Abstract
Background and Purpose
A major gap in the field of ischemic preconditioning (IPC) is whether or not long-lasting neuroprotection can be achieved. Moreover, the specific mechanisms underlying IPC and how they can be translated into the clinic remain uncertain. To fill these gaps, we tested the hypothesis that IPC exerts long-lasting structural and functional neuroprotection against ischemic stroke through the master gatekeeper of antioxidant defenses, nuclear factor erythroid 2-related factor 2 (Nrf2). We also tested whether the brain could be pharmaceutically preconditioned with a potent and blood-brain barrier-permeable Nrf2 activator, 2-cyano-3,12-dioxo-oleana-1,9(11)-dien-28-trifluoethyl amide (CDDO-TFEA).
Methods
IPC was induced by transient middle cerebral artery occlusion (MCAO) for 12 minutes, and ischemic stroke was generated by MCAO for 60 minutes in wild-type (WT) or Nrf2 knockout (KO) mice. Sensorimotor function, learning/memory skills, and brain tissue loss were measured up to 35 days after stroke. Primary rodent cortical neurons from wildtype (WT) and Nrf2 KO mice were subjected to lethal oxygen-glucose deprivation (OGD) or a brief OGD episode as a preconditioning (PC) stimulus before OGD. Cell viability/death, lipid electrophile generation, and Nrf2 activation were measured. CDDO-TFEA or its vehicle was administered in vivo for three consecutive days before MCAO. Tissue loss and neurological tests were performed 35 days after stroke.
Results
IPC significantly reduced sensorimotor deficits, post-stroke cognitive impairments, and brain tissue loss, 35 days after MCAO in WT mice. These enduring protective effects of IPC were inhibited in Nrf2 KO mice. In neuronal cultures, PC also endowed primary neurons with ischemic tolerance against OGD-induced cell death, an effect that was abolished by loss of Nrf2 expression in KO neurons. PC induced the generation of low levels of lipid electrophiles and led to activation of the Nrf2 pathway. The mechanism underlying IPC may be translatable, as exogenous administration of the Nrf2 activator CDDO-TFEA significantly reduced neurological dysfunction and ischemic brain damage after MCAO.
Conclusions
IPC provides long-lasting neuroprotection against ischemic brain injury and post-stroke cognitive dysfunction. Nrf2 activation plays a key role in this beneficial outcome and is a promising therapeutic target for the attenuation of ischemic brain injury.
Keywords: Stroke, neuroprotection, ischemic tolerance, electrophile, post-stroke cognitive impairment
Visible abstract
(A) Ischemic stroke is associated with severe oxidative stress, which induces the generation of large amounts of lipid electrophiles and produces long-term cognitive deficits and brain tissue loss. (B) We discovered that ischemic preconditioning (IPC) provides enduring protection against long-term stroke-induced sensorimotor deficits, cognitive deficits, and brain tissue loss. In comparison with stroke, IPC only creates mild oxidative stress, which leads to the generation of subtoxic levels of lipid electrophiles followed by Nrf2 activation. This mechanism has translational potential, as the Nrf2 inducer CDDO elicits comparable protection in vivo.
Introduction
It has been known for many decades that ischemic preconditioning (IPC) of the brain is an effective approach to elicit ischemic tolerance against subsequent lethal stroke 1–3. However, whether IPC provides short-lasting or long-lasting neuroprotection is still debated. Two timeframes of IPC-mediated ischemic tolerance have been reported in the literature 3: 1) a rapid timeframe that emerges within minutes and provides transient protection with a duration of several hours, and 2) a delayed timeframe that emerges within hours to days and confers a relatively longer protection period (> 7 days). Rapid preconditioning only provides short-lasting protection that wanes within 1–3 days 4, 5. Although several studies have reported that delayed non-ischemic preconditioning offers long-lasting protection against ischemic injuries 6–9, it remains unclear if delayed ischemic preconditioning offers similarly long-lasting neuroprotection. As long-lasting protection would be more clinically meaningful, there is an urgent need to determine if IPC simply delays ischemic brain injury or reduces the magnitude of the injury in a persistent fashion.
Post-stroke cognitive impairments affect up to one-third of stroke survivors 10 and are becoming increasingly prevalent in the elderly population 11. However, whether IPC can reduce post-stroke cognitive impairments is not known. As there is no treatment available against post-stroke cognitive impairment and dementia, it is important to investigate if IPC can offer some degree of protection against post-stroke cognitive impairment.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a master transcription factor regulating cellular redox equilibrium by inducing the expression of myriad antioxidant genes 2, 12–16. In our previous study 17, we discovered that delayed IPC in mice activates the Nrf2 pathway, as indicated by the upregulation of heme oxygenase 1 (HO-1) in neurons, astrocytes, and endothelial cells. Among the ten categories of Nrf2 activators 18, the Michael reaction acceptors are the only endogenous activators of this transcription factor. Michael reaction acceptors are electrophiles, and the majority are end products of lipid peroxidation, including 4-hydroxynonenal (4-HNE) from omega-6 fatty acids and 4-hydroxy-hexenal (4-HHE) from omega-3 fatty acids 19–22.
Previous in vitro studies have demonstrated that preconditioning (PC) of astrocytes activated astrocytic Nrf2, which protected neurons against ischemic injury in astrocyte-neuron co-cultures 23, 24; however, it is unknown if direct PC of neurons can also prevent ischemic injury. It is well known that neurons are highly sensitive to oxidative stress and that PC is associated with sublethal oxidative stress 23, 25–27. On this backdrop, we hypothesize that PC may lead to mild oxidative stress in neurons via the generation of lipid electrophiles, followed by induction of self-defense mechanisms through electrophile-mediated Nrf2 activation and Nrf2-mediated transcription of Phase 2 antioxidant genes.
To address the abovementioned gaps and explore the potential mechanisms behind IPC, we choose delayed IPC in the present study, as it confers more robust neuroprotection than rapid IPC 28 and is dependent upon genetic reprogramming and de novo protein synthesis 2, 25, 29. We report that IPC can protect the brain against long-term sensorimotor and cognitive dysfunction and tissue loss after experimental ischemic stroke through the activation of Nrf2. Finally, to emphasize the importance of Nrf2 activation in long-lasting neuroprotection, we also performed a translational study with a potent and blood-brain barrier-permeable Nrf2 activator 2-cyano-3,12-dioxo-oleana-1,9(11)-dien-28-trifluoethyl amide (CDDO-TFEA), which exerted similar robust and enduring neuroprotective effects.
Methods
Animals and blinding
This study was designed in accordance with ARRIVE guidelines. All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) and carried out in accordance with the Stroke Treatment and Academic Roundtable (STAIR) guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male C57BL/6 mice and Nrf2 knockout (KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed with a 12:12 hr light-dark cycle at 22–24°C. The mice were randomly assigned to sham surgery (Sham), Stroke, and IPC+Stroke using a lottery-drawing box. All the outcome assessments were performed by investigators blinded to the group assignments.
Middle cerebral artery occlusion (MCAO)
Male wild-type (WT) and Nrf2 KO mice underwent MCAO on the left side for 12 min to induce IPC 3, 30, and 60 min to induce preclinical stroke 20, 31. These procedures were followed by reperfusion for the indicated durations. The interval between IPC and stroke was three days. The effects of IPC on ischemic outcomes in female mice have been reported in our previous study 17.
MCAO was performed with standard, previously published procedures 17, 20, 31. In brief, mice were anesthetized with 1.5% isoflurane in a 30% O2/70% N2O mixture under spontaneous breathing, and rectal temperature was maintained at 37.0 ± 0.5°C with a temperature-regulated heating pad. Mean arterial blood pressure was monitored with a tail-cuff. Under a surgical microscope, the left external, internal, and common carotid arteries were exposed through a midline neck incision. After coagulating and cutting the branches of the external carotid arteries, a 7–0 monofilament nylon suture with a silicone coat was inserted in the lumen of the external carotid artery and advanced to the origin of the middle cerebral artery via the internal carotid artery. The success of ischemia was confirmed by the measurement of regional cortical cerebral blood flow (rCBF) and the examination of neurological deficits 17.
Drug preparation and administration
2-cyano-3,12-dioxo-oleana-1,9(11)-dien-28-trifluoethyl amide (CDDO-TFEA) was purchased from Cayman Chemical (Ann Arbor, MI) and prepared according to previous reports 32. The mice received CDDO-TFEA (25 μmol/kg) or vehicle by gastric gavage in 200-μL volumes for three consecutive days before MCAO.
Behavioral tests
Sensorimotor functions were assessed with the Rotarod and adhesive removal tests beginning in the first week after stroke, and long-term cognitive function was assessed using the Morris water maze test for five weeks after stroke.
Rotarod test
Mice were trained for three consecutive days before surgery and were examined up to 35 days after stroke. After the mice were placed on the rods (IITC Life Science Inc., Woodland Hills, CA), the rods began to rotate and accelerate to 40 rpm within 300 s. The maximal length of each trial was 300 s. Results are presented as the duration that the mice were able to remain upright on the rods 17, 33, 34.
Adhesive removal test
Mice were trained for five consecutive days before surgery and were tested up to 7 days after stroke. An adhesive paper patch (3 × 3 mm) was attached to the distal radial region of the right forelimb. The time to remove the patch from the forelimb was recorded 17, 20, 35.
Morris water maze test
Mice were first trained in the Morris water maze for three days before surgery, and the tests were initiated 31 days after stroke when motor deficits have largely recovered in this model. The pool was 110 cm in diameter (SDI, San Diego Instruments, San Diego, CA), whereas the platform was 11 cm in diameter and submerged 1 cm under the surface of opaque water filled with non-toxic paint. Mice underwent one hidden platform test per day for five days, during which each mouse was released from one of 4 quadrants and allowed to search for the hidden platform for 90 seconds (learning phase of the test). At the end of each trial, the mouse was placed on the platform or allowed to remain on the platform for 30 seconds. Prominent spatial cues were arranged around the room. Four trials were performed per day for five consecutive days, with the location of the platform kept constant. Spatial learning abilities were measured as the time (in seconds) for the animal to reach the submerged platform on each day (latency). At day 35, a single, 60-s probe trial was performed in which the platform was removed. The time spent in the target quadrant where the platform was previously located was recorded (memory phase of the test). Swim speed was also recorded to assess gross locomotor function 20, 36.
Immunohistochemistry
Brains were sectioned in the coronal plane at 20 μm thickness and stained with anti-microtubule associated protein 2 (MAP2, 1:200; Santa Cruz Biotechnology, Dallas, TX). Tissue loss was calculated relative to the contralateral hemisphere by the following edema-corrected equation: (viable area of contralateral hemisphere - viable area of ipsilateral hemisphere) / viable area of the contralateral hemisphere). The viable areas in this equation were defined as the MAP2-immunoreactive zones 20, 34. Sections at the level of the dorsal hippocampus were immunostained with an antibody against the specific neuronal marker NeuN (1:200, Abcam). For cell counting, the numbers of NeuN-positive CA1 neurons per mm2 were counted by NIH Image J.
Primary neuronal cultures and oxygen-glucose deprivation (OGD)
Primary cultures of the rodent cortical neurons were dissected from E16–18 fetuses and maintained in Neurobasal media supplemented with B27 (Gibco, ThermoFisher Scientific, Pittsburgh, PA), as previously described 20, 31, 37. The experiments were performed 10–14 days after seeding cells in culture plates.
OGD was used to mimic in vitro ischemia with standard, previously published procedures 31. A brief duration of OGD (12 minutes) was used as a PC stimulus, prior to 1-h lethal OGD. The interval between PC and OGD was 16 h, and cells were returned to normal culture media and normal oxygenation for 24 h after OGD before harvest.
Western blots and Western slot blots
Total cell lysates from primary rat neurons were subjected to standard Western blot assays, as previously described 20, 31. Protein concentrations from total neuronal lysates were determined by Bradford protein assay (Bio-Rad, Hercules, CA). Equal amounts of proteins were subjected to electrophoresis, followed by transfer to polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA). Membranes were probed with antibodies recognizing HO-1 (1:1000, Enzo Life Science, Farmingdale, NY) and β-actin (1:3000, Sigma-Aldrich, St. Louis, MO). After incubation in secondary antibodies (Santa Cruz Biotechnology, Dallas, TX), membranes were incubated with chemiluminescent substrates (Pierce, ThermoFisher Scientific, Pittsburgh, PA) and developed with X-ray film. ImageJ software was used for gel analyses.
For the Western slot blot assays, proteins were loaded directly onto PVDF membranes using a Bio-Dot Microfiltration apparatus (Bio-Rad, Hercules, CA). Anti-4-HNE (1:1000, R&D, Minneapolis, MN) and anti-tubulin (1:2000, Abcam, Cambridge, MA) were used as the primary antibodies 17, 38. The remaining procedures were the same as described above for the Western blots.
Cell death and viability
Cell death and viability were measured by the Live/Dead cell viability assay (Molecular Probes, ThermoFisher Scientific, Pittsburgh, PA), the lactate dehydrogenase (LDH) release assay (Pointe Scientific Inc., Canton, MI), and the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis, MO). These assays were performed in triplicate, on at least three independent occasions.
The Live/Dead cell viability assay was performed according to the manufacturer’s instructions (Molecular Probes, Eugene, OR), as previously described 17, 20. In this assay, red dots (fluorescent ethidium homodimer-1) represent dead cells with compromised membranes, whereas green dots (fluorescent membrane-permeant calcein AM) represent live cells. The numbers of red dots were expressed as a function of red plus green dots and were presented as “cell death percentages”. For cell counting, three random fields were photographed per well by a blinded observer. Four to six wells per condition per experiment were selected, and the experiments were repeated on at least three independent occasions.
For the MTT assay, cells were incubated with 0.5 mg/mL MTT solution for 40 min, when purple precipitates became visible. After two washes, cells were lysed with dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO), in which the purple precipitates dissolved. Cell viabilities were then determined by absorbance at 595 nm, and data were expressed relative to the control group.
Extracellular LDH release by damaged cells was measured by an LDH detection kit (Pointe Scientific, Inc., Canton, MI), according to the manufacturer’s instructions. All data were expressed as the percentage LDH release relative to the control group.
Statistical analyses
The results are presented as the mean ± SD for the in vivo studies and mean ± SEM for in vitro studies. The difference between means was assessed by Student’s t-test (single comparisons) or by the appropriate analysis of variance (ANOVA, for multiple comparisons). GraphPad Prism software (version 7.0, La Jolla, CA, USA) was used for statistical analyses. A p value ≤ 0.05 was deemed statistically significant.
Results
IPC protects against long-term cognitive defects and brain tissue loss
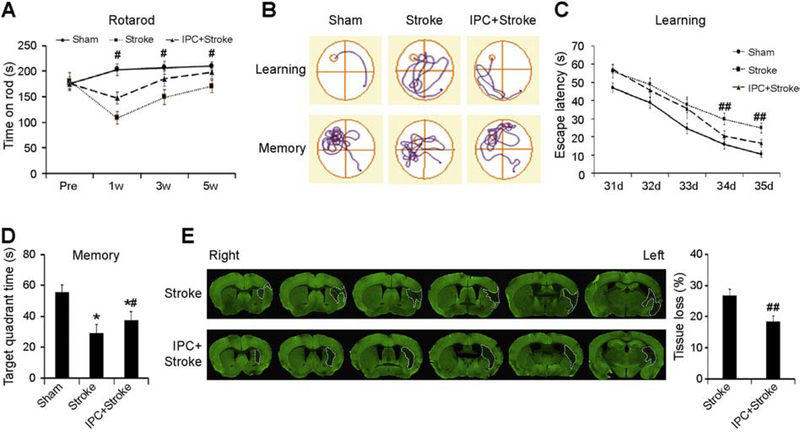
To determine if IPC offers long-term neuroprotection in mice, we performed the Rotarod test up to 5 weeks after stroke. As shown in Fig. 1A, IPC significantly improved sensorimotor function in this assay, for at least five weeks post-injury. To assess post-stroke cognitive function, we performed the Morris water maze tests in the fifth week after stroke. As shown in Fig. 1B–D, IPC significantly improved learning and memory capacities after stroke. Notably, swim speeds did not differ across groups, suggesting that the water maze results could not be attributed to differences in gross motor skills (Supplementary Fig. 1A). Immediately following the memory test at 35 d post-stroke, the mice were sacrificed, and the brains were harvested for immunostaining with the neuronal marker MAP2 to assess brain tissue loss. As shown in Fig. 1E, IPC significantly reduced tissue loss compared to the stroke group, indicating a long-term neuroprotective effect of IPC. Hippocampal neurons play an important role in learning and memory, particularly those in the CA1 fields. To investigate whether stroke and IPC affect CA1 neuron viability, we counted neurons immunostained for the specific neuronal marker NeuN. As shown in Supplementary Fig. 2, there were no significant differences between control and preconditioned groups in NeuN+ cell counts after MCAO. We also performed TUNEL staining to detect dead cells. While TUNEL-positive signals were visible at the rim of the infarction in the cortex, little signals could be detected in the hippocampi of both groups (Data not shown).
Figure 1. IPC improves long-term stroke outcomes in vivo.
IPC was induced in mice by 12-min MCAO, and stroke was induced by 60-min MCAO. (A) The latency to fall off the rod was recorded up to 5 weeks post-stroke in the Rotarod test. (B-D) Morris water maze tests were performed at 31–35 d after stroke. (B) Representative traces of swim paths of mice attempting to find a submerged platform (upper panels, defined as “learning”), and searching for the platform after its removal (lower panels, defined as “memory”). (C) The escape latency was recorded on days 31–35 after stroke, and (D) the time spent in the target quadrant after the platform was removed was plotted to reflect spatial memory. (E) Representative MAP2 staining and analyses of brain tissue loss at 35 d after stroke. Data are shown as mean ± SD, n=7–9 per group. *p≤0.05 vs Sham, #, ## p≤0.05, 0.01 for IPC+Stroke vs Stroke.
Nrf2 plays a key role in IPC-mediated long-term neuroprotection in vivo
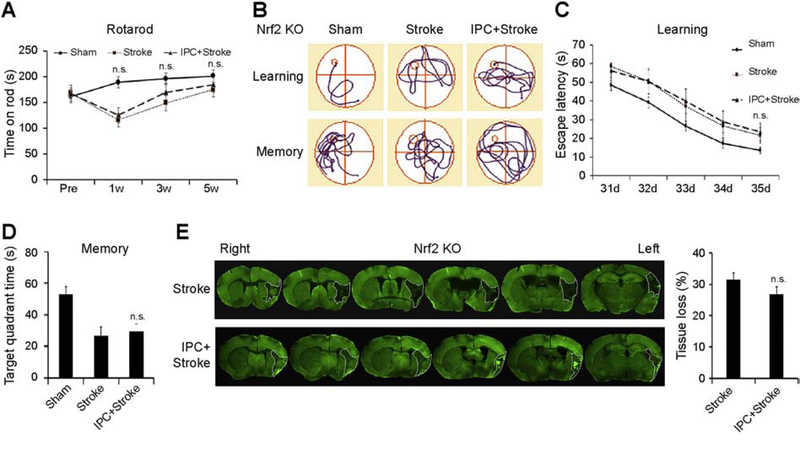
Next, we tested the hypothesis that Nrf2 is essential for IPC-mediated neuroprotection. For these mechanistic experiments, we preconditioned Nrf2 knockout (KO) mice and induced stroke three days later. As expected, neither sensorimotor function (Fig. 2A) nor cognitive function (Fig. 2B–D and Supplementary Fig. 1B) was preserved by IPC in Nrf2 KO mice. The measurements of brain tissue loss at 35 d post-stroke (Fig. 2E) further confirmed the reduction of IPC-mediated protection in Nrf2 KO mice. Collectively, these findings indicate that Nrf2 plays an important role in IPC-mediated long-term ischemic tolerance in vivo.
Figure 2. Key role of Nrf2 in IPC-mediated long-term neuroprotection in vivo.
IPC was induced in Nrf2 KO mice by 12-min MCAO, and stroke was induced by 60-min MCAO. (A) The latency to fall off the rod was recorded up to 5 weeks post-stroke in the Rotarod test. (B-D) Morris water maze tests were performed at 31–35 d after stroke. (B) Representative traces of swim paths of mice attempting to find a submerged platform (upper panels, defined as “learning”), and searching for the platform after its removal (lower panels, defined as “memory”). (C) The escape latency was recorded on days 31–35 after stroke, and (D) the time spent in the target quadrant after the platform was removed was plotted to reflect spatial memory. (E) Representative MAP2 staining and analyses of brain tissue loss at 35 d after stroke in mice. Data are shown as mean ± SD, n=7–9 per group. *p≤0.05 vs Sham. n.s. no significant difference between Stroke and IPC+Stroke mice.
In vitro PC neuroprotection against OGD is associated with mild oxidative stress
As mentioned in the Introduction, among the ten categories of Nrf2 activators 18, the only endogenous activators are lipid electrophiles, the end products of natural lipid peroxidation. Our previous work confirmed that IPC leads to the generation of sublethal amounts of 4-HNE, which then activates Nrf2 in mouse brains 17. In the present study, we examined whether this outcome also mediates PC-afforded neuronal protection in vitro.
Consistent with previous reports, PC protected primary neuronal cultures from lethal OGD, as indicated by live/dead staining and cell counting (Fig. 3A–B), as well as the MTT and LDH release assays (Fig. 3C–D) 39. In addition, PC also increased the levels of 4-HNE modified proteins (Fig. 3E) and HO-1, a reliable marker of Nrf2 activation 20, 31 (Fig. 3F). Notably, the increase in 4-HNE and HO-1 by PC was lower than with lethal OGD (Fig. 3E–F), suggesting that PC only elicits mild or sublethal oxidative stress, consistent with our in vivo findings 17. These data support the hypothesis that PC elicits a sublethal degree of oxidative stress and protects against OGD-induced neuron death.
Figure 3. Preconditioning (PC) protects against OGD-induced neuronal death and is associated with moderate oxidative stress.
Primary rat neuronal cultures were subjected to PC (12-min OGD) and lethal OGD (1-h OGD). (A) Representative Live/Dead cell staining, (B) dead neuron counting, (C) MTT assay, and (D) LDH release assay demonstrate that PC reduces neuronal death induced by lethal OGD. Total cell lysates were harvested for Western blots or Western slot blots. (E) Western slot blot showing a moderate and robust generation of 4-HNE modified proteins by PC and OGD, respectively. (F) Western blot showing a moderate and robust increase in HO-1 expression after PC and OGD, respectively. n=3–6 per group. *, **, *** p≤0.05, 0.01. 0.001 vs Ctrl. #, ### p≤0.05, 0.001 vs OGD. &&, &&& p≤0.01, 0.001 vs PC. Scale bar: 10 μm.
Nrf2 plays a key role in PC-mediated neuroprotection in vitro
Next, we determined whether Nrf2 was critical in PC-afforded neuroprotection using neurons derived from Nrf2 KO fetal mice. As expected, PC-mediated protection was abolished in Nrf2 KO neurons, as evidenced by Live/Dead staining and cell counting (Fig. 4A–B), as well as MTT (Fig. 4C) and LDH assays (Fig. 4D), indicating an indispensable role for Nrf2 in PC.
Figure 4. Key role of Nrf2 in PC-mediated neuroprotection in vitro.
Primary mouse neurons from Nrf2 KO mice were subjected to PC (12-min OGD) and lethal OGD (1-h OGD). (A) Live/Dead cell staining, (B) dead neuron counting, (C) MTT assay, and (D) LDH release assay demonstrate that PC fails to elicit neuroprotection in Nrf2 KO neurons. n=3–6, *** p≤0.001 vs Ctrl; n.s.: not significant. Scale bar: 10 μm.
Pharmacological activation of Nrf2 robustly preconditions the brain in vivo
A major goal in researching the mechanisms behind IPC is to translate the corresponding mechanism through pharmaceutical approaches 40, 41. To determine if the Nrf2-mediated protective mechanism is clinically translatable, we activated Nrf2 using the compound CDDO-TFEA, a potent triterpenoid with high blood-brain barrier permeability 42. As shown in Fig. 5, CDDO-TFEA pretreatment improved sensorimotor function (Fig. 5A–B) and learning and memory up to 35 days post-injury (Fig. 5C–D), without affecting gross motor functions (Supplementary Fig 1C). As expected, these neurological improvements were associated with reduced brain tissue loss (Fig. 5E). These results suggest that Nrf2 is an attractive target for pharmacological preconditioning against stroke in vivo.
Figure 5. Pharmacological activation of the Nrf2 pathway provides long-term protection against cerebral ischemia in vivo.
Mice were pretreated with CDDO-TFEA for three days and then subjected to stroke. (A) The Rotarod test and (B) adhesive removal test show that CDDO improves sensorimotor functions. (C, D) In the Morris water maze test, both learning and memory abilities are improved by CDDO. (E) Representative MAP2 staining and quantitative analyses of brain tissue loss at 35 d. Data are shown as mean ± SD, n=7–9 per group. *, **, ***p<0.05, 0.01, 0.001 vs. vehicle (Veh).
Discussion
The present study is the first to demonstrate that IPC provides long-lasting neuroprotection up to 35 days after stroke. IPC preserves both the functional and structural integrity of the brain in an Nrf2-dependent manner. At the cellular level, PC confers neuronal ischemic tolerance by lipid electrophile generation and Nrf2 activation. Finally, the use of CDDO-TFEA revealed that the Nrf2-mediated protective mechanism might be clinically translatable.
Previous studies have reported that both rapid and delayed IPC only elicit short-term protection against global ischemia 4, 43. In focal cerebral ischemia models of embolic or thrombotic stroke, long-lasting protection has only been reported following non-ischemic preconditioning, such as lipopolysaccharide 7, spreading depression 8, and asphyxia 6, 9; thus, whether long-lasting protection can be elicited by delayed ischemic PC was not known. Here, we show that delayed IPC confers long-term protection against stroke at both the structural and functional levels, as indicated by cognitive tests and infarct volume measurements up to 35 days after stroke.
Our findings of spatial memory tests are particularly important because post-stroke cognitive impairment and dementia are increasingly prevalent in the elderly population and pose a heavy socioeconomic burden 11, 44. The mechanisms of cognitive protection are unclear. Our results show that there no obvious CA1 neuronal loss, consistent with reports that the hippocampus is supplied by the posterior cerebral artery but not by the middle cerebral artery (MCA), and that MCAO does not cause frank hippocampal damage. However, MCAO causes white matter damage 45–47, and IPC reduces white matter injury, including the fibers that connect the hippocampus and thalamus 48, 49. Aside from the hippocampus, other brain regions are also critical for learning and memory, such as the frontal, parietal, and temporal lobes 50, and the MCA supplies the blood to these regions. As IPC significantly reduces infarct volumes after MCAO, the preserved brain regions likely contribute to the improvement of cognitive function. However, further experiments are needed to elucidate the exact mechanisms underlying cognitive protection in IPC.
Upon exploration of the mechanism underlying IPC-afforded neuroprotection, we focused on oxidative signaling and de novo protein synthesis. We observed that several Nrf2-controlled proteins are upregulated after IPC, including HO-1, glutamate-cysteine ligase, and the thioredoxins. When Nrf2 gene expression was ablated, ischemic tolerance was lost in the present study, consistent with previous studies revealing an indispensable role for Nrf2 23. Nrf2 has also been reported to mediate preconditioning with hyperbaric oxygen 51, limb ischemia 52, and inhaled anesthetic gas 53. Thus, the engagement of the Nrf2 pathway serves as a generalizable mechanism underlying ischemic tolerance against stroke.
We previously found that IPC leads to a mild increase in 4-HNE and widespread Nrf2 activation in the brain. Cortical neurons displayed a diffuse, albeit mild increase in HO-1 expression after IPC in mice 17. In the present study, we further reported that PC protected neurons via Nrf2 activation and that this may be mediated by the generation of lipid electrophiles. To our knowledge, we are the first to report the involvement of low levels of electrophiles in PC-mediated neuronal Nrf2 activation and self-protection against ischemic injury.
It is not fully understood how Nrf2 activation after IPC offers long-lasting protection against ischemic brain injury. De novo protein synthesis, controlled by transcription factors, is essential for the emergence of ischemic tolerance 2, 54. A key component of IPC is the interval between IPC and lethal stroke, which is necessary for subsiding the sublethal injury of IPC and the synthesis of new proteins. Nrf2 is a transcription factor that upregulates anti-oxidative enzymes 13, 55. Unlike small molecular antioxidants (glutathione, vitamin C, vitamin E) that are consumed during their anti-oxidative actions, anti-oxidative enzymes are not consumed during anti-oxidative reactions, as they operate catalytically; therefore, they exert long-term anti-oxidative effects 56. In addition to these enzymes, Nrf2 also upregulates anti-apoptotic proteins and several cell-type-specific proteins, which may also contribute to the long-lasting protection of IPC. These pre-existing cytoprotective proteins may reduce ischemic brain injury after lethal MCAO, especially in the penumbra regions. However, it is important to note that Nrf2 may not be the only transcription factor that contributes to IPC-mediated protection. Peroxisome proliferator-activated receptor-gamma (PPARγ) 57, heat shock factor-1 58, and hypoxia-inducible factor-1 also serve to reestablish redox and proteostatic equilibrium 59. Other transcriptions factors, such as PPARγ, can also be activated by lipid electrophiles 60, 61
There are two major goals of IPC studies. First, a superior understanding of the biological mechanisms underlying preconditioning is expected to accelerate the development of new neuroprotective strategies. To this end, pharmacological activation of Nrf2 with CDDO-TFEA for three days significantly reduced neurobehavioral dysfunction and infarct volume induced by focal ischemic stroke. These findings support our previous report that pretreatment with CDDO-Im protects hippocampal CA1 neurons after global ischemia in rats 31. Pharmaceutical preconditioning with alternative Nrf2 activators such as sulforaphane, triterpenoid derivatives, or fish oil 31, 62 also holds translational promise. Triterpenoid derivatives or fish oil might be especially useful in patients with a history of transient ischemic attacks or at high risk for stroke. Post-ischemic treatment with potent electrophiles to expedite and enhance Nrf2 activation has translational potential and may be more effective in combination with reperfusion therapy 31
The second goal of IPC studies is to guide its application in the clinic. Clinical investigations have shown that IPC can induce ischemic tolerance in humans 63–65. Based on current diagnostic approaches and techniques of ischemic stroke, however, it is difficult to predict when a stroke will occur, even in persons with obvious risk factors. Although this limits the use of IPC before the stroke onset to attenuate ischemic brain injury, there are also several scenarios in which IPC can be purposefully induced in the clinic. For example, IPC can be induced in patients with anticipated ischemic events, such as vascular neurosurgery 3, 66. Another tool to reduce stroke morbidity and infarct size in the clinic is remote preconditioning, which can be administered non-invasively and inexpensively with a blood pressure cuff 67. Finally, pharmaceutical preconditioning and alternative electrophiles may also offer promise in the clinic, especially those with strong potency, such as triterpenoid derivatives, sulforaphane, resveratrol, or fish oil 20, 31, 68–70. The latter method can be especially useful in patients with a history of transient ischemic attacks or at high risk for stroke. Future studies to determine Nrf2 activation in fibroblasts or plasma cells harvested from remotely preconditioned patients might also shed light on the underlying mechanisms.
Summary
In conclusion, we report here that IPC-mediated ischemic tolerance provides long-lasting protection against ischemic brain injury. Based on this study and our previous work, it seems likely that IPC elicits the generation of lipid electrophiles that activate the Nrf2 pathway and induce long-lasting protection. These mechanisms are applicable to the clinic, as oral administration of CDDO-TFEA provided a similar degree of long-term protection. Therefore, targeting Nrf2 is a promising strategy to protect the mammalian brain against stroke.
Supplementary Material
Supplementary Figure 1. Swim speeds in Morris Water Maze tests.
IPC and stroke did not alter swim speeds in (A) WT or (B) Nrf2 mice. (C) CDDO treatment also failed to change swim speeds. These results indicate that changes in learning and memory were not due to alterations in gross motor function.
Supplementary Figure 2. Hippocampal NeuN+ neurons after MCAO in mice.
Immunostaining of NeuN in the ipsilateral hippocampus from MCAO and IPC+MCAO groups in WT mice. High power images of CA1 neurons are shown in the lower right inset. NeuN+ cell counting failed to reveal any difference between the two groups. Scale bar = 200 μm.
Highlights.
Ischemic preconditioning provides long-lasting neuroprotection against sensorimotor and cognitive impairments as well as tissue loss after ischemic stroke; the protection is reduced in Nrf2 knockout mice.
Mechanistically, preconditioning leads to sublethal generation of lipid electrophiles, which then mediate endogenous activation of the Nrf2 pathway.
Pharmacological activation of Nrf2 provides a similar degree of long-term protection in translational studies.
Nrf2 is a promising therapeutic target with enduring protective effects against ischemic stroke.
Acknowledgements
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS092810. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jun Chen was supported by VA merit review grants and the VA Senior Research Career Scientist Award. We thank Pat Strickler for the administrative support.
Footnotes
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Tuo Yang, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA.
Yang Sun, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA.
Qianqian Li, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA.
Senmiao Li, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA.
Yejie Shi, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA.
Rehana K. Leak, Division of Pharmaceutical Sciences, Duquesne University, Pittsburgh, PA.
Jun Chen, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA; Geriatric Research, Educational and Clinical Center, Veterans Affairs Pittsburgh Health Care System, PA.
Feng Zhang, Department of Neurology, Pittsburgh Institute of Brain Disorders and Recovery, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, et al. ‘Ischemic tolerance’ phenomenon found in the brain. Brain research. 1990;528:21–24 [DOI] [PubMed] [Google Scholar]
- 2.Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. Journal of neurochemistry. 2004;89:73–89 [DOI] [PubMed] [Google Scholar]
- 3.Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, et al. Preconditioning provides neuroprotection in models of cns disease: Paradigms and clinical significance. Progress in neurobiology. 2014;114:58–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cerebr Blood F Met. 1997;17:175–182 [DOI] [PubMed] [Google Scholar]
- 5.Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:757–761 [DOI] [PubMed] [Google Scholar]
- 6.Ma D, Hossain M, Pettet GK, Luo Y, Lim T, Akimov S, et al. Xenon preconditioning reduces brain damage from neonatal asphyxia in rats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:199–208 [DOI] [PubMed] [Google Scholar]
- 7.Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J Neurosurg. 2005;103:715–723 [DOI] [PubMed] [Google Scholar]
- 8.Yanamoto H, Xue JH, Miyamoto S, Nagata I, Nakano Y, Murao K, et al. Spreading depression induces long-lasting brain protection against infarcted lesion development via bdnf gene-dependent mechanism. Brain research. 2004;1019:178–188 [DOI] [PubMed] [Google Scholar]
- 9.Vannucci RC, Towfighi J, Vannucci SJ. Hypoxic preconditioning and hypoxic-ischemic brain damage in the immature rat: Pathologic and metabolic correlates. Journal of neurochemistry. 1998;71:1215–1220 [DOI] [PubMed] [Google Scholar]
- 10.Mijajlovic MD, Pavlovic A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, et al. Post-stroke dementia - a comprehensive review. BMC medicine. 2017;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guekht A, Skoog I, Edmundson S, Zakharov V, Korczyn AD. Artemida trial (a randomized trial of efficacy, 12 months international double-blind actovegin): A randomized controlled trial to assess the efficacy of actovegin in poststroke cognitive impairment. Stroke; a journal of cerebral circulation. 2017;48:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shokeir AA, Hussein AM, Barakat N, Abdelaziz A, Elgarba M, Awadalla A. Activation of nuclear factor erythroid 2-related factor 2 (nrf2) and nrf-2-dependent genes by ischaemic pre-conditioning and post-conditioning: New adaptive endogenous protective responses against renal ischaemia/reperfusion injury. Acta Physiol (Oxf). 2014;210:342–353 [DOI] [PubMed] [Google Scholar]
- 13.Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of nrf2 and phase ii antioxidant enzymes in neuroprotection. Progress in neurobiology. 2013;100:30–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuadrado A, Kugler S, Lastres-Becker I. Pharmacological targeting of gsk-3 and nrf2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The keap1-nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shih AY, Li P, Murphy TH. A small-molecule-inducible nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10321–10335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang T, Sun Y, Mao L, Zhang M, Li Q, Zhang L, et al. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and nrf2 activation. Redox Biol. 2018;17:323–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtzclaw WD, Dinkova-Kostova AT, Talalay P. Protection against electrophile and oxidative stress by induction of phase 2 genes: The quest for the elusive sensor that responds to inducers. Adv Enzyme Regul. 2004;44:335–367 [DOI] [PubMed] [Google Scholar]
- 19.Guichardant M, Chantegrel B, Deshayes C, Doutheau A, Moliere P, Lagarde M. Specific markers of lipid peroxidation issued from n-3 and n-6 fatty acids. Biochemical Society transactions. 2004;32:139–140 [DOI] [PubMed] [Google Scholar]
- 20.Zhang M, Wang S, Mao L, Leak RK, Shi Y, Zhang W, et al. Omega-3 fatty acids protect the brain against ischemic injury by activating nrf2 and upregulating heme oxygenase 1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1903–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapple SJ, Cheng X, Mann GE. Effects of 4-hydroxynonenal on vascular endothelial and smooth muscle cell redox signaling and function in health and disease. Redox Biol. 2013;1:319–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in hbe1 cells. Redox Rep. 2007;12:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, Tsujita T, et al. Mild oxidative stress activates nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E1–2; author reply E3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning protects astrocytes against oxygen glucose deprivation via the nuclear erythroid 2-related factor 2 pathway. Translational stroke research. 2018;9:99–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puisieux F, Deplanque D, Bulckaen H, Maboudou P, Gele P, Lhermitte M, et al. Brain ischemic preconditioning is abolished by antioxidant drugs but does not up-regulate superoxide dismutase and glutathion peroxidase. Brain research. 2004;1027:30–37 [DOI] [PubMed] [Google Scholar]
- 26.Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigo R, Fernandez-Gajardo R, Gutierrez R, Matamala JM, Carrasco R, Miranda-Merchak A, et al. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol Disord Drug Targets. 2013;12:698–714 [DOI] [PubMed] [Google Scholar]
- 28.Durukan A, Tatlisumak T. Preconditioning-induced ischemic tolerance: A window into endogenous gearing for cerebroprotection. Experimental & translational stroke medicine. 2010;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nature reviews. Neuroscience. 2006;7:437–448 [DOI] [PubMed] [Google Scholar]
- 30.Stevens SL, Leung PY, Vartanian KB, Gopalan B, Yang T, Simon RP, et al. Multiple preconditioning paradigms converge on interferon regulatory factor-dependent signaling to promote tolerance to ischemic brain injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:8456–8463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Wang S, Zhang M, Weng Z, Li P, Gan Y, et al. Pharmacological induction of heme oxygenase-1 by a triterpenoid protects neurons against ischemic injury. Stroke; a journal of cerebral circulation. 2012;43:1390–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu M, Reddy NM, Higbee EM, Potteti HR, Noel S, Racusen L, et al. The nrf2 triterpenoid activator, cddo-imidazolide, protects kidneys from ischemia-reperfusion injury in mice. Kidney Int. 2014;85:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, et al. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death signaling. The Journal of neuroscience. 2008;28:13038–13055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stetler RA, Gao Y, Leak RK, Weng Z, Shi Y, Zhang L, et al. Ape1/ref-1 facilitates recovery of gray and white matter and neurological function after mild stroke injury. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E3558–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat. Protocols. 2009;4:1560–1564 [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun. 2016;7:10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sciarretta C, Minichiello L. The preparation of primary cortical neuron cultures and a practical application using immunofluorescent cytochemistry. Methods Mol Biol. 2010;633:221–231 [DOI] [PubMed] [Google Scholar]
- 38.Weber D, Milkovic L, Bennett SJ, Griffiths HR, Zarkovic N, Grune T. Measurement of hne-protein adducts in human plasma and serum by elisa-comparison of two primary antibodies. Redox Biol. 2013;1:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bae ON, Rajanikant K, Min J, Smith J, Baek SH, Serfozo K, et al. Lymphocyte cell kinase activation mediates neuroprotection during ischemic preconditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7278–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crippa JAD, Guimaraes FS, Joca S. Translational neuroscience: Narrowing distances for future discoveries. CNS Neurol Disord Drug Targets. 2015;14:962–962 [DOI] [PubMed] [Google Scholar]
- 41.De Souza Crippa JA, Guimaraes FS, Joca S. Editorial: Translational neuroscience: Narrowing distances for future discoveries. CNS Neurol Disord Drug Targets. 2015;14:962. [DOI] [PubMed] [Google Scholar]
- 42.Stack C, Ho D, Wille E, Calingasan NY, Williams C, Liby K, et al. Triterpenoids cddo-ethyl amide and cddo-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of huntington’s disease. Free radical biology & medicine. 2010;49:147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corbett D, Crooks P. Ischemic preconditioning: A long term survival study using behavioural and histological endpoints. Brain research. 1997;760:129–136 [DOI] [PubMed] [Google Scholar]
- 44.Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South london stroke register 1995–2010. Stroke; a journal of cerebral circulation. 2013;44:138–145 [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Povysheva N, Rose ME, Mi Z, Banton JS, Li W, et al. Role of uchl1 in axonal injury and functional recovery after cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Xia J, Zhang F, Shi Y, Wu Y, Pu H, et al. Galectin-1-secreting neural stem cells elicit long-term neuroprotection against ischemic brain injury. Sci Rep. 2015;5:9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, et al. Apurinic/apyrimidinic endonuclease ape1 is required for pacap-induced neuroprotection against global cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3204–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamner MA, Ye Z, Lee RV, Colman JR, Le T, Gong DC, et al. Ischemic preconditioning in white matter: Magnitude and mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35:15599–15611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumgartner P, El Amki M, Bracko O, Luft AR, Wegener S. Sensorimotor stroke alters hippocampo-thalamic network activity. Sci Rep. 2018;8:15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesner RP. Exploration of the neurobiological basis for a three-system, multiattribute model of memory. Curr Top Behav Neurosci. 2018;37:325–359 [DOI] [PubMed] [Google Scholar]
- 51.Zhai X, Lin H, Chen Y, Chen X, Shi J, Chen O, et al. Hyperbaric oxygen preconditioning ameliorates hypoxia-ischemia brain damage by activating nrf2 expression in vivo and in vitro. Free radical research. 2016;50:454–466 [DOI] [PubMed] [Google Scholar]
- 52.Chen M, Zhang M, Zhang X, Li J, Wang Y, Fan Y, et al. Limb ischemic preconditioning protects endothelium from oxidative stress by enhancing nrf2 translocation and upregulating expression of antioxidases. PloS one. 2015;10:e0128455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang T, Sun Y, Zhang F. Anti-oxidative aspect of inhaled anesthetic gases against acute brain injury. Medical gas research. 2016;6:223–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, et al. Ischemic preconditioning and brain tolerance: Temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke; a journal of cerebral circulation. 1998;29:1937–1950; discussion 1950–1931 [DOI] [PubMed] [Google Scholar]
- 55.Sun Y, Yang T, Leak RK, Chen J, Zhang F. Preventive and protective roles of dietary nrf2 activators against central nervous system diseases. CNS Neurol Disord Drug Targets. 2017;16:326–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52 Suppl 1:S128–138 [DOI] [PubMed] [Google Scholar]
- 57.Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, et al. Ischemic preconditioning reveals that glt1/eaat2 glutamate transporter is a novel ppargamma target gene involved in neuroprotection. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1327–1338 [DOI] [PubMed] [Google Scholar]
- 58.Tucker NR, Middleton RC, Le QP, Shelden EA. Hsf1 is essential for the resistance of zebrafish eye and brain tissues to hypoxia/reperfusion injury. PloS one. 2011;6:e22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valsecchi V, Pignataro G, Del Prete A, Sirabella R, Matrone C, Boscia F, et al. Ncx1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke; a journal of cerebral circulation. 2011;42:754–763 [DOI] [PubMed] [Google Scholar]
- 60.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-delta 12, 14-prostaglandin j2 is a ligand for the adipocyte determination factor ppar gamma. Cell. 1995;83:803–812 [DOI] [PubMed] [Google Scholar]
- 61.Ou Z, Zhao X, Labiche LA, Strong R, Grotta JC, Herrmann O, et al. Neuronal expression of peroxisome proliferator-activated receptor-gamma (ppargamma) and 15d-prostaglandin j2--mediated protection of brain after experimental cerebral ischemia in rat. Brain research. 2006;1096:196–203 [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neuroscience Letters. 2006;393:108–112 [DOI] [PubMed] [Google Scholar]
- 63.Schaller B Ischemic preconditioning as induction of ischemic tolerance after transient ischemic attacks in human brain: Its clinical relevance. Neurosci Lett. 2005;377:206–211 [DOI] [PubMed] [Google Scholar]
- 64.Weber R, Diener HC, Weimar C. Why do acute ischemic stroke patients with a preceding transient ischemic attack present with less severe strokes? - insights from the german stroke study. Eur Neurol. 2011;66:265–270 [DOI] [PubMed] [Google Scholar]
- 65.Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, et al. Transient ischemic attacks before ischemic stroke: Preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke; a journal of cerebral circulation. 2004;35:616–621 [DOI] [PubMed] [Google Scholar]
- 66.Narayanan SV, Dave KR, Perez-Pinzon MA. Ischemic preconditioning and clinical scenarios. Curr Opin Neurol. 2013;26:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861 [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Hu X, Yang W, Gao Y, Chen J. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic–ischemic brain injury through anti-inflammatory actions. Stroke; a journal of cerebral circulation. 2010;41:2341–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alfieri A, Srivastava S, Siow RCM, Cash D, Modo M, Duchen MR, et al. Sulforaphane preconditioning of the nrf2/ho-1 defense pathway protects the cerebral vasculature against blood–brain barrier disruption and neurological deficits in stroke. Free Radical Biology and Medicine. 2013;65:1012–1022 [DOI] [PubMed] [Google Scholar]
- 70.Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1141–1147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Swim speeds in Morris Water Maze tests.
IPC and stroke did not alter swim speeds in (A) WT or (B) Nrf2 mice. (C) CDDO treatment also failed to change swim speeds. These results indicate that changes in learning and memory were not due to alterations in gross motor function.
Supplementary Figure 2. Hippocampal NeuN+ neurons after MCAO in mice.
Immunostaining of NeuN in the ipsilateral hippocampus from MCAO and IPC+MCAO groups in WT mice. High power images of CA1 neurons are shown in the lower right inset. NeuN+ cell counting failed to reveal any difference between the two groups. Scale bar = 200 μm.