Summary
Introduction
Chronic infections and infestations represent one of the leading causes of cancer. Eleven agents have been categorized by the International Agency for Research on Cancer (IARC) in Group 1, 3 in Group 2A and 4 in Group 2B. We previously estimated that the incidence of cancers associated with infectious agents accounted for the 8.5% of new cancer cases diagnosed in Italy in 2014.
Methods
In the present study we evaluated the incidence of cancer in Italy and in the 20 Italian regions in 2018, based on the data of Cancer Registries, and calculated the fraction attributable to infectious agents.
Results
Cancers of infectious origin contributed to the overall burden of cancer in Italy with more than 27,000 yearly cases, the 92% of which was attributable to Helicobacter pylori, human papillomaviruses, and hepatitis B and C viruses. With the exception of papillomavirus-related cancers, the incidence of cancers of infectious origin was higher in males (16,000 cases) than in females (11,000 cases). There were regional and geographical variations of cancers depending on the type of cancer and on the gender. Nevertheless, the overall figures were rather similar, the infection-related cancers accounting for the 7.2, 7.6, and 7.1% of all cancers in Northern, Central, and Southern Italy, respectively.
Conclusions
The estimate of the incidence of cancers attributable to infectious agents in Italy in 2018 (7.3% of all cancer cases) is approximately half of the worldwide burden, which has been estimated by IARC to be the 15.4% of all cancer cases in 2012.
Keywords: Cancer incidence, Chronic infections and cancer, Infection-related cancers, Italy, Regional distribution
Introduction
Altogether, chronic infections and infestations represent one of the leading causes of cancer worldwide. Eleven agents have been categorized by IARC (International Agency for Research on Cancer) in Group 1 (sufficient evidence of carcinogenicity to humans), 3 in Group 2A (probably carcinogenic), and 4 in Group 2B (possibly carcinogenic) [1]. Several other viruses, bacteria and protozoa have been suspected to be associated with various human cancers [2, 3].
The global burden of infection-related cancers has been estimated and periodically updated at IARC. The population attributable fraction (PAF) in the world population, in terms of incidence, was estimated to be the 15.6% in 1990 [4], 17.8% in 2002 [5], 16.1% in 2008 [6], and 15.4% in 2012 [7]. The last figure corresponds to 2 million new cancer cases of infectious origin out of 14 million cases of all cancers, with broad variations depending on the geographical region and on the developmental status. In fact, the PAFs varied from less than 5% in North America, Australia-New Zealand and some Western and Northern Europe countries to more than 50% in sub-Saharian Africa countries [7].
The PAF, indicating the proportion of cancers of infectious and parasitic origin, has been estimated in various countries based either on morbidity and/or on mortality data. Thus, mortality PAF estimates have been made in the USA in 1981 (10%) [8], in the UK both in 1998 (10-20%) [9] and 2005 (5%) [10], in France in 2000 (3.6%) [11], and in China in 2005 (29.4%) [12]. Other studies estimated incidence data or both incidence and mortality data. For instance, the 4.1% of all new cancer cases occurring in France in 2015 were attributable to infectious agents [13]. In China, infection-related cancers accounted in 2005 for 25.9 and 29.4% of the overall cancer cases and deaths, respectively [14]. In another study in China, cancers related to the main cancer-related pathogens in men and women accounted for 17.7 and 15.4% (incidence) and 20.0 and 16.9% (mortality), respectively [15]. In the Korean population, for 2007 the fractions of all cancers attributable to infection were 25.1% and 16.8% for cancer incidence in men and women, and 25.8% and 22.7% for cancer mortality in men and women, respectively [16]. The 2.9% of cancers diagnosed in Australia in 2010 were attributable to infectious agents [17].
We previously estimated the PAF of infection-related cancers in Italy, based on 2014 national incidence data [2]. That analysis demonstrated that cancers associated with 6 pathogens accounted for 31,000 out of 365,000 new yearly cases (8.5%), 42.0% of which was attributable to Helicobacter pylori (Hp), 34.7% to hepatitis B virus (HBV) and hepatitis C virus (HCV), 19.8% to human papillomaviruses (HPV), 2.9% to human herpesvirus 8 (HHV8) or Kaposi’s sarcoma virus (KSHV), and 0.2% to human herpesvirus 4 (HHV4) or Epstein-Barr virus (EBV). The objectives of the present study were to reassess the incidence of infection-related cancers in Italy 4 years later and to evaluate the geographical distribution of these cancers in the 20 Italian regions. To this purpose, we made estimates of the incidence and of the attributable fractions (AFs) of cancers associated with IARC Group 1 pathogens, excepting the infestations by trematodes, which are rare in Italy, and HIV-1 infections, because HIV-related immunodeficiency requires the concomitant infection with other carcinogenic infectious agents [1].
Methods
MONITORED REGIONS AND COVERAGE BY CANCER REGISTRIES (CR)
The study covered the whole Italian territory, which includes 20 regions whose localization is shown in the map (Fig. S1). The regions were grouped according to geographic areas (North, Central or South) [18]. Northern regions include, in alphabetical order, Emilia Romagna, Friuli Venezia Giulia, Liguria (Ligury), Lombardia (Lombardy), Piemonte (Piedmont), Trentino Alto Adige (Trentino South-Tyrol), Valle d’Aosta (Aosta Valley), and Veneto, having an overall population of 27,746,158 residents as to January 2018 (Tab. SI). Central regions include Lazio (Latium), Marche (Marches), Toscana (Tuscany), and Umbria, having an overall population of 12,050,054 residents (Tab. SII). Southern and Insular regions include Abruzzo, Basilicata, Calabria, Molise, Puglia (Apulia), Sardegna (Sardinia), and Sicilia (Sicily), having an overall population of 20,697,761 residents (Tab. SIII).
Fig. S1.
Geographical distribution of the 20 Italian regions.
Tab. S1.
Estimated incidence (new cases/100,000 residents) of cancers attributable to infectious agents in Northern Italy regions in 2018.
| Gender | Emilia Romagna | Friuli Venezia Giulia | Liguria | Lombardia | Piemonte | Trentino Alto Adige | Valle d’Aosta | Veneto | All Northern regions | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resident population as to January 2018 | M | 2,162,684 | 589,785 | 743,755 | 4,907,685 | 2,123,610 | 525,523 | 61,695 | 2,395,801 | 13,510,538 | |
| F | 2,289,945 | 625,753 | 813,226 | 5,128,573 | 2,252,255 | 542,125 | 64,507 | 2,509,236 | 14,225,620 | ||
| M+F | 4,452,629 | 1,215,538 | 1,556,981 | 10,036,258 | 4,375,865 | 1,067,648 | 126,202 | 4,905,037 | 27,746,158 | ||
| Population covered by Cancer Registries (%) | M+F | 80 | 100 | 55 | 92 | 30 | 100 | 100 | 53 | 71 | |
| Cancer site (ICD-10) | Infectious agent | ||||||||||
| Non-cardia stomach (C16.1-C16.9) | Hp | M | 30.6 | 35.8 | 25.9 | 28.2 | 27.1 | 19.7 | 26.0 | 20.2 | 26.7 |
| F | 19.6 | 17.8 | 14.3 | 15.1 | 14.0 | 13.0 | 12.4 | 10.9 | 14.6 | ||
| M+F | 50.3 | 53.6 | 40.2 | 43.3 | 41.1 | 32.7 | 38.4 | 31.1 | 41.3 | ||
| MALT (C88.4) | Hp | M | 0.4 | 0.0 | 0.4 | 0.5 | 0.4 | 1.1 | 0.0 | 0.4 | 0.4 |
| F | 0.5 | 0.0 | 0.3 | 0.3 | 0.2 | 0.3 | 0.0 | 0.2 | 0.2 | ||
| M+F | 0.9 | 0.0 | 0.7 | 0.8 | 0.6 | 1.4 | 0.0 | 0.6 | 0.6 | ||
| Total Hp-associated cancers | Hp | M | 31.0 | 35.8 | 26.3 | 28.6 | 27.5 | 20.8 | 26.0 | 20.6 | 27.1 |
| F | 20.1 | 17.8 | 14.6 | 15.3 | 14.2 | 13.2 | 12.4 | 11.1 | 14.9 | ||
| M+F | 51.1 | 53.6 | 40.9 | 43.9 | 41.7 | 34.0 | 38.4 | 31.7 | 42.0 | ||
| Liver (C22) | HBV
(± HDV) + HCV |
M | 18.8 | 22.5 | 24.0 | 24.5 | 24.6 | 20.8 | 29.8 | 22.3 | 23.4 |
| F | 7.8 | 7.5 | 10.1 | 9.5 | 9.3 | 4.9 | 5.3 | 7.8 | 7.8 | ||
| M+F | 26.6 | 30.0 | 34.1 | 34.0 | 33.9 | 25.7 | 35.1 | 30.1 | 31.2 | ||
| Uterine cervix
(C53) |
HPV | M | - | - | - | - | - | - | - | - | - |
| F | 7.6 | 9.1 | 10.7 | 8.7 | 9.4 | 5.5 | 9.3 | 7.5 | 8.5 | ||
| M+F | 7.6 | 9.1 | 10.7 | 8.7 | 9.4 | 5.5 | 9.3 | 7.5 | 8.5 | ||
| Vulva
(C51) |
HPV | M | - | - | - | - | - | - | - | - | - |
| F | 1.2 | 1.4 | 1.1 | 1.0 | 1.0 | 0.8 | 0.8 | 1.1 | 1.0 | ||
| M+F | 1.2 | 1.4 | 1.1 | 1.0 | 1.0 | 0.8 | 0.8 | 1.1 | 1.0 | ||
| Vagina
(C52) |
HPV | M | - | - | - | - | - | - | - | - | - |
| F | 0.7 | 0.8 | 0.8 | 0.7 | 0.7 | 1.0 | 0.0 | 0.7 | 0.7 | ||
| M+F | 0.7 | 0.8 | 0.8 | 0.7 | 0.7 | 1.0 | 0.0 | 0.7 | 0.7 | ||
| Penis
(C60) |
HPV | M | 0.8 | 0.8 | 1.3 | 0.8 | 1.0 | 0.7 | 0.8 | 0.8 | 0.9 |
| F | - | - | - | - | - | - | - | - | - | ||
| M+F | 0.8 | 0.8 | 1.3 | 0.8 | 1.0 | 0.7 | 0.8 | 0.8 | 0.9 | ||
| Oral cavity
(C00-C08) |
HPV | M | 0.4 | 0.6 | 0.4 | 0.4 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| F | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | ||
| M+F | 0.7 | 0.9 | 0.7 | 0.6 | 0.7 | 0.7 | 0.8 | 0.8 | 0.8 | ||
| Oropharynx
(C09-C10, C12-C14) |
HPV | M | 1.4 | 3.2 | 1.6 | 1.7 | 1.8 | 2.4 | 2.0 | 1.6 | 1.9 |
| F | 0.4 | 0.8 | 0.4 | 0.5 | 0.4 | 0.4 | 0.7 | 0.4 | 0.5 | ||
| M+F | 1.8 | 4.0 | 2.0 | 2.2 | 2.2 | 2.8 | 2.7 | 2.0 | 2.4 | ||
| Larynx
(C32) |
HPV | M | 0.6 | 1.0 | 0.8 | 0.7 | 0.7 | 0.5 | 1.1 | 0.8 | 0.8 |
| F | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | ||
| M+F | 0.7 | 1.1 | 0.9 | 0.8 | 0.8 | 0.6 | 1.3 | 0.9 | 0.9 | ||
| Total HPV-associated cancers | HPV | M | 3.3 | 5.6 | 4.2 | 3.6 | 3.9 | 4.0 | 4.3 | 3.6 | 4.1 |
| F | 10.2 | 12.5 | 13.5 | 11.1 | 11.9 | 8.1 | 11.3 | 10.0 | 11.1 | ||
| M+F | 13.5 | 18.1 | 17.7 | 14.7 | 15.8 | 12.2 | 15.6 | 13.6 | 15.2 | ||
| Nasopharynx
(C11) |
EBV (HHV4) | M | 1.2 | 0.5 | 1.2 | 1.2 | 1.3 | 0.8 | 1.3 | 0.8 | 1.0 |
| F | 0.4 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.0 | 0.4 | 0.4 | ||
| M+F | 1.6 | 1.0 | 1.6 | 1.6 | 1.7 | 1.2 | 1.3 | 1.2 | 1.4 | ||
| Burkitt’s lymphoma
(C83.7) |
EBV (HHV4) ± Pf | M | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 |
| F | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | ||
| M+F | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.0 | 0.0 | 0.1 | 0.1 | ||
| Hodgkin’s lymphoma
(C81) |
EBV (HHV4) | M | 1.6 | 1.3 | 1.8 | 1.4 | 1.4 | 1.2 | 0.0 | 1.6 | 1.3 |
| F | 1.1 | 1.2 | 0.8 | 1.2 | 1.2 | 1.0 | 0.6 | 1.4 | 1.1 | ||
| M+F | 2.7 | 2.5 | 2.6 | 2.6 | 2.6 | 2.2 | 0.6 | 3.0 | 2.4 | ||
| Total EBV-associated cancers | EBV | M | 2.9 | 2.0 | 3.1 | 2.8 | 2.8 | 1.9 | 1.3 | 2.5 | 2.4 |
| F | 1.5 | 1.8 | 1.2 | 1.6 | 1.6 | 1.4 | 0.6 | 1.8 | 1.4 | ||
| M+F | 4.4 | 3.8 | 4.3 | 4.4 | 4.4 | 3.3 | 1.9 | 4.3 | 3.8 | ||
| Kaposi’s sarcoma
(C46) |
KSHV (HHV8) | M | 2.2 | 1.4 | 1.6 | 2.2 | 2.6 | 1.0 | 1.6 | 1.3 | 1.7 |
| F | 0.8 | 0.0 | 1.1 | 0.8 | 0.9 | 0.6 | 0.0 | 0.2 | 0.6 | ||
| M+F | 3.0 | 1.4 | 2.7 | 3.0 | 3.5 | 1.6 | 1.6 | 1.5 | 2.3 | ||
| Adult T cell lymphoma/leukemia (C91.5) | HTLV1 | M | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| M+F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| All the above cancers | M | 58.2 | 67.1 | 59.0 | 61.6 | 61.4 | 48.5 | 63.0 | 50.2 | 58.7 | |
| F | 40.4 | 39.6 | 30.3 | 38.3 | 37.9 | 28.3 | 29.5 | 31.0 | 35.7 | ||
| M+F | 98.6 | 106.7 | 89.3 | 99.9 | 99.3 | 76.8 | 92.5 | 81.2 | 94.4 |
Hp: Helicobacter pylori; HBV: hepatitis B virus; HCV: hepatitis C virus; HDV: hepatitis D virus (Delta agent); HPV: human papillomavirus; EBV: Epstein-Barr virus; HHV4: human herpesvirus 4; PF: Plasmodium falciparum; KSKV: Kaposi’s sarcoma virus; HHV8: human herpesvirus 8; HTLV: human T-lymphotropic virus; M: males; F: females.
Tab. SII.
Estimated incidence (new cases/100,000 residents) of cancers attributable to infectious agents in Central Italy regions in 2018.
| Gender | Lazio | Marche | Toscana | Umbria | All Central regions | ||
|---|---|---|---|---|---|---|---|
| Resident population as to January 2018 | M | 2,848,727 | 743,645 | 1,803,203 | 425,547 | 5,821,122 | |
| F | 3, 047,966 | 788,108 | 1,933,765 | 459,093 | 6,228,932 | ||
| M+F | 5,896,693 | 1,531,753 | 3,736,968 | 884,640 | 12,050,054 | ||
| Population covered by Cancer Registries (%) | M+F | 15 | 0 | 33 | 100 | 25 | |
|
Cancer site
(ICD-10) |
Infectious agent | ||||||
| Non-cardia stomach (C16.1-C16.9) | Hp | M | 24.0 | 34.1 | 34.3 | 37.2 | 32.4 |
| F | 14.4 | 20.9 | 20.9 | 21.9 | 19.6 | ||
| M+F | 38.4 | 55.0 | 55.2 | 59.1 | 52.0 | ||
| MALT (C88.4) | Hp | M | 0.4 | 0.6 | 0.6 | 0.5 | 0.6 |
| F | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 | ||
| M+F | 0.7 | 1.0 | 0.9 | 0.8 | 0.9 | ||
| Total Hp-associated cancers | Hp | M | 24.4 | 34.7 | 34.9 | 37.8 | 33.0 |
| F | 14.7 | 21.3 | 21.3 | 22.2 | 19.9 | ||
| M+F | 39.1 | 56.0 | 56.2 | 60.0 | 52,9 | ||
| Liver (C22) | HBV (± HDV) +HCV | M | 18.8 | 16.4 | 15.1 | 18.4 | 17.2 |
| F | 7.9 | 6.9 | 6.4 | 7.6 | 7.2 | ||
| M+F | 26.7 | 23.3 | 21.5 | 26.0 | 24.4 | ||
| Uterine cervix (C53) | HPV | M | - | - | - | - | |
| F | 7.6 | 8.1 | 8.8 | 8.7 | 8.3 | ||
| M+F | 7.6 | 8.1 | 8.8 | 8.7 | 8.3 | ||
| Vulva (C51) | HPV | M | - | - | - | - | |
| F | 1.0 | 1.2 | 1.1 | 1.3 | 1.2 | ||
| M+F | 1.0 | 1.2 | 1.1 | 1.3 | 1.2 | ||
| Vagina (C52) | HPV | M | - | - | - | - | |
| F | 0.6 | 0.6 | 0.5 | 0.7 | 0.6 | ||
| M+F | 0.6 | 0.6 | 0.5 | 0.7 | 0.6 | ||
| Penis (C60) | HPV | M | 0.9 | 1.2 | 1.1 | 1.3 | 1.1 |
| F | - | - | - | - | |||
| M+F | 0.9 | 1.2 | 1.1 | 1.3 | 1.1 | ||
| Oral cavity (C00-C08) | HPV | M | 0.4 | 0.4 | 0.4 | 0.5 | 0.4 |
| F | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||
| M+F | 0.6 | 0.6 | 0.6 | 0.7 | 0.6 | ||
| Oropharynx (C09-C10, C12-C14) | HPV | M | 1.2 | 0.9 | 1.0 | 0.9 | 1.0 |
| F | 0.3 | 0.2 | 0.2 | 0.3 | 0.3 | ||
| M+F | 1.5 | 1.1 | 1.2 | 1.2 | 1.3 | ||
| Larynx (C32) | HPV | M | 0.7 | 0.6 | 0.7 | 0.6 | 0.7 |
| F | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||
| M+F | 0.8 | 0.7 | 0.8 | 0.7 | 0.8 | ||
| Total HPV-associated cancers | HPV | M | 3.1 | 3.2 | 3.2 | 3.3 | 3.2 |
| F | 9.7 | 10.4 | 11.0 | 11.3 | 10.6 | ||
| M+F | 12.8 | 13.6 | 14.2 | 14.6 | 13.8 | ||
| Nasopharynx (C11) | EBV (HHV4) | M | 1.2 | 0.9 | 1.0 | 0.8 | 1.0 |
| F | 0.4 | 0.5 | 0.4 | 0.7 | 0.5 | ||
| M+F | 1.6 | 1.4 | 1.4 | 1.5 | 1.5 | ||
| Burkitt’s lymphoma (C83.7) | EBV (HHV4) ± Pf | M | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 |
| F | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | ||
| M+F | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | ||
| Hodgkin’s lymphoma (C81) | EBV (HHV4) | M | 1.6 | 1.8 | 1.9 | 1.7 | 1.7 |
| F | 1.2 | 1.2 | 1.0 | 1.4 | 1.2 | ||
| M+F | 2.8 | 3.0 | 2.9 | 3.1 | 2.9 | ||
| Total EBV-associated cancers | EBV | M | 2.8 | 2.7 | 2.9 | 2.5 | 2.7 |
| F | 1.5 | 1.8 | 1.4 | 2.2 | 1.7 | ||
| M+F | 4.31 | 4.5 | 4.3 | 4.7 | 4.4 | ||
| Kaposi’s sarcoma (C46) | KSHV (HHV8) | M | 2.0 | 1.1 | 1.6 | 0.7 | 1.3 |
| F | 0.8 | 0.3 | 0.2 | 0.2 | 0.4 | ||
| M+F | 2.8 | 1.4 | 1.8 | 0.9 | 1.7 | ||
| Adult T cell lymphoma/leukemia (C91.5) | HTLV1 | M | 0.0 | 0.1 | 0.2 | 0.2 | 0.1 |
| F | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | ||
| M+F | 0.0 | 0.1 | 0.3 | 0.2 | 0.1 | ||
| All the above cancers | M | 51.3 | 58.2 | 57.8 | 62.8 | 57.5 | |
| F | 34.6 | 40.6 | 40.4 | 43.4 | 39.7 | ||
| M+F | 85.9 | 98.8 | 98.2 | 106.2 | 97.2 |
Hp: Helicobacter pylori; HBV: hepatitis B virus; HCV: hepatitis C virus; HDV: hepatitis D virus (Delta agent); HPV: human papillomavirus; EBV: Epstein-Barr virus; HHV4: human herpesvirus 4; PF: Plasmodium falciparum; KSKV: Kaposi’s sarcoma virus; HHV8: human herpesvirus 8; HTLV: human T-lymphotropic virus; M: males; F: females.
Tab. SIII.
Estimated incidence (new cases/100,000 residents) of cancers attributable to infectious agents in Southern Italy regions in 2018.
| Gender | Abruzzo | Basilicata | Calabria | Campania | Molise | Puglia | Sardegna | Sicilia | All Southern regions | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Resident population as to January 2018 | M | 641,185 | 278,882 | 959,437 | 2,841,049 | 152,228 | 1,967,751 | 810,072 | 2,445,343 | 10,095,947 | |
| F | 674,011 | 288,236 | 997,250 | 2,985,811 | 156,265 | 838,104 | 2,581,646 | 10,601,814 | |||
| M+F | 1,315,196 | 567,118 | 1,956,687 | 5,826,860 | 308,493 | 4,048,242 | 1,648,176 | 5,026,929 | 20,697,761 | ||
| Population covered by Cancer Registries (%) | M+F | 0 | 100 | 64 | 71 | 0 | 54 | 42 | 91 | 65 | |
|
Cancer site
(ICD-10) |
Infectious agent | ||||||||||
| Non-cardia stomach (C16.1-C16.9) | Hp | M | 20.0 | 30.0 | 24.4 | 19.9 | 20.5 | 17.6 | 17.8 | 16.1 | 20.8 |
| F | 13.1 | 17.9 | 12.5 | 12.8 | 13.7 | 11.9 | 11.8 | 10.2 | 13.0 | ||
| M+F | 33.1 | 47.9 | 36.9 | 32.7 | 34.2 | 29.5 | 29.6 | 26.3 | 33.8 | ||
| MALT (C88.4) | Hp | M | 04 | 0.00 | 0.2 | 0.3 | 0.5 | 0.5 | 0.7 | 0.3 | 0.4 |
| F | 0.2 | 0.5 | 0.4 | 0.2 | 0.0 | 0.3 | 0.4 | 0.2 | 0.3 | ||
| M+F | 0.6 | 0.5 | 0.6 | 0.5 | 0.5 | 0.8 | 1.1 | 0.5 | 0.7 | ||
| Total Hp-associated cancers | Hp | M | 20.3 | 30.0 | 24.6 | 20.2 | 21.0 | 18.1 | 18.5 | 16.4≤ | 21.1 |
| F | 13.3 | 18.4 | 12.9 | 13.0 | 13.7 | 12.1 | 12.1 | 10.3 | 13.2 | ||
| M+F | 33.6 | 48.4 | 37.5 | 33.2 | 34.7 | 30.2 | 30.6 | 26.7 | 34.3 | ||
| Liver (C22) | HBV (± HDV) + HCV | M | 20.8 | 21.5 | 20.4 | 22.2 | 21.0 | 19.4 | 22.5 | 14.3 | 20.3 |
| F | 10.7 | 9.9 | 10.1 | 10.8 | 11.3 | 9.2 | 10.7 | 7.5 | 10.0 | ||
| M+F | 31.5 | 31.4 | 30.5 | 33.0 | 32.3 | 28.6 | 33.2 | 21.8 | 30.3 | ||
| Uterine cervix (C53) | HPV | M | - | - | - | - | - | - | - | - | - |
| F | 7.1 | 8.0 | 7.4 | 6.8 | 7.0 | 6.9 | 7.0 | 6.9 | 7.1 | ||
| M+F | 7.1 | 7.98 | 7.42 | 6.80 | 7.04 | 6.87 | 7.04 | 6.86 | 7.14 | ||
| Vulva (C51) | HPV | M | - | - | - | - | - | - | - | - | - |
| F | 1.0 | 0.9 | 1.1 | 0.9 | 1.1 | 1.1 | 0.8 | 0.9 | 1.0 | ||
| M+F | 1.0 | 0.9 | 1.1 | 0.9 | 1.1 | 1.1 | 0.8 | 0.9 | 1.0 | ||
| Vagina (C52) | HPV | M | - | - | - | - | - | - | - | - | |
| F | 0.5 | 0.3 | 0.2 | 0.5 | 0.5 | 0.5 | 0.4 | 0.3 | 0.4 | ||
| M+F | 0.5 | 0.3 | 0.2 | 0.5 | 0.5 | 0.5 | 0.4 | 0.3 | 0.4 | ||
| Penis (C60) | HPV | M | 1.2 | 0.7 | 1.2 | 1.0 | 1.3 | 1.0 | 1.0 | 1.1 | 1.1 |
| F | - | - | - | - | - | - | - | - | - | ||
| M+F | 1.2 | 0.7 | 1.2 | 1.0 | 1.3 | 1.0 | 1.0 | 1.1 | 1.1 | ||
| Oral cavity (C00-C08) | HPV | M | 0.4 | 0.5 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | 0.4 | 0.4 |
| F | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||
| M+F | 0.6 | 0.6 | 0.5 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | ||
| Oropharynx
(C09-C10. C12-C14) |
HPV | M | 0.8 | 1.3 | 0.4 | 0.8 | 0.8 | 0.8 | 1.8 | 0.6 | 0.9 |
| F | 0.2 | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | ||
| M+F | 1.00 | 1.4 | 0.6 | 1.0 | 1.0 | 1.0 | 2.0 | 0.8 | 1.01 | ||
| Larynx (C32) | HPV | M | 0.8 | 0.5 | 0.7 | 0.8 | 0.8 | 0.6 | 0.8 | 0.6 | 0.7 |
| F | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | ||
| M+F | 0.9 | 0.5 | 0.8 | 0.9 | 0.9 | 0.7 | 0.9 | 0.7 | 0.8 | ||
| Total HPV-associated cancers | HPV | M | 3.2 | 3.0 | 2.6 | 2.9 | 3.3 | 2.8 | 4.1 | 2.6 | 3.0 |
| F | 9.0 | 9.3 | 9.1 | 8.6 | 9.1 | 8.9 | 8.7 | 8.4 | 8.9 | ||
| M+F | 12.2 | 12.3 | 11.7 | 11.5 | 12.4 | 11.7 | 12.8 | 11.0 | 11.9 | ||
| Nasopharynx (C11) | EBV (HHV4) | M | 1.6 | 2.3 | 1.5 | 1.2 | 1.6 | 1.3 | 1.2 | 2.0 | 1.6 |
| F | 0.5 | 0.6 | 0.2 | 0.4 | 0.5 | 0.4 | 0.3 | 0.6 | 0.4 | ||
| M+F | 2.1 | 2.9 | 1.7 | 1.6 | 2.1 | 1.7 | 1.5 | 2.6 | 2.0 | ||
| Burkitt’s lymphoma
(C83.7) |
EBV (HHV4) ± Pf | M | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 |
| F | 0.00 | 0.0 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| M+F | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | ||
| Hodgkin’s lymphoma (C81) | EBV (HHV4) | M | 1.4 | 1.0 | 1.4 | 1.5 | 1.4 | 1.3 | 1.6 | 1.5 | 1.4 |
| F | 1.1 | 1.6 | 1.6 | 1.2 | 1.2 | 1.3 | 1.3 | 1.0 | 1.3 | ||
| M+F | 2.5 | 2.6 | 3.0 | 2.7 | 2.6 | 2.6 | 2.9 | 2.5 | 2.7 | ||
| Total EBV-associated cancers | EBV | M | 3.1 | 3.4 | 3.0 | 2.7 | 3.0 | 2.8 | 2.9 | 3.5 | 3.0 |
| F | 1.5 | 2.2 | 1.8 | 1.6 | 1.7 | 1.7 | 1.6 | 1.6 | 1.7 | ||
| M+F | 4.6 | 5.6 | 4.8 | 4.3 | 4.7 | 4.5 | 4.5 | 5.1 | 4.7 | ||
| Kaposi’s sarcoma
(C46) |
KSHV (HHV8) | M | 3.0 | 3.6 | 3.0 | 2.4 | 2.6 | 4.3 | 3.2 | 2.0 | 3.0 |
| F | 1.3 | 1.0 | 0.8 | 1.1 | 1.3 | 2.2 | 1.4 | 0.7 | 1.2 | ||
| M+F | 4.3 | 4.6 | 3.8 | 3.5 | 3.9 | 6.4 | 4.6 | 2.7 | 4.2 | ||
| Adult T cell lymphoma/leukemia (C91.5) | HTLV1 | M | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 |
| F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| M+F | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | ||
| All the above cancers | M | 50.3 | 61.4 | 53.5 | 50.3 | 50.8 | 47.4 | 51.4 | 38.8 | 50.5 | |
| F | 35.9 | 40.9 | 34.6 | 35.0 | 37.0 | 34.0 | 34.6 | 28.5 | 35.1 | ||
| M+F | 86.2 | 102.3 | 88.1 | 85.4 | 87.8 | 81.4 | 86.0 | 67.3 | 85.6 |
Hp: Helicobacter pylori; HBV: hepatitis B virus; HCV: hepatitis C virus; HDV: hepatitis D virus (Delta agent); HPV: human papillomavirus; EBV: Epstein-Barr virus; HHV4: human herpesvirus 4; Pf: Plasmodium falciparum; KSKV: Kaposi’s sarcoma virus; HHV8: human herpesvirus 8; HTLV: human T-lymphotropic virus; M: males; F: females.
The estimate of the incidence of cancers associated with chronic infections in the male and female population of whole Italy and of individual regions was based on the data available from Italian CRs, which in 2018 covered the 68% of the national population. In particular, the coverage in Northern regions was on an average 71% (Tab. SI), the coverage in Central regions was 25% (Tab. SII), and the coverage in Southern and Insular regions was 65% (Tab. SIII).
ESTIMATES OF THE INCIDENCE OF CANCERS OF INFECTIOUS ORIGIN
The evaluated agents covered DNA viruses, including HPV, HHV4/EBV, HHV8/KSHV, and HBV, either alone or in coinfection/superinfection with hepatitis D virus (HDV); RNA viruses, including HCV and human T-lymphotropic virus-I (HTLV-I); and bacteria, including Hp. The main cancers associated with the above pathogens are shown in Tables SI-SIII. The incidence estimates were selected from the AIOM-AIRTUM publication [19] for the available cancer sites (oropharynx, liver, uterine cervix, vulva, vagina, penis, Hodgkin’s lymphoma, Kaposi’s sarcoma). The remaining sites (non-cardia stomach, MALT, oral cavity, larynx, nasopharynx, Burkitt’s lymphoma, adult T cell lymphoma/leukemia) were estimated by applying the previously described methodology [19], that is: a) preliminary time trend analysis; b) computation of regional incidence rates (with correction for incomplete coverage when necessary); c) projection of incidence rates to 2018; and d) application of rates to the resident population. A preliminary time trend analysis was made by selecting data from CRs with complete information on incidence during the 2003-2014 period. Cancer sites, three major age group (0-49, 50-69, 70+ years) and sex were considered. The average annual percent change (APC) of age-standardized incidence rates was estimate by a Joinpoint analysis [20]. APCs for the most recent period were considered for projections.
REGIONAL INCIDENCE RATES
Site-, sex-, age- and region-specific rates were computed in the calendar period 2010-14 (observed rate). Also site-, sex-, age- and geographic area- specific rates were computed for the same calendar period. Then regional rates were obtained as the weighted average between the observed rate and the area specific rates: Regional rate = α*Observed rate + (1-α)*Area specific rate, where α is the regional coverage. Consequently, in case the proportion of population covered by CRs is 0%, as it was the case for 3 regions (Marche, Abruzzo, and Molise), the regional rate equals the area specific rate, in case such a proportion is 100% the regional rate equals the observed rate. In Lazio and Campania the average rate was computed between observed rates and national pool data, instead of area specific ones.
PROJECTION OF INCIDENCE RATES TO 2018 AND APPLICATION OF RATES TO THE RESIDENT POPULATION
We assumed that incidence observed in the period 2010-2014 represent on an average the incidence of the year 2012 and that the time trend observed in the most recent time interval between 2003 and 2014 does not vary during the subsequent six years. Moreover, in order to take into account the random variability, when the site-, sex- and age- trend variation was not statistically significant (that is 95% confidence intervals include 0), the APC was constrained to 1. Therefore, incidence rates were projected to 2018 by multiplying the site-, sex-, age- and region- specific rates. The site-, sex-, age- and region- specific projected rates were multiplied by the region and age specific resident population in 2018, based on prevision from the National Statistical Institute [21].
ATTRIBUTABLE FRACTIONS
The fraction of each cancer attributable to infectious agents was inferred from data available in the literature. In particular, as detailed in Table I, the data for HBV (± HDV) + HCV-related liver cancer made reference to the Italian population [22]. The data for HPV-related oropharynx cancer were related to the South Europe population and those for EBV-related Hodgkin’s lymphoma were related to the European population [7]. Those related to Burkitt’s lymphoma were related to the USA and European populations [6]. All the other cancers were related to the world population [6, 7, 23-25]. As shown in Table I, the AFs for individual cancers ranged from a minimum of 0.04 for HPV-related oral cancer to a maximum of 1 for KSHV/HHV8-related Kaposi’s sarcoma, HTLV-1-related adult T cell lymphoma/leukemia, and HPV-related uterine cervix cancer. However, it is known that lifestyle, environmental and genetic factors can affect the susceptibility to HPV-related cervical cancer [26].
Tab. I.
Estimates of total incident cancer cases and of incident cancer cases attributable to the main cancer-associated infectious agents in Italy in 2014 and 2018.
| Italy, 2014 [2] | Italy, 2018 [present study] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer site
(ICD-10) |
Infectious
agent |
AF | Gender | Total
incident cases |
Infection-related
cases |
AF | Gender | Total
incident cases |
Infection-related
cases |
| Non-cardia stomach (C16.1-C16.9) | Hp | 0.90
(World [6]) |
M
F M+F |
7,500
5,500 13,000 |
6,750
4,950 11,700 |
0.89
(World [7]) |
M
F M+F |
8,156
5,060 13,216 |
7,259
4,503 11,762 |
| MALT
(C88.4) |
Hp | 0.86
(World [6]) |
M
F M+F |
700
850 1,550 |
602
731 1,333 |
0.74
(World [7]) |
M
F M+F |
169
112 281 |
125
83 208 |
| Total Hp-associated cancers | Hp | M
F M+F |
8,200
6,350 14,550 |
7,352
5,681 13,033 |
M
F M+F |
8,325
5,172 13,497 |
7,384
4,586 11,970 |
||
| Liver
(C22) |
HBV
(± HDV) +HCV |
0.87
(Italy [29]) |
M
F M+F |
8,600
3,800 12,400 |
7,465
3,298 10,763 |
0.68
(Italy [22]) |
M
F M+F |
8,929
3,966 12,895 |
6,071
2,697 8,768 |
| Uterine cervix
(C53) |
HPV | 1.00
(World [6]) |
F | 2,200 | 2,200 | 1.00
(World [23]) |
F | 2,241 | 2,241 |
| Vulva
(C51) |
HPV | 0.40
(World [24]) |
F | 900 | 364 | 0.25
(World [24]) |
F | 1,266 | 317 |
| Vagina
(C52) |
HPV | 0.61
(World [24]) |
F | 200 | 122 | 0.78
(World [23]) |
F | 226 | 176 |
| Penis
(C60) |
HPV | 0.47
(World [24]) |
M | 182 | 85 | 0.50
(World [23]) |
M | 563 | 282 |
| Oral cavity
(C00-C08) |
HPV | 0.24
(World [25]) |
M
F M+F |
2,283
1,524 3,807 |
537
358 895 |
0.04
(World [7]) |
M
F M+F |
3,034
1,721 4,755 |
121
69 190 |
| Oropharynx
(C09-C10, C12-C14) |
HPV | 0.36
(World [25]) |
M
F M+F |
1,214
348 1,562 |
432
124 566 |
0.24
(South Europe [7]) |
M
F M+F |
1,552
407 1,959 |
372
98 470 |
| Larynx
(C32) |
HPV | 0.24
(World [25]) |
M
F M+F |
3,714
335 4,049 |
891
80 971 |
0.05
(World [7]) |
M
F M+F |
4,076
500 4,576 |
204
25 229 |
| Total HPV-associated cancers | HPV | M
F M+F |
7,393
5,507 12,900 |
1,945
3,248 5,203 |
M
F M+F |
9,225
6,561 15,786 |
979
3,126 4,105 |
||
| Nasopharynx
(C11) |
EBV (HHV4) | 0.80 (Low
incidence regions [6]) |
M
F M+F |
315
124 439 |
252
99 351 |
0.80
(Low incidence regions [7]) |
M
F M+F |
452
156 608 |
362
125 487 |
| Burkitt’s lymphoma
(C83.7) |
EBV (HHV4) ±
Pf |
0.20
(USA & Europe [6]) |
M
F M+F |
200
100 300 |
40
20 60 |
0.20
(USA & Europe [6]) |
M
F M+F |
115
44 159 |
23
9 32 |
| Hodgkin’s lymphoma
(C81) |
EBV (HHV4) | NA | NA | 0.36
(Europe [7]) |
M
F M+F |
1,228
1,013 2,241 |
442
365 807 |
||
| Total EBV-associated cancers | EBV (HHV4) | M
F M+F |
515
224 739 |
292
119 411 |
M
F M+F |
1,795
1,213 3,008 |
839
503 1,342 |
||
| Kaposi’s sarcoma
(C46) |
KSHV (HHV8) | 1.00
(World [6]) |
M
F M+F |
600
300 900 |
600
300 900 |
1.00
(World [23]) |
M
F M+F |
646
280 926 |
646
280 926 |
| Adult T cell lymphoma/leukemia (C91.5) | HTLV1 | NA | NA | 1.00
(World [23]) |
M F M+F |
12
2 14 |
12
2 14 |
||
| All the above cancers | M
F M+F |
25,758
16,831 42,589 |
18,034
13,194 31,238 |
M
F M+F |
28,932
17,194 46,126 |
15,894
11,178 27,072 |
|||
Hp: Helicobacter pylori; HBV: hepatitis B virus; HCV: hepatitis C virus; HDV: hepatitis D virus (Delta agent); HPV: human papillomavirus; EBV: Epstein-Barr virus; HHV4: human herpesvirus 4; PF: Plasmodium falciparum; KSKV: Kaposi’s sarcoma virus; HHV8: human herpesvirus 8; HTLV: human T-lymphotropic virus; AF: attributable fraction; M: males; F: females.; NA: not available.
Results
COMPARISON OF THE INCIDENCE OF INFECTION-RELATED CANCERS IN ITALY IN 2014 AND 2018
Table I compares the incidence of infection-related cancers in Italy as estimated in 2014 [2] with the one estimated in 2018 (present study). According to the more recent estimate, there was a 13% decrease in the incidence of all cancers associated with chronic infections, which accounted for a total of 31,238 cases in 2014 and a total of 27,072 cases in 2018. Such a decrease depends both on methodological issues and on variations in the AFs adopted during that period. In particular, in the case of Hp-related cancers the incidence of non-cardia stomach was very similar in 2014 and 2018. In contrast, there was a sharp drop in Hp-related MALT cases especially because of a different methodological approach. As to the liver cancers attributable to HBV and HCV, the 1.23-fold decrease in the incidence from 2014 to 2018 parallels a 1.28-fold decrease in the adopted AFs. The estimates of HPV-related cancers of female genitals were very similar in 2014 and 2018. In contrast, there was an apparent increase in HPV-related penis cancer in 2018 because of a different methodological approach. The estimate of the incidence of HPV-related cancers of the upper aerodigestive tract was much lower in 2018 because in the meantime a considerable drop of the AFs had been proposed. Some variations also occurred for EBV-related cancers because Hodgkin’s lymphoma had not been included in 2014. The estimate of KSHV (HHV8)-related Kaposi’s sarcoma was almost identical in 2014 and 2018, whereas very few cases of HTLV1-related adult T cell lymphoma/leukemia had not been computed in the previous study.
INCIDENCE OF INFECTION-RELATED CANCERS IN THE ITALIAN REGIONS IN 2018
Tables SI, SII and SIII report the resident male and female population in 2018 [27], the percentage of the population covered by accredited CRs, and the estimated incidence of cancers, expressed as cases/100,000 residents, attributable to infectious agents in Northern, Central and Southern Italian regions, respectively. The panels in Figure 1 display maps that show the estimated incidence of cancers of infectious origin in the male and female population of the 20 Italian regions in 2018. As to all cancers of infectious origin (Fig. 1A), there was an evident intergender difference, which among males was characterized by a general trend to a gradient from North to South, with a maximum of 67 cases/100,000 in Veneto and a minimum of 39 cases/100,000 in Sicily. A similar picture was apparent in the female population, but with some regional exceptions. In fact, the maximum incidence was in Umbria (42 cases/100,000) but with close data in a Southern region (Basilicata). On the whole, by combining the two genders, the total number of cases/100,000 residents in Northern, Central and Southern regions were 94.4, 97.2 and 85.6, respectively. A strong difference between males and females was also evident for HBV/HCV-related liver cancer (Fig. 1B), with sharp interregional variations in the male population, where the maximum incidence values were recorded in Northwestern regions, while a more homogeneous regional distribution occurred in the female population. By combining the two genders, the number of HBV/HCV-related liver cancer cases/100,000 residents in Northern, Central and Southern regions were 31.2, 24.4 and 30.3, respectively.
Fig. 1.
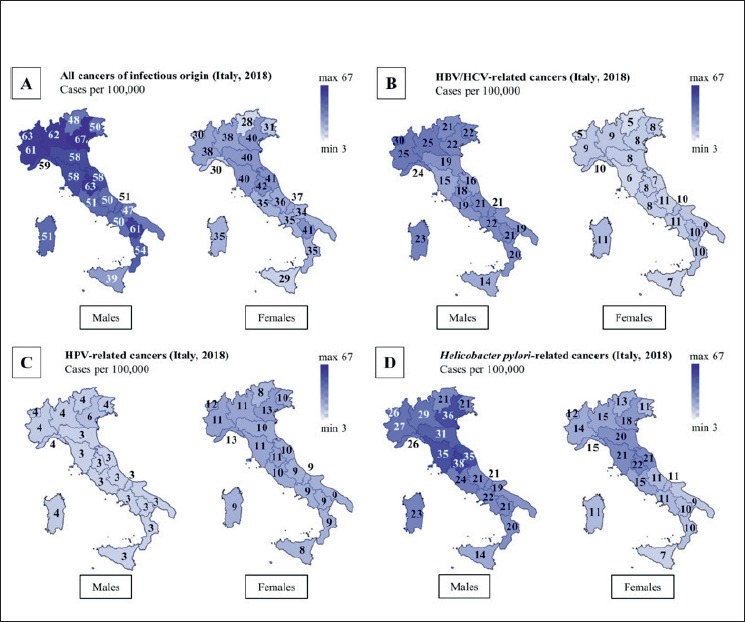
Maps showing the estimated incidence of all cancers of infectious origin (A), of HBV/HCV-related cancers (B), of HPV-related cancers (C), and of Hp-related cancers (D) in the male and female population of the 20 Italian regions in 2018. The incidence is drawn with a continuous tonal gradation, ranging on a scale between a minimum of 3 and a maximum of 67. The number of new cases/100,000 is reported in each region.
The higher incidence of all HPV-related cancers in females, compared to males, is evident from the values reported in Fig. 1C. The interregional differences were not sharp, although there was a regular gradient from North to South, especially in the female population. By combining the two genders, the number of HPV- related cancer cases/100,000 residents in Northern, Central and Southern regions were 15.2, 13.8 and 11.9, respectively. The incidence of Hp-related cancers was considerably higher in males than in females (Fig. 1D). The highest incidence values were clustered in Central Italy regions. By combining the two genders, the number of Hp-related liver cancer cases/100,000 residents in Northern, Central and Southern regions were 42.0, 52.9 and 34.3, respectively.
Table SIV reports the estimates of total incident cancer cases for the whole Italy and by region and area. By cumulating the two genders, the infection-related cancers in Northern, Central, and Southern Italy accounted in 2018 for the 7.2, 7.6, and 7.1% of all cancers, respectively. The national figure was 7.3%, corresponding to 27,381 cases of infection-related cancers out of a total of 373,300 incident cases.
Tab. SIV.
Estimates of total incident cancer cases and proportion of incident cancer cases attributable to the main cancer-associated infections in Italian regions in 2018.
| Region | Gender | Total incident casesa | Infection-related casesb |
|---|---|---|---|
| Northern Italy | |||
| Emilia Romagna | M | 15,550 | 1,270 (8.2%) |
| F | 15,350 | 943 (6.1%) | |
| Friuli Venezia Giulia | M | 4,750 | 399 (8.4%) |
| F | 4,300 | 253 (5.9%) | |
| Liguria | M | 6,150 | 441 (7.2%) |
| F | 5,800 | 336 (5.8%) | |
| Lombardia | M | 33,550 | 3,050 (9.1%) |
| F | 30,650 | 2,001 (6.5%) | |
| Piemonte | M | 16,300 | 1,216 (8.1%) |
| F | 14,550 | 870 (6.0%) | |
| Trentino Alto Adige | M | 3,100 | 257 (8.3%) |
| F | 2,800 | 156 (5.6%) | |
| Valle d’Aosta | M | 450 | 40 (8.9%) |
| F | 350 | 20 (5.7%) | |
| Veneto | M | 16,759 | 1,215 (7.3%) |
| F | 15,100 | 789 (5.2%) | |
| All Northern regions | M | 96,600 | 7,987 (8.3%) |
| F | 88,900 | 5,369 (6.0%) | |
| Central Italy | |||
| Lazio | M | 17,150 | 1,472 (8.6%) |
| F | 16,700 | 1,074 (6.4%) | |
| Marche | M | 5,000 | 437 (8.7%) |
| F | 4,800 | 325 (6.8%) | |
| Toscana | M | 12,900 | 1,053 (8.2%) |
| F | 12,000 | 796 (6.6%) | |
| Umbria | M | 3,050 | 270 (8.9%) |
| F | 2,900 | 202 (7.0%) | |
| All Central regions | M | 38,100 | 3,233 (8.5%) |
| F | 36,400 | 2,397 (6.6%) | |
| Southern Italy and Islands | |||
| Abruzzo | M | 4,200 | 324 (7.7%) |
| F | 3,800 | 245 (6.4%) | |
| Basilicata | M | 1,850 | 174 (9.4%) |
| F | 1,400 | 118 (8.4%) | |
| Calabria | M | 5,850 | 514 (8.8%) |
| F | 4,500 | 346 (7.7%) | |
| Campania | M | 16,350 | 1,420 (8.7%) |
| F | 13,700 | 1,056 (7.7%) | |
| Molise | M | 1,000 | 77 (7.7%) |
| F | 900 | 59 (6.6%) | |
| Puglia | M | 10,850 | 936 (7.9%) |
| F | 10,750 | 716 (6.7%) | |
| Sardegna | M | 5,200 | 417 (8.0%) |
| F | 4,800 | 292 (6.1%) | |
| Sicilia | M | 13,900 | 956 (6.9%) |
| F | 13,250 | 746 (5.6%) | |
| All Southern Italy and Islands | M | 60,200 | 4,418 (8.0%) |
| F | 53,100 | 3,577 (6.7%) | |
| All Italian regions | M | 194,900 | 16,038 (8.2%) |
| F | 178,400 | 11,223 (6.4%) | |
| M+F | 373,300 | 27,381 (7.3%) | |
a From AIOM/AIRTUM [19].
b From Tables SI-SIII.
Discussion
The present study estimated the incidence of the most relevant cancers associated with chronic infections over the whole national territory and, individually, in the 20 Italian regions. Drawing an up-to-date picture of the epidemiological situation is important because of the evolving estimates of PAFs and of our knowledge about the infectious origin of cancer. For instance, Hp role in gastric cancer was established in the nineties, the role of HPV in head and neck cancer has recently been assessed, and recent studies are suggesting a role for other pathogens, such as the involvement of EBV in gastric cancer development, either alone or in coinfection with Hp [28].
The overall national estimates of infection-related cancers in 2018 (present study) were slightly lower than those made in 2014 [2], with a decrease from a total of 31,238 new cases to 27,072 cases (-13%). For some cancers, such as Hp-related non-cardia stomach cancer, HPV-related uterine cervix cancer and KSHV-related Kaposi’s sarcoma, the estimates were virtually overlapping in 2014 and 2018. For other cancers there were variations, mainly in the sense of a decrease, which depended either on technical issues or on the lower AFs adopted recently.
The most paradigmatic example of AF resizing is provided by liver cancer attributable to hepatotropic viruses. In the previous study, we adopted an AF of 0.87, which was based on an estimate made in Italy in 2001 [29]. Already at that time, HCV infections overwhelmed HBV infections in the causation of primary hepatocellular carcinoma in Italy [29]. Later on, the scenario has further evolved, with a progressively increasing contribution of non-viral factors and a decrease of the role of the viral etiology, which went down to 67.6% in the quinquennium 2010-2014, with the contribution of 8.6% by HBV (± HDV), 47.3% by HCV, 1.5% by HBV + HCV, and 10.5% classified as multi-etiology [22]. Indeed, as inferred from 770,000 cases of liver cancer occurring worldwide in 2012, the contribution of these hepatotropic viruses to liver cancer is sharply variable according to the geographical area, HBV causing approximately 2 of 3 liver cancers in developing countries but 1 in 4 cases in more developed countries [30]. Such a trend appears to be even more pronounced in Italy. Screening for HBV and HCV was introduced to reduce transmission among high risk groups. HCV screening has gained increasing diffusion because of the potential of antiviral drugs to eliminate the infection, which led WHO to pose the objective of eradication in 2030 [31].
Hp, HPV, HBV and HCV together accounted for the 92% of all infection-attributable cancers diagnosed both in the world in 2012 [7] and in Italy in 2018. However, there are sharp differences in the overall contribution of the above agents to the incident cancers of infectious origin, which in the world and in Italy was 14.8% and 6.8%, respectively. In particular, Hp, HBV + HCV, and HPV were responsible on a global scale for the 5.4, 4.8, and 5.0% of all incident cancer cases [7], whereas in Italy they were responsible for the 3.2, 2.3, and 1.3% of cases, respectively. Another important difference is that the total numbers of infection-related cancers worldwide was equal in males and females, i.e. 1,100,000 cases each in 2012 [7], whereas in Italy there were in 2018 many more cases in males (16,000) than in females (11,000). This is likely to be ascribed to the fact that the most pronounced difference between the world and the Italian situation is related to HPV-related cancers, which displayed a female/male ratio of 3.2 in Italy and of 8.6 in the world [7]. Such a difference reflects the fact that in Italy there is a broad application both of primary prevention measures (anti-HPV vaccination) and of secondary prevention by means of oncological screenings (PAP test and HPV-DNA test).
There were evident variations in cancer incidence between the North, Centre and South of Italy and among the 20 regions, with some characteristic clusters of cancers according to the geographical localization. Thus, there was a general trend to a North to South downward gradient for all cancers of infectious origin, and especially to a lower incidence in Southern Italy. As to HBV/HCV-related liver cancer cancers, the maximum incidence was detected in Northwestern regions, at least in males, and the lowest incidence was in Central Italy. It should be noted that sub-regional variations may also occur. For instance, within the Campania region in 1998-2002 the incidences of liver cancer recorded by the Naples CR in the male and female populations were 34.8 and 10.2 cases/100,000 residents, respectively, whereas the corresponding values recorded in the nearby Salerno CR were 14.6 and 6.2 cases/100,000 residents, respectively [32, 33]. A moderate North to South downward gradient occurred in the incidence of HPV-related cancers. The incidence of Hp-related cancers was remarkably high in Central Italy regions, which confirms the observation that gained the definition of “gastric cancer belt” in Central Italian regions [34].
A statistically significant correlation (r = 0.893, P < 0.001) was detected between the estimated 2018 incidence data of HBV/HCV and Hp-related cancers in the male and female population of Northern, Central and Southern Italy, as detected in the present study, and the corresponding mortality data for liver and stomach cancers for the period 2010-2014, standardized on the European population [19]. It is exceedingly difficult to relate cancer incidence to the incidence of the related infections in each region, also because these cancers have a long latency time, and the information about the geographical distribution of the corresponding infections in the past is scanty. Furthermore, many of these infections are not clinically apparent and therefore they are just detectable by implementing ad hoc designed screening programs. For instance, HPV may be harbored in a latent state for 20 years or longer before manifesting as a precancerous lesion of the cervix [35], and the latent period between infection with hepatotropic viruses and primary hepatocellular carcinoma is in the order of decades [36].
Assessing the incidence of infection-related cancers has two intrinsic limitations. The first critical point is that we had to extrapolate incidence data to the whole population. The second one is that, for each infection-related cancer, AFs may vary depending on the regional area and may not necessarily be uniform over the whole national territory, depending on several local variables.
Conclusions
In conclusion, our estimates suggest that cancers of infectious origin contributed to the overall burden of cancer in Italy with more than 27,000 new cases, which represent the 7.3% of all incident cancer cases in 2018. This figure is comparable to the fraction of new cancer cases diagnosed in Europe in 2012 that was ascribed to infections, which accounted for the 7.2% of all cancer cases [7].
There were differences highlighting a higher incidence of these cancers in males, with the exception of HPV-related cancers. Regional variations and geographical clusters of cancer cases were evident, depending on the type of cancer and on the gender. Nevertheless, the overall figures were rather similar, the infection-related cancers accounting for the 7.2, 7.6, and 7.1% of all cancers in Northern, Central, and Southern Italy, respectively.
Figures and tables
Acknowledgements
The study was carried out under the auspices of the Cancer Prevention/Oncological Screenings Working Group of the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI).
Funding sources: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
SDF: study conception, design, and writing of the manuscript; SLM: data analysis and interpretation and statistical analysis; EC: interpretation of data and manuscript review; LM: contribution to the design of the study; FB: statistical analysis; FS: interpretation of data and manuscript review; CB: collection of data, estimate of incidence and interpretation.
References
- [1].IARC/WHO. Biological agents. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, IARC 2012;100B:1-441. [PMC free article] [PubMed] [Google Scholar]
- [2].De Flora S, Crocetti E, Bonanni P, Ferro A, Vitale F. Vaccines and Cancer Prevention/Screening Working Groups of the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI). Incidence of infection-associated cancers in Italy and prevention strategies. Epidemiol Prev 2015;39:14-20. [PubMed] [Google Scholar]
- [3].De Flora S, La Maestra S. Epidemiology of cancers of infectious origin and prevention strategies. J Prev Med Hyg 2015;56:E15-20. [PMC free article] [PubMed] [Google Scholar]
- [4].Pisani P, Parkin D., Muñoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev 1997;6:387-400. [PubMed] [Google Scholar]
- [5].Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-44. https://doi.org/10.1002/ijc.21731 10.1002/ijc.21731 [DOI] [PubMed] [Google Scholar]
- [6].de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607-15. https://doi.org/10.1016/S1470-2045(12)70137-7 10.1016/S1470-2045(12)70137-7 [DOI] [PubMed] [Google Scholar]
- [7].Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Healt 2016;4:e609-16. https://doi.org/10.1016/S2214-109X(16)30143-7 10.1016/S2214-109X(16)30143-7 [DOI] [PubMed] [Google Scholar]
- [8].Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981;66:1191-308. [PubMed] [Google Scholar]
- [9].Doll R. Epidemiological evidence of the effects of behaviour and the environment on the risk of human cancer. Recent Results Cancer Res 1998;154:3-21. [DOI] [PubMed] [Google Scholar]
- [10].Doll R, Pet R. Epidemiology of cancer. Warrel DA, Cox TM, Firth J, eds. Oxford Textbook of Medicine. 4th edn. New York, NY: Oxford University Press; 2005;3:193-218. [Google Scholar]
- [11].Boffetta P, Tubiana M, Hill C, Boniol M, Aurengo A, Masse R, Valleron AJ, Monier R, de Thé G, Boyle P, Autier P. The causes of cancer in France. Ann Oncol 2009;20:550-5. https://doi.org/10.1093/annonc/mdn597 10.1093/annonc/mdn597 [DOI] [PubMed] [Google Scholar]
- [12].Wang JB, Jiang Y, Liang H, Li P, Xiao HJ, Ji J, Xiang W, Shi JF, Fan YG, Li L, Wang D, Deng SS, Chen WQ, Wei WQ, Qiao YL, Boffetta P. Attributable causes of cancer in China. Ann Oncol 2012;23:2983-9. https://doi.org/10.1093/annonc/mds139 10.1093/annonc/mds139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Shield KD, Marant Micallef C, de Martel C, Heard I, Megraud F, Plummer M, Vignat J, Bray F, Soerjomataram I. New cancer cases in France in 2015 attributable to infectious agents: a systematic review and meta-analysis. Eur J Epidemiol 2018;33:263-74. https://doi.org/10.1007/s10654-017-0334-z 10.1007/s10654-017-0334-z [DOI] [PubMed] [Google Scholar]
- [14].Xiang W, Shi JF, Li P, Wang JB, Xu LN, Wei WQ, Zhao FH, Qiao YL, Boffetta P. Estimation of cancer cases and deaths attributable to infection in China. Cancer Causes Control 2011;22:1153-61. https://doi.org/10.1007/s10552-011-9791-y 10.1007/s10552-011-9791-y [DOI] [PubMed] [Google Scholar]
- [15].Islami F, Chen W, Yu XQ, Lortet-Tieulent J, Zheng R, Flanders WD, Xia C, Thun MJ, Gapstur SM, Ezzati M, Jemal A. Cancer deaths and cases attributable to lifestyle factors and infections in China, 2013. Ann Oncol 2017;28:2567-74. https://doi.org/10.1093/annonc/mdx342 10.1093/annonc/mdx342 [DOI] [PubMed] [Google Scholar]
- [16].Shin A, Park S, Shin HR, Park EH, Park SK, Oh JK, Lim M, Choi BY, Boniol M, Boffetta P. Population attributable fraction of infection-related cancers in Korea. Ann Oncol 2011;22:1435-42. https://doi.org/10.1093/annonc/mdq592 10.1093/annonc/mdq592 [DOI] [PubMed] [Google Scholar]
- [17].Antonsson A, Wilson LF, Kendall BJ, Bain CJ, Whiteman DC, Neale RE. Cancers in Australia in 2010 attributable to infectious agents. Aust N Z J Public Health 2015;39:446-51. https://doi.org/10.1111/1753-6405.12445 10.1111/1753-6405.12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].ISTAT Demografia in cifre. Available at: www.demo.istat.it. Access on 20/07/2019.
- [19].AIOM (Associazione Italiana di Oncologia Medica) / AIRTUM (Associazione Italiana dei Registri Tumori)/Fondazione Passi. The Numbers of Cancer in Italy 2018 (in Italian). Brescia, Italy: Intermedia Editore; 2018, pp. 1-344. [Google Scholar]
- [20].Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for join-point regression with applications to cancer rates. Statistics in Medicine 2000;19:335-51. [DOI] [PubMed] [Google Scholar]
- [21].ISTAT central Hypothesis. Available at: www.demo.istat.it. Accessed on 20/07/2019.
- [22].Bucci L, Garuti F, Lenzi B, Pecorelli A, Farinati F, Giannini EG, Granito A, Ciccarese F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Borzio F, Sacco R, Cammà C, Virdone R, Marra F, Felder M, Morisco F, Benvegnù L, Gasbarrini A, Svegliati-Baroni G, Foschi FG, Missale G, Masotto A, Nardone G, Colecchia A, Bernardi M, Trevisani F; Italian Liver Cancer (ITA.LI.CA) group. The evolutionary scenario of hepatocellular carcinoma in Italy: an update. Liver Int 2017;37:259-70. https://doi.org/10.1111/liv.13204 10.1111/liv.13204 [DOI] [PubMed] [Google Scholar]
- [23].de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017;141:664-70. https://doi.org/10.1002/ijc.30716 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 2009;124:1626-36. https://doi.org/10.1002/ijc.24116 10.1002/ijc.24116 [DOI] [PubMed] [Google Scholar]
- [25].Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467-75. https://doi.org/10.1158/1055-9965.EPI-04-0551 10.1158/1055-9965.EPI-04-0551 [DOI] [PubMed] [Google Scholar]
- [26].Au WW. Life style, environmental and genetic susceptibility to cervical cancer. Toxicology 2004;198:117-20. https://doi.org/10.1016/j.tox.2004.01.022 10.1016/j.tox.2004.01.022 [DOI] [PubMed] [Google Scholar]
- [27].ISTAT. Available at: https://www.istat.it/it/dati-analisi-e-prodotti/contenuti-interattivi/popolazione-residente (Accessed on 20/07/2019).
- [28].Singh S, Jha HC. Status of Epstein-Barr virus coinfection with Helicobacter pylori in gastric Cancer. J Oncol 2017:3456264 https://doi.org/10.1155/2017/3456264 10.1155/2017/3456264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stroffolini T, Sagnelli E, Mele A, Almasio P. Trends of aetiological factors of hepatocellular carcinoma in Italy. Dig Liver Dis 2005;37:985-6. https://doi.org/10.1016/j.dld.2005.07.002 10.1016/j.dld.2005.07.002 [DOI] [PubMed] [Google Scholar]
- [30].Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer 2018;142:2471-77. https://doi.org/10.1002/ijc.31280 10.1002/ijc.31280 [DOI] [PubMed] [Google Scholar]
- [31].World Health Organization. Combating hepatitis B and C to reach elimination by 2030. Advocacy brief. 2016. Available at https://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/
- [32].Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer incidence in five continents, Vol. IX. IARC Sci Publ 2007 No. 160, Lyon: IARC. [Google Scholar]
- [33].Fusco M, Girardi E, Piselli P, Palombino R, Polesel J, Maione C, Scognamiglio P, Pisanti FA, Solmone M, Di Cicco P, Ippolito G, Franceschi S, Serraino D, Collaborating Study Group. Epidemiology of viral hepatitis infections in an area of southern Italy with high incidence rates of liver cancer. Eur J Cancer 2008;44:847-53. https://doi.org/10.1016/j.ejca.2008.01.025 10.1016/j.ejca.2008.01.025 [DOI] [PubMed] [Google Scholar]
- [34].Cislaghi C, Decarli A, La Vecchia C, Laverda N, Mezzanotte G, Smans Dati, indicatori e mappe di mortalità tumorale: Italia, 1975-1977 (in Italian). Bologna: Pitagora; 1986. [Google Scholar]
- [35].Watson RA. Human papillomavirus: confronting the epidemic - A urologist’s perspective. Rev Urol 2005;7:135-44. [PMC free article] [PubMed] [Google Scholar]
- [36].de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190-200. https://doi.10.1002/hep.27969 10.1002/hep.27969 [DOI] [PMC free article] [PubMed] [Google Scholar]


