Abstract
The niche is the environment in which stem cells reside and is responsible for the maintenance of unique stem cell properties such as self-renewal and an undifferentiated state. The heterogeneous populations which constitute a niche include both stem cells and surrounding differentiated cells. This network of heterogeneity is responsible for the control of the necessary pathways that function in determining stem cell fate. The concept that cancer stem cells, a subpopulation of cells responsible for tumor initiation and formation, reside in their own unique niche is quickly evolving and it is of importance to understand and identify the processes occurring within this environment. The necessary intrinsic pathways that are utilized by this cancer stem cell population to maintain both self-renewal and the ability to differentiate are believed to be a result of the environment where cancer stem cells reside. The ability of a specific cancer stem cell niche to provide the environment in which this population can flourish is a critical aspect of cancer biology that mandates intense investigation. This review focuses on current evidence demonstrating that homeostatic processes such as inflammation, epithelial to mesenchymal transition, hypoxia and angiogenesis contribute to the maintenance and control of cancer stem cell fate by providing the appropriate signals within the microenvironment. It is necessary to understand the key processes occurring within this highly specialized cancer stem cell niche to identify potential therapeutic targets that can serve as the basis for development of more effective anticancer treatments.
Keywords: cancer stem cell, niche, microenvironment, inflammation, hypoxia, angiogenesis, vascular niche, EMT, tumor dormancy
Stem cell populations are enriched in specific anatomical locations and require both distinctive and specific microenvironments. The niche is the microenvironment in which stem cells reside and is responsible for the maintenance of unique stem cell properties such as self-renewal and an undifferentiated state. Niches are composed of heterogeneous populations including stem cells and surrounding differentiated cells that control critical intrinsic factors necessary in determining stem cell fate. These critical factors include stromal support cells, soluble factors, extracellular matrix proteins and blood vessels.1 The concept involving the existence and role of cancer stem cells was first described in 1994 by Lapidot et al. in a model of acute myeloid leukemia (AML).2 To date, cancer stem cell populations have been identified in numerous solid tumors including those of the breast,3 brain,4 prostate,5-7 colon,8 pancreas9 and liver.10 Cancer stem cells (CSCs) are characterized by their ability to self-renew, differentiate and initiate tumors.10-12 The concept of a CSC niche is derived from the similarities that exist between normal stem cells and CSCs. The signaling pathways utilized by both normal stem cells and CSCs overlap and are based on embryonic signaling pathways which allow self-renewal. To date, our understanding regarding stem cell regulation has expanded due to intense investigation of the processes occurring within this unique microenvironment.
To begin to understand the processes that may occur within the CSC niche, it is necessary to understand the components which define a normal stem cell niche. Stem cells are the foundation for developmental hierarchy and are controlled within their highly specialized microenvironments that function to maintain and promote stem cells. The intrinsic factors that are necessary in determining cell fate are crucial in maintaining and defining stem cell fate. Embryonic stem cells (ESC) are conventionally derived from the inner cell mass (ICMs) of blastocysts during embryonic development. However, recent reports demonstrate that ESCs can be derived from single blastomeres and these ESCs are capable of differentiating into the three germ layers.13-15 ESCs are pluripotent cells that have the ability to differentiate into all of the cells and tissues of the body (reviewed in Ref. 16). The molecular mechanisms used to maintain both the ability of an ESC to self-renew and remain pluripotent are not completely defined, but it is clear that the microenvironment is responsible for providing the necessary extrinsic cues. It is well established that the transcription factors Oct4, Nanog and Sox2 are essential regulators of ESC pluripotency. These transcription factors are responsible for the transcription of genes necessary to maintain stemness and also responsible for ensuring genes involved in cellular differentiation are transcriptionally silenced.17 The proper maintenance and regulation of the ESC microenvironment is crucial to normal development. Interestingly, there have been studies performed that demonstrate the proteins contained within the blastocyst have a cancer inhibitory effect if exposed to tumor cells derived from various carcinomas.18 It has also been proven that injection of carcinomas into mouse blastocysts can result in a healthy chimera of normal mouse and human cells in all tissues (reviewed in Ref. 17). This inhibitory effect by the blastocysts niche occurs by activation of programmed cell death pathways and by epigenetic reprogramming of tumor cells19 thereby, demonstrating the significance of the ESC microenvironment in controlling stem cell fate. However, although key genes have been identified to maintain pluripotency and it has been shown that the mouse ESC niche has the capability of reprogramming tumor cells, the exact biological mechanisms occurring within the ESC niche still remain largely unknown.
Niches for mammalian adult stem cells have been identified in the intestinal, neural, epidermal and hematopoietic systems.20 Adult stem cells (ASC) are defined by their ability to replenish dying cells and regenerate damaged tissue. ASCs, unlike ESCs, are multipotent as they are lineage restricted to the tissue in which they reside. The extracellular matrix (ECM) is responsible for retaining stem cells within a specialized environment and in combination with support cells initiates signal transduction events and presents immobilized growth factors including cytokines such as stem cell factor (SCF) and leukemia inhibitory factor (LIF).21 Cell to cell interactions between the stem cells and support cells, interactions between stem cells and ECM, and both the composition of ECM and the physicochemical environment are all key contributing factors in proper stem cell maintenance. The identification of the mechanisms and signaling processes occurring within the niche have been complicated by the fact that stem cells represent such a rare population and are difficult to identify in vivo. However, despite this difficulty, the hematopoietic system is the most understood in terms of niche maintenance and serves as a useful model in identifying and understanding stem cell niches in other mammalian tissues. Various signaling pathways have been identified within several mammalian adult niches including Activin/Nodal, Akt/PTEN, BMP, JAK/STAT, PI3K, TGF-β, Wnt and cell cycle pathways.22 These pathways have been shown to function in the maintenance and control of ESCs, ASCs and CSCs demonstrating the similarities which exist between SC niches.
In regards to the development of treatment and the role stem cells may play is best described in terms of pediatric cancers. It has been proven and shown that for the treatment of pediatric hematologic malignancies; allogeneic stem cell transplantation (SCT) is the best form and most curative of the cellular immunotherapies available for childhood cancers.23 Hence, the relationship between stem cells and pediatric cancers is well understood and established. The connection between CSCs or TICs and childhood cancers we believe can serve as a model for the function of CSCs in adult cancers. Neuroblastoma, a pediatric tumor believed to derive from the embryonic neural crest, has been shown to contain a CSC population isolated from both tumors and bone marrow metastases.24 The ability to isolate and identify this unique population from high-risk neuroblastoma patients demonstrated a role for these cells in tumor development. In addition, Hansford et al. demonstrated that CSCs could be isolated from the bone marrow of patients in clinical remission suggesting CSCs could be useful in the prediction of patient prognosis and serve as a bio-marker.24 The ability to identify these cells in patients considered under clinical remission provides further evidence for their ability to withstand traditional treatments and provide new avenues for therapeutic development.
Medulloblastoma is the most frequent type of solid tumor and the leading cause of cancer related deaths in early childhood. The identification of CSCs within this cancer type has given new targets for therapeutic development. The tie between medulloblastoma development and CSCs had led to intense investigations determining the signaling pathways which mediate the switch from normal neurogenesis to tumorigenesis. Specifically, the TGFβ pathway has been shown to regulate neural stem cell proliferation and tumor development providing a target for drug development in pediatric brain cancer.25 Additionally, using medulloblastoma as a model for investigating CSCs has served as extremely useful in identifying the Notch pathway, known to function in differentiation and metastasis, as a contributor to osteosarcoma metastasis. Notch signaling has also been shown to function as a promoter of medulloblastoma stem cell survival thereby, contributing to angiogenesis in neuroblastoma.26 Lastly, Wilms tumor, a type of pediatric kidney cancer, has been used to compare the whole genome between undifferentiated “blastemal” cells representing embryonic renal tissue derived from tumors, normal kidney, fetal kidney and human ES cells. Analysis and comparison of these whole genomes highlighted the importance of an interconnected network which drives Wilms tumor formation and overlaps with critical genes that function as regulators of kidney development. Specifically, the identification of Polycomb repression as a critical player in both ESCs and Wilms tumors was shown. The use of whole genome analysis in this context further demonstrates the similarities which are present between ESC and tumor tissue and expansion of these types of studies to additional cancers can function to identify additional mechanism(s) contributing to tumor development and CSC maintenance.27 These data suggest that the presence of CSCs within cancers, specifically pediatric cancers, provide the tumor with an advantage to resist traditional therapies and their ability to do so may lie within the similarities they have with ESCs. The regulatory mechanism(s) which are used by both ESCs and CSCs are not fully known and we believe to understand this, the environment in which these cells flourish must be investigated.
To date, our understanding of the microenvironment is limited and under intense investigation. To further understand and elucidate the biology occurring within the CSC niche, it is necessary to understand the key processes occurring within the microenvironment which define stem cell fate to potentially identify targets which function to sustain this population. Additionally, it is of great interest to define the processes which allows the niche to provide survival signals to CSCs in response to both chemo- and radiotherapies (reviewed in Ref. 28) as they can serve as potential therapeutic targets. It is hypothesized that CSCs may arise from aberrant signaling from the microenvironment and flourish due to a deregulated environment conducive to the preservation of malignancy. The ability of the CSC niche to sustain this rare and lethal population by manipulating homeostatic processes such as inflammation, epithelial to mesenchymal transition (EMT), hypoxia and angiogenesis is the focus of this review (Fig. 1). Herein, we will focus on these various biological mechanisms that may be occurring within the CSC niche that contribute and participate to CSC maintenance.
Figure 1.
Cancer stem cells are maintained by biological processes occurring within the microenvironment. There is a heterogeneity that is observed in solid tumors and within this hierarchical organization of cells, there is a subpopulation of cells termed cancer stem cells (CSCs), represented in blue. It is hypothesized and there is evidence to suggest that these CSCs are influenced by a unique microenvironment termed the CSC niche. Within the CSC niche and microenvironment, there are homeostatic processes that function in determining CSC fate such as inflammation, epithelial to mesenchymal transition (EMT), hypoxia and angiogenesis.
Epithelial to Mesenchymal Transition and Inflammation as Regulators of the CSC Niche
Epithelial to mesenchymal transition or EMT is a process that was first observed by Betty Hay to occur during normal organismal development.29,30 Early in embryogenesis, certain lineages of epithelial cells give rise to cells with a more mesenchymal phenotype.31 This transformation results in a loss of cell polarity, loss of cell-to-cell adhesion contacts and more importantly, an enhanced ability to migrate and invade the local tissue.29-31 In recent years, the process of EMT has been significantly investigated as a contributing factor to the progression of cancer.32 Even more recent is the hypothesis that CSCs demonstrate very similar properties to these transitioned mesenchymal cells33,34 and furthermore, these CSCs also are much more invasive than their nonstem cell counterpart.35,36
The process of EMT is controlled by the presence of specific signaling molecules within the microenvironment or niche, and is ultimately what triggers this transition to occur. Similarly, it is thought that the expansion of the CSC compartment is also controlled by the molecules present in its niche, and perhaps that overlap exists between the two microenvironments.37-43 Further complicating this idea is the presence of normal adult stem cells (ASCs), which can be also be activated for their expansion using similar signals.44
The connection between EMT and cancer progression with regards to microenvironment is strongly tied to the inflammatory process and wound healing.45 Recent data suggests that one in five cancer deaths are due to malignancies triggered by chronic inflammation and it has been linked to the proliferation and metastasis of tumors 46 The most common inflammatory molecules present within the microenvironment that can regulate both processes include IL-6, IL-8, TGF-β1, NFκβ, TNFα and HIF-1α31,32,34 (Fig. 2). The binding of these signaling molecules to their respective receptors results in massive changes in downstream signaling molecules, which also overlap between the processes of EMT, inflammation and the expansion of the CSC population.44,47 The downstream pathways known to regulate CSCs include: Wnt, SHH, Notch, TGF-β and RTKs-EGF, FGF, IGF and HGF.48-51 These pathways are controlled by cues from the microenvironment and have been reviewed intensely over the last few years.
Figure 2.
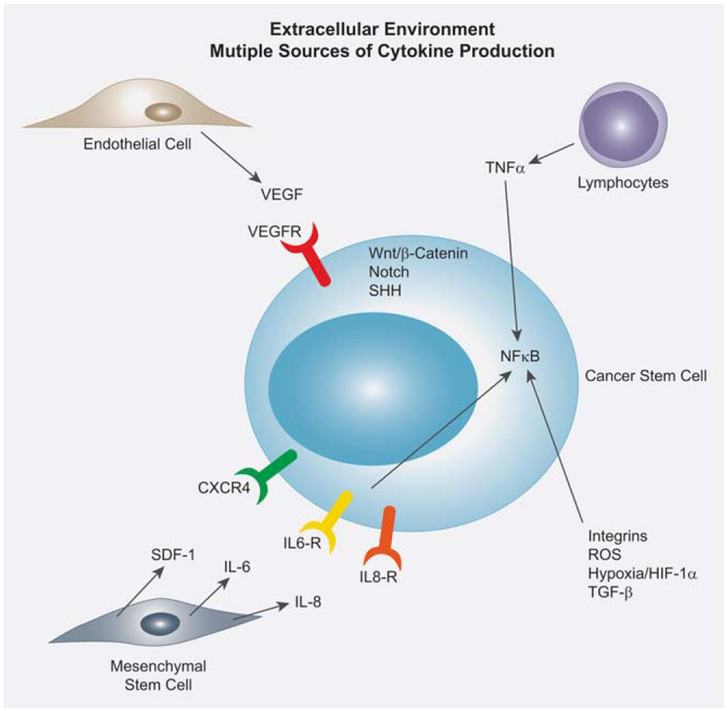
The cancer stem cell extracellular environment contains multiple sources of cytokine production. Endothelial cells produce vast amounts of VEGF which bind to the VEGFR on the CSC. Activated lymphocytes produce TNFα and utilize the NFκB pathway to confer downstream signaling in the CSC. Additionally, integrins, ROS, hypoxia/HIF-1α and TGF-β also utilize the NFκB pathway to confer downstream signaling. Bone marrow derived mesenchymal stem cells produce a variety of cytokines which regulate CSCs, including SDF-1, IL-6 and IL-8. The stem cell pathways Wnt, Notch and SHH is all active in the CSC, yet the effect from the extracellular environment is complex and not well defined.
In support of this connection between EMT and inflammation is the observation that a strong association exists between hypoxia and EMT. The key downstream regulators of EMT, Snail and Twist are in fact activated by hypoxia-inducible factor-1 α (HIF-1α).52 Hypoxia has been shown to activate EMT in tumors by increasing expression of CSC genes which regulate their properties of self-renewal including Wnt and Notch (as reviewed in Ref. 34). Another inflammatory mediator, TNFα has also been recently shown to affect the process of EMT 53 In line with this evidence, TGF-β-induced EMT is accelerated dramatically by the presence of activated macrophages and TNFα is the critical factor produced by macrophages that accelerates the EMT.54
The Role of NFκβ in Maintaining CSCs
Many of the converging inflammatory cytokines first introduced to regulate the CSC microenvironment have been shown to activate the NFκβ signaling pathway and recent evidence demonstrates that NFκβ regulates the EMT inducers Slug, Snail and Twist (as reviewed in Ref. 34). This interesting connection led us to examine the role NFκβ may be playing in maintaining the CSC population by conferring signals from the extracellular environment. For example, in pancreatic cancer cells, NFκβ promotes not only EMT, but also migration and invasion of these cells.55 In mouse skin cells, inhibition of reactive oxygen species or ROS-induced NFκβ can abrogate TGF-β-induced EMT.56 Breast CSCs demonstrate that TNFα up-regulates SLUG with a dependency on canonical NFκB /HIF-1α signaling, which is strongly enhanced by p53 inactivation.57 Interestingly, in ovarian cancer stem cells (OCSCs), a major characteristic of CD44+ cells is the presence of constitutively active NFκB,58 which is enhanced by the ligation of TLR4 and TNFα. By inhibiting NFκB, the group could promote apoptosis of OCSCs and inhibit both the constitutive and TNFα-induced NFκβ activity. Additionally, when prostate CSCs were treated with parthenolide, a drug known to inhibit NFκB, the cells were not able to survive or form xenografts in mice.59 Likewise, in myeloid-derived precursor cells (MDSCs), the precursors to gastric cancer, activation of NFκβ leads to an increase in the production of IL-6, TNFα and stromal-derived growth factor-1 (SDF-1).60 A final piece of evidence linking NFκβ and CSCs is that NFκB is hypothesized to regulate secretion of growth factors from the bone marrow environment61 and each of these cell types mentioned above responds to growth factor signaling. Thus, the regulation of CSCs and other stem cell systems by activation of NFκB from the microenvironment is of significant interest, especially when trying to understand the molecular regulation governing these cell types. Thus, NFκB signaling maintains not only the process of inflammation and EMT, but also the signals conveyed by the CSCs niche.
Within the stem cell niche, there also exist normal mesenchymal stem cells (MSCs) derived from either stromal tissue or recruited from the bone marrow.62 It is thought that the MSCs reside in a perivascular niche where they associate with blood vessels.63 The relationship of normal stem cells and CSCs are their close interrelationship with the vasculature.64 It is thought that the CSCs are regulated by their perivascular micromilieu now referred to as the vascular CSC niche of which VEGF seems to be the most important molecule.
The Role of IL-6 in Maintaining CSCs
The inflammation-based pathway that activates the STAT transcription factors mentioned previously is interleukin-6 (IL-6) signaling. IL-6 is a pleiotropic molecule most notably regulating proliferation, differentiation and functional maturation of multiple hematopoietic cell lineages.65,66 IL-6 functions by binding to its receptor IL-6R present on target cells and further associating with an affinity converter gp130 (β-chain, CD130).67 In addition to contributing to cancer regulation, an increased production of IL-6 has also been implicated in Alzheimer’s disease, autoimmune diseases including rheumatoid arthritis and chronic inflammation.68 Additionally, the IL-6 cytokine family contains interleukin-11 (IL-11), oncostatin-M (OSM) and leukemia inhibitory factor (LIF). This makes IL-6 an attractive target for therapy since neutralizing antibodies or competitive inhibitors are being synthesized to directly target their activation.
With regard to stem cells, LIF has been found to support the undifferentiated state of mouse ES cells by activation STAT3;69 however, LIF alone cannot maintain this state in human ES cells.70 It has been shown that FGF and TGF-β are key regulators in self-renewal and maintenance of these cells.71-74 One explanation to this obvious disconnect between mice and man is that mouse cells might have temporary cell surface expression of gp130 and the LIF receptor (LIF-R) and although human ES cells do express gp130, they may not express LIF-R at its cell surface. Thus, stimulation with LIF would not be able to block their differentiation.75 In the hematopoietic system, however, functional activation of gp-130-mediated STAT1/3 signaling is required for the normal balance of hematopoietic progenitors during fetal and adult development.76 Early hematopoietic stem cells express low levels of gp130 receptor; however they do not express IL-6R,77 further demonstrating the role IL-6/IL-6R plays in maintaining early progenitor cells. In later stages of hematopoietic development, stimulation with IL-6 induces differentiation of murine ES cells by down-regulating the Wnt/β-catenin pathway.
With regards to CSCs, in breast CSCs, IL-6 induces malignant features in Notch-3 expressing cells isolated from human ductal breast carcinoma and normal mammary gland.78 Furthermore, in glioblastoma stem cells (GBM-SC), targeting the expression of IL6R or IL6 ligand expression with short hairpin RNAs (shRNAs) significantly reduces growth and neurosphere formation capacity and induces apoptosis.79 The role of STAT3 in CSC regulation has only recently been investigated, yet higher levels of STAT3 have been demonstrated in CSCs isolated from liver, bone, cervical and brain cancers.79-84 Treatment of putative GBM-SC with an inhibitor of STAT3 called Stattic results in a dramatic reduction in their formation.84 In addition, in prostate cancer, the production of IL-6 can confer a survival advantage to local stem cells, which is a proposed method to facilitate tumorigenesis.85 Stattic has been shown to inhibit invasive prostate cancer cells which demonstrate a stem-like phenotype.86 Clearly, the above evidence demonstrates a significant role for IL-6 signaling in regulating not only normal but also CSC populations in various cancer models. The many sources of IL-6 in the microenvironment contribute to the regulation of this unique population of cells.
Additional Chemokines Regulating CSCs
CXCR4
Additional evidence also suggests the chemokine SDF-1α (SDF-1α/CXCL12) and its receptor CXCR4 are key regulators in facilitating breast cancer metastasis to distant organs such as bone marrow, liver and lung tissues (reviewed in Ref. 87). Presence of CXCR4 on breast cancer cells promotes cell proliferation, migration and invasion, but recently has also has been shown to mark the CSC population of cells within a heterogeneous tumor.88-91 The main source of SDF-1α is secreted from MSCs present in such tissues as the liver, lungs, lymphatic tissues and the marrow,92 and it is widely accepted that its predominant role is for homing and maintenance of HSC in the marrow niches. It is not surprising then that CXCR4 expression is also found on CSCs and could function as a major regulator of CSC homing and dissemination within the niche environment.
ROS
Furthermore, a connection between ROS itself and EMT has also recently been made.93 It is thought that ROS contribute to EMT due to increased levels of TGF-β, TNF-α, HIF-1 α, MMP-3 and TPA, as well as HGF, EGF and certain micro-RNAs.93 As reviewed by Pani et al., ROS can also contribute to the CSC.94 Pani et al. point out that normal stem cells prefer a glycolytic microenvironment to maintain homeostasis within their niche, whereas CSCs seem to have a preference for a hypoxic microenvironment. Although The Warburg effect has since been demonstrated in different types of tumors and a vast number of these cells do display an increase in glucose uptake, it is well known that not all cells in the tumor react in this manner.95 It is possible that the CSC populations are these nonreactive cells. Interestingly, it has also recently been shown that the tumor stroma of human breast cancers shows a transcriptional shift towards oxidative stress, DNA damage/repair, inflammation and hypoxia, consistent with the “Reverse Warburg Effect.”96 In addition CSCs isolated from mouse and human mammary glands demonstrate a lower content of ROS compared to their more differentiated progeny.97 For example, increased levels of the antioxidant genes GSH and FOXO-1 in breast CSCs actually leads to resistance to radiomimetic and pro-oxidant chemotherapies. Thus, to eradicate the CSC compartment completely, new cancer treatments will have to be developed with the lower ROS levels in mind. Perhaps targeting of ROS could lead to the reversal of EMT features and selectively kill the CSCs.
The Role of HIFs and Hypoxia In the Maintenance of a CSC Niche
The ability of the stem cell niche, embryonic, adult or cancer, to maintain cells in an undifferentiated state are critical and although not fully characterized, it is hypothesized that a hypoxic state is necessary. In human development, organogenesis occurs under physiological hypoxic conditions that are maintained by multiple processes and pathways including hypoxia-inducible transcription factors (HIFs), mammalian target of rapamycin (mTOR) and the endoplasmic reticulum (ER) stress response.98 In culture, it has been demonstrated that hypoxia, a level of ~3% O2, is necessary for the maintenance of an undifferentiated state of ESCs and in a normoxic environment, a level of ~21% O2, there is a decrease in the percentage of single-cell embryos developing to blastocysts.99 ESC colonies maintained at low O2 levels have better morphology and lower spontaneous differentiation as well.100 It has previously been shown that the maintenance of an undifferentiated state can be reversed by culturing in normoxic conditions or by Notch inhibition.99 Notch signaling is critical in stem cell maintenance and in the regulation of angiogenesis as well. The ability to inhibit maintenance of an undifferentiated state by changing oxygen availability demonstrates the critical balance deemed necessary within the microenvironment. Additionally, within the ESC niche, it appears that the major signaling pathways involved in maintenance of an undifferentiated state in hypoxic conditions include FGF, TGF-β/BMP and Wnt pathways.100 ESC cells cultured in normoxic conditions do maintain pluripotent ability, but over time lose this phenotype and begin to differentiate demonstrating that a hypoxic environment is necessary for full pluripotency.98
Stem cell niches are often located in anatomical regions characterized by hypoxic conditions as they require low levels of oxygen to minimize damage caused by DNA oxidation. The effect of oxygen levels on stem cells is best understood using HSC as a model as the bone marrow microenvironment is hypoxic. It is believed that the advantage HSCs have in residing in a hypoxic niche is the ability to undergo slow-cycle proliferation without undergoing DNA damage. Mesenchymal stem cells (MSCs), a major component of the stromal cell system, reside within the marrow and have the ability to maintain self-renewal properties and can differentiate into various connective tissue lineages including osteogenic, chon-drogenic and adipogenic differentiation.101,102 The relationship between oxygen and MSCs is under intense investigation as MSCs reside in locations close to the vascular structures but the tissues where MSCs are found exhibit low oxygen levels.101 The exact mechanism(s) by which oxygen regulates MSCs is unknown but it is clear that oxygen is a critical regulator of MSC fate. Additionally, a link between the role of oxygen in the regulation of the neural stem cell (NSC) niche has come to light. The human brain appears to reside within a physiological oxygen gradient in which the NSCs are located in a hypoxic environment that maintains NSCs in their undifferentiated state. NSCs have the classic stem cell characteristics which include self-renewal and the ability to differentiate into astrocytes, oligodendrocytes and neurons.101 As stated, hypoxic conditions and oxygen levels are key to the maintenance of an undifferentiated state for stem cells from various origins, importantly, common regulators of hypoxic conditions within these niches are the HIFs. Stem cells exhibit an increased capability for DNA damage response and are protected from oxidative damage by expressing HIF-1α, a transcriptional regulator of critical growth factors.103
It is established that solid tumors are characterized by their poorly vascularized regions and flourish under hypoxic conditions. The ability of malignant cells to survive hypoxic conditions is under the regulation of HIFs (HIF-1α and HIF2α) that are capable of mediating transcriptional responses and activate specific signaling pathways, such as Notch and Oct4, which are known to regulate stem cell fate (Fig. 3). Specifically, it is advantageous for the tumor to survive under hypoxic conditions; therefore, it is proposed that CSCs specifically thrive within the hypoxic tumor microenvironment as necessary stem cell regulators are activated.
Figure 3.
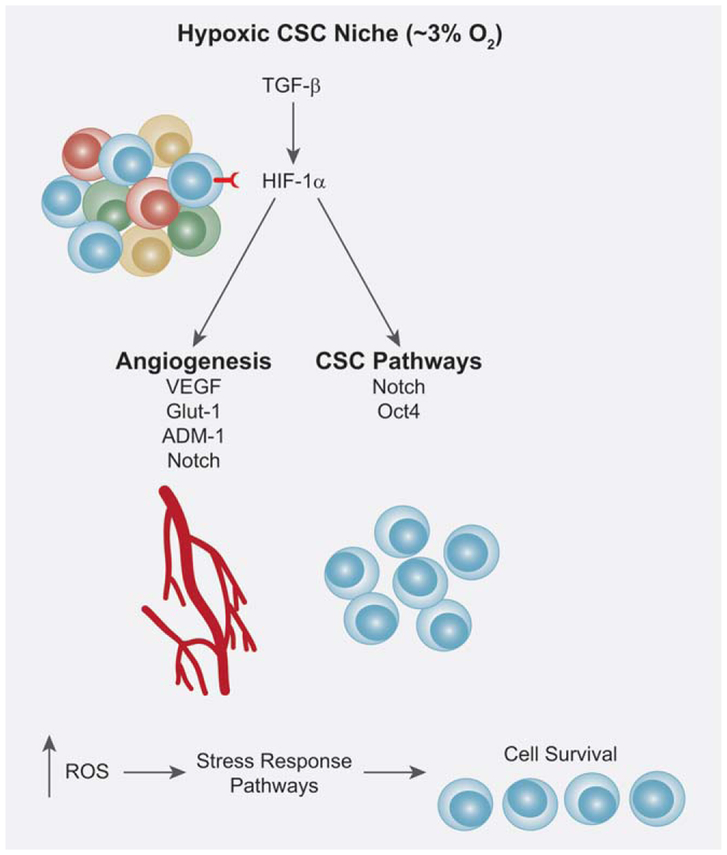
The hypoxic cancer stem cell niche. In human development, a hypoxic environment is key for organismal development and the maintenance of an undifferentiated stem cell state. It is established that hypoxia inducible factors (HIFs) are responsible for the regulation of hypoxic conditions and are regulated and stabilized by TGF-β. HIF-1α expression by CSCs results in promotion of tumor progression, survival and metastasis. HIF-1α is able to regulate genes involved in CSC pathways such as Notch and Oct4 that promote CSC survival and self-renewal. HIF-1α is also capable of regulating target genes involved in angiogenesis such as VEGF, GLUT-1, ADM-1 and Notch. Additionally, within a hypoxic environment, there is an increase in ROS that results in the activation of stress response pathways that promote CSC survival as well. The ability of CSCs to thrive in a hypoxic environment is advantageous in the promotion of tumorigenesis.
HIFs contribute to tumor progression, cell survival and metastasis. Hypoxic conditions also play a key role in therapeutic resistance. HIF targets genes such as VEGF, GLUT-1, ADAM-1, Oct4 and Notch, crucial regulators of angiogenesis and the maintenance of stem cells (Fig. 3). Hypoxia and the HIFs have been shown to increase proliferation, increase self-renewal and increase tumorigenicity, all established characteristics of CSCs.104 Hypoxia has been shown to regulate and increase expression of CSC surface markers such as CD133, CD44 and CSC related genes such as Sox2.104 Additionally, TGF-β, a regulator in the maintenance of stem cells, has also been shown to induce HIF stabilization, further demonstrating the importance of CSCs to reside and maintain a hypoxic environment for survival by interacting with the microenvironment. Recently, Das et al. have shown that side population (SP) cells, another method used to isolate CSCs, localize in hypoxic areas of solid tumors in vivo and that SP cells migrate to areas of hypoxia in nude nice,105 showing further evidence for the hypothesis that a CSC niche is characterized by a hypoxic environment.
Another advantage CSCs have by residing in hypoxic conditions is that there is irregular activation of the HIF complex resulting in disruptive changes in the ‘angiogenic factor gradients’ contributing to the formation of necrotic regions. Additionally, hypoxic conditions increases intracellular ROS resulting in the induction of an integrated stress response, which promotes cellular survival106 leading to an advantage for tumor cells.
An additional factor that may serve to be critical in CSC maintenance is the relationship that exists between hypoxia and ECM remodeling. Both embryonic and tumor cells invade peripheral tissues and migrate though various microenvironment, therefore, ECM degradation is key in tumor invasion. The upregulation of proteolytic enzyme expression or activity as a result of an hypoxic environment leads to an increase in invasion.107 In regards to CSCs and metastasis, this is a key survival mechanism and process necessary for tumor cells to continue proliferation and survive under stressful conditions. Additionally, it also provides an environment in which tumor cells can continue to undergo genetic and adaptive changes that favor a malignant phenotype and increased aggressiveness, a property of CSCs. Hypoxia also regulates molecules involved in cell migrations including cytoskeleton proteins, integrins and chemo-attractants. For example, CXCR4, as previously mentioned, is a key regulator in breast cancer metastasis and its presence on breast cancer cells promotes cell proliferation, migration and invasion. The role of CXCR4 as a chemokine receptor and its suggested role as a regulator of the homing process, as previously described, are under investigation. Recently, CXCR4 has been shown to mark the CSC population of cells specifically in pancreatic cancer.88-91,108 CXCR4 expression has also been shown to correlate with HIF-1α in oral squamous cell carcinoma (OSCC)109 and colon cancer.110 Therefore, the expression of CXCR4 may also function as a means of CSC survival within a normally toxic environment, such as a hypoxic one, thereby, providing a survival advantage to this population. The possible dual role of CXCR4 as a mediator of homing and as a survival advantage within the CSC niche must be further investigated.
The role sof HIFs and hypoxia in CSC maintenance is becoming increasingly attractive as a potential therapeutic target. The evidence suggests that HIF pathways are key regulators of CSCs and by reducing their activity, CSCs can possibly be driven into a differentiated state resulting in a reduction of tumor repopulation. The regulation of genes critical to CSC maintenance by hypoxia and HIFs further confirm that the microenvironment or CSC niche must be taken into account in the development of potential therapeutics.
Angiogenesis in the Maintenance of a CSC Niche
Angiogenesis is defined as the formation of new vessels from a pre-existing vascular network which is typical during development, growth and wound healing in normal healthy individuals. Hypoxia is a major stimulant of angiogenesis and HIF-1α is its key mediator. The formation of new vessels is a tightly regulated process involving angiogenic activators including VEGF, fibroblast growth factors (FGFs), platelet-derived growth factor (PDGF) and epidermal growth factor (EGF). The process of vessel formation requires activators which allow for proper development, however, this process must be tightly controlled, thus, there is a need for molecules which function as angiogenic inhibitors as well. These inhibitors include thrombospondin 1, angiostatin, endostatin and tumstatin (discussed in Ref. 111). VEGF participates in both physiological and pathological processes by allowing new vessel growth as a result of releasing growth factors anchored to the ECM proteins. The networks of capillaries that are formed distribute supply to the tumors metabolic needs and express high levels of VEGF.112 It has been shown that within a glioblastoma model, CD133+ cells, representative of the CSC population, produce high levels of VEGF in comparison to the CD133− population.113 Bao et al. showed that CD133+ cells readily form highly-vascular haemorrhagic tumors in the brains of immunocompromised mice and additionally showed that by using a VEGF inhibitor CD133+ induced endothelial cell migration, tube formation and tumor initiation can be blocked.113 This study demonstrated that CSCs derived from glioblastoma possess proangiogenic properties via high VEGF expression and contribute to the angiogenic process, possibly by communicating with the microenvironment. The ability of CSCs to express high levels of VEGF and manipulate the angiogenic process by favoring proangiogenesis contributes to tumor expansion and formation by enhancing survival in a normally stressful environment.
It is established that for primary tumor growth, angiogenesis is a necessary process and CSCs reside in close proximity to tumor blood vessels and has been termed the vascular CSC niche (Fig. 4), this has specifically been demonstrated in the brain tumor niche.114 NSCs require a vascular niche for growth that is formed by endothelial cells, a requirement for angiogenesis. NSC mediated tumor growth in immunodeficient mice is accelerated by coinjection with endothelial cells, further demonstrating that factors within the microenvironment promote growth and progression of cancer cells.17 Additionally, Folkins et al. have shown that a combination of antiangiogenic anticancer therapy is effective in targeting ‘brain tumor stem-like cells.’115 This study further supports the belief that by targeting and disrupting the angiogenic processes within a tumor, there is disruption of the CSC population and its ability to self-renew.
Figure 4.
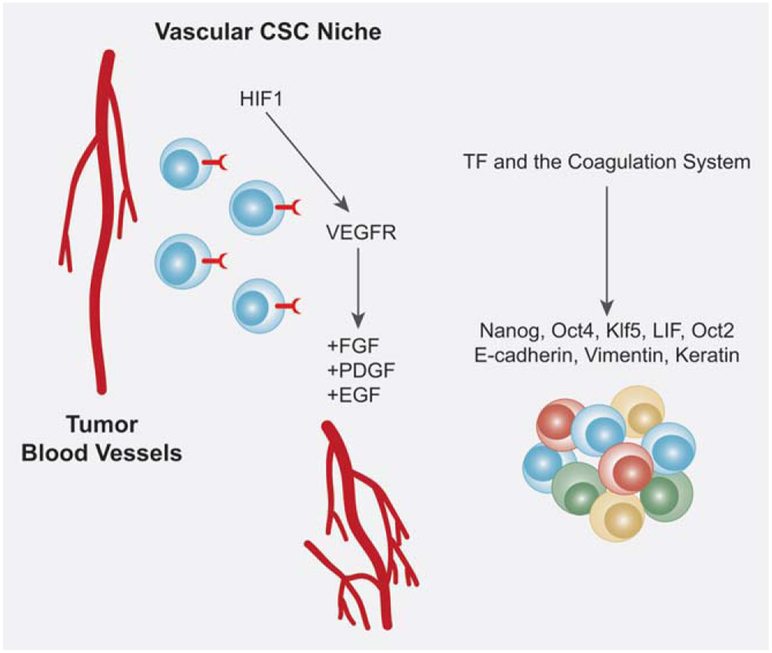
The vascular cancer stem cell niche. The process of angiogenesis is a normal homeostatic process in healthy individuals; however, it is also a necessary process for primary tumor growth. CSCs, as demonstrated in the neural stem cell niche, reside within in close proximity to tumor blood vessels and promote angiogenesis. Angiogenesis is stimulated by a hypoxic environment as well, therefore, HIF1, is capable of mediating VEGF receptors expressed on the surface of CSCs. The activation of VEGF results in the activation of angiogenic regulators such as FGFs, PDGF and EGFs as well. Additionally, tissue factor (TF) receptors, hypothesized to exist on the surface of CSCs, promote growth, survival, migration and a proangiogenic phenotype. TF has been shown to upregulate critical CSC regulators such as Nanog, Oct4, Kl5 and LIF which can promote CSC maintenance within a vascular CSC niche.
Recently, there is support demonstrating the importance of vasculogenic mimicry (VM) in cancer development. VM is the process by which tumor cells can form extravascular networks that contribute to blood circulation for the tumor. The formation of an extensive network which can provide the tumor environment with the proper nutrients for survival must have a key role in sustaining the microenvironment. Interestingly, VM networks have a close resemblance to embryonic vasculogenic networks, suggesting that the tumors cells which are contributing to VM development are capable of acquiring a phenotype reminiscent of undifferentiated ESCs.116
VM has been reported in various cancers such as breast, ovarian, prostate, lung and clear renal cell carcinoma (reviewed in Ref. 116) and is associated with both metastasis and poor clinical outcome.116 The signaling pathways which are associated with VM development include the vascular endothelial(VE)-cadherin cascade, the cAMP signaling pathway, galectin molecules, Nodal/Notch and Wnt signaling (reviewed in Ref. 117). VE-cadherin is an adhesive protein often associated with aggressive cancer cells and metastasis.117 VE-cadherin is transcriptionally upregulated via initiation by HIF-2a, a key regulator of hypoxia. The cAMP signaling pathway, which functions in endothelial cell differentiation, is also associated with Notch signaling as well.117 The galectins are carbohydrate-binding proteins that which are associated with tumor survival, angiogenesis and metastasis.117 The Nodal/Notch signaling, a key upregulated pathway during embryogenesis, is associated with CSC maintenance and in the context of VM, has been shown to over-expressed.117 The Wnt signaling pathway, a key pathway in both ESC and CSCs, is suggested to play a role in VM formation as it functions in embryonic vasculogenesis.117 These signaling pathways which are suggested to function in mediating VM development are also implicated in the maintenance of CSCs as well.
Recently, in a model of hepatocellular carcinoma, Twist1, an inducer of EMT and a promoter of CSC formation in breast cancer,118 head and neck cancers118,119 has been shown to be linked to VM formation. Twist1 was shown to correlate with both invasion and shorter survival time in patients.120 The function of Twist in reference to CSCs is often associated with the concept of EMT. Specifically, in the breast CSC model, Twist has been shown to enrich for breast CSCs and it is this population which displays characteristics of cells that have undergone EMT (reviewed in Ref. 121). The newly defined role of Twist in VM development and its known role in breast CSCs combined, leads us to speculate that expression of Twist to induce VM development within the microenvironment can enrich and propagate CSC formation as well.
Hence, these data give evidence to the concept that the tumor microenvironments, specifically the angiogenic processes which occur within a tumor, encourage CSC maintenance and possibly CSC propagation. The processes of both angiogenesis and VM development appear to be a result of several pathways which are interwined and coregulated. It is the relationship between these various signaling molecules within the microenvironment that sustain the milieu necessary for enhancement of tumor formation and quite possibly, promote the CSC population. Although the exact mechanism(s) by which this occurs is unknown, it appears to be a critical factor in the processes governing CSC maintenance.
Tissue Factor, the Coagulation System and the CSC Niche
There is increasing evidence defining the link between tissue factor (TF), a cell-associated receptor for coagulation factor VIIa (FVIIa) that functions as an initiator of blood coagulation, and its role in maintaining CSC niches. The TF pathway includes TF, protease activated receptors (PARs), agonists and effectors. TF expression is normally absent in the vascular lumen but its interaction with FVIIa is increased during vascular injury and during angiogenesis on endothelial cells. TF expressing cells are often correlated with an increase in growth, survival, migration and a proangiogenic phenotype, often seen in cancer cells.122 TF is expressed by both cancer cells and the surrounding vascular tumor stroma and it is believed that the specific role of TF in cancer is reliant on crosstalk between the cancer cells and stroma.
TF and its role in tumor cell behavior have recently been demonstrated in a variety of cancers including stomach, breast, colon and pancreatic.123-125 It is hypothesized that cancer growth can result in a hyperactive TF pathway via several mechanisms including: the accumulation of cells with procoagulant properties; abnormal expression of TF by the activated endothelial cells of the tumor vasculature; leaking of coagulation factors including TF into the perivascular space; and entry of TF-expressing cells via invasion and metastasis (discussed in Ref. 126). The expression of TF by cancer cells may contribute to tumor cell behavior as the procoagulant environment which is often seen in tumors can result in the accumulation of growth factors that promote the stimulation of tumor growth and enhancement of tumor cell survival. This environment which is created by an overexpressing TF pathway can initiate the development of a surrounding rich in growth factors that are utilized by the cancer cells to thrive upon. Interestingly, the TF pathway inhibitor (TFPI) has been implicated as a potential plasma biomarker candidate for pancreatic cancer. TFPI functions to inhibit the activation of proteases by TF-VIIa which ultimately lead to the formation of a clot. In a pancreatic model, TFPI has been shown to be significantly increased in patients at the time of diagnosis and decrease to normal levels post surgical resection of the tumor.127 TFPI has been shown to exert control over endothelial cell migration via inhibition of the ERK pathway128 and the interaction of TFPI with the TF-VIIa complex has been shown to enhance cancer cell migration and adhesion in primary bladder carcinoma cells which can result in metastasis.129 The data suggests that the role of the TF-VIIa complex in modulating tumor cell behavior is key to tumor formation in providing an environment in which tumor cells can efficiently expand.
In terms of CSCs, crucial regulators of self-renewal, Nanog, Oct4, Klf5 and LIF, have all been shown to either induce factors involved in the coagulation system or become upregulated as a consequence of coagulation system stimulation (Fig. 4) (extensively reviewed in Refs. 122 and 126). Interestingly, these same factors are deemed necessary in the process of reprogramming fibroblasts to ES cells, also known as induced pluripotent cells (iPS cells).130,131 We believe it is plausible to speculate that these very same factors which are necessary for ESC and CSC maintenance are utilized during development to induce factors that contribute to the development of the coagulation system during organismal development. TF expressing cells are associated with growth, survival, migration and proangiogenesis,122 cellular processes which are necessary and critical for proper organismal development. Hence, the ability of these factors to function as versatile players and their ability to act on a global level should be taken into consideration.
The role of TF and the coagulation system has also been shown to affect differentiation pathways in NSCs, HSCs and MSCs by upregulating and inducing expression of well-known differentiation markers such as Oct-2 in NSCs, GM-SCF, M-CSF in HSCs, and CCN1, CCN2 and vimentin in MSCs.122,126 Additionally, there is a link connecting EMT and the coagulation system as E-cadherin, vimentin and keratin has been shown to be induced by TF expression changes.122,126
Although there is definitive experimental evidence lacking that proves the TF pathway directly affects CSC niches, there is a clear role for TF in cell signaling, angiogenesis, cancer and CSC related mechanisms, i.e., self-renewal, differentiation and EMT. Whether the TF pathway directly or indirectly affects CSC niches is an appealing concept for further examination as anticoagulant agents directed against the TF pathways may serve as potential targets.
Angiogenic Dormancy and CSCs
The mechanisms regarding tumor dormancy and the ability of CSCs to remain quiescent are intertwined with the concept of angiogenic dormancy. Angiogenic dormancy occurs when tumors are unable to expand due to poor vascularization and the fraction of dying cells equals the dividing ones, thus, resulting in a dormant stage where there is no increase in tumor mass over time.132 The mechanism(s) which function in inducing this state of tumor dormancy are believed to be rooted within the microenvironment of the tumor itself. The interaction of the tumor cells and niche in which they reside can result in growth arrest of tumor cells. For example, it was shown in a head and neck carcinoma model that blocking of the metastasis-associated urokinase receptor (u-PAR), EGFR, integrins or FAK, resulted in a state of tumor dormancy (discussed in Ref. 133). Interestingly, our laboratory has recently shown in prostate CSCs that by blocking the integrin αVβ3 receptor, a vitronectin specific receptor, we can block CSC differentation by human serum and maintain the prostate CSCs in an undifferentiated state.134 Additionally our study also showed that by blocking this receptor, we could also inhibit vivo tumor formation as well.134 This in fact, could serve as an example of how the microenvironment can contribute to tumor dormancy. Additionally, it has previously been demonstrated a loss of dormancy can occur by the overexpression of angiogenic stimulators including Myc,135 VEGF and bFGF.136 Therefore, loss of expression of these key angiogenic factors can result in the tumor dormancy phenotype. The ability to block specific receptors and signaling cascades which are contained within the immediate environment can result in inhibition of tumorigenesis by holding the cells in a nontumorigenic state. Tumor dormancy can be supported by what is considered in a nonpermissive niche in which there is a lack of proper signals to induce tumorigenesis and the cells are maintained in a quiescent state. However, if exposed to a permissive niche where the cells interact with molecules and products encouraging tumorigenesis and proliferation, the state of tumor dormancy is lost.
It is hypothesized that this state of tumor dormancy may promote CSC dormancy. The process of tumor initiation by CSCs is dependent upon their activation, therefore, to remain in a dormant environment implies that this population may be held in an “inactive state,”137 as demonstrated by our laboratory in the prostate CSC model.134 The concept of ‘angiogenic switch’ is reliant on the induction of proangiogenic gene expression by stimuli which favor an increase in tissue mass. As discussed, angiogenic processes have been shown to function in CSC maintenance therefore, it is reasonable to speculate that antiangiogenic processes can function to inhibit or prevent CSC function as well. In a state of angiogenic dormancy, the niche may not provide the necessary growth factors, inflammatory molecules or signals required by a CSC to initiate tumor formation or metastasis. However, once the niche begins to generate signals and factors necessary for CSC function, as seen under hypoxic conditions for example, the CSC may exit a state of dormancy and function as a driver of tumorigenesis.
The exact mechanism(s) by which a dormant state can allow persistence of CSCs in an undifferentiated, nontumorigenic state is unclear. As discussed, the vasculature system functions as a critical regulator of CSCs, the processes by which this system can influence various stimulation or inhibition of CSCs are under investigation. However, it is clear that there is a regulatory role for the vascular system within the CSC niche.
Conclusions
To date, our understanding of the microenvironment or niche and its role in CSCs are limited and under intense investigation. It is of great interest to define the mechanism(s) the CSC niche uses to define CSC fate. There is emerging evidence for the existence of a CSC niche that utilizes cell signaling pathways traditionally used to maintain homeostatic processes such as inflammation, EMT, hypoxia and angiogenesis as discussed here. The ability of a CSC niche to provide an environment in which a CSC can flourish is a critical process which must be further investigated to enhance our understanding of the basic biology behind cancer and to possibly identify potential therapeutic targets.
Acknowledgement
The authors thank the NCI-Frederick Scientific Publications, Graphics and Media department for their help with the figure production. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Grant sponsor: National Cancer Institute, National Institutes of Health (federal funds); Grant number: HHSN261200800001E; Grant sponsor: Intramural Research Program (NIH)
References
- 1.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 2008;9: 11–21. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645–8. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003;100: 3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63: 5821–8. [PubMed] [Google Scholar]
- 5.Kasper S. Identification, characterization, and biological relevance of prostate cancer stem cells from clinical specimens. Urol Oncol 2009;27:301–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer 2008;98:756–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006;25: 1696–708. [DOI] [PubMed] [Google Scholar]
- 8.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445:111–5. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res 2007;67: 1030–7. [DOI] [PubMed] [Google Scholar]
- 10.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. Faseb J 2007;21:3777–85. [DOI] [PubMed] [Google Scholar]
- 11.Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers— therapeutic implications. Trends Mol Med 2008;14:450–60. [DOI] [PubMed] [Google Scholar]
- 12.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol 2007;23:675–99. [DOI] [PubMed] [Google Scholar]
- 13.Chung Y, Klimanskaya I, Becker S, Marh J, Lu SJ, Johnson J, Meisner L, Lanza R. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature 2006;439:216–9. [DOI] [PubMed] [Google Scholar]
- 14.Geens M, Mateizel I, Sermon K, De Rycke M, Spits C, Cauffman G, Devroey P, Tournaye H, Liebaers I, Van de Velde H. Human embryonic stem cell lines derived from single blastomeres of two 4-cell stage embryos. Hum Reprod 2009;24: 2709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorthongpanich C, Yang SH, Piotrowska-Nitsche K, Parnpai R, Chan AW. Development of single mouse blastomeres into blastocysts, outgrowths and the establishment of embryonic stem cells. Reproduction 2008;135:805–13. [DOI] [PubMed] [Google Scholar]
- 16.Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 2004;23:7150–60. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Vela A, Aguilar-Gallardo C, Simon C. Building a framework for embryonic microenvironments and cancer stem cells. Stem Cell Rev 2009;5:319–27. [DOI] [PubMed] [Google Scholar]
- 18.Pierce GB, Pantazis CG, Caldwell JE, Wells RS. Specificity of the control of tumor formation by the blastocyst. Cancer Res 1982;42:1082–7. [PubMed] [Google Scholar]
- 19.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE, Hendrix MJ. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA 2008;105:4329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol 2005;21: 605–31. [DOI] [PubMed] [Google Scholar]
- 21.Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr Opin Biotechnol 2008;19:534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Li H, Yang H, Rao MS, Zhan M. Mechanisms controlling embryonic stem cell self-renewal and differentiation. Crit Rev Eukaryot Gene Exp 2006;16:211–31. [DOI] [PubMed] [Google Scholar]
- 23.Wayne AS, Capitini CM, Mackall CL. Immunotherapy of childhood cancer: from biologic understanding to clinical application. Curr Opin Pediatr 2010;22: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansford LM, McKee AE, Zhang L, George RE, Gerstle JT, Thorner PS, Smith KM, Look AT, Yeger H, Miller FD, Irwin MS, Thiele CJ, et al. Neuroblastoma cells isolated from bone marrow metastases contain a naturally enriched tumor-nitiating cell. Cancer Res 2007;67: 11234–43. [DOI] [PubMed] [Google Scholar]
- 25.Rodini CO, Suzuki DE, Nakahata AM, Pereira MC, Janjoppi L, Toledo SR, Okamoto OK. Aberrant signaling pathways in medulloblastomas: a stem cell connection. Arq Neuropsiquiatr 2010;68: 947–52. [DOI] [PubMed] [Google Scholar]
- 26.Zweidler-McKay PA. Notch signaling in pediatric malignancies. Curr Oncol Rep 2008;10:459–68. [DOI] [PubMed] [Google Scholar]
- 27.Aiden AP, Rivera MN, Rheinbay E, Ku M, Coffman EJ, Truong TT, Vargas SO, Lander ES, Haber DA, Bernstein BE. Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network. Cell Stem Cell 2010;6:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghotra VP, Puigvert JC, Danen EH. The cancer stem cell microenvironment and anti-cancer therapy. Int J Radiat Biol 2009;85:955–62. [DOI] [PubMed] [Google Scholar]
- 29.Hay ED. Organization and fine structure of epithelium and mesenchyme in the developing chick embryo In: Fleischmajer R and Billingham RE, Editors, 1968. Epithelial-Mesenchymal Interactions, Williams and Wilkins, Baltimore, pp. 31–55. [Google Scholar]
- 30.Hay ED. Collagen and other matrix glycoproteins in embryogenesis In Hay ED, editor, Cell Biology of Extracellular Matrix. 2nd edn. Plenum Press, New York, USA, 1991, pp. 419–62. [Google Scholar]
- 31.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871–90. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 2009;1:303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell Mol Life Sci 2010;67:2605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005;65:5506–11. [DOI] [PubMed] [Google Scholar]
- 36.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, Thomas SB, Farrar WL. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis 2009;26:433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS One 2010;5:e12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raimondi C, Gianni W, Cortesi E, Gazzaniga P. Cancer stem cells and epithelial-mesenchymal transition: revisiting minimal residual disease. Curr Cancer Drug Targets 2010;10:496–508. [DOI] [PubMed] [Google Scholar]
- 39.Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res 2010;70:7360–64. [DOI] [PubMed] [Google Scholar]
- 40.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Pluijm G Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone 2010;48:37–43. [DOI] [PubMed] [Google Scholar]
- 42.Xia H, Cheung WK, Sze J, Lu G, Jiang S, Yao H, Bian XW, Poon WS, Kung HF, Lin MC. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and β-catenin signaling. J Biol Chem 2010;285:36995–37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang MH, Hsu DSS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OKS., and Wu KJ. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 2010;12:982–992. [DOI] [PubMed] [Google Scholar]
- 44.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. JMammary Gland Biol Neoplasia 2010;15:117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dvorak HF, Galli SJ, Dvorak AM. Cellular and vascular manifestations of cell-mediated immunity. Hum Pathol 1986;17: 122–37. [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 47.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29:4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crea F, Mathews LA, Farrar WL, Hurt EM. Targeting prostate cancer stem cells. Anticancer Agents Med Chem 2009;9: 1105–13. [DOI] [PubMed] [Google Scholar]
- 49.Ischenko I, Seeliger H, Schaffer M, Jauch KW, Bruns CJ. Cancer stem cells: how can we target them? Curr Med Chem 2008;15:3171–84. [DOI] [PubMed] [Google Scholar]
- 50.Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S, Russo P. Cancer stem cells: a new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem 2009;16:1688–703. [DOI] [PubMed] [Google Scholar]
- 51.Trosko J Cancer stem cells and cancer non-stem cells: from adult stem cells or from re-programming of differentiated somatic cells. Vet Pathol 2009;46:176–193. [DOI] [PubMed] [Google Scholar]
- 52.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol 2003;163: 1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamauchi Y, Kohyama T, Takizawa H, Kamitani S, Desaki M, Takami K, Kawasaki S, Kato J, Nagase T. Tumor necrosis factor-alpha enhances both epithelial-mesenchymal transition and cell contraction induced in A549 human alveolar epithelial cells by transforming growth factor-beta1. Exp Lung Res 2010;36:12–24. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, Tanihara H, Saya H. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem 2010;285:4060–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett 2010;295:214–28. [DOI] [PubMed] [Google Scholar]
- 56.Tobar N, Villar V, Santibanez JF. ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem 2010;340: 195–202. [DOI] [PubMed] [Google Scholar]
- 57.Storci G, Sansone P, Mari S, D’Uva G, Tavolari S, Guarnieri T, Taffurelli M, Ceccarelli C, Santini D, Chieco P, Marcu KB, Bonafe M. TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol 2010;225:682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leizer AL, Alvero AB, Fu HH, Holmberg JC, Cheng YC, Silasi DA, Rutherford T, Mor G. Regulation of Inflammation by the NF-kappaB Pathway in Ovarian Cancer Stem Cells. AmJ Reprod Immunol 2010;65: 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawasaki BT, Hurt EM, Kalathur M, Duhagon MA, Milner JA, Kim YS, Farrar WL. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: an integrated molecular profiling approach. Prostate 2009;69:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 2008;14:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moharita AL, Taborga M, Corcoran KE, Bryan M, Patel PS, Rameshwar P. SDF-1alpha regulation in breast cancer cells contacting bone marrow stroma is critical for normal hematopoiesis. Blood 2006;108: 3245–52. [DOI] [PubMed] [Google Scholar]
- 62.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121: 335–48. [DOI] [PubMed] [Google Scholar]
- 63.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–13. [DOI] [PubMed] [Google Scholar]
- 64.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 2007;7:733–6. [DOI] [PubMed] [Google Scholar]
- 65.Barton BE. IL-6: insights into novel biological activities. Clin Immunol Immunopathol 1997;85:16–20. [DOI] [PubMed] [Google Scholar]
- 66.Peters M, Muller AM, Rose-John S. Interleukin-6 and soluble interleukin-6 receptor: direct stimulation of gp130 and hematopoiesis. Blood 1998;92:3495–504. [PubMed] [Google Scholar]
- 67.Gerhartz C, Heesel B, Sasse J, Hemmann U, Landgraf C, Schneider-Mergener J, Horn F, Heinrich PC, Graeve L. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I. Definition of a novel phosphotyrosine motif mediating STAT1 activation. J Biol Chem 1996;271:12991–8. [DOI] [PubMed] [Google Scholar]
- 68.Klein B, Lu ZY, Bataille R. Clinical applications of IL6 inhibitors. Res Immunol 1992;143:774–6. [DOI] [PubMed] [Google Scholar]
- 69.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 1998;12:2048–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daheron L, Opitz SL, Zaehres H, Lensch MW, Andrews PW, Itskovitz-Eldor J, Daley GQ. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2004;22:770–8. [DOI] [PubMed] [Google Scholar]
- 71.Xu RH, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 2002;20:1261–4. [DOI] [PubMed] [Google Scholar]
- 72.Xu RH, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods 2005;2:185–90. [DOI] [PubMed] [Google Scholar]
- 73.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005;118:4495–509. [DOI] [PubMed] [Google Scholar]
- 74.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 2005;132:1273–82. [DOI] [PubMed] [Google Scholar]
- 75.Rose-John S GP130 stimulation and the maintenance of stem cells. Trends Biotechnol 2002;20:417–9. [DOI] [PubMed] [Google Scholar]
- 76.Jenkins BJ, Quilici C, Roberts AW, Grail D, Dunn AR, Ernst M. Hematopoietic abnormalities in mice deficient in gp130-mediated STAT signaling. Exp Hematol 2002;30:1248–56. [DOI] [PubMed] [Google Scholar]
- 77.Kollet O, Aviram R, Chebath J, ben-Hur H, Nagler A, Shultz L, Revel M, Lapidot T. The soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro maintenance and proliferation of human CD34(+)CD38(− low) cells capable of repopulating severe combined immunodeficiency mice. Blood 1999;94:923–31. [PubMed] [Google Scholar]
- 78.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest 2007;117:3988–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, MacSwords J, Cao Y, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells 2009;27:2393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng D, Peng C, Li C, Zhou Y, Li M, Ling B, Wei H, Tian Z. Identification and characterization of cancer stem-like cells from primary carcinoma of the cervix uteri. Oncol Rep 2009;22:1129–34. [DOI] [PubMed] [Google Scholar]
- 81.Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS, Jessup JM, Shetty K, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA 2008;105:2445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson H, Huelsmeyer M, Chun R, Young KM, Friedrichs K, Argyle DJ. Isolation and characterisation of cancer stem cells from canine osteosarcoma. Vet J 2008;175:69–75. [DOI] [PubMed] [Google Scholar]
- 83.Nilsson CL, Dillon R, Devakumar A, Shi SD, Greig M, Rogers JC, Krastins B, Rosenblatt M, Kilmer G, Major M, Kaboord BJ, Sarracino D, et al. Quantitative phosphoproteomic analysis of the STAT3/IL-6/HIF1alpha signaling network: an initial study in GSC11 glioblastoma stem cells. J Proteome Res 2010;9:430–43. [DOI] [PubMed] [Google Scholar]
- 84.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells 2009; 27:2383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maitland NJ, Collins AT. Inflammation as the primary aetiological agent of human prostate cancer: a stem cell connection? J Cell Biochem 2008;105:931–9. [DOI] [PubMed] [Google Scholar]
- 86.Mathews LA, Hurt EM, Zhang X, Farrar WL. Epigenetic regulation of CpG promoter methylation in invasive prostate cancer cells. Mol Cancer 2010;9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel SA, Heinrich AC, Reddy BY, Rameshwar P. Inflammatory mediators: parallels between cancer biology and stem cell therapy. J Inflamm Res 2009;2: 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koblas T, Zacharovova K, Berkova Z, Mindlova M, Girman P, Dovolilova E, Karasova L, Saudek F. Isolation and characterization of human CXCR4-positive pancreatic cells. Folia Biol (Praha) 2007;53:13–22. [PubMed] [Google Scholar]
- 89.Hwang-Verslues WW, Kuo WH, Chang PH, Pan CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, Lee EY, Chang KJ, et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS One 2009;4:e8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mueller MT, Hermann PC, Heeschen C. Cancer stem cells as new therapeutic target to prevent tumour progression and metastasis. Front Biosci (Elite Ed) 2010;2: 602–13. [DOI] [PubMed] [Google Scholar]
- 91.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313–23. [DOI] [PubMed] [Google Scholar]
- 92.Burger JA, Peled A. CXCR4 antagonists: targeting the microenvironment in leukemia and other cancers. Leukemia 2009;23:43–52. [DOI] [PubMed] [Google Scholar]
- 93.Wang Z, Li Y, Sarkar FH. Signaling mechanism(s) of reactive oxygen species in epithelial-mesenchymal transition reminiscent of cancer stem cells in tumor progression. Curr Stem Cell Res Ther 2010;5:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell’s escape from oxidative stress. Cancer Metastasis Rev 2010;29:351–78. [DOI] [PubMed] [Google Scholar]
- 95.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell 2008;134:703–7. [DOI] [PubMed] [Google Scholar]
- 96.Pavlides S, Tsirigos A, Vera I, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, Martinez-Outschoorn UE, Howell A, Sotgia F, Lisanti MP. Transcriptional evidence for the “Reverse Warburg Effect” in human breast cancer tumor stroma and metastasis: similarities with oxidative stress, inflammation, Alzheimer’s disease, and “Neuron-Glia Metabolic Coupling.” Aging (Albany NY) 2010;2:185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009;458:780–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 2008;9:285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ezashi T, Das P, Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA 2005;102:4783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen HF, Kuo HC, Lin SP, Chien CL, Chiang MS, Ho HN. Hypoxic culture maintains self-renewal and enhances embryoid body formation of human embryonic stem cells. Tissue Eng Part A 2010;16:2901–13. [DOI] [PubMed] [Google Scholar]
- 101.Mohyeldin A, Garzon-Muvdi T, Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 2010;7:150–61. [DOI] [PubMed] [Google Scholar]
- 102.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000;28:875–84. [DOI] [PubMed] [Google Scholar]
- 103.Milas L, Hittelman WN. Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin Radiat Oncol 2009;19:96–105. [DOI] [PubMed] [Google Scholar]
- 104.Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer 2010;102:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das B, Tsuchida R, Malkin D, Koren G, Baruchel S, Yeger H. Hypoxia enhances tumor stemness by increasing the invasive and tumorigenic side population fraction. Stem Cells 2008;26:1818–30. [DOI] [PubMed] [Google Scholar]
- 106.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem 2008;283:31153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Silvan U, Diez-Torre A, Arluzea J, Andrade R, Silio M, Arechaga J. Hypoxia and pluripotency in embryonic and embryonal carcinoma stem cell biology. Differentiation 2009;78:159–68. [DOI] [PubMed] [Google Scholar]
- 108.Billadeau DD, Chatterjee S, Bramati P, Sreekumar R, Shah V, Hedin K, Urrutia R. Characterization of the CXCR4 signaling in pancreatic cancer cells. Int J Gastrointest Cancer 2006;37:110–9. [DOI] [PubMed] [Google Scholar]
- 109.Ishikawa T, Nakashiro K, Klosek SK, Goda H, Hara S, Uchida D, Hamakawa H. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncol Rep 2009;21:707–12. [PubMed] [Google Scholar]
- 110.Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L, Zhang Y. Clinicopathologic significance of HIF-1alpha, CXCR4, and VEGF expression in colon cancer. Clin Dev Immunol 2010:537531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta 2009; 1796:293–308. [DOI] [PubMed] [Google Scholar]
- 112.Okazaki K, Maltepe E. Oxygen, epigenetics and stem cell fate. Regen Med 2006;1:71–83. [DOI] [PubMed] [Google Scholar]
- 113.Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 2006;66:7843–8. [DOI] [PubMed] [Google Scholar]
- 114.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. [DOI] [PubMed] [Google Scholar]
- 115.Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel RS. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res 2007;67: 3560–4. [DOI] [PubMed] [Google Scholar]
- 116.Paulis YWJ, Soetekouw PMMB, Verheul HMW, Tjan-Heijnen VCG, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochim Biophys Acta 2010; 1806:18–28. [DOI] [PubMed] [Google Scholar]
- 117.Blaschuk OW, Devemy E. Cadherins as novel targets for anti-cancer therapy. Eur J Pharmacol 2009;625:195–8. [DOI] [PubMed] [Google Scholar]
- 118.Vesuna F, Lisok A, Kimble B, Raman V. Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia 2009;11:1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 2010;12:982–92. [DOI] [PubMed] [Google Scholar]
- 120.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 2010; 51:545–56. [DOI] [PubMed] [Google Scholar]
- 121.Cakouros D, Raices RM, Gronthos S, Glackin CA. Twist-ing cell fate: mechanistic insights into the role of twist in lineage specification/differentiation and tumorigenesis. J Cell Biochem 2010;110: 1288–98. [DOI] [PubMed] [Google Scholar]
- 122.Milsom C, Magnus N, Meehan B, Al-Nedawi K, Garnier D, Rak J. Tissue factor and cancer stem cells: is there a linkage? Arterioscler Thromb Vasc Biol 2009;29: 2005–14. [DOI] [PubMed] [Google Scholar]
- 123.Iversen N, Lindahl AK, Abildgaard U. Elevated plasma levels of the factor Xa-TFPI complex in cancer patients. Thromb Res 2002;105:33–6. [DOI] [PubMed] [Google Scholar]
- 124.Yasuda T, Ueda T, Kamei K, Shinzaki W, Sawa H, Shinzeki M, Ku Y, Takeyama Y. Plasma tissue factor pathway inhibitor levels in patients with acute pancreatitis. J Gastroenterol 2009;44:1071–9. [DOI] [PubMed] [Google Scholar]
- 125.Sierko E, Wojtukiewicz MZ, Zimnoch L, Kisiel W. Expression of tissue factor pathway inhibitor (TFPI) in human breast and colon cancer tissue. Thromb Haemost 2010;103:198–204. [DOI] [PubMed] [Google Scholar]
- 126.Garnier D, Milsom C, Magnus N, Meehan B, Weitz J, Yu J, Rak J. Role of the tissue factor pathway in the biology of tumor initiating cells. Thromb Res 2010; 125 (Suppl 2):S44–50. [DOI] [PubMed] [Google Scholar]
- 127.Balasenthil S, Chen N, Lott ST, Chen J, Carter J, Grizzle WE, Frazier ML, Sen S, Killary AM. A migration signature and plasma biomarker panel for pancreatic adenocarcinoma. Cancer Prev Res (Phila) 2011;4:137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Provencal M, Michaud M, Beaulieu E, Ratel D, Rivard GE, Gingras D, Beliveau R. Tissue factor pathway inhibitor (TFPI) interferes with endothelial cell migration by inhibition of both the Erk pathway and focal adhesion proteins. Thromb Haemost 2008;99:576–85. [DOI] [PubMed] [Google Scholar]
- 129.Fischer EG, Riewald M, Huang HY, Miyagi Y, Kubota Y, Mueller BM, Ruf W. Tumor cell adhesion and migration supported by interaction of a receptor-protease complex with its inhibitor. J Clin Invest 1999;104:1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 131.Yamanaka S, Takahashi K. [Induction of pluripotent stem cells from mouse fibroblast cultures]. Tanpakushitsu Kakusan Koso 2006;51:2346–51. [PubMed] [Google Scholar]
- 132.Almog N Molecular mechanisms underlying tumor dormancy. Cancer Lett 2010;294:139–46. [DOI] [PubMed] [Google Scholar]
- 133.Favaro E, Amadori A, Indraccolo S. Cellular interactions in the vascular niche: implications in the regulation of tumor dormancy. APMIS 2008;116:648–59. [DOI] [PubMed] [Google Scholar]
- 134.Hurt EM, Chan K, Serrat MA, Thomas SB, Veenstra TD, Farrar WL. Identification of vitronectin as an extrinsic inducer of cancer stem cell differentiation and tumor formation. Stem Cells 2010;28:390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, Bachmann MH, Borowsky AD, Ruebner B, Cardiff RD, Yang Q, Bishop JM, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature 2004;431: 1112–7. [DOI] [PubMed] [Google Scholar]
- 136.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 2006;5: 1779–87. [DOI] [PubMed] [Google Scholar]
- 137.Rak J, Milsom C, Yu J. Vascular determinants of cancer stem cell dormancy—do age and coagulation system play a role? APMIS 2008;116: 660–76. [DOI] [PubMed] [Google Scholar]