Abstract
Purpose.
To describe the characteristics of pediatric subjects who enroll in phase I trials, to determine the associations between pre-enrollment characteristics and the risk for toxicity, and to analyze response and survival outcomes.
Experimental Design.
Pre-enrollment characteristics and study outcomes were retrospectively analyzed for children with refractory solid tumors treated in one of 16 phase I trials with similar eligibility criteria at the National Cancer Institute between 1992 and 2005.
Results.
The 262 subjects analyzed had received a median of two (range, 0–9) prior chemotherapy regimens, and were on one (range, 0–12) concomitant medication. The Eastern Cooperative Oncology Group performance status scores for subjects were 0 (29%), 1 (48%), and 2 (19%); 19% had received a prior stem cell transplantation and 73% had received prior radiation. Approximately 90% of subjects were evaluable for the primary trial endpoints (toxicity and pharmacokinetics). Seventeen percent of subjects experienced a dose-limiting toxicity (DLT), 5% discontinued the study drug because of toxicity, and a drug-related death occurred in one subject (0.4%). Variables associated with a higher risk for developing a DLT, by multiple logistic regression analysis, were drug dose and prior radiation, for myelosuppressive agents, and drug dose and performance status, for nonmyelosuppressive agents. The complete and partial response rate was 4%; however, 17% of subjects had stable disease (received three or more cycles). The median overall survival time from the time of enrollment was five months.
Conclusions.
Primary trial objectives are achieved in approximately 90% of subjects with the standard phase I trial design and eligibility criteria despite the intensification of frontline and salvage therapies in pediatric subjects with cancer.
Keywords: Phase I, Pediatric oncology, Toxicity, Survival
Introduction
Phase I trials of new anticancer drugs are conducted separately in children with refractory cancers in order to identify a safe dose (usually the maximum-tolerated dose [MTD]) of the new agent in children and to describe the toxicity profile and pharmacokinetics of the drug in this population [1, 2]. The validity and generalizability of the dose and safety profile of new agents determined in pediatric phase I trials are influenced in part by the characteristics of the patients enrolled in the trials.
Frontline and retrieval chemotherapy regimens for many childhood cancers have become more complex and intensive in an effort to improve long-term cure rates. The intensification of treatment regimens has been facilitated by improvements in supportive care; however, the more intensive frontline regimens may render patients more intolerant of subsequent treatments and their cancers more refractory to any form of therapy [3]. The pediatric-to-adult MTD ratio in 1974 was 2.02, but it was only 0.76 20 years later in 1995 [4]. The intensification of therapy for childhood cancers may underlie the increasing similarity in tolerability between adult and pediatric patients over time, because prior therapy in pediatric patients can limit tolerance to investigational new agents, especially myelosuppressive drugs. In addition, the fraction of patients enrolled with certain types of tumors may have shifted over time as cure rates have improved.
These changes in pediatric cancer therapeutics may require new approaches to the clinical development of new agents for childhood cancers. Previous analyses of pediatric phase I trials, which were based on a retrospective review of published phase I trials, have been useful for identifying trends and have focused on the development of standardized recommendations for the design of pediatric phase I trials [1], the response rate and toxic death rate observed [5], and the safety and efficiency of pediatric phase I trials compared with adult studies [6]. However, there are few published reports examining the clinical characteristics of pediatric subjects at the time of entry into investigational drug studies and how these factors may impact primary trial outcomes. In addition, although most pediatric phase I trials require a minimum life expectancy of at least 8 weeks as an entry criterion, the survival time of children entered in pediatric phase I trials has not previously been analyzed.
Knowledge of the characteristics and outcomes of children enrolled in pediatric phase I trials may aid in the selection of subjects and design of future phase I trials and contribute to a better informed consent discussion on the risks and benefits for pediatric subjects who are considering enrollment in phase I trials. We retrospectively reviewed subjects enrolled at the National Cancer Institute (NCI) Pediatric Oncology Branch (POB) in phase I trials from 1992 to 2005. We describe the enrollment characteristics of these subjects and their association with the outcomes of the phase I trials, and radiographic response and overall survival.
Materials and Methods
Pediatric Phase I Clinical Trials
Sixteen pediatric phase I trials of systemically administered drugs conducted in patients with refractory solid tumors were analyzed. Phase I trials studying intrathecal agents (n = 3) were excluded in order to have a more homogeneous population. Multi-institutional trials not coordinated by the NCI were excluded. The trials were categorized according to whether the agent undergoing dose escalation was myelosuppressive (n = 7 trials), nonmyelosuppressive (n = 6 trials), or a combination of a myelosuppressive agent with a modulating agent (n = 3 trials). The individual trials are described in Table 1 [7–23]. Eleven of the investigational agents were administered i.v. and five were administered orally.
Table 1.
Pediatric Oncology Branch solid tumor phase I trials included in analysis
| Dose level |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study no. | Drug | Mechanism of action | Schedule | MTD/recommended dose | At MTD | Total n | n of patientsa | DLT | Study |
| Myelosuppressive agent | |||||||||
| 1 | Docetaxelb–d | Microtubule-stabilizing agent | 1-hour i.v. × 1 day every 21 days | 65 mg/m2 | 3e | 9 | 10 | M | Blaney et al. [10], Seibel et al. [11] |
| 2 | Ixabepilone | Microtubule-stabilizing agent | 1-hour i.v. daily × 5 days every 21 days | 8 mg/m2 | 4 | 5 | 13 | M, F | Widemann et al. [19] |
| 3 | Lipodoxb | Liposomal doxorubicin | 1-hour i.v. × 1 day every 21 days | 60 mg/m2 | 2 | 3 | 15 | M | Lowe et al. [8] |
| 4 | Paclitaxel | Microtubule-stabilizing agent | 3-hour i.v. × 1 day every 21 days | 250 mg/m2 | 2 | 3 | 9 | Ne, M | Berg et al. [20] |
| 5 | Pyrazoloacridined | DNA-binding agent | 1-hour i.v. × 1 day or 24-hour i.v. × 1 day every 21 days | 640 mg/m2 | 3 | 4 | 19 | M | Berg et al. [9] |
| 6 | Tipifarnibb | Farnesyltransferase inhibitor | Orally twice daily × 21 days every 28 days | 200 mg/m2 | 2 | 4 | 14 | M, R, GI | Widemann et al. [12] |
| 7 | Tomudexb | Antifolate, TS inhibition | 15-minute i.v. × 1 day every 21 days | 6 mg/m2 | 7 | 8 | 9 | M, H, GI, R | Widemann et al. [18] |
| Nonmyelosuppressive agent | |||||||||
| 8 | 9-Cis-retinoic acidb,d | Differentiating agent | Orally, three times a day × 28 days | ≤ 12 yrs, 35 mg/m2; >12 yrs, 85 mg/m2 |
1 3 |
2 4 |
15 | CNS | Adamson et al. [14] |
| 9 | ABT-751b | Microtubule-destabilizing agent | Orally daily × 7 days every 21 days Orally daily × 21 days |
200 mg/m2 100 mg/m2 |
4 2 |
5 4 |
20 7 |
Ne, HTN, F Ne, HTN, M, F, GI |
Fox et al. [13] Fox et al. [23] |
| 10 | ATRAf/INF-α2Ab,d | Differentiating agent/immunomodulator | Orally 3 days/wk/ 3 × 106 U/m2 s.c. 5 days/wk |
90 mg/m2 | 2 | 3 | 14 | CNS | Adamson et al. [32] |
| 11 | Phenylacetateb | Differentiating agent | 24-hour i.v. every 28 days | 9 gm/m2 | 4 | 5 | 20 | S | Serabe et al. [22] |
| 12 | Phenylbutyrateb | Differentiating agent | 24-hour i.v. every 28 days | 12.5 gm/m2 | 2 | 3 | 4 | S | Serabe et al. [21] |
| 13 | SU101d | PDGF receptor inhibitor | 96-hour i.v. every 21 days | 390 mg/m2 | 3 | 4 | 16 | CNS | Adamson et al. [15] |
| Modulating agent plus standard chemotherapy | |||||||||
| 14 | Lobradimilf/carboplatin | Bradykinin analogue/platination | 10-minute i.v. with carboplatin infusion × 2 days every 28 days | 600 ng/kg IBW | 4 | 4 | 21 | Noneg | Warren et al. [17] |
| 15 | Tariquidarf + CT | P-glycoprotein inhibitor/CT | 30-minute i.v. prior to CT dose every 21 days | 2 mg/kg | 3 | 3 | 19 | Noneg | Fox et al. [7] |
| 16 | Temozolomidef/O6BG | Alkylator/AGT inactivator | Orally/i.v. × 5 days every 28 days | 75 or 120 mg/m2 | 6h | 7h | 37 | M | Warren et al. [33] |
Number of patients enrolled in the trial at the NCI.
Multi-institutional trial.
Study included in Carlson et al. [4] review.
Study included in Lee et. al. [6] review.
MTD originally determined to be 65 mg/m2 (dose level 3). After establishing new eligibility criteria, excluding heavily pretreated patients, the MTD was determined to be 125 mg/m2 (dose level 6). After adding filgrastim, dose level 8 (185 mg/m2) was defined as the MTD.
Investigational agent used in stratification for regression analysis.
None observed for modulating agent.
First three dose levels required to achieve biologically active dose of O6BG at constant temozolomide dose. Abbreviations: AGT, 06 alklyguanine-DNA aklytransferase; ATRA, all trans retinoic acid; CNS, central nervous system; CT, chemotherapy (either docetaxel, vinorelbine, or doxorubicin); DLT, dose-limiting toxicity; F, fatigue; GI, nausea, vomiting, diarrhea, abdominal pain, or constipation; H, hepatotoxicity; HTN, hypertension; IBW, ideal body weight; INF, interferon; M, myelosuppression; MTD, maximum-tolerated dose; NCI, National Cancer Institute; Ne, neuropathy; O6BG, O6-benzylguanine; PDGF, platelet-derived growth factor; R, rash; S, somnolence; TS, thymidylate synthase.
The trials had similar eligibility criteria, which usually required an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 (or equivalent for the ixabepilone and ABT-751 trials that used Karnofsky/Lansky performance status scores), an expected survival duration of at least 8 weeks (except for the phase I trials of ABT-751, ixabepilone, and tariquidar, which did not include life expectancy as a criterion), serum transaminases ≤2 to 2.5 times the upper limit of normal and bilirubin ≤1.5 to 2.0 times the upper limit of normal, and an absolute neutrophil count >1,500/mm3 and platelet count >100,000/mm3. All studies required that subjects had recovered from the toxicity of any prior therapy prior to enrollment, but specific criteria varied. The majority of studies requested that at least 3 weeks had to have elapsed since the last dose of myelosuppressive chemotherapy and up to 6 weeks had to have elapsed if they had previously received a nitrosurea.
The protocol eligibility criteria that addressed prior radiation therapy varied in the 16 trials and included (refer to Table 1 for study numbers in parentheses):
Six protocols required that at least 4 weeks had to have passed prior to the last dose of radiation (studies 2, 3, 6, 9, 15, and 16).
Five protocols did not define time limitations since the completion of radiation therapy but did not consider subjects with prior extensive radiation evaluable for hematological toxicity (studies 4, 11, 12, 13, and 14).
Three protocols prohibited enrollment of subjects with extensive prior radiation, defined as central axis or hemipelvic radiation (studies 1, 5, and 7).
Two protocols did not address the timing or extent of prior radiation therapy (studies 8 and 10).
Eligibility criteria that addressed prior bone marrow or peripheral stem cell transplantation also varied by protocol and included:
Four studies required that at least 4 months had to have elapsed since transplant before enrollment (studies 3, 6, 15, and 16).
One study required that 6 months had to have elapsed since transplant (study 2).
One study required that 4 months had to have elapsed since an allogeneic transplant and 2 months had to have elapsed since an autologous transplant (study 9).
Five studies did not define time limitations following a prior transplant, but subjects who had been previously transplanted were not evaluable for hematological toxicity (studies 4, 11, 12, 13, and 14).
Two studies prohibited the enrollment of subjects who had a prior transplant (studies 1 and 5).
Three studies did not include eligibility criteria that addressed prior bone marrow or stem cell transplantation (studies 8, 7, and 10).
None of the studies analyzed had eligibility criteria that included graft versus host disease as an exclusion criterion.
Subject Selection
We reviewed the characteristics and outcomes of 262 subjects who had malignant solid tumors, who were enrolled in one of the pediatric phase I trials described in Table 1, and who were treated at the National Institutes of Health (NIH) Clinical Center between June 1992 and December 2005. Subjects with leukemia or nonmalignant solid tumors were excluded from the analysis. For 50 subjects who participated in more than one phase I trial, only their participation in their first phase I trial at the NCI was included in this analysis. Phase I protocol registries managed and updated by the clinical research staff at the NCI POB, hospital charts, and research charts were used for review and collection of data.
Pre-enrollment characteristics that were collected on each patient included age, sex, performance status, diagnosis, tumor location at diagnosis and at the time of enrollment in the phase I trial, baseline CBC, hepatic enzymes and bilirubin, number of prior myelosuppressive regimens, prior radiation therapy, prior stem cell transplantation, daily narcotic use, and concomitant medications. Concomitant medications were defined as regularly scheduled medications that subjects were taking prior to starting the phase I trial and excluded medications that were administered on an as needed schedule, vitamin supplements, and topical medications.
The outcomes collected on each subject included the highest grade (NCI Common Toxicity Criteria) of any toxicity that was experienced by the subject and judged to be at least possibly related to the investigational agent, the dose-limiting toxicities (DLTs) as defined by each protocol, hospitalizations, RBC and platelet transfusions during study, date of enrollment and date that off-study criteria were met, participation in pharmacokinetic studies, radiographic response, and date of death. For this analysis, subjects were considered evaluable for toxicity if they completed a treatment cycle or if they experienced a DLT during the first treatment cycle. Response was evaluated using the standard solid tumor response criteria (World Health Organization or Response Evaluation Criteria in Solid Tumors) [24, 25] specified by the phase I protocol. For this analysis, stable disease was defined as the absence of radiographic or clinical evidence of progressive disease, complete response, or partial response for three or more cycles.
Statistical Analysis
Pre-enrollment characteristics were analyzed as descriptive variables and summary statistics were generated. The relationship of DLT to pre-enrollment characteristics was evaluated by stratifying subjects into those who received myelosuppressive investigational agents and those who received nonmyelosuppressive agents. Three studies evaluated cytotoxic chemotherapy in combination with an investigational modulating agent described below. Subjects enrolled in the phase I trial of temozolomide (TMZ) in combination with the alkylguanine alkyltransferase inhibitor O6-benzylguanine (O6BG) were stratified to the myelosuppressive cohort, because the dose of TMZ, which is a myelosuppressive agent, was escalated after the previously defined optimal biological dose of O6BG was achieved. Subjects enrolled in the phase I trials of the P-glycoprotein inhibitor tariquidar in combination with cytotoxic chemotherapy (docetaxel, vinorelbine, or doxorubicin) and the blood-brain barrier modulator lobradimil in combination with carboplatin were stratified to the nonmyelosuppressive cohort, because the myelosuppressive agents were administered at a dose that was previously demonstrated to be tolerable and the doses of the nonmyelosuppressive investigational agents tariquidar and lobradimil were escalated. Therefore, only toxicities attributed to TMZ, tariquidar, or lobradimil were included in this analysis.
A logistic regression analysis assessing the relationship of DLT to each pre-enrollment characteristic was performed. A multiple logistic regression analysis stratified by myelosuppressive agent versus nonmyelosuppressive agent was performed using variables that were statistically significant (p ≤ .10) in the univariate analysis (included variables: age, sex, prior regimen, drug dose, performance status) or that were assessed to be clinically significant (included variables: concomitant medications, prior radiation, prior stem cell transplant, and narcotic use). Our analysis included age in years, drug dose expressed as a fraction of the MTD, concomitant medications, and number of prior regimens as continuous variables. Sex, prior radiation (any prior radiation versus none), performance status (ECOG performance status score 0 versus 1 or 2), prior stem cell transplant, and narcotic use were included as binary variables.
Using Kaplan–Meier survival analysis, overall survival was calculated from the time of enrollment to death. Statistical analyses were performed using STATA version 9.0 (College Station, TX).
Results
Pre-Enrollment Characteristics
Of the 262 subjects included in this analysis, 89 were enrolled in phase I trials of a myelosuppressive agent (n = 7 trials), 96 were enrolled in phase I trials of a nonmyelosuppressive (n = 6 trials) agent, and 77 were enrolled in phase I trials of a myelosuppressive agent combined with a modulating agent (n = 3 trials).
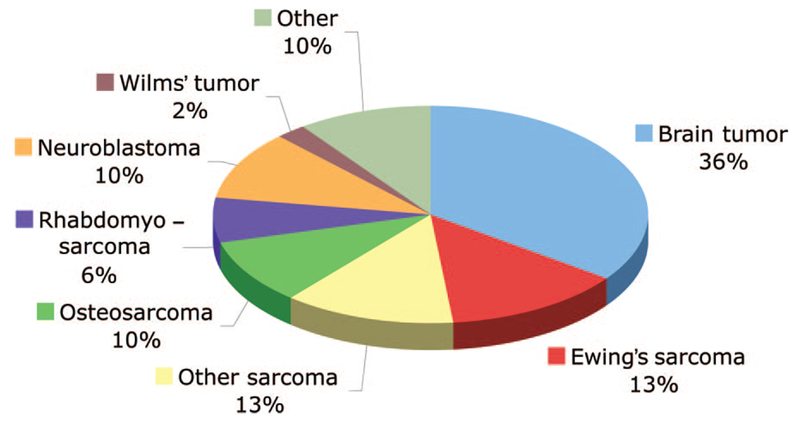
Pre-enrollment characteristics are described in detail in Table 2. The subjects were a heterogeneous group, and a variety of sarcomas (41%) and brain tumors (35%) were the most frequent diagnoses (Fig. 1). The median age of subjects was 14 years (range, 1–25) and more boys than girls were enrolled in phase I trials (62% versus 38%). The majority of subjects had a good performance status, the median number of concomitant medications was one, and only 16% of subjects required daily narcotics for pain. At diagnosis, only one third of subjects had metastatic disease; however, at the time of study enrollment, two thirds presented with metastases. The majority of subjects had received two or more prior myelosuppressive regimens (range, 0–9), 73% had received radiation therapy, and 19% had received a prior stem cell transplantation.
Table 2.
Baseline patient characteristics
| Characteristic | n = 262 | Frequency (%) |
|---|---|---|
| Sex | ||
| Male | 163 | 62 |
| Female | 99 | 38 |
| Age (years) | ||
| Median | 14 | |
| Range | 1–25 | |
| Performance status score | ||
| ECOG 0 | 75 | 29 |
| ECOG 1 | 127 | 48 |
| ECOG 2 | 50 | 19 |
| Unavailable | 10 | 4 |
| Tumor location | ||
| At diagnosis | ||
| Local | 171 | 65 |
| Metastatic | 88 | 34 |
| Unavailable | 3 | 1 |
| At study enrollment | ||
| Local | 84 | 32 |
| Metastatic | 173 | 66 |
| Unavailable | 5 | 2 |
| Baseline laboratory values (mean, standard deviation) | ||
| ANC (× 103/mm3) | 4.7 | 3.1 |
| Hemoglobin (g/dl) | 11.8 | 1.8 |
| Platelets (× 103/mm3) | 253 | 112 |
| Total bilirubin (mg/dl) | 0.5 | 0.46 |
| Prior regimens | ||
| Median | 2 | |
| Range | 0–9 | |
| Prior radiation | ||
| Yes | 190 | 73 |
| No | 68 | 26 |
| Unavailable | 4 | 1 |
| Prior stem cell transplantation | ||
| Yes | 50 | 19 |
| No | 210 | 80 |
| Unavailable | 2 | 1 |
| Daily narcotic use | ||
| Yes | 43 | 16 |
| No | 207 | 79 |
| Unavailable | 12 | 5 |
| Concomitant medication | ||
| Median | 1 | |
| Range | 0–12 | |
Abbreviations: ANC, absolute neutrophil count; ECOG, Eastern Cooperative Oncology Group.
Figure 1.
Diagnoses of the 262 patients enrolled in pediatric phase I trials at the National Cancer Institute. The diagnoses were: brain tumor (n = 92); Ewing’s sarcoma (n = 35); other sarcoma (n = 33), which includes alveolar soft part sarcoma, desmoplastic round cell tumor, synovial sarcoma, and undifferentiated sarcoma; osteosarcoma (n = 26); rhabdomyosarcoma (n = 17); neuroblastoma (n = 27); Wilms’ tumor (n = 6); and other (n = 26), which includes carcinoma (n = 15), lymphoma (n = 1), germ-cell tumor (n = 2), and other embryonal tumors (n = 8), such as hepatoblastoma, pancreatoblastoma, and pleuropulmonary blastoma.
Forty-two subjects (16%) were enrolled in two separate phase I trials at the NCI, and eight subjects (2%) were enrolled in three trials. Each trial enrolled at least one subject who had previously been in another phase I trial. The pre-enrollment characteristics of subjects who were enrolled in two or more studies (n = 50) were not statistically different from the characteristics of the subjects who were enrolled in only one study (n = 212).
Outcome Characteristics
Outcome characteristics are described in detail in Table 3. Approximately 90% of subjects who were enrolled in a phase I trial were evaluable for the primary endpoints of toxicity and pharmacokinetics. DLTs at any time during trial participation were observed in 17% of subjects (n = 44). Seventy-three percent of DLTs occurred during cycle 1 (n = 32), compared with 18% in the second cycle (n = 8) and 9% in the third cycle or beyond (n = 4). Grade 3 toxicities were experienced by 16% of subjects and were predominantly gastrointestinal and neurological. Neurotoxicities included sensory neuropathy, somnolence, pseudotumor cerebri, and ataxia. Grade 4 toxicities were experienced by 22% of subjects; 90% of these were hematological (83% of grade 4 toxicities were a result of neutropenia). One subject (0.4%) died from respiratory failure that was possibly related to the study drug, and 5% of subjects discontinued treatment with the study drug because of toxicity.
Table 3.
Study outcome characteristics
| Outcome | n = 262 | Frequency (%) |
|---|---|---|
| Participation in pharmacokinetic studies | ||
| Yes | 231 | 88 |
| No | 30 | 11 |
| Unavailable | 1 | <1 |
| Dose-limiting toxicity | ||
| Yes | 44 | 17 |
| No | 200 | 76 |
| Not evaluable | 18 | 7 |
| Grade 3 toxicity | ||
| Totala | 42 | 16 |
| Hematological | 4 | 10 |
| Gastrointestinal | 12 | 29 |
| Hepatobiliary | 5 | 12 |
| Neurological | 13 | 31 |
| Pain | 5 | 12 |
| Other | 3 | 7 |
| Unavailable | 8 | 3 |
| Grade 4 toxicity | ||
| Totala | 58 | 22 |
| Hematological | 52 | 90 |
| Gastrointestinal | 1 | 2 |
| Hepatobiliary | 1 | 2 |
| Neurological | 2 | 3 |
| Pain | 1 | 2 |
| Other | 1 | 2 |
| Unavailable | 4 | 2 |
| Response | ||
| Complete response | 4 | 2 |
| Partial response | 4 | 2 |
| Stable disease | 45 | 17 |
| Progressive disease | 183 | 70 |
| Not evaluable | 19 | 7 |
| Unavailable | 7 | 3 |
| n of cycles | ||
| Median | 1 | |
| Range | 0–31 | |
| Hospitalizations | ||
| Totala | 112 | 43 |
| Drug relatedb | 32 | 29 |
| Drug unrelated | 80 | 71 |
| Unavailable | 10 | 4 |
| Off-study reasons | ||
| Progressive disease | 222 | 85 |
| Withdrawal | 9 | 3 |
| Toxicity | 12 | 5 |
| Toxic deathb | 1 | <1 |
| Other | 10 | 4 |
| Unavailable | 8 | 3 |
Total n of subjects out of 262.
At least possibly related to study drug.
Twenty-one percent of subjects (n = 54) required a packed RBC transfusion and 13% (n = 33) required platelet transfusions while receiving an investigational agent in a phase I trial. Forty-three percent of subjects were hospitalized at least once while on study, but only 29% of hospital admissions were attributed to the study drug. Reasons for hospitalization unrelated to the study drug included infections, gastrointestinal events, neurological deterioration, and procedures.
Approximately 90% of subjects in these trials were evaluable for response to the study drug (Table 3). A complete or partial response was documented in 4% of subjects. All subjects who had an objective radiographic response had received a myelosuppressive agent. In addition, 17% of subjects had stable disease for ≥3 months. The median number of treatment cycles administered was one (range, 0–31) across all of the trials.
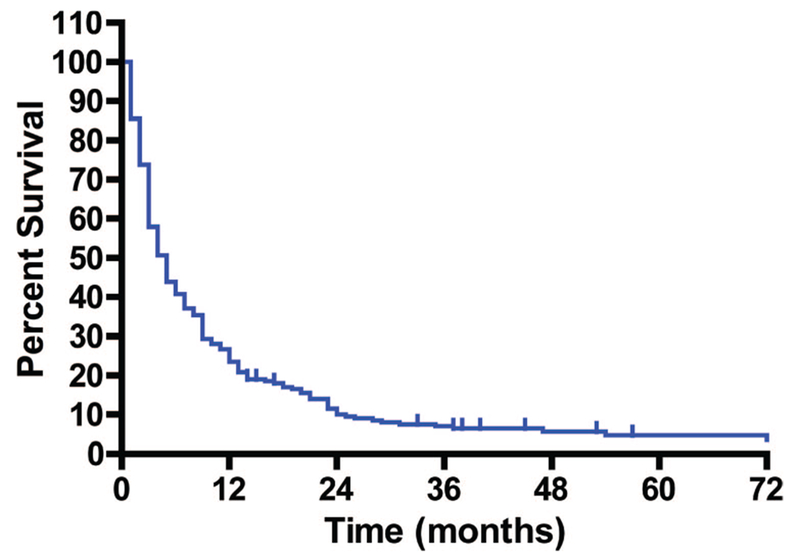
The median overall survival time from the time of enrollment in a phase I trial was 5 months (Fig. 2). Thirteen subjects survived >36 months. The diagnoses of these subjects were brain tumor (glioblastoma, n = 1; meningioma, n = 1; astrocytoma, n = 3; glioma, n = 2), malignant peripheral nerve sheath tumor (n = 2), and one of each with pancreatoblastoma, papillary thyroid carcinoma, squamous cell carcinoma, and alveolar soft part sarcoma.
Figure 2.
Overall survival in 262 pediatric subjects with refractory solid tumors who were enrolled in a phase I trial at the National Cancer Institute (NCI). Survival is plotted from the time of enrollment in the first phase I trial at the NCI.
Relationship of Toxicity to Pre-Enrollment Characteristics
The multiple logistic regression analysis to evaluate the relationship of DLT to pre-enrollment characteristics (Table 4) demonstrated that drug dose (expressed as a fraction of the MTD) was statistically significantly associated with the risk for a DLT regardless of the type of agent. Subjects who received myelosuppressive agents and who had received prior radiation therapy were 9.3-fold (95% confidence interval, 1.6–54.8) more likely to experience a DLT than those who did not receive prior radiation. However, the number of prior treatment regimens did not influence the risk of experiencing a DLT. The odds ratio for experiencing a DLT from a myelosuppressive agent was tenfold higher in girls than in boys, but there was no gender difference for nonmyelosuppressive agents. A separate analysis comparing differences in pre-enrollment characteristics between boys and girls revealed age (boys, 14 years versus girls, 11 years; p = .01) as the only statistically significant difference, and age was not associated with a higher risk for a DLT. There were no appreciable differences between the distributions of boys and girls across dose levels (p = .5). The primary difference was that grade 4 neutropenia occurred more frequently in girls. Among all DLTs observed in girls, 47% were related to neutropenia, compared with only 18% in boys. Subjects who received nonmyelosuppressive agents and who had an ECOG performance status score of 1 or 2 at the time of enrollment in the study were over three times more likely to develop a DLT than those with an ECOG performance status score of 0 at enrollment.
Table 4.
Multiple logistic regression model for risk of DLT
| Myelosuppressive agents |
Nonmyelosuppressive agents |
|||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Age | 1.0 | (0.9–1.2) | 1.0 | (0.9–1.1) |
| n of concomitant medications | 1.0 | (0.7–1.3) | 0.9 | (0.6–1.3) |
| Drug dosea | 93.9b | (4.2–2114) | 34.0b | (1.9–595) |
| n of prior regimens | 1.0 | (0.6–1.4) | 1.1 | (0.8–1.5) |
| Gender | ||||
| Male | 1.0 | - | 1.0 | - |
| Female | 10.1b | (2.3–44.8) | 0.8 | (0.2–2.5) |
| Prior radiation | ||||
| None | 1.0 | - | 1.0 | - |
| Yes | 9.3b | (1.6–54.8) | 1.2 | (0.2–6.7) |
| ECOG performance status score | ||||
| 0 | 1.0 | - | 1.0 | - |
| 1 or 2 | 0.6 | (0.2–1.6) | 3.4b | (1.3–8.8) |
| Prior transplant | ||||
| None | 1.0 | - | 1.0 | - |
| Yes | 1.9 | (0.2–21) | 1.4 | (0.4–5.2) |
| Narcotic use | ||||
| None | 1.0 | - | 1.0 | - |
| Yes | 3.7 | (0.6–22) | 0.8 | (0.1–4.9) |
Dose is expressed as a fraction of the MTD.
Significant at <5% significance level.
Abbreviations: CI, confidence interval; DLT, dose-limiting toxicity; ECOG, Eastern Cooperative Oncology Group; MTD, maximum-tolerated dose; OR, odds ratio.
Discussion
The clinical development of new drugs for childhood cancers is challenging because of the rarity of the diseases, improvements in standard treatments that have led to higher cure rates, and the availability of active retrieval regimens for children who experience a relapse [3,26]. The condition of subjects entering investigational drug studies and their ability to tolerate the investigational agent are likely to be affected by the nature of their prior therapy, and this may have an impact on the generalizability of the results (MTD) of the trial.
Our primary objective for this analysis was to describe the characteristics of pediatric subjects who were enrolled in our phase I trials in the current of era of intensive frontline and retrieval therapy and to determine whether pre-enrollment subject characteristics, including prior therapy, had an impact on the outcomes of phase I trials using a multivariate analysis. Our study represents a single institutional experience, which allowed for the analysis of detailed subject information generally not possible to obtain from a review of published phase I trials.
Pediatric subjects enrolled in our phase I trials were heavily pretreated, with 68% of subjects having received both prior chemotherapy and radiation therapy and more than half having previously received two or more myelosuppressive regimens. Among 82 subjects who received one or no prior myelosuppressive regimens, 77% (n = 63) had received radiation therapy. Only 1.5% (n = 4) of the subjects had received no prior therapy. This is identical to a previous study, in which 68% of pediatric subjects received both radiation and chemotherapy before phase I trial entry [4].
Despite extensive pretreatment, the majority of subjects had a good performance status, and did not require regular narcotic medications for tumor-related pain. Pediatric subjects were on a median of one concomitant medication (range, 0–12) at the time of enrollment; in contrast to adult subjects who receive a median of four (range, 0–22) concomitant medications at the time of enrollment in phase I trials [27]. Although a higher number of concomitant medications could increase the risk for drug interactions, they do not appear to influence the outcome of phase I trials in adults [27].
Approximately 90% of subjects enrolled in our phase I trials were evaluable for toxicity and pharmacokinetic endpoints. This serves as validation for the standard eligibility criteria used in these trials. Phase I trials were safe, with manageable toxicities and a DLT rate of 17%, which is similar to the DLT rate of 24% described by Lee et al. [6]. Most grade 3 drug-related toxicities were nonhematological, whereas grade 4 toxicities were almost exclusively hematological. The toxic death rate was 0.4%, which is similar to previously published reports of toxic death rates of 0.5% [6] and 0.7% [5] for other pediatric phase I trial reviews. The eligibility criteria used in our trials selected a population of subjects who tolerated the investigational agent with acceptable toxicity despite having substantial prior treatment, and 20% of the subjects subsequently were enrolled in one or more additional phase I trials at the NCI.
The low number of treatment cycles (median of one) received by subjects did not allow us to evaluate the investigational agents for chronic or cumulative toxicities. This limited drug exposure may impact the development of molecularly targeted agents, which typically require oral administration on a chronic continuous dosing schedule and may require multiple cycles to exhibit toxic or therapeutic effects. This limitation should be considered when designing phase II or upfront trials of these agents. Investigators need to carefully consider patient selection criteria, definition of response, and timing for restaging. They should also be aware of potential cumulative toxicities and design dose-modification strategies during the study, although this will unlikely affect the starting dose recommended from the phase I trial.
Another objective of this study was to determine whether pre-enrollment subject characteristics were associated with the risk of experiencing a DLT. For both myelosuppressive and nonmyelosuppressive investigational agents, drug dose, which was expressed as a fraction of the MTD, was the characteristic most strongly associated with the risk of experiencing a DLT. Although this confirmed relationship between drug dose and DLT is intuitive on the surface, a number of factors could have obscured the dose–response relationship in these trials, including:
The design of pediatric phase I trials, which typically use a starting dose that is 80% of the adult MTD, study a relatively narrow dose range compared with adult trials, and use smaller dose-escalation increments (25%–30%);
The small numbers of patients on each dose level; and
The inherent pharmacokinetic and pharmacodynamic variability of most anticancer drugs.
The fact that dose was associated with the risk of developing a DLT validates the trial design, which uses DLT as the primary determinant of the recommended dose of these agents in children.
This study is not amenable for the analysis of the relationship between pharmacokinetic parameters and toxicity across studies as it is not possible to normalize pharmacokinetic parameters for studies with different drugs. Of the 10 studies with available analyzed pharmacokinetic data, preliminary evidence of a relationship between a drug exposure parameter (area under the concentration–time curve [AUC]) and DLT was observed in two trials [14, 28], although two other trials did correlate AUC and degree of neutropenia [12, 9]. Given the relative infrequency of observing a DLT during a phase 1 trial, and the small number of subjects studied, it is not surprising that statistically significant relationships do not necessarily emerge.
The median number of dose levels studied per study was four (range, 3–8), similar to the findings of Lee et al. [6] (median, 4; range, 2–13). Although the dose level at which the MTD occurred usually translated to one dose level below the total number of dose levels studied, there were some exceptions. In the tipifamib study, for example, the dose was de-escalated a second time because of a DLT in the expanded cohort of patients.
Prior treatment with radiation therapy was associated with a greater risk for a DLT for subjects receiving myelosuppressive investigational agents (odds ratio, 9.3). Radiation therapy to marrow-containing bones may cause acute myelosuppression and decrease bone marrow reserve when a substantial fraction of marrow-producing bone is radiated [29, 30]. The observations that prior radiation was a risk factor for developing a DLT with myelosuppressive agents and that the number of prior chemotherapies was not a risk factor in our studies are consistent with a longer-lasting effect of radiation on bone marrow reserve.
Prior therapy, especially radiation, should be carefully delineated in the study eligibility criteria for investigational agents that are myelosuppressive. This is exemplified by a pediatric phase I trial of docetaxel [10]. In an initial cohort that included heavily pretreated subjects, myelosuppression was dose limiting and the MTD was 65 mg/m2. In a subsequent cohort that excluded subjects who had previously received central axis (pelvis, spine, cranium, ribs) radiation or more than two prior chemotherapy regimens, the docetaxel dose was safely escalated to 125 mg/m2.
For subjects receiving myelosuppressive agents, being female was associated with a statistically significantly higher risk for a DLT, primarily neutropenia. No statistical differences in measured pre-enrollment characteristics between boys and girls other than age were detected, and age was not associated with a higher risk for a DLT. This illustrates the need for looking at global differences in gender.
For subjects on nonmyelosuppressive agents, a worse performance status was associated with a greater risk for a DLT. The primary DLT seen for nonmyelosuppressive agents was neurological toxicity. Many neurological adverse events are graded in the NCI Common Toxicity Criteria by the degree to which the event interferes with “activities of daily living.” Subjects with a poorer baseline performance status as a result of the underlying disease that may already compromise daily life activities may be at a greater risk for developing dose-limiting neurological toxicities.
The likelihood of achieving an objective response (complete or partial response) was 4%, which is slightly lower than the previously reported rates of 9.6% [6] and 7.9% [5]. Our study population included a greater proportion of subjects with sarcoma and brain tumors (Fig. 1) than previously reported distributions [6]. Subjects with osteosarcoma and rhabdomyosarcoma had response rates of <3% in the review by Shah et al. [5]. In addition, our review included a larger subset (40%) of trials with nonmyelosuppressive agents than previous reviews, and these agents may be less likely to produce objective responses. Data on symptom relief and disease stabilization as measures of clinical benefit have not historically been captured in pediatric phase I trials [6]. Seventeen percent of our subjects had stable disease (received three or more cycles). In the era of molecularly targeted therapies where objective radiologic response may not be an appropriate end point for assessing activity, the evaluation of time to progression [31] or the use of more sensitive imaging methods to assess drug effects may be warranted.
Subjects who enroll in phase I trials are generally considered to have a very poor prognosis. We evaluated the overall survival time for our subjects from the time of enrollment in a phase I trial. The median survival time was 5 months, but 13 subjects with a variety of diagnoses survived >36 months, which is unexpected for subjects with refractory incurable disease. In some cases, the investigational agent appeared to contribute to the longer survival.
Conclusion
Continued progress in the treatment of pediatric cancer is dependent on the development of new therapeutic agents in an efficient and safe manner. Our analysis demonstrates that investigational agents can be administered safely and that the current primary trial endpoints for phase I trials are achievable, despite the changing paradigm of frontline and retrieval therapies for childhood cancers. This report may aid in the selection of subjects and design of phase I trials in pediatric oncology and allow more complete and informed discussions for physicians and patients on the risks and benefits of enrolling in phase I trials.
Acknowledgments
We thank Seth Steinberg for providing advice on the design of the study and analysis of the data. We thank the research nurses, Alberta Aikin, Andy Gillespie, Wendy Goodspeed, Anne Goodwin, Michelle O’Brien, and Patricia Whitcomb, for their dedication and support, without which none of our work would be possible.
This study was presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1–5, 2007, Chicago, IL. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The views expressed do not necessarily represent views of the NIH or the U.S. government.
Footnotes
Disclosure: The authors disclose that this article discusses the investigational use of the following products in pediatric phase I trials: docetaxel, ixabepilone, paclitaxel, pyrazoloacridine, tomudex, 9-cis-retinoic acid, ATRA/INF-α2A, phenylacetate, phenylbutyrate, and temozolomide/O6benzylguanine, provided by CTEP; lipodox, provided by Elan Pharmaceuticals; tipifarnib, provided by Janssen Research Foundation; ABT-751, provided by Abbott Laboratories; SU101, provided by SUGEN; lobradimil, provided by Alkermes; and tariquidar, provided by Xenova. No potential conflicts of interest were reported by the authors, planners, reviewers, or staff managers of this article.
References
- 1.Smith M, Bernstein M, Bleyer WA et al. Conduct of phase I trials in children with cancer. J Clin Oncol 1998;16:966–978. [DOI] [PubMed] [Google Scholar]
- 2.Adamson PC, Blaney SM. New approaches to drug development in pediatric oncology. Cancer J 2005;11:324–330. [DOI] [PubMed] [Google Scholar]
- 3.Balis FM. The Challenge of developing new therapies for childhood cancers. The Oncologist 1997;2:I–II. [PubMed] [Google Scholar]
- 4.Carlson L, Ho P, Smith M et al. Pediatric phase I drug tolerance: A review and comparison of recent adult and pediatric phase I trials. J Pediatr Hematol Oncol 1996;18:250–256. [DOI] [PubMed] [Google Scholar]
- 5.Shah S, Weitman S, Langevin AM et al. Phase I therapy trials in children with cancer. J Pediatr Hematol Oncol 1998;20:431–438. [DOI] [PubMed] [Google Scholar]
- 6.Lee DP, Skolnik JM, Adamson PC. Pediatric phase I trials in oncology: An analysis of study conduct efficiency. J Clin Oncol 2005;23:8431–8441. [DOI] [PubMed] [Google Scholar]
- 7.Fox E, Widemann BC, Chen CC et al. Pediatric phase I trial and pharmacokinetic study of P-glycoprotein inhibitor, tariquidar, in combination with doxorubicin, vinorelbine, or docetaxel. Proc Am Soc Clin Oncol 2004;23: 805. [Google Scholar]
- 8.Lowe EA, Adamson PC, Widemann BC et al. Phase 1 trial and pharmacokinetic study of liposomal doxorubicin (TLC-D99, Myocet™) in children withrefractory solid tumors. Proc Am Soc Clin Oncol 2002;21:109a. [Google Scholar]
- 9.Berg SL, Blaney SM, Adamson PC et al. Phase I trial and pharmacokinetic study of pyrazoloacridine in children and young adults with refractory cancers.J Clin Oncol 1998;16:181–186. [DOI] [PubMed] [Google Scholar]
- 10.Blaney SM, Seibel NL, O’Brien M et al. Phase I trial of docetaxel administered as a 1-hour infusion in children with refractory solid tumors: A collaborative pediatric branch, National Cancer Institute and Children’s Cancer Group trial. J Clin Oncol 1997;15:1538–1543. [DOI] [PubMed] [Google Scholar]
- 11.Seibel NL, Blaney SM, O’Brien M et al. Phase I trial of docetaxel with filgrastim support in pediatric patients with refractory solid tumors: A collaborative Pediatric Oncology Branch, National Cancer Institute and Children’s Cancer Group trial Clin Cancer Res 1999;5:733–737. [PubMed] [Google Scholar]
- 12.Widemann BC, Salzer WL, Arceci RJ et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol 2006;24:507–516. [DOI] [PubMed] [Google Scholar]
- 13.Fox E, Maris JM, Widemann BC et al. A phase 1 study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 7 days every 21 days in pediatric patients with solid tumors. Clin Cancer Res 2006;12: 4882–4887. [DOI] [PubMed] [Google Scholar]
- 14.Adamson PC, Widemann BC, Reaman GH et al. A phase I trial and pharmacokinetic study of 9-cis-retinoic acid (ALRT1057) in pediatric patients with refractory cancer: A joint Pediatric Oncology Branch, National Cancer Institute, and Children’s Cancer Group study. Clin Cancer Res 2001;7:3034–3039. [PubMed] [Google Scholar]
- 15.Adamson PC, Blaney SM, Widemann BC et al. Pediatric phase I trial and pharmacokinetic study of the platelet-derived growth factor (PDGF) receptor pathway inhibitor SU101. Cancer Chemother Pharmacol 2004;53:482–488. [DOI] [PubMed] [Google Scholar]
- 16.Adamson PC. Clinical and pharmacokinetic studies of all-trans-retinoic acid in pediatric patients with cancer. Leukemia 1994;8(suppl 3):S22–S25. [PubMed] [Google Scholar]
- 17.Warren KE, Patel MC, Aikin AA et al. Phase I trial of lobradimil (RMP-7) and carboplatin in children with brain tumors. Cancer Chemother Pharmacol 2001;48:275–282. [DOI] [PubMed] [Google Scholar]
- 18.Widemann B, Balis F, Reaman G et al. Pediatric phase I trial and pharmacokinetic (PK) study of ralitrexed (ZD1694, tomudex). Proc Am Soc Clin Oncol 1999;18:563a. [Google Scholar]
- 19.Widemann B, Fox E, Goodspeed W et al. Phase I trial of the epothilone B analog BMS-247550 (ixabepilone) in children with refractory solid tumors. Proc Am Soc Clin Oncol 2005;23:807s. [Google Scholar]
- 20.Berg SL, Blaney SM, Adamson PC et al. Phase I trial and pharmacokinetics of paclitaxel administered as a 3 hour infusion to children with refractory cancers. Proc Am Assoc Cancer Res 1998;39:322. [Google Scholar]
- 21.Serabe B, Adamson PC, Jakacki R et al. Phase I trial and pharmacokinetic study of sodium phenylbutyrate in children with refractory cancer. Child’s Nerv Syst 1998;14:512a. [Google Scholar]
- 22.Serabe B, Adamson PC, Wolfe R et al. Phase I trial and pharmacokinetic study of phenylacetate given as a 28-day continuous infusion in children. Proc Am Assoc Cancer Res 1997;38:224. [Google Scholar]
- 23.Fox E, Maris JM, Widemann BC et al. A phase I study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 21 days every 28 days in pediatric patients with solid tumors. Clin Cancer Res 2008;14: 1111–1115. [DOI] [PubMed] [Google Scholar]
- 24.Miller AB, Hoogstraten B, Staquet M et al. Reporting results of cancer treatment. Cancer 1981;47:207–214. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92: 205–216. [DOI] [PubMed] [Google Scholar]
- 26.Kurmasheva R, Morton C, Houghton PJ. Developing new agents for the treatment of childhood cancer. Curr Opin Investig Drugs 2005;6:1215–1227. [PubMed] [Google Scholar]
- 27.Genre D, Viens P, Von Hoff DD et al. Patients who are receiving concomitant medications should not systematically be excluded from phase I studies. Anticancer Drugs 1999;10:1–7. [DOI] [PubMed] [Google Scholar]
- 28.Fox E, Widemann BC, Maris JM et al. The pharmacokinetics and pharmacodynamics of ABT-751 in children with recurrent neuroblastoma and other solid tumors. Proc Am Soc Clin Oncol 2007;25:540s. [Google Scholar]
- 29.Young NS, Maciejewski JP. Aplastic anemia In: Hoffman R, Benz E, Shattil S et al. , eds. Hematology: Basic Principles and Practice, Fourth Edition Philadelphia: Churchill Livingstone, 2005:381–407. [Google Scholar]
- 30.Champlin R Bone marrow aplasia due to radiation accidents: Pathophysiology, assessment and treatment. Baillieres Clin Haematol 1989;2:69–82. [DOI] [PubMed] [Google Scholar]
- 31.Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. The Oncologist 2002;7:401–409. [DOI] [PubMed] [Google Scholar]
- 32.Adamson PC, Reaman G, Finklestein JZ et al. Phase I trial and pharmacokinetic study of all-trans-retinoic acid administered on an intermittent schedule in combination with interferon-alpha2a in pediatric patients with refractory cancer. J Clin Oncol 1997;15:3330–3337. [DOI] [PubMed] [Google Scholar]
- 33.Warren KE, Aikin AA, Libucha M et al. Phase I study of O6-benzylguanine and temozolomide administered daily for 5 days to pediatric patients with solid tumors. J Clin Oncol 2005;23:7646–7653. [DOI] [PubMed] [Google Scholar]