Abstract
Objective:
To understand the factors associated with glycemic control after starting insulin in youth with type 2 diabetes following glycemic failure (persistent HbA1c ≥8%) with metformin alone, metformin+rosiglitazone or metformin+lifestyle in the TODAY study.
Methods:
Change in HbA1c after add-on insulin therapy and the factors predictive of glycemic response were evaluated. At one-year post-insulin initiation, 253 youth had a mean of 3.9±1.0 visits since the time of insulin initiation. Participants were divided into three groups according to glycemic control: consistent decrease in HbA1c by ≥0.5%, change < 0.5%, or consistent increase in HbA1c ≥0.5%, at 75% or more of the visits.
Results:
Within one-year post-insulin initiation, 33.2% of participants had a consistent HbA1c decrease of ≥0.5%, 46.2% changed HbA1c <0.5%, and 20.6% had an increase ≥0.5%. At randomization into TODAY and at time of insulin initiation, the three glycemia groups were similar in age, sex, race-ethnicity, pubertal stage, BMI z-score, diabetes duration, and insulin secretion indices. Consistent HbA1c improvement was associated with higher insulin sensitivity (1/fasting insulin) at randomization and at time of failure, higher adiponectin at randomization, and was not associated with indices of β-cell function.
Conclusions:
Response to add-on insulin was highly variable among youth in TODAY. Greater insulin sensitivity and higher adiponectin concentrations at randomization were associated with improved glycemic control after initiation of insulin. Due to limited information on adherence to insulin injections, the roles of adherence to the prescribed insulin regimen or psychosocial factors are unknown.
Keywords: Adolescent, type 2 diabetes mellitus, insulin, glycemic control
INTRODUCTION
TODAY (Treatment Options for type 2 Diabetes in Adolescents and Youth) was a multicenter, randomized clinical trial designed to compare the effect of three treatment regimens to maintain glycemic control in youth with recent-onset type 2 diabetes. Treatment groups (metformin monotherapy [M], metformin plus rosiglitazone [M+R], metformin plus intensive lifestyle intervention [M+L]) were compared by time to primary outcome, defined as HbA1c ≥8% for six months or inability to wean the participant from insulin for metabolic decompensation within 3 months of its initiation.1 The TODAY trial showed high therapeutic failure rates in response to oral antidiabetic treatment with deterioration in glycemic control associated with rapid loss of β-cell function2; 45.6 % of participants reached primary outcome necessitating initiation of insulin therapy with a median time to treatment failure of 11.5 months (range <1 to 60) . Initially, insulin glargine 0.2 units/kg was started and titrated based on self-monitoring of blood glucose. Add-on glargine insulin therapy was considered successful if HbA1c was <7.0% at 6 months.
In adult studies, long-acting insulin glargine initiation after deterioration of glycemic control on oral medications is associated with improved glycemia without increased hypoglycemia or excessive weight gain.3, 4 Studies in youth are limited. We found that mean HbA1c remained elevated at 9% at 1 and 2 years after insulin initiation in TODAY.5 In the Pediatric Diabetes Consortium clinical registry, youth with type 2 diabetes who required insulin therapy continued to have worse diabetes control over time compared with those who maintained glycemic control on metformin alone.6 The factors associated with glycemic control on insulin therapy in youth with type 2 diabetes and poor glycemic control on metformin are not well understood.
The TODAY cohort provides a unique opportunity to examine the effects of insulin therapy in youth with type 2 diabetes. Our previous evaluation of risk factors for glycemic failure in TODAY indicated that higher failure rates were associated with β-cell dysfunction, higher HbA1c at randomization2, and African-American race.7 In addition, the change in total and high molecular weight adiponectin concentrations over the first 6 months post randomization in TODAY was predictive of the responsiveness to oral diabetes therapy.7
The objective of the current study was to determine the factors associated with improved glycemic control or lack thereof following initiation of insulin therapy in the TODAY participants who reached primary outcome, to help guide comprehensive and individualized management of youth type 2 diabetes. We hypothesized that failure to improve glycemic control after add-on insulin would be related to lower β-cell function, younger age at diagnosis, longer duration of diabetes, African-American race, and lower high molecular weight adiponectin.
METHODS
Study design:
Details regarding the TODAY study design and methods have been previously reported.1,8 In brief, 699 youth aged 10–17 were enrolled in the study between July 2004 and February 2009. Participants had type 2 diabetes of <2 years duration, diagnosed based on the American Diabetes Association criteria, a BMI ≥85th percentile for age and sex, with negative pancreatic autoantibodies. After randomization, participants were seen every two months in the first year and quarterly thereafter.
Demographic data were collected at randomization, as were blood samples for HbA1c, total and high molecular weight (HMW) adiponectin, inflammatory markers, and fasting lipids.5,7 Race-ethnicity was determined by self-report and participants were categorized as non-Hispanic Black, non-Hispanic White, Hispanic or ‘Other’ (including Asian, American Indian and Alaska Native). Only the three main racial-ethnic groups are compared due to heterogeneity and small numbers in the ‘Other’ race-ethnicity group. Height, weight and BMI were obtained at randomization and every study visit thereafter. HbA1c was measured at every visit while other laboratory measures (lipids, adiponectin, inflammatory markers) were collected at randomization, 6 months, and annually. Oral glucose tolerance tests (OGTTs), after 10–14 hours of overnight fasting, were performed at randomization, 6 months, 24 months, and annually thereafter. OGTT blood samples were obtained at 0, 30, 60, 90 and 120 minutes for glucose, insulin, and C-peptide.9 The TODAY protocol was approved by the Institutional Review Boards at each participating institution. Parents of children and adolescents provided written informed consent; children and adolescents provided assent.
Post-failure treatment:
Participants who reached glycemic failure defined as: a) HbA1c ≥8.0% over a 6-month period, or b) inability to wean from temporary insulin therapy within 3 months of acute metabolic decompensation were started on insulin glargine 0.2 units/kg in the evening, titrated based on self-monitoring of blood glucose. When participants reached primary outcome, all subjects were “rescued” with glargine but remained in their assigned treatment group. This design was used in order to continue to assess the efficacy of each treatment group on the numerous important secondary outcomes in TODAY. Metformin was continued in the three arms of the study, rosiglitazone was discontinued in the M+R group, and insulin was added. After initiation of insulin therapy, participants and clinicians remained masked to the original treatment assignment, but were unmasked to HbA1c.
Insulin glargine was titrated up to 1.0 U/kg/d (maximum 100 U) until fasting glucose levels measured by self-monitoring reached 70–150 mg/dl. Add-on insulin glargine therapy was considered unsuccessful if (1) 1.0 U/kg/day (to a maximum of 100 units) did not bring fasting blood glucose to target within 1 month or (2) HbA1c > 8% at 3 months or (3) HbA1c > 7% at 6 months. At that point, insulin therapy—including adding rapid, short, or intermediate acting insulin—was provided at the clinician’s discretion. The type of insulin and prescribed dose are presented in Table 2. All other types of insulin, mostly NPH, regular, or other unspecified, were included as ‘Other’. The prescribed insulin dose is reported as the total units per day of any insulin. The protocol included assessments of adherence for oral medications only; adherence to insulin was not assessed. Hypoglycemia events were captured at study visits. Mild hypoglycemia was defined as “episode(s) of low blood sugar” and assessed via self-report at every visit, while severe hypoglycemia was defined as the “presence of low blood sugar episodes that required help from someone else to bring the blood sugar back to normal”.10
Table 2.
Participant characteristics (n=253) according to their glycemic change pattern within one year post-insulin initiation†
| Consistent HbA1c decrease of ≥0.5% (A, n=84) | <0.5% change in HbA1c (B, n=117) | Consistent HbA1c increase of ≥0.5% (C, n=52) | P-value | |
|---|---|---|---|---|
| At randomization | ||||
| Female (%) | 63.1% | 66.7% | 57.7% | P=ns |
| Race-ethnicity (%) | ||||
| Non-Hispanic Black | 40.5% | 38.5% | 40.4% | P=ns |
| Hispanic | 39.3% | 34.2% | 40.4% | |
| Non-Hispanic White | 17.9% | 17.9% | 11.5% | |
| Other | 2.4% | 9.4% | 7.7% | |
| Age (years) | 13.5 ± 2.0 | 14.3 ± 2.0 | 14.0 ± 2.1 | P=ns |
| Diabetes duration (months) | 9.7 ± 6.9 | 8.6 ± 6.5 | 8.5 ± 5.5 | P=ns |
| HbA1c (%) | 6.3 ± 0.7 | 6.5 ± 0.8 | 6.4 ± 0.7 | P=ns |
| BMI (kg/m2) | 34.2 ± 6.8 | 35.2 ± 7.7 | 36.2 ± 8.5 | P=ns |
| BMI Z-score | 2.2 ± 0.5 | 2.2 ± 0.5 | 2.3 ± 0.5 | P=ns |
| Total adiponectin (ng/mL)1 | 5979 ± 2541 | 5198 ± 2239 | 5293 ± 2211 | P=0.040 A vs B |
| HMW adiponectin (ng/mL)1 | 3202 ± 1849 | 2770 ± 1744 | 2738 ± 1710 | P=0.046 A vs B,C |
| 1/ fasting insulin (mL/uU) | 0.052 ± 0.036 | 0.043 ± 0.036 | 0.047 ± 0.041 | P=0.020 A vs B |
| oDI | 0.0025 ± 0.0025 | 0.0020 ± 0.0021 | 0.0023 ± 0.0031 | ns |
| At time of glycemic failure (insulin initiation) | ||||
| Diabetes duration (years) | 2.7 ± 1.2 | 2.3 ± 1.1 | 2.4 ± 1.0 | ns |
| HbA1c (%) | 11.1 ± 2.0 | 9.8 ± 1.7 | 9.1 ± 1.4 | P=0.0001 ALL |
| BMI (kg/m2) | 34.1 ± 7.6 | 36.0 ± 7.8 | 36.5 ± 8.5 | P=ns |
| BMI Z-score | 2.0 ± 0.6 | 2.1 ± 0.6 | 2.2 ± 0.6 | P=ns |
| Total adiponectin (ng/mL)1 | 5737 ± 2452 | 5225 ± 3875 | 5241 ± 2204 | P=ns |
| HMW adiponectin (ng/mL)1 | 3025 ± 1973 | 2770 ± 2536 | 2693 ± 1590 | P=ns |
| 1/ fasting insulin (mL/uU)2 | 0.060 ± 0.043 | 0.042 ± 0.035 | 0.045 ± 0.032 | P=0.002 A vs B,C |
| Change from baseline | 0.012 ± 0.035 | 0.001 ± 0.024 | 0.003 ± 0.028 | (P=0.062 A vs B) |
| oDI2 | 0.0012 ± 0.0013 | 0.0009 ± 0.0008 | 0.0012 ± 0.0011 | P=ns |
| Change from baseline | −0.0011 ± 0.0019 | −0.0008 ± 0.0017 | −0.0008 ± 0.0015 | P=ns |
| At one year ± 3 months post-insulin initiation | ||||
| Diabetes duration (years) | 3.5 ± 1.2 | 3.2 ± 1.1 | 3.3 ± 1.0 | P=ns |
| HbA1c (%) | 9.1 ± 2.4 | 9.9 ± 1.9 | 11.5 ± 1.5 | P<0.0001 ALL |
| BMI (kg/m2) | 35.6 ± 7.2 | 35.9 ± 7.2 | 36.1 ± 8.2 | P=ns |
| BMI Z-score | 2.1 ± 0.5 | 2.1 ± 0.6 | 2.0 ± 0.7 | P=ns |
| Total adiponectin (ng/mL)1 | 5970 ± 2532 | 5344 ± 4233 | 5648 ± 1526 | P=ns |
| HMW adiponectin (ng/mL)1 | 3392 ± 1908 | 3139 ± 3943 | 2817 ± 1135 | P=ns |
| Prescribed insulin dose3 (units/kg/day) | 0.8 ± 0.5 | 0.6 ± 0.4 | 0.7 ± 0.5 | P=ns |
| Insulin dose (total daily units)3 | 73.6 ± 47.9 | 65.2 ± 48.7 | 68.8 ± 46.6 | P=ns |
| Type of insulin | ||||
| Glargine only | 54.2% | 65.6% | 60.0% | P=ns |
| Glargine+Humalog | 26.4% | 15.6% | 8.9% | |
| Other insulin | 11.1% | 12.5% | 26.7% | |
| Any combination | 8.3% | 6.3% | 4.4% |
HMW: high molecular weight; oDI: c-peptide based oral disposition index;
P-value from model adjusted for race/ethnicity;
P-value from model adjusted for HbA1c at time of failure;
P-value from model adjusted for HbA1c at year 1.
Laboratory Assays and Calculations:
All assays were performed at the TODAY central laboratory at the Northwest Lipid Research Laboratory, University of Washington (Seattle, WA).1 HbA1c, C-peptide, and insulin were measured as previously reported.9 Total adiponectin concentrations were measured using latex beads based assay reagents on a Modular P Chemistry analyzer (Roche Hitachi, Inc., Indianapolis, IN) and HMW adiponectin was measured using ELISA (R&D Systems, Inc., Minneapolis, MN).7 Inflammatory markers, including high sensitivity C reactive protein (hs-CRP), homocysteine, plasminogen activator inhibitor-1(PAI-1), and interleukin-6 (IL-6), were determined using standard methods.5
Insulin sensitivity was calculated as [1/fasting insulin (1/IF)], and OGTT-derived measures of insulin secretion C-peptide index (ΔCpep30/ΔG30) was calculated as the ratio of the incremental C-peptide and glucose responses over the first 30 min of the test.2,9 The oral disposition index (oDI), a measure of β-cell function relative to insulin sensitivity, was calculated as the product of insulin sensitivity multiplied by the C-peptide index oDI= 1/IF × ΔC30/ΔG30.2,11
Analysis sample:
Of the 699 TODAY participants, 319 reached the primary outcome. Participants who reached the primary outcome during TODAY but never received add-on insulin (owing to primary outcome right before the end of the study or loss to follow-up after reaching primary outcome) were excluded (n=23). An additional 43 participants with less than two HbA1c measurements available post-insulin initiation were also excluded from analyses. Data collected from female participants (n=47) in the year after a pregnancy were excluded from the analyses. Thus, data were available for 253 out of 319 participants. These participants attended an average of 3.9±1.0 visits (min-max: 2–6) post-insulin initiation. Comparison of the 253 participants included in the analysis with the 66 excluded showed no difference with respect to sex, race-ethnicity, duration of diabetes, randomization BMI or HbA1c.
Data analysis:
Data are presented as mean (±standard deviation) or percent. Paired t-tests were used to compare anthropometrics and laboratory measures for the 253 participants at randomization and time of failure. Participants were classified into 3 groups of glycemic change pattern within the first year post-insulin initiation: group (A) had consistent decrease in HbA1c by ≥0.5% at 75% or more of the visits during the first year on insulin after glycemic failure; group (B) had HbA1c change <0.5%; and group (C) had consistent increases in HbA1c ≥0.5% at 75% or more of the visits during the first year on insulin. Chi-square ANOVA tests were used for comparison among the three groups at randomization, time of failure, and at one year ±3 months post-insulin initiation. Differences among the groups in the change from baseline (randomization), defined as the value at time of insulin initiation minus randomization value, for select variables were also evaluated. Unadjusted p-values from models are presented unless otherwise indicated. A generalized linear mixed model was used to compare the HbA1c slopes among the three groups before and post-initiation of insulin. The model consisted of regressing HbA1c as a function of time in the study relative to insulin start, thereby obtaining one intercept at ‘time 0’ (insulin initiation) and two slopes (one before and one after ‘time 0’) for each participant. The intercept and the two times relative to ‘time 0’ were included in the model as random effects. Testing was performed using the log-transformed value due to departure from normality for the following variables: total adiponectin, HMW adiponectin, and all the inflammatory markers and OGTT-derived outcomes. All analyses were considered exploratory with p-values <0.05 considered statistically significant.
RESULTS
Demographic and Metabolic Characteristics:
The 253 participants who reached the primary outcome and were started on insulin were 64% female, with mean age of 14.0 years at the time of randomization (Table 1). Mean HbA1c was 10.1% at insulin initiation, 20.6±12.6 months post randomization (29.5±13.6 months after diagnosis of diabetes). At the time of primary outcome, participants had slightly higher BMI but lower BMI Z-score, and no significant difference in insulin sensitivity (1/fasting insulin) or in adiponectin concentrations compared with values at randomization. Participants had higher HbA1c (10.1±1.9 vs. 6.4±0.8, p <0.001) and worsening measures of β-cell function (i.e., lower oDI; 0.0010±0.0011 vs. 0.0022±0.0025, p <0.0001) at the time of glycemic failure compared with randomization. Inflammatory markers, apart from IL-6, were significantly worse at the time of glycemic failure compared with randomization.
Table 1.
Participant characteristics (n= 253) at randomization and time of failure†
|
|
At randomization | At time of failure | P-value |
|---|---|---|---|
| Female (%) | 63.6% | -- | -- |
| Race-ethnicity (%) | |||
| Non-Hispanic Black | 39.5% | -- | -- |
| Hispanic | 37.2% | -- | |
| Non-Hispanic White | 16.6% | -- | |
| Other | 6.7% | -- | |
| Pubertal stage (%) | |||
| Stages 4–5 | 90.1% | 97.3% | -- |
| Stages 1–3 | 9.9% | 2.6% | |
| Diabetes duration (months) | 8.9 ± 6.4 | 29.5 ± 13.6 | P<0.0001 |
| Age (years) | 14.0 ± 2.1 | 15.6 ± 2.3 | P<0.0001 |
| HbA1c (%) | 6.4 ± 0.8 | 10.1 ± 1.9 | P<0.0001 |
| Weight (kg) | 95.9 ± 26.0 | 99.4 ± 27.0 | P<0.0001 |
| BMI (kg/m2) | 35.1 ± 7.6 | 35.4 ± 7.9 | P=0.002 |
| BMI Z-score | 2.2 ± 0.5 | 2.1 ± 0.6 | P<0.0001 |
| Total adiponectin (ng/mL) | 5478 ± 2356 | 5390 ± 3163 | P=ns |
| HMW adiponectin (ng/mL) | 2907 ± 1777 | 2834 ± 2187 | P=ns |
| 1/ fasting insulin (mL/uU) | 0.047 ± 0.037 | 0.048 ± 0.038 | P=ns |
| C-peptide index (ng/mL per mg/dL) | 0.0540 ± 0.0588 | 0.0284 ± 0.0283 | P<0.0001 |
| oDI | 0.0022 ± 0.0025 | 0.0011 ± 0.0010 | P<0.0001 |
| Hs-CRP (mg/dL) | 0.43 ± 0.96 | 0.49 ± 0.57 | P=0.004 |
| Homocysteine (μmol/L) | 6.09 ± 1.88 | 6.47 ± 1.95 | P=0.0001 |
| PAI-1 (ng/mL) | 21.8 ± 15.5 | 28.4 ± 20.9 | P<0.0001 |
| IL-6 (pg/mL) | 2.32 ± 1.89 | 2.49 ± 1.89 | P=ns |
HMW: high molecular weight; oDI: c-peptide based oral disposition index; hs-CRP: high sensitivity C reactive protein; PAI-1: plasminogen activator inhibitor-1; IL-6: interleukin-6; P-value from paired t-test comparing the characteristics of participants at randomization and time of failure.
Characteristics of participants according to their glycemic change pattern after one year of insulin therapy:
In the 253 participants who reached primary outcome, 84 (33.2%) had a consistent HbA1c decrease ≥0.5%, 117 (46.2%) had change <0.5%, and 52 (20.6%) had a consistent HbA1c increase ≥0.5%. Post-insulin initiation, HbA1c fell slightly from the 10.1% at the time of failure to 9.7% at 6 months and 10.0% at one year post-insulin initiation. At 12 months post-insulin start, 6.6% of all participants had HbA1c <7%, 12.4% had a value between 7–8%, and 81.0% had HbA1c >8%. In participants with a consistent decrease in HbA1c, 13.0% had HbA1c <7%, 25.3% had HbA1c 7–8%, and 61.3% had HbA1c >8%. In participants with a change in HbA1c < 0.5%, 5.0% had HbA1c <7%, 9.0% had HbA1c 7–8%, and 86.0% had HbA1c >8%. All subjects with consistent increase in HbA1c had HbA1c >8% at 12 months post-insulin start.
At randomization and at time of glycemic failure, the three groups did not differ in age, sex, race-ethnicity, BMI, diabetes duration, or insulin secretion indices (Table 2). The group with consistently lower (improved) HbA1c after starting insulin therapy had higher insulin sensitivity (as reflected by 1/fasting insulin) at randomization and at time of failure, and higher total and HMW adiponectin at randomization compared with the other two groups. The group with greater than 0.5% decrease in HbA1c was prescribed a larger mean total daily insulin dose compared with the other two groups (0.8 units/kg/day vs. 0.6 [<0.5% change in HbA1c] and 0.7 [consistent HbA1c increase ≥0.5%] units/kg/day; p=ns) but the difference was not statistically significant. There was no difference in treatment randomization among the three response groups. The proportion of participants randomized to receive rosiglitazone at study entry was similar among the three response groups: 27.4% of n=84 with consistent HbA1c decrease ≥0.5% vs. 26.5% of n=117 with change <0.5% vs. 34.6% of n=52 with consistent HbA1c increase ≥0.5%. There were no differences among the groups in the change from baseline (randomization to insulin initiation) for BMI, BMI Z-score, and total and HMW adiponectin (data not shown).
Overall, the frequency of mild hypoglycemia was 5.4% and 7.8% at 6 and 12 months after add-on insulin therapy, respectively, with no significant difference among the three groups at month 6 (p=ns). However, a higher proportion of mild hypoglycemia was found in the consistent HbA1c decrease group compared with the no change and consistent HbA1c increase groups at one year post add-on insulin (16.2% vs 2.1% and 6.7% respectively, p=0.0029). There were 5 participants with severe hypoglycemia events (loss of consciousness, seizure) across the entire TODAY study period; one of those five participants reached glycemic failure and received add-on insulin therapy after experiencing the event.
Time to glycemic failure and HbA1c trajectory before and after insulin initiation:
The mean (±standard deviation) time to glycemic failure was not significantly different among the three groups: 14.5±11.9 months in those with consistent HbA1c decrease ≥0.5%, 11.8±11.5 months in those with <0.5% change, and 13.3±11.5 in those with consistent ≥0.5% increase in HbA1c. Overall, there was no significant difference in the rate of increase of HbA1c in the year prior to initiation of insulin among the three groups (Figure 1). However, in the 6 months prior to insulin start, the rates of increase did differ (p=0.0008), with the consistent HbA1c decrease group slope increasing more rapidly than the < 0.5% change group (p=0.0009) and the consistent increase group (p=0.0015).
Figure 1.
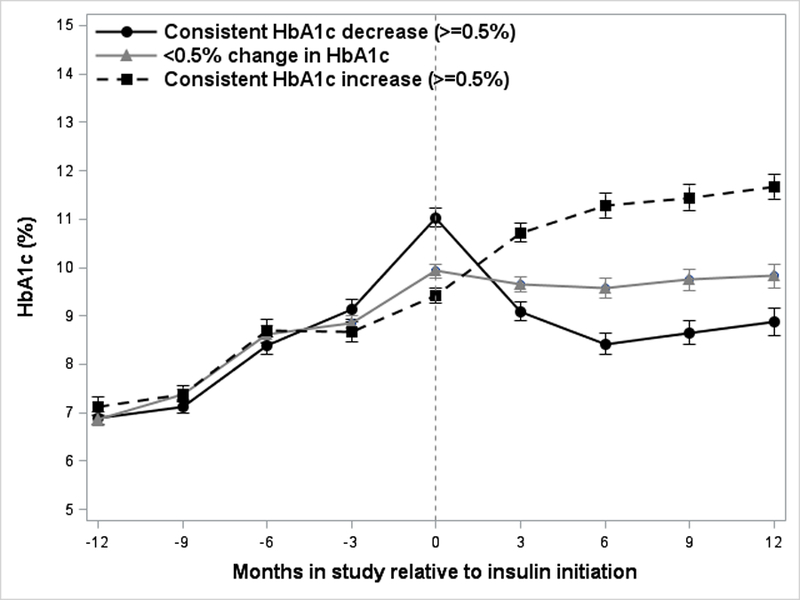
Trajectory of HbA1c before and after initiation of insulin therapy in the 3 glycemic response groups showing consistent HbA1c decrease (≥0.5%), <0.5% change in HbA1c, and consistent HbA1c increase (≥0.5%) within 1 year of insulin initiation
DISCUSSION
Our findings illustrate that the response to add-on insulin therapy in youth with type 2 diabetes after failure of oral antidiabetic agents, is highly variable. Only 33% of TODAY youth had a consistent reduction of HbA1c by ≥0.5% by 6 months after add-on insulin therapy. Among the physiologic variables that may affect responsiveness to insulin therapy, greater insulin sensitivity and higher adiponectin levels at randomization were associated with improved glycemic control after insulin initiation.
Several adult studies assessing glargine as a once-a-day basal insulin add-on therapy to oral glucose-lowering drugs demonstrated clinically important improvements in glycemic control, with HbA1c reduction of 1 to 2%.12,13 In a randomized treat-to-target study, addition of either NPH or glargine over 24 weeks resulted in HbA1c <7% in 60% of patients with 25% less frequent hypoglycemia with glargine use.12 Similarly, in a parallel group study of poorly-controlled adults with type 2 diabetes, glargine or NPH therapy resulted in improvement in glycemia with decrease mean HbA1c from ~9% to 7% at 36 months.13 Greater weight gain was observed in those who reached target HbA1c <7%.13 In treat-to-target trials with once-a-day glargine or detemir insulin, HbA1c typically decreased by approximately 1.5% within the first 12 weeks, sustained up to 52 weeks, with 52% of participants achieving HbA1c ≤7.0%.14 In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, the standard (vs. intensive treatment) arm targeted an HbA1c level of 7.0–7.9%. In that trial, ~70% of participants in the standard treatment arm achieved target HbA1c of <8%.15 These data in adults contrast with only 33% of our youth achieving consistent 0.5% reduction in HbA1c over the 12 months after insulin initiation, and only 19% had HbA1c less than or equal to 8%, with mean HbA1c levels remaining greater than 8%.
There were no differences in the response to insulin in relation to age, sex, race, BMI or duration of diabetes. This is in contrast to our previous reports of sex and race differences in response to oral hypoglycemic agents in TODAY participants.7,8 These findings are also somewhat different than in adult studies. In the ACCORD study, patients who were younger, African-American, female sex, with higher BMI, longer duration of diabetes, higher randomization HbA1c, who were on insulin, and had a history of cardiovascular disease were less likely to achieve target HbA1c (≤8%) after 12 months in the standard arm of the study.15 A report by Riddle et al. that included analyses from participants in 12 studies using insulin glargine showed male sex, White race, shorter duration of diabetes, lower randomization HbA1c (p < 0.0001), and metformin use and not using sulfonylurea at randomization were significantly associated with reaching target HbA1c of 7%, after adjustment for covariates.16 On the other hand, in the UKPDS, no race-related differences were reported in glycemic response to diabetes treatments that included insulin.17
The lower responsiveness to insulin in our study vs. adult data could be related to the higher HbA1c levels at time of insulin initiation in our participants. In the report by Riddle et al., the randomization HbA1c category was independently associated with reaching HbA1c target, with 75% of those with randomization HbA1c <8.0%, vs. 38% with randomization HbA1c ≥9.0% achieving HbA1c ≤7.0% by 24 weeks of intervention.16 This has also been noted in studies of adults with type 2 diabetes treated with insulin18 and in adults and children with intensification of therapeutic regimen in type 1 diabetes19,20. Unlike the adult studies, in our youth, the group with the highest HbA1c at initiation of insulin had the greatest response to insulin. The effect of insulin has been attributed to improvement of glucotoxicity and lipotoxicity21. Given that the group with consistent decrease of HbA1c also had the most rapid increase in HbA1c acutely (6 months) prior to insulin start, the decreasing gluco/lipotoxicity at the liver/muscle tissue level could potentially have led to the more significant improvement in glycemia in this group with greater insulin sensitivity at randomization and at insulin start.
Nevertheless, despite greater sensitivity to insulin in this group that may have contributed to greater improvement in HbA1c compared with the other two groups, the fact that they started with a higher HbA1c translated to less improvement in HbA1c than that reported in adults.16,18 A greater endogenous insulin production could be hypothesized to contribute to the greater improvement in HbA1c. However, insulin secretion indices were not different among the three groups. This is in contrast to glycemic control on oral agents which was related to residual β-cell function and not insulin sensitivity.9 Also, β-cell reserve and HbA1c at randomization were independent predictors of glycemic durability on randomized therapy prior to failure.22 However, at glycemic failure, β-cell dysfunction is evident and does not affect response to insulin. Consistent with our findings, in an adult study, randomization C-peptide and glycemia were not different between patients who achieved good glycemic control (HbA1c <7.0%) on add-on insulin therapy compared with those who did not.13 In the recent RISE study23, insulin therapy in early youth-onset type 2 diabetes did not rescue β-cell function, which continued to deteriorate over time. Therefore, it is unlikely that insulin therapy in diabetes of longer duration than the RISE study, as in this TODAY group, will have a major effect in improving β-cell function.
On the other hand, insulin sensitivity index and adiponectin concentrations were higher in the group with consistent improvement in HbA1c ≥0.5%. Insulin sensitivity improvement was noted in TODAY in association with rosiglitazone therapy in the first 6 months of the trial and was related to the more durable glycemic control observed in the metformin+ rosiglitazone arm compared with the other two arms of the study.2 Nonetheless, the proportion of individuals who were randomized to the metformin+rosiglitazone arm was similar in the 3 response groups. Therefore, it is unlikely that the assignment to rosiglitazone contributed to the observed differences in response to insulin. Adiponectin is known to be a biomarker of insulin sensitivity in youth24, as well as in adults. In TODAY, adiponectin concentrations were predictive of glycemic efficacy on the randomized treatment prior to failure7, consistent with adult data.25 Lower increment in HMW adiponectin levels in non-Hispanic Blacks compared with the other racial groups seemed to explain the racial differences observed in TODAY with higher failure rates in non-Hispanic Black youth.7 It is interesting that these physiologic variables of insulin sensitivity and higher total and HMW adiponectin were related to a better response to insulin in youth with type 2 diabetes at similar duration of diabetes and similar degree of obesity, independent of race or randomized treatment arm. This suggests that adiponectin may be useful as a prognostic biomarker of response to insulin therapy in individuals with type 2 diabetes.
Given this novel observation of a relationship of insulin sensitivity to response to insulin therapy, it could be advanced that the lower insulin sensitivity in youth compared with adults26,27 may contribute to the lower degree of responsiveness to insulin therapy in youth compared with adults with type 2 diabetes. Similar insulin doses (~0.7 units/kg/day) resulted in significant reduction in HbA1c in adult studies.13 Higher insulin doses are thus likely to be needed in adolescents compared with adults. This is supported by the borderline higher insulin dose in the group with consistent HbA1c improvement in our study. On the other hand, adherence to the diabetes care regimen is likely the most important determinant of response to therapy and is difficult to accurately assess with the use of insulin injections. Adherence is known to be a challenge in the adolescent age group including in type 1 diabetes with higher HbA1c observed in adolescents compared with other age groups regardless of the modality of treatment.28
We did not observe significant weight gain with insulin therapy in youth with type 2 diabetes. This may be related to overall suboptimal glycemic control. The rates of mild hypoglycemia were low as reported before29, although higher in the group with consistent HbA1c improvement, suggesting possible greater adherence to therapy in that group. The group with glycemic failure had significantly higher inflammatory markers at the time of failure compared with randomization. In our previous analyses, we reported that rates of dyslipidemia and inflammatory markers were related to change in HbA1c and rose before and after start of insulin therapy, raising concern for premature atherosclerosis in this population.5
Overall, this study provides novel information about the clinical course of youth with type 2 diabetes after add-on insulin therapy following failure of oral antidiabetic treatment administered in a randomized controlled trial, and the characteristics of youth who improve glycemic control vs. those who do not. A strength is the long duration of follow-up, with data available longitudinally up to 12 months after insulin start and evaluation of trajectory of change in HbA1c. Some limitations need to be acknowledged. The trial was not designed to test an intensive insulin dosing regimen. Insulin therapy was individualized according to the treating physician discretion rather than a target HbA1c and insulin dose cannot be verified. Assessment of adherence to insulin regimen and psychosocial variables related to insulin use were not measured. Medication and lifestyle adherence may explain in part the differences in our outcomes compared to adult trials.12
Future studies are needed to test more intensive insulin regimens, perhaps initiated earlier in the disease course. Adjunct therapies are now available and several pediatric phase 3 trials are ongoing. In addition, measures to monitor and encourage adherence need to be evaluated in this population.
In summary, there is significant variability in the response to add-on insulin therapy in TODAY youth. Among the physiologic variables, higher insulin sensitivity and adiponectin levels and not residual beta cell function, BMI, sex, ethnicity or diabetes duration, appear to be associated with improved glycemic control on insulin therapy. Due to limited information on adherence to insulin injections, it remains unknown to what extent adherence to the prescribed insulin regimen and psychosocial factors played a role. Our findings support the need for more aggressive therapy in youth with type 2 diabetes. Initiation of insulin therapy at HbA1c lower than 8% may need to be considered if glycemic control is deteriorating on oral agents.
Supplementary Material
ACKNOWLEDGMENTS
A complete list of participants in the TODAY Study Group is presented in the Supplementary Material.
The TODAY Study Group thanks the following companies for their donations: Becton, Dickinson and Company; Bristol-Myers Squibb; Eli Lilly and Company; GlaxoSmithKline; LifeScan, Inc.; Pfizer; Sanofi Aventis. We also gratefully acknowledge the participation and guidance of the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service; the opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the respective Tribes and the Indian Health Service.
Materials developed and used for the TODAY standard diabetes education program and the intensive lifestyle intervention program are available to the public at https://today.bsc.gwu.edu/.
Funding. The study was funded by National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health which did not have any input on the study design or data analyses. This work was completed with funding grant numbers U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, U01-DK61254, and T32-DK063687; from the National Center for Research Resources General Clinical Research Centers Program grant numbers M01-RR00036 (Washington University School of Medicine), M01-RR00043-45 (Children’s Hospital Los Angeles), M01-RR00069 (University of Colorado Denver), M01-RR00084 (Children’s Hospital of Pittsburgh), M01-RR01066 (Massachusetts General Hospital), M01-RR00125 (Yale University), and M01-RR14467 (University of Oklahoma Health Sciences Center); and from the NCRR Clinical and Translational Science Awards grant numbers UL1-RR024134 (Children’s Hospital of Philadelphia), UL1-RR024139 (Yale University), UL1-RR024153 (Children’s Hospital of Pittsburgh), UL1-RR024989 (Case Western Reserve University), UL1-RR024992 (Washington University in St Louis), UL1-RR025758 (Massachusetts General Hospital), and UL1-RR025780 (University of Colorado Denver).
Footnotes
Conflict of Interest. No potential conflicts of interest relevant to this article were reported.
Trial registration: ClinicalTrials.gov
REFERENCES
- 1.TODAY Study Group, Zeitler P, Epstein L, Grey M, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007. April;8(2):74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013. June;36(6):1749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddle MC. Timely initiation of basal insulin. Am J Med. 2004. February 2;116 Suppl 3A:3S–9S. Review. [DOI] [PubMed] [Google Scholar]
- 4.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR; U.K. Prospective Diabetes Study Group. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care. 2002. February;25(2):330–6. Erratum in: Diabetes Care 2002 Jul;25(7):1268. [DOI] [PubMed] [Google Scholar]
- 5.Levitt Katz LE, Bacha F, Gidding SS, et al. ; TODAY Study Group. Lipid Profiles, Inflammatory Markers, and Insulin Therapy in Youth with Type 2 Diabetes. J Pediatr. 2018. May;196:208–216.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacha F, Cheng P, Gal RL, et al. ; for the Pediatric Diabetes Consortium. Initial Presentation of Type 2 Diabetes in Adolescents Predicts Durability of Successful Treatment with Metformin Monotherapy: Insights from the Pediatric Diabetes Consortium T2D Registry. Horm Res Paediatr. 2018;89(1):47–55. [DOI] [PubMed] [Google Scholar]
- 7.Arslanian S, El Ghormli L, Bacha F, et al. ; TODAY Study Group. Adiponectin, Insulin Sensitivity, β-Cell Function, and Racial/Ethnic Disparity in Treatment Failure Rates in TODAY. Diabetes Care. 2017. January;40(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TODAY Study Group; Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012. June 14;366(24):2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacha F, Pyle L, Nadeau K, et al. ; TODAY Study Group. Determinants of glycemic control in youth with type 2 diabetes at randomization in the TODAY study. Pediatr Diabetes. 2012. August;13(5):376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaquist ER, Anderson J, Childs B, et al. ; American Diabetes Association; Endocrine Society. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. J Clin Endocrinol Metab. 2013. May;98(5):1845–59. [DOI] [PubMed] [Google Scholar]
- 11.Sjaarda LA, Michaliszyn SF, Lee S, et al. HbA(1c) diagnostic categories and β-cell function relative to insulin sensitivity in overweight/obese adolescents. Diabetes Care. 2012. December;35(12):2559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003. November;26(11):3080–6. [DOI] [PubMed] [Google Scholar]
- 13.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia. 2006. March;49(3):442–51. [DOI] [PubMed] [Google Scholar]
- 14.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naïve people with type 2 diabetes. Diabetologia. 2008. March;51(3):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake TC, Hsu FC, Hire D, et al. Factors associated with failure to achieve a glycated haemoglobin target of <8.0% in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Obes Metab. 2016. January;18(1):92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddle MC, Vlajnic A, Zhou R, Rosenstock J. Baseline HbA1c predicts attainment of 7.0% HbA1c target with structured titration of insulin glargine in type 2 diabetes: a patient-level analysis of 12 studies. Diabetes Obes Metab. 2013. September;15(9):819–25. [DOI] [PubMed] [Google Scholar]
- 17.Davis TM, Cull CA, Holman RR; U.K. Prospective Diabetes Study (UKPDS) Group. Relationship between ethnicity and glycemic control, lipid profiles, and blood pressure during the first 9 years of type 2 diabetes: U.K. Prospective Diabetes Study (UKPDS 55). Diabetes Care. 2001. July;24(7):1167–74. [DOI] [PubMed] [Google Scholar]
- 18.Cummings MH, Cao D, Hadjiyianni I, Ilag LL, Tan MH. Characteristics of insulin-Naïve people with type 2 diabetes who successfully respond to insulin glargine U100 after 24 weeks of treatment: a meta-analysis of individual participant data from 3 randomized clinical trials. Clin Diabetes Endocrinol. 2018. May 8;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004. November;27(11):2590–6. [DOI] [PubMed] [Google Scholar]
- 20.Chase HP, Arslanian S, White NH, Tamborlane WV. Insulin glargine versus intermediate-acting insulin as the basal component of multiple daily injection regimens for adolescents with type 1 diabetes mellitus. J Pediatr. 2008. October;153(4):547–53. [DOI] [PubMed] [Google Scholar]
- 21.Andrews WJ, Vasquez B, Nagulesparan M, et al. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984. July;33(7):634–42. [DOI] [PubMed] [Google Scholar]
- 22.Zeitler P, Hirst K, Copeland KC, et al. ; TODAY Study Group. HbA1c After a Short Period of Monotherapy With Metformin Identifies Durable Glycemic Control Among Adolescents With Type 2 Diabetes. Diabetes Care. 2015. December;38(12):2285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.RISE Consortium. Impact of Insulin and Metformin Versus Metformin Alone on β-Cell Function in Youth With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes. Diabetes Care. 2018. August;41(8):1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004. February;27(2):547–52. [DOI] [PubMed] [Google Scholar]
- 25.Wagner JA, Wright EC, Ennis MM, et al. Utility of adiponectin as a biomarker predictive of glycemic efficacy is demonstrated by collaborative pooling of data from clinical trials conducted by multiple sponsors. Clin Pharmacol Ther. 2009. December;86(6):619–25. [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: Who is worse off? Pediatr Diabetes. 2018. March;19(2):205–211. [DOI] [PubMed] [Google Scholar]
- 27.RISE Consortium. Metabolic Contrasts Between Youth and Adults With Impaired Glucose Tolerance or Recently Diagnosed Type 2 Diabetes: I. Observations Using the Hyperglycemic Clamp. Diabetes Care. 2018. August;41(8):1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paris CA, Imperatore G, Klingensmith G, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009. August;155(2):183–9.e1. [DOI] [PubMed] [Google Scholar]
- 29.Imperatore G, Boyle JP, Thompson TJ, et al. ; SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012. December;35(12):2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


