Abstract
PURPOSE
Asparaginase-associated pancreatitis (AAP) is common in patients with acute lymphoblastic leukemia (ALL), but risk differences across age groups both in relation to first-time AAP and after asparaginase re-exposure have not been explored.
PATIENTS AND METHODS
We prospectively registered AAP (n = 168) during treatment of 2,448 consecutive ALL patients aged 1.0-45.9 years diagnosed from July 2008 to October 2018 and treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol.
RESULTS
Compared with patients aged 1.0-9.9 years, adjusted AAP hazard ratios (HRa) were associated with higher age with almost identical HRa (1.6; 95% CI, 1.1 to 2.3; P = .02) for adolescents (10.0-17.9 years) and adults (18.0-45.9 years). The day 280 cumulative incidences of AAP were 7.0% for children (1.0-9.9 years: 95% CI, 5.4 to 8.6), 10.1% for adolescents (10.0 to 17.9 years: 95% CI, 7.0 to 13.3), and 11.0% for adults (18.0-45.9 years: 95% CI, 7.1 to 14.9; P = .03). Adolescents had increased odds of both acute (odds ratio [OR], 5.2; 95% CI, 2.1 to 13.2; P = .0005) and persisting complications (OR, 6.7; 95% CI, 2.4 to 18.4; P = .0002) compared with children (1.0-9.9 years), whereas adults had increased odds of only persisting complications (OR, 4.1; 95% CI, 1.4 to 11.8; P = .01). Fifteen of 34 asparaginase-rechallenged patients developed a second AAP. Asparaginase was truncated in 17/21 patients with AAP who subsequently developed leukemic relapse, but neither AAP nor the asparaginase truncation was associated with increased risk of relapse.
CONCLUSION
Older children and adults had similar AAP risk, whereas morbidity was most pronounced among adolescents. Asparaginase re-exposure should be considered only for patients with an anticipated high risk of leukemic relapse, because multiple studies strongly indicate that reduction of asparaginase treatment intensity increases the risk of relapse.
INTRODUCTION
Because adult patients now more frequently receive pediatric-inspired acute lymphoblastic leukemia (ALL) treatment, their tolerance to such therapy has become an important issue.1,2 Depending on the extent of asparaginase (ASP) exposure, larger trials report asparaginase-associated pancreatitis (AAP) in up to 11% of children with ALL.3-16 Furthermore, premature withdrawal of ASP reduces cure rates,3,4,17 and one of the commonest causes of ASP truncation in children is AAP because many experience a second AAP after ASP rechallenge.3,5,18
ASP depletes the body of asparagine,19 interfering with the highly active pancreatic protein synthesis. Acute pancreatitis arises from premature activation of trypsin within pancreatic acinar cells, acinar cell destruction, concomitant local inflammation, and ultimately pancreatic autodigestion.20 However, the direct mechanism behind AAP is unknown, and treatment is mainly supportive.21 Although mortality is reported in only a small percentage of patients,3,6,7,13,18,22 both acute and long-term morbidities after childhood AAP are common.13,18,22-24
Some clinical3-9,15,18,25 and genetic5,9,26 risk factors for acute pancreatitis have been proposed, including adolescent age. However, comparative studies of pediatric and adult patients with ALL, as well as studies exploring the AAP-related morbidity and impact on leukemic relapse risk, are missing. We investigated the cumulative incidence, clinical characteristics, and relapse risk in patients with ALL with AAP aged 1.0-45.9 years uniformly treated according to the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol.
PATIENTS AND METHODS
Study Population
A total of 2,448 patients (including 168 patients with AAP) aged 1.0-45.9 years with a diagnosis of either B-cell precursor or T-cell Philadelphia chromosome-negative ALL between July 2008 and October 2018 were included. All patients were treated according to the NOPHO ALL2008 protocol in Denmark, Estonia, Finland, Iceland, Lithuania, Norway, and Sweden. An inclusion flowchart is presented in the Data Supplement.
All centers complied with mandatory registration of AAP 4 times a year throughout the study period.27,28 We identified patients with AAP in the NOPHO ALL registry on April 2, 2019, which provided data on patient, disease, and antileukemic treatment. Detailed questionnaires in relation to the diagnosis, complications, and outcome of AAP were subsequently completed by local clinicians. The data were merged with previously published data from 60% (80/134) of the pediatric cohort.5
AAP-Related Definitions
The diagnosis of acute pancreatitis required fulfillment of at least 2 of 3 diagnostic criteria: (1) abdominal pain; (2) serum amylase (total or pancreas specific) or lipase ≥ 3 times the upper normal limit; and (3) ultrasound, computed tomography, or magnetic resonance imaging compatible with pancreatitis according to the international Ponte di Legno consensus criteria.29 The definition of AAP in this study required a diagnosis within 4 weeks after the last ASP injection—the cutoff time point of measurable polyethylene glycol conjugated Escherichia coli–derived ASP (PegASP) acitivity.30 Definitions of grading and complications are presented in the Data Supplement and Tables 1 and 2.
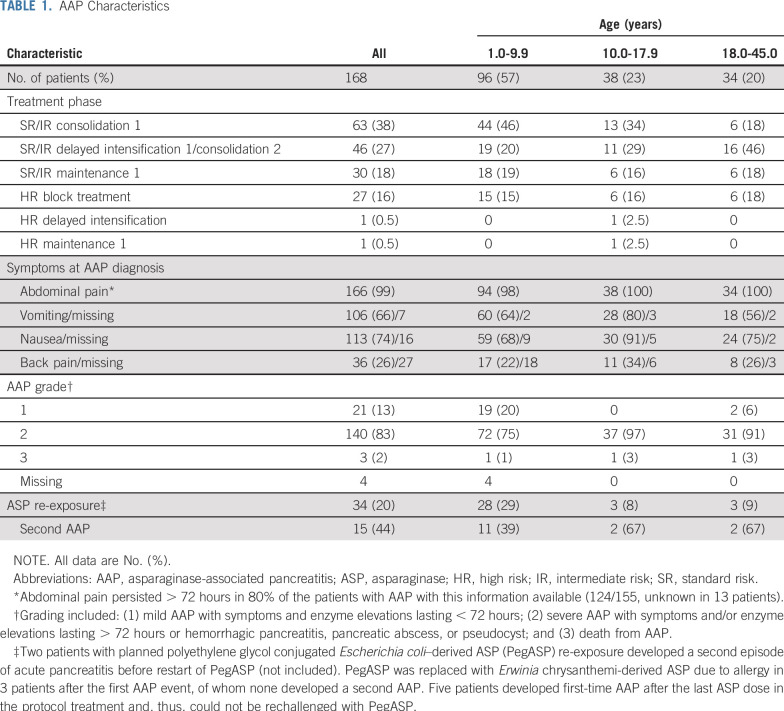
TABLE 1.
AAP Characteristics
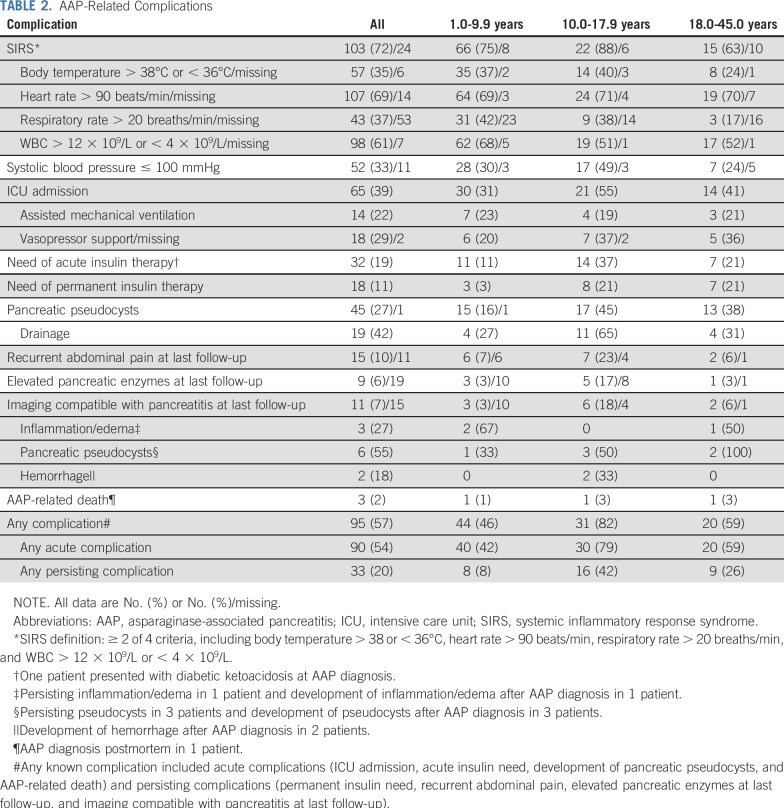
TABLE 2.
AAP-Related Complications
ALL2008 Protocol Treatment
Therapy details on the NOPHO ALL2008 protocol have been reported previously.2,27,28,31 Treatment was based on stratification into 4 risk groups: standard risk (SR); intermediate risk (IR); high risk (HR); and high risk with hematopoietic stem cell transplantation (HR-SCT) in first complete remission (CR1), guided by tumor burden at diagnosis, immunophenotype, cytogenetics, CNS involvement, and minimal residual disease levels on treatment days 15, 29, and 79 (or after the second HR block). SR and IR patients received identical PegASP treatment. Of note, children (SR and IR) were randomly assigned to receive PegASP, 1,000 IU/m2/dose intramuscularly, either at 2-week (control arm) or 6-week (experimental arm) intervals from weeks 14 to 33 (ClinicalTrials.gov identifier: NCT00819351). All children received PegASP at 6-week intervals from week 14 after the randomization closed on March 1, 2016.32 The randomization did not influence cure rates.32 Patients aged ≥ 18 years and treated at an adult clinic were not included in the PegASP randomization; however, adults from Estonia, Lithuania, and Sweden received PegASP at 6-week intervals from March, September, and April 2016, respectively. The adult centers from the remaining countries continued PegASP treatment according to the control arm. The NOPHO ALL2008 protocol is presented in the Data Supplement.
The study was approved by the regional research ethics committee of the Capital Region of Denmark (Protocol No. H-2-2010-002) and the Danish Data Protection Agency (Journal No. 2012-58-0004). All patients gave written informed consent, and the study was conducted in accordance with the Declaration of Helsinki (version 2008; www.wma.net).
Statistical Analyses
Patients were followed from the time of ALL diagnosis until the date of the first event (relapse, death during induction or in CR1, or second malignant neoplasm [SMN]); SCT in CR1; loss to follow-up/abandonment of therapy; or last follow-up in the registry or April 2, 2019, whichever came first. When exploring AAP, patients were followed from day 30 (the time of the first ASP dose) until the date of the first event including censoring 4 weeks after the last planned ASP injection or 4 weeks after the ASP truncation date, if available, respectively. The reversed Kaplan-Meier method was used to estimate the follow-up time. Cumulative incidences were estimated using the Aalen-Johansen estimator considering relapse, death, and SMN as competing events; the estimates were compared using Gray’s test. The body mass index z-scores were calculated accounting for age and sex according to Danish references.33 Time to first AAP was analyzed in a Cox proportional hazards regression model including relevant preselected clinical characteristics. As a sensitivity analysis of the predefined age groups (1.0-9.9 years, 10.0-17.9 years, and 18.0-45.9 years), new age groups were explored, each containing approximately 25% of the AAP events (1.0-4.9 years, 5.0-8.9 years, 9.0-16.9 years, and 17.0-45.9 years). Investigating potential risk factors of AAP-related complications, preselected clinical variables were included in a multiple logistic regression model of development of any AAP-related complication within ≥ 100 days after the AAP diagnosis. To investigate the association between AAP and time to death in CR1 and relapse, we used Cox models with AAP as a time-dependent variable and delayed entry on the CR1 date, respectively. As sensitivity analyses, the models were stratified by risk group on day 29 with delayed entry on day 29 or the CR1 date, if later than day 29. In all Cox models, relevant interactions and the proportional hazards assumption were explored. Two-sided P values < .05 were regarded as statistically significant.
RESULTS
Patient and Treatment Characteristics
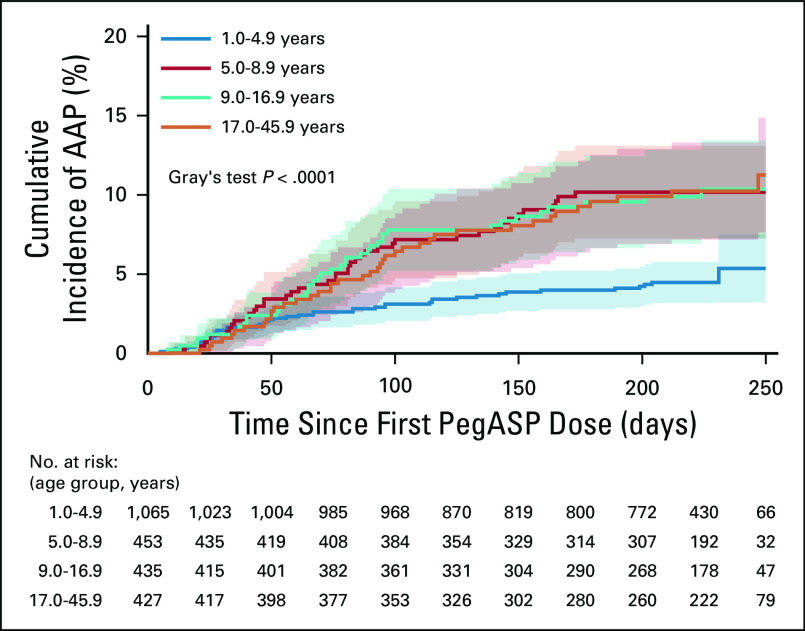
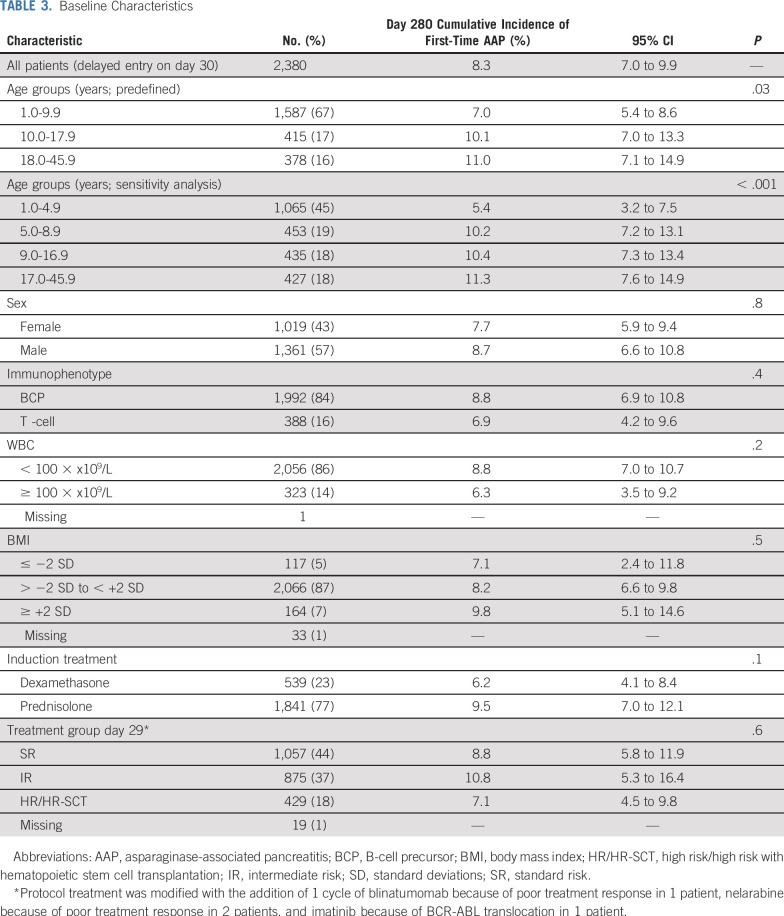
Following all patients for a median of 245 days (interquartile range [IQR], 186-259), the day 280 cumulative incidence of first-time AAP (168/2,448) was 8.3% (95% CI, 7.0 to 9.9) with all but 1 late AAP included at this time point. The day 280 cumulative incidences were 7.0% (95% CI, 5.4 to 8.6), 10.1% (95% CI, 7.0 to 13.3), and 11.0% (95% CI, 7.1 to 14.9) in patients aged 1.0-9.9 years, 10.0-17.9 years, and 18.0-45.9 years, respectively (P = .03; Data Supplement). However, when analyzing the 4 new age groups, the risk of AAP already rose at 5 years of age, being 5.4% (95% CI, 3.2 to 7.5) in patients aged 1.0-4.9 years, 10.2% (95% CI, 7.2 to 13.1) in patients aged 5.0-8.9 years, 10.4% (95% CI, 7.3 to 13.4) in patients aged 9.0-16.9 years, and 11.3% (95% CI, 7.6 to 14.9) in patients aged 17.0-45.9 years (P < .001; Fig 1). The clinical characteristics of the patients appear in Tables 1 and 3. None of the patients had a prior history of pancreatitis or any known comorbidity, particularly of the liver and pancreas.
FIG 1.
The cumulative incidence of first-time asparaginase-associated pancreatitis (AAP) by age groups with 95% CIs and patients at risk. The day 280 cumulative incidences were 5.4% (95% CI, 3.2 to 7.5) for patients aged 1.0-4.9 years; 10.2% (95% CI, 7.2 to 13.1) for patients aged 5.0-8.9 years; 10.4% (95% CI, 7.3 to 13.4) for patients aged 9.0-16.9 years; and 11.3% (95% CI, 7.6 to 14.9) for patients aged 17.0-45.9 years. Of note, day 280 from acute lymphoblastic leukemia diagnosis to event corresponds to day 250 on the x-axis because of entry for all patients on day 30 (the time of the first polyethylene glycol conjugated Escherichia coli–derived ASP [PegASP] dose).
TABLE 3.
Baseline Characteristics
AAP occurred within a median of 10 days (IQR, 6-13; range, 0-28) from last PegASP exposure after a median number of 5 PegASP doses in total (IQR, 3-7; range, 1-14). PegASP was replaced with Erwinia chrysanthemi-derived ASP because of allergy in 1 patient before the AAP event. The PegASP activity was measurable in 98% of the AAP patients during treatment with this information available (86/88, unknown in 80 patients). Of note, in 6 of the excluded 8 patients with acute pancreatitis occurring more than 4 weeks after the last PegASP dose, acute pancreatitis occurred within 32-90 days (median, 38 days) after the last PegASP dose. The remaining 2 patients developed acute pancreatitis 210 and 589 days, respectively, after the last PegASP dose (Data Supplement).
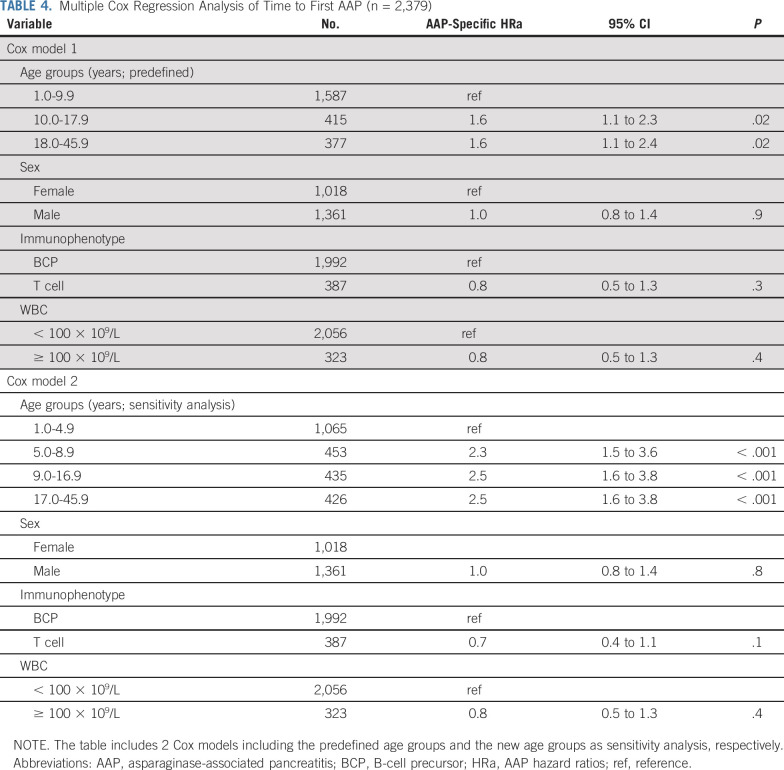
In a multiple Cox regression analysis (including sex, immunophenotype, and WBC), the hazard of AAP was doubled already since the age of 5 years (Table 4). Hence, patients aged 5.0-8.9 years demonstrated an HRa of 2.3 (95% CI, 1.5-3.6; P < .0001), patients aged 9.0-16.9 years demonstrated an HRa of 2.5 (95% CI, 1.6-3.8, P < .0001), and patients aged 17.0-45.9 years demonstrated an HRa of 2.5 (95% CI, 1.6 to 3.8; P < .0001; Table 4). No difference in the estimates was observed when including the initially excluded 8 patients diagnosed with acute pancreatitis more than 4 weeks after the last PegASP dose as a sensitivity analysis (results not shown). Additionally, the abovementioned associations remained unchanged when stratifying by induction glucocorticoids (prednisolone v dexamethasone) or by day 29 minimal residual disease-guided risk group (SR v IR v HR/HR-SCT), respectively (results not shown).
TABLE 4.
Multiple Cox Regression Analysis of Time to First AAP (n = 2,379)
AAP-Related Complications and Mortality
Of the 9 patients who presented with hemorrhagic and/or necrotizing pancreatitis at AAP diagnosis, 33% (3/9) had persisting symptoms and signs of chronic pancreatitis at last follow-up, and 1 of these died as a result of AAP (Table 2). Forty-five patients developed pseudocysts (27%; 45/167 with this information available), of whom 21% (9/43; unknown in 2 patients) had recurrent abdominal pain at last follow-up (Table 2).
In a sex-adjusted multiple logistic regression of any AAP-related complication (ie, AAP-related death; intensive care unit admission; acute and permanent need for insulin therapy; development of pancreatic pseudocysts; recurrent abdominal pain; elevated pancreatic enzymes at last follow-up; and imaging at last follow-up showing pancreatic inflammation/edema, pseudocysts, or hemorrhage), only patients with ≥ 100 days of follow-up after the AAP diagnosis (160/168) were included (median follow-up, 2.3 years; IQR, 1.3-4.2). Patients aged 10.0-17.9 years demonstrated more than 4-fold increased odds of developing any of these AAP-related complications (OR, 4.4; 95% CI, 1.7 to 11.2; P = .002), compared with patients aged 1.0-9.9 years (Table 5). Neither age ≥ 18.0 years (OR, 1.5; 95% CI, 0.7 to 3.5; P = .3) compared with children aged 1.0-9.9 years nor sex was associated with development of any AAP-related complication (Table 5). When including the 4 new age groups as a sensitivity analysis, both adolescents aged 9.0-16.9 years and adults aged 17.0-45.9 years had increased odds of developing any AAP-related complication (9.0-16.9 years: OR, 7.3, 95% CI, 2.7 to 19.7; P = .0001; and 17.0-45.9 years: OR, 2.6; 95% CI, 1.1 to 6.4; P = .04; Data Supplement), compared with children aged 1.0-4.9 years.
TABLE 5.
Multiple Logistic Regression of AAP-Related Complications (n = 160)
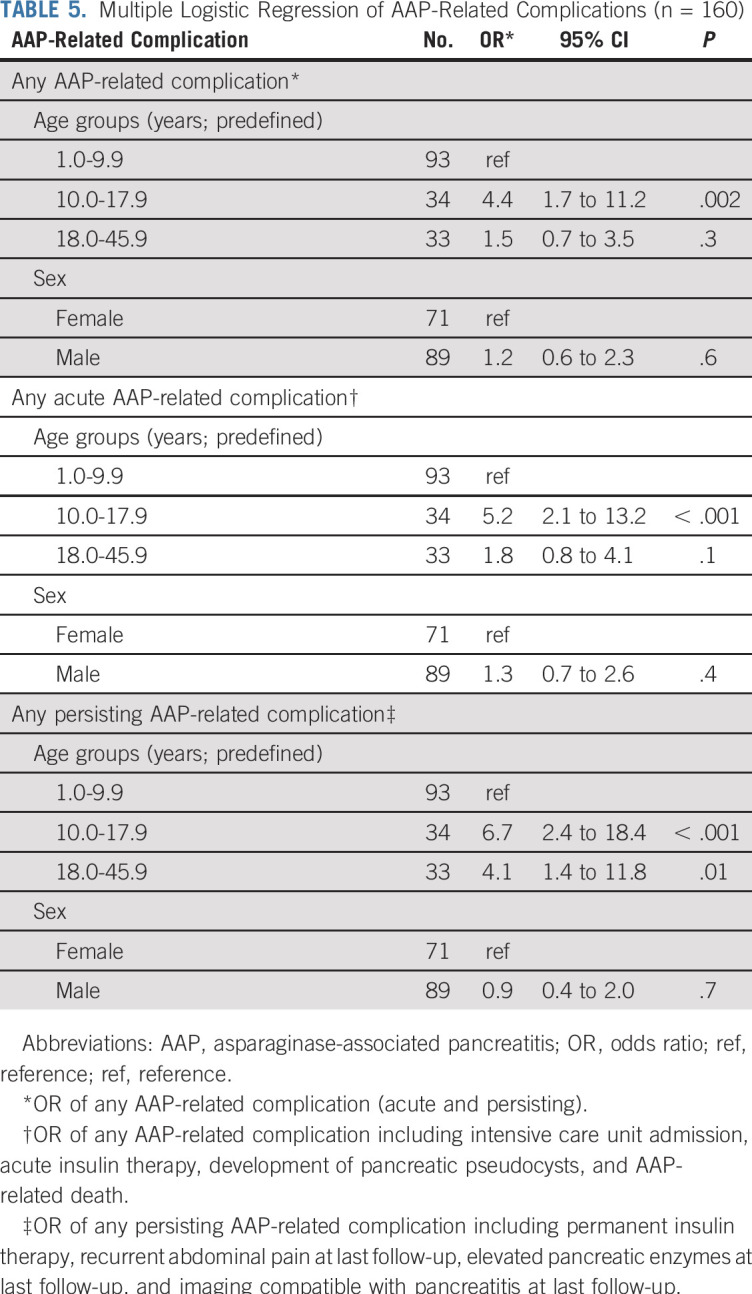
When analyzing development of any acute AAP-related complication (ie, AAP-related death, acute insulin need, intensive care unit admission, and pancreatic pseudocyst development), only patients aged 10.0-17.9 years had increased odds of developing any acute complication (OR, 5.2; 95% CI, 2.1 to 13.2; P = .0005), compared with patients aged 1.0-9.9 years (Table 5; Data Supplement). Odds of developing any persisting AAP-related complication (ie, elevated pancreatic enzymes at last follow-up; imaging at last follow-up showing pancreatic inflammation/edema, pseudocysts, or hemorrhage; permanent insulin need; and recurrent abdominal pain) were increased for patients aged 10.0-17.9 years (OR, 6.7; 95% CI, 2.4 to 18.4; P = .0002) and patients aged 18.0-45.9 years (OR, 4.1; 95% CI, 1.4 to 11.8; P = .01), compared with patients aged 1.0-9.9 years (Table 5; Data Supplement). Notably, in the sensitivity analysis, patients aged 5.0-8.9 years did not have increased odds of developing any persisting AAP-related complication (OR, 1.7; 95% CI, 0.3 to 10.8; P = .6), compared with patients aged 1.0-4.9 years (Data Supplement).
Death from any cause occurred as the first event in 81 of 2,448 patients, including 5 AAP patients, of whom 3 aged 8.6, 17.3, and 18.6 years died as a result of first-time AAP within 0-29 days from AAP diagnosis (AAP was an unexpected autopsy finding in 1 patient). In an age- and sex-adjusted Cox analysis of time to death in CR1, no difference was found when comparing AAP patients with non-AAP patients (HRa, 0.4; 95% CI, 0.05 to 2.6; P = .3; remaining results not shown). Stratifying the abovementioned models by risk group on day 29 did not change the results.
Asparaginase Re-Exposure and Relapse
Approximately one fifth (34/168) of the patients with AAP were rechallenged with ASP, of whom 44% (15/34) developed a second episode of AAP after a median of 2 ASP doses (IQR, 1-3; range, 1-7); 40% (6/15) were severe (Table 1). Development of a second AAP episode after ASP re-exposure was not significantly associated with age (Data Supplement). No patient with a second AAP episode was further re-exposed to ASP.
Leukemic relapse in CR1 occurred in 196 of 2,448 patients during the study period, including 21 AAP patients, among whom PegASP was truncated in 81% (17/21) because of AAP. The age- and sex-adjusted hazard of relapse among patients with AAP who were truncated in ASP was not significantly increased when comparing with patients with AAP who were re-exposed to ASP (5.0-year cumulative incidence of relapse: 13.2% v 14.2%; HRa, 1.0; 95% CI, 0.3 to 3.1; P = .97). Moreover, no difference between patients with AAP versus patients without AAP was found in an age- and sex-adjusted Cox analysis (HRa, 1.7; 95% CI, 0.8 to 3.3; P = .1; remaining results not shown). Stratifying the abovementioned models by risk group on day 29 did not change the results.
DISCUSSION
Despite superior cure rates for children versus adults with ALL, many centers do not use pediatric ALL protocols, partly because of worries about risk of toxicities. Because ASP plays a crucial role in pediatric ALL protocols, the present findings of no increased AAP risk in adults compared with children down to the age of 5 years—in spite of an ASP-heavy protocol—are comforting and in accordance with previous studies.34,35 Moreover, our findings are compatible with previous pediatric studies reporting more than a 2-fold increased AAP risk in patients older than 9 years of age.3,4,8,15,25 This similarity between older children and young adults with ALL has also recently been demonstrated for thromboembolic complications.2
Additionally, the odds of any AAP-related complication were increased by more than 7-fold in adolescents aged 9.0-16.9 years and more than 2-fold in adults aged 17.0-45.9 years, compared with younger children aged 1.0-4.9 years. In fact, adolescents had the most pronounced increase in odds, demonstrating more than 7-fold increased odds of any acute complication and more than 12-fold increased odds of any persisting complication, compared with the youngest children. This emphasizes the striking vulnerability of this age group, although the reasons hereof are unknown. Changes in endogenous sex hormones may give rise to the increased frequency of insulin resistance and (pre)metabolic syndrome during puberty,36 which has been associated with dyslipidemia and decreased antioxidant capacity.37 Oxidative stress and inflammation play a pivotal role in the pathogenesis of pancreatitis—and probably also in the development of pancreatitis-related complications.38,39
In contrast to age, the role of genetic predisposition is less clear. Recent genome-wide association studies have found different candidate single-nucleotidepolymorphisms associated with pancreatitis in patients with ALL5,9,26; however, only rs13228878 and rs10273639 associated with elevated expression of the PRSS1 gene encoding for trypsinogen have been validated.26
The early onset of AAP coincided with the PegASP administration, yet the cumulative PegASP dose up to the time of AAP ranged from 1,000 to 14,000 IU/m2. Substantial evidence supports that the AAP risk is proportional to the number of doses administered.9,32 Notably, this is in contrast to ASP hypersensitivity, which in general occurs after the first few doses.40 Although premature withdrawal of ASP has been linked to inferior survival4 and PegASP was truncated in the majority of patients with AAP (80%) in our study, neither AAP nor truncation of ASP because of AAP was associated with increased risk of relapse, potentially reflecting low study power in this regard. When looking at ASP re-exposure, 44% of those who were rechallenged with PegASP in our study developed a second event of acute pancreatitis (40% being severe cases), which is in accordance with previous findings in pediatric studies.5,18 Thus, current guidelines for children with ALL recommend ASP re-exposure only in patients who within 48 hours have no symptoms, normalized pancreatic enzymes, and no evidence of pseudocysts or necrosis.23 Of note, these guidelines are based on classification of acute pancreatitis according to the original Atlanta criteria—distinguishing between severe (lasting > 48 hours) and nonsevere (lasting ≤ 48 hours) acute pancreatitis.41,42 For adolescents and adults with ALL, some expert panel guidelines recommend permanent discontinuation of ASP for clinically acute pancreatitis (vomiting and severe abdominal pain with elevated pancreatic enzymes above 3 times the upper normal limit and/or pseudocyst development).43 However, the pressing question in relation to (1) the AAP risk in AAP-naive patients, (2) the risk of a second AAP episode after ASP re-exposure, and (3) overall survival after AAP-related ASP truncation remains unanswered: Who needs more, less, or no ASP? In that respect, the lack of association between characteristics of the first and second AAP is important, which supports that the decision on re-exposure primarily should reflect the anticipated risk of leukemic relapse—except for patients having persisting symptoms from their first AAP.
The main strengths of this study include the international, multicenter, and population-based design and the inclusion of uniformly treated patients with the same diagnosis across a wide age span. Additionally, the online mandatory prospective and systematic registration of 20 predefined treatment-related toxicities strengthens the reliability of the findings.27
The limitations include the lack of power regarding the analyses of leukemic relapse and second AAP event. Moreover, a potential introduction of selection bias in favor of patients with clear-cut symptoms exists because no systematic screening of AAP was performed in patients with abdominal pain or systemic inflammatory response syndrome. Thus, AAP can easily be misinterpreted as sepsis, unless pancreatic enzyme levels are measured.18 Still, the impact of age on AAP incidence and risk of complications stands strong, and it is unlikely that these potential weaknesses would have markedly influenced the findings.
In conclusion, older children and adults had similar AAP risk, whereas morbidity was most pronounced among adolescents. ASP re-exposure should be considered only for patients with an anticipated high risk of leukemic relapse, because multiple studies strongly indicate that reduction of ASP treatment intensity increases the risk of relapse.4,44,45
ACKNOWLEDGMENT
We sincerely thank the acute lymphoblastic leukemia (ALL) patients, colleagues, and research nurses at the ALL centers for contributing to the study, reporting data to the NOPHO ALL registry, and completing the questionnaires. We also thank Kirsten Kørup Rasmussen, Louise Rold Helt, and Pernille Rudebeck Mogensen (Pediatric Research Laboratory, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark) for data extraction help.
Footnotes
Supported by research grants from the Research Foundation of Rigshospitalet (University of Copenhagen; CUR), Krista and Viggo Petersen’s Foundation (Litra D/6034-29; CUR), the Danish Childhood Cancer Foundation (KS), and the Danish Cancer Society (KS; BOW).
AUTHOR CONTRIBUTIONS
Conception and design: Cecilie U. Rank, Benjamin O. Wolthers, Thomas L. Frandsen, Nina Toft, Peder S. Wehner, Arja Harila-Saari, Petter Quist-Paulsen, Ulla Wartiovaara-Kautto, Ólafur G. Jónsson, Kjeld Schmiegelow
Administrative support: Birgitte K. Albertsen
Provision of study materials or patients: Birgitte K. Albertsen, Ulrik M. Overgaard, Arja Harila-Saari, Johan Malmros, Jonas Abrahamsson, Ulrika Norén-Nyström, Beata Tomaszewska-Toporska, Bendik Lund, Laimonas Griškevičius, Ulla Wartiovaara-Kautto, Mari Punab, Kjeld Schmiegelow
Collection and assembly of data: Cecilie U. Rank, Benjamin O. Wolthers, Birgitte K. Albertsen, Thomas L. Frandsen, Ulrik M. Overgaard, Nina Toft, Peder S. Wehner, Arja Harila-Saari, Johan Malmros, Mats M. Heyman, Jonas Abrahamsson, Ulrika Norén-Nyström, Beata Tomaszewska-Toporska, Bendik Lund, Kirsten B. Jarvis, Petter Quist-Paulsen, Goda E. Vaitkevičienė, Laimonas Griškevičius, Mervi Taskinen, Ulla Wartiovaara-Kautto, Kristi Lepik, Mari Punab, Ólafur G. Jónsson, Kjeld Schmiegelow
Data analysis and interpretation: Cecilie U. Rank, Benjamin O. Wolthers, Kathrine Grell, Birgitte K. Albertsen, Thomas L. Frandsen, Nina Toft, Ove J. Nielsen, Mats M. Heyman, Bendik Lund, Petter Quist-Paulsen, Ulla Wartiovaara-Kautto, Kjeld Schmiegelow
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Asparaginase-Associated Pancreatitis in Acute Lymphoblastic Leukemia: Results From the NOPHO ALL2008 Treatment of Patients 1-45 Years of Age
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Cecilie U. Rank
Travel, Accommodations, Expenses: Lundbeck
Benjamin O. Wolthers
Employment: Novo Nordisk
Birgitte K. Albertsen
Honoraria: Shire
Research Funding: Erytech Pharma (Inst)
Mats M. Heyman
Honoraria: Pfizer (Inst), Servier (Inst)
Research Funding: Pfizer (Inst), Servier (Inst)
Ulla Wartiovaara-Kautto
Honoraria: Pfizer, Sanofi
Consulting or Advisory Role: Pfizer, Celgene
Travel, Accommodations, Expenses: Roche, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–615. doi: 10.1038/leu.2017.265. [DOI] [PubMed] [Google Scholar]
- 2.Rank CU, Toft N, Tuckuviene R, et al. Thromboembolism in acute lymphoblastic leukemia: Results of NOPHO ALL2008 protocol treatment in patients aged 1 to 45 years. Blood. 2018;131:2475–2484. doi: 10.1182/blood-2018-01-827949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney SL, Dahlberg SE, Levy DE, et al. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2009;53:162–167. doi: 10.1002/pbc.22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 5.Wolthers BO, Frandsen TL, Abrahamsson J, et al. Asparaginase-associated pancreatitis: A study on phenotype and genotype in the NOPHO ALL2008 protocol. Leukemia. 2017;31:325–332. doi: 10.1038/leu.2016.203. [DOI] [PubMed] [Google Scholar]
- 6.Samarasinghe S, Dhir S, Slack J, et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013;162:710–713. doi: 10.1111/bjh.12407. [DOI] [PubMed] [Google Scholar]
- 7.Raja RA, Schmiegelow K, Albertsen BK, et al. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the NOPHO ALL2008 protocol. Br J Haematol. 2014;165:126–133. doi: 10.1111/bjh.12733. [DOI] [PubMed] [Google Scholar]
- 8.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109:896–904. doi: 10.1182/blood-2006-06-027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;34:2133–2140. doi: 10.1200/JCO.2015.64.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): A randomised controlled trial. Lancet Oncol. 2014;15:809–818. doi: 10.1016/S1470-2045(14)70243-8. [DOI] [PubMed] [Google Scholar]
- 11.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: Results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–1210. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: Results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trial. Blood. 2002;99:2734–2739. doi: 10.1182/blood.v99.8.2734. [DOI] [PubMed] [Google Scholar]
- 13.Sahu S, Saika S, Pai SK, et al. L-asparaginase (Leunase) induced pancreatitis in childhood acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1998;15:533–538. doi: 10.3109/08880019809018315. [DOI] [PubMed] [Google Scholar]
- 14.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 15.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol. 2007;25:813–819. doi: 10.1200/JCO.2006.08.6397. [DOI] [PubMed] [Google Scholar]
- 16.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338:1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 17. Aldoss I, Pullarkat V, Martinez D, et al: The number of PEG-asparaginase doses administered is a determinant of relapse risk in adult ALL treated with a pediatric-like regimen. Blood 122:3915, 2013. [Google Scholar]
- 18.Wolthers BO, Frandsen TL, Baruchel A, et al. Asparaginase-associated pancreatitis in childhood acute lymphoblastic leukaemia: An observational Ponte di Legno Toxicity Working Group study. Lancet Oncol. 2017;18:1238–1248. doi: 10.1016/S1470-2045(17)30424-2. [DOI] [PubMed] [Google Scholar]
- 19.Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28:97–113. doi: 10.1016/s1040-8428(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 20.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 21.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375:1972–1981. doi: 10.1056/NEJMra1505202. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Calderón J, Exiga-Gonzaléz E, Morán-Villota S, et al. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol. 2009;31:790–793. doi: 10.1097/MPH.0b013e3181b794e8. [DOI] [PubMed] [Google Scholar]
- 23.Raja RA, Schmiegelow K, Frandsen TL. Asparaginase-associated pancreatitis in children. Br J Haematol. 2012;159:18–27. doi: 10.1111/bjh.12016. [DOI] [PubMed] [Google Scholar]
- 24.Wolthers BO, Mogensen PR, Frandsen TL, et al. Insulin-dependent diabetes: A chronic complication to acute pancreatitis in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2019;66:e27437. doi: 10.1002/pbc.27437. [DOI] [PubMed] [Google Scholar]
- 25.Knoderer HM, Robarge J, Flockhart DA. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2007;49:634–639. doi: 10.1002/pbc.21037. [DOI] [PubMed] [Google Scholar]
- 26.Wolthers BO, Frandsen TL, Patel CJ, et al. Trypsin-encoding PRSS1-PRSS2 variations influence the risk of asparaginase-associated pancreatitis in children with acute lymphoblastic leukemia: A Ponte di Legno toxicity working group report. Haematologica. 2019;104:556–563. doi: 10.3324/haematol.2018.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frandsen TL, Heyman M, Abrahamsson J, et al. Complying with the European Clinical Trials directive while surviving the administrative pressure - An alternative approach to toxicity registration in a cancer trial. Eur J Cancer. 2014;50:251–259. doi: 10.1016/j.ejca.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Toft N, Birgens H, Abrahamsson J, et al. Toxicity profile and treatment delays in NOPHO ALL2008-comparing adults and children with Philadelphia chromosome-negative acute lymphoblastic leukemia. Eur J Haematol. 2016;96:160–169. doi: 10.1111/ejh.12562. [DOI] [PubMed] [Google Scholar]
- 29.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: A Delphi consensus. Lancet Oncol. 2016;17:e231–e239. doi: 10.1016/S1470-2045(16)30035-3. [DOI] [PubMed] [Google Scholar]
- 30. doi: 10.1002/pbc.26686. Tram Henriksen L, Gottschalk Højfeldt S, Schmiegelow K, et al: Prolonged first-line PEG-asparaginase treatment in pediatric acute lymphoblastic leukemia in the NOPHO ALL2008 protocol-pharmacokinetics and antibody formation. Pediatr Blood Cancer 64:e26686, 2017. [DOI] [PubMed] [Google Scholar]
- 31.Toft N, Birgens H, Abrahamsson J, et al. Risk group assignment differs for children and adults 1-45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL-2008 protocol. Eur J Haematol. 2013;90:404–412. doi: 10.1111/ejh.12097. [DOI] [PubMed] [Google Scholar]
- 32.Albertsen BK, Grell K, Abrahamsson J, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: A NOPHO ALL2008 randomized study. J Clin Oncol. 2019;37:1638–1646. doi: 10.1200/JCO.18.01877. [DOI] [PubMed] [Google Scholar]
- 33.Nysom K, Mølgaard C, Hutchings B, et al. Body mass index of 0 to 45-y-old Danes: Reference values and comparison with published European reference values. Int J Obes Relat Metab Disord. 2001;25:177–184. doi: 10.1038/sj.ijo.0801515. [DOI] [PubMed] [Google Scholar]
- 34.DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia. 2015;29:526–534. doi: 10.1038/leu.2014.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribera JM, Oriol A, Sanz MA, et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol. 2008;26:1843–1849. doi: 10.1200/JCO.2007.13.7265. [DOI] [PubMed] [Google Scholar]
- 36.Agirbasli M, Agaoglu NB, Orak N, et al. Sex hormones and metabolic syndrome in children and adolescents. Metabolism. 2009;58:1256–1262. doi: 10.1016/j.metabol.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrijevic-Sreckovic V, Colak E, Djordjevic P, et al. Prothrombogenic factors and reduced antioxidative defense in children and adolescents with pre-metabolic and metabolic syndrome. Clin Chem Lab Med. 2007;45:1140–1144. doi: 10.1515/CCLM.2007.259. [DOI] [PubMed] [Google Scholar]
- 38.Verlaan M, Roelofs HM, van-Schaik A, et al. Assessment of oxidative stress in chronic pancreatitis patients. World J Gastroenterol. 2006;12:5705–5710. doi: 10.3748/wjg.v12.i35.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robles L, Vaziri ND, Ichii H. Role of oxidative stress in the pathogenesis of pancreatitis: Effect of antioxidant therapy. Pancreat Disord Ther. 2013;3:112. doi: 10.4172/2165-7092.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123:2026–2033. doi: 10.1182/blood-2013-10-534347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bradley EL., III A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 42.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 43.Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: Recommendations of an expert panel. Leuk Lymphoma. 2011;52:2237–2253. doi: 10.3109/10428194.2011.596963. [DOI] [PubMed] [Google Scholar]
- 44.Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer. 2011;117:238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Højfeldt SG, Grell K, Abrahamsson J, et al: Relapse following truncation of asparaginase in NOPHO ALL2008, Nordic Society of Paediatric Haematology and Oncology 37th Annual Meeting. Aalborg, Denmark, May 3-7, 2019. [Google Scholar]