes is very uncertain. No data was available on the use of vasopressor or inotrope administration to correct haemodynamic instability and on the cost of treatment.
Because of limited evidence on complications in the included studies, especially non‐surgery‐related complications, we decided to perform a post‐hoc analysis of the average number of complications per person. Six trials with a total of 544 participants contributed data to this outcome. Trial results showed that the average number of non‐surgery‐related complications per person was higher in the RFT group (Poisson regression model: 0.5 vs 0.36; P = 0.01), and the average total number of complications per person was higher in the RFT group (Poisson regression model: 0.69 vs 0.54; P = 0.02). We judged the certainty of evidence to be very low for this outcome.
Overall completeness and applicability of evidence
This systematic review includes published trials comparing RFT with GDFT in adults undergoing major non‐cardiac surgery. This review has a number of limitations.
One of its crucial limitations is that most of the studies included in the review refer to abdominal surgery. Only one study examined the intervention in orthopaedic surgery. No studies addressed trauma patients or those undergoing emergency surgery, urological surgery, trauma, burn surgery, or other types of surgery. Moreover, it was not possible to select a group of participants undergoing laparoscopic surgery, who may be subject to different fluid requirements. It should be mentioned that the Colantonio 2015 study, which was performed in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC), was assigned the highest weight in Peto OR analysis of mortality, and its exclusion changed the significance of the results of the review, indicating that Colantonio 2015 had a great influence on these results. It has to be taken into consideration that this procedure is associated with significant perioperative risk and requires extensive surgical dissection associated with possible blood loss and long duration. It also has a great impact on fluid balance due to the instillation of chemotherapeutic agent into the peritoneal cavity at high temperature (41°C to 43°C) with its possible vasodilatory effect (Garg 2018). Hence the evidence does not fully address the review question, and conclusions cannot be generalized to the whole population of adults undergoing major non‐cardiac surgery.
Moreover, the population of participants included in the studies consisted mainly of ASA II participants (60.4%). Only 16.9% of participants were ASA III, and 22.7% ASA I. None of the participants were ASA IV. This is a serious limitation of the review because the review does not give information on restrictive fluid therapy (RFT) compared with goal‐directed fluid therapy (GDFT) in a higher‐risk population.
For all outcomes, we observed that the overall number of participants was rather low, and the optimal information size was not met.
Another limitation is that there is no clear definition of RFT, also referred to as 'zero‐fluid therapy' in the medical literature. For the purpose of this review, we based the definition of RFT on study authors' classification, provided that it fit within the general criteria of RFT, which include mainly covering of fluid losses in a 1:1 ratio and a small amount of fluid given to cover basal metabolic rate and limited perspiration losses. Such an approach is subject to bias and heterogeneity due to between‐study variation in the fluid protocols used in the RFT group, resulting in differences in the total amount of fluid received by participants. Additionally, in most of the included studies, anaesthesiologists were allowed by protocol to give additional fluid boluses without limits based on traditional clinical parameters such as heart rate, blood pressure, central venous pressure, or diuresis.
There was also between‐study variation in the approaches to fluid therapy protocols used in the GDFT groups and in the type of haemodynamic monitoring used, which resulted in differences in the duration and amount of fluid received by participants, as well as the timing of fluid infusion.
Evidence was insufficient to perform the subgroup analyses planned in the protocol of this review. Hence, one has to bear in mind that the results of this review address a heterogeneous population of participants and varying approaches to restrictive and goal‐directed fluid therapy.
Quality of the evidence
We included in this review a total of six publications reporting results from six randomized controlled trials (RCTs) enrolling a total of 562 participants. We used GRADEpro GDT to assess the certainty of the evidence. Overall, we judged the evidence to be of very low certainty for all‐cause mortality, major complications, surgery‐related complications, non‐surgery‐related complications, renal failure, quality of surgical recovery, and length of hospital stay. Thus, the evidence on effects of RFT on clinical outcomes in adults undergoing major non‐cardiac surgery is very uncertain.
Overall, there was high risk of bias in the included studies. Only one study was at low risk of bias in all domains (Phan 2014). Two studies were judged at high risk of bias in the blinding of participants and personnel domains (Colantonio 2015; Zhang 2012). We judged that mortality and major complications are not likely to be influenced by lack of blinding, whereas lack of blinding may influence other outcomes, and this was taken into consideration when the certainty of evidence was assessed per outcome. Two studies were judged as having high risk of bias due to the incomplete outcome data domain (Colantonio 2015; Srinivasa 2013). Benes 2015 was judged at unclear risk of bias for random sequence generation, and Brandstrup 2012 was judged at unclear risk of bias in blinding of participants and personnel, blinding of outcome assessment, and other bias.
Another potential problem for which we downgraded the certainty of evidence in this review is indirectness of evidence. Most of the studies examined performance of abdominal surgery. Only Benes 2015 was performed on orthopaedic surgery, and Colantonio 2015 included participants undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC). Such a highly selected group of included participants reduces the generalizability of observed outcomes to the broader surgical population. Most of the participants included in these studies were low‐risk ASA II participants (60.4%); 22.7% were ASA I, and only 16.9% were ASA III. This is an important drawback of the review because it does not reveal information on the effectiveness of RFT compared with GDFT in a high‐risk population (ASA III and IV), which is the population for which GDFT is mainly recommended. Another drawback is that fluid protocols in the RFT groups were generally imprecise. The basal infusion rate was specified; however additional boluses were allowed at the discretion of the anaesthesiologist, based on traditional clinical target parameters including heart rate, blood pressure, central venous pressure, or diuresis, which could result in higher final amounts of fluids given in RFT ‐ above the assumptions of the study protocol.
We further downgraded the certainty of evidence in the review due to imprecision of the results. For all outcomes, the optimal information size was not met, the event rate was low, and results of all meta‐analyses had wide confidence intervals crossing the line of no effect and including both benefit and harm.
Post‐hoc analysis conducted on the average number of non‐surgery‐related complications per person, which showed a higher rate of complications in the RFT group compared with the GDFT group (Poisson regression model), is subject to high risk of bias because the analysis was not planned.
Potential biases in the review process
We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we took measures to reduce bias in the review process. We used a comprehensive and sensitive search strategy to identify RCTs meeting the inclusion criteria. We did not restrict our search by language or publication status. Additionally, we searched the reference lists of potentially relevant studies and reviews, and we searched trial registries. Two review authors independently assessed the eligibility of studies against inclusion criteria. The probability that any important studies were omitted in the search process is low.
Two review authors worked independently to assess bias and extract data from the included studies. When necessary, a third review author was consulted. We attempted to contact study authors whenever we encountered missing information, but we were not always successful in these attempts. Thus, in many cases, we were not able to obtain information for comprehensive data extraction or bias assessment.
Even though we tried to minimize publication bias in the review process, the review may be subject to this type of bias. We planned to create and examine funnel plots to explore possible small‐study and publication biases if we were able to pool more than 10 studies; however, we found only six studies during the review process. Additionally, not all trials were actually completed. Martini 2009 was primarily classified as meeting the inclusion criteria based on the published abstract. However, additional information from study authors revealed that randomization was started without ethical committee full approval (some missing papers), and the study was eventually completed as a retrospective analysis.
This review may be subject to additional reporting bias because there is no clear definition of RFT, and we based the definition of RFT on study authors' classifications, provided that it fit within the general criteria of RFT. Such an approach is subjective, and study authors were not always precise in classifying intervention groups as restrictive, as was done in the case of the Colantonio 2015 study. This could lead to bias in deciding which studies to include in the review.
Some of the statistical methods used may impose limitations on the review process. For continuous measures, such as hospital length of stay, we calculated mean differences when means and standard deviations were available; however, for some studies, such data were not available. Thus, when the distribution of variables was presented as median and range or interquartile range, or both, we converted these values to means and standard deviations using algorithms described by Wan 2014. Additionally, the evidence on non‐surgery‐related complications was limited because study authors rarely reported the number of participants with non‐surgery‐related complications. To address this issue, we performed a post‐hoc analysis assessing the average number of complications per person using a Poisson regression model. Moreover, in the Zhang 2012 study, two GDFT groups were used, which differed by type of fluid given for boluses (crystalloids or colloids). For the purpose of this review, these groups were combined in the analysis.
Agreements and disagreements with other studies or reviews
Several systematic reviews with meta‐analyses have been conducted recently to compare GDFT with conventional fluid therapy protocols in various clinical settings. The conventional fluid therapy protocols usually included various fluid regimens, ranging from restrictive to liberal fluid approaches depending on the inclusion criteria of the review. To our best knowledge, however, none of the reviews exclusively compared RFT with GDFT. The results of the other reviews are consistent with our findings and show benefit of GDFT compared with alternative fluid regimens.
A recent systematic review by Xu and colleagues comparing GDFT with conventional fluid therapy in colorectal surgery showed a lower complication rate in the GDFT group (Xu 2018). However, no significant differences in mortality were found. A meta‐analysis by Sun and colleagues comparing GDFT with conventional fluid therapy in major abdominal surgery showed benefit of GDFT for short‐ and long‐term mortality, overall complication rate, and gastrointestinal function recovery (Sun 2017). Som and colleagues conducted a meta‐analysis of RCTs comparing GDFT based on non‐invasive flow‐based haemodynamic measurements with conventional fluid therapy in major non‐cardiac surgery (Som 2017). This review did not show benefit of GDFT for mortality, hospital, and ICU LOS, but showed benefit of GDFT for rates of postoperative complications, abdominal complications, and frequency of wound infection. Ripolles‐Melchor and colleagues conducted two systematic reviews with meta‐analyses comparing GDFT with conventional fluid therapy (Ripolles 2016), and GDFT based on oesophageal Doppler flow parameters with conventional fluid therapy (Ripolles‐Melchor 2016a), in adult non‐cardiac surgery. Both reviews showed a significant reduction in the number of participants with complications, but no differences in mortality. Another review conducted by the same author group compared GDFT (performed intraoperatively and postoperatively or only postoperatively) with conventional fluid therapy in adult non‐cardiac surgery (Ripolles‐Melchor 2016b). This review showed a significant reduction in mortality but no difference in the number of participants with complications. A meta‐analysis performed by Rollins and colleagues comparing GDFT with conventional fluid therapy in participants undergoing elective, major abdominal surgery was designed to determine whether there was a difference in outcomes between studies that did and did not use enhanced recovery after surgery (ERAS) protocols (Rollins 2016). GDFT was associated with a significant reduction in morbidity, hospital and intensive care length of stay (ICU LOS), and time to passage of faeces. If participants were managed in the ERAS pathway, the benefit of GDFT was less pronounced (possibly because of the lower number of studies included) and was observed only in intensive care LOS and time to passage of faeces ‐ not in morbidity or hospital LOS.
Our hypothesis that RFT may offer benefits comparable with GDFT to people undergoing major surgery was not confirmed by this review. Goal‐directed fluid therapy may offer benefit not only compared with conventional fluid regimens but also in settings where fluid therapy is designed to be restrictive; however, the evidence is very uncertain.
Authors' conclusions
Implications for practice.
Based on very low‐certainty evidence, we are uncertain whether restrictive fluid therapy (RFT) is inferior to goal‐directed fluid therapy (GDFT) in selected populations of adults undergoing major non‐cardiac surgery. The evidence is derived mainly from studies on elective abdominal surgery in a low‐risk population. Results of the review should not, therefore, be generalized to higher‐risk populations and other surgery types.
Implications for research.
Data in our review were mainly derived from low‐quality studies in participants undergoing elective abdominal surgery. Most of the participants were low risk (ASA I and II). Some of the outcomes were assessed in only a few studies, and these studies may have been underpowered to detect differences. Larger, higher‐quality RCTs, including a wider spectrum of surgery types and a wider spectrum of participant groups, which include high‐risk and emergency surgery participants, are needed to assess the intervention in other settings and to confirm our results. Moreover, a more accurate definition of restrictive fluid therapy, referring also to the preoperative and postoperative periods, is needed to make further research transparent and replicable. Well‐designed RFT protocols should be used in new trials, ensuring real fluid limitation in the RFT group.
What's new
| Date | Event | Description |
|---|---|---|
| 11 December 2019 | Amended | Minor change to Declarations of interest |
Acknowledgements
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia, at the time of review preparation) for her support.
We would like to thank Janne Vendt (Information Specialist) for help with the searches and in retrieving full‐text reports or publications of potentially eligible studies.
We would like to thank Ann Møller (Content Editor); Jing Xie (Statistical Editor); the Cochrane Anaesthesia and Cochrane Emergency and Critical Care Editorial Team; Birgitte Brandstrup, Benjamin G Chousterman, and Barbara Kabon (Peer Reviewers); Jenny Negus (Consumer Reviewer); Teo Quay (Managing Editor); and Andrew Smith (Co‐ordinating Editor) for help and editorial advice provided during preparation of the final version of this review.
We would like to thank Nicola Petrucci (Content Editor), the Cochrane Anaesthesia and Cochrane Emergency and Critical Care screening team, and Birgitte Brandstrup and Benjamin G Chousterman (Peer Reviewers) for help and editorial advice provided during preparation of the protocol of this review (Wrzosek 2017).
We would like to thank Anna Witkowska for her contributions in retrieving full‐text reports or publications or potentially eligible studies.
We would like to thank Agata Smoleń for her contributions to early work on the protocol (Wrzosek 2017).
Appendices
Appendix 1. Modified Johns Hopkins surgical criteria*
| General | Includes | Excludes | |
| Grade I | Minimal to mild risk independent to anaesthesia Minimally to moderately invasive procedure Potential blood loss < 500 mL |
Breast biopsy Removal of minor skin or subcutaneous lesions Myringotomy tubes Hysteroscopy Cystoscopy Vasectomy Circumcision Fibre‐optic bronchoscopy Diagnostic laparoscopy Dilatation and curettage Fallopian tube ligation Arthroscopy Inguinal hernia repair Laparoscopic lysis of adhesion Tonsillectomy/rhinoplasty |
Open exposure of internal body organs Repair of vascular or neurological structures Placement of prosthetic devices Postoperative monitored care setting Open exposure of abdomen, thorax, neck, cranium Resection of major body organs |
| Grade II | Moderately to significantly invasive procedures Potential blood loss 500 to 1500 mL Moderate risk to patient independent of anaesthesia |
Thyroidectomy Hysterectomy Myomectomy Cystectomy Cholecystectomy Laminectomy Hip/knee replacement Nephrectomy Major laparoscopic procedures Resection/reconstructive surgery of the digestive tract |
Open thoracic or intracranial procedure Major vascular repair (e.g. aortofemoral bypass) Planned postoperative monitored care setting (ICU, ACU) |
| Grade III | Highly invasive procedure Potential blood loss > 1500 mL Major to critical risk to patient independent of anaesthesia Usual postoperative ICU stay with invasive monitoring |
Major orthopaedic/spinal reconstruction Major reconstruction of the gastrointestinal tract Major genitourinary surgery (e.g. radical retropubic prostatectomy) Major vascular repair without postoperative ICU stay Cardiothoracic procedure Intracranial procedure Major procedure on the oropharynx Major vascular, skeletal, neurological repair |
* Donati 2004
Appendix 2. Clavien‐Dindo classification of surgical complications*
| Grades | Definition |
| Grade I | Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions. Allowed therapeutic regimens are drugs such as antiemetics, antipyretics, analgesics, diuretics, and electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside |
| Grade II | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included |
| Grade III | Requiring surgical, endoscopic, or radiological intervention |
| ‐ IIIa | Intervention not under general anaesthesia |
| ‐ IIIb | Intervention under general anaesthesia |
| Grade IV | Life‐threatening complication (including CNS complications) requiring IC/ICU management |
| ‐ IVa | Single organ dysfunction (including dialysis) |
| ‐ IVb | Multi‐organ dysfunction |
| Grade V | Death of a patient |
CNS: central nervous system.
IC: intensive care.
ICU: intensive care unit.
Appendix 3. Search strategy
MEDLINE All (Ovid SP)
1 (Surgical Procedures, Operative or Perioperative Care).sh. or surgery.hw,fs. or (per?operat* or peri‐operat* or per‐operat* or intraoperat* or intra‐operat* or surg*).af.
2 (fluid therapy or plasma volume or plasma substitutes).sh. or (((fluid* or h?emodynamic*) adj3 (therap* or restrict* or loading or administrat* or manag* or maintenance or intravenous* or IV)) or hydration or ((iv or intravenous) adj5 infusion*) or (cr?stall* or volume replacement or fluid titration or cristal* or colloid*)).af. or (plasma adj2 (substitute* or volume)).af.
3 (restrict* or limit* or reduct* or low or small or little or zero fluid* or RFT or standard or conventional or routin* or usual* or less or traditional or (fast and track) or fast‐track or ERAS or ERP or (enhanced and recovery and (surg* or program*)) or ((multimodal or enhanced or accelerated) and (optimi?ation or management or rehabilitation or protocol or package or program or pathway))).af.
4 exp hemodynamics/ or ((goal adj3 (directed or oriented or target*)) or GDT or GDFT or goaldirected or plethysmograph* or h?emodynamic* or ((per?operat* or peri‐operat* or per‐operat* or intraoperat* or intra‐operat*) adj2 monitor*) or heart function or ((systolic or pulse or blood) adj2 pressure) or (cardiac adj2 (output or volume or index)) or stroke index or pulse pressure or arterial pulse or vigileo or flotrac or proAQT or blood pressure or thermodilution or dilution technique* or lithium or impedance or masimo or pleth or echocardiography or echo or doppler or cardioQ or pulmonary arter* or swan ganz or flow time).af.
5 1 and 2 and 3 and 4
6 ((randomized controlled trial or controlled clinical trial).pt. or randomi?ed.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (exp animals/ not humans.sh.)
7 5 and 6
Embase (Ovid SP)
1 (surgery or perioperative period).hw. or surgery.fs. or (per?operat* or peri‐operat* or per‐operat* or intraoperat* or intra‐operat* or surg*).af.
2 fluid therapy.hw. or plasma volume.sh. or plasma substitute.sh. or (((fluid* or h?emodynamic*) adj3 (therap* or restrict* or loading or administrat* or manag* or maintenance or intravenous* or IV)) or hydration or ((iv or intravenous) adj5 infusion*) or (cr?stall* or volume replacement or fluid titration or cristal* or colloid*)).af. or (plasma adj2 (substitute* or volume)).af.
3 (restrict* or limit* or reduct* or low or small or little or zero fluid* or RFT or standard or conventional or routin* or usual* or less or traditional or (fast and track) or fast‐track or ERAS or ERP or (enhanced and recovery and (surg* or program*)) or ((multimodal or enhanced or accelerated) and (optimi?ation or management or rehabilitation or protocol or package or program or pathway))).af.
4 exp hemodynamics/ or ((goal adj3 (directed or oriented or target*)) or GDT or GDFT or goaldirected or plethysmograph* or h?emodynamic* or ((per?operat* or peri‐operat* or per‐operat* or intraoperat* or intra‐operat*) adj2 monitor*) or heart function or ((systolic or pulse or blood) adj2 pressure) or (cardiac adj2 (output or volume or index)) or stroke index or pulse pressure or arterial pulse or vigileo or flotrac or proAQT or blood pressure or thermodilution or dilution technique* or lithium or impedance or masimo or pleth or echocardiography or echo or doppler or cardioQ or pulmonary arter* or swan ganz or flow time).af.
5 1 and 2 and 3 and 4
6 ((crossover procedure or double blind procedure or single blind procedure).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab,sh. or (doubl* adj blind*).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or allocat*.ti,ab. or trial*.ti,ab. or randomized controlled trial.sh. or random*.ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.))
7 5 and 6
Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Surgical Procedures, Operative] explode all trees
#2 MeSH descriptor: [Perioperative Care] explode all trees
#3 (peroperat* or perioperat* or intraoperat* or intra‐operat* or per‐operat* or peri‐operat* or surg*):ti,ab,kw
#4 #1 or #2 or #3
#5 MeSH descriptor: [Fluid Therapy] explode all trees
#6 MeSH descriptor: [Plasma Volume] explode all trees
#7 MeSH descriptor: [Plasma Substitutes] explode all trees
#8 ((fluid* or hemodynamic* or haemodynamic*) near/3 (therap* or restrict* or loading or administrat* or manag* or maintenance or intravenous* or IV)):ti,ab,kw
#9 hydration:ti,ab,kw
#10 ((iv or intravenous) near/5 infusion*):ti,ab
#11 (cr?stall* or volume replacement or fluid titration or cristal* or colloid*):ti,ab
#12 (plasma near/2 (substitute* or volume)):ti,ab
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 restrict* or limit* or reduct* or low or small or little or zero fluid* or RFT or standard or conventional or routin* or usual* or less or traditional or (fast and track) or fast‐track or ERAS or ERP or (enhanced and recovery and surg*) or (enhanced and recovery and program*) or ((multimodal or enhanced or accelerated) and (optimization or management or rehabilitation or protocol or package or program or pathway))
#15 MeSH descriptor: [Hemodynamics] explode all trees
#16 ((goal NEAR/3 (directed or oriented or target*)) or GDT or GDFT or goaldirected or plethysmograph* or hemodynamic* or haemodynamic* or ((peroperat* or perioperat* or peri‐operat* or intraoperat* or intra‐operat*) NEAR/2 monitor*) or heart function or ((systolic or pulse or blood) NEAR/2 pressure) or (cardiac NEAR/2 (output or volume or index)) or stroke index or pulse pressure or arterial pulse or vigileo or flotrac or proAQT or blood pressure or thermodilution or dilution technique* or lithium or impedance or masimo or pleth or echocardiography or echo or doppler or cardioQ or pulmonary arter* or swan ganz or flow time)
#17 #15 or #16
#18 #4 and #13 and #14 and #17, in Trials
Data and analyses
Comparison 1. Restrictive versus goal‐directed fluid therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major complications | 5 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.78, 3.34] |
| 2 All‐cause mortality | 6 | 544 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 3 Peto OR all‐cause mortality | 6 | 544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.81 [1.38, 16.84] |
| 4 Length of hospital stay | 5 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.55, 0.50] |
| 5 Surgery‐related complications | 4 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.87, 2.72] |
| 6 Non‐surgery‐related complications | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.52, 1.93] |
| 7 Renal failure | 4 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.57, 3.36] |
Comparison 2. Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis for risk of bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major complications | 5 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.78, 3.34] |
| 1.1 Low risk of bias studies | 4 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.63, 3.36] |
| 1.2 High risk of bias studies | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.64, 13.98] |
| 2 All‐cause mortality | 6 | 544 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.06] |
| 2.1 Low risk of bias studies | 5 | 464 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.00, 0.06] |
| 2.2 High risk of bias studies | 1 | 80 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
Comparison 3. Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis for missing data, worst‐case scenario.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major complications | 5 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.96, 3.98] |
| 2 All‐cause mortality | 6 | 561 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.09] |
Comparison 4. Restrictive versus goal‐directed fluid therapy ‐ sensitivity analysis as per exclusion of Colantonio 2015 study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major complications | 4 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.60, 2.10] |
| 2 All‐cause mortality | 5 | 464 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.01, 0.04] |
| 3 Peto OR all‐cause mortality | 5 | 464 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.70 [0.74, 18.44] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benes 2015.
| Methods |
Study type: double‐blinded, parallel RCT Location: Czech Republic ‐ Department of Anesthesia and Intensive Care Medicine of the Faculty of Medicine, and Charles University Hospital in Plzen Number of centres: 1 Duration of study: late November 2012 to early March 2013 Follow‐up: 30 days Protocol: ACTRN12612001014842 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: 80 randomized (40 in RFT and 40 in GDFT); 80 analysed (40 in RFT and 40 in GDFT) Characteristics
Comorbidities, n (%)
Intraoperative fluids (mL)
Preoperative fluid deficit: absent: participants were fasted before the procedure, small amounts of liquids were allowed for those later on the operating schedule and for chronic medication ingestion. During fasting, all participants received an infusion of Hartmann solution (2 mL/kg/h) from the morning of the operative day |
|
| Interventions |
Treatment groups
Concomitant treatment in both groups
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
|
| Notes |
Funding: supported by the Charles University Research Fund (project number P36), the open access fee was granted by the CNSystems Graz, Austria. The CNAP® Monitor and Task Force® Monitor software were supplied by CNSystems, Graz, Austria Conflict of interest (COI): COI reported ‐ JB is an advisory board member for Edwards Lifesciences Inc.; all other co‐authors declare no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | NR |
| Allocation concealment (selection bias) | Low risk | Technique of envelopes stored in non‐transparent containers (1 per stratum). Each envelope, holding 1 participant’s identification, was then placed in another non‐transparent container, which remained sealed until the end of the study, when the concealment was broken for statistical analysis |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome) ‐ all study members as well as the surgeon and other healthcare staff apart from the anaesthesiologist were blinded to individual participant’s allocation. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome) ‐ all study members as well as the surgeon and other healthcare staff apart from the anaesthesiologist were blinded to individual participant’s allocation. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Length of hospital stay | Low risk | All study members as well as the surgeon and other healthcare staff apart from the anaesthesiologist were blinded to individual participant's allocation. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | All study members as well as the surgeon and other healthcare staff apart from the anaesthesiologist were blinded to individual participant's allocation. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Renal failure | Low risk | All study members as well as the surgeon and other healthcare staff apart from the anaesthesiologist were blinded to individual participant's allocation. Anaesthesiologist had a protocol of fluid management |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). 2 investigators blinded to study group allocation and not participating in anaesthesia care and randomization evaluated the state of participants during regular visits |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). 2 investigators blinded to study group allocation and not participating in anaesthesia care and randomization evaluated the state of participants during regular visits |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Length of hospital stay | Low risk | 2 investigators blinded to study group allocation and not participating in anaesthesia care and randomization evaluated the state of participants during regular visits |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | 2 investigators blinded to study group allocation and not participating in anaesthesia care and randomization evaluated the state of participants during regular visits |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Renal failure | Low risk | 2 investigators blinded to study group allocation and not participating in anaesthesia care and randomization evaluated the state of participants during regular visits |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis; no loss to follow‐up (LTFU) |
| Selective reporting (reporting bias) | Low risk | All outcome data provided as described in the protocol |
| Other bias | Low risk | None identified |
Brandstrup 2012.
| Methods |
Study type: parallel RCT Location: Denmark ‐ 5 Danish hospitals Number of centres: 5 Duration of study: March 2008 to July 2009 Follow‐up: 30 days Protocol: NR |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: 151 randomized (79 in RFT and 72 in GDFT); 150 analysed (79 in RFT and 71 in GDFT) Characteristics
Comorbidities, n (%)
Intraoperative fluids (mL)
Preoperative fluid deficit: absent ‐ participants did not receive preoperative oral gut irrigation and were allowed to drink clear fluids until 2 hours before surgery |
|
| Interventions |
Treatment groups
Concomitant treatment in both groups: NR |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: funded by Aase and Einar Danielsen’s Fond. Deltex Medical A/S and Neovitalis A/S supported the trial by lending us the CardioQ‐ODMTM Doppler monitors and a training programme for the anaesthesiologists. A discount was given on the Doppler probes. Fresenius Kabi supported the trial by printing the case report forms, generating the randomization sequence, and providing and packing the randomization envelopes. BB received a travel grant from Fresenius Kabi Conflict of interest: COI reported ‐ no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomization sequence was made by Fresenius Kabi and was delivered in sealed, opaque, consecutively numbered envelopes |
| Allocation concealment (selection bias) | Low risk | The randomization sequence was made by Fresenius Kabi and was delivered in sealed, opaque, consecutively numbered envelopes. Block randomization with 6 participants in each block was performed to ensure an equal number of participants in the 2 groups from each centre. The number of participants in each block was kept secret for all investigators until the concealment was broken at the end of the trial |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome) ‐ Surgeons and participants were kept blinded. Anaesthesiologist was not blinded. No information on blinding of staff that cared for participants after operation. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome) ‐ Surgeons and participants were kept blinded. Anaesthesiologist was not blinded. No information on blinding of staff that cared for participants after operation. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Length of hospital stay | Unclear risk | Surgeons and participants were kept blinded. Anaesthesiologist was not blinded. No information on blinding of staff that cared for participants after operation. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Surgery‐related complications | Unclear risk | Surgeons and participants were kept blinded. Anasthesiologist was not blinded. No information on blinding of staff that cared for participants after operation. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Non‐surgery‐related complications | Unclear risk | Surgeons and participants were kept blinded. Anasthesiologist was not blinded. No information on blinding of staff that cared for participants after operation. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Renal failure | Unclear risk | Surgeons and participants were kept blinded. Anaesthesiologist was not blinded. No information on blinding of staff that cared for participants after operation. Anaesthesiologist had a protocol of fluid management |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). No information on blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). No information on blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Length of hospital stay | Unclear risk | No information on blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Surgery‐related complications | Unclear risk | No information on blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Non‐surgery‐related complications | Unclear risk | No information on blinding of outcome assessors |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Renal failure | Unclear risk | No information on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis; 1 participant randomized to GDFT group excluded from analysis because the planned surgery was cancelled |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but no concerns were raised |
| Other bias | Unclear risk | The presence of both the anaesthetist and the surgeon was mandatory for inclusion of participants; hence strictly consecutive participant inclusion was not preserved |
Colantonio 2015.
| Methods |
Study type: parallel RCT Location: Italy ‐ National Cancer Institute Number of centres: 1 Duration of study: June 2010 to September 2012 Follow‐up: up to 30 days Protocol: NR |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: 86 randomized (44 in RFT and 42 in GDFT); 80 analysed (42 in RFT and 38 in GDFT) Characteristics
Comorbidities, n (%)
Intraoperative fluids (mL)
Intraoperative fluids (mL/kg/h)
Preoperative fluid deficit: no information ‐ study authors did not report any information on preoperative fluid deficit |
|
| Interventions |
Treatment groups
Concomitant treatment in both groups In both groups, participants were transfused with concentrated red cells for Hb values < 8 mg/dL (9 mg/dL in participants with congestive heart failure or coronary heart disease). In both groups during the HIPEC (duration 90 minutes), fresh frozen plasma (FFP) was administered (1 U/15 min) for a total of 6 units, in accordance with the standardized technique applied at our institute. Diuresis was maintained at values ≥ 120 mL/15 min; administration of diuretics (furosemide) was free up to a maximum of 250 mg |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: departmental funding Conflict of interest: COI reported ‐ no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Process of randomization was carried out according to specific dedicated software, developed in‐house by GW Basic programmer, which generated an assignment code verified immediately before induction of anaesthesia |
| Allocation concealment (selection bias) | Low risk | An operator who is not directly involved in the study randomly divided participants into 2 treatment groups |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). Surgeons were not blinded. No information on blinding of other personnel and participants Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). Surgeons were not blinded. No information on blinding of other personnel and participants Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Length of hospital stay | High risk | Surgeons were not blinded. No information on blinding of other personnel and participants. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Surgery‐related complications | High risk | Surgeons were not blinded. No information on blinding of other personnel and participants. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Non‐surgery‐related complications | High risk | Surgeons were not blinded. No information on blinding of other personnel and participants. Anaesthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Renal failure | High risk | Surgeons were not blinded. No information on blinding of other personnel and participants. Anaesthesiologist had a protocol of fluid management |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). The incidence of postoperative complications was rated by anaesthesiologists who were not involved in the intraoperative management of participants ‐ a blinded observer recorded the outcomes |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). The incidence of postoperative complications was rated by anaesthesiologists who were not involved in the intraoperative management of participants ‐ a blinded observer recorded the outcomes |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Length of hospital stay | Low risk | The incidence of postoperative complications was rated by anaesthesiologists who were not involved in the intraoperative management of participants ‐ a blinded observer recorded the outcomes |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Surgery‐related complications | Low risk | The incidence of postoperative complications was rated by anaesthesiologists who were not involved in the intraoperative management of participants ‐ a blinded observer recorded the outcomes |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | The incidence of postoperative complications was rated by anaesthesiologists who were not involved in the intraoperative management of participants ‐ a blinded observer recorded the outcomes |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Renal failure | Low risk | The incidence of postoperative complications was rated by anaesthesiologists who were not involved in the intraoperative management of participants ‐ a blinded observer recorded the outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per‐protocol analysis: Randomized: intervention: 44, control: 42 Analysed: intervention: 42, control: 38 Exclusions More participants excluded from the GDFT group versus the RFT group (4 vs 2) Exclusion reasons GDFT: surgery cancelled due to deterioration of participant's clinical condition ‐ 3; intraoperative anaesthesiological complications ‐ 1 RFT: surgery cancelled due to deterioration of participant's clinical condition ‐ 1; intraoperative anaesthesiological complications ‐ 1 'Worst case scenario' analysis influences the results of analysis for major complications and mortality |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but no concerns were raised |
| Other bias | Low risk | None identified |
Phan 2014.
| Methods |
Study type: parallel RCT Location: Australia ‐ St Vincent’s Hospital campus, St Vincent’s Public Hospital, and St Vincent’s Private Hospital, Fitzroy, Victoria Number of centres: 3 Duration of study: June 2012 to December 2013 Follow‐up: 30 days Protocol: ACTRN12612000717853 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: 100 randomized (50 in RFT and 50 in GDFT); 100 analysed (50 in RFT and 50 in GDFT) Characteristics
Comorbidities, n (%)
Intraoperative fluids (mL)
Preoperative fluid deficit: absent ‐ Nutricia PreOp* 2 × 200 mL carbohydrate drinks were given to participants the day before surgery and 2 hours before surgery |
|
| Interventions |
Treatment groups
Concomitant treatment in both groups Postoperative fluids for both groups followed an identical regimen: a maintenance rate of 0.5 mL/kg/h Hartmann’s solution (minimum 40 mL/h) for the first 24 hours, with additional boluses allowed for hypotension or urine output < 30 mL/h for 4 hours |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: supported by St Vincent’s Hospital Research Endowment Fund 2012, AUD $20,000 Conflict of interest: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization with sealed opaque envelopes was done through a computer‐generated randomization sequence and occurred on the day of surgery just before the anaesthetic was administered |
| Allocation concealment (selection bias) | Low risk | Randomization with sealed opaque envelopes was done through a computer‐generated randomization sequence and occurred on the day of surgery just before the anaesthetic was administered |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). The anaesthetist was not blinded. However, the participant, the surgical team, and data collectors were blinded. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). The anaesthetist was not blinded. However, the participant, the surgical team, and data collectors were blinded. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Length of hospital stay | Low risk | The anaesthetist was not blinded. However, the participant, the surgical team, and data collectors were blinded. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Surgery‐related complications | Low risk | The anaesthetist was not blinded. However, the participant, the surgical team, and data collectors were blinded. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | The anaesthetist was not blinded. However, the participant, the surgical team, and data collectors were blinded. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Renal failure | Low risk | The anaesthetist was not blinded. However, the participant, the surgical team, and data collectors were blinded. Anasthesiologist had a protocol of fluid management |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). All postoperative data were collected by a research nurse or a research registrar who was blinded to the allocation |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). All postoperative data were collected by a research nurse or a research registrar who was blinded to the allocation |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Length of hospital stay | Low risk | All postoperative data were collected by a research nurse or a research registrar who was blinded to the allocation |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Surgery‐related complications | Low risk | All postoperative data were collected by a research nurse or a research registrar who was blinded to the allocation |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | All postoperative data were collected by a research nurse or a research registrar who was blinded to the allocation |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Renal failure | Low risk | All postoperative data were collected by a research nurse or a research registrar who was blinded to the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis; no loss to follow‐up (LTFU) |
| Selective reporting (reporting bias) | Low risk | All outcome data provided as described in protocol |
| Other bias | Low risk | None identified |
Srinivasa 2013.
| Methods |
Study type: parallel RCT Location: New Zealand ‐ Manukau Surgery Centre ‐ Middlemore Hospital Auckland, North Shore Hospital Auckland Number of centres: 1 Duration of study: November 2009 to September 2011 Follow‐up: up to 30 days Protocol: NCT00911391 |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: 85 randomized (43 in RFT and 42 in GDFT); 74 analysed (37 in RFT and 37 in GDFT) Characteristics
Comorbidities, n (%): NR Intraoperative fluids (mL)
Preoperative fluid deficit: absent ‐ oral bowel preparation was used at the discretion of the operating surgeon for participants having left‐sided colonic operations, but was otherwise avoided. Participants undergoing bowel preparation received 1 litre of crystalloid before surgery. All participants received 400 mL of oral carbohydrate loading on the morning of surgery up to 2 hours before their operation |
|
| Interventions |
Treatment groups
Concomitant treatment in both groups
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: the oesophageal Doppler monitor was lent by Pharmaco NZ for the duration of the study. All disposable probes were purchased at regular cost, and Pharmaco NZ had no input into study design, data collection, interpretation of results, or decision to publish Conflict of interest: COI reported ‐ no COI. SS and PPS are recipients of the Auckland Medical Research Foundation Ruth Spencer Medical Research Fellowship. T‐CY is a recipient of a New Zealand Health Research Council Clinical Training Scholarship |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was conducted using random numbers obtained from an open‐source computer‐based random number generator (http://www.random.org). The randomization sequence was generated by a third party not involved in the conduct of the study |
| Allocation concealment (selection bias) | Low risk | Allocation details were concealed in opaque envelopes that were opened on the day of surgery, when patients were randomized. The allocation was performed by a research assistant after insertion of the ODM probe before the start of surgery and before colloid administration |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). The patient, study investigators, the surgeon, and other medical staff responsible for patient care were blinded to patient allocation. An unblinded research assistant and the consultant anaesthetist were aware of patient allocation. The research assistant was not involved in any postoperative data collection or perioperative care of patients. A drape was placed to prevent surgeons from observing fluid administration, and the research assistant was instructed to attach intravenous fluids periodically for patients in the fluid restriction group without actually administering them, to mimic the anticipated practice of fluid boluses in the GDFT group. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). The patient, study investigators, the surgeon, and other medical staff responsible for patient care were blinded to patient allocation. An unblinded research assistant and the consultant anaesthetist were aware of patient allocation. The research assistant was not involved in any postoperative data collection or perioperative care of patients. A drape was placed to prevent surgeons from observing fluid administration, and the research assistant was instructed to attach intravenous fluids periodically for patients in the fluid restriction group without actually administering them, to mimic the anticipated practice of fluid boluses in the GDFT group. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Length of hospital stay | Low risk | The patient, study investigators, the surgeon, and other medical staff responsible for patient care were blinded to patient allocation. An unblinded research assistant and the consultant anaesthetist were aware of patient allocation. The research assistant was not involved in any postoperative data collection or perioperative care of patients. A drape was placed to prevent surgeons from observing fluid administration, and the research assistant was instructed to attach intravenous fluids periodically for patients in the fluid restriction group without actually administering them, to mimic the anticipated practice of fluid boluses in the GDFT group. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Surgery‐related complications | Low risk | The patient, study investigators, the surgeon, and other medical staff responsible for patient care were blinded to patient allocation. An unblinded research assistant and the consultant anaesthetist were aware of patient allocation. The research assistant was not involved in any postoperative data collection or perioperative care of patients. A drape was placed to prevent surgeons from observing fluid administration, and the research assistant was instructed to attach intravenous fluids periodically for patients in the fluid restriction group without actually administering them, to mimic the anticipated practice of fluid boluses in the GDFT group. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | The patient, study investigators, the surgeon, and other medical staff responsible for patient care were blinded to patient allocation. An unblinded research assistant and the consultant anaesthetist were aware of patient allocation. The research assistant was not involved in any postoperative data collection or perioperative care of patients. A drape was placed to prevent surgeons from observing fluid administration, and the research assistant was instructed to attach intravenous fluids periodically for patients in the fluid restriction group without actually administering them, to mimic the anticipated practice of fluid boluses in the GDFT group. Anasthesiologist had a protocol of fluid management |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Quality of surgical recovery | Low risk | The patient, study investigators, the surgeon, and other medical staff responsible for patient care were blinded to patient allocation. An unblinded research assistant and the consultant anaesthetist were aware of patient allocation. The research assistant was not involved in any postoperative data collection or perioperative care of patients. A drape was placed to prevent surgeons from observing fluid administration, and the research assistant was instructed to attach intravenous fluids periodically for patients in the fluid restriction group without actually administering them, to mimic the anticipated practice of fluid boluses in the GDFT group. Anasthesiologist had a protocol of fluid management |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ Major complications | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). Outside the intraoperative phase, all data were collected prospectively by a single‐blinded investigator |
| Blinding of outcome assessment (detection bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by lack of blinding (objective outcome). Outside the intraoperative phase, all data were collected prospectively by a single‐blinded investigator |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Length of hospital stay | Low risk | Outside the intraoperative phase, all data were collected prospectively by a single‐blinded investigator |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Surgery‐related complications | Low risk | Outside the intraoperative phase, all data were collected prospectively by a single‐blinded investigator |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | Outside the intraoperative phase, all data were collected prospectively by a single‐blinded investigator |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Quality of surgical recovery | Low risk | Outside the intraoperative phase, all data were collected prospectively by a single‐blinded investigator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As‐treated analysis: Randomized: intervention: 42, control: 43 Analysed: intervention: 37, control: 37 Exclusions Intervention: did not receive allocated intervention (stoma created) n = 5: (a) stapler misfire n = 1, (b) rectal lesion found at operation n = 2, (c) poor vascularity of bowel on clinical assessment n = 2 Control: did not receive allocated intervention (stoma created) n = 6: (a) unresectable lesion n = 1, (b) rectal lesion found at operation n = 4, (c) poor vascularity of bowel on clinical assessment n = 1 'Worst case scenario' analysis influences results of the analysis for major complications and mortality Other: 3 patients (2 fluid restriction, 1 GDFT) had an intraoperative protocol violation |
| Selective reporting (reporting bias) | Low risk | All outcome data provided as described in the protocol |
| Other bias | Low risk | None identified |
Zhang 2012.
| Methods |
Study type: parallel RCT Location: China ‐ Fudan University, Department of Anesthesiology, Huashan Hospital, Shanghai Number of centres: 1 Duration of study: NR Follow‐up: until discharge of patient Protocol: NR |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: 60 randomized (20 in RFT, 20 in GDFT Ringer’s lactate group, and 20 in GDFT colloid group); 60 analysed (20 in RFT, 20 in GDFT Ringer’s lactate group, and 20 in GDFT colloid group) Characteristics
Comorbidities, n (%)
Intraoperative fluids (mL)
Preoperative fluid deficit: present ‐ surgery was preceded by an 8‐hour fasting period |
|
| Interventions |
Treatment groups
Concomitant treatment in both groups Blood loss was replaced with HES at a 1:1 ratio, and blood transfusion was started when clinically indicated and supported by laboratory evidence of haematocrit < 28% |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
Funding: NR Conflict of interest: COI reported ‐ no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 3 groups according to the intraoperative fluid protocol using a random number generator in sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to 1 of 3 groups according to the intraoperative fluid protocol using a random number generator in sealed envelopes |
| Blinding of participants and personnel (performance bias) Primary outcome ‐ All‐cause mortality | Low risk | Outcome unlikely to be influenced by the lack of blinding (objective outcome). No information on blinding of surgeons, participants , staff. Quote. "the same surgical team was in charge of the postoperative care of the patients, including fluid infusion and postoperative analgesia" |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Length of hospital stay | High risk | No information on blinding of surgeons, participants , staff. Quote. "the same surgical team was in charge of the postoperative care of the patients, including fluid infusion and postoperative analgesia" |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Surgery‐related complications | High risk | No information on blinding of surgeons, patients, staff: "The same surgical team was in charge of the postoperative care of the patients, including fluid infusion and postoperative analgesia" |
| Blinding of participants and personnel (performance bias) Secondary outcome ‐ Non‐surgery‐related complications | High risk | No information on blinding of surgeons, participants , staff. Quote. "the same surgical team was in charge of the postoperative care of the patients, including fluid infusion and postoperative analgesia" |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Length of hospital stay | Low risk | Once the participants were sent to the ward, follow‐up was conducted by an independent researcher who was unaware of the randomization of the participant until the participant was discharged from the hospital |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Surgery‐related complications | Low risk | Once the participants were sent to the ward, follow‐up was conducted by an independent researcher who was unaware of the randomization of the participant until the participant was discharged from the hospital |
| Blinding of outcome assessment (detection bias) Secondary outcome ‐ Non‐surgery‐related complications | Low risk | Once the participants were sent to the ward, follow‐up was conducted by an independent researcher who was unaware of the randomization of the participant until the participant was discharged from the hospital |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis; no exclusions or drop‐outs |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but no concerns raised |
| Other bias | Low risk | None identified |
ASA: American Society of Anaesthesiology.
BMI: body mass index.
CO: cardiac output.
COI: conflict of interest.
COPD: chronic obstructive pulmonary disease.
Cr: creatinine.
CVP: central venous pressure.
DVT: deep venous thrombosis.
FFP: fresh frozen plasma.
GDFT: goal‐directed fluid therapy.
Hb: haemoglobin.
HES: hydroxyethyl starch.
HIPEC: hyperthermic intraperitoneal chemotherapy.
HR: heart rate.
ICU: intensive care unit.
INR: international normalized ratio.
IQR: interquartile range.
ITT: intention‐to‐treat.
IV: intravenous.
LOS: length of stay.
LTFU: lost to follow‐up.
MAP: mean arterial pressure.
NR: not reported.
ODM: oesophageal doppler monitor.
PPV: pulse pressure variation.
RCT: randomized controlled trial.
RFT: restrictive fluid therapy.
SD: standard deviation.
SRS: surgical recovery score.
SV: stroke volume.
SVI: stroke volume index.
SVV: stroke volume variation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ackland 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Benes 2010 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Bisgaard 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Bloom 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Buettner 2008 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Bundgaard‐Nielsen 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Calvo 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Calvo‐Vecino 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Cecconi 2011 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Cesur 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Challand 2012 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Chattopadhyay 2012 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Chytra 2007 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Concha 2011 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Corbella 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Cordero‐Rochet 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Correa‐Gallego 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Demirel 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Dhawan 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Elgendy 2017 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Foppa 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Forget 2009 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Forget 2010 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Forget 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Fukui 2009 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Funcke 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Funk 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Futier 2010 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Gerent 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Gómez‐Izquierdo 2017 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Hand 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Hughes 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Johnson 2011 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Joosten 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Kaufmann 2017 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Kellman 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Kulkarni 2012 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Kumar 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Lai 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Li 2011 | Wrong comparison. The control group did not meet the criteria for GDFT |
| Liang 2017 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Lilot 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Liu 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Lobo 2011 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Lopes 2007 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Luo 2017 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Martini 2009 | The study was classified as meeting the inclusion criteria primarily on the basis of the published abstract. However, additional information from study authors revealed that randomization was started without ethical committee full approval (some missing papers), and the study was finally completed as a retrospective analysis |
| McKenny 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Minkovich 2012 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Minto 2011 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Moppett 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Muller 2009 | Wrong comparison. The intervention group did not meet the criteria for RFT |
| Munoz 2012 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| NCT03193320 | The study has not been started. Study authors were not able to conduct the study ‐ information was obtained through email contact with study authors |
| Noblett 2006 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Park 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Peng 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Pillai 2011 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Rath 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Schmid 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Sundaram 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Szturz 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Van der Linden 2010 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Vanakas 2012 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Venn 2002 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Wakeling 2005 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Wen 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Wilmin 2009 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Wilson 1999 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Xiao 2015 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Xu 2017 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Yu 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Zakhaleva 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Zeng 2014 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Zhao 2018 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Zheng 2013 | Wrong intervention. The intervention group did not meet the criteria for RFT |
| Zheng 2016 | Wrong intervention. The intervention group did not meet the criteria for RFT |
RFT: restrictive fluid therapy.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800014777.
| Trial name or title | Comparison of three different liquid therapies in colorectal surgery |
| Methods |
Study type: parallel RCT Location: Shenyang, China Number of centres: 1 Duration of study: NR Follow‐up: NR Protocol: ChiCTR1800014777 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Treatment groups
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | February 2018 |
| Contact information | Zhao Xiaochun; +86 18940257646; zhaoxc@sj‐hospital.org |
| Notes |
Funding: NR Conflict of interest: NR |
NCT02625701.
| Trial name or title | Perioperative fluid management: goal‐directed therapy versus restrictive approach: a randomized controlled trial |
| Methods |
Study type: parallel, single‐blinded (participants) RCT Location: Geneva, Switzerland Number of centres: NR Duration of study: NR Follow‐up: up to 15 weeks after date of surgery Protocol: NCT02625701 |
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: NR Characteristics: NR Comorbidities, n (%): NR |
| Interventions |
Treatment groups
Concomitant treatment in both groups
|
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | January 2012 |
| Contact information | Marc Licker; +41223827439; marc‐joseph.licker@hcuge.ch |
| Notes |
Funding: University Hospital, Geneva Conflict of interest: NR When we contacted study authors, we were informed that the study will be finished within a few months and the results will be published in the upcoming year |
NCT03039946.
| Trial name or title | Automated closed‐loop versus restrictive fluid therapy in abdominal surgery: a pilot randomized controlled trial |
| Methods |
Study type: parallel, single‐blinded (participants) RCT Location: Brussels, Belgium Number of centres: 1 Duration of study: NR Follow‐up: up to 90 days after hospitalization Protocol: NCT03039946 |
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: NR Characteristics: NR Comorbidities, n (%): NR |
| Interventions |
Treatment groups
Concomitant treatment in both groups: NR |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | January 2017 |
| Contact information | NR |
| Notes |
Funding: Erasme University Hospital Conflict of interest: NR |
NCT03519165.
| Trial name or title | Restrictive or individualized goal‐directed fluid replacement strategy in ovarian cancer cytoreductive surgery (RIGoROCS) |
| Methods |
Study type: parallel, double‐blinded (participants, outcomes assessor) RCT Location: Kolkata, India Number of centres: 1 Duration of study: NR Follow‐up: up to approximately 2 years Protocol: NCT03519165 |
| Participants |
Inclusion criteria
Exclusion criteria
Total number of participants: NR Characteristics: NR Comorbidities, n (%): NR |
| Interventions |
Treatment groups
Concomitant treatment in both groups: NR |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | June 2016 |
| Contact information | Jyotsna Goswami; 03366057000 ext 7179; jyotsnagoswami@gmail.com |
| Notes |
Funding: Tata Medical Center Conflict of interest: NR |
APTT: activated partial thromboplastin time.
ASA: American Society of Anesthesiology.
BMI: body mass index.
COPD: chronic obstructive pulmonary disease.
CRP: C‐reactive protein.
CVP: central venous pressure.
FEV₁: forced expiratory volume in one second.
GDFT: goal‐directed fluid therapy.
GDT: goal‐directed therapy.
HIPEC: hyperthermic intraperitoneal chemotherapy.
IDS: interval debulking surgery.
IL‐6: interleukin‐6.
INR: international normalized ratio.
LOS: length of stay.
LVEF: left ventricular ejection fraction.
MAP: mean arterial pressure.
MELD: model for end‐stage liver disease.
MI: myocardial infarction.
NR: not reported.
PDS: primary debulking surgery.
PPV: pulse pressure variation.
RCT: randomized controlled trial.
RFT: restrictive fluid therapy.
RIFLE: risk, injury, failure, loss of function, and end‐stage renal disease.
SIRS: systemic inflammatory response syndrome.
SOFA: sequential organ failure assessment.
SVI: stroke volume index.
SVRI: systemic vascular resistance index.
SVV: stroke volume variation.
TNF: tumour necrosis factor.
Differences between protocol and review
We made the following changes to the published protocol (Wrzosek 2017).
Because data on the number of participants with at least one non‐surgery‐related complication were very limited (reported only by Srinivasa 2013), we decided to perform a post‐hoc analysis on the average number of non‐surgery‐related complications per person. Additionally, we compared the average total number of complications per person and the average number of complications per person grouped by type. The average number of complications per person with 95% CI was estimated, and it was compared using a Poisson regression model.
For continuous measures, such as hospital LOS, we calculated mean differences when means and standard deviations were available; however, for some studies, such data were not available. Thus, in a case when the distribution of variables was presented as the median and range or interquartile range, or both, these values were converted to means and standard deviations using algorithms described by Wan 2014.
We modified the search strategy to make it more sensitive and precise; we added additional keywords referring to RFT and GDFT.
Because the studies reported low event rates for all‐cause mortality, and due to many zero‐event groups, we performed an additional post‐hoc Peto odds ratio analysis and Poisson regression analysis, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and by the Statistical Editor. Also, we presented the results for this outcome as the risk difference (RD) because it better reflects the magnitude of the treatment effect for low event rates.
We presented NNTB values only for statistically significant outcomes. For non‐significant outcomes, we decided not to present NNTB values. Very wide confidence intervals approaching infinity do not bring clinically important information to the reader and may lead to misinterpretation.
We decided to perform sensitivity analysis and to test how the exclusion of the Colantonio 2015 study influenced the results of the review. We chose to do this because, in most of the included studies, the total volume of fluid finally received by participants intraoperatively was smaller in the RFT group compared with the GDFT group. Exceptions to this included the Benes 2015 study, where the volumes were comparable, and the Colantonio 2015 study, where participants in the RFT group received more fluid than those in the GDFT group. Moreover, in Colantonio 2015, study authors declare that the fluid protocol in the intervention group was 'mainly restrictive' and participants received a basal infusion of crystalloid ranging from 4 to 10 mL/kg/h. This overlaps with infusion rates set in other included studies; however, the upper limit is higher, which could result in less rigorous fluid restriction in this study compared with other included studies.
Contributions of authors
Anna Wrzosek (AW), Joanna Jakowicka‐Wordliczek (JJW), Renata Zajaczkowska (RZ), Milosz Jankowski (MJ), Malgorzata M Bala (MMB), Wojciech Serednicki (WS), Matusz J Swierz (MJS), Maciej Polak (MP), Jerzy Wordliczek (JW).
Conceiving the review: AW, JJW, RZ, WS, MJ, MJS, MMB, MP, JW.
Designing the review: AW, MMB, RZ, WS, MJ, JW.
Co‐ordinating the review: AW.
Undertaking manual searches: AW, JJW, MJS, RZ, WS.
Screening search results: AW, JJW, RZ, WS, MJ, MJS, MMB, MP, JW.
Organizing retrieval of papers: AW, MJS, MMB.
Screening retrieved papers against inclusion criteria: AW, JJW, RZ, WS, MJ, JW.
Appraising quality of papers: AW, JJW, RZ, WS, MJ, MJS, MMB.
Abstracting data from papers: AW, JJW, RZ, WS, MJ, MJS.
Writing to authors of papers for additional information: AW, MJS.
Providing additional data about papers: AW, MJS.
Obtaining and screening data on unpublished studies: AW, JJW, RZ, WS, MJ, MJS.
Managing data for the review: AW, MJ, MJS, MMB.
Entering data into RevMan5: AW, MJS, MJ.
Analysing RevMan statistical data: AW, MJS, MP, MMB.
Performing other statistical analysis not using RevMan5: AW, MP.
Performing double entry of data (data entered by person one; data entered by person two): MJS, AW.
Interpreting data: AW, JJW, RZ, WS, MJ, MJS, MMB, MP, JW.
Making statistical inferences: MJS, AW, MMB.
Writing the review: AW, MJS, JJW, RZ, WS, MJ, MMB, MP, JW.
Providing guidance on the review: AW, MMB.
Securing funding for the review: no funding provided.
Performing previous work that was the foundation of the present study: not applicable.
Serving as guarantor for the review (one author): AW.
Taking responsibility for reading and checking the review before submission: AW, JJW, RZ, WS, MJ, JW, MMB.
Declarations of interest
Anna Wrzosek is employed as an Assistant in the Department of Interdisciplinary Intensive Care. Dr Wrzosek has no known conflicts of interest.
Maciej Polak is an Assistant in the Department of Epidemiology and Population Studies in the Institute of Public Health. Dr Polak has no known conflicts of interest.
Malgorzata M Bała is acting head of the Chair of Epidemiology and Preventive Medicine and the Head of the Department of Hygiene and Dietetics. Prof Bala has no known conflicts of interest.
Mateusz J Swierz is an Assistant in the Department of Surgery of a Specialist Hospital in Gorlice and a PhD student at the Department of Hygiene and Dietetics. Dr Swierz has no known conflicts of interest.
Joanna Jakowicka‐Wordliczek is employed as a Specialist in Anaesthesiology and Intensive Care. Dr Jakowicka‐Wordliczek has no known conflicts of interest.
Renata Zajaczkowska is employed as a Specialist in Anaesthesiology and Intensive Care. Dr Zajaczkowsk has no known conflicts of interest.
Milosz Jankowski is employed as a Specialist in Anaesthesiology and Intensive Care. He receives honoraria (as a freelancer) from a publishing company that draws its revenue from pharmaceutical companies in unrelated indications. He is not aware of any direct conflict of interest.
Jerzy Wordliczek is a Head of the Department of Interdisciplinary Intensive Care. Prof Wordliczek has no known conflicts of interest.
Wojciech T Serednicki is employed as a Consultant in Anaesthesia and Critical Care. Dr Serednicki has no known conflicts of interest.
New
References
References to studies included in this review
Benes 2015 {published data only}
- Benes J, Haidingerova L, Pouska J, Stepanik J, Stenglova A, Zatloukal J, et al. Fluid management guided by a continuous non‐invasive arterial pressure device is associated with decreased postoperative morbidity after total knee and hip replacement. BMC Anesthesiology 2015;15(1):148‐58. [PUBMED: 26471495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandstrup 2012 {published data only}
- Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near‐maximal stroke volume or zero fluid balance?. British Journal of Anaesthesia 2012;109(2):191‐9. [PUBMED: 22710266] [DOI] [PubMed] [Google Scholar]
Colantonio 2015 {published data only}
- Colantonio L, Claroni C, Fabrizi L, Marcelli ME, Sofra M, Giannarelli D, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Journal of Gastrointestinal Surgery 2015;19(4):722‐9. [PUBMED: 25595308] [DOI] [PubMed] [Google Scholar]
Phan 2014 {published data only}
- Phan TD, D'Souza B, Rattray MJ, Johnston MJ, Cowie BS. A randomised controlled trial of fluid restriction compared to oesophageal Doppler‐guided goal‐directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesthesia and Intensive Care 2014;42(6):752‐60. [PUBMED: 25342408] [DOI] [PubMed] [Google Scholar]
Srinivasa 2013 {published data only}
- Srinivasa S, Taylor MH, Singh PP, Yu TC, Soop M, Hill AG. Randomized clinical trial of goal‐directed fluid therapy within an enhanced recovery protocol for elective colectomy. British Journal of Surgery 2013;100(1):66‐74. [PUBMED: 23132508] [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang J, Qiao H, He Z, Wang Y, Che X, Liang W. Intraoperative fluid management in open gastrointestinal surgery: goal‐directed versus restrictive. Clinics (Sao Paulo, Brazil) 2012;67(10):1149‐55. [PUBMED: 23070341] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ackland 2015 {published data only}
- Ackland GL, Iqbal S, Paredes LG, Toner A, Lyness C, Jenkins N, et al. Individualised oxygen delivery targeted haemodynamic therapy in high‐risk surgical patients: a multicentre, randomised, double‐blind, controlled, mechanistic trial. Lancet Respiratory Medicine 2015;3(1):33‐41. [PUBMED: 25523407] [DOI] [PubMed] [Google Scholar]
Benes 2010 {published data only}
- Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Critical Care (London, England) 2010;14(3):R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisgaard 2013 {published data only}
- Bisgaard J, Gilsaa T, Rønholm E, Toft P. Optimising stroke volume and oxygen delivery in abdominal aortic surgery: a randomised controlled trial. Acta Anaesthesiologica Scandinavica 2013;57(2):178‐88. [PUBMED: 22897633] [DOI] [PubMed] [Google Scholar]
Bloom 2015 {published data only}
- Bloom M, Cuff G, Plichta A. Goal‐directed fluid therapy in neurosurgical cases. Journal of Neurosurgical Anesthesiology 2015;27(4):424‐5. [Google Scholar]
Buettner 2008 {published data only}
- Buettner M, Schummer W, Huettemann E, Schenke S, Hout N, Sakka SG. Influence of systolic‐pressure‐variation‐guided intraoperative fluid management on organ function and oxygen transport. British Journal of Anaesthesia 2008;101(2):194‐9. [PUBMED: 18511439] [DOI] [PubMed] [Google Scholar]
Bundgaard‐Nielsen 2013 {published data only}
Calvo 2014 {published data only}
- Calvo Vecino JM, Ripolles Melchor J, Martinez Hurtado E, Abad Gurumeta A, Casans Frances R, Serrano A. Efficacy of intraoperatory optimisation of fluids guided with transoesophageal doppler monitorisation: a multicentre randomised controlled trial. European Journal of Anaesthesiology 2014; Vol. 31:13.
Calvo‐Vecino 2018 {published data only}
- Calvo Vecino JM, Ripolles Melchor J, Mythen MG, Casans Frances R, Balik A, Artacho JP, et al. Effect of goal‐directed haemodynamic therapy on postoperative complications in low‐moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial). British Journal of Anaesthesia 2018;120(4):734‐44. [PUBMED: 29576114] [DOI] [PubMed] [Google Scholar]
Cecconi 2011 {published data only}
- Cecconi M, Fasano N, Langiano N, Divella M, Costa MG, Rhodes A, et al. Goal‐directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Critical Care 2011;15(3):R132. [PUBMED: 21624138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cesur 2018 {published data only}
- Cesur S, Cardakozu T, Kus A, Turkyilmaz N, Yavuz O. Comparison of conventional fluid management with PVI‐based goal‐directed fluid management in elective colorectal surgery. Journal of Clinical Monitoring and Computing 2018:Epub ahead of print. [DOI: 10.1007/s10877-018-0163-y; PUBMED: 29948666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Challand 2012 {published data only}
- Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, et al. Randomized controlled trial of intraoperative goal‐directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. British Journal of Anaesthesia 2012; Vol. 108, issue 1:53‐62. [PUBMED: 21873370] [DOI] [PubMed]
Chattopadhyay 2012 {published data only}
- Chattopadhyay S, Patel A, Mittal S, Biliatis I, Christian S, Terblanche A, et al. Role of intra‐operative fluid optimisation using oesophageal doppler in advanced ovarian cancer; early postoperative recovery and fitness for discharge. International Journal of Gynecological Cancer 2012;3:E286. [DOI] [PubMed] [Google Scholar]
Chytra 2007 {published data only}
- Chytra I, Pradl R, Bosman R, Pelnár P, Kasal E, Zidková A. Esophageal Doppler‐guided fluid management decreases blood lactate levels in multiple‐trauma patients: a randomized controlled trial. Critical Care (London, England) 2007;11(1):R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Concha 2011 {published data only}
- Concha PM, Mertz KV, Cortínez FL, Zúñiga DA, Pinedo MG. Transesophageal echocardiography to monitor fluid administration during the perioperative period. Revista Medica de Chile 2011;139(9):1157‐62. [PUBMED: 22215394] [PubMed] [Google Scholar]
Corbella 2018 {published data only}
- Corbella D, Toppin PJ, Ghanekar A, Ayach N, Schiff J, Rensburg A, et al. Cardiac output‐based fluid optimization for kidney transplant recipients: a proof‐of‐concept trial. Canadian Journal of Anesthesia 2018;65(8):873‐83. [PUBMED: 29637407] [DOI] [PubMed] [Google Scholar]
Cordero‐Rochet 2014 {published data only}
- Cordero‐Rochet MJ, McCluskey SA, Minkovich L, Gilbert R. Goal directed fluid management in free flap reconstructive surgery. Canadian Journal of Anesthesia 2014;61:S75. [Google Scholar]
Correa‐Gallego 2015 {published data only}
- Correa‐Gallego C, Tan KS, Arslan‐Carlon V, Gonen M, Denis SC, Langdon‐Embry L, et al. Goal‐directed fluid therapy using stroke volume variation for resuscitation after low central venous pressure‐assisted liver resection: a randomized clinical trial. Journal of the American College of Surgeons 2015; Vol. 221, issue 2:591‐601. [PUBMED: 26206652] [DOI] [PMC free article] [PubMed]
Demirel 2018 {published data only}
- Demirel I, Bolat E, Altun AY, Ozdemir M, Bestas A. Efficacy of goal‐directed fluid therapy via pleth variability index during laparoscopic Roux‐en‐Y gastric bypass surgery in morbidly obese patients. Obesity Surgery 2018;28(2):358‐63. [PUBMED: 28762023] [DOI] [PubMed] [Google Scholar]
Dhawan 2018 {published data only}
- Dhawan R, Shahul S, Roberts JD, Smith ND, Steinberg GD, Chaney MA. Prospective, randomized clinical trial comparing use of intraoperative transesophageal echocardiography to standard care during radical cystectomy. Annals of Cardiac Anaesthesia 2018;21(3):255‐61. [PUBMED: 30052211] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elgendy 2017 {published data only}
- Elgendy MA, Esmat IM, Kassim DY. Outcome of intraoperative goal‐directed therapy using Vigileo/FloTrac in high‐risk patients scheduled for major abdominal surgeries: a prospective randomized trial. Egyptian Journal of Anaesthesia 2017; Vol. 33, issue 3:263‐9.
Foppa 2014 {published data only}
- Foppa C, Zakhaleva J, Tam J, Denoya PI, Bishawi M, Bergamaschi R. The impact of intravenous fluid administration on complication rates in bowel surgery with enhanced recovery protocol: a randomized controlled trial. Techniques in Coloproctology 2014;18(1):91. [DOI] [PubMed] [Google Scholar]
Forget 2009 {published data only}
- Forget P, Lois F, Kock M. Does the pleth variability index improve fluid management during major abdominal surgery?. Critical Care (London, England) 2009;13(Suppl 1):P204. [Google Scholar]
Forget 2010 {published data only}
- Forget P, Lois F, Kock M. Goal‐directed fluid management based on the pulse oximeter‐derived pleth variability index reduces lactate levels and improves fluid management. Anesthesia and Analgesia 2010;111(4):910‐4. [PUBMED: 20705785] [DOI] [PubMed] [Google Scholar]
Forget 2013 {published data only}
- Forget P, Lois F, Kartheuser A, Leonard D, Remue C, Kock M. The concept of titration can be transposed to fluid management. But does it change the volumes? Randomised trial on pleth variability index during fast‐track colonic surgery. Current Clinical Pharmacology 2013; Vol. 8, issue 2:110‐4. [PUBMED: 23061978] [DOI] [PubMed]
Fukui 2009 {published data only}
- Fukui K, Markstaller K, Leibundgut D, Pestel G. Timing of intraoperative fluid management by difference in pulse pressure (dPP). European Journal of Anaesthesiology 2009;45:68. [Google Scholar]
Funcke 2018 {published data only}
- Funcke S, Saugel B, Koch C, Schulte D, Zajonz T, Sander M, et al. Individualized, perioperative, hemodynamic goal‐directed therapy in major abdominal surgery (iPEGASUS trial): study protocol for a randomized controlled trial. Trials 2018;19(1):273. [PUBMED: 29743101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Funk 2015 {published data only}
- Funk DJ, HayGlass KT, Koulack J, Harding G, Boyd A, Brinkman R. A randomized controlled trial on the effects of goal‐directed therapy on the inflammatory response open abdominal aortic aneurysm repair. Critical Care (London, England) 2015;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Futier 2010 {published data only}
- Futier E, Constantin JM, Petit A, Chanques G, Kwiatkowski F, Flamein R, et al. Conservative vs restrictive individualized goal‐directed fluid replacement strategy in major abdominal surgery: a prospective randomized trial. Archives of Surgery 2010; Vol. 145, issue 12:1193‐200. [PUBMED: 21173294] [DOI] [PubMed]
Gerent 2018 {published data only}
- Gerent AR, Almeida JP, Fominskiy E, Landoni G, Oliveira GQ, Rizk SI, et al. Effect of postoperative goal‐directed therapy in cancer patients undergoing high‐risk surgery: a randomized clinical trial and meta‐analysis. Critical Care 2018;22(1):133. [PUBMED: 29792232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gómez‐Izquierdo 2017 {published data only}
- Gómez‐Izquierdo JC, Trainito A, Mirzakandov D, Stein BL, Liberman S, Charlebois P, et al. Goal‐directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: a randomized controlled trial. Anesthesiology 2017;127(1):36‐49. [PUBMED: 28459732] [DOI] [PubMed] [Google Scholar]
Hand 2016 {published data only}
- Hand WR, Stoll WD, McEvoy MD, McSwain JR, Sealy CD, Skoner JM, et al. Intraoperative goal‐directed hemodynamic management in free tissue transfer for head and neck cancer. Head and Neck 2016;38(Suppl 1):E1974‐80. [PUBMED: 26829494] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2013 {published data only}
- Hughes T, Cottam S, Heaton N, Bernal W, Auzinger G, Wendon J, et al. Peri‐operative haemodynamic optimisation using Pulsioflex monitoring in Whipple's surgery. Anaesthesia 2013;68:33. [Google Scholar]
Johnson 2011 {published data only}
- Johnson E, Nunoo R, Al‐Abbad J, Senagore A, Emery T, Dujovny N, et al. Intraoperative fluid management and its correlation with the surgical Apgar score in laparoscopic segmental colectomy. Diseases of the Colon and Rectum 2011;54(5):e200. [Google Scholar]
Joosten 2018 {published data only}
- Joosten A, Raj Lawrence S, Colesnicenco A, Coeckelenbergh S, Vincent JL, Linden P, et al. Personalized versus protocolized fluid management using noninvasive hemodynamic monitoring (clearsight system) in patients undergoing moderate‐risk abdominal surgery. Anesthesia and Analgesia 2018:Epub ahead of print. [DOI: 10.1213/ANE.0000000000003553; PUBMED: 29878939] [DOI] [PubMed] [Google Scholar]
Kaufmann 2017 {published data only}
- Kaufmann KB, Stein L, Bogatyreva L, Ulbrich F, Kaifi JT, Hauschke D, et al. Oesophageal doppler guided goal‐directed haemodynamic therapy in thoracic surgery ‐ a single centre randomized parallel‐arm trial. British Journal of Anaesthesia 2017;118(6):852‐61. [PUBMED: 28575331] [DOI] [PubMed] [Google Scholar]
Kellman 2014 {published data only}
- Kellman S, Roberts JD, Chaney M, Negron E. Prospective randomized clinical trial comparing routine intraoperative transesophageal echocardiography to standard care during radical cystectomy. Anesthesia and Analgesia 2014;118(5 Suppl 1):S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulkarni 2012 {published data only}
- Kulkarni R, Craske DA, Abdel‐Galil K, Hatfield A, Liu A, Pick A, et al. Haemodynamic optimisation in head and neck cancer surgery: pilot randomised controlled trial of LiDCO rapid. European Archives of Oto‐Rhino‐Laryngology 2012;269(4):1370. [Google Scholar]
Kumar 2016 {published data only}
- Kumar L, Rajan S, Baalachandran R. Outcomes associated with stroke volume variation versus central venous pressure guided fluid replacements during major abdominal surgery. Journal of Anaesthesiology, Clinical Pharmacology 2016; Vol. 32, issue 2:182‐6. [PUBMED: 27275046] [DOI] [PMC free article] [PubMed]
Lai 2015 {published data only}
- Lai CW, Starkie T, Creanor S, Struthers RA, Portch D, Erasmus PD, et al. Randomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitness. British Journal of Anaesthesia 2015;115(4):578‐89. [PUBMED: 26385666] [DOI] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li H, Afzal A, Lian Q, Kramer GC, Svensen C, Prough D. Restricted fluid therapy decreases surgical blood loss ‐ a clinical study of two fluid regimens during cesarean section under spinal anesthesia. Anesthesia and Analgesia 2011;112(5 Suppl 1):291. [Google Scholar]
Liang 2017 {published data only}
- Liang M, Li Y, Lin L, Lin X, Wu X, Gao Y, et al. Effect of goal‐directed fluid therapy on the prognosis of elderly patients with hypertension receiving plasmakinetic energy transurethral resection of prostate. International Journal of Clinical and Experimental Medicine 2017; Vol. 10, issue 1:1290‐6.
Lilot 2018 {published data only}
- Lilot M, Bellon A, Gueugnon M, Laplace MC, Baffeleuf B, Hacquard P, et al. Comparison of cardiac output optimization with an automated closed‐loop goal‐directed fluid therapy versus non standardized manual fluid administration during elective abdominal surgery: first prospective randomized controlled trial. Journal of Clinical Monitoring and Computing 2018;32(6):993‐1003. [PUBMED: 29380190] [DOI] [PubMed] [Google Scholar]
Liu 2018 {published data only}
- Liu TJ, Zhang JC, Gao XZ, Tan ZB, Wang JJ, Zhang PP, et al. Clinical research of goal‐directed fluid therapy in elderly patients with radical resection of bladder cancer. Journal of Cancer Research and Therapeutics 2018;14(Suppl 1):S173‐9. [PUBMED: 29578169] [DOI] [PubMed] [Google Scholar]
Lobo 2011 {published data only}
- Lobo SM, Ronchi LS, Oliveira NE, Brandão PG, Froes A, Cunrath GS, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high‐risk surgery. Critical Care (London, England) 2011; Vol. 15, issue 5:R226. [DOI] [PMC free article] [PubMed]
Lopes 2007 {published data only}
- Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Michard F. Goal‐directed fluid management based on pulse pressure variation monitoring during high‐risk surgery: a pilot randomized controlled trial. Critical Care (London, England) 2007;11(5):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2017 {published data only}
- Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal‐directed fluid restriction during brain surgery: a prospective randomized controlled trial. Annals of Intensive Care 2017; Vol. 7, issue 1:16. [PUBMED: 28211020] [DOI] [PMC free article] [PubMed]
Martini 2009 {published data only}
- Martini A, Menestrina N, Simion D, Filetici L, Schweiger V, Gottin L. Perioperative fluid administration in pancreatic surgery: comparison of three regimens. Critical Care 2009;13:S80‐1. [Google Scholar]
McKenny 2013 {published data only}
- McKenny M, Conroy P, Wong A, Farren M, Gleeson N, Walsh C, et al. A randomised prospective trial of intra‐operative oesophageal Doppler‐guided fluid administration in major gynaecological surgery. Anaesthesia 2013;68(12):1224‐31. [PUBMED: 24116747] [DOI] [PubMed] [Google Scholar]
Minkovich 2012 {published data only}
- Minkovich L, McCluskey SA, Djaiani G, Goldstein D, Gilbert R. Goal directed fluid therapy in surgery for head and neck cancers. Canadian Journal of Anesthesia 2012;59:1344381. [Google Scholar]
Minto 2011 {published data only}
- Minto G, Challand C, Sneyd JR, Mellor N, Hosie KB, Erasmus P, et al. Is the impact of intraoperative goal‐directed fluid therapy on length of stay after major elective colorectal surgery related to patients' aerobic fitness?. British Journal of Anaesthesia 2011;106(3):440. [Google Scholar]
Moppett 2015 {published data only}
- Moppett IK, Rowlands M, Mannings A, Moran CG, Wiles MD, NOTTS Investigators. LiDCO‐based fluid management in patients undergoing hip fracture surgery under spinal anaesthesia: a randomized trial and systematic review. British Journal of Anaesthesia 2015; Vol. 114, issue 3:444‐59. [PUBMED: 25500940] [DOI] [PubMed]
Muller 2009 {published data only}
Munoz 2012 {published data only}
- Munoz CA, Rojas JLT, Bermudez OIG. Intraoperative oesophageal doppler during emergency abdominal surgery. British Journal of Anaesthesia 2012;108:ii347. [Google Scholar]
NCT03193320 {unpublished data only}
- NCT03193320. Management of intraoperative fluids in ambulatory surgery [Intraoperative fluid therapy management in low‐risk patients under general anesthesia ‐ a randomized controlled trial comparing liberal, restrictive and pleth variability index (PVI)‐guided fluid administration in a day surgery setting]. https://clinicaltrials.gov/ct2/show/record/NCT03193320 (first received 15 June 2017).
Noblett 2006 {published data only}
- Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler‐optimized fluid management on outcome after elective colorectal resection. British Journal of Surgery 2006;93(9):1069‐76. [PUBMED: 16888706] [DOI] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park S, Kim H, Koo B. Effect of goal‐directed fluid therapy using stroke volume variation in free flap reconstruction. Anesthesia and Analgesia 2016;122(5 Suppl 3):S302. [Google Scholar]
Peng 2014 {published data only}
- Peng K, Li J, Cheng H, Ji FH. Goal‐directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Medical Principles and Practice 2014;23(5):413‐20. [PUBMED: 24994571] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pillai 2011 {published data only}
- Pillai P, McEleavy I, Gaughan M, Snowden C, Nesbitt I, Durkan G, et al. A double‐blind randomized controlled clinical trial to assess the effect of Doppler optimized intraoperative fluid management on outcome following radical cystectomy. Journal of Urology 2011; Vol. 186, issue 6:2201‐6. [PUBMED: 22014804] [DOI] [PubMed]
Rath 2018 {published data only}
- Rath G, Mishra N, Chaturvedi A, Bithal P. Effect of goal‐directed intraoperative fluid therapy on hospital stay in patients undergoing excision of supratentorial tumors. Journal of Neurosurgical Anesthesiology 2018;30(4):421‐2. [Google Scholar]
Schmid 2014 {published data only}
- Schmid S, Heim M, Kapfer B, Blobner M, Kochs E, Jungwirth B. Can goal‐directed hemodynamic management improve renal outcome after major non‐cardiac surgery?. European Journal of Anaesthesiology 2014;31:57. [Google Scholar]
Sundaram 2016 {published data only}
- Sundaram SC, Salins SR, Nandha Kumar A, Korula G. Intra‐operative fluid management in adult neurosurgical patients undergoing intracranial tumour surgery: randomised control trial comparing pulse pressure variance (PPV) and central venous pressure (CVP). Journal of Clinical and Diagnostic Research 2016;10(5):UC01‐5. [PUBMED: 27437329] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szturz 2018 {published data only}
- Szturz P, Folwarczny P, Kula R, Neiser J, Sevcik P, Benes J. Multi‐parametric functional hemodynamic optimization improves postsurgical outcome after intermediate risk open gastrointestinal surgery, a randomized controlled trial. Minerva Anestesiologica 2018:Epub ahead of print. [DOI: 10.23736/S0375-9393.18.12467-9; PUBMED: 29756693] [DOI] [PubMed] [Google Scholar]
Vanakas 2012 {published data only}
- Vanakas T, Asouhidou I, Samaras A, Diminikos G, Ioannou P. Implementation of goal‐directed protocol in elderly patients undergoing femoral fracture repair. European Journal of Anaesthesiology 2012; Vol. 29:67.
Van der Linden 2010 {published data only}
- Linden PJ, Dierick A, Wilmin S, Bellens B, Hert SG. A randomized controlled trial comparing an intraoperative goal‐directed strategy with routine clinical practice in patients undergoing peripheral arterial surgery. European Journal of Anaesthesiology 2010;27(9):788‐793. [PUBMED: 20613538] [DOI] [PubMed] [Google Scholar]
Venn 2002 {published data only}
- Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. British Journal of Anaesthesia 2002;88(1):65‐71. [PUBMED: 11881887] [DOI] [PubMed] [Google Scholar]
Wakeling 2005 {published data only}
Wen 2016 {published data only}
- Wen XL, Jing GX, He P, Hou JR. Clinical study on the capacity management guided by stroke volume variation in elderly patients with laparoscopic radical gastrectomy for gastric cancer. Journal of Xi'an Jiaotong University (Medical Sciences) 2016;37(6):851‐6. [Google Scholar]
Wilmin 2009 {published data only}
- Wilmin S, Dierick A, Hert S, Linden P. Optimization of oxygen delivery in vascular surgery with Flotrac System, a prospective double blind randomized study. European Journal of Anaesthesiology 2009;45:38. [Google Scholar]
Wilson 1999 {published data only}
- Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, et al. Reducing the risk of major elective surgery: randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ 1999;318(7191):1099‐1103. [PUBMED: 10213716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiao 2015 {published data only}
- Xiao W, Duan QF, Fu WY, Chi XZ, Wang FY, Ma DQ, et al. Goal‐directed fluid therapy may improve hemodynamic stability of parturient with hypertensive disorders of pregnancy under combined spinal epidural anesthesia for cesarean delivery and the well‐being of newborns. Chinese Medical Journal 2015;128(14):1922‐31. [PUBMED: 26168834] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2017 {published data only}
- Xu H, Shu SH, Wang D, Chai XQ, Xie YH, Zhou WD. Goal‐directed fluid restriction using stroke volume variation and cardiac index during one‐lung ventilation: a randomized controlled trial. Journal of Thoracic Disease 2017;9(9):2992‐3004. [PUBMED: 29221272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2016 {published data only}
- Yu X, Yan J, Zhai Z, Ma X. Optimized management of fluid volume guided by PiCCO parameters in skull base tumor resection. Critical Care Medicine 2016;44(12 Suppl 1):473. [Google Scholar]
Zakhaleva 2013 {published data only}
- Zakhaleva J, Tam J, Denoya P I, Bishawi M, Bergamaschi R. The impact of intravenous fluid administration on complication rates in bowel surgery within an enhanced recovery protocol: a randomized controlled trial. Colorectal Disease 2013;15(7):892‐9. [PUBMED: 23905554] [DOI] [PubMed] [Google Scholar]
Zeng 2014 {published data only}
- Zeng K, Li Y, Liang M, Gao Y, Cai H, Lin C. The influence of goal‐directed fluid therapy on the prognosis of elderly patients with hypertension and gastric cancer surgery. Drug Design, Development and Therapy 2014; Vol. 8:2113‐9. [PUBMED: 25378913] [DOI] [PMC free article] [PubMed] [Retracted]
Zhao 2018 {published data only}
- Zhao G, Peng P, Zhou Y, Li J, Jiang H, Shao J. The accuracy and effectiveness of goal directed fluid therapy in plateau‐elderly gastrointestinal cancer patients: a prospective randomized controlled trial. International Journal of Clinical and Experimental Medicine 2018;11(8):8516‐22. [Google Scholar]
Zheng 2013 {published data only}
- Zheng H, Guo H, Ye JR, Chen L, Ma HP. Goal‐directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. World Journal of Surgery 2013;37(12):2820‐9. [PUBMED: 24048581] [DOI] [PubMed] [Google Scholar]
Zheng 2016 {published data only}
References to ongoing studies
ChiCTR1800014777 {published data only}
- Comparison of three different liquid therapies in colorectal surgery. Ongoing study February 2018.
NCT02625701 {unpublished data only}
- NCT02625701. Perioperative fluid management: goal‐directed versus restrictive strategy [Perioperative fluid management: goal‐directed therapy vs. restrictive approach, a randomized controlled trial]. https://clinicaltrials.gov/ct2/show/NCT02625701 (first received 22 March 2012).
NCT03039946 {unpublished data only}
- NCT03039946. Automated closed‐loop versus restrictive fluid therapy in abdominal surgery [Automated closed‐loop versus restrictive fluid therapy in abdominal surgery: a pilot randomized controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT03039946 (first received 31 January 2017).
NCT03519165 {unpublished data only}
- NCT03519165. Restrictive or individualized goal‐directed fluid replacement strategy in ovarian cancer cytoreductive surgery [Restrictive or individualized goal‐directed fluid replacement strategy in ovarian cancer cytoreductive surgery‐ a prospective randomized controlled trial]. https://clinicaltrials.gov/ct2/show/record/NCT03519165 (first received 22 March 2016).
Additional references
Awad 2013
- Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta‐analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clinical Nutrition (Edinburgh, Scotland) 2013;32(1):34‐44. [PUBMED: 23200124] [DOI] [PubMed] [Google Scholar]
Barbieri 2009
- Barbieri A, Vanhaecht K, Herck P, Sermeus W, Faggiano F, Marchisio S, et al. Effects of clinical pathways in the joint replacement: a meta‐analysis. BMC Medicine 2009;7:32. [PUBMED: 19570193] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandstrup 2003
- Brandstrup B, Tonnesen H, Beier‐Holgersen R, Hjortso E, Ording H, Lindorff‐Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor‐blinded multicenter trial. Annals of Surgery 2003;238(5):641‐8. [PUBMED: 14578723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandstrup 2006
- Brandstrup B, Svensen C, Engquist A. Hemorrhage and operation cause a contraction of the extracellular space needing replacement ‐ evidence and implications? A systematic review. Surgery 2006;139(3):419‐32. [PUBMED: 16546507] [DOI] [PubMed] [Google Scholar]
Bruegger 2005
- Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. American Journal of Physiology. Heart and Circulatory Physiology 2005;289(5):H1993‐9. [PUBMED: 15964925] [DOI] [PubMed] [Google Scholar]
Cao 2012
- Cao F, Li J, Li F. Mechanical bowel preparation for elective colorectal surgery: updated systematic review and meta‐analysis. International Journal of Colorectal Disease 2012;27(6):803‐10. [PUBMED: 22108902] [DOI] [PubMed] [Google Scholar]
Cecconi 2013
- Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: goal‐directed therapy ‐ what is the evidence in surgical patients? The effect on different risk groups. Critical Care (London, England) 2013;17(2):209. [PUBMED: 23672779] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chappell 2008
- Chappell D, Jacob M, Hofmann‐Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology 2008;109(4):723‐40. [PUBMED: 18813052] [DOI] [PubMed] [Google Scholar]
Corcoran 2012
- Corcoran T, Rhodes JE, Clarke S, Myles PS, Ho KM. Perioperative fluid management strategies in major surgery: a stratified meta‐analysis. Anesthesia and Analgesia 2012;114(3):640‐51. [PUBMED: 22253274] [DOI] [PubMed] [Google Scholar]
Cruz 2010
- Cruz DN, Bagshaw SM, Ronco C, Ricci Z. Acute kidney injury: classification and staging. Contributions to Nephrology 2010;164:24‐32. [PUBMED: 20427990] [DOI] [PubMed] [Google Scholar]
Della Rocca 2014
- Della Rocca G, Vetrugno L, Tripi G, Deana C, Barbariol F, Pompei L. Liberal or restricted fluid administration: are we ready for a proposal of a restricted intraoperative approach?. BMC Anesthesiology 2014;14:62. [PUBMED: 25104915] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [PUBMED: 15273542] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donati 2004
- Donati A, Ruzzi M, Adrario E, Pelaia P, Coluzzi F, Gabbanelli V, et al. A new and feasible model for predicting operative risk. British Journal of Anaesthesia 2004;93(3):393‐9. [PUBMED: 15220171] [DOI] [PubMed] [Google Scholar]
Egholm 2018
- Egholm JW, Pedersen B, Moller AM, Adami J, Juhl CB, Tonnesen H. Perioperative alcohol cessation intervention for postoperative complications. Cochrane Database of Systematic Reviews 2018;11:CD008343. [PUBMED: 30408162] [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote [Computer program]
- EndNote X9. Available at www.endnote.com/ (accessed March 2019).
Feldheiser 2012
- Feldheiser A, Conroy P, Bonomo T, Cox B, Garces TR, Spies C. Development and feasibility study of an algorithm for intraoperative goal directed haemodynamic management in noncardiac surgery. Journal of International Medical Research 2012;40(4):1227‐41. [PUBMED: 22971475] [DOI] [PubMed] [Google Scholar]
Feldheiser 2016
- Feldheiser A, Aziz O, Baldini G, Cox BP, Fearon KC, Feldman LS, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiologica Scandinavica 2016;60(3):289‐334. [PUBMED: 26514824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gan 2002
- Gan TJ, Soppitt A, Maroof M, El‐Moalem H, Robertson KM, Moretti E, et al. Goal‐directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002;97(4):820‐6. [PUBMED: 12357146] [DOI] [PubMed] [Google Scholar]
Garcia 2009
- Garcia S, Moritz TE, Goldman S, Littooy F, Pierpont G, Larsen GC, et al. Perioperative complications after vascular surgery are predicted by the revised cardiac risk index but are not reduced in high‐risk subsets with preoperative revascularization. Circulation. Cardiovascular Quality and Outcomes 2009;2(2):73‐7. [PUBMED: 20031818] [DOI] [PubMed] [Google Scholar]
Garg 2018
- Garg R. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: fluid and temperature remain the culprit!. Indian Journal of Anaesthesia 2018; Vol. 62, issue 3:162‐5. [PUBMED: 29643548] [DOI] [PMC free article] [PubMed]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greco 2014
- Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta‐analysis of randomized controlled trials. World Journal of Surgery 2014;38(6):1531‐41. [PUBMED: 24368573] [DOI] [PubMed] [Google Scholar]
Guay 2018
- Guay J, Ochroch EA, Kopp S. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in adults without acute lung injury. Cochrane Database of Systematic Reviews 2018;7:CD011151. [PUBMED: 29985541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gustafsson 2013
- Gustafsson UO, Scott MJ, Schwenk W, Demartine N, Roulin D, Francis N, et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS) society. World Journal of Surgery 2013;37(2):259‐84. [PUBMED: 23052794] [DOI] [PubMed] [Google Scholar]
Güenaga 2011
- Güenaga KF, Matos D, Wille‐Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD001544.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 2017
- Hahn RG. Adverse effects of crystalloid and colloid fluids. Anaesthesiology Intensive Therapy 2017;49(4):303‐8. [PUBMED: 28953310] [DOI] [PubMed] [Google Scholar]
Hamilton 2011
- Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta‐analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high‐risk surgical patients. Anesthesia and Analgesia 2011;112(6):1392‐402. [PUBMED: 20966436] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.handbook.cochrane.org.
Jacob 2008
- Jacob M, Chappell D, Conzen P, Finsterer U, Rehm M. Blood volume is normal after pre‐operative overnight fasting. Acta Anaesthesiologica Scandinavica 2008;52(4):522‐9. [PUBMED: 18339157] [DOI] [PubMed] [Google Scholar]
Jacob 2009
- Jacob M, Chappell D, Rehm M. The 'third space' ‐ fact or fiction?. Best Practice & Research. Clinical Anaesthesiology 2009;23(2):145‐57. [PUBMED: 19653435] [DOI] [PubMed] [Google Scholar]
Jewer 2019
- Jewer JK, Wong MJ, Bird SJ, Habib AS, Parker R, George RB. Supplemental peri‐operative intravenous crystalloids for postoperative nausea and vomiting: an abridged Cochrane systematic review. Anaesthesia 2019:Epub ahead of print. [PUBMED: 31536172] [DOI] [PubMed] [Google Scholar]
Jhanji 2008
- Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high‐risk surgical patients in a large NHS trust. Anaesthesia 2008;63(7):695‐700. [PUBMED: 18489613] [DOI] [PubMed] [Google Scholar]
Joosten 2015
- Joosten A, Alexander B, Delaporte A, Lilot M, Rinehart J, Cannesson M. Perioperative goal directed therapy using automated closed‐loop fluid management: the future?. Anaesthesiology Intensive Therapy 2015;47(5):517‐23. [PUBMED: 26578397] [DOI] [PubMed] [Google Scholar]
Khuri 2005
- Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long‐term survival after major surgery and the adverse effect of postoperative complications. Annals of Surgery 2005;242(3):326‐41; discussion 341‐3. [PUBMED: 16135919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamke 1977a
- Lamke LO, Nilsson GE, Reithner HL. Water loss by evaporation from the abdominal cavity during surgery. Acta Chirurgica Scandinavica 1977;143(5):279‐84. [PUBMED: 596094] [PubMed] [Google Scholar]
Lamke 1977b
- Lamke LO, Nilsson GE, Reithner HL. Insensible perspiration from the skin under standardized environmental conditions. Scandinavian Journal of Clinical and Laboratory Investigation 1977;37(4):325‐31. [PUBMED: 616059] [DOI] [PubMed] [Google Scholar]
Lange 2009
- Lange CP, Ploeg AJ, Lardenoye JW, Breslau PJ. Patient‐ and procedure‐specific risk factors for postoperative complications in peripheral vascular surgery. Quality & Safety in Health Care 2009;18(2):131‐6. [PUBMED: 19342528] [DOI] [PubMed] [Google Scholar]
Lewis 2018
- Lewis SR, Pritchard MW, Schofield‐Robinson OJ, Alderson P, Smith AF. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery. Cochrane Database of Systematic Reviews 2018;7:CD012584. [PUBMED: 30019463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowell 1990
- Lowell JA, Schifferdecker C, Driscoll DF, Benotti PN, Bistrian BR. Postoperative fluid overload: not a benign problem. Critical Care Medicine 1990;18(7):728‐33. [PUBMED: 2364713] [DOI] [PubMed] [Google Scholar]
Miller 2015
- Miller TE, Roche AM, Mythen M. Fluid management and goal‐directed therapy as an adjunct to Enhanced Recovery After Surgery (ERAS). Canadian Journal of Anaesthesia 2015;62(2):158‐68. [PUBMED: 25391735] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009 2009;6(7):e1000097. [PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molina 2015
- Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the national surgical quality improvement program in orthopaedic surgery. Clinical Orthopaedics and Related Research 2015;473(5):1574‐81. [PUBMED: 24706043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mythen 2012
- Mythen MG, Swart M, Acheson N, Crawford R, Jones K, Kuper M, et al. Perioperative fluid management: consensus statement from the enhanced recovery partnership. Perioperative Medicine (London, England) 2012;1:2. [PUBMED: 24764518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nisanevich 2005
- Nisanevich V, Felsenstein I, Almogy G, Weissman C, Einav S, Matot I. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005;103(1):25‐32. [PUBMED: 15983453] [DOI] [PubMed] [Google Scholar]
Nygren 2012
- Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clinical Nutrition (Edinburgh, Scotland) 2012;31(6):801‐16. [PUBMED: 23062720] [DOI] [PubMed] [Google Scholar]
Odor 2018
- Odor PM, Bampoe S, Dushianthan A, Bennett‐Guerrero E, Cro S, Gan TJ, et al. Perioperative administration of buffered versus non‐buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures: a Cochrane systematic review. Perioperative Medicine (London, England) 2018;7:27. [PUBMED: 30559961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pearse 2014
- Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output–guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311(21):2181‐90. [PUBMED: 24842135] [DOI] [PubMed] [Google Scholar]
Rahbari 2009
- Rahbari NN, Zimmermann JB, Schmidt T, Koch M, Weigand MA, Weitz J. Meta‐analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. British Journal of Surgery 2009;96(4):331‐41. [PUBMED: 19283742] [DOI] [PubMed] [Google Scholar]
Reithner 1980
- Reithner L, Johansson H, Strouth L. Insensible perspiration during anaesthesia and surgery. Acta Anaesthesiologica Scandinavica 1980;24(5):362‐6. [PUBMED: 7468126] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ripolles 2016
- Ripolles J, Espinosa A, Martinez‐Hurtado E, Abad‐Gurumeta A, Casans‐Frances R, Fernandez‐Perez C, et al. Intraoperative goal directed hemodynamic therapy in noncardiac surgery: a systematic review and meta‐analysis. Brazilian Journal of Anesthesiology 2016;66(5):513‐28. [PUBMED: 27591466] [DOI] [PubMed] [Google Scholar]
Ripolles‐Melchor 2016a
- Ripolles‐Melchor J, Casans‐Frances R, Espinosa A, Abad‐Gurumeta A, Feldheiser A, Lopez‐Timoneda F, et al. Goal directed hemodynamic therapy based in esophageal Doppler flow parameters: a systematic review, meta‐analysis and trial sequential analysis [Terapia hemodinamica guiada por objetivos basada en parametros de flujo con Doppler esofagico: revision sistematica, metaanalisis y analisis secuencial de ensayos.]. Revista Espanola de Anestesiologia y Reanimacion 2016;63(7):384‐405. [PUBMED: 26873025] [DOI] [PubMed] [Google Scholar]
Ripolles‐Melchor 2016b
- Ripolles‐Melchor J, Espinosa A, Martinez‐Hurtado E, Abad‐Gurumeta A, Casans‐Frances R, Fernandez‐Perez C, et al. Perioperative goal‐directed hemodynamic therapy in noncardiac surgery: a systematic review and meta‐analysis. Journal of Clinical Anesthesia 2016;28:105‐15. [PUBMED: 26440438] [DOI] [PubMed] [Google Scholar]
Rollins 2016
- Rollins KE, Lobo DN. Intraoperative goal‐directed fluid therapy in elective major abdominal surgery: a meta‐analysis of randomized controlled trials. Annals of Surgery 2016;263(3):465‐76. [PUBMED: 26445470] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salicath 2018
- Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient‐controlled intravenous analgesia for pain following intra‐abdominal surgery in adults. Cochrane Database of Systematic Reviews 2018;8:CD010434. [PUBMED: 30161292] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shabsigh 2009
- Shabsigh A, Korets R, Vora KC, Brooks CM, Cronin AM, Savage C, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. European Urology 2009;55(1):164‐74. [PUBMED: 18675501] [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith I, Kranke P, Murat I, Smith A, O'Sullivan G, Soreide E, et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. European Journal of Anaesthesiology 2011;28(8):556‐69. [PUBMED: 21712716] [DOI] [PubMed] [Google Scholar]
Som 2017
- Som A, Maitra S, Bhattacharjee S, Baidya DK. Goal directed fluid therapy decreases postoperative morbidity but not mortality in major non‐cardiac surgery: a meta‐analysis and trial sequential analysis of randomized controlled trials. Journal of Anesthesia 2017;31(1):66‐81. [PUBMED: 27738801] [DOI] [PubMed] [Google Scholar]
Soni 2009
- Soni N. British consensus guidelines on intravenous fluid therapy for adult surgical patients – GIFTASUP: Cassandra's view. Anaesthesia 2009;64(3):235‐8. [PUBMED: 19302633] [DOI] [PubMed] [Google Scholar]
Sun 2017
- Sun Y, Chai F, Pan C, Romeiser JL, Gan TJ. Effect of perioperative goal‐directed hemodynamic therapy on postoperative recovery following major abdominal surgery ‐ a systematic review and meta‐analysis of randomized controlled trials. Critical Care (London, England) 2017;21(1):141. [PUBMED: 28602158] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svatek 2010
- Svatek RS, Fisher MB, Matin SF, Kamat AM, Grossman HB, Nogueras‐Gonzalez GM, et al. Risk factor analysis in a contemporary cystectomy cohort using standardized reporting methodology and adverse event criteria. Journal of Urology 2010;183(3):929‐34. [PUBMED: 20083264] [DOI] [PubMed] [Google Scholar]
Vallet 2013
- Vallet B, Blanloeil Y, Cholley B, Orliaguet G, Pierre S, Tavernier B. Guidelines for perioperative haemodynamic optimization. Annales Françaises d'Anesthèsie et de Rèanimation 2013;32(10):e151‐8. [PUBMED: 24126197] [DOI] [PubMed] [Google Scholar]
Varadhan 2010
- Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta‐analysis of randomized controlled trials. Clinical Nutrition (Edinburgh, Scotland) 2010;29(4):434‐40. [PUBMED: 20116145] [DOI] [PubMed] [Google Scholar]
Voldby 2016
- Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting ‐ a clinical review. Journal of Intensive Care 2016;4:27. [PUBMED: 27087980] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Lui J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PUBMED: 25524443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodcock 2012
- Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. British Journal of Anaesthesia 2012;108(3):384‐94. [PUBMED: 22290457] [DOI] [PubMed] [Google Scholar]
Xu 2018
- Xu C, Peng J, Liu S, Huang Y, Guo X, Xiao H, et al. Goal‐directed fluid therapy versus conventional fluid therapy in colorectal surgery: a meta analysis of randomized controlled trials. International Journal of Surgery (London, England) 2018;56:264‐73. [PUBMED: 29972762] [DOI] [PubMed] [Google Scholar]
Zhuang 2013
- Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta‐analysis of randomized controlled trials. Diseases of the Colon and Rectum 2013;56(5):667‐78. [PUBMED: 23575408] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wrzosek 2017
- Wrzosek A, Jakowicka‐Wordliczek J, Zajaczkowska R, Serednicki WT, Jankowski M, Bala MM, et al. Perioperative restrictive versus goal‐directed fluid therapy for adults undergoing major non‐cardiac surgery. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD012767] [DOI] [PMC free article] [PubMed] [Google Scholar]


