Abstract
Background
Ovarian cancer commonly presents at a late stage and is associated with poor prognosis. The most common histological subtype is serous ovarian carcinoma. Dual-specificity phosphatase 2 (DUSP2) is a protein phosphatase and substrate for mitogen-activated protein kinases (MAPKs) with increased expression levels in malignancy. This study aimed to evaluate the expression of DUSP2 in tumor tissues from patients with serous ovarian carcinoma and the association with tumor grade, stage, and patient survival and to investigate the effects of DUSP2 expression in SKOV3 and OVCAR3 cells in vitro.
Material/Methods
Tumor tissue and adjacent normal ovarian tissue from 127 patients with histologically confirmed serous ovarian carcinoma underwent quantitative real-time polymerase chain reaction (qRT-PCR) and immunohistochemistry to measure DUSP2 mRNA and protein expression, respectively. Tumor grade, stage, and clinicopathological data underwent correlation analysis with DUSP2 expression, and survival data were assessed with Kaplan-Meier and Cox regression analysis. The effects of DUSP2 expression on the proliferation and migration of SKOV3 and OVCAR3 cells were evaluated.
Results
Immunohistochemistry showed that DUSP2 was down-regulated in serous ovarian carcinoma tissues compared with adjacent ovarian tissues, and was significantly correlated with tumor stage. Survival analysis showed that DUSP2 expression was an independent risk factor for patient survival. DUSP2 expression in SKOV3 and OVCAR3 cells in vitro suppressed cell proliferation and migration.
Conclusions
Down-regulation of DUSP2 expression in serous ovarian carcinoma was an independent risk factor for patient survival, and its expression in SKOV3 and OVCAR3 cells inhibited cell proliferation and migration in vitro.
MeSH Keywords: Cell Proliferation, Ovarian Neoplasms, Prognosis
Background
Worldwide, ovarian carcinoma is one of the most prevalent gynecologic malignancies and includes four major histological subtypes, clear cell carcinoma, endometrioid carcinoma, mucinous adenocarcinomas, and serous carcinoma [1]. The different subtypes of ovarian carcinoma have distinct pathological characteristics and require different treatments [2,3]. Although there have been recent clinical advances to improve patient quality of life, the clinical outcome for patients with all subtypes of advanced-stage ovarian carcinoma has not improved. Serous ovarian carcinoma is the most frequent histological subtype of ovarian carcinoma [4]. Due to the lack of clinical symptoms associated with early-stage serous ovarian carcinoma and the lack of diagnostic biomarkers, most patients present with late-stage disease, which results in poor clinical prognosis. Serous ovarian carcinoma is a tumor that shows molecular heterogeneity, and gene expression profiling data have shown that gene expression profiles affect clinical outcome [5]. Therefore, the identification of diagnostic and prognostic biomarkers in serous ovarian carcinoma is clinically important.
Dual-specificity phosphatases (DUSPs) are members of the protein phosphatase family that can dephosphorylate serine, threonine, and tyrosine. The major substrates of DUSPs are mitogen-activated protein kinases (MAPKs), which include a Thr-Xaa-Tyr (TXY) motif [6]. MAPKs regulate cell proliferation and cell migration of malignant cells, and the roles of DUSPs have recently been studied in tumor progression. Inhibition of DUSP1 and DUSP6 have been shown to suppress the growth of malignant peripheral nerve sheath tumors (MPNSTs) [7], and DUSP1 has a role in the progression of small cell carcinoma of the prostate [8]. DUSP1 also has roles in the development of drug resistance in several tumor types [9–11]. Also, DUSP3 is highly expressed in prostate cancer [12], and silencing DUSP3 was shown to result in tumor cell-cycle arrest [13]. In contrast, dual-specificity phosphatase 2 (DUSP2) was reported to be down-regulated in bladder cancer and colon cancer [14–16]. However, the expression patterns and functions of DUSP2 in other tumor types remain unknown, and its potential role as a tumor suppressor remain to be investigated.
Therefore, this study aimed to evaluate the expression of DUSP2 in tumor tissues from patients with serous ovarian carcinoma and the association with tumor grade, stage, and patient survival and to investigate the effects of DUSP2 expression in SKOV3 and OVCAR3 cells in vitro.
Material and Methods
Patients with primary serous ovarian carcinoma
This study retrospectively enrolled 127 patients who had a histological diagnosis of primary serous ovarian carcinoma at the Yidu Central Hospital of Weifang, China and Jining No. 1 Peoples’ Hospital, Weifang, China. Ovarian tissue specimens, including fresh-frozen tissues and formalin-fixed, paraffin-embedded tissue samples, were obtained from the Department of Pathology. Written informed consent was obtained from all patients enrolled in this study. The study protocol complied with the Helsinki Declaration and was approved by the Research Ethics Committee of the Yidu Central Hospital of Weifang, China.
The median age of the 127 patients with serous ovarian carcinoma was 54 years, and the median follow-up from diagnosis was 55 months. All ovarian tumors were histologically graded, and the patients underwent staging according to the International Federation of Gynecology and Obstetrics (FIGO) criteria [17]. There were 38 patients with well-differentiated (G1) serous ovarian carcinoma, 41 patients with moderately-differentiated (G2) serous ovarian carcinoma, and 48 patients with poorly-differentiated (G3) serous ovarian carcinoma. Sixty-six patients had negative lymph nodes at the time of diagnosis, and 61 patients had lymph node metastasis. There were 85 patients (66.9%) who were FIGO stage I/II, and 42 patients (33.1%) who were FIGO stage III/IV. The serum CA-125 levels before surgical resection were recorded. The clinicopathological features of the 127 patients studied are summarized in Table 1.
Table 1.
Correlation between clinicopathologic characteristics and the expression of dual-specificity phosphatase 2 (DUSP2) in the 127 patients with serous ovarian carcinoma.
| Variables | Cases (n=127) | DUSP2 expression | P-value | |
|---|---|---|---|---|
| Low (n=48) | High (n=79) | |||
| Age (years) | 0.476 | |||
| ≤54 yrs | 66 | 23 (34.8%) | 43 (65.2%) | |
| >54 yrs | 61 | 25 (41.0%) | 36 (59.0%) | |
| Histopathological grade | 0.068 | |||
| G1 | 38 | 9 (23.7%) | 29 (76.3%) | |
| G2 | 41 | 20 (48.8%) | 21 (51.2%) | |
| G3 | 48 | 19 (39.6%) | 29 (60.4%) | |
| CA-125 level | 0.414 | |||
| ≤400 U/mL | 72 | 23 (31.9%) | 47 (65.3%) | |
| >400 U/mL | 55 | 23 (41.8%) | 32 (58.2%) | |
| Lymph node metastasis | <0.001* | |||
| Negative | 66 | 14 (21.2%) | 52 (78.8%) | |
| Positive | 61 | 34 (55.7%) | 27 (44.3%) | |
| FIGO stage | 0.017* | |||
| I–II | 85 | 26 (30.6%) | 59 (69.4%) | |
| III–IV | 42 | 22 (52.4%) | 20 (47.6%) | |
P<0.05 represented statistical significance.
FIGO – International Federation of Gynecology and Obstetrics.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from clinical tissues from 22 patients with serous ovarian carcinoma using TRIzol. The cDNA was reverse-transcribed using 1 μg of total RNA using the cDNA Synthesis Kit, according to the manufacturer’s instructions (Takara, Minato-ku, Tokyo, Japan). The qRT-PCR was then performed using the ABI Prism 7700 sequence detector system (Applied Biosystems, Branchburg, NJ, USA), according to the manufacturer’s protocol. The mRNA levels of dual-specificity phosphatase 2 (DUSP2) in tumor tissues and adjacent tissues were normalized to GAPDH and quantified using the ΔCt method [18]. The primers used included:
DUSP2, forward: 5′-TTTGAGGGCCTTTTCCGCTACAAGAG-3′;
DUSP2, reverse: 5′-GCCTCCGCTGTTCTTCACCCAGTC-3′;
GAPDH, forward: 5′-CCACCCATGGCAAATTCCATGGCA-3′;
GAPDH, reverse: 5′-TCTAGACGGCAGGTCAGGTCCAC-3′.
Immunohistochemistry
Immunohistochemical staining for DUSP2 was performed using 4 μm tissue sections prepared from archived formalin-fixed, paraffin-embedded tissue samples. Briefly, the tissue sections were deparaffinized, rehydrated, and processed in EDTA antigen retrieval buffer and then autoclaved at 121°C for 4 min for antigen retrieval. Tissue sections were incubated in 3% H2O2 for 10 min to block endogenous peroxidase activity. Nonspecific antigen binding was blocked by incubation with normal goat serum. Tissue sections were incubated with a primary antibody to DUSP2 (dilution, 1: 200) (Cat. No. LS-B14289) (Lifespan Bioscience, Seattle, WA, USA) and counterstained with hematoxylin.
Two pathologists, who were unaware of the patient clinicopathological and outcome data, reviewed and evaluated the immunohistochemistry results independently. Immunohistochemistry staining was assessed by light microscopy as the percentage of positively stained tumor cells and staining intensity. The percentage of positively stained cells was scored as 0 (negative), 1 (0–25% positive), 2 (25–50% positive), 3 (50–75% positive), or 4 (75–100% positive). The staining intensity was scored as 0 (negative), 1 (weak staining), 2 (medium staining), or 3 (high staining). The final immunohistochemistry score was calculated by multiplying the two scores (range, 0–12). The patients were then grouped into two subgroups based on the receiver operating characteristic (ROC) curve analysis. Patients with an immunohistochemistry score >7 were the high DUSP2 expression group, and the low DUSP2 expression group had an immunohistochemistry score of <7.
Cell culture and transfection
Two serous ovarian carcinoma cell lines, SKOV3 and OVCAR3, were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA), Nontumorous ovarian surface epithelial (OSE) cells were obtained from ScienCell Research Laboratories (Carlsbad, CA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) medium containing 10% fetal bovine serum (FBS) and maintained at 37°C in 95% air and 5% CO2.
Transfection with a DUSP2-plasmid or DUSP2-siRNA were performed using Lipofectamine 3000 reagent (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The transfection reagent was used as the control. The siRNA target sequence for DUSP2 was 5′-GCAUCACAGCCGUCCUCAATT-3′. To further explore the role of ERK1/2 in the anti-tumor effects of DUSP2, cells transfected with DUSP2-siRNA were simultaneously treated with the ERK1/2 inhibitor, PD98059 [19].
Western blot
Transfected cells at 80–90% confluence were washed with ice-cold phosphate-buffered saline (PBS) and lysed on ice using RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) containing a cocktail of a protease inhibitor and a phosphatase inhibitor (Roche Applied Sciences, Mannheim, Germany). The protein concentration was measured using the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Approximately 20 μg of protein was used for Western blot. Equal amounts of protein were separated by electrophoresis on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% BSA for 1h at room temperature, the membranes were incubated at 4°C overnight in primary antibodies to DUSP2 (#LS-B14289, Lifespan Bioscience, Seattle, WA, USA), GAPDH (#sc-47724, Santa Cruz Biotechnology Inc., Dallas, TX, USA), ERK1/2 (#4695, Cell Signaling Technology, Danvers, MA, USA), anti-pTpY-ERK1/2 (#9101, Cell Signaling Technology, Danvers, MA, USA). After washing with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein detection was performed using enhanced chemiluminescence (ECL) reagent.
Cell proliferation and migration assays
At 48 hours after transfection, SKOV3 and OVCAR3 cells were seeded into 96-well plates at a density of 104 cells/well. Cell viability was evaluated with the cell counting kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Mashiki, Japan) at directed time points by adding 10 μl of CCK-8 reagent into each well and incubated for another 4 h at 37°C. Absorbance was determined with a microplate reader at 450 nm. Proliferation curves were plotted using GraphPad version 6.0 software (GraphPad Software, La Jolla, CA, USA). All experiments were performed in triplicate.
Cell migration capacity was evaluated using a wound-healing assay. Cells were plated into six-well plates and incubated until they reached 100% confluence. Artificial wounds were created with a 10 μl pipette tip and washed with PBS to remove the detached cells. The medium was replaced with fresh culture medium without serum. The wound closure was monitored at 0 h and 24 h using microscopy, and the wound area was quantified by image analysis.
Statistical analysis
Data were analyzed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). Comparison between groups was performed with a paired or unpaired Student’s t-test. The chi-squared (χ2) test was used to compare clinical variables. Survival curves were plotted using the Kaplan-Meier method, and differences were compared using the log-rank test. Cox regression analysis was used to evaluate the independent prognostic factors for overall survival (OS). Data were expressed as the mean±standard deviation (SD) from at least three different experiments. A P-value <0.05 was considered to be statistically significant.
Results
Clinicopathological findings in patients with serous ovarian carcinoma and the expression of dual-specificity phosphatase 2 (DUSP2)
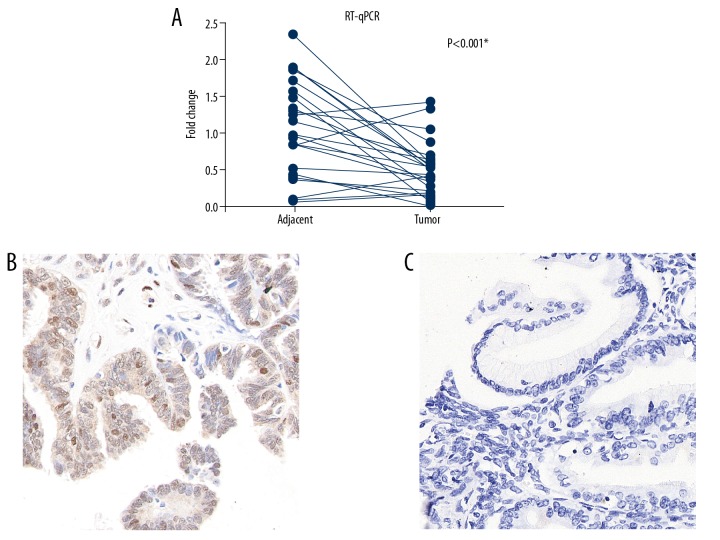
This study included 22 pairs of tissue from serous ovarian carcinoma and adjacent nontumorous tissues from surgical resection specimens. The mRNA levels of DUSP2 were compared by quantitative real-time polymerase chain reaction (qRT-PCR). DUSP2 mRNA expression was significantly down-regulated in 17/22 patients (77.3%) (Figure 1A, P<0.001).
Figure 1.
Dual-specificity phosphatase 2 (DUSP2) was down-regulated in serous ovarian carcinoma. (A) Comparison of the mRNA level of DUSP2 measured by quantitative real-time polymerase chain reaction (qRT-PCR) from 27 paired serous ovarian carcinoma and adjacent normal ovarian tissues show that DUSP2 transcription was down-regulated in serous ovarian carcinoma. * P<0.05 (paired Student’s t-test). (B) Representative photomicrograph of the immunohistochemistry findings for high expression of DUSP2 in serous ovarian carcinoma tissue showing both nuclear and cytoplasmic immunostaining. Magnification, ×400. (C) Representative photomicrograph of the immunohistochemistry findings for low expression of DUSP2 in serous ovarian carcinoma tissue. Magnification, ×400.
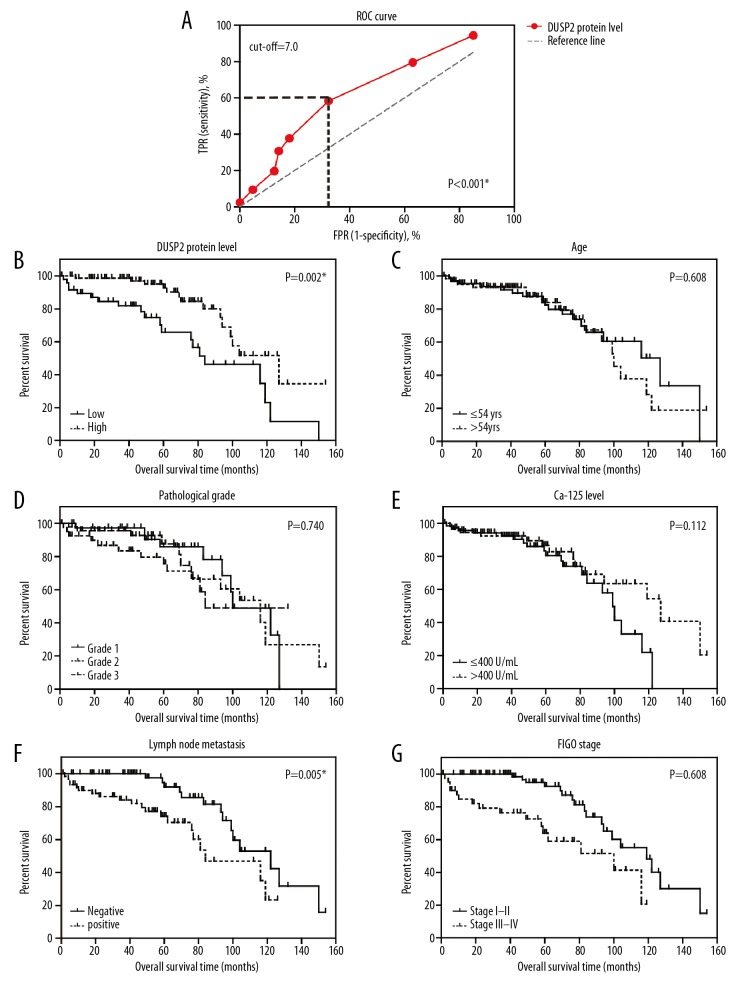
The protein levels of DUSP2 were evaluated using immunohistochemistry (Figure 1B, 1C). According to the immunostaining results, DUSP2 was localized to both the cell nucleus and the cytoplasm. There were different immunostaining patterns for DUSP2 expression in the tumor tissues from different patients, and so the patients were divided into a low DUSP2 expression group and a high DUSP2 expression group, based on the immunohistochemistry scores. The cut-off immunohistochemistry score was established at 7.0, according to the findings of the receiver operating characteristic (ROC) curve analysis (Figure 2A).
Figure 2.
Low expression levels of dual-specificity phosphatase 2 (DUSP2) were associated with reduced overall survival (OS) in patients with serous ovarian carcinoma. (A) The receiver operating characteristic (ROC) curve analysis for the immunohistochemistry scores, plotted to identify the cut-off value for dividing patients into the low DUSP2 group and the high DUSP2 group. The cut-off immunohistochemistry score was 7.0, with a Youden index of 0.26. (B) The clinical significance of DUSP2 expression was evaluated by Kaplan-Meier survival analysis, and compared by the log-rank test, showing that high expression of DUSP2 was associated with improved overall survival. The prognostic effects of age (C), histopathological tumor grade (D), serum CA-125 level (E), lymph node metastasis (F), and tumor stage (G), were assessed by the Kaplan-Meier method.
There was no significant correlation between the protein expression levels of DUSP2 and patient age, histological tumor grade, or preoperative serum level of CA-125 (Table 1). However, lower levels of DUSP2 were significantly correlated with lymph node metastases (P<0.001), indicating that DUSP2 might inhibit tumor invasion. Patients with significantly lower DUSP2 expression levels were more frequently diagnosed at stage III–IV serous ovarian carcinoma, while those with significantly higher DUSP2 expression levels were more frequently diagnosed at stage I–II serous ovarian carcinoma (P=0.017). The negative correlation between DUSP2 protein expression and disease progression implied that DUSP2 might have a tumor suppressor role in serous ovarian carcinoma.
Low DUSP2 expression levels were associated with reduced overall survival (OS) in patients with serous ovarian carcinoma
The 5-year overall survival (OS) rate of the study cohort was 83.23%, with a median survival time of 116 months. The clinical significance of each clinicopathological factor evaluated was further analyzed using the Kaplan-Meier method (Figure 2B), and showed that patients with increased expression levels of DUSP2 had an improved overall survival time. The overall survival time for patients in the low DUSP2 group was 85.3±8.5 months, compared with 115.2±7.5 months for patients in the high DUSP2 group (P=0.002). The five-year overall survival rate of the low DUSP2 group (65.9%) was also significantly lower than that of the high DUSP2 group (92.8%).
In addition to the expression levels of DUSP2, the effects of conventional clinicopathological characteristics on patient prognosis were assessed (Figure 2C–2G) (Table 2). Lymph node metastasis was a significantly unfavorable prognostic factor (P=0.005), as was the tumor stage (P=0.002). Patients with advanced advanced-stage (III–IV) serous ovarian carcinoma had significantly reduced overall survival time (77.8±7.7 months) compared with patients with early-stage (I–II) serous ovarian carcinoma (113.1±6.3 months).
Table 2.
Univariate analysis of clinicopathological characteristics and patient prognosis.
| Variables | Cases (n=127) | Overall survival | P-value | |
|---|---|---|---|---|
| Mean±SD (months) | 5-year OS (%) | |||
| Age (years) | 0.608 | |||
| ≤54 yrs | 66 | 107.3±7.9 | 82.5% | |
| >54 yrs | 61 | 99.4±7.9 | 84.0% | |
| Histopathological grade | 0.740 | |||
| G1 | 38 | 102.5±6.7 | 86.0% | |
| G2 | 41 | 98.1±9.7 | 75.7% | |
| G3 | 48 | 98.9±8.4 | 87.6% | |
| CA-125 level | 0.112 | |||
| ≤400 U/mL | 72 | 90.5±5.2 | 86.0% | |
| >400 U/mL | 55 | 112.4±8.3 | 86.6% | |
| Lymph node metastasis | 0.005* | |||
| Negative | 66 | 115.1±6.9 | 92.0% | |
| Positive | 61 | 85.2±6.7 | 74.2% | |
| FIGO stage | 0.001* | |||
| I–II | 85 | 113.1±6.3 | 92.5% | |
| III–IV | 42 | 77.8±7.7 | 64.0% | |
| DUSP2 expression | 0.002* | |||
| Low | 48 | 85.3±8.5 | 65.9% | |
| High | 79 | 115.2±7.5 | 92.8% | |
P<0.05 represented statistical significance.
FIGO – International Federation of Gynecology and Obstetrics; OS – overall survival; SD – standard deviation; DUSP2 – dual-specificity phosphatase 2.
Cox regression analysis was used to identify independent prognostic factors in patients with serous ovarian carcinoma. (Table 3). Advanced stage (P=0.007) and low DUSP2 levels (P=0.048) were identified as independent risk factors for the overall survival of patients with serous ovarian carcinoma. Although lymph node metastasis showed no significant difference (P=0.055), the hazard ratio (HR) was 2.12 for patients with positive lymph nodes (95% CI, 0.98–4.55).
Table 3.
Multivariate analysis of clinicopathological characteristics and patient prognosis.
| Variables | Hazard ratio (HR) | 95% confidence interval (CI) | P-value |
|---|---|---|---|
| Lymph node metastasis (positive vs. negative) | 2.12 | 0.98–4.55 | 0.055 |
| FIGO stage (III/IV vs. I/II) | 2.68 | 1.31–5.51 | 0.007* |
| DUSP2 expression (high vs. low) | 0.48 | 0.23–0.99 | 0.048* |
P<0.05 represented statistical significance.
FIGO – International Federation of Gynecology and Obstetrics; DUSP2 – dual-specificity phosphatase 2.
DUSP2 expression inhibited cell proliferation and migration in SKOV3 and OVCAR3 cells in vitro
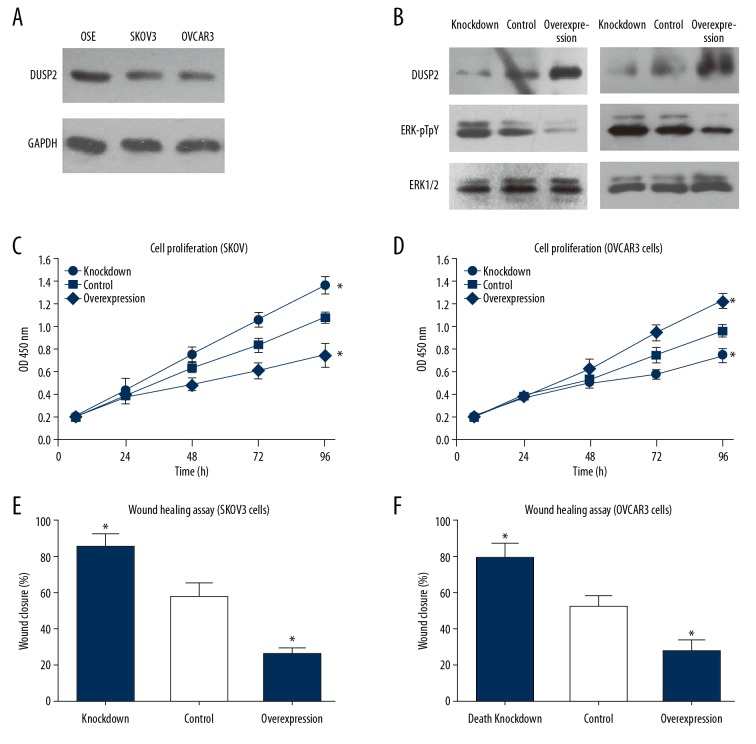
The protein expression levels of DUSP2 were lower the ovarian carcinoma cell lines, SKOV3 and OVCAR3, compared with benign ovarian surface epithelial (OSE) cells (Figure 3A). Also, siRNA and overexpression plasmids targeting DUSP2 were transiently transfected into serous ovarian carcinoma cell lines using Lipofectamine 3000 reagent. The major substrates of DUSPs are MAPK proteins. In this study, silencing DUSP2 increased the phosphorylation levels of ERK1/2, while overexpression of DUSP2 decreased the ERK1/2 phosphorylation levels (Figure 3B). Silencing DUSP2 expression promoted cell proliferation of both SKOV3 and OVCAR3 cell lines (Figure 3C, 3D). However, overexpression of DUSP2 inhibited SKOV3 and OVCAR3 cell proliferation. Consistent with the clinical findings, the cell migration capacity was enhanced by knockdown of DUSP2, while overexpression of DUSP2 had an inhibitory effect (Figure 3E, 3F).
Figure 3.
Dual-specificity phosphatase 2 (DUSP2) inhibited ERK1/2 activation and cell proliferation of SKOV3 and OVCAR3 cells in vitro. (A) The protein expression level of DUSP2 in OSE, SKOV3, and OVCAR3 cells were compared by Western blot. DUSP2 was down-regulated in SKOV3 and OVCAR3 cell lines. (B) Both SKOV3 and OVCAR3 cells were transfected with either siRNA targeting DUSP2 or plasmid overexpressing DUSP2. The transfection efficiency was evaluated by Western blot and compared with the control cells treated with transfection reagents. (C, D) The proliferation capacity of SKOV3 and OVCAR3 cells was estimated by the cell counting kit-8 (CCK-8) assay. (E, F) The wound-healing assay was conducted to evaluate the effects of silencing or overexpression of DUSP2 on cell migration. Data are shown as the mean±standard deviation (SD) from three independent experiments (* P<0.05).
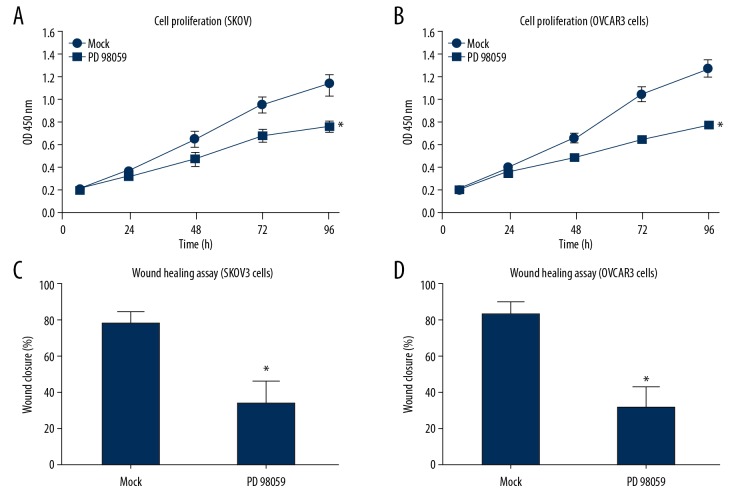
Cells transfected with DUSP2-siRNA were simultaneously treated with the ERK1/2 inhibitor, PD98059 to further explore the role of ERK1/2 in the anti-tumor effects of DUSP2. According to the findings from the cell proliferation assays, ERK inhibition significantly blocked the effects of knockdown of DUSP2 (Figure 4).
Figure 4.
The ERK inhibitor, PD98059, blocked the oncogenic effects of silencing dual-specificity phosphatase 2 (DUSP2) in SKOV3 and OVCAR3 cells in vitro. SKOV3 and OVCAR3 cells transfected with DUSP2 siRNA were further treated with either 25 μM of the ERK1/2 inhibitor (PD98059) or with dimethyl sulfoxide (DMSO) (control). The cell proliferation and migration capacities were evaluated by the cell counting kit-8 (CCK-8) assay (A, B) and wound-healing assay (C, D), respectively. Data are shown as the mean±standard deviation (SD) from three independent experiments (* P<0.05).
Discussion
Due to the lack of diagnostic biomarkers for early-stage ovarian carcinoma, most patients with serous ovarian carcinoma are diagnosed with late-stage disease, which results in high patient mortality. Although different dual-specificity phosphatase (DUSP) proteins may exert either oncogenic or anti-tumor functions, recently published studies have shown that dual-specificity phosphatase 2 (DUSP2) has anti-tumor effects. The protein expression level of DUSP2 has been reported to be down-regulated in colon cancer [14]. Loss of DUSP2 is associated with reduced prognosis in patients with bladder cancer [16]. Also, suppression of DUSP2 by hypoxia-inducible factor 1-α (HIF-1α) increased chemoresistance and malignancy in several human cancer cell lines [14]. The DUSP2 gene mutation was identified in large B cell lymphoma [20]. Epigenetic alterations of the DUSP2 gene, including hypermethylation, have been demonstrated in patients with head and neck cancer [21,22]. The aim of this study was to evaluate the expression of dual-specificity phosphatase 2 (DUSP2) in tumor tissues from 127 patients with serous ovarian carcinoma and the association with tumor grade, stage, and patient survival and to investigate the effects of DUSP2 expression in SKOV3 and OVCAR3 cells in vitro.
The findings of the present study showed that DUSP2 was down-regulated in serous ovarian carcinoma tissues, which is supported by the findings in other malignancies. Reduced expression levels of DUSP2 were significantly correlated with tumor metastasis and advanced tumor stage in patients with serous ovarian carcinoma. Lower levels of DUSP2 were associated with reduced overall survival in patients with serous ovarian carcinoma. In addition to clinicopathological associations, downstream mechanisms of DUSP2 in modulating tumor progression were also investigated. A previous study showed that DUSP2 inhibited angiogenesis and metastasis of colon cancer by the down-regulation of interleukin-8 [14]. The findings from the present study showed that DUSP2 could negatively regulate the phosphorylation status of ERK1/2 proteins, which determine ERK1/2 activity. ERK1/2 proteins have multiple roles in cancer development and progression by promoting cell proliferation and metastasis and enhancing gene transcription [23]. However, nuclear translocation of ERK1/2 acts as a cancer agent and facilitates cell apoptosis [24]. In the present study, ERK1/2 was inhibited using the specific inhibitor, PD98059, which reduced the proliferation and migration of SKOV3 and OVCAR3 cells in vitro. Therefore, DUSP2 expression might inhibit the progression of serous ovarian carcinoma by dephosphorylating ERK1/2. This hypothesis requires further studies to validate this possible mechanism for the tumor-suppressive effects of DUSP2.
This study had several limitations. Firstly, all the patients included in this study were from two medical centers in Shandong, China, which might have introduced regional bias. Therefore, future multicenter studies that include more tissue samples from patients with serous ovarian carcinoma are recommended. Secondly, the main aim of this study was to evaluate the clinicopathological and prognostic associations of DUSP2 expression in patients with serous ovarian carcinoma, rather than studying the molecular mechanisms involved. Future in vivo studies using animal models may be required to investigate further the mechanisms involved in the effects of DUSP2 in the progression of serous ovarian carcinoma. Finally, the upstream and downstream signaling pathways of DUSP2 require investigation with future molecular studies.
Conclusions
This study aimed to evaluate the expression of dual-specificity phosphatase 2 (DUSP2) in tumor tissues from patients with serous ovarian carcinoma and the association with tumor grade, stage, and patient survival and to investigate the effects of DUSP2 expression in SKOV3 and OVCAR3 cells in vitro. DUSP2 expression inhibited the proliferation and migration of SKOV3 and OVCAR3 cells by dephosphorylating ERK1/2. Low expression of DUSP2 in tumor tissues was associated with reduced overall survival in patients with serous ovarian carcinoma.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 3.Tan W, Pan M, Liu H, et al. Ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. Onco Targets Ther. 2017;10:3467–74. doi: 10.2147/OTT.S139009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. Cancer J Clin. 2018;68(4):284–96. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natanzon Y, Goode EL, Cunningham JM. Epigenetics in ovarian cancer. Semin Cancer Biol. 2018;51:160–69. doi: 10.1016/j.semcancer.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27(2):253–61. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 7.Ramkissoon A, Chaney KE, Milewski D, et al. Targeted inhibition of the dual specificity phosphatases DUSP1 and DUSP6 suppress MPNST growth via JNK. Clin Cancer Res. 2019;25(13):4117–27. doi: 10.1158/1078-0432.CCR-18-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhang Y, Chen M, et al. DUSP1 is involved in the progression of small cell carcinoma of the prostate. Saudi J Biol Sci. 2018;25(5):858–62. doi: 10.1016/j.sjbs.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng F, Xu Z, Chen J, et al. DUSP1 induces apatinib resistance by activating the MAPK pathway in gastric cancer. Oncol Rep. 2018;40(3):1203–22. doi: 10.3892/or.2018.6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Martinez D, Soto A, Gil-Araujo B, et al. Resveratrol promotes apoptosis through the induction of dual specificity phosphatase 1 and sensitizes prostate cancer cells to cisplatin. Food Chem Toxicol. 2019;124:273–79. doi: 10.1016/j.fct.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Lin P, Ren Y, Yan X, et al. The high NRF2 expression confers chemotherapy resistance partly through upregulated DUSP1 in myelodysplastic syndromes. Haematologica. 2019;104(3):485–96. doi: 10.3324/haematol.2018.197749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnoldussen YJ, Lorenzo PI, Pretorius ME, et al. The mitogen-activated protein kinase phosphatase vaccinia H1-related protein inhibits apoptosis in prostate cancer cells and is overexpressed in prostate cancer. Cancer Res. 2008;68(22):9255–64. doi: 10.1158/0008-5472.CAN-08-1224. [DOI] [PubMed] [Google Scholar]
- 13.Rahmouni S, Cerignoli F, Alonso A, et al. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nature Cell Biol. 2006;8(5):524–31. doi: 10.1038/ncb1398. [DOI] [PubMed] [Google Scholar]
- 14.Lin SC, Hsiao KY. Loss of dual-specificity phosphatase-2 promotes angiogenesis and metastasis via up-regulation of interleukin-8 in colon cancer. J Pathol. 2017;241(5):638–48. doi: 10.1002/path.4868. [DOI] [PubMed] [Google Scholar]
- 15.Dong W, Li N, Pei X, Wu X. Differential expression of DUSP2 in left- and right-sided colon cancer is associated with poor prognosis in colorectal cancer. Oncol Lett. 2018;15(4):4207–14. doi: 10.3892/ol.2018.7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin H, He W, Li Y, et al. Loss of DUSP2 predicts a poor prognosis in patients with bladder cancer. Human Pathol. 2019;85:152–61. doi: 10.1016/j.humpath.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Cho KR, Shih I-M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Ma B, Malik AB, et al. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol. 2012;13(5):457–64. doi: 10.1038/ni.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuhmacher B, Bein J, Rausch T, et al. JUNB, DUSP2, SGK1, SOCS1 and CREBBP are frequently mutated in T-cell/histiocyte-rich large B-cell lymphoma. Haematologica. 2019;104(2):330–37. doi: 10.3324/haematol.2018.203224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo Nigro C, Vivenza D, Denaro N, et al. DUSP2 methylation is a candidate biomarker of outcome in head and neck cancer. Ann Transl Med. 2018;6(13):271. doi: 10.21037/atm.2018.06.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haag T, Richter AM, Schneider MB, et al. The dual specificity phosphatase 2 gene is hypermethylated in human cancer and regulated by epigenetic mechanisms. BMC Cancer. 2016;16:49. doi: 10.1186/s12885-016-2087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Zhang Q, Li K, et al. Prognostic significance of USP33 in advanced colorectal cancer patients: New insights into beta-arrestin-dependent ERK signaling. Oncotarget. 2016;7(49):81223–40. doi: 10.18632/oncotarget.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotnikov A, Flores K, Maik-Rachline G, et al. The nuclear translocation of ERK1/2 as an anticancer target. Nat Commun. 2015;6:6685. doi: 10.1038/ncomms7685. [DOI] [PubMed] [Google Scholar]