Abstract
Background
Although the oncogenic roles of multiple copies in T-cell malignancy 1 (MCT-1) have been revealed in multiple cancers, its effects on non-small cell lung cancer (NSCLC) progression are still uncertain. This study aimed to reveal the effects of MCT-1 on the stem cell-like traits of NSCLC cells.
Material/Methods
Western blot, real-time quantitative polymerase chain reaction (RT-qPCR), spheroid forming ability, and ALDH1 (aldehyde dehydrogenase 1) activity analysis were carried out to examine the effects of MCT-1/micrRNa-34 (miR-34a)/interleukin-6 (IL-6) on the stem cell-like characteristics of lung cancer cells.
Results
MCT-1 knockdown reduced the spheroid forming ability, characterized as the decreased spheroid size and number. Additionally, MCT-1 knockdown decreased the expression of the NSCLC stemness markers and the activity of ALDH1. Moreover, MCT-1 knockdown decreased IL-6 secretion that promotes NSCLC cell stemness. Furthermore, MCT-1 knockdown increased the level of miR-34a, which attenuated the stemness of NSCLC cells through targeting IL-6R (IL-6 receptor) expression.
Conclusions
These results suggest MCT-1/miR-34a/IL-6/IL-6R axis is responsible for MCT-1-mediated effects on NSCLC cell stemness.
MeSH Keywords: Lung Neoplasms; MicroRNAs; Neoplastic Stem cells; Receptors, Interleukin-6
Background
Lung cancer is one of the most dangerous malignant tumors to human health and life. In the United States, the incidence of lung cancer ranks the second among males and females, and the mortality rate ranks first [1]. In China, the morbidity and mortality of lung cancer are in first place in males, the second in females, and first place in mortality [2]. At present, the treatment methods of lung cancer mainly include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy [3]. However, the annual survival rate of lung cancer patients is still relatively low. The main reason might be that patients have resistance to conventional treatment methods, such as radiotherapy and chemotherapy, in the course of their treatment [3]. At present, drug resistance is often solved using new drugs, or chemotherapy alone or in combination with radiotherapy. However, the specific mechanism of drug resistance remains to be studied.
At present, more and more scholars believe that drug resistance in lung cancer treatment might be due to the existence of cancer stem cells (CSCs) [4]. CSCs are different from normal cancer cells. They can self-renew and differentiation and they play an important role in tumorigenesis, progression, metastasis, and drug resistance [4]. In addition, cell stress conditions, such as chemotherapy, can promote the symmetrical division and proliferation of CSCs [5]. This effect becomes larger in the course of continuous chemotherapy, and eventually results in chemotherapy-resistant tumors. Targeted drugs for CSCs are very important for chemotherapy resistance therapy, inhibition of tumor progression, and tumor recurrence. At present, the intrinsic mechanism of chemotherapy resistance induced by lung CSCs is not clear, but many markers of lung CSCs have been confirmed, including ALDH1 (aldehyde dehydrogenase 1), CD133, CD166, CD87, and CD117 [6]. These markers are associated with lung cancer therapeutic resistance. The oncogenic roles of T-cell malignancy 1 (MCT-1), originally identified in T-cell lymphoma, have been found in various tumors, for example, knockdown of MCT-1 by short hairpin RNA markedly represses xenograft tumorigenicity of the phosphatase and tensin homolog (PTEN)-null breast cancer cells [7]; PKC (protein kinase C) knockdown or inhibition arrests the cell cycle in G1 phase of diffuse large B-cell lymphomas (DLBCL) potentially by downregulating MCT-1 expression [8]. Notably, overexpression of MCT-1 promotes lung cancer cell progression, angiogenesis, and necrosis through the YY1-EGFR-MnSOD signaling [9], and its roles of MCT-1 in regulating tumor stemness were first revealed in breast cancer cells [10]. However, its roles in lung cancer stemness are still unclear.
More and more studies have found that microRNA (miRNA) can limit the differentiation potential of pluripotent stem cells and act as a barrier for somatic cell reprogramming. Therefore, miRNA plays an important regulatory role in CSCs-mediated tumor metastasis and drug resistance, and it has potential clinical significance. Recent studies have found several kinds of miRNAs expressed in lung CSCs, and studies have established the mechanisms by which they regulate the stemness of lung CSCs, for example, studies of miR-146 [11], miR-20b [12], and miR-150-5p [13]. The suppressive roles of the miR-34 family have been revealed in lung adenocarcinomas [14]. Additionally, miR-34a was shown to sensitize lung cancer cells to chemotherapeutic drugs and ionizing radiation [15,16]; these 2 effects are tightly associated with CSCs. Therefore, we speculate that miR-34a has similar effects in lung cancer. Additionally, in the current work, we explored the upstream and downstream effectors of miR-34a in regulating the stem cell-like traits of lung cancer cells.
Material and Methods
Cell culture and cytokines
Human lung adenocarcinoma cell line A549 was cultured in DMEM medium (containing 10% fetal bovine serum, 1% streptomycin and penicillin) and placed in an incubator with 37°C, 5% carbon dioxide and saturated humidity. Recombinant human interleukin-6 (IL-6) protein was purchased from R&D Systems (Minneapolis, MN, USA), the concentration 0.5 ng/mL was used in this work.
Real-time quantitative PCR (RT-qPCR)
TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) was used to extract total RNA from cells. The operation steps were referred to the instructions of RNA extraction kit (Transgen, Beijing, China). Nanodrop spectrophotometer was used to detect the concentration and purity of total RNA (the ratio of A260/A280 was between 1.8 and 2.0). The reverse transcription kit (Transgen) was used for reverse transcription. The product of the cDNA was stored at −20°C. The reaction conditions were as follows: pre-denaturation: 95°C 30 seconds; PCR cycle: 95°C denaturation 15 seconds; 60°C annealing 20 seconds; 70°C extension 20 seconds for 40 cycles; melting curve analysis: 95°C 5 seconds, 60°C 30 seconds. The relative expression of RNA was expressed by 2−ΔΔct method. After 3 independent repetitive experiments, each sample was analyzed statistically.
Western blot
About 5×105 cells were collected from each group. RIPA protein lysate (Transgen) was added to extract the whole cell protein. BCA protein quantitative kit (Transgen) was used to detect the protein concentration. After 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) protein isolation, the protein was transferred to polyvinylidene difluoride (PVDF) membrane (Merck Millipore, Billerica, MA, USA) by wet transformation, oscillated by 5% skimmed-milk powder at room temperature for 1 hour and washed with TBST (tris-buffered saline and Tween 20). Primary antibodies were added respectively. After incubation at 4°C overnight, membranes were washed by TBST, horseradish peroxidase (HRP)-labeled secondary antibodies were added, incubated at room temperature for 1 hour, and enhanced chemiluminescence (ECL) luminescent solution was added for chemiluminescence. Development and gel imager were used to observe the protein bands, and the images were obtained. Gray scale analysis of the images was performed by ImageJ software, and the gray ratio between the target protein and the internal reference protein was calculated. After repeating 3 times, statistical analysis was carried out.
Plasmid construction
The lentivirus vectors of MCT-1 knockdown (Len-MCT-1-kd), miR-34a overexpression (miR-34a), miR-34a knockdown (miR-34a-kd), IL-6R overexpression (IL-6R) and corresponding control vectors were purchased from VectorBuilder Inc., (Chicago, IL, USA).
Spheroid forming analysis
Cells in logarithmic growth phase were digested by trypsinase, centrifuged, and suspended in serum-free conditioned medium (B27 reagent, epidermal growth factor [EGF] with final concentration of 20 ng/mL, bFGF with final concentration of 20 ng/mL) again and transferred to 10 cm ultra-low adsorption culture dish or 6-well plate pre-added with serum-free conditioned medium, and appropriate amount of serum-free conditioned culture medium was added every other day; 7–10 days later, spheroid forming was observed under a microscope. Spheres (>50 um) were counted and photographed.
ALDH1 activity detection
ALDH1 assay kit (Solarbio, Beijing, China) was used to determine ALDH1 activity in lung cancer cells following the manufacturer’s protocols.
Statistical assay
Results were presented as the mean±standard deviation (SD). Student’s t-test or the Tukey-Kramer post-hoc test was used to assess the differences using SPSS 13.0 statistical analysis software. Every experiment was performed at least three times. P<0.05 showed significant difference.
Results
Knockdown of MCT-1 attenuated the stemness-like traits of A549 cells
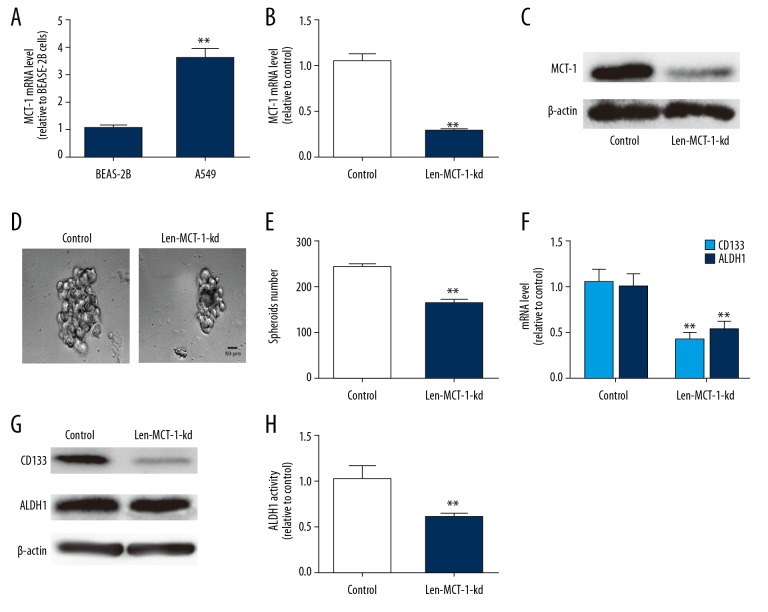
Since CSCs have been regarded as the origin of tumor progression, we first detected the effects MCT-1 on the stemness-like traits of NSCLC A549 cells. MCT-1 was knocked down in A549 cells because it is confirmed to hold a higher level in NSCLC cells compared with that in normal lung epithelial cells (Figure 1A). The knockdown efficiency of MCT-1 was confirmed by qPCR and western blot analysis (Figure 1B, 1C). As shown in Figure 1D and 1E, MCT-1 knockdown reduced the spheroid size and number of A549 cells. Additionally, MCT-1 knockdown decreased the expression of lung cancer stemness markers (CD133 and ALDH1) (Figure 1F, 1G). Moreover, ALDH1 activity was reduced in A549 cells with MCT-1 knockdown (Figure 1H). Thus, we demonstrate that MCT-1 knockdown attenuates the stemness-like traits of A549 cells.
Figure 1.
Knockdown of MCT-1 attenuates the stemness-like traits of A549 cells. (A) RT-qPCR analysis on MCT-1 mRNA expression in BEAS-2B and A549 cells. (B, C) The knockdown efficiency of Len-MCT-1-kd was confirmed in A549 cells via RT-qPCR and western blot assay. (D) Spheroid forming analysis on A549 cells infected with or without Len-MCT-1-kd lentivirus. (E) RT-qPCR analysis on ALDH1 and CD133 in A549 cells with or without MCT-1 knockdown. (F) The mRNA levels of CD133 and ALDH1 were detected in A549 cells with MCT-1 knockdown or not. (G) The protein levels of CD133 and ALDH1 were measured in A549 cells with MCT-1 knockdown or not. (H) ALDH1 activity detection in A549 cells infected with Len-MCT-1-kd or not. ** P<0.01 versus control. RT-qPCR – real-time quantitative polymerase chain reaction; ALDH1 – aldehyde dehydrogenase 1; MCT-1 – multiple copies in T-cell malignancy 1.
Knockdown of MCT-1 decreased IL-6 secretion which promotes stemness-like traits of A549 cells
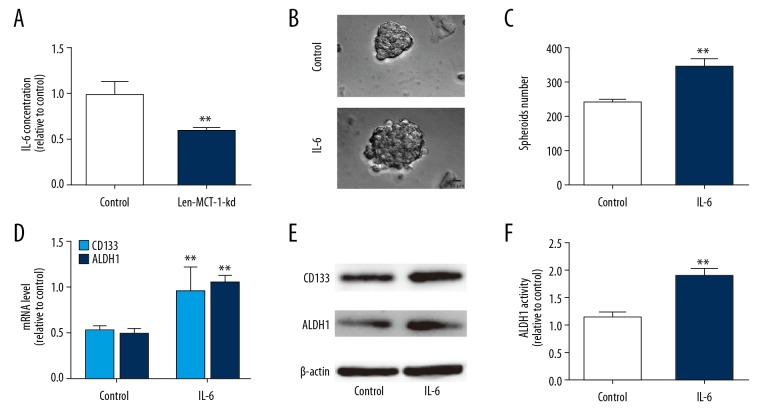
Since the cytokine IL-6 communicates between cancer cells and microenvironment, and promotes CSC progression, we determined the effects of MCT-1 knockdown on IL-6 secretion in A549 cells. As expected, MCT-1 knockdown decreased IL-6 secretion in A549 cells (Figure 2A). Notably, IL-6 enhanced the stemness of A549 cells, which is evident by the decreased spheroid size, number, expression of lung cancer stemness markers and ALDH1 activity (Figure 2B–2F).
Figure 2.
Knockdown of MCT-1 decreased IL-6 secretion which promotes stemness-like traits of A549 cells. (A) The IL-6 concentration was determined in A549 cells infected with Len-MCT-1-kd or not. (B, C) Spheroid forming analysis on A549 cells with Len-MCT-1-kd infection or not. (D, E) RT-qPCR and western blot analysis on A549 cells with Len-MCT-1-kd infection or not. (F) ALDH1 activity was evaluated in A549 cells with or without MCT-1 knockdown. ** P<0.01 versus control. IL-6 – interleukin-6; RT-qPCR – real-time quantitative polymerase chain reaction; MCT-1 – multiple copies in T-cell malignancy 1; ALDH1 – aldehyde dehydrogenase 1.
MiR-34a overexpression attenuated the stemness-like traits of A549 cells via targeting IL-6R
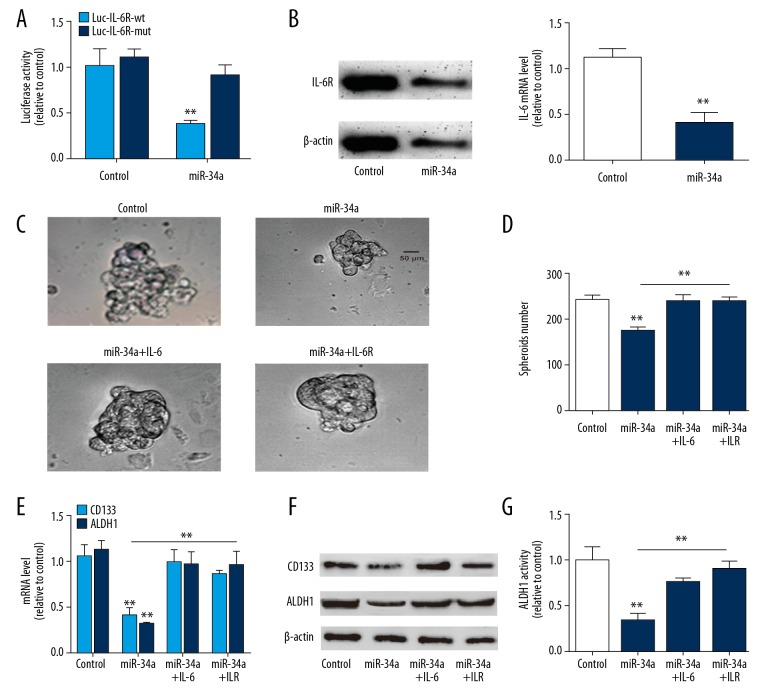
We further explored the upstream regulators of IL-6-mediated effects on A549 cell stemness. As miR-34a, the tumor suppressor, has been confirmed to target IL-6R in breast cancer [10], we wondered whether miR-34a could hold the opposite effects with MCT-1 via targeting IL-6R. Firstly, we confirmed the binding of miR-34a on IL-6R via luciferase reporter assay, RT-qPCR and western blot analysis in A549 cells. As shown in Figure 3A, miR-34a overexpression reduced the activity of Luc-IL-6R-wt (with miR-34a binding site) but had no effect on Luc-IL-6R-mut (with mutant miR-34a binding site) activity. Additionally, miR-34a overexpression significantly decreased IL-6R expression (Figure 3B). It was found that miR-34a overexpression decreased the spheroid size and number, which was rescued by IL-6R overexpression or IL-6 treatment (Figure 3C, 3D). Additionally, the expression of lung cancer stemness markers was reduced by miR-34a overexpression, this effect was partially reversed by IL-6 treatment or IL-6R overexpression (Figure 3E, 3F). Moreover, miR-34a overexpression decreased ALDH1 activity, which was partially diminished by IL-6 treatment or IL-6R overexpression (Figure 3G).
Figure 3.
MiR-34a overexpression attenuates the stemness-like traits of A549 cells via targeting IL-6R. (A) The activity of Luc-IL-6R-wt and Luc-IL-6R-mut was evaluated in A549 cells with or without miR-34a overexpression. (B) IL-6R expression was measured in A549 cells with or without miR-34a overexpression. (C) Sphere size was measured in A549 cells with miR-34a overexpression as well as IL-6 treatment or IL-6R overexpression or not. (D) Sphere number was determined in A549 cells with miR-34a overexpression plus IL-6 treatment or IL-6R overexpression or not. (E, F) RT-qPCR and western blot analysis on the A549 cells described in (C). (G) ALDH1 activity was examined in A549 cells with miR-34a overexpression as well as IL-6 treatment or IL-6R overexpression or not. ** P<0.01 versus control. MiR-34a – microRNA-34a; IL-6 – interleukin-6; MCT-1 – multiple copies in T-cell malignancy 1; RT-qPCR – real-time quantitative polymerase chain reaction; ALDH1 – aldehyde dehydrogenase 1.
MCT-1 regulated the stemness-like traits of A549 cells through the miR-34a/IL-6/IL-6R axis
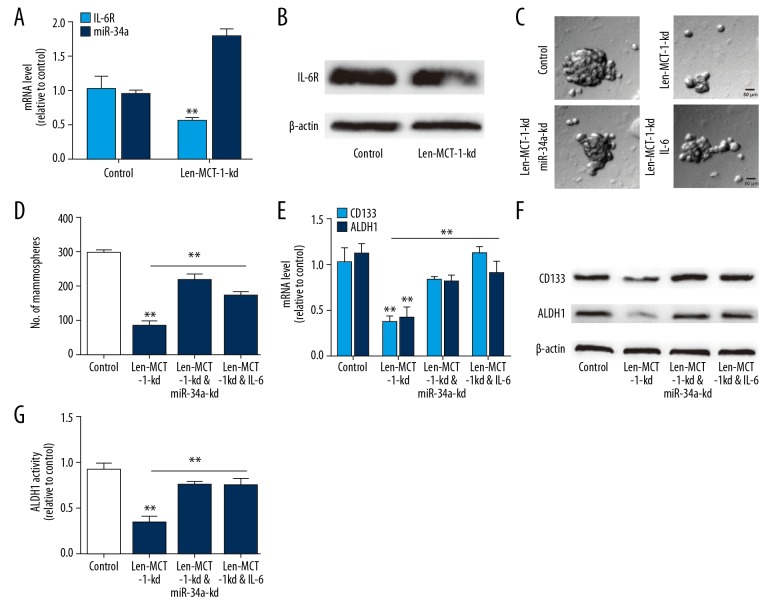
Finally, we investigated whether MCT-1 exerts its effects on A549 cell stemness through the miR-34a/IL-6/IL-6R axis. Firstly, it was found that MCT-1 knockdown indeed upregulated miR-34a level and decreased IL-6R expression in A549 cells (Figure 4A, 4B). Then miR-34a was knocked down or IL-6 was added in A549 cells with MCT-1 knockdown. As shown in Figure 4C and 4D, the decreased spheroid formation ability mediated by MCT-1 knockdown was partially reversed by miR-34a knockdown or IL-6 treatment. MCT-1 knockdown-induced the downregulation of lung cancer stemness markers was rescued by miR-34a knockdown or IL-6 treatment (Figure 4E, 4F). A consistent result was obtained on ALDH1 activity (Figure 4G). Therefore, these results indicate that MCT-1 regulates the stemness of A549 cells through the miR-34a/IL-6/IL-6R axis.
Figure 4.
MCT-1 regulates the stemness-like traits of A549 cells through the miR-34a/IL-6/IL-6R axis. (A) RT-qPCR analysis on IL-6R and miR-34a levels in A549 cells with MCT-1 knockdown or not. (B) Western blot analysis on IL-6R level in A549 cells infected with Len-MCT-1-kd or not. (C, D) Spheroid forming ability was evaluated in A549 cells infected with Len-MCT-1-kd plus miR-34a knockdown or IL-6R overexpression. (E, F) CD133 and ALDH1 expression was evaluated in A549 cells with MCT-1 knockdown as well miR-34a knockdown or IL-6R overexpression. (G) ALDH1 activity was examined in A549 cells with MCT-1 knockdown plus miR-34a knockdown or IL-6R overexpression. ** P<0.01 versus control. MCT-1 – multiple copies in T-cell malignancy 1; MiR-34a – microRNA-34a; RT-qPCR – real-time quantitative polymerase chain reaction; IL-6 – interleukin-6; ALDH1 – aldehyde dehydrogenase 1.
Discussion
In the current study, we first indicate MCT-1 roles in regulating the stem cell-like characteristics of lung cancer cells, which is evident by the decreased ALDH1 activity, expression of stemness modulators and spheroid forming ability led by MCT-1 knockdown.
Recent studies have found that the IL-6/JAK2/STAT3 signaling pathway is involved in the growth regulation of CSC. Marotta et al. confirm that JAK2/STAT3 is necessary for the growth of breast cancer stem cells. Further studies found that blocking JAK2/STAT3 signaling pathway can make breast cancer stem cells lose their stemness characteristics. More importantly, IL-6 stimulation can significantly increase the amount of CSC in breast cancer cells, suggesting that it may promote the de-differentiation of adult stem cells into CSCs. Here, we used A549 lung adenocarcinoma cell line as a model to study the effect of IL-6 stimulation on stem cell-like characteristics of adult lung adenocarcinoma cells and found that IL-6 indeed enhanced the stemness of A549 cells. However, the concrete mechanisms by which IL-6 exerts its effects are still unclear. We speculate that IL-6 might exert its effects on A549 cell stemness through the JAK2/STAT3 signaling, this should be evaluated in the following study. Additionally, it was found that miR-34a inhibited the stemness-like characteristics of A549 cells through targeting IL-6R. Although the miR-34a-IL-6R axis has been identified before [10], this is the first work demonstrating the miR-34a/IL-6R axis in regulating the stemness of A549 cells. Further mechanistic studies reveal that MCT-1 could negatively regulate miR-34a expression, this is responsible for MCT-1-mediated effects on A549 cell stemness. However, the detailed mechanisms by which MCT-1 downregulates miR-34a level need to be explored in the future work. Moreover, this work just performs the in vitro experiments, further in vivo experiments should be constructed to confirm the conclusion.
Conclusions
This work reveals a novel MCT-1/miR-34a/IL-6R axis in regulating the stem cell-like characteristics of A549 cells, providing a potential target for lung adenocarcinoma treatment.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Liu Z, Liang H, Lin J, et al. The incidence of lymph node metastasis in patients with different oncogenic driver mutations among T1 non-small-cell lung cancer. Lung Cancer. 2019;134:218–24. doi: 10.1016/j.lungcan.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 2.Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. 2019;11:943–53. doi: 10.2147/CMAR.S187317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owen DH, Wei L, Bertino EM, et al. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin Lung Cancer. 2018;19(6):e893–900. doi: 10.1016/j.cllc.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke MF. Clinical and therapeutic implications of cancer stem cells. N Engl J Med. 2019;380(23):2237–45. doi: 10.1056/NEJMra1804280. [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, Xiang C, Li X, et al. STARD13-correlated ceRNA network-directed inhibition on YAP/TAZ activity suppresses stemness of breast cancer via co-regulating Hippo and Rho-GTPase/F-actin signaling. J Hematol Oncol. 2018;11(1):72. doi: 10.1186/s13045-018-0613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Codony-Servat J, Codony-Servat C, Cardona AF, et al. Cancer stem cell biomarkers in egfr-mutation-positive non-small-cell lung cancer. Clin Lung Cancer. 2019;20(3):167–77. doi: 10.1016/j.cllc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Wu MH, Chen YA, Chen HH, et al. MCT-1 expression and PTEN deficiency synergistically promote neoplastic multinucleation through the Src/p190B signaling activation. Oncogene. 2014;33(43):5109–20. doi: 10.1038/onc.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang G, Zheng J, Xiao W, et al. PKC inhibition of sotrastaurin has antitumor activity in diffuse large B-cell lymphoma via regulating the expression of MCT-1. Acta Biochim Biophys Sin (Shanghai) 2018;50(4):399–407. doi: 10.1093/abbs/gmy021. [DOI] [PubMed] [Google Scholar]
- 9.Tseng HY, Chen YA, Jen J, et al. Oncogenic MCT-1 activation promotes YY1-EGFR-MnSOD signaling and tumor progression. Oncogenesis. 2017;6(4):e313. doi: 10.1038/oncsis.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng YS, Tseng HY, Chen YA, et al. MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol Cancer. 2019;18(1):42. doi: 10.1186/s12943-019-0988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G, Wang M, Li X, et al. TUSC7 suppression of Notch activation through sponging miR-146 recapitulated the asymmetric cell division in lung adenocarcinoma stem cells. Life Sci. 2019;232:116630. doi: 10.1016/j.lfs.2019.116630. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Li W, Luo S, et al. Adipose derived stem cells promote tumor metastasis in breast Cancer cells by stem cell factor inhibition of miR20b. Cell Signal. 2019;62:109350. doi: 10.1016/j.cellsig.2019.109350. [DOI] [PubMed] [Google Scholar]
- 13.Dai FQ, Li CR, Fan XQ, et al. MiR-150-5p inhibits non-small-cell lung cancer metastasis and recurrence by targeting HMGA2 and beta-catenin signaling. Mol Ther Nucleic Acids. 2019;16:675–85. doi: 10.1016/j.omtn.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JS, Kim EJ, Lee S, et al. MiR-34a and miR-34b/c have distinct effects on the suppression of lung adenocarcinomas. Exp Mol Med. 2019;51(1):9. doi: 10.1038/s12276-018-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song C, Lu P, Sun G, et al. MiR-34a sensitizes lung cancer cells to cisplatin via p53/miR-34a/MYCN axis. Biochem Biophys Res Commun. 2017;482(1):22–27. doi: 10.1016/j.bbrc.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 16.He X, Yang A, McDonald DG, Riemer EC, et al. MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget. 2017;8(41):69797–807. doi: 10.18632/oncotarget.19267. [DOI] [PMC free article] [PubMed] [Google Scholar]